Abstract
Background
Interleukin 2 receptor antagonists (IL2Ra) are used as induction therapy for prophylaxis against acute rejection in kidney transplant recipients. Use of IL2Ra has increased steadily since their introduction, but the proportion of new transplant recipients receiving IL2Ra differs around the globe, with 27% of new kidney transplant recipients in the United States, and 70% in Australasia receiving IL2Ra in 2007.
Objectives
To systematically identify and summarise the effects of using an IL2Ra, as an addition to standard therapy, or as an alternative to another immunosuppressive induction strategy.
Search methods
We searched the Cochrane Renal Group’s specialised register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE to identify new records, and authors of included reports were contacted for clarification where necessary.
Selection criteria
Randomised controlled trials (RCTs) in all languages comparing IL2Ra to placebo, no treatment, other IL2Ra or other antibody therapy.
Data collection and analysis
Data was extracted and assessed independently by two authors, with differences resolved by discussion. Dichotomous outcomes are reported as relative risk (RR) and continuous outcomes as mean difference (MD) with 95% confidence intervals (CI).
Main results
We included 71 studies (306 reports, 10,520 participants). Where IL2Ra were compared with placebo (32 studies; 5,854 patients) graft loss including death with a functioning graft was reduced by 25% at six months (16 studies: RR 0.75, 95% CI 0.58 to 0.98) and one year (24 studies: RR 0.75, 95% CI 0.62 to 0.90), but not beyond this. At one year biopsy‐proven acute rejection was reduced by 28% (14 studies: RR 0.72, 95% CI 0.64 to 0.81), and there was a 19% reduction in CMV disease (13 studies: RR 0.81, 95% CI 0.68 to 0.97). There was a 64% reduction in early malignancy within six months (8 studies: RR 0.36, 95% CI 0.15 to 0.86), and creatinine was lower (7 studies: MD ‐8.18 µmol/L 95% CI ‐14.28 to ‐2.09) but these differences were not sustained.
When IL2Ra were compared to ATG (18 studies, 1,844 participants), there was no difference in graft loss at any time point, or for acute rejection diagnosed clinically, but the was benefit of ATG therapy over IL2Ra for biopsy‐proven acute rejection at one year (8 studies:, RR 1.30 95% CI 1.01 to 1.67), but at the cost of a 75% increase in malignancy (7 studies: RR 0.25 95% CI 0.07 to 0.87) and a 32% increase in CMV disease (13 studies: RR 0.68 95% CI 0.50 to 0.93). Serum creatinine was significantly lower for IL2Ra treated patients at six months (4 studies: MD ‐11.20 µmol/L 95% CI ‐19.94 to ‐2.09). ATG patients experienced significantly more fever, cytokine release syndrome and other adverse reactions to drug administration and more leucopenia but not thrombocytopenia. There were no significant differences in outcomes according to cyclosporine or tacrolimus use, azathioprine or mycophenolate, or to the study populations baseline risk for acute rejection. There was no evidence that effects were different according to whether equine or rabbit ATG was used.
Authors' conclusions
Given a 38% risk of rejection, per 100 recipients compared with no treatment, nine recipients would need treatment with IL2Ra to prevent one recipient having rejection, 42 to prevent one graft loss, and 38 to prevent one having CMV disease over the first year post‐transplantation. Compared with ATG treatment, ATG may prevent some experiencing acute rejection, but 16 recipients would need IL2Ra to prevent one having CMV, but 58 would need IL2Ra to prevent one having malignancy. There are no apparent differences between basiliximab and daclizumab. IL2Ra are as effective as other antibody therapies and with significantly fewer side effects.
Plain language summary
Interleukin 2 receptor antagonists (IL2Ra) reduce the risk of acute rejection episodes at six and twelve months after kidney transplantation
Acute rejection is a major problem in the early period following kidney transplantation. Immunosuppressive drugs are used to prevent this. IL2Ra, a newer antibody therapy, can be added to a patient's existing immunosuppression to further reduce the risk of rejection. This review found that adding IL2Ra reduced the risk of graft loss or death with a functioning transplant, acute rejection, and early malignancy, but did not improve patient survival. Compared to ATG, another possible antibody option, IL2Ra treatment caused less CMV disease and malignancy and had fewer side effects, but although there was no difference in clinically diagnosed acute rejection, IL2Ra treatment resulted in more biopsy proven rejection at 1 year.
Background
Kidney transplantation is the treatment of choice for patients with end‐stage kidney disease (ESKD). In the developed world there are approximately 280 patients per million population (pmp) with a functioning kidney transplant. The transplant rate is around 30 pmp and between 30‐50% of transplanted organs come from living donors. Graft survival beyond five years has remained unchanged since the 1970s, with an average annual decline of approximately 5%. Waiting lists for transplantation continue to grow, demand exceeding organ availability. Strategies to increase donor organ availability and to prolong kidney allograft survival have become priorities in kidney transplantation (ANZDATA 2008; OPTN/SRTR 2008; UK National Transplant Database 2009; UK Renal Registry report 2007).
Transplant outcome is influenced by many factors. In the absence of immunosuppression, transplanted organs undergo progressive immune mediated injury (rejection). Standard immunosuppressive therapy consists of initial induction and then maintenance regimens to prevent rejection, with short courses of more intensive immunosuppressive therapy to treat episodes of acute rejection. Standard protocols in use typically involve three drug groups each directed to a site in the T‐cell activation and proliferation cascade which is central to the rejection process: calcineurin inhibitors (e.g. cyclosporin, tacrolimus), anti‐proliferative agents (e.g. azathioprine, mycophenolate mofetil) and steroids (prednisolone) (Hong 2000).
Short‐term graft survival is related to control of the acute rejection process. The risk of graft rejection is greatest in the immediate post‐transplant period, and immunosuppression is therefore initiated at high levels. This is either by using higher doses of the agents used in maintenance therapy, or by adding an additional immunosuppressive induction agent. The potential induction agents are an anti‐T cell antibody preparation, either a polyclonal anti‐lymphocyte antibody (e.g. anti‐thymocyte globulin (ATG)) or a monoclonal antibody (e.g. muromonab‐CD3), or an interleukin 2 receptor antibody (IL2Ra, also sometimes called anti‐CD25 antibodies).
IL2Ra are humanised or chimeric (murine/human) IgG monoclonal antibodies to the alpha subunit of the IL2 receptor present only on activated T lymphocytes. The binding of IL2 to its receptor induces second messenger signals to stimulate the T cell to enter the cell cycle and proliferate, resulting in clonal expansion and differentiation. IL2Ra inhibit this IL2 mediated activation. The rationale for use of IL2Ra has been as induction agents in combination with standard agents to try to prevent acute rejection, or to minimise exposure to the calcineurin inhibitors (particularly in recipients deemed at high risk of delayed initial graft function) thereby ameliorating their short and long‐term nephrotoxic side effects (so called calcineurin inhibitor sparing regimes) (Cibrik 2001; Goebel 2000)
Current opinion favours minimising early graft injury by using induction therapy (including IL2Ra) to prevent acute rejection, particularly in patients at high risk of early acute rejection . High‐risk groups include young adults and children, recipients of kidney with pancreas transplant, African‐Americans, and immunologically 'sensitised' patients. Sensitised patients are those with high titres of preformed circulating anti‐HLA antibodies, which can be estimated by testing Panel Reactive Antibodies (PRA) and other related tests. These circulating anti‐HLA antibodies may come about as a result of underlying illness, previous transplantation, previous pregnancy or blood transfusion. However there is no evidence that a decrease in early rejection rates translates into a uniform increase in long‐term graft survival for all. There is concern that newer drugs or combinations of drugs, whilst apparently improving early graft outcome by reducing early acute rejection episodes, may in fact increase the risk of malignant or cardiovascular disease in the medium and longer term, thereby curtailing patient survival (i.e. increasing death with a functioning allograft). (Pascual 2001; Vanrenterghem 2001)
There is considerable variability in the use of immunosuppressive agents both geographically and within patient groups. There is also variation in terms of the combinations of agents chosen and the dosage regimens employed. This variation is partly, but not completely, explained by different perceptions of the relative potency and specificity of different immunosuppressive regimens. In the Unites States in 2007, 27% of new kidney recipients received an IL2Ra as induction therapy, and 45% received an ATG preparation, whereas in Australia 70% received an IL2Ra and only 5% an ATG preparation (ANZDATA 2008; OPTN/SRTR 2008).
We originally reviewed the randomised control trial (RCT) evidence of benefits and harms of IL2Ra, compared with no treatment, or compared with another immunosuppressive strategy, in 2004 (Webster 2004). The aim of this review was to update the short and longer‐term benefits and harms of IL2Ra in kidney transplant recipients with new evidence from RCTs.
Objectives
To update the evidence and evaluate the benefits and harms of IL2Ra in kidney transplant recipients, when they are added to a standard dual or triple therapy regimen or when compared to another induction agent or immunosuppressive strategy.
To determine whether the benefits and harms vary in absolute or relative terms dependant on the type of IL2Ra (basiliximab or daclizumab), the co‐interventions used, or the population sub group of transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs, whether published or unpublished, in which IL2Ra were used to treat kidney transplant recipients.
Types of participants
Adults and children with ESKD that are the recipient of a first or subsequent cadaveric or living donor kidney transplant. Recipients who received another solid organ in addition to a kidney transplant (e.g. kidney and pancreas) were excluded.
Types of interventions
IL2Ra given in the intra operative period or at any time post‐transplantation, in combination with any other immunosuppressive agents for any declared rationale (e.g. induction therapy, or prophylaxis against rejection, or calcineurin sparing etc). All dosage regimens were included.
Control patients receive no IL2Ra, placebo, a different IL2Ra or a different dosage of IL2Ra, or another agent that the IL2Ra arm did not receive.
Types of outcome measures
The outcome measures relate to those used by transplant registries to assess patient and graft survival. Outcome events were assessed at one, three and six months, one year, and two to five years post‐transplant.
Primary outcomes
Patient mortality (all‐cause)
Graft loss or death with a functioning allograft
Graft loss censored for death with a functioning graft (loss of graft function resulting in dependence on dialysis)
Incidence of acute rejection (classified as clinically suspected and treated, or biopsy proven, or steroid resistant)
Secondary outcomes
Incidence of malignancy (all‐site)
Incidence of post‐transplant lymphoproliferative disease (PTLD) and lymphoma
Incidence of Cytomegalovirus (CMV) disease, diagnosed by culture, serology, antigen or antibody testing, or as specified by authors.
Incidence of new onset post‐transplant diabetes mellitus (PTDM)
Incidence of treatment related adverse reactions (including reactions to drug administration, and also haematological adverse reactions)
NEW OUTCOMES added for the review update, but not present in the original review
-
Transplant function, measured by
serum creatinine
directly measured or estimated glomerular filtration rate (GFR)
Search methods for identification of studies
Initial review
The literature search from the original review used search strategies detailed in Appendix 1, and consisted of;
Cochrane Renal Group specialised register of RCTs (June 2003). Cochrane Central Register of Controlled Trials (CENTRAL ‐ issue 3, 2003 in The Cochrane Library) for any "New" records not yet incorporated in the specialised register,
MEDLINE and Pre MEDLINE (1966 to November 2002) were searched using the above terms, combined with the optimally sensitive strategy for the identification of RCTs Dickersin 1994.
EMBASE (1980 to November 2003) was searched using terms similar to those used for MEDLINE and combined with a search strategy for the identification of RCTs Lefebvre 1996.
Reference lists of nephrology textbooks, review articles and relevant studies.
Conference proceeding's abstracts from nephrology scientific meetings.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Review update
For the update of this review, the following sources were used.
Cochrane Renal Group specialised register of RCTs.
Cochrane Central Register of Controlled Trials (CENTRAL ‐ issue 4, 2009) in The Cochrane Library) for any "New" records not yet incorporated in the specialised register.
MEDLINE (2009) were searched using the above terms, combined with the optimally sensitive strategy for the identification of RCTs (Glanville 2006).
EMBASE (2009) was searched using terms similar to those used for MEDLINE and combined with a search strategy for the identification of RCTs (Lefebvre 2008)
Note: The Cochrane Renal Group's specialised register contains studies identified from:
Quarterly searched of CENTRAL
Weekly searches of MEDLINE
Handsearched results of journals and the proceedings of major conferences (Renal Group 2009).
The electronic search strategies used are in Appendix 1.
Data collection and analysis
The review update was undertaken by seven authors (ACW, LPR, RMG, SLM, GYH, NSW, JCC).
Selection of studies
The search strategy described was performed to identify eligible studies (GYH). The titles and abstracts were independently screened by two authors (of ACW, LPR, SLM, RMG). Where necessary, the full text was independently assessed by two authors. Disagreement about inclusion was resolved by discussion (ACW, NSW).
Where duplicate reports of the same study were suspected, where necessary authors were contacted for clarification. If duplication was confirmed, the initial first complete publication was selected (the 'index' publication) and was the primary data source, but any other additional prior or subsequent reports were also included. These additional prior or subsequent reports containing additional outcome data (such as longer‐term follow‐up, or different outcomes) also contributed to the meta‐analysis. Studies were named using the family name of the first author of the earliest full report of the study to appear in a peer‐reviewed journal, together with the year of publication. Where no peer‐reviewed journal article was identified, the study was named using the family name of the first author of the earliest report, and the calendar year of that report.
Data extraction and management
Data extraction was performed independently by two authors (of ACW, LPR, SLM, RMG, NSW) using a standardised form. Authors of published work were contacted for clarification of unclear data, and any data they provided was incorporated (see acknowledgements). Data was entered into RevMan (AW, SLM, RMG).
Assessment of risk of bias in included studies
Quality of studies was assessed independently by two authors (of ACW, LPR, SLM, RMG) without blinding to journal or authorship. Discrepancies were resolved by discussion (ACW, JCC, NSW). The quality items were assessed using the risk of bias assessment tool (Higgins 2008) (seeAppendix 2), with each of the six risk of bias domain assessed as yes, no or unclear.
Was there adequate sequence generation?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study (objective and subjective outcomes)?
Were incomplete outcome data adequately addressed (intention‐to‐treat analysis)?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. malignancy or no malignancy) results were expressed as risk ratio (RR), and continuous outcomes were expressed as mean difference (MD), both with 95% confidence intervals (CI).
Dealing with missing data
Where a study reported outcome data after excluding some randomised participants from the denominator, if sufficient information was reported elsewhere, or was supplied by the study authors, we re‐included missing data in the analyses.
In studies where the standard deviation was not reported, it was calculated where possible (e.g. from the standard error) or inferred from available data by imputation (Higgins 2008).
Assessment of heterogeneity
Heterogeneity amongst study results was analysed using a Cochran Q test (n ‐1 degrees of freedom), with P < 0.05 used to denote statistical significance, and with I² calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity beyond chance (Higgins 2003).
Assessment of reporting biases
Potential for publication bias was assessed for the primary outcomes and for CMV disease and malignancy, using funnel plots of the log odds ratio (OR) (Egger 1997).
Data synthesis
Data was extracted first from individual studies and then pooled for summary estimates using a random effects model. The random effects model was chosen as it provides a more conservative estimate of effect in the presence of known or unknown potential heterogeneity (Deeks 2001).
Meta‐regression was performed for the following outcomes: all‐cause mortality, graft loss (death censored), acute rejection, CMV disease and malignancy, using data from all studies reporting these outcomes at any time within the first year post‐transplantation, with a priori subgroups listed above as explanatory variables (see below). Meta‐regression was undertaken on the log RR scale using STATA software (Stata11, StataCorp LP, Texas, USA), each study weighting equal to the inverse of the variance of the estimate for that study, with between study variance estimated using the restricted maximum‐likelihood method.
Subgroup analysis and investigation of heterogeneity
Stratified meta‐analysis and meta‐regression were used to explore important clinical differences among the studies that might potentially be expected to alter the magnitude of treatment effect, using restricted maximum‐likelihood to estimate the between study variance. Subgroups were defined a priori and included.
Baseline immunological risk for acute rejection of study population (low, mixed, or high)
Type of calcineurin inhibitor used (cyclosporin or tacrolimus)
Type of antimetabolite used (azathioprine or mycophenolate)
Intervention IL2Ra used (basiliximab or daclizumab)
Whether the calcineurin inhibitor was given from the time of transplantation at standard dose or used differently (e.g. delayed introduction or given in different dosages across the IL2Ra and control arms)
Sensitivity analysis
Sensitivity analyses based on publication type (conference abstract or peer reviewed journal) and study methodological quality (whether the study was conducted using an intention to treat analysis judged as adequate versus inadequate/unclear) were undertaken, aiming to establish whether the estimated treatment effects were robust to reasonable assumptions of the influence of these potential biases.
Results
Description of studies
The process of identifying reports of RCT for inclusion in the original review and in the review update are outlined in Figure 1. The review update contributed 189 reports from 33 studies. 41 were new reports of studies already included in the original review, 148 were reports of new studies.
1.

A total of 306 reports (publications and abstracts) of 71 studies qualified for inclusion in the review (Figure 1). The 71 combined studies represented a total of 10,520 randomised participants. Sixteen of these studies (Bernarde 2004; Cerrillos 2006; Chen 2003; de Boccardo 2002; Fangmann 2004; Flechner 2000; Garcia 2002; Hanaway 2008; Khan 2000; Locke 2008; Philosophe 2002; Pourfarziani 2003; Sandrini 2002; Shidban 2000; Shidban 2003; Yussim 2004) were available in abstract form only (1,705 participants), whilst the remaining 55 (8,815 participants) were published in 15 different journals. Basiliximab was used in 36 studies, daclizumab in 31, and other IL2Ra were used in six studies (either Anti‐tac, BT563, 33B3.1 or Lo‐tac‐1)
IL2Ra versus placebo/ no treatment
Thirty‐two studies (5,854 participants) compared an IL2Ra with placebo or no treatment in a calcineurin inhibitor based treatment regimen (Ahsan 2002; Baczkowska 2002; Bernarde 2004; Bingyi 2003; Cerrillos 2006; Chen 2003; Daclizumab double 1999; Daclizumab triple 1998; de Boccardo 2002; CAESAR (Ekberg) 2007; Fangmann 2004; Folkmane 2001; Grenda 2006; Ji 2007; Kahan 1999; Kirkman 1989; Kirkman 1991; Kyllonen 2007; Lawen 2003; Martin Garcia 2003; Nashan 1997; Offner 2008; Parrott 2005; Pescovitz 2003; Pisani 2001; Ponticelli 2001; Sandrini 2002; Sheashaa 2003; SYMPHONY (Ekberg) 2007; Tan 2004; van Gelder 1995; Yussim 2004).
IL2Ra versus ATG
Eighteen studies (1,844 participants) compared IL2Ra to an ATG preparation. Of these 12 studies (1,286 participants) used rabbit ATG ("thymoglobulin") (Abou‐Ayache 2008; Brennan 2006; Ciancio 2005; Kim 2008a; Lebranchu 2002; Locke 2008; Mourad 2004; Noel 2009; Pourfarziani 2003; Soulillou/Cant 1990; Hernandez 2007) and 7 (558 participants) used equine ATG (e.g. "ATGam") (Hourmant 1994; Kriaa 1993; Ruggenenti 2006; Shidban 2003; Sollinger 2001; Tullius 2003; Kyllonen 2007).
IL2Ra versus other antibody
Four studies (165 participants) compared IL2Ra with muromonab‐CD3 (OKT3) and one study (13 participants) compared IL2Ra with rituximab (Clatworthy 2009). Two studies (395 participants) compared IL2Ra with alemtuzumab (Ciancio 2005; Hanaway 2008).
IL2Ra versus other immunosuppressive strategy
Five studies (293 participants) (Grego 2007; Khan 2000; Lin 2006; Nair 2001; Perrea 2006) compared basiliximab with daclizumab. Four studies (345 participants) (Bernarde 2004; Kumar 2005; Matl 2001; Vincenti 2003) compared different doses of IL2Ra. Four studies (208 participants) (Asberg 2006; Garcia 2002; Gelens 2006; Wilson 2004) compared an IL2Ra with a calcineurin inhibitor, although study design for these four studies was heterogeneous, with co‐interventions varying across study arms (Characteristics of included studies). Three studies (1,372 participants) (ATLAS 2003; CARMEN (Rostaing) 2005; ter Meulen 2002) compared IL2Ra with steroids. One study (31 participants) compared IL2Ra with MMF (Kaplan 2003).
Two studies which had more than two arms were able to contribute data to more than one of the above comparisons (Bernarde 2004; Kyllonen 2007).
Baseline immunosuppression
Baseline immunosuppression varied both within studies (where three arms were investigated) and amongst studies. Cyclosporin was used in 55 studies (including 29 studies in the IL2Ra with placebo/ no treatment comparison and 14 studies in the IL2Ra with ATG comparison). In 20 of these studies the cyclosporin was stated to be the microemulsion (Neoral) formulation (Abou‐Ayache 2008; Asberg 2006; de Boccardo 2002; Grego 2007; Kahan 1999; Kaplan 2003; Lawen 2003; Lebranchu 2002; Lin 2006; Mourad 2004; Nashan 1997; Offner 2008; Parrott 2005; Ponticelli 2001; Sandrini 2002; Shidban 2000; Shidban 2003; Sollinger 2001; SYMPHONY (Ekberg) 2007; Tan 2004). In the remaining studies the cyclosporin formulation was not stated or was in solution (sandimune). Tacrolimus was used in 22 studies (Ahsan 2002; ATLAS 2003; CARMEN (Rostaing) 2005; Cerrillos 2006; Ciancio 2005; Clatworthy 2009; Garcia 2002; Gelens 2006; Grenda 2006; Hanaway 2008; Hernandez 2007; Khan 2000; Martin Garcia 2003; Noel 2009; Perrea 2006; Philosophe 2002; SYMPHONY (Ekberg) 2007; ter Meulen 2002; Tullius 2003; Vincenti 2003; Wilson 2004; Yussim 2004).
Reported outcome measures
The reporting of outcome measures was variable across studies (56/71 studies reported patient mortality, 30/71 reported CMV disease, see Figure 1). Reporting of harms was more limited and inconsistent among studies and frequently studies reported incomplete data for harm outcomes. Participants with any serious infection were reported in 32 (45%) studies, however a further 15 (21%) studies also assessed infection, but expressed their results as 'infectious episodes', and so this data could not be easily meaningfully combined.
Risk of bias in included studies
Reporting of details of study methodology was incomplete for the majority of studies, and are summarised in Figure 2.
2.
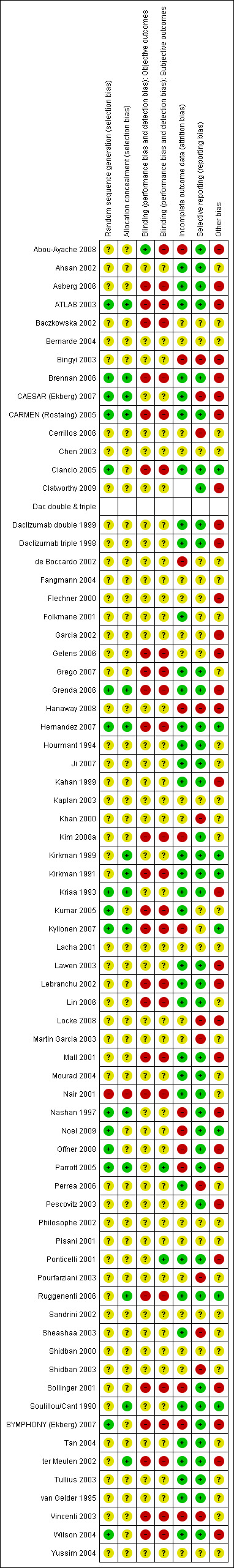
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Sequence generation and allocation concealment
Sixteen studies reported adequate sequence generation, and 15 studies reported adequate allocation concealment. One study (Nair 2001) used inadequate methods of sequence generation and allocation concealment. The remainder (54 studies for sequence generation and 55 for allocation concealment) used unclear methodology.
Blinding of objective and subjective outcomes
One study (Abou‐Ayache 2008) adequately reported blinding of objective outcomes, and two studies (Parrott 2005; Ponticelli 2001) adequately reported blinding of subjective outcomes. Twenty four had inadequate blinding of objective and 25 inadequate blinding of subjective outcomes. The remainder had unclear methods.
Incomplete outcome data and selective reporting
Incomplete outcome data was adequately addressed in 36 studies, and inadequately in 13 (the remainder were unclear). Forty one studies were free of selective reporting, but 12 were inadequate, the remainder unclear.
Other biases
Eight studies (Kirkman 1989; Kirkman 1991; Hernandez 2007; Ciancio 2005; Kumar 2005; Kyllonen 2007; Noel 2009; Soulillou/Cant 1990) declared their funding source to be an independent or academic funding body, and so were judged free of potential other bias. The remainder either declared sponsorship by a pharmaceutical industry company, or included an author who declared a pharmaceutical company as an affiliation, and so were judged as not free of potential bias. Others did not disclose the funding source of the study (judged unclear).
Effects of interventions
IL2Ra versus placebo/no treatment
Results can be found in comparison 1, Analyses 1.1 to 1.21. In general, all effects were homogeneous across all outcomes.
There was no difference in mortality, but graft loss including death with a functioning graft (Analysis 1.2) was reduced by 25% at six months (16 studies, 3017 participants: RR 0.75, 95% CI 0.58 to 0.98) and at one year after transplantation (24 studies, 4672 participants: RR 0.75, 95% CI 0.62 to 0.90). Graft loss censored for death with function showed similar significant reduction favouring IL2Ra (Analysis 1.3) at 6 months and 1 year. Beyond one year, there were fewer studies reporting graft loss outcomes, and so there was uncertainty whether the reduction was sustained beyond the first post‐transplant year (Analysis 1.2; Analysis 1.3). Incidence of biopsy‐proven acute rejection was reduced by 69% at three months, 32% at six months, and 28% at one year post‐transplantation for those treated with an IL2Ra (Analysis 1.5: at 3 months (2 studies): RR 0.31, 95% CI 0.14 to 0.68; at 6 months (15 studies): RR 0.68, 95% CI 0.62 to 0.76; at one year (14 studies): RR 0.72, 95% CI 0.64 to 0.81). This advantage was similar for clinically suspected acute rejection (Analysis 1.4). Treatment with an IL2Ra showed a pronounced effect in preventing early steroid‐resistant rejection, reducing incidence at six months by 48% (Analysis 1.6 (9 studies, 1928 participants): RR 0.52, 95% CI 0.39 to 0.68).
1.2. Analysis.
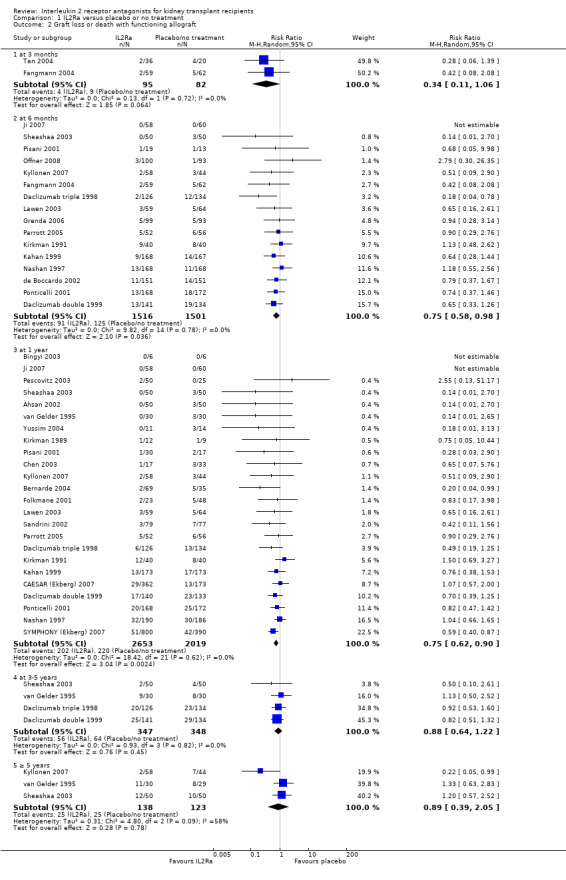
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 2 Graft loss or death with functioning allograft.
1.3. Analysis.
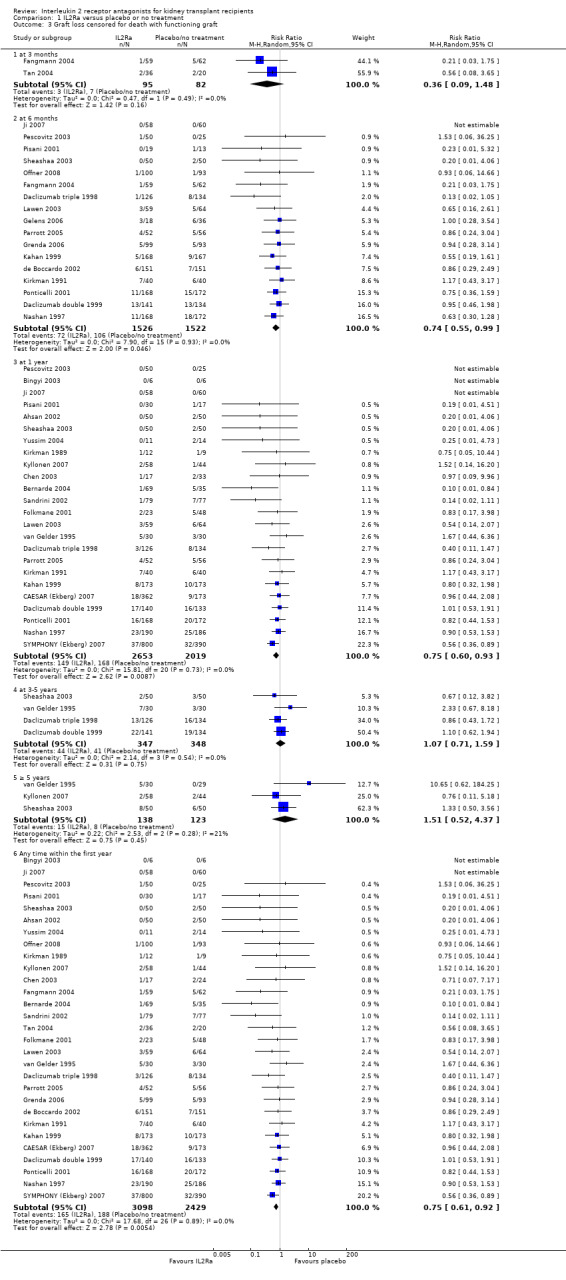
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 3 Graft loss censored for death with functioning graft.
1.5. Analysis.
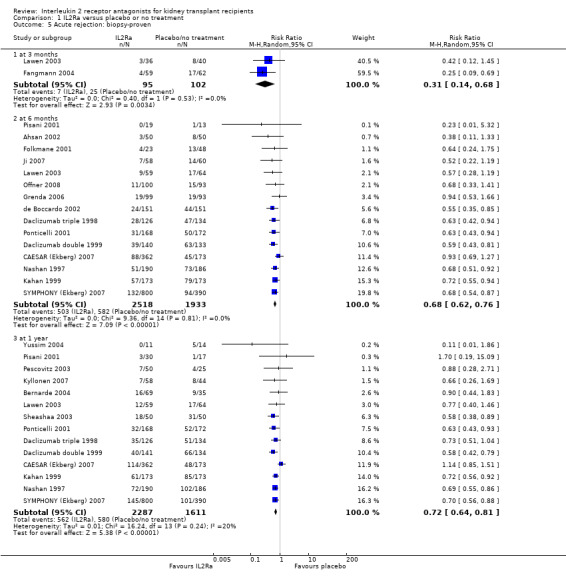
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 5 Acute rejection: biopsy‐proven.
1.4. Analysis.
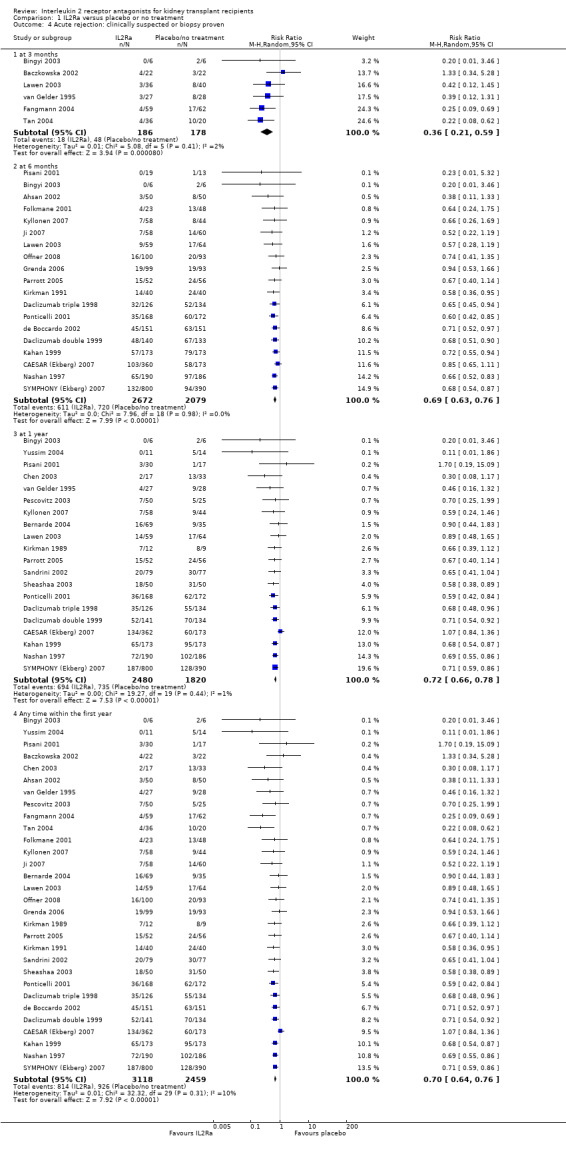
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 4 Acute rejection: clinically suspected or biopsy proven.
1.6. Analysis.
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 6 Acute rejection: steroid resistant.
Use of IL2Ra resulted in a 64% reduction in early malignancy within six months of transplantation (Analysis 1.7 (8 studies, 1878 participants): RR 0.36, 95% CI 0.15 to 0.86), but the effect was not sustained beyond six months. CMV infection was reduced in IL2Ra treated patients at three and six months, but not significantly so (Analysis 1.10). At one year, when more studies reported CMV outcomes, there was a 19% reduction in CMV disease for IL2Ra treated recipients (Analysis 1.9 (13 studies, 3169 participants): RR 0.81, 95% CI 0.68 to 0.97).
1.7. Analysis.
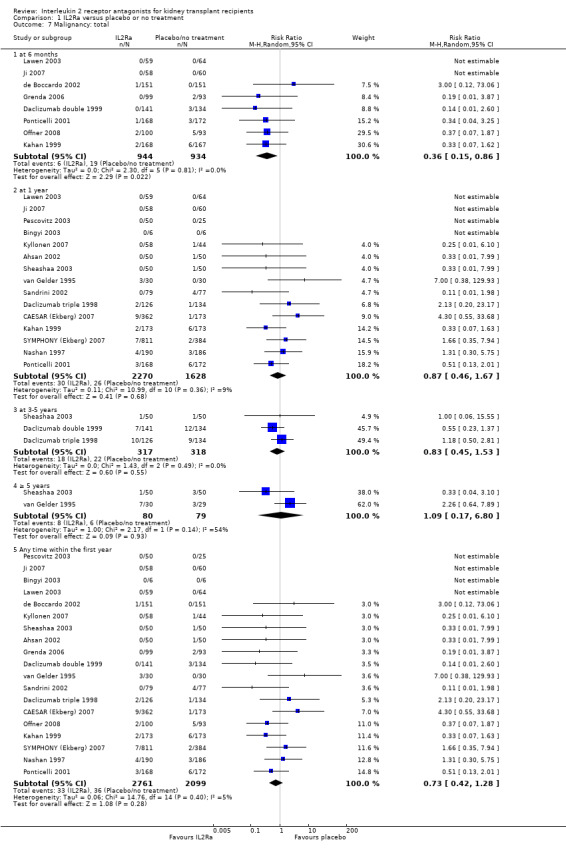
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 7 Malignancy: total.
1.10. Analysis.
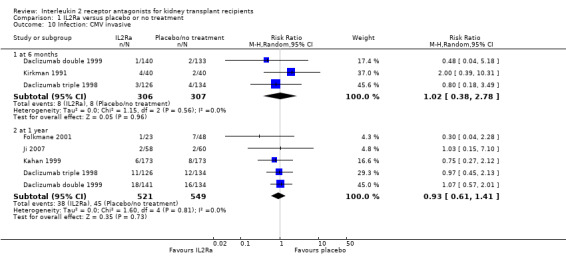
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 10 Infection: CMV invasive.
1.9. Analysis.
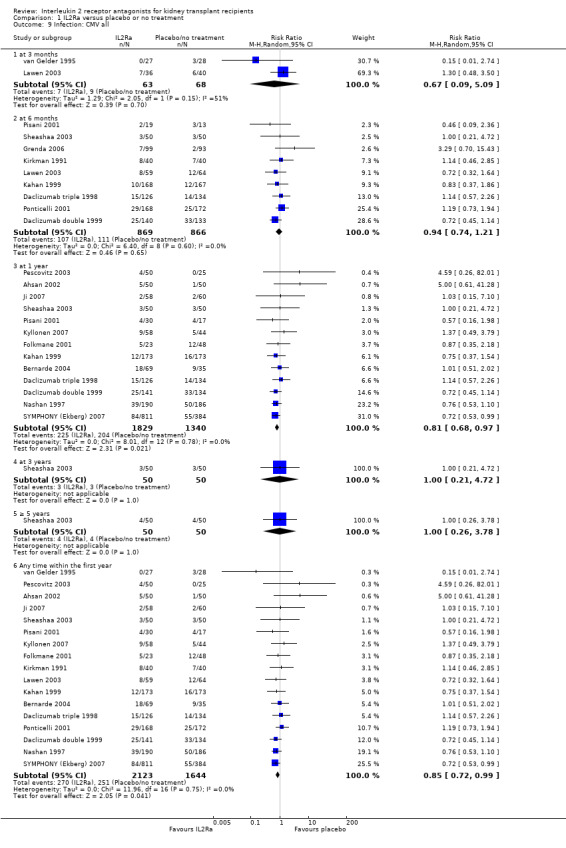
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 9 Infection: CMV all.
Serum creatinine was significantly lower for IL2Ra treated patients at one, three and six months post‐transplantation (Analysis 1.15: at 1 month (4 studies, 646 participants) MD ‐21.45 µmol/L 95% CI ‐33.03 to ‐9.38; at 3 months, (7 studies, 820 participants) MD ‐7.33 µmol/L 95% CI ‐13.58 to ‐1.08; and at 6 months (7 studies, 1231 participants) MD ‐8.18 µmol/L 95% CI ‐14.28 to ‐2.09), but this effect was not sustained at one year (Analysis 1.15 (8 studies, 1135 participants): MD ‐5.31 µmol/L 95% CI ‐13.90 to 3.28) or beyond, where there was no difference in creatinine. Few studies reported GFR, and there was no evidence of difference for IL2Ra or placebo (Analysis 1.16). Data was sparse for other outcomes, and there was no difference demonstrated for PTDM (Analysis 1.12), total serious infections (Analysis 1.11) or for adverse reaction to drug administration (Analysis 1.13).
1.15. Analysis.
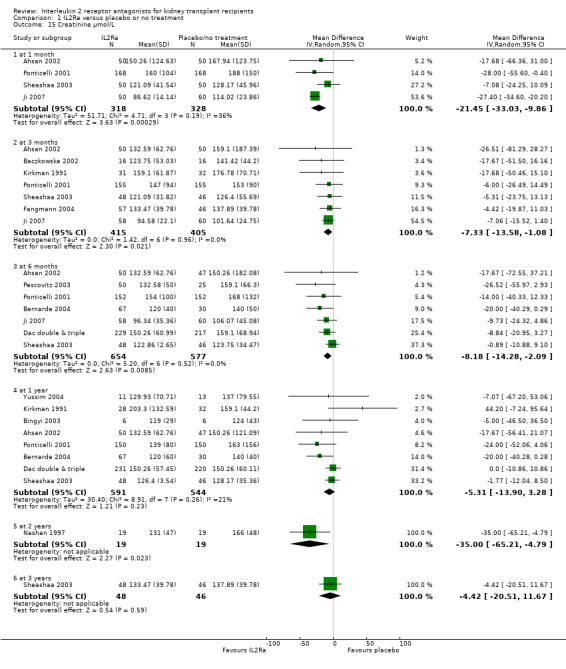
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 15 Creatinine µmol/L.
1.16. Analysis.
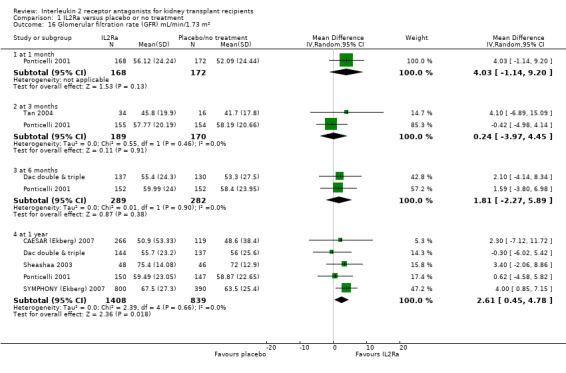
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 16 Glomerular filtration rate (GFR) mL/min/1.73 m².
1.12. Analysis.
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 12 Post‐transplant diabetes mellitus (PTDM).
1.11. Analysis.
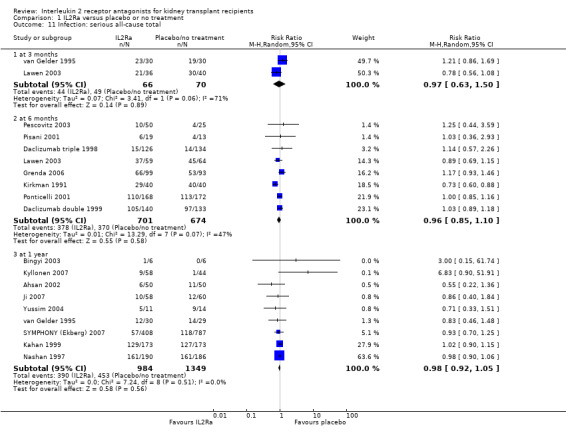
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 11 Infection: serious all‐cause total.
1.13. Analysis.
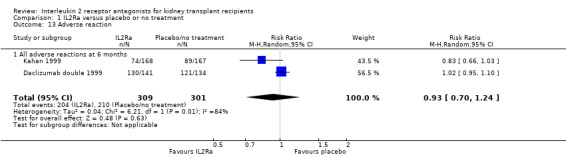
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 13 Adverse reaction.
There was no significant heterogeneity of effects for any outcomes when IL2Ra was compared with placebo/no treatment. We performed sensitivity analysis to examine the effect of studies methodology (whether intention‐to‐treat analysis was used, or not) and publication status (whether the study results were published in a peer‐reviewed journal, or not) on the outcomes death, graft loss censored for death, acute rejection (diagnosed clinically or by biopsy), CMV and malignancy, using data from studies reporting these outcomes at any time within the first post‐transplant year. Results are summarised in Table 1 and Table 2. There was no evidence to suggest difference in estimates of effect for studies that did not use intention‐to‐treat analysis or were unclear in how they analysed data. For studies published in non‐peer reviewed journals or as conference abstracts, there was a greater benefit in reduction of graft loss using IL2Ra (10 studies, RR 0.36 95% CI 0.18 to 0.71) than for those studies published in peer reviewed journals (19 studies, RR 0.81 95%CI 0.66 to 1.01 (P for difference 0.02), but no significant difference for other outcomes. Figure 3 shows the funnel plot for graft loss within the first year post‐transplantation.
1. IL2Ra compared with placebo/no treatment: stratified meta‐analysis (death, graft loss, acute rejection).
|
|
Death | Graft loss | Acute rejection | ||||||
| N | RR (95% CI) | P | N | RR (95% CI) | P | N | RR (95% CI) | P | |
| Publication status | |||||||||
| Abstract | 8 | 0.70 (0.22, 2.20) | 0.81 | 10 | 0.36 (0.18, 0.71) | 0.02 | 10 | 0.61 (0.48, 0.77) | 0.20 |
| Journal | 16 | 0.81 (0.54, 1.21) | 19 | 0.81 (0.66, 1.01) | 20 | 0.72 (0.66, 0.79) | |||
| ITT analysis | |||||||||
| ITT used | 13 | 0.80 (0.46, 1.32) | 0.90 | 15 | 0.87 (0.65, 1.15) | 0.17 | 15 | 0.69 (0.59, 0.80) | 0.80 |
| No/ unclear | 11 | 0.81 (0.48, 1.40) | 14 | 0.65 (0.48, 0.88) | 15 | 0.69 (0.62, 0.78) | |||
| Risk for AR | |||||||||
| Low | 10 | 0.80 (0.42, 1.50) | 0.74 | 10 | 0.84 (0.59, 1.20) | 0.39 | 11 | 0.68 (0.60, 0.76) | 0.02† |
| Mixed | 7 | 0.83 (0.52, 1.35) | 9 | 0.73 (0.55, 0.97) | 9 | 0.75 (0.64, 0.88) | |||
| High | 2 | 0.08 (0.01, 7.20) | 2 | 0.61 (0.14, 2.63) | 2 | 0.25 (0.11, 0.56) | |||
| Unclear | 5 | 0.42 (0.03, 7.31) | 8 | 0.57 (0.26, 1.23) | 8 | 0.66 (0.50, 0.88) | |||
| CNI | |||||||||
| Cyclosporine | 21 | 0.90 (0.57, 1.42) | 0.37 | 25 | 0.82 (0.64, 1.03) | 0.17 | 26 | 0.69 (0.63, 0.77) | 0.69 |
| Tacrolimus | 2 | 0.10 (0.01, 9.76) | 3 | 0.77 (0.24, 2.48) | 3 | 0.66 (0.28, 1.57) | |||
| Unclear/mixed | 1 | 0.63 (0.32, 1.25) | 10 | 0.56 (0.36, 0.89) | 1 | 0.71 (0.59, 0.86) | |||
| Antimetabolite | |||||||||
| Azathioprine | 8 | 0.97 (0.44, 2.14) | 0.80 | 10 | 0.78 (0.52, 1.16) | 0.38 | 10 | 0.66 (0.57, 0.76) | 0.69 |
| Mycophenolate | 12 | 0.71 (0.42, 1.21) | 14 | 0.59 (0.42, 0.83) | 15 | 0.69 (0.55, 0.88) | |||
| Unclear/mixed | 4 | 0.61 (0.18, 2.12) | 5 | 0.95 (0.67, 1.35) | 5 | 0.69 (0.60. 0.79) | |||
| IL2Ra | |||||||||
| Basiliximab | 12 | 0.93 (0.51, 1.71) | 0.95 | 16 | 0.77 (0.57, 1.03) | 0.67 | 16 | 0.68 (0.61, 0.76) | 0.88 |
| Daclizumab | 9 | 0.65 (0.39, 1.08) | 10 | 0.68 (0.92, 0.93) | 11 | 0.70 (0.57, 0.87) | |||
| Other | 3 | 1.88 (0.42, 8.48) | 3 | 1.26 (0.59, 2.72) | 3 | 0.60 (0.43, 0.84) | |||
N = total number of studies reporting given outcome, RR = risk ratio, P = P for difference among strata, ITT = analysis by intention‐to‐treat principle, CNI= calcineurin inhibitor, IL2Ra= interleukin 2 receptor antibody
† Test for low/mixed risk versus high risk
2. IL2Ra compared with placebo/no treatment: stratified meta‐analysis (cytomegalovirus disease, malignancy).
| Cytomegalovirus disease | Malignancy | |||||
| N | RR (95% CI) | P | N | RR (95% CI) | P | |
| Publication status | ||||||
| Abstract | 5 | 0.97 (0.65, 1.44) | 0.47 | 4 | 1.18 (0.15, 9.62) | 0.70 |
| Journal | 12 | 0.82 (0.69, 0.98) | 15 | 0.76 (0.41, 1.42) | ||
| ITT analysis | ||||||
| ITT used | 11 | 0.93 (0.73, 1.18) | 0.30 | 11 | 0.71 (0.31, 1.61) | 0.72 |
| No/ unclear | 6 | 0.78 (0.63, 0.97) | 8 | 0.89 (0.37, 2.10) | ||
| Risk of AR | ||||||
| Low | 8 | 0.82 (0.65, 1.05) | 0.47 | 9 | 0.70 (0.28, 1.81) | 0.82 |
| Mixed | 5 | 0.83 (0.66, 1.06) | 6 | 0.93 (0.41, 2.11) | ||
| High | 0 | No data | 0 | No data | ||
| Unclear | 4 | 1.02 (0.63, 1.65) | 4 | 0.22 (0.01, 5.99) | ||
| CNI | ||||||
| Cyclosporine | 15 | 0.88 (0.73, 1.06) | 0.34 | 16 | 0.73 (0.38, 1.40) | 0.43 |
| Tacrolimus | 1 | 5.00 (0.61, 41.3) | 2 | 0.06 (0.01, 5.84) | ||
| Unclear/mixed | 1 | 0.72 (0.53,0.99) | 1 | 1.66 (0.35, 7.94) | ||
| Antimetabolite | ||||||
| Azathioprine | 5 | 1.18 (0.84, 1.65) | 0.05 | 8 | 0.58 (0.20,1.72) | 0.81 |
| Mycophenolate | 7 | 0.78 (0.60, 1.02) | 7 | 1.10 (0.42, 2.87) | ||
| Unclear/mixed | 5 | 0.75 (0.58, 0.97) | 4 | 0.70 (0.18, 2.74) | ||
| IL2Ra | ||||||
| Basiliximab | 9 | 0.88 (0.71, 1.10) | 0.74 | 11 | 0.51 (0.25, 1.05) | 0.05 |
| Daclizumab | 6 | 0.81 (0.61, 1.08) | 7 | 1.81 (0.63, 5.20) | ||
| Other | 2 | 0.81 (0.10, 6.32) | 1 | 7.00 (0.38, 129.9) | ||
N = total number of studies reporting given outcome, RR = risk ratio, P = P for difference among strata, ITT = analysis by intention to treat principle, CNI= calcineurin inhibitor, IL2Ra= interleukin 2 receptor antibody
3.
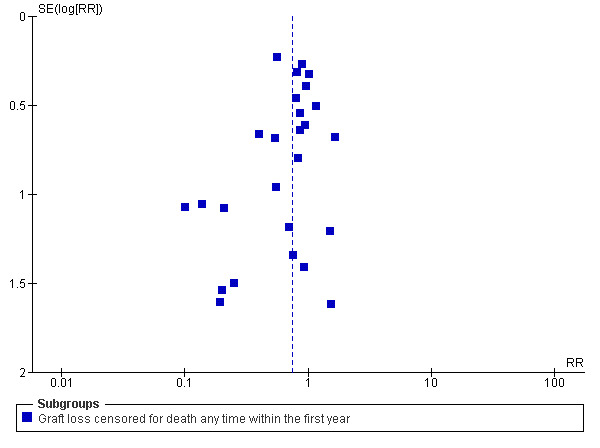
IL2Ra vs Placebo/no treatment. Graft loss censored for death at any time within the first year
To investigate the effect of calcineurin inhibitor and antimetabolite co‐intervention, and the study population background risk for acute rejection, we performed subgroup analysis using the same outcomes. The results are summarised in Table 1 and Table 2 (forest plots not shown). There was no evidence that effects of IL2Ra were different for any outcome when used with either cyclosporin or tacrolimus, or when used with azathioprine or mycophenolate, except for the outcome CMV disease. For CMV disease, there was more evidence of benefit for reducing CMV disease when used with mycophenolate (7 studies, RR 0.78 95% CI 0.60 to 1.02) than when used with azathioprine (5 studies, RR 1.18 95% CI 0.84 to 1.65) (P for difference 0.05). There was no evidence that the effects of IL2Ra were different depending on the study population baseline risk for acute rejection for death, graft loss, CMV or malignancy, but there was some evidence that higher risk populations benefited more in reduction of acute rejection than those at lower baseline risk (Table 1, respectively 2 studies RR 0.25 95% CI 0.11 to 0.56 and 11 studies RR 0.68 95% CI 0.60 to 0.76; P for difference 0.02)
IL2Ra versus ATG
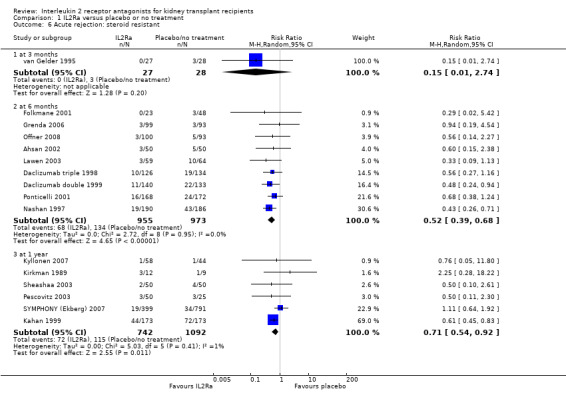
When IL2Ra were compared to ATG, there was no evidence of a difference in death (Analysis 2.1), graft loss whether including death with function (Analysis 2.2) or censored for death (Analysis 2.3), at any time point post‐transplantation. There was no difference for acute rejection diagnosed clinically at any time point (Analysis 2.4), at any time within the first year (15 studies, 1571 participants: RR 1.12 95% CI 0.93 to 1.33) or for biopsy‐proven rejection at three or six months (Analysis 2.5), but there was benefit of ATG therapy over IL2Ra for biopsy‐proven acute rejection at one year, where there was a 30% increase in those treated with IL2Ra (Analysis 2.5 8 studies, 1106 participants: RR 1.30 95% CI 1.01 to 1.67). This effect was not seen for steroid‐resistant rejection and any time point, although fewer studies reported this outcome (Analysis 2.6). Recipients treated with IL2Ra showed a 75% reduction in malignancy at one year compared with ATG treated (Analysis 2.7 7 studies, 1067 participants: RR 0.25 95% CI 0.07 to 0.87), although not at other time points. CMV disease was reduced, but not significantly so, for IL2Ra treated recipients at three and six months and one year (Analysis 2.9). When considering CMV disease occurring at any time within the first year post‐transplant, IL2Ra treated recipients showed a 32% reduction compared to the ATG treated (Analysis 2.9 13 studies, 1647 participants: RR 0.68 95% CI 0.50 to 0.93). Serum creatinine was significantly lower for IL2Ra treated patients at six months and one year post‐transplantation (Analysis 2.15, respectively 4 studies, 244 participants: MD ‐11.20 µmol/L 95% CI ‐19.94 to ‐2.09; and 6 studies, 586 participants: MD ‐8.84 µmol/L 95% CI ‐17.23 to ‐0.45) but this effect was not certain at other time points where there was no difference demonstrated in mean creatinine. Few studies reported GFR, and there was no evidence of difference for IL2Ra or ATG (Analysis 2.16). Compared with IL2Ra, ATG patients experienced significantly more fever, cytokine release syndrome and other adverse reactions to drug administration (Analysis 2.12), and more leucopenia but not thrombocytopenia (Analysis 2.13).
2.1. Analysis.
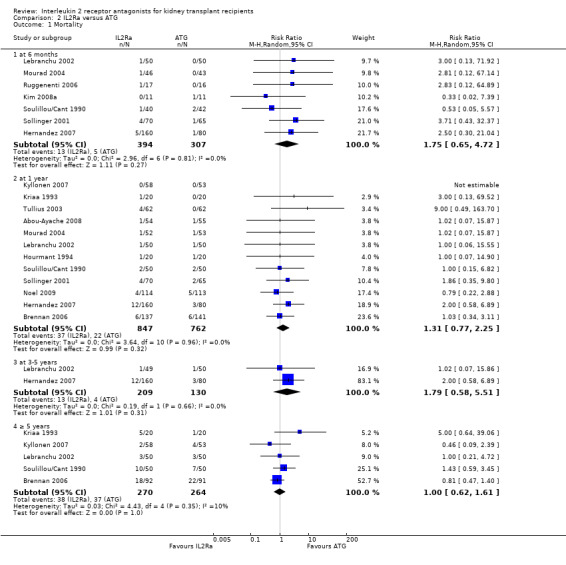
Comparison 2 IL2Ra versus ATG, Outcome 1 Mortality.
2.2. Analysis.
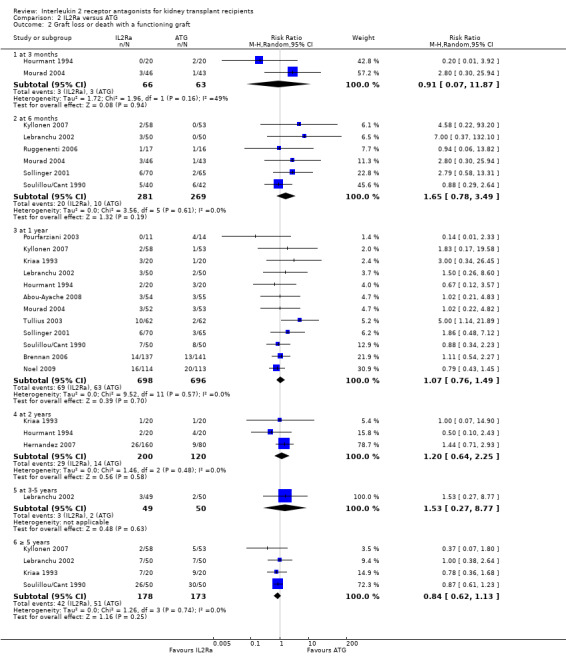
Comparison 2 IL2Ra versus ATG, Outcome 2 Graft loss or death with a functioning graft.
2.3. Analysis.
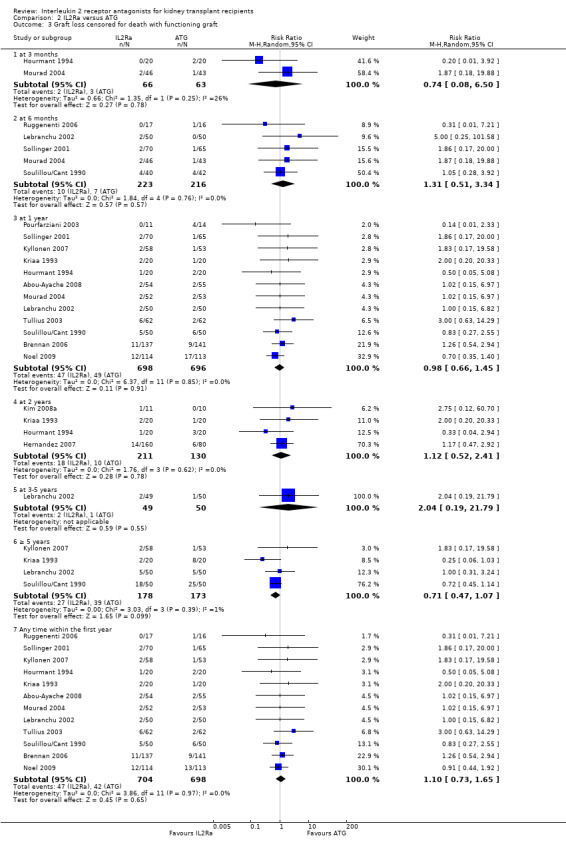
Comparison 2 IL2Ra versus ATG, Outcome 3 Graft loss censored for death with functioning graft.
2.4. Analysis.
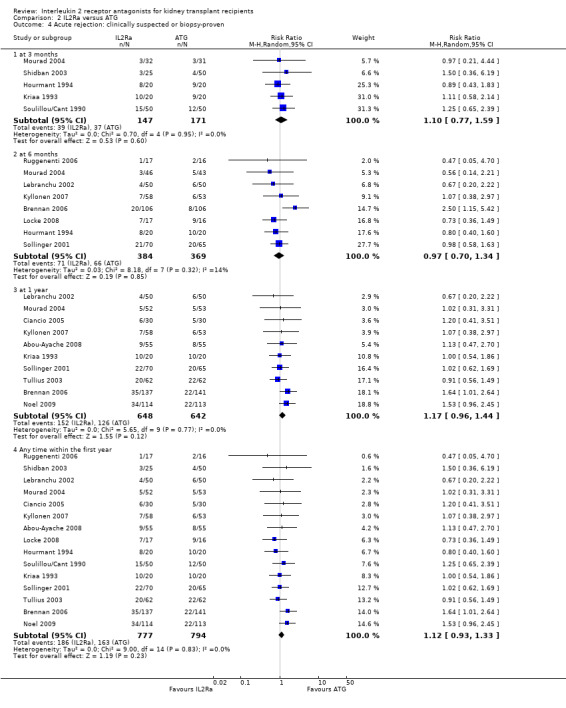
Comparison 2 IL2Ra versus ATG, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
2.5. Analysis.
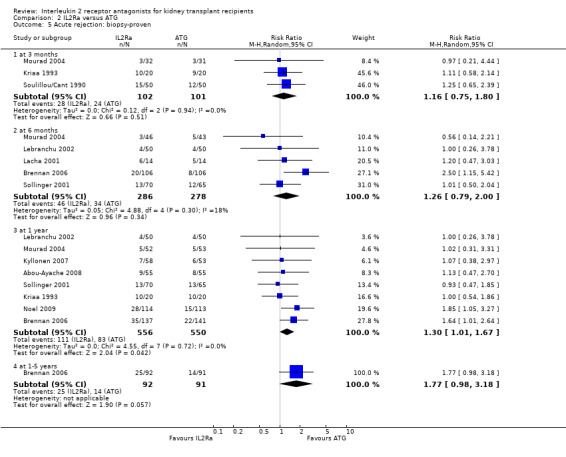
Comparison 2 IL2Ra versus ATG, Outcome 5 Acute rejection: biopsy‐proven.
2.6. Analysis.
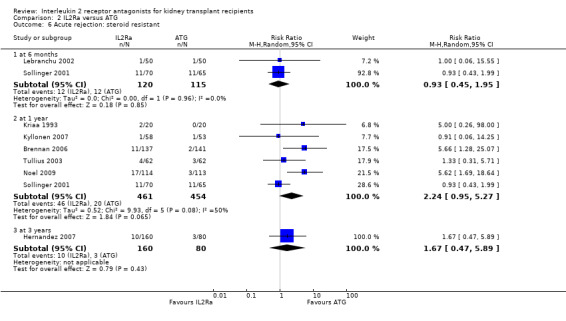
Comparison 2 IL2Ra versus ATG, Outcome 6 Acute rejection: steroid resistant.
2.7. Analysis.
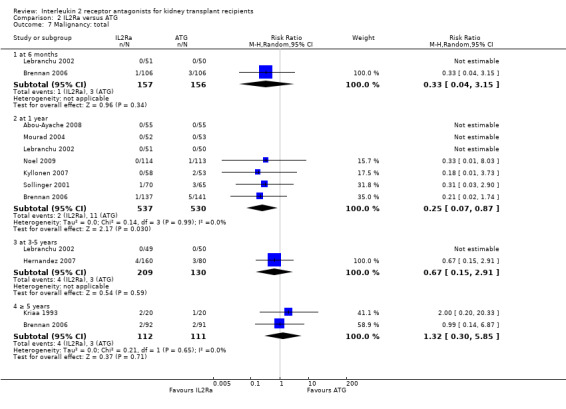
Comparison 2 IL2Ra versus ATG, Outcome 7 Malignancy: total.
2.9. Analysis.
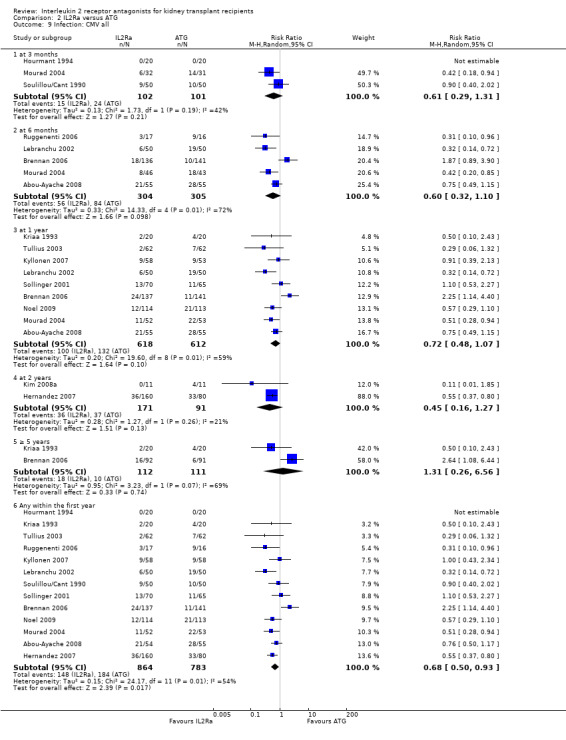
Comparison 2 IL2Ra versus ATG, Outcome 9 Infection: CMV all.
2.15. Analysis.
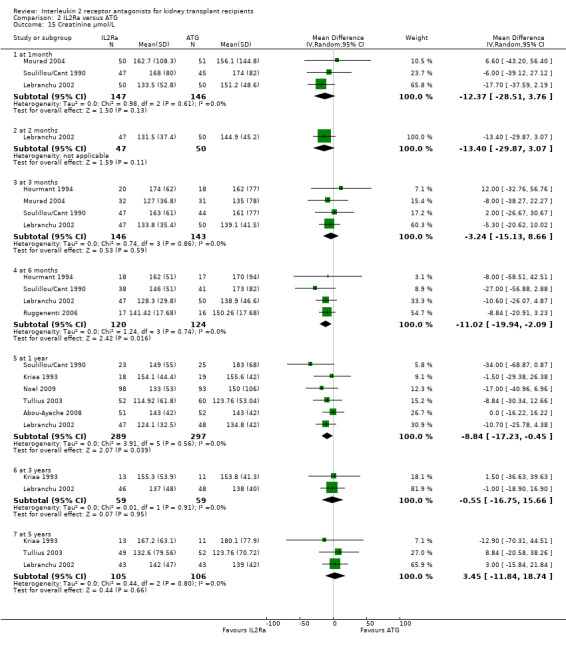
Comparison 2 IL2Ra versus ATG, Outcome 15 Creatinine µmol/L.
2.16. Analysis.
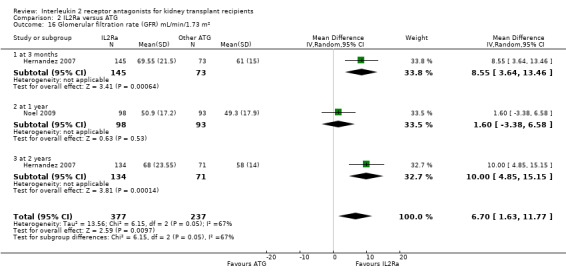
Comparison 2 IL2Ra versus ATG, Outcome 16 Glomerular filtration rate (GFR) mL/min/1.73 m².
2.12. Analysis.
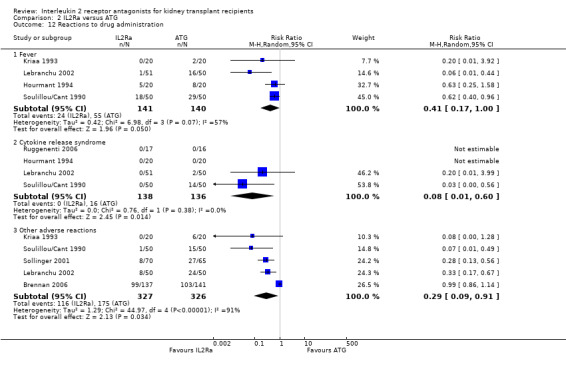
Comparison 2 IL2Ra versus ATG, Outcome 12 Reactions to drug administration.
2.13. Analysis.
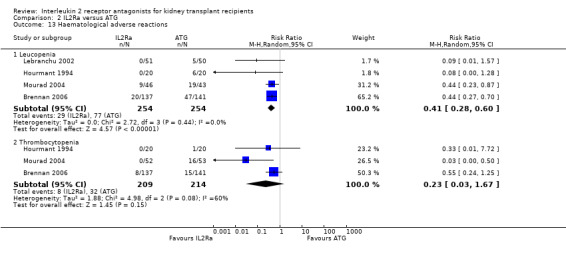
Comparison 2 IL2Ra versus ATG, Outcome 13 Haematological adverse reactions.
Overall, effects among studies were homogeneous. However, as in the original version of the review, significant heterogeneity was demonstrated for the outcome of CMV disease at six months (5 studies: RR 0.60, 95% CI 0.32 to 1.10; Chi² = 14.33, df = 4; P = 0.006, I² =72%) and similarly at one year (5 studies: RR 0.60, 95% CI 0.32 to 1.10; Chi² = 14.33, df = 4; P = 0.006, I² =72%) or at any time point within the first year (13 studies: RR 0.68, 95% CI 0.50 to 0.93; Chi² = 24.17, df = 11; P = 0.01, I² =54%). As in the original review, heterogeneous results were largely attributable to one study (Brennan 2006). Sensitivity analysis, by removal of this study from each analysis, showed more homogeneous results strongly favouring IL2Ra (at six months: RR 0.47, 95% CI 0.29 to 0.77; P = 0.13; I² = 46%; at any time within the first year: RR 0.62 95% CI 0.49 to 0.77; P = 0.34; I² = 11%). Sensitivity analysis for outcomes death, graft loss censored for death, acute rejection, CMV disease and malignancy (all reported within the first post‐transplant year), demonstrated no differences of effect for intention‐to‐treat analysis or for publication status (Table 3 and Table 4). There were also no significant differences for the same outcomes, between subgroup analyses when stratified according to whether the studies used cyclosporin or tacrolimus, or azathioprine or mycophenolate, or according to the study population baseline risk for acute rejection (Table 3 and Table 4; forest plots not shown). When comparing the effects of IL2Ra with ATG, there was no evidence that effects were different according to the formulation of ATG used, specifically whether equine or rabbit (Table 3 and Table 4).
3. IL2Ra versus ATG: stratified meta‐analysis (death, graft loss, acute rejection).
|
|
Death | Graft loss | Acute rejection | ||||||
| N | RR (95% CI) | P | N | RR (95% CI) | P | N | RR (95% CI | P | |
| Publication status | |||||||||
| Abstract | 2 | 21.64 (0.24, 930.90) | 0.21 | 2 | 2.64 (0.72, 9.65) | 0.17 | 4 | 0.92 (0.66, 1.27) | 0.16 |
| Journal | 10 | 1.19 (0.68, 2.07) | 10 | 1.01 (0.66. 1.55) | 11 | 1.21 (0.98, 1.50) | |||
| ITT analysis | |||||||||
| ITT used | 8 | 1.33 (0.68, 2.62) | 0.56 | 8 | 1.18 (0.69, 2.00) | 0.62 | 10 | 1.10 (0.87, 1.39) | 0.42 |
| No/ unclear | 4 | 1.08 (0.42, 2.79) | 4 | 1.03 (0.54, 1.94) | 4 | 0.96 (0.68, 1.37) | |||
| Risk for AR | |||||||||
| Low | 5 | 1.53 (0.62, 3.78) | 0.56 | 4 | 0.99 (0.44, 2.23) | 0.82 | 6 | 1.10 (0.78, 1.55) | 0.96† |
| Mixed | 3 | 1.20 (0.44, 3.28) | 4 | 1.15 (0.61, 2.18) | 4 | 1.12 (0.84, 1.49) | |||
| High | 2 | 1.03 (0.37, 2.85) | 2 | 1.13 (0.51, 2.50) | 3 | 1.04 (0.60, 1.80) | |||
| Unclear | 2 | 1.01 (0.07, 13.86) | 2 | 1.29 (0.29, 5.72) | 2 | 1.05 (0.48, 2.27) | |||
| CNI | |||||||||
| Cyclosporine | 10 | 1.33 (0.72, 2.45) | 0.62 | 10 | 1.10 (0.65, 1.84) | 0.93 | 11 | 1.12 (0.90, 1.41) | 0.76 |
| Tacrolimus | 2 | 1.65 (0.08, 33.44) | 2 | 1.35 (0.45, 4.01) | 3 | 1.19 (0.84, 1.70) | |||
| Unclear/mixed | 0 | No data | 0 | No data | 1 | 0.73 (0.36, 1.50) | |||
| Antimetabolite | |||||||||
| Azathioprine | 4 | 1.14 (0.25, 5.08) | 0.41 | 4 | 0.97 (0.41, 2.30) | 0.42 | 4 | 1.02 (0.71, 1.45) | 0.57 |
| Mycophenolate | 6 | 1.05 (0.54, 2.07) | 6 | 1.07 (0.66, 1.74) | 8 | 1.30 (1.02, 1.66) | |||
| Unclear/mixed | 2 | 2.24 (0.67, 7.53) | 0 | No data | 3 | 0.83 (0.56, 1.24) | |||
| IL2Ra | |||||||||
| Basiliximab | 7 | 1.45 (0.73, 2.88) | 0.58 | 7 | 1.41 (0.77, 2.58) | 0.34 | 8 | 1.12 (0.87, 1.44) | 0.76 |
| Daclizumab | 2 | 0.83 (0.26, 2.66) | 2 | 0.93 (0.47, 1.85) | 4 | 1.21 (0.86, 1.70) | |||
| Other | 3 | 1.14 (0.25, 5.23) | 3 | 0.88 (0.35, 2.23) | 3 | 1.01 (0.69, 1.47) | |||
| ATG formulation | |||||||||
| Equine | 5 | 1.95 (0.51, 7.42) | 0.47 | 6 | 1.69 (0.67, 4.27) | 0.33 | 7 | 0.96 (0.73, 1.24) | 0.12 |
| Rabbit ‐thymoglobulin | 7 | 1.13 (0.62, 2.07) | 6 | 1.00 (0.64, 1.58) | 8 | 1.27 (1.00, 1.62) | |||
N = total number of studies reporting given outcome, RR = risk ratio, P = P for difference among strata, ITT = analysis by intention to treat principle, CNI= calcineurin inhibitor, IL2Ra= interleukin 2 receptor antibody, N/A = not applicable. † Test for low/mixed risk versus high risk
4. IL2Ra versus ATG: stratified meta‐analysis (cytomegalovirus disease, malignancy).
| Cytomegalovirus disease | Malignancy | |||||
| N | RR (95% CI) | P | N | RR ((95% CI) | P | |
| Publication status | ||||||
| Abstract | 2 | 0.38 (0.13. 1.13) | 0.33 | 0 | No data | N/A |
| Journal | 11 | 0.71 (0.52, 0.98) | 7 | 0.25 (0.07, 0.87) | ||
| ITT analysis | ||||||
| ITT used | 9 | 0.60 (0.37, 0.97) | 0.65 | 3 | 0.24 (0.03, 1.83) | 0.91 |
| No/ unclear | 4 | 0.79 (0.59, 1.07) | 4 | 0.24 (0.03, 1.72) | ||
| Risk of AR | ||||||
| Low | 5 | 0.61 (0.45, 0.82) | 0.34 | 2 | 0.99 (0.01, 84.05) | 0.64 |
| Mixed | 4 | 0.58 (0.32, 1.04) | 2 | 0.27 (0.03, 2.25) | ||
| High | 2 | 2.24 (1.14, 4.38) | 1 | 0.21 (0.02, 1.74) | ||
| Unclear | 2 | 0.67 (0.35, 1.28) | 2 | 0.13 (0.01, 21.76) | ||
| CNI | ||||||
| Cyclosporine | 11 | 0.72 (0.51, 1.02) | 0.39 | 6 | 0.26 (0.06, 1.08) | 0.76 |
| Tacrolimus | 2 | 0.51 (0.28, 0.93) | 1 | 0.09 (0.01, 58.07) | ||
| Unclear/mixed | 0 | No data | 0 | No data | ||
| Antimetabolite | ||||||
| Azathioprine | 4 | 0.88 (0.51, 1.52) | 0.81 | 1 | 0.04 (0.01, 24.49) | 0.59 |
| Mycophenolate | 6 | 0.76 (0.47, 1.23) | 6 | 0.27 (0.06, 1.12) | ||
| Unclear/mixed | 3 | 0.50 (0.35, 0.71) | 0 | No data | ||
| IL2Ra | ||||||
| Basiliximab | 8 | 0.66 (0.41, 1.07) | 0.86 | 5 | 0.25 (0.06, 1.06) | 0.96 |
| Daclizumab | 2 | 0.70 (0.49, 1.00) | 2 | 0.21 (0.01, 38.31) | ||
| Other | 3 | 0.80 (0.39, 1.64) | 0 | No data | ||
| ATG formulation | ||||||
| Equine | 6 | 0.70 (0.43, 1.15) | 0.86 | 2 | 0.25 (0.03, 2.05) | 0.97 |
| Rabbit ‐thymoglobulin | 7 | 0.69 (0.47, 1.03) | 5 | 0.24 (0.04, 1.57) | ||
N = total number of studies reporting given outcome, RR = risk ratio, P = P for difference among strata, ITT = analysis by intention to treat principle, CNI= calcineurin inhibitor, IL2Ra= interleukin 2 receptor antibody, N/A = not applicable.
IL2Ra versus other mono‐ or polyclonal antibody preparations
There was no difference in effect for IL2Ra compared with muromonab‐CD3 (OKT3) for all outcomes other than adverse reactions to study drug administration. No statistically significant differences in treatment effect were demonstrated for mortality, graft loss, acute rejection, or CMV infection (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7). Lacha 2001 (28 participants) showed significantly increased adverse reactions to muromonab‐CD3 administration over IL2Ra (Analysis 3.8).
3.1. Analysis.
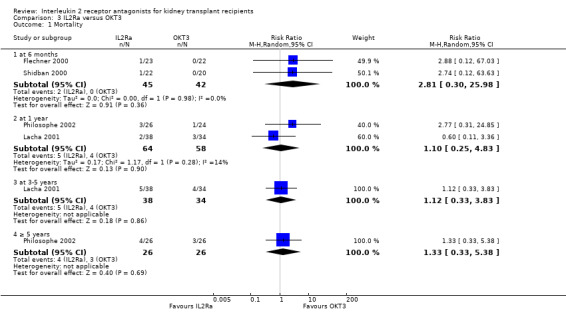
Comparison 3 IL2Ra versus OKT3, Outcome 1 Mortality.
3.2. Analysis.
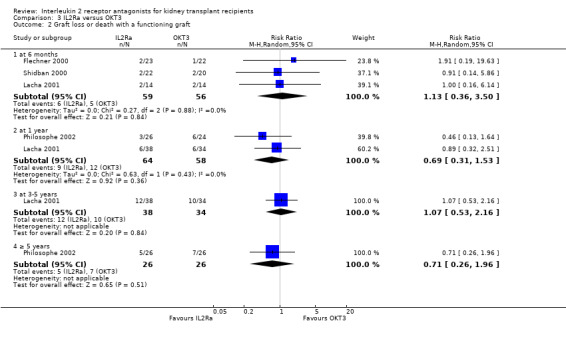
Comparison 3 IL2Ra versus OKT3, Outcome 2 Graft loss or death with a functioning graft.
3.3. Analysis.
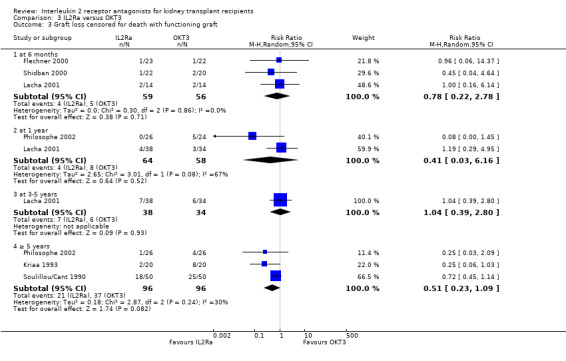
Comparison 3 IL2Ra versus OKT3, Outcome 3 Graft loss censored for death with functioning graft.
3.4. Analysis.
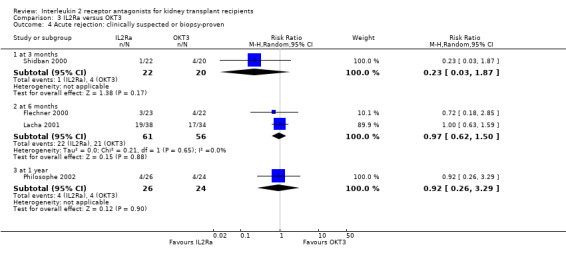
Comparison 3 IL2Ra versus OKT3, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
3.5. Analysis.
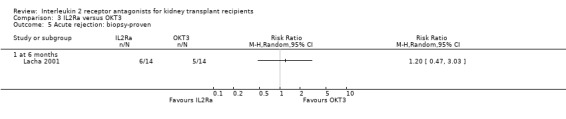
Comparison 3 IL2Ra versus OKT3, Outcome 5 Acute rejection: biopsy‐proven.
3.6. Analysis.
Comparison 3 IL2Ra versus OKT3, Outcome 6 Acute rejection: steroid resistant.
3.7. Analysis.
Comparison 3 IL2Ra versus OKT3, Outcome 7 Infection: CMV all.
3.8. Analysis.
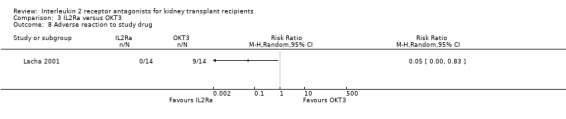
Comparison 3 IL2Ra versus OKT3, Outcome 8 Adverse reaction to study drug.
There was no difference in effect demonstrated for IL2Ra compared versus alemtuzumab for mortality, graft loss, acute rejection or CMV infection (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5).
4.1. Analysis.
Comparison 4 IL2Ra versus alemtuzumab, Outcome 1 Mortality.
4.2. Analysis.
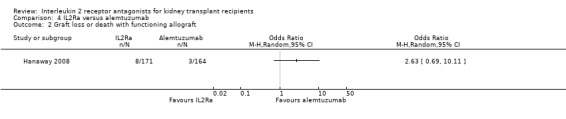
Comparison 4 IL2Ra versus alemtuzumab, Outcome 2 Graft loss or death with functioning allograft.
4.3. Analysis.
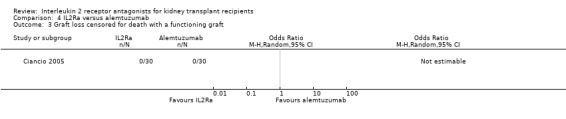
Comparison 4 IL2Ra versus alemtuzumab, Outcome 3 Graft loss censored for death with a functioning graft.
4.4. Analysis.
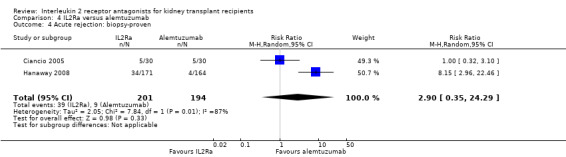
Comparison 4 IL2Ra versus alemtuzumab, Outcome 4 Acute rejection: biopsy‐proven.
4.5. Analysis.
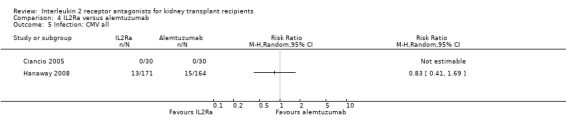
Comparison 4 IL2Ra versus alemtuzumab, Outcome 5 Infection: CMV all.
The remaining unique study comparing IL2Ra with rituximab did not show any difference in effect for any reported outcome (forest plots not shown; Clatworthy 2009).
The effect of dose of IL2Ra
The effect of one single dose versus two doses of IL2Ra and of standard versus extended dosing of IL2Ra showed no significant differences for any reported outcome (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 5.10; Analysis 5.11; Analysis 6.1; Analysis 6.2Analysis 6.3Analysis 6.4Analysis 6.5Analysis 6.6).
5.1. Analysis.
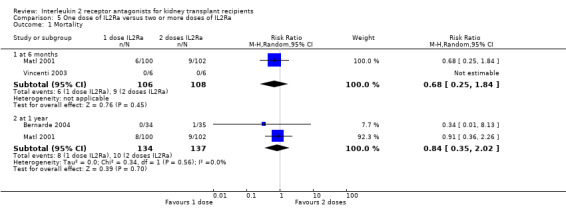
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 1 Mortality.
5.2. Analysis.
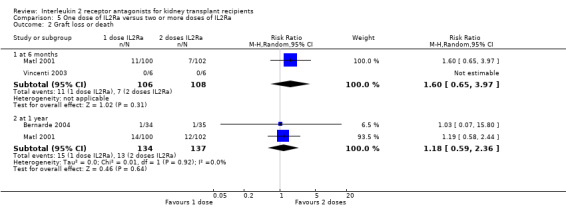
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 2 Graft loss or death.
5.3. Analysis.
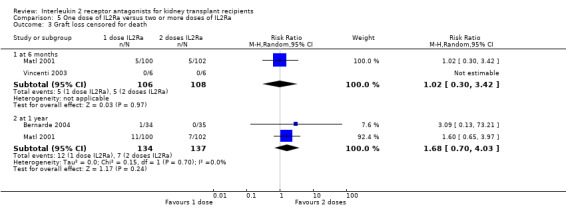
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 3 Graft loss censored for death.
5.4. Analysis.
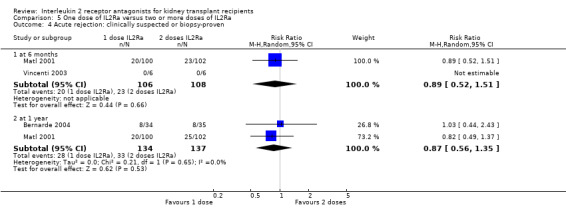
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
5.5. Analysis.
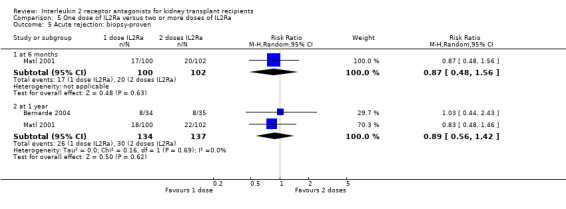
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 5 Acute rejection: biopsy‐proven.
5.6. Analysis.
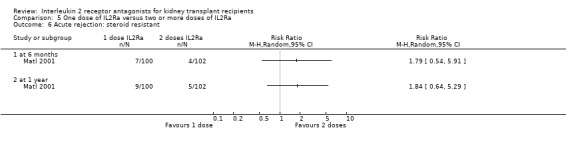
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 6 Acute rejection: steroid resistant.
5.7. Analysis.
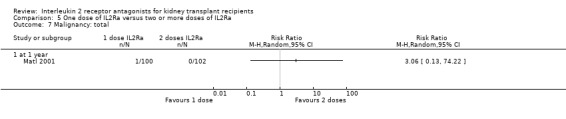
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 7 Malignancy: total.
5.8. Analysis.
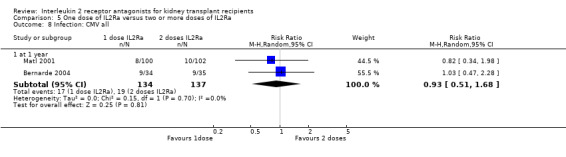
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 8 Infection: CMV all.
5.9. Analysis.
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 9 Post‐transplant diabetes mellitus (PTDM).
5.10. Analysis.
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 10 Creatinine mg/dL.
5.11. Analysis.
Comparison 5 One dose of IL2Ra versus two or more doses of IL2Ra, Outcome 11 Creatinine µmol/L.
6.1. Analysis.
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 1 Mortality.
6.2. Analysis.
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 2 Graft loss or death.
6.3. Analysis.
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 3 Graft loss censored for death.
6.4. Analysis.
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
6.5. Analysis.
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 5 Post‐transplant diabetes mellitus (PTDM).
6.6. Analysis.
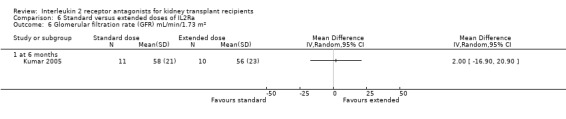
Comparison 6 Standard versus extended doses of IL2Ra, Outcome 6 Glomerular filtration rate (GFR) mL/min/1.73 m².
The comparative efficacy of different IL2Ra preparations
The five studies (Grego 2007; Khan 2000; Lin 2006; Nair 2001; Perrea 2006) comparing basiliximab and daclizumab head‐to‐head were small (total 293 participants). Outcomes were synthesised where they were reported at the same time point (Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5Analysis 7.6; Analysis 7.7; Analysis 7.8; Analysis 7.9). There were no significant differences demonstrated between basiliximab and daclizumab in head‐to‐head comparison.
7.1. Analysis.
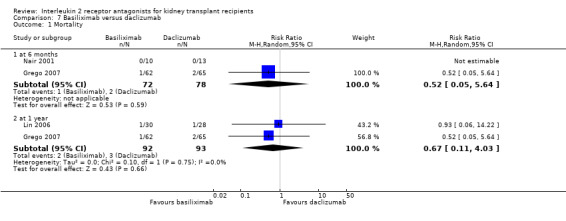
Comparison 7 Basiliximab versus daclizumab, Outcome 1 Mortality.
7.2. Analysis.
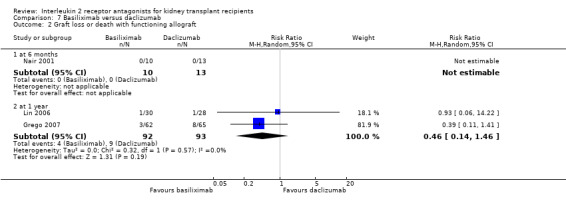
Comparison 7 Basiliximab versus daclizumab, Outcome 2 Graft loss or death with functioning allograft.
7.3. Analysis.
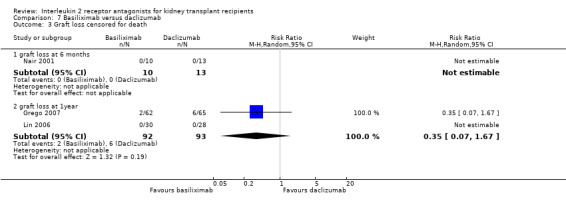
Comparison 7 Basiliximab versus daclizumab, Outcome 3 Graft loss censored for death.
7.4. Analysis.
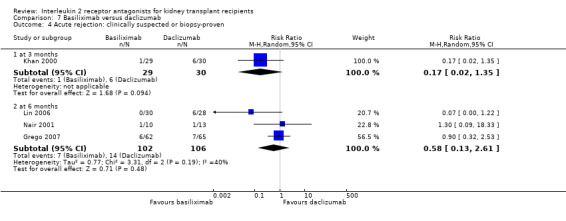
Comparison 7 Basiliximab versus daclizumab, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
7.5. Analysis.
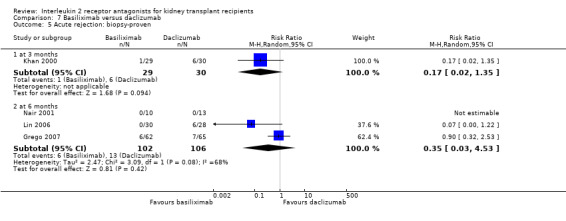
Comparison 7 Basiliximab versus daclizumab, Outcome 5 Acute rejection: biopsy‐proven.
7.6. Analysis.
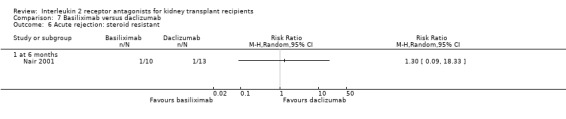
Comparison 7 Basiliximab versus daclizumab, Outcome 6 Acute rejection: steroid resistant.
7.7. Analysis.
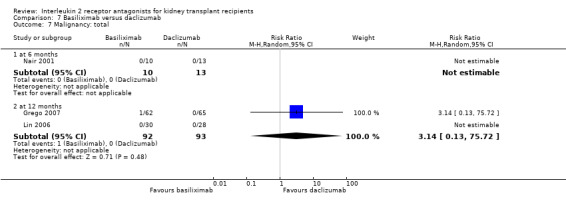
Comparison 7 Basiliximab versus daclizumab, Outcome 7 Malignancy: total.
7.8. Analysis.
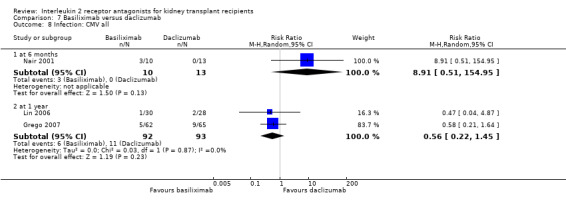
Comparison 7 Basiliximab versus daclizumab, Outcome 8 Infection: CMV all.
7.9. Analysis.
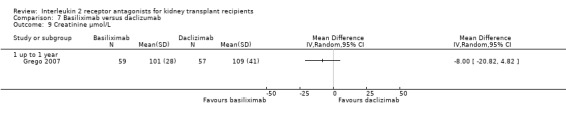
Comparison 7 Basiliximab versus daclizumab, Outcome 9 Creatinine µmol/L.
Indirect comparison, by stratifying studies according to their intervention (daclizumab or basiliximab), showed no clear difference for any outcomes. Indirect comparison of basiliximab versus daclizumab when compared to placebo/no treatment are shown in Figure 3. An indirect comparison of basiliximab versus daclizumab when compared to ATG is shown in Table 3 and Table 4 (stratified forest plots not shown).
Additional comparisons
Although four studies compared IL2Ra with calcineurin inhibitors, they were small (total 208 participants), heterogeneous in design and no more than two studies reported any outcomes and the same time point (see Characteristics of included studies for more details of Asberg 2006; Garcia 2002; Gelens 2006; Wilson 2004). There were no differences demonstrated for mortality or graft loss (Analysis 8.1; Analysis 8.2). For acute rejection there was overall benefit favouring the control arms using calcineurin inhibitors compared with IL2Ra (Analysis 8.3: RR 2.26 95% CI 1.50 to 3.41), and at six months and one year, and for study reporting GFR at one year (Analysis 8.7). There were no demonstrated differences in other outcomes (Analysis 8.4; Analysis 8.5; Analysis 8.6).
8.1. Analysis.
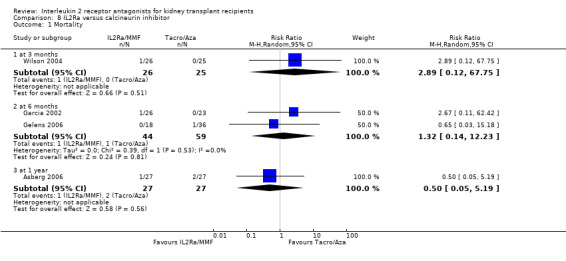
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 1 Mortality.
8.2. Analysis.
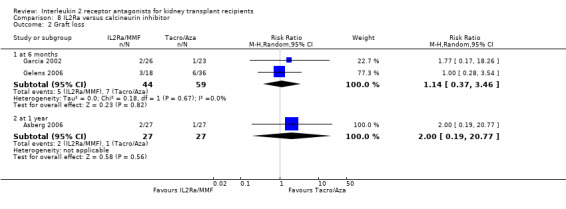
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 2 Graft loss.
8.3. Analysis.
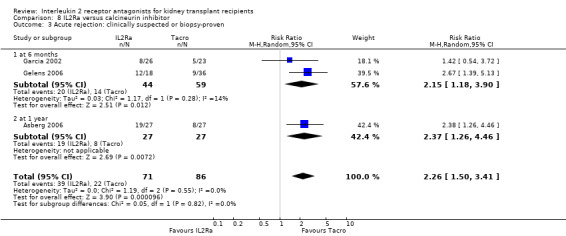
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 3 Acute rejection: clinically suspected or biopsy‐proven.
8.7. Analysis.
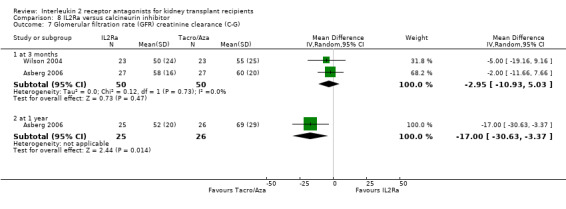
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 7 Glomerular filtration rate (GFR) creatinine clearance (C‐G).
8.4. Analysis.
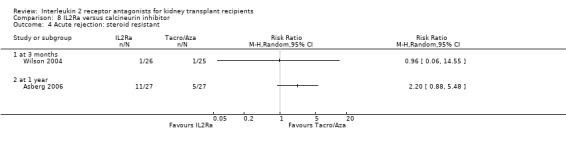
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 4 Acute rejection: steroid resistant.
8.5. Analysis.
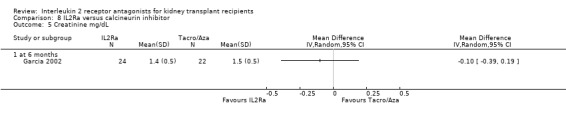
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 5 Creatinine mg/dL.
8.6. Analysis.
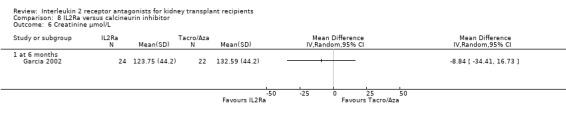
Comparison 8 IL2Ra versus calcineurin inhibitor, Outcome 6 Creatinine µmol/L.
Where studies compared IL2Ra with steroids there was no difference in mortality or graft loss (Analysis 9.1; Analysis 9.2; Analysis 9.3), but there was a significant difference in acute rejection at one year favouring use of steroids (Analysis 9.4, 2 studies: RR 1.31 95% CI 1.03 to 1.67), although this was not evident when considering only biopsy‐proven (Analysis 9.5) or steroid‐resistant rejection (Analysis 9.6). There were no differences in malignancy or GFR ( Analysis 9.7 and Analysis 9.8 respectively).
9.1. Analysis.
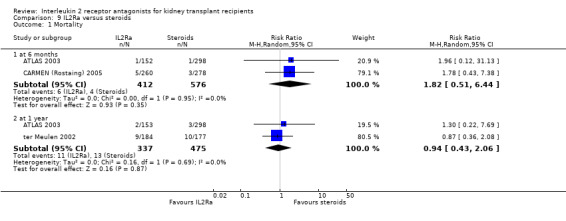
Comparison 9 IL2Ra versus steroids, Outcome 1 Mortality.
9.2. Analysis.
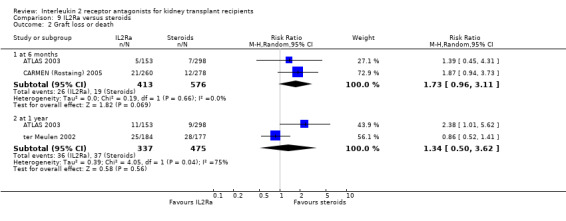
Comparison 9 IL2Ra versus steroids, Outcome 2 Graft loss or death.
9.3. Analysis.
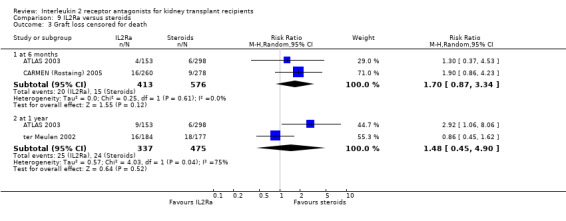
Comparison 9 IL2Ra versus steroids, Outcome 3 Graft loss censored for death.
9.4. Analysis.
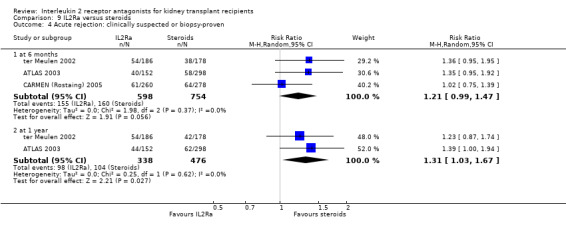
Comparison 9 IL2Ra versus steroids, Outcome 4 Acute rejection: clinically suspected or biopsy‐proven.
9.5. Analysis.
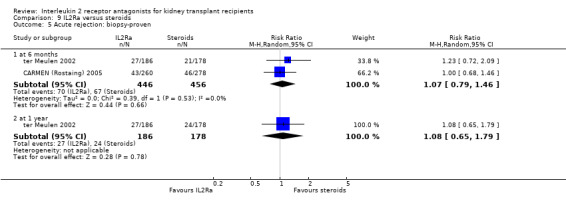
Comparison 9 IL2Ra versus steroids, Outcome 5 Acute rejection: biopsy‐proven.
9.6. Analysis.
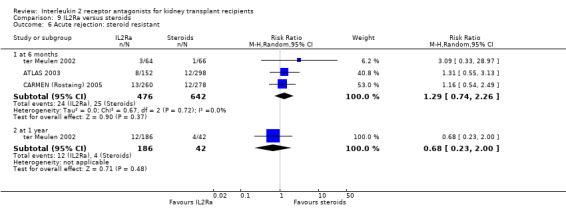
Comparison 9 IL2Ra versus steroids, Outcome 6 Acute rejection: steroid resistant.
9.7. Analysis.
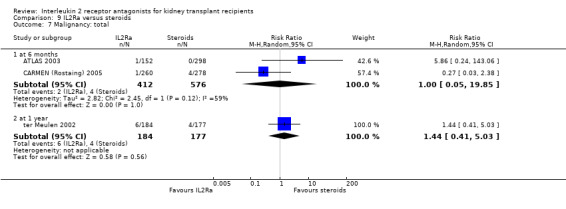
Comparison 9 IL2Ra versus steroids, Outcome 7 Malignancy: total.
9.8. Analysis.
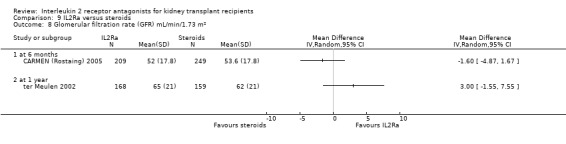
Comparison 9 IL2Ra versus steroids, Outcome 8 Glomerular filtration rate (GFR) mL/min/1.73 m².
The remaining study examined the effect of IL2Ra in a unique comparison (versus MMF, Kaplan 2003), and showed no difference in any outcomes reported, and so no further summary was possible (forest plots not shown).
Discussion
The use of an IL2Ra in addition to standard calcineurin inhibitor‐based dual or triple therapy significantly reduces graft loss, acute rejection and CMV disease within the first year post‐transplantation. At six months IL2Ra reduce early malignancy and improve graft function. This is a class effect, as there was no evidence that the effects of basiliximab and daclizumab were different. The use of an IL2Ra in place of ATG showed no difference in graft loss or in clinically diagnosed acute rejection, but did show an increase in biopsy‐proven acute rejection at one year (but not at other time points). Compared with ATG, IL2Ra use reduced incidence of CMV disease and malignancy, and improved mean serum creatinine. Recipients receiving ATG had more adverse reactions to drug administration. There was no evidence that the effects differed dependent on immunosuppressive co‐interventions, or whether the ATG was raised in horses or in rabbits. The lack of consistent outcome definitions and varied time of outcome reporting among studies hampered many more meaningful comparisons that could potentially be made.
Strengths and limitations
This meta‐analysis was undertaken with deliberately broad inclusion criteria, to better explore the totality of evidence available, and to make pragmatic comparisons that related to common clinical practice decisions. We undertook an extensive literature search, and sought data from all reports of each study we identified. This update re‐organised data comparisons from their presentation in the original review (Webster 2004), by splitting ATG comparisons away from those with other mono‐ or polyclonal antibodies. We added a succinct exploration of subgroup effects to explore potential differences that might results from other study design features or settings such as co‐interventions or population baseline immunological risk. We also added new outcomes relating to transplant function (serum creatinine and GFR). The results demonstrated a remarkable consistency and homogeneity of effect for IL2Ra over a large number of diverse outcomes. The review update was able to confirm differences in effect for important clinical outcomes that were hinted at, but not proven, in the original review. An example is graft loss which moved from 14 studies showing RR of 0.83 (95% CI 0.66 to 1.04) in the original review, to 24 studies showing RR of 0.75 (95% CI 0.62 to 0.90). Hence, new findings include a significant reduction in graft loss, and in CMV disease and malignancy for those treated with IL2Ra compared to placebo/no treatment. Similarly, with new evidence, the comparison of IL2Ra with ATG was more informative.
Despite these strengths, there was still insufficient power to show definite reduction in some important outcomes through all time points, and inconsistent reporting of important outcomes hampered interpretation. Although 16 studies with 2,211 participants compared IL2Ra with ATG, only 10 studies reported acute rejection diagnosed clinically or by biopsy at one year, only eight studies reported biopsy‐proven rejection, and only six studies reported steroid‐resistant rejection. Hence, we cannot be sure what outcomes were experienced by participants in the studies that provided no data. Although we believe this is the most comprehensive evidence summary on this topic, use of these results must acknowledge the evident limitations of the data available from this study cohort.
As in the original review, the applicability of the meta‐analysis results to other populations and settings may be limited by the circumstances of the constituent studies. This update included more data for recipients at higher baseline risk of acute rejection than the original review, but many studies included participants of mixed immunological risk and did not provide stratified results, so power to investigate potential differences was thus reduced. One possible way to clarify these residual doubts and uncertainties, would be through increased access to transparent study outcome dataset, and by use of standardised outcome definitions. Individual patient data meta‐analysis would likely be informative. However, the high level of homogeneity of results among RCTs for the majority of outcomes, particularly the primary outcomes of graft loss and acute rejection, suggests that the results are likely to be generalisable to populations of greater and lesser risk. The relative under‐reporting of treatment harms compared with treatment benefits, and the incomplete data presented is not a problem peculiar to this review, and is widely recognised as common to many RCTs and systematic reviews (Cuervo 2003).
In an attempt to minimise publication bias, this meta‐analysis included both unpublished data and data from conference abstracts. We also made strenuous efforts to include non‐English language sources. In the update, 25/189 (13.2%) new reports came from handsearching conference proceedings over and above those already searched by the specialised register of the Cochrane Renal Group. We examined funnel plots of the key outcomes (mortality, graft loss censored for death, acute rejection, CMV disease and malignancy) for asymmetry that might suggest potential publication bias (not all included other than Figure 3 because of size and complexity constraints on the review as a whole). This was done in recognition that confining a meta‐analysis to published data or English language alone has been demonstrated to over‐estimate positive treatment effects (Egger 1997).
The internal validity of the design, conduct and analysis of the included RCTs was difficult to assess because of the omission of important methodological details in the study reports. No single study adequately reported all domains of the risk of bias assessment (Figure 2), despite using information from many data sources and attempting author contact to try to clarify these details. Thus it is impossible to exclude the possibility that elements of internal biases may be present in the results of the meta‐analysis (Begg 1996; Moher 1999).
Authors' conclusions
Implications for practice.
IL2Ra show significant benefit in reducing acute rejection, graft loss, CMV disease and early malignancy, but not mortality in kidney transplant recipients when added to standard calcineurin‐based therapy. IL2Ra compared with ATG reduce CMV disease, malignancy and cause significantly fewer side effects, with no differences in graft loss or clinically diagnosed or steroid‐resistant rejection, but an increase at one year of biopsy‐proven rejection. Basiliximab and daclizumab are equally effective. Thus, the benefits and harms of adding IL2Ra use outweigh standard therapy alone, but choice of IL2Ra over ATG may be different for different patients. The applicability of the findings of this updated review are summarised in Table 5, which demonstrates that in adding IL2Ra to standard calcineurin based therapy, for every 100 people treated, within the first year, two fewer will loose their graft, 11 fewer will experience acute rejection, and two fewer will experience CMV disease. The number needed to treat with IL2Ra to prevent one person losing their graft is 42, nine for acute rejection, and 38 for CMV disease.
5. Applicability in clinical practice.
| Graft loss | Acute rejection | |||||||
| IL2Ra | Control | Difference | NNT# | Il2Ra | Control | Difference | NNT | |
| IL2Ra versus placebo | 6 | 8 | ↓ 2 | 42 | 27 | 38 | ↓ 11 | 9 |
| IL2Ra versus ATG | 7 | 6 | ns | ‐ | 24 | 21 | ns | ‐ |
| Cytomegalovirus disease | Malignancy | |||||||
| IL2Ra versus placebo | 13 | 15 | ↓ 2 | 38 | 1 | 2 | ns | ‐ |
| IL2Ra versus ATG | 16 | 24 | ↓ 8 | 16 | 0 | 2 | 2 | 58 |
Projected numbers of transplant recipients* experiencing graft loss censored for death, acute rejection, experiencing cytomegalovirus disease and their malignancy within 1 year of transplantation per hundred patients treated with IL2Ra.
* calculated as absolute risk reduction/increase per 100 people treated with IL2Ra using summary rate in control (comparator) arms of studies compared to that in the investigative (IL2Ra) arm of studies. ‘ns’ = difference not statistically significant (i.e. summary RR confidence intervals cross 1.00).
# number needed to be treated with IL2Ra to cause 1 person to experience difference in the direction noted. Number needed not given where difference between IL2Ra and comparator arms was not significantly different.
In using IL2Ra over ATG, when treating 100 people, there will be no difference in graft loss or overall rejection, but eight fewer with CMV disease (number needed to treat to prevent one case is 16). However, although differences in malignancy are significantly different, within the first year the absolute risk of early malignancy is small, so per 100 people treated there will be no a difference of two, and the number needed to treat to prevent one case of cancer is 58.
In using these relative and absolute measures of effect it is clear that different treatment decisions may be appropriate for different patients.
Implications for research.
The updated review findings will permit a further economic evaluation, using more recent and precise evidence than was previously possible Morton 2009.
Despite the homogeneity of results across the populations of the pooled studies, there was under representation of high risk participants and in particular of children. The availability of the full study datasets would permit individual patient data meta‐analysis, and would be an economical way of using existing data more effectively. Failing this, future studies involving younger patients, and those at higher baseline risk of acute rejection would enhance certainty of benefit in this subgroup. The importance of follow‐up prolonged beyond one year cannot be over emphasised, particularly to clarify the risks and eventual outcome of harms from differing immunosuppressive treatment strategies. Where this cannot be achieved in an RCT, inclusion of information that could form a linkage key, would permit a hybrid design of RCT with an observational cohort, allowing later linkage with longer term follow‐up data, perhaps from a registry or from administrative hospital records. This is an under‐exploited method to gain valuable medium and longer term data that would otherwise be unknown.
Many of the uncertainties of the meta‐analysis might be clarified if meta‐analysis of individual patient data were possible. This would increase the statistical power of the analysis, and thus might clarify the estimates of effect which approach, but do not reach, statistical significance, and clarify subgroups effects are consistent with overall findings. Individual data analysis would also allow time‐to‐event data to be incorporated more easily, and allow more flexible analysis of patient subgroups and outcomes. However, if complete data were not available from all RCTs, then analysis of only selected data would obviously risk the introduction of bias to the estimates (Clarke 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 2 May 2014 | Amended | Study names amended to match the Renal Group's Specialised Register |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 18 February 2010 | Amended | New data available, no change to conclusions |
| 31 October 2009 | New citation required and conclusions have changed | Complete update of review, 33 new studies added |
Notes
Issue 3, 2010: New data available, no change to conclusions
Issue 1, 2010: The risk of bias assessment tool was used for this update and applied to all 71 studies (38 from original review and 33 new studies)
Acknowledgements
We are grateful to Dr EG Playford who contributed to the original iteration of this review (Issue 1, 2004), contributing to the design, quality assessment, data collection, entry, analysis and interpretation, and writing.
The authors wish to thank all report authors who responded to our enquiries about their work, and especially Drs N Ahsan, D Brennan, H Ekberg, I Folkmane, J Kovarik, A Kumar, G Mourad, B Nashan, S Sandrini, H Sheashaa, H Shidban, R Stratta and LB Zimmerhackl, who were particularly helpful in providing additional information and data.
Appendices
Appendix 1. Electronic search strategies
| Database searched | Search terms |
| Cochrane Renal Group Specialised Register | The following terms were used: Kidney transplant, kidney allograft, graft rejection, interleukin 2 receptor antagonists, basiliximab, daclizumab, simulect, zenapax together with register codes used to identify studies relevant to this review. |
| CENTRAL |
|
| MEDLINE | 1. Kidney Transplantation/ 2. basiliximab.tw. 3. daclizumab.tw. 4. zenapax.tw. 5. cd25.tw. 6. cd 25.tw. 7. bt563.tw. 8. simulect.tw. 9. exp Receptors, Interleukin‐2/ 10. exp Antibodies, Monoclonal/ 11. interleukin‐2 receptor$.tw. 12. (interleukin 2 adj10 antagoni$).tw. 13. il2.tw. 14. il 2.tw. 15. il2R.tw. 16. il 2R.tw. 17. il 2 R.tw. 18. monoclonal antibod$.tw. 19. or/2‐18 20. 1 and 19 |
| EMBASE | 1. exp Interleukin 2 Receptor Antibody/ 2. basiliximab.tw. 3. daclizumab.tw. 4. dacliximab.tw. 5. cd25.tw. 6. cd 25.tw. 7. bt563.tw. 8. simulect.tw. 9. zenapax.tw. 10. interleukin‐2 receptor$.tw. 11. (interleukin 2 adj10 antagonist$).tw. 12. (interleukin‐2 adj10 antibod$).tw. 13. il2.tw. 14. il‐2.tw. 15. il2r.tw. 16. il‐2r.tw. 17. il‐2‐r.tw. 18. or/1‐17 19. exp Kidney Transplantation/ 20. 18 and 19 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Was there adequate sequence generation? | Yes (low risk of bias): Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| No (high risk of bias): Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Was allocation adequately concealed? | Yes (low risk of bias): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| No (high risk of bias): Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Was knowledge of the allocated interventions adequately prevented during the study? | Yes (low risk of bias): No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. |
| No (high risk of bias): No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Were incomplete outcome data adequately addressed? | Yes (low risk of bias): No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| No (high risk of bias): Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Are reports of the study free of suggestion of selective outcome reporting? | Yes (low risk of bias): The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| No (high risk of bias): Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Was the study apparently free of other problems that could put it at a risk of bias? | Yes (low risk of bias): The study appears to be free of other sources of bias. |
| No (high risk of bias): Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. |
Data and analyses
Comparison 1. IL2Ra versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 3 months | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.13, 75.82] |
| 1.2 at 6 months | 15 | 2919 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.45, 1.40] |
| 1.3 at 1 year | 24 | 4647 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.54, 1.10] |
| 1.4 at 3 years | 4 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.30, 1.29] |
| 1.5 at ≥ 5 years | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 2 Graft loss or death with functioning allograft | 29 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 3 months | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.06] |
| 2.2 at 6 months | 16 | 3017 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.58, 0.98] |
| 2.3 at 1 year | 24 | 4672 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.62, 0.90] |
| 2.4 at 3‐5 years | 4 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.22] |
| 2.5 ≥ 5 years | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.39, 2.05] |
| 3 Graft loss censored for death with functioning graft | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 3 months | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.48] |
| 3.2 at 6 months | 17 | 3048 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 3.3 at 1 year | 24 | 4672 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.93] |
| 3.4 at 3‐5 years | 4 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.59] |
| 3.5 ≥ 5 years | 3 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.52, 4.37] |
| 3.6 Any time within the first year | 29 | 5527 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 4 Acute rejection: clinically suspected or biopsy proven | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 3 months | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.21, 0.59] |
| 4.2 at 6 months | 19 | 4751 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.63, 0.76] |
| 4.3 at 1 year | 20 | 4300 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.66, 0.78] |
| 4.4 Any time within the first year | 30 | 5577 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.64, 0.76] |
| 5 Acute rejection: biopsy‐proven | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 at 3 months | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.68] |
| 5.2 at 6 months | 15 | 4451 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.62, 0.76] |
| 5.3 at 1 year | 14 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.64, 0.81] |
| 6 Acute rejection: steroid resistant | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 at 3 months | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.74] |
| 6.2 at 6 months | 9 | 1928 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.39, 0.68] |
| 6.3 at 1 year | 6 | 1834 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.92] |
| 7 Malignancy: total | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 8 | 1878 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.86] |
| 7.2 at 1 year | 15 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 7.3 at 3‐5 years | 3 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.45, 1.53] |
| 7.4 ≥ 5 years | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.17, 6.80] |
| 7.5 Any time within the first year | 19 | 4860 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.42, 1.28] |
| 8 PTLD/lymphoma | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 at 3 months | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 at 6 months | 6 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.17] |
| 8.3 at 1 year | 8 | 2481 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.10, 2.12] |
| 8.4 at 3 years | 1 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.71] |
| 8.5 ≥ 5 years | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.12, 68.50] |
| 8.6 Any time within the first year | 13 | 3864 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.29] |
| 9 Infection: CMV all | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 at 3 months | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 5.09] |
| 9.2 at 6 months | 9 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.21] |
| 9.3 at 1 year | 13 | 3169 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.97] |
| 9.4 at 3 years | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.72] |
| 9.5 ≥ 5 years | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.26, 3.78] |
| 9.6 Any time within the first year | 17 | 3767 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 10 Infection: CMV invasive | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 at 6 months | 3 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.38, 2.78] |
| 10.2 at 1 year | 5 | 1070 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.61, 1.41] |
| 11 Infection: serious all‐cause total | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 at 3 months | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.63, 1.50] |
| 11.2 at 6 months | 8 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.10] |
| 11.3 at 1 year | 9 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 12 Post‐transplant diabetes mellitus (PTDM) | 4 | 1372 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.51, 2.12] |
| 12.1 at 1 year | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.43, 5.33] |
| 12.2 at 5 years | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.18, 1.83] |
| 13 Adverse reaction | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
| 13.1 All adverse reactions at 6 months | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
| 14 Creatinine mg/dL | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 at 1 month | 4 | 654 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.37, ‐0.11] |
| 14.2 at 3 months | 7 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.18, ‐0.03] |
| 14.3 at 6 months | 7 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.16, ‐0.02] |
| 14.4 at 1 year | 8 | 1135 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.15, 0.04] |
| 14.5 at 2 years | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 14.6 at 3 years | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.13] |
| 15 Creatinine µmol/L | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 at 1 month | 4 | 646 | Mean Difference (IV, Random, 95% CI) | ‐21.45 [‐33.03, ‐9.86] |
| 15.2 at 3 months | 7 | 820 | Mean Difference (IV, Random, 95% CI) | ‐7.33 [‐13.58, ‐1.08] |
| 15.3 at 6 months | 7 | 1231 | Mean Difference (IV, Random, 95% CI) | ‐8.18 [‐14.28, ‐2.09] |
| 15.4 at 1 year | 8 | 1135 | Mean Difference (IV, Random, 95% CI) | ‐5.31 [‐13.90, 3.28] |
| 15.5 at 2 years | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐35.0 [‐65.21, ‐4.79] |
| 15.6 at 3 years | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐4.42 [‐20.51, 11.67] |
| 16 Glomerular filtration rate (GFR) mL/min/1.73 m² | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 at 1 month | 1 | 340 | Mean Difference (IV, Random, 95% CI) | 4.03 [‐1.14, 9.20] |
| 16.2 at 3 months | 2 | 359 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐3.97, 4.45] |
| 16.3 at 6 months | 2 | 571 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐2.27, 5.89] |
| 16.4 at 1 year | 5 | 2247 | Mean Difference (IV, Random, 95% CI) | 2.61 [0.45, 4.78] |
1.1. Analysis.
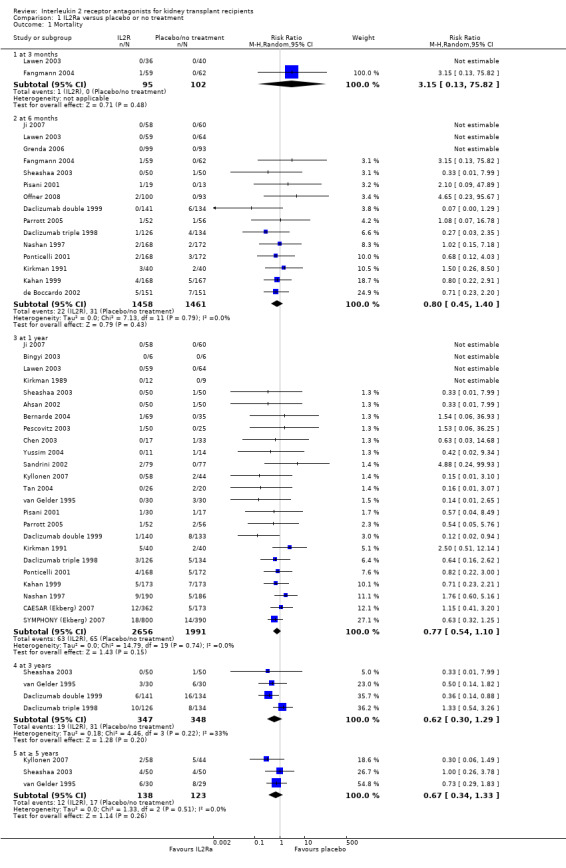
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 1 Mortality.
1.8. Analysis.
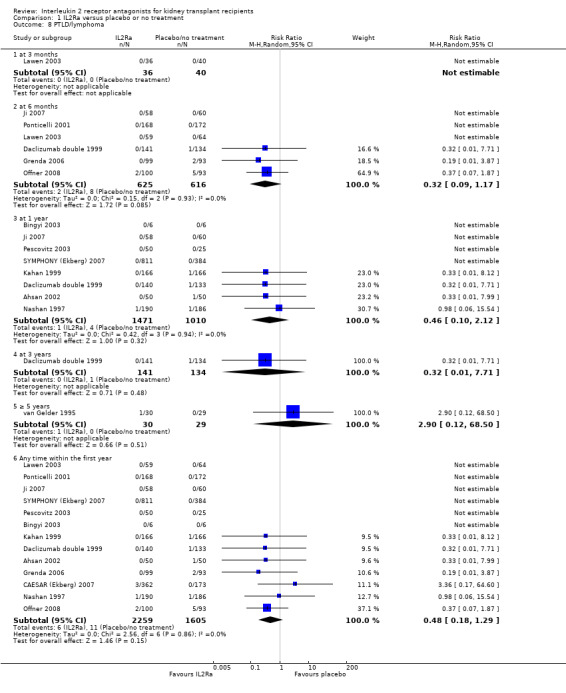
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 8 PTLD/lymphoma.
1.14. Analysis.
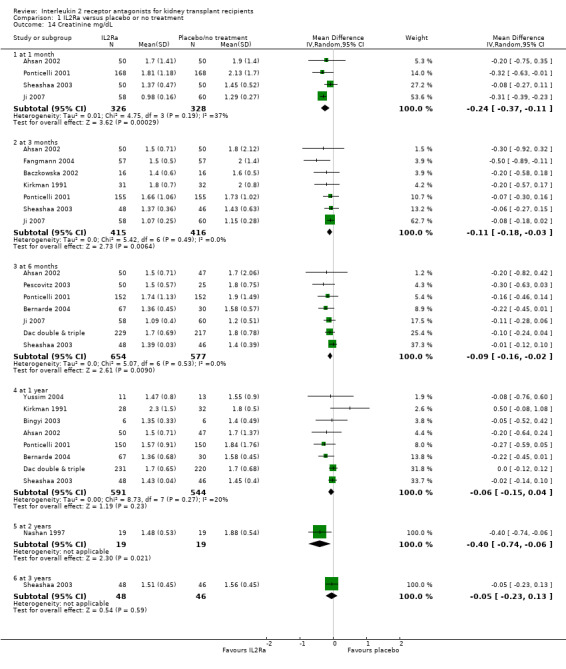
Comparison 1 IL2Ra versus placebo or no treatment, Outcome 14 Creatinine mg/dL.
Comparison 2. IL2Ra versus ATG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 7 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.65, 4.72] |
| 1.2 at 1 year | 12 | 1609 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.77, 2.25] |
| 1.3 at 3‐5 years | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.58, 5.51] |
| 1.4 ≥ 5 years | 5 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.61] |
| 2 Graft loss or death with a functioning graft | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 3 months | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.07, 11.87] |
| 2.2 at 6 months | 6 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.78, 3.49] |
| 2.3 at 1 year | 12 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.49] |
| 2.4 at 2 years | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.64, 2.25] |
| 2.5 at 3‐5 years | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.27, 8.77] |
| 2.6 ≥ 5 years | 4 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.13] |
| 3 Graft loss censored for death with functioning graft | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 3 months | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.08, 6.50] |
| 3.2 at 6 months | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.34] |
| 3.3 at 1 year | 12 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 3.4 at 2 years | 4 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.52, 2.41] |
| 3.5 at 3‐5 years | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.19, 21.79] |
| 3.6 ≥ 5 years | 4 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 3.7 Any time within the first year | 12 | 1402 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.73, 1.65] |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 3 months | 5 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.59] |
| 4.2 at 6 months | 8 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.34] |
| 4.3 at 1 year | 10 | 1290 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.96, 1.44] |
| 4.4 Any time within the first year | 15 | 1571 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.33] |
| 5 Acute rejection: biopsy‐proven | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 at 3 months | 3 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.75, 1.80] |
| 5.2 at 6 months | 5 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.79, 2.00] |
| 5.3 at 1 year | 8 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.01, 1.67] |
| 5.4 at 1‐5 years | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.98, 3.18] |
| 6 Acute rejection: steroid resistant | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 at 6 months | 2 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.95] |
| 6.2 at 1 year | 6 | 915 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.95, 5.27] |
| 6.3 at 3 years | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.47, 5.89] |
| 7 Malignancy: total | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.15] |
| 7.2 at 1 year | 7 | 1067 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.07, 0.87] |
| 7.3 at 3‐5 years | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.15, 2.91] |
| 7.4 ≥ 5 years | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.30, 5.85] |
| 8 PTLD/lymphoma | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 At 1 year | 5 | 855 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.82] |
| 8.2 At 3 years | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.00, 2.07] |
| 8.3 At ≥ 5 years | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.35] |
| 9 Infection: CMV all | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 at 3 months | 3 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.31] |
| 9.2 at 6 months | 5 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.10] |
| 9.3 at 1 year | 9 | 1230 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.48, 1.07] |
| 9.4 at 2 years | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.16, 1.27] |
| 9.5 ≥ 5 years | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.26, 6.56] |
| 9.6 Any within the first year | 13 | 1647 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 10 Infection: CMV invasive | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 at 3 months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.02, 1.65] |
| 10.2 at 6 months | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.35, 15.46] |
| 10.3 at 1 year | 2 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.59, 6.09] |
| 10.4 at 3 years | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.35, 2.09] |
| 11 Post‐transplant diabetes mellitus (PTDM) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 at 1 year | 3 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.28, 3.72] |
| 12 Reactions to drug administration | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Fever | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 1.00] |
| 12.2 Cytokine release syndrome | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.60] |
| 12.3 Other adverse reactions | 5 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.09, 0.91] |
| 13 Haematological adverse reactions | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Leucopenia | 4 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.60] |
| 13.2 Thrombocytopenia | 3 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.67] |
| 14 Creatinine mg/dL | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 at 1 month | 3 | 293 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.32, 0.04] |
| 14.2 at 2 months | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.04] |
| 14.3 at 3 months | 3 | 226 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.18, 0.11] |
| 14.4 at 6 months | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.23, ‐0.02] |
| 14.5 at 1 year | 6 | 580 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.20, ‐0.01] |
| 14.6 at 3 years | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.19, 0.18] |
| 14.7 at 5 years | 3 | 207 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.13, 0.22] |
| 15 Creatinine µmol/L | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 at 1month | 3 | 293 | Mean Difference (IV, Random, 95% CI) | ‐12.37 [‐28.51, 3.76] |
| 15.2 at 2 months | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐13.40 [‐29.87, 3.07] |
| 15.3 at 3 months | 4 | 289 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐15.13, 8.66] |
| 15.4 at 6 months | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐11.02 [‐19.94, ‐2.09] |
| 15.5 at 1 year | 6 | 586 | Mean Difference (IV, Random, 95% CI) | ‐8.84 [‐17.23, ‐0.45] |
| 15.6 at 3 years | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐16.75, 15.66] |
| 15.7 at 5 years | 3 | 211 | Mean Difference (IV, Random, 95% CI) | 3.45 [‐11.84, 18.74] |
| 16 Glomerular filtration rate (GFR) mL/min/1.73 m² | 2 | 614 | Mean Difference (IV, Random, 95% CI) | 6.70 [1.63, 11.77] |
| 16.1 at 3 months | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 8.55 [3.64, 13.46] |
| 16.2 at 1 year | 1 | 191 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐3.38, 6.58] |
| 16.3 at 2 years | 1 | 205 | Mean Difference (IV, Random, 95% CI) | 10.0 [4.85, 15.15] |
2.8. Analysis.
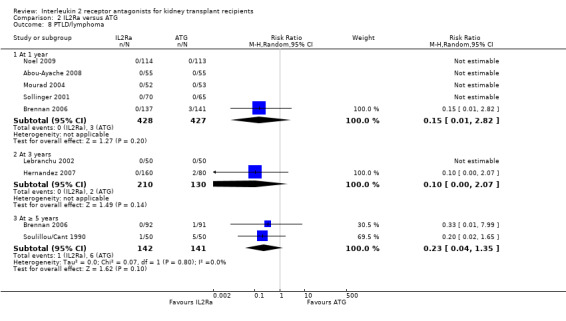
Comparison 2 IL2Ra versus ATG, Outcome 8 PTLD/lymphoma.
2.10. Analysis.
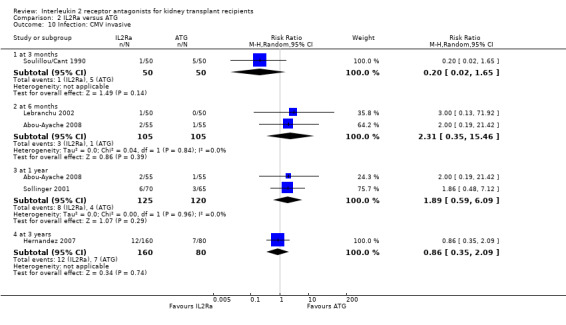
Comparison 2 IL2Ra versus ATG, Outcome 10 Infection: CMV invasive.
2.11. Analysis.
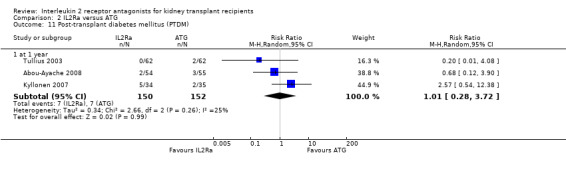
Comparison 2 IL2Ra versus ATG, Outcome 11 Post‐transplant diabetes mellitus (PTDM).
2.14. Analysis.
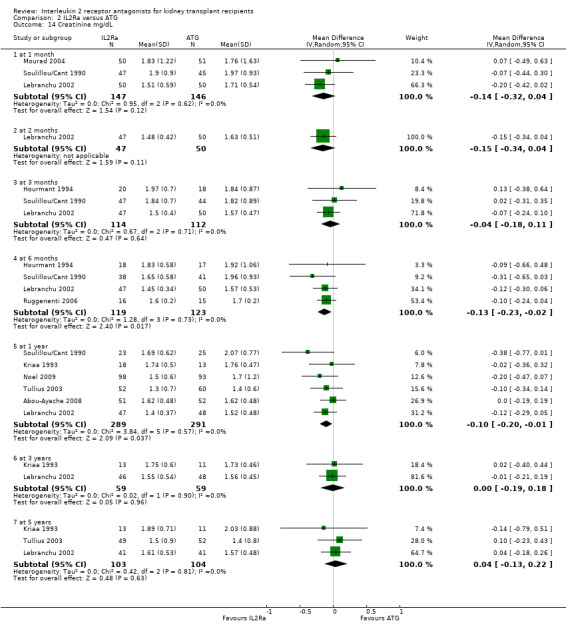
Comparison 2 IL2Ra versus ATG, Outcome 14 Creatinine mg/dL.
Comparison 3. IL2Ra versus OKT3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.30, 25.98] |
| 1.2 at 1 year | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.25, 4.83] |
| 1.3 at 3‐5 years | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.33, 3.83] |
| 1.4 ≥ 5 years | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.38] |
| 2 Graft loss or death with a functioning graft | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.36, 3.50] |
| 2.2 at 1 year | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.31, 1.53] |
| 2.3 at 3‐5 years | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.53, 2.16] |
| 2.4 ≥ 5 years | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.26, 1.96] |
| 3 Graft loss censored for death with functioning graft | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 6 months | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.22, 2.78] |
| 3.2 at 1 year | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.03, 6.16] |
| 3.3 at 3‐5 years | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.39, 2.80] |
| 3.4 ≥ 5 years | 3 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.09] |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 3 months | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.87] |
| 4.2 at 6 months | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.62, 1.50] |
| 4.3 at 1 year | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.26, 3.29] |
| 5 Acute rejection: biopsy‐proven | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Acute rejection: steroid resistant | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Infection: CMV all | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.83] |
| 7.2 Any within the first year | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.83] |
| 8 Adverse reaction to study drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Creatinine mg/dL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Creatinine µmol/L | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.9. Analysis.
Comparison 3 IL2Ra versus OKT3, Outcome 9 Creatinine mg/dL.
3.10. Analysis.
Comparison 3 IL2Ra versus OKT3, Outcome 10 Creatinine µmol/L.
Comparison 4. IL2Ra versus alemtuzumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.29, 12.87] |
| 2 Graft loss or death with functioning allograft | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Graft loss censored for death with a functioning graft | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Acute rejection: biopsy‐proven | 2 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.35, 24.29] |
| 5 Infection: CMV all | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. One dose of IL2Ra versus two or more doses of IL2Ra.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.84] |
| 1.2 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.02] |
| 2 Graft loss or death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.65, 3.97] |
| 2.2 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.59, 2.36] |
| 3 Graft loss censored for death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 6 months | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 3.2 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.70, 4.03] |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 6 months | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.52, 1.51] |
| 4.2 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.35] |
| 5 Acute rejection: biopsy‐proven | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 at 6 months | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.56] |
| 5.2 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 6 Acute rejection: steroid resistant | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Malignancy: total | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Infection: CMV all | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 at 1 year | 2 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.51, 1.68] |
| 9 Post‐transplant diabetes mellitus (PTDM) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Creatinine mg/dL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 at 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Creatinine µmol/L | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 at 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Standard versus extended doses of IL2Ra.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Graft loss or death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Graft loss censored for death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Post‐transplant diabetes mellitus (PTDM) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Glomerular filtration rate (GFR) mL/min/1.73 m² | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Basiliximab versus daclizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.64] |
| 1.2 at 1 year | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.03] |
| 2 Graft loss or death with functioning allograft | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 at 1 year | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.46] |
| 3 Graft loss censored for death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 graft loss at 6 months | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 graft loss at 1year | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.67] |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 3 months | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.35] |
| 4.2 at 6 months | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.13, 2.61] |
| 5 Acute rejection: biopsy‐proven | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 at 3 months | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.35] |
| 5.2 at 6 months | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.03, 4.53] |
| 6 Acute rejection: steroid resistant | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Malignancy: total | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 at 12 months | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 75.72] |
| 8 Infection: CMV all | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 at 6 months | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 8.91 [0.51, 154.95] |
| 8.2 at 1 year | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.45] |
| 9 Creatinine µmol/L | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 up to 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. IL2Ra versus calcineurin inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 3 months | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 67.75] |
| 1.2 at 6 months | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.14, 12.23] |
| 1.3 at 1 year | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.19] |
| 2 Graft loss | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.37, 3.46] |
| 2.2 at 1 year | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.77] |
| 3 Acute rejection: clinically suspected or biopsy‐proven | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.50, 3.41] |
| 3.1 at 6 months | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.18, 3.90] |
| 3.2 at 1 year | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.26, 4.46] |
| 4 Acute rejection: steroid resistant | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 at 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Creatinine mg/dL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Creatinine µmol/L | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Glomerular filtration rate (GFR) creatinine clearance (C‐G) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 at 3 months | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐10.93, 5.03] |
| 7.2 at 1 year | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐17.0 [‐30.63, ‐3.37] |
Comparison 9. IL2Ra versus steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 2 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.51, 6.44] |
| 1.2 at 1 year | 2 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.43, 2.06] |
| 2 Graft loss or death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 2 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.96, 3.11] |
| 2.2 at 1 year | 2 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.50, 3.62] |
| 3 Graft loss censored for death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 at 6 months | 2 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.87, 3.34] |
| 3.2 at 1 year | 2 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.45, 4.90] |
| 4 Acute rejection: clinically suspected or biopsy‐proven | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 at 6 months | 3 | 1352 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.47] |
| 4.2 at 1 year | 2 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.03, 1.67] |
| 5 Acute rejection: biopsy‐proven | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 at 6 months | 2 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.79, 1.46] |
| 5.2 at 1 year | 1 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.65, 1.79] |
| 6 Acute rejection: steroid resistant | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 at 6 months | 3 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.74, 2.26] |
| 6.2 at 1 year | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.23, 2.00] |
| 7 Malignancy: total | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 2 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.05, 19.85] |
| 7.2 at 1 year | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.41, 5.03] |
| 8 Glomerular filtration rate (GFR) mL/min/1.73 m² | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 at 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abou‐Ayache 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "centrally randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Open‐label, however for acute rejection "a blinded centralized analysis was carried out by two pathologists". |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis reported, however (1) 3 patients excluded post‐randomisation due to no transplant and or treatment, (2) 2 patients excluded after one dose of intervention but no transplant, (3) 1 excluded due to receipt of poor quality graft due to ecstasy abuse, and (4) 8 excluded form "on therapy population" due to protocol violation |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | High risk | "...supported by a grant from ROCHE ‐ France *Trial name: ECTAZ; protocol identification: 010624; date of registration: 16 July 2001, without restrictions on publication." |
Ahsan 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly selected" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | For acute rejection "All biopsies were reviewed by a pathologist unaware of the protocol" |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated who assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for at 6 months (acute rejection) and 12 months (death and graft loss) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Unclear risk | Funding source not stated |
Asberg 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized in a 1:1 ratio", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Supported by a grant from Roche Norway AS |
ATLAS 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group 1
Control group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “the randomisation list was generated by the Data Operations department” "stratified by centre” |
| Allocation concealment (selection bias) | Low risk | “each centre received a unique sequence of patient numbers and a set of sealed envelopes” ”the corresponding envelopes were opened providing the information for the allocated treatment” |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for. 6/457 excluded ‐ never received transplant or study drug ‐ unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Sponsored by a grant from Fujisawa GmbH |
Baczkowska 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised, controlled study" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open label |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients followed or accounted for, however additional patients reported in 2008 abstract |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however additional patients reported in 2008 abstract. No study protocol available to assess secondary outcomes of study |
| Other bias | Unclear risk | Funding source not stated |
Bernarde 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment groups
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data only available from conference proceedings abstract |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) reported, however data only available from conference proceedings abstract |
| Other bias | Unclear risk | Funding source not stated, data only available from conference proceedings abstract |
Bingyi 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly allocated", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers of patients at end of study not reported |
| Selective reporting (reporting bias) | High risk | For this review, only acute rejection was reported at 12 months, death and graft loss not stated and numbers at end of study not reported |
| Other bias | High risk | Supported by Novartis |
Brennan 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment 1 group
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated "1:1 variable‐block randomization" used |
| Allocation concealment (selection bias) | Low risk | Stated "The treatment assignments were randomized at an independent centre" |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for at 12 months |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | High risk | "The design, data collection, and analysis were performed by a sponsor, Genzyme, which hold the primary data" |
CAESAR (Ekberg) 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment groups (group 1 and 2)
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code... generated in the Oracle Clinical randomization module" |
| Allocation concealment (selection bias) | Low risk | "Treatment assignment, corresponding to patient number, was provided on a sheet sealed inside a randomization envelope" |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Outcomes assessed locally, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Outcomes assessed locally, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for. Excluded 1 randomised patient form group 2 because of refusal to take medications. Not likely to influence results |
| Selective reporting (reporting bias) | High risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study and not all outcomes outlined in method are reported in results. There is no breakdown of numbers for 18 month data, and many outcomes reported as percentages |
| Other bias | High risk | Funded by Hoffmann‐LaRoche, 4/9 authors are employees of Roche |
CARMEN (Rostaing) 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “the randomisation schedule was generated by the Data Operations department, stratified by centre” |
| Allocation concealment (selection bias) | Low risk | “each patient number having a corresponding sealed envelope containing the randomisation details for that patient" "once assigned the envelope was opened” |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding or outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for. 13/551 excluded as never transplanted and/or received study drug. Unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Supported by a grant from Fujisawa GmbH |
Cerrillos 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly assigned", no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 52 patients randomised, however unclear how many per group and no numbers reported anywhere in the abstract |
| Selective reporting (reporting bias) | High risk | Primary outcomes for this review reported (death, graft loss and acute rejection), however only percentages given |
| Other bias | Unclear risk | No funding source provided Abstract only data |
Chen 2003.
| Methods | RCT | |
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. Data only available from 2 conference proceedings abstracts |
| Other bias | Unclear risk | Funding source not stated. Data only available from 2 abstracts |
Ciancio 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Treatment group 3
Baseline immunosuppression
Co‐interventions CMV prophylaxis |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Standard randomised block design" was used |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, biopsy reading for acute rejection not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for at 2 years |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Low risk | Study supported by the University of Miami and registered at clinical.trials.gov. Unlikely to significantly influence on results |
Clatworthy 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | "open‐label" |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | "open‐label" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Supported by Roche. Authors have received grants and fees from Roche, Wyeth, Astellas and GlaxoSmithKline |
Dac double & triple.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See Daclizumab double 1999 and Daclizumab triple 1998. Appears here as artefact of data entry ‐ not a separate trial | |
Daclizumab double 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | Pooled analysis of Daclizumab double and triple therapy studies published after primary studies. Data used only when presented separately for each study. 3 year follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised, double‐blind placebo‐controlled" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported for the primary analyses of efficacy and safety, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | High risk | Not stated, but author list includes employees of Hoffmann‐LaRoche Inc |
Daclizumab triple 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | Pooled analysis of Daclizumab double and triple therapy studies published after primary studies. Data used only when presented separately for each study. 3 year follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised, double‐blind placebo‐controlled" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported for the primary analyses of efficacy and safety, all patients followed up or accounted for. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | High risk | Supported by a grant from Hoffmann‐LaRoche Inc |
de Boccardo 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | "Double blind", blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | "Double blind", blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Stated ITT, but not all randomised patients were analysed. Data only available from conference proceedings abstract |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however data only available as percentages from conference proceedings abstract |
| Other bias | Unclear risk | Funding source not stated, data only available from conference proceedings abstract |
Fangmann 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data only available from 3 conference proceedings abstracts |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. Data only available for 3 conference proceedings abstracts |
| Other bias | Unclear risk | Funding source not stated. Data only available from 3 abstracts |
Flechner 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not reported |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however data only available from abstract |
| Other bias | High risk | Funding source not stated; abstract only data available |
Folkmane 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group (group 3)
Control groups (group 1 and group 2)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results presented as mixture of numbers and percentages, however all patients appear to be accounted for |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes, graft loss and acute rejection, were reported. Death not reported or mentioned |
| Other bias | Unclear risk | Funding source not stated |
Garcia 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data only available from conference proceedings abstract |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however data only available from conference proceedings abstract |
| Other bias | High risk | No funding source stated, however 1 author is an employee of Produtos Roche Quimicos e Farmaceuticos |
Gelens 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group (groups 1 and 2)
All groups received 135 mg methylprednisolone on days 0 and 1 only |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized (1‐1‐1)", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis reported, however interim analysis at the request of the Ethical Committee |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however results are from an interim analysis |
| Other bias | High risk | Supported by grants from Fujisawa Benelux and Roche Pharmaceuticals |
Grego 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized in a 1:1 ratio", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | Funding source not stated |
Grenda 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The "randomisation was stratified by centre using the method of permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "Allocation to treatment was performed locally using sealed randomisation envelopes" |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Yes: Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | "study was supported by Astellas Pharma, Munich, Germany" |
Hanaway 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group (CIH‐LR/CIH‐HR/Thymo‐HR)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patient numbers listed under parameters in results table, however dichotomous results presented as percentages and no SD for continuous outcomes |
| Selective reporting (reporting bias) | High risk | Primary outcomes only reported as percentages |
| Other bias | High risk | Funding source not stated, 1 author employee of the pharmaceutical company Astellas Pharma U.S. Abstract only data available |
Hernandez 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1 (B+C)
Treatment group 2 (A)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computer generated random number sequence" was used |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered sealed envelopes...concealed from the members who were involved in the enrolment of patients" |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | "Neither patients nor clinicians were blinded to therapy". Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | "Neither patients nor clinicians were blinded to therapy". Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, 25% of randomised patients were excluded, however data for all patients has been reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Low risk | Supported by grants FIS 02/1350 and FIS 04/0988 from Instituto de Salud Carlos III and RTIC (C03/03), Spanish Ministry of Health |
Hourmant 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" and "allocation stratified" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | Funding source not stated |
Ji 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up and accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Unclear risk | No funding source stated |
Kahan 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, 2 patients (1 from each group) were not transplanted. All other patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Supported by a grant from Novartis Pharmaceuticals |
Kaplan 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | Not stated |
Khan 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", not further information provided |
| Allocation concealment (selection bias) | Unclear risk | No stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | No stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | No stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients followed up or accounted for, however results only available for conference proceedings abstract |
| Selective reporting (reporting bias) | High risk | Only the primary outcome of acute rejection reported in conference proceedings abstract |
| Other bias | Unclear risk | Funding source not stated |
Kim 2008a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", not further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | "open‐label" |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients excluded from analysis post randomisation |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Not stated |
Kirkman 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression (2 regimens)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "patients randomized", no further information provided |
| Allocation concealment (selection bias) | Low risk | “patients were randomized to experimental or control groups by a sealed envelope technique” |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up for 12 months |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Low risk | Study funded by NIH. Unlikely to significantly influence on results |
Kirkman 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "patients randomized", no further information provided |
| Allocation concealment (selection bias) | Low risk | “patients were randomized to either the experimental or control groups by a sealed envelope technique” |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | "study was not blinded to either participants or investigators" |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | "study was not blinded to either participants or investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for and/or data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Low risk | Study funded by NIH contract No. I‐A1‐82512. Unlikely to significantly influence on results |
Kriaa 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
Cointerventions: Prophylactic antibiotics (ampicillin, oxacillin and gentamycin on days 0 and 2; sulfamethozazole‐trimethoprim for first month) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated using a "randomization table" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Binding or outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of this review (death, graft loss and acute rejection) were reported |
| Other bias | High risk | Funding source not stated, 1/7 authors employee of Technopharm |
Kumar 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was completed using the First Generator Plan from randomization.com" (http://www.randomization.com/) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | After 77 enrolments, patients were shown the results of the interim analyses ‐ no further enrolments took place. Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | After 77 enrolments, patients were shown the results of the interim analyses ‐ no further enrolments took place. Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | "Funded internally from clinical revenue. The manuscript was supported by an unrestricted educational grant from Novartis Pharmaceuticals Corporation" |
Kyllonen 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 1
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation 5:5:4 then changed to 8:4:2 |
| Allocation concealment (selection bias) | Low risk | Computer‐generated numbered randomisation slips were sealed into consecutively numbered envelopes by a person not connected with the study |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not reported |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT, patients were withdrawn after randomisation |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) were reported |
| Other bias | Low risk | Supported by the Helsinki university Hospital Research Fund |
Lacha 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear reporting of numbers in each group |
| Selective reporting (reporting bias) | Unclear risk | Death not reported, discussion states "...graft outcomes, survival rates and graft function is similar in both groups" |
| Other bias | Unclear risk | Source of funding not stated |
Lawen 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" on 1:1 basis and "stratified" based on 1st or 2nd transplant, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | "Participating centres and patients remained blinded up to the end of the 12 month database lock", blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | "Participating centres and patients remained blinded up to the end of the 12 month database lock", blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported ‐ all patients randomised were analysed (8 did not receive 2nd dose) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death graft loss and acute rejection) have been reported |
| Other bias | High risk | Supported by Novartis, 2/12 authors employees of Novartis |
Lebranchu 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Basiliximab group
ATG group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "open randomised" but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for and/or data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study. |
| Other bias | High risk | Study supported by Novartis, France |
Lin 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) were reported |
| Other bias | Unclear risk | Funding source not stated |
Locke 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Interim results only available from conference proceedings abstract |
| Selective reporting (reporting bias) | High risk | Acute rejection results at 6 months only available. States "no significant differences at 6 months between the two treatment arms with regards to patient and graft survival, infection, adverse drug events, malignancy, delayed graft function, and length of stay." No numbers provided |
| Other bias | High risk | Funding source not stated; abstract only data available |
Martin Garcia 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group (groups II and III)
Control group (group I)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states "patients were included in a random way" and main text states "3 groups were separated, according to the immunosuppressive treatment" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear on initial numbers, if the study was randomised, and only 2 outcomes were reported |
| Selective reporting (reporting bias) | High risk | Only acute rejection and lip herpes were reported at 1 year |
| Other bias | Unclear risk | Funding source not stated |
Matl 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Used "code‐breaker envelopes" no further information provided |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis stated, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Funding source not stated, however 1/7 authors employee of Novartis Pharma |
Mourad 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, 2 patients excluded post randomisation because they never received a transplant, unlike to influence results |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study. |
| Other bias | Unclear risk | Funding source not stated |
Nair 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “randomly used in alternate patients” ‐ quasi‐RCT |
| Allocation concealment (selection bias) | High risk | Alternate patients assigned |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for the this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | Funding source not stated |
Nashan 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation was done separately within each centre according to a randomisation code generated by Novartis" |
| Allocation concealment (selection bias) | Low risk | "The trial pharmacist and the principal investigator each held a set of sealed envelopes containing the randomisation code" |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis stated, 42 patients were withdrawn post‐transplantation but included in analyses |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Funded by Novartis |
Noel 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation procedure stratified for PRA>80% |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis reported, however percentages only reported for some outcomes |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) reported |
| Other bias | Low risk | Disclosure statement 'none' |
Offner 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization numbers were computer generated" |
| Allocation concealment (selection bias) | Unclear risk | "Investigators were notified by fax" |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double‐blind "Investigators remained blinded until all patients had completed the 12‐month visit and the database was locked". Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double‐blind "Investigators remained blinded until all patients had completed the 12‐month visit and the database was locked". Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 192/202 analysed. All patients accounted for, however 5 in placebo group were give study drug and analysed in the treatment group, so not ITT as stated |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Study funded by Novartis Pharma |
Parrott 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | 1 year follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | "Study medication was packed sequentially and numbered... and patients were allocated to the next available treatment pack" |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double‐blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double‐blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 108/113 analysed. Five excluded, 4 with no transplant, 1 with transplant but no drug. Not ITT analysis as stated |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Funded by Novartis Pharmaceuticals, 1/5 authors Novartis employee |
Perrea 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | High risk | Primary outcomes for this review not reported |
| Other bias | Unclear risk | Funding source not stated |
Pescovitz 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
Cointervention: CMV prophylaxis |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Double blind, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The data set included all randomized patients who received at least one dose of study medication ‐ Numbers not given for those randomised who did not receive one dose |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No protocol but outcomes specified in method reported in results |
| Other bias | High risk | Funded by 1) Hoffmann‐LaRoche Inc. 2) Grant HSMOIRR750 |
Philosophe 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Interim results only, results presented as percentages and unsure of numbers |
| Selective reporting (reporting bias) | Unclear risk | Interim 1 year results presented |
| Other bias | Unclear risk | Funding source not stated. Abstract only data available |
Pisani 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment groups (group 1 and 2)
Control group (group 3)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly allocated", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | No stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | No stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of patients by group not reported for outcomes, preliminary data only available |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported (death, graft loss and acute rejection), however preliminary data only available |
| Other bias | Unclear risk | Funding source not stated |
Ponticelli 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised according to a "central list of randomisation" no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Double blind, blinding of outcomes assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Low risk | Double blind, blinding of outcomes assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, 5 patients were not transplanted. All other patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | Supported by Novartis, 2/15 authors employees of Novartis |
Pourfarziani 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only data, unclear reporting of events and numbers |
| Selective reporting (reporting bias) | High risk | Death not reported, abstract only data available |
| Other bias | Unclear risk | Funding source not stated. |
Ruggenenti 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned on a 1:1 basis", no further information provided |
| Allocation concealment (selection bias) | Low risk | Patient allocation was centralized (at the Unit of Biostatistics) under the responsibility of an independent investigator who was not involved in study design or conduct |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, all patients accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) were reported |
| Other bias | Low risk | "No pharmaceutical company involvement", study was initiated and internally funded |
Sandrini 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ confirmed randomised by author email but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Confirmed double blind by author email, blinding of outcome assessors not confirmed |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Confirmed double blind by author email, blinding of outcome assessors not confirmed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients followed up and accounted for, however data only available from conference proceedings abstract |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however data only available from conference proceedings abstract |
| Other bias | Unclear risk | Funding source not stated |
Sheashaa 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | High risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported, however graft loss reported as percentages |
| Other bias | Unclear risk | Funding source not stated |
Shidban 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear reporting |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review reported (death, graft loss, acute rejection) however data reported as a mixture of numbers and percentages |
| Other bias | Unclear risk | Funding source not stated. Abstract only data |
Shidban 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Interim analysis, abstract only data available |
| Selective reporting (reporting bias) | High risk | Death and graft loss not reported |
| Other bias | Unclear risk | Funding source not stated. Abstract only data available |
Sollinger 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment Group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT. Six patients excluded: 3 did not receive treatment, 2 withdrew consent and 1 lost to follow‐up ‐ all for ATG group |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | High risk | "Supported by Novartis Pharmaceuticals", one author an employee of Novartis |
Soulillou/Cant 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly assigned" no further information provided |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes” “containing the treatment assignments were prepared” |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Blinding of outcomes assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Low risk | Grant form the Caisse Nationale d'Assurance Maladie |
SYMPHONY (Ekberg) 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients underwent randomisation... with the use of a centralized interactive voice response system (ClinIT) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Open‐label, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Open label, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | States ITT analysis for main outcomes, however some patients randomised were not included in analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No protocol but outcomes specified in method reported in results |
| Other bias | High risk | Funding for the study was provided by Hoffmann‐La Roche, which had advisory input into the study design, collected the data, monitored the conduct of the study, performed the statistical analyses, and coordinated the writing of the manuscript with all authors |
Tan 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | No stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | No stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | Funding source not stated |
ter Meulen 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly assigned" but no further information provided |
| Allocation concealment (selection bias) | Low risk | “randomisation was carried out by opening a sealed opaque envelope with the lowest available study number at each participating centre” |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | “both clinicians and patients were aware of the randomised assignment”, blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | “both clinicians and patients were aware of the randomised assignment”, blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes, ITT analysis reported, all patients followed up or accounted for (3 patients lost to follow up at 12 months) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) were reported |
| Other bias | High risk | Supported by grants from Roche Pharmaceuticals, Mijdrecht, and Fujisawa, Houten |
Tullius 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) were reported |
| Other bias | Unclear risk | Funding source not stated |
van Gelder 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | 1, 3 and 10 year follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "recipients were randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Stated "double‐blind placebo‐controlled study", blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Stated "double‐blind placebo‐controlled study" blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for and/or data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported. No study protocol available to assess secondary outcomes of study |
| Other bias | Unclear risk | No funding source stated |
Vincenti 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Not stated, however 1 group received 1 dose and the other 2 doses. Blinding of outcome assessors not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Not stated, however 1 group received 1 dose and the other 2 doses. Blinding of outcome assessors not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results only reported as percentages and no final numbers indicated |
| Selective reporting (reporting bias) | High risk | Only acute rejection reported and only as a percentage |
| Other bias | Unclear risk | Funding source not stated |
Wilson 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a balanced block‐of‐four scheme |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Unblinded |
| Blinding (performance bias and detection bias) Subjective outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 patients were excluded from the analysis due to graft primary non‐function, however all data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review (death, draft loss and acute rejection) have been reported |
| Other bias | High risk | "Funded jointly by Fujisawa and Roche; neither organisation contributed to the preparation of this manuscript" |
Yussim 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized", no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only data available, not all outcome numbers were reported |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes for this review (death, graft loss and acute rejection) have been reported |
| Other bias | Unclear risk | Funding source not stated. Abstract only data available |
CSA‐ME ‐ cyclosporin micro emulsion; DGF ‐ delayed graft function; IV ‐ intravenous; NHBD ‐ non‐heart beating donors; MF ‐ mycophenolate mofetil; NS ‐ not stated; TAC ‐ tacrolimus
Unless otherwise stated in notes, no significant differences in demographic characteristics are reported for any comparative group.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andres 2009 | IL2Ra received in both treatment arms |
| Budde 2005 | RCT including IL2Ra, but not directly testing IL2Ra |
| Burke 2005 | RCT including IL2Ra, but not directly testing IL2Ra |
| Chadban 2006 | RCT including IL2Ra, but not directly testing IL2Ra |
| Chan 2008 | RCT including IL2Ra, but not directly testing IL2Ra |
| Flechner‐318 2002 | RCT including IL2Ra, but not directly testing IL2Ra |
| FREEDOM Study | RCT including IL2Ra, but not directly testing IL2Ra |
| Hamdy 2005 | RCT including IL2Ra, but not directly testing IL2Ra |
| Hiesse 1992 | NOT RCT or quasi‐RCT |
| Hirose 2004 | RCT including IL2Ra, but not directly testing IL2Ra |
| Kovarik 2003 | RCT including IL2Ra, but not directly testing IL2Ra |
| Kramer‐2307 2003 | RCT including IL2Ra, but not directly testing IL2Ra |
| Kreis 2003 | RCT including IL2Ra, but not directly testing IL2Ra |
| Light 2002 | RCT including IL2Ra, but not directly testing IL2Ra |
| Martinez‐Mier 2006 | RCT including IL2Ra, but not directly testing IL2Ra |
| McDonald 2008 | RCT including IL2Ra, but not directly testing IL2Ra |
| Meier‐Kriesche 2004 | RCT including IL2Ra, but not directly testing IL2Ra |
| Montagnino 2005 | RCT including IL2Ra, but not directly testing IL2Ra |
| Mourad 2005 | RCT including IL2Ra, but not directly testing IL2Ra |
| MyPROMS Study | RCT including IL2Ra, but not directly testing IL2Ra |
| Nematalla 2007 | RCT including IL2Ra, but not directly testing IL2Ra |
| Painter 2003 | Steroid withdrawal not induction study |
| Pescovitz 2004 | RCT including IL2Ra, but not directly testing IL2Ra |
| Provenzano 2000 | RCT including IL2Ra, but not directly testing IL2Ra |
| Scholten 2006 | RCT including IL2Ra, but not directly testing IL2Ra |
| Tian 2007 | IL2Ra laboratory study |
| Vincenti 2005b | RCT including IL2Ra, but not directly testing IL2Ra |
| Wang 2008 | Not IL2Ra RCT |
| Zarkhin 2008 | Not IL2Ra RCT |
Contributions of authors
ACW: Developed protocol, developed search strategy, screened titles and abstracts, identified studies and coordinated study results, resolved disagreement about study inclusion, performed data abstraction, assessed study quality, RevMan data entry, and authored final review
LPR: Screened titles and abstracts, performed data abstraction and assessed study quality
RMG: Screened titles and abstracts, performed data abstraction, RevMan data entry and assessed study quality,
SLM: Screened titles and abstracts, performed data abstraction, RevMan data entry and assessed study quality,
GYH: Reviewed search strategy, performed search and combined search results, identified studies and resolved disagreement about study inclusion
NSW: Resolved disagreement about study inclusion and performed data abstraction
JRC: Reviewed protocol, final results, and co‐authored manuscript
JCC: Reviewed protocol, identified studies, final results, and review and resolved disagreement about study inclusion
Declarations of interest
Dr Jeremy Chapman: has advisory board and clinical trial involvement with Novartis, Roche, Janssen‐Cilag, Fujisawa and Wyeth, and has also been an invited speaker at national and international meetings sponsored by these companies.
ACW, JCC, NW, GYH, LPR, RMG, SLM ‐ none declared
Edited (no change to conclusions)
References
References to studies included in this review
Abou‐Ayache 2008 {published data only}
- Abou‐Ayache R, Buchler M, Lepogamp P, Westeel PF, Meur Y, Etienne I, et al. CMV infections after two doses of daclizumab versus thymoglobulin in renal transplant patients receiving mycophenolate mofetil, steroids and delayed cyclosporine A. Nephrology Dialysis Transplantation 2008;23(6):2024‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abou‐Ayache R, Lebranchu Y, Leopgamp P, Westeel J, Meur Y, Etienne I, et al. Two‐dose daclizumab induction treatment versus thymoglobuline in renal transplant patients receiving a mycophenolate mofetil based immunosuppression [abstract no: 121]. American Journal of Transplantation 2005;5(Suppl 11):187. [CENTRAL: CN‐00644200] [Google Scholar]
Ahsan 2002 {published data only}
- Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC. Limited dose monoclonal IL‐2R antibody induction protocol after primary kidney transplantation. American Journal of Transplantation 2002;2(6):568‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ahsan N, Holman MJ, Yang HC. Limited dose monoclonal IL‐2R antibody induction in kidney transplantation ‐ a prospective, randomized, controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):469. [CENTRAL: CN‐00400019] [DOI] [PubMed] [Google Scholar]
Asberg 2006 {published data only}
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR‐matched de novo kidney transplant recipients. Transplantation 2006;82(1):62‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor‐avoidance with daclizumab, mycophenolate mofetil and prednisolone in DR matched de novo kidney transplant recipients [abstract no: SP737]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv264. [DOI] [PubMed] [Google Scholar]
ATLAS 2003 {published data only}
- Klinger M, Vitko S, Salmela K, Wlodarczyk Z, Tyden G, the ATLAS Study Group. Large, prospective study evaluating steroid‐free immunosuppression with tacrolimus/basiliximab and tacrolimus/mmf compared with tacrolimus/mmf/steroids in renal transplantation [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788‐9. [CENTRAL: CN‐00446121] [Google Scholar]
- Kramer BK, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Vitko S. Two steroid‐free immunosuppressive regimens (basiliximab/tacrolimus and tacrolimus/mmf) in comparison to tacrolimus/MMF/steroid therapy after renal transplantation [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):9A. [CENTRAL: CN‐00583329] [Google Scholar]
- Kramer BK, Kruger B, Hoffmann U, Wlodarczyk Z, Tyden G, Senatorski G, et al. 1‐year‐follow‐up of two steroid‐free immunosuppressive regimens ‐ basiliximab/tacrolimus and tacrolimus/MMR ‐ in comparison to tacrolimus/MMF/steroids after renal transplantation [abstract no: F‐PO1026]. Journal of the American Society of Nephrology 2004;15(Oct):289A. [CENTRAL: CN‐0058338] [Google Scholar]
- Kramer BK, Kruger B, MacK M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: Focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, et al. Two corticosteroids‐free regimens ‐ tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil ‐ in comparison with a standard triple regimen in renal transplantation: results of the Atlas Study. Transplantation 2005;80(12):1734‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, the ATLAS Study Group. Comparison of two steroid‐free regimens ‐ basiliximab/tacrolimus and tacrolimus/mmf ‐ with tacrolimus/mmf/steroid therapy after renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):312. [CENTRAL: CN‐00433656] [Google Scholar]
Baczkowska 2002 {published data only}
- Baczkowska T, Durlik M, Perkowska A, Sadowska A, Cieciura T, Nowacka‐Cieciura E, et al. [Cytokines and growth factors serum level and renal allograft function (preliminary report)] [Polish]. Polski Merkuriusz Lekarski 2003;15(88):356‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska A, Cieciura T, Wierzbicki P, Klosowka D, Matlosz B, et al. Daclizumab allows for a protocol with low‐dose cyclosporine in low rejection‐risk kidney recipients ‐ preliminary data [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):309. [CENTRAL: CN‐00400168] [Google Scholar]
- Baczkowska T, Perkowska‐Francka A, Durlik M, Cieciura T, Nowacka‐Cieciura E, Pazik J, et al. The role of the protocol biopsies in renal allograft recipients. Transplantation Proceedings 2003;35(6):2179‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Cieciura T, Pazik J, Nowacka‐Cieciura E, Lewandowski Z, et al. Untreated subclinical, borderline rejection in 12 and 36‐month protocol biopsies is not associated with progressive loss of GFR at 5‐year's follow‐up [abstract no: 1755]. Transplantation 2008;86(Suppl 2):581. [CENTRAL: CN‐00671803] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, Pazik J, et al. Serum TGF‐b1 correlates with chronic histopathological lesions in protocol biopsies in kidney allograft recipients [abstract]. Transplantation. 2004;78(2 Suppl):319. [CENTRAL: CN‐00583398] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Pazik J, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, et al. The role of the protocol biopsies in renal allograft recipients: three‐years’ follow‐up [abstract no: SP736]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00602089] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Sadowska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura t, et al. Serum TGF‐beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplantation Proceedings 2005;37(2):773‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernarde 2004 {published data only}
- Bernarde K, Folkmane I, Rozentals R, Bicans J. Induction immunosuppression with interleukin‐2 receptor antibodies (basiliximab) in renal transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):467. [CENTRAL: CN‐00509086] [Google Scholar]
Bingyi 2003 {published data only}
- Bingyi S, Ming C, Yeyong Q, Chunbai M, Wenqiang Z. The effect of anti‐CD25 monoclonal antibody (Simulect) to the lymphocytes in the peripheral blood of the recipients of kidney transplantation. Transplantation Proceedings 2003;35(1):243‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bingyi S, Yeyong Q, Ming C, Chunbai M, Wenqiang Z. Randomised trial of Simulect versus placebo for control of acute rejection in renal allograft recipients. Transplantation Proceedings 2003;35(1):192‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brennan 2006 {published and unpublished data}
- Brennan DC, Daller JA, Lake KD, Cibrik D, Castillo D, Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. New England Journal of Medicine 2006;355(19):1967‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Schnitzler M. 5 Year outcomes in a randomized trial comparing rabbit antithymocyte globulin and basiliximab in kidney transplant recipients: Linking clinical trial data with registry data: 798. [Abstract]. Transplantation 2008;86(2 Suppl):279. [Google Scholar]
- Brennan DC, Schnitzler MA. Long‐Term results of rabbit antithymocyte globulin and basiliximab induction. New England Journal of Medicine 2008;359(16):1736‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Thymoglobulin Induction Study Group. A prospective randomized multicenter comparison of thymoglobulin versus Simulect for induction therapy in high risk renal transplant recipients [abstract no:398]. American Journal of Transplantation 2002;2(Suppl 3):238. [CENTRAL: CN‐00400376] [Google Scholar]
- Brennan DC, Thymoglobulin Induction Study Group. Thymoglobulin versus Simulect for induction immunosuppression in cadaveric renal transplant recipients: expanded results from a prospective, randomized, multicenter trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):438‐9. [CENTRAL: CN‐00444533] [Google Scholar]
- Brennan DC, Thymoglubulin Induction Study Group. A prospective, randomized, multicenter study of thymoglobulin compared to Simulect for induction immunosuppression: preliminary results [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00400375]
- Brennan DC, Willoughby LM, Buchanan PM, Dzebisashvili N, Ercole P, Schnitzler MA. Novel approach to obtain long‐term outcomes of patients in a randomized trial comparing thymoglobulin and basiliximab in kidney transplant using registry data [abstract no: 334]. American Journal of Transplantation 2007;7(Suppl 2):234. [CENTRAL: CN‐00644216] [Google Scholar]
- Hardinger KL, Brennan DC, Schnitzler M. Thymoglobulin has its greatest efficacy in recipients of standard criteria donors and donors without hypertension [abstract no: 536]. American Journal of Transplantation 2008;8(Suppl 2):321. [Google Scholar]
- Josephson MA. Rabbit antithymocyte globulin or basiliximab for induction therapy?. New England Journal of Medicine 2006;355(19):2033‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Killen JP, Chadban S, Brennan DC, Buchanan P, Schnitzler MA. Antithymocyte globulin versus basiliximab in renal transplantation. New England Journal of Medicine 2007;356(6):634‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnitzler MA, Buchanan PM, Willoughby LM. Cost‐effectiveness of thymoglobulin compared to basiliximab in kidney transplant using multicenter randomized trial data [abstract no: 326]. American Journal of Transplantation 2007;7(Suppl 2):232. [CENTRAL: CN‐00644279] [Google Scholar]
CAESAR (Ekberg) 2007 {published data only}
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, CAESAR Study Group. Low‐dose cyclosporine in conjunction with daclizumab, mycophenolate mofetil and corticosteroids is safe and effective in contrast to early cyclosporine withdrawal [abstract]. Transplantation 2004;78(Suppl 2):458. [Google Scholar]
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Calleja E, et al. The use of daclizumab and mycophenolate mofetil in combination with corticosteroids and cyclosporine (low dose versus low dose followed by withdrawal) to optimize renal function in recipients of renal allografts [abstract]. Transplantation 2004;78(2 Suppl):458. [CENTRAL: CN‐00509171] [Google Scholar]
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. American Journal of Transplantation 2007;7(3):560‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Spleiss O, Rashford M, Nasmyth‐Miller C, Essioux L. Association of three polymorphisms with acute kidney transplantation: an exploratory pharmacogenetic analysis of a randomized mulitcenter clinical trial (The CAESaR study). American Journal of Transplantation 2006;6(Suppl 2):410. [Google Scholar]
- Vincenti F, Vanrenterghem Y, Nashan B, Grinyo J, Ekberg H, Nasmyth‐Miller C, et al. The use of mycophenolate mofetil, daclizumab and corticosteroids with cyclosporine (low dose, low dose/withdrawal and standard dose) to optimize renal function in renal allograft recipients ‐ 18 month results [abstract no: 1507]. American Journal of Transplantation 2005;5(Suppl 11):539. [CENTRAL: CN‐00644286] [Google Scholar]
CARMEN (Rostaing) 2005 {published data only}
- Budde K, Neumayer HH, Rostaing L, Catarovich D, Mourad G, Rigotti P, et al. Steroid‐free immunosuppression with daclizumab, tacrolimus and mmf is efficacious and improves cholesterol, glucose and bone mineral density ‐ the CARMEN study [abstract]. Transplantation 2004;78(2 Suppl):168. [CENTRAL: CN‐00509111] [Google Scholar]
- Cantarovich D, Rostaing L, Mourad G, Neumayer HH, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. The combination of daclizumab, tacrolimus, and MMF is an effective and safe steroid‐free immunosuppressive regimen after renal transplantation. Results of a large multicentre trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788. [CENTRAL: CN‐00444672] [Google Scholar]
- Kramer BK, Kruger B, Mack M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: Focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐1791. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad GL, Rostaing D, Cantarovich H, Neumayer H, Rigotti P, the Tacrolimus Steroidfree Study Group. Immunosuppression without steroids: daclizumab/tacrolimus/MMF vs. tacrolimus/MMF/steroids in renal transplantation [abstract no: 12]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003.
- Pascual J, Rigotti P, Vialtel P, Sanchez‐Rructuoso A, Escuin F, The Bone Density Study Group. Immunosuppression without steroids: a daclizumab, tacrolimus and MMF regimen prevents loss of bone mass following renal transplantation [abstract no 369]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003.
- Rigotti P, Vialtel P, Pascual J, Sanchez‐Fructuoso A, Escuin F, the Bone Mineral Density Study Group. Immunosuppression without maintenance steroids prevents decline of bone mineral density following renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):199. [CENTRAL: CN‐00447406] [Google Scholar]
- Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, et al. Corticosteroid‐free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 2005;79(7):807‐814. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Catarovich D, Mourad G, Neumayer HH, Rigotti P, the CARMEN Study Group. Steroid‐free immunosuppression with a combination of daclizumab, tacrolimus and MMF is efficacious and safe: results of a large multicenter trial in renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):312. [CENTRAL: CN‐00447473] [Google Scholar]
- Zaoui P, Vialtel P, Rigotti PP, Sanchez‐Fructuoso A, Escuin F, the Bone Mineral Density Study Group. A steroid‐free immunosuppressive regimen of daclizumab, tacrolimus and MMF prevents loss of bone mass following renal transplantation [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):495. [CENTRAL: CN‐00448519] [Google Scholar]
Cerrillos 2006 {published data only}
- Cerrillos I, Gomez‐Navarro B, Cueto A, Ramos F, Monteon F. Daclizumab two doses 0 and 4 days is efficacious to prevent rejection after kidney transplantation [abstract no: PUB163]. Journal of the American Society of Nephrology 2006;17(Abstracts):850A. [CENTRAL: CN‐00615829] [Google Scholar]
Chen 2003 {published data only}
- Chen J, Huang H, Peng W, Wu J. Double filtration plasmapheresis with/without daclizumab induction in the sensitized candidates of cadaveric renal transplantation: a randomized prospective trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):494. [CENTRAL: CN‐00444777] [Google Scholar]
- Chen J, Peng W, Wu J. The effect of daclizumab in highly sensitive kidney recipients [abstract no: SA‐PO652]. Journal of the American Society of Nephrology 2003;14(Nov):440A. [CENTRAL: CN‐00583413] [Google Scholar]
Ciancio 2005 {published data only}
- Carreno MR, Ciancio G, Burke GW, Rosen A, Ricordi C, Tzakis A, et al. Cellular phenotypes affected by induction therapy with campath‐1h vs thymoglobulin vs Zenapax in kidney allograft recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):405. [CENTRAL: CN‐00509121] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune‐monitoring. Transplantation 2005;80(4):457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi AD, Carreno MR, Rosen A, et al. Randomized trial of three different induction regimens to prevent acute renal allograft rejection: early results [abstract]. American Journal of Transplantation 2004;4(Suppl 8):266. [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow‐up. Clinical Transplantation 2008;22(2):200‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Mattiazzi A, Illanes HG, Gaynor JJ, Carreno MR, et al. A randomized trial of three different antibody induction regimens in renal transplantation. American Journal of Transplantation 2005;5(Suppl 11):569. [CENTRAL: CN‐00644195] [Google Scholar]
Clatworthy 2009 {published data only}
- Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B‐cell‐depleting induction therapy and acute cellular rejection. New England Journal of Medicine 2009;360(25):2683‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dac double & triple {published data only}
- See Dacilizumab Double and Triple studies.
Daclizumab double 1999 {published data only}
- Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3‐year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation 2001;72(5):839‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bumgardner GL, Ramos E, Lin A, Vincenti F, Daclizumab Triple Therapy and Double Therapy Groups. Daclizumab (humanized anti‐IL2R alpha mAb) prophylaxis for prevention of acute rejection in renal transplant recipients with delayed graft function. Transplantation 2001;72(4):642‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B, Thervet E. Placebo‐controlled study of a humanized anti‐TAC monoclonal antibody in dual therapy for prevention of acute rejection after renal transplantation. Transplantation Proceedings 1998;30(4):1331‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G. Zenapax (daclizumab) reduces the incidence of acute rejection episodes and improves patient survival following renal transplantation. No 14874 and No 14393 Zenapax Study Groups. Transplantation Proceedings 1999;31(1‐2):267‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G, Nashan B, Vincenti F. Daclizumab prevents acute rejection and improves patient survival post transplantation: 1 year pooled analysis. Transplant International 2000;13(2):151‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G, on behalf of the NO 14874 and NO 14393 Zenapax Study Groups. Daclizumab (Zenapax) reduces the incidence of acute rejection episodes following renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00400813]
- Hardie I R, Zenepax Dual Therapy Study Group. A randomized clinical trial of Zenapax for preventing acute rejection in renal transplantation [abstract]. Nephrology 1997;3(Suppl 1):S71. [CENTRAL: CN‐00460899] [Google Scholar]
- Hengster P, Pescovitz MD, Hyatt D, Margreiter R. Cytomegalovirus infections after treatment with daclizumab, an anti IL‐2 receptor antibody, for prevention of renal allograft rejection. Roche Study Group. Transplantation 1999;68(2):310‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nashan B, Light S, Hardie IR, Lin A, Johnson JR. Reduction of acute renal allograft rejection by daclizumab. Daclizumab Double Therapy Study Group. Transplantation 1999;67(1):110‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nashan B, Zenapax Dual Therapy Study Group. Incidence of CMV infections during daclizumab treatment in renal allograft patients [abstract]. Transplantation 1998;65(12):93. [MEDLINE: ] [Google Scholar]
- Vincenti F, Nashan B, Bumgardner G, Hardie I, Pescovitz M, Johnson RWG, et al. Three year outcome of the phase III clinical trials with Daclizumab [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):750A. [CENTRAL: CN‐00403007] [Google Scholar]
- Vincenti F, Nashan B, Bumgardner G, Hardie I, Pescovitz M, Johnson RWG, et al. Three year outcome of the phase III clinical trials with daclizumab [abstract]. Transplantation 2000;69(8 Suppl):S261. [CENTRAL: CN‐00403006] [Google Scholar]
- Vincenti F, Nashan B, Light S. Daclizumab: Outcome of phase III trials and mechanism of action. Transplantation Proceedings 1998;30(5):2155‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zenapax Double and Triple Therapy Study Group. Pooled analysis of phase III studies of Zenapax (Daclizumab), a humanized anti‐IL‐2R antibody [abstract]. Transplantation 2002;65(8):S180. [CENTRAL: CN‐00403195] [Google Scholar]
Daclizumab triple 1998 {published data only}
- Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3‐year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation 2001;72(5):839‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bumgardner GL, Ramos E, Lin A, Vincenti F, Daclizumab Triple Therapy and Double Therapy Groups. Daclizumab (humanized anti‐IL2Ralpha mAb) prophylaxis for prevention of acute rejection in renal transplant recipients with delayed graft function. Transplantation 2001;72(4):642‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G. Zenapax (daclizumab) reduces the incidence of acute rejection episodes and improves patient survival following renal transplantation. No 14874 and No 14393 Zenapax Study Groups. Transplantation Proceedings 1999;31(1‐2):267‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G, Nashan B, Vincenti F. Daclizumab prevents acute rejection and improves patient survival post transplantation: 1 year pooled analysis. Transplant International 2000;13(2):151‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Backman L, Tufveson G, Tyden G, on behalf of the NO 14874 and NO 14393 Zenapax Study Groups. Daclizumab (Zenapax) reduces the incidence of acute rejection episodes following renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00400813]
- Hengster P, Pescovitz MD, Hyatt D, Margreiter R. Cytomegalovirus infections after treatment with daclizumab, an anti IL‐2 receptor antibody, for prevention of renal allograft rejection. Roche Study Group. Transplantation 1999;68(2):310‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kirkman RL, Vincenti F, Pescovitz MD, Bumgardner G, Gaston RS, Light S. A Phase I/II Randomized, double blind, placebo controlled study of Zenapax in combination with cellCept, neoral and steroids. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago, ILL. 1997:260. [CENTRAL: CN‐00509281]
- Vincenti F, Bi‐Continental Triple Therapy HAT Study Group. A phase III multicenter study of humanized anti‐tac (HAT) for the prevention of rejection in primary cadaveric renal allograft recipients. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago, ILL. 1997:260. [CENTRAL: CN‐00509543]
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin‐2‐receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. New England Journal of Medicine 1998;338(3):161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Nashan B, Bumgardner G, Hardie I, Pescovitz M, Johnson RWG, et al. Three year outcome of the phase III clinical trials with Daclizumab [abstract]. Journal of the American Society of Nephrology 1999;10(Program and Abstracts):750A. [CENTRAL: CN‐00403007] [Google Scholar]
- Vincenti F, Nashan B, Bumgardner G, Hardie I, Pescovitz M, Johnson RWG, et al. Three year outcome of the phase III clinical trials with daclizumab [abstract]. Transplantation 2000;69(8 Suppl):S261. [CENTRAL: CN‐00403006] [Google Scholar]
- Vincenti F, Nashan B, Light S. Daclizumab: Outcome of phase III trials and mechanism of action. Transplantation Proceedings 1998;30(5):2155‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zenapax Double and Triple Therapy Study Group. Pooled analysis of phase III studies of Zenapax (Daclizumab), a humanized anti‐IL‐2R antibody [abstract]. Transplantation 2002;65(8):S180. [CENTRAL: CN‐00403195] [Google Scholar]
de Boccardo 2002 {published data only}
- Boccardo G. Latin American study of the efficacy and safety of Simulect in kidney transplant recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00400671]
Fangmann 2004 {published data only}
- Fangmann J, Arns W, Marti H, Budde K, Beckurts T, Hauss J. Low dose cyclosporine regimen with daclizumab induction and mycophenolate mofetil after kidney transplantation ‐ impact on renal function and rejection episodes [abstract no: 113]. American Journal of Transplantation 2005;5(Suppl 11):185. [CENTRAL: CN‐00644197] [Google Scholar]
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):353. [CENTRAL: CN‐00509182] [Google Scholar]
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):280. [Google Scholar]
Flechner 2000 {published data only}
- Flechner SM, Goldfarb DA, Fairchild R, Cook D, Mastroianni B, Fisher R, et al. A randomized prospective trial of OKT3 vs basiliximab for induction therapy in renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S157. [CENTRAL: CN‐00400926] [DOI] [PubMed] [Google Scholar]
Folkmane 2001 {published data only}
- Folkmane I, Bicans J, Amerika D, Chapenko S, Murovska M, Rosentals R. Low rate of acute rejection and cytomegalovirus infection in kidney transplant recipients with basiliximab. Transplantation Proceedings 2001;33(7‐8):3209‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Folkmane I, Bicans J, Chapenko S, Murovska M, Rosentals R. Results of renal transplantation with different immunosuppressive regimens. Transplantation Proceedings 2002;34(2):558‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Folkmane I, Chapenko S, Murovska M, Rosental R. Low rate of acute rejection and cytomegalovirus infection in renal transplant recipients with basiliximab [abstract no:1037]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [DOI] [PubMed]
Garcia 2002 {published data only}
- Garcia R, Hanzawa NM, Machado PGP, Moreira SR, Prismich G, Felipe CR, et al. A calcineurin inhibitor‐free regimen for low risk kidney transplant recipients [abstract no:2379]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
Gelens 2006 {published data only}
- Gelens M, Christiaans M, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grego 2007 {published data only}
- Grego K, Arnol M, Bren AF, Kmetec A, Tomazic J, Kandus A. Basiliximab versus daclizumab combined with triple immunosuppression in deceased donor renal graft recipients. Transplantation Proceedings 2007;39(10):3093‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grego K, Kandus A, Bren AF. Basiliximab versus daclizumab for prevention of acute renal allograft rejection [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2006;17(Abstracts):223A. [CENTRAL: CN‐00602013] [Google Scholar]
Grenda 2006 {published data only}
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J, Fitzpatrick M, et al. A prospective, randomized, multicenter trial of tacrolimus‐based therapy with or without basiliximab in pediatric renal transplantation. American Journal of Transplantation 2006;6(7):1666‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J, Paediatric Tacrolimus Study Group. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomised study in paediatric kidney transplantation [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
- Vondrak K, Grenda R, Watson AR, Webb NJA, Beattie J, Pediatric Tacrolimus Study Group. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomized study in pediatric kidney transplantation [abstract no: 964]. American Journal of Transplantation 2005;5(Suppl 11):401. [Google Scholar]
- Webb N, Prokurat S, Vondrak K, Watson A, Hughes D, Hamer C, et al. Multicentre randomized prospective trial of tacrolimus, azathioprine and prednisolone with or without basiliximab; two year follow‐up data [abstract no: 121 (FC)]. Paediatric Nephrology 2007;22(9):1446. [CENTRAL: CN‐00653717] [DOI] [PubMed] [Google Scholar]
Hanaway 2008 {published data only}
- Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (Alemtuzumab, Thymoglobulin and Basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 135]. American Journal of Transplantation 2008;8(Suppl 2):215. [CENTRAL: CN‐00653740] [Google Scholar]
- Holman J, Harrison G, Vandeputte K, First R, Fitzsimmons W. Immune cell activation comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 553]. Transplantation 2008;86(2 Suppl):194. [CENTRAL: CN‐00676047] [Google Scholar]
- Woodle S, Hanaway M, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 876]. Transplantation 2008;86(2 Suppl):306. [CENTRAL: CN‐00653740] [Google Scholar]
Hernandez 2007 {published data only}
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hourmant 1994 {published data only}
- Hourmant M, Mauff B, Cantarovich D, Dantal J, Baatard R, Denis M, et al. Prevention of acute rejection episodes with an anti‐interleukin 2 receptor monoclonal antibody. II. Results after a second kidney transplantation. Transplantation 1994;57(2):204‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ji 2007 {published data only}
- Ji SM, Li LS, Cheng Z, Cheng DR, Sun QQ, Chen JS, et al. A single‐dose daclizumab induction protocol in renal allograft recipients: a Chinese single center experience. Transplantation Proceedings 2007;39(5):1396‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kahan 1999 {published data only}
- Hall M, Kovarik J, Gerbeau C, Schmidt AG. Influence of the duration of IL‐2 receptor (IL‐2R) blockade on the incidence of acute rejection episodes in renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998.
- Kahan BD, Rajagopalan PR, Hall M, United States Simulect Renal Study Group. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti‐interleukin‐2‐receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation 1999;67(2):276‐284. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD, Rajagopalan PR, Hall ML. Reduction of acute cellular rejection in renal allograft patients with basiliximab (Simulect). 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:260.
- Kahan BD, Rajagopalan PR, Hall ML, Kovarik JM, US Simulect Study Group. Basiliximab (Simulect) is efficacious in reducing the incidence of acute rejection episodes in renal allograft patients: results at 12 months [abstract]. Transplantation 1998;65(12):S189. [CENTRAL: CN‐00401446] [Google Scholar]
- Kahan BD, Rajagopalan PR, Hall ML, Kovarik JM, US Simulect Study Group. Basiliximab (Simulect) is efficacious in reducing the incidence of acute rejection episodes in renal allograft patients: results at 12 months [abstract]. Transplantation 1998;66(8):S1. [Google Scholar]
- Keown P, Balshaw R, Kalo Z, Khorasheh S, Matthisson M. Economic analysis of basiliximab (Simulect) in renal transplantation. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00583133]
- Kovarik J, Kahan BD, Rajagopalan PR, Bennett W, Mulloy LL, Gerbeau C, et al. Population pharmacokinetics and exposure‐response relationships for basiliximab in kidney transplantation. The U.S. Simulect Renal Transplant Study Group. Transplantation 1999;68(9):1288‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Gerbeau C, Hall M, Schmidt AG. Influence of the duration of IL‐2 receptor (IL‐2R) blockade on the incidence of acute rejection episodes in renal transplantation [abstract]. Transplantation 1998;65(12):S179. [CENTRAL: CN‐00402057] [Google Scholar]
- Lorber MI, Fastenau J, Wilson D, DiCesare J, Hall ML. A prospective economic evaluation of basiliximab (Simulect) therapy following renal transplantation. Clinical Transplantation 2000;14(5):479‐485. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mulloy LL, Wright F, Hall ML, Moore M. Simulect (basiliximab) reduces acute cellular rejection in renal allografts from cadaveric and living donors. Transplantation Proceedings 1999;31(1‐2):1210‐1213. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mulloy LL, Wright F, Hall ML, Moore M, US Simulect Study Group. Basiliximab (Simulect) reduces acute cellular rejection in renal allografts from cadaveric and living donors [abstract]. Transplantation 1998;66(8):S1. [DOI] [PubMed] [Google Scholar]
- Mulloy LL, Wright F, Hall ML, Moore M, on behalf of the US Simulect Study Group. Basiliximab (Simulect) reduces acute cellular rejection in renal allografts from cadaveric and living donors [abstract]. Transplantation 1998;65(12):S190. [DOI] [PubMed] [Google Scholar]
- Nashan B, Thistlethwaite R, Schmidt AG, Hall M, Chodoff L, Global Simulect Study Group. Reduced acute rejection and superior one‐year renal allograft survival with basiliximab (Simulect) in patients with diabetes mellitus [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998.
- Nashan B, Thistlewaite R, Schmidt AG, Hall M, Chodoff L, the Global Simulect Study Group. Reduced acute rejection and superior one‐year renal allograft survival with basiliximab (Simulect) in patients with diabetes mellitus [abstract]. Transplantation 1998;65(12):S179. [CENTRAL: CN‐00402057] [DOI] [PubMed] [Google Scholar]
- Soulillou JP, Kahan BD, Hall ML, Schmidt AG, CHIB 352/201 Simulect Study Groups. Basiliximab (Simulect) significantly reduced the incidence of acute rejection episodes in renal allograft patients: pooled data US/Europe/Canada Studies [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00402717]
- Thistlethwaite JR, Nashan B, Hall M, Chodoff L, Lin TH. Reduced acute rejection and superior 1‐year renal allograft survival with basiliximab in patients with diabetes mellitus. The Global Simulect Study Group. Transplantation 2000;70(5):784‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaplan 2003 {published data only}
- Kaplan B, Cibrik DM, Schold JD, Mulgaonkar S, Magee J, Howell T, et al. Pilot randomized prospective study of dual vs triple immunosuppression in older renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):212. [Google Scholar]
Khan 2000 {published data only}
- Khan AJ, Sarkissian N, Brennen TS, Gonzalez JM, Nassar GM, Achkar K, et al. Comparison of two IL‐2 receptor blockers in decreasing the incidence of acute rejection in early post‐transplant time in renal transplant recipients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):694A. [CENTRAL: CN‐00433633] [Google Scholar]
Kim 2008a {published data only}
- Kim MJ, Tsinalis D, Franz S, Binet I, Gurke L, Mihatsch MJ, et al. ATG‐Fresenius or daclizumab induction therapy in immunologically high risk kidney recipients: a prospective randomized pilot trial. Annals of Transplantation 2008;13(4):21‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Kirkman 1989 {published data only}
- Carpenter CB, Kirkman RL, Shapiro ME, Milford EL, Tiney NL, Waldmann TA, et al. Prophylactic use of monoclonal anti‐IL‐2 receptor antibody in cadaveric renal transplantation. American Journal of Kidney Diseases 1989;14(5 Suppl 2):54‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Kirkman RL, Shapiro ME, Carpenter CB, Milford EL, Ramos EL, Tilney NL, et al. Early experience with anti‐Tac in clinical renal transplantation. Transplantation Proceedings 1989;21(1 Pt 2):1766‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Ramos EL, Leggat JE, Milford EL, Kirkman RL, Tilney NL, Strom TB, et al. In vivo anti‐interleukin‐2 receptor (anti‐Tac) therapy is immunosuppressive, but not tolerogenic. Transactions of the Association of American Physicians 1989;102:231‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ramos EL, Milford EL, Kirkman RL, Tilney NL, Strom TB, Shapiro ME, et al. Differential IL‐2 receptor expression in renal allograft recipients treated with an anti‐IL‐2‐receptor antibody. Transplantation 1989;48(3):415‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirkman 1991 {published data only}
- Carpenter CB, Kirkman RL, Shapiro ME, Milford EL, Tiney NL, Waldmann TA, et al. Prophylactic use of monoclonal anti‐IL‐2 receptor antibody in cadaveric renal transplantation. American Journal of Kidney Diseases 1989;14(5 Suppl 2):54‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Kirkman RL, Shapiro ME, Carpenter CB, McKay DB, Milford EL, Ramos EL, et al. A randomized prospective trial of anti‐Tac monoclonal antibody in human renal transplantation. Transplantation 1991;51(1):107‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kirkman RL, Shapiro ME, Carpenter CB, McKay DB, Milford EL, Ramos EL, et al. A randomized prospective trial of anti‐Tac monoclonal antibody in human renal transplantation. Transplantation Proceedings 1991;23(1 Pt 2):1066‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Ramos EL, Leggat JE, Milford EL, Kirkman RL, Tilney NL, Strom TB, et al. In vivo anti‐interleukin‐2 receptor (anti‐Tac) therapy is immunosuppressive, but not tolerogenic. Transactions of the Association of American Physicians 1989;102:231‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ramos EL, Milford EL, Kirkman RL, Tilney NL, Strom TB, Shapiro ME, et al. Differential IL‐2 receptor expression in renal allograft recipients treated with an anti‐IL‐2‐receptor antibody. Transplantation 1989;48(3):415‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kriaa 1993 {published data only}
- Beaudreuil S, Durrbach A, Noury J, Ducot B, Kriaa F, Bazin H, et al. Long‐term results (10 years) of a prospective trial comparing Lo‐tact‐1 monoclonal antibody and anti‐thymocyte globulin induction therapy in kidney transplantation. Transplant International 2006;19(10):814‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beaudreuil S, Durrbach A, Noury J, Kriaa F, Bazin H, Charpentier B. Long term follow‐up (10 years) of a prospective trial assay comparing lo‐tact‐1 antibody versus anti‐thymocyte globulin induction therapy in kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):467‐8. [CENTRAL: CN‐00509085] [DOI] [PubMed] [Google Scholar]
- Kriaa F, Hiesse C, Alard P, Lantz O, Noury J, Charpentier B, et al. Prophylactic use of the anti‐IL‐2 receptor monoclonal antibody LO‐Tact‐1 in cadaveric renal transplantation: results of a randomized study. Transplantation Proceedings 1993;25(1 Pt 1):817‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Kumar 2005 {published data only}
- Fa K, Kode RK, Lu Q, Kumar MSA, Laftavi MR, Pankewycz OG. Value of one month protocol biopsies combined with a molecular analysis in predicting efficacy of rapid steroid withdrawal after renal transplantation [abstract]. American Journal of Transplantation 2002;2(Suppl 3):171. [Google Scholar]
- Fa K, Laftavi MR, Ferry E, Kumar AMS, Fyfe B, Pankewycz OG. The predictive value of subclinical rejection in a steroid free immunosuppressive regimen [abstract]. American Journal of Transplantation 2003;3(Suppl 5):480. [Google Scholar]
- Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, et al. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 2006;81(6):832‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kumar MSA, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract no:2440]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00416079]
- Kumar MSA, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):393. [Google Scholar]
- Kumar MSA, Xiao SG, Fyfe B, Sierka D, Heifets M, Moritz MJ, et al. Steroid avoidance in renal transplantation using basiliximab induction, cyclosporine‐based immunosuppression and protocol biopsies. Clinical Transplantation 2005;19(1):61‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kyllonen 2007 {published data only}
- Kyllonen L, Eklund B, Matinlauri I, Salmela K. Induction with single bolus ATG or basiliximab in cadaveric kidney transplantation with cyclosporin immunosuppression [abstract]. XIXth International Congress of the Transplantation Society, Miami, Florida. 2002 Aug 25‐30. [CENTRAL: CN‐00401573]
- Kyllonen L, Eklund B, Matinlauri I, Salmela K. Induction with single bolus ATGor basiliximab in cadaveric kidney transplantation with cyclosporin immunosuppression [abstract no: 2330]. Transplantation 2002;74(4 Suppl):466. [CENTRAL: CN‐00401573] [Google Scholar]
- Kyllonen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84(1):75‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matinlauri IH, Kyllonen LE, Eklund BH, Koskimies SA, Salmela KT. Weak humoral posttransplant alloresponse after a well‐HLA‐matched cadaveric kidney transplantation. Transplantation 2004;78(2):198‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matinlauri IH, Kyllonen LE, Salmela KT, Helin H, Pelzl S, Susal C. Serum sCD30 in monitoring of alloresponse in well HLA‐matched cadaveric kidney transplantations. Transplantation 2005;80(12):1809‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turunen AJ, Fernandez JA, Lindgren L, Salmela KT, Kyllonen LE, Makisalo H, et al. Activated protein C reduces graft neutrophil activation in clinical renal transplantation. American Journal of Transplantation 2005;5(9):2204‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turunen AJ, Lindgren L, Salmela KT, Kyllonen LE, Makisalo H, Siitonen SM, et al. Association of graft neutrophil sequestration with delayed graft function in clinical renal transplantation. Transplantation 2004;77(12):1821‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lacha 2001 {published data only}
- Lacha J, Bartosova K, Lyerova L, Burgelova M, Teplan V, Vitko S. Long‐term effect of zenapax versus okt‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):265. [CENTRAL: CN‐00509303] [DOI] [PubMed] [Google Scholar]
- Lacha J, Simova M, Noskova L, Teplan V, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients. Transplantation Proceedings 2001;33(3):2273‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lacha J, Simova M, Noskova L, Teplan V, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. Transplantation 2000;69(8):S158. [CENTRAL: CN‐00401578] [DOI] [PubMed] [Google Scholar]
- Lacha J, Viklicky O, Noskova L, Kalanin J, Striz I, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00401579]
Lawen 2003 {published data only}
- Davies E, Lawen J, Mourad G, Oppenheimer F, Durand D, Gonzalez‐Molina M, et al. Basiliximab (Simulect) is safe and effective in combination with neoral, steroids and cellcept for the prevention of acute rejection episodes in renal transplantation. Interim results of a double blind, randomized clinical trial [abstract]. American Society of Nephrology 1999;10(Program & Abstracts):725A‐6A. [CENTRAL: CN‐00400659] [Google Scholar]
- Lawen J, Davies E, Mourad G, Oppenheimer F, Gonzalez‐Molina M, Bourbigot B, et al. Basiliximab (Simulect) is safe and effective in combination with triple therapy of neoral steroids and cellcept in renal transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S260. [CENTRAL: CN‐00401599] [Google Scholar]
- Lawen JG, Davies EA, Mourad G, Oppenheimer F, Molina MG, Rostaing L, et al. Randomized double‐blind study of immunoprophylaxis with basiliximab, a chimeric anti‐interleukin‐2 receptor monoclonal antibody, in combination with mycophenolate mofetil‐containing triple therapy in renal transplantation. Transplantation 2003;75(1):37‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lebranchu 2002 {published data only}
- Al Najjar A, Etienne I, Pogamp P, Bridoux F, Meur Y, Toupance O, et al. Long‐term results of monoclonal anti‐Il2‐receptor antibody versus polyclonal antilymphocyte antibodies as induction therapy in renal transplantation. Transplantation Proceedings 2006;38(7):2298‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brun C, Al Najjar A, Buchler M, Pen C, Lebranchu Y, Lilliu H. Cost‐minimisation study comparing simulect versus thymoglobuline in renal transplant induction. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00509107] [DOI] [PubMed]
- Buchler M, Benfatma L, Lepogamp P, Bridoux F, Lemeur Y, Toupance O, et al. Three year results of a randomized study comparing as induction treatment simulect® and thymoglobuline®. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):349. [CENTRAL: CN‐00509108] [Google Scholar]
- Lebranchu Y, Bridoux F, Buchler M, Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF‐containing triple therapy. American Journal of Transplantation 2002;2(1):48‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Bridoux F, Etienne I, Buchler M, Toupance O, Meur Y, et al. A multicenter, randomized trial of Simulect versus thymoglobuline in renal transplantation [abstract no:1598]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00401605]
- Lebranchu Y, Bridoux F, Lemeur Y, Bouchoule I, Lavaud S, Lobbedez T, et al. A multicenter randomized trial of Simulect versus thymoglobuline in renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00644240]
- Lebranchu Y, Hurault LB, Toupance O, Touchard G, Lemeur Y, Etienne I, et al. A multicenter randomized trial of Simulect versus thymoglobuline in renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S258. [CENTRAL: CN‐00644238] [Google Scholar]
- Lilliu H, Brun C, Pen C, Buchler M, Al Najjar A, Reigneau O, et al. Cost‐minimization study comparing Simulect versus Thymoglobulin in renal transplant induction. Transplantation Proceedings 2001;33(7‐8):3197‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lilliu H, Brun‐Strang C, Pen C, Buchler M, Al Najjar A, Priol G, et al. Cost‐minimization study comparing Simulect vs. Thymoglobulin in renal transplant induction. Clinical Transplantation 2004;18(3):247‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin M, Ming A, Zhao M. The clinical study of two‐dose basiliximab compared with two‐dose daclizumab in renal transplantation [abstract]. Transplantation 2004;78(2):466. [CENTRAL: CN‐00509323] [DOI] [PubMed] [Google Scholar]
- Lin M, Ming A, Zhao M. Two‐dose basiliximab compared with two‐dose daclizumab in renal transplantation: a clinical study. Clinical Transplantation 2006;20(3):325‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locke 2008 {published data only}
- Leffell MS, Kopchliiska D, Lucas DP, Jackson AM, Montgomery RA, Locke JE, et al. Effect of induction agent on cellular and humoral responses to renal transplants in sensitized patients [abstract no: 14]. American Journal of Transplantation 2008;8(Suppl 2):182. [Google Scholar]
- Locke J, Simpkins C, Leffell MS, Zacary A, Collins V, Warren D, et al. Results of a randomized prospective study of induction therapy with daclizumab versus thymoglobulin among crossmatch positive renal transplant recipients [abstract no: 521]. Transplantation 2008;86(Suppl 2):182‐3. [Google Scholar]
Martin Garcia 2003 {published data only}
- Martin GD, Martin GJ, Mendiluce A, Gordillo R, Bustamente J. Tacrolimus‐basiliximab versus cyclosporine‐basiliximab in renal transplantation "de novo": acute rejection and complications. Transplantation Proceedings 2003;35(5):1694‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matl 2001 {published data only}
- Matl I, Bachleda P, Lao M, Michalsky R, Navratil P, Treska V. Basiliximab (Simulect) can be administered safely and effectively by IV bolus in a single dose on day 1 post renal transplantation in patients receiving triple therapy with azathioprine [abstract no:1107]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00401865] [DOI] [PubMed]
- Matl I, Bachleda P, Lao M, Michalsky R, Navratil P, Treska V, et al. Safety and efficacy of an alternative basiliximab (Simulect) regimen after renal transplantation: administration of a single 40‐mg dose on the first postoperative day in patients receiving triple therapy with azathioprine. Transplant International 2003;16(1):45‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matl I, Bachleda P, Michalsky R, Navratil P, Lao M, Treska V, et al. Basiliximab can be administered safely and effectively in a single dose on day 1 postrenal transplantation in patients receiving triple therapy with azathioprine. Transplantation Proceedings 2001;33(7‐8):3205‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mourad 2004 {published data only}
- Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal‐transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78(4):584‐590. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C, Lorho R, Therver E, Fares N. Simulect versus thymoglobulin with delayed introduction of neoral in renal transplantation: three month results of a French multicenter randomized trial [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00402018]
- Mourad GJ, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. A sequential protocol using simulect vs thymoglobulin in low immunological risk renal transplant recipients: six‐month results of a French multicenter, randomized trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00446849] [Google Scholar]
Nair 2001 {published data only}
- Nair MP, Nampoory MR, Johny KV, Costandi JN, Abdulhalim M, Reshaid W, et al. Induction immunosuppression with interleukin‐2 receptor antibodies (basiliximab and daclizumab) in renal transplant recipients. Transplantation Proceedings 2001;33(5):2767‐2769. [DOI] [PubMed] [Google Scholar]
- Nampoory MR, Abdulhalim M, Johny KV, Jawad Donia FA, Nair MP, Said T, et al. Bolus anti‐thymocyte globulin induction in renal transplant recipients: a comparison with conventional ATG or anti‐interleukin‐2 receptor antibody induction. Transplantation Proceedings 2002;34(7):2916‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nampoory NMR, Nair MP, Johny KV, Said T, El‐Reshaid W, Samhan M, et al. Induction immunosuppression with anti interleukin (IL‐2) receptor antibodies and anti thymocyte globulin in renal transplantation ‐ a comparative study [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):699A‐700A. [CENTRAL: CN‐00433639] [Google Scholar]
Nashan 1997 {published data only}
- Akehurst R, Chilcott J, Holmes M. The economic implications of the use of Basiliximab versus placebo for the control of acute cellular rejection in renal allograft recipients [abstract]. Transplantation 1999;67(7):S155. [CENTRAL: CN‐00400025] [Google Scholar]
- Breidenbach T, Korn A, Maibucher A, Schlitt HJ, Oldhafer KJ, Kliem V, et al. Two years results of a clinical trial with basiliximab (Simulect) in renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00400373]
- Breidenbach T, Korn A, Schlitt HJ, Kliem V, Brunkhorst R, Schmidt AG, et al. Basiliximab (Simulect) reduces acute rejections, CMV infections and duration of hospital stay in renal allograft patients [abstract]. Transplantation 1998;65(12):S180. [CENTRAL: CN‐00400374] [Google Scholar]
- Chilcott J, Akehurst R, Whitfield M. Economics of Basiliximab (Simulect) in preventing acute rejection in renal transplantation [abstract no:1453]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400541]
- Chilcott JB, Holmes MW, Walters S, Akehurst RL, Nashan B. The economics of basiliximab (Simulect) in preventing acute rejection in renal transplantation. Transplant International 2002;15(9‐10):486‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keown P, Balshaw R, Kalo Z, Khorasheh S, Matthisson M. Economic analysis of basiliximab (simulect) in renal transplantation [abstract]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00583133]
- Keown PA, Balshaw R, Baladi JF, International Simulect Study Group. Canadian economic analysis of basiliximab (Simulect) in renal transplantation [abstract no: P1041]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00401481]
- Keown PA, Balshaw R, Krueger H, Baladi JF. Economic analysis of basiliximab in renal transplantation. Transplantation 2001;71(11):1573‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Koch M, Korn A, Lueck R, Becker T, Klempnauer J, Nashan B. Long term results of basiliximab in renal transplantation [abstract no:1020]. American Journal of Transplantation 2002;2(Suppl 3):395. [CENTRAL: CN‐00401523] [Google Scholar]
- Kovarik JM, Moore R, Wolf P, Abendroth D, Landsberg D, Soulillou JP, et al. Screening for basiliximab exposure‐response relationships in renal allotransplantation. Clinical Transplantation 1999;13(1 Pt 1):32‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. [erratum appears in Lancet 1997 Nov 15;350(9089):1484]. Lancet 1997;350(9086):1193‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nashan B, Moore R, Schmidt AG, Abeywickrama K, Soulillou JP, CHIB201 International Study Group. Reduction of acute cellular rejection by basiliximab (simulect), in renal allograft recipients [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:261. [CENTRAL: CN‐00520365]
- Nashan B, Thistlethwaite R, Schmidt AG, Hall M, Chodoff L, Global Simulect Study Group. Reduced acute rejection and superior one‐year renal allograft survival with basiliximab (Simulect) in patients with diabetes mellitus [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998.
- Nashan B, Thistlewaite R, Schmidt AG, Hall M, Chodoff L, Global Simulect Study Group. Reduced acute rejection and superior one‐year renal allograft survival with basiliximab (Simulect) in patients with diabetes mellitus [abstract]. Transplantation 1998;65(12):S179. [CENTRAL: CN‐00402057] [DOI] [PubMed] [Google Scholar]
- Soulillou JP, Kahan BD, Hall ML, Schmidt AG, CHIB 352/201 Simulect Study Groups. Basiliximab (Simulect) significantly reduced the incidence of acute rejection episodes in renal allograft patients: pooled data US/Europe/Canada Studies [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00402717]
- Thistlethwaite JR, Nashan B, Hall M, Chodoff L, Lin TH. Reduced acute rejection and superior 1‐year renal allograft survival with basiliximab in patients with diabetes mellitus. The Global Simulect Study Group. Transplantation 2000;70(5):784‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noel 2009 {published data only}
- Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus antithymocyte globulin in high‐immunological‐risk renal transplant recipients. Journal of the American Society of Nephrology 2009;20(6):1385‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus thymoglobulin in renal transplant recipients with a high immunological risk: a French and Belgian prospective randomized trial [abstract no: 331]. American Journal of Transplantation 2007;7(Suppl 2):233. [CENTRAL: CN‐00644178] [Google Scholar]
Offner 2008 {published data only}
- Hocker B, Kovarik J, Offner GF, Zimmerhack LB, Jungraithmayr TC, Koepf S, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients under CsA, MMF and corticosteroids [abstract no: COD. PP 210]. Pediatric Nephrology 2006;21(10):1574. [Google Scholar]
- Hocker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation 2008;86(9):1234‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Offner G, Toenshoff B, Hocker B, Krauss M, Bulla M, Cochat P, et al. Efficacy and safety of basiliximab in pediatric renal transplant patients receiving cyclosporine, mycophenolate mofetil, and steroids. Transplantation 2008;86(9):1241‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tönshoff B, Offner G, Hoecker B, Pape L, Rascher W, Neuhaus T, et al. A multicenter, placebo‐controlled trial evaluating the efficacy and safety of Basiliximab (Simulect) in combination with CsA, MMF and steroids in pediatric renal allograft recipients: 12 months results [abstract no: COD. OP 25]. Pediatric Nephrology 2006;21(10):1513. [CENTRAL: CN‐00583475] [Google Scholar]
- Zimmerhackl LB, Grossmann A, Jungraithmayr TC, Pedevilla P, Cochat P, Doetsch J, et al. Basiliximab as induction therapy in pediatric renal transplantation: 5 year results [abstract no: SA‐PO2534]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):679A. [Google Scholar]
- Zimmerhackl LB, Toenshoff B, Offner G, Mihatsch M, Fischer W. First multicenter, placebo controlled trial of basiliximab (Simulect) in pediatric renal allograft recipients: efficacy results including a 6‐month biopsy (for the pediatric simulect® study group) [abstract no: SA‐PO451]. Journal of the American Society of Nephrology 2006;17(Abstracts):671A. [CENTRAL: CN‐00644290] [Google Scholar]
Parrott 2005 {published data only}
- Parrott NR, Hammad AQ, Watson CJ, Lodge JP, Andrews CD. Multicenter, randomized study of the effectiveness of basiliximab in avoiding addition of steroids to cyclosporine a monotherapy in renal transplant recipients. Transplantation 2005;79(3):344‐348. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parrott NR, Hammad AQ, Watson CJE, Lodge PJA, Andrews C. Basiliximab (simulect) with ciclosporin (neoral) as a strategy for steroid avoidance in renal transplantation. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):350. [CENTRAL: CN‐00509403] [Google Scholar]
Perrea 2006 {published data only}
- Perrea DN, Moulakakis KG, Poulakou MV, Vlachos IS, Papachristodoulou A, Kostakis AI. Correlation between oxidative stress and immunosuppressive therapy in renal transplant recipients with an uneventful postoperative course and stable renal function. International Urology & Nephrology 2006;38(2):343‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pescovitz 2003 {published data only}
- Kirkman RL, Vincenti F, Pescovitz MD, Bumgardner GL, Gaston RS, Light SE. A phase I/II randomized, double blind, placebo controlled study of zenapax in combination with cellcept, neoral, and steroids. [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:260.
- Pescovitz MD, Bumgardner G, Gaston RS, Kirkman RL, Light S, Patel IH, et al. Pharmacokinetics of daclizumab and mycophenolate mofetil with cyclosporine and steroids in renal transplantation. Clinical Transplantation 2003;17(6):511‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Philosophe 2002 {published data only}
- Philosophe B, Schweitzer EJ, Foster CE, Campos L, Myers S, Bartlett ST. Long term results of a prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no: 126]. American Journal of Transplantation 2005;5(Suppl 11):188. [CENTRAL: CN‐00644144] [Google Scholar]
- Philosophe B, Wiland AM, Mann DL, Farney AC, Schweitzer EJ, Colonna JO, et al. Prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no:2063]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
- Philosophe B, Wiland AM, Mann DL, Farney AC, Schweitzer EJ, Colonna JO, et al. Prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no:402]. American Journal of Transplantation 2002;2(Suppl 3):239. [CENTRAL: CN‐00402238] [Google Scholar]
Pisani 2001 {published data only}
- Coppelli A, Buonomo O, Iaria G, Pisani F, Pollicita S, Rizzello A. Preliminary results of a prospective randomized study of basiliximab and steroid withdrawal in kidney transplantation [abstract no:1617]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400600]
- Pisani F, Buonomo O, Iaria G, Tisone G, Mazzarella V, Pollicita S, et al. Preliminary results of a prospective randomized study of basiliximab in kidney transplantation. Transplantation Proceedings 2001;33(1‐2):2032‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 2001 {published data only}
- Chilcott, JB. Economics of basiliximab (Simulect) in preventing acute rejection in renal transplantation. ISOT 2001. [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Cambi V, Shapira Z, Monteon F, Salmela K, Kahn D, et al. A multicenter, double blind, placebo controlled study of basiliximab (simulect) in combination with triple therapy including azathioprine for the prevention of acute rejection episodes in renal allograft patients [abstract]. Transplantation 1999;67(7):S158. [CENTRAL: CN‐00402269] [Google Scholar]
- Ponticelli C, Yusim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab (Simulect) significantly reduces the incidence of acute rejection in renal transplant patients receiving triple therapy with azathioprine [abstract]. Transplantation 2000;69(8 Suppl):S156. [CENTRAL: CN‐00402270] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab (Simulect) significantly reduces the incidence of acute rejection in renal transplant patients receiving a triple therapy with azathioprine [abstract]. International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000.
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. A randomized, double‐blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients. Transplantation 2001;72(7):1261‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab (Simulect) significantly reduces the incidence of acute rejection in renal transplant patients receiving a triple therapy with azathioprine [abstract no:0114]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00583373]
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab significantly reduces acute rejection in renal transplant patients given triple therapy with azathioprine. Transplantation Proceedings 2001;33(1‐2):1009‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walters SJ. Economics of basiliximab (Simulect) in preventing acute rejection in renal transplantation [abstract no:441]. Proceedings.10th ESOT & 12th ETCO Congress; 2001 Oct 6 ‐ 11; Lisboa, Portugal. 2001.
- Walters SJ, Whitfield M, Akehurst RL, Chilcott JB. Economic implications of the use of basiliximab in addition to triple immunosuppressive therapy in renal allograft recipients: a UK perspective. Pharmacoeconomics 2003;21(2):129‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walters SJ, Whitfield M, Akehurst RL, Chilcott JB. Pharmacoeconomic evaluation of Simulect prophylaxis in renal transplant recipients. Transplantation Proceedings 2001 Nov;33(7‐8):3187‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pourfarziani 2003 {published data only}
- Pourfarziani V, Lesanpezeshki M, Einollahi B, et al. Zenapax versus ALG prophylaxis in immunologically high‐risk group of renal allograft recipients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):494. [CENTRAL: CN‐00447271] [Google Scholar]
- Pourfarziani V, Lesanpezeshki M, Eyn EB. Zenapax versus anti‐lymphocyte globulin prophylaxis in immunologically high‐risk group of renal allograft recipients. Kowsar Medical Journal 2007;12(1):69‐73. [Google Scholar]
Ruggenenti 2006 {published data only}
- Codreanu I, Cravedi P, Ruggenenti P, Remuzzi G. Antilymphocyte therapy in kidney transplantation: a prospective randomized trial of full‐dose rabbit anti‐human thymocyte globulin (ratg) versus low‐dose RATG and basiliximab. [abstract]. Transplantation 2004;78(2 Suppl):276. [CENTRAL: CN‐00509138] [Google Scholar]
- Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G. Basiliximab combined with low‐dose rabbit anti‐human thymocyte globulin: A possible further step toward effective and minimally toxic T cell‐targeted therapy in kidney transplantation. Clinical Journal of the American Society of Nephrology ‐ CJASN 2006;1(3):546‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sandrini 2002 {published data only}
- Boggi U, Arisi L, Valente U, Greca G, Calconi G, Donati D, et al. Basiliximab facilitates steroid withdrawal after primary kidney transplantation: results of a placebo‐controlled study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00400327]
- Sandrini S, Arisi L, Rizzo G, Valente U, Greca G, Calconi G, et al. Simulect facilitates steroid withdrawal after renal transplantation: results of an Italian, multicentre, placebo‐controlled study [abstract]. 5th International Conference on New Trends in Clinical and Experimental Immunosuppression; 2002 Feb 7‐10; Geneva, Switzerland. 2002. [CENTRAL: CN‐00402503]
- Sandrini S, Rizzo G, Valente U, Greca G, Calconi G, Donati D, et al. Basiliximab facilitates steroid withdrawal after renal transplantation: results of an Italian, multicentre, placebo‐controlled study (Swiss study) [abstract]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00403504] [Google Scholar]
Sheashaa 2003 {published data only}
- Sheashaa HA, Bakr MA, Ismail AM, Gheith OE, Dahshan KF, Sobh MA, et al. Long‐term evaluation of basiliximab induction therapy in live donor kidney transplantation: a five‐year prospective randomized study. American Journal of Nephrology 2005;25(3):221‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sheashaa HA, Bakr MA, Ismail AM, Gheith OE, El‐Dahshan KF, Sobh MA, et al. Basiliximab reduces the incidence of acute cellular rejection in live related donor kidney transplantation, results of five years prospective randomized trial [abstract no:SP425]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v161. [CENTRAL: CN‐00644283] [Google Scholar]
- Sheashaa HA, Bakr MA, Ismail AM, Mahmoud KM, Sobh MA, Ghoneim MA. Basiliximab induction therapy for live donor kidney transplantation: a long‐term follow‐up of prospective randomized controlled study. Clinical & Experimental Nephrology 2008;12(5):376‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sheashaa HA, Bakr MA, Ismail AM, Sobh MA, Ghoneim MA. Basiliximab reduces the incidence of acute cellular rejection in live‐related‐donor kidney transplantation: a three‐year prospective randomized trial. Journal of Nephrology 2003 May;16(3):393‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Shidban 2000 {published data only}
- Shidban H, Sabawi M, Aswad S, Chambers G, Castillon I, Naraghi R, et al. Controlled trial of IL2R antibody basiliximab (Simulect) vs low dose OKT3 in cadaver kidney transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S156. [CENTRAL: CN‐00402633] [Google Scholar]
Shidban 2003 {published data only}
- Aswad S, Shidban H, Naraghi R, Puhawan M, Sabawi M, Mendez RG, et al. A prospective, randomized, phase IV comparative trial of Thymoglobulin® versus Simulect® for the prevention of delayed graft function and acute allograft rejection in renal transplant recipients. [abstract no: SA‐PO551]. Journal of the American Society of Nephrology 2003;14(Nov):417A. [CENTRAL: CN‐00447713] [Google Scholar]
- Shidban H, Sabawi M, Puhawan M, Aswad S, Mendez RG, Mendez R. A prospective, randomized, phase IV comparative trial of thymoglobulin versus simulect for the prevention of delayed graft function and acute allograft rejection in renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):352. [CENTRAL: CN‐00447713] [Google Scholar]
Sollinger 2001 {published data only}
- Kaplan B, Polsky D, Weinfurt K, Fastenau J, Kim J, Ryu S, et al. Quality of life improvement and lower costs associated with Simulect based induction therapy [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):733A. [CENTRAL: CN‐00401459] [Google Scholar]
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pescovitz M, Kovarik JM, Gerbeau C, Simulect US‐O1 Study Group. Pharmacokinetics of basiliximab when coadministered with MMF in kidney transplantation [abstract no: 0112]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00402225]
- Pescovitz MD, Barbeito R. Effect of "C2" Cyclosporine Levels and Time to Initiation of Cyclosporine Therapy on Outcomes in Patients Receiving Neoral and Simulect [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):703A. [CENTRAL: CN‐00433641] [Google Scholar]
- Pescovitz MD, Barbeito R, Simulect US. Two‐hour post‐dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clinical Transplantation 2002;16(5):378‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Polsky D, Weinfurt KP, Kaplan B, Kim J, Fastenau J, Schulman KA. An economic and quality‐of‐life assessment of basiliximab vs antithymocyte globulin immunoprophylaxis in renal transplantation. Nephrology Dialysis Transplantation 2001;16(5):1028‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sollinger H, Kaplan B, Pescovitz M, Philosophe B, Roza A, Brayman K, et al. A multicenter randomized trial of Simulect with early neoral vs atgam with delayed neoral in renal transplantation [abstract no:0113]]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00402698]
- Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation 2001;72(12):1915‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sollinger H, Pescovitz M, Philosophe B, Roza A, Brayman K, Somberg K. A multicenter, randomized trial of simulect with early neoral vs atgam with delayed neoral in renal transplantation. A 6 month interim analysis [abstract]. Transplantation 1999;67(7):S151. [CENTRAL: CN‐00402699] [Google Scholar]
Soulillou/Cant 1990 {published data only}
- Cantarovich D, Giral M, Hourmant M, Dantal J, Blancho G, Soulillou JP. 15‐year results of a randomized study comparing anti‐CD25 monoclonal antibody and antithymocyte globulin induction in kidney transplantation [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
- Cantarovich D, Mauff B, Hourmant M, Giral M, Denis M, Jacques Y, et al. Anti‐IL2 receptor monoclonal antibody (33B3.1) in prophylaxis of early kidney rejection in humans: a randomized trial versus rabbit antithymocyte globulin. Transplantation Proceedings 1989;21(1 (Pt 2)):1769‐71. [MEDLINE: ] [PubMed] [Google Scholar]
- Cantarovich M, Giral M, Hourmant M, Dantal J, Blancho G, Soulillou JP. 15‐year results of a randomized study comparing anti‐cd25 monoclonal antibody and antithymocyte globulin induction in kidney transplantation. [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):308‐9. [CENTRAL: CN‐00415383] [Google Scholar]
- Soulillou JP, Cantarovich D, Mauff B, Giral M, Robillard N, Hourmant M, et al. Randomized controlled trial of a monoclonal antibody against the interleukin‐2 receptor (33B3.1) as compared with rabbit antithymocyte globulin for prophylaxis against rejection of renal allografts. New England Journal of Medicine 1990;322(17):1175‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SYMPHONY (Ekberg) 2007 {published data only}
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981] [Google Scholar]
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐00602015] [Google Scholar]
- Ekberg H, Bernasconi C, Halloran P. CNI minimisation with 2 G mycophenolate mofetil ‐ what have we learned from the Symphony Study [abstract no: 964]. Transplantation 2008;86(2 Suppl):334. [CENTRAL: CN‐00671785] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retained their distinct toxicity profiles despite low doses in the Symphony Study [abstract no: 55]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653721] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Mamelok R, Bernasconi C, Vincenti F, Tedesco‐Silva H, Daloze P, et al. The challenge of meeting target drug concentrations: experience from the Symphony study [abstract no:58]. American Journal of Transplantation 2007;7(Suppl 2):161. [CENTRAL: CN‐00615834] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Daloze P, PearsonT. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: 691]. American Journal of Transplantation 2006;6(Suppl 2):300. [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Bitko S, Klempnauer J, Gurkan A, et al. Improved outcomes after de novo renal transplantation: 2‐year results from the Symphony Study [abstract no: 531]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00653754] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: LB04]. American Journal of Transplantation 2008;8(Suppl 2):336. [CENTRAL: CN‐00653755] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing with mycophenolate mofetil in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: 623]. Transplantation 2008;86(2 Suppl):218. [CENTRAL: CN‐00676055] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. New England Journal of Medicine 2007;357(25):2562‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Symphony ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: 49]. American Journal of Transplantation 2006;6(Suppl 2):83. [CENTRAL: CN‐00602018] [Google Scholar]
- Ekberg H, Vincenti F, Tedesco da Silva H, Daloze P, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the Symphony Study [abstract no: 691]. American Journal of Transplantation 2006;6(Suppl 2):300. [CENTRAL: CN‐00602015] [Google Scholar]
- Frei U, Daloze P, Vitko S, Klempnauer J, Reyes‐Acevedo R, Titiz I, et al. Characterization of acute rejections and associated relative risk factors in the Symphony Study [abstract no: 245]. American Journal of Transplantation 2007;7(Suppl 2):210. [CENTRAL: CN‐00653713] [Google Scholar]
- Frei U, Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, et al. SYMPHONY ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: F‐FC152]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Gentil MA, Hernandez D, Plumed JS, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study [abstract no: P342]. Transplant International 2007;20(Suppl 2):177. [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the Symphony PK substudy [abstract no: 824]. American Journal of Transplantation 2006;6(Suppl 2):345. [CENTRAL: CN‐00602016] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study. American Journal of Transplantation 2007;7(Suppl 2):443. [CENTRAL: CN‐00653737] [Google Scholar]
- Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard‐dose or low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony pharmacokinetic substudy. Nephrology Dialysis Transplantation 2009;24(7):2269‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Halloran P, Meier‐Kriesche HU, Schold J, Vanrenterghem Y. Blood pressure in the first year following renal transplantation is associated with immunosuppressive regimens: evidence from the Symphony study [abstract no:1137]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615835] [Google Scholar]
- Kuypers D, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the SYMPHONY PK substudy [abstract no: TH‐PO564]. Journal of the American Society of Nephrology 2006;17(Abstracts):227A. [CENTRAL: CN‐00602016] [Google Scholar]
- Lloberas N, Brunet M, Torras J, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. Influence of MRP2 and UGT1A9 polimorphisms in the MPA pharmacokinetics in renal transplant substudy within the Symphony Study [abstract no: 2243]. Transplantation 2008;86(2 Suppl):733. [CENTRAL: CN‐00671787] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Llaudo I, Torras J, Caldes A, Cruzado JM. Polymorphisms of the MDR1 gene in renal transplant recipients affect the PGP activity. Results of the pharmacogenetic substudy within the Symphony Study [abstract no: 2244]. Transplantation 2008;86(2 Suppl):734. [CENTRAL: CN‐00671786] [Google Scholar]
- Oppenheimer F, Rebello P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Health‐related quality of life of patients receiving low toxicity immunosuppressive regimens [abstract no: TH‐PO566]. Journal of the American Society of Nephrology 2006;17(Abstracts):228A. [CENTRAL: CN‐00602017] [DOI] [PubMed] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Cost‐effectiveness analysis of adverse events in low toxicity immunosuppressive regimens, preliminary results of the quality of life substudy within the Symphony Study [abstract no: 107]. Transplantation 2008;86(2 Suppl):38. [CENTRAL: CN‐00677744] [Google Scholar]
Tan 2004 {published data only}
- Tan J, Yang S, Wu W. Basiliximab (Simulect) reduces acute rejection among sensitized kidney allograft recipients. Transplantation Proceedings 2005;37(2):903‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tan J, Yang S, Wu W. Basiliximab (simulect®) reduces acute rejection among sensitized kidney allograft recipients [abstract]. Transplantation 2004;78(2 Suppl):266. [DOI] [PubMed] [Google Scholar]
ter Meulen 2002 {published data only}
- Hendrikx TK, Klepper M, IJzermans J, Weimar W, Baan CC. Clinical rejection and persistent immune regulation in kidney transplant patients. Transplant Immunology 2009;21(3):129‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. British Journal of Clinical Pharmacology 2003;56(3):327‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselink DA, Ngyuen H, Wabbijn M, Smak Gregoor PJH, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids [abstract]. American Journal of Transplantation 2003;3(Suppl 5):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Meulen CG, Riemsdijk IC, Hene RJ, Christiaans MHL, Gelder T, Hilbrands LB, et al. A prospective randomized trial comparing steroid‐free immunosuppression with limited steroid exposure on bone mineral density in the first year after renal transplantation [abstract no:0344]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00402832]
- ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin‐2 receptor alpha after treatment with daclizumab. Kidney International 2003;64(2):697‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Hilbrands LB, Bergh JP, Hermus AR, Hoitsma AJ. The influence of corticosteroids on quantitative ultrasound parameters of the calcaneus in the 1st year after renal transplantation. Osteoporosis International 2005;16(3):255‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Riemsdijk I, Hene RJ, Christiaans MH, Borm GF, Corstens FH, et al. No important influence of limited steroid exposure on bone mass during the first year after renal transplantation: a prospective, randomized, multicenter study. Transplantation 2004;78(1):101‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Riemsdijk I, Hene RJ, Christiaans MH, Borm GF, Gelder T, et al. Steroid‐withdrawal at 3 days after renal transplantation with anti‐IL‐2 receptor alpha therapy: a prospective, randomized, multicenter study. American Journal of Transplantation 2004;4(5):803‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gelder T, ter Meulen CG, Hene RJ, Christiaans MhL, Borm GF, Riemsdijk IC, et al. Steroid withdrawal at three days after renal transplantation with anti il‐2 receptor therapy: a prospective randomized multicenter trial. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509529] [DOI] [PubMed] [Google Scholar]
- Riemsdijk IC, Termeulen RG, Christiaans MH, Hene RJ, Hoitsma AJ, Hooff JP, et al. Anti‐CD25 prophylaxis allows steroid‐free renal transplantation in tacrolimus‐based immunosuppression [abstract no: 133]. American Journal of Transplantation 2002;2(Suppl 3):171. [CENTRAL: CN‐00402963] [Google Scholar]
Tullius 2003 {published data only}
- Pascher A, Ulrich F, Kohler S, Weiss S, Tullius S, Reinke P, et al. ATG versus basiliximab induction therapy in kidney allograft recipients receiving dual immunosuppressive regimen: six‐year results [abstract no: 800]. Transplantation 2008;86(2 Suppl):279. [CENTRAL: CN‐00676048] [Google Scholar]
- Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. ATG versus basiliximab induction therapy in renal allograft recipients receiving a dual immunosuppressive regimen: one‐year results. Transplantation Proceedings 2003;35(6):2100‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. Induction therapy with ATG vs basiliximab (simulect) in renal allograft recipients: 1‐year results of a prospective randomized, single center study [abstract]. American Journal of Transplantation 2003;3(Suppl 5):478. [CENTRAL: CN‐00520398] [Google Scholar]
- Ulrich F, Niedzwiecki S, Pascher A, Fellmer P, Weiss S, Kohler S, et al. ATG versus basiliximab induction therapy in kidney allograft recipients receiving a dual immunosuppressive regimen: six‐year results [abstract no: 540]. American Journal of Transplantation 2008;8(Suppl 2):322. [CENTRAL: CN‐00653718] [Google Scholar]
van Gelder 1995 {published data only}
- Wabbijn M, Balk AH, Domburg RT, Vantrimpont PJ, Riemsdijk IC, Baan CC, et al. Ten‐year follow‐up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin‐2 receptor. Experimental & Clinical Transplantation 2004;2(1):201‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Gelder T, Zietse R, Mulder AH, Yzermans JN, Hesse CJ, Vaessen LM, et al. A double‐blind, placebo‐controlled study of monoclonal anti‐interleukin‐2 receptor antibody (BT563) administration to prevent acute rejection after kidney transplantation. Transplantation 1995;60(3):248‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gelder T, Zietse R, Yzermans JN, Rischen‐Vos J, Vaessen LM, Weimar W. Long‐term follow‐up after induction treatment with monoclonal anti‐interleukin‐2 receptor antibody (BT563) in kidney allograft recipients: a double‐blind, placebo‐controlled trial. Transplantation Proceedings 1996;28(6):3221‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Vincenti 2003 {published data only}
- Vincenti F, Pace D, Birnbaum J, Lantz M. Pharmacokinetic and pharmacodynamic studies of one or two doses of daclizumab in renal transplantation. American Journal of Transplantation 2003;3(1):50‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 2004 {published data only}
- Asher JF, Wilson CH, Gupta A, Gok MA, Talbot D. Use of daclizumab in preventing delayed graft function in non‐heart beating donor kidney transplantation in Newcastle upon Tyne. Transplantationsmedizin ‐ Organ der Deutschen Transplantationsgesellschaft 2004;16(2):96‐100. [EMBASE: 2004360749] [Google Scholar]
- Asir L, Wilson CH, Talbot D. Interleukin 2 receptor blockers may directly inhibit lymphocyte mediated ischaemia reperfusion injury. Transplant International 2005 Sep;18(9):1116. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilson C, Brook NR, Gok MA, Gupta A, Asher JF, Nicholson ML, et al. Evaluation of daclizumab to reduce delayed graft function in non‐heart‐beating renal transplantation: a prospective, randomized trial. Transplantation Proceedings 2005;37(4):1774‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilson C, Brook NR, Gok MA, Gupta AJ, Asher J, Nicholson ML, et al. Evaluation of daclizumab to reduce delayed graft function in non‐heartbeating renal transplantation: a prospective randomised trial [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550743]
- Wilson CH, Brook NR, Gok MA, Asher JF, Nicholson ML, Talbot D. Randomized clinical trial of daclizumab induction and delayed introduction of tacrolimus for recipients of non‐heart‐beating kidney transplants. British Journal of Surgery 2005;92(6):681‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilson CH, Brook NR, Gok MA, Gupta A, Asher JF, Nicholson ML, et al. A randomised controlled trial of daclizumab to reduce the incidence of delayed graft function in recipients of non‐heart‐beating renal grafts. [abstract]. Transplantation 2004;78(2 Suppl):466. [CENTRAL: CN‐00509562] [Google Scholar]
- Wilson CH, Brook NR, Gok MA, Gupta AJ, Nicholson ML, Talbot D. Prospective randomised trial of the use of Daclizumab in renal transplantation using kidneys from non heart beating donors. Annals of Transplantation 2004;9(2):29‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Yussim 2004 {published data only}
- Yussim A, Bielsky V, Bar‐Nathan N, Shaharabani E, Burstein I, Lustig S, et al. Two‐dose daclizumab in conjunction with tacrolimus‐based protocol in kidney transplantation ‐ prospective, randomized study. [abstract]. Transplantation 2004;78(2 Suppl):466. [CENTRAL: CN‐00509575] [Google Scholar]
References to studies excluded from this review
Andres 2009 {published data only}
- Andres A, Marcen R, Valdes F, Plumed JS, Sola R, Errasti P, et al. A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function. Clinical Transplantation 2009;23(1):23‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Budde 2005 {published data only}
- Budde K, Bosmans J, Zeier M, Sennesael J, Hopt U, Fischer WH, et al. Safety and efficacy of reduced or full dose of cyclosporine (neoral®) in combination with mycophenolatesodium (myfortic®), basiliximab (simulect®), and steroids in de novo kidney transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):83. [CENTRAL: CN‐00527096] [Google Scholar]
- Budde K, Bosmans JL, Sennesael J, Zeier M, Hopt U, Fischer W, et al. Reduced cyclosporine exposure is safe and efficacious in combination with basiliximab, enteric‐coated mycophenolate‐sodium, and steroids [abstract no: 1195]. American Journal of Transplantation 2005;5(Suppl 11):461. [CENTRAL: CN‐00644165] [Google Scholar]
- Budde K, Bosmans JL, Sennesael J, Zeier M, Pisarski P, Schutz M, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab. Clinical Nephrology 2007;67(3):164‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Zeier M, Bosmans JL, Sennesael J, Glander P, Fischer W, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab [abstract no: F‐PO1088]. Journal of the American Society of Nephrology 2006;17(Abstracts):565A. [CENTRAL: CN‐00644166] [DOI] [PubMed] [Google Scholar]
Burke 2005 {published data only}
- Burke GW III, Ciancio G, Figueiro J, Olson L, Gomez C, Rosen A, et al. Can acute rejection be prevented in SPK transplantation?. Transplantation Proceedings 2002;34(5):1913‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burke GW, Ciancio G, Mattaiazzi A, Gomez C, Rosen A, Suzart K, et al. Can acute rejection be prevented in SPK transplantation? A randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance, comparing rapamycin with mycophenolate mofetil [abstract]. American Journal of Transplantation 2003;3(Suppl 5):322. [CENTRAL: CN‐00444587] [Google Scholar]
- Burke GW, Ciancio G, Mattiazzi A, Gomez C, Rosen A, Miller J. Can acute rejection be prevented in SPK transplantation? a randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycophenolate mofetil. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):562. [CENTRAL: CN‐00509113] [Google Scholar]
- Burke GW, Ciancio G, Mattiazzi A, Gomez C, Rosen A, Miller J. Lower rate of acute rejection with rapamycin than with mycophenolate mofetil in kidney pancreas transplantation: a randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycopenolate mofetil [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550658]
- Burke GW, Ciancio G, Mattiazzi A, Illanes H, Gomez C, Rosen A, et al. Lower rate of acute rejection with rapamycin than with mycophenolate mofetil in kidney pancreas transplantation. A randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycophenolate mofetil. [abstract no: 789]. American Journal of Transplantation 2005;5(Suppl 11):357. [CENTRAL: CN‐00644167] [Google Scholar]
Chadban 2006 {published data only}
- Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one‐year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric‐coated mycophenolate sodium (EC‐MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA‐ME), basiliximab and steroids in de novo kidney transplantation. [abstract no: 32]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:51. [CENTRAL: CN‐00583470]
Chan 2008 {published data only}
- Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85(6):821‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Everolimus (RAD001) concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients [abstract no: SA‐PO2529]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):678A. [Google Scholar]
Flechner‐318 2002 {published data only}
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351] [Google Scholar]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00415657]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches. [abstract no:1317]. American Journal of Transplantation 2002;2(Suppl 3):470. [Google Scholar]
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil‐based immunosuppression: 5‐year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83(7):883‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Kurian S, Solez K, Cook DJ, Burke JT, Rollin H, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function at two years compared to calcineurin inhibitor drugs. [abstract]. Transplantation 2004;78(2 Suppl):141. [CENTRAL: CN‐00509193] [Google Scholar]
- Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. American Journal of Transplantation 2004;4(11):1776‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Solez K, Cook DJ, Burke JT, Rollin H, Mastoianni B, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function compared to calcineurin inhibitor (cni) drugs. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):296. [CENTRAL: CN‐00509194] [Google Scholar]
FREEDOM Study {published data only}
- Schena FP, Vincenti F, Paraskevas S, Hauser I, FREEDOM Study Group. Renal function and rejection incidence in de novo renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids [abstract no: F‐FC153]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601969] [Google Scholar]
- Schena FP, Vincenti F, Paraskevas S, Hauser I, Grinyo J, FREEDOM Study Group. 12‐month results of a prospective, randomized trial of steroid avoidance, steroid withdrawal and standard steroids in de novo renal transplant patients receiving cyclosporine, enteric‐coated mycophenolate sodium (EC‐MPS, myfortic®) and basiliximab [abstract no: 54]. American Journal of Transplantation 2006;6(Suppl 2):84‐5. [CENTRAL: CN‐00644263] [Google Scholar]
- Vincenti F, Schena FP, Paraskevas S, Hauser I, FREEDOM Study Group. Comparison of metabolic parameters in renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids with a 12‐month, randomized, multicenter trial [abstract no: F‐PO1076]. Journal of the American Society of Nephrology 2006;17(Abstracts):562A. [CENTRAL: CN‐00602097] [Google Scholar]
- Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. American Journal of Transplantation 2008;8(2):307‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walker R, Campbell S, Chadban S, Kanellis J, Pilmore H, Russ G. Preliminary results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS), basiliximab, and neoral C‐2 comparing a regimen without steroids or short‐term use of steroids with standard steroid treatment in de novo kidney recipients. [abstract no: 34]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:52. [CENTRAL: CN‐00583481]
- Walker R, Vincenti F, Schena FP, Pescovitz MD, Shoker A, Grinyo J, et al. Preliminary results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS), basiliximab, and neoral C‐2 comparing two investigational steroid regimens (without steroids or short‐term use of steroids) with standard steroid treatment in de novo kidney recipients [abstract no: T‐PO50027]. Nephrology 2005;10(Suppl):A214. [CENTRAL: CN‐00583480] [Google Scholar]
Hamdy 2005 {published data only}
- Hamdy AF, Bakr MA, Ghoneim MA. Long‐term efficacy and safety of a calcineurin inhibitor‐free regimen in live‐donor renal transplant recipients. Journal of the American Society of Nephrology 2008;19(6):1225‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy e, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiesse 1992 {published data only}
- Hiesse C, Kriaa F, Alard P, Lantz O, Noury J, Bensadoun H, et al. Prophylactic use of the IL‐2 receptor‐specific monoclonal antibody LO‐Tact‐1 with cyclosporin A and steroids in renal transplantation. Transplant International 1992;5 Suppl 1:S444‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hirose 2004 {published data only}
- Hirose K, Posselt AM, Stock PG, Hirose R, Vincenti F. Treatment of kidney transplant patients with the novel co‐stimulatory blocker LEA29y (BMS‐224818) and antiil2 receptor antibody does not impede the development of regulatory t cells. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):442. [CENTRAL: CN‐00509233] [Google Scholar]
Kovarik 2003 {published data only}
- Kovarik JM, Dantal J, Civati G, Rizzo G, Rouilly M, Bettoni‐Ristic O, et al. Influence of delayed initiation of cyclosporine on everolimus pharmacokinetics in de novo renal transplant patients. American Journal of Transplantation 2003;3(12):1576‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kramer‐2307 2003 {published data only}
- Campbell S, Eris J, Brown F, Russ G, Caicedo L, Walker R, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 24 month result [abstract no: FC‐50002]. Nephrology 2005;10(Suppl):A1. [CENTRAL: CN‐00602128] [Google Scholar]
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CsA and basiliximab at 24 months. [abstract no: 36]. 24th Annual Scientific Meeting.Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:53. [CENTRAL: CN‐00583469]
- Eris J, Campbell S, Burbigott B, Leone J, Kraemer B, Rigotti P, et al. Excellent graft function in de novo kidney transplant recipients treated with certican®, simulect® and reduced neoral® exposure: 12‐month results [abstract]. Transplantation 2004;78(2 Suppl):31. [Google Scholar]
- Eris J, Campbell S, Walker R, Russ G, Stambe C, RAD2307 International Study Group. Excellent graft function in de novo transplant recipients treated with everolimus, reduced dose neoral and simulect: 6 months analysis. [abstract]. 22nd Annual Scientific Meeting Transplantation Society of Australia & New Zealand; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:33. [CENTRAL: CN‐00509178]
- Kraemer BK, Bourbigot B, Vitko S, Rigotti P, Caicedo L, Boccardo G. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CSA and basiliximab at 24 months [abstract no: PO‐437]. 12th Congress of the European Society for Organ Transplantation (ESOT); 2005 Oct 15‐19; Geneva, Switzerland. 2005. [CENTRAL: CN‐00653704]
- Kramer BK, Neumayer HH, Stahl R, Pietrzyk M, Kruger B, Pfalzer B, et al. Graft function, cardiovascular risk factors, and sex hormones in renal transplant recipients on an immunosuppressive regimen of everolimus, reduced dose of cyclosporine, and basiliximab. Transplantation Proceedings 2005;37(3):1601‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Whelchel J, Eris J, Campbell S, Vitko S, Haas T, et al. Excellent graft function in de novo kidney transplant recipients treated with concentration controlled everolimus, reduced neoral exposure and basiliximab: 6 months analysis [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786. [CENTRAL: CN‐00446190] [Google Scholar]
- Leone J, Vitko S, Whelchel J, Eris J, Campbell S, Boubigott B, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican©, Simulect© and reduced Neoral© exposure: 24 month result [abstract no: 1011]. American Journal of Transplantation 2005;5(Suppl 11):414. [Google Scholar]
- Rigotti P, et al. Excellent Graft Function in De Novo Kidney Transplant Recipients Treated with Concentration Controlled Everolimus, Reduced Neoral Exposure and Simulect: 6 Months Analysis [abstract no:2]. European Society of Transplantation; 2003 Sept; Venice, Italy. 2003. [CENTRAL: CN‐00527155]
- Tedesco H, Pascual J, Civati G, Filho G, Garcia V, Haas T. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral in de novo kidney transplant recipients: 6 months analysis [abstract]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00447959] [Google Scholar]
- Tedesco‐Silva H Jr, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12‐month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transplant International 2007;20(1):27‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6‐month safety and efficacy results of two randomized studies. American Journal of Transplantation 2004;4(4):626‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Whelchel J, Vitko S, Eris J, Campbell S, Burbigott B, Leone J, et al. Excellent graft function in de novo kidney transplant recipients treated with certican®, simulect® and reduced neoral® exposure: 12‐month results. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):297. [CENTRAL: CN‐00509179] [Google Scholar]
Kreis 2003 {published data only}
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Lowering cyclosporine dose is not associated with an increased risk of gastro‐intestinal adverse events nor the need for dosage decrease of mycophenolate mofetil [abstract no: P743]. Transplantation 2004;78(2 Suppl):462. [CENTRAL: CN‐00509292] [Google Scholar]
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse m, et al. Daclizumab and mycophenolate mofetil in renal transplant recipients: 2‐year outcome after early reduction of cyclosporine [abstract]. American Journal of Transplantation 2003;3(Suppl 5):476. [CENTRAL: CN‐00446199] [Google Scholar]
Light 2002 {published data only}
- Light JA, Sasaki TM, Ghasemian R, Barhyte DY, Fowlkes DL. Daclizumab induction/tacrolimus sparing: a randomized prospective trial in renal transplantation. Clinical Transplantation 2002;16(Suppl 7):30‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martinez‐Mier 2006 {published data only}
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: Initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [EMBASE: 2006628001] [DOI] [PubMed] [Google Scholar]
McDonald 2008 {published data only}
- McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. American Journal of Transplantation 2008;8(5):984‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meier‐Kriesche 2004 {published data only}
- Bresnahan B, Cibrik D, Jensik S, Whelchel J, Klintmalm G, Cohen D, et al. Treatment of high‐risk renal transplant recipients with EC‐MPS (Myfortic®) is safe and efficacious [abstract no: PUB216]. Journal of the American Society of Nephrology 2005;16:829A. [CENTRAL: CN‐00644277] [Google Scholar]
- Cibrik D, Jensik S, Bresnahan B, Whelchel J, Klintmalm G, ERL2405‐US01 Study Group. Safety and efficacy of EC‐MPS in combination with simulect and neoral in de novo renal transplant high‐risk recipients [abstract no: 135]. American Journal of Transplantation 2005;5(Suppl 11):190. [CENTRAL: CN‐00644170] [Google Scholar]
- Cibrik D, Jensik S, Meier‐Kriesche H, Bresnahan B, Lieberman B, Myfortic US01 renal Transplant Group. Enteric‐coated mycophenolate sodium in combination with optimized neoral dosing, basiliximab, and steroids results in good efficacy and renal function in renal transplant recipients in the first six months [abstract no:220]. American Journal of Transplantation 2008;4(Suppl 8):218. [CENTRAL: CN‐00644278] [Google Scholar]
- Cibrik D, Meier‐Kriesche HU, Bresnahan B, Wu YM, Klintmalm G, Kew CE, et al. Renal function with cyclosporine C2 monitoring, enteric‐coated mycophenolate sodium and basiliximab: a 12‐month randomized trial in renal transplant recipients. Clinical Transplantation 2007;21(2):192‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meier‐Kriesche H, Cibrik D, Bresnahan B, Cohen D, Lieberman B. Optimized Neoral C2 monitoring in combination with enteric‐coated mycophenolic acid, basiliximab and steroids is effective, safe and tolerable: 12‐month results of a multicenter, randomized, prospective trial. [abstract no: F‐PO1068]. Journal of the American Society of Nephrology 2004;15(Oct Abstracts Issue):299A. [CENTRAL: CN‐00583408] [Google Scholar]
Montagnino 2005 {published data only}
- Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplantation Proceedings 2005;37(2):788‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, et al. A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low‐dose cyclosporine. Nephrology Dialysis Transplantation 2008;23(2):707‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mourad 2005 {published data only}
- Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. American Journal of Transplantation 2006;6(5 Pt 1):1042‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Karras A, Kamar N, Garrigue V, Legendre C, Lefrancois N, et al. Renal function with delayed or immediate cyclosporine microemulsion in combination with enteric‐coated mycophenolate sodium and steroids: results of follow up to 30 months post‐transplant. Clinical Transplantation 2007;21(3):295‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C. Assessment of two strategies of neoral® administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic®, steroids and anti‐il2r antibodies: 6 months interim results. [abstract]. Transplantation 2004;78(2 Suppl):454. [CENTRAL: CN‐00509366] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C, Myriade FR. Assessment of two strategies of neoral administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic, steroids, and anti‐IL2R antibodies: 12‐month results of a randomized, multicentre, open, prospective controlled study. Transplantation Proceedings 2005;37(2):920‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing, Rostaing L, Legendre C. Assessment of two neoral® administration strategies on renal function and efficacy in de novo renal transplant patients receiving enteric‐coated mycophenolate sodium, steroids and anti‐il2r antibodies: 6 months interim analysis of a randomized, multicentre, open, prospective controlled study.[abstract]. American Journal of Transplantation 2004;4(Suppl 8):219. [CENTRAL: CN‐00509367] [Google Scholar]
- Rostaing L, Mourad G, Kamar N, Garrigue V, Karras A, Lefrancois N, et al. Tolerability of enteric‐coated mycophenolate sodium to 1 year in combination with cyclosporine and corticosteroids in renal transplant recipients. Transplantation Proceedings 2006;38(9):2860‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Mourad G, Legendre C. Safety and tolerability of enteric‐coated mycophenolate sodium in combination with steroids and two regimen of neoral®, in denovo kidney transplant recipients: 6 months interim results. A randomized, multicentre, open, prospective controlled study. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):219. [CENTRAL: CN‐00583362] [Google Scholar]
MyPROMS Study {published data only}
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nematalla 2007 {published data only}
- Neamatalla A, Bakr A, Elagroudy A, Elshehawy E, Shokier A, Ghoneim M. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: FP222]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi93. [CENTRAL: CN‐00653762] [PubMed] [Google Scholar]
- Nematalla AH, Bakr MA, Elagrody AE, Elshehawy E, Salim M, Shokier AA. Steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (one year follow up) [abstract no: SP734]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00653763] [PubMed] [Google Scholar]
- Nematalla AH, Bakr MA, Gheith OA, Elagroudy AE, Elshahawy e, Aghoneim M. Steroid‐avoidance immunosuppression regimen in live‐donor renal allotransplant recipients: a prospective, randomized, controlled study. Experimental & Clinical Transplantation 2007;5(2):673‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Painter 2003 {published data only}
- Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, et al. Health‐related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney International 2003;63(6):2309‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pescovitz 2004 {published data only}
- Pescovitz M, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety and efficacy of mycophenolate mofetil in combination with sirolimus vs cyclosporine in renal transplant patients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):251. [CENTRAL: CN‐00509411] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. British Journal of Clinical Pharmacology 2007;64(6):758‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Provenzano 2000 {published data only}
- Provenzano R, Tayeb J, Thackkar R, Morrison L. Analysis of patient and graft outcomes in daclizumab based induction immunosuppression using neoral vs tacrolimus [abstract]. Journal of the American Society of Nephrology 2000;11(Sept Program & Abstracts):703A. [CENTRAL: CN‐00433643] [Google Scholar]
Scholten 2006 {published data only}
- Scholten EM, Rowshani AT, Cremers S, Bemelman FJ, Eikmans M, Kan E, et al. Untreated rejection in 6‐month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of graft function. Journal of the American Society of Nephrology 2006;17(9):2622‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tian 2007 {published data only}
- Tian J, Zhang JY, Hu D, Hu WF, Huang J. Influences of single‐dose Basiliximab on Foxp3mRNA, CD4+CD25+ regulatory T cells, interleukin‐2 and interleukin‐2 receptor in the peripheral blood of renal transplantation recipients. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(25):4861‐5. [EMBASE: 2007338028] [Google Scholar]
Vincenti 2005b {published data only}
- Vincenti F, Schena F, Walker R, Pescovitz M, Shoker A. 3 months interim results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS, Myfortic©), basiliximab, and neoral C‐2 comparing different steroid protocols in de novo kidney recipients [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2005;16(Oct):236A. [Google Scholar]
- Vincenti F, Schena FP, Walker R, Pescovitz MD, Shoker A, Grinyo J, et al. Preliminary 3‐month results comparing immunosuppressive regimens of enteric‐coated mycophenolate sodium (EC‐MPS) without steroids vs short‐term use of steroids vs standard steroid treatment including basiliximab, and neoral C‐2 in de novo kidney recipients [abstract no: 1542]. American Journal of Transplantation 2005;5(Suppl 11):548. [Google Scholar]
Wang 2008 {published data only}
- Wang Z, Xiao L, Shi BY, Qian YY, Bai HW, Chang JY, et al. Short‐term anti‐CD25 monoclonal antibody treatment and neogenetic CD4(+)CD25(high) regulatory T cells in kidney transplantation. Transplant Immunology 2008;19(1):69‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zarkhin 2008 {published data only}
- Sarwal M, Zarkhin V, Mohile S, Kambham N, Li L, Martin J, et al. Randomized trial of Rituximab vs standard of care for B cell dense acute renal transplant rejection [abstract no: 538]. American Journal of Transplantation 2007;7(Suppl 2):287. [CENTRAL: CN‐00644179] [Google Scholar]
- Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. American Journal of Transplantation 2008;8(12):2607‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
ANZDATA 2008
- Campbell S, McDonald S, Excell L, Livingston B. Chapter 8 Transplantation. In: McDonald S, Excell L, Livingston B editor(s). ANZDATA Registry Report 2008. available from http://www.anzdata.org.au/v1/annual_reports_download.html. Adelaide, South Australia.: Australia and New Zealand Dialysis and Transplant Registry, 2008. [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cibrik 2001
- Cibrik Dm, Kaplan B, Meier‐Kriesche H. Role of anti‐interleukin‐2 receptor antibodies in kidney transplantation. Biodrugs 2001;15(10):655‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clarke 2001
- Clarke MJ. Obtaining individual patient data from randomised controlled trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care. BMJ books, 2001:109‐21. [Google Scholar]
Cuervo 2003
- Cuervo LG, Clarke M. Balancing benefits and harms in health care. BMJ 2003;327(7406):65‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Publishing Group, 2001:285‐312. [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JNV, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Goebel 2000
- Goebel J, Stevens E, Forrest K, Roszman TL. Daclizumab (Zenapax) inhibits early interleukin‐2 receptor signal transduction events. Transplant Immunology 2000;8(3):153‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons Inc, 2008. [Google Scholar]
Hong 2000
- Hong J, Kahan B. Immunosuppressive agents in organ transplantation: past, present and future. Seminars in Nephrology 2000;20(2):108‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium, Adelaide, Australia, 20‐24 October. 1996.
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morton 2009
- Morton RL, Howard K, Webster AC, Wong G, Craig JC. The cost‐effectiveness of induction immunosuppression in kidney transplantation. Nephrology Dialysis Transplantation 2009;24(7):2258‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPTN/SRTR 2008
- Rockville M, Richmond V. Annual Report of the US Scientific Registry of Transplant Recipients and the Organ Procurement and transplantation Network: Transplant data 1989‐1998. 2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998‐2007 http://optn.transplant.hrsa.gov/data/annualReport.asp. Rockville, MD, USA: U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2008.
Pascual 2001
- Pascual J, Marcen R, Ortuno J. Anti‐interleukin‐2 receptor antibodies: basiliximab and daclizumab. Nephrology Dialysis Transplantation 2001;16(9):1756‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Renal Group 2009
- Willis NS, Mitchell R, Higgins GY, Webster AC, Craig JC. Cochrane Renal Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2009, Issue 4. Art. No.: RENAL (accessed November 2009).
UK National Transplant Database 2009
- Transplant Activity report 2008‐2009, section 3 Kidney Activity. NHS Blood and Transplant, Transplant Activity in the UK. available at: http://www.organdonation.nhs.uk/ukt/statistics/statistics.jsp.
UK Renal Registry report 2007
- Ravanan R, Udayaraj U, Steenkamp R, Ansel D, Tomson C. Chapter 11: Measures of Care in Adult Renal Transplant Recipients in the UK. In: Ansell D, Feehally J, Feest T, Tomson C, Williams AJ, Warwick G editor(s). The Renal Association UK Renal Registry Tenth Annual Report. Bristol, UK: The Renal Association, December 2007. [Google Scholar]
Vanrenterghem 2001
- Vanrenterghem Y. Tailoring immunosuppressive therapy for renal transplant recipients. Pediatric Transplantation 2001;5(6):467‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Webster 2003
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig J. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003897] [DOI] [PubMed] [Google Scholar]
Webster 2004
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig J. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003897.pub2] [DOI] [PubMed] [Google Scholar]


