Caenorhabditis is a group of nematodes that contains the important model organism C. elegans. Several chromosome-level genome assemblies exist for species within this group, but it has been a challenge to fully assemble the genome...
Keywords: Caenorhabditis remanei, Caenorhabditis elegans, chromosome-level assembly, comparative genomics
Abstract
The nematode Caenorhabditis elegans is one of the key model systems in biology, including possessing the first fully assembled animal genome. Whereas C. elegans is a self-reproducing hermaphrodite with fairly limited within-population variation, its relative C. remanei is an outcrossing species with much more extensive genetic variation, making it an ideal parallel model system for evolutionary genetic investigations. Here, we greatly improve on previous assemblies by generating a chromosome-level assembly of the entire C. remanei genome (124.8 Mb of total size) using long-read sequencing and chromatin conformation capture data. Like other fully assembled genomes in the genus, we find that the C. remanei genome displays a high degree of synteny with C. elegans despite multiple within-chromosome rearrangements. Both genomes have high gene density in central regions of chromosomes relative to chromosome ends and the opposite pattern for the accumulation of repetitive elements. C. elegans and C. remanei also show similar patterns of interchromosome interactions, with the central regions of chromosomes appearing to interact with one another more than the distal ends. The new C. remanei genome presented here greatly augments the use of the Caenorhabditis as a platform for comparative genomics and serves as a basis for molecular population genetics within this highly diverse species.
THE free-living nematode Caenorhabditis elegans is one of the most-used and best-studied model organisms in genetics, developmental biology, and neurobiology (Brenner 1973, 1974; Blaxter 1998). C. elegans was the first multicellular organism with a complete genome sequence (C. elegans Sequencing Consortium 1998), and the C. elegans genome currently has one of the best-described functional annotations among metazoans, as well as possessing hundreds of large-scale data sets focused on functional genomics (Gerstein et al. 2010). The genome of C. elegans is compact, roughly 100 Mb [100.4 Mb is the “classic” N2 assembly (C. elegans Sequencing Consortium 1998); 102 Mb is the V2010 strain genome (Yoshimura et al. 2019)], and consists of six holocentric chromosomes, five of which are autosomes and one that is a sex chromosome (X). All chromosomes of C. elegans have a similar pattern of organization: a central region occupying about one-half of the chromosome that has a low recombination rate, low transposon density, and high gene density, and the “arms” display the characteristics exactly opposite to this (Waterston et al. 1992; Barnes et al. 1995; Rockman and Kruglyak 2009).
About 65 species of the Caenorhabditis genus are currently known (Kiontke et al. 2011), and for many of them genomic sequences are available (Stevens et al. 2019) (http://www.wormbase.org/ and https://evolution.wormbase.org). Most of the Caenorhabditis nematodes are outcrossing species with females and males (gonochoristic), but three species — C. elegans, C. briggsae, and C. tropicalis — reproduce primarily via self-fertilizing (“selfing”) hermaphrodites with rare males (androdioecy) (Kiontke et al. 2011). Caenorhabditis species have the XX/XO sex determination: females and hermaphrodites carry two copies of the X chromosomes, while males have only one X chromosome (Pires-daSilva 2007).
C. remanei is an obligate outcrossing nematode, a member of the “Elegans” supergroup, which has become an important model for natural variation (Jovelin et al. 2003; Reynolds and Phillips 2013), experimental evolution (Sikkink et al. 2014a, 2015, 2019; Castillo et al. 2015), and population genetics (Graustein et al. 2002; Cutter and Charlesworth 2006; Cutter et al. 2006; Dolgin et al. 2007; Jovelin et al. 2009; Dey et al. 2012). Whole-genome data are available for three strains of C. remanei (Table 1), but all of these assemblies are fragmented. To improve genomic precision for experimental studies and to facilitate the analysis of chromosome-wide patterns of genome organization, recombination, and diversity, the complete assembly for this species is required. We generated a chromosome-level genome assembly of the C. remanei PX506 inbred strain using a long-read/Hi-C approach, and used this new chromosome-level resolution in a comparative framework to reveal global similarities in genome organization and spatial chromosome interactions between C. elegans and C. remanei.
Table 1. Available genome assemblies of C. remanei.
| Strain | NCBI ID | Total size (Mb) | Number of scaffolds | Scaffold N50 (Mb) | Scaffold L50 | GC% | Number of genes |
|---|---|---|---|---|---|---|---|
| PB4611 | GCA_000149515.1 | 145.443 | 3,670 | 0.435 | 70 | 38.50 | 32,412 |
| PX356 | GCA_001643735.2 | 118.549 | 1,591 | 1.522 | 10 | 35.90 | 24,977 |
| PX439 | GCA_002259225.1 | 124.542 | 912 | 1.765 | 13 | 35.30 | 24,867 |
| PX506a | GCA_010183535.1 | 124.870 | 7 | 21.502 | 3 | 37.96 | 26,308 |
NCBI ID, National Center for Biotechnology Information identifier.
This study.
Materials and Methods
Nematode strains
Nematodes were maintained under standard laboratory conditions as described previously (Brenner 1974). C. remanei isolates were originally derived from individuals living in association with terrestrial isopods (family Oniscidea) collected from Koffler Scientific Reserve at Jokers Hill, King City, Toronto, Ontario, as described in Sikkink et al. (2014b). Strain PX393 was founded from a cross between single female and male C. remanei individuals isolated from isopod Q12. This strain was propagated for two to three generations before freezing. PX506, the source of the genome described here, is an inbred strain derived from PX393 following sibling mating for 30 generations to reduce within-strain heterozygosity. This strain was frozen and subsequently recovered at large population size for several generations before further experimental analysis.
Sequencing and genome assembly of the C. remanei reference
Strain PX506 was grown on 20 110-mm plates until its entire Escherichia coli food source (strain OP50) was consumed. Worms were washed 5× in M9 using 15-ml conical tubes and spun at a low speed to concentrate. The worm pellet was flash frozen and genomic DNA was isolated using a Genomic-tip 100/G column (QIAGEN, Valencia, CA). Next, 4 μg (average size 23 kb) was frozen and shipped to Dovetail Genomics (Santa Cruz, CA; https://dovetailgenomics.com), along with frozen whole animals for subsequent Pacific Biosciences (PacBio) and Hi-C analysis. The C. remanei PX506 inbred strain was sequenced and assembled by Dovetail Genomics. The primary contigs were generated from two PacBio single-molecule real-time (SMRT) Cells using the FALCON assembly (Chin et al. 2016) followed by Arrow polishing (https://github.com/PacificBiosciences/GenomicConsensus). The final scaffolds were constructed with Dovetail Genomics Hi-C library sequences and the HiRise software pipeline (Putnam et al. 2016). Additionally, we performed whole-genome sequencing of the PX506 strain using the Nextera kit (Illumina) for 100-bp paired-end read sequencing on the Illumina Hi-Seq 4000 platform (University of Oregon Sequencing Facility, Eugene, OR).
We then performed a BLAST (Basic Local Alignment Search Tool) search (Altschul et al. 1990) against the National Center for Biotechnology Information (NCBI) GenBank nucleotide database (Benson et al. 2012) and filtered any scaffolds (E-value <1e−15) of bacterial origin. Short scaffolds with good matches to Caenorhabditis nematodes were aligned to six chromosome-sized scaffolds by GMAP v.2018-03-25 (Wu and Watanabe 2005) and visualized in IGV v.2.4.10 (Thorvaldsdóttir et al. 2013) to examine whether they represent alternative haplotypes.
The final filtered assembly was compared to the “recompiled” version of the C. elegans reference genome generated from strain VC2010, a modern strain derived from the classical N2 strain (Yoshimura et al. 2019), and C. briggsae genomes (available under accession numbers PRJEB28388 from the NCBI Genome database and PRJNA10731 from WormBase WS260) by MUMmer3.0 (Kurtz et al. 2004). The names and orientations of the C. remanei chromosomes were defined by the longest total nucleotide matches in proper orientation to C. elegans chromosomes. Dot plots with these alignments were plotted using the ggplot2 package (Wickham 2016) in R (R Core Team 2018). The completeness of the C. remanei genome assembly was assessed by BUSCO v.3.0.2 (Simão et al. 2015) with the Metazoa odb9 and Nematoda odb9 databases. Results were visualized with generate_plot_xd_v2.py script (https://github.com/xieduo7/my_script/blob/master/busco_plot/generate_plot_xd_v2.py).
The mitochondrial genome was generated using a reference mitochondrial genome of C. remanei (KR709159.1) from the NCBI database (http://www.ncbi.nlm.nih.gov/nucleotide/) and Illumina reads of the C. remanei PX506 inbred strain. The reads were aligned with bwa mem v.0.7.17 (Li and Durbin 2009), filtered with samtools v.1.5 (Li et al. 2009a). We marked PCR duplicates in the mitochondrial assembly with MarkDuplicates from picard-tools v.2.0.1 (http://broadinstitute.github.io/picard/), realigned insertions/deletions and called variants with IndelRealignment and HaplotypeCaller in the haploid mode from GATK tools v.3.7 (McKenna et al. 2010), filtered low-quality sites, and then used bcftools consensus v.1.5 (Li 2011) to generate the new reference mitochondrial genome. To estimate the residual heterozygosity throughout the rest of the genome, we implemented a similar read-mapping protocol but used the default parameters to call genotypes and then filtered variants using standard hard filters (residual_heterozygosity.sh and plot_residual_heterozygosity.R).
Repeat masking in C. remanei and C. elegans
For repeat masking, we created a comprehensive repeat library (Coghlan et al. 2018; see also instructions at http://avrilomics.blogspot.com) and masked sequence-specific repeat motifs, as described in Woodruff and Teterina (2019 preprint). De novo repeat discovery was performed by RepeatModeler v.1.0.11 (Smit and Hubley 2008) with the NCBI engine. Transposon elements were detected by transposonPSI (http://transposonpsi.sourceforge.net), with sequences shorter than 50 bases filtered out. Inverted transposon elements were located with detectMITE v.2017-04-25 (Ye et al. 2016) with default parameters. Transfer RNAs were identified with tRNAscan-SE v.1.3.1 (Lowe and Eddy 1997) and their sequences were extracted from a reference genome by the getfasta tool from the BEDTools package v.2.25.0 (Quinlan and Hall 2010). We searched for LTR retrotransposons as described at http://avrilomics.blogspot.com/2015/09/ltrharvest.html, by LTRharvest and LTRdigest from GenomeTools v.1.5.11 (Gremme et al. 2013) with domains from the Gypsy Database (Llorens et al. 2010), and several models of Pfam protein domains (Finn et al. 2015), listed in Tables SB1 and SB2 of Steinbiss et al. (2009). To filter LTRs, we used two scripts: https://github.com/satta/ltrsift/blob/master/filters/filter_protein_match.lua and https://gist.github.com/avrilcoghlan/4037d6b8cca32eaf48b0.
Additionally, we uploaded nematode repeats from the Dfam database (Hubley et al. 2015) using the queryRepeatDatabase.pl script from the RepeatMasker v.4.0.7 (Smit et al. 2015) utilities with the “–species rhabditida” option, and C. elegans and ancestral repetitive sequences from Repbase v.23.03, (Bao et al. 2015). We then combined all repetitive sequences obtained from these tools and databases in one redundant repeat library. We clustered those sequences with < 80% identity by uclust from the USEARCH package v.8.0, (Edgar 2010) and classified them via the RepeatMasker Classify tool v.4.0.7, (Smit et al. 2015). Potential protein matches with C. remanei (PRJNA248911) or C. elegans protein sequences (PRJNA13758) from WormBase W260 were detected with BLASTX (Altschul et al. 1990). The repetitive sequences classified as “unknown” and having BLAST hits with E-value ≤ 0.001 with known protein-coding genes were removed from the final repeat libraries.
For C. remanei, the final repeat library was used by RepeatMasker with “–s” and “–gff” options. An additional round of masking was performed with the “–species caenorhabditis” option. The genome was also masked with the redundant repeat library acquired before the clustering step. Regions that were masked with the redundant library but not masked with the final library were extracted using BEDTools subtract and classified by RepeatMasker Classify. Additionally, we checked the depth coverage with the Illumina reads in these regions, as regions classified as a known type of repeat and displaying coverage > 70 were masked in the reference genome by BEDTools maskfasta. The masked regions were extracted to a bed file with a bash script (https://gist.github.com/danielecook/cfaa5c359d99bcad3200), and the same regions were soft masked by BEDTools maskfasta with the “–soft” option.
Using the same approach, we masked the C. elegans reference N2 strain (PRJNA13758 from WormBase W260) and then extracted all regions that were masked in the “official” masked version of the genome but not masked by our final repeat library. These regions were extracted, classified by RepeatMasker with default parameters, and searched against C. elegans proteins with the BLASTX algorithm and the C. elegans reference genome with BLASTN. Regions with unknown class and a match with C. elegans proteins (see above) were removed. Regions with > 5 matches and an E-value ≤ 0.001 with the C. elegans genome were added to the final database, and used to mask the C. elegans reference genome generated from strain VC2010 (Yoshimura et al. 2019). The same regions were soft-masked with BEDTools maskfasta.
Full-length transcript sequencing
We used single-molecule long-read RNA sequencing (Iso-Seq) to obtain high-quality transcriptomic data. We used the Clonetech SMARTer PCR complementary DNA (cDNA) Synthesis kit for cDNA synthesis and PCR amplification with no size selection starting with 500 ng of total RNA from a mixed-staged population of C. remanei strain PX506 (Cat#634925; Clonetech). PacBio library generation was performed on-site at the University of Oregon Genomics and Cell Characterization Core Facility and sequenced on a PacBio Sequel I platform utilizing four SMRT cells of data.
We generated circular consensus reads using the ccs tool with “–noPolish –minPasses 1” options from PacBio SMRT link tools v.5.1.0 (https://www.pacb.com/support/software-downloads/) and obtained full-length transcripts with lima from the same package with “–isoseq –no-pbi” options. Next, trimmed reads from all SMRT cells were merged together, clustered, and polished with isoseq3 tools v.3.2 (https://github.com/PacificBiosciences/IsoSeq), and mapped to the C. remanei reference genome with GMAP. Redundant isoforms were collapsed by collapse_isoforms_by_sam.py from Cupcake ToFU (https://github.com/Magdoll/cDNA_Cupcake). The longest ORFs were predicted with TransDecoder v.5.0.1 (Haas et al. 2013) and used as coding sequence (CDS) hints in the genome annotation (see below).
Genome annotation
We performed de novo annotation of the C. remanei genome using the following hybrid approach. For ab initio gene prediction, we applied the GeneMark-ES algorithm v.4.33 (Ter-Hovhannisyan et al. 2008) with default parameters. De novo gene prediction with the MAKER pipeline v.2.31.9 (Holt and Yandell 2011) was carried out with C. elegans (PRJNA13758), C. briggsae (PRJNA10731), and C. latens (PRJNA248912) proteins from WormBase 260, excluding the repetitive regions identified above. To implement gene prediction using the BRAKER pipeline v.2.1.0 (https://github.com/Gaius-Augustus/BRAKER), we included RNA-sequencing (RNA-seq) from our previous C. remanei studies (SRX3014311 and SRP049403).
Annotations from BRAKER2, MAKER2, and GeneMark-ES were combined in EVidenceModeler v.1.1.1 (Haas et al. 2008) with weights 6, 3, and 1, correspondingly. CDS from the EVidenceModeler results were used to train AUGUSTUS version 3.3 (Stanke et al. 2006) as described on http://bioinf.uni-greifswald.de/augustus/binaries/tutorial/training.html. Next, models were optimized and retrained again, then we created a file with extrinsic information with factor 1000 and malus 0.7 for CDS, and all other options as in “extrinsic.E.cfg” for annotation with est database hits from the AUGUSTUS supplemental files. The final annotation was executed with Iso-Seq data as the hints file and EVidenceModeler -trained models with “–singlestrand=true –gff3=on –UTR=off”. Scanning for known protein domains and the functional annotation were conducted with InterProScan v.5.27-66.0 (Quevillon et al. 2005). We validated/filtered final gene models according to coverage with RNA-seq and Iso-Seq data, matches with known Caenorhabditis proteins, and protein/transposon domains.
We identified one-to-one orthologs of C. remanei and C. elegans proteins using orthofinder2 (Emms and Kelly 2019); for C. elegans we used only proteins validated in the VC2010 (Yoshimura et al. 2019). The identities of the proteins were estimated by pairwise global alignments using calc_pc_id_between_seqs.pl script (https://gist.github.com/avrilcoghlan/5311008). Gene synteny plots were made in R with a custom script (synteny_plot.R).
Genome activity and features
We studied patterns of genome activity in C. elegans and C. remanei using C. elegans Hi-C data from the (Brejc et al. 2017) study (SRR5341677–SRR5341679) and the Hi-C reads produced in the current study, as well as available RNA-seq data from the L1 larval stage for C. elegans (SRR016680, SRR016681, and SRR016683) and C. remanei (SRP049403). Hi-C reads were mapped to the reference genomes with bwa mem and RNA-seq read with STAR v.2.5 (Dobin et al. 2013) using the default parameters and gene annotations; to count reads for transcripts, we used htseq-count from the HTSeq package v.0.9.1 (Anders et al. 2015) and corrected by the total lengths of the gene CDSs [reads per kilobase of transcript per million mapped reads (RPKM)] using a bash script (https://gist.github.com/darencard/fcb32168c243b92734e85c5f8b59a1c3) and a custom R script (RNA_seq_R_analysis_and_figures.R). For Hi-C interactions, we applied the Arima pipeline (https://github.com/ArimaGenomics/mapping_pipeline), BEDTools, and a custom bash script (Hi-C_analysis_with_ARIMA.sh and Hi-C_R_analysis_and_figures.R).
We calculated the fraction of exonic/intronic DNA and the number of genes per 100-kb windows from the genome annotations using the BEDtools coverage tool. GC content and the percent of repetitive regions were estimated, correspondingly, from the unmasked and hard-masked genomes via BEDtools nuc, also on 100-kb windows by a custom script (get_genomic_fractions.sh). For the formal statistical tests, we defined chromosome “centers” to be the central one-half of a chromosome and the “arms” to be the peripheral one-quarter of each length on either side of the center. To measure the positional effect of these genomic features, we conducted the Cohen’s d effect size test with package “lsr” in R (Navarro 2013) and calculated statistical differences using the Wilcoxon–Mann–Whitney test using basic R (see a custom script fractions_stats_and_figures.R).
Data availability
Strain PX506 is available from the Caenorhabditis Genetic Center. All raw sequencing data generated in this study have been submitted to the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/) under accession number PRJNA577507. This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under the accession WUAV00000000; the version described in this paper is version WUAV01000000. The reference genome assembly is available at the NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome/) under accession number GCA_010183535.1. Supplementary custom scripts to estimate statistics, and generate main and supplemental figures, are available on GitHub (https://github.com/phillips-lab/C.remanei_genome). Supplemental material available at figshare: https://doi.org/10.25386/genetics.11889099. All online resources mentioned in the manuscript were accessed in March 2020.
Results
New reference genome assembly and annotation
We generated a high-quality chromosome-level assembly of the C. remanei PX506 inbred line with deep PacBio whole-genome sequencing (∼100× coverage by 1.3 million reads) and Hi-C (∼900× with 418 million paired-end Illumina reads). Assembly of the PacBio sequences resulted in 135.85 Mb of genome and bacterial sequences with 298 scaffolds. The Hi-C data dramatically improved the PacBio assembly, and the HiRise scaffolding increased the N50 from 4.042 to 21.502 Mb by connecting scaffolds from the PacBio assembly together, resulting in 235 scaffolds (see the summary statistics in Table 1 and Supplemental Material, Tables S1–3). After the filtering of scaffolds of bacterial origin, six chromosome-sized scaffolds were obtained, as expected (Figure S1). Additionally, there were 180 short scaffolds that are alternative haplotypes or unplaced scaffolds (the average length is 31,169 nt with SD of 48,700 and a median length of 19,076 nt). Because only the long-sized fraction of total DNA was selected in the long-read library, the mitochondrial DNA was not covered by PacBio sequencing. The mitochondrial genome was therefore generated independently using the Illumina whole-genome data of the reference strain (see Materials and Methods). The total length of the new C. remanei reference genome without alternative haplotypes is 124,870,449 bp, which is very close in size to previous assemblies of other C. remanei strains (Table 1). After 30 generations of inbreeding, the residual heterozygosity of the PX506 line remained at 0.02% of SNPs (a 100-fold decrease relative to population-level variability; Dey et al. 2012). Most of the remaining polymorphic sites in PX506 are located in the peripheral parts of chromosomes, with one-half of all sites on the X chromosome (Figure S2).
To assess the quality of the new reference, we performed a standard BUSCO analysis (Simão et al. 2015). The new assembly of PX506 presented here has 975 of 982 BUSCO genes for completeness (97.9% based on the Nematode database) and displays fewer missed and duplicated genes than the previous assembly (PX356), but for the most part the BUSCO scores are very similar (see Figure S3).
We used full-length transcripts, RNA-seq data from previous C. remanei studies, known Caenorhabditis proteins, and ab initio predictions to annotate the C. remanei genome (see Materials and Methods). The final annotation contains 26,308 protein-coding genes, which is close to the number of annotated genes in other C. remanei strains (Table 1). Each of the genes predicted by AUGUSTUS has been validated by at least one type of evidence: 25,380 genes have hits with known Caenorhabditis proteins (23,840 with C. elegans, C. briggsae, and C. latens), including 25,373 that have matches with the previously annotated genes of C. remanei; 19,285 contain known protein domains or functional annotation; 18,662 were supported by RNA-seq data; and 8870 have full-transcript evidence derived from 19,410 high-quality isoforms from the Iso-Seq data. In addition, 27 genes were predicted from the full-transcript data.
Synteny of C. remanei and C. elegans
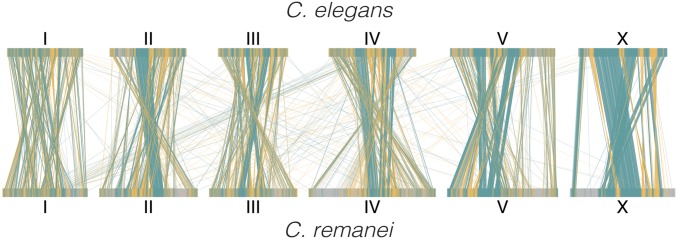
We identified 11,160 one-to-one orthologs of C. remanei and C. elegans protein-coding genes, which, after additional filtering on the global-alignment identity, resulted in 9247 ortholog pairs. Comparison of our new chromosome-level assembly to that of C. elegans revealed that the C. remanei and C. elegans genomes are in high synteny, despite having a very large number of within-chromosome rearrangements (only 120 of ortholog pairs are not located on homologous chromosomes). The distribution of orthologs across chromosomes is fairly uniform (chromosome I contains 1511 orthologs; II contains 1498; III contains 1473; IV contains 1472; V contains 1703; and X contains 1470). The central domains of autosomes and most regions of the X chromosomes are more highly conserved than the rest of the genome (Figure 1). Orthologs located on the X chromosome have greater global identity than ones located on autosomes (W = 532,160, P-value = 0.0128).
Figure 1.
Gene synteny plot of the 9247 one-to-one orthologs of C. elegans (the top row) and C. remanei (the bottom row). The lines connect locations of the orthologs on the C. elegans and C. remanei reference genomes. Teal lines represent genes in the same orientation, whereas orange lines show genes in an inverted orientation.
We chose the orientation of the C. remanei chromosomes based on the same/inverted directions of nucleotide alignments and one-to-one orthologs in C. elegans. However, it appears that the ancestral orientation of chromosome III is actually inverted relative to the C. elegans standard based on syntenic blocks between C. briggsae and C. remanei (e.g., C. elegans chromosome III has undergone large-scale inversion since divergence from the common ancestor of these three species, see the dot plots in Figure S4).
Organization of C. remanei and C. elegans chromosomes
To compare the genomic organization of C. remanei and C. elegans, we identified repetitive sequences in the C. remanei PX506 genome and the updated reference of C. elegans (Yoshimura et al. 2019). In total, 22.04% from 124.8 Mb of the C. remanei genome and 20.77% from 102 Mb of the C. elegans genome were repetitive. All homologous chromosomes of C. remanei are, on average, 22% longer than corresponding homologous chromosomes in C. elegans; the physical sizes of chromosomes I, II, III, IV, V, and X are 15.3, 15.5, 14.1, 17.7, 21.2, and 18.1 Mb in C. elegans and 17.2, 19.9, 17.8, 25.7, 22.5, and 21.5 Mb in C. remanei, respectively. These findings are consistent with the conclusions of Fierst et al. (2015) that the differences in the genome sizes of outcrossing and selfing Caenorhabditis species cannot be explained solely by an increase in transposable element abundance.
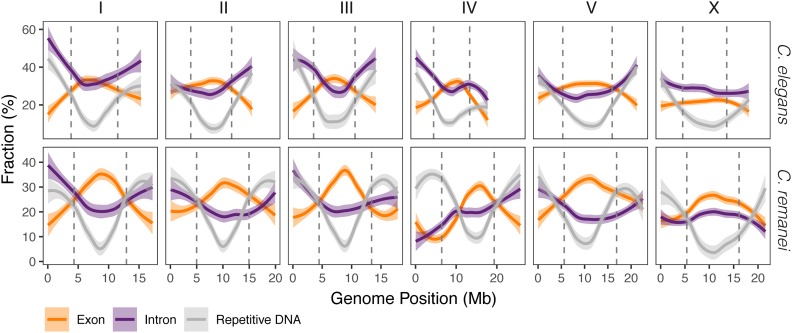
To identify finer-scale patterns displayed across each chromosome, we estimated fractions of exons, introns, and repetitive DNA per 100-kb windows (Figure 2), as well as GC content, gene counts, and gene fractions (Figure S5). In general, C. elegans and C. remanei display analogous patterns of organization across all chromosomes. Repetitive DNA was found in greater quantities in the peripheral parts of chromosomes of C. elegans (Cohen’s d = 1.58, W = 232,780, P-value < 2.2e−16) and C. remanei (Cohen’s d = 1.44, W = 332,810, P-value < 2.2e−16). Repetitive regions of C. elegans (VC2010) and C. remanei (PX506) genomes are available in Files S3 and S4.
Figure 2.
Genomic landscape of genetic elements for C. elegans and C. remanei. Vertical dashed lines show the boundaries of the central domain. Colored lines represent the smoothed means of the fraction of repetitive DNA (gray), exons (orange), and introns (purple) calculated from 100-kb windows. Shaded areas show 95% C.I.s of the mean.
Further, the fractions of the repetitive DNA in both species are negatively correlated with number of genes (r = −0.26 in C. elegans and r = −0.45 in C. remanei) and the exonic fractions (r = −0.43 and −0.63). There is an inverse positional effect with respect to the number of genes: more genes are located in the central domain than in the peripheral parts of chromosomes in C. elegans (Cohen’s d = 0.44, W = 93,950, P-value = 2.9e−15) and in the C. remanei genome (Cohen’s d = 0.72, W = 116,470, P-value < 2.2e−16), as has long been noted in C. elegans (Barnes et al. 1995). Both species have a similarly high density of genes (211.2 and 216.2 genes per megabase for C. elegans and C. remanei, respectively), which is one order of magnitude higher than for humans (Dunham et al. 2004). Not surprisingly, then, genes occupy a large fraction of the genome in both C. elegans (the mean fraction per 100 kb is 0.58, 95% C.I. 0.569–0.5836) and C. remanei (the mean fraction equals 0.44, 95% C.I. 0.436–0.452). Genes on the arms have longer total intron sizes then in the central domains (C. elegans: W = 53,932,000, P-value < 2.2e−16; C. remanei: W = 82,764,000, P-value < 2.2e−16). GC content, number genes, and gene fraction also differ between central and peripheral parts of chromosomes, as shown in Figure S5 and Table S4.
In both species, there is more intronic DNA in the peripheries of chromosomes than in their centers (Cohen’s d = 0.68, W = 177,730, P-value < 2.2e−16 for C. elegans; Cohen’s d = 0.32, W = 227,220, P-value = 1e−06 for C. remanei), although for C. remanei this effect is strongly driven by the different distributions of introns on chromosomes IV and X (Figure 2). Overall, 28.5 and 27.3% of total intron lengths consist of repetitive elements in C. elegans and C. remanei. Additionally, we investigated the transcriptional landscapes of the C. elegans and C. remanei genomes at the L1 larval stage, and found that the expression of genes in the central domain is very slightly, yet significantly, larger than gene expression in the peripheral domains (Cohen’s d = 0.06, W = 9,796,800, P-value < 2.2e−16 for C. elegans; Cohen’s d = 0.04, W = 29,629,000, P-value = 2.9e−14 for C. remanei); the chromosome-wise distribution of RPKM is shown in Figure S6.
Similar patterns of within-genome interactions
In examining the pattern of read mapping of Hi-C data across the C. remanei genome, we noted that the central domain of each chromosome appears to be enriched for interactions with the central domains of all other chromosomes (Figure S1). To explore this further, we examined the distances of three-dimensional (3D) interactions within chromosomes and proportions of interchromosomal contacts in C. remanei and C. elegans genomes. This analysis should be considered preliminary, as the data are likely noisy since they were obtained from mixed tissues and the C. remanei sample was collected from mixed developmental stages (including adult worms), whereas the C. elegans results are derived from a reanalysis of data from embryos (Brejc et al. 2017). At the moment, Hi-C data for different developmental stages of C. elegans and/or C. remanei are not publicly unavailable.
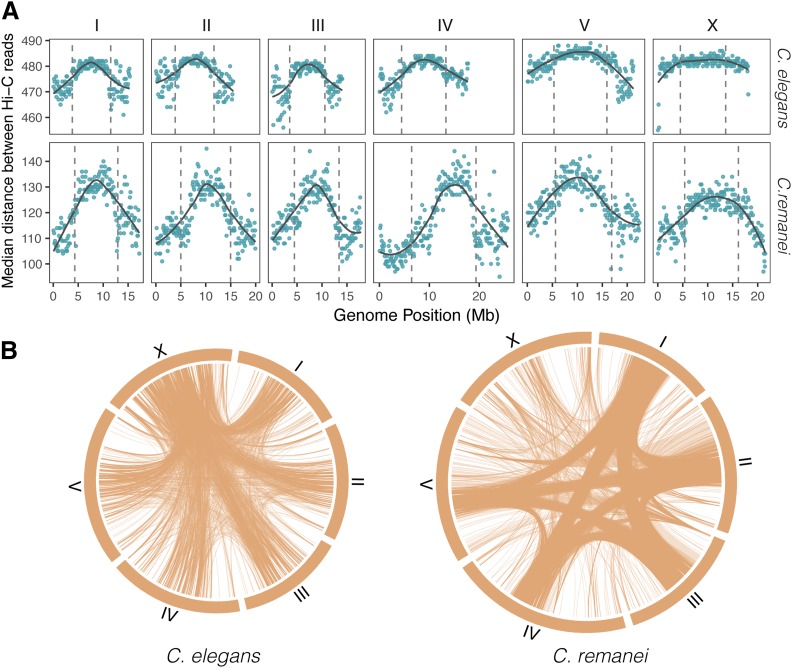
A total of 12% of the 199.2 million read pairs mapped to different chromosomes of C. remanei, which indicates a high level of potential trans-chromosome interactions. We observed an even higher proportion (32.7% from 123.9 million read pairs) of trans-chromosome contacts in the C. elegans sample. When we consider interactions within rather than between chromosomes, we find that the central domains tend to have a larger median distance between interaction pairs compared to the arms. This difference is significant within both species (Figure 3A; Cohen’s d = 1.46, W = 39,418, P-value < 2.2e−16 for C. elegans; Cohen’s d = 1.74, W = 45,396, P-value < 2.2e−16 for C. remanei).
Figure 3.
Genome landscape of median distances in Hi-C read pairs in C. elegans and C. remanei samples. (A) Distributions of distances between paired reads of cis-chromosome interactions. The vertical dashed lines show the boundaries of the central domain. Medians are estimated per 100-kb windows, with the gray lines representing the smoothed means of the values. The differences between values for C. elegans and C. remanei are due to the library size selection for Illumina sequencing in these two Hi-C experiments, and so only the relative patterns and not the absolute values are relevant here. (B) Trans-chromosome interactions in C. elegans and C. remanei. Lines represent contacts between 100-kb windows. For the C. elegans data set, only contacts with > 200 pairs of reads are shown; for the C. remanei data set, only contacts with > 100 pairs of reads are shown (the C. remanei Hi-C data set is two times smaller than the C. elegans data set). These filters emphasize differences in interaction density/location; the actual total number of interactions is approximately the same for all chromosomes.
Central domains tend to interact with other central domains in C. remanei (Figure 3A; 36.2% center–center contacts, 40.6% arm–center, and 19.3% arm–arm), but the proportion of center–center contacts in C. elegans is lower (27.8% center–center, 49.7% arm–center, and 22.5% arm–arm). The deviation from the expected uniform distribution (one center–center: two center–arm/arm–center: one arm–arm) of trans-chromosome interactions is larger in C. remanei than in C. elegans (χ2 = 330,220, d.f. = 2, P-value < 2.2e−16 for C. elegans; χ2 = 1,643,800, d.f. = 2, P-value < 2.2e−16 for C. remanei). All chromosomes, both in the C. elegans and C. remanei samples, have almost even numbers of contacts with other chromosomes (C. elegans: chromosome I has 15.1% from all interchromosomal contacts, II has 15.3%, III has 14.2%, IV has 17%, V has 19.5%, and X has 18.8%; C. remanei: I has 16.1%, II has 16.7%, III has 16.1%, IV has 18.2%, V has 18.2%, and X has 14.7%). However, if we focus specifically on windows with localized contacts we see that within C. remanei, interactions are more dispersed on X and V chromosomes and there are areas of thick contacts in the central parts of autosomes, whereas in the C. elegans sample all chromosomes actively interact (Figure 3B).
Discussion
We have generated a high-quality reference genome of the C. remanei line PX506, which is now one of the five currently available chromosome-level assemblies of Caenorhabditis nematodes of the Elegans supergroup, including two selfing species, C. elegans (C. elegans Sequencing Consortium 1998) and C. briggsae (Stein et al. 2003), and outcrossing C. inopinata (Kanzaki et al. 2018) and C. nigoni (Yin et al. 2018). C. remanei is an outcrossing nematode with high genetic diversity in comparison with C. elegans, C. briggsae, and C. tropicalis (Jovelin et al. 2003; Cutter et al. 2006). Therefore, to reduce the diversity and improve the quality of assembly, we constructed a highly inbred line from wild isolates collected from a forest near Toronto (see Materials and Methods). As expected, we assembled a genome consisting of six chromosomes, each of which is largely syntenic at a macro level with the genome assemblies from the other Caenorhabditis species. The difference in the genome lengths between C. elegans and C. remanei is quite large, from 102 Mb to well over 124 Mb. However, this degree of size variation appears to be typical for Caenorhabditis nematodes. For example, Stevens et al. (2019) showed that the genome sizes across the genus can vary from 65 to 140 Mb and that, overall, the size of the genome correlates with the number of genes but not necessarily the mode of reproduction.
This is the third C. remanei genome assembly generated by our group (PX439, PX356, and PX506; Table 1). The two previous chromosome-scale assemblies of other C. remanei strains (PX439 and PX356) were constructed with Illumina data and multiple mate-pair libraries (Fierst et al. 2015). However, the C. remanei genome has extended repetitive regions that failed to assemble using short reads. Further, strong segregation distortion among strains made it very difficult to construct the genetic map and definitively align shorter contigs to specific putative chromosomes. In this study, we used deep PacBio sequencing and Hi-C linkage information to overcome the repetitive regions and achieve better assembly characteristics. The combination of long-read and linkage data are a powerful toolset to produce chromosome-level assemblies, which are currently being increasingly used in a large number of species (e.g., Gordon et al. 2016; Gong et al. 2018; VanBuren et al. 2018; Low et al. 2019).
In addition to genome assembly, we performed annotation of the new C. remanei reference genome, using full-length transcript data (Iso-Seq), which has proven to be an effective technique to create high-quality annotations (Gonzalez-Garay 2016), short-read transcriptome sequencing, protein sequences of related species, and a hybrid annotation pipeline. To validate predicted gene models, we additionally used the previous annotation of C. remanei, since it was manually curated and was mostly supported by RNA-seq data (Fierst et al. 2015). The genes that were not present in the previous annotation are supported by other lines of evidence, including genes predicted from the Iso-Seq data. We found a total of 26,308 genes in the C. remanei genome, a slight increase over previous estimates (Fierst et al. 2015) and reconfirmation that C. remanei appears to have more genes than the selfing species.
We compared the genomic organization of C. remanei and C. elegans using the latest available version of the VC2010 C. elegans genome, which is based on a modern strain derived from the classical N2 strain and which led to an enlargement of the N2-based genome by an additional 1.8 Mb of repetitive sequences (Yoshimura et al. 2019). C. remanei and C. elegans genomes are in high synteny in spite of multiple intrachromosomal rearrangements (Figure 1). We observed many more intra- than interchromosomal rearrangements, which is consistent with first comparative observations of the C. elegans and C. briggsae genomes, which saw a 10-fold difference in these rates (Stein et al. 2003). This overall pattern remains consistent even when comparing C. elegans to more distantly related genera of nematodes (Guiliano et al. 2002; Whitton et al. 2004; Mitreva et al. 2005).
One plausible explanation for this pattern is that the low rate of interchromosomal translocations is generated by the multilevel control of meiotic recombination in Caenorhabditis. Pairing of chromosomes during meiosis in C. elegans is initiated from specific regions (“pairing centers”) located on the ends of homologous chromosomes (MacQueen et al. 2005; Tsai and McKee 2011), followed by chromosome synapsis via assembly of the synaptonemal complex along coupled chromosomes (MacQueen et al. 2002; Rog and Dernburg 2013). Crossovers in C. elegans can be formed only between properly synapsed regions (Lui and Colaiácovo 2013; Cahoon et al. 2019). Taken together, these molecular mechanisms permit meiotic recombination only between homologous regions linked in cis to the pairing centers, which presumably reduces the number of interchromosomal rearrangements, thereby resulting in the evolutionary stability of the nematode karyotype (Rog and Dernburg 2013).
The central domains of autosomes and a large portion of the X chromosome have more extended conservative regions between C. remanei and C. elegans. The similar pattern has been observed in comparative genomic studies of C. elegans and C. briggsae (Stein et al. 2003; Hillier et al. 2007). Apparently, the stability and conservation of the central regions is also connected to the recombinational landscape, as the central half chromosomes in C. elegans display a recombination rate that is several times lower than that observed on chromosome ends (Rockman and Kruglyak 2009), C. briggsae (Ross et al. 2011), as well as in C. remanei (A. A. Teterina, J. H. Willis, P. C. Phillips personal communication), without definitive hotspots of recombination (Kaur and Rockman 2014). Variation in recombination rate on the X chromosome is less than that on autosomes (Bernstein and Rockman 2016) and, because of the XX/X0 sex determination system of nematodes, the population size of the sex chromosome is three-quarters that of the autosomes (Wright 1931). So, orthologs of C. elegans and C. remanei located on the X chromosome are more conserved on average, likely because selection against deleterious mutations on the sex chromosome is greater than on autosomes (Montgomery et al. 1987; Coghlan and Wolfe 2002).
The chromosomes of C. elegans (C. elegans Sequencing Consortium 1998) and C. remanei also have a very similar pattern of gene organization, with a central region (the central domain or “central gene cluster”) (Barnes et al. 1995) characterized by high gene density, shorter genes and introns, lower GC content (Figure S5 and Table S4), and almost two times lower abundance of repetitive elements compared to chromosome arms. Repetitive elements in C. elegans and C. remanei are more abundant in the peripheries of chromosomes and, respectively, leave less room for protein-coding genes in those regions. About 28% of the total intron lengths in these nematodes are occupied by transposable elements, which could partially explain the increase of the gene lengths and intron fractions on the arms. The positive correlation of intron size with recombination rate and transposable elements has been previously observed in C. elegans (Prachumwat et al. 2004; Li et al. 2009b). The central gene clusters and transposable elements enriched in the arms are common, and are likely the ancestral pattern observed in C. elegans, C. briggsae, C. tropicalis, and C. remanei, yet distinct in C. inopinata (Woodruff and Teterina, 2019 preprint).
Use of Hi-C data in the genome assembly allows us to perform a preliminary analysis of the 3D chromatin organization across mixed developmental stages in C. elegans and C. remanei. The central domains show more cis-chromosome interactions than the peripheral parts of chromosomes in C. remanei (Figure 3). In C. elegans, variation in interaction intensity across the chromosome is somewhat less perceptible, probably because of minor differences in the fractions of genes on the central domains vs. arms. In both species, central regions show more distant interactions than arms. All chromosomes have numerous trans-chromosome interactions that are more tightly localized in the central regions. This pattern can be explained both by the densities of genes in the central domains and by technical issues with mapping of the reads to the repetitive regions. In contrast to the autosomes, the pattern of trans-chromosome activity is more dissimilar in C. elegans and C. remanei. This could be caused by species-specific differences or by the fact that the developmental stages of the samples do not strictly correspond for the two species (the C. elegans data set used early embryos whereas the C. remanei sample included all stages of the life cycle). In this case, both X chromosomes are active in hermaphrodites (XX), but their activity is reduced by one-half by a dosage-compensation mechanism in all tissues in C. elegans after the 30-cell stage (gastrulation) (Meyer 2005; Strome et al. 2014; Crane et al. 2015; Brejc et al. 2017). The presences of individuals at the early developmental stages could therefore potentially affect the extents of interactions with X chromosome observed within the C. elegans sample. Dosage compensation suppresses gene expression on both X chromosomes, modulates chromatin conformation by forming topologically associated domains, and partially compresses both X chromosomes (Meyer 2010; Lau et al. 2014; Brejc et al. 2017). All of these structural changes could potentially affect the relative intensities and availabilities of interactions between the X chromosome and autosomes.
What might drive these interchromosomal interactions? Cis- and trans-chromosome interactions could mediate transcriptional activity through colocalization of transcriptional factors on gene regulatory regions (Miele and Dekker 2008; Pai and Engelke 2010; Maass et al. 2019). Genome activity and the spatial organization of a genome are dynamic properties, and chromatin accessibility in C. elegans is tissue-specific, changing over developmental time (Daugherty et al. 2017; Jänes et al. 2018). However, C. elegans tends to have active euchromatin in the central parts of chromosomes and silent heterochromatin in the arms, which are anchored to the nuclear membrane (Ikegami et al. 2010; Liu et al. 2011; Mattout et al. 2015; Solovei et al. 2016; Cabianca et al. 2019). This pattern of regulation is consistent with the pattern of interactions that we observe. Nevertheless, much more work needs to be conducted, particularly aimed at stage- and tissue-specific effects, before the role and dynamics of spatial chromosome interaction in Caenorhabditis can be fully revealed.
Overall, despite numerous within-chromosome rearrangements, C. elegans and C. remanei show similar patterns of chromosomal structure and activity. The chromosome-level assembly of C. remanei presented here provides a solid new platform for experimental evolution, comparative and population genomics, and the study of genome function and architecture.
Acknowledgments
We thank members of the Phillips laboratory for helpful discussions, the University of Oregon Genomics and Cell Characterization Core Facility (GC3F) for advice and support, and Tim Ahearne and Scott Sholtz for helping to generate the C. remanei PX506 inbred line. This work was supported by grants from the National Institutes of Health (R01 GM-102511 and R35 GM-131838) to P.C.P.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11889099.
Communicating editor: V. Reinke
Literature Cited
- Altschul S. F., Gish W., Miller W., Myers E. W., and Lipman D. J., 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., and Huber W., 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Kojima K. K., and Kohany O., 2015. Repbase update, a database of repetitive elements in eukaryotic genomes. Mob. DNA 6: 11 10.1186/s13100-015-0041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T., Kohara Y., Coulson A., and Hekimi S., 1995. Meiotic recombination, noncoding DNA and genomic organization in Caenorhabditis elegans. Genetics 141: 159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D. J. et al. , 2012. GenBank. Nucleic Acids Res. 41: D36–D42. 10.1093/nar/gks1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M. R., and Rockman M. V., 2016. Fine-scale crossover rate variation on the Caenorhabditis elegans X chromosome. G3 (Bethesda) 6: 1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M., 1998. Caenorhabditis elegans is a nematode. Science 282: 2041–2046. 10.1126/science.282.5396.2041 [DOI] [PubMed] [Google Scholar]
- Brejc K., Bian Q., Uzawa S., Wheeler B. S., Anderson E. C. et al. , 2017. Dynamic control of X chromosome conformation and repression by a histone H4K20 demethylase. Cell 171: 85– 102.e23 10.1016/j.cell.2017.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1973. The genetics of behaviour. Br. Med. Bull. 29: 269–271. 10.1093/oxfordjournals.bmb.a071019 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabianca D. S., Muñoz-Jiménez C., Kalck V., Gaidatzis D., Padeken J. et al. , 2019. Active chromatin marks drive spatial sequestration of heterochromatin in C. elegans nuclei. Nature 569: 734–739. 10.1038/s41586-019-1243-y [DOI] [PubMed] [Google Scholar]
- Cahoon C. K., Helm J. M., and Libuda D. E., 2019. Synaptonemal complex central region proteins promote localization of pro-crossover factors to recombination events during Caenorhabditis elegans meiosis. Genetics 213: 395–409. 10.1534/genetics.119.302625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo D. M., Burger M. K., Lively C. M., and Delph L. F., 2015. Experimental evolution: assortative mating and sexual selection, independent of local adaptation, lead to reproductive isolation in the nematode Caenorhabditis remanei. Evolution 69: 3141–3155. 10.1111/evo.12815 [DOI] [PubMed] [Google Scholar]
- C. elegans Sequencing Consortium, 1998 Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282: 2012–2018 [corrigenda: Science 283: 35 (1999)]; [corrigenda: Science 283: 2103 (1999)]; [corrigenda: Science 285: 1493 (1999)]. [DOI] [PubMed]
- Chin C.-S., Peluso P., Sedlazeck F. J., Nattestad M., Concepcion G. T. et al. , 2016. Phased diploid genome assembly with single-molecule real-time sequencing. Nat. Methods 13: 1050–1054. 10.1038/nmeth.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan A., and Wolfe K. H., 2002. Fourfold faster rate of genome rearrangement in nematodes than in Drosophila. Genome Res. 12: 857–867. 10.1101/gr.172702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan A., Tsai I. J., and Berriman M., 2018. Creation of a comprehensive repeat library for a newly sequenced parasitic worm genome. Protoc. Exch. DOI: 10.1038/protex.2018.054. 10.1038/protex.2018.054 [DOI] [Google Scholar]
- Crane E., Bian Q., McCord R. P., Lajoie B. R., Wheeler B. S. et al. , 2015. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature 523: 240–244. 10.1038/nature14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter A. D., and Charlesworth B., 2006. Selection intensity on preferred codons correlates with overall codon usage bias in Caenorhabditis remanei. Curr. Biol. 16: 2053–2057. 10.1016/j.cub.2006.08.067 [DOI] [PubMed] [Google Scholar]
- Cutter A. D., Baird S. E., and Charlesworth D., 2006. High nucleotide polymorphism and rapid decay of linkage disequilibrium in wild populations of Caenorhabditis remanei. Genetics 174: 901–913. 10.1534/genetics.106.061879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A. C., Yeo R. W., Buenrostro J. D., Greenleaf W. J., Kundaje A. et al. , 2017. Chromatin accessibility dynamics reveal novel functional enhancers in C. elegans. Genome Res. 27: 2096–2107. 10.1101/gr.226233.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Jeon Y., Wang G.-X., and Cutter A. D., 2012. Global population genetic structure of Caenorhabditis remanei reveals incipient speciation. Genetics 191: 1257–1269. 10.1534/genetics.112.140418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C. et al. , 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. S., Charlesworth B., Baird S. E., and Cutter A. D., 2007. Inbreeding and outbreeding depression in Caenorhabditis nematodes. Evolution 61: 1339–1352. 10.1111/j.1558-5646.2007.00118.x [DOI] [PubMed] [Google Scholar]
- Dunham A., Matthews L., Burton J., Ashurst J., Howe K. et al. , 2004. The DNA sequence and analysis of human chromosome 13. Nature 428: 522–528. 10.1038/nature02379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C., 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Emms D. M., and Kelly S., 2019. OrthoFinder2: fast and accurate phylogenomic orthology analysis from gene sequences. Genome Biol. 20: 238 10.1186/s13059-019-1832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L., Willis J. H., Thomas C. G., Wang W., Reynolds R. M. et al. , 2015. Reproductive mode and the evolution of genome size and structure in Caenorhabditis nematodes. PLoS Genet. 11: e1005323 (erratum PLoS Genet. 11: e1005497). 10.1371/journal.pgen.1005323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J. et al. , 2015. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44: D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein M. B., Lu Z. J., Van Nostrand E. L., Cheng C., Arshinoff B. I. et al. , 2010. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330: 1775–1787. 10.1126/science.1196914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Dan C., Xiao S., Guo W., Huang P. et al. , 2018. Chromosomal-level assembly of yellow catfish genome using third-generation DNA sequencing and Hi-C analysis. Gigascience 7: giy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garay M. L., 2016. Introduction to isoform sequencing using Pacific Biosciences technology (Iso-Seq), pp. 141–160 in Transcriptomics and Gene Regulation. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Gordon D., Huddleston J., Chaisson M. J., Hill C. M., Kronenberg Z. N. et al. , 2016. Long-read sequence assembly of the gorilla genome. Science 352: aae0344 10.1126/science.aae0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graustein A., Gaspar J. M., Walters J. R., and Palopoli M. F., 2002. Levels of DNA polymorphism vary with mating system in the nematode genus Caenorhabditis. Genetics 161: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremme G., Steinbiss S., and Kurtz S., 2013. GenomeTools: a comprehensive software library for efficient processing of structured genome annotations. IEEE/ACM Trans. Comput. Biol. Bioinform. 10: 645–656. 10.1109/TCBB.2013.68 [DOI] [PubMed] [Google Scholar]
- Guiliano D., Hall N., Jones S., Clark L., Corton C. et al. , 2002. Conservation of long-range synteny and microsynteny between the genomes of two distantly related nematodes. Genome Biol. 3: RESEARCH0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B. J., Salzberg S. L., Zhu W., Pertea M., Allen J. E. et al. , 2008. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 9: R7 10.1186/gb-2008-9-1-r7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B. J., Papanicolaou A., Yassour M., Grabherr M., Blood P. D. et al. , 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8: 1494–1512. 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier L. W., Miller R. D., Baird S. E., Chinwalla A., Fulton L. A. et al. , 2007. Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol. 5: e167 10.1371/journal.pbio.0050167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C., and Yandell M., 2011. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics 12: 491 10.1186/1471-2105-12-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubley R., Finn R. D., Clements J., Eddy S. R., Jones T. A. et al. , 2015. The Dfam database of repetitive DNA families. Nucleic Acids Res. 44: D81–D89. 10.1093/nar/gkv1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Egelhofer T. A., Strome S., and Lieb J. D., 2010. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 11: R120 10.1186/gb-2010-11-12-r120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänes J., Dong Y., Schoof M., Serizay J., Appert A. et al. , 2018. Chromatin accessibility dynamics across C. elegans development and ageing. Elife 7: e37344. 10.7554/eLife.37344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovelin R., Ajie B., and Phillips P., 2003. Molecular evolution and quantitative variation for chemosensory behaviour in the nematode genus Caenorhabditis. Mol. Ecol. 12: 1325–1337. 10.1046/j.1365-294X.2003.01805.x [DOI] [PubMed] [Google Scholar]
- Jovelin R., Dunham J. P., Sung F. S., and Phillips P. C., 2009. High nucleotide divergence in developmental regulatory genes contrasts with the structural elements of olfactory pathways in Caenorhabditis. Genetics 181: 1387–1397. 10.1534/genetics.107.082651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N., Tsai I. J., Tanaka R., Hunt V. L., Liu D. et al. , 2018. Biology and genome of a newly discovered sibling species of Caenorhabditis elegans. Nat. Commun. 9: 3216 10.1038/s41467-018-05712-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T., and Rockman M. V., 2014. Crossover heterogeneity in the absence of hotspots in Caenorhabditis elegans. Genetics 196: 137–148. 10.1534/genetics.113.158857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K. C., Félix M.-A., Ailion M., Rockman M. V., Braendle C. et al. , 2011. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 11: 339 10.1186/1471-2148-11-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Phillippy A., Delcher A. L., Smoot M., Shumway M. et al. , 2004. Versatile and open software for comparing large genomes. Genome Biol. 5: R12 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. C., Nabeshima K., and Csankovszki G., 2014. The C. elegans dosage compensation complex mediates interphase X chromosome compaction. Epigenetics Chromatin 7: 31 10.1186/1756-8935-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., 2011. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27: 2987–2993. 10.1093/bioinformatics/btr509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., and Durbin R., 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009a The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu G., and Xia X., 2009b Correlations between recombination rate and intron distributions along chromosomes of C. elegans. Prog. Nat. Sci. 19: 517–522. 10.1016/j.pnsc.2008.06.019 [DOI] [Google Scholar]
- Liu T., Rechtsteiner A., Egelhofer T. A., Vielle A., Latorre I. et al. , 2011. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 21: 227–236. 10.1101/gr.115519.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens C., Futami R., Covelli L., Domínguez-Escribá L., Viu J. M. et al. , 2010. The Gypsy Database (GyDB) of mobile genetic elements: release 2.0. Nucleic Acids Res. 39: D70–D74. 10.1093/nar/gkq1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low W. Y., Tearle R., Bickhart D. M., Rosen B. D., Kingan S. B. et al. , 2019. Chromosome-level assembly of the water buffalo genome surpasses human and goat genomes in sequence contiguity. Nat. Commun. 10: 260 10.1038/s41467-018-08260-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T. M., and Eddy S. R., 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25: 955–964. 10.1093/nar/25.5.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui D. Y., and Colaiácovo M. P., 2013. Meiotic development in Caenorhabditis elegans, pp. 133–170 in Germ Cell Development in C. elegans. Springer-Verlag, New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass P. G., Barutcu A. R., and Rinn J. L., 2019. Interchromosomal interactions: a genomic love story of kissing chromosomes. J. Cell Biol. 218: 27–38. 10.1083/jcb.201806052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen A. J., Colaiácovo M. P., McDonald K., and Villeneuve A. M., 2002. Synapsis-dependent and-independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 16: 2428–2442. 10.1101/gad.1011602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen A. J., Phillips C. M., Bhalla N., Weiser P., Villeneuve A. M. et al. , 2005. Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell 123: 1037–1050. 10.1016/j.cell.2005.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A., Cabianca D. S., and Gasser S. M., 2015. Chromatin states and nuclear organization in development—a view from the nuclear lamina. Genome Biol. 16: 174 10.1186/s13059-015-0747-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K. et al. , 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. J., 2005 X-chromosome dosage compensation (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.8.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Meyer B. J., 2010. Targeting X chromosomes for repression. Curr. Opin. Genet. Dev. 20: 179–189. 10.1016/j.gde.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele A., and Dekker J., 2008. Long-range chromosomal interactions and gene regulation. Mol. Biosyst. 4: 1046–1057. 10.1039/b803580f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitreva M., Blaxter M. L., Bird D. M., and McCarter J. P., 2005. Comparative genomics of nematodes. Trends Genet. 21: 573–581. 10.1016/j.tig.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Montgomery E., Charlesworth B., and Langley C. H., 1987. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet. Res. 49: 31–41. 10.1017/S0016672300026707 [DOI] [PubMed] [Google Scholar]
- Navarro D., 2013. Learning Statistics with R: A Tutorial for Psychology Students and Other Beginners: Version 0.5. Available at: https://open.umn.edu/opentextbooks/textbooks/learning-statistics-with-r-a-tutorial-for-psychology-students-and-other-beginners. Accessed: March 2020. [Google Scholar]
- Pai D. A., and Engelke D. R., 2010. Spatial organization of genes as a component of regulated expression. Chromosoma 119: 13–25. 10.1007/s00412-009-0236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-daSilva A., 2007. Evolution of the control of sexual identity in nematodes. Semin Cell Dev. Biol. 18: 362–370. [DOI] [PubMed] [Google Scholar]
- Prachumwat A., DeVincentis L., and Palopoli M. F., 2004. Intron size correlates positively with recombination rate in Caenorhabditis elegans. Genetics 166: 1585–1590. 10.1534/genetics.166.3.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N. H., O’Connell B. L., Stites J. C., Rice B. J., Blanchette M. et al. , 2016. Chromosome-scale shotgun assembly using an in vitro method for long-range linkage. Genome Res. 26: 342–350. 10.1101/gr.193474.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon E., Silventoinen V., Pillai S., Harte N., Mulder N. et al. , 2005. InterProScan: protein domains identifier. Nucleic Acids Res. 33: W116–W120. 10.1093/nar/gki442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R., and Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Reynolds R. M., and Phillips P. C., 2013. Natural variation for lifespan and stress response in the nematode Caenorhabditis remanei. PLoS One 8: e58212 10.1371/journal.pone.0058212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman M. V., and Kruglyak L., 2009. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 5: e1000419 10.1371/journal.pgen.1000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog O., and Dernburg A. F., 2013. Chromosome pairing and synapsis during Caenorhabditis elegans meiosis. Curr. Opin. Cell Biol. 25: 349–356. 10.1016/j.ceb.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A., Koboldt D. C., Staisch J. E., Chamberlin H. M., Gupta B. P. et al. , 2011. Caenorhabditis briggsae recombinant inbred line genotypes reveal inter-strain incompatibility and the evolution of recombination. PLoS Genet. 7: e1002174 10.1371/journal.pgen.1002174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink K. L., Ituarte C. M., Reynolds R. M., Cresko W. A., and Phillips P. C., 2014a The transgenerational effects of heat stress in the nematode Caenorhabditis remanei are negative and rapidly eliminated under direct selection for increased stress resistance in larvae. Genomics 104: 438–446. 10.1016/j.ygeno.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink K. L., Reynolds R. M., Ituarte C. M., Cresko W. A., and Phillips P. C., 2014b Rapid evolution of phenotypic plasticity and shifting thresholds of genetic assimilation in the nematode Caenorhabditis remanei. G3 (Bethesda) 4: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink K. L., Reynolds R. M., Cresko W. A., and Phillips P. C., 2015. Environmentally induced changes in correlated responses to selection reveal variable pleiotropy across a complex genetic network. Evolution 69: 1128–1142. 10.1111/evo.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink K. L., Reynolds R. M., Ituarte C. M., Cresko W. A., and Phillips P. C., 2019. Environmental and evolutionary drivers of the modular gene regulatory network underlying phenotypic plasticity for stress resistance in the nematode Caenorhabditis remanei. G3 (Bethesda) 9: 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F. A., Waterhouse R. M., Ioannidis P., Kriventseva E. V., and Zdobnov E. M., 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31: 3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Smit A. F., and Hubley R., 2008. RepeatModeler Open-1.0. Available at: http://www.repeatmasker.org. Accessed: March 2020.
- Smit A. F., Hubley R., and Green P., 2015. RepeatMasker Open-4.0. 2013–2015. Available at: http://www.repeatmasker.org. Accessed: March 2020.
- Solovei I., Thanisch K., and Feodorova Y., 2016. How to rule the nucleus: divide et impera. Current Op. Cell Biol. (Henderson NV) 40: 47–59. [DOI] [PubMed] [Google Scholar]
- Stanke M., Keller O., Gunduz I., Hayes A., Waack S. et al. , 2006. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34: W435–W439. 10.1093/nar/gkl200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. D., Bao Z., Blasiar D., Blumenthal T., Brent M. R. et al. , 2003. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1: e45 10.1371/journal.pbio.0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbiss S., Willhoeft U., Gremme G., and Kurtz S., 2009. Fine-grained annotation and classification of de novo predicted LTR retrotransposons. Nucleic Acids Res. 37: 7002–7013. 10.1093/nar/gkp759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L., Félix M. A., Beltran T., Braendle C., Caurcel C. et al. , 2019. Comparative genomics of 10 new Caenorhabditis species. Evol. Lett. 3: 217–236. 10.1002/evl3.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S., Kelly W. G., Ercan S., and Lieb J. D., 2014. Regulation of the X chromosomes in Caenorhabditis elegans. Cold Spring Harb. Perspect. Biol. 6: a018366 10.1101/cshperspect.a018366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Hovhannisyan V., Lomsadze A., Chernoff Y. O., and Borodovsky M., 2008. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 18: 1979–1990. 10.1101/gr.081612.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H., Robinson J. T., and Mesirov J. P., 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.-H., and McKee B. D., 2011. Homologous pairing and the role of pairing centers in meiosis. J. Cell Sci. 124: 1955–1963. 10.1242/jcs.006387 [DOI] [PubMed] [Google Scholar]
- VanBuren R., Wai C. M., Colle M., Wang J., Sullivan S. et al. , 2018. A near complete, chromosome-scale assembly of the black raspberry (Rubus occidentalis) genome. Gigascience 7: giy094 10.1093/gigascience/giy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A. et al. , 1992. A survey of expressed genes in Caenorhabditis elegans. Nat. Genet. 1: 114–123. 10.1038/ng0592-114 [DOI] [PubMed] [Google Scholar]
- Whitton C., Daub J., Quail M., Hall N., Foster J. et al. , 2004. A genome sequence survey of the filarial nematode Brugia malayi: repeats, gene discovery, and comparative genomics. Mol. Biochem. Parasitol. 137: 215–227. 10.1016/j.molbiopara.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Wickham H., 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
- Woodruff G. C., and Teterina A. A., 2019. Degradation of the repetitive genomic landscape in a close relative of C. elegans. bioRxiv. (Preprint posted October 8, 2019). 10.1101/797035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S., 1931. Evolution in Mendelian populations. Genetics 16: 97–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. D., and Watanabe C. K., 2005. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21: 1859–1875. 10.1093/bioinformatics/bti310 [DOI] [PubMed] [Google Scholar]
- Ye C., Ji G., and Liang C., 2016. detectMITE: a novel approach to detect miniature inverted repeat transposable elements in genomes. Sci. Rep. 6: 19688 10.1038/srep19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Schwarz E. M., Thomas C. G., Felde R. L., Korf I. F. et al. , 2018. Rapid genome shrinkage in a self-fertile nematode reveals sperm competition proteins. Science 359: 55–61. 10.1126/science.aao0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura J., Ichikawa K., Shoura M. J., Artiles K. L., Gabdank I. et al. , 2019. Recompleting the Caenorhabditis elegans genome. Genome Res. 29: 1009–1022. 10.1101/gr.244830.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strain PX506 is available from the Caenorhabditis Genetic Center. All raw sequencing data generated in this study have been submitted to the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/) under accession number PRJNA577507. This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under the accession WUAV00000000; the version described in this paper is version WUAV01000000. The reference genome assembly is available at the NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome/) under accession number GCA_010183535.1. Supplementary custom scripts to estimate statistics, and generate main and supplemental figures, are available on GitHub (https://github.com/phillips-lab/C.remanei_genome). Supplemental material available at figshare: https://doi.org/10.25386/genetics.11889099. All online resources mentioned in the manuscript were accessed in March 2020.