Abstract
Background
Renal artery stenosis (RAS) can lead to hypertension and renal failure. Nevertheless, its treatment by percutaneous transluminal renal angioplasty (PTRA) remains controversial. It is unknown, whether patients with global kidney ischemia (GKI), that means patients with bilateral RAS or RAS with a single functioning kidney, may benefit from PTRA or not.
Methods
We retrospectively analyzed 93 patients with RAS (25 bilateral or single functioning kidney) undergoing PTRA. Patients had refractory hypertension (≥3 medications). Blood pressure, antihypertensive drugs and serum-creatinine were compared pre-/post-intervention and at 1 year’s follow-up.
Results
At 1 year after PTRA of patients with GKI, systolic and diastolic blood pressure were significantly reduced compared to patients with unilateral PTRA (systolic: −19.1 ± 10.5 [bilateral] vs. −11.4 ± 12.1 mmHg [unilateral], P < 0.01; diastolic: −10.1 ± 6.8 mmHg vs. −6.3 ± 6.6 mmHg, P < 0.05). The number of antihypertensive drugs was reduced by −0.8 ± 3.0 at 1 year in patients with GKI, while it increased by +0.1 ± 3.5 in the unilateral RAS group (P < 0.001). Furthermore, post-interventional serum-creatinine decreased by −34.6 ± 31.4 μmol/I after of patients with GKI (P < 0.001 vs. baseline). In patients with unilateral PTRA, a non-significant increase in serum-creatinine was observed (+8.3 ± 2 μmol/l).
Conclusion
PTRA in patients with GKI led to improved blood pressure and renal function. A large, well-designed, randomized clinical trial targeting this population is still needed. The benefit of PTRA should be measured with the risks in each patient individually.
Keywords: Hypertension, Renal artery stenosis, Percutaneous transluminal renal angioplasty, Serum-creatinine
1. Introduction
In Western populations, incidence and prevalence of atherosclerosis and thus the most common pathogenetic cause of renal artery stenosis (RAS) are decreasing since the more widespread use of statins [1]. Prevalence of RAS is approximately 0.5% in North America [2]. Atherosclerotic RAS is much more common in up to 50% of elderly people and in patients with hypertension, diabetes, renal disease and also nicotine abuse, as well as in patients with atherosclerosis in other vascular systems [1], [3].
Means to identify and treat RAS are endorsed in guidelines for diagnosis and treatment of arterial hypertension [4], [5]. PTRA can be performed with excellent technical success rate, a low rate of complications and good morphological 1-year results [6]. Whether PTRA translates into clinical benefit with regard to an improvement in morbidity and mortality is still controversial [7]. Neutral results of percutaneous renal artery angioplasty (PTRA) in recent large randomized, controlled trials (“Angioplasty and Stenting for Renal Artery Lesions” [ASTRAL] and the “Cardiovascular Outcomes in Renal Atherosclerotic Lesions” [CORAL]) have led to more reluctant indication for PTRA [8], [9], [10], [11], but not every clinical scenario was covered in those trials. There are potential areas for improvement focusing mainly on procedural details and patient selection with respect to PTRA of RAS. Particularly, subgroups of patients with GKI were unrepresented in these trials and accordingly the potential benefit of PTRA in this subgroup cannot be addressed.
The present analysis was performed to determine if such patients may be a representable population to benefit from PTRA.
2. Methods
2.1. Study design
We retrospectively studied all subsequent patients with RAS undergoing PTRA between 2000 and 2016 at St. Josefs-Hospital in Wiesbaden, Germany. The study was approved by the ethical committee of the State Chamber of Medicine in Hessen (Nr. FF86/2017).
2.2. Data collection
Data was collected 1 year pre-, immediately pre-, immediately post and 1 year post-PTRA. Baseline was defined as 3–6 months pre-interventionally. Demographic characteristics (age, gender), comorbidities (e.g., diabetes, hypertension, heart failure, and coronary artery disease), and a complete list of antihypertensive drugs, systolic and diastolic blood pressure from ambulatory 24-hour blood pressure measurements, office blood pressure and also serum creatinine were obtained from St. Josefs-Hospital’s patient data and electronic patient charts from Nierenzentrum Wiesbaden.
2.3. Diagnosis of RAS
All patients were repeatedly evaluated by a nephrologist and referred for treatment after repeated clinical evaluation and measurement of renal resistive index (RI). An RI > 0.5 was considered relevant and served as indicator for presence of a hemodynamically relevant unilateral RAS. The Doppler-derived renal resistive index (RI) is a well-established parameter and has been used for years to detect renal artery stenosis and estimate its hemodynamic relevance. The pulse wave reflects the resistance to blood flow caused by microvascular bed distal to the site of measurement. The RI calculation is calculated automatically by the duplex sonography device using the following formula: (peak systolic velocity – end diastolic velocity)/peak systolic velocity [12], [13], [14].
As suggested by others RIs were measured in three areas within the renal cortex. A side difference of RI > 0.5 between kidneys indicates a hemodynamic relevant stenosis in the preceding renal artery stenosis (reduced RI on the stenotic side). In case of bilateral stenosis a comparison between both sides cannot be applied. The RIs can be compared to age-matched values of a hypertensive control population In addition direct duplex-measurements within the renal artery need to be applied. A Vmax of >2 m/s indicates a relevant stenosis [12], [13], [14].
2.4. Criteria of patients selection, or indication of this treatment
Patients were selected for angioplasty when the following criteria were fulfilled:
Hemodynamic relevant renal artery stenosis (one side or both sides) diagnosed by renal duplex sonography and either rapid decline in renal function, difficult to control hypertension or hospitalization due to flash pulmonary edema / cardial decompensation.
2.5. PTRA procedure
Under sterile conditions a femoral sheath (1.78 mm, 6 French) was inserted with local anesthesia and mild sedation with midazolam. Angiography was performed to verify presence of RAS and determine morphology. Stenting was performed after passage of a guide wire and predilation of RAS (In 92% of the cases) unless predilation yielded a “stent-like” result in seven cases. A RX Herculink Elite Renal Stent (Abbott Vascular International BVBA) was used in all cases. The diameter was chosen according the non-stenosed vessel diameter. An oversizing of plus 1 mm was preferred. All stents covered the stenotic areal and protruded approximately 2 mm in the abdominal aorta. After angioplasty a control angiogram with contrast media was performed to document a successful procedure.
Antiplatelet therapy given before the intervention was acetyl salicylic acid (ASA) 100 mg/day and clopidogrel 300 mg/day starting the day prior to the intervention. Alternatively, a bolus dose of 500 mg of ASA and clopidogrel 600 mg had to be administered on the day of the procedure. Prior to the intervention, a bolus dose of 2500–10,000 IU of heparin was given. Dual antiplatelet therapy had to be administered for at least 4 weeks and ASA infinitely.
2.6. Statistical analysis
Analyses were performed using Statistical Package for the Social Sciences (SPSS) statistical software package, version 20 (Apache Software Foundation, USA). As most of the parameters had non-Gaussian distributions, nonparametric tests were used throughout the analysis (T-test). All tests were performed two-tailed. P < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
A total of 115 RAS were treated and 127 stents were implanted in 93 patients (48 male, mean age: 68.9 ± 9.8 years). Unilateral RAS was present in 68 patients, while 25 patients had bilateral RAS or RAS of a single functioning kidney.
Groups differed with regard to prevalence of coronary artery disease and heart failure. Among patients with unilateral RAS 19% had coronary artery disease compared to 59% from the bilateral RAS group (P < 0.01). Heart failure with reduced ejection fraction (<35%) was more prevalent in the group with bilateral RAS (40% vs. 12%, P < 0.001). Arterial hypertension, peripheral artery disease and diabetes mellitus were distributed equally in both groups (Table 1).
Table 1.
Baseline characteristics of the study population with Procedural data.*
| unilateral RAS N = 68 | bilateral RAS N = 25 | P value | |
|---|---|---|---|
| Age (years) | 67.1 ± 10.2 | 73.7 ± 7.1 | <0.001 |
| Male (n) | 48 (70.6%) | 15 (60%) | n.s. |
| Systolic blood pressure (mmHg) | 156.3 ± 10.9 | 157.9 ± 13.2 | n.s. |
| Diastolic blood pressure (mmHg) | 93.3 ± 6.4 | 94.6 ± 7.2 | n.s. |
| Serum-creatinine (µmol/l) | 111.5 ± 41.5 | 192.4 ± 75.8 | <0.001 |
| Medical history and risk factors (n%) | |||
| Arterial Hypertension | 66 (97%) | 24 (96%) | n.s. |
| Diabetes mellitus | 36 (52%) | 14 (56%) | n.s. |
| LVEF < 45% | 8 (11%) | 10 (40%) | <0.001 |
| Previous stroke | 5 (7%) | 2 (8%) | n.s. |
| Nicotine abuse | 42 (61%) | 19 (76%) | n.s. |
| Coronary artery disease | 12 (17%) | 13 (52%) | <0.01 |
| Peripheral artery disease | 17 (25%) | 7 (28%) | n.s. |
| Sudden pulmonary edema | 0 | 5 (20%) | <0.001 |
| Number of stents | 74 | 53 | |
| Stent-size (diameter mm × length mm) | 6.0 ± 0.5 × 18 ± 1 | 6.0 ± 0.5 × 16 ± 1 | n.s. |
| Contrast agent (ml) | 62 ± 2.4 | 94 ± 4.5 | n.s. |
Data are given as mean ± SD or number.
3.2. Procedure
Stenting was performed in 92% of cases. Predilation yielded a stent-like result in seven cases. In those cases plain balloon angioplasty without stenting was performed if no dissection was documented and patients had severe bleeding complications in their past medical history. Interventions were technically successful and without any complications in all cases. Table 1 illustrates procedural data.
3.3. Change in blood pressure
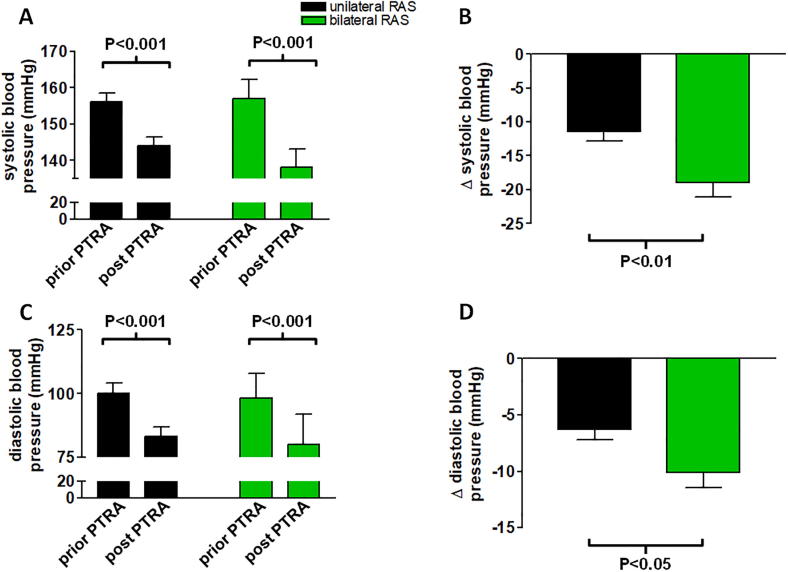
Post-interventionally, at 1 year there was a statistically significant reduction in systolic and diastolic blood pressure in both groups compared to baseline values (Fig. 1A, C). However, this decrease was much more prominent among patients with PTRA of bilateral RAS in comparison to patients with PTRA of unilateral RAS (Fig. 1B, D). On average the reduction in systolic blood pressure was 19.1 ± 10.5 mmHg in patients undergoing bilateral PTRA, while patients with unilateral RAS experienced a reduction by 11.4 ± 12.1 mmHg (P < 0.01, Fig. 1B). Similarly, reduction in diastolic blood pressure was more profound for patients with PTRA of bilateral RAS compared to unilateral RAS (10.1 ± 6.8 mmHg vs. 6.3 ± 6.6 mmHg, P < 0.05, Fig. 1D).
Fig. 1.
Panels A and B illustrate changes in systolic and diastolic (C, D) blood pressure prior to and post PTRA compared into groups of patients with uni- vs. bilateral RAS. There was a significant absolute reduction in systolic and diastolic blood pressure in both groups (P < 0.001). (B, D). However, the effect was more pronounced for both, systolic and diastolic blood pressure in patients having undergone bilateral PTRA (P < 0.05). Data are mean ± SD.
3.4. Changes in serum creatinine levels and number of antihypertensive drugs
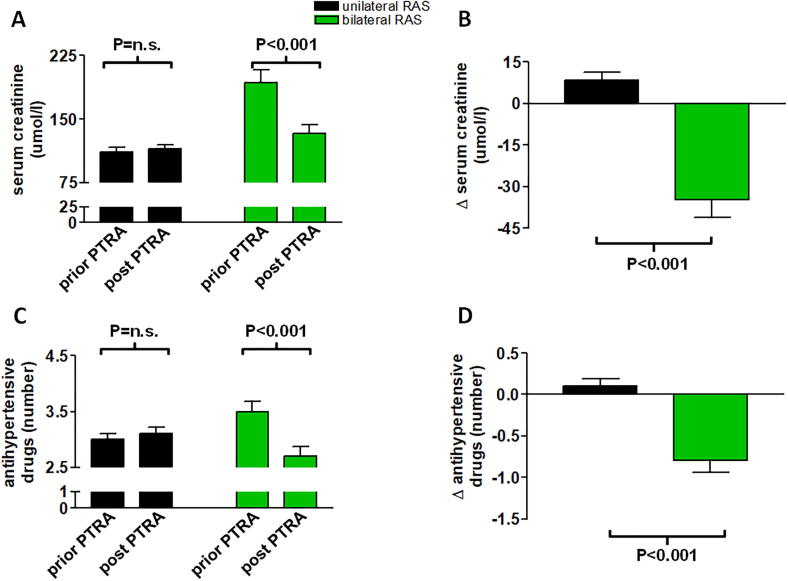
Patients with PTRA of bilateral RAS had higher baseline creatinine at the time of PTRA compared with patients with unilateral stenosis (192.4 ± 75.8 μmol/I vs. 111.5 ± 41.5 μmol/I, P < 0.001). The bilateral group experienced significant improvement in renal function post-interventionally (Fig. 2A, B). Serum creatinine levels decreased by 34.6 ± 6.4 μmol/I (P < 0.001 vs. baseline), whereas in patients with PTRA of unilateral RAS there was a mild increase over the period of follow-up + 8.3 ± 3.1 μmol/l, (P = n.s., Fig. 2A, B).
Fig. 2.
This figure illustrates changes in serum creatinine (panels A, B) and number of antihypertensive drugs (C, D) compared into patients with uni- vs. bilateral RAS. There was a significant reduction in serum creatinine and antihypertensive drugs in the bilateral RAS group (P < 0.05). On the other hand, there was a numerical increase in serum creatinine as well as number of antihypertensive drugs in patients with unilateral PTRA (P = n.s. and P = n.s., respectively, panels B, D). The relative difference between the two groups was highly significant (P < 0.001). Data are mean ± SD.
3.5. Changes in number of antihypertensive drugs
The number of antihypertensive drugs was reduced by −0.8 ± 3.0 post-interventionally in patients with bilateral RAS, while the number of drugs slightly increased by +0.1 ± 3.5 in the unilateral RAS group (P < 0.001, Fig. 2C, D).
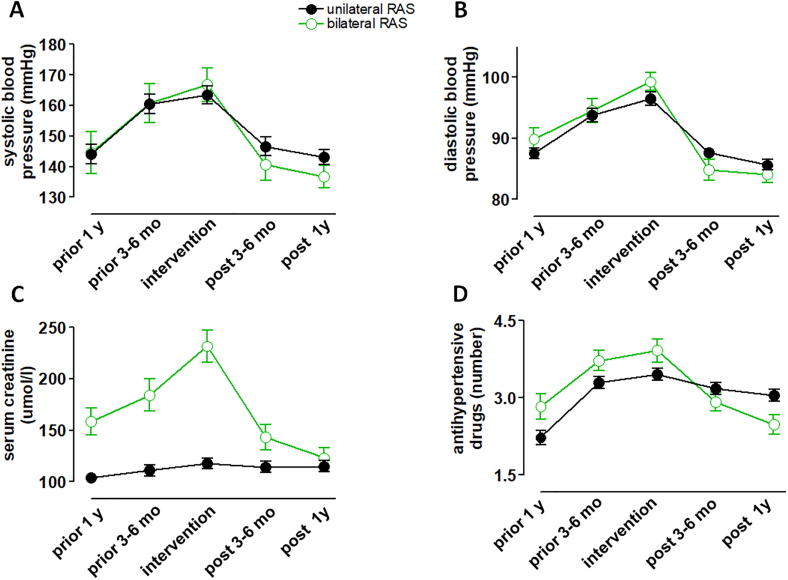
3.6. 1-year changes
Development of blood pressure elevations during the year prior to intervention was qualitatively similar between patients with bilateral vs. unilateral RAS (Fig. 3A, B). Worsening of renal function prior to PTRA was more evident in patients with bilateral RAS (Fig. 3C) and serum creatinine significantly decreased after intervention in the unilateral group only. The mean number of antihypertensive drugs in the bilateral group decreased below the level of drugs in the unilateral group (Fig. 3D).
Fig. 3.
This figure illustrates the 1-year courses of systolic panel A) and diastolic (B) blood pressure, serum creatinine (C) and numbers of antihypertensive drugs (D) in both groups. Data are mean ± SD.
The long term outcome in terms of renal function (serum creatinine) was 114.9 ± 45.8 μmol/I (n = 25) after 1 year in the unilateral group. In the in the bilateral group long term outcome was 123.9 ± 47.9 μmol/I (n = 68) after 1 year, accordingly. The systolic blood pressure was 136.8 ± 3.6 mmHg (n = 68) after 1 year in the unilateral group and 143.1 ± 2.4 mmHg (n = 25) after 1 year in the in the bilateral group, accordingly. The diastolic blood pressure was 84.1 ± 1.3 mmHg (n = 68) after 1 year in the unilateral group and 85.7 ± 0.9 mmHg (n = 25) after 1 year in the in the bilateral group, accordingly. The number of antihypertensive drugs was 2.5 ± 0.2 (n = 68) after 1 year in the unilateral group and 3.0 ± 0.1 (n = 25) after 1 year in the in the bilateral group, accordingly.
During the follow up there were no deaths of any cause observed and no stent thrombosis or restenosis were detected in the duplex sonographic assessments. Also no patient had a target lesion revascularization (TLR) during the follow up.
4. Discussion
4.1. Main findings
The key finding of our study was that in comparison to patients with unilateral RAS patients with GKI benefitted to a significant extent in terms of blood pressure reduction and improvement of renal function.
The key for the management of RAS lies in establishing the functional significance of the stenosis, that is, when the RAS is actually responsible for activation of the renin angiotensin system or induces renal tissue ischemia that is still amenable for reversal by reperfusion techniques. The ongoing uncertainty regarding the benefit of revascularization reinforces that this is not an easy task needing individual assessment.
The results of the ASTRAL and CORAL trials have answered part of the question of when to intervene in the case of RAS. These trials have shown, that in patients with stable kidney function and adequate blood pressure control, the mere presence of RAS does not justify PTRA [9], [10]; confirmed by recent meta-analyses [15]. On the other hand, clinical evidence for the benefit or lack of benefit in patients with GKI with uncontrolled blood pressure or chronic kidney disease progression is in small studies documented [16], [17]. A previous work in 33 patients had already demonstrated a favorable outcome of kidney function in patients with GKI [16].
A previous, non-randomized, prospective study showed that in patients with chronic renal insufficiency and RAS, PTRA improved or stabilized renal function and preserved kidney size [16]. The CORAL study included patients with 67 ± 11% diameter RAS and aimed to include patients with difficulty to control blood pressure or renal insufficiency [10]. However, due to recruitment problems, blood pressure criteria were relaxed, ultimately resulting in a mean number of antihypertensive drugs of 2.1 ± 1.6 in the drug group - which does not meet the definition of “difficult to treat high blood pressure” [18]. The second inclusion criterion was impaired renal function with glomerular filtration rate (GFR) <60 ml/min. The estimated GFR of patients enrolled in the study was 58 ml/min, which is the expected value in the predominantly male patient group with a mean age of 70 years according to the chronic kidney disease epidemiology collaboration (CKD-EPI) formula. On average there was no clinically relevant impairment of kidney function. The overall CORAL population was therefore on average a cohort of patients without specific renal problems [10]. A mild increase in serum creatinine was documented in the overall population during the period of the trial, resembling the mild increase in the group of unilateral RAS in our study.
In contrast to CORAL, a key inclusion criterion of the ASTRAL study (in addition to presence of atherosclerotic RAS) was the uncertainty of the treating physician whether the patient would benefit from PTRA. The study accordingly showed no benefit of PTRA over optimal medical therapy in such selected patients. Furthermore, contrasting to our study, a low but relevant number in serious complications in PTRA treated patients occurred in ASTRAL. It can be concluded from this study that if there is uncertainty about the benefits of PTRA, such should not be performed.
The study group that contributed the largest number of patients to the ASTRAL trial (n = 72 patients) analyzed patients that had been screened for but had not been included in the ASTRAL study [19]. These were 467 patients with ≥50% diameter RAS. Of these, 51% had significant clinical conditions (including 37 patients with pulmonary edema, 116 with refractory hypertension, 46 with rapidly worsening kidney function and 31 both with hypertension and kidney disease). Approximately, two-thirds of patients with sudden pulmonary edema and one-third of patients with other clinical characteristics received PTRA while the remainder was treated by medication. Over a mean follow-up of 3.8 years the hazard ratio (HR) for death in patients with sudden pulmonary edema was 0.4 (95% confidence interval [CI] 0.2–0.9) if receiving PTRA compared to not receiving PTRA. If only one characteristic of treatment-refractory hypertension or rapid deterioration of renal function alone was present, PTRA conferred no benefit. However, if both refractory hypertension and rapidly deteriorating renal function were present, HR was 0.15 (95%CI: 0.02–09) in the PTRA group and 0.23 (95% CI: 0.1–0.6) for cardiovascular events. In summary, PTRA in the patients without such relevant clinical conditions did not lead to reduction of events.
Vassallo et al. demonstrated results of 131 “high-risk” patients with at least 70% diameter RAS [20]. These “high-risk” patients had previous sudden pulmonary edema, difficult-to-control hypertension despite triple antihypertensive therapy or had rapid renal impairment. Authors compared these patients with a control group of 144 patients with comparable angiographic degree of stenosis but no clinical problems.
In the control group, PTRA was performed in 30% of cases while patients in the “high-risk” group received PTRA in 42% of cases. The control group did not experience any improvement in mortality or progression to dialysis. Among “high-risk” patients, PTRA reduced the event rate per 100 patient-years significantly by 36% (mortality) and 36% (progression to dialysis). Patients with bilateral RAS (n = 40) experienced reductions by 70% (mortality) and 69% (progression to dialysis) confirming that a clinical benefit of PTRA is present with appropriate patient selection – consistent with our results [20].
The high drop of blood pressure in our study might somehow be explained be the selection bias of this retrospective analysis and some data collection from office blood pressure. Only patients with refractory hypertension were chosen for analysis in, Diagnosis of RAS and indication for PTRA”. The rather sudden increase in blood pressure values prior intervention (as seen in Fig. 1,3) might overestimate the blood pressure reducing effect of the intervention. However, significant blood pressure reductions were seen in another retrospective study recently published [21]. The study by Courand and coworkers demonstrated a similarly strong reduction in ambulatory blood pressure measurements [21].
In summary, the currently available clinical trials have some limitations in their abilities to screen and adequately help identify patients with significant RAS in order to decide for PTRA. Additionally, these trials have excluded patients with the highest likelyhood of having clinically significant disease. This highly restricts their applicability to low-risk cohorts with stable or slowly progressive renal failure and amenable to control of hypertension. Our analysis emphazises that the subgroup of patients with GKImay indeed benefit from PTRA. This is accordingly stated in a recent update of the German guidelines for PTRA [22].
4.2. Study limitation
The primary limitation of this study lies in its retrospective character with a modest sample size. The other main study limitation besides the small study cohort is the limited follow‐up period of 1 year. The effect of PTRA of patients with GKI should be investigated in a larger randomized multicenter clinical trial – although this might be almost impossible to conduct due to slow recruitment.
4.3. Conclusion
PTRA of patients with GKI led to improved blood pressure and renal function. A large, well-designed, randomized clinical trial targeting this population is still needed. The benefit of PTRA should be measured with the risks in each patient individually.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- 1.Aboyans V., Ricco J.B., Bartelink M.E.L., Bjorck M., Brodmann M., Cohnert T. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur. Heart J. 2018;39(9):763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 2.Lewin A., Blaufox M.D., Castle H., Entwisle G., Langford H. Apparent prevalence of curable hypertension in the Hypertension Detection and Follow-up Program. Arch. Intern. Med. 1985;145(3):424–427. [PubMed] [Google Scholar]
- 3.Anderson J.L., Halperin J.L., Albert N.M., Bozkurt B., Brindis R.G., Curtis L.H. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(13):1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Bohm M. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur. Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 5.Kintscher U., Böhm M., Goss F., Kolloch R., Kreutz R., Schmieder R.E. Kommentar zur 2013-ESH/ESC-Leitlinie zum Management der arteriellen Hypertonie. Kardiologe. 2014;8:223–230. [Google Scholar]
- 6.Lanzer P., Weser R., Prettin C. Coronary-like revascularization for atherosclerotic renal artery stenosis–results in 181 consecutive patients. Clin. Res. Cardiol. 2006;95(11):584–590. doi: 10.1007/s00392-006-0429-0. [DOI] [PubMed] [Google Scholar]
- 7.Jenks S., Yeoh S.E., Conway B.R. Balloon angioplasty, with and without stenting, versus medical therapy for hypertensive patients with renal artery stenosis. Cochrane Database Syst. Rev. 2014;12(12):CD002944. doi: 10.1002/14651858.CD002944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bax L., Woittiez A.J., Kouwenberg H.J., Mali W.P., Buskens E., Beek F.J. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann. Intern. Med. 2009;150(12) doi: 10.7326/0003-4819-150-12-200906160-00119. 840–8, w150-1. [DOI] [PubMed] [Google Scholar]
- 9.Wheatley K., Ives N., Gray R., Kalra P.A., Moss J.G., Baigent C. Revascularization versus medical therapy for renal-artery stenosis. New Engl. J. Med. 2009;361(20):1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C.J., Murphy T.P., Cutlip D.E., Jamerson K., Henrich W., Reid D.M. Stenting and medical therapy for atherosclerotic renal-artery stenosis. New Engl. J. Med. 2014;370(1):13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy T.P., Cooper C.J., Cutlip D.E., Matsumoto A., Jamerson K., Rundback J. Roll-in experience from the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study. J. Vasc. Interv. Radiol. 2014;25(4):511–520. doi: 10.1016/j.jvir.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumme B., Hollenbeck M. Doppler sonography in renal artery stenosis–does the Resistive Index predict the success of intervention? Nephrol. Dialysis Transplant. : Off. Publ. Eur. Dialysis Transplant Associat. – Eur. Renal Associat. 2007;22(3):692–696. doi: 10.1093/ndt/gfl686. [DOI] [PubMed] [Google Scholar]
- 13.Krumme B. Renal Doppler sonography–update in clinical nephrology. Nephron Clin. Pract. 2006;103(2):c24–c28. doi: 10.1159/000090605. [DOI] [PubMed] [Google Scholar]
- 14.Krumme B., Blum U., Schwertfeger E., Flugel P., Hollstin F., Schollmeyer P. Diagnosis of renovascular disease by intra- and extrarenal Doppler scanning. Kidney Int. 1996;50(4):1288–1292. doi: 10.1038/ki.1996.440. [DOI] [PubMed] [Google Scholar]
- 15.Bavry A.A., Kapadia S.R., Bhatt D.L., Kumbhani D.J. Renal artery revascularization: updated meta-analysis with the CORAL trial. JAMA Intern. Med. 2014;174(11):1849–1851. doi: 10.1001/jamainternmed.2014.4332. [DOI] [PubMed] [Google Scholar]
- 16.Watson P.S., Hadjipetrou P., Cox S.V., Piemonte T.C., Eisenhauer A.C. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation. 2000;102(14):1671–1677. doi: 10.1161/01.cir.102.14.1671. [DOI] [PubMed] [Google Scholar]
- 17.Zeller T., Muller C., Frank U., Burgelin K., Schwarzwalder U., Horn B. Survival after stenting of severe atherosclerotic ostial renal artery stenoses. J. Endovasc. Ther. : Off. J. Int. Soc. Endovasc. Specialists. 2003;10(3):539–545. doi: 10.1177/152660280301000320. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun D.A. Resistant or difficult-to-treat hypertension. J. Clin. Hypertens. (Greenwich). 2006;8(3):181–186. doi: 10.1111/j.1524-6175.2006.04747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie J., Green D., Chrysochou C., Chalmers N., Foley R.N., Kalra P.A. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am. J. Kidney Dis. 2014;63(2):186–197. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Vassallo D., Ritchie J., Green D., Chrysochou C., Kalra P.A. The effect of revascularization in patients with anatomically significant atherosclerotic renovascular disease presenting with high-risk clinical features. Nephrol. Dialysis Transplant. : Off. Publ. Eur. Dialysis Transplant Associat. – Eur. Renal Associat. 2018;33(3):497–506. doi: 10.1093/ndt/gfx025. [DOI] [PubMed] [Google Scholar]
- 21.Courand Pierre-Yves, Dinic Miriana, Lorthioir Aurélien, Bobrie Guillaume, Grataloup Christine, Denarié Nicolas, Soulat Gilles, Mousseaux Elie, Sapoval Marc, Azizi Michel, Amar Laurence. Resistant hypertension and atherosclerotic renal artery stenosis: effects of angioplasty on ambulatory blood pressure. a retrospective uncontrolled single-center study. Hypertension. 2019;74(6):1516–1523. doi: 10.1161/HYPERTENSIONAHA.119.13393. [DOI] [PubMed] [Google Scholar]
- 22.Oberhuber A., Hupp T., Richter G.M., Nitzsche E.U., Radermacher J., Rump L.C. Kurzfassung der S2k-Leitlinie Erkrankungen der Nierenarterie. Gefässchirurgie. 2019;24(3):251–257. [Google Scholar]