Abstract
Background
Invasive fungal infection is an important cause of mortality and morbidity in very preterm or very low birth weight infants. Uncertainty exists about the effect of prophylactic oral/topical non‐absorbed antifungals to reduce mucocutaneous colonisation and so limit the risk of invasive fungal infection in this population.
Objectives
To assess the effect of prophylactic oral/topical non‐absorbed antifungal therapy on the incidence of invasive fungal infection, mortality and morbidity in very preterm or very low birth weight infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group. This included searches of the Cochrane Central Register of Controlled Trials (CENTRAL: The Cochrane Library, 2015, Issue 7), MEDLINE, EMBASE, and CINAHL (to May 2015), conference proceedings, and previous reviews.
Selection criteria
Randomised controlled trials or quasi‐randomised controlled trials that compared the effect of prophylactic oral/topical non‐absorbed antifungal therapy versus placebo or no drug or another antifungal agent or dose regimen in very preterm or very low birth weight infants.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Review Group with separate evaluation of trial quality and data extraction by two review authors.
Main results
Four trials, in which a total of 1800 infants participated, compared oral/topical non‐absorbed antifungal prophylaxis (nystatin or miconazole) with placebo or no drug. These trials had various methodological weaknesses including quasi‐randomisation, lack of allocation concealment, and lack of blinding of intervention and outcomes assessment. The incidence of invasive fungal infection was very high in the control groups of three of these trials. Meta‐analysis found a statistically significant reduction in the incidence of invasive fungal infection (typical risk ratio 0.20, 95% confidence interval 0.14 to 0.27; risk difference −0.18, −0.21 to −0.15) but substantial statistical heterogeneity was present. We did not find a statistically significant effect on mortality (typical risk ratio 0.87, 0.72 to 1.05; risk difference −0.03, −0.06 to 0.01). None of the trials assessed posthospital discharge outcomes. Three trials (N = 326) assessed the effect of oral/topical non‐absorbed versus systemic antifungal prophylaxis. Meta‐analyses did not find any statistically significant differences in the incidences of invasive fungal infection or all‐cause mortality.
Authors' conclusions
The finding of a reduction in risk of invasive fungal infection in very low birth weight infants treated with oral/topical non‐absorbed antifungal prophylaxis should be interpreted cautiously because of methodological weaknesses in the included trials. Further large randomised controlled trials in current neonatal practice settings are needed to resolve this uncertainty. These trials might compare oral/topical non‐absorbed antifungal agents with placebo, with each other, or with systemic antifungal agents and should include an assessment of effect on long‐term neurodevelopmental outcomes.
Plain language summary
Prophylactic oral/topical non‐absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants
Review question: In very preterm or very low birth weight (VLBW) infants, does prophylactic oral/topical non‐absorbed antifungal therapy reduce the risk of invasive fungal infection, mortality and adverse neurodevelopmental outcomes?
Background: Fungi such as candida (the organism that causes thrush) can cause bloddstream and other severe infections in VLBW infants (birth weight less than 1500 grams). These infections are often difficult to diagnose and frequently cause death or disability. Therefore, it may be appropriate to attempt to prevent such infections by giving VLBW infants antifungal drugs as a routine part of their care. This review assessed specifically the effect of giving infants antifungal drugs that reduce skin and gut carriage of fungi to reduce the chances of a severe infection developing.
Study characteristics: Four trials, in which a total of 1800 infants participated, examined whether giving VLBW infants a drug to prevent fungi growing on the skin or in the gut reduced the risk of bloodstream or other severe infection. The trials used one of two commonly available drugs (nystatin or miconazole) and compared these with either a placebo ("dummy" drug) or no drug. These trials, however, had some design weaknesses that make it less certain that their results can be taken at face value.
Key results: The overall analysis suggested that this treatment might reduce severe infection rates in VLBW infants but there was no evidence of a reduction in the risk of dying.
Conclusions: Larger and higher quality trials are needed to resolve this uncertainty.
Background
Description of the condition
Invasive fungal infection accounts for 10% of all cases of late‐onset invasive infection in very preterm or very low birth weight (VLBW) infants (Stoll 2002; Shane 2013). Invasive fungal infection is an important cause of morbidity and mortality in very preterm (less than 32 weeks) and VLBW (less than 1500 grams) infants (Kossoff 1998;Benjamin 2006;Robinson 2009;Wynn 2012). The reported mortality rates of greater than 25% are higher than those attributed to invasive bacterial infection in VLBW infants (Saiman 2000; Makhoul 2002; Stoll 2002; Benjamin 2003, Ascher 2012). Invasive fungal infection is also associated with short‐ and long‐term morbidity, including adverse neurodevelopmental outcomes (Lee 1998; Friedman 2000; Saiman 2000; Benjamin 2006; Wynn 2012 ; Adams‐Chapman 2013; Barton 2014).
The incidence of invasive fungal infection in VLBW infants is between about 1% and 4%, but the risk of infection is inversely related to gestational age and birth weight. In extremely preterm (less than 28 weeks) or extremely low birth weight (ELBW) infants (less than 1000 grams), reported incidences are between about 2% and 8%. Much higher incidences, up to 20%, have been reported for infants of birth weight less than 750 grams or gestational age at birth less than 26 weeks (Saiman 2000; Makhoul 2002; Horbar 2002; Karlowicz 2002; Clerihew 2006; Vergnano 2011;Oeser 2014). It has been reported that the incidence of invasive fungal infection in preterm and low birth weight infants is decreasing, especially among ELBW infants. This coincides with an increase in the use of antifungal prophylaxis, a reduction in the use of broad spectrum antibiotics, and the introduction of central venous catheter care bundles (Oeser 2013;Aliaga 2014; Oeser 2014).
Observational studies suggest that mucocutaneous or tracheal fungal colonisation is a risk factor for invasive infection (Faix 1989; Pappu‐Katikaneni 1990; Rowen 1994; Huang 1998). However, multivariate analyses that account for potential confounding variables have not confirmed this association (Saiman 2000). Other putative risk factors for invasive fungal infection in VLBW infants include severity of illness at birth, the use of multiple courses of antibiotics (particularly third‐generation cephalosporins), the use of parenteral nutrition, the presence of a central venous catheter, and exposure to histamine receptor subtype 2 antagonists (Rowen 1994; Benjamin 2006; Cotten 2006; Manzoni 2006; Barton 2014 ; Oeser 2014).
The clinical presentation of invasive fungal infection in VLBW infants is similar to that of bacterial infection and this may cause delays in diagnosis and treatment. The diagnosis may be further delayed due to an inability to recover the organism from microbiological culture of blood, cerebrospinal fluid, or urine. A high index of suspicion and the use of additional laboratory and clinical tests may be needed to confirm the suspected diagnosis (Benjamin 2003; Oeser 2014).
Description of the intervention
Given the difficulty in establishing an early diagnosis and the high level of associated morbidity and mortality, there is a need to assess the effect of strategies to prevent invasive fungal infection in VLBW infants (Brecht 2009). In addition to generic infection control practices and avoidance of modifiable risk factors, two broad chemoprophylactic strategies are employed in current clinical practice (Burwell 2006; Clerihew 2008; Ganesan 2008; O'Grady 2008; Howell 2009; Kaguelidou 2012):
Prophylaxis using systemically‐absorbed antifungal drugs that achieve fungicidal concentrations in tissue, blood, cerebrospinal fluid, and urine. Evidence exists that systemic antifungal prophylaxis using fluconazole reduces the incidence of invasive fungal infection, but there is concern about toxicity (Frattarelli 2004); as well as the effect that its widespread use may have on the emergence of antifungal resistance (Brion 2007; Austin 2007).
Prophylaxis using oral/topical non‐absorbed agents such as nystatin or miconazole. Observational studies have suggested that oral/topical non‐absorbed antifungal prophylaxis reduces mucocutaneous fungal colonisation and the risk of invasive infection in VLBW infants (Ganesan 2008; Howell 2009). However, the specific effect of antifungal prophylaxis independently of other confounding interventions and variables is unable to be determined from these studies. Another concern is that hyper‐osmolar nystatin preparations may increase the risk of adverse gastrointestinal events in VLBW infants (Ernst 1983;Radmacher 2012).
Why it is important to do this review
This review focuses on randomised comparisons of oral/topical non‐absorbed antifungal prophylaxis compared with no antifungal prophylaxis or compared with systemic antifungal prophylaxis. The effect of systemic antifungal prophylaxis compared with no prophylaxis is addressed in another Cochrane review (Austin 2007).
Objectives
To assess the effect of prophylactic oral/topical non‐absorbed antifungal therapy on the incidence of invasive fungal infection, mortality and adverse neurodevelopmental outcomes in very preterm or VLBW infants.
We examined the following interventions:
oral/topical antifungal prophylaxis versus placebo or no drug;
oral/topical antifungal prophylaxis versus systemic antifungal prophylaxis;
one oral/topical antifungal regimen versus another oral/topical antifungal regimen.
We pre‐specified these subgroup analyses:
extremely preterm (less than 28 weeks) or ELBW infants (less than 1000 grams);
trials in which participants were infants with fungal colonisation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials, including cluster randomised trials.
Types of participants
VLBW infants (less than 1500 grams) or very preterm infants (less than 32 weeks at birth).
Types of interventions
Antifungal prophylaxis with oral/topical non‐absorbed drugs versus placebo or nothing or another antifungal drug regimen.
Types of outcome measures
Primary outcomes
-
Confirmed invasive fungal infection as determined by:
culture of fungus from a normally sterile site: cerebrospinal fluid, blood, urine, bone or joint, peritoneum, pleural space. Samples should have been collected using methods to minimise contamination with surface‐colonising organisms;
findings on autopsy examination consistent with invasive fungal infection;
findings on ophthalmological examination consistent with fungal ophthalmitis or retinitis;
pathognomonic findings on renal ultrasound examination such as 'renal fungal balls'.
Death prior to hospital discharge.
Neurodevelopmental outcomes assessed beyond infancy (neurological evaluations, developmental scores, and classifications of disability, including auditory and visual disability, non‐ambulant cerebral palsy, developmental delay); and cognitive and educational outcomes at five years or older (intelligence quotient and/or indices of educational achievement measured using a validated tool including school examination results).
Secondary outcomes
Bronchopulmonary dysplasia (oxygen supplementation at 36 weeks postmenstrual age);
Necrotising enterocolitis (Bell stage 2 or 3);
Retinopathy of prematurity: a) any stage; b) requiring treatment;
Duration of intensive care unit or hospital admission (days);
Emergence of organisms resistant to antifungal agents, as detected in individual infants enrolled in the study or, in the case of cluster randomised studies, on surveillance of other infants in the same unit in the study centre (including infants who were admitted to the unit following completion of the study);
Adverse drug reactions attributed to the antifungal agent, such as rash (including Stevens–Johnson reactions), gastrointestinal disturbance, abnormal hepatic or renal function, cardiac arrhythmias, thrombophlebitis, seizures, and anaphylaxis or toxicity sufficient to cease drug administration.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2015, Issue 7), MEDLINE (1966 to May 2015), EMBASE (1980 to May 2015), and CINAHL (1982 to May 2015), using a combination of the following text words and MeSH terms: [Infant, Newborn OR Infant, Premature OR Infant, Low Birth Weight OR LBW OR infan* OR neonat*] AND [Mycoses/ OR fung* OR candid* OR Candida albicans OR Antifungal Agents/ OR Triazoles/ OR fluconazole OR azole OR amphotericin B OR nystatin OR nystan OR mycostatin OR nilstat OR nystex OR miconazole OR daktarin OR ketoconazole OR clotrimazole]. The search outputs were limited with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply any language restriction [See Appendix 1; Appendix 2 for search strategy].
We searched ClinicalTrials.gov and Current Controlled Trials for completed or ongoing trials.
Searching other resources
We examined the references in studies identified as potentially relevant. We also searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2015), the European Society for Paediatric Research (1995 to 2014), the UK Royal College of Paediatrics and Child Health (2000 to 2015), and the Perinatal Society of Australia and New Zealand (2000 to 2015). We considered trials reported only as abstracts to be eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
Two review authors screened the title and abstract of all studies identified by the above search strategy. We reassessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until we achieved consensus.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements until we achieved consensus. We asked the investigators for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Group to assess the methodological quality of any included trials. We requested additional information from the trial authors to clarify methodology and results as necessary. We evaluated and reported the following issues in the 'Risk of bias' tables:
Sequence generation (the method used to generate the allocation sequence):
low risk: any truly random process, e.g. random number table; computer random number generator;
high risk: any non‐random process, e.g. odd or even date of birth; hospital or clinic record number;
unclear risk: no or unclear information provided.
Allocation concealment (the method used to conceal the allocation sequence):
low risk: e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes;
high risk: open random allocation, e.g. unsealed or non‐opaque envelopes, alternation; date of birth;
unclear: no or unclear information provided.
Blinding (the methods used to ensure blinding of participants, clinicians and caregivers, and outcome assessors):
low risk;
high risk;
unclear.
Incomplete outcome data (completeness of data including attrition and exclusions from the analysis for each outcome and any reasons for attrition or exclusion where reported): We will assess whether missing data are balanced across groups or are related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will reinstate missing data in the analyses. We will categorise completeness as:
low risk: adequate (less than 10% missing data);
high risk: inadequate (more than 10% missing data);
unclear risk: no or unclear information provided.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and weighted mean difference (WMD) for continuous data, with respective 95% confidence intervals (CI). We determined the number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis is the participating infant in individually randomised trials, and the neonatal unit for cluster randomised trials.
Assessment of heterogeneity
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each RR analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected substantial heterogeneity (I² more than 50%), we explored the possible causes (for example, differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
If more than five trials were included in a meta‐analysis, we examined a funnel plot for asymmetry.
Data synthesis
We used the fixed‐effect model in Review Manager 5.3 for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We prespecified the following subgroup analyses:
extremely preterm (less than 28 weeks) or ELBW infants (less than 1000 grams);
infants with fungal colonisation at trial entry.
Results
Description of studies
We included seven eligible trials: Sims 1988; Wainer 1992: Ozturk 2006; Violaris 2010; Aydemir 2011a; Aydemir 2011b; Mersal 2013 (see Characteristics of included studies).
Included studies
Oral/topical non‐absorbed antifungal prophylaxis versus placebo or no drug (comparison 1):
Four trials compared oral/topical non‐absorbed antifungal prophylaxis with placebo or no drug:
Sims 1988 quasi‐randomly allocated 67 infants of birth weight less than 1250 grams to receive either oral nystatin or no treatment until one week after endotracheal extubation (average five weeks).
Wainer 1992 recruited 600 infants of birth weight less than 1750 grams. We made a consensus decision to include the trial because most participating infants were less than 1500 grams. Participants were randomised to receive either oral miconazole or placebo until discharge. The study was undertaken in the late 1980s in South Africa. Due to limited resources mechanical ventilation was not offered to ELBW infants (12% of the participants).
Ozturk 2006 randomly allocated 938 VLBW infants to receive either prophylactic oral nystatin (100,000 IU three times daily) or no treatment. Infants in the control group who had oral fungal colonisation detected at trial entry or on surveillance cultures were treated with nystatin (100,000 IU three times daily).
Aydemir 2011a randomly allocated 185 VLBW infants to receive either oral nystatin 100,000 IU three times daily or "equal volumes of intravenous or oral normal saline" placebo every third day until the 30th day after birth (or 45th day in ELBW infants).
The primary outcomes of all studies were fungal colonisation and invasive fungal infection. All provided data on in‐hospital mortality but none assessed any postdischarge outcomes.
Oral/topical non‐absorbed versus systemic antifungal prophylaxis (comparison 2):
Two trials compared oral/topical antifungal prophylaxis with systemic antifungal prophylaxis:
Violaris 2010 randomised 80 VLBW infants to receive either oral nystatin or fluconazole beginning between days five to seven after birth. Outcome data on invasive fungal infection and mortality were reported.
Aydemir 2011b randomly allocated 187 VLBW infants to receive either oral nystatin 100,000 IU eight hourly or intravenous fluconazole 3 mg/kg every third day until 30 days after birth (or 45 days after birth in ELBW infants).
Mersal 2013 randomly allocated 59 preterm infants of birth weight less than 1200 grams to receive either oral nystatin 100,000 IU eight hourly for six weeks (N = 24) or intravenous fluconazole 6 mg/kg every 72 hours at end of first week of life, then every 48 hours from second week to sixth week of life (N = 35).
One oral/topical non‐absorbed antifungal regimen versus another oral/topical non‐absorbed antifungal regimen (comparison 3):
We did not find any trials that compared different dose regimens of oral/topical non‐absorbed antifungal prophylaxis.
Excluded studies
We excluded five studies (Harris 1960; Damjanovic 1993; Herruzo‐Cabrera 1994; Demirel 2013; Oncel 2015: see Characteristics of excluded studies).
Risk of bias in included studies
Quality assessments are described in the table Characteristics of included studies and displayed in Figure 1. One trial was quasi‐randomised and lacked allocation concealment (Sims 1988). The most common methodological weakness was lack of blinding of caregivers and investigators and assessors to the nature of the intervention. Only one trial is likely to have been truly placebo‐controlled (Wainer 1992). All of the trials reported complete or near‐complete assessment for primary outcomes.
1.
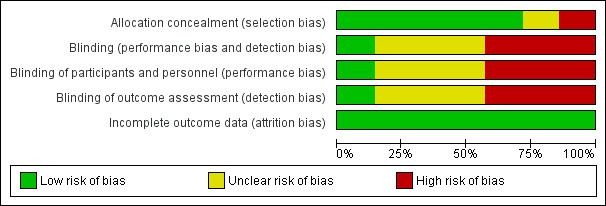
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Oral/topical non‐absorbed antifungal prophylaxis versus placebo or no drug (comparison 1):
Primary outcomes:
Invasive fungal infection (Outcome 1.1): Meta‐analysis of data from four trials found a statistically significant reduction in the intervention group but with significant and substantial statistical heterogeneity (Figure 2): typical RR 0.20 (95% CI 0.14 to 0.27), I² = 80%; typical RD −0.18 (95% CI −0.21 to −0.15); NNTB five infants.
2.
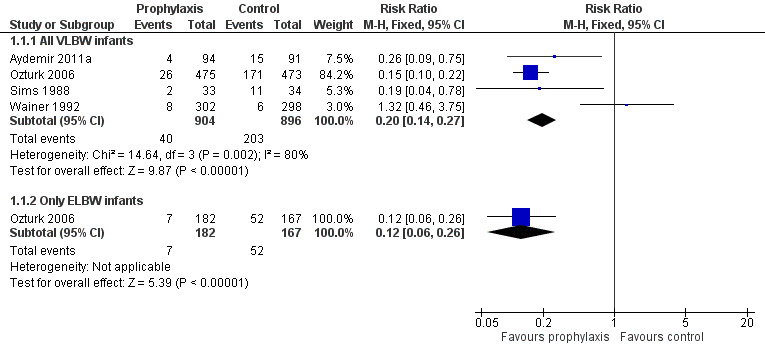
Forest plot of comparison: 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, outcome: 1.1 Incidence of invasive fungal infection.
Forest plot inspection suggested that the direction of the effect size estimate from Wainer 1992 was inconsistent with those of the other three trials. Meta‐analysis omitting data from Wainer 1992 removed heterogeneity, but did not change the pooled effect size estimate [revised typical RR 0.16 (95% CI 0.11 to 0.23), I² = 0%].
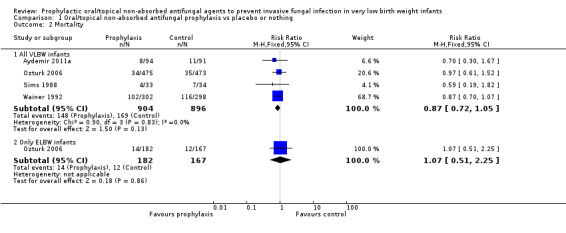
Death prior to hospital discharge (Outcome 1.2): None of the individual trials or a meta‐analysis of data from all four trials found a statistically significant effect. We found no evidence of statistical heterogeneity (Figure 3): typical RR 0.87 (95% CI 0.72 to 1.05), I² = 0%; typical RD −0.03 (95% CI −0.06 to 0.01).
3.
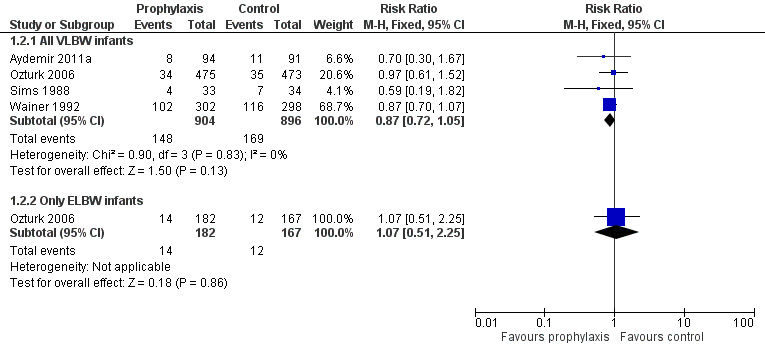
Forest plot of comparison: 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, outcome: 1.2 Mortality.
Neurodevelopmental outcomes: Not reported by any trials.
Secondary outcomes:
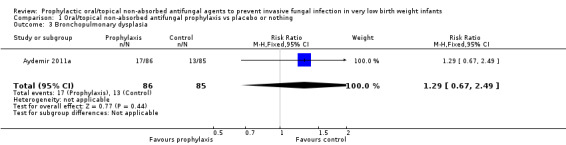
Bronchopulmonary dysplasia (Outcome 1.3):Aydemir 2011a did not find a statistically significant difference: RR 1.29 (95% CI 0.67 to 2.49). Outcome not reported in the other trials.
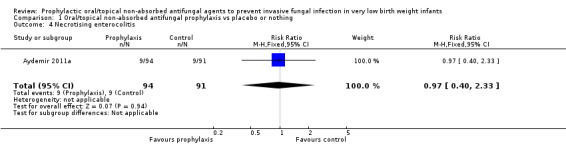
Necrotising enterocolitis (Outcome 1.4):Aydemir 2011a did not find a statistically significant difference: RR 0.97 (95% CI 0.40 to 2.33). Outcome not reported in the other trials.
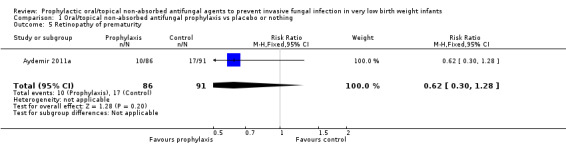
Retinopathy of prematurity (Outcome 1.5):Aydemir 2011a did not find a statistically significant difference in the incidence of retinopathy requiring surgery: RR 0.62 (95% CI 0.30 to 1.28). Outcome not reported in the other trials.
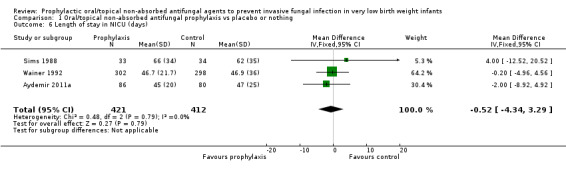
Duration of intensive care unit stay (Outcome 1.6): Three trials reported length of stay in intensive care (Sims 1988; Wainer 1992; Aydemir 2011a). None of the trials individually, or a meta‐analysis of data from all trials, found a statistically significant difference: WMD −0.52 (95% CI −4.34 to 3.29) days (I² = 0%) (Figure 4).
4.
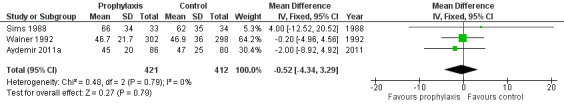
Forest plot of comparison: 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, outcome: 1.6 Length of stay in NICU (days).
Adverse events attributed to drug reactions or toxicity sufficient to cease drug administration: Not reported by any trials.
Subgroup analyses
-
Extremely preterm or ELBW infants: Data were available from one trial (Ozturk 2006).
invasive fungal infection: Ozturk 2006 found a statistically significant reduction: RR 0.12 (95% CI 0.06 to 0.26); RD −0.27 (95% CI −0.35 to −0.20).
mortality: Ozturk 2006 did not find a statistically significant effect: RR 1.07 (95% CI 0.51 to 2.25); RD 0.01 (95% CI −0.05 to 0.06).
Trials in which participants were infants with fungal colonisation: None of the trials restricted participation to infants with fungal colonisation at trial entry.
Oral/topical non‐absorbed versus systemic antifungal prophylaxis (comparison 2):
Three trials compared oral or topical antifungal prophylaxis (nystatin) with systemic antifungal prophylaxis (fluconazole) (Violaris 2010; Aydemir 2011b; Mersal 2013).
Primary outcomes:
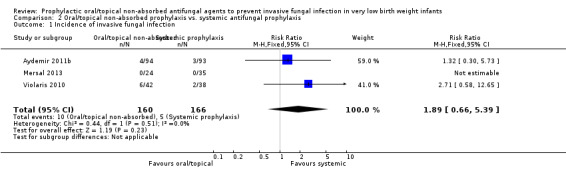
Invasive fungal infection (Outcome 2.1): Meta‐analysis did not detect a statistically significant difference (Figure 5): typical RR 1.89 (95% CI 0.66 to 5.39), I² = 0%; typical RD 0.03 (95% CI −0.02 to 0.07).
5.
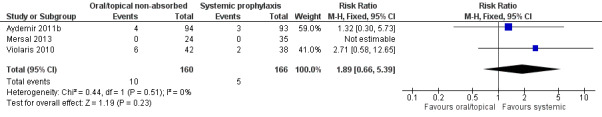
Forest plot of comparison: 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, outcome: 2.1 Incidence of invasive fungal infection.
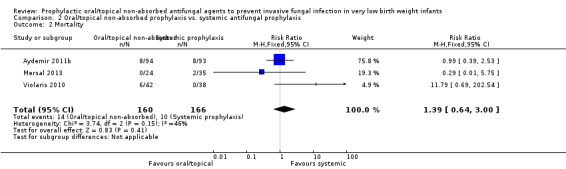
Death prior to hospital discharge (Outcome 2.2): Meta‐analysis did not detect a statistically significant difference (Figure 6): typical RR 1.39 (95% CI 0.64 to 3.00), I² = 46%; typical RD 0.02 (95% CI −0.03 to 0.08).
6.
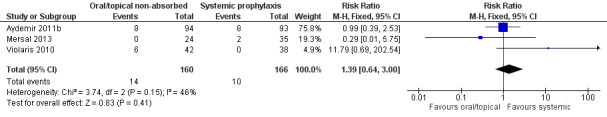
Forest plot of comparison: 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, outcome: 2.2 Mortality.
Neurodevelopmental outcomes: None of the trials reported any neurodevelopmental outcomes.
Secondary outcomes:
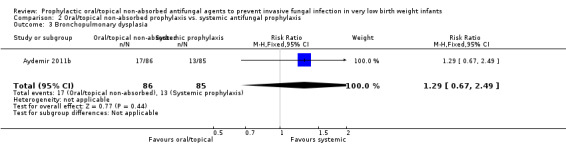
Bronchopulmonary dysplasia in surviving infants:Aydemir 2011b did not find a statistically significant difference: RR 1.29 [95% CI 0.67 to 2.49]; RD 0.04 [95% CI −0.07 to 0.16]. Not reported by Violaris 2010 or Mersal 2013.
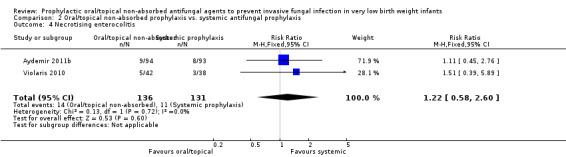
Necrotising enterocolitis: Meta‐analysis of data from Violaris 2010 and Aydemir 2011b did not detect a statistically significant difference (Figure 7): typical RR 1.22 (95% CI 0.58 to 2.60), I² = 0%; RD 0.02 (95% CI −0.05 to 0.09). Not reported by Mersal 2013.
7.
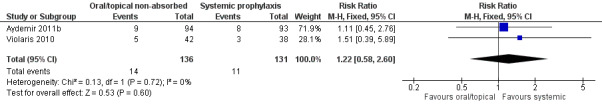
Forest plot of comparison: 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, outcome: 2.4 Necrotising enterocolitis.
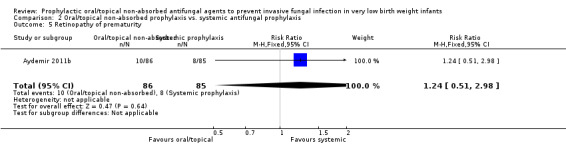
Retinopathy of prematurity:Aydemir 2011b did not find a statistically significant difference in the incidence of retinopathy requiring surgery in surviving infants: RR 1.24 [95% CI 0.51 to 2.98]; RD 0.02 [95% CI −0.07 to 0.11]. Not reported by Violaris 2010 or Mersal 2013.
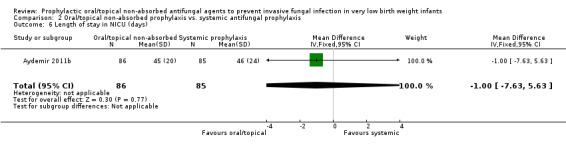
Duration of intensive care unit stay:Aydemir 2011b did not find a statistically significant difference: MD −1.00 (95% CI −7.63 to 5.63) days. Not reported by Violaris 2010 or Mersal 2013.
Adverse events attributed to drug reactions or toxicity sufficient to cease drug administration: Not reported by any of the trials.
Subgroup analyses
Extremely preterm or ELBW infants: None of the trials provided subgroup data.
Trials in which participants were infants with fungal colonisation: None of the trials restricted participation to infants with fungal colonisation at trial entry.
Discussion
Summary of main results
Meta‐analysis of data from four trials suggests that oral/topical non‐absorbed prophylaxis reduces the risk of invasive fungal infection in VLBW infants significantly and substantially. None of the trials or a meta‐analysis of their data found a statistically significant effect on mortality. Meta‐analysis of data from three trials did not detect an effect on the duration of intensive care. The trials reported only limited data on other neonatal morbidities that may be associated with invasive fungal infection. None of the trials assessed long‐term neurodevelopmental outcomes.
Three trials assessed the effect of oral/topical non‐absorbed antifungal prophylaxis (nystatin) versus systemic antifungal prophylaxis (fluconazole). Meta‐analyses did not find any statistically significant effects on the incidence of invasive fungal infection or all‐cause mortality but much larger studies would be needed to exclude more modest but important effect sizes.
Overall completeness and applicability of evidence
The finding that oral/topical non‐absorbed antifungal prophylaxis reduces the risk of invasive fungal infection in VLBW infants should be interpreted and applied with caution. The existence of substantial statistical heterogeneity in the meta‐analysis raises concern that the estimate of effect is not robust. The applicability of the finding is also limited by the very high incidence of invasive fungal infection in the control populations in the three trials that found a statistically significant effect on the incidence of invasive fungal infection (Sims 1988; Ozturk 2006; Aydemir 2011a). About one‐sixth to one‐third of infants in the control groups developed invasive fungal infection, much higher than the less than 5% incidence estimated in large cohort studies (Saiman 2000; Horbar 2002; Karlowicz 2002; Makhoul 2002; Clerihew 2006; Howell 2009). This limits the applicability of the NNTB estimate (five infants), since in clinical settings with lower incidences of invasive fungal infection a much larger number of infants would need treatment to prevent a single extra case of invasive fungal infection.
Quality of the evidence
The largest trial (N = 948) contributed 84% of the weighted estimate of risk ratio effect on invasive fungal infection (Ozturk 2006). This trial of nystatin prophylaxis was undertaken in Turkey within the past decade. More than one‐third of participants were ELBW infants receiving intensive care interventions. The criteria for diagnosing invasive fungal infection appear to be have been robust. Efforts to limit contamination of microbiological cultures by surface colonising organisms were made; for example, fungal urinary tract infection was based on culture of organisms from two separate supra‐pubic bladder aspirates. However, caregivers or assessors were not blinded to the intervention and this may have caused surveillance and ascertainment bias if thresholds for investigation and diagnosis of suspected invasive fungal infection were adjusted according to treatment status. Although 25% of control VLBW infants received nystatin to treat oral fungal colonisation detected at trial entry or during the trial period, this is likely to have reduced the effect size of the primary intervention.
The second largest trial (N = 600) did not detect a statistically significant effect of miconazole prophylaxis on the incidence of invasive fungal infection (Wainer 1992). This trial was placebo‐controlled and therefore less prone to surveillance bias. The trial was undertaken in South Africa 25 years ago in a settling with few intensive care resources. Twelve per cent of participants were ELBW and the overall incidence of invasive fungal infection was 2% in the control group. This lower incidence may be related to the fact that because of resource limitations ELBW infants did not receive intensive care interventions. Two‐thirds of ELBW infants died. The applicability of the trial's findings to modern neonatal intensive care settings in high‐income countries is therefore likely to be limited.
A subgroup analysis of outcomes for infants colonised with fungi at trial entry was not possible. None of the trials prespecified fungal colonisation as an entry criterion. Between 25% and 45% of participating infants had fungal colonisation, but subgroup data for these infants were not available in the published reports of the included trials. Even if these data become available for analysis, those from the largest trial would be of limited value since infants in the control group received antifungal treatment if oral fungal colonisation was detected (Ozturk 2006).
Potential biases in the review process
The existence of substantial statistical heterogeneity in the meta‐analysis of the effect of oral/topical non‐absorbed antifungal prophylaxis versus placebo or no drug on the incidence of invasive fungal infection raises concern that the estimate is not robust (Figure 2). The heterogeneity may be due to differences between the trials including population characteristics (proportion of ELBW infants), nature of the intervention (miconazole in one trial, nystatin in the others), methodological quality issues (particularly unblinded allocation and intervention) and the effect of other co‐interventions (availability of intensive care for ELBW infants). Forest plot inspection suggested that the direction of the effect size estimate from Wainer 1992 was inconsistent with those of the other three trials. In a post hoc sensitivity analysis, removal of this trial from the meta‐analysis removed statistical heterogeneity from the RR estimate and but did not change the direction or size of the estimate.
Authors' conclusions
Implications for practice.
The available trial data remain insufficient to guide clinical practice. Although meta‐analysis suggests that oral/topical non‐absorbed antifungal agents (nystatin or miconazole) reduce the risk of invasive fungal infection, methodological weaknesses limit the validity and applicability of this finding.
Implications for research.
Further randomised controlled trials of oral/topical non‐absorbed antifungal prophylaxis are needed to provide more valid and precise estimates of effect size. Because most neonatologists who currently use antifungal prophylaxis target infants thought to be at greatest risk, mainly ELBW or extremely preterm infants with additional risk factors, a trial restricted to this population of infants or even smaller or lower gestation infants may be appropriate and acceptable (Burwell 2006; Clerihew 2008; Howell 2009). Oral/topical non‐absorbed antifungal prophylaxis may be compared with placebo or with systemic prophylaxis (Austin 2007; Isaacs 2008). Any trial should aim to assess long‐term outcomes, particularly disability‐free survival, as well as the effect on invasive fungal infection.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2015 | New citation required but conclusions have not changed | This updates the review "Prophylactic oral/topical non‐absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants" published in the Cochrane Library (Austin 2013). |
| 3 September 2015 | New search has been performed | Updated search identified one new trial for inclusion (Mersal 2013). |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 27 January 2013 | New search has been performed | Updated search identified new trials for inclusion (Violaris 2010; Aydemir 2011a; Aydemir 2011b). |
| 4 June 2009 | New citation required and conclusions have changed | Substantive update. |
| 14 October 2007 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
David Henderson‐Smart for his guidance.
Rocio Rodriguez‐Lopez for updating the electronic search strategy.
Appendices
Appendix 1. Update detailed electronic search strategy
Information Specialist: Rocio Rodriguez Lopez [rocio.lopez@york.ac.uk]
Databases:
· MEDLINE (Ovid SP), 1946 – current;
· EMBASE (Ovid SP), 1974 – current;
· Cumulative Index to Nursing and Allied Health Literature Plus (CINAHL Plus) (EBSCO), 1937 – current;
· Cochrane Central Register of Controlled Trials (CENTRAL)
· The International Clinical Trials Registry Platform (ICTRP)
· Clinical Trials.gov
We applied a date limit July 2012 onwards to the bibliographic databases.
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
exp infant, premature/ (41486)
exp infant, low birth weight/ (26342)
Infant, Premature, Diseases/ (17779)
(preterm* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW).ti,ab,hw. (189775)
or/1‐4 (192144)
exp Mycoses/ (104604)
exp Fungi/ (302448)
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s).ti,ab,hw. (459779)
or/6‐8 (604641)
and/5,9 (4150)
exp Antifungal Agents/ (134995)
exp azoles/ (523420)
(fungicid* or antifungal or azole*).ti,ab,rn. (66796)
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol).ti,ab,rn. (10206)
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine).ti,ab,rn. (4594)
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec).ti,ab,rn. (17395)
or/11‐16 (655015)
and/10,17 (820)
limit 18 to ed=20120601‐20140717 (100)
100 total results saved to Endnote library marked MEDLINE_17/07/2014 in Custom 4 field.
Database: EMBASE <1974 to 2014 May 21>
Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
prematurity/ (72156)
exp low birth weight/ (39615)
(preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW).ti,ab,hw. (232091)
or/1‐3 (235476)
exp mycosis/ (146509)
fungal colonization/ (2535)
exp fungus/ (386952)
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s).ti,ab,hw. (564479)
or/5‐8 (746245)
and/4,9 (5666)
exp antifungal agent/ (267508)
exp pyrrole derivative/ (57174)
(fungicid* or antifungal or azole*).ti,ab,rn. (51286)
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol).ti,ab,rn. (31540)
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine).ti,ab,rn. (12465)
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec).ti,ab,rn. (21126)
or/11‐16 (335058)
and/10,17 (1268)
limit 18 to em=201220‐201429 (225)
225 total results saved to Endnote library marked EMBASE_17/067/2014 in Custom 4 field.
CINAHL Plus Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
S18 S11 AND S17 34 S17 S12 OR S13 OR S14 OR S15 OR S16 5,975
S16 TX (Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec) 1,244
S15 TX (Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine) 195
S14 TX (Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol) 1,038
S13 TX (fungicid* or antifungal or azole*) 4,679
S12 (MH "Antifungal Agents+") 4,935
S11 S5 AND S10 535
S10 S6 OR S7 OR S8 OR S9 32,460
S9 TX (fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s) 27,232
S8 (MH "Fungi+") 7,672
S7 (MH "Mycosis Fungoides") 228
S6 (MH "Mycoses+") 10,176
S5 S1 OR S2 OR S3 OR S4 38,598
S4 TX (preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW) 37,954
S3 (MH "Infant, Premature, Diseases") 2,438
S2 (MH "Infant, Low Birth Weight+") 8,128
S1 (MH "Infant, Premature") 13,445
34 total results saved to Endnote library marked CINAHL_18/07/2014 in Custom 4 field.
Cochrane Library (CENTRAL) Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
ID Search Hits
MeSH descriptor: [Infant, Premature] explode all trees 2753
MeSH descriptor: [Infant, Low Birth Weight] explode all trees 1814
MeSH descriptor: [Infant, Premature, Diseases] explode all trees 2186
(preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW):ti,ab,kw (Word variations have been searched) 15350
#1 or #2 or #3 or #4 15681
MeSH descriptor: [Mycoses] explode all trees 2223
MeSH descriptor: [Fungi] explode all trees 1068
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s):ti,ab,kw (Word variations have been searched) 7137
#6 or #7 or #8 8166
#5 and #9 197
MeSH descriptor: [Antifungal Agents] explode all trees 1647
MeSH descriptor: [Azoles] explode all trees 29830
(fungicid* or antifungal or azole*):ti,ab,kw (Word variations have been searched) 2260
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol):ti,ab,kw (Word variations have been searched) 862
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine):ti,ab,kw (Word variations have been searched) 336
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec):ti,ab,kw (Word variations have been searched) 813
#11 or #12 or #13 or #14 or #15 or #16 31769
#10 and #17 Online Publication Date from Jan 2012 to Jul 2014, in Trials 7
7 total results saved to Endnote library marked CENTRAL_17/07/2014 in Custom 4 field.
The International Clinical Trials Registry Platform (ICTRP) http://www.who.int/ictrp/en/ Searched online 17/07/14 Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(premature OR preterm OR "low birth weight") AND ( Nystatin OR Fluconazole OR Amphotericin)
5 total results saved to Endnote library marked ICTRP 17/07/2014 in Custom 4 field.
ClinicalTrials.gov https://clinicaltrials.gov/ Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(premature OR preterm OR "low birth weight") AND ( Nystatin OR Fluconazole OR Amphotericin)
7 total results saved to Endnote library marked CLINICALTRIALS.GOV 17/07/2014 in Custom 4 field.
Total Results
| Database | Results | After deduplication | Custom 4 field |
| MEDLINE and MEDLINE In‐Process | 100 | 90 | MEDLINE 17/07/2014 |
| EMBASE | 225 | 170 | EMBASE 17/07/2014 |
| CINAHL | 34 | 12 | CINAHL 17/07/2014 |
| CENTRAL | 7 | 3 | CENTRAL 17/07/2014 |
| The International Clinical Trials Registry Platform (ICTRP) | 5 | 5 | ICTRP 17/07/2014 |
| ClinicalTrials.gov | 7 | 7 | CLINICALTRIALS.GOV 17/07/2014 |
| Total | 378 | 287 |
Appendix 2. Addendum to search (May 2015)
Search Date: May 19, 2015 (N = 16)
Search Terms: (nystatin OR nystan OR mycostatin OR nilstat OR nystex OR miconazole OR daktarin OR ketoconazole OR clotrimazole) AND
Plus the following database‐specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of invasive fungal infection | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All VLBW infants | 4 | 1800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.14, 0.27] |
| 1.2 Only ELBW infants | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.06, 0.26] |
| 2 Mortality | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All VLBW infants | 4 | 1800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 2.2 Only ELBW infants | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.51, 2.25] |
| 3 Bronchopulmonary dysplasia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.67, 2.49] |
| 4 Necrotising enterocolitis | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.40, 2.33] |
| 5 Retinopathy of prematurity | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.28] |
| 6 Length of stay in NICU (days) | 3 | 833 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐4.34, 3.29] |
1.1. Analysis.
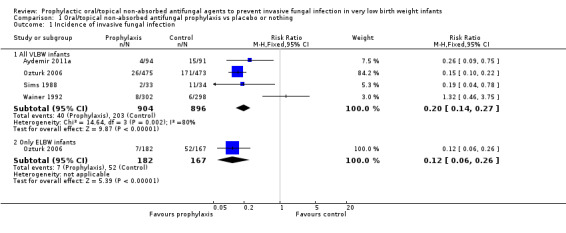
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 1 Incidence of invasive fungal infection.
1.2. Analysis.
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 2 Mortality.
1.3. Analysis.
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 3 Bronchopulmonary dysplasia.
1.4. Analysis.
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 4 Necrotising enterocolitis.
1.5. Analysis.
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 5 Retinopathy of prematurity.
1.6. Analysis.
Comparison 1 Oral/topical non‐absorbed antifungal prophylaxis vs placebo or nothing, Outcome 6 Length of stay in NICU (days).
Comparison 2. Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of invasive fungal infection | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.66, 5.39] |
| 2 Mortality | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 3.00] |
| 3 Bronchopulmonary dysplasia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.67, 2.49] |
| 4 Necrotising enterocolitis | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.58, 2.60] |
| 5 Retinopathy of prematurity | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.51, 2.98] |
| 6 Length of stay in NICU (days) | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.63, 5.63] |
2.1. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 1 Incidence of invasive fungal infection.
2.2. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 2 Mortality.
2.3. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 3 Bronchopulmonary dysplasia.
2.4. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 4 Necrotising enterocolitis.
2.5. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 5 Retinopathy of prematurity.
2.6. Analysis.
Comparison 2 Oral/topical non‐absorbed prophylaxis vs. systemic antifungal prophylaxis, Outcome 6 Length of stay in NICU (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aydemir 2011a.
| Methods | Randomised controlled trial | |
| Participants | 185 VLBW infants | |
| Interventions | Oral nystatin 100,000 U/ml 8 hourly (N = 94) versus normal saline placebo* (N = 91) every third day versus until the 30th day after birth (or 45th day in ELBW infants) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fungi with native azole resistance Adverse drug reactions |
|
| Notes | Setting: Zekai Tahir Burak Maternity Hospital, Ankara, Turkey; 2008 to 2009 *Report states placebo‐controlled but unclear how this was achieved The same infants form the oral nystatin group in both Aydemir 2011a and Aydemir 2011b |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Aydemir 2011b.
| Methods | Randomised controlled trial | |
| Participants | 187 VLBW infants. | |
| Interventions | Oral nystatin 100,000 U/ml 8 hourly (N = 94) versus fluconazole 3 mg/kg (N = 93) every third day versus until the 30th day after birth (or 45th day in ELBW infants) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fungi with native azole resistance Adverse drug reactions |
|
| Notes | Setting: Zekai Tahir Burak Maternity Hospital, Ankara, Turkey; 2008 to 2009 The same infants form the oral nystatin group in both Aydemir 2011a and Aydemir 2011b |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Mersal 2013.
| Methods | Randomised controlled trial | |
| Participants | 59 preterm infants < 30 weeks, birth weight < 1200 grams. Exclusion criteria: severe congenital anomalies, severe sepsis, intraventricular haemorrhage, persistent pulmonary hypertension, coagulopathy. |
|
| Interventions | Oral nystatin 1 ml (100,000 IU) every 8 h for first six weeks of life (N = 24) or intravenous fluconazole 6 mg/kg every 72 hours at end of first week of life, then every 48 hours from second week to sixth week of life (N = 35). Interventions commenced at one week of age. | |
| Outcomes | Invasive fungal infection Mortality |
|
| Notes | Location: Jeddah, Saudi Arabia. Participants enrolled February 2011 to February 2012. 60 infants were enrolled in the study. 3 were withdrawn from analysis (2 severe bacterial sepsis in fluconazole group, 1 Edwards syndrome). 57 were included in final analysis (24 in nystatin group, 33 in fluconazole group). The allocation group for the infant with Edwards syndrome is unknown. It is unclear as to whether the two infants with bacterial sepsis were withdrawn from analysis from the initial N = 35 (see above) or the N = 33 as the report quotes "2/33...in fluconazole group died because of bacterial sepsis...excluded from the study". The study author has been contacted and clarification if awaited. We have therefore assumed that there were a total of two deaths of N = 35 in the fluconazole group, and no deaths of N = 24 in the nystatin group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See notes above |
Ozturk 2006.
| Methods | Randomised controlled trial | |
| Participants | 938 VLBW infants with subgroup report for ELBW infants (N = 349) | |
| Interventions | Nystatin 100000 IU orally, 8 hourly (N = 475) versus no drug (N = 463) | |
| Outcomes | Fungal colonisation and invasive fungal infection | |
| Notes | Setting: Division of Neonatology, Erciyes University Hospital, Turkey, 2002 to 2005 25% of control VLBW infants received nystatin (100,000 IU orally, 8 hourly) to treat oral fungal colonisation detected at trial entry or during the trial period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by "someone not directly involved in the study" using random number tables |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Sims 1988.
| Methods | Quasi‐randomised controlled trial; odd or even hospital number allocation | |
| Participants | 67 infants of birth weight < 1250 grams | |
| Interventions | Nystatin 1 ml orally, 8 hourly (N = 33) versus no drug (N = 34) Treatment from inclusion until one week after endotracheal extubation |
|
| Outcomes | Fungal colonisation and invasive fungal infection Duration of mechanical ventilation and duration of intensive care admission |
|
| Notes | Setting: Los Angeles County+University of Southern California Medical Centre, 1985 to 1986 The study took place during a period of overcrowding in the intensive care unit; 222 infants with a birthweight < 1250 grams were born during a 12‐month period; 55 died within 48 hours, 88 relatively healthy infants were transferred elsewhere and 67 of the remaining 88 infants were recruited to the study. One infant in the control group had Candida albicans pneumonia supported by postmortem evidence. All the other affected infants had positive urine and blood cultures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Violaris 2010.
| Methods | Randomised controlled trial | |
| Participants | 80 VLBW infants Haemodynamically unstable infants and infants with severe congenital anomalies or abnormal liver function tests were not eligible to participate |
|
| Interventions | Nystatin (100,000 units/kg/day) in each side of the mouth (N = 42) versus fluconazole (4 mg/kg) orally (N = 38) beginning on day five after birth Medications were continued until full oral feedings were attained or systemic fungal infection was diagnosed |
|
| Outcomes | Invasive fungal infection, invasive bacterial infection, biochemical indices related to liver function, mortality | |
| Notes | Setting: Brooklyn Hospital Center, New York; 1997 to 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation and allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unable to blind interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unable to blind interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up |
Wainer 1992.
| Methods | Randomised controlled trial | |
| Participants | 600 infants of birth weight < 1750 grams. | |
| Interventions | Miconazole 0.75 ml orally 3 times daily (N = 302) vs. placebo (N = 298) | |
| Outcomes | Fungal colonisation and invasive fungal infection. Duration of mechanical ventilation and duration of intensive care admission | |
| Notes | Setting: Baragwanath Hospital, South Africa from October 1989 to July 1990 Due to limited resources, mechanical ventilation was not offered to infants with birth weight < 1000 grams. This group made up 12% (73/600) of the infants and had a high mortality rate (67%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
ELBW: extremely low birth weight VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Damjanovic 1993 | Not a randomised controlled trial |
| Demirel 2013 | Trial of nystatin versus probiotic |
| Harris 1960 | The gestational age or birth weight of participants was not reported – assumed to include term and preterm infants |
| Herruzo‐Cabrera 1994 | A prospective cohort study but not a randomised controlled trial |
| Oncel 2015 | Trial of nystatin versus probiotic |
Differences between protocol and review
None.
Contributions of authors
William McGuire and Jemma Cleminson screened the title and abstract of all studies identified by the search strategy, screened the full text of the report of each study identified as of potential relevance, extracted the data separately, compared data, and resolved differences by consensus. All authors completed the final review.
Sources of support
Internal sources
Christchurch Women's Hospital, Christchurch, New Zealand.
Centre for Reviews and Dissemination, University of York, UK.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
NIHR, UK.
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aydemir 2011a {published data only}
- Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F164‐8. [PUBMED: 20659937] [DOI] [PubMed] [Google Scholar]
Aydemir 2011b {published data only}
- Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F164‐8. [PUBMED: 20659937] [DOI] [PubMed] [Google Scholar]
Mersal 2013 {published data only}
- Mersal A, Alzahrani I, Azzouz M, Alsubhi A, Alsawaigh H, Albshri N, et al. Oral nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: non‐inferiority trial. Journal of Clinical Neonatology 2013;2(2):88‐92. [PUBMED: 24049751] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozturk 2006 {published data only}
- Ozturk MA, Gunes T, Koklu E, Cetin N, Koc N. Oral nystatin prophylaxis to prevent invasive candidiasis in Neonatal Intensive Care Unit. Mycoses 2006;49(6):484‐92. [PUBMED: 17022766] [DOI] [PubMed] [Google Scholar]
Sims 1988 {published data only}
- Sims ME, Yoo Y, You H, Salminen C, Walther FJ. Prophylactic oral nystatin and fungal infections in very‐low‐birthweight infants. American Journal of Perinatology 1988;5(1):33‐6. [PUBMED: 3276336] [DOI] [PubMed] [Google Scholar]
Violaris 2010 {published data only}
- Violaris K, Carbone T, Bateman D, Olawepo O, Doraiswamy B, LaCorte M. Comparison of fluconazole and nystatin oral suspensions for prophylaxis of systemic fungal infection in very low birthweight infants. American Journal of Perinatology 2010;27(1):73‐8. [PUBMED: 19504425] [DOI] [PubMed] [Google Scholar]
- Violaris K, Doraiswamy B, Olawepo O, Gulrajani‐LaCorte M. Fluconazole versus nystatin prophylaxis for fungal infection in very low birth weight (VLBW) infants. Pediatric Research. 1998; Vol. 44:254A.
Wainer 1992 {published data only}
- Wainer S, Cooper PA, Funk E, Bental RY, Sandler DA, Patel J. Prophylactic miconazole oral gel for the prevention of neonatal fungal rectal colonization and systemic infection. Pediatric Infectious Disease Journal 1992;11(9):713‐6. [PUBMED: 1448310] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Damjanovic 1993 {published data only}
- Damjanovic V, Connolly CM, Saene HK, Cooke RW, Corkill JE, Belkum A, et al. Selective decontamination with nystatin for control of a Candida outbreak in a neonatal intensive care unit. Journal of Hospital Infection 1993;24(4):245‐59. [PUBMED: 8104984] [DOI] [PubMed] [Google Scholar]
Demirel 2013 {published data only}
- Demirel G, Celik IH, Erdeve O, Saygan S, Dilmen U, Canpolat FE. Prophylactic Saccharomyces boulardii versus nystatin for the prevention of fungal colonization and invasive fungal infection in premature infants. European Journal of Paediatrics 2013;172(10):1321‐6. [PUBMED: 23703468] [DOI] [PubMed] [Google Scholar]
Harris 1960 {published data only}
- Harris LJ. Further observations on a simple procedure to eliminate thrush from hospital nurseries. American Journal of Obstetrics and Gynecology 1960;80:30‐1. [PUBMED: 14399978] [DOI] [PubMed] [Google Scholar]
Herruzo‐Cabrera 1994 {published data only}
- Herruzo‐Cabrera R, Garcia Gonzalez JI, Garcia‐Magan P, Rey‐Calero J. Nosocomial infection in a neonatal intensive care unit and its prevention with selective intestinal decolonization. A multivariant evaluation of infection reduction. European Journal of Epidemiology 1994;10(5):573‐80. [PUBMED: 7859857] [DOI] [PubMed] [Google Scholar]
Oncel 2015 {published data only}
- Oncel MY, Arayici S, Sari FN, Simsek GK, Yurttutan S, Erdeve O, et al. Comparison of Lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. The Journal of Maternal‐Fetal & Neonatal Medicine 2015;28:1790‐4. [PUBMED: 25245226] [DOI] [PubMed] [Google Scholar]
Additional references
Adams‐Chapman 2013
- Adams‐Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. The Journal of Pediatrics 2013;163(4):961‐7. [PUBMED: 23726546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aliaga 2014
- Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics 2014;133(2):236‐42. [PUBMED: 24446441] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascher 2012
- Ascher SB, Smith PB, Watt K, Benjamin DK, Cohen‐Wolkowiez M, Clark RH, et al. Antifungal therapy and outcomes in infants with invasive Candida infections. Pediatric Infectious Disease Journal 2012;31(5):439‐43. [PUBMED: 22189522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 2007
- Clerihew L, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003850.pub3] [DOI] [PubMed] [Google Scholar]
Barton 2014
- Barton M, O'Brien K, Robinson JL, Davies DH, Simpson K, Asztalos E, et al. Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infectious Diseases 2014;14:327. [PUBMED: 24924877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2003
- Benjamin DK Jr, Poole C, Steibach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end‐organ damage: a critical appraisal of the literature using meta‐analytic techniques. Pediatrics 2003;112(3 Pt 1):634‐40. [PUBMED: 12949295] [DOI] [PubMed] [Google Scholar]
Benjamin 2006
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84‐92. [PUBMED: 16396864] [DOI] [PubMed] [Google Scholar]
Brecht 2009
- Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(1):F65‐9. [PUBMED: 18838467] [DOI] [PubMed] [Google Scholar]
Brion 2007
- Brion LP, Uko SE, Goldman DL. Risk of resistance associated with fluconazole prophylaxis: systematic review. Journal of Infection 2007;54(6):521‐9. [PUBMED: 17239952] [DOI] [PubMed] [Google Scholar]
Burwell 2006
- Burwell LA, Kaufman D, Blakely J, Stoll BJ, Fridkin SK. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics 2006;118(4):e1019‐26. [PUBMED: 16982807] [DOI] [PubMed] [Google Scholar]
Clerihew 2006
- Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(3):F188‐92. [PUBMED: 16332924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clerihew 2008
- Clerihew L, McGuire W. Antifungal prophylaxis for very low birthweight infants: UK national survey. Archives of Disease in Childhood Fetal and Neonatal Edition 2008;93(3):F238‐9. [DOI] [PubMed] [Google Scholar]
Cotten 2006
- Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr, National Institute for Child Health and Human Development Neonatal Research Network. The association of third‐generation cephalosporin use and invasive candidiasis in extremely low birth‐weight infants. Pediatrics 2006;118(2):717‐22. [PUBMED: 16882828] [DOI] [PubMed] [Google Scholar]
Ernst 1983
- Ernst JA, Williams JM, Glick MR, Lemons JA. Osmolality of substances used in the intensive care nursery. Pediatrics 1983;72(3):347‐52. [PUBMED: 6889039] [PubMed] [Google Scholar]
Faix 1989
- Faix RG, Kovarik SM, Shaw TR, Johnson RV. Mucocutaneous and invasive candidiasis among very low birth weight (<1,500 grams) infants in intensive care nurseries: a prospective study. Pediatrics 1989;83(1):101‐7. [PUBMED: 2909957] [PubMed] [Google Scholar]
Frattarelli 2004
- Frattarelli DA, Reed MD, Giacoia GP, Aranda JV. Antifungals in systemic neonatal candidiasis. Drugs 2004;64(9):949‐68. [PUBMED: 15101785] [DOI] [PubMed] [Google Scholar]
Friedman 2000
- Friedman S, Richardson SE, Jacobs SE, O'Brien K. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatric Infectious Disease Journal 2000;19(6):499‐504. [PUBMED: 10877162] [DOI] [PubMed] [Google Scholar]
Ganesan 2008
- Ganesan K, Harigopal S, Neal T, Yoxall CW. Prophylactic oral nystatin for preterm babies under 33 weeks' gestation decreases fungal colonisation and invasive fungaemia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(4):F275‐8. [PUBMED: 19036756] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horbar 2002
- Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Members of the Vermont Oxford Network. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002;110(1 Pt 1):143‐51. [PUBMED: 12093960] [DOI] [PubMed] [Google Scholar]
Howell 2009
- Howell AJ, Isaacs D, Halliday R, Australasian Study Group for Neonatal Infections. Oral nystatin prophylaxis and neonatal fungal infections. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(6):F429‐33. [PUBMED: 19321509] [DOI] [PubMed] [Google Scholar]
Huang 1998
- Huang YC, Li CC, Lin TY, Lien RI, Chou YH, Wu JL, et al. Association of fungal colonization and invasive disease in very low birth weight infants. Pediatric Infectious Disease Journal 1998;17(9):819‐22. [PUBMED: 9779769] [DOI] [PubMed] [Google Scholar]
Isaacs 2008
- Isaacs D. Fungal prophylaxis in very low birth weight neonates: nystatin, fluconazole or nothing?. Current Opinion in Infectious Diseases 2008;21(3):246‐50. [PUBMED: 18448968] [DOI] [PubMed] [Google Scholar]
Kaguelidou 2012
- Kaguelidou F, Pandolfini C, Manzoni P, Choonara I, Bonati M, Jacqz‐Aigrain E. European survey on the use of prophylactic fluconazole in neonatal intensive care units. European Journal of Pediatrics 2012;171(3):439‐45. [PUBMED: 21912893] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlowicz 2002
- Karlowicz MG, Rowen JL, Barnes‐Eley ML, Burke BL, Lawson ML, Bendel CM, et al. The role of birth weight and gestational age in distinguishing extremely low birth weight infants at high risk of developing candidemia from infants at low risk: a multicenter study. Pediatric Research 2002;51:301A. [Google Scholar]
Kossoff 1998
- Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatric Infectious Disease Journal 1998;17(6):504‐8. [PUBMED: 9655543] [DOI] [PubMed] [Google Scholar]
Lee 1998
- Lee BE, Cheung PY, Robinson JL, Evanochko C, Robertson CM. Comparative study of mortality and morbidity in premature infants (birth weight < 1,250g) with candidemia or candidal meningitis. Clinical Infectious Diseases 1998;27(3):559‐65. [PUBMED: 9770157] [DOI] [PubMed] [Google Scholar]
Makhoul 2002
- Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late‐onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109(1):34‐9. [DOI] [PubMed] [Google Scholar]
Manzoni 2006
- Manzoni P, Farina D, Leonessa M, d'Oulx EA, Galletto P, Mostert M, et al. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics 2006;118(6):2359‐64. [DOI] [PubMed] [Google Scholar]
O'Grady 2008
- O'Grady MJ, Dempsey EM. Antifungal prophylaxis for the prevention of neonatal candidiasis?. Acta Paediatrica 2008;97(4):430‐3. [PUBMED: 18363952] [DOI] [PubMed] [Google Scholar]
Oeser 2013
- Oeser C, Lamagni T, Heath PT, Sharland M, Ladhani S. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000‐2009. The Pediatric Infectious Disease Journal 2013;32:23‐6. [PUBMED: 23241987] [DOI] [PubMed] [Google Scholar]
Oeser 2014
- Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal invasive fungal infection in England 2004‐2010. Clinical Microbiology and Infection 2014;20:936‐41. [PUBMED: 24479862] [DOI] [PubMed] [Google Scholar]
Pappu‐Katikaneni 1990
- Pappu‐Katikaneni LD, Rao KP, Banister E. Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses 1990;33(1):20‐3. [PUBMED: 2342516] [DOI] [PubMed] [Google Scholar]
Radmacher 2012
- Radmacher PG, Adamkin MD, Lewis ST, Adamkin DH. Milk as a vehicle for oral medications: hidden osmoles. Journal of Perinatology 2012;32(3):227‐9. [PUBMED: 21701446] [DOI] [PubMed] [Google Scholar]
Robinson 2009
- Robinson JL, Davies HD, Barton M, O'Brien K, Simpson K, Asztalos E, et al. Characteristics and outcome of infants with candiduria in neonatal intensive care ‐ a Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infectious Diseases 2009;9:183. [PUBMED: 19930662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowen 1994
- Rowen JL, Rench MA, Kozinetz CA, Adams JM, Baker CJ. Endotracheal colonization with candida enhances risk of systemic candidiasis in very low birth weight neonates. Journal of Pediatrics 1994;124(5 Pt 1):789‐94. [PUBMED: 8176570] [DOI] [PubMed] [Google Scholar]
Saiman 2000
- Saiman L, Ludington E, Pfaller M, Rangel‐Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The national epidemiology of mycosis survey study group. Pediatric Infectious Disease Journal 2000;19(4):319‐24. [PUBMED: 10783022] [DOI] [PubMed] [Google Scholar]
Shane 2013
- Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. American Journal of Perinatology 2013;30(2):131‐41. [PUBMED: 23297182] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285‐91. [PUBMED: 12165580] [DOI] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9‐14. [PUBMED: 20876594] [DOI] [PubMed] [Google Scholar]
Wynn 2012
- Wynn JL, Tan S, Gantz MG, Das A, Goldberg RN, Adams‐Chapman I, et al. Outcomes following candiduria in extremely low birth weight infants. Clinical Infectious Diseases 2012;54(3):331‐9. [PUBMED: 22144537] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Austin 2004
- Austin NC, Darlow B. Prophylactic oral antifungal agents to prevent systemic candida infection in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003478.pub2] [DOI] [PubMed] [Google Scholar]
Austin 2009
- Austin N, Darlow BA, McGuire W. Prophylactic oral/topical non‐absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003478.pub3] [DOI] [PubMed] [Google Scholar]
Austin 2013
- Austin N, Darlow BA, McGuire W. Prophylactic oral/topical non‐absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD003478.pub4] [DOI] [PubMed] [Google Scholar]


