Abstract
Transfer RNA (tRNA) genes are widely studied sites of replication-fork pausing and genome instability in the budding yeast Saccharomyces cerevisiae. tRNAs are extremely highly transcribed and serve as constitutive condensin binding sites. tRNA transcription by RNA polymerase III has previously been identified as stimulating replication-fork pausing at tRNA genes, but the nature of the block to replication has not been incontrovertibly demonstrated. Here, we describe a systematic, genome-wide analysis of the contributions of candidates to replication-fork progression at tDNAs in yeast: transcription factor binding, transcription, topoisomerase activity, condensin-mediated clustering, and Rad18-dependent DNA repair. We show that an asymmetric block to replication is maintained even when tRNA transcription is abolished by depletion of one or more subunits of RNA polymerase III. By contrast, analogous depletion of the essential transcription factor TFIIIB removes the obstacle to replication. Therefore, our data suggest that the RNA polymerase III transcription complex itself represents an asymmetric obstacle to replication even in the absence of RNA synthesis. We additionally demonstrate that replication-fork progression past tRNA genes is unaffected by the global depletion of condensin from the nucleus, and can be stimulated by the removal of topoisomerases or Rad18-dependent DNA repair pathways.
Keywords: Replication-fork stalling, Replication-transcription conflicts, DNA damage
THE maintenance of eukaryotic genomes is a challenging task: replicating even the highly compact ∼12 MB genome of the budding yeast Saccharomyces cerevisiae requires the establishment and progression of hundreds of replication forks . The passage of replisomes through the genome can be impeded by a variety of protein-DNA complexes and DNA secondary structures, including transcribed genes (Deshpande and Newlon 1996; Ivessa et al. 2003; Azvolinsky et al. 2009; Bermejo et al. 2009; Alzu et al. 2012; Sabouri et al. 2012; Osmundson et al. 2017; Tran et al. 2017), unfired replication origins (Ivessa et al. 2003), centromeres (Chen et al. 2019), R loops (Hamperl et al. 2017; Lang et al. 2017), and G quadruplexes (Paeschke et al. 2011). Unresolved collisions between such obstacles on the DNA template and the replication fork will transiently stall or permanently arrest the replisome; this can lead to replication-fork collapse (Lambert and Carr 2013; Ait Saada et al. 2018), DNA damage (Ait Saada et al. 2018), and dissociation of the replisome from the DNA (Cortez 2015). However, widespread replication-fork stalling or arrest is detrimental even in the absence of replication-fork collapse, since the impairment of two convergent forks without a licensed origin in the intervening sequence will lead to underreplication of this region (Blow et al. 2011).
Transcription represents both a widespread and extensively investigated impediment to genome duplication (Hamperl and Cimprich 2016; Hamperl et al. 2017). Replication-transcription conflicts have been studied since the early 1980s, when it was first shown in vitro that DNA primer extension by the bacteriophage T4 replication machinery could be stopped by addition of RNA polymerase (Bedinger et al. 1983). S. cerevisiae transfer RNA (tRNA) genes (hereafter referred to as tDNAs) are a long-standing model system for replication-transcription conflicts. tRNAs are highly transcribed, accounting for ∼15% of cellular RNA (Warner 1999) despite representing <0.2% of the yeast genome. tDNAs are prominent sites of polar replication-fork pausing (Deshpande and Newlon 1996; Ivessa et al. 2003; Azvolinsky et al. 2009), whereby a tDNA oriented such that transcription occurs in the head-on orientation relative to replication impedes a greater proportion of replisomes genome-wide than tDNAs cooriented with replication (Osmundson et al. 2017). Replication-fork pausing and genome instability at these sites are mitigated by the redundant activity of the Pif1 family helicases Pif1 and Rrm3 (Osmundson et al. 2017; Tran et al. 2017). Consistent with head-on replication-transcription conflicts at tDNAs being deleterious, tDNAs show a strong bias toward the codirectional orientation in the yeast genome (Osmundson et al. 2017). An analogous statistical coorientation of replication and transcription is not observed for protein-coding genes in yeast (Raghuraman et al. 2001), but is prevalent in prokaryotes (Rocha and Danchin 2003), and has been described for both the Caenorhabditis elegans (Pourkarimi et al. 2016) and human replication programs (Petryk et al. 2016; Chen et al. 2019). Thus, replication-transcription conflicts can shape genome architecture and replication dynamics across biological kingdoms.
Replication-transcription conflicts at both tDNAs and protein-coding genes are associated with genome instability in yeast (Prado and Aguilera 2005; Tran et al. 2017). Interestingly, replisome pausing and the onset of DNA damage at tDNAs appear to be mechanistically separable: cooriented collisions in the absence of both Pif1 helicases impede a substantial fraction of replisomes (Osmundson et al. 2017; Tran et al. 2017), but only head-on conflicts lead to a dramatic increase in R-loop-dependent gross chromosomal rearrangements (GCRs) (Tran et al. 2017). Similar to tDNAs, transcription-dependent mitotic recombination is strongly biased to the head-on orientation at a model protein-coding gene in yeast (Prado and Aguilera 2005). In humans, codirectional and head-on conflicts with RNA polymerase II (RNAPII) both impede the replisome, but elicit distinct signaling and damage outcomes, which are modulated by R-loop levels (Hamperl et al. 2017). Replication termination is enriched at the 3′-end of transcribed genes in humans (Chen et al. 2019), which are sites of RNAPII pausing (Glover-Cutter et al. 2008). This is consistent with RNAPII conflicts impeding fork movement more globally. R-loop-dependent genome instability has also been demonstrated at common fragile sites (Helmrich et al. 2011), and transcription-dependent instability is observed at highly transcribed genes (Barlow et al. 2013). Consistent with defects in RNA metabolism exacerbating genome instability in the context of a stalled replication fork, mutations that impair cotranscriptional RNA processing increase DNA damage in both yeast and humans (Paulsen et al. 2009; Stirling et al. 2012). While R loops are associated with DNA damage at tDNAs, they do not substantially impede replisome progression at these sites (Osmundson et al. 2017). In prokaryotes, head-on conflicts are mutation hotspots (Srivatsan et al. 2010; Merrikh et al. 2011) and unresolved R loops impede replisome passage at a highly transcribed head-on gene (Lang et al. 2017). In addition, a cooriented conflict leads to DNA damage if RNA polymerase backtracking is increased (Dutta et al. 2011). Thus, there exists a complex relationship between the ultimate outcome of a conflict, the orientation of the transcribed gene, and the transcriptional state of RNA polymerase.
Although tDNAs in yeast serve as a model for eukaryotic replication-transcription conflicts, our knowledge of how these sites asymmetrically impede fork passage is incomplete. tRNAs, along with other short noncoding RNAs including 5S ribosomal RNA and U6 small nuclear RNA, are transcribed by RNA polymerase III (RNAPIII). RNAPIII is a 17-subunit complex that shares some structural and functional homology with RNA polymerase I and RNAPII (Arimbasseri et al. 2014). De novo tRNA transcription is initiated by TFIIIC binding to internal A and B box promoter elements (Orioli et al. 2012), which subsequently recruits TFIIIB to a position ∼40 bp upstream of the transcription start site (Paule and White 2000). Bdp1, one of three subunits of TFIIIB, facilitates the formation of a stable TFIIIB-DNA complex (Colbert and Hahn 1992; Shah et al. 1999; Cloutier et al. 2001; Kassavetis et al. 2005; Abascal-Palacios et al. 2018) resistant to dissociation by heparin and molar salt concentrations (Kassavetis et al. 1990). TFIIIB recruits and positions RNAPIII over the transcription initiation region, where Bdp1 stimulates allosteric rearrangement of RNAPIII subunits C37 and C34 in a manner that promotes template melting, resulting in a transcription-competent RNAPIII (Abascal-Palacios et al. 2018). RNAPIII recruitment to tDNAs requires all three subunits of TFIIIB (Khoo et al. 2014), as well as the C82-C34-C31 heterotrimeric subcomplex of RNAPIII (Brun et al. 1997).
Eliminating TFIIIC binding by a B-block point mutation (Baker et al. 1986) removed fork pausing in wild-type and rrm3Δ yeast (Ivessa et al. 2003). Furthermore, deleting terminator sequences tripled the length of the tRNA transcript (Allison 1985) without affecting replisome pausing (Ivessa et al. 2003). Based on these findings, it was suggested that replication-fork pausing at tDNAs is caused by the transcription initiation complex (Ivessa et al. 2003).
tDNAs in S. cerevisiae are also constitutive condensin binding sites (D’Ambrosio et al. 2008; Haeusler et al. 2008). By FISH, tDNAs are sequestered in the nucleolus throughout the cell cycle, dependent on interactions between condensin subunits Smc2 and Smc4, TFIIIB subunit Brf1, and TFIIIC subunit Tfc1 (Haeusler et al. 2008). Depletion of the condensin subunit Brn1 leads to a decrease in intrachromosomal interactions genome-wide, and a loss of interchromosomal homotypic interactions at a subset of tDNAs (Paul et al. 2018). Apart from tDNAs, condensin is also enriched at centromeres and rDNA genes (Wang et al. 2016; Lazar-Stefanita et al. 2017; Schalbetter et al. 2017).
Unsurprisingly given their high transcriptional output (Moir and Willis 2013), tDNAs are sites of high RNAPIII transcription complex occupancy and abundant R loops (El Hage et al. 2014). Convergent DNA and RNA polymerases may generate high levels of supercoiling between them, forming a topological barrier with a small footprint that makes it difficult for topoisomerases to resolve (Wang 2002). Any combination of these factors (the transcription initiation complex, RNAPIII occupancy, R loops, and supercoiling) could contribute to the polar barrier at tDNAs. Here, we systematically deplete tDNA-associated protein complexes and assay replication-fork progression genome-wide by sequencing Okazaki fragments.
We find that transcription impedes replication-fork progression, but observe that TFIIIB creates a strong polar replication barrier even in the absence of transcription. Disruption of condensin binding does not stimulate replisome progression past tDNAs. However, impaired fork progression can be partially suppressed via inactivation of the Rad18-mediated postreplicative repair pathway or the loss of either type 1 or type 2 topoisomerase activity.
Materials and Methods
Yeast strains and propagation
All strains were derived from W303 RAD5+, and most have the anchor away background: tor1-1::HIS3, fpr1::NatMX4, RPL13A-2xFKBP12::TRP1. The exceptions are TLSΔ rrm3Δ, rad18Δ rrm3Δ, and their experimental controls (Figure 3, rrm3Δ, wild type). All sequenced strains have a doxycycline-repressible DNA ligase (cdc9::tetO7-CDC9 cmv-LacI-URA3). Protein tagging and gene knockout was carried out by PCR and lithium acetate transformations. Strains were grown in YPD at 30°. For spot tests, cells were grown to log phase and plated in a 1:5 dilution series. All experiments were carried out in three biological replicate strains derived from one or more crosses, except forBRN1-FRB; rrm3Δ, which has two biological and one technical replicate.
Figure 3.
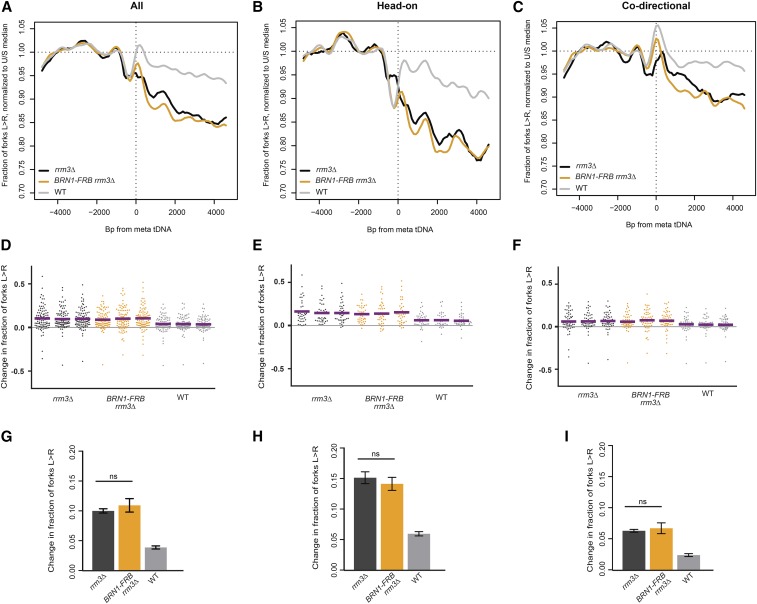
Loss of condensin binding via Brn1 depletion has no effect on replisome progression at tDNAs. (A) Replisome direction around tDNAs transcribed in either direction (n = 93), (B) head-on (n = 39), or (C) codirectional (n = 54) relative to the replication fork, and >5 kb away from the closest origin. (D–F) Change in fork direction around tDNAs transcribed in either direction (n = 93), (E) only head-on (n = 39), or (F) only codirectional (n = 54) with respect to replication forks. Mean change from each replicate is indicated by a purple line. (G–I) Grand mean and SD of the change in replication direction from above. Significance was determined by Monte Carlo resampling. tDNAs are >5 kb away from the closest origin. WT, dox-repressible CDC9 strain congenic with rrm3∆.
FACS analysis
Cells were released and collected from G1 arrest and fixed in 70% ethanol. Fixed cells were then spun down and resuspended in 50 mM sodium citrate with RNase A (50-153-8126; Fisher Scientific) for 1 hr at 50°. Samples were subsequently incubated with proteinase K (219350480; MP Biomedicals) for 1 hr at 50°, and stained with SYTOX green (S7020; Fisher Scientific). Samples were sonicated before processing with a Becton Dickinson Accuri.
Northern blots
Total RNA was extracted by phenol freeze. Briefly, 10 µg of total RNA was loaded onto 10% denatured PAGE gels and run for 1 hr. Samples were semidry-transferred onto zeta-probe membrane (1620159; Bio-Rad) and blocked overnight with 125 µg/ml denatured fish sperm in 6× SSPE, 0.1% SDS, 2× Denhardt’s. Membranes were subsequently probed overnight with 20 pmol of radiolabeled oligos against U4 RNA (5′-CCATGAGGAGACGGTCTGG-3′) and tS(CGA)C (5′-TATTCCCACAGTTAACTGCGGTCAAGATATTT-3′). All blocking and probing steps were carried out at 37°. Membranes were washed twice with 6× SSPE, 0.1% SDS before exposing to phosphoimager screens.
Okazaki fragment enrichment and library generation
Asynchronous cultures were grown to midlog phase, and rapamycin (1 µg/ml) was added for 1 hr as required. DNA ligase was repressed for 2.5 hr with 40 µg/ml doxycycline. Cells were pelleted and Okazaki fragments were purified and deep-sequenced as previously described with some changes (Smith and Whitehouse 2012). Briefly, genomic DNA was extracted from spheroblasts using zymolyase-20T (NC0516655; Sunrise Science Products) in sorbitol-sodium citrate-EDTA buffer (1 M sorbitol, 100 mM sodium citrate, 60 mM EDTA, pH 7.0). Spheroblasts were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0, 50 mM EDTA, 100 mM NaCl, 1.5% sarkosyl) and digested with proteinase K (219350480; MP Biomedicals) at 42° for 2–2.5 hr. DNA was precipitated and treated with RNase (RNase Cocktail Enzyme Mix; Thermo Fisher Scientific) overnight at 4° in sodium chloride-Tris HCl-EDTA buffer (1M NaCl, 200 mM Tris-HCl, 100 mM EDTA). Okazaki fragments were enriched by sequential elutions from Source15Q ion exchange columns (89128-854; Avantor VWR). Up to 1 µg of fragments were ligated to adaptor primer pairs overnight at 16° and cleaned (GeneJET PCR Purification; Thermo Fisher Scientific) before second-strand synthesis with Taq polymerase (New England Biolabs) at 50° for 15min. Products between 200 and 600 bp were purified from 2% agarose gels run in 0.5× TAE using QIAquick kits (QIAGEN, Valencia, CA), and excess adaptors were removed by magnetic beads (HighPrep PCR; MagBio Genomics) before and after amplification (Phusion polymerase; New England Biolabs).
Sequencing was carried out on the Illumina NextSeq500 platform. All libraries are paired-end except for one biological replicate from each of the following: rrm3Δ in the anchor away background, RPC25-FRB RPC82-FRB rrm3Δ, and BDP1-FRB rrm3Δ. Sequencing data for rrm3Δ and wild-type replicates in Figure 4 are from Osmundson et al. (2017).
Figure 4.
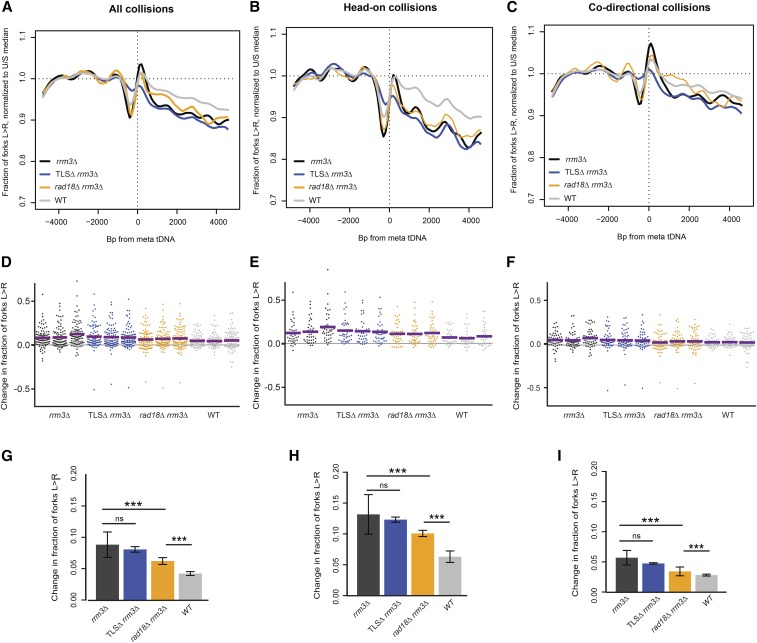
Rad18 impedes fork progression at tDNAs. (A and D) Replisome direction around tDNAs transcribed in either direction (n = 93), (B and E) head-on (n = 39), or (C and F) codirectional (n = 54) relative to the replication fork, and >5 kb away from the closest origin. Wild-type and rrm3Δ data are from Osmundson et al. (2017). (G) Grand mean of the change in fork direction around tDNAs transcribed in either direction (n = 93), (H) only head-on (n = 39), or (I) only codirectional (n = 54) with respect to replication forks. Significance was determined by Monte Carlo resampling; *** P < 0.0001. tDNAs are >5 kb away from the closest origin. Wild-type and rrm3Δ sequencing data in A–C are from Osmundson et al. (2017). WT, congenic dox-repressible CDC9 strain.
Computational methods
bowtie2 (version 2.2.9) was used to align FASTQ files to the S. cerevisiae reference genome (Saccharomyces Genome Database, R64-2-1), and samtools (version 1.3.1) was used to select and sort reads with MAPQ score ≥40. BEDPE/BED files were generated using bedtools (version 2.26.0); genomecov was used to calculate Watson and Crick read depth with one-based coordinates. The fraction of total reads mapping to either the Watson or Crick strand was calculated in 100-bp bins using an in-house Python script. Rightward-moving forks were quantified as the fraction of reads mapping to the Crick strand and vice versa. Leftward- and rightward-moving forks were first quantified separately, then leftward forks were flipped bioinformatically such that all forks could be represented as “rightward”-moving forks. Using R, the average fraction of rightward-moving forks over a 10-kb window around the meta-tDNA = [rev (fraction of Watson reads from leftward-moving forks) + [(1−(fraction of Watson reads from rightward-moving forks))]/2. Line graphs were smoothed over 200 bp. The analysis focused on fork progression ∼93 tDNAs (Osmundson et al. 2017) that were neither near sequence gaps nor replication origins.
Monte Carlo resampling
We evaluated the significance of the change in replication direction between data sets by Monte Carlo resampling, as previously described (Osmundson et al. 2017). Briefly, data across two different strains (e.g., wild type vs. TLSΔ) were randomly sampled 10,000 times, and the grand mean of the change in fork direction was calculated. The P-value is the number of times that the resampled data recreated a difference in means greater than or equal to the actual difference in means between the data sets.
Data availability
Sequencing data have been submitted to the Gene Expression Omnibus under accession number GSE139860. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11862216.
Results
Quantifying replication-fork progression at tDNAs in mutant strains
The Pif1 and Rrm3 helicases were previously shown to facilitate replication-fork progression through tDNAs oriented in either direction relative to replication in yeast (Osmundson et al. 2017; Tran et al. 2017), but the precise nature of the obstacle to replication has not been clearly defined. To dissect the individual contributions of transcription per se, transcription factors, condensin, and DNA supercoiling to the impediment of replication forks, we designed a panel of S. cerevisiae strains where potential replisome blocks at tDNAs were depleted, either by gene knockout or using the anchor away system (Haruki et al. 2008) for essential genes. To elucidate the impact of TFIIIB binding and RNAPIII recruitment on fork progression, we depleted TFIIIB subunit Bdp1. We also depleted RNAPIII subunit C25 (RPC25), which is required for transcription initiation (Zaros and Thuriaux 2005), and subunit C82 (RPC82), which facilitates recruitment by TFIIIB (Brun et al. 1997) and tDNA promotor opening (Brun et al. 1997; Abascal-Palacios et al. 2018). Condensin subunit Brn1 was depleted to assess the effect of higher-order clustering on fork progression, and topoisomerases Top1 and Top2 were depleted to determine the impact of supercoiling around replication-transcription conflicts.
All mutants were combined with deletion of RRM3 and a repressible allele of CDC9, encoding DNA ligase I. The use of rrm3Δ as a strain background ensures that the impediment of fork progression at tDNAs is pronounced but not maximal, such that both increased and decreased fork progression should be detectable. In the presence of wild-type Rrm3, the stalling at tDNAs is sufficiently small that there are no detectable differences in fork progression upon depletion of the candidate barriers (Supplemental Material, Figure S1A). The dox-repressible CDC9 allele allows unligated Okazaki fragments to be enriched for sequencing as the basis to assay replication-fork progression.
We first validated anchor-away-mediated depletion of essential proteins. Conditional depletion of essential factors recapitulated the lethal phenotypes of null mutants (Figure S1B). To assay the effects of gene deletion or protein depletion on tRNA transcription, we carried out Northern blots against the intron of tS(CGA)C (SUP61) such that only nascent pre-tRNAs are detected (Sethy-Coraci et al. 1998). Northern blotting confirmed that tRNA transcription was quickly reduced below readily detectable levels within 20 min of nuclear depletion of C25 or Bdp1 (Figure 1A), and could be continually repressed for at least 3.5 hr—the maximum duration of experiments described here. Transcription shut-off in RPC25-FRB RPC82-FRB was equally fast (data not shown), and there was no visible difference in effect on transcription between C25 depletion alone or C25 and C82 double depletion.
Figure 1.
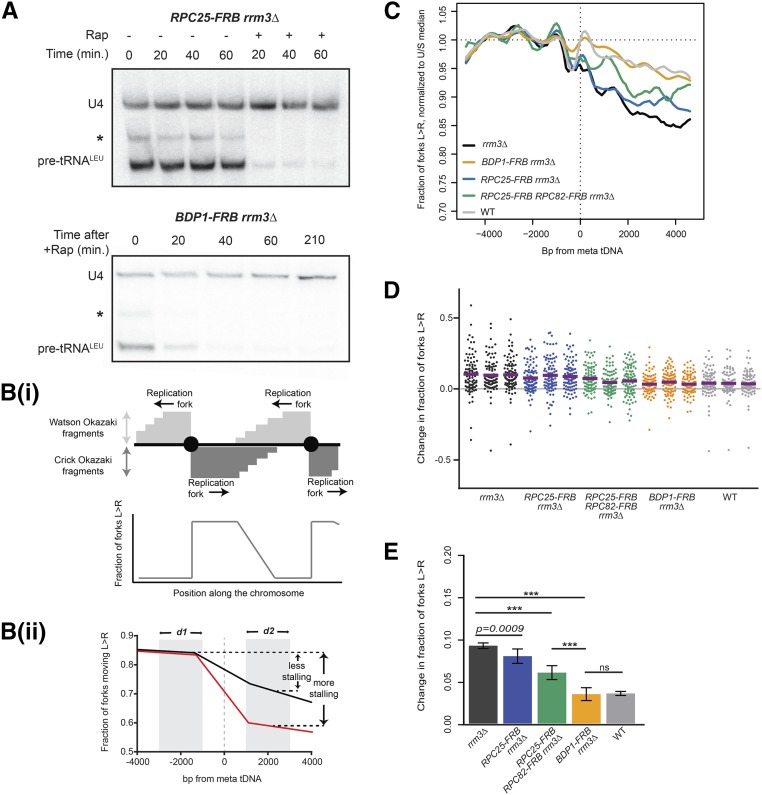
The RNAPIII transcription complex impedes the replisome at tDNAs. (A) Northern blots using an intronic probe against tRNALeu from RPC25-FRB;rrm3∆ and BDP1-FRB;rrm3∆ upon rapamycin-induced nuclear depletion of FRB-tagged proteins. * indicates a background band that we presume to be a longer pre-tRNA isoform or intermediate, 10 μg total RNA/lane. Loading control: U4 small nuclear RNA. (B) Unligated Okazaki fragments map to either the Watson or Crick strand. (Bi) Leftward-moving replication forks generate Watson fragments, and rightward-moving forks generate Crick fragments. (Bottom) The average direction of replication forks at every position across the genome can be quantified as the ratio of Crick:Watson reads. (Bii) A schematic of average fork direction ±5 kb around a meta-tDNA. tDNAs replicated by predominantly leftward or rightward-moving forks were analyzed separately, then superimposed such that fork direction is always presented as rightward-moving forks (Materials and Methods). Shaded areas ±1–3 kb around the meta-tDNA (d1 and d2) indicate the regions used to quantify the change in fork direction around the meta-tDNA. (C) Replisome direction around tDNAs (n = 93) that are at least 5 kb away from origins. Values are normalized to the median fraction of rightward-moving forks upstream of the tDNA, and smoothed over 200 bp. Leftward-moving forks were bioinformatically “flipped” such that they are represented as rightward-moving forks; see Materials and Methods. (D) Change in fork direction at individual tDNAs (n = 93) from three biological replicates of each strain background. Mean change indicated by purple line. (E) Grand mean and SD of the change in replication direction from above. Significance was determined by Monte Carlo resampling; *** P < 0.0001. WT, congenic anchor away strain (Haruki et al. 2008).
We quantified replication-fork progression genome-wide by repressing DNA ligase (CDC9) for 2.5 hr following 1 hr of rapamycin treatment to deplete the protein of interest from the nucleus, and sequenced Okazaki fragments from an asynchronous population in a strand-specific manner (Figure 1Bi) (Smith and Whitehouse 2012). Rapamycin treatment was maintained during ligase repression to ensure continued depletion of the protein of interest. As previously established, Okazaki fragment sequencing captures replication fork pauses that allow convergent forks to complete replication downstream of the impediment (Osmundson et al. 2017). Convergent forks are captured because fork direction is quantified from the strand bias of Okazaki fragments around the meta-tDNA, which includes fragments from convergent forks downstream of the tDNA. Thus, fork progression around any genomic element can be visualized as a decrease in the number of forks moving in a given direction, which is proportional to the number of Okazaki fragments mapping to a particular strand (Figure 1B; see Materials and Methods). This decrease can be quantified as the difference between the average number of rightward-moving forks upstream vs. downstream of the meta-tDNA (Figure 1Bii, d1 and d2). As previously described, we used the regions ±1–3 kb up- and downstream of the meta-tDNA, and focused our analysis on the 93 of 275 nuclear tDNAs in S. cerevisiae that are not within 5 kb of an origin or sequence gap (Osmundson et al. 2017).
TFIIIB (Bdp1) impedes replisome passage at tDNAs in a transcription-independent manner in rrm3Δ cells
In the absence of Rrm3 helicase, we observed a pronounced change in replication direction around tDNAs compared to wild-type cells, consistent with our previous analysis of these sites (Osmundson et al. 2017) (Figure 1, C–E). Replication direction data for a meta-tDNA are shown in Figure 1C for one representative replicate, and the fraction of replication forks converging within ±1 kb of each individual tDNA for each replicate is shown in Figure 1D; the mean percentage of fork convergence within ±1 kb of these tDNAs across replicates is shown in Figure 1E. In wild-type cells with the anchor away background, 3.9% of replication termination events occur within the analyzed region, compared with 9.9% in rrm3Δ.
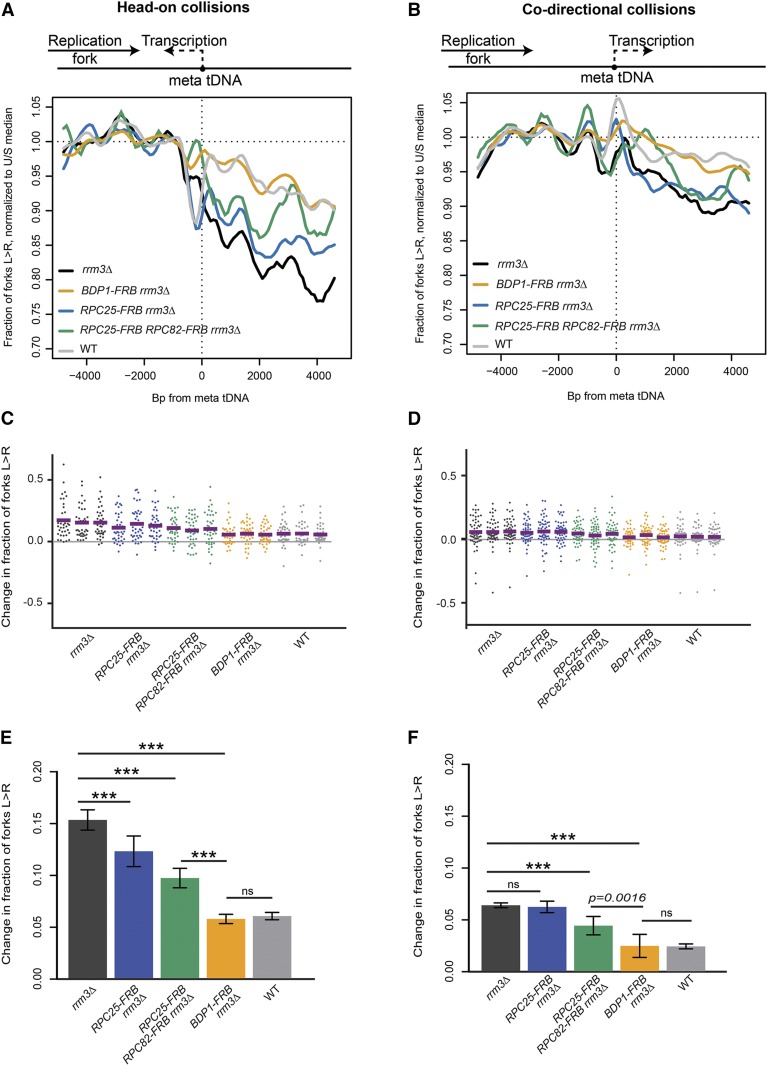
Depletion of the RNAPIII subunit C25 prior to Okazaki fragment collection in the rrm3Δ background moderately reduced fork progression at tDNAs, with 7.7% (P = 0.0009) of forks converging at these sites (Figure 1E). However, concurrent depletion of C82 and C25 yielded a significant reduction in fork stalling (6.5%, P < 0.0001), although it was not a full rescue compared to the termination events in wild-type cells. Since C25 depletion abrogates detectable transcription (Figure 1A), this suggests that transcribing RNAPIII acts as a barrier to fork progression but is unlikely to be the sole obstacle. By contrast, analogous nuclear depletion of the TFIIIB subunit Bdp1 dramatically reduced fork convergence at tDNAs from 10 to 3.3% (P < 0.0001), essentially restoring a wild-type phenotype (Figure 1, C–E). This result is consistent with the fact that TFIIIB is recruited to tDNAs before RNAPIII, and stays bound to the DNA for repeated rounds of transcription (Arimbasseri et al. 2014). Therefore, in the absence of C25 or a transcribing RNAPIII, fork progression is still hindered by the presence of TFIIIB and/or associated factors. The same result was obtained when all 275 nuclear tDNAs were included in the analysis (Figure S1C); note that the apparent loss of strand bias upstream of the meta-tDNA is caused by the presence of proximal replication origins. For this reason, all subsequent analyses was limited to tDNAs located >5 kb away from replication origins.
TFIIIB (Bdp1) and RNAPIII (C25, C82) present an asymmetric obstacle to replication-fork progression in rrm3Δ
We and others have previously found that fork stalling at tDNAs is orientation dependent (Deshpande and Newlon 1996; Osmundson et al. 2017; Tran et al. 2017). As expected, head-on collisions were more likely to cause fork stalling than cooriented collisions in rrm3Δ strains (Figure 2). Bdp1 depletion in rrm3Δ reduced fork convergence at tDNAs from 15.1 to 5.6% (P < 0.0001) for head-on collisions (Figure 2, A, C, and E), and from 6.3 to 2.4% (P < 0.0001) for codirectional collisions (Figure 2, B, D, and F), in both cases reducing the impediment to fork progression to a level that is indistinguishable from an RRM3 wild-type strain. Consistent with the weaker effect of RNAPIII depletion on fork progression, C82 and C25 dual depletion reduced fork stalling to 9.6% (P < 0.0001) for head-on collisions and 4.3% (P < 0.0001) for codirectional collisions. We conclude that the asymmetric replication-fork pausing observed at tDNAs is maintained in the absence of ongoing transcription, and that TFIIIB itself is an asymmetric impediment to fork progression in rrm3Δ.
Figure 2.
TFIIIB and RNAPIII are asymmetric obstacles to the replication fork. (A and B) Replisome direction around tDNAs transcribed (A) head-on (n = 39) or (B) codirectionally (n = 54) relative to the replication fork, and >5 kb away from the closest origin. (C and D) Change in fork direction around tDNAs as shown in A and B. (E and F) Grand mean and SD of the change in replication direction from above. Significance was determined by Monte Carlo resampling; *** P < 0.0001. WT, congenic anchor away strain.
Loss of condensin (Brn1) binding has no effect on replisome progression at tDNAs in rrm3Δ cells
tDNAs are cis-acting sites of condensin binding, and the maintenance of tDNA clustering requires direct interaction between condensin and TFIIIB/TFIIIC (D’Ambrosio et al. 2008; Haeusler et al. 2008). We depleted condensin subunit Brn1 from the nucleus to determine whether the role of condensin in DNA compaction impedes replication-fork progression at tDNAs. The Brn1-FRB construct has previously been described, and rapamycin treatment of this strain leads to dramatic loss of higher-order chromosome structure consistent with substantial loss of condensin binding (Paul et al. 2018). Nuclear depletion of Brn1 in the rrm3Δ background did not significantly rescue replication-fork progression at the 93 tDNAs analyzed, with 10.9% of forks converging at these sites compared to 10% in rrm3Δ alone (P = 0.6713) (Figure 3, A, D, and G). Analysis of fork progression around tDNAs separated by orientation relative to replication confirmed that condensin binding has no effect on fork pausing at tDNAs (Figure 3, B, C, E, F, H, and I). Fork progression did not change at the subset of tDNAs that lose interchromosomal interactions upon Brn1 depletion (Figure S2) (Paul et al. 2018). Since a global loss of condensin binding and interchromosomal interactions does not rescue fork progression, we conclude that neither condensin nor the long-range chromosomal interactions it facilitates present a significant obstacle to replisome passage at tDNAs in rrm3Δ.
Preventing Rad18-dependent template switching partially suppresses replication-fork pausing at tDNAs in rrm3Δ cells
DNA damage tolerance pathways ensure the completion of genome replication in the presence of replisome-blocking DNA damage (Cipolla et al. 2016). The two main pathways include error-prone lesion bypass by translesion synthesis (TLS) polymerases, and error-free template switching, where the sister chromatid is used as a template to bypass fork blocks and DNA damage. Both pathways require the ubiquitylation of PCNA by E3 ligase Rad18 (Ulrich 2011). To assay the effect of DNA damage tolerance pathways on replication through tDNAs, we deleted RAD18 and analyzed replisome progression. The absence of Rad18 in rrm3Δ cells significantly reduced head-on fork pausing at tDNAs from 13.2 to 10.1% (Figure 4B, P < 0.0001), and from 5.7 to 3.4% for codirectional collisions (Figure 4C, P < 0.0001). We conclude that the presence of functional DNA damage tolerance pathways impedes the replisome at S. cerevisiae tDNAs in rrm3Δ.
One possible underlying cause for the increased replication-fork pausing in the presence of Rad18 could be slow replication at these sites by TLS polymerases. Even though TLS has been suggested to predominantly act on damaged DNA behind replication forks (Karras and Jentsch 2010), TLS polymerases could be recruited to stalled or paused forks (Marians 2018). To test whether any TLS polymerase was responsible for modulating replication past tDNAs, we analyzed replisome progression in a rev1Δ rev3Δ rad30Δ rrm3Δ strain lacking all TLS polymerases (TLSΔ). In contrast to deletion of RAD18, loss of TLS polymerases had no effect on replisome progression at tDNAs (Figure 4). Fork progression analysis at our 93 tDNAs showed an average of 8.8 and 8.1% fork stalling in rrm3Δ and TLSΔ rrm3Δ, respectively, but this difference was not statistically significant (P = 0.0934). Given that TLSΔ had no effect on replisome progression at tDNAs, our data suggests that factors recruited via the Rad18 pathway for Rad5-dependent template switch may directly or indirectly hinder replisome passage through tDNAs.
Topoisomerases 1 and 2 redundantly facilitate replication-fork pausing
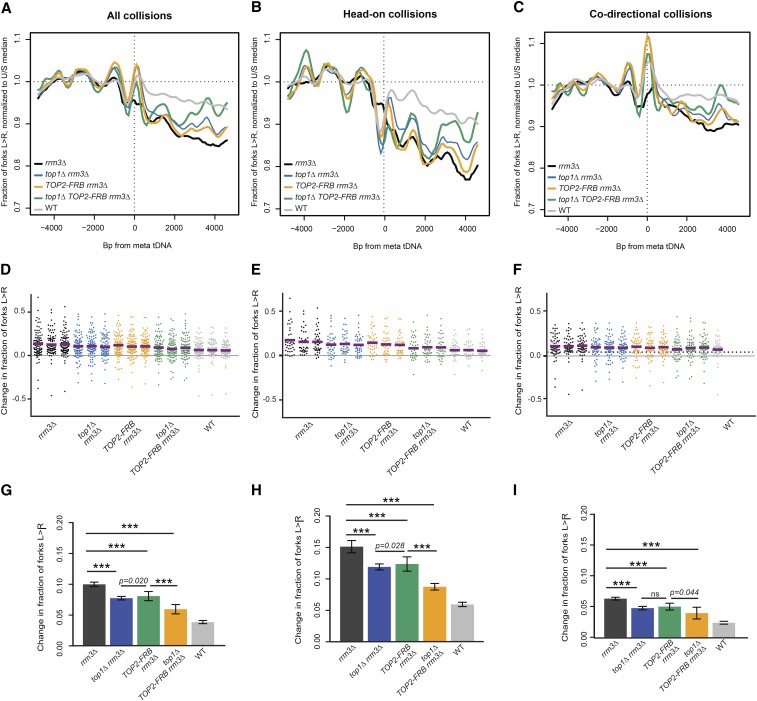
The unwinding of the DNA duplex during replication creates supercoils in the unreplicated portions of the genome ahead of the fork. This mechanical strain is also transmitted behind the fork, where sister chromatids intertwine, forming precatenanes that prevent accurate chromatid segregation during mitosis. Type 1 topoisomerases (e.g., yeast Top1) makes a transient nick on a single strand to relax positively supercoiled DNA accumulated at unreplicated regions. Type 2 topoisomerases (e.g., Top2) cut both strands of DNA to resolve precatenanes behind the replication fork. DNA replication can be completed with either Top1 or Top2, but loss of Top2 leads to the formation of cruciform structures that activate the checkpoint at the M/G1 transition (Bermejo et al. 2007). The absence of both Top1 and Top2 can cause an accumulation of positive supercoiling (Bermejo et al. 2007; Yeeles et al. 2015; Deegan et al. 2019), leading to persistent Rad9-dependent checkpoint activation and, ultimately, S-phase arrest (Bermejo et al. 2007). We constructed top1Δ rrm3Δ, TOP2-FRB rrm3Δ and top1Δ TOP2-FRB rrm3Δ strains with repressible CDC9 for Okazaki fragment analysis. Rapamycin-mediated nuclear depletion of Top2-FRB has previously been described (Bermejo et al. 2007), and our nuclear depletion of Top2 in a top1Δ strain recapitulated the expected S-phase accumulation (Figure S3A). The absence of Top1 and Top2 activity did not affect tRNA transcription (Figure S3B), as expected from the previously described phenotype of a top1-1; top2-1 mutant at the restrictive temperature (Brill et al. 1987).
Our hypothesis was that the simultaneous loss of Top1 and Top2 would exacerbate fork stalling at tDNAs in rrm3Δ by increasing the buildup of supercoils adjacent to the gene. However, contrary to this expectation, the absence of topoisomerases in rrm3Δ significantly reduced fork stalling near tDNAs (Figure 5). Only 7.8 and 7.7% of forks were stalled after Top1 deletion or Top2 depletion, respectively, compared to 10.4% in rrm3Δ (P < 0.0001). Simultaneous Top1 and Top2 depletion further reduced fork stalling to 5.1% (P < 0.0001), indicating that Top1 and Top2 act redundantly to increase rather than decrease the extent of fork stalling around tDNAs (Figure 5). Interestingly, unlike the effect of Bdp1 (TFIIIB) depletion, the change in fork progression in topoisomerase-deficient cells was not precisely centered on the tDNA itself, but rather manifested more diffusely between 1 and 5 kb downstream from the tDNAs (Figure 5, A–C, compare to Figure 1C and Figure 2, A and B).
Figure 5.
Topoisomerases facilitate fork stalling at tDNAs. (A and D) Replisome direction around tDNAs transcribed in either direction (n = 93), (B and E) head-on (n = 39), or (C and F) codirectional (n = 54) relative to the replication fork, and >5 kb away from the closest origin. Wild-type and rrm3∆ data are from Osmundson et al. (2017). (G) Grand mean of the change in fork direction around tDNAs transcribed in either direction (n = 93), (H) only head-on (n = 39), or (I) only codirectional (n = 54) with respect to replication forks. Significance was determined by Monte Carlo resampling; *** P < 0.0001. tDNAs are >5 kb away from the closest origin. Wild-type and rrm3∆ sequencing data in A–C are from Osmundson et al. (2017). WT, congenic anchor away strain.
Discussion
The progression of replication forks through a compact genome, especially at hard-to-replicate sites, is modulated by many factors. Our data indicate that TFIIIB is an asymmetric barrier to replication forks at tDNAs, whereas RNAPIII-mediated transcription is less of an impediment. We also observed that topoisomerases and Rad18 maintain fork pausing at tDNAs in wild-type cells, possibly to promote DNA repair at these sites. Replication-fork progression is the result of complex interplay between factors that cause fork stalling, and those that maintain this stalling to protect genome integrity.
Implications for replication-transcription conflicts
When tDNAs were first identified as polar blocks to replication more than two decades ago (Deshpande and Newlon 1996), transcription by RNAPIII was determined to be integral for this impediment to replication via the use of a temperature-sensitive rpc160 allele. It was subsequently shown by two-dimensional gel that preventing TFIIIC binding restored fork progression at tDNAs, but transcription read-through did not affect replisome pausing (Ivessa et al. 2003). Our data support the idea that the most significant barrier to replisome progression is the occupancy of transcription factors, namely TFIIIB, rather than transcription.
Replication-fork progression is only slightly reduced when C25 is sequestered outside the nucleus, and remains prominent when C82 is additionally depleted (Figure 1 and Figure 2). C25 depletion leads to impaired transcription initiation (Zaros and Thuriaux 2005), and as expected, that alone was sufficient to virtually shut off tRNA transcription within 20 min (Figure 1B). This indicates that transcription per se is not required to impede the replication fork. Double depletion of C25 and C82 led to a greater reduction in fork progression, presumably because RNAPIII is not bound to TFIIIB without C82 (Brun et al. 1997), so the replication fork was only impeded by TFIIIB and not TFIIIB-RNAPIII. We saw the greatest rescue of fork progression when Bdp1 was depleted, leaving only TFIIIC bound at tDNAs. Since Bdp1 facilitates TFIIIB’s resistance to dissociation (Colbert and Hahn 1992; Shah et al. 1999; Cloutier et al. 2001; Kassavetis et al. 2005; Abascal-Palacios et al. 2018), we infer that TFIIIB is the primary impediment to the replication fork at tDNAs in the absence of Rrm3. TFIIIC is more labile compared to TFIIIB; tRNA transcription in S. cerevisiae involves extensive recycling of RNAPIII (Dieci and Sentenac 2003), mediated by the continued association of TFIIIB with the promoter after TFIIIC displacement during active transcription (Ferrari et al. 2004; Soragni and Kassavetis 2008; Acker et al. 2013). Our data suggest that TFIIIC is not a substantial impediment to the replication fork in the absence of Rrm3 (Figure 1, C–E).
Although tRNA transcription levels during S phase can be modulated by Maf1 (Upadhya et al. 2002) and Cdk1 (Herrera et al. 2018), high transcription is constitutive, and maintaining tDNAs in a transcriptionally competent state is apparently sufficient to impede replication. However, because gross chromosomal rearrangements at tDNA-proximal replication forks are caused by transcription-dependent R loops (Tran et al. 2017), continued TFIIIB association itself is unlikely to induce genome instability. We propose that TFIIIB occupancy has the greatest effect on replication fork progression, and RNAPIII transcription leads to genome instability.
In contrast to tDNAs in S. cerevisiae, which are dispersed throughout the genome but preferentially oriented in the direction of replication (Osmundson et al. 2017), human tDNAs are predominantly organized in large clusters with no apparent orientation bias (Mungall et al. 2003). S. cerevisiae and other unicellular eukaryotes experience strong selective pressure for increased rates of protein synthesis, while mammalian cells are subject to distinct evolutionary constraints and are therefore less likely to maximize the transcription of individual tRNAs. Therefore, these loci are unlikely to be as relevant for genome integrity in higher eukaryotes than in yeast and other unicellular models. However, replication termination in the human genome is enriched at the ends of RNAPII-transcribed genes (Chen et al. 2019); these sites are notable as sites of transcriptional pausing (Proudfoot 2016), which suggests that collisions between the replication fork and transcriptionally inactive polymerases are frequent in mammals, albeit with RNAPII rather than RNAPIII. It is unclear why S. cerevisiae RNAPII does not induce such localized replication-fork stalling: possibly the higher processivity required to allow mammalian polymerases to transcribe megabase-scale genes renders mammalian RNAPII a harder-to-displace obstacle. Regardless, because at least a fraction of relevant collisions with the replication fork appear to occur with polymerases that are not actively transcribing at the time of the collision, transcription per se might represent an imperfect measure of how a given locus will affect replication-fork progression.
Replisome progression through a structured chromatin environment
tDNAs are highly enriched for condensin binding in S. cerevisiae (D’Ambrosio et al. 2008; Haeusler et al. 2008). The association of condensin at these sites is downstream of TFIIIC recruitment and mediates the clustering of condensin-associated tDNAs (Haeusler et al. 2008). tDNA clustering is lost along with other intra- and interchromosomal interactions upon condensin depletion (Paul et al. 2018), but this destruction of chromosome architecture does not increase the likelihood of replisome passage at tDNAs (Figure 3). It is likely that the higher-order structure maintained by condensin is more labile than the protein-DNA interactions that facilitate tRNA transcription, at least during S phase.
Accessory factors that enhance replication-fork stalling at tDNAs
Our data demonstrate that the absence of topoisomerases significantly decreases the extent to which replication forks stall at tDNAs (Figure 5). The role of topoisomerases at these loci is likely to be complex. Topoisomerase 1 has been shown to promote fork progression by removing R loops in mammalian cells (Tuduri et al. 2009) and tDNAs were sites of increased Top2 binding in a defective chromatin-remodeling background in S. cerevisiae (Swanston et al. 2019). Top2 was required for the transcription of long (>3 kb) transcripts by RNAPII (Joshi et al. 2012) in yeast, but not required for transcription of short transcripts like U4 small nuclear RNA or RNAPIII-transcribed genes (Figure S3B). In support of this, inhibition of Top1 and Top2 had no effect on 5S RNA and tRNA transcription in HeLa cells in vitro (Gottesfeld 1986). Topoisomerases play a role in facilitating programmed fork arrest and chromatin stabilization, which allows for the binding of potential fork impediments (Teves and Henikoff 2014). The promotion of fork stalling around tDNAs by topoisomerases is supported by recent work demonstrating that topoisomerases are recruited by Tof1 to stabilize forks (Shyian et al. 2019), though it is unclear how the supercoiling-related activity of topoisomerases is balanced and regulated with respect to its fork stabilization activity.
Similar to topoisomerases, loss of Rad18 decreases replication-fork pausing at tDNAs (Figure 4). Deletion of all three TLS polymerases did not recapitulate this phenotype, indicating that the decreased fork progression observed in rad18Δ was not due to the slow activity of these polymerases. Components of the Rad5-Ubc13-Mms2-dependent template switching pathway represent plausible candidates for modulating fork stalling. This is supported by the fact that Rad5-dependent template switching suppresses duplication-mediated GCRs but not single-copy sequence-mediated GCRs (Putnam et al. 2010), which are elevated in head-on conflicts at tDNAs in rrm3Δ (Tran et al. 2017) . Template switching up to 75 kb downstream of collapsed forks was detected in S. pombe (Jalan et al. 2019), and an alternative method of recombination-dependent fork restart, known as HoRReR, has also been reported downstream of a polar replication-fork block (Lambert et al. 2010; Miyabe et al. 2015).
Rad5 also recruits TLS polymerases to single-strand DNA gaps in S phase by PCNAK164Ub in response to replication stress by HU treatment or in pol32Δ (Gallo et al. 2019). However, given that the TLSΔ strain had no effect on fork progression, it appears unlikely that replication-transcription conflicts at tDNAs create substrates for TLS-mediated repair. This suggests that Rad5-dependent template switching could be the primary mechanism for maintaining fork progression at replication-transcription conflicts. Slower fork progression at tDNAs may allow for the recruitment of DNA repair factors and subsequent processing of DNA damage. It will be interesting to determine the mechanism(s) by which Rad18 directly or indirectly impedes replication-fork progression at hard-to-replicate loci.
Acknowledgments
We thank members of the Smith laboratory for helpful discussions and critical review of the paper. This work was funded by the National Institute of General Medical Sciences (grant R01GM127336 to D.J.S.).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11862216.
Communicating editor: J. Surtees
Literature Cited
- Abascal-Palacios G., Ramsay E. P., Beuron F., Morris E., and Vannini A., 2018. Structural basis of RNA polymerase III transcription initiation. Nature 553: 301–306. 10.1038/nature25441 [DOI] [PubMed] [Google Scholar]
- Acker J., Conesa C., and Lefebvre O., 2013. Yeast RNA polymerase III transcription factors and effectors. Biochim. Biophys. Acta 1829: 283–295. 10.1016/j.bbagrm.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Ait Saada A., Lambert S. A. E., and Carr A. M., 2018. Preserving replication fork integrity and competence via the homologous recombination pathway. DNA Repair (Amst.) 71: 135–147. 10.1016/j.dnarep.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. S., 1985. Effects of alterations in the 3′ flanking sequence on invivo and in vitro expression of the yeast SUP4-o tRNA Tyr gene. EMBO J. 4: 2657–2664. 10.1002/j.1460-2075.1985.tb03984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzu A., Bermejo R., Begnis M., Lucca C., Piccini D. et al. , 2012. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell 151: 835–846. 10.1016/j.cell.2012.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri A. G., Rijal K., and Maraia R. J., 2014. Comparative overview of RNA polymerase II and III transcription cycles, with focus on RNA polymerase III termination and reinitiation. Transcription 5: e27639 10.4161/trns.27369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky A., Giresi P. G., Lieb J. D., and Zakian V. A., 2009. Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol. Cell 34: 722–734. 10.1016/j.molcel.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. R., Gabrielsen O., and Hall B. D., 1986. Effects of tRNA Tyr point mutations on the binding of yeast RNA polymerase III transcription factor C. J. Biol. Chem. 261: 5275–5282. [PubMed] [Google Scholar]
- Barlow J. H., Faryabi R. B., Callen E., Wong N., Malhowski A. et al. , 2013. Identification of early replicating fragile sites that contribute to genome instability. Cell 152: 620–632. 10.1016/j.cell.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P., Hochstrasser M., Jongeneel C. V., and Alberts B. M., 1983. Properties of the T4 bacteriophage DNA replication apparatus: the T4 dda DNA helicase is required to pass a bound RNA polymerase molecule. Cell 34: 115–123. 10.1016/0092-8674(83)90141-1 [DOI] [PubMed] [Google Scholar]
- Bermejo R., Doksani Y., Capra T., Katou Y. M., Tanaka H. et al. , 2007. Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation. Genes Dev. 21: 1921–1936. 10.1101/gad.432107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo R., Capra T., Gonzalez-Huici V., Fachinetti D., Cocito A. et al. , 2009. Genome-organizing factors Top2 and Hmo1 prevent chromosome fragility at sites of S phase transcription. Cell 138: 870–884. 10.1016/j.cell.2009.06.022 [DOI] [PubMed] [Google Scholar]
- Blow J. J., Ge X. Q., and Jackson D. A., 2011. How dormant origins promote complete genome replication. Trends Biochem. Sci. 36: 405–414. 10.1016/j.tibs.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., and Sternglanz R., 1987. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. Nature 326: 414–416 (erratum: Nature 326: 812). 10.1038/326414a0 [DOI] [PubMed] [Google Scholar]
- Brun I., Sentenac A., and Werner M., 1997. Dual role of the C34 subunit of RNA polymerase III in transcription initiation. EMBO J. 16: 5730–5741. 10.1093/emboj/16.18.5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Keegan S., Kahli M., Tonzi P., Fenyo D. et al. , 2019. Transcription shapes DNA replication initiation and termination in human cells. Nat. Struct. Mol. Biol. 26: 67–77. 10.1038/s41594-018-0171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla L., Maffia A., Bertoletti F., and Sabbioneda S., 2016. The regulation of DNA damage tolerance by ubiquitin and ubiquitin-like modifiers. Front. Genet. 7: 105 (erratum: Front. Genet. 7: 184). 10.3389/fgene.2016.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier T. E., Librizzi M. D., Mollah A. K. M. M., Brenowitz M., and Willis I. M., 2001. Kinetic trapping of DNA by transcription factor IIIB. Proc. Natl. Acad. Sci. USA 98: 9581–9586. 10.1073/pnas.161292298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert T., and Hahn S., 1992. A yeast TFIIB-related factor involved in RNA polymerase III transcription. Genes Dev. 6: 1940–1949. 10.1101/gad.6.10.1940 [DOI] [PubMed] [Google Scholar]
- Cortez D., 2015. Preventing replication fork collapse to maintain genome integrity. DNA Repair (Amst.) 32: 149–157. 10.1016/j.dnarep.2015.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio C., Schmidt C. K., Katou Y., Kelly G., Itoh T. et al. , 2008. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 22: 2215–2227. 10.1101/gad.1675708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan T. D., Baxter J., Ortiz Bazán M. Á., Yeeles J. T. P., and Labib K. P. M., 2019. Pif1-family helicases support fork convergence during DNA replication termination in eukaryotes. Mol. Cell 74: 231–244.e9. 10.1016/j.molcel.2019.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A. M., and Newlon C. S., 1996. DNA replication fork pause sites dependent on transcription. Science 272: 1030–1033. 10.1126/science.272.5264.1030 [DOI] [PubMed] [Google Scholar]
- Dieci G., and Sentenac A., 2003. Detours and shortcuts to transcription reinitiation. Trends Biochem. Sci. 28: 202–209. 10.1016/S0968-0004(03)00054-9 [DOI] [PubMed] [Google Scholar]
- Dutta D., Shatalin K., Epshtein V., Gottesman M. E., and Nudler E., 2011. Linking RNA polymerase backtracking to genome instability in E. coli. Cell 146: 533–543. 10.1016/j.cell.2011.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A., Webb S., Kerr A., and Tollervey D., 2014. Genome-wide distribution of RNA-DNA hybrids identifies RNase H targets in tRNA genes, retrotransposons and mitochondria. PLoS Genet. 10: e1004716 10.1371/journal.pgen.1004716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R., Rivetti C., Acker J., and Dieci G., 2004. Distinct roles of transcription factors TFIIIB and TFIIIC in RNA polymerase III transcription reinitiation. Proc. Natl. Acad. Sci. USA 101: 13442–13447. 10.1073/pnas.0403851101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Kim T., Szakal B., Saayman X., Narula A. et al. , 2019. Rad5 recruits error-prone DNA polymerases for mutagenic repair of ssDNA gaps on undamaged templates. Mol. Cell 73: 900–941.e9. 10.1016/j.molcel.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Glover-Cutter K., Kim S., Espinosa J., and Bentley D. L., 2008. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat. Struct. Mol. Biol. 15: 71–78. 10.1038/nsmb1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., 1986. Novobiocin inhibits RNA polymerase III transcription in vitro by a mechanism distinct from DNA topoisomerase II. Nucleic Acids Res. 14: 2075–2088. 10.1093/nar/14.5.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler R. A., Pratt-Hyatt M., Good P. D., Gipson T. A., and Engelke D. R., 2008. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 22: 2204–2214. 10.1101/gad.1675908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamperl S., and Cimprich K. A., 2016. Conflict resolution in the genome: how transcription and replication make it work. Cell 167: 1455–1467. 10.1016/j.cell.2016.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamperl S., Bocek M. J., Saldivar J. C., Swigut T. and Cimprich K. A., 2017. Transcription-replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell 170: 774–786.e19. 10.1016/j.cell.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H., Nishikawa J., and Laemmli U. K., 2008. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol. Cell 31: 925–932. 10.1016/j.molcel.2008.07.020 [DOI] [PubMed] [Google Scholar]
- Helmrich A., Ballarino M., and Tora L., 2011. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell 44: 966–977. 10.1016/j.molcel.2011.10.013 [DOI] [PubMed] [Google Scholar]
- Herrera M. C., Chymkowitch P., Robertson J. M., Eriksson J., Boe S. O. et al. , 2018. Cdk1 gates cell cycle-dependent tRNA synthesis by regulating RNA polymerase III activity. Nucleic Acids Res. 46: 11698–11711 (erratum: Nucleic Acids Res. 46: 12188–12189). 10.1093/nar/gky846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa A. S., Lenzmeier B. A., Bessler J. B., Goudsouzian L. K., Schnakenberg S. L. et al. , 2003. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol. Cell 12: 1525–1536. 10.1016/S1097-2765(03)00456-8 [DOI] [PubMed] [Google Scholar]
- Jalan M., Oehler J., Morrow C. A., Osman F., and Whitby M. C., 2019. Factors affecting template switch recombination associated with restarted DNA replication. eLife 8: e41697. 10.7554/eLife.41697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. S., Piña B., and Roca J., 2012. Topoisomerase II is required for the production of long Pol II gene transcripts in yeast. Nucleic Acids Res. 40: 7907–7915. 10.1093/nar/gks626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras G. I., and Jentsch S., 2010. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 141: 255–267. 10.1016/j.cell.2010.02.028 [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., and Geiduschek E. P., 1990. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell 60: 235–245. 10.1016/0092-8674(90)90739-2 [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Soragni E., Driscoll R., and Geiduschek E. P., 2005. Reconfiguring the connectivity of a multiprotein complex: fusions of yeast TATA-binding protein with Brf1, and the function of transcription factor IIIB. Proc. Natl. Acad. Sci. USA 102: 15406–15411. 10.1073/pnas.0507653102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo S. K., Wu C. C., Lin Y. C., Lee J. C., and Chen H. T., 2014. Mapping the protein interaction network for TFIIB-related factor Brf1 in the RNA polymerase III preinitiation complex. Mol. Cell. Biol. 34: 551–559. 10.1128/MCB.00910-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S., and Carr A. M., 2013. Impediments to replication fork movement: stabilisation, reactivation and genome instability. Chromosoma 122: 33–45. 10.1007/s00412-013-0398-9 [DOI] [PubMed] [Google Scholar]
- Lambert S., Mizuno K., Blaisonneau J., Martineau S., Chanet R. et al. , 2010. Homologous recombination restarts blocked replication forks at the expense of genome rearrangements by template exchange. Mol. Cell 39: 346–359. 10.1016/j.molcel.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Lang K. S., Hall A. N., Merrikh C. N., Ragheb M., Tabakh H. et al. , 2017. Replication-transcription conflicts generate R-loops that orchestrate bacterial stress survival and pathogenesis. Cell 170: 787–799.e18. 10.1016/j.cell.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar-Stefanita L., Scolari V. F., Mercy G., Muller H., Guerin T. M. et al. , 2017. Cohesins and condensins orchestrate the 4D dynamics of yeast chromosomes during the cell cycle. EMBO J. 36: 2684–2697. 10.15252/embj.201797342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians K. J., 2018. Lesion bypass and the reactivation of stalled replication forks. Annu. Rev. Biochem. 87: 217–238. 10.1146/annurev-biochem-062917-011921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikh H., Machon C., Grainger W. H., Grossman A. D., and Soultanas P., 2011. Co-directional replication-transcription conflicts lead to replication restart. Nature 470: 554–557. 10.1038/nature09758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe I., Mizuno K., Keszthelyi A., Daigaku Y., Skouteri M. et al. , 2015. Polymerase δ replicates both strands after homologous recombination-dependent fork restart. Nat. Struct. Mol. Biol. 22: 932–938. 10.1038/nsmb.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir R. D., and Willis I. M., 2013. Regulation of pol III transcription by nutrient and stress signaling pathways. Biochim. Biophys. Acta 1829: 361–375. 10.1016/j.bbagrm.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungall A. J., Palmer S. A., Sims S. K., Edwards C. A., Ashurst J. L. et al. , 2003. The DNA sequence and analysis of human chromosome 6. Nature 425: 805–811. 10.1038/nature02055 [DOI] [PubMed] [Google Scholar]
- Orioli A., Pascali C., Pagano A., Teichmann M., and Dieci G., 2012. RNA polymerase III transcription control elements: themes and variations. Gene 493: 185–194. 10.1016/j.gene.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Osmundson J. S., Kumar J., Yeung R., and Smith D. J., 2017. Pif1-family helicases cooperatively suppress widespread replication-fork arrest at tRNA genes. Nat. Struct. Mol. Biol. 24: 162–170. 10.1038/nsmb.3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeschke K., Capra J. A., and Zakian V. A., 2011. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell 145: 678–691. 10.1016/j.cell.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M. R., Markowitz T. E., Hochwagen A., and Ercan S., 2018. Condensin depletion causes genome decompaction without altering the level of global gene expression in Saccharomyces cerevisiae. Genetics 210: 331–344. 10.1534/genetics.118.301217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule M. R., and White R. J., 2000. Transcription by RNA polymerases I and III. Nucleic Acids Res. 28: 1283–1298. 10.1093/nar/28.6.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen R. D., Soni D. V., Wollman R., Hahn A. T., Yee M. C. et al. , 2009. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol. Cell 35: 228–239. 10.1016/j.molcel.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk N., Kahli M., d’Aubenton-Carafa Y., Jaszczyszyn Y., Shen Y. et al. , 2016. Replication landscape of the human genome. Nat. Commun. 7: 10208 10.1038/ncomms10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourkarimi E., Bellush J. M., and Whitehouse I., 2016. Spatiotemporal coupling and decoupling of gene transcription with DNA replication origins during embryogenesis in C. elegans. eLife 5: e21728. 10.7554/eLife.21728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F., and Aguilera A., 2005. Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J. 24: 1267–1276. 10.1038/sj.emboj.7600602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., 2016. Transcriptional termination in mammals: stopping the RNA polymerase II juggernaut. Science 352: aad9926 10.1126/science.aad9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam C. D., Hayes T. K., and Kolodner R. D., 2010. Post-replication repair suppresses duplication-mediated genome instability. PLoS Genet. 6: e1000933 10.1371/journal.pgen.1000933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M. K., Winzeler E. A., Collingwood D., Hunt S., Wodicka L. et al. , 2001. Replication dynamics of the yeast genome. Science 294: 115–121. 10.1126/science.294.5540.115 [DOI] [PubMed] [Google Scholar]
- Rocha E. P., and Danchin A., 2003. Gene essentiality determines chromosome organisation in bacteria. Nucleic Acids Res. 31: 6570–6577. 10.1093/nar/gkg859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri N., McDonald K. R., Webb C. J., Cristea I. M., and Zakian V. A., 2012. DNA replication through hard-to-replicate sites, including both highly transcribed RNA Pol II and Pol III genes, requires the S. pombe Pfh1 helicase. Genes Dev. 26: 581–593. 10.1101/gad.184697.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalbetter S. A., Goloborodko A., Fudenberg G., Belton J. M., Miles C. et al. , 2017. SMC complexes differentially compact mitotic chromosomes according to genomic context. Nat. Cell Biol. 19: 1071–1080. 10.1038/ncb3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethy-Coraci I., Moir R. D., Lopez-de-Leon A., and Willis I. M., 1998. A differential response of wild type and mutant promoters to TFIIIB70 overexpression in vivo and in vitro. Nucleic Acids Res. 26: 2344–2352. 10.1093/nar/26.10.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. M. A., Kumar A., Geiduschek E. P., and Kassavetis G. A., 1999. Alignment of the B” subunit of RNA polymerase III transcription factor IIIB in its promoter complex. J. Biol. Chem. 274: 28736–28744. 10.1074/jbc.274.40.28736 [DOI] [PubMed] [Google Scholar]
- Shyian M., Albert B., Zupan A. M., Ivanitsa V., Charbonnet G. et al. , 2020. Fork pausing complex engages topoisomerases at the replisome. Genes. Dev. 34: 87–98. 10.1101/gad.331868.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., and Whitehouse I., 2012. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature 483: 434–438. 10.1038/nature10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soragni E., and Kassavetis G. A., 2008. Absolute gene occupancies by RNA polymerase III, TFIIIB, and TFIIIC in Saccharomyces cerevisiae. J. Biol. Chem. 283: 26568–26576. 10.1074/jbc.M803769200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan A., Tehranchi A., MacAlpine D. M., and Wang J. D., 2010. Co-orientation of replication and transcription preserves genome integrity. PLoS Genet. 6: e1000810 10.1371/journal.pgen.1000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling P. C., Chan Y. A., Minaker S. W., Aristizabal M. J., Barrett I. et al. , 2012. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 26: 163–175. 10.1101/gad.179721.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanston A., Zabrady K., and Ferreira H. C., 2019. The ATP-dependent chromatin remodelling enzyme Uls1 prevents Topoisomerase II poisoning. Nucleic Acids Res. 47: 6172–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves S. S., and Henikoff S., 2014. Transcription-generated torsional stress destabilizes nucleosomes. Nat. Struct. Mol. Biol. 21: 88–94. 10.1038/nsmb.2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P. L. T., Pohl T. J., Chen C. F., Chan A., Pott S. et al. , 2017. PIF1 family DNA helicases suppress R-loop mediated genome instability at tRNA genes. Nat. Commun. 8: 15025 10.1038/ncomms15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuduri S., Crabbé L., Conti C., Tourrière H., Holtgreve-Grez H. et al. , 2009. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 11: 1315–1324 [corrigenda: Nat. Cell Biol. 12: 1122 (2010)]. 10.1038/ncb1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H. D., 2011. Timing and spacing of ubiquitin-dependent DNA damage bypass. FEBS Lett. 585: 2861–2867. 10.1016/j.febslet.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Upadhya R., Lee J., and Willis I. M., 2002. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol. Cell 10: 1489–1494. 10.1016/S1097-2765(02)00787-6 [DOI] [PubMed] [Google Scholar]
- Wang J. C., 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3: 430–440. 10.1038/nrm831 [DOI] [PubMed] [Google Scholar]
- Wang D., Mansisidor A., Prabhakar G., and Hochwagen A., 2016. Condensin and Hmo1 mediate a starvation-induced transcriptional position effect within the ribosomal DNA array. Cell Rep. 14: 1010–1017 (erratum: Cell Rep. 17: 624). 10.1016/j.celrep.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., 1999. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24: 437–440. 10.1016/S0968-0004(99)01460-7 [DOI] [PubMed] [Google Scholar]
- Yeeles J. T., Deegan T. D., Janska A., Early A., and Diffley J. F., 2015. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature 519: 431–435. 10.1038/nature14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaros C., and Thuriaux P., 2005. Rpc25, a conserved RNA polymerase III subunit, is critical for transcription initiation. Mol. Microbiol. 55: 104–114. 10.1111/j.1365-2958.2004.04375.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data have been submitted to the Gene Expression Omnibus under accession number GSE139860. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11862216.