ABSTRACT
This network meta-analysis (NMA), based on one phase II and nine phase III studies, involving 6,124 patients with metastatic NSCLC, indirectly compares Atezolizumab + Bevacizumab + chemotherapy (ABC), Atezolizumab + chemotherapy (AC), Pembrolizumab + chemotherapy (PC), Pembrolizumab alone, Bevacizumab + chemotherapy (BC) and chemotherapy alone. Each of these is recommended as front-line interventions, according to the US FDA and the European Medicines Agency (EMA) for advanced NSCLC without EGFR mutation or ALK rearrangement. Studies were identified through PubMed, EMBASE, the Cochrane Library, Medline, and abstracts found in oncology articles. Primary endpoints, i.e., progression-free survival (PFS) and overall survival (OS) with corresponding hazard ratios (HR), objective response rates (ORR) and adverse event (AEs) with odds risk (OR) were pooled according to frequentist network meta-analytical techniques. PD-L1 expression thresholds, as well as non-squamous/squamous were used to determine subgroups. Immunotherapy plus chemotherapy appeared superior to Pembrolizumab alone for PD-L1-high (i.e., TPS≥50%) NSCLC patients. BC might also be specifically recommended as an initial first-line treatment for PD-L1-high, non-squamous NSCLC patients, since BC was not inferior to Pembrolizumab alone. PC and ABC might be preferred for NSCLC patients with intermediate PD-L1 (1% ≤PD-L1, TPS<50%) expression. BC can also be tentatively recommended specifically for PD-L1-intermediate, non-squamous NSCLC patients. Combined immunotherapies can all be recommended for PD-L1-negative (i.e., TPS<1%) NSCLC patients, although especially the ABC combination for non-squamous NSCLC patients, which was superior to PC in regards of PFS. However, PC performed comparable to ABC in the whole population and in all subgroup save this one. More predictive biomarkers could be factored into further analyses to help identifying the most effective treatment regimens for specific patient groups.
KEYWORDS: Non-Small-Cell-Lung-Carcinoma, cancer, PD-L1, expression, adverse events, survival, combined, interventions, systematic review, network meta-analysis
Introduction
Advanced non-small-cell-lung cancer (NSCLC) is one of the leading causes of death worldwide and is the leading cause of cancer-related mortality. All cancers can develop through a variety of mechanisms and are capable of escaping both specific and nonspecific immune attacks. The known immune evasion mechanisms are usually similar, and can be identical to the mechanisms which govern tolerance. This makes it incredibly difficult to disentangle antitumor responses from treatment-related adverse events. As such, there are a number of approved therapies although efficacy varies substantially. So, while new technologies and medicines emerge, researchers and practitioners are looking to identify indicators which can be used to ensure specific-combined therapies will maximize the benefit for each patient.
Unfortunately, the predictive effect of interventions which target and then block PD-1 (programmed cell death 1) and PD-L1 (programmed cell death – ligand 1) pathways have been inconclusive, overall. However, a modicum of evidence is available which suggests that those diagnosed with metastatic NSCLC and displaying PD-(L)1 over-expression may encounter an increased benefit to combinations which include immune checkpoint inhibitors that target PD-(L)1 pathways.1 At present, the optimal combination therapy for NSCLC remains illusive which has led some to consider the prospective application of PD-(L)1 expression as a predictive biomarker, thereby narrowing target populations.
Immune checkpoint inhibitors emerged with some positive results in earlier-stage clinical studies which brought new optimism for both patients and practitioners.2 Currently, evidence suggests the single-agent Pembrolizumab (i.e. Keytruda), or Pembrolizumab + chemotherapy (PC) are the most effective first-line therapies for advanced NSCLC without oncogenic drivers and in patients with PD-L1-high (PD-L1 TPS≥50%) expression.3,4 Whereas for patients with PD-L1-intermediate expression (1%≤PD-L1 < 50%), PC is generally considered the best option and Pembrolizumab alone is thought to be only an acceptable option for patients who may be either unfit or unwilling to receive chemotherapeutic interventions.5 Based on IMpower 150, Atezolizumab (i.e., Tecentiq) + Bevacizumab (i.e. Avastin), + chemotherapy (frequently referred to as ABC) are also recommended by the US FDA and the European Medicines Agency (EMA) as the first-line treatment of patients without EGFR mutation or ALK rearrangement.
There are, of course, a number of approved alternative combinations. For example, AC is also commonly discussed with patients as a first-line option.6 Likewise, BC, or chemotherapy alone are considered standard approaches, implemented in an effort to inhibit disease progression and to prolong the overall survival of PD-L1-negative patients without oncogenic drivers.7 However, we are yet to determine which is the optimal intervention for NSCLC, according to PD-L1 expression. Therefore, this study is an attempt to identify the optimal intervention for NSCLC by comparing the efficacy and safety of ABC, PC, Pembrolizumab alone, BC, and chemotherapy alone. We adopted a frequentist meta-analytical approach to compare these approved first-line treatments for advanced NSCLC. Subgroup analysis was conducted according to PD-L1 expression across the entire cohort. Non-squamous or squamous NSCLC was categorized for further subgroup analysis.
Materials & methods
Study eligibility
Pubmed, Embase, the Cochrane Library and Medline, as well as abstracts from major conference proceedings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (EMSO), the American Association for Cancer Research (AACR), and the World Conference on Lung Cancer (WCLC) were searched from inception until September 10, 2019. Eligible randomized controlled trials analyzing Pembrolizumab alone, or PC or AC, or ABC, or BC, with chemotherapy alone, as first-line treatments for advanced NSCLC were identified.
Randomized phase II or III studies focusing on Pembrolizumab, PC, AC, or ABC treatments were eligible for inclusion. The target population consisted of previously untreated advanced/metastatic NSCLC patients. Outcomes needed to be expressed as an objective response rate (ORR), progression-free survival (PFS) or overall survival (OS) in order to be included. Trials involving pretreated patients were excluded.
Data extraction
Two investigators independently examined titles, abstracts, and full texts to assess eligibility. Data were extracted into a predefined spreadsheet. As well as extracting OS, PFS, and ORR this network meta-analysis also includes an analysis of adverse events associated with each intervention. Hazard ratio (HRs) and corresponding 95% confidence intervals (CIs) for OS and PFS were extracted. Likewise, dichotomous ORR data, grade 1–5 adverse events (AEs) and grade ≥3 AEs were clustered.
Trial characteristics information were also extracted for critical appraisal. This included the number of participants, demographics, clinicopathological characteristics and of course, PD-L1 expression.
Quality assessment
Two independent reviewers evaluated selective outcome reporting bias. Disagreements were discussed with a third author. Quality judgment of selected reports were made following the Cochrane Collaboration’s risk of bias tool.8 Discrepancies were resolved through discussion with a third independent reviewer.
Data analysis
Heterogeneity was analyzed across the eligible studies in terms of trial characteristics. This NMA satisfies the technical requirement that each treatment should be represented by at least one clinical study which thereby creates viable comparative network connections. A fixed-effect model was applied because ABC and BC were evaluated in only one trial each.
Pooled PFS and OS estimates are presented with corresponding HRs and 95%CI. Measures for dichotomous data (i.e., ORR) and categorical data (i.e., AEs) were pooled with odds ratio (ORs) and corresponding 95%CI. When the upper or lower limit of 95%CI were both greater than one or alternatively less than one, this was considered statistically significant. This is equivalent to p values<.05, which is generally considered the threshold for statistical significance.
Additional subgroups were determined according to histology (i.e., squamous/non-squamous) and levels of PD-L1expression (i.e., PD-L1-high: PD-L1 ≥ 50%; PD-L1-negative: PD-L1 < 1%; PD-L1-intermediate: 50%>PD-L1 ≥ 1%).
NMA is an open loop of evidence without direct comparison between experimental groups, therefore we did not assess statistical inconsistencies. Statistical analysis was carried out using R.V3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Studies included in the NMA
Ten trials, involving 6,124 patients met our eligibility criteria (see the trial selection process presented in Additional file: search strategies).9–22 The assessment of risk of bias is also provided in Additional file: Figure S1 and Table S1. The heterogeneity from the direct comparison in the Meta-analysis was provided in Additional file: Table S2.
Study characteristics and outcomes of included trials are summarized in Tables 1 and 2. Three trials investigated Pembrolizumab versus chemotherapy, three trials investigated PC versus chemotherapy alone and three trials investigated AC versus chemotherapy. All trials, except IMpower 150, used standardized chemotherapeutic regimens according to practice guidelines. Only the IMpower 150 study, investigated ABC versus BC versus AC.
Table 1.
Study characteristics.
| Source | Histology | PD-L1 Expression | Treatment Regimen | Median ages (years) | mPFS (months) | mOS (months) | Median Follow-up Time (months) |
|---|---|---|---|---|---|---|---|
| KEYNOTE-0219,19 | Non-squamous | All | PC | 62.50 | 13.00 | NR | 23.90 |
| Chemo | 63.20 | 8.90 | NR | 23.90 | |||
| KEYNOTE-02411,20 | Squamous and Non-squamous | ≥50% | Pembro | 64.50 | 10.30 | 30.00 | 25.20 |
| Chemo | 66.00 | 6.00 | 14.20 | 25.20 | |||
| KEYNOTE-04212 | Squamous and Non-squamous | ≥1% | Pembro | 63.00 | 7.10 | 20.00 | 12.80 |
| Chemo | 63.00 | 6.40 | 12.20 | 12.80 | |||
| KEYNOTE-042 in China23 | Squamous and Non-squamous | ≥1% | Pembro | NR | NR | 20.00 | 11.30 |
| Chemo | NR | NR | 13.70 | 11.30 | |||
| KEYNOTE-18910 | Non-squamous | All | PC | 65.00 | 8.80 | NR | 10.50 |
| Placebo+Chemo | 63.50 | 4.90 | 11.30 | 10.50 | |||
| KEYNOTE-40713 | Squamous | All | PC | 65.00 | 6.40 | 15.90 | 7.80 |
| Placebo+Chemo | 65.00 | 4.80 | 11.30 | 7.80 | |||
| IMpower-13014 | Non-squamous | All | AC | 64.00 | 7.00 | 18.60 | 18.50 |
| Chemo | 65.00 | 5.50 | 13.90 | 18.80 | |||
| IMpower-13117,21 | Squamous | All | AC | 65.00 | 6.30 | 14.20 | 25.50 |
| Chemo | 65.00 | 5.60 | 13.50 | 25.50 | |||
| IMpower-13218 | Non-squamous | All | AC | 64.00 | 7.60 | 18.10 | 14.80 |
| Chemo | 63.00 | 5.20 | 13.60 | 14.80 | |||
| IMpower-15016,22 | Non-squamous | All | ABC | 63.00 | 8.40 | 19.80 | 13.50 |
| AC | 63.00 | 6.90 | 19.50 | 19.60 | |||
| BC | 63.00 | 6.80 | 14.90 | 19.70 |
Abbreviation: Pembro: pembrolizumab; Chemo: chemotherapy; Placebo+Chemo: placebo plus chemotherapy; PC: pembrolizumab plus chemotherapy; AC: atezolizumab plus chemotherapy; ABC: atezolizumab plus bevacizumab plus chemotherapy; BC: bevacizumab plus chemotherapy. NR: not reported; PFS: progression-free survival; OS: overall survival.
Table 2.
Information on primary outcome of the studies included in network meta-analysis.
| Source | Overall |
PD-L1 ≥ 50% |
1%≤PD-L1 < 50% |
PD-L1 < 1% |
HR for PFS (95%CI) |
HR for OS (95%CI) |
Incidence of AEs (%) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients |
No. of Response |
No. of Patients |
No. of Response |
No. of Patients |
No. of Response |
No. of Patients |
No. of Response |
Grade 1–5 AEs |
Grade 3–5 AEs |
|||||||||||||||||||
| EM | CM | EM | CM | EM | CM | EM | CM | EM | CM | EM | CM | EM | CM | EM | CM | Overall | PD-L1 ≥ 50% | 1%≤PD-L1 < 50% | PD-L1 < 1% | Overall | PD-L1 ≥ 50% | 1%≤PD-L1 < 50% | PD-L1 < 1% | EM | CM | EM | CM | |
| KEYNOTE-0219,19 | 60 | 63 | - | - | 20 | 17 | 16 | 6 | 19 | 23 | 5 | 9 | 21 | 23 | 12 | 3 | 0.53(0.33–0.86) | - | - | - | 0.56(0.32–0.95) | - | - | - | 94.2 | 95.7 | 59.5 | 50.8 |
| KEYNOTE-02411,20 | 154 | 151 | - | - | 154 | 151 | 70 | 45 | - | - | - | - | - | - | - | - | - | 0.50(0.37–0.68) | - | - | - | 0.65(0.50–0.86) | - | - | 76.6 | 90 | 31.2 | 53.3 |
| KEYNOTE-04212 | 637 | 637 | - | - | 299 | 300 | 118 | 96 | - | - | - | - | - | - | - | - | - | 0.81(0.67–0.99) | - | - | - | 0.69(0.56–0.85) | 0.92(0.77–1.11) | - | 96.2 | 92.7 | 74.8 | 60.8 |
| KEYNOTE-042 in China23 | 128 | 134 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.62(0.38–1.00) | 0.69(0.40–1.20) | - | - | - | - | - |
| KEYNOTE-18910 | 410 | 206 | 195 | 39 | 132 | 70 | 81 | 16 | 128 | 58 | 62 | 12 | 127 | 63 | 41 | 9 | 0.52(0.43–0.64) | 0.36(0.25–0.52) | 0.55(0.37–0.81) | 0.75(0.53–1.05) | 0.49(0.38–0.64) | 0.42(0.26–0.68) | 0.55(0.34–0.90) | 0.59(0.38–0.92) | 94.6 | 90.7 | 69.2 | 57.8 |
| KEYNOTE-40713 | 278 | 281 | 161 | 108 | 73 | 73 | 44 | 24 | 103 | 104 | 51 | 43 | 95 | 99 | 60 | 40 | 0.56(0.45–0.70 | 0.37(0.24–0.58) | 0.56(0.39–0.80) | 0.68(0.47–0.98) | 0.64(0.49–0.85) | 0.64(0.37–1.10) | 0.57(0.36–0.90) | 0.61(0.38–0.98) | 91.8 | 87.2 | 57.4 | 41.6 |
| IMpower-13014 | 447 | 226 | 220 | 72 | - | - | - | - | - | - | - | - | - | - | - | - | 0.65(0.54–0.77) | 0.51(0.34–0.77) | 0.61(0.43–0.85) | 0.72(0.56–0.91) | 0.80(0.65–0.99) | 0.84(0.51–1.39) | 0.70(0.45–1.08) | 0.81(0.61–1.08) | 99.8 | 99 | 67.2 | 65.8 |
| IMpower-13117,21 | 169 | 140 | 83 | 57 | 32 | 16 | 19 | 5 | 67 | 53 | 35 | 23 | 70 | 71 | 31 | 30 | 0.71(0.60–0.85) | 0.44(0.27–0.71) | 0.70(0.53–0.92) | 0.81(0.64–1.03) | 0.88(0.73–1.05) | 0.48(0.29–0.81) | 1.08(0.81–1.45) | 0.87(0.67–1.13) | 98.2 | 97.9 | 69.8 | 68.2 |
| IMpower-13218 | 292 | 286 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.60(0.494–0.72) | 0.46(0.22–0.96) | 0.80(0.56–1.16) | 0.45(0.31–0.64) | 0.81(0.64–1.03) | - | - | - | 93.2 | 90.3 | 39 | 22.6 |
| IMpower-15016,22 | 359 | 337 | - | - | 74 | 71 | 51 | 35 | 134 | 126 | 78 | 51 | 189 | 196 | 95 | 72 | 0.59(0.5–0.69) | 0.33(0.22–0.51) | 0.55(0.42–0.73) | 0.75(0.60–0.94) | 0.76(0.63–0.93) | 0.67(0.42–1.06) | 0.76(0.54–1.08) | 0.83(0.64–1.08) | 62.7 | 89.9 | 17.8 | 41 |
| 349 | 337 | - | - | 68 | 71 | 42 | 35 | 147 | 126 | 64 | 51 | 185 | 196 | 57 | 72 | 0.91(0.78–1.06) | 0.63(0.43–0.92) | 0.79(0.61–1.03) | 1.10(0.89–1.36) | 0.85(0.71–1.03) | 0.75(0.47–1.20) | 0.74(0.52–1.03) | 0.98(0.76–1.27) | 73.4 | 90 | 26.6 | 53.3 | |
Abbreviation: EM: experimental arm; CM: control arm; Pembro: pembrolizumab; Chemo: chemotherapy; Placebo+Chemo: placebo plus chemotherapy; PC: pembrolizumab plus chemotherapy; AC: atezolizumab plus chemotherapy; ABC: atezolizumab plus bevacizumab plus chemotherapy; BC: bevacizumab plus chemotherapy. NR: not reported; PFS: progression-free survival; OS: overall survival. HR: hazard ratio
All trials assessed PD-L1 expression with immunohistochemical methods. The median follow-up time ranged from 7.8 months to 23.9 months. Nine trials provided PFS, OS, and necessary toxicity data. ORR data was not reported in the IMpower-132 trial and the KEYNOTE-042 study which took place in China. ORR subgroup analysis according to PD-L1 expression was not reported in the IMpower-13014 and PFS and OS subgroup analysis according to PD-L1 expression were not reported in the KEYNOTE-021 trial.9,19 The KEYNOTE-042 study conducted in China only analyzed OS according to PD-L1 expression.23 A presentation of the network is provided in Figure 2. Indirect comparisons between ABC and PC or Pembrolizumab alone were connected through the delivery of AC.
Figure 2.
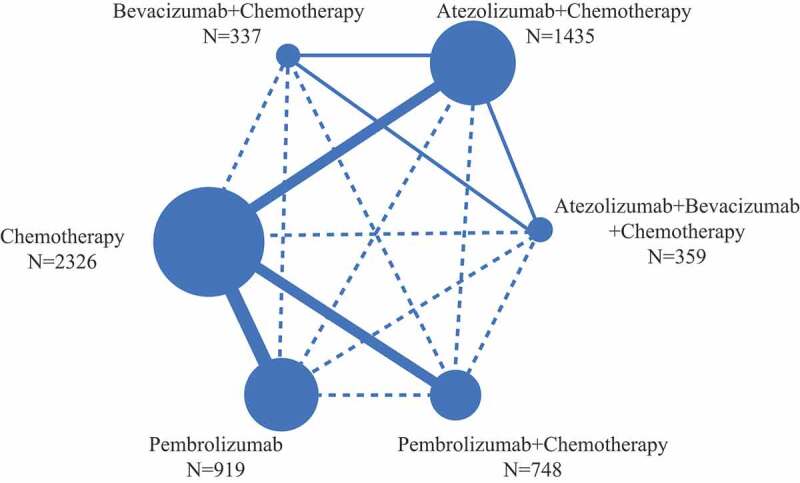
Network structure for all the included trials. Each circular node represents a treatment type. The circle size is proportional to the total number of patients. The width of lines is proportional to the number of studies performing head-to-head comparisons in the same study, and the dotted line is the indirect comparison which was shown in this NWM.
NMA for overall study cohort
The results and forest plots for indirect comparison are presented in Figure 1. and Additional file: Figure S1, 2, 3.
Figure 1.
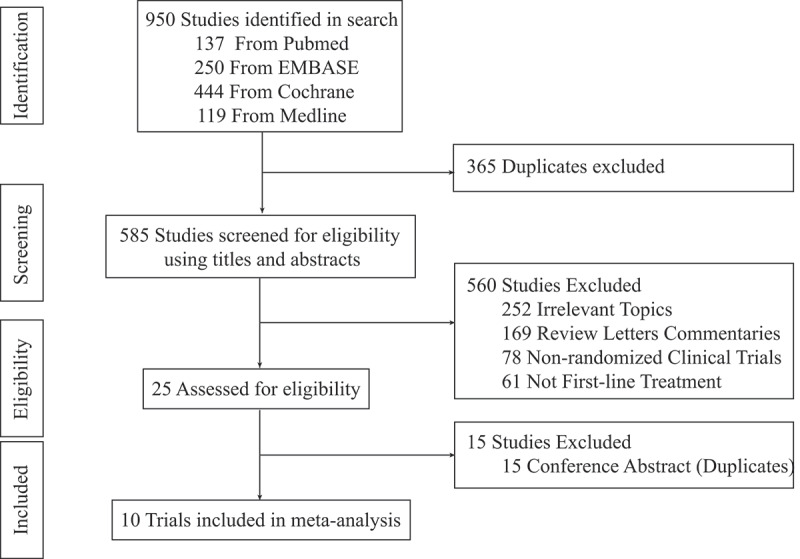
Flow chart of study selection.
Figure 3.
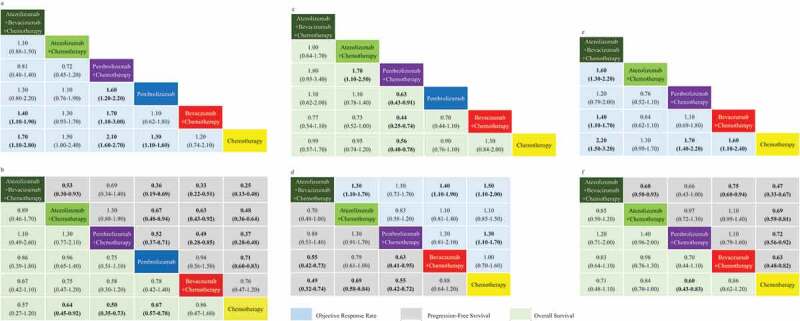
ORR, PFS, and OS profiles for study cohort in the subgroup analysis based on PD-L1 expression. PD-L1-high (a,b), PD-L1-intermediate (c,d) and PD-L1-negative (e,f) subgroup analysis according to network meta-analysis (NMA). Each cell contains the pooled odds ratios (OR) and 95% credibility intervals for ORR, and the Hazard-Radio (HR) and 95% credibility intervals for PFS and OS; significant results are emboldened.
PD-L1 TPS≥50% cohort
For PD-L1-high patients, the ORR-NMA was based on seven trials, whereas the PFS-NMA and OS-NMA are based on eight separate trials. In terms of ORR, ABC appears inferior to PC, but superior to AC and Pembrolizumab alone. ABC, PC, and Pembrolizumab alone all performed significantly better than BC and chemotherapy alone, expect Pembrolizumab versus BC (OR 1.10, 95% CI 0.62–1.80). PC performed significantly better than Pembrolizumab alone (OR 1.60, 95% CI 1.20–2.20). ABC and AC had inferior trends to PC but appears superior to Pembrolizumab alone.
For PFS, all treatment groups were significantly superior to BC and chemotherapy alone, expect Pembrolizumab versus BC (HR 0.94, 95% CI 0.56–1.50) and BC versus chemotherapy alone (HR 0.76, 95% CI 0.47–1.20). ABC, AC, and PC all appear significantly superior to Pembrolizumab alone. ABC performed significantly better than AC (HR 0.53, 95% CI 0.30–0.93) and appears superior to PC (HR 0.69, 95% CI 0.34–1.40), while AC appears inferior to PC, overall.
For OS, AC, PC, and Pembrolizumab alone performed significantly better than chemotherapy alone (HR 0.64, 95% CI 0.0.45–0.92 for AC, HR 0.50, 95% CI 0.35–0.73 for PC, HR 0.67, 95% CI 0.57–0.80 for Pembrolizumab alone). Although there was no significant difference in effect between subgroups. In terms of overall survival, PC appears superior to ABC, AC, BC, and Pembrolizumab alone for patients with high PD-L1 expression.
Intermediate PD-L1 (1%≤PD-L1 < 50%) cohort
For PD-L1-intermediate patients, the ORR-NMA was based on five trials, the PFS-NMA was based on six and OS-NMA was based on seven trials. Pembrolizumab alone was not analyzed using ORR or PFS due to the lack of data from KEYNOTE-024, KEYNOTE-042 and the KEYNOTE-042 study conducted in China.
For ORR, ABC appears superior to PC, and was significantly more effective than AC (OR 1.30, 95% CI 1.10–1.70), BC (OR 1.40, 95% CI 1.10–1.90) and chemotherapy alone (OR 1.50, 95% CI 1.10–2.00). PC performed significantly better than chemotherapy alone (OR 1.30, 95% CI 1.10–1.70), but appears only marginally superior to BC (ORR 1.30, 95% CI 0.81–2.10). AC appears inferior to PC but superior to BC and chemotherapy alone.
In terms of PFS, ABC and PC both performed significantly more effectively than BC (HR 0.55, 95% CI 0.42–0.73 for ABC; HR 0.63, 95% CI 0.41–0.95 for PC) and chemotherapy alone (HR 0.49, 95% CI 0.32–0.74 for ABC; HR 0.55, 95% CI 0.42–0.72 for PC). AC was also appears superior to chemotherapy alone (HR 0.69, 95% CI 0.58–0.84); however,; ABC appears superior to both PC and AC, and AC can be considered inferior to PC.
For OS, ABC appears inferior to both PC and Pembrolizumab alone, but appears superior to BC. AC was significantly inferior to PC (HR 1.70, 95% CI 1.10–2.50), with only marginal superiority over BC and chemotherapy alone. PC was significantly more effective than Pembrolizumab alone (HR 0.63, 95% CI 0.43–0.91), BC (HR 0.44, 95% CI 0.25–0.74) and chemotherapy alone (HR 0.56, 95% CI 0.40–0.78). Pembrolizumab alone appears only marginally superior to BC and chemotherapy alone.
PD-L1 < 1% cohort
For patients with low PD-L1 expression, the ORR-NMA was based upon five trials, the PFS-NMA based on six and the OS-NMA based on five trials. Pembrolizumab alone was not analyzed using ORR and PFS could not be analyzed due to the lack of data from KEYNOTE-024, KEYNOTE-042, and KEYNOTE-042 conducted in China. In terms of ORR, ABC appears superior to PC, and was significantly more effective than AC (OR 1.60, 95% CI 1.30–2.20), BC (OR 1.40, 95% CI 1.10–1.70) and chemotherapy alone (OR 2.20, 95% CI 1.50–3.20). PC appears superior to BC, and was significantly better than chemotherapy alone (OR 1.70, 95% CI 1.40–2.20). AC also appears inferior to both PC and BC, but appears superior to chemotherapy alone.
For PFS, ABC (HR 0.47, 95% CI 0.33–0.67), AC (HR 0.69, 95% CI 0.59–0.81), PC (HR 0.72, 95% CI 0.56–0.92) and BC (HR 0.63, 95% CI 0.48–0.82) were significantly more effective than chemotherapy alone. ABC was significantly more effective than BC (HR 0.75, 95% CI 0.60–0.94), but not PC (HR 0.66, 95% CI 0.43–1.00). Overall, PC also appears inferior to BC.
There was no significant difference in OS between ABC, AC, PC, BC, and chemotherapy alone. Although PC appears to be superior to chemotherapy alone (HR 0.60, 95% CI 0.43–0.83).
NMA for non-squamous NSCLC
ORR-NMA was not conducted for non-squamous NSCLC due to the lack of data. The results and forest plots providing indirect comparisons between treatments are however presented in Figure 4 and Additional file: Figure S4. 5.
Figure 4.
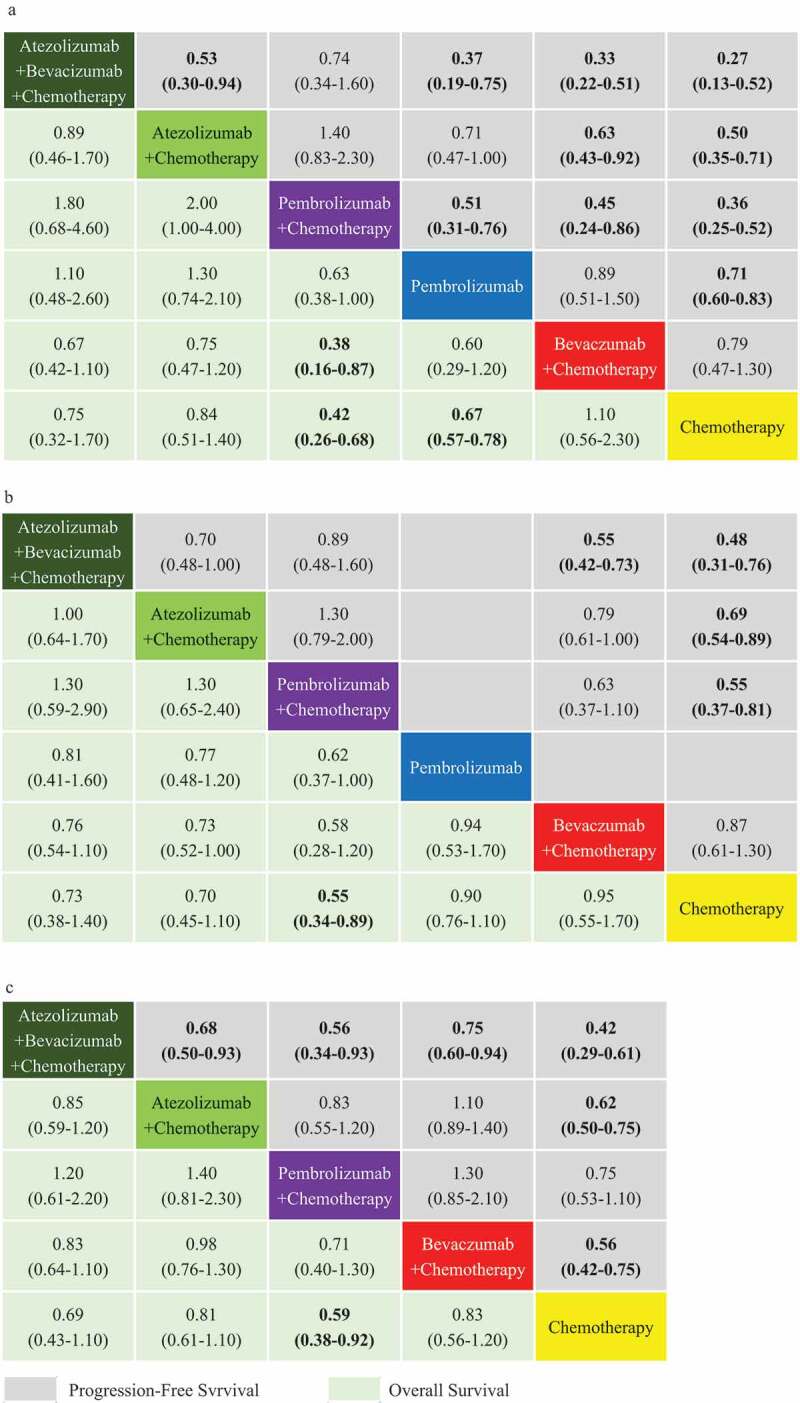
PFS and OS comparison profiles for non-squamous NSCLC under subgroup analysis are based on PD-L1 expression. PD-L1-high (a), PD-L1-intermediate (b) and PD-L1-negative (c) subgroup analysis according to network meta-analysis (NMA). Each cell contains the Hazard-Radio (HR) and 95% credibility intervals for PFS and OS; significant results are emboldened.
PD-L1 ≥ 50% cohort
For PD-L1-high patients, the PFS-NMA and the OS-NMA were based on six separate trials. ORR-NMA was not possible, between ABC and PC or Pembrolizumab alone, because connections could not be established due to the lack of AC data. For PFS, ABC appears superior to PC; however,; these intervention strategies were both significantly more effective than Pembrolizumab alone (HR 0.37, 95% CI 0.19–0.75 for ABC; HR 0.51, 95% CI 0.31–0.76 for PC), BC (HR 0.33, 95% CI 0.22–0.51 for ABC; HR 0.45, 95% CI 0.24–0.86 for PC) and chemotherapy alone (HR 0.27, 95% CI 0.13–0.52 for ABC; HR 0.36, 95% CI 0.25–0.52 for PC). AC was significantly superior to BC (HR 0.63, 95% CI 0.43–0.92) and chemotherapy alone (HR 0.50, 95% CI 0.35–0.71). Pembrolizumab alone was marginally superior to BC (HR 0.89, 95% CI 0.51–1.50), but was substantially more effective than chemotherapy alone (HR 0.71, 95% CI 0.60–0.83).
For OS, PC performed significantly better than BC (HR 0.38, 95% CI 0.16–0.87) and chemotherapy alone (HR 0.42, 95% CI 0.26–0.68). Pembrolizumab alone performed significantly better than chemotherapy alone (HR 0.67, 95% CI 0.57–0.78). Although there were no statistically significant difference between treatment groups, except for those previously mentioned.
Intermediate PD-L1 (1%≤PD-L1 < 50%) cohort
For PD-L1-intermediate patients, the PFS-NMA was based on four trials and OS-NMA on five trials. ORR-NMA was not analyzed for PD-L1-high patients analysis due to the missing AC connection. It was also not possible to analyze Pembrolizumab alone in this cohort due to the lack of PFS data.
For PFS, ABC appears superior to PC, AC, and was significantly more effective than BC (HR 0.55, 95% CI 0.42–0.73) and chemotherapy alone (HR 0.48, 95% CI 0.31–0.76). AC (HR 0.69, 95% CI 0.54–0.89) and PC (HR 0.55, 95% CI 0.37–0.81) were significantly more effective than chemotherapy, although there was only a marginal improvement compared to BC (HR 0.79, 95% CI 0.61–1.00 for AC; HR 0.63, 95% CI 0.37–1.10 for PC). There were no significant differences among ABC, AC, and PC in terms of progression-free survival.
For OS, PC appears superior to chemotherapy alone (HR 0.55, 95% CI 0.34–0.89). Although there was no significant difference when comparing ABC, AC, PC, pembrolizumab alone, BC, and chemotherapy.
PD-L1 < 1% cohort
For PD-L1-low patients, the PFS-NMA was based on four trials and OS-NMA on three. ORR-NMA was not analyzed due to the missing AC connection, for the same reason as for the PD-L1-high expression analysis. Pembrolizumab alone was also not analyzed due to the lack of data.
For PFS, ABC appears to provide a significant improvement compared with AC (HR 0.68, 95% CI 0.50–0.93), PC (HR 0.56, 95% CI 0.34–0.93), BC (HR 0.75, 95% CI 0.60–0.94) and chemotherapy alone (HR 0.42, 95% CI 0.29–0.61). AC (HR 0.62, 95% CI 0.50–0.75) performed significantly better than chemotherapy and appears superior to PC. Although PC appears inferior to BC while being superior to chemotherapy alone. BC was significantly more effective than chemotherapy alone (HR 0.56, 95% CI 0.42–0.75). PC appears superior to chemotherapy in terms of OS (HR 0.59, 95% CI 0.38–0.92). However, there was no significant difference among other interventions in terms of overall survival.
NMA for squamous non-small cell lung cancer
For PD-L1-high patients with squamous NSCLC, the ORR-NMA, PFS-NMA, and OS-NMA were both based on separate five trials. The results and forest plots for indirect comparison among treatment groups are presented in Figure 5. and in Additional file: Figure S6,7, 8. NMA profiles for PD-L1-intermediate and PD-L1-low are presented in Additional file: Figure S6, 7, and 8. For ORR, PC (OR 1.80, 95% CI 1.30–2.70) and Pembrolizumab alone (OR 1.30, 95% CI 1.10–1.60) performed significantly better than chemotherapy alone. PC and AC also appear superior to Pembrolizumab alone.
Figure 5.
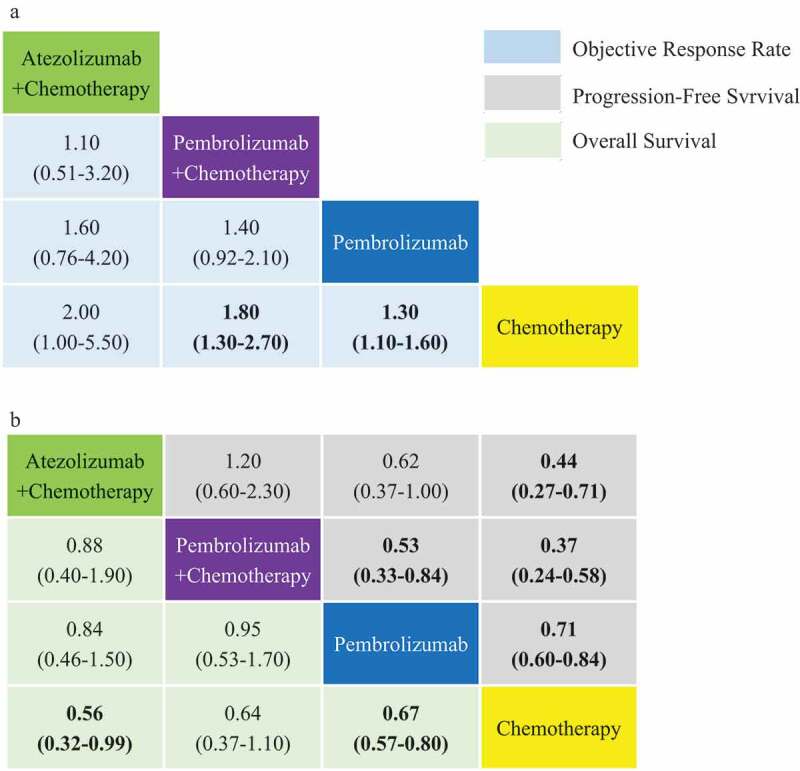
ORR, PFS, and OS comparative profiles for PD-L1-high squamous NSCLC according to network meta-analysis (NMA). Each cell contains the pooled odds ratios (OR) and 95% credibility intervals for ORR (a), and the Hazard-Radio (HR) and 95% credibility intervals for PFS and OS (b); significant results are emboldened.
For PFS, PC was significantly more effective than Pembrolizumab alone (HR 0.53, 95% CI 0.33–0.84) and chemotherapy alone (HR 0.37, 95% CI 0.24–0.58). Pembrolizumab appears to provide a significant benefit compared to chemotherapy alone (HR 0.71, 95% CI 0.60–0.84). AC on the other hand appears inferior to PC, yet superior to Pembrolizumab alone.
For OS, PC appears superior to Pembrolizumab alone. Both AC (HR 0.56, 95% CI 0.32–0.99) and Pembrolizumab alone (HR 0.67, 95% CI 0.57–0.80) performed significantly more effectively than chemotherapy alone.
For patients with intermediate PD-L1 expression, AC (HR 0.70, 95% CI 0.53–0.92) and PC (HR 0.56, 95% CI 0.39–0.80) were significantly more effective than chemotherapy in terms of PFS and PC appears significantly superior to both chemotherapy alone (HR 0.57, 95% CI 0.36–0.90) and AC in terms of overall survival.
For PD-L1-negative patients, PC appears significantly superior to chemotherapy alone in terms of ORR (OR 1.50, 95% CI 1.20–2.10), PFS (HR 0.68, 95% CI 0.47–0.98) and OS (HR 0.61, 95% CI 0.38–0.98). There was no identifiable difference among the other regimens included.
NMA for safety analysis
OR-NMAs were based on all 10 trials according to grade 1–5 AEs and clustered grade 3–5 AEs, as stipulated in the design. Figure 6. highlights that those with low grade and grade 3–5 AEs perhaps benefit more from PC and Pembrolizumab alone compared to BC (OR 0.95, 95% CI 0.91–0.99 for PC, OR 0.69, 95% CI 0.64–0.74 for Pembrolizumab alone for grade 1–5 AEs; OR 0.73, 95% CI 0.61–0.88 for PC, OR 0.33, 95% CI 0.26–0.42 for Pembrolizumab alone for grade 3–5 AEs).
Figure 6.
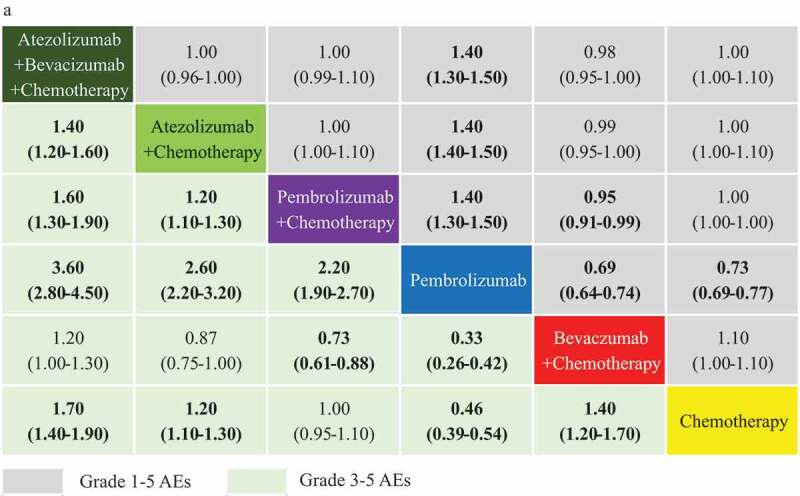
Incidence of grade 1–5 AEs and grade ≥3 AEs comparative profiles according to network meta-analysis (NMA). Each cell contains the pooled odds ratios (OR) and 95% credibility intervals for the incidence of AEs; significant results are emboldened. AEs: adverse events AE.
ABC and AC appear significantly less safe than PC with an OR 1.60 (95% CI 1.30–1.90 for grade 3–5 AEs for ABC) and an OR 1.20 (95% CI 1.10–1.30 for grade 3–5 AEs for AC). Pembrolizumab alone appears to be the safest intervention among the regimens analyzed. All forest plots for indirect comparisons are provided, in the Additional file: Figure S9.
Discussion
This indirect network meta-analysis was conducted to analyze approved first-line treatments for advanced metastatic NSCLC in patients, without EGFR mutation or ALK rearrangement, according to PD-L1 expression. In patients with PD-L1-high expression, we found PC, AC, and ABC are significantly superior to Pembrolizumab alone, BC and chemotherapy alone, in terms of progression-free survival. However, Pembrolizumab alone appears marginally superior to BC in terms of ORR, PFS and OS, while being significantly superior to chemotherapy alone. There were no clear differences among the three combined immunotherapies, expect for ABC, which appears significantly superior to the AC in terms of progression-free survival.
These findings subtly changed when comparing these combinations for patients presenting with PD-L1-high expression and non-squamous NSCLC. PC appears significantly superior to Pembrolizumab alone according to PFS, while AC appears comparatively less beneficial. However, these three combinations all appear to be superior to chemotherapy alone. For patients with PD-L1-high expression, immunotherapies combined with chemotherapy should be generally preferred rather than Pembrolizumab alone. However, BC may also be recommended as the initial first-line treatment for PD-L1-high patients with advanced non-squamous NSCLC since this regime appears, at least equal to Pembrolizumab alone.
For patients with advanced NSCLC and intermediate PD-L1 expression, chemotherapy appears to add benefit to Pembrolizumab alone. PC also appears to provide an additional benefit compared to both BC and chemotherapy alone in terms of overall survival. Although both ABC and PC appear superior to BC and chemotherapy alone in progression-free survival. This further confirms that chemotherapy alone is generally less effective across all sub-populations suffering both non-squamous and squamous NSCLC. However, the timing was not intercalated here and these sequential data may provide more insight in further research of this type.
When comparing the three included combination therapies, AC appears to provide less benefit in overall survival compared with PC and ABC in objective response rates. However, for those with advanced non-squamous NSCLC and intermediate PD-L1 expression, PC, and Pembrolizumab alone performed only marginally better than BC but the ABC combination appears superior to the BC combination. PC and ABC may generally be preferred first-line treatments for PD-L1-intermediate, advanced NSCLC patients; however,; this evidence suggests that BC may also be recommended as an initial first-line intervention for those with intermediate PD-L1 expression and advanced, non-squamous NSCLC.
For PD-L1-negative, advanced NSCLC, all four combined treatments once again appear significantly superior to chemotherapy alone, in terms of progression-free survival. That said, ABC appears statistically superior to PC, AC, BC, and chemotherapy alone in terms of PFS. There was only marginal superiority compared to PC in ORR and OS, and this is NMA highlighted no statistical differences in efficacy between PC and BC. The same appears to be true for PD-L1-negative patients with advanced non-squamous NSCLC. Combined immunotherapies should still be recommended for PD-L1-negative patients with advanced NSCLC, but specifically ABC ought to be administered as the initial first-line intervention for PD-L1-negative patients with advanced non-squamous NSCLC, which conflicts with previous conclusions.
Of course, the interventions included in this investigation do not include the most recent additions to the NSCLC treatment landscape. This is a rapidly evolving evidence base and formally approved interventions are constantly being added. For example, researchers at the 2019 World Conference on Lung Cancer (WCLC), presented data for a new potential first-line intervention. Their findings suggest that Camrelizumab plus chemotherapy may result in a substantial clinical benefit for those diagnosed with advanced non-squamous NSCLC and with negative oncogenic drivers, in terms of PFS, ORR and OS.24 This combination may become an approved first-line therapy, although, further clinical research is required because our forthcoming network meta-analysis of PD-L1-high patients, appears to suggest that Camrelizumab plus chemotherapy maybe inferior to both ABC and PC, and appears only marginally superior to both Pembrolizumab alone, and AC (data not provided here).
Likewise, reported in the 2019 European Society for Medical Oncology (ESMO), researchers found that Nivolumab plus Ipilimumab (NI) can improve overall survival for both PD-L1-high and PD-L1-negative patients in the Checkmate 227 study.25 They also found that Atezolizumab alone improved PFS and OS for PD-L1-high patients with NSCLC in IMpower 110.26 However, findings from a related network meta-analysis suggest that NI is inferior to other immunotherapeutic combinations for PD-L1-high patients, yet appears superior to other combinations for patients with PD-L1-negative expression. They also found Atezolizumab to be inferior to ABC and PC while appearing superior to chemotherapy alone and NI in PFS and OS. These data were not included here but maybe included in an update, pending approval. What this does perhaps highlight, is the diversity of responses to immune checkpoint inhibitors and chemotherapy.
Chemotherapeutics tend to either enhance immunogenicity or can eliminate immunosuppressive cells while potentially reprogramming certain elements of the tumor milieu. It is important for us to differentiate these effects as we move forward because chemotherapeutics can delay the occurrence of drug resistance, thereby potentially redefining survival. Consistently, meta-analytical studies confirm that adding chemotherapy to first-line, immune checkpoint inhibitors such as, Pembrolizumab improves treatment efficacy for patients with advanced NSCLC.27,28 This can be partially explained by the synergistic effects of combined immunotherapies with chemotherapy-induced neoantigen release.29 Although not all immune checkpoint inhibitors are the same. Nivolumab and Pembrolizumab are specifically PD-1 antagonists whereas Durvalumab and Atezolizumab were designed to bind the PD-1, PD-L1 ligand. This could be further used to attenuate effects for specific NSCLC subpopulations although further research is necessary.
Meta-analyses have demonstrated that combining chemotherapy with either Atezolizumab or Pembrolizumab shown consistently improved benefit compared to any other immune checkpoint inhibitor combination, such as Ipilimumab plus chemotherapy,30 Ipilimumab plus Nivolumab, Nivolumab plus chemotherapy,31 and Durvalumab plus tremelimumab,32 as initial first-line treatments for advanced NSCLC patient. BC is also not inferior to Pembrolizumab for PD-L1-high patients and not inferior to PC for PD-L1-intermediate patients30 although this may be because Bevacizumab has an anti-angiogenic capacity with immunomodulatory effects. This may encourage immune reprogramming of the tumor micro-environment which may convert an immune-suppressive action to an immune-permissive one. Again though, it appears necessary to link studies more closely so that basic medical knowledge can be used consequentially for selecting participants for global phase II and III trials.
Another example of when biomarkers could be incorporated to align patients with specific treatments to maximize benefit can be seen in Bevacizumab-induced tumor vascular normalization, which theoretically, promotes T cells infiltration, thereby increasing sensitivity to checkpoint inhibitor-based therapies. This mechanism of action is particularly prominent in the ABC combined intervention, which improves progression-free survival for PD-L1-negative patients with advanced non-squamous NSCLC.33,34 However, previous studies have shown there is no additional benefit in immune checkpoint inhibitor treatments which block PD-1 and PD-L1 compared to chemotherapy in EGFR-positive patients.35-37 Also, lower prevalence of PD-L1 expression in EGFR-positive patients compared to those with wild-type expression suggests clinical benefit may not be determined by high PD-L1 expression alone. The US FDA and the EMA recommend the included interventions for advanced NSCLC without EGFR mutations and ALK rearrangements. This further acknowledges the possibility of aligning patients to specific therapeutic combinations according to predictive biomarkers, although further systematic studies are required.
While evidence appears to confirm that there is a benefit to applying anti-PD1/PD-L1 inhibitors according to PD-L1 expression;9 PD-L1 expression alone may not be the most effective biomarker for selecting patients for immunotherapy.37 Pembrolizumab (a PD-1 inhibitor) plus chemotherapy appears superior to Atezulizumab (a PD-L1 inhibitor) plus chemotherapy in this indirect comparison. A potential biological explanation for this is that Pembrolizumab simultaneously blocks binding between PD1 receptors and both corresponding ligands, PD-L1 and PD-L2, while Atezolizumab was designed to block only the PD-L1. This biological knowledge could be combined and used in conjunction with phase III clinical evidence to further explore the subtle differences within study cohorts. Again, this appears to be the next logical step if we are to align specific-combined interventions, with sequencing, to specific populations.
This study revealed a significantly lower incidence of grade 3–5 AEs among those administer with Pembrolizumab alone and PC compared with ABC, AC, and BC combinations. Pembrolizumab alone appears to be the best tolerated among all the combinations analyzed here, with a lower incidence of all grade, and grade 3–5 AEs. As such, Pembrolizumab might also be recommended specifically for the older patients or for those considered to be in relatively poor-condition with high PD-L1 expression. Also based on this study, PC maybe for those patients in poor condition, with intermediate or negative PD-L1 expression. However, it is important to mention the limitations associated with approved anti-PD-L1 IHC assays.
KEYNOTE studies utilize the 22C3 pharmDx assay to detect TPS, which is defined according to the percentage of tumor cells found within membranous PD-L1 staining.38 While, IMpower studies implement SP142 assaying technologies to detect PD-L1 expression on tumor cells and tumor-infiltrating immune cells.14,16,39 The difference between these detection methods and the corresponding thresholds might lead to patient misclassification, impacting on outcomes overall.
This was not the only limitation we encountered during this meta-analysis. Our study relied on published results rather than on individual patient data which actually reduces precision. There is also presently only one IMpower 150 study which randomized participants directly to either ABC and BC treatments. Unfortunately, this exception highlights the lack of data from head-to-head comparisons of these immunotherapeutic combinations. BC is currently recommended as the initial first-line therapy to be tried for advanced NSCLC, based on improved survival observed in the BEYOND and AVAIL studies.40,41 However, this NMA suggests BC will only improve progression-free survival for patients with PD-L1-negative expression. This discrepancy might have manifested through low reporting quality, because several studies failed to report sub-grouping and outputs such as ORR, PFS, or OS. The studies included were heterogeneous in terms of study location, population, number of patients of different studies, basal conditions and in the difference between PD-L1 detection methods and the corresponding thresholds. This is something we must address as a research community, because subtle differences may be used to align both combinations and treatment regimens to specific populations, and perhaps in the future to individuals.
Conclusion
Evidence from this study suggests combined immunotherapies are superior to Pembrolizumab alone for PD-L1 ≥ 1% but especially for PD-L1 ≥ 50%. For advanced non-squamous NSCLC, BC can also be recommended as an initial first-line treatment for PD-L1 ≥ 1%. Combined immunotherapies can still be recommended for PD-L1-negative patients with advanced NSCLC, but ABC can be recommended specifically for those with non-squamous NSCLC. This study suggests PD-L1 expression may shed light on individual response differences although there are other potential predictive biomarkers which could be factored into identify and target specific populations who respond best to specific combinations. This new collaborative, biomarker-driven phase in research, necessitates bridging traditional boundaries between basic medical and clinical research, where interdisciplinary research teams record and report more sophisticated data. This additional knowledge will help to align specific combinations to specific patient groups, although of course, further research is required.
Funding Statement
This work was supported by National Natural Science Foundation of China (81972864) and Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001) and Science and Technology Plan of Jinan (201907113).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Authors’ contributions
Jie Liu searched the literature and wrote the manuscript. Chengming Li helped to collect literature and participated in discussions. Xue Meng and Jinming Yu designed the study. Samuel Seery examined and verified the study. All authors read and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Huang Q, Hua Z, Hai J, Socinski MA, Lim E, Chen H, Stebbing J.. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology 2018;2017(12):e1396403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuai W, Hao J, Hao W, Yong F, Tan L. Efficacy and safety of immune checkpoint inhibitors in non-small cell lung cancer. J. Oncoimmunology, 2018;7(8):e1457600. doi: 10.1080/2162402X.2018.1457600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguezabreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y, Fang W, Yang Y, Hou X, Huang Y. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J ImmunoTher Cancer. 2018;6. doi: 10.1186/s40425-018-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low JL, Walsh RJ, Ang Y, Chan G, Soo RA. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Ther Adv Med Oncol. 2019;11:175883591987036. doi: 10.1177/1758835919870360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Liu N, Gao S, et al. Can an ¹⁸F-ALF-NOTA-PRGD2 PET/CT Scan Predict Treatment Sensitivity to Concurrent Chemoradiotherapy in Patients with Newly Diagnosed Glioblastoma? Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2016;57(4):524-529. doi: 10.2967/jnumed.115.165514 [DOI] [PubMed]
- 7.Rossi A. New options for combination therapy for advanced non-squamous NSCLC. Expert Rev Respir Med. 2019;13(11):1095–12. doi: 10.1080/17476348.2019.1667233. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br Med J. 2011;343:889–893. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadgeel SM, Stevenson JP, Langer CJ, Leena G, Hossein B, Amita P, Villaruz LC, Matthew G, Ralph H, Chih-Hsin YJ. Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non–small-cell lung cancer: phase 1 cohorts from the KEYNOTE-021 study. J. Lung cancer 2018;125:273–281. doi: 10.1016/j.lungcan.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, Rodríguezabreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18:1600–1609. doi: 10.1016/S1470-2045(17)30690-3. [DOI] [PubMed] [Google Scholar]
- 12.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 14.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 15.Socinski MA, `Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodrã-Guez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:NEJMoa1716948. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 17.Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein MA, Soo RA, Conter HJ, Kozuki T, Silva C, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36(18_suppl):LBA9000LBA9000. doi: 10.1200/JCO.2018.36.18_suppl.LBA9000. [DOI] [Google Scholar]
- 18.Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, Novello S, Orlandi F, Ball S, Goldschmidt J, et al. IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thoracic Oncol. 2018;13:S332S333. doi: 10.1016/j.jtho.2018.08.262. [DOI] [Google Scholar]
- 19.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:14971508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck DR-AM, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, et al., KEYNOTE-024 3-year survival update: pembrolizumab VS platinum-based chemotherapy for advanced non-small non-small-cell lung cancer.. 2019 World Conference on Lung Cancer Abstract Book. [accessed 2019 September8. https://wclc2019.iaslc.org/onsite-information/. [Google Scholar]
- 21.Jotte FCR, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein M, Soo R, Conter H, Kozuki T, Huang K, Graupner V, et al., IMpower131: final os results of Carboplatin + Nab-Paclitaxel ± Atezolizumab in advanced Squamous NSCLC. 2019 World Conference on Lung Cancer Abstract Book. [accessed 2019 September8]. https://wclc2019.iaslc.org/onsite-information/. [Google Scholar]
- 22.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 23.Wu -LZY-L, Fan Y, Zhou J, Zhang L, Zhou Q, Li W, Hu C, Chen G, Zhang X, Zhou C, et al., KEYNOTE-042 China Study: first-line pembrolizumab VS chemotherapy in Chinese patients with advanced NSCLC with PD-L1 TPS≥1%. 2019 World Conference on Lung Cancer Abstract Book. [accessed 2019 September8]. https://wclc2019.iaslc.org/onsite-information/. [Google Scholar]
- 24.Zhou GCC, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, Wang Q, et al., A randomized phase 3 study of camrelizumab plus chemotherapy as 1st line therapy for advanced/metastatic nonsquamous non-small cell lung cancer. 2019 World Conference on Lung Cancer Abstract Book. [accessed 2019 September8]. https://wclc2019.iaslc.org/onsite-information/. [Google Scholar]
- 25.Peters SRS, Paz-Ares L, Caro RB, Zurawski B, Kim S-W, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, et al., Nivolumab+low-dose ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: checkmate 227 part 1 final analysis. (2019 European Society for Medical Oncology (ESMO)).
- 26.David FDM, Spigel R, Giaccone G, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, et al., IMpower 110: interimOS analysis of a phase III study of atezolizumab vs platinum-based chemotherapy as 1L treatment in PD-L1-selected NSCLC. 2019ESMO congress.
- 27.Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, Zhang L. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J ImmunoTher Cancer. 2019;7. doi: 10.1186/s40425-019-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhoum H, Zhang Y, Chen G, Zhao S, Liu J, Hong S, Zhang L. Which is the optimal immunotherapy for advanced non-squamous non-small-cell lung cancer in combination with chemotherapy? J Thoracic Oncol. 2018;13:S1059–S1060. doi: 10.1016/j.jtho.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Govindan R, Szczesna A, Ahn MJ, Schneider CP, Mella PFG, Barlesi F, Han B, Ganea DE, Pawel JV, Vladimirov V, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non–small-cell lung cancer. J Clin Oncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 31.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters S, Antonia S, Goldberg SB, Heymach JV, Kim ES, Nakagawa K, Papadimitrakopoulou V, Mukhopadhyay P, McIntosh S, Rizvi NA. MYSTIC: A global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy or durvalumab monotherapy versus platinum-based chemotherapy (CT) in the first-line treatment of patients (pts) with advanced stage IV NSCLC. J Thoracic Oncol. 2016;11:S139–S140. doi: 10.1016/S1556-0864(16)30300-8. [DOI] [Google Scholar]
- 33.Hegde PS, Wallin JJ. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. J. Seminars in cancer biology 2018;52(2):117–124. doi: 10.1016/j.semcancer.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J (United States). 2018;24:193–204. [DOI] [PubMed] [Google Scholar]
- 35.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):123–135. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 37.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 38.Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D, Kulangara K. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non–small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24(6):392–397. doi: 10.1097/PAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):NEJMoa1716948. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol Off J Am Soci Clin Oncol. 2015;33:2197. [DOI] [PubMed] [Google Scholar]
- 41.Reck M, von Pawel J, Zatloukal P,R, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: aVAil. J Clin Oncol Off J Am Soci Clin Oncol. 2009;27(8):1227. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


