Abstract
AIM
To investigate the potential effect and mechanism of leucine-rich α-2-glycoprotein-1 (LRG1) on corneal angiogenesis and lymphangiogenesis.
METHODS
Corneal neovascularization and lymphatics were induced by establishing alkali burn mouse model. Immunofluorescence staining was performed to detect the location of LRG1 in cornea tissues and to verify the source of LRG1-positive cells. Corneal whole-mount staining for CD31 (a panendothelial cell marker) and lymphatic endothelial hyluronan receptor-1 (LYVE-1; lymphatic marker) was performed to detect the growth of blood and lymphatic vessels after local application of exogenous LRG1 protein or LRG1 siRNA. In addition, expressions of the proangiogenic vascular endothelial growth factor (VEGF) related proteins were detected using Western blot analysis.
RESULTS
LRG1 was dramatically increased in alkali burned corneal stroma in both the limbal and central areas. LRG1-positive cells in the corneal stroma were mainly derived from Vimentin-positive cells. Local application of exogenous LRG1 protein not only aggravated angiogenesis but also lymphangiogenesis significantly (P<0.01). LRG1 group upregulated the levels of VEGF and the vascular endothelial growth factor receptor (VEGFR) family when compared with the phosphate-buffered saline (PBS) control group. We also found that LRG1-specific siRNA could suppress corneal angiogenesis and lymphangiogenesis when compared with the scramble siRNA-treated group (P<0.01).
CONCLUSION
LRG1 can facilitate corneal angiogenesis and lymphangiogenesis through heightening the stromal expression of VEGF-A, B, C, D and VEGFR-1, 2, 3; LRG1-specific siRNA can suppress corneal angiogenesis and lymphangiogenesis in corneal alkali burn mice.
Keywords: leucine-rich α-2-glycoprotein-1, angiogenesis, lymphangiogenesis, cornea, alkali burn, vascular endothelial growth factor
INTRODUCTION
The normal cornea is an avascular connective tissue that is lack of both blood and lymphatic vessels, which is termed “corneal angiogenic and lymphangiogenic privilege”. Angiogenic and lymphangiogenic privilege actively maintains corneal transparency and provides the cornea with special protection against the immune rejection of corneal transplantation, which is an important part of “immune privilege”[1]. However, under some pathological situations (e.g., inflammation, ischemia, infection, and trauma), the delicate homeostasis of vessel growth and inhibition is disturbed, and the ocular immune privilege is disrupted[2]–[3]. Subsequently, capillaries invade from the limbal vascular plexuses to the previously avascular areas of the cornea, causing corneal neovascularization (CNV)[4]. Some studies have revealed that in the CNV, there is a parallel outgrowth of corneal lymphangiogenesis (CL)[5]–[6]. CNV and CL can compromise corneal transparency, weaken corneal allograft acceptance, diminish visual acuity, and may ultimately result in blindness, which severely impacts patient quality of life and increases the public health burden[7]. Unlike corneal angiogenesis, CL is distinct under microscopic observation[8]. In the past, a lack of markers of lymphatic vessels meant that studies of lymphatic vessels lagged behind studies of CNV[9]. Recently, however, with the development of lymphatic vessel markers, some studies have confirmed that lymphangiogenesis plays an equally important role in corneal transplantation and orbital tumors[5],[10]. Importantly, the search for the common regulator of CNV and lymphatic vessels provides a broader therapeutic opportunity for the study of blinding eye disease.
Leucine-rich α-2-glycoprotein-1 (LRG1) is a highly conserved protein member of the leucine-rich repeat family[11]. Recently, several studies have demonstrated that LRG1 was significantly related to the formation of pathological blood vessels in different tissues and also to certain pathophysiological statuses, such as in retinal vascular disease and choroidal neovascularization[12], ischemic rat brain[13], colorectal cancer[14], osteoarthritis[15], and diabetic kidney disease[16]. Collectively, these studies have shown that LRG1 is a therapeutic target for pathogenic angiogenic-related diseases with bright prospect. However, the effects of LRG1 on CNV remains unclear. And more importantly, to date, it is still unknown whether LRG1 plays a critical role in lymphangiogenesis as well as in angiogenesis. Therefore, in this study, we aimed to investigate the potential mechanism of LRG1 on corneal angiogenesis and lymphangiogenesis using a corneal alkali burn model in mice.
MATERIALS AND METHODS
Ethical Approval
C57BL/6J mice (male, 6-8wk) were purchased from Charles River (Beijing, China) and were raised in the Animal Center of Shandong Eye Institute. All animal experiments were performed according to the guidelines and statement on the use of animals in ophthalmic and vision research from the Association for Research in Vision and Ophthalmology. All animal experimental protocols were approved by the Animal Center of Shandong Eye Institute. Mice were randomly divided into 7 groups (n=15, each): normal control, 3, 7d after alkali burning, phosphate-buffered saline (PBS) treated and recombinant LRG1 protein (rLRG1) treated groups, scramble siRNA-treated and LRG1 siRNA-treated groups. All operations were performed on the right eye. Three independent experiments were performed.
Mouse Corneal Alkali Burn Model and its Treatment
The alkali burn induced corneal angiogenesis and lymphangiogenesis were established as previously described[17]. Mice were anesthetized with an intraperitoneal injection of 0.6% pentobarbital (10 mg/kg; Abbott Japan, Tokyo, Japan). After the topical anesthesia with a drop of proparacaine hydrochloride (Santen, Suzhou, China) on their corneal surfaces, a 2 mm diameter filter paper (soaked in 1 mol/L NaOH for 20s) was placed on the center of the right cornea for 40s, with the help of a surgical microscope. The ocular surface was then gently rinsed with a 0.9% saline solution for 40s, and subsequently, ofloxacin ointments (Santen) were applied to avoid infection. All the alkali burn model was grad I of Dua et al's[18] classification. The exogenous protein rLRG1 (R&D Systems, Abingdon, UK) was applied topically (5 µL, 500 ng/mL) to the burned eyes six times daily for seven consecutive days. LRG1 proteins in topical application based on the reason that it is a gentle way to worked on the cornea after alkali burning to minimize suffering of the experimental animal. For the knockdown of LRG1, mouse LRG1-specific siRNA (5 µL/eye; Dharmacon, Denver, Colorado, USA) were injected in the alkali burned corneas subconjunctivally at concentrations of 20 µmol/L at 24h before the injury and at 0, 24 and 72h after injury. siRNA is easy to decompose and expensive, in order to make full use of LRG1 specific siRNA, we adopted the way of subconjunctival injection. The control animals were treated with PBS topically or with subconjunctivally injection of control scramble siRNA. CNV was monitored and photographed under a Topcon slit lamp, SL-D701 (Topcon, Tokyo, Japan), on postoperative days 0, 3, and 7. At day 7, all mice were sacrificed, and their eyeballs were collected for further examination.
Isolation of Murine Keratocytes
After euthanasia, the eyes with and without alkali burns were enucleated and incubated in Dulbecco's Modified Eagle's medium (DMEM) containing 15 mg/mL dispase II (Roche Diagnostics, Indianapolis, IN, USA) at 4°C for 18h to remove the entire corneal epithelium. Then the corneal stroma was carefully separated from the sclera under the dissecting microscope. Isolated corneal stroma was digested using collagenase A (Roche) at 37°C for 2h, and the obtained cell suspension was centrifuged. Keratocytes were resuspended in a DMEM/F12 medium, seeded on a 96-well plate, and incubated overnight at 37°C in a 5% CO2 atmosphere. The adherent cells were used for further immunofluorescence staining.
Immunofluorescence Staining
To monitor the expression of LRG1 in the cornea at days 0, 3, and 7 and the immunofluorescence co-localization of LRG1, Vimentin, and CD31 at day 7, the mouse eyeballs were enucleated and embedded in Tissue-Tek optimum cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan). Cryosections (7 µm) prepared from OCT-embedded eyeballs and cultured stromal cells were fixed in 4% paraformaldehyde at room temperature for 10min, permeabilized with 0.5% Triton X-100 for 5min, and blocked with 5% bovine serum albumin (BSA) at room temperature for 1h. Sample sections were incubated with primary antibodies, anti-LRG1 (ABclonal, Wuhan, China), anti-Vimentin (Abcam, Cambridge, UK) or anti-CD31 (BD Biosciences, New Jersey, USA) overnight at 4°C, after which the sections were incubated with fluorescein-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) at 37°C for 1h. Each step was followed by three washes with PBS. The images were examined under a fluorescence microscope (E800; Nikon, Tokyo, Japan) after counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI).
Cornea Whole-Mount Staining for Blood and Lymphatic Vessels
Cornea whole-mount staining was performed as previously described[19]. Briefly, the sample corneas were fixed in Zamboni fixative for 2h and blocked with 0.2% Triton X-100 combined with 2% goat serum and 1% BSA for 1h at room temperature. Subsequently, corneal flat mounts were incubated with rabbit anti-mouse LYVE-1 (Abcam), and phycoerythrin (PE)-conjugated mouse anti-CD31 antibody (BD Biosciences) at 4°C overnight, then washed and incubated with fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit secondary antibody (Invitrogen). After washing, the flat mount images were examined under the fluorescence microscope (E800; Nikon, Tokyo, Japan).
Western Blot Assay
Protein samples were extracted from corneal stroma in radioimmunoprecipitation (RIPA) assay buffer. Equal amounts (25 µg) of total protein were run on SDS-PAGE gels (EpiZyme, Shanghai, China) and then transferred to PVDF membrane (Millipore, Billerica, MA, USA). Subsequently, the membranes were incubated with antibodies against VEGF-A, VEGF-B, VEGF-C, VEGFR-1, and VEGFR-2 (Santa Cruz Biotechnology, Dallas, TX, USA), as well as VEGF-D (Affinity Biosciences, Cincinnati, OH, USA) and VEGFR-3 (Abcam), followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Proteintech, Wuhan, China), and mouse IgGκ light chain binding protein (Santa Cruz). HRP-conjugated GAPDH (Proteintech) was detected as a loading control. The protein bands were detected with the Enhanced Super Signal Chemiluminescent Substrate (Thermo Fisher, Waltham, MA, USA), and the images were acquired using a Kodak 4000R Pro Image Station (Kodak, Rochester, NY, USA).
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean±SEM. Experiments with two groups were analyzed using two-tailed unpaired Student's t-tests, and experiments with multiple groups were analyzed using one-way analysis. Differences with P-values of less than 0.05 were considered statistically significant.
RESULTS
LRG1 Expression in Alkali Burned Corneal Stroma
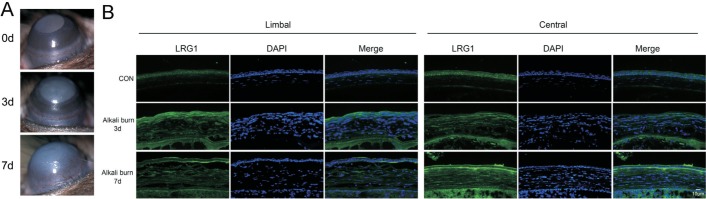
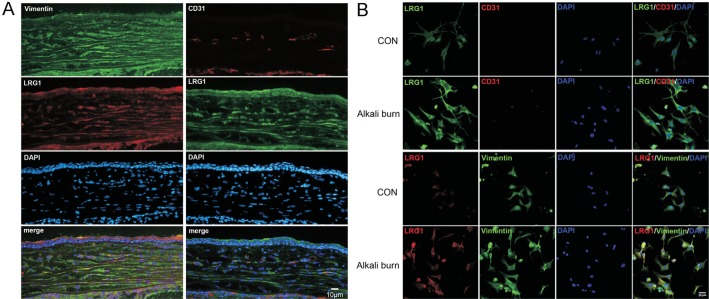
To confirm that the alkali burn model in mice was well established, the CNV was photographed under a slit lamp. At day 3, blood vessels sprouted from the limbus. At day 7, the growth of new blood vessels was more pronounced (Figure 1A). To clarify the change of LRG1 expression during CNV, corneas from normal control mice (avascular corneas) and corneas from alkali burn mice (CNV corneas) were analyzed by immunofluorescence staining of LRG1 at different time points. In the avascular corneas, LRG1 was mainly expressed in the epithelial layer; however, the expression in the corneal stroma was weak. In the CNV corneas, the positive staining of LRG1 showed a dramatic increase in the corneal stroma at both the limbal and central areas (Figure 1B). Next, we determined the cell source of the positively expressed LRG1 in the corneal stroma and found that, at day 7, only a small fraction of LRG1-positive cells were CD31-positive vascular endothelial cells, while most of them were Vimentin-positive cells (Figure 2A). Through an in vitro culture of stromal keratocytes, we also found that the expression of LRG1 was greater in cells from the CNV corneas than in the avascular corneas. In addition, co-localization staining showed that nearly all of the LRG1-positive cells were Vimentin-positive cells (Figure 2B). These results suggest that the LRG1 positive cells were mainly from Vimentin-positive cells.
Figure 1. LRG1 was increased in alkali burned corneas.
A: Representative slit lamp microscopic photographs of the CNV at 0, 3, and 7d after alkali burn; B: Representative images of immunohistochemistry for LRG1 in limbal and central cornea at 0, 3, and 7d after alkali burn; CON: Control group; DAPI: 4′,6-diamidino-2-phenylindole, the nuclear staining; LRG1: Recombinant LRG1 protein treated group.
Figure 2. LRG1 expression was mainly localized to Vimentin-positive cells in the stroma.
A: Representative images of the co-localization staining of LRG1 with CD31 and Vimentin; Bar=10 µm; B: The co-localization staining of LRG1 with CD31 and Vimentin in stromal cells in vitro. CON: Control group; DAPI: 4′,6-diamidino-2-phenylindole, the nuclear staining; LRG1: Recombinant LRG1 protein treated group; Bar=50 µm.
Effect of LRG1 on Corneal Angiogenesis and Lymphangiogenesis
The effects of LRG1 on CNV and CL were evaluated by local application of exogenous LRG1 protein in the corneal alkali burn mice. The slit lamp examination revealed severe angiogenesis in the corneas treated with LRG1 as compared to the control corneas at day 7 (Figure 3A), and the whole-mounted staining for CD31 also showed that LRG1 promoted the outgrowth of blood vessels after alkali burn (Figure 3B). The neovascularized areas of the corneas from the LRG1 group was larger, 60.27%±0.58%, than PBS treated group, at 37.83%±1.20% (Figure 3C). Remarkably, lymphatic vessels were prominently increased by LRG1 treatment as quantified from the corneal whole mounts stained for LYVE-1 (Figure 3B). We found that the LYVE-1-positive area, which indicates the region of lymphangiogenesis, was about 65.92%±0.87% in the LRG1 groups, significantly higher than the 51.10%±1.56% in the PBS group (Figure 3D). These findings indicate that LRG1 promoted not only angiogenesis but also, notably, lymphangiogenesis in the cornea.
Figure 3. LRG1 promoted corneal angiogenesis and lymphangiogenesis in a mouse model of alkali burn.

A: Representative slit lamp microscopic photographs of CNV at 7d after burning. Topical application of rLRG1 increased the outgrowth of new blood vessels. B: Whole-mounted double staining of corneal angiogenesis (red: CD31) and corneal lymphangiogenesis (green: LYVE-1) at 7d after burning. Upper panels are representative of the whole cornea, and lower panels are a partial enlargement of the upper panels. C: Corneal angiogenesis analysis by measuring area covered by CD31 positive staining of neovascularization. D: Corneal lymphangiogenesis analysis by measuring area covered by LYVE-1 positive staining of lymphangiogenesis. bP<0.01. PBS: Phosphate-buffered saline treated group; LRG1: Recombinant LRG1 protein treated group; LYVE-1: Lymphatic endothelial hyluronan receptor-1; CNV: Corneal neovascularization; CL: Corneal lymphangiogenesis; Bar=100 µm.
Effect of LRG1 on the Levels of VEGF and the VEGF Receptor Family
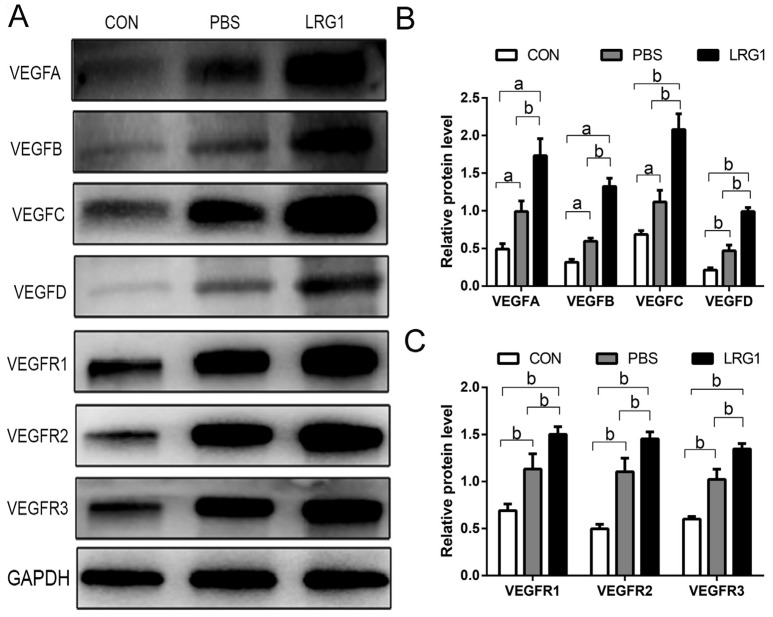
VEGF and its receptor signaling play crucial roles in both corneal angiogenesis and lymphangiogenesis. The protein levels of VEGF-A, B, C, D and VEGFR-1, 2, 3 were determined by Western blot analysis to explore the potential molecular mechanism of LRG1 promoting corneal angiogenesis and lymphangiogenesis. We found that the upregulation of VEGF and VEGFR induced by alkali burns was more severe after LRG1 treatment (Figure 4A). The corneal stromal expression of VEGF-A, B, C, and D in the LRG1 group increased by approximately 75%±1.44%, 88%±1.15%, 78%±3.57%, and 74.18%± 2.73%, respectively, when compared with the PBS group (Figure 4B). Similarly, the corneal stromal expression of VEGFR-1, 2, and 3 in the LRG1 group increased by approximately 29.89%±1.61%, 27.36%±3.83%, and 24.23%±2.05%, respectively, when compared with the PBS control group (Figure 4C).
Figure 4. LRG1 upregulated the expression of the VEGF and VEGFR family.
A: Expression of VEGF-A, B, C, D, and VEGFR-1, 2, 3 was examined by Western blot analysis; B: Western blot analysis of VEGF-A, B, C, D in the indicated groups; C: Western blot analysis of VEGFR-1, 2, 3 in the indicated groups. aP<0.05, bP<0.01. CON: Control group; PBS: Phosphate-buffered saline treated group; LRG1: Recombinant LRG1 protein treated group.
LRG1-Specific siRNA Suppressed Corneal Angiogenesis and Lymphangiogenesis
To further verify whether targeting LRG1 has therapeutic implications for corneal angiogenesis and lymphangiogenesis, we applied LRG1 siRNA subconjunctivally to knockdown LRG1 expression in the cornea, and the length of new corneal blood vessel outgrowth from the limbal edge was decreased in the LRG1 siRNA-treated group compared with the scramble siRNA-treated group (Figure 5A). The whole-mounted staining for CD31 and LYVE-1 revealed that knockdown of LRG1 notably suppressed the outgrowth of lymphatic and blood vessels (Figure 5B), with the angiogenesis and lymphangiogenesis areas reduced by approximately 21.71%±1.06% and 22.39%±2.13% in the LRG1 siRNA-treated group and the scramble siRNA-treated group (Figure 5C-5D).
Figure 5. Specific siRNA of LRG1 attenuated corneal angiogenesis and lymphangiogenesis in a mouse model of alkali burn.
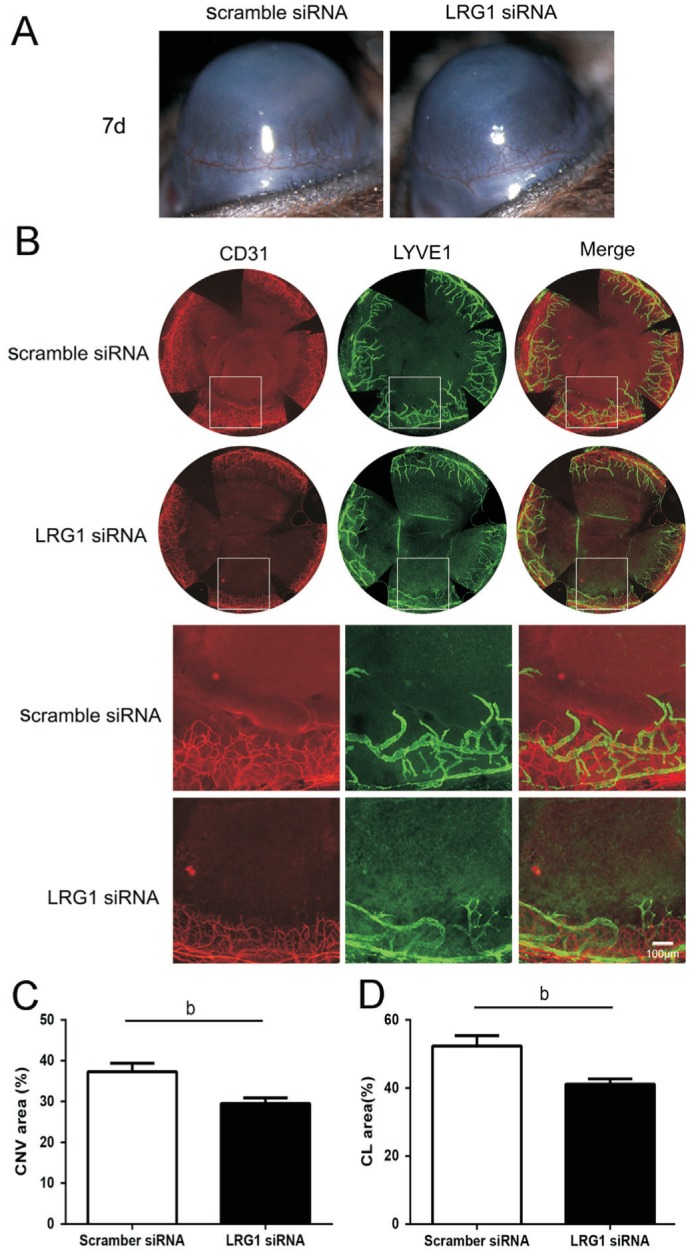
A: Representative slit lamp microscopic photographs of CNV at 7d after burning. Subconjunctival injection of LRG1 siRNA suppressed the outgrowth of corneal angiogenesis. B: Whole-mounted double staining of corneal angiogenesis (Red: CD31) and corneal lymphangiogenesis (Green: LYVE-1) at 7d after burning. Upper panels are representative of the whole cornea, and lower panels are a partial enlargement of the upper panels. C: Corneal angiogenesis analysis by measuring area covered by CD31 positive staining of neovascularization. D: Corneal lymphangiogenesis analysis by measuring area covered by LYVE-1 positive staining of lymphangiogenesis. bP<0.01. LYVE-1: Lymphatic endothelial hyluronan receptor-1; CNV: Corneal neovascularization; CL: Corneal lymphangiogenesis; Bar=100 µm.
DISCUSSION
Earlier studies showed that CNV have some positive effects in clearance of infections, because it compensates for non-healing epithelial defects and prevents corneal melting[20]. However, the disadvantages of excessive CNV are also notable. CNV can compromise corneal transparency, weaken corneal allograft acceptance, shorten the graft longevity and may ultimately result in permanent vision loss[10],[21]. In the present study, we demonstrated that LRG1 contributed to corneal angiogenesis and lymphangiogenesis in a corneal alkali burn mouse model by regulating the VEGF and VEGFR family. Specific knockdown of LRG1 reduced the outgrowth of corneal angiogenesis and lymphangiogenesis, indicating that LRG1 is a potential therapeutic target for corneal diseases involving angiogenesis and lymphangiogenesis.
Being a novel angiogenic factor, the acceleration effect of LRG1 on neovascularization has been reported in several other tissues, such as retina[12], brain[13], colorectal cancer[14], subchondral bone[15], and diabetic kidney[16]. However, its biological functions in the cornea remain unknown. The most important finding of this study is that LRG1 also promotes lymphangiogenesis. These findings expand our understanding of the multiple functions of LRG1 and enrich our knowledge about corneal angiogenesis and lymphangiogenesis.
Some earlier studies showed that corneal chemical injury model can simulate the complex microenvironment of human diseases[22], and this Dua et al's[18] classification grad I model generally does not cause corneal perforation and does not affect the measurement of new blood vessels. The corneal tissue can be better preserved, which is beneficial to immunofluorescence staining, and this modeling method is low-cost and easy to operate[23], it is widely used in the related studies of CNV[7],[24]–[26]. So this study used alkali burning of the cornea and found increased expression of LRG1 in corneal stroma after alkali burning.
CD31 served as a specific endothelial cell marker to detect neovascularization[27], and the co-localization of cryosections and in vitro cultures of stromal keratocytes showed that nearly all of the LRG1-positive cells were Vimentin-positive; Only a small fraction were CD31-positive cells. However, previous studies showed LRG1 expression predominantly in CD31-positive endothelial cells[13],[28]. The difference of LRG1 origin may be due to its tissue specificity and microenvironment. The whole-mounted staining of CD31 showed that LRG1 aggravated alkali burn induced corneal angiogenesis, which is consistent with a mouse model study of hypoxia-driven retinal angiogenesis[12]. These results demonstrate the promoting effect of LRG1 on ocular angiogenesis.
Pathological CL mediates disease states such as dry eye disease and malignant melanoma[29]–[30] as well as corneal transplant rejection. Lymphangiogenesis plays a significant role in the regulation of corneal edema and transparency[6]. Studies have shown that CL and CNV play equally important roles in graft rejection[31], and some researchers found that CL, not CNV, to be the primary mediator of immune rejection after corneal transplantation[32]. Therefore, a better understanding of CL could open new avenues for promoting graft survival and preventing vision loss in ocular diseases. Previous research found that LRG1 is related to lymphatic metastasis in tumor tissue. But there is no any research reported whether LRG1 play an important role in lymphangiogenesis. The relationship between corneal angiogenesis and lymphangiogenesis is complex and have not been clarified. Both corneal hemangiogenesis and lymphangiogenesis can be induced by inflammation, ischemia, infection, and trauma. In the micropoket model of mouse cornea, pro-angiogenic factors can also induce the growth of lymphangiogenesis[33] suggesting that the generation of hemangiogenesis and lymphangiogenesis may have similar mechanisms. Some studies have shown that CNV always occurs simultaneously with lymphangiogenesis[5]–[6]. But the lymphatic vessels recede earlier than the blood vessels[2]. Here, we first found that while promoting CNV, LRG1 can also promote CL, suggesting that LRG1 contributes to both hemangiogenic and lymphangiogenic responses.
VEGFs and their VEGFRs are central regulators in the processes of angiogenesis and lymphangiogenesis in cancer and vascular anomalies[34]. There are five homologues of VEGF: VEGF-A, B, C, D, and placental growth factor. VEGFs perform their functions through three tyrosine kinase receptors: VEGFR-1, 2, and 3[35]. VEGF-A and VEGF-B bind to VEGFR-1 and VEGFR-2 to drive angiogenesis, while VEGF-C and VEGF-D bind to VEGFR-2 and VEGFR-3 to drive lymphangiogenesis[34],[36]–[38]. Therefore, we detected the effects of LRG1 on VEGF-A, B, C, D and VEGFR-1, 2, 3 to determine whether LRG1 functions by regulating VEGF signaling. The results indicated that exogenous application of LRG1 significantly promoted the expression of VEGFs and their receptors in alkali burned corneas. Similarly, Wang et al[12] found LRG1 induced secretion of VEGF through TGF β/Smads signaling, and Zhang et al[14] found that LRG1 promoted VEGF-A expression in colorectal cancer cells via HIF-1α activation. The formation of blood and lymphatic vessels requires a concerted effort using complex signaling pathways. Our studies have more comprehensively demonstrated the effects of LRG1 on VEGF signaling because it covered more types of VEGFs and VEGFRs, indicating that LRG1 may promote corneal angiogenesis by upregulating VEGF-A, B and their receptors, VEGFR-1 and VEGFR-2, and may promote CL by upregulating VEGF-C, D and their receptors, VEGFR-2 and VEGFR-3.
Finally, we evaluated the potential therapeutic value of targeting LRG1 for corneal angiogenesis and lymphangiogenesis using a complementary approach: siRNA-mediated downregulation. We found that specific inhibition of LRG1 suppressed both corneal angiogenesis and CL. Recently, due to the crucial roles of LRG1 in retinal and cancerous neovascularization related diseases, researchers began investigating the use of drugs targeting LRG1 for the treatment of angiogenesis-related diseases. Magacizumab, a humanized monoclonal antibody against LRG1 that prevents formation of abnormal blood vessels, is now in Phase I and Phase IIa clinical trials (J. Greenwood, The pathogenic role of LRG1 in ocular neovascularization: From discovery to targeted therapy, EVER-Acta lecture presented at EVER Meeting, October 6, 2016). We propose that the blocking of LRG1 is not only a treatment for angiogenesis but also a new target for the treatment of lymphangiogenesis. In conclusion, our work on corneal angiogenesis and lymphangiogenesis not only supports the existing view that LRG1 promotes angiogenesis, but also suggests that LRG1 can promote lymphangiogenesis through modulating VEGF signaling. Targeting of LRG1 may be a potential therapeutic approach against angiogenesis and lymphangiogenesis related diseases. Although our present study did not clarify the different roles and mechanism of LRG1 in promoting corneal angiogenesis and lymphangiogenesis, to some extent, our results provide some new theoretical basis for CNV and CL. In future studies, we will deepen the research on the mechanism of LRG1 promoting CL.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81670828); the Shandong Provincial Key Research and Development Program (No.2017GSF18141).
Conflicts of Interest: Song S, None; Cheng J, None; Yu BJ, None; Zhou L, None; Xu HF, None; Yang LL, None.
REFERENCES
- 1.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 2.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25(4):443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 3.Yu HY, Sun LY, Cui J, Li Y, Yan Y, Wei X, Wang C, Song FQ, Jiang WT, Liu YF, Ge HY, Qian H, Li XG, Tang XL, Liu P. Three kinds of corneal host cells contribute differently to corneal neovascularization. EBioMedicine. 2019;44:542–553. doi: 10.1016/j.ebiom.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelfattah NS, Amgad M, Zayed AA, Salem H, Elkhanany AE, Hussein H, Abd El-Baky N. Clinical correlates of common corneal neovascular diseases: a literature review. Int J Ophthalmol. 2015;8(1):182–193. doi: 10.3980/j.issn.2222-3959.2015.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Hos D, Bukowiecki A, Horstmann J, Bock F, Bucher F, Heindl LM, Siebelmann S, Steven P, Dana, Eming SA, Cursiefen C. Transient ingrowth of lymphatic vessels into the physiologically avascular cornea regulates corneal edema and transparency. Sci Rep. 2017;7(1):7227. doi: 10.1038/s41598-017-07806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun MM, Chan AM, Law SM, Duarte S, Diaz-Aguilar D, Wadehra M, Gordon LK. Epithelial membrane protein-2 (EMP2) antibody blockade reduces corneal neovascularization in an in vivo model. Invest Ophthalmol Vis Sci. 2019;60(1):245–254. doi: 10.1167/iovs.18-24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le VNH, Hou YH, Horstmann J, Bock F, Cursiefen C. Novel method to detect corneal lymphatic vessels in vivo by intrastromal injection of fluorescein. Cornea. 2018;37(2):267–271. doi: 10.1097/ICO.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 9.Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol. 2009;24(3):135–138. doi: 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Montana M, Santosa SM, Isjwara ID, Huang YH, Han KY, O'Neil C, Wang A, Cortina MS, de la Cruz J, Zhou Q, Rosenblatt MI, Chang JH, Azar DT. Angiogenesis and lymphangiogenesis in corneal transplantation-a review. Surv Ophthalmol. 2018;63(4):453–479. doi: 10.1016/j.survophthal.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druhan LJ, Lance A, Li S, Price AE, Emerson JT, Baxter SA, Gerber JM, Avalos BR. Leucine rich α-2 glycoprotein: a novel neutrophil granule protein and modulator of myelopoiesis. PLoS One. 2017;12(1):e0170261. doi: 10.1371/journal.pone.0170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XM, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai ZH, Arthur HM, Bainbridge J, Moss SE, Greenwood J. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013;499(7458):306–311. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng HM, Song YJ, Zhu JY, Liu Q, Lu PT, Ye N, Zhang Z, Pang YX, Qi JP, Wu H. LRG1 promotes angiogenesis through upregulating the TGF-β1 pathway in ischemic rat brain. Mol Med Rep. 2016;14(6):5535–5543. doi: 10.3892/mmr.2016.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JJ, Zhu LY, Fang JY, Ge ZZ, Li XB. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res. 2016;35:29. doi: 10.1186/s13046-016-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YY, Xu JJ, Zhang XD, Wang CD, Huang Y, Dai KR, Zhang XL. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8(3):e2715. doi: 10.1038/cddis.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Q, Zhang L, Fu J, Verghese DA, Chauhan K, Nadkarni GN, Li ZZ, Ju WJ, Kretzler M, Cai GY, Chen XM, D'Agati VD, Coca SG, Schlondorff D, He JC, Lee K. LRG1 promotes diabetic kidney disease progression by enhancing TGF-β-induced angiogenesis. J Am Soc Nephrol. 2019;30(4):546–562. doi: 10.1681/ASN.2018060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Jeong H, Yang MS, Lim CW, Kim B. Therapeutic effects of zerumbone in an alkali-burned corneal wound healing model. Int Immunopharmacol. 2017;48:126–134. doi: 10.1016/j.intimp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85(11):1379–1383. doi: 10.1136/bjo.85.11.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XP, Di GH, Dong MC, Qu ML, Zhao XW, Duan HY, Hu XL, Liu T, Zhou QJ, Shi WY. Epithelium-derived miR-204 inhibits corneal neovascularization. Exp Eye Res. 2018;167:122–127. doi: 10.1016/j.exer.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37(12):2485–2494. [PubMed] [Google Scholar]
- 21.Tshionyi M, Shay E, Lunde E, Lin A, Han KY, Jain S, Chang JH, Azar DT. Hemangiogenesis and lymphangiogenesis in corneal pathology. Cornea. 2012;31(1):74–80. doi: 10.1097/ICO.0b013e31821dd986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou ZJ, Chang JH. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29(3):208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giacomini C, Ferrari G, Bignami F, Rama P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: an overview of two common animal models of corneal neovascularization. Exp Eye Res. 2014;121:1–4. doi: 10.1016/j.exer.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Tai PWL, Ai JZ, Gessler DJ, Su Q, Yao XY, Zheng Q, Zamore PD, Xu X, Gao GP. Transcriptome profiling of neovascularized corneas reveals miR-204 as a multi-target biotherapy deliverable by rAAVs. Mol Ther Nucleic Acids. 2018;10:349–360. doi: 10.1016/j.omtn.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XP, Li KR, Yu Q, Yao MD, Ge HM, Li XM, Jiang Q, Yao J, Cao C. Ginsenoside Rh2 inhibits vascular endothelial growth factor-induced corneal neovascularization. FASEB J. 2018;32(7):3782–3791. doi: 10.1096/fj.201701074RR. [DOI] [PubMed] [Google Scholar]
- 26.Liu D, Wu QN, Zhu YQ, Liu YJ, Xie XL, Li SH, Lin HT, Chen WR, Zhu FM. Co-delivery of metformin and levofloxacin hydrochloride using biodegradable thermosensitive hydrogel for the treatment of corneal neovascularization. Drug Deliv. 2019;26(1):522–531. doi: 10.1080/10717544.2019.1609623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livnat T, Weinberger Y, Budnik I, Deitch I, Dahbash M, Sella R, Dardik R, Kenet G, Nisgav Y, Weinberger D. Activated protein C induces suppression and regression of choroidal neovascularization-A murine model. Exp Eye Res. 2019;186:107695. doi: 10.1016/j.exer.2019.107695. [DOI] [PubMed] [Google Scholar]
- 28.Amer R, Tiosano L, Pe'er J. Leucine-rich α-2-glycoprotein-1 (LRG-1) expression in retinoblastoma. Invest Ophthalmol Vis Sci. 2018;59(2):685–692. doi: 10.1167/iovs.17-22785. [DOI] [PubMed] [Google Scholar]
- 29.Chen WS, Cao ZY, Sugaya S, Lopez MJ, Sendra VG, Laver N, Leffler H, Nilsson UJ, Fu JX, Song JH, Xia LJ, Hamrah P, Panjwani N. Erratum: Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat Commun. 2016;7:12063. doi: 10.1038/ncomms12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji YW, Lee JL, Kang HG, Gu N, Byun H, Yeo A, Noh H, Kim S, Choi EY, Song JS, Lee HK. Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul Surf. 2018;16(3):306–313. doi: 10.1016/j.jtos.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Rho CR, Choi JS, Seo M, Lee SK, Joo CK. Inhibition of lymphangiogenesis and hemangiogenesis in corneal inflammation by subconjunctival Prox1 siRNA injection in rats. Invest Ophthalmol Vis Sci. 2015;56(10):5871–5879. doi: 10.1167/iovs.14-14433. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184(2):535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao RH, Lim S, Ji H, Zhang Y, Yang YL, Honek J, Hedlund EM, Cao YH. Mouse corneal lymphangiogenesis model. Nat Protoc. 2011;6(6):817–826. doi: 10.1038/nprot.2011.359. [DOI] [PubMed] [Google Scholar]
- 34.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454(7204):656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 36.Coso S, Bovay E, Petrova TV. Pressing the right buttons: signaling in lymphangiogenesis. Blood. 2014;123(17):2614–2624. doi: 10.1182/blood-2013-12-297317. [DOI] [PubMed] [Google Scholar]
- 37.Wong BW, Zecchin A, García-Caballero M, Carmeliet P. Emerging concepts in organ-specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell. 2018;45(3):289–301. doi: 10.1016/j.devcel.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Rahman HNA, Dong YZ, et al. Epsin deficiency promotes lymphangiogenesis through regulation of VEGFR3 degradation in diabetes. J Clin Investig. 2018;128(9):4025–4043. doi: 10.1172/JCI96063. [DOI] [PMC free article] [PubMed] [Google Scholar]