Abstract
We report a case of an adult patient suffering from leukoencephalopathy with brainstem and spinal cord involvement and elevated white matter lactate (LBSL) caused by a DARS2 polymorphism. DARS2 mutation was identified by combining MRI and genetic analysis. Our patient was affected by compound heterozygosity for a pathogenic mutation and a common variant, but with reduced aspartyl-tRNA synthetase activity. Brain and spinal cord magnetic resonance imaging revealed extensive white matter abnormalities; spectroscopy revealed no lactate elevation. A new compound heterozygous DARS2 variant combined with a polymorphism in the other allele in an adult patient with LBSL was identified, resulting in reduced DARS2 activity. This combination is rare and has consequences on how we should consider benign variant polymorphisms in the future.
Keywords: DARS2, LBSL, Adult leukodystrophy, Polymorphism
Introduction
Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL) is a rare autosomal recessive disease caused by mutations in the DARS2 gene that encodes mitochondrial aspartyl-tRNA synthetase [1]. Almost all the patients are compound heterozygous for two DARS2 mutations and one of them is almost invariably a splice site mutation in intron 2, upstream of exon 3 [1]. The prevalence data are scarce and conflicting [2], but LBSL is present in 2.6% of general population according the Exome Aggregation Consortium (ExAC) database [3].
LBSL is most often a relatively mild disorder, characterized by juvenile onset of slowly progressive ataxia, cerebellar, pyramidal, spasticity, and dorsal column dysfunction [2, 4]. However, the clinical course of LBSL is not uniform, and there is a lack of longitudinal data on these patients [2]. LBSL is defined based on characteristic findings observed on magnetic resonance imaging (MRI) such as inhomogeneous abnormalities of the periventricular cerebral white matter and specific involvement of brainstem and spinal cord tracts [1, 5, 6]. Lactate elevation is a common but not uniform finding in the affected white matter on spectroscopy [7]. Currently, there is no treatment available for LBSL.
To our knowledge, we report here a new DARS2 mutation discovered in an adult patient with LBSL presenting compound heterozygosity for a novel pathogenic mutation and a common so far considered benign variant. Aspartyl-tRNA synthetase activity in mitochondria was reduced.
Methods
The patient has been followed since July 2014 for walking disorders. The brain and medullar MRIs were performed at the Hôpital Erasme, Brussels, and the Centre Hospitalier de Wallonie picarde, Tournai, Belgium. The DNA of the mitochondrial aspartyl-tRNA synthetase (DARS2) gene and its functional characteristics were investigated in our patient and her mother at the VU Medical Center, Amsterdam, the Netherlands. Appropriate informed consent was obtained.
Results
Clinical Presentation
Our patient (female), with no significant medical history except a treated hypertension and one episode of gestational diabetes at her last pregnancy, was referred to investigations, at the age of 45 years, because of tingling sensations in the legs for the last 3 years, increasing with time. At the first consultation, she also had the following complaints: troubles to synchronize movements; while walking, she experienced bilateral weakness in the legs with pain or a sensation of tightness when walking more than 15 min that limited the capacity to walk and also stand; episodes described as vertigo for the last 3 months, mainly with closed eyes, with postural instability limiting the standing capacity; and finally, urinary urgency for the last 6 months.
Clinical examination revealed bilateral exhaustible horizontal gaze nystagmus when looking to the left, no dysarthria nor dysphasia, light bilateral dysmetria at finger-nose and heel-knee testing, positive Romberg with open eyes, worsening with closed eyes, dysdiadochokinesia, loss of vibration sensation in right leg and decreased in left leg. The rest of the sensory examination, including touch, pain and proprioception, was within normal limits. Tendon reflexes were at 3/4 symmetrically, only Achilles tendon reflexes were at 1/4 symmetrically. Plantar reflexes were downgoing. She had a normal gait.
A spastic paraparesis with leg cramps, but no urinary incontinence progressively appeared during follow-up. The Mini-Mental State Examination score was 28/30, revealing no cognitive disorders.
Electromyography revealed a sensorimotor axonal polyneuropathy. Plasma lactate was not investigated, while the cerebrospinal fluid analysis was normal with normal lactate (1.6 mmol/L). At the end of 2014, we decided to stop the diabetes therapy initiated by the general practitioner, and a diabetic diet was initiated in addition to physiotherapy.
DARS2 Mutations
Because LBSL was suspected, the DARS2 gene was sequenced, and two variants were identified, the novel variant c.374G>A (p.Arg125His) and the known variant that is considered benign (c.1013G>A, p.Gly338Glu) (rs141298312). Patient mother's DNA was sequenced and revealed the same mutation c.374G>A (p.Arg125His) but not the “benign” variant, and she does not suffer from LBSL. DNA from the father was not available for testing. Due to the fact that on the second allele only the “benign variant” was detected, aspartyl-tRNA synthetase (DARS2) activity was measured in mitochondria isolated from lymphoblasts from the index patient. DARS2 activity was 14% of a healthy control. To confirm that the c.1013G>A results in decreased DARS2 activity, HEK293 cells were transfected with the wild-type DARS2 and with the DARS2 containing the polymorphism. Determination of DARS2 activity in lysates of the transfected HEK293 cells showed an enzymatic activity for the polymorphism of 53% of the wild type.
MRI Abnormalities
Brain and spinal cord MRI revealed extensive white matter abnormalities (Fig. 1); spectroscopy revealed no lactate elevation (Fig. 2). A brain and medullar control MRI were performed in 2017 (Fig. 3) because of increasing complaints of paraparesis and cramps. However, the patient is clinically stable. The medullar MRI images are not displayed in Figure 3 because they showed no change in comparison to 2014.
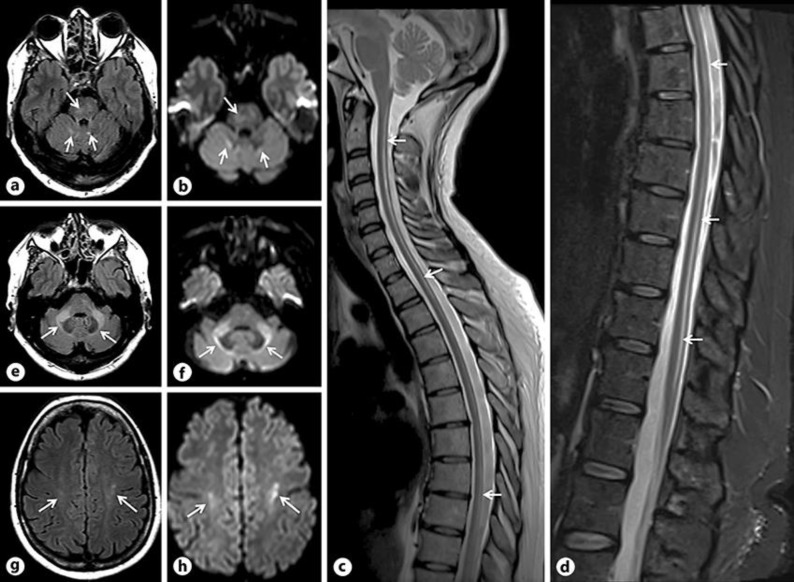
Fig. 1.
First brain and spinal cord MRI from 2014. On axial T2-FLAIR (a, e, g) and DWI (b, f, h), hyperintensities are seen in the basis pontis (a, b, long arrows), in the deep cerebellar white matter at the level of the superior cerebellar peduncles (a, b, short arrows), in the middle cerebellar peduncles, extending deeply to surround the dentate nuclei (e, f, arrows), and in the central white matter at the level of the central semi-ovale. On sagittal T2 (c) and T2-fat sat (d) spinal cord images, hyperintensities are seen, involving the dorsal columns along the entire cord (arrows).
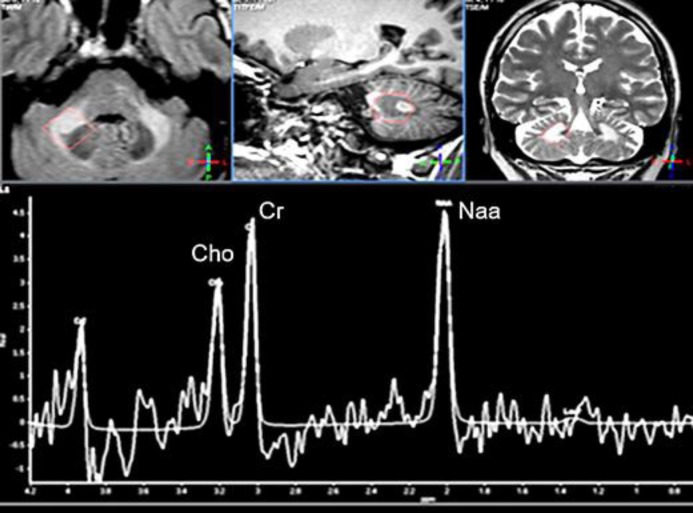
Fig. 2.
Cerebellar spectroscopy images obtained in 2015. The voxel is acquired in the right deep middle cerebellar peduncle (see anatomical images in the upper row). The spectrum shows obviously decreased NAA/Cr and Cho/Cr ratios, reflecting the neuronal loss or dysfunction as well as the impaired myelin turnover.
Fig. 3.
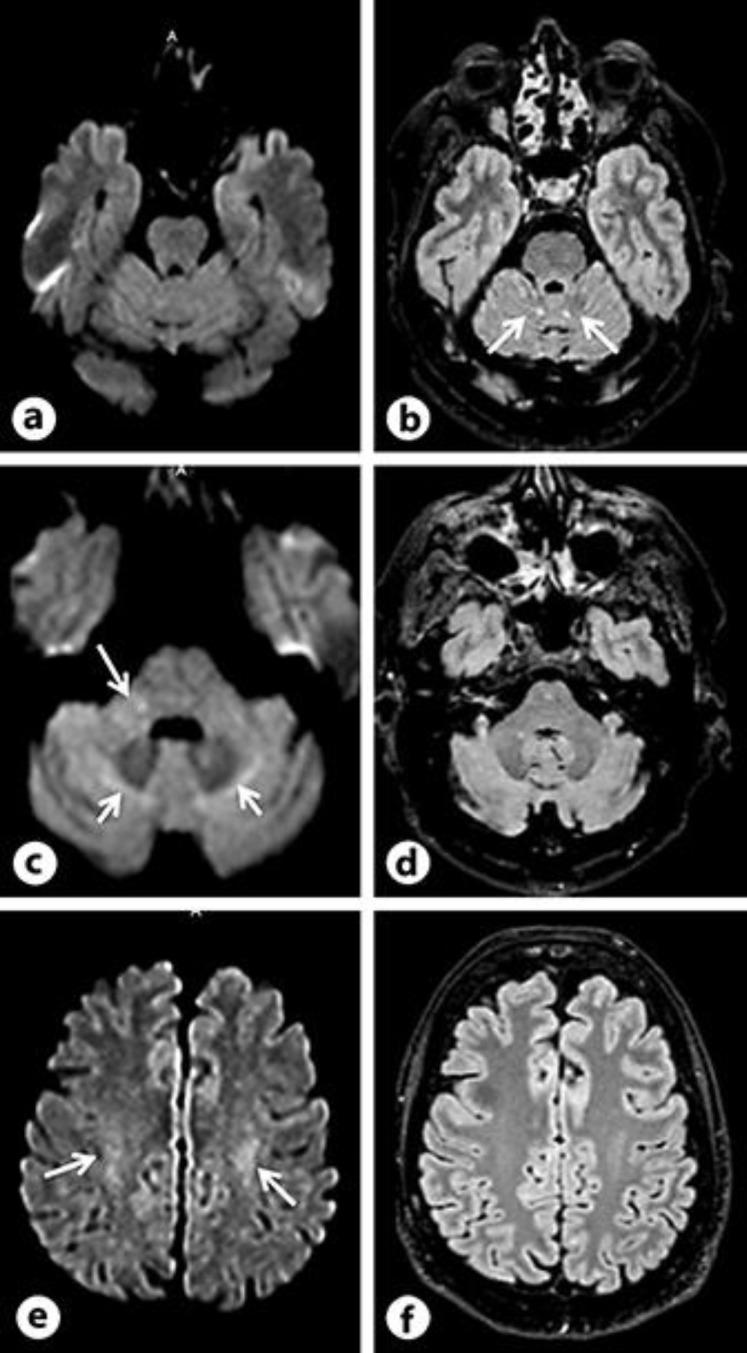
Brain control MRI from 2017. Notice the regression of white matter signal abnormalities as compared to the initial MRI both on DWI (a, c, e) and FLAIR (b, d, f) images. Some FLAIR and DWI hyperintensities are still seen, at the level of the superior cerebellar peduncles (b, arrows), the middle cerebellar peduncles (c, long arrows), around the dentate nuclei (c, short arrows), and in the central semi ovale white matter (e, arrows), but overall, images have improved.
Discussion
Most LBSL patients have compound heterozygous DARS2 mutations [1], but homozygous DARS2 mutations may also cause the disease [8]. The clinical course of LBSL is variable. While the mean age at onset is 8 years (range 0.4–40 years) [2], our patient reached adult age without any clinical symptoms until the age of 43 years. Early infantile, severe form has been reported with some patients becoming wheelchair-dependent in their late teens or early 20s [9] and some dying before reaching adult age.
The diagnosis of LBSL is based on the characteristic findings in brain and spinal cord MR imaging. The severity of these findings does not seem to correlate with the clinical course of the disease [2]. These studies suggest that compound heterozygosity for the pathogenic mutation and the “benign variant” cause LBSL in our patient. In our case, the regression of white matter abnormalities (Fig. 3) could be attributed to withdrawal of oral antidiabetic drugs.
In conclusion, we report a new compound heterozygous DARS2 variant combined with a polymorphism in the other allele in an adult patient with LBSL, resulting in reduced DARS2 activity.
This combination has caused disease in our patient, which reveals the importance of now taking into consideration the benign variant. Furthermore, this shows the importance of functional enzymatic analysis.
Statement of Ethics
The patient provided both oral and written informed consent for the publishing of this report.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding support.
Data Availability Statement
The authors state that anonymized data will be shared by request from any qualified investigator.
Author Contributions
N.N.I.R. followed the patient, examined the MRI images, contributed to the diagnosis, physical examination, and testing of the patient and wrote the paper. M.C. contributed to the writing and revision of the manuscript. J.P. provided the figures, their legends. L.M. achieved the MRI protocols. V.I. contributed to the genetic analysis. All authors read and approved the final manuscript.
Acknowledgement
The authors thank the patient, Marjo van der Knaap from VU University Medical Center for advice, and the Amsterdam UMC Metabolic Laboratory for the genetic analysis.
References
- 1.Scheper GC, van der Klok T, van Andel RJ, van Berkel CG, Sissler M, Smet J, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007 Apr;39((4)):534–9. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 2.van Berge L, Hamilton EM, Linnankivi T, Uziel G, Steenweg ME, Isohanni P, et al. LBSL Research Group Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain. 2014 Apr;137((Pt 4)):1019–29. doi: 10.1093/brain/awu026. [DOI] [PubMed] [Google Scholar]
- 3. http://exac.broadinstitute.org/
- 4.Alibas H, Koytak PK, Ekinci G, Uluc K. A case with leukoencephalopathy with brainstem and spinal cord involvement and elevated lactate (LBSL) with Its Characteristic Clinical and Neuroimaging Findings. Clin Neuroradiol. 2014 Sep;24((3)):297–300. doi: 10.1007/s00062-013-0250-x. [DOI] [PubMed] [Google Scholar]
- 5.van der Knaap MS, van der Voorn P, Barkhof F, Van Coster R, Krägeloh-Mann I, Feigenbaum A, et al. A new leukoencephalopathy with brainstem and spinal cord involvement and high lactate. Ann Neurol. 2003 Feb;53((2)):252–8. doi: 10.1002/ana.10456. [DOI] [PubMed] [Google Scholar]
- 6.Steenweg ME, Pouwels PJ, Wolf NI, van Wieringen WN, Barkhof F, van der Knaap MS. Leucoencephalopathy with brainstem and spinal cord involvement and high lactate: quantitative magnetic resonance imaging. Brain. 2011 Nov;134((Pt 11)):3333–41. doi: 10.1093/brain/awr254. [DOI] [PubMed] [Google Scholar]
- 7.Martikainen MH, Ellfolk U, Majamaa K. Impaired information-processing speed and working memory in leukoencephalopathy with brainstem and spinal cord involvement and elevated lactate (LBSL) and DARS2 mutations: a report of three adult patients. J Neurol. 2013 Aug;260((8)):2078–83. doi: 10.1007/s00415-013-6940-0. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita S, Miyake N, Matsumoto N, Osaka H, Iai M, Aida N, et al. Neuropathology of leukoencephalopathy with brainstem and spinal cord involvement and high lactate caused by a homozygous mutation of DARS2. Brain Dev. 2013 Apr;35((4)):312–6. doi: 10.1016/j.braindev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Steenweg ME, van Berge L, van Berkel CG, de Coo IF, Temple IK, Brockmann K, et al. Early-onset LBSL: how severe does it get? Neuropediatrics. 2012 Dec;43((6)):332–8. doi: 10.1055/s-0032-1329395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that anonymized data will be shared by request from any qualified investigator.