Abstract
AIM
To estimate the prevalence of blindness and visual impairment resulting from cataract in the population aged ≥50y in Hungary, and to assess the cataract surgical services.
METHODS
A rapid assessment of avoidable blindness (RAAB) was conducted. A total of 3523 eligible people were randomly selected and examined. Each participant underwent surgery for cataract was interviewed with regard to the year, place, and costs of the surgery. Participants with obvious cataract were asked why they had not yet undergone surgery (barriers to surgery).
RESULTS
An estimated 12 514 people were bilaterally blind; the visual acuity (VA) in 19 293 people was <6/60, and the VA in 73 962 people was <6/18 in the better eye due to cataract. An estimated 77 933 eyes are blind; 98 067 eyes had a VA of <6/60, and an estimated 277 493 eyes had a VA of <6/18 due to cataract. Almost all cataract surgeries were conducted in government hospitals. The age- and sex-adjusted cataract surgical coverage with VA<3/60 in eyes was 90.0%. The rate of good visual outcome after surgery was 79.5%. Ocular comorbidity was the main cause of poor outcome (78.1%), followed by late complications (such as posterior capsule opacification) (17.2%), inadequate optical correction (3.1%), and surgical complications (1.6%). The main barrier to surgery in people with bilateral cataract and VA of <6/60 was ‘need not felt’.
CONCLUSION
The prevalence of visual impairment resulting from cataract is slightly higher than expected. The quality of the cataract surgical service seems adequate in Hungary. However, the number of cataract operations per year should continue to increase due to the increasing patient demands and the aging population.
Keywords: cataract prevalence, blindness, visual impairment, rapid assessment of avoidable blindness
INTRODUCTION
Globally, in 2015 an estimated 36 million people were blind, and 216.6 million people had moderate or severe visual impairment (MVI and SVI, respectively)[1]. Among individuals in the global population who were blind in 2015, the leading cause was cataract, and cataract was the second leading cause among the population with MVI or SVI[2]. The burden of this eye disease is particularly high in developing countries. Although the proportion of blindness due to cataract decreased from 1990 to 2010 worldwide[3]–[9] (and in the period between 1990 and 2015, as well[2]), cataract is expected to remain the major cause of blindness in 2020[2].
Hungary is situated in Central Europe, bordering Austria, Slovakia, Ukraine, Romania, Serbia, Croatia, and Slovenia. Hungary is a medium-sized member state of the European Union with approximately 10 million inhabitants. Hungary is an established market economy in Europe (GDP=28 799 USD, GINI coefficient=30.4).
The ratio of ophthalmologists to the general population is approximately 10 eye doctors/10 000 inhabitants. Every second doctor is surgically active. In total, 92 units (university departments, hospitals, and 1-day surgery units) can be found in the country, wherein cataract operations are performed (22 units in the capital and 70 units in regional cities). In 2015, the cataract surgical rate was estimated to be 8544 (unpublished data based on the National Cataract Register, personal communication with HJ. Kiss, Semmelweis University, 2018).
A rapid assessment of avoidable blindness (RAAB) survey with a diabetic retinopathy module (DRM) was conducted by our research group in Hungary in 2015. We found that “the prevalence of blindness was relatively low, with age-related macular degeneration (AMD) and other posterior segment diseases being the leading causes”[10]. We reported the experiences, challenges, and lessons learned in conducting such a population-based survey in this industrialized country. The study was difficult to perform, but successful. The results of the RAAB provide a solid base for the development of a national program for universal eye health and to prepare active media campaigns[11]. We also reported the prevalence of diabetes mellitus and diabetic retinopathy (DR) and the coverage of diabetic eye care services. The prevalence of DR (20.1%, an estimated 755 000 people) was slightly lower than expected, but the number of ophthalmologically uncontrolled diabetic eyes was high[12]–[14].
One important finding was that cataract is still a significant cause of blindness and visual impairment in this established market economy of Europe; it was the main cause of SVI, MVI, and early visual impairment (EVI) and the third major cause of blindness[10]. These findings are similar to the previous estimations for Central Europe[2],[15], wherein Hungary is situated. However, there were some differences in that Central Europe cataract was estimated to be the leading cause of blindness and second leading cause of SVI and MVI in Central Europe. One possible explanation for this discrepancy is the scarce reliable epidemiological data from this European subregion (only one survey exists from Bulgaria)[15].
Following the RAAB study, population-based data are currently available, and further analysis to increase the accuracy of the estimates is possible. Such analysis is also essential to plan cataract surgical services properly.
Therefore, the purpose of this current study was to estimate the prevalence of blindness and visual impairment resulting from cataract in the population aged ≥50y in Hungary, and to evaluate the cataract surgical services and the barriers to surgery.
SUBJECTS AND METHODS
Ethical Approval
The study was conducted in compliance with the tenets of the Declaration of Helsinki and with applicable country and local requirements regarding ethics committees, institutional review boards, and other statutes or regulations regarding protection of the rights and welfare of human subjects participating in biomedical research. Permission to conduct the study was granted by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (No.234/2014) Committee. Written informed consent was obtained from all participants prior to examination.
A nationwide RAAB+DRM was conducted in Hungary between 2014 and 2016. This population-based survey was organized by the Semmelweis University Budapest, with co-funding from the Lions Sight First Program. The main goal was to assess the current situation regarding blindness and visual impairment in Hungary.
The details of the methods were reported elsewhere[11]. Briefly, a sample size was estimated based on the expected prevalence of blindness of 2.5% among adults aged ≥50y, with a required confidence interval of 95%, precision of 25%, design effect of 1.4%, and noncompliance rate of 10%. A total of 105 clusters of 35 people aged ≥50y were randomly selected, and 3675 people were enrolled. Finally, a total of 3523 people were examined in their households (coverage of 95.9%). The examined clusters included persons from vulnerable populations also (such as Roma ethnic population, homeless people), but we did not identify them in the survey data collection.
Five study teams were formed, with each composed of a senior ophthalmic resident or eye specialist, a nurse, an assistant, a driver, and a local guide. Doctors, nurses, and assistants received training for the survey and eye examination protocol for 5d. Data entry and cleaning were performed by the survey teams directly after the examination. An automatic data check was also implemented. The teams then sent the completed data files to the survey coordinator, who merged the data from all teams into the main survey database.
Visual acuity (VA) was measured using a Snellen tumbling E-chart with optotype sizes of 6/12, 6/18, and 6/60 at 6 and 3 m (6/60 only) with available correction. If the VA was <6/12 in either eye, pinhole vision was also measured for each eye. Participants were categorized according to the VA in the better eye as follows: normal vision was indicated by VA ≥6/12, EVI was indicated by VA <6/12-6/18, MVI was indicated by VA <6/18-6/60, SVI was indicated by VA <6/60-3/60, and blindness was indicated by VA<3/60.
The status of the lens was examined with a direct ophthalmoscope under semi-dark conditions (in unlit rooms or in the shadowed areas of the yards). We graded the lens status as follows: normal lens/minimal lens opacity, apparent lens opacity, lens absent (aphakia), pseudophakia without posterior capsule opacification (PCO), pseudophakia with PCO, and no view of the lens.
Every participant with a history of cataract surgery was interviewed: the date of operation, place of operation (government hospital or private hospital), and type of surgery [with or without intraocular lens (IOL)] were recorded.
The cataract surgical coverage (CSC) was calculated as follows: CSC for persons=(x+y/x+y+z)×100%, where x=persons with unilateral (pseudo)aphakia and operable cataract in the other eye, y=persons with bilateral (pseudo)aphakia, and z=persons with bilateral operable cataract; CSC for eyes VA=(a/a+b)×100%, where a=(pseudo)aphakic eyes, and b=eyes with operable cataract[16].
If the VA was <6/12, we determined whether the cause was ocular comorbidity, surgical complications, late complications (such as PCO), or inadequate optical correction.
All of the participants whose bilateral VA was <6/60 due to cataract were asked why they had not had cataract surgery and provided the following possible responses: ‘Need not felt’, ‘Fear of surgery’, ‘Cannot afford operation’, ‘Treatment denied by provider’ (due to other comorbidity), ‘Unaware that treatment is possible’, ‘No access to treatment’, or ‘Local reason’ (‘On a waiting list’). Participants who needed further ophthalmic or diabetic examination were referred to their general practitioner or ophthalmologist.
RESULTS
The survey included 3675 people aged ≥50y, of whom 3523 were finally examined (1273 males and 2250 females). The coverage was 95.9%. Seventy-one persons (1.9%) were absent, 80 (2.2%) refused to participate in the study, and one (0.03%) was not able to perform the tests.
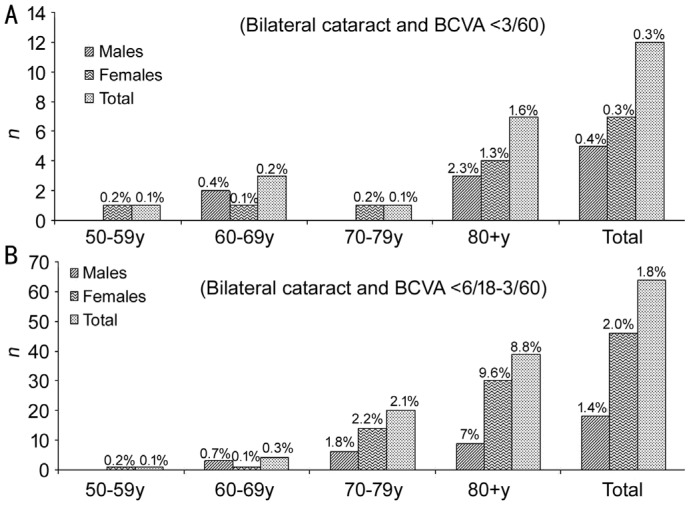
The age- and sex-adjusted prevalence of bilateral blindness due to cataract in the population of patients aged ≥50y in 2015 are provided in Table 1 (The prevalence could be similar in male as well as in female and therefore statistically not significantly different. However, the difference might be still relevant due to the difference in absolute numbers.). Figure 1 presents the age- and sex-specific prevalence of bilateral cataract blindness (Figure 1A), SVI, and MVI (Figure 1B).
Table 1. Adjusted results for cataract and blindness, severe visual impairment, moderate visual impairment, and early visual impairment.
| Parameters | Males |
Females |
Total |
|||
| n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | |
| Blindness (VA<3/60) due to cataract | ||||||
| Bilateral cataract | 5638 (0.4) | 0-0.7 | 6876 (0.3) | 0.1-0.5 | 12514 (0.3) | 0.1-0.5 |
| Cataract eyes | 30899 (1.0) | 0.5-1.4 | 47034 (1.1) | 0.8-1.4 | 77933 (1.0) | 0.8-1.3 |
| SVI (VA<6/60-3/60) due to cataract in better eye | ||||||
| Bilateral cataract | 1041 (0.1) | 0-0.2 | 5738 (0.3) | 0.1-0.5 | 6779 (0.2) | 0-0.3 |
| Cataract eyes | 2983 (0.1) | 0-0.2a | 17151 (0.4) | 0.2-0.6a | 20134 (0.3) | 0.1-0.4 |
| MVI (VA<6/18-6/60) due to cataract in better eye | ||||||
| Bilateral cataract | 17505 (1.1) | 0.4-1.8 | 37164 (1.7) | 1.2-2.3 | 54669 (1.5) | 1.0-1.9 |
| Cataract eyes | 57036 (1.8) | 1.1-2.5 | 122390 (2.8) | 2.2-3.4 | 179426 (2.4) | 1.9-2.9 |
| EVI (VA<6/12-6/18) due to cataract in better eye | ||||||
| Bilateral cataract | 31911 (1.0) | 0.2-1.8 | 88119 (2.0) | 1.2-2.8 | 120030 (1.6) | 1.0-2.2 |
| Cataract eyes | 109609 (3.5) | 2.5-4.4a | 234600 (5.4) | 4.6-6.2a | 344209 (4.6) | 3.9-5.3 |
CI: Confidence interval; EVI: Early visual; SVI: Severe visual impairment; MVI: Moderate visual impairment; VA: Visual acuity. aSignificant difference in prevalence between men and women.
Figure 1. Age- and sex-specific prevalence of bilateral cataract blindness (A), severe visual impairment, and moderate visual impairment (B).
The age- and sex-adjusted prevalence of bilateral (pseudo)aphakia was 7.5%, which equated to an estimated 280 097 people in Hungary: 83 202 males (4.2%) and 196 895 females (9.1%). The estimated number of (pseudo)aphakic eyes was 699 840 in Hungary: 228 314 in males (7.2%) and 471 526 in females (10.9%).
Of all cataract operations, 98.8% were conducted in government hospitals and 1.2% in private hospitals. Almost all of the eyes that underwent surgery had an IOL implanted (98.6%).
In eyes with a VA of <3/60, 96 of every 100 bilateral cataract-blind persons underwent surgery in one or both eyes. Of all cataract-blind eyes, 90% underwent surgery. The coverage was only slightly lower for eyes with VAs of <6/60 and 6/18 (Table 2).
Table 2. Age- and sex-adjusted results for cataract surgical coverage.
| VA | Males (%) | Females (%) | Total (%) |
| CSC (eyes) | |||
| VA<3/60 | 88.1 | 90.9 | 90.0 |
| VA<6/60 | 87.1 | 88.0 | 87.7 |
| VA<6/18 | 71.5 | 71.7 | 71.6 |
| CSC (persons) | |||
| VA<3/60 | 94.6 | 97.1 | 96.3 |
| VA<6/60 | 93.7 | 94.8 | 94.5 |
| VA<6/18 | 82.2 | 83.7 | 83.2 |
CSC: Cataract surgical coverage; VA: Visual acuity.
Of all eyes that underwent cataract surgery, 79.5% had a VA of 6/18 (good outcome) or better (68.7% had a VA of 6/12 or better), and in 8.7% of the eyes, the VA was <6/60 with available correction (poor outcome). Data regarding outcome disaggregated by sex and the World Health Organization (WHO) recommendations regarding the outcome categories are provided in Table 3. With pinhole vision, the results improved to 85.4% with good outcome and decreased to 7.6% with poor outcome. The visual outcome of eyes undergoing surgery during the past 3y (84.4% good, 4.1% poor) was better than those who underwent surgery 4-6y prior (81.3% good, 9.4% poor) and ≥7y prior (71.3% good, 15.0% poor).
Table 3. VA in operated eyes in sample with available correction by stratified by sex.
| VA | Males | Females | Total | WHO recommendation |
| Very good: can see 6/12 | 147 (68.4) | 360 (68.8) | 507 (68.7) | >80% |
| Good: can see 6/18 | 22 (10.2) | 58 (11.1) | 80 (10.8) | |
| Borderline: can see 6/60 | 23 (10.7) | 64 (12.2) | 87 (11.8) | <15% |
| Poor: cannot see 6/60 | 23 (10.7) | 41 (7.8) | 64 (8.7) | <5% |
| Total | 215 (100) | 523 (100) | 738 (100) |
VA: Visual acuity; WHO: World Health Organization.
Ocular comorbidity was the main cause of poor outcome (78.1%), followed by late complications (such as PCO) (17.2%), inadequate optical correction (3.1%), and surgical complications (1.6%).
Barriers to cataract surgery in bilateral blind participants due to cataract were quite similar for males and females: ‘Need not felt’ was the most common barrier (75%), followed by ‘Fear of surgery’ (15%), and ‘Other comorbidity’ (10%). ‘Cannot afford operation’, ‘Unaware that treatment is possible’, ‘No access to treatment’, and ‘Local reason’ (‘On a waiting list’) were not mentioned (all 0).
DISCUSSION
Prevalence of Visual Impairment due to Cataract
We conducted the RAAB+DRM study in 2015 in Hungary (the first Central European country in which this method was applied) and found that the major causes of blindness were AMD (27.3%) and other posterior segment diseases (27.3%), followed by cataract (21.2%). Moreover, cataract was the main cause of SVI, MVI, and EVI[10].
According to previous estimates, in 2015, cataract was the most common cause of blindness and the second cause of MVI and SVI in Central Europe[2],[15]. According to earlier results, the age-standardized prevalence in those aged ≥50y was 0.2% for blindness and 0.6% for MVI and SVI in 2010 in this European sub-region[17]; however, the prevalence we observed were slightly higher than expected. One possible explanation for these different results is that only one study was identified from Central Europe[17]; thus, the prevalence might be underestimated due to the relatively small sample size.
The results of our RAAB survey indicated that blindness and impaired vision due to unoperated cataract were markedly more frequent than expected based on based on tacit knowledge.
Output of Cataract Services
Despite the higher than expected prevalence of cataract blindness and visual impairment, it seems that the quality of cataract surgical services is adequate in Hungary. An estimated 700 000 eyes had undergone surgery for cataract in the country by the time of the survey. There was a remarkable increase in the number of cataract surgeries in the past two decades. While 39 508 operations were performed in 2000, this number increased to 60 665 in 2010; in the year 2015, a total of 85 440 cataract operations were performed (unpublished data based on the National Cataract Register, personal communication with HJ. Kiss, Semmelweis University, 2018). While the hospitalization period due to cataract surgery was 3.9d on average in 2000, this was decreased to <1d in 2010 and almost all operations are currently performed as an out-patient 1-day surgery[18].
Quality of Cataract Services and Visual Outcome after Cataract Surgery
Almost all cataract extractions were performed in government hospitals or 1-day surgery departments. Only eight participants (11 eyes) underwent surgery in private hospitals. Ten eyes had good visual outcome and one had poor outcome due to ocular comorbidity. This proportion is similar to the results seen in government hospitals.
Almost all of the eyes that underwent surgery received an IOL (98.6%). This result is not surprising, because in 1998 the percentage of IOL implantation was already 97% in Hungary[19], which increased up to 99% in 2015 (unpublished data based on the National Cataract Register, personal communication with HJ. Kiss, Semmelweis University, 2018).
The CSC rate for VA<3/60 was higher than that in Moldova (the nearest country to Hungary in which a RAAB study was conducted)[20], and is higher or similar to current findings worldwide[21]–[22].
The VA of all subjects who underwent surgery earlier was also measured with available correction and with a pinhole. This report provides population-based data regarding visual outcome, which is not specific to one surgeon or one hospital and with follow-up periods ranging from 1mo to several decades. When cataract surgery was performed several years earlier, the likelihood of vision loss due to other causes than cataract increased. This vision loss was expected because the more time that has passed since the cataract surgery and the higher the age, the higher the risk of development of other sight-threatening eye diseases.
Although the visual outcome after cataract surgery was close to the WHO norm[23], and the proportion of eyes with a VA of <6/60 was <10%, research into the possible causes of poor visual outcome may be indicated. Analysis of current surgical practices to reduce surgical complications and analysis of current practice of IOL power calculation may be needed. Considerable improvement could be achieved with more accurate biometry or adequate optical correction. Nevertheless, a detailed preoperative examination of patients with cataracts may reduce the number of patients with concurrent blindness who may not regain vision after surgery. These patients may need counselling to provide them with realistic expectations of the future in terms of their vision. Review of the surgical procedures may lead to further improvements in visual outcome. It is important to keep in mind, that a poor outcome according to WHO norm might be actually a significant improvement and result in a patient being much more independent.
Patients with low vision postoperatively might benefit from referral to low vision services, which does not happen often even in high-income countries.
New Demands and the Aging Population
The more developed a society, the greater the demand for good eyesight. With economic improvements, the public demand for good eyesight and cataract surgery at earlier stages will also increase. Due to this demand and demographic changes (aging population), the number of cataract operations per year needs to increase further.
The indication for cataract surgery has recently changed. Patients' need for good vision has increased and cataract surgery should be performed in the early stages (this is occurring in Hungary, as indicated by the CSC for VA<6/18). If the indication for cataract surgery is a VA of <6/60, then 19 293 people (6 679 males and 12 614 females) aged ≥50y would require surgery in both eyes. Overall, an estimated 98 067 eyes (33 882 male eyes and 64 185 female eyes) would require surgery. If the indication for cataract surgery is a VA of <6/18, then 73 962 (24 184 males and 49 778 females) people aged ≥50y would require surgery in both eyes. Overall, an estimated 277 493 eyes (90 918 male eyes and 186 575 female eyes) would require surgery.
The population is aging in Hungary. The proportion of the total population of people aged ≥50y will increase from 32.7% in 2000 to 45.5% in 2030. The average life expectancy in Hungary will increase from 71.5y in 2000 to 78.5 in 2030. The population aged ≥50y will increase from 3.32 million in 2000 to 4.28 million in 2030. (https://www.census.gov/data-tools/demo/idb/informationGateway.php.). Thus, the number of cataract operations per year must continue to increase to account for the demographic changes and increased demand, because people wish to continue to live an active life as they grow older. They wish to drive a car, read books and newspapers, watch TV, and see their (grand) children.
‘There is an ongoing reduction in the age-standardized prevalence of blindness and visual impairment, yet the growth and ageing of the world's population is causing a substantial increase in number of people affected’[1], thus there may be needed to scale up cataract surgical services globally.
Barriers to Cataract Surgery
To meet this challenge, it is essential to understand the potential barriers to surgery. The RAAB is designed to be a rapid procedure and there is not sufficient time during the survey to conduct in-depth interviews to determine why people blinded by cataract have not yet undergone surgery. Hence, the data on barriers should be regarded as an indication of whether more detailed qualitative studies are required. In Hungary, the major barrier to cataract surgery was ‘need not felt’. One of the most important experiences during the study was that many people who believed that there was no need to undergo cataract surgery only realized their visual impairment for the first time when the ‘better eye’ was covered during examination.
Here, we found that the main reason why non-operated cataract is still the main cause of SVI, MVI, and EVI in Hungary is the patients not feeling the need to undergo surgery. This suggests that this is a patient-centric problem that may be solved by the provision of better health-related information, but the issue could also derive from eye surgeons who prefer to operate on more mature cases only. Regular self-checks may be useful in the recognition of the presence or progression of visual impairment and may reduce the number of non-operated cataract cases in Hungary. Furthermore, the relatively high proportion of patients with a ‘fear of surgery’ highlights the fact that eye health education should be improved. The barriers we found differ from the findings of previous studies in Kenya[24], Rwanda[25], Eritrea[26], Guatemala[27], or Turkmenistan[28]. In Hungary, the health care system is financed by the state; thus, the entire cataract surgery procedure is performed at no cost to the patient (the health insurance supports the transportation and the medication also, and therefore, the cost of the surgery was not mentioned as a reason to not undergo surgery by the participants. Our quantitative survey is too limited to develop any further conclusions, qualitative studies needed for deep analysis of barriers to cataract surgery[29].
Limitations
Our participation rate was high, but Ramke et al[30] suggested that even high participation rates do not completely nullify response bias. We were unable to completely avoid bias, but the reliability of our results was sufficient to infer definite conclusions.
In conclusion, the purpose of this study was to estimate the prevalence of blindness and visual impairment resulting from cataract in the population aged ≥50y in Hungary, and to evaluate the cataract surgical services and the barriers to surgery. Our data may serve as a useful basis for further estimations. Moreover, we found that cataract is still a common disease in this industrialized country in Europe.
Acknowledgments
We would like to thank the Hungarian National Institute for the Blind, the Lions Clubs Association of Hungary, and 77 Elektronika Kft for their active support during this study.
Authors' contributions: Sándor GL conducted the survey, analyzed the data, and wrote the article. Tóth G, Szabó D, Szalai I, Lukács R, Pék A and Tóth GZ conducted the survey and analyzed the data. Papp A and Nagy ZZ planned the survey and reviewed the manuscript. Limburg H and Németh J designed the survey, edited and reviewed the manuscript. All authors agree with the final version of the manuscript and agree to be accountable for all aspects of the work.
Foundation: Supported by SightFirst grant (No.SF 1825/UND) from Lions Clubs International Foundation, Oak Brook, IL, USA.
Conflicts of Interest: Sándor GL, None; Tóth G, None; Szabó D, None; Szalai I, None; Lukács R, None; Pék A, None; Tóth GZ, None; Papp A, None; Nagy ZZ, None; Limburg H, None; Németh J, None.
REFERENCES
- 1.Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Resnikoff S, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 3.Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Parodi MB, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014;98(5):629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, George R, Asokan R, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, Vijaya L, White RA, Wong TY, Resnikoff S, Taylor HR, Bourne RR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in Central and South Asia: 1990-2010. Br J Ophthalmol. 2014;98(5):592–598. doi: 10.1136/bjophthalmol-2013-303998. [DOI] [PubMed] [Google Scholar]
- 5.Keeffe J, Taylor HR, Fotis K, Pesudovs K, Flaxman SR, Jonas JB, Leasher J, Naidoo K, Price H, White RA, Wong TY, Resnikoff S, Bourne RR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in Southeast Asia and Oceania: 1990-2010. Br J Ophthalmol. 2014;98(5):586–591. doi: 10.1136/bjophthalmol-2013-304050. [DOI] [PubMed] [Google Scholar]
- 6.Khairallah M, Kahloun R, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Bourne RR, Vision Loss Expert Group Prevalence and causes of vision loss in north Africa and the middle east: 1990-2010. Br J Ophthalmol. 2014;98(5):605–611. doi: 10.1136/bjophthalmol-2013-304068. [DOI] [PubMed] [Google Scholar]
- 7.Leasher JL, Lansingh V, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, Silva JC, White RA, Wong TY, Resnikoff S, Taylor HR, Bourne RR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in Latin America and the Caribbean: 1990-2010. Br J Ophthalmol. 2014;98(5):619–628. doi: 10.1136/bjophthalmol-2013-304013. [DOI] [PubMed] [Google Scholar]
- 8.Naidoo K, Gichuhi S, Basáñez MG, Flaxman SR, Jonas JB, Keeffe J, Leasher JL, Pesudovs K, Price H, Smith JL, Turner HC, White RA, Wong TY, Resnikoff S, Taylor HR, Bourne RR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in sub-Saharan Africa: 1990-2010. Br J Ophthalmol. 2014;98(5):612–618. doi: 10.1136/bjophthalmol-2013-304081. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Zheng Y, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, White RA, Resnikoff S, Taylor HR, Bourne RR, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in East Asia: 1990-2010. Br J Ophthalmol. 2014;98(5):599–604. doi: 10.1136/bjophthalmol-2013-304047. [DOI] [PubMed] [Google Scholar]
- 10.Szabó D, Sándor GL, Tóth G, Pék A, Lukács R, Szalai I, Tóth GZ, Papp A, Nagy ZZ, Limburg H, Németh J. Visual impairment and blindness in Hungary. Acta Ophthalmol. 2018;96(2):168–173. doi: 10.1111/aos.13542. [DOI] [PubMed] [Google Scholar]
- 11.Németh J, Szabó D, Tóth G, Sándor G, Lukács R, Pék A, Szalai I, Papp A, Resnikoff S, Limburg H. Feasibility of the rapid assessment of avoidable blindness with diabetic retinopathy module (RAAB+DR) in industrialised countries: challenges and lessons learned in Hungary. Ophthalmic Epidemiol. 2018;25(4):273–279. doi: 10.1080/09286586.2018.1438634. [DOI] [PubMed] [Google Scholar]
- 12.Tóth G, Szabó D, Sándor GL, Pék A, Szalai I, Lukács R, Tóth GZ, Papp A, Nagy ZZ, Limburg H, Németh J. Regional disparities in the prevalence of diabetes and diabetic retinopathy in Hungary in people aged 50 years and older. Orv Hetil. 2017;158(10):362–367. doi: 10.1556/650.2017.30692. [DOI] [PubMed] [Google Scholar]
- 13.Tóth G, Szabó D, Sándor GL, Szalai I, Lukács R, Pék A, Tóth GZ, Papp A, Nagy ZZ, Limburg H, Németh J. Diabetes and diabetic retinopathy in people aged 50 years and older in Hungary. Br J Ophthalmol. 2017;101(7):965–969. doi: 10.1136/bjophthalmol-2016-309016. [DOI] [PubMed] [Google Scholar]
- 14.Tóth G, Szabó D, Sándor GL, Nagy ZZ, Karadeniz S, Limburg H, Németh J. Diabetes and blindness in people with diabetes in Hungary. Eur J Ophthalmol. 2019;29(2):141–147. doi: 10.1177/1120672118811738. [DOI] [PubMed] [Google Scholar]
- 15.Bourne RRA, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, Friedman DS, Keeffe JE, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Peto T, Saadine J, Silvester AJ, Tahhan N, Taylor HR, Varma R, Wong TY, Resnikoff S, Vision Loss Expert Group of the Global Burden of Disease Study Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102(5):575–585. doi: 10.1136/bjophthalmol-2017-311258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limburg H, Foster A. Cataract surgical coverage: an indicator to measure the impact of cataract intervention programmes. Community Eye Health. 1998;11(25):3–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762–6769. doi: 10.1167/iovs.15-17201. [DOI] [PubMed] [Google Scholar]
- 18.Salacz G, Ferencz M. 20 éves a hazai Cataracta- és Refraktív Sebészeti Regiszter. In: Kovács B, Nagy Z, Kerényi Á, Salacz G, editors. Emlékkönyv, A Magyar Műlencse Implantációs és Refraktív Sebészeti Társaság 25 éve. Budapest: Tudomány Kiadó; 2014. pp. 90–98. [Google Scholar]
- 19.Kocur I, Resnikoff S, Foster A, International Study Group Eye healthcare services in eastern Europe: Part 1. Cataract surgery. Br J Ophthalmol. 2002;86(8):847–850. doi: 10.1136/bjo.86.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zatic T, Bendelic E, Paduca A, Rabiu M, Corduneanu A, Garaba A, Novac V, Curca C, Sorbala I, Chiaburu A, Verega F, Andronic V, Guzun I, Căpăţină O, Zamă-Mardari I. Rapid assessment of avoidable blindness and diabetic retinopathy in Republic of Moldova. Br J Ophthalmol. 2015;99(6):832–836. doi: 10.1136/bjophthalmol-2014-305824. [DOI] [PubMed] [Google Scholar]
- 21.Lee L, D'Esposito F, Garap J, Wabulembo G, Koim SP, Keys D, Cama AT, Limburg H, Burnett A. Rapid assessment of avoidable blindness in Papua New Guinea: a nationwide survey. Br J Ophthalmol. 2019;103(3):338–342. doi: 10.1136/bjophthalmol-2017-311802. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan S, Deshmukh A, Giri Shrestha P, Basnet P, Kandel RP, Lewallen S, Sapkota YD, Bassett K, Yin VT. Prevalence of blindness and cataract surgical coverage in Narayani Zone, Nepal: a rapid assessment of avoidable blindness (RAAB) study. Br J Ophthalmol. 2018;102(3):291–294. doi: 10.1136/bjophthalmol-2017-310716. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organisation, Programme for the Prevention of Blindness and Deafness. Geneva: Feb 16-18, 1998. Informal Consultation on Analysis of Blindness Prevention Outomes, ANNEX III. [Google Scholar]
- 24.Mathenge W, Kuper H, Limburg H, Polack S, Onyango O, Nyaga G, Foster A. Rapid assessment of avoidable blindness in Nakuru district, Kenya. Ophthalmology. 2007;114(3):599–605. doi: 10.1016/j.ophtha.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Mathenge W, Nkurikiye J, Limburg H, Kuper H. Rapid assessment of avoidable blindness in Western Rwanda: blindness in a postconflict setting. PLoS Med. 2007;4(7):e217. doi: 10.1371/journal.pmed.0040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller A, Zerom M, Limburg H, Ghebrat Y, Meresie G, Fessahazion K, Beyene K, Mathenge W, Mebrahtu G. Results of a rapid assessment of avoidable blindness (RAAB) in Eritrea. Ophthalmic Epidemiol. 2011;18(3):103–108. doi: 10.3109/09286586.2010.545932. [DOI] [PubMed] [Google Scholar]
- 27.Beltranena F, Casasola K, Silva JC, Limburg H. Cataract blindness in 4 regions of Guatemala: results of a population-based survey. Ophthalmology. 2007;114(8):1558–1563. doi: 10.1016/j.ophtha.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Amansakhatov S, Volokhovskaya ZP, Afanasyeva AN, Limburg H. Cataract blindness in Turkmenistan: results of a national survey. Br J Ophthalmol. 2002;86(11):1207–1210. doi: 10.1136/bjo.86.11.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nijkamp MD, Ruiter RA, Roeling M, van den Borne B, Hiddema F, Hendrikse F, Nuijts RM. Factors related to fear in patients undergoing cataract surgery: a qualitative study focusing on factors associated with fear and reassurance among patients who need to undergo cataract surgery. Patient Educ Couns. 2002;47(3):265–272. doi: 10.1016/s0738-3991(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 30.Ramke J, Palagyi A, Kuper H, Gilbert CE. Assessment of response bias is neglected in cross-sectional blindness prevalence surveys: a review of recent surveys in low- and middle-income countries. Ophthalmic Epidemiol. 2018;25(5-6):379–385. doi: 10.1080/09286586.2018.1500613. [DOI] [PubMed] [Google Scholar]