Abstract
Background
Anaemia is a nearly universal complication of chronic kidney disease (CKD). Erythropoiesis-stimulating agents (ESAs) have been demonstrated to improve clinical outcomes and quality of life (QOL) in renal patients with anaemia. Patient-reported outcome measures (PROMs) are increasingly being used to evaluate the patient-centred impact of medical therapy. Here, we describe a systematic review of studies that evaluated patient-centred outcomes (PCOs) in renal patients undergoing anaemia treatment.
Methods
We conducted a search of Medline (Ovid), EMBASE (Ovid), PsychINFO, and CINAHL databases for studies published until March 2018 that investigated an intervention to treat anaemia in renal patients and used at least one PROM. We also performed a quality assessment for all included studies. Statistical analyses characterized each study, PROMs used, the quality of PCO reporting, and the association between haematological outcomes and PCOs.
Results
Of the 3,533 studies identified in the database search, 21 met all eligibility criteria. Fourteen (67%) of the studies were randomized-controlled trials. Most studies (81%) investigated CKD patients, 14% investigated post-renal transplant patients and 5% assessed patients with heart disease on haemodialysis. The most common anaemia intervention, used in 95% of studies, was ESAs. Forty-three percent of studies utilized one PROM, most commonly the SF-36, a measure of QOL not specifically created for use in nephrology patients. About a third of studies selectively reported PROM subscales, rather than reporting all subscales. Notable biases among included studies included lack of blinding, selective outcome reporting, and lack of power estimates for PCOs. We did not find a statistically significant association between improvements in haemoglobin and QOL.
Conclusions
Future studies employing anaemia and nephrology-specific PROMs and conducted with greater rigour, standardization in the research methods, and reporting of PCOs in renal populations will improve understanding of PCOs in this patient group and hopefully improve patient outcomes and experiences.
Keywords: Anaemia, Chronic kidney disease, Patient-centred outcomes, Quality of life, Systematic review
Introduction
Anaemia is a nearly universal sequela of chronic kidney disease (CKD) typically due to underproduction of erythropoietin, although other aetiologies may also be present [1, 2, 3]. In CKD, anaemia is independently associated with a heightened risk of cardiovascular disease [4], increase in hospitalizations, mortality, and reduced quality of life (QOL) [5, 6, 7]. Therapies to treat anaemia in these patients include erythropoiesis-stimulating agents (ESAs), iron replacement and blood transfusion. Management of anaemia, and use of ESAs in particular, has been shown to reduce mortality and cardiovascular complications, while improving anaemia symptoms [8] and QOL [9, 10]. Though initially shown to have a clinical benefit, ESAs in patients with renal disease are associated with an increased occurrence of stroke, heart failure, myocardial infarction, worsening renal function, and death [11, 12, 13, 14]. ESAs should, therefore, be used sparingly in renal populations. Current recommendations for ESA use in nephrology use haemoglobin (Hb) levels to direct therapy [15]. Using patient symptoms and QOL, patient-centred outcomes (PCOs) may be more appropriate thresholds for initiation and modification of treatment.
Patient-reported outcome measures (PROMs) are one class of methodological tools that are increasingly being employed to evaluate the impact of disease burden and the effects of medical therapies on QOL and patient well-being [16]. PROMs typically measure multiple domains of well-being with one or more questions in each domain subsection, including physical, functional, emotional, and social components of well-being. PCOs are becoming recognized as a critical aspect of patient-centred research and are increasingly being utilized as endpoints in clinical trials [17, 18]. In this study, we aimed to characterize the quality of PROM usage and PCO reporting in studies of CKD patients with anaemia and to determine whether improvements in haematological outcomes are associated with improvements in PCOs, as measured by PROMs.
Materials and Methods
Database Search
A search strategy was developed in conjunction with an experienced information specialist. The databases Medline (Ovid), Embase (Ovid), CINAHL, and PsychINFO were searched without limits from inception until March 2018. The search strategy is presented in online supplementary Appendix A (for all online suppl. material, see www.karger.com/doi/10.1159/000502208).
Eligibility Criteria
Studies addressing interventions to manage anaemia in persons with renal disease of any age were included. Interventions administered at any dose, frequency, and for any duration were eligible. Studies had to report at least one haematological outcome (e.g., Hb levels) and at least one PCO measured by one or more PROM tools. Comparator groups for the anaemia intervention could be placebo, a different anaemia therapy, or no comparator (i.e., patients compared before and after the anaemia intervention). In the case of blood transfusion, we also accepted comparator groups of different transfusion thresholds. Randomized and non-randomized clinical trials, observational cohort studies, and case series with >10 subjects were all accepted.
Article Selection and Data Abstraction
A detailed description of the study screening, selection and abstraction processes was provided in a previous study [19]. In the current study, we also abstracted data concerning PCOs and haematological outcomes. All stages of full-text review and data abstraction were performed independently by 3 reviewers (J.L., P.S., and I.P.) without duplication using a standardized and piloted form. Any discrepancies were resolved by a fourth, senior investigator (E.S.).
Statistical Analyses
Descriptive analysis of included studies was performed to synthesize study and patient characteristics, the PROMs used by these studies, and the quality of PCO reporting. We evaluated the completeness of PROM subscale reporting before and after anaemia intervention. We defined complete PROM subscale reporting as the reporting of all validated subscales for the chosen PROM.
Next, we assessed whether an improvement in haematological outcomes was associated with an improvement in PCOs following an intervention for the treatment of anaemia in renal disease patients. While we wished to analyse studies by PROM and PROM subscales, this was not feasible due to small sample sizes, and variability in PROMs used and subscales reported. Instead, we analysed according to various dimensions of PCOs, which we classified as follows: (a) physical functioning and QOL, (b) mental and emotional QOL, (c) social QOL (e.g., relationships and social interactions), (d) fatigue, vitality, and energy levels, and (e) global or overall QOL and well-being. Studies reporting PROM subscales relevant to one PCO dimension were grouped together for analysis. Due to the heterogeneity in the reporting of haematological outcomes and PCOs, it was not possible to perform a meta-analysis. Instead, data was analysed dichotomously using 2 × 2 tables to determine whether an association existed between haematological outcomes and PCOs following an anaemia intervention.
Fisher's exact tests were performed to test for associations between haematological outcome improvement and PCO improvement, as is appropriate for small sample sizes. For unclear improvements in haematological outcomes (e.g., if no p value was given), we looked at either the mean change in Hb levels, the difference in Hb levels between study groups, or the difference in Hb before and after anaemia intervention. We considered a significant change in Hb to be a statistically significant improvement (i.e., p < 0.05) in Hb levels, or an Hb change of 10 g/L or more, as this is likely clinically and statistically significant. For an unclear improvement in a subscale of a PROM tool (e.g., if no measure of statistical significance was provided), we assessed whether the magnitude of improvement in the scale met minimal clinical significance, as defined by each PROM tool. In cases where it was not possible to ascertain whether there was a significant change in haematological outcomes or in PCOs, the study was excluded from this analysis, and the reason for exclusion was noted.
A risk-of-bias assessment was also completed for each included study. For randomized-controlled trials (RCTs), the Revised Cochrane Risk-of-Bias Tool for RCTs (RoB 2.0) was used, and for prospective studies we utilized the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [20, 21].
Results
Database Search Results
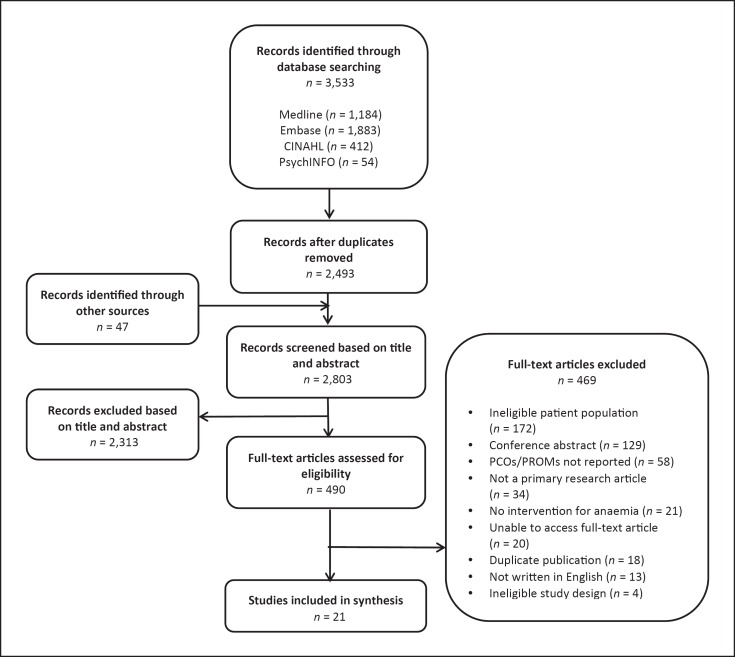
Of 3,533 articles obtained from database searching, 21 articles met all eligibility criteria. Figure 1 shows the study selection process.
Fig. 1.
Flowchart of the study selection process.
Study Characteristics
Baseline characteristics of the included studies are presented in Table 1. Studies were published between 1991 and 2017, with 6 (29%) published after 2010. It is relevant to note that recombinant human erythropoietin was approved for use in CKD in 1989 and all included articles in this study were published after that time [22]. The majority of studies were RCTs (67%) and 33% were prospective cohort studies.
Table 1.
Summary of included studies (n = 21) by study design
| Study | Patient population | Study design | Number | Blinding | Follow-up duration | Anaemia intervention | Comparator group | PROMs used | Study quality assessmentb |
|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al. (2006) [41] | Adult (18+) patients with CKD stages 3–5 and anaemia (Hb <120 g/L), not on dialysis | RCT | 89 | Open-label | 10 weeks | Intravenous iron (sodium ferric gluconate complex, given in doses of 250 mg/250 mL over 1 h on days 1, 8, 15, and 22) | Oral iron (325 mg ferrous sulphate tablets given 3 times daily for 6 weeks) | KDQoL | Moderate |
| Akizawa et al. (2011) [42] | Adult (20+) patients with CKD and anaemia (Hb <100 g/L), not on dialysis | RCT | 322 | Open-label | 48 weeks | Darbepoetin alfa (given as needed to achieve Hb of 110–130 g/L) | rHuEPO (given as needed to achieve Hb of 90–110 g/L) | FACIT-F, SF-36 | Moderate |
| Alexander et al. (2007) [43] | Adult (18+) patients with CKD and anaemia (Hb <100 g/L), not on dialysis | RCT | 81 | Open-label | 24 weeks | Darbepoetin alfa (0.45 g/kg initial subcutaneous dose, then dose adjusted to achieve Hb increase of 10–30 g/L per month) | No intervention | SF-36, KDQoL, FACT-An and FACT-F, instrumental activities of daily living scale | High |
| Foley et al. (2000) [44] | Adult (18+) patients with end-stage renal disease, cardiomyopathy, and anaemia (90< Hb <110 g/L), on haemodialysis | RCT | 146 | Open-label | 48 weeks | Epoetin alfa (given subcutaneously as needed to achieve Hb of 130–140 g/L) | Epoetin alfa (given as needed to achieve Hb of 95–105 g/L) | SF-36, KDQ, Health Utilities Index | Low |
| Furuland et al. (2003) [45] | Adult (18+) patients with end stage renal disease and anaemia (90< Hb <120 g/L), with or without dialysis | RCT | 416 | Open-label | 48 weeks | Epoetin alfa (50 U/kg initial subcutaneous dose, then dose adjusted to achieve Hb of 135–150 g/L in females and 145–160 g/L in males) | Epoetin alfa (given as needed to maintain Hb of 90–120 g/L) | KDQ | High |
| Laupacis et al. (1991) [46] | Adult (18–75 years) patients with end-stage renal disease and anaemia (Hb <90 g/L), on haemodialysis | RCT | 118 | Double-blind | 6 months | Erythropoietin (given intravenously 3 times per week over 6 months, to maintain Hb of 95–110 g/L (low Hb group), or Hb of 115–130 g/L (high Hb group) | Placebo | KDQ, sickness impact profile, time trade-off technique, VAS, 6MWT, exercise stress test | Moderate |
| Muirhead et al. (1992) [47] | Adult (18+) patients with end-stage renal disease and at least one other major comorbidity, anaemia (Hb <95 g/L), on haemodialysis | RCT | 128 | Single-blinda | Unclear, >48 weeks | rHuEPO (intravenous, dose adjusted to achieve Hb of 105–125 g/L over 8–24 weeks) | rhuEPO (subcutaneous, dose adjusted to achieve Hb of 105–125 g/L over 8–24 weeks) | KDQ | Moderate |
| Parfrey et al. (2005) [48] | Adult (18+) patients with renal failure and anaemia (80< Hb <120 g/L), on haemodialysis | RCT | 596 | Double-blind | 96 weeks | Epoetin alfa (intravenous, dose adjusted to achieve Hb levels of 135–145 g/L by 24 weeks) | Epoetin alfa (given as needed to maintain Hb of 95–115 g/L) | FACIT-F, KDQoL vitality and social interaction subscales, 6MWT | Low |
| Pfeffer et al. (2009) [49] | Adult patients with CKD, type 2 diabetes, and anaemia (Hb<110 g/L), not on dialysis | RCT | 4,038 | Double-blind | Unclear, >49 weeks | Darbepoetin alfa (dose adjusted to maintain Hb of 130 g/L) | Placebo | FACT-F, SF-36 | Low |
| Provenzano et al. (2005) [50] | Adult (18+) patients with CKD and anaemia (with stable Hb levels, Hb 110 g/L), not on dialysis | RCT | 519 | Open-label | 16 weeks | Epoetin alfa (4 treatment groups: (1) 10,000 units weekly, (2) 20,000 units biweekly, (3) 30,000 units every 3 weeks, (4) 40,000 units every 4 weeks, all for 16 weeks) | No specified comparator; 4 treatment groups compared to each other | KDQ, LASA | High |
| Roger et al. (2014) [51] | Patients aged 70 years and over with CKD stages 3–5 and anaemia (Hb <110 g/L) | RCT | 51 | Double-blind | 24 weeks | Darbepoetin alfa (0.75 g/kg biweekly, then monthly to achieve Hb of 130 g/L) | Placebo (biweekly for 16 weeks, then monthly) | SF-36, FACT-An, EQ-5D | Moderate |
| Rossert et al. (2006) [52] | Adult (18–75 years) patients with CKD and anaemia (Hb<125 g/L for females and <130 g/L for males) | RCT | 390 | Open-label | 40 months | Epoetin alfa (25–100 IU/kg weekly subcutaneous dose, then dose adjusted to achieve Hb of 130–150 g/L over 4–6 months) | Epoetin alfa (given as needed to achieve Hb of 110–120 g/L) | SF-36, Katz index of activities of daily living | High |
| Saglimbene et al. (2017) [53] | Adult (18+) patients with CKD and anaemia (Hb <100 g/L without treatment or <130 g/L if had treatment), on haemodialysis | RCT | 656 | Single-blinda | 1 year | ESAs (epoetin alfa or epoetin beta 18,000 IU weekly, or darbepoetin alfa 90 µg weekly) | ESAs (epoetin alfa or epoetin beta 4,000 IU weekly, or darbepoetin alfa 20 µg weekly) | KDQoL-SF | Moderate |
| Singh et al. (2006) [11] | Adult (18+) patients with CKD and anaemia (Hb <110 g/L), not on dialysis | RCT | 1,432 | Open-label | 3 years | Epoetin alfa (given subcutaneously weekly or biweekly, as needed, to achieve Hb of 135 g/L) | Epoetin alfa (given as needed to achieve Hb of 113 g/L) | SF-36, KDQ, LASA | Moderate |
| Abu-Alfa et al. (2008) [54] | Adult (18+) patients with CKD and anaemia (Hb <110 g/L), not on dialysis | Prospective cohort, single arm | 911 | Open-label | 1 year | Darbepoetin alfa (0.45 g/kg subcutaneous dose every 2 weeks, then dose adjusted to achieve Hb of 110–120 g/L) | None (patients compared before and after intervention) | KDQoL-CRI | Fair |
| Bloom et al. (2011) [55] | Adult (18+) renal transplant patients with anaemia (Hb <110 g/L), not on dialysis | Prospective cohort, single arm | 66 | Open-label | 24 weeks | Darbepoetin alfa (0.75 g/kg initial subcutaneous dose, then dose adjusted to maintain Hb of 110–125 g/L) | None (patients compared before and after intervention) | SF-36 | Good |
| Bonner et al. (2013) [56] | Adult (18+) patients with CKD and anaemia (Hb<100 g/L) | Prospective cohort, single arm | 28 | Open-label | 1 year | ESAs (any ESA, dosage regimen determined by physician) | None (patients compared before and after intervention) | SF-36, human activity profile, fatigue severity scale | Poor |
| Islam et al. (2015) [57] | Pre-dialysis chronic renal failure patients with anaemia (Hb <95 g/L) | Prospective cohort, single arm | 45 | Open-label | 6 months | rHuEPO (80–100 IU/kg weekly subcutaneous dose) | None (patients compared before and after intervention) | PROM tool name not specified; tool measured the following QoL aspects: physical ability, sense of well-being, appetite | Poor |
| Kawada et al. (2009) [58] | Adult (18+) renal transplant patients with anaemia (Hb <120 g/L) | Prospective cohort, single arm | 24 | Open-label | 6 months | rHuEPO (6,000 IU weekly dose given subcutaneously to achieve Hb of 133 g/L, then as needed to maintain Hb levels) | None (patients compared before and after intervention) | SF-36 | Good |
| Provenzano et al. (2004) [59] | Adult (18+) patients with CKD (any stage) and anaemia (Hb <110 g/L), not on dialysis | Prospective cohort, single arm | 1,557 | Open-label | 16 weeks | Epoetin alfa (10,000 U subcutaneous weekly dose for 16 weeks) | None (patients compared before and after intervention) | KDQ, LASA | Good |
| Rebollo et al. (2004) [60] | Adult (18+) renal transplant patients with chronic renal insufficiency and anaemia (Hb <110 g/L), not on dialysis | Prospective cohort, single arm | 24 | Open-label | 1 year | rHuEPO (2,000 IU subcutaneous weekly dose for 4 weeks, then adjusted to achieve haematocrit of 35%, total of 6 months of treatment) | None (patients compared before and after intervention) | SF-36 | Fair |
chronic kidney disease; ESAs, erythropoiesis-stimulating agents; EQ-5D, quality of life instrument developed by the EuroQoL Association; FACT-An, Functional Assessment of Cancer Therapy Anaemia scale; FACT-F, Functional Assessment of Cancer Therapy Anaemia scale; FACIT-F, Functional Assessment of Chronic Illness Therapy Fatigue scale; KDQ, Kidney Disease Questionnaire; KDQoL, Kidney Disease Quality of Life instrument; KDQoL-CRI, KDQoL questionnaire for chronic renal insufficiency; KDQoL-SF, KDQoL Short Form questionnaire; LASA, linear analogue scale assessment; PROMs, patient-reported outcome measures; QoL, quality of life; RCT, randomized-controlled trial; rHuEPO, recombinant human erythropoietin; SF-36, 36-item Short Form Health Survey; VAS, visual analogue scale; 6MWT, 6-min walking test.
Single blinding: outcome assessors were blinded.
Revised Cochrane Risk-of-Bias Tool for RCTs (RoB 2.0) was used to evaluate RCTs and the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used for prospective studies.
Double-blinded studies accounted for 19% of the total, 10% were single-blinded, and the majority of studies were open-label (71%). All studies included both male and female patients. Nearly all (81%) studies investigated adults (≥18 years old), one study looked at older adults only (≥65 years old), and 14% of studies did not clearly report an age criterion for study inclusion. Across all studies, sample size ranged from 24 to 4,038 participants and mean study duration was 54 weeks (range 6–209 weeks).
We found that 81% of studies investigated patients with CKD, while 14% of studies investigated patients following renal transplantation surgery, and one study (5%) assessed patients with heart failure on haemodialysis. In the studies investigating patients with CKD, patients were categorized according to various measures of renal function, including glomerular filtration rate (GFR), creatinine clearance, and serum creatinine levels. Of the 38% of studies reporting GFR, patients with GFR ranging from 70 to 10 mL/min/1.73 m2 were included, corresponding to CKD stages of 2–5. About one third of studies did not clearly report renal function criteria for inclusion, but many of these studies only included patients on haemodialysis, indicating advanced renal dysfunction. In 33% of studies, some or all patients were receiving dialysis, while in 57% of all studies, none of the patients were on dialysis.
All patients in all studies were anaemic at the start of the study period, with the Hb level used to diagnose anaemia varying between 80 and 130 g/L. A little over half of the studies defined anaemia as an Hb level of either <110 or <100 g/L. The World Health Organization (WHO) defines anaemia as Hb ≤120 g/L in females and ≤130 g/L in males. The Canadian Society of Nephrology recommends investigating Hb below 135 g/L in males and 120 g/L in females [23]. The rationale for Hb cut-offs in the included studies was not explained by the authors.
Nineteen percent of studies compared an anaemia intervention to placebo or no intervention, or to another therapy (10% of studies), and 33% had no distinct comparator group. Many studies (33%) compared different Hb targets. The intervention to treat anaemia was ESAs for most studies (95%), while one study (5%) investigated iron supplementation and none examined the role of red blood cell (RBC) transfusion. Thirty-five percent of studies used Epoetin alfa, 30% Darbepoetin alfa, and 35% of studies did not clearly define the formulation of the ESA that was used. Dosing and duration of therapy varied widely across studies (Table 1).
Use of PROMs
Table 1 shows the PROMs used by each study. Almost half of the studies (43%) utilized one PROM, 24% of studies used 2 different PROMs, another 24% used 3 different PROMs, and 10% reported 4 or more different PROMs. The most commonly used PROM tool was the general QOL tool, Short Form Health Survey (SF-36). Tools specific to renal disease, including the Kidney Disease Questionnaire (KDQ) and the Kidney Disease Quality of Life Questionnaire, were used in 33 and 24% of studies, respectively. Linear scales (i.e., linear analogue scale assessment, visual analogue scale) were used to assess QOL and functioning in 19% of studies. The Functional Assessment of Cancer Therapy scales for anaemia and fatigue were utilized in 14% of studies, despite not all included patients having malignant diagnoses. The Functional Assessment of Chronic Illness Therapy fatigue scale was used in 10% of studies. Other PROMs for QOL and functioning were employed infrequently (Table 1). The rationale for the selection of PROMs was not clearly described in the majority of studies.
In all studies, PROMs were described in the methods section, but these descriptions varied in the level of detail, including descriptions of the PROM subscales, the measurement scales for these tools, and whether the PROM was validated in the patient population under investigation. All studies consistently defined the time points at which the PROM was administered to patients (e.g., at baseline, at 1 month after intervention, at 6-month follow-up). However, reporting of how PROM data was collected was poor. More than half of the studies identified that the PROM was self-administered by the patient and 1 study had their PROMs administered by healthcare professionals; however, 38% did not describe how their PROMs were administered. Additionally, blinding of study personnel to PROM and haematological outcomes were not consistently described in included studies.
Quality of PCO Reporting
About a third (29%) of studies included PCOs as a primary outcome, 43% included PCOs as a secondary outcome, and 29% did not report prioritization of study outcomes. All studies administered and reported their PROMs prior to commencement of and following anaemia treatment. Most studies (81%) had clear reporting of PCOs, which we defined as presenting numeric values of PROM scores (i.e., absolute values of scores or change in score), as well as some measure of statistical significance when comparing 2 or more study groups. Another 19% of studies presented scores from PROMs graphically, without any numerical values. Evaluation of whether studies reported the entirety of a PROM, including all verified subscales, or were selective in their PROM subscale employment and reporting found that 71% of studies reported the PROM tool in its entirety, leaving 29% of the studies that selectively reported subscales from their chosen PROMs. In addition, the results of PCO assessments afforded limited discussion in the text, and non-significant results were largely ignored in the discussion sections of these papers.
Association between Haematological Outcomes and PCOs
We assessed whether there was an association between improvement in haematological outcomes and PCOs, as measured by PROMs, following an anaemia intervention. The haematological outcomes of all studies were related to patients achieving and maintaining a certain Hb level. All PROMs included in this analysis had questions related to the following 5 domains: (1) physical functioning and QOL, (2) mental and emotional QOL, (3) social QOL, (4) fatigue, vitality, and energy levels, and (5) global well-being and QOL. Twenty of 21 included studies (95%) evaluated ESAs as the intervention for anaemia; the one study looking at iron therapy was not eligible for this analysis.
Table 2 shows the results of this analysis. While many patients appeared to have improvement in PCO measures following ESA treatment, we found no significant association between improvement in haematological outcomes and improvement in any of the 5 QOL domains described above (p > 0.05 for each QOL analysis). Thus, it is hard to attribute improvement in quality of life to treatment of anaemia and raises questions about other therapeutic effects of ESAs and possible placebo effect of knowing one is receiving therapy for anaemia.
Table 2.
Association between haematological outcome improvement and improvement in PCOs following ESA treatment in nephrology patients
| Haematological outcome |
Studies, n | p value | |||
|---|---|---|---|---|---|
| Improvement | No change | ||||
| Physical QOL | |||||
| Improvement | 10 | 2 | 18 | >0.05 | |
| No change | 6 | 0 | |||
| Mental and emotional QOL | |||||
| Improvement | 9 | 1 | 16 | >0.05 | |
| No change | 5 | 1 | |||
| Social QOL | |||||
| Improvement | 4 | 0 | 16 | >0.05 | |
| No change | 10 | 2 | |||
| Fatigue/energy levels/vitality | |||||
| Improvement | 9 | 1 | 18 | >0.05 | |
| No change | 7 | 1 | |||
| Global well-being and QOL | |||||
| Improvement | 5 | 0 | 13 | >0.05 | |
| No change | 7 | 1 | |||
PCO, patient-centred outcomes; ESA, erythropoietin-stimulating agent; QOL, quality of life.
Risk of Bias
We performed a risk-of-bias assessment for all included studies (n = 21). Final results of bias and quality assessments are reported in Table 1. A breakdown of the bias assessment for each RCT is included in online supplementary Appendix B. In summary, we found that 79% of RCTs demonstrated a moderate-to-high risk of bias due largely to open-label study designs, high number of participant withdrawals, the subjective nature of PCOs, a paucity of available published protocols, and reduced statistical power for PCOs. Among the prospective studies, we found that most studies (57%) were of fair or poor quality, due in part to lack of blinding, poor reporting of PROM outcomes, loss of participants to follow-up, and lack of power estimates for PCOs, which were largely defined as secondary outcomes.
Discussion
We performed a systematic review of the use of PCOs in anaemic renal disease patients receiving anaemia treatment. The majority of studies were RCTs, but most were open-label and therefore prone to information bias. We identified a number of poor study design characteristics, including a large number of open-label studies [24]. Biases may be especially prevalent in the assessment of PCOs given their inherent subjectivity [19]. Open-label RCTs evaluating inherently subjective patient outcomes have been shown to exaggerate both OR and effect sizes; hence, inadequate blinding of patient-centred research may interfere with the validity of conclusions [25]. Moreover, a recent systematic review of ESAs in renal disease suggested a publication bias that likely favours ESAs, but could not be measured due to a lack of accurate instruments [26]. Another methodologic limitation of included studies was that the majority of studies either reported QOL as a secondary outcome or did not clearly define the prioritization of PCOs; hence, it is difficult to determine whether these RCTs were adequately powered to evaluate changes in PCOs and detect significant differences.
ESAs were the most commonly studied anaemia therapy, with only one study that evaluated iron as an intervention for anaemia. Given the growing use of IV iron in managing anaemia in CKD patients, this lack of evidence regarding the impact of IV iron on PCOs is concerning [27]. This has been recognized and future trials of IV iron in CKD patients, including the PIVOTAL trial, have defined QOL as a secondary efficacy outcome [28, 29]. We did not identify any studies that evaluated the use of RBC transfusion. This may be owing to the decreased use of this therapy for fear of HLA alloimmunization which can compromise a patient's eligibility for renal transplant, as well as the availability of ESAs [30]. Yet, in older non-dialysis-CKD patients, RBC transfusions occur in 11–14/100 person-years [31], and in younger non-dialysis-CKD patients, RBC transfusion remains a relevant, if less frequently used, treatment strategy [32]. Patients with renal dysfunction are known to be at increased risk of transfusion-associated circulatory overload and thus transfusion may actually impair their feelings of well-being [33]. Due to these known clinical risks, PCOs related to transfusion in management of anaemia in patients with renal dysfunction should be considered for future study.
Methodologic rigor was lacking in the assessment of PCOs in the studies included. Some inadequacies we encountered included failure to describe how PROMs were administered, no clear descriptions of blinding procedures with respect to data collection for PCOs, and use of PROMs not validated for kidney disease patients or anaemic populations. We also observed suboptimal and incomplete reporting of PCOs. Approximately 30% of studies did not employ an entire PROM with all associated subscales, but selectively chose subscales to administer and report. Worse yet, some indicated that an entire PROM would be used, but did not report outcomes for all domains. Since these PROMs have been validated in patient populations in their entirety, selective administration and reporting of certain subscales may inadequately capture the impact of an intervention on overall QOL. Furthering the difficulty in determining impact of anaemia treatment on PCOs is the fact that 19% of studies had unclear reporting of PCOs. In some of these, data was presented graphically only, without any numerical values, which made abstracting PCOs for empirical analysis challenging and would hinder future attempts at meaningful meta-analyses. In addition, few studies made any mention of clinically important differences when reporting PCOs, but instead focused on statistical differences even though evaluation of minimally important clinical differences are known to better measure the impact of an intervention on PCOs [34]. In agreement with our conclusions, authors of a recent meta-analysis that investigated ESAs and QOL in renal populations noted that meta-analysis could be considered an inappropriate method of analysis due to significant clinical and statistical heterogeneity amongst included studies [26].
A wide variety of PROMs were used in the included studies. As each PROM evaluates somewhat different aspects of QOL, and some tools are specific to certain patient populations, the result is heterogeneous outcomes that make comparisons of PCOs across studies challenging. Standardization of the types of PROMs used in renal disease patients and the development of a rigorous set of methodological guidelines for use of PCOs in research contexts may promote advancement of patient-centred research initiatives in nephrology. Better PCO research can lead to changes in practice that aim to improve patient QOL. Additionally, with more sound studies, clinical practice guidelines for the management of this universal complication of CKD can be developed, as it has previously been shown that clinical practice guidelines have a positive impact on medical practice [35]. Evidence-based guidelines that include studies of PCOs that are methodologically sound would result in the most robust recommendations regarding when to initiate ESA therapy and how to monitor it. This would allow for more rational use of this expensive therapy, and at times risky therapy.
Consistent with previous findings, the majority of studies employed one PROM tool [19]. It is not known if the use of fewer PROMs is associated with incomplete assessment of the multiple facets of patient well-being in the presence of renal disease, anaemia, and additional comorbidities. The 2 most commonly used PROM tools in our studies were the SF-36 and KDQ. The KDQ has been shown to reliably assess the patient-centred impact of kidney disease, but is not specific to anaemia [36, 37]. Similarly, the SF-36 has been validated in numerous patient populations but is neither anaemia-specific nor nephrology-specific. As a result, these tools may not adequately assess anaemia symptoms, which may be indistinguishable from symptoms of renal impairment and other comorbidities known to occur in this patient population. In addition, unsuitable tools such as the Functional Assessment of Cancer Therapy tool were employed to assess QOL in numerous studies, even though such tools are intended for use in other patient populations. Future studies in anaemic renal disease populations should consider COSMIN assessment of possible tools to determine which is the most suitable for a given study [38]. Such evaluation may indicate the need for development of PROMs specific for the study of anaemia due to renal disease.
We found no significant association between improvements in Hb levels and any of the 5 dimensions of QOL. As a result of the aforementioned heterogeneity in reporting PROMs, our sample size for each analysis was small and consequently underpowered. Thus, this finding only suggests the need for additional evaluation. However, it does raise questions about the merit of current anaemia strategies in CKD patients. Rather notoriously, a recent reanalysis of the Normal Hematocrit Cardiac Trial [39] demonstrated methodological errors and discrepant reporting of results, including an erroneous conclusion of improved QOL within the ESA-treated group [40]. Consistent with this study, a recent systematic review of PCOs in renal populations found that treatment with ESAs was not associated with improvements in health-related QOL [26]. These authors also noted how significant heterogeneity amongst included studies, few good-quality studies, and possible publication bias may have limited their analysis and validity of their conclusions. Future large-scale analyses of the relationship between PCOs and anaemia management in renal patients that evaluate and report PCOs using rigorous study methods are required. Such studies should be powered to show clinically and statistically significant outcomes measured by PCO tools.
Limitations of this study include variation in ESA dosing regimens, differences in follow-up length, and heterogeneity in patient populations, which all may have contributed to sources of heterogeneity that were not accounted for in the analysis. These limitations reflect the substandard quality of much of the research in this area, rather than poor conduct in the present study.
Progress in our understanding of QOL in renal patients with anaemia will allow clinicians to determine which patients will respond optimally to ESA therapy, while ensuring that ESAs are administered in a cost-effective manner on a population level. Perhaps most importantly, physicians will be able to more accurately advise patients of what they can expect, in terms of symptom management and QOL if they elect to begin or continue therapies to treat anaemia.
Conclusion
The majority of studies reporting PCOs in anaemic renal populations investigated ESAs for treatment of anaemia. Overall, evaluation of PCOs in these studies was hindered by poor methodology and reporting. Future directions for research in this area include the revision of, and/or the development of kidney-specific PROMs to include questions able to discriminate the effects of symptoms of anaemia on QOL. Improvement of our understanding of the patient-centred impact of anaemia management in nephrology patients may help to ensure that anaemia therapy is standardized in such a way as to enable varying management plans based on unique patient characteristics which are also cost-effective on a population level.
Disclosure Statement
The authors have no conflicts of interest to report.
Funding Sources
P.S. received funding for this research through the Hematology Opportunities for the Next Generation of Research Scientists (HONORS) award offered by the American Society of Hematology.
Author Contributions
The results presented in this paper have not been published previously in whole or part, except in abstract form. All authors listed contributed in varying capacities to the design, data collection, analysis, and drafting of the current manuscript. P.S. and E.S. contributed to the design, data collection, analysis, and drafting of the manuscript. I.P. contributed to the data collection, analysis, and manuscript drafting. J.L. and C.D. contributed to data collection and manuscript drafting. A.D. contributed to the library search and data collection. A.T. contributed to analysis and manuscript drafting. M.C. contributed to the analysis and manuscript drafting. All authors approved the final version of the manuscript.
Availability of Data and Material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
We would like to thank Dr. Todd Fairhead, MD, MSc, FRCPC for his help in editing and providing helpful suggestions for the manuscript. We would like to thank Dr. Elianna Saidenberg for her dedication to this research project. She was a guiding light to all of her students. She was a fearless patient advocate and had an unwavering passion for hematology and transfusion medicine. Although she is no longer present physically, her inspirational words and steadfast commitment to medicine will always be felt by those who had the pleasure of crossing paths with her beautiful personality.
References
- 1.McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004 Sep;20((9)):1501–10. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 2.Dowling TC. Prevalence, etiology, and consequences of anemia and clinical and economic benefits of anemia correction in patients with chronic kidney disease: an overview. Am J Health Syst Pharm. 2007 Jul;64((13 Suppl 8)):S3–7. doi: 10.2146/ajhp070181. [DOI] [PubMed] [Google Scholar]
- 3.Pinevich AJ, Petersen J. Erythropoietin therapy in patients with chronic renal failure. West J Med. 1992 Aug;157((2)):154–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Zalunardo N, Levin A. Anemia and the heart in chronic kidney disease. Semin Nephrol. 2006 Jul;26((4)):290–5. doi: 10.1016/j.semnephrol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Soni RK, Weisbord SD, Unruh ML. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010 Mar;19((2)):153–9. doi: 10.1097/MNH.0b013e328335f939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayat A, Haria D, Salifu MO. Erythropoietin stimulating agents in the management of anemia of chronic kidney disease. Patient Prefer Adherence. 2008 Feb;2:195–200. doi: 10.2147/ppa.s2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009 Apr;14((2)):240–6. doi: 10.1111/j.1440-1797.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 8.Hörl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013 May;9((5)):291–301. doi: 10.1038/nrneph.2013.21. [DOI] [PubMed] [Google Scholar]
- 9.O'Mara NB. Anemia in patients with chronic kidney disease. Diabetes Spectr. 2008;21((1)):12–9. [Google Scholar]
- 10.Manavalan M, Majumdar A, Harichandra Kumar KT, Priyamvada PS. Assessment of health-related quality of life and its determinants in patients with chronic kidney disease. Indian J Nephrol. 2017 Jan-Feb;27((1)):37–43. doi: 10.4103/0971-4065.179205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. CHOIR Investigators Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov;355((20)):2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 12.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. CREATE Investigators Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov;355((20)):2071–84. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 13.Garimella PS, Katz R, Patel KV, Kritchevsky SB, Parikh CR, Ix JH, et al. Health ABC Study Association of serum erythropoietin with cardiovascular events, kidney function decline, and mortality: the health aging and body composition study. Circ Heart Fail. 2016 Jan;9((1)):e002124. doi: 10.1161/CIRCHEARTFAILURE.115.002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seliger SL, Zhang AD, Weir MR, Walker L, Hsu VD, Parsa A, et al. Erythropoiesis-stimulating agents increase the risk of acute stroke in patients with chronic kidney disease. Kidney Int. 2011 Aug;80((3)):288–94. doi: 10.1038/ki.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, Evans J, et al. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017 Nov;18((1)):345. doi: 10.1186/s12882-017-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015 Feb;350(feb10 14):g7818. doi: 10.1136/bmj.g7818. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel SE, Normand SL. Getting the methods right—the foundation of patient-centered outcomes research. N Engl J Med. 2012 Aug;367((9)):787–90. doi: 10.1056/NEJMp1207437. [DOI] [PubMed] [Google Scholar]
- 18.Washington AE, Lipstein SH. The Patient-Centered Outcomes Research Institute—promoting better information, decisions, and health. N Engl J Med. 2011 Oct;365((15)):e31. doi: 10.1056/NEJMp1109407. [DOI] [PubMed] [Google Scholar]
- 19.Staibano P, Perelman I, Lombardi J, Davis A, Tinmouth A, Carrier M, et al. Patient-centered outcomes in the management of anemia: A scoping review. Transfus Med Rev. 2018 doi: 10.1016/j.tmrv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct;343(oct18 2):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quality assessment tool for observational cohort and cross-sectional studies NIH, 2019
- 22.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989 Jul;321((3)):158–63. doi: 10.1056/NEJM198907203210305. [DOI] [PubMed] [Google Scholar]
- 23.White CT, Barrett BJ, Madore F, Moist LM, Klarenbach SW, Foley RN, et al. Canadian Society of Nephrology Clinical practice guidelines for evaluation of anemia. Kidney Int Suppl. 2008 Aug;74((110)):S4–6. doi: 10.1038/ki.2008.268. [DOI] [PubMed] [Google Scholar]
- 24.Inrig JK, Califf RM, Tasneem A, Vegunta RK, Molina C, Stanifer JW, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis. 2014 May;63((5)):771–80. doi: 10.1053/j.ajkd.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014 Aug;43((4)):1272–83. doi: 10.1093/ije/dyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collister D, Komenda P, Hiebert B, Gunasekara R, Xu Y, Eng F, et al. The effect of erythropoietin-stimulating agents on health-related quality of life in anemia of chronic kidney disease: A systematic review and meta-analysis. Ann Intern Med. 2016 Apr;164((7)):472–8. doi: 10.7326/M15-1839. [DOI] [PubMed] [Google Scholar]
- 27.Del Vecchio L, Longhi S, Locatelli F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J. 2016 Apr;9((2)):260–7. doi: 10.1093/ckj/sfv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdougall IC. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J. 2017 Dec;10(Suppl 1):i16–24. doi: 10.1093/ckj/sfx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in ckd: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016 Nov;68((5)):677–90. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Mota MA. Red cell and human leukocyte antigen alloimmunization in candidates for renal transplantation: a reality. Rev Bras Hematol Hemoter. 2013;35((3)):160–1. doi: 10.5581/1516-8484.20130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim HN, Ishani A, Guo H, Gilbertson DT. Blood transfusion use in non-dialysis-dependent chronic kidney disease patients aged 65 years and older. Nephrol Dial Transplant. 2009 Oct;24((10)):3138–43. doi: 10.1093/ndt/gfp213. [DOI] [PubMed] [Google Scholar]
- 32.Gill KS, Muntner P, Lafayette RA, Petersen J, Fink JC, Gilbertson DT, et al. Red blood cell transfusion use in patients with chronic kidney disease. Nephrol Dial Transplant. 2013 Jun;28((6)):1504–15. doi: 10.1093/ndt/gfs580. [DOI] [PubMed] [Google Scholar]
- 33.Murphy EL, Kwaan N, Looney MR, Gajic O, Hubmayr RD, Gropper MA, Koenigsberg M, Wilson G, Matthay M, Bacchetti P, Toy P, Group TS. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013 Apr;126((4)):357. doi: 10.1016/j.amjmed.2012.08.019. e329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000 Nov;18((5)):419–23. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 35.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993 Nov;342((8883)):1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 36.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994 Oct;3((5)):329–38. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 37.Rao S, Carter WB, Mapes DL, Kallich JD, Kamberg CJ, Spritzer KL, et al. Development of subscales from the symptoms/problems and effects of kidney disease scales of the kidney disease quality of life instrument. Clin Ther. 2000 Sep;22((9)):1099–111. doi: 10.1016/S0149-2918(00)80087-9. [DOI] [PubMed] [Google Scholar]
- 38.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010 May;19((4)):539–49. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998 Aug;339((9)):584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 40.Fishbane S, Wish JB. A physician's perseverance uncovers problems in a key nephrology study. Kidney Int. 2012 Jul;82((2)):135–7. doi: 10.1038/ki.2012.122. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R, Rizkala AR, Bastani B, Kaskas MO, Leehey DJ, Besarab A. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26((5)):445–54. doi: 10.1159/000096174. [DOI] [PubMed] [Google Scholar]
- 42.Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. KRN321 STUDY Group Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Ther Apher Dial. 2011 Oct;15((5)):431–40. doi: 10.1111/j.1744-9987.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 43.Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Curr Med Res Opin. 2007 Dec;23((12)):2997–3008. doi: 10.1185/030079907X242502. [DOI] [PubMed] [Google Scholar]
- 44.Foley RN, Parfrey PS, Morgan J, Barré PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000 Sep;58((3)):1325–35. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 45.Furuland H, Linde T, Ahlmén J, Christensson A, Strömbom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003 Feb;18((2)):353–61. doi: 10.1093/ndt/18.2.353. [DOI] [PubMed] [Google Scholar]
- 46.Laupacis A, Wong C, Churchill D, The Canadian Erythropoietin Study Group The use of generic and specific quality-of-life measures in hemodialysis patients treated with erythropoietin. Control Clin Trials. 1991 Aug;12((4 Suppl)):168S–79S. doi: 10.1016/s0197-2456(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 47.Muirhead N, Churchill DN, Goldstein M, Nadler SP, Posen G, Wong C, et al. Comparison of subcutaneous and intravenous recombinant human erythropoietin for anemia in hemodialysis patients with significant comorbid disease. Am J Nephrol. 1992;12((5)):303–10. doi: 10.1159/000168464. [DOI] [PubMed] [Google Scholar]
- 48.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005 Jul;16((7)):2180–9. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov;361((21)):2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 50.Provenzano R, Bhaduri S, Singh AK, Group PS, PROMPT Study Group Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: the PROMPT study. Clin Nephrol. 2005 Aug;64((2)):113–23. doi: 10.5414/cnp64113. [DOI] [PubMed] [Google Scholar]
- 51.Roger SD, Jassal SV, Woodward MC, Soroka S, McMahon LP. A randomised single-blind study to improve health-related quality of life by treating anaemia of chronic kidney disease with Aranesp® (darbepoetin alfa) in older people: STIMULATE. Int Urol Nephrol. 2014 Feb;46((2)):469–75. doi: 10.1007/s11255-013-0512-1. [DOI] [PubMed] [Google Scholar]
- 52.Rossert J, Levin A, Roger SD, Hörl WH, Fouqueray B, Gassmann-Mayer C, et al. Effect of early correction of anemia on the progression of CKD. Am J Kidney Dis. 2006 May;47((5)):738–50. doi: 10.1053/j.ajkd.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 53.Saglimbene V, Palmer SC, Craig JC, Ruospo M, Nicolucci A, Tonelli M, et al. CE-DOSE Study Investigators Low versus high dose erythropoiesis-stimulating agents in hemodialysis patients with anemia: A randomized clinical trial. PLoS One. 2017 Mar;12((3)):e0172735. doi: 10.1371/journal.pone.0172735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abu-Alfa AK, Sloan L, Charytan C, Sekkarie M, Scarlata D, Globe D, et al. The association of darbepoetin alfa with hemoglobin and health-related quality of life in patients with chronic kidney disease not receiving dialysis. Curr Med Res Opin. 2008 Apr;24((4)):1091–100. doi: 10.1185/030079908x280653. [DOI] [PubMed] [Google Scholar]
- 55.Bloom RD, Bolin P, Gandra SR, Scarlata D, Petersen J. Impact on health-related quality of life in kidney transplant recipients with late posttransplant anemia administered darbepoetin alfa: results from the STRATA study. Transplant Proc. 2011 Jun;43((5)):1593–600. doi: 10.1016/j.transproceed.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Bonner A, Caltabiano M, Berlund L. Quality of life, fatigue, and activity in Australians with chronic kidney disease: a longitudinal study. Nurs Health Sci. 2013 Sep;15((3)):360–7. doi: 10.1111/nhs.12038. [DOI] [PubMed] [Google Scholar]
- 57.Islam S, Rahman H, Rashid HU. Effect rHuEpo on predialysis CRF patients: study of 45 cases. Bangladesh Med Res Counc Bull. 2005 Aug;31((2)):83–7. [PubMed] [Google Scholar]
- 58.Kawada N, Moriyama T, Ichimaru N, Imamura R, Matsui I, Takabatake Y, et al. Negative effects of anemia on quality of life and its improvement by complete correction of anemia by administration of recombinant human erythropoietin in posttransplant patients. Clin Exp Nephrol. 2009 Aug;13((4)):355–60. doi: 10.1007/s10157-009-0170-x. [DOI] [PubMed] [Google Scholar]
- 59.Provenzano R, Garcia-Mayol L, Suchinda P, Von Hartitzsch B, Woollen SB, Zabaneh R, et al. POWER Study Group Once-weekly epoetin alfa for treating the anemia of chronic kidney disease. Clin Nephrol. 2004 Jun;61((6)):392–405. doi: 10.5414/cnp61392. [DOI] [PubMed] [Google Scholar]
- 60.Rebollo P, Baltar JM, Campistol JM, Ortega T, Ortega F. Quality of life of patients with chronic renal allograft rejection and anemia. J Nephrol. 2004 Jul-Aug;17((4)):531–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.