Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with significant morbidity, increased risk of stroke, reduced quality of life, and increased mortality [1], [2], [3], [4]. Concomitant modifiable risk factors are associated with increased incident AF [5], and risk factor modification programs have been shown to increase the likelihood of maintaining sinus rhythm and preventing AF progression [6]. Interestingly, in a study of 1295 individuals with symptomatic AF, the majority were able to identify lifestyle-related conditions as triggers for their episodes of AF. The most commonly reported triggers were alcohol (35%), caffeine (28%), exercise (23%), and lack of sleep (21%) [7]. In this context, Siland et al. studied the relationship between self-reported lifestyle components, obtained by standardized questionnaires at inclusion, and incident AF in the large multi-disciplinary prospective population-based cohort study (“Lifelines“) including 98,966 individuals [8]. Although traditional risk factors such as advanced age, male sex, body mass index, heart failure and previous stroke were associated with incident AF in this study, none of the self-reported lifestyle components such as physical activity, nutritional status and sleep quality were associated with incident AF. Siland et al. are congratulated for performing this analysis in an extremely large cohort. The new finding of this study is that lifestyle components, when they are assessed by standardized questionnaires obtained at the beginning of the study, are not associated with incident AF. The design of the study as well as the used questionnaires may partially explain these unexpected findings.
Physical activity was assessed by a questionnaire interrogating a combination of physical activity and intensity and Metabolic Equivalent of Task (MET) scores in minutes per week. Some, but not all, studies reporting the association between self-reported physical activity and incident AF have described a modest reduction in AF incidence amongst physically active participants [9], [10], [11]. However, recent studies have shown the association between objectively-measured exercise capacity and AF incidence to be much stronger than for subjectively-reported physical activity [12]. Moreover, the relationship between physical activity and AF appears to be both gender-dependent and non-linear, thus adding additional nuance to the association [13]. It appears clear that methods to track physical activity both objectively and continuously are needed to truly assess the magnitude of AF risk reduction amongst physically active individuals. Wearable devices look likely to be the way forward with regards to this, although expecting a one size fits all recommendation regarding physical activity is unlikely to be achievable. It is likely that factors such as gender, background comorbidities and the nature of the physical activity (type, intensity) may contribute to this story. Additionally, delineating between the effects of physical activity and cardiorespiratory fitness is likely to be important.
Nutritional status was inventoried once at the beginning of the study by the Mini Nutritional Assessment screening score including six questions related to appetite, weight loss, mobility, (neuro) psychological stress and anthropomorphic measures such as body mass index or calf circumference. Spot-assessment of diet may not provide a representative summary about the temporal variety of diet and therefore obviously simplifies the dynamic and complex dietary intake and actual nutritional status. However, a more detailed assessment of dietary intake is challenging. More intense questionnaires such as the food frequency questionnaires (FFQ) and diet quality indexes (DQI) discourage patients from completing, which results in missing or random data. Additionally, FFQ and DQI mainly represent the current diet of the participants, as it requires participants to remember their past diet. Additionally, repeated measures of overall dietary composition (micro- and macronutrients) or biomarkers specific for food intake (e.g. urinary hydroxytyrosol for extra-virgin olive oil or α-linolenic for walnut consumption) may be another strategy to document dietary intake and provide a better and more representative insight into the actual diet composition and nutritional status [14]. Theoretically, the composition of the gut microbiota may also provide worthful information about long-term stability of diet or compliance of certain dietary interventions [15], [16]. Theoretically, diet diaries supported by mHealth technologies may allow a more longitudinal assessment of diet, capturing intermittent changes or fluctuations in dietary patterns.
In this report of the Lifelines study, sleep quality was assessed by the Pittsburgh Sleep Quality Index (PSQI), a validated tool which measures several aspects of sleep, including subjective quality, sleep latency, efficiency and daytime dysfunction, offering a composite score ranging from 0 to 21 (lower scores denote healthier sleep quality). Notably the study participants reported generally healthy sleep quality as indicated by a relatively low PSQI score of 4 [IQR:3–6] with no difference between those who developed AF and those who did not. Self-reported questionnaires may deviate from objective measurements but these findings are in contrast to a comparable study (Health eHeart) which reported that reduced sleep quality in 4553 participants enrolled electronically was associated with increased AF incidence. Also utilising the PSQI, and independent of the presence of sleep-disordered breathing, increased global PSQI score, increased sleep onset latency, reduced efficiency, reduced sleep quality and increased night time awakenings – were all associated with increased AF risk [17]. Further, the objectively measured reduction in rapid eye movement (REM) sleep was also associated with increased AF incidence. The relationship between self-reported sleep duration and mortality has been shown to be very non-linear (i.e. more of a U-curve) [18]. However, although subjective long sleepers with heart failure have poor sleep efficiency, objectively measured sleep duration strongly predicts mortality in heart failure patients in a linear manner [19]. Additionally, in a very recently published study of 385,292 participants from the UK Biobank, a bespoke questionnaire aimed at quantifying sleep quality within five domains found that a healthy sleep pattern was associated with reduced risks of cardiovascular disease, coronary heart disease and stroke [20]. On the other hand, the discord between subjective and objective measures of sleep quality (namely excessive daytime sleepiness) has been increasingly established in patients with sleep-disordered breathing and AF [21], [22], [23]. While the mechanisms of this discord remain unclear, the findings of this study and their contrast to previous studies underscore the importance of objective evaluation of sleep in the context of cardiovascular assessment [24]. Given the potentially resource-intensive requirements of sleep studies, sleep trackers implemented in mobile devices or wearables may provide a more practical means for objective measurements of sleep duration and potentially also of sleep quality [25], [26], [27].
The study by Siland et al. showed that self-reported assessment of lifestyle components may be challenging [8]. The results of questionnaires may not always reflect the “true” lifestyle and self-reported physical activity, nutritional status and sleep quality in a specific patient may deviate from objective measurements. Several factors related to the lifestyle assessment and/or AF assessment may contribute to the discrepancy between self-reported and objectively assessed lifestyle components and incident AF. The majority of studies, including Lifelines, quantified lifestyle components by self-report on study entry, leaving the potential for reporting bias and time-varying changes. However, lifestyle may vary (seasonal variation) during the follow-up of a study resulting in a dynamic exposure to lifestyle-related conditions which may critically impact the timepoint and extent of incident AF episodes. Additionally, the detection of AF by hospital admissions or medical records lacks sensitivity for asymptomatic or subclinical AF, or for cases requiring minimal management. In the case of the study Lifelines, missing ECGs, unavailable information about AF-related therapies and limited follow-up may result in a low detection rate of new incident AF cases. Study design and AF ascertainment method in observational studies relating to AF incidence may critically impact the result of correlation analyses.
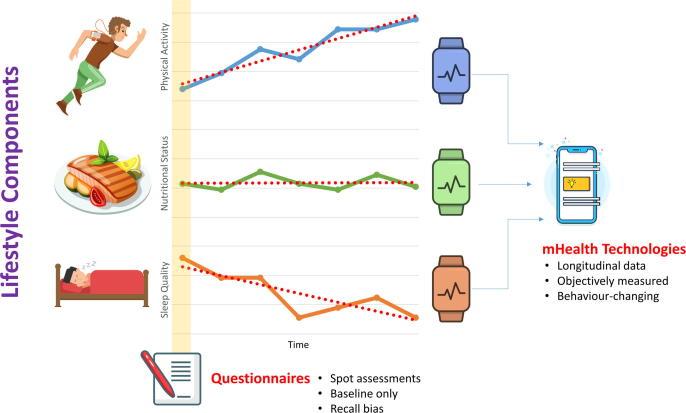
The optimal approach to assess the impact of lifestyle in large population-based cohort studies remains unclear. Various studies have aimed to adopt the multi-generational longitudinal design of the seminal Framingham Heart Study, which effectively shaped our understanding of the effect of lifestyle on cardiovascular outcomes [28]. However, this type of studies requires a great amount of resources and remain reliant in large on subjective reports. Therefore, new mHealth technologies may provide a promising infrastructure for a more objective and longitudinal assessment of lifestyle components. Additionally, the mHealth based assessment of lifestyle components could be coupled with longitudinal rhythm monitoring to determine the relationship between lifestyle and AF (Fig. 1.).
Fig. 1.
New mHealth technologies may provide a promising infrastructure for a more objective and longitudinal assessment of lifestyle components.
References
- 1.Chang T.Y., Liao J.N., Chao T.F., Vicera J.J., Lin C.Y., Tuan T.C., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Chung F.P., Chen S.A. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayinde H., Schweizer M.L., Crabb V., Ayinde A., Abugroun A., Hopson J. Age modifies the risk of atrial fibrillation among athletes: A systematic literature review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2018;18:25–29. doi: 10.1016/j.ijcha.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int. J. Cardiol. Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prochnau D., von Knorre K., Figulla H.R., Schulze P.C., Surber R. Efficacy of temperature-guided cryoballoon ablation without using real-time recordings – 12-Month follow-up. Int. J. Cardiol. Heart Vasc. 2018;21:50–55. doi: 10.1016/j.ijcha.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas D., Christ T., Fabritz L., Goette A., Hammwöhner M., Heijman J., Kockskämper J., Linz D., Odening K.E., Schweizer P.A., Wakili R., Voigt N. German cardiac society working group on cellular electrophysiology state-of-the-art paper: impact of molecular mechanisms on clinical arrhythmia management. Clin. Res. Cardiol. 2019;108:577–599. doi: 10.1007/s00392-018-1377-1. [DOI] [PubMed] [Google Scholar]
- 6.Middeldorp M.E., Ariyaratnam J., Lau D., Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart. 2020;106:325–332. doi: 10.1136/heartjnl-2019-315327. [DOI] [PubMed] [Google Scholar]
- 7.Groh C.A., Faulkner M., Getabecha S., Taffe V., Nah G., Sigona K., McCall D., Hills M.T., Sciarappa K., Pletcher M.J., Olgin J.E., Marcus G.M. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. 2019;16:996–1002. doi: 10.1016/j.hrthm.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Siland J.E., Zwartkruis V., Geelhoed B., de Boer R.A., van Gelder I.C., van der Harst P., Rienstra M. Lifestyle components: self-reported physical activity, nutritional status, sleep quality and incident atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2020 doi: 10.1016/j.ijcha.2020.100492. (IJCHA_2020_13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D., Furberg C.D., Psaty B.M., Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett B.M., Conen D., Buring J.E., Moorthy M.V., Lee I.M., Albert C.M. Physical activity and the risk of incident atrial fibrillation in women. Circ. Cardiovasc. Qual. Outcomes. 2011;4:321–327. doi: 10.1161/CIRCOUTCOMES.110.951442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi W.T., Alirhayim Z., Blaha M.J., Juraschek S.P., Keteyian S.J., Brawner C.A., Al-Mallah M.H. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the henry ford exercise testing (FIT) project. Circulation. 2015;131:1827–1834. doi: 10.1161/CIRCULATIONAHA.114.014833. [DOI] [PubMed] [Google Scholar]
- 12.Elliott A.D., Linz D., Verdicchio C.V., Sanders P. Exercise and atrial fibrillation: prevention or causation? Heart Lung Circ. 2018;27:1078–1085. doi: 10.1016/j.hlc.2018.04.296. [DOI] [PubMed] [Google Scholar]
- 13.Elliott A.D., Linz D., Mishima R., Kadhim K., Gallagher C., Middeldorp M.E., Verdicchio C.V., Hendriks J.M.L., Lau D.H., La Gerche A., Sanders P. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehz897. ehz897. [DOI] [PubMed] [Google Scholar]
- 14.Leeming E.R., Johnson A.J., Spector T.D., Le Roy C.I. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11:2862. doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishima R.S., Elliott A.D., Sanders P., Linz D. Gastrointestinal sodium absorption, microbiome, and hypertension. Nat. Rev. Cardiol. 2017;14:693. doi: 10.1038/nrcardio.2017.159. [DOI] [PubMed] [Google Scholar]
- 16.Mishima R.S., Elliott A.D., Sanders P., Linz D. Microbiome and atrial fibrillation. Int. J. Cardiol. 2018;255:103–104. doi: 10.1016/j.ijcard.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 17.Christensen M.A., Dixit S., Dewland T.A., Whitman I.R., Nah G., Vittinghoff E., Mukamal K.J., Redline S., Robbins J.A., Newman A.B., Patel S.R., Magnani J.W., Psaty B.M., Olgin J.E., Pletcher M.J., Heckbert S.R., Marcus G.M. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. doi: 10.1016/j.hrthm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linz D., Kadhim K., Kalman J.M., McEvoy R.D., Sanders P. Sleep and cardiovascular risk: how much is too much of a good thing? Eur. Heart J. 2019;40:1630–1632. doi: 10.1093/eurheartj/ehy772. [DOI] [PubMed] [Google Scholar]
- 19.Reinhard W., Plappert N., Zeman F., Hengstenberg C., Riegger G., Novack V., Maimon N., Pfeifer M., Arzt M. Prognostic impact of sleep duration and sleep efficiency on mortality in patients with chronic heart failure. Sleep Med. 2013;14:502–509. doi: 10.1016/j.sleep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Fan M., Sun D., Zhou T., Heianza Y., Lv J., Li L., Qi L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 2020;41:1182–1189. doi: 10.1093/eurheartj/ehz849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadhim K., Middeldorp M.E., Elliott A.D., Jones D., Hendriks J.M.L., Gallagher C., Arzt M., McEvoy R.D., Antic N.A., Mahajan R., Lau D.H., Nalliah C., Kalman J.M., Sanders P., Linz D. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can. J. Cardiol. 2019;35:1457–1464. doi: 10.1016/j.cjca.2019.07.627. [DOI] [PubMed] [Google Scholar]
- 22.Kadhim K., Lau D.H., Sanders P., Linz D. Sleep apnea in atrial fibrillation - highly prevalent, highly relevant, but most patients are not somnolent! Int. J. Cardiol. Heart Vasc. 2020;26 doi: 10.1016/j.ijcha.2019.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traaen G.M., Øverland B., Aakerøy L., Hunt T.E., Bendz C., Sande L., Aakhus S., Zaré H., Steinshamn S., Anfinsen O.G., Loennechen J.P., Gullestad L., Akre H. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2019;26 doi: 10.1016/j.ijcha.2019.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linz D., Baumert M., Catcheside P., Floras J., Sanders P., Lévy P., Cowie M.R., Doug McEvoy R. Assessment and interpretation of sleep disordered breathing severity in cardiology: clinical implications and perspectives. Int. J. Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 25.Linz D., Kadhim K., Brooks A.G., Elliott A.D., Hendriks J.M.L., Lau D.H., Mahajan R., Gupta A.K., Middeldorp M.E., Hohl M., Nalliah C.J., Kalman J.M., McEvoy R.D., Baumert M., Sanders P. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int. J. Cardiol. 2018;272:155–161. doi: 10.1016/j.ijcard.2018.07.124. [DOI] [PubMed] [Google Scholar]
- 26.Linz D., Brooks A.G., Elliott A.D., Nalliah C.J., Hendriks J.M.L., Middeldorp M.E., Gallagher C., Mahajan R., Kalman J.M., McEvoy R.D., Lau D.H., Sanders P. Variability of sleep apnea severity and risk of atrial fibrillation: the VARIOSA-AF study. JACC Clin. Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Linz D., Baumert M., Desteghe L., Kadhim K., Vernooy K., Kalman J.M., Dobrev D., Arzt M., Sastry M., Crijns H.J.G.M., Schotten U., Cowie M.R., McEvoy R.D., Heidbuchel H., Hendriks J., Sanders P., Lau D.H. Nightly sleep apnea severity in patients with atrial fibrillation: potential applications of long-term sleep apnea monitoring. Int. J. Cardiol. Heart Vasc. 2019;24 doi: 10.1016/j.ijcha.2019.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]