Abstract
Prior to maturation, mouse oocytes are arrested at the germinal vesicle (GV) stage during which they experience constitutive calcium (Ca2+) influx and spontaneous Ca2+ oscillations. The oscillations cease during maturation but Ca2+ influx continues, as the oocytes’ internal stores attain maximal content at the culmination of maturation, the metaphase II stage. The identity of the channel(s) that underlie this Ca2+ influx has not been completely determined. GV and matured oocytes are known to express three Ca2+ channels, CaV3.2, TRPV3 and TRPM7, but females null for each of these channels are fertile and their oocytes display minor modifications in Ca2+ homeostasis, suggesting a complex regulation of Ca2+ influx. To define the contribution of these channels at the GV stage, we used different divalent cations, pharmacological inhibitors and genetic models. We found that the three channels are active at this stage. CaV3.2 and TRPM7 channels contributed the majority of Ca2+ influx, as inhibitors and oocytes from homologous knockout (KO) lines showed severely reduced Ca2+ entry. Sr2+ influx was promoted by CaV3.2 channels, as Sr2+ oscillations were negligible in CaV3.2-KO oocytes but robust in control and Trpv3-KO GV oocytes. Mn2+ entry relied on expression of CaV3.2 and TRPM7 channels, but Ni2+ entry depended on the latter. CaV3.2 and TRPV3 channels combined to fill the Ca2+ stores, although CaV3.2 was the most impactful. Studies with pharmacological inhibitors effectively blocked the influx of divalent cations, but displayed off-target effects, and occasionally agonist-like properties. In conclusion, GV oocytes express channels mediating Ca2+ and other divalent cation influx that are pivotal for fertilization and early development. These channels may serve as targets for intervention to improve the success of assisted reproductive technologies.
Keywords: Calcium, influx, signaling, TRPV3, TRPM7, Cav3.2, meiosis
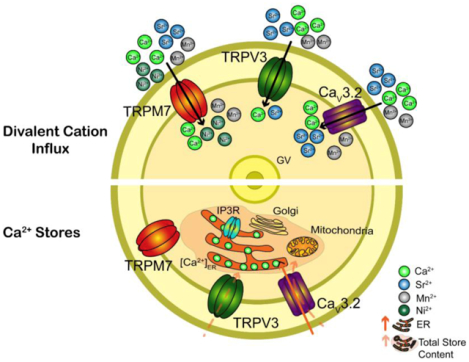
Graphical Abstract
1. Introduction
A wide range of cellular processes such as gene expression, muscle contraction, secretion, cell division, fertilization and apoptosis are regulated by changes in the intracellular concentration of free calcium ([Ca2+]i) [1]. To accomplish this, cells devote significant amounts of their energy reserves to create and maintain Ca2+ gradients between the extracellular and intracellular environments as well as between cellular compartments such that brief changes in [Ca2+]i can have profound signaling effects [2].
Transient rises in [Ca2+]i as well as Ca2+ influx from the extracellular environment ([Ca2+]o) play important roles in unfertilized and fertilized oocytes in all animal species from marine invertebrates to mammals [3–5]. In mammals, [Ca2+]i rises in MII oocytes, henceforth called eggs, induce exit from the meiotic arrest triggering egg activation and initiation of embryo development [6]. Prior to this stage, changes in Ca2+ homeostasis also occur during maturation, including spontaneous oscillations at the GV stage, and accumulation of Ca2+ in the endoplasmic reticulum (ER), the main internal Ca2+ store ([Ca2+]ER) [7]. The function of these Ca2+ changes prior to fertilization is not well established, although Ca2+ content in the ER is necessary to support the protein synthesis required for meiosis progression, and for the initial [Ca2+]i rises post fertilization [8, 9]. Remarkably, even though extracellular Ca2+ underlies both the spontaneous oscillations and the filling of [Ca2+]ER stores [10–12], the plasma membrane (PM) channel(s) that underpin Ca2+ influx at GV stage have remained uncharacterized.
Cells have at their disposal multiple PM channel(s) and transporters to maintain Ca2+ homeostasis [11, 13]. Oocytes and eggs are not an exception, and divalent cation permeable PM channel(s) were detected early on in these cells using electrophysiology [14, 15]. It was noted that voltage-dependent Ca2+ currents were present in GV oocytes and in MII eggs [16, 17], and that the presence of this current was associated with acquisition of meiotic competence [15]. Predictably, CaV3.2 (member of the T-type Cav sub-group [18]), which are voltage gated Ca2+ channels, were the first whose functional expression was demonstrated in eggs and zygotes [19, 20]. Although readily detectable, genetic studies found that mice lacking CaV3.2 are fertile and their oocytes and eggs display only slightly reduced [Ca2+]ER content [21]. Importantly, how CaV3.2 channels affected spontaneous oscillations or divalent cation plasma membrane fluxes in GV oocytes and during maturation was not examined. Given that CaV3.2-KO mice are fertile, other Ca2+ influx mechanism(s) must be functional in mammalian oocytes and eggs. A proposed mechanism of Ca2+ influx was the Store Operated Ca2+ Entry (SOCE), which is composed of STIM1, that acts as the sensor of ER Ca2+ levels, and ORAI1, which is the PM channel [22, 23]. Although STIM1 and ORAI1 appear to be expressed at the molecular level in mouse oocytes [10], the functional expression of ORAI1 via electrophysiology has not been confirmed, and single or combined genetic deletion of Stim1/2 and Orai1 failed to affect Ca2+ influx in mouse oocytes and/or eggs [24]. The transient receptor potential (TRP) family of channels could also mediate Ca2+ influx in mouse oocytes and eggs. TRP channels are grouped into six subfamilies: TRPC, TRPM, TRPV, TRPA, TRPML, and TRPP (or PKD) [25, 26]. These channels are, in general, nonselective, weakly voltage-dependent, and display widespread expression. We have previously reported functional expression of TRPV3 and TRPM7 in mouse oocytes and eggs [27, 28], and showed that they are under distinct regulation during oocyte maturation. For example, TRPV3 expression was undetectable by electrophysiology at the GV stage, but its activity increased gradually during maturation and peaked at the MII stage [27]. It was not examined however if TRPV3 impacts [Ca2+]ER content in GV oocytes, and whether it mediates entry of other divalent cations at this stage. Importantly, just like CaV3.2 null mice, Trpv3-KO mice are fertile [27]. Unlike TRPV3, the functional expression of TRPM7 is more prominent in GV oocytes than in MII eggs, and its functional expression experiences a rebound after fertilization in 2-cell embryos [28]. Whole-animal deletion of Trpm7 is embryonic lethal, which has prevented the study of its function in gametes of Trpm7-null mice. However, a recently generated conditional allele showed that TRPM7 mediates Ca2+ influx in GV oocytes and in eggs post-fertilization, although conditional KO (cKO) females displayed normal fertility [29]. TRPM7 displays permeability to a range of trace metal ions [30] including Mg2+ and Zn2+. It has been shown that Zn2+ plays a critical role in oocyte maturation and fertilization [31]. Therefore, it cannot be ruled out that besides permeating Ca2+, this channel plays a primary role in permeating other divalent cations in mouse oocytes and eggs [28].
In this study, we used a combination of techniques including fluorescence imaging, genetic models and pharmacological agents to identify the contribution of the three known channels to divalent cation influx in mouse GV oocytes. We aimed to determine their preference for specific divalent cations measured by fluorescence, establish their contributions to [Ca2+]ER content, and test the specificity of commonly used pharmacological inhibitors. We found that all three channels CaV3.2, TRPV3, and TRPM7 are active at this stage, although CaV3.2 and TRPM7 are largely responsible for Ca2+ influx. These channels supply the [Ca2+]ER of GV oocytes, albeit CaV3.2 seems the most important. Lastly, multiple divalent cations pass through these channels, but not to the same extent, as determined by the inability of certain divalent cations to induce intracellular responses in oocytes from KO models and/or in the presence of inhibitors, some of which displayed off-target effects and/or agonist-like properties.
2. Materials and Methods
2.1. Mouse strains and collection of mouse oocytes
CaV3.2-KO mice were purchased from Jackson Laboratory (JAX stock #013770; Bar Harbor, ME) [32]. Trpv3-KO mice with mixed background of C57BL/6 and 129/SvEvTac, were the generous gift from Dr. Cheng (University of Michigan, Ann Arbor, MI) [33]. To generate Trpv3-CaV3.2-KO (double knockout) colony, single KO mice were mated together. Double heterozygotes males and females mated to achieve double knockout animals. CD1 females and WT C57BL/6 females were used as controls.
GV oocytes were collected from ovaries of 6- to 10-week-old female mice 44–46 h after injection of 5 IU of pregnant mare serum gonadotropin (PMSG; Sigma; Saint Louis, MO). Immediately after euthanasia, MII eggs were collected from the oviducts (CD1 females) 12–14 h after administration of 5 IU of human chorionic gonadotropin (hCG), which was administered 46–48 h after PMSG. Cumulus cells were removed with 0.1% bovine testes hyaluronidase (Sigma). HEPES–buffered Tyrode’s lactate solution (TL-HEPES) containing 5% heat-treated fetal calf serum (FCS; Gibco/ThermoFisher; Waltham, MA) and 100 μM IBMX was used to collect cumulus intact GV oocytes. Cumulus cells were removed by repetitive pipetting and denuded oocytes were kept in Chatot, Ziomek, and Bavister (CZB) media supplemented with 3 mg/ml bovine serum albumin (BSA) and IBMX and were incubated under mineral oil at 37°C in a humidified atmosphere of 5% CO2. All animal procedures were performed according to research animal protocols approved by the University of Massachusetts Institutional Animal Care and Use Committee.
2.2. PCR Genotyping
PCR genotyping were performed using ear punch samples to confirm Trpv3-KO line, using primers as previously described [33]. Jackson Laboratory protocol was followed to confirm CaV3.2-KO line [32]. Both sets of primers were used to confirm Trpv3-CaV3.2-KO line.
2.3. Total RNA extraction and Real Time PCR
High Pure RNA Isolation Kit (11828665001; Roche Life Sciences) was used to extract total RNA from GV oocytes (#50) and MII eggs (#50) of CD1 females. iScript cDNA Synthesis kit (1708890; Bio Rad) was used for cDNA synthesis. Taqman qPCR was performed using gene expression assays to detect the three ion channels in both GV oocytes and MII eggs using following primers: TRPV3 (Mm00455003_m1), TRPM7 (Mm00457998_m1), CaV3.2 (Mm00445382_m1) (ThermoFisher SCIENTIFIC).
2.4. Western blot
Mouse GV oocytes and MII eggs (n=200 or 400) were collected from CD1 females as described above. Whole lysates were prepared by the immediate addition of 2X-Laemmli sample buffer and frozen at −80 °C until use. Whole tissue lysates were prepared as previously described by our laboratory (Wu et al., 1998, and He et al., 1997). Thawed samples were boiled for 3 min, mixed well and loaded onto 6.0-% SDS-PAGE gels, and the resolved polypeptides were transferred onto Nitrocellulose membranes (FisherScientific, Cat # 45-004-002) using a Mini Trans-Blot Cell (Bio-Rad, Hercules, CA). Transfer was done using transfer buffer (4X) without methanol. The membranes were blocked in 6% nonfat dry milk in PBS– 0.1% Tween, and incubated overnight at 4 °C with the selected primary antibody; this was followed by multiple washes and finally for 1 h of incubation with a horseradish peroxidase–labeled secondary antibody (BioRad, Cat # 170–6515, 1:2000). Immunoreactivity was detected using chemiluminescence reagents (NEN Life Science Products, Boston, MA) and visualized using a G-box imaging system (Syngene). The primary antibodies were a rabbit anti-TRPM7 monoclonal (Abcam, Cat # EPR4582, 1:1000 dilution); a rabbit anti-CaV3.2 polyclonal antibody (Alomone, Cat # ACC-025, 1:500); and an anti-TRPV3 (N15/4) (Neuromab Cat # 75–043). Western blots were repeated at least two times.
2.5. Imaging procedures and monitoring of divalent cation influx
Ca2+ imaging was performed as previously described by our laboratory [34], using a Ca2+ sensitive dye Fura-2-acetoxymethyl ester (Fura 2-AM, Invitrogen/ThermoFisher). Oocytes were loaded with 1.25 μM Fura-2AM supplemented with 0.02% Pluronic acid (Invitrogen) for 20 min at room temperature (RT). Oocytes were placed in micro-drops of TL-HEPES on glass bottom dish (Mat-Tek Corp., Ashland, MA) under mineral oil. Oocytes were monitored simultaneously using an inverted microscope (Nikon, Melville, NY) outfitted for fluorescence measurements. Fura 2AM was excited between 340 and 380 nm wavelengths using a 75 W Xenon arc lamp and a filter wheel (Ludl Electronic Products Ltd., Hawthorne, NY), and fluorescence was captured every 20s. The emitted light above 510 nm was collected by a cooled Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ). Nikon Element software was used to coordinate the filter wheel and data acquisition. The acquired data were saved and analyzed using Microsoft Excel and GraphPad Prism Version 5.0 (GraphPad Software, La Jolla, CA). Ca2+ influx and spontaneous Ca2+ oscillations were measured using TL-HEPES media containing the standard concentration of 2 mM CaCl2. We also induced Ca2+ influx and oscillations by adding extra Ca2+ to a final concentration of 5 mM, 10 min after the initiation of Ca2+ monitoring. Assessment of Sr2+ influx and oscillations were carried out in Ca2+ free TL-HEPES supplemented with 10 mM Sr2+ throughout the monitoring period. Mn2+ influx studies were performed in TL-HEPES media containing 2 mM CaCl2, which 100 μM Mn2+ were added 5 min after the initiation of monitoring. Mn2+ influx was estimated by the quenching of Fura-2’s fluorescence intensity at the 360 nm wavelength, which is the isosbestic point. To detect Ni2+ influx, oocytes were loaded with 0.5 μM Mag-Fura-2AM (Invitrogen) supplemented with 0.02% Pluronic acid for 10 min at RT. Monitoring of Ni2+ influx, which caused a decrease in the intensity of Mag-Fura-2 at both wavelengths was performed in nominal Divalent Free Media (DFM, nominal Ca2+ and Mg2+ free TL-HEPES). Mag-Fura-2 was excited as for Fura-2 and fluorescence captured every 20s. Ca2+ influx studies following emptying of the stores by thapsigargin (TG) were performed in nominal Ca2+ free TL-HEPES. Ca2+ was added to a final concentration of 5 mM at the indicated time point several minutes after the initial Ca2+ rise caused by TG, had reached baseline values.
2.6. Measurement of Ca2+ Store content
GV oocytes were kept in CZB media supplemented with IBMX and 0.01% polyvinyl alcohol (PVA; Sigma) at 37°C in a humidified atmosphere of 5% CO2 for 1 h in the presence of specific inhibitors. The specific inhibitors were kept during loading of Fura-2AM and Ca2+ monitoring. Emptying of the stores was performed in nominal Ca2+ free TL-HEPES following the addition of Ionomycin (IO) (2.5 μM) or thapsigargin (10 μM) at the indicated time points. Amplitude and area under the curve (AUC) of Ca2+ rise was assessed.
2.7. Pharmacological agents
NS8593 (N2538), NiCl2.6H2O (N6136) and MgCl2.6H2O (M2393) were purchased from Sigma Aldrich. Mibefradil (2198) was from Tocris. 2-APB (100065), Thapsigargin (586005) and Ionomycin (407592) were from Calbiochem. Waixenicin A was provided as a generous gift from Dr. F. David Horgen (Hawaii Pacific University; Kaneohe, HI). Stock solutions were made following manufacturers’ recommendations and kept in −80 °C.
2.8. Slope calculations
The slope of the decline of the fluorescence intensity caused by the addition of Mn2+ and Ni2+ was used to estimate the influx of these ions. The rate of quenching (ΔF360 and ΔF380) was measured by analyzing the slope of the fluorescence decline for 4 min between X1: the first value after addition of the divalent cation where noticeable quenching was observed, and X2: the value after 4 min had elapsed from X1. ΔF360 and ΔF380 was normalized relative to the fluorescence level at inflection point. This method was chosen as it permitted to compare the effects of all the inhibitors on the rate of influx of Mn2+ and Ni2+ influx with a single slope. Corresponding values for each oocyte were collected, averaged and statistically compared.
2.9. Statistical analysis
Values from at least three different experiments were used for evaluation of statistical significance for each of the comparisons performed. The Prism software (GraphPad Software) was used to perform the statistical comparisons using Student’s t-test or one-way ANOVA or Chi Square test, according to the experiment analyzed. All bar graphs are presented as mean ± SEM. Significant differences were considered at P values < 0.05.
3. Results
3.1. Assessing divalent cation influx in GV oocytes
Mouse GV oocytes display spontaneous oscillations in the presence of [Ca2+]o (7) or [Sr2+]o [7, 35], which suggests the expression of nonselective cation channel(s) in these cells. This is in agreement with reports describing functional expression in oocytes and/or eggs of CaV3.2 and TRPM7 channels, which are all capable of permeating a variety of divalent cations [21, 24, 27, 29]. However, whether the level of expression of Cav3.2 and TRPM7 channels change during maturation, and which or if all these channel(s) are responsible for the influx of divalent cations in GV oocytes is unknown. To gain insight into these questions, we tested the expression of the channels in GV oocytes and MII eggs. We first used quantitative reverse transcriptase PCR, and our results show that the expression of these channels does not change between these stages (Supp. Fig. 1). Next, we performed western blotting with commercially available antibodies and whole cell lysates containing between 200 to 400 oocytes/eggs. A prominent immunoreactive band corresponding to TRPM7, approximate MW of 220 kDa (Fig. 1A), was observed in oocyte and eggs, whereas we failed to detect immunoreactivity for the other two channels in these cells (Supp. Fig. 2A and data not shown). Remarkably, the TRPM7 levels seemed to increase during maturation, and its relative MW in MII eggs appeared higher than in GV oocytes, which suggests that TRPM7 undergoes phosphorylation during this process. The antibody used to detect TRMP7, was also reactive against TRPM6. Whereas we detected TRPM6 prominently in three different tissues (Fig. 1B), we did not detect it in oocytes and/or eggs (Fig. 1A), in agreement with previous reports [28], although a faint band corresponding to TRPM7 was apparent in the kidney lane (Fig. 1B). Brain showed immunoreactivity against Cav3.2 (Suppl. Fig. 2B), although immunoreactivity to TRPV3 was difficult to judge due to many non-specific bands (data not shown); noteworthy, TRPV3 expression in eggs was previously reported by us using electrophysiology and immunofluorescence [27]. It is worth noting that CaV3.2 has been functionally tested by Ca2+ imaging and electrophysiology in MII eggs [21].
Figure 1. TRPM7 protein is expressed in mouse GV oocytes and MII eggs.
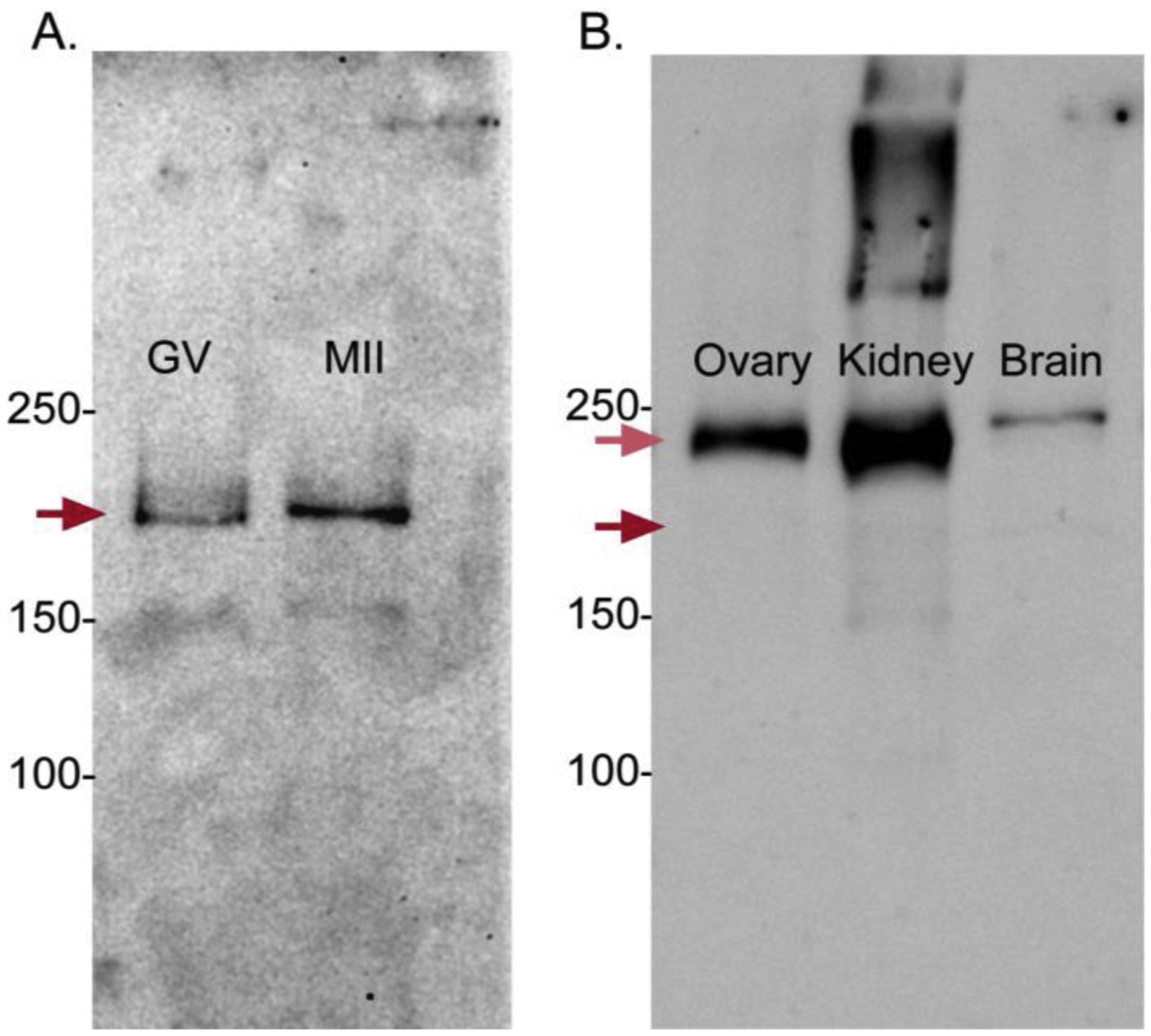
Immunoreactivity of TRPM7 was detected using an anti-TRPM7 + TRPM6 antibody. (A) The immunoreactive band in oocytes and eggs is likely TRPM7 given the ~MW of 210 kDa, and because its functional expression was confirmed by electrophysiology [28]. (B) In all tissues tested here, TRPM6 expression (~MW 240 kDa) appears to be clearly predominant over TRPM7. Red arrows indicate the expected locations of TRPM7 (dark red) and TRPM6 (light red). Representative blots are shown.
Given the higher sensitivity of Ca2+ imaging to detect functional expression of channels vs. western blotting, and the need to ascertain the active channels in GV oocytes, we used this approach to probe the active channels in oocytes. We monitored the influx of various divalent cations besides Ca2+ and Sr2+ such as Mn2+ and Ni2+. Mn2+ has been widely used to test divalent influx in cells and eggs [36, 37], whereas Ni2+, which effectively inhibits voltage-gated Ca2+ channels especially CaV3.2 [38], efficiently permeates through TRPM7 [30]. We used fluorometric dyes to monitor the influx of these cations [39]. Our results show that all the aforementioned divalent cations persistently enter the ooplasm. The influx of Ca2+ and Sr2+ induced oscillations, although of different frequencies (Fig. 2A, B), while Mn2+ and Ni2+ caused sustained fluorescence quenching (Fig. 2C, D). In addition, whereas Ca2+, Sr2+, and Mn2+ gained rapid access into the ooplasm, Ni2+ required several min (Fig. 2D). These results show that the PM of GV oocytes is permeable to multiple divalent cations, and suggest that the level(s) of expression and function of the underlying channels is closely regulated.
Figure 2. Divalent cations permeate the plasma membrane of GV oocytes.
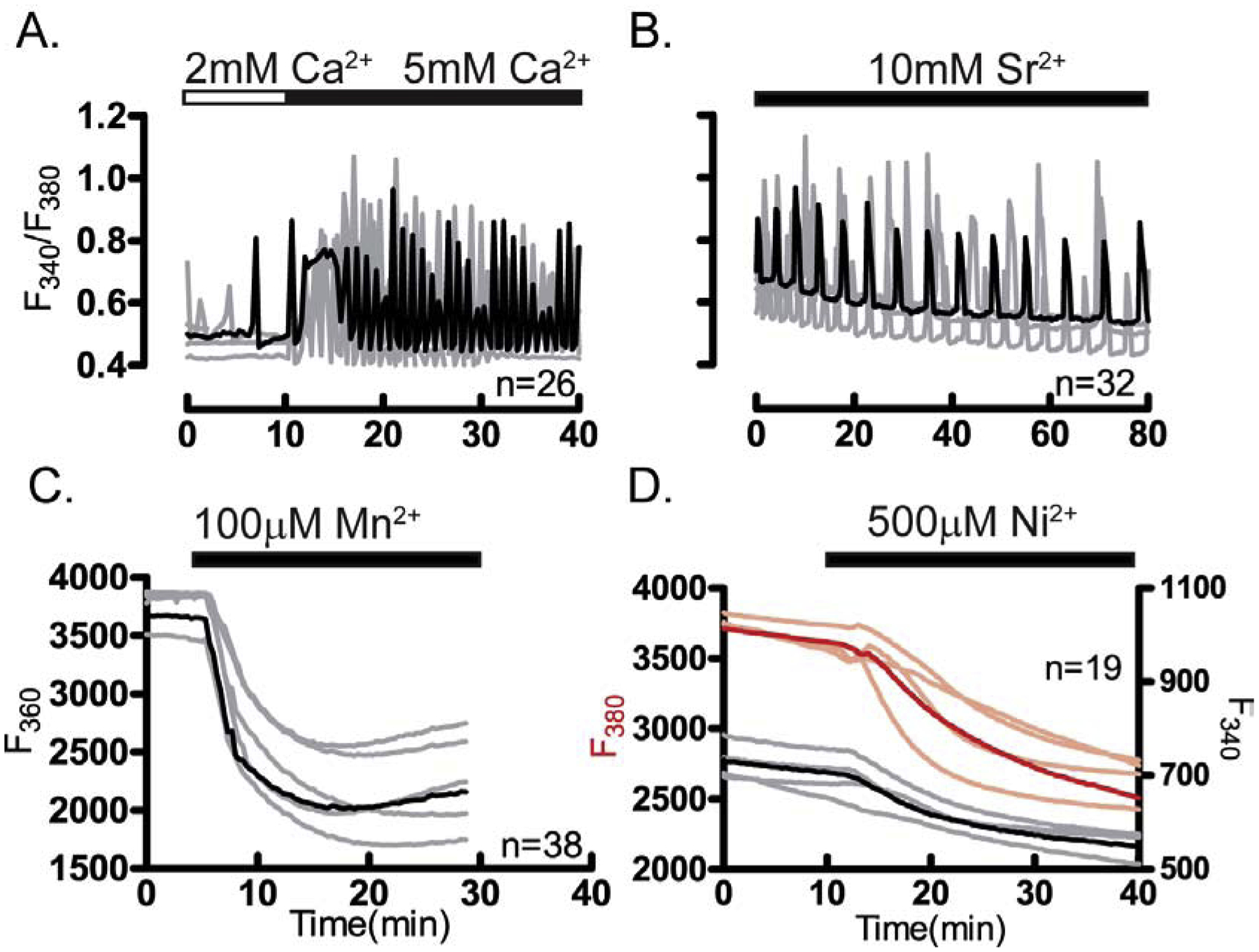
Divalent cation influx was examined in GV oocytes. (A) Representative traces of Ca2+ oscillations caused by enhanced influx after increasing [Ca2+]o from 2 mM to 5 mM. (B) Sr2+ induced oscillations. (C) Fluorescence quenching at F360 following Mn2+ addition (100 μM). (D) Fluorescence quenching at F380 following Ni2+ addition (500 μM) in oocytes loaded with Mag-Fura-2AM in DFM media. Horizontal bars above each panel show the time during which divalent cations were added to the media. All experiments were replicated 4 times. In all panels in this figure and throughout the manuscript, representative traces are shown; darker traces represent the most common response; n: total number of oocytes examined.
3.2. Several channels contribute to spontaneous and induced Ca2+ influx in GV oocytes
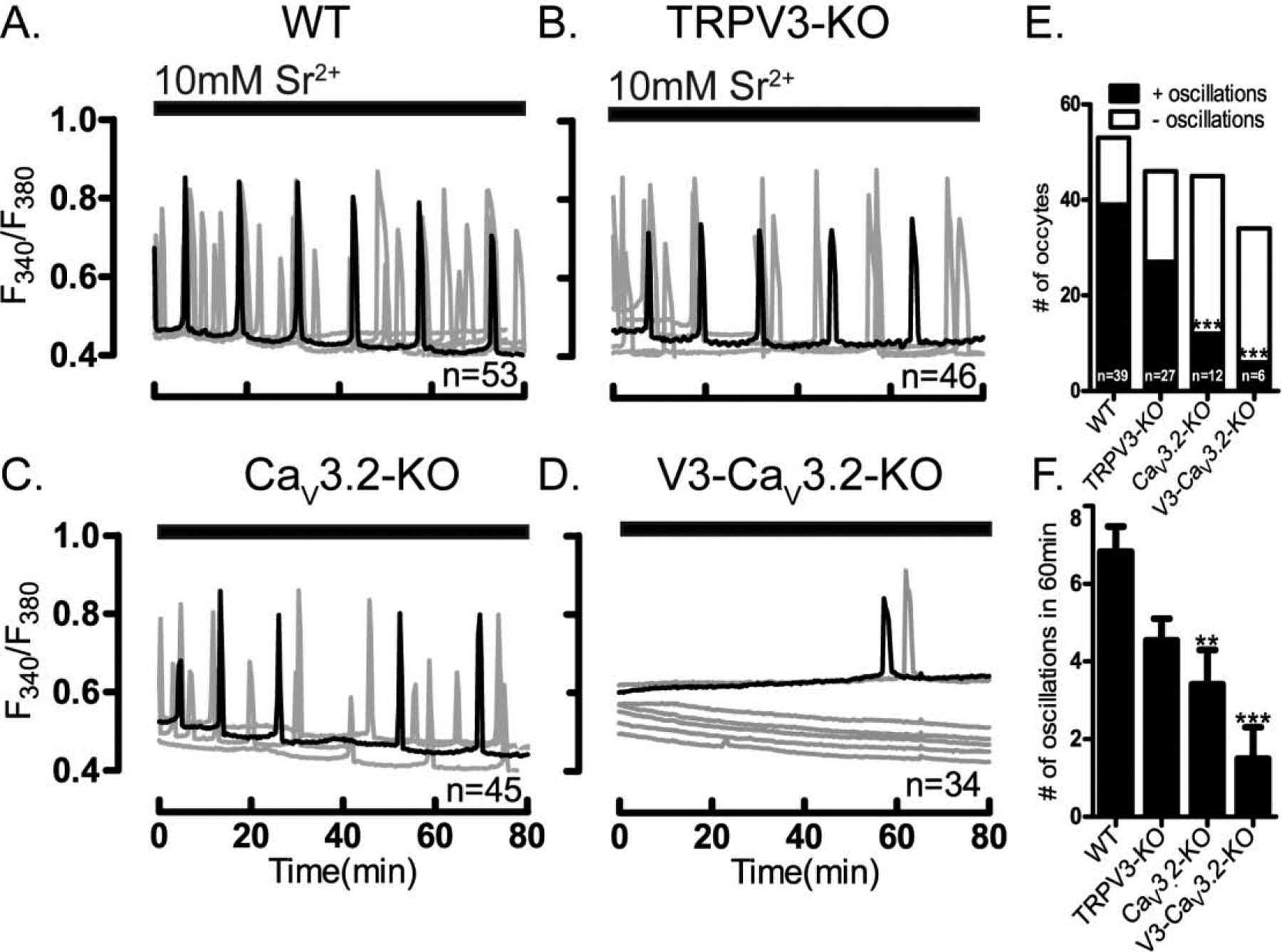
We next examined which of the aforementioned PM channels underlies the spontaneous [Ca2+]i oscillations in GV oocytes. For this we first used oocytes from mice null for specific channels. We tested [Ca2+]i oscillations in oocytes from CaV3.2, Trpv3 and Trpv3-CaV3.2 null mice (see Materials and Methods). We found that Trpv3-KO oocytes showed spontaneous oscillations similar to those of WT and CD1 oocytes, whereas fewer oocytes from CaV3.2 and Trpv3-CaV3.2-KO mice exhibited oscillations, which also were of much shorter duration (Fig. 3A). These results suggest that expression of CaV3.2 channels is necessary to support the spontaneous Ca2+ oscillations in GV oocytes (Fig. 3D; P<0.5).
Figure 3. Ca2+ influx in GV oocytes is mediated by multiple channels.
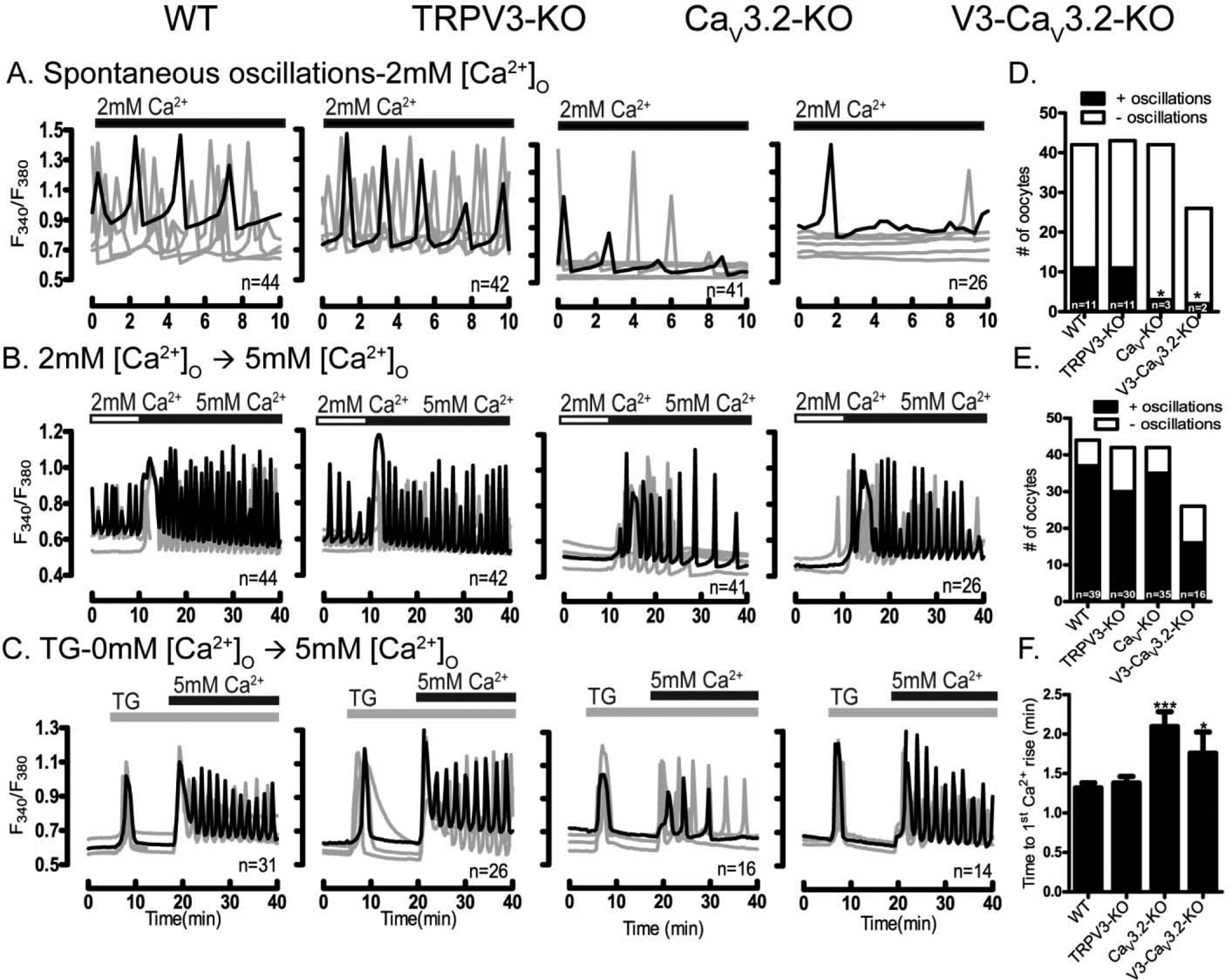
Presence of spontaneous oscillations and Ca2+ influx examined in WT oocytes and oocytes lacking TRPV3, CaV3.2 & V3-CaV3.2 channels. (A-four upper panels) Spontaneous Ca2+ oscillations in WT and KO oocytes in media containing basal Ca2+ levels (2 mM). Fewer oocytes of the CaV3.2 and V3-CaV3.2 KO lines display oscillations, which are also of decreased frequency (D) (P< 0.05). (B-four medium panels) Ca2+ influx triggered by increasing [Ca2+]o from 2 mM to 5 mM induces oscillations in all WT and KO lines, which appear of equal initial frequency (E). (C-four lower panels) After addition of TG (10 μM), Ca2+ influx resulted in reduced responses in CaV3.2 and V3-CaV3.2 KO oocytes, especially in the interval from Ca2+addition to the 1st Ca2+ peak (P< 0.05) (F). Filled horizontal bars above each panel show the time of addition of Ca2+ (black) or TG (grey) to the media. All experiments were 4 replicated for times. Asterisk(s) above columns in bar graphs denote significant differences from other conditions here and throughout the manuscript.
Ca2+ influx can be abruptly induced in GV oocytes, and we therefore examined if this type of influx was mediated by the same channel(s). To accomplish this we used two well established methods [40]: first, we raised [Ca2+]o from 2 mM to 5 mM, and second, after emptying the stores with TG, we raised [Ca2+]o from 0 mM to 5 mM. Baseline [Ca2+]i levels were monitored prior to increasing [Ca2+]o, and monitoring continued after the addition of Ca2+. We found that raising [Ca2+]o stimulated Ca2+ influx in oocytes of all strains (Fig. 3B, E), although with slightly greater initial rise (amplitude) and persistence in WT, Trpv3, and Trpv3-CaV3.2-KO oocytes than in oocytes of CaV3.2-KO mice. Significant differences were detected when applying the TG protocol (Fig. 3C); we observed a time delay to the first [Ca2+]i rise in both CaV3.2- and Trpv3-CaV3.2-KO oocytes (Fig. 3F; P <0.05), and in CaV3.2-KO oocytes [Ca2+]i levels quickly returned to the original baseline values, which was not the case for oocytes of the other strains that continued to oscillate above the baseline. These results suggest that CaV3.2 channels play an important role in the influx of Ca2+ in GV oocytes, although other channels, especially following abrupt increases, are also involved in Ca2+ entry in these cells. How CaV3.2 channels participate in this influx is unknown, but it is possible that they mediate an initial influx of Ca2+ that causes changes in membrane potential and/or activate nearby channels triggering the opening of these Ca2+ channels that are ultimately responsible for the large influx, but these possibilities remain to be established.
To identify the channel(s) capable of mediating the remaining influx associated with abrupt Ca2+ increases, we made use of pharmacological inhibitors (see Suppl. Table 1 for summary of inhibitors and suggested specificity). We first examined the effects of common CaV3.2 channel inhibitors such as Mibefradil (1μM) and Ni2+ (100μM) [41]. These inhibitors failed to prevent the initiation of oscillations following addition of extra [Ca2+]o, confirming that Ca2+ can permeate through other channel(s) (Fig. 4A, B). It is worth noting that whereas mibefradil eventually terminated the oscillations, Ni2+ had negligible effect and it might have slightly prolonged the responses. We next examined the participation of TRPM7 channels, as we have previously shown that its inhibition abrogated Ca2+ influx in GV oocytes [28]. We first used two general inhibitors: 2-APB (50 μM) and MgCl2 (10 mM). These inhibitors prevented oscillations, although MgCl2 was more effective (Fig. 4C). We then examined more specific inhibitors of TRPM7, and showed that NS8593 (10 μM) had a strong inhibitory effect (Fig. 4D), which is consistent with our previous results [28]. We also used waixenicin-A (WA, 5 μM), which purportedly has the highest specificity against TRPM7 [42, 43]; WA also effectively suppressed Ca2+ influx and oscillations (Fig. 4D). We repeated these studies using the TG approach (Suppl. Fig. 3). Our results were mostly consistent with the previous data in that CaV3.2 inhibitors were ineffective at reducing the initial Ca2+ influx, and 2-APB (100 μM) and MgCl2 (10 mM) were very potent inhibitors, especially the latter. Of the TRPM7 inhibitors, NS8593 (10 μM) successfully inhibited the responses, but WA was not effective, although due to cell toxicity we only used low concentrations of it (5 μM). Altogether, the data suggest that besides CaV3.2, TRPM7 is an important contributor to Ca2+ influx in GV oocytes. We further confirmed this by demonstrating that Ca2+ influx in TRPV3-CaV3.2-KO oocytes is abrogated by treatment with the TRPM7 inhibitor NS8593 (10 μM) (Suppl. Fig. 4). These results are consistent with a recent report showing greatly reduced TG-induced Ca2+ influx in Trpm7-cKO GV oocytes [29, 44, 45].
Figure 4. Pharmacological inhibitors disrupt Ca2+ influx/oscillations in GV oocytes.
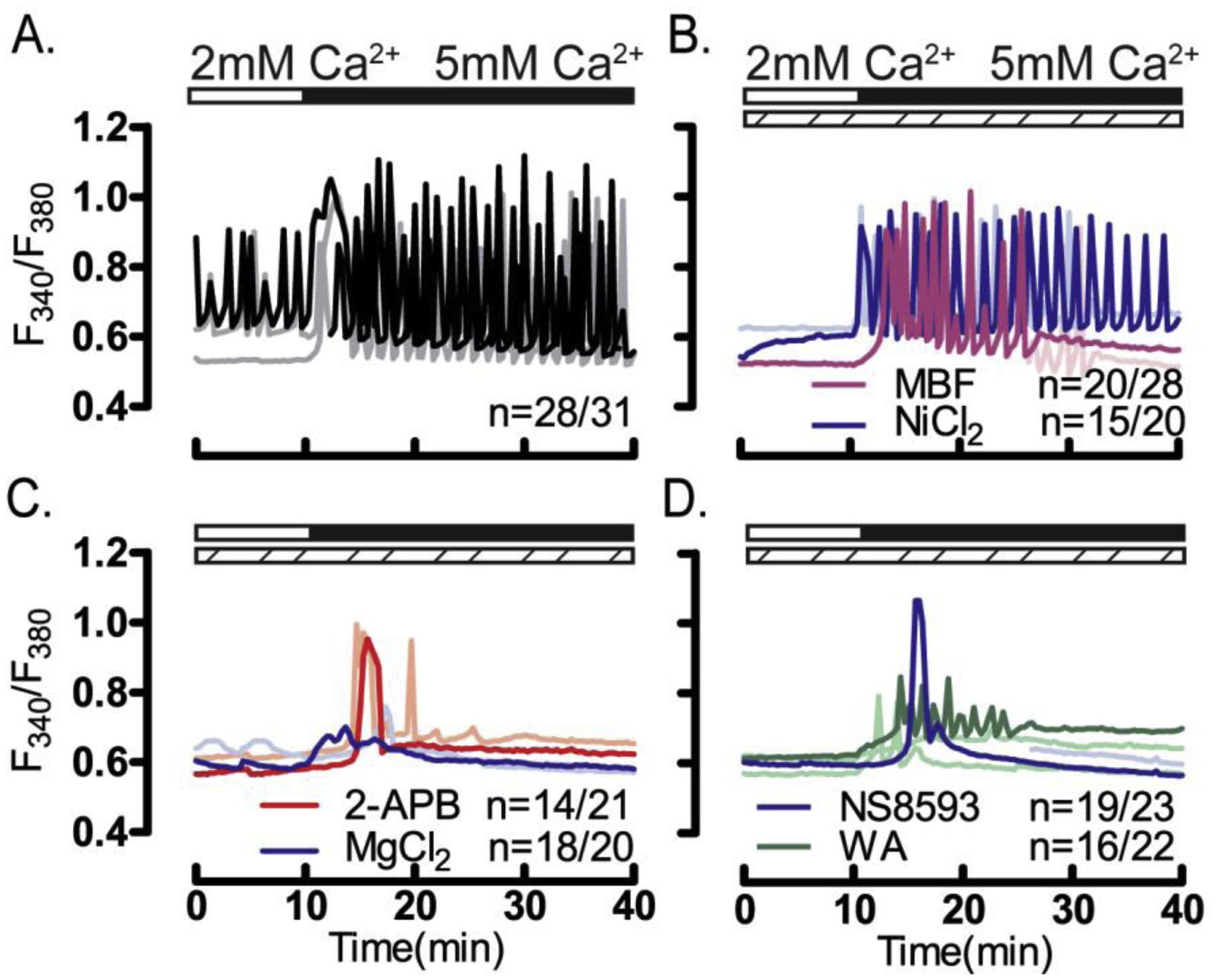
Ca2+ influx in GV oocytes was tested by increasing [Ca2+]o from 2- to 5 mM (white/black horizontal bars above each panel) in the presence of pharmacological inhibitors (dashed bars). Increasing [Ca2+]o in (A) control, untreated oocytes, or oocytes treated with (B) CaV3.2 inhibitors: MBF (1 μM) or Ni2+ (100 μM) induced oscillations in the majority of oocytes. Conversely, Increasing [Ca2+]o in the presence of the (C) general TRP channel inhibitors 2-APB (50 μM) or MgCl2 (10 mM), or (D) in the presence of the TRPM7 inhibitors NS8593 (10 μM) or waixenicin-A (5 μM) failed to induce oscillations (P <0.05). All experiments were performed in 4 replicates.
3.3. Sr2+ induced oscillations in GV oocytes are greatly diminished in the absence of CaV3.2 channels
[Sr2+]o was shown to generate action potentials in mouse MII eggs [17], and later studies showed that it is capable of inducing spontaneous oscillations in GV oocytes and MII eggs [12, 46]. These oscillations likely represent, at least initially, intracellular changes in the concentrations of both Ca2+ and Sr2+, although they are induced by the influx of Sr2+ from the extracellular media, which for these experiments is nominally Ca2+ free. However, the channel(s) responsible for mediating Sr2+ influx were unknown until recently when we showed TRPV3 mediates Sr2+ influx in MII eggs [27]. This study also reported that the functional expression of TRPV3 was low in GV oocytes, raising doubts whether it could mediate Sr2+ influx at this stage. We examined this question using Trpv3-KO GV oocytes, which showed robust Sr2+ oscillations, as we reported previously [28], although of lower frequency than WT oocytes (Fig. 5A, B, F; P<0.05). Conversely, Sr2+ oscillations were severely curtailed in CaV3.2-KO, and almost absent in Trpv3-CaV3.2-KO oocytes suggesting that CaV3.2 channels play a pivotal role in mediating Sr2+ influx in GV oocytes (Fig. 5C–F; P<0.05). Nevertheless, the fact that a few oscillations were still detectable in Trpv3-CaV3.2-KO oocytes suggests the presence of an additional channel(s).
Figure 5. Sr2+-induced oscillations are diminished in CaV3.2- and V3-CaV3.2-KO oocytes.
Sr2+ induced oscillations were tested in WT and in oocytes lacking TRPV3, or CaV3.2 or V3-CaV3.2 channels. Experiments were performed in nominal Ca2+-free media containing 10 mM Sr2+. (A) Sr2+ induced the expected oscillations in WT oocytes, and largely similar responses in (B) TRPV3-KO oocytes. Sr2+ induced oscillations were reduced in (C) CaV3.2-KO and in (D) V3-CaV3.2-KO oocytes both in the (E) number of cells showing oscillations, and in the (F) number of rises during the first 1h of imaging (P<0.05). Horizontal bars above each panel denote the time during which Sr2+ was present in the media. All experiments were performed in 3–4 replicates.
To identify other channel(s) mediating Sr2+ influx in WT GV oocytes, we used the same series of pharmacological inhibitors. Consistent with genetic studies, the CaV3.2 inhibitors, mibefradil (1 μM) and Ni2+ (100 μM), immediately terminated Sr2+ oscillations in all treated oocytes (Fig. 6A–B). Among the broad-range inhibitors, MgCl2 (10 mM) also immediately stopped the oscillations, although the non-specific inhibitor 2-APB (50 μM) only protractedly attenuated the oscillations (Fig. 6C). Remarkably, higher concentrations of 2-APB caused a large Sr2+ influx (100 μM, Suppl Fig. 5A–C), which is consistent with its role as an agonist of TRPV3 channels [47]; it also demonstrated for the first time functional expression, albeit incipient, of TRPV3 channels in GV oocytes. The inhibitors of TRPM7 channels showed dissimilar effects, as whereas addition of NS8593 (10 μM) terminated the oscillations after a short delay, WA (5 μM), the most specific TRPM7 inhibitor, was almost without effect (Fig. 6D). It is worth noting that we did not use inhibitors against TRPV3 channel in this study or throughout the manuscript, as none of the inhibitors tested blocked Sr2+ oscillations in MII eggs (data not shown). Collectively, our results show that CaV3.2 and to a lesser extent TRPV3 channels, contribute to Sr2+ influx in GV oocytes. The delayed inhibition of Sr2+ influx caused by NS8593, suggests this compound displays some off-target effects even when used at the recommended concentrations.
Figure 6. CaV3.2 channels inhibitors rapidly terminate Sr2+ induced oscillations.
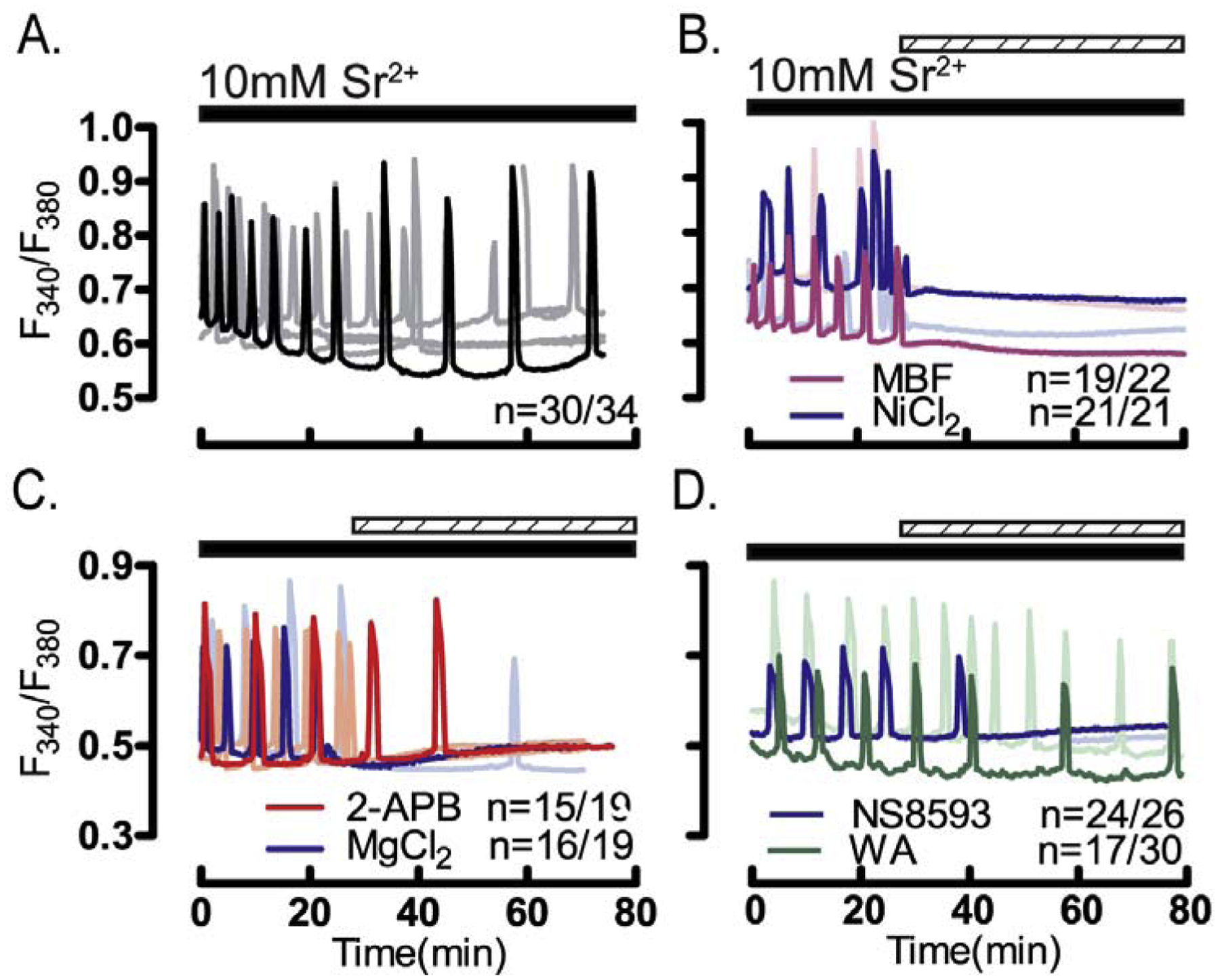
Experiments were performed in nominal Ca2+-free media containing 10mM Sr2+ (black bar) and pharmacological inhibitors (dashed horizontal bars) were added 25 min following initiation of imaging. (A) Control, untreated oocytes showed normal oscillations, which were immediately terminated (B) by the addition of the CaV3.2 inhibitors MBF (1 μM) and Ni2+ (100 μM). (C) The general TRP channels inhibitors 2-APB (50 μM) & MgCl2 (5 mM) and (D) the TRPM7 inhibitor NS8593 (10 μM) also terminated oscillations but more protractedly especially by the latter; waixenicin-A (5 μM) was without effect. All experiments were replicated 4 times.
3.4. Mn2+ influx causes persistent quenching of Fura-2AM fluorescence
Mn2+ has long been used to study general pathways of divalent ion influx in cells, as its entry causes marked quenching of Fura-2 fluorescence that is easily detectable [48]. Mn2+ influx has been observed in both unfertilized and fertilized eggs [36], although the channel(s) that mediate(s) this influx remain(s) unknown. To address this question, we combined analysis of genetic KO models with results from pharmacological inhibitors. The addition of Mn2+ caused the expected quenching of Fura-2 fluorescence intensity in all oocytes, although the rate of decrease of the slope, assessed by percent of change in fluorescence, was reduced in CaV3.2-KO oocytes compared to WT, Trpv3-KO and Trpv3-CaV3.2-KO oocytes (Fig. 7A–D), suggesting that CaV3.2 channels are involved in Mn2+ influx at this stage. Remarkably, not only the rate of Mn2+ influx was increased in Trpv3-CaV3.2-KO oocytes, but also the time from addition to inflection was significantly shortened (Fig. 7E–F), suggesting that in these oocytes the expression of other channel(s) contributing to Mn2+ influx might be enhanced.
Figure 7. Multiple channels enable Mn2+ entry.
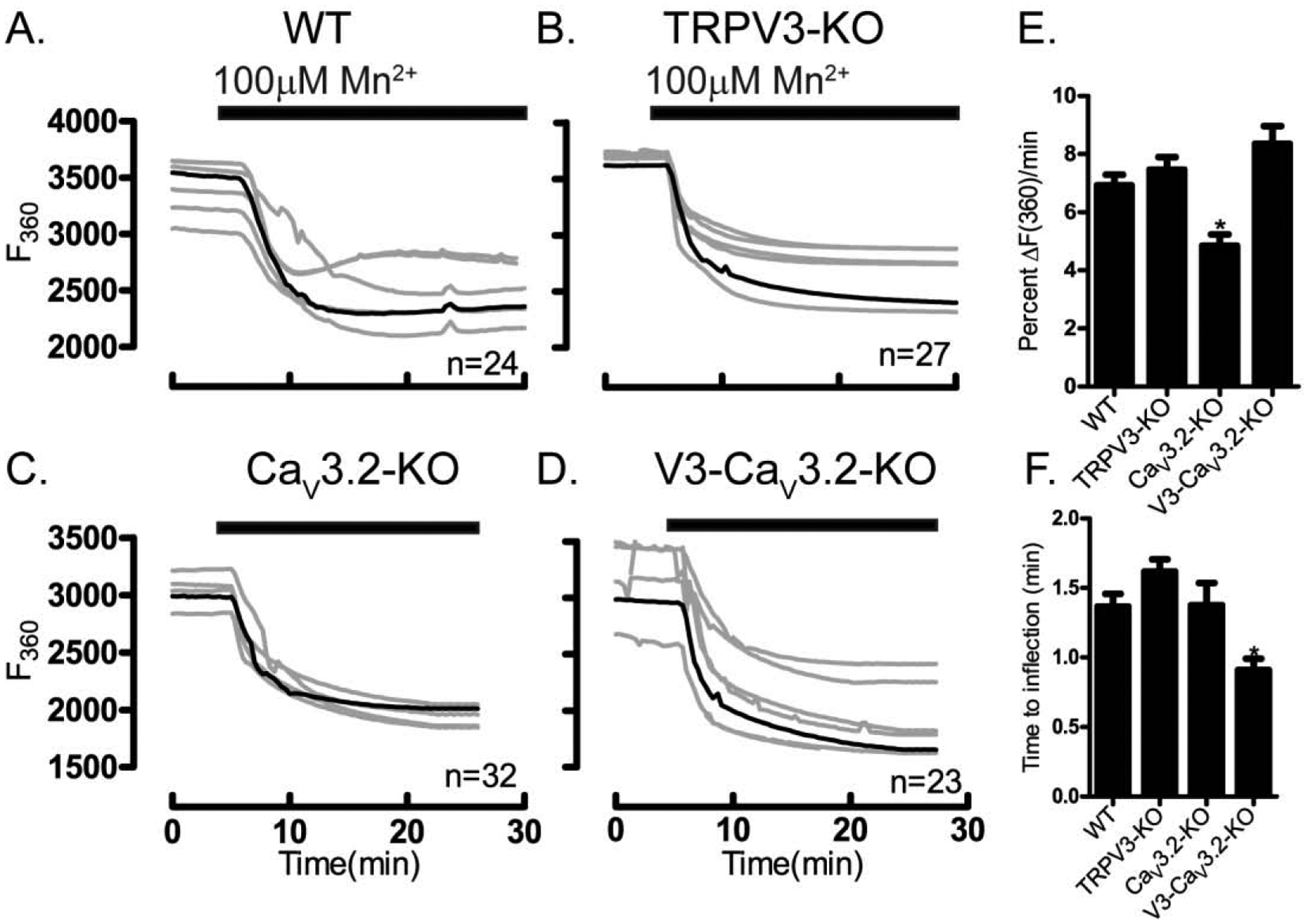
Mn2+ influx was tested in WT and in oocytes lacking TRPV3, or CaV3.2 or V3-CaV3.2 channels. Experiments were performed in media containing 2 mM Ca2+. (A) Mn2+ influx in WT oocytes, (B) TRPV3-KO oocytes, (C) CaV3.2-KO oocytes, (D) V3-CaV3.2-KO oocytes. (E) Fluorescence quenching assessed by the rate of F360 nm fluorescence decrease per minute relative to baseline fluorescence (P<0.05). (F) Time to inflection measured from the time of Mn2+ addition (5 min) to the time of first significant change in fluorescence values, which shortened in V3-CaV3.2-KO oocytes (P<0.05). Black horizontal bars denote addition of 100 μM Mn2+. Experiments were replicated 4 times.
Further studies using mibefradil (1 μM) and Ni2+ (100 μM) confirmed the role of CaV3.2 channels, as they delayed and reduced the rate of fluorescence quenching compared to that of controls (Fig. 8A–B). 2-APB (50 μM) and MgCl2 (10 mM) provided maximal inhibition, as they nearly eliminated influx, especially MgCl2 (Fig. 8C), whereas TRPM7 inhibitors, delayed and reduced Mn2+ influx but did not abolish it (Fig. 8D). Altogether, these results suggest that Mn2+ influx in GV oocytes occurs through several channels mostly CaV3.2 [49] and TRPM7 [50], although their individual contributions still needs to be parsed out (Fig. 8E & F). The role of these channels was confirmed using NS8593 (10 μM) in TRPV3-CaV3.2-KO oocytes, which abrogated Mn2+ influx (Suppl. Fig. 6).
Figure 8. Inhibitors of CaV3.2 & TRPM7 channels suppress Mn2+ influx.
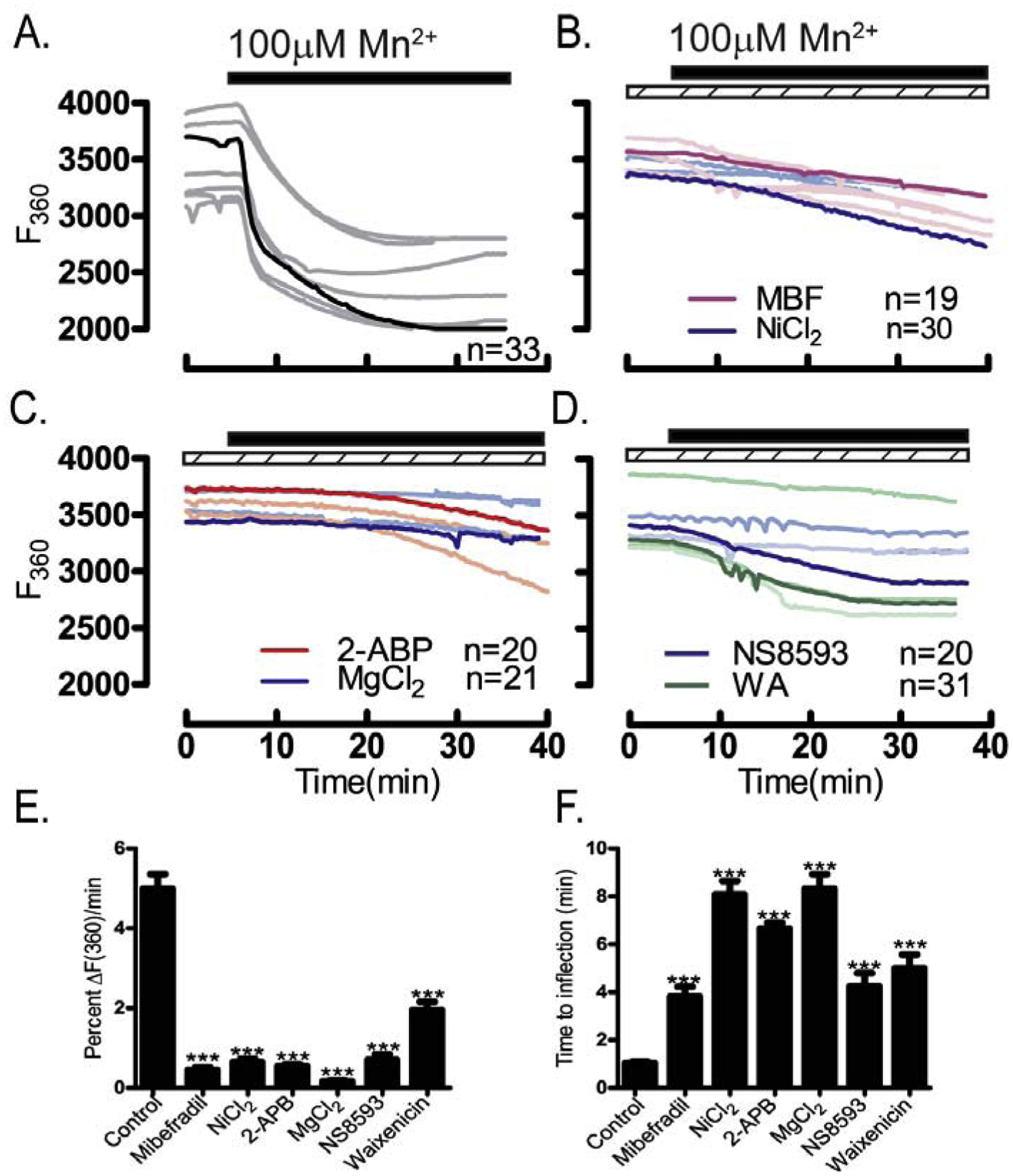
Mn2+ influx monitored using traces depicting quenching of F360 nm fluorescence. Experiments were performed in media containing 2 mM Ca2+ in the absence/presence of specific pharmacological inhibitors (dashed bars) after addition of Mn2+ (black bars): (A) control untreated oocytes, (B) oocytes treated with the CaV3.2 inhibitors MBF (1 μM) or Ni2+ (100 μM), (C) or with the general TRP inhibitors 2-APB (50 μM) and MgCl2 (10 mM), or (D) with the TRPM7 inhibitors NS8593 (10 μM) or waixenicin-A (1 μM). (E) Rate of fluorescence quenching per minute at F360 nm relative to baseline fluorescence was reduced by all inhibitors examined (P<0.05), and (F) time to inflection measured from the time of addition (5 min) to the time fluorescence quenching begins was prolonged by all inhibitors examined(P<0.05). Experiments were replicated 3 times.
3.5. Ni2+ influx causes protracted and persistent quenching of Mag-Fura-2AM fluorescence
Ni2+ selectively blocks T-type channels [51] but is known to permeate other channels [30]. Ni2+ influx can be followed by the quenching it produces on the fluorescence of Fura-2 and Mag-Fura-2 [37, 39, 52]. We selected Mag-Fura-2 because is much less sensitive to the [Ca2+]i increases induced by a variety of methods used in cells (Suppl. Fig. 7). Addition of Ni2+ caused a marked and comparable reduction in Mag-Fura-2 intensity in WT, Trpv3-KO and CaV3.2-KO oocytes suggesting that these channels are unlikely to participate in its influx (Fig. 9A–C). However, Ni2+ induced quenching was significantly increased in Trpv3-CaV3.2-KO oocytes (Fig. 9D–E), which is also reflected by the shortened time to inflection, implying that the responsible channel(s) permeating Ni2+ is still present in these KO oocytes (Fig. 9F). Our pharmacological studies showed that mibefradil (1 μM) had no effect on blocking Ni2+ influx (Fig. 10A, B) and that whereas both general inhibitors of TRP channels markedly reduced Ni2+ influx, MgCl2 (10 mM) outperformed 2-APB (100 μM) (Fig. 10C). TRPM7 inhibitors abrogated Ni2+ influx, with NS8593 (10 μM) being the most potent of all inhibitors tested (Fig. 10D). Altogether, our results show that TRPM7 is likely to serve as the major pathway for Ni2+ influx in GV oocytes (Fig. 10E & F).
Figure 9. Enhanced Ni2+ influx in V3-CaV3.2-KO oocytes.
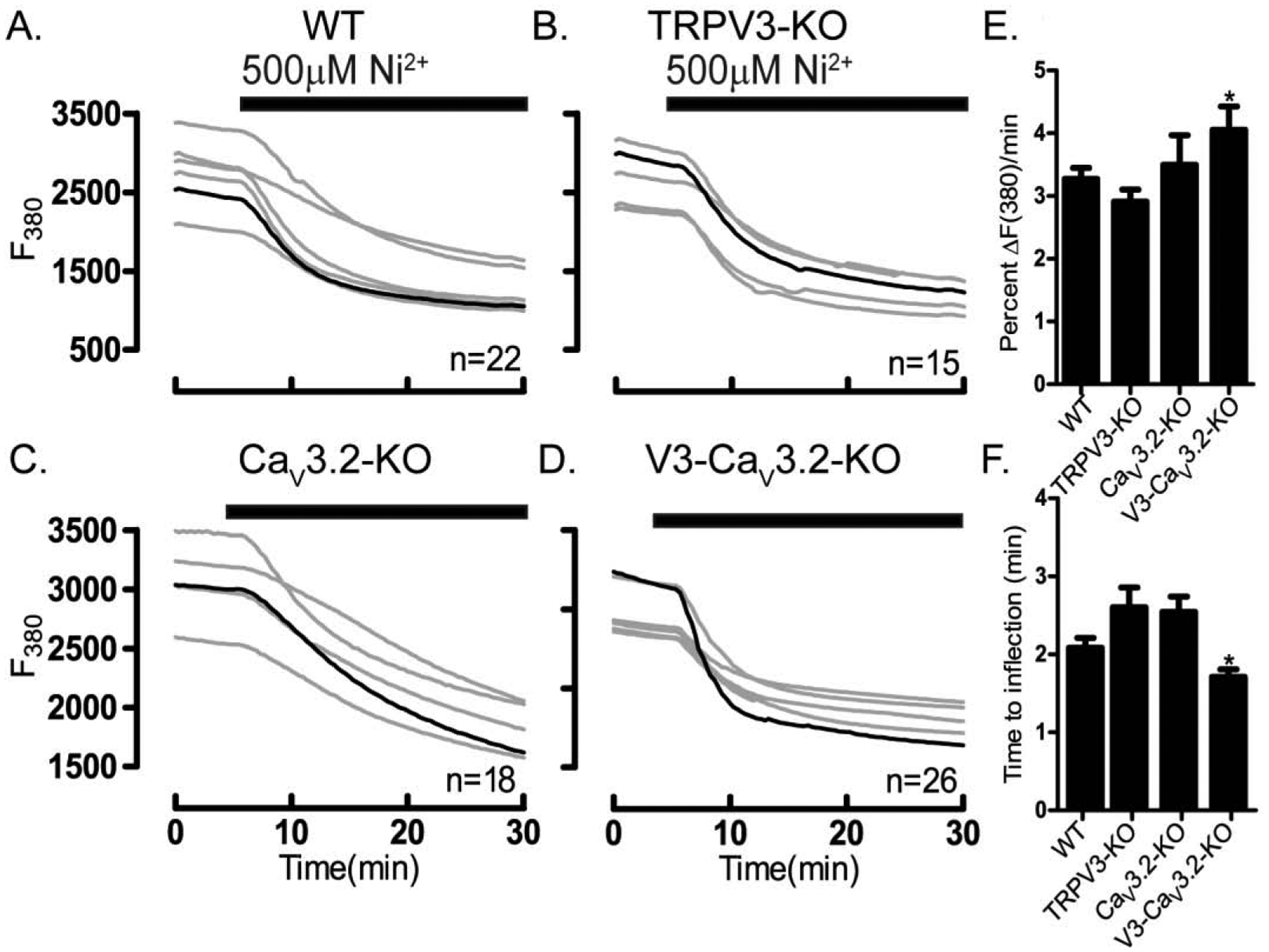
Ni2+ influx was tested in WT and oocytes lacking TRPV3, CaV3.2 or V3-CaV3.2 channels. Experiments were performed using Mag-Fura-2AM loaded oocytes in divalent free medium (DFM). Ni2+ influx in (A) WT oocytes, (B) TRPV3-KO oocytes, (C) CaV3.2-KO oocytes, (D) V3-CaV3.2-KO oocytes. (E) Fluorescence quenching at F380 assessed by the rate of fluorescence decrease per minute relative to baseline fluorescence (*P<0.05). (F) Time to inflection measured from the time of addition (5 min) to the time fluorescence quenching begins (*P<0.05). Black bars show the time at which Ni2+ was added. Experiments were replicated three times.
Figure 10. TRPM7 channel inhibitors block Ni2+ influx.
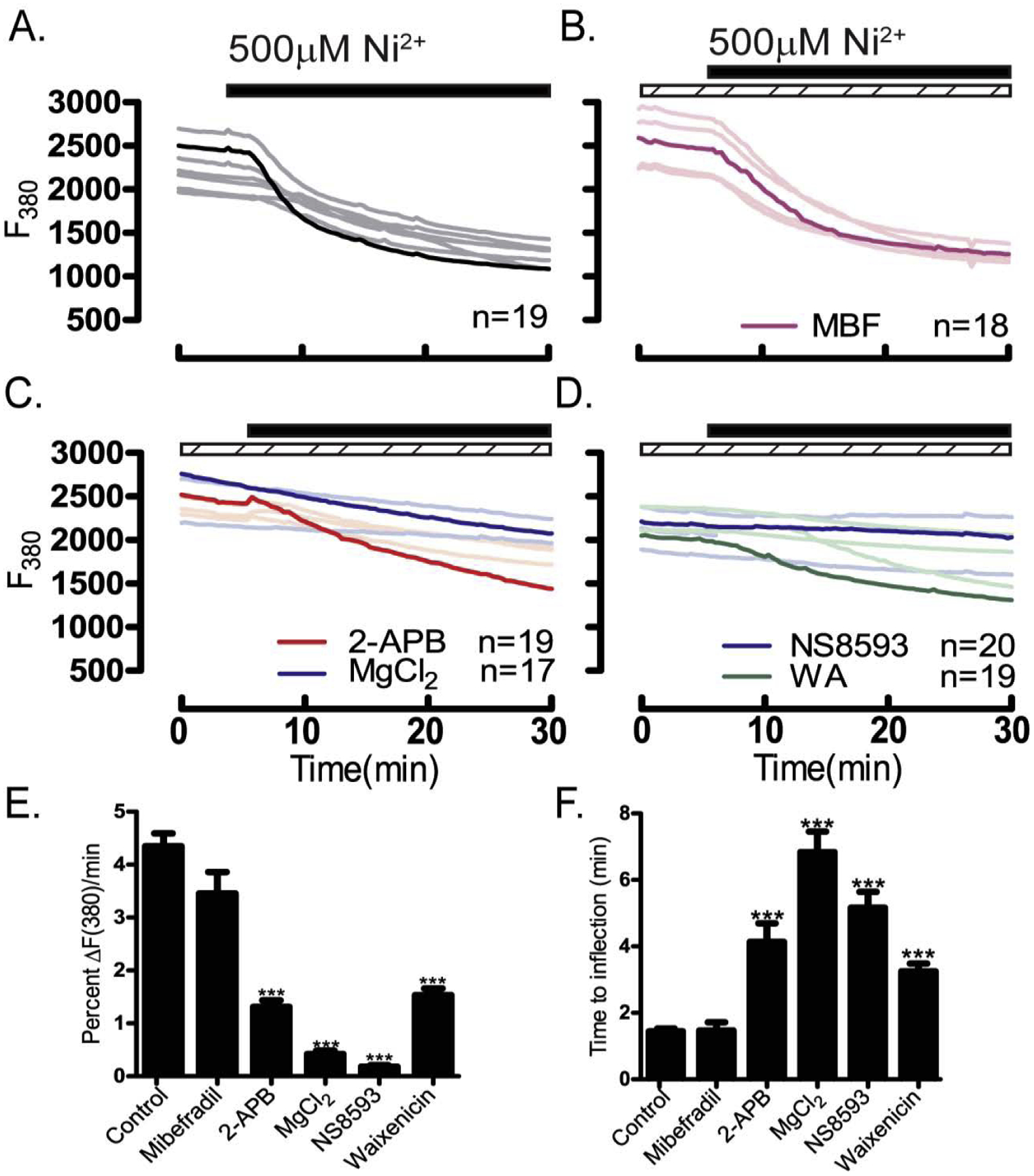
Inhibition of Ni2+ influx (black bars) was examined in the absence/presence of pharmacological inhibitors (dashed bars) and assessed by the rate of fluorescence quenching per minute at F380 relative to baseline fluorescence. Experiments were performed using Mag-Fura-2AM loaded oocytes in divalent free medium. (A) Control, untreated group, (B) CaV3.2 inhibitor MBF (1 μM), (C) general inhibitors of TRP channels 2-APB (100 μM) or MgCl2 (10 mM), (D) TRPM7 inhibitors NS8593 (10 μM) or waixenicin-A (5 μM). (E) Maximal inhibition of fluorescence quenching of Mag-Fura by general TRP inhibitors, and more specifically by the TRPM7 inhibitor NS8593 (P<0.05). (F) Time to inflection measured from the time of addition (5 min) to the time of fluorescence quenching begins, was also maximally inhibited by TRP inhibitors and more specifically by NS8593 (P<0.05). Experiments were replicated 3 times.
3.6. Different contribution of Ca2+ channels to the Ca2+ store content of GV oocytes
We next examined how the previously identified divalent cation permeable channels affect the intracellular Ca2+ content of GV oocytes. We used TG to evaluate the ER content in the null mouse lines described above. A significant reduction in the amplitude of the response was only observed in CaV3.2-KO oocytes (Fig. 11A & C), whereas the AUC measurements were not different for any of the groups (P >0.05; data not shown). Similar results were obtained by Bernhardt et al, 2015 [21]. To measure total store content, we used IO in a similar cohort of oocytes. We found that the amplitude of the response was reduced in oocytes of all lines, but significantly in Trpv3- and Trpv3-CaV3.2-KO oocytes (Fig. 11B & D; P <0.05), whereas the AUC response was reduced in all KO oocytes (Fig. 11E; P <0.05).
Figure 11. The absence of TRPV3- and V3-CaV3.2 channels reduces internal Ca2+ stores.
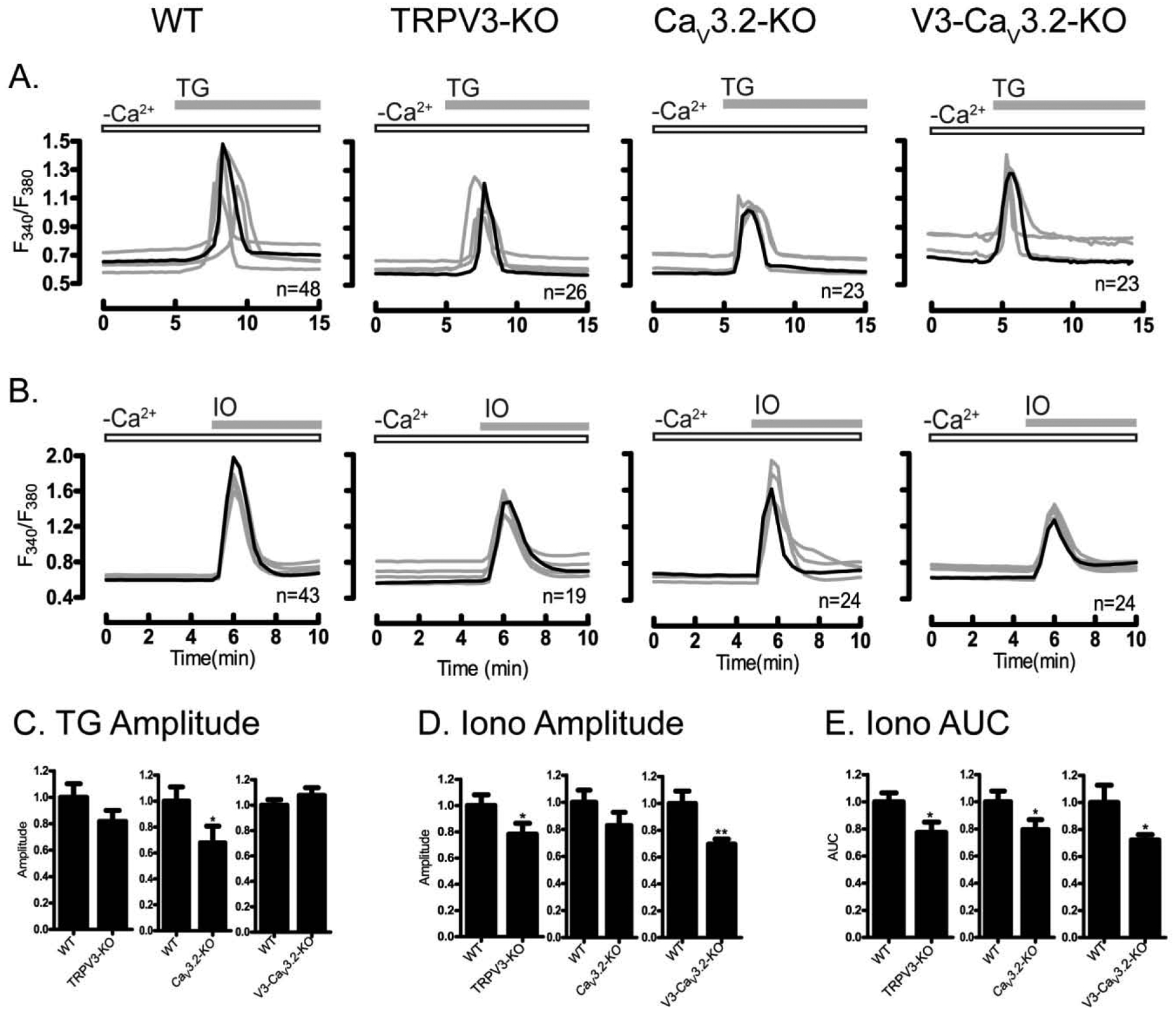
Ca2+ release from ER and total stores was measured using TG (10 μM) and IO (2.5 μM) in WT and in oocytes lacking TRPV3, CaV3.2, or V3-CaV3.2 channels. Experiments were performed in nominal Ca2+-free medium. (A) TG responses in WT, TRPV3-, CaV3.2- and V3-CaV3.2-KO oocytes. (B) Ionomycin responses in WT, TRPV3-, CaV3.2- and V3-CaV3.2-KO oocytes. (C) Peak amplitude of TG-induced Ca2+ release was reduced in CaV3.2 (P<0.05). (D) Peak amplitude of ionomycin-induced Ca2+ release was reduced V3-CaV3.2 (P<0.05), whereas (E) AUC response induced by IO was reduced in all KO lines (P<0.05). Open bars indicate nominal Ca2+ free media; filled gray bars show the time at which TG/IO was added. Experiments were replicated 3 times.
We extended the above studies by incubating GV oocytes with the aforementioned inhibitors for 1 hr, after which TG or IO was added and Ca2+ responses were monitored. The CaV3.2 inhibitors, mibefradil (1 μM) and Ni2+ (100 μM) significantly reduced the response to TG, and similar effects were observed in the presence of the general inhibitors 2-APB (100 μM) and MgCl2 (10 mM) (Fig. 12A, B). The TRPM7 inhibitors were less effective (Fig. 12C). The responses to IO displayed a similar trend, with CaV3.2 inhibitors and general inhibitors providing the strongest inhibition, whereas the other inhibitors only displayed marginal effects, although NS8593 (10 μM) still reduced the amplitude of the response (Fig. 12, D–F). Altogether, these results reveal that under steady-state conditions, CaV3.2 channels are mostly responsible for the Ca2+ store content of GV oocytes, but contributions by TRPV3 and TRPM7 channels are also evident.
Figure 12. Internal Ca2+ stores are reduced in the presence of CaV3.2 and general TRP channels inhibitors.
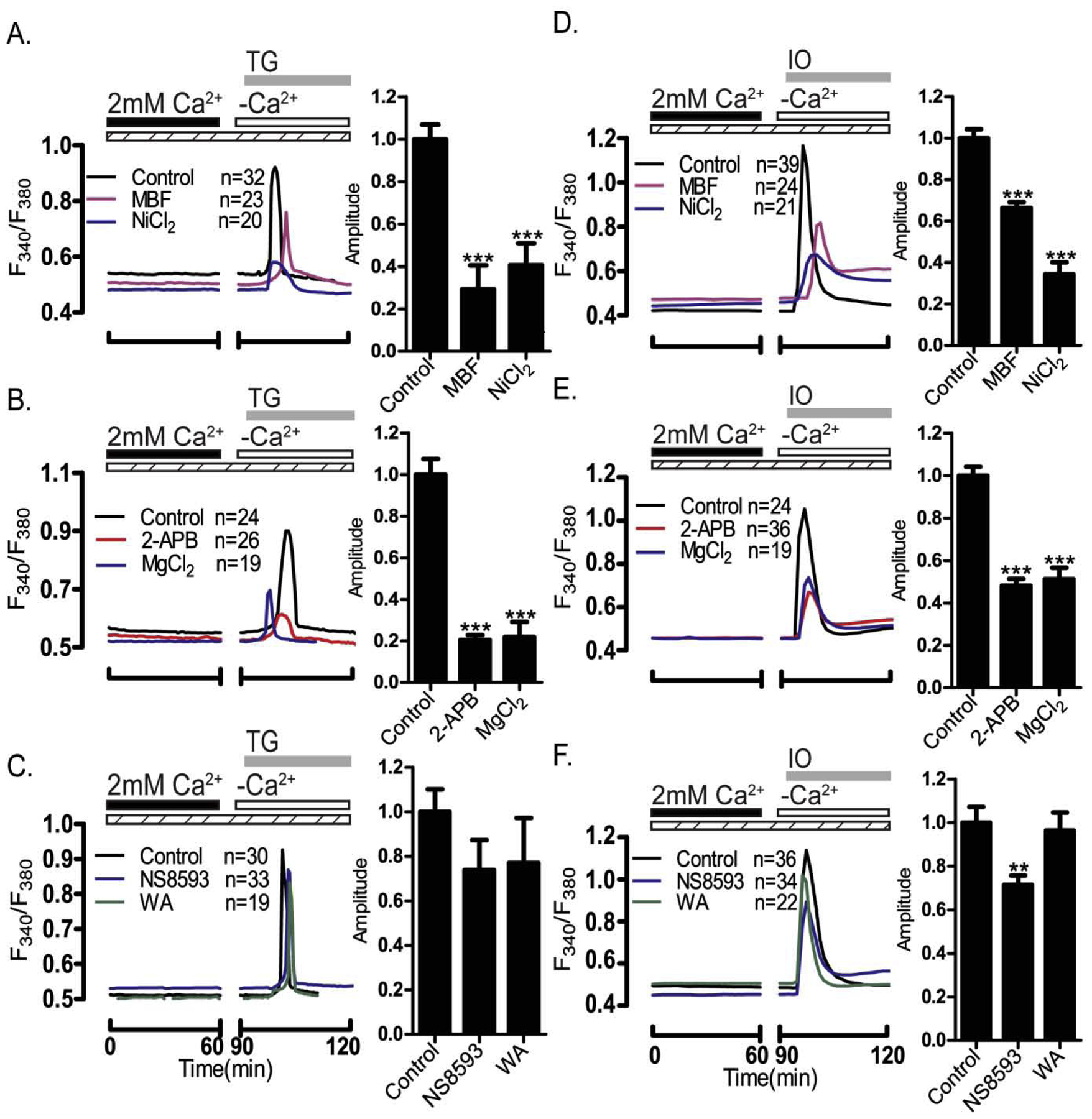
Oocytes were incubated with pharmacological inhibitors for 1 h after which internal Ca2+ store content was tested following addition of TG and IO-induced. Peak amplitude of intracellular Ca2+ release was graphed and quantified. (A and D) the CaV3.2 inhibitors MBF (1 μM) and Ni2+ (100 μM) reduced responses to both treatments (P<0.05), and similar effects were observed after the addition of (B and E) the TRP channels general inhibitors 2-APB (100 μM) and MgCl2 (5 mM) (P<0.05). (C & F) The TRPM7 inhibitors NS8593 (10 μM) & waixenicin-A (1 μM) were without effect (P>0.05) regarding TG responses, while NS8593 affected the amplitude of the IO response (P>0.05). Dashed bars indicate the presence of pharmacological inhibitors; black and white bars indicate the presence/absence of Ca2+; gray bars show the time at which IO/TG was added.
4. Discussion
In this study we examined the presence and function of channels that mediate divalent cation influx in mouse GV oocytes. We found that the three channels whose expression has been identified by electrophysiology in mouse oocytes and/or eggs are active at the GV stage. However, we observed that they respond distinctively to divalent cation exposure. We found for example that Ca2+ and Mn2+ influx is mostly dependent on CaV3.2 and TRPM7 channels, whereas Sr2+ induced oscillations largely rely on CaV3.2 expression and much less on TRPV3, and TRPM7 appears to mediate the totality of Ni2+ influx. Under steady-state conditions, CaV3.2 channels are primarily responsible for maintaining Ca2+ store content, although TRPM7 is able to mediate acute, induced Ca2+ influx demands. Lastly, as reported in other systems, broadly used inhibitors such as 2-APB and Ni2+ showed dual properties, as they effectively inhibited the influx of certain cations/channel(s), while simultaneously working as agonists for other channels; other inhibitors showed more uniform and specific effects. Identification of the channels responsible for Ca2+ and divalent cation homeostasis in GV oocytes and their molecular regulation during oocyte maturation could be used to improve fertility and also to prevent conception.
4.1. Non-selective channels mediate divalent cation influx in GV oocytes
The changes in Ca2+ homeostasis that occur in GV oocytes and the mechanisms that underpin them are less characterized than those in MII eggs. Not that they are less significant, as they contribute to maintain the mitochondrial function of these cells [53] as well as to increase the ER Ca2+ content during maturation [54]. Therefore, it is not surprising that several channels sustain Ca2+ influx at the GV stage. One of these channels is CaV3.2, which was recently suggested to be the sole functional T-type channel in mouse eggs [21]. Here, we found that oocytes from CaV3.2 null mice failed to show the spontaneous oscillations that are stereotypical of this stage, a finding that was also replicated using specific inhibitors. Surprisingly, Ca2+ influx induced by increasing [Ca2+]o or by re-addition of [Ca2+]o after TG was only somewhat affected by the absence of CaV3.2 channels, and specific pharmacological inhibitors also caused modest inhibition of this influx. These results suggest that the expression of CaV3.2 channels mainly contributes to Ca2+ influx under basal, steady conditions whereas other channels, two of which are examined here, are capable of mediating Ca2+ influx when greater or acute demands of Ca2+ arise; these channels may maintain Ca2+ homeostasis in oocytes null for CaV3.2.
Consistent with the above results and with findings from the literature using heterologous expression and electrophysiology, CaV3.2 channels showed permeability to several divalent cations in the following order: Mn2+>Ca2+>Sr2+. Therefore, it is not surprising that CaV3.2 channels appear indispensable for Sr2+ influx at the GV stage, which may be due in part to its higher permeability ratio for Sr2+ in relation to other cations [49]. It is intriguing nevertheless that despite the presence of CaV3.2 channels in mouse MII eggs [19, 20, 55], Sr2+ influx in these cells requires the expression of TRPV3 channels [27]. The difference between the GV and MII stages could be attributed to several factors such as higher expression of TRPV3 channels in MII eggs compared to CaV3.2, or an increase in the open probability of TRPV3 at the MII stage in relation to CaV3.2, or higher unitary conductance of TRPV3 channels for Sr2+ than CaV3.2 channels. These results raise the question of what regulates the expression and function of CaV3.2 channels in GV oocytes and throughout maturation. Whether or not these channels experience rearrangements in the channel pore that reshape the selectivity filter, as it is proposed to occur for certain channels [56], or are partly internalized [57], and/or differentially affect the function of neighboring channels or proteins during oocyte maturation will require additional investigation.
Another channel expressed in mouse oocytes and eggs is TRPV3 [27]. Remarkably, electrophysiological recordings and Ca2+ imaging studies in the presence of extracellular Ca2+ failed to detect functional expression of TRPV3 channels in GV oocytes [27]. Nevertheless, here using Sr2+, 100 μM 2-APB and genetic models, we found that TRPV3 channels are functionally expressed in GV oocytes, although they are not the main mediators of Sr2+ influx at this stage, consistent with previous findings. The difference between our previous report and the present, is that the functional expression of TRPV3 in GV oocytes was tested by patch-clamping and imaging in the presence of Ca2+ but not of Sr2+[27], which probably has a higher permeability ratio than Ca2+. Further, in the present study, we estimated Sr2+ influx by monitoring Sr2+-induced intracellular oscillations. These oscillations not only depend on the influx of Sr2+ across the membrane, but also rely on the modifications of IP3R1 by Ca2+ or Sr2+. Importantly, given that at the MII stage the sole mediator of Sr2+ influx and oscillations is TRPV3, and that its PM functional expression experiences a steady increase during maturation, future studies should examine the mechanism(s) regulating these changes.
We recently reported functional expression of a third channel in mouse oocytes and eggs, TRPM7 [28]. In that study we did not examine its role on Ca2+ influx or its impact on the content of the internal Ca2+ stores. This function however was addressed using a TRPM7 cKO line [29], which showed that cKO GV oocytes had comparable intracellular Ca2+ content to those of control eggs suggesting that TRPM7 channels are not responsible for the basal, steady Ca2+ influx. Remarkably, TRPM7 cKO oocytes showed greatly reduced influx following addition of extra Ca2+ or re-addition of [Ca2+]o after TG, which is indicative of the so-called SOCE mechanism [29]. Our findings with pharmacological inhibitors are consistent with these results, as the broad inhibitors, MgCl2 and 2-APB, and also the TRPM7 inhibitors, NS8593 and WA, drastically reduced Ca2+ influx stimulated by TG exposure. It is worth noting that SOCE influx is usually mediated by the combined actions of Stim1 and Orai1 [58]. However, studies using GV oocytes from Orai1-KO or Stim1/2-cKO mice failed to show alterations of Ca2+ influx or spontaneous oscillations [24], which suggests that canonical SOCE might not play a significant role in mouse GV oocytes. Instead, TRPM7, which has been shown to modulate SOCE in lymphocytes [44, 45], might be responsible for modulating acute Ca2+ influx and/or demands in GV oocytes. Future studies should ascertain the precise mechanism(s) of action whereby TRPM7 modulates this type of Ca2+ influx in GV oocytes as well as the influx of Ca2+ and other divalent cations during later stages of maturation, fertilization and early embryonic development. It is noteworthy that our western blotting results reveal possible phosphorylation of TRPM7 channels in mouse oocytes and eggs. It is well known that TRPM7 is phosphorylated in mammalian cells and it also undergoes autophosphorylation [59]. The effects of phosphorylation on TRPM7 are varied, and have been shown to alter the channel function as well as the stability and distribution of TRPM7 [60, 61]. Future studies should examine the site(s) that are possibly modified in mouse oocytes and eggs and their impact on TRPM7 function(s).
The apparent minor role of TRPM7 in supporting basal Ca2+ influx in our study was also evidenced by the near absence of spontaneous Ca2+ oscillations in oocytes from Trpv3-CaV3.2-KO mice, where TRPM7 should feature prominently. Similarly, TRPM7 did not appear to support steady Sr2+influx in GV oocytes, as Trpv3-CaV3.2-KO oocytes display greatly reduced oscillations in its presence. The TRPM7-like channel nevertheless mediated Mn2+ influx, and it appears to be the sole mediator of Ni2+ influx, which is consistent with its known permeability ratio to Ca2+ from electrophysiological studies in somatic cells [30]. Consistent with this, the influx of Mn2+ and Ni2+ was only somewhat decreased in oocytes of single channel KO lines, and it was enhanced in Trpv3-CaV3.2-KO oocytes. Lastly, Mn2+ and Ni2+ influx were abrogated by NS8593 and greatly diminished by WA – the more specific of the TRPM7 inhibitors [43]. Our results therefore suggest that although TRPM7 channels do not contribute to steady-state Ca2+ influx in GV oocytes, at least under our conditions that contained [Mg2+]o, they may play an important role when abrupt Ca2+ demands arise. TRPM7 channels might also mediate the influx of other divalent cations such as Zn2+, Co2+ and Mg2+, which are all important enzyme-cofactors [62], and in the case of Zn2+ is also important for post-implantation embryo development [63]. Future studies should determine whether the early embryonic lethality associated with the loss of TRPM7 is due to reduced Ca2+ influx or reflects a combined deficiency in the influx of one or more of the aforementioned divalent cations. Indeed, genetic ablation of the channel function of TRPM7 in the intestine impacts the homeostasis of Zn2+, Mg2+, and Ca2+ at the organismal level leading to deficiencies that are incompatible with post-natal survival [64].
4.2. Ca2+ influx and store content in GV oocytes
The increase in the content of the internal Ca2+ stores throughout oocyte maturation is a well-documented phenomenon [2, 8, 65]. In GV oocytes, the internal Ca2+ stores contain low Ca2+, and the content remains low even if oocytes are arrested at this stage for prolonged periods of time, which is all the most remarkable because it happens despite persistent Ca2+ influx [10]. Importantly, GV oocytes contain some Ca2+ in the stores and if one of the channels under consideration here, or if other undiscovered channel(s) contribute to their filling is not presently known. Recent studies used genetic models to address this question. Unexpectedly, it was found that oocytes lacking the molecular components of SOCE showed unaltered store content [24], and the same conclusion was drawn from studies using TRPM7 cKO oocytes [29]. Conversely, the Ca2+ store of oocytes lacking CaV3.2 was unevenly affected, although the TG- sensitive stores were reduced, the total Ca2+ store content as assessed by IO was unaffected [21]. In the present study, we found that absence of CaV3.2 channels diminished both the TG-sensitive stores and the total stores, whereas the absence of Trpv3-KO channels reduced the total store content. Consistent with these results, simultaneous deletion of CaV3.2 and Trpv3 synergistically diminished total store content, findings that were largely replicated by the addition of pharmacological inhibitors. Of these, the general and CaV3.2 channel-specific inhibitors maximally reduced Ca2+ store content, whereas TRPM7 inhibitors had lesser impact, findings that are consistent with a published genetic study [29]. In those studies, simultaneous deletion of CaV3.2 and TRPM7 channels synergistically reduced TG-sensitive stores in MII eggs and the number of pups per litter [29]. Together, our studies show that despite functional expression of at least three divalent cation permeable channels on the PM of GV oocytes, these channels contribute distinctly to the filling of the internal stores. Future studies should examine why and how this is determined, and whether the distribution of these channels on the PM and/or their relationship to the ER organization impacts the contribution to the filling of the stores at the GV stage and during maturation.
4.3. Ca2+ channel inhibitors in GV oocytes
Although inhibitors of PM channels have been used for decades to identify Ca2+ channels and their functions in neurons and other somatic cells [37], the use of these blockers in mammalian oocytes and eggs has been limited. Here we employ a combination of inhibitors to identify the expression and contribution of PM channels to the Ca2+ homeostasis of GV oocytes. Further, because we contrasted their effectiveness with cation influx in oocytes null for two of these channels, we can make inferences about their specificity. Of the two broad inhibitors used, MgCl2 seemed to be the ideal compound, as it affects the conduction properties of all the three channels whose expression is known in oocytes and eggs [66, 67]. Consistent with these reports, 10 mM Mg2+ effectively blocked influx of all divalent cations examined, although a range of concentrations would be necessary to determine if differential sensitivity to it exists among these channels. The other broad inhibitor, 2-APB, produced less consistent responses, as it effectively inhibited Ca2+ influx at all concentrations, while at higher concentrations acted as a TRPV3 agonist promoting Sr2+ influx [47]. Thus, 2-APB has disparate effects on divalent cation influx in GV oocytes that depends on the concentration of the drug, as it was reported in somatic cells, and the divalent cation under consideration. The inhibitors of CaV3.2 channels also produced some unexpected results. While 1 μM mibefradil consistently inhibited the influx of Ca2+, Sr2+ and Mn2+, 100 μM Ni2+ did not attenuate Ca2+ influx when promoted by addition of extra Ca2+ or following re-addition of Ca2+ after TG. These results are consistent with Ni2+’s high permeability through TRPM7 [30], which may also be relieving the block in TRPM7’s pore region induced by Ca2+ and/or Mg2+ [30], causing Ca2+ to permeate this channel as well as other present active channel(s) more effectively. Thus, the Ca2+ responses after the addition of Ni2+ should be interpreted with caution, as its presence simultaneously inverses the functional activity of two channels expressed in mouse oocytes and eggs. Both TRPM7 inhibitors used here proved effective at blocking TRPM7-like channels, although WA was the most specific, as it blocked Ni2+ influx and curtailed stimulated Ca2+ influx without affecting Sr2+ influx; we could not test the full effectiveness of WA, as the concentrations recommended in somatic cells of ~10 μM [43] proved toxic for GV oocytes. NS8593 displayed stronger inhibitory properties, although it also protractedly inhibited Sr2+ influx suggesting residual effects on CaV3.2 channels, which may explain its stronger effect on the content of the internal Ca2+ stores.
4.4. Conclusions
In conclusion, we show that divalent cations permeate GV oocytes through at least three different channels. These channels are all nonselective, although they might display different permeability ratios for the ions tested here. Similarly, we found these channels differentially contribute to the constitutive, steady-state Ca2+ influx vs. stimulated Ca2+ influx, and to the filling of internal Ca2+ stores, although the underlying cellular and/or molecular reasons behind these differences are unknown. Lastly, we characterized inhibitors and concentrations that can reliably prevent influx of specific divalent cations and identified the agonist properties of other inhibitors. Future studies should examine how changes in Ca2+ homeostasis at the GV stage impact maturation and oocyte developmental competence.
Supplementary Material
Highlights.
CaV3.2, TRPM7 and TRPV3 channels differentially mediate the influx of Ca2+ and other divalent cations in GV oocytes.
CaV3.2 and TRPM7 are responsible for most of the steady-state and acute Ca2+ influx, respectively.
CaV3.2 supports the majority of Sr2+ influx, TRPM7 the majority of Ni2+ influx, and both channels mediate Mn2+ influx.
CaV3.2 maintains Ca2+ levels in the Thapsigargin-sensitive ER stores, but expression of CaV3.2 and TRPV3 is necessary to sustain the total internal Ca2+ stores, which are emptied by Ionomycin.
2-APB and Ni2+ act as both agonists and antagonists of divalent cation influx in GV oocytes.
Acknowledgments:
This study was supported in part by a NIH grant (NICHD092499) to R.A.F. G.A. was supported in part by the Frances and Chou-Chu Hong Graduate Fellowship in Veterinary and Animal Sciences, UMass Amherst. We thank Banyoon Cheon for technical assistance in primers design and Changli He for overall technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and doesn’t necessarily represent the official views of the National Institute of Health.
Declarations of interest: None.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Berridge MJ, Lipp P, and Bootman MD, The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol, 2000. 1(1): p. 11–21. [DOI] [PubMed] [Google Scholar]
- [2].Wakai T, Vanderheyden V, and Fissore RA, Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations. Cold Spring Harb Perspect Biol, 2011. 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cuthbertson KS, Whittingham DG, and Cobbold PH, Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature, 1981. 294(5843): p. 754–7. [DOI] [PubMed] [Google Scholar]
- [4].Ridgway EB, Gilkey JC, and Jaffe LF, Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A, 1977. 74(2): p. 623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steinhardt R, Zucker R, and Schatten G, Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol, 1977. 58(1): p. 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stricker SA, Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol, 1999. 211(2): p. 157–76. [DOI] [PubMed] [Google Scholar]
- [7].Carroll J and Swann K, Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem, 1992. 267(16): p. 11196–201. [PubMed] [Google Scholar]
- [8].Jones KT, Carroll J, and Whittingham DG, Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem, 1995. 270(12): p. 6671–7. [DOI] [PubMed] [Google Scholar]
- [9].Tombes RM, et al. , Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol, 1992. 117(4): p. 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cheon B, et al. , Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol Biol Cell, 2013. 24(9): p. 1396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wakai T, et al. , Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs. J Cell Sci, 2013. 126(Pt 24): p. 5714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kline D and Kline JT, Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem, 1992. 267(25): p. 17624–30. [PubMed] [Google Scholar]
- [13].Clapham DE, Calcium signaling. Cell, 2007. 131(6): p. 1047–58. [DOI] [PubMed] [Google Scholar]
- [14].Hagiwara S and Miyazaki S, Ca and Na spikes in egg cell membrane. Prog Clin Biol Res, 1977. 15: p. 147–58. [PubMed] [Google Scholar]
- [15].Murnane JM and DeFelice LJ, Electrical maturation of the murine oocyte: an increase in calcium current coincides with acquisition of meiotic competence. Zygote, 1993. 1(1): p. 49–60. [DOI] [PubMed] [Google Scholar]
- [16].Peres A, Resting membrane potential and inward current properties of mouse ovarian oocytes and eggs. Pflugers Arch, 1986. 407(5): p. 534–40. [DOI] [PubMed] [Google Scholar]
- [17].Yoshida S, Permeation of divalent and monovalent cations through the ovarian oocyte membrane of the mouse. J Physiol, 1983. 339: p. 631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Catterall WA, Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol, 2000. 16: p. 521–55. [DOI] [PubMed] [Google Scholar]
- [19].Day ML, Johnson MH, and Cook DI, Cell cycle regulation of a T-type calcium current in early mouse embryos. Pflugers Arch, 1998. 436(6): p. 834–42. [DOI] [PubMed] [Google Scholar]
- [20].Kang D, et al. , Acetylcholine increases Ca2+ influx by activation of CaMKII in mouse oocytes. Biochem Biophys Res Commun, 2007. 360(2): p. 47682. [DOI] [PubMed] [Google Scholar]
- [21].Bernhardt ML, et al. , CaV3.2 T-type channels mediate Ca(2)(+) entry during oocyte maturation and following fertilization. J Cell Sci, 2015. 128(23): p. 4442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gomez-Fernandez C, et al. , Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Hum Reprod, 2012. 18(4): p. 194–203. [DOI] [PubMed] [Google Scholar]
- [23].Machaty Z, et al. , Fertility: Store-Operated Ca(2+) Entry in Germ Cells: Role in Egg Activation. Adv Exp Med Biol, 2017. 993: p. 577–593. [DOI] [PubMed] [Google Scholar]
- [24].Bernhardt ML, et al. , Store-operated Ca(2+) entry is not required for fertilization-induced Ca(2+) signaling in mouse eggs. Cell Calcium, 2017. 65: p. 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Desai BN and Clapham DE, TRP channels and mice deficient in TRP channels. Pflugers Arch, 2005. 451(1): p. 11–8. [DOI] [PubMed] [Google Scholar]
- [26].Wu LJ, Sweet TB, and Clapham DE, International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev, 2010. 62(3): p. 381–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carvacho I, et al. , TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep, 2013. 5(5): p. 1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carvacho I, et al. , TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep, 2016. 6: p. 34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bernhardt ML, et al. , TRPM7 and CaV3.2 channels mediate Ca(2+) influx required for egg activation at fertilization. Proc Natl Acad Sci U S A, 2018. 115(44): p. E10370–E10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Monteilh-Zoller MK, et al. , TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol, 2003. 121(1): p. 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kong BY, et al. , Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway. Biol Reprod, 2012. 87(1): p. 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen CC, et al. , Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science, 2003. 302(5649): p. 1416–8. [DOI] [PubMed] [Google Scholar]
- [33].Cheng X, et al. , TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell, 2010. 141(2): p. 331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee HC, et al. , TRPV3 channels mediate Ca(2)(+) influx induced by 2-APB in mouse eggs. Cell Calcium, 2016. 59(1): p. 21–31. [DOI] [PubMed] [Google Scholar]
- [35].Zhang D, et al. , Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum Reprod, 2005. 20(11): p. 3053–61. [DOI] [PubMed] [Google Scholar]
- [36].Cheek TR, et al. , Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development, 1993. 119(1): p. 179–89. [DOI] [PubMed] [Google Scholar]
- [37].Merritt JE, Jacob R, and Hallam TJ, Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem, 1989. 264(3): p. 1522–7. [PubMed] [Google Scholar]
- [38].Lee JH, et al. , Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J, 1999. 77(6): p. 303442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tashiro M, Inoue H, and Konishi M, Physiological pathway of magnesium influx in rat ventricular myocytes. Biophys J, 2014. 107(9): p. 2049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bird GS, et al. , Methods for studying store-operated calcium entry. Methods, 2008. 46(3): p. 204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCloskey C, et al. , Voltage-dependent Ca2+ currents contribute to spontaneous Ca2+ waves in rabbit corpus cavernosum myocytes. J Sex Med, 2009. 6(11): p. 3019–31. [DOI] [PubMed] [Google Scholar]
- [42].Chubanov V, et al. , Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol, 2012. 166(4): p. 1357–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zierler S, et al. , Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem, 2011. 286(45): p. 39328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Beesetty P, et al. , Inactivation of TRPM7 kinase in mice results in enlarged spleens, reduced T-cell proliferation and diminished store-operated calcium entry. Sci Rep, 2018. 8(1): p. 3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Faouzi M, et al. , The TRPM7 channel kinase regulates store-operated calcium entry. J Physiol, 2017. 595(10): p. 3165–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bos-Mikich A, Swann K, and Whittingham DG, Calcium oscillations and protein synthesis inhibition synergistically activate mouse oocytes. Mol Reprod Dev, 1995. 41(1): p. 84–90. [DOI] [PubMed] [Google Scholar]
- [47].Hu HZ, et al. , 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem, 2004. 279(34): p. 35741–8. [DOI] [PubMed] [Google Scholar]
- [48].Luo D, et al. , Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem, 2001. 276(8): p. 561321. [DOI] [PubMed] [Google Scholar]
- [49].Kaku T, et al. , The gating and conductance properties of Cav3.2 low-voltage-activated T-type calcium channels. Jpn J Physiol, 2003. 53(3): p. 165–72. [DOI] [PubMed] [Google Scholar]
- [50].Li M, Jiang J, and Yue L, Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol, 2006. 127(5): p. 525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bhattacharjee A, et al. , T-type calcium channels facilitate insulin secretion by enhancing general excitability in the insulin-secreting beta-cell line, INS-1. Endocrinology, 1997. 138(9): p. 3735–40. [DOI] [PubMed] [Google Scholar]
- [52].Golynskiy MV, et al. , Metal binding studies and EPR spectroscopy of the manganese transport regulator MntR. Biochemistry, 2006. 45(51): p. 15359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wakai T and Fissore RA, Constitutive IP3R1-mediated Ca(2+) release reduces Ca(2+) store content and stimulates mitochondrial metabolism in mouse GV oocytes. J Cell Sci, 2019. 132(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jones KT and Nixon VL, Sperm-induced Ca(2+) oscillations in mouse oocytes and eggs can be mimicked by photolysis of caged inositol 1,4,5trisphosphate: evidence to support a continuous low level production of inositol 1, 4,5-trisphosphate during mammalian fertilization. Dev Biol, 2000. 225(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- [55].Peres A, The calcium current of mouse egg measured in physiological calcium and temperature conditions. J Physiol, 1987. 391: p. 573–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Khakh BS and Lester HA, Dynamic selectivity filters in ion channels. Neuron, 1999. 23(4): p. 653–8. [DOI] [PubMed] [Google Scholar]
- [57].Shukla AK, et al. , Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem, 2010. 285(39): p. 30115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Smyth JT, et al. , Activation and regulation of store-operated calcium entry. J Cell Mol Med, 2010. 14(10): p. 2337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kim TY, et al. , Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem Biophys Res Commun, 2012. 417(3): p. 1030–4. [DOI] [PubMed] [Google Scholar]
- [60].Cai N, et al. , The kinase activity of the channel-kinase protein TRPM7 regulates stability and localization of the TRPM7 channel in polarized epithelial cells. J Biol Chem, 2018. 293(29): p. 11491–11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schmitz C, et al. , The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem, 2005. 280(45): p. 37763–71. [DOI] [PubMed] [Google Scholar]
- [62].Knape MJ, et al. , Divalent metal ions control activity and inhibition of protein kinases. Metallomics, 2017. 9(11): p. 1576–1584. [DOI] [PubMed] [Google Scholar]
- [63].Tian X, et al. , Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol Reprod, 2014. 90(4): p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mittermeier L, et al. , TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc Natl Acad Sci U S A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mehlmann LM and Kline D, Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod, 1994. 51(6): p. 1088–98. [DOI] [PubMed] [Google Scholar]
- [66].Luo J, et al. , Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. J Invest Dermatol, 2012. 132(9): p. 2158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Demeuse P, Penner R, and Fleig A, TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol, 2006. 127(4): p. 421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


