Summary
Epithelial cells spontaneously form acini (also known as cysts or spheroids) with a single, fluid-filled central lumen, when grown in 3D matrices. The size of the lumen is dependent on apical secretion of chloride ions, most notably by the CFTR channel, which has been suggested to establish pressure in the lumen due to water influx. To study the cellular biomechanics of acini morphogenesis and homeostasis we used MDCK-2 cells. Using FRET-force biosensors for E-cadherin we observed significant increases in the average tension per molecule for each protein in mature 3D acini as compared to 2D monolayers. Increases in CFTR activity resulted in increased E-cadherin forces, indicating that ionic gradients affect cellular tension. Direct measurements of pressure revealed that mature acini experience significant internal hydrostatic pressure (37 +/− 10.9 Pa). Changes in CFTR activity resulted in pressure and/or volume changes, both which affect E-cadherin tension. Increases in CFTR chloride secretion also induced YAP signaling and cellular proliferation. In order to recapitulate disruption of acinar homeostasis, we induced epithelial to mesenchymal transition (EMT). During the initial stages of EMT there was a gradual decrease in E-cadherin force and lumen pressure that correlated with lumen infilling. Strikingly, increasing CFTR activity was sufficient to block EMT. Our results show that ion secretion is an important regulator of morphogenesis and homeostasis in epithelial acini. Furthermore, this work demonstrates that for closed 3D cellular systems, ion gradients can generate osmotic pressure or volume changes, both of which result in increased cellular tension.
Keywords: epithelial morphogenesis, E-cadherin, mechanobiology, biophysics, osmotic pressure, 3D live FRET imaging, acini culture, CFTR activity
Blurb:
Narayanan et al. describe the role of ion secretion in the generation of osmotic pressure and changes in mechanical tension across E-cadherin in epithelial acini. Increasing the osmotic gradient increased force exerted across E-cadherin, induced cellular proliferation and also blocked EMT progression.
Introduction
While some epithelial tissues exist as continuous sheets of cells, in many organs they form more elaborate 3D structures, such as tubes or ducts with single lumens that transport gases and liquids. Epithelial cells cultured in reconstituted basement membranes or collagen gels spontaneously form acini (also known as cysts or spheroids) with a single hollow lumen that resemble 3D epithelial structures found in normal tissue [1] Acinar cells exhibit differences in both apical-basal polarity and responses to growth factors when compared to cells grown as 2D monolayers [1]. Differences in cell-cell junctions, cytoskeletal organization, and nuclear shape [2] have also been reported, suggesting that cellular response to mechanical cues could be different in 3D acini.
Ionic gradients established across cellular membranes facilitate fluid secretion into epithelial lumens. The resulting hydrostatic pressure that arises from fluid influx has been identified as a key regulator of embryogenesis and organ development [3, 4]. Prior studies have established that a chloride channel known as cystic fibrosis transmembrane conductance regulator (CFTR) regulates the lumen size of epithelial acini [5, 6]. CFTR contains numerous protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites that regulate activity of the channel [7]. Based on the relationship between CFTR activity and lumen size, it has been hypothesized that changes in apical delivery of ions by pumps and channels would create an osmotic pressure within the lumen of acini [8]. Recent computational models of lumenogenesis have shown that hydrostatic pressure (formed by the equilibrium of osmotic pressure, fluid influx, and paracellular leak) contribute to lumen growth and homeostasis [9, 10].
An essential aspect of 3D epithelial morphogenesis and homeostasis is attributed to the formation and maintenance of cell-cell junctions [11, 12]. E-cadherin has been shown to mediate lumen formation in acinar structures [13, 14]. Modulation of E-cadherin adhesion [15], and over-expression of cadherin-6 have shown to impair tubulogenesis [13]. Significant rearrangements in adherens junctions, desmosomes, and tight junctions have been observed during tubulogenesis [16]. Lastly, changes in expression of cell-cell adhesion molecules has been established as one of the hallmarks of EMT progression. Taken together, these observations point to cell-cell contacts as an important mediator of acini homeostasis and morphogenesis.
Recent work by our group and others has shown that E-cadherin is subjected to significant mechanical force in 2D monolayers [17, 18]. Given the significant differences in epithelial cell behavior between 2D and 3D, as well as the important role of cell-cell contacts in 3D epithelial morphogenesis and homeostasis, we hypothesized that the mechanical forces at adherens junctions in 3D acini would be substantially different from the forces in 2D monolayers. In this study we used two approaches to study the biomechanics of acini, using Madin-Darby canine kidney (MDCK) epithelial cells as a model system. First, using previously developed and validated FRET-force biosensors, we observed a two-fold increase in tension across E-cadherin in 3D acini compared to 2D monolayers. E-cadherin tension was proportional to CFTR activity, indicating that ionic gradients increase mechanical forces between cells. Secondly, using a micropipette device we directly measured the internal pressure in the lumen of acini. Mature acini had substantial pressure (37 Pa), which was dependent on CFTR activity. We also show that changes in osmotic gradients are correlated to lumen formation, cell proliferation, and EMT progression. Taken together, our data show that ionic gradients induce cellular forces that regulate the morphogenesis and homeostasis of epithelial acini.
Results
Significant mechanical forces are present in mature epithelial acini
To study the cellular biomechanics of epithelial acini, we developed a MDCK-2 cell line stably expressing previously developed E-cadherin [18]. The FRET biosensors use the TSmod sensor (Figures S1A and S1B) that has an inverse FRET-force relationship [19]. Initial experiments were performed with cells growing in or on top of Matrigel™ in order to form acini and 2D monolayers, respectively, over a period of 7 to 10 days. The acinar structures consisted of a sphere with a single hollow lumen with distribution of the E-cadherin force sensor similar to that of endogenous E-cadherin (Figures S1C and S1D), which is consistent with previous publications [20, 21]. Acini expressing the E-cadherin tension sensor displayed reduced FRET compared to 2D monolayers grown on Matrigel™, indicating an increase in force across E-cadherin in 3D structures (Figure 1A). While we felt it is best to compare the biophysical behavior in 2D vs 3D using the same matrix, we note that Matrigel™ is a soft matrix (estimated to be 450 Pa) [22]. We have previously shown that cells grown on 1kPa gels have reduced E-cadherin tension [17], and therefore, 2D monolayer data in Figure 1A should not be compared to prior 2D work of cells cultured on plastic or glass [17, 18, 23]. Additionally, FRET measurements made using a force-insensitive control for E-cadherin (EcadΔcyto) did not show significant differences in forces exerted across E-cadherin in 3D compared to 2D (Figure 1B and Figures S1A and S1B).
Figure.1. Epithelial acini experience significant osmotic stretch in 3D. MDCK-2 cells expressing the E-cadherin tension sensor modules were used in all experiments.
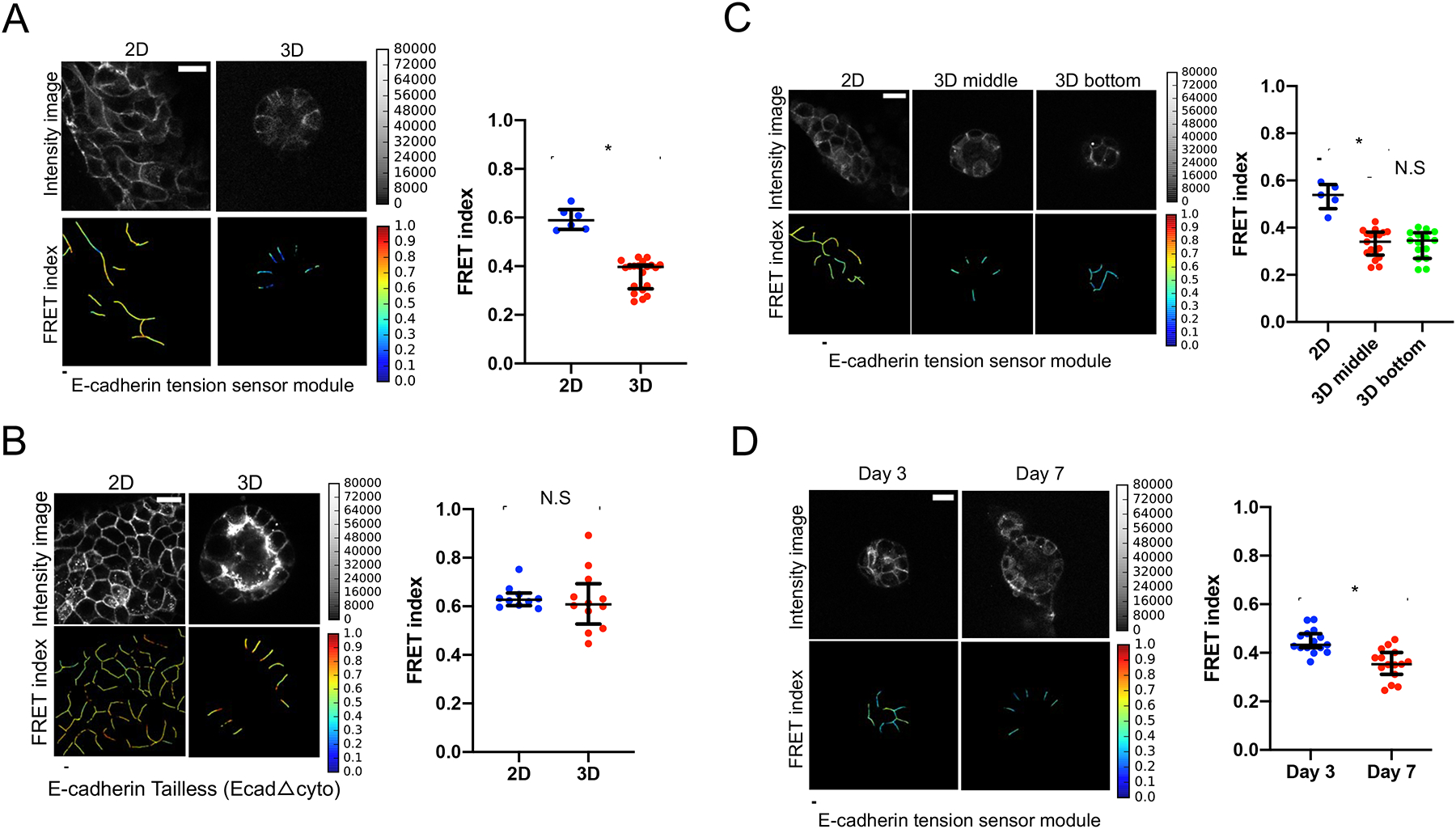
(A) Cells expressing the full-length E-cadherin tension sensor module were grown in Matrigel™ to form 3D acini or on top of Matrigel™ to form 2D monolayer (Scale bar=20 μm). The scatter dot plot shows FRET index measurements across multiple acini depicting a significantly higher force across E-cadherin in 3D as compared to monolayers. The horizontal line (black) in the scatter dot plot denotes the median of the FRET index estimates and the whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment out of a total of three independent experiments performed on separate days, *p<0.05, Mann-Whitney test).
(B) FRET index measurements in the force insensitive tailless control (EcadΔcyto) shows no difference in force across E-cadherin in acini compared to monolayers. The horizontal line (black) denotes the median of the FRET index estimates and the whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment out of a total of three independent experiments performed on separate days, N.S (not significant, p>0.05), Mann-Whitney test).
(C) FRET index measurements to analyze E-cadherin FRET based on cell-cell junction orientation in acini. Data shown as FRET index images and scatter dot plot depicts no significant difference in FRET across cell-cell junctions in a bottom slice of the acinus compared to the middle section. The horizontal line (black) denotes the median of the FRET index estimates and the whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment out of a total of three independent experiments performed on separate days, N.S (not significant, p>0.05) and *p<0.05, One-way ANOVA test and Mann-Whitney test).
(D) Multiple acini expressing the full-length E-cadherin tension sensor showed a significant decrease in FRET (higher force) on day 7 of acini morphogenesis (indicating higher E-cadherin force) wherein acini exhibited single lumen morphology as compared to day 3 that depicted a multiple lumen phenotype (Scale bar=20 μm).The scatter dot plot of the FRET index measurements depicts a higher force (lower FRET) on day 7 as compared to day 3 of acini morphogenesis. The horizontal line (black) denotes the median of the FRET index estimates and the whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment out of a total of three independent experiments performed on separate days, *p<0.05, Mann-Whitney test).
Also see Figure S1 for validation of the tension sensor modules used in the experiments.
To further confirm that reduced FRET in 3D was due to increased force, we performed a number of control experiments. First, we examined if the cell junction orientation in 3D (flipped 90 degrees from 2D) contributed to the FRET differences. We imaged both equatorial and bottom regions of individual acini, noting that the bottom regions of the acinus were more similar to the junction orientation in 2D. There were no significant differences between equatorial and bottom junctions in 3D acini (Figure 1C). Secondly, we considered the possibility that changes in cadherin clustering could affect intermolecular FRET (non-force FRET occurring between adjacent sensors). We developed a stable cell line expressing both an E-cadherin tension sensor with a “dark” donor (mTFP1) and also an E-cadherin tension sensor with a “dark” acceptor (mEYFP) to measure intermolecular FRET, similar to [24]. Intermolecular FRET was only a minimal component of FRET, and importantly, it did not change between 2D and 3D conditions (Figure S1E).
We speculated that tension across E-cadherin would change during the development of the acinus. We observed an increase in E-cadherin tension from immature acini (Day 3) to mature acini (Day 7) (Figure 1D). The increased forces correlated with the transition from a multiple lumen phenotype to the formation of a single lumen.
CFTR activity regulates mechanical tension across E-cadherin
To further test the hypothesis that ionic gradients affect E-cadherin tension, we examined how alterations in CFTR activity affected E-cadherin tension. To activate CFTR, cells were treated with forskolin to elevate cAMP (cyclic adenosine monophosphate) levels. Elevated cAMP levels activate PKA, ultimately leading to activation of CFTR [25]. Forskolin treatment for 24 hours resulted in a significant increase in mechanical tension across E-cadherin (Figure 2A). In parallel experiments we also used CFTRinh-172 as a direct inhibitor of CFTR activity [26]. Treatment with CFTRinh-172 reduced force across E-cadherin (Figure 2A). When cells were pre-treated with CFTRinh-172 prior to addition of forskolin, the force across E-cadherin was not affected by forskolin (Figure 2A), confirming that the effect of forskolin on E-cadherin force requires CFTR activity. Treatment of cells with CFTRinh-172 after forskolin treatment did not completely reverse the forskolin-induced increase in E-cadherin force (Figure S2A), suggesting that established ionic gradients can persist in the absence of CFTR activity. Furthermore, FRET measurements made using the force insensitive control for E-cadherin (EcadΔcyto) did not show any significant differences in force across E-cadherin, in response to activation and/or inhibition of CFTR activity (Figure 2B).
Figure.2. Osmotic pressure drives force across E-cadherin in epithelial acini. MDCK-2 cells expressing the E-cadherin tension sensor modules were used in all experiments.
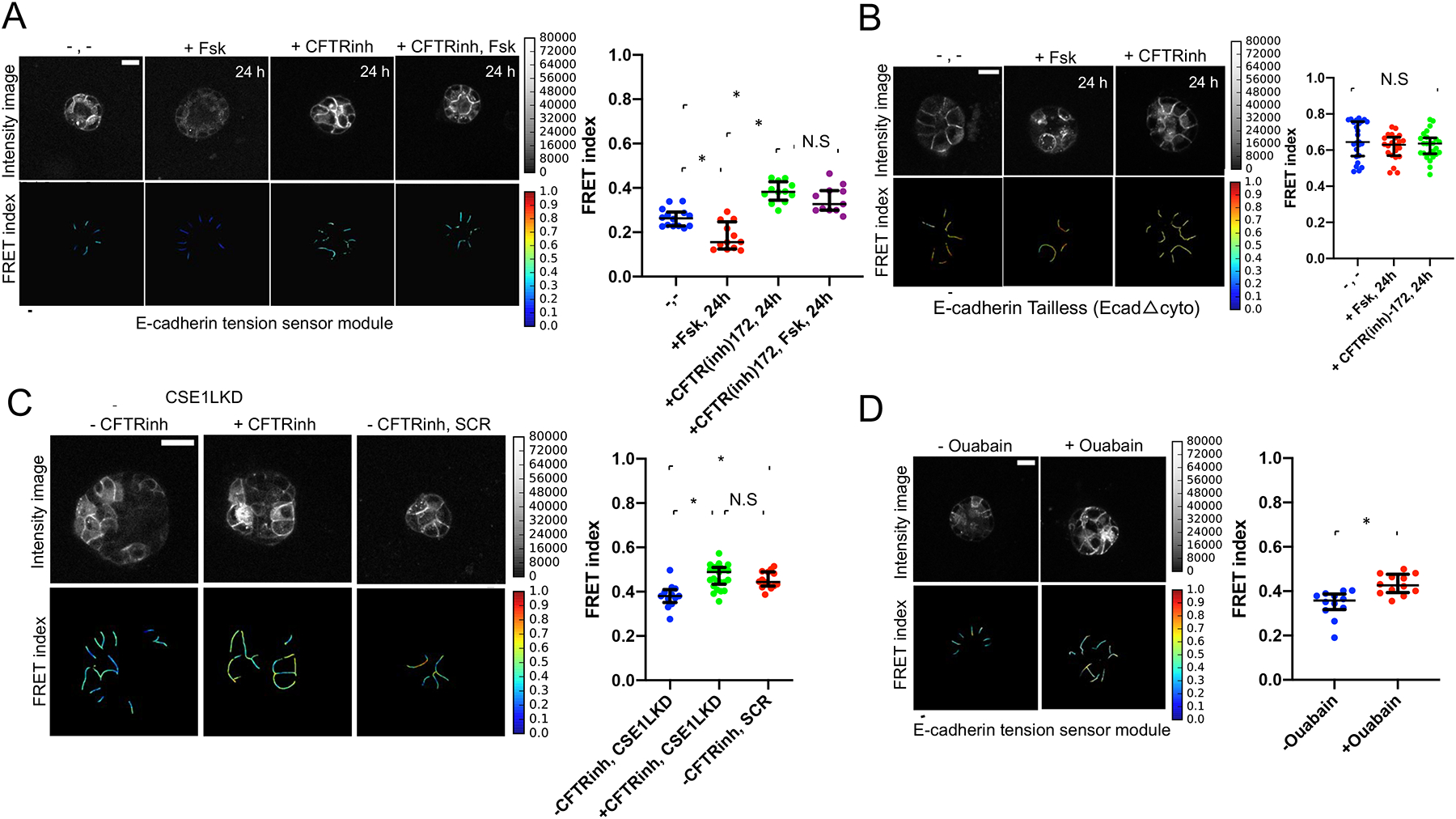
(A) Acini expressing the E-cadherin tension sensor module were treated with forskolin (10 μM) for 24 hours and showed significant decrease in FRET indicating higher force. CFTRinh-172 treatment (10 μM) significantly reduced force across E-cadherin. The scatter dot plot shows FRET index measurements across multiple acini depicting a significantly higher force across E-cadherin upon forskolin treatment over a period of 24 hours as compared to the untreated control and acini that were CFTR inhibited. Forskolin treatment following CFTR inhibition seldom caused any change in the reduced force. The horizontal line (black) denotes the median of the FRET index estimates and the whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of three independent experiments performed on separate days, *p<0.05, N.S (not significant, p>0.05), One-way ANOVA test and Mann-Whitney test). Scale bar = 20 μm.
(B) Treatment of the truncated E-cadherin mutant (EcadΔcyto) with forskolin (Fsk) and CFTR inhibitor (CFTR(inh)-172) for 24 hours did not yield any significant changes to FRET when compared to the untreated control (−,−), as shown in the FRET index images and scatter dot plot. The horizontal line (black) denotes the median of the FRET index estimates and the black whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of three independent experiments that were performed on separate days, p>0.05 (N.S), One-way ANOVA test and Mann-Whitney test).
(C) Significant increase in mechanical tension across E-cadherin (decreased FRET) and larger lumen size was detected in CSE1LKD (−CFTRinh, CSE1LKD) compared to control cells that were infected with a non-targeting shRNA (−CFTRinh, SCR) as well as the CFTR-inhibited CSE1LKD control (+CFTRinh, CSE1LKD), as shown in the scatter dot plot. The CFTR-inhibited control (+CFTRinh, CSE1LKD) was treated with the CFTR inhibitor (CFTR(inh)-172) on day 0, with media replenishment containing CFTR(inh)-172 every day until the 5th day of acinar development. The horizontal line (black) denotes the median of the FRET index estimates and the black whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of three independent experiments that were performed on separate days, *p<0.05, p>0.05 (N.S), One-way ANOVA test and Mann-Whitney test). Scale bar=50 μm.
(D) Effect of Na+/ K+-ATPase inhibition on force across E-cadherin in epithelial acini. Acini treated with Ouabain (Na+/ K+-ATPase inhibitor, 0.1 μM) showed significant increase in FRET (reduced force across E-cadherin) 48 hours post-treatment compared to the untreated control. The Ouabain-treated acini exhibited epithelial stratification thereby showing a reduction in lumen size. The horizontal line (black) in the scatter dot plot denotes the median of the FRET index estimates and the black whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of three independent experiments that were performed on separate days, *p<0.05, Mann-Whitney test). Scale bar=20 μm.
Also see Figure S2 that shows how perturbation of CFTR activity can modulate tension across E-cadherin in glandular acini.
To genetically increase CFTR activity we knocked down CSE1L, a known inhibitor of CFTR [27] (Figure S2D). After only 5 days, MDCK-2 cells expressing the E-cadherin tension sensor module with reduced CSE1L had a larger lumen and higher E-cadherin force compared to its non-silenced scramble control (Figure 2C), in agreement with prior results [27]. Additionally, treatment with CFTRinh-172 rescued the effects of CSE1L knockdown, restoring E-cadherin forces to normal levels (Figure 2C). Acini with reduced CSE1L levels also had a multilayered structure, likely attributed to other functions of CSE1L, such as apoptosis [28] or cell polarity [29].
As an alternate approach to modulate the ionic gradient present in the lumen, we used the inhibitor Ouabain to inhibit the basal Na+/K+-ATPase pump, which is essential for regulating solute and fluid transport into the cell and acini lumen size [30, 31]. Inhibition of the Na+/K+-ATPase via Ouabain reduced force across E-cadherin compared to the untreated control (Figure 2D).
We also observed variation in the localization of E-cadherin with a number of pharmacological treatments (Figures 2A and 2D). Masking of cells was carefully performed to remove intracellular E-cadherin from FRET analysis. Minimal intermolecular FRET was observed in all treatments with no significant differences between groups (Figure S2B). Additionally, we also examined the effects of forskolin, PKA agonist (8-bromo cAMP), and CFTRinh-172 in 2D monolayer cultures, which also similarly affected E-cadherin localization and expression. These treatments did not affect FRET in 2D monolayers (Figure S2C), suggesting that a closed 3D system is necessary for these conditions to affect force exerted across E-cadherin.
CFTR activity affects acini pressure and deformability
To directly measure hydrostatic pressure within the acini lumen, we used a custom micropipette system in which the relative internal lumen pressure of a single acinus was measured (see example trace; Figure S3A). In mature acini, we measured pressure of 37 +/− 10.9 Pa (Figure 3A). Inhibition of CFTR (CFTRinh-172) or the basal Na+/K+-ATPase (Ouabain) for 24 hours reduced pressure approximately 50 percent (Figure 3A and Figure S3B), indicating that ionic gradients significantly contribute to lumen pressure. Surprisingly, activation of CFTR via forskolin or PKA agonist (8-bromo cAMP) for 24 hours did not result in a significant increase in pressure (Figure 3A and Figure S3B). However, treatment with forskolin resulted in a significant increase in lumen diameter (Figures 3B and 3C). This suggests that further increases in ionic gradients act to increase lumen size rather than lumen pressure.
Figure 3. CFTR activity affects lumen pressure and deformability in epithelial acini.
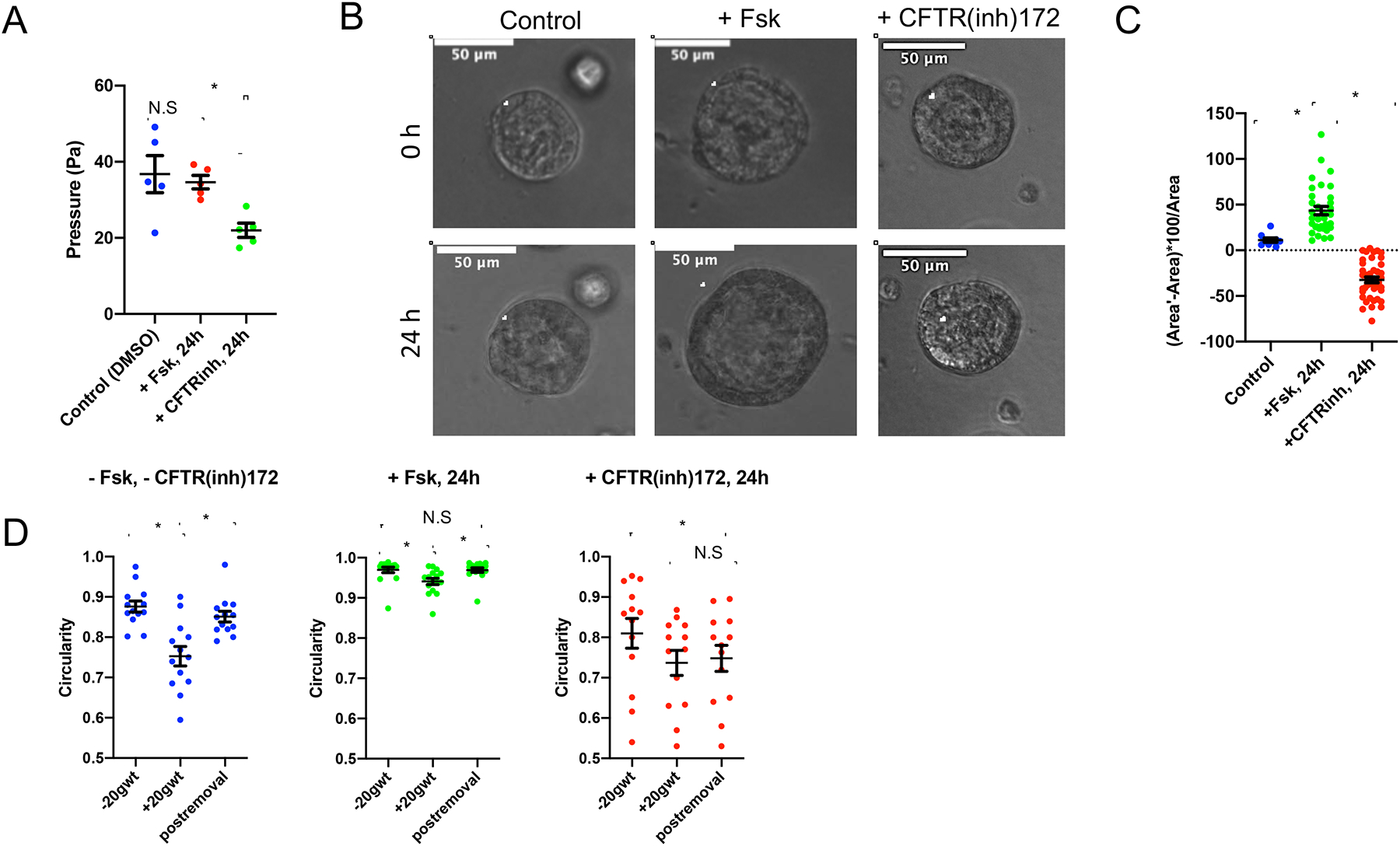
(A) Lumen pressure was directly measured in acini. Acini were cultured in Matrigel™ until they reached a luminal diameter of approximately 200 μm and then pressure was measured following culture for 24 hours in media containing forskolin (Fsk, 10 μM), CFTR inhibitor (CFTR(inh)-172, 10 μM), and vehicle control (DMSO). Atleast 5 acini were measured for pressure in all three cases (*p<0.001, N.S (not significant, p>0.05), One-way ANOVA, Tukey (HSD) test).
(B) MDCK-2 acini grown in Matrigel™, on 8-well chamber slides for 6 days, were imaged prior to treatment, and the same acinus was located post the 24 hour-forskolin (Fsk, 10 μM) and 24 hour-CFTR(inh)-172 (10 μM) treatment for lumen area changes. Representative image shows the central cross-section of acini with the lumen circumference outlined (white). Scale bar=50 μm.
(C) Percentage change in luminal area depicts a significant increase in luminal size after 24 hours of forskolin treatment (+ Fsk, 24hrs), and a significant reduction in size after 24 hours of CFTR(inh)-172 treatment, compared to the untreated control. At least 15 acini were scored for each group over two independent experiments (*p<0.001, paired t-test).
(D) Significant reduction in circularity upon compression of acini expressing the E-cadherin tension sensor module with a 20g calibrated weight, and higher elastic recoil to the original shape was observed in the untreated group ([–] CFTRinh-172) compared to the forskolin-treated (+Fsk, 24h) and the CFTR-inhibited (+CFTR(inh)-172, 24h) groups. − wt indicates prior to compression, + wt denotes compression with weight, and − wt* denotes recovery after removal of weight. Data represents paired analysis (13 acini were scored for each group for all three cases, *p<0.001, paired t-test).
Refer to Figure S3 to see how lumen pressure and acinar plasticity are affected through modulation of CFTR activity.
To further investigate if ionic gradients had a significant mechanical effect on the acinar structure, we compared the responses of control (−Fsk, −CFTR(inh)172), forskolin-treated (+Fsk, 24h) and CFTRinh-172-treated (+CFTR(inh)172, 24h) acini to compression (Figure 3D, and Figures S3C–S3H). We measured both the deformation under compression and elastic recoil when the weight was removed to qualitatively assess the role of internal pressure in mediating the spherical shape. We observed larger deformations for control acini during compression (measured as changes in circularity and mean edge strain, respectively) (Figure 3D, and Figures S3D, S3G and S3H) as compared to acini treated with forskolin (Fsk) and CFTRinh-172 (Figures S3E, S3F and S3H). Additionally, we observed reduced recoil (measured as circularity) for CFTR-inhibited acini as compared to control and forskolin-treated acini (Figure 3D and Figure S3H). The lack of recoil in the CFTR inhibited acini, the reduced deformation in forskolin-treated acini, and the increased circularity in uncompressed forskolin-treated acini indicates that lumen pressure is important for maintaining acinar shape, elasticity and resisting deformation by external forces.
Osmotic stretch regulates E-cadherin force induced proliferation
Based on prior studies, we hypothesized that the increased tension across E-cadherin, as a result of ionic gradients, induces proliferation in an E-cadherin dependent manner [32]. To assess cellular proliferation, acini were stained with Ki-67. Forskolin induced nuclear localization of Ki-67 between 24 to 48 hours in the non-transfected parental MDCK-2 acini. Ki-67 expression, however, was minimized in cells expressing a cytoskeletal decoupled “tailless” E-cadherin (EcadΔcyto) (Figure 4A, and Figures S1A and S1B) indicating that cytoskeleton-connected E-cadherin is necessary for the response. Acini treated with forskolin for a period of 6 hours showed a significant increase in positive YAP1 nuclear staining as early as 6 hours compared to the control (Figures 4C and 4D). Forskolin also did not increase Ki-67 as well as YAP1 expression in 2D monolayers (Figure S4A–S4D), indicating that the forskolin-mediated effects require a 3D environment to induce proliferation. Lastly, knockdown of CSE1L to increase CFTR activity also increased Ki-67 and YAP1 expression compared to the non-silenced control (Figures 4B, 4E and 4F).
Figure.4. Osmotic pressure regulates cellular proliferation in an E-cadherin dependent manner. MDCK-2 cells were used in all experiments.
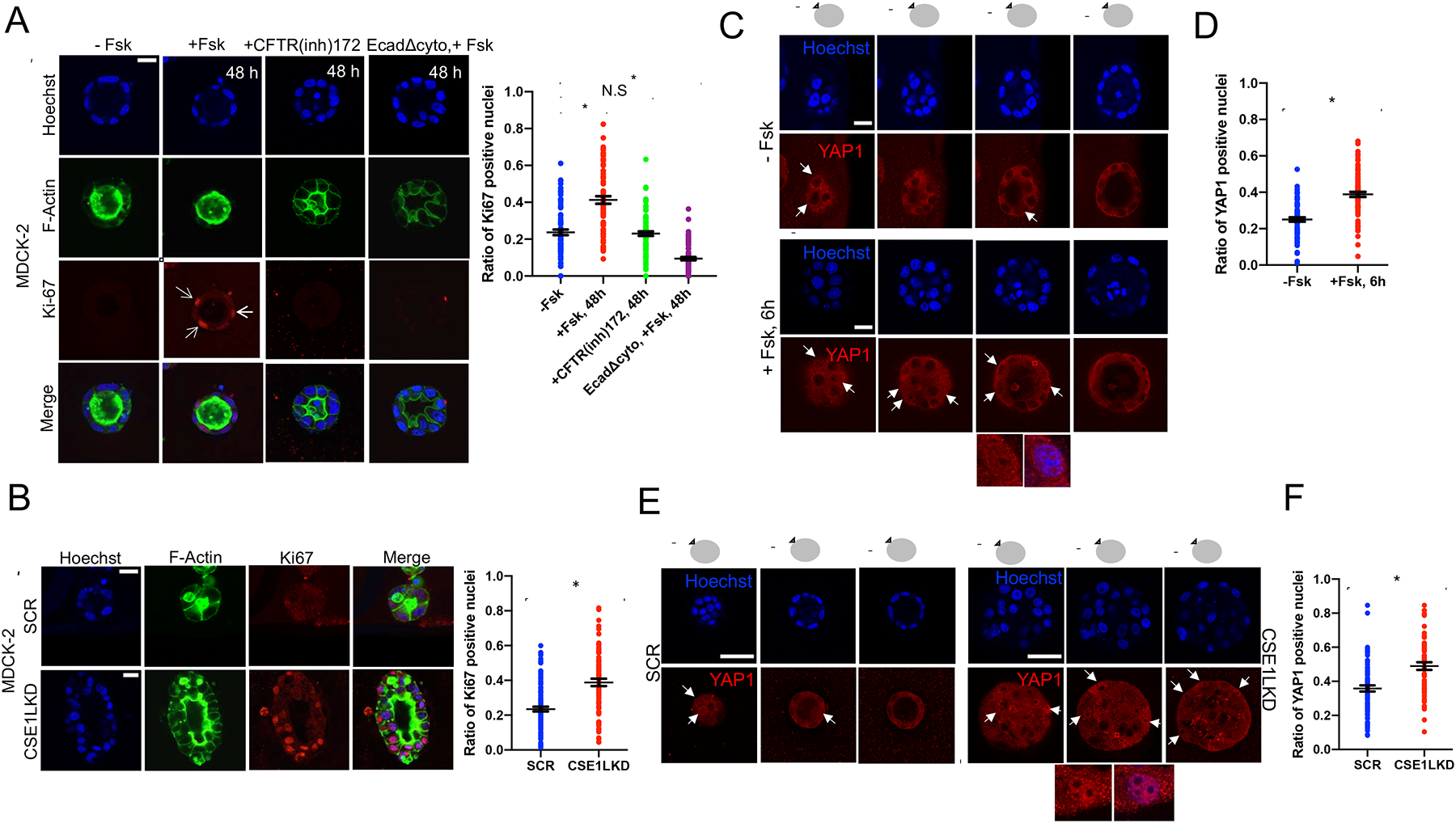
(A) MDCK-2 parental cells were ki67 (red) positive after 48 hours of treatment with forskolin. Forskolin treatment of EcadΔcyto expressing cells and CFTR inhibition did not induce cellular proliferation. Actin-488 was used to stain the actin cytoskeleton (green) and the nuclei were counterstained with Hoechst 33342 (blue). Quantification of immuno-positive nuclei (Ki67) normalized against the total nuclei count is as shown. Representative data shows the central cross-section of acini with and without treatment. Atleast 30 acini were scored over three independent experiments. The mean±SEM is indicated. *p<0.001 (One-way ANOVA, Tukey (HSD) test). Scale bar=20 μm.
(B) Cells with CSE1L knockdown had increased ki67 (red) staining as compared to scramble control cells. All acini were fixed on day 5, co-stained for actin (green) and the nuclei (blue) were counterstained with Hoechst 33342 (Scale bar=20 μm). Quantitative analysis of the number of proliferating cells as indicated by positive Ki67 nuclear staining depicts a higher proportion of proliferating cells in the CSE1LKD as compared to its non-silenced scramble control (SCR). Representative data shows the central cross-section of acini with and without treatment. Atleast 30 acini were scored over three independent experiments. The mean±SEM is indicated. *p<0.001 (Student t-test).
(C) MDCK-2 parental cells showed an increase in nuclear YAP localization (red) as early as 6 hours post treatment with forskolin. Representative data shows four cross-sections of the acinus (from top to center) indicating active-YAP localization. The nuclei (blue) were counterstained with Hoechst 33342. Scale bar=20 μm.
(D) Quantification of immuno-positive nuclei (YAP1) normalized against the total nuclei count depicts a higher proportion of stretch-induced cells in the forskolin-treated group (+Fsk, 6h) as compared to the untreated control (−Fsk). About 40 acini were scored over three independent experiments. The mean±SEM is indicated. *p<0.001 (One-way ANOVA, Tukey (HSD) test).
(E) Cells with CSE1L knockdown (CSE1LKD) had increased YAP1 (red) staining as compared to scramble control cells (SCR). Representative data shows three cross-sections of the acinus (from top to center) indicating active-YAP localization. All acini were fixed on day 5, and the nuclei (blue) were counterstained with Hoechst 33342 (Scale bar= 50 μm).
(F) Quantitative analysis of the number of YAP-positive nuclei as indicated by positive YAP nuclear staining depicts a higher proportion of stretch-induced cells in the CSE1LKD as compared to its non-silenced scramble control (SCR). Atleast 30 acini were scored over three independent experiments. The mean±SEM is indicated. *p<0.001 (Student t-test).
Refer to Figure S4 to see how osmotic pressure-driven stretch is absent in epithelial monolayers.
Mechanical force across E-cadherin is down regulated during EMT progression
Because EMT is known to affect acini lumen morphology by inducing lumen filling, we wanted to understand how force exerted on E-cadherin is affected during the early stages of EMT, prior to down regulation of E-cadherin. First, we observed that mechanical force across E-cadherin, measured via FRET ratio analysis (Figures 5A and 5B) was gradually reduced from over the course of 48 hours of TGF-β1 induced EMT. We also observed a decrease in tensile force exerted across E-cadherin during EMT progression in 2D monolayers (Figure S5A). Measurement of lumen pressure showed an approximately 50 percent decrease in acini after 24 hours of TGF-β1 treatment (Figure 5C). To test the hypothesis that higher forces exerted on E-cadherin might prevent progression through EMT, we pre-treated the cells with forskolin prior to TGF-β1 treatment. Forskolin prevented TGF-β1 induced changes in lumen morphology, force across E-cadherin, and expression of N-cadherin, α-smooth muscle actin, vimentin and E-cadherin (Figures 5D–5F, and Figures S5B and S5C). Additionally, forskolin treatment also minimized twist nuclear localization (Figure S5D), suggesting TGF-β signaling is also regulated by changes in osmotic gradients. Because cAMP signaling has been shown to affect EMT [33], we compared the effects of PKA and Epac1 agonists on monolayers and acini to decouple the effects of osmotically driven stretch (present in acinar structures) from signaling effects (present in both monolayers and acini). Epac1 activation blocked TGFβ1-induced N-cadherin expression in both 2D and 3D, suggesting its effects are independent of osmotic pressure (Figures S5E and S5F). However, activation of PKA with 6-Bnz-cAMP (an agonist that selectively activates cAMP-dependent PKA but not Epac signaling pathways [34]) blocked EMT in 3D but not 2D, indicating its effect on EMT is likely due to increased osmotic pressure (Figures S5E and S5F). Knockdown of CSE1L also prevented TGFβ1-induced expression of N-cadherin and lumen filling in acini (Figures 5G and 5H).
Figure.5. Force across E-cadherin is reduced during EMT progression.
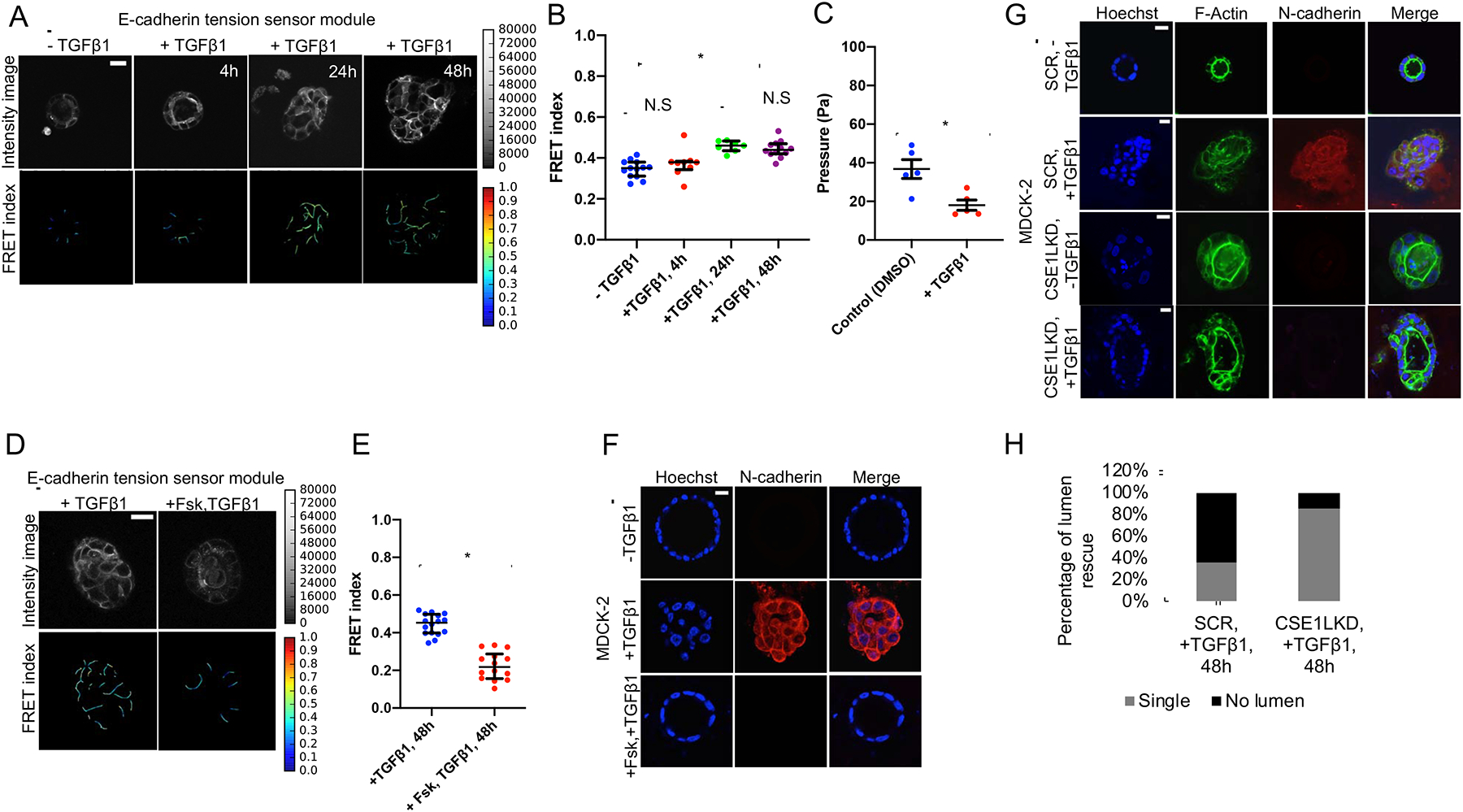
(A) Time course of TGF-β1 treatment shows a gradual decrease in force across E-cadherin. Separate acini (expressing the E-cadherin tension sensor module) were imaged for each case (Scale bar=20 μm).
(B) FRET index measurements as depicted in the scatter dot plot showed significant reduction in force across E-cadherin after 24 and 48 hours TGF-β1 treatment (2ng-ml−1) in the E-cadherin tension sensor module. The horizontal line (black) denotes the median of the FRET index estimates and the black whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of at least three independent experiments that were performed on separate days, *p<0.05, p>0.05 (N.S), One-way ANOVA test and Mann-Whitney test).
(C) Acini were cultured in Matrigel™ until they reached a luminal diameter of approximately 200 μm and then pressure was measured following culture for 24 hours in TGF-β1 (2ng-ml−1) and vehicle control (DMSO). Atleast 5 acini were measured for pressure in both cases (*p<0.001, Student t-test).
(D) Treatment of E-cadherin tension sensor module acini simultaneously with 10 μM forskolin (beginning 30 minutes prior to 48 hours of 2ng-ml−1 TGF-β1 treatment) prevented the decrease in force across E-cadherin (as depicted by reduced FRET index) and preserved the single-lumen morphology (Scale bar=20 μm).
(E) Mechanical tension across E-cadherin is signficantly increased (decreased FRET index) with simultaneous treatment with forskolin, as shown in the scatter dot plot. The horizontal line (black) denotes the median of the FRET index estimates and the black whiskers denote the largest observation within 1.5xIQR (representative data shown is from one experiment chosen from a pool of three independent experiments that were performed on separate days, *p<0.05, Mann-Whitney test).
(F) Simultaneous treatment with 10 μM forskolin prevented TGF-β1(2ng-ml−1) induced expression of N-cadherin (red), as well as acinar luminal filling (performed on the non-transfected parental MDCK-2 cells). Scale bar=20 μm.
(G) The CSE1LKD prototype of the non-transfected parental MDCK-2 cell-line displayed minimal N-cadherin (red) expression at the cell-cell junctions in the presence of TGF-β1 (2ng-ml−1) for 48 hours, compared to its non-silenced scramble control (SCR) that was characterized by acinar luminal filling and increased N-cadherin expression (Scale bar=20 μm).
(H) Quantification of the CSE1LKD prototype for single lumen morphology in the presence of TGF-β1 (2ng-ml−1) for 48 hours. Majority of the CSE1LKD acini exhibited single lumen in the presence of TGF-β1 as opposed to its non-silenced scramble control (Atleast 15 acini were scored).
Also see Figure S5 to further understand how increased CFTR activity blocks EMT progression in epithelial acini.
Discussion
It is well established that epithelial acini behave differently than 2D epithelial monolayers, which includes changes in morphology, apico-basal polarity, and responses to growth factors [1, 2]. In addition, innovative work by Valerie Weaver’s group has shown that acini are responsive to changes in the extracellular environment [35]. However, it is not yet well understood how cells in acini experience and generate forces, in particular when grown in soft/compliant matrices, like Matrigel™. Traditionally, resistance from substrate stiffness is thought to be necessary for cells to generate large forces. Our data suggest that hydrostatic pressure can serve a similar resistance function, acting as a balance for intracellular myosin forces.
Our work in this study demonstrates that epithelial acini have significant lumen pressure, and that this pressure (sensed as tensile forces by cells) affects the morphogenesis and homeostasis of acini. Our work in this manuscript adds to a growing body showing that pressure impacts cellular behavior. Within individual cells, changes in intracellular pressure have already been shown to be important for driving cell rounding during mitosis [36] and cell migration [37]. Likewise forces from inter-blastocoelic hydrostatic pressure have shown to be important for fibronectin fibril assembly [38], formation and positioning of the blastocoel [39], and the control of blastocyst size [40]. Perhaps, most directly related to our studies of epithelial cells is a recent report showing the regulation of branching morphogenesis by transmural pressure in a developing lung [3]. Our study clearly demonstrates that mechanical forces (between and within cells) are affected by changes in ion secretion (Figure 2). Additionally, a recent report showed that basally formed hemicysts (also known as epithelial domes) also experience significant hydrostatic pressure [41]. The contribution of ionic gradients (osmotic pressure) to cellular forces should therefore be considered in any closed 3D cellular system in which fluid is present.
A number of studies have established that CFTR activity regulates the lumen size of epithelial acini [5, 6]. Based on the relationship between CFTR activity and lumen size, a previous hypothesis was that increases in apical delivery of ions by pumps and channels increases pressure within the lumen [8]. In Figure 3, by directly measuring pressure, we are able to directly demonstrate that CFTR chloride secretion contributes to the formation of lumen pressure, providing experimental validation of this prior hypothesis. Interestingly, further increases of CFTR did not result in increased lumen pressure, despite increasing E-cadherin force (Figure 2). Instead, increased CFTR activity resulted in a large increase in lumen size. According to the physical principle known as the Law of Laplace, an increase in circumferential tension (E-cadherin force) can occur from either increased pressure or increased lumen volume. However, because the cells in the wall can also actively respond in ways to affect both pressure (e.g. ion secretion, changes in barrier function) and wall expansion (e.g. cell division, cytoskeletal changes, myosin contractility), the physical principles governing pressure, circumferential tension and lumen expansion, and their correlations is likely more complex than predicted by the Law of Laplace.
Given our observation of a plateau in pressure, it is tempting to speculate that acini may possess an internal pressure set-point, which is mediated through an active feedback mechanism in which circumferential tension regulates cellular expansion (through changes in cellular elasticity, contractility, and cellular division), thereby establishing a homeostatic equilibrium. The mechanisms that could actively regulate acini expansion are not yet clear. Of note, recent work by Xavier Trepat’s group has shown that epithelial cell expansion is not homogeneous, and that some cells may possess super-elastic properties, allowing for some cells to rapidly expand into a super-stretched state [41]. It will be interesting to determine how cellular changes can affect the relationship between pressure, tension, and expansion during more complex 3D morphological changes, such as tubulogenesis or branching morphogenesis.
Interestingly, despite mature acini being relatively homeostatic, our data also show variations in internal pressure (ranging from 21 to 49 Pa) (Figure 3A), and E-cadherin FRET measurements. We hypothesize that the pressure and circumferential tension of epithelial acini may be dynamic, being affected by a number of processes that occur even in mature acini (cell division, apoptosis, lumen size, paracellular leak, and changes in ion secretion).
Our work identifies the adherens junction as a potential sensor of tension arising from CFTR-driven ionic gradients. E-cadherin forces appear necessary for epithelial cells to respond to changes in osmotic pressure, including cellular proliferation (Figure 4). We also wish to note that our 3D studies of CFTR-induced proliferation (Figure 4) complement a number of existing studies by James Nelson’s group, in which it was shown that mechanical forces across E-cadherin are responsible for stretch-induced proliferation of epithelial 2D monolayers [32]. Given recent work by this group showing that increased tension on E-cadherin orients the direction of cell division [42], it is an attractive hypothesis that osmotic pressure-induced E-cadherin forces could be a principal mechanism for maintaining symmetric division of cells in acini.
Although our data support the hypothesis wherein circumferential stretch is sensed by the adherens junction, other mechanosensitive structures could ultimately be the primary mechanosensor. Our efforts to manipulate ionic gradients likely also affect forces across other structures in the cell-cell junction, such as desmosomes [43] and tight junctions [44], as well as other cytoskeletal connected structures, such as focal adhesions [19] and/or the nuclear LINC complex [45].
Our finding of reduced force on E-cadherin, as well as lumen pressure, during the earliest stages of EMT (Figures 5A–5C) is surprising given prior work suggesting that higher external forces (e.g. matrix stiffness) and intracellular forces (e.g. myosin contractility) promote EMT [35, 46, 47]. However, these prior results are not necessarily contradictory to our observations; rather it is possible that EMT induces a transfer of force from cell-cell junctions to cell-matrix adhesions. Our findings are also supported by recent work showing that HGF stimulation (also known to induce EMT) decreases E-cadherin force [23]. In addition, it may be necessary to reduce force across E-cadherin to induce disassembly of adherens junctions, as E-cadherin has been shown to exhibit catch bond behavior [48]. Interestingly, loss of CFTR activity has also been shown to promote EMT [49]. Our work, along with other reports [50], indicates that cellular tension is an important factor in the propensity of cells to undergo EMT.
In conclusion, we show that epithelial acini experience significantly higher forces across E-cadherin when grown in 3D, which arise from the effect of ionic gradients in the closed lumen. These forces are necessary for the formation and homeostasis of epithelial acini. Force biosensors, such as TSmod, are well suited to study mechanical forces in acinar structures and can be applied in future studies involving more complex 3D cellular systems.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents (including stable cell lines and other plasmids) should be directed to and will be fulfilled by the Lead Contact, Daniel E. Conway (dconway@vcu.edu). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Stable cell lines
To generate stable cell lines expressing canine full-length E-cad TSmod, and EcadΔcyto, cells were transfected with lipofectamine 2000 and selected using 500 ug/ml G418 (ThermoFisher). Full-length canine E-cad TSmod and EcadΔcyto, consisting of a modified TSmod (eYFP A206K instead of venus A206K) were gifted by Alex Dunn [18]. Dark mTFP1 and dark mEYFP versions of E-cad TSmod for intermolecular FRET controls were generated by site directed mutagenesis to change a single tyrosine (equivalent to Y66 in eGFP) to leucine [51].
ShRNA knockdown:
CSE1L was knocked down using lentivirus shRNA canine cse1L (pLKO.1 vector, sequence CCGG CCCTGCTGCTGTTGTAAAT CTCGAG ATTTACAA CAGCAGCAGGGTTTTTG, gift of Michel Bagnat) [27]. The non-silencing scramble shRNA pLKO.1 (addgene #1864, gift of Michel Bagnat) was used as a control. Cells were infected and then selected with 1.0 ug/ul puromycin. Knockdown was verified by western blotting, using rabbit CSE1L antibody (A300–473A, Bethyl Laboratories). Because CSE1L knockdown was not found to be stable, cells were used within 2–4 passages of infection.
Cell culture and drug treatment
Madin-Darby canine kidney cells (MDCK-2) were a gift from Rob Tombes (VCU Biology) and were maintained in high glucose DMEM (ThermoFisher) to which was added 10% fetal bovine serum (ThermoFisher) and 1% penicillin/streptomycin (ThermoFisher) under standard cell culture conditions. Madin-Darby canine kidney cells (MDCK-2) were grown in Matrigel™ for 7–10 days to form acini using the protocol of Debnath et al. [52]. Phenol-free reduced growth factor reduced Matrigel™ (Corning) was used for all 3D acini experiments. For experiments involving immunofluorescence staining, 8-well chamber slides were used (Lab-Tek, Rochester, NY) and for live-cell imaging experiments p-35 10 mm glass bottom dishes (#1.5 glass, Cell Vis, Mountain View, California). The pipettes and centrifuge tubes used for Matrigel™ and preparation of the seeding media were kept at 4°C. The matrigel bed was created from 45μL of Matrigel™, maintained at 4°C, followed by incubation at 37°C for 30 minutes to allow cross-linking of the gelatinous protein mixture [52]. The cells were sub-cultured and re-suspended in media and Matrigel™ and added to the chamber slides or glass bottom dishes. Cells were then incubated at 37°C for a period of 7–10 days, with media replacement every 3 days. Because CSE1L knockdown cells developed larger lumens in a shorter time span, these acini were imaged at an earlier time point (5 days), as indicated. For perturbation of CFTR activity, Forskolin (F6886, Tocris Bioscience, UK), the chloride channel inhibitor CFTRinh-172 (Tocris Bioscience, UK), Epac1 activator 8-pCPT-2-O-Me-cAMP-AM (Tocris Bioscience, UK), and protein kinase A activators; 8-Bromo-cAMP, sodium salt and 6-Bnz-cAMP, sodium salt (Tocris Bioscience, UK) were each used at a working concentration of 10 μM in all experiments. To inhibit the basal Na+/K+-ATPase pump, Ouabain (Tocris Bioscience, UK) was used at a working concentration of 0.1 μM in all experiments. For experiments involving EMT induction, recombinant human TGF-β1 (R&D systems) was used at a concentration of 2 ng/ml in all experiments.
METHOD DETAILS
Seeding in Matrigel™ for 3D live FRET imaging
For live-cell imaging experiments, p-35 10 mm glass bottom dishes (#1.5 glass, Cell Vis, Mountain View, California) were used. In order to compare FRET-based force measurements in epithelial acini, 50 μl of Matrigel™ maintained at 4°C was used to create the matrigel b ed, in such a way that the bed is thicker at the center and thinner along the rim of the p-35 10mm glass cut-out dishes. By tapering the bed toward the rim starting from the center in a circular pattern, a gradient in depth is created such that acini are formed in the areas that are deep enough for the cells to burrow in as a result of matrigel overlay. At the same time, the thinner areas of the bed form monolayers, thereby preventing acini formation, and were used for 3D vs 2D comparison studies. The matrigel bed was allowed to solidify as a result of cross-linking of the gelatinous protein mixture by incubation at 37°C for 30 minutes. The pipettes and centrifuge tubes used for Matrigel™, and preparation of the seeding media were precooled at 4°C. The cells were sub-cultured and re-suspended in media and Matrigel™, and about 300 ul of the seeding media containing the cells was added onto the 10mm cut-out area of the glass bottom dishes. DMEM media replenished with FBS and pen-strep was added 1–2 hours later and the dishes were incubated at 37°C for a period of 7–10 days, with media replacement every 3 days.
FRET imaging and analysis
Living cells in glass bottom dishes expressing the force sensors were imaged using a plan-apochromat 40x water immersion NA 1.1 objective lens on an inverted Zeiss LSM 710 laser scanning microscope (Oberkochen, Germany). All the images were acquired at 458 nm excitation wavelength from an argon laser source. Using the online-unmixing mode, both mTFP1 (donor) and mEYFP (acceptor) channels were collected via spectral unmixing as previously described [45].
Intensity images were further processed and analyzed using a custom Python code, which involved background subtraction and removal of saturated pixels, as previously described [45]. For each data set, the data was acquired for at least 7–8 epithelial acini. Images were masked manually on Image J (Fiji). Because it is difficult to discern from the confocal image section if the E-cadherin is truly basal (or out of plane lateral), due to the acinus orientation in matrigel, all of the E-cadherin-positive cell membranes were included in masked images. For some data sets, a more stringent masking approach (in which only obvious lateral junctions were masked) was employed compared to the more lenient masking (in which all E-cadherin-positive cell membranes were included). While it was observed that the strict masking approach was more precise, data presented in this manuscript are from images that were masked so as to include all possible E-cadherin-positive cell membrane.
The FRET index images were obtained by taking the ratio of the acceptor fluorophore channel (mEYFP) to the donor fluorophore channel (mTFP1), which was then multiplied with the binary image masks that outlined the cell-cell junctions in order to inspect FRET pixels of interest. When comparing multiple groups, all pixels of interest were aggregated following which, an upper and lower bound for the intensity was chosen in order to exclude dim pixels. Low intensity/dim pixels can bias the FRET index due to noise and auto-fluorescence, and bright pixels, on the other hand, can bias the FRET index due to detector saturation, as previously described by Arsenovic et al. [45]. The upper and lower bounds on the intensity were chosen based on the slope of the FRET index vs intensity behavior such that the plateau region of the plot is isolated and the same intensity bounds are applied to all groups in an experiment to prevent the choice of bounds from affecting the relative differences between groups.
Immunostaining
Immunofluorescence experiments were performed using the 8-well chamber slides. Antibodies used were: mouse monoclonal anti-N-Cadherin (clone 32, BD Biosciences, working dilution 1:200), rabbit polyclonal anti-Twist1 (ABD29, EMD Millipore, working dilution 1:50), rabbit monoclonal anti-E-Cadherin (24E10, Cell Signaling Technology, working dilution 1:200), rabbit polyclonal anti-CSE1L (A300–473A, Bethyl Laboratories, working dilution 1:1000), mouse monoclonal anti-α-Smooth Muscle Actin (A5228, Sigma, working dilution 1:200), mouse monoclonal anti-Vimentin (sc-32322, Santa Cruz Biotechnologies, working dilution 1:200), mouse monoclonal anti-YAP (sc-101199, Santa Cruz Biotechnologies, working dilution 1:100); and M01, clone 2F12, Abnova, working dilution 1:100), and rabbit polyclonal anti-Ki67 (PA5–19462, ThermoFisher; and ab15580, Abcam; working dilution 1:500). Cells were fixed with 2% para-formaldehyde in Ca2+PBS and incubated at 37°C overnight. The samples we re then rinsed thrice with PBS-glycine (0.1M) to quench the aldehyde groups and permeabilized with 0.5% Triton-X 100 (Sigma) for 30 minutes, followed by three additional 0.1M PBS-glycine rinses and blocking with 5% BSA (Sigma), all at room temperature. Primary antibodies were added and incubated at 4°C overnight and allowed to return to room temperature prior to the next series of steps that were performed in the dark at room temperature. Following three PBS rinses, the relevant secondary antibodies; Alexa Fluor 568 donkey anti-rabbit antibody, Alexa Fluor 647 donkey anti-mouse antibody, Alexa Fluor 647 chicken anti-rabbit antibody, and Alexa Fluor 488 goat anti-rabbit antibody (Invitrogen, working dilution 1:250) were added and incubated at room temperature for one hour. In the indicated experiments, acini were co-stained with Acti-stain 488, phalloidin (Cytoskeleton, Inc.) and Hoechst 33342 (ThermoFisher). Slides were mounted and imaged with a Zeiss LSM 710 confocal laser-scanning microscope.
Western Blotting
For the immunoblot, cells were plated on six wells and allowed to grow to confluency following which, the protein was extracted through cell lysis and quantified. Standard polyacrylamide gel electrophoresis and Western blot procedures were employed using the BioRad Trans-Blot 20 Mini-Protean Tetra System, 4–20% bis-acrylamide crosslinked gels, and polyvinylidene fluoride (PVDF) microporous membranes. Analysis of protein expression was conducted using either chemiluminescent images of PVDF membranes captured on the BioRad ChemiDoc Touch Gel Imaging System and associated Image Lab software.
Acini Pressure Measurements
Luminal pressure of MDCK acini was measured using a micropipette connected to a pressure transducer, as adapted from Jelinek and Pexieder [53]. Briefly, borosilicate glass micropipettes were pulled to an internal diameter of 40–45 μm with a 30° beveled tip and connected via polyethylene tubing to a differential pressure transducer (Honeywell; CPCL04DFC). A signal conditioner (Omega; DMD4059-DC) was used to amplify the transducer signal, and a data acquisition module (National Instruments; USB-6009) and custom LabVIEW program were used to record voltage over time. The transducer was calibrated using a water manometer and all data were processed in MATLAB.
MDCK-2 acini were cultured on Matrigel™ in PDMS wells adhered to glass coverslips until a diameter of approximately 200 μm was achieved. Upon growing to the appropriate size, acini were treated with DMSO (control), forskolin (10 μM), or CFTRinh-172 (10 μM), PKA (10 μM), Ouabain (0.1μM) and TGF- β1 (2 ng/ml) for 24 hours. Following culture, the micropipette tip was inserted into each spheroid after a five-minute voltage baseline was collected. The luminal pressure was recorded for at least five minutes before the micropipette was removed and pressure was recorded as the average pressure value over the time period. Pressures reported are relative pressures; the internal lumen pressure being relative to the pressure at the same height in the media outside of the acinus.
Acini size measurement
MDCK-2 acini were cultured in Matrigel™ and analyzed for luminal size changes upon forskolin treatment over a period of 24 hours compared to the untreated control. 5 × 5 grids were drawn out on the base of the 8-well chamber slides prior to seeding in order to aid in locating the same acinus over a 24-hour time frame. The major and minor axes were recorded using Image J and the corresponding elliptical area measurements for the central cross-section of an acinus was calculated. The percentage change in area (before and after treatment) compared to the untreated control, was recorded and graphed in Microsoft excel. Since the same acinus was imaged over a period of 24 hours, a paired student t-test statistical analysis was performed using the R software.
Acini compression
MDCK-2 cells expressing the wild-type E-cadherin sensor was allowed to form into acini over a period of 7 days in glass bottom p-35 dishes (as described in Seeding in Matrigel™ for 3D live FRET imaging). Spheroids closer to the 10mm cut-out wall were located using the Zeiss LSM 710 confocal laser-scanning microscope, with an image captured of the acinus prior to compression. The located acinus was compressed using a calibrated 20g weight by applying it onto the matrigel bed, perpendicular to the xy-plane (Figure S3C). Acini compression was accomplished by moving the weight along the x- direction, against the 10mm cut-out wall. The linear force used to move the weight was applied by hand. A second image was captured of the same acinus while compressed. In order to detect the recoil, the weight was moved away, and a third image of the unloaded acinus was acquired. Each image was processed using Fiji (Image J), and area outlines as well as circularity measurements were obtained as a result, which included the major and minor axes measurements. The circularity measurements recorded for the two experiments involving the untreated control (− Fsk, CFTR(inh)172), CFTR-inhibited (+ CFTR(inh)172, 24h) and the forskolin-treated (+ Fsk, 24h) acini were plotted on MS Excel (Figures S3D, S3E and S3F).
To measure strain during compression and with release of compression, the (x,y) coordinates of 24 points were captured around the circumference of the acini with points 1, 7, 13, 19 at the four major poles and 5 points equidistant in between, using Fiji (xyclick macro). The mean edge strain was determined using MATLAB based on coordinate geometry, and the (x,y) points were aligned based on acini centroid and by eye to minimize overall difference between conditions (compression and release). Root mean squared displacements between conditions were then determined and averaged for acini (Figures S3G and S3H).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance was measured using unpaired, two-tailed student t-test, two-tailed Mann-Whitney test and the Analysis of Variance (ANOVA) test. The One-way ANOVA test was followed by a further comparison of the groups using the Tukey (HSD) test so as to obtain significant differences between multiple groups, if any. In the case of observations that are paired and imaged under varying conditions, a paired t-test was conducted to obtain statistical significance. All statistical tests were conducted at a 5% significance level (p<0.05). The R statistical software was used for statistical analyses.
DATA AND CODE AVAILABILITY
All data and code supporting the findings of this study are available from the corresponding author upon request.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-N-Cadherin, clone 32 | BD Biosciences | Cat#610920 |
| Rabbit polyclonal anti-Twist1 | Sigma | Cat#ABD29 |
| Rabbit monoclonal anti-E-Cadherin (24E10) | Cell Signaling Technology | Product#3195S |
| Mouse monoclonal anti-α-Smooth Muscle Actin, clone 1A4 | Sigma | Cat#A5228 |
| Santa Cruz Biotechnologies | Santa Cruz Biotechnologies | Cat#sc-32322 |
| Mouse monoclonal anti-YAP | Santa Cruz Biotechnologies | Cat#sc-101199 |
| Mouse monoclonal anti-YAP (M01), clone 2F12 | Abnova | Cat#H00010413-M01 |
| Rabbit polyclonal anti-Ki67 | Invitrogen | Cat# PA5–19462 |
| Rabbit polyclonal anti-Ki67 | Abcam | Cat#ab15580 |
| Rabbit polyclonal anti-CSE1L | Bethyl Laboratories | Cat#A300–473A |
| Alexa Fluor 568 donkey anti-rabbit antibody | Invitrogen | Cat#A-10042 |
| Alexa Fluor 647 donkey anti-mouse antibody | Invitrogen | Cat#A-31571 |
| Alexa Fluor 647 chicken anti-rabbit antibody | Invitrogen | Cat#A-21443 |
| Alexa Fluor 488 goat anti-rabbit antibody | Invitrogen | Cat#A-11008 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Matrigel Matrix Basement Membrane | Corning | Product#35623 |
| Forskolin | Tocris | Cat#1099 |
| CFTRinh 172 | Tocris | Cat#3430 |
| Ouabain | Tocris | Cat#1076 |
| 8-Bromo-cAMP, sodium salt | Tocris | Cat#1140 |
| 6-Bnz-cAMP, sodium salt | Tocris | Cat#5255 |
| 8-pCPT-2-O-Me-cAMP-AM | Tocris | Cat#4853 |
| Recombinant Human TGF-β1 | R&D Systems | Cat#240-B-002 |
| Experimental Models: Cell Lines | ||
| MDCK-2 | Gifted by Rob Tombes (VCU Biology) | N/A |
| Recombinant DNA | ||
| Full length canine E-cadherin TSmod | Gifted by Alex Dunn [18] | N/A |
| Canine E-cadherin EcadΔcyto | Gifted by Alex Dunn [18] | N/A |
| Full length canine E-cadherin TSmod, dark mTFP1 (Y72L in mTFP1) | Generated by site directed mutagenesis | N/A |
| Full length canine E-cadherin TSmod, dark mEYFP (Y66L in mEYFP) | Generated by site directed mutagenesis | N/A |
| lentivirus shRNA canine cse1L | Gifted by Michel Bagnat [27] | N/A |
| non-silencing scramble shRNA pLKO.1 | Gifted by Michel Bagnat [27] | Addgene#1864 |
| Software and Algorithms | ||
| Image J | [54] | https://imagej.nih.gov/ij/ |
| R Software | [55] | https://www.R-project.org/ |
Highlights:
E-cadherin experiences higher mechanical tension in acini compared to monolayers
E-cadherin tension is positively correlated to CFTR activity
Epithelial acini have significant lumen pressure, in part due to CFTR activity
Increased CFTR activity increases cell proliferation and blocks EMT progression.
Acknowledgements
We wish to thank Christopher Lemmon, Rebecca Heise, Gregory Walsh, Lewis Scott, Lauren Griggs, Kevin Denis, Shaston Newman, Jolene Cabe, Lynne Elmore, Venkat Maruthamuthu, Tanmay Lele, Alex Dunn, and Valerie Weaver for helpful discussions and Michel Bagnat, Alex Dunn, and Rob Tombes for providing reagents. We also wish to especially acknowledge Andrei Ivanov for providing the initial suggestion of osmotic pressure affecting E-cadherin force and his guidance during the initial stages of the project. This project was supported by Institutional Research Grant IRG-73-001-37 from the American Cancer Society (to DEC), Burrows Welcome Fund grant 1017521 (to JPG), National Science Foundation CAREER Award CMMI 1653299 (to DEC), National Science Foundation grant CMMI 1537256 (to JPG) and grants R03AR068096 (to DEC), R35GM119617 (to DEC), R01HL133163 (to JPG) and T32EB003392 (to TJA) from the National Institutes of Health. Services and products in support of the research project were generated by the VCU Massey Cancer Center Biological Macromolecule Shared Resource supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References:
- 1.Zegers MMP, O’Brien LE, Yu W, Datta A, and Mostov KE. 2003. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 13: 169–76. [DOI] [PubMed] [Google Scholar]
- 2.Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, and Nickerson JA. 2006. The ultrastructure of MCF-10A acini. J. Cell. Physiol 208: 141–8. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CM, Gleghorn JP, Pang M-F, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, and Stone HA. 2017. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development. 144: 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmond ME, and Jacobson AG. 1977. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev. Biol 57: 188–98. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, and Welsh MJ. 1991. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 253: 202–5. [DOI] [PubMed] [Google Scholar]
- 6.Navis A, and Bagnat M. 2015. Developing pressures: fluid forces driving morphogenesis. Curr. Opin. Genet. Dev 32: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzamora R, King JD, and Hallows KR. 2011. CFTR Regulation by Phosphorylation. . pp. 471–488. [DOI] [PubMed]
- 8.Datta A, Bryant DM, and Mostov KE. 2011. Molecular regulation of lumen morphogenesis. Curr. Biol 21: R126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Herrero T, Alessandri K, V Gurchenkov B, Nassoy P, and Mahadevan L. 2017. Organ size control via hydraulically gated oscillations. Development. 144: 4422–4427. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta S, Gupta K, Zhang Y, Viasnoff V, and Prost J. 2018. Physics of lumen growth. Proc. Natl. Acad. Sci. U. S. A 115: E4751–E4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Maio A, Vega VL, and Contreras JE. 2002. Gap junctions, homeostasis, and injury. J. Cell. Physiol 191: 269–282. [DOI] [PubMed] [Google Scholar]
- 12.Green KJ, Getsios S, Troyanovsky S, and Godsel LM. 2010. Intercellular Junction Assembly, Dynamics, and Homeostasis. Cold Spring Harb. Perspect. Biol 2: a000125–a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia L, Liu F, Hansen SH, Ter Beest MBA, and Zegers MMP. 2011. Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Mol. Biol. Cell 22: 2031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciano DK, Brakeman PR, Lee C-Z, Spivak N, Eastburn DJ, Bryant DM, Beaudoin GM, Hofmann I, Mostov KE, and Reichardt LF. 2011. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 138: 2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrova YI, Spano MM, and Gumbiner BM. 2012. Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol. Biol. Cell 23: 2092–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack AL, Runyan RB, and Mostov KE. 1998. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol 204: 64–79. [DOI] [PubMed] [Google Scholar]
- 17.Mohan A, Schlue KT, Kniffin AF, Mayer CR, Duke AA, Narayanan V, Arsenovic PT, Bathula K, Danielsson BE, Dumbali SP, Maruthamuthu V, and Conway DE. 2018. Spatial Proliferation of Epithelial Cells Is Regulated by E-Cadherin Force. Biophys. J 115: 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, and Dunn AR. 2012. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. U. S. A 109: 12568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, and Schwartz MA. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466: 263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xian W, Schwertfeger KL, Vargo-Gogola T, and Rosen JM. 2005. Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J. Cell Biol 171: 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson JF, Hughes S, Chambers K, and Lang SH. 2009. Polarized fluid movement and not cell death, creates luminal spaces in adult prostate epithelium. Cell Death Differ. 16: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soofi SS, Last JA, Liliensiek SJ, Nealey PF, and Murphy CJ. 2009. The elastic modulus of Matrigel as determined by atomic force microscopy. J. Struct. Biol 167: 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayrard C, Bernaudin C, Déjardin T, Seiler C, and Borghi N. 2018. Src- and confinement-dependent FAK activation causes E-cadherin relaxation and β-catenin activity. J. Cell Biol 217: 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, and Schwartz MA. 2013. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, and Wine JJ. 1996. Delta F508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am. J. Physiol 270: C1544–55. [DOI] [PubMed] [Google Scholar]
- 26.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJV, and Verkman AS. 2002. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin–induced intestinal fluid secretion. J. Clin. Invest 110: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnat M, Navis A, Herbstreith S, Brand-Arzamendi K, Curado S, Gabriel S, Mostov K, Huisken J, and Stainier DYR. 2010. Cse1l Is a Negative Regulator of CFTR-Dependent Fluid Secretion. Curr. Biol 20: 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens P, Brinkmann U, and Wellmann A. 2003. CSE1L/CAS: its role in proliferation and apoptosis. Apoptosis. 8: 39–44. [DOI] [PubMed] [Google Scholar]
- 29.Jiang M-C, Liao C-F, and Tai C-C. 2002. CAS/CSE 1 stimulates E-cadhrin-dependent cell polarity in HT-29 human colon epithelial cells. Biochem. Biophys. Res. Commun 294: 900–905. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Findlay IA, and Sheppard DN. 2004. The relationship between cell proliferation, Cl– secretion, and renal cyst growth: A study using CFTR inhibitors. Kidney Int. 66: 1926–1938. [DOI] [PubMed] [Google Scholar]
- 31.Grantham JJ, Uchic M, Cragoe EJ, Kornhaus J, Grantham JA, Donoso V, Mangoo-Karim R, Evan A, and McAteer J. 1989. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int. 35: 1379–89. [DOI] [PubMed] [Google Scholar]
- 32.Benham-Pyle BW, Pruitt BL, and Nelson WJ. 2015. Mechanical strain induces E-cadherin-dependent Yap1 and −catenin activation to drive cell cycle entry. Science (80-.). 348: 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D, and Aroonsakool N. 2012. cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol 166: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang X-B, Christensen AE, Schwede F, Genieser H-G, Bos JL, Doskeland SO, Beavo JA, and Butt E. 2008. Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods 5: 277–278. [DOI] [PubMed] [Google Scholar]
- 35.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, and Weaver VM. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8: 241–54. [DOI] [PubMed] [Google Scholar]
- 36.Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, and Hyman AA. 2011. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 469: 226–230. [DOI] [PubMed] [Google Scholar]
- 37.Petrie RJ, Koo H, and Yamada KM. 2014. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science (80-.). 345: 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, and DeSimone DW. 2009. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev. Cell 16: 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, Turlier H, and Maître J-L. 2019. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science. 365: 465–468. [DOI] [PubMed] [Google Scholar]
- 40.Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie RJ, Bergert M, Diz-Muñoz A, Mahadevan L, and Hiiragi T. 2019. Hydraulic control of mammalian embryo size and cell fate. Nature. 571: 112–116. [DOI] [PubMed] [Google Scholar]
- 41.Latorre E, Kale S, Casares L, Gómez-González M, Uroz M, Valon L, Nair RV, Garreta E, Montserrat N, del Campo A, Ladoux B, Arroyo M, and Trepat X. 2018. Active superelasticity in three-dimensional epithelia of controlled shape. Nature. 563: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart KC, Tan J, Siemers KA, Sim JY, Pruitt BL, Nelson WJ, and Gloerich M. 2017. E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc. Natl. Acad. Sci 114: E5845–E5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baddam S, Arsenovic P, Narayanan V, Duggan N, Mayer C, Newman S, Abutaleb D, Mohan A, Kowalczyk A, and Conway D. 2018. The Desmosomal Cadherin Desmoglein-2 Experiences Mechanical Tension as Demonstrated by a FRET-Based Tension Biosensor Expressed in Living Cells. Cells. 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J, and Citi S. 2017. Tension-Dependent Stretching Activates ZO-1 to Control the Junctional Localization of Its Interactors. Curr. Biol 27: 3783–3795.e8. [DOI] [PubMed] [Google Scholar]
- 45.Arsenovic PT, Ramachandran I, Bathula K, Zhu R, Narang JD, Noll NA, Lemmon CA, Gundersen GG, and Conway DE. 2016. Nesprin-2G, a Component of the Nuclear LINC Complex, Is Subject to Myosin-Dependent Tension. Biophys. J 110: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butcher DT, Alliston T, and Weaver VM. 2009. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9: 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, and Weaver VM. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139: 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manibog K, Li H, Rakshit S, and Sivasankar S. 2014. Resolving the molecular mechanism of cadherin catch bond formation. Nat. Commun 5: 3941. [DOI] [PubMed] [Google Scholar]
- 49.Zhang JT, Jiang XH, Xie C, Cheng H, Da Dong J, Wang Y, Fok KL, Zhang XH, Sun TT, Tsang LL, Chen H, Sun XJ, Chung YW, Cai ZM, Jiang WG, and Chan HC. 2013. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim. Biophys. Acta 1833: 2961–9. [DOI] [PubMed] [Google Scholar]
- 50.Gomez EW, Chen QK, Gjorevski N, and Nelson CM. 2010. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J. Cell. Biochem 110: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, and Schwartz MA. 2013. Fluid Shear Stress on Endothelial Cells Modulates Mechanical Tension across VE-Cadherin and PECAM-1. Curr. Biol 23: 1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debnath J, Muthuswamy SK, and Brugge JS. 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 30: 256–68. [DOI] [PubMed] [Google Scholar]
- 53.Jelínek R, and Pexiedner T. 1968. The pressure of encephalic fluid in chick embryos between the 2nd and 6th day of incubation. Physiol. Bohemoslov 17: 297–305. [PubMed] [Google Scholar]
- 54.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Team RC (2018). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing; ). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code supporting the findings of this study are available from the corresponding author upon request.


