Abstract
Vaccines against infectious bronchitis of chickens (Gallus gallus domesticus) have arguably been the most successful, and certainly the most widely used, of vaccines for diseases caused by coronaviruses, the others being against bovine, canine, feline and porcine coronaviruses. Infectious bronchitis virus (IBV), together with the genetically related coronaviruses of turkey (Meleagris gallopavo) and ring-necked pheasant (Phasianus colchicus), is a group 3 coronavirus, Severe acute respiratory syndrome (SARS) coronavirus being tentatively in group 4, the other known mammalian coronaviruses being in groups 1 and 2. IBV replicates not only in respiratory tissues (including the nose, trachea, lungs and airsacs, causing respiratory disease), but also in the kidney (associated with minor or major nephritis), oviduct, and in many parts of the alimentary tract—the oesophagus, proventriculus, duodenum, jejunum, bursa of Fabricius, caecal tonsils, rectum and cloaca, usually without clinical effects. The virus can persist, being re-excreted at the onset of egg laying (4 to 5 months of age), believed to be a consequence of the stress of coming into lay. Genetic lines of chickens differ in the extent to which IBV causes mortality in chicks, and in respect of clearance of the virus after the acute phase. Live attenuated (by passage in chicken embryonated eggs) IBV strains were introduced as vaccines in the 1950s, followed a couple of decades later by inactivated vaccines for boosting protection in egg-laying birds. Live vaccines are usually applied to meat-type chickens at 1 day of age. In experimental situations this can result in sterile immunity when challenged by virulent homologous virus. Although 100% of chickens may be protected (against clinical signs and loss of ciliary activity in trachea), sometimes 10% of vaccinated chicks may not respond with a protective immune response. Protection is short lived, the start of the decline being apparent 9 weeks after vaccination with vaccines based on highly attenuated strains. IBV exists as scores of serotypes (defined by the neutralization test), cross-protection often being poor. Consequently, chickens may be re-vaccinated, with the same or another serotype, two or three weeks later. Single applications of inactivated virus has generally led to protection of <50% of chickens. Two applications have led to 90 to 100% protection in some reports, but remaining below 50% in others. In practice in the field, inactivated vaccines are used in laying birds that have previously been primed with two or three live attenuated virus vaccinations. This increases protection of the laying birds against egg production losses and induces a sustained level of serum antibody, which is passed to progeny. The large spike glycoprotein (S) comprises a carboxy-terminal S2 subunit (approximately 625 amino acid residues), which anchors S in the virus envelope, and an amino-terminal S1 subunit (approximately 520 residues), believed to largely form the distal bulbous part of S. The S1 subunit (purified from IBV virus, expressed using baculovirus or expressed in birds from a fowlpoxvirus vector) induced virus neutralizing antibody. Although protective immune responses were induced, multiple inoculations were required and the percentage of protected chickens was too low (<50%) for commercial application. Remarkably, expression of S1 in birds using a non-pathogenic fowl adenovirus vector induced protection in 90% and 100% of chickens in two experiments. Differences of as little as 5% between the S1 sequences can result in poor cross-protection. Differences in S1 of 2 to 3% (10 to 15 amino acids) can change serotype, suggesting that a small number of epitopes are immunodominant with respect to neutralizing antibody. Initial studies of the role of the IBV nucleocapsid protein (N) in immunity suggested that immunization with bacterially expressed N, while not inducing protection directly, improved the induction of protection by a subsequent inoculation with inactivated IBV. In another study, two intramuscular immunizations of a plasmid expressing N induced protective immunity. The basis of immunity to IBV is not well understood. Serum antibody levels do not correlate with protection, although local antibody is believed to play a role. Adoptive transfer of IBV-infection-induced αβ T cells bearing CD8 antigen protected chicks from challenge infection. In conclusion, live attenuated IBV vaccines induce good, although short-lived, protection against homologous challenge, although a minority of individuals may respond poorly. Inactivated IBV vaccines are insufficiently efficacious when applied only once and in the absence of priming by live vaccine. Two applications of inactivated IBV are much more efficacious, although this is not a commercially viable proposition in the poultry industry. However, the cost and logistics of multiple application of a SARS inactivated vaccine would be more acceptable for the protection of human populations, especially if limited to targeted groups (e.g. health care workers and high-risk contacts). Application of a SARS vaccine is perhaps best limited to a minimal number of targeted individuals who can be monitored, as some vaccinated persons might, if infected by SARS coronavirus, become asymptomatic excretors of virus, thereby posing a risk to non-vaccinated people. Looking further into the future, the high efficacy of the fowl adenovirus vector expressing the IBV S1 subunit provides optimism for a live SARS vaccine, if that were deemed to be necessary, with the possibility of including the N protein gene.
RÉSUMÉ
On peut dire que les vaccins contre la bronchite infectieuse (IB) des poulets (Gallus gallus domesticus) ont été ceux qui, parmi les vaccins contre les maladies à coronavirus, ont eu le plus de succès; et certainement ceux qui ont été les plus utilisés au niveau mondial, les autres étant ceux à coronavirus bovins, canins, félins et porcins.
Le virus de l'IB (IBV), le coronavirus de la dinde (Meleagridis gallopavo) et celui du faisan de Colchide (Phasianus Colchicus) avec lesquels il y a des relations génétiques font partie du groupe 3 des coronavirus; le coronavirus SARS a été provisoirement classé dans le groupe 4 et les autres coronavirus des mammifères ont été classés dans les groupes 1 et 2.
L'IBV se multiplie au niveau des tissus respiratoires (incluant le nez, la trachée, les poumons, les sacs aériens) causant des symptômes respiratoires, mais également au niveau des reins (associé à une néphrite mineure ou majeure), de l'oviducte et en différents endroits du tractus alimentaire : œsophage, proventricule, duodénum, jéjunum, bourse de Fabricius, amygdales caecales rectum et cloaque, sans causer de troubles cliniques. Le virus peut persister puis être re-excrété au début de la ponte (à l'âge de 4 à 5 mois) passant pour être une conséquence du stress au moment de l'entrée en ponte.
Des lignées génétiques de poulet réagissent différemment à l'IBV notamment en ce qui concerne la mortalité chez les poulets et la clearance du virus après la phase aiguë.
Les souches d'IBV atténuées (par passage sur œufs embryonnés) ont été utilisées comme vaccins dans les années 1950 puis une vingtaine d'années plus tard des vaccins inactivés ont été développés pour booster la protection des animaux en ponte. Les vaccins à virus vivants sont généralement administrés à l'âge d'un jour chez les poulets de chair. Dans les conditions expérimentales, ceci peut entraîner une immunité stérile lors d'une épreuve à virus virulent homologue. Bien que 100% des poulets peuvent être protégés (contre les signes cliniques et la perte de l'activité ciliaire au niveau de la trachée), il arrive que 10% des poulets vaccinés ne présentent pas de réponse immune protectrice. La protection conférée par les souches vivantes est courte, elle commence à décliner neuf semaines après la vaccination pour les vaccins préparés avec des souches très atténuées. L'IBV présente de nombreux sérotypes (définis par séroneutralisation), la protection croisée est souvent faible. En conséquence, les poulets doivent être revaccinés avec le même ou un autre sérotype deux ou trois semaines plus tard.
Une simple administration de vaccin inactivé entraîne généralement une protection des sujets inférieure à 50%. D'après certaines publications, deux administrations entraînent 90 à 100% de protection, mais pour d'autres la protection est inférieure à 50%. Sur le terrain, les vaccins inactivés sont administrés aux futures adultes qui ont reçu au préalable deux ou trois vaccins à virus vivants atténués. Ceci augmente la protection des animaux adultes contre les pertes de protection en œufs et induit des anticorps sériques à un niveau soutenu qui sont transmis à la descendance.
La glycoprotéine de spicule (S) comprend une sous-unité S2 carboxyterminal (environ 625 résidus d'acides aminés) qui permet l'encrage de S dans l'enveloppe du virus et une sous-unité S1 amino-terminal (environ 520 résidus) considérée être la forme bulbeuse importante et distale de S. La sous-unité S1 (purifiée du IBV, exprimée en baculovirus ou chez des oiseaux ayant reçu le vecteur viral variole aviaire) induit des anticorps neutralisants (VN). Des réponses immunes et protectrices ont été induites, mais plusieurs inoculations sont nécessaires et pour autant le pourcentage de poulets protégés est trop faible (< 50%) pour être utilisés sur le terrain. Il est à souligner que l'expression de S1 chez des oiseaux après administration d'un vecteur adénovirus aviaire non pathogène a induit une protection de 90% et de 100% des poulets au cours de deux expérimentations. Des différences aussi faibles que 5% entre des séquences de S1 peuvent entraîner une protection croisée faible. Des différences de 2 à 3% au niveau de S1 (correspondant à 10 ou 15 acides aminés) peuvent changer le sérotype, suggérant qu'un petit nombre d'épitopes sont immunodominants en ce qui concerne les anticorps neutralisants.
Des études préliminaires sur le rôle de la protéine de Capside (N) de l'IBV au niveau immunitaire ont suggéré que l'immunisation avec N exprimé en bactérie, bien que n'induisant pas directement une protection, a amélioré l'induction de la protection lors de l'inoculation ultérieure d'un IBV inactivé. Dans une autre étude, deux immunisations intramusculaires d'un plasmide exprimant N ont induit une immunité protectrice.
La base de l'immunité de l'IBV n'est pas encore parfaitement connue. Les taux d'anticorps sériques ne sont pas corrélés avec la protection, même si les anticorps locaux jouent un rôle. Des transferts de cellules T alphabéta induisant l'infection de l'IBV, portant l'antigène CD8 ont protégé des poulets contre une épreuve virulente.
En conclusion, les vaccins vivants atténués de l'IBV induisent une bonne protection bien que de courte durée vis-à-vis d'une épreuve homologue, même si une minorité d'individus ont des réponses faibles. Les vaccins inactivés de l'IBV sont insuffisamment efficaces quand ils sont administrés une seule fois en absence de vaccins à virus vivants administrés préalablement. Deux administrations de vaccin inactivé d'IBV sont beaucoup plus efficaces bien que cela ne soit pas une proposition valable pour l'industrie avicole. Quoi qu'il en soit, le coût et la logistique de l'administration multiple de vaccin inactivé du SARS devraient être beaucoup acceptables pour la protection des populations humaines principalement s'il s'agit de groupes ciblés, tels que le personnel soignant et les contacts à risque élevé. L'administration d'un vaccin contre le SARS est certainement mieux si elle est limitée à un petit nombre d'individus ciblés qui pourront être suivis. En effet, les personnes vaccinées peuvent, si elles sont infectées par le coronavirus du SARS, devenir asymptomatiques excrétrices de virus et ainsi présenter un risque vis-à-vis des personnes non vaccinées. Pour l'avenir, le vecteur adénovirus aviaire exprimant la sous-unité S1 de l'IBV qui présente une bonne efficacité, permet d'être optimiste pour un vaccin vivant contre le SARS, si ceci s'avère nécessaire, avec la possibilité d'inclure le gène de la protéine N.
ZUSAMMENFASSUNG
Von den Impfstoffen gegen Coronavirus-induzierte Erkrankungen sind wohl die Impfstoffe gegen die infektiöse Bronchitis (IB) des Huhnes (Gallus gallus domesticus) die erfolgreichsten und sicherlich die am häufigsten eingesetzten, wobei die anderen Impfstoffe gegen Rinder-, Kaninchen-, Katzen- und Schweinecoronaviren gerichtet sind. Das IB-Virus (IBV) ist zusammen mit den genetisch verwandten Coronaviren der Pute (Meleagris gallopavo) und des Ringfasans (Phasianus colchicus) ein Coronavirus der Gruppe 3, während das SARS Coronavirus vorläufig in die Gruppe 4 eingeordnet wurde und die anderen bekannten Mammalier-Coronaviren zu den Gruppen 1 und 2 gehören.
Das IBV vermehrt sich nicht nur im Respirationstrakt (einschließlich Nase, Trachea, Lungen und Luftsäcken verbunden mit Verursachung einer respiratorischen Erkrankung) sondern auch in Niere (assoziiert mit gering- bis hochgradiger Nephritis), Ovidukt und in vielen Teilen des Verdauungstrakts - Ösophagus, Proventriculus, Duodenum, Jejunum, Bursa Fabricii, Zäkaltonsillen, Rektum und Kloake, dort jedoch gewöhnlich ohne klinische Auswirkungen. Das Virus kann persistieren und wird mit Beginn der Legetätigkeit (im Alter von 4-5 Monaten) wieder ausgeschieden, wobei die Virusaktivierung als Konsequenz aus dem damit verbundenen Stress angesehen wird.
Verschiedene genetische Hühnerlinien unterscheiden sich im Ausmaß der durch IBV verursachten Mortalität sowie hinsichtlich der Virus-Clearance nach der akuten Infektionsphase.
In den 1950iger Jahren wurden (durch Passagen in embryonierten Hühnereiern) attenuierte IBV-Stämme als Lebendvirusvakzinen eingeführt. Einige Jahrzehnte später folgten Inaktivatvakzinen zur Boosterung der Schutzwirkung bei Legehennen. Lebendvirusimpfstoffe werden gewöhnlich bei Masthühnern am ersten Lebenstag appliziert. Unter experimentellen Bedingungen kann dies zu einer sterilen Immunität führen, wenn die Tiere mit einem virulenten homologen Virus belastungsinfiziert werden. Obwohl 100 % der Tiere geschützt sein können (gegen klinische Symptome und Zilienverlust in der Trachea), zeigen manchmal 10 % der Küken keine schützende Immunantwort. Die Schutzwirkung ist kurzfristig. Neun Wochen nach der Vakzination mit hoch attenuierten Impfstämmen wird der Beginn des Abfalls sichtbar. Das IBV besitzt eine Vielzahl von Serotypen (definiert durch Neutralisationstests), wobei die Kreuzschutzwirkung oft gering ist. Daraus folgt, dass Hühner zwei bis drei Wochen nach der Erstvakzination mit dem gleichen oder einem anderen Serotyp revakziniert werden sollten.
Einmalige Applikation von inaktiviertem Virus hat allgemein zum Schutz von 50 % der Hühner geführt. Zwei Applikationen riefen laut einiger Veröffentlichungen einen 90-100%igen Schutz hervor, in anderen Untersuchungen blieb er jedoch unter 50 %. Unter Praxisbedingungen im Feld werden Inaktivatimpfstoffe bei Legehennen eingesetzt, die vorher zwei- oder dreimal mit attenuierten Lebendvirusvakzinen geimpft worden sind. Dies erhöht die Schutzwirkung bei den Legehennen gegen Legeleistungseinbrüche und induziert einen anhaltenden Antikörperlevel, der an die Nachkommen weitergegeben wird.
Das große Spikeprotein (S) umfasst eine S2-Untereinheit am Karboxy-Ende (ungefähr 625 Aminosäuren), die das S in der Virushülle verankert, und eine S1-Untereinheit am Amino-Ende ( ungefähr 520 Aminosäuren), von der angenommen wird, dass sie größtenteils den distalen Bulbusteil des S formt. Die S1-Untereinheit (gereinigt aus dem IBV, exprimiert unter Verwendung von Baculovirus oder exprimiert in Hühnern mit Hilfe eines Vektor-Hühner-Pockenvirus) induzierte Virusneutralisierende (VN) Antikörper. Obwohl eine schützende Immunantwort induziert wurde, waren mehrfache Inokulationen erforderlich und der Prozentsatz geschützter Hühner war zu niedrig ( < 50 %) für die kommerzielle Anwendung. Bemerkenswerterweise induzierte die Exprimierung von S1 in Hühnern unter Verwendung eines apathogenen Vektor-Hühner-Adenovirus in zwei Experimenten eine Schutzwirkung bei 90 bzw. 100 % der Tiere. Unterschiede von weniger als 5 % in den S1-Sequenzen können in geringen Kreuzschutzwirkungen resultieren. Unterschiede im S1 von 2-3% (10-15 Aminosäuren) können den Serotyp ändern, was vermuten lässt, dass eine geringe Anzahl von Epitopen immunodominant ist hinsichtlich der neutralisierenden Antikörper.
Anfangsuntersuchungen zur Bedeutung des IBV-Nukleokapsidproteins (N) für die Immunität legten nahe, dass die Immunisierung mit in Bakterien exprimiertem N, obwohl nicht direkt Schutz auslösend, die Induktion der Schutzwirkung bei einer nachfolgenden Inokulation mit inaktiviertem IBV verbesserte. In einer anderen Studie führten zwei intramuskuläre Immunisierungen mit einem N exprimierenden Plasmid zu einer schützenden Immunität.
Die Grundlagen der Immunität gegen IBV sind nicht gut erforscht. Serumantikörper korrelieren nicht mit der Schutzwirkung, doch lokalen Antiköpern wird eine wichtige Rolle zugesprochen. Die adoptive Übertragung von durch IBV-Infektion induzierten alpha/beta-T-Zellen mit CD8-Oberflächenantigen schützten Hühnerküken gegen eine Belastungsinfektion.
Zusammenfassend lässt sich sagen, dass attenuierte Lebendvirusimpfstoffe einen guten, aber kurzfristigen Schutz gegen homologe Belastungsinfektionen hervorrufen, wobei eine Minderheit der Tiere eine schlechte Immunantwort zeigen können. IBV-Inaktivatvakzinen sind nur ungenügend wirksam, wenn sie nur einmalig und ohne Vorvakzination mit einer Lebendvirusvakzine angewendet werden. Zwei Applikationen von inaktiviertem IBV sind viel effizienter, doch dies ist ein kommerziell nicht rentabler Vorschlag für die Geflügelindustrie. Für den Schutz der menschlichen Bevölkerung jedoch dürften die Kosten und die Logistik für eine mehrfache Applikation einer SARS-Inaktivatvakzine akzeptabler sein, zumal wenn die Anwendung auf bestimmte Zielgruppen wie Beschäftigte im Gesundheitswesen und hoch gefährdete Kontaktpersonen beschränkt wird. Die Applikation eines SARS-Impfstoffs sollte möglichst limitiert bleiben auf eine minimale Anzahl von Zielpersonen, die überwacht werden können, da einige geimpfte Individuen, wenn sie sich mit dem SARS-Coronavirus infizieren, asymptomatische Virusausscheider werden können und somit ein Risiko für die nicht vakzinierte Bevölkerung darstellen können. Beim Blick auf die Zukunft stimmt die hohe Wirksamkeit des IBV-S1-Subunit-exprimierenden Vektor-Hühner-Adenovirus optimistisch für eine SARS-Lebendvirus-Vakzine mit der Möglichkeit der Inkorporierung des N-Proteingens, falls das für nötig erachtet werden sollte.
REVISION
Las vacunas frente a la bronquitis infecciosa (IB) de pollos (Gallus gallus domesticus) han sido las más exitosas y ciertamente las más comúnmente usadas de las vacunas frente a enfermedades causadas por coronavirus, siendo las otras frente a coronavirus bovino, canino, felino y porcino. El virus de IB (IBV), junto con otros coronavirus relacionados genéticamente como el del pavo (Meleagridis gallopavo) y faisán común (Phasianus colchicus), forman el grupo 3 coronavirus, mientras que el coronavirus del SARS se encuentra en el grupo 4 y el resto de coronavirus de mamíferos en los grupos 1 y 2.
El IBV se replica no únicamente en tejidos del aparato respiratorio (incluída la cavidad nasal, tráquea, pulmones y sacos aéreos, causando enfermedad respiratoria), sino también en el riñón (asociado con nefritis de diferente intensidad), oviducto, y en varias partes del tracto digestivo — esófago, proventrículo, duodeno, yeyuno, bolsa de Fabricio, tonsilas cecales, recto y cloaca, habitualmente sin efectos clínicos. El virus puede persistir, siendo reexcretado al inicio de la puesta (a los 4 o 5 meses de edad), probablemente como consecuencia del estrés de la entrada en puesta.
Las diversas líneas genéticas de pollos presentan diferencias respecto a la mortalidad causada por la infección con IBV en pollos y respecto a la eliminación del virus tras la fase aguda.
Las cepas vivas atenuadas de IBV (mediante pases en huevos de pollo embrionados) fueron introducidas como vacunas en 1950s, y un par de décadas después fueron seguidas por las vacunas inactivadas para prolongar la protección en aves de puesta. Las vacunas vivas se aplican normalmente en aves de carne al día de edad. En condiciones experimentales esto puede resultar en una inmunidad estéril cuando los animales son desafiados con un virus homólogo. Aunque el 100% de los pollos puede estar protegido (frente a la sintomatología clínica y a la pérdida de actividad ciliar de la tráquea), en ocasiones el 10% de las aves vacunadas no responden con una respuesta inmune protectora. La protección suele durar poco y empieza a declinar a las nueve semanas tras la vacunación con vacunas basadas en cepas muy atenuadas. Existen varios serotipos de IBV (definidos mediante técnicas de neutralización), pero la protección cruzada suele ser pobre. En consecuencia los pollos son revacunados con el mismo u otro serotipo, dos o tres semanas después.
Las aplicaciones únicas de virus inactivado normalmente protegen a < 50% de los pollos. Se ha descrito que dos aplicaciones pueden llegar a proteger del 90 al 100% de las aves, pero en otras ocasiones se ha descrito una protección por debajo del 50%. En la práctica de campo, las vacunas inactivadas se utilizan en aves de puesta que han sido previamente vacunadas con dos o tres vacunas de virus vivo atenuado. Esto aumenta la protección en las aves de puesta frente a las pérdidas de producción de huevos e induce una nivel de anticuerpos séricos sostenido y que pasa a la progenie.
La glicoproteína de la espícula (S) comprende una subunidad carboxi/terminal S2 (de aproximadamente 625 aminoácidos), que enclava la S en el envoltorio viral, y una subunidad aminoterminal S1 (de aproximadamente 520 aminoácidos) que forma la parte distal de la parte bulbosa de la S. La subunidad S1 (purificada de un virus de IBV, expresada en baculovirus o en aves mediante un vector de virus de viruela aviar) induce anticuerpos neutralizante (VN). Aunque se indujeron respuestas inmunes protectoras, se requirieron múltiples inoculaciones y el porcentaje de pollos protegidos fue demasiado bajo ( < 50%) para poder ser aplicada comercialmente. Cabe remarcar, que la expresión de S1 en aves mediante un vector de adenovirus aviar no patógeno indujo una protección de entre 90 y 100% de los pollos en dos experimentos. Diferencias de tan sólo el 5% en las secuencias de la S1 resultaron en una protección cruzada baja. Diferencias de entre el 2 y el 3% (de 10 a 15 aminoácidos) en la S1 pueden cambiar el serotipo, lo que sugiere que un número bajo de epítopos son inmunodominantes respecto a los anticuerpos neutralizantes.
Los estudios iniciales sobre el papel que juega la proteína de la nucleocápside (N) de IBV en la inmunidad sugieren que la inmunización con proteína N expresada en bacterias, aunque no induce protección directamente, mejoró la protección inducida por una inoculación subsiguiente con IBV inactivado. En otro estudio, dos inmunizaciones intramusculares con un plásmido que expresaba N indujeron una inmunidad protectora.La base de la inmunidad frente a IBV no está totalmente clara. Los niveles de anticuerpos en suero no se correlacionan con la protección, aunque se reconoce que los anticuerpos locales juegan un papel importante. La transferencia de linfocitos T CD8 alfabeta inducidos mediante una infección con IBV, protegieron a los pollos frente a una infección experimental.
En conclusión, las vacunas vivas atenuadas inducen una buena protección, aunque de corta duración, frente a la infección experimental homóloga, pero una minoría de individuos puede responder pobremente. Las vacunas de IBV inactivadas no son suficientemente eficaces cuando se administran únicamente una vez y no se realiza una primovacunación con una vacuna viva. Dos aplicaciones de una vacuna inactivada de IBV son mucho más eficaces, aunque no parece una propuesta viable desde el punto de vista comercial para la industria avícola. Aún así, el coste y la logística de una aplicación múltiple de una vacuna inactivada frente al SARS serían más aceptables para la protección de una población humana, especialmente si está limitada a grupos de alto riesgo, como trabajadores relacionados con temas sanitarios o personas con alto riesgo de contacto. La aplicación de la vacuna frente al SARS es quizás mejor limitarla a un número de individuos de riesgo que puedan ser monitorizados, ya que algunas personas vacunadas podrían, al estar infectadas con el coronavirus del SARS, volverse excretores asintomáticos del virus, siendo un riesgo para la población no vacunada. De cara a un futuro próximo, la alta eficacia de un vector de adenovirus aviar que expresa la subunidad S1 del IBV proporciona optimismo con respecto a una vacuna viva de SARS, y si se cree necesario podría haber la posibilidad de incluir el gen de la proteína N.
Introduction
Severe acute respiratory syndrome (SARS) emerged in humans in Guangdong Province, China, in late 2002 and subsequently spread to other continents early the following year (Chan-Yeung & Yu, 2003; Lee et al., 2003; Peiris et al., 2003; Poutanen et al., 2003; Tsang et al., 2003). Shortly after the spread of the virus beyond China the causative agent was quickly identified as being a species of coronavirus (SARS coronavirus) that was previously unknown (Drosten et al., 2003; Ksiazek et al., 2003; Marra et al., 2003; Peiris et al., 2003; Rota et al., 2003). Indeed, the proteins of SARS coronavirus had such low amino acid identity with those of known coronaviruses that it has been tentatively assigned to a new coronavirus group 4. The three existing coronavirus groups (Table 1) were initially devised on the basis of a lack of antigenic relationships between the species of different groups (Pedersen et al., 1978; Sánchez et al., 1990; Enjuanes et al., 2000; González et al., 2003). It is probable that the vast majority of the human population is susceptible to SARS coronavirus. The SARS epidemic was contained at ‘only’ 8460 cases by the enormous effort, and sacrifice, of a large number of individuals associated with many public authorities. So severe were the infections, in a fully susceptible population, that 808 died; doubtless a greater proportion would have died had it not been for the medical attention focussed upon them. Sadly, a large proportion of the deaths in some countries were sustained by health care workers. In addition to the human misery, there were dire economic consequences for those countries most affected.
Table 1. Coronavirus species.
| Group 1 | Porcine transmissible gastroenteritis coronavirus |
| Canine enteric coronavirus | |
| Feline coronavirus | |
| Porcine epidemic diarrhoea coronavirus | |
| Human coronavirus 229E | |
| Group 2 | Murine hepatitis coronavirus |
| Human coronavirus OC43 | |
| Bovine coronavirus | |
| Canine respiratory coronavirus | |
| Porcine haemagglutinating encephalomyelitis coronavirus | |
| Group 3 | Infectious bronchitis coronavirus |
| Turkey coronavirus | |
| Pheasant coronavirus | |
| Group 4 | SARS coronavirusa |
SARS coronavirus has provisionally been placed in group 4, based on the criteria previously used to place the other coronaviruses into groups (i.e. extremely low amino acid identity between its proteins and those of the other three groups, and nature and organization of its non-structural protein genes).
The last SARS cases of the early 2003 epidemic were in June 2003. The disease might arise again by further transmission from the suspected animal reservoir, or transmission from human, possibly asymptomatic, carriers. There is evidence from studies of avian infectious bronchitis virus (IBV) and feline coronavirus that coronaviruses establish persistent infections. Chicks that had been experimentally infected with IBV at 1 day of age re-excreted virus at around 19 weeks of age (Jones & Ambali, 1987). It is suspected that the stressor of the start of egg production caused the release of the virus. Approximately 10% of cats that had been naturally infected with feline coronavirus became asymptomatic carriers, excreting virus for over 1 year (Addie & Jarrett, 2001; Addie et al., 2003). Others excreted virus for periods of several months.
The World Health Organisation has called for the development of vaccines against SARS. This short review looks at the experiences with IBV vaccines over half a century, which might be instructive with regard to the development of SARS vaccines.
Infectious bronchitis
As suggested by its name, IBV causes respiratory disease, although its replication is not limited to the respiratory tract. It is the major respiratory virus of the chicken (the domestic fowl), as it is endemic in probably all countries that raise chickens. Its host range is considered to be limited to the chicken (Cavanagh & Naqi, 2003), although genetically very similar coronaviruses cause disease in turkeys (enteric disease; Guy, 2000; Cavanagh et al., 2001) and pheasants (respiratory and kidney disease; Lister et al., 1985; Gough et al., 1996; Cavanagh et al., 2002). IBV occurs globally, both in chickens kept on a large and a small scale (Wunderwald & Hoop, 2002). It exists as scores of serotypes/genotypes (Cavanagh, 2001; Meulemans et al., 2001; Farsang et al., 2002), which are problematic with regard to prophylaxis.
IBV causes deciliation of the ciliated epithelia of the nose and trachea, clinical signs being similar to those of the common cold caused in humans by the group 1 and group 2 human coronaviruses (HCoV-229E and HCoV-OC43, respectively; Cavanagh, 2000, 2004). Chickens, especially those of only a few days or weeks of age, exhibit nasal discharge, snicking (similar to sneezing), watery eyes and lethargy (Dhinakar Raj & Jones, 1997; Cavanagh & Naqi, 2003). Some chickens exhibit râles, a vibration emanating from lower in the respiratory tract, and small areas of pneumonia may be observed in the lungs (Dhinakar Raj & Jones, 1997). Notwithstanding this, and the fact that titres of virus in the lungs can be similar to those in the nose and trachea, bronchitis is not considered to cause pneumonia.
From a welfare point of view the hardest hit are chicks of only a few days or weeks of age. Some may die directly from the viral infection but a greater number die following secondary bacterial infection. An overall slowing down of growth causes further economic losses. Juvenile and mature birds suffer less from IBV infection although the economic consequences of infection in egg-laying stock can be disastrous, as egg production drops precipitously and usually does not rise back to normal in the flock as a whole.
As discussed further later, IBV grows at many epithelial surfaces beyond the respiratory tract (reviewed by Dhinakar Raj & Jones, 1997). Although many alimentary tract tissues are susceptible to IBV, infection of enteric tissues usually does not manifest itself clinically. Recently a strain of IBV has been associated with disease of the proventriculitis (the cranial glandular compartment of the stomach, adjacent to the gizzard, which is the caudal muscular compartment) (Yu et al., 2001a). Nephritis is not uncommon in a proportion of naturally IBV-infected meat-type birds, environmental factors playing a role. Some IBV strains are intrinsically nephropathogenic; that is, they reproducibly cause nephritis when inoculated experimentally into specific pathogen free chickens (Lambrechts et al., 1993; Pensaert & Lambrechts, 1994; Cook et al., 2001; Li & Yang, 2001). The oviduct is also susceptible to IBV, which may contribute to diminished egg production.
Infectious bronchitis virus
IBV contains the same number of structural proteins (i.e. those present in virons) as the group 1 coronaviruses; that is, a nucleocapsid protein associated with the 27.6 kb single-stranded, positive-sense RNA genome, a large spike glycoprotein (S), a smaller integral membrane glycoprotein (M) and a few molecules of the envelope protein (Lai & Cavanagh, 1997; Enjuanes et al., 2000; González et al., 2003). It does not contain a haemagglutinin esterase glycoprotein such as is found in group 2 coronaviruses. The coronavirus S protein is a dimer or trimer (Cavanagh, 1983; Gallagher & Buchmeier, 2001; Lewicki & Gallagher, 2002). The S glycopolypeptide of IBV, like that of group 2 coronaviruses, is cleaved into amino-terminal S1 (approximately 520 amino acid residues) and carboxy-terminal S2 (approximately 625 residues) glycopolypeptides (Lai & Cavanagh, 1997). The IBV S protein has been demonstrated to be a determinant of the host cell range in vitro. When the S protein gene of an infectious cDNA clone of the Beaudette strain (Casais et al., 2001), which replicates in mammalian Vero and BHK cells, was replaced by that of the M41 strain, which does not replicate in those cell lines, the recombinant virus was unable to replicate in them (Casais et al., 2003). The recombinant did replicate in primary chick kidney cells, as did both the Beaudette and M41 strains. Whether the S protein of IBV is a determinant of tissue tropism in vivo remains to be determined.
Tissue tropism of IBV
IBV initially infects the upper respiratory tract. Titres of live virus are maximal in the nose and trachea by 3 days post inoculation (by eye-drop and intranasally) and remain so for 2 to 5 days further, depending on the strain (Table 2) (Ambali & Jones, 1990; Hofstad & Yoder, 1966). Thereafter the titre falls to below detectable levels, although virus was still detectable at 14 days in some studies (Ambali & Jones, 1990). The virus is restricted to the ciliated and mucus-secreting cells (reviewed by Dhinakar Raj & Jones, 1997). Similar virus titres occur in the lungs and airsacs, sometimes peaking a little after those in the nose and trachea. Small areas of pneumonia may be observed in the lungs (Dhinakar Raj & Jones, 1997).
Table 2. Titres of IBV (strain G) in respiratory and non-respiratory tissues following intranasal and eye-drop inoculation of 1-day-old chicks (from Ambali & Jones, 1990).
| Virus titre (log10 CD50/g)a on the following days after inoculation | ||||||
|---|---|---|---|---|---|---|
| Tissue | 1 day | 3 days | 5 days | 7 days | 10 days | 14 days |
| Non-enteric | ||||||
| Trachea | 5 | 5 | 5 | 5 | 3 | 2 |
| Kidney | 3 | 4 | 5 | 5 | 4 | 3 |
| Upper gut | ||||||
| Proventriculus | 5 | 3 | 3 | 3 | 3 | 3 |
| Duodenum | 4 | 4 | 3 | 3 | 3 | 3 |
| Jejunum | 0 | 2 | 2 | 0 | 0 | 0 |
| Lower gut | ||||||
| Rectum | 3 | 5 | 6 | 4 | 4 | 4 |
| Bursa | 2 | 3 | 5 | 4 | 3 | 3 |
| Caecal tonsil | 2 | 3 | 4 | 4 | 4 | 4 |
| Ileum | 3 | 5 | 3 | 3 | 3 | 3 |
CD, ciliostatic dose; the virus was titrated in tracheal organ cultures, the presence of virus being indicated by cessation of ciliary activity. For simplicity, titres have been rounded to the nearest log value.
Lucio & Fabricant (1990) investigated the growth of several isolates of IBV in chickens. Although they did not quantify the virus that they recovered, they showed that infectious virus was recovered from the trachea, bronchus, lung, oesophagus, proventriculus, duodenum, jejunum, caeca (caecal tonsils), kidney and cloaca. The longest periods of isolation were in respect of the kidney and caecal tonsils, the latter being at the distal end of the alimentary tract in birds.
Ambali & Jones (1990) used another strain (strain G) of IBV that produced high titres in the kidney, similar to those in the trachea (Table 1). Various alimentary tract tissues (proventriculus, duodenum, ileum, caecal tonsils, bursa of Fabricius and rectum) had peak titres the same as in the trachea and kidney, or within 10-fold of that amount (Table 1). Fluorescence revealed the location of the virus, in epithelial cells. Several other isolates were compared with the G strain; they were isolated much less frequently or not at all from kidney and rectal contents (El-Houadfi et al., 1986). Organ culture studies showed that while all strains studied by Bhattacharjee & Jones (1997) grew in the proventriculus, bursa and kidney explants, some did not grow in the caecal tonsil or rectal explants.
In the experiments of Hofstad & Yoder (1966), titres in the kidney and bursa of Fabricius were approximately two orders of magnitude lower than in the respiratory tissues for a number of IBV strains of low passage number in embryos. High egg-passaged virus (85 passages) was almost undetectable in the kidney and bursa, and gave reduced titres in the trachea and lung.
The most intensively studied tropism, apart from the upper respiratory tract, has been that for the kidney. Although only a small proportion of IBV isolates exhibit high nephropathogenicity, these can cause up to 44% mortality within 3 weeks of intratracheal inoculation (Pensaert & Lambrechts, 1994) in the absence of vaccination. Histopathological and immunochemical studies of the process showed that the MA-87 strain of virus attacked mainly the lower nephron down to the collecting duct epithelial cells (Chen & Itakura, 1996; Chen et al., 1996). An ultrastructural investigation revealed that the virus replicated in all segments of tubules and ducts, but more frequently in the epithelial cells of the collecting ducts, collecting tubules, distal convoluted tubules and Henle's loops (Chen, 1996). The extent of mortality is age related (greatest in chicks; Lambrechts et al., 1993) and varies according to the line of bird; for example, 18% and 44% of an egg layer-type bird and a meat-type bird, respectively (Pensaert & Lambrechts, 1994).
Birds have a small lymphoid organ, the Harderian gland, in the eye-socket that is a major contributor to locally produced antibody for protecting oculonasal mucosae (reviewed by Dhinakar Raj & Jones, 1997). Inoculation of a mild vaccinal strain of IBV by eye-drop resulted in replication of virus in the gland and partial damage to it (Toro et al., 1996). There was an increase in the number of plasma cells and enlargement of lymphoid foci. IBV has also been isolated from another lymphoid organ, the bursa of Fabricius (Table 1) (El-Houadfi et al., 1986; Ambali & Jones, 1990), with gross and histopathological lesions occurring, even after infection with vaccinal strains.
Most recently, Yu et al. (2001a) have studied the pathogenesis of three isolates of IBV from the proventriculus of chicken flocks that had affected proventriculi. Experimental infections with these strains resulted in age-dependent mortality (75 to 100% in 2-week-old birds, 0 to 25% in 16-week-old chickens). Necropsy of sick chickens revealed that the mucosa of the proventriculus was thickened and exuded a milky fluid when squeezed, and the entire proventriculus was enlarged.
Thus, IBV strains as a group productively infect a large range of epithelial surfaces, literally from the top to the bottom of the chicken. Isolates differ in their extent of replication in the non-respiratory tissues, and some produce clinical disease in non-respiratory tissues, most notably the kidney and proventriculus. The pantropism of IBV might be the case for SARS coronavirus, as the latter has not only been associated with pneumonia, but also with diarrhoea (although it remains to be demonstrated whether the SARS virus is replicating in enteric tissues; Peiris et al., 2003). A point of difference is that whereas SARS virus is associated with severe clinical signs in both the respiratory and enteric tracts, IBV is usually limited to disease in the respiratory tract.
IBV exists as dozens of serotypes
It is not appropriate in this short review to go into detail about the extensive antigenic variation exhibited by IBV (Darbyshire et al., 1979; Ignjatovic & Mcwaters; 1991; Cook et al., 1999; Cavanagh, 2001; Meulemans et al., 2001; Farsang et al., 2002); it is an issue that may not arise with SARS coronavirus. That said, only a few amino acid differences in the S1 protein of two strains of IBV are sufficient to change the serotype.
The large spike glycoprotein (S) comprises a carboxy-terminal S2 subunit (approximately 625 amino acid residues), which anchors S in the virus envelope, and an amino-terminal S1 subunit (approximately 520 residues), believed to largely form the distal bulbous part of S (Cavanagh, 1995; Lai & Cavanagh, 1997). It is the S1 subunit that induces virus-neutralizing (VN) antibody. This has been demonstrated in various ways: failure to induce VN by virus from which S1 had been removed (Cavanagh et al., 1986); induction of VN antibody by S1 released from virions by treatment with urea (Cavanagh et al., 1986), by non-ionic detergent, followed by affinity chromatography (Ignjatovic & Galli, 1994) and by expression from a vaccinia virus recombinant (Tomley et al., 1987); by production of monoclonal antibody-resistant mutants, the mutations being in the S1 part of the spike protein gene (Cavanagh et al., 1988; Koch et al., 1990; Kant et al., 1992).
Failure of serotypes to induce cross-protective immunity
Experimental vaccination studies usually involve application of virus to the nostrils and by eye-drop, sometimes by direct application to the trachea. Protection is determined by challenge, 3 weeks after vaccination, the challenge virus being applied by the same route. Three main approaches to the assessment of protection have been: (1) observation of clinical signs (not commonly used); and removal of the trachea at 4 or 5 days after challenge followed by either (2) quantitative assessment of ciliary activity or (3) detection of live challenge virus, usually by inoculation of embryonated eggs. The second and third methods result in similar deductions being made as regards protection (Marquardt et al., 1982), although in some studies challenge virus was isolated from some individuals whose ciliary activity was unaffected or little affected. Arguably, declaring a chicken as non-protected because of the recovery of small amounts of challenge virus, when ciliary activity is normal, is being too stringent.
Most IBV serotypes differ from each other by 20 to 25% of S1 amino acids (Adzhar et al., 1997; Kingham et al., 2000), although some differ by up to 50% (Cavanagh et al., 1997; Gelb et al., 1997). (Differences between the other IBV proteins are in the region of 10%, rarely exceeding 15%; Cavanagh et al., 2001, 2002.) Generally speaking, the immunity induced by inoculation with one serotype protects poorly against infection by heterologous serotypes.
With regard to protection of the respiratory tract, in some experiments some heterologous challenge viruses broke through in 100% of the chickens (i.e. 0% cross-protection) (Hofstad, 1981; Marquardt et al., 1982; Picault et al., 1986). Other challenge viruses, known now to differ by 20% or so in S1 from the virus used to initially infect the chickens, broke through immunity in 30 to 70% of cases (Rosenberger et al., 1976; Hofstad, 1981). Collectively these and other reports show that cross-protection can range from very poor to moderate (poor for commercial purposes), although in some cases the differences among these reports may be also be due to differences in stringency by which protection was assessed (discussed later). When Winterfield et al. (1976) used clinical criteria to assess cross-protection, a vaccinal strain of the Massachusetts serotype was concluded to protect all chickens (in groups of 10) against challenge with serotypes now known to differ by 20% or so in their S1 proteins. Protection was assessed as being less when re-isolation of challenge virus was used as the criterion for protection. There is no doubt that in the field the application of a vaccine that is homologous to the prevailing field strain has given better protection than an heterologous vaccine.
Cross-protection against nephritis is also usually poor, although some strains are more cross-protective than others (Pensaert & Lambrechts, 1994; Cook et al., 2001).
Monoclonal antibody-resistant mutant investigations have revealed that many of the amino acids involved in the formation of VN epitopes are located within the first and third quarters of the linear S1 polypeptide (Cavanagh et al., 1988; Koch et al., 1990; Kant et al., 1992). Sequence analysis of variants that are genetically very similar (>95% amino acid identity in S1) has shown that most of the differences are within these two regions (Cavanagh et al., 1992; Adzhar et al., 1997). Moreover, some variants that differed by only 2 to 3% of S1 amino acids (10 to 15 residues) behaved as different serotypes (Cook, 1984; Cook & Huggins, 1986; Cavanagh et al., 1992).
Would small S1 differences between SARS coronavirus isolates be of practical significance?
It is conceivable that there are variants (e.g. with small differences in S1) of SARS coronavirus in the field (i.e. within its natural host(s)). If this were to be the case, and if variants infected humans, would the variants be able to break through the immunity induced by a vaccine based on a strain isolated from humans in early 2003? Unfortunately there have been few protection studies based on closely related variants of IBV.
Cavanagh et al. (1997) inoculated groups of 10 chickens with the virulent UK/6/82 isolate and challenged with isolates that differed by up to 4% of S1 amino acids (some of which were different serotypes; Cook, 1984; Cook & Huggins, 1986), protection being assessed on retention of ciliary activity in trachea. Challenge with two variants (98% S1 identity with UK/6/82) resulted in challenge scores (a relative measure of retention of ciliary activity) virtually the same as with the homologous challenge. Challenge with two others isolates (96% and 98% S1 identity, respectively), resulted in a higher challenge score, indicative of less cross-protection, although the numbers were not statistically significantly different. Also, in three experiments one of each group of 10 birds initially inoculated with the UK/6/82 virus was clearly not protected against homologous challenge. The chickens had been individually inoculated with a high dose (5 to 6 log10 infectious virus) of the primary inoculum. Therefore, this lack of protection in 10% of birds was unlikely to have been due to a failure to initiate infection with the primary virus, but rather to heterogeneity of the immune response.
In other studies approximately 10% of chickens in a group, which had been inoculated and challenged with the same strain or with one of the same serotype, were not fully protected (Winterfield et al., 1976; Hofstad, 1981; Picault et al., 1986; Parsons et al., 1992; Cavanagh et al., 1997; Cook et al., 1999; Nix et al., 2000) (using virus isolation or tracheal ciliary activity as the criterion). These results show that chickens (out-bred, although with restricted sets of parental breeding stocks) are not uniform in their response to IBV vaccination. One would imagine that the human population might be even more heterogeneous in response to vaccination against SARS.
Nix et al. (2000) inoculated chickens with several virulent isolates that had 94% or greater amino acid identity in S1. All were assessed as being of the same serotype, Arkansas, but some cross-reacted in a VN test much less than others. Some isolates induced 100% protection, assessed by re-isolation of challenge virus, a stringent test, against challenge, including against challenge with a strain that was of lower serological identity. However, two other isolates induced cross-protection of only 55 to 58% of the chickens when the challenge virus was of lower serological identity.
Clearly, the induction of protective immunity to IBV is complex. It not only depends on the antigenic relatedness of the primary infecting strain and a subsequent challenge strain, but also on the response of individual chickens; approximately 10% of chickens in some experiments failed to develop a protective immune response. Furthermore, while breeds of chicken are similar in that they are all efficiently infected by IBV, resulting in similar titres of virus, they differ in their capacity to clear the infection (Otsuki et al., 1990). Small (∼ 5%) differences in S1 amino acids would appear to be sometimes responsible for poor cross-protection. It should be borne in mind that while the focus has been on the relatedness of the S1 protein, other viral proteins may have a role in protective immunity; for example, the nucleocapsid protein, of which more later. Recently, we (T. Hodgson, R. Casais, B. Dove, P. Britton & D. Cavanagh, manuscript in preparation) have substituted (swapped) the spike protein gene of our Beaudette infectious clone (receiver strain) with that of the M41 strain (donor strain). Both strains are of the Massachusetts serotype and have 95.0% S1 amino acid identity. The Beaudette and M41 strains are attenuated and pathogenic for chickens, respectively. When chickens were inoculated with the donor, receiver or spike-swapped recombinant IBV and challenged with M41, the receiver strain induced almost no tracheal protection (assessed by ciliary activity) while the recombinant IBV induced protection almost as good as the donor strain. This was not due to an increased virulence of the receiver strain, as the spike-swapped recombinant was as non-pathogenic as the Beaudette receiver strain, and both replicated poorly. As the receiver strain and recombinant virus had identical proteins except for the spike protein, it would appear that the poor immunity induced by the receiver strain against the donor strain was due to some of the 5.0% of amino acid differences (27 different residues) in S1. This supports the findings of earlier work that small differences in S1 can contribute to poor cross-protection (Cavanagh et al., 1997; Nix et al., 2000).
Vaccination with live-attenuated vaccines
Meat-type chicks (broilers) are usually vaccinated on the day of hatch, in the hatchery, by spray. The birds are vaccinated at this very early age in part for logistical/economic reasons. Also, because IBV is endemic and ever-present in many poultry-rearing parts of the world, protection is needed as early as possible. The consequences of IBV infection are greater for chicks than older birds. Also, the modern broiler goes for processing at only 6 weeks of age, so vaccination has to be early.
Many studies have been undertaken during the past 30 years or so to examine the degree of protection induced by single inoculations of various serotypes of IBV. In many of the studies referred to in the previous section, the initial inocula were virulent strains, not attenuated derivatives. This was an advantage in that the degree of protective immunity induced by a virus is in part dependent on its virulence. Notwithstanding, the overall conclusions reached, already described, were similar, irrespective of whether the initial inoculum virus was virulent or attenuated.
Duration of respiratory tract immunity following live attenuated vaccination
Protection of the respiratory tract following a single live attenuated virus vaccination has been found to be short-lived; Gough & Alexander (1979) and Darbyshire & Peters (1984) reported a decline in the number of protected chickens at 6 and 9 weeks after vaccination, respectively. In regions where a given serotype of IBV vaccine is consistently not giving acceptable protection against field challenge, the birds might be revaccinated 2 to 3 weeks after the first application. Sometimes this is with the same vaccine preparation, sometimes with another product of the same serotype, and sometimes with a vaccine of a heterologous serotype. The latter approach sometimes gives protection against a broader range of serotypes (Cook et al., 1999).
Protection against nephritis by live vaccines
Some strains of IBV are markedly nephropathogenic. For example, experimental tracheal infection of commercial meat-type birds with the B1648 nephropathogenic strain resulted in the death of approximately one-third of a group of 68 chickens within 9 days. Vaccination, by coarse spray, with the homologous attenuated strain completely protected against mortality upon challenge 4 weeks later (Pensaert & Lambrechts, 1994). Vaccination with non-homologous, commercially available vaccines markedly, although not completely, reduced mortality. The homologous vaccine had prevented detectable growth of the challenge virus in the trachea, as ascertained by virus isolation, but the heterologous vaccines did not do so. Challenge virus in the kidney was assessed by immunofluorescence. By this criterion the number of chicks with detectable IBV in the kidney was reduced by 84% by vaccination with the homologous vaccine, and not at all by the heterologous vaccines.
Vaccination with inactivated vaccines
Inactivated oil-emulsion IBV vaccines were developed during the 1960s and 1970s. The objective was to make a vaccine that would give long-lasting immunity to the hen bird, to protect against drops in egg production. Single applications of inactivated virus induced little or no protection against egg loss (McDougall, 1969; Box et al., 1980; Muneer et al., 1987) and no protection against loss of ciliary activity in the trachea (Martins et al., 1991).
However, immunization of 11 chickens with a single dose of inactivated IBV strain M41 resulted in 36 to 45% (depending on the criterion used) developing respiratory tract protection (Cavanagh et al., 1986). A dose of 100 μg was not more efficacious than 5 μg. In a second experiment, 29% (based on retention of ciliary activity in the trachea) to 76% (based on not isolating challenge virus) of 17 chickens were protected after a dose of 10 μg. In a third experiment, 40% of 10 chickens were judged protected (retention of ciliary activity) following a single 10 μg dose. It is possible that larger amounts of virus was used in these three experiments than by previous workers, and that this was the reason for the partial success.
As part of a study of the role of the IBV nucleocapsid protein (N) protein in protective immunity (discussed later), Boots et al. (1992) immunized chickens with inactivated IBV at 4 weeks of age and again 6 weeks later. After challenge, 1/8 and 8/8 of the two groups of chickens, respectively, were protected, by assessment of ciliary activity in trachea. The degree of protection was assessed as being slightly less (88%) when immunofluorescence was used to detect infected tracheal cells.
Ignjatovic & Galli (1994) gave four doses, each of 200 μg, of inactivated IBV; 40% and 20% of 10 birds had developed protection (assessed by non-re-isolation of challenge virus) in the trachea and kidney, respectively, after three doses. These percentages did not increase after four doses. Song et al. (1998) immunized chickens up to three times with an inactivated nephropathogenic strain. Protection (assessed by non-re-isolation of challenge virus) of the kidney and trachea was 50% and 13%, respectively, of eight chickens, after two inoculations, rising to 88% and 50%, respectively, after three inoculations.
To see whether two doses of inactivated vaccine would be protective against loss of egg production, McDougall (1969) vaccinated birds at an interval of 8 weeks and challenged them 18 weeks later. Only a few of the vaccinated birds had respiratory signs and these lasted for less than 3 days, compared with 8 days for the unvaccinated controls. The birds that had received the inactivated vaccine were largely protected against a drop in egg production. Gough et al. (1977) demonstrated a poor serum antibody response to a single application of inactivated infectious bronchitis vaccine but titres increased markedly upon revaccination 10 weeks later.
The approach commonly used in the poultry industry today is to vaccinate young females two or more times with live vaccine, followed by one dose of inactivated vaccine as the birds come into lay. The live vaccines serve to give protection to the young bird and to prime the immune response to the later inactivated vaccine (Gough et al., 1977; Box et al., 1980; Finney et al., 1990). Gough et al. (1977) demonstrated that the chickens that had received one dose of live vaccine followed by inactivated vaccine 10 weeks later, were protected against challenge 10 weeks later, as assessed by re-isolation of challenge virus from trachea, oviduct and kidney. Box & Ellis (1985) reported that the response to the inactivated vaccine was poor if given within 8 weeks of a live vaccination.
Passive immunization
Macdonald et al. (1981) inoculated 12-week-old chickens with 2 ml convalescent IBV serum. Two days later they were challenged by intravenous application of the H52 vaccinal strain, a procedure known to result in nephritis. The birds were protected against kidney disease, as assessed by lack of watery droppings, macroscopic and microscopic pathology in the kidney, in contrast to control birds to which non-immune serum had been given. The passive application of immune serum did not protect against respiratory infection, although onset was delayed and of shorter duration.
Variation among genetic lines of chicken in their susceptibility to IBV
Smith et al. (1985) and Cook et al. (1986) demonstrated varying mortality among different breeds of chicken, both when inoculated with a mixture of IBV isolates alone and when in conjunction with a pool of Escherichia coli strains. The IBV predisposed the birds to infection with the bacterium, resulting in increased mortality. Bumstead et al. (1989) continued with this IBV/E. coli infection, showing marked variation in mortality among several inbred lines of White Leghorn chickens. More detailed analysis of two of these lines, line 15I (highly susceptible) and line C (relatively resistant), showed that the rate of virus production and titres in the respiratory tract were similar during the first 4 days after infection (Otsuki et al., 1990). Thereafter, however, titres declined much more slowly in the susceptible line 15I, taking two-fold longer to decline to undetectable levels. In tracheal explants of the two lines there was no difference in the replication profile of the virus, showing that the difference in the birds themselves was not because of an intrinsic difference in the capacity of the trachea to support the replication of IBV. The underlying cause of the difference in vivo might be immunological.
The extent to which infection by a nephropathogenic strain caused mortality was also dependent on the type of chicken (Pensaert & Lambrechts, 1994). Thus, 0% and 2% of a specified pathogen free flock died within the periods 5 to 7 days and 7 to 9 days, respectively, after tracheal inoculation of the B1648 strain. In contrast, 15% and 21% of commercial meat-type birds died in these periods.
Presence and duration of antibody in the serum, trachea and nasal secretions
The humoral immune response to IBV vaccination has mostly been investigated by measuring antibody levels in serum, using enzyme-linked immunosorbent assay (ELISA), VN or haemagglutination-inhibition (HAI) tests. (IBV haemagglutinates very poorly until treated with enzyme preparations containing neuraminidases; Alexander et al., 1983; Schultze et al., 1992, and references therein.). However, there have also been a few studies of IBV antibodies in the nose and trachea. It should be stated at the outset that many studies have shown that the presence or absence or titre of serum antibody to IBV does not correlate with protection; that is, vaccinated chickens may be protected against respiratory disease IBV irrespective of the titre of serum antibody (for example, Raggi & Lee, 1965; Ignjatovic & Galli, 1994).
The profile of the serum antibody response depends on the method used to detect it. Following infection of chickens with a virulent strain of IBV, specific antibody was first detected by ELISA (plates coated with IBV), a majority of the birds having detectable antibody by 6 days after infection, with titres maximal within 21 days and tending to decline shortly afterwards (Mockett & Darbyshire, 1981). VN antibody was delayed in comparison with ELISA-detected antibody, being first detected in the period 9 to 21 days after infection, peaking within 35 days and remaining level for the remainder of the sampling period (63 days after inoculation), although in 25% (2/8) of the birds the VN antibody was still increasing at 63 days, when sampling ended. The HAI antibodies were first detected at 9 days, peaking at 14 to 17 days and then declining, although in one bird the HAI titre did not peak until much later (as with the VN titre in the same bird).
Mockett (1985) also compared ELISAs using purified S and M proteins with the conventional virus-coated ELISA; the primary and secondary antibody response profiles were very similar, VN antibody again later than ELISA antibody.
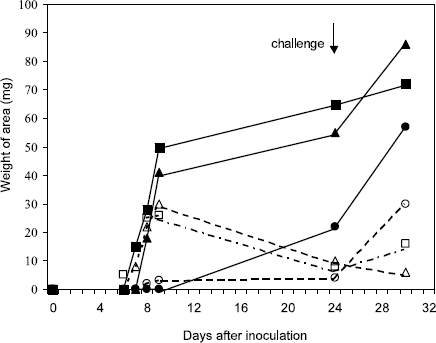
Following infection with a live IBV vaccinal strain there was a good primary immunoglobulin (Ig)M response (Mockett & Cook, 1986; Martins et al., 1991). As expected, the primary IgM response peaked, and declined, before that of the IgG response (Figure 1) (Martins et al., 1991). The secondary IgM response (i.e. in response to a second (challenge) infection) peaked at the same time as that of IgG, but declined faster (Figure 1). Mockett & Cook (1986) suggested that measurement specifically of serum IgM would be useful in defining recent infection. In contrast to the response after IBV infection, vaccination with inactivated virus produced almost no IgM response, as well as a poor IgG response (Figure 1) (Martins et al., 1991).
Figure 1. Antibody induction by inactivated and live IBV vaccination, from Martins et al. (1991). Profile of IBV-specific IgM and IgG responses of unprimed 14-week-old chickens to live attenuated vaccine (H120) given by eye-drop or intramuscularly (i.m.), and to inactivated vaccine (oil emulsion) given i.m. The IgM and IgG were measured using an ELISA. The ELISA results (as absorbance values) aere plotted graphically, and the areas corresponding to each immunoglobulin class were cut out and weighed. The chickens were challenged with virulent IBV 24 days after vaccination. IgG: live vaccine, eye-drop (▄); live vaccine, i.m. (▴); inactivated vaccine, i.m. (•). IgM: live vaccine, eye-drop (□); live vaccine, i.m. (△); inactivated vaccine, i.m. (○).
A single and double vaccination with inactivated IBV resulted in HAI titres of 1/28 and 1/219, respectively, 4 and 2 weeks after the respective vaccinations (Gough et al., 1977). A single inoculation of 3 μg purified IBV induced maximum titres of HAI and ELISA antibody of 6 log2 and 12 log2, respectively, by 3 to 4 weeks after vaccination (Cavanagh et al., 1986). VN antibodies peaked 1 week later, at 5 log2. Titres decreased eight-fold within 4 weeks of peaking.
Holmes (1973) measured VN antibody in nasal washings after challenge of chickens that had received either a single live virus infection or two intra-muscular inoculations (4 weeks apart) with inactivated IBV. Serum antibody levels rose rapidly in both groups. Nasal antibody rose more slowly in the group that had received inactivated virus than in the group that had received a primary inoculum of live virus.
Induction of protection by subviral and vectored vaccines
There is no doubt that the IBV S protein, when inoculated on its own, can induce protective immunity. The proportion of chickens being protected may be dependent on the manner by which the S protein is presented to the host. There is also evidence that the N protein can prime protective immune responses, and one report that the N protein on its own induced protective immunity.
Immunization with the S protein
Whereas a single inoculation of 10 μg IBV induced tracheal protection in 40% of 10 chickens, all birds were susceptible after immunization with IBV from which the S1 subunit had been removed by urea, indicating that the S1 subunit was required to induce protective immunity (Cavanagh et al., 1986). The S1 that had been released by treatment with urea was dialysed against decreasing concentrations of urea, and eventually in the absence of urea; this might have enabled some renaturation of the protein. Sedimentation analysis indicated, as expected, that the S1 that had been released by urea was in monomeric form; that is, it was no longer a multimeric (dimer or trimer) molecule with quaternary structure. Notwithstanding, 22% and 44% of 10 chickens that had been inoculated on four occasions with 3 μg S1 developed VN and HAI antibody, respectively. There were no positives following three inoculations. Protective immunity was not assessed in that experiment.
Ignjatovic & Galli (1994) dispersed the proteins of a nephropathogenic IBV using non-ionic detergent and purified S1, N and M by affinity chromatography using monoclonal antibodies. Chickens were inoculated on four occasions, 50 μg for each dose. Chickens that had been immunized with N or M proteins did not develop protective immunity (assessed by non-recovery of challenge virus) to either the trachea or kidney following challenge. However, 71% and 86% of 10 chickens inoculated with S1 developed tracheal and kidney immunity after four inoculations. The respective percentages were 70% and 10% after three inoculations.
The S1 protein has also been expressed in Spodoptera frugiperda cells from a recombinant Autographa californica baculovirus (Song et al., 1998). When chickens were inoculated with these cells containing expressed S1, 43% and 0% of seven birds were protected (assessed by non-re-isolation of challenge nephropathogenic virus) in the kidney and trachea, respectively. Protection rose to 50% and 25%, respectively, of eight chickens after three inoculations.
The S1 protein has also been expressed in situ from fowlpoxvirus and adenovirus vectors. Three inoculations, in the wing web, of a recombinant fowlpoxvirus expressing S1 induced IBV-specific antibodies (Wang et al., 2002). After challenge with IBV, there was somewhat less recovery of challenge virus and less tracheal damage than in controls, and only mild clinical signs. Greater protection was achieved following a single oral application of a fowl adenovirus expressing S1 (Johnson et al., 2003). Protection (assessed by non-re-isolation of challenge virus) was obtained in 90% and 100% of 10 to 13 chickens in two experiments.
Immunization with the N protein
The first study of the role of the IBV N protein in immunity was by Boots et al. (1992). The N protein was in the form of a bacterially expressed fusion protein with β-galactosidase. Initial experiments were with mice, inoculated in the footpad. A single inoculation resulted in popliteal lymph node cell proliferative responses to IBV. Delayed-type hypersensitivity responses were obtained when mice had been inoculated with: (a) inactivated IBV on two occasions; (b) inactivated IBV followed by N protein; and (c) N protein followed by inactivated IBV.
Experiments were subsequently undertaken with chickens. Following a single intramuscular inoculation of inactivated IBV, 1/8 birds were protected against respiratory challenge, as assessed by ciliary activity in the trachea, and none were protected in a group inoculated with N protein. Other chickens were inoculated with either inactivated virus or N protein, and then inoculated a second time, 6 weeks later, with inactivated IBV. After challenge, 8/8 and 8/10 chickens in the two groups, respectively, developed protective immunity (Table 3). The extent of protection was assessed as being slightly less when immunofluorescence was used to detect challenge virus in tracheal cells (Table 3). In a third group in which birds had been inoculated with β-galactosidase without any N protein, and then inoculated with inactivated IBV, some chickens had protective immunity, induced by the secondary inoculation with inactivated IBV (Table 3). Linear regression analysis showed that this was statistically less than in the group that had been primed with the N protein. The authors concluded that immunization with the N protein had induced protective immunity by activation of cytotoxic or helper T-cell responses.
Table 3. Priming of tracheal protection by primary immunization of chickens with IBV N protein (modified from Boots et al., 1992).
| Number of protected chickens | ||||
|---|---|---|---|---|
| Ciliary activity assay | Immunofluorescence assay | |||
| Primary immunization | Challenge after primary immunizationa | Challenge after secondary immunizationb | Challenge after primary immunizationa | Challenge after secondary immunizationb |
| Inactivated IBV | 1/8 | 8/8 | NDc | 7/8 |
| β-galactosidase–IBV N protein | 0/8 | 8/10 | ND | 7/10 |
| fusion protein | ||||
| β-galactosidase | 0/8 | 8/12 | ND | 4/12 |
Chickens were challenged by eye-drop 4 weeks after primary immunization.
The secondary immunization was inactivated IBV. Chickens were challenged by eye-drop 4 weeks after secondary immunization.
ND, not done.
Two intramuscular immunizations of chickens with a plasmid expressing the N protein, or a fragment of the N protein, induced immune responses that protected the birds from infection by IBV (applied by eye-drop and intranasally), as evidenced by mark reduction in replication of the challenge virus (Seo et al., 1997b). A fragment of the N protein comprising the carboxyterminal 120 amino acid residues was sufficient to induce protection. Yu et al. (2001b) expressed this fragment in chickens using a single inoculation of a fowlpoxvirus recombinant. Upon challenge with a homologous strain and one heterologous strain of IBV, the birds did not develop clinical signs, although some replication of challenge virus was detected by an ELISA. The birds were not protected against challenge with a different heterologous strain.
Nature of protective immune responses to IBV
Collisson and colleagues (Seo & Collisson, 1997b; Pei et al., 2003) have shown that cytotoxic T-cell responses in chickens to IBV infection correlated with initial decreases in infection and clinical signs. Cytotoxic T-cell activity was major histocompatibility complex restricted, and lysis was mediated by CD8+CD4– cells. Adoptive transfer of IBV-infection-induced αβ T cells bearing CD8 antigen protected chicks from challenge infection (Seo et al., 2000; reviewed in Collisson et al., 2000). Earlier work has been reviewed by Dhinakar Raj & Jones (1997). Tissues from chickens infected with two field strains of IBV were assayed for interferon, which was readily detected in the trachea and lung but at only low levels in the plasma, kidney, liver and spleen (Otsuki et al., 1987).
Final comments
If there were to be a resurgence of SARS in humans, one would anticipate that the authorities would rapidly put into operation the various surveillance and control measures that proved successful in early 2003. If vaccines had been developed, vaccination might be included, especially for health care workers and contacts in the wider community. Coronaviruses can establish persistent infections, in at least a proportion of their hosts, resulting in chronic asymptomatic shedders, with subsequent problems for containment of the disease. For this and other reasons, a decision to apply a SARS vaccine would not be taken lightly. Notwithstanding, what type of vaccine might be used?
The poultry industry prefers to use live vaccines rather than inactivated ones. The former are cheaper to make and buy, and easier/cheaper to apply. These financial considerations would presumably not be paramount in the case of a resurgence of SARS in humans. Efficacy would be an important criterion for use of a particular vaccine, although that also applies in the poultry industry, of course. Safety of a SARS vaccine might be deemed more important than efficacy, assuming that a vaccine had an acceptable, if not complete, efficacy. In general, inactivated vaccines are considered to be safer than live attenuated vaccines, although the safety of inactivated vaccines cannot be taken for granted.
Unless a future SARS outbreak were to get rapidly out of control, it would seem unlikely that a conventional live attenuated vaccine would be used; the potential risk of vaccine-related problems (reactions) might be deemed too high. Genetic manipulation, in addition to traditional approaches, will be used as a means of attenuating SARS coronavirus (Kuo et al., 2000; Thiel et al., 2001; de Haan et al., 2002a,b; Casais et al., 2003; Ortego et al., 2003). However, a desirable outcome cannot be guaranteed and fears of vaccine-related problems will still be harboured. However, given the almost total susceptibility of the global human population, the lethal outcome of 10% of cases, and the huge strain put on the health care services, and economies, a killed vaccine, subunit vaccine or vectored vaccine might be used in the first instance, among selected groups of people.
The experience of single applications of inactivated IBV is not greatly promising in the context of SARS; a single application of inactivated IBV protected <50% of chickens, sometimes much less than 50%. However, even a poorly efficacious single application of an inactivated SARS vaccine might help to reduce the spread of the virus, by reducing the number of susceptible people. At the first sign of a resurgence of SARS, the authorities might decide to vaccinate key personnel, with a view to revaccinating them. The outcome of some experiments with IBV in this regard is promising. In a number of studies, two vaccinations with inactivated IBV produced protective immunity in >85% of chickens. That said, in some studies even >2 inoculations of high doses of inactivated IBV gave protection in only 50% or so of chickens. It might be that the criterion of protection used in these studies—non-re-isolation of challenge virus—might be too stringent. That is, some amelioration of the clinical effects of infection might be obtained even if there is some detectable replication of the challenge virus. In the case of infection by SARS coronavirus, if a vaccine only protected against the worst outcomes—pneumonia, death—then it might be deemed to have been successful.
Experimental IBV subunit vaccines, in the form of the S1 protein, were efficacious, but, as expected, not more so than inactivated virus. In two studies, protection of the trachea was achieved after three vaccinations in 70% and 59% of chickens, respectively. Protection of the kidney was less efficient (10% and 25%, respectively).
Vectored SARS vaccines might be considered likely to be less potentially problematic, with regard to safety, than attenuated SARS coronavirus. Perhaps such a vaccine might be used if an outbreak developed into an epidemic or pandemic. In this regard the results of the investigation by Johnson et al. (2003) are very promising; a single oral application of a non-pathogenic fowl adenovirus expressing the IBV S1 protein gave 90+% protection, as good as is obtained by conventional attenuated IBV.
In the event that any type of live vaccine was used against SARS, an inactivated SARS vaccine might subsequently be applied, to build upon the initial vaccination and perhaps provide at least medium-term protection. The experience with IBV is very good in this regard.
If SARS coronavirus were to re-emerge in humans, its S1 protein might not be the same as that of the 2002/2003 outbreak. Research with IBV has indicated that differences of only 5% of S1 protein amino acid can reduce cross-protection. Consequently, S1 differences among SARS coronavirus isolates must not be viewed complacently. The finding that the IBV N protein plays a beneficial role in immunity suggests that the SARS virus N protein should not be overlooked in a SARS vaccine development programme.
References
- Addie D.D. & Jarrett O. (2001). Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Veterinary Record, 148, 649–653. [DOI] [PubMed] [Google Scholar]
- Addie D.D., Schaap I.A.T., Nicolson O. & Jarrett O. (2003). Persistence and transmission of natural type 1 feline coronavirus infection. Journal of General Virology, 84, 2735–2744. [DOI] [PubMed] [Google Scholar]
- Adzhar A., Gough R.E., Haydon D., Shaw K., Britton P. & Cavanagh D. (1997). Molecular analysis of the 793/B serotype of infectious bronchitis virus in Great Britain. Avian Pathologyogy, 26, 625–640. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Allan W.H., Biggs P.M., Bracewell C.D., Darbyshire J.H., Dawson P.S., Harris A.H., Jordan F.T.W., Macpherson I., McFerran J.B., Randall C.J., Stuart J.C., Swarbrick O. & Wilding G.P. (1983). A standard technique for haemagglutination inhibition test for antibodies to avian infectious bronchitis virus. The Veterinary Record, 113, 64. [DOI] [PubMed] [Google Scholar]
- Ambali A.G. & Jones R.C. (1990). Early pathogenesis in chicks with an enterotropic strain of infectious bronchitis virus. Avian Diseases, 34, 809–817. [PubMed] [Google Scholar]
- Bhattacharjee P.S. & Jones R.C. (1997). Susceptibility of organ cultures from chicken tissues for strains of infectious bronchitis virus isolated from the intestine. Avian Pathology, 26, 553–563. [DOI] [PubMed] [Google Scholar]
- Boots A.M.H., Benaissa-Trouw B.J., Hesselink W, Rijke E., Schrier G. & Hensen E.J. (1992). Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine, 10, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box P.G., Beresford A.W. & Roberts B. (1980). Protection of laying hens against infectious bronchitis with inactivated emulsion vaccines. The Veterinary Record, 106, 264–268. [DOI] [PubMed] [Google Scholar]
- Box P.G. & Ellis K.R. (1985). Infectious bronchitis in laying hens: interference with response to emulsion vaccine by attenuated live vaccine. Avian Pathologyogy, 14, 9–22. [DOI] [PubMed] [Google Scholar]
- Bumstead N., Huggins M.B. & Cook J.K.A (1989). Genetic differences in susceptibility to a mixture of avian infectious bronchitis virus and Escherichia coli. British Poultry Science, 30, 39–48. [DOI] [PubMed] [Google Scholar]
- Casais R., Thiel V., Siddell S., Cavanagh D. & Britton P. (2001). A reverse genetics system for the avian coronavirus infectious bronchitis virus. Journal of Virology, 75, 12359–12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D. & Britton P. (2003). A recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. Journal of Virology, 77, 9084–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. (1983). Coronavirus IBV: structural characterisation of the spike protein. Journal of General Virology, 64, 2577–2583. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. (1995). The coronavirus surface glycoprotein. In Siddell S.G. (Ed.), The Coronaviridae (pp. 73–113). New York: Plenum Press. [Google Scholar]
- Cavanagh D. (2000). Coronaviruses and toroviruses. In Zuckerman A.Z., Banatvala J.E. & Pattison J.R. (Eds.), Principles and Practice of Clinical Virology, 4th edn (pp. 345–356). Chichester: John Wiley and Sons. [Google Scholar]
- Cavanagh D. (2001). Innovation and discovery: the application of nucleic acid-based technology to avian virus detection and characterization. Avian Pathology, 30, 581–598. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. (2004). Coronaviruses and toroviruses. In Zuckerman A.J., Banatvala J.E., Griffiths P.D., Pattison J.R. & Schoub B.D. (Eds.), Principles and Practice of Clinical Virology, (5th edn) (in press). Chichester: John Wiley & Sons. [Google Scholar]
- Cavanagh D. & Naqi S. (2003). Infectious bronchitis. In Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R. & Swayne D.E. (Eds.), Diseases of Poultry, 11th edn (pp. 101–119). Ames: Iowa State Press. [Google Scholar]
- Cavanagh D., Davis P.J., Darbyshire J.H. & Peters R.W. (1986). Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralising or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. Journal of General Virology, 67, 1435–1442. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J. & Mockett A.P.A. (1988). Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Research, 11, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K.A., Li D., Kant A. & Koch G. (1992). Location of the amino-acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious-bronchitis virus. Avian Pathology, 21, 33–43. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Ellis M.M. & Cook J.K.A. (1997). Relationship between variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection. Avian Pathology, 26, 63–74. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Sharma M., Drury S.E., Ainsworth H.L., Britton P. & Gough R.E. (2001). Detection of a coronavirus from turkey poults in Europe genetically related to infectious bronchitis virus of chickens. Avian Pathology, 30, 365–378. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Welchman D.d.B., Britton P. & Gough R.E. (2002). Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathology, 31, 81–93. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M. & Yu W.C. (2003). Outbreak of severe acute respiratory syndrome in Hong Kong Special Administrative Region: a case report. British Medical Journal, 326, 850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.Y. (1996). CytoPathology of chick renal epithelial cells experimentally infected avian infectious bronchitis virus. Avian Pathology, 25, 675–690. [DOI] [PubMed] [Google Scholar]
- Chen B.Y. & Itakura C. (1996). Cytopathology of chick renal epithelial cells experimentally infected with avian infectious bronchitis virus. Avian Pathology, 25, 675–690. [DOI] [PubMed] [Google Scholar]
- Chen B.Y., Hosi S., Nunoya T. & Itakura C. (1996). Histopathology and immunohistochemistry of renal lesions due to infectious bronchitis virus in chicks. Avian Pathology, 25, 269–283. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Pei J., Dzielawa J. & Seo S.H. (2000). Cytotoxic T cells are critical in the control of infectious bronchitis virus in poultry. Developmental and Comparative Immunology, 24, 187–200. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A. (1984). The classification of new serotypes of infectious bronchitis virus isolated from poultry flocks in Great Britain between 1981 and 1983. Avian Pathology, 13, 733–741. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A. & Huggins M.B. (1986). Newly isolated serotypes of infectious bronchitis virus: Their role in disease. Avian Pathology, 15, 129–138. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Smith H.W. & Huggins M.B. (1986). Infectious bronchitis immunity: its study in chickens experimentally infected with mixtures of infectious bronchitis virus and Escherichia coli. Journal of General Virology, 67, 1427–1434. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Orbell S.J., Woods M.A. & Huggins M.B. (1999). Breadth of protection of therespiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous types. Avian Pathology, 28, 471–479. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Chesher J., Baxendale W., Greenwood N., Huggins M.B. & Orbell S.J. (2001). Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathology, 30, 423–426. [DOI] [PubMed] [Google Scholar]
- Darbyshire J.H. & Peters R.W. (1984). Sequential development of humoral immunity and assessement of protection in chickens following vaccination and challenge with avian infectious bronchitis virus. Research in Veterinary Science, 37, 77–86. [PubMed] [Google Scholar]
- Darbyshire J.H., Rowell J.G., Cook J.K.A. & Peters R.W. (1979). Taxonomic studies on strains of avian infectious bronchitis virus using neutralisation tests in tracheal organ cultures. Archives of Virology, 61, 227–238. [DOI] [PubMed] [Google Scholar]
- de Haan C.A.M., Masters P.S., Shen X-L., Weiss S. & Rottier P. (2002a). The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology, 296, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A.M., Volders H., Koetzner C.A., Masters P.S. & Rottier P. (2002b). Coronaviruses maintain viability despite dramatic rearrangments of the strictly conserved genome organization. Journal of Virology, 76, 12491–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhinakar Raj G. & Jones R.C. (1997). Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathology, 26, 677–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguiere A-M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J-C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H-D., Osterhaus A.D.M.E., Schmitz H. & Doerr H.W. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine, 348, 1967–1976. [DOI] [PubMed] [Google Scholar]
- El-Houadfi M., Jones R.C., Cook J.K.A. & Ambali A.G. (1986). The isolation and characterisation of six avian infectious bronchitis viruses isolated in Morocco. Avian Pathology, 15, 93–105. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Brian D., Cavanagh D., Holmes K., Lai M.M.C., Laude H., Masters P., Rottier P., Siddell S., Spaan W.J.M., Taguchi F. & Talbot P. (2000). Coronaviridae. In Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R. & Wickner R.B. (Eds.), Virus Taxonomy (pp. 835–849). New York: Academic Press. [Google Scholar]
- Farsang A., Ros C., Renström L.H.M., Baule C., Soós T. & Belák S. (2002). Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathology, 31, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney P.M., Box P.G. & Holmes H.C. (1990). Studies with a bivalent infectious bronchitis killed virus vaccine. Avian Pathology, 19, 435–450. [DOI] [PubMed] [Google Scholar]
- Gallagher T.M. & Buchmeier M.J. (2001). Coronavirus spike proteins in viral entry and pathogenesis. Virology, 279, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J. Jr, Keeler C.L. Jr, Nix W.A.K., Rosenberger J.K. & Cloud S.S. (1997). Antigenic and S-1 genomic characterization of the Delaware variant serotype of infectious bronchitis virus. Avian Diseases, 41, 661–669. [PubMed] [Google Scholar]
- González J.M., Gomez-Puertas P., Cavanagh D., Gorbalenya A.E. & Enjuanes L. (2003). A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Archives of Virology (in press). [DOI] [PMC free article] [PubMed]
- Gough R.E. & Alexander D.J. (1979). Comparison of duration of immunity in chickens infected with a live infectious bronchitis vaccine by three different routes. Research in Veterinary Science, 26, 329–332. [PubMed] [Google Scholar]
- Gough R.E., Allan W.H. & Nedelciu D. (1977). Immune response to monovalent and bivalent Newcastle disease and infectious bronchitis inactivated vaccines. Avian Pathology, 6, 131–142. [DOI] [PubMed] [Google Scholar]
- Gough R.E., Cox W.J., Winkler C.E., Sharp M.W. & Spackman D. (1996). Isolation and identification of infectious bronchitis virus from pheasants. Veterinary Record, 138, 208–209. [DOI] [PubMed] [Google Scholar]
- Guy J.S. (2000). Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses. Avian Pathology, 29, 206–212. [DOI] [PubMed] [Google Scholar]
- Hofstad M.S. (1981). Cross-immunity in chickens using seven isolates of avian infectious bronchitis virus. Avian Diseases, 25, 650–654. [PubMed] [Google Scholar]
- Hofstad M.S. & Yoder H.W. Jr (1966). Avian infectious bronchitis—virus distribution in tissues of chicks. Avian Diseases, 10, 230–239. [PubMed] [Google Scholar]
- Holmes H.C. (1973). Neutralizing antibody in nasal secretions of chickens following administration of avian infectious bronchitis virus. Archives de Gesamte Virusforschung, 43, 235–241. [DOI] [PubMed] [Google Scholar]
- Ignjatovic J. & Galli L. (1994). The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Archives of Virology, 138, 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J. & Mcwaters P.G. (1991). Monoclonal antibodies to three structural proteins of avian infectious bronchitis virus: characterization of epitopes and antigenic differentiation of Australian strains. Journal of General Virology, 72, 2915–2922. [DOI] [PubMed] [Google Scholar]
- Johnson M.A., Pooley C., Ignjatovic J. & Tyack S.G. (2003). A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine, 21, 2730–2736. [DOI] [PubMed] [Google Scholar]
- Jones R.C. & Ambali A.G. (1987). Re-excretion of an enterotropic infectious bronchitis virus by hens at point of lay after experimental infection at day old. Veterinary Record, 120, 617–620. [DOI] [PubMed] [Google Scholar]
- Kant A., Koch G., van Roozelaar D.J., Kusters J.G., Poelwijk J.G. & van der Zeijst B.A.M. (1992). Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. Journal of General Virology, 73, 591–596. [DOI] [PubMed] [Google Scholar]
- Kingham B.F., Keeler C.L. Jr, Nix W.A., Ladman B.S. & Gelb J. Jr (2000). Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Diseases, 44, 325–335. [PubMed] [Google Scholar]
- Koch G., Hartog L., Kant A. & van Roozelaar D.J. (1990). Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. Journal of General Virology, 7, 1929–1935. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C., Zaki S.R., Peret T., Emery S., Tong S-X., Urbani C., Corner J.A., Lim W., Rollin P.F., Dowell S., Ling A-E., Humphrey C., Shieh W-J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J., LeDuc J.W., Bellini W. & Anderson L.J. (2003). A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine, 348, 1953–1966. [DOI] [PubMed] [Google Scholar]
- Kuo L., Godeke G.J., Raamsman M.J., Masters P.S. & Rottier P.J. (2000). Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. Journal of Virology, 74, 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C. & Cavanagh D. (1997). The molecular biology of coronaviruses. Advances in Virus Research, 48, 1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts C., Pensaert M. & Ducatelle R. (1993). Challenge experiments to evaluate cross-protection induced at the trachea and kidney level by vaccine strains and Belgian nephropathogenic isolates of avian infectious bronchitis virus. Avian Pathology, 22, 577–590. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S. & Sung J.Y. (2003). A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine, 348, 1986–1994. [DOI] [PubMed] [Google Scholar]
- Lewicki D.N. & Gallagher T.M. (2002). Quaternary structure of coronavirus spikes in complex with carcinoembryonic antigen-related cell adhesion molecule cellular receptors. The Journal of Biological Chemistry, 277, 19727–19734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister S.A., Beer J.V., Gough R.E., Holmes R.G., Jones J.M.W & Orton R.G. (1985). Outbreaks of nephritis in pheasants (Phasianus colchicus) with a possible coronavirus aetiology. Veterinary Record, 117, 612–613. [DOI] [PubMed] [Google Scholar]
- Li H. & Yang H.C. (2001). Sequence analysis of nephropathogenic infectious bronchitis virus strains of the Massachusetts genotype in Beijing. Avian Pathology, 30, 535–541. [DOI] [PubMed] [Google Scholar]
- Lucio B. & Fabricant J. (1990). Tissue tropism of three cloacal isolates and Massachusetts strain of infectious bronchitis virus. Avian Diseases, 34, 865–870. [PubMed] [Google Scholar]
- Macdonald J.W., Randall C.J., McMartin D.A., Dagless M.D. & Gazdzinski P. (1981). Active and passive immunisation against nephritis induced by an avian infectious bronchitis virus. Avian Pathology, 10, 121–129. [DOI] [PubMed] [Google Scholar]
- Marquardt W.W., Kadavil S.K. & Snyder D.B. (1982). Comparison of ciliary activity and virus recovery from tracheas of chickens and humoral immunity after inoculation with serotypes of avian infectious bronchitis virus. Avian Diseases, 26, 828–834. [PubMed] [Google Scholar]
- Marra M.A., Jones S.J.M., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Drebot M., Fernanda L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C. & Roper R.L. (2003). The genome sequence of the SARS-associated coronavirus. Science, 300, 1399–1404. [DOI] [PubMed] [Google Scholar]
- Martins N.R. da Silva, Mockett A.P.A., Barrett A.D.T. & Cook J.K.A. (1991). IgM responses in chicken serum to live and inactivated infectious bronchitis virus vaccines. Avian Diseases, 35, 470–475. [PubMed] [Google Scholar]
- McDougall J.S. (1969). Avian infectious bronchitis virus: the protection afforded by an inactivated virus vaccine. Veterinary Record, 85, 378–381. [DOI] [PubMed] [Google Scholar]
- Meulemans G., Boschmans M., Decaesstecker M., van den Berg T.P., Denis P. & Cavanagh D. (2001). Epidemiology of infectious bronchitis virus in Belgian broilers: a retrospective study, 1986 to 1995. Avian Pathology, 30, 411–421. [DOI] [PubMed] [Google Scholar]
- Mockett A.P.A. (1985). Envelope proteins of avian infectious bronchitis virus: purification and biological properties. Journal of Virological Methods, 12, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett A.P.A. & Cook J.K.A. (1986). The detection of specific IgM to infectious bronchitis virus in chicken serum using an ELISA. Avian Pathology, 15, 437–446. [DOI] [PubMed] [Google Scholar]
- Mockett A.P.A. & Darbyshire J.H. (1981). Comparative studies with an enzyme-linked immunosorbent assay (ELISA) for antibodies to avian infectious bronchitis virus. Avian Pathology, 10, 1–10. [DOI] [PubMed] [Google Scholar]
- Muneer M.A., Newman J.A., Halvorson D.A., Sivanandan V. & Coon C.N. (1987). Effects of avian infectious bronchitis virus (Arkansas strain) on vaccinated laying chickens. Avian Diseases, 31, 820–828. [PubMed] [Google Scholar]
- Nix W.A., Troeber D.S., Kingham B.F., Keeler C.L. Jr & Gelb J. Jr (2000). Emergence of subtype strains of the Arkansas serotype of infectious bronchitis virus in Delmarva broiler chickens. Avian Diseases, 44, 568–581. [PubMed] [Google Scholar]
- Ortego J., Sola I., Almazan F., Ceriani J.E., Riquelme C., Balach M., Plana-Durán J. & Enjuanes L. (2003). Transmissible gastroenteritis coronavirus gene 7 is not essential but influences in vivo virus replication and virulence. Virology, 308, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki K., Nakamura T., Kubota N., Kawaoka Y. & Tsubokura M. (1987). Comparison of two strains of avian infectious bronchitis virus for their interferon induction, viral growth and development of virusneutralising antibody in experimentally-infected chickens. Veterinary Microbiology, 15, 31–40. [DOI] [PubMed] [Google Scholar]
- Otsuki K., Huggins M.B. & Cook J.K.A. (1990). Comparison of the susceptibility to infectious bronchitis virus infection of two inbred lines of White Leghorn chickens. Avian Pathology, 19, 467–475. [DOI] [PubMed] [Google Scholar]
- Parsons D., Ellis M.M., Cavanagh D. & Cook J.K.A. (1992). Characterisation of an avian infectious bronchitis virus isolated from IB-vaccinated broiler breeder flocks. The Veterinary Record, 131, 408–411. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Ward J. & Mengeling W.L. (1978). Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Archives of Virology, 58, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J., Elwood Briles W & Collisson E.W. (2003). Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology, 306, 376–384. [DOI] [PubMed] [Google Scholar]
- Peiris M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C., Chan K.H., Tsang D.N.C., Yung R.W.H., Ng T.K. & Yuen K.Y. (2003). Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet, 361, 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M. & Lambrechts C. (1994). Vaccination of chickens against a Belgian nephropathogenic strain of infectious bronchitis virus B1648 using attenuated homologous and heterologous strains. Avian Pathology, 23, 631–641. [DOI] [PubMed] [Google Scholar]
- Picault J.P., Drouin P., Guittet M., Bennejean G., Protais J., L'Hosptialier R., Gillet J.P., Lamande J. & Le Bachelier A. (1986). Isolation, characterisation and preliminary cross-protection studies with a new pathogenic avian infectious bronchitis virus (strain PL-84084). Avian Pathology, 15, 367–383. [DOI] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajen M., Petric M., Brunham R.C. & McGeer A.J. (2003). Identification of severe acute respiratory syndrome in Canada. New England Journal of Medicine, 348, 1995–2005. [DOI] [PubMed] [Google Scholar]
- Raggi L.G. & Lee G.G. (1965). Lack of correlation between infectivity, serological response and challenge results in immunization with an avian infectious bronchitis vaccine. Journal of Immunology, 94, 538–543. [PubMed] [Google Scholar]
- Rosenberger J.K., Alphin R.L. & Krauss W.C. (1976). Cross-protection studies with a Holland strain (Nobilis H-52) of infectious bronchitis virus. Avian Diseases, 20, 199–201. [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.-H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C.T., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rassmussen M., Fouchier R., Gunther S., Osterhaus A.D.M.E., Drosten C., Pallansch M.A., Anderson L.J. & Bellini W.J. (2003). Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science, 300, 1394–1399. [DOI] [PubMed] [Google Scholar]
- Sánchez C.M., Jiménez G., Laviada M.D., Correa I., Suñé C., Bullido M.J., Gebauer F., Smerdou C., Callebaut P., Escribano J.M. & Enjuanes L. (1990). Antigenic homology among coronaviruses related to transmissible gastroenteritis virus. Virology, 174, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Cavanagh D. & Herrler G. (1992). Neuraminidase treatment of avian infectious bronchitis coronavirus reveals a hemagglutinin activity that is dependent on sialic acid-containing receptors on erythrocytes. Virology, 189, 792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S. & Collisson E.W. (1997a). Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. Journal of Virology, 71, 5173–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S. & Collisson E.W. (1997b). The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protect chickens from acute infection. Journal of Virology, 71, 7889–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S., Pei J., Briles W.E., Dzielawa J. & Collisson E.W. (2000). Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology, 269, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.W, Cook J.K.A. & Parsell Z.E. (1985). The experimental infection of chickens with mixtures of infectious bronchitis virus and Escherichia coli. Journal of General Virology, 66, 777–786. [DOI] [PubMed] [Google Scholar]
- Song C.-S., Lee Y.-J., Lee C.-W., Sung H.-W., Kim J.-H., Mo I.-P., Izumiya Y., Jang H.-K. & Mikami T. (1998). Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. Journal of General Virology, 79, 719–723. [DOI] [PubMed] [Google Scholar]
- Thiel V., Herold J., Schelle B. & Siddell S.G. (2001). Infectious RNA transcribed in vitro from a cDNA copy of the human coronavirus genome in vaccinia virus. Journal of Virology, 82, 1273–1281. [DOI] [PubMed] [Google Scholar]
- Tomley F.M., Mockett A.P.A., Boursnell M.E.G., Binns M.M., Cook J.K.A., Brown T.D.K. & Smith G.L. (1987). Expression of the infectious bronchitis virus spike protein by recombinant vaccinia virus and induction of neutralising antibodies in vaccinated mice. Journal of General Virology, 68, 2291–2298. [DOI] [PubMed] [Google Scholar]
- Toro H., Godoy V., Larenas J., Reyes E. & Kaleta E.F. (1996). Avian infectious bronchitis viral persistence in the Harderian gland and histological changes after eyedrop vaccination. Avian Diseases, 40, 114–120. [PubMed] [Google Scholar]
- Tsang K.W., Ho P.K., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y. & Lai K.N. (2003). A cluster of cases of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine, 348, 1977–1985. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Schnitzlein WM., Tripathy D.N., Girshick T. & Khan M.I. (2002). Construction and immunogenicity studies of recombinant fowlpox containing the S1 gene of Massachusetts M41 strain of infectious bronchitis virus. Avian Diseases, 46, 831–838. [DOI] [PubMed] [Google Scholar]
- Winterfield R.W., Fadly A.M. & Hoerr F.J. (1976). Immunity to infectious bronchitis virus from spray vaccination with derivatives of a Holland strain. Avian Diseases, 20, 42–48. [PubMed] [Google Scholar]
- Wunderwald C. & Hoop R.K. (2002). Serological monitoring of 40 Swiss fancy breed poultry flocks. Avian Pathology, 31, 157–162. [DOI] [PubMed] [Google Scholar]
- Yu L., Jian Y., Low S., Wang Z., Nam S.J., Liu W. & Kwang J. (2001a). Characterisation of three infectious bronchitis virus isolates from China associated with proventriculitis in vaccinated chickens. Avian Diseases, 45, 416–424. [PubMed] [Google Scholar]
- Yu L., Liu W., Schnitzlein W.M., Tripathy D.N. & Kwang J. (2001b). Study of protection by recombinant fowlpox virus expressing C-terminal nucleocapsid protein of infectious bronchitis virus against challenge. Avian Diseases, 45, 340–348. [PubMed] [Google Scholar]


