Abstract
Clostridioides difficile typing is invaluable for the investigation of both institution-specific outbreaks as well as national surveillance. While the epidemic ribotype 027 (RT027) has received a significant amount of resources and attention, ribotype 106 (RT106) has become more prevalent throughout the past decade. The purpose of this systematic review was to comprehensively summarize the genetic determinants, antimicrobial susceptibility, epidemiology, and clinical outcomes of infection caused by RT106. A total of 68 articles published between 1999 and 2019 were identified as relevant to this review. Although initially identified in the United Kingdom in 1999, RT106 is now found worldwide and became the most prevalent strain in the United States in 2016. Current data indicate that RT106 harbors the tcdA and tcdB genes, lacks binary toxin genes, and does not contain any deletions in the tcdC gene, which differentiates it from other epidemic strains, including ribotypes 027 and 078. Interestingly, RT106 produces more spores than other strains, including RT027. Overall, RT106 is highly resistant to erythromycin, clindamycin, fluoroquinolones, and third-generation cephalosporins. However, the MIC90 in most studies are one to two fold dilutions below the epidemiologic cut-off values of metronidazole and vancomycin, suggesting both are acceptable treatment options from an in vitro perspective. The few clinical outcomes studies available concluded that RT106 causes less severe disease than RT027, but patients were significantly more likely to experience multiple CDI relapses when infected with a RT106 strain. Specific areas warranting future study include potential survival advantages provided by genetic elements as well as a more robust investigation of clinical outcomes associated with RT106.
Keywords: Anaerobe, molecular surveillance, strain typing, spores, recurrence
Introduction
Clostridioides difficile is an anaerobic Gram-positive bacillus that can cause life-threatening illness ranging from diarrhea to pseudomembranous colitis and toxic megacolon.[1] C. difficile infection (CDI) is the most common health care-associated infection in the United States (US) and carries a large burden on the national health care system.[2–5] As awareness of CDI has increased globally, several C. difficile strains have been identified as causing epidemics and/or more severe disease, most notably ribotype 027 (RT027).[6–9]
Polymerase chain reaction (PCR) ribotyping is a common typing method to categorize C. difficile strains.[10, 11] C. difficile typing is valuable for the investigation of both institution-specific outbreaks as well as national surveillance.[12] RT106 was initially identified in the United Kingdom (UK) in 1999[13] but was not reported outside of the UK until 2009.[14] Since then, RT106 has been found many other areas worldwide and became the most prevalent strain in the US in 2016.[15, 16] Despite its increasing prevalence, the degree to which RT106 has been studied so far is limited. This article summarizes the available literature on RT106 genetic determinants, antimicrobial susceptibility, epidemiology, and clinical outcomes.
Literature search and selection
A systematic search for relevant literature published on or before 23 May 2019 was conducted in PubMed and Scopus. This date was chosen as an arbitrary cutoff given the timeline of the investigators. The following combination of search terms was used to identify relevant articles: (“ribotype 106” OR “ribotypes 106” OR “type 106” OR RT106 OR “ARL 106” OR 106 OR DH OR NAP11 OR “DH/NAP11/106”) AND (“Clostridium difficile” OR “Clostridioides difficile”). Articles were included if they mentioned RT106 (or related types as discussed below); all others were excluded. Two investigators (DB and FA) independently screened each abstract for inclusion, and full text was reviewed after concordance of the two investigators. The references of the included articles were studied to identify additional relevant articles for inclusion. Any discrepancies between the authors were collaboratively resolved with two other investigators (AG and TC).
Results
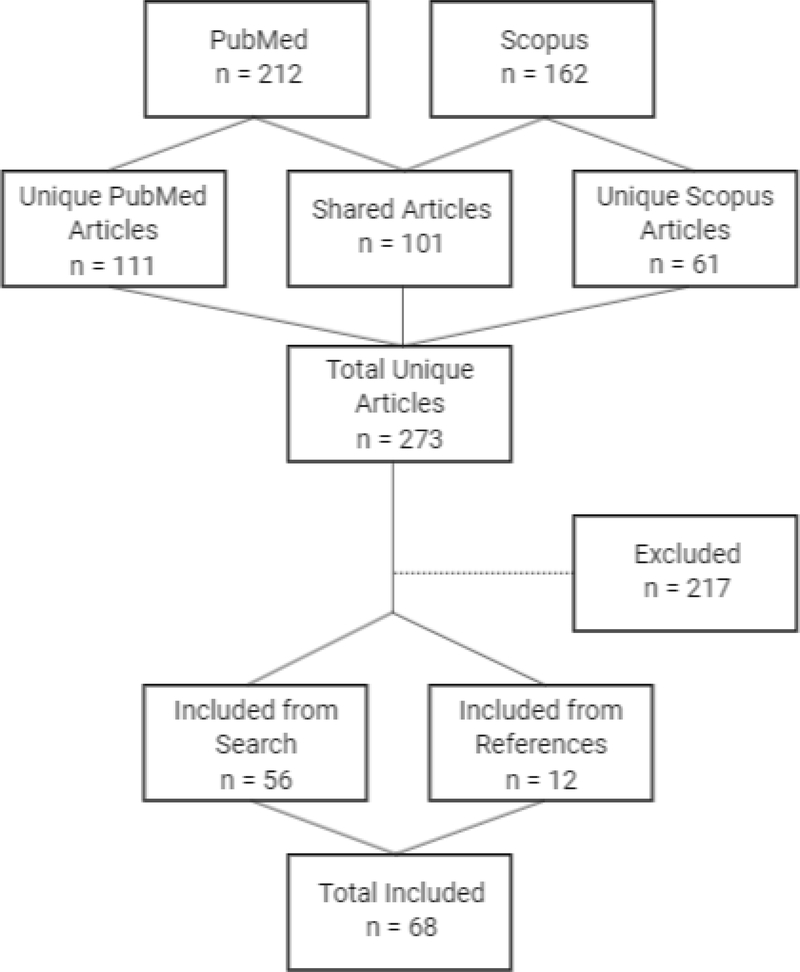
The literature selection process is summarized in Figure 1. After initial screening, 212 articles in PubMed and 162 articles in Scopus met our inclusion criteria. All but 61 articles identified in Scopus were also identified in PubMed, leaving a total of 273 unique publications. There were 56 articles published between 2005 and 2019 that were deemed relevant by abstract review and 217 that were excluded after full text review. An additional 12 articles were identified in the references of the included articles bringing the total number of included articles to 68 published between 1999 and 2019.
Figure 1.
Systematic review flowchart
Typing, Detection, and Genomic Overview
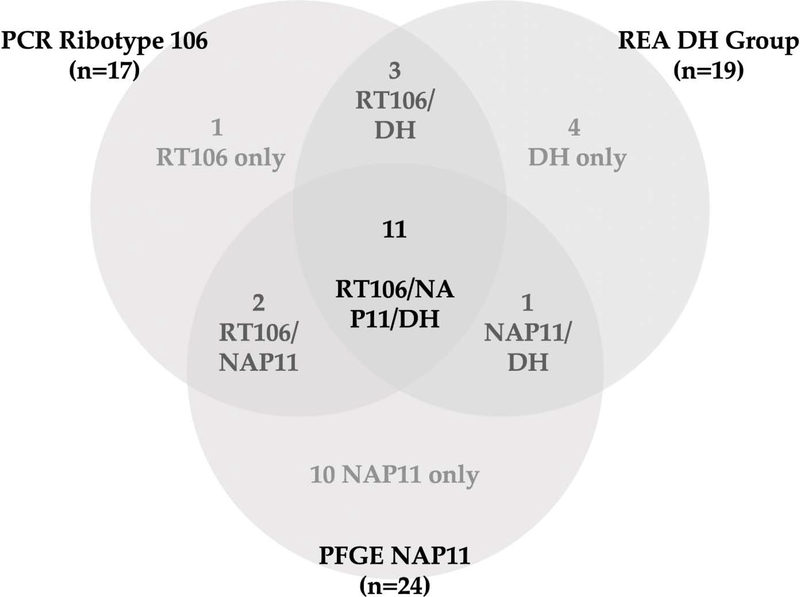
Typing of C. difficile strains is important for understanding the epidemiology and transmission dynamics of CDI. PCR ribotyping is considered the standard method to classify variants among C. difficile, although other techniques, such as restriction endonuclease analysis (REA) of chromosomal deoxyribonucleic acid (DNA) and pulsed-field gel electrophoresis (PFGE) also provide discrimination between strains.[17] Multilocus sequence typing (MLST) can also be used by indexing the sequences of nucleotides at seven loci. For RT106, the allelic profile for the loci adk, atpA, dxr, glyA, recA, sodA, and tpi is 1, 1, 2, 1, 1, 7, and 1, respectively.[18] MLST is less commonly used than PCR, REA, and PFGE for epidemiologic investigations.[19–21] RT106 is most commonly associated with REA group DH, PFGE type NAP11, and sequence type 42 (ST-42).[19, 22, 23] Although there is a large degree of overlap among the identification assigned by each of the methods, the type assigned to a given C. difficile isolate may differ between methods (Figure 2).[19]
Figure 2.
Differences in C. difficile strain identification assigned by common typing methods
*Data are derived from reference [19]
The C. difficile toxin genes, toxins A (TcdA0 and B (TcdB) are the most common targets for diagnostics using either an enzyme immunoassay (EIA) or for the gene that encodes TcdA or TcdB through polymerase chain reaction (PCR)-based nucleic acid amplification tests (NAAT) [24]. Furthermore, glutamate dehydrogenase (GDH) assays detect an enzyme present in the cell wall of both toxigenic and nontoxigenic strains of C. difficile, and can therefore be used for screening [25, 26], but must be paired with another diagnostic test for diagnosis of CDI..[27] For diagnosis of RT106 specifically, multiple brands of EIA tests were determined to be less sensitive than a commercially available NAAT (n = 16) and GDH/EIA algorithms were less sensitive than NAAT for detecting all non-RT027 ribotypes including RT106.[28] A subsequent investigation confirmed that a commercial GDH/EIA algorithm (C DIFF CHECKTM-60, TechLab, Blacksburg, VA) was less sensitive than NAAT overall (GeneXpert, Cepheid, Sunnyvale, CA), but no differences were seen between ribotypes (RT106 n = 10).[29] Strain specific sensitivity should be reassessed in future trials given the small sample sizes in these studies.
The genome of C. difficile has a high degree of mosaicism due to several mobile genetic elements which create a high degree of variability between ribotypes.[30] The complete genome sequence of RT106 was reported in 2017 under the accession number CP022524; the genome length is 4,087,127 base pairs and contains 3,562 coding sequences.[31] A comparative genomic study found that RT106 had only 41% conservation of the core genes present in the reference strain CD630 (GenBank: AM180355.1), the lowest of all ribotypes tested. However, RT106 had 100% conservation of the divergent sequences present in RT027 that were absent from reference strain CD630, which may represent genes associated with increased virulence. RT106 strains notably lacked a conjugative transposon, CTn5, which would limit its ability to transfer genes to neighboring bacteria.[30] The existing data suggest that RT106 has conserved genes also present in RT027, though future studies should attempt to more comprehensively define gene sequences associated with virulent phenotypes and/or strains known to cause epidemics.
Toxin, Spore Production, and Germination
The two major virulence factors of C. difficile are toxin A (TcdA) and toxin B (TcdB), which are encoded by the tcdA and tcdB genes, respectively.[32, 33] Some strains produce a binary toxin, C. difficile transferase (CDT), encoded by genes cdtA and cdtB.[34] PCR analysis has identified the presence of the tcdA and tcdB genes in most RT106 strains, however, RT106 lacks cdtA and cdtB genes as well as deletions in the tcdC gene.[20, 22, 35, 36] However, other variants have been reported, including six RT106 isolates collected from symptomatic patients in Medellin, Colombia that were tcdA−/tcdB+.[36] It is unclear whether this discrepancy is due to a genetic mutation or simply an experimental error.
While qualitative toxin characterization is helpful to predict phenotype, quantitative characterization has been used to try to explain hypervirulent phenotypes in the epidemic setting.[37] Investigators in the UK sought to quantify toxin production in three C. difficile ribotypes (001, 027, 106) and two reference strains (CD630 and VPI 10463, a known high toxin producer).[38] RT106 produced a lower amount of toxins than RT027 or VPI 10463 over 24 hours, but more toxin than ribotypes 001 and CD630. They also measured spo0A expression, which serves as the master regulator of sporulation, and the number of spores produced between strains over 24 hours. They concluded that RT106 produced significantly more spores than all other strains (P < 0.05). The high level of sporulation combined with moderate toxin production may help explain the growing geographic distribution of RT106, but multiple other factors have been shown to play a role as well. Such data should be interpreted with caution as high sporulation and high toxin production is present in many different ribotypes, including uncommon ribotypes.
Another factor impacting virulence is germination efficiency, which is the proportion of and rate at which spores give rise to vegetative cells that are able to form new colonies. Germination efficiency was compared among several ribotypes (027, 078, and 106), and RT106 was found to have the lowest germination efficiency of all isolates tested while RT027 had the highest. The authors concluded that high germination efficiency was associated with severe CDI and treatment failure.[39] A human gut model of CDI was used to demonstrate between-strain differences in total viable C. difficile (vegetative cell plus spore) counts and cytotoxin levels over time. While RT027 and RT106 total viable counts decreased by a similar magnitude (~2 log10 cfu/mL), the decrease was less rapid (4 days in RT027 versus 7 days in RT106) given the relatively greater proportion of spores seen in the RT106 model.[40] In addition, there was a sustained increase in spore count while germination commenced and proliferation of vegetative cells proceeded in the RT106 model which was not observed in the RT027 model. Furthermore, cytotoxin levels decreased more slowly in the RT106 model when compared to the RT027 model (14 versus 9 days) following treatment with vancomycin. Although the investigators could not rule out experimental variation, it is plausible that the relatively greater proportion of spores seen in the RT106 model led to continued germination and toxin production throughout vancomycin treatment given vancomycin’s inactivity against C. difficile spores. Two years later, the same investigators used the human gut model of CDI to assess the utility of linezolid for the treatment of CDI.[41] Unlike RT027, RT106 total viable counts increased 9 days after cessation of linezolid instillation, and subsequently C. difficile cytotoxin was detectable.
Other Virulence Factors
Although the direct cytotoxic effects of TcdA and TcdB and subsequent induction of pro-inflammatory cytokine production is primarily responsible for the acute inflammation observed in CDI, C. difficile produces other proteins that may also contribute to disease progression.[33] Such proteins include surface-layer proteins (SLPs), flagella, and heat-shock proteins (HSPs). When produced by RT106, these proteins induced similar levels of cytokine production as those seen when produced by other ribotypes.[42] These data suggest that while these proteins may mediate the immune response during CDI, they are not responsible for the range of virulence seen between C. difficile ribotypes. Therefore, the between-strain variation in toxin production could be a more distinguishing factor in vivo.
RT106 also has ten accessory genomic elements (AGEs) with unique sequences, although the function of the proteins transcribed from these sequences is not well understood.[22] When observed in other species of bacteria, several of these protein products play a role in intestinal mucosal adhesion, biofilm formation, and sporulation.[43, 44] They may also confer protection from threats such as oxidative stress and bacteriophages that exist in the intestinal tract.[45, 46] Some may also account for the differences in the phenotypic antimicrobial resistance profile of RT106, although further study is warranted.[22]
In addition to bacterial properties, a difference in host immune response in the gut to RT106 has been observed and may impact disease presentation and progression. Humans produce various antimicrobial peptides in response to CDI.[33] These include human neutrophil peptide 1 (HNP1), which is expressed by neutrophils and kills pathogens at sites of inflammation, and human α-defensin 5 (HD5), which is secreted by Paneth cells in the small intestine and acts within the intestinal mucosa.[47, 48] In vitro, ribotype 106 was less susceptible to direct killing by HNP1 and HD5 than hypervirulent ribotypes 027 and 078 and epidemic ribotypes 017 and 020.[49] While the authors recognized the broad antibacterial effects of both HNP1 and HD5 on multiple C. difficile ribotypes, they suggested that between-ribotype differences may be due to differences in the molecular wall composition of various ribotypes.
Biofilm formation is another factor influencing antimicrobial activity and host response to infection. Although little is described regarding the ability of RT106 to produce biofilm, one in vitro dose-finding study tested the activity of various Manuka honey concentrations on biofilm formation in three C. difficile strains (RT027, RT106, and ATCC 9689).[50] All strains produced biofilm and the anti-biofilm effects of Manuka honey were concentration dependent with optimal effects observed with concentrations between 40 and 50% (w/v). The authors concluded that Manuka honey may be useful if incorporated into sanitizers or disinfectants in health care settings.
Antimicrobial Susceptibility
Overall, RT106 is highly resistant to erythromycin,[23, 51, 52] clindamycin,[16, 23, 51, 53] fluoroquinolones,[16, 51, 53–55] and third-generation cephalosporins.[16, 23, 51, 54, 56] While nearly all RT106 isolates tested were resistant to erythromycin,[16, 23, 51] clindamycin resistance varied among studies.[16, 23, 51, 54, 56] Although methylation of the 23S ribosomal DNA gene by erythromycin ribosomal methylase (Erm) is a common mechanism of macrolide-lincosamide resistance, only one fourth of isolates in one study harbored the ermB gene.[54, 57] Rates of moxifloxacin resistance were very high in most studies [23, 51, 53–55] but RT106 was less resistant than the other four most prevalent ribotypes (002, 014, 020, and 027) in a recent Canadian study.[56] Data regarding RT106 susceptibility are also available for imipenem, piperacillin-tazobactam, cadazolid, amoxicillin/clavulanic acid, penicillin, and tetracycline although these data are less easily interpreted.[16, 23, 51, 55, 58]
A recent outbreak at an Illinois hospital in 2017 provided some mechanistic insight for resistance development in RT106.[59] One RT106 clone caused seven CDI episodes between two patients over 337 days, and the isolates from each sequential relapse displayed increased antibiotic resistance. Specifically, the clones acquired a 46,000 bp AGE that included 39 genes, including ermB, a rpoB missense mutation, associated with rifaximin resistance, and a penicillin binding protein (PBP) gene missense mutation, associated with ampicillin resistance. Another study identified a mutation in the DNA gyrase subunit, gyrA, which is associated with fluoroquinolone resistance.[54]
The reported susceptibility of RT106 to common CDI treatment agents vary by geographic region, but clinical applications of susceptibility have not been well established. The EUCAST ECVs for metronidazole and vancomycin are 2 mg/L, while CLSI has established a breakpoint of 8 mg/L for metronidazole and an ECV of 2 mg/L for vancomycin.[60, 61] In a study utilizing spiral gradient endpoint analysis for MIC determination, the RT106 MIC range for metronidazole was 0.36–2.57 mg/L (MIC50/MIC90; 1.03/1.82 mg/L).[62, 63] Two other large studies that included 80 and 137 RT106 isolates reported MIC50/MIC90 (range) of 0.5/1 (0.25–2 mg/L) and 0.5/1 (0.064–3 mg/L), respectively.[56, 58] Geometric mean MICs for metronidazole across three years in 22 European countries ranged from 0.37–0.65 mg/L.[53] Lastly, the geometric mean MIC of metronidazole among 475 RT106 isolates obtained in Scotland was 0.21 mg/L.[51] Geometric mean MICs for vancomycin across three years in 22 European countries ranged from 0.71–0.90 mg/L.[53] Only two of 475 RT106 isolates collected in Scotland displayed reduced susceptibility to vancomycin with MICs of 3 mg/L.[51] The MIC90 values for vancomycin among two groups of RT106 isolates (n =10 with reduced susceptibility to metronidazole and n=11 that were susceptible to metronidazole) were both 2 mg/L.[55] Furthermore, the MIC50/MIC90 (range) values for vancomycin against 137 RT106 isolates collected in England were 0.5/1 (0.25–2) mg/L, which was comparable to the other four most prevalent ribotypes tested (001, 002, 015, 027).[58] Taken together, these data suggest that both metronidazole and vancomycin are reasonable options for treating CDI caused by RT106 from an in vitro standpoint. However, existing in vivo trials analyzing the association of MIC with clinical outcome are absent, therefore more robust data are desperately needed.
Epidemiology
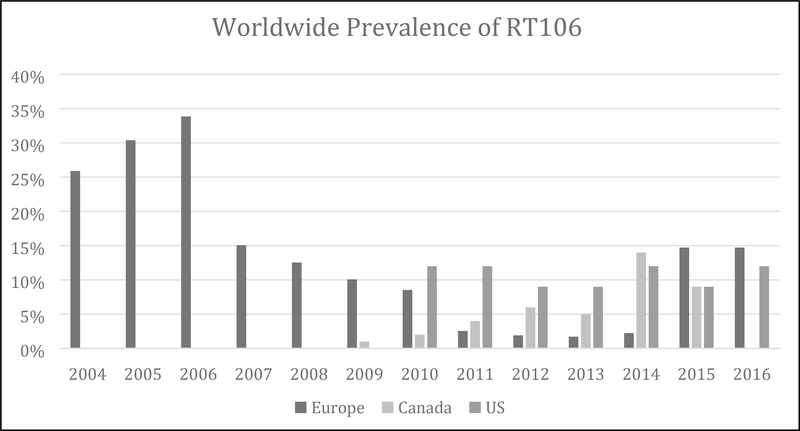
Although RT106 has a broad international distribution, the majority of reports have come from the UK where it has predominated as one of the region’s most prevalent ribotypes over the past decade.[16, 58, 64–69] England established one of the earliest centralized surveillance and ribotyping networks in the mid-2000s, and RT106 was identified as the most common ribotype in the network’s first reports between 2004–2005.[53, 58, 69–71] More recently, the prevalence of RT106 has decreased in the UK and was no longer among the ten most prevalent ribotypes in 2013.[53, 64] RT106 has been isolated from patients in a number of other European countries, including Scotland[72], Ireland[53, 68], France[53, 68, 73], Spain[53, 74–78], Portugal[53, 73], Belgium[53, 73], Cyprus[53, 73], Germany[53, 68], Switzerland[53, 79], Italy[53, 80], and Greece.[53, 81] More recently, RT106 has also been isolated in other parts of the Eurasian continent in countries including Lebanon[20], Israel[16], and Bangladesh.[82]
As the prevalence of RT106 has decreased across much of Europe, there has been a sharp rise in many other parts of the world including North America.[15, 16, 56, 83, 84] RT106 was first identified in the US between 2008–2009 [85] and quickly rose in prevalence from 1% to 11%, surpassing the epidemic RT027 in 2014.[15, 16] During that same time, the prevalence increased from 0% to 8% in Canada making RT106 the fourth most common ribotype between 2013–2015 (Figure 3).[16, 56] Based on the most recent surveys, RT106 remains the most commonly identified strain in the United States amongst community-associated infections in adults, and has been identified in both community- and health care-associated infections among pediatric patients.[15, 86] While its presence is highest in North America, RT106 has been isolated from patients in multiple South and Central American countries, including Costa Rica,[87] Colombia,[36, 88] and Brazil,[14, 18, 89–92] confirming its global distribution. Although the cause for such widespread dissemination of RT106 remains unknown, one contributor may be its resistance to environmental decontamination. A study comparing five disinfectants found that RT106 was among several C. difficile strains (ribotypes 001 and 027, CD630, and VPI 10463) that persisted after cleaning at the recommended concentration and duration.[93] Furthermore, RT106 has been found in hospital rooms following standardized cleaning with detergent and 1% hypochlorite solution.[94] Another potential contributor may be the number of environmental reservoirs identified for RT106. It has been isolated from multiple animal species, including symptomatic dogs in Spain and Brazil [76, 77, 91, 92], wild coati in Brazil [90], and bivalve mollusks in Italy.[18] In addition, RT106 was the most prevalent ribotype found in various types of commercially available compost and manure products, and fertilizers were shown to carry C. difficile even after processing.[35] RT106 was also isolated from children’s sandboxes in Madrid and samples of treated water from a municipal wastewater treatment plant in Switzerland, representing potential sources of transmission.[78, 79]
Figure 3.
Prevalence of ribotype 106 in Canada, Europe, and the United States over time
*Data are derived from references [15, 53, 58, 62, 66, 68–70, 74, 83, 86]
Clinical outcomes
Clinical outcomes of interest in CDI trials include clinical cure, recurrence, and mortality. A combination of clinical cure without recurrence at 30 days is an endpoint that is commonly used in clinical trials and referred to as “sustained response” or “global cure”.[95–97] Many CDI outcomes studies are retrospective and, given the heterogeneity of strains included, it is difficult to make strain-specific comparisons. Strain-specific outcome studies are scant in the literature and are limited to large outbreaks, most recently the hypervirulent RT027.[6, 95, 96, 98, 99] Despite the emergence of RT106 as a dominant strain in the US in the 2010s, little is known about the clinical outcomes associated with this strain.[15]
The first published report of RT106 clinical outcomes was derived from a group of 97 adults who were diagnosed with CDI >48 hours after admission to a single center in the UK.[100] Only 37 (38%) cases were caused by RT106, while the remainder were caused by ribotypes 027 (45%), 001 (10%), and 012, 005, 014, 026, 078, 094 (<10%, each). Although the investigators deemed the number of cases within each ribotype too small for statistical comparison, RT106 was associated with numerically less mortality. The all-cause 28-day mortality was 10.8% and 22.7% for patients infected with RT106 and RT027, respectively. Furthermore, the 3-day attributable mortality was 2.7% and 11.4% for RT106 and RT027, respectively. Patients infected with RT106 also experienced numerically fewer episodes of severe CDI (defined as white blood cells (WBC) >20,000 cells/μL and/or creatinine clearance <30 mL/min) and toxic megacolon. The frequency of relapse within 60 days was 8% for RT106 and 11% for RT027.
A case series from a single center in the UK describes the outcomes of nine adults infected with RT106 and hospitalized in a surgical ward.[72] The mean age of these patients was 73 years (range: 38–90 years). While much of the article was dedicated to infection control practices implemented to control the outbreak, there was a descriptive analysis of the clinical outcomes. Five patients were treated and discharged without complications. Two patients experienced a CDI recurrence and two patients died, but the CDI was not considered a factor in one case.
A large, nationwide outcomes study in the US included a convenience sample of cases identified through the Centers for Disease Control and Prevention (CDC) Emerging Infections Program (EIP) C. difficile surveillance network.[101] This network consists of 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. The only outcome reported for RT106 was disease severity. Severe disease was defined as the development of ileus, toxic megacolon, or pseudomembranous colitis within 5 days of CDI diagnosis or serum WBC ≥15,000 cells/μL within 1 calendar day of stool sample collection. Of the 2,057 isolates available for typing, 187 (9.1%) were RT106, which represented the third most common type. The investigators found RT106 to cause significantly less severe disease than RT027 (OR, 0.28; 95% CI, 0.18–0.48; P <0.05).[101]
A pediatric-focused outcomes study details clinical outcomes associated with RT106 in patients admitted to a single center in Chicago, US.[102] The primary outcome was CDI recurrence within 8 weeks of the previous episode. Interestingly, 16% (19/117) were RT106 making it the predominant strain. RT027 was only found in one patient. The investigators found RT106 to have a similar risk of recurrence compared with non-RT106 strains (RR, 1.7; 95% CI, 0.7–4.2, P = 0.3). However, those whose initial infection was caused by RT106 were significantly more likely to experience multiple CDI relapses (40% vs 8%; P = 0.05).
The most recent publication represents the only prospective analysis of RT106 clinical outcomes.[103] Unfortunately, of the 123 typed isolates, only four were identified as RT106. Two of these patients went on to develop complications: one patient developed pancolitis and one developed toxic megacolon and required a colectomy. Two patients died, one of which was deemed to be caused by CDI.
Taken together, these data highlight the need for further investigation of RT106 clinical outcomes. Future studies should focus on comparing RT106 to virulent strains, such as RT027, and other prevalent strains, such as RT014–020. Outcomes should include multiple relevant clinical outcomes such as clinical cure, mortality (preferably attributable mortality), recurrence, and sustained response. Lastly, other patient covariates including age, validated comorbidity scores (e.g. Charlson comorbidity index), and treatment agent should be obtained and accounted for. These data are invaluable to clinicians and epidemiologists and must become a priority given the increasing prevalence of RT106 in relation to historical epidemic strains.
Discussion and Conclusion
By conducting a systematic review of the literature, we were able to identify 68 articles published between 1999 and 23 May 2019. Notably, very few of these articles focused solely on RT106 but were rather identified as part of a larger collection of C. difficile isolates. Therefore, much is yet to be learned about this epidemic strain. The ability of C. difficile to infect patients is largely dependent on two characteristics; toxin production and the formation of spores.[33] Current data suggest RT106 harbors the tcdA and tcdB genes, lacks binary toxin genes, and does not contain any deletions in the tcdC gene, which differentiates it from other epidemic strains, including ribotypes 027 and 078.[6, 8, 20, 22, 35, 36] One differentiating feature of RT106 is its ability to produce high levels of spores.[40] This characteristic could feasibly contribute to its ability to cause hospital outbreaks given the difficulty in eradicating C. difficile spores from the hospital environment.[104, 105] Since no therapeutic agent used to treat CDI has the ability to kill C. difficile in its spore form, RT106 may be able to cause recurrent disease more effectively than other ribotypes. While these thoughts are purely speculative, they warrant further investigation.
Of the 68 articles that met inclusion criteria in this review, 12 articles reported MICs for various antibiotics with the number of RT106 isolates ranging from one to 475.[14, 23, 36, 51, 52, 54, 56, 58, 62, 77, 78, 100] Investigators have used multiple methods to illustrate antibiotic susceptibility, including geometric mean MIC, percentage susceptible, MIC50/MIC90, and MIC range. This limited our ability to illustrate these data visually. Methods such as percentage susceptible and percentage resistant have significant limitations when interpreting given the lack of standardized breakpoints for C. difficile. Future studies should report a combination of MIC50/MIC90 and MIC range to allow for easier interpretation across studies. This will give different investigators the opportunity to apply different breakpoints as appropriate.
Lastly, given the widespread adoption of prospective C. difficile ribotyping by prominent research groups, the next logical step in CDI clinical outcomes research is ribotype-stratified outcomes analysis.[69, 104] Specifically, RT106 outcomes research should include 30- and 90-day recurrence as well as duration of diarrhea as outcomes to determine the effect of high spore production on clinical outcome.
In conclusion, ribotype 106 has been a predominant strain in Europe for decades and has now been identified in most countries conducting C. difficile surveillance. Investigations over the past 10 years have shed light on several factors that could contribute to the widespread prevalence of RT106, such as higher levels of sporulation and antibiotic resistance. However, more research is needed to clarify conflicting results and to confirm findings observed in studies with small sample sizes. Specific areas warranting future study include potential survival advantages provided by genetic elements as well as a more robust investigation of clinical outcomes associated with RT106. Although this review aimed to summarize the evidence available regarding RT106, it also elucidates the effort that still needs to be put forth to better understand this common C. difficile ribotype.
Highlights.
Clostridioides difficile ribotype 106 (RT106) has been identified worldwide and became the most prevalent strain in the United States in 2016.
RT106 produces more spores than the epidemic ribotype 027 (RT027).
Available clinical outcomes data suggest that RT106 is not hypervirulent but patients are significantly more likely to experience a C. difficile recurrence if infected with RT106.
Acknowledgements
This study was funded in part by the National Institutes of Health NIAID (U01AI124290-01)
Footnotes
All authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson S and Gerding DN, Clostridium difficile--associated diarrhea. Clin Infect Dis, 1998. 26(5): p. 1027–34; quiz 1035–6. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, et al. , Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med, 2018. 379(18): p. 1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessa FC, et al. , Burden of Clostridium difficile infection in the United States. N Engl J Med, 2015. 372(9): p. 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AJ, et al. , The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis, 2012. 55(2): p. 216–23. [DOI] [PubMed] [Google Scholar]
- 5.Dubberke ER and Olsen MA, Burden of Clostridium difficile on the healthcare system. Clin Infect Dis, 2012. 55 Suppl 2: p. S88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald LC, et al. , An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med, 2005. 353(23): p. 2433–41. [DOI] [PubMed] [Google Scholar]
- 7.He M, et al. , Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet, 2013. 45(1): p. 109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goorhuis A, et al. , Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis, 2008. 47(9): p. 1162–70. [DOI] [PubMed] [Google Scholar]
- 9.Lim SK, et al. , Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin Infect Dis, 2014. 58(12): p. 1723–30. [DOI] [PubMed] [Google Scholar]
- 10.Huber CA, et al. , Challenges for standardization of Clostridium difficile typing methods. J Clin Microbiol, 2013. 51(9): p. 2810–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuijper E, van den Berg RJ, and Brazier J, Comparison of Molecular Typing Methods Applied to Clostridium difficile Molecular Epidemiology of Microorganisms: Methods and Protocols, ed. Caugant DA. 2009: Springer Dordrecht Heidelberg; 159–172. [DOI] [PubMed] [Google Scholar]
- 12.Endres BT, et al. , Epidemic Clostridioides difficile Ribotype 027 Lineages: Comparisons of Texas Versus Worldwide Strains. Open Forum Infect Dis, 2019. 6(2): p. ofz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stubbs SLJ, et al. , PCR Targeted to the 16S-23S rRNA Gene Intergenic Spacer Region of Clostridium difficile and Construction of a Library Consisting of 116 Different PCR Ribotypes. J Clin Microbiol, 1999. 37(2): p. 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balassiano IT, et al. , Characterization of Clostridium difficile strains isolated from immunosuppressed inpatients in a hospital in Rio de Janeiro, Brazil. Anaerobe, 2009. 15(3): p. 61–4. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Clostridioides difficile Infection (CDI) Tracking. 2019. [cited 2019 June 3]; Available from: https://www.cdc.gov/hai/eip/cdiff-tracking.html#reports.
- 16.Cheknis A, et al. , Molecular epidemiology of Clostridioides (Clostridium) difficile strains recovered from clinical trials in the US, Canada and Europe from 2006–2009 to 2012–2015. Anaerobe, 2018. 53: p. 38–42. [DOI] [PubMed] [Google Scholar]
- 17.Killgore G, et al. , Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol, 2008. 46(2): p. 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diniz AN, et al. , Molecular epidemiology of Clostridioides (previously Clostridium) difficile isolates from a university hospital in Minas Gerais, Brazil. Anaerobe, 2019. 56: p. 34–39. [DOI] [PubMed] [Google Scholar]
- 19.Tenover FC, et al. , Comparison of strain typing results for Clostridium difficile isolates from North America. J Clin Microbiol, 2011. 49(5): p. 1831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger FK, et al. , Molecular characterization, toxin detection and resistance testing of human clinical Clostridium difficile isolates from Lebanon. Int J Med Microbiol, 2018. 308(3): p. 358–363. [DOI] [PubMed] [Google Scholar]
- 21.Manzoor SE, et al. , Extended multilocus variable-number tandem-repeat analysis of Clostridium difficile correlates exactly with ribotyping and enables identification of hospital transmission. J Clin Microbiol, 2011. 49(10): p. 3523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kociolek LK, et al. , Comparative genomics analysis of Clostridium difficile epidemic strain DH/NAP11/106. Microbes Infect, 2018. 20(4): p. 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutlu E, et al. , Molecular characterization and antimicrobial susceptibility patterns of Clostridium difficile strains isolated from hospitals in south-east Scotland. J Med Microbiol, 2007. 56(Pt 7): p. 921–9. [DOI] [PubMed] [Google Scholar]
- 24.Lyras D, et al. , Toxin B is essential for virulence of Clostridium difficile. Nature, 2009. 458(7242): p. 1176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyerly DM, Barroso LA, and Wilkins TD, Identification of the Latex Test-Reactive Protein of Clostidium difficile as Glutamate Dehydrogenase. Journal of Clinical Microbiology, 1991. 29(11): p. 2639–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyerly DM and Wilkins TD, Commercial Latex Test for Clostridium difficile Toxin A Does Not Detect Toxin A. Journal of Clinical Microbiology, 1986. 23(3): p. 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald LC, et al. , Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis, 2018. 66(7): p. e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover FC, et al. , Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol, 2010. 48(10): p. 3719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg SD, et al. , Lack of effect of strain type on detection of toxigenic Clostridium difficile by glutamate dehydrogenase and polymerase chain reaction. Diagn Microbiol Infect Dis, 2011. 70(3): p. 417–9. [DOI] [PubMed] [Google Scholar]
- 30.Marsden GL, et al. , Array comparative hybridisation reveals a high degree of similarity between UK and European clinical isolates of hypervirulent Clostridium difficile. BMC Genomics, 2010. 11: p. 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozer EA, et al. , Complete Genome Sequence of Clostridioides difficile Epidemic Strain DH/NAP11/106/ST-42, Isolated from Stool from a Pediatric Patient with Diarrhea. Genome Announc, 2017. 5(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voth DE and Ballard JD, Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev, 2005. 18(2): p. 247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vedantam G, et al. , Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes, 2012. 3(2): p. 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerding DN, et al. , Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes, 2014. 5(1): p. 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dharmasena M and Jiang X, Isolation of Toxigenic Clostridium difficile from Animal Manure and Composts Being Used as Biological Soil Amendments. Appl Environ Microbiol, 2018. 84(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar CL, et al. , Subtyping of Clostridium difficile PCR ribotypes 591, 106 and 002, the dominant strain types circulating in Medellin, Colombia. PLoS One, 2018. 13(4): p. e0195694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warny M, et al. , Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. The Lancet, 2005. 366(9491): p. 1079–1084. [DOI] [PubMed] [Google Scholar]
- 38.Vohra P and Poxton IR, Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology, 2011. 157(Pt 5): p. 1343–53. [DOI] [PubMed] [Google Scholar]
- 39.Moore P, et al. , Germination efficiency of clinical Clostridium difficile spores and correlation with ribotype, disease severity and therapy failure. J Med Microbiol, 2013. 62(Pt 9): p. 1405–13. [DOI] [PubMed] [Google Scholar]
- 40.Baines SD, et al. , Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother, 2009. 63(3): p. 520–5. [DOI] [PubMed] [Google Scholar]
- 41.Baines SD, et al. , Evaluation of linezolid for the treatment of Clostridium difficile infection caused by epidemic strains using an in vitro human gut model. J Antimicrob Chemother, 2011. 66(7): p. 1537–46. [DOI] [PubMed] [Google Scholar]
- 42.Vohra P and Poxton IR, Induction of cytokines in a macrophage cell line by proteins of Clostridium difficile. FEMS Immunol Med Microbiol, 2012. 65(1): p. 96–104. [DOI] [PubMed] [Google Scholar]
- 43.Hendrickx AP, et al. , LPxTG surface proteins of enterococci. Trends Microbiol, 2009. 17(9): p. 423–30. [DOI] [PubMed] [Google Scholar]
- 44.Eichenberger P, et al. , The σE Regulon and the Identification of Additional Sporulation Genes in Bacillus subtilis. Journal of Molecular Biology, 2003. 327(5): p. 945–972. [DOI] [PubMed] [Google Scholar]
- 45.Dai D, et al. , DNA Phosphorothioate Modification Plays a Role in Peroxides Resistance in Streptomyces lividans. Front Microbiol, 2016. 7: p. 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu T, et al. , A novel host-specific restriction system associated with DNA backbone S-modification in Salmonella. Nucleic Acids Res, 2010. 38(20): p. 7133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salzman NH, Microbiota-immune system interaction: an uneasy alliance. Curr Opin Microbiol, 2011. 14(1): p. 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehrer RI and Lu W, alpha-Defensins in human innate immunity. Immunol Rev, 2012. 245(1): p. 84–112. [DOI] [PubMed] [Google Scholar]
- 49.Furci L, et al. , New role for human alpha-defensin 5 in the fight against hypervirulent Clostridium difficile strains. Infect Immun, 2015. 83(3): p. 986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammond EN, Donkor ES, and Brown CA, Biofilm formation of Clostridium difficile and susceptibility to Manuka honey. BMC Complement Altern Med, 2014. 14: p. 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiuff C, et al. , The epidemiology of Clostridium difficile in Scotland. J Infect, 2011. 62(4): p. 271–9. [DOI] [PubMed] [Google Scholar]
- 52.John R and Brazier JS, Antimicrobial susceptibility of polymerase chain reaction ribotypes of Clostridium difficile commonly isolated from symptomatic hospital patients in the UK. J Hosp Infect, 2005. 61(1): p. 11–4. [DOI] [PubMed] [Google Scholar]
- 53.Freeman J, et al. , The ClosER study: results from a three-year pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011–2014. Clin Microbiol Infect, 2018. 24(7): p. 724–731. [DOI] [PubMed] [Google Scholar]
- 54.Solomon K, et al. , PCR ribotype prevalence and molecular basis of macrolide-lincosamide-streptogramin B (MLSB) and fluoroquinolone resistance in Irish clinical Clostridium difficile isolates. J Antimicrob Chemother, 2011. 66(9): p. 1976–82. [DOI] [PubMed] [Google Scholar]
- 55.Chilton CH, et al. , In vitro activity of cadazolid against clinically relevant Clostridium difficile isolates and in an in vitro gut model of C. difficile infection. J Antimicrob Chemother, 2014. 69(3): p. 697–705. [DOI] [PubMed] [Google Scholar]
- 56.Karlowsky JA, et al. , PCR ribotyping and antimicrobial susceptibility testing of isolates of Clostridium difficile cultured from toxin-positive diarrheal stools of patients receiving medical care in Canadian hospitals: the Canadian Clostridium difficile Surveillance Study (CAN-DIFF) 2013–2015. Diagn Microbiol Infect Dis, 2018. 91(2): p. 105–111. [DOI] [PubMed] [Google Scholar]
- 57.Arthur M, Brisson-Noel A, and Courvalin P, Origin and evolution of genes specifying resistance to macrolide, lincosamide and streptogramin antibiotics: data and hypotheses. J Antimicrob Chemother, 1987. 20(6): p. 783–802. [DOI] [PubMed] [Google Scholar]
- 58.Brazier JS, et al. , Distribution and antimicrobial susceptibility patterns of Clostridium difficile PCR ribotypes in English hospitals, 2007–08. Euro Surveill, 2008. 13(41). [DOI] [PubMed] [Google Scholar]
- 59.Kociolek LK, et al. , Whole-genome analysis reveals the evolution and transmission of an MDR DH/NAP11/106 Clostridium difficile clone in a paediatric hospital. J Antimicrob Chemother, 2018. 73(5): p. 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clinical and Laboratory Standards Institute (CLSI), Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI standard M100. 2019, Clinical Laboratory and Standards Institute: Wayne, Pennsylvania. [Google Scholar]
- 61.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0,. 2019; Available from: http://www.eucast.org/clinical_breakpoints/.
- 62.Baines SD, et al. , Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother, 2008. 62(5): p. 1046–52. [DOI] [PubMed] [Google Scholar]
- 63.Pong R, et al. , Spiral gradient endpoint susceptibility testing: a fresh look at a neglected technique. J Antimicrob Chemother, 2010. 65(9): p. 1959–63. [DOI] [PubMed] [Google Scholar]
- 64.Public Health England. Clostridium difficile reporting network (CDRN) report. 2019. [cited 2019 June 3]; Available from: https://www.gov.uk/government/publications/clostridium-difficile-ribotyping-network-cdrn-report.
- 65.Behar L, et al. , Toxigenic Clostridium difficile colonization among hospitalised adults; risk factors and impact on survival. J Infect, 2017. 75(1): p. 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanek J, et al. , Epidemiological survey of Clostridium difficile ribotypes in the North East of England during an 18-month period. J Hosp Infect, 2012. 81(3): p. 209–12. [DOI] [PubMed] [Google Scholar]
- 67.Miyajima F, et al. , Characterisation and carriage ratio of Clostridium difficile strains isolated from a community-dwelling elderly population in the United Kingdom. PLoS One, 2011. 6(8): p. e22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauer MP, et al. , Clostridium difficile infection in Europe: a hospital-based survey. The Lancet, 2011. 377(9759): p. 63–73. [DOI] [PubMed] [Google Scholar]
- 69.Wilcox MH, et al. , Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis, 2012. 55(8): p. 1056–63. [DOI] [PubMed] [Google Scholar]
- 70.Brazier JS, Patel B, and Pearson A, Distribution of Clostridium difficile PCR ribotype 027 in British hospitals. Euro Surveill, 2007. 12(4): p. E070426 2. [DOI] [PubMed] [Google Scholar]
- 71.Freeman J, et al. , The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev, 2010. 23(3): p. 529–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ratnayake L, et al. , Control of an outbreak of diarrhoea in a vascular surgery unit caused by a high-level clindamycin-resistant Clostridium difficile PCR ribotype 106. J Hosp Infect, 2011. 79(3): p. 242–7. [DOI] [PubMed] [Google Scholar]
- 73.Freeman J, et al. , Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin Microbiol Infect, 2015. 21(3): p. 248 e9–248 e16. [DOI] [PubMed] [Google Scholar]
- 74.Suarez-Bode L, et al. , Increasing prevalence of the epidemic ribotype 106 in healthcare facility-associated and community-associated Clostridioides difficile infection. Anaerobe, 2019. 55: p. 124–129. [DOI] [PubMed] [Google Scholar]
- 75.Alcala L, et al. , Impact of clinical awareness and diagnostic tests on the underdiagnosis of Clostridium difficile infection. Eur J Clin Microbiol Infect Dis, 2015. 34(8): p. 1515–25. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez-Perez S, et al. , Prevalence and characteristics of Clostridium perfringens and Clostridium difficile in dogs and cats attended in diverse veterinary clinics from the Madrid region. Anaerobe, 2017. 48: p. 47–55. [DOI] [PubMed] [Google Scholar]
- 77.Orden C, et al. , Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole-resistant strains. Anaerobe, 2017. 43: p. 78–81. [DOI] [PubMed] [Google Scholar]
- 78.Orden C, et al. , Recreational sandboxes for children and dogs can be a source of epidemic ribotypes of Clostridium difficile. Zoonoses Public Health, 2018. 65(1): p. 88–95. [DOI] [PubMed] [Google Scholar]
- 79.Romano V, et al. , Toxigenic Clostridium difficile PCR ribotypes from wastewater treatment plants in southern Switzerland. Appl Environ Microbiol, 2012. 78(18): p. 6643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pasquale V, et al. , Occurrence of toxigenic Clostridium difficile in edible bivalve molluscs. Food Microbiol, 2012. 31(2): p. 309–12. [DOI] [PubMed] [Google Scholar]
- 81.Kachrimanidou M, et al. , Clostridium difficile infections in a university hospital in Greece are mainly associated with PCR ribotypes 017 and 126. J Med Microbiol, 2017. 66(12): p. 1774–1781. [DOI] [PubMed] [Google Scholar]
- 82.Islam MA, et al. , Clostridioides difficile ribotypes isolated from domestic environment and from patients in Bangladesh. Anaerobe, 2019. 56: p. 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katz KC, et al. , The evolving epidemiology of Clostridium difficile infection in Canadian hospitals during a postepidemic period (2009–2015). CMAJ, 2018. 190(25): p. E758–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tickler IA, et al. , Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother, 2014. 58(7): p. 4214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sambol SP, et al. Variable fluoroquinolone resistance between emerging North American and European isolates of Clostridium difficile REA group DH (PCR ribotype 106). in ClostPath Int. Conf. 2009. Rome, Italy. [Google Scholar]
- 86.Wendt JM, et al. , Clostridium difficile infection among children across diverse US geographic locations. Pediatrics, 2014. 133(4): p. 651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopez-Urena D, et al. , Predominance and high antibiotic resistance of the emerging Clostridium difficile genotypes NAPCR1 and NAP9 in a Costa Rican hospital over a 2-year period without outbreaks. Emerg Microbes Infect, 2016. 5: p. e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salazar CL, et al. , Molecular, microbiological and clinical characterization of Clostridium difficile isolates from tertiary care hospitals in Colombia. PLoS One, 2017. 12(9): p. e0184689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopes Cancado GG, et al. , Clinical epidemiology of Clostridium difficile infection among hospitalized patients with antibiotic-associated diarrhea in a university hospital of Brazil. Anaerobe, 2018. 54: p. 65–71. [DOI] [PubMed] [Google Scholar]
- 90.Silva RO, et al. , Carriage of Clostridium difficile in free-living South American coati (Nasua nasua) in Brazil. Anaerobe, 2014. 30: p. 99–101. [DOI] [PubMed] [Google Scholar]
- 91.Silva RO, et al. , Clostridium difficile ribotypes in humans and animals in Brazil. Mem Inst Oswaldo Cruz, 2015. 110(8): p. 1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silva ROS, et al. , Clostridioides difficile infection in dogs with chronic-recurring diarrhea responsive to dietary changes. Anaerobe, 2018. 51: p. 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vohra P and Poxton IR, Efficacy of decontaminants and disinfectants against Clostridium difficile. J Med Microbiol, 2011. 60(Pt 8): p. 1218–24. [DOI] [PubMed] [Google Scholar]
- 94.Shapey S, et al. , Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J Hosp Infect, 2008. 70(2): p. 136–41. [DOI] [PubMed] [Google Scholar]
- 95.Louie TJ, et al. , Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med, 2011. 364(5): p. 422–31. [DOI] [PubMed] [Google Scholar]
- 96.Cornely OA, et al. , Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. The Lancet Infectious Diseases, 2012. 12(4): p. 281–289. [DOI] [PubMed] [Google Scholar]
- 97.Carlson TJ, et al. , Ridinilazole for the treatment of Clostridioides difficile infection. Expert Opin Investig Drugs, 2019. 28(4): p. 303–310. [DOI] [PubMed] [Google Scholar]
- 98.Johnson S, et al. , Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med, 1999. 341(22): p. 1645–51. [DOI] [PubMed] [Google Scholar]
- 99.Loo VG, et al. , A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med, 2005. 353(23): p. 2442–9. [DOI] [PubMed] [Google Scholar]
- 100.Sundram F, et al. , Clostridium difficile ribotypes 027 and 106: clinical outcomes and risk factors. J Hosp Infect, 2009. 72(2): p. 111–8. [DOI] [PubMed] [Google Scholar]
- 101.See I, et al. , NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis, 2014. 58(10): p. 1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kociolek LK, et al. , Molecular epidemiology of Clostridium difficile infections in children: a retrospective cohort study. Infect Control Hosp Epidemiol, 2015. 36(4): p. 445–51. [DOI] [PubMed] [Google Scholar]
- 103.Khanafer N, et al. , Outcomes of Clostridium difficile-suspected diarrhea in a French university hospital. Eur J Clin Microbiol Infect Dis, 2018. 37(11): p. 2123–2130. [DOI] [PubMed] [Google Scholar]
- 104.Alam MJ, et al. , Community Environmental Contamination of Toxigenic Clostridium difficile. Open Forum Infect Dis, 2017. 4(1): p. ofx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gerding DN, Muto CA, and Owens RC Jr., Measures to control and prevent Clostridium difficile infection. Clin Infect Dis, 2008. 46 Suppl 1: p. S43–9. [DOI] [PubMed] [Google Scholar]