Summary
Background
Selective dorsal rhizotomy (SDR) is an irreversible surgical procedure involving the division of selected sensory nerve roots, followed by intensive physiotherapy. The aim is to improve function and quality of life in children with cerebral palsy and a Gross Motor Function Classification System (GMFCS) level of II or III (walks with or without assistive devices, respectively). We assessed gross motor function before and after SDR and postoperative quality of life in a study commissioned by NHS England.
Methods
We did a prospective observational study in five hospitals in England who were commissioned to perform SDR on children aged 3–9 years with spastic diplegic cerebral palsy. The primary outcome was score changes in the 66-item Gross Motor Function Measure (GMFM-66) and seven domains of the Cerebral Palsy Quality of Life Questionnaire ([CP-QoL] social wellbeing and acceptance, feelings about functioning, participation and physical health, emotional wellbeing and self-esteem, access to services, family health, and pain and impact of disability) from before to 24 months after SDR.
Findings
From Sept 4, 2014, to March 21, 2016, 137 children underwent SDR. The mean age was 6·0 years (SD 1·8). The mean GMFM-66 score increased after SDR with an annual change of 3·2 units (95% CI 2·9 to 3·5, n=137). Of the seven CP-QoL domains, five showed significant improvements over time: feelings about functioning mean annual change 3·0 units (95% CI 2·0 to 4·0, n=133), participation and physical health 3·9 units (2·5 to 5·3, n=133), emotional wellbeing and self-esteem 1·3 units (0·2 to 2·3, n=133), family health 2·0 units (0·7 to 3·3, n=132), and pain and impact of disability −2·5 units (−3·9 to −1·2, n=133). 17 adverse events were reported in 15 children, of which none were severe and 15 (88%) resolved.
Interpretation
SDR improved function and quality of life in the 24 months after surgery in children with cerebral palsy classified as GMFCS levels II and III. On the basis of these findings, an interim national policy decision was made that SDR would be funded for eligible children in England from 2018.
Funding
National Institute for Health and Care Excellence, National Institute for Health Research Biomedical Research Centre, NHS England.
Introduction
The worldwide incidence of cerebral palsy is 2–3 per 1000 livebirths,1 and approximately 80% of affected children have spasticity in their lower limbs.2 With no cure, treatment options aim to alleviate symptoms and improve quality of life. Medical treatments include intramuscular injections of botulinum toxin, oral baclofen, or both, to relieve muscle stiffness, anti-inflammatories for pain, and orthotic devices and physiotherapy to aid walking. Surgical interventions include operations on bony or soft tissue, orthopaedic surgery to restore lower-limb alignment, and neurosurgery such as selective dorsal rhizotomy (SDR).3 SDR is an irreversible neurosurgical procedure in which sensory nerve roots are divided. The aim is to reduce spasticity in the lower limbs and improve gross motor function and quality of life.4, 5, 6, 7 Intensive physiotherapy is provided for several months after SDR.4
Evidence from randomised controlled trials (RCTs) relating to SDR is limited. Three RCTs were done in Canada and the USA but were published between 1997 and 1998.6, 8, 9 These compared SDR plus physiotherapy with physiotherapy alone. Data from the 90 children assessed were summarised in a meta-analysis in 2002,10 which showed consistent overall improvement in function in children receiving SDR compared with those receiving physiotherapy alone (2·7 unit mean difference in scores in the 66-item Gross Motor Function Measure (GMFM-66) units at a median follow-up of 12 months; appendix). The RCTs were well reported and included robust methods of randomisation and allocation concealment, but follow-up was short (9–12 months) and no data were collected on quality of life. Two of the three RCTs reported no adverse events8, 9 and the third reported one spinal epidural abscess and one transient urinary retention.6
Research in context.
Evidence before this study
Selective dorsal rhizotomy (SDR) for children with cerebral palsy has not been routinely available via the National Health Service (NHS) in England. Although some international evidence indicates positive long-term outcomes, NHS England judged that the evidence base was insufficient to support universal funding. We searched the Cochrane database, EMBASE, PubMed, Web of Science, and grey literature on Oct 15, 2018, for randomised controlled trials that compared SDR and non-SDR outcomes in patients with cerebral palsy. We found three small randomised controlled trials that involved 90 patients. A meta-analysis provided evidence of improved mean gross motor function 9–12 months after SDR with no suggestion of harm. All three studies, however, were done in the 1990s, since when surgical techniques have changed, and they captured no data on quality of life or long-term follow-up. A new randomised trial was impossible in the UK because of the lack of clinical equipoise among physicians and patients (including parents), yet firmer evidence of benefit without harm was needed to inform a policy decision. Therefore, in 2014, NHS England commissioned a robust evaluation of SDR with standardised outcomes and with 24 months of follow-up after surgery in five NHS paediatric neurosurgical centres in England.
Added value of this study
This assessment of SDR followed by intensive physiotherapy was done in a large contemporary group of 137 children. The study obtained prospective data on a wide range of validated outcomes collected in a standardised way, and particularly assessed gross motor function (66-item Gross Motor Function Measure) and quality of life (Cerebral Palsy Quality of Life Questionnaire for Children parent version), including pain and adverse events, over the 2-year follow-up. Assessment of multiple data points allowed patients' trajectories to be modelled statistically and showed clear improvement in function and quality of life, including reduced pain, with no evidence of harm. Furthermore, we compared our data with age-specific and severity-specific norm centiles for gross motor function that were developed in Canada for children with cerebral palsy who had not had SDR. These showed that the observed benefit of SDR among children in this study exceeded the expected function without SDR.
Implications of all the available evidence
This evaluation provides robust evidence that SDR plus intensive physiotherapy improves gross motor function, quality of life, and pain to greater degrees than would be expected without SDR. The evidence was used to inform a policy review in 2018 by NHS England, which resulted in funding of SDR being supported for eligible children aged 3–9 years with cerebral palsy.
SDR has not been routinely available via the National Health Service (NHS) in England and has been possible to obtain only through individual funding requests.11 Although the findings of several cohort studies (including the RCTs) have suggested positive long-term outcomes after SDR,5, 6, 8, 9, 10, 12, 13, 14 NHS England judged the evidence base to be inadequate to permit universal funding because studies were more than 20 years old and quality of life was not measured in the RCTs4 and requested further evidence for the effects of SDR on longer-term outcomes. A further RCT was not possible in the UK because of the lack of equipoise among physicians and parents who strongly believed that there was a need for SDR. Therefore, in 2014, NHS England commissioned a prospective evaluation of SDR in a series of eligible children with standardised outcomes and 2 years of follow-up. We report on the gross motor function and quality of life outcomes after SDR.
Methods
Study design and oversight
Five NHS paediatric neurosurgical centres in England (appendix) were commissioned by NHS England Commissioning through Evaluation programme to provide a package of care according to a standardised protocol. The Commissioning through Evaluation programme enables a limited number of patients to access treatments that are promising but not yet universally funded by the NHS to allow new data to be collected on clinical outcomes and patients' experiences. The SDR care package included surgery and physiotherapy for up to 24 months for eligible children. Data were collected in the Research Electronic Data Capture application15 and outcomes were reported to a national database.4 Planning and oversight of the study was provided by a multidisciplinary SDR steering committee, which included clinical and academic experts, representatives of patients, and NHS England commissioners. The study did not include a control group and, therefore, age-specific and severity-specific normalised GMFM-66 centiles developed in Canada and based on children who had not had SDR were used to provide comparative data.16, 17, 18
Ethics approval for the collation of data and its subsequent analysis was given by the UK Health Research Authority. Written informed consent for data collection and analysis was obtained from the parents or primary caregivers for all children.
Selection of patients
Each centre had a multidisciplinary team that selected children for inclusion in the study according to a rigorous process. The teams were experienced in the delivery of SDR surgery and other accepted antispasticity treatments and the neurosurgeons performing surgery had previous experience and training in the SDR procedure. The assessment process was designed to balance functional abilities and impairments. A minimum list of assessments was provided by NHS England to be done for each patient before and after surgery (appendix).
Eligible children had spastic diplegic cerebral palsy that limited functional abilities and sufficient strength to rehabilitate after SDR surgery. Other inclusion criteria were age 3–9 years; MRI confirmation of no damage to key areas of the brain controlling posture or coordination (eg, lesions in the basal ganglia or cerebellum, which are associated with dyskinetic or ataxic cerebral palsy types) and typical cerebral palsy changes (white-matter damage of immaturity, namely periventricular leukomalacia); Gross Motor Function Classification System (GMFCS)19 level II (walks without assistive devices) or III (walks with assistive devices); no evidence of genetic or neurological progressive illness; mild to moderate lower-limb weakness with ability to maintain antigravity postures, no significant scoliosis or hip dislocation (Reimer's index20 <40%); confirmed financial or resource commitment for community-based postoperative physiotherapy; and no other relevant medical or personal contraindications. Patients with progressive neurological conditions and those with dystonia were excluded (appendix).
Treatment and follow-up
SDR was performed with a single-level laminectomy or laminoplasty approach with neurophysiology-guided partial section of the dorsal (sensory) nerve roots. Intraoperative neurophysiology was mandated to guide the selection of the nerve rootlets being divided. Each nerve root from L1 to S1 was divided into smaller portions (rootlets) of approximately equal size, which were tested with neurophysiology to elicit a reflex motor response. The responses were recorded and graded for extent of spread to adjacent myotome levels.21 The rootlets responsible for the greatest neurophysiology responses were cut. The physiotherapy regimen after surgery was specified and recommended for 24 months after discharge from hospital. Follow-up assessments were done at 4–6 months, 12 months, and 24 months after surgery.
Outcomes
The primary outcomes were change in gross motor function, measured with the GMFM-66 (a 66-item tool, with higher scores representing greater motor function, which is measured in five key motor domains: lying and rolling; sitting; crawling and kneeling; standing; and walking, running and jumping), which has been validated for assessment of children with cerebral palsy;22, 23 change in GMFM-66 centiles measured with the normalised version of GMFM-66, which is adjusted for age and GMFCS level; change in quality of life measured with seven domains of the parent or caregiver version of the Cerebral Palsy Quality of Life Questionnaire for Children ([CP-QoL] social wellbeing and acceptance, feelings about functioning, participation and physical health, emotional wellbeing and self-esteem, access to services, family health, and pain and impact of disability);24 and adverse events, measured for intensity, duration, outcomes, and relation to SDR. GMFCS was used to categorise the severity of involvement,19 and we used GMFM-66 as an objective assessment of neurological impairment during follow-up. Prespecified secondary outcomes were assessment of spasticity using the Modified Ashworth Scale,25 the Boyd and Graham Scale,26 gait analyses, and physiotherapy assessments (appendix).
Statistical analysis
The study was originally intended to include 163 patients, but due to a delay in the launch of the Commissioning through Evaluation programme, the overall number treated was 137. GMFM-66 scores, GMFM-66 centiles, and CP-QoL scores were used to follow patients' trajectories based on within-patient changes from before to 24 months after SDR. We used a linear mixed model in which the patient was the random effect and time was modelled using time from SDR surgery to each assessment. Difference in changes over time was assessed by GMFCS level by fitting an interaction term in the model with a likelihood ratio test. All results were scaled to mean annual change with 95% CIs. Model fit and model assumptions were checked with residual plots. Changes in the Modified Ashworth Scale, Boyd and Graham Scale, and Gait Profile Score over time were analysed with non-parametric tests. Sensitivity analysis of all children and all children excluding the three children aged older than 9 years at the time of SDR surgery found no material difference in results. To account for the multiplicity of outcomes and sensitivity analysis, we adjusted for multiple testing using a Bonferroni correction for the eight-variable composite outcome of GMFM-66 score and the seven CP-QoL domain scores. The modified cutoff for significance for this composite outcome was 0·0064 (1 – 0·950·125). Stata version 15 was used for all statistical analyses.
Role of the funding source
The funders of the study had a role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Between Sept 4, 2014, and March 21, 2016, 137 patients underwent SDR in the five study centres. No patients withdrew from the programme, but one patient did not complete the final assessment at 24 months. Most patients (61%) were boys and had GMFCS level III (62%; table 1). The mean length of hospital stay following SDR for all patients was 19·3 days (SD 7·1), although the time varied by centre (range 3–39 days). All patients had nerve rootlets cut during SDR in L2–S1, and 125 had rootlets cut in L1 (appendix). For most patients the percentage of rootlets cut for L1–S1 was between 60% and less than 70%.
Table 1.
Baseline and operative characteristics
| All patients (n=137) | ||
|---|---|---|
| Baseline characteristics | ||
| Age at SDR (years) | ||
| Mean (SD) | 6·0 (1·8) | |
| Range | 3–9* | |
| Boys | 83/137 (61%) | |
| Girls | 54/137 (39%) | |
| GMFCS level | ||
| II | 52/137 (38%) | |
| III | 85/137 (62%) | |
| Height before SDR (cm) | ||
| Mean (SD) | 112·1 (12·7) | |
| Range | 87·0–139·0 | |
| Weight before SDR (kg) | ||
| Mean (SD) | 21·3 (7·0) | |
| Range | 11·4–45·5 | |
| Body-mass index before SDR (kg/m2) | ||
| Mean (SD) | 16·9 (4·0) | |
| Range | 12·8–48·9 | |
| Body-mass index centile before SDR | ||
| Mean (SD) | 55·2 (31·9) | |
| Range | 0–100 | |
| Pregnancy duration (weeks) | ||
| Mean (SD) | 32 (4·0) | |
| Range | 26–42 | |
| Birthweight (kg) | ||
| Mean (SD) | 1·9 (0·8) | |
| Range | 0·8–4·2 | |
| Medication in previous 6 months | ||
| Oral baclofen | 23/129 (18%) | |
| Diazepam | 3/129 (2%) | |
| Botulinum toxin | 15/129 (12%) | |
| Previous surgery | ||
| Gastrocnemius or heel cord | 0/129 | |
| Bone | 2/129 (2%) | |
| Adductor | 0/129 | |
| Hamstring | 1/129 (1%) | |
| SEN or education, health, and care plan | ||
| Learning difficulties | 19/137 (14%) | |
| Behaviour, emotional, and social difficulty | 20/137 (15%) | |
| Speech, language, and communication needs | 6/137 (4%) | |
| Autistic spectrum disorder | 5/137 (4%) | |
| Hearing impairment | 1/137 (1%) | |
| Visual impairment | 4/137 (3%) | |
| Physical disability other than cerebral palsy | 17/137 (12%) | |
| No SEN disability | 28/137 (20%) | |
| No SEN support | 57/137 (42%) | |
| Operative characteristics | ||
| Length of stay in hospital after SDR (days) | ||
| Mean (SD) | 19·3 (7·1) | |
| Range | 3–39 | |
| Intraoperative neurophysiology | 137/137 (100%) | |
| Sphincter monitoring | 114/122 (93%) | |
| Mean proportion of nerve rootlet cut from L1 to S1 left and right† | 64·6 | |
| Range of the mean proportion of the centre of nerve rootlet cut from L1 to S1 left and right† | 57·1–66·0 | |
SDR=selective dorsal rhizotomy. GMFCS=Gross Motor Function Classification System. SEN=special educational needs.
Three children were aged 9 years at the time of the assessment before SDR and 10 years at time of surgery. A sensitivity analysis of all children and excluding the three children aged older than 9 years at the time for surgery showed no difference in results.
Excludes rootlets with 0% cut.
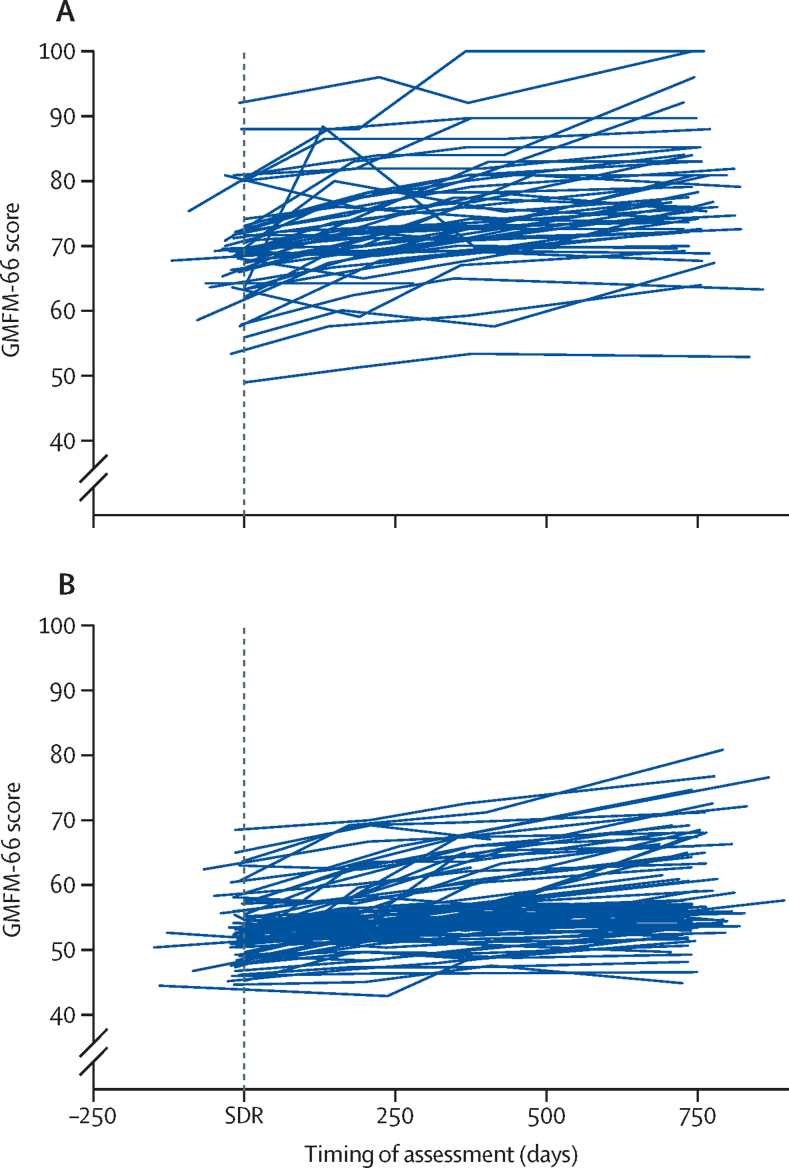
GMFM-66 scores increased in almost all children from before surgery to 24 months after surgery (figure, appendix). Mean annual increases were significant (table 2), with the overall increase being 3·2 units per year (95% CI 2·9–3·5, p<0·0001). The estimated increase was higher in patients with GMFCS level II than in those with GMFCS level III (table 2), which was a significant difference (pinteraction=0·006). Similarly, significant changes were seen for normalised GMFM-66 centiles over 2 years (table 2). The estimated norms for GMFM-66 calculated from the Canadian cohort who had not undergone SDR were lower than the modelled change and entirely below the 95% CIs estimated from the current SDR data. This was true for all children and in the GMFCS II and III subgroups (appendix).
Figure.
Observed trajectories of gross motor function for all patients by disease severity
137 children were assessed at baseline and all points before 24 months. (A) Gross Motor Function Classification System level II. (B) Gross Motor Function Classification System level III. GMFM-66=66-item Gross Motor Function Measure. SDR=selective dorsal rhizotomy.
Table 2.
Primary outcomes
| Before SDR | 24 months after SDR | Modelled mean change per year (95% CI) | ||
|---|---|---|---|---|
| GMFM-66* | ||||
| GMFCS level II | 69·0 (7·9), n=52 | 77·6 (8·9), n=51 | 3·8 (3·2 to 4·2, p<0·0001) | |
| GMFCS level III | 52·8 (4·6), n=85 | 58·8 (7·5), n=82 | 2·9 (2·5 to 3·2, p<0·0001) | |
| All patients | 59·0 (9·9), n=137 | 66·0 (12·2), n=133 | 3·2 (2·9 to 3·5, p<0·0001) | |
| Normalised GMFM-66 centiles† | ||||
| GMFCS level II | 67·3 (28·0), n=51 | 78·8 (22·2), n=46 | 3·7 (2·0 to 5·2, p<0·0001) | |
| GMFCS level III | 54·6 (21·1), n=85 | 69·7 (23·3), n=81 | 7·3 (6·0 to 8·7, p<0·0001) | |
| CP-QoL Child domains‡ | ||||
| Social wellbeing and acceptance§ | ||||
| GMFCS level II | 82·4 (15·3), n=51 | 84·7 (15·6), n=49 | 0·7 (−0·9 to 2·3, p=0·405) | |
| GMFCS level III | 81·5 (12·7), n=82 | 82·1 (11·3), n=79 | −0·01 (−1·1 to 1·1, p=0·979) | |
| All patients | 81·8 (13·7), n=133 | 83·1 (13·1), n=128 | 0·3 (−0·7 to 1·2, p=0·580) | |
| Feelings about functioning§ | ||||
| GMFCS level II | 74·2 (16·4), n=51 | 81·2 (14·0), n=48 | 2·4 (0·6 to 4·2, p=0·008) | |
| GMFCS level III | 68·1 (11·3), n=82 | 76·5 (12·1), n=79 | 3·4 (2·3 to 4·5, p<0·0001) | |
| All patients | 70·5 (13·8), n=133 | 78·3 (13·0), n=128 | 3·0 (2·0 to 4·0, p<0·0001) | |
| Participation and physical health§ | ||||
| GMFCS level II | 59·1 (17·4), n=51 | 70·6 (18·8), n=49 | 4·1 (1·7 to 6·6, p=0·001) | |
| GMFCS level III | 53·7 (16·6), n=82 | 63·6 (17·3), n=79 | 3·7 (2·0 to 5·5, p<0·0001) | |
| All patients | 55·7 (17·1), n=133 | 66·3 (18·1), n=128 | 3·9 (2·5 to 5·3, p<0·0001) | |
| Emotional wellbeing and self-esteem§ | ||||
| GMFCS level II | 77·8 (18·5), n=51 | 82·7 (16·7), n=49 | 1·5 (−0·4 to 3·5, p=0·121) | |
| GMFCS level III | 78·5 (10·7), n=82 | 82·1 (11·3), n=79 | 1·1 (−0·1 to 2·3, p=0·072) | |
| All patients | 78·2 (14·2), n=133 | 82·3 (13·6), n=128 | 1·3 (0·2 to 2·3, p=0·018) | |
| Access to services§ | ||||
| GMFCS level II | 47·4 (13·0), n=51 | 49·7 (11·9), n=49 | 0·5 (−1·2 to 2·3, p=0·563) | |
| GMFCS level III | 48·5 (11·3), n=82 | 52·1 (14·2), n=79 | 0·5 (−0·9 to 1·9, p=0·465) | |
| All patients | 48·1 (11·9), n=133 | 51·2 (13·3), n=128 | 0·5 (−0·6 to 1·6, p=0·351) | |
| Pain and impact of disability§ | ||||
| GMFCS level II | 35·8 (17·8), n=51 | 23·9 (16·7), n=49 | −1·7 (−3·4 to 0·1, p=0·06) | |
| GMFCS level III | 36·8 (19·4), n=82 | 30·4 (16·8), n=78 | −3·9 (−6·1 to −1·8, p<0·0001) | |
| All patients | 36·4 (18·7), n=133 | 27·9 (17·0), n=127 | −2·5 (−3·9 to −1·2, p<0·0001) | |
| Family health¶ | ||||
| GMFCS level II | 70·2 (19·6), n=51 | 79·9 (14·4), n=49 | 4·1 (2·2 to 5·9, p<0·0001) | |
| GMFCS level III | 67·9 (18·4), n=81 | 69·9 (19·8), n=79 | 0·7 (−1·1 to 2·5, p=0·458) | |
| All patients | 68·8 (18·8), n=132 | 73·8 (18·5), n=128 | 2·0 (0·7 to 3·4, p=0·003) | |
SDR=selective dorsal rhizotomy. GMFM-66=66-item Gross Motor Function Measure. GMFCS=Gross Motor Function Classification System. CP-QoL=Cerebral Palsy Quality of Life Questionnaire.
There was evidence of an interaction between GMFCS level and GMFM-66 score (p=0·006).
GMFM-66 centiles are GMFCS level-specific and, therefore, no aggregated score is calculated.
Results are from the child primary caregiver or parent version.
No evidence of an interaction between GMFCS levels (p>0·05).
There was evidence of an interaction between GMFCS level and CP-QoL Child family health domain (p=0·0127).
The CP-QoL results for all children improved significantly over 2 years for all domains assessed except social wellbeing and acceptance, emotional wellbeing and self-esteem, and access to services, although these did also improve slightly (table 2).
17 adverse events were reported for 15 children, with most having one event only (table 3). All but two were thought to be definitely or probably related to SDR, but none was a serious safety concern. The most common events were wound infection and persisting dysaesthesia in the feet and legs. No severe adverse events were reported and most resolved.
Table 3.
Adverse events
|
Adverse event |
Concomitant medication | Outcome | Additional information | |||
|---|---|---|---|---|---|---|
| Type | Intensity or severity | Related to SDR | ||||
| Patient 1 | ||||||
| Unknown | Uncovered dystonia | Mild | Unknown | Yes | Not resolved | Uncovered by SDR surgery |
| Patient 2 | ||||||
| 400 days | Persisting dysaesthesia of feet and legs | Mild | Definitely | No | Resolved | Required hamstring lengthening 24 months after SDR |
| Patient 3 | ||||||
| 30 days | Wound infection | Mild | Definitely | No | Resolved | ·· |
| Patient 4 | ||||||
| 191 days | Persisting dysaesthesia of feet and legs | Mild | Definitely | No | Resolved | Treated with dabapentin |
| Patient 5 | ||||||
| 2 days | Diarrhoea and vomiting | Mild | Unlikely | No | Resolved | Patient isolated and recovered quickly |
| Patient 6 | ||||||
| 1 day | Constipation | Mild | Unlikely | No | Resolved with laxative | Related to pain medication |
| Patient 7 | ||||||
| 22 days | Wound infection | Mild | Possible or likely | Yes | Resolved | Resolved with antibiotic treatment |
| Patient 8 | ||||||
| Unknown | Persisting dysaesthesia of feet and legs | Mild | Possible or likely | No | Not resolved | Hypersensitivity in right foot |
| Patient 9 | ||||||
| Unknown | Back pain | Mild | Possible or likely | Unknown | Resolved | ·· |
| Patient 10 | ||||||
| 6 days | Wound infection | Mild | Definitely | No | Resolved | ·· |
| Patient 11 | ||||||
| 55 days | Urgency | Mild | Unknown | No | Resolved | ·· |
| Patient 12 | ||||||
| 28 days | Wound infection | Mild | Definitely | No | Resolved | ·· |
| Patient 13 | ||||||
| 64 days | New weakness | Mild | Definitely | No | Resolved | ·· |
| Patient 14 | ||||||
| 1 day | Urinary retention after removal of indwelling urinary catheter | Moderate | Definitely | No | Resolved | Also had previously implanted intrathecal baclofen pump removed at SDR surgery while tube remained in situ; catheter reinserted later |
| 34 days | Persisting dysaesthesia of feet and legs | Mild | Definitely | No | Resolved | ·· |
| 60 days | Swelling reported under wound site after discharge | Mild | Definitely | No | Resolved | Intrathecal baclofen pump removed at surgery |
| Patient 15 | ||||||
| Unknown | Granulation of wound | Mild | Definitely | Unknown | Resolved | ·· |
Durations of adverse events are shown. SDR=selective dorsal rhizotomy.
The mean Gait Profile Score improved significantly from before to 24 months after SDR (p<0·001). Physiotherapists reported that the post-SDR physiotherapy recommendations had been implemented for 126 (95%) of 134 children at 24 months.
Five of the eight composite variables fulfilled the criterion for significance of p<0·0064, and even with adjustment for multiple testing, the primary outcomes were significant.
Discussion
This prospective multicentre study followed up children with spastic diplegia for 24 months after they underwent SDR and found consistent improvements in patients' outcomes that increased annually over this period. In particular, GMFM-66 scores and normalised GMFM-66 centiles increased significantly with reasonably narrow 95% CIs. The estimated increase in GMFM-66 scores was higher in children classified as GMFCS level II than in those classified as GMFCS level III. All changes were greater than those that would be expected without SDR,16, 17, 18 validating the improvement in function. The findings were also consistent with a meta-analysis of RCTs, which showed a greater improvement in mean GMFM-66 with SDR plus physiotherapy than with physiotherapy alone with median follow-up of 12 months.10 Compared with the GMFM-66 centiles for children who had not undergone SDR, those in our study showed a similar trend towards an improvement from before to 24 months after SDR, with greater change seen in children classified as GMFCS level III than in those classified as GMFCS level II. Specifically, quality of life improved in terms of feelings about functioning, participation and physical health, emotional wellbeing and self-esteem, pain and impact of disability, and family health. The pain score was reduced by 2·5 units per year, which although a small change, was significant. These findings are consistent with previously reported improved quality of life 20–28 years after SDR14 and reduced pain after 17 years.5
No serious safety concerns related to SDR occurred in the 24-month postoperative follow-up period. SDR surgery and postoperative care have evolved since the RCTs,6, 8, 9 but they also showed no short-term safety concerns. Nevertheless, complications following SDR have been reported in other studies. Grunt and colleagues7 did a systematic review of 21 observational studies among which six studies reported spinal abnormalities, although no strong association was found with SDR. In a study with 10 years of follow-up, all 19 patients assessed had transient flexor spasm in the calves and hypotonia of the legs after SDR, one patient had transient urinary incontinence, and ten patients had hyperaesthesia.27
This study has several strengths. First, it represents a contemporary clinical series of prospectively enrolled patients with defined characteristics who underwent SDR in five paediatric neurosurgical centres. All the children underwent rigorous and standardised assessments before and after surgery. In 2017, NHS England requested an interim analysis of results to feed into policy discussions. All children were included in these analyses, which drew upon the primary outcomes and formed part of the evidence base used to support the introduction of SDR into specialised NHS commissioning in July, 2018, pending completion of the final analyses.28 Second, this study included a larger study sample than previous studies, which allowed prospective collection of a wide range of specific clinical data. Third, the primary outcomes of gross motor function and quality of life were measured with validated instruments that are widely used in research. Fourth, we used linear mixed models that account for the longitudinal measurements and allow the inclusion of all patients in the analyses despite a small number of missing values.
The main limitation of the study is the absence of a comparison group, which means that direct comparisons of outcomes cannot be made in children who did and did not undergo SDR. Given the evidence for the effectiveness of SDR from the 1990s,6, 8, 9 a further trial is unlikely to be acceptable and, therefore, no concurrent comparator patients can be recruited in England. To mitigate this limitation, we normalised our raw GMFM-66 scores based on an external standard. Another limitation is that the length of follow-up was 24 months, which does not allow assessment of outcomes into young adulthood. However, several long-term cohort studies with different groups of patients and outcomes suggest sustained (15–28 years) positive effects of SDR on quality of life5, 14 and function.12, 13
The clinical interpretation of the size of change in GMFM-66 is challenging because to our knowledge there is no established consensus on the minimum clinically important difference. The annual change of 2·3 GMFM-66 units we calculated is in broad agreement with the mean improvement in GMFM-66 reported by Bolster and colleagues,29 who found a change of 7·6 units after 5 years in 19 children. Further comparison is difficult due to differences in follow-up periods and populations of patients. However, this finding, along with the accompanying improvement in quality of life in our study (including reduction in pain) and improvement in gross motor function beyond that expected without SDR, strongly support the effectiveness of SDR.
Our study enrolled children aged 3–9 years with GMFCS levels II or III, which is estimated to occur in 15% of children with cerebral palsy and a diagnosis of spastic diplegia.4 The study was not designed to disentangle age effects or to extrapolate findings to children and young people of other ages or other GMFCS levels. There are also other unmeasurable factors, such as the quantity of physiotherapy received, which are confounders that cannot be accounted for. However, in this sense, our study reflects real-world clinical practice and provides a valuable picture of how the service would function and how patients would respond at least in the 24 months after SDR. NHS England has recommended that physicians continue to capture outcomes on this cohort of children.
In conclusion, this multicentre prospective study provided assessed current SDR practice with a standardised approach in 137 children and five centres. We could demonstrate consistent evidence of improvement in function beyond the expected trajectory of children with cerebral palsy not treated with SDR. This improvement in function was seen alongside clear improvement in quality of life, including pain reduction. The observed benefits were seen for children with GMFCS levels II and III cerebral palsy over a 2-year period and mirrored those reported in earlier RCTs. Finally, this study led directly to an interim national policy decision that SDR would be funded for eligible children with cerebral palsy in England from 2018.
Acknowledgments
Acknowledgments
Patients' treatment costs were funded by NHS England. The views expressed are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research (NIHR), or UK Department of Health. JLP is an NIHR Senior Investigator.
Contributors
JSu, BC, SE, ME, CB, EB, MP, and JLP contributed to the systematic review. All authors contributed to the study design. JSu, BC, SE, ME, CB, EB, MP, KA, SC, RE, JG, SH, KM, BP, JSm, CS, MV, and JLP collected the data. JSu, BC, SE, ME, CB, EB, MP, and JLP did the data analysis. All authors contributed to data interpretation and writing of the report.
Declaration of interests
KA, JG, BP, and MV provide selective dorsal rhizotomy (SDR) and KM and JSm provide physiotherapy to patients who have received SDR outside of this study as either a privately funded or self-funded treatment. JSm had provided privately funded physiotherapy to some patients included in the current study before enrolment. SC has received private fees for seeing patients from abroad who wanted an opinion about whether SDR was a suitable treatment for their child. HPa and HPo are employed by the National Institute for Health and Care Excellence (NICE) and were contracted by NHS England to oversee the study. CV has received payments or expenses for acting as the clinical chair for NHS England Commissioning through Evaluation for SDR. JSu, BC, SE, ME, CB, EB, MP, and JLP were employed by King's College London, London, UK, in partnership with NICE and NHS England, funded by NICE, and supported by the National Institute for Health Research Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. MP has received personal fees from Merck. The other authors declare no competing interests.
Supplementary Material
References
- 1.Surveillance of Cerebral Palsy in Europe Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; Manchester: 2010. NICE interventional procedures guidance [IPG373]: selective dorsal rhizotomy for spasticity in cerebral palsy. [Google Scholar]
- 3.Farmer JP, Sabbagh AJ. Selective dorsal rhizotomies in the treatment of spasticity related to cerebral palsy. Childs Nerv Syst. 2007;23:991–1002. doi: 10.1007/s00381-007-0398-2. [DOI] [PubMed] [Google Scholar]
- 4.NHS England . NHS England Commissioning Board; Leeds: 2013. Commissioning Board. Clinical commissioning policy statement: selective dorsal rhizotomy for spasticity in children with cerebral palsy. Reference: NHSCB/E09/PS/a. [Google Scholar]
- 5.Tedroff K, Löwing K, Åström E. A prospective cohort study investigating gross motor function, pain, and health-related quality of life 17 years after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2015;57:484–490. doi: 10.1111/dmcn.12665. [DOI] [PubMed] [Google Scholar]
- 6.Steinbok P, Reiner AM, Beauchamp R. A randomized clinical trial to compare posterior rhizotomy plus physiotherapy with physiotherapy alone in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 1997;39:178–184. doi: 10.1111/j.1469-8749.1997.tb07407.x. [DOI] [PubMed] [Google Scholar]
- 7.Grunt S, Becher JG, Vermeulen RJ. Long-term outcome and adverse effects of selective dorsal rhizotomy in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2011;53:490–498. doi: 10.1111/j.1469-8749.2011.03912.x. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin JF, Bjornson KF, Astley SJ. Selective dorsal rhizotomy: efficacy and safety in an investigator-masked randomized clinical trial. Dev Med Child Neurol. 1998;40:220–232. doi: 10.1111/j.1469-8749.1998.tb15454.x. [DOI] [PubMed] [Google Scholar]
- 9.Wright FV, Sheil EMH, Drake JM. Evaluation of selective dorsal rhizotomy for the reduction of spasticity in cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 1998;40:239–247. doi: 10.1111/j.1469-8749.1998.tb15456.x. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin JF, Bjornson KF, Steinbok P. Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol. 2002;44:17–25. doi: 10.1017/s0012162201001608. [DOI] [PubMed] [Google Scholar]
- 11.NHSEngland Standard operating procedures: individual funding requests. June 20, 2018. https://www.england.nhs.uk/publication/standard-operating-procedures-individual-funding-requests/
- 12.Dudley RW, Parolin M, Gagnon B. Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr. 2013;12:142–150. doi: 10.3171/2013.4.PEDS12539. [DOI] [PubMed] [Google Scholar]
- 13.Langerak NG, Lamberts RP, Fieggen AG. Functional status of patients with cerebral palsy according to the International Classification of Functioning, Disability and Health model: a 20-year follow-up study after selective dorsal rhizotomy. Arch Phys Med Rehabil. 2009;90:994–1003. doi: 10.1016/j.apmr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Park TS, Liu JL, Edwards C. Functional outcomes of childhood selective dorsal rhizotomy 20 to 28 years later. Cureus. 2017;9:e1256. doi: 10.7759/cureus.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCaP)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna SE, Bartlett DJ, Rivard LM, Russell DJ. Tabulated reference percentiles for the 66-item Gross Motor Function Measure for use with children having cerebral palsy. September, 2008. https://canchild.ca/system/tenon/assets/attachments/000/000/222/original/tabulated_gmfm66_percentiles.pdf
- 17.Hanna SE, Bartlett DJ, Rivard LM, Russell DJ. Reference curves for the Gross Motor Function Measure: percentiles for clinical description and tracking over time among children with cerebral palsy. Phys Ther. 2008;88:596–607. doi: 10.2522/ptj.20070314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell DJ, Rosenbaum PL, Wright M, Avery LM. 2nd edn. MacKeith Press; Canada: 2013. Gross Motor Function Measure (GMFM-66 & GMFM-88) user's manual. [Google Scholar]
- 19.Palisano R, Rosenbaum P, Bartlett D, Livingston M. GMFCS-E&R: gross motor function classification system: expanded and revised. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 20.Reimers J, Poulsen S. Adductor transfer versus tenotomy for stability of the hip in spastic cerebral palsy. J Pediatr Orthop. 1984;4:52–54. doi: 10.1097/01241398-198401000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Park TS, Johnston JM. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Neurosurg. 2006;21:e7. [PubMed] [Google Scholar]
- 22.CanChild Resources: Gross Motor Function Measure (GMFM) 2018. https://canchild.ca/en/resources/44-gross-motor-function-measure-gmfm
- 23.Hanna SE, Rosenbaum PL, Bartlett DJ. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 2008;51:295–302. doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen KL, Wang HY, Tseng MH. The Cerebral Palsy Quality of Life for Children (CP QOL-Child): evidence of construct validity. Res Dev Disabil. 2013;34:994–1000. doi: 10.1016/j.ridd.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 26.Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol. 1999;6:S23–S35. [Google Scholar]
- 27.Tedroff K, Löwing K, Jacobsen DNO, Åström E. Does loss of spasticity matter? A 10-year follow-up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2011;53:724–729. doi: 10.1111/j.1469-8749.2011.03969.x. [DOI] [PubMed] [Google Scholar]
- 28.NHS England Specialised commissioning team, clinical commissioning policy statement: selective dorsal rhizotomy (SDR) for the treatment of spasiticty in cerebral palsy (children aged 3–9 years): reference 170063P. July, 2018. https://www.england.nhs.uk/wp-content/uploads/2018/07/1769-selective-dorsal-rhizotomy.pdf
- 29.Bolster EA, Van Schie PE, Becher JG. Long-term effect of selective dorsal rhizotomy on gross motor function in ambulant children with spastic bilateral cerebral palsy, compared with reference centiles. Dev Med Child Neurol. 2013;55:610–616. doi: 10.1111/dmcn.12148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.