Abstract
Background:
Lofexidine is a non-opioid treatment for opioid withdrawal syndrome. Its sympatholytic actions counteract the noradrenergic hyperactivity that occurs during abrupt opioid withdrawal.
Methods:
The effect of lofexidine 2.16 and 2.88 mg/day on QTcF (QT interval, heart-rate corrected, Fridericia formula) was studied as part of a large, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier: NCT01863186). ECGs were time-matched to blood sampling for lofexidine concentration and were collected at prespecified time points over a 7-day inpatient period. Analyses included mean change-from-baseline QTcF and exposure-response modeling to predict QTcF at relevant lofexidine concentrations.
Results:
A total of 681 adult men and women received at least 1 dose of study drug; 566 qualified for inclusion in the concentration-QTcF analysis. Most subjects were withdrawing from heroin. During the first 24 hours (Days 1c2) post-baseline, small increases in QTcF were observed in all groups: 4.7 ms for lofexidine 2.16 mg, 7.4 ms for lofexidine 2.88 mg and 1.4 ms for placebo. These increases were transient; by Day 4, when lofexidine levels had reached steady-state, QTcF increases were not present. By Day 7, QTcF was decreased from baseline in all groups. Exposure-response modeling predicted <10 ms increases in QTcF at lofexidine concentrations 3 times those obtained at maximal recommended dose.
Conclusions:
Lofexidine was associated with small, transient QTcF increases. Decreases in QTcF that occurred with higher lofexidine concentrations argue for an indirect QTcF effect, potentially from changes in autonomic tone. Both opioid withdrawal and lofexidine’s sympatholytic actions would be expected to alter sympathetic outflow over the 7-day withdrawal.
Keywords: Lofexidine, QTcF, QTc, Exposure-Response Modeling, Opioid Withdrawal
1. Introduction
1.1. Opioid Withdrawal
Management of opioid withdrawal is a crucial treatment step in managing patients with underlying opioid use disorder (OUD). Chronic opioid administration induces adaptation of the noradrenergic neurons in the brainstem locus coeruleus (LC) to upregulate cAMP pathways and norepinephrine (NE) production to counteract mu opioid receptor suppression of NE signaling. Abrupt opioid discontinuation results in unopposed noradrenergic hyperactivity that drives the majority of opioid withdrawal symptoms, often referred to as opioid withdrawal syndrome (OWS) (Mazei-Robison and Nestler, 2012; Kosten and George, 2002). OWS is a constellation of extremely disturbing symptoms including uncontrollable anxiety, irritability, insomnia, sweating, diarrhea, nausea/vomiting, temperature dysregulation, and pain (Tetrault and O’Connor, 2009). Alleviating withdrawal symptoms is an important step toward assisting OUD patients successfully transition into long-term management (Kosten and Baxter, 2019).
1.2. Lofexidine for Treatment of Opioid Withdrawal Symptoms
Lofexidine (LUCEMYRA™) is a central α2-adrenergic receptor agonist that was approved by the Food and Drug Administration (FDA) in May 2018 for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults (US WorldMeds LLC, 2018). The sympatholytic action of lofexidine counteracts the increased LC noradrenergic outflow that drives OWS. Lofexidine has slightly different pharmacological properties than clonidine including moderate affinity for 5HT1A receptors (Raffa et al., 2019). In a Cochrane review of historical head-to-head comparison studies and placebo-controlled studies, Gowing et al. concluded that lofexidine has a better safety profile than clonidine. The likelihood of completing withdrawal treatment was higher with either lofexidine or clonidine compared with placebo (Gowing et al., 2016).
In the two pivotal clinical trials of lofexidine for registration, subjects receiving lofexidine as compared with placebo had significantly reduced severity of opioid withdrawal symptoms and a greater likelihood of completing a 5-day (2.88 mg/day) or 7-day (2.16 mg or 2.88 mg/day) treatment period (Gorodetzky et al., 2017; Fishman et al., 2018). The most common adverse reactions (incidence ≥ 10% and notably more frequent than placebo) were orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth (US WorldMeds LLC, 2018). The recommended starting dose of lofexidine is 0.54 mg QID (2.16 mg/day). The maximum recommended dose is 0.72 mg QID (2.88 mg/day). Lofexidine should be taken orally at 5- to 6-hour intervals and may be continued for up to 14 days with dosing guided by symptoms and adverse effects.
1.3. Objectives
Lofexidine treatment was previously reported to be associated with increases in QTc interval (i.e., prolonging cardiac repolarization) in a small number of patients receiving concomitant methadone (Schmittner et al., 2009; Schmittner et al., 2004). Therefore, a robust exposure-response (ER) analysis of QTc interval and lofexidine plasma concentration was prospectively planned for the 7-day pivotal study. ECG parameters, including concentration-QTc (C-QTc) analysis of the relationship between lofexidine plasma concentrations and change-from-baseline in QT, heart-rate corrected, Fridericia formula (ΔQTcF) were analyzed.
2. Materials and Methods
2.1. Trial Overview
Full details of the study design and efficacy and safety results have been published (Fishman et al., 2018). Briefly, the study was a double-blind, placebo-controlled efficacy and safety study in opioid-dependent adults undergoing abrupt discontinuation from short-acting opioids. Subjects received placebo or lofexidine 2.16 mg or 2.88 mg/day for a total of 7 days in a blinded fashion. The study was conducted at 18 sites in the US from June of 2013 until December of 2014. All sites obtained protocol approval by a local or central institutional review board. All subjects provided written informed consent.
2.2. Major Enrollment Criteria
Men or women ≥18 years old and meeting criteria for current dependence according to the Mini International Neuropsychiatric Interview (MINI) on any opioid with a half-life similar to heroin or morphine with use for ≥21 of the past 30 days were eligible. A baseline score ≥2 on the Objective Opiate Withdrawal Scale, and if female, agreement to use an acceptable method of contraception was required. Subjects with unstable medical conditions, self-reported use of methadone or buprenorphine in the past 14 days (confirmed by urine drug screen), or self-reported need for use of psychotropics, antihypertensives, antiarrhythmics, or anticonvulsant medications within the past 4 weeks were excluded. A positive urine screen for use of other illicit drugs (cannabinoids, cocaine, amphetamines, methamphetamines, benzodiazepines, or barbiturates) prior to study entry was not a basis for exclusion; however, evidence of use (e.g., positive urine screen) during the study required subject discontinuation. Subjects with clinically significant abnormal ECG (e.g., second- or third-degree heart block, uncontrolled arrhythmia, or QTcF interval >450 ms for males and >470 ms for females) were also excluded.
2.3. Study Design
Qualifying subjects were randomized (3:3:2 ratio) to receive lofexidine 2.16 mg/day [0.54 mg 4 times daily (QID)], lofexidine 2.88 mg/day (0.72 mg QID), or matching placebo QID; doses were administered at 8 AM, 1 PM, 6 PM, and 11 PM during the double-blind treatment period of 7 days. During the study subjects were retained as inpatients to assure compliance with treatment and availability for study measurements. Supportive medications including guaifenesin, alumina, magnesia, simethicone, dioctyl sodium sulfosuccinate, psyllium hydrocolloid suspension, bismuth sulfate, acetaminophen, multivitamins, zolpidem and nicotine replacement therapy were permitted. Any other medications deemed necessary by the Investigator required approval of the Sponsor’s Medical Monitor.
2.4. Randomization and Blinding
A stratified randomization procedure was used to ensure gender balance. Study-site personnel, subjects, sponsor, and clinical research personnel were blinded to study drug assignment.
2.5. ECG and Lofexidine Pharmacokinetic (PK) Measurements
Serial ECGs were recorded and transferred to a central ECG laboratory (Cardiocore Lab Inc.). At each timepoint, duplicate recordings were interpreted in a uniform fashion, with readers blinded to treatment. Finger-prick blood samples for PK analysis were collected immediately following completion of the ECG recordings. This sparse sampling schedule was focused on initial exposure (Stage 1), the early accumulation phase (Stage 2), transition to steady state (Stage 3), and the steady-state phase (Stage 4). Table 1 shows the timing of ECGs, lofexidine dosing and blood sampling for pharmacokinetic/pharmacodynamic (PK/PD) analysis.
Table 1.
Schedule of selected study events.
| Study Day | |||||||
|---|---|---|---|---|---|---|---|
| Study Event | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 12-lead ECG | pre 8 am dose pre 1 pm dose 4pm 5pm |
pre 8 am dose | pre 8 am dose | pre 8 am dose pre 1 pm dose 4 pm 5 pm |
|||
| Lofexidine or placebo administration | 8 am 1pm 6pm 11pm |
8 am 1pm 6pm 11pm |
8 am 1pm 6pm 11pm |
8 am 1pm 6pm 11pm |
8 am 1pm 6pm 11pm |
8 am 1pm 6pm 11pm |
8 am 1 pm 6 pm 11 pm |
| PK blood sample for lofexidine | pre 8 am dose pre 1 pm dose 4 pm 5 pm |
pre 8 am dose | 9 pma 10 pma |
pre 8 am dose | 9 pma 10 pma |
pre 8 am dose pre 1 pm dose 4 pm 5 pm |
|
Not used in concentration-QTcF analysis.
ECG, electrocardiogram; PK, pharmacokinetics.
The maximum concentration (Cmax) was determined for each subject from all the existing concentrations for which there was matching QT information.
Subjects with QTcF >500 ms or a >25% increase from baseline required discontinuation from the study.
2.6. Statistical Analysis
The safety population included all subjects who received at least one dose of study treatment (lofexidine or placebo); the C-QTcF population was a subset of the safety population and included subjects with at least 1 ΔQTcF value (i.e., baseline and at least 1 postdosing value) and, for subjects on active treatment, with a lofexidine plasma concentration value at the same time point. Subjects with measurable lofexidine plasma concentration at baseline and time points with a >30-minute difference between ECG and blood sampling were excluded from the C-QTcF analysis.
Statistical analysis was performed using R for Windows (v3.2.2 or later). In addition to descriptive statistics given by dose group and timepoint (“by timepoint” analysis) of ΔQTcF, an ER analysis was performed investigating the relationship of change in QTcF to lofexidine plasma concentrations. This analysis followed the principles laid down in Garnett et al., 2018. In particular, it followed the approach of specifying the principles for model selection in the analysis plan without prespecifying one primary hypothesis test in all detail or adjusting the type-I error level (α-level) for the model selection.
The base model was a linear mixed effects model with fixed effects defined as: ΔQTcF ~ C + time + treatment + BL where ΔQTcF is the change from the 8 AM predose value of Day 1, C is the lofexidine plasma concentration and time is a factor with one level for each of the 9 postdose timepoints and BL is the baseline QTcF value of each subject. Treatment is a factor with two levels: “Active” and “Placebo”. It was included as a diagnostic against misspecification of the model. A significant treatment effect, corresponding to the intercept, in a model that has concentration as covariate is not physiologic and therefore an indication that the model is inappropriate. The random effects of this model were an intercept and a concentration effect (“slope”) per subject, and an unstructured covariance matrix was allowed. To test if a linear relationship between ΔQTcF and concentration was sufficient, a quadratic effect in concentration was added to the primary linear model and tested.
In case linearity did not hold, a nonlinear Emax model was also to be fitted. In such a model the effect of concentration cannot exceed an asymptotic value allowing saturation of the QTcF effect with increasing concentration to be modelled.
Modifications of the above basic model were also considered. On the one hand, a simplified model without baseline was considered. A model without the time effect was also investigated in a prospective way. An exploratory model including Day (Day 1 and Day 7 only) as additional factor and its interaction with concentration was fitted retrospectively to shed light on the differences seen between these days.
The models were used to predict the effect of lofexidine on the placebo-corrected ΔQTcF at several concentrations of interest.
A subgroup analysis was performed in a post-hoc fashion. In this analysis, subjects were grouped by completer status, i.e., subjects with data on Days 1 and 2 only, those with data beyond Day 2 but not beyond Day 7, 1 PM, and subjects with data beyond Day 7 1 PM.
3. Results
3.1. Subjects
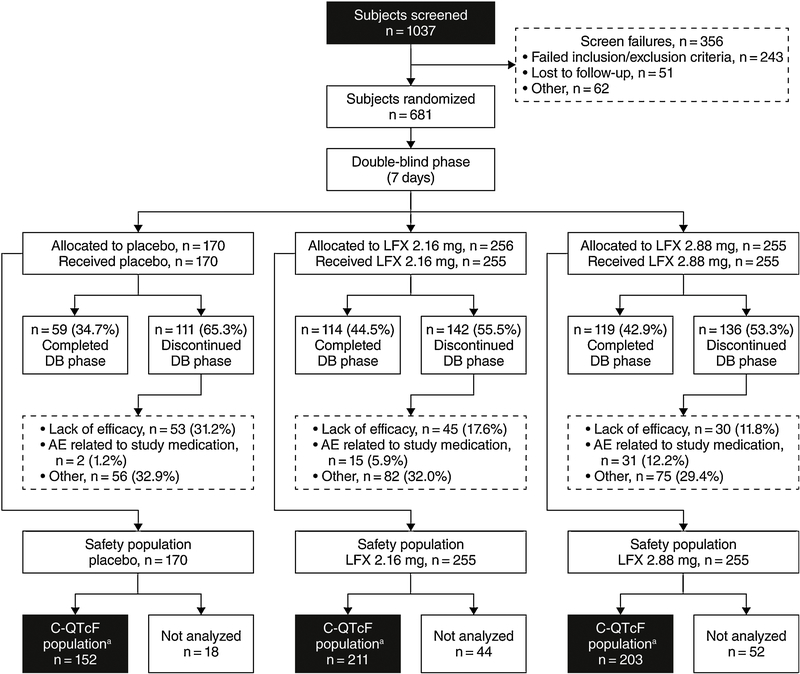
A total of 681 subjects comprised the safety population, and 566 subjects were included in the C-QTcF analysis (Figure 1). The safety population for the current analysis included an additional 78 subjects who were not reported in the published efficacy/safety analysis (Fishman et al., 2018). The C-QTcF analysis population included 211, 203 and 152 subjects who received lofexidine 2.16 mg/day, lofexidine 2.88 mg/day, or placebo. Table 2 displays the number of included subjects by timepoint. Background characteristics as reported in the efficacy/safety analysis (Fishman et al., 2018) revealed that the majority of the study population was white (73.8%), male (70.9%) and used heroin as their primary opioid (83.2%). A small proportion (<20%) primarily used oxycodone, hydrocodone or other short-acting opioids. Mean age was 35.0 ±11 years. Concomitant use of drugs with a known association with prolonged QTc was relatively sparse. One lofexidine-treated subject received concomitant ciprofloxacin, 2 subjects received ondansetron (1 placebo, 1 lofexidine), 3 subjects received methadone (a protocol deviation; 2 placebo, 1 lofexidine) and 6 subjects reported using cocaine (a protocol deviation; 3 placebo, 3 lofexidine). No subjects received concomitant antiarrhythmic medications. Use of cocaine at the last visit prior to study Day 1 (based on urine drug screens) was similar among treatment groups (13%−15%).
Figure 1.
Study flow diagram.
a The C-QTcF population included subjects with baseline and at least 1 on-treatment QTcF value with a plasma concentration value from the same time point (within 30 minutes) and with no measurable lofexidine plasma concentration at baseline.LFX, lofexidine.
Table.2.
Number of subjects with ECG and PK data at each study time point (C-QTcF population).
| Time | Number of subjects | ||
|---|---|---|---|
| LFX 2.16 mg | LFX 2.88 mg | Placebo | |
| DAY 1: pre 1 pm dose | 202 | 194 | 151 |
| DAY 1: 4 pm | 200 | 187 | 139 |
| DAY 1: 5 pm | 191 | 182 | 135 |
| DAY 2: pre 8 am dose | 177 | 172 | 118 |
| DAY 4: pre 8 am dose | 114 | 119 | 74 |
| DAY 7: pre 8 am dose | 91 | 99 | 58 |
| DAY 7: pre 1 pm dose | 54 | 68 | 37 |
| DAY 7: 4 pm | 54 | 63 | 37 |
| DAY 7: 5 pm | 54 | 65 | 37 |
ECG, electrocardiogram; LFX, lofexidine; PK, pharmacokinetics.
3.2. Plasma Lofexidine Concentration Analysis
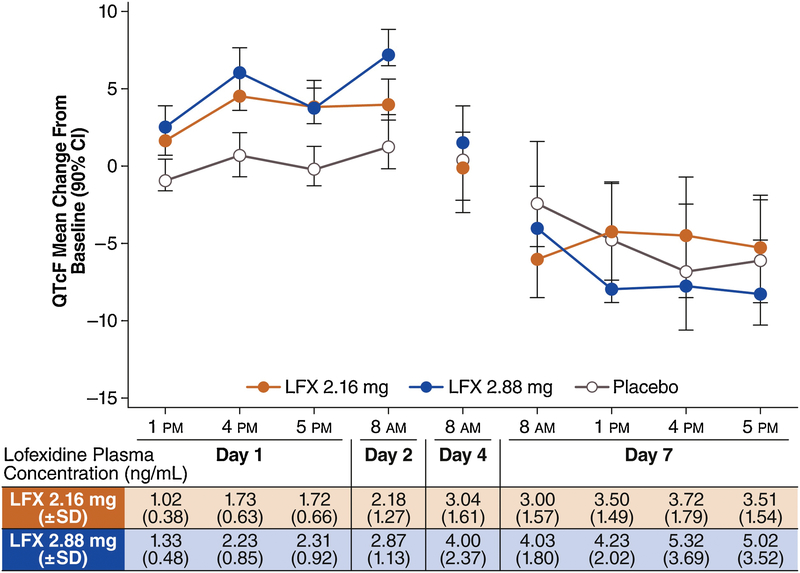
Mean plasma lofexidine concentration increased from Day 1 and reached steady state levels by Day 4, consistent with lofexidine’s 15- to 20-hour half-life (Figure 2). Mean concentration before the morning dose on Day 7 was similar to that observed on Day 4 at the same timepoint. The non-uniform dosing schedule used (8 am, 1 pm, 6 pm, 11 pm) led to slight increases in lofexidine plasma concentration as the treatment day progressed (see Day 7 predose 1 pm, 4 pm and 5 pm concentrations in Figure 2).
Figure 2.
Change-from-baseline QTcF (ΔQTcF, ms) and mean lofexidine plasma concentration by timepoint. On Day 1 and in the morning of Day 2, a small prolongation of the QTcF interval is seen in both lofexidine groups; with continued dosing, ΔQTcF is reduced and prolongation is not observed on Day 7. LFX, lofexidine.
3.3. “By timepoint” Analysis of the Effect of Lofexidine on the QTcF Interval and other ECG Parameters
The largest increase of mean ΔQTcF was seen on Day 1 or before the morning dose on Day 2 in all treatment groups: 4.7 ms (90% CI: 3.4 to 6.0) at 04:00 PM on Day 1 in the lofexidine 2.18 mg group and 7.4 ms (90% CI: 5.8 to 9.1) and 1.4 ms (90% CI: −0.3 to 3.0) before the morning dose on Day 2 in the lofexidine 2.88 mg/day and placebo groups, respectively. In all treatment groups, mean QTcF thereafter decreased to levels below baseline values by Day 7, despite plasma lofexidine concentrations being higher on Days 4 and 7 than on Days 1 or 2 in the lofexidine treatment groups (Table 3 and Figure 2).
Table 3.
Change-from-baseline QTcF (ΔQTcF, ms) across study treatments and time points (C-QTcF population).
| Timepoint | Treatment | N | Mean | 90 % Confidence Interval | |
|---|---|---|---|---|---|
| DAY 1: pre 1 pm dose | LFX 2.16 | 202 | 1.9 | 0.8 | 3.1 |
| LFX 2.88 | 194 | 2.9 | 1.7 | 4.2 | |
| Placebo | 151 | −0.7 | −2.0 | 0.6 | |
| DAY 1: 4 pm | LFX 2.16 | 200 | 4.7 | 3.4 | 6.0 |
| LFX 2.88 | 187 | 6.2 | 4.6 | 7.9 | |
| Placebo | 139 | 0.8 | −0.8 | 2.3 | |
| DAY 1: 5 pm | LFX 2.16 | 191 | 3.9 | 2.6 | 5.2 |
| LFX 2.88 | 182 | 3.9 | 2.2 | 5.5 | |
| Placebo | 135 | 0.0 | −1.4 | 1.4 | |
| DAY 2: pre 8 am dose | LFX 2.16 | 177 | 4.2 | 2.7 | 5.6 |
| LFX 2.88 | 172 | 7.4 | 5.8 | 9.1 | |
| Placebo | 118 | 1.4 | −0.3 | 3.0 | |
| DAY 4: pre 8 am dose | LFX 2.16 | 114 | 0.0 | −2.2 | 2.3 |
| LFX 2.88 | 119 | 1.5 | −0.9 | 4.0 | |
| Placebo | 74 | 0.4 | −2.9 | 3.8 | |
| DAY 7: pre 8 am dose | LFX 2.16 | 91 | −6.0 | −8.5 | −3.6 |
| LFX 2.88 | 99 | −4.0 | −6.8 | −1.3 | |
| Placebo | 58 | −2.4 | −6.4 | 1.7 | |
| DAY 7: pre 1 pm dose | LFX 2.16 | 54 | −4.1 | −7.2 | −1.1 |
| LFX 2.88 | 68 | −7.8 | −11.1 | −4.6 | |
| Placebo | 37 | −4.7 | −8.5 | −1.0 | |
| DAY 7: 4 pm | LFX 2.16 | 54 | −4.2 | −7.8 | −0.6 |
| LFX 2.88 | 63 | −7.6 | −10.6 | −4.5 | |
| Plascebo | 37 | −6.6 | −10.7 | −2.4 | |
| DAY 7: 5 pm | LFX 2.16 | 54 | −5.3 | −8.6 | −2.0 |
| LFX 2.88 | 65 | −8.1 | −11.6 | −4.6 | |
| Placebo | 37 | −6.0 | −10.3 | −1.7 | |
LFX, lofexidine; QTcF, QT interval corrected, Fridericia formula.
In the lofexidine groups, mean heart rate was moderately reduced post-dosing with the largest mean change-from-baseline heart rate (ΔHR) of −10.0 bpm for the 2.16 mg group and −12.8 bpm for the 2.88 mg group, both occurring on Day 1 at 5 PM. The reduction of heart rate was somewhat smaller by Day 7, less than 9 bpm in both lofexidine groups. In the placebo group, mean heart rate generally increased from Day 1 to Day 7 with a largest mean increase of 12.3 bpm on Day 7, at 4 PM. There were no clinically relevant effects on cardiac conduction, i.e., the PR and QRS intervals.
3.4. Concentration-QTcF Modeling
None of the primary, prospectively-defined models provided a good fit to the observed data. The linear C-QTc model with baseline as covariate and the Emax model with baseline as covariate, however, provided an acceptable fit, and both models were used to characterize the relationship between lofexidine plasma concentration and placebo-corrected ΔQTcF. The predicted QT effect (placebo-corrected ΔQTcF) was small with both models, and an effect exceeding 10 ms could be excluded across the observed plasma concentration range (Figure 3). At the geometric mean Cmax of 2.9 ng/mL in the 2.16-mg/day group, the predicted placebo-corrected ΔQTcF effect was 3.7 ms and 3.8 ms with the linear and the Emax ER models, respectively. At the geometric mean Cmax of 3.7 ng/mL in the 2.88-mg/day group, the predicted effect was greater: 4.0 ms and 4.2 ms, respectively (Table 4).
Figure 3.
Predicted QT effect (placebo-corrected ΔQTcF, ms) across lofexidine plasma concentrations based on the (A) linear model with baseline and (B) Emax model with baseline.Goodness-of-fit plot with predicted effect on ΔQTcF, with 90% confidence intervals (90% CI; black line with grey shaded area). The ΔQTcF values are adjusted for the placebo response and correspond to placebo-corrected ΔQTcF as used in other analyses. The horizontal bars near the lower edge of the figures show the lofexidine plasma concentration decile breakpoints (hatch marks). The vertical bars and whiskers show the interquartile ranges about the observed median placebo-corrected ΔQTcF (solid circles) within each concentration decile. The shaded areas between the two curvilinear lines in each graph show the 90% CI for the ΔQTcF as calculated from the two models. Both models predict a small QT effect with increasing lofexidine plasma concentrations. With the linear model, an effect on placebo-corrected ΔQTcF larger than 10 ms can be excluded up to lofexidine plasma concentrations of ~10 ng/mL.
Table 4.
Model-based predictions of QT effect (placebo-corrected ΔQTcF) at lofexidine geometric mean Cmax
| Model | Days | Dose (mg/day) | Conca (ng/mL) | Prediction (ms) | SE (ms) | DF | t-value | 90% CI (ms) | |
|---|---|---|---|---|---|---|---|---|---|
| Linear with baseline | Allb | LFX 2.16 | 2.89 | 3.7 | 0.95 | 507.9 | 3.91 | 2.1 | 5.3 |
| LFX 2.88 | 3.68 | 4.0 | 1.04 | 564.0 | 3.86 | 2.3 | 5.7 | ||
| Day 7 | LFX 2.16 | 3.73 | 4.0 | 1.04 | 564.8 | 3.85 | 2.3 | 5.7 | |
| LFX 2.88 | 4.71 | 4.4 | 1.20 | 527.5 | 3.63 | 2.16 | 6.4 | ||
| Emax with baseline | Allb | LFX 2.16 | 2.89 | 3.8 | 0.96 | 3.98 | 2.2 | 5.4 | |
| LFX 2.88 | 3.68 | 4.2 | 1.05 | 3.98 | 2.16 | 5.9 | |||
| Day 7 | LFX 2.16 | 3.73 | 3.8 | 1.05 | 3.64 | 2.1 | 5.6 | ||
| LFX 2.88 | 4.71 | 4.2 | 1.22 | 3.43 | 2.2 | 6.2 | |||
Geometric mean Cmax
The “All Days” concentration value is lower than the Day 7 value because it includes a substantial number of Day 1 Cmax values for subjects who discontinued the study early, prior to lofexidine concentrations having accumulated to their final steady state levels.
CI, confidence interval; Cmax, maximum concentration; Conc, concentration; DF, degrees of freedom; LFX, lofexidine; QTcF, QT interval corrected, Fridericia formula; SE, standard error.
3.5. Supportive Analyses
In the analyses by completer status, ΔQTcF was compared between subjects with ECG data on Day 1 and 2 only and subjects who completed Day 7. Table 2 gives the number of subjects by time point and day, and in Figure 4 ΔQTcF is shown for both groups. Subjects who discontinued the study before Day 4 had slightly higher ΔQTcF values on Day 1, but the marked decrease in ΔQTcF from Day 1 to Day 7 is also seen in subjects with data from all days.
Figure 4.
Change-from-baseline QTcF (ΔQTcF, ms) by completer status. The same pattern of mild QTc prolongation on Day 1 that disappears with continued dosing was seen in subjects with data from all days, i.e., up to and including Day 7, as compared to the full study population (Figure 2). In subjects with data on Days 1 and 2 only, the effect on ΔQTcF was somewhat more pronounced in the lofexidine 2.88 mg group. LFX, lofexidine.
The ER analyses using a linear model with “day” as an additional factor also supported the conclusion that the effect of lofexidine on ΔQTc was small on Day 1 and disappeared on Day 7 despite substantially higher lofexidine plasma concentrations (data not shown).
3.5.1. Subjects with Increases in QTcF >60 ms.
Two subjects (0.3 %) had QTcF increases from baseline of >60 ms. One subject receiving lofexidine 2.88 mg/day demonstrated QTcF intervals of 465 and 489 ms on Day 2; both were recorded as adverse events. A second subject receiving placebo demonstrated QTcF values 513 ms on Day 4 and 541 ms on Day 7: these were recorded as a serious adverse event per protocol prespecified criteria. ECG data from both subjects were included in the QTcF analyses. Polymorphic ventricular tachycardia or Torsades de Pointes was not observed in any subjects.
4. Discussion
In this trial, the effect of lofexidine on QTcF interval was carefully evaluated in 566 subjects in a 7-day, double-blind, placebo-controlled trial. Both the “by time point” analysis of lofexidine’s effect on ΔQTcF and the C-QTcF analysis with a linear and an Emax model indicated that an effect on the mean QTcF interval exceeding 10 ms can be excluded at doses up to the maximum recommended daily dose of 2.88 mg and up to plasma concentrations of ∼10 ng/mL, i.e., concentrations far exceeding therapeutic levels.
Opioids are known to prolong QTc interval via blockade of the hERG (human ether à-go-go related gene) cardiac potassium channel (Wedam and Haigney 2016), and methadone, as an example, is associated with significant QTc increases at therapeutic doses and has been shown to cause Torsades de Pointes (Florian et al., 2012; Behzadi et al., 2018). There is also some evidence suggesting that oxycodone at therapeutic doses may prolong QTcF (Fanoe et al., 2009). Based on patch-clamp testing in cell cultures, the effect of codeine, morphine, fentanyl, and heroin on hERG potassium channels is believed to be substantially less than that of methadone and potentially not clinically relevant at typical maximal doses; however, these opioids have not been well-studied for their effects on QTc interval in human subjects (Katchman et al., 2002; Wedam and Haigney 2016).
Lofexidine is administered during opioid withdrawal, a time frame when opioids may be present at significant plasma concentrations, and it is therefore important to evaluate the effect of the drug on cardiac repolarization, i.e., the QTc interval. Subjects in this trial were withdrawing from short-acting opioids, most from heroin, with a small proportion withdrawing from oxycodone, hydrocodone and others. Multiple analyses found small, clinically irrelevant increases of ΔQTcF related to lofexidine administration. This effect occurred early, at Day 1 or 2, with QTcF values decreasing below baseline by Day 7. This pattern was also seen when analyzing subjects who completed the full treatment length of 7 days, suggesting that the decrease in mean QTcF over time was not caused by subjects with higher QTcF values discontinuing the study early. Similarly, it likely did not result from use of other drugs that cause QTc prolongation. Subjects who used drugs known to increase QTc were balanced among treatment groups.
Although the pre-specified C-QTcF models did not provide a good fit to the data, the negative findings of these models are supported by the “By timepoint” results. The relatively poor fit, in particular of the linear model, is further explained by the additional models involving Day as a factor. In the spirit of Garnett et al. (2018), this gives additional credibility to the results.
The mechanism of the small QTcF mean increases in the lofexidine groups within the first 24 hours of opioid discontinuation is unknown. QTc prolongation based on inhibition of the hERG cardiac ion channel is, in the vast majority of known cases, concentration-dependent (Garnett et al., 2008). The observed QTc pattern with lofexidine cannot therefore be explained only based on this mechanism because the QTcF increase was attenuated with continued dosing and higher concentrations. Changes in the autonomic nervous system that affect QTcF presumably played a role in the mean QTcF changes over time (Bexton et al., 1986). It can be speculated that the mild QTc prolongation seen on Day 1 and 2 in the lofexidine groups is an indirect effect of changes in autonomic tone created by the interactions of opioid withdrawal and lofexidine’s antiadrenergic effects. While the placebo group also demonstrated a decrease in QTcF interval on Day 7 as compared with Days 1 and 2, there was essentially no change from baseline (maximum increase of 1.4 ms) during the first 24 hours. The placebo response also suggests changing autonomic tone over the 7-day withdrawal period. Although increased heart rate and blood pressure are known to occur after abrupt opioid withdrawal (Kienbaum et al., 2001; Tompkins et al., 2014), the effect of withdrawal on cardiac repolarization has not been well studied. Further research on the effects of opioid withdrawal on autonomic tone and the QTc interval are needed to answer questions raised by the findings of this study.
While the mean QTc prolongation observed in this study on Day 1 and 2 on treatment with lofexidine was small, it seems prudent to make prescribing physicians aware of this effect when considering treating subjects at high risk of proarrhythmias. Lofexidine prescribing information therefore contains warnings and precautions including risk of QT prolongation and recommends avoiding use in patients with congenital long QT syndrome and monitoring ECG in patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias, hepatic or renal impairment, or in patients taking other medicinal products that lead to QT prolongation. There are no contraindications to lofexidine use (US WorldMeds LLC, 2018).
5. Conclusions
An effect of lofexidine on the QTcF interval exceeding 10 ms can be excluded up to lofexidine concentrations of ∼10 ng/mL, which is 3-fold higher than mean steady-state concentrations seen in subjects on the maximum recommended daily dose.
In subjects experiencing acute opioid withdrawal, lofexidine at steady state concentrations was not associated with QTcF prolongation. Adaptation of the autonomic nervous system to opioid withdrawal and the sympatholytic action of lofexidine may underlie this observation.
Highlights.
Small mean QTcF (QT interval, heart-rate corrected, Fridericia formula) increases were observed with lofexidine (<8 ms) on Days 1–2.
QTcF mean increases for lofexidine were transient and not present Day 4 or later.
Modeling predicted an effect <10 ms at 3-fold maximal therapeutic concentration.
QTcF means were below baseline for all groups, including placebo, by Day 7.
Acknowledgments
Role of Funding Source
This work was supported by US WorldMeds, LLC, and the National Institute on Drug Abuse [grant number U01DA033276]. The Curry Rockefeller Group, LLC, provided editorial support, funded by US WorldMeds, LLC.
ClinicalTrials.gov identifier: NCT01863186
Footnotes
Author Disclosures
Conflicts of Interest
Börje Darpö is a consultant for iCardiac/ERT and owns stock in ERT. Mark Pirner is an employee of US WorldMeds. James Longstreth is a consultant to US WorldMeds, LLC. Georg Ferber is an independent consultant working for clinical research organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behzadi M, Joukar S, Beik A, 2018. Opioids and cardiac arrhythmia: A literature review. Med. Princ. Pract 27, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexton RS, Vallin HO, Camm AJ, 1986. Diurnal variation of the QT interval—Influence of the autonomic nervous system. Br. Heart J 55, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanoe S, Jensen GB, Sjøgren P, Korsgaard MP, Grunnet M, 2009. Oxycodone is associated with dose-dependent QTc prolongation in patients and low-affinity inhibiting of hERG activity in vitro. Br. J. Clin. Pharmacol 67, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M, Tirado C, Alam D, Gullo K, Clinch T, Gorodetzky CW, CLEEN-SLATE Team., 2018. Safety and efficacy of lofexidine for medically managed opioid withdrawal: A randomized controlled clinical trial. J. Addict. Med 13, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC, 2012. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin. Pharmacol. Ther 91, 666–672. [DOI] [PubMed] [Google Scholar]
- Garnett CE., Beasley N., Bhattaram VA., Jadhav PR., Madabushi R., Stockbridge N., Tornøe CW., Wang Y., Zhu H., Gobburu JV., 2008. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J. Clin. Pharmacol 48, 13–18. [DOI] [PubMed] [Google Scholar]
- Garnett C, Bonate PL, Dang Q, Ferber G, Huang D, Liu J, Mehrotra D, Riley S, Sager P, Tornoe C, Wang Y, 2018. Scientific white paper on concentration-QTc modeling. J. Pharmacokinet. Pharmacodyn 45, 383–397. [DOI] [PubMed] [Google Scholar]
- Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K, 2017. A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend. 176, 79–88. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White JM, 2016. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev 5, CD002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, Ebert SN, 2002. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J. Pharmacol. Exp. Ther 303, 688–694. [DOI] [PubMed] [Google Scholar]
- Kienbaum P., Heuter T., Michel MC., Scherbaum N., Gastpar M., Peters J., 2001. Chronic mu-opioid receptor stimulation in humans decreases muscle sympathetic nerve activity. Circulation. 103, 850–855. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Baxter LE, 2019. Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am. J. Addict 28, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, George TP, 2002. The neurobiology of opioid dependence: Implications for treatment. Sci. Pract. Perspect 1, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Nestler EJ, 2012. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb. Perspect. Med 2, a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JV Jr., Taylor R Jr., James RP, Pirner M, 2019. Differences in the receptor binding profile of lofexidine compared to clonidine. Pharmacol. Pharm 10, 1–10. [Google Scholar]
- Schmittner J, Schroeder JR, Epstein DH, Krantz MJ, Eid NC, Preston KL, 2009. Electrocardiographic effects of lofexidine and methadone coadministration: Secondary findings from a safety study. Pharmacotherapy. 29, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittner J, Schroeder JR, Epstein DH, Preston KL, 2004. QT interval increased after single dose of lofexidine. BMJ. 329, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, O’Connor PG, 2009. Management of opioid intoxification and withdrawal In: Ries RK, Fiellin DA, Miller SC, Saitz R (Eds.), Principles of addiction medicine, fourth ed Lippincott Williams and Wilkins, Philadelphia, PA: pp. 589–606. [Google Scholar]
- Tompkins DA, Smith MT, Mintzer MZ, Campbell CM, Strain EC, 2014. A double blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine. J. Pharmacol. Exp. Ther 348, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US WorldMeds LLC, 2018. Lucemyra [package insert]. US WorldMeds, LLC, Louisville, KY. [Google Scholar]
- Wedam EF, Haigney MC, 2016. The impact of opioids on cardiac electrophysiology. Curr. Cardiol. Rev 12, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]