Abstract
AIM
To evaluate the efficacy and safety of ruthenium-106 (106Ru) plaque radiotherapy at a dose (>50 Gy) higher than recommended (29-50 Gy) for treatment of circumscribed choroidal hemangioma (CCH) in Chinese patients.
METHODS
This retrospective study included 25 symptomatic CCH patients undergoing 106Ru plaque brachytherapy involving 25 eyes between January 2005 and August 2016. Ophthalmic examination was performed at the baseline and at each post-treatment follow-up visit, using best-corrected visual acuity (BCVA), dilated fundus examination, and B-scan ultrasonography. The primary efficacy outcome measures included the changes in BCVA and hemangioma dimensions at the last followup visit from the baseline.
RESULTS
The mean follow-up duration was 28.0±26.6 (range, 12-110)mo. All the hemangiomas were located in the posterior pole except for two involving the fovea. The mean apex dose of 106Ru plaque radiotherapy was 84.4±19.7 Gy. The mean BCVA improved from 41.4±29.3 (0-97) at the baseline to 53.0±33.8 (0-97) ETDRS letters at the last visit (P=0.01). The mean hemangioma height declined from 3.98±0.88 (2.40-5.50) mm to 0.84±1.63 (0-6.47) mm (P≤0.001), and the greatest linear diameter (GLD) reduced from 9.36±2.23 (6.80-15.00) to 7.40±2.45 (0-13.00) mm (P≤0.001). Hemangioma size increased in one (4%) eye with a worsened vision, and subretinal fluid completely resolved in all but one patient (4%). Radiation-related retinopathy was observed in two patients at post-treatment 9 and 11mo, respectively.
CONCLUSION
106Ru plaque brachytherapy at a dose (>50 Gy) higher than recommended (29-50 Gy) is an effective treatment regimen for symptomatic CCH associated with significantly improved visual acuity and a favorable safety profile in Chinese patients.
Keywords: circumscribed choroidal hemangioma, radiotherapy, ruthenium-106, Chinese patients, retrospective study
INTRODUCTION
Circumscribed choroidal hemangioma (CCH) is a rare benign vascular hamartoma and generally arises unilaterally in the choroidal tissue of the posterior pole[1]. Accumulation of serous subretinal fluid (SRF) and intraretinal cystoid edema result in visual loss. Approximately 40% of CCH cases will become progressively symptomatic, while no intervention is generally required for asymptomatic cases[2]. For symptomatic CCH cases, effective treatment modalities include transpupillary thermotherapy, laser photocoagulation, photodynamic therapy, radiotherapy, and anti-vascular endothelial growth factor (VEGF) medication[3]–[6].
Radiation therapy has been recommended for treating choroidal hemangiomas, especially for patients with extensive subretinal exudation and retinal detachment refractory to photodynamic therapy[7]. Multiple radiation therapies, including plaque radiotherapy (brachytherapy), external beam, proton beam radiation and stereotactic radiosurgery, have been reported to be effective for treating symptomatic CCH[5],[7]–[9]. Among these modalities, plaque brachytherapy is thought to be a more targeted treatment for hemangioma, as a higher dose acts on the base of the hemangioma rather than the apex with minimized radiation-induced side effects[5],[8].
As a safe and effective therapeutic approach for choroidal hemangiomas, the efficacy and safety ruthenium-106 (106Ru) plaque brachytherapy has been studied in Caucasian CCH patients[5],[9], with a knowledge gap in Eastern Asian populations including Chinese patients. Therefore, the objective of the present study is to evaluate the efficacy and safety of 106Ru plaque brachytherapy at a dose (>50 Gy) higher than recommended (29-50 Gy) for treating symptomatic CCH in Chinese patients, aiming to decrease hemangioma recurrence without increasing radiation retinopathy, in comparison with those in Caucasian population reported from previous literature.
SUBJECTS AND METHODS
Ethical Approval
This study retrospectively reviewed a cohort of 25 symptomatic patients undergoing 106Ru plaque brachytherapy for unilateral CCH at the Ophthalmology Center of Peking University People's Hospital between January 2005 and August 2016. All the included patients were consecutively followed up for 1y or more. The study protocol was approved by the Ethics Committee at Peking University People's Hospital, Beijing, China in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient before receiving 106Ru plaque brachytherapy.
CCH was diagnosed using a combination of binocular indirect ophthalmoscopy by a single board-certified ophthalmologist (Liang JH), B-scan ultrasonography (USG), optical coherence tomography (OCT), fluorescein angiography (FA) and indocyanine green angiography (ICGA) prior to plaque brachytherapy. The inclusion criteria were presence of CCH with extensive subretinal exudation and/or retinal detachment and receiving no treatment for CCH, regardless of baseline best-corrected visual acuity (BCVA). Patients with complicating ophthalmic conditions, including glaucoma, diabetic retinopathy, rhegmatogenous retinal detachment, and macular hole, were excluded from analysis.
Plaque Brachytherapy
A 106Ru ophthalmic applicator (BEBIG Isotopen und Medizintechnik GmbH, Berlin, Germany) was used for plaque brachytherapy with the size and shape adjusted to the dimension and location of the CCH. The hemangioma location and dimension were determined using a combination of indirect binocular ophthalmoscopy, USG, FA, and ICGA, and reconfirmed before implantation of the plaque. An additional 2-mm healthy margin was included for the choice of plaque size. Upon completion of the implantation, indentation and transillumination was done to confirm plaque placement and positioning. An in-house calibration protocol was used for plaque quality control. The target radiation dose for all the CCH patients was calculated by the single board-certified ophthalmologist (Liang JH), and the plaque was removed after delivering the target dose. Insertion and removal of the plaque was performed by Liang JH in all patients under general anesthesia.
Follow-up Visits
Follow-up ophthalmic examination were performed at an interval of 3-6mo in the first year and every 6-12mo afterwards. The examination included BCVA, slit-lamp biomicroscopy, dilated funduscopy, USG, OCT, FA, and ICGA (HRA-2, Heidelberg Retina Angiograph System; Heidelberg Engineering Inc., Vista, CA, USA). Dilated fundus examination was performed by the single board-certified ophthalmologist (Liang JH) for a consistent clinical evaluation. USG was used to determine the greatest basal diameter and the apical height of the hemangioma. OCT was used to measure the foveal center thickness and the height of the SRF for hemangiomas located near the fovea.
Outcome Measures
The primary efficacy outcome measures included the changes in BCVA and hemangioma dimensions at the last follow-up visit from the baseline. Significant improvement in BCVA was defined as an at least two-line improvement in visual acuity of letters on the Early Treatment Diabetic Retinopathy Study (ETDRS) acuity chart at the last visit. Major safety outcome measures included occurrences of treatment-emergent radiation retinopathy and cataract formation.
Statistical Analysis
All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The paired t-test was used to compare the changes in BCVA and hemangioma dimension on USG between the baseline and the last follow-up visit. A two-sided P value of less than 0.05 was considered statistically significant unless otherwise specified.
RESULTS
Twenty-five symptomatic CCH patients were included for analysis in this study with 25 unilateral eyes involved. This cohort included 12 men and 13 women at a mean age of 41.1±12.3 (range 16-67)y, and the mean follow-up duration was 28.0±26.6 (range 12-110)mo. The hemangiomas were located under the retina in all patients, with 23/25 (92%) located in the posterior pole and 2/25 (8%) involving the fovea. The demographic and clinical characteristics are shown in Table 1 and individual patient characteristics are listed in Table 2. The situation before and after the treatment of a patient were exhibited including fundus photography, B-scan USG, and FA (Figure 1).
Table 1. Demographic and clinical characteristics of CCH patients.
| Characteristics | Statistics |
| No. of patients | 25 |
| No. of eyes | 25 |
| Male/female | 12/13 |
| Age (y, range) | 41.1±12.3 (16-67) |
| Follow-up time (mo, range) | 28.0±26.6 (12-110) |
| Hemangioma location, n (%) | |
| Foveal | 2/25 (8) |
| Extra-foveal | 23/25 (92) |
| Exudative retinal detachment, n (%) | 25/25 (100) |
| Involvement of macular area, n (%) | 24/25 (96) |
CCH: Circumscribed choroidal hemangioma.
Table 2. Summary of treatment and clinical characteristics of CCH patients.
| Pt No. | Sex | Age (y) | Laterality | Location | Pre-treatment dimension (mm) |
Post-treatment dimension (mm) |
Plaque | Apex dose (Gy) | Follow-up (mo) | BCVA (ETDRS) |
Complication | |||
| D | H | D | H | BL | LFUV | |||||||||
| 1 | M | 46 | OS | F | 9.26 | 4.28 | 7.32 | 0.12 | CCA | 95 | 12 | 53 | 90 | None |
| 2 | F | 67 | OD | Ex-F | 10.11 | 4.39 | 9.03 | 0.20 | CCA | 106 | 70 | 37 | 63 | None |
| 3 | M | 34 | OS | Ex-F | 10.86 | 5.33 | 7.8 | 0.22 | CCA | 95 | 13 | 47 | 47 | None |
| 4 | M | 53 | OD | Ex-F | 7.56 | 4.18 | 6.88 | 0.01 | CCA | 78 | 12 | 30 | 33 | None |
| 5 | M | 44 | OD | Ex-F | 9.7 | 4.3 | 8.13 | 2.61 | COB | 95 | 12 | 0 | 0 | None |
| 6 | M | 43 | OD | Ex-F | 6.8 | 3.6 | 5.88 | 0.03 | CCA | 83 | 12 | 47 | 83 | None |
| 7 | F | 36 | OD | F | 7.16 | 3.4 | 6.89 | 0.18 | COB | 75 | 39 | 5 | 5 | None |
| 8 | M | 29 | OS | Ex-F | 7.73 | 3.23 | 5.56 | 0.03 | CCA | 85 | 110 | 87 | 87 | None |
| 9 | M | 65 | OS | Ex-F | 8.1 | 3.5 | 7.44 | 0.06 | CCA | 73 | 12 | 33 | 33 | None |
| 10 | F | 35 | OS | Ex-F | 9.47 | 4.02 | 8.07 | 0.11 | CCA | 60 | 12 | 24 | 47 | RP |
| 11 | M | 49 | OS | Ex-F | 15 | 5.5 | 13 | 6.47 | COB | 93 | 12 | 63 | 5 | None |
| 12 | F | 24 | OD | Ex-F | 8.75 | 4.04 | 7.77 | 0.04 | CCA | 100 | 12 | 63 | 63 | None |
| 13 | F | 30 | OS | Ex-F | 7.8 | 3.1 | 7.00 | 0.05 | COB | 103 | 12 | 33 | 57 | None |
| 14 | M | 60 | OD | Ex-F | 7.1 | 2.4 | 6.99 | 0.08 | CCA | 73 | 30 | 33 | 47 | None |
| 15 | M | 42 | OS | Ex-F | 7.45 | 3.12 | 6.64 | 0.12 | CCA | 56 | 48 | 73 | 93 | None |
| 16 | F | 38 | OS | Ex-F | 11.44 | 4.97 | 10.08 | 0.19 | COB | 122 | 15 | 0 | 0 | None |
| 17 | F | 41 | OD | Ex-F | 6.95 | 3.01 | 5.88 | 0.01 | CCA | 56 | 87 | 77 | 97 | None |
| 18 | M | 37 | OD | Ex-F | 7.64 | 2.89 | 6.01 | 0.09 | CCA | 86 | 12 | 77 | 77 | None |
| 19 | F | 16 | OD | Ex-F | 13.79 | 5.32 | 10.11 | 0.11 | COB | 59 | 49 | 27 | 77 | None |
| 20 | M | 45 | OS | Ex-F | 9.07 | 4.04 | 6.4 | 2 | CCA | 69 | 12 | 5 | 30 | None |
| 21 | F | 30 | OS | Ex-F | 9.2 | 4.2 | 0 | 0 | CCB | 129 | 12 | 0 | 0 | None |
| 22 | F | 53 | OS | Ex-F | 11.2 | 5.2 | 11.3 | 3.4 | CCA | 67 | 12 | 5 | 30 | None |
| 23 | F | 39 | OD | Ex-F | 10.3 | 3.8 | 8.83 | 0.02 | CCB | 105 | 34 | 97 | 97 | RP |
| 24 | F | 42 | OD | Ex-F | 8.3 | 2.8 | 7.09 | 0.64 | CCA | 71 | 36 | 67 | 97 | None |
| 25 | F | 29 | OD | Ex-F | 13.36 | 5.03 | 3.9 | 4.05 | COB | 77 | 12 | 53 | 67 | None |
F: Female; M: Male; F: Foveal; EX-F: Extra-foveal; D: Diameter; H: Height; BL: Baseline; LFUV: Last follow-up visit; RP: Retinopathy.
n=25
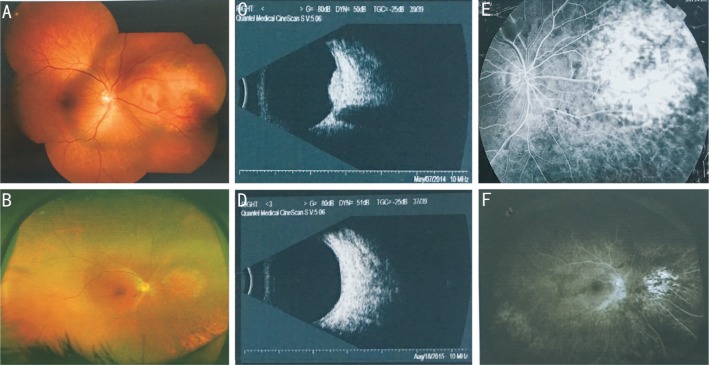
Figure 1. Treatment outcome in a patient with CCH pretreatment and post-treatment 15mo using 106Ru plaque radiotherapy.
An orange-red colored (A), CCH located in the posterior polar and tumor reduction (B) in post-treatment 15mo on color funduscopy. C, D: Before and after treatment on B-scan USG. E, F: Before and after treatment on FA, in the same eye.
Treatment outcomes are shown in Table 3. The mean apex dose of 106Ru plaque radiotherapy was 84.4±19.7 Gy. The mean BCVA improved from 41.4±29.3 (0-97) at the baseline to 53.0±33.8 (0-97) ETDRS letters at the last visit (P=0.01). Visual acuity significantly improved in 14/25 (56%) eyes; 10/25 (40%) eyes remained unchanged but 1/25 eye (4%) worsened. At the last visit, 22/25 (88%) eyes with the disease located in the posterior pole had an improved or unchanged visual acuity, while 2/25 (8%) eyes with the disease involving the foveal area showed no improvement. The mean hemangioma height declined from 3.98±0.88 (2.40-5.50) to 0.84±1.63 (0-6.47) mm (P≤0.001), and the greatest linear diameter reduced from 9.36±2.23 (6.80-15.00) to 7.40±2.45 (0-13.00) mm (P≤0.001). In one patient, the hemangioma height increased from 5.50 mm at the baseline to 6.47 mm at the last visit with a worsening BCVA. SRF completely resolved in all cases except for one patient.
Table 3. Treatment outcomes of 106Ru plaque therapy.
| Treatment outcomes | n=25 |
| BCVA, ETDRS (letters) | |
| Baseline | 41.4±29.3 |
| The last follow-up visit | 53.0±33.8 |
| Pa | 0.01 |
| BCVA improved or stable, n (%) | 24 (96) |
| Tumor dimension on USG | |
| Height, mm | |
| Baseline | 3.98±0.88 |
| The last follow-up visit | 0.84±1.63 |
| Pa | ≤0.001 |
| GLD, mm | |
| Baseline | 9.36±2.23 |
| The last follow-up visit | 7.40±2.45 |
| Pa | ≤0.001 |
| Complete SRF resolution, n (%) | 24 (96) |
| Recurrence, n (%) | 0 |
BCVA: Best-corrected visual acuity; ETDRS: The Early Treatment Diabetic Retinopathy Study; USG: Ultrasonography; GLD: The greatest linear diameter; SRF: Subretinal fluid. aP value using the paired Student's t-test.
Radiation-related retinopathy on funduscopy was observed in two (8%) patients at post-treatment 9 (dose=60 Gy) and 11mo (105 Gy), respectively. These two patients with radiation related retinopathy responded to treatment with between two and three intravitreal bevacizumab injections with one having resolved two years after occurrence and the other lost to follow-up. None of patients developed any other complications, such as glaucoma, choroidal ischemia, or secondary choroidal neovascularization.
DISCUSSION
The present study demonstrated the efficacy and safety of 106Ru plaque brachytherapy at a dose (>50 Gy) higher than recommended (29-50 Gy) for treating symptomatic CCH in Chinese patients. The clinical benefits included improvement in visual acuity and reduction in hemangioma dimension, without significantly increasing the risk of radiation-induced retinopathy. To the best of our knowledge, the present work was the first report regarding 106Ru aplaque brachytherapy for treating CCH in Chinese population.
Multiple treatment modalities have been available for treatment of symptomatic CCH. Laser photocoagulation has long been used as the first-line therapy for CCH but results in a limited visual recovery[10]. Transpupillary thermotherapy is more effective in pathologic response but requires repeated performance, which is therefore mainly indicated for hemangiomas with a large retinal detachment[11]. Use of oral propranolol may be partially effective for infantile hemangioma[12], and intravitreous injection with anti-VEGF medication and photodynamic therapy had also been used for treating CCH with complicating SRF[13]. Photodynamic therapy, however, showed no significant visual acuity improvement although the hemangioma became downsized only if a double-dose therapy was given[14].
Multiple radiotherapy techniques have been used for treating symptomatic CCH with a high efficacy and a favorable safety profile[15]–[21]. Plaque brachytherapy achieves a more targeted delivery and results in less frequent radiation-induced retinopathy, using a variety of isotopes including palladium-103, cobalt-60, 106Ru and iodine-125[2],[17]–[20]. A low-dose radiation therapy achieves significantly improved visual acuity, complete SRF resolution and hemangioma regression in a high proportion of symptomatic CCH[15]–[16]. However, a major limitation regarding plaque brachytherapy is the inconsistence in radiation dose between the base and the apex of the hemangioma at a risk of recurrence from the base[21].
In this study, 106Ru was given at a dose higher than recommended (normally at a dose range of 29-50 Gy) for plaque brachytherapy in a Chinese CCH population, due to the risk of disease recurrence from a low-dose radiotherapy, with patient demographic and clinical characteristics similar to those from previous studies (Table 4)[5],[21]. A large proportion of patients showed significant (56%) improvement in visual acuity, similar to those (12/21, 57%) reported by Naseripour et al[9]. A major difference in patient characteristics was the variation in hemangioma location, more frequently (92%) located in the extra-foveal area in this study compared to previous ones[8],[13]. Hemangioma dimension also became significantly downsized to an extent similar to or greater than those in previous reports[4],[8],[16],[18]–[19],[21]–[22]. An additional benefit of high-dose 106Ru plaque brachytherapy was achievement of complete SRF resolution in the great majority (96%) of patients.
Table 4. Comparative treatment outcomes of 106Ru plaque therapy for CCH.
| Parameters | Present study | Medraperla et al[5] | Naseripour et al[9] | Joshi et al[21] |
| No. of patients | 25 | 8 | 21 | 8 |
| Radioactive isotope | 106Ru | 106Ru and iodine-125 | 106Ru | 106Ru |
| Dose, Gy, mean | 84.4 | 50 | 38.5 | 32.5 |
| Radiation-related complication, n (%) | ||||
| Retinopathy | 2 (8) | 0 | 5 (23.8) | 0 |
| Papillopathy | 0 | 0 | 1 (4.8) | 0 |
| Mean tumor height, mm | ||||
| Baseline | 3.99 | 4.8 | 3.87 | 5.0 |
| Last visit | 0.84 | 2.1 | 0.7 | Not reported |
| GLD, mm, mean | ||||
| Baseline | 9.36 | 10.6 | 10.0 | 12.7 |
| Last visit | 7.40 | Not reported | 8.33 | Not reported |
| BCVA, mean | ||||
| Baseline | ≈20/200 | 20/80 | 20/80 | Not reported |
| Last visit | ≈20/160 | 20/30 | 20/50 | Not reported |
| Visual acuity improvement, % | Two or more lines, 52 | Three or more lines, 63 | Two or more lines, 57 | Not reported |
| Follow-up period, mo, mean | 28.0 | 25.0 | 38.6 | Not reported |
CCH: Circumscribed choroidal hemangioma; BCVA: Best-corrected visual acuity; GLD: The greatest linear diameter.
Major safety concern is radiation-induced retinopathy in the scenario of high-dose therapy. Within a follow-up duration of at least 1y and up to 9y, only a small portion (2/25, 4%) of patients developed radiation-induced retinopathy within the first year of 106Ru plaque therapy, significantly lower than that reported by Naseripour et al[9]; Schemia and hypoxia are still recognized as one of the important causes of radiation retinopathy, so intravitreal anti-VEGF medication is the treatment regimen of choice for radiation-induced retinopathy.
There were some limitations in our study. First, the present work was not in a randomized or controlled design due to rarity of symptomatic CCH. Second, the sample size was relatively small but comparable to those of previous proof-of-concept studies. Third, the efficacy outcomes might be biased by confounding factors, including patient's characteristics, operator's expertise and follow-up procedure, which were controlled in the study design, such as operation by a single ophthalmologist.
In conclusion, the present work demonstrated that 106Ru plaque radiotherapy at a dose (>50 Gy) higher than recommended (29-50 Gy) was effective for treatment of symptomatic CCH in Chinese patients. The clinical benefits included significant improvement in visual acuity and marked hemangioma regression similar to or greater than those in previous studies. Radiation-induced retinopathy occurred at a relatively low frequency but remained manageable and responsive to medical intervention. A randomized, dose-controlled study powered with a sufficient sample size is yet to be done for validation of the long-term efficacy and safety of high-dose 106Ru plaque radiotherapy for treating symptomatic CCH.
Acknowledgments
Conflicts of Interest: Li J, None; Jin EZ, None; Liang JH, None.
REFERENCES
- 1.Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108(12):2237–2248. doi: 10.1016/s0161-6420(01)00812-0. [DOI] [PubMed] [Google Scholar]
- 2.López-Caballero C, Saornil MA, De Frutos J, Bianciotto C, Muiños Y, Almaraz A, López-Lara F, Contreras I. High-dose iodine-125 episcleral brachytherapy for circumscribed choroidal haemangioma. Br J Ophthalmol. 2010;94(4):470–473. doi: 10.1136/bjo.2009.160184. [DOI] [PubMed] [Google Scholar]
- 3.García-Arumí J, Ramsay LS, Guraya BC. Transpupillary thermotherapy for circumscribed choroidal hemangiomas. Ophthalmology. 2000;107(2):351–356. discussion 357. doi: 10.1016/s0161-6420(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 4.Jamison A, Cauchi P, Gilmour DF. Photodynamic therapy for circumscribed choroidal haemangioma in a Scottish cohort. Ocul Oncol Pathol. 2018;4(5):322–330. doi: 10.1159/000486340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madreperla SA, Hungerford JL, Plowman PN, Laganowski HC, Gregory PT. Choroidal hemangiomas: visual and anatomic results of treatment by photocoagulation or radiation therapy. Ophthalmology. 1997;104(11):1773–1778. discussion 1779. doi: 10.1016/s0161-6420(97)30027-x. [DOI] [PubMed] [Google Scholar]
- 6.Lasave AF, Serrano MA, Arevalo JF. Photodynamic therapy with verteporfin plus intravitreal bevacizumab for circumscribed choroidal hemangioma: 4 years of follow-up. Retin Cases Brief Rep. 2017 doi: 10.1097/ICB.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 7.Nourinia R, Karimi S, Mashayekhi A. Circumscribed choroidal hemangioma. J Ophthalmic Vis Res. 2015;10(3):320. doi: 10.4103/2008-322X.170353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilling H, Sauerwein W, Lommatzsch A, Friedrichs W, Brylak S, Bornfeld N, Wessing A. Long-term results after low dose ocular irradiation for choroidal haemangiomas. Br J Ophthalmol. 1997;81(4):267–273. doi: 10.1136/bjo.81.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naseripour M, Maleki A, Astaraki A, Sedaghat A, Jaberi R, Lee S, Azma Z, Silpa-Archa S. Ruthenium-106 brachytherapy in the treatment of circumscribed choroidal hemangioma. Retina. 2018;38(5):1024–1030. doi: 10.1097/IAE.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 10.Mahdjoubi A, Dendale R, Desjardins L, Lemaitre S, Lumbroso-Le Rouic L, Goudjil F, Cassoux N, Levy-Gabriel C. Treatment of exudative circumscribed choroidal hemangioma: efficacy of fractionated proton therapy (20 gray relative biological effectiveness in 8 fractions) Retina. 2019;39(4):692–699. doi: 10.1097/IAE.0000000000002002. [DOI] [PubMed] [Google Scholar]
- 11.Hirakata A, Okada AA, Asakawa M, Mitsui K, Hida T. A case of choroidal hemangioma with bullous exudative retinal detachment treated successfully by transpupillary thermotherapy. Nippon Ganka Gakkai Zasshi. 2001;105(6):415–420. [PubMed] [Google Scholar]
- 12.Tanabe H, Sahashi K, Kitano T, Tomita Y, Saito AM, Hirose H. Effects of oral propranolol on circumscribed choroidal hemangioma: a pilot study. JAMA Ophthalmol. 2013;131(12):1617–1622. doi: 10.1001/jamaophthalmol.2013.5669. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lee CS, Kim M, Lee SC. Retinal fluid changes and therapeutic effects in symptomatic circumscribed choroidal hemangioma patients: a long-term follow up study. BMC Ophthalmol. 2018;18(1):321. doi: 10.1186/s12886-018-0993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Lee CS, Lee SC. Efficacy of double dose photodynamic therapy for circumscribed choroidal hemangioma. Retina. 2019;39(2):392–397. doi: 10.1097/IAE.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 15.Gilda C, Claudia R, Maria BA, Nunzio V, Antonio F, Raffaele L, Giovanni C. Evaluation of vascular changes with optical coherence tomography angiography after ruthenium-106 brachytherapy of circumscribed choroidal hemangioma. Eye (Lond) 2018;32(8):1401–1405. doi: 10.1038/s41433-018-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy-Gabriel C, Rouic LL, Plancher C, Dendale R, Delacroix S, Asselain B, Habrand JL, Desjardins L. Long-term results of low-dose proton beam therapy for circumscribed choroidal hemangiomas. Retina. 2009;29(2):170–175. doi: 10.1097/IAE.0b013e31818bccfb. [DOI] [PubMed] [Google Scholar]
- 17.Aizman A, Finger PT, Shabto U, Szechter A, Berson A. Palladium 103 (103Pd) plaque radiation therapy for circumscribed choroidal hemangioma with retinal detachment. Arch Ophthalmol. 2004;122(11):1652–1656. doi: 10.1001/archopht.122.11.1652. [DOI] [PubMed] [Google Scholar]
- 18.Chao AN, Shields CL, Shields JA, Krema H. Plaque radiotherapy for choroidal hemangioma with total retinal detachment and iris neovascularization. Retina. 2001;21(6):682–684. doi: 10.1097/00006982-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Zografos L, Bercher L, Chamot L, Gailloud C, Raimondi S, Egger E. Cobalt-60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121(2):190–199. doi: 10.1016/s0002-9394(14)70584-7. [DOI] [PubMed] [Google Scholar]
- 20.Frau E, Rumen F, Noel G, Delacroix S, Habrand JL, Offret H. Low-dose proton beam therapy for circumscribed choroidal hemangiomas. Arch Ophthalmol. 2004;122(10):1471–1475. doi: 10.1001/archopht.122.10.1471. [DOI] [PubMed] [Google Scholar]
- 21.Joshi S, Reddy VAR, Ganesa P, et al. Ruthenium 106 plaque brachytherapy: indications and outcome in ocular tumors. J Cancer Res Ther. 2009;5:S88–S89. [Google Scholar]
- 22.Ritland JS, Eide N, Tausjø J. External beam irradiation therapy for choroidal haemangiomas. Visual and anatomical results after a dose of 20 to 25 Gy. Acta Ophthalmol Scand. 2001;79(2):184–186. doi: 10.1034/j.1600-0420.2001.079002184.x. [DOI] [PubMed] [Google Scholar]