Abstract
Living organisms sense and respond to light, a crucial environmental factor, using photoreceptors, which rely on bound chromophores such as retinal, flavins or linear tetrapyrroles for light sensing. The discovery of photoreceptors that sense light using 5′-deoxyadenosylcobalamin, a form of vitamin B12 that is best known as an enzyme cofactor, has expanded the number of known photoreceptor families and unveiled a new biological role of this vitamin. The prototype of these B12-dependent photoreceptors, CarH, is widespread in bacteria and mediates light-dependent gene regulation in a photoprotective cellular response. CarH activity as a transcription factor relies on the modulation of its oligomeric state by 5′-deoxyadenosylcobalamin and light. This review surveys current knowledge about these B12-dependent photoreceptors, their distribution and mode of action, and the structural and photochemical basis of how they orchestrate signal transduction and control gene expression.
Keywords: photoregulation, photochemistry, CarH, transcriptional repressor, chromophore, optogenetics
INTRODUCTION: LIGHT AND PHOTORECEPTORS
Sunlight is essential for life on Earth. Conversion of the energy contained in sunlight into chemical energy by photosynthetic bacteria, algae, and plants accounts for the majority of fixed biomass and molecular oxygen (1). A crucial environmental factor, the ability to sense and respond to light is vital for most living organisms. Human vision is based on the eye detecting visible light (or simply light), which corresponds to the 380-760 nm wavelength range of the electromagnetic spectrum (2, 3). Light can directly or indirectly signal diverse biological processes including DNA repair, circadian rhythms, taxis, development, morphology, physiology, and virulence, and also mediates biosynthetic reactions such as in vitamin D3 synthesis (4-13). The pervasive role of light in biology, however, comes with a price: light absorption by photosensitive biomolecules like porphyrins, chlorophyll, or flavins can generate highly reactive oxygen species (ROS) that cause photooxidative damage of DNA, proteins, membranes, and other cellular components, and ultraviolet (UV) light triggers formation of mutagenic thymidine dimer lesions in DNA (14-18). Consequently, various cellular strategies have evolved to avoid, minimize, or repair light-induced damage (11, 14-16, 19).
Light, beneficial or harmful, has to be detected and converted to a cellular signal to elicit the appropriate response. In all domains of life, this fundamental task is carried out by photoreceptor proteins (or photoreceptors) that directly sense light through a chromophore component. For example, specific tryptophans in the UVR8 (UV Resistance Locus 8) protein sense UV light (20, 21). Visible light photoreceptors, however, must rely on noncovalently or covalently associated nonprotein chromophores, since no protein component absorbs in this wavelength range. Known chromophores include retinal, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), p-coumaric acid (4-hydroxycinnamic acid), and 3′-hydroxyechinenone ketocarotenoid, all of which absorb in the blue-green region (2, 3, 6, 22-28), and linear tetrapyrroles (biliverdins, phycocyanobilin, phytochromobilin, and phycoviolobilin), which absorb red/far-red light (29, 30). These unsaturated molecules have a conjugated π system with delocalized electrons, which favors light absorption (31). This type of system enables electron transfer, cis-trans isomerization about double bonds, or covalent bond formation-disruption to alter chromophore conformation and chemistry, which is then transmitted to the protein part of the photoreceptor to modulate function.
Photoreceptors have been classified into distinct families based on the chromophore and protein sequence/structure conservation. Rhodopsins have retinal as chromophore; cryptochromes, LOV (light-oxygen-voltage), and BLUF (blue-light utilizing FAD) sensors use FMN or FAD; phytochromes rely on linear tetrapyrroles; xanthopsins employ p-coumaric acid; and orange carotenoid protein has ketocarotenoid (12, 26, 28, 31, 32). Photoreceptors can be standalone proteins or part of larger proteins with multiple domains, each endowed with a defined activity, such as directing specific interactions (with proteins, DNA, RNA, membranes, small molecules) or enzymatic catalysis (kinases, diguanylate cyclases, phosphodiesterases). In multidomain photoreceptors, one or more modules sense the light input, undergo molecular changes, and transmit the signal to output effector domains to carry out a specific light-dependent function. The photosensory module frequently is an autonomous unit that can be swapped between different proteins, as is exemplified by the natural occurrence of a given type of photoreceptor module in functionally distinct proteins. In nature, this modularity allows for combinatorial module mixing as an effective strategy in the evolution of signaling and regulatory complexity (33). Biological engineers have also harnessed this modularity and created artificial genetic fusions of specific photoreceptor modules and proteins of interest, whose functions can then be optically monitored or controlled with high temporal and spatial precision (32). These advances have led to the vibrant field of optogenetics and the development of powerful tools and applications in cell biology and neurobiology (34-36).
The recent discovery of photoreceptors with a novel light-sensing chromophore, the vitamin B12 derivative 5′-deoxyadenosylcobalamin (simply, adenosylcobalamin or AdoCbl), has enlarged the number of known photoreceptor families and unveiled a new biological facet of this vitamin (37). Significant progress has been achieved in understanding how this new type of photoreceptor works. High-resolution structures of the functionally relevant states have revealed the molecular architecture of the photoreceptor and provided detailed snapshots of its light-dependent mechanism of action (38). Some fascinating features in the photochemistry have also been observed (39, 40). These aspects will be covered here together with an overview of the discovery, functions, distribution, and evolution of these B12-dependent photoreceptors.
VITAMIN B12: CHEMISTRY AND BIOLOGY
Cobalamin Forms and Chemistry
Since its first discovery as the anti-pernicious anemia factor (41, 42), vitamin B12 has seen a rich and storied scientific history. A decade after its first isolation in 1948 (43, 44), the description of its crystal structure by Hodgkin et al. (45) marked a heroic moment for small-molecule crystallography, revealing one of nature’s most complex cofactors in three dimensions, and its total chemical synthesis in the early ´70s capped a classic effort by Woodward, Eschenmoser, and coworkers (46). In parallel, extensive studies into the chemistry and biology of vitamin B12 and its derivatives, collectively referred to as the cobalamins or often just “B12”, revealed their intricate chemical structure, complex reactivity, and rich spectroscopic properties as well as their biosynthetic origins and roles in living systems (47-52). Their best-characterized biological functions in fatty acid and folate metabolism make them essential micronutrients for humans and other animals, although curiously not for plants or fungi. Fitting for B12’s storied history, more recent studies have revealed new and unanticipated biological functions, for example in modulating the structure of microbial communities (53) and as a light sensor in light-dependent gene regulation, the main focus of this review.
Chemically, the principal feature of B12 is its corrin ring, whose four pyrrolic nitrogens coordinate a central cobalt atom that is usually in the Co3+ or Co(III) oxidation state. The corrin pyrroline and pyrrolidine groups are adorned with methyl, acetamide, and propionamide groups, one of which links the ring to the 5,6-dimethylbenzimidazole (DMB) ribonucleoside tail characteristic of B12 (Figure 1). In free B12 at physiological pH, a nitrogen of this DMB base occupies the lower, or α, axial coordination site on the central cobalt, a conformation described as “base-on” or “DMB-on”. The octahedral coordination sphere of Co(III) is completed by an upper, or β, axial ligand, which can take a variety of forms depending on the B12 derivative (Figure 1).
Figure 1.
Vitamin B12 and derivatives. (Left) General chemical structure of B12 in the base-on conformation with cobalt formally in the Co(III) state, the lower axial dimethylbenzimidazole ligand in blue, and the upper axial ligand denoted by “R” in red. (Right) Selected upper axial ligands and the corresponding B12 forms are shown.
The two major biological forms of B12, methylcobalamin (MeCbl) and AdoCbl (also known as coenzyme B12), both have an alkyl group as the upper axial ligand: MeCbl has a methyl group (Me) and AdoCbl has a 5′-deoxyadenosyl group (Ado) that is bonded to the cobalt through its 5′-carbon (Figure 1) (54, 55). Thus, both MeCbl and AdoCbl feature a covalent Co-C bond, as revealed by their respective structures (56, 57), and represent rare examples of naturally occurring organometallic compounds. Not surprisingly, these Co-C bonds have intriguing chemical properties: chemically inert in the absence of light, their relatively low bond dissociation energies [reported as 32-40 kcal mol−1 for MeCbl and 24-35 kcal mol−1 for AdoCbl; (58-62)] allow them to be cleaved rather easily. This feature underlies the use of MeCbl and AdoCbl as cofactors in enzyme catalysis, although their exact chemical properties and biological functions are distinct. Other B12 forms with different upper axial ligands are known (Figure 1). Cyanocobalamin (CNCbl or vitamin B12) with a cyanide upper axial ligand is found frequently free in nature, but is nonfunctional and has to be converted in vivo to MeCbl or AdoCbl for biological use. In the absence of other ligands, B12 in aqueous solution ligates a water molecule to form hydroxocobalamin or aquocobalamin (AqCbl), which predominates at physiological pH. Regardless of the upper axial ligand, free B12 forms are generally found in the DMB-on conformation at physiological pH. Protonation of DMB at low pH leads to its replacement by water, yielding the “base-off” (DMB-off) conformation and a less rigid B12 molecule with altered chemistry (63). Various proteins, including the B12-dependent photoreceptors reviewed here and many B12-using enzymes, bind B12 with a His side chain replacing the DMB ligand, a B12-binding mode known as “base-off/His-on” (64).
Under aerobic conditions, the central cobalt of the various B12 forms is generally in the +3 oxidation state and relatively redox-inactive. Homolytic or heterolytic cleavage of the β-axial bond or reduction of AqCbl with a strong reductant can transiently generate cobalt in the Co2+/Co(II) or Co1+/Co(I) states, the coordination number decreasing from 6 in Co(III) to 5 in Co(II) and 4 in Co(I). The one-electron-reduced Co(II) form of B12, termed cob(II)alamin or cob(II), is paramagnetic and can be detected by electron paramagnetic resonance (EPR) spectroscopy. This form is relatively inert under anaerobic conditions but rapidly oxidizes in the presence of molecular oxygen. Reduction by an additional electron to Co(I) yields cob(I)alamin or cob(I), a supernucleophile and potent reductant that decomposes even under anaerobic conditions. The unique chemical properties of B12 in each oxidation state are harnessed in various ways by different B12-dependent enzymes.
Cobalamin Photochemistry
Cobalamins exhibit vibrant colors that originate from strong absorption in the UV-visible range, mostly from π-π* transitions. The spectral features of each form depend on the cobalt oxidation state and the nature of the upper and lower axial ligands (65-67). As a result of this absorption, cobalamins exhibit rich and complex photochemistry, which is again modulated by the axial ligands (61, 62). Although the photochemical properties of CNCbl and AqCbl have been studied (68, 69), the discussion here will center on the biologically relevant alkylcobalamins MeCbl and AdoCbl, whose light sensitivity has been known since their first isolation. In particular, photolytic cleavage of the AdoCbl Co-C bond has been regarded as a model system for its cleavage in enzyme active sites (70), and therefore has been studied extensively. Early studies focused on determining the products of light-induced decomposition (70-73). The underlying photochemical processes are controlled by electronic relaxation dynamics that occur on picosecond time scales. Paired ultrafast transient absorption spectroscopy and theoretical calculations have emerged as powerful approaches for the study of B12 photochemistry and have provided detailed insight into the electronic processes and the intermediates following excitation (62, 74-78). Near-UV and visible light of wavelengths <530 nm (photon energies >40 kcal mol−1) cleave the Co-C bond of both MeCbl and AdoCbl on a time scale of 10-100 ps. The quantum yields for MeCbl Co-C photolysis are wavelength-dependent, high at 400 nm but much lower at 522 nm, whereas near unit quantum yields are observed for AdoCbl over this wavelength range (61, 62). The initial cleavage events are generally homolytic, generating a caged alkyl radical:cob(II) pair that can either recombine to regenerate the Co-C bond or dissociate. Competition between geminate recombination and radical escape determines the ultimate photolysis yield (79). Net photolysis is reduced by solvent cage effects around Cbl and accelerated by compounds such as molecular oxygen that can intercept the radicals formed and suppress recombination pathways (74, 80).
The fates of the alkyl radical and cob(II) generated upon photolysis of alkylcobalamins depend on the environmental conditions. In the case of MeCbl, the highly reactive methyl radical rapidly reacts through complex pathways to form formaldehyde and smaller amounts of methanol, formic acid, and carbon dioxide under aerobic conditions, and under anaerobic conditions it forms a mixture of formaldehyde, methane, ethane, as well as smaller amounts of methanol and formic acid (72). Upon AdoCbl photolysis, the 5′-deoxyadenosyl radical (Ado•) rapidly reacts in the presence of molecular oxygen to form 5′-peroxyadenosine, which in turn decomposes to adenosine-5′-aldehyde and minor amounts of adenosine and adenine (39, 70). In the absence of oxygen, Ado• undergoes intramolecular addition of the radical to the adenine base and forms 5′-deoxy-5′,8-cycloadenosine (71, 73). The second major photolysis product of both MeCbl and AdoCbl, cob(II), is stable under anaerobic conditions, but is rapidly oxidized in the presence of oxygen to AqCbl, a reaction further enhanced by 5′-peroxyadenosine (70). The reactivity of these photolysis products underscores a central dichotomy of B12 biology: although AdoCbl and MeCbl can mediate unique chemistry, their light sensitivity and reactivity require exquisite control to suppress inadvertent side reactions.
Cobalamins as Cofactors in Enzymes and Riboswitches
Although B12 is essential in animals and in many prokaryotes, only some of the latter can synthesize it de novo (51, 52). Plants, fungi, and many prokaryotes bypass the need for B12 by using only alternative enzymes or metabolic pathways that do not require this cofactor, and many other species contain both B12-dependent and B12-independent enzymes for the same reaction (48). Not surprisingly for such a complex molecule, the two B12 biosynthetic pathways (aerobic or anaerobic) are amongst the most intricate known in nature. Each pathway involves more than 30 genes and steps subject to genetic and enzymatic controls. The earliest steps lead to the formation of a tetrapyrrole precursor, uroporphyrinogen III, which is also common to heme and chlorophyll biosynthesis (48, 50, 81-84). The two B12 biosynthetic pathways then diverge, using different routes to convert the precursor into cobyrinic acid a,c-diamide, at which point they converge again to generate the B12 corrin ring and to attach the DMB. Given the high genetic and metabolic cost, organisms that require B12, even ones capable of its biosynthesis, have evolved mechanisms to acquire and assimilate trace amounts of exogenous B12 and to salvage and regenerate the intracellular pool of this valuable cofactor (48, 85-89). Generally, external B12 in diverse forms (including ones lacking DMB, the cobinamides) is captured and transported into cells using dedicated proteins and sophisticated mechanisms in bacteria and animals. In the cell, imported B12 is usually processed (eg. decyanation of CNCbl, dealkylation) by specialized enzymes and escorted with the aid of chaperones (to prevent side reactions and dilution) to the AdoCbl or MeCbl generation/utilization pathways (48, 85-90). MeCbl is directly produced and used by methionine synthase/methyltransferase; AdoCbl is produced by ATP-dependent adenosyltransferases (ATRs) for use by other factors (86, 89, 91, 92). Three classes of sequence-unrelated ATRs are known in bacteria: CobA (BtuR or CobO), which acts in de novo AdoCbl biosynthesis, and EutT and PduO, which convert imported B12 to AdoCbl (89, 92). The most widely prevalent and studied is PduO, whose human ortholog (MMAB) is associated with mitochondrial B12 metabolism (89).
B12-dependent enzymes have been extensively surveyed elsewhere (54, 55, 63, 93-97), but a few aspects relevant to the theme of this review are highlighted here. B12 is used for three major types of reactions in biology: MeCbl is used for methyl transfer reactions, AdoCbl is used for radical-based transformations in mutases, dehydratases, deaminases and class II ribonucleotide reductases, and cobalamin without an upper ligand is used by reductive dehalogenases and by the tRNA-modifying enzyme epoxyqueuosine reductase, whose structures and mechanisms have only started to emerge (98-101). The activities of these enzymes, which bind to their cofactor with KD in the nM-mM range (97), are based on Co-C bond cleavage and formation of highly reactive species. The modes of Co-C bond cleavage and the resulting reaction mechanisms, however, are very distinct.
MeCbl-dependent enzymes catalyze heterolytic cleavage of the Co-C bond to form highly reactive cob(I), which retains both bonding electrons, and a methyl carbocation that is transferred to a nucleophilic acceptor. The prototypical member of this class, MeCbl-binding methionine synthase (called MetH in bacteria), catalyzes transfer of a methyl group from 5-methyltetrahydrofolate (MeTHF) to homocysteine (Hcy) for methionine synthesis (54, 55). This large monomeric enzyme has four structurally and functionally distinct domains, one each to bind Hcy, MeTHF, B12, and S-adenosylmethionine (102). The crystal structure of its isolated MeCbl-binding domain provided the first visualization of B12 bound to a protein and of the base-off/His-on B12-binding mode (64), which has since been observed in many other B12-dependent enzymes. Structural and biochemical studies have provided a framework to understand the workings of this enzyme and the mechanistic safeguards that protect the reactive MeCbl cofactor (55, 103). MeCbl is sandwiched between a Rossmann-fold domain, which binds the B12 lower face and the DMB tail, and a four-helix bundle, which caps the B12 upper face and prevents inadvertent loss of the methyl group (64, 104). Thus, MeCbl is sequestered, requiring that the MeTHF and the Hcy binding domains of MetH displace the four-helix bundle to gain access during catalysis (55, 103).
In the presence of the corresponding substrate, AdoCbl-dependent enzymes catalyze homolytic cleavage of the AdoCbl Co-C bond to generate cob(II) and Ado•, which initiates a chemical transformation by abstracting a hydrogen atom from the substrate (93). At the end of the catalytic cycle, Ado• is regenerated and recombines with cob(II) to restore AdoCbl for another round of catalysis. AdoCbl is thus a radical reservoir in AdoCbl-dependent enzymes, allowing for reversible access to the working species, Ado•, as required. Examples of these enzymes include methylmalonyl-CoA mutase and its relatives, which interconvert branched and linear acyl groups through carbon skeleton transformations; aminomutases, which migrate the terminal amino groups of lysine or ornithine; and eliminases, which mediate migration and elimination of a hydroxyl or amino group. Not surprisingly, these radical-based reactions must take place under carefully controlled conditions to prevent side reactions and oxidative quenching of intermediates. To achieve these conditions, AdoCbl-dependent enzymes bind AdoCbl in a buried cavity between two domains: a substrate-binding domain, usually an (α/β)8 triose phosphate isomerase barrel, and a B12-binding domain that is either a Rossmann-fold domain homologous to that in MetH or a distinct domain that binds AdoCbl base-on (93). In this architectural context, Ado• generation and the ensuing chemical transformations occur in a controlled environment, thereby enabling the difficult radical-based chemistry of AdoCbl-dependent enzymes.
Beyond its function as an enzyme cofactor, B12 can also bind to RNA-based regulatory elements called riboswitches (105, 106). The first such RNA element to be discovered, the AdoCbl riboswitch, spans the ≥200-nt 5′ untranslated region of mRNA from genes involved in B12 metabolism (105, 107). Both the AdoCbl and the more recently discovered AqCbl riboswitches bind tightly (KD≈10-250 nM) to the respective base-on B12 form using similar RNA structural cores, with additional peripheral extensions in the AdoCbl riboswitch conferring cofactor specificity (108, 109). The less common AqCbl riboswitch was speculated to have evolved in marine bacteria due to their high light exposure, which would favor greater availability of AqCbl over the less light-stable AdoCbl (108). However, the photochemistry and photobiology of these B12 riboswitches remain unexplored. Interestingly, B12 riboswitches typically sense the presence of AdoCbl (or AqCbl) to regulate expression of proteins involved in B12 uptake, biosynthesis, or use, whereas the photoreceptors reviewed here sense a light-dependent change in the state of B12 to control expression of genes apparently unrelated to B12 metabolism.
DISCOVERY OF CarA AND CarH, AND A ROLE FOR B12 IN LIGHT RESPONSE
Even though the light sensitivity of AdoCbl and MeCbl was known for years, this property was thought to serve no physiological function. The light sensitivity is actually undesired in enzyme catalysis as it leads to cofactor inactivation. The discovery that B12, specifically AdoCbl, can serve as a light sensor thus represented a major surprise. This new facet of B12 biology emerged from studies in the Gram-negative soil bacterium Myxococcus xanthus, in which light induces a transcriptional response leading to carotenoid biosynthesis (11, 110). Carotenoids protect cells against photooxidative damage by quenching singlet oxygen (1O2) and other ROS (14, 16, 110, 111), and light has been shown to generate 1O2 and trigger the response in M. xanthus (112). Yet, despite its well-established light response, genetic and bioinformatic studies failed to identify conventional photoreceptors in M. xanthus (11).
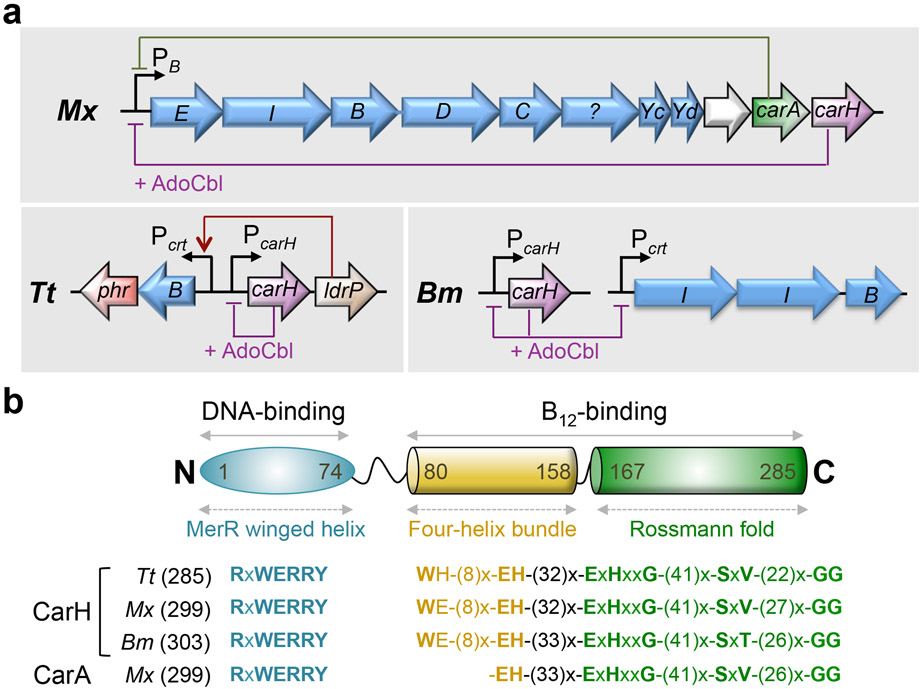
A resolution to this conundrum and the earliest hints for a role of B12 in a cellular response to light came from the identification of the first putative B12-binding transcription factors: the paralogous M. xanthus repressors of carotenoid (car) gene expression CarA and CarH, encoded by the two most downstream genes of the light-inducible carB operon, which groups all but one of the structural genes for carotenoid biosynthesis (Figure 2) (11, 110, 113). Their N-terminal sequences resembled the DNA-binding domains (DBD) of MerR transcription factors (114-117), suggesting a direct involvement in regulating gene expression (118). Their C-terminal segments were noteworthy, as they resembled the MeCbl-binding domain of MetH in sequence, size, predicted secondary structure, and in the presence of the base-off/His-on B12-binding motif Figure 2b (113), first described by Drennan et al. (64) and until then found only in enzymes that use B12 as a cofactor. As predicted, CarA did bind to B12 but, paradoxically, functioned independently of the cofactor. Whether or not B12 was present, CarA could bind to DNA using its autonomous, N-terminal MerR-type DBD (119, 120), and dimerize via its C-terminal module (37, 121, 122). To repress transcription, the CarA dimer appears to bind to a bipartite operator in a stepwise cooperative manner, first to a high-affinity palindrome and then to a second similar but lower-affinity one downstream, which overlaps with the −35 element of the carB promoter (PB), to block RNA polymerase access to PB (Figures 2a and 3a) (123). Light abolishes CarA-mediated repression by inducing expression of CarS, an antirepressor that structurally mimics operator DNA to bind tightly to the CarA DNA recognition helix and physically sequester it from operator binding (Figure 3a) (122-125).
Figure 2.
Experimentally studied CarH and CarA proteins. (a) Genomic context of the carH/carA genes in M. xanthus (Mx), T. thermophilus (Tt) and B. megaterium (Bm). Other genes and their corresponding products are as follows (carotenoid synthesis genes are in blue): crtE (E), farnesyltransferase; crtI (I), phytoene desaturase; crtB (B), phytoene synthase; crtD (D), hydroxyneurosporene dehydrogenase; crtC (C), neurosporene hydroxylase; ?, putative carotenoid biosynthesis protein; crtYc (Yc), crtYd (Yd), components of a heterodimeric lycopene cyclase; white arrow, predicted acyltransferase domain-containing protein; phr, DNA photolyase; ldrP, CRP/FNR family transcriptional regulator. (b) Domain architecture of CarH/CarA proteins. Numbers delimiting the domains are from the crystal structure of CarH from Tt. Characteristic motifs (x, any residue) are shown below for each protein (size in residues is indicated in brackets).
Figure 3.
Light-dependent gene regulation mechanisms by B12-binding transcription factors. (a) M. xanthus CarA and CarS. Cooperative binding of CarA dimers to a bipartite operator overlapping the −35 promoter region blocks access to RNA polymerase and represses transcription in the dark. Light induces production of CarS, which sequesters CarA, prevents operator DNA-binding, and activates transcription. (b) M. xanthus/T. thermophilus CarH. CarH monomers in the apo form bind to AdoCbl (filled blue asterisk) to form stable AdoCbl-bound CarH tetramers in the dark, which bind to operator DNA overlapping the −35 promoter region to block access to RNA polymerase and repress transcription. Light disrupts AdoCbl-CarH tetramers to monomers by photolysing bound AdoCbl (unfilled blue asterisks and blue circles correspond to products after photolysis described in the text), loss of operator-binding, and activation of transcription. (c) B. megaterium CarH. ApoCarH tetramers, which do not bind DNA, yield AdoCbl-bound tetramers that bind to operator DNA overlapping the −35 region of PcarH (another operator that overlaps the −10 region of Pcrt is not shown) to repress transcription in the dark. Light disrupts AdoCbl-CarH tetramers to dimers (by photolysing bound AdoCbl), abolishing operator binding and relieving repression. (d) R. capsulatus CrtJ and AerR. CrtJ dimers repress transcription in the dark by binding to two sites overlapping the −10 and −35 promoter regions. On exposure to light, binding of AqCbl (produced by AdoCbl or MeCbl photolysis) to AerR enables its association with CrtJ to disrupt CrtJ dimers and DNA-binding, activating transcription.
Repression of PB in the dark was abolished on deleting carA but, intriguingly, it was restored on supplying B12 exogenously (M. xanthus cannot synthesize B12 de novo but has the cellular machinery for its uptake and assimilation), as long as the CarA operator was intact (113, 121). Detailed genetic analysis in a carA-deleted genetic background demonstrated that downregulation of PB by B12 required CarH and its B12-binding motif (121). These findings thus revealed that light-induced carotenogenesis in M. xanthus was regulated by two parallel and distinct pathways, orchestrated by a pair of paralogous factors, of which one (CarH), but not the other (CarA), required vitamin B12 (121). The study also established a firm link between B12 (the form used was unknown at this point) and CarH in light-dependent gene regulation and marked the discovery of a role for B12 as a light sensor.
M. xanthus CarA and CarH were the only known transcription factors with a B12-binding domain fused to a DBD until a later study reported a protein with a similar domain architecture acting in light-induced carotenogenesis in the Gram-positive soil bacterium Streptomyces coelicolor (126). Molecular details on how this protein, named LitR (for light-induced transcription regulator), functions and whether or not it requires B12 remain unaddressed. The subsequent outpouring of microbial genome data revealed many bacterial species with genes for CarA/CarH homologs of unknown function, often amidst ones for carotenogenesis or light-related responses (37, 121). Studies of a selected few of these are now revealing the molecular basis for their distinct modes of action.
MOLECULAR MECHANISMS OF B12-BASED PHOTORECEPTORS
Adenosylcobalamin in Light-dependent Gene Regulation by CarH
The mechanistic basis for the combined action of CarH and B12, the link to light, and the specific form of B12 involved emerged in a pioneering study reported in 2011 (37). Domain swap and bacterial two-hybrid analyses firmly established that the C-terminal domain of CarH conferred the B12 dependence in vivo, with B12 controlling the oligomeric state of this domain (37). In-depth analysis of the molecular interplay between CarH and B12 was, however, thwarted by the inability to purify native M. xanthus CarH. The existence of a homolog of unknown function in Thermus thermophilus (a Gram-negative bacterium that can synthesize B12 de novo) not only enabled comparative studies but also, since it was easily purified, its biochemical, hydrodynamic, structural, and photochemical characterization (37-40, 127). The T. thermophilus protein and a chimera with its DBD replaced by that of M. xanthus CarH (which could functionally replace M. xanthus CarH in vivo) exhibited, in vitro, B12-dependent oligomerization and DNA-binding in the dark that was disrupted by light. Surprisingly, the form of B12 required turned out to be AdoCbl, despite the similarity of the CarH B12-binding domain to that in MeCbl-dependent MetH (37). Only AdoCbl rapidly transformed the monomeric apoprotein into a stable tetramer that has 1:1 AdoCbl:CarH stoichiometry and binds tightly to operator DNA (KD≈70 nM) (37, 38). Photolysis of AdoCbl-CarH by exposure to near-UV, blue, or green light swiftly provoked disassembly of the tetramer to monomers, with concomitant loss of operator-binding (Figure 3b) (37, 127). Interestingly, T. thermophilus CarH retains the photolysed AdoCbl as a stable adduct, refractory to exchange with fresh AdoCbl (38, 40). That AdoCbl is also the B12 form required by M. xanthus CarH was evident when the deletion of the only gene encoding an ATR (of the PduO type) in this bacterium, which would cause an abrogation of intracellular AdoCbl generation, resulted in the loss of B12-dependent repression of carotenogenesis. Moreover, even in the absence of the CarS antirepressor, light still relieved repression by M. xanthus CarH. These studies thus established thatM. xanthus CarH also directly senses light using AdoCbl to regulate gene expression (37).
The location of the gene for T. thermophilus CarH next to one for carotenogenesis, as in M. xanthus (Figure 2a), suggested a function in light-regulated carotenoid synthesis, which was indeed demonstrated (128). However, while M. xanthus CarH acts at a promoter that drives expression of the genes for carotenoid synthesis as well as its own, the one in T. thermophilus represses its own promoter and that of LdrP, a transcriptional activator of the carotenogenic gene transcribed in the opposite direction (Figure 2a) (128). In another variation recently found in Bacillus megaterium (a Gram-positive bacterium that can synthesize B12 de novo), the CarH homolog regulates its own expression and that of target carotenogenic genes present at an unlinked genetic locus (Figure 2a) (129). Here, the active “dark” state AdoCbl-bound repressor is again a tetramer but its inactivation by light yields a dimer rather than a monomer (Figure 3c) (129).
Another Mode of Gene Regulation Dependent on B12 and Light: AerR
A new twist to B12-dependent light-regulated gene expression was reported with AerR, a small, standalone B12-binding protein found in Rhodobacter capsulatus and related α-proteobacteria (130, 131). AerR (also termed PpaA) is an antirepressor of CrtJ (the aerobic repressor of carotenoid (crt) gene expression; also termed PpsR), a dimeric, redox-regulated repressor of genes for carotenoid, heme, and bacteriochlorophyll biosynthesis and for structural proteins of the light-harvesting complex II (130, 132, 133). Like CarA, the CrtJ dimer binds cooperatively to two tandem palindromes in the promoter region to repress transcription, and requires an antirepressor (AerR) for derepression in the light (132). But unlike the CarA antirepressor CarS, AerR is not transcriptionally activated by light but instead binds to AqCbl, which is produced in the light by photolysis of AdoCbl (or MeCbl). AqCbl-bound AerR then targets CrtJ to disrupt its oligomerization, DNA-binding and repressor activity (Figure 3d) (130, 131). As in T. thermophilus CarH, photolysed B12 is tightly bound to AerR. Two His in AerR were implicated in this tight binding, one corresponding to the base-off/His-on B12-binding motif and another that is not conserved in its homologs (130).
The CarH Tetramer: Structural Comparisons with Known DNA- and B12-binding Proteins
A series of crystal structures of the functionally relevant states of T. thermophilus CarH and structure-based mutational analysis by our groups recently provided detailed insight into the architecture and the light-dependent conformational changes of the CarH photoreceptor (38). The “dark” AdoCbl-CarH tetramer is a dimer of two dimers, each with a striking head-to-tail arrangement of two monomers. The N-terminal domain of each monomer structurally matches the winged-helix DBD found in CarA (Figure 4a) (119) and MerR-type transcription factors (114-116). It has the canonical DNA recognition α-helix, with a conserved RxWERRY motif in CarH, its homologs, and CarA (Figure 2b), and the β-hairpin wing. The CarH DBD conserves most of the residues that contact DNA in CarA (119) and in MerR proteins (114-116), but employs a distinct DNA-binding mode described in the next section. The DBD adopts different orientations relative to the C-terminal domain due to a flexible linker of ~6-residues observed in the T. thermophilus CarH structure. Remarkably, the C-terminal AdoCbl-bound light-sensing module is structurally more similar to the MetH MeCbl-binding domain (64) than to any known AdoCbl-binding protein (Figure 4a). As in MetH, this CarH module has a four-helix bundle, which contacts the upper face of the Cbl, followed by a five-stranded α/β Rossmann domain that binds the lower face in the base-off/His-on mode using the conserved His of the Glu/Asp-x-His or E/DxH (where x is any residue) B12-binding motif (Figures 2b and 4a). But in contrast to the MetH module, in which only the Me of MeCbl can fit snugly as the upper axial ligand, the CarH B12-binding pocket accommodates the far bulkier Ado group of AdoCbl. Also, AdoCbl-CarH is a tetramer whose B12-binding modules act as light sensors (37, 127), whereas the large multidomain MetH is a monomer (102) whose B12-binding module suppresses undesired light-induced side reactions (104).
Figure 4.
Dark state AdoCbl-bound CarH compared to structurally similar DNA- and B12-binding domains. (a) CarH protomer structure [Protein Data Bank (PDB) accession code 5C8D] showing the modules for DNA-binding (compared with that in CarA, top left; PDB accession code 2JML) and B12-binding (compared to that in MetH, bottom left; PDB accession code 1BMT). (b) Close-up of the B12-binding site showing residues contacting the upper axial ligand (in cyan) of MeCbl in MetH and cobalt-coordinating His (H759). (c) Close-up of the B12-binding site showing residues contacting the upper axial ligand (in cyan) of AdoCbl in CarH and cobalt-coordinating His (H177). For a better view, the orientation in b and c is slightly different from that in a. (d) Comparison (in stereo) of the orientations of the AdoCbl Ado group in CarH, in free AdoCbl, and in selected enzymes whose structures have been determined with intact AdoCbl: IcmF (PDB accession code 4XC6) and related mutases; ornithine-aminomutase (OAM) in its resting state (PDB accession code 3KP1).
The CarH structure reveals how an enlarged B12-binding pocket and the substitution of four hydrophobic residues in the four-helix bundle, Phe708, Leu715, Val718, and Val719 in MetH to Trp131, Val138, Glu141, and His142, respectively, in CarH (Figure 4b,c), allowed for the repurposing of the MetH MeCbl-binding module into one that binds AdoCbl (38). These changes produce a larger cavity, a more polar environment, and a H-bond between Glu141 and the Ado ribose group. Moreover, the Phe in MetH is directly above the Me group of MeCbl to protect it from photolysis (104), whereas the larger side chain of the equivalent Trp in CarH is to the side of the Ado group in AdoCbl, contacting its ribose moiety. Although the AdoCbl Co-C bond length in CarH (2.2 Å; (38)) is similar to that in the free form or in the substrate-free resting state of AdoCbl-based enzymes (93), the relative orientation of the Ado group differs (Figure 4d). Consistent with their importance, the aforementioned Trp, Glu, and His form a conserved W-9(x)-EH motif in CarH homologs, and mutating any of these residues impairs AdoCbl-binding and tetramerization (38). Tellingly, M. xanthus CarA, which binds to B12 but does not require it for oligomerization or function (37), retains the Glu and His but lacks the Trp and four other adjacent residues (Figure 2b).
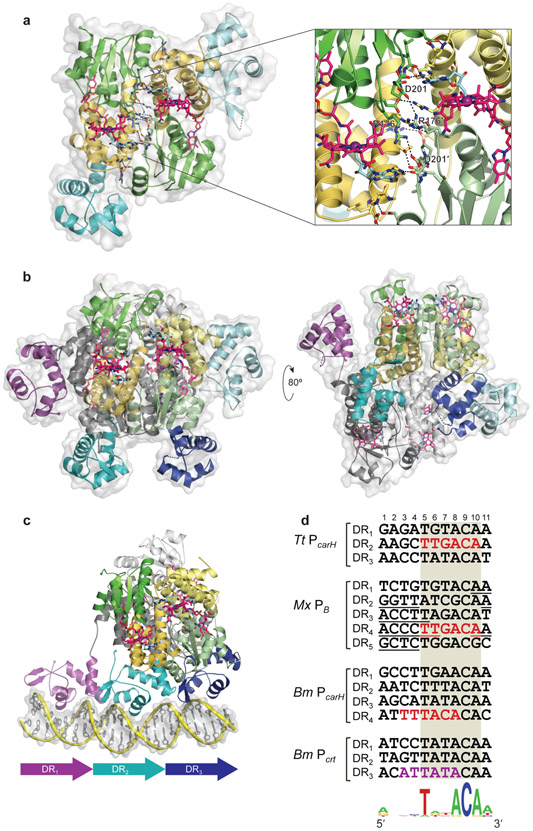
The presence of the Ado group maintains the AdoCbl-bound protomer in an extended conformation, which allows the helix bundle of one monomer unit to pack against the Rossmann fold of another unit to form a head-to-tail dimer. The dimer interface between the head-to-tail monomeric units is extensive, with over twenty H-bonds and salt bridges, some involving the Ado group (Figure 5a). Tetramers form by packing two head-to-tail dimers in a staggered manner (Figure 5b). Assembly of the tetramer from apoprotein monomers upon adding AdoCbl is so rapid and favorable that dimers are detected only if the dimer-dimer interface is disrupted by mutation (37, 38, 127). Residues at the dimer and tetramer interfaces are not highly conserved, but key interactions appear to be retained. For example, an Arg-Asp salt bridge between Arg176 in one monomer and Asp201 in the other is crucial for dimerization (Figure 5a), as its disruption produces monomers, but can be swapped for an Asp-Arg or a Glu-Arg pair (38). In M. xanthus CarH, this pair is already swapped in the form of a Glu-Arg pair, but is also indispensable for oligomerization, and can be replaced by Arg-Glu in vivo (J. Fernández-Zapata, M.C. Polanco, S. Padmanabhan & M. Elías-Arnanz, unpublished findings). It is intriguing that CarA, unlike CarH, forms dimers independently of B12 or light (37), and some AdoCbl-dependent CarH homologs, such as that in Bacillus megaterium, are active as repressors in the tetrameric form but become dimers instead of monomers in the light (Figure 3) (129). Structures of these homologs can provide insights into why their oligomerization behavior differs from that observed for T. thermophilus CarH.
Figure 5.
The CarH tetramer and its unexpected DNA binding mode. (a) The head-to-tail packing of the two protomers in a CarH dimer, with the helix bundle colored yellow, the AdoCbl-binding domain in green, and the two DBDs in cyan. On the right is a close-up of the extensive interface of the head-to-tail dimer with several H-bonds and ionic interactions indicated. D201 and R176 indicate residues whose interaction was shown to be crucial by mutational analysis. (b) The CarH tetramer of two head-to-tail dimers with the four DBDs (in purple, cyan, light cyan, and dark blue) [Protein Data Bank (PDB) accession code 5C8D]. Two alternative views of the tetramer are shown to better appreciate the complex quaternary structure of CarH. The view on the left shows the distribution of the DBDs on the protein surface (DBDs shown in pink and dark blue correspond to the head-to-tail dimer at the back, whose B12-binding domains are shown in gray). (c) CarH tetramer (colored as in panel b) in complex with DNA (PDB accession code 5C8E) is shown. Three DBDs are reoriented and contact three direct repeats (DR) in the DNA sequence, depicted schematically below (structures in b and d are redrawn versions of those previously reported in (38)). (d) Direct repeats recognized by CarH at PcarH in T. thermophilus (Tt) determined from the structure of the complex, and those inferred in M. xanthus (Mx) and B. megaterium (Bm) from footprinting data and sequence inspection. Base pairs covered by the recognition helix, as deduced from the CarH-DNA structure, are shaded. The −35 promoter region is shown in red. In Mx PB, the two CarA binding site palindromes are underlined. The repeats in Bm Pcrt and its −10 region (in purple) correspond to the noncoding strand. The DNA sequence logo for a probable consensus CarH direct repeat recognition site from the sequences above is shown (bottom).
CarH´s architecture of a tetramer formed by two head-to-tail dimers is rather unusual for a transcription factor. This arrangement results in neighbouring DBDs being pointed away from each other (Figure 5b). Very few structures of transcription factors with such a head-to-tail assembly are known. One example, Bacillus subtilis GabR, has an N-terminal winged-helix domain connected by a long, flexible 29-residue linker to a C-terminal domain with an aminotransferase (AT) family fold that binds to pyridoxal 5′-phosphate (PLP), the vitamin B6 coenzyme (134, 135). With or without bound PLP, two AT-domains pack head-to-tail to form a stable GabR dimer (134). The GabR-DNA complex structure is not known but it has been proposed that a GabR dimer binds to two direct ATACCA repeats, separated by a 29-bp AT-rich spacer that is bent in the complex and includes the −35 promoter region (134, 135). The DNA-binding mode of CarH, however, is distinct.
The Surprising Mode of DNA-binding by CarH
The crystal structure of the CarH-DNA complex revealed a surprising mode of DNA-binding, with three out of the four DBDs in the CarH tetramer contacting three adjacent sites (Figure 5c). This binding mode was corroborated by hydroxyl radical footprinting and systematic analysis of CarH-binding to mutant operators (38). The CarH tetramer, alone or DNA-bound, has the same overall architecture except for a reorientation of three DBDs to contact three adjacent direct 11-bp repeats, the central one containing the TTGACA of the −35 promoter element (Figure 5c, d) (38). H-bonds and electrostatic interactions position the recognition helix of each DBD (involving the conserved RxWERRY motif) in the major groove, and the β-hairpin wing in the minor groove. Mutating DNA contacts in any two repeats or all three, but not in just one, abolishes binding by CarH. Tight DNA binding requires a tetramer, since mutants that form only dimers bind DNA with reduced affinity and cooperativity, and light-induced monomers do so poorly (37, 38).
It is intriguing that the T. thermophilus CarH tetramer uses three out of four DBDs to contact three adjacent DNA sites and, accordingly, only three direct repeats can be identified in its operator segment (Figure 5d) (38). Curiously, within the ~50 bp M. xanthus CarH operator (which also spans that of CarA) mapped using the chimera with the M. xanthus CarH DBD fused to the T. thermophilus CarH AdoCbl-binding domain mentioned earlier (37), five direct repeats similar to those of T. thermophilus can be identified (Figure 5d). Future studies will help establish the actual number of repeats and DBDs required for DNA-binding and function in this case. Interestingly, AdoCbl-bound B. megaterium CarH has been reported to bind to two DNA sites located at unlinked genetic loci (Figure 2a). One is an ~45-bp DNA site that overlaps with the −35 element of its own promoter (PcarH), and the other is about 10 bp shorter and includes the −10 element of the promoter for the carotenogenic operon (Pcrt); in both cases, an imperfect interrupted palindrome with two 6-bp half-sites separated by 16 bp was proposed as the binding site (129). However, four and three similar direct repeats can be discerned in the longer and shorter sites, respectively (Figure 5d). Thus, the B. megaterium CarH tetramer could conceivably target direct repeats, as does T. thermophilus CarH, rather than a palindrome as proposed, although this possibility remains to be established experimentally. The activities of signaling proteins such as photoreceptors often depend dramatically on the properties of the linker between the receptor and effector modules (136). The intrinsically unstructured linker between the CarH DBD and the light-sensing oligomerization module, whose length and sequence vary in different homologs (~6 residues in T. thermophilus, ~20 in M. xanthus, ~14 in B. megaterium), could underlie the flexible DNA-binding mode of CarH, an aspect that needs to be explored. The finding that the B. megaterium homolog can bind to a site that overlaps with the −10 promoter element (Figure 5d) suggests that CarH can target the −35 as well as the −10 promoter elements to achieve transcriptional repression. The CarH proteins that have been studied, although from distantly related bacteria, nonetheless appear to recognize similar 11-bp repeats, with highly conserved bases at positions 5 (T), 8 (A), 9 (C) and 10 (A) (Figure 5d). Occurrence of these conserved bases in adjacent repeats could thus help identify other CarH-regulated promoters.
Interestingly, in binding as a tetramer to direct repeats, CarH conserves most of the DNA contacts of CarA and MerR proteins, even though the latter bind as dimers to (pseudo)palindromic sequences (114-117, 119, 123). But unlike MerR factors, CarH and CarA are only known to repress transcription by binding to sites overlapping a promoter with optimal spacer length (38, 119, 121, 123). Whereas CarA does this as a dimer, and independently of B12, by stepwise cooperative binding, T. thermophilus CarH only represses as an AdoCbl-bound tetramer using a DBD from one head-to-tail dimer to bind the −35 promoter element in the central repeat, and the DBDs from the other dimer to bind the outer repeats (Figure 5c) (38).
Structure of Light-exposed CarH Suggests Mechanism of Photoregulation
The structure of light-exposed AdoCbl-CarH (Figure 6a) clearly revealed bound Cbl without an Ado group, and provided molecular insights into how light triggers tetramer disassembly (38). In the dark, the Ado group functions as a “molecular doorstop” by stacking against Trp131 (of the W-9(x)-EH motif) (Figures 4c, 6b). This interaction maintains the CarH protomer in the extended conformation required to assemble the head-to-tail dimer and thereby the tetramer. Once the Ado group dissociates upon exposure to light, Trp131 moves into the void caused by the loss of the Ado group, leading to a sizable shift (>8 Å) of the four-helix bundle relative to the Rossmann fold (Figure 6a). The resulting bent conformation of the protomer disrupts the head-to-tail dimer interface, causing tetramer collapse and loss of DNA binding. The helix bundle shift repositions Trp131 and the adjacent His (His132), the latter of which ends up as the upper axial ligand of the Co in Cbl, forming a very stable bis-His ligation (Figure 6b,c) (38). The structure of photolysed CarH provided the first visualization of a bis-His ligation involving Cbl. The very stable bis-His bond explains why fresh AdoCbl cannot replace Cbl in photolysed T. thermophilus CarH (38, 40). The biologically expensive Cbl is thus securely retained for recovery and reuse in vivo, although the details of the recovery process are not yet known. The lower axial His is the most strictly conserved residue of the canonical B12-binding motif and is indispensable for cofactor binding and activity (37, 121, 129). By contrast, the upper axial His is not strictly required for tetramer assembly or its light-induced collapse. This His is conserved in thermophilic (38) and many other CarH homologs, but not all (for example, it is a Glu in M. xanthus and B. megaterium CarH; Figure 2b). A similar bis-His cobalt ligation was proposed in AerR, but the expected upper axial His is neither conserved nor is it in the putative four-helix bundle (130). Whether other residues can coordinate the Co on the upper site or another ligand such as water is involved remains to be explored.
Figure 6.
Molecular basis of photoregulation by CarH. (a) Structure of light-exposed CarH [solid, Protein Data Bank (PDB) accession code 5C8F] with the arrow indicating the major shift in the helix bundle relative to the dark structure (transparent, PDB accession code 5C8D). (b) Close-up of the AdoCbl-binding site highlighting the role of the upper axial Ado group (in cyan) of AdoCbl as a molecular doorstop in the dark state. The arrow indicates the helix bundle shift that occurs on exposure to light leading to relocation of W131 and the other indicated residues. The nonconserved E129 was suggested to be involved in forming the bis-His adduct by deprotonating His132 based on homology modeling and molecular dynamics studies (40), but this may be unlikely given its positioning in the crystal structure. (c) Close-up of the bis-His Co coordination in light-exposed CarH. (d) Scheme of the proposed mechanism for CarH photolysis adapted from (39), with additional details from the high-resolution structures (38) and apparent rates, kapp, from transient kinetics data (40). The chemical structure of 4′,5′-anhydroadenosine, the product of AdoCbl photolysis in CarH (39), is shown in the middle (and to the right) of the scheme.
Photochemical Basis of CarH Function
The use of AdoCbl as a light sensor posed additional interesting questions regarding the photochemistry involved in the process. In particular, it seemed highly counterintuitive that a cell response to mitigate photooxidative damage by highly reactive ROS would rely on AdoCbl photolysis, as this process usually generates Ado•, which can itself produce ROS and trigger radical-induced cell damage. This apparent paradox prompted studies aimed at characterizing the products of CarH photolysis and the underlying photochemistry. In one study, the products of AdoCbl-CarH photolysis formed under aerobic or anaerobic conditions were analyzed using liquid chromatography-mass spectrometry and UV–Vis, EPR, and NMR spectroscopies after sample workup on the time scale of minutes to hours (39). The other study involved transient kinetics analysis over femtoseconds to seconds using ultrafast spectroscopy, in which photolysis triggered by laser pulse excitation was followed by time-resolved acquisition of absorption spectra (40). These studies indicated that CarH orchestrates a distinct photochemistry to safeguard the use of AdoCbl as a light sensor and set the stage for further studies to elucidate the details.
Photolysis of free AdoCbl generates Ado•, which then forms a set of well-characterized products described earlier (5´-peroxyadenosine, adenosine-5′-aldehyde, and minor amounts of adenosine and adenine in the presence of oxygen, and 5′-deoxy-5′,8-cycloadenosine in the absence of oxygen) (71, 72). However, photolysis of CarH-bound AdoCbl did not yield any of these products but instead generated 4′,5′-anhydroadenosine (Figure 6d), which had not been previously detected as a photolysis product of AdoCbl (39). 4′,5′-anhydroadenosine had been observed as an inactivation product of some AdoCbl-dependent enzymes, the relevance and mechanism of which remain unclear (137, 138). More importantly, it was known to be a minor product of AdoCbl thermolysis via β-H elimination from the ribose C4′ of Ado•, primarily in viscous solvents when radical escape is slowed by solvent cage effects (139). The observation of 4′,5′-anhydroadenosine as the sole photolysis product thus directly suggested that CarH alters the photochemistry of AdoCbl and that the reactive Ado• is not released free into solution. In principle, two mechanistic routes could lead to formation of 4′,5′-anhydroadenosine (Figure 6d). Photolysis could occur by Co-C bond homolysis to form cob(II) and Ado•, followed by β-H transfer from Ado• to cob(II) to form 4′,5′-anhydroadenosine and cob(III) hydride (hydridocobalamin), or by Co-C bond heterolysis to form cob(III) and an Ado− anion, which could undergo β-hydride elimination to again yield 4′,5′-anhydroadenosine and cob(III) hydride (39). Under aerobic conditions, cob(III) hydride would get oxidized to the final bis-His-ligated cob(III) species observed in the crystal structure. Thus, either pathway yields the same end products, requiring characterization of the intermediates to distinguish between the pathways.
Analysis of the photochemical mechanism (over fs to s) of CarH-bound AdoCbl using ultrafast spectroscopy led to the conclusion that Co-C bond photolysis occurs primarily by a heterolytic mechanism (Figure 6d), providing further evidence that CarH alters the photochemistry of AdoCbl (40). Although a small amount of homolytic cleavage was also observed, the resulting cob(II):Ado• radical pair underwent quantitative recombination. Thus, this study suggested that the photochemistry of CarH-bound AdoCbl is altered in two ways: formation of Ado• is suppressed by activation of a heterolytic Co-C bond cleavage pathway, and any Ado• formed through homolytic Co-C bond cleavage is not released into solution (40).
The mechanistic basis for this altered photochemistry is still largely unclear. The UV-visible spectrum of CarH-bound AdoCbl, compared to free AdoCbl, exhibits some changes in the α and β absorption bands, including a shift of the α band to higher energy (38-40). Although these changes could point to a specific effect of the binding environment on the photochemistry, more studies are needed. In addition, CarH might exert a cage effect following photolytic cleavage of the AdoCbl Co-C bond to suppress dissociation of Ado• and Ado−, and to instead favor β-H elimination. Similar cage effects have been observed in MetH and in the AdoCbl-dependent enzyme glutamate mutase, although in both cases these cage effects favor radical pair recombination and not a β-H elimination (104, 140, 141). Additionally, the observation that cob(II) is formed upon anaerobic photolysis of CarH seems at odds with heterolytic Co-C bond cleavage, although formation of cob(II) could be explained as a downstream consequence of cob(III) hydride decay (39). The contributions of specific amino acids are similarly unclear. The lack of conservation of His132, whose side chain occupies the B12 upper face following photolysis, suggests that its role may be specific to formation of bis-His-ligated cob(III). Instead, the conserved Trp, Glu, and His of the W-9(x)-EH motif, which interact with the Ado group in the dark-state CarH tetramer and orient the Ado group differently than is usually observed in enzyme-bound or free AdoCbl (Figure 4d), could be involved in reprogramming AdoCbl photochemistry (37-40, 142), but again more work is needed. Detailed mechanistic studies using experimental as well as quantum mechanics/molecular mechanics approaches will help further elucidate the novel and promisingly rich photochemistry of CarH.
DISTRIBUTION AND EVOLUTIONARY ASPECTS OF B12-DEPENDENT PHOTORECEPTORS
Genome searches yield hundreds of proteins with a MerR-type DBD fused to a B12-binding domain in bacteria, only some of which are capable of de novo B12 biosynthesis. We could identify ~600 proteins with the characteristic motifs found in experimentally studied CarH proteins, and hence likely to be AdoCbl- and light-dependent: RxWxxR in the DBD and W-(9)x-EH (or W-(10)x-EH in many actinobacterial homologs, notably of the genus Streptomyces) preceding the E/DxH motif in the B12-binding domain (Figure 7). Various other surrounding residues in the DBD and the B12-binding domain are also conserved (Figure 7b), hinting at their structural and functional importance. However, the His observed as the upper axial ligand in the bis-His cobalt of light-exposed T. thermophilus CarH (His132) is conserved only in some phyla, like Deinococcus-Thermus, suggesting a restricted functional role. One noteworthy feature is that the His of the W-(9)x-EH motif (His142 in T. thermophilus) and the Glu/Asp of the E/DxH of the B12-binding motif (Glu175 in T. thermophilus) are typically spaced 32-39 residues apart (33-36 being most common). Another is the rather wide distribution of linker size (~6-120 residues) between the DBD and the B12-binding domain, which possibly underlies, as noted before, a rather flexible DNA-binding mode. Altogether, the motifs shown in Figure 7b may serve as a signature for an AdoCbl-binding light-sensor module. The various putative CarH proteins are distributed across many bacterial phyla, being most abundant in Actinobacteria, Bacteroidetes, Chloroflexi, Deinococcus-Thermus, firmicutes, and β- and δ-Proteobacteria (Figure 1a). In most species, the corresponding genes are present as a single copy that, consistent with a light-response function, frequently occurs in the vicinity of genes for carotenogenesis or DNA photolyase. However, as observed in B. megaterium (129), carH can be involved in light-dependent regulation even when spatially decoupled in the genome from its target genes.
Figure 7.
Phylogenetic distribution of CarH and of its B12-binding domain in other proteins. (a) Distribution of the indicated domain architectures across different bacterial phyla, with the number of genomes for each phylum indicated (from ~8400 bacterial genomes available at http://www.ncbi.nlm.nih.gov/genomes; for simplicity, only Candidatus phyla with relevant B12-binding domains are shown). The number of proteins in each phylum (H, CarH; A, CarA) correspond to the color heat map on the bottom right. InterPro (protein sequence analysis & classification, https://www.ebi.ac.uk/interpro/) was used to search for proteins with a B12-binding domain (IPR006158) alone, or in combination with a MerR-type DNA-binding (IPR000551) domain or other domains [IPR007024, globin-like; IPR005467, histidine kinase; IPR029016, GAF (cGMP-specific phosphodiesterases, adenylyl cyclase, FhlA)]. Protein sequences retrieved from UniProt (Universal Protein Resource, http://www.uniprot.org/) were aligned using MUSCLE in MEGA7 (Molecular Evolutionary Genetics Analysis, http://www.megasoftware.net/). Those with the E/DxH B12-binding motif preceded by a W(9/10)xEH motif were selected by manual curation. The ones fused to a MerR-type DBD with the RxWxxR motif were classified as CarH or, if they lacked the Trp but not the EH of the W(9/10)xEH motif, as CarA. The 200-260 residue size range was used to select for standalone proteins. (b) Sequence logo created using WebLogo (http://weblogo.berkeley.edu/) for 498 CarH proteins with the W-(9)x-EH motif (those with a W-(10)x-EH motif, mostly in actinobacteria, were omitted). Shown are segments around signature residues (asterisks above) used for curation in the DBD (top left, cyan border), the four-helix bundle (top right, gold border) and the Rossmann fold (bottom, green border). In the sequences (x, any residue) below each logo, conserved residues (≥95%) are highlighted in bold and larger font.
Proteins with CarH domain architecture but which lack the Trp of the W-(9)x-EH motif, like CarA, and hence probably B12-independent, are less abundant (~60) and more narrowly distributed (Actinobacteria, Firmicutes, and δ-Proteobacteria) (Figure 1a). The predominance of CarH proteins and their wider distribution suggests an ancestral protein that was more like CarH, a hypothesis in line with the distribution of CarH and CarA in the Myxococcales order of δ-Proteobacteria (25 and 10 proteins, respectively). Members of its Sorangiineae and Nannocystineae suborders have just one carH gene. M. xanthus and related species in the Cystobacterineae, however, have two tandem paralogous genes (carA and carH), as well as CarS and the full repertoire of other factors required in the B12-independent CarA-CarS pathway, with Cystobacter fuscus standing out as the only one lacking CarA (M.C. Polanco, J. Fernández-Zapata, S. Padmanabhan, M. Elías-Arnanz, unpublished data). Taken together with the ancient origin proposed for B12 (48), the most parsimonious hypothesis is that the gene for an ancestral AdoCbl-dependent CarH duplicated and functionally diverged in Cystobacterineae to evolve into carA. Presence of carH, alone or in tandem with carA, in the neighbourhood of genes for carotenogenesis in myxobacteria, suggests a common AdoCbl-dependent mode of regulation of this light-induced process, the alternative B12-independent pathway co-existing in species with CarA. Having both pathways likely confers an evolutionary advantage, since the photoprotective carotenogenic response is maintained even in the absence of available B12. It mirrors the coexistence of B12-dependent and independent isozymes in many organisms that ensures crucial enzyme activities on limited B12 availability (48).
Besides B12-dependent enzymes and CarH/CarA, genome data reveal the B12-binding domain in a large number of proteins, standalone or linked to other effector/output domains (37, 143). In particular, a CarH-type AdoCbl-binding module with the characteristic W-(9)x-EH motif (again, W-(10)x-EH in Streptomyces) can be found in ~170 widely distributed standalone proteins (200-260 residues) (Figure 7a). Notably, B12-binding AerR/PpaA antirepressors, which are chromosomally coupled to CrtJ/PpsR and restricted to purple nonsulfur bacteria, appear to be distinct from CarH, lacking the characteristic motif (130, 131). In some cases, a CarH-type AdoCbl-binding module is associated with domains typically involved in signal transduction (Figure 7a), such as globin (heme-based oxygen sensor), histidine kinase, or GAF (cGMP-specific phosphodiesterases, adenylyl cyclase, FhlA) (144). Their functions and modes of action remain uncharted, but it is tempting to speculate that these exhibit AdoCbl- and light-dependent protein-protein interactions with themselves (like CarH) and/or with other effector proteins. The stage has thus been set for future studies of the function and evolution of these putative B12-binding proteins.
CONCLUDING REMARKS AND OUTLOOK
The discovery that AdoCbl is the chromophore of a new family of photoreceptors changed the perception that a basic property of AdoCbl, its light sensitivity, has no direct biological function. Major landmarks have been reached in understanding the mechanism of action of the first known photoreceptor of this class, CarH, including characterization of its distinct photochemistry and high-resolution structural descriptions of its functionally relevant states. These studies have illustrated how nature has assembled a light- and B12-dependent transcription factor by repurposing structural modules of enzymes and DNA-binding proteins and repurposing AdoCbl from an enzyme cofactor to a light sensor. Genome data reveal similar B12-binding domains in a plethora of organisms, standalone or linked to other modules that could allow for integration of signals and regulation of diverse biological processes. Their study will surely be a rich source to identify new modes of B12- and light-dependent regulation and possible cross-talk between distinct signaling pathways. Future surprises are certainly in store, given the precedent from the studies thus far. Undoubtedly, innovative experimental and theoretical approaches will address the various remaining questions on the molecular mechanisms of B12-based photoreception. Whereas light induces reversible molecular changes in the chromophore of other photoreceptors (31, 32), it alters the chromophore irreversibly for the AdoCbl-dependent photoreceptor. The latter suggests that specialized cellular salvage and repair pathways may exist that remain to be discovered. Another exciting research avenue concerns possible applications of these photoreceptors. In initial studies, CarH has been exploited for light- and B12-dependent conditional expression of essential bacterial genes (145). Whether it can be optimized for additional optogenetic applications remains to be seen. In any case, the study of these photoreceptors has already uncovered fascinating new biology and chemistry, with many more surprises likely ahead.
SUMMARY POINTS.
A new family of photoreceptors uses 5′-deoxyadenosylcobalamin (AdoCbl) or coenzyme B12 as a chromophore to sense near-UV, blue, and green light.
The first characterized AdoCbl-dependent photoreceptor, the CarH transcription factor, is a tetramer that represses transcription of genes involved in carotenoid biosynthesis in the dark. Light causes the CarH-bound AdoCbl to photolyse, leading to a large protein conformational change that disrupts the tetramer, impairing DNA-binding and abolishing transcription repression.
The photochemistry of the AdoCbl-based photoreceptor appears to be distinct from that of free and enzyme-bound AdoCbl. The altered photochemistry provides a means to safeguard the use of AdoCbl as a light sensor. The underlying molecular basis for the difference in photochemistry remains an open question.
In another mode of light- and B12-dependent gene regulation, light induces cobalamin-binding to an antirepressor, which then binds to its target repressor to activate transcription.
Genome data reveal B12-binding modules similar to the one in CarH associated with various other domains or existing as standalone proteins, the functions of which are unknown.
FUTURE ISSUES.
The structural basis for the variations in the oligomerization behaviour of AdoCbl-dependent photoreceptors is not known. For example, it is not clear why some tetramers disassemble to dimers whereas others disassemble to monomers.
More evidence is needed to support the proposal that the variation in linker length between the CarH DNA-binding domains and light-sensing domains explains the apparently flexible modes of DNA-binding of CarH homologs.
The exact role(s) of the protein residues around the Ado group in CarH remain to be determined. It is not clear which residues, if any, are responsible for the distinct photochemistry of CarH.
Theoretical (quantum mechanics, molecular mechanics) approaches will be helpful in understanding the photochemistry of AdoCbl-bound CarH.
The intracellular fates of the photolysed photoreceptor and its associated chromophore remain to be established, as do the cellular machineries involved in determining these fates.
Whether other physiological processes are regulated by AdoCbl-based light-sensing is an open question.
It remains to be determined if B12-based photoreceptors can be exploited as a useful optogenetic tool.
Photoreceptor: a protein that directly senses light via a chromophore component, which is an organic noncovalently or covalently bound molecule that undergoes photochemical transformation and triggers conformational changes in the protein for signal propagation. Various classes of photoreceptors have been established based on the chromophore and the protein component. A “sensor” or “input” domain in a photoreceptor senses light producing molecular changes that are transmitted to an “effector” or “output” domain that has a defined activity, which can be binding to DNA, RNA, or proteins, or be enzymatic.
Optogenetics: a technology that combines genetic and optical methods to achieve rapid and precise control of biological processes. Optogenetics uses natural or genetically-modified light-sensitive proteins or domains to spatiotemporally modulate protein-protein interactions, cell signaling, enzyme activities, or gene expression.
Adenosyltransferases (ATRs): enzymes that convert cob(II)alamin to AdoCbl. Three classes of sequence-unrelated ATRs are known in bacteria: CobA (BtuR or CobO), which acts in de novo AdoCbl biosynthesis, and EutT and PduO, which convert imported B12 to AdoCbl. The most prevalent and well studied is PduO, whose human ortholog (MMAB) is associated with mitochondrial B12 metabolism.
Riboswitches: RNA-based elements, found mostly in bacteria and archaea (a few in plants), which mediate a mode of gene regulation based on RNA conformational changes upon binding to ligands like vitamins, amino acids, nucleotides, aminosugars, or metals. Riboswitches can regulate transcription or translation, and generally act in cis, although some that act in trans are also known. The first discovered riboswitch and the second most-widespread is the AdoCbl riboswitch.
MerR proteins: Transcription factors that regulate responses to heavy metals, drugs, and other stresses in bacteria. They are dual-function regulators that can repress or activate a promoter by binding as dimers to a site with (pseudo)palindromic sequences, which is located within a spacer of suboptimal length between the −10 and −35 promoter elements.
ACKNOWLEDGMENTS
We apologize for any relevant work not cited due to space constraints. This work was supported by grants from the Ministerio de Economía and Competitividad-Spain (BFU2015-67968-C2-1-P co-financed by FEDER to M.E.-A.; BFU2015-67968-C2-2-P to S.P.) and Fundación Séneca-Spain (19429/PI/14 to M.E.-A). C.L.D. is an HHMI Investigator with support from the National Institutes of Health (GM69857). M.J. was funded by National Institutes of Health Grant F32 GM116331.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Croce R, van Amerongen H. 2014. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol 10: 492–501 [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K. 2012. Chemistry and biology of vision. J. Biol. Chem 287: 1612–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald G. 1968. The molecular basis of visual excitation. Nature 219: 800–07 [DOI] [PubMed] [Google Scholar]
- 4.Cohen SE, Golden SS. 2015. Circadian rhythms in cyanobacteria. Microbiol. Mol. Biol. Rev 79: 373–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel DH, Kay SA. 2012. Complexity in the wiring and regulation of plant circadian networks. Curr. Biol 22: R648–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, et al. 2011. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol 62: 335–64 [DOI] [PubMed] [Google Scholar]
- 7.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. 2010. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 584: 2618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fankhauser C, Christie JM. 2015. Plant phototropic growth. Curr. Biol 25: R384–89 [DOI] [PubMed] [Google Scholar]
- 9.Ballare CL. 2014. Light regulation of plant defense. Annu. Rev. Plant Biol 65: 335–63 [DOI] [PubMed] [Google Scholar]
- 10.Swartz TE, Tseng TS, Frederickson MA, Paris G, Comerci DJ, et al. 2007. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317: 1090–93 [DOI] [PubMed] [Google Scholar]
- 11.Elías-Arnanz M, Padmanabhan S, Murillo FJ. 2011. Light-dependent gene regulation in nonphototrophic bacteria. Curr. Opin. Microbiol 14: 128–35 [DOI] [PubMed] [Google Scholar]
- 12.Purcell EB, Crosson S. 2008. Photoregulation in prokaryotes. Curr. Opin. Microbiol 11: 168–78 [DOI] [PubMed] [Google Scholar]
- 13.Crane BR, Young MW. 2014. Interactive features of proteins composing eukaryotic circadian clocks. Annu. Rev. Biochem 83: 191–219 [DOI] [PubMed] [Google Scholar]
- 14.Ziegelhoffer EC, Donohue TJ. 2009. Bacterial responses to photo-oxidative stress. Nat. Rev. Microbiol 7: 856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Wakao S, Fischer BB, Niyogi KK. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol 60: 239–60 [DOI] [PubMed] [Google Scholar]
- 16.Glaeser J, Nuss AM, Berghoff BA, Klug G. 2011. Singlet oxygen stress in microorganisms. Adv. Microb. Physiol 58: 141–73 [DOI] [PubMed] [Google Scholar]
- 17.Latifi A, Ruiz M, Zhang CC. 2009. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev 33: 258–78 [DOI] [PubMed] [Google Scholar]
- 18.Setlow RB. 1966. Cyclobutane-type pyrimidine dimers in polynucleotides. Science 153: 379–86 [DOI] [PubMed] [Google Scholar]
- 19.Erickson E, Wakao S, Niyogi KK. 2015. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 82: 449–65 [DOI] [PubMed] [Google Scholar]
- 20.Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, et al. 2012. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D, Hu Q, Yan Z, Chen W, Yan C, et al. 2012. Structural basis of ultraviolet-B perception by UVR8. Nature 484: 214–9 [DOI] [PubMed] [Google Scholar]
- 22.Genick UK, Soltis SM, Kuhn P, Canestrelli IL, Getzoff ED. 1998. Structure at 0.85 A resolution of an early protein photocycle intermediate. Nature 392: 206–9 [DOI] [PubMed] [Google Scholar]
- 23.Herrou J, Crosson S. 2011. Function, structure and mechanism of bacterial photosensory LOV proteins. Nat. Rev. Microbiol 9: 713–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leverenz RL, Sutter M, Wilson A, Gupta S, Thurotte A, et al. 2015. PHOTOSYNTHESIS. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science 348: 1463–6 [DOI] [PubMed] [Google Scholar]
- 25.Losi A, Gartner W. 2012. The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu. Rev. Plant Biol 63: 49–72 [DOI] [PubMed] [Google Scholar]
- 26.Masuda S 2013. Light detection and signal transduction in the BLUF photoreceptors. Plant Cell Physiol. 54: 171–9 [DOI] [PubMed] [Google Scholar]
- 27.Sancar A 2003. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev 103: 2203–37 [DOI] [PubMed] [Google Scholar]
- 28.Wilson A, Punginelli C, Gall A, Bonetti C, Alexandre M, et al. 2008. A photoactive carotenoid protein acting as light intensity sensor. Proc. Natl. Acad. Sci. U S A 105: 12075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders K, Essen LO. 2015. The family of phytochrome-like photoreceptors: diverse, complex and multi-colored, but very useful. Curr. Opin. Struct. Biol 35: 7–16 [DOI] [PubMed] [Google Scholar]
- 30.Rockwell NC, Su YS, Lagarias JC. 2006. Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol 57: 837–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moglich A, Yang X, Ayers RA, Moffat K. 2010. Structure and function of plant photoreceptors. Annu. Rev. Plant Biol 61: 21–47 [DOI] [PubMed] [Google Scholar]
- 32.Shcherbakova DM, Shemetov AA, Kaberniuk AA, Verkhusha VV. 2015. Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools. Annu. Rev. Biochem 84: 519–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharyya RP, Remenyi A, Yeh BJ, Lim WA. 2006. Domains, motifs, and scaffolds: the role of modular interactions in the evolution and wiring of cell signaling circuits. Annu. Rev. Biochem 75: 655–80 [DOI] [PubMed] [Google Scholar]
- 34.Fenno L, Yizhar O, Deisseroth K. 2011. The development and application of optogenetics. Annu. Rev. Neurosci 34: 389–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miesenbock G 2011. Optogenetic control of cells and circuits. Annu. Rev. Cell. Dev. Biol 27: 731–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Cui B. 2015. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 33: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz-Guerrero JM, Polanco MC, Murillo FJ, Padmanabhan S, Elías-Arnanz M. 2011. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA 108: 7565–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jost M, Fernandez-Zapata J, Polanco MC, Ortiz-Guerrero JM, Chen PY, et al. 2015. Structural basis for gene regulation by a B12-dependent photoreceptor. Nature 526: 536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jost M, Simpson JH, Drennan CL. 2015. The transcription factor CarH safeguards use of adenosylcobalamin as a light sensor by altering the photolysis products. Biochemistry 54: 3231–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutta RJ, Hardman SJ, Johannissen LO, Bellina B, Messiha HL, et al. 2015. The photochemical mechanism of a B12-dependent photoreceptor protein. Nat. Commun 6: 7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whipple GH, Robscheit-Robbins FS. 1925. Favourable influence of liver, heart and skeletal muscle in diet on blood regeneration in anemia. Am. J. Physiol 72: 408–18 [Google Scholar]
- 42.Minot GR, Murphy WP 1926. Treatment of pernicious anemia by a special diet. J. Am. Med. Assoc 87: 470–76 [PMC free article] [PubMed] [Google Scholar]
- 43.Rickes EL, Brink NG, Koniuszy FR, Wood TR, Folkers K. 1948. Crystalline vitamin B12. Science 107: 396–97 [DOI] [PubMed] [Google Scholar]
- 44.Smith EL. 1948. Purification of anti-pernicious anaemia factors from liver. Nature 161: 638. [DOI] [PubMed] [Google Scholar]
- 45.Hodgkin DC, Kamper J, Mackay M, Pickworth J, Trueblood KN, White JG. 1956. Structure of vitamin B12. Nature 178: 64–66 [DOI] [PubMed] [Google Scholar]
- 46.Eschenmoser A, Wintner CE. 1977. Natural product synthesis and vitamin B12. Science 196: 1410–20 [DOI] [PubMed] [Google Scholar]
- 47.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–3 [DOI] [PubMed] [Google Scholar]
- 48.Roth JR, Lawrence JG, Bobik TA. 1996. Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol 50: 137–81 [DOI] [PubMed] [Google Scholar]
- 49.Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, Walker GC. 2007. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature 446: 449–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep 19: 390–412 [DOI] [PubMed] [Google Scholar]
- 51.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem 278: 41148–59 [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN. 2009. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 10: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degnan PH, Taga ME, Goodman AL. 2014. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 20: 769–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem 72: 209–47 [DOI] [PubMed] [Google Scholar]
- 55.Ludwig ML, Matthews RG. 1997. Structure-based perspectives on B12-dependent enzymes. Annu. Rev. Biochem 66: 269–313 [DOI] [PubMed] [Google Scholar]
- 56.Lenhert PG, Hodgkin DC. 1961. Structure of the 5,6-dimethyl-benzimidazolylcobamide coenzyme. Nature 192: 937–8 [DOI] [PubMed] [Google Scholar]
- 57.Rossi R, Glusker JP, Randaccio L, Summers MF, Toscano PJ, Marzilli LG. 1985. The structure of a B12 coenzyme: methylcobalamin studies by x-ray and NMR methods. J. Am. Chem. Soc 107: 1729–38 [Google Scholar]
- 58.Hung RR, Grabowski JJ. 1999. Listening to reactive intermediates: application of photoacoustic calorimetry to vitamin B12 compounds. J. Am. Chem. Soc 121: 1351–64 [Google Scholar]
- 59.Hay BP, Finke RG. 1986. Thermolysis of the cobalt-carbon bond of adenosylcobalamin. 2. Products, kinetics, and cobalt-carbon bond dissociation energy in aqueous solution. J. Am. Chem. Soc 108: 4820–29 [Google Scholar]
- 60.Randaccio L, Geremia S, Demitri N, Wuerges J. 2010. Vitamin B12: unique metalorganic compounds and the most complex vitamins. Molecules 15: 3228–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozlowski PM, Garabato BD, Lodowski P, Jaworska M. 2016. Photolytic properties of cobalamins: a theoretical perspective. Dalton Trans. 45: 4457–70 [DOI] [PubMed] [Google Scholar]
- 62.Rury AS, Wiley TE, Sension RJ. 2015. Energy cascades, excited state dynamics, and photochemistry in cob(III)alamins and ferric porphyrins. Acc. Chem. Res 48: 860–67 [DOI] [PubMed] [Google Scholar]
- 63.Gruber K, Puffer B, Krautler B. 2011. Vitamin B12-derivatives-enzyme cofactors and ligands of proteins and nucleic acids. Chem. Soc. Rev 40: 4346–63 [DOI] [PubMed] [Google Scholar]
- 64.Drennan CL, Huang S, Drummond JT, Matthews RG, Ludwig ML. 1994. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science 266: 1669–74 [DOI] [PubMed] [Google Scholar]
- 65.Liptak MD, Brunold TC. 2006. Spectroscopic and computational studies of Co1+cobalamin: spectral and electronic properties of the "superreduced" B12 cofactor. J. Am. Chem. Soc 128: 9144–56 [DOI] [PubMed] [Google Scholar]
- 66.Stich TA, Brooks AJ, Buan NR, Brunold TC. 2003. Spectroscopic and computational studies of Co3+-corrinoids: spectral and electronic properties of the B12 cofactors and biologically relevant precursors. J. Am. Chem. Soc 125: 5897–914 [DOI] [PubMed] [Google Scholar]
- 67.Stich TA, Buan NR, Brunold TC. 2004. Spectroscopic and computational studies of Co2+corrinoids: spectral and electronic properties of the biologically relevant base-on and base-off forms of Co2+cobalamin. J. Am. Chem. Soc 126: 9735–49 [DOI] [PubMed] [Google Scholar]
- 68.Shell TA, Lawrence DS. 2011. A new trick (hydroxyl radical generation) for an old vitamin (B12). J. Am. Chem. Soc 133: 2148–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiley TE, Miller WR, Miller NA, Sension RJ, Lodowski P, et al. 2016. Photostability of hydroxocobalamin: ultrafast excited state dynamics and computational studies. J. Phys. Chem. Lett 7: 143–47 [DOI] [PubMed] [Google Scholar]
- 70.Schwartz PA, Frey PA. 2007. 5'-Peroxyadenosine and 5'-peroxyadenosylcobalamin as intermediates in the aerobic photolysis of adenosylcobalamin. Biochemistry 46: 7284–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hogenkamp HP. 1963. A cyclic nucleoside derived from coenzyme B12. J. Biol. Chem 238: 477–80 [PubMed] [Google Scholar]
- 72.Hogenkamp HP. 1966. The photolysis of methylcobalamin. Biochemistry 5: 417–22 [DOI] [PubMed] [Google Scholar]
- 73.Law PY, Wood JM. 1973. The photolysis of 5'-deoxyadenosylcobalamin under anaerobic conditions. Biochim. Biophys. Acta 331: 451–4 [DOI] [PubMed] [Google Scholar]
- 74.Chen E, Chance MR. 1990. Nanosecond transient absorption spectroscopy of coenzyme B12. Quantum yields and spectral dynamics. J. Biol. Chem 265: 12987–94 [PubMed] [Google Scholar]
- 75.Endicott JF, Netzel TL. 1979. Early events and transient chemistry in the photohomolysis of alkylcobalamins. J. Am. Chem. Soc 101: 4000–02 [Google Scholar]
- 76.Jones AR, Russell HJ, Greetham GM, Towrie M, Hay S, Scrutton NS. 2012. Ultrafast infrared spectral fingerprints of vitamin B12 and related cobalamins. J. Phys. Chem. A 116: 5586–94 [DOI] [PubMed] [Google Scholar]
- 77.Walker LA, Jarett JT, Anderson NA, Pullen SH, Matthews RG, Sension RJ. 1998. Time-resolved spectroscopic studies of B12 coenzymes: the identification of a metastable cob(III)alamin photoproduct in the photolysis of methylcobalamin. J. Am. Chem. Soc 120: 3597–603 [Google Scholar]
- 78.Walker LA, Shiang JJ, Anderson NA, Pullen SH, Sension RJ. 1998. Time-resolved spectroscopic studies of B12 coenzymes: the photolysis and geminate recombination of adenosylcobalamin. J. Am. Chem. Soc 120: 7286–92 [Google Scholar]
- 79.Stickrath AB, Carroll EC, Dai X, Harris DA, Rury A, et al. 2009. Solvent-dependent cage dynamics of small nonpolar radicals: lessons from the photodissociation and geminate recombination of alkylcobalamins. J. Phys. Chem. A 113: 8513–22 [DOI] [PubMed] [Google Scholar]
- 80.Yamada R, Shimizu S, Fukui S. 1966. Factors affecting the anaerobic photolysis of the cobalt-carbon bond of cobalt-methylcobalamin. Biochim. Biophys. Acta 124: 195–7 [DOI] [PubMed] [Google Scholar]
- 81.Deery E, Schroeder S, Lawrence AD, Taylor SL, Seyedarabi A, et al. 2012. An enzyme-trap approach allows isolation of intermediates in cobalamin biosynthesis. Nat. Chem. Biol 8: 933–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hazra AB, Han AW, Mehta AP, Mok KC, Osadchiy V, et al. 2015. Anaerobic biosynthesis of the lower ligand of vitamin B12. Proc. Natl. Acad. Sci. U S A 112: 10792–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, et al. 2013. Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc. Natl. Acad. Sci. U S A 110: 14906–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin L, Bauer CE. 2013. Controlling the delicate balance of tetrapyrrole biosynthesis. Philos. Trans. R. Soc. Lond. B Biol. Sci 368: 20120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banerjee R, Gherasim C, Padovani D. 2009. The tinker, tailor, soldier in intracellular B12 trafficking. Curr. Opin. Chem. Biol 13: 484–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cracan V, Banerjee R. 2013. Cobalt and corrinoid transport and biochemistry. Met. Ions Life Sci 12: 333–74 [DOI] [PubMed] [Google Scholar]
- 87.Fedosov SN. 2012. Physiological and molecular aspects of cobalamin transport. Subcell. Biochem 56: 347–67 [DOI] [PubMed] [Google Scholar]
- 88.Nielsen MJ, Rasmussen MR, Andersen CB, Nexo E, Moestrup SK. 2012. Vitamin B12 transport from food to the body's cells-a sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol 9: 345–54 [DOI] [PubMed] [Google Scholar]
- 89.Yamanishi M, Vlasie M, Banerjee R. 2005. Adenosyltransferase: an enzyme and an escort for coenzyme B12? Trends Biochem. Sci 30: 304–8 [DOI] [PubMed] [Google Scholar]
- 90.Jost M, Cracan V, Hubbard PA, Banerjee R, Drennan CL. 2015. Visualization of a radical B12 enzyme with its G-protein chaperone. Proc. Natl. Acad. Sci. U S A 112: 2419–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gherasim C, Lofgren M, Banerjee R. 2013. Navigating the B12 road: assimilation, delivery, and disorders of cobalamin. J. Biol. Chem 288: 13186–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson CL, Pechonick E, Park SD, Havemann GD, Leal NA, Bobik TA. 2001. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J. Bacteriol 183: 1577–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dowling DP, Croft AK, Drennan CL. 2012. Radical use of Rossmann and TIM barrel architectures for controlling coenzyme B12 chemistry. Annu. Rev. Biophys 41: 403–27 [DOI] [PubMed] [Google Scholar]
- 94.Giedyk M, Goliszewska K, Gryko D. 2015. Vitamin B12 catalysed reactions. Chem. Soc. Rev 44: 3391–404 [DOI] [PubMed] [Google Scholar]
- 95.Ludwig ML, Drennan CL, Matthews RG. 1996. The reactivity of B12 cofactors: the proteins make a difference. Structure 4: 505–12 [DOI] [PubMed] [Google Scholar]
- 96.Jones AR, Levy C, Hay S, Scrutton NS. 2013. Relating localized protein motions to the reaction coordinate in coenzyme B12-dependent enzymes. FEBS J. 280: 2997–3008 [DOI] [PubMed] [Google Scholar]
- 97.Sukumar N 2013. Crystallographic studies on B12 binding proteins in eukaryotes and prokaryotes. Biochimie 95: 976–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bommer M, Kunze C, Fesseler J, Schubert T, Diekert G, Dobbek H. 2014. Structural basis for organohalide respiration. Science 346: 455–8 [DOI] [PubMed] [Google Scholar]
- 99.Payne KA, Quezada CP, Fisher K, Dunstan MS, Collins FA, et al. 2015. Reductive dehalogenase structure suggests a mechanism for B12-dependent dehalogenation. Nature 517: 513–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dowling DP, Miles ZD, Kohrer C, Maiocco SJ, Elliott SJ, et al. 2016. Molecular basis of cobalamin-dependent RNA modification. Nucleic Acids Res. 44: 9965–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Payne KA, Fisher K, Sjuts H, Dunstan MS, Bellina B, et al. 2015. Epoxyqueuosine reductase structure suggests a mechanism for cobalamin-dependent tRNA modification. J. Biol. Chem 290: 27572–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goulding CW, Postigo D, Matthews RG. 1997. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry 36: 8082–91 [DOI] [PubMed] [Google Scholar]
- 103.Matthews RG, Koutmos M, Datta S. 2008. Cobalamin-dependent and cobamide-dependent methyltransferases. Curr. Opin. Struct. Biol 18: 658–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jarrett JT, Drennan CL, Amaratunga M, Scholten JD, Ludwig ML, Matthews RG. 1996. A protein radical cage slows photolysis of methylcobalamin in methionine synthase from Escherichia coli. Bioorg. Med. Chem 4: 1237–46 [DOI] [PubMed] [Google Scholar]
- 105.Mandal M, Breaker RR. 2004. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol 5: 451–63 [DOI] [PubMed] [Google Scholar]
- 106.Serganov A, Patel DJ. 2012. Metabolite recognition principles and molecular mechanisms underlying riboswitch function. Annu. Rev. Biophys 41: 343–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nahvi A, Barrick JE, Breaker RR. 2004. Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes. Nucleic Acids Res. 32: 143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson JE Jr., Reyes FE, Polaski JT, Batey RT. 2012. B12 cofactors directly stabilize an mRNA regulatory switch. Nature 492: 133–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peselis A, Serganov A. 2012. Structural insights into ligand binding and gene expression control by an adenosylcobalamin riboswitch. Nat. Struct. Mol. Biol 19: 1182–4 [DOI] [PubMed] [Google Scholar]
- 110.Elías-Arnanz M, Fontes M, Padmanabhan S. 2008. In Myxobacteria: Multicellularity and Differentiation ed. Whitworth DE, pp. 211–25: ASM Press [Google Scholar]
- 111.Armstrong GA. 1997. Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu. Rev. Microbiol 51: 629–59 [DOI] [PubMed] [Google Scholar]
- 112.Galbis-Martinez M, Padmanabhan S, Murillo FJ, Elías-Arnanz M. 2012. CarF mediates signaling by singlet oxygen, generated via photoexcited protoporphyrin IX, in Myxococcus xanthus light-induced carotenogenesis. J. Bacteriol 194: 1427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cervantes M, Murillo FJ. 2002. Role for vitamin B12 in light induction of gene expression in the bacterium Myxococcus xanthus. J. Bacteriol 184: 2215–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang CC, Lin LY, Zou XW, Huang CC, Chan NL. 2015. Structural basis of the mercury(II)-mediated conformational switching of the dual-function transcriptional regulator MerR. Nucleic Acids Res. 43: 7612–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heldwein EE, Brennan RG. 2001. Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409: 378–82 [DOI] [PubMed] [Google Scholar]
- 116.Philips SJ, Canalizo-Hernandez M, Yildirim I, Schatz GC, Mondragon A, O'Halloran TV. 2015. TRANSCRIPTION. Allosteric transcriptional regulation via changes in the overall topology of the core promoter. Science 349: 877–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martell DJ, Joshi CP, Gaballa A, Santiago AG, Chen TY, et al. 2015. Metalloregulator CueR biases RNA polymerase's kinetic sampling of dead-end or open complex to repress or activate transcription. Proc. Natl. Acad. Sci. U S A 112: 13467–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Botella JA, Murillo FJ, Ruiz-Vazquez R. 1995. A cluster of structural and regulatory genes for light-induced carotenogenesis in Myxococcus xanthus. Eur. J. Biochem 233: 238–48 [DOI] [PubMed] [Google Scholar]
- 119.Navarro-Avilés G, Jiménez MA, Pérez-Marín MC, González C, Rico M, et al. 2007. Structural basis for operator and antirepressor recognition by Myxococcus xanthus CarA repressor. Mol. Microbiol 63: 980–94 [DOI] [PubMed] [Google Scholar]
- 120.Pérez-Marín MC, López-Rubio JJ, Murillo FJ, Elías-Arnanz M, Padmanabhan S. 2004. The N terminus of Myxococcus xanthus CarA repressor is an autonomously folding domain that mediates physical and functional interactions with both operator DNA and antirepressor protein. J. Biol. Chem 279: 33093–103 [DOI] [PubMed] [Google Scholar]
- 121.Pérez-Marín MC, Padmanabhan S, Polanco MC, Murillo FJ, Elías-Arnanz M. 2008. Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol. Microbiol 67: 804–19 [DOI] [PubMed] [Google Scholar]
- 122.López-Rubio JJ, Elías-Arnanz M, Padmanabhan S, Murillo FJ. 2002. A repressor-antirepressor pair links two loci controlling light-induced carotenogenesis in Myxococcus xanthus. J. Biol. Chem 277: 7262–70 [DOI] [PubMed] [Google Scholar]
- 123.López-Rubio JJ, Padmanabhan S, Lázaro JM, Salas M, Murillo FJ, Elías-Arnanz M. 2004. Operator design and mechanism for CarA repressor-mediated down-regulation of the photoinducible carB operon in Myxococcus xanthus. J. Biol. Chem 279: 28945–53 [DOI] [PubMed] [Google Scholar]
- 124.Whitworth DE, Hodgson DA. 2001. Light-induced carotenogenesis in Myxococcus xanthus: evidence that CarS acts as an anti-repressor of CarA. Mol. Microbiol 42: 809–19 [DOI] [PubMed] [Google Scholar]
- 125.León E, Navarro-Avilés G, Santiveri CM, Flores-Flores C, Rico M, et al. 2010. A bacterial antirepressor with SH3 domain topology mimics operator DNA in sequestering the repressor DNA recognition helix. Nucleic Acids Res. 38: 5226–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takano H, Obitsu S, Beppu T, Ueda K. 2005. Light-induced carotenogenesis in Streptomyces coelicolor A3(2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J. Bacteriol. 187: 1825–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diez AI, Ortiz-Guerrero JM, Ortega A, Elias-Arnanz M, Padmanabhan S, Garcia de la Torre J. 2013. Analytical ultracentrifugation studies of oligomerization and DNA-binding of TtCarH, a Thermus thermophilus coenzyme B12-based photosensory regulator. Eur. Biophys. J 42: 463–76 [DOI] [PubMed] [Google Scholar]
- 128.Takano H, Kondo M, Usui N, Usui T, Ohzeki H, et al. 2011. Involvement of CarA/LitR and CRP/FNR family transcriptional regulators in light-induced carotenoid production in Thermus thermophilus. J. Bacteriol 193: 2451–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takano H, Mise K, Hagiwara K, Hirata N, Watanabe S, et al. 2015. Role and function of LitR, an adenosyl B12-bound light-sensitive regulator of Bacillus megaterium QM B1551, in regulation of carotenoid production. J. Bacteriol 197: 2301–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheng Z, Li K, Hammad LA, Karty JA, Bauer CE. 2014. Vitamin B12 regulates photosystem gene expression via the CrtJ antirepressor AerR in Rhodobacter capsulatus. Mol. Microbiol 91: 649–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vermeulen AJ, Bauer CE. 2015. Members of the PpaA/AerR antirepressor family bind cobalamin. J. Bacteriol 197: 2694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ponnampalam SN, Bauer CE. 1997. DNA binding characteristics of CrtJ. A redox-responding repressor of bacteriochlorophyll, carotenoid, and light harvesting-II gene expression in Rhodobacter capsulatus. J. Biol. Chem 272: 18391–6 [DOI] [PubMed] [Google Scholar]
- 133.Ponnampalam SN, Buggy JJ, Bauer CE. 1995. Characterization of an aerobic repressor that coordinately regulates bacteriochlorophyll, carotenoid, and light harvesting-II expression in Rhodobacter capsulatus. J. Bacteriol 177: 2990–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Edayathumangalam R, Wu R, Garcia R, Wang Y, Wang W, et al. 2013. Crystal structure of Bacillus subtilis GabR, an autorepressor and transcriptional activator of gabT. Proc. Natl. Acad. Sci. U S A 110: 17820–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Al-Zyoud WA, Hynson RM, Ganuelas LA, Coster AC, Duff AP, et al. 2016. Binding of transcription factor GabR to DNA requires recognition of DNA shape at a location distinct from its cognate binding site. Nucleic Acids Res. 44: 1411–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ohlendorf R, Schumacher CH, Richter F, Moglich A. 2016. Library-Aided Probing of Linker Determinants in Hybrid Photoreceptors. ACS Synth. Biol 5: 1117–26 [DOI] [PubMed] [Google Scholar]
- 137.Katz RN, Vickrey TM, Schrauzer GN. 1976. Detection of 4',5'-anhydroadenosine as the cleavage product of coenzyme B12 in functional holoenzymes. Angew. Chem. Int. Ed. Engl 15: 542–3 [DOI] [PubMed] [Google Scholar]
- 138.Krouwer JS, Schultz RM, Babior BM. 1978. The mechanism of action of ethanolamine ammonia-lyase, an adenosylcobalamin-dependent enzyme. Reaction of the enzyme.cofactor complex with 2-aminoacetaldehyde. J. Biol. Chem 253: 1041–7 [PubMed] [Google Scholar]
- 139.Garr CD, Finke RG. 1993. Adocobalamin (AdoCbl or coenzyme B12) cobalt-carbon bond homolysis radical-cage effects: product, kinetic, mechanistic, and cage efficiency factor (Fc) studies, plus the possibility that coenzyme B12-dependent enzymes function as "ultimate radical cages" and "ultimate radical traps". Inorg. Chem 32: 4414–21 [Google Scholar]
- 140.Sension RJ, Cole AG, Harris AD, Fox CC, Woodbury NW, et al. 2004. Photolysis and recombination of adenosylcobalamin bound to glutamate mutase. J. Am. Chem. Soc 126: 1598–9 [DOI] [PubMed] [Google Scholar]
- 141.Sension RJ, Harris DA, Stickrath A, Cole AG, Fox CC, Marsh EN. 2005. Time-resolved measurements of the photolysis and recombination of adenosylcobalamin bound to glutamate mutase. J. Phys. Chem. B 109: 18146–52 [DOI] [PubMed] [Google Scholar]
- 142.Gruber K, Krautler B. 2016. Coenzyme B12 Repurposed for Photoregulation of Gene Expression. Angew. Chem. Int. Ed. Engl 55: 5638–40 [DOI] [PubMed] [Google Scholar]
- 143.Cheng Z, Yamamoto H, Bauer CE. 2016. Cobalamin's (vitamin B12) surprising function as a photoreceptor. Trends Biochem. Sci 41: 647–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinkova M, Kitanishi K, Shimizu T. 2013. Heme-based globin-coupled oxygen sensors: linking oxygen binding to functional regulation of diguanylate cyclase, histidine kinase, and methyl-accepting chemotaxis. J. Biol. Chem 288: 27702–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.García-Moreno D, Polanco MC, Navarro-Avilés G, Murillo FJ, Padmanabhan S, Elías-Arnanz M. 2009. A vitamin B12-based system for conditional expression reveals dksA to be an essential gene in Myxococcus xanthus. J. Bacteriol 191: 3108–19 [DOI] [PMC free article] [PubMed] [Google Scholar]