Abstract
Objectives:
Gender differences may modify symptoms, disease expression, and treatment effects. The objective was to evaluate the link between life impact and gender in psoriatic arthritis (PsA).
Methods:
ReFlaP ( NCT03119805) was a study in 14 countries of consecutive adult patients with definite PsA. Participants underwent comprehensive PsA assessment: Disease Activity in PSoriatic Arthritis (DAPSA), Minimal Disease Activity (MDA), and Psoriatic Arthritis Impact of Disease (PsAID). Disease activity was compared by gender using t-tests or Wilcoxon tests. The association of PsAID with gender was analyzed using hierarchical generalized linear models.
Results:
Of 458 participants 50.2% were male, mean age (SD) 53.1 (12.6) years, PsA duration 11 (8.2) years, and 51.5% taking bDMARDs. Women versus men had worse Leeds enthesitis index: 0.8 (1.7) / 0.3 (0.9), pain [numerical rating scale 0–10 (NRS)]: 4.7 (2.7) / 3.5 (2.7), HAQ-DI: 0.9 (0.7) / 0.5 (0.6), fatigue NRS: 5.2 (3) / 3.3 (2.8), PsAID: 4.1 (2.4) / 2.8 (2.3), p<0.001 for all, and were less frequently at treatment target (T2T): DAPSA (DAPSA cut-offs ≤4 remission, >4 and ≤14 low disease activity): 16.9 (14.9) / 12.6 (16.6), MDA: 25.7% / 50.0%, p<0.001 for all. High life impact (PsAID≥4) was associated with female gender [odds ratio (OR) 2.3], enthesitis (OR 1.34), tender joints (OR 1.10) p<0.001 for all, and comorbidities (OR 1.22, p=0.002).
Conclusions:
High life impact was independently associated with female gender, enthesitis, comorbidities, and tender joints. At T2T, women vs men had higher life impact. Life impact needs to become part of PsA T2T strategies.
Keywords: psoriatic arthritis, gender, sex, life impact, treatment target, patient reported outcomes
Psoriatic arthritis (PsA) occurs in one of four people with the autoimmune skin disease psoriasis (1). Although PsA has equal prevalence among men and women, several national registries and longitudinal observational studies have shown phenotypic and outcomes differences between the sexes. These differences can be summarized as follows: women had more frequently polyarthritis (2–5), enthesitis (6), elevated inflammatory markers (5,8), and worse pain (4–6), fatigue (3, 5–7), physical and work disability (4–7); while men had more frequently oligoarthritis, axial disease (2–4, 8), nail psoriasis (3), worse PASI scores (5), and higher radiographic progression(3, 9). Biologic DMARD (bDMARD) use, while appearing similar in men and women with PsA, seems to have higher effectiveness for men who responded better to TNF-inhibitor treatment (4, 7, 8), and had longer bDMARD persistence (7, 8, 12, 13) as shown in several studies. Interestingly, in psoriasis, similar to PsA, a negative association of female sex with treatment response and biologic drug survival was also documented (14–17).
Examination of disease activity, response to treatment and contextual factors is needed to evaluate if the male and female PsA phenotypes are distinct and to optimize treatment approaches within a personalized medicine framework. Understanding factors underlying differences in reporting and outcomes between men and women will enable more effective implementation and maintenance of treatment targets in both women and men.
Recently we performed an international study of patients with established PsA (18). PsA-specific disease activity and life impact measures were systematically collected in accordance with treatment targets (19) and outcomes (20) recently established in PsA through consensus. The objective of this analysis was to assess gender specific treat-to-target status, disease activity and patient reported outcomes (PROs), and to evaluate the association of PsA life impact with gender.
METHODS
Study population
Consecutive patients with rheumatologist-diagnosed PsA and more than two years disease duration were enrolled in 21 centers in 14 countries as part of the Remission and Flare in PsA Study (ReFlaP, NCT03119805). The study design has been previously described (18). The ReFlaP study was approved by the Institutional Review Board at the coordinating site (Sorbonne Universite, Paris, France) and at each participating site. All patients gave written informed consent for their participation in the study.
Data collection
In addition to demographics, comorbidities (21, 22) and disease characteristics, a PsA-specific data collection framework was used. Investigators recorded 66 swollen joint counts (SJC66, range 0–66) and 68 tender joint counts (TJC68, 0–68), tender entheseal points using the Leeds Enthesitis Index (LEI, 0–6), active psoriasis body surface area (BSA, range 0–100%), physician global assessment [numeric rating scale (NRS), 0–10 cm], and biologic use. PROs collected included pain, patient global assessment of skin and joints (numeric rating scales, 11 point NRS, 0–10), the Health Assessment Questionnaire Disability Index (HAQ-DI, 0–3), and Psoriatic Arthritis Impact of Disease- 12 items (PsAID, 0–10) (23). Higher PROs scores reflect worse patient status. For PsAID, a score of <=4 represents a patient acceptable symptom state (23). Disease activity was calculated using Disease activity in Psoriatic Arthritis (DAPSA, continuous score) (24) and Minimal Disease Activity (MDA, yes/no) (25). DAPSA is calculated as the sum of SJC66, TJC68, patient-reported pain [numeric rating scale (NRS) 0–10], patient global assessment (PGA) of PsA (NRS 0–10), and CRP (C-reactive protein, mg/dL). Higher DAPSA scores represent worse disease activity. A DAPSA values of ≤4 corresponds to remission, >4 and ≤14 to low disease activity, >14 and ≤28 to moderate disease activity and >28 to high disease activity (24). MDA is a cutoff based checklist of seven PsA disease activity criteria which includes 66/68 joint counts, enthesitis, physical function/disability, pain, patient global, and psoriasis assessment [SJC66≤1, TJC68≤1, LEI≤1, HAQ-DI≤0.5, Pain≤1.5, Patient global≤2, and psoriasis body surface area (BSA)<3%]; if five out of seven are met the patient is considered in MDA (23). DAPSA remission or low disease activity, or MDA are the current treatment targets in PsA (19). The PsAID instrument was recently provisionally endorsed by Outcome Measures in Rheumatology (OMERACT) for the measurement of PsA specific health-related quality of life in clinical trials and longitudinal studies (26).
Statistical analysis
Descriptive analyses were performed to compare men and women for PsA characteristics, disease activity, and PROs. Group means for continuous variables were compared using t-tests or Wilcoxon tests, and proportions for categorical variables were compared using chi-square tests or Fisher’s exact test if sample size was inadequate. We hypothesized higher life impact in higher PsA disease activity states and compared PsAID12 mean scores in men and women separately by disease activity categories (DAPSA low disease activity and remission, corresponding to being at treatment target (19), and separately in DAPSA moderate and high disease activity). We similarly compared change scores in participants who intensified therapy at baseline for active disease.
We used hierarchical generalized linear models to evaluate the association between PsAID (outcome) and gender (predictor), and whether trends over time were differential between gender, including an interaction between gender and visit and a random patient-level intercept. We used a logistic model for PsAID life impact score as a categorical variable, where a PsAID score >4 was defined as the threshold for high life impact (23). We constructed multivariate regression models including gender, number of comorbidities [Groll Functional Comorbidity index (21)], age, and disease duration. We then added to the multivariate models, musculoskeletal disease activity (SJC66, TJC68, LEI), skin disease activity (BSA>5%), systemic inflammation [CRP (mg/dL), continuous value], and biologic use (yes/no). We also used a hierarchical linear model to estimate the association of PsAID score as a continuous variable with gender from multivariate linear regression models using the same covariates as described above. We also applied these models separately in each gender group.
RESULTS
Of 466 patients, 458 had complete data on gender (see Table 1): 230 (50.2%) were men, mean age (standard deviation, SD) was 53.1 (12.6) years, mean disease duration was 11 (8.2) years, and 51.5% were taking a bDMARD. Mean (SD) PROs were PGA 4.2 (2.7), HAQ-DI 0.7 (0.7), and PsAID 3.4 (2.5). Psoriatic skin disease affecting BSA>5% was present in 13.8% of participants. Average Groll Functional Comorbidity index was higher in women, 1.3 (1.8) vs 0.8 (1.1), p<0.001. Osteoporosis, depression, anxiety, upper gastrointestinal disorders, degenerative disc disease, and obesity were significantly more frequent in women (Supplement Table 1). Average time between visits was 20 (10) weeks, among 398 (87%) with follow-up at the second visit. There were 61 women and 52 men who intensified therapy due to active disease at baseline and had a follow-up visit.
Table 1.
Disease characteristics of 458 patients, overall and by gender
| Patient characteristic Mean (SD) or N(%) | Overall n=458 | Women n=228 | Men n=230 | P value men vs women** |
|---|---|---|---|---|
| Age, years | 53.1 (12.6) | 51.7 (13.1) | 54.6 (11.9) | 0.017 |
| Disease duration, years | 10.9 (8.2) | 11.0 (8.6) | 10.9 (7.9) | 0.828 |
| Number of comorbidities* | 1.0 (1.5) | 1.3 (1.8) | 0.8 (1.1) | < 0.001 |
| TJC68 | 4.6 (9.4) | 5.4 (9.2) | 3.8 (9.5) | 0.069 |
| SJC66 | 2.0 (6.2) | 2.1 (5.6) | 2.0 (6.8) | 0.857 |
| LEI | 0.6 (1.4) | 0.8 (1.7) | 0.3 (0.9) | < 0.001 |
| BSA >5% | 63 (13.8) | 31 (13.6) | 32 (13.9) | 0.922 |
| Patient global NRS | 4.2 (2.7) | 4.8 (2.6) | 3.5 (2.7) | < 0.001 |
| Pain NRS | 4.1 (2.8) | 4.7 (2.7) | 3.5 (2.7) | < 0.001 |
| Fatigue NRS | 4.2 (3.1) | 5.2 (3.0) | 3.2 (2.8) | < 0.001 |
| HAQ-DI | 0.7 (0.7) | 0.9 (0.7) | 0.5 (0.6) | < 0.001 |
| PsAID | 3.4 (2.5) | 4.1 (2.4) | 2.8 (2.3) | < 0.001 |
| DAPSA | 14.8 (15.9) | 16.9 (14.9) | 12.6 (16.6) | 0.004 |
| DAPSA remission (≤4) | 87 (19.8) | 27 (12.3) | 60 (27.1) | < 0.001 |
| DAPSA low disease activity (>4 and ≤14) | 164 (37.3) | 76 (34.7) | 88 (39.8) | 0.268 |
| DAPSA moderate disease activity (>14 and ≤28) | 104 (23.6) | 57 (26.0) | 47 (21.3) | 0.241 |
| DAPSA high disease activity (>28), | 72 (16.4) | 49 (22.4) | 23 (10.4) | 0.001 |
| MDA 5/7 met | 171 (37.8) | 58 (25.7) | 113 (50.0) | < 0.001 |
| MDA 7/7 met (VLDA) | 57 (12.6) | 13 (5.8) | 44 (19.5) | < 0.001 |
| CRP >5 mg/L | 175 (39.5) | 100 (45.2) | 75 (33.8) | 0.014 |
| Current bDMARD | 236 (51.5) | 124 (54.4) | 112 (48.7) | 0.224 |
Notations:
The Functional (Groll) comorbidity index, includes obesity (19).
Significance for difference between men and women determined by Wilcoxon Rank test. Bold font designates significant p-values.
Abbreviations: SD standard deviation, TJC68 tender joint count range 0–68, SJC66 swollen joint count range 0–66, LEI Leeds enthesitis index range 0–6, BSA body surface area range 0–100%, NRS numeric rating scale range 0–10, HAQ-DI Health Assessment Questionnaire Disability index range 0–3, PsAID Psoriatic Arthritis Impact of Disease range 0–10, DAPSA Disease activity in Psoriatic Arthritis range 0–270, MDA Minimal Disease Activity, VLDA Very Low Disease Activity, CRP C-Reactive Protein, bDMARD biologic Disease Modifying Rheumatic Drug. Missing values: 9 women and 9 men had missing values for DAPSA; missing values for (women, men): TJC68 (0,2), SJC66 (0,2), Pain VAS (1,1), Patient global VAS (1,2), CRP (7,8)
PsA measures in men and women
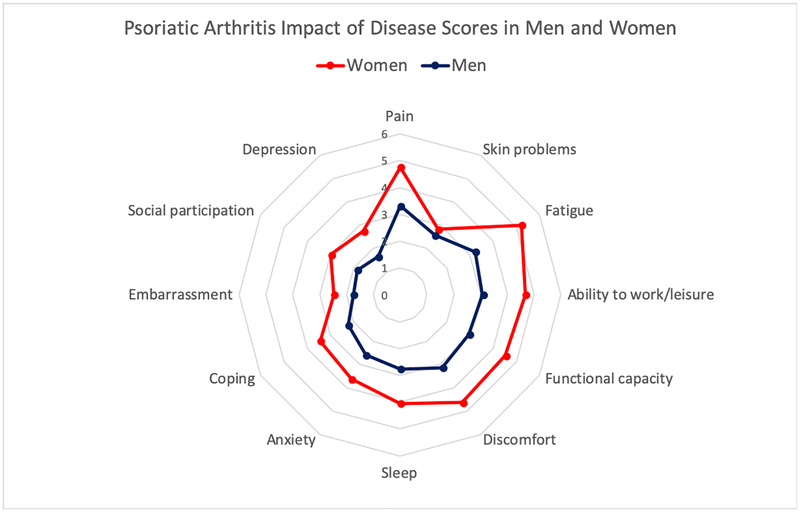
Musculoskeletal disease activity was moderate: mean (SD) TJC68 was 4.6 (9.4), SJC66 was 2.0 (6.2), LEI was 0.6 (1.4), and CRP>5mg/L was present in 39.5%. Swollen and tender joint counts were similar in men and women, while enthesitis was significantly worse in women as a group (see Table 1). Percentages with psoriasis BSA> 5% were not different between men and women, similar to other PsA populations in rheumatology practices. PROs were significantly higher in women versus men: PGA 4.8 (2.6) versus 3.6 (2.7), HAQ-DI 0.9 (0.7) versus 0.5 (0.6) and PsAID 4.1 (2.4) versus 2.8 (2.3), p <0.001 for all (Table 1). For individual NRS scale components of the PsAID, scores were systematically higher in women (all p <0.01) except for skin problems (2.8 (3.0) females, 2.6 (2.6) males, p=0.38) (Figure 1). In the subgroup of participants who intensified treatment for active disease at baseline, group-level improvements were larger in women than men at the second visit for both HAQ-DI and PsAID (Table 2).
Figure 1:
Psoriatic Arthritis Impact of Disease (PsAID) individual numerical rating scale (NRS) mean scores in women (n=228) versus men (n=230). All mean scores were significantly different between women and men (p<0.01) except for the ‘Skin problems’ NRS (p=0.32). Score ranges are 0–10 where 0 is best and 10 is worst.
Table 2.
Change scores in men and women who intensified treatment for active disease at baseline
| Change scores (visit1-visit2) * Mean (SD) | Men (n=52) | Women (n=61) | p-value |
|---|---|---|---|
| DAPSA | 9.5 (25.0) | 13.4 (19.0) | 0.423 |
| TJC68 | 2.9 (16.8) | 4.1 (14.1) | 0.709 |
| SJC66 | 2.2 (5.5) | 2.4 (4.6) | 0.807 |
| LEI | 0.1 (1.8) | 0.6 (2.0) | 0.168 |
| Pain VAS | 1.5 (4.0) | 2.3 (3.5) | 0.317 |
| Patient global VAS | 1.5 (4.1) | 1.9 (3.4) | 0.613 |
| HAQ-DI | 0.0 (0.9) | 0.5 (0.9) | 0.003 |
| PsAID | 0.6 (3.5) | 2.1 (3.1) | 0.031 |
| CRP (mg/L) | 0.4 (7.2) | 0.4 (2.5) | 0.948 |
Positive change scores show improvement. Bold font designates significant p-values.
Abbreviations: SD standard deviation, TJC68 tender joint count range 0–68, SJC66 swollen joint count range 0–66, LEI Leeds enthesitis index range 0–6, BSA body surface area range 0–100%, VAS visual analog scale range 0–10, HAQ-DI Health Assessment Questionnaire Disability index range 0–3, PsAID Psoriatic Arthritis Impact of Disease range 0–10, CRP C-Reactive Protein.
Missing values for (women, men): TJC68 (0,2), SJC66 (0,2), Pain VAS (1,1), Patient global VAS (1,2), CRP (7,8)
PsA treat-to-target state and life impact
Overall, 57.1% participants had DAPSA levels ≤14 and 37.8% were in MDA, fulfilling remission and low disease activity criteria. MDA was less often reached in women: 25.7% females versus 50.0% males (p<0.001). Mean DAPSA disease activity was higher in women versus men: 16.9 (14.9) versus 12.6 (16.6) (p=0.004). There were gender differences in the unique components of treatment targets between men and women. Women at DAPSA treatment target (score ≤14) had higher TJC68, pain and patient global assessment scores than men at the same treatment target. There was no gender difference for DAPSA components when the treatment target was not met (Table 3). Women versus men at MDA treatment target were less likely to meet the patient global criterion (score ≤2) and the HAQ-DI criterion (score ≤0.5). When not in MDA women were still less likely than men to meet a HAQ-DI score ≤0.5 (Table 4).
Table 3.
DAPSA components in women (n=219) and men (n=221) with PsA by treatment target state
| Target status | DAPSA≤14 (at target) | DAPSA>14 (not at target) | ||||
|---|---|---|---|---|---|---|
| DAPSA variables Mean (SD) | Women (n=103) | Men (n=148) | p-value | Women (n=116) | Men (n=73) | p-value |
| TJC68 | 0.8 (1.2) | 0.5 (1.0) | 0.028 | 9.6 (11.3) | 10.3 (14.6) | 0.745 |
| SJC66 | 0.5 (1.1) | 0.3 (0.8) | 0.193 | 3.6 (7.5) | 5.4 (11.2) | 0.214 |
| Pain VAS | 2.7 (1.9) | 2.1 (1.7) | 0.018 | 6.6 (2.0) | 6.0 (2.3) | 0.112 |
| Patient global VAS | 3.0 (2.0) | 2.2 (1.9) | 0.003 | 6.4 (2.0) | 6.0 (2.3) | 0.167 |
| CRP (mg/L) | 0.6 (1.3) | 0.6 (1.3) | 0.665 | 3.1 (8.9) | 2.3 (5.7) | 0.452 |
Bold font designates significant p-values. Abbreviations: SD standard deviation, TJC68 tender joint count range 0–68, SJC66 swollen joint count range 0–66, LEI Leeds enthesitis index range 0–6, BSA body surface area range 0–100%, VAS visual analog scale range 0–10, HAQ-DI Health Assessment Questionnaire Disability index range 0–3, PsAID Psoriatic Arthritis Impact of Disease range 0–10, CRP C-Reactive Protein.
Missing values (9 women and 9 men had missing values for DAPSA, missing for (women, men): TJC68 (0,2), SJC66 (0,2), Pain VAS (1,1), Patient global VAS (1,2), CRP (7,8)
Table 4.
MDA components in women (n=226) and men (n=226) with PsA by treatment target state
| Target status | MDA met (at target) | MDA not met (not at target) | ||||
|---|---|---|---|---|---|---|
| MDA variables N(%) | Women (n=58) | Men (n=113) | p-value | Women (n=168) | Men (n=113) | p-value |
| TJC68 ≤1 | 56 (96.6) | 105 (92.9) | 0.341 | 41 (36.3) | 41 (36.3) | 0.174 |
| SJC66 ≤1 | 54 (93.1) | 106 (93.8) | 0.863 | 58 (51.3) | 59 (50.4) | 0.248 |
| LEI ≤1 | 56 (96.6) | 110 (97.3) | 0.320 | 120 (71.4) | 92 (81.4) | 0.087 |
| Pain VAS ≤1.5 | 32 (55.2) | 65 (57.5) | 0.771 | 4 (2.4) | 1 (0.9) | 0.355 |
| Patient global VAS≤2 | 33 (56.9) | 85 (75.2) | 0.011 | 16 (9.5) | 13 (11.5) | 0.594 |
| HAQ-DI ≤0.5 | 50 (86.2) | 110 (97.3) | 0.005 | 34 (20.2) | 41 (36.3) | 0.003 |
| BSA =0 | 29 (50.0) | 48 (42.5) | 0.352 | 53 (31.5) | 30 (26.5) | 0.369 |
| BSA >0 and ≤5 | 26 (44.8) | 63 (55.8) | 0.178 | 87 (51.8) | 57 (50.4) | 0.826 |
Bold font designates significant p-values. Abbreviations: TJC68 tender joint count range 0–68, SJC66 swollen joint count range 0–66, LEI Leeds enthesitis index range 0–6, VAS visual analog scale range 0–10, HAQ-DI Health Assessment Questionnaire Disability index range 0–3, BSA body surface area range 0–100%, bDMARD biologic Disease Modifying Rheumatic Drug.
Missing values for (women, men): TJC68 (0,2), SJC66 (0,2), Pain VAS (1,1), Patient global VAS (1,2)
In DAPSA remission and low disease activity, mean PsAID (SD) scores were 2.68 (1.96) in females and 1.65 (1.38) in males (p<0.001). In moderate and high disease activity, mean PsAID (SD) scores were 5.32 (2.16) in females and 4.80 (2.28) in males (p=0.117) (Supplement Figure 2).
Link between gender and life impact
In the simple regression model adjusted for age and PsA disease duration, female gender was significantly associated with high PsAID score independent of follow-up time between the consecutive visits [OR 2.71; 95%CI (1.85–3.97), p<0.001]. In the more complex multivariate regression model, built on the initial model, high life impact was associated with female gender [OR 2.30; 95%CI (1.49–3.55), p<0.001], LEI [OR 1.34; 95%CI (1.14–1.57), p<0.001], TJC68 [OR 1.10; 95%CI (1.06–1.14), p<0.001], and comorbidity score [OR 1.22; 95%CI (1.07–1.39), p=0.002]; and was independent of SJC66, psoriasis, CRP, biologic use, and follow-up time between the consecutive visits. We identified a small interaction term between gender and follow-up time, significant in the linear regression, suggesting that the PsAID score decreased by 0.18 points more per month in women than in men. This coefficient became smaller (0.12) after adjustment for covariates. Predictors identified were otherwise consistent between the logistic regression and linear regression models (Table 5).
Table 5.
The association of gender with PsA life impact and disease activity over time among 458 psoriatic arthritis patients.
| Model covariates | Logistic regression Outcome PsAID≥4, OR (95% CI), p-value | Linear regression Outcome PsAID Beta coefficient (95% CI), p-value | ||
|---|---|---|---|---|
| Simple models adjusted for age and PsA duration | ||||
| Gender | 2.71 (1.85, 3.97) | <0.001 | 1.37 (0.94, 1.79) | <0.001 |
| Follow-up time (months) | 0.96 (0.88, 1.04) | 0.306 | −0.01 (−0.09, 0.07) | 0.769 |
| Follow-up time × Gender* | 0.91 (0.81, 1.02) | 0.095 | −0.18 (−0.29, −0.06) | 0.003 |
| Multivariable models adjusted for age and PsA duration | ||||
| Gender* | 2.3 (1.49, 3.55) | <0.001 | 0.99 (0.60, 1.38) | <0.001 |
| Follow-up time (months) | 0.93 (0.85, 1.03) | 0.170 | −0.02 (−0.10, 0.05) | 0.598 |
| Follow-up time × Gender | 0.94 (0.82, 1.07) | 0.335 | −0.12 (−0.23, −0.02) | 0.023 |
| Comorbidity | 1.22 (1.07, 1.39) | 0.002 | 0.18 (0.08, 0.28) | 0.001 |
| TJC68 | 1.10 (1.06, 1.14) | < 0.001 | 0.08 (0.06, 0.10) | < 0.001 |
| SJC66 | 1.02 (0.98, 1.07) | 0.399 | 0.02 (−0.01, 0.06) | 0.125 |
| LEI | 1.34 (1.14, 1.57) | < 0.001 | 0.37 (0.24, 0.50) | < 0.001 |
| Psoriasis BSA >5% | 0.99 (0.96, 1.02) | 0.567 | 0.00 (−0.03, 0.03) | 0.949 |
| CRP (mg/dL) | 1.04 (0.26, 1.75) | 0.891 | 0.45 (−0.01, 0.91) | 0.054 |
| Current bDMARD | 1.23 (0.87, 1.75) | 0.244 | −0.06 (−0.36, 0.25) | 0.722 |
Notations:
interaction term between follow-up time in weeks and gender. Bold font designates significant p-values. Abbreviations: TJC68 tender joint count range 0–68, SJC66 swollen joint count range 0–66, LEI Leeds enthesitis index range 0–6, VAS visual analog scale range 0–10, HAQ-DI Health Assessment Questionnaire Disability index range 0–3, BSA body surface area range 0–100%, bDMARD biologic Disease Modifying Rheumatic Drug.
Missing values for (women, men): TJC68 (0,2), SJC66 (0,2), Pain VAS (1,1), Patient global VAS (1,2)
In separate regression models for each gender we observed that life impact was independently associated with the TJC68 in both men [OR 1.07; 95%CI (1.03–1.12), p=0.002] and women [OR 1.13; 95%CI (1.08–1.19), p<0.001], consistent with the general model above, however the association was stronger for women. In women as a group, but not in men, life impact was inversely independently associated with follow-up time [OR 0.88; 95%CI (0.81–0.96), p=0.005] (Supplement Table 2). In men as a group, but not in women, life impact was independently associated with LEI [OR 1.63; 95%CI (1.25–2.13), p<0.001], comorbidities [OR 1.31; 95%CI (1.06–1.63), p=0.013], and biologic use [OR 1.85; 95%CI (1.06–3.23), p=0.029] (Supplement Table 3). In linear regression models life impact increased with enthesitis, comorbidities, and the 68 tender joint count for both men and women; while for women but not for men, life impact increased with body surface area affected by psoriasis, and decreased with biologic use and follow-up time (Supplement Tables 2 and 3).
DISCUSSION
PsA is a heterogeneous rheumatologic disease that affects men and women in equal numbers but not necessarily resulting in equal disease burden or treatment responses. In this study, women were less likely than men to be at PsA treatment targets, and had higher PsA disease activity, comorbidities, and life impact. Separate treat-to-target components were more difficult to achieve in women than men for the tender joint count, pain, patient global and physical function/disability as measured by the HAQ-DI. In the treat-to-target state, PsA specific life impact, measured by the PsAID, was worse in women compared to men, showing that women were disadvantaged for life impact even when they achieved treatment targets. Our findings are consistent with other studies (2–9) and confirm that our current treatment strategies are not sufficient to bridge the life impact gap between women and men.
We further examined associations of life impact in multivariate logistic and linear regression models including disease activity, comorbidities, treatment, and follow-up time. The association remained significant with excess life impact in women. In addition to gender, the TJC68, LEI, and comorbidities were independent predictors of life impact. While women in the ReFlaP study, on average, were not on less bDMARD treatment than men, we have to consider this in context of their disease burden, which raises the question of either differential response to treatment, gender-based treatment bias, or both (27). Differential response to treatment between women and men is an intriguing hypothesis (7). A national PsA registry study in Denmark showed incremental responses to TNF inhibitors were consistently higher in men versus women and that men had significantly higher odds of achieving treatment response across a range of response definitions (7). Women had higher disease activity and worse physical function/disability (HAQ-DI) at baseline; as a consequence, women not only did not overcome their disadvantage present at baseline, instead the outcomes gap for both disease activity and physical function/disability became wider over time. In addition to less treatment effectiveness women also had more adverse events than men (7). Not surprisingly, average biologic retention (median TNF inhibitor persistence) in this study was 3.8 years (95% CI: 3.0, 5.7) in men versus 1.4 (1.1, 1.8) in women (p<0.001), and has been confirmed in other studies (7, 8, 12, 13). In the Swedish PsA registry, five-year improvements in treated PsA favored men for the tender joint count and HAQ-DI, and women for pain, however women were still disadvantaged across outcomes at the end of follow up (4). Regarding gender-based treatment-bias, a study in rheumatoid arthritis (RA) showed that, at biologic treatment initiation, women had higher levels of disease activity by patient reported measures than men (28) and that physician measures were better aligned with patient reported measures in men versus women. In RA a dual treat-to-target strategy is being explored (29) which would consider symptoms concomitantly with traditional remission definitions. In the ReFlaP study women had worse status than men, while at the same time, women who changed treatment for active disease improved significantly more than men on physical function and life impact scores. However, our study was not designed to assess treatment specific effects.
The study has limitations. Although estimates are adjusted for comorbidities, disease factors and treatment, comorbidities were only assessed through a simple list (30). Disease duration in ReFlaP was on average 11 years and therefore findings may not be generalizable to early PsA populations. Roughly half of the patients were treated with bDMARDs which limits generalizability to PsA cohorts with smaller prevalence of bDMARD treatment. The prevalence of moderate/severe psoriasis was low in this study which is consistent with other rheumatology clinic populations but may limit generalizability to those with more significant skin disease. There was a single follow-up visit and therefore long-term trends could not be assessed. Strengths of the study consist in the multicenter international sample, representative of the spectrum of PsA disease burden and treatment patterns, and collection of comprehensive PsA clinical data and validated disease activity measures for PsA.
The present findings have practical clinical implications. Treat-to-target in clinical practice may reduce outcome differences between men and women, and improve life impact in both genders. However, we found differential life impact in men and women who were at treatment target, with higher life impact in women. This is an important finding identifying the need to diversify PsA management through inclusion of life impact as a treatment goal, concomitantly with disease activity specific treat-to-target strategies.
Supplementary Material
Supplement Figure 2: DASPA and PsAID score distributions in men and women with PsA. Panel a) DAPSA scores by gender; b) PsAID scores by gender; c) PsAID scores in DAPSA low disease activity and remission (DAPSA ≤14); Panel d) PsAID scores in DAPSA moderate and high disease activity (DAPSA >14).
Significance and Innovation.
Women were less likely to be at PsA-specific treatment targets of MDA and DAPSA remission/low disease activity than men.
Female gender, enthesitis, comorbidities, and tender joints were independently linked to high PsA life impact.
Gender needs to be considered in the implementation of treat-to-target in clinical practice: while women as a group had higher disease activity and life impact, they responded to change in therapy for active disease with significantly more improvement than men in physical function and life impact.
Life impact needs to be incorporated with the treat-to-target strategy in PsA in order to be addressed separately from disease activity.
Acknowledgments:
this study was funded by Pfizer.
AMO is a Jerome L. Greene Foundation Scholar and is supported in part by a research grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award number P30-AR070254. JP is supported in part by a research grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award number P30-AR070254. LCC is funded by a National Institute for Health Research Clinician Scientist award. The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. YYL is funded by the Clinician Scientist award of the National Medical Research Council, Singapore (NMRC/CSA-INV/0022/2017). The views expressed are those of the author(s) and not necessarily those of the NMRC.
Contributor Information
Ana-Maria Orbai, Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, USA.
Jamie Perin, Johns Hopkins Bloomberg School of Public Health, Department of International Health, Baltimore, MD, USA.
Clémence Gorlier, Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris France,; Pitié Salpêtrière hospital, AP-HP, Rheumatology department, Paris, France.
Laura C. Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Uta Kiltz, Rheumazentrum Ruhrgebiet, Herne and Ruhr-University Bochum, Germany.
Ying Ying Leung, Singapore General Hospital, Duke-NUS Medical School, Singapore.
Penelope E. Palominos, Hospital de Clinicas de Porto Alegre, Porto Alegre, Brazil.
Juan D Cañete, Hospital Clínic and IDIBAPS, Arthritis Unit, Rheumatology Department, Barcelona, Spain.
Rossana Scrivo, Rheumatology Unit, Department of Internal Medicine and Medical Specialties, Sapienza Università di Roma, Rome, Italy.
Andra Balanescu, Sf Maria Hospital, University of Medicine and Pharmacy Carol Davila, Bucharest, Romania.
Emmanuelle Dernis, Le Mans Central Hospital, Le Mans, France.
Sandra Tälli, East-Tallinn Central Hospital, Tallinn, Estonia.
Adeline Ruyssen-Witrand, Rheumatology Unit, Toulouse University Hospital, UMR 1027, Inserm, Université Paul Sabatier Toulouse III, Toulouse, France.
Martin Soubrier, Gabriel Montpied Hospital, Clermont Ferrand, France.
Sibel Aydin, University of Ottawa, the Ottawa Hospital Research Institute, Ottawa, Canada.
Lihi Eder, Women’s College Hospital, University of Toronto, Toronto, ON, Canada.
Inna Gaydukova, North-western State medical university, St.Petersburg, Russia.
Ennio Lubrano, Academic Rheumatology Unit, Dipartimento di Medicina e Scienze della Salute “Vincenzo Tiberio”, University of Molise,Campobasso, Italy..
Umut Kalyoncu, Hacettepe University Faculty of Medicine, Department of Internal Medicine, Division of Rheumatology, Ankara, Turkey.
Pascal Richette, Hopital Lariboisiere Centre Viggo Petersen, service de Rhumatologie, Paris, France; Universite Paris Diderot UFR de Medecine, Inserm UMR1132 Bioscar, Paris France.
M. Elaine Husni, Cleveland Clinic, Department of rheumatic and Immunologic Diseases, Cleveland, USA.
Josef S. Smolen, Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Vienna, Austria.
Maarten de Wit, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, Netherlands.
Laure Gossec, Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris France,; Pitié Salpêtrière hospital, AP-HP, Rheumatology department, Paris, France.
REFERENCES
- 1.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65 e19. [DOI] [PubMed] [Google Scholar]
- 2.Queiro R, Sarasqueta C, Torre JC, Tinture T, Lopez-Lagunas I. Comparative analysis of psoriatic spondyloarthropathy between men and women. Rheumatol Int. 2001;21(2):66–8. [DOI] [PubMed] [Google Scholar]
- 3.Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis. 2013;72(4):578–82. [DOI] [PubMed] [Google Scholar]
- 4.Theander E, Husmark T, Alenius GM, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis. 2014;73(2):407–13. [DOI] [PubMed] [Google Scholar]
- 5.Nas K, Capkin E, Dagli AZ, Cevik R, Kilic E, Kilic G, et al. Gender specific differences in patients with psoriatic arthritis. Mod Rheumatol 2017;27:345–349. [DOI] [PubMed] [Google Scholar]
- 6.Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical Characteristics, Disease Activity, and Patient-Reported Outcomes in Psoriatic Arthritis Patients With Dactylitis or Enthesitis: Results From the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res (Hoboken). 2017;69(11):1692–9. [DOI] [PubMed] [Google Scholar]
- 7.Hojgaard P, Ballegaard C, Cordtz R, Zobbe K, Clausen M, Glintborg B, et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology (Oxford). 2018;57(9):1651–60. [DOI] [PubMed] [Google Scholar]
- 8.Vieira-Sousa E, Eusébio M, Ávila-Ribeiro P, Khmelinskii N, Cruz-Machado R, Rocha TM, et al. Real-World Long-Term Effectiveness of Tumor Necrosis Factor Inhibitors in Psoriatic Arthritis Patients from the Rheumatic Diseases Portuguese Register. J Rheumatol 2019. [DOI] [PubMed] [Google Scholar]
- 9.Wallenius M, Skomsvoll JF, Koldingsnes W, Rodevand E, Mikkelsen K, Kaufmann C, et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis. 2009;68(5):685–9. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Brubacher B, Buskila D, Langevitz P, Farewell VT. Psoriatic spondyloarthropathy in men and women: a clinical, radiographic, and HLA study. Clin Invest Med. 1992;15(4):371–5. [PubMed] [Google Scholar]
- 11.Geijer M, Lindqvist U, Husmark T, Alenius GM, Larsson PT, Teleman A, et al. The Swedish Early Psoriatic Arthritis Registry 5-year Followup: Substantial Radiographic Progression Mainly in Men with High Disease Activity and Development of Dactylitis. J Rheumatol. 2015;42(11):2110–7. [DOI] [PubMed] [Google Scholar]
- 12.Stober C, Ye W, Guruparan T, Htut E, Clunie G, Jadon D. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology (Oxford). 2018;57(1):158–63. [DOI] [PubMed] [Google Scholar]
- 13.Fagerli KM, Kearsley-Fleet L, Watson KD, Packham J, Contributors Group BR, Symmons DPM, et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. Data from the British Society for Rheumatology Biologics Register. RMD Open. 2018;4(1):e000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren RB, Marsden A, Tomenson B, Mason KJ, Soliman MM, Burden AD, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–52. [DOI] [PubMed] [Google Scholar]
- 16.van den Reek J, van Vugt LJ, van Doorn MBA, van der Kraaij GE, de Kort WJA, Lucker GPH, et al. Initial Results of Secukinumab Drug Survival in Patients with Psoriasis: A Multicentre Daily Practice Cohort Study. Acta Derm Venereol. 2018;98(7):648–54. [DOI] [PubMed] [Google Scholar]
- 17.Hagg D, Sundstrom A, Eriksson M, Schmitt-Egenolf M. Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am J Clin Dermatol. 2017;18(4):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlier C, Orbai AM, Puyraimond-Zemmour D, Coates LC, Kiltz U, Leung YY, et al. Comparing patient-perceived and physician-perceived remission and low disease activity in psoriatic arthritis: an analysis of 410 patients from 14 countries. Ann Rheum Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. [DOI] [PubMed] [Google Scholar]
- 22.Shan J, Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: A systematic review and meta-analysis. Joint Bone Spine. 2018. [DOI] [PubMed] [Google Scholar]
- 23.Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–9. [DOI] [PubMed] [Google Scholar]
- 24.Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–8. [DOI] [PubMed] [Google Scholar]
- 25.Coates LC, Navarro-Coy N, Brown SR, Brown S, McParland L, Collier H, et al. The TICOPA protocol (TIght COntrol of Psoriatic Arthritis): a randomised controlled trial to compare intensive management versus standard care in early psoriatic arthritis. BMC Musculoskelet Disord. 2013;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orbai AM, Holland R, Leung YY, Tillett W, Goel N, Christensen R, et al. PsAID12 Provisionally Endorsed at OMERACT 2018 as Core Outcome Measure to Assess Psoriatic Arthritis-specific Health-related Quality of Life in Clinical Trials. J Rheumatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reygaerts T, Mitrovic S, Fautrel B, Gossec L. Effect of biologics on fatigue in psoriatic arthritis: A systematic literature review with meta-analysis. Joint Bone Spine. 2018;85(4):405–10. [DOI] [PubMed] [Google Scholar]
- 28.Arkema EV, Neovius M, Joelsson JK, Simard JF, van Vollenhoven RF. Is there a sex bias in prescribing anti-tumour necrosis factor medications to patients with rheumatoid arthritis? A nation-wide cross-sectional study. Ann Rheum Dis. 2012;71(7):1203–6. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira RJO, Ndosi M, de Wit M, Santos EJF, Duarte C, Jacobs JWG, et al. Dual target strategy: a proposal to mitigate the risk of overtreatment and enhance patient satisfaction in rheumatoid arthritis. Ann Rheum Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Daien CI, Tubery A, Beurai-Weber M, du Cailar G, Picot MC, Jaussent A, et al. Relevance and feasibility of a systematic screening of multimorbidities in patients with chronic inflammatory rheumatic diseases. Joint Bone Spine. 2019;86(1):49–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 2: DASPA and PsAID score distributions in men and women with PsA. Panel a) DAPSA scores by gender; b) PsAID scores by gender; c) PsAID scores in DAPSA low disease activity and remission (DAPSA ≤14); Panel d) PsAID scores in DAPSA moderate and high disease activity (DAPSA >14).