Abstract
Bullous pemphigoid (BP), a chronic autoimmune subepidermal blistering skin disease, has been described in end-stage renal disease patients requiring dialysis after the placement of an artero-venous fistula. We report a case of a novel onset of BP following a peritoneal dialysis abdominal Tenckhoff catheter placement. The 3-month treatment with systemic doxycycline and topical clobetasol propionate allowed a rapid disappearing of the blisters and left the patient free of symptoms in the follow-up. To our knowledge, this is the first case describing a new BP onset after a peritoneal dialysis catheter placement.
Keywords: Bullous pemphigoid, Peritoneal dialysis, Haemodialysis, End-stage renal disease, Tenckhoff catheter
Introduction
Bullous pemphigoid (BP), a chronic autoimmune subepidermal blistering skin disease, has been described in end-stage renal disease patients requiring dialysis after the placement of an artero-venous fistula. We report a case of a novel onset of BP following a peritoneal dialysis abdominal Tenckhoff catheter placement.
Case Report
We present the case of a 76-year-old Caucasian patient with a diagnosis of BP. The clinical history is remarkable for a patient with end-stage renal disease on renal replacement therapy with automated peritoneal dialysis, diabetes mellitus known for 15 years, and arterial hypertension. Two months before the diagnosis the patient developed numerous bullous lesions (mean diameter of 3 cm) in the abdominal region surrounding the exit site of the Tenckhoff catheter. The lesions were very pruritic, with a serous content, and in differential diagnosis we considered traumatic lesions or a contact dermatitis due to the plaster used for the catheter medication. Despite the change of the plaster and the education to the patient not to scratch, we observed the development of several new lesions always in the same area (Fig. 1a). We performed a skin biopsy that described a subepithelial split with a lymphohistiocytic infiltrate with abundant eosinophils. Direct immunofluorescence identified linear deposits of IgG and C3 along the basement membrane zone. Indirect immunofluorescence performed on the salt-split skin disclosed linear staining of circulating IgG on the epidermal side of the artificial blister. Moreover, the patient's serum revealed positivity for anti-BP180 NC16A antibodies (ELISA) while the anti-BP230 antibody was also detectable but below the positivity threshold (7.1 U/mL; normal values <9 U/mL). A diagnosis of BP was made according to both the 2015 European Dermatology Forum consensus recommendations [1] and the 2015 German guidelines [2], as well as to the recent criteria proposed by Meijer et al. [3].
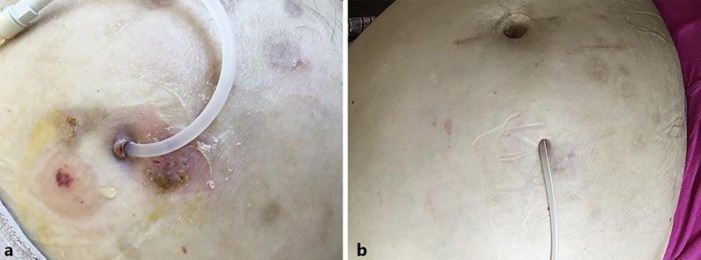
Fig. 1.
Patient with bullous pemphigoid. Burst tense blisters located on the abdominal wall in the area surrounding the exit site of the Tenckhoff catheter before treatment (a) and after treatment (b).
The patient was treated with doxycycline 100 mg twice a day and topical 0.05% clobetasol propionate twice a day; doxycycline was tapered after 6 weeks and discontinued after 8 weeks, while clobetasol propionate was discontinued after 12 weeks. The blisters and erosions disappeared within 2 weeks (Fig. 1b). The patient has remained free of symptoms in the 18 months of follow-up.
Discussion
BP is a rare autoimmune condition, more common in older patients, with a not completely defined pathophysiology; some cases have been deemed as secondary to drug exposure (like loop diuretics and gliptins, also called dipeptidyl peptidase-4 − DPP4 − inhibitors) or secondary to skin injuries induced by trauma or surgical procedures. The prodromal phase can last from weeks to years. In this case, our patient was treated with linagliptin and torasemide for many years, while no other plausible drug has been recently introduced. An abdominal Tenckhoff catheter was placed 12 months before the onset of the bullous lesions.
Patients with end-stage renal disease requiring dialysis show a high prevalence of diabetes mellitus [4]. Due to the risk of drug accumulation and therefore of severe hypoglycaemic episodes, the use of many oral hypoglycaemic drugs, like metformin and sulphonylureas, is precluded. Therefore, most of these patients are treated with gliptins that competitively inhibit an enzyme (DDP4 or CD26), ubiquitously expressed in almost every organ system including the skin, that acts by preventing glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) inactivation, thus increasing the secretion of insulin and suppressing the release of glucagon by the pancreas. In the last years there is growing epidemiological evidence of the association between BP onset and gliptin therapy [5], which could represent, like diabetes mellitus, a risk factor rather than an aetiological factor. Despite the large percentage of dialysis patients exposed to gliptins, BP remains a rare disease, leaving unsolved the question of whether the correlation between gliptins and BP is causative or simply a random association.
Furosemide was first associated with BP in 1969 [6] and several similar cases have been reported since then. Yet the aetiological association has so far not been established, nor the pathophysiological mechanism explained. Moreover, almost all dialysis patients with a residual renal function, thus a residual capacity to produce urine, are treated with loop diuretics (torasemide, furosemide), highlighting the same difficulty in differentiating between a random and an aetiological association with BP. It is also noteworthy that our patient was treated with torasemide because, even potentially considering the association between furosemide and BP a class effect of all loop diuretics, to date no case has been reported associating the use of torasemide with BP onset. The current guidelines advise stopping the therapy with loop diuretics [2] at least temporarily after the diagnosis of BP, but in our case the risk of volume overload was considered too high and therapy was continued. In most cases where an association between gliptins or loop diuretics and BP has been established, the drug had to be discontinued to obtain the resolution of BP or avoid the recurrence of the disease [7, 8, 9].
Therefore, in our case, considering the area of skin involved by the lesions, the good response to the therapy without discontinuing the aforementioned drugs, the timing of onset of the lesions, and the absence of recurrence of the disease 18 months after the discontinuation of the BP therapy, we hypothesize a correlation with the peritoneal catheter placement. Retrospectively, the patient developed papular lesions in the legs 1 year previously; a punch biopsy showed an aspecific pattern of lymphocytic infiltrate with eosinophils, deemed as a chronic lymphocytic vasculitis. We suppose that these lesions were already due to the pemphigoid and that the catheter placement together with the skin injury due to the daily plaster removal for the medication of the exit site could have represented a traumatic “second-hit”. In the literature 6 cases are described [10] of new BP onset after the placement of an artero-venous fistula. To the best of our knowledge, this is the first case describing a new BP onset after a peritoneal dialysis catheter placement.
Statement of Ethics
The subject of the case report gave his written informed consent to publish his case (including publication of images). The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Disclosure Statement
The author has no conflicts of interest to declare.
Funding Sources
The author has no funding sources to disclose.
Author Contributions
Davide Giunzioni treated the patient and wrote this article.
Acknowledgements
The author thanks Prof. Katarzyna Woźniak, from the Department of Dermatology and Immunodermatology of the Medical University of Warsaw, for her help in the diagnosis and treatment of the patient. She received no grant for her contribution.
References
- 1.Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015 Apr;172((4)):867–77. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E, Goebeler M, Hertl M, Sárdy M, Sitaru C, Eming R, et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015 Jul;13((7)):713–27. doi: 10.1111/ddg.12612. [DOI] [PubMed] [Google Scholar]
- 3.Meijer JM, Diercks GF, de Lang EW, Pas HH, Jonkman MF. Assessment of Diagnostic Strategy for Early Recognition of Bullous and Nonbullous Variants of Pemphigoid. JAMA Dermatol. 2019 Feb;155((2)):158–65. doi: 10.1001/jamadermatol.2018.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer A, Pippias M, Noordzij M, Stel VS, Andrusev AM, Aparicio-Madre MI, et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J. 2019 Feb;12((5)):702–20. doi: 10.1093/ckj/sfz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Front Immunol. 2019 Jun;10:1238. doi: 10.3389/fimmu.2019.01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebringer A, Adam WR, Parkin JD. Bullous haemorrhagic eruption associated with frusemide. Med J Aust. 1969 Apr;1((15)):768–71. doi: 10.5694/j.1326-5377.1969.tb105512.x. [DOI] [PubMed] [Google Scholar]
- 7.Attaway A, Mersfelder TL, Vaishnav S, Baker JK. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors. A case report and review of literature. J Dermatol Case Rep. 2014 Mar;8((1)):24–8. doi: 10.3315/jdcr.2014.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magdaleno-Tapial J, Valenzuela-Oñate C, Esteban Hurtado Á, Ortiz-Salvador JM, Subiabre-Ferrer D, Ferrer-Guillén B, et al. Association Between Bullous Pemphigoid and Dipeptidyl Peptidase 4 Inhibitors: A Retrospective Cohort Study. Actas Dermosifiliogr. 2019 Dec;7310((19)):30365–5. doi: 10.1016/j.ad.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018 Oct;154((10)):1152–8. doi: 10.1001/jamadermatol.2018.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osipowicz K, Kalinska-Bienias A, Kowalewski C, Wozniak K. Development of bullous pemphigoid during the haemodialysis of a young man: case report and literature survey. Int Wound J. 2017 Feb;14((1)):288–92. doi: 10.1111/iwj.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]