Abstract
Background
Several studies have suggested that prophylactic antibiotics given during pregnancy improved maternal and perinatal outcomes, while others have shown no benefit and some have reported adverse effects.
Objectives
To determine the effect of prophylactic antibiotics on maternal and perinatal outcomes during the second and third trimester of pregnancy for all women or women at risk of preterm delivery.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2015) and reference lists of retrieved articles.
Selection criteria
Randomised controlled trials comparing prophylactic antibiotic treatment with placebo or no treatment for women in the second or third trimester of pregnancy before labour.
Data collection and analysis
We assessed trial quality and extracted data.
Main results
The review included eight randomised controlled trials. Approximately 4300 women were recruited to detect the effect of prophylactic antibiotic administration on pregnancy outcomes.
Primary outcomes
Antibiotic prophylaxis did not reduce the risk of preterm prelabour rupture of membranes (risk ratio (RR) 0.31; 95% confidence interval (CI) 0.06 to 1.49 (one trial, 229 women), low quality evidence) or preterm delivery (RR 0.88; 95% CI 0.72 to 1.09 (six trials, 3663 women), highquality evidence). However, preterm delivery was reduced in the subgroup of pregnant women with a previous preterm birth who had bacterial vaginosis (BV) during the current pregnancy (RR 0.64; 95% CI 0.47 to 0.88 (one trial, 258 women)), but there was no reduction in the subgroup of pregnant women with previous preterm birth without BV during the pregnancy (RR 1.08; 95% CI 0.66 to 1.77 (two trials, 500 women)). A reduction in the risk of postpartum endometritis (RR 0.55; 95% CI 0.33 to 0.92 (one trial, 196 women)) was observed in high‐risk pregnant women (women with a history of preterm birth, low birthweight, stillbirth or early perinatal death) and in all women (RR 0.53; 95% CI 0.35 to 0.82 (three trials, 627 women), moderate quality evidence). There was no difference in low birthweight (RR 0.86; 95% CI 0.53 to 1.39 (four trials; 978 women)) or neonatal sepsis (RR 11.31; 95% CI 0.64 to 200.79) (one trial, 142 women)); and blood culture confirming sepsis was not reported in any of the studies.
Secondary outcomes
Antibiotic prophylaxis reduced the risk of prelabour rupture of membranes (RR 0.34; 95% CI 0.15 to 0.78 (one trial, 229 women), low quality evidence) and gonococcal infection (RR 0.35; 95% CI 0.13 to 0.94 (one trial, 204 women)). There were no differences observed in other secondary outcomes (congenital abnormality; small‐for‐gestational age; perinatal mortality), whilst many other secondary outcomes (e.g. intrapartum fever needing treatment with antibiotics) were not reported in included trials.
Regarding the route of antibiotic administration, vaginal antibiotic prophylaxis during pregnancy did not prevent infectious pregnancy outcomes. The overall risk of bias was low, except that incomplete outcome data produced high risk of bias in some studies. The quality of the evidence using GRADE was assessed as low for preterm prelabour rupture of membranes, high for preterm delivery, moderate for postpartum endometritis, low for prelabour rupture of membranes, and very low for chorioamnionitis. Intrapartum fever needing treatment with antibiotics was not reported in any of the included studies.
Authors' conclusions
Antibiotic prophylaxis did not reduce the risk of preterm prelabour rupture of membranes or preterm delivery (apart from in the subgroup of women with a previous preterm birth who had bacterial vaginosis). Antibiotic prophylaxis given during the second or third trimester of pregnancy reduced the risk of postpartum endometritis, term pregnancy with pre‐labour rupture of membranes and gonococcal infection when given routinely to all pregnant women. Substantial bias possibly exists in the review's results because of a high rate of loss to follow‐up and the small numbers of studies included in each of our analyses. There is also insufficient evidence on possible harmful effects on the baby. Therefore, we conclude that there is not enough evidence to support the use of routine antibiotics during pregnancy to prevent infectious adverse effects on pregnancy outcomes.
Plain language summary
Antibiotic prophylaxis during the second and third trimester in pregnancy to reduce adverse pregnancy outcomes and morbidity
Antibiotics are administered to pregnant women during the second and third trimester of pregnancy (before labour) to prevent bacteria in the vagina and cervix affecting the pregnancy. Infection by some infectious organisms in a woman’s genital tract can cause health problems for the mother and her baby, and has been associated with preterm births. This review of eight randomised trials involved approximately 4300 women in their second or third trimester. We found that antibiotics did not reduce the risk of preterm prelabour rupture of the membranes (one trial, low quality of evidence), or the risk of preterm birth (six trials, highquality of evidence). Preterm delivery was reduced in pregnant women who had a previous preterm birth and an imbalance of bacteria in the vagina (bacterial vaginosis) during the current pregnancy. There was no reduction in preterm delivery in pregnant women with previous preterm birth without a bacterial imbalance during the current pregnancy (two trials). Postpartum endometritis, or infection of the uterus following birth, was reduced overall (three trials, moderate quality of evidence), as well as in a trial of high‐risk women who had a previous preterm birth (one trial, moderate quality of evidence). No reduction in neonatal illness was observed. Outcomes of interest were available in trials with high losses to follow‐up. We could not estimate the side effects of antibiotics since side effects were rare; however, antibiotics may still have serious side effects on women and their babies.
There is, therefore, no justification to give antibiotics to all pregnant women during the second or third trimester to prevent adverse infectious effects on pregnancy outcomes.
Summary of findings
Summary of findings for the main comparison. Prophylactic antibiotics versus placebo for preventing infectious morbidity and mortality.
| Prophylactic antibiotics versus placebo for preventing infectious morbidity and mortality | ||||||
| Population: women in the second or third trimester of pregnancy before labour and delivery Settings: hospitals in Kenya, Belgium, USA, India, The Netherlands Intervention: prophylactic antibiotics versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic antibiotics versus placebo | |||||
| Preterm prelabour rupture of membranes | Study population | RR 0.31 (0.06 to 1.49) | 229 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 84 per 1000 | 60 per 1000 (16 to 224) | |||||
| Moderate | ||||||
| 55 per 1000 | 40 per 1000 (10 to 147) | |||||
| Prelabour rupture of membranes | Study population | RR 0.34 (0.15 to 0.78) | 229 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 173 per 1000 | 59 per 1000 (26 to 135) | |||||
| Moderate | ||||||
| 173 per 1000 | 59 per 1000 (26 to 135) | |||||
| Preterm delivery | Study population | RR 0.88 (0.72 to 1.09) | 3663 (6 studies) | ⊕⊕⊕⊕ high | ||
| 155 per 1000 | 150 per 1000 (127 to 178) | |||||
| Moderate | ||||||
| 101 per 1000 | 98 per 1000 (83 to 116) | |||||
| Chorioamnionitis | Study population | RR 0.62 (0.10 to 3.62) | 229 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 27 per 1000 | 17 per 1000 (3 to 99) | |||||
| Moderate | ||||||
| 27 per 1000 | 17 per 1000 (3 to 98) | |||||
| Puerperal sepsis/postpartum endometritis | Study population | RR 0.53 (0.35 to 0.82) | 627 (3 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 161 per 1000 | 85 per 1000 (56 to 132) | |||||
| Moderate | ||||||
| 104 per 1000 | 55 per 1000 (36 to 85) | |||||
| Intrapartum fever needing treatment with antibiotics | Not estimable | (0 study) | See comment | This outcome was not reported in any of the included studies. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect, few events & small sample size. 2 One study with design limitations. 3 Few events and small sample size. 4 Small sample size.
Background
Description of the condition
Female genital tract infection can be caused by various organisms and could be due to acquisition, overgrowth, or ascending of the normal flora from the lower genital tract into the uterine cavity.
Maternal genital tract infection or colonisation by some infectious organisms can cause maternal and perinatal mortality and morbidity. Preterm delivery is the most common cause of perinatal morbidity and mortality in the world. Moreover, prematurity is implicated in at least two‐thirds of early infant deaths (Cunningham 1997).
A large number of medical and demographic factors have been implicated in the aetiology of preterm birth. These can be categorised into four groups:
medical and obstetric complications (e.g. hypertensive disorders, placental haemorrhage);
lifestyle factors (e.g. cigarette smoking, poor nutrition);
amniotic fluid infection caused by a variety of micro‐organisms located in the genital tract;
cervical incompetence.
Approximately one‐third of preterm births have been associated with chorioamniotic infection (Lettieri 1993). Many micro‐organisms have been suggested as the cause of preterm prelabour rupture of membranes, preterm labour, or both; for example, bacterial vaginosis, Trichomonas vaginalis, Neisseria gonorrhoeae, Ureaplasma urealyticum, Chlamydia trachomatis and Group B streptococci (Braun 1971; Gravett 1986; Hardy 1984; Hillier 1995; Regan 1981). Case detection and treatment in pregnant women is problematic and expensive, emphasising the need for other strategies. Considering the association between preterm births and infection, prophylactic antibiotics seem to be a logical strategy for preventing preterm births, although the effectiveness of prophylactic antibiotics for which population should be given is still not clear (e.g., all women or only high‐risk women).
Description of the intervention
Antibiotic prophylaxis is used for prevention of infection and reduces the risk of sequelae of infection. Antibiotics used for prophylaxis should be initiated before documented infection. Antibiotic use during pregnancy is not without possible risk. Long‐term follow‐up of children whose mothers were randomly allocated to antibiotics versus placebo for suspected preterm labour with intact membranes were found to have an increased risk of cerebral palsy at seven years of age (Kenyon 2008).
How the intervention might work
Infections and related complications in pregnancy and childbirth are potentially preventable. However, the appropriate intervention is yet to be identified. Routine antenatal detection and treatment of infections, especially in countries with a high prevalence, would seem the most reasonable approach. Limited laboratory facilities make this strategy unrealistic in low‐resource settings. Diagnosis algorithms, including clinical signs and symptoms, and behavioural patterns, are sometimes used for quick identification of infections for prompt care. Unfortunately, despite the fact that this approach may be useful in countries with limited resources, diagnostic algorithms have low sensitivity, predictive values and validity. In a situation where realistic options are few, a strategy of routine antibiotic prophylaxis might be a worthwhile alternative.
Why it is important to do this review
The available body of literature on prophylactic antibiotics in pregnancy has yielded conflicting results. While some studies demonstrated that prophylactic antibiotic administration in pregnancy improved maternal and perinatal morbidity and mortality, other studies could not confirm this finding (Eschenbach 1991; McCormack 1987; Morales 1994; Newton 1989; Oleszczuk 2000; Romero 1988; Romero 1993). It is in view of this uncertainty that there is a need for a systematic review of the results of randomised controlled trials of antibiotic prophylaxis in pregnancy.
Objectives
To determine whether the routine administration of prophylactic antibiotics in the second or third trimester of pregnancy for all women or women at risk of preterm delivery reduces adverse pregnancy and infant outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) including cluster‐RCTs were eligible for inclusion. Quasi‐RCTs were not eligible for inclusion.
Types of participants
Women in the second or third trimester of pregnancy before labour and delivery. We included routine prophylaxis for all women and prophylaxis for women with high risk of preterm birth. High risk was defined as women at risk of preterm delivery, for example, having a previous spontaneous preterm delivery, history of having an infant with a low birthweight, having bacterial vaginosis (BV) in the current pregnancy, or a pre‐pregnancy weight less than 50 kg. We excluded trials in which antibiotics were given to women for treatment purposes or if they had a positive test for fetal fibronectin (fFN) as the trial entry criteria.
Types of interventions
Prophylactic prenatal antibiotics versus placebo or no treatment. We excluded interventions at intrapartum.
Types of outcome measures
Primary outcomes are directly related to infectious morbidity/mortality.
Primary outcomes
Maternal outcomes
Preterm prelabour rupture of membranes (membrane rupture before gestational age of 37 weeks and before labour)
Preterm delivery
Puerperal sepsis/postpartum endometritis, wound infection, urinary tract infection
Neonatal outcomes
Low birthweight
Clinical neonatal sepsis
Blood culture confirming sepsis
Secondary outcomes
Maternal outcomes
Prelabour rupture of membranes (membrane rupture after gestational age of 37 weeks but before labour)
Chorioamnionitis
Intrapartum fever needing treatment with antibiotics
Serious maternal complications of puerperal infection (requiring laparotomy for infection or hysterectomy), death
Gonococcal cervicitis (postpartum detected) (not prespecified)
Maternal side effects of antibiotic prophylaxis, such as allergy, gastrointestinal tract disturbance, etc
Duration of hospital stay
Satisfaction with care
Compliance with medication regimens, such as taking all medication according to doctor's instructions
Neonatal outcomes
Mean gestational age
Admission to neonatal intensive care unit
Mean birthweight
Ophthalmia neonatorum
Congenital abnormality
Small‐for‐gestational age
Abnormal neurological development
Perinatal mortality
Childhood allergies
Childhood gastrointestinal problems
Childhood functional impairment
Childhood cerebral palsy
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 April 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of all retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review,seeThinkhamrop 2015a.
For this second update in 2015, we used the following methods when assessing the trials identified by the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving the third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence
For this update, we assessed the quality of the evidence using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison.
Maternal outcomes
Preterm prelabour rupture of membranes (membrane rupture before gestational age of 37 weeks and before labour)
Prelabour rupture of membranes (membrane rupture after gestational age of 37 weeks but before labour)
Preterm delivery
Chorioamnionitis
Intrapartum fever needing treatment with antibiotics
Puerperal sepsis/postpartum endometritis, wound infection, urinary tract infection
We used GRADEprofiler (GRADEpro 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. If necessary, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We intended to include cluster‐randomised controlled trials (RCTs) in the analyses along with individually‐randomised trials. Although we could not find any cluster‐RCTs for this version of the review. In future updates, if identified, we will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐RCTs and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We would also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We planned to exclude cross‐over design since it is unlikely to be a valid study design for this review.
Other unit of analysis issues
We planned to exclude outcomes for multiple pregnancies since there is a different mechanism for preterm labour.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out subgroup analyses on the high‐risk group: that is, the group of pregnant women who may be at risk of having the primary outcomes, including those with a history of preterm delivery, a history of genital tract and urinary tract infection.
For the previous update (Thinkhamrop 2015a), 'high risk' was defined as women who had a previous spontaneous preterm delivery, history of low birthweight, a diagnosis of bacterial vaginosis (BV) in the current pregnancy (BV identified after enrolment and antibiotics used only for prophylaxis before knowing if the participant had BV or not), or a pre‐pregnancy weight less than 50 kg.
All outcomes were used in subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not conduct sensitivity analysis in this version of the review. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
We searched up to 30 April 2015 and assessed at total of 46 reports of 20 trials. In this 2015 update, we included eight trials (Gichangi 1997; Hauth 1995; McGregor 1990; Paul 1997; Sen 2005; Temmerman 1995; Van den Broek 2009; Vermeulen 1999) and excluded 11 trials (Andrews 2003; Andrews 2006; Audebert 1989; Goldenberg 2006; Gray 2001; Larsson 2006; Luntamo 2010; Peters 1995; Shennan 2006; Tripathi 2008; Unger 2015). We have grouped Rathjen 2010, a previously ongoing report, under the newly excluded trial (Unger 2015). One study is ongoing (Hoffman 2013).
In this 2015 update, we have included a previously excluded trial (Van den Broek 2009). In this trial there are two interventions (azithromycin prophylaxis for preterm labour and sulphadoxine‐pyrimethamine as an antimalarial prophylaxis). However, the antimalarial prophylaxis was given to all recruited women and so the trial meets our inclusion criteria for antibiotic prophylaxis (azithromycin compared with placebo). In the previous update (Thinkhamrop 2015a), we excluded two previously included studies (Lin 2005; Shennan 2006), because the trials included pregnant women with positive fetal fibronectin using antibiotic for treatment but not for prophylaxis. Lin 2005 is an additional report of the excluded trial Andrews 2003. Two previously excluded studies are now incorporated into the excluded trial Goldenberg 2006 and one previously excluded study (Kurtzman 2008) is now incorporated into the excluded trial Shennan 2006. One previously ongoing study (Ashorn 2006) is now incorporated in the newly excluded trial Luntamo 2010.
Included studies
Eight trials (nine reports) met the inclusion criteria for this review. For a detailed description of the included studies, seeCharacteristics of included studies. Three of the studies (Hauth 1995; McGregor 1990; Vermeulen 1999), were conducted in high‐income countries (USA, Netherlands) while the other five (Gichangi 1997; Paul 1997; Sen 2005; Temmerman 1995, Van den Broek 2009) were reports from low‐ and middle‐income countries (Kenya, India, Malawi). Three trials (Gichangi 1997; Hauth 1995; Vermeulen 1999), enrolled only high‐risk pregnant women. All studies adequately described the characteristics of the women admitted into the study.
The antibiotics used in these studies were oral erythromycin, azithromycin, metronidazole, cephalexin, cefetamet‐pivoxil, and parenteral ceftriaxone, and clindamycin vaginal cream.
The earliest of the studies reviewed was published in 1990, five others were published from 1995 to 1999, one in 2005, and the latest one was published in 2009.
Excluded studies
We excluded 11 trials (36 reports) for the following reasons.
Antibiotic administration took place during the first half of the pregnancy and not during the second and third trimesters of pregnancy, which is the focus of this review (Audebert 1989; Gray 2001; Larsson 2006).
One study focused on twin gestation, which has a higher risk of adverse pregnancy outcome with some different mechanisms from single pregnancy (Peters 1995).
Antibiotics were administered before the current pregnancy, when the women were not pregnant (Andrews 2006).
Antibiotics were given prenatally and during labour, which was not relevant to the review's objective to assess the effect of prophylactic antibiotics given prenatally (Goldenberg 2006).
The study compared one intervention with another intervention without a placebo/no treatment arm (Luntamo 2010; Unger 2015).
Antibiotic usage was for treatment after having identified the infection, not for prophylaxis (Andrews 2003; Shennan 2006; Tripathi 2008).
For a detailed description of the excluded studies,seeCharacteristics of excluded studies. Trials with more than one report can be found in the reference list of the excluded studies.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of all 'Risk of bias' assessments.
1.
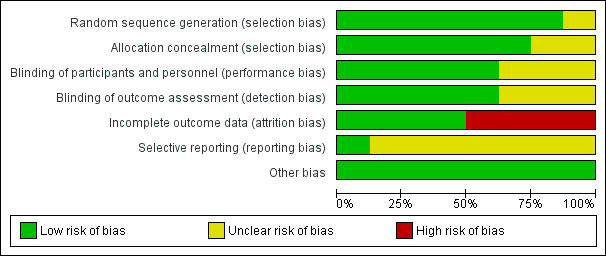
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
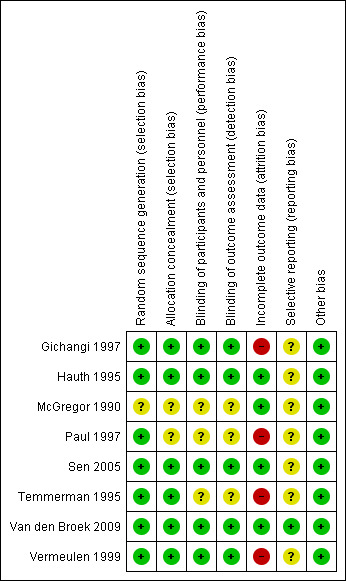
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
For the detailed information on methods, seeCharacteristics of included studies.
Allocation
The methodological quality of the trials based on selection bias (method of randomisation, allocation concealment) was mainly adequate. Only two studies had unclear allocation concealment (McGregor 1990; Paul 1997) and one unclear methods of randomisation (McGregor 1990).
Blinding
Eight studies were described as being double‐blind randomised trials, although three studies had no information on methods for blinding in the study reports (McGregor 1990; Paul 1997; Temmerman 1995). Outcome assessors were blind to treatment groups in five studies (Gichangi 1997; Hauth 1995; Sen 2005; Van den Broek 2009; Vermeulen 1999).
Incomplete outcome data
One study (Temmerman 1995) had a high drop‐out rate (166 (41.5%) out of 400 women enrolled). The losses for some outcomes were higher than the figures given in the characteristics of included studies tables (Gichangi 1997; Temmerman 1995). This might have influenced the results. However, there was no evidence that these drop‐outs occurred preferentially in one or the other arm of the trial. There were high drop‐out rates in the other studies too (Gichangi 1997 21%; Paul 1997 22%; Vermeulen 1999 15.5%). These high loss rates might have the potential to introduce bias. Fours studies were assessed as low risk of bias, due to few drop‐outs with reasons given (Hauth 1995; McGregor 1990; Sen 2005; Van den Broek 2009).
Selective reporting
We cannot assess reporting bias of most included studies since we did not have the studies' protocols in seven trials. Only one trial protocol was available online (Van den Broek 2009).
Other potential sources of bias
We did not identify any other potential sources of bias in the included studies.
Effects of interventions
See: Table 1
We included eight randomised controlled trials with a total of approximately 4300 women to evaluate the effect of prophylactic antibiotic administration in the second or third trimester on pregnancy outcomes. We found many studies on the topic of antibiotic use to prevent preterm delivery, but unlike the included studies, these studies focused on antibiotic treatment given after there was evidence of infection or complications of pregnancy; for example, detection of bacterial vaginosis (BV) or prelabour rupture of membranes before administration of antibiotics. These studies were therefore identified as trials of treatment and not prophylaxis. Publication of the included studies took place over more than 15 years (1990 to 2009). Three trials with a total of 1019 women (Gichangi 1997; Hauth 1995; Vermeulen 1999) enrolled only high‐risk pregnant women. 'High risk' was defined as women who had a previous spontaneous preterm delivery, history of low birthweight, a diagnosis of BV in the current pregnancy (BV identified after enrolment and antibiotics used only for prophylaxis before knowing if the participant had BV or not). or a pre‐pregnancy weight less than 50 kg. Seven studies used oral antibiotics: erythromycin alone (McGregor 1990; Paul 1997); erythromycin plus metronidazole (Hauth 1995); cefetamet‐pivoxil (Gichangi 1997); or a combination of metronidazole and cephalexin (Sen 2005), and azithromycin (Van den Broek 2009). One study used ceftriaxone intramuscular injection (Temmerman 1995); and one used clindamycin vaginal cream application (Vermeulen 1999).
The subgroup analysis was conducted for outcomes of preterm delivery (Analysis 1.2), preterm delivery in all high‐risk pregnancies (Analysis 1.3), puerperal sepsis (Analysis 1.4), low birthweight (Analysis 1.5), mean birthweight (Analysis 1.12), and perinatal mortality (Analysis 1.15).
1.2. Analysis.
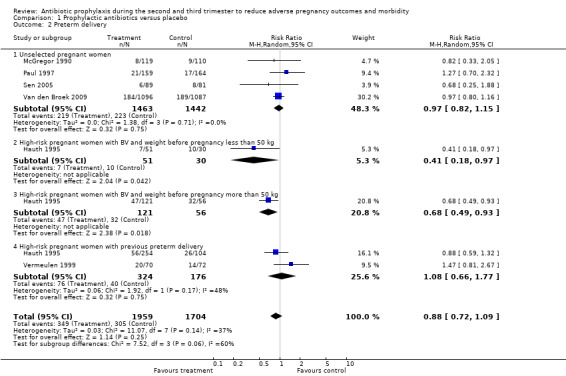
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 2 Preterm delivery.
1.3. Analysis.
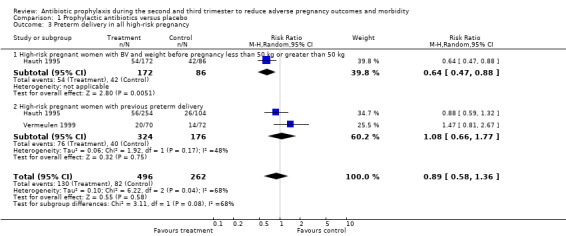
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 3 Preterm delivery in all high‐risk pregnancy.
1.4. Analysis.
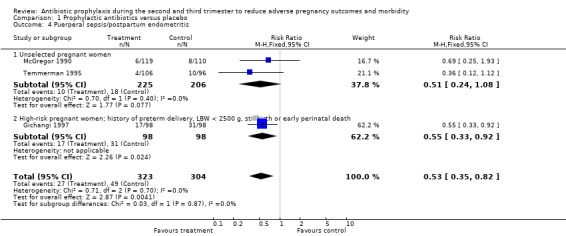
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 4 Puerperal sepsis/postpartum endometritis.
1.5. Analysis.
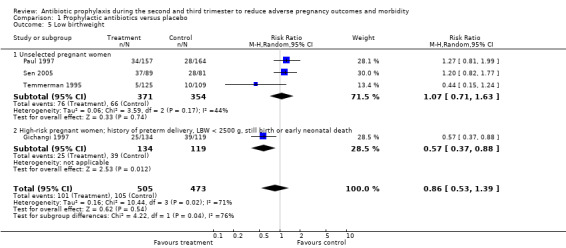
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 5 Low birthweight.
1.12. Analysis.
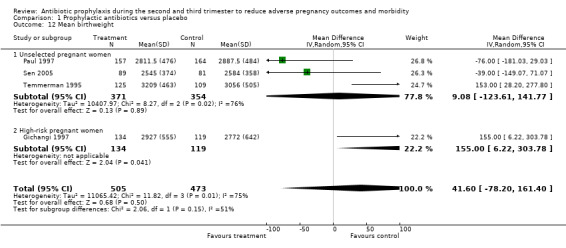
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 12 Mean birthweight.
1.15. Analysis.
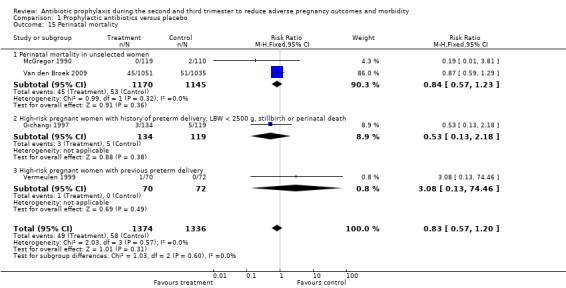
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 15 Perinatal mortality.
The interaction was significant between the subgroups in preterm delivery in all high‐risk pregnancies (P = 0.08) (Analysis 1.3), and low birthweight (P = 0.04) (Analysis 1.5). There was no significant interaction identified in the other analyses (Analysis 1.2; Analysis 1.4; Analysis 1.12; Analysis 1.15).
Prophylactic antibiotics versus placebo
Primary outcomes
Studies of antibiotic prophylaxis during the second or third trimester (ranging from 14 to 34 weeks of gestational age) in pregnant women reported the primary outcomes of interest as the following: preterm prelabour rupture of membranes, preterm delivery, postpartum endometritis, low birthweight and neonatal sepsis. Blood culture confirming sepsis was not reported in any of the studies.
Antibiotic prophylaxis did not reduce the risk of preterm prelabour rupture of membranes (average risk ratio (RR) 0.31; 95% confidence interval (CI) 0.06 to 1.49 (one trial, 229 women) Analysis 1.1) or preterm delivery (average RR 0.88; 95% CI 0.72 to 1.09 (six trials, 3663 women) Analysis 1.2 all women; average RR 0.97; 95% CI 0.82 to 1.15 (four trials, 2905 women) Analysis 1.2.1 unselected women). However, preterm delivery was reduced in the subgroup of pregnant women with a previous preterm birth who had BV during the current pregnancy (average RR 0.64, 95% CI 0.47 to 0.88; one trial, 258 women, subgroup differences P = 0.08; Analysis 1.3.1), but there was no reduction in the subgroup of pregnant women with previous preterm birth without BV during the pregnancy (average RR 1.08; 95% CI 0.66 to 1.77 (two trials, 500 women) Analysis 1.3,2), or in the whole group of high‐risk women (average RR 0.89; 95% CI 0.58 to 1.36 (two trials, 758 women) Analysis 1.3), and a difference between these subgroups of high‐risk women was observed (Test for subgroup differences: Chi² = 3.11, df = 1 (P = 0.08), I² = 67.8%).
1.1. Analysis.
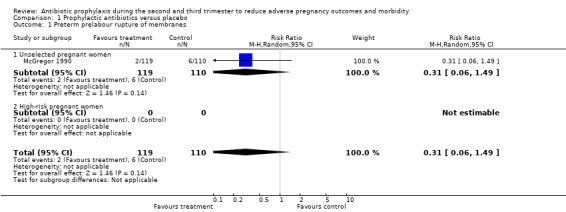
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 1 Preterm prelabour rupture of membranes.
A reduction in the risk of postpartum endometritis (RR 0.55; 95% CI 0.33 to 0.92 (one trial, 196 women)) was observed in high‐risk pregnant women (women with a history of preterm birth, low birthweight, stillbirth or early perinatal death) Analysis 1.4,2, and in all women (RR 0.53; 95% CI 0.35 to 0.82 (three trials, 627 women) Analysis 1.4), but not in unselected women (RR 0.51; 95% CI 0.24 to 1.08 (two trials, 431 women) Analysis 1.4.1. There was no difference in low birthweight in all women (average RR 0.86; 95% CI 0.53 to 1.39 (four trials; 978 women) (Analysis 1.5), or unselected pregnant women (average RR 1.07; 95% CI 0.71 to 1.63 (three trials; 725 women) Analysis 1.5.1, although a difference was observed in high‐risk women (average RR 0.57; 95% CI 0.37 to 0.88 (one trial; 253 women) Analysis 1.5.2 and a difference in subgroups observed (Test for subgroup differences: Chi² = 4.22, df = 1 (P = 0.04), I² = 76.3%). There was no difference in neonatal sepsis (RR 11.31; 95% CI 0.64 to 200.79) (one trial, 142 women) Analysis 1.6, and blood culture confirming sepsis was not reported in any of the studies.
1.6. Analysis.
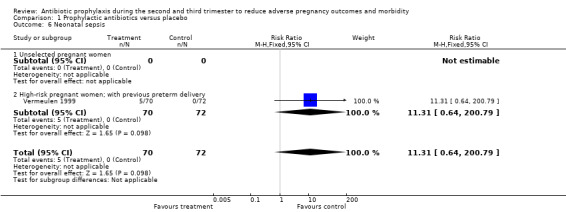
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 6 Neonatal sepsis.
Secondary outcomes
A reduction was observed in preterm rupture of membranes (RR 0.34; 95% CI 0.15 to 0.78; one trial; 229 women; Analysis 1.7) and gonococcal infection (postpartum detected) (RR 0.35, 95% CI 0.13 to 0.94; one trial; 204 women; Analysis 1.9) in the group of women who received antibiotic prophylaxis. Other secondary outcomes did not show any significant effects (chorioamnionitis; compliance; mean gestational age; mean birthweight; congenital abnormality; small‐for‐gestational age; perinatal mortality). The included studies did not report any serious adverse effects of antibiotic prophylaxis, and no data were reported on some maternal outcomes that we had planned to assess, including intrapartum fever requiring treatment with antibiotics, serious maternal complications (puerperal infection requiring laparotomy for infection or hysterectomy; death), maternal side effects, duration of hospital stay and satisfaction with care. We observed a lack of data to assess congenital abnormality and perinatal mortality. We also found limited data to evaluate the effect of antibiotics on low birthweight in unselected women. There were four trials in this analysis: three reported on an unselected population and the other reported in a high‐risk group, which had effects in opposite directions. We found no data on the following neonatal outcomes: admission to neonatal intensive care unit; ophthalmia neonatorum; and abnormal neurological development.
1.7. Analysis.
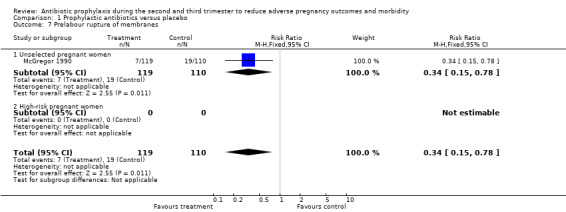
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 7 Prelabour rupture of membranes.
1.9. Analysis.
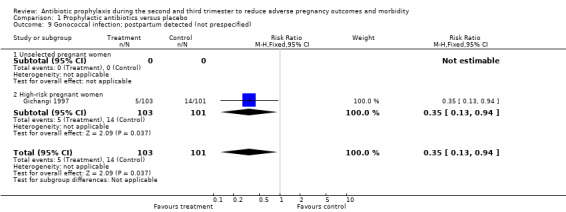
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 9 Gonococcal infection; postpartum detected (not prespecified).
There was only one included study (Vermeulen 1999) that used vaginal application for antibiotic prophylaxis. It did not prevent infectious morbidity outcomes in terms of preterm delivery (average RR 1.47; 95% CI 0.81 to 2.67; 142 women) Analysis 1.2.4 and neonatal sepsis (RR 11.31; 95% CI 0.64 to 200.79; 142 women) Analysis 1.6.2.
One study (McGregor 1990) reported that compliance with medication was different between groups (73% in the treatment group versus 84% in the control group). In this trial, the treatment and control group received treatment bottles that looked identical, but which contained either an erythromycin base tablet or a placebo.
Discussion
Summary of main results
The results of this review showed that antibiotic prophylaxis during the second or third trimester of pregnancy was effective in reducing risk of preterm delivery in pregnant women with bacterial vaginosis (BV) in the current pregnancy, prelabour rupture of membranes, postpartum endometritis and gonococcal infection (detected postpartum). However, some analyses are based on studies with high risk of bias or only one trial. Limited data showed that routine use of antibiotics during pregnancy might prevent infectious morbidity for the mother, but could not reduce neonatal morbidity and mortality. We could not estimate the side effects of prophylactic antibiotics from these data, since side effects from prophylactic antibiotics are rare events.
Overall completeness and applicability of evidence
None of the included studies reported on preterm labour, serious maternal complications of puerperal infection requiring laparotomy, maternal side effects of antibiotic prophylaxis (severe side effect), duration of hospitalisation, satisfaction with care, blood culture confirming neonatal sepsis or opthalmia neonatorum. However, these outcomes are not the outcomes we expected when evaluating the effectiveness of the intervention. Some included studies reported the expected outcomes, such as preterm delivery, preterm prelabour rupture of membranes, prelabour rupture of membranes, chorioamnionitis, puerperal sepsis, postpartum endometritis, mean gestational age, low birthweight, admission to neonatal intensive care unit and perinatal mortality. Nevertheless, the power of the available studies is inadequate to provide conclusions about some rare but serious outcomes such as chorioamnionitis, intrapartum fever requiring antibiotic treatment, neonatal sepsis, admission to the intensive neonatal care unit and perinatal mortality. However, an ongoing study (Hoffman 2013) plans to enrol 1726 women which, once completed, might add more power to the assessment of these rare outcomes.
Quality of the evidence
Overall, the methodological quality of the included trials was satisfactory. Of the eight trials included, six described methods which were at low risk for selection bias, five were at low risk for performance bias, and four had low risk of incomplete outcome data. However, some outcomes of interest were only available in trials with high risk of incomplete outcome data and high rate of loss to follow‐up. Furthermore, most outcomes of interest were found in only one to two studies, meaning that the sample size might not be large enough to demonstrate key differences.
The rate of loss to follow‐up in the included studies was quite high (20% to 40%), especially for studies that reported on puerperal sepsis/postpartum endometritis. Since puerperal sepsis/postpartum endometritis had the significant beneficial effect of antibiotic prophylaxis administration during pregnancy, we are reluctant to recommend the use of this intervention due to this potential bias.
The quality of the evidence as assessed using GRADE was moderate for postpartum endometritis, and was downgraded due to a small sample size in one study. After including Van den Broek 2009 trial, the quality of evidence for preterm delivery was upgraded to high quality. Low‐quality evidence was also observed for preterm prelabour rupture of membranes, and prelabour rupture of membranes due to small sample size, wide confidence intervals crossing the line of no effect, and the design limitations of one study. Very low quality of evidence was also found for chorioamnionitis due to the design limitations of one study, few events and small sample size and wide confidence intervals crossing the line of no effect. (Table 1).
Potential biases in the review process
Our search strategy was supported by the Pregnancy and Childbirth Review Group. All review authors contributed to assessing the appropriateness of inclusion or exclusion of studies, so potential bias in the review process is unlikely.
Agreements and disagreements with other studies or reviews
Most outcomes of interest in this review revealed no evidence of effectiveness of the intervention, with the same direction of effect as individual studies. However, those outcome assessments were available in only one or two studies. Of the outcomes of interest that revealed evidence of effectiveness (preterm delivery in pregnant women with BV in the current pregnancy, prelabour rupture of membranes, postpartum endometritis and postpartum detection of gonococcal infection), only postpartum endometritis had three included studies relevant to our analysis, while two of these three individual studies showed no significant effectiveness.
Authors' conclusions
Implications for practice.
Routine use of antibiotic prophylaxis in pregnant women during the second and third trimester could prevent maternal infectious morbidity by reducing postpartum endometritis. We observed risk reduction in preterm delivery only in pregnant women with bacterial vaginosis (BV) during the current pregnancy, but there was a lack of evidence showing any benefits for neonatal morbidity and mortality. We also noted a possible substantial bias in the review's results due to a high rate of loss to follow‐up. The evidence is not strong enough to support routine use of antibiotics in the second and third trimester to prevent infectious complications.
Implications for research.
The results of this review suggest that antibiotic prophylaxis might only be effective in reducing maternal puerperal infection. Due to limited data, we could not evaluate any benefits for neonatal morbidity and mortality. In addition, data were lacking on some health outcomes such as short‐ and long‐term effects on children. Therefore, there is a need for further studies to address these missing gaps in the evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2015 | New citation required but conclusions have not changed | Review updated. This update now includes eight studies. At the last update the review included seven studies. The review's conclusions remain unchanged. |
| 30 April 2015 | New search has been performed | Search updated. One trial previously excluded (Van den Broek 2009), after further scrutiny, has now been included. One trial (Unger 2015) previously an ongoing study (published in Rathjen 2010) has been excluded. Please note that blinding has now been divided into two assessments: 1. Blinding of participants and personnel (performance bias); and 2. Blinding of outcome assessors (detection bias) ‐ tables have been updated. A 'Summary of findings' table has been incorporated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 30 August 2009 | New search has been performed | New search conducted in June 2009 which identified 11 new studies. We have included three (Lin 2005a; Sen 2005; Shennan 2006a) and excluded eight (Andrews 2006; Audebert 1989; Goldenberg 2006; Goldenberg 2005b; Kurtzman 2008; Larsson 2006; Tripathi 2008). One is ongoing (Ashorn 2006a). Another new search on 2 September 2010 identified four new reports (Aboud 2009; Kafulafula 2009; Stringer 2010a; Van den Broek 2009a). These trials will be incorporated into the next update of this review. |
| 5 March 2008 | Amended | Converted to new review format. |
| 29 February 2004 | New search has been performed | February 2004: search repeated, identifying one new report of an existing excluded study. |
Acknowledgements
We would like to express our special thanks to Dr Metin Gulmezoglu for his suggestions and encouragement to complete the first version of this review, and the Pregnancy and Childbirth Review Group for their technical support and advice.
As part of the pre‐publication editorial process, this review update has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser (previous update, Thinkhamrop 2015a).
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Prophylactic antibiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm prelabour rupture of membranes | 1 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.49] |
| 1.1 Unselected pregnant women | 1 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.49] |
| 1.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Preterm delivery | 6 | 3663 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.72, 1.09] |
| 2.1 Unselected pregnant women | 4 | 2905 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.15] |
| 2.2 High‐risk pregnant women with BV and weight before pregnancy less than 50 kg | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.97] |
| 2.3 High‐risk pregnant women with BV and weight before pregnancy more than 50 kg | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.93] |
| 2.4 High‐risk pregnant women with previous preterm delivery | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.77] |
| 3 Preterm delivery in all high‐risk pregnancy | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.58, 1.36] |
| 3.1 High‐risk pregnant women with BV and weight before pregnancy less than 50 kg or greater than 50 kg | 1 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.88] |
| 3.2 High‐risk pregnant women with previous preterm delivery | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.77] |
| 4 Puerperal sepsis/postpartum endometritis | 3 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.82] |
| 4.1 Unselected pregnant women | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.08] |
| 4.2 High‐risk pregnant women; history of preterm delivery, LBW < 2500 g, stillbirth or early perinatal death | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.33, 0.92] |
| 5 Low birthweight | 4 | 978 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 5.1 Unselected pregnant women | 3 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.63] |
| 5.2 High‐risk pregnant women; history of preterm delivery, LBW < 2500 g, still birth or early neonatal death | 1 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.88] |
| 6 Neonatal sepsis | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.31 [0.64, 200.79] |
| 6.1 Unselected pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High‐risk pregnant women; with previous preterm delivery | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.31 [0.64, 200.79] |
| 7 Prelabour rupture of membranes | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 7.1 Unselected pregnant women | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 7.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Chorioamnionitis | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.62] |
| 8.1 Unselected pregnant women | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.62] |
| 8.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Gonococcal infection; postpartum detected (not prespecified) | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.94] |
| 9.1 Unselected pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 High‐risk pregnant women | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.94] |
| 10 Compliance | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 10.1 Unselected pregnant women | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 10.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Mean gestational age (weeks) | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.01, 1.39] |
| 11.1 Unselected pregnant women | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 High‐risk pregnant women | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.01, 1.39] |
| 12 Mean birthweight | 4 | 978 | Mean Difference (IV, Random, 95% CI) | 41.60 [‐78.20, 161.40] |
| 12.1 Unselected pregnant women | 3 | 725 | Mean Difference (IV, Random, 95% CI) | 9.08 [‐123.61, 141.77] |
| 12.2 High‐risk pregnant women | 1 | 253 | Mean Difference (IV, Random, 95% CI) | 155.0 [6.22, 303.78] |
| 13 Congenital abnormality | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.20, 11.14] |
| 13.1 Unselected pregnant women | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.20, 11.14] |
| 13.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Small‐for‐gestational age | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.42, 3.96] |
| 14.1 Unselected pregnant women | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.42, 3.96] |
| 14.2 High‐risk pregnant women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Perinatal mortality | 4 | 2710 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.20] |
| 15.1 Perinatal mortality in unselected women | 2 | 2315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.23] |
| 15.2 High‐risk pregnant women with history of preterm delivery, LBW < 2500 g, stillbirth or perinatal death | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.13, 2.18] |
| 15.3 High‐risk pregnant women with previous preterm delivery | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
1.8. Analysis.
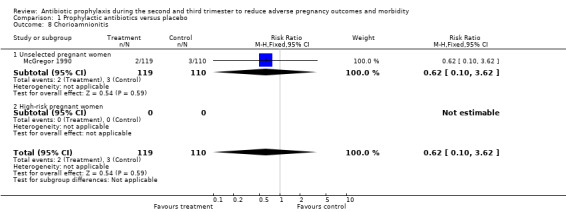
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 8 Chorioamnionitis.
1.10. Analysis.
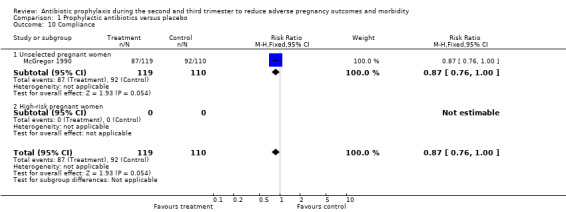
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 10 Compliance.
1.11. Analysis.
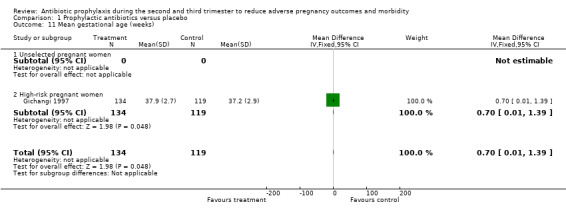
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 11 Mean gestational age (weeks).
1.13. Analysis.
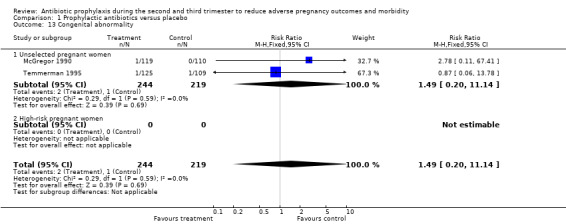
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 13 Congenital abnormality.
1.14. Analysis.
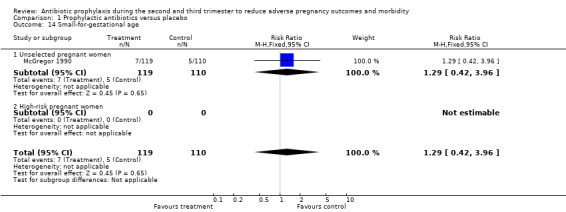
Comparison 1 Prophylactic antibiotics versus placebo, Outcome 14 Small‐for‐gestational age.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gichangi 1997.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 320 pregnant women during GA 28 to 32 wks with a history of LBW (less than 2500 g), stillbirth or early perinatal death. (High risk.) | |
| Interventions | Treatment group received a single dose of 2 g cefetamet‐pivoxil and the control group received a placebo. There was no information on the appearance of the placebo tablet. | |
| Outcomes | A total of 253 of 320 women delivered in the study centre. Out of these 253 women, there were 134 in the treatment group and 119 in the placebo group. The mean birthweight in the treatment group was higher than in the placebo group. | |
| Notes | Nairobi, Kenya and Ghent, Belgium. November 1995 to February 1996. 83% of the treatment group and 74% of the placebo group delivered at the study centre, the rest were delivered elsewhere and could not be traced for follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors mentioned that they use randomised allocation. |
| Allocation concealment (selection bias) | Low risk | The patients were randomised by means of sealed envelopes into either the intervention group or placebo group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were enrolled by one investigator who was blinded to the study medication, and the women received a single oral dose of 2 g of cefetamet‐pivoxil or a placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The persons who assessed the outcomes did not know to which group the women were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 83% of the treatment group and 74% of the placebo group delivered at the study centre, the rest delivered elsewhere and could not be traced for follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
Hauth 1995.
| Methods | A 2:1 double‐blind, randomised, placebo‐controlled trial. | |
| Participants | The study randomised 624 pregnant women during GA 22 to 24 weeks, who were at risk of preterm delivery due to having had a previous preterm delivery or a pre‐pregnancy weight of less than 50 kg. 433 were in the treatment group and 191 were in the placebo group. (High risk.) | |
| Interventions | The treatment group were given 250 mg metronidazole 3 times a day for 7 days, and erythromycin 333 mg 3 times a day for 14 days, while an identical preparation containing lactose was given to the placebo group. | |
| Outcomes | 26% of the trial group delivered preterm, as compared with 68% of the placebo group. | |
| Notes | Birmingham, Alabama. May 1989 to December 1993. 8 participants were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was used for allocation. |
| Allocation concealment (selection bias) | Low risk | Our Investigational Drug Service generated a blocked randomisation scheme in a ratio of 2:1 (i.e., 2 women were assigned to the study treatment for every 1 woman assigned to placebo), with blocks of randomly chosen sizes. At 22 to 24 weeks’ gestation (mean, 22.9), each woman was assigned to take either metronidazole (250 mg 3 times a day for 7 days) and erythromycin base (333 mg 3 times a day for 14 days) or an identical‐appearing placebo containing a lactose filler. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All the women were seen every 2 weeks for antepartum care by the same nursing team, and the importance of adherence to treatment was emphasised. Pills were counted at each visit, and each woman kept a log of medications. If a woman’s compliance was less than 80%, she was counselled again about the importance of taking the pills. All follow‐up visits were scheduled for the same day of the week, but women who presented at unscheduled times still received care from the same nursing team. Patients who missed their regular clinic visits were called on the telephone and seen at the next convenient time. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment was a hard outcome (delivery before 37 weeks). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 of 624 pregnant women were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
McGregor 1990.
| Methods | Double‐blind, placebo‐controlled trial. | |
| Participants | 235 pregnant women during GA 26 to 30 weeks. (Unselected pregnant women.) | |
| Interventions | They were given identical prepared bottles and tablets that were either erythromycin base 333 mg or placebo taking 1 tablet 3 times a day for 1 week. | |
| Outcomes | 229 of 235 women were analysed. Prelabour rupture of membranes occurred less frequently (P < 0.01) among women who received erythromycin (6%) versus placebo (16%). | |
| Notes | Denver, Colorado and Seattle, Washington. October 1985 to August 1988. 4 participants were lost to follow‐up. Only 73% of women randomised to receive erythromycin, and 84% of women who receive placebo completed 4 or more days of study treatment (P = 0.04) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no information about randomisation in the article. |
| Allocation concealment (selection bias) | Unclear risk | There was no information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no information about blinding to the participant and attending physicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information about blinding to whom assess the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 235 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
Paul 1997.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 437 pregnant women during gestational age 26 to 34 weeks. (Unselected pregnant women.) | |
| Interventions | The treatment group received erythromycin sterate 500 mg and placebo (no description of placebo tablet) in the control group twice a day for 6 wks. | |
| Outcomes | Of the 437 women enrolled in the trial, 219 were in the erythromycin group and 218 in the placebo group. There were no differences in their mean birthweight, incidence of LBW or incidence of preterm delivery in the treatment and control groups. | |
| Notes | 29 participants were lost to follow‐up. 66 participants dropped out with a specified reason. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised to receive tablets of either erythromycin stearate 500 mg or placebo twice a day. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the trial report. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the trial report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 437 participants, 29 participants were lost to follow‐up. 66 participants dropped out with a specified reason. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
Sen 2005.
| Methods | A non‐placebo, randomised controlled trial. | |
| Participants | 224 pregnant women in their second trimester (between 14 and 24 weeks) were recruited during February to July 2001. | |
| Interventions | Women in the intervention group were treated with a course of antimicrobials and provided with iron‐folic acid tablets and women in the control group received iron‐folic acid tablets only. A combination of metronidazole and cephalexin was used for antimicrobial therapy. | |
| Outcomes | 112 women in the intervention group and 112 women in the control group were analysed to assess the pregnancy outcomes. | |
| Notes | Only 170 of the total 224 enrolled women were analysed. The study was conducted among pregnant women attending the antenatal clinic of a government hospital in Kolkata, India, that serves the urban poor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into intervention and control groups. |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment or control according to the randomisation was sealed in serial‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians who evaluated the women and their babies were blinded to the treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians who evaluated the women and their babies were blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 224 participants, 112 women were allocated to the intervention group and 112 women to the control group. Only 89 participants in the intervention group and 81 in the control group were analysed to assess pregnancy outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
Temmerman 1995.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 400 pregnant women during GA 28 to 32 wks. (Unselected pregnant women.) | |
| Interventions | Single dose of 250 mg ceftriaxone IM versus placebo 3.5 mL 0.9% NaCl IM. | |
| Outcomes | Mean birthweight in the ceftriaxone group 153 g higher than in the placebo group, i.e. 3209 versus 3056 (P = 0.01). | |
| Notes | Nairobi, Kenya. 60% of the treatment group and 57% of the placebo group were delivered at the study centre; the rest were delivered elsewhere. 166 participants were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by means of sealed envelopes into treatment and control groups. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised by means of sealed envelopes into treatment and control groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the trial report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 400 participants, 60% of the treatment group and 57% of the placebo group delivered at the study centre; the rest delivered elsewhere. 166 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
Van den Broek 2009.
| Methods | A randomised controlled trial. | |
| Participants | Pregnant women, gestational age less than 24 weeks, in three rural and one peri‐urban antenatal clinic in Southern Malawi. | |
| Interventions | Recruited women were randomly allocated to receive either 1g azithromycin or placebo at both 16‐24 and 28‐32 wks. of gestation. All women received iron tablets daily 60 mg + 0.25 mg folic acid and antimalarial prophylaxis (two doses of Fansidar: 500 mg sulphadoxine with 25 mg phrimethamine). | |
| Outcomes | Preterm delivery, mean gestational age at delivery, mean birthweight, perinatal mortality, maternal malaria and anaemia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was prepared by a statistician not involved in the trial analysis using a random generation procedure with variable block size to assign to treatments equally within each block of consecutive numbers. |
| Allocation concealment (selection bias) | Low risk | The azithromycin and placebo treatments allocated were provided in identical capsules and packed in pairs of sealed envelopes for each individual study number, according to the randomised schedule, by staff who were not involved in the conduct of the trial. The randomisation schedule was placed in sealed envelopes and not disclosed to anyone involved in the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participant and the midwives who allocated the random numbers were blinded to the study assignment. At no time during the study was there cause to unblind the treatment allocation for any of the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: intervention group 1096 (95.4%); control group 1087 (94.7%). Both groups balanced and few drop‐outs with reasons. |
| Selective reporting (reporting bias) | Low risk | Trial protocol was available online at doi: 10.1371/journal.pmed.1000191.s001 |
| Other bias | Low risk | None known. |
Vermeulen 1999.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 168 pregnant women during GA 26 to 32 wks with a history of preterm delivery in the preceding pregnancy (high risk). | |
| Interventions | Clindamycin 2% vaginal cream, or placebo (identical‐looking cream), applied daily for 7 days. | |
| Outcomes | 142 of 168 enrolled women were analysed. No difference was found in overall preterm birth between the treatment and the control groups. | |
| Notes | 12 hospitals in The Netherlands January 1, 1994 to December 31, 1996. The lost to follow‐up rate, or incomplete medication taken, was 13 out of 83 in the treatment group and 13 out of 85 in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 4 and was stratified by centre and by bacterial vaginosis. |
| Allocation concealment (selection bias) | Low risk | A research co‐ordinator allocated medication or placebo using a pre‐determined randomisation list so that care providers were blinded to medication and the presence of bacterial vaginosis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participating women collected their medication at the pharmaceutical department of each hospital. The medication, or an identical looking placebo, had to be applied for seven days intravaginally at 26 and 32 weeks of gestation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The care providers were blinded to medication and the presence of bacterial vaginosis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The lost to follow‐up rate or incomplete medication taken was 13 out of 83 in the treatment group and 13 out of 85 in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Low risk | None. |
GA: gestational age IM: intramuscular LBW: low birthweight NaCl: sodium chloride wks: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2003 | The study included pregnant women with positive fetal fibronectin. The authors mentioned in the study's objective that they planned to estimate the antibiotic treatment effects in asymptomatic women with a positive cervical or vaginal fetal fibronectin test to reduce the risk of spontaneous preterm delivery. Fetal fibronectin is a placental and membrane protein that is a unique epitope of the fibronectins. One hypothesis holds that intrauterine infection causes disruption of the extracellular choriodecidual basement membrane, causing leakage of fetal fibronectin into cervical or vaginal secretions, in which it can then be detected. This might imply that antibiotic usage in this situation is for treatment not for prophylaxis. |
| Andrews 2006 | Prophylactic antibiotics were administered during the interpregnancy interval in non‐pregnant women with a prior early (< 34 weeks') spontaneous preterm birth, and not during the second and third trimesters, which is the objective of this review. |
| Audebert 1989 | A randomised study designed to assess the efficacy of Polygynax in preventing vaginal infections at the start of pregnancy. However, the study outcomes assessment was only on the eradication rate of vaginal infection. They did not assess the pregnancy outcomes for mothers and newborns. |
| Goldenberg 2006 | Prophylactic antibiotics were given prenatally and during labour, which was not relevant to the review's objective to assess the effect of prophylactic antibiotics given prenatally. |
| Gray 2001 | Pregnant women were enrolled at varying gestations, and treatment could not be provided on a fixed schedule during pregnancy. In this trial, the intervention was given to 529 women in the first half of gestation and to 851 women in the second half of gestation. This is unlikely to have introduced bias to the comparison between randomisation arms because the trimester of enrolment was similar in the two arms. Nevertheless, the variable timing of treatment during pregnancy may have reduced the efficacy of antibiotics on adverse pregnancy outcomes, especially if the antibiotic prophylaxis was given in the first trimester. |
| Larsson 2006 | Participants recruited for prophylactic antibiotics were between 10 and 14 weeks of gestational age, which was not relevant to the review's objective to assess the effect of antibiotic prophylaxis given in the second or third trimester. |
| Luntamo 2010 | We planned to include only studies that compared any intervention with placebo or no treatment arm. However, this study compared 3 treatment arms without a placebo arm. |
| Peters 1995 | The objective of this study was to determine whether prophylactic treatment with oral broad‐spectrum antimicrobial therapy improves pregnancy outcomes in twin gestations. The perinatal morbidity and mortality in twin gestations is higher than in singleton gestations because of an increased incidence of preterm labour, which is mainly due to mechanical distention of the uterus, or is combined with other factors. |
| Shennan 2006 | This study randomised pregnant women with positive fetal fibronectin, who were screened for fetal fibronectin at 24 and 27 weeks' gestation, to receive a week’s course of oral metronidazole or placebo. Women who received a positive test received antibiotics. Antibiotic usage in this situation is considered a form of treatment rather than prophylaxis. |
| Tripathi 2008 | This study assessed antibiotic treatment effects on pregnancy outcomes in pregnant women with abnormal vaginal flora, which was not relevant to our objective to assess antibiotic prophylaxis (not as a treatment of a documented infection). |
| Unger 2015 | The trial had two treatment arms. The intervention arm women received three courses of sulphadoxine‐pyrimethamine and azithromycin.The women assigned to the control arm received one course of sulphadoxine‐pyrimethamine and chloroquin. Since there is no placebo arm this study does not meet the review inclusion criteria. |
Characteristics of ongoing studies [ordered by study ID]
Hoffman 2013.
| Trial name or title | Clindamycin to reduce preterm birth in a low resource setting [official title: Clindamycin to reduce preterm birth in a low resource setting: A randomized placebo‐controlled trial] |
| Methods | A randomised placebo‐controlled trial in pregnant women between 13‐20 gestational weeks with an elevated vaginal pH ≥ 5 (evidence of this type of infection), will be randomised to 300 mg oral clindamycin 2 times per day for 5‐days administered at 13‐20 weeks of gestation or placebo (starch). |
| Participants | Pregnant women between 13‐20 gestational weeks with an elevated vaginal pH ≥ 5. |
| Interventions | 300 mg oral clindamycin two times per day for 5‐days administered at 13‐20 weeks of gestation or placebo (starch). |
| Outcomes | Preterm birth prior to 37 weeks. Preterm birth prior to 34 weeks. Late miscarriage. Low birthweight. Very low birthweight. Neonatal complications through 42 days after delivery. Maternal complications through 42 days postpartum. The utility of vaginal pH tests for identification of women at elevated risk for preterm delivery. |
| Starting date | April 2013. |
| Contact information | ClinicalTrials.gov (accessed 21 May 2013) 2013 Contact: Mrutyunjaya Bellad, MD, mbbellad@hotmail.com Contact: Shivaprasad Goudar, MD, sgoudar@jnmc.edu |
| Notes | Estimated primary completion date: August 2015 (final data collection date for primary outcome measure). |
Hb: haemoglobin IPTp: intermittent preventive treatment in pregnancy LLIN: long‐lasting insecticidal nets (S)AE: (serious) adverse event SP: sulfadoxine‐pyrimethamine
Differences between protocol and review
We have performed subgroup analysis for pregnancies with previous preterm delivery and bacterial vaginosis in the current pregnancy; this subgroup analysis was not prespecified in our protocol.
The outcome of gonococcal infection detected postpartum was not prespecified in our protocol.
The title was changed for the previous update (Thinkhamrop 2015a) to: Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity.
Contributions of authors
For this update, June 2015, J Thinkhamrop extracted data, analysed and interpreted the data, drafted and approved the final version of the update review. P Lumbiganon, GJ Hofmeyr and O Adetoro commented and approved the final version. E Ota verified the data, evaluated the quality of the data and summarised findings.
For the second version of this review (Thinkhamrop 2015a), J Thinkhamrop conducted the literature search under the supervision of the Pregnancy and Childbirth Group's Trials Search Co‐ordinator, extracted data, analysed and interpreted the data, drafted and approved the final version of the update review. P Lumbiganon extracted and interpreted the data, and approved the final version of the review. GJ Hofmeyr and O Adetoro commented and approved the final version. E Ota verified the data, evaluated the quality of the data and summarised findings.
For the first version of this review (Thinkhamrop 2002), GJ Hofmeyr and O Adetoro prepared the original protocol, commented on the draft of the review and approved the final version of the review. J Thinkhamrop revised the protocol, conducted the literature search, analysed and interpreted the data, drafted and approved the final version of the review. P Lumbiganon revised the protocol, interpreted the data, drafted and approved the final version of the review.
Sources of support
Internal sources
Khon Kaen University, Thailand.
University of the Witwatersrand, South Africa.
Ogun State University, Nigeria.
External sources
Thailand Research Fund/Senior Research Scholar, Thailand.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gichangi 1997 {published data only}
- Gichangi PB, Ndinya‐Achola JO, Ombete J, Nagelkerke NJ, Temmerman M. Antimicrobial prophylaxis in pregnancy: a randomized placebo‐controlled trial with cefetamet‐pivoxil in pregnant women with a poor obstetric history. American Journal of Obstetrics and Gynecology 1997;177:680‐4. [DOI] [PubMed] [Google Scholar]
Hauth 1995 {published data only}
- Hauth JC, Goldenberg LR, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. New England Journal of Medicine 1995;333:1732‐6. [DOI] [PubMed] [Google Scholar]
McGregor 1990 {published data only}
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Franco‐Buff A, et al. Prospective, double‐blinded, randomized, placebo‐controlled trial of short‐course erythromycin (E) base in women at high risk for preterm birth. Proceedings of the Society for Gynecologic Investigation; 1988. 1988.
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double‐blind, placebo‐controlled trial of erythromycin treatment. American Journal of Obstetrics and Gynecology 1990;163:1580‐91. [DOI] [PubMed] [Google Scholar]
Paul 1997 {published data only}
- Paul VK, Singh M, Buckshee K. Erythromycin treatment of pregnant women to reduce the incidence of low birth weight and preterm deliveries. International Journal of Gynecology & Obstetrics 1998;62:87‐8. [DOI] [PubMed] [Google Scholar]
Sen 2005 {published data only}
- Sen A, Mahalanabis D, Mukhopadhyay S, Chakrabarty K, Singh AK, Bisai S, et al. Routine use of antimicrobials by pregnant Indian women does not improve birth outcome: a randomized controlled trial. Journal of Health, Population & Nutrition 2005;23(3):236‐44. [PubMed] [Google Scholar]
Temmerman 1995 {published data only}
- Temmerman M, Njagi E, Nagelkerke N, Ndinya‐Achola J, Plummer FA, Meheus A. Mass antimicrobial treatment in pregnancy, a randomized, placebo‐controlled trial in a population with high rates of sexually transmitted diseases. Journal of Reproductive Medicine 1995;40:176‐80. [PubMed] [Google Scholar]
Van den Broek 2009 {published data only}
- Broek NR, White SA, Goodall M, Ntonya C, Kayira E, Kafulafula G, et al. The APPLe study: a randomized, community‐based, placebo‐controlled trial of azithromycin for the prevention of preterm birth, with meta‐analysis. PLoS Medicine 2009;6(12):e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 1999 {published data only}
- Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo‐controlled double‐blind trial. British Journal of Obstetrics and Gynaecology 1999;106:652‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2003 {published data only}
- Anderson B, Zhao Y, Andrews WW, Dudley DJ, Sibai B, Iams JD, et al. Effect of antibiotic exposure on Nugent score among pregnant women with and without bacterial vaginosis. Obstetrics & Gynecology 2011;117(4):844‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin‐positive women. Obstetrics & Gynecology 2003;101(5 Pt 1):847‐55. [DOI] [PubMed] [Google Scholar]
- Hendler I, Andrews WW, Carey CJ, Klebanoff MA, Noble WD, Sibai BM, et al. The relationship between resolution of asymptomatic bacterial vaginosis and spontaneous preterm birth in fetal fibronectin‐positive women. American Journal of Obstetrics and Gynecology 2007;197(5):488.e1‐488.e5. [DOI] [PubMed] [Google Scholar]
- Lin M, for the NICHD MFMU Network. Impact of quantitative fetal fibronectin (FFN) change of efficacy of antibiotic vs. placebo treatment of asymptomatic FFN positive women to prevent subsequent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S56. [Google Scholar]
- Ramsey P. Relationship of midtrimester fetal fibronectin (FFN) concentration to antibiotic efficacy for the prevention of spontaneous preterm birth in asymptomatic FFN positive women [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S11. [Google Scholar]
Andrews 2006 {published data only}
- Andrews W, Goldenberg R, Hauth J, Cliver S. Interconceptional antibiotics to prevent spontaneous preterm birth (SPTB): a randomized trial [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):s57. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2006;194(3):617‐23. [DOI] [PubMed] [Google Scholar]
- Tita A, Cliver S, Goepfert A, Goldenberg R, Conner M, Andrews W. Impact of interconceptional antibiotics on the "endometrial flora". American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S17. [Google Scholar]
- Tita A, Cliver S, Goepfert A, Goldenberg R, Conner M, Andrews W. Interconceptional antibiotics and preterm delivery: subgroup analyses. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S76. [DOI] [PubMed] [Google Scholar]
- Tita AT, Cliver SP, Goepfert AR, Conner M, Goldenberg RL, Hauth JC, et al. Impact of interconception antibiotics on the endometrial microbial flora. American Journal of Obstetrics and Gynecology 2007;196(3):226.e1‐226.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tita AT, Cliver SP, Goepfert AR, Conner M, Goldenberg RL, Hauth JC, et al. Clinical trial of interconceptional antibiotics to prevent preterm birth: subgroup analyses and possible adverse antibiotic‐microbial interaction. American Journal of Obstetrics and Gynecology 2007;197(4):367.e1‐6. [DOI] [PubMed] [Google Scholar]
Audebert 1989 {published data only}
- Audebert AJM. Use of polygynax in pregnant women for preventing cervico‐vaginal infections. Revue Francaise de Gynecologie et d Obstetrique 1989;84:287‐90. [PubMed] [Google Scholar]
Goldenberg 2006 {published data only}
- Aboud S, Msamanga G, Read JS, Wang L, Mfalila C, Sharma U, et al. Effect of prenatal and perinatal antibiotics on maternal health in Malawi, Tanzania, and Zambia. International Journal of Gynecology & Obstetrics 2009;107(3):202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R, Andrews W, Taha T, Fawzi W, Brown E. HIVNET 024: fetal fibronectin (FFN), preterm birth (PTB) and mother to child transmission of HIV (MTCT) in African women with HIV [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S52. [Google Scholar]
- Goldenberg R, Mudenda V, Brown E, Taha T, Kaaya E. HIVNET 024: neutrophilic and mononuclear placental infiltration, antibiotics and outcome in an African population [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S188. [Google Scholar]
- Goldenberg R, Taha T, Hoffman I, Fawzi W, Mwatha A. HPTN 024: antibiotics do not prevent preterm birth (PTB) in an HIV‐infected African population [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S3. [Google Scholar]
- Goldenberg RL, Mudenda V, Read JS, Brown ER, Sinkala M, Kamiza S, et al. HPTN 024 study: histologic chorioamnionitis, antibiotics and adverse infant outcomes in a predominantly HIV‐1‐infected African population. American Journal of Obstetrics & Gynecology 2006;195(4):1065‐74. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Mwatha A, Read JS, Adeniyi‐Jones S, Sinkala M, Msmanga G, et al. The HPTN 024 study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. American Journal of Obstetrics and Gynecology 2006;194(3):650‐61. [DOI] [PubMed] [Google Scholar]
- Kafulafula G, Mwatha A, Chen YQ, Aboud S, Martinson F, Hoffman I, et al. Intrapartum antibiotic exposure and early neonatal, morbidity, and mortality in Africa. Pediatrics 2009;124(1):e137‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub‐Saharan Africa does not appear to be associated with low birth weight or preterm birth. South African Medical Journal 2010;100(1):58‐64. [PMC free article] [PubMed] [Google Scholar]
- Taha TE, Brown ER, Hoffman IF, Fawzi W, Read JS, Sinkala M, et al. A phase III clinical trial of antibiotics to reduce chorioamnionitis‐related perinatal HIV‐1 transmission. AIDS 2006;20(9):1313‐21. [DOI] [PubMed] [Google Scholar]
Gray 2001 {published data only}
- Gray RH, Wabwire‐Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. American Journal of Obstetrics & Gynecology 2001;185:1209‐17. [DOI] [PubMed] [Google Scholar]
- Kigozi GG, Brahmbhatt H, Wabwire‐Mangen F, Wawer MJ, Serwadda D, Sewankambo N, et al. Treatment of trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. American Journal of Obstetrics and Gynecology 2003;189:1398‐400. [DOI] [PubMed] [Google Scholar]
Larsson 2006 {published data only}
- Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U, the premature study group of the southeast health care region of Sweden. Late miscarriage and preterm birth after treatment with clindamycin: a randomised consent design study according to Zelen. BJOG: an international journal of obstetrics and gynaecology 2006;113(6):629‐37. [DOI] [PubMed] [Google Scholar]
Luntamo 2010 {published data only}
- Ashorn P. Gestational sulfadoxine‐pyrimethamine and azithromycin treatment to prevent preterm birth (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
- Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Tropical Medicine & International Health 2013;18(4):386‐97. [DOI] [PubMed] [Google Scholar]
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine‐ pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2010;83(6):1212‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 1995 {published data only}
- Peter MT, Brown CEL, Buam A, Risser R. Randomized, double‐blind, placebo‐controlled trial of amoxycillin/clavulanic acid to prevent preterm delivery in twin gestation. Infectious Diseases in Obstetrics and Gynecology 1995;3:158‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shennan 2006 {published data only}
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Minimal quantitative levels of fetal fibronectin (1‐49 NG/ML) at 24‐27 weeks are associated with an increased risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S47. [DOI] [PubMed] [Google Scholar]
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Quantitative fetal fibronectin screening at 24 weeks substantially discriminates the risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. [DOI] [PubMed] [Google Scholar]
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET study. BJOG: an international journal of obstetrics and gynaecology 2006;113(1):65‐74. [DOI] [PubMed] [Google Scholar]
- Thompson C. Taken before their time. Nursing Times 2002;98(3):29. [Google Scholar]
Tripathi 2008 {published data only}
- Gupta S, Tripathi R, Singh N, Bhalla P, Ramji S, Mala YM. Pregnancy outcome in asymptomatic women with abnormal vaginal flora without any treatment and after treatment with vaginal clindamycin and clotrimazole: a randomised controlled trial. South African Journal of Obstetrics and Gynaecology 2013;19(2):35‐8. [Google Scholar]
- Tripathi R, Gupta S, Bhalla P, Mala YM, Tyagi S, Ramji S. Maternal and fetal outcome in patients with abnormal vaginal flora after treatment with vaginal clindamycin in early pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):161. [Google Scholar]
Unger 2015 {published data only}
- Rathjen P. Intermittent preventive treatment with azithromycin‐containing regimens in pregnant women in Papua New Guinea (IPTp in PNG). ClinicalTrials.gov (http://clinicaltrials.gov) [accessed April 2013) 2010.
- Unger HW, Ome‐Kaius M, Wangnapi RA, Umbers AJ, Hanieh S, Suen CS, et al. Sulphadoxine‐pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Medicine 2015;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Hoffman 2013 {published data only}
- Hoffman M. Clindamycin to reduce preterm birth in a low resource setting. ClinicalTrials.gov (accessed 21 May 2013) 2013.
Additional references
Ashorn 2006
- Ashorn P. Gestational sulfadoxine‐pyrimethamine and azithromycin treatment to prevent preterm birth (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Braun 1971
- Braun P, Lee Y, Klein JO, Marcy M, Klein TA, Charles D. Birth weight and genital mycoplasma in pregnancy. New England Journal of Medicine 1971;284:167‐74. [DOI] [PubMed] [Google Scholar]
Cunningham 1997
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC, Hankins GDV, et al. Williams Obstetrics. 20th Edition. Norwalk, CT: Appleton & Lange, 1997. [Google Scholar]
Eschenbach 1991
- Eschenbach DA, Nugent RP, Rao VA, Cotch MF, Gibbs RS, Lipscomb KA, et al. A randomized placebo controlled trial of erythromycin for the treatment of ureaplasma urealyticum in preventing premature delivery. American Journal of Obstetrics and Gynecology 1991;164:734‐42. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Gravett 1986
- Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 1986;256:1899‐905. [PubMed] [Google Scholar]
Hardy 1984
- Hardy PH, Nell EE, Spence MR, Hardy JB, Graham DA, Rosenbaum RC. Prevalence of six sexually transmitted disease agents among pregnant innercity adolescents and pregnancy outcome. Lancet 1984;8:333‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillier 1995
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of low‐birth weight infant. New England Journal of Medicine 1995;333:1737‐42. [DOI] [PubMed] [Google Scholar]
Kenyon 2008
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet 2008;372(9646):1319‐27. [DOI] [PubMed] [Google Scholar]
Kurtzman 2008
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Quantitative fetal fibronectin screening at 24 weeks substantially discriminates the risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. [DOI] [PubMed] [Google Scholar]
Lettieri 1993
- Lettieri L, Vintzileos AM, Rodis JF, Albini SM, Saladia CM. Does idiopathic preterm labor resulting in preterm birth exist?. American Journal of Obstetrics and Gynecology 1993;168:1480‐5. [DOI] [PubMed] [Google Scholar]
Lin 2005
- Lin M, for the NICHD MFMU Network. Impact of quantitative fetal fibronectin (FFN) change of efficacy of antibiotic vs. placebo treatment of asymptomatic FFN positive women to prevent subsequent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S56. [Google Scholar]
McCormack 1987
- McCormack WM, Rosner B, Lee YH, Munoz A, Kass CD. Effect on birth weight of erythromycin in treatment of pregnant women. Obstetrics & Gynecology 1987;69:202‐7. [PubMed] [Google Scholar]
Morales 1994
- Morales JW, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo controlled double blind study. Obstetrics & Gynecology 1994;171:345‐9. [DOI] [PubMed] [Google Scholar]
Newton 1989
- Newton ER, Dinsmoor MJ, Gibbs RS. A randomised blinded placebo controlled trial of antibiotics in idiopathic preterm labour. Obstetrics & Gynecology 1989;74:562‐6. [PubMed] [Google Scholar]
Oleszczuk 2000
- Oleszczuk JJ, Keith LG. Vaginal infection: prophylaxis and perinatal outcome‐a review of the literature. International Journal of Fertility 2000;45(6):358‐67. [PubMed] [Google Scholar]
Rathjen 2010
- Rathjen P. Intermittent preventive treatment with azithromycin‐containing regimens in pregnant women in Papua New Guinea (IPTp in PNG). ClinicalTrials.gov (http://clinicaltrials.gov) [accessed April 2013) 2010.
Regan 1981
- Regan JA, Chao S, James SL. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. American Journal of Obstetrics and Gynecology 1981;141:184‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 1988
- Romero R, Mazor M. Infection and preterm labor. Clinical Obstetrics and Gynecology 1988;31:553‐83. [DOI] [PubMed] [Google Scholar]
Romero 1993
- Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, et al. Antibiotic treatment of preterm labour with intact membranes: a multicenter randomised double blinded controlled trial. American Journal of Obstetrics and Gynecology 1993;169:764‐74. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thinkhamrop 2002
- Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration during second and third trimester in pregnancy for preventing infectious morbidity and mortality. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002250] [DOI] [PubMed] [Google Scholar]
Thinkhamrop 2015a
- Thinkhamrop J, Hofmeyer GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD002250.pub2] [DOI] [PubMed] [Google Scholar]


