Developmental phenotypes are often consistent across individuals within a population in the face of environmental and genetic challenges. However, these challenges can exceed the level of system robustness and change developmental...
Keywords: seam cells, epidermis, temperature, Wnt pathway, cryptic genetic variation, robustness, C. elegans, stem cell, V6pappa, V6pappp
Abstract
Populations often display consistent developmental phenotypes across individuals despite inevitable biological stochasticity. Nevertheless, developmental robustness has limits, and systems can fail upon change in the environment or the genetic background. We use here the seam cells, a population of epidermal stem cells in Caenorhabditis elegans, to study the influence of temperature change and genetic variation on cell fate. Seam cell development has mostly been studied so far in the laboratory reference strain (N2), grown at 20° temperature. We demonstrate that an increase in culture temperature to 25° introduces variability in the wild-type seam cell lineage, with a proportion of animals showing an increase in seam cell number. We map this increase to lineage-specific symmetrization events of normally asymmetric cell divisions at the fourth larval stage, leading to the retention of seam cell fate in both daughter cells. Using genetics and single-molecule imaging, we demonstrate that this symmetrization occurs via changes in the Wnt asymmetry pathway, leading to aberrant Wnt target activation in anterior cell daughters. We find that intrinsic differences in the Wnt asymmetry pathway already exist between seam cells at 20° and this may sensitize cells toward a cell fate switch at increased temperature. Finally, we demonstrate that wild isolates of C. elegans display variation in seam cell sensitivity to increased culture temperature, although their average seam cell number is comparable at 20°. Our results highlight how temperature can modulate cell fate decisions in an invertebrate model of stem cell patterning.
DURING development, organisms must withstand environmental and genetic perturbations to produce consistent phenotypes (Félix and Barkoulas 2012). These phenotypes are often a product of complex developmental events that require a tight balance between cell division and cell differentiation (Soufi and Dalton 2016). A key example is stem cell divisions, consisting of highly controlled asymmetric and symmetric patterns, which are vital for generating cell diversity, as well as maintaining cell numbers in tissues and organs (Morrison and Kimble 2006; Knoblich 2008). Developmental robustness has inherent limits and certain perturbations can push a system outside its buffering zone (Braendle and Felix 2008; Barkoulas et al. 2013). In these cases, it is also important to understand how systems fail by investigating how perturbations precisely modulate developmental processes. Here, we address the question of how changes in environmental temperature can affect cell fate outcomes using the nematode Caenorhabditis elegans as a model system. While it is well known that increasing or decreasing environmental temperature can change the development speed in C. elegans, the effect of temperature on specific cell division and fate acquisition events is less well understood. The C. elegans adult hermaphrodite consists of 959 somatic cells with their complete and stereotypical lineage characterized (Sulston and Horvitz 1977); this, alongside the isogenic nature of C. elegans populations, makes it an attractive model to study environmental effects on development.
We focus here on the seam cells, which are a population of epidermal cells that are found along the two lateral sides of the animal body. Seam cell development has been used as a system to study mechanisms of stem cell patterning in an invertebrate model (Joshi et al. 2010). This is because seam cells show stem cell behavior during larval development as they go through reiterative asymmetric divisions, where usually the posterior daughter retains the seam cell fate, while the anterior daughter differentiates to a neuroblast or acquires hyp7 fate and joins the syncytial epidermis (also known as the hypodermis) (Figure 1A) (Chisholm and Hsiao 2012). C. elegans hatch with 10 seam cells on each lateral side and a symmetric division increases the total seam cell number from 10 cells to 16 during the second larval (L2) stage (Figure 1A). The exact pattern of seam cell divisions differs between each lineage in the head (H0–H2), midbody (V1–V6), and tail (T) region and over developmental time (Figure 1A). The balance between seam cell proliferation and differentiation is controlled through transcription factor activity (Koh and Rothman 2001; Cassata 2005; Nimmo et al. 2005; Kagoshima et al. 2007; Huang et al. 2009; Brabin et al. 2011; Gorrepati et al. 2013; Hughes et al. 2013) and the Wnt/β-catenin asymmetry pathway (Mizumoto and Sawa 2007b; Sawa and Korswagen 2013; Gorrepati et al. 2015), which is an adaptation of the conserved canonical Wnt signaling pathway in the context of an asymmetric division. In this case, selective activation of Wnt-dependent transcription in one of the two seam cell daughters relies on asymmetric localization of Wnt components in mother cells that are polarized before division (Takeshita and Sawa 2005; Goldstein et al. 2006; Mizumoto and Sawa 2007a; Gleason and Eisenmann 2010; Baldwin et al. 2016).
Figure 1.
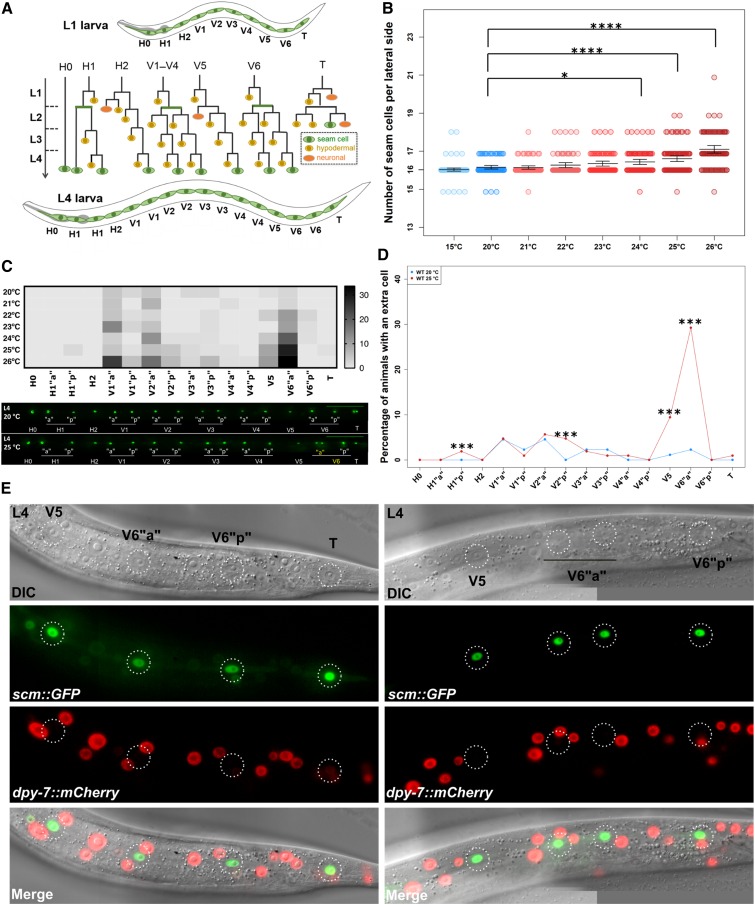
Lineage-specific seam cell duplications occur at higher growth temperatures. (A) Schematic showing WT seam cell division patterns across larval stages (green = seam cell, yellow = hypodermal cell, and orange = neuroblast). (B) Counts of seam cell nuclei in JR667 (wIs51 [scm::GFP] used as WT reference) when animals were grown at temperatures from 15° (blue dots) to 26° (red dots) (* P < 0.05 and ****P < 0.0001, with one-way ANOVA followed by Dunnett’s multiple comparison test, n ≥ 78). Error bars represent 95% C.I.s of the mean. (C) Heat map displaying occurrence of errors along the seam axis as temperature increases (upper panel). Lower panel shows representative images of animals grown at 20° and 25° at the L4 stage, yellow labels indicate extra seam cells in the anterior V6 lineage at 25°. Bar, 100 µm. For simplicity, the anterior and posterior branches of each seam cell lineage at the L4 stage are depicted in all figures as “a” and “p,” and their daughter cells after division as “aa”/“ap” and “pa”/“pp”. (D) Quantification of percentage of seam cell duplications per seam cell lineage at the L4 stage. Note that the highest proportion of duplications was seen in the anterior V6 lineage at 25° (***P < 0.001 with a binomial test, n = 88 and 106 for 20° and 25°, respectively). (E) In instances of extra nuclei in the anterior V6 lineage at the L4 stage, V6pappa cells do not express the hypodermal marker dpy-7p::mCherry and retain scm::GFP expression. L4, fourth larval; WT, wild-type.
Despite progress made over the last few years in identifying key factors contributing to epidermal development, most studies have been conducted using a single C. elegans isolate grown in a single environment, which is the laboratory reference strain N2 grown at 20°. It therefore remains unknown whether changes in the growth environment or the genetic background would have an impact on seam cell patterning. In this study, we start to address this question by investigating the effect of different growth temperatures, as well as genetic backgrounds, on seam cell development. We demonstrate that as culture temperature is increased within physiological limits (e.g., 25°), populations become more variable and start producing one extra seam cell on average. We show that this increase in seam cell number occurs via a cell fate switch that is observed reproducibly in specific cell lineages. This cell fate switch is dependent upon the Hox gene mab-5 and the β-catenin gene bar-1, both previously unknown to play a role in the hermaphrodite seam cell lineage at 20°. We show that at high temperatures, an impaired Wnt asymmetry pathway leads to ectopic Wnt pathway activation in anterior daughters of specific seam cells that may already be sensitized regardless of the growth temperature. Finally, we study here seam cell development for the first time outside N2 and find that wild isolates of C. elegans show a conserved seam cell number at 20°. Nevertheless, by raising animals at 25°, we reveal cryptic genetic variation between isolates and show that the sensitivity of the seam cell lineage to higher temperature evolves within C. elegans, as certain isolates show an enhanced or suppressed response in comparison to N2. Together, these findings expand our knowledge of developmental system behavior upon environmental and genetic perturbation.
Materials and Methods
Nematode culture and genetics
Strains used in this study were maintained and handled according to standard protocols (Brenner 1974). Strain JR667, containing a scm::GFP transgene (wIs51) in the N2 background, was used as a reference strain and was maintained with OP50 as a food source on standard NGM plates. The scm::GFP marker was introgressed from JR667 into wild isolates JU775, JU2519, and CB4856 together with a dat-1::GFP marker using a two-step cross repeated five times to produce 10-times backcrossed strains. In the first step, hermaphrodites carrying the vtIs1[dat-1p::GFP] + wIs51[scm::GFP] transgenes linked on chromosome V were mated to wild-isolate males. In the second step, F1 males were crossed to wild-isolate hermaphrodites. F1 hermaphrodites carrying both wIs51 and vtIs1 were allowed to self, and homozygous progeny for the marker were considered backcrossed twice. The linkage between the two transgenes (vtIs1 and wIs51) was broken at the last step when F1 hermaphrodites, from a cross between wild-isolate hermaphrodites carrying these transgenes and wild-isolate males, were allowed to self. Recombinant progeny carrying only wIs51 were picked and maintained. scm::GFP was introgressed into wild isolates (JU2007 and XZ1516) by crossing wild-isolate males to JR667 hermaphrodites. F1 males carrying the transgene were crossed to wild-isolate hermaphrodites. This last step was repeated nine times to produce 10-times backcrossed wild isolates. A complete list of strains used in this study is presented in Supplemental Material, Table S1.
Microscopy and phenotypic characterization
Standard seam cell scorings were performed by mounting animals on 2% agar pads containing 100 µM sodium azide (NaN3). These were covered with a coverslip and viewed under a compound microscope (AxioScope A1; Zeiss [Carl Zeiss], Thornwood, NY). Seam cell numbers were scored at the late fourth larval (L4) or early adult stage, focusing on one lateral side per animal.
To perform temperature treatments, synchronized animals were prepared by bleaching adults and placing eggs on standard NGM plates at different temperatures. To quantify seam cells, animals were collected between 44 and 48 hr for L4s grown at 20°, and between 36 and 40 hr for L4s at 25°. Seam cell duplications were scored based on the stereotypical position of seam cells in relation to the vulva at the L4 or early adult stage, and in relation to each other. More specifically, eight seam cell lineages (H0, H1a, H1p, H2, V1a, V1p, V2a, and V2p) are anterior to the vulva, two are adjacent to the vulva (V3a and V3p), and six seam cell lineages are found posterior to the vulva (V4a, V4p, V5, V6a, V6p, and T). In cases of increased seam cell number, the extra seam cell was assigned, when possible, to the nearest seam cell neighbor at the L4 stage, also taking into account the position of the corresponding seam cells on the opposite lateral side.
POP-1 levels were characterized using a strain carrying the transgene qIs74[sys-1p::GFP::POP-1]. Images of seam cells were analyzed using ImageJ. The following formula was used to calculate the corrected total cell fluorescence in cell nuclei: integrated density − (area of selected cell × mean fluorescence of background readings), with three background readings around the animal taken for each cell pair. Cells with an anterior/posterior intensity ratio > 1.1 or < 0.9 were classified as anterior > posterior or anterior < posterior respectively, while cell pairs that had a ratio between 0.9 and 1.1 were classified as equal in fluorescence intensity.
RNA interference by feeding
Animals were fed double-stranded RNA-expressing bacteria as a food source. HT115 bacteria containing RNA interference (RNAi) clones or an empty-vector control were grown overnight, and seeded directly on NGM plates that contained 1 μM IPTG, 25 μg/ml ampicillin, and 6.25 μg/ml tetracycline. All RNAi clones used in this study were sequence validated and came from the Ahringer RNAi library (Source Bioscience).
Cloning
A dpy-7p::mCherry::H2B:unc-54 cassette was assembled in vector pCFJ906 using standard three-fragment Gateway cloning (Invitrogen, Carlsbad, CA). A recovered miniMos insertion was crossed to JR667 to generate strain MBA227. To construct a seam cell-driven GFP::CAAX transgene, the GFP sequence was amplified using pPD93.65 as template and fused to the following sequence containing the CAAX motif using nested PCR: 5′-AAGGACGGAAAGAAGAAGAAGAAGAAGTCCAAGACCAAGTGCGTCATCATG-3′. The GFP::CAAX fragment was then cloned into pIR5 (Katsanos et al. 2017) via Gibson assembly. This vector contains a shorter version of the same promoter fragment that is also present in pMF1, originally used to make the scm::GFP marker. A stable integrant was obtained via transgenesis and γ irradiation. The resulting line was backcrossed 10 times to N2 before crossing to JR667 to generate strain MBA237.
Single-molecule fluorescence in situ hybridization
Populations of nematodes were synchronized by bleaching and subsequently fixed in 4% formaldehyde at appropriate stages for the experiment (17 hr to image larval stage 1 worms, and 40 and 44 hr to capture the L4 division at 20° and 25°, respectively). Single-molecule fluorescence in situ hybridization (smFISH) was performed as previously described (Katsanos et al. 2017) using a pool of 27–48 oligos fluorescently labeled with Cy5 (Biomers). Imaging was performed using a motorized epifluorescence Ti-eclipse microscope (Nikon, Garden City, NY) and a DU-934 CCD-17291 camera (Andor Technology, Belfast, UK) acquiring 0.8-μm step z-stacks. Image analysis and spot quantification were performed on raw data using a MATLAB (MathWorks, Natick, MA) routine as previously described (Barkoulas et al. 2013). For all images presented in this study, the probe signal channel was inverted for clarity [black spots correspond to messenger RNAs (mRNAs)] and merged to the seam cell channel (GFP) using ImageJ (NIH, Rockville, MD).
Data availability
Data were analyzed and presented with the R programming environment or GraphPad Prism 7. Two-sample Student’s t-tests were performed for differences in mean seam cell number. Binomial tests were performed to test for differences in the proportions of seam cell symmetrization events between strains and/or treatments. All statistics were carried out in R version 3.2.0. All reagents and strains are available upon request. Nematode strains are listed in Table S1. Table S2 contains oligo sequences used as smFISH probes in this study. Table S3 contains raw counts from smFISH experiments presented in Figure 2, Figure 3, Figure 4, and Figure S3. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11687178.
Figure 2.
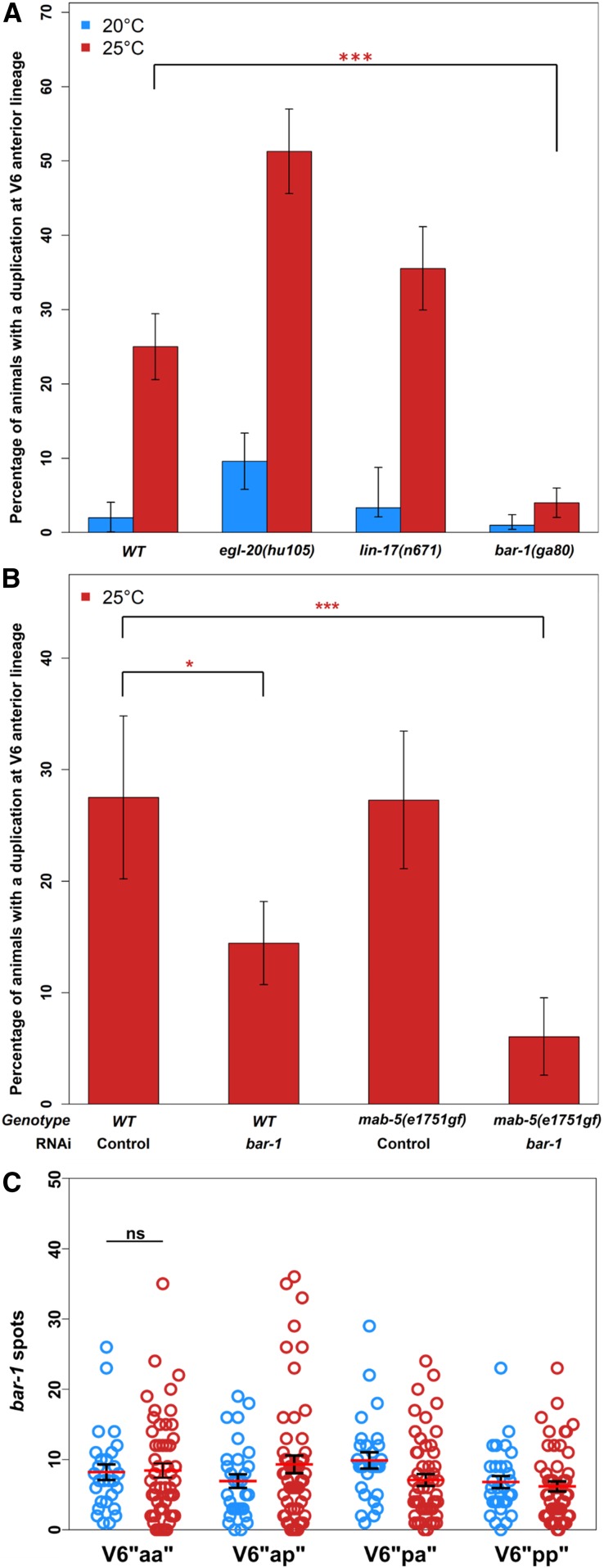
The Hox gene mab-5 is necessary and sufficient for seam cell duplications. (A) The loss-of-function allele mab-5(e1239) suppresses anterior V6 lineage seam cell duplications when compared to WT (N2) at 25° (***P < 0.001 with a binomial test), while the lin-39(n709) or egl-5(n945) loss-of-function mutations do not display a similar effect, n ≥ 78. Error bars indicate SE of the proportion. (B and C) Gain-of-function mab-5(e1751) mutants (triangle points) show significant increases in duplications at the L4 stage across seam cell lineages, at both 20° and 25° compared to WT (circle points) animals (*** correspond to P < 0.001 with a binomial test, n ≥ 40). (D) mab-5 mRNA levels at the L4 stage in V5ppppa/V5ppppp, V6pappa/V6pappp, and V6ppppa/V6ppppp cells measured by smFISH in animals grown at 25° (red circles, n = 28) compared to 20° animals (blue circles, n = 29). Significance of changes was tested with a two-sample Student’s t-test (*P < 0.05, **P < 0.01). Error bars indicate SEM. L4, fourth larval; mRNA, messenger RNA; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.
Figure 3.
The β-catenin bar-1 is necessary for anterior V6 lineage seam cell duplications. (A) Anterior V6 lineage seam cell duplications were significantly suppressed in bar-1(ga80) mutants at 25° compared to WT animals grown at 20° (***P < 0.001, binomial test, n ≥ 120). No suppression was seen in egl-20(hu105) and lin-17(n671) mutants, n ≥ 30. (B) The proportion of animals displaying anterior V6 lineage seam cell duplications in WT and mab-5(e1751gf) animals was significantly decreased when grown at 25° on bar-1 RNAi (*P < 0.05 and ***P < 0.001, n ≥ 40). (C) mRNA levels of bar-1, measured by smFISH at the L4 stage. Counts in V6pappa cells are not significantly different between animals grown at 20° and 25° (n ≥ 30). Error bars indicate SE of the proportion (A and B) or mean (C). L4, fourth larval; mRNA, messenger RNA; RNAi, RNA interference; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.
Figure 4.
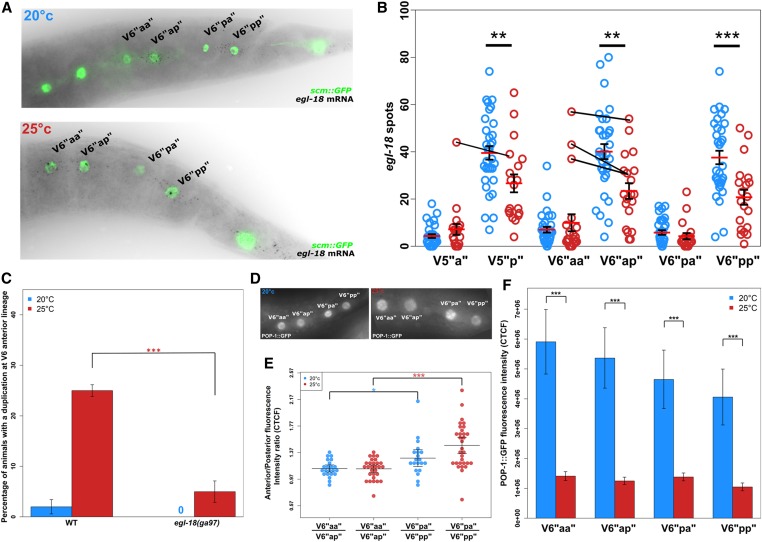
Wnt pathway asymmetry characterization in posterior seam cell lineages at the L4 stage. (A) Representative images of egl-18 smFISH (black dots correspond to mRNAs) in V6papp and V6pppp daughter cells at 20° and 25°. Seam cells are marked green due to expression of the scm::GFP marker. (B) Quantification of egl-18 mRNA levels in V5pppp, V6papp, and V6pppp daughters at 20° and 25°. Expression was significantly lower in posterior cells, i.e., V5ppppp, V6pappp, and V6ppppp at 25° (red circles) compared to the same seam cells grown at 20° (blue circles, **P < 0.01 and ***P < 0.001 with a two-sample Student’s t-test, n > 20 per cell). At low frequency, animals grown at 25° showed extreme expression values in V6pappa and V5ppppa cells, which were even higher than their posterior counterparts (cell pairs are indicated by black lines). (C) Seam cell duplications at anterior V6 lineage are suppressed in the egl-18(ga97) mutant at 25° (***P < 0.001 with a binomial test, n = 120). (D) Representative images of nuclear POP-1::GFP expression at 20° and 25° at the L4 stage. (E) The ratio of nuclear POP-1::GFP expression between V6papp and V6pppp daughter cells at 20° and 25°, n ≥ 22 (*P < 0.05 and ***P < 0.001, two-sample Student’s t-test, error bars indicate 95% C.I.s of the mean). In both cases, anterior/posterior cell fluorescence intensity ratio is shown (simplified as “aa”/“ap” for V6papp daughters and “pa”/“pp” for V6pppp). (F) Average POP-1::GFP intensity in V6papp and V6pppp daughter cells at 20° and 25° (error bars represent SEM, *P < 0.05 and **P < 0.001, with a two-sample Student’s t-test, n ≥ 22). L4, fourth larval; mRNA, messenger RNA; RNAi, RNA interference; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.
Results
Increase in growth temperature leads to extra seam cells in specific lineages
Seam cell development has been mostly studied so far at the standard growth temperature of C. elegans in the laboratory, which is 20°. We therefore decided to investigate whether varying the growth temperature could affect seam cell development. To this end, we cultured C. elegans at a range of temperatures ranging from 15° to 26° and scored seam cell number based on the expression of an scm::GFP marker (wIs51). We placed eggs to hatch at different temperatures and scored terminal seam cell numbers at the end of the L4 stage. At this stage, all somatic divisions are completed, and so the terminal seam cell number acts as a potential readout for seam cell defects that have accumulated over postembryonic development.
As expected, populations grown at 20° showed an average seam cell number of 16 cells per lateral side and low phenotypic variance, because of the rare occurrence of animals displaying 15 or 17 seam cells (Figure 1B). While we found no statistically significant difference in seam cell number when the culture temperature was decreased to 15°, we were surprised to see that populations grown at ≥ 23° showed a mild, yet statistically significant, increase in terminal seam cell number (Figure 1B, P < 0.05, two-sample Student’s t-test). This increase was most frequent at 25° and 26°. We decided to use 25° for all subsequent experiments, which is considered to be a viable physiological temperature for C. elegans growth and is commonly used in laboratories as an alternative temperature to 20°, for example to accelerate development or study temperature-sensitive mutants.
We first sought to investigate the developmental basis underlying the increase in seam cell number at 25°. While scoring seam cell number at different temperatures, we observed that a frequent error at 25° was the tight clustering of two seam cell nuclei, a phenotype that we refer to here as “seam cell duplication” for simplicity. We mapped the frequency of extra seam cells along the axes of the larvae and assigned them to seam cell lineages based on their position relative to the position of their closest seam cell neighbor. This highlighted a significant hotspot for seam cell duplications in the anterior V6 lineage, with around 30% of animals in the population showing this phenotype when grown at 25° in contrast to only 2% at 20° (Figure 1, C and D, P < 0.001, binomial test). Seam cell duplications were not exclusive to the V6 lineage, but were also observed, albeit with lower frequency, in the V5, V1, and V2 lineages (Figure 1, C and D). This finding indicates that the seam cell lineages display different sensitivities to temperature increase.
To understand when these duplications occur during postembryonic development, we transferred animals at different developmental stages from culture at 20° to 25°. We found that transferring animals at any stage before L2 resulted in a similar increase in seam cell number, suggesting that seam cell lineage errors occur after the L2 developmental stage (Figure S1A). By scoring the frequency of extra cells in the anterior V6 lineage at the later larval stages in animals raised entirely at 25°, we found that seam cell duplications occurred during the L4 division (Figure S1B). This observation is consistent with the close clustering of pairs of nuclei observed at the end of the L4 stage, indicative of a recent developmental event.
The seam cell duplications observed may have been a consequence of defects in cell division or cell differentiation during the L4 stage. With regard to cell division defects, one possibility was that the extra nuclei are a result of a failure of cytokinesis at 25°. To address this possibility, we used a marker of seam cell membranes to look for multinucleate cells at the L4 stage, but we did not observe any defects in cytokinesis in instances of extra nuclei in the anterior V6 lineage (Figure S1C). Although we have not formally ruled out the possibility of an ectopic seam cell division, we believe this is unlikely due to the number of observations throughout the L4 stage at 25°, during which we have never obtained evidence of a cell division in addition to the wild-type seam cell division pattern. We then explored whether, despite the seam cells dividing successfully, the anterior daughters fail to differentiate into hyp7, but instead retain the seam cell fate. Indeed, using a strain that carries both the seam cell and a hyp7 cell marker (dpy-7p::mCherry), we found that both cells of the duplicated anterior V6 lineage (V6pappa and V6pappp) expressed the seam cell marker alone, with neither cell expressing the hyp7 cell marker (Figure 1E). Finally, we reasoned that the additional seam cell may reflect a timing constraint for V6pappa cells to differentiate before terminal seam cell fusion occurs, since seam cells terminally fuse at the end of the L4 stage and development is accelerated at 25°. We argue that this is unlikely to be the case because we found that V6papp cells are not the last seam cells to divide at the L4 stage, despite the fact that this lineage displays the highest sensitivity to temperature (Figure S1D). Furthermore, anterior V6 duplications at the L4 stage were still observed in an aff-1 mutant background, which is impaired in terminal seam cell fusion (Figure S1E). Taken together, these results indicate that an increase in culture temperature induces seam cell duplications during the L4 division, due to conversion of asymmetric cell divisions to symmetric wherein both cells adopt the seam cell fate.
The Hox gene mab-5 is necessary for seam cell duplications at 25°
Ectopic seam cells at 25° were most frequently found in specific lineages, which highlights differences in sensitivity to temperature along the nematode body axis. We therefore decided to investigate whether factors involved in anterior–posterior patterning can influence the seam cell phenotype in response to temperature increase. Hox genes are known to be involved in the specification of the animal body axis and play a role in seam cell patterning as well (Salser and Kenyon 1996; Arata et al. 2006). A posterior body Hox gene, mab-5, has been reported to be required for V5 and V6 seam cell lineages in males to generate sensory rays, while loss of mab-5 leads to a switch from ray formation to alae (Salser and Kenyon 1996). To investigate the role of Hox genes in anterior V6 lineage defects at 25°, we compared terminal seam cell number and frequency of anterior V6 lineage duplication at the L4 stage in three strains carrying individual loss-of-function mutations for the mid and posterior Hox genes lin-39, egl-5, and mab-5 (Austin and Kenyon 1994; Salser and Kenyon 1996; Maloof and Kenyon 1998) (Figure 2A and Figure S2A). We found that only loss of mab-5 function significantly suppressed the number of V6pappa cells remaining as seam cells at 25° (Figure 2A, P < 0.001, binomial test), indicating that mab-5 is necessary for anterior V6 lineage seam cell duplication events. In addition, we found that mab-5 is required for the maintenance of posterior seam cell fates in hermaphrodites, as mab-5 loss-of-function animals lost posterior seam cells at low frequency (Figure S3A). This seam cell loss is unlikely to affect the comparison between temperatures because the frequency of anterior V6 lineage loss was similar between 20° and 25° (Figure S3A). To investigate whether mab-5 is also sufficient for seam cell duplications, we quantified seam cell number in the mab-5 gain-of-function mutant mab-5(e1751gf). In this background, mab-5 expression is thought to expand from the posterior to the anterior end due to a mab-5 genomic locus duplication (Salser and Kenyon 1992). We confirmed mab-5 expansion at the mRNA level with smFISH (Figure S3, B–E). Interestingly, we found that mab-5(e1751gf) mutants showed a significant increase in ectopic seam cells, which was observed even at 20° and became more pronounced at 25°, with multiple lineages showing seam cell duplications (Figure 2, B and C, P < 0.001, binomial test).
Based on the mab-5 gain-of-function seam cell phenotype, we then hypothesized that an increase in mab-5 mRNA levels may underlie the seam cell duplications we observed at 25°. To address this hypothesis, we quantified mab-5 expression by smFISH in wild-type animals, focusing on V5 and V6 cell lineages at the L4 stage, which are either very sensitive (like V6pappa cells) or less sensitive (V6ppppa cells) to fate change at higher temperature. Surprisingly, we found a significant decrease in mRNA expression at 25° compared to 20° (Figure 2D and Figure S3F, P < 0.05, two-sample Student’s t-test), although the expression of mab-5 showed a peak in V6pappa cells at both 20° and 25° degrees. Taken together, we conclude that mab-5 is necessary and sufficient for seam cell lineage fate changes that occur at the L4 stage. However, seam cell duplications in response to higher temperature are unlikely to be driven at the level of an increase in mab-5 mRNA expression.
Seam cell duplications require the canonical Wnt pathway component BAR-1
Because of the role of Wnt signaling in controlling seam cell division patterns, we went on to investigate whether mutations in candidate Wnt components may suppress the anterior V6 lineage seam cell symmetrization at the L4 stage at 25°. For example, the Frizzled receptor LIN-17 and the posteriorly produced Wnt ligand EGL-20 have previously been shown to polarize seam cell divisions, and interact with mab-5 during QL neuroblast migration (Mizumoto and Sawa 2007b; Middelkoop and Korswagen 2014). However, we found that loss-of-function mutations in either of these factors do not suppress the anterior V6 lineage seam cell duplication at the L4 stage (Figure 3A and Figure S2B). Instead, loss of the canonical Wnt pathway β-catenin bar-1 led to a significant decrease in the number of anterior V6 seam cell duplications at 25° compared to wild-type (Figure 3A, P < 0.001, binomial test). To validate this result, we knocked down bar-1 by RNAi and found that this treatment also significantly reduced the number of anterior V6 seam cell duplications observed at 25° at the L4 stage (Figure 3B, P < 0.05, binomial test). This bar-1 RNAi treatment also suppressed the anterior V6 seam cell duplications in the mab-5 gain-of-function (Figure 3B, P < 0.001, binomial test), indicating that bar-1 is either required for mab-5 expression in this cell or acts in parallel to mab-5 to facilitate seam cell symmetrization at 25°.
The dependence of seam cell duplication on bar-1 was surprising as this gene was not previously thought to play a major role in seam cell development studied at 20°. We therefore tested if bar-1 expression could be detected in seam cells and, subsequently, if bar-1 mRNA levels were changed when animals were cultured at 25°. We were able to detect bar-1 expression by smFISH in both anterior and posterior V6 seam cell lineages at the L4 stage (Figure S3G). However, we found no consistent change in mean number of bar-1 transcripts at 25° vs. 20°, for example there was no significant change in V6pappa cells that show the most frequent seam cell fate retention (Figure 3C). These results indicate that bar-1 is expressed in seam cells at the time when asymmetric division defects occur, suggesting that these defects may happen through temperature-driven local activation of the Wnt pathway.
Impaired Wnt pathway asymmetry underlies seam cell fate changes
One of the main pathways involved in the maintenance of seam cell fate and regulation of the asymmetric cell division is the Wnt pathway (Gleason and Eisenmann 2010; Sawa and Korswagen 2013). Following an asymmetric seam cell division, activation of the Wnt pathway is usually restricted to posterior cell daughters, which express key downstream genes such as the GATA transcription factor egl-18 and maintain the seam cell fate (Gleason and Eisenmann 2010; Gorrepati et al. 2013). Ectopic activation of the Wnt pathway in anterior cell daughters has been shown to be sufficient for these cells to adopt the seam cell fate, in a similar manner to their posterior counterparts. For example, this occurs upon pop-1/tcf downregulation and leads to a dramatic increase in the average seam cell number (Gleason and Eisenmann 2010). To address whether defects in asymmetric seam cell division were associated with changes in Wnt pathway activity localization, we quantified egl-18 mRNA expression in animals grown at 20° and 25° during the L4 division. As expected, we found that at 20° and following the L4 asymmetric division the posterior cell daughters (V5ppppp, V6pappp, and V6ppppp) of the V5 and V6 lineages all expressed egl-18 at a higher level than their anterior sister cells (V5ppppa, V6pappa, and V6ppppa, respectively) (Figure 4, A and B). Consistent with our findings of ectopic seam fate retention at 25°, we found that a subset of anterior cells showed, at low frequency, expression values near or beyond those anticipated for posterior daughter cells. In particular, V6pappa cells showed a noticeable increase in extreme egl-18 expression values at 25° compared to 20° (Figure 4B, see black lines connecting anterior to posterior cell pairs), which is likely to underpin the seam cell duplications observed. In addition, we observed that the posterior daughter cells that are fated to remain seam cells at 25° showed significantly less egl-18 expression than the same cells at 20° (Figure 4B). These results are indicative of a molecular shift in the L4 division at 25° from an asymmetric toward a more symmetric mode.
To test whether egl-18 plays a functional role in the symmetrization of L4 seam cell divisions at 25°, we scored seam cell duplications in an egl-18 loss-of-function mutant. We focused on the anterior V6 lineage at the L4 stage, which is largely unaffected in this mutant background at 20°, to assess seam cell fate symmetrization frequency at 25°. We found that loss of egl-18 activity suppressed the seam cell duplication frequency (Figure 4C, P < 0.001, binomial test). Taken together, these data support the idea that seam cell duplications may occur due to ectopic activation of the Wnt pathway in anterior seam cell daughters following the L4 division at 25°.
Nuclear levels of POP-1 are a good indicator of post cell division asymmetry in seam cells, with high POP-1 levels (usually in anterior daughters) associated with a nonseam cell fate due to repression of Wnt targets, and lower levels (in posterior daughters) associated with activation of Wnt targets and retention of the seam cell fate (Mizumoto and Sawa 2007b; Gleason and Eisenmann 2010). To investigate whether the distribution of POP-1 is changed in seam cell daughters at 25°, we used a strain carrying POP-1::GFP, and compared the levels of nuclear GFP expression between V6papp and V6pppp seam cell daughters after the L4 division. We found that one-third of V6papp daughter cell pairs (V6pappa/V6pappp) had equivalent POP-1::GFP levels at both 20 and 25°. This was in contrast to V6pppp daughter cell pairs (V6ppppa/V6ppppp), which maintained significantly higher levels of POP-1 expression in anterior vs. posterior daughters at both 20° and 25° (P-value < 0.05, two-sample Student’s t-test) (Figure 4, D and E). We also found that the overall level of POP-1::GFP expression was significantly decreased in all V6 daughter cells at 25° following the L4 division (Figure 4F). These results suggest that V6 cells may have intrinsic differences in Wnt pathway asymmetry, which make them more sensitive to temperature perturbations. When this is combined with a lowering of the overall amount of nuclear POP-1 in V6 lineage cells, this sensitivity may lead to a greater chance of V6pappa cells retaining a seam cell fate.
The genetic background modifies the pattern and frequency of seam cell fate changes at 25°
Seam cell development has never been studied in any other C. elegans isolate except for the laboratory reference strain N2. Over the last few years, several divergent C. elegans strains have been isolated from various locations throughout the world, offering the opportunity to study the effect of the genetic background on seam cell patterning and its robustness to various perturbations, including temperature increase.
Here, we sought to investigate whether seam cell number is robust to standing genetic variation and whether the observed seam cell duplications in response to higher temperature could also be observed in other natural isolates. To be able to visualize the seam cells, we first genetically introgressed the seam cell marker scm::GFP into five wild isolates by repeated backcrossing. We included strains that are known to be significantly divergent from N2, such as the commonly used Hawaiian isolate CB4856 (Andersen et al. 2012). We found that all five isolates tested had an average of 16 seam cells per lateral side at 20°, which is the same as N2 (Figure 5A and Figure S4A). However, they responded differently to temperature increase (Figure 5A and Figure S4A). In particular, isolate XZ1516 was much more sensitive and showed higher frequency of duplications in various seam cell lineages when cultured at 25° (Figure 5B and Figure S4, B–D). Isolate JU2519 showed frequent duplications in the V3 lineage, which is not common in N2. Interestingly, the anterior V6 lineage remained the most sensitive to temperature increase in all isolates that were sensitive to temperature. At the other end of the spectrum, seam cells in CB4856 were robust to temperature increase, with the frequency of seam cell lineage defects being the same between the two growth temperatures of 20° and 25° (Figure 5, A and B). Together, these results indicate that variation in the genetic background can both enhance and suppress the seam cell fate changes observed upon temperature increase.
Figure 5.
Evolution of the frequency of seam cell duplications in wild isolates of C. elegans. (A) Plot showing average seam cell number at 20° and 25° in N2 (depicted in green), JU775 (in purple), JU2519 (in pink), JU2007 (in light blue), XZ1516 (in yellow), and CB4856 (in orange). Error bars show 95% C.I.s of the mean. (B) Quantification of seam cell duplications in these isolates at 20° and 25° at the L4 stage. Note that some isolates are more (JU2519 and XZ1516) or less sensitive (CB4856) than N2 to display temperature-driven seam cell duplications. Statistical comparisons are within isolates between 20° and 25° [***P < 0.001 with Student’s t-test (A) or binomial test (B), n = 120 for all strains]. L4, fourth larval.
Discussion
Higher temperatures lead to reproducible cell fate changes in the epidermis
Changes in temperature can have remarkable effects on both the physiology and development of organisms. For example, temperature decrease is known to extend life span in C. elegans, and a recent study showed that this acts through the reduction of age-mediated exhaustion of germline stem cells (Lee et al. 2019). Temperature can affect core cellular processes, such as the timing of cell division, which may be directly linked to temperature-imposed fitness barriers (Begasse et al. 2015). We report here that increasing growth temperature, yet within the physiological range of temperatures for C. elegans culture, leads to a gradual increase in the average number of seam cells. Interestingly, additional seam cells do not occur at random along the body axis of the animal. For example, we report that the anterior V6, V5, anterior V1, and V2 lineages are hotspots for seam cell duplications in the N2 background at the L4 stage. Among all cells, V6pappa shows the highest frequency of seam cell fate retention, while its neighbor V6ppppa is almost insensitive to cell fate change upon temperature increase. This is of note because V6pappa and V6ppppa cells are related in lineage and diverge only at the L2 stage, when their common precursor (V6p) divides symmetrically to generate the two sublineages. Therefore, differences in seam cell sensitivities to temperature increase are unlikely to depend exclusively on early lineage delimitation.
With regard to cell fate patterning, how environmental changes can affect developmental fidelity has been previously studied in the context of vulva development. Vulva formation is very robust to temperature change, with the frequency of cell fate patterning errors remaining extremely low within the physiological range of temperatures, while the exact type of errors depends on the exact environmental pressure (Braendle and Felix 2008). For example, growth at 25° results in a low frequency (usually < 1%) of undivided P4.p and P8.p cells, which are part of the vulval competence group, although these cells do not contribute directly to the formation of the vulva by acquiring (primary or secondary) vulval cell fates (Braendle and Felix 2008). Vulva development can be substantially debuffered via subjecting animals to more extreme nonphysiological temperature challenges, such as transient culture at 30° (Grimbert and Braendle 2014). Similar to seam cell lineages, vulval cells also display cell-specific sensitivities to high temperature, with secondary cells being more affected than primary cells through a decrease in Notch pathway activity (Grimbert and Braendle 2014). Therefore, it will be interesting in the future to establish a mechanistic framework describing the outcome of cell fate decisions as a function of temperature, both within and beyond the seam cell lineage.
Seam cell lineage defects in response to high temperature are dependent upon the Hox gene mab-5
The cell-specific sensitivity to duplication along the body axis at 25° led us to investigate whether Hox genes may be involved in the manifestation of this developmental phenotype. We found that the posterior Hox gene and Antennapedia homolog mab-5 plays a role in hermaphrodite seam cell patterning, because it is required for seam cell maintenance, as well as anterior V6 lineage seam cell duplications, in response to temperature increase. Interestingly, expansion of the mab-5 expression domain to the anterior end of the animal leads to a high frequency of anterior seam cell duplications even at 20°, indicating that mab-5 may directly trigger or sensitize epidermal cells to convert from any asymmetric mode of division to a symmetric one. The sensitivity in lineages such as V2, which is outside the endogenous mab-5 expression domain but produces extra seam cells at 25°, highlights that other factors must act in parallel to mab-5. For example, although the Hox gene lin-39 is not required for V6papp seam cell division symmetrization at 25°, it might still play a role in anterior seam cell duplications because it mildly suppressed seam cell number increase at 25° (Figure S2A). It was previously reported that mab-5 plays a role in the seam cell lineage in males, where dynamic mab-5 expression in the V5 and V6 lineages is required for sensory ray formation by regulating cell proliferation and differentiation (Salser and Kenyon 1996; Hunter et al. 1999). The posterior V5 and V6 seam cells divide symmetrically in late postembryonic development in males (Sulston et al. 1980), so it could be argued that the sex-dependent expansion of seam cells may result in a less-fixed cell fate pattern in hermaphrodites, leading to the seam cell duplication phenotype. We consider this unlikely to be a main driver because the frequency of seam cell duplications is much lower in the posterior V6 lineage compared to other seam cell lineages.
Our findings support the idea that mab-5 is required for the V6pappa cell fate change in response to temperature, presumably by creating a permissive environment within the posterior region of the animal for such cell fate changes to occur. Notably, mab-5 may act in a cell-autonomous manner, as mRNA expression was detected in anterior seam cell daughters following the L4 division and before these cells differentiated to become hyp7 at 20°. Interestingly, mab-5 mRNA levels showed a maximum at the L4 stage at V6pappa, which is the cell that displays the highest frequency of symmetrization at 25°. This raises the possibility that mab-5 levels may relate to the sensitivity of cells undergoing a cell fate change. Higher temperatures are thought to generally increase levels of gene expression, but this relationship varies from one gene to another (Gómez-Orte et al. 2017). We found no increase in the expression of mab-5 when temperature increases. Taken together, these results are consistent with a model in which mab-5 is required for the symmetrization of division at 25°, although these errors do not arise due to changes in the levels of mab-5 transcription. It is interesting that mab-5 has been previously reported to be involved in the competence of other epidermal cells to respond to developmental signals, namely the ventral epidermal precursor cells in males, the most posterior of which give rise to the hook sensillum group [P (9–11).p]. In this context, mab-5 is necessary for P (9–11).p cell specification, and overexpression in anterior P (1–8).p cells makes them competent to generate posterior epidermal fates depending on the activity of the Notch and EGF pathway (Yu et al. 2010).
Seam cell fate changes in response to temperature increase may be due to both induced and intrinsic differences in Wnt pathway asymmetry
We present here evidence that symmetrization of Wnt pathway activity may underlie the seam cell defects in response to growth at higher temperatures. First, we found that V6pappa cells show higher egl-18 expression values at 25° at a frequency that matches the penetrance of the seam cell duplication phenotype. Furthermore, the posterior daughter cells of the L4 division (V5ppppp, V6pappp, and V6ppppp) exhibited overall decreased expression at 25° compared to 20°. This pattern of egl-18 expression has been previously reported in lin-22 mutants, which also show stochastic symmetrization of seam cell divisions along the body axis at late larval stages (Katsanos et al. 2017). In addition to ectopic egl-18 expression, low POP-1 levels are thought to correlate with a high potential for seam cell division symmetrization toward the seam cell fate (Gleason and Eisenmann 2010). This is because low POP-1 levels may fall under a threshold that is required for POP-1 repressor function in anterior cell daughters, while remaining sufficient for transcriptional activation in posterior daughter cells (van der Horst et al. 2019). Interestingly, low POP-1 levels can override the effect of the Wnt asymmetry pathway, as demonstrated during symmetric L2 divisions when the RNT-1/BRO-1 module represses pop-1 expression, without affecting nuclear POP-1 asymmetric distribution in daughter cells (van der Horst et al. 2019). We found an overall decrease in nuclear POP-1 levels at the L4 stage in seam cells at 25° in comparison to 20°, which is consistent with the symmetrization of L4 divisions at 25°. At the same time, V6papp daughter cell pairs appeared more symmetric in nuclear POP-1 levels compared to V6pppp daughters at the L4 stage, both at 20° and 25°. This result highlights that intrinsic cell-to-cell differences in the Wnt asymmetry pathway may sensitize certain seam cells to divide symmetrically. Identifying factors that modulate the Wnt asymmetry pathway in specific seam cells will be the focus of future work.
With regard to upstream signaling components, we found that a loss-of-function mutation in bar-1 suppressed the seam cell lineage defect at 25°. This is unexpected because asymmetric seam cell divisions are thought to depend on the β-catenins WRM-1 and SYS-1, which regulate POP-1 subcellular redistribution and transcriptional activity respectively (Rocheleau et al. 1999; Lo et al. 2004; Shetty et al. 2005; Phillips et al. 2007). The potential contributions of WRM-1 and SYS-1 to the extra seam cells observed in response to temperature were not investigated here due to the temperature sensitivity of the available mutants, and their strong seam cell defects. While bar-1 is known to modulate Wnt expression through the canonical molecular pathway (Sawa and Korswagen 2013), its role in seam cell development has been questioned, partly because bar-1 loss-of-function mutants only show very mild seam cell defects at 20°. However, we found that bar-1 is expressed in anterior and posterior seam cells, and facilitates the seam cell duplication phenotype at 25°. The activation of BAR-1 has been shown to increase Wnt target genes such as egl-18 in the epidermis (Gorrepati et al. 2013; Jackson et al. 2014). This raises the possibility that BAR-1-dependent ectopic activation of the Wnt pathway in V6pappa cells may underlie the cell fate change observed. Another possibility is that BAR-1 modulates seam cell competence to respond to temperature increase through mab-5 regulation (Middelkoop and Korswagen 2014). It will be interesting to dissect in the future how BAR-1 relates to mab-5 expression, POP-1 levels, and POP-1 nuclear asymmetry in seam cells.
Background genetic variation influences seam cell development
Cryptic genetic variation refers to genetic variation that is phenotypically silent under wild-type conditions, yet can have phenotypic consequences when a biological system is perturbed (Gibson and Dworkin 2004; Paaby and Rockman 2014). Cryptic genetic variation can therefore remain neutral in populations, but become adaptive or deleterious upon perturbation, which is thought to relate to the increased prevalence of certain human diseases in modern times (Gibson and Dworkin 2004; Gibson and Reed 2008). Cryptic genetic variation can be revealed in model organisms via controlled system perturbations, such as introducing mutations into divergent wild-isolate backgrounds or subjecting animals to various environmental treatments. Recent efforts have therefore succeeded in detecting cryptic genetic variation affecting molecular (Snoek et al. 2017) or developmental processes in C. elegans, including embryogenesis (Paaby et al. 2015), germ layer specification (Torres Cleuren et al. 2019), and vulva development (Milloz et al. 2008; Duveau and Felix 2012; Grimbert and Braendle 2014).
We reveal here, for the first time, cryptic genetic variation underlying seam cell development by demonstrating that divergent wild C. elegans isolates show significant differences in seam cell number when grown at 25°, while they show a comparable average seam cell number when grown at the standard growth temperature of 20°. Our results suggest that differences in the frequency of seam cell duplications over various cell lineages among isolates at 25° largely account for the differences in mean seam cell number. Among all isolates, XZ1516 is the most sensitive strain and displays seam cell duplications in various cell lineages at 25°. On the other hand, CB4856 is the least sensitive strain and its average seam cell number was not significantly affected by temperature. It is currently unclear if CB4856 also shows higher tolerance to temperature increase in other developmental tissues; however, this strain has been reported to show a preference for colder temperatures (Anderson et al. 2007). Interestingly, XZ1516 and CB4856 were both sampled at the same geographic location (Hawaii), which highlights that the evolution of the seam cell duplication phenotype is unlikely to reflect some specific ecological adaptation. It is of note that certain isolates displayed changes in the frequencies of seam cell duplications, both within or outside the lineages that are affected in the N2 background. For example, isolates JU775 and JU2007 showed enhanced sensitivity in the anterior V1 and anterior V6 lineages, which is comparable to N2, whereas JU2519 and XZ1516 showed novel duplications in the H1, H2, V3, and T cell lineages. The broad expansion of cell-specific sensitivity observed in strain XZ1516 is reminiscent of the mab-5 gain-of-function phenotype in N2, and thus may reflect evolution in similar morphogenetic factors.
It will be interesting in the future to discover the genetic architecture of cryptic genetic variation underlying seam cell development. Given the importance of the Wnt signaling pathway in regulating seam cell development, it is intriguing to speculate that differences in Wnt pathway activity or sensitivity of the response to Wnt activation among isolates may underlie the cryptic genetic variation observed. Previous studies have revealed cryptic genetic variation in cell-specific Ras and Notch pathway activity outputs in the context of the vulval signaling network (Milloz et al. 2008). More recently, cryptic variation was detected in the contribution of the Wnt input in the gene regulatory network underlying endoderm specification (Torres Cleuren et al. 2019). Quantifying changes in the abundance of Wnt signaling components among isolates is likely to be challenging at the whole-organism level (Singh et al. 2016). Seam cell development may thus offer a suitable tissue-specific readout to facilitate the discovery of genetic modifiers influencing the conserved Wnt signaling pathway, with possible implications in human development and disease.
Acknowledgments
We thank Michael Fasseas and Iqrah Razzaq for help with experiments. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD-010440). This work was supported by the European Research Council (639485-ROBUSTNET) and the Leverhulme Trust (RPG-2015-235).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11687178.
Communicating editor: M. Sundaram
Literature Cited
- Andersen E. C., Gerke J. P., Shapiro J. A., Crissman J. R., Ghosh R. et al. , 2012. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat. Genet. 44: 285–290. 10.1038/ng.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Albergotti L., Proulx S., Peden C., Huey R. B. et al. , 2007. Thermal preference of Caenorhabditis elegans: a null model and empirical tests. J. Exp. Biol. 210: 3107–3116. 10.1242/jeb.007351 [DOI] [PubMed] [Google Scholar]
- Arata Y., Kouike H., Zhang Y., Herman M. A., Okano H. et al. , 2006. Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev. Cell 11: 105–115. 10.1016/j.devcel.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Austin J., and Kenyon C., 1994. Cell contact regulates neuroblast formation in the Caenorhabditis elegans lateral epidermis. Development 120: 313–323. [DOI] [PubMed] [Google Scholar]
- Baldwin A. T., Clemons A. M., and Phillips B. T., 2016. Unique and redundant β-catenin regulatory roles of two Dishevelled paralogs during C. elegans asymmetric cell division. J. Cell Sci. 129: 983–993. 10.1242/jcs.175802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoulas M., van Zon J. S., Milloz J., van Oudenaarden A., and Felix M. A., 2013. Robustness and epistasis in the C. elegans vulval signaling network revealed by pathway dosage modulation. Dev. Cell 24: 64–75. 10.1016/j.devcel.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Begasse M. L., Leaver M., Vazquez F., Grill S. W., and Hyman A. A., 2015. Temperature dependence of cell division timing accounts for a shift in the thermal limits of C. elegans and C. briggsae. Cell Reports 10: 647–653. 10.1016/j.celrep.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Brabin C., Appleford P. J., and Woollard A., 2011. The Caenorhabditis elegans GATA factor ELT-1 works through the cell proliferation regulator BRO-1 and the Fusogen EFF-1 to maintain the seam stem-like fate. PLoS Genet. 7: e1002200 10.1371/journal.pgen.1002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendle C., and Felix M. A., 2008. Plasticity and errors of a robust developmental system in different environments. Dev. Cell 15: 714–724. 10.1016/j.devcel.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorahabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassata G., 2005. ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development 132: 739–749. 10.1242/dev.01638 [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., and Hsiao T. I., 2012. The Caenorhabditis elegans epidermis as a model skin. I: development, patterning, and growth. Wiley Interdiscip. Rev. Dev. Biol. 1: 861–878. 10.1002/wdev.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duveau F., and Felix M. A., 2012. Role of pleiotropy in the evolution of a cryptic developmental variation in Caenorhabditis elegans. PLoS Biol. 10: e1001230 10.1371/journal.pbio.1001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., and Barkoulas M., 2012. Robustness and flexibility in nematode vulva development. Trends Genet. 28: 185–195. 10.1016/j.tig.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Gibson G., and Dworkin I., 2004. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5: 681–690. 10.1038/nrg1426 [DOI] [PubMed] [Google Scholar]
- Gibson G., and Reed L. K., 2008. Cryptic genetic variation. Curr. Biol. 18: R989–R990. 10.1016/j.cub.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason J. E., and Eisenmann D. M., 2010. Wnt signaling controls the stem cell-like asymmetric division of the epithelial seam cells during C. elegans larval development. Dev. Biol. 348: 58–66. 10.1016/j.ydbio.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B., Takeshita H., Mizumoto K., and Sawa H., 2006. Wnt signals can function as positional cues in establishing cell polarity. Dev. Cell 10: 391–396. 10.1016/j.devcel.2005.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Orte E., Cornes E., Zheleva A., Sáenz-Narciso B., de Toro M. et al. , 2017. Effect of the diet type and temperature on the C. elegans transcriptome. Oncotarget 9: 9556–9571. 10.18632/oncotarget.23563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrepati L., Thompson K. W., and Eisenmann D. M., 2013. C. elegans GATA factors EGL-18 and ELT-6 function downstream of Wnt signaling to maintain the progenitor fate during larval asymmetric divisions of the seam cells. Development 140: 2093–2102. 10.1242/dev.091124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrepati L., Krause M. W., Chen W., Brodigan T. M., Correa-Mendez M. et al. , 2015. Identification of Wnt pathway target genes regulating the division and differentiation of larval seam cells and vulval precursor cells in Caenorhabditis elegans. G3 (Bethesda) 5: 1551–1566. 10.1534/g3.115.017715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbert S., and Braendle C., 2014. Cryptic genetic variation uncovers evolution of environmentally sensitive parameters in Caenorhabditis vulval development. Evol. Dev. 16: 278–291. 10.1111/ede.12091 [DOI] [PubMed] [Google Scholar]
- Huang X., Tian E., Xu Y., and Zhang H., 2009. The C. elegans engrailed homolog ceh-16 regulates the self-renewal expansion division of stem cell-like seam cells. Dev. Biol. 333: 337–347. 10.1016/j.ydbio.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Hughes S., Brabin C., Appleford P. J., and Woollard A., 2013. CEH-20/Pbx and UNC-62/Meis function upstream of rnt-1/Runx to regulate asymmetric divisions of the C. elegans stem-like seam cells. Biol. Open 2: 718–727. 10.1242/bio.20134549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. P., Harris J. M., Maloof J. N., and Kenyon C., 1999. Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit Wnt signaling. Development 126: 805–814. [DOI] [PubMed] [Google Scholar]
- Jackson B. M., Abete-Luzi P., Krause M. W., and Eisenmann D. M., 2014. Use of an activated beta-catenin to identify Wnt pathway target genes in Caenorhabditis elegans, including a subset of collagen genes expressed in late larval development. G3 (Bethesda) 4: 733–747. 10.1534/g3.113.009522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. M., Riddle M. R., Djabrayan N. J., and Rothman J. H., 2010. Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 239: 1539–1554. 10.1002/dvdy.22296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoshima H., Nimmo R., Saad N., Tanaka J., Miwa Y. et al. , 2007. The C. elegans CBF beta homologue BRO-1 interacts with the Runx factor, RNT-1, to promote stem cell proliferation and self-renewal. Development 134: 3905–3915. 10.1242/dev.008276 [DOI] [PubMed] [Google Scholar]
- Katsanos D., Koneru S. L., Mestek Boukhibar L., Gritti N., Ghose R. et al. , 2017. Stochastic loss and gain of symmetric divisions in the C. elegans epidermis perturbs robustness of stem cell number. PLoS Biol. 15: e2002429 10.1371/journal.pbio.2002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. A., 2008. Mechanisms of asymmetric stem cell division. Cell 132: 583–597. 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Koh K., and Rothman J. H., 2001. ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development 128: 2867–2880. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Noormohammadi A., Koyuncu S., Calculli G., Simic M. S. et al. , 2019. Prostaglandin signals from adult germ stem cells delay somatic aging of Caenorhabditis elegans. Nat. Metab. 1: 790–810. 10.1038/s42255-019-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M. C., Gay F., Odom R., Shi Y., and Lin R., 2004. Phosphorylation by the beta-catenin/MAPK complex promotes 14–3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell 117: 95–106. 10.1016/S0092-8674(04)00203-X [DOI] [PubMed] [Google Scholar]
- Maloof J. N., and Kenyon C., 1998. The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development 125: 181–190. [DOI] [PubMed] [Google Scholar]
- Middelkoop T. C., and Korswagen H. C., 2014. Development and migration of the C. elegans Q neuroblasts and their descendants (October 15, 2014), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.173.1, http://www.wormbook.org. 10.1895/wormbook.1.173.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloz J., Duveau F., Nuez I., and Felix M. A., 2008. Intraspecific evolution of the intercellular signaling network underlying a robust developmental system. Genes Dev. 22: 3064–3075. 10.1101/gad.495308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., and Sawa H., 2007a Cortical beta-catenin and APC regulate asymmetric nuclear beta-catenin localization during asymmetric cell division in C. elegans. Dev. Cell 12: 287–299. 10.1016/j.devcel.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Mizumoto K., and Sawa H., 2007b Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 17: 465–473. 10.1016/j.tcb.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., and Kimble J., 2006. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068–1074. 10.1038/nature04956 [DOI] [PubMed] [Google Scholar]
- Nimmo R., Antebi A., and Woollard A., 2005. mab-2 encodes RNT-1, a C. elegans Runx homologue essential for controlling cell proliferation in a stem cell-like developmental lineage. Development 132: 5043–5054. 10.1242/dev.02102 [DOI] [PubMed] [Google Scholar]
- Paaby A. B., and Rockman M. V., 2014. Cryptic genetic variation: evolution’s hidden substrate. Nat. Rev. Genet. 15: 247–258. 10.1038/nrg3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaby A. B., White A. G., Riccardi D. D., Gunsalus K. C., Piano F. et al. , 2015. Wild worm embryogenesis harbors ubiquitous polygenic modifier variation. Elife 4: e09178. 10.7554/eLife.09178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. T., Kidd A. R. III, King R., Hardin J., and Kimble J., 2007. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104: 3231–3236. 10.1073/pnas.0611507104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau C. E., Yasuda J., Shin T. H., Lin R., Sawa H. et al. , 1999. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell 97: 717–726. 10.1016/S0092-8674(00)80784-9 [DOI] [PubMed] [Google Scholar]
- Salser S. J., and Kenyon C., 1992. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature 355: 255–258. 10.1038/355255a0 [DOI] [PubMed] [Google Scholar]
- Salser S. J., and Kenyon C., 1996. A C. elegans Hox gene switches on, off, on and off again to regulate proliferation, differentiation and morphogenesis. Development 122: 1651–1661. [DOI] [PubMed] [Google Scholar]
- Sawa H., and Korswagen H. C., 2013. Wnt signaling in C. elegans (December 9, 2013), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.7.2, http://www.wormbook.org. 10.1895/wormbook.1.7.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P., Lo M. C., Robertson S. M., and Lin R., 2005. C. elegans TCF protein, POP-1, converts from repressor to activator as a result of Wnt-induced lowering of nuclear levels. Dev. Biol. 285: 584–592. 10.1016/j.ydbio.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Singh K. D., Roschitzki B., Snoek L. B., Grossmann J., Zheng X. et al. , 2016. Natural genetic variation influences protein abundances in C. elegans developmental signalling pathways. PLoS One 11: e0149418 10.1371/journal.pone.0149418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek B. L., Sterken M. G., Bevers R. P. J., Volkers R. J. M., Van’t Hof A. et al. , 2017. Contribution of trans regulatory eQTL to cryptic genetic variation in C. elegans. BMC Genomics 18: 500 10.1186/s12864-017-3899-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A., and Dalton S., 2016. Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development 143: 4301–4311. 10.1242/dev.142075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., and Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Albertson D. G., and Thomson J. N., 1980. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 78: 542–576. 10.1016/0012-1606(80)90352-8 [DOI] [PubMed] [Google Scholar]
- Takeshita H., and Sawa H., 2005. Asymmetric cortical and nuclear localizations of WRM-1/beta-catenin during asymmetric cell division in C. elegans. Genes Dev. 19: 1743–1748. 10.1101/gad.1322805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Cleuren Y. N., Ewe C. K., Chipman K. C., Mears E. R., Wood C. G. et al. , 2019. Extensive intraspecies cryptic variation in an ancient embryonic gene regulatory network. Elife 8: e48220. 10.7554/eLife.48220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst S. E. M., Cravo J., Woollard A., Teapal J., and van den Heuvel S., 2019. C. elegans Runx/CBFβ suppresses POP-1 TCF to convert asymmetric to proliferative division of stem cell-like seam cells. Development 146: dev180034. 10.1242/dev.180034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Seah A., and Sternberg P. W., 2010. Re-programming of C. elegans male epidermal precursor fates by Wnt, Hox, and LIN-12/Notch activities. Dev. Biol. 345: 1–11. 10.1016/j.ydbio.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were analyzed and presented with the R programming environment or GraphPad Prism 7. Two-sample Student’s t-tests were performed for differences in mean seam cell number. Binomial tests were performed to test for differences in the proportions of seam cell symmetrization events between strains and/or treatments. All statistics were carried out in R version 3.2.0. All reagents and strains are available upon request. Nematode strains are listed in Table S1. Table S2 contains oligo sequences used as smFISH probes in this study. Table S3 contains raw counts from smFISH experiments presented in Figure 2, Figure 3, Figure 4, and Figure S3. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11687178.
Figure 2.
The Hox gene mab-5 is necessary and sufficient for seam cell duplications. (A) The loss-of-function allele mab-5(e1239) suppresses anterior V6 lineage seam cell duplications when compared to WT (N2) at 25° (***P < 0.001 with a binomial test), while the lin-39(n709) or egl-5(n945) loss-of-function mutations do not display a similar effect, n ≥ 78. Error bars indicate SE of the proportion. (B and C) Gain-of-function mab-5(e1751) mutants (triangle points) show significant increases in duplications at the L4 stage across seam cell lineages, at both 20° and 25° compared to WT (circle points) animals (*** correspond to P < 0.001 with a binomial test, n ≥ 40). (D) mab-5 mRNA levels at the L4 stage in V5ppppa/V5ppppp, V6pappa/V6pappp, and V6ppppa/V6ppppp cells measured by smFISH in animals grown at 25° (red circles, n = 28) compared to 20° animals (blue circles, n = 29). Significance of changes was tested with a two-sample Student’s t-test (*P < 0.05, **P < 0.01). Error bars indicate SEM. L4, fourth larval; mRNA, messenger RNA; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.
Figure 3.
The β-catenin bar-1 is necessary for anterior V6 lineage seam cell duplications. (A) Anterior V6 lineage seam cell duplications were significantly suppressed in bar-1(ga80) mutants at 25° compared to WT animals grown at 20° (***P < 0.001, binomial test, n ≥ 120). No suppression was seen in egl-20(hu105) and lin-17(n671) mutants, n ≥ 30. (B) The proportion of animals displaying anterior V6 lineage seam cell duplications in WT and mab-5(e1751gf) animals was significantly decreased when grown at 25° on bar-1 RNAi (*P < 0.05 and ***P < 0.001, n ≥ 40). (C) mRNA levels of bar-1, measured by smFISH at the L4 stage. Counts in V6pappa cells are not significantly different between animals grown at 20° and 25° (n ≥ 30). Error bars indicate SE of the proportion (A and B) or mean (C). L4, fourth larval; mRNA, messenger RNA; RNAi, RNA interference; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.
Figure 4.
Wnt pathway asymmetry characterization in posterior seam cell lineages at the L4 stage. (A) Representative images of egl-18 smFISH (black dots correspond to mRNAs) in V6papp and V6pppp daughter cells at 20° and 25°. Seam cells are marked green due to expression of the scm::GFP marker. (B) Quantification of egl-18 mRNA levels in V5pppp, V6papp, and V6pppp daughters at 20° and 25°. Expression was significantly lower in posterior cells, i.e., V5ppppp, V6pappp, and V6ppppp at 25° (red circles) compared to the same seam cells grown at 20° (blue circles, **P < 0.01 and ***P < 0.001 with a two-sample Student’s t-test, n > 20 per cell). At low frequency, animals grown at 25° showed extreme expression values in V6pappa and V5ppppa cells, which were even higher than their posterior counterparts (cell pairs are indicated by black lines). (C) Seam cell duplications at anterior V6 lineage are suppressed in the egl-18(ga97) mutant at 25° (***P < 0.001 with a binomial test, n = 120). (D) Representative images of nuclear POP-1::GFP expression at 20° and 25° at the L4 stage. (E) The ratio of nuclear POP-1::GFP expression between V6papp and V6pppp daughter cells at 20° and 25°, n ≥ 22 (*P < 0.05 and ***P < 0.001, two-sample Student’s t-test, error bars indicate 95% C.I.s of the mean). In both cases, anterior/posterior cell fluorescence intensity ratio is shown (simplified as “aa”/“ap” for V6papp daughters and “pa”/“pp” for V6pppp). (F) Average POP-1::GFP intensity in V6papp and V6pppp daughter cells at 20° and 25° (error bars represent SEM, *P < 0.05 and **P < 0.001, with a two-sample Student’s t-test, n ≥ 22). L4, fourth larval; mRNA, messenger RNA; RNAi, RNA interference; smFISH, single-molecule fluorescence in situ hybridization; WT, wild-type.