Abstract
Exercise can exert anti-inflammatory effects in an intensity-dependent manner; however, the mechanisms mediating these effects are continually being established. Programme Death Receptor-1 (PD-1) is a membrane bound receptor that maintains immune tolerance by dampening immune cell interactions, such as those mediated by cytotoxic T-cell lymphocytes (CD8+). The aim of this study was to characterise sub-populations of CD8+ T-cells with regards to their expression of PD-1 before and immediately after exercise. Interleukin (IL)-6, soluble PD-1 (sPD-1) and its ligand (sPD-L1) were also quantified in plasma. Eight individuals (mean ± SD: age 29 ± 5 years; BMI 24.2 ± 3.4 kg m2; O2max 44.5 ± 6.4 ml kg−1·min−1) undertook two time and energy-matched cycling bouts in a counterbalanced study design: one of moderate intensity (MOD) and a bout of high intensity interval exercise (HIIE). Both MOD and HIIE increased the number, but not the proportion of circulating CD8+ PD-1+ cells, with no differences between trials. Within the CD8+ PD-1+ pool, the expression of PD-1 increased on central memory cells following HIIE only (fold change: MOD 1.0 vs HIIE +1.4), as well the concentration of CD8+PD-1+ memory cells within the circulation (cells/uL: MOD -0.4 vs HIIE +5.8). This response composed a very small part of the exercise-induced CD8+ lymphocytosis (Pre-Ex: 0.38% to Post-Ex: 0.69%; p > 0.05). sPD-L1 and IL-6 concentration increased in tandem following MOD and HIIE (r = 0.57; P = 0.021), with a reciprocal decline in sPD-1 observed. The current data demonstrate that PD-1+ CD8+ lymphocytes were mobilised following both MOD and HIIE. Both the number of central memory CD8+ T-cells expressing PD-1 and the expression level on these cells were increased following HIIE only. This intensity-dependent phenotypic response, in conjunction with increased circulatory sPD-L1 may represent an aspect of the anti-inflammatory response to exercise and warrants further investigation.
Keywords: Immune checkpoints, Immune tolerance, sPD-1, sPD-L1, Exercise
Highlights
-
•
PD-1 is a membrane-bound T-cell receptor that regulates immune tolerance.
-
•
We explored phenotypic changes in PD-1+ T-cells after exercise.
-
•
Circulating PD-1+ CD8+ T-cells increased after moderate and high intensity interval exercise (HIIE).
-
•
Central memory CD8+ T-cell number and expression increased after HIIE only.
-
•
Post-exercise levels of soluble PD-1 Ligand increased and correlated with IL-6.
1. Introduction
Regular exercise can lower systemic inflammation in both healthy and clinical populations (Hamer et al., 2014, 2012). The cumulative impact of acute inflammatory responses that follow individual exercise bouts is believed to be a major stimulus for creating this anti-inflammatory environment, which appears more marked at higher exercise intensities (Wang et al., 2012). The mechanisms underpinning these effects are primarily attributed to: 1) increased release of myokines from skeletal muscle (e.g. IL-6), 2) phenotypic shifts in cell composition (blood monocytes and adipose tissue-resident macrophages) and 3) a reduction in the expression of membrane-bound immune receptors that govern immune cell activity, migration and inflammatory cytokine production (Gleeson et al., 2011). The independent contribution of these factors is far from clear and a myriad of other mechanisms likely contribute. For example, programmed-death receptor-1 (PD-1) is an immune checkpoint receptor with an established role in regulating circulating inflammatory signals (Coles et al., 2014) but is yet to be extensively characterised following acute exercise or periods of exercise training.
PD-1 is a membrane-bound receptor primarily expressed on lymphoid cells that provides a ‘brake’ or checkpoint for the immune system (Francisco et al., 2010). PD-1 interacts specifically with its co-stimulatory ligands, PD-Ligand 1 (PD-L1) and 2 (PD-L2), which are ubiquitously expressed. These ligand-receptor interactions result in reduced immune cell activity and thus provide an anti-inflammatory environment that is critical for the maintenance of immunological homeostasis. It is now well established that aberrant expression of PD-1 can dramatically affect immune function and health (Bartosińska et al., 2018; Granados et al., 2017; Iijima et al., 2017; Ilie et al., 2016; Li et al., 2014; Lu et al., 2017; Novák et al., 2015). High expression of PD-1 on certain immune cells (e.g. cytotoxic T-cells (CD8+)) is known to severely suppress immune function in numerous blood and tissue cancers (Ilie et al., 2016; Lu et al., 2017; Novák et al., 2015). Conversely, reduced expression of PD-1 has been documented on CD8+ T-cells from patients with autoimmune diseases, such as type-1 diabetes (Granados et al., 2017; Iijima et al., 2017), rheumatoid arthritis (Li et al., 2014) and Psoriasis (Bartosińska et al., 2018), driving autoimmunity. These studies underpin the central role that PD-1 has in regulating immunological homeostasis, and yet, alterations in PD-1 expression have only recently been explored for the first time in the context of exercise (Gustafson et al., 2017).
Gustafson et al. (2017) reported that a single session of maximal cycling marginally increased the blood composition of PD-1+ T-cells within CD4+ (+3.15%) and CD8+ (+5.53%) subsets (Gustafson et al., 2017). It is well documented that the influx of lymphocytes into the circulation during exercise is largely driven by CD8+, rather than CD4+ T-cells (Turner et al., 2016), and is dependent on the intensity of the bout (Campbell et al., 2009). The composition of this CD8+ T-cell pool is markedly altered to favour increased immune surveillance for antigen-presentation at secondary lymphoid organs and inflamed tissues (Dhabhar et al., 2012). PD-1 is primarily expressed on memory cells (Claireaux et al., 2018) and functions to limit the inflammatory response by increasing apoptosis of antigen-specific effector T-cells (Francisco et al., 2010); however, how antigen-specific frequencies of CD8+ T-cells expressing PD-1 change following exercise bouts of different intensities is currently unknown. Further to the membrane-bound forms of PD-1 and PD-L1, soluble (s) PD-1 and sPD-L1 are detectable within the extracellular environment of blood (Chen et al., 2018; Wei et al., 2018), but have yet to be explored in the context of exercise, despite their role in modulating T-cell activity (Kuipers et al., 2006; Song et al., 2011).
Our collective understanding of both membrane and soluble PD-1 in the context of exercise and immunity is currently lacking, particularly regarding how exercise alters the specific memory phenotype of T-cells expressing PD-1, their physiological relevance in blood (i.e. number of cells expressing PD-1) and relationships to sPD-1 and sPD-L1. Characterisation of these responses could provide important insight into the anti-inflammatory effects that underpin exercise, with important subsequent implications for health and immunity. The aim of the present study was to characterise changes in the expression of PD-1 in circulating naïve, central memory, effector memory and terminally differentiated CD8+ T-cells following two time and energy-matched bouts of moderate and high intensity cycling. To better understand the link between cellular PD-1 and plasma sPD-1/sPD-L1 in the context of inflammation, sample-matched IL-6 measurements were made.
2. Materials and methods
2.1. Participants
Following ethical approval from the University of Worcester Research Ethics Committee, eight healthy males (mean ± SD: age 29 ± 5 years; BMI 24.2 ± 3.4 kg m2; O2max 44.5 ± 6.4 ml kg−1·min−1) were recruited to take part in the study. All participants completed questionnaires addressing health history and habitual levels of weekly physical activity using the International Physical Activity Questionnaire (IPAQ). All participants gave their written informed consent and the study was carried out in accordance with the Declaration of Helsinki (2008). Participants were non-smokers and reported that they had not taken any antioxidant vitamin supplements or anti-inflammatory drugs for 8 weeks prior to the laboratory visit. In addition, participants reported to be free from any viral or bacterial infections for at least 4 weeks prior to taking part. Participants were also required to refrain from any strenuous physical activity, consumption of alcoholic beverages or caffeine for two days prior to each experimental session.
2.2. Experimental sessions
Participants reported to the laboratory on three separate occasions, all carried out under stable climatic conditions (18–20 °C and humidity between 45 – 55%). Following a 30-min period of rest, resting heart rate (RS400, Polar Electro, Finland), height (Seca Alpha, Hamburg, Germany) and body mass (Tanita, Tokyo, Japan) were determined on each visit. On participants first visit, cardiorespiratory fitness (max) was assessed using a ramp test to exhaustion on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Groningen, Netherlands). Workload commenced at 100 Watts and was increased by 30 Watts every 4 min. Heart rate and oxygen uptake was assessed continuously using a breath-by-breath system (Cortex Biophysik Metalyzer, Germany). max was determined if two of the following criteria were met in conjunction with a plateau in oxygen consumption after an increase in workload: volitional exhaustion; a respiratory exchange ratio of ≥1.15; heart rate within 10 beats·min−1 of the age-predicted maximal heart rate (220 - age); a drop in cadence below 60 revolutions per minute (Howley et al., 1995). A final obtained value of rate of oxygen consumption relative to body mass was accepted as max (ml.kg−1min−1) and used to inform the workload for the subsequent two trials.
At least one week following the max test, participants undertook two energy and time-matched cycling trials in a counterbalanced order: a moderate intensity bout of continuous cycling at 60% max for 58 min (MOD) and a bout of high intensity interval exercise (HIIE), consisting of 10 x 4-min intervals at 85% max, with 2-min rest intervals. Both trials took place following an identical overnight fast at least one week apart. In both studies, oxygen uptake was assessed continuously to maintain target and ensure equal energy expenditure between MOD and HIIE. Ratings of perceived exertion (RPE) were measured every 6 min during the trials.
2.3. Blood sampling
In both MOD and HIIE, a catheter (Appleton Woods, Birmingham, UK) was inserted into the antecubital vein of the arm prior to exercise to obtain a baseline sample after 30 min of rest (Pre-Ex). Additional blood samples were taken immediately (Post-Ex), 30 min (Post-Ex+30) and 60 min (Post-Ex+60) following completion of each cycling trial. At Pre and Post+0 timepoints, 18 ml of blood was drawn into two separate vacutainer tubes containing potassium ethylene diaminetetraacetic acid (EDTA) (Becton, Dickson & Company, Oxford, UK) for independent isolation or peripheral blood monocular cells (PBMCs) and plasma. At Post-Ex+30 and Post-Ex+60 timepoints, 9 ml of blood was collected for plasma isolation only. The catheter was flushed every 30 min with isotonic saline solution (0.9% sodium chloride) to prevent blood clotting.
2.4. Plasma analyses
A cytometric bead array technique was used to quantify plasma interleukin (IL)-6 on a BD C6 Accuri Flow Cytometer (BD Biosciences, Berkshire). Plasma PD-L1 (BMS2212; sensitivity: 0.6 pg/ml, intraassay CV: 2.1%, interassay CV: 3.4%) and PD-1 (BMS2214; sensitivity 1.14 pg/ml, intraassay CV: 3.2%, interassay CV: 6.4%) were quantified using commercially available ELISA kits (Thermo Fisher Scientific, Loughborough). All concentrations were corrected for changes in plasma volume based upon established criteria (Dill and Costill, 1974).
2.5. Isolation of CD8+ T-cells
Whole blood from Pre-Ex and Post-Ex timepoints only were used to isolate PBMCs using density gradient centrifugation. Blood was diluted 1:1 with Hanks Balance Salt Solution (HBSS), and then layered carefully on top of Histopaque 1077 (Sigma, Dorset), before centrifuging at 400g for 40 min at 21 °C. The PBMC layer was aspirated and then washed three times with HBSS, by centrifuging steps at 300g for 10 min. Approximately 3 million cells per time point were then used to enrich CD8+ T-cells by negative selection using MACS® bead separation (Miltenyi Biotec, Surrey, UK). PBMCs were incubated with a biotin-antibody cocktail (anti-CD4, CD15, CD16, CD19, CD34, CD36, CD56, CD123, TCRγ/δ, and CD235a), followed by a CD8+ T-cell microbead cocktail (anti-CD14, CD61 and anti-biotin) for 10 min at 4 °C, with intermittent washes using column wash buffer (PBS supplemented with 0.5% bovine serum albumin and 2 mM EDTA; pH = 7.2). CD8+ cells from both time-points were eluted by negative selection using three separate MiniMACS LD-column passes, using an excess of de-gassed column buffer (Miltenyi Biotec, Surrey, UK). CD8+ T-cell enrichment was confirmed by flow cytometry staining analysing the CD3+CD8+ lymphocyte population (>95% purity).
2.6. Flow cytometry
Approximately 1,000,000 viable PBMCs were used for determination of CD8+ T-cell subsets and expression of PD-1 using four-colour flow cytometry (Guava EasyCyte, Millipore UK Ltd, Hertfordshire, UK). Cells were incubated with fluorescently conjugated antibodies – CD3-FITC (clone: HIT3a), CD279-PE (clone: EH12.2H7), CD45RA-PerCP (clone: HI100), CD27-APC (clone: M-T271) (Biolegend, Cambridge, UK) for 30 min at 4 °C followed by intermittent washes with PBS for 5 min at 300×g. Compensation was adjusted daily by using single stained controls and gates established using fluorescence minus one controls. Confirmation of non-specific antibody binding was determined by using isotope-matched controls.
Flow cytometry data were analysed using GuavaSoft 3.1 (Millipore UK Ltd, Hertfordshire, UK). Briefly, lymphocytes were gated on forward versus side scatter and CD3+ and CD8+ T-cell proportions used to determine MACS enrichment efficacy. MACS enriched CD8+ T-cells were identified as naïve (CD27+ CD45RA+), central memory (CD27+ CD45RA-), effector memory (CD27− CD45RA-), or terminally differentiated effector memory cells (TEMRA: CD27− CD45RA+) accordingly (Di Mitri et al., 2011). Within each sub-population, mean fluorescence intensity (MFI) and percentage positive cells was established for PD-1 (CD279). The percentage of PD-1+ cells were used with whole blood cell counts to determine the circulating number of PD-1+ cells in peripheral blood for each sub-population, and adjusted for changes in plasma volume.
2.7. Statistical analysis
The Shapiro Wilk test was used to check for normality in scale data at all time points. Variables with a non-normal distribution were log transformed if necessary. Changes in sub-populations of immune cells, CD8+ cells expressing PD-1, PD-1 expression levels and plasma markers (sPD-1, sPD-L1 and IL-6) were assessed over time (Pre, Post, Post-Ex+30 and Post-Ex+60) and between Trials (MOD, HIIE) by a 2∗2 (cellular data) or 2∗4 (plasma data) repeated-measures analysis of variance (ANOVA) or Wilcoxon Signed Rank Tests, depending on variable normality. Post hoc analysis of any ANOVA interaction effects (Trial∗Time) were performed by a test of simple effects by pairwise comparisons, with Bonferroni correction. Mann Whitney U tests were performed to determine differences between MOD and HIIE at different timepoints for non-parametric data. Effect sizes for main effects and interaction effects of ANOVA are presented as partial eta2 (η2p), using Cohen’s definition of η2p of 0.01, 0.06 and 0.14 for ‘small’, ‘medium’ and ‘large’ effects respectively (Cohen, 1988). Pearson correlation and Spearman rank were used to assess the relationship between parametric and non-parametric data respectively. All values are presented as means ± standard deviation or error (indicated throughout manuscript). Data were back transformed for ease of presentation in figures. Statistical significance was accepted at the P < 0.05 level. Statistical analyses were performed using SPSS (PASW Statistics, release 23.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Physiological response during MOD and HIIE
The physiological responses during each exercise bout are reported in detail elsewhere (Wadley et al., 2019). Briefly, Peak and RPE were significantly greater in HIIE compared to MOD (P < 0.00001), but there were no statistically significant differences in mean and energy expenditure.
3.2. Changes in lymphocyte sub-populations after MOD and HIIE in blood
Changes in lymphocyte and CD8+ T-cell subset concentrations are reported in Table 1, Table 2 respectively. Total lymphocyte concentration increased after both trials (P = 0.014). Within the lymphocyte pools, CD3+ (P = 0.011) and CD8+ (P = 0.006) T-cell concentrations also increased, with no differences between MOD and HIIE for any lymphocyte subset. Within the CD8+ T-cell pool, naïve (P = 0.050), central memory (P = 0.036), effector memory (P = 0.050) and TEMRA (P = 0.012) all increased following MOD, but only TEMRA increased following HIIE (P = 0.049). There were no statistically significant differences in CD8+ T-cell concentrations between MOD and HIIE at any timepoint.
Table 1.
Mean changes (SD) in total lymphocytes, CD3+ and CD8+ T-cells before and after MOD and HIIE.
| Pre-Exercise | Post-Exercise | Main Effects of Time | Time × Trial Interaction | |
|---|---|---|---|---|
| Lymphocytes (cells/uL) | ||||
| MOD | 1720.2 (651.1) | 2761.3 (1292.3) ∗ | F(1,7) = 10.7; P = 0.014; η2 = 0.6 | F(1,7) = 0.03; P = NS |
| HIIE | 1559.4 (456.9) | 2523.7 (1165.7) ∗ | ||
| CD3+T-cells (cells/uL) | ||||
| MOD | 1272.8 (515.1) | 2022.19 (515.1) ∗ | F(1,7) = 11.7; P = 0.011; η2 = 0.6 | F(1,7) = 0.02; P = NS |
| HIIE | 1180.9 (365.9) | 1877.2 (870.9) ∗ | ||
| CD8+T-cells (cells/uL) | ||||
| MOD | 419.5 (161.2) | 717.8 (337.4) ∗ | F(1,7) = 15.1; P = 0.006; η2 = 0.7 | F(1,7) = 0.22; P = NS |
| HIIE | 413.0 (101.7) | 688.9 (310.6) ∗ | ||
Legend: ∗P < 0.05; NS P > 0.05.
Table 2.
Mean changes (SD) in CD8+ T-cell subsets before and after MOD and HIIE.
| Naïve CD8+ T-cells (cells/uL) | |||
|---|---|---|---|
| Pre-Ex | Post-Ex | Time: Pre vs. Post | |
| MOD | 84.8 (120.3) | 161.7 (209.8) ∗ | Z = -1.96; P = 0.05, η2 = 0.24 |
| HIIE | 81.0 (63.1) | 116.8 (82.8) | Z = -1.12; P = NS |
|
Trial: MOD vs HIIE |
Z = -0.42; P = NS |
Z = 0; P = NS |
|
| Central Memory CD8+T-cells (cells/uL) | |||
|
Pre-Ex |
Post-Ex |
Time: Pre vs. Post |
|
| MOD | 50.3 (46.2) | 126.0 (142.1) ∗ | Z = -2.10; P = 0.036, η2 = 0.28 |
| HIIE | 89.9 (75.8) | 159.8 (158.6) | Z = -1.26; P = NS |
|
Trial: MOD vs HIIE |
Z = -1.05; P = NS |
Z = -0.84; P = NS |
|
| Effector Memory CD8+T-cells (cells/uL) | |||
|
Pre-Ex |
Post-Ex |
Time: Pre vs. Post |
|
| MOD | 113.1 (72.5) | 166.9 (59.0) ∗ | Z = -1.96 P = 0.05, η2 = 0.24 |
| HIIE | 123.6 (96.1) | 168.0 (127.0) | Z = -1.12; P = NS |
|
Trial: MOD vs HIIE |
Z = 0; P = NS |
Z = -0.63; P = NS |
|
| Terminally Differentiated Effector Memory CD8+T-cells (cells/uL) | |||
|
Pre-Ex |
Post-Ex |
Time: Pre vs. Post |
|
| MOD | 171.3 (65.4) | 263.2 (94.1) ∗ | Z = -2.52; P = 0.012, η2 = 0.40 |
| HIIE | 118.4 (98.1) | 244.3 (184.5) ∗ | Z = -1.82; P = 0.049, η2 = 0.21 |
| Trial: MOD vs HIIE | Z = -1.26; P = NS | Z = -0.84; P = NS | |
Legend: ∗P < 0.05; NS P > 0.05.
3.3. Changes in PD-1+ cell concentration, expression and composition within the CD8+ T-cell pool after MOD and HIIE
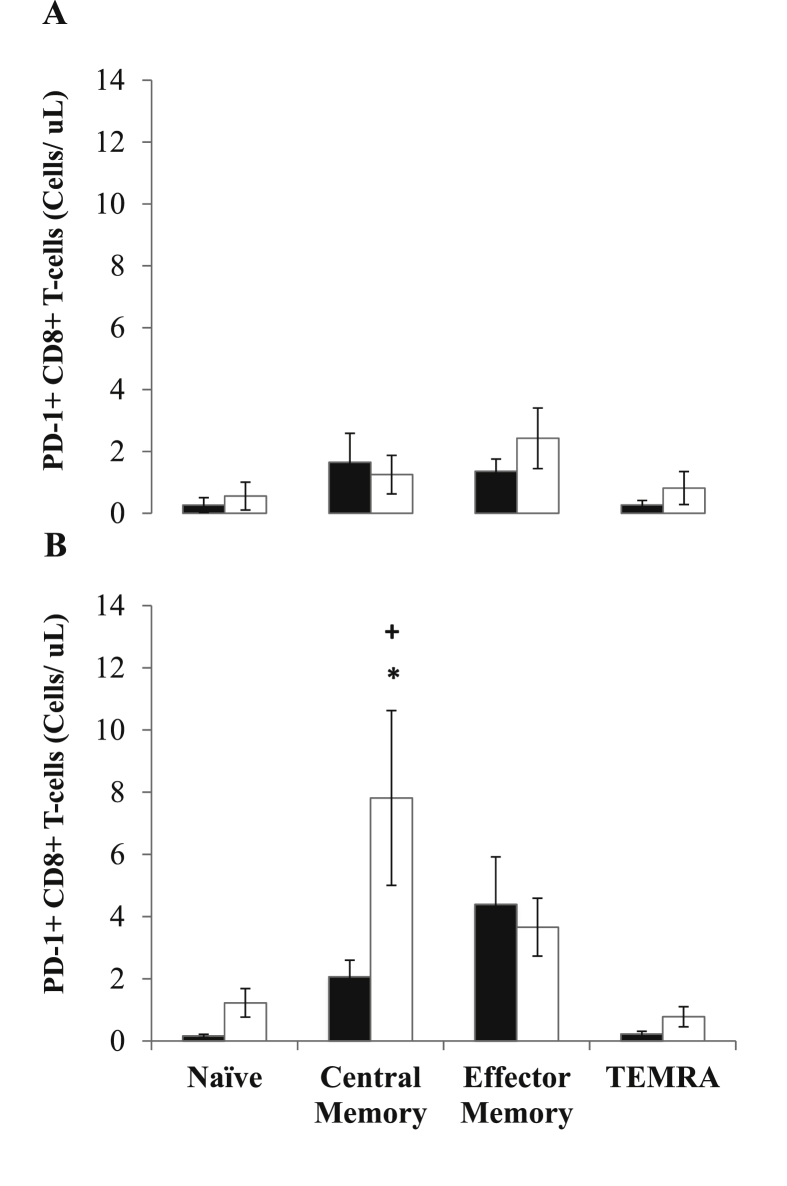
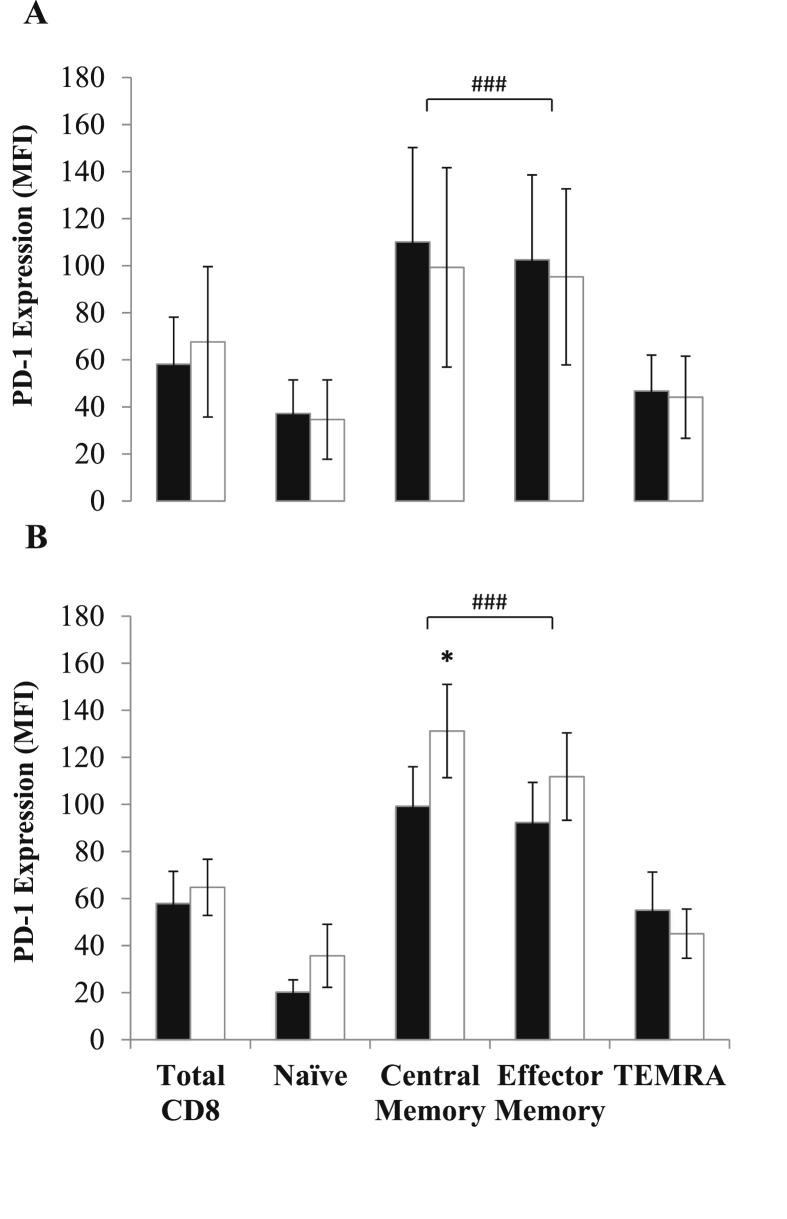
PD-1 expression was significantly higher on central and effector memory T-cell subsets when compared to naïve or TEMRA (P’s < 0.0001). The circulating concentration of CD8+ PD-1+ T-cells was significantly higher after both trials (MOD: Z = −2.38, P = 0.017, η2 = 0.35 and HIIE: Z = −1.26, P = 0.234), however there were no significant differences between trials (Z = −2.10, P = 0.035, η2 = 0.28). Changes in PD-1+ cell concentration and expression within CD8+ T-cell subsets are reported in Fig. 1, Fig. 2 respectively. Within the CD8+ T-cell pool, there was a significant increase in PD-1+ central memory cells following HIIE only (Z = −2.31, P = 0.021, η2 = 0.33), with cell number significantly higher than MOD Post-Ex (Z = −2.52, P = 0.010. η2 = 0.40). In addition, PD-1 MFI was significantly greater in central memory cells following HIIE, indicating an increase in receptor expression (Z = −2.24, P = 0.025, η2 = 0.31).
Fig. 1.
Changes in the concentrations of CD8+ T-cell subsets (naïve, central memory, effector memory and terminally differentiated effector memory (TEMRA) cells) before (black bars) and after (white bars) MOD (panel A) and HIIE (panel B). Values are means ± standard error. ∗ indicates significant differences relative to Pre-Ex: ∗p < 0.05.+indicates a significant difference between MOD and HIIE:+p < 0.05.
Fig. 2.
Changes in the expression of PD-1 in CD8+ T-cells and their subsets before (black bars) and after (white bars) MOD (panel A) and HIIE (panel B). Values are means ± standard error. ∗ indicates a significant difference relative to Pre-Ex: ∗p < 0.05. # indicates significantly higher expression levels in memory T-cells subsets, compared to naïve and TEMRA: ###p < 0.0001.
3.4. Changes in sPD-1, sPD-L1 and IL-6 in response to MOD and HIIE and associations with cellular variables
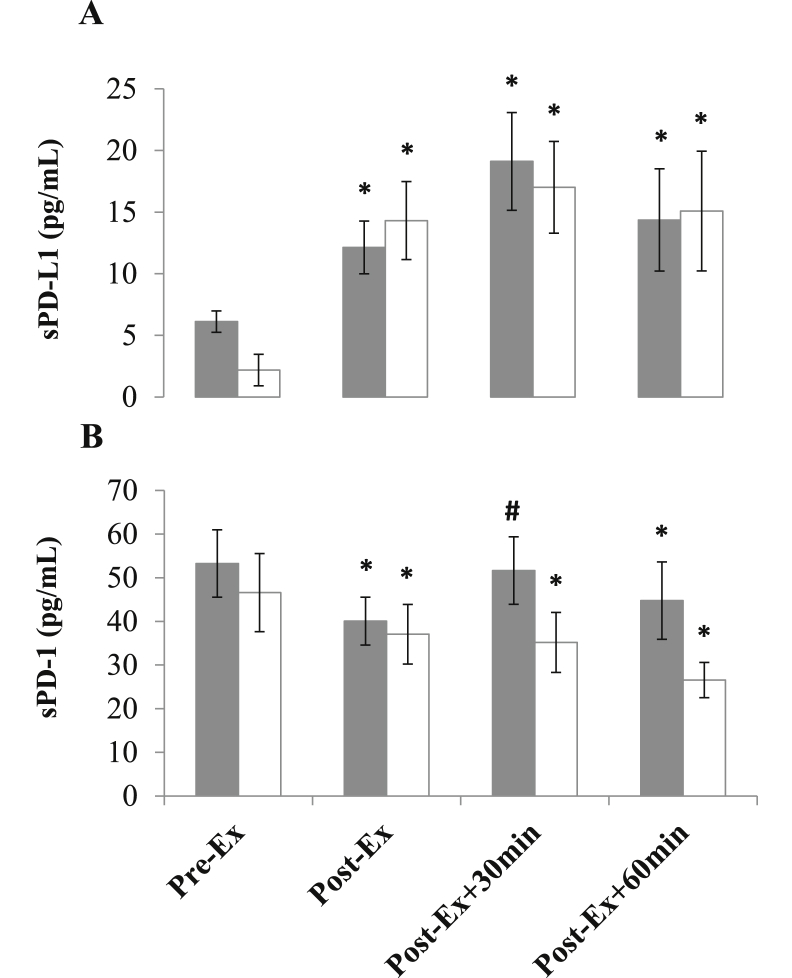
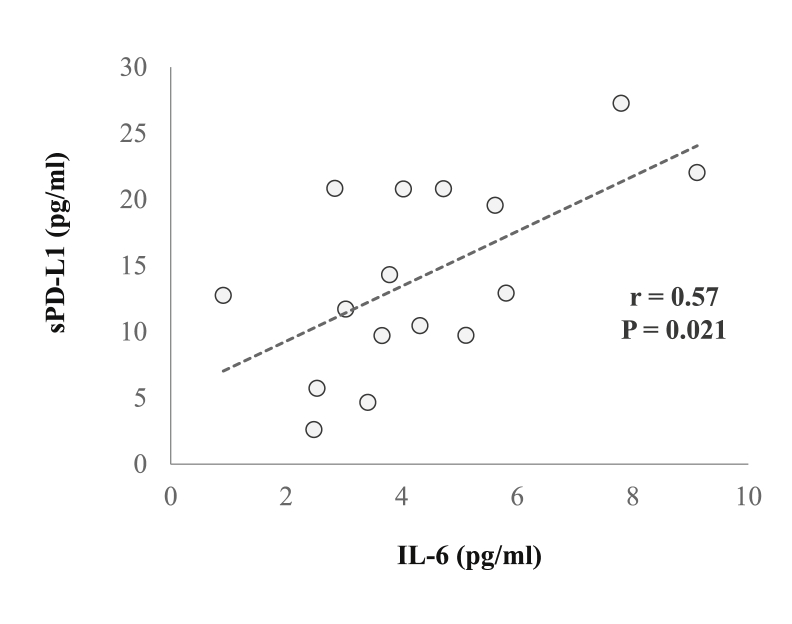
Changes in plasma levels of sPD-1 and sPD-L1 are reported in Fig. 3 sPD-L1 concentration increased immediately (MOD: Z = −2.38, P = 0.017, η2 = 0.35 and HIIE: Z = −2.38, P = 0.017, η2 = 0.35) after exercise and was elevated Post-Ex+30 and Post-Ex+60 (MOD: Z = −2.52, P = 0.012, η2 = 0.40 and HIIE: Z = −2.52, P = 0.012, η2 = 0.40). A decrease in sPD-1 concentration was observed immediately after both trials (MOD: Z = −2.52, P = 0.012, η2 = 0.40 and HIIE: Z = −2.24, P = 0.025, η2 = 0.31), and remained below Pre-Ex levels at Post-Ex+60 (MOD: Z = −2.38, P = 0.017, η2 = 0.35 and HIIE: Z = −2.10, P = 0.036, η2 = 0.28). IL-6 concentration increased above Pre-Ex at all post-exercise timepoints in both trials (Time effect: F (3) = 15.5, P < 0.0001, η2 = 0.66), with the magnitude of increase significantly greater following HIIE (Time x Condition effect: F (3) = 7.0, P < 0.001, η2 = 0.47). A positive correlation was noted between concentrations of IL-6 and PD-L1 Post-Ex (r = 0.57; P = 0.021). No statistically significant correlations were noted between plasma and cellular variables.
Fig. 3.
Changes in the concentrations of PD-L1 (A) and PD-1 (B) before (Pre-Ex) and after (Post-Ex, Post-Ex+30 and Post-Ex+60) MOD (grey bars) and HIIE (white bars). Values are means ± standard error. ∗ indicates a significant difference relative to Pre-Ex: ∗p < 0.05. # indicates a significant difference relative to Post-Ex: #p < 0.05.
4. Discussion
The current study highlights that single bouts of moderate and high intensity interval exercise evoke an increase in the circulating concentration of PD-1+ CD8+ T-cells in young, untrained, healthy males. As part of this response, the number of PD-1+ CD8+ central memory T-cells increased following HIIE only, with PD-1 expression also increased on these cells. Plasma sPD-L1 and sPD-1 increased and decreased respectively after both trials, with a positive association noted between IL-6 and PD-L1. Taken together, these results highlight a phenotypic specific change in the regulation of PD-1 on CD8+ T-cells that is exercise intensity-dependent. Furthermore, we highlight for the first time that soluble forms of the PD-1 receptor and ligand are reciprocally regulated after exercise, and relate to changes in IL-6. This study provides evidence of a potentially novel aspect of the anti-inflammatory response to exercise and warrants further investigation to contextualise the findings amongst other established mechanisms.
A single session of exercise induces large haemodynamic and β2-adrenergic receptor-mediated changes that drive the mobilisation of lymphocytes into the bloodstream (Graff et al., 2018). This response is not uniform, with lymphocytes such as CD8+ T-cells with high antigen specificity, activation and migratory capacity being preferentially mobilised (e.g. effector T-cells) (Campbell et al., 2009). The general consensus is that this response is highly functional, and operates to enhance immunosurveillance and tissue remodelling in the period after exercise (Campbell and Turner, 2018). As a result, exercise immunology research has largely focussed on characterising CD8+ T-cells with regards to their activation, migration and effector functions (e.g. cytotoxicity). Conversely, relatively little attention has been paid to negative regulators of T-cell function, which might act to suppress or modulate this functional redistribution of T-cells. The results of the current study demonstrate that both MOD and HIIE evoke an increase in PD-1+ CD8+ T-cell concentration into the bloodstream. The magnitude of this response was comparable between trials; however, the phenotypic responses differed, with the concentration of central memory CD8+ T-cells expressing PD-1 (Fig. 1) and the surface expression level (MFI) on these cells increasing after HIIE only (Fig. 2). Coupled to the finding that CD8+ PD-1- central memory T-cells were not significantly elevated after HIIE (data not shown), this indicates a specific phenotypic change with regards to PD-1 regulation within the central memory T-cell pool, rather than just changes in T-cell trafficking into the periphery.
PD-1 is a critical regulator of T-cell tolerance, modulating changes in cell activation (Keir et al., 2007), differentiation (Ahn et al., 2018) and migration into tissues (Brunner-Weinzierl and Rudd, 2018). Expression of PD-1 is primarily limited to memory T-cells (Gustafson et al., 2017) (Fig. 2), with progressive loss of the PD-1 receptor documented on T-cells with higher effector functions, such as TEMRA, which are typically the most sensitive to exercise-induced mobilisation (Campbell et al., 2009). Whereas effector memory T-cells circulate between blood and peripheral tissues, central memory T-cells circulate between lymph and blood (Drouillard et al., 2018), awaiting antigen presentation. We speculate that an increase in CD8+ central memory T-cell PD-1 expression after HIIE may be a homeostatic mechanism to limit T-cell receptor activation, differentiation and redistribution of antigen-specific T-cells from the lymphatic system after exercise. This change is directly related to the intensity of exercise, given that the HIIE protocol controlled for both total exercise duration and the energy cost of exercise, compared to MOD.
The only previous study to investigate changes in immune checkpoint expression after exercise reported an increase in the percentage of PD-1+, but not CTLA-4+ CD8+ T-cells after maximal cycling exercise (Gustafson et al., 2017). A compositional increase in PD-1+ CD8+ T-cells was not observed in the present study, and as such, suggests that preferential trafficking of PD-1+ CD8+ T-cells may only increase in response to maximal cycling exercise. Notably however, the current study subsequently phenotyped CD8+ T-cells, as well as determining physiologically relevant changes within the bloodstream in response to exercise. Importantly, the increase in CD8+ PD-1+ central memory T-cells composed a very small component of the total exercise-induced lymphocytosis (Pre-Ex: 0.38% to Post-Ex: 0.69%). Our data therefore provide novel insight into changes in PD-1 within T-cells after different intensities of exercise, both with regards to cell phenotype and physiologically relevant changes within the circulation. Both are undoubtedly important in understanding changes in T-cell function and their redistribution after exercise. Future work should take this approach to establish whether a small increase in CD8+ PD-1+ central memory T-cells has physiological relevance in governing the immune responses to exercise. For a more complete picture, other immune checkpoints, such as CTLA-4 should be explored in this context.
Another novel finding from the present study was that exercise evoked an increase in the circulating plasma concentration of sPD-L1 and decrease in sPD-1 immediately, and up to 60 min post-exercise (Fig. 3). These responses were not dependent on the intensity of exercise, nor associated with changes in membrane-bound PD-1 expression or circulating PD-1+ cell numbers. The exact cellular origin and functional actions of these soluble immune checkpoints have yet to be fully established. Both sPD-1 (primarily released from T-cells) and sPD-L1 (released from dendritic cells and tissues) (Gu et al., 2018) can be produced through alternate mRNA splicing (Nielsen et al., 2005) or cleavage of the membrane-bound form directly (Zanto et al., 2011). There is some evidence to suggest that sPD-1 and sPD-L1 may act in an antagonistic manner, such that sPD-1 competes and sPD-L1 engages with membrane-bound PD-1 (Gu et al., 2018; He et al., 2005). This is supported by studies suggesting that sPD-1 enhances (Yuan et al., 2004) and sPD-L1 inhibits (Li et al., 2016) T-cell activity. From the current study, we can therefore speculate that an increase in sPD-L1 after exercise may form a component of the anti-inflammatory response to exercise, acting to engage and activate membrane-bound PD-1. This is supported by a reduction in sPD-1 concentration and a positive association between post-exercise sPD-L1 and IL-6 (Fig. 4), a pleiotropic cytokine with a role in anti-inflammatory signalling (Ellingsgaard et al., 2019). It must be noted that the cellular mechanisms mediating the removal of sPD-1 from the circulation in this short timeframe after exercise is far from clear at present. Further work is needed to fully understand the kinetics of soluble immune checkpoints after exercise and their functional role is regulating communication between immune cells and tissues.
Fig. 4.
Pearson’s correlation between Post-Ex IL-6 and sPD-L1 concentrations across both trials (n = 16).
PD-1 and other immune checkpoints have attracted significant attention in biomedical and clinical research because of their profound impact on regulating immune tolerance in diseases associated with immune dysfunction. Immune checkpoint expression is commonly elevated on T-cells isolated from patients with blood and tissue cancers (Ilie et al., 2016; Lu et al., 2017; Novák et al., 2015) and conversely reduced on T-cells from patients with various autoimmune diseases (Bartosińska et al., 2018; Granados et al., 2017; Iijima et al., 2017; Li et al., 2014). How an increase in PD-1 expression after acute exercise translates into changes with regular exercise training, particularly in these populations is currently unknown. It is conceivable that exercise-induced upregulation of PD-1 expression could enhance immune tolerance in patients with certain autoimmune diseases; however, more work is needed to investigate the effects of regular exercise on immune checkpoints in a clinical context.
5. Conclusion
The results of the current study have highlighted phenotypic and physiological relevant changes in PD-1+ CD8+ T-cells after bouts of MOD and HIIE. PD-1+ CD8+ T-cells are mobilised in response to both types of exercise, however, central memory CD8+ T-cells expressing PD-1 increased after HIIE only. Despite this being a very small component of the exercise-induced lymphocytosis, PD-1 expression also increased on these cells, highlighting a specific phenotypic response associated with exercise at higher intensities. We have also highlighted for the first time that soluble immune checkpoints are altered in response to exercise, with sPD-L1 and sPD-1 increasing and decreasing up to 60 min after exercise respectively. Further work is needed to examine the changes in membrane and soluble immune checkpoints after exercise, and their relevance to regulating health in the context of autoimmunity.
Funding
This research was supported by the University of Worcester and the National Institute for Health Research NIHR) Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
AJW, SJC and TC were involved in the conception and design of the experiments. AJW, JV, TC and GK carried out all data acquisition at the University of Worcester. AJW and SJC carried out data analysis and interpretation. Drafting of the article for important intellectual content was undertaken by AJW and all authors undertook revision and final approval of the manuscript.
References
- Ahn E., Araki K., Hashimoto M., Li W., Riley J.L., Cheung J., Sharpe A.H., Freeman G.J., Irving B.A., Ahmed R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 2018;115:4749–4754. doi: 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosińska J., Zakrzewska E., Purkot J., Michalak-Stoma A., Kowal M., Krasowska D., Chodorowska G., Giannopoulos K. Decreased blood CD4+PD-1+ and CD8+PD-1+ T cells in psoriatic patients with and without arthritis. Postep. Dermatologii i Alergol. 2018;35:344–350. doi: 10.5114/ada.2018.75609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner-Weinzierl M.C., Rudd C.E. CTLA-4 and PD-1 control of T-cell motility and migration: implications for tumor immunotherapy. Front. Immunol. 2018;9:1–8. doi: 10.3389/fimmu.2018.02737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.P., Riddell N.E., Burns V.E., Turner M., van Zanten J.J.C.S.V., Drayson M.T., Bosch J. a. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Campbell J.P., Turner J.E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 2018;9:1–21. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Guo H., Li S., Liu C., Ding S., Huang Y., Fang C., Hu J. Soluble programmed death-1 ligand 1(sPD-L1) is significantly reduced in the serum of type 1 diabetes patients. Acta Diabetol. 2018;55:515–517. doi: 10.1007/s00592-017-1081-z. [DOI] [PubMed] [Google Scholar]
- Claireaux M., Galperin M., Benati D., Nouël A., Mukhopadhyay M., Klingler J., de Truchis P., Zucman D., Hendou S., Boufassa F., Moog C., Lambotte O., Chakrabarti L.A. A high frequency of HIV-Specific circulating follicular helper T cells is associated with preserved memory B cell responses in HIV Controllers. mBio. 2018;9:1–22. doi: 10.1128/mBio.00317-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. second ed. 1988. Statistical Power Analysis for the Behavioural Sciences. [Google Scholar]
- Coles S., Gilmore M.N., Reid R., Knapper S., Burnett A.K., Man S., Tonks A., Darley R.L. CD200 and PD1-L1 in AML are associated with expanded PD-1+ late differentiated CD8+ T cells and a decreased CD4:CD8 ratio: a new link between distinct immunosuppressive pathways. Blood. 2014;124:992. [Google Scholar]
- Dhabhar F.S., Malarkey W.B., Neri E., McEwen B.S. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones. Psychoneuroendocrinology. 2012;37:1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D., Azevedo R.I., Henson S.M., Libri V., Riddell N.E., Macaulay R., Kipling D., Soares M.V.D., Battistini L., Akbar A.N. Reversible senescence in human CD4 + CD45RA + CD27 − memory T cells. J. Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- Dill D.B., Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Drouillard A., Neyra A., Mathieu A.-L., Marçais A., Wencker M., Marvel J., Belot A., Walzer T. Human naive and memory T cells display opposite migratory responses to sphingosine-1 phosphate. J. Immunol. 2018;200:551–557. doi: 10.4049/jimmunol.1701278. [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H., Hojman P., Pedersen B.K. Exercise and health — emerging roles of IL-6. Curr. Opin. Physiol. 2019;10:49–54. doi: 10.1016/j.cophys.2019.03.009. [DOI] [Google Scholar]
- Francisco L.M., Sage P.T., Sharpe A.H. PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. (The) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Graff R.M., Kunz H.E., Agha N.H., Baker F.L., Laughlin M., Bigley A.B., Markofski M.M., LaVoy E.C., Katsanis E., Bond R.A., Bollard C.M., Simpson R.J. β 2 -Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Granados H.M., Draghi A., Tsurutani N., Wright K., Fernandez M.L., Sylvester F.A., Vella A.T. Programmed cell death-1, PD-1, is dysregulated in T cells from children with new onset type 1 diabetes. PloS One. 2017;12:1–11. doi: 10.1371/journal.pone.0183887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D., Ao X., Yang Y., Chen Z., Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J. Immunother. Cancer. 2018;6:132. doi: 10.1186/s40425-018-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson M.P., DiCostanzo A.C., Wheatley C.M., Kim C.H., Bornschlegl S., Gastineau D.A., Johnson B.D., Dietz A.B. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer. 2017;5:1–14. doi: 10.1186/s40425-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Hackett R.A., Bostock S., Lazzarino A.I., Carvalho L.A., Steptoe A. Objectively assessed physical activity, adiposity, and inflammatory markers in people with type 2 diabetes. BMJ Open Diabetes Res. Care. 2014;2 doi: 10.1136/bmjdrc-2014-000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Sabia S., Batty G.D., Shipley M.J., Tabak A.G., Singh-Manoux A., Kivimaki M. Physical activity and inflammatory markers over 10 years follow up in men and women from the Whitehall II cohort study. Circulation. 2012;126:928–933. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Zhang G., He Y., Zhu H., Zhang H., Feng Z. Blockade of B7-H1 with sPD-1 improves immunity against marine hepatocarcinoma. Anticancer Res. 2005;25:3309–3313. [PubMed] [Google Scholar]
- Howley E.T., Bassett D.R., Jr., Welch H.G. Criteria for maximal oxygen uptake: a review and commentary. Med. Sci. Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- Iijima T., Kato K., Jojima T., Tomotsune T., Fukushima M., Suzuki K., Aso Y. Circulating CD4+PD-1+ and CD8+PD-1+ T cells are profoundly decreased at the onset of fulminant type 1 diabetes and are restored by treatment, contrasting with CD4+CD25+FoxP3+ regulatory T cells. Diabetes Res. Clin. Pract. 2017;133:10–12. doi: 10.1016/j.diabres.2017.07.036. [DOI] [PubMed] [Google Scholar]
- Ilie M., Falk A.T., Butori C., Chamorey E., Bonnetaud C., Long E., Lassalle S., Zahaf K., Vénissac N., Mouroux J., Cohen C., Brambilla E., Marquette C.H., Hofman V., Hofman P. PD-L1 expression in basaloid squamous cell lung carcinoma: relationship to PD-1+ and CD8+ tumor-infiltrating T cells and outcome. Mod. Pathol. 2016;29:1552–1564. doi: 10.1038/modpathol.2016.149. [DOI] [PubMed] [Google Scholar]
- Keir M.E., Freeman G.J., Sharpe A.H. PD-1 regulates self-reactive CD8 + T cell responses to antigen in lymph nodes and tissues. J. Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- Kuipers H., Muskens F., Willart M., Hijdra D., van Assema F.B., Coyle A.J. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur. J. Immunol. 2006;36 doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- Li S., Liao W., Chen M., Shan S., Song Y., Zhang S., Song H., Author Z.Y. Expression of programmed death-1 (PD-1) on CD4+ and CD8+ T cells in rheumatoid arthritis. Inflammation. 2014;37:116–121. doi: 10.1007/s10753-013-9718-8. [DOI] [PubMed] [Google Scholar]
- Li Y., Xiao Y., Su M., Zhang R., Ding J., Hao X., Ma Y. Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp. Ther. Med. 2016;11:251–256. doi: 10.3892/etm.2015.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Yang L., Yao D., Wu X., Li J., Liu X., Deng L., Huang C., Wang Y., Li D., Liu J. Tumor antigen-specific CD8+ T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancer. Cell. Immunol. 2017;313:43–51. doi: 10.1016/j.cellimm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Nielsen C., Ohm-Laursen L., Barington T., Husby S., Lillevang S.T. Alternative splice variants of the human PD-1 gene. Cell. Immunol. 2005;235:109–116. doi: 10.1016/j.cellimm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Novák M., Procházka V., Turcsányi P., Papajík T. Numbers of CD8+PD-1+ and CD4+PD-1+ cells in peripheral blood of patients with chronic lymphocytic leukemia are independent of binet stage and are significantly higher compared to healthy volunteers. Acta Haematol. 2015;134:208–214. doi: 10.1159/000381468. [DOI] [PubMed] [Google Scholar]
- Song M.Y., Park S.H., Nam H.J., Choi D.H., Sung Y.C. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J. Immunother. 2011;34:297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]
- Turner J.E., Wadley A.J., Aldred S., Fisher J.P., Bosch J.A., Campbell J.P. Intensive exercise does not preferentially mobilize skin-homing T cells and NK cells. Med. Sci. Sports Exerc. 2016;48 doi: 10.1249/MSS.0000000000000914. [DOI] [PubMed] [Google Scholar]
- Wadley A.J., Keane G., Cullen T., James L., Vautrinot J., Davies M., Hussey B., Hunter D.J., Sarabjit M., Holliday A., Petersen S.V., Bishop N.C., Lindley M.R., Coles S.J. Characterisation of extracellular redox enzyme concentrations in response to exercise in humans. J. Appl. Physiol. 2019 doi: 10.1152/japplphysiol.00340.2019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Song H., Tang X., Yang Y., Vieira V.J., Niu Y., Ma Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand. J. Med. Sci. Sports. 2012;22:643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- Wei W., Xu B., Wang Y., Wu Chen, Jiang J., Wu Changping. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors. Med. (United States) 2018;97:1–6. doi: 10.1097/MD.0000000000009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., He Y., Wang X., Zhang H., Li D., Feng Z., Zhang G. Investigation on the effects of soluble Programmed Death-1 (sPD-1) enhancing anti-tumor immune response. J. Huazhong Univ. Sci. Technol. - Med. Sci. 2004;24:531–534. doi: 10.1007/bf02911345. [DOI] [PubMed] [Google Scholar]
- Zanto T.P., Hennigan K., Östberg M., Clapp W.C., Gazzaley A. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol. Lett. 2011;46:564–574. doi: 10.1016/j.cortex.2009.08.003.Predictive. [DOI] [Google Scholar]