Abstract
Background:
African Americans have lower rates of kidney transplantation (KT) compared to Whites, even after adjusting for demographic and medical factors. In this study, we examined whether racial disparity in KT wait-listing persists after adjusting for social determinants of health (e.g. cultural, psychosocial, knowledge).
Methods:
We prospectively followed a cohort of 1055 patients who were evaluated for KT between 3/10–10/12 and followed through 8/18. Participants completed a semi-structured telephone interview shortly after their first KT evaluation appointment. We used Wilcoxon rank-sum and Pearson chi-square tests to examine race differences in the baseline characteristics. We then assessed racial differences in the probability of wait-listing while accounting for all predictors using cumulative incidence curves and Fine & Gray proportional subdistribution hazards models.
Results:
There were significant differences in the baseline characteristics between non-Hispanic African Americans (AA) and non-Hispanic Whites (WH). AA were 25% less likely (95% confidence interval, 0.60–0.96) to be wait-listed than WH even after adjusting for medical factors and social determinants of health. In addition, being older, having lower income, public insurance, more comorbidities, and being on dialysis decreased the probability of wait-listing while having more social support and transplant knowledge increased the probability of wait-listing.
Conclusion:
Racial disparity in kidney transplant wait-listing persisted even after adjusting for medical factors and social determinants of health, suggesting the need to identify novel factors that impact racial disparity in transplant wait-listing. Developing interventions targeting cultural and psychosocial factors may enhance equity in access to transplantation.
1. Introduction
Kidney transplantation (KT) is the treatment of choice for patients with end stage renal disease (ESRD) and has been associated with improved patient survival and quality of life compared to remaining on dialysis.1 African Americans (AA) are four times more likely than Whites (WH) to develop ESRD but only half as likely to receive KT.2 This disparity exists in every stage of the transplant process (from referral to receipt of KT).3 Although there have been numerous prior studies demonstrating AA versus WH racial disparities in referral for KT in dialysis patients, besides our work,4,5 only Sequist et al.6 and Patzer et al.7 examined disparities that occur after referral for KT (but before KT acceptance). Both found that minorities were less likely than WH to be placed on a waiting list and undergo KT. This finding speaks to the importance of our work focusing on disparities in processes occurring after referral to a transplant center rather than only on the referral itself. We aimed to identify factors and strategies that can be targeted in the transplant clinic to facilitate completing the transplant work up and increase the probability of wait-listing.
Medical factors such as medical comorbidities and Human Leukocyte Antigen (HLA) matching, as well as social determinants of health including geography, insurance type and socio-economic status (SES),3–5,7–9 have been shown to impact racial disparity between WH and racial minorities (AA, Hispanics, etc.) in KT. In 2003, the United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) changed the kidney allocation policy to eliminate points for HLA-B matching. This policy change resulted in a 23% reduction in the disparity of KT rates between AA and WH.10 The new kidney allocation system (KAS) implemented in December 2014 includes time on dialysis in the calculation of KT wait-time with the aim of increasing equity in allocation, particularly for ethnic minorities who have been disproportionately affected by delayed referrals.11,12 Since this policy change, the difference in the wait-listing rates between AA and WH decreased from 19% pre-KAS to 12% post KAS. This difference is mainly due to the decrease in early wait-listing of WH patients as there is no longer an incentive for early wait-listing in dialysis patients. Of note, racial disparity between WH and racial minorities (AA, Hispanics, etc.) in kidney transplant wait-listing have persisted even under the new KAS13 suggesting that there are other factors that may be perpetuating racial disparity in kidney transplant wait-listing.
Using a biopsychosocial model14 to inform our conceptual approach (Figure 1) and informed by the Center for Health Equity Research and Promotion model15 of key potential determinants of health disparities within the healthcare system, we selected culturally-related factors4,5 and psychosocial characteristics16 that have been shown to contribute to healthcare disparities across racial/ethnic groups. Although such factors have been studied in other clinical populations,17,18 ours was the first to use a prospective study design to examine these factors as potential contributors to racial disparity in KT wait-listing.19,20 Thus, we wanted to examine whether these variables account for the relationship between race and wait-listing.
Figure 1:
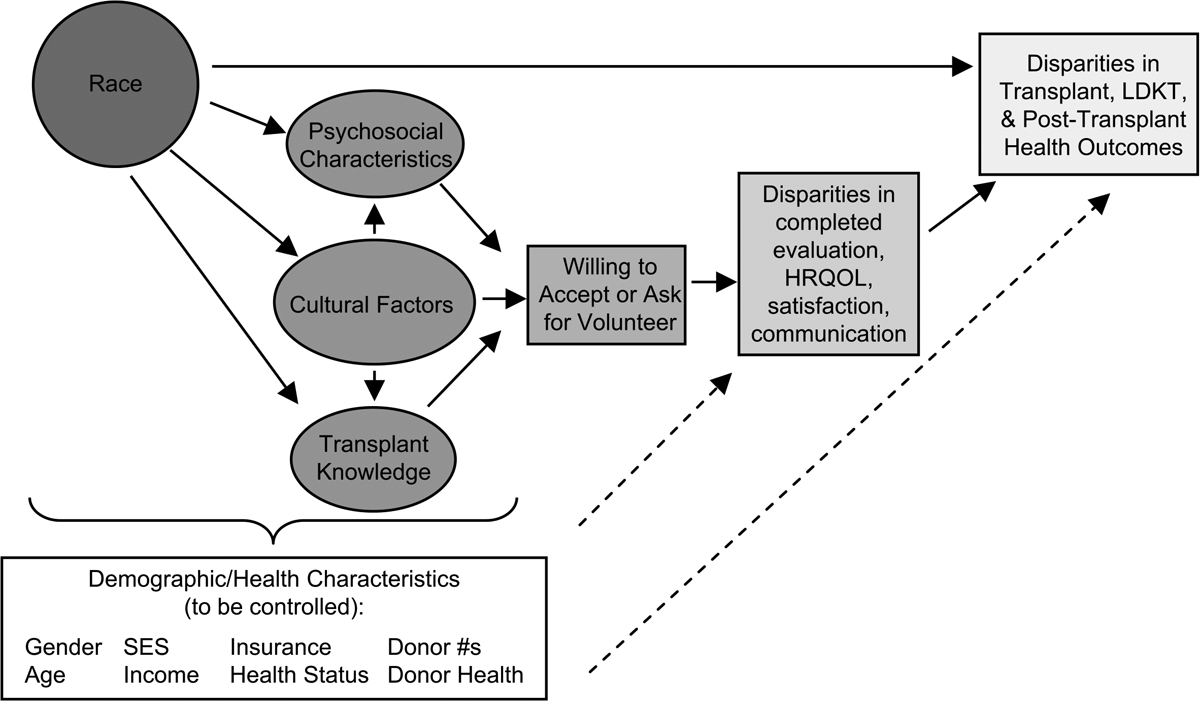
Conceptual model of the relationship between non-medical factors and transplant outcomes
Our previous pilot work demonstrated that psychosocial and cultural factors, including perceived racism, medical mistrust, religiosity and family loyalty, were more prevalent in AA and were associated with longer time to wait-listing.5 That study, however, was limited by a small sample size. In the current study, we conducted a large-scale prospective cohort study of ESRD patients, who were being evaluated for KT, to assess whether racial disparity persists after accounting for culturally-related factors, transplant-related beliefs and psychosocial characteristics (while controlling for demographics and medical factors).
2. Materials and Methods:
Study design
This prospective cohort study was conducted at the Starzl Transplant Institute at the University of Pittsburgh Medical Center (UPMC) kidney transplant clinic. All participants who were evaluated in clinic and provided informed consent were recruited in the study. Participants completed a semi-structured telephone interview (~1 hour) shortly after their first KT evaluation appointment. The interview included several existing valid measures4,5 and was conducted by research interviewers from the Survey Research Program (SRP) at the University of Pittsburgh Center for Social and Urban Research (UCSUR). We prospectively tracked participants via medical record until they were accepted, found ineligible for transplant, or the end of the follow up period (08/18). Data analysis for this study was performed at the University of New Mexico. This study was approved by the Institutional Review Boards (IRB) at the University of Pittsburgh (PRO09060113) and the University of New Mexico (17–084) and a data use agreement was signed between the two institutions. The study was conducted in accordance to the Declaration of Helsinki and are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Study sample
Inclusion criteria were: 1) age 18 and over; 2) English speaking; 3) referred for KT. Patients were excluded if they had received a KT previously (to reflect national data on the majority of transplant recipients who are first-time recipients21 and prevent patients’ previous experience with KT from influencing current outcomes), had a cognitive or sensory impairment (such as blindness or deafness) that prevented them from completing an interview, or if they were judged by the clinic staff at the time of their initial clinic appointment to be ineligible to continue with the KT evaluation process (i.e., because they were too ill - in these cases, patients were triaged from the clinic and never initiated transplant evaluation, thus we could not approach them to participate in the study).
In total, 1726 KT candidates were referred to UPMC transplant center for transplant evaluation. Of these, 389 were not eligible for participation (301 had had a previous kidney transplant, 52 were determined to be ineligible for transplantation at the time of their initial clinic appointment and were triaged before initiating the KT evaluation process, 21 had a cognitive or sensory impairment, and 15 did not speak English). The remaining 1337 patients were eligible to participate in our study. Of these, 185 were not able to be enrolled (176 refused, 6 had no workable contact information and were thus lost, 2 were too ill and died prior to the first telephone interview, and 1 was inadvertently not approached by the transplant team personnel to gain permission for the research team to describe the study). Thus, 1152 patients (86.2% of those eligible) consented and completed the first interview. Due to small numbers (97; 8.42%) and significant heterogeneity within the “Other” group, we excluded them from further analysis, leaving 1055 WH and AA in the remaining sample.
Study measures
Predictor Variables
We provide extended descriptions, ranges, and psychometric properties of all predictor variables in Table 1.
Table 1.
Potential predictors of transplant outcomes
| Predictor Categories | Variables | Description and scoring |
|---|---|---|
| Demographic characteristics | ||
| Race/ethnicity | During the first interview, respondents could select as many of the following race categories as appropriate: “American Indian or Alaska Native”, “Asian”, “Black or African American”, “Native Hawaiian or Other Pacific Islander”, “White”, or “Other”. If the “Other” category was specified, respondents had the opportunity to provide a verbatim response that was used to re-categorize any “Other” responses into a known category, if possible. For the purposes of this article, “Non-Hispanic African American” refers to respondents who self-identified with only one race group (“Black or African American”) and were also non-Hispanic. “Non-Hispanic White” refers to respondents who self-identified with only one race group (“White”) and were also non-Hispanic. “Hispanic” refers to respondents who were Hispanic (regardless of race). “Other Minorities” refers to non-Hispanic respondents who could not be directly classified as either “Non-Hispanic African American” or “Non-Hispanic White”. | |
| Gender | Categories listed in Table 2 | |
| Age | This variable was defined as the difference (in years) between the completion date of the first interview and the date of birth. | |
| Marital status | Married versus not | |
| Education | Categories listed in Table 2 | |
| Income | Categories listed in Table 2 | |
| Insurance status | During the first interview, respondents could select as many of the following current healthcare coverage categories as appropriate: “VA”, “Medicare”, “Medicaid”, “Private Health Insurance”, “Self-pay”, “None”, and “Other”. If the “Other” category was specified, respondents had the opportunity to provide a verbatim response that was used to re-categorize any “Other” responses into a known category, if possible. For purposes of this article, “Public” coverage included any mention of “VA”, “Medicare”, or “Medicaid” without any mention of “Private Health Insurance”. “Private Only” coverage included the reporting of “Private Health Insurance” without the mention of any public coverage (“VA”, “Medicare”, “Medicaid”). “Private and Public” coverage included the mention of “Private Health Insurance” along with at least one mention of “VA”, “Medicare”, or “Medicaid”. | |
| Occupation | During the first interview, respondents were asked if they currently had paid employment. If the answer was “yes”, the respondent was asked to provide a verbatim response describing the kind of the work they currently perform. If the answer was “no”, the respondent was asked to provide a verbatim response describing the kind of work they performed when they last worked. These verbatim responses were used to classify the occupation verbatim responses into a categorization based on the Hollingshead Occupational Scale as follows:
|
|
| Kidney Allocation System (KAS) changes | We added KAS as a co-variate to control for its potential effect in our model. | |
| Medical/Health Factors | ||
| Dialysis type | Center-based hemodialysis or peritoneal dialysis (at time of evaluation) | |
| Dialysis duration | Time on dialysis (at time of evaluation). Because dialysis duration was skewed, we used established literature39 to determine the following categories for dialysis duration:
|
|
| Body mass index (BMI) | Calculated with patient height and weight using NHLBI’s calculator available at: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm | |
| Perceived burden of kidney disease40 | Participants rated the extent to which they felt burdened by their kidney disease on a scale of 1 (definitely true) to 5 (definitely false) for each item (e.g., “My kidney disease interferes with my life”). We calculated an overall mean score for this variable. Cronbach’s alpha for the current sample = 0.772. | |
| Charlson Comorbidity Index41 | For all study participants, inpatient and outpatient medical utilization records were examined for the purposes of calculating the Charlson Comorbidity Index. Any applicable ICD-9-CM code occurring no more than 12 months prior to presentation for evaluation at the Starzl kidney transplant center was utilized. | |
| Number of potential living donors in the patient’s social network42 | The network of potential living donors available for evaluation was determined by asking participants to indicate how many living relatives and friends they had aged 18–70 years, the age range of living kidney donors. Actual living donors were individuals who were undergoing, had already undergone, or were planning to undergo evaluation for living donation to a specific patient. For our analyses, we summed across these three groups for an overall number of living donors. | |
| Culturally-Related Factors | ||
| Experience of discrimination43 | Assessed with an adapted version of the perceived discrimination in healthcare measure. For this 7-item measure, participants indicate the extent to which they have experienced a set of discriminatory practices (e.g., “When getting healthcare, I was treated with less respect than other people because of my race or color.”), with a range of 1 (never) to 5 (always). We summed across these items for an overall experience of discrimination score. Cronbach’s alpha for the current sample = 0.909. | |
| Perceived racism44 | These items assess the extent to which patients believe that racism is common in healthcare, as opposed to having personal experience with racism in healthcare (e.g., “Doctors treat African American and White people the same.”). Item responses range from 1 (strongly disagree) to 5 (strongly agree). An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0746. | |
| Medical mistrust45 | Assesses the degree to which participants believe their hospital to be trustworthy, competent, and acting in their best interests (e.g., “I trust hospitals”; 1 = strongly disagree to 5 = strongly agree)]. An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.844. | |
| Trust in physicians46 | Assesses the degree of patient trust in their physician [e.g., “I doubt that my doctor really cares about me as a person”; range=1 (totally disagree) to 5 (totally agree)]. An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.796 | |
| Family loyalty47 | Assesses feelings of loyalty and mutual support regarding the family (e.g., “The family should consult close relatives (uncles, aunts, first cousins) concerning its important decisions”; range=1 (strongly disagree) to 5 (strongly agree)]. An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.802 | |
| Religious objections to LDKT48 | Assessed with a revised subscale of the Organ Donation Attitude Survey (ODAS). The ODAS was created by experts in the psychological evaluation of religious beliefs as a measure of individuals’ attitudes towards organ donation. We revised this 8-item scale to assess religious beliefs as they relate to living donor kidney transplantation (e.g., “I believe that living donor kidney transplantation is against my religion”; 1 (strongly disagree) to 5 (strongly agree)). | |
| Psychosocial Characteristics | ||
| Emotional distress49 | Measured with the anxiety and depression subscales of the Brief Symptom Inventory (BSI). Each subscale comprises 6 items related to either anxiety or depression (e.g., “Please indicate how bothered or distressed you have been by that feeling during the past two weeks: nervousness or shakiness inside”; 1 (not at all) to 5 (extremely)). An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.820 for anxiety, 0.815 for depression, and 0.829 for combined anxiety + depression. | |
| Social support50,51 | Measured with a 12-item version of the Interpersonal Support Evaluation List (ISEL-12). The ISEL assesses patients’ perceived availability of 3 separate functions of social support. The “tangible” subscale is intended to measure perceived availability of material aid; the “appraisal” subscale, the perceived availability of someone to talk to about one’s problems; and the “belonging” subscale, the perceived availability of people with whom one can do things [e.g., “I feel that there is no one I can share my most private worries and fears with;” 1(definitely false) to 4 (definitely true)]. An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.850. | |
| Self-esteem52 | Measured using the Rosenberg Self-Esteem Scale. The self-esteem scale assesses patients’ feelings of self-worth and self-respect (e.g., “I feel that I am a person of worth, at least on an equal plane with others”). Individual responses range from 1(strongly agree) to 4 (strongly disagree). An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.852. | |
| Locus of control53 | Assessed with the 18-item Multidimensional Health Locus of Control (MHLC) scales, Form C. The scale includes separate subscales to assess the extent to which recipients view their health condition is due to their own behavior (Internal Locus of Control) or the behavior of doctors, other people not including doctors, chance, luck, or fate (External Locus of Control). Responses to items range from 1 (strongly disagree) to 6 (strongly agree). An overall mean score was calculated for this variable. Cronbach’s alpha for the current sample = 0.760 for Internal Locus of Control and 0.813 for External Locus of Control. | |
| Transplant Knowledge, Concerns, and Preference | ||
| Transplant knowledge40,54 | Assessed with items adapted from the KT Knowledge Survey and the KT Questionnaire. This measure includes 27 multiple choice and true-false items. A summative score is created for the total number of items that patients answered correctly. | |
| Transplant learning activities | The type, number, and time spent in each educational activity were assessed by self-report. Patients were asked to indicate whether they had engaged in any of a list of activities to learn or think about transplantation (e.g., “Read brochures about kidney transplant from living donors”). Then, patients were asked to indicate how much time was spent on each of the activities that they checked. A summative score was calculated for the total number of items checked and total time spent on all learning activities. | |
| Transplant concerns40,54 | Assessed using 30 items adapted from the KT Questionnaire. This measure asks patients to indicate whether any of a list of concerns affected their decisions about getting a transplant, including concerns about transplant for themselves and concerns about the potential donors future health status. The items can be summed to indicate overall level of concern about transplantation, or examined individually in order to determine particular concern items that vary by race. | |
Note:
We included these measures because they (a) are widely used in organ donation and/or transplantation studies, other medical populations, or both; (b) have known psychometric properties, including (for scaled measures) Cronbach’s α’s of ~.80-.92 (see references cited with each instrument for psychometric data); and (c) used in our previous research.
Outcome variable: Acceptance for transplant wait-listing
The primary outcome variable, acceptance for transplant wait-listing was determined by chart review. Patients who were not wait-listed at the end of follow-up (08/18), were censored. To determine patients’ outcome status, we accounted for all possible transplant outcomes, calculated time from evaluation to time of outcome, and identified 7 potential categories:
Wait-listed = patients wait-listed after evaluation. (Endpoint = date of listing)
Deceased prior to wait-list = patients who were enrolled in the study but passed away before completion of evaluation. (Endpoint = date of death)
Closed patient choice prior to wait-list = patients who specifically verbalized desire not to pursue transplant any longer. (Endpoint = date record was closed)
Closed due to incomplete evaluation = patients who started an evaluation but were closed before being accepted or rejected- due to incomplete evaluation. (Endpoint = date of closure)
Clinic rejected prior to wait-list = patients who started an evaluation but were rejected for transplant or closed for reasons other than patient choice or incomplete evaluation, i.e. did not meet medical requirements, social requirements, etc. (Endpoint = date of rejection)
Still undergoing evaluation. (Endpoint = date of last follow-up on August 2018)
Transplanted at another center = a patient who did not complete their evaluation at UPMC because they received transplant from another center. (Endpoint= date of transplant)
Transplant at another center and death were considered as competing risks, and all other outcomes, other than wait-listing, were censored.
3. Statistical Analyses
We compared descriptive statistics of baseline and outcome characteristics between the two racial groups using Wilcoxon rank sum tests for continuous variables and Chi-square tests for categorical variables. Prior to statistical modeling via time-to-wait-listing analysis with competing risks, we assessed for multi-collinearity by computing correlation coefficients and multivariable variance inflation factors for all variables of interest. We identified no major concerns. We plotted cumulative incidence curves of time to acceptance for transplant wait-listing by race.
To address missing data in the multivariable analysis, we deleted individuals with missing data for any single variable from analysis. We verified this case-deletion strategy by including the data from all participants and incorporating missing data indicators. Because the results from both analyses were comparable, we reported the results from analyses on data from all participants.
For our primary multivariable analyses, to determine the degree to which each of the patient characteristics was associated with transplant wait-listing, we used multivariable Fine & Gray proportional subdistribution hazards regression models.22 We included all baseline characteristics in the multivariable models that were previously statistically significant (p<0.1) in bivariate models against time to wait-listing. To test our main hypothesis that social determinants of health account for the relationship between race and KT wait-listing in the presence of competing risks, we fit three nested models using a SAS macro.23,24 Model 1 was an unadjusted Fine & Gray model with race/ethnicity, for which we constructed a cumulative incidence curve. Model 2 was a multivariable Fine & Gray model including race/ethnicity, demographics, and medical factors. Model 3 included all variable in Model 2 as well as cultural, psychosocial, and transplant knowledge. Before testing our multivariable models, we assessed the proportional hazards modelling assumption. It is important to note that we are not making the case that social determinants of health operate differently for AA versus WH patients, as an interaction analysis would imply. Instead, we hypothesize that AA patients tend to have more of the variables that make KT wait-listing less likely and fewer of the variables that make KT more likely. Therefore, we hypothesize that differences in these key variables should account for differences in time to wait-listing. Thus a test of interaction effect would not be appropriate for this analysis.
4. Results
Baseline Characteristics
We found significant differences in the baseline characteristics between AA and WH in our sample (Table 2). In general, AA patients were younger, had lower status occupations, lower income, relied on public insurance and were less likely to be married compared to WH patients. AA patients also had more comorbidities, were more likely to be on dialysis with higher dialysis vintage, but had more potential donors at the time of evaluation compared to WH patients. Culturally, they reported experiencing more racism, discrimination in healthcare, had higher medical mistrust and religious objections to LDKT, although they had greater trust in physicians, family loyalty than WH. Psychosocially, AA reported less social support, but greater internal and external locus of control than WH. AA candidates had lower transplant knowledge and spent less time engaging in fewer learning activities than WH. A higher percentage of AA patients were wait-listed after the implementation of the new KAS compared to WH.
Table 2:
| Full Cohort (n=1,055) | Whites (n=788) | African Americans (n=267) | P value | |
|---|---|---|---|---|
| Age | 56.7 ± 13.4 | 57.5 ± 13.6 | 54.2± 12.6 | <0.001 |
| Female | 405 (38.4) | 301 (38.2) | 104 (39.0) | 0.827 |
| Education (HS+ or less)44,46 |
495 (46.9) | 358 (45.4) | 137 (51.3) | 0.096 |
| Occupation (≤ lower status occupation) | 542 (51.5) | 374 (47.5) | 168 (63.2) | <0.001 |
| Income (< $25,000) | 485 (48.4) | 313 (41.5) | 172 (69.6) | <0.001 |
| Insurance | <0.001 | |||
| Public | 370 (35.4) | 246 (31.4) | 124 (47.2) | |
| Private | 277 (26.5) | 223 (28.5) | 54 (20.5) | |
| Both public/private | 399 (38.2) | 314 (40.1) | 85 (32.3) | |
| Married | 543 (51.5) | 456 (57.9) | 87 (32.6) | <0.001 |
| Medical factors | ||||
| Body Mass Index at evaluation | 29.6 ± 6.3 | 29.6 ± 6.2 | 29.5 ± 6.5 | 0.413 |
| Charlson co-morbidity score | 4.2 ± 1.8 | 4.1 ± 1.7 | 4.4 ± 1.9 | 0.020 |
| Burden of Kidney Disease | 3.6 ± 1.1 | 3.7 ± 1.1 | 3.6 ± 1.1 | 0.399 |
| Number of potential donors | 22.7 ± 17.8 | 21.6 ± 16.5 | 26.2 ± 20.9 | 0.004 |
| Living Donor | 556 (52.8) | 423 (53.8) | 133 (50.0) | 0.290 |
| Duration of dialysis | <.0001 | |||
| 0 years on dialysis | 365 (34.6) | 312 (39.6) | 53 (19.9) | |
| <1 year on dialysis | 470 (44.5) | 346 (43.9) | 124 (46.4) | |
| 1 - ≤5 years on dialysis | 163 (15.5) | 101 (12.8) | 62 (23.2) | |
| >5 years on dialysis | 57 (5.4) | 29 (3.7) | 28 (10.5) | |
| Cultural factors | ||||
| Racism in healthcare | 2.3 ± 0.7 | 2.2 ± 0.7 | 2.7 ± 0.8 | <0.001 |
| Medical mistrust | 2.4 ± 0.5 | 2.4 ± 0.5 | 2.6 ± 0.5 | <0.001 |
| Trust in physician | 2.2 ± 0.5 | 2.2 ± 0.5 | 2.3 ± 0.5 | <0.001 |
| Family loyalty | 49.8 ± 9.4 | 49.0 ± 8.7 | 52.3 ± 10.9 | <0.001 |
| Any Religious objection to LDKT+++@ | 679 (65.3) | 491 (63.2) | 188 (71.5) | 0.015 |
| Experienced discrimination in healthcare | 268 (25.6) | 133 (17.0) | 135 (51.1) | <0.001 |
| Psychosocial factors | ||||
| Social support – Total | 42.4 ± 5.9 | 42.8 ± 5.5 | 41.3 ± 6.7 | 0.007 |
| Self-esteem | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.2 ± 0.5 | 0.277 |
| Internal locus of control | 4.0 ± 1.1 | 3.9 ± 1.1 | 4.1 ± 1.1 | 0.012 |
| External locus of control | 3.4 ± 0.8 | 3.4 ± 0.8 | 3.7 ± 0.9 | <0.001 |
| Anxiety (≥moderate)* | 47 (4.5) | 34 (4.3) | 13 (4.9) | 0.704 |
| Depression (≥moderate)* | 42 (4.0) | 27 (3.4) | 15 (5.6) | 0.113 |
| Transplant knowledge | ||||
| Transplant knowledge | 21.3 ± 2.8 | 21.6 ± 2.7 | 20.2 ± 3.0 | <0.001 |
| Number of learning activities | 4.5 ± 1.6 | 4.6 ± 1.6 | 4.3 ± 1.6 | 0.002 |
| Hours engaged in learning activities | 19.0 ± 23.9 | 20.2 ± 24.1 | 15.4 ± 22.7 | <0.001 |
| Total transplant concerns | 10.9 ± 4.7 | 10.8 ± 4.6 | 11.2 ± 4.9 | 0.258 |
| Outcomes | ||||
| Wait-listed after KAS++ | 29 (4.9) | 16 (3.4) | 13 (11.3) | <0.001 |
| Outcome status | <0.001 | |||
| Wait-listed | 591 (56.0) | 476 (60.4) | 115 (43.1) | |
| Deceased prior to waitlist | 287 (27.2) | 203 (25.8) | 84 (31.5) | |
| Closed patient choice prior to wait-list | 13 (1.2) | 10 (1.3) | 3 (1.1) | |
| Closed due to incomplete evaluation | 116 (11.0) | 64 (8.1) | 52 (19.5) | |
| Clinic rejected prior to wait-list | 26 (2.5) | 18 (2.3) | 8 (3.0) | |
| Still undergoing evaluation | 6 (0.6) | 3 (0.4) | 3 (1.1) | |
| Transplanted at another center | 16 (1.5) | 14 (1.8) | 2 (0.8) |
Patients were recruited after beginning transplant evaluation, 3/10–10/12, and followed through the end of study period, 8/18; Among eligible patients, those enrolled showed no large or significant differences from those not enrolled on any available demographic characteristic (age, sex, race/ethnicity).
Continuous variables were assessed using the Wilcoxon rank sum test and are expressed as mean ± SD while categorical variables were assessed using the Chi-square tests and are expressed as n (%)
HS = High school
KAS = Kidney allocation system
LDKT = Live donor kidney transplantation
Anxiety & depression were measured using the Brief Symptom Inventory (BSI) [Scale 1–5 with >3 being moderate)
Among patients wait-listed (n=591). Remaining n=464 were neither wait-listed active nor wait-listed inactive.
Includes mixed objection (Combination of Neutral and No Objection)
n=2 missing for occupation, transplant knowledge, having living donor; n=3 missing for transplant concerns; n=4 missing for family loyalty score; n=5 missing in medical mistrust index, trust in physician; n=6 missing in total social support; n=7 missing for internal and external locus of control, experienced discrimination in healthcare; n=8 missing for income (45 don’t know/refused), self-esteem scale, total hours engaged in learning activities, n=9 missing for insurance type; n=11 missing for racism in healthcare; n=15 missing for religious objection to LDKT.
Acceptance for transplant wait-listing
Figure 2 shows the cumulative incidence curves of the probability of being wait-listed for KT by race. AA patients were less likely to be wait-listed compared to WH patients (Hazard Ratio (HR): 0.56, 95% Confidence Interval (CI): 0.46–0.68, p <0.001). Table 3 shows the Fine & Gray proportional subdistribution hazards models for time to wait-listing. The probability of wait-listing increased for AA patients after adjusting for demographic and medical factors (HR 0.69, 95% CI: 0.55–0.84, p <0.001) as well as for psychosocial factors (HR 0.75, 95% CI: 0.59–0.96, p = 0.021) although the disparity persisted. Being older, having lower income, public insurance, more comorbidities, or being on dialysis at the time of kidney transplant evaluation decreased the probability of wait-listing while having more social support or more transplant knowledge increased the probability of wait-listing. We checked for proportionality of hazards assumptions for each model and found no issues.
Figure 2:
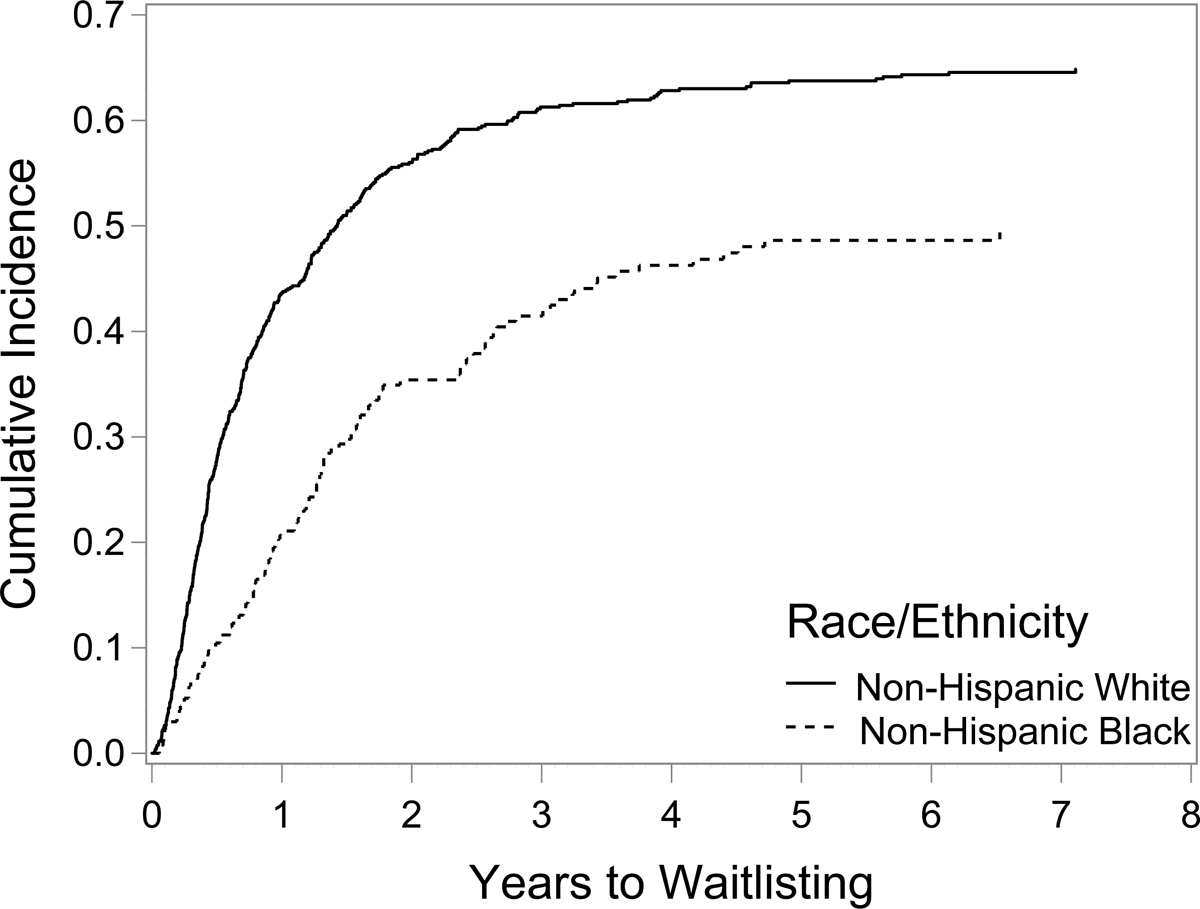
Cumulative incidence curves of time to acceptance for transplant wait-listing by race (Unadjusted)
African Americans were less likely to be wait-listed compared to Whites in the unadjusted model.
Table 3:
Unadjusted and adjusted Fine & Gray proportional subdistribution hazards models for years to wait-listing for kidney transplant.
| Unadjusted (Model 1) | Adjusted (Model 2) | Adjusted (Model 3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables1 | SHR2 | 95% CI | P value | SHR2 | 95% CI | P value | SHR2 | 95% CI | P value |
| Race/ethnicity | |||||||||
| Non-Hispanic White | Reference | Reference | Reference | ||||||
| Non-Hispanic African American | 0.56 | 0.46–0.68 | <0.0001 | 0.68 | 0.55–0.84 | <0.001 | 0.75 | 0.59–0.96 | 0.021 |
| Other demographic characteristics | |||||||||
| Age (years) | 0.99 | 0.98–0.99 | 0.001 | 0.99 | 0.98–0.99 | 0.025 | |||
| Education (High School or less) | 0.99 | 0.82–1.19 | 0.877 | 1.05 | 0.86–1.28 | 0.642 | |||
| Occupation (<= lower status) | 1.01 | 0.84–1.22 | 0.911 | 1.06 | 0.87–1.28 | 0.572 | |||
| Household income (< US $25,000) | 0.64 | 0.52–0.79 | <0.001 | 0.66 | 0.54–0.82 | <0.001 | |||
| Insurance status | 0.008 | 0.006 | |||||||
| Private only | Reference | Reference | |||||||
| Public only | 0.63 | 0.49–0.80 | 0.002 | 0.67 | 0.52–0.86 | 0.002 | |||
| Public and private | 0.79 | 0.63–0.97 | 0.028 | 0.79 | 0.64–0.99 | 0.041 | |||
| Marital status (married) | 0.96 | 0.80–1.15 | 0.651 | 0.96 | 0.79–1.15 | 0.636 | |||
| Medical factors | |||||||||
| Charlson Comorbidity index | 0.89 | 0.85–0.94 | <0.001 | 0.91 | 0.86–0.96 | <0.001 | |||
| Burden of Kidney Disease | 0.98 | 0.91–1.06 | 0.593 | 0.97 | 0.89–1.06 | 0.516 | |||
| Have living donor | 1.09 | 0.92–1.30 | 0.333 | 1.02 | 0.85–1.22 | 0.827 | |||
| Dialysis (yes) | 0.61 | 0.50–0.75 | <0.001 | 0.62 | 0.50–0.76 | <0.001 | |||
| Cultural factors | |||||||||
| Racism in healthcare | 1.03 | 0.89–1.18 | 0.698 | ||||||
| Medical mistrust | 0.96 | 0.75–1.21 | 0.715 | ||||||
| Trust in physician | 1.11 | 0.90–1.35 | 0.339 | ||||||
| Any religious objection to *LDKT | 0.99 | 0.82–1.17 | 0.814 | ||||||
| Experienced discrimination in healthcare | 0.88 | 0.69–1.13 | 0.316 | ||||||
| Psychosocial factors | |||||||||
| Total social support | 1.03 | 1.01–1.05 | 0.001 | ||||||
| Self-esteem | 0.90 | 0.73–1.11 | 0.320 | ||||||
| Internal locus of control | 0.98 | 0.90–1.06 | 0.561 | ||||||
| External locus of control | 1.04 | 0.93–1.16 | 0.536 | ||||||
| Transplant knowledge | |||||||||
| Transplant Knowledge | 1.06 | 1.02–1.10 | 0.006 | ||||||
| Number of learning activities | 1.02 | 0.96–1.09 | 0.453 | ||||||
| Hours engaged in learning activities | 1.00 | 0.99–1.00 | 0.851 | ||||||
Sample size n=1002 for unadjusted model and adjusted model
SHR = subdistribution hazard ratio
LDKT = Live donor kidney transplantation
5. Discussion
This study is innovative because we offered a comprehensive prospective examination of the influence of social determinants of health on KT wait-listing (controlling for demographic and medical factors) in a large population being evaluated for KT. We found significant differences in the baseline characteristics between AA and WH patients, with AA being younger and having lower SES compared to WH. Our sample of KT candidates was older and had a larger proportion of WH patients than the US population of KT candidates but was equivalent in the proportion of women and those who were on dialysis.25 As found in other clinical and community dwelling populations,26 AA reported more discrimination, perceived racism in healthcare, and medical mistrust compared to WH. Even after adjusting for these differences, racial disparity in KT wait-listing persisted.
The strength of this study lies in its large sample size and the use of a multi-pronged approach to understand the racial disparity in KT. The meticulous collection of data and chart reviews resulted in a significant amount of data available to test for the persistence of racial disparity in KT wait-listing after accounting for social determinants of health. Using the time of first KT evaluation as the starting point eliminated the barriers relating to transplant referral and allowed us to focus on racial disparity during the evaluation period, identify factors that impact wait-listing and possibly channel resources to address these factors.
Our findings support previous work examining the effects of discrimination and medical mistrust on referral for KT27–29 although ours is the first to examine these variables as predictors of KT wait-listing. Although significant differences were noted in perceived discrimination and medical mistrust between the two groups, these factors did not account for the racial disparity in KT wait-listing, which contradicted the findings of our pilot work.5 This finding could be explained by the smaller sample size (n = 127) used in the pilot study resulting in potential sampling bias. The study may have been underpowered to detect a significant difference in racial disparity hence justifying the need to conduct this current larger study.
In the current study, we confirmed previous work that racial disparity exists in KT wait-listing between AA and WH.3,5,8,30 Consistent with previous studies that showed that patients with lower SES are less likely to be referred for transplant, wait-listed or receive a KT,3,5,8 we found that being older, AA, having lower income, being on public insurance, having more comorbidities, or being on dialysis at the time of kidney transplant evaluation negatively impacted wait-listing. We believe these variables can be considered risk factors for taking longer to complete the KT evaluation process. Because these are basic assessments done at the first KT evaluation clinic appointment, these patients can be flagged for increased assistance to complete the evaluation process. Identifying this at-risk population will allow us to channel more resource including the use of peer navigators,31,32 fast-track clinics33 as well as education programs34 to help these patients complete their work up and be wait-listed.
Similarly, we found that greater social support and transplant knowledge positively impacted wait-listing. Although it may not be feasible to assess these variables in transplant centers nationwide, we believe knowing that these factors have a positive influence on KT wait-listing helps transplant centers identify ways to improve patients’ likelihood of completing evaluation; namely, targeting efforts at improving KT education for all patients at initiation of evaluation and assessing for and ensuring strong social support during the evaluation period from the transplant team’s social worker.
The results of this study differed from our previously published Veterans Affairs (VA) study. In the VA study, we looked at the effects of the same set of predictors on race differences in KT wait-listing among Veterans, but did not find any racial disparity in the time to wait-listing.4 This finding could be explained by the difference in the evaluation process used by VA. At VA facilities, patients are referred to the transplant centers only after the required transplant work up has been completed, hence eliminating patients who have yet to complete their work up and cannot be wait-listed. In contrast, at UPMC (and most non-VA transplant centers), the work up starts only after the first transplant clinic evaluation. Furthermore, the Veteran patient population with ESRD tends to be more homogenous than non-Veterans (e.g., age, income, education, SES, comorbidities). Despite the uniqueness of the Veteran population, the success of the VA system in eliminating racial disparity in wait-listing should serve as an example to the non-VA transplant centers, especially in light of the current study findings of persistent racial disparity. The VA KT evaluation process provides full coverage for: (a) all ancillary testing that can be scheduled and tracked within the same electronic health record; (b) all transport and lodging in connection with KT evaluation for patients and their primary caregiver; and, (c) post transplant immunosuppression. Thus, extending the Medicare ESRD entitlement to cover these expenses may help reduce disparities in non-VA transplant centers where racial disparities exists.3,8,35
The results of this study should be interpreted in light of some limitations. First, this was a single-center study. Every transplant center has its unique evaluation process and patient population; the barriers noted in this study may not apply to other transplant centers. However, this study highlighted the need to focus on social determinants of health to reduce racial disparity. This finding likely will be applicable to transplant centers that experience racial disparity regardless of racial/ethnic composition. Another limitation of this study is the persistence of racial disparity in spite of adjustments for all measured variables suggesting that there may be variables that we did not measure and adjust for accounting for the persistent disparity. Novel factors will need to be considered in future studies on racial disparity.
In conclusion, we found that racial disparity exists in transplant wait-listing even after correcting for social determinants of health. Efforts to identify novel factors that continue to contribute to racial disparity are needed. In the meantime, clinically, the identification of high-risk KT candidates in this study (older patients, AA patients, patients with lower SES, patients on dialysis, or who have multiple co-morbidities) will allow us to target patients on whom to intensify interventions that facilitate the completion of their work up promptly. At the same time, increasing transplant education programs36–38 to enhance transplant knowledge and ensuring that patients have adequate social support will increase the probability that patients complete their work up and get wait-listed for transplant.
Acknowledgments
The SRTR data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding
This research is supported in part by NIDDK Grants #R01DK081325 and R01DK101715; and, by the National Institutes of Health through Grant Number UL1 TR001857.
Abbreviations:
- AA
African Americans
- BMI
Body Mass Index
- CCI
Charlson Comorbidity Index
- DDKT
Deceased donor kidney transplant
- ESRD
End stage renal disease
- HLA
Human Leukocyte Antigen
- HR
Hazard ratio
- IRB
Institutional Review Board
- KAS
Kidney allocation system
- KT
Kidney transplantation
- LDKT
Living donor kidney transplantation
- SES
Socioeconomic status
- SRP
Survey Research Program
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement and Transplantation Network
- UCSUR
University of Pittsburgh Center for Social and Urban Research
- UPMC
University of Pittsburgh Medical Center
- VA
Veterans Affair
- WH
Whites
Footnotes
Disclosure
The authors of this manuscript declare no financial conflict of interest.
References
- 1.Fujisawa M, Ichikawa Y, Yoshiya K, et al. Assessment of Health-Related Quality of Life in Renal Transplant and Hemodialysis Patients Using the SF-36 Health Survey. Urology. 2000;56(2):201–206. [DOI] [PubMed] [Google Scholar]
- 2.Taber D, Gebregziabher M, Hunt K, et al. Twenty years of evolving trends in racial disparities for adult kidney transplant recipients. Kidney Int. 2016;90(4):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi S, Gaynor J, Bayers S, et al. Disparities Among Blacks, Hispanics, and Whites in Time from Starting Dialysis to Kidney Transplant Waitlisting. Transplantation. 2013;95(2):309–318. [DOI] [PubMed] [Google Scholar]
- 4.Freeman M, Pleis J, Bornemann K, et al. Has the Department of Veterans Affairs found a way to avoid racial disparities in the evaluation process for kidney transplantation? Transplantation. 2017;101(6):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myaskovsky L, Doebler D, Posluszny D, et al. Perceived Discrimination Predicts Longer Time to be Accepted for Kidney Transplant. Transplantation. 2012;93(4):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequist TD, Narva AS, Stiles SK, et al. Access to renal transplantation among American Indians and Hispanics. Am J Kidney Dis. 2004;44(2):344–352. [DOI] [PubMed] [Google Scholar]
- 7.Patzer RE, Perryman JP, Schrager JD, et al. The Role of Race and Poverty on Steps to Kidney Transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen KL, Zhang R, Huang Y, et al. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7(9):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders MR, Cagney KA, Ross LF, et al. Neighborhood Poverty, Racial Composition and Renal Transplant Waitlist. Am J Transplant. 2010;10(8):1912–1917. [DOI] [PubMed] [Google Scholar]
- 10.Hall E, Massie A, James N, et al. Effect of Eliminating Priority Points for HLA-B Matching on Racial Disparities in Kidney Transplant Rates. Am J Kidney Dis. 2011;58(5):813–816. [DOI] [PubMed] [Google Scholar]
- 11.Melanson T, Hockenberry J, Plantinga L, et al. New kidney allocation system associated with increased rates of transplants among Black and Hispanic patients. Health Aff(Millwood). 2017;36(6):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald JJ, Turgeon N. Early Experience with the New Kidney Allocation System: A Perspective from a Transplant Center. Clin J Am Soc Nephrol. 2017;12(12):2060–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Melanson TA, Plantinga LC, et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant. 2018;18(8):1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper LA, Hill MN, Powe NR. Designing and evaluating interventions to eliminate racial and ethnic disparities in health care. J Gen Intern Med. 2002;17(6):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilbourne AM, Switzer GE, Hyman KB, et al. Advancing health disparities research within the healthcare system: A conceptual framework. Am J Public Health. 2006;96(12):2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noohi S, Khaghani-Zadeh M, Javadipour M, et al. Anxiety and depression are correlated with higher morbidity after kidney transplantation. Transplantation proceedings. 2007;39(4):1074–1078. [DOI] [PubMed] [Google Scholar]
- 17.Paradies Y A systematic review of empirical research on self-reported racism and health. Int J Epidemiol. 2006;35(4):888–901. [DOI] [PubMed] [Google Scholar]
- 18.Myaskovsky L, Switzer GE, Crowley-Matoka M, et al. Psychosocial factors associated with ethnic differences in transplantation. Curr Opin Organ Transplant. 2007;12(2):182–187. [Google Scholar]
- 19.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubbs V, Gregorich SE, Perez-Stable EJ, et al. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4(1):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System. USRDS annual data report: Epidemiology of kidney disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD; 2018. [Google Scholar]
- 22.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am State Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Kohl M, Plischke M, Leffondré K, et al. PSHREG: A SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed. 2015;118(2):218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noordzij M, Leffondré K, van Stralen K, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–2677. [DOI] [PubMed] [Google Scholar]
- 25.Scientific Registry of Transplant Recipients: Standard Analysis File. 2002 2017. Available at srtr.transplant.hrsa.gov.
- 26.Bird ST, Bogart LM, Delahanty DL. Health-related correlates of perceived discrimination in HIV care. AIDS Patient Care STDs. 2004;18(1):19–26. [DOI] [PubMed] [Google Scholar]
- 27.Tong A, Hanson CS, Chapman JR, et al. ‘Suspended in a paradox’—patient attitudes to wait-listing for kidney transplantation: systematic review and thematic synthesis of qualitative studies. Transpl Int. 2015;28(7):771–787. [DOI] [PubMed] [Google Scholar]
- 28.Wachterman M, McCarthy E, Marcantonio E, et al. Mistrust, Misperceptions and Miscommunication: A Qualitative Study of Preferences about Kidney Transplantation Among African Americans. Transplant Proc. 2015;47(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klassen AC, Hall AG, Saksvig B, et al. Relationship between patients’ perceptions of disadvantage and discrimination and listing for kidney transplantation. Am J Public Health. 2002;92(5):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patzer R, Amara S, Wasse H, et al. Neighborhood Poverty and Racial Disparities in Kidney Transplant Waitlisting. J Am Soc Nephrol. 2009;20(6):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan C, Leon J, Sayre S, et al. Impact of Navigators on Completion of Steps in the Kidney Transplant Process: A Randomized, Controlled Trial. Clin J Am Soc Nephrol. 2012;7(10):1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu M, Petgrave-Nelson L, Smith KD, et al. Transplant Center Patient Navigator and Access to Transplantation among High-Risk Population: A Randomized, Controlled Trial. Clin J Am Soc Nephrol. 2018;13(4):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bornemann K, Croswell E, Abaye M, et al. Protocol of the KTFT-TALK study to reduce racial disparities in kidney transplant evaluation and living donor kidney transplantation. Contemp Clin Trials. 2017;53(Supplement C):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patzer RE, Perryman JP, Pastan S, et al. Impact of a Patient Education Program on Disparities in Kidney Transplant Evaluation. Clin J Am Soc Nephrol. 2012;7(4):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keith D, Ashby VB, Port FK. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol. 2008;3(2):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strigo TS, Ephraim PL, Pounds I, et al. The TALKS Study to Improve Communication, Logistical and Financial Barriers to Live Donor Kidney Transplantation in African Americans: Protocol of a Randomized Clinical Trial. BMC Nephrol. 2015;16:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patzer R, Paul S, Plantinga L, et al. A Randomized Trial to Reduce Disparities in Referral for Transplant Evaluation. J Am Soc Nephrol. 2016;28(3):935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigue J, Paek M, Egbuna O, et al. Making House Calls Increases Living Donor Inquiries and Evaluations for Blacks on the Kidney Transplant Waiting List. Transplantation. 2014;98(9):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart DE, Kucheryavaya AY, Klassen DK, et al. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am J Transplant. 2016;16(6):1834–1847. [DOI] [PubMed] [Google Scholar]
- 40.Waterman A, Barrett A, Stanley S. Optimal Transplant Education for Recipients to Increase Pursuit of Living Donation. Prog Transplant. 2008;18(1):55–62. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales K, et al. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 42.Delmonico F, Council of the Transplantation Society. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53–66. [PubMed] [Google Scholar]
- 43.Williams D, Yan Y, Jackson JS, et al. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 44.Boulware LE, Ratner LE, Ness PM, et al. The contribution of sociodemographic, medical, and attitudinal factors to blood donation among the general public. Transfusion. 2002;42(6):669–678. [DOI] [PubMed] [Google Scholar]
- 45.LaVeist T, Issac L, Williams KP. Mistrust of Health Care Organizations Is Associated with Underutilization of Health Services. Health Serv Res. 2009;44(6):2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson L, Dedrick R. Development of the Trust in Physician Scale: A Measure to Assess Interpersonal Trust in Patient-Physician Relationships. Psychol Rep. 1990;67(3 Pt 2):1091–1100. [DOI] [PubMed] [Google Scholar]
- 47.Bardis P A Familism Scale. Marriage and Family Living. 1959;21:340–341. [Google Scholar]
- 48.Rumsey S, Hurford D, Cole A. Influence of Knowledge and Religiousness on Attitudes toward Organ Donation. Transplant Proc. 2003;35(8):2845–2850. [DOI] [PubMed] [Google Scholar]
- 49.Derogatis L, Spencer P. The Brief Symptom Inventory (BSI): Administration, Scoring and Procedure Manual. Baltimore, MD: Clinical Psychometric Research;1975. [Google Scholar]
- 50.Cohen S, Hoberman HM. Positive Events and Social Supports as Buffers of Life Change Stress1. Journal of Applied Social Psychology. 1983;13(2): 99–125. [Google Scholar]
- 51.Cohen S, Mermelstein R, Kamarck T, et al. Measuring the Functional Components of Social Support In: Sarason IG, Sarason BR, eds. Social Support: Theory, Research and Applications. Dordrecht: Springer Netherlands; 1985:73–94. [Google Scholar]
- 52.Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press;1965. [Google Scholar]
- 53.Wallston K, Stein M, Smith CA. Form C of the MHLC Scales: a Condition-specific Measure of Locus of Control. J Pers Assess. 1994;63(3):534–553. [DOI] [PubMed] [Google Scholar]
- 54.Waterman A, Stanley S, Covelli T, et al. Living Donation Decision Making: Recipients’ Concerns and Educational Needs. Prog Transplant. 2006;16(1):17–23. [DOI] [PubMed] [Google Scholar]


