The Surviving Sepsis Campaign panel recently recommended that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU [1].”
Yet, COVID-19 pneumonia [2], despite falling in most of the circumstances under the Berlin definition of ARDS [3], is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance (more than 50% of the 150 patients measured by the authors and further confirmed by several colleagues in Northern Italy). This remarkable combination is almost never seen in severe ARDS. These severely hypoxemic patients despite sharing a single etiology (SARS-CoV-2) may present quite differently from one another: normally breathing (“silent” hypoxemia) or remarkably dyspneic; quite responsive to nitric oxide or not; deeply hypocapnic or normo/hypercapnic; and either responsive to prone position or not. Therefore, the same disease actually presents itself with impressive non-uniformity.
Based on detailed observation of several cases and discussions with colleagues treating these patients, we hypothesize that the different COVID-19 patterns found at presentation in the emergency department depend on the interaction between three factors: (1) the severity of the infection, the host response, physiological reserve and comorbidities; (2) the ventilatory responsiveness of the patient to hypoxemia; (3) the time elapsed between the onset of the disease and the observation in the hospital. The interaction between these factors leads to the development of a time-related disease spectrum within two primary “phenotypes”: Type L, characterized by Low elastance (i.e., high compliance), Low ventilation-to-perfusion ratio, Low lung weight and Low recruitability and Type H, characterized by High elastance, High right-to-left shunt, High lung weight and High recruitability.
COVID-19 pneumonia, Type L
At the beginning, COVID-19 pneumonia presents with the following characteristics:
Low elastance. The nearly normal compliance indicates that the amount of gas in the lung is nearly normal [4].
Low ventilation-to-perfusion (VA/Q) ratio. Since the gas volume is nearly normal, hypoxemia may be best explained by the loss of regulation of perfusion and by loss of hypoxic vasoconstriction. Accordingly, at this stage, the pulmonary artery pressure should be near normal.
Low lung weight. Only ground-glass densities are present on CT scan, primarily located subpleurally and along the lung fissures. Consequently, lung weight is only moderately increased.
Low lung recruitability. The amount of non-aerated tissue is very low; consequently, the recruitability is low [5].
To conceptualize these phenomena, we hypothesize the following sequence of events: the viral infection leads to a modest local subpleural interstitial edema (ground-glass lesions) particularly located at the interfaces between lung structures with different elastic properties, where stress and strain are concentrated [6]. Vasoplegia accounts for severe hypoxemia. The normal response to hypoxemia is to increase minute ventilation, primarily by increasing the tidal volume [7] (up to 15–20 ml/kg), which is associated with a more negative intrathoracic inspiratory pressure. Undetermined factors other than hypoxemia markedly stimulate, in these patients, the respiratory drive. The near normal compliance, however, explains why some of the patients present without dyspnea as the patient inhales the volume he expects. This increase in minute ventilation leads to a decrease in PaCO2.
The evolution of the disease: transitioning between phenotypes
The Type L patients may remain unchanging for a period and then improve or worsen. The possible key feature which determines the evolution of the disease, other than the severity of the disease itself, is the depth of the negative intrathoracic pressure associated with the increased tidal volume in spontaneous breathing. Indeed, the combination of a negative inspiratory intrathoracic pressure and increased lung permeability due to inflammation results in interstitial lung edema. This phenomenon, initially described by Barach in [8] and Mascheroni in [9] both in an experimental setting, has been recently recognized as the leading cause of patient self-inflicted lung injury (P-SILI) [10]. Over time, the increased edema increases lung weight, superimposed pressure and dependent atelectasis. When lung edema reaches a certain magnitude, the gas volume in the lung decreases, and the tidal volumes generated for a given inspiratory pressure decrease [11]. At this stage, dyspnea develops, which in turn leads to worsening P-SILI. The transition from Type L to Type H may be due to the evolution of the COVID-19 pneumonia on one hand and the injury attributable to high-stress ventilation on the other.
COVID-19 pneumonia, Type H
The Type H patient:
High elastance. The decrease in gas volume due to increased edema accounts for the increased lung elastance.
High right-to-left shunt. This is due to the fraction of cardiac output perfusing the non-aerated tissue which develops in the dependent lung regions due to the increased edema and superimposed pressure.
High lung weight. Quantitative analysis of the CT scan shows a remarkable increase in lung weight (> 1.5 kg), on the order of magnitude of severe ARDS [12].
High lung recruitability. The increased amount of non-aerated tissue is associated, as in severe ARDS, with increased recruitability [5].
The Type H pattern, 20–30% of patients in our series, fully fits the severe ARDS criteria: hypoxemia, bilateral infiltrates, decreased the respiratory system compliance, increased lung weight and potential for recruitment.
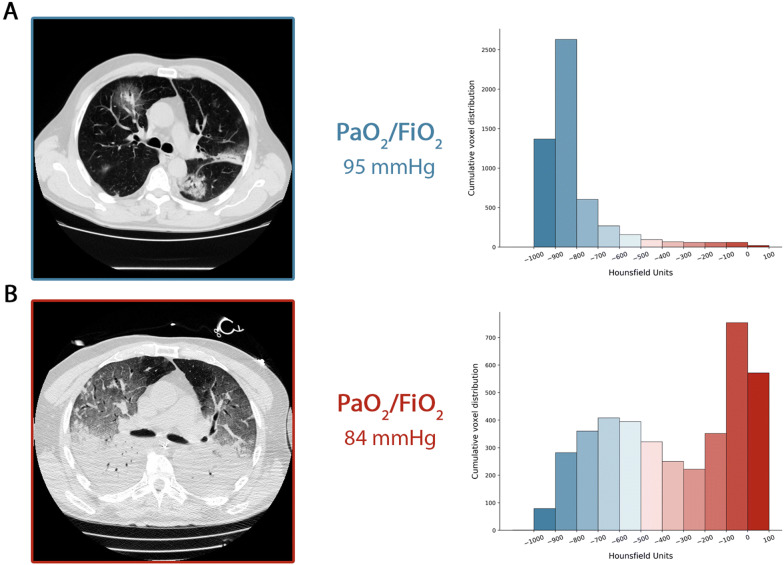
Figure 1 summarizes the time course we described. In panel a, we show the CT in spontaneous breathing of a Type L patient at admission, and in panel b, its transition in Type H after 7 days of noninvasive support. As shown, a similar degree of hypoxemia was associated with different patterns in lung imaging.
Fig. 1.
a CT scan acquired during spontaneous breathing. The cumulative distribution of the CT number is shifted to the left (well-aerated compartments), being the 0 to − 100 HU compartment, the non-aerated tissue virtually 0. Indeed, the total lung tissue weight was 1108 g, 7.8% of which was not aerated and the gas volume was 4228 ml. Patient receiving oxygen with venturi mask inspired oxygen fraction of 0.8. b CT acquired during mechanical ventilation at end-expiratory pressure at 5 cmH2O of PEEP. The cumulative distribution of the CT scan is shifted to the right (non-aerated compartments), while the left compartments are greatly reduced. Indeed, the total lung tissue weight was 2744 g, 54% of which was not aerated and the gas volume was 1360 ml. The patient was ventilated in volume controlled mode, 7.8 ml/kg of tidal volume, respiratory rate of 20 breaths per minute, inspired oxygen fraction of 0.7
Respiratory treatment
Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different. The proposed treatment is consistent with what observed in COVID-19, even though the overwhelming number of patients seen in this pandemic may limit its wide applicability.
The first step to reverse hypoxemia is through an increase in FiO2 to which the Type L patient responds well, particularly if not yet breathless.
In Type L patients with dyspnea, several noninvasive options are available: high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV). At this stage, the measurement (or the estimation) of the inspiratory esophageal pressure swings is crucial [13]. In the absence of the esophageal manometry, surrogate measures of work of breathing, such as the swings of central venous pressure [14] or clinical detection of excessive inspiratory effort, should be assessed. In intubated patients, the P0.1 and Pocclusion should also be determined. High PEEP, in some patients, may decrease the pleural pressure swings and stop the vicious cycle that exacerbates lung injury. However, high PEEP in patients with normal compliance may have detrimental effects on hemodynamics. In any case, noninvasive options are questionable, as they may be associated with high failure rates and delayed intubation, in a disease which typically lasts several weeks.
The magnitude of inspiratory pleural pressures swings may determine the transition from the Type L to the Type H phenotype. As esophageal pressure swings increase from 5 to 10 cmH2O—which are generally well tolerated—to above 15 cmH2O, the risk of lung injury increases and therefore intubation should be performed as soon as possible.
Once intubated and deeply sedated, the Type L patients, if hypercapnic, can be ventilated with volumes greater than 6 ml/kg (up to 8–9 ml/kg), as the high compliance results in tolerable strain without the risk of VILI. Prone positioning should be used only as a rescue maneuver, as the lung conditions are “too good” for the prone position effectiveness, which is based on improved stress and strain redistribution. The PEEP should be reduced to 8–10 cmH2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. An early intubation may avert the transition to Type H phenotype.
Type H patients should be treated as severe ARDS, including higher PEEP, if compatible with hemodynamics, prone positioning and extracorporeal support.
In conclusion, Type L and Type H patients are best identified by CT scan and are affected by different pathophysiological mechanisms. If not available, signs which are implicit in Type L and Type H definition could be used as surrogates: respiratory system elastance and recruitability. Understanding the correct pathophysiology is crucial to establishing the basis for appropriate treatment.
Acknowledgements
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflicts of interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus I, Research T A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Force ARDSDT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. The American review of respiratory disease. Am Rev Respir Dis. 1987;136:730–736. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 6.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 7.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201:20–32. doi: 10.1164/rccm.201903-0596SO. [DOI] [PubMed] [Google Scholar]
- 8.Al B, Martin J, Eckman M. Positive pressure respiration and its application to the treatment of acute pulmonary edema. Ann Intern Med. 1938;12:754–795. doi: 10.7326/0003-4819-12-6-754. [DOI] [Google Scholar]
- 9.Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15:8–14. doi: 10.1007/BF00255628. [DOI] [PubMed] [Google Scholar]
- 10.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi P, D'Andrea L, Vitale G, Pesenti A, Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- 12.Maiolo G, Collino F, Vasques F, Rapetti F, Tonetti T, Romitti F, Cressoni M, Chiumello D, Moerer O, Herrmann P, Friede T, Quintel M, Gattinoni L. Reclassifying acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:1586–1595. doi: 10.1164/rccm.201709-1804OC. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L, Giosa L, Bonifazi M, Pasticci I, Busana M, Macri M, Romitti F, Vassalli F, Quintel M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med. 2019;13:737–746. doi: 10.1080/17476348.2019.1638767. [DOI] [PubMed] [Google Scholar]
- 14.Walling PT, Savege TM. A comparison of oesophageal and central venous pressures in the measurement of transpulmonary pressure change. Br J Anaesth. 1976;48:475–479. doi: 10.1093/bja/48.5.475. [DOI] [PubMed] [Google Scholar]