Abstract
Background
Hypertension is associated with an increased risk of stroke, myocardial infarction and congestive heart failure. Methyldopa is a centrally acting antihypertensive agent, which was commonly used in the 1970's and 80's for blood pressure control. Its use at present has largely been replaced by antihypertensive drug classes with less side effects, but it is still used in developing countries due to its low cost. A review of its relative effectiveness compared to placebo on surrogate and clinical outcomes is justified.
Objectives
To quantify the effect of methyldopa compared to placebo in randomized controlled trials (RCTs) on all cause mortality, cardiovascular mortality, serious adverse events, myocardial infarctions, strokes, withdrawals due to adverse effects and blood pressure in patients with primary hypertension.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (1960‐June 2009), MEDLINE (2005‐June 2009), and EMBASE (2007‐June 2009). Bibliographic citations from retrieved studies were also reviewed. No language restrictions were applied.
Selection criteria
We selected RCTs studying patients with primary hypertension. We excluded studies of patients with secondary hypertension or gestational hypertension.
Data collection and analysis
Two reviewers independently extracted data and assessed trial quality using the risk of bias tool. Data synthesis and analysis was performed using RevMan 5. Data for blood pressure were combined using the generic inverse variance method.
Main results
Twelve trials (N=595) met the inclusion criteria for this review. None of these studies evaluated the effects of methyldopa compared to placebo on mortality and morbidity outcomes. Data for withdrawals due to adverse effects were not reported in a way that permitted meaningful meta‐analysis. Data from six of the twelve trials (N=231) were combined to evaluate the blood pressure lowering effects of methyldopa compared to placebo. This meta‐analysis shows that methyldopa at doses ranging from 500‐2250 mg daily lowers systolic and diastolic blood pressure by a mean of 13 (95%CI 6‐20) / 8 (95% CI 4‐13) mmHg. Overall, the risk of bias was considered moderate.
Authors' conclusions
Methyldopa lowers blood pressure to varying degrees compared to placebo for patients with primary hypertension. Its effect on clinical outcomes, however, remains uncertain.
Plain language summary
Methyldopa reduces blood pressure in people with high blood pressure
Methyldopa is a medication that has been used to treat high blood pressure since the 1960s. While there is some belief methyldopa reduces blood pressure, there are concerns due to the potential for this drug to cause adverse effects. The aim of this review was to determine the extent to which methyldopa reduces blood pressure, the nature of methyldopa's adverse effect profile, and to determine the clinical impact of its use for hypertension.The search revealed 12 trials with a total of 595 patients that were randomized to either a methyldopa treatment arm (296 patients) or a placebo treatment arm (299 patients). The daily doses of methyldopa used in these studies ranged 500‐2250 mg daily. The most commonly studied daily dose of methyldopa was 750 mg daily. Most studies followed patients for four to six weeks of therapy. None of the studies reported on the clinical impact of methyldopa (e.g. if methyldopa reduced the risk of having a stroke compared to placebo). Overall reporting of adverse effects was poor so no conclusions can be drawn about the adverse effect profile. This meta‐analysis shows that methyldopa reduces systolic/diastolic blood pressure by approximately 13/8 mmHg compared to placebo.
Background
Description of the condition
Hypertension is associated with structural changes in the heart and blood vessels which may lead to cardiovascular mortality and morbidity (i.e. cardiovascular disease, stroke, peripheral vascular disease, and renal disease). Hypertension is typically defined as having a systolic blood pressure (SBP) ≥ 140 mm Hg and a diastolic blood pressure (DBP) ≥ 90 mm Hg (CHEP 2008, Chobanian 2003). Worldwide, approximately 1 billion people are affected by hypertension (Chobanian 2003) and seven million deaths per year may be attributed to hypertension (WHO 2003). In addition, for every 20 mm Hg increase in SBP and 10 mm Hg increase in DBP (through the range from 115/75 to 185/115 mm Hg) in people aged 40 to 70 years, the risk of cardiovascular disease morbidity doubles (Chobanian 2003). This emphasizes the importance of finding safe and effective treatments for the prevention of the associated mortality and morbidity in hypertensive patients.
Description of the intervention
Methyldopa (α‐methyl‐3,4‐dihydroxy‐L‐phenylalanine) is an analog of DOPA (3,4‐hydroxyphenylanine) and is a prodrug which requires metabolism to an active metabolite in order to exert its effects in the central nervous system. It was discovered over five decades ago (Stein 1955) and its blood pressure lowering effects were discovered shortly after, in 1959 (Sjoerdsma 1982). In the 1970's and 80's methyldopa was considered an effective antihypertensive agent especially in the elderly, patients with renal insufficiency, and pregnancy. The JNC hypertension guidelines in 1977 recommended methyldopa as add on therapy after diuretics (JNC 1977).
Methyldopa has been associated with a wide spectrum of adverse events including CNS depressant effects (drowsiness, fatigue, lethargy, depression), decreased libido, dry mouth, hepatitis, myocarditis, and haemolytic anaemia (Webster 1996, Brunton 2006).
How the intervention might work
Methyldopa is metabolized to α‐methylnorepinephrine, which acts as an agonist at presynaptic α2 adrenergic receptors in the brainstem and results in the inhibition adrenergic neuronal outflow. The attenuation of norepinephrine release in the brainstem reduces the output of vasoconstrictor adrenergic signals to the peripheral sympathetic nervous system, leading to blood pressure reduction (Brunton 2006).
Why it is important to do this review
The side effect profile of methyldopa, combined with introduction of newer antihypertensives that claim to produce an improved quality of life has resulted in methyldopa being removed from most treatment guidelines for hypertension (Croog 1986).
Despite these changes in the guidelines, methyldopa is still widely used in developing countries. Possible reasons for its continued use include: no adverse effect on biochemistry, compatibility with other antihypertensive agents and low cost compared to newer, more expensive agents. It is important to review the evidence of benefit and harm of methyldopa since clinicians in developing countries continue to prescribe methyldopa despite its absence from treatment guidelines. The primary purpose of this systematic review is to evaluate the relative effectiveness of methyldopa compared to placebo in lowering blood pressure, morbidity, and mortality.
Objectives
-
To determine the effect of methyldopa as monotherapy compared to placebo in adults (of varying age and race) with primary (essential) hypertension (with and without co‐morbidities) on the following:
mortality
morbidity
systolic and diastolic blood pressure
To determine whether methyldopa is associated with an increased incidence of withdrawals due to adverse effects compared to placebo.
Methods
Criteria for considering studies for this review
Types of studies
Included studies must be randomized controlled trials that compare oral methyldopa to oral placebo. Data from cross‐over trials were included.
Types of participants
Participants must have primary (essential) hypertension defined by a systolic BP greater than 140 mmHg or a diastolic BP greater than 90 mm Hg or both, and no secondary cause found for the high blood pressure. Patients must not have significant renal insufficiency as evidenced by documented serum creatinine levels greater than 1.5 times normal values to exclude patients with hypertension secondary to renal failure. Participants who were taking medications that affect blood pressure other than oral methyldopa were excluded. Participants were not restricted by age, gender, baseline risk, or any other co‐morbid conditions.
Types of interventions
The intervention of interest is oral methyldopa monotherapy. The comparative intervention is oral placebo. No restrictions were set for initial and final doses of methyldopa used, nor for duration of therapy of methyldopa or placebo control.
Types of outcome measures
Primary outcomes
All cause mortality
Cardiovascular mortality
Non‐cardiovascular mortality
Number of patients experiencing at least one serious adverse event
Fatal and non‐fatal stroke
Fatal and non‐fatal myocardial infarction
Secondary outcomes
Number of patients who withdrew due to adverse events
Number of patients with at least one adverse event
Change in systolic blood pressure
Change in diastolic blood pressure
Search methods for identification of studies
The Database of Abstracts of Reviews of Effectiveness (DARE) and the Cochrane Database of Systematic Reviews were searched for related reviews.
The following electronic databases were searched for primary studies:
The Cochrane Central Register of Controlled Trials (CENTRAL) (1960‐2009)
English language databases, including MEDLINE (2005‐June 2009) and EMBASE (2007‐June 2009)
Electronic databases were searched using a strategy combining a variation of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms relating to methyldopa and hypertension. No language restrictions were used. The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary as applicable. Full electronic database search strategies are in Appendix 1, Appendix 2, Appendix 3, and Appendix 4.
Searching other resources
Reference lists of all papers and relevant reviews were identified.
Authors of trials reporting incomplete information were contacted to provide the missing information.
The manufacturer of methyldopa (previously Merck Sharp and Dohme, now Merck Frosst) was contacted for published and unpublished studies.
Data collection and analysis
Selection of studies
The initial screen of titles and abstracts of all identified studies was conducted independently by two reviewers (GM, AT) and those articles which clearly did not meet the predefined inclusion criteria were excluded. Full text articles of potentially relevant studies were retrieved and translated to English where required. Studies which fulfilled the inclusion criteria were examined in detail. Reasons for excluding any study were documented. Trials with more than one publication were counted only once.
Data extraction and management
Study characteristics and the outcome measures of interest were collected independently by the two reviewers using a pre‐formed standardized data extraction sheet. Data was then cross‐checked and any differences in interpretation of the data was resolved through further examination and consensus between the reviewers. The data extracted from each study included the following: patient characteristics including gender, age, ethnicity, and co‐morbid conditions; methods including means of random allocation of participants to trial interventions, allocation concealment, blinding of patients, health care providers, and outcomes assessors, losses to follow‐up and how they were handled, and duration of trial follow‐up; interventions including dose and duration of methyldopa used; outcome measures as described above. All data, regardless of compliance or completion of follow up, was collected in order to allow for analysis by intention to treat.
The position of the patient during blood pressure measurement may affect the blood pressure lowering effect. However, in order not to lose valuable data, if only one position was reported, data from that position were extracted. When blood pressure measurement data were available in more than one position, data were extracted in accordance with the following order of preference: 1)sitting; 2) standing; and 3) supine. In the case of missing information in the included studies, investigators were contacted (by email, letter and/or fax) to obtain the missing information. In the case of missing values for standard deviation of the change in blood pressure or heart rate, the standard deviation was imputed based on the information in the same trial or from other trials using the same dose. The following hierarchy (listed from high to low preference) was used to impute standard deviation values:
Pooled standard deviation calculated either from the statistic corresponding to an exact p‐value reported or from the 95% confidence interval of the mean difference between treatment group and placebo.
Standard deviation of change in blood pressure/heart rate from a different position than that of the blood pressure data/heart rate used.
Standard deviation of blood pressure/heart rate at the end of treatment
Standard deviation of blood pressure/heart rate at the end of treatment measured from a different position than that of the blood pressure/heart rate data used.
Standard deviation of blood pressure/heart rate at baseline (except if this measure was used for entry criteria).
Weighted mean standard deviation of change in blood pressure/heart rate from other trials.
Assessment of risk of bias in included studies
The following parameters were evaluated to assess the overall methodological quality of each study:
Method used for randomization of trial participants
Method used for concealment of treatment allocation
Whether or not the individuals involved in the study (including health care providers, assessors and patients) were blinded to the treatment allocation
Whether or not all participants were accounted for at the end of trial when reporting outcomes
Whether or not the study was free of selective reporting of outcomes
Measures of treatment effect
For evaluation of the primary outcomes (e.g. mortality, serious adverse events, cerebrovascular events, and cardiac events), the total number of patients with at least one event within each trial were to be recorded as a percent. Proportions were to be calculated for these dichotomous outcomes, and comparisons between groups were to be presented as relative risk ratios (with corresponding 95% confidence intervals). This was, however, not done as none of the included trials reported on these outcomes.
One of the included cross‐over RCTs (Fernandez 1980) was considered appropriate for inclusion in the meta‐analysis of blood pressure effect because pooled standard error (SE) of mean blood pressure and end of study mean blood pressure in the methyldopa and placebo treatment periods were provided. This data was entered using the generic inverse variance outcome method. Subsequently all other parallel group RCTs' blood pressure data was entered in the same way. One parallel group RCT (Aronow 1977) provided SE of the mean for end of study blood pressure in each treatment group. These SEs were converted to standard deviations (SD). In Aronow 1978 and Mroczek 1974 both end of study mean blood pressures and SDs were provided for each treatment group. End of study mean blood pressures and SDs were then entered into RevMan 5 to determine the mean difference and 95% CI for end of study BP between methyldopa and placebo. The boundaries of the 95% CI were subtracted from each other and the difference was divided by 3.92 in order to calculate the pooled standard error for the difference in end of study blood pressure between groups. This data was then entered using the generic inverse variance method.
In the Tiwari 1982 study randomized patients were further divided into Group I (mild hypertension) and Group II (moderately severe hypertension) in each treatment group. For each Group I and each Group II, end of study mean blood pressures and SDs were provided. For the methyldopa patients, Group I was combined with Group II by calculating a weighted mean blood pressure and weighted mean SD. This was also done for the placebo patients. The difference in mean blood pressure was calculated using the end of study mean blood pressure and SD for the combined methyldopa group and the combined placebo group. The pooled standard error and difference in end of study mean blood pressure between groups was calculated using the method described above and entered using the generic inverse variance method.
In the Schnaper 1975 study only mean change in blood pressure at end of study was reported for each group. The mean change for methyldopa was subtracted from the mean change in the placebo group and this was then entered as the difference in end of study mean blood pressure between groups using the generic inverse variance method. Paran 1993 only reported end of study mean blood pressures in each treatment group. Neither the Paran 1993 nor the Schnaper 1975 studies reported information to allow the calculation of pooled standard errors for the treatment blood pressure differences. An imputed pooled standard error for the difference in end of study mean blood pressure difference was used for both trials. The imputed pooled standard error was calculated by using the pooled standard errors from Aronow 1977, Aronow 1978, Mroczek 1974, Fernandez 1980, and Tiwari 1982 to determine a weighted pooled standard error.
All analyses were initially conducted using a fixed effects model.
Unit of analysis issues
Data from all patients individually randomized to each intervention were used in the analyses. Care was taken to identify situations in which data had been censored or excluded or if data presented was the total number of events or the total number of patients with a first event. Authors were contacted for clarification when necessary.
Dealing with missing data
In general if there were missing data, the authors of the study were contacted using e‐mail for clarification. In cases where missing information was ultimately not available, the best estimate was included based on information in the same trial or information from other trials using similar doses. For instance, If standard error of the change was not provided for blood pressure, the value was imputed using the pooled standard error of change data from other similar trials and by calculating a weighted pooled standard error.
Assessment of heterogeneity
Assessment for heterogeneity across the studies was done using the I2 statistic test (a threshold of 30‐60% was used to define important heterogeneity) and the chi‐squared statistic test (with statistical significance being set at p<0.10). If heterogeneity was detected for outcomes, a random effects model was used to determine if the effects of methyldopa were still statistically significantly different from placebo. Clinical and methodological sources of heterogeneity were explored and characteristics for consideration included: baseline risk factors for the outcomes of interest, duration of studies, age, race, and sex distribution of patients across the studies.
Assessment of reporting biases
In the event that missing data was assumed to be a poor outcome or was imputed, sensitivity analyses were performed to see if results were sensitive to the assumptions being made. The potential impact of missing data was reviewed in the discussion section.
Data synthesis
Cochrane Review Manager software, RevMan 5, was used for all data syntheses and analyses. Relative risks and risk differences were to be calculated for dichotomous clinical outcomes but was not done as none of the trials provided this data. Data for blood pressure reduction was combined using a the generic inverse variance method which entailed entering the end of study mean blood pressure difference and pooled standard error of the difference.
Subgroup analysis and investigation of heterogeneity
No planned subgroup analyses were conducted as data in trials was limited and poorly reported. Any subgroup differences would have been unreliable estimates and very difficult to interpret.
Sensitivity analysis
The planned sensitivity analyses were not conducted as few trials were found and data within those trials was limited. Instead, post‐hoc sensitivity analyses were performed using the following parameters:
The effects of methyldopa with inclusion of trials where blood pressure pooled standard errors were imputed
The analysis of blood pressure differences without the data from the Tiwari 1982 study due to the fact that its blood pressure differences compared to all other included trials in the meta‐analysis was inexplicably greater.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies
Results of the search
The search strategy identified 785 citations in CENTRAL, MEDLINE, EMBASE. Following a review of their titles and abstracts, 734 citations that clearly did not meet our inclusion criteria were excluded while 51 citations were selected for further review. There are two articles that have not been retrieved to date and two articles awaiting translation (see Characteristics of studies awaiting classification). The manufacturer of methyldopa was not able to provide any additional clinical trials of interest for this review. Accordingly, the full articles of 47 potentially eligible citations were reviewed, and from their reference lists, an additional ten studies were identified and reviewed. Of these 57 citations, we excluded 43. Most of the excluded trials were excluded because participants were not randomized to a methyldopa only or a placebo only treatment arm during the study period. Of the 14 citations that met our inclusion criteria, two proved to be duplicate publications (Bar‐On 1993, Yodfat 1996) of Yodfat 1993. Thus, 12 studies were included in the final review.
Included studies
See: Characteristics of included studies
Out of the 12 included studies, seven studies were randomized controlled parallel trials, four studies were randomized cross‐over trials, and one study was a single‐dose trial. From these trials, a total of 595 patients were randomized to either a methyldopa treatment arm (296 patients) or a placebo treatment arm (299 patients). The daily doses of methyldopa used in these studies ranged 500‐2250 mg daily. The most commonly studied daily dose of methyldopa was 750 mg daily. Treatment durations with methyldopa of the included studies ranged from three to 52 weeks (excluding the single‐dose study). Most studies evaluated the effects of methyldopa given over four to six weeks.
A summary of each of the 12 trials which met our inclusion criteria is presented below.
This randomized trial compared the effects of either methyldopa, trimazosin, or placebo on supine and standing blood pressure and heart rate in men with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline standing blood pressure of the patients was 164.2/104.0 mm Hg. The trial followed up 18 patients with an average age of 53.8 years (+/‐ 7.8 years) for 17 weeks. At the end of the trial, one patient from the methyldopa treatment arm dropped out secondary to a drug‐related adverse effect.
This randomized trial was similar in design to Aronow 1977. Again, clinical cardiovascular outcomes were not investigated. The mean baseline standing blood pressure of the patients was 152.2/104.7 mm Hg. The trial followed up 57 patients with an average age of 49.6 years (+/‐ 10.7 years) for 16 weeks. At the end of the trial, ten patients dropped out of the trial: two patients developed methyldopa‐induced drug fever, three patients failed to meet the study's inclusion criteria following randomization, two patients were lost to follow up, two patients were non‐compliant with taking trial medications, and one patient dropped out of the study without any specified adequate reason.
This randomized cross‐over trial was designed to evaluate the effects of four interventions on supine and standing blood pressure in patients with essential hypertension: methyldopa alone, chlorothiazide alone, placebo alone, or combination therapy with methyldopa and chlorothiazide. Clinical cardiovasuclar outcomes were not investigated. Patients were assessed in each treatment arm for four weeks and then entered a two‐week washout period before crossing over to the next treatment arm. The mean baseline standing blood pressure of the patients was 163.9/109.5 mm Hg. The trial followed up 24 patients aged 21‐68 for 25 weeks. At the end of the trial, one patient dropped from study secondary to a drug‐related adverse effect while in the chlorothiazide arm.
This randomized trial compared the effects of either methyldopa, captopril, indapamide, or placebo on supine blood pressure, arterial blood flow, and peripheal resistance in patients with essential hypertension. Patients randomized to the methyldopa and captopril arms received only a single dose during the steady, whereas patients receiving indapamide and placebo remained on the medication for four weeks. Clinical cardiovascular outcomes were not investigated. The baseline mean arterial pressure of the patients ranged 120.8‐128.0 mm Hg. For patients stratified to the methyldopa arm, supine blood pressure was measured at baseline, and then at 30 minutes and four hours following the single dose. The trial followed up 24 patients aged 26‐60 years and all of these patients were accounted for at the end of the study period.
This randomized cross‐over trial was designed to evaluate the effects of methyldopa or placebo on supine and standing blood pressure and various psychometric tests in patients with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline untreated diastolic blood pressure ranged 90‐105 mm Hg. Patients were assessed in each treatment arm for three weeks with no washout period between the two treatment arms. The trial followed up 16 patients aged 26‐67 years for ten weeks. Patient withdrawals and patients lost to follow up were not reported.
This randomized cross‐over trial was designed to evaluate the effects of methyldopa, metoprolol, or placebo on supine blood pressure, heart rate, and calf blood flow in patients with essential hypertension and intermittent claudication. Clinical cardiovascular outcomes were not investigated. The mean baseline blood pressure during the placebo run‐in period was 190/99 mm Hg. Patients were assessed in each treatment arm for three weeks with no washout period between the three treatment arms. The trial followed up 17 patients aged 41‐73 years for 12 weeks. At the end of the trial, three patients were lost to follow up with no adequate reason given.
This randomized trial compared methyldopa, prazosin, or placebo on supine and standing blood pressure in patients with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline standing blood pressure ranged 163/104‐168/106 mm Hg. The trial followed up 60 patients with an average age of 46 years (+/‐ 9 years) for 20 weeks. Patient withdrawals and patients lost to follow up were not reported.
This randomized trial compared methyldopa, isradipine, or placebo on sitting blood pressure in men with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline sitting blood pressure was 155/102 mm Hg. The trial followed up 48 male patients aged 40‐65 years for one year. At the end of the trial, 14 patients from the placebo treatment arm were censored from the final reporting of outcomes secondary to deviation from protocol due to treatment failure.
This randomized cross‐over trial was designed to evaluate methyldopa alone, propranolol alone, practolol alone, placebo alone, methyldopa and propranolol, or methyldopa and practolol on supine and standing blood pressure, heart rate, weight, and treatment‐emergent side effects in patients with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline standing blood pressure was 182.9/123.3 mm Hg. The trial followed up 24 patients aged 24‐61 years for for 24 weeks. At the end of the trial, two patients were withdrawn from the study secondary to non‐fatal cardiovascular events (cerebral thrombosis and myocardial infarction) and a third patients withdrew secondary to "domestic circumstances". "Substitute patients" were enrolled in place of these original three patients to maintain the balanced design of the study.
This randomized trial compared methyldopa, prazosin, or placebo on supine and standing blood pressure and treatment‐emergent side effects in patients with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline blood pressure of the trial participants were not reported. The trial followed up 50 patients (age not reported) for 15 weeks. At the end of the trial, two patients from the methyldopa treatment arm withdrew secondary febrile reactions and their outcome data were censored. Also, two patients from the placebo treatment arm were lost to follow up without any adequate reason given.
This randomized trial compared methyldopa, propranolol, or placebo on supine blood pressure in patients with essential hypertension. Clinical cardiovascular outcomes were not investigated. The patients were stratified into two groups: mild hypertensives with baseline diastolic blood pressure 95‐114 mm Hg and moderate to severe hypertensives with baseline diastolic blood pressure 115‐130 mm Hg. The trial followed up 62 patients (age not reported) for six weeks. At the end of the trial, five patients dropped out of the study without any adequate reason given. No details were given with regards to which treatment arm these five patients were randomized and their outcomes data were censored.
This randomized trial compared methyldopa, isradipine, or placebo on sitting blood pressure and heart rate and treatment‐emergent side effects in men with essential hypertension. Clinical cardiovascular outcomes were not investigated. The mean baseline blood pressure ranged 150.7/99.3‐154.5/99.8 mm Hg. The trial followed up 368 patients aged 40‐65 years for one year. At the end of the trial, 21 patients withdrew from the study for reasons not specified. An additional 70 patients discontinued therapy either due to a "critical cardiac event", lack of efficacy, or adverse reactions. Details with regards to which treatment arm these patients were randomized were not given. It was also reported that 60 of these 70 patients were followed until the end of the study period; however, the remaining ten patients were not addressed. Also, the number of patients used to calculate outcomes data in each treatment arm were not reported.
Excluded studies
Risk of bias in included studies
For the overall assessment of the risk of bias in included studies see Figure 1 and Figure 2.
1.
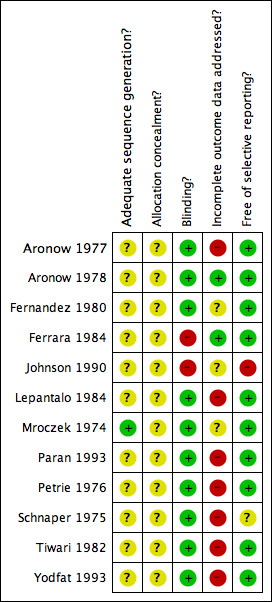
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
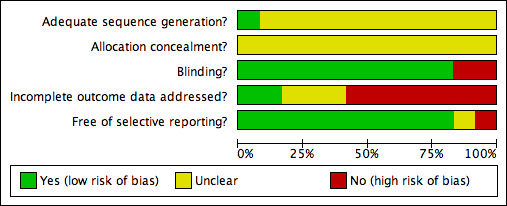
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Information pertaining to allocation concealment from all 12 included studes was insufficient to evaluate this aspect of reporting bias. Also, in all but one study (Mroczek 1974), details concerning methods of sequence generation for participant randomization were not provided. The authors recognize that poor reporting of study methodology does not necessarily imply that the study is methodologically flawed. Accordingly, it is our interpretation that since this aspect of quality reporting is unknown, the results from the majority of the studies included for this review may overestimate or underestimate the true effect of methyldopa for the prespecified outcomes of interest, or the results may be accurate.
In general, blinding of participants and investigators was adequate in 10 of the 12 included studies for this review based on simple reporting. However, the methods of blinding were adequately described in only four of these 10 trials (Mroczek 1974, Petrie 1976, Schnaper 1975, Tiwari 1982). Morever, blinding may have been compromised during the trials in view of the fact that limited details were provided with regards to treatment‐emergent adverse effects with methyldopa. For instance, CNS depressant effects and gastrointestinal side effects may incidentally reveal which patients were randomized to receive methyldopa during the study. Two studies (Ferrara 1984, Johnson 1990) were open label studies.
Overall, the quality of the majority of included trials were compromised by incomplete reporting of outcomes data. Specifically, of the 12 included studies, seven trials (Aronow 1977, Lepantalo 1984, Paran 1993, Petrie 1976, Schnaper 1975, Tiwari 1982, Yodfat 1993) either did not adequately report outcomes data for all randomized patients, failed to explain reasons for censoring results data of certain patients, and/or did not provide any details with regards to patients lost to follow up. Most trials did not report their results using the intention‐to‐treat principle. As such, the poor quality of outcomes data reporting in the included trials may again result in errors of estimation of the true effect of methyldopa compared to placebo on the prespecified outcomes of interest in this review.
Most of the included studies were free of selective reporting. One study (Johnson 1990) did not report on certain prespecified outcomes and another study (Schnaper 1975) reported outcomes that were not prespecified. It is important to note, however, that most studies did not state predefined primary and secondary outcomes in their trial methodology. Moreover, most trials did not report treatment‐emergent adverse effects or serious adverse events in any systematic manner that can be used in a meaningful meta‐analysis for this review.
Effects of interventions
Meta‐analyses (Analysis 1.1, Analysis 1.2) of methyldopa's blood pressure lowering efficacy compared to placebo were performed for seven of the 12 included studies (Aronow 1977, Aronow 1978, Fernandez 1980, Mroczek 1974, Paran 1993, Schnaper 1975, Tiwari 1982). From these seven trials, a total of 231 patients were randomized to either methyldopa (N=116) or placebo (N=115) treatment arms. These patients received either methyldopa or placebo control for treatment durations that ranged from 4 to 52 weeks. Doses of methyldopa used in these seven studies ranged from 500 mg daily to 2250 mg daily.
1.1. Analysis.
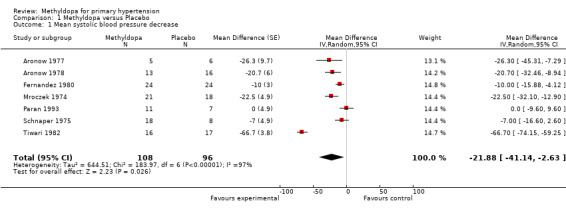
Comparison 1 Methyldopa versus Placebo, Outcome 1 Mean systolic blood pressure decrease.
1.2. Analysis.
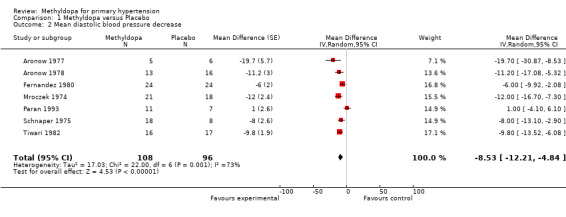
Comparison 1 Methyldopa versus Placebo, Outcome 2 Mean diastolic blood pressure decrease.
The analysis of mean difference in SBP (Analysis 1.1, Figure 3) found that methyldopa reduced SBP by 22.73 mmHg (95%CI 19.39‐26.08, p<0.00001) however there was significant heterogeneity (I2=97%). When the data from the Tiwari 1982 study (the effect size of this particular trial was large relative to other trials) was de‐selected, the analysis found that methyldopa reduced SBP by 11.64 mmHg (95%CI 7.90‐15.38, p<0.00001) with significant but relatively less heterogeneity (I2=69%). When the random effects model was used for this analysis the statistical significance did not change. Specifically, using the random effects model, methyldopa reduced SBP by 21.88 mmHg (95%CI 2.63‐41.14, p<0.03, I2=97%) when the Tiwari 1982 study was included, and 13.09 mmHg (95%CI 5.77‐20.41, p=0.0005, I2=69%) when the Tiwari 1982 study was not included. A sensitivity analysis was conducted to determine the impact of removing trials for which pooled standard error was imputed. This analysis found that methyldopa produced a reduction in SBP of 15.18mmHg (95% CI 10.70‐19.67, p<0.00001), a result similar to the original analysis. This analysis also detected significant heterogeneity (I2=59%) and when the data was re‐analyzed using a random effects model the reduction in SBP remained statistically significant (p<0.0001).
3.
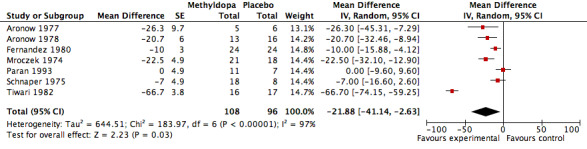
Forest plot of comparison: 1 Methyldopa versus Placebo, outcome: 1.1 Mean systolic blood pressure decrease.
The analysis of mean difference in DBP (Analysis 1.2, Figure 4) found that methyldopa reduced DBP by 8.07 mmHg (95%CI 6.23‐9.90, p<0.00001) however there was significant heterogeneity (I2=73%). When the data from the Tiwari 1982 study (the effect size of this particular trial was large relative to other trials) was de‐selected, the analysis found that methyldopa reduced DBP by 7.51 mmHg (95%CI 5.40‐9.62, p<0.00001) with significant and similar heterogeneity (I2=76%). When the random effects model was used for this analysis the statistical significance did not change. Specifically, using the random effects model, methyldopa reduced DBP by 8.53 mmHg (95%CI 4.84‐12.21, p<0.00001, I2=73%) when the Tiwari 1982 study was included, and 8.39 mmHg (95%CI 3.87‐12.92, p=0.0003, I2=76%) when the Tiwari 1982 study was not included. A sensitivity analysis was conducted to determine the impact of removing trials for which pooled standard error was imputed. This analysis found that methyldopa produced a reduction in DBP of 9.61 mmHg (95% CI 7.00‐12.22, p<0.00001), a result similar to the original analysis. This analysis also detected significant heterogeneity (I2=61%) and when the data was re‐analyzed using a random effects model the reduction in DBP remained statistically significant (p<0.0001).
4.
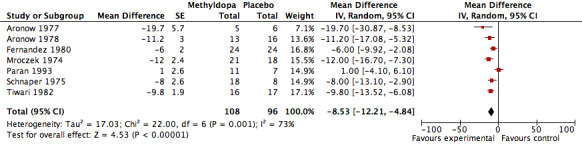
Forest plot of comparison: 1 Methyldopa versus Placebo, outcome: 1.2 Mean diastolic blood pressure decrease.
Three cross‐over studies (Johnson 1990, Lepantalo 1984, Petrie 1976) were not included in the meta‐analyses of methyldopa's blood pressure lowering efficacy because they did not include an adequate washout period between treatment periods. Thus, one could not rule out overlapping antihypertensive effects when patients were transferred between methyldopa and placebo treatment arms. One randomized trial (Yodfat 1993) was not included in this meta‐analysis because the authors did not specify the final number of patients who completed each treatment arm when reporting their results. One randomized trial (Ferrara 1984) did not have any useable data because it was a single dose study.
Johnson 1990 crossover study involving 16 patients found that standing systolic blood pressure was decreased from 142 (+/‐ 14) to 132 (+/‐ 20) mm Hg and that diastolic pressure decreased from 100 (+/‐ 5) to 90 (+/‐ 8) after three weeks of treatment with methyldopa 750 mg daily compared to placebo. Lepantalo 1984 crossover study involving 14 patients found that supine systolic blood pressure was decreased from 187 (+/‐ 21) to 167 (+/‐ 20) and that diastolic pressure decreased from 98 (+/‐ 10) to 88 (+/‐ 10) after three weeks of treatment with methyldopa 500‐1000 mg daily. Petrie 1976's crossover study involving 24 patients found that standing systolic blood pressure decreased from 175.2 to 159.1 (SDs not reported) and that diastolic pressure decreased from 122.1 to 111.2 after four weeks of treatment with methyldopa 750 mg daily. Yodfat 1993 trial, which originally randomized 244 patients to either methyldopa or placebo arms found that sitting diastolic blood pressure decreased from 90 to 87 (visual interpretation from graphic, SDs not reported) after 52 weeks of treatment with methyldopa 500‐1000 mg daily. However, as mentioned, it is unknown how many of the original 244 patients completed either treatment arm at the end of the study. Lastly, Ferrara 1984 single dose study in 12 patients found that methyldopa 500 mg daily decreased supine mean blood pressure 126.1 (+/‐ 14) to 124.8 (+/‐ 8) after 30 minutes and that 122.9 (+/‐ 12) to 119.4 (+/‐ 4) after four hours.
Unfortunately, none of the included studies for this review reported results for the following clinical outcomes: all cause mortality, cardiovascular mortality, non‐cardiovascular mortality, serious adverse events, fatal and non‐fatal myocardial infarction, and fatal and non‐fatal stroke. Also, the trials did not report the numbers of patients experiencing at least one adverse event or the numbers of patients with withdrawals due to adverse effects in a manner that would permit a meaningful meta‐analysis.
Discussion
Summary of main results
There is insufficient evidence to conclude on the effects of methyldopa versus placebo for mortality, morbidity, withdrawals due to adverse effects, or total adverse effects. Although not included in this review, other randomized trials which tested methyldopa against non‐placebo controls also did not find any differences in clinical outcomes. For instance, in Sprackling 1981, 123 elderly subjects (mean age 80 years) with a single casual diastolic blood pressure of 100 mmHg or more were randomized to treatment with methyldopa 250 mg twice daily (which was subsequently adjusted as necessary to bring the standing diastolic blood pressure towards target of 90 mmHg) and was compared to no treatment (i.e. did not receive medication over and above any treatment that their general practitioner deemed to be necessary for other aspects of their health). Standing SBP/DBP was reduced significantly by ‐18.3/ ‐7.8 mmHg but there were no significant differences between the groups in mortality or morbidity.
Methyldopa was commonly used in the 1970's and 80's for blood pressure control. Even though its use at present has largely been replaced by newer antihypertensive drugs with more acceptable tolerability profiles, it is still widely used in developing countries due to its lower cost. Although there is insufficient information to make conclusions about adverse effects from this review, it is important to note that adverse effects of methyldopa are not uncommon and can be serious. They include immune mediated haemolytic anemia (20% Coombs positive), hepatotoxicity (5% increased liver enzymes) and a lupus‐like syndrome (Goodman & Gilman 1996). Thus, in addition to the fact that this review did not find any evidence of clinical outcomes benefit for the use of methyldopa in patients with primary hypertension, healthcare practitioners should also be aware that there are potential serious side effects associated with the use of methyldopa.
The analysis of six trials that provided data that was amenable for meta‐analysis found that methyldopa reduced SBP by 13.09 (5.77‐20.41) mmHg and reduced DBP by 8.39 (2.87‐12.92) mmHg over and above reductions in blood pressure due to placebo. The imputation of pooled standard error for two trials did not impact the finding as the confidence intervals for blood pressure reductions overlapped when data from these trials was removed. Similar reductions were also seen in trials that were not included in the meta‐analysis (i.e. methyldopa was associated with SBP reductions of from approximately 15‐20 mmHg and DBP reductions of approximately 10 mmHg).
Overall completeness and applicability of evidence
While contact was made with certain authors (Johnson 1990, Yodfat 1993), further study information was not made available secondary to the dated nature of the trials. In general, reporting of outcomes was incomplete in all trials. The applicability of the results is therefore limited as the data may not be reliable (i.e. results are likely to represent an overestimate of the effects of methyldopa versus placebo).
Quality of the evidence
Overall, the quality of evidence was compromised secondary to the unclear nature of random sequence generation and allocation concealment procedures of almost all trials. Moreover, many of the trials did not report complete outcomes data for all randomized patients. Thus, the estimation of the true effect of methyldopa on outcomes such as BP effects is likely an overestimate.
Authors' conclusions
Implications for practice.
Methyldopa lowers blood pressure in patients with primary (essential) hypertension, when given at doses 500‐2250 mg daily compared to placebo. Clinicians who wish to recommend methyldopa for their patients should understand, however, that while methyldopa may reduce blood pressure, to the best of our knowledge, there are no known clinical studies which have associated use of methyldopa with a reduction in all cause mortality, myocardial infarction, or stroke. In addition, despite poor reporting of treatment‐emergent adverse effects, clinicians must weigh the risks of potential serious side effects with use of methyldopa that include hemolytic anemia, hepatotoxicity as well as lupus‐like syndrome against the benefits of blood pressure reduction with no proven beneficial effect on adverse cardiovascular outcomes.
Implications for research.
Despite methyldopa's use as an antihypertensive agent for patients with essential hypertension since the 1970's, the prescribing of this agent has been based solely on blood pressure reduction studies. Because of the relatively high incidence of adverse effects associated with this drug, large trials comparing methyldopa with other classes of antihypertensive drugs are not recommended.
Acknowledgements
We would like to acknowledge the original authors of this protocol (Pillay A, O' Reagan L) who identified the topic and contributed extensive background work on the protocol.
We would also like to acknowledge the assistance provided by the Cochrane Hypertension Group.
Special thanks to Stephen Adams for all his efforts with retrieving articles for this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials search strategy
2nd Quarter 2009
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. (767) 2. exp hypertension/ (10978) 3. hypertens$.mp. (22124) 4. exp blood pressure/ (18927) 5. bloodpressure.tw. (12) 6. ((diastolic or systolic or arterial or blood) adj pressure).mp. (36962) 7. or/2‐6 (45779) 8. 1 and 7 (495)
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) 1950 to Present with Daily Update
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. methyldopa/ (3389) 2. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. (13603) 3. or/1‐2 (13603) 4. exp hypertension/ (182296) 5. hypertens$.tw. (245258) 6. exp blood pressure/ (217493) 7. blood pressure.mp. (297950) 8. ((diastolic or systolic or arterial) adj pressure).tw. (57456) 9. bloodpressure.tw. (29) 10. or/4‐8 (510419) 11. randomized controlled trial.pt. (273041) 12. controlled clinical trial.pt. (79457) 13. random$.mp. (585582) 14. placebo$.mp. (129736) 15. dt.fs. (1319017) 16. trial.tw. (230419) 17. groups.ab. (910688) 18. (doubl$ adj3 blind$).mp. (121952) 19. or/11‐18 (2545966) 20. animals/ not (humans/ and animals/) (3292945) 21. 19 not 20 (2153905) 22. 3 and 10 and 21 (1787) 23. limit 22 to yr="2005 ‐ 2009" (34)
Appendix 3. EMBASE search strategy
1980 to 2009 Week 23
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. methyldopa/ (9231) 2. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. (17074) 3. or/1‐2 (17074) 4. exp hypertension/ (217537) 5. hypertens$.tw. (194611) 6. exp blood pressure/ (182591) 7. blood pressure.mp. (206734) 8. bloodpressure.tw. (80) 9. ((diastolic or systolic or arterial) adj pressure).tw. (47531) 10. or/4‐9 (435730) 11. controlled clinical trial$.mp. (70720) 12. random$.mp. (440389) 13. placebo$.mp. (178765) 14. dt.fs. (1568096) 15. trial.ab. (170210) 16. groups.ab. (776954) 17. (doubl$ adj3 blind$).mp. (107021) 18. or/11‐17 (2490472) 19. animals/ not (humans/ and animals/) (14488) 20. 18 not 19 (2489216) 21. 3 and 10 and 20 (3145) 22. limit 21 to yr="2007 ‐ 2009" (267)
Appendix 4. Database of Abstracts of Reviews of Effects search strategy
2nd Quarter 2009
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).tw. (13) 2. hypertens$.tw. (454) 3. ((diastolic or systolic or arterial or blood) adj pressure).tw. (395) 4. 1 and (2 or 3) (12)
Data and analyses
Comparison 1. Methyldopa versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean systolic blood pressure decrease | 7 | 204 | Mean Difference (Random, 95% CI) | ‐21.88 [‐41.14, ‐2.63] |
| 2 Mean diastolic blood pressure decrease | 7 | 204 | Mean Difference (Random, 95% CI) | ‐8.53 [‐12.21, ‐4.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aronow 1977.
| Methods | Single‐centre study Randomization: "Double‐blind randomized study...." Blinding: "Double‐blind randomized study...." Withdrawals: "Of the six patients on methyldopa, one was dropped from the study because of methyldopa‐induced drug fever...." Lost to follow‐up: 0% Treatment duration: 8 weeks on active treatment period Analysis type: per protocol |
|
| Participants | Geographic region: not reported Study setting: not reported N=18 Age range: 53.8 +/‐ 7.8 Gender: males only Race: not reported Blood pressure at entry: Supine (164.2/103.6); Standing (164.2/104.0) Co‐morbid conditions: not reported Inclusion criteria: essential hypertension Exclusion criteria: coronary artery disease; cerebrovascular disease; heart failure; renal disease; hepatic disease |
|
| Interventions | All anti‐hypertensives discontinued for a two week washout period before trial entry and patients did not take any other medications besides study medications. Then all patients received single‐blind placebo for four weeks.
All patients then entered single blind placebo three capsules three times daily x 3 weeks |
|
| Outcomes | Supine blood pressure Standing blood pressure Supine heart rate Standing heart rate Side effects |
|
| Notes | Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: Methyldopa arm (5); Placebo arm (6); Trimazosin arm (6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | High risk | 1 patient dropped from Methyldopa group but no explanation regarding what was done with that patient's data |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Aronow 1978.
| Methods | Multi‐centre study Randomization: "...in a double‐blind, randomized study, 20 patients were randomized to trimazosin for eight weeks, 18 patients were randomized to methyldopa for eight weeks, and 19 patients were randomized to placebo for eight weeks." Blinding: "...single blind placebo and double‐bliind trimazosin, methyldopa, or placebo...." Withdrawals: "47 patients completed the study....Two patients with methyldopa‐induced fever were discontinued from the study. One of these patients also developed laboratory evidence of hepatotoxicity on methtyldopa. Three patients were discontinued from because of normal blood pressure at the end of the first single‐bind placebo period. Two patients were discontinued from the study because they did not return for follow‐up visits at the proper time. Two patients were discontinued from the study because they were unreliable and took their medication intermittently. One patient dropped out of the study." Lost to follow‐up: The study did not report on results of patients who were discontinued from the study. "Two patients were discontinued from the study because they did not return for follow‐up visits at the proper time....One patient dropped out of the study" Treatment duration: 8 weeks on active treatment period Analysis type: Per protocol |
|
| Participants | Geographic region: United States (California, Alabama, Georgia) Study setting: not reported N=57 Age range: 49.6 +/‐ 10.7 Gender: 41 males; 16 females Race: not reported Blood pressure at entry: Methyldopa ‐ Standing 152.2/104.7 (+/‐ 15.7/6.8); Placebo ‐ Standing 158.4/104.9 (+/‐ 19.2/8.7); Trimazosin ‐ Standing 155.7/104.8 (+/‐ 14.6/6.4) Co‐morbid conditions: not reported Inclusion criteria: Essential hypertension Exclusion criteria: not reported |
|
| Interventions | All anti‐hypertensives discontinued for a two week washout period before trial entry and patients did not take any other medications besides study medications. Then all patients received single‐blind placebo for four weeks (one capsule three times daily)
All patients then entered single blind placebo three capsules three times daily x 2 weeks |
|
| Outcomes | Supine blood pressure Standing blood pressure Supine heart rate Standing heart rate Side effects |
|
| Notes | Assessment of medication compliance: Final number of patients included in each arm when reporting results: Methyldopa arm (13); Placebo arm (16); Trimazosin arm (18) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | Low risk | All 57 patients accounted for during trial |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Fernandez 1980.
| Methods | Single‐centre study Randomization: "The patients were numbered in the order they entered the study and were randomly assigned to one of four groups. Each group of six patients received all four treatments...in a different sequence determined by random assortment in a Latin square design." Blinding: "Randomized double blind trial...."; "The agents (tablets) were identical in appearance and taste." Withdrawals: "One patient was dropped from the study because of severe abdomenal cramps and diarrhea that developed one hour after 150 mg of chlorothiazide was taken and disappeared when this treatment was stopped." Lost to follow‐up: 0% Treatment duration: 16 weeks of active treatment with each of the four treatment periods lasting four weeks Analysis type: per protocol |
|
| Participants | Geographic region: not reported Study setting: outpatient clinic N=24 Age range: 21‐68 Gender: 22 males; 2 females Race: Caucasian Blood pressure at entry: Supine (165.0/105.8); Standing (163.9/109.5) Co‐morbid conditions: not reported Inclusion criteria: essential hypertension; supine or standing diastolic blood pressure 90‐120 mm Hg Exclusion criteria: grade III or IV hypertensive retinopathy; heart failure; acute myocardial infarction; arrhythmias; angina pectoris; impaired liver or kidney function; blood dyscrasias; positive results of Coomb's test; allergy to any study drug; previous stroke; insulin‐dependent diabetes mellitus; serum potassium less than 3.5 mmol/L; pregnant patients; malignant diseases; any other condition at investigator's discretion |
|
| Interventions | All anti‐hypertensives discontinued and replaced with placebo for a three week washout period before trial entry.
***Cross over trial: Each group of six patients received all four treatments for four weeks each with each treatment period separate by a two week washout |
|
| Outcomes | Supine blood pressure Standing blood pressure Standing heart rate Weight Side effects |
|
| Notes | All patients instructed to limit dietary salt intake to less than 2.3 grams daily Assessment of medication compliance: tablet counts of medication bottles weekly during study periods (all patients achieved over 80% compliance overall in study) Final number of patients included in each arm when reporting results: All four groups (23) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear 1 patient dropped from study secondary to side effect while on chlorothiazide arm but no explanation regarding what was done with that patient's data |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Ferrara 1984.
| Methods | Single‐centre study Randomization: "...patient were randomly given a single dose of placebo, captopril 50 mg, methyldopa 500 mg, or indapamide 2.5 mg." Blinding: not reported Withdrawals: none Lost to follow‐up: none Treatment duration: single dose study Analysis type: unclear |
|
| Participants | Geographic region: Naples, Italy Study setting: outpatient clinic N=24 Age range: 26‐60 Gender: 18 males, 6 females Race: not reported Blood pressure at entry: Mean supine blood pressure ‐ Methyldopa arm (127.7), Placebo arm (125.2), Captopril arm (128.0), Indapamide arm (120.8) Co‐morbid conditions: not reported Inclusion criteria: essential hypertension Exclusion criteria: target organ damage secondary to hypertension |
|
| Interventions | All anti‐hypertensives and any other drugs were discontinued for a two week washout period before trial entry
|
|
| Outcomes | Supine blood pressure Supine heart rate Arterial blood flow |
|
| Notes | Single dose study (blood pressure was measured every 30 minutes for 5 hour after the dose was given). Reported data comparing methyldopa and placebo on reduction in blood pressure 30 minutes and four hours after single dose given; thus, no real useable data from this study. Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: Methyldopa arm (6); Placebo arm (6); Captopril arm (6); Indapamide arm (6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Open label |
| Incomplete outcome data addressed? All outcomes | Low risk | All randomized patients accounted for at end of study |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Johnson 1990.
| Methods | Single‐centre study Randomization: "In a cross‐over design study, patients were randomly assigned to receive either methyldopa...for three weeks followed by matching placebo tablets for three more weeks, or the reverse sequence of treatments." Blinding: not reported Withdrawals: not reported Lost to follow‐up: not reported Treatment duration: patients took both methyldopa and placebo each for three weeks Analysis type: not reported |
|
| Participants | Geographic region: Study setting: not reported N=16 Age range: 26‐67 Gender: 5 males, 11 females Race: not reported Blood pressure at entry: not reported Co‐morbid conditions: not reported Inclusion criteria: essential hypertension; untreated supine diastolic blood pressure 90‐105 mm Hg Exclusion criteria: not reported |
|
| Interventions | All anti‐hypertensives and any other drugs were discontinued for a four week washout period before trial entry
***Cross over trial: All 16 patients rotated throughout the above treatment arms without washout periods between treatment arms |
|
| Outcomes | Supine blood pressure Standing blood pressure |
|
| Notes | Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Open label |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear ‐ final number of patients in each treatment arm was not reported in results |
| Free of selective reporting? | High risk | Did not report data for supine blood pressures |
Lepantalo 1984.
| Methods | Single‐centre study Randomization: "Three treatment periods...in random order." Blinding: "The study was double blind...placebo...." Withdrawals: none Lost to follow‐up: 3 patients Treatment duration: each treatment period was three weeks Analysis type: not reported |
|
| Participants | Geographic region: not reported Study setting: not reported N=17 Age range: 41‐73 Gender: 9 males; 5 females Race: not reported Blood pressure at entry: Supine (190/99) Co‐morbid conditions: no coronary artery disease, heart failure, stroke, or advanced limb ischemia Inclusion criteria: essential hypertension; intermittent claudication Exclusion criteria: not reported |
|
| Interventions | All patients entered three week run‐in period with placebo
***Cross over trial: All 14 patients rotated throughout the above three treatment arms without washout periods between treatment arms |
|
| Outcomes | Supine blood pressure Supine heart rate Supine calf blood flow |
|
| Notes | Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: All three treatment arms (14) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | High risk | 17 patients recruited but only 14 patients completed the trial. No data given for 3 missing patients |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Mroczek 1974.
| Methods | Single‐centre study Randomization: "Random assignment was made to one of the three treatment groups by a computer using a pseudo‐random number generator assigned to the list of drugs." Blinding: "The double blind aspect of the study was maintained by having the evaluating physician indicate dosage increases by prescription to an experienced drug monitor who was acquainted with the study design, the patient drug assignment, and dosage schedule."; "Placebo...was supplied in identical matching capsules to prazosin and methyldopa." Withdrawals: not reported Lost to follow‐up: not reported Treatment duration: blood pressures measured at two week intervals Analysis type: not reported |
|
| Participants | Geographic region: not reported Study setting: not reported N=60 Age range: Methyldopa arm (47.2 +/‐ 9.4), Placebo arm (45.7 +/‐ 11.1), Prazosin arm (44.7 +/‐ 9.9) Gender: 8 males, 52 females Race: all blacks Blood pressure at entry: Methyldopa arm ‐ Supine (160/101), Standing (168/106); Placebo arm ‐ Supine (156/100), Standing (163/104); Prazosin arm ‐ Supine (156/101), Standing (164/105) Co‐morbid conditions: not reported Inclusion criteria: essential hypertension; diastolic blood pressure greater than 95 mm Hg Exclusion criteria: not reported |
|
| Interventions | All anti‐hypertensives discontinued and replaced with single blind placebo for an eight week washout period before trial entry.
|
|
| Outcomes | Supine blood pressure Standing blood pressure Side effects (postural dizziness, headache, other) |
|
| Notes | Assessment of medication compliance: medications were dispensed as 14‐day supplies and patients returned with medication bottles for capsule counts (level of achievement of medication compliance not reported) Final number of patients included in each arm when reporting results: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Random assignment...by computer using a pseudo‐random number generator assigned to the list of drugs." |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear ‐ final number of patients in each treatment arm was not reported in results |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Paran 1993.
| Methods | Single‐centre study Randomization: two parallel treatment groups and a placebo group; "...patients were double blindly randomized into three treatment groups...." Blinding: "The study was double‐blind...."; no other details provided Withdrawals: 14 patients from placebo arm withdrew due to treatment failure. Lost to follow‐up: 0% Treatment duration: follow‐up for one year. Analysis type: per protocol. |
|
| Participants | Geographic region: Israel. Study setting: outpatient clinic N=48 Age range: 40‐65 Gender: all males Race: not reported Blood pressure at entry: Sitting blood pressure ‐ Methyldopa arm (155/102); Placebo arm (154/101) Co‐morbid conditions: not reported Inclusion criteria: Average sitting diastolic blood pressure of 95‐119 mm Hg on two consecutive visits while on placebo for first two to four weeks Exclusion criteria: secondary hypertension; "hypertensive complications" |
|
| Interventions | All anti‐hypertensives discontinued and placebo given for two to four weeks.
"Titration period lasted eight weeks or until DBP of 90 mm Hg or less was achieved." |
|
| Outcomes | Sitting blood pressure (monthly evaluations in the clinic for one year). Pre and post‐exercise (treadmill) blood pressure, heart rate, EKG (two evaluations: one during placebo run‐in period and one at end of year). Heart rate |
|
| Notes | Only seven patients originally randomized to the placebo arm were included in the reporting of outcomes secondary to treatment failure. All patients in the methyldopa arm and in the isradipine arm were included in the reporting of outcomes. Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: methyldopa arm (11), placebo arm (7), isradipine arm (16) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...patients were double blindly randomized into three treatment groups...." |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | High risk | "The placebo included 21 patients at the start; however, only seven of these completed the whole course of one year, the rest having deviated from protocol due to treatment failure....Subjects who did not stay on the same treatment for a year could not be included in the comparative analysis." Study did not provide results data for the 14 patients who received placebo and did not complete the trial |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Petrie 1976.
| Methods | Single‐centre study Randomization: "A double‐blind crossover method was used to assess the effects...of six treatments, each given three times a day...."; "Each treatment was given for four weeks, and each of the 24 patients received the six treatments." Blinding: "A double‐blind crossover method was used...."; "The drug supplies for each patient were pre‐packed (in duplicate) and new containers were issued at the start of each new treatment period."; matching placebo tablets were used; "The double‐placebo technique ensured that patients took the same number of tablets throughout the trial."; "The observer not recording the blood pressure completed a questionnaire on symptoms in another room." Withdrawals: "Two of the original patients were withdrawn from the study while on active treatment because of non‐fatal cardiovascular events (cerebral thrombosis, myocardial infarction). A third patient withdrew because of domestic disturbances. Reserve duplicate drug supplies were used for their substitutes to maintain the balanced design of the trial." Lost to follow‐up: 0% Treatment duration: Patients were assessed every two weeks at clinic for blood pressure recording; total duration 24 weeks comprising of six separate four‐week treatment periods. Analysis type: per protocol |
|
| Participants | Geographic region: not reported Study setting: outpatient clinic N=24 Age range: 48.5 (24‐61) Gender: 13 male, 11 female Race: Not recorded Blood pressure at entry: Supine (189/117); Standing (183/123) Co‐morbid conditions: not reported Inclusion criteria: age 21‐65; supine DBP greater than 105 mm Hg and less than 125 mm Hg Exclusion criteria: history of recent myocardial infarction; evidence of cardiac failure, heart block, or gross ischemia; grade III or IV retinopathy; diabetes mellitus; gout; impaired liver function; creatinine clearance less than 60 mL/min; on any other drug treatment |
|
| Interventions | All medications discontinued 14 days before trial entry.
***Cross over trial: All 24 patients rotated throughout the above six treatment arms without washout periods between treatment arms |
|
| Outcomes | Supine blood pressure Standing blood pressure Post‐exercise blood pressure Heart rate Symptom questionnaire: general wellbeing, dizziness, headache, energy, tiredness, mood, sleep, dreams, bowel habit, Tablet counts Body weight |
|
| Notes | According to the authors, "one month on each treatment was chosen to allow for adequate time for the effects to become evident and for the influence of any cross‐over effects to be minimized." Unknown at which point in treatment period the three patients mentioned above withdrew from the study. Assessment of medication compliance: capsule counts (patients achieved >90% compliance throughout trial) Final number of patients included in each arm when reporting results: 24 patients rotated through each treatment arm (3 patients were substituted) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Each patient received methyldopa, propranolol, practolol, methyldopa combined with propranolol, methyldopa combined with practolol, and placebo for four weeks each according to a random sequence." |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | "A double‐blind crossover method was used...."; "The drug supplies for each patient were pre‐packed (in duplicate) and new containers were issued at the start of each new treatment period."; matching placebo tablets were used; "The double‐placebo technique ensured that patients took the same number of tablets throughout the trial."; "The observer not recording the blood pressure completed a questionnaire on symptoms in another room." |
| Incomplete outcome data addressed? All outcomes | High risk | "Two of the original patients were withdrawn from the study while on active treatment because of non‐fatal cardiovascular events (cerebral thrombosis, myocardial infarction). A third patient withdrew because of domestic disturbances. Reserve duplicate drug supplies were used for their substitutes to maintain the balanced design of the trial."; "A complete set of observations was available for each of the 24 patients...." Outcomes data for the three patients who withdrew from therapy were not made available |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Schnaper 1975.
| Methods | Single‐centre study Randomization: "Random assignment was made...in a balanced manner so that after ten patients were admitted, the number in each group was approximately the same." Blinding: "Medication was dispensed in identical capsules, and each patient was given an individually coded bottle."; "15‐week double‐blind comparison of three groups of patients...." Withdrawals: "Two patients in the methyldopa group were dropped from the study because of febrile reactions. Initially the medication was withdrawn when the reaction occurred, and the fever subsided. However, twice when medication was given again, fever recurred after two to three weeks." Lost to follow‐up: 2 patients from placebo group not accounted for in results Treatment duration: Patients in methyldopa arm received treatment for an average of 38.2 days Analysis type: per protocol |
|
| Participants | Geographic region: Alabama, US Study setting: Outpatient clinic N=50 Age range: not recorded Gender: not recorded Race: not recorded Blood pressure at entry: not recorded Co‐morbid conditions: not recorded Inclusion criteria: at least 21 years old; diagnosis of essential hypertension or renal hypertension not amenable to surgical treatment; presence of sustained baseline supine diastolic blood pressure between 95‐115 mm Hg Exclusion criteria: labile hypertension; pregnant women; cerebrovascular accident or acute myocardial infarction in past year; receiving a sedative or a tranquillizer; receiving an investigational drug; secondary hypertension |
|
| Interventions | Patients who were taking antihypertensives were first entered into an "extended washout period" of at least two weeks. Patients whose diastolic blood pressure remained above 95 mm Hg were then entered in a single‐blind placebo period for two weeks. Patients then entered an 11‐week double blind study period
|
|
| Outcomes | Reduction in supine blood pressure Reduction in standing blood pressure Side effects (dry mouth, headache, postural dizziness, lack of energy, nasal congestion, urinary frequency, constipation, drowsiness, febrile reactions) Weight |
|
| Notes | Two studies were published in this article, but only one study's methodology and results are described here as the other study did not have a methyldopa arm. Measured sitting, supine, and standing blood pressures. Assessment of medication compliance: done via capsule counts of medication bottles (level of achievement of medication compliance not reported) Final number of patients included in each arm when reporting results: methyldopa arm (18), placebo arm (8), prazosin arm (20) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Random assignment was made...in a balanced manner...." |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | "15‐week double‐blind comparison of three groups of patients...."; "Medication was dispensed in identical capsules, and each patient was given an individually coded bottle." |
| Incomplete outcome data addressed? All outcomes | High risk | 2 patients from placebo group not accounted for in results |
| Free of selective reporting? | Unclear risk | Did not indicate which specific side effects would be reported in the methodology, and reported various side effects in the results in a non‐systematic manner Weight was reported as a measured outcome in the methodology, but not actually reported in the results |
Tiwari 1982.
| Methods | Single‐centre study Randomization: "These capsules were given in randomized order and doses were increased after every two weeks...." Blinding: "This was a double blind placebo controlled study...."; "Placebo, methyldopa, and propranolol were put in identical looking capsules and coded without knowledge of either the observer or subject." Withdrawals: "Of the 62 cases included in the study, five cases dropped out due to different reasons and 57 patients completed the trial." Lost to follow‐up: "Of the 62 cases included in the study, five cases dropped out due to different reasons and 57 patients completed the trial." Treatment duration: Six weeks total on active treatment period Analysis type: per protocol |
|
| Participants | Geographic region: not reported Study setting: not reported N=62 Age range: not reported Gender: not reported Race: not reported Blood pressure at entry: Methyldopa arm ‐ Group I (?16.0/106.4), Group II (179.7/118.8); Placebo arm ‐ Group I (263.3/103.6), Group II (190.3/121.6); Propranolol arm ‐ Group I (165.2/106.8), Group II (182.9/120.5) Co‐morbid conditions: not reported Inclusion criteria: essential hypertension; sustained diastolic blood pressure greater than 95 mm Hg Exclusion criteria: secondary hypertension; pregnant patients; myocardial infarction or cerebrovascular accident in past six months; accelerated hypertension; hepatic or renal dysfunction |
|
| Interventions | All medications were discontinued during a two week washout period before trial entry
|
|
| Outcomes | Supine blood pressure | |
| Notes | Patients were stratified into two groups based on severity of hypertension: Group I "Mild Hypertension" ‐ diastolic blood pressure 95‐114 mmHg (30 patients); Group II "Moderate‐Severe Hypertension" ‐ 115‐130 mm Hg (27 patients) Doses were increased every two weeks up to the maximum dose and patients who responded to drug therapy was maintained at the same dose for a further two weeks Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: Methyldopa arm (9 + 7 patients); Placebo arm (11 + 6 patients); Propranolol arm (10 + 14 patients) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "These capsules were given in randomized order...." |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | "This was a double blind placebo controlled study...."; "Placebo, methyldopa, and propranolol were put in identical looking capsules and coded without knowledge of either the observer or subject." |
| Incomplete outcome data addressed? All outcomes | High risk | "Of the 62 cases included in the study, five cases dropped out due to different reasons and 57 patients completed the trial." Did not report from which treatment arms these patients dropped out and did not report reasons for dropping out |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Yodfat 1993.
| Methods | Multi‐centre study Randomization: "...all patients were randomly assigned to receive one of the three treatments...." Blinding: "...double blind active treatment....placebo...." Withdrawals: "Twenty‐one patients withdrew from the study, the majority during the titration period. Seventy patients discontinued the double‐blind treatment, of whom 60 were followed until the end of the study."; Reasons for discontinuation of therapy (e.g.. critical cardiac events, lack of efficacy, adverse reaction) in each treatment arm was described in the study for the 70 patients in the double‐blind treatment. No details provided regarding the 21 patients who withdrew from the study in terms of which treatment arm they withdrew from and reasons for withdrawing from the study. Lost to follow‐up: not reported (actual number of patients included in each treatment arm when describing results of study was not reported) Treatment duration: active treatment period and follow‐up for one year; patients visited clinic every two weeks during placebo run‐in and dose titration periods, then monthly until end of study period Analysis type: reported as "intention to treat" but unable to assess and confirm (actual number of patients included in each treatment arm when describing results of study was not reported) |
|
| Participants | Geographic region: Israel Study setting: not reported N=368 Age range: 40‐65 Gender: males only Race: not reported Blood pressure at entry: Methyldopa arm (152.0/99.3); Placebo arm (150.7/99.8); Isradipine arm (154.5/99.7) Co‐morbid conditions: none of the patients had a history of alcohol abuse, mental disorder, or insulin‐dependent diabetes mellitus Inclusion criteria: essential hypertension; sitting diastolic blood pressure 95‐105 mm Hg Exclusion criteria: secondary hypertension; malignant hypertension; unstable angina; recent myocardial infarction; any clinically relevant cardiovascular disease; any abnormal laboratory findings (including liver function tests and creatinine levels up to 1.5 mg/100 mL) |
|
| Interventions | Patients had two to four week single‐blinded placebo washout period before entering trial
Patients entered final one month single‐blind placebo period at end of trial |
|
| Outcomes | Sitting blood pressure Sitting heart rate Clinical symptoms Side effects (most frequently reported: shortness of breath, chest pain palpitations, sleep disorders, sexual disorders, headache, fatigue, heartburn, nausea, vomiting, diarrhoea, constipation, abdominal pain) |
|
| Notes | No useable data obtained from this study as the actual number of patients in each treatment arm when describing outcomes was not reported. Assessment of medication compliance: not reported Final number of patients included in each arm when reporting results: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...all patients were randomly assigned to receive one of the three treatments...." |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double blind |
| Incomplete outcome data addressed? All outcomes | High risk | Actual number of patients in each treatment arm when describing outcomes was not reported No details provided regarding the 20 patients who withdrew from the study in terms of reasons or from which treatment arm they withdrew No details provided for the 10 patients who discontinued therapy with a reason but were not followed until the end of the study |
| Free of selective reporting? | Low risk | Reported on all pre‐specified outcomes of interest |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alfonzo Guerra 1980 | No placebo only arm during randomization (compared methyldopa versus clonidine versus clonidine and thiazide diuretic) |
| Anonymous 1986 | No methyldopa arm (trial focused on enalapril versus placebo) |
| Aoki 1970 | No placebo only arm during randomization (compared methyldopa versus hydralazine in patients already receiving hydrochlorothiazide) |
| Avigdor 1983 | Methyldopa, placebo, debrisoquine, and propranolol were added randomly to patients already receiving the diuretic mefruside |
| Bayliss 1962 | Not a randomized study comparing methyldopa versus placebo |
| Beanlands 1978 | No placebo only arm during randomization (compared methyldopa versus pindolol) |
| Belleau 1977 | No placebo only arm during randomization (compared methyldopa versus debrisoquine in patients already receiving hydrochlorothiazide) |
| Bradley 1977 | No placebo only arm during randomization (compared methyldopa versus prazosin) |
| Bune 1981 | No placebo only arm during randomization (compared methyldopa versus guanfacine in patients already receiving bendrofluazide) |
| Cannon 1962 | Not a randomized study comparing methyldopa versus placebo |
| Co‐operative 1973 | Treatment groups received varying combinations of methyldopa, bendrofluazide, and/or debrisoquine (could not differentiate patients who received only methyldopa and therefore cannot compare against patients who received only placebo) |
| Corea 1983 | No placebo arm during randomization (both treatment groups received methyldopa) |
| Daley 1962 | Not a randomized study comparing methyldopa versus placebo |
| De Divitiis 1981 | No placebo only arm during randomization (compared methyldopa versus atenolol) |
| Deschamps 1992 | Patients were not diagnosed with essential hypertension (trial of methyldopa in patients with pregnancy‐related hypertension) |
| Dollery 1962 | No placebo only arm during randomization (all treatment groups received methyldopa) |
| Dunn 1978 | Primarily assessed effect of antihypertensives to maintain blood pressure in presence of external stressor (no outcomes of interest) Unknown if randomized trial |
| Frederiksen 1974 | Patients were not diagnosed with essential hypertension (trial of methyldopa in patients with angina) |
| Glassock 1982 | No placebo only arm during randomization (compared methyldopa vs pindolol) |
| Glazer 1975 | No placebo only arm during randomization (compared methyldopa versus guanethidine in patients already receiving hydrochlorothiazide) |
| Gonasun 1982 | No placebo only arm during randomization (compared pindolol versus methyldopa) |
| Guidi 1981 | No placebo only arm during randomization (compared methyldopa versus metoprolol in patients already receiving chlorthalidone) |
| Hamilton 1963 | No placebo only arm during randomization (all patients received methyldopa) |
| Horwitz 1966 | Not a randomized study comparing methyldopa versus placebo |
| Innes 1992 | No placebo only arm during randomization (compared methyldopa versus labetalol) Patients were not diagnosed with essential hypertension (trial of methyldopa in patients with hypertension secondary to various renal disorders) |
| Irvine 1962 | Not a randomized study comparing methyldopa versus placebo |
| Johnson 1966 | No placebo only arm during randomization (all patients received methyldopa) |
| Klapper 1964 | Not a randomized study comparing methyldopa versus placebo |
| Kuokkanen 1979 | No placebo only arm during randomization (compared methyldopa versus prazosin) |
| Kuschke 1963 | Not a randomized study comparing methyldopa versus placebo |
| Levine 1968 | No placebo only arm during randomization (compared methyldopa versus guanethidine) |
| Masso 1979 | No placebo only arm during randomization (compared methyldopa versus bromazepam) |
| McAreavey 1983 | Methyldopa or placebo were added in patients already receiving atenolol and bendrofluazide if blood pressure was not controlled |
| McAreavey 1984 | Methyldopa or placebo were added in patients already receiving atenolol and bendrofluazide if blood pressure was not controlled |
| Murad 1991 | No placebo only arm during randomization (compared methyldopa versus lisinopril) |
| Oates 1960 | Not a randomized study comparing methyldopa versus placebo |
| Oates 1965 | No placebo only arm during randomization (compared methyldopa versus guanethidine versus pargyline) |
| Okanga 1978 | No placebo only during randomization (compared methyldopa versus atenolol) |
| Onesti 1962 | No placebo only arm during randomization (all patients received methyldopa) |
| Sermswan 2003 | Methyldopa or placebo were added in patients already receiving three other antihypertensives (e.g.. thiazide diuretic, calcium channel blocker, beta blocker, ACE inhibitor) |
| Smirk 1963 | Not a randomized study comparing methyldopa versus placebo |
| Smith 1966 | No placebo only arm during randomization (compared methyldopa versus methyldopa and thiazide versus thiazide and herbal product) |
| Wright 1982 | Some trial patients continued with diuretic therapy (bendrofluazide) during the study period. Cannot distinguish results details relating to patients receiving only methyldopa versus those who received methyldopa and diuretic therapy. Duration of effect of single dose of methyl dopa was studied in a double blind cross over study in 10 patients who were selected because methyldopa had proven to be effective and well tolerated in these patients. Methyldopa was given once daily up to 4 g as a single dose either in the morning or evening and compared to placebo. At the end of every 2 weeks BP was measured every hour from 08.30h to 22.30 h. |
Characteristics of studies awaiting assessment [ordered by study ID]
Arnold 1962.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article translation (text in German) |
Brahm 1973.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article translation (text in German) |
Cid‐Troncoso 1992.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Epstein 1978.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval (retrieved article was abstract only) |
Differences between protocol and review
The protocol did not state that randomized cross‐over trials would be included. This type of trial was included as the search did not retrieve many parallel group RCTs and it was thought that properly done randomized cross‐over RCTs would add to the knowledge of the effects of methyldopa on blood pressure. In addition a hierarchy of standard deviation data to be used for imputation was added keeping in line with previous reviews from the Cochrane Hypertension Review Group.
Michael Kandler was a co‐author of the protocol but was unable to participate in the conduct of the full review therefore his name does not appear in the list of authors. Vijaya Musini was added as an author as her expertise was needed in terms of analyzing data using generic inverse variance.
Contributions of authors
GM and AT were responsible for searching for trials, data extraction, data analyses, interpretation of data, and writing/editing of the final report.
VM provided assistance with data analyses, interpretation of data, and writing/editing of the final report.
Sources of support
Internal sources
Fraser Health Authority, Canada.
Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Canada.
External sources
No sources of support supplied
Declarations of interest
Greg T Mah has no perceived or actual conflicts of interest to declare.
Aaron M Tejani has no perceived or actual conflicts of interest to declare for the past 6 years.
Vijaya M Musini has no perceived or actual conflicts of interest to declare.
New
References
References to studies included in this review
Aronow 1977 {published data only}
- Aronow WS, Tobis J, Hughes D, Siegel J, Easthope J. Comparison of trimazosin and methyldopa in hypertension. Clinical Pharmacology and Therapeutics 1977;22(4):425‐9. [DOI] [PubMed] [Google Scholar]
Aronow 1978 {published data only}
- Aronow WS, Oberman A, Pool PE, Schnaper HW, Seagren SC, Tobis J, et al. Effect of trimazosin, methyldopa, and placebo on hypertension. Current Therapeutic Research 1978;23(4):448‐54. [Google Scholar]
Fernandez 1980 {published data only}
- Fernandez PG, Zachariah PK, Bryant DG, Missan SS. Antihypertensive efficacy of alpha‐methyldopa, chlorothiazide, and Supres‐150 (alpha‐methyldopa‐chlorothiazide). Canadian Medical Association Journal 1980;123:284‐7. [PMC free article] [PubMed] [Google Scholar]
Ferrara 1984 {published data only}
- Ferrara LA, Rubba P, Iannuzzi A, Fasano ML. Haemodynamic changes in peripheral arterial circulation during antihypertensive treatment with captopril, methyldopa and indapamide. International Journal of Clinical Pharmacology Research 1984;4(5):389‐93. [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson B, Hoch K, Errichetti A, Johnson J. Effects of methyldopa on psychometric performance. Journal of Clinical Pharmacology 1990;30:1102‐5. [DOI] [PubMed] [Google Scholar]
Lepantalo 1984 {published data only}
- Lepantalo M. Chronic effects of metoprolol and methyldopa on calf blood flow in intermittent claudication. British Journal of Clinical Pharmacology 1984;18:90‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mroczek 1974 {published data only}
- Mroczek WJ, Fotiu S, Davidov ME, Finnerty, FA. Prazosin in hypertension: a double‐blind evaluation with methyldopa and placebo. Current Therapeutic Research 1974;16(8):769‐77. [PubMed] [Google Scholar]
Paran 1993 {published data only}
- Paran E, Neumann L. The effects of isradipine and alpha‐methyldopa on exercise haemodynamics in hypertensive patients. Journal of Drug Delivery 1993;6(1):11‐4. [Google Scholar]
Petrie 1976 {published data only}
- Petrie JC, Galloway DB, Jeffers TA, Millar HR, Smith MC, Wood RA, et al. Methyldopa and propranolol or practolol in moderate hypertension. British Medical Journal 1976;2:137‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnaper 1975 {published data only}
- Schnaper HW, Oberman A. Double‐blind studies of the clinical effectiveness of prazosin. Postgraduate Medicine 1975:81‐7. [PubMed] [Google Scholar]
Tiwari 1982 {published data only}
- Tiwari HK, Sundar S, Kumar A, Rao NSN, Valsh SK. Propranolol and methyldopa in hypertension: a double blind placebo controlled study. Indian Medical Gazette 1982;116(12):361‐5. [Google Scholar]
Yodfat 1993 {published data only}
- Bar‐On D, Amir M. Reexamining the quality of life of hypertensive patients ‐ a new self‐structured measure. American Journal of Hypertension 1993;6:62S‐66S. [DOI] [PubMed] [Google Scholar]
- Yodfat Y, Bar‐On D, Amir M, Cristal N. Quality of life in normotensives compared to hypertensive men treated with isradipine or methyldopa as monotherapy or in combination with captopril: the LOMIR‐MCT‐IL study. Journal of Human Hypertension 1996;10:117‐122. [PubMed] [Google Scholar]
- Yodfat Y, Cristal N (LOMIR‐MCT‐IL Research Group). A multicenter, double‐blind, randomized, placebo‐controlled study of isradipine and methyldopa as monotherapy or in combination with captopril in the treatment of hypertension. American Journal of Hypertension 1993;6(3 Part 2):57S‐61S. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alfonzo Guerra 1980 {published data only}
- Alfonza Guerra JP, Lopez MA, Suarez JT. A double blind comparative study of two hypotensive drugs: clonidine and alpha‐methyl‐dopa [Estudio comparativo a doble ciegas de dos drogas hipotensoras: clonidina y alfa‐metil‐dopa]. Revista Cubana de Medicina 1980;19:109‐19. [Google Scholar]
Anonymous 1986 {published data only}
- First‐step treatment of mild to moderate hypertension ‐ comparison with conventional stepped‐care treatment [Basistherapeutikum bei leichten hypertonieformen ‐ randomiserte doppelblindstudien: vergleich standardtherapie versus enalapril]. Fortschritte der Medizin 1986;104:820‐1. [Google Scholar]
Aoki 1970 {published data only}
- Aoki VS, WIlson WR. Hydralazine and methyldopa in thiazide‐treated hypertensive patients. American Heart Journal 1970;79(6):798‐804. [DOI] [PubMed] [Google Scholar]
Avigdor 1983 {published data only}
- Avigdor L, Waeber B, Brunner HR. Evaluation by practicing physicians of the antihypertensive efficacy of debrisoquin, methyldopa and propranolol [Evaluation par des medicins installes de l'efficacite antihypertensive de la debrisoquine, de la methyldopa et du propranolol]. Schweizerische Medizinische Wochenschrift 1983;113(9):331‐8. [PubMed] [Google Scholar]
Bayliss 1962 {published data only}
- Bayliss RIS, Harvey‐Smith, EA. Methyldopa in the treatment of hypertension. Lancet 1962;1:763‐8. [DOI] [PubMed] [Google Scholar]
Beanlands 1978 {published data only}
- Beanlands DS, Allard PP, Wilson M, Orbeck KW, Helman AB, Lefebvre R. Comparison of efficacy and safety of pindolol and alpha‐methyldopa in treatment of mild to moderate hypertension: results of a double‐blind evaluative study. Clinical and Investigative Medicine 1978;1:139‐45. [PubMed] [Google Scholar]
Belleau 1977 {published data only}
- Belleau L, Lebel M, Lachance JG. Debrisoquine versus methyldopa in the treatment of thiazide‐resistant hypertension. Current Therapeutic Research 1977;22(1):134‐42. [Google Scholar]
Bradley 1977 {published data only}
- Bradley WF, Hoffman FG, Hutchison JC, Kalams Z, Waldron SL. Comparison of prazosin and methyldopa in mild to moderate hypertension, a multicenter cooperative study. Current Therapeutic Research 1977;21(1):28‐35. [PubMed] [Google Scholar]
Bune 1981 {published data only}
- Bune AJ, Chalmers JP, Graham JR, Howe PRC, West MJ, Wing LMH. Double‐blind trial comparing guanfacine and methyldopa in patients with essential hypertension. European Journal of Clinical Pharmacology 1981;19:309‐15. [DOI] [PubMed] [Google Scholar]
Cannon 1962 {published data only}
- Cannon PJ, Whitlock RT, Morris C, Angers M, Laragh JH. Effect of alpha‐methyl DOPA in severe and malignant hypertension. JAMA 1962;179(9):673‐81. [DOI] [PubMed] [Google Scholar]
Co‐operative 1973 {published data only}
- Control of moderate raise blood pressure ‐ report of a Co‐operative randomized controlled trial. British Medical Journal 1973;3:434‐6. [PMC free article] [PubMed] [Google Scholar]
Corea 1983 {published data only}
- Corea L, Bentivoglio M, Provvidenza M, Panebianco G. Two low doses of methyldopa in mild to moderate hypertension: a comparative double‐blind study and a long‐term follow up. Current Therapeutic Research 1983;34(1):217‐26. [Google Scholar]
Daley 1962 {published data only}
- Daley D, Evans B. Another hypotensive agent ‐ methyldopa. British Medical Journal 1962;2:156‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Divitiis 1981 {published data only}
- Divitiis O, Petitto M, Somma S, Fazio S. Atenolol and methyldopa in the treatment of mild‐moderate hypertension: a double‐blind comparison and combination with single doses. International Journal of Clinical Pharmacology Research 1981;1(4):245‐53. [Google Scholar]
Deschamps 1992 {published data only}
- Deschamps C, Guzman P, Mejia M. Comparison of hydralazine and alpha methyldopa in hypertension in pregnancy [Comparacion de hidralazina y alfa metildopa en la hipertension durante el embarazo]. Acta Medica Dominicana 1992;14(6):222‐4. [Google Scholar]
Dollery 1962 {published data only}
- Dollery CT, Harington M. Methyldopa in hypertension clinical and pharmacological stdies. Lancet 1962:759‐63. [DOI] [PubMed] [Google Scholar]
Dunn 1978 {published data only}
- Dunn FG, Melville DI, Jones JV, Lorimer AR, Lawrie TDV. Standardized stress and hypertension: comparison of effect of propranolol and methyldopa. British Journal of Clinical Pharmacology 1978;5(3):223‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frederiksen 1974 {published data only}
- Frederiksen RT, Cheitlin MD, Ferguson DR. The treatment of angina pectoris with alpha methyldopa. American Heart Journal 1974;88(1):47‐50. [DOI] [PubMed] [Google Scholar]
Glassock 1982 {published data only}
- Glassock RJ, Weitzman RE, Bennett CM, Maxwell M, Hamilton B, Winer N, et al. Pindolol: effects on blood pressure and plasma renin activity. American Heart Journal 1982;104(2):421‐5. [DOI] [PubMed] [Google Scholar]
Glazer 1975 {published data only}
- Glazer N. Comparison of guanethidine and methyldopa in essential hypertension: a controlled study. Current Therapeutic Research 1975;17(3):249‐56. [PubMed] [Google Scholar]
Gonasun 1982 {published data only}
- Gonasun LM. Antihypertensive effects of pindolol. American Heart Journal 1982;104(2):374‐87. [DOI] [PubMed] [Google Scholar]
Guidi 1981 {published data only}
- Guidi G, Giuntoli F, Galeone F, Checchi M, Locci P, Saba GC, et al. A double blind trial on hypotensive effect of an association of chlorthalidone and metoprolol or methyldopa [Studio comparativo in doppio cieco dell'effetto ipotensivo di un trattamento combinato con clortalidone e metoprololo o alfa‐metildopa]. Giornale di Clinica Medica 1981;62(8):558‐66. [PubMed] [Google Scholar]
Hamilton 1963 {published data only}
- Hamilton M, Kopelman H. Treatment of severe hypertension with methyldopa. British Medical Journal 1963;1:151‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horwitz 1966 {published data only}
- Horwitz D, Pettinger WA, Orvis H, Thomas RE, Sjoerdsma A. Effects of methyldopa in fifty hypertensive patients. Clinical Pharmacology and Therapeutics 1966;8(2):224‐34. [DOI] [PubMed] [Google Scholar]
Innes 1992 {published data only}
- Innes A, Gemmell HG, Smith FW, Edward N, Catto GRD. The short term effects of oral labetalol in patients with chronic renal disease and hypertension. Journal of Human Hypertension 1992;6:211‐4. [PubMed] [Google Scholar]
Irvine 1962 {published data only}
- Irvine ROH, O'Brien KP, North JDK. Alpha methyl dopa in treatment of hypertension. Lancet 1962;1:300‐3. [Google Scholar]
Johnson 1966 {published data only}
- Johnson P, Kitchin AH, Lowther CP, Turner RW. Treatment of hypertension with methyldopa. British Medical Journal 1966;1:133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klapper 1964 {published data only}
- Klapper MS, Richard L, Chazan JA. The use of methyldopa in hypertension. Southern Medical Journal 1964;57:1437‐9. [DOI] [PubMed] [Google Scholar]
Kuokkanen 1979 {published data only}
- Kuokkanen K, Mattila MJ. Antihypertensive effect of prazosin in combination with methyldopa, clonidine, or propranolol. Annals of Clinical Research 1979;11:18‐24. [PubMed] [Google Scholar]
Kuschke 1963 {published data only}
- Kuschke HJ, Wolfer HJ, Igata A, Becker G. Treatment of hypertension with alpha‐methyldopa ‐ clinical and hemodynamical studies [Die behandlung der hypertonie mit l‐a‐methyl‐dopa ‐ klinische und hamodynamische untersuchungen]. Munchener Medizinische Wochenschrift 1963;105(25):1305‐8. [Google Scholar]
Levine 1968 {published data only}
- Levine PR, Rosenbloom SE, Shapera RP, Shapiro AP. Technique of controlled drug assay ‐ Comparison of guanethidine, methyldopa, and a placebo in the hypertensive negro woman. Archives of Internal Medicine 1968;122:305‐10. [DOI] [PubMed] [Google Scholar]
Masso 1979 {published data only}
- Masso M, Perez P. Double‐blind clinical trial of bromazepam and alpha‐methyldopa in arterial hypertension. Pharmatherapeutica 1979;2(3):195‐204. [Google Scholar]
McAreavey 1983 {published data only}
- McAreavey D, Ramsay LE, Lorimer AR, McLaren D, Reid JL, Robertson JIS, et al. The 'third drug' trial: a comparative study of anti‐hypertensive agents added to treatment when blood pressure is uncontrolled by a beta‐blocker plus thiazide diuretic. Journal of Hypertension 1983;1(Suppl 2):116‐9. [PubMed] [Google Scholar]
McAreavey 1984 {published data only}
- McAreavey D, Ramsey LE, Latham L, McLaren AD, Lorimer AR, Reid JL, et al. "Third drug" trial: a comparative study of antihypertensive agents added to treatment when blood pressure remains uncontrolled by a beta blocker plus thiazide diuretic. British Medical Journal 1984;288:106‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murad 1991 {published data only}
- Murad EE, Chavez de los Rios M, Lopez AP, Carrasco FG, Sanchez AG. Lisinopril vs methyl‐dopa in essential hypertension: A comparative study [Estudio comparativo entre el lisinopril y la metildopa en el tratamiento de la hipertension arterial esencial]. Investigacion Medica Internacional 1991;18(4):163‐8. [Google Scholar]
Oates 1960 {published data only}
- Oates JA, Gillespie L, Udenfriend S, Sjoerdsma A. Decarboxylase inhibition and blood pressure reduction by alpha‐methyl‐3,4‐dihydroxy‐DL‐phenylalanine. Science 1960;131(3417):1890‐1. [DOI] [PubMed] [Google Scholar]
Oates 1965 {published data only}
- Oates JA, Seligmann AW, Clark MA, Rousseau P, Lee RE. The relative efficacy of guanethidine, methyldopa and pargyline as antihypertensive agents. New England Journal of Medicine 1965;273(14):729‐34. [DOI] [PubMed] [Google Scholar]
Okanga 1978 {published data only}
- Okanga JBO. Atenolol (Tenormin) compared with methyldopa (Aldomet) in the treatment of hypertension. East African Medical Journal 1978;55(9):447‐52. [PubMed] [Google Scholar]
Onesti 1962 {published data only}
- Onesti G, Brest AN, Novack P, Moyer JH. Pharmacodynamic effects and clinical use of alpha methyldopa in the treatment of essential hypertension. American Journal of Cardiology 1962;9:863‐7. [DOI] [PubMed] [Google Scholar]
Sermswan 2003 {published data only}
- Sermswan A, Archawarak N. Methyldopa supplement for resistant essential hypertension: a prospective randomized placebo control crossover study. Journal of the Medical Association of Thailand 2003;86(12):1156‐61. [PubMed] [Google Scholar]
Smirk 1963 {published data only}
- Smirk H. Hypotensive action of methyldopa. British Medical Journal 1963;1:146‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1966 {published data only}
- Smith WM, Bachman B, Galante JG, Hanowell EG, Johnson WP, Koch CE, et al. Co‐operative clinical trial of alpha‐methyldopa ‐ double‐blind control comparison of alpa‐methyldopa and chlorothiazide, and chlorothiazide and rauwolfia. Annals of Internal Medicine 1966;65(4):657‐71. [DOI] [PubMed] [Google Scholar]
Wright 1982 {published data only}
- Wright JM, Orozco‐Gonzalez M, Polak G, Dollery CT. Duration of effect of single daily dose methyldopa therapy. British Journal of Clinical Pharmacology 1982;13:847‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Arnold 1962 {published data only}
Brahm 1973 {published data only}
Cid‐Troncoso 1992 {published data only}
Epstein 1978 {published data only}
Additional references
Brunton 2006
- Brunton LL, Lazo JS, Parker KL. Goodman & Gilmans: The pharmacological basis of therapeutics. 11th Edition. New York: McGraw‐Hill, 2006. [Google Scholar]
CHEP 2008
- Canadian Hypertension Education Program (CHEP). The 2008 Canadian Hypertension Education Program recommendations for the management of hypertension. Canadian Journal of Cardiology 2008;24(6):455‐475. [Google Scholar]
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
Croog 1986
- Croog SH, Levine S, Testa MA. The effects of antihypertensive therapy on quality of life. New England Journal of Medicine 1986;314:1657‐1664. [DOI] [PubMed] [Google Scholar]
Goodman & Gilman 1996
- Goodman LS, Gilman AG. The Pharmacological Basis of Therapeutics. Ninth. McGraw‐Hill, 1996. [Google Scholar]
JNC 1977
- Joint National Committee. Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 1977;237(3):255‐261. [PubMed] [Google Scholar]
Sjoerdsma 1982
- Sjoerdsma A. Methyldopa. British Journal of Clinical Pharmacology 1982;13:45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprackling 1981
- Sprackling ME, Mitchell JRA, Short AH, Watt G. Blood pressure reduction in the elderly: a randomized controlled trial of methyldopa. British Medical Journal 1981;283:1151‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1955
- Stein GA, Bronner E. Alpha‐methyl‐alpha‐amino acids: Derivatives of DL ‐ phenylalanine. Journal of the American Chemical Society 1955;77:700‐703. [Google Scholar]
Webster 1996
- Webster J, Koch HF. Aspects of tolerability of central acting antihypertensive drugs. Journal of Cardiovascular Pharmacology 1996;27(13):S49‐S54. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of Hypertension 2003;21:1983‐1992. [DOI] [PubMed] [Google Scholar]


