Abstract
Bone mass and quality in humans are controlled by numerous genetic and environmental factors that are not fully understood. Increasing evidence has indicated that maternal metabolic dysregulation impairs multiple physiological processes in the adult offspring, but a similar effect on bone health is yet to be established. Here, we have analyzed the bones of first-generation offspring from murine dams that present metabolic syndrome due to a high-fat and high-sugar (HF/HS) diet. Micro-CT analyses show that the long bones of HF/HS offspring possess lower cortical bone mass and weaker mechanical strength than normal, even though the trabecular bone is not affected. Histomorphometry and serum biochemistry indicate that both bone formation and resorption are diminished in the HF/HS offspring. In vitro, both osteoblast and osteoclast progenitors from the HF/HS offspring are deficient in differentiation, likely due to impairment of mitochondrial respiration. The study, therefore, identifies maternal metabolic health as an important environmental factor influencing bone volume and strength.
Keywords: obesity, bone metabolism, maternal effect, mitochondria, osteoblast, osteoclast
Fragility fractures caused by osteoporosis and osteopenia represent a significant health burden among the senior population, resulting in increased mortality and health care costs [1]. Current osteoporosis therapies include both antiresorptives such as bisphosphonates (eg, zoledronic acid) and denosumab (humanized monoclonal antibody against RANKL) and the bone anabolic agents teriparatide and abaloparatide targeting parathyroid hormone-related protein receptor type 1 signaling [2]. Although the current therapies are effective in reducing vertebral fractures, they are less so with nonvertebral fractures, which account for 80% of all fractures in the community [3]. For example, zoledronic acid reduced clinical vertebral fracture by 77% but reduced nonvertebral fracture by 25% in postmenopausal women with osteoporosis [4]. Denosumab treatment for 3 years resulted in a 68% decrease in vertebral fracture but a decrease of 20% in nonvertebral fractures [5]. Similarly, teriparatide or abaloparatide was more effective against vertebral versus nonvertebral fractures [6, 7]. Romosozumab, a humanized monoclonal antibody against sclerostin (an endogenous Wnt inhibitor) recently approved by the Food and Drug Administration, decreased vertebral versus nonvertebral fractures by 73% or 24%, respectively, after 12 months of use [8]. Thus, there remains a great unmet clinical need for therapies effectively reducing nonvertebral fragility fracture. Because cortical bone strength is a main determinant of nonvertebral fractures, it is of great clinical relevance to understand the genetic and environmental factors controlling cortical bone mass and quality.
Mounting evidence has implicated maternal well-being in the health of the offspring. Based on epidemiological evidence, David Barker first proposed adverse fetal environment as a cause for adult chronic diseases, a hypothesis commonly known as “developmental origins of health and disease [9-11].” Later studies of adult children from mothers exposed to extreme famine during the Nazi occupation of the Netherlands revealed multiple transgenerational health consequences including increased obesity, cardiovascular diseases, and reduced cognitive function in late adulthood [12, 13]. Remarkably, 6 decades later when compared with their unexposed, sex-matched siblings, the individuals prenatally exposed to the famine exhibited less deoxyribonucleic acid (DNA) methylation of the imprinted insulin-like growth factor 2, indicating that intrauterine conditions can cause life-long epigenetic changes in humans [14]. A similar link between intrauterine food restriction and adult obesity has also been documented in rats [15]. Conversely, maternal obesity increased obesity in the offspring in rodents [16, 17]. In addition, maternal exposure to high-fat diet leads to hyperproliferation in prostates and also increases mammary cancer risk in adult offspring [18, 19]. At least a part of the adverse maternal effect may be explained by the inferior oocyte quality, including abnormal mitochondria associated with high-fat diet–induced obese females [20, 21]. More recently, the aberrant oocyte mitochondria were shown to persist for at least 3 generations in the mouse, resulting in transmission of metabolic dysfunction through the female germline [22]. Thus, both nuclear epigenetic modifications and mitochondrial changes may mediate maternal effects on the offspring.
Maternal diet exposure has also been implicated in offspring bone health. A prospective study of 53922 mother-child pairs from the Danish National Birth Cohort indicates that consumption of Western diet (high intake of fat, meat, and potatoes and low intake of fruits and vegetables) in midpregnancy is associated with a significant increase in forearm fractures before age 16 years [23]. Similarly, in rodents, maternal high-fat diet has been shown to impair bone formation in both embryos and adult offspring [24-26]. The underlying mechanisms, however, are not fully understood.
In this study, we studied the bones of mice that were born to dams fed an HF/HS diet but were weaned onto regular chow diet. The HF/HS progenies exhibited a notable suppression in both bone formation and resorption, resulting in a net decrease in cortical bone mass and strength. In vitro studies showed that mitochondrial activities were compromised in the progenitors of either osteoblasts or osteoclasts, indicating that maternally transmitted mitochondrial dysfunction may underlie the bone defect in the adult offspring.
1. Materials and Methods
A. Mouse Breeding and Feeding Scheme
All procedures in this study were approved by the Animal Studies Committee at Washington University School of Medicine and conformed to National Institutes of Health guidelines. The HF/HS feeding regimen for females has been previously reported [22]. Briefly, 4-week-old C57BL6/J female mice were fed either a high-fat/ high-sugar (HF/HS) diet (test diet 58R3; 59% fat, 26% carbohydrates [17% sucrose], 15% protein) or regular chow (chow, PicoLab Rodent diet 20; 13% fat, 62% carbohydrates [3.2% sucrose], 25% protein) and continued on the diet through pregnancy and until weaning of the pups (F1). The stud males were fed regular chow except when they were housed with females for mating, at which time they shared the same diet with the females. The F1 progenies were fed regular chow after weaning until sacrifice. For the HF/HS-fed stud male experiment, 4-week-old C57BL6/J males were fed either the HF/HS diet or the regular chow for 6 weeks before they were switched to regular chow diet and mated with chow-fed females. Fasting glucose levels were measured at the end of the 6-week-long HF/HS feeding and before mating.
B. Analyses of Bones
Femora were analyzed for bone phenotypes. The thresholds for microcomputed tomography (micro-CT) quantification of trabecular and cortical bone parameters were set at a lower/upper threshold of 200/1000 and 250/1000, respectively. The cortical bone parameters were derived from 50 micro-CT slices (0.8 mm total) at the middle shaft of the femur; trabecular bone parameters were assessed in 100 micro-CT slices (1.6 mm total) immediately below the distal-end growth plate of the femur. For dynamic histomorphometry, calcein green (10 mg/kg; Sigma, Saint Louis, MO, USA) was injected intraperitoneally 7 days and 2 days before sacrifice. All dynamic and static histomorphometric analyses were performed on a minimum of 3 sections each from 3 animals per group. Quantification of calcein labeling was performed with the endocortical bone surface at the mid-diaphysis of the tibia on 30μm-thick longitudinal sections (Osteo II, BIOQUANT, Nashville, TN, USA). Hematoxylin and eosin and tartrate-resistant acid phosphatase (TRAP) staining were performed with standard protocols. TRAP+ cells were counted on all trabecular bone surfaces of the distal femur with the BIOQUANT software (Nashville, TN, USA). Adipocytes were defined by their round morphology and counted within the marrow space spanning 5 mm proximally from the distal growth plate of the femur. The total adipocyte number was divided by marrow area in arbitrary unit. CTX-I and P1NP assays were performed with sera collected from mice after 6 hours of fasting, and with the RatLaps enzyme-linked immunosorbent assay kit and the Rat/Mouse P1NP enzyme immunoassay kit (both from Immunodiagnostic Systems, Ltd.), respectively.
C. Cell Culture and Differentiation Assays
Bone marrow stromal cells (BMSCs) were isolated and cultured from 8-week-old F1 male mice as previously described [27]. BMSCs were isolated from the femur and the tibia and were plated in alpha minimum essential medium with 10% fetal bovine serum; medium was changed at day 3 and day 6. Cells were passaged once at days 7 through 8 and reseeded at 1x105 cells/cm2 for osteoblast differentiation in osteogenic media (alpha minimum essential medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, 50 µg/ml L-ascorbic acid, and 10 mM ß-glycerophosphate). The cells were cultured for 3 days before ribonucleic acid (RNA) was isolated with the QIAGEN RNeasy kit (cat# 74104).
For osteoclastogenesis, bone marrow macrophages (BMMs) were isolated from 8-week-old F1 males and cultured in osteoclastogenic media (alpha-MEM containing 10% FBS, 1% penicillin/streptomycin, supernatant from the CMG 14-12 cell line, purified glutathione-S-transferase RANKL (GST-RANKL)) based on published methods [28, 29] for 3 days before RNA isolation.
D. RNA Analyses
Total RNA was isolated from tibial and femoral bone shafts (without bone marrow) of 8-week-old F1 males. The bone shafts were pulverized at 2000 rpm for 20seconds with a Mikro-Dismembrator in liquid nitrogen before being extracted with Trizol (Invitrogen); RNA was then purified with the RNeasy RNA extraction kit (Qiagen). For reverse transcription RNA was reverse-transcribed into complementary DNA by using iScript complementary DNA synthesis kit (Bio-Rad). Quantitative polymerase chain reaction (qPCR) was performed with Fast-start SYBR Green (Bio-Rad) in a Step-One machine (ABI). Relative expression was calculated with the 2–(ΔΔCt) method and normalized to ribosomal 18S RNA. qPCR primers are listed in Table 1.
Table 1.
Nucleotide Sequences for Quantitative Polymerase Chain Reaction Primers
| Gene | qPCR Sequences 5ʹ to 3ʹ |
|---|---|
| 18S | F: CGGCTACCACATCCAAGGAA R: GCTGGAATTACCGCGGCT |
| Runx2 | F: CCAACCGAGTCATTTAAGGCT R: GCTCACGTCGCTCATCTTG |
| Osx | F: GGAAAGGAGGCACAAAGAAGC R: CCCCTTAGGCACTAGGAG |
| Alp | F: CCAACTCTTTTGTGCCAGAGA R: GGCTACATTGGTGTTGAGCTTTT |
| Col1a1 | F: TGCTTCCACGTTTACAGCTCTAAAG R: GTCAGGAAAGGGTCATCTGTAGTCC |
| Ocn | F: TGCTGGAGTGGTCTCTATGA R: ACCCTCTTCCCACACTGT |
| Atf4 | F: GCATGCTCTGTTTCGAATGGA R: CCAACGTGGTCAAGAGCTCAT |
| Tars | F: CCCTGGCCTGAATACATTAACAC R: CGGCTTGCTATCTTTTGCTGC |
| Asns | F: CAAGGAGCCCAAGTTCAGTAT R: GGCTGTCCTCCAGCCAAT |
| NFATc1 | F: GGTAACTCTGTCTTTCTAACCTTAAGCTC R: GTGATGACCCCAGCATGCACCAGTCACAG |
| CatK | F: GAAGAAGACTCACCAGAAGCAG R: TCCAGGTTATGGGCAGAGATT |
| Cox2 | F: GCCGACTAAATCAAGCAACA R: CAATGGGCATAAAGCTATGG |
| Cox5 | F: GGGTCACACGAGACAGATGA R: GGAACCAGATCATAGCCAACA |
| ATP5γ1 | F: AGTTGGTGTGGCTGGATCA R: GCTGCTTGAGAGATGGGTTC |
| Idh3a | F: CCTCCTGCTTAGTGCTGTGA R: CGTTGCCTCCCAGATCTTT |
Abbreviation: qPCR, quantitative polymerase chain reaction.
E. Bone Mechanical Testing
The testing was performed as a service by the Washington University Musculoskeletal Research Center Structure and Strength Core. Three-point bending was performed on an Instron Dynamight (Instron, Norwood, MA) hydraulic testing machine. Clearly dissected femurs were placed on a 7-mm span support such that the geometric center of the bone was midway between them and contacted on the posterior side. The actuator was set to displacement control and moved downward toward the bone at 0.1 mm/second. Data were collected using a custom MATLAB 2018a (Mathworks, Natick, MA) program collecting at 100 Hz. Force and displacement data were analyzed in conjunction with imaging data to estimate material properties in addition to gross calculations.
F. Seahorse Cellular Flux Analyses
BMSCs and BMMs were plated in XF96 plates at 100000 cells per well precoated with Cell-Tak (BD Biosciences). On the following day, the cells were switched to XF assay medium modified with Dulbecco’s modified Eagle medium (Seahorse cat#102353-100) supplemented with 5.5 mM glucose, and further incubated in a CO2-free incubator for 1 hour. Oligomycin and trifluoromethoxy carbonylcynide phenylhydrazone (FCCP) (Seahorse Stress kit) were prepared in the XF assay medium with final concentration of 5 mM and 1 mM, respectively. At the end of the assays, cell numbers were measured for normalization.
G. Mitochondrial DNA Measurement
Total cellular DNA was isolated from cells or tissues with DNAeasy Blood and Tissue Kit (Qiagen). Mitochondrial DNA content was determined by qPCR by comparing the mitochondrially encoded Cox2 gene to an intron of the nuclear-encoded ß-globin gene [30]. The following primers were used. Cox2 F: GCCGACTAAATCAAGCAACA;
Cox2 R: CAATGGGCATAAAGCTATGG. ß-globin F: GAAGCGATTCTAGGGAGCAG; ß-globin R: GGAGCAGCGATTCTGAGTAGA.
H. Statistical Analyses
All quantitative data are presented as mean ± SD with a minimum of 3 independent samples. Statistical significance is determined by the Student t test or 1-way analysis of variance followed by the Tukey post hoc test by using the software GraphPad Prism v6.
2. Results
A. Diet-Induced Maternal Metabolic Syndrome Impairs Cortical Bone in the Offspring
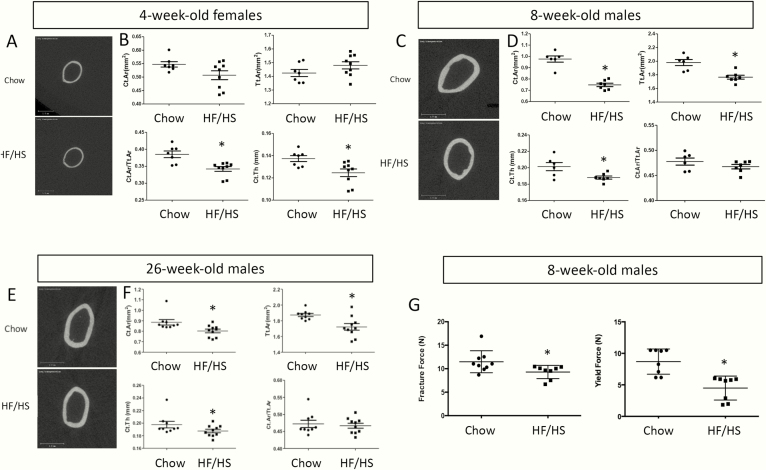
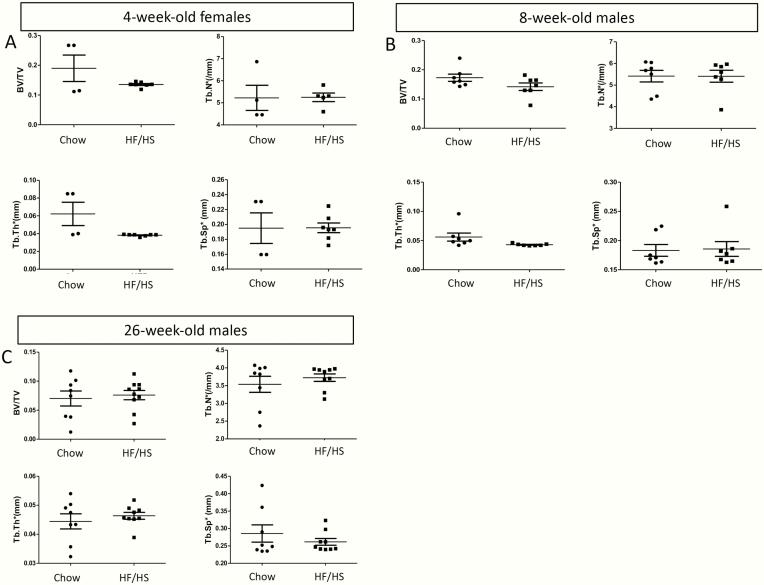
We have previously shown that C57BL6/J females fed an HF and HS diet from 4 through 10 weeks of age develop obesity and metabolic syndrome [22]. Importantly, offspring for at least 3 generations (F1-F3) from the HF/HS-fed dams develop mitochondrial dysfunction in skeletal muscle even when the offspring is raised on regular chow. To determine whether the transgenerational deleterious effect extends to bone, we have analyzed the femurs of the F1 offspring by micro-CT. At 4 weeks of age, the F1 females from HF/HS-fed dams exhibited thinner cortical bone thickness (Ct.Th) despite a normal overall size (total area [Tt.Ar]), resulting in a lower ratio of bone over total cross-sectional area (Ct.Ar/Tt.Ar) than those born to the chow-fed dams (Fig. 1A, B). Because most of the F1 females were used for other studies, we have mainly analyzed the F1 males for the current study [22]. Similar to the 4-week-old females, males born to the HF/HS-fed dams at either 8 or 26 weeks of age exhibited thinner cortices (cortical bone thickness) than normal (Fig. 1C-F). However, unlike the females, the males also showed a reduction in both total cross-sectional area (Tt.Ar) and the cortical bone area (Ct.Ar) while maintaining a normal ratio between the two (Fig. 1C-F). Three-point bending experiments showed that the femurs from 8-week-old F1 males born to HF/HS-fed dams displayed a smaller fracture force and yield force (Fig. 1G). Interestingly, the trabecular parameters were indistinguishable between the chow-fed progenies or HF/HS-fed progenies, in either 4-week-old females or in 8- or 26-week-old males (Fig. 2A-C). Thus, maternal metabolic syndrome induced by HF/HS diet diminishes cortical bone accrual and weakens bone strength in the progenies.
Figure 1.
HF/HS-induced maternal metabolic syndrome diminishes cortical bone in F1 offspring. (A, C, E) Micro-CT 3-D reconstruction images of the midshaft region of the femur in 4-week-old females (A), 8- (C) or 26-week-old males (E). (B, D, F) Quantification of cortical bone parameters from micro-CT scans of femurs in 4-week-old females (B), 8- (D) or 26-week-old males (F). Ct.Ar., cortical area; Ct.Th., cortical thickness; F1, offspring; HF, high-fat; HS, high-sugar; micro-CT, microcomputed tomography; Tt.Ar., total area. *P < 0.05; n = 7 or 9 for chow or HF/HS (4-week-old), respectively; n = 6 or 7 for chow or HF/HS (8-week-old), respectively; n = 9 or 10 for chow or HF/HS (26-week-old), respectively. (G) Mechanical testing results from the femurs of 8-week-old males. *P < 0.05; n = 8 or 9 for chow or HF/HS, respectively.
Figure 2.
Maternal metabolic syndrome does not affect trabecular bone in F1 offspring. Femurs were analyzed by micro-CT in 4-week-old females (A), 8-week-old males (B), or 26-week-old males (C). Each dot represents 1 animal. BV/TV, bone volume/tissue volume; HF, high-fat; HS, high-sugar; Tb.N*, trabecular number; Tb.Sp*, trabecular spacing; Tb.Th*, trabecular thickness.
B. Maternal Metabolic Syndrome Causes Low Turnover Osteopenia in the Offspring
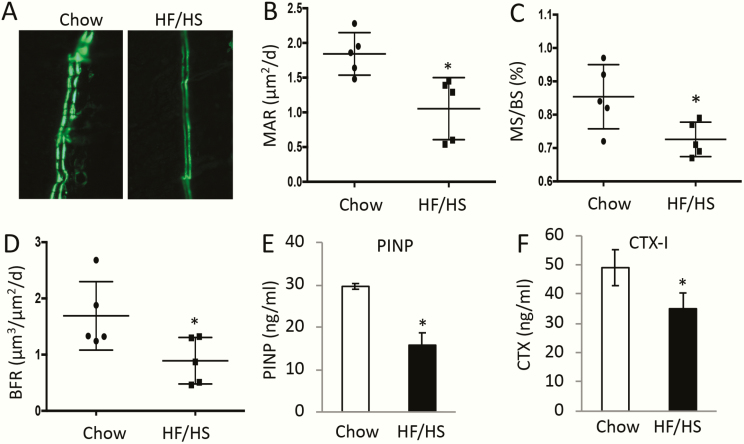
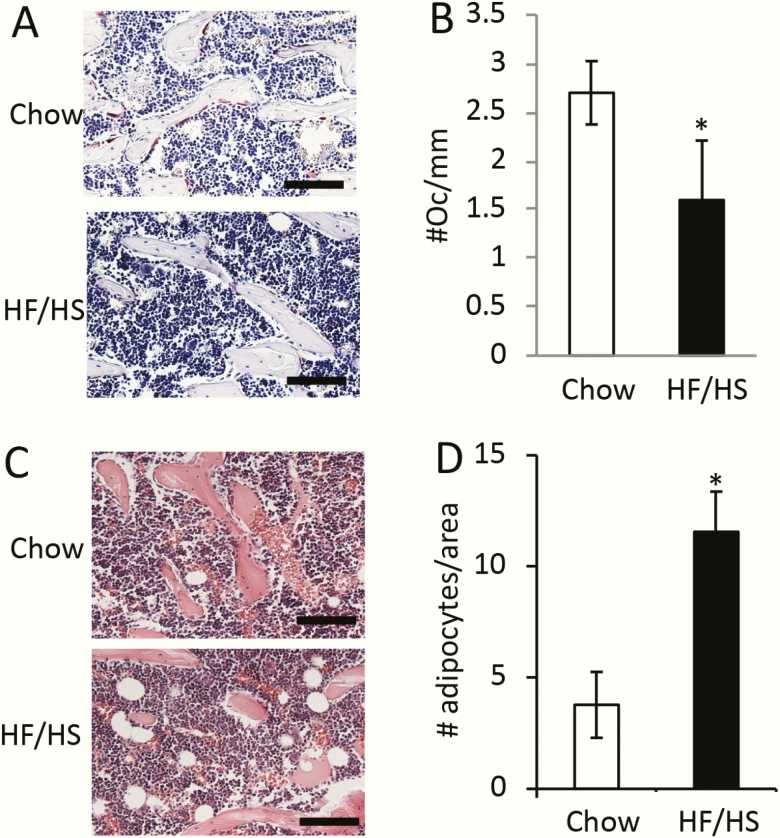
To investigate the cellular basis for the cortical bone phenotype, we performed double labeling experiments in 8-week-old F1 males. The distance between the 2 fluorescent labels on the endocortical bone surfaces was notably reduced in the animals descended from HF/HS-fed dams over the normal controls (Fig. 3A). Quantification confirmed a significant decrease in both mineral apposition rate and mineralizing bone surface at both periosteal and endosteal surfaces, resulting in a marked decrease in bone formation rate in the offspring of HF/HS-fed dams (Fig. 3B-D). Consistent with the double labeling results, the levels of aminoterminal propeptide of type I procollagen in the serum, an indicator for the overall bone formation activity, were approximately 50% lower in the offspring of HF/HS-fed dams than those of chow-fed dams (Fig. 3E). Somewhat unexpectedly, the circulating levels of CTX-I, a cleavage product of type I collagen reflecting total bone resorption activity, was also reduced in the HF/HS offspring (Fig. 3F). In keeping with the decrease in bone resorption activity, histological staining of bone sections showed that the number of TRAP+ osteoclasts was diminished in the mice born to HF/HS-fed dams (Fig. 4A, B). On the other hand, histology detected more adipocytes in the bone marrow of the HF/HS progenies (Fig. 4C, D). Thus, the bone phenotype in the offspring of HF/HS-fed dams may be characterized as low turnover osteopenia.
Figure 3.
Maternal metabolic syndrome suppresses bone formation and resorption in the offspring. F1 males were analyzed at 8 weeks of age. (A) Representative images for double labeling at the endosteal surface of the femur. (B-D) Quantification of double labeling results. MAR, mineral apposition rate (B); MS/BS, mineralizing surface over total bone surface (C); BFR, bone formation rate (D). *P < 0.05; n = 5. (E, F) Serum levels of procollagen type I N-terminal propeptide (P1NP) (E) or C-terminal telopeptide of collagen I (CTX-I) (F). *P < 0.05; n = 3.
Figure 4.
HF/HS offspring data exhibit decreased osteoclast number and increased bone marrow adipocytes. F1 males were analyzed at 8 weeks of age. Scale bar, 100 µm. (A) Representative images of TRAP staining on femur sections. TRAP+ osteoclasts stained dark red. (B) Quantification of osteoclast numbers normalized to trabecular bone surface. *P < 0.05; n = 3. (C) Representative H&E staining of femur sections showing marrow adipocytes. (D) Quantification of bone marrow adipocytes on H&E-stained femur sections. *P < 0.05; n = 3. F1, offspring; H&E, hematoxylin and eosin; HF, high-fed; HS, high-sugar; TRAP, tartrate-resistant acid phosphatase.
C. Osteoblast and Osteoclast Progenitors are Impaired in HF/HS Offspring
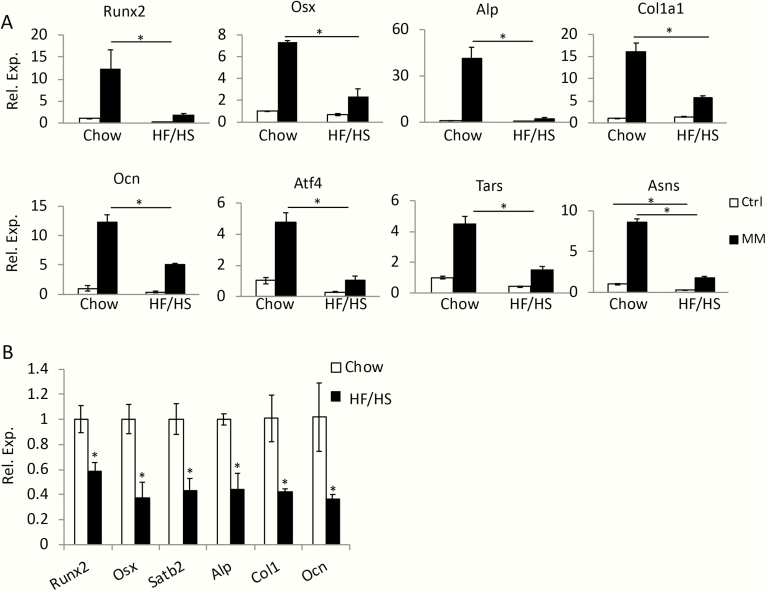
To examine the potential maternal effect on osteoblast progenitors, we isolated BMSCs (known to contain osteoprogenitors) from 8-week-old F1 males born to either HF/HS-fed or chow-fed dams (referred to as HF/HS or chow offspring) and assessed their differentiation potential in vitro. After 3 days of culture in mineralization media instead of growth media, BMSCs from the chow offspring significantly upregulated not only the common osteoblast markers Runx2, Osx, Alpl. Cola1, and Ocn, but also the protein anabolism genes Atf4, Tars, and Asns recently shown to increase with osteoblast differentiation [31] (Fig. 5A). Although the same genes were also induced in BMSCs from the HF/HS offspring by the mineralization media, their expression level was clearly diminished (Fig. 5A). In keeping with the in vitro results, the expression levels of the osteoblast marker genes in bone were significantly lower in HF/HS than chow offspring (Fig. 5B). Thus, maternal metabolic syndrome reduces the differentiation potential of osteoblast progenitors.
Figure 5.
Maternal metabolic syndrome impairs bone marrow osteoblast progenitors in the offspring. (A) Analyses for osteoblast differentiation in BMSCs isolated from chow or HF/HS offspring. mRNA levels normalized to 18S rRNA were expressed as relative values to those in cells from chow offspring and cultured in growth media, which was designated as 1. *P < 0.05, 1-way factorial ANOVA followed by Tukey post hoc test, n = 3. Alp, alakine phosphatase, official name Alpl; ANOVA, analysis of variance; Asns, asparagine synthetase Atf4, activating transcription factor 4; BMSCs, bone marrow stromal cells; Cola1, collagen type I alpha 1 chain; Ctrl, regular growth media; HF, high-fat; HS, high-sugar; MM, mineralization media; mRNA, messenger RNA; Ocn, osteocalcin, official name Bglap; Osx, osterix, official name Sp7; RT-qPCR, reverse transcriptase–quantitative polymerase chain reaction; RNA, ribonucleic acid; rRNA, ribosomal RNA; Runx2, runt-related transcription factor 2; Tars, threonyl-tRNA synthetase; tRNA, transer RNA. (B) RT-qPCR analyses of osteoblast marker genes in the bones of chow or HF/HS 8-week-old offspring. The mRNA levels in chow offspring were designated 1. *P < 0.05; n = 3.
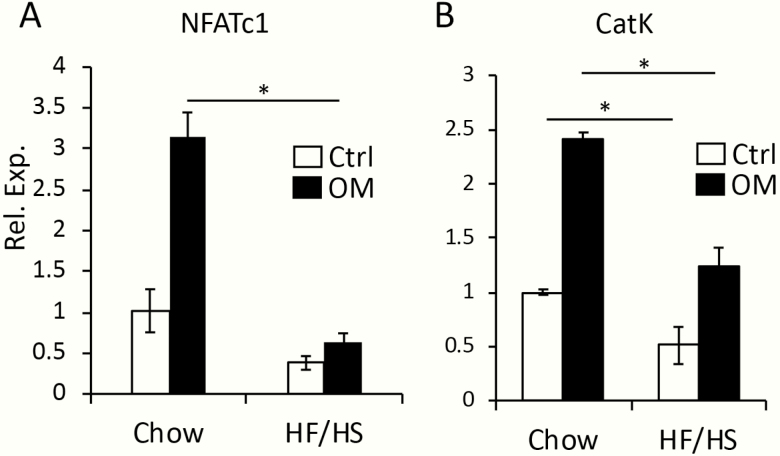
We next investigated whether osteoclast progenitors were similarly affected. For this, we isolated BMMs from the F1 males and performed osteoclastogenesis assays in vitro. Nfatc1 and cathepsin K (CatK), representing early and late stages of osteoclast differentiation respectively, were significantly induced in BMMs from the chow offspring following incubation in the osteoclastogenic media (Fig. 6A, B). However, the levels of either gene following induction of differentiation was much reduced in BMMs from HF/HS offspring. Thus, both osteoblast and osteoclast progenitors were negatively impacted by maternal metabolic syndrome.
Figure 6.
Maternal metabolic syndrome impairs bone marrow osteoclast progenitors in the offspring. (A, B) RT-qPCR analyses for osteoclast marker genes Nfatc1 (A) and CatK (B). mRNA levels normalized to 18S rRNA were expressed as relative values to those in BMMs from chow offspring and cultured in growth media (designated 1). BMMs, bone marrow macrophages; CatK, cathepsin K; Ctrl, regular growth media; HF, high-fat; HS, high-sugar; mRNA, messenger RNA; OM, osteoclastogenic medium; RNA, ribonucleic acid; rRNA, ribosomal RNA; RT-qPCR, reverse transcriptase–quantitative polymerase chain reaction. *P < 0.05; 1-way factorial ANOVA followed by Tukey post hoc test, n = 3.
D. Osteoblast and Osteoclast Progenitors Exhibit Mitochondrial Dysfunction in HF/HS Offspring
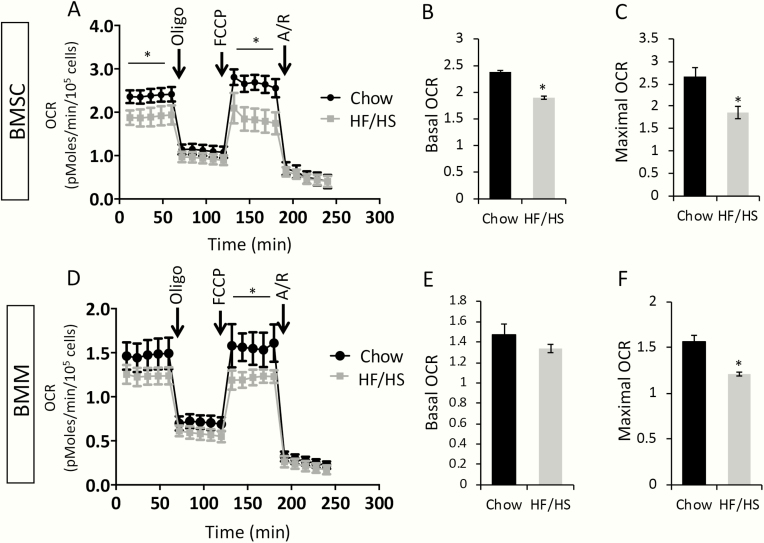
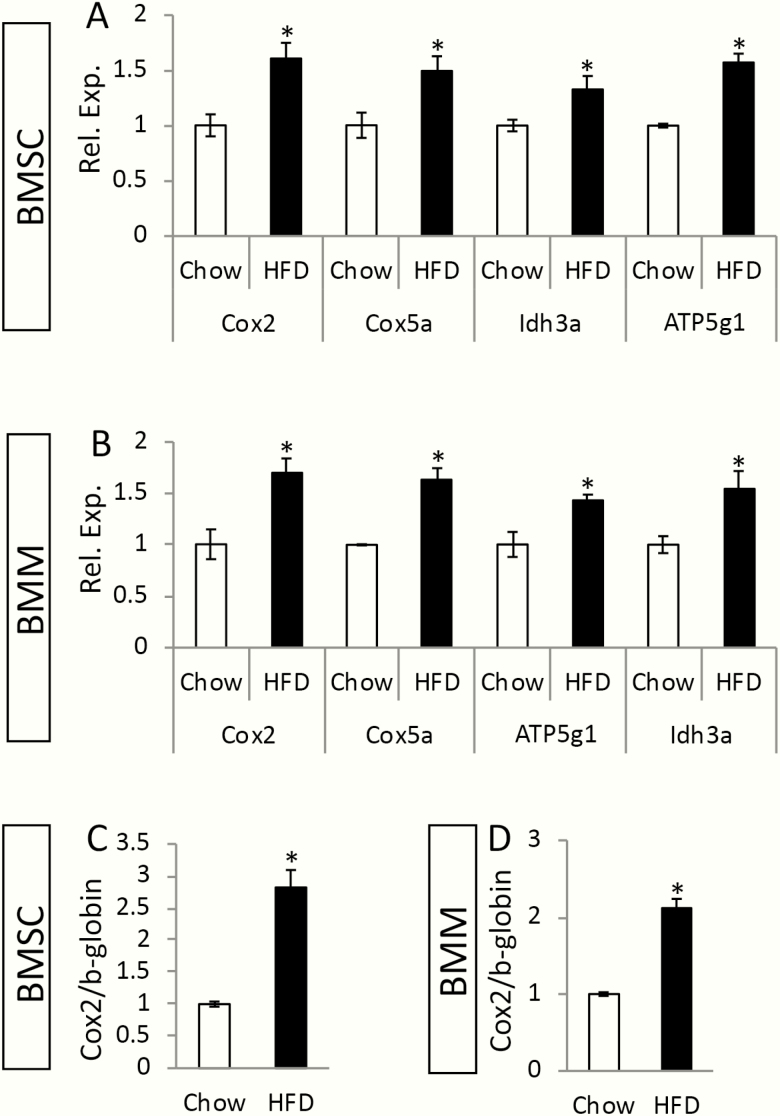
As our previous studies have shown that maternal metabolic syndrome causes mitochondrial dysfunction to the offspring, we next tested whether this applied to the osteoblast or osteoclast progenitors. To this end, we measured the oxygen consumption rate (OCR) in BMSCs or BMMs isolated from 8-week-old F1 males with Seahorse technology. Both basal and maximal OCR in BMSCs were reduced in HF/HS offspring compared with the chow counterparts (Fig. 7A-C). In the BMMs of HF/HS offspring, although the basal OCR was relatively normal, the maximal OCR was suppressed (Fig. 7D-F). Interestingly, despite the decreased OCR, the genes that participate in the electron transport chain or the tricarboxylic acid (TCA) cycle, such as Cox2, Cox5a, Idh3a ,or ATP5g1, were expressed at a higher level in the BMSCs or BMMs from HF/HS offspring than those of chow offspring (Fig. 8A, B). Moreover, the Cox2 gene in the mitochondrial genome, when normalized to the nuclear gene b-globin, presented a higher copy number in BMSCs or BMMs from the HF/HS offspring than normal (Fig. 8C, D). The increased mitochondrial number likely reflects a compensatory response in the face of impaired mitochondrial function.
Figure 7.
HF/HS offspring data exhibit mitochondrial dysfunction in BMSCs and BMMs. (A) OCR by Seahorse analyses in BMSCs. (B, C) Quantification of basal or maximal OCR in BMSCs. *P < 0.05; n = 3. (D) OCR by Seahorse analyses in BMMs. (E, F) Quantification of basal or maximal OCR in BMMs. *P < 0.05; n = 3. A/R, antimycin A/rotenone; BMMs, bone marrow macrophages; BMSCs, bone marrow stromal cells; HF, high-fat; HS, high-sugar; OCR, oxygen consumption rate; Oligo, oligomycin; FCCP: trifluoromethoxy carbonylcyanide phenylhydrazone.
Figure 8.
HF/HS offspring data exhibit increased mitochondria abundance in BMSCs and BMMs. (A, B) RT-qPCR analyses of relative mRNA levels normalized to 18S mRNA expression in BMSCs (A) or BMMs (B). Levels in cells from chow offspring were designated 1. *P < 0.05; n = 3. ATP5g1, ATP synthase, H+ transporting, mitochondrial F0 complex, subunit C1 (subunit 9); BMMs, bone marrow macrophages; BMSCs, bone marrow stromal cells; Cox2, cytochrome c oxidase subunit 2; Cox5a, cytochrome c oxidase subunit 5a; HF, high-fat; HS, high-sugar; Idh3a, isocitrate dehydrogenase 3 (NAD+) alpha; mRNA, messenger RNA; RNA, ribonucleic acid; RT-qPCR, reverse transcriptase–quantitative polymerase chain reaction. (C, D) Relative abundance of mitochondrial Cox2 gene to nuclear ß-globin gene in BMSCs (C) or BMMs (D). *P < 0.05; n = 3.
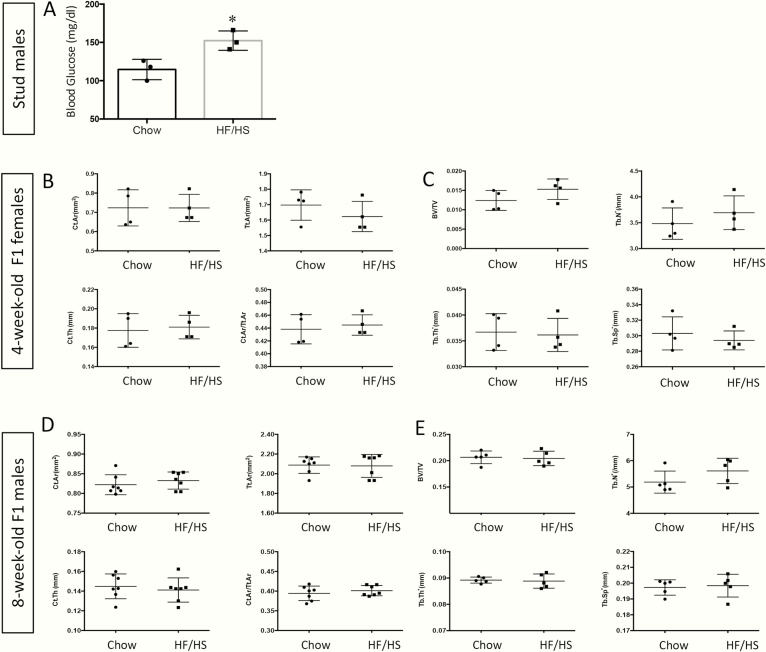
The results so far suggest that maternal transmission of mitochondrial dysfunction underlies the bone defects in the offspring. To test this notion further, we investigated whether males, which do not transmit their mitochondria to the next generation, could cause a similar bone defect in the offspring. Specifically, we raised stud males on the HF/HS diet from 4 weeks until 10 weeks of age before mating them with chow-fed females to produce progenies fed with normal chow. Measurements of fasting blood glucose levels confirmed that the males fed with an HF/HS diet were hyperglycemic (Fig. 9A). In fact, the extent of hyperglycemia in the HF/HS-fed stud males was higher than that observed in the HS/HF-fed females, as we have previously reported [22]. However, micro-CT analyses detected no defects in either trabecular or cortical bone of the male or female offspring at either 4 or 8 weeks of age (Fig. 9B-E). Thus, the transgenerational effect of HF/HS diet on bone is transmitted only through females, perhaps partly via the passage of dysfunctional mitochondria.
Figure 9.
Paternal exposure to HF/HS diet does not affect the bones of the offspring. (A) Blood glucose levels after overnight fasting in chow-fed or HF/HS-fed males. Four-week-old C57BL6/J male mice were fed chow or HS/HF diet for 6 weeks before fasting glucose levels were measured. (B-E) Femurs were analyzed by micro-CT in 4-week-old F1 females (B, C) or 8-week-old F1 males (D, E) for both cortical (B, D) and trabecular (C, E) bone parameters. BV/TV, bone volume fraction; Ct.Ar., cortical area; Ct.Th., cortical thickness; HF, high-fat; HS, high-sugar; micro-CT, microcomputed tomography; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation Tt.Ar., total area. n = 3 (A), 4 (B, C), 7 (D), or 5 (E). Each dot represents 1 animal.
3. Discussion
We have shown that mothers with diet-induced metabolic dysregulation transmit a notable bone defect to their adult offspring. Specifically, both male and female offspring from the affected dams exhibit reduced cross-sectional size and cortical thickness in the long bones, resulting in a clear decrease in mechanical strength. The findings, therefore, reinforce the notion that intrauterine or early postnatal exposure to an adverse nutritional environment negatively impacts bone health in the long term.
Our findings appear to be at odds with those from a recent study [25]. There, the progenies born to high-fat diet–fed dams continued on an HF diet after weaning until 6 weeks of age before they were switched to regular chow and analyzed at 20 weeks of age. Those authors analyzed the tibia by micro-CT and reported that the male progenies had lower trabecular bone mass but increased cortical bone total cross-sectional area than normal. In contrast, we see in the femur no change in the trabecular bone but a decrease in cortical bone total cross-sectional area at either 8 or 24 weeks of age. Although the exact reason for the discrepancy is unknown at present, it is possible that continued feeding of the HF diet to the progenies after weaning in the previous study might have compounded with the maternal effect to cause a different bone phenotype. Moreover, we cannot rule out that slight differences in diet compositions between the studies or potential differences between tibias and femora might account for the different outcome. Finally, as our analyses of the trabecular bone were limited to micro-CT studies, detailed static and dynamic histomorphometry might uncover additional defects transmitted by the HF/HS-fed dams.
The bone phenotype in our study appears to be caused by defects in both bone formation and resorption. This notion is supported by the observation that the serum biochemical markers for both processes (P1NP and CTX-1, respectively) are suppressed in the affected offspring versus the control offspring. Whereas the cross-sectional size is determined mainly by outward (periosteal) de novo bone growth, commonly known as modeling, the cortical bone thickness depends on the opposing activities of endosteal bone formation and resorption, or bone remodeling. Because both cortical cross-sectional size and thickness are reduced in the affected offspring, the maternal factors likely affect both modeling and remodeling of the cortical bone. Interestingly, however, bone length, which is largely driven by the growth of growth plate cartilage, appears to be normal. Similarly, the trabecular bone is not affected, indicating that the net balance from bone remodeling remains relatively normal in that compartment even though both resorption and formation are compromised. The different outcomes between cortical and trabecular bone are not known at present, but they may reflect distinct remodeling dynamics between the 2 compartments.
The bone defects are likely due to a direct effect on osteoblasts and osteoclasts. In vitro studies showed that BMSCs or BMMs from the affected offspring were less efficient in osteoblast or osteoclast differentiation, respectively. Although the precise mechanism for the defects in BMSCs or BMMs is not certain as present, both cell types exhibit deficiency in mitochondrial respiration despite increased mitochondrial DNA and gene expression, indicating that mitochondrial defects may be at least partially responsible. Since we have previously shown that the HF/HS-fed 4-week-old C57BL6/J female mice transmit abnormal mitochondria through oocytes to skeletal muscles of the offspring for multiple generations, we suspect that the mitochondrial defects observed here in BMSCs and BMMs are similarly inherited through the female germ line [22]. However, the abnormal intrauterine metabolic conditions and indirect exposure to the HF/HS diet either in utero or through the milk could also cause mitochondrial defects in somatic tissues in the offspring. Moreover, besides the mitochondrial defects, the aforementioned adverse conditions could lead to epigenetic changes in the nuclear genome that contribute to the differentiation defects of BMSC and BMMs. The HS/HF-fed stud males did not transmit a bone defect to the next generation, lending further support to the notion that the maternally transmitted mitochondrial defects may be responsible for the transgenerational bone defect. However, we could not rule out that the indirect exposure of the offspring to HS/HF in utero and before weaning, neither of which occurred in the HS/HF-fed stud male experiment, might also explain the lack of a transgenerational effect. Nonetheless, the current study provides evidence that the maternal metabolic state is an important factor influencing bone health of the next generation and potentially their predisposition to osteoporosis in adulthood.
Acknowledgments
Financial Support: The work is partially supported by National Institute of Diabetes and Digestive and Kidney Diseases R01 DK 111212 (F.L.).
Glossary
Abbreviations
- BMMs
bone marrow macrophages
- BMSCs
bone marrow stromal cells
- DNA
deoxyribonucleic acid
- F1
offspring
- HF
high-fat
- HS
high-sugar
- micro-CT
microcomputed tomography
- OCR
oxygen consumption rate
- qPCR
quantitative polymerase chain reaction
- RNA
ribonucleic acid
- TRAP
tartrate-resistant acid phosphatase
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364-376. [DOI] [PubMed] [Google Scholar]
- 2. Ramchand SK, Seeman E. Advances and unmet needs in the therapeutics of bone fragility. Front Endocrinol (Lausanne). 2018;9:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726-1733. [DOI] [PubMed] [Google Scholar]
- 4. Black DM, Delmas PD, Eastell R, et al. ; HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809-1822. [DOI] [PubMed] [Google Scholar]
- 5. Cummings SR, San Martin J, McClung MR, et al. ; FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756-765. [DOI] [PubMed] [Google Scholar]
- 6. Miller PD, Hattersley G, Riis BJ, et al. ; ACTIVE Study Investigators Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722-733. [DOI] [PubMed] [Google Scholar]
- 7. Neer RM, Arnaud CD, Zanchetta JR, et al. . Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441. [DOI] [PubMed] [Google Scholar]
- 8. Cosman F, Crittenden DB, Adachi JD, et al. . Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543. [DOI] [PubMed] [Google Scholar]
- 9. Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A. 2010;107(39):16757-16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595-601. [DOI] [PubMed] [Google Scholar]
- 11. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412-417. [DOI] [PubMed] [Google Scholar]
- 12. Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485-491. [DOI] [PubMed] [Google Scholar]
- 13. de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci U S A. 2010;107(39):16881-16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heijmans BT, Tobi EW, Stein AD, et al. . Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046-17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215(4539):1518-1519. [DOI] [PubMed] [Google Scholar]
- 16. Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275(4):R1374-R1379. [DOI] [PubMed] [Google Scholar]
- 17. Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R528-R538. [DOI] [PubMed] [Google Scholar]
- 18. de Assis S, Warri A, Cruz MI, et al. . High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benesh EC, Humphrey PA, Wang Q, Moley KH. Maternal high-fat diet induces hyperproliferation and alters Pten/Akt signaling in prostates of offspring. Sci Rep. 2013;3:3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luzzo KM, Wang Q, Purcell SH, et al. . High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS One. 2012;7(11):e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27(4):716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saben JL, Boudoures AL, Asghar Z, et al. . Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 2016;16(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen SB, Rasmussen MA, Olsen SF, et al. . Maternal dietary patterns during pregnancy in relation to offspring forearm fractures: prospective study from the Danish National Birth Cohort. Nutrients. 2015;7(4):2382-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen JR, Zhang J, Lazarenko OP, et al. . Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. Faseb J. 2012;26(3):1131-1141. [DOI] [PubMed] [Google Scholar]
- 25. Chen JR, Lazarenko OP, Zhao H, Alund AW, Shankar K. Maternal obesity impairs skeletal development in adult offspring. J Endocrinol. 2018;239(1):33-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang C, Oest ME, Jones JC, Prater MR. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res B Dev Reprod Toxicol. 2009;86(5):362-369. [DOI] [PubMed] [Google Scholar]
- 27. Tu X, Joeng KS, Nakayama KI, et al. . Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12(1):113-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15(8):1477-1488. [DOI] [PubMed] [Google Scholar]
- 29. Zeng R, Faccio R, Novack DV. Alternative NF-κB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res. 2015;30(12):2287-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24(14):1507-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karner CM, Lee SY, Long F. Bmp induces osteoblast differentiation through both Smad4 and mTORC1 signaling. Mol Cell Biol. 2017;37(4):e00253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]