ABSTRACT
The molecular cargo of tumor-cell-derived exosomes (TEX) mimics that of parental tumor cells. Thus, TEX could potentially serve as noninvasive biomarkers of cancer progression. However, separation of TEX from non-TEX in patients’ plasma requires tumor antigen-specific detection reagents. CD44v3 has been of interest as a potential biomarker of disease progression in HNSCC, because its overexpression in tumor cells associates with poor outcome. Here, CD44v3+ TEX immunocaptured from plasma of 44 HNSCC patients and 7 healthy donors (HDs) were evaluated as potential biomarkers of disease activity and stage. Exosomes were isolated from plasma of by size exclusion chromatography. Using anti-CD44v3 or anti-CD3 mAbs on beads, CD44v3+ TEX CD3(-)TEX-enriched exosomes were immunocaptured from supernatants of nonmalignant or HNSCC cell lines and from patients’ plasma. On-bead flow cytometry was used for the detection of FAS-L, PD-L1, TGFF-β. CSPG4 or EGFR on exosomes. The TEX expression profiles were correlated to clinicopathological parameters. Relative florescence intensity (RFI) values for CD44v3 were higher (p < .01) on TEX from HNSCC cell lines or on CD44v3+ CD3(-) plasma-derived exosomes. RFI values of CD44v3 on CD3(-) exosomes were higher (p < .005) in patients than in HDs and correlated (p < .05) with the UICC stage and lymph node metastasis. In HNSCC patients, CD44v3+ exosomes higher levels of immunosuppressive proteins compared to CD44v3(-) exosomes (p < .05-p < .005), and RFI values for these markers correlated with higher disease stages and lymph node metastasis. Isolation of CD44v3+ exosomes by immunocapture allowed for enrichment of TEX which are potentially promising liquid biomarkers of the tumor burden and disease stage in HNSCC.
KEYWORDS: CD44v3 protein, HNSCC, tumor-derived exosomes (TEX), biomarkers, disease activity
Introduction
Head and Neck Squamous Cell Carcinoma (HNSCC) is an aggressive and highly immunosuppressive malignancy.1–3 Consistently low survival rates of the patients diagnosed with advanced HNSCC are due to a high frequency of locoregional recurrence and resistance of HNSCC cancers to conventional therapies.4 Most patients with HNSCC fail to respond to immunotherapy with immune checkpoint inhibitors, such as nivolumab or pembrolizumab,5 and therapy with these antibodies does not lead to expected “normalization” of immune anti-tumor responses.5 Patients’ poor responsiveness to therapy might also be due to the presence of cancer stem cells (CSC), a subset of tumor cells which remain viable after therapy, retain their ability to proliferate and differentiate and proceed to re-populate the tumor.6 Tumors, including HNSCC, take advantage of various resistance mechanisms to escape and progress. The absence of biomarkers that could differentiate tumors likely to respond from those that remain resistant to therapy has interfered with the development of effective therapeutic strategies for HNSCC. A search for disease-associated biomarkers in HNSCC is an ongoing quest.
Among a number of biomarkers considered potentially useful for diagnosis or prognosis of HNSCC, such as EGFR,7 PD-L1,8 TGF-β,9 FasL10 or CSPG4,11,12 CD44 has emerged as an interesting molecule found to be overexpressed in tumors and also decorating CSCs.13 Thus, in HNSCC, CD44+ cancer cells, but not CD44(-) cancer cells, were shown to promote the formation of new tumors.14 CD44 is a transmembrane glycoprotein expressed in various cell types, including hematopoietic, epithelial or endothelial cells.15,16 Alternative splicing of 1–10 exons on the chromosome 11p13 results in at least 20 variants of CD44.17,18 Some of the isoforms are expressed on various normal cell types, while other variants are overexpressed on cancer cells. For example, CD44v6 was identified on tumor cells in various cancer tissues, including HNSCC.19–22 CD44v3 has been of special interest as a potential biomarker of disease progression in HNSCC, because its overexpression in HNSCC cells associates with increased proliferation, migration and a greater metastatic potential of HNSCC cancer cell lines and tumor tissues.22,23 CD44v3 overexpression in HNSCC correlates with poor outcome, and similar associations were reported for colorectal cancer and malignant melanoma.23–25 Further, increased mRNA and protein levels of CD44v3 in HNSCC tumor tissues correlated to poor overall patient survival.23,24 The CD44v3 protein binds to VEGF, and through this interaction, it mediates pro-angiogenic effects.25,26 In addition to cell-associated CD44 isoforms, soluble CD44 proteins are also detectable in patients’ plasma, although no reliable correlations of soluble CD44 with HNSCC progression have been established.27–29 The available data on the role of cell-associated and/or soluble CD44 and CD44 variants in tumor progression were performed mostly in cancer cell lines and only rarely in patient-derived samples. Few studies investigating soluble CD44v3 levels in HNSCC have been reported.30,31 In contrast, newer data in the literature suggest that cell membrane associated CD44v3 in tumor cells might serve as a potential biomarker of HNSCC progression and outcome.21,22,32 These data provided the rationale for our investigation of CD44v3 in tumor cell-derived exosomes.
Exosomes are a subset of small extracellular vesicles (EVs) that are produced by all cells and serve as an intercellular communication network.33,34 Because exosomes originate from the multivesicular bodies (MVBs), these small vesicles (30–150 nm) are considered as molecular mimics of the parental cells.33 Tumor cells produce large numbers of exosomes dubbed as “TEX,” and TEX circulate freely in all body fluids35 and could serve as readily accessible surrogates for tumor cells which produced them. Thus, plasma-derived TEX mimicking the content of the parent tumor cell and carrying CD44v3 protein emerge as potentially ideal biomarkers of HNSCC progression/response to therapy.
To capitalize on our capability to isolate TEX and non-TEX exosomes of HNSCC progression/response to therapy from supernatants of tumor cell lines or from plasma of cancer patients by immune capture,36,37 we evaluated the role of CD44v3 carried by exosomes from plasma of HNSCC patients as liquid biomarkers of disease activity and progression.
Results
Characterization of exosomes
Captured and non-captured exosome fractions were characterized by on-bead flow cytometry for the presence of selected antigens; western blots for tetraspanins and TSG101; TEM for morphology; and TRPS for the vesicle sizearps (SFigure 1). To illustrate the efficiency of immune capture, results of two capture experiments are shown in SFigure 1. In the first, there were 2.7 × 1011 exosomes/mL plasma before immune capture with anti- CD44v3 Abs and 1.6 × 1011 exosomes/mL in the non-captured fraction. Thus, 1.1 × 1011 CD44v3(+) exosomes/mL (~45%) were immunocaptured. In the second experiment, the immunocapture efficiency was ~90%. By TRPS, exosomes in the captured and non-captured fractions were similar in size, but TME indicated a somewhat greater size heterogeneity of the non-captured exosomes. TSG101 was carried by captured and non-captured exosomes.
Expression levels of CD44 and CD44v3 in cell lines and cell line-derived exosomes
Nearly all cells in the three cultured HNSCC lines and THP1 cells were CD44+ (Figure 1a). However, the percentages of cells positive for CD44v3 were significantly lower in these cultured cells and varied from 60% to 25% in the three HNSCC cell lines. THP1 cells were only weakly positive, and COS-7 control cells were negative, as expected. Thus, at the cell surface, the CD44v3 protein expression discriminated between HNSCC cells and nonmalignant cells, while CD44 did not.
Figure 1.
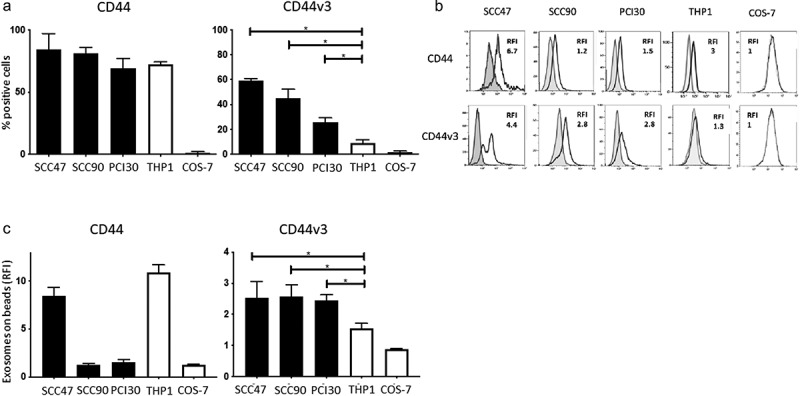
CD44 and CD44v3 expression on cell lines and on exosomes produced by these cell lines. In a: Percentages of CD44+ and CD44v3+ cells in cultures of various cell lines as determined by flow cytometry. The data are means ± SD of four experiments performed with each cell line. In b: Representative flow cytometry results for CD44+ and CD44v3+ exosomes stained with labeled anti-CD44 or anti-CD44v3 Abs, respectively, and examined by on-bead flow cytometry. Note higher RFI values for CD44v3 exosomes, except in case of SSC47 exosomes. *p < .01. In c: Comparisons of RFI values for CD44+ and CD44v3+ exosomes produced by the cell lines shown in a. Note that exosomes produced by HNSCC cell lines are uniformly positive for CD44v3. The data are means ± SD of three flow cytometry assays for each exosome preparation.
When exosomes isolated from supernatants of the same cell lines were interrogated by on-bead flow cytometry for levels of CD44 and CD44v3 expression (Figure 1b, c) all were found to be positive, but the RFI values varied considerably for CD44+ and CD44v3+ exosomes (Figure 1b). While THP1-derived exosomes carried high levels of CD44 (RFI = 9.6), their CD44v3 protein content was minimal (RFI = 1.3). In contrast, all HNSCC-derived exosomes were CD44v3+, and only SCC47-derived exosomes carried more CD44 than CD44v3 protein. The data show that HNSCC cell-derived exosomes are positive/enriched in the CD44v3 protein (Figure 1c) just like the HNSCC parent cells. These results provided the basis for investigating the presence/levels of these proteins in exosomes from HNSCC patients’ plasma and the association of CD44+ and CD44v3+ exosomes with disease activity.
Clinicopathological characteristics of HNSCC patients
The clinicopathological characteristics of the patients whose plasma was used for exosome isolation are listed in Table 1. The patients were predominantly male and the mean age was 61 yrs. Anatomical locations of the primary tumors were: oral cavity (45%), pharynx (16%) and larynx (36%). Seventeen patients (68%) donated blood at the time of diagnosis prior to any therapy. These patients had an active disease (AD). Eight patients (32%) donated blood after completing curative therapy at the time when they had no evidence of disease (NED) as determined by clinical evaluations. Fourteen patients (56%) presented with an advanced tumor stage (T3, T4) and 56% had a positive nodal status. Forty percent of the patients were UICC stage I or II and 60% were UICC III or IV. The majority of patients had a moderate histological differentiation grade by histopathology. Eighty percent of the patients consumed tobacco and/or alcohol (60%) at the time of diagnosis. HDs were matched for gender and age (+/−5 years).
Table 1.
Clinicopathological parameters.
| Patients (n = 44) |
||
|---|---|---|
| Characteristics | n | % |
| Age (years) | ||
| ≤63 | 26 | 59 |
| >63 | 18 | 41 |
| (range: 41–99) | ||
| Gender | ||
| Male | 33 | 75 |
| Female | 11 | 25 |
| Disease status | ||
| AD | 31 | 70 |
| NED | 13 | 30 |
| Primary tumor side | ||
| Oral cavity | 20 | 46 |
| Pharynx | 8 | 18 |
| Larynx | 16 | 36 |
| Tumor stage | ||
| T1 | 10 | 23 |
| T2 | 12 | 27 |
| T3 | 10 | 23 |
| T4 | 12 | 27 |
| Nodal status | ||
| N0 | 20 | 46 |
| N ≥ 1 | 24 | 54 |
| Distant metastasis | ||
| M0 | 43 | 98 |
| M1 | 1 | 2 |
| UICC stage | ||
| I | 15 | 34 |
| II | 6 | 14 |
| III | 10 | 23 |
| IV | 13 | 29 |
| Alcohol consumption | ||
| Yes | 26 | 59 |
| No | 18 | 41 |
| Tobacco consumption | ||
| Yes | 35 | 80 |
| No | 9 | 20 |
Increased CD44 and CD44v3 levels in plasma-derived exosomes of HNSCC patients compared to HDs
To study levels of CD44+ and CD44v3+ exosomes in plasma of HNSCC patients, immunocapture was performed using anti-CD3 mAbs. As previously reported, 36,37 this separated total plasma exosomes into two fractions: CD3(+) T-cell derived exosomes and CD3(-) exosomes, which in cancer patients are enriched in tumor-derived exosomes (TEX). Each of the fractions was next analyzed by on-bead flow cytometry for expression levels of CD44 and CD44v3 proteins, and Figure 2a shows that the CD3(+) fraction was significantly enriched in CD44+ exosomes and contained significantly lower levels of the CD44v3 protein than the CD3(-) fraction. The enrichment of CD3(-) exosomes in CD44v3 protein was significant (p < .005) and confirmed that this enrichment occurred in the exosome fraction derived, in part, from tumor cells.
Figure 2.
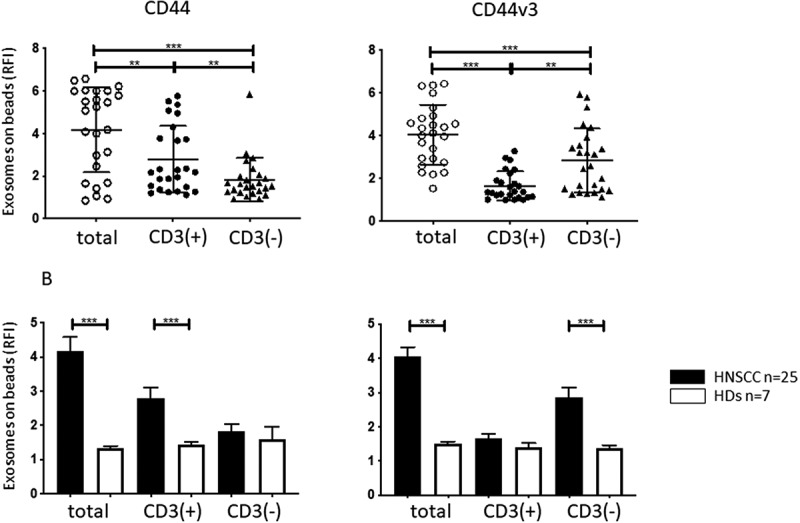
CD44 and CD44v3 expression on plasma-derived exosomes from HNSCC patients and HDs. In a: RFI values for CD44+ and CD44v3+ exosomes in total (prior to capture), CD3(+) and CD3(-) fractions captured from plasma of HNSCC patients. Note elevated CD44v3 levels in total and CD3(-) exosome fractions. In contrast, significantly higher CD44 levels seen on total and CD3(+) exosomes. In b: Mean RFI values (± SD) for expression levels of CD44 or CD44v3 on the exosome fractions obtained from plasma of 25 HNSCC patients or 7 HDs. Note that the same low levels of expression of both proteins in exosomes from plasma of HDs contrasts with high expression levels of CD44v3 in total and CD3(-) exosome fractions from patients’ plasma. **p < .005; ***p < .0005.
Similar immune capture was then performed with plasma of HDs. Figure 2b shows that the CD3(-) and CD3(+) exosome fractions of HDs contained significantly lower and equivalent levels of CD44 and CD44v3 proteins relative to the same fractions in HNSCC patients’ plasma.
Correlation of CD44 and CD44v3 levels in exosomes with clinicopathological parameters
The RFI values for the CD44+ and CD44v3+ proteins in exosomes in the CD3(+) and CD3(-) fractions immunocaptured from patients’ plasma as described above were assessed by on-bead flow cytometry. Patients were divided into those with early (stage I/II) vs late (stage III/IV) disease and those with/without evidence of nodal metastases (Figure 3a). No differences in RFI values for the CD44 protein were seen in the total, CD3(+) or CD3(-) fractions in HNSCC patients who were sorted based on disease stage. In contrast, RFI values for CD44v3+ exosomes were significantly higher in total and CD3(-) fractions, but not in the CD3(+) fraction, of patients with stage III/IV disease. Similar results were obtained when patients were stratified into those with N0 and N > 1 disease. The data suggest that expression levels of the CD44v3, but not of CD44 protein, on total and CD3(-) plasma-derived exosomes correlate with clinicopathological variables in HNSCC patients, and that CD44v3 could be, therefore, considered as a potential target for selective immune capture of HNSCC-derived exosomes from plasma.
Figure 3.
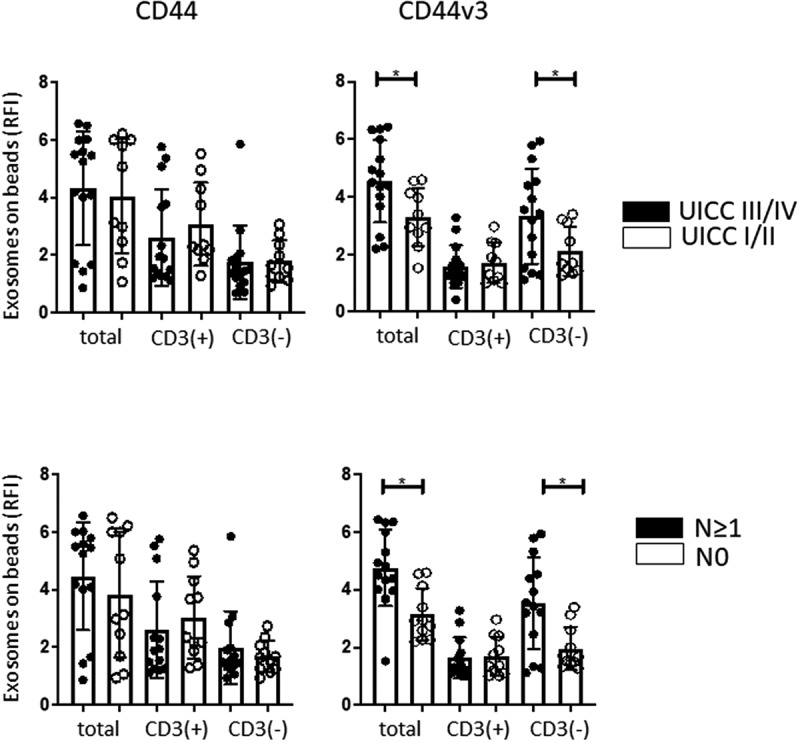
Correlations of the CD44 and CD44v3 protein levels (in RFI values) on exosomes in total, CD3(+) and CD3(-) fractions derived from plasma of HNSCC patients with clinicopathological data. No significant correlations were observed between UICC stage or nodal status and expression levels of CD44 on exosomes. In contrast, significant correlations (*p < .05) were noted between CD44v3 expression levels on exosomes in the total or CD3(-) fractions and UICC status as well as nodal status.
Immune capture of plasma exosomes using anti-CD44v3 mAbs
To evaluate the potential role of CD44v3 on tumor-derived exosomes (TEX) as a biomarker of disease progression in HNSCC, we next immunocaptured plasma exosomes with biotinylated anti-CD44v3 Ab. In parallel, immunocapture of the same plasma exosomes was performed with anti-CD45 mAb using CD45, a pan-hematopoietic marker, as a non-tumor control. As shown in SFigure 2A, B, the majority of exosomes in the captured fractions were CD44v3+ (RFI = 3.4) or CD45+ (RFI = 5.2), and the respective non-captured exosomes were minimally reactive for CD44v3 or CD45. The results confirm the selective enrichment of CD44v3(+) or CD45(+) exosomes in the captured fractions. Detection analysis of the captured exosomes by on-bead flow cytometry showed significantly higher levels for EpCAM in CD44v3(+) and CD45(-) exosome fractions (Figure 4). Expression of CD44v3 was high in the CD44v3(+) fraction and minimal in the CD45(+) fraction. Conversely, and as expected, the CD45(-) fraction enriched in EPCAM contained high levels of CD44v3+ exosomes. Expression levels of CD44+ exosomes were low in CD44v3(+) and CD45(+) fractions. The data show that immune capture targeting CD44v3 on HNSCC exosomes reproducibly separates TEX from non-tumor-derived exosomes. Conversely, immune capture targeting CD45 on plasma-derived exosomes separates hematopoietic cell-derived exosomes from TEX, which are CD44v3+.
Figure 4.
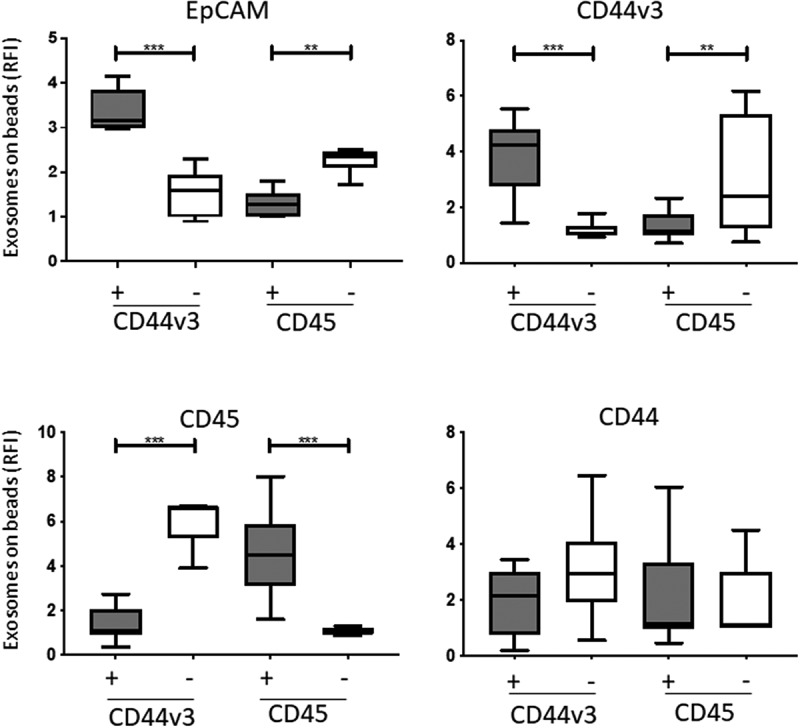
Detection of antigens carried by immunocaptured CD44v3 exosomes from plasma of HNSCC patients (n = 12). In parallel, exosomes were also captured with anti-CD45 mAb as a control. Flow cytometry-based analysis showed the highest levels of EPCAM on CD44v3(+) exosomes. Expression levels of CD44v3 and EPCAM were also high in the CD45(-) fraction enriched in TEX; they were low in CD45(+) and CD44v3(-) exosomes. CD44 expression levels were comparable in all exosome fractions (*p < .05, **p < .005). Gray bars indicate CD44v3+ or CD45+ exosomes, while white bars indicate CD44V3(-) and CD45(-) exosomes.
Molecular content of CD44v3(+) exosomes in HNSCC plasma
Flow cytometry analysis of the CD44v3(+) exosomes immunocaptured from plasma of HNSCC patients showed relatively high RFI values for EGFR, TGF-β1, PD-L1 and CSPG4, as compared to CD44v3(-) exosomes (Figure 5a). Further, in CD44v3(+) exosomes, RFl values of EGFR, PD-L1, FasL, TGF-β and CSPG4 correlated with clinicopathological parameters: CD44v3(+) exosomes from patients with high stage disease (high UICC stage and lymph node metastasis) had higher levels of the immunosuppressive markers and EGFR than CD44v3(-) exosomes (Figure 5b). The data are consistent with our previous reports demonstrating expression of immunosuppressive protein on TEX in HNSCC.25,26
Figure 5.
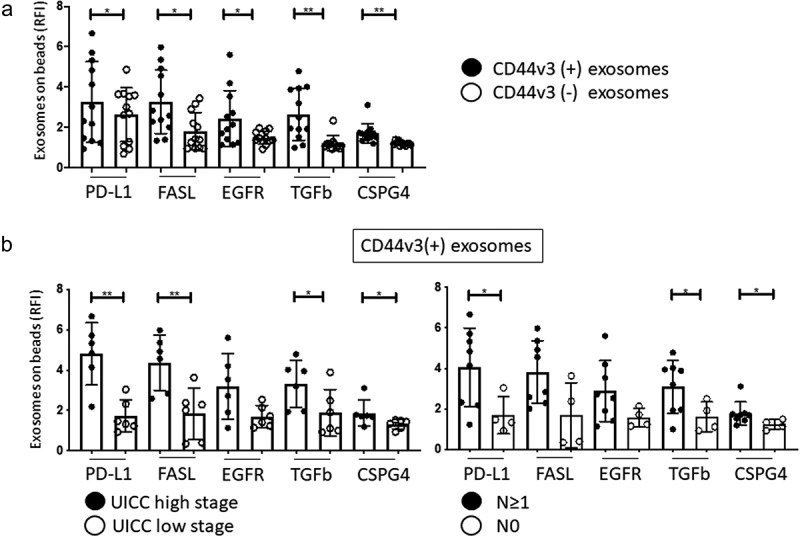
Molecular content of captured CD44v3(+) and non-captured CD44v3(-) exosome fractions determined by on-bead flow cytometry. In a: comparative expression of exosome markers on CD44v3(+) and CD44v3(-) exosomes. Note elevated levels of immunosuppressive markers (PD-L1, FasL, TGF-β1) and tumor markers (EGFR, CSPG4) on CD44v3(+) exosomes. In b, correlations between expression of immunosuppressive and tumor markers on CD44v3(+) exosomes and UICC tumor stage or patients’ lymph node status. Note significantly (*p < .05, **p < .005) increased expression levels of almost all markers in CD44v3(+) exosomes and captured fraction.
Discussion
The presence and levels of CD44 isoforms in tumor cells have been associated with worse outcome in various types of cancer.38,39 The CD44v3 isoform is differentially expressed in tumor tissues of HNSCC patients, and it has been associated with tumor progression.24 Because the CD44v3 isoform expression on HNSCC cells has been linked to poor outcome,23,24,30 we focused on this tumor-associated protein that is carried by tumor-derived exosomes as a potential biomarker of HNSCC activity and progression.
Emerging data suggest that plasma of cancer patients is rich in TEX, which potentially could serve as noninvasive liquid biopsies of the tumor.40,41 We, therefore, asked whether: (a) CD44 and/or CD44v3 proteins are present on exosomes in plasma of HNSCC patients; (b) CD44v3 is a selective marker for HNSCC cells and (c) CD44v3(+) TEX in patients’ plasma correlate with the clinicopathological profile and disease activity. To be able to study TEX present in cancer patients’ plasma, it is necessary to separate them from non-tumor-derived exosomes. We have described an immune-based separation of melanoma cell-derived exosomes36 and of CD3(+) T cell-derived exosomes37 from plasma by using mAbs specific for antigens selectively carried by these exosomes. While CD3 is only expressed on T cells, and anti-CSPG4 mAb we used for immune capture has been shown to be specific for melanoma, 11 no mAbs specific for antigens expressed on HNSCC exist. It was, therefore, important to ask whether the CD44v3 protein overexpressed on HNSCC cells might qualify as a potential biomarker of HNSCC. Although not specific for HNSCC, CD44v3 might serve as HNSCC-associated antigen that is enriched in TEX and, therefore, useful for their isolation from patients’ plasma. Indeed, in contrast to CD44, the CD44v3 isoform carried by TEX might fit in the category of a biomarker of disease activity in HNSCC, because its expression levels on TEX correlated with disease stage and activity. Comparing the characteristics of CD44v3(+) exosomes with those of immunocaptured CD3(-) or CD45(-) exosomes, all of which are partially enriched in TEX, we demonstrated that CD44v3(+) exosomes in plasma of HNSCC patients meet the criteria for a potential biomarker of disease activity in HNSCC.
To date, a majority of studies performed with cancer tissues, including HNSCC, involve tumor biopsies,23,27 and serial needle biopsies are difficult and often unreliable due to the tumor heterogeneity. Such biopsies often do not reflect the entire tumor microenvironment, and the false positive or negative results for CD44 and CD44v3 expression on tumor cells may be common, depending on the ratios of tumor/immune/stromal cells in each tumor. Clearly, a blood derived noninvasive `liquid biopsy` eliminates these concerns. Here, we provide a novel method of separating CD44v3(+) exosomes from patient`s plasma which originate mainly from tumor cells and can be used to “track” the tumor load and the tumor stage in HNSCC patients. The correlative data linking disease activity in HNSCC patients to levels of CD44v3 expression on TEX are preliminary, but given promising data reported in this pilot study, further exploration is warranted to confirm the role of CD44v3(+) TEX as a reliable biomarker of tumor progression in HNSCC.
Not unexpectedly, CD44v3+ TEX were found to be enriched in the immunosuppressive and tumor growth-promoting cargo, including proteins such as PD-L1, FasL, TGF-β and EGFR. Further, quantification of the TEX immunosuppressive cargo by on-bead flow cytometry indicated that the RFI values for these proteins were significantly elevated in CD44v3+ TEX of patients with stage III/IV HNSCC and in patients with lymph node metastases relative to patients with stage I/II disease or no LN metastases. These results are consistent with the data previously reported by us for immunocaptured CD3(-) TEX in patients with HNSCC.37,42 The data represent additional supportive evidence for the immunosuppressive role of TEX in cancer, and for the importance of TEX-mediated immunosuppression in cancer progression.40,43
It might be instructive to consider why CD44v3 on TEX emerges as better biomarker of HNSCC activity than soluble CD44 isoforms. Soluble factors in plasma are susceptible to enzymatic degradation, while proteins associated with and carried by exosomes may be resistant to enzymatic hydrolysis. We have previously shown that PD-L1 expression on plasma exosomes in HNSCC patients significantly correlated to clinicopathological parameters, whereas soluble PD-L1 levels did not.8 This and other similar findings suggest that exosomes are efficient transporters of intercellular messages without interference from the external environment. This feature of exosomes emphasizes their importance as biomarkers relative to soluble factors in body fluids.
In this communication, we not only provide evidence that CD44v3 is detectable on plasma-derived exosomes in HNSCC patients, but that elevated RFI values of CD44v3 in TEX might serve as potential biomarkers of disease stage and nodal metastasis as well as biomarkers of immune dysfunction frequently seen in patients with advanced cancers.3
Materials and methods
Cell lines
CD44 negative COS-7 cell line was purchased from the ATCC and cultured in DMEM complete media. HNSCC cell lines: PCI-30 (HPV-) was established in our laboratory39 and SCC47 and SCC90 (HPV+) were obtained from Dr. Robert Ferris (University of Pittsburgh) and were cultured in DMEM complete media. The monocytic THP1 cell line was obtained from Dr. Saumendra Sarkar (University of Pittsburgh) and was maintained in RPMI complete medium.
Patients
Peripheral blood specimens were randomly obtained from 44 HNSCC patients seen at the UPMC Otolaryngology Clinic between years 2008 and 2017. All samples were obtained from patients prior to any therapy. The blood samples were delivered to the laboratory and were immediately centrifuged at 1,000 x g for 10 min. Plasma specimens were stored in 1 mL aliquots at −80°C and were thawed immediately prior exosome isolation. The collection of blood samples and access to clinical data for research were approved by the Institutional Review Board of the University of Pittsburgh (IRB #960279, IRB #0403105 and IRB #0506140). Clinicopathological characteristics of the patients enrolled in the study are shown in Table 1. Plasma from seven healthy donors (HDs) was obtained and processed as described above.
Exosome isolation by mini size-exclusion chromatography (mini-SEC)
The mini-SEC method for exosome isolation was established and optimized in our laboratory as previously described44 (EV-TRACK ID: EV1600078). Briefly, plasma samples or cell culture supernatants were thawed and were centrifuged first at 2,000xg for 10 min at room temperature (RT) and then for 30 min at 10,000xg at 4°C. Next, plasma was ultrafiltrated using a 0.22 μm filter (EMD Millipore, Cat # SLGPO33RB). An aliquot of plasma (1 mL) was placed on a mini-SEC column and eluted with PBS. Sequential 1 mL fractions were collected, and fraction #4 was highly enriched in exosomes. The fraction #4 contained the majority of non-aggregated plasma-derived exosomes as previously reported.44
Characteristics of plasma-derived exosomes
Exosomes isolated by mini-SEC were evaluated for their size by Tunable Resistive Pulse Sensing (TRPS), morphology and size by transmission electron microscopy (TEM), and the cellular origin by Western blots to confirm the presence of endosomal markers (e.g., TSG101) and other vesicle-associated proteins such as CD63, CD81 or CD9. These methods for confirmation of the exosome nomenclature are routinely followed as described in detail in our previous publications.40,45,46 They follow the MISEV (2018) guidelines for the definition of extracellular vesicle (EV) size, vesicular morphology, cellular origin, biochemical composition and functionality.47 Using these criteria, we are confident that the extracellular vesicles (EVs) we isolate from plasma or cell supernatants are exosomes (SFigure 1).
BCA protein assay and exosome concentration
The protein concentration of the isolated exosome fraction #4 was determined using a Pierce BCA protein assay kit (Pierce Biotechnology, Cat# 23225) according to the manufacturer’s instructions. Exosomes were concentrated using Vivaspin 500 (VS0152, 300,000 MWCO, Sartorius, Cat# UFC510096). For FACS and other analyses, 10 μg of protein in 100 μL aliquots of PBS were used.
Flow cytometry of cells
Cells were stained for surface antigens using 200.000 cells/sample. After blocking with 2% bovine serum albumin (BSA), for 15 min, conjugated antibodies CD44-FITC (BD Biosciences) CD44v3-PE (R&D) or their matching isotypes were added and a 30-min incubation at 4 C followed. Cells were washed with FACS buffer (eBioscience) and immediately scanned in a Gallios flow cytometer (Beckman Coulter). Analysis was performed as recommended using Kaluza 1.5a software.
Immune capture of exosomes and detection of proteins on the surface of captured exosomes
For detection of exosome-associated surface proteins by flow cytometry, exosomes were first captured on ExoCap™ Streptavidin magnetic beads were purchased from MBL International, Cat # MEX-SA) and used as previously described.37,42 Briefly, exosomes were co-incubated with biotin-labeled anti-CD63 mAb (clone 353018, Biolegend) for measuring the total exosome fraction. For capture of T-cell derived CD3+ exosomes, anti-CD3 mAb (clone Hit3a, Biolegend) adjusted to the concentration of 1ug was used for 2 h incubation with exosomes at RT as described.38 Next, an aliquot of beads (10uL for CD63 capture, 50uL for CD3 capture) was added, and the tubes were again incubated for 2 h at RT. Samples were washed 1x with dilution buffer from the kit using a magnet.
For detection of antigens carried by the captured exosomes, the bead/anti-CD63 or anti-CD3 Ab/exosome complexes were co-incubated with the selected detection Abs labeled with a fluorochrome or with the labeled isotype control Ab for 1 h at RT. Next, the complexes were washed 3x using a magnet and were re-suspended in 300uL of PBS for antigen detection by flow cytometry. The non-captured exosome fractions were collected and re-captured with anti-CD63 mAb as described above for detection of their cargo.
Immune capture of exosomes carrying CD44v3 was performed using anti-CD44v3 biotinylated Ab (clone BBA11, R & D Systems). Anti-CD44v3 Ab was purchased from R&D systems and was biotinylated with the Lightning-Link Biotin Antibody Labeling Kit (Novus Biologicals). Biotinylation was confirmed by flow cytometry. The immune capture procedure was the same as described above with the following modifications. CD44v3 biotinylated antibody (1 mg/mL) was used at the ratio of 1:25 (2ug) in 100 uL PBS and 100 uL beads were added.
Immune capture of exosomes carrying CD45 was also performed as described above with the following modifications: biotinylated CD45 Ab (clone HI30, Biolegend) was used at a dilution of 1:25 (2 ug) in 100uL PBS and 25uL beads were added. The incubation with the CD45 biotinylated Ab was performed O/N at 4 C. The non-captured fraction was re-captured with anti-CD63-biotinylated antibody for antigen detection by on-bead flow cytometry.
Antigen detection by on-bead flow cytometry using fluorochrome-conjugated Abs was performed following blocking with mouse serum for 30 min at RT. The following detection Abs were used: PD-L1 PE (eBioscience), TGF-b LAP PE (R&D), CD45 PE-Cy7 (Beckman Coulter), CD63 APC (Biolegend), EGFR PE (Biolegend), Fas ligand PE (all Biolegend), EpCAM PE (Abcam). Anti-CSPG4 Ab was a gift from Dr. Soldano Ferrone (Harvard Medical School, Cambridge, USA); it was conjugated to APC using the Lightning-Link APC Antibody Labeling Kit (Novus Biologicals).
To confirm the efficiency of immune capture of CD44v3(+) and CD45(+) exosomes, the non-captured fractions were re-captured using biotinylated anti-CD63 antibody, stained for CD44v3 or CD45, respectively, and analyzed by on-bead flow cytometry. In these assays, CD44v3(+) or CD45(+) exosomes were always under 10% (SFigure 1).
Funding Statement
This work has been supported in part by NIH grants RO-1CA 168628 and R21-C205644 to TLW and by the Deutsche Forschungsgemeinschaft to MNT (research fellowship # TH 2172/1-1).
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL.. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67(18):8865–9. doi: 10.1158/0008-5472.can-07-0767. [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12(13):3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside TL. Head and neck carcinoma immunotherapy: facts and hopes. Clin Cancer Res. 2017. doi: 10.1158/1078-0432.ccr-17-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. doi: 10.1200/jco.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380(23):2237–2245. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 7.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39(10):1348–1354. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–5364. [PubMed] [Google Scholar]
- 11.Wang X, Wang Y, Yu L, Sakakura K, Visus C, Schwab JH, Ferrone CR, Favoino E, Koya Y, Campoli MR, et al. CSPG4 in cancer: multiple roles. Curr Mol Med. 2010;10(4):419–429. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- 12.Ilieva KM, Cheung A, Mele S, Chiaruttini G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE, et al. Chondroitin sulfate proteoglycan 4 and its potential as an antibody immunotherapy target across different tumor types. Front Immunol. 2017;8:1911. doi: 10.3389/fimmu.2017.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Zuo X, Xie K, Wei D. The role of CD44 and cancer stem cells. Methods Mol Biol. 2018;1692:31–42. doi: 10.1007/978-1-4939-7401-6_3. [DOI] [PubMed] [Google Scholar]
- 14.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourguignon LY, Lokeshwar VB, He J, Chen X, Bourguignon GJ. A CD44-like endothelial cell transmembrane glycoprotein (GP116) interacts with extracellular matrix and ankyrin. Mol Cell Biol. 1992;12(10):4464–4471. doi: 10.1128/MCB.12.10.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 18.Iida N, Bourguignon LY. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995;162(1):127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- 19.Wang JL, Su WY, Lin YW, Xiong H, Chen YX, Xu J, Fang JY. CD44v6 overexpression related to metastasis and poor prognosis of colorectal cancer: A meta-analysis. Oncotarget. 2017;8(8):12866–12876. doi: 10.18632/oncotarget.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian C, Wang Y, Chen Y, Zeng L, Zhang Q, Shuai X, Huang K. Suppression of pancreatic tumor growth by targeted arsenic delivery with anti-CD44v6 single chain antibody conjugated nanoparticles. Biomaterials. 2013;34(26):6175–6184. doi: 10.1016/j.biomaterials.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 21.Sagawa K, Uwa N, Daimon T, Sakagami M, Tsujimura T. Expression of CD44 variant isoforms, CD44v3 and CD44v6, are associated with prognosis in nasopharyngeal carcinoma. J Laryngol Otol. 2016;130(9):843–849. doi: 10.1017/s0022215116008525. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelberg D, Kuku G, Selvaraju R, Nestor M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumour Biol. 2014;35(3):2053–2062. doi: 10.1007/s13277-013-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todoroki K, Ogasawara S, Akiba J, Nakayama M, Naito Y, Seki N, Kusukawa J, Yano H. CD44v3+/CD24- cells possess cancer stem cell-like properties in human oral squamous cell carcinoma. Int J Oncol. 2016;48(1):99–109. doi: 10.3892/ijo.2015.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzmann EJ, Weed DT, Civantos FJ, Goodwin WJ, Bourguignon LY. A novel CD44 v3 isoform is involved in head and neck squamous cell carcinoma progression. Otolaryngol Head Neck Surg. 2001;124(4):426–432. doi: 10.1067/mhn.2001.114674. [DOI] [PubMed] [Google Scholar]
- 25.Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Heroult M, Augustin HG, Ponta H, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114(25):5236–5244. doi: 10.1182/blood-2009-04-219204. [DOI] [PubMed] [Google Scholar]
- 26.Forster-Horvath C, Meszaros L, Raso E, Dome B, Ladanyi A, Morini M, Albini A, Timar J. Expression of CD44v3 protein in human endothelial cells in vitro and in tumoral microvessels in vivo. Microvasc Res. 2004;68(2):110–118. doi: 10.1016/j.mvr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Faber A, Barth C, Hormann K, Kassner S, Schultz JD, Sommer U, Stern-Straeter J, Thorn C, Goessler UR. CD44 as a stem cell marker in head and neck squamous cell carcinoma. Oncol Rep. 2011;26(2):321–326. doi: 10.3892/or.2011.1322. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, von Au A, Schnolzer M, Hackert T, Zoller M. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget. 2016;7(34):55409–55436. doi: 10.18632/oncotarget.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Hal NL, Van Dongen GA, Ten Brink CB, Heider KH, Rech-Weichselbraun I, Snow GB, Brakenhoff RH. Evaluation of soluble CD44v6 as a potential serum marker for head and neck squamous cell carcinoma. Clin Cancer Res. 1999;5:3534–3541. [PubMed] [Google Scholar]
- 30.Wang SJ, Wreesmann VB, Bourguignon LY. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck. 2007;29(6):550–558. doi: 10.1002/hed.20544. [DOI] [PubMed] [Google Scholar]
- 31.Franzmann EJ, Reategui EP, Pedroso F, Pernas FG, Karakullukcu BM, Carraway KL, Hamilton K, Singal R, Goodwin WJ. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1348–1355. doi: 10.1158/1055-9965.EPI-06-0011. [DOI] [PubMed] [Google Scholar]
- 32.Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7(1):1435138. doi: 10.1080/20013078.2018.1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theodoraki MN, Hoffmann TK, Whiteside TL. Separation of plasma-derived exosomes into CD3((+)) and CD3((-)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol. 2018;192(3):271–283. doi: 10.1111/cei.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood). 2013;238(3):324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orian-Rousseau V, Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res. 2014;123:231–254. doi: 10.1016/b978-0-12-800092-2.00009-5. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23(16):4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theodoraki MN, Hoffmann TK, Jackson EK, Whiteside TL. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin Exp Immunol. 2018;194(1):67–78. doi: 10.1111/cei.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theodoraki MN, Yerneni S, Gooding WE, Ohr J, Clump DA, Bauman JE, Ferris RL, Whiteside TL. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology. 2019;8(7):1593805. doi: 10.1080/2162402X.2019.1593805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5(1):29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig N, Razzo BM, Yerneni SS, Whiteside TL. Optimization of cell culture conditions for exosome isolation using mini-size exclusion chromatography (mini-SEC). Exp Cell Res. 2019;378(2):149–157. doi: 10.1016/j.yexcr.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Ludwig N, Hong C-S, Ludwig S, Azambuja JH, Sharma P, Theodoraki M-N, Whiteside TL. Isolation and analysis of tumor-derived exosomes. Cur Protoc Immunol. 2019;127(1):e91. doi: 10.1002/cpim.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thery C, Witwer KW, Alcaraz E. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


