Abstract
Background
The delivery of combination contraceptive steroids from a transdermal contraceptive patch or a contraceptive vaginal ring offers potential advantages over the traditional oral route. The transdermal patch and vaginal ring could require a lower dose due to increased bioavailability and improved user compliance.
Objectives
To compare the contraceptive effectiveness, cycle control, compliance (adherence), and safety of the contraceptive patch or the vaginal ring versus combination oral contraceptives (COCs).
Search methods
Through February 2013, we searched MEDLINE, POPLINE, CENTRAL, LILACS, ClinicalTrials.gov, and ICTRP for trials of the contraceptive patch or the vaginal ring. Earlier searches also included EMBASE. For the initial review, we contacted known researchers and manufacturers to identify other trials.
Selection criteria
We considered randomized controlled trials comparing a transdermal contraceptive patch or a contraceptive vaginal ring with a COC.
Data collection and analysis
Data were abstracted by two authors and entered into RevMan. For dichotomous variables, the Peto odds ratio (OR) with 95% confidence intervals (CI) was calculated. For continuous variables, the mean difference was computed. We also assessed the quality of evidence for this review.
Main results
We found 18 trials that met our inclusion criteria. Of six patch studies, five examined the marketed patch containing norelgestromin plus ethinyl estradiol (EE); one studied a patch in development that contains levonorgestrel (LNG) plus EE. Of 12 vaginal ring trials, 11 examined the same marketing ring containing etonogestrel plus EE; one studied a ring being developed that contains nesterone plus EE.
Contraceptive effectiveness was not significantly different for the patch or ring versus the comparison COC. Compliance data were limited. Patch users showed better compliance than COC users in three trials. For the norelgestromin plus EE patch, ORs were 2.05 (95% CI 1.83 to 2.29) and 2.76 (95% CI 2.35 to 3.24). In the levonorgestrel plus EE patch report, patch users were less likely to have missed days of therapy (OR 0.36; 95% CI 0.25 to 0.51). Of four vaginal ring trials, one found ring users had more noncompliance (OR 3.99; 95% CI 1.87 to 8.52), while another showed more compliance with the regimen (OR 1.67; 95% CI 1.04 to 2.68).
More patch users discontinued early than COC users. ORs from two meta‐analyses were 1.59 (95% CI 1.26 to 2.00) and 1.56 (95% CI 1.18 to 2.06) and another trial showed OR 2.57 (95% CI 0.99 to 6.64). Patch users also had more discontinuation due to adverse events than COC users. Users of the norelgestromin‐containing patch reported more breast discomfort, dysmenorrhea, nausea, and vomiting. In the levonorgestrel‐containing patch trial, patch users reported less vomiting, headaches, and fatigue.
Of 11 ring trials with discontinuation data, two showed the ring group discontinued less than the COC group: OR 0.32 (95% CI 0.16 to 0.66) and OR 0.52 (95% CI 0.31 to 0.88). Ring users were less likely to discontinue due to adverse events in one study (OR 0.32; 95% CI 0.15 to 0.70). Compared to the COC users, ring users had more vaginitis and leukorrhea but less vaginal dryness. Ring users also reported less nausea, acne, irritability, depression, and emotional lability than COC users.
For cycle control, only one trial study showed a significant difference. Women in the patch group were less likely to have breakthrough bleeding and spotting. Seven ring studies had bleeding data; four trials showed the ring group generally had better cycle control than the COC group.
Authors' conclusions
Effectiveness was not significantly different for the methods compared. Pregnancy data were available from half of the patch trials but two‐thirds of ring trials. The patch could lead to more discontinuation than the COC. The patch group had better compliance than the COC group. Compliance data came from half of the patch studies and one‐third of the ring trials. Patch users had more side effects than the COC group. Ring users generally had fewer adverse events than COC users but more vaginal irritation and discharge.
The quality of the evidence for this review was considered low for the patch and moderate for the ring. The main reasons for downgrading were lack of information on the randomization sequence generation or allocation concealment, the outcome assessment methods, high losses to follow up, and exclusions after randomization.
Keywords: Female; Humans; Pregnancy; Consumer Behavior; Contraceptive Agents, Female; Contraceptive Agents, Female/administration & dosage; Contraceptive Agents, Female/adverse effects; Contraceptive Devices, Female; Contraceptive Devices, Female/adverse effects; Contraceptives, Oral, Combined; Contraceptives, Oral, Combined/administration & dosage; Contraceptives, Oral, Combined/adverse effects; Drug Implants; Drug Implants/adverse effects; Medication Adherence; Menstrual Cycle; Menstrual Cycle/drug effects; Randomized Controlled Trials as Topic
Plain language summary
Skin patch or vaginal ring compared to pills for birth control
The skin patch and the vaginal (birth canal) ring are two methods of birth control. Both methods contain the hormones estrogen and progestin. The patch is a small, thin, adhesive square that is applied to the skin. The contraceptive vaginal ring is a flexible, lightweight device that is inserted into the vagina. Both methods release drugs like those in birth control pills. These methods could be used more consistently than pills because they do not require a daily dose. This review looked at how well the methods worked to prevent pregnancy, if they caused bleeding problems, if women used them as prescribed, and how safe they were.
Through February 2013, we did computer searches for randomized controlled trials of the skin patch or vaginal ring compared to pills for birth control. Pills included types with both estrogen and progestin. We wrote to researchers to find other trials.
We found 18 trials. Of six patch trials, five compared the marketed patch to birth control pills and one studied a patch being developed. Of 12 ring trials, 11 looked at the marketed ring and pills while one studied a ring being developed. The methods compared had similar pregnancy rates. Patch users reported using their method more consistently than the pill group did. Only half of the patch studies had data on pregnancy or whether the women used the method correctly. However, most of the ring studies had those data.
Patch users were more likely than pill users to drop out early from the trial. Ring users were not more likely to drop out early. Compared to pill users, users of the marketed patch had more breast discomfort, painful periods, nausea, and vomiting. Ring users had more vaginal irritation and discharge than pill users but less nausea, acne, irritability, depression, and emotional changes. Ring users often had fewer bleeding problems than pill users.
The quality of information was classed as low for the patch trials and moderate for the ring studies. Lower quality was due to not reporting how groups were assigned or not having good outcome measures. Other issues were high losses and taking assigned women out of the analysis. Studies of the patch and ring should provide more detail on whether women used the method correctly.
Background
Description of the condition
The transdermal skin patch and the vaginal ring were developed as alternative methods of delivering contraceptive steroids to women. Both methods could increase user compliance by avoiding the need for daily pill‐taking of combination oral contraceptives (COCs). Many women using oral contraceptives do not follow the pill regimen consistently. Effectiveness of any contraceptive method depends on compliance with the regimen and continuation (Grimes 2009). An estimated 47% to 81% of oral contraceptive users in the United States miss at least one pill per cycle (Potter 1996; Rosenberg 1998). A study in Spain showed that recent users of OCs, the patch, or the ring reported missing or delaying use of their chosen method at high rates: 65%, 30%, 20%, respectively (Lete 2008). Even in a randomized trial with electronic monitoring of compliance, 57% of participants missed three or more pills per cycle (Hou 2010). This included both the control and the intervention group, which had text‐message reminders. In a trial of structured counseling, having at least 12 years of education predicted continuation of an effective contraceptive method at three months (Langston 2010). Failure rates of the skin patch and the vaginal ring appear similar to those of COCs (Trussell 2011). The typical‐use estimate for pills (9%) was based on the National Survey of Family Growth 1995 and 2002; perfect use for pills (0.3%) was based on clinical trials. For the transdermal patch and vaginal ring, estimates for typical and perfect use were based on those for the pill. Randomized trials had not shown superiority of these methods over OCs (Trussell 2011).
Description of the intervention
The approved devices in these categories are the Ortho Evra® transdermal patch and the vaginal rings NuvaRing® and Progering® (Sitruk‐Ware 2013). The regimens for these devices mimic that of most COCs: 21 days of active hormones, followed by 7 days without hormones. The marketed transdermal patch is a small, thin adhesive square with daily delivery to the systemic circulation of the progestin norelgestromin (17‐deacetyl norgestimate) 150 µg and the estrogen ethinyl estradiol (EE) 20 µg. The patch should be applied for one week to the lower abdomen, upper torso, buttocks, or the upper lower arm (Ortho Evra® only) and then replaced immediately by a new one. Three weekly patches are followed by one patch‐free week. Transdermal patches in development include those containing gestodene and EE (Bayer 2012a), levonorgestrel and EE (Stanczyk 2011), and a levonorgestrel‐only product (NICHD 2012).
Two contraceptive rings have been marketed and others are in development (Brache 2013; Sitruk‐Ware 2013). The NuvaRing® vaginal ring is a flexible, lightweight device made of polymer tubing that releases an almost constant rate of the progestin etonogestrel and the estrogen ethinyl estradiol (EE). Etonogestrel is the biologically active form of desogestrel (3‐keto desogestrel), which is used in some oral contraceptive formulations. Desogestrel belongs to the gonane class of progestins, which are derived from testosterone and include levonorgestrel and gestodene (Henzl 2000). A cycle of ring use consists of three weeks with the ring in place, followed by a ring‐free week. The progesterone‐only vaginal ring, Progering®, is approved for use by lactating women in several Latin America countries (Brache 2013). This three‐month ring can be used up to one year, i.e., four rings for three months each. A ring containing nesterone and ethinyl estradiol has been in development (Pop Council 2012; Sitruk‐Ware 2013). The progestin nestorone is derived from progesterone, in particular, 19‐norprogesterone. Unlike the etonogestrel ring that is replaced each cycle, the nestorone ring is intended for one‐year use. The regimen still involves 21 days of use followed by a 7‐day break, but the nesterone ring would be re‐used for 13 cycles. Studies in India will evaluate the efficacy, safety, and acceptability of the progesterone‐only ring within the various cultures of India (Pop Council 2012). In addition, a ring containing ulipristal acetate is being developed and is designed for continuous use over three months (Brache 2013; Jensen 2013). This ring could meet the needs for women who cannot use, or prefer to avoid, estrogens (Jensen 2013). Other prototypes in development include combined rings releasing estradiol instead of ethinyl estradiol for a better safety profile (Brache 2013). In addition, dual protection rings are being developed to provide both contraception and protection against HIV transmission and other sexually transmitted infections (Sitruk‐Ware 2013).
How the intervention might work
The administration of combination contraceptive steroids from a skin patch or vaginal ring offers other advantages over the traditional oral route. Since the gastrointestinal tract and, subsequently, the first‐pass effect are avoided, drug bioavailability is higher with these two delivery systems. Increased bioavailability, along with the ability to provide a sustained drug release rate, allows a lower dose to be used. The decreased dosage could improve effectiveness and cycle control while reducing side effects. Also, neither the patch nor the ring has to be fitted by a health care provider. Finally, unlike other vaginal barrier methods, the vaginal ring does not have to be placed to cover the cervix, which simplifies its use.
Why it is important to do this review
Possible drawbacks to these methods include incomplete ovulation inhibition, suboptimal cycle control, and spontaneous detachment of the skin patch or expulsion of the vaginal ring. Also, the ring could potentially lead to vaginal infections, cervical changes, coital interference, unpleasant odor and difficulties or inconvenience related to ring insertion or removal. This systematic review examines the effectiveness, cycle control, compliance (adherence), and adverse events associated with these methods.
Objectives
The objective is to compare the contraceptive effectiveness, cycle control, compliance, and safety of the contraceptive skin patch versus combination oral contraceptives and the contraceptive vaginal ring versus COCs.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) in any language comparing the combination contraceptive skin patch with a COC or the combination contraceptive vaginal ring with a COC. Treatment length had to be at least 3 cycles or 84 days.
Types of participants
Women of reproductive age without medical contraindications to either trial contraceptive method were eligible.
Types of interventions
All types of contraceptive skin patches and vaginal rings were eligible for the review. Any COC could be the comparison method.
Types of outcome measures
The main outcomes were measured as follows:
1) Contraceptive effectiveness
Cumulative life‐table or Kaplan‐Meier pregnancy rate
Proportion of pregnancies by women or cycles
2) Discontinuation (overall and due to adverse events)
Cumulative life‐table or Kaplan‐Meier discontinuation rates
Proportion of women who discontinued early
3) Cycle control ‐ proportion of women or cycles with
Breakthrough bleeding
Spotting
Amenorrhea
4) Compliance (regimen adherence) ‐ Proportion of women or cycles with self‐reported correct use of assigned device
5) Safety ‐ Proportion of women who reported a side effect or adverse event (AE). Side effects include, but are not limited to, abdominal cramps or pain, acne, breast tension or discomfort, depression or mood changes, diarrhea, dizziness, dysmenorrhea, edema, headache, nausea, vomiting, and weight gain. Events determined to be related to the study product were used where available; otherwise, the totals reported were included.
Search methods for identification of studies
Electronic searches
Through February 2013, we searched the computerized databases MEDLINE, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and LILACS for trials of the contraceptive skin patch or the contraceptive ring. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The recent search strategies are given in Appendix 1. The previous strategy also included EMBASE and is shown in Appendix 2.
Searching other resources
We searched the references of the publications identified for inclusion. For the initial review, we contacted known researchers and the manufacturers of the skin patch and the contraceptive ring to identify published or unpublished trials that we might have missed.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches with no language limitations. One author reviewed the search results and identified reports for inclusion or exclusion. A second author also examined the reports identified for appropriate categorization.
Data extraction and management
One author abstracted the data and entered the information into RevMan. Another author conducted a second data abstraction and verified correct data entry. Any discrepancies were resolved by discussion.
Assessment of risk of bias in included studies
The data in the present review were based on the analytic method (e.g., intention to treat, per protocol) used in the trial report. Studies were examined for methodological quality, according to recommended principles (Higgins 2011). The following factors were assessed: the study design, blinding, randomization method, group allocation concealment, exclusions after randomization, loss to follow up, and early discontinuation.
Measures of treatment effect
For dichotomous outcomes, the Peto odds ratio (OR) with 95% confidence interval (CI) was calculated. An example is the proportion of women who discontinued early. The Peto OR is useful when treatment effects are small and when events are not very common (Higgins 2011). This approach performs well under many circumstances, except when the study arms are severely unbalanced, which rarely occurs in RCTs (Deeks 2001).
We calculated crude risk for deep venous thrombosis for the ring users in the seven studies that reported serious adverse events (SAEs). We also calculated the risk of venous thromboembolism in the five patch trials; one pulmonary embolism was reported. The denominator was the number of women who used the ring or the patch. Confidence intervals were calculated using the Poisson distribution.
For continuous variables, the mean difference was computed with 95% confidence interval (CI) using a fixed effect model. RevMan uses the inverse variance approach (Higgins 2011). An example is the mean number of bleeding or spotting days. Kaplan‐Meier rates for pregnancy were also entered into tables.
Assessment of heterogeneity
We tested for statistical heterogeneity using the chi‐square test in RevMan. If the P value was < 0.1, we attempted to identify reasons for the heterogeneity, such as methodological differences across the studies. If a meta‐analysis included heterogeneous studies, we also discussed the results of each trial separately.
Data synthesis
We did not combine data from studies with different doses. Results were not combined in a meta‐analysis if the eligible trials differed in their comparison oral contraceptive.
A fixed effect model does not require the assumption of normal distribution for the effects (Deeks 2001). Fixed and random effects will give the same result if no heterogeneity exists, which is also the case if a comparison includes a single study. There is no consensus regarding the use of either model.
We assessed the quality of evidence for this review and summarized the results. Quality could be high, moderate, low, or very low. RCTs were considered to be high quality then downgraded for each of the following:
no information on randomization sequence generation or allocation concealment or no concealment used;
outcome assessment (no pregnancy test or no structured questionnaire or definition for contraceptive use or cycle control);
loss to follow up greater than 20%;
exclusions after randomization.
We did not downgrade on the basis of blinding. The interventions were visibly different (patch or ring versus COC). Therefore, blinding of investigators and participants to assignment was difficult, although blinding of outcome assessors was more feasible.
Results
Description of studies
Results of the search
The 2012 to 2013 searches resulted in 209 unduplicated citations. This includes 144 from MEDLINE, CENTRAL, POPLINE, and LILACS. Searches of Clinical trials sites resulted in 61 references (ClinicalTrials.gov and ICTRP). Other sources provided four citations (conference abstracts and a pharmaceutical company site).
We included three new trials that had a total of six reports (Gilliam 2010; Mohamed 2011; Kaunitz 2012). We excluded two new studies (Bustillos‐Alamilla 2010; Guazzelli 2011); reasons can be found in Characteristics of excluded studies. After 17 references were reserved as background literature, the remaining 183 citations were discarded due to ineligibility.
Included studies
Eighteen randomized controlled trials met the eligibility criteria for this review; 6 compared the contraceptive skin patch to a combination oral contraceptive (COC) and 12 compared the vaginal ring to a COC. The total number of women randomized in these trials was 9238; the patch studies had 5322 and the ring studies included 3916.
Transdermal patch trials
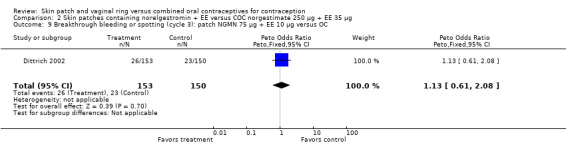
Of the six patch trials, five used the transdermal patch that has been marketed (Audet 2001; Dittrich 2002; Urdl 2005; Boonyarangkul 2007; Kluft 2008). The Ortho Evra® skin patch is produced by Ortho‐McNeil‐Janssen Pharmaceuticals Inc., Titusville, NJ. It consists of a thin, medicated, adhesive layer between a protective polyester layer and a polyester release liner that is removed before application of the patch. The 20 cm2 patch is applied to the upper outer arm, lower abdomen, upper torso or buttocks where it transdermally releases norelgestromin 150 μg and ethinyl estradiol 20 μg daily. Each patch should be attached for one week, after which it should be removed and replaced immediately by a new patch. Three weekly patches are to be followed by one patch‐free week. Dittrich 2002 also included two study groups that were assigned to use smaller patches with decreased drug dosages. The latter patches have not been marketed. The sixth trial used a transdermal patch in development, AG200‐15, which releases levonorgestrel 120 µg plus EE 30 µg (Kaunitz 2012). The regimen for use is the same as that for the norelgestromin plus EE patch.
Most of these trials were multicenter trials, conducted in the USA and Canada (Audet 2001); the USA, Europe and South Africa (Dittrich 2002); Europe and South Africa (Urdl 2005); the USA (Kaunitz 2012); and The Netherlands (Kluft 2008). Boonyarangkul 2007 had one center in Thailand. The planned trial length varied from 3 treatment cycles (Boonyarangkul 2007) to a combination of 6 and 13 treatment cycles (Audet 2001; Urdl 2005; Kaunitz 2012). Participants were to follow the recommended patch or COC regimens except for women in one trial (Dittrich 2002), who were to delay the use of patch or COC by one day at the start of the fourth cycle.
The trials that examined the norelgestromin plus EE patch had various comparison COCs, including a monophasic contraceptive containing desogestrel (Urdl 2005; Kluft 2008), levonorgestrel (Boonyarangkul 2007), or norelgestromin (Dittrich 2002); and a triphasic contraceptive with levonorgestrel (Audet 2001; Kluft 2008). Kaunitz 2012, which studied a patch releasing levonorgestrel plus EE, compared the patch to a COC containing levonorgestrel.
Vaginal ring trials
Of the 12 ring trials, 11 examined the same marketed vaginal ring. The NuvaRing® (NV Organon, Oss, The NetherlandsMerck, Whitehouse Station, NJ, USA) is a flexible, lightweight ring, made of ethylene vinyl acetate copolymer, 54 mm in diameter with a cross‐section of 4 mm. The device releases etonogestrel 120 μg and ethinyl estradiol 15 μg daily. The ring is inserted into the vagina where it is to remain in place for three weeks. Each ring should be followed by a ring‐free week, and a new ring is to be used for each cycle. Rad 2006 studied a newer, unmarketed, contraceptive vaginal ring made of silicone rubber. That ring releases nestorone 150 µg and ethinyl estradiol 15 µg daily (Pop Council 2012). While the ring uses the same regimen as its predecessor (21 days of use and 7 days of nonuse), the nesterone ring is intended for one year of use.
Eleven trials used the same ring but different COCs, which included pills containing drospirenone (Ahrendt 2006; Mohamed 2011), levonorgestrel (Duijkers 2004a; Veres 2004; Oddsson 2005; Sabatini 2006; Elkind‐Hirsch 2007), desogestrel (Guida 2005), gestodene (Sabatini 2006), or norgestimate (Westhoff 2005;Stewart 2007; Gilliam 2010). For Rad 2006, which studied a ring being developed for marketing (Pop Council 2012), the comparison COC contained levonorgestrel.
The 12 trials of the vaginal ring included multicenter studies in Europe (Duijkers 2004a; Ahrendt 2006), Europe and South America (Oddsson 2005), and the USA (Gilliam 2010). Single‐center trials were from Europe (Guida 2005; Rad 2006), the USA (Veres 2004; Westhoff 2005; Elkind‐Hirsch 2007; Stewart 2007), Egypt (Mohamed 2011), and Italy (Sabatini 2006). Trial length for these vaginal ring studies were 3 cycles or 84 days (Veres 2004; Westhoff 2005; Rad 2006; Stewart 2007; Gilliam 2010), 5 or 6 cycles (Duijkers 2004a; Guida 2005; Elkind‐Hirsch 2007), or 12 to 13 cycles (Oddsson 2005; Ahrendt 2006; Sabatini 2006; Mohamed 2011).
Data limitations
By sample size, these studies can be grouped as:
small (up to 130) (Duijkers 2004a; Veres 2004; Guida 2005; Rad 2006; Boonyarangkul 2007; Elkind‐Hirsch 2007; Stewart 2007; Kluft 2008)
mid‐sized (200 to 600) (Dittrich 2002; Oddsson 2005; Westhoff 2005; Sabatini 2006; Gilliam 2010; Mohamed 2011) and
large (1000 or more) (Audet 2001; Urdl 2005; Ahrendt 2006; Kaunitz 2012).
Some trials had limited outcome data relevant to this review. Because Dittrich 2002 emphasized ovulation suppression and Guida 2005 focused on sexual life satisfaction, only discontinuation data were included for those trials. Rad 2006 emphasized coagulation tests, so only pregnancy and discontinuation data could be used in this review. Elkind‐Hirsch 2007 focused on insulin sensitivity; adverse events were the only data analyzed here. Kluft 2008 examined hemostasis variables; this review included discontinuation and total adverse events. Veres 2004 reported means without standard deviations for vaginal symptoms. Compliance data were lacking in several studies (Westhoff 2005; Ahrendt 2006; Boonyarangkul 2007; Elkind‐Hirsch 2007; Kluft 2008). Also in Westhoff 2005, means were provided for bleeding data but without a variance measure for use in analysis; however, the report provided proportions of women with specific bleeding patterns. In Sabatini 2006, adverse events were given for three time points rather than the total study.
Two crossover trials reported data for the whole study rather than per study period (Veres 2004; Kaunitz 2012). For Veres 2004, this includes data on compliance and satisfaction. The conference presentation of Kaunitz 2012 showed most results as percentages in figures without absolute numbers and reported Pearl Indices without confidence intervals. However, we were able to analyze data from Kaunitz 2012 on discontinuation, noncompliance, and adverse events.
Some of the trial reports included only percentages, rather than absolute numbers for several outcomes. Kaunitz 2012 reported most data in figures capturing percentages but the lack of numerators and denominators precluded outcome calculations. Since the precision of the estimate is unknown when only percentages are reported, the interpretation of the estimate is limited. Attempts were made to obtain additional data from the researchers.
Risk of bias in included studies
Allocation
For method of randomization, several trials used a centralized phone system. Most others reported the sequence as 'computer‐generated'. Other details such as block size are given in Characteristics of included studies.
Adequate methods for allocation concealment include a centralized telephone system (interactive voice response system) and the use of sequentially‐numbered, opaque, sealed envelopes (Schulz 1995; Schulz 2002a). The use of an automated centralized treatment assignment via telephone greatly reduces the risk of inadvertent or intended assignment errors that can occur with an inadequate process of allocation concealment (Haag 1998). Pharmacy distribution of pill bottles is another good method. Of the included trials, 11 used some concealment as follows: five had interactive phone systems (Audet 2001; Oddsson 2005; Urdl 2005; Ahrendt 2006; Kluft 2008); one had pharmacy distribution (Rad 2006); three used sequentially‐numbered, opaque envelopes (Veres 2004; Westhoff 2005; Gilliam 2010); one used envelopes but did not provide much detail (Stewart 2007); and one had an unspecified method of concealment (Guida 2005).
Of the remaining seven trials, two did not use concealment (Duijkers 2004a; Sabatini 2006) and five did not provide any information about concealment (Dittrich 2002; Boonyarangkul 2007; Elkind‐Hirsch 2007; Mohamed 2011; Kaunitz 2012).
Blinding
Sixteen trials were open‐label. The differences in treatment made double‐blinding unfeasible. Two did not specify if the investigators or assessors were blinded (Audet 2001; Elkind‐Hirsch 2007).
Incomplete outcome data
Losses greater than 20% threaten trial validity (Strauss 2005). Five trials had overall losses between 22% and 32% (Audet 2001; Ahrendt 2006; Oddsson 2005; Westhoff 2005; Sabatini 2006). However, four of these studies with high losses had 12 or 13 treatment cycles versus 3 to 6 cycles for most of the other trials. In addition, two trials had differential losses between the study groups with one group losing as much as 30%. In Duijkers 2004a, the ring group lost 30% while the COC lost 7%. For Sabatini 2006, losses ranged from 12% for the ring group to 32% for the gestodene COC group.
Most of the studies used a modified intent‐to‐treat analysis, in which only the women who received the study treatment for at least one day were included in the analysis. This definition of 'intention to treat' is common in trials sponsored by pharmaceutical companies but conflicts with the CONSORT guidelines and regulatory recommendations (Moher 2001; CONSORT 2009).
Six studies excluded women after randomization (Audet 2001; Dittrich 2002; Sabatini 2006; Elkind‐Hirsch 2007; Gilliam 2010; Mohamed 2011). In Audet 2001, eight women in the patch group and six in the COC group were excluded after randomization due to discovery of ineligibility. In Dittrich 2002, women who missed three consecutive pills in any cycle were to be excluded. One woman assigned to the patch was excluded due to a protocol violation. Sabatini 2006 reportedly excluded two women due to pregnancy from the gestodene‐containing group. Elkind‐Hirsch 2007 excluded 35% of each group, i.e., the women who did not use the study product and those who discontinued early. Gilliam 2010 excluded one woman from each group who did not use the study medication and those who terminated early, including for adverse events. Mohamed 2011 also excluded women who terminated early due to adverse events, the desire to become pregnant, and pregnancy.
Other potential sources of bias
Most of the studies used a modified intent‐to‐treat analysis, in which only the women who received the study treatment for at least one day were included in the analysis. This definition of 'intention to treat' is common in trials sponsored by pharmaceutical companies but conflicts with the CONSORT guidelines and regulatory recommendations (Moher 2001; CONSORT 2009).
Trials sponsored by pharmaceutical companies are more likely to have outcomes favoring the industry than studies funded by other sources (Als‐Nielsen 2003; Lexchin 2003). Of the six patch trials, all but one (Boonyarangkul 2007) mentioned industry sponsorship. Of the 12 ring trials, seven were sponsored by industry; one reported only government support (Rad 2006), and four did not mention their source of support (Guida 2005; Sabatini 2006; Stewart 2007; Mohamed 2011).
Effects of interventions
Skin patch versus COC
Effectiveness
The skin patch containing norelgestromin plus EE and the control COC did not differ significantly in contraceptive effectiveness in the three trials reporting pregnancy data. In Audet 2001 and Urdl 2005, the 6‐cycle and 13‐cycle Kaplan‐Meier cumulative probabilities of pregnancy for the patch versus the COC arm were not significantly different (Analysis 1.2; Analysis 4.2). In three trials, the odds ratio (OR) of pregnancy for the patch versus the COC showed the groups were similar (Audet 2001; Urdl 2005; Boonyarangkul 2007).
1.2. Analysis.
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 2 Kaplan‐Meier cumulative pregnancy rates (95% CI).
| Kaplan‐Meier cumulative pregnancy rates (95% CI) | |||
|---|---|---|---|
| Study | Outcome | Skin patch | COC |
| Audet 2001 | 6‐cycle rate | 0.6 (0 to 1.2) | 1.2 (0.2 to 2.1) |
| Audet 2001 | 13‐cycle rate | 1.3 (0 to 2.7) | 1.8 (0.2 to 3.4) |
4.2. Analysis.
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 2 Kaplan‐Meier cumulative pregnancy rates (95% CI).
| Kaplan‐Meier cumulative pregnancy rates (95% CI) | |||
|---|---|---|---|
| Study | Outcome | Skin patch | COC |
| Urdl 2005 | 6‐cycle rate | 0.5 (0 to 1.0) | 0.3 (0 to 0.8) |
| Urdl 2005 | 13‐cycle rate | 0.5 (0 to 1.0) | 0.3 (0 to 0.8) |
The study of the levonorgestrel (LNG) plus EE patch (Kaunitz 2012) reported the Pearl Indices for in‐treatment pregnancies: 4.96 for the patch (6 or 13 cycles) and 4.02 for the COC (6 cycles). Confidence intervals were not provided.
Discontinuation
More patch users discontinued early from the trials reporting those data than women assigned to use the COC. The ORs for overall discontinuation were: for Audet 2001 and Kluft 2008 combined, 1.59 (95% CI 1.26 to 2.00; Analysis 1.3); for Urdl 2005 and Kluft 2008 combined, 1.56 (95% CI 1.18 to 2.06; Analysis 4.3); and for the norelgestromin 150 μg patch in Dittrich 2002, 2.57 (95% CI 0.99 to 6.64; Analysis 2.1).
1.3. Analysis.
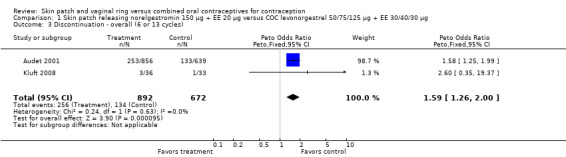
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 3 Discontinuation ‐ overall (6 or 13 cycles).
4.3. Analysis.
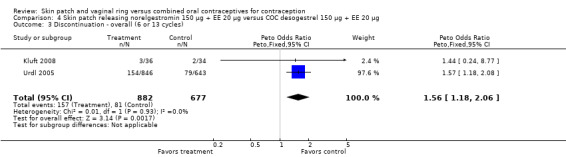
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 3 Discontinuation ‐ overall (6 or 13 cycles).
2.1. Analysis.
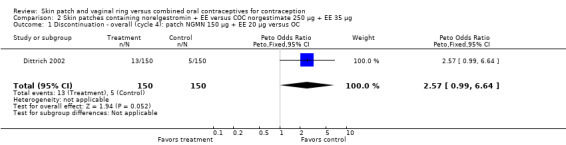
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 1 Discontinuation ‐ overall (cycle 4): patch NGMN 150 µg + EE 20 µg versus OC.
Patch users were also more likely to discontinue due adverse events (AEs) than COC users: for Audet 2001 and Kluft 2008 combined, OR 2.28 (95% CI 1.61 to 3.25; Analysis 1.4); for Urdl 2005 and Kluft 2008 combined, OR 2.11 (95% CI 1.44 to 3.11; Analysis 4.4); and for Kaunitz 2012, OR 1.82 (95% CI 1.19 to 2.79; Analysis 5.1).
1.4. Analysis.
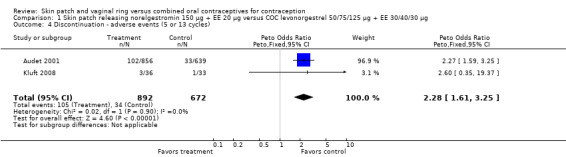
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 4 Discontinuation ‐ adverse events (5 or 13 cycles).
4.4. Analysis.
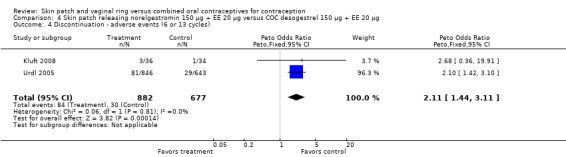
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 4 Discontinuation ‐ adverse events (6 or 13 cycles).
5.1. Analysis.
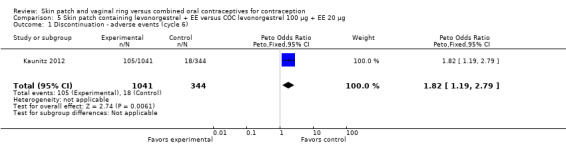
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 1 Discontinuation ‐ adverse events (cycle 6).
Compliance (regimen adherence)
Patch users showed better compliance to the regimen per cycle than COC users in the three trials providing such data. In Audet 2001, the OR for compliance was 2.05 (95% CI 1.83 to 2.29; Analysis 1.5) and in Urdl 2005, the OR was 2.76 (95% CI 2.35 to 3.24) (Analysis 4.5). Also, in Kaunitz 2012, women in the levonorgestrel patch group were less likely to have had cycles with missed days of therapy compared to the COC group (OR 0.36; 95% CI 0.25 to 0.51; Analysis 5.2). In addition, Dittrich 2002 reported compliance by cycle that ranged from 94% to 97% for the group with the 20‐cm2 patch versus 77% to 80% for the OC group. Data were insufficient for analysis here.
1.5. Analysis.
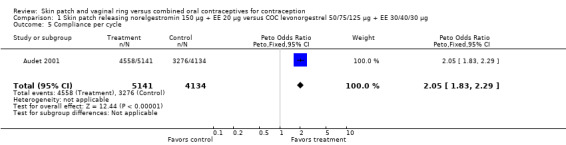
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 5 Compliance per cycle.
4.5. Analysis.
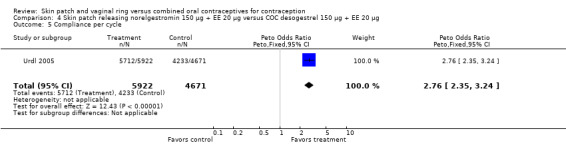
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 5 Compliance per cycle.
5.2. Analysis.
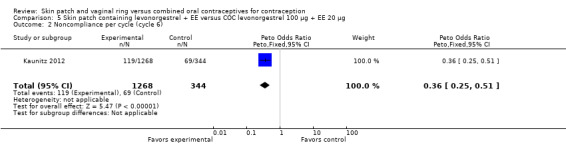
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 2 Noncompliance per cycle (cycle 6).
The percentages with application site reactions varied. In Audet 2001, approximately 20% of patch users reported skin reactions and 3% reported early trial discontinuation as a result. Patch detachment was rare, with women reporting about 5% of the patches required replacement due to complete (2%) or partial (3%) detachment (Audet 2001). In Urdl 2005, about 14% of patch users had application site reactions. For the levonorgestrel patch, Kaunitz 2012 reported 3% of diaries indicated patch detachment. Also, the diaries reportedly indicated moderate skin irritation in 2.6% of cycles and severe irritation in 0.1% of cycles (Kaunitz 2012).
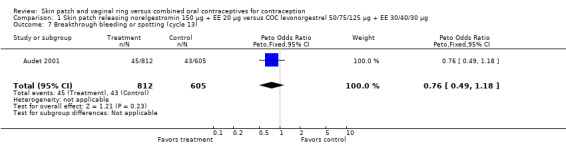
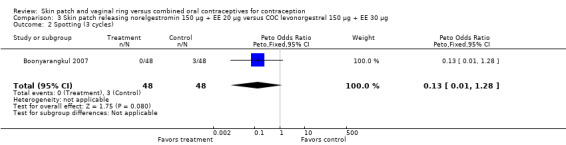
Cycle control
Four of the six patch trials had data on cycle control that we could analyze, but only one significant difference was noted. In Urdl 2005, breakthrough bleeding and spotting was less common within the patch group than the COC group at cycle 13 (OR 0.65; 95% CI 0.46 to 0.92; Analysis 4.7).
4.7. Analysis.
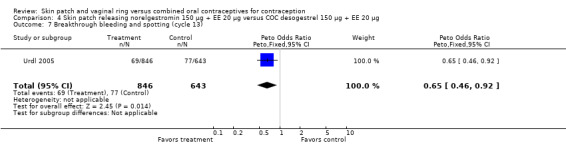
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 7 Breakthrough bleeding and spotting (cycle 13).
Adverse events
AE data were reported for five patch trials. A few comparisons showed differences between the study groups.
-
Patch users more often reported breast discomfort or pain compared to the COC group in three trials:
Urdl 2005 (OR 2.98; 95% CI 2.29 to 3.90; Analysis 4.8),
Audet 2001 (OR 3.09; 95% CI 2.26 to 4.22; Analysis 1.8), and
Boonyarangkul 2007 (OR 9.11; 95% CI 2.48 to 33.52; Analysis 3.4).
-
Nausea and vomiting:
Urdl 2005 found norelgestromin patch users reported more nausea (OR 2.08; 95% CI 1.46 to 2.95; Analysis 4.10), as well as vomiting (OR 1.88; 95% CI 1.12 to 3.16; Analysis 4.14).
The trial of a levonorgestrel patch found vomiting less likely among patch users (OR 0.35; 95% CI 0.14 to 0.91; Analysis 5.9) (Kaunitz 2012).
-
Other AEs:
Dysmenorrhea was more common among norelgestromin patch users in Audet 2001 (OR 1.43; 95% CI 1.03 to 1.99; Analysis 1.11).
LNG patch users were less likely to report headache (OR 0.23; 95% CI 0.10 to 0.51; Analysis 5.4) and fatigue (OR 0.18; 95% CI 0.04 to 0.82; Analysis 5.13) than the COC users (Kaunitz 2012).
4.8. Analysis.
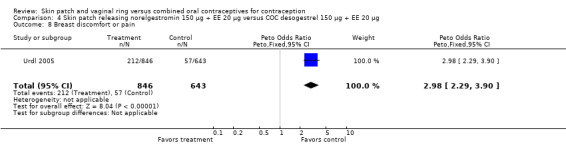
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 8 Breast discomfort or pain.
1.8. Analysis.
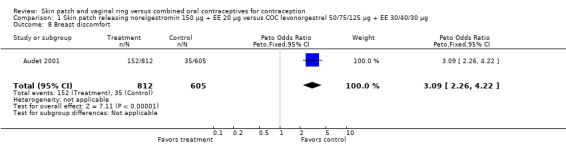
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 8 Breast discomfort.
3.4. Analysis.
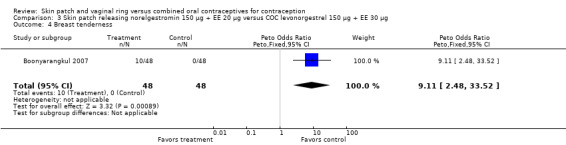
Comparison 3 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 4 Breast tenderness.
4.10. Analysis.
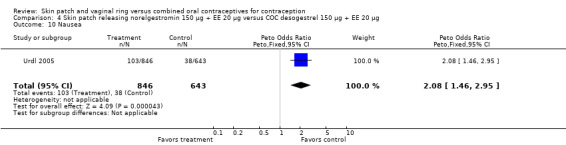
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 10 Nausea.
4.14. Analysis.
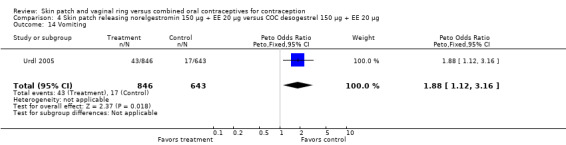
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 14 Vomiting.
5.9. Analysis.
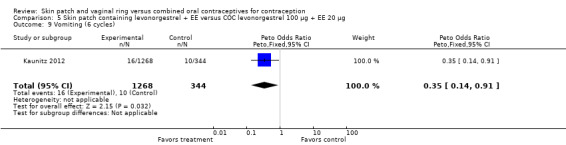
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 9 Vomiting (6 cycles).
1.11. Analysis.
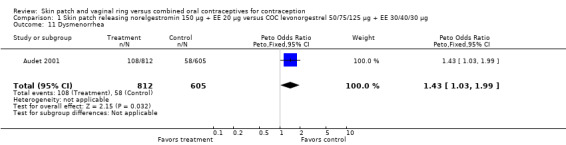
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 11 Dysmenorrhea.
5.4. Analysis.
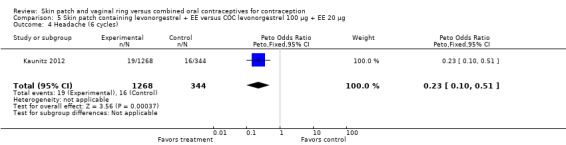
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 4 Headache (6 cycles).
5.13. Analysis.
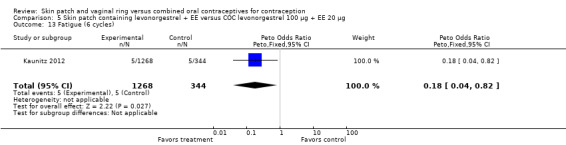
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 13 Fatigue (6 cycles).
Few serious adverse events (SAEs) occurred that were considered related to the study product. For patch users, one each of the following occurred: pain and paraesthesia in the left arm, migraine, and cholecystitis (Audet 2001); blood clot left subclavian vein, uncontrollable nausea and vomiting, and drug overdose (Benadryl) (Kaunitz 2012); breast nodule with no malignancy (Dittrich 2002); and pulmonary embolism (Urdl 2005). In the five trials of the norelgestromin‐containing patch, one venous thromboembolism among 1892 patch users yields an estimated frequency of 53 per 100,000 women (95% CI 1 to 294). If the LNG patch study were included, one venous thromboembolism among 3160 patch users would yield an estimated frequency of 32 per 100,000 women (95% CI 1 to 176). The COC users had one related SAE each of intracranial pressure and severe depression (Audet 2001), breast cancer (Urdl 2005), and liver problem (unspecified) (Kaunitz 2012).
Satisfaction
One patch study had satisfaction data. In Urdl 2005, patch users were more likely to be very satisfied with their method than COC users (OR 1.35; 95% CI 1.09 to 1.68; Analysis 4.16).
4.16. Analysis.
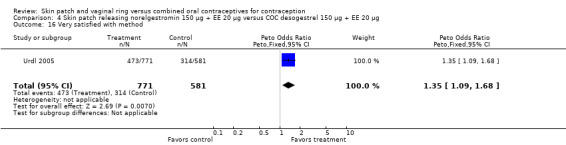
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 16 Very satisfied with method.
Vaginal ring versus COC
Effectiveness
Contraceptive effectiveness was not significantly different for the vaginal ring and comparison COCs in the eight trials that reported pregnancy.
Discontinuation
Eleven of the 12 trials provided data on discontinuation and only two showed any significant difference. In Sabatini 2006, when the gestodene COC was the comparison, the OR was 0.32 (95% CI 0.16 to 0.66; Analysis 7.2) at 12 cycles. For Gilliam 2010, in which the comparison was a triphasic norgestimate COC, the OR was 0.52 (95% CI 0.31 to 0.88; Analysis 10.2) at 6 months. The ring users also had lower odds for discontinuation due to AEs in Sabatini 2006 (OR 0.32; 95% CI 0.15 to 0.70; Analysis 7.3). Women who discontinued due to 'loss of desire' are not included here, as those data may have been assessed differently than AEs.
7.2. Analysis.
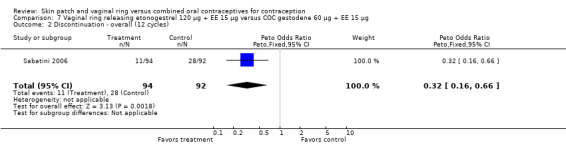
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 2 Discontinuation ‐ overall (12 cycles).
10.2. Analysis.
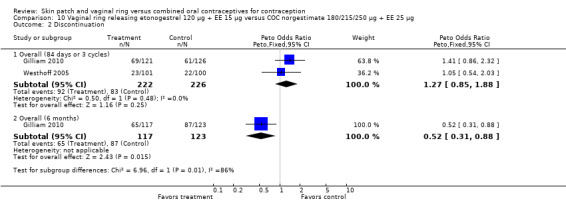
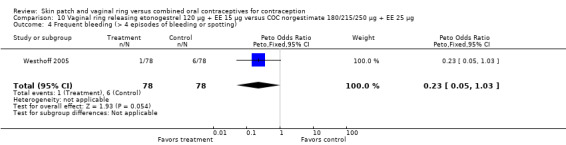
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 2 Discontinuation.
7.3. Analysis.
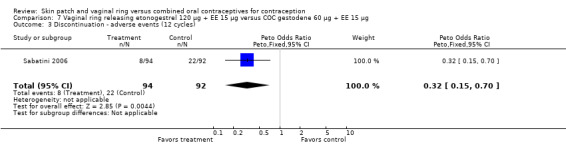
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 3 Discontinuation ‐ adverse events (12 cycles).
Compliance (adherence)
Data were available from four ring trials and findings were mixed (Veres 2004; Oddsson 2005; Stewart 2007; Gilliam 2010). Two trials showed no major differences (Oddsson 2005; Stewart 2007). In the crossover trial of Veres 2004, the ring users reported more noncompliance (OR 3.99; 95% CI 1.87 to 8.52; Analysis 6.4). Conversely, reports of 'perfect use' over 3 months in Gilliam 2010 were more likely for vaginal ring users versus COC users (OR 1.67; 95% CI 1.04 to 2.68; Analysis 10.3). In addition, Ahrendt 2006 reported full compliance for 89.2% of ring cycles and 85.5% of COC cycles; data were not sufficient for analysis here.
6.4. Analysis.
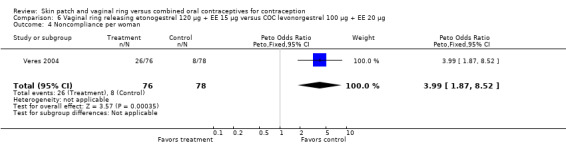
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 4 Noncompliance per woman.
10.3. Analysis.
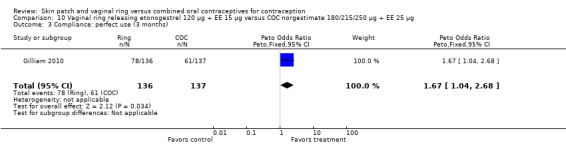
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 3 Compliance: perfect use (3 months).
Cycle control
Seven ring trials reported bleeding data (Oddsson 2005; Westhoff 2005; Ahrendt 2006; Sabatini 2006; Elkind‐Hirsch 2007; Stewart 2007; Mohamed 2011). Five obtained the data from diaries, while Elkind‐Hirsch 2007 gathered bleeding data with AEs and Stewart 2007 included a bleeding item in the questionnaire. The significant differences shown between the study groups include the following:
Ahrendt 2006: For most cycles, the means were not significantly different, including cycle 13 shown here. However, the mean number of breakthrough bleeding or spotting days was higher for the ring group at cycle 6 (MD 2.00; 95% CI 1.57 to 2.43; Analysis 9.4).
Oddsson 2005: Breakthrough bleeding was less likely for ring users than COC users at cycle 6 (OR 0.22; 95% CI 0.05 to 0.88; Analysis 11.6) but not at cycle 13.
Westhoff 2005: Prolonged bleeding (bleeding or spotting episode lasting at least 10 days) was less likely for the ring group (OR 0.42; 95% CI 0.20 to 0.89; Analysis 10.6). Frequent bleeding, defined as more than four episodes of bleeding or spotting, was also less likely for the ring users (OR 0.23; 95% CI 0.05 to 1.03; Analysis 10.4).
-
-
Spotting and breakthrough bleeding were less common among ring users.
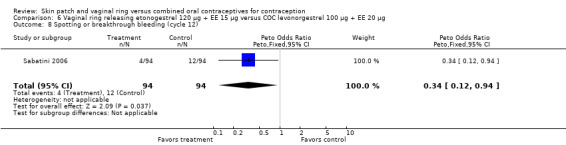
Compared to the levonorgestrel COC, the OR at cycle 6 was 0.36 (95% CI 0.15 to 0.87; Analysis 6.7); at cycle 12, the OR was 0.34 (95% CI 0.12 to 0.94; Analysis 6.8).
Versus the gestodene COC, the OR at cycle 6 was 0.26 (95% CI 0.11 to 0.57; Analysis 7.6); at cycle 12, the OR was 0.33 (95% CI 0.12 to 0.91; Analysis 7.7).
-
Early or late withdrawal bleeding was less likely among ring users than COC users.
Compared to the levonorgestrel COC, the OR at cycle 6 was 0.23 (95% CI 0.07 to 0.70; Analysis 6.5), and at cycle 12 the OR was 0.21 (95% CI 0.05 to 0.86; Analysis 6.6).
Versus the gestodene COC, the OR at cycle 6 was 0.18 (95% CI 0.07 to 0.46; Analysis 7.4); at cycle 12 the OR was 0.19 (95% CI 0.05 to 0.73; Analysis 7.5).
-
9.4. Analysis.
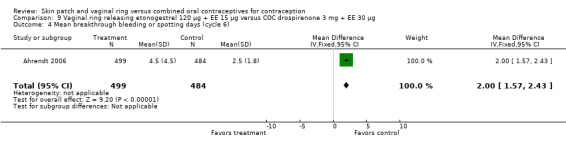
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 4 Mean breakthrough bleeding or spotting days (cycle 6).
11.6. Analysis.
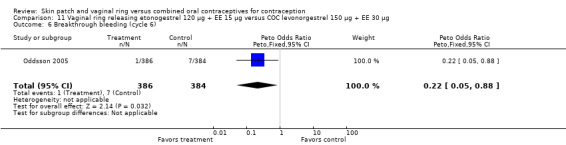
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 6 Breakthrough bleeding (cycle 6).
10.6. Analysis.
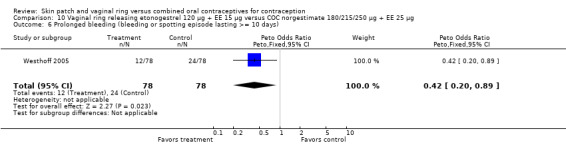
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 6 Prolonged bleeding (bleeding or spotting episode lasting >= 10 days).
10.4. Analysis.
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 4 Frequent bleeding (> 4 episodes of bleeding or spotting).
6.7. Analysis.
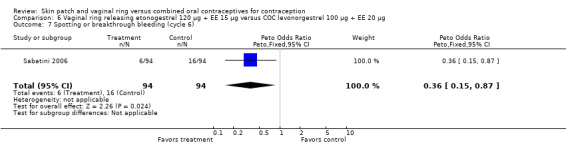
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 7 Spotting or breakthrough bleeding (cycle 6).
6.8. Analysis.
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 8 Spotting or breakthrough bleeding (cycle 12).
7.6. Analysis.
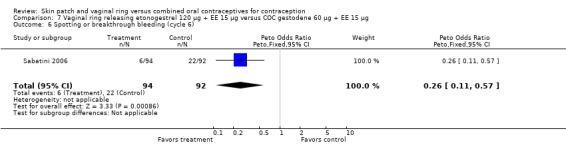
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 6 Spotting or breakthrough bleeding (cycle 6).
7.7. Analysis.
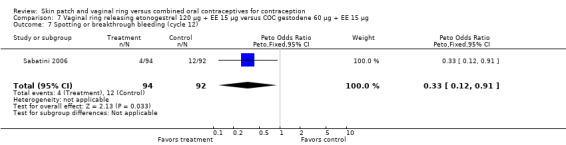
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 7 Spotting or breakthrough bleeding (cycle 12).
6.5. Analysis.
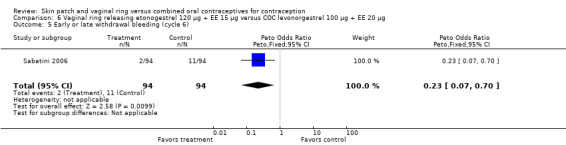
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 5 Early or late withdrawal bleeding (cycle 6).
6.6. Analysis.
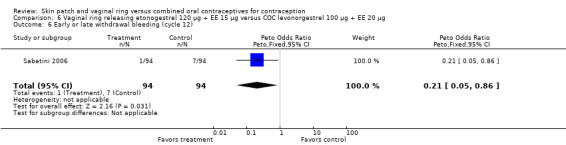
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 6 Early or late withdrawal bleeding (cycle 12).
7.4. Analysis.
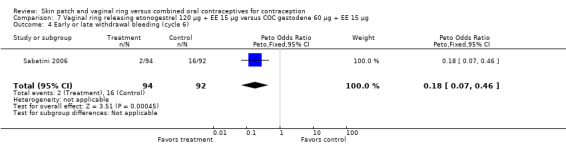
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 4 Early or late withdrawal bleeding (cycle 6).
7.5. Analysis.
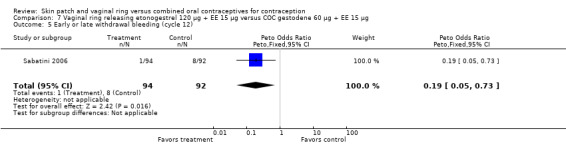
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 5 Early or late withdrawal bleeding (cycle 12).
Adverse events
Seven trials reported on AEs or side effects (Duijkers 2004a; Oddsson 2005; Ahrendt 2006; Sabatini 2006; Elkind‐Hirsch 2007; Stewart 2007; Mohamed 2011). Stewart 2007 used a questionnaire to elicit side effects; results for the side effects being 'worse' are reported here. Few comparisons showed any significant differences between the groups and those are the focus here.
Vaginal or genital symptoms differed between the groups in several comparisons.
Vaginitis was reported more frequently by the ring users in the meta‐analysis of Duijkers 2004a and Oddsson 2005 (OR 2.84; 95% CI 1.34 to 6.01; Analysis 11.15). This effect was largely due to the difference in Oddsson 2005. In Ahrendt 2006 and Mohamed 2011 combined, vaginitis was again more common in the ring arm (OR 2.48; 95% CI 1.39 to 4.43; Analysis 9.12).
Differences in leukorrhea were noted in two meta‐analyses and they favored the COC group: Duijkers 2004a and Oddsson 2005 (OR 6.42; 95% CI 2.71 to 15.22; Analysis 11.17); Ahrendt 2006 and Mohamed 2011 (OR 3.21; 95% CI 1.61 to 6.40; Analysis 9.13).
Sabatini 2006 showed less vaginal dryness among the ring users: compared to the levonorgestrel COC, the OR at cycle 6 was 0.12 (95% CI 0.03 to 0.47; Analysis 6.16) and at cycle 12 the OR was 0.13 (95% CI 0.03 to 0.65; Analysis 6.17). For vaginal dryness in the gestodene comparison, the OR at cycle 6 was 0.11 (95% CI 0.04 to 0.32; Analysis 7.14); at cycle 12, the OR was 0.12 (95% CI 0.03 to 0.50; Analysis 7.15).
In Oddsson 2005, ring users were more likely to report genital pruritus (OR 4.58; 95% CI 1.14 to 18.41; Analysis 11.16).
11.15. Analysis.
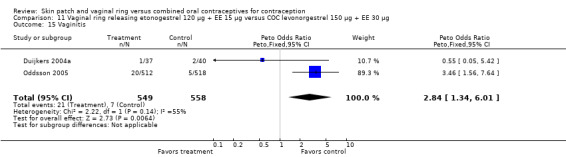
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 15 Vaginitis.
9.12. Analysis.
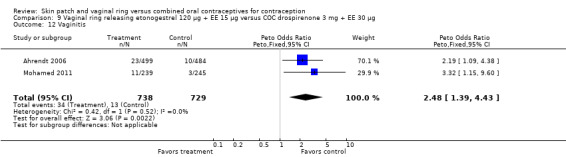
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 12 Vaginitis.
11.17. Analysis.
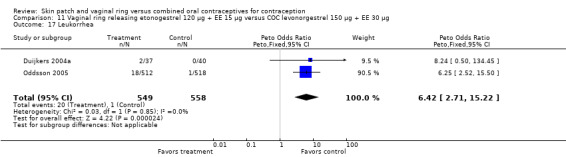
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 17 Leukorrhea.
9.13. Analysis.
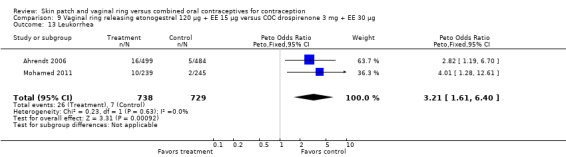
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 13 Leukorrhea.
6.16. Analysis.
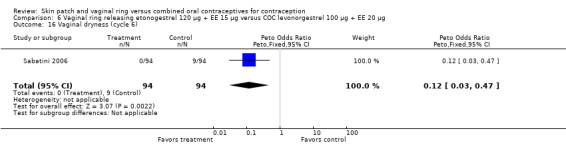
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 16 Vaginal dryness (cycle 6).
6.17. Analysis.
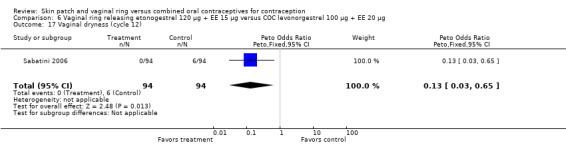
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 17 Vaginal dryness (cycle 12).
7.14. Analysis.
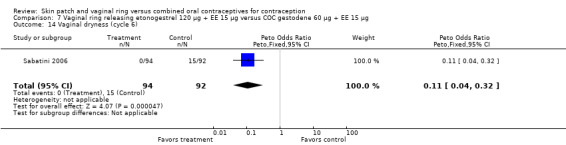
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 14 Vaginal dryness (cycle 6).
7.15. Analysis.
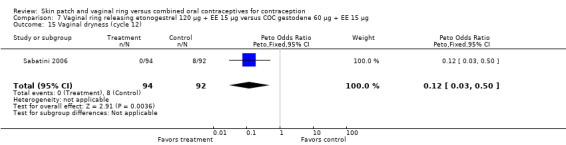
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 15 Vaginal dryness (cycle 12).
11.16. Analysis.
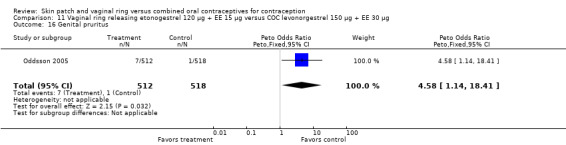
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 16 Genital pruritus.
Emotional symptoms differed by study arm in two trials (Sabatini 2006; Stewart 2007). In Sabatini 2006, irritability and depression were less common among the ring users than the COC users. For the ring versus levonorgestrel COC comparisons, the OR for irritability at cycle 6 was 0.26 (95% CI 0.08 to 0.88; Analysis 6.19); at cycle 12, the OR was 0.28 (95% CI 0.08 to 1.01; Analysis 6.20). For depression, the OR at cycle 12 for ring versus levonorgestrel COC was 0.23 (95% CI 0.05 to 1.03; Analysis 6.23). For the comparisons of the ring versus gestodene COC, the OR for irritability at cycle 6 was 0.28 (95% CI 0.08 to 0.99; Analysis 7.16). For depression, the OR at cycle 12 was 0.21 (95% CI 0.05 to 0.84; Analysis 7.19). Emotional lability was reported less among ring users in Mohamed 2011 (OR 0.19; 95% CI 0.06 to 0.59; Analysis 9.16).
6.19. Analysis.
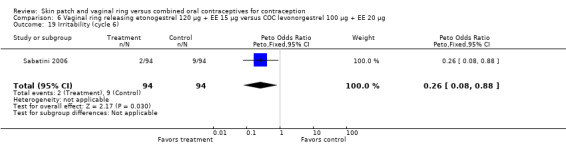
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 19 Irritability (cycle 6).
6.20. Analysis.
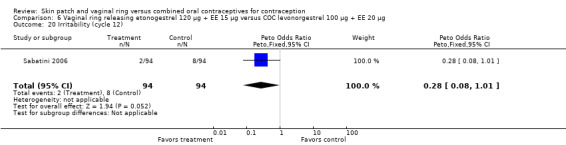
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 20 Irritability (cycle 12).
6.23. Analysis.
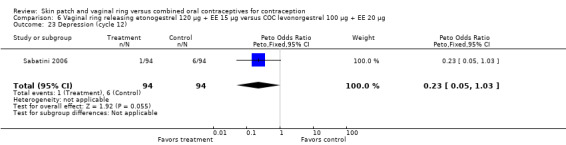
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 23 Depression (cycle 12).
7.16. Analysis.
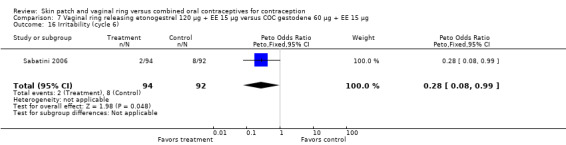
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 16 Irritability (cycle 6).
7.19. Analysis.
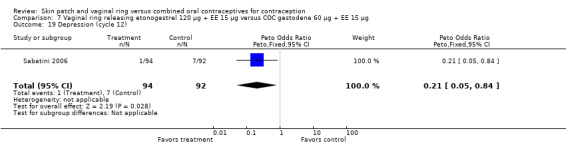
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 19 Depression (cycle 12).
9.16. Analysis.
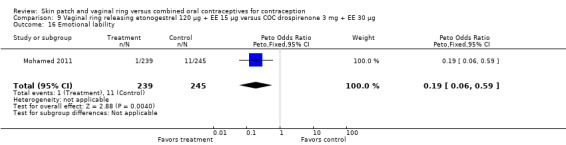
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 16 Emotional lability.
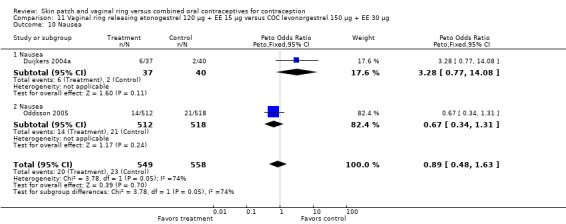
Other AEs showed some differences between study groups. Nausea was less likely among ring users in Ahrendt 2006 and Mohamed 2011 combined (OR 0.39; 95% CI 0.21 to 0.74; Analysis 9.10) and in Stewart 2007 (OR 0.31; 95% CI 0.12 to 0.79; Analysis 12.6). Acne was also reported less often for the ring group compared to the COC group in Mohamed 2011 (OR 0.18; 95% CI 0.06 to 0.54; Analysis 9.14), Stewart 2007 (OR 0.19; 95% CI 0.05 to 0.69; Analysis 12.10), and Oddsson 2005 (OR 0.23; 95% CI 0.08 to 0.63; Analysis 11.10). Also in Stewart 2007, women in the ring group were less likely than the OC users to report worsening of body weight (OR 0.25; 95% CI 0.11 to 0.54; Analysis 12.14), headaches (OR 0.25; 95% CI 0.10 to 0.63; Analysis 12.5), moodiness (OR 0.34; 95 CI 0.15 to 0.76; Analysis 12.11), or sex drive (OR 0.22; 95 CI 0.05 to 0.91; Analysis 12.13). 'Ring‐related problems' were reported for 4.6% and 6.7% of the ring users in Oddsson 2005 and Mohamed 2011, respectively. In Ahrendt 2006, 'method‐related events' occurred in 6.6% of ring users and in 0.4% of COC users.
9.10. Analysis.
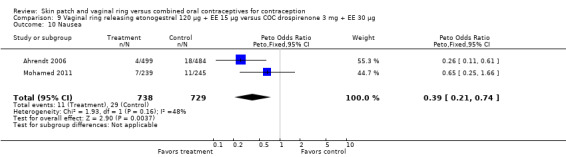
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 10 Nausea.
12.6. Analysis.
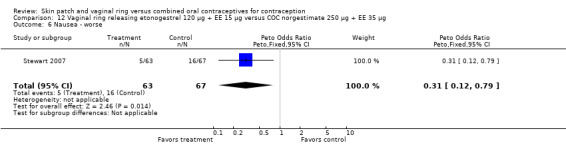
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 6 Nausea ‐ worse.
9.14. Analysis.
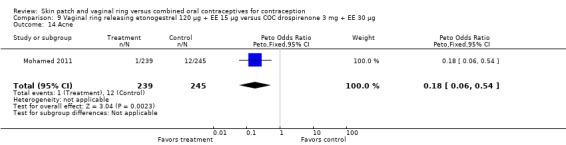
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 14 Acne.
12.10. Analysis.
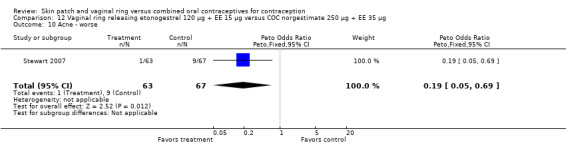
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 10 Acne ‐ worse.
11.10. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 10 Nausea.
12.14. Analysis.
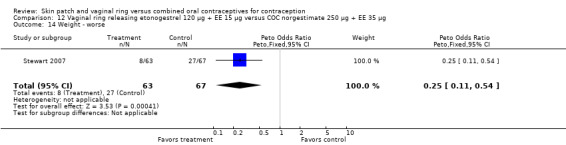
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 14 Weight ‐ worse.
12.5. Analysis.
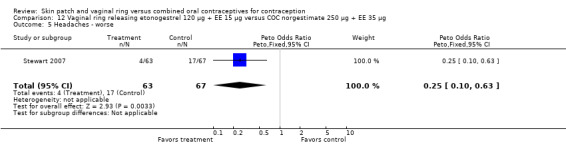
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 5 Headaches ‐ worse.
12.11. Analysis.
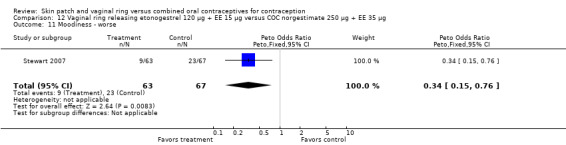
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 11 Moodiness ‐ worse.
12.13. Analysis.
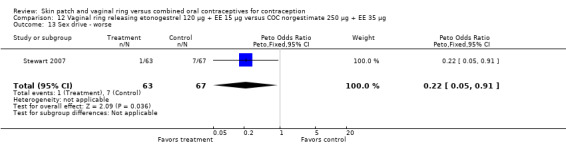
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 13 Sex drive ‐ worse.
For breast tenderness or pain in the meta‐analysis of Duijkers 2004a and Oddsson 2005, the comparison groups were not significantly different, but heterogeneity was present. Within Oddsson 2005, the results favored the COC group (OR 2.25; 95% CI 0.99 to 5.14; Analysis 11.11).
11.11. Analysis.
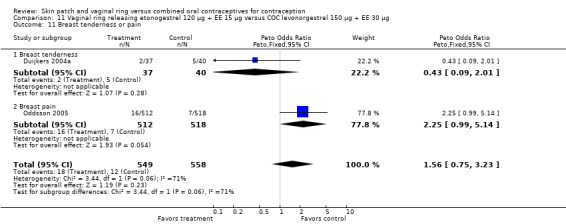
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 11 Breast tenderness or pain.
SAEs related to the study product were few. However, two vaginal ring users had deep venous thrombosis (Ahrendt 2006; Oddsson 2005). For the seven trials reporting SAEs, the estimated risk for deep venous thrombosis in the ring users was 149 (95% CI 18 to 538) per 100,000 women. The woman with thrombosis in Ahrendt 2006 was found to be heterozygous for Factor V Leiden, which is associated with increased risk for venous thrombosis. Of the COC users in the ring trials, one had abdominal pain and cholelithiasis (Ahrendt 2006) and one had hypertension (Oddsson 2005).
Satisfaction and future use
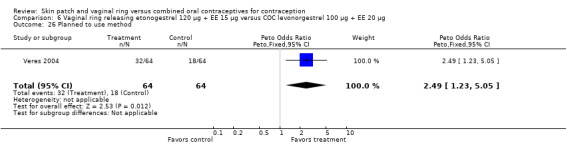
The ring users appeared more satisfied with their method than the COC users. Four ring studies had such data. The meta‐analysis of Westhoff 2005 and Gilliam 2010 showed significant heterogeneity with I2 = 88% (OR 1.50; 95% CI 1.01 to 2.24; Analysis 10.7). The study groups were not significantly different in Gilliam 2010 but the results for Westhoff 2005 favored the ring group. Also, in Westhoff 2005, more of the ring group planned to use the method after the study (OR 2.51; 95% CI 1.32 to 4.77; Analysis 10.8). In Veres 2004, the mean score for satisfaction with method was higher for ring users (MD 0.70; 95% CI 0.37 to 1.03; Analysis 6.25). The ring users were more likely to report planning to use the method after the study (OR 2.49; 95% CI 1.23 to 5.05; Analysis 6.26) (Veres 2004). No significant difference was noted in Ahrendt 2006.
10.7. Analysis.
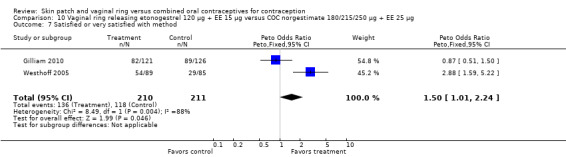
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 7 Satisfied or very satisfied with method.
10.8. Analysis.
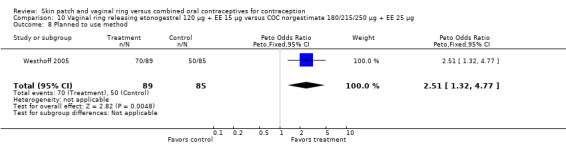
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 8 Planned to use method.
6.25. Analysis.
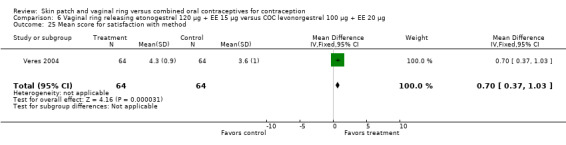
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 25 Mean score for satisfaction with method.
6.26. Analysis.
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 26 Planned to use method.
Discussion
Summary of main results
Contraceptive effectiveness was similar for the contraceptive skin patch and comparison oral contraceptive. Effectiveness was also similar for the vaginal ring and the comparison COC. As mentioned earlier, failure rates for the skin patch and the vaginal ring appear similar to those for COCs (Trussell 2011). Effectiveness of contraceptives depends on compliance with the regimen and continued use (Grimes 2009):
Compliance data were limited. Patch users showed better compliance than COC users in the three trials with such data. Compliance results varied across the four vaginal ring studies with those data. One found poorer compliance among the ring users, two showed no difference, and one showed ‘perfect’ use was more common for the vaginal ring users compared to COC users. The accuracy and consistency of compliance data depend on the data collection instrument as well as self‐reports; the latter can be affected by social desirability. The different reporting across trials as compliance or noncompliance makes makes comparing results difficult. However, differences in reported compliance did not lead to significantly lower pregnancy rates. The sample sizes of the trials could have been insufficient to detect an actual difference in effectiveness.
More patch users discontinued early than women assigned to use the COC. Furthermore, women in the patch group were more likely to discontinue due to adverse events. Of 11 vaginal ring trials with discontinuation data, two showed significant differences between the study groups. Vaginal ring users were less likely to discontinue (overall or due to AEs) than COC users.
Satisfaction with the contraceptive method may influence continuation. In one study, patch users were more likely than COC users to be very satisfied with their method. Of the four ring trials that reported satisfaction, three found ring users more likely than the COC group to be satisfied or planning to use the method. Varying results may be due to differences in the comparison COCs, the instruments used to assess satisfaction, and the duration of the trials.
For cycle control, the groups were not significantly different in three of four patch studies with such data. In one trial, breakthrough bleeding or spotting was less common among patch users. The US Food and Drug Administration warns that women using the patch may be exposed to more estrogen on average than women taking a pill with EE 35 μg (FDA 2012). The ring trials with bleeding data generally showed fewer problems for the ring group compared to the COC group. In three of seven trials with such data, the ring group had fewer episodes of breakthrough bleeding and spotting. One study showed the ring group had a greater mean for breakthrough bleeding and spotting days. Fewer problems may relate to steady‐state hormone levels, in contrast to peaks and troughs with oral contraceptives. The better bleeding patterns did not translate into less discontinuation, though.
For adverse events, five patch trials and seven ring trials reported data. Where significant differences were noted, norelgestromin patch users generally had more AEs than the COC users. These included breast discomfort or pain, dysmenorrhea, nausea, and vomiting. Patch users had less moodiness. Users of a levonorgestrel patch had less nausea, headache, and fatigue. Ring users had more vaginal irritation and discharge than COC users in several trials, but less nausea and acne. One trial showed ring users had fewer reports of worsening body weight, headaches, or sex drive. Another found less irritability, depression, and vaginal dryness among the ring users than the COC users. However, that trial investigated certain tolerability issues, presumably actively, whereas other researchers usually summarized participants’ reports of adverse events. Participants may report more problems if prompted, but the effect should be similar in both groups. Since these trials were not blinded, the results could be biased. The estimated risk for deep venous thrombosis in the users of the norelgestromin patch was 53 per 100,000 women (95% CI 1 to 294). For the etonogestrel ring users, the risk was 149 (95% CI 18 to 538) per 100,000 women, but one woman with deep venous thrombosis had an additional risk factor. Because of the rarity of venous thromboembolism, these randomized controlled trials are not informative concerning comparative risk. In surveillance of women in Olmsted County, Minnesota (USA), the overall incidence of venous thromboembolism (VTE) from 1966 to 1990 ranged from 27 to 84 per 100,000 women per year, for ages 15 to 19 and 40 to 44 years, respectively (Heit 2005). VTE included both deep venous thrombosis and pulmonary embolism. The risk for VTE was higher in pregnancy (96) and even higher in the postpartum period (511) from 1966 to 1995 (Heit 2005). The increased risk may only affect 5 to 10 per 10,000 users of the patch or certain COCs per year (Raymond 2012).
Quality of the evidence
The quality of the evidence for this review was considered low for the patch and moderate for the ring, based on the evidence from the studies (Table 14). Of the six patch trials, two provided moderate or high quality evidence. The main reasons for downgrading patch studies were not having information on randomization sequence generation or allocation concealment as well as limitations of the outcome assessment method, i.e., no pregnancy test or no structured questionnaire or definition for contraceptive use or cycle control. Of 12 ring trials, seven had moderate or high quality evidence. The main limitations ring trials were the randomization and allocation issues, followed by losses to follow up greater than 20% and exclusions after randomization.
1. Quality of evidence.
| Study | Experimental intervention | Allocation | Outcome assessment | Losses | Exclusion | Quality of evidence1 |
| Patch | ||||||
| Urdl 2005 | Patch2 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | high |
| Kluft 2008 | Patch | ‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | moderate |
| Audet 2001 | Patch | ‐‐‐ | ‐‐‐ | ‐1 | ‐1 | low |
| Boonyarangkul 2007 | Patch | ‐1 | ‐1 | ‐‐‐ | ‐‐‐ | low |
| Dittrich 2002 | Patch | ‐1 | ‐‐‐ | ‐‐‐ | ‐1 | low |
| Kaunitz 2012 | LNG patch | ‐1 | ‐1 | ‐‐‐ | ‐‐‐ | low |
| RIng | ||||||
| Stewart 2007 | Ring3 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | high |
| Rad 2006 | Nesterone ring | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | high |
| Ahrendt 2006 | Ring | ‐‐‐ | ‐‐‐ | ‐1 | ‐‐‐ | moderate |
| Gilliam 2010 | Ring | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | moderate |
| Guida 2005 | Ring | ‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | moderate |
| Oddsson 2005 | Ring | ‐‐‐ | ‐‐‐ | ‐1 | ‐‐‐ | moderate |
| Westhoff 2005 | Ring | ‐‐‐ | ‐‐‐ | ‐1 | ‐‐‐ | moderate |
| Duijkers 2004a | Ring | ‐1 | ‐‐‐ | ‐1 | ‐‐‐ | low |
| Elkind‐Hirsch 2007 | Ring | ‐1 | ‐1 | ‐‐‐ | ‐1 | low |
| Mohamed 2011 | Ring | ‐1 | ‐‐‐ | ‐‐‐ | ‐1 | low |
| Veres 2004 | Ring | ‐‐‐ | ‐1 | ‐1 | ‐‐‐ | low |
| Sabatini 2006 | Ring | ‐1 | ‐‐‐ | ‐1 | ‐1 | very low |
1Quality could be high, moderate, low, or very low. RCTs were considered to be high quality then downgraded for the following: a) no information on randomization sequence generation or allocation concealment, or no concealment; b) outcome assessment (no pregnancy test, or no structured questionnaire or definition for contraceptive use or cycle control; crossover data presented for whole study rather than per period); c) losses to follow up > 20%; d) exclusion after randomization.
2Patch: unless otherwise specified, contains norelgestromin + EE
3Ring: unless otherwise specified, contains etonogestrel + EE
CONSORT guidelines for the reporting of randomized controlled trials recommend the reporting of outcome data in absolute numbers, rather than percentages (CONSORT 2009). Several trials reported some outcomes only as percentages, which prevented the inclusion of those data in the review.
Authors' conclusions
Implications for practice.
These trials showed similar effectiveness rates for a contraceptive skin patch or a vaginal ring compared a combined oral contraceptive (COC). The patch could lead to more discontinuation than the COC. Patch users had increased risk for breast discomfort, painful periods, nausea, and vomiting. Ring users had more vaginal irritation and discharge than COC users but better cycle control and less nausea, acne, and emotional problems.
Implications for research.
Randomized controlled trials of the skin patch and vaginal ring should be reported in a manner consistent with CONSORT guidelines. The quality of the evidence for this review was low for the patch and moderate for the ring. Quality could be improved by providing information on randomization sequence generation and allocation concealment, using more reliable outcome measures, reducing losses to follow up, and including all randomized participants in the analysis. More reporting of pregnancy data from patch studies would strengthen the evidence regarding effectiveness. In addition, assessment and reporting of compliance would aid the interpretation of results.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2013 | New search has been performed | Search updated. |
| 17 January 2013 | New citation required but conclusions have not changed | Three new studies were included (Mohamed 2011; Gilliam 2010; Kaunitz 2012). Added summary of evidence quality (Table 14). |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 4 January 2010 | New citation required but conclusions have not changed | Four new trials were incorporated: 2 on skin patch (Boonyarangkul 2007; Kluft 2008) and 2 on vaginal ring (Elkind‐Hirsch 2007; Stewart 2007) |
| 30 December 2009 | New search has been performed | Searches were updated |
| 15 April 2008 | Amended | Converted to new review format |
| 18 September 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
From FHI 360: Carol Manion helped develop and conduct the literature searches.
Appendices
Appendix 1. Search 2013
MEDLINE via PubMed (01 Jul 2009 to 07 Mar 2013)
(contraceptive patch or (contraceptive and patch)) or ((vagina and ring) or (vagina* and ring) or (contraceptive agents and ring) or (contraceptive devices and (ring or patch))) AND (Clinical Trial[ptyp])
CENTRAL (2009 to 07 Mar 2013)
(contrac* AND patch) OR ((contracept* OR vagina*) AND ring) in title, abstract, or keywords
POPLINE (2009 to 12 Nov 2012)
((oral OR pill) AND contracep*) AND ((contraceptive AND patch) OR ((vagina* OR contracep*) AND ring))
LILACS (25 Jan 2013)
contraceptive agents, female or agentes anticonceptivos or anticoncepcionais [Words]
AND patch or parche or placa or emplastro or ring or anillo or anel [Words]
ClinicalTrials.gov (01 Jul 2009 to 12 Nov 2012)
Study type: Interventional Condition: NOT (polycystic OR in vitro) Intervention: ((contraception OR contraceptive) AND (patch OR ring) AND oral) NOT (implanon OR IUS OR IUD)
ICTRP (2009 to 12 Nov 2012)
oral contraceptive AND (skin patch OR vaginal ring)
Appendix 2. Search 2009
MEDLINE via PubMed (2007 to 02 Dec 2009)
(contraceptive patch or (contraceptive and patch)) or ((vagina and ring) or (vagina* and ring) or (contraceptive agents and ring) or (contraceptive devices and (ring or patch))) AND (randomized controlled trial[pt] or controlled clinical trial[pt]or randomized controlled trials[mh] or random allocation[mh] or double‐blind method[mh] or single‐blind method[mh] or clinical trial[pt] or clinical trials[mh] or (clinical trial(tw) or ((singl* or doubl* or trebl* or tripl*) and (mask* or blind*))) or "latin square" or placebos[mh]or placebo* or random* or research design[mh] or comparative stud* or evaluation studies[pt] or follow‐up studies[mh] or prospective studies[mh] or cross‐over studies[mh] or control* or prospectiv* or volunteer*) NOT (animal[mh] NOT human[mh])
POPLINE (2007 to 01 Dec 2009)
(compar* / clinical trials / comparative studies / random / double‐blind studies) & ((contraceptive & patch) / ((vagina / contraceptive agents) & ring))
CENTRAL (2007 to 23 Oct 2009)
(contrac* AND patch) OR ((contracept* OR vagina*) AND ring) in title, abstract, or keywords
EMBASE (22 Jan 2007 to 09 Dec 2009)
(s contracept? and patch) OR (s ring?(W)(nuva OR vagina? OR contracept?) OR (((oestradiol OR estradiol) AND ethinyl) OR ee) AND (desogestrel OR etonogestrel)) AND ring?)
LILACS (through 02 Dec 2009)
contraceptive agents, female or agentes anticonceptivos or anticoncepcionais [Words]
AND patch or parche or placa or emplastro or ring or anillo or anel [Words]
ClinicalTrials.gov (to 28 Oct 2009)
Intervention: (contraception OR contraceptive) AND (patch OR ring) AND oral
ICTRP (to 29 Oct 2009)
oral contraceptive AND (skin patch OR vaginal ring)
Data and analyses
Comparison 1. Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per cycle | 1 | 9407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.18, 1.77] |
| 2 Kaplan‐Meier cumulative pregnancy rates (95% CI) | Other data | No numeric data | ||
| 3 Discontinuation ‐ overall (6 or 13 cycles) | 2 | 1564 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.26, 2.00] |
| 4 Discontinuation ‐ adverse events (5 or 13 cycles) | 2 | 1564 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [1.61, 3.25] |
| 5 Compliance per cycle | 1 | 9275 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [1.83, 2.29] |
| 6 Breakthrough bleeding or spotting (cycle 6) | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.93, 1.98] |
| 7 Breakthrough bleeding or spotting (cycle 13) | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.49, 1.18] |
| 8 Breast discomfort | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.09 [2.26, 4.22] |
| 9 Headache | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.77, 1.27] |
| 10 Nausea | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.88, 1.49] |
| 11 Dysmenorrhea | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [1.03, 1.99] |
| 12 Abdominal pain | 1 | 1417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 13 Adverse events ‐ total (6 cycles) | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.28, 3.06] |
1.1. Analysis.
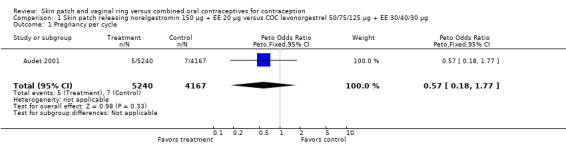
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 1 Pregnancy per cycle.
1.6. Analysis.
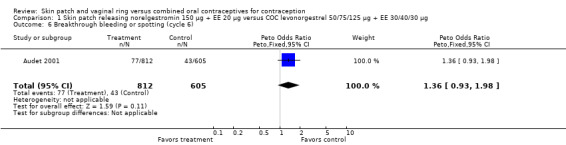
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 6 Breakthrough bleeding or spotting (cycle 6).
1.7. Analysis.
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 7 Breakthrough bleeding or spotting (cycle 13).
1.9. Analysis.
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 9 Headache.
1.10. Analysis.
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 10 Nausea.
1.12. Analysis.
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 12 Abdominal pain.
1.13. Analysis.
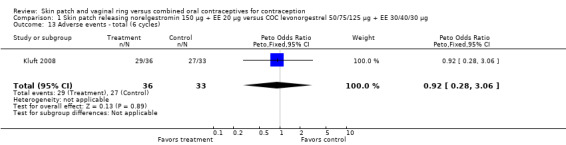
Comparison 1 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 50/75/125 µg + EE 30/40/30 µg, Outcome 13 Adverse events ‐ total (6 cycles).
Comparison 2. Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg.
2.2. Analysis.
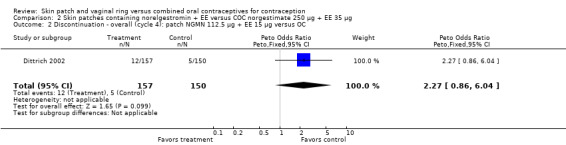
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 2 Discontinuation ‐ overall (cycle 4): patch NGMN 112.5 µg + EE 15 µg versus OC.
2.3. Analysis.
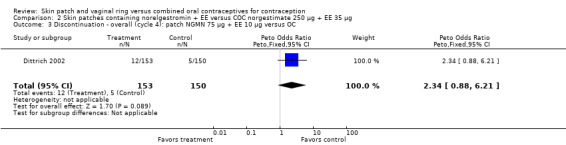
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 3 Discontinuation ‐ overall (cycle 4): patch NGMN 75 µg + EE 10 µg versus OC.
2.4. Analysis.
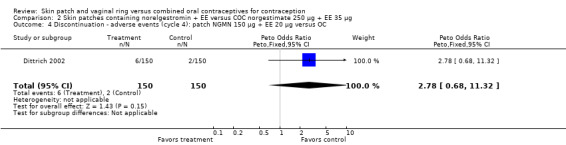
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 4 Discontinuation ‐ adverse events (cycle 4): patch NGMN 150 µg + EE 20 µg versus OC.
2.5. Analysis.
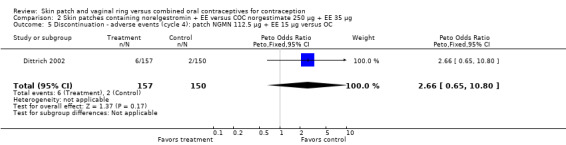
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 5 Discontinuation ‐ adverse events (cycle 4): patch NGMN 112.5 µg + EE 15 µg versus OC.
2.6. Analysis.
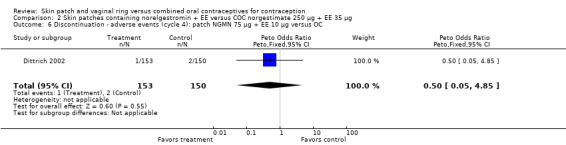
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 6 Discontinuation ‐ adverse events (cycle 4): patch NGMN 75 µg + EE 10 µg versus OC.
2.7. Analysis.
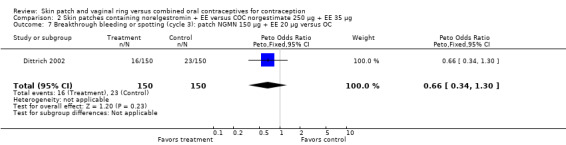
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 7 Breakthrough bleeding or spotting (cycle 3): patch NGMN 150 µg + EE 20 µg versus OC.
2.8. Analysis.
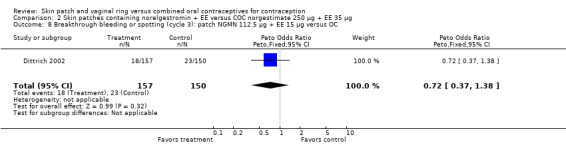
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 8 Breakthrough bleeding or spotting (cycle 3): patch NGMN 112.5 µg + EE 15 µg versus OC.
2.9. Analysis.
Comparison 2 Skin patches containing norelgestromin + EE versus COC norgestimate 250 µg + EE 35 µg, Outcome 9 Breakthrough bleeding or spotting (cycle 3): patch NGMN 75 µg + EE 10 µg versus OC.
Comparison 3. Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman (3 cycles) | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Spotting (3 cycles) | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.28] |
| 3 Amenorrhea | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Breast tenderness | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.11 [2.48, 33.52] |
| 5 Nausea | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.42 [0.66, 8.90] |
3.1. Analysis.
Comparison 3 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 1 Pregnancy per woman (3 cycles).
3.2. Analysis.
Comparison 3 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 2 Spotting (3 cycles).
3.3. Analysis.
Comparison 3 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 3 Amenorrhea.
3.5. Analysis.
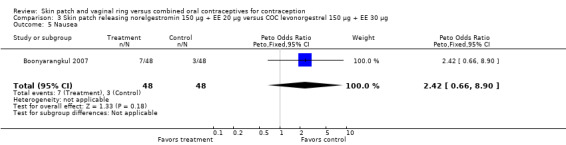
Comparison 3 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 5 Nausea.
Comparison 4. Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 1484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.30, 7.53] |
| 2 Kaplan‐Meier cumulative pregnancy rates (95% CI) | Other data | No numeric data | ||
| 3 Discontinuation ‐ overall (6 or 13 cycles) | 2 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [1.18, 2.06] |
| 4 Discontinuation ‐ adverse events (6 or 13 cycles) | 2 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.44, 3.11] |
| 5 Compliance per cycle | 1 | 10593 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.76 [2.35, 3.24] |
| 6 Breakthrough bleeding and spotting (cycle 3) | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 7 Breakthrough bleeding and spotting (cycle 13) | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.46, 0.92] |
| 8 Breast discomfort or pain | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.98 [2.29, 3.90] |
| 9 Headache | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.64, 1.05] |
| 10 Nausea | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.46, 2.95] |
| 11 Dysmenorrhea | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.72, 1.83] |
| 12 Abdominal pain | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.71, 1.36] |
| 13 Vaginitis | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.62, 1.46] |
| 14 Vomiting | 1 | 1489 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [1.12, 3.16] |
| 15 Adverse events ‐ total | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.67, 5.65] |
| 16 Very satisfied with method | 1 | 1352 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
4.1. Analysis.
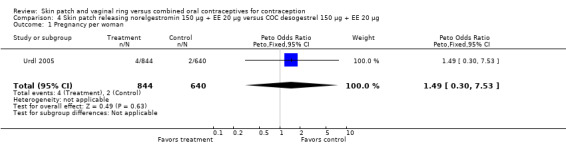
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 1 Pregnancy per woman.
4.6. Analysis.
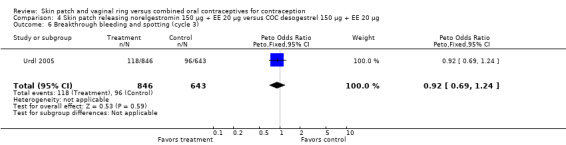
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 6 Breakthrough bleeding and spotting (cycle 3).
4.9. Analysis.
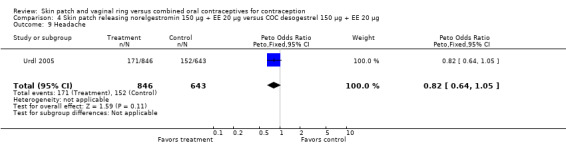
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 9 Headache.
4.11. Analysis.
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 11 Dysmenorrhea.
4.12. Analysis.
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 12 Abdominal pain.
4.13. Analysis.
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 13 Vaginitis.
4.15. Analysis.
Comparison 4 Skin patch releasing norelgestromin 150 µg + EE 20 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 15 Adverse events ‐ total.
Comparison 5. Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation ‐ adverse events (cycle 6) | 1 | 1385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.82 [1.19, 2.79] |
| 2 Noncompliance per cycle (cycle 6) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.25, 0.51] |
| 3 Breast tenderness (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.50, 3.07] |
| 4 Headache (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.10, 0.51] |
| 5 Migraine (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.47, 5.59] |
| 6 Nausea (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.51, 1.75] |
| 7 Dysmenorrhea (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.19, 1.22] |
| 8 Acne (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.24, 1.53] |
| 9 Vomiting (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.14, 0.91] |
| 10 Mood swings (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.38, 5.42] |
| 11 Depression (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [0.55, 5.71] |
| 12 Dizziness (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.28, 3.59] |
| 13 Fatigue (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.04, 0.82] |
| 14 Hypertension (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.09, 1.36] |
| 15 Libido decreased (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.11, 3.97] |
| 16 Weight increased (6 cycles) | 1 | 1612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.19 [0.82, 5.87] |
5.3. Analysis.
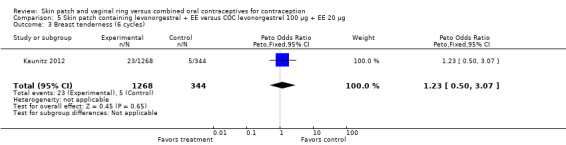
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 3 Breast tenderness (6 cycles).
5.5. Analysis.
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 5 Migraine (6 cycles).
5.6. Analysis.
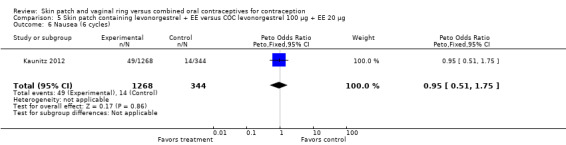
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 6 Nausea (6 cycles).
5.7. Analysis.
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 7 Dysmenorrhea (6 cycles).
5.8. Analysis.
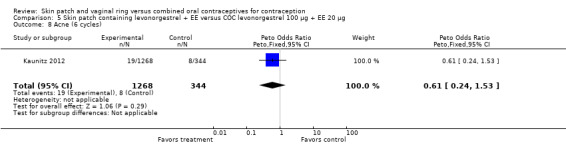
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 8 Acne (6 cycles).
5.10. Analysis.
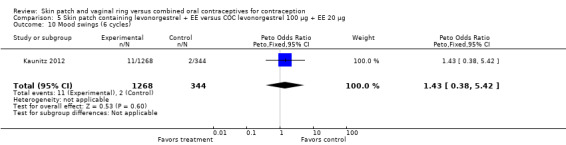
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 10 Mood swings (6 cycles).
5.11. Analysis.
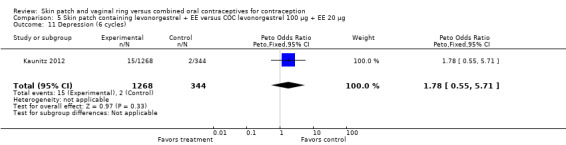
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 11 Depression (6 cycles).
5.12. Analysis.
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 12 Dizziness (6 cycles).
5.14. Analysis.
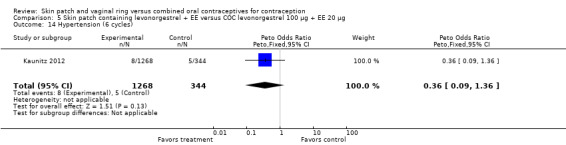
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 14 Hypertension (6 cycles).
5.15. Analysis.
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 15 Libido decreased (6 cycles).
5.16. Analysis.
Comparison 5 Skin patch containing levonorgestrel + EE versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 16 Weight increased (6 cycles).
Comparison 6. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 2 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.00] |
| 2 Discontinuation ‐ overall (3 to 12 cycles) | 3 | 407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.39, 1.11] |
| 3 Discontinuation ‐ adverse events (3 or 12 cycles) | 2 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.20, 1.11] |
| 4 Noncompliance per woman | 1 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.99 [1.87, 8.52] |
| 5 Early or late withdrawal bleeding (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.07, 0.70] |
| 6 Early or late withdrawal bleeding (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.05, 0.86] |
| 7 Spotting or breakthrough bleeding (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.15, 0.87] |
| 8 Spotting or breakthrough bleeding (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.12, 0.94] |
| 9 Breakthrough bleeding (cycle 5) | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.42] |
| 10 Breast tenderness (cycles 5 & 6) | 2 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.22, 1.79] |
| 11 Breast tenderness (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.18, 2.34] |
| 12 Headache (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.32, 2.02] |
| 13 Headache (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.23, 1.86] |
| 14 Nausea (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.14, 1.35] |
| 15 Nausea (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.08, 1.19] |
| 16 Vaginal dryness (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.47] |
| 17 Vaginal dryness (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.03, 0.65] |
| 18 Vaginal yeast infection/discomfort (cycle 5) | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.02 [0.30, 122.32] |
| 19 Irritability (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.08, 0.88] |
| 20 Irritability (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.08, 1.01] |
| 21 Mood swings (cycle 5) | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.05, 12.92] |
| 22 Depression (cycle 6) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.08, 1.19] |
| 23 Depression (cycle 12) | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.03] |
| 24 Hot flashes (cycle 5) | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 6.38] |
| 25 Mean score for satisfaction with method | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.37, 1.03] |
| 26 Planned to use method | 1 | 128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [1.23, 5.05] |
6.1. Analysis.
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 1 Pregnancy per woman.
6.2. Analysis.
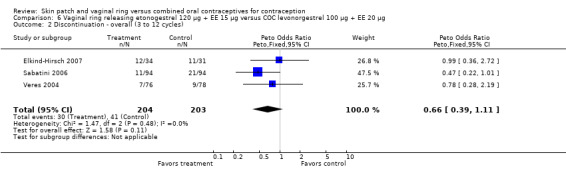
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 2 Discontinuation ‐ overall (3 to 12 cycles).
6.3. Analysis.
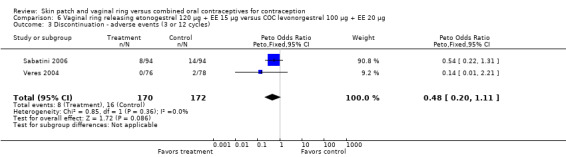
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 3 Discontinuation ‐ adverse events (3 or 12 cycles).
6.9. Analysis.
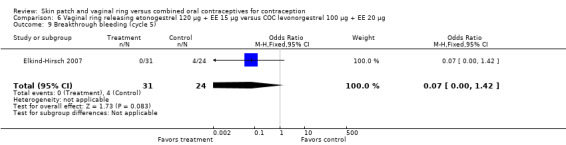
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 9 Breakthrough bleeding (cycle 5).
6.10. Analysis.
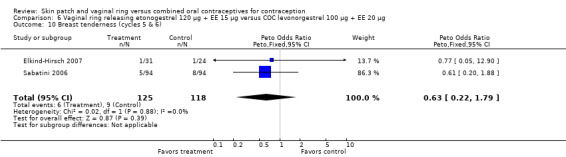
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 10 Breast tenderness (cycles 5 & 6).
6.11. Analysis.
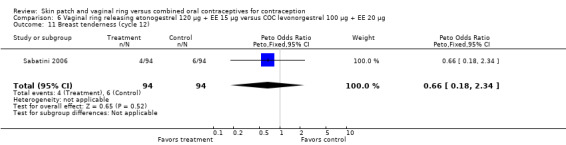
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 11 Breast tenderness (cycle 12).
6.12. Analysis.
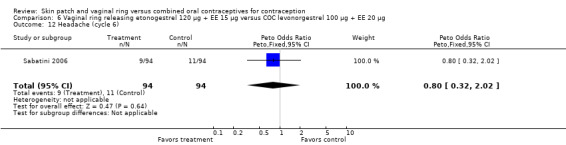
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 12 Headache (cycle 6).
6.13. Analysis.
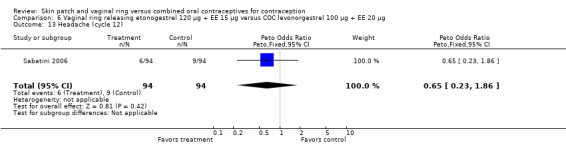
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 13 Headache (cycle 12).
6.14. Analysis.
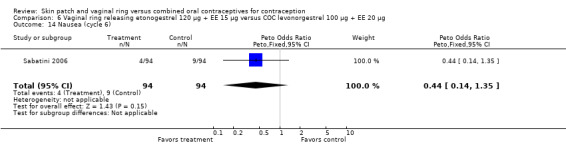
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 14 Nausea (cycle 6).
6.15. Analysis.
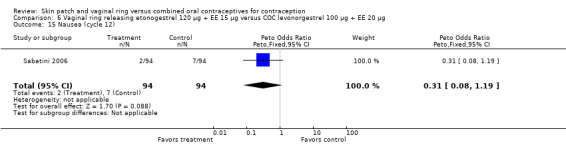
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 15 Nausea (cycle 12).
6.18. Analysis.
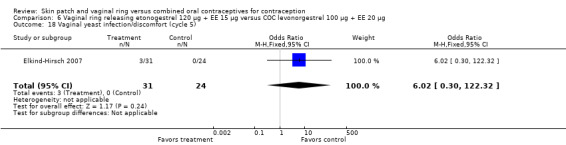
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 18 Vaginal yeast infection/discomfort (cycle 5).
6.21. Analysis.
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 21 Mood swings (cycle 5).
6.22. Analysis.
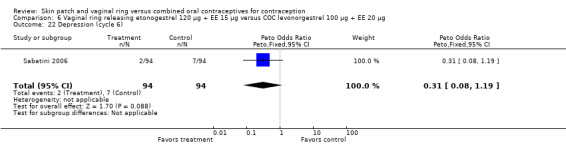
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 22 Depression (cycle 6).
6.24. Analysis.
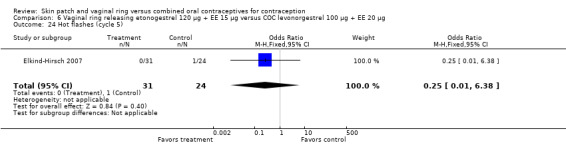
Comparison 6 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 100 µg + EE 20 µg, Outcome 24 Hot flashes (cycle 5).
Comparison 7. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Discontinuation ‐ overall (12 cycles) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.16, 0.66] |
| 3 Discontinuation ‐ adverse events (12 cycles) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.15, 0.70] |
| 4 Early or late withdrawal bleeding (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.07, 0.46] |
| 5 Early or late withdrawal bleeding (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.05, 0.73] |
| 6 Spotting or breakthrough bleeding (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.11, 0.57] |
| 7 Spotting or breakthrough bleeding (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.12, 0.91] |
| 8 Breast tenderness (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.21, 2.20] |
| 9 Breast tenderness (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.29] |
| 10 Headache (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.34, 2.24] |
| 11 Headache (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.22, 1.82] |
| 12 Nausea (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.16, 1.85] |
| 13 Nausea (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.09, 1.82] |
| 14 Vaginal dryness (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.04, 0.32] |
| 15 Vaginal dryness (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 16 Irritability (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.08, 0.99] |
| 17 Irritability (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.08, 1.16] |
| 18 Depression (cycle 6) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.08, 1.42] |
| 19 Depression (cycle 12) | 1 | 186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.05, 0.84] |
7.1. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 1 Pregnancy per woman.
7.8. Analysis.
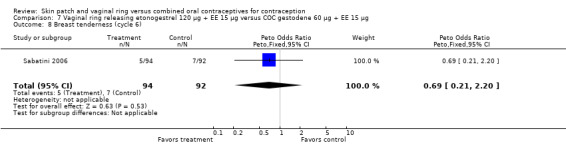
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 8 Breast tenderness (cycle 6).
7.9. Analysis.
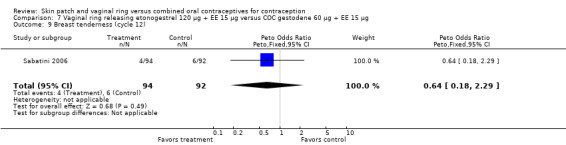
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 9 Breast tenderness (cycle 12).
7.10. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 10 Headache (cycle 6).
7.11. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 11 Headache (cycle 12).
7.12. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 12 Nausea (cycle 6).
7.13. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 13 Nausea (cycle 12).
7.17. Analysis.
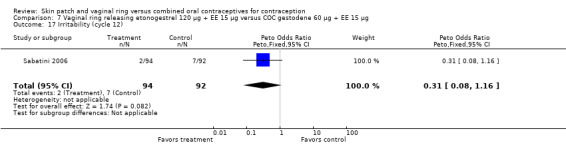
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 17 Irritability (cycle 12).
7.18. Analysis.
Comparison 7 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC gestodene 60 µg + EE 15 µg, Outcome 18 Depression (cycle 6).
Comparison 8. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC desogestrel 150 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation ‐ overall (cycle 6) | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.11, 4.01] |
8.1. Analysis.
Comparison 8 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC desogestrel 150 µg + EE 20 µg, Outcome 1 Discontinuation ‐ overall (cycle 6).
Comparison 9. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 716 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.05, 1.76] |
| 2 Discontinuation ‐ overall (cycle 12 or 13) | 2 | 1583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.93, 1.48] |
| 3 Discontinuation ‐ adverse events (cycle 12 or 13) | 2 | 1583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.95, 1.84] |
| 4 Mean breakthrough bleeding or spotting days (cycle 6) | 1 | 983 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [1.57, 2.43] |
| 5 Mean breakthrough bleeding or spotting days (cycle 13) | 1 | 983 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 6 Breakthrough bleeding or spotting (12 cycles) | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.44, 1.26] |
| 7 No withdrawal bleeding (12 cycles) | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.23, 2.29] |
| 8 Breast pain | 2 | 1467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.47, 1.40] |
| 9 Headache | 2 | 1467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.65, 1.43] |
| 10 Nausea | 2 | 1467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.21, 0.74] |
| 11 Dysmenorrhea | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.32 [0.66, 8.10] |
| 12 Vaginitis | 2 | 1467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.48 [1.39, 4.43] |
| 13 Leukorrhea | 2 | 1467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.21 [1.61, 6.40] |
| 14 Acne | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.54] |
| 15 Vomiting | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.05, 2.68] |
| 16 Emotional lability | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.06, 0.59] |
| 17 Decreased libido | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [1.00, 12.18] |
| 18 Weight increase | 1 | 484 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.14, 1.10] |
| 19 Total number of adverse events (cycle 12) | 1 | 600 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.92, 1.82] |
| 20 Satisfied or very satisfied with method | 1 | 983 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.55, 1.12] |
9.1. Analysis.
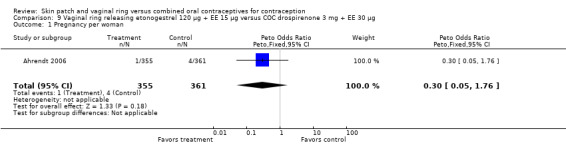
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 1 Pregnancy per woman.
9.2. Analysis.
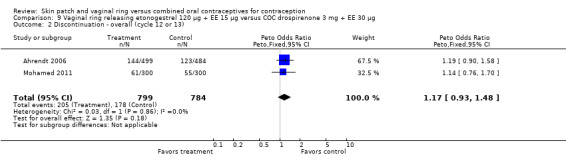
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 2 Discontinuation ‐ overall (cycle 12 or 13).
9.3. Analysis.
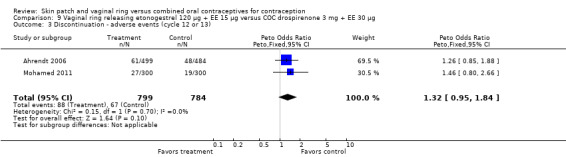
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 3 Discontinuation ‐ adverse events (cycle 12 or 13).
9.5. Analysis.
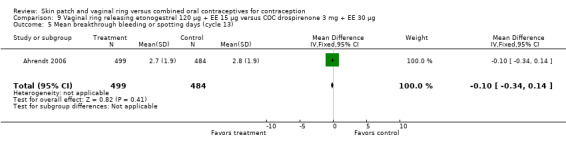
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 5 Mean breakthrough bleeding or spotting days (cycle 13).
9.6. Analysis.
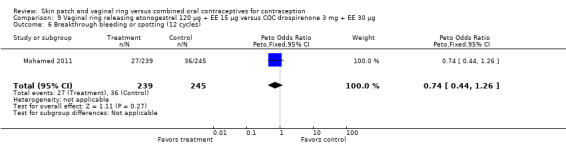
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 6 Breakthrough bleeding or spotting (12 cycles).
9.7. Analysis.
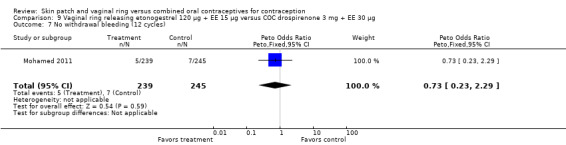
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 7 No withdrawal bleeding (12 cycles).
9.8. Analysis.
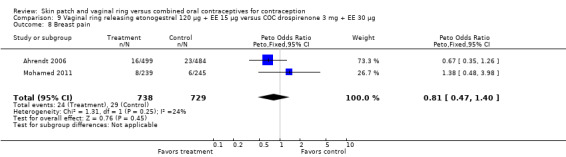
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 8 Breast pain.
9.9. Analysis.
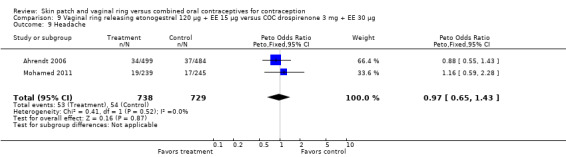
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 9 Headache.
9.11. Analysis.
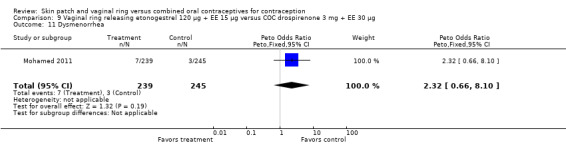
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 11 Dysmenorrhea.
9.15. Analysis.
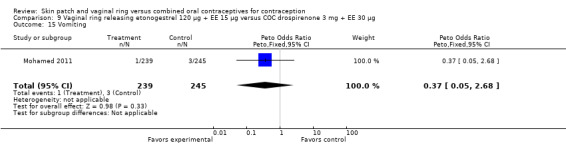
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 15 Vomiting.
9.17. Analysis.
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 17 Decreased libido.
9.18. Analysis.
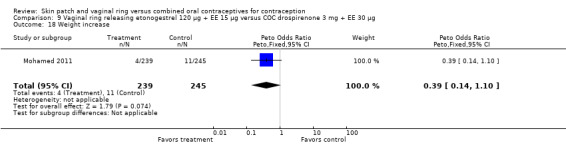
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 18 Weight increase.
9.19. Analysis.
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 19 Total number of adverse events (cycle 12).
9.20. Analysis.
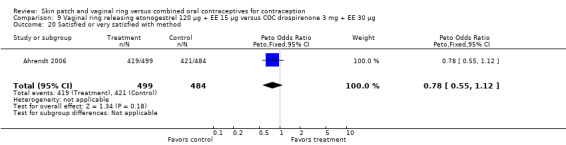
Comparison 9 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC drospirenone 3 mg + EE 30 µg, Outcome 20 Satisfied or very satisfied with method.
Comparison 10. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Discontinuation | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Overall (84 days or 3 cycles) | 2 | 448 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.85, 1.88] |
| 2.2 Overall (6 months) | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.31, 0.88] |
| 3 Compliance: perfect use (3 months) | 1 | 273 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [1.04, 2.68] |
| 4 Frequent bleeding (> 4 episodes of bleeding or spotting) | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.03] |
| 5 Irregular bleeding (bleeding‐free interval > 17 days) | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.33, 1.75] |
| 6 Prolonged bleeding (bleeding or spotting episode lasting >= 10 days) | 1 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 7 Satisfied or very satisfied with method | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [1.01, 2.24] |
| 8 Planned to use method | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.32, 4.77] |
10.1. Analysis.
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 1 Pregnancy per woman.
10.5. Analysis.
Comparison 10 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 180/215/250 µg + EE 25 µg, Outcome 5 Irregular bleeding (bleeding‐free interval > 17 days).
Comparison 11. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.29, 3.51] |
| 2 Pregnancy per cycle | 1 | 10783 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.30, 3.55] |
| 3 Discontinuation ‐ overall (6 or 13 cycles) | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.81, 1.38] |
| 4 Discontinuation ‐ adverse events (6 or 13 cycles) | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.89, 2.00] |
| 5 Compliance per cycle | 1 | 10783 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.96, 1.20] |
| 6 Breakthrough bleeding (cycle 6) | 1 | 770 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.88] |
| 7 Breakthrough spotting (cycle 6) | 1 | 770 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.36, 1.24] |
| 8 Breakthrough bleeding (cycle 13) | 1 | 425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.15 [0.01, 2.45] |
| 9 Breakthrough spotting (cycle 13) | 1 | 425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.38, 2.67] |
| 10 Nausea | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.48, 1.63] |
| 10.1 Nausea | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.28 [0.77, 14.08] |
| 10.2 Nausea | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.31] |
| 11 Breast tenderness or pain | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [0.75, 3.23] |
| 11.1 Breast tenderness | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.09, 2.01] |
| 11.2 Breast pain | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.25 [0.99, 5.14] |
| 12 Headache | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.80, 2.10] |
| 13 Dysmenorrhea | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [0.77, 4.52] |
| 14 Abdominal pain | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.63, 4.57] |
| 15 Vaginitis | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.84 [1.34, 6.01] |
| 16 Genital pruritus | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.58 [1.14, 18.41] |
| 17 Leukorrhea | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.42 [2.71, 15.22] |
| 18 Urinary tract infection | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.51 [0.78, 72.32] |
| 19 Acne | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.08, 0.63] |
| 20 Nervousness | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.24 [0.50, 134.45] |
| 21 Depression | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.32] |
| 22 Dizziness | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.05, 5.42] |
| 23 Libido decrease | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.32, 2.05] |
| 24 Weight increase | 2 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.41, 2.13] |
| 25 Increased glucocorticoids | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.05, 5.42] |
| 26 Sinusitis | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.48 [0.15, 376.80] |
| 27 Leg pain | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [0.65, 7.88] |
11.1. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 1 Pregnancy per woman.
11.2. Analysis.
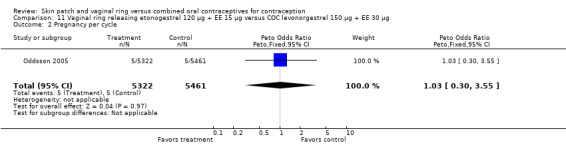
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 2 Pregnancy per cycle.
11.3. Analysis.
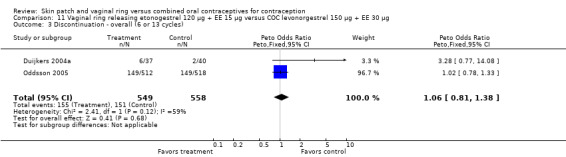
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 3 Discontinuation ‐ overall (6 or 13 cycles).
11.4. Analysis.
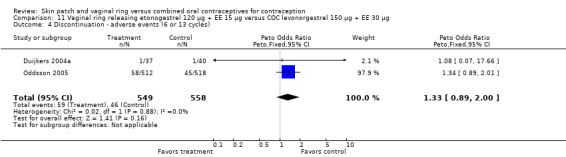
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 4 Discontinuation ‐ adverse events (6 or 13 cycles).
11.5. Analysis.
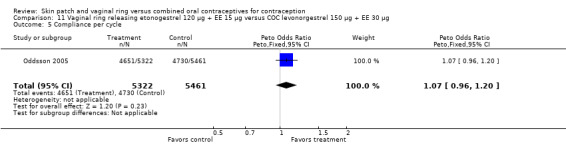
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 5 Compliance per cycle.
11.7. Analysis.
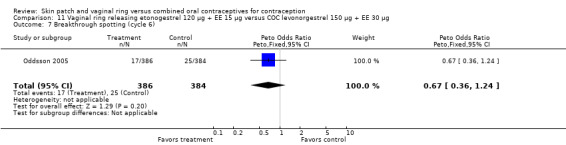
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 7 Breakthrough spotting (cycle 6).
11.8. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 8 Breakthrough bleeding (cycle 13).
11.9. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 9 Breakthrough spotting (cycle 13).
11.12. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 12 Headache.
11.13. Analysis.
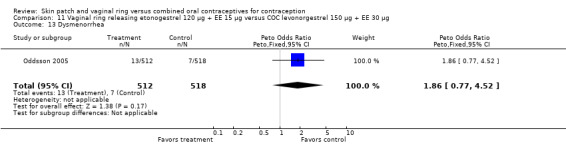
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 13 Dysmenorrhea.
11.14. Analysis.
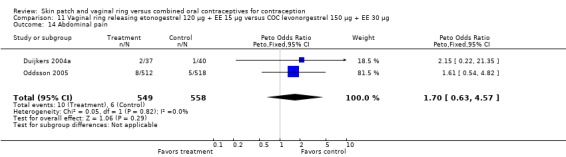
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 14 Abdominal pain.
11.18. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 18 Urinary tract infection.
11.19. Analysis.
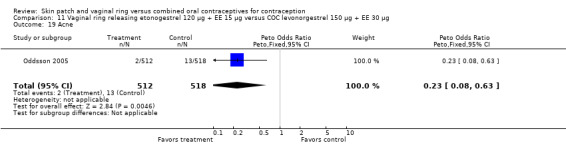
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 19 Acne.
11.20. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 20 Nervousness.
11.21. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 21 Depression.
11.22. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 22 Dizziness.
11.23. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 23 Libido decrease.
11.24. Analysis.
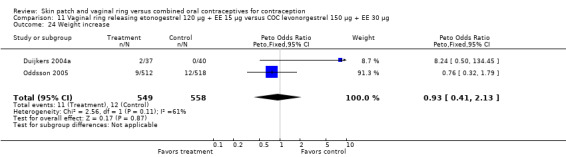
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 24 Weight increase.
11.25. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 25 Increased glucocorticoids.
11.26. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 26 Sinusitis.
11.27. Analysis.
Comparison 11 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 27 Leg pain.
Comparison 12. Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman (3 cycles) | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.15, 1.43] |
| 2 Compliance per woman | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.27, 1.09] |
| 3 Amount of blood in period ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.24, 1.73] |
| 4 Bothersome side effects ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.17, 1.03] |
| 5 Headaches ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.10, 0.63] |
| 6 Nausea ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.12, 0.79] |
| 7 Cramping in periods ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.30, 1.74] |
| 8 PMS ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.18, 1.34] |
| 9 Discharge ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.52, 2.69] |
| 10 Acne ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.05, 0.69] |
| 11 Moodiness ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.15, 0.76] |
| 12 Depresssion ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.47, 5.03] |
| 13 Sex drive ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.91] |
| 14 Weight ‐ worse | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.11, 0.54] |
12.1. Analysis.
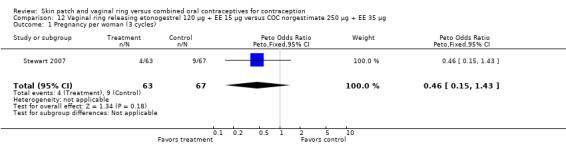
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 1 Pregnancy per woman (3 cycles).
12.2. Analysis.
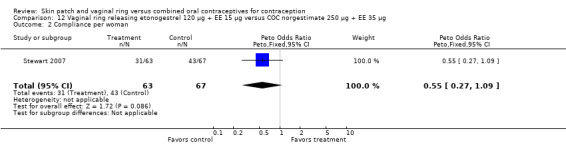
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 2 Compliance per woman.
12.3. Analysis.
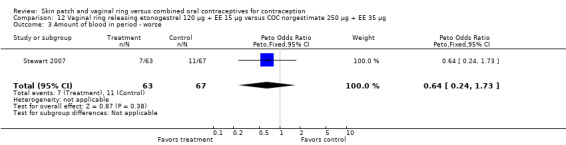
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 3 Amount of blood in period ‐ worse.
12.4. Analysis.
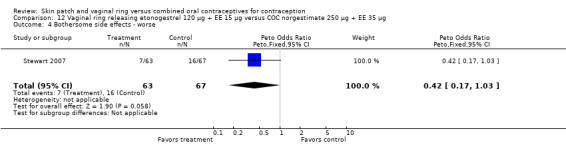
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 4 Bothersome side effects ‐ worse.
12.7. Analysis.
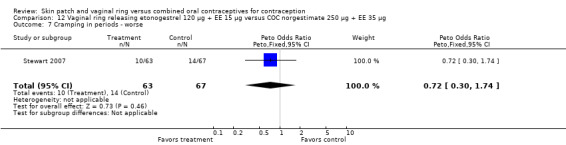
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 7 Cramping in periods ‐ worse.
12.8. Analysis.
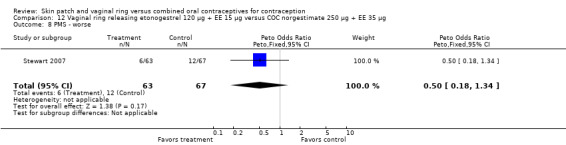
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 8 PMS ‐ worse.
12.9. Analysis.
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 9 Discharge ‐ worse.
12.12. Analysis.
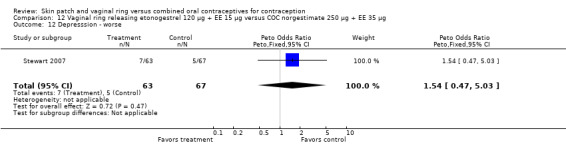
Comparison 12 Vaginal ring releasing etonogestrel 120 µg + EE 15 µg versus COC norgestimate 250 µg + EE 35 µg, Outcome 12 Depresssion ‐ worse.
Comparison 13. Vaginal ring releasing nestorone 150 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy | 1 | 47 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Discontinuation ‐ overall (cycle 3) | 1 | 47 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.23] |
13.1. Analysis.
Comparison 13 Vaginal ring releasing nestorone 150 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 1 Pregnancy.
13.2. Analysis.
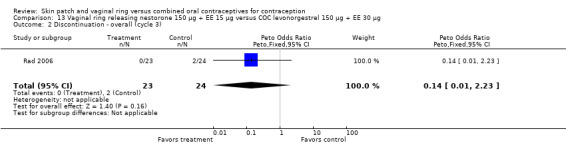
Comparison 13 Vaginal ring releasing nestorone 150 µg + EE 15 µg versus COC levonorgestrel 150 µg + EE 30 µg, Outcome 2 Discontinuation ‐ overall (cycle 3).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahrendt 2006.
| Methods | Randomized trial in 10 European countries from May 2002 to Apr 2004. | |
| Participants | 1017 women, at least 18 years old, seeking contraception. Exclusion criteria: contraindication for hormonal contraception, abortion or breastfeeding in past 2 months, injectable hormonal contraceptive use in past 6 months, abnormal cervical smear during screening, and use in past 2 months of drugs that interfere with metabolism of hormonal contraceptives. | |
| Interventions | Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily versus COC containing drospirenone 3 mg + EE 30 µg; 13 treatment cycles. |
|
| Outcomes | Contraceptive efficacy, compliance, acceptability, tolerability (adverse events), continuation. Diary cards used to record ring or pill use; used to determine exposure and dosing compliance. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted via an Interactive Voice Response System. |
| Allocation concealment (selection bias) | Low risk | Interactive Voice Response System |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization and before treatment : 1017 ‐ 983 = 34 Loss after treatment: 267/983 = 27%; ring 29% and COC 25% Total loss: (34 + 267) / 1017 = 30%; ring 31% and COC 28% |
Audet 2001.
| Methods | 39 centers in the United States and 6 centers in Canada. A priori sample size determination reported. | |
| Participants | Sexually active, healthy women aged 18 to 45 years with a normal body weight and regular menses. Exclusions included pregnancy; lactation; high blood pressure; dermal hypersensitivity; squamous intraepithelial lesions, adenocarcinoma or malignancy; smoking among those over age 35 years; recent alcohol or substance abuse; recent use of injectable progestin; recent experimental drug or device use. | |
| Interventions | Patch (releasing norelgestromin 150 µg + EE 20 µg daily; N=591 for 6 cycles and N=265 for 13 cycles) Oral contraceptive (levonorgestrel 50/75/125 µg + EE 30/40/30 µg; N=439 for 6 cycles and N=200 for 13 cycles). First third of women enrolled were to receive 13 treatment cycles and the remaining women were to receive 6 cycles. |
|
| Outcomes | Contraceptive efficacy, cycle control and compliance. Compliance determined by daily dosing (and patch replacement) noted on diary cards. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | interactive voice‐activated randomization system with permuted blocks stratified by study center. |
| Allocation concealment (selection bias) | Low risk | interactive voice‐activated randomization system |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant unblinded. Blinding of investigator and outcome assessor not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization and before treatment: 78/1495 = 5%
Loss after receiving treatment product: 388/1417 = 27%; patch 30% and COC 24%
Total loss: (78 + 388)/1495 = 31%; patch 33% and COC 28% Exclusions after randomization (due to discovery of ineligibility): patch 8/856 (0.9%); COC 6/639 (0.9%). |
Boonyarangkul 2007.
| Methods | RCT conducted at university hospital in Bangkok (Thailand) from Dec 2005 to Dec 2006. | |
| Participants | 96 women, 35 to 48 years old. Inclusion criteria: multiparity, age >=35 years, no contraindication for hormonal contraceptive use, normal physical and pelvic exams, no OC or implant use in past 3 months and no DMPA in past year. Exclusion criteria: breastfeeding, postpartum period (undefined), or pregnancy. | |
| Interventions | 1) Transdermal patch (delivers norelgestromin 150 µg + EE 20 µg) (N=48) 2) COC levonorgestrel 150 µg + EE 30 µg (N=48) Treatment duration: 3 months; monthly follow up visits in clinic | |
| Outcomes | Menstrual patterns (via diary) and side effects | |
| Notes | No information on sample size calculation; no mention of funding source. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization in "simple random technique" |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding (performance bias and detection bias) All outcomes | High risk | open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: none reported |
Dittrich 2002.
| Methods | RCT conducted at 32 centers in the United States, Europe and South Africa. | |
| Participants | Healthy, ovulatory women aged 18 to 45 years with a normal body weight and regular menses. Exclusions included pregnancy; lactation; heavy smoking; recent alcohol or substance abuse; current cervical dysplasia; recent select drug or device use. | |
| Interventions | 1) 10 cm2 patch (norelgestromin 75 µg + EE 10 µg; N=153) 2) 15 cm2 patch (releasing norelgestromin 112.5 µg + EE 15 µg; N=157) 3) 20 cm2 patch (releasing norelgestromin 150 µg + EE 20 µg; N=150) 4) Oral contraceptive (releasing norelgestromin 250 µg + EE 35 µg; N=150) Duration: 4 treatment cycles. | |
| Outcomes | Ovulation suppression, cycle control, pituitary‐ovarian activity, compliance, patch adhesion, safety (adverse events). Compliance determined by daily dosing (and patch replacement) noted on diary cards. Reported compliance and AEs as percentages without absolute numbers for analysis. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a computer‐generated schedule with permuted blocks of four and stratified by study center. |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding (performance bias and detection bias) All outcomes | High risk | unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total loss: 42/610 = 7%; by group, 8% for 10 cm2 and 15 cm2 patches, 9% for 20 cm2 patch, and 3% for COC. Participants who missed 3 consecutive pills were to be withdrawn from study. |
Duijkers 2004a.
| Methods | Randomized trial at 3 centers in the Netherlands, England, and Scotland from Sep 1998 to Jul 1999. | |
| Participants | 85 women, 18 to 40 years, seeking contraception. Inclusion criteria: menstrual cycle 24 to 35 days (+/‐ 3 days), body mass index (BMI) from 18 and to 29 kg/m2. Exclusion criteria: abnormal carbohydrate metabolism or adrenal or thyroid function; contraindication to hormonal contraceptives; use of hormonal contraceptive in past 2 months (2 months wash‐out was acceptable) or injectable in past 6 months; genital prolapse, vaginitis or bleeding cervical "erosion," Papanicolaou smear class III‐V, severe or chronic constipation, dyspareunia or other coital problems, use of drug that interferes with sex steroid metabolism, and history of drug or alcohol abuse. | |
| Interventions | Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily (N=44) versus COC containing levonorgestrel 150 µg + EE 30 µg (N=41); 6 treatment cycles |
|
| Outcomes | Contraceptive efficacy, safety (adverse events), discontinuation. Compliance was reported assessed with diaries in both groups, but not reported. Researchers reported no differences in analysis of intent‐to‐treat and per‐protocol populations, so intent to treat was reported. Study focused on metabolic issues. |
|
| Notes | Corresponding investigator provided design information for an earlier Cochrane review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to one of the researchers, a computer‐generated list was used for randomization. Block size of 4 for either ring or OC. |
| Allocation concealment (selection bias) | High risk | According to one of the researchers, no allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization and before treatment: 85 ‐ 77 = 8 (7 ring; 1 COC). Loss after treatment: 8/77 = 10%; ring 6/37 = 16% and COC 2/40 = 5%. Total loss: (8 + 8) / 85 = 19%; ring 30% and COC 7%. Eight women withdrew prior to study medication (6 due to abnormal lab values); intent‐to‐treat population included 40 (OC) and 37 (ring). |
Elkind‐Hirsch 2007.
| Methods | Randomized trial conducted in Louisiana (USA). Modified intent‐to‐treat analysis used (at least one treatment dose) with the available data. Reported post hoc power analysis. | |
| Participants | 65 healthy women, 18 to 40 years old, seeking contraception or contraceptive steroids for cycle control. All were nonsmokers or had not smoked for 3 months. Exclusion criteria: contraindication to hormonal contraceptive, used drugs that interfered with carbohydrate metabolism, used injectable contraceptive in past 6 months or hormonal IUD or OC in past 2 months; had condition relevant to ring use, such as cervicitis or vaginitis; non‐normal Papanicolaou smear; prolapse of cervix, cystocele, or rectocele. | |
| Interventions | 1) Vaginal ring (releasing etonogestrel 120 µg plus EE 15 µg daily) (N=34) 2) OC containing levonorgestrel 100 µg plus 20 µg (N=31) Treatment duration: 5 cycles | |
| Outcomes | Primary: insulin sensitivity; also adverse events. Compliance to regimen reportedly documented during study; no further information provided. |
|
| Notes | Grant received from Organon USA Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated program" |
| Allocation concealment (selection bias) | Unclear risk | no information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses: none reported. Exclusions: 35% ring and 35% COC; includes women who never used study product and who discontinued early. |
Gilliam 2010.
| Methods | Randomized trial conducted at two Chicago universities (USA) from Mar 2006 to Dec 2008. A priori sample size determination reported. | |
| Participants | 273 healthy women, aged 18 to 45 years, full‐time college or university students. Inclusion criteria: daily access to electronic mail address to fulfill tracking objectives; agreed to avoid vaginal product use unless instructed by physician or study clinician. Exclusion criteria: known or suspected pregnancy, pregnancy in past 2 months, plans for pregnancy in next 6 months, contraindications to combined hormonal contraceptive, allergy or hypersensitivity to either drug intervention, investigational drug use in past 2 months, family or personal history of blood clots, prior use of contraceptive vaginal ring, contraceptive patch or OC use within 1 month or injectable contraceptive use in past 6 months, and any other contraindication in medication labeling. |
|
| Interventions | 1) Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily (N=136) 2) COC containing norgestimate 180/215/250 µg + EE 25 µg) (N=137) Duration: 3 cycles. Participants were sent daily reminders with link to online diaries and surveys. |
|
| Outcomes | 'Perfect use' during first 3 months; daily diary entries indicated never missed a pill or never removed the vaginal ring for more than 2 hours during days 1 to 21 of all 3 monthly cycles. Imperfect use: discontinued early, lost to follow up (did not complete 3‐month survey and made no diary entries in month 3), or did not complete 3‐month survey. Online, internet‐based diaries and surveys (baseline; 3 and 6 months) for adherence to regimen and acceptability of assigned method. Continuation at 6 months. |
|
| Notes | Same contraceptive methods as Westhoff 2005. Perfect‐use analysis was based on all randomized participants in assigned group. Per‐protocol population used to assess secondary aims (compare rates of satisfaction and intention to continue the contraceptive method at 3 months). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization algorithm in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Sequentially‐number, opaque, sealed envelopes containing 3‐month supply of contraceptive method. Individual at university, not associated with clinical portion of study, created and sealed the randomization envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Research staff, study physicians, statisticians, and participants were unblinded to contraceptive method group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization and before treatment: 1 ring; 1 COC. Participants that started a contraceptive method completed usage diaries: 135 ring; 136 COC. 3 months 26/273 (10%); ring 15/136 (11%); COC 11/137 (8%). Total loss (6 months): 36/273 (12%); ring 19/136 (14%), COC 14/137 (10%) Lost to follow up at 3 months: ring 8/136 (6%), COC 8/137 (6%). Exclusions after randomization:
|
Guida 2005.
| Methods | Randomized unblinded trial conducted in Italy from August 2003 to December 2003. | |
| Participants | 56 women attending the family planning clinic. Inclusion criteria: 22 to 34 years old, reached menarche at 12 to 14 years old, BMI between 20 and 22 kg/m2, sexually active with habitual partner, no abnormal menstrual cycles, and no abnormal dietary requirements. Exclusion criteria: pregnancy or breastfeeding in past year, hepatic disease, vascular or metabolic disorder, > 10 cigarettes/day, history of migraine, use of psychotropic drugs or drugs that affect pharmacokinetics of contraceptive steroids, use of hormonal contraceptives in past year, hysterectomy or oophorectomy, and other contraindications for hormonal contraceptives. | |
| Interventions | Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily versus COC containing desogestrel 150 µg + EE 20 µg; 6 treatment cycles. |
|
| Outcomes | Continuation. Report focused on sexual life satisfaction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was "computer‐generated". |
| Allocation concealment (selection bias) | Low risk | Report states that randomization sequence was concealed until after assignment, but does not state how it was concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomized = 56 Total loss: 5/56 = 9%; ring 7% and COC 11% |
Kaunitz 2012.
| Methods | Multicenter crossover study, presumably conducted in USA, dates not specified. | |
| Participants | 1503 sexually active women. Inclusion criteria: any body weight, requesting contraception, 17 to 40 years of age, smokers < 35 years of age, well‐controlled hypertension and diabetes mellitus without vascular disease, regular menses, and appropriate candidate for combination estrogen‐progestin contraception were randomized (AG200‐15, n=1128; COC n=375). | |
| Interventions | 1) Transdermal patch, AG200‐15, releasing levonorgestrel 120 µg + EE 30 µg; 2) COC containing LNG 100 µg + EE 20 µg; AG200‐15 group treated for 1 year (13 cycles); COC group initially treated for 6 cycles with COC then 7 cycles of AG200‐15. |
|
| Outcomes | Pregnancy (Pearl Index), breakthrough bleeding and spotting, noncompliance (cycles with reported missing days of drug‐taking), patch wearability, and adverse events. Percentages presented in figures without absolute numbers; denominators not provided for cycle control data or breakthrough bleeding or spotting. |
|
| Notes | Conference presentation. Requested data for cycles 1 to 6 prior to crossover. Investigators communicated they were preparing a manuscript for publication and were unable to share further data at the time. Funding: study sponsored by Agile Therapeutics (patch manufacturer). All 3 researchers have relevant financial interests with Agile: consultant, Medical Advisory Board member, and Chief Medical Officer. Study features: minorities were highly represented; 60% of participants had not used hormonal contraception immediately prior to enrolling in the study (report says 60% and 68%); 30% of participants had BMI >= 30 kg/m2; half of that group had BMI >= 35. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized 3:1 |
| Allocation concealment (selection bias) | Unclear risk | Not provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were difficult to estimate given presentation and crossover: overall, 232/1500 (15%) did not complete the study, which includes 39% of COC group that did not crossover. Without the crossover, loss estimates: patch‐only 84/1125 (7%); COC‐only 31/375 (8%). |
Kluft 2008.
| Methods | Open label RCT conducted at 2 centers in The Netherlands from 1998 to 1999. Sample size calculation based on ability to differentiate between patch and monophasic OC groups in prothrombin fragment 1+2 from baseline to 6 months. | |
| Participants | 104 healthy non‐smoking women, aged 18 to 45 years old. Inclusion criteria: regular menstrual cycles, normal prothrombin and activated partial thromboplastin times, no cervical dysplasia, not pregnant, BMI <= 35% of ideal, blood pressure <140/90. Exclusion criteria: lactating, pregnant in past 6 months, contraindication to hormonal therapy, use of steroid hormones in past 2 months or injectable contraceptive in past 6 months, abuse of alcohol or drugs in past 12 months; use of experimental drug or device, hepatic enzyme‐inducing drugs, or drug affecting coagulation in past 30 days; Factor V Leiden mutation. | |
| Interventions | 1) Transdermal patch (containing norelgestromin 6 mg/ EE 0.75 mg) (N=36) 2) Monophasic COC (desogestrel 150 µg/ EE 20 µg) (N=35) 3) Triphasic COC (levonorgestrel 50/75/125 µg/ EE 30/40/30 µg) (N=33) Treatment duration: 6 cycles | |
| Outcomes | Primary: hemostasis variables. Also reported adverse events. | |
| Notes | Funded by Ortho Women's Health and Urology. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization balanced by permuted blocks (size not specified) and stratified by center |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐activated system |
| Blinding (performance bias and detection bias) All outcomes | High risk | open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow up: 1/104 (1%) overall (from monophasic group) |
Mohamed 2011.
| Methods | Randomized trial conducted at Kasr El‐Aini Hospital in Cairo, Egypt between 01 May 2008 and 31 July 2010. | |
| Participants | 600 women. Inclusion criteria: 17 to 42 years of age, at risk of becoming pregnant, regular menstrual cycles, seeking contraception. Exclusion criteria: contraindications for contraceptive steroid use, use of injectable hormonal contraceptive in past 6 months, use of hormonal IUD or other hormonal contraceptive in past 2 months, abortion or breastfeeding within 2 months before starting trial medication. Also excluded if abnormal cervical smear was diagnosed during screening and prolapse of the uterine cervix, cystocele, or rectocele was diagnosed before or during screening. |
|
| Interventions | 1) NuvaRing (N=300), releasing etonogestrel 120 µg + EE 15 µg daily 2) COC (drospirenone 3 mg + EE 30 μg) Duration: 12 consecutive cycles with each treatment cycle consisting of 3 weeks of ring or pill treatment followed by 1‐week ring‐free or pill‐free period. Women were seen 5 times: at screening visit and within 1 week after the ring‐free or pill‐free periods of cycles 3, 6, 9, and 12 or at premature discontinuation. |
|
| Outcomes | Cycle control (via diary cards)
Adverse events, reported by participants or observed by physician during examination. Particular attention to blood pressure, acne, body weight, and vaginal discharge. Metabolic effects (not included in this review) |
|
| Notes | Unable to obtain information from investigator regarding randomization, allocation concealment, and funding. Also requested data on AEs and bleeding for all study women; report only provides data on women who completed the study. Discontinued due to AE: 27 ring and 19 COC women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized in 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow up: 65/600 (11%); ring 32/300 (11%); COC 33/300 (11%). Excluded those who discontinued due to AE and other reasons.
|
Oddsson 2005.
| Methods | Randomized trial in 11 countries in Europe and South America. | |
| Participants | 1030 healthy women, 18 or more years old Exclusion criteria: contraindication to hormonal contraception, injectable contraceptive use in past 6 months, postpartum or postabortion in past 2 months, breastfeeding in past 2 months, abnormal cervical smear in screening, or drugs that interfere with contraceptive metabolism. | |
| Interventions | Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily (N=512) versus COC containing levonorgestrel 150 µg + EE 30 µg (N=518); 13 treatment cycles. |
|
| Outcomes | Contraceptive efficacy, compliance, safety, cycle control. Subjects used diary cards to record ring or pill use; data used to determine exposure and compliance. |
|
| Notes | Corresponding investigator provided bleeding data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted with interactive voice response system, which gave treatment group and medication number. |
| Allocation concealment (selection bias) | Low risk | interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | High risk | open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization and before treatment: 1079 ‐1030 = 49 Loss after treatment = 298/1030 = 29%; ring 29% and COC 29% Total loss = (49 + 298) /1079 = 32%; ring 33% and COC 31% |
Rad 2006.
| Methods | Open‐label, randomized trial conducted in the Netherlands. Wash‐out period of at least 2 normal menstrual cycles prior to study admission. Report included information on a priori power determination. | |
| Participants | 48 healthy premenopausal women between 18 to 34 years old with at least 2 regular menstrual cycles prior to study admission. Eligibility was assessed with medical history, physical and gynecological exam including cervical smear, vital signs, electrocardiogram, blood chemistry, serology, and test on Factor V Leiden. Exclusion criteria: carrier of Factor V Leiden and personal or family history of venous thromboembolism. | |
| Interventions | Vaginal ring releasing nestorone 150 µg + EE 15 µg daily versus COC containing levonorgestrel 150 µg + EE 30 µg; 3 treatment cycles |
|
| Outcomes | Pregnancy and continuation. Study focused on hemostasis variables. Report noted there were no serious adverse events but did not provide AE data. |
|
| Notes | Investigator provided some information on design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to correspondence with the researcher, the randomization sequence was "generated by computer"; study packets were distributed to investigators by the pharmacy by prescription with subject and randomization numbers. |
| Allocation concealment (selection bias) | Low risk | According to correspondence with the researcher, the treatments were concealed in 'large thick" envelopes; the study packets were distributed to investigators by the pharmacy by prescription with subject and randomization numbers. |
| Blinding (performance bias and detection bias) All outcomes | High risk | open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss after randomization and before treatment: 48 ‐ 47 = 1 (ring). Loss after treatment: 2 (COC) /47 = 4%. Total loss: 3/48 = 6%; ring 4% and COC 8%. |
Sabatini 2006.
| Methods | Randomized trial conducted from December 2003 to June 2005. Study location not reported; researchers were from Italy. | |
| Participants | 280 women with regular menstrual cycles, sexually active, and seeking contraception. Inclusion criteria: same partner throughout the study, which was reportedly based on the investigators' determination of 'harmony between partners'. Exclusion criteria: contraindication to COC use, psychotropic drug use, obesity, abnormal Papanicolaou test, and dyspareunia. | |
| Interventions | Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily versus COC containing levonorgestrel (LNG) 100 µg + EE 20 µg versus COC containing gestodene (GSD) 60 µg + EE 15 µg; 12 treatment cycles |
|
| Outcomes | Pregnancy, continuation, cycle control, and side effects (adverse events). | |
| Notes | Investigator provided information on design and side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to the researcher, the randomization was conducted by sequentially numbering the women and then drawing lots for the three study arms. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment was apparent. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Researcher communicated that no blinding was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss after randomization (possibly before treatment): 2/282 = 1%
Loss after treatment: 60/280 = 21%; ring 12% ring, COC LNG 22%, COC GSD 30%.
Total loss: 62/282 = 22%; ring 12%, LNG 22%, GSD 32% According to investigator, 2 women were excluded after randomization due to pregnancy, apparently from the gestodene group. |
Stewart 2007.
| Methods | Randomized crossover study conducted at urban family planning clinic in USA from Apr 2003 to Feb 2004. Pilot study to develop and validate measures of acceptability and use; sample size was based on available budget. | |
| Participants | 130 women attending clinic requesting contraception. Inclusion criteria: no contraindication to hormonal contraceptives, age 15 to 21 years, at least 1 regular menstrual cycle before enrollment or within 7 days of abortion, speak either English or Spanish. Exclusion criteria: hormonal contraceptive use in past month. | |
| Interventions | 1) Vaginal ring ‐ NuvaRing® (releasing etonogestrel 120 µg + EE 15 µg) (N=63) 2) COC norgestimate 250 µg + EE 35 µg (N=67) Treatment duration: one method for 3 cycles then switched to other method for 3 cycles | |
| Outcomes | Primary: acceptability of ring versus COC including compliance. Data from surveys (computer‐assisted self‐interviews). Pregnancy was assessed by urine test at baseline and after each method was used for 3 cycles. |
|
| Notes | No mention of funding source | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence by "random number generator" |
| Allocation concealment (selection bias) | Low risk | Envelope sequence provided by Department of Epidemiology and Biostatistics; no mention of whether envelopes were opaque or sealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses: 9% overall (12/130). Flow chart did not distinguish the losses (N=12) from the discontinuations (N=44) within the study arms. |
Urdl 2005.
| Methods | Open‐label, randomized trial in 65 centers in Europe and South Africa. Randomization done in 4:3 ratio (patch versus OC). Report provided information on a priori power calculation. | |
| Participants | 1517 healthy women aged 18 to 45 years. Inclusion criteria: sexually active and at risk of pregnancy, regular menstrual cycles, at least one normal menses after removal of any IUD or progestin implant, not pregnant, blood pressure < 140/90, within 35% of ideal body weight, and agree to use only the study drug as contraception and not use any other systemic steroid. Exclusion criteria: lactation or pregnancy in past 42 days, contraindication to hormonal therapy, uncontrolled thyroid disorder, abnormal Papanicolaou test, dermal hypersensitivity to topical application, smoking if > 35 years old and if OC use is restricted in this population locally, alcohol or substance abuse in past 12 months, injectable hormone use in past 6 months, use of experimental drug or device or hepatic enzyme‐inducing drug in past 30 days. | |
| Interventions | 1) 20 cm2 patch releasing norelgestromin 150 µg + EE 20 µg daily versus 2) COC containing desogestrel 150 µg + EE 20 µg. First third of women were enrolled for 13 treatment cycles and the remaining women were enrolled for 6 cycles. | |
| Outcomes | Pregnancy, continuation, compliance, cycle control, satisfaction, adverse events. Compliance determined by daily dosing (and patch replacement information) noted on diary cards. |
|
| Notes | Corresponding investigator provided Ns for calculating satisfaction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted via interactive voice‐activated system; randomized to treatment within centers by permuted blocks. |
| Allocation concealment (selection bias) | Low risk | interactive voice‐activated system |
| Blinding (performance bias and detection bias) All outcomes | High risk | open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss after randomization and before treatment: 15 + 13 = 28; 28 / 1517 = 1.8% Loss to follow up: 14 + 14 = 28; 28 / 1517 = 1.8% Total loss: (28 + 261) / 1517 = 19%; patch 21% and COC 16%. |
Veres 2004.
| Methods | Randomized crossover trial conducted during 2002 at a metropolitan university‐affiliated clinic in the USA. | |
| Participants | 80 women recruited by flyer and newspaper. Exclusion criteria: < 18 or > 45 years old, contraindication to COC use, inability to speak or read English, current use of contraceptive implant or IUD, contraceptive injection use in past 6 months, current diagnosis of uterine infection or fibroid or cervical dysplasia. In addition: risk factors for genital infection, such as diabetes, chronic use of immune suppressors, autoimmune disorder such as HIV, > 3 genital infections in past 12 months, > 3 herpes outbreaks in past 12 months, or sexually transmitted infection in past 12 months; and abnormal pelvic exam or cervical cytology at baseline. | |
| Interventions | Crossover: Vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily (N=40) versus COC containing levonorgestrel 100 µg + EE 20 µg (N=40); 3 cycles for each treatment. |
|
| Outcomes | Pregnancy, continuation, compliance, satisfaction. Investigator clarified the denominators for noncompliance. Vaginal symptoms were also listed in the report, but only means and percentiles. The investigator did not have standard deviations any longer; without any variance measure, the data could not be analyzed. Symptoms were collected with daily diary cards; no information on how compliance was assessed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Off‐site research pharmacy provided the randomization schedule and allocation verification. Computerized random number generator used balance blocks of 10 (concealed from study staff doing the enrollment). |
| Allocation concealment (selection bias) | Low risk | Opaque randomization envelopes had sequential randomization number on label and contained study assignment information (did not specify if envelopes were sealed). |
| Blinding (performance bias and detection bias) All outcomes | High risk | After randomization, no blinding was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss by crossover period: period 1, ring first 2/40 = 5%; COC first 4/40 = 10%; period 2, crossed to COC 5/38 = 13%; crossed to ring 5/36 = 14%. Total loss: 16/80 = 20%; ring 7/40 = 18%; COC 9/40 = 23% |
Westhoff 2005.
| Methods | Open‐label randomized trial in metropolitan university‐affiliated clinic in the USA; conducted from May 2003 to March 2004. Report provided information on a priori power calculation. | |
| Participants | 201 women recruited through flyers and internet postings. Inclusion criteria: English‐speaking, 18 to 40 years old, regular menstrual cycles, no contraindication to hormonal contraception, no hormonal contraceptive use in past 2 menses (or 6 menses for injectables), > 2 menses since pregnancy, no recent use of emergency contraception, and no unprotected sex in past 10 days. | |
| Interventions | Immediate start: vaginal ring releasing etonogestrel 120 µg + EE 15 µg daily versus triphasic COC containing norgestimate 180/215/250 µg + EE 25 µg; 84 treatment days |
|
| Outcomes | Pregnancy, continuation, cycle control, satisfaction. Diaries used to collect bleeding data. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Researcher not involved in study generated assignments with random number table and simple randomization. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. Study coordinator and interviewers were blinded to assignment before opening the envelope. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses after randomization: none reported. Loss (after treatment): 45/201 (22%); ring 23/101 (23%) and COC 22/100 (22%). |
AE = adverse event COC = combined oral contraceptive EE = ethinyl estradiol IUD = intrauterine device
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Archer 2002 | Pooled results from two eligible trials (Audet 2001; Urdl 2005) and one nonrandom trial (Smallwood 2001) |
| Archer 2004 | Sub‐group analysis of data from Audet 2001 |
| Bednarek 2008 | Choice of contraceptive method; randomized to observed or Sunday start method |
| Bjarnadottir 2002 | Pooled results from two eligible trials ((Audet 2001; Urdl 2005) and one nonrandom trial (Smallwood 2001) |
| Bustillos‐Alamilla 2010 | Bleeding data does not meet review criteria; participants compared their bleeding to usual and recorded as 'more, same, or less'. Spotting was reported as 'selected event.' Adverse events were selected and reported by quarter; some reportedly persisted over time. |
| Creasy 2000 | Abstract reported on the three eligible trials (Audet 2001; Dittrich 2002; Urdl 2005) but did not include any additional information. |
| Duijkers 2004b | Pharmacodynamic data only |
| Guazzelli 2011 | Not RCT; women chose contraceptive method |
| Mulders 2001 | Only pharmacodynamic data available |
| Pierson 2000 | Only pharmacodynamic data available |
| Pierson 2003 | Only pharmacodynamic data available |
| Roumen 2006 | Study duration was only one cycle. |
| Sibai 2002 | Pooled results from two randomized controlled trials (Audet 2001; Urdl 2005) and one nonrandom trial (Smallwood 2001). |
| Smallwood 2001 | Non‐comparative study |
| Timmer 2000 | Only pharmacokinetic data available |
| Van den Heuvel 2005 | Treatment duration was one cycle |
| White 2005 | Only pharmacokinetic data available |
| Zacur 2002 | Pooled results from two eligible trials (Audet 2001; Urdl 2005) and one nonrandom trial (Smallwood 2001) |
| Zieman 2002 | Pooled results from two eligible trials (Audet 2001; Urdl 2005) and one nonrandom trial (Smallwood 2001) |
Characteristics of studies awaiting assessment [ordered by study ID]
Agile 2011.
| Methods | RCT: open‐label comparative evaluation |
| Participants | 400 sexually active women 18 to 40 years with regular menses 24 to 35 days and in good general health; Exclusion criteria: pregnancy or lactation; significant skin reaction to transdermal preparations or sensitivity to surgical/medical tape; any disease that may worsen under hormonal treatment; use of other contraceptive methods other than study medication |
| Interventions | 1) AG200‐15 contraceptive patch; one patch per week for 3 weeks followed by a 7‐day patch free period for 6 months 2) COC containing levonorgestrel 150 µg + EE 30 µg; one tablet each day for 28‐day cycle; Duration: 6 months |
| Outcomes | Safety; contraceptive efficacy; hormone related adverse events; cycle control (bleeding pattern); subject compliance and serum concentration of EE and levonorgestrel |
| Notes |
Bayer 2012b.
| Methods | RCT: double‐blind, double‐dummy, parallel‐group study; |
| Participants | 342 women 18 to 45 years old; smokers not > 35 years at the time of informed consent; normal cervical smear not requiring follow up; history of regular cyclic menstrual periods; Exclusion criteria: pregnancy or lactation; significant skin reaction to transdermal preparations or sensitivity to surgical/medical tape; any disease that may worsen under hormonal treatment; use of other contraceptive methods other than study medication |
| Interventions | 1) transdermal contraceptive patch containing gestodene 2.1 mg + EE 0.55 mg; one patch per week for 3 weeks followed by a 7‐day patch free period for 7 cycles and placebo tablets 2) oral contraceptive containing levonorgestrel 100 µg + EE 20 µg; 1 tablet a day for 3 weeks followed by a 7‐day tablet‐free period for 7 cycles and placebo patches |
| Outcomes | Primary: cycle control parameters and bleeding pattern indices; Secondary: pregnancies while on treatment up to 14 days after removal of last patch; blood pressure changes during dosing‐free interval |
| Notes | 06 Nov 2012: Bayer representative communicated that the trial was completed. However, the findings are part of much larger file on a product not yet approved. They could not share the study report at this time. |
Weisberg 2012.
| Methods | RCT: sequence generated by a computer program; allocation concealed in opaque envelope; blinded |
| Participants | 140 women, 18 to 45 years old; new users, restarters or switchers; no contraindications to combined hormonal contraceptive use |
| Interventions | 1) oral contraceptive pill containing ethinyl estradiol 20 µg and levonorgestrel 100 µg; once daily for 12 months 2) contraceptive vaginal ring releasing etonogestrel 150 µg + EE 15 µg over 24 hours; replaced every 4 weeks for 12 months |
| Outcomes | Primary: number of bleeding or spotting days over 12 months from daily bleeding diary; verified by telephone and face‐to‐face interviews |
| Notes | 23 Oct 2012: Investigator communicated that the trial was completed. They are analyzing the data and expect to have a manuscript by Jan 2013. |
Contributions of authors
L Lopez led the updates from 2007 to 2013 (reviewed the search results, abstracted data, updated the literature, and drafted the revised review). L Stockton extracted data for the new studies in 2012 and helped revise the manuscript. D Grimes did the second data abstraction through 2010. M Gallo drafted the original review in 2003 and abstracted the data for that version. K Schulz provided statistical expertise. All authors reviewed and edited the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
For conducting the review: LML (2007 to 2013); DAG (2001 to 2010); MFG (2001 to 2002); LLS (2012); KFS (2001 to 2010)
-
U.S. Agency for International Development, USA.
For conducting the review: LML (2007 to 2013); DAG (2001 to 2010); MFG (2001 to 2002); LLS (2012); KFS (2001 to 2010)
Declarations of interest
D Grimes has consulted with Bayer Healthcare Pharmaceuticals and Merck & Co., Inc.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahrendt 2006 {published data only}
- Ahrendt H‐J, Nisand I, Bastianelli C, Gómez AM, Gemzell‐Danielsson K, Urdl W, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 3 µg of ethinyl estradiol and 3 mg of drospirenone. Contraception 2006;74(6):451‐7. [DOI] [PubMed] [Google Scholar]
- Milsom I, Lete I, Bjertnaes A, Rokstad K, Lindh I, Gruber CJ, et al. Effects on cycle control and bodyweight of the combined contraceptive ring, NuvaRing, versus an oral contraceptive containing 30 µg ethinyl estradiol and 3 mg drospirenone. Human Reproduction 2006;21(9):2304‐11. [DOI] [PubMed] [Google Scholar]
Audet 2001 {published data only}
- Audet MC, Moreau M, Koltun WD, Waldbaum AS, Shangold G, Fisher AC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. Journal of the American Medical Association 2001;285(18):2347‐54. [DOI] [PubMed] [Google Scholar]
Boonyarangkul 2007 {published data only}
- Boonyarangkul A, Taneepanichskul S. Comparison of cycle control and side effects between transdermal contraceptive patch and an oral contraceptive in women older than 35 years. Journal of the Medical Association of Thailand 2007;90(9):1715‐9. [PubMed] [Google Scholar]
Dittrich 2002 {published data only}
- Dittrich R, Parker L, Rosen JB, Shangold G, Creasy GW, Fisher AC. Transdermal contraception: evaluation of three transdermal norelgestromin/ethinyl estradiol doses in a randomized, multicenter, dose‐response study. American Journal of Obstetrics and Gynecology 2002;186(1):15‐20. [DOI] [PubMed] [Google Scholar]
Duijkers 2004a {published and unpublished data}
- Duijkers I, Killick S, Bigrigg A, Dieben TO. A comparative study on the effects of a contraceptive vaginal ring NuvaRing and an oral contraceptive on carbohydrate metabolism and adrenal and thyroid function. European Journal of Contraception and Reproductive Health Care 2004;9(3):131‐40. [DOI] [PubMed] [Google Scholar]
Elkind‐Hirsch 2007 {published data only}
- Elkind‐Hirsch KE, Darensbourg C, Ogden B, Ogden LF, Hindelang P. Contraceptive vaginal ring use for women has less adverse metabolic effects than an oral contraceptive. Contraception 2007;76(5):348‐56. [DOI] [PubMed] [Google Scholar]
Gilliam 2010 {published data only}
- Gilliam M L, Neustadt A, Kozloski M, Mistretta S, Tilmon S, Godfrey E. Adherence and acceptability of the contraceptive ring compared with the pill among students: a randomized controlled trial. Obstetrics and Gynecology 2010;115(3):503‐10. [DOI] [PubMed] [Google Scholar]
- Hughey A B, Neustadt A B, Mistretta S Q, Tilmon S J, Gilliam M L. Daily context matters: predictors of missed oral contraceptive pills among college and graduate students. American Journal of Obstetrics and Gynecology 2010;203(4):323 e1‐7. [DOI] [PubMed] [Google Scholar]
Guida 2005 {published data only}
- Guida M, Spiezio Sardo A, Bramante S, Sparice S, Acunzo G, Tommaselli GA, et al. Effects of two types of hormonal contraception‐‐oral versus intravaginal‐‐on the sexual life of women and their partners. Human Reproduction 2005;20(4):1100‐6. [DOI] [PubMed] [Google Scholar]
Kaunitz 2012 {published data only (unpublished sought but not used)}
- Kaunitz A, Archer DF, Foegh M. Increased compliance with a low‐dose combination contraceptive patch (AG200‐15) compared with a low‐dose combination oral contraceptive (COC) in a Phase 3 clinical trial (abstract). Contraception 2012;86(2):178. [Google Scholar]
- Kaunitz A, Foegh M, Mishell D. Increased compliance independent of patient demographics with a low‐dose combination contraceptive patch (AG200‐15) compared with a low‐dose combination oral contraceptive in a Phase 3 clinical trial (abstract). Contraception 2012;86(3):314. [Google Scholar]
- Kaunitz AM, Mishell DR, Foegh M. Comparative Phase 3 study of AG200‐15, a low‐dose estrogen and levonorgestrel contraceptive patch. http://www.agiletherapeutics.com/Clinical_support.html (accessed 26 Sep 2012).
Kluft 2008 {published data only}
- Kluft C, Mayer G, Helmerhorst FM, Hall H, Creasy G. Comparison of the effects of a contraceptive patch and oral contraceptives on coagulation parameters [abstract no. FC2.30.04]. International Journal of Gynaecology and Obstetrics 2000;70(Suppl 2):B77. [Google Scholar]
- Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception 2008;77(2):77‐83. [DOI] [PubMed] [Google Scholar]
Mohamed 2011 {published data only (unpublished sought but not used)}
- Mohamed A M, El‐Sherbiny W S, Mostafa W A. Combined contraceptive ring versus combined oral contraceptive (30‐mug ethinylestradiol and 3‐mg drospirenone). International Journal of Gynaecology and Obstetrics 2011;114(2):145‐8. [DOI] [PubMed] [Google Scholar]
Oddsson 2005 {published data only}
- Oddsson K, Leifels‐Fischer B, Wiel‐Masson D, Melo NR, Benedetto C, Verhoeven CHJ, et al. Superior cycle control with a contraceptive vaginal ring compared with an oral contraceptive containing 30 µg ethinylestradiol and 150 µg levonorgestrel: a randomized trial. Human Reproduction 2005;20(2):557‐62. [DOI] [PubMed] [Google Scholar]
- Oddsson K, Leifels‐Fischer B, Melo NR, Wiel‐Masson D, Benedetto C, Verhoeven CHJ, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1‐year randomized trial. Contraception 2005;71(3):176‐82. [DOI] [PubMed] [Google Scholar]
Rad 2006 {published data only}
- Rad M, Kluft C, Menard J, Burggraaf J, Kam ML, Meijer P, et al. Comparative effects of a contraceptive vaginal ring delivering a nonandrogenic progestin and continuous ethinyl estradiol and a combined oral contraceptive containing levonorgestrel on hemostasis variables. American Journal of Obstetrics and Gynecology 2006;195(1):72‐7. [DOI] [PubMed] [Google Scholar]
- Sitruk‐Ware RL, Menard J, Rad M, Burggraaf J, Kam ML, Tokay BA, et al. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception 2007;75(6):430‐7. [DOI] [PubMed] [Google Scholar]
Sabatini 2006 {published data only}
- Sabatini R, Cagiano R. Comparison profiles of cycle control, side effects and sexual satisfaction of three hormonal contraceptives. Contraception 2006;74(3):220‐3. [DOI] [PubMed] [Google Scholar]
Stewart 2007 {published data only}
- Stewart FH, Brown BA, Raine TR, Weitz TA, Harper CC. Adolescent and young women's experience with the vaginal ring and oral contraceptive pills. Journal of Pediatric and Adolescent Gynecology 2007;20(6):345‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urdl 2005 {published data only}
- Hedon B, Helmerhorst FM, Cronje HS, Shangold G, Fisher A, Creasy G. Comparison of efficacy, cycle control, compliance and safety in users of a contraceptive patch vs an oral contraceptive. International Journal of Gynaecology and Obstetrics 2000;70(Suppl 2):B78. [Google Scholar]
- Urdl W, Apter D, Alperstein A, Koll P, Schönian S, Bringer J, et al. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once‐weekly transdermal compared with oral contraception. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;121(2):202‐10. [DOI] [PubMed] [Google Scholar]
Veres 2004 {published data only}
- Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstetrics and Gynecology 2004;104(3):555‐63. [DOI] [PubMed] [Google Scholar]
Westhoff 2005 {published data only}
- O'Connell KJ, Osborne LM, Westhoff C. Measured and reported weight change for women using a vaginal contraceptive ring vs. a low‐dose oral contraceptive. Contraception 2005;72(5):323‐7. [DOI] [PubMed] [Google Scholar]
- Schafer JE, Osborne LM, Davis AR, Westhoff C. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception 2006;73(5):488‐92. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Osborne LM, Schafer JE, Morroni C. Bleeding patterns after immediate initiation of an oral compared with a vaginal hormonal contraceptive. Obstetrics and Gynecology 2005;106(1):89‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Archer 2002 {published data only}
- Archer D, Bigrigg A, Smallwood G, Shangold G, Creasy G. An integrated assessment of patient compliance with a weekly contraceptive patch (ORTHO EVRA / EVRA). Fertility and Sterility 2001; Vol. 76 (3 Suppl 1):20. [DOI] [PubMed]
- Archer DF, Bigrigg A, Smallwood GH, Shangold GA, Creasy GW, Fisher AC. Assessment of compliance with a weekly contraceptive patch (Ortho Evra™/Evra™) among North American women. Fertility and Sterility 2002;77:27‐31. [DOI] [PubMed] [Google Scholar]
Archer 2004 {published data only}
- Archer DF, Cullins V, Creasy GW, Fisher AC. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception 2004;69:189‐95. [DOI] [PubMed] [Google Scholar]
Bednarek 2008 {published data only}
- Bednarek PH, Nichols MD, Carlson N, Edelman AB, Creinin MD, Truitt S, et al. Effect of "observed start" vs. traditional "Sunday start" on hormonal contraceptive continuation rates after medical abortion. Contraception 2008;78(1):26‐30. [DOI] [PubMed] [Google Scholar]
Bjarnadottir 2002 {published data only}
- Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. American Journal of Obstetrics and Gynecology 2002;186:389‐95. [DOI] [PubMed] [Google Scholar]
Bustillos‐Alamilla 2010 {published data only}
- Bustillos‐Alamilla E, Zepeda‐Zaragoza J, Hernandez‐Ruiz M A, Briones‐Landa C H. Combined hormonal contraception in cycles artificially extended [Anticoncepción con hormonales combinados en ciclos extendidos artificialmente]. Ginecología de Obstetricia de México 2010;78(1):37‐45. [PubMed] [Google Scholar]
Creasy 2000 {published data only}
- Creasy G, Hall N, Shangold G. Patient adherence with the contraceptive patch dosing schedule versus oral contraceptives. Obstetrics and Gynecology 2000; Vol. 95:S60.
Duijkers 2004b {published data only}
- Duijkers IJM, Klipping C, Verhoeven CHJ, Dieben TOM. Ovarian function with the contraceptive vaginal ring or an oral contraceptive: a randomized study. Human Reproduction 2004;19:2668‐73. [DOI] [PubMed] [Google Scholar]
- Duijkers IJM, Verhoeven CHJ, Dieben TOM, Klipping C. Follicular growth during contraceptive pill or vaginal ring treatment depends on the day of ovulation in the pretreatment cycle. Human Reproduction 2004;19:2674‐9. [DOI] [PubMed] [Google Scholar]
Guazzelli 2011 {published data only}
Mulders 2001 {published data only}
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertility and Sterility 2001;75:865‐70. [DOI] [PubMed] [Google Scholar]
Pierson 2000 {published data only}
- Pierson RA, Hess AR, Archer DF, Parsons K, Shangold G, Fisher A, et al. Effects of a contraceptive patch and 3 oral contraceptives on follicular development following incorrect dosing [abstract no. FC2.30.08]. International Journal of Gynaecology and Obstetrics 2000;70:78. [Google Scholar]
Pierson 2003 {published data only}
- Pierson RA, Archer DF, Moreau M, Shangold GA, Fisher AC, Creasy GW. Ortho Evra/Evra versus oral contraceptives: follicular development and ovulation in normal cycles and after an intentional dosing error. Contraception 2003;80:34‐42. [DOI] [PubMed] [Google Scholar]
Roumen 2006 {published data only}
- Roumen FJ, Dieben TO. Comparison of uterine concentrations of ethinyl estradiol and etonogestrel after use of a contraceptive vaginal ring and an oral contraceptive. Fertility and Sterility 2006;85:57‐62. [DOI] [PubMed] [Google Scholar]
Sibai 2002 {published data only}
- Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra™/Evra™). Fertility and Sterility 2002;77:19‐26. [DOI] [PubMed] [Google Scholar]
Smallwood 2001 {published data only}
- Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. Efficacy and safety of a transdermal contraceptive system. Obstetrics and Gynecology 2001;98(5 Pt 1):799‐805. [DOI] [PubMed] [Google Scholar]
Timmer 2000 {published data only}
- Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clinical Pharmacokinetics 2000;39:233‐42. [DOI] [PubMed] [Google Scholar]
Van den Heuvel 2005 {published data only}
- Heuvel MW, Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005;72:168‐74. [DOI] [PubMed] [Google Scholar]
White 2005 {published data only}
- White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. American Journal of Obstetrics and Gynecology 2005;192:2055‐9. [DOI] [PubMed] [Google Scholar]
- White T, Özel B, Jain JK, Stanczyk FZ. Effects of transdermal and oral contraceptives on estrogen‐sensitive hepatic proteins. Contraception 2006;74:293‐6. [DOI] [PubMed] [Google Scholar]
Zacur 2002 {published data only}
- Zacur HA, Hedon B, Mansour D, Shangold GA, Fisher AC, Creasy GW. Integrated summary of Ortho Evra™/Evra™ contraceptive patch adhesion in varied climates and conditions. Fertility and Sterility 2002;77:32‐5. [DOI] [PubMed] [Google Scholar]
Zieman 2002 {published data only}
- Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra™/Evra™ transdermal system. Fertility and Sterility 2002;77:13‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Agile 2011 {published data only (unpublished sought but not used)}
- Agile Therapeutics. Transdermal contraceptive delivery system (TCDS) of levonorgestrel and ethinyl estradiol (ATI‐CL13). http://clinicaltrials.gov/ct2/show/NCT01236768 (accessed 27 Sep 2012). [NCT01236768]
Bayer 2012b {published and unpublished data}
- Bayer Healthcare AG. US cycle control and blood pressure study. http://clinicaltrials.gov/ct2/show/NCT00920985 (accessed 27 Sep 2012). [NCT00920985]
Weisberg 2012 {published data only}
- Weisberg E. Comparative 12 month study of menstrually‐signalled use of a combined contraceptive pill versus a combined contraceptive vaginal ring. http://www.anzctr.org.au/ACTRN12609000391279.aspx (accessed 26 Jul 2012).
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. Journal of the American Medical Association 2003;290:921‐8. [DOI] [PubMed] [Google Scholar]
Bayer 2012a
- Bayer Healthcare AG. Inhibition of ovulation and pharmacokinetics of transdermal ethinylestradiol (EE) and gestodene (GSD). http://clinicaltrials.gov/ct2/show/NCT00915915 (accessed 01 Oct 2012). [DOI: ]
Brache 2013
- Brache V, Payan LJ, Faundes A. Current status of contraceptive vaginal rings. Contraception 2013;87(3):264‐72. [DOI] [PubMed] [Google Scholar]
CONSORT 2009
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 15 Jul 2009).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001:285‐312. [Google Scholar]
FDA 2012
- US Food, Drug Administration. Ortho Evra (norelgestromin/ethinyl estradiol) information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm110402.htm (accessed 08 Nov 2012).
Grimes 2009
- Grimes DA. Forgettable contraception. Contraception 2009;80:497‐9. [DOI] [PubMed] [Google Scholar]
Haag 1998
- Haag U. Technologies for automating randomized treatment assignment in clinical trials. Drug Information Journal 1998;32:7‐11. [Google Scholar]
Heit 2005
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ III. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Annals of Internal Medicine 2005;143:697‐706. [DOI] [PubMed] [Google Scholar]
Henzl 2000
- Henzl MR, Edwards JA. Pharmacology of progestins: 17alpha‐hydroxyprogesterone derivatives and progestins of the first and second generation. In: Sitruk‐Ware R, Mishell DR editor(s). Progestins and Antiprogestins in Clinical Practice. New York (NY): Marcel Dekker, 2000:101‐32. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 24 October 2012).
Hou 2010
- Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text‐message reminders to improve adherence with oral contraceptives. Obstetrics and Gynecology 2010;116(3):633‐40. [NCT00733707] [DOI] [PubMed] [Google Scholar]
Jensen 2013
- Jensen JT. Vaginal ring delivery of selective progesterone receptor modulators for contraception. Contraception. 2012/10/09 2013; Vol. 87, issue 3:314‐8. [DOI] [PMC free article] [PubMed]
Langston 2010
Lete 2008
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
NICHD 2012
- Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). A multi‐center, open‐label, randomized, parallel group study to evaluate pharmacokinetic profile, effects on the mechanisms of contraceptive efficacy and safety of two progestin‐only patches containing different doses of levonorgestrel (LNG) (CCN009). http://clinicaltrials.gov/ct2/show/NCT01166412 (accessed 23 Sep 2012). [DOI: ]
Pop Council 2012
- Population Council. Licensing Opportunities. http://www.popcouncil.org/what/licensing.asp#/jQueryUITabs1‐2 (accessed 12 Nov 2012).
Potter 1996
- Potter L, Oakley D, Leon‐Wong E, Canamar R. Measuring compliance among oral contraceptive users. Family Planning Perspectives 1996;28:154‐8. [PubMed] [Google Scholar]
Raymond 2012
Rosenberg 1998
- Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling and satisfaction with oral contraceptives: a prospective evaluation. Family Planning Perspectives 1998;30:89‐92,104. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. [DOI] [PubMed] [Google Scholar]
Sitruk‐Ware 2013
- Sitruk‐Ware R, Nath A, Mishell DR. Contraception technology: past, present and future. Contraception 2013;87(3):319‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanczyk 2011
- Stanczyk FZ, Rubin A, Flood L, Foegh M. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinylestradiol and levonorgestrel. Hormone Molecular Biology and Clinical Investigation 2011;6(2):231‐40. [DOI] [PubMed] [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83(5):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]


