Abstract
Objectives
The rapid emergence of hypervirulent C. difficile isolates and the paucity of effective anticlostridial antibiotics call for extensive research to identify new treatment options. The objective of the present study was to test the anticlostridial activity of bioactive extracts of turmeric, a natural herb widely known for its profound medicinal properties.
Methods
The minimum inhibitory concentrations of turmeric derivatives were determined against 27 C. difficile strains, including hypervirulent (BI/NAP1/027) and clinical toxigenic isolates. Additionally, their ability to inhibit C. difficile toxin production and spore formation was investigated. Furthermore, the safety profiles of turmeric derivatives regarding their effects on human gut microflora, such as Bacteroides, Lactobacillus, and Bifidobacterium was evaluated.
Results
Curcuminoids, the major phytoconstituents of turmeric including curcumin, demethoxycurcumin, and bisdemethoxycurcumin inhibit growth of C. difficile at concentrations ranging from 4 to 32 μg/ml. Additionally, curcuminoids showed no negative effect on major populating species of the human gut. Curcumin was more effective than fidaxomicin in inhibiting C. difficile toxin production, but less so in inhibiting spore formation.
Conclusion
Our findings suggest that curcumin has potential as an anticlostridial agent. More work is needed to further investigate the efficacy of curcumin as a standalone drug or as a supplement of current drugs of choice, as it has no antagonistic activities but might overcome their drawbacks.
Keywords: Clostridium difficile, anticlostridial drugs, turmeric, curcuminoids, curcumin
1. INTRODUCTION
Clostridium difficile is an anaerobic, Gram-positive spore-forming and toxin-producing bacillus that causes severe infections in humans. Clostridium difficile infection (CDI) or Clostridium difficile-associated diarrhea (CDAD) are major public health problems identified by watery diarrhea, fever, nausea, and abdominal pain. CDI symptoms can evince into pseudomembranous colitis, toxic megacolon, colon perforation, sepsis, or fulminant colitis [1]. These severe manifestations are attributed to toxins, which are the main virulence factors of C. difficile. C. difficile produces two major toxins, TcdA and TcdB, that inactivate Rho and Rac GTPases in the colonic epithelial cells, leading to loss of tight junctions, compromised epithelial integrity and promotion of mucosal inflammation [2]. In addition, endospores produced during germination are another virulence factor associated with CDI. C. difficile spores play a crucial role in disease transmission by virtue of their ability to persist in a dormant state for up to five months on inanimate surfaces. These spores germinate in the gut to form toxin-producing vegetative cells upon discovering suitable environments, serving as the major cause of dissemination and recurrence of infection [3, 4]
The CDI problem is both persistent and growing. In the past two decades, a dramatic upsurge in CDI cases has been observed, resulting in nearly half a million cases, more than 29,000 deaths and an economic burden of nearly $5 billion in the United States alone [5]. This rapid escalation can be attributed to the emergence of hypervirulent fluoroquinolone-resistant strains. These strains are characterized as Restriction Endonuclease Analysis (REA) group BI, pulsed-field gel electrophoresis (PFGE) North American pulsotype 1 (NAP1), and PCR ribotype 027 (BI/NAP1/027) [6, 7]. Hypervirulent strains show augmented infectivity along with an increased symptomatic disease rate relative to endemic strains. In addition, they possess the ability to outcompete endemic strains in the gut of their host [8]. Analysis of these hypervirulent isolates revealed an 18-bp deletion in the tcdC gene, a proposed negative regulator of the two toxins, which is purportedly related to the greater production of toxins A and B [9, 10].
As treatment options for CDI decline, there is an urgent need for new anticlostridial drugs. The current list of antimicrobials used in the treatment of CDI is restricted to vancomycin, metronidazole and fidaxomicin. While metronidazole, a non-FDA-approved drug, is no longer recommended for CDI, vancomycin is the preferred FDA-approved option for severe cases [6]. Fidaxomicin, the only anticlostridial drug approved by the FDA in the past 3 decades, proved to be non-inferior to vancomycin in the clinical cure of CDI. Fidaxomicin had similar activity in patients with first recurrence of CDI but was superior in preventing a second recurrence in 28 days. However, fidaxomicin was not superior in cases of patients infected with BI/NAP1/027 hypervirulent strains [11, 12]. Therapeutic antimicrobial choices have become limited due to increasing resistance and reduced susceptibility to first-line drugs, making the development of new anticlostridial drugs more imperative and crucial [13].
With the evolution of bacterial pathogens resistant to conventional antibiotics, natural phytochemicals derived from medicinal plants can be explored as a promising alternative. Plants have been a source of traditional treatment agents against several human diseases for centuries in many parts of the world. Bioactive components present in many medicinal plants have the potential to be exploited as therapeutic alternatives against CDI [14, 15]. Turmeric, extracted from rhizomes of Curcuma longa, is a natural herb with diverse therapeutic properties, ranging from anti-cancer to treating neurodegenerative diseases. Several components from turmeric, including curcuminoids, mainly curcumin, bisdemethoxycurcumin, and demethoxycurcumin, and various volatile oils, such as turmerone, atlantone and zingibirone, make up medicinal turmeric powder. Of these compounds, curcumin comprises 0.3–5.4% of raw turmeric and is the most investigated constituent [16]. Curcumin is the subject of more than 100 clinical trials due to its versatile properties, including antitumor, anti-inflammatory, and antioxidant properties. However, its anticancer activities are the most widely studied [17].The antibacterial activities of curcumin were first reported by E. Schraufstatter and H. Bernt in 1949 [18]. The other two curcuminoids, bisdemethoxycurcumin and demethoxycurcumin, were reported to possess similar antibacterial activities [19].
In view of the imperative requirement of new anticlostridial drugs, we report the activity of turmeric’s derivatives, with curcumin being the line of focus, against a wide panel of C. difficile clinical isolates. Additionally, we investigate their ability to inhibit C. difficile toxin production and spore formation. We discuss their safety profiles regarding their effects on human gut microflora, such as Bacteroides, Lactobacillus, and Bifidobacterium. Our results show that curcumin has the potential to be used as a standalone drug as well as individually as a supplement with present drugs of choice to combat CDI.
2. MATERIALS AND METHODS
2.1. Bacterial strains, chemicals, and media
C. difficile isolates (Table 1) were obtained from the American Type Culture Collection (ATCC) and Biodefense and Emerging Infections Research Resources Repository (BEI Resources). Curcumin, bisdemethoxycurcumin, demethoxycurcumin, tetrahydrocurcumin (Cayman chemical, Ann Arbor, MI), curcumol (Sigma-Aldrich, St. Louis, MO), metronidazole (Beantown Chemical Corporation, Hudson, NH), vancomycin hydrochloride (Gold Biotechnology, St. Louis, MO), and fidaxomicin (Apexbio, Houston, TX) were procured from commercial vendors. Phosphate-buffered saline, fetal bovine serum and nonessential amino acids (NEAA) were purchased from Fisher Scientific (Waltham, MA). Brain heart infusion (BHI) was purchased from Becton, Dickinson and Company (Cockeysville, MD). Yeast extract, L-cysteine, vitamin K, hemin, Dulbecco’s Modified Eagle’s medium (DMEM), and penicillin/streptomycin were obtained from Sigma-Aldrich (St. Louis, MO).
Table 1:
Bacterial strains used in the study
| Bacterial Strain | Alternate designation | Description |
|---|---|---|
| C. difficile P3 | NR-32884 | Toxin producing strain isolated in 2001 from the stool sample of a CDI case in Western Pennsylvania, USA. |
| C. difficile P13 | NR-32891 | Toxin producing strain isolated in 2005 from the stool sample of a CDI case in Western Pennsylvania, USA. |
| C. difficile NAP07 (CDC#2007054) | HM-88 | Strain obtained from a human stool sample. |
| C. difficile Isolate 4 | NR-13430 | Isolated in 2008/2009 from a human patient diagnosed with CDI in the Mid-Atlantic region, USA. |
| C. difficile Isolate 5 | NR-13431 | Isolated in 2008/2009 from a human patient diagnosed with CDI in the Mid-Atlantic region, USA. |
| C. difficile Isolate 9 | NR-13435 | Isolated in 2008/2009 from a human patient diagnosed with CDI in the Mid-Atlantic region, USA. |
| C. difficile ATCC 9689 | 90556-M6S | tcdB+,Ribotype 001 isolate |
| C. difficile ATCC 700057 | VPI 11186 |
tcdA-/tcdB-,(Non-toxin producing)
Ribotype 038 isolate |
| C. difficile ATCC 43598 | 1470 | tcdB+, Ribotype 017 isolated from stool sample, asymptomatic neonate, Belgium |
| C. difficile ATCC BAA 1801 | 3232 | Non-toxin producing Ribotype 010 isolated from human feces (adult with diarrhea), Belgium. |
| C. difficile ATCC BAA 1870 | 4118 | Clinically isolated tcdA+/tcdB+ toxinotype IIIb, Ribotype 027, from Maine, USA |
| C. difficile ATCC BAA 1871 | 4111 | tcdA-/tcdB- toxinotype 0, NAP2, Ribotype 001 |
| C. difficile ATCC 43255 | VPI 10463 | tcdA+/tcdB+ toxinotype 0, Ribotype 087 |
| C. difficile Isolate 20100207 | NR-49278 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2010 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in New York, USA. |
| C. difficile Isolate 20100502 | NR-49277 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2010 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in Colorado, USA. |
| C. difficile Isolate 20100211 | NR-49279 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 iolated in 2010 from a fecal sample of a pediatric female patient diagnosed with healthcare-associated CDI in New York, USA. |
| C. difficile Isolate 20100221 | NR-49280 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2010 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in New York, USA. |
| C. difficile Isolate 20110052 | NR-49281 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2010 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in New York, USA. |
| C. difficile Isolate 20110870 | NR-49288 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of a young adult female patient diagnosed with healthcare-associated CDI in Tennessee, USA. |
| C. difficile Isolate 20120015 | NR-49284 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in New York, USA. |
| C. difficile Isolate 20110979 | NR-49285 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an elderly female patient diagnosed with community-associated CDI in midwestern USA. |
| C. difficile Isolate 20110999 | NR-49286 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an elderly female patient diagnosed with healthcare-associated CDI in western/midwestern USA. |
| C. difficile Isolate 20120013 | NR-49283 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of a young male patient diagnosed with community-associated CDI in northeastern USA. |
| C. difficile Isolate 20120184 | NR-49289 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an elderly female patient diagnosed with fatal healthcare-associated CDI in Tennessee, USA. |
| C. difficile Isolate 20120187 | NR-49290 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an elderly male patient diagnosed with healthcare-associated CDI in Tennessee, USA. |
| C. difficile Isolate 20120236 | NR-49291 | NAP1a, tcdA+b, tcdB+c and tcdC+d PaLoce operon, CDTf Ribotype 027 isolated in 2011 from a fecal sample of an older female patient diagnosed with community-associated CDI in midwestern, USA. |
| Bacteroides fragilis HM-711 | CL05T00C42 | Isolated from healthy adult feces in Boston, Massachusetts, USA |
| Lactobacillus casei ATCC® 334 | -- | Isolated from dairy products; Emmental cheese |
| Lactobacillus gasseri HM-641 | SJ-9E-US | Isolated in 2007 from the vaginal mucosa of a healthy US woman |
| Lactobacillus gasseri HM-400 | EX336960VC03 | Isolated in March 2010 from a human mid-vaginal wall in Richmond, Virginia |
| Lactobacillus gasseri HM-407 | EX336960VC13 | Isolated in March 2010 from a human mid-vaginal wall in Richmond, Virginia |
| Bifidobacterium l ongum HM-848 | 2–2B | Isolated from a six-year-old human patient |
| Bacteroides dorei HM-719 | CL02T12C06 | Isolated from healthy adult human feces in Boston, Massachusetts, USA |
| Bifidobacterium longum HM-846 | 1–6B | Isolated in 2006 from feces of a six-year-old healthy human child in Russia |
| Bifidobacterium bifidum ATCC-11863 | 212A | -- |
| Lactobacillus crispatus HM-103 | JV-V01 | Isolated from normal human vaginal flora |
NAP= North American pulsed-field gel electrophoresis type.
tcdA= C. difficile toxin A gene.
tcdB= C. difficile toxin B gene.
tcdC= Anti-sigma factor gene.
PaLoc= Pathogenicity locus.
CDT= C. difficile binary toxin.
2.2. In vitro antibacterial activity of turmeric derivatives against C. difficile
Minimum Inhibitory Concentrations (MICs) were determined by a broth microdilution assay according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, with slight modifications [20]. Each concentration of turmeric derivatives and control antibiotic (fidaxomicin, vancomycin and metronidazole) was added to 96-well plates in triplicate. A bacterial suspension of ~105 CFU/mL initial bacterial titer in BHIS broth (BHI supplemented with yeast extract, L-cysteine, vitamin K and hemin) was prepared and seeded into the 96-well plates. Plates were then incubated for 48 hours at 37 °C under anaerobic conditions. MICs were determined as the lowest concentration of a given drug that suppressed the visual growth of bacteria. MIC50 and MIC90 were calculated, which are the minimum concentrations of each agent that inhibited the visual growth of 50% and 90% of the tested isolates, respectively.
2.3. Activity of turmeric derivatives against gut microflora
In vitro antibacterial activity of turmeric derivatives against commensal microflora of the human gut was assessed by determining their MICs against representative microbial species by the broth microdilution assay. Each drug concentration was added to 96-well plates in triplicate. A bacterial suspension of ~105 CFU/mL initial bacterial titer was prepared and seeded into 96-well plates containing the required concentrations of test compounds and available anticlostridial antibiotics. Bacterial suspensions were prepared in MRS broth for Lactobacillus species and BHIS broth for Bacteroides and Bifidobacterium species. Plates for Bacteroides and Bifidobacterium species were incubated anaerobically, and those of Lactobacillus were incubated in the presence of 5% CO2 for 48 hours at 37 °C. MICs were determined as the lowest concentration of a drug that suppressed the visual growth of bacteria [21].
2.5. Effect of turmeric derivatives on toxin production from a toxigenic C. difficile strain
The ability of curcumin to inhibit toxin production by C. difficile was determined as previously reported with slight modifications [2]. Briefly, a late exponential phase culture of a toxigenic hypervirulent strain, C. difficile ATCC BAA1870, was used to determine the total amounts of toxins A and B produced in the presence and absence of different subinhibitory concentrations of curcumin and control anticlostridial drugs. Briefly, ~ 2.6 × 105 CFU/mL of C. difficile ATCC BAA1870 was suspended in BHIS broth and aliquoted into 500 μL tubes. Drugs at (1/8 ×, 1/4 × and 1/2 × MIC) were added to each tube in triplicate, and the tubes were then incubated anaerobically (using BD GasPak™ EZ Container Systems) at 37 °C for 12 hours. One portion of each suspension was serially diluted by 10-fold, plated onto BHIS agar and incubated anaerobically at 37 °C for 14 hours to assess bacterial counts. The second portion was centrifuged at 10,000 rpm for 5 minutes to extract the cell-free supernatant containing toxins. The total concentration of C. difficile toxins A and B was measured in the supernatant using an enzyme-linked immunosorbent assay (ELISA) kit (Meridian Bioscience, Inc, Cincinnati, OH), following the manufacturer’s instructions. Optical density (450 nm), corresponding to the toxin concentration, was measured and compared for curcumin and the control drugs. The total toxin produced in the supernatant of the untreated control was considered 100%, and the reduction as a result of antibiotic treatment was expressed as the percentage inhibition.
2.6. Effect of turmeric derivatives on C. difficile spore formation
To assess the ability of curcumin to inhibit spore formation by C. difficile, a bacterial suspension of C. difficile strain HM-88, in late exponential phase, was prepared and aliquoted into tubes with drugs added (in triplicate) at concentrations equivalent to 1/2 × and 1 × MIC. Tubes were then incubated anaerobically for 5 days at 37 °C to allow spore formation. Post-incubation, one portion of each tube was serially diluted and plated onto BHIS agar supplemented with 0.1% taurocholic acid to count the total number of bacteria (vegetative cells + spores). The second part was centrifuged to collect the cells and re-suspended in PBS followed by overnight storage at 4 °C. The bacterial suspensions in PBS were shock-heated at 70 °C for 25 minutes to kill vegetative cells followed by serial dilution and plating to determine heat-resistant spore counts [3]. The results were reported as an average of two independent experiments performed in triplicate.
2.7. Synergism of curcumin with anticlostridial drugs
Investigation of synergistic effects between curcumin and current drugs of choice was determined by performing a checkerboard assay [22–25]. Two-fold serial dilutions of curcumin, with final concentrations ranging from 2 to 64 μg/ml, were assorted with either fidaxomicin, ranging from 0.0005 – 0.5 μg/ml, or vancomycin and metronidazole, at a range of 0.015–8 μg/ml, in 96-well plates. Bacterial suspensions of 105 CFU/mL of C. difficile ATCC BAA1870 and C. difficile Isolate 20100207 were seeded into 96-well plates containing the compounds, followed by anaerobic incubation at 37 °C for 48 hours. The drug combinatory effects were evaluated by calculating fractional inhibitory concentration (FIC) indices according to the following equation: FIC index = FICA + FICB, where FICA = MICA+B/MICA and FICB = MICB+A/MICB. MICA+B is the MIC of compound A in the combination with compound B, MICB+A is the MIC of compound B in combination with compound A, and MICA or MICB are the individual MICs of the compounds. The combination effect is defined as synergistic when FIC ≤ 0.5, additive when 0.5 < FIC ≤ 1.0, indifferent when 1.0 < FIC ≤ 4.0, and antagonistic when FIC > 4.0 [26–28].
2.8. Statistical Analysis
GraphPad Prism version 6.0 for Windows (GraphPad Software, La Jolla CA) was utilized for statistical analyses. Two-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparisons test was utilized to analyze the spore inhibition data.
3. RESULTS
3.1. In vitro susceptibilities of C. difficile isolates to turmeric compounds
Evaluation of the anticlostridial activity of turmeric’s derivatives against a large panel of clinical C. difficile isolates, mainly hypervirulent strains (ribotype 027), revealed potent activity of 3 main curcuminoids, curcumin, bisdemethoxycurcumin and demethoxycurcumin over tetrahydrocurcumin and curcumol, by inhibiting growth at concentrations ranging from 4 to 32 μg/mL. As depicted in Table 2, curcumin, the drug of interest due to its relative cost-effectiveness and wide availability, inhibited 50% of the tested isolates (MIC50) at a concentration of 16 μg/mL and 90% (MIC90) at 32 μg/mL. The MIC50 and MIC90 for bisdemethoxycurcumin were 16 μg/mL, while they were 16 and 32 μg/mL for demethoxycurcumin, respectively. In contrast, the MIC50 and MIC90 were 128 and >128 for tetrahydrocurcumin, respectively, and both values were over 128 μg/mL for curcumol. Control anticlostridial drugs Fidaxomicin, Vancomycin and Metronidazole displayed MIC ranges of 0.015 – 0.125 μg/mL, 0.125 – 2 μg/mL and 0.125 – 1 μg/mL, respectively, against the same set of isolates.
Table 2:
The minimum inhibitory concentration (MIC, μg/mL) of turmeric’s derivatives and control anticlostridial drugs against C. difficile isolates
| Strain ID | Curcumin | Bisdemethoxycurcumin | Demethoxycurcumin | Tetrahydrocurcumin | Curcumol | Fidaxomicin | Vancomycin | Metronidazole |
|---|---|---|---|---|---|---|---|---|
| NR-49277 | 32 | 16 | 16 | >128 | >128 | 0.06 | 0.5 | 0.25 |
| NR-49278 | 32 | 16 | 16 | 128 | >128 | 0.03 | 0.25 | 0.25 |
| NR-49279 | 16 | 16 | 16 | >128 | >128 | 0.06 | 0.25 | 1 |
| NR-49281 | 32 | 16 | 32 | 128 | 128 | 0.015 | 0.5 | 0.125 |
| NR-49282 | 16 | 16 | 32 | >128 | >128 | 0.03 | 1 | 0.25 |
| NR-49283 | 16 | 8 | 16 | 128 | >128 | 0.015 | 1 | 0.25 |
| NR-49284 | 16 | 32 | 32 | 128 | >128 | 0.03 | 0.25 | 0.125 |
| NR-49285 | 16 | 8 | 16 | 64 | >128 | 0.03 | 0.25 | 0.25 |
| NR-49286 | 16 | 8 | 32 | 128 | >128 | 0.015 | 0.25 | 0.125 |
| NR-49287 | 16 | 8 | 16 | 128 | >128 | 0.015 | 0.5 | 0.25 |
| NR-49288 | 16 | 16 | 32 | 128 | >128 | 0.03 | 0.5 | 0.125 |
| NR-49290 | 16 | 8 | 16 | >128 | >128 | 0.015 | 0.5 | 0.25 |
| NR-49291 | 16 | 8 | 16 | 128 | >128 | 0.015 | 1 | 0.25 |
| P-3 | 32 | 16 | 32 | >128 | >128 | 0.06 | 1 | 0.5 |
| P-11 | 16 | 8 | 16 | 128 | >128 | 0.015 | 0.125 | 0.125 |
| P-19 | 16 | 32 | 32 | >128 | >128 | 0.125 | 1 | 0.25 |
| Isolate 4 | 32 | 16 | 32 | 128 | >128 | 0.06 | 0.5 | 0.125 |
| Isolate 7 | 32 | 32 | 32 | >128 | >128 | 0.125 | 0.5 | 0.25 |
| Isolate 9 | 32 | 16 | 32 | >128 | >128 | 0.03 | 0.5 | 0.06 |
| HM-88 | 16 | 16 | 8 | 128 | >128 | 0.007 | 0.25 | 0.25 |
| ATCC 9689 | 32 | 16 | 32 | >128 | >128 | 0.125 | 1 | 0.125 |
| ATCC BAA1801 | 32 | 16 | 64 | 128 | >128 | 0.06 | 0.5 | 0.125 |
| ATCC BAA1870 | 32 | 8 | 16 | 128 | >128 | 0.015 | 2 | 0.25 |
| ATCC BAA1871 | 32 | 16 | 16 | 128 | >128 | 0.06 | 0.5 | 0.125 |
| ATCC 43255 | 16 | 4 | 8 | 64 | >128 | 0.06 | 0.5 | 0.25 |
| ATCC 43598 | 32 | 16 | 16 | 64 | 128 | 0.03 | 1 | 0.25 |
| ATCC 700057 | 32 | 16 | 64 | >128 | >128 | 0.125 | 0.5 | 0.25 |
| MIC50 | 16 | 16 | 16 | 128 | >128 | 0.03 | 0.5 | 0.25 |
| MIC90 | 32 | 16 | 32 | >128 | >128 | 0.125 | 1 | 0.25 |
3.2. In vitro susceptibility of gut microflora to curcumin
For curcumin to be considered as an anticlostridial option, it is imperative to establish its harmless effect on gut microflora. Assessment of the survival of major inhabitants of gut microflora, namely, Lactobacillus, Bifidobacterium and Bacteroides species, upon treatment with turmeric derivatives revealed stringency in their correlative activity against C. difficile. The assessment revealed that none of the derivatives inhibited growth of the tested species against C. difficile isolates, except for curcumin constraining the growth of Lactobacillus gasseri HM-407 at 32 μg/ml. By contrast, control antibiotics hampered the growth of the majority of tested gut microflora species, with MICs dropping down to ≤1 μg/mL in certain cases (Table 3).
Table 3:
The minimum inhibitory concentration (MIC, μg/ml) of turmeric’s derivatives and control anticlostridial drugs against normal microflora.
| Strain ID | Curcumin | Bisdemethoxycurcumin | Demethoxycurcumin | Tetrahydrocurcumin | Curcumol | Fidaxomicin | Vancomycin | Metronidazole |
|---|---|---|---|---|---|---|---|---|
| Bacteroides fragilis HM-711 | >128 | 32 | 64 | >128 | 128 | >128 | 8 | 1 |
| Lactobacillus casei ATCC® 334 | >128 | >128 | >128 | >128 | >128 | 4 | >128 | >128 |
| Lactobacillus gasseri HM-641 | >128 | >128 | >128 | >128 | >128 | ≤1 | ≤1 | >128 |
| Lactobacillus gasseri HM-400 | 64 | >128 | 64 | >128 | 128 | ≤1 | 8 | >128 |
| Lactobacillus gasseri HM-407 | 32 | >128 | 128 | 128 | >128 | ≤1 | ≤1 | >128 |
| Bifidobacterium longum HM-848 | >128 | >128 | >128 | >128 | >128 | ≤1 | ≤1 | 64 |
| Bacteroides dorei HM-719 | >128 | 128 | 128 | >128 | >128 | >128 | 16 | ≤1 |
| Bifidobacterium longum HM-846 | >128 | >128 | >128 | 64 | >128 | ≤1 | 1 | >128 |
| Bifidobacterium bifidum ATCC-11863 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| Lactobacillus crispatus HM-103 | >128 | >128 | >128 | >128 | >128 | ≤1 | ≤1 | >128 |
3.4. Measurement of C. difficile toxin inhibition mediated by curcumin
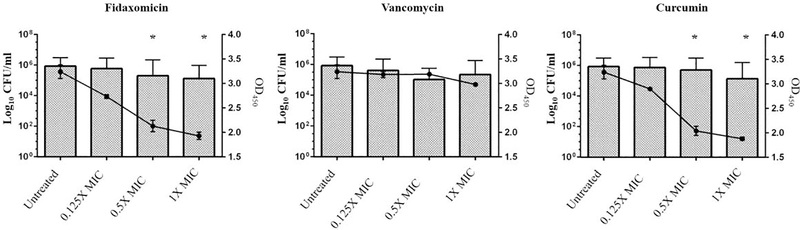
After preliminary evaluation of the antibacterial activity of selective derivatives of turmeric against C. difficile isolates and assessing gut safety prospects, curcumin was selected for further in vitro assessments due its availability and economic applicability. Curcumin exhibited a dose-dependent inhibition of C. difficile toxin production compared to the DMSO untreated control (diluent for drugs). As depicted in Figure 1, curcumin at 1/8 ×, 1/4 × and 1/2 × MIC inhibited 10.57%, 37.05% and 41.97% of total toxin production, respectively. Fidaxomicin, an FDA-approved anticlostridial antibiotic known to inhibit toxin production, inhibited toxin production by 15.62%, 30.47% and 40.44% at 1/8x, 1/4x and 1/2 × MIC, respectively. Vancomycin, on the other hand, showed no toxin inhibition. Our findings concerning the effect of fidaxomicin and vancomycin on toxin inhibition is consistent with previous reports [29].
Figure 1. Toxin inhibition by curcumin and control anticlostridial drugs (vancomycin and fidaxomicin).
Drugs at concentrations of 1/8 ×, 1/4 × and 1/2 × MIC were incubated with a hypervirulent, toxigenic strain of C. difficile (strain ATCC BAA1870). Bacterial counts were determined for each sample, and toxin levels were assessed in the supernatant using an enzyme-linked immunosorbent assay (ELISA). Error bars represent the standard deviation for values obtained from triplicate samples. The asterisk (*) represents a significant difference (p<0.01) from the untreated control sample.
3.3. Curcumin-mediated inhibition of C. difficile spore formation
To investigate its effects on spore formation, curcumin and control anticlostridial drugs were added at 0.5 × and 1 × MIC to a culture of C. difficile HM-88 in late exponential growth phase and incubated for 5 days. Curcumin significantly inhibited spore formation at 1 × MIC, by ∼0.6 log10, and some inhibition of spore formation was also observed at 0.5x MIC, ~0.4 log10, while fidaxomicin, a known spore inhibitor for C. difficile, significantly inhibited spore formation at both 1 × and 0.5 × MIC by ~1.5 log10 and ~0.8 log10, respectively (Figure 2). In contrast, vancomycin did not inhibit spore formation at either concentration [30].
Figure 2. Spore inhibition by curcumin compared with control anticlostridial drugs, fidaxomicin and vancomycin.
Drugs (1/2 x and 1 x MIC) were incubated with bacteria for five days followed by serial dilution and plating to count both total bacteria and heat-resistant spores. Error bars represent the standard deviation values from triplicate samples for two individual treatments. % represents non-spore forming cells. An asterisk (*) indicates a significant difference (p<0.01) from the untreated control sample.
3.6. Synergism of curcumin with existing anticlostridial drugs
Synergistic interactions between curcumin and current drugs of choice are depicted in Table 4. Curcumin did not show any synergistic activity when combined with control anticlostridial drugs but also did not interfere with the activity of any of the tested drugs. The FIC indices for both tested strains varied from 1.25 to 2.0, falling into the category of indifference. Notably, there were no antagonistic effects observed between curcumin and antibiotics.
Table 4:
The minimum inhibitory concentration (MIC, μg/ml) of turmeric’s derivatives and control anticlostridial drugs against human gut species.
| Strain | Anticlostridial | MIC (μg/ml) | FICI | Interpretation | |||
|---|---|---|---|---|---|---|---|
| Anticlostridial (Alone) | Curcumin (Alone) | Anticlostridial (Combination) | Curcumin (Combination) | ||||
| C. difficile ATCCBAA1870 | Fidaxomicin | 0.015 | 32 | 0.015 | 32 | 2 | Indifferent |
| Vancomycin | 2 | 32 | 2 | 32 | 2 | Indifferent | |
| Metronidazole | 0.25 | 16 | 0.25 | 4 | 1.25 | Indifferent | |
| C. difficile Isolate 20100207 | Fidaxomicin | 0.03 | 32 | 0.03 | 16 | 1.5 | Indifferent |
| Vancomycin | 0.25 | 32 | 0.25 | 16 | 1.5 | Indifferent | |
| Metronidazole | 0.125 | 32 | 0.25 | 16 | 1.5 | Indifferent |
DISCUSSION
The rapid emergence of hypervirulent C. difficile isolates and the paucity of effective anticlostridial antibiotics call for extensive research to identify more drugs to battle infections caused by this pathogen. The issue is complicated by the scarcity of treatment options. Currently, there are only three available drugs for the treatment of CDI, and only two have been approved by the FDA in last five decades. Additionally, high recurrence rates and potential colonization of other drug-resistant pathogens due to hampered gut microflora upon treatment are still prevalent. Although previous reports suggested minimal anticlostridial activity of raw powdered turmeric, an indigenous Indian spice known for its medicinal properties, further analysis of its fractions for activity against C. difficile had yet to be explored [14]. In this study, we evaluated the activity of reported bioactive extracts of turmeric against 27 C. difficile strains, including hypervirulent (BI/NAP1/027) and clinical toxigenic isolates. Curcuminoids are diarylheptanoids representing the major phytoconstituents of turmeric, consisting essentially of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Our findings showed that curcuminoids inhibit growth of C. difficile at concentrations ranging from 4 to 32 μg/ml. Additionally, curcuminoids are of key interest due to their status characterized as “safe” by various food and health administrations, such as the FDA, Joint Expert Committee of the Food and Agriculture Organization/World Health Organization (FAO/WHO), the Natural Health Products Directorate of Canada and the Codex Alimentarius, with a 2.8–3.0 mg/kg/day generally recognized as safe (GRAS) [31]. A significant consideration for an ideal therapy for CDI would be specificity of the antimicrobial agent used and any potential negative impact on gut microflora. Thus, it was essential to investigate the selective activity of turmeric on the gut microflora species, considering the supremacy of narrow-spectrum antibiotics with limited effect on normal gut inhabitants as an important factor for sustained success of CDI treatment and reduced relapses. In an in vitro model containing major populating species of the human gut, curcuminoids had an edge over vancomycin and metronidazole, which have been reported to cause declines in the counts of gut microflora upon treatment, as well as fidaxomicin, which is exemplified clinically for protecting the natural inhabitants of the gut [32, 33]. While fidaxomicin had deleterious effects against Lactobacilli and Bifidobacteria, curcuminoids spared all species, affirming gut microflora enriching properties of curcuminoids, as reported previously [31].
We narrowed the selective derivatives to just curcumin for investigating its inhibitory activity against C. difficile’s major virulence factors, toxin production and spore formation. Toxins play a pivotal role in inducing inflammation and provoking disease; thus, their inhibition can be linked to effective treatment of CDI [2]. Curcumin has previously been reported to have a concentration-based mechanism of action, i.e., inducing membrane damage at relatively high concentrations and displaying apoptotic markers at lower concentrations in Escherichia coli [34]. Therefore, we tested the effect of curcumin at sub-inhibitory concentrations against C. difficile ATCC BAA1870, a hypervirulent toxigenic strain of C. difficile. Curcumin inhibited the total toxin production at 0.5 × and 0.25 × better than fidaxomicin, the anticlostridial known for its toxin inhibiting properties, but no such effect was observed with vancomycin, consistent with previous reports. In addition to toxin production, spores formed by hypervirulent and epidemic strains of C. difficile provide resistance to disinfection procedures, with the potential to germinate post-treatment and resulting in higher relapse rates [2]. Curcumin resulted in spore reduction at 1 × MIC, as tested in C. difficile HM-88, but not as efficiently as fidaxomicin; in contrast, no reduction in spore formation was observed upon treatment with vancomycin. The ability of curcumin to interfere with spore formation may translate into reduced CDI relapses. Curcumin’s ability to address other deleterious effects related to CDI have been addressed in previous reports about its anti-inflammatory activities and ability to block IL-8 synthesis triggered in the case of Helicobacter pylori infection [35]. The anti-inflammatory property of curcumin can protect inflammation and rounding of human colonic epithelial cells (Caco-2) exposed to C. difficile toxins. Additionally, curcumin’s ability to block multiple signaling pathways enables it to reduce synthesis of the cytokine IL-8, which is strongly associated with the prognostic parameters of severe CDI [36]. Further study is needed to improve the bioavailability of curcumin, especially the poor solubility, to enhance the therapeutic efficacy and clinical applications.
In conclusion, we report anticlostridial activity of curcuminoids from a GRAS accredited natural spice, turmeric, against C. difficile, with special emphasis on curcumin. We also describe the ability of curcumin to inhibit the two main virulence factors of anaerobic pathogens, namely, toxin production and spore formation. Taking into consideration the safety profile of curcumin and previous reports of its ability to prevent inflammation and to reduce IL-8 secretion, our findings suggest that curcumin has potential as an anticlostridial agent. More work is needed to further investigate the efficacy of curcumin as a standalone drug or as a supplement of current drugs of choice, as it has no antagonistic activities but might overcome their drawbacks.
Highlights.
The study proposes curcumin, a bioactive extracts of turmeric, as a potential standalone natural
antibiotic for the treatment of Clostridium difficile infection. Synergistic results support the
likely use of curcumin as a supplement to current lines of drugs to enhance their activity.
Acknowledgements
We would like to thank Ahmed Hassan, Purdue University, for his assistance in carrying out this research.
Funding: No funding
Footnotes
Declarations
Competing Interests: None
Ethical Approval: Not required
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kachrimanidou M and Malisiovas N, Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol, 2011. 37(3): p. 178–87. [DOI] [PubMed] [Google Scholar]
- 2.AbdelKhalek A, et al. , Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int J Antimicrob Agents, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns DA and Minton NP, Sporulation studies in Clostridium difficile. J Microbiol Methods, 2011. 87(2): p. 133–8. [DOI] [PubMed] [Google Scholar]
- 4.Fekety R, et al. , Epidemiology of antibiotic-associated colitis; isolation of Clostridium difficile from the hospital environment. Am J Med, 1981. 70(4): p. 906–8. [DOI] [PubMed] [Google Scholar]
- 5.Lessa FC, et al. , Burden of Clostridium difficile infection in the United States. N Engl J Med, 2015. 372(9): p. 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickers RJ, et al. , Ridinilazole: a novel therapy for Clostridium difficile infection. Int J Antimicrob Agents, 2016. 48(2): p. 137–43. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor JR, Johnson S, and Gerding DN, Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology, 2009. 136(6): p. 1913–24. [DOI] [PubMed] [Google Scholar]
- 8.Yakob L, et al. , Mechanisms of hypervirulent Clostridium difficile ribotype 027 displacement of endemic strains: an epidemiological model. Sci Rep, 2015. 5: p. 12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald LC, et al. , An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med, 2005. 353(23): p. 2433–41. [DOI] [PubMed] [Google Scholar]
- 10.Warny M, et al. , Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet, 2005. 366(9491): p. 1079–84. [DOI] [PubMed] [Google Scholar]
- 11.Cornely OA, et al. , Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis, 2012. 55 Suppl 2: p. S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie TJ, et al. , Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med, 2011. 364(5): p. 422–31. [DOI] [PubMed] [Google Scholar]
- 13.Harnvoravongchai P, et al. , Insights into drug resistance mechanisms in Clostridium difficile. Essays Biochem, 2017. 61(1): p. 81–88. [DOI] [PubMed] [Google Scholar]
- 14.Roshan N, Riley TV, and Hammer KA, Antimicrobial activity of natural products against Clostridium difficile in vitro. J Appl Microbiol, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Num M, S. and Useh NM, Clostridium : Pathogenic Roles, Industrial Uses and Medicinal Prospects of Natural Products as Ameliorative Agents against Pathogenic Species. Vol. 7 2014. 81–94. [Google Scholar]
- 16.Nagpal M and Sood S, Role of curcumin in systemic and oral health: An overview. J Nat Sci Biol Med, 2013. 4(1): p. 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta SC, Patchva S, and Aggarwal BB, Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J, 2013. 15(1): p. 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schraufstatter E and Bernt H, Antibacterial action of curcumin and related compounds. Nature, 1949. 164(4167): p. 456. [DOI] [PubMed] [Google Scholar]
- 19.Karimi N, et al. , Antioxidant, Antimicrobial and Physicochemical Properties of Turmeric Extract-Loaded Nanostructured Lipid Carrier (NLC). Colloid and Interface Science Communications, 2018. 22: p. 18–24. [Google Scholar]
- 20.CLSI, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard - Eighth edition, in CLSI document M11-A8. 2012, Wayne, PA: Clinical and Laboratory Standard Institute. [Google Scholar]
- 21.Goldstein EJ, et al. , Comparative in vitro activities of SMT19969, a new antimicrobial agent, against Clostridium difficile and 350 gram-positive and gram-negative aerobic and anaerobic intestinal flora isolates. Antimicrob Agents Chemother, 2013. 57(10): p. 4872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thangamani S, et al. , Antibacterial activity and mechanism of action of auranofin against multidrug resistant bacterial pathogens. Sci Rep, 2016. 6: p. 22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammad H, et al. , Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. J Antibiot (Tokyo), 2015. 68(4): p. 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammad H, et al. , Antibacterial Characterization of Novel Synthetic Thiazole Compounds against Methicillin-Resistant Staphylococcus pseudintermedius. PLoS One, 2015. 10(6): p. e0130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammad H, Cushman M, and Seleem MN, Antibacterial Evaluation of Synthetic Thiazole Compounds In Vitro and In Vivo in a Methicillin-Resistant Staphylococcus aureus (MRSA) Skin Infection Mouse Model. PLoS One, 2015. 10(11): p. e0142321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harnvoravongchai P, et al. , Antimicrobial Effect of Asiatic Acid Against Clostridium difficile Is Associated With Disruption of Membrane Permeability. Front Microbiol, 2018. 9: p. 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldesouky HE, et al. , Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int J Antimicrob Agents, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Eldesouky HE, et al. , Reversal of Azole Resistance in Candida albicans by Sulfa Antibacterial Drugs. Antimicrob Agents Chemother, 2018. 62(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babakhani F, et al. , Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother, 2013. 68(3): p. 515–22. [DOI] [PubMed] [Google Scholar]
- 30.Chilton CH, et al. , Association of Fidaxomicin with C. difficile Spores: Effects of Persistence on Subsequent Spore Recovery, Outgrowth and Toxin Production. PLoS One, 2016. 11(8): p. e0161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zam W, Gut Microbiota as a Prospective Therapeutic Target for Curcumin: A Review of Mutual Influence. J Nutr Metab, 2018. 2018: p. 1367984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locher HH, et al. , In vitro and in vivo antibacterial evaluation of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob Agents Chemother, 2014. 58(2): p. 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chilton CH, et al. , Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. J Antimicrob Chemother, 2014. 69(2): p. 451–62. [DOI] [PubMed] [Google Scholar]
- 34.Yun DG and Lee DG, Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl Microbiol Biotechnol, 2016. 100(12): p. 5505–14. [DOI] [PubMed] [Google Scholar]
- 35.Foryst-Ludwig A, et al. , Curcumin blocks NF-kappaB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun, 2004. 316(4): p. 1065–72. [DOI] [PubMed] [Google Scholar]
- 36.Czepiel J, et al. , The presence of IL-8 +781 T/C polymorphism is associated with the parameters of severe Clostridium difficile infection. Microb Pathog, 2018. 114: p. 281–285. [DOI] [PubMed] [Google Scholar]