Abstract
A precise temporal and spatial control of intracellular Ca2+ concentration is essential for a coordinated contraction of the heart. Following contraction, cardiac cells need to rapidly remove intracellular Ca2+ to allow for relaxation. This task is performed by two transporters: the plasma membrane Na+-Ca2+ exchanger (NCX) and the sarcoplasmic reticulum (SR) Ca2+− ATPase (SERCA). NCX extrudes Ca2+ from the cell, balancing the Ca2+entering the cytoplasm during systole through L-type Ca2+ channels. In parallel, following SR Ca2+ release, SERCA activity replenishes the SR, reuptaking Ca2+ from the cytoplasm.
The activity of the mammalian exchanger is fine-tuned by numerous ionic allosteric regulatory mechanisms. Micromolar concentrations of cytoplasmic Ca2+ potentiate NCX activity, while an increase in intracellular Na+ levels inhibits NCX via a mechanism known as Na+-dependent inactivation. Protons are also powerful inhibitors of NCX activity. By regulating NCX activity, Ca2+, Na+ and H+ couple cell metabolism to Ca2+ homeostasis and therefore cardiac contractility. This review summarizes the recent progress towards the understanding of the molecular mechanisms underlying the ionic regulation of the cardiac NCX with special emphasis on pH modulation and its physiological impact on the heart.
Keywords: Na+-Ca2+ exchanger, protons, pH regulation, calcium binding domains, excitation contraction coupling, Ca2+ dynamics
Graphical abstract
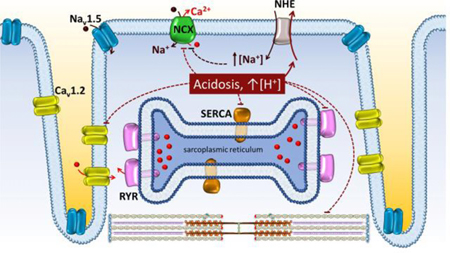
Impact of cytoplasmic acidosis on proteins mediating excitation-contraction coupling
1. Introduction
Cytoplasmic protons play pivotal roles in cardiac cells: from powering mitochondrial respiration to modulating the activities of enzymes, contractile proteins, ion channels and transporters. Because of the multifaceted effects of protons, intracellular pH is tightly regulated within a narrow range as any untoward alterations in proton levels can lead to drastic changes in metabolism, enzyme function and ionic balances that power both the contractile and electrical activities of cardiac cells. Whereas small fluctuations of intracellular pH frequently occur during changes in cardiac workload [1], drastic changes in intracellular pH are seen during respiratory and metabolic acidosis, and myocardial ischemia [2, 3]. These conditions can be associated with decreased heart rate and contractile force, and if left untreated can ultimately lead to arrhythmia [2–7]. The detrimental effects of these pathological conditions are in large part due to the accompanying cellular acidosis and the direct interaction of protons with many of the Ca2+ handling proteins in the cell [7]. Of particular interest is the effects that pH exerts on the plasma membrane transporter: the Na+-Ca2+ exchanger (NCX) [8], the activity of which is strongly inhibited by changes in cytoplasmic protons within the physiological range [9–12].
NCX is considered the main Ca2+ extrusion mechanism in cardiomyocytes [13–16]. It accomplishes this task by moving three Na+ into the cells while transporting one Ca2+ in the opposite direction. This results in an electrogenic transport that can be measured as ionic current. By extruding Ca2+ from the cell in exchange for Na+ influx, NCX helps restoring intracellular [Ca2+] after each contraction to its diastolic levels. Although the normal mode of the exchanger is to extrude Ca2+ (forward mode) [13, 14, 17, 18], in certain instances set by Na+ and Ca2+ gradients and membrane potential, NCX can promote Ca2+ influx. This “reverse mode” seems relevant during pathophysiological situations such as ischemia-reperfusion [19–21].
Due to the powerful inhibitory effect that pH exerts on NCX, intracellular protons are important regulators of Ca2+ dynamics. This review summarizes our current knowledge on the regulatory properties of NCX with emphasis on NCX pH regulation: the molecular determinants underlying its regulation; its effects on NCX activity; and the potential physiological impact of NCX pH regulation on heart function. We will focus on the cardiac isoform of the exchanger (NCX1.1) as most studies have been conducted using this exchanger.
2. Topology of the cardiac Na+-Ca2+ exchanger
The cardiac Na+-Ca2+ exchanger (NCX1.1) is composed by 970 amino acids with the first 32 cleaved off as part of an N-terminal signal sequence, which is not required for function [8, 22]. The current model predicts these residues to be organized into two groups of 5 transmembrane segments (10 total TMSs) separated by a large cytoplasmic loop (Figure 1). This membrane topology is based on the crystal structure of the archaebacterial homolog NCX_Mj [23] and biochemical studies conducted on the cardiac isoform [24, 25].
Figure 1: Schematic topology of NCX1.1.
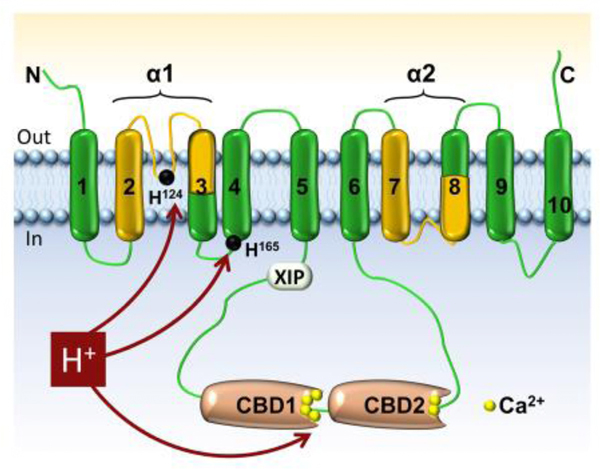
Topology of the cardiac exchanger NCX1.1 as modeled by Ren and coworkers [24]. The transmembrane segments (TMS) involved in ion transport are shown in yellow. These regions, which are highly conserved among the Cation/Ca2+ exchanger superfamily, are named α-repeats [26] and host the 12 residues that coordinate the transported Na+ and Ca2+ ions (as demonstrated in NCX_Mj by [23], not shown). Between TMSs 5 and 6 there is a large cytoplasmic loop which includes the XIP region and two Ca2+-binding domains (CBD). The XIP region controls the inactivation of NCX due to high levels of cytosolic Na+ [75], while the Ca2+-binding domains confer Ca2+ regulation to NCX. Protons can displace Ca2+ from these sites decreasing NCX activity [10]. The two histidines found relevant for NCX pH regulation are indicated. Mutations at these sites decrease the sensitivity of NCX to cytoplasmic protons [9].
Among the transmembrane segments of special interest are TMSs 2, 3, 7 and 8 as they contain two sequences known as α-repeats (Figure 1). These regions, which span residues 97–150 in TMSs 2–3 (α 1-repeat) and 799–849 in TMSs 7–8 (α 2-repeat) of the cardiac isoform, show intramolecular homology, suggestive of gene duplication [26]. Moreover, they are highly conserved among other exchangers and are regarded as the signature sequence of the cation/Ca2+ transporter superfamily [26, 27]. There is a great deal of evidence to support their involvement in ion transport as mutations at these sites have been shown to dramatically affect the activity of eukaryotic exchangers [28–37]. The crystal structure of the archaebacterial exchanger (NCX_Mj) has further solidified their role in ion translocation by showing that the α-repeats contain 12 residues that coordinate the transported ions [23]. These amino acids are highly conserved between the archaebacterial and mammalian homologs [23] and they share functional similarity [30, 33, 35, 38–40].
Between TMSs 5 and 6 is a ~540 amino acid cytoplasmic loop, which confers extensive regulatory properties to NCX. At its N-terminus lies a stretch of 20 hydrophobic and positive amino acids (aa 219–238) termed the exchanger inhibitory peptide (XIP) region, as a peptide with identical sequence inhibits NCX activity when exogenously applied to the cytoplasmic surface of the exchanger [41]. It is well established that this region is involved in the regulation of NCX by intracellular Na+ [41, 42] (see Section 3.1) and PIP2 [43, 44].
A recent NMR study showed the presence of a structured domain downstream of XIP. This region (aa 284–322) has been identified as a two helix bundle domain (THB) since it forms two tandem α-helices connected by a short linker [45]. Despite the conserved amino acid sequence of this domain, the functional role of THB remains to be determined.
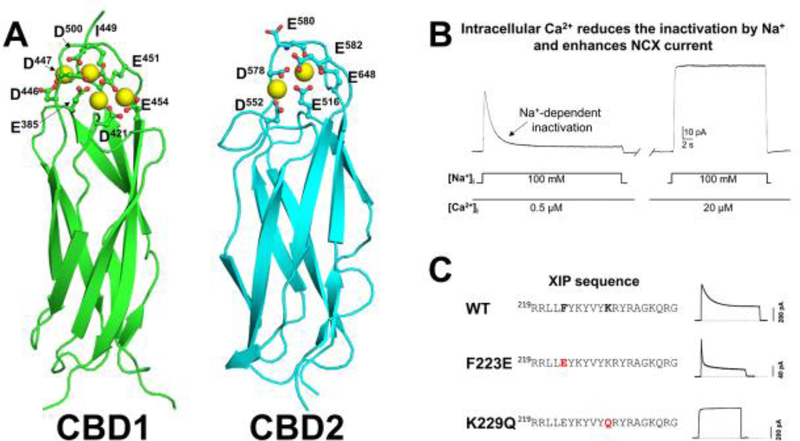
Forty-nine amino acids downstream of THB lies two sequential regions responsible for Ca2+ regulation. They are identified as Ca2+-binding domain 1 (CBD1; aa 371–501) and Ca2+-binding domain 2 (CBD2; aa 501–678). These domains share the same β-sandwich architecture (Figure 2A) and form conjoint structures connected by a short linker within the full length exchanger (Figure 1). Several CBDs structures are available including the conjunct domains from the drosophila exchanger and the mammalian CBD1 mutated at position E454 [46–51].
Figure 2: Cytoplasmic Ca2+ and Na+ regulate the cardiac Na+-Ca2+ exchanger.
A. Structure of NCX1 Ca2+ regulatory domains (CBD1–2DPK and CBD2–2QVM) [52, 136]. Ca2+ bound to either CBD1 or CBD2 is not transported, but activates NCX by increasing the turnover rate and removing the Na+-dependent inactivation [78]. B. Typical outward current recorded using the giant excised patch technique. cRNA encoding for the cardiac exchanger (NCX1.1) is injected into Xenopus laevis oocytes. Four to five days after injection, exchange currents are recorded using the giant patch technique (20–30 μm tip diameter) in the inside out mode. 8 mM Ca2+ is present within the pipette (external side) while the cytoplasmic side of the patch is bathed either in 100 mM Cs+ or Na+ and the indicated cytoplasmic free Ca2+ concentrations. Cs+ cannot bind to the transport sites, preventing activity. Replacement of Cs+ with Na+ generates an outward current (reverse mode) that peaks and then declines over several seconds due to the Na+-dependent inactivation. This process is not observed in the presence of micromolar Ca2+ concentrations. C. The amino acid sequence of the XIP region of NCX1.1 is shown on the left. Two single-site substitutions within this domain either accelerate (F223E) or abolish (K229Q) the Na+-dependent inactivation, as shown by the corresponding exchanger outward current traces (right) (modified from [42]).
CBD1 coordinates four Ca2+ ions, while CBD2 undergoes alternative splicing providing different Ca2+ coordinating properties [48–55]. Evidence shows that CBD2 expressing exon A coordinate two Ca2+ ions (Figure 2A), while exchangers expressing exon B in CBD2 do not bind Ca2+ [56]. The latter splice variant is mainly expressed in non-excitable cells, while the Ca2+ coordinating CBD2 isoforms are found in excitable cells, including the heart [57]. This suggests that the Ca2+-binding domain properties of CBD2 are tailored to meet the specific needs of the cells where they are expressed. Several extensive reviews have covered this subject and the reader is referred to these for further information [48, 56, 58–60].
Finally, within the large cytoplasmic loop distal to the CBDs is an amphipathic α-helix encompassing residues 740–756. This region is found to be essential for palmitoylation of NCX at residue C739 [61–63].
As an atomic structure of a mammalian exchanger is not available, the three-dimensional organization of these regulatory regions as well as their molecular interactions with TMSs 1–5 and TMSs 6–10 remain unknown. Any such interactions are likely to translate the signal from the large regulatory loop to the transport sites embedded within the TMS to control the activity of NCX. This intramolecular level of regulation may be complicated by evidence indicating that the mammalian NCX exists at least as a dimer [64, 65] and that the large cytoplasmic loops of the adjacent proteins undergo conformational changes induced by cytoplasmic Ca2+ [64]. Clearly this information depicts a much more complex organization for the mammalian exchangers when compared to its archaebacterial homolog and it suggests that the mammalian NCX has evolved a complex regulatory system to ensure dynamic control of Ca2+ inside excitable cells in general and cardiac cells in particular, vital for efficient and timely contraction.
3. Allosteric regulation of the cardiac Na+-Ca2+ exchanger
The most extensively studied regulatory mechanisms of NCX are its modulation by cytosolic ions: Na+, Ca2+ and protons. Other non-ionic modulators have been shown to affect NCX activity which will be briefly mentioned herein. Among them are calmodulin [66], phosphorylation [67–69] and calpain which, by cleaving NCX within the large cytoplasmic loop, either activates [70] or inhibits [71] NCX activity, in a NCX isoform-dependent manner. Strong evidence also demonstrates that NCX activity is enhanced by lipids [72–74], while more recent findings show that Cys 739 within the large cytoplasmic loop of NCX is palmitoylated and that this is a prerequisite process for the Na+-dependent inactivation to take place [63].
Together these regulatory mechanisms portray a multilevel and intricate control system to modulate NCX activity. This complicates determining the extent of NCX activity in both physiological and pathophysiological settings and more investigations are necessary to determine the relative contributions of each modulation to cardiac function.
3.1. Regulation by cytoplasmic Na+ and Ca2+
In addition to being transported, cytoplasmic Na+ and Ca2+ allosterically regulate NCX activity. High concentrations of intracellular Na+ (half inactivation ~15–20 mM) results in a slow decay of exchanger current (Figure 2B), via a process known as Na+-dependent inactivation [42, 75]. The current model predicts that Na+ bound to its cytoplasmic transport sites triggers conformational changes that inhibit activity [75]. The XIP region is strongly implicated in these molecular rearrangements as point mutations within this stretch of amino acids drastically alter the extent of Na+-dependent inactivation, as shown in Figure 2C [41, 42]. Since high levels of Na+ [42, 75, 76] are required to initiate the inactivation of NCX, its physiological relevance has been questioned [77]. Thus, experimental studies directly addressing the possible impact of this regulation in cardiac function are needed.
In contrast to the inhibitory effect of intracellular Na+, an elevation of cytoplasmic Ca2+ stimulates NCX activity. The site for this “regulatory” Ca2+ is distinct from that of transported Ca2+, as it binds to two domains within the large cytoplasmic loop (see Section 2). The binding of Ca2+ at these sites increases the NCX turnover rate and attenuates any inactivation caused by cytoplasmic Na+ (Figure 2B) [42, 78–80].
Experiments conducted with the isolated conjoint Ca2+-binding domains indicates a Ca2+ affinity in the lower nanomolar range (~10 nM) [64, 81], while the apparent Ca2+ affinity obtained from the full length exchanger tends to be higher (22 to 800 nM) [78, 80, 82, 83]. In spite of this large variability, electrophysiological studies in isolated cardiomyocytes provided strong evidence that NCX is regulated by cytoplasmic Ca2+ under physiological settings [84].
Although cytosolic Ca2+ is generally known as an activator of NCX, in certain instances it can act as a suppressor of exchanger activity. This is observed in mammalian cells in which repetitive Ca2+ influx results in decreased NCX1 activity [85], which seems to be due to a Ca2+-dependent endocytotic process, i.e. removal of NCX from the plasma membrane [86].
NCX1 shares significant sequence and functional similarity with its two isoforms NCX2 and NCX3, including regulation by intracellular Ca2+. As NCX2 and NCX3 are not the focus of this review, their Ca2+ regulatory properties are only briefly mentioned herein. Electrophysiological studies indicate that NCX2 currents are less sensitive to Ca2+ when compared to NCX1 [87], while the Ca2+ dependent regulation of NCX3 ionic currents is poorly characterized. This is mainly due to the limited expression of this protein in Xenopus oocytes which has prevented a detailed analysis of its biophysical properties. NCX3 appears to have a dual response to cytoplasmic Ca2+ by showing an initial activation, as seen in NCX1 [88], followed by a Ca2+-driven inactivation process [85, 89]. This inactivation process may be responsible for the decline in NCX3 ionic currents observed upon repetitive Ca2+ influx [85].
Studies conducted with the isolated Ca2+ binding domains indicated that the properties of CBD1 are quite conserved among the three exchanger isoforms. Instead, CBD2 shows distinct features between the three isoforms, which likely contribute to the different responses of these exchangers to Ca2+ [48, 90]. We refer the reader to other references for a more detailed analysis of this subject [48, 90–92].
3.2. Modulation by cytoplasmic protons
Proton regulation of NCX was first identified in squid axons more than twenty years ago [93]. Subsequently, using highly purified sarcolemmal vesicles isolated from canine ventricles, Philipson and his collaborators demonstrated that Na+-dependent Ca2+ uptake was inhibited at pH 6 and stimulated at pH 9 [12], providing the first demonstration that protons act directly on NCX activity. However, as the isolated vesicles contained both outside-out (extracellular surface- outward) and inside-out (cytoplasmic surface-outward), it was not possible to definitively assign the sidedness of action of protons on NCX. Thus, Philipson further characterized NCX proton modulation by analyzing the effects of pH exclusively on inside-out vesicles. It was established that protons interact with the cytoplasmic portion of the cardiac exchanger, inhibiting its forward mode [12].
With the development of the giant excised patch technique and its application to NCX [94], it was possible to directly monitor the regulation of NCX by cytoplasmic pH. Using this approach, Hilgemann and colleagues observed [95] that half inhibition of the exchanger outward current occurred at physiologically relevant pH levels. Moreover, it was shown that alkaline pH could relieve the Na+-dependent inactivation process, thereby increasing NCX activity. This result provided the first insight into a potential molecular process governing NCX pH regulation: namely demonstrating a link between proton and Na+ regulation. This concept was further developed by Lederer’s group which by performing giant patch excised experiments on adult guinea pig ventricular cardiac myocytes investigated how intracellular Na+ influenced NCX pH modulation [96]. It was demonstrated that NCX proton inhibition could be partially relieved by removing cytoplasmic Na+ [11, 96]. As protons were not competing with Na+ at its transport sites, it was concluded that the Na+-dependent inactivation of NCX may be at least in part due to Na+ acting as cofactor in proton block and therefore enhancing proton inhibition. This hypothesis was supported by two additional lines of evidence: first, proton inhibition was largely insensitive to changes in intracellular Ca2+, ruling out the regulatory Ca2+ binding sites as potential proton targets and second, that the exposure of the cytoplasmic side of NCX to α-chymotrypsin not only abolished Na+ and Ca2+ regulation, but also proton inhibition. As α-chymotrypsin cleaves a portion of the large cytoplasmic loop, the removal of both Na+-dependent inactivation and pH regulation further supported an intimate connection between these two regulatory mechanisms.
This working hypothesis was, however, disputed by subsequent electrophysiological studies showing that acidic pH decreased the sensitivity of NCX current measured from cardiac myocytes to cytoplasmic Ca2+ [10]. These findings shifted the focus of pH regulation to the Ca2+ regulatory domains instead of the Na+-dependent inactivation process. To validate the role of the CBDs in pH regulation, Boyman and colleagues directly measured Ca2+ binding to the purified CBDs at different pH values [10]. Results demonstrated that protons significantly decreased the Ca2+ sensitivity of the isolated Ca2+ regulatory domains, further supporting their role in NCX pH regulation. Based on these results, it was proposed that protons act by displacing Ca2+ ions from the coordinating sites of CBD1 and CBD2 resulting in an inhibition of NCX transport activity [10].
To gain further understandings into the molecular mechanisms that govern NCX pH modulation, a detailed analysis utilizing mutagenesis and giant patch technologies was conducted on the cardiac exchanger [9]. The approach was to use site-specific mutagenesis to remove individually, or in combination, the Na+- or Ca2+-regulatory mechanism of NCX, allowing the investigation of their respective roles in NCX pH sensitivity.
Strikingly, these investigations revealed that proton block persisted after replacement of key residues coordinating Ca2+ within both of the Ca2+-binding domains. This demonstrated that protons were interacting with exchanger regions outside of the Ca2+-binding domains [9]. Moreover, it was shown that an exchanger lacking the Na+-dependent inactivation was still sensitive to cytoplasmic pH, although with a significantly reduced sensitivity [9]. Two important conclusions were made: first, Ca2+ and Na+ regulation are not required for proton block to occur, indicating that NCX pH regulation is a distinct molecular process; and second, intracellular Na+ is an important cofactor in pH modulation as it enhances the sensitivity of NCX for protons, as previously reported [96].
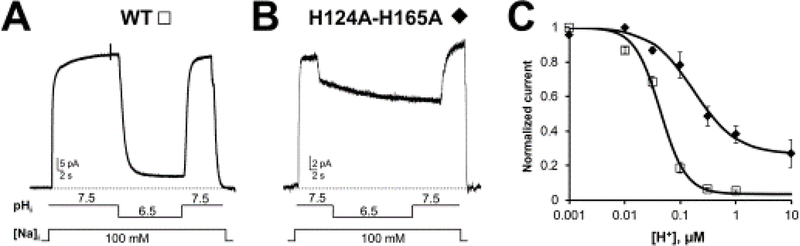
To hunt down the residues responsible for protein inhibition extensive single site mutagenesis was undertaken. It was revealed that two amino acids significantly contributed to NCX pH regulation: namely, histidine 124 and 165. Individual replacement of these two histidines decreased the apparent affinity of NCX for protons by 2 and 4 fold, respectively, underlying their relevance in proton regulation [9]. As shown in Figure 3, the concomitant replacement of these two histidines, with alanine, almost completely abolished NCX sensitivity to pH.
Figure 3: Two histidines are important for NCX pH regulation.
Exchanger currents were recorded using the giant excised patch technique in the inside out configuration from Xenopus laevis oocytes expressing the indicated constructs (modified from [9]). A. The WT cardiac exchanger is highly sensitive to changes in intracellular pH and its activity is almost completely abolished in the presence of cytoplasmic pH 6.5. B. Mutation of histidine 124 and 165 to alanine drastically decreased the exchanger pH sensitivity, underlying the role of these two residues in the regulation of NCX by protons. C. Dose response curves for cytoplasmic H+ for WT (cardiac isoform NCX1.1) and H124A-H165A mutant exchangers. Replacement of histidine 124 and 165 drastically decreased the sensitivity of NCX to cytoplasmic protons and significantly changed the cooperativity of binding.
Histidine 124 is located in the linker between TMSs 2 and 3 within the α1-repeat (Figure 1), a region previously characterized as important for ion binding and transport [34, 35, 97]. This region of cardiac exchanger has been modeled to form a reentrant loop as a mutant with a cysteine placed at position 125 generated an ionic current that was subsequently blocked by cytoplasmically applied membrane impermeant sulfhydryl reagents [29, 30]. In contrast, the corresponding region in the archaebacterial homolog is extracellular. Given this discrepancy between the two exchangers, whether His 124 affects NCX pH regulation by being directly protonated, which would require access from the cytoplasmic side, remains to be established. Another possibility is that histidine 124 perturbs other regions involved in pH inhibition. Indeed, previous investigations reported that replacement of His 124 with an asparagine, significantly slowed down the Na+-dependent inactivation [35]. As previously discussed, Na+ regulation enhances pH inhibition, and as such a mutation at this site may affect the affinity of NCX for protons indirectly.
Histidine 165 is located at the cytoplasmic end of TMS 4 (Figure 1) within a short sequence particularly enriched with positively charged amino acids (161RKIKHLR). Replacement of this histidine with alanine removed both Na+ and Ca2+ regulation and drastically decreased the apparent affinity of NCX for cytoplasmic protons and the cooperativity of proton binding [9]. Undoubtedly, this residue is highly strategic and essential for NCX ionic allosteric regulation. A speculative role for this residue/region is to transduce the regulatory signals from the large cytoplasmic loop to the to the transmembrane segments responsible for ion transport and protonation may be an effective way of altering this transfer of information. Independently of the mechanisms by which these residues control NCX pH regulation, these findings reveal their essential contribution to NCX pH regulation. These two histidines are highly conserved within the exchanger family by being present across vertebrate species, further highlighting their role in NCX regulation.
The studies highlighted in this section clearly depict conflicting mechanisms of action of protons on NCX activity, calling for further investigations to fully decipher how pH modulates NCX. Similarly, the physiological impact of this regulation remains elusive as its investigation remains technically challenging in native tissue. Nonetheless, in the following section we review and discuss how inhibition of NCX by cytosolic protons may impact cardiac function.
4. NCX role in cardiac function
4.1. Role of the Na+-Ca2+ exchanger in excitation-contraction coupling
Cardiac myocytes express only one exchanger isoform, NCX1.1 [57, 98, 99]. In mammals this isoform is the dominant Ca2+ efflux mechanism. This is particularly relevant during excitation-contraction coupling when NCX helps to remove the excess intracellular Ca2+, therefore maintaining contractility [13, 14, 100].
Because of the essential role that NCX plays in Ca2+ dynamics, alterations in its activity or expression are associated with various cardiac pathologies including systolic heart failure [101–105] and post-ischemic cardiac injury [19, 20, 106]. The increased NCX expression and function observed in some studies during the course of heart failure could have two major effects. Depending on intracellular Na+ concentration, it could promote Ca2+ efflux, which will act over time to deplete the sarcoplasmic reticulum Ca2+ content, thereby causing decreased contractility. Concomitantly, the increased depolarizing current generated via NCX can induce delayed afterdepolarizations (DAD). Such aberrant electrical activity is known to contribute to the arrhythmogenicity frequently associated with heart failure [107–111]. The effect of NCX on DADs and triggered arrhythmias and has been well demonstrated in several studies using genetically modified mice [112, 113]. Conversely, Ca2+ influx via NCX acting in the reverse mode has been shown to be a major contributor to Ca2+ overload during reperfusion after an ischemic event, which can ultimately lead to myocyte death [19, 20, 106].
Numerous reviews have covered the role of NCX in excitation-contraction coupling, ischemia-reperfusion injury and arrhythmia and the reader is referred to them [16, 20, 105, 114–118].
4.2. Contribution of NCX to the detrimental effects linked to cardiac acidosis
The detrimental effects of acidosis on cardiac performance have long been known. Isaac Newton in the 17th century noticed that the heart of an eel stopped beating when exposed to a drop of vinegar [119]. Pathophysiologically, numerous conditions cause alterations in the acid-base balance of cardiac muscle: metabolic and respiratory acidosis, malignant hyperthermia and hypercapnia, sleep apnea/hypopnea syndrome, and most notably ischemia. Many of these conditions have been linked to decreased contractility and heart rate, increased cell depolarization, ventricular fibrillation and propensity for arrhythmia [3, 5, 6, 120]. Acidosis can be transient or persistent and the heart responds differently to the two insults. Within a few seconds of the onset of acidosis there is a reduction in contractility. Although the increased levels of protons inhibits key proteins involved in Ca2+ handling such as ryanodine receptors, (RyRs) [121], L-type Ca2+ channels [121–123], the sarcoplasmic reticulum Ca2+-ATPase (SERCA) [124–126], and NCX itself, this initial decrease in contractility is mainly due to the effects of protons on the contractile proteins themselves, namely by reducing the binding of Ca2+ to troponin C (TnC) (Graphical Abstract) [127–131].
A different scenario is observed in conditions of persistent acidosis. As an adaptive response, both diastolic and systolic Ca2+ levels increase to compete off the protons from TnC, thereby partially recovering heart contractility. Among the mechanisms proposed for this rise in intracellular Ca2+ is a functional coupling between NCX and the myocardial Na+-H+ exchanger (NHE) [3, 132]. With acidosis NHE is activated, increasing intracellular Na+ levels leading to two potential outcomes regarding NCX: the increased Na+ reduces the Na+ driving force, which will act to decrease the Ca2+ efflux; or NCX is pushed into the reverse mode, thereby leading to Ca2+ entry. While such an interaction between NHE and NCX is generally accepted and theoretically plausible, some studies provide a contrarian viewpoint. Allen and Xiao have shown that although intracellular Ca2+ rises during late acidosis, it occurs independently of changes in intracellular Na+ [133]. Possibly, the elevated intracellular Na+ and protons may both act on NCX to suppress its activity via their regulatory roles, leading to increased diastolic Ca2+. Further investigations are needed to dissect the contribution of NCX to the Ca2+ aberrations triggered by acidosis and the role of the direct inhibition of NCX activity by cytoplasmic protons.
In addition to contributing to the Ca2+ imbalance seen during acidosis, NCX may also play a role in acidosis induced arrhythmia by triggering DADs [6, 134]. These abnormal depolarizations are more prominent during recovery from acidosis since it coincides with the complete relief of NCX proton block. In this condition, the Ca2+ released from the overloaded sarcoplasmic reticulum [134, 135] triggers NCX depolarizing current, thereby creating the opportunity for ectopic beats.
Although such evidence makes NCX an important player in the detrimental effects linked to cardiac acidosis, it has to be underscored that experimentally it is difficult to assess its direct role in these processes due to the lack of specific blockers or potential adaptations encountered in transgenic animals. New strategies are needed to determine the contribution of NCX to cardiac function in both physiological and pathophysiological settings.
5. Conclusions
In this review, we have summarized the current knowledge of allosteric regulation of NCX focusing upon ionic regulation in general and pH regulation in particular. We have highlighted the progress towards understanding NCX pH regulation and its potential physiological and pathophysiological implications. As one of the most prominent controlling mechanisms for Ca2+ dynamics, NCX has the ability to greatly influence cardiac contractility and excitability. With its relatively high sensitivity to protons and the frequent association of acidosis with detrimental effects on cardiac function, NCX could be a vital point of pharmacological intervention in reducing the negative effects of such conditions. It is therefore essential that we continue to tease apart the complex interplay that ionic regulations play in the mechanistic understanding of NCX under both normal and pathological settings.
HIGHLIGHTS.
The cardiac Na+-Ca2+ exchanger (NCX) controls Ca2+ homeostasis
Cytoplasmic Ca2+ enhances NCX activity via two cytoplasmic Ca2+-binding domains
Intracellular Na+ inactivates NCX and this process involves the XIP region
Intracellular protons strongly inhibit NCX activity
NCX pH regulation involves histidines 124 and 165 and two Ca2+-binding domains
Acknowledgments
Funding:
This work was supported by the NIH/NHLBI grants R01 HL130308 (MO) and MPI R01 HL147569 (JG/MO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bountra C, Kaila K, Vaughan-Jones RD, Effect of repetitive activity upon intracellular pH, sodium and contraction in sheep cardiac Purkinje fibres, J Physiol, 398 (1988) 341–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steenbergen C, Deleeuw G, Rich T, Williamson JR, Effects of acidosis and ischemia on contractility and intracellular pH of rat heart, Circ Res, 41 (1977) 849–858. [DOI] [PubMed] [Google Scholar]
- [3].Vaughan-Jones RD, Spitzer KW, Swietach P, Intracellular pH regulation in heart, J Mol Cell Cardiol, 46 (2009) 318–331. [DOI] [PubMed] [Google Scholar]
- [4].Gerst PH, Fleming WH, Malm JR, A quantitative evaluation of the effects of acidosis and alkalosis upon the ventricular fibrillation threshold, Surgery, 59 (1966) 1050–1060. [PubMed] [Google Scholar]
- [5].Orchard CH, Kentish JC, Effects of changes of pH on the contractile function of cardiac muscle, Am J Physiol, 258 (1990) C967–981. [DOI] [PubMed] [Google Scholar]
- [6].Orchard CH, Cingolani HE, Acidosis and arrhythmias in cardiac muscle, Cardiovasc Res, 28 (1994) 1312–1319. [DOI] [PubMed] [Google Scholar]
- [7].Crampin EJ, Smith NP, Langham AE, Clayton RH, Orchard CH, Acidosis in models of cardiac ventricular myocytes, Philos Trans A Math Phys Eng Sci, 364 (2006) 1171–1186. [DOI] [PubMed] [Google Scholar]
- [8].Nicoll DA, Longoni S, Philipson KD, Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger, Science, 250 (1990) 562–565. [DOI] [PubMed] [Google Scholar]
- [9].John S, Kim B, Olcese R, Goldhaber JI, Ottolia M, Molecular determinants of pH regulation in the cardiac Na+-Ca+ exchanger, J Gen Physiol, 150 (2018) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boyman L, Hagen BM, Giladi M, Hiller R, Lederer WJ, Khananshvili D, Proton-sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger, J Biol Chem, 286 (2011) 28811–28820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Doering AE, Lederer WJ, The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells, J Physiol, 466 (1993) 481–499. [PMC free article] [PubMed] [Google Scholar]
- [12].Philipson KD, Bersohn MM, Nishimoto AY, Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles, Circ Res, 50 (1982) 287–293. [DOI] [PubMed] [Google Scholar]
- [13].Goldhaber JI, Philipson KD, Cardiac sodium-calcium exchange and efficient excitation-contraction coupling: implications for heart disease, Adv Exp Med Biol, 961 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ottolia M, Torres N, Bridge JH, Philipson KD, Goldhaber JI, Na+/Ca2+ exchange and contraction of the heart, J Mol Cell Cardiol, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Philipson KD, Nicoll DA, Sodium-calcium exchange: a molecular perspective, Annu Rev Physiol, 62 (2000) 111–133. [DOI] [PubMed] [Google Scholar]
- [16].Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, Goldhaber J, Kaplan J, Lingrel JB, Pavlovic D, Philipson K, Sipido KR, Xie ZJ, Na+/Ca2+ exchange and Na+/K+-ATPase in the heart, J Physiol, 593 (2015) 1361–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blaustein MP, Lederer WJ, Sodium/calcium exchange: its physiological implications, Physiol Rev, 79 (1999) 763–854. [DOI] [PubMed] [Google Scholar]
- [18].Philipson KD, Nicoll DA, Ottolia M, Quednau BD, Reuter H, John S, Qiu Z, The Na+/Ca2+ exchange molecule: an overview, Ann N Y Acad Sci, 976 (2002) 1–10. [DOI] [PubMed] [Google Scholar]
- [19].Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E, Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury, Circ Res, 97 (2005) 916–921. [DOI] [PubMed] [Google Scholar]
- [20].Murphy E, Cross H, Steenbergen C, Sodium regulation during ischemia versus reperfusion and its role in injury, Circ Res, 84 (1999) 1469–1470. [DOI] [PubMed] [Google Scholar]
- [21].Lee C, Dhalla NS, Hryshko LV, Therapeutic potential of novel Na+-Ca2+ exchange inhibitors in attenuating ischemia-reperfusion injury, Can J Cardiol, 21 (2005) 509–516. [PubMed] [Google Scholar]
- [22].Sahin-Tóth M, Nicoll DA, Frank JS, Philipson KD, Friedlander M, The cleaved N-terminal signal sequence of the cardiac Na+-Ca2+ exchanger is not required for functional membrane integration, Biochemical and Biophysical Research Communications, 212 (1995) 968–974. [DOI] [PubMed] [Google Scholar]
- [23].Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y, Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger, Science, 335 (2012) 686–690. [DOI] [PubMed] [Google Scholar]
- [24].Ren X, Philipson KD, The topology of the cardiac Na+/Ca(2)+ exchanger, NCX1, J Mol Cell Cardiol, 57 (2013) 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Szerencsei RT, Kinjo TG, Schnetkamp PP, The topology of the C-terminal sections of the NCX1 Na + /Ca 2+ exchanger and the NCKX2 Na +/Ca2+-K + exchanger, Channels (Austin), 7 (2013) 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schwarz EM, Benzer S, Calx, a Na+-Ca2+ exchanger gene of Drosophila melanogaster, Proc Natl Acad Sci U S A, 94 (1997) 10249–10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cai X, Lytton J, The cation/Ca2+ exchanger superfamily: phylogenetic analysis and structural implications, Mol Biol Evol, 21 (2004) 1692–1703. [DOI] [PubMed] [Google Scholar]
- [28].Altimimi HF, Fung EH, Winkfein RJ, Schnetkamp PP, Residues contributing to the Na+-binding pocket of the SLC24 Na+/Ca2+-K+ Exchanger NCKX2, J Biol Chem, 285 (2010) 15245–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iwamoto T, Nakamura TY, Pan Y, Uehara A, Imanaga I, Shigekawa M, Unique topology of the internal repeats in the cardiac Na+/Ca2+ exchanger, Febs Letters, 446 (1999) 264–268. [DOI] [PubMed] [Google Scholar]
- [30].Iwamoto T, Uehara A, Imanaga I, Shigekawa M, The Na+/Ca2+ exchanger NCX1 has oppositely oriented reentrant loop domains that contain conserved aspartic acids whose mutation alters its apparent Ca2+ affinity, J Biol Chem, 275 (2000) 38571–38580. [DOI] [PubMed] [Google Scholar]
- [31].Jalloul AH, Cai S, Szerencsei RT, Schnetkamp PPM, Residues important for K+ ion transport in the K+-dependent Na+-Ca2+ exchanger (NCKX2), Cell Calcium, 74 (2018) 61–72. [DOI] [PubMed] [Google Scholar]
- [32].Jalloul AH, Liu G, Szerencsei RT, Schnetkamp PPM, Residues important for Ca2+ ion transport in the neuronal K+-dependent Na+-Ca2+ exchanger (NCKX2), Cell Calcium, 74 (2018) 187–197. [DOI] [PubMed] [Google Scholar]
- [33].John SA, Liao J, Jiang Y, Ottolia M, The cardiac Na+-Ca2+ exchanger has two cytoplasmic ion permeation pathways, Proc Natl Acad Sci U S A, 110 (2013) 7500–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nicoll DA, Hryshko LV, Matsuoka S, Frank JS, Philipson KD, Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+-Ca2+ exchanger, Journal of Biological Chemistry, 271 (1996) 13385–13391. [DOI] [PubMed] [Google Scholar]
- [35].Ottolia M, Nicoll DA, Philipson KD, Mutational analysis of the alpha-1 repeat of the cardiac Na+-Ca2+ exchanger, J Biol Chem, 280 (2005) 1061–1069. [DOI] [PubMed] [Google Scholar]
- [36].Schnetkamp PP, Jalloul AH, Liu G, Szerencsei RT, The SLC24 family of K+-dependent Na+-Ca(2)+ exchangers: structure-function relationships, Curr Top Membr, 73 (2014) 263–287. [DOI] [PubMed] [Google Scholar]
- [37].Winkfein RJ, Szerencsei RT, Kinjo TG, Kang K, Perizzolo M, Eisner L, Schnetkamp PP, Scanning mutagenesis of the alpha repeats and of the transmembrane acidic residues of the human retinal cone Na/Ca-K exchanger, Biochemistry, 42 (2003) 543–552. [DOI] [PubMed] [Google Scholar]
- [38].Liao J, Marinelli F, Lee C, Huang Y, Faraldo-Gomez JD, Jiang Y, Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger, Nat Struct Mol Biol, 23 (2016) 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van Dijk L, Giladi M, Refaeli B, Hiller R, Cheng MH, Bahar I, Khananshvili D, Key residues controlling bidirectional ion movements in Na+/Ca2+ exchanger, Cell Calcium, 76 (2018) 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Giladi M, Tal I, Khananshvili D, Structural Features of Ion Transport and Allosteric Regulation in Sodium-Calcium Exchanger (NCX) Proteins, Front Physiol, 7 (2016) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li ZP, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD, Identification of a Peptide Inhibitor of the Cardiac Sarcolemmal Na+-Ca2+ Exchanger, Journal of Biological Chemistry, 266 (1991) 1014–1020. [PubMed] [Google Scholar]
- [42].Matsuoka S, Nicoll DA, He Z, Philipson KD, Regulation of cardiac Na+-Ca2+ exchanger by the endogenous XIP region, Journal of General Physiology, 109 (1997) 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD, Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger, Am J Physiol Cell Physiol, 278 (2000) C661–666. [DOI] [PubMed] [Google Scholar]
- [44].Hilgemann DW, Ball R, Regulation of cardiac Na+/Ca2+ exchange and KATP potassium channels by PIP2, Science, 273 (1996) 956–959. [DOI] [PubMed] [Google Scholar]
- [45].Yuan J, Yuan C, Xie M, Yu L, Bruschweiler-Li L, Bruschweiler R, The Intracellular Loop of the Na+/Ca2+ Exchanger Contains an “Awareness Ribbon”-Shaped Two-Helix Bundle Domain, Biochemistry, 57 (2018) 5096–5104. [DOI] [PubMed] [Google Scholar]
- [46].Chaptal V, Ottolia M, Mercado-Besserer G, Nicoll DA, Philipson KD, Abramson J, Structure and functional analysis of a Ca2+ sensor mutant of the Na+/Ca2+ exchanger, J Biol Chem, 284 (2009) 14688–14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Giladi M, Hiller R, Hirsch JA, Khananshvili D, Population shift underlies Ca2+-induced regulatory transitions in the sodium-calcium exchanger (NCX), J Biol Chem, 288 (2013) 23141–23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hilge M, Aelen J, Foarce A, Perrakis A, Vuister GW, Ca2+ regulation in the Na+/Ca2+ exchanger features a dual electrostatic switch mechanism, Proc Natl Acad Sci U S A, 106 (2009) 14333–14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hilge M, Aelen J, Vuister GW, Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors, Mol Cell, 22 (2006) 15–25. [DOI] [PubMed] [Google Scholar]
- [50].Wu M, Le HD, Wang M, Yurkov V, Omelchenko A, Hnatowich M, Nix J, Hryshko LV, Zheng L, Crystal structures of progressive Ca2+ binding states of the Ca2+ sensor Ca2+ binding domain 1 (CBD1) from the CALX Na+/Ca2+ exchanger reveal incremental conformational transitions, J Biol Chem, 285 (2009) 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu M, Wang M, Nix J, Hryshko LV, Zheng L, Crystal structure of CBD2 from the Drosophila Na+/Ca2+ exchanger: diversity of Ca2+ regulation and its alternative splicing modification, J Mol Biol, 387 (2009) 104–112. [DOI] [PubMed] [Google Scholar]
- [52].Besserer GM, Ottolia M, Nicoll DA, Chaptal V, Cascio D, Philipson KD, Abramson J, The second Ca2+-binding domain of the Na+/Ca2+ exchanger is essential for regulation: crystal structures and mutational analysis, Proc Natl Acad Sci U S A, 104 (2007) 18467–18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Giladi M, Sasson Y, Fang X, Hiller R, Buki T, Wang YX, Hirsch JA, Khananshvili D, A common Ca2+-driven interdomain module governs eukaryotic NCX regulation, PLoS One, 7 (2012) e39985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hilge M, Aelen J, Perrakis A, Vuister GW, Structural basis for Ca2+ regulation in the Na+/Ca2+ exchanger, Ann N Y Acad Sci, 1099 (2007) 7–15. [DOI] [PubMed] [Google Scholar]
- [55].Johnson E, Bruschweiler-Li L, Showalter SA, Vuister GW, Zhang F, Bruschweiler R, Structure and dynamics of Ca2+-binding domain 1 of the Na+/Ca2+ exchanger in the presence and in the absence of Ca2+, J Mol Biol, 377 (2008) 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hilge M, Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger, J Biol Chem, 287 (2012) 31641–31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Quednau BD, Nicoll DA, Philipson KD, Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat, Am J Physiol, 272 (1997) C1250–1261. [DOI] [PubMed] [Google Scholar]
- [58].Chaptal V, Besserer GM, Ottolia M, Nicoll DA, Cascio D, Philipson KD, Abramson J, How does regulatory Ca2+ regulate the Na+-Ca2+ exchanger?, Channels (Austin), 1 (2007) 397–399. [DOI] [PubMed] [Google Scholar]
- [59].Giladi M, Khananshvili D, Molecular determinants of allosteric regulation in NCX proteins, Adv Exp Med Biol, 961 (2013) 35–48. [DOI] [PubMed] [Google Scholar]
- [60].Khananshvili D, Structure-Dynamic Coupling Through Ca2+-Binding Regulatory Domains of Mammalian NCX Isoform/Splice Variants, Adv Exp Med Biol, 981 (2017) 41–58 [DOI] [PubMed] [Google Scholar]
- [61].Fuller W, Reilly L, Hilgemann DW, S-palmitoylation and the regulation of NCX1, Channels (Austin), 10 (2016) 75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Plain F, Congreve SD, Yee RSZ, Kennedy J, Howie J, Kuo CW, Fraser NJ, Fuller W, An amphipathic alpha-helix directs palmitoylation of the large intracellular loop of the sodium/calcium exchanger, J Biol Chem, 292 (2017) 10745–10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reilly L, Howie J, Wypijewski K, Ashford ML, Hilgemann DW, Fuller W, Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling, FASEB J, 29 (2015) 4532–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].John SA, Ribalet B, Weiss JN, Philipson KD, Ottolia M, Ca2+-dependent structural rearrangements within Na+-Ca2+ exchanger dimers, Proc Natl Acad Sci U S A, 108 (2011) 1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ren X, Nicoll DA, Galang G, Philipson KD, Intermolecular cross-linking of Na+-Ca2+ exchanger proteins: evidence for dimer formation, Biochemistry, 47 (2008) 6081–6087. [DOI] [PubMed] [Google Scholar]
- [66].Chou AC, Ju YT, Pan CY, Calmodulin Interacts with the Sodium/Calcium Exchanger NCX1 to Regulate Activity, PLoS One, 10 (2015) e0138856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang YH, Hancox JC, Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation--still a paradox?, Cell Calcium, 45 (2009) 1–10. [DOI] [PubMed] [Google Scholar]
- [68].Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M, Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C, J Biol Chem, 271 (1996) 13609–13615. [DOI] [PubMed] [Google Scholar]
- [69].Iwamoto T, Pan Y, Nakamura TY, Wakabayashi S, Shigekawa M, Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation, Biochemistry, 37 (1998) 17230–17238. [DOI] [PubMed] [Google Scholar]
- [70].Hnatowich M, Le HD, DeMoissac D, Ranson K, Yurkov V, Gilchrist JS, Omelchenko A, Hryshko LV, mu-Calpain-mediated deregulation of cardiac, brain, and kidney NCX1 splice variants, Cell Calcium, 51 (2012) 164–170. [DOI] [PubMed] [Google Scholar]
- [71].Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P, Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity, Cell, 120 (2005) 275–285. [DOI] [PubMed] [Google Scholar]
- [72].Philipson KD, Bers DM, Nishimoto AY, The role of phospholipids in the Ca2+ binding of isolated cardiac sarcolemma, J Mol Cell Cardiol, 12 (1980) 1159–1173. [DOI] [PubMed] [Google Scholar]
- [73].Riedel MJ, Baczko I, Searle GJ, Webster N, Fercho M, Jones L, Lang J, Lytton J, Dyck JR, Light PE, Metabolic regulation of sodium-calcium exchange by intracellular acyl CoAs, Embo J, 25 (2006) 4605–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vemuri R, Philipson KD, Phospholipid composition modulates the Na+-Ca2+ exchange activity of cardiac sarcolemma in reconstituted vesicles, Biochim Biophys Acta, 937 (1988) 258–268. [DOI] [PubMed] [Google Scholar]
- [75].Hilgemann DW, Matsuoka S, Nagel GA, Collins A, Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation, Journal of General Physiology, 100 (1992) 905–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Matsuoka S, Hilgemann DW, Inactivation of Outward Na+-Ca2+ Exchange Current in Guinea-Pig Ventricular Myocytes, Journal of Physiology-London, 476 (1994) 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Despa S, Bers DM, Na+ transport in the normal and failing heart - remember the balance, J Mol Cell Cardiol, 61 (2013) 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hilgemann DW, Collins A, Matsuoka S, Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP, Journal of General Physiology, 100 (1992) 933–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD, Regulation of the cardiac Na+-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca2+-binding domain, Journal of General Physiology, 105 (1995) 403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ottolia M, Nicoll DA, Philipson KD, Roles of two Ca2+-binding domains in regulation of the cardiac Na+-Ca2+ exchanger, J Biol Chem, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Giladi M, Boyman L, Mikhasenko H, Hiller R, Khananshvili D, Essential role of the CBD1-CBD2 linker in slow dissociation of Ca2+ from the regulatory two-domain tandem of NCX1, J Biol Chem, 285 (2010) 28117–28125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Miura Y, Kimura J, Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca, J Gen Physiol, 93 (1989) 1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reeves JP, Condrescu M, Allosteric activation of sodium-calcium exchange activity by calcium: persistence at low calcium concentrations, J Gen Physiol, 122 (2003) 621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Weber CR, Ginsburg KS, Philipson KD, Shannon TR, Bers DM, Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes, J Gen Physiol, 117 (2001) 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lariccia V, Amoroso S, Calcium- and ATP-dependent regulation of Na/Ca exchange function in BHK cells: Comparison of NCX1 and NCX3 exchangers, Cell Calcium, 73 (2018) 95–103. [DOI] [PubMed] [Google Scholar]
- [86].Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, Hilgemann DW, Massive calcium-activated endocytosis without involvement of classical endocytic proteins, J Gen Physiol, 137 (2011) 111–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li Z, Matsuoka S, Hryshko LV, Nicoll DA, Bersohn MM, Burke EP, Lifton RP, Philipson KD, Cloning of the NCX2 isoform of the plasma membrane Na+-Ca2+ exchanger, J Biol Chem, 269 (1994) 17434–17439. [PubMed] [Google Scholar]
- [88].Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, Philipson KD, Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3), Am J Physiol, 274 (1998) C415–423. [DOI] [PubMed] [Google Scholar]
- [89].Matsuoka S, Forefront of Na+/Ca2+ exchanger studies: regulation kinetics of Na+/Ca2+ exchangers, J Pharmacol Sci, 96 (2004) 12–14. [DOI] [PubMed] [Google Scholar]
- [90].Giladi M, Lee SY, Ariely Y, Teldan Y, Granit R, Strulovich R, Haitin Y, Chung KY, Khananshvili D, Structure-based dynamic arrays in regulatory domains of sodium-calcium exchanger (NCX) isoforms, Sci Rep, 7 (2017) 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tal I, Kozlovsky T, Brisker D, Giladi M, Khananshvili D, Kinetic and equilibrium properties of regulatory Ca2+-binding domains in sodium-calcium exchangers 2 and 3, Cell Calcium, 59 (2016) 181–188. [DOI] [PubMed] [Google Scholar]
- [92].Breukels V, Touw WG, Vuister GW, NMR structure note: solution structure of Ca(2)+ binding domain 2B of the third isoform of the Na+/Ca(2)+ exchanger, J Biomol NMR, 54 (2012) 115–121. [DOI] [PubMed] [Google Scholar]
- [93].Dipolo R, Beauge L, The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons, Biochim Biophys Acta, 688 (1982) 237–245. [DOI] [PubMed] [Google Scholar]
- [94].Hilgemann DW, Giant excised cardiac sarcolemmal membrane patches: sodium and sodium-calcium exchange currents, Pflugers Arch, 415 (1989) 247–249. [DOI] [PubMed] [Google Scholar]
- [95].Hilgemann DW, Matsuoka S, Nagel GA, Collins A, Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation, J Gen Physiol, 100 (1992) 905–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Doering AE, Lederer WJ, The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+-Ca2+ exchanger in the guinea-pig, J Physiol, 480 ( Pt 1) (1994) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shigekawa M, Iwamoto T, Uehara A, Kita S, Probing ion binding sites in the Na+/Ca2+ exchanger, Ann N Y Acad Sci, 976 (2002) 19–30. [DOI] [PubMed] [Google Scholar]
- [98].Kofuji P, Hadley RW, Kieval RS, Lederer WJ, Schulze DH, Expression of the Na-Ca exchanger in diverse tissues: a study using the cloned human cardiac Na-Ca exchanger, Am J Physiol, 263 (1992) C1241–1249. [DOI] [PubMed] [Google Scholar]
- [99].Lee SL, Yu AS, Lytton J, Tissue-specific expression of Na+-Ca2+ exchanger isoforms, J Biol Chem, 269 (1994) 14849–14852. [PubMed] [Google Scholar]
- [100].Goldhaber JI, Henderson SA, Reuter H, Pott C, Philipson KD, Effects of Na+-Ca2+ exchange expression on excitation-contraction coupling in genetically modified mice, Ann N Y Acad Sci, 1047 (2005) 122–126. [DOI] [PubMed] [Google Scholar]
- [101].Flesch M, Schwinger RH, Schiffer F, Frank K, Sudkamp M, Kuhn-Regnier F, Arnold G, Bohm M, Evidence for functional relevance of an enhanced expression of the Na+-Ca2+ exchanger in failing human myocardium, Circulation, 94 (1996) 992–1002. [DOI] [PubMed] [Google Scholar]
- [102].Hobai IA, O’Rourke B, Enhanced Ca2+-activated Na+-Ca2+ exchange activity in canine pacing-induced heart failure, Circ Res, 87 (2000) 690–698. [DOI] [PubMed] [Google Scholar]
- [103].Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H, Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure, Circ Res, 75 (1994) 443–453. [DOI] [PubMed] [Google Scholar]
- [104].Hasenfuss G, Pieske B, Calcium cycling in congestive heart failure, J Mol Cell Cardiol, 34 (2002) 951–969. [DOI] [PubMed] [Google Scholar]
- [105].Sipido KR, Volders PG, Vos MA, Verdonck F, Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy?, Cardiovasc Res, 53 (2002) 782–805. [DOI] [PubMed] [Google Scholar]
- [106].Schafer C, Ladilov Y, Inserte J, Schafer M, Haffner S, Garcia-Dorado D, Piper HM, Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury, Cardiovasc Res, 51 (2001) 241–250. [DOI] [PubMed] [Google Scholar]
- [107].Bers DM, Pogwizd SM, Schlotthauer K, Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure, Basic Res Cardiol, 97 Suppl 1 (2002) I36–42. [DOI] [PubMed] [Google Scholar]
- [108].Pogwizd SM, Bers DM, Na/Ca exchange in heart failure: contractile dysfunction and arrhythmogenesis, Ann N Y Acad Sci, 976 (2002) 454–465. [DOI] [PubMed] [Google Scholar]
- [109].Pogwizd SM, Bers DM, Cellular basis of triggered arrhythmias in heart failure, Trends Cardiovasc Med, 14 (2004) 61–66. [DOI] [PubMed] [Google Scholar]
- [110].Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM, Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure, Circ Res, 85 (1999) 1009–1019. [DOI] [PubMed] [Google Scholar]
- [111].Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ, Vos MA, Enhanced Ca2+ release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis, Circulation, 102 (2000) 2137–2144. [DOI] [PubMed] [Google Scholar]
- [112].Bogeholz N, Pauls P, Bauer BK, Schulte JS, Dechering DG, Frommeyer G, Kirchhefer U, Goldhaber JI, Muller FU, Eckardt L, Pott C, Suppression of Early and Late Afterdepolarizations by Heterozygous Knockout of the Na+/Ca2+ Exchanger in a Murine Model, Circ Arrhythm Electrophysiol, 8 (2015) 1210–1218. [DOI] [PubMed] [Google Scholar]
- [113].Bogeholz N, Pauls P, Kaese S, Schulte JS, Lemoine MD, Dechering DG, Frommeyer G, Goldhaber JI, Seidl MD, Kirchhefer U, Eckardt L, Muller FU, Pott C, Triggered activity in atrial myocytes is influenced by Na+/Ca2+ exchanger activity in genetically altered mice, J Mol Cell Cardiol, 101 (2016) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goldhaber JI, Philipson KD, Cardiac sodium-calcium exchange and efficient excitation-contraction coupling: implications for heart disease, Adv Exp Med Biol, 961 (2013) 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Khananshvili D, The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease, Mol Aspects Med, 34 (2013) 220–235. [DOI] [PubMed] [Google Scholar]
- [116].Khananshvili D, Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions, Pflugers Arch, 466 (2014) 43–60. [DOI] [PubMed] [Google Scholar]
- [117].Bogeholz N, Eckardt L, Pott C, Advantages and limitations of transgenic mice: the role of the Na+/Ca2+ exchanger in cardiac electrophysiology and arrhythmia, Curr Med Chem, 21 (2014) 1330–1335. [DOI] [PubMed] [Google Scholar]
- [118].Sipido KR, Volders PG, Schoenmakers M, De Groot SH, Verdonck F, Vos MA, Role of the Na/Ca exchanger in arrhythmias in compensated hypertrophy, Ann N Y Acad Sci, 976 (2002) 438–445. [DOI] [PubMed] [Google Scholar]
- [119].Roos A, Boron WF, Intracellular pH, Physiol Rev, 61 (1981) 296–434. [DOI] [PubMed] [Google Scholar]
- [120].Vaughan-Jones RD, Wu ML, Bountra C, Sodium-hydrogen exchange and its role in controlling contractility during acidosis in cardiac muscle, Mol Cell Biochem, 89 (1989) 157–162. [DOI] [PubMed] [Google Scholar]
- [121].Rousseau E, Pinkos J, pH modulates conducting and gating behaviour of single calcium release channels, Pflugers Arch, 415 (1990) 645–647. [DOI] [PubMed] [Google Scholar]
- [122].Balnave CD, Vaughan-Jones RD, Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes, J Physiol, 528 Pt 1 (2000) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Komukai K, Pascarel C, Orchard CH, Compensatory role of CaMKII on ICa and SR function during acidosis in rat ventricular myocytes, Pflugers Arch, 442 (2001) 353–361. [DOI] [PubMed] [Google Scholar]
- [124].Fabiato A, Fabiato F, Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles, J Physiol, 276 (1978) 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kohmoto O, Spitzer KW, Movsesian MA, Barry WH, Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes, Circ Res, 66 (1990) 622–632. [DOI] [PubMed] [Google Scholar]
- [126].Choi HS, Trafford AW, Orchard CH, Eisner DA, The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes, J Physiol, 529 Pt 3 (2000) 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Holroyde MJ, Robertson SP, Johnson JD, Solaro RJ, Potter JD, The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofibrillar adenosine triphosphatase, J Biol Chem, 255 (1980) 11688–11693. [PubMed] [Google Scholar]
- [128].Godt RE, Baumgarten CM, Potential and K+ activity in skinned muscle fibers. Evidence against a simple Donnan equilibrium, Biophys J, 45 (1984) 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lee JA, Allen DG, Mechanisms of acute ischemic contractile failure of the heart. Role of intracellular calcium, J Clin Invest, 88 (1991) 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Metzger JM, Parmacek MS, Barr E, Pasyk K, Lin WI, Cochrane KL, Field LJ, Leiden JM, Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice, Proc Natl Acad Sci U S A, 90 (1993) 9036–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D’Alecy LG, Ingwall JS, Metzger JM, Histidine button engineered into cardiac troponin I protects the ischemic and failing heart, Nat Med, 12 (2006) 181–189. [DOI] [PubMed] [Google Scholar]
- [132].Garciarena CD, Youm JB, Swietach P, Vaughan-Jones RD, H+-activated Na+ influx in the ventricular myocyte couples Ca(2)+-signalling to intracellular pH, J Mol Cell Cardiol, 61 (2013) 51–59. [DOI] [PubMed] [Google Scholar]
- [133].Allen DG, Xiao XH, Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion, Cardiovasc Res, 57 (2003) 934–941. [DOI] [PubMed] [Google Scholar]
- [134].Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A, Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II, Am J Physiol Heart Circ Physiol, 295 (2008) H1669–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Valverde CA, Kornyeyev D, Ferreiro M, Petrosky AD, Mattiazzi A, Escobar AL, Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion, Cardiovasc Res, 85 (2010) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nicoll DA, Sawaya MR, Kwon S, Cascio D, Philipson KD, Abramson J, The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif, J Biol Chem, 281 (2006) 21577–21581. [DOI] [PubMed] [Google Scholar]