Abstract
Epithelial cells form intercellular junctions to strengthen cell–cell adhesion and limit diffusion, allowing epithelia to function as dynamic tissues and barriers separating internal and external environments. Junctions form as epithelial cells differentiate; clusters of junction proteins first concentrate apically, then mature into continuous junctional belts that encircle and connect each cell. In mammals and Drosophila, atypical protein kinase C (aPKC) is required for junction maturation, although how it contributes to this process is poorly understood. A role for the Caenorhabditis elegans aPKC homolog PKC-3 in junction formation has not been described previously. Here, we show that PKC-3 is essential for junction maturation as epithelia first differentiate. Using a temperature-sensitive allele of pkc-3 that causes junction breaks in the spermatheca and leads to sterility, we identify intragenic and extragenic suppressors that render pkc-3 mutants fertile. Intragenic suppressors include an unanticipated stop-to-stop mutation in the pkc-3 gene, providing evidence for the importance of stop codon identity in gene activity. One extragenic pkc-3 suppressor is a loss-of-function allele of the lethal(2) giant larvae homolog lgl-1, which antagonizes aPKC within epithelia of Drosophila and mammals, but was not known previously to function in C. elegans epithelia. Finally, two extragenic suppressors are loss-of-function alleles of sups-1—a previously uncharacterized gene. We show that SUPS-1 is an apical extracellular matrix protein expressed in epidermal cells, suggesting that it nonautonomously regulates junction formation in the spermatheca. These findings establish a foundation for dissecting the role of PKC-3 and interacting genes in epithelial junction maturation.
Keywords: adherens junction, cell polarity, suppressor, stop codon, aPKC, kinase
EPITHELIAL cells sculpt and line our organs, providing an interactive and protective interface that separates internal from external environments. Each cell within an epithelium links to its neighbors through different types of intercellular junctions. Adherens junctions (AJs) are composed of the transmembrane adhesion protein E-cadherin and its associated catenins, which couple the E-cadherin cytoplasmic domain to the underlying actomyosin cytoskeleton (Mege and Ishiyama 2017). Homophilic E-cadherin interactions link AJs on adjacent cells, providing an intercellular adhesive and mechanical coupling that enables tissue formation and morphogenesis (Takeichi 2014; Pinheiro and Bellaiche 2018). Consequently, in both vertebrate and invertebrate embryos, disruption of core AJ components such as E-cadherin leads to developmental arrest as the first epithelia form (Costa et al. 1998; Harris and Peifer 2004; Stephenson et al. 2010). Tight junctions (TJs), which include membrane-spanning proteins, such as claudins, occludins and JAM-A, form a semipermeable diffusion barrier between cells that limits water loss and excludes pathogens (Van Itallie and Anderson 2014; Balda and Matter 2016).
Junctions form along the lateral surfaces of epithelia at characteristic asymmetric positions that vary depending on the organism and cell type (St Johnston and Ahringer 2010; Chen et al. 2018). Junction biogenesis requires the prior establishment of apicobasal polarity, which is elaborated by a network of interacting proteins that collaborate to define distinct apical, junctional, and basolateral plasma membrane domains. The PAR polarity proteins Par3, Par6, and aPKC (atypical protein kinase C) promote the formation of the junctional (Par3) and apical (Par6 and aPKC) domains. Par3 is a large multi-PDZ domain scaffolding protein that helps concentrate junction proteins such as E-cadherin into clusters at an apical region of the lateral surfaces of cells (Etemad-Moghadam et al. 1995; Harris and Peifer 2005; Achilleos et al. 2010; Franz and Riechmann 2010). Par3 also promotes the apical localization of Par6, which, in turn, mediates the maturation of junctions into continuous belts that encircle the cell (Totong et al. 2007; Franz and Riechmann 2010).
The role of Par6 in epithelia is highly conserved. For example, removing Par6B function from human bronchial epithelial cells, or PAR-6 from Caenorhabditis elegans epidermal cells, produces a remarkably similar defect in junction maturation (Totong et al. 2007; Wallace et al. 2010). Par6 likely functions by localizing aPKC and promoting its kinase activity. Par6 and aPKC interact directly through their PB1 domains (Joberty et al. 2000; Lin et al. 2000; Noda et al. 2001; Suzuki et al. 2001). In addition, Par6 contains a semi-CRIB (Cdc42/Rac interactive binding) domain and adjacent PDZ domain that bind active Cdc42 Rho GTPase (Joberty et al. 2000; Lin et al. 2000; Noda et al. 2001). Binding of active Cdc42 to Par6 is thought to stimulate the kinase activity of aPKC (Yamanaka et al. 2001; Atwood et al. 2007). However, at least in vitro, Par6 is able to activate aPKC even in the absence of Cdc42 (Graybill et al. 2012), suggesting that aPKC kinase activity may be regulated by layered mechanisms.
It remains poorly understood how aPKC promotes junction maturation. In cultured mammalian cells, aPKC phosphorylates the TJ proteins JAM-A and occludin, which promotes junction maturation through an unknown mechanism (Jain et al. 2011; Iden et al. 2012). aPKC can also regulate junctions by phosphorylating the myosin activator ROCK, inhibiting its function to prevent apical constriction (Ishiuchi and Takeichi 2011), although it is not known whether aPKC regulates ROCK in a similar fashion during junction maturation. It remains possible that aPKC contributes to junction maturation in large part indirectly, through its well-established regulation of other polarity proteins, such as the basolateral polarity regulator Lgl (Yamanaka et al. 2003; Hutterer et al. 2004; Dong et al. 2015; Visco et al. 2016; Almagor et al. 2019).
Despite the importance of C. elegans as a model for understanding mechanisms of cell polarity and epithelial cell formation, the role of the sole aPKC homolog PKC-3 has been well-defined only in the zygote, where it functions with PAR-6 to mediate anterior–posterior polarization (Tabuse et al. 1998; Lang and Munro 2017). Epithelial cells first differentiate midway through embryogenesis when junction proteins and polarity proteins cluster at cell contacts and move toward the apical surface (Köppen et al. 2001; McMahon et al. 2001; Achilleos et al. 2010). C. elegans AJs and TJs are present in overlapping zones near the apicolateral surface, with AJs forming at a slightly more apical position (Köppen et al. 2001; McMahon et al. 2001). PAR-6 localizes to apical surfaces and promotes the maturation of nascent junctions from clusters of proteins into continuous belts (Totong et al. 2007). PKC-3 colocalizes with PAR-6 within epithelial cells (Leung et al. 1999; Bossinger et al. 2001; McMahon et al. 2001), but its role in junction formation has not been examined. Surprisingly, CDC-42 is not required for junction maturation (although it is needed for junction remodeling during elongation) (Zilberman et al. 2017), suggesting either that CDC-42 is not essential for PKC-3 activation in epithelial cells, or that PAR-6 regulates junction maturation in C. elegans, at least in part, independently of PKC-3.
Here, we use targeted protein degradation to show that PKC-3, like PAR-6, is required for junction maturation in C. elegans epithelial cells. Taking advantage of a conditional hypomorphic allele of pkc-3 that causes discontinuities in spermathecal junctions and results in sterility, we perform a large-scale suppressor screen to identify pkc-3 modifier mutations. Our characterization of several suppressor mutations reveals an unexpected role for pkc-3 stop codon choice; for the first time, establishes a function for the lethal(2) giant larvae homolog lgl-1 in regulating pkc-3 in C. elegans epithelial cells; and identifies a surprising link between pkc-3 and an epidermal apical extracellular matrix (aECM) protein that nonautonomously suppresses spermathecal junction defects caused by pkc-3 reduction of function. These findings establish a foundation for investigating the role of C. elegans PKC-3 and interacting genes in promoting epithelial junction maturation.
Materials and Methods
Strains
N2 was used as the wild type. Strain and transgene genotypes are listed in Table 1.
Table 1. Transgenes and strains.
| Name | Genotype | Reference |
|---|---|---|
| Transgenes | ||
| naIs8 | lim-7p::mCherry, (Cb)unc-119(+) | Voutev et al. (2009) |
| sIs10089 | pgp-12p::GFP, dpy-5(+), pCeh361 | Zhao et al. (2005) |
| xnIs131 | pie-1p::pkc-3, unc-119(+) | This study |
| xnEx487 | sups-1p::sups-11−351::gfp, unc-119(+) | This study |
| xnEx531 | sups-1p::sups-11−44::gfp, unc-119(+) | This study |
| xnEx533 | pkc-3(+), sur-5p::sur-5::gfp | This study |
| xnEx534 | pkc-3(ne4250), sur-5p::sur-5::gfp | This study |
| xnEx536 | sups-1p::gfp::H2B, pRF4 | This study |
| Strains | ||
| CB398 | mec-8(e398) | Chalfie and Sulston (1981) |
| DP38 | unc-119(ed3) | Maduro and Pilgrim (1995) |
| FT328 | pkc-3(ne4250); unc-119(ed3); xnIs131 | This study |
| FT355 | sups-1(xn7); pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT382 | pkc-3(ne4250xn8); xnIs131; unc-119(ed3) | This study |
| FT383 | pkc-3(ne4250xn9); xnIs131; unc-119(ed3) | This study |
| FT384 | pkc-3(ne4250xn10); xnIs131; unc-119(ed3) | This study |
| FT385 | pkc-3(ne4250xn11); xnIs131; unc-119(ed3) | This study |
| FT386 | pkc-3(ne4250xn12); xnIs131; unc-119(ed3) | This study |
| FT436 | pkc-3(ne4250); naIs8 | This study |
| FT1430 | pkc-3(ne4250); sIs10089 | Armenti et al. (2014a) |
| FT1351 | sups-1(xn20); pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1352 | xn21; pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1353 | xn22; pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1354 | xn23; pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1356 | xn25; pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1358 | xn27; pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1360 | pkc-3(ne4250xn28); xnIs131; unc-119(ed3) | This study |
| FT1377 | pkc-3(ne4250xn35); xnIs131; unc-119(ed3) | This study |
| FT1382 | lgl-1(xn37); pkc-3(ne4250); xnIs131; unc-119(ed3) | This study |
| FT1931 | pkc-3(xn84[zf1::gfp::pkc-3]) | This study |
| FT1949 | sups-1(xn87[sups-1::yfp]) | This study |
| FT1950 | pkc-3(ne4250); lgl-1(dd21) | This study |
| FT1952 | unc-119(ed3); xnEx487 | This study |
| FT1991 | dpy-10(cn64) pkc-3(it309[gfp::pkc-3]); lgl-1(xn103[lgl-1::zf1::mScarlet]) | This study |
| FT2143 | pkc-3(ne4250); xnEx533 | This study |
| FT2144 | pkc-3(ne4250); xnEx534 | This study |
| FT2145 | hmr-1(cp21[hmr-1::gfp]); pkc-3(ne4250) | This study |
| FT2146 | hmr-1(cp21[hmr-1::gfp]); pkc-3(ne4250); sups-1(xn20CRISPR) | This study |
| FT2147 | pkc-3(ne4250xn8CRISPR) | This study |
| FT2148 | pkc-3(ne4250); sups-1(xn20CRISPR) | This study |
| FT2149 | unc-119(ed3); xnEx531 | This study |
| FT2150 | noah-1(mc68[noah-1::mCherry]); sups-1(xn87[sups-1::yfp]) | This study |
| FT2161 | sups-1(xn20CRISPR) | This study |
| FT2162 | pkc-3(it309xn8CRISPR[gfp::pkc-3TAA]) | This study |
| FT2165 | xnEx536 | This study |
| FT2184 | pkc-3(ne4250); sym-1(mn601); him-8(e1489) | This study |
| JJ1610 | pkc-3(ok544)/mIn1[dpy-10(e128) mIs14]; him-8(e1489) | This study |
| KK1228 | pkc-3(it309[gfp::pkc-3TGA]) | A gift from Ken Kemphues |
| LP172 | hmr-1(cp21[hmr-1::gfp]) | Marston et al. (2016) |
| SP2163 | sym-1(mn601) | Davies et al. (1999) |
| WM150 | lin-11(n566); pkc-3(ne4246) | A gift from Craig Mello |
| WM151 | pkc-3(ne4250) | A gift from Craig Mello |
Suppressor screen
pkc-3(ne4250); xnIs131 [pie-1p::pkc-3] worms grown at 15° were mutagenized with ethyl methanesulfonate as described (Brenner 1974). F1 embryos were collected and split onto multiple plates at 15° so that independent mutations could be isolated from the same mutagenesis. F2 embryos from each F1 plate were plated at 25.5° onto several 10 cm peptone-enriched plates seeded with NA22 Escherichia coli. Plates were kept at 25.5° for 2 weeks. Plates containing worms with a suppressor mutation had numerous larvae, whereas plates without a suppressor mutation contained sterile F2 worms. Only one suppressor was saved from the progeny of a given F1 plate and was maintained at 25.5° to establish a suppressor strain. Three independent mutagenesis screens were performed.
To initially quantify the penetrance of each suppressor, L1 worms were singled to individual wells in a seeded NGM agar 24-well plate, grown at 25.5° for 48 hr, and scored for the presence or absence of F1. Worms were scored as sterile only if no F1 embryos or larvae were present in the well. This assay was used in subsequent experiments to quantify suppression or rescue phenotypes.
Genetic analysis of suppressors
The dominance of extragenic suppressors was assessed by crossing pkc-3(ne4250); sIs10089 males to each suppressor strain (in the pkc-3(ne4250); xnIs131 genetic background) at 15°. Outcross F1 hermaphrodites (recognized by GFP expression in the excretory cell) were singled to individual plates at the L1 stage and allowed to grow at 25° for 48 hr, then scored for sterility as described above. Control uncrossed suppressors were grown simultaneously under the same conditions. Suppressors were classified as recessive if heterozygotes had significantly lower fertility than homozygous suppressors (P ≤ 0.05, Fisher’s exact test).
Linkage of xn8 to pkc-3(ne4250) was tested by crossing pkc-3(ne4250xn8); xnIs131 [pie-1p::pkc-3] hermaphrodites to naIs8 males. Outcross hermaphrodite F1 progeny (those expressing mCherry) were allowed to self-fertilize, and F2 hermaphrodites raised at 25° were analyzed for sterility. All pkc-3 homozygotes were fertile (27/27), indicating that the xn8 suppressor mutation is linked to pkc-3(ne4250).
Complementation tests between xn7 and either xn7 (positive control), xn20, or xn37 (negative control) were performed by crossing pkc-3(ne4250); xnIs131 [pie-1p::pkc-3]; xn7/Ø; sIs10089/+ males with pkc-3(ne4250); xnIs131 [pie-1p::pkc-3]; sup/sup hermaphrodites (where sup is xn7, xn20, or xn37). Outcross F1 hermaphrodites (those expressing GFP) were raised at 25.5° and scored for sterility. F1 suppressor genotype: xn7/xn7 (positive control): 88% fertile (38/43), xn7/xn20: 84% fertile (56/67), + xn7/xn37 + (negative control): 5% fertile (1/19), no suppressor [pkc-3(ne4250) alone]: 0% fertile (0/59). Probability of complementation (Fisher’s exact test): xn7 vs. xn7: P ≤ 0.001, xn7 vs. xn20: P ≤ 0.001, xn7 vs. xn37: P = 0.25.
Amplifying and sequencing pkc-3 and lgl-1 in suppressor strains
To identify intragenic suppressor mutations, the complete pkc-3 genomic locus was amplified using primers 5′-CAGTTTCGCAGTTTTCGTTCAG-3′ and 5′-CCACGAGTTAGTGTGTCATAG-3′ (3803 bp) and sequenced. In addition to the H390Q mutation in the kinase domain, pkc-3(ne4250) contains an E9K missense mutation near the N-terminus outside of any known functional domains. However, we consider it unlikely that this mutation contributes to the temperature-sensitive sterile phenotype, since the pkc-3(ne4246) D386V mutation (Fievet et al. 2013) also causes temperature-sensitive sterility when worms are upshifted to 25° at the L1 stage (24/24 worms). The lgl-1 locus was amplified in two overlapping fragments in all remaining extragenic suppressors using primers 5′-ATGAGCAGCATCTTACGATTTAT-3′ and 5′-CAAAACTAGATGCGCCGT-3′ (3512 bp); 5′-GCCAGCAAATCCTATTGG-3′ and 5′-CTATGACTTGTGCGTACTGCTC-3′) (2639 bp).
Whole genome sequencing and mutation identification
Genomic DNA was isolated from washed worms using the Puregene Core Kit A (Qiagen). gDNA libraries were constructed using the KAPA library preparation kit (KAPA Biosystems), and samples were sequenced using an Illumina HiSeq 2500 Sequencer and 50- or 100-bp paired-end reads. Coverage ranged from 24x to 50x. The workflow for variant calling used the following software: FastQC for quality control, BWA for mapping (Li and Durbin 2010), SAMTOOLS for sorting (Li et al. 2009), PICARD to remove duplicates, GATK to call and filter variants (McKenna et al. 2010), and Bedtools to compare to reference (Quinlan and Hall 2010). Integrative Genomics Viewer (IGV) was used to browse alignments and verify mutations. For xn7 and xn20, MAQGene (Bigelow et al. 2009) was also used to annotate the variant call list.
CRISPR/Cas9 genome editing
For genome editing with RNP complexes, Cas9 protein (UC-Berkeley), tracrRNA (IDT), and custom-designed crRNAs (IDT) were injected into worm gonads with PCR product or ssDNA oligo (IDT) repair templates as described (Paix et al. 2016). Co-CRISPR targeting the dpy-10 locus was used to identify candidate edited F1 worms, which were screened by PCR for the desired edit and verified by sequencing.
pkc-3(xn84[zf1::gfp::pkc-3]):
pkc-3(it309[gfp::pkc-3]) worms were injected using a crRNA with target homology sequence 5′-CCGGTAGAAAAAATGAGTAA-3′ and a repair ssDNA oligo containing sequences encoding the ZF1 domain and GGGCCC linker: 5′-AGTTCAATTTTTATTTCAGAGTACCGGTAGAAAAAATGACAGAATACAAAACGCGACTTTGTGATGCGTTCCGCCGTGAAGGATACTGCCCGTACAACGACAATTGCACATATGCTCACGGACAAGATGAGCTGAGAGTTCCGAGGGTAGGGCCCATGAGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCC-3′. pkc-3(xn84[zf1::gfp::pkc-3]) was outcrossed to N2 to remove a linked dpy-10(cn64) mutation that was introduced by co-CRISPR during genome editing.
pkc-3(ne4250xn8CRISPR):
pkc-3(ne4250) worms were injected using a crRNA with target homology sequence 5′-TGGGACTCGGGGGATGGTGG-3′ and ssDNA oligo repair template 5′-TGAATCCTCTACAAATGAGTCGGGAAGATTCAGTCTAATTGCCCCACCATCCCCCGAGTCCCAAATATTTATTTTC-3′.
lgl-1(xn103[lgl-1::zf1::mScarlet]):
pkc-3(it309[gfp::pkc-3]) worms were injected using a crRNA with target homology sequence 5′-ACGGTGAATTTGAACTTTCG-3′. zf1::mScarlet dsDNA repair template with ∼35 bp homology arms was prepared using primers 5′-TCCGAACTACAGCGAAGTACGGTGAATTTGAACTTTAGAGGTTGGTCATCCACCCATAAAAGCGGGCCCACAGAATACAAAACGCGACTTTGTG-3′ and 5′-CAACAGGAAAACGATTTTTAAAAAAAATGCATCTACTTGTAGAGCTCGTCCATTCCTC-3′ and plasmid pJA047 as a template (a gift from Joshua Abrams). F1 worms with the co-CRISPR dpy-10(cn64) mutation were screened by fluorescence and verified by PCR and sequencing.
sups-1(xn20CRISPR):
pkc-3(ne4250) worms or N2 worms were injected using a crRNA with target homology sequence 5′-AGCTTTCGAAGCATTAGTCT-3′ and ssDNA oligo 5′-GTAATCAAGCATACCTCGCGGAGCTTTCGAAGCATTAGTCTGAACTGAATGCGTACAGCGCTAAACAGAT-3′.
To create sups-1(xn87[sups-1::yfp]), a plasmid derived from pDD122 (Dickinson et al. 2013) expressing Cas9 and a sgRNA with target homology sequence 5′-GATACGGATGACTTTAATAGG-3′, and repair template derived from pSA122 (Armenti et al. 2014b) containing yfp and 1.5 kb homology arms, were injected into unc-119(ed3) worms as described (Dickinson et al. 2013). A strain carrying the insertion was genotyped by sequencing PCR product amplified with primers 5′-CTTTAATAGGCGACACTTTGATGATGTCTC-3′ and 5′-ATGTCAGAAGTGATTTGGTATGCCGTACTC-3′.
RNAi
RNAi was performed by the bacterial feeding method as described (Kamath et al. 2001). For L4 larvae feeding experiments, larvae were placed on RNAi plates seeded with HT115 bacteria transformed with RNAi clones and allowed to feed for 48 hr at 25.5° before scoring progeny for sterility as described above. For L1 feeding experiments, larvae were placed on feeding plates at the L1 stage, grown at 25.5°, and scored for fertility in the same generation. Efficacy of sups-1 RNAi in L1 feeding experiments was determined by verifying that simultaneous L4 feeding experiments suppressed the sterility of pkc-3(ts). lgl-1 RNAi clone F56F10.4 from the Ahringer RNAi Library (Kamath et al. 2003), sups-1 RNAi clone F22F4.1 from the Vidal RNAi Library (Rual et al. 2004), and negative control vector pPD129.36 (Timmons and Fire 1998) were used to express dsRNAs.
Transgene construction
pkc-3p::pkc-3 plasmid pJN299 was generated by cloning a genomic fragment containing the pkc-3 gene, 7723 bp upstream of the ATG, and 1541 bp downstream of the stop codon, into pBluescript. pkc-3p::pkc-3(ts) plasmid pSA001 was generated from pJN299 by PCR-mediated site-directed mutagenesis and the following primers: 5′-GTTCTGATTGACGCTGAAGGACAAATAAAACTGACAGATTATGG-3′ and 5′-CCATAATCTGTCAGTTTTATTTGTCCTTCAGCGTCAATCAGAAC-3′.
sups-1p::sups-11–44::gfp plasmid pJM31 and sups-1p::sups-11–351::gfp plasmid pJM32 were generated by Gibson assembly (Gibson et al. 2009) from sups-1p::sups-1::gfp plasmid pJM25, which contains 4310 bp 5′ of the sups-1 ATG and 842 bp 3′ of the sups-1 stop codon. For sups-1p::sups-11–44::gfp, all of sups-1 coding sequence except 179 bp downstream of the ATG were removed. For sups-1p::sups-11−351::gfp, all of sups-1 coding sequences except 1476 bp downstream of the ATG were removed.
sups-1p::gfp::H2B plasmid (pJM43) was generated by Gibson assembly by flanking GFP-H2B coding sequences with the 5′ and 3′ sups-1 genomic regulatory sequences from plasmid pJM25 (see above).
Worm transformation
Injections to create extrachromosomal arrays were performed as described (Mello et al. 1991). sups-1p::sups-11–44::gfp and sups-1p::sups-11–351::gfp were microinjected at 20 ng/µl together with unc-119(+) rescue plasmid (80 ng/μl) pJN254 (Nance et al. 2003) into unc-119(ed3) young adult worms. pkc-3p::pkc-3 and pkc-3p::pkc-3(ne4250) were microinjected at 20 ng/µl with sur-5::gfp (80 ng/μl) into pkc-3(ne4250) young adult worms.
Imaging and image analysis
Images were taken on a Leica confocal microscope (SP5II or SP8), using a 63 × 1.2 NA water-immersion objective lens, or, alternatively, on a Zeiss AxioImager using a 40x 1.3NA oil-immersion objective lens and Axiocam MRM camera. Measurements were performed using ImageJ (National Institutes of Health). Images were cropped, rotated, and levels were adjusted in ImageJ and Adobe Photoshop.
To immobilize embryos expressing SUPS-1::YFP fusion proteins during late embryogenesis (when twitching), embryos were placed in a custom-designed mounting chamber and perfused with 3–5 p.s.i. nitrogen gas. The top of the chamber was fitted with a #1.5 coverslip. Embryos were pipetted onto the coverslip and covered with a 3% agarose pad to prevent desiccation. After ∼10 min in nitrogen, embryos ceased moving, but resumed movement and completed development once the chamber was vented with room air.
Spermathecal junction analysis of HMR-1::GFP
L1 larvae were isolated from a 3-hr hatch off at 20° and transferred to new plates at 25.5° for 37–40 hr, until reaching the late L4 stage. Worms were then mounted in 5 μM levamisole on a 5% agar pad and imaged 20 min later. Control and experimental worms were grown and analyzed simultaneously.
DAPI staining of adult worms
L1 larvae were isolated from a 3-hr hatch off at 20° and transferred to new plates at 25.5° for 37–40 hr, until reaching the late L4 stage. Worms were then collected in siliconized 1.5-ml Eppendorf tubes, supernatant was aspirated, then worms were resuspended in 95% ethanol and fixed for 5 min. Supernatant was aspirated and a drop of Vectashield mounting medium containing DAPI was added, as described (Pepper et al. 2003). Worms in mounting medium were placed on 5% agar pads, covered for microscopy, and imaged on a Zeiss AxioImager with an Apotome to obtain confocal sections.
Quantifying expression levels of GFP::PKC-3
Images were acquired using a Leica SP8 confocal microscope, 63 × 1.4 NA oil-immersion objective, 488 nm laser, 4x zoom, and 0.7 μm z-interval and photon-counting mode. Intensities were measured using the FIJI(NIH) software. For vulva measurements, a 1.2 µm line was drawn along the apical membranes of the vulF cells, and the average intensity was measured. Both measurements were averaged per vulva/worm. Stages L4.4 through L4.8 were used for intensity measurements (Mok et al. 2015).
Antibody staining
Embryos were fixed for 20 min in −20° methanol and 5 min in 3.7% paraformaldehyde in salts, and immunostained as described (Anderson et al. 2008). L4 larvae were dissected onto poly-l-lysine coated slides to liberate the gonad, and fixed in methanol and aldehydes as above. Primary antibodies used were Rabbit anti-PAR-6 1:20 (Schonegg and Hyman 2006), Mouse anti-PSD-95 (recognizes DLG-1) 1:200 (Affinity BioReagents), Chicken anti-GFP 1:1000 (Aves), and Rabbit anti-GFP 1:1000 (Abcam). Species-specific secondary antibodies conjugated with Alexa488 (Molecular Probes) or Cy-3 (Jackson ImmunoResearch) dyes were used for detection.
Aligning and mapping PKC-3 residues onto PKC-3ι structure
Sequences of PKC-3 and aPKC orthologs were obtained from Wormbase (https://wormbase.org) and Universal Protein Resource (UniProt) (https://www.uniprot.org/) databases. Sequences were aligned using the Multiple Sequence Comparison by Log-Expectation (MUSCLE) tool (Madeira et al. 2019). We utilized kinase domain alignments to map PKC-3 mutations onto the homologous resides of the human aPKCι kinase domain structure (Messerschmidt et al. 2005), which was visualized and exported using Protean 3D software (DNASTAR).
Statistical analysis
Statistical analysis was performed in GraphPad Prism 8. Statistical tests and sample sizes are indicated in Results, and in the figures and figure legends. Corrections for comparison of multiple samples are indicated when used. No statistical method was used to predetermine sample size.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental Data (Movie 1) has been uploaded to the GSA Figshare portal. WGS of sup-1(xn7) and sups-1(xn20) are deposited at NCBI as Sequence Read Archive data (xn7: SRR9969397; xn20: SRR9988268). Supplemental material available at figshare: https://doi.org/10.25386/genetics.9637079.
Results
PKC-3 promotes junction maturation in epithelial cells
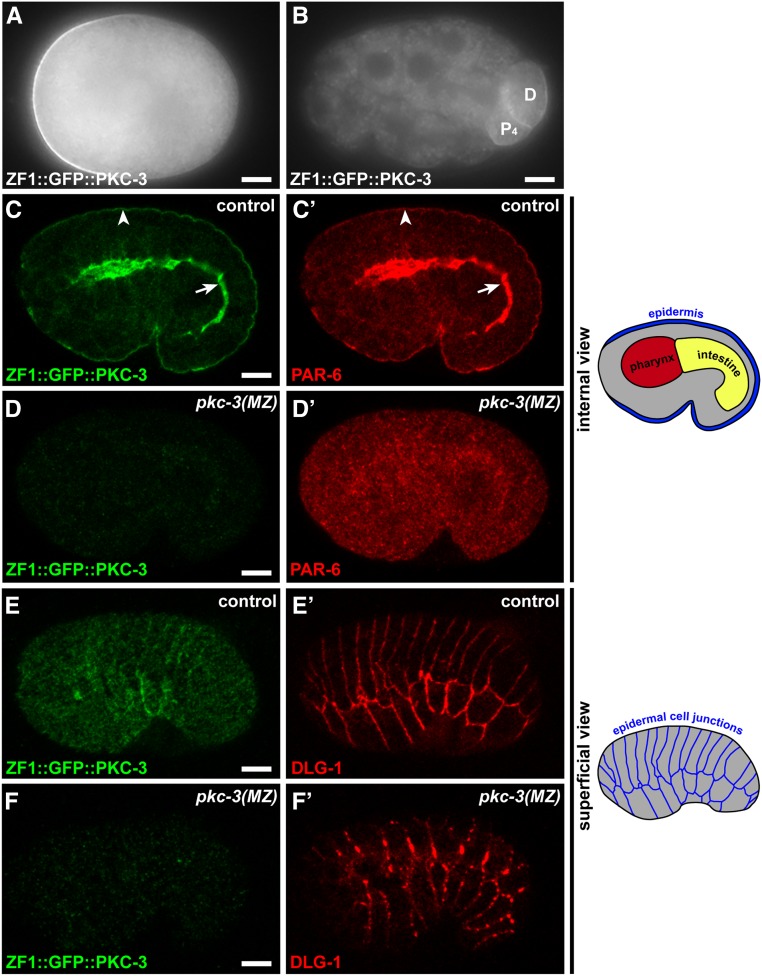
To determine whether PKC-3 is required for epithelial junction formation, we employed a degron strategy to selectively remove PKC-3 protein from embryos after its role in anterior–posterior polarization of the one-cell embryo was complete. Maternally provided proteins containing the ZF1 degron are rapidly degraded from early embryonic somatic cells when they are recognized by the E3 ubiquitin ligase substrate adaptor protein ZIF-1 (Reese et al. 2000; DeRenzo et al. 2003; Nance et al. 2003). Because endogenous ZIF-1 activity is limited to very early embryos, ZF1-tagged proteins that are also expressed zygotically reappear in later embryos and are not degraded (Nance et al. 2003; Armenti et al. 2014b). Using CRISPR/Cas9, we inserted sequences encoding the ZF1 degron into a functional gfp::pkc-3 knock-in allele (Rodriguez et al. 2017; Wang et al. 2017). zf1::gfp::pkc-3 worms were fertile and produced viable embryos (embryonic lethality: zf1::gfp::pkc-3, 3/501; wild type, 8/647), indicating that zf1::gfp::pkc-3 is a functional pkc-3 allele. In the one-cell embryo, maternally provided ZF1::GFP::PKC-3 enriched at the anterior cortex (Figure 1A), like endogenous PKC-3 (Tabuse et al. 1998), but by the 24-cell stage ZF1::GFP::PKC-3 had degraded to undetectable levels in somatic cells (Figure 1B). Zygotically expressed ZF1::GFP::PKC-3 reappeared during the middle stages of embryogenesis at the apical surfaces of differentiating epithelia, including the epidermis (Figure 1C, arrowhead), pharynx, and intestine (Figure 1C, arrow), where it colocalized with PAR-6 (Figure 1C’). Thus, the zf1::gfp::pkc-3 allele provides a mechanism for clearing endogenously tagged maternal PKC-3 protein from the early embryo.
Figure 1.
PKC-3 is required for PAR-6 localization and epithelial junction maturation. (A) One-cell stage embryo. ZF1::GFP::PKC-3 is enriched at the anterior cortex. (B) 24-cell stage embryo. ZF1::GFP::PKC-3 has degraded from all embryonic cells except for the germline precursor (“P4”) and its recently born sister cell (“D”). (C–C’) ZF1::GFP::PKC-3 and PAR-6 localization in a central plane of a fixed and immunostained comma-stage embryo. Both proteins colocalize at apical surfaces of epithelia, including the epidermis (arrowhead) and intestine (arrow); see schematic at right for positions of major classes of epithelial cells. (D–D’) ZF1::GFP::PKC-3 is not detected in a pkc-3(MZ) embryo and PAR-6 apical enrichment is lost. (E–E’) ZF1::GFP::PKC-3 and DLG-1 in a superficial view of the epidermis in a comma stage embryo. DLG-1 forms continuous belts around each epidermal cell; see schematic at right. (F–F’) ZF1::GFP::PKC-3 and DLG-1 in a pkc-3(MZ) embryo; maternal ZF1::GFP::PKC-3 is degraded and DLG-1 accumulates in apicolateral clusters rather than continuous belts. Bar, 10 µm.
To generate embryos lacking both maternal and zygotic PKC-3, we self-fertilized hermaphrodites that were heterozygous for the zf1::gfp::pkc-3 allele and the pkc-3(ok544) deletion allele (Alan et al. 2013), which lacks sequences encoding the kinase domain, and examined homozygous pkc-3(ok544) mutant progeny [hereafter pkc-3(MZ) embryos]. pkc-3(MZ) embryos showed no detectable maternal ZF1::GFP::PKC-3 at the stage when epithelial cells first differentiate (Figure 1D), and PAR-6 protein, which normally colocalizes with PKC-3 at apical surfaces, was instead present within the cytoplasm (30/30 embryos) (Figure 1D’). We used three-dimensional time-lapse differential interference contrast imaging to determine if pkc-3(MZ) embryos had defects in epithelial morphogenesis. Midway through embryogenesis, wild-type embryos elongate fourfold in length when epidermal cells change shape to squeeze the embryo (Vuong-Brender et al. 2016). In contrast to control sibling embryos [zf1::gfp::pkc-3/zf1::gfp::pkc-3 and zf1::gfp::pkc-3/pkc-3(ok544)], which completed elongation when imaged on the same slide (50/51 embryos), all pkc-3(MZ) embryos arrested before the twofold stage of elongation, and developed ruptures in the epidermis that allowed internal cells to extrude from the embryo surface (9/9 embryos) (Movie 1). The arrest phenotype of pkc-3(MZ) embryos is the same as that described previously for par-6(MZ) embryos (Totong et al. 2007).
Given the defects of pkc-3(MZ) embryos in elongation and epidermal cell integrity, we immunostained embryos to determine if they had defective junctions. Whereas the junction protein DLG-1 formed continuous junctional belts around epidermal cells in control sibling embryos (Figure 1E’), DLG-1 in pkc-3(MZ) embryos accumulated within discontinuous clusters at the apicolateral region of epidermal cells (Figure 1F’) (30/30 embryos)—a phenotype indistinguishable from that of par-6(MZ) mutant embryos (Totong et al. 2007). Taken all together, these results indicate that PKC-3 is required for junction maturation, and the similarity in phenotype of pkc-3(MZ) and par-6(MZ) embryos suggests that PKC-3 and PAR-6 function together in this process.
A hypomorphic temperature-sensitive pkc-3 mutation causes sterility
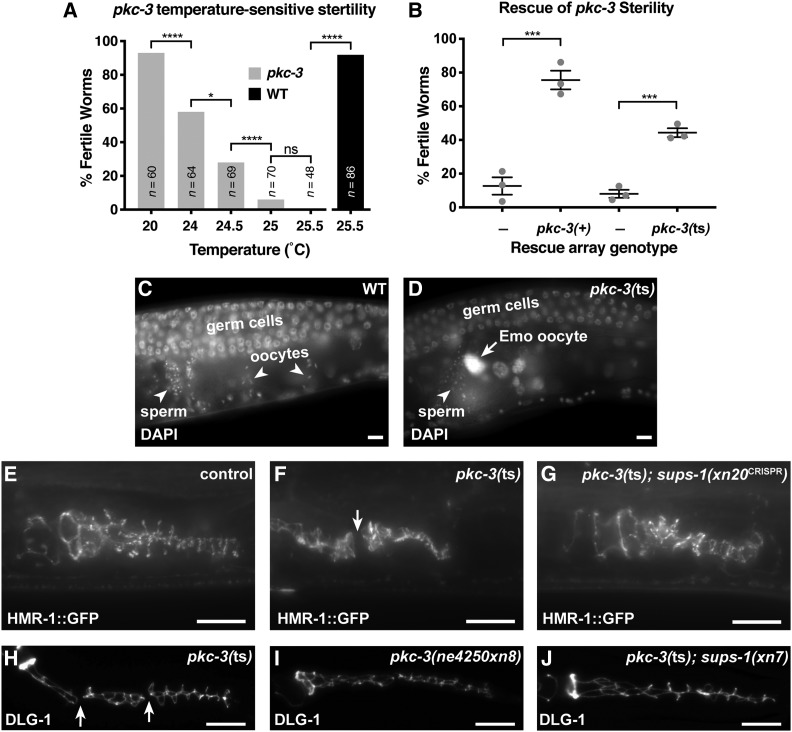
The pkc-3(ne4250) temperature-sensitive missense mutation, which alters the kinase domain of PKC-3, inhibits anterior–posterior polarization of the zygote (Fievet et al. 2013). To determine whether pkc-3(ne4250) mutants [hereafter pkc-3(ts) mutants] have epithelial defects, we allowed pkc-3(ts) mutant embryos to develop at the permissive temperature, and upshifted them to the restrictive temperature at the L1 stage. Several types of epithelial cells form during larval stages, including those of the somatic gonad and reproductive organs (Newman et al. 1996; McCarter et al. 1997; Aono et al. 2004). Upshifted pkc-3(ts) mutants developed a sterile phenotype that increased markedly in penetrance with small changes in temperature (Figure 2A). To confirm that the temperature-sensitive sterile phenotype was caused by the pkc-3(ts) mutation, we performed rescue experiments with extrachromosomal arrays. Wild-type pkc-3 genomic DNA rescued the sterile phenotype of most pkc-3(ts) mutants grown at the restrictive temperature (Figure 2B). An extrachromosomal array expressing the pkc-3(ts) allele was also able to rescue the sterility of pkc-3(ts) mutants at the restrictive temperature (Figure 2B), albeit to a lesser extent than wild-type pkc-3, indicating that the ne4250 mutation does not abolish all pkc-3 activity. These findings show that pkc-3 is required for fertility, and that the pkc-3(ts) mutant is hypomorphic even at the restrictive temperature.
Figure 2.
pkc-3(ts) causes sterility and spermathecal junction gaps that are rescued by suppressors. (A) Fertility of pkc-3(ne4250) worms shifted at the L1 stage to the indicated temperature (gray bars), and fertility of wild-type worms at 25.5° (black bar). ****P ≤ 0.001, *P ≤ 0.05, ns, not significant, Fisher’s exact test. (B) Rescue of sterility of pkc-3(ne4250) worms, shifted at the L1 stage to 25.5°, by extrachromosomal arrays expressing pkc-3(+) (xnEx533) or pkc-3(ne4250) (xnEx534) with sur-5::gfp cotransformation marker. Array-negative control worms are the siblings of array-bearing worms. Gray dots indicate mean fertility from one of three individual experiments, horizontal line is the mean of means, and error bars are the SEM. Sample sizes from three experiments: xnEx533 control (n = 98, 113, 87); xnEx533 (n = 101, 105, 110); xnEx534 control (n = 89, 111, 98); xnEx534, (n = 93, 104, 85). ***P ≤ 0.001, unpaired Welch’s t-test. (C and D) DAPI-stained gonads in fixed young adult wild-type and pkc-3(ts) hermaphrodites. Germ cells (mitotic or early meiotic), oocytes, and sperm are indicated. An endomitotic (Emo) oocyte nucleus in pkc-3(ts) is shown in (D, arrow). (E–G) Endogenously tagged HMR-1::GFP in adherens junctions of the spermatheca of live L4 stage worms of the indicated genotype at 25.5°; control worms are wild-type worms expressing HMR-1::GFP. A break in junctional HMR-1::GFP in a pkc-3(ts) worm is indicated (F, arrow). (H–J). Spermathecal junctions in fixed L4 larvae of the indicated genotype stained for DLG-1; all genotypes also contain the pie-1p::pkc-3 (xnIs131) transgene to circumvent maternal-effect lethality. Breaks in the spermathecal junctions of a pkc-3(ts) mutant are indicated (H, arrow). Bar, 10 µm.
We stained nuclei of sterile pkc-3(ts) young adult hermaphrodites with DAPI to determine if the sterile phenotype could be explained by defects in the germ line or somatic gonad. The gonad is a symmetrical U-shaped tube capped by a distal tip cell at the end of each arm (Hubbard and Schedl 2019). Proliferative germ cells occupying distal regions of each gonad arm enter meiosis and differentiate into gametes in more proximal regions of the gonad. During the last larval stage, germ cells differentiate into sperm, which are stored in the spermatheca (Figure 2C)—an accordion-like epithelial tube that connects the somatic gonad with the uterus and is the site of fertilization. Adults produce only oocytes, which reside in the proximal region of the gonad before they mature, are ovulated into the spermatheca, become fertilized, and exit into the uterus. Gonads of sterile young adult pkc-3(ts) mutants contained proliferating and meiotic germ cells, as well as mature sperm and oocytes (54/54 worms) (Figure 2D). However, in contrast to oocytes in wild type, in which individual chromosomes were visible (38/38 worms) (Figure 2C), most sterile pkc-3(ts) mutants contained polyploid endomitotic (Emo) oocyte nuclei (50/54 worms), which are recognized by intense DAPI staining that fills the nucleus (Figure 2D, arrow). These findings are consistent with a previous report showing that postembryonic RNAi of pkc-3 results in a partially penetrant Emo phenotype (Aono et al. 2004).
The Emo phenotype occurs when oocytes fail to ovulate and become trapped in the proximal gonad (Iwasaki et al. 1996). Given the requirement for pkc-3 in junction maturation in the embryo, we examined pkc-3(ts) mutants to determine if they had defects in spermathecal junctions that might inhibit ovulation. Using a functional hmr-1::gfp knock-in allele (Marston et al. 2016), we visualized spermathecal junctions in late L4 stage control and pkc-3(ts) hermaphrodites. Whereas HMR-1::GFP was enriched in continuous junctions between spermathecal cells in control L4 larvae (38/38 worms) (Figure 2E), nearly half of pkc-3(ts) L4 larvae showed one or more prominent gaps in spermathecal HMR-1::GFP junctional localization (23/48 worms; P < 0.00001, Fisher’s exact test) (Figure 2F, arrow). Since we examined junctions in L4 larvae, before oocyte ovulation begins, spermathecal junction discontinuities in pkc-3(ts) mutants are not a result of mechanical strain induced by oocyte ovulation, but, rather, likely reflect defects in the establishment or maintenance of spermathecal AJs.
To address whether pkc-3 is likely required in spermathecal cells, we examined pkc-3(ts) worms expressing a pkc-3(+) extrachromosomal array. Extrachromosomal arrays are lost at low frequency during mitotic divisions, producing mosaic animals in which descendant cells lacking the array can be recognized by the lack of a nuclear SUR-5::GFP cotransformation marker (Yochem and Herman 2003). We assessed whether the pkc-3(+) rescuing array was present in spermathecal cells in 31 fertile and 23 sterile blindly selected pkc-3(ts); xnEx533 [pkc-3(+), sur-5::gfp] worms. In 30/31 fertile worms, but only 7/23 sterile worms, the array was present within the spermathecal lineage (P = 0.015, Fisher’s exact test), consistent with a requirement for pkc-3 in the spermatheca or lineally related cells such as the sheath cells of the somatic gonad.
A selection screen for suppressors of the pkc-3 sterile phenotype
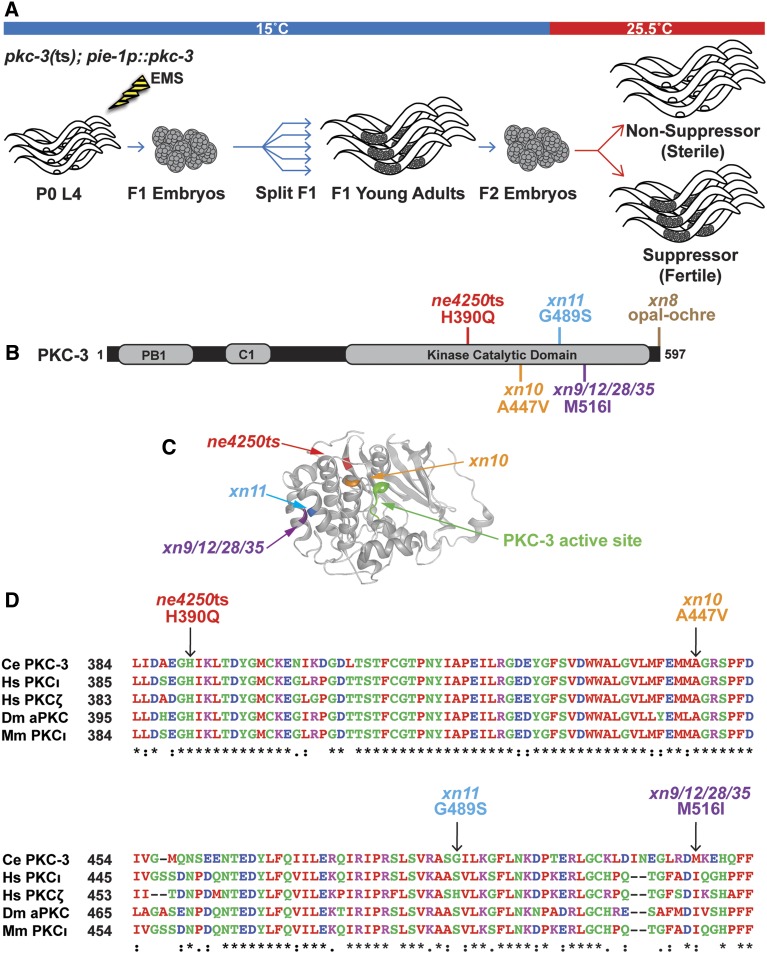
Given that the pkc-3(ts) allele produces a conditional sterile phenotype and is a hypomorphic allele that can be rescued by overexpressing the mutant protein, it provided an ideal mutation to use in a suppressor screen for interacting genes that contribute to junction formation. Since pkc-3(ts) mutants also have a conditional maternal-effect lethal phenotype caused by defects in anterior–posterior polarization of the one-cell embryo (Fievet et al. 2013), we simplified the screen by specifically rescuing the maternal-effect lethal phenotype with a transgene that expresses pkc-3(+) only maternally (xnIs131 [pie-1p::pkc-3]). pkc-3(ts); pie-1p::pkc-3(+) adults grown at the restrictive temperature laid viable eggs that grew to become sterile adults (Table 2), which provided additional evidence that the sterile phenotype does not arise from loss of pkc-3 function in the germ line. For the selection screen, we mutagenized pkc-3(ts); pie-1p::pkc-3 worms grown at the permissive temperature, split F1s into separate populations, and upshifted F2 eggs from each population to the restrictive temperature, placing them on large, bacteria-rich plates that would allow populations to expand if fertile worms were present (Figure 3A). Whereas most plates contained only sterile adults, and populations did not expand, plates with putative suppressors expanded in population and contained numerous fertile worms. Independent suppressor strains (those derived from different F1 plates) were founded from a single fertile worm and rescreened by scoring individual progeny grown at 25.5° for the presence of eggs laid on the plate. From three independent screens, we isolated 15 suppressor strains that had a penetrance of ≥75% fertile adults, compared to 3% fertile adults in unmutagenized pkc-3(ts); pie-1p::pkc-3 worms (Table 2) grown side-by-side at the same temperature.
Table 2. Suppressors of pkc-3(ts) sterility.
| Genotypea | Fertility | n | Dominanceb |
|---|---|---|---|
| Intragenic suppressors | |||
| pkc-3(ne4250) | 3% | 24 | N/A |
| pkc-3(ne4250xn8) | 85% | 59 | N/D |
| pkc-3(ne4250xn9) | 79% | 59 | N/D |
| pkc-3(ne4250xn10) | 98% | 83 | N/D |
| pkc-3(ne4250xn11) | 89% | 83 | N/D |
| pkc-3(ne4250xn12) | 98% | 81 | N/D |
| pkc-3(ne4250xn28) | 75% | 32 | N/D |
| pkc-3(ne4250xn35) | 80% | 20 | N/D |
| Extragenic suppressors | |||
| pkc-3(ne4250); xn7 | 100% | 24 | Recessive |
| pkc-3(ne4250); xn7/+ | 17% | 35 | |
| pkc-3(ne4250); xn20 | 100% | 19 | Recessive |
| pkc-3(ne4250); xn20/+ | 10% | 29 | |
| pkc-3(ne4250); xn21 | 91% | 22 | Recessive |
| pkc-3(ne4250); xn21/+ | 4% | 45 | |
| pkc-3(ne4250); xn22 | 96% | 23 | Dominant |
| pkc-3(ne4250); xn22/+ | 78% | 55 | |
| pkc-3(ne4250); xn23 | 100% | 24 | Recessive |
| pkc-3(ne4250); xn23/+ | 3% | 32 | |
| pkc-3(ne4250); xn25 | 92% | 24 | Recessive |
| pkc-3(ne4250); xn25/+ | 3% | 31 | |
| pkc-3(ne4250); xn27 | 100% | 23 | Recessive |
| pkc-3(ne4250); xn27/+ | 6% | 35 | |
| pkc-3(ne4250); xn37 | 96% | 24 | Recessive |
| pkc-3(ne4250); xn37/+ | 11% | 19 | |
All strains are also homozygous for the xnIs131 [pie-1p::pkc-3] transgene.
Fisher’s Exact test, P < 0.05.
Figure 3.
Intragenic pkc-3(ts) suppressors are in the kinase domain and stop codon. (A) Design of the pkc-3 suppressor screen. The pie-1p::pkc-3 (xnIs131) transgene expresses pkc-3 only maternally and was used to bypass the requirement for pkc-3 in anterior–posterior polarity in the early embryo. (B) PKC-3 protein depicting conserved domains and locations of the temperature-sensitive mutation and intragenic suppressors. (C) Structure of the catalytic domain of human aPKCι with superimposed PKC-3 mutations mapped to the homologous location. (D) Sequence alignment of a portion of the kinase domain from PKC-3 and paralogues in human (Hs), fruit fly (Dm), and mouse (Mm). Arrows indicate amino acid changes in the pkc-3(ne4250) allele and intragenic missense suppressors. Residues that are identical (“*”), conserved with strongly similar amino acids (“:”), and conserved with weakly similar amino acids (“.”) are indicated below.
Intragenic suppressors are missense mutations within the kinase domain
Anticipating that some of the suppressors would be second-site mutations within the pkc-3(ne4250) allele that increase its activity or expression, we first sequenced pkc-3 within each suppressor strain. Of the 15 suppressors, 6 contained second-site mutations within pkc-3 that resulted in missense changes to a total of three residues within the kinase domain, including 4 suppressors (from two separate mutagenesis screens) that harbored the same amino acid change (M516I) (Figure 3B). Mapping the three missense mutations onto homologous positions of the highly similar human aPKCι kinase domain revealed that they are predicted to lie within three different α-helices, and are not immediately adjacent to the H390Q change of the pkc-3(ne4250) allele (Figure 3C). All of the mutations produce relatively mild amino acid substitutions: two of the three missense mutations converted one conserved hydrophobic residue to another (A447V and M516I), whereas the third (G489S) converted the PKC-3 amino acid to that found in the homologous position in human aPKCι (Figure 3D). Given the nature of these mutations and their distinct locations, we consider it likely that they suppress the H390Q change of the pkc-3(ne4250) allele by modulating the stability or activity of the mutant PKC-3 protein. The finding that 6 of 15 suppressors we analyzed contained intragenic missense mutations within pkc-3, and that 4 of the independently isolated suppressors have the same missense mutation, indicates that the suppressor screen approached saturation.
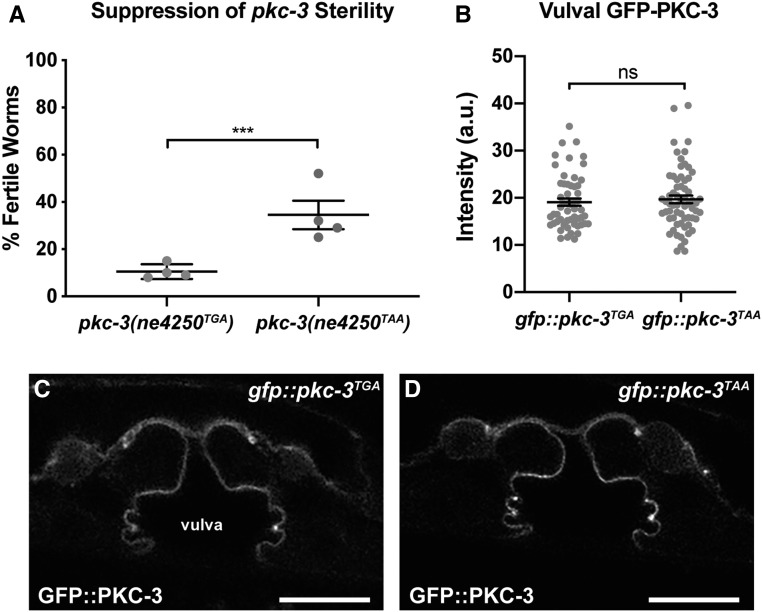
An opal to ochre stop codon substitution suppresses pkc-3 sterility
A seventh suppressor, xn8, changed the endogenous opal (TGA) stop codon of the pkc-3 gene to an ochre (TAA) stop codon (Figure 3B). Because we were unable to unlink the suppression phenotype in the strain containing xn8 from pkc-3(ts) (see Materials and Methods), we tested whether the opal to ochre stop codon change could be the causative suppressor mutation. Using CRISPR/Cas9, we engineered the xn8 TGA to TAA stop codon change directly into pkc-3(ne4250). Strikingly, a significantly higher percentage of pkc-3(ne4250TAA) worms grown at 25° were fertile compared to pkc-3(ne4250TGA) worms (Figure 4A). To determine if suppression could be explained by increased levels of the mutant PKC-3 protein, we engineered the xn8 TGA to TAA stop codon mutation into the pkc-3(it309[gfp::pkc-3]) knock-in allele, in which gfp is inserted at the 5′ end of pkc-3, leaving the pkc-3 coding sequence, stop codon, and 3′ UTR unaltered. Changing the stop codon of pkc-3(it309[gfp::pkc-3]) did not result in significantly higher levels GFP::PKC-3 protein in L4 stage epithelial cells (Figure 4, B–D) (we analyzed vulval cells since the apical surfaces of spermathecal cells are typically in contact with other cells), although a small increase in expression would be difficult to detect given the range of expression levels we observed in both genotypes (Figure 4B). Although we do not yet know the mechanism by which the xn8 stop codon mutation suppresses pkc-3(ne4250) (see Discussion), the identification of an opal to ochre suppressor demonstrates that stop codon choice can regulate gene function. In addition, isolation of the xn8 mutation suggests that the pkc-3(ne4250) genetic background is likely to be highly sensitized, and further emphasizes the extent of saturation of the selection screen.
Figure 4.
An intragenic stop-to-stop substitution suppresses pkc-3(ts) sterility. (A) Suppression of the sterile phenotype of pkc-3(ne4250) mutants by the xn8CRISPR TGA to TAA stop codon mutation. Sample sizes from four independent experiments: pkc-3(ne4250TGA) (n = 95, 105, 108, 101), pkc-3(ne4250TAA) (n = 64, 114, 112, 106). Gray dots indicate mean fertility from each experiment, horizontal line is the mean of means, and error bars are the SEM (∗∗∗P < 0.001, Fisher’s exact test). (B) GFP-PKC-3 levels at the apical surfaces of vulval cells in gfp::pkc-3TGA worms (n = 55) and gfp::pkc-3TAA worms (n = 64), which contain the xn8 TGA to TAA stop codon substitution. Gray circles indicate the GFP intensity measurements from individual worms; the average (line) and SEM (bars) from four combined imaging experiments are shown. ns, not significant (P = 0.487, unpaired Welch’s t-test). (C and D) L4 stage vulval cells of the indicated genotype expressing GFP-PKC-3. Bar, 10 µm.
The basolateral polarity regulator lgl-1 suppresses pkc-3 sterility
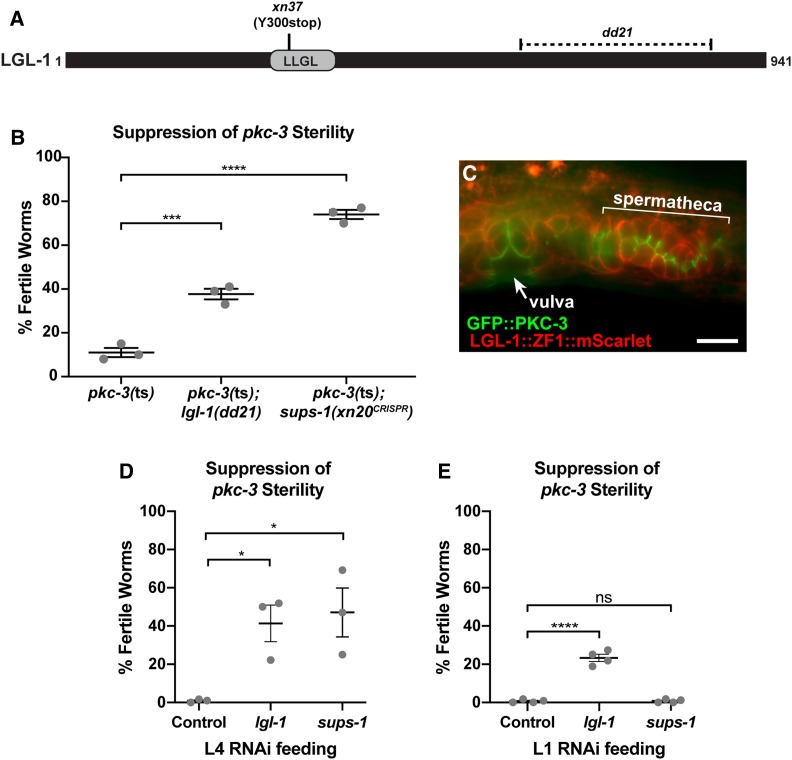
The remaining eight suppressors contained no second-site mutation within the pkc-3 gene and were presumed to be extragenic. Seven of the extragenic suppressors were recessive and one was dominant (Table 2). One candidate gene we suspected could function as a pkc-3 suppressor was lgl-1, which encodes a homolog of the basolateral polarity regulator and aPKC antagonist Lethal(2) giant larvae/Lgl (Beatty et al. 2010; Hoege et al. 2010). We sequenced the lgl-1 coding sequence in the extragenic suppressors, and identified an early nonsense mutation in the xn37 suppressor strain. The xn37 mutation is predicted to truncate the 941 amino acid LGL-1 protein at amino acid 300, deleting most of the LLGL domain, which is conserved among Lgl homologs (Figure 5A). In the one-cell embryo, LGL-1 binds to, and negatively regulates, PKC-3 (Beatty et al. 2010; Hoege et al. 2010), and transgenic LGL-1::GFP localizes to basolateral surfaces of embryonic epithelial cells (Beatty et al. 2010). However, no function for LGL-1 has been described after the one-cell stage. To test whether inhibition of lgl-1 suppresses pkc-3(ts) sterility, we crossed the lgl-1(dd21) deletion allele (Hoege et al. 2010) into the pkc-3(ts) background, and, in separate experiments, depleted lgl-1 by RNAi in pkc-3(ts) worms. lgl-1(dd21) and lgl-1 RNAi both significantly suppressed the sterility of pkc-3(ts) worms (Figure 5B and Figure 5, D and E), confirming that lgl-1 is a suppressor of pkc-3(ts). To determine if endogenous LGL-1 is expressed within the spermatheca and other epithelia, we used CRISPR/Cas9 to insert mScarlet and zf1 at the 3′ end of the lgl-1 locus. LGL-1::ZF1::mScarlet localized to the basolateral surfaces of spermathecal cells and other epithelial cells in the reproductive tract, in a pattern complementary to apical GFP::PKC-3 (Figure 5C). Together, these findings strongly suggest that the antagonistic relationship between PKC-3 and LGL-1 that occurs in the early embryo (Beatty et al. 2010; Hoege et al. 2010), and in Drosophila homologs within epithelial cells (Bilder et al. 2003; Tanentzapf and Tepass 2003), is preserved in C. elegans epithelia.
Figure 5.
An lgl-1 mutation suppresses pkc-3(ts) sterility. (A) LGL-1 protein showing position of the conserved LLGL domain and regions affects by the xn37 nonsense mutation and the dd21 deletion (dashed area). (B) Suppression of the sterile phenotype of pkc-3(ne4250) mutants by the lgl-1(dd21) allele and by the sups-1(xn20CRISPR) mutation. Samples sizes from three independent experiments: pkc-3(ne4250) (n = 96, 105, 108), pkc-3(ne4250); lgl-1(dd21) (n = 69, 113, 106), pkc-3(ne4250); sups-1(xn20) (n = 57, 104, 110). (C) Endogenously tagged apical GFP::PKC-3 and basolateral LGL-1::mScarlet in epithelial cells of the vulva (arrow) and spermatheca (bracket). (D) Suppression of pkc-3(ts) sterility by L4 RNAi feeding of lgl-1 or sups-1 dsRNA. All worms were fed at 25.5° and contained the pie-1p::pkc-3 (xnIs131) transgene to circumvent maternal-effect lethality. Samples sizes from three independent experiments: control RNAi (n = 109, 96, 644), lgl-1(RNAi) (n = 128, 144, 459), sups-1(RNAi) (n = 78, 76, 455). (E) Suppression of pkc-3(ts) sterility by L1 RNAi feeding of lgl-1 or sups-1 dsRNA. All worms were fed at 25.5° and contained the pie-1p::pkc-3 (xnIs131) transgene. Samples sizes from four independent experiments: control RNAi (n = 402, 168, 116, 190), lgl-1(RNAi) (n = 211, 168, 160, 185), sups-1(RNAi) (n = 558, 171, 157, 145). For graphs in (B,D, and E) gray dots indicate average fertility from an independent experiment, bar is the mean of means, and error bars are the SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001, ns = not significant, Bonferroni’s multiple comparison test. Bar, 10 µm.
sups-1 encodes a novel apical epidermal protein that suppresses pkc-3 sterility
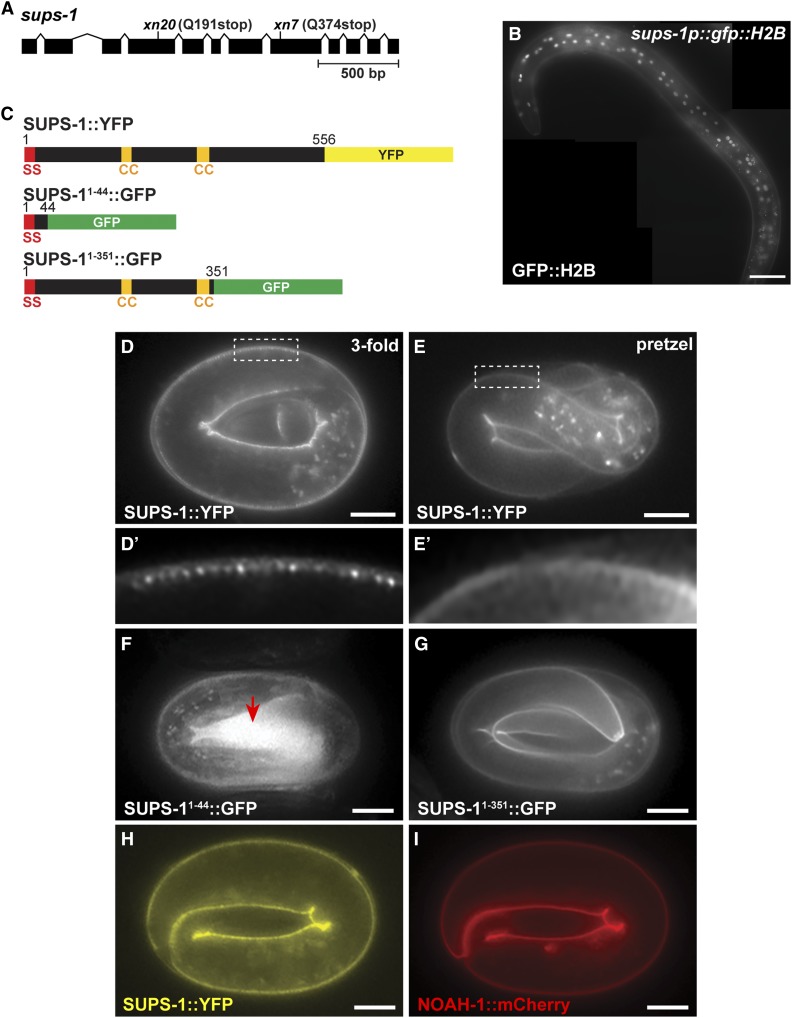
To begin to determine the identity of the remaining extragenic suppressors, we performed whole genome sequencing (WGS) on each strain and cataloged the unique EMS-induced mutations that could potentially disrupt gene function. We considered nonsense and missense mutations, as well as mutations altering invariant splice donor and acceptor sequences. WGS confirmed that none of the remaining six suppressors contained second-site mutations within pkc-3 or mutations that disrupt lgl-1. Comparing the identities of genes harboring mutations in each strain, we found that two suppressors, xn7 and xn20, each had a nonsense mutation in the previously uncharacterized gene F22F4.1 (Figure 6A), which we renamed sups-1 (suppressor of pkc-3 sterility). Consistent with mutations in sups-1 causing the suppression phenotype, xn7 and xn20 failed to complement (see Materials and Methods), and RNAi of sups-1 suppressed the sterility of pkc-3(ts) mutants when fed at the L4 stage and examined in the next generation (Figure 5D). However, in contrast to lgl-1 RNAi, sups-1 RNAi did not suppress the sterility of pkc-3(ts) mutants when feeding was performed at the L1 stage and worms were examined in the same generation (Figure 5E). To confirm that sups-1 is a pkc-3 suppressor, we introduced the xn20 nonsense mutation directly into pkc-3(ts) worms using CRISPR/Cas9. sups-1(xn20CRISPR) strongly suppressed the sterility of pkc-3(ts) (Figure 5B). By contrast, the sups-1(xn20CRISPR) mutation on its own, introduced directly into wild-type worms by CRISPR/Cas9 genome editing, caused no obvious phenotypes—worms had normal fecundity [sups-1(xn20CRISPR), 279 +/− 24 (n = 15); wild type, 227 ± 41 (n = 15), error is standard deviation (SD)] and produced viable broods [embryonic lethality: sups-1(xn20CRISPR) 4/3381; wild type, 19/2970]. Taken together, these findings indicate that sups-1 is a nonessential gene with no obvious phenotype when mutated alone, but that its loss strongly suppresses the sterile phenotype of pkc-3(ts) mutants.
Figure 6.
SUPS-1 is an apical epidermal protein. (A) sups-1 gene showing location of xn7 and xn20 nonsense mutations. Boxes represent exons and chevrons indicate introns. (B) GFP::H2B in a young adult worm expressed from sups-1 regulatory sequences. GFP::H2B is present in epidermal cell nuclei, which are near the surface of the worm. Partially overlapping images were stitched together using FIJI (Bar, 50 µm). (C) Schematics of endogenously tagged SUPS-1::YFP and GFP-tagged deletions expressed from transgenes. Numbers indicate amino acid positions; SS, signal sequence; CC, predicted coiled-coil domain. (D–E’) SUPS-1::YFP localization at the apical surfaces of epidermal cells at the indicated stages. Boxed regions are magnified in (D’ and E’) to show the striated apical pattern of SUPS-1::YFP in a threefold embryo and smoother apical localization in a pretzel-stage embryo. (F) SUPS-11–44::GFP, which is secreted into the extraembryonic space (red arrow). (G) SUPS-11–351::GFP, which shows a similar localization pattern as endogenously tagged SUPS-1::YFP. (H and I) Localization of SUPS-1::YFP (H) and NOAH-1::mCherry (I) in the same embryo. Bar in (D–I), 10 µm.
sups-1 is predicted to encode a 556 amino acid protein that is widely conserved among nematodes but lacks clear orthologs in other phyla. The C. elegans SUPS-1 protein, like SUPS-1 orthologs in the distantly related nematodes Pristionchus pacificus (PPA10264, BLASTP score 5e−129) and Trichuris muris (TMUE_0000000498, BLASTP score 1e−28), contains a predicted signal sequence at its N-terminus and two predicted coiled-coil domains (Lupas et al. 1991) in a central region of the protein, but no other domains of known function (Figure 6C). To determine when and where SUPS-1 is expressed, we used CRISPR/Cas9 to insert yfp at the 3′ end of the endogenous sups-1 gene (Figure 6C). SUPS-1::YFP was expressed transiently, first appearing during late embryogenesis (∼threefold stage) specifically on apical surfaces of epidermal cells (Figure 6, D and E), but was no longer visible after hatching. When initially expressed, SUPS-1::YFP appeared in a striated pattern at the apical surface (Figure 6D’) but transitioned to a smoother apical localization before hatching (pretzel stage, Figure 6E’). sups-1 mRNA detected by RNAseq is present in late embryos and larvae (Hillier et al. 2009), suggesting either that sups-1 mRNA is translated only during embryogenesis or that SUPS-1 protein is processed after embryogenesis such that the YFP in SUPS-1::YFP cannot be detected. However, we note that the failure of sups-1 RNAi begun during the L1 stage to suppress the sterility of pkc-3(ts) mutants, in contrast to lgl-1 RNAi, is consistent with embryo-specific expression (Figure 5E).
We tested whether SUPS-1 is a secreted protein by fusing the first 44 amino acids of SUPS-1 to GFP and expressing the fusion protein from the sups-1 promoter (Figure 6C). SUPS-11–44::GFP accumulated in late embryos within the extraembryonic space (Figure 6F, arrow), indicating that it is secreted. Since SUPS-1 is not predicted to contain an additional transmembrane domain, the presence of a functional signal sequence suggests that SUPS-1 is secreted and subsequently accumulates on the surfaces of epidermal cells. A longer GFP fusion protein containing the signal sequence and the two predicted coiled-coil domains showed the same apical epidermal localization as full-length SUPS-1 (Figure 6, C and G), suggesting that the coiled-coil domains, and/or sequences between the coiled-coil domains and the signal sequence, mediate binding of SUPS-1 to apical epidermal surfaces.
The localization of SUPS-1::YFP, and the timing of its expression, suggest that SUPS-1 may be a component of the epidermal apical extracellular matrix (aECM), which forms as embryos elongate (Priess and Hirsh 1986; Mancuso et al. 2012). In addition, the striated localization pattern of SUPS-1::YFP that we observe at earlier stages of its expression (Figure 6D’) has been noted for other epidermal aECM proteins, such as NOAH-1 (Vuong-Brender et al. 2017). SUPS-1::YFP and NOAH-1::mCherry colocalized in the epidermis (Figure 6, H and I), suggesting that SUPS-1 is a component of, or associates with, the aECM. However, SUPS-1 is not a critical component of the aECM; whereas mutations in several aECM components inhibit elongation of the embryo and lead to lethality (Mancuso et al. 2012; Vuong-Brender et al. 2017), sups-1 embryos are viable (see above). sups-1 RNAi also did not result in synthetic lethality with two other genes that function redundantly to regulate the aECM during embryo elongation, sym-1 and mec-8 (Davies et al. 1999) [embryonic lethality: sym-1(mn601) control(RNAi): 2.1% (n = 866), sym-1(mn601) sups-1(RNAi): 2.9% (n = 976), mec-8(e398) control(RNAi): 2.6% (n = 983), mec-8(e398) sups-1(RNAi): 2.4% (n = 905)]. We also tested whether a mutation in sym-1, which encodes an aECM component (Davies et al. 1999), could suppress pkc-3 sterility, but it did not (Table 3). Together, these results indicate that sups-1 is a nonessential aECM component, and that there is some specificity among aECM components in the ability to suppress pkc-3.
Table 3. Specificity of pkc-3 suppression by aECM genes.
| Genotype | Fertility | n | pa |
|---|---|---|---|
| pkc-3(ne4250) | 4.8% | 62 | N/A |
| pkc-3(ne4250); sups-1(xn20CRISPR) | 62% | 66 | <0.00001 |
| pkc-3(ne4250); sym-1(mn601) | 1.5% | 135 | 0.18 |
| sym-1(mn601) | 99% | 73 | N/A |
Compared to pkc-3(ne4250), Fisher’s exact test.
To determine if SUPS-1 is expressed by epidermal cells, as suggested by its localization, we used sups-1 regulatory sequences to drive GFP-tagged histone H2B. GFP::H2B was present in epidermal nuclei in late embryos, larvae and young adults (Figure 6B, n = 29/29), but we could not detect GFP::H2B in other tissues, including the spermatheca. The pattern of sups-1 expression suggests that SUPS-1 influences junction formation in the spermatheca nonautonomously. Consistent with nonautonomous regulation of junctions, significantly fewer pkc-3(ne4250); sups-1(xn20CRISPR) L4 stage worms (Figure 2G, 4/30) showed gaps in spermathecal cell AJs, which we visualized with endogenously tagged HMR-1::GFP, than did pkc-3(ne4250) worms (Figure 2F, 23/48) (P = 0.003, Fisher’s exact test). Prominent junction gaps present in the L4 stage spermathecae of pkc-3(ts) worms (54/64 worms), as detected by DLG-1 immunostaining, were also significantly suppressed by the xn8 stop-to-stop (gaps in 8/29 worms, P < 0.0001) and sups-1 mutations (gaps in 11/43 worms, P < 0.0001, Fisher’s exact test with Bonferroni’s correction). Taken together, these results indicate that sups-1 is an epidermally expressed gene that nonautonomously suppresses the spermathecal junction defects of pkc-3(ts) mutants to restore fertility.
Discussion
PKC-3 in C. elegans epithelial junction maturation
The role of pkc-3 in C. elegans epithelial cells has remained elusive. This is in large part because maternal PKC-3 protein has an essential role in polarizing the one-cell embryo, yet the protein persists into later developmental stages, potentially masking phenotypes that would otherwise arise in zygotic pkc-3 mutants (Tabuse et al. 1998; Alan et al. 2013). Here, we used a degron strategy to degrade maternal PKC-3 after its essential role in early embryonic polarity was complete, allowing us to obtain embryos lacking detectable maternal and zygotic PKC-3 protein before epithelial cells differentiate. pkc-3(MZ) embryos showed phenotypes identical to par-6(MZ) embryos (Totong et al. 2007)—junction maturation failed and embryos arrested by the twofold stage with ruptures in the epidermis. This observation is perhaps not surprising given that PAR-6 and PKC-3 bind one another, localize interdependently to the cortex, and cause similar polarity defects in the one-cell embryo upon depletion (Watts et al. 1996; Tabuse et al. 1998; Hung and Kemphues 1999; Li et al. 2010). However, the finding that PKC-3 and PAR-6 depletion causes the same defect in junction maturation helps to resolve the role of the PKC-3 regulator CDC-42 in the same process. cdc-42(MZ) embryos have considerably milder phenotypes than par-6(MZ) and pkc-3(MZ) embryos, and junction maturation still occurs (Zilberman et al. 2017). This finding contrasts with a study from the early embryo suggesting that CDC-42 both localizes and activates PKC-3 (Rodriguez et al. 2017), presumably through binding to the PAR-6 semi-CRIB domain, and raised the possibility that PAR-6 regulates junction maturation, at least in part, independently of PKC-3. However, the identical phenotypes of par-6(MZ) and pkc-3(MZ) mutants suggest an alternative possibility—that PAR-6 and PKC-3 promote junction maturation together but do so, at least in part, independently of CDC-42. This interpretation is consistent with in vitro structural studies showing that Par6 binding can activate aPKC kinase activity in the absence of Cdc42 (Graybill et al. 2012). An important goal for future studies will be to learn how PAR-6 and PKC-3 activity is controlled in time and space to promote junction maturation, given that CDC-42 is not essential for this process.
Why the hypomorphic pkc-3(ts) mutant causes such a specific junction phenotype in the spermatheca without obvious defects in other epithelial cell junctions remains unclear. In contrast to the small gaps between junction proteins that we observed in pkc-3(MZ) embryos, AJs between spermathecal cells in pkc-3(ts) larvae often contained one or more prominent gaps, suggesting that a subset of spermathecal cells lost junctional connections with one another. These defects were already present at the L4 stage, before ovulation of oocytes into the spermatheca begins, ruling out the possibility that ovulation itself caused the spermatheca to tear. It is possible that spermathecal cells differ from other cells in the repertoire of PKC-3 regulators or effectors that they express, making them more sensitive to PKC-3 inhibition. It is also plausible that spermathecal cells, which are myoepithelial cells with aligned contractile microfilaments (Strome 1986), have biomechanical properties that make junctions more prone to break when compromised by partial PKC-3 inactivation.
LGL-1 and junction formation
Our identification and validation of a suppressor mutation in the lgl-1 gene provides the first evidence that lgl-1 has a function in C. elegans epithelial cells. lgl-1 mutants are viable and healthy (Beatty et al. 2010; Hoege et al. 2010), in contrast to Drosophila lethal(2) giant larvae mutants and mouse Lgl1 mutants, which have defects in cell polarity and proliferation (Bilder et al. 2000; Hutterer et al. 2004; Klezovitch et al. 2004). Similar to its homologs in flies and mammals, LGL-1 physically interacts with PKC-3 (Beatty et al. 2010; Hoege et al. 2010). In the one-cell embryo, LGL-1 antagonizes PKC-3 function but does so redundantly with the RING domain protein PAR-2 (Beatty et al. 2010; Hoege et al. 2010). We showed that endogenously tagged LGL-1::ZF1::mScarlet localizes to basolateral surfaces of epithelial cells, including those of the spermatheca, consistent with previous findings utilizing an lgl-1::gfp transgene (Beatty et al. 2010). Thus, whereas the function of LGL-1 appears to be conserved in C. elegans epithelial cells, other polarity regulators likely function redundantly with LGL-1 to restrict PKC-3 to the apical surface.
Nonautonomous suppression of pkc-3 junction defects by sups-1
The identification of two independent suppressor mutations in the previously uncharacterized sups-1 gene establishes sups-1 for the first time as a regulator of junctions in C. elegans epithelial cells. We were surprised to find that sups-1 encodes a secreted protein that is likely a component of, or that associates with, the aECM of embryonic epidermal cells. Other than a functional signal sequence and two predicted coiled-coil domains that are conserved in other nematode SUPS-1 homologs, the SUPS-1 protein contains no obvious functional domains. sups-1 expression, determined both by visualizing endogenously tagged SUPS-1::YFP as well as GFP::H2B expressed from sups-1 regulatory sequences, appears to be specific to epidermal cells; we could detect no SUPS-1::YFP in the spermatheca, and the sups-1p::gfp-H2B reporter was not visibly expressed in spermathecal cells. SUPS-1::YFP expression disappeared after embryonic stages, suggesting that it is a transient component of the epidermal aECM. Thus, it remains somewhat of a mystery how epidermal SUPS-1 in the embryo contributes to junction formation in the spermatheca—an organ that forms near the end of larval development. One possibility is that secreted SUPS-1 diffuses to other tissues in quantities below our level of detection, perhaps binding to spermathecal cell surfaces and influencing junction formation. Precedent for this hypothesis comes from studies of other secreted apical ECM proteins—the C. elegans lipocalins LPR-1 and LPR-3—that can travel between cells and function nonautonomously (Forman-Rubinsky et al. 2017; Pu et al. 2017). Another possibility is that SUPS-1 function in embryonic epidermal cells is important for the subsequent production or regulation of epidermally derived signals that nonautonomously influence spermathecal cells. This idea also has some precedent, as cuticular collagens expressed in the epidermis as effectors of BMP signaling regulate whole worm body size and growth (Madaan et al. 2018), and function of the lin-4 and let-7 developmental timing miRNA pathway in the epidermis can alter mTOR signaling and vitellogenin production in the intestine (Dowen et al. 2016). There is also precedent for nonautonomous control of somatic gonad development by the epidermis, as mutations in the heterochronic gene lin-28 disrupt spermathecal development nonautonomously by affecting the timing of epidermal differentiation (Choi and Ambros 2019). The identification of sups-1 as a pkc-3 suppressor highlights a previously unknown connection between the aECM of the epidermis and epithelial cells of the somatic gonad that will be interesting to pursue. There appears to be some specificity to this suppression, as mutations in another aECM component gene, sym-1, had no effect on pkc-3 sterility.
Stop codon identity can regulate pkc-3 activity
One of the reasons that forward genetic mutagenesis screens are remarkable, particularly those utilizing mutagens that mostly result in single nucleotide changes, is that they can produce unexpected mutations. One such example is our identification and validation of the xn8 suppressor, which causes a synonymous TGA to TAA substitution in the pkc-3 stop codon. While the mechanistic basis of suppression remains to be determined, we were unable to detect an increase in GFP::PKC-3 levels within vulval epithelial cells upon introducing the identical stop codon change into the pkc-3(it309[gfp::pkc-3]) allele, which is otherwise identical to pkc-3 downstream of the inserted gfp. We consider two mechanisms of suppression most likely. First, it is possible that higher levels of PKC-3 are produced when the stop codon is changed to TAA, for example because of increased mRNA stability or translation. Such an increase would necessarily be small, and beyond our ability to detect easily in vulval cells, or limited to other cells such as spermathecal cells, in which we did not quantify GFP::PKC-3 (the apical surfaces of these cells are obscured by contact with other cells). While we did not observe any qualitative differences in endogenously tagged GFP::PKC-3 levels in any tissue upon changing the stop codon to TAA, it is possible that the TAA stop codon slightly increases PKC-3 protein levels, or causes the protein to accumulate to steady-state levels more quickly, and that this increase is responsible for pkc-3(ts) suppression. Consistent with this possibility, we showed that overexpressing the pkc-3(ts) mutant allele from an extrachromosomal array suppresses the pkc-3(ts) sterile phenotype. Moreover, C. elegans genes have a strong bias for TAA stop codons, particularly those that are more highly expressed (Stenico et al. 1994). This trend is true in humans as well, where TAA stop codons are favored in highly expressed genes (Trotta 2016). A second possibility is that the xn8 TGA to TAA stop codon mutation affects translational readthrough. Once thought to be rare outside of viruses, stop codon readthrough is likely to occur fairly frequently in Drosophila, where it has been demonstrated to occur in several genes (Jungreis et al. 2011). The last amino acid of PKC-3 (valine) is highly conserved, raising the possibility that extension of the PKC-3 protein through readthrough could have significant effects on its activity. In this scenario, the TGA to TAA change would either reduce readthrough to yield a small increase in active PKC-3 protein, or, alternatively, would increase readthrough to create a small population of PKC-3 variants that are more active than wild-type PKC-3. Arguing against the latter possibility, a bioinformatics analysis of Drosophila genes with evolutionary signatures of readthrough showed that TGA stop codons occur much more frequently in readthrough candidates than do TAA stop codons (Jungreis et al. 2011). Regardless of how the xn8 TGA to TAA stop codon substitution affects PKC-3 activity, the isolation and validation of this suppressor allele provides a striking demonstration that stop codon mutations can contribute to gene activity, and should not be discounted when considering candidate mutations that cause or modify human diseases.
Acknowledgments
We thank Joshua Abrams, Jane Hubbard, Ken Kemphues, Michel Labouesse, and Craig Mello for generous gifts of worm strains and plasmids; Igor Dolgalev, Luke Noble, and Luis A. Martinez for assistance with whole genome sequence analysis; Annita Achilleos and Sameer Shah for initial characterization of pkc-3 alleles and assistance with screens; Stevan Hubbard for insights on structural analysis of PKC-3 variants, and Andrew Fire and members of the Nance laboratory for comments on the manuscript. Some strains were provided by the Caenorhabditis Genome Center (CGC), which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This work was supported by grants from the National Science Foundation Graduate Research Fellowship to J.G.M.-R. and from the NIH to J.N. (R01GM098492, R35GM118081).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.9637079.
Communicating editor: M. Sundaram
Literature Cited
- Achilleos A., Wehman A. M., and Nance J., 2010. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137: 1833–1842. 10.1242/dev.047647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alan J. K., Struckhoff E. C., and Lundquist E. A., 2013. Multiple cytoskeletal pathways and PI3K signaling mediate CDC-42-induced neuronal protrusion in C. elegans. Small GTPases 4: 208–220. 10.4161/sgtp.26602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagor L., Ufimtsev I. S., Ayer A., Li J., and Weis W. I., 2019. Structural insights into the aPKC regulatory switch mechanism of the human cell polarity protein lethal giant larvae 2. Proc. Natl. Acad. Sci. USA 116: 10804–10812. 10.1073/pnas.1821514116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Gill J. S., Cinalli R. M., and Nance J., 2008. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320: 1771–1774. 10.1126/science.1156063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono S., Legouis R., Hoose W. A., and Kemphues K. J., 2004. PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development 131: 2865–2874. 10.1242/dev.01146 [DOI] [PubMed] [Google Scholar]
- Armenti S. T., Chan E., and Nance J., 2014a Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Dev. Biol. 394: 110–121. 10.1016/j.ydbio.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenti S. T., Lohmer L. L., Sherwood D. R., and Nance J., 2014b Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 141: 4640–4647. 10.1242/dev.115048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood S. X., Chabu C., Penkert R. R., Doe C. Q., and Prehoda K. E., 2007. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J. Cell Sci. 120: 3200–3206. 10.1242/jcs.014902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., and Matter K., 2016. Tight junctions as regulators of tissue remodelling. Curr. Opin. Cell Biol. 42: 94–101. 10.1016/j.ceb.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Beatty A., Morton D., and Kemphues K., 2010. The C. elegans homolog of Drosophila Lethal giant larvae functions redundantly with PAR-2 to maintain polarity in the early embryo. Development 137: 3995–4004. 10.1242/dev.056028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow H., Doitsidou M., Sarin S., and Hobert O., 2009. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat. Methods 6: 549 10.1038/nmeth.f.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Li M., and Perrimon N., 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289: 113–116. 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., and Perrimon N., 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5: 53–58. 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- Bossinger O., Klebes A., Segbert C., Theres C., and Knust E., 2001. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230: 29–42. 10.1006/dbio.2000.0113 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., and Sulston J., 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82: 358–370. 10.1016/0012-1606(81)90459-0 [DOI] [PubMed] [Google Scholar]
- Chen J., Sayadian A. C., Lowe N., Lovegrove H. E., and St Johnston D., 2018. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol. 16: e3000041 10.1371/journal.pbio.3000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., and Ambros V., 2019. The C. elegans heterochronic gene lin-28 coordinates the timing of hypodermal and somatic gonadal programs for hermaphrodite reproductive system morphogenesis. Development 146: pii: dev164293. 10.1242/dev.164293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Raich W., Agbunag C., Leung B., Hardin J. et al. , 1998. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 141: 297–308. 10.1083/jcb.141.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. G., Spike C. A., Shaw J. E., and Herman R. K., 1999. Functional overlap between the mec-8 gene and five sym genes in Caenorhabditis elegans. Genetics 153: 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRenzo C., Reese K. J., and Seydoux G., 2003. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature 424: 685–689. 10.1038/nature01887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., and Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. 10.1038/nmeth.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Zhang X., Liu W., Chen Y. J., Huang J. et al. , 2015. A conserved polybasic domain mediates plasma membrane targeting of Lgl and its regulation by hypoxia. J. Cell Biol. 211: 273–286. 10.1083/jcb.201503067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowen R. H., Breen P. C., Tullius T., Conery A. L., and Ruvkun G., 2016. A microRNA program in the C. elegans hypodermis couples to intestinal mTORC2/PQM-1 signaling to modulate fat transport. Genes Dev. 30: 1515–1528. 10.1101/gad.283895.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad-Moghadam B., Guo S., and Kemphues K. J., 1995. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83: 743–752. 10.1016/0092-8674(95)90187-6 [DOI] [PubMed] [Google Scholar]
- Fievet B. T., Rodriguez J., Naganathan S., Lee C., Zeiser E. et al. , 2013. Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat. Cell Biol. 15: 103–112. 10.1038/ncb2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman-Rubinsky R., Cohen J. D., and Sundaram M. V., 2017. Lipocalins are required for apical extracellular matrix organization and remodeling in Caenorhabditis elegans. Genetics 207: 625–642. 10.1534/genetics.117.300207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A., and Riechmann V., 2010. Stepwise polarisation of the Drosophila follicular epithelium. Dev. Biol. 338: 136–147. 10.1016/j.ydbio.2009.11.027 [DOI] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R. Y., Venter J. C., Hutchison C. A. III et al. , 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6: 343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Graybill C., Wee B., Atwood S. X., and Prehoda K. E., 2012. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J. Biol. Chem. 287: 21003–21011. 10.1074/jbc.M112.360495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., and Peifer M., 2004. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167: 135–147. 10.1083/jcb.200406024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., and Peifer M., 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170: 813–823. 10.1083/jcb.200505127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier L. W., Reinke V., Green P., Hirst M., Marra M. A. et al. , 2009. Massively parallel sequencing of the polyadenylated transcriptome of C. elegans. Genome Res. 19: 657–666. 10.1101/gr.088112.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C., Constantinescu A. T., Schwager A., Goehring N. W., Kumar P. et al. , 2010. LGL can partition the cortex of one-cell Caenorhabditis elegans embryos into two domains. Curr. Biol. 20: 1296–1303. 10.1016/j.cub.2010.05.061 [DOI] [PubMed] [Google Scholar]
- Hubbard E. J., and Schedl T., 2019. Biology of the Caenorhabditis elegans germline stem cell system. Genetics 213: 1145–1188. 10.1534/genetics.119.300238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T. J., and Kemphues K. J., 1999. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126: 127–135. [DOI] [PubMed] [Google Scholar]
- Hutterer A., Betschinger J., Petronczki M., and Knoblich J. A., 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell 6: 845–854. 10.1016/j.devcel.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Iden S., Misselwitz S., Peddibhotla S. S., Tuncay H., Rehder D. et al. , 2012. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J. Cell Biol. 196: 623–639. 10.1083/jcb.201104143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi T., and Takeichi M., 2011. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat. Cell Biol. 13: 860–866. 10.1038/ncb2274 [DOI] [PubMed] [Google Scholar]
- Iwasaki K., McCarter J., Francis R., and Schedl T., 1996. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134: 699–714. 10.1083/jcb.134.3.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Suzuki T., Seth A., Samak G., and Rao R., 2011. Protein kinase Cζ phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem. J. 437: 289–299. 10.1042/BJ20110587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Petersen C., Gao L., and Macara I. G., 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2: 531–539. 10.1038/35019573 [DOI] [PubMed] [Google Scholar]
- Jungreis I., Lin M. F., Spokony R., Chan C. S., Negre N. et al. , 2011. Evidence of abundant stop codon readthrough in Drosophila and other metazoa. Genome Res. 21: 2096–2113. 10.1101/gr.119974.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Martinez-Campos M., Zipperlen P., Fraser A. G. and Ahringer J., 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002 10.1186/gb-2000-2-1-research0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R. et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- Klezovitch O., Fernandez T. E., Tapscott S. J., and Vasioukhin V., 2004. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18: 559–571. 10.1101/gad.1178004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppen M., Simske J. S., Sims P. A., Firestein B. L., Hall D. H. et al. , 2001. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3: 983–991. 10.1038/ncb1101-983 [DOI] [PubMed] [Google Scholar]
- Lang C. F., and Munro E., 2017. The PAR proteins: from molecular circuits to dynamic self-stabilizing cell polarity. Development 144: 3405–3416. 10.1242/dev.139063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung B., Hermann G. J., and Priess J. R., 1999. Organogenesis of the Caenorhabditis elegans intestine. Dev. Biol. 216: 114–134. 10.1006/dbio.1999.9471 [DOI] [PubMed] [Google Scholar]
- Li H., and Durbin R., 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kim H., Aceto D. G., Hung J., Aono S. et al. , 2010. Binding to PKC-3, but not to PAR-3 or to a conventional PDZ domain ligand, is required for PAR-6 function in C. elegans. Dev. Biol. 340: 88–98. 10.1016/j.ydbio.2010.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Edwards A. S., Fawcett J. P., Mbamalu G., Scott J. D. et al. , 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2: 540–547. 10.1038/35019582 [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., and Stock J., 1991. Predicting coiled coils from protein sequences. Science 252: 1162–1164. 10.1126/science.252.5009.1162 [DOI] [PubMed] [Google Scholar]
- Madaan U., Yzeiraj E., Meade M., Clark J. F., Rushlow C. A. et al. , 2018. BMP signaling determines body size via transcriptional regulation of collagen genes in Caenorhabditis elegans. Genetics 210: 1355–1367. 10.1534/genetics.118.301631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F., Park Y. M., Lee J., Buso N., Gur T. et al. , 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47: W636–W641. 10.1093/nar/gkz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M., and Pilgrim D., 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso V. P., Parry J. M., Storer L., Poggioli C., Nguyen K. C. et al. , 2012. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 139: 979–990. 10.1242/dev.075135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston D. J., Higgins C. D., Peters K. A., Cupp T. D., Dickinson D. J. et al. , 2016. MRCK-1 drives apical constriction in C. elegans by linking developmental patterning to force generation. Curr. Biol. 26: 2079–2089. 10.1016/j.cub.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T., and Schedl T., 1997. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181: 121–143. 10.1006/dbio.1996.8429 [DOI] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K. et al. , 2010. The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L., Legouis R., Vonesch J. L., and Labouesse M., 2001. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J. Cell Sci. 114: 2265–2277. [DOI] [PubMed] [Google Scholar]
- Mege R. M., and Ishiyama N., 2017. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb. Perspect. Biol. 9: pii: a028738 10.1101/cshperspect.a028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., and Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. 10.1002/j.1460-2075.1991.tb04966.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt A., Macieira S., Velarde M., Badeker M., Benda C. et al. , 2005. Crystal structure of the catalytic domain of human atypical protein kinase Cι reveals interaction mode of phosphorylation site in turn motif. J. Mol. Biol. 352: 918–931. 10.1016/j.jmb.2005.07.060 [DOI] [PubMed] [Google Scholar]
- Mok D. Z., Sternberg P. W., and Inoue T., 2015. Morphologically defined sub-stages of C. elegans vulval development in the fourth larval stage. BMC Dev. Biol. 15: 26 10.1186/s12861-015-0076-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J., Munro E. M., and Priess J. R., 2003. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 130: 5339–5350. 10.1242/dev.00735 [DOI] [PubMed] [Google Scholar]
- Newman A. P., White J. G., and Sternberg P. W., 1996. Morphogenesis of the C. elegans hermaphrodite uterus. Development 122: 3617–3626. [DOI] [PubMed] [Google Scholar]
- Noda Y., Takeya R., Ohno S., Naito S., Ito T. et al. , 2001. Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells 6: 107–119. 10.1046/j.1365-2443.2001.00404.x [DOI] [PubMed] [Google Scholar]
- Paix A., Schmidt H., and Seydoux G., 2016. Cas9-assisted recombineering in C. elegans: genome editing using in vivo assembly of linear DNAs. Nucleic Acids Res. 44: e128 10.1093/nar/gkw502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper A. S., Lo T. W., Killian D. J., Hall D. H., and Hubbard E. J., 2003. The establishment of Caenorhabditis elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev. Biol. 259: 336–350. 10.1016/S0012-1606(03)00203-3 [DOI] [PubMed] [Google Scholar]
- Pinheiro D., and Bellaiche Y., 2018. Mechanical force-driven adherens junction remodeling and epithelial dynamics. Dev. Cell 47: 3–19 (erratum: Dev. Cell 47: 391). 10.1016/j.devcel.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Priess J. R., and Hirsh D. I., 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117: 156–173. 10.1016/0012-1606(86)90358-1 [DOI] [PubMed] [Google Scholar]
- Pu P., Stone C. E., Burdick J. T., Murray J. I., and Sundaram M. V., 2017. The lipocalin LPR-1 cooperates with LIN-3/EGF signaling to maintain narrow tube integrity in Caenorhabditis elegans. Genetics 205: 1247–1260. 10.1534/genetics.116.195156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R., and Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese K. J., Dunn M. A., Waddle J. A., and Seydoux G., 2000. Asymmetric segregation of PIE-1 in C. elegans is mediated by two complementary mechanisms that act through separate PIE-1 protein domains. Mol. Cell 6: 445–455. 10.1016/S1097-2765(00)00043-5 [DOI] [PubMed] [Google Scholar]
- Rodriguez J., Peglion F., Martin J., Hubatsch L., Reich J. et al. , 2017. aPKC cycles between functionally distinct PAR protein assemblies to drive cell polarity. Dev. Cell 42: 400–415.e49. 10.1016/j.devcel.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J. F., Ceron J., Koreth J., Hao T., Nicot A. S. et al. , 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14: 2162–2168. 10.1101/gr.2505604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonegg S., and Hyman A. A., 2006. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development 133: 3507–3516. 10.1242/dev.02527 [DOI] [PubMed] [Google Scholar]
- St Johnston D., and Ahringer J., 2010. Cell polarity in eggs and epithelia: parallels and diversity. Cell 141: 757–774. 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Stenico M., Lloyd A. T., and Sharp P. M., 1994. Codon usage in Caenorhabditis elegans: delineation of translational selection and mutational biases. Nucleic Acids Res. 22: 2437–2446. 10.1093/nar/22.13.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. O., Yamanaka Y., and Rossant J., 2010. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 137: 3383–3391. 10.1242/dev.050195 [DOI] [PubMed] [Google Scholar]
- Strome S., 1986. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J. Cell Biol. 103: 2241–2252. 10.1083/jcb.103.6.2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Yamanaka T., Hirose T., Manabe N., Mizuno K. et al. , 2001. Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 152: 1183–1196. 10.1083/jcb.152.6.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y., Izumi Y., Piano F., Kemphues K. J., Miwa J. et al. , 1998. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 125: 3607–3614. [DOI] [PubMed] [Google Scholar]
- Takeichi M., 2014. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15: 397–410. 10.1038/nrm3802 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., and Tepass U., 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5: 46–52. 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- Timmons L., and Fire A., 1998. Specific interference by ingested dsRNA. Nature 395: 854 10.1038/27579 [DOI] [PubMed] [Google Scholar]
- Totong R., Achilleos A., and Nance J., 2007. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 134: 1259–1268. 10.1242/dev.02833 [DOI] [PubMed] [Google Scholar]
- Trotta E., 2016. Selective forces and mutational biases drive stop codon usage in the human genome: a comparison with sense codon usage. BMC Genomics 17: 366 10.1186/s12864-016-2692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., and Anderson J. M., 2014. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 36: 157–165. 10.1016/j.semcdb.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visco I., Hoege C., Hyman A. A., and Schwille P., 2016. In vitro reconstitution of a membrane switch mechanism for the polarity protein LGL. J. Mol. Biol. 428: 4828–4842. 10.1016/j.jmb.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Voutev R., Keating R., Hubbard E. J., and Vallier L. G., 2009. Characterization of the Caenorhabditis elegans Islet LIM-homeodomain ortholog, lim-7. FEBS Lett. 583: 456–464. 10.1016/j.febslet.2008.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong-Brender T. T., Yang X., and Labouesse M., 2016. C. elegans embryonic morphogenesis. Curr. Top. Dev. Biol. 116: 597–616. 10.1016/bs.ctdb.2015.11.012 [DOI] [PubMed] [Google Scholar]
- Vuong-Brender T. T. K., Suman S. K., and Labouesse M., 2017. The apical ECM preserves embryonic integrity and distributes mechanical stress during morphogenesis. Development 144: 4336–4349. 10.1242/dev.150383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S. W., Durgan J., Jin D., and Hall A., 2010. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol. Biol. Cell 21: 2996–3006. 10.1091/mbc.e10-05-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. C., Low T. Y. F., Nishimura Y., Gole L., Yu W. et al. , 2017. Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization. Nat. Cell Biol. 19: 988–995. 10.1038/ncb3577 [DOI] [PubMed] [Google Scholar]
- Watts J. L., Etemad-Moghadam B., Guo S., Boyd L., Draper B. W. et al. , 1996. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development 122: 3133–3140. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Suzuki A., Sugiyama Y., Kitamura K. et al. , 2001. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731. 10.1046/j.1365-2443.2001.00453.x [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Sugiyama Y., Ishiyama C., Suzuki A. et al. , 2003. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13: 734–743. 10.1016/S0960-9822(03)00244-6 [DOI] [PubMed] [Google Scholar]
- Yochem J., and Herman R. K., 2003. Investigating C. elegans development through mosaic analysis. Development 130: 4761–4768. 10.1242/dev.00701 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Fang L., Chen N., Johnsen R. C., Stein L. et al. , 2005. Distinct regulatory elements mediate similar expression patterns in the excretory cell of Caenorhabditis elegans. J. Biol. Chem. 280: 38787–38794. 10.1074/jbc.M505701200 [DOI] [PubMed] [Google Scholar]
- Zilberman Y., Abrams J., Anderson D. C., and Nance J., 2017. Cdc42 regulates junctional actin but not cell polarization in the Caenorhabditis elegans epidermis. J. Cell Biol. 216: 3729–3744. 10.1083/jcb.201611061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental Data (Movie 1) has been uploaded to the GSA Figshare portal. WGS of sup-1(xn7) and sups-1(xn20) are deposited at NCBI as Sequence Read Archive data (xn7: SRR9969397; xn20: SRR9988268). Supplemental material available at figshare: https://doi.org/10.25386/genetics.9637079.