Abstract
Since the incidence of mucosal melanoma is higher in the Japanese population compared to Caucasians, and since mucosal melanoma possesses a lower mutation burden compared to cutaneous melanoma, the efficacy of anti-PD1 antibody (Ab) monotherapy for mucosal melanoma is limited. Therefore, other targeting molecules that enhance the anti-tumor effects of immune checkpoint inhibitors are needed. In this report, we present a case with anti-PD1 Ab-resistant recurrent malignant melanoma of the nasal cavity successfully treated with nivolumab, ipilimumab plus denosumab combination therapy.
Keywords: Mucosal melanoma, Bone metastasis, Nivolumab plus ipilimumab combination therapy, Denosumab
Introduction
Since the incidence of mucosal melanoma is higher in the Japanese population compared to Caucasians [1], and since mucosal melanoma possesses a lower mutation burden compared to cutaneous melanoma [2], the efficacy of anti-PD1 antibody (Ab) monotherapy for mucosal melanoma is limited [3, 4]. In addition, the efficacy of ipilimumab monotherapy in patients with nivolumab-resistant melanoma is extremely low after objective tumor progression [5]. Therefore, other targeting molecules that enhance the anti-tumor effects of immune checkpoint inhibitors (ICIs) are needed. In this report, we present a case with anti-PD1 Ab-resistant recurrent malignant melanoma of the nasal cavity successfully treated with nivolumab, ipilimumab plus denosumab combination therapy.
Case Report
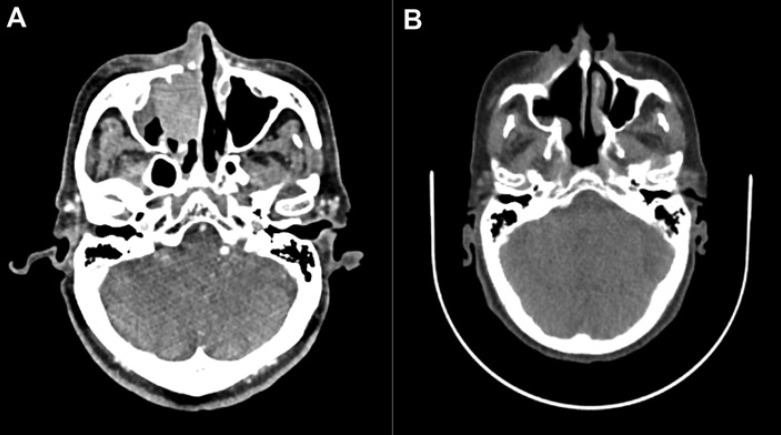
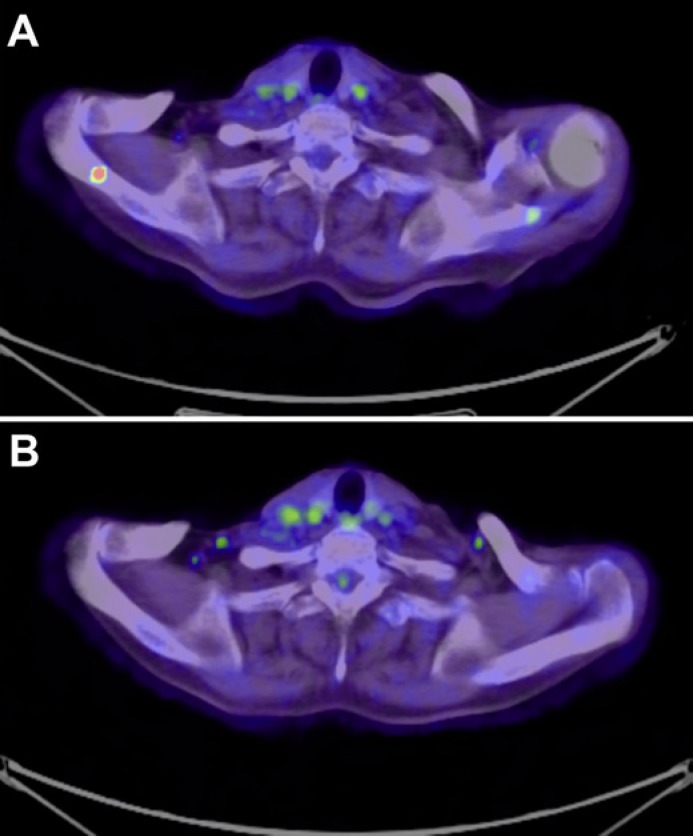
The patient was an 81-year-old Japanese woman described in the Journal of Dermatology in 2019 [6]. Twenty-one months after the administration of pembrolizumab monotherapy, follow-up computed tomography (CT) scan revealed the local recurrence of melanoma, 36.80 × 26.78 mm in size, in the nasal cavity (Fig. 1A). Since the recurrent tumor was limited to the nasal cavity, we employed intensity-modulated radiotherapy (IMRT) using CyberKnife with 45 Gy in 9 fractions. Two months after the radiotherapy, magnetic resonance imaging (MRI) revealed regression of the tumor (Fig. 1B). However, 3 months after tumor regression, follow-up positron emission tomography (PET)-CT revealed multiple metastases in the lungs, scapula, and subcutaneous lesions (Fig. 2A). Since the melanoma was BRAFV600E mutation negative, nivolumab (80 mg/kg/every 3 weeks) was given in combination with ipilimumab (3 mg/kg/every 3 weeks) for 4 cycles without any adverse events. In addition, since this patient showed metastatic melanoma of the bone, we administered denosumab 120 mg every month. Three months after the first administration of nivolumab plus ipilimumab combination therapy, the multiple metastases in the lungs, scapula, and subcutaneous lesions had regressed (Fig. 2B). We continued to administer pembrolizumab (240 mg/kg/every 3 weeks), and there was no evidence of recurrence 6 months after achieving complete remission.
Fig. 1.
CT scan before radiotherapy: local recurrence of melanoma, 36.80 × 26.78 mm in size, in the nasal cavity (A). MRI at 2 months after IMRT treatment: regression of the tumor (B).
Fig. 2.
PET-CT image: metastasis at the scapula before (A) and after (B) combination therapy.
Discussion
The combination or sequential administration of nivolumab and ipilimumab with a planned switch is among the most effective chemotherapies against advanced melanoma [7, 8], but the efficacy of ipilimumab monotherapy in patients with nivolumab-resistant cutaneous and mucosal melanoma is low after objective tumor progression compared to its efficacy in patients with planned-switched treatment [5, 9]. These reports suggested that the efficacy of nivolumab plus ipilimumab combination therapy in anti-PD1 Ab therapy-resistant patients is lower than that in anti-PD1 Ab therapy-naïve patients. In addition, recently, Hamid et al. [4] has reported the results of a case series with mucosal melanoma treated with pembrolizumab monotherapy. The objective response rate to pembrolizumab for ipilimumab therapy-naïve mucosal melanoma patients was 22%, suggesting a poor prognosis for mucosal melanoma compared to cutaneous melanoma patients [10]. Therefore, additional methods to enhance the anti-tumor effects of ICIs in patients with mucosal melanomas are needed.
To enhance the anti-tumor effects of anti-PD1 Abs, not only the induction of CD8+ T cells in the tumor lesion [11, 12], but also other targeting molecules that enhance the anti-tumor effects of ICIs should be taken into account [13]. Recently, Ahern et al. [14, 15] have highlighted the therapeutic effects of co-administration of anti-RANKL Abs with ICIs, such as anti-PD1 Abs and anti-CTLA4 Abs, against melanoma by the suppression of RANKL+ PD1highCD8 T cells in a B16F10 mouse melanoma model. They concluded that anti-RANKL Abs could enhance the anti-melanoma effects of ICIs. Indeed, in clinics, anti-RANKL Abs enhanced the therapeutic effects of ipilimumab in patients with terminal-stage metastatic melanoma [16, 17]. These reports suggested that denosumab might improve the therapeutic effects of nivolumab plus ipilimumab combination therapy against advanced anti-PD1 Ab-resistant mucosal melanoma.
In this report, we described a case of anti-PD1 Ab-resistant advanced mucosal melanoma treated with nivolumab, ipilimumab plus denosumab combination therapy. Our present case suggested that nivolumab, ipilimumab plus denosumab combination therapy is not only useful for conventional cutaneous melanoma as we previously reported [17], but also useful for recurrent anti-PD1 Ab-resistant mucosal melanoma as a second-line therapy.
Statement of Ethics
The patient gave written informed consent.
Disclosure Statement
The authors have no conflicting interests to declare.
Funding Sources
This study was supported in part by the Japan Agency for Medical Research and Development (19cm0106434h0002).
Author Contributions
Taku Fujimura designed the research study. Taku Fujimura, Yumi Kambayashi, Ohuchi Kentaro, Ryo Amagai, Sato Yota, Tanita Kayo, and Akira Hashimoto treated the patient and acquired the clinical data. Taku Fujimura wrote the manuscript. Taku Fujimura and Setsuya Aiba supervised the study.
References
- 1.Fujisawa Y, Yoshikawa S, Minagawa A, Takenouchi T, Yokota K, Uchi H, et al. Clinical and histopathological characteristics and survival analysis of 4594 Japanese patients with melanoma. Cancer Med. 2019;8:2146–56. doi: 10.1002/cam4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelo SP, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226–35. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Ribas A, Hodi FS, Walpole E, Daud A, et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: a post-hoc analysis of KEYNOTE-001, 002, 006. Br J Cancer. 2018;119:670–4. doi: 10.1038/s41416-018-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Uchi H, Fujimura T, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci. 2018;89:60–6. doi: 10.1016/j.jdermsci.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Fujimura T, Kambayashi Y, Sato Y, Tanita K, Tono H, Lyu C, et al. Successful treatment of unresectable recurrent programmed death ligand 1 moderately-expressing malignant melanoma of the nasal cavity with pembrolizumab monotherapy. J Dermatol. 2019;46:e260. doi: 10.1111/1346-8138.14786. [DOI] [PubMed] [Google Scholar]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17:943–55. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saijo K, Imai H, Ouchi K, Okada Y, Sato Y, Komine K, et al. Therapeutic benefits of ipilimumab among Japanese patients with nivolumab-refractory mucosal melanoma: a case series study. Tohoku J Exp Med. 2019;248:37–43. doi: 10.1620/tjem.248.37. [DOI] [PubMed] [Google Scholar]
- 10.Carlino MS, Long GV, Schadendorf D, Robert C, Ribas A, Richtig E, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur J Cancer. 2018;101:236–43. doi: 10.1016/j.ejca.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura T, Okuyama R, Ohtani T, Ito Y, Haga T, Hashimoto A, et al. Perilesional treatment of metastatic melanoma with interferon-beta. Clin Exp Dermatol. 2009;34:793–9. doi: 10.1111/j.1365-2230.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 12.Kakizaki A, Fujimura T, Furudate S, Kambayashi Y, Yamauchi T, Yagita H, et al. Immunomodulatory effect of peritumorally administered interferon-beta on melanoma through tumor-associated macrophages. Oncoimmunology. 2015;4:e1047584. doi: 10.1080/2162402X.2015.1047584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam PA, Verhoeven Y, Trinh XB, Wouters A, Lardon F, Prenen H, et al. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2019;133:85–91. doi: 10.1016/j.critrevonc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Ahern E, Harjunpää H, O'Donnell JS, Allen S, Dougall WC, Teng MWL, et al. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology. 2018;7:e1431088. doi: 10.1080/2162402X.2018.1431088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, et al. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res. 2017;23:5789–801. doi: 10.1158/1078-0432.CCR-17-0606. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Yagita H, McArthur GA. Combination anti-CTLA-4 and anti-RANKL in metastatic melanoma. J Clin Oncol. 2016;34:e104–6. doi: 10.1200/JCO.2013.51.3572. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida S, Fujimura T, Kambayashi Y, Amagai M, Hashimoto A, Aiba S. Successful treatment of multiple metastatic melanoma with nivolumab, ipilimumab plus denosumab combined therapy. Case Rep Oncol. 2019;12:829–33. doi: 10.1159/000504019. [DOI] [PMC free article] [PubMed] [Google Scholar]