Abstract
Obtaining multiple single-unit recordings in particular neural networks from behaving animals is crucial for the understanding of cognitive functions of the brain. Attaining stable, chronic recordings from the brain is also the foundation to develop effective cortical prosthetic devices. However, severe immune response caused by micromotion between stiff implants and surrounding brain tissue often limits the lifetime of penetrating, neural recording devices. To reduce the stiffness mismatch between recording devices and brain tissue, we developed a flexible, polymer based multi-electrode array for recording single neuron activities from the rat hippocampus, a major subcortical structure of the rat brain. Parylene C, a biocompatible polymer, was used as the structural and insulation material of the multi-electrode array. 64 platinum (Pt) recording electrodes were placed in groups along each shank to conform to the anatomical distribution of hippocampal principle neurons. The multi-electrode array was chronically implanted in three animals. After recovery, neural activity together with movement traces were collected from the behaving animals.
I. Introduction
Parylene C is one of the most popular polymers in the microelectromechanical systems (MEMS) field. It possesses high dielectric strength and is biocompatible. In addition, the Young’s modulus of the Parylene is 102× lower than that of metal and silicon, materials commonly used in penetrating, neural recording devices. These properties make it an ideal insulation and coating material for many implantable devices [1][2]. Thin Parylene films are vapor deposited under room temperature conditions and devices are fabricated using a combination of standard processes such as surface micromachining and photolithography [3]. With advanced micro- and nano-fabrication techniques, a Parylene-based surface electrode with high-density recording sites and complex layout was previously developed and used to record from and stimulate the salamander retina [4]. Penetrating neural probes made with Parylene were also developed and applied to obtain high resolution electromyograms from tobacco hornworm [5] and single unit activity from the rat cortex [6]. We demonstrated that sham Parylene arrays having eight 20 μm thick (10 μm base layer and 10 μm insulation layer) shanks but lacking the metal layer (nonfunctional devices) elicited minimal immune response [7]. Here we utilize fully-functional, customized Parylene multi-electrode arrays to record from multiple sub-regions of the hippocampus, a deep subcortical structure, in behaving rats.
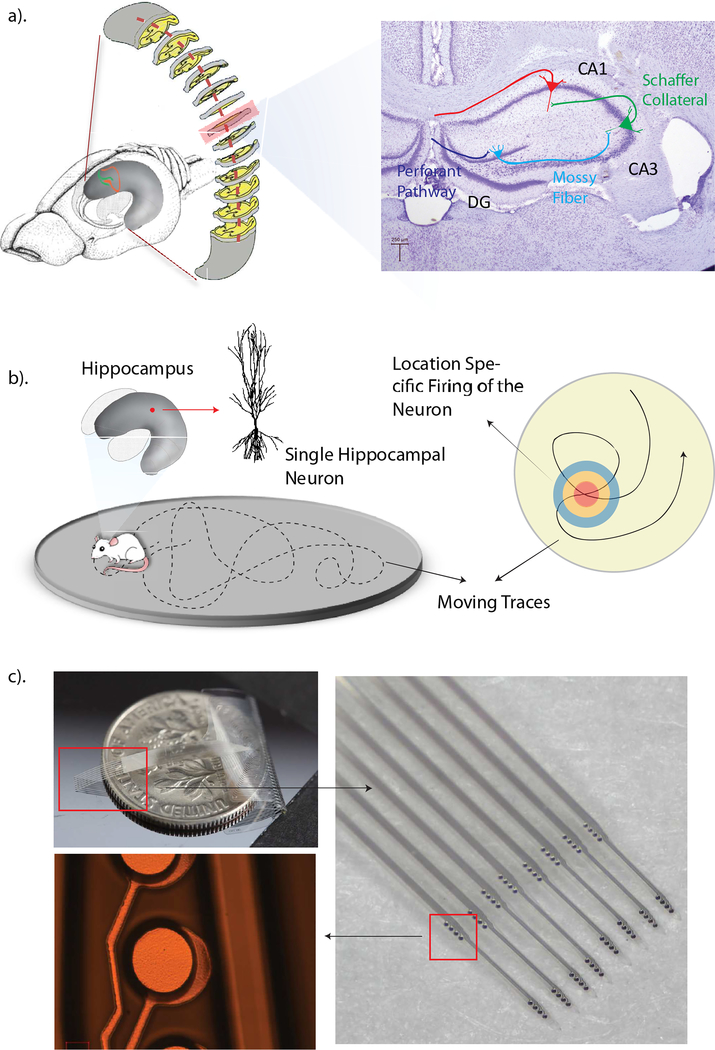
The hippocampus is a subcortical brain structure that is essential for the formation of new, long-term declarative memories. Three sub-regions of the hippocampus consist of a cascading neural network which converts sensory and associative cortical information into streams suitable for long-term storage in downstream cortical regions. Activation of certain hippocampal neurons are clearly related to the animal’s location in the environment. Following from this observation, these neurons were named place cells [8]. Recordings of place cell activity from multiple sub-regions of the hippocampal circuit will help to characterize space coding in different hippocampal sub-regions. In addition, simultaneous recording of single unit activity from multiple stages of the hippocampal circuit will provide insights to the identification of network dynamics and long-term plasticity during spatial information processing [9].
II. MATERIALS AND METHODS
A. Electrodes and Packaging
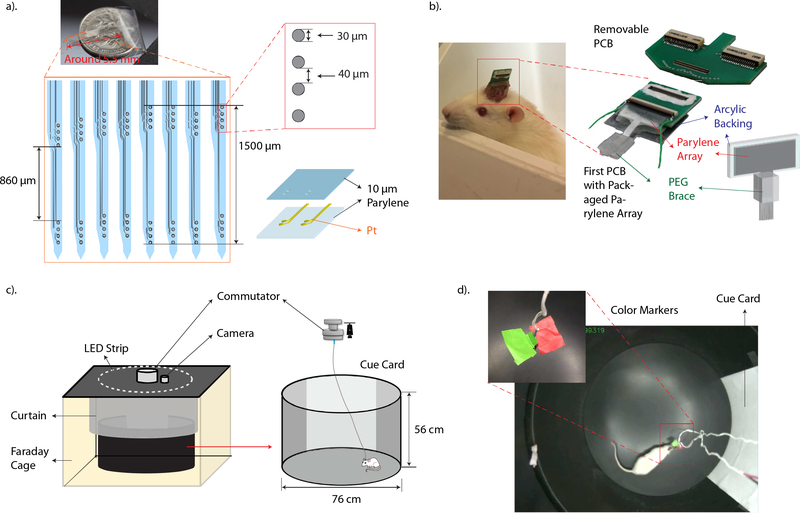
The Parylene multi-electrode array has eight individual shanks arranged in series, 250 μm apart. Thin recording electrodes and metal traces were sandwiched between two 10 μm thick Parylene films. On each shank, eight recording electrodes of 30 μm diameter were arranged into two recording groups with the bottom group targeting at the CA3 and the top group targeting at the CA1 cell body layer. The layout of all recording groups was specially designed to conform to the curvature of the hippocampus [10]. Prior to implantation, each recording site and corresponding connection trace was potentiostatically cleaned by cyclic voltammetry (CV) to remove residual scum remaining after fabrication. The connectivity and electrochemical impedance of each recording electrode at 1 kHz was also measured with electrode impedance spectroscopy (EIS). To interface with the data acquisition system, two PCBs were used. The Parylene array was first inserted into a zero-insertion force (ZIF) connector that attached to the first PCB which will be chronically mounted onto the animal’s head. Another removable PCB with connectors compatible with the recording system was used to mate the animal to the recording system. A special method for the insertion of the flexible recording array was also developed [11]. To support the insertion of the flexible, bare Parylene array, the array was attached to an acrylic backing with a polyethylene glycol (PEG) block. Prior to implantation, only the tip of the array was exposed. The layout of the Parylene multi-electrode array is shown in Figure 2a. and a packaged Parylene array ready for implantation is shown in Figure 2b.
Figure 2.
a). A detailed sketch of the layout of the Parylene array. The diameter of each electrode, spacing between electrodes and 3D structure of the Parylene array are shown on the right. b). The left photo shows an animal implanted with the Parylene array with only half of the first PCB exposed for connection. Right side photos show a packaged-Parylene array attached to the first PCB and a photo of the removable, second PCB. c). Schematic representation of the recording environment and the dimension of the recording chamber. A 76 cm x 56 cm round recording chamber is placed inside a customized Faraday cage and the interior isolated from the lab environment with a black curtain. A white cue card covering ¼ of the chamber’s wall is used as a visual cue to the animal. A LED strip is placed on top of the Faraday cage to provide illumination to the recording area. A commutator is used to prevent twisting of connect cables. d). Shows a top view of the recording chamber captured with the over-head camera. The photo on top left corner shows two color tabs attached to the headstage which was affixed to the animal’s head. The tabs served as markers for the behavioral recording system to track during recording sessions.
B. Electrode Implantation
All animal preparation and care were in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines and pre-approved by the Department of Animal Resource of the University of Southern California. Male Sprague-Dawley (SD) rats weighting between 300 g and 450 g were used for array implantations. The detailed surgical procedure was described in our previous paper [7]. In summary, after the animal was anesthetized, the skull, dura layer and blood vessels above the right hippocampus were removed to expose the brain surface for the implantation of the Parylene array. The tip of the Parylene array, which was attached to a micro-manipulator, was inserted into the brain surface slowly. Following dissolution of the PEG brace with saline, the exposed Parylene array was fully inserted. Neural activities were monitored while advancing the Parylene array to provide guidance for localization and targeting. After the Parylene array reached the desired depth, the cranial window was sealed with dental cement and the Parylene array was permanently mounted to three anchor screws pre-attached to the skull with more dental cement. The top part of the customized PCB and electrical connections were left un-covered for future connection.
C. Data Acquisition
After the animal recovered from the implantation surgery, the PCB was connected to a 64 channel, Plexon (Plexon Inc., Dallas, TX) data acquisition system. Neural activities were recorded and digitized at 40 kHz while the animal was running in an open field. During each five-minute recording session, small food pellets were randomly scattered onto the floor of the recording chamber to encourage the rat to move frequently and explore the whole environment. Two small colored tabs, one red and one green, were attached to the headstage connecting the PCB. Using a video tracking system (CinePlex, Plexon Inc., Dallas, TX), the position of the color markers, indicating the animal’s location in the field, was also tracked and recorded with a camera that overlooks the recording chamber. Figure 2c. and 2d. show a diagram and photograph of the behavioral and neural recording setups. Post-recording, spike activities collected from different hippocampal neurons were distinguished with the principal component analysis method using offline spike sorting software (Offline Sorter, Plexon Inc., Dallas, TX). Correlations between simultaneously recorded moving traces of the animal and neural activities from the hippocampus were also analyzed.
III. RESULTS
A. Implantation Results
Three Parylene multi-electrode arrays were chronically implanted in three rats. Electrodes and connective traces that showed any defects during inspection prior to implantation were considered as non-functional electrodes. In addition, electrodes that had extremely high impedance as determined by electrochemical impedance spectroscopy were considered open circuits. Recording groups with more than two defective electrodes or open circuits were considered as non-functional groups. All three implanted Parylene arrays simultaneously recorded single-unit activity from CA1 and the CA3 cell body layers during the implantation procedure. After the animal recovered, 33 units (29 units from the CA3 region and 4 units from the CA1 region) were recorded from three implantations. Among three implantations, one Parylene array recorded neural activity from both sub-regions of the hippocampus. Number of functional electrodes, functional recording groups implanted in each animal, and units recorded from during surgery and behaving animals are listed in Table I.
TABLE I.
Number of functional electrodes and recording groups of each implanted Parylene array and number of active units recording during and after the implantation.
| Array # | # of Functional Electrodes | # of Groups | Active Units during Surgery (CA1+CA3) | Active Units in Behaving Animal (CA1+CA3) |
|---|---|---|---|---|
| 1 | 45 | 11 | 6 (4+2) | 7 (0+7) |
| 2 | 63 | 14 | 11 (7+4) | 11 (7+4) |
| 3 | 61 | 16 | 10(4+6) | 16 (0+16) |
B. Place Cells and Place Fields
The spatial distribution of firing rates of single units was calculated with data analysis software (Neuro Explorer, Nex Technologies, Madison, AL). Over-head views of the open field were divided into 0.2 cm × 0.2 cm bins and the firing rate of each unit in a bin was computed as the total number of spikes divided by the total amount of time the rat spent in that bin. Place cells which showed location-specified receptive fields were recorded from all three behaving rats that were chronically implanted with the Parylene multi-electrode arrays. The array that recorded unitary activities from both the CA1 and the CA3 cell body layers recorded place cells from both sub-regions. Theta cells, which increase their firing rate when there is a theta rhythm in the hippocampus, are another major type of neurons that commonly seen in the hippocampus [12]. Units whose activation locked to the theta rhythm were observed with the Parylene array while the rat was walking in the environment.
IV. DISCUSSION AND FUTURE WORK
In this paper, we show neural activity recorded from behaving animals with a flexible, Parylene based multi-electrode array. On average, 11 units were recorded with each 64 channel Parylene array. Compared to the number of recording sites implanted, the number of units recorded is low. In the current design, four closely spaced electrodes were considered one recording group and this layout was purposefully selected to compensate for small variations between animals allowing for the relative ease of targeting the two thin cell body layers with one recording array simultaneously. However, as a tradeoff, neighboring electrodes were likely to pick up signals from the same unit which lowered the overall number of units recorded from each implantation. In future work we will optimize the implantation location, as only one Parylene array recorded from both the CA1 and the CA3 sub-region in behaving animal of the three implanted. More Parylene devices will be implanted to determine the location which can maximize the number of units recorded from both sub-regions from behaving animals.
Figure 1.
a). Schematic diagram of the rat brain, the curved hippocampus and a coronal slice of the hippocampus. b). Diagrammatic drawing of the position-specified firing property of hippocampal neurons. c). Photographs of a fabricated, fully functional Parylene multi-electrode array. The photo on top left shows the size of the Parylene array. The right photo shows a zoomed-in view of eight Parylene shanks and sixteen recording groups positioned on those shanks. The bottom left photo shows a representative recording electrode.
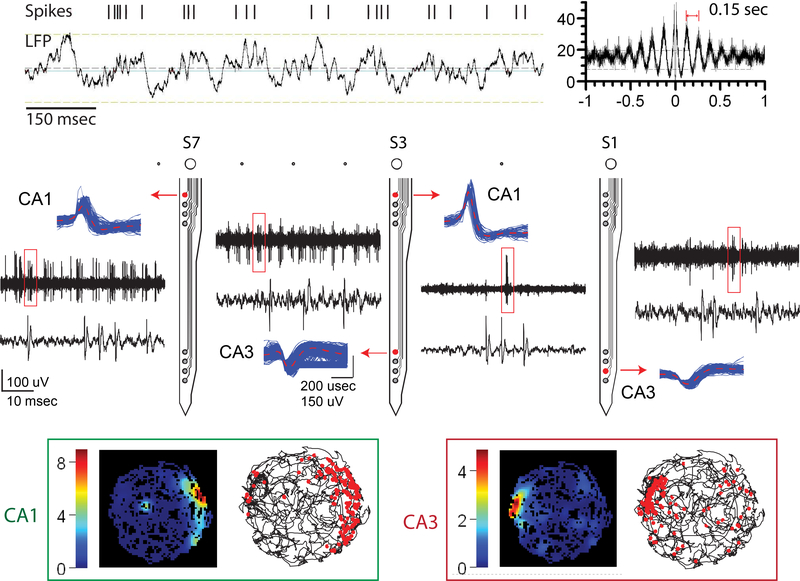
Figure 3.
Recording results from behaving animals. The middle panel shows both unitary activities and high-pass filtered, continuously recorded signals from one chronically implanted Parylene array while the animal ran freely in the open field. Neural activities were recorded from both the CA1 and the CA3 sub-regions. The top panel shows an example of a theta cell, whose firing rate increases when there is theta rhythm (4 to 7 Hz) presents. The autocorrelation of the same unit is shown on the right side. The bottom panel shows two place cells recorded with this Parylene array from the CA1 and the CA3 sub-regions respectively. Two color maps on the left side plot the firing rates of these units regarding to the animal’s position in the open field. Pictures on the right side show the moving trace of the animal and each red dot represents one spike firing in that particular location.
Acknowledgment
The authors would like to thank Min-Chi Hsiao for his expert guidance and insightful suggestions. We also thank Dr. Donghai Zhu of the Keck Photonics Laboratory for help with fabrication.
This work was funded by NSF INSPIRE (CBET-134193).
Contributor Information
Huijing Xu, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089 USA.
Ahuva W. Hirschberg, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA90089 USA
Kee Scholten, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA90089 USA.
Ellis Meng, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA90089 USA.
Theodore W. Berger, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA90089 USA.
Dong Song, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA90089 USA.
References
- [1].Brydon HL, Keir G, Thompson EJ, Bayston R, Hayward R, and Harkness W, “Protein adsorption to hydrocephalus shunt catheters: CSF protein adsorption.,” J. Neurol. Neurosurg. Psychiatry, vol. 64, no. 5, pp. 643–7, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dickerman RD, McConathy WJ, Morgan J, Stevens QE, Jolley JT, Schneider S, and Mittler MA, “Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: Revisited,” J. Clin. Neurosci, vol. 12, no. 7, pp. 781–783, 2005. [DOI] [PubMed] [Google Scholar]
- [3].Crowell JE, “Chemical methods of thin film deposition: Chemical vapor deposition, atomic layer deposition, and related technologies,” J. Vac. Sci. Technol. A Vacuum, Surfaces, Film, vol. 21, no. 5, pp. S88–S95, 2003. [Google Scholar]
- [4].Rodger DC, Fong AJ, Li W, Ameri H, Ahuja AK, Gutierrez C, Lavrov I, Zhong H, Menon PR, Meng E, Burdick JW, Roy RR, Edgerton VR, Weiland JD, Humayun MS, and Tai YC, “Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording,” Sensors Actuators, B Chem, vol. 132, no. 2, pp. 449–460, 2008. [Google Scholar]
- [5].Metallo C, White RD, and Trimmer B. a., “Flexible parylene-based microelectrode arrays for high resolution EMG recordings in freely moving small animals,” J. Neurosci. Methods, vol. 195, no. 2, pp. 176–184, 2011. [DOI] [PubMed] [Google Scholar]
- [6].Kuo JTW, Kim BJ, Hara S. a., Lee CD, Yu L, Gutierrez C. a., Hoang TQ, Pikov V, and Meng E, “3D Parylene sheath probes for reliable, long-term neuroprosthetic recordings,” Proc. IEEE Int. Conf. Micro Electro Mech. Syst., pp. 1073–1076, 2013. [Google Scholar]
- [7].Xu H, Hirschberg AW, Scholten K, Berger TW, Song D, and Meng E, “Acute in vivo testing of a conformal polymer microelectrode array for multi-region hippocampal recordings,” J. Neural Eng, no. Accepted Manuscript, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keefe JO, “Units in the Hippocampus Moving Rat,” vol. 109, 1976. [Google Scholar]
- [9].Song D, Robinson B, Hampson R, Marmarelis V, Deadwyler S, and Berger T, Sparse large-scale nonlinear dynamical modeling of human hippocampus for memory prostheses, vol. PP, no. 99 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu H, Weltman A, Scholten K, Meng E, Berger TW, and Song D, “Chronic multi-region recording from the rat hippocampus in vivo with a flexible Parylene-based multi-electrode array,” Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, pp. 1716–1719, 2017. [DOI] [PubMed] [Google Scholar]
- [11].Weltman A, Xu H, Scholten K, Berger TW, Song D, and Meng E, “Deep Brain Targeting Strategy for Bare Parylene Neural Probe Arrays,” Hilt. Head Conf., pp. 3–6, 2016. [Google Scholar]
- [12].Behavioral I and Ranck B, “Studies on Single Neurons and Septum in Dorsal Hippocampal in Unrestrained Rats,” 1973. [DOI] [PubMed] [Google Scholar]