Abstract
Background
The use of antibiotic prophylaxis for hernia repair is currently a controversial issue given the disparity among study results in this area.
Objectives
The objective of this systematic review was to clarify the effectiveness of antibiotic prophylaxis in reducing postoperative wound infection rates in elective open inguinal hernia repair.
Search methods
We searched the Cochrane Colorectal Cancer Group specialized register, by crossing the terms herni* and inguinal or groin and the terms antimicr* or antibiot* , as free text and MeSH terms. A similar search were performed in Medline using the following terms: #1 antibiotic* OR antimicrob* OR anti infecti* OR antiinfecti*; #2 prophyla* OR prevent*; #3 #1 AND #2; #4 clean AND (surgery OR tech* OR proced*); #5 herni*; #6 (wound infection) AND #4; #7 #3 AND (#4 or #5 or #6). National Research Register, ISI‐Web, DARE, Scirus, TRIPDATABASE, NHS EED, reference list of the included studies and web of clinical trials register (www.controlled‐trials.com and clinicaltrials.gov) were checked to identify further studies.
Selection criteria
Only randomised clinical trials were included.
Data collection and analysis
In the present review, we searched for eligible trials in October 2011. This revealed four new included trials, so seventeen trials are included in the meta‐analysis. Eleven of them used prosthetic material for hernia repair (hernioplasty) whereas the remaining studies did not (herniorrhaphy). Pooled and subgroup analysis were conducted depending on whether prosthetic material was or not used. A fixed effects model was used in the analysis.
Main results
The total number of patients included was 7843 (prophylaxis group: 4703, control group: 3140). Overall infection rates were 3.1% and 4.5% in the prophylaxis and control groups, respectively (OR 0.64, 95% CI 0.50 ‐ 0.82). The subgroup of patients with herniorrhaphy had infection rates of 3.5% and 4.9% in the prophylaxis and control groups, respectively (OR 0.71, 95% CI 0.51 ‐ 1.00). The subgroup of patients with hernioplasty had infection rates of 2.4% and 4.2% in the prophylaxis and control groups, respectively (OR 0.56, 95% CI 0.38 ‐ 0.81).
Authors' conclusions
Based on the results of this systematic review the administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended. Neither can the administration be recommended against when high rates of wound infection are observed.
Plain language summary
Administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended.
The use of antibiotic prophylaxis for elective hernia repair is currently a controversial issue. Although elective hernia repair is considered a clean procedure, the rate of postoperative wound infection in many countries exceeds the one expected for clean surgery, increasing discomfort in patients and health care expenses. In addition, antibiotics administration is not exempt of potential risks. Controlled clinical trials on the use of antibiotic prophylaxis for hernia repair are scarce, the number of patients studied is low and the results are diverse. Based on the results of this meta‐analysis of randomised clinical trials, administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended. Neither can the administration be recommended when high rates of wound infection are observed.
Background
Wound infection is one of the most commonly occurring surgical complications. Infection of a wound may result from a number of factors both intrinsic and extrinsic to the patient. Although many of the intrinsic factors cannot be modified, the external ones can certainly be influenced. In particular those related to aseptic conditions, surgical technique and peri‐operative care. However even under the most scrupulous aseptic conditions and with a careful technique, post‐operative wound infection still presents a very serious problem.
The use of antibiotic prophylaxis to avoid infectious complications of surgery is very common in surgical practice. However, indiscriminate use of antibiotics can lead to problems including an increase in costs and the emergence of resistant micro‐organisms. The benefits of antibiotic prophylaxis either in clean‐contaminated, contaminated and dirty surgery are universally accepted. Antibiotic prophylaxis is generally accepted in clean surgery (i.e. surgery with no inflammation, no contact with septic material, or interruption of aseptic technique where hollow viscera is not opened) when the placement of prosthetic materials, or the presence of infection poses a significant risk to the patient. Nonetheless, controversy remains about the use of antibiotics in some types of clean surgery.
Surgery for inguinal hernia is one of the most common techniques performed in a general surgical service making up approximately a third of total interventions (Cainzos 1990; Rodriguez 2005). This type of surgery is considered clean and it has been estimated that the rate of postoperative infection should not be greater than 2% (Condon 1991; Page 1993; Dellinger 1994; Woods 1998).
Currently, the use of antibiotic prophylaxis is recommended for elective open mesh inguinal hernia repair (Condon 1991; Page 1993; Woods 1998). However, this treatment is not universally accepted. For hernia repair not involving prosthetic material, the antibiotic prophylaxis is not recommended in the absence of risk factors, but the controversy arises when wound infection rates exceed the expected figures (Bailey 1992; Ranaboldo 1993; Holmes 1994). Contradictory results from clinical trials investigating the effectiveness of antibiotic prophylaxis have complicated this situation (Wittmann 1995; Leaper 1998).
Objectives
The objective of this systematic review was to clarify the effectiveness of antibiotic prophylaxis in reducing postoperative wound infection rates in elective open inguinal hernia repair.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials on antibiotic prophylaxis for hernia repair were included. Randomised clinical trials of antibiotic prophylaxis in patients subject to clean surgical techniques were also included in the review when the report allowed the extraction of data on hernia repair.
Types of participants
Adult patients undergoing open elective inguinal or femoral hernia repair, with or without the use of prosthetic material. Laparoscopic repairs were excluded from this review. We performed an overall analysis of the studies, stratified by whether herniorrhaphies (non‐mesh repair) or hernioplasties (mesh repair) was used.
Types of interventions
Treatment group: administration of prophylactic antibiotics, irrespective of the type of administered antibiotic or the route of administration. Control group: placebo or no treatment. Studies using antiseptics for prophylaxis were not included in the review.
Types of outcome measures
Wound infection rate assessed at least at 30 days after the prophylactic antibiotic treatment was given. The criteria of infection were as defined by the authors of each primary study: discharge of pus from the wound; a wound that was opened and not closed; spreading erythema indicative of cellulitis or definitions established by associations as Centers for Disease Control (CDC) (Horan 1992).
Search methods for identification of studies
A search in the Cochrane Colorectal Cancer Group specialised register was conducted crossing the terms herni* and inguinal or groin and the terms antimicr* or antibiot* as free text and MeSH terms.
A similar search was conducted in Medline with the following search strategy:
#1 antibiotic* or antimicrob* or anti infecti* or antiinfecti* #2 prophyla* or prevent* #3 #1 and #2 #4 clean and (surgery or tech* or proced*) #5 herni* #6 (wound infection) and #4 #7 #3 and (#4 or #5 or #6)
National Research Register, ISI‐Web, DARE, Scirus, TRIPDATABASE, NHS EED, reference list of the included studies and web of clinical trials register (www.controlled‐trials.com and clinicaltrials.gov) were checked to identify further studies.
Controlled clinical trials were sought as a source of supplementary evidence.
In the previous update, the searches were performed until June 2009. In this update, the searches were performed October 2011.
Data collection and analysis
Data extraction
Authors independently performed the selection of studies and data extraction. Disagreements were resolved through consensus, or by the input of a third party. The following data were extracted from each study: study design; type of allocation and allocation concealment; number of patients included; mesh or non‐mesh hernia repair; antibiotics used; dose, mode and timing of antibiotic administration; wound infection rates in prophylaxis and control groups, respectively. When the paper was considered eligible and data in the publication were incomplete, we contacted with the author to obtain the necessary information.
Assessment of methodological quality
The methodological quality of the included studies was assessed as recommended by the Cochrane Collaboration.
Statistical analysis
Odds ratios and 95% confidence intervals were computed for included studies. Since heterogeneity between studies due to differences in study design and populations was expected, the analysis was initially performed with a random effects model. Nevertheless, chi‐square and I2 tests for heterogeneity were conducted to test whether significant heterogeneity precluded a meta‐analysis. Heterogeneity assessment was performed according to Higgins 2003. If significant heterogeneity was found to be present, the use of meta‐regression techniques was considered to assess the impact of prophylaxis on the following factors: * Number and type of antibiotics used. * Quality of study design * Use of mesh.
We expected that a small number of events would be observed in the included studies. Therefore, a fixed‐effect meta‐analysis using Peto odds ratios was performed as a sensitivity analysis, as a tool to reduce bias in the analysis of scarce data (Deeks 1999). Sensitivity analyses were also conducted excluding highly influential studies, as well as poor quality studies.
Numbers needed to treat (NNT) with 95% confidence intervals were estimated for each study, for the herniorrhaphies and hernioplasties sub‐groups, and for the pooled results, if there was a statistically significant benefit with the use of antibiotic prophylaxis. Numbers needed to treat were computed as the inverse of risk differences. In the studies where statistical signification was not reached, numbers needed to treat and their confidence intervals were computed according to Altman 1998.
Results
Description of studies
From the initial search strategy we identified 39 potentially eligible studies, and after further analyses 7 studies met the inclusion criteria of the first published version of this review. After updating the search strategy, one additional study was identified, so eight studies met the above criteria and were included.
The first update of this review (August 2006) identified 159 additional studies. After reading the abstract, eight studies were selected for a more detailed revision. Four studies were excluded for different reasons. Finally, four additional studies were included in the meta analysis, making a total of twelve studies.
The second update (June 2009) the search revealed additional 99 studies, of which one was selected for inclusion in the meta analysis (Jain 2008). So, thirteen studies composed the meta‐analysis.
The present update (October 2011) identified 213 additional studies, of which 23 studies were selected for a more detailed revision. One study was excluded (Praveen 2009). Four studies met inclusion criteria and were included (Ergul 2011; Othman 2011; Shankar 2010; Tzovaras 2007). In total, seventeen studies are included in this update. Studies were described according to the antibiotic used, timing and route of administration, number of doses given, technique of hernia repair, criteria for the diagnosis of infection and follow‐up time.
In one study on the use of different surgical techniques including hernia repair, Evans 1973 used intra‐muscular (IM) cephaloridine 1 gr in the anaesthesia induction and two further intra‐muscular doses in the postoperative period. Definition of infection was: purulent exudates at the wound site or need for pus drainage from the wound. Follow‐up was 4 weeks.
Andersen 1980 used a single dose of 1 gr topical subfascial ampicillin before closure of non‐mesh hernia repair and cholecystectomy. The criteria for wound infection considered in this study was the collection of pus in the wound site requiring revision. Patients in the study were followed for one year.
In the study on antimicrobial prophylaxis for mastectomy and non‐mesh hernia repair, Platt 1990 used a single dose of 1g cefonicid, administered intra‐venously 90 minutes before surgery. Wound infection was defined as the presence of erythema and drainage, purulent exudates, or non‐closing open wound. Probable infection was considered when erythema extending at least 2 cm to any direction was present, or when wound infection was diagnosed even if established infection criteria were not met.
Lazorthes 1992 combined cefamandole 750 mg to local anaesthesia for patients undergoing non‐mesh hernia repair. Wound abscess was defined as all wounds with discharge in which pathogens grew regardless of whether or not the discharge was purulent or serohematic. Discharge of pus, even when germs were not found, was also considered a wound abscess. Patients were assessed one month after the intervention.
Taylor 1997 administered amoxicillin‐clavulanic acid 1.2 gr intra‐venously before the incision in patients undergoing non‐mesh hernia repair. Infection was defined as purulent wound discharge or spreading erythema indicative of cellulites; wound breakdown; or dehiscence with clinical evidence of infection. Patients were assessed 4 and 6 weeks after surgery.
Pessaux 2006 analysed patients undergoing elective hernia inguinal repair from 3 previous clinical trials on antibiotics in clean surgical techniques. The intervention groups were homogenous. Several antibiotics were administered by endovenous route during anaesthetic induction : cefotaxime, cefazolin, ceftriaxone and amoxacillin‐clavulanic acid. The surgical technique was Shouldice herniorrhaphy. Since the publication didn't report enough information to include the study in the meta analysis, we contacted the principal author to complete them. The minimum follow‐up was of 6 weeks. Morales 2000 is a multicenter study that assessed the efficacy of a single dose of cefazolin 2 gr administered intra‐venously during anaesthetic induction for patients undergoing mesh hernia repair. The authors defined the following criteria for wound infection: a) cutaneous erythema greater than 2 cm on both sides of the incision, b) purulent exudates through the wound, c) organism isolated from culture of non‐ purulent exudates, and d) an open wound that was not closed afterwards.
The wound was assessed 30 days after the surgery.
Yerdel 2001 used ampicillin‐sulbactam 1.5 gr IV before the incision in patients undergoing mesh hernia repair. The criteria of the CDC (1992) for wound infection were applied. One year follow‐up period was considered.
Oteiza 2004 used amoxacillin‐clavulanic acid 2 gr IV 15‐30 minutes before the incision in patients undergoing elective mesh hernia repair, Lichtenstein or plug‐mesh technique. Wound infection was defined as the purulent exudate or non purulent exudate with positive culture or the surgeon declares that incisional infection is present. A month follow‐up period was considered.
Aufenacker 2004 used cefuroxime 1,5 gr IV during anaesthetic induction in patients undergoing mesh hernia repair (Lichtenstein technique). The patients were assessed at one, two and twelve weeks. The criteria of the CDC for wound infection were applied. Celdran 2004 used cefazolin 1 gr IV 30 minutes before the surgery. Lichtenstein technique was carried out. Assessment was performed at two year follow‐up after surgery. The authors used the CDC criteria for wound infection. Perez 2005 administered cefazolin 1 gr IV before the incision in patients undergoing Lichtentein technique. The authors used the CDC criteria for wound infection.
Tzovaras 2007 administered 1.2 gr IV amoxicillin‐clavulanic acid or placebo to patients underwent tension‐free hernioplasty between January 2000 to June 2004. CDC criteria were applied. They were followed 1 month after the operation.
Jain 2008 administered placebo or 1.2 gr IV amoxicillin‐clavulanic acid before incision. PHS mesh repair was carried out. The patients were assessed at 7‐9 days, two weeks and four weeks after discharge. CDC criteria was used for wound infection.
Shankar 2010 between November 2006 and June 2008 performed a study with 334 patients underwent to tension free mesh repair using a polypropylene mesh to receive cefazolin 1 gr IV at the time of induction. Patients were followed for 30 days. CDC criteria were applied.
Ergul 2011 between July 2008 and October 2010 administered cefazolin 1 gr IV before induction to 200 patients underwent to Lichtenstein repair. The wound was assessed at 3, 5, 7 and 30 days after discharge. The authors used the CDC criteria for wound infection.
Othman 2011 during a period from July 2006 to April 2010 administered 1.2 gr IV amoxicillin and clavulanic acid 30 minutes or placebo before incision to 98 patients underwent to Lichtenstein hernioplasty. Follow‐up was 30 days. CDC criteria were applied.
The remaining studies were excluded for several reasons:
studies focused on clean surgical techniques including hernia pathology, but data for this subgroup of patients could not be collected (Houck 1989; Lewis 1995; Nundy 1983; Dixon 2006; Karran 1992; Esposito 2006).
studies reported results of patients series having no antibiotic prophylaxis (Hedawoo 1995; Wantz 1996), otherwise, they were non‐controlled studies of antibiotic prophylaxis (Angio 2001; Dazzi 1994; Gervino 2000; Massaioli 1995; Spallitta 1999; Van‐Damme 1981; Sultan 1989; Deysine 2005).
both study arms received antibiotic prophylaxis (Musella 2001; Shwed 1991; Reggiori 1996; Kuzu 2005; Terzi 2005; Praveen 2009).
studies used historical controls (Abo‐Rahmy 1998), they were comparative retrospective or non‐randomised studies, or mis balances in patients and techniques used in both treatment groups were present (Barreca 2000; Escartín 1999; Gilbert 1993; Hair 2000; Platt 1992; Ryan 1967; Vara 1993).
studies did not use antibiotic but local antiseptic treatment (Gilmore 1977).
Risk of bias in included studies
Assessment of methodological quality is presented in Figure 1 and Figure 2.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Three studies did not provide information on the random allocation concealment procedures (Andersen 1980; Lazorthes 1992; Pessaux 2006). Consequently, they are considered as "unclear". The concealment procedure of Evans 1973 (coin tossing) and Celdran 2004 (number list) was inappropriate. The remaining studies used appropriated concealment methods (sealed envelopes: Morales 2000; Ergul 2011; Shankar 2010, computer programs: Platt 1990; Taylor 1997; Yerdel 2001;Oteiza 2004, Aufenacker 2004, Perez 2005; Tzovaras 2007; Jain 2008; Othman 2011).
Eleven of the trials were blinded (Morales 2000; Platt 1990; Taylor 1997; Yerdel 2001; Aufenacker 2004; Perez 2005;Celdran 2004; Tzovaras 2007; Jain 2008; Ergul 2011; Othman 2011). Andersen 1980 was described by the author as a triple‐blinded trial despite the fact that the control group received no intervention, and the remaining trials were open (Evans 1973,Lazorthes 1992, Pessaux 2006; Oteiza 2004; Shankar 2010).
The two comparison groups were homogeneous in the majority of included studies, with respect to epidemiological characteristics, techniques used in the hernia repair, and associated co‐morbilities. However, the two groups in Andersen 1980 differed with regard to one factor, and in Evans 1973 they differed by more than one factor. In the study of Jain 2008 the surgery was significantly longer in the placebo group.
Apart from Evans 1973 and Lazorthes 1992, the statistical methods were clearly reported in the studies.
All studies except Lazorthes 1992, clearly described a set of wound infection criteria.
All studies used a penicillin derivative antibiotic. The route of administration was intravenous in fourteen studies, subcutaneous/subfascial in two studies (Andersen 1980; Lazorthes 1992) and intramuscularly in one study (Evans 1973). Lazorthes 1992; Morales 2000; Platt 1990; Taylor 1997; Yerdel 2001; Oteiza 2004; Aufenacker 2004; Celdran 2004, Pessaux 2006, Perez 2005; Tzovaras 2007; Jain 2008; Ergul 2011; Othman 2011; Shankar 2010 used a single preoperative dose. Andersen 1980 administered subfascial antibiotic before closing the aponeurosis. Evans 1973 used one preoperative and two postoperative intramuscularly doses.
Five trials included patients with no drug intervention as the control group (Andersen 1980; Evans 1973; Lazorthes 1992, Pessaux 2006 and Oteiza 2004). The remaining studies used placebo.
Effects of interventions
The heterogeneity analysis did not reach statistical significance neither for the overall analysis nor in the sub‐groups analysis, considering separately trials with herniorrhaphy and hernioplasty Figure 3. For this reason, the main analysis was performed with the fixed effects model and a sensitivity analysis was performed with the random effects model .
3.
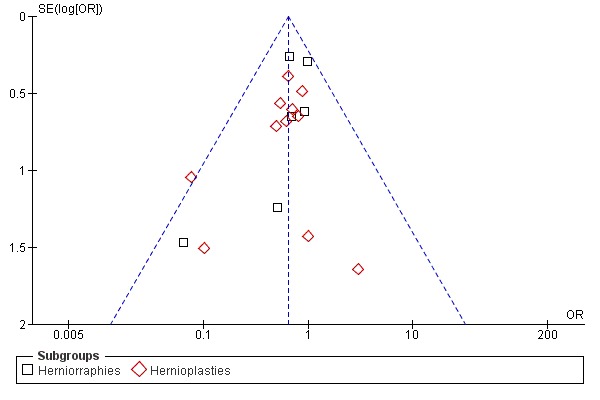
Funnel plot of comparison.
The total number of patients included in the meta‐analysis was 7843 (prophylaxis group: 4703, control group: 3140). The overall infection rates were 3.1% in the prophylaxis group, and 4.5% in the control group (OR 0.64, 95% CI 0.5 ‐ 0.82).Figure 4
4.

Forest plot of comparison: 1 Antibiotic prophylaxis vs Placebo, outcome: 1.1 Wound infection.
Analysis of the group of patients with herniorrhaphy showed no evidence of heterogeneity (P=0.51, I2=0%). The number of patients treated with prophylaxis was 2932 and the infection rate for this group was 3.5%. The number of patients in the control group was 1337 and the infection rate was 4.9% (OR 0.71, 95% CI 0.51 ‐ 1.00).
Analysis of the group of patients with hernioplasty showed no evidence of heterogeneity (P=0.66, I2=0%). The number of patients treated with prophylaxis was 1771 and the infection rate for this group was 2.4%. The number of patients in the control group was 1803 and the infection rate was 4.2% (OR 0.56, 95% CI 0.38 ‐ 0.81).
In Table 2 the NNT of the studies including in the meta analysis are exposed.
1. Numbers need to treat (NNT) with 95% CI for each study, for the herniorrhaphies and hernioplasties sub‐groups, and for pooled results.
| AUTHOR | INFECTED PROPHYLAXIS | TOTAL PROPHYLAXIS | % | INFECTED CONTROL | TOTAL CONTROL | % | NNT CI 95% |
| Andersen 1980 | 5 | 137 | 3.65 | 6 | 150 | 4 | 285 (20 to infinite to NNTH 24) |
| Evans 1973 | 1 | 48 | 2.08 | 2 | 49 | 4.08 | 50 (11 to infinite to NNTH 20) |
| Lazorthes 1992 | 0 | 155 | 0 | 7 | 153 | 4.57 | 22 (12 , 79) |
| Pessaux 2006 | 68 | 2008 | 3.38 | 20 | 394 | 5.07 | 59 (25 to infinite to NNTH 162) |
| Platt 1990 | 4 | 301 | 1.32 | 6 | 311 | 1.9 | 167 (38 to infinite to NNTH 71) |
| Taylor 1997 | 25 | 283 | 8.8 | 25 | 280 | 8.9 | 1057 (20 to infinite to NNTH 21) |
| SUBTOTAL HR | 103 | 2932 | 3.5 | 66 | 1337 | 4.9 | 70 (36 , 1181) |
| Aufenacker 2004 | 8 | 475 | 1.68 | 9 | 472 | 1.9 | 449 (52 to infinite to NNTH 68) |
| Celdran 2004 | 0 | 50 | 0 | 4 | 49 | 8.16 | 12 (6 , 201) |
| Ergul 2011 | 5 | 100 | 5 | 7 | 100 | 7 | 50 (12 to infinite to NNTH 22 |
| Jain 2008 | 1 | 60 | 1.6 | 1 | 60 | 1.6 | infinite |
| Morales 2000 | 4 | 237 | 1.68 | 6 | 287 | 2.09 | 248 (37 to infinite to NNTH 52) |
| Oteiza 2004 | 1 | 124 | 0.8 | 0 | 123 | 0 | ‐124 (130 to infinite to NNTH 42) |
| Othman 2011 | 4 | 50 | 8 | 6 | 48 | 12.5 | 22 (6 to infinite to NNTH 13) |
| Perez 2005 | 3 | 174 | 1.72 | 6 | 176 | 3.4 | 59 (20 to infinite to NNTH 61) |
| Shankar 2010 | 12 | 172 | 6.9 | 17 | 162 | 10.5 | 28 (10 to infinite to NNTH 39) |
| Tzovaras 2007 | 5 | 193 | 2.6 | 9 | 193 | 4.6 | 48 (17 to infinite to NNTH 61) |
| Yerdel 2001 | 1 | 136 | 0.7 | 12 | 133 | 9.02 | 12 (7 , 31) |
| SUBTOTAL HP | 44 | 1771 | 2.48 | 77 | 1803 | 4.2 | 56 (34, 165) |
| TOTAL | 147 | 4703 | 3.1 | 143 | 3140 | 4.5 | 70 (43, 183) |
(NNTH= number needed to treat‐harm).
Sensitivity analyses In the sensitivity analysis performed with the random effects model, the overall analysis still shows statistical significance but the herniorrhaphy subgroup estimation become non significant. Similarly, sensitivity analysis performed with risk differences present borderline signification. These results require cautiousness in interpretation, because they could be congruent both with benefit and no effect from antibiotic prophylaxis administration.
Sensitivity analysis performed excluding any of the trials did not show any qualitative differences with respect to the main analysis. See results in Figure 5.
5.
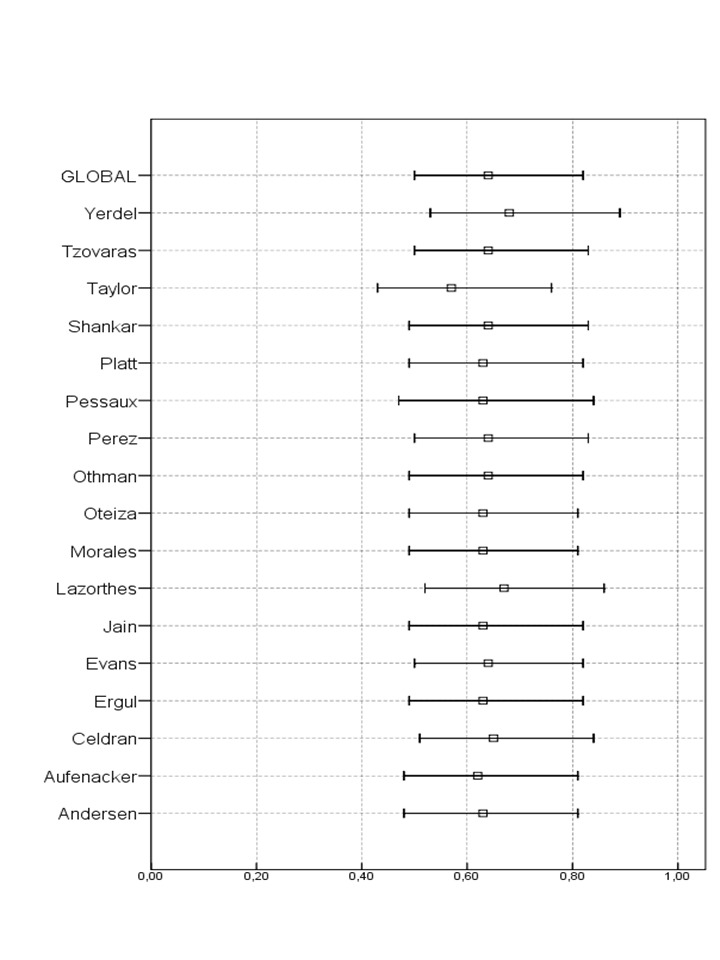
Sensitivity analysis excluding each of the studies and their relationship to main analysis.
Metaregression Factors like number and type of antibiotics administered, methodological quality and use of mesh for hernia repair are not related to differences in the treatment effect between the included studies, as shown by the non significant results of the meta regression model (details can be sought from the authors).
Discussion
A systematic review is important, considering that hernia repair is a commonly used technique in any general hospital. As a clean procedure, the wound infection rate should not exceed 2%. However, follow‐up studies have shown figures as low as 0.1% (Wantz 1996; Rutkow 1993), and close to 10% (Bailey 1992). The mean wound infection rate in general hospitals has been estimated around 4% (Cainzos 1990; Holmes 1994). Surgeons do not usually assess wound infection after hernia repair because in most cases, the patient is discharged from the hospital under an outpatient‐based major surgical regimen, or in the first 48 hours after the procedure. Therefore, wound abscess drainage is usually performed in emergency rooms several days after discharge, without attaining any control. This gives the impression that infection rates are lower than the actual values. It has been calculated that 72% of patients are diagnosed after discharge during a 4‐6 week follow‐up period, once the intervention has taken place (Ranaboldo 1993).
Wound infection after hernia repair is not a devastating event as in other types of clean surgery (i.e. Neurosurgery), where antibiotic prophylaxis is given to avoid case fatality. In general, simple drainage with or without antibiotic therapy is enough to resolve the problem in such a way that vital risk is not a major problem for the patient. Nonetheless, wound infection can lead to significant discomfort and inconvenience, and leading to use of more potent antibiotics, to a higher risk of hernia relapse and even to re‐intervention, raising significantly the costs. Therefore, even though wound infection is not a severe condition, it is a common event that constitutes an important health problem. A study conducted to assess the postoperative infection‐related costs, found that annual expenses for infections after hernia repair (a very often performed procedure) were similar to those for colon surgery (a less frequent technique) (Davey 1998).
Several factors that may increase the infection rate after hernia repair have been analysed (NRC 1964; Haley 1985; Wittmann 1995; Porcu 1996; Pessaux 2006). Although it may not be possible to modify the patient‐related factors, it could be possible to modify factors related to the environment and the surgical technique, in such a way that the administration of antibiotic prophylaxis does not involve the detriment of either sanitary conditions or the surgical technique. Current recommendations suggest the administration of antibiotic prophylaxis when prosthetic material is being used or when risk factors are present (Condon 1991; Page 1993; Woods 1998; Mangram 1999,Simons 2009). Controversy arises when greater series using synthetic material show up infection rates around 0% (Gilbert 1993; Wantz 1996) whereas series without prosthetic material provide rates around 10% (Bailey 1992; Ranaboldo 1993; Taylor 1997). Alternatively, benefits of antibiotic prophylaxis to prevent infections after the first week from the intervention have been questioned, as it would not be covered by the prophylaxis administration of antibiotics (Sanderson 1999). Surveys conducted among surgeons have reported that about half of them use antibiotic prophylaxis for hernia repair (Mozillo 1988; Codina 1999; Heineck 1999).
There are several studies on the use of antibiotics for hernia repair, but most of them compare new antibiotics versus antibiotics whose efficacy has already been established. Other studies are conducted with too few patients and insufficient statistical power to draw firm conclusions. Many of them are retrospective series and in some instances there is a lack of control groups. In order to detect a 50% difference between both groups (reduction of the actual rate from 4% to an appropriate rate of 2% in clean surgical procedures) and to have sufficient statistical power, a prospective, randomised blinded study should include at least 800 patients in each treatment arm. This involves performing multicenter studies or studies with longer recruitment periods.
From those studies considered for further analysis after reading the abstracts, there were only seventeen studies that met the criteria to be included in the review. They were well‐designed comparative, randomised and often blinded studies. However, conclusions cannot be drawn due to disparity of results. Evans 1973; Andersen 1980; Platt 1990; Taylor 1997; Morales 2000; Aufenacker 2004; Oteiza 2004; Perez 2005; Tzovaras 2007; Jain 2008; Ergul 2011; Othman 2011 and Shankar 2010 concluded that antibiotic prophylaxis is not efficacious, whereas Lazorthes 1992; Yerdel 2001 (this study was finished early due to the incidence of high infection rates in the control group); Celdran 2004 (finished early for ethical reasons) and Pessaux 2006 (in high risk patients) did so.
Meta‐analysis is a non‐perfect technique that is no substitute for a large and well‐designed randomised controlled study. Nonetheless, the technique is indicated for similar situations where the number of patients in the studies is low, or when results are conflicting, as it provides pooled estimates with narrower confidence intervals and greater statistical power (DerSimonian 1982; Sacks 1987; Sackett 1997; Imperiale 1999). Unlike the early version of this review (Sanchez‐Manuel 2001) where studies should be only controlled trials to meet the inclusion criteria, the current review has been restricted to randomised prospective trials to improve the quality of the review, reducing biases produced by lack of randomisation, as well as the level of heterogeneity.
When the analysis included all the comparative and controlled studies, the observed trend in the confidence intervals of the randomised clinical trials becomes more evident, with a higher statistical significance at the expense of greater heterogeneity. That is to say, a greater benefit from antibiotic prophylaxis is detected, particularly when mesh repair is used. Heterogeneity in the set of comparative studies came from the herniorrhaphy group. Variations in the results among the studies were statistically significant, whereas they were absent in the hernioplasty group. However, caution should be exercised when interpreting these results since non‐randomised and unblinded studies tend to overestimate the effects of treatment or prophylaxis.
Meta‐analysis is only as strong as the primary data on which it is based (Imperiale 1999). Therefore, non‐randomised studies, even not included in a statistical analysis, should be taken into account. The current meta‐analysis has only addressed the use of antibiotic (whatever the type) for prophylaxis. The antibiotics considered in the included studies were beta lactamic agents, which are commonly used for antibiotic prophylaxis. They are able to attack Gram‐positive cocci, commonly responsible for infections after hernia repair. All the included studies used antibiotic prophylaxis according to clinical management norms (Condon 1991,Page 1993,Woods 1998,Mangram 1999). In every case, patients were followed‐up for longer than 30 days, the time required to follow up postoperative infections (Ranaboldo 1993).
A separate analysis of subgroups herniorrhaphies and hernioplasties showed that it was possible to combine these (no statistically significant heterogeneity). In the herniorrhaphies subgroup, the results showed that prophylaxis might reduce the postoperative wound infection, although statistical significance is borderline. In the hernioplasties subgroup, the antibiotic prophylaxis show a significant reduction of wound infection rates. The overall analysis show a significant albeit small reduction of wound infection rates with a low level of heterogeneity. However, sensitivity analysis with a random effects model shows marginal significance.There are several reasons, widely known, that explain this phenomenon: under the random effects model, confidence intervals are wider than under a fixed effects one, and smaller studies have more weight. In addition, the incidence of infections in the populations studied is low, thus being more difficult to show a significant reduction caused by the prophylaxis. It is possible that this sum of factors is masking a small, significant benefit of antibiotic prophylaxis, that might be of interest to clinicians in settings of high incidence of infections.
In conclusion, the results of this meta‐analysis show that antibiotic prophylaxis may be useful to prevent wound infection in open elective hernia repair. However, the data are not sufficiently strong neither to recommend its universal administration nor to recommend against its use when high rates of wound infections are observed. When assessing this results it is important to take into account the setting of the included studies. Neither individual patient risk factors nor hospital‐related risk factors (outpatient surgery, hospitals of different level) that might change the conclusions from this meta‐analysis were considered in the included studies. These results should also be considered within their context; that is to say, the applicability of the results is related to the studies included in this meta‐analysis. Therefore, to make generalizations of the findings inclusion of studies conducted in other settings should be carefully considered.
Authors' conclusions
Implications for practice.
Based on the results of this systematic review, administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended. Nevertheless, its administration cannot either be recommended against when high rates of wound infection are observed.
Implications for research.
Identification of risk factors for infection would be useful to identify those groups of patients that may benefit from antibiotic prophylaxis. A cost‐effectiveness analysis to evaluate the advantages of antibiotic prophylaxis is needed to appropriately appraise the economic implications.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2012 | New citation required but conclusions have not changed | Four new trials added |
| 25 October 2011 | New search has been performed | Four new trials included. Conclusions not changed. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 28 June 2009 | New citation required but conclusions have not changed | One new trial included. Conclusions not changed. |
| 8 August 2006 | Amended | Administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended. Nevertheless, its administration cannot either be recommended against when high rates of wound infection are observed. |
| 17 March 2004 | New search has been performed | Administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be firmly recommended or discarded. |
Acknowledgements
My special thanks to the Iberoamerican Cochrane Centre, particularly to Marta Roqué, for providing statistical advice.
Data and analyses
Comparison 1. Antibiotic prophylaxis vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 17 | 7843 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.82] |
| 1.1 Herniorraphies | 6 | 4269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 1.00] |
| 1.2 Hernioplasties | 11 | 3574 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.81] |
1.1. Analysis.
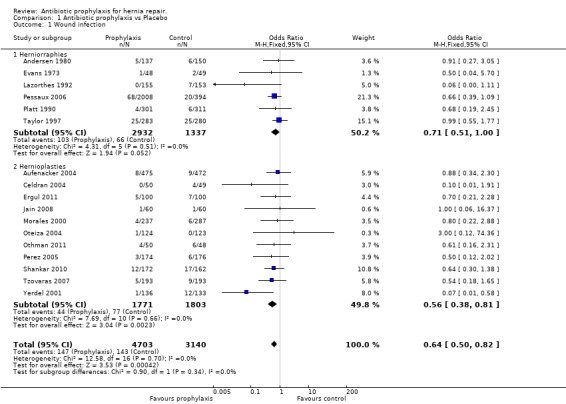
Comparison 1 Antibiotic prophylaxis vs Placebo, Outcome 1 Wound infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 1980.
| Methods | Study: prospective, randomised. Allocation: NM. Blinding: yes. | |
| Participants | 287 patients: 137 P / 150 C | |
| Interventions |
Herniorrhaphy ATB: ampicillin 1 gr subfascial. MA: before closure fascial. Control: no treatment |
|
| Outcomes | IP= 5; IC= 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A triple‐blind, random‐allocation design was used." Comment: Information not provided about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...were assigned at the end of the operation to one of the following four regimens:..." Comment: Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
Aufenacker 2004.
| Methods | Study: prospective, randomised, multicenter. Allocalion: computer. Blinding: yes. | |
| Participants | 1008 patients: 505 P / 503 C | |
| Interventions |
Hernioplasty. ATB: cefuroxime 1.500 mg IV. MA: induction. Control= placebo. |
|
| Outcomes | IP= 9; IC= 8 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A pharmacist carried out randomizations according to a computer generated list in blocks of 10 patients with stratification for each hospital." |
| Allocation concealment (selection bias) | Low risk | Quote: "A pharmacist prepared the trial medication under laminar airflow condition and it was packed in nontransparent material to exclude optical differences." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Probably yes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probably yes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In most cases, the surgeon who performed the operation did not perform the follow‐up." Comment: Probably yes. |
Celdran 2004.
| Methods | Study: prospective, randomised. Allocaton: number list. Blinding: yes | |
| Participants | 99 patients: 50 P / 49 C. | |
| Interventions |
Hernioplasty ATB: cephazolin 1 gr. MA: 30 minutes before the incision. Control: placebo |
|
| Outcomes | IP= 0; IC= 4 | |
| Notes | The interim analysis, performed after the first 91 patients had been included, recommended ending the study due to ethical reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A list of random numbers was generated to assign the treatment." |
| Allocation concealment (selection bias) | High risk | Quote: "A prospective, double‐blind trial was performed ..." Comment: Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned. Comment: Probably not done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Comment: Probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients were examined 1 week, 1, 3, and 6 months, and 1 and 2 years postoperatively by trained impartial surgeon." |
Ergul 2011.
| Methods | Study: prospective, randomised. Allocation: sealed envelopes. Blinding: yes | |
| Participants | 200 patients: 100 P / 100 C. | |
| Interventions |
Hernioplasty ATB: cefazolin 1 gr IV. MA: induction. Control: sterile saline. |
|
| Outcomes | IP=5; IC=7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Quote: "The anaesthesiologist administered the trial medication (antibiotic or sterile saline in coded syringes..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Probably yes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Probably yes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The surgeon who performed the follow‐up frequently was not the surgeon who performed the operation." |
Evans 1973.
| Methods | Study: prospective, randomised. Allocation: coin. Blinding: no. | |
| Participants | 97 patients: 48 P / 49 C. | |
| Interventions |
Herniorrhaphy ATB: cefaloridine 1 gr IM. MA: induction and two dosis postoperatives. Control: no treatment. |
|
| Outcomes | IP= 1;IC= 2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of a coin |
| Allocation concealment (selection bias) | High risk | Quote: "To avoid bias the details were entered not in the patients' case notes but on punch cards which were kept separately and analysed manually." Comment: Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Concealment not mentioned. Comment: Probably not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Comment: Probably not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. Comment: Probably not. |
Jain 2008.
| Methods | Study: prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 120 patients: 60 P / 60 C. | |
| Interventions |
Hernioplasty ATB: amoxicillin‐clavulanic acid 1.2 gr IV. MA: before incision. Control: placebo. |
|
| Outcomes | IP= 1; IC= 1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated code |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a computer‐generated code by a junior resident who was not involved in the surgery, data compilation or patient follow‐up. The same resident also prepared the antibiotic or the placebo syringes containing normal saline." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The surgeon who performed the operation was not allowed to follow up their patient." |
Lazorthes 1992.
| Methods | Study: prospective, randomised. Allocation: NM. Blinding: no. | |
| Participants | 308 patients: 155 P / 153 C. | |
| Interventions |
Herniorrhaphy ATB: cefamandole 750 mg subcutaneous. MA: added to local anaesthesia. Control: no treatment. |
|
| Outcomes | IP= 0; IC= 7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two groups of 162 patients were randomly allotted to receive ..." Information not provided. |
| Allocation concealment (selection bias) | High risk | Not mentioned. Comment: Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned. Comment: Probably not done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Comment: Probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. Comment: Probably not done. |
Morales 2000.
| Methods | Study: multicenter, prospective, randomised. Allocation: envelopes. Blinded: yes. | |
| Participants | 524 patients: 237 P / 287 C. | |
| Interventions |
Hernioplasty ATB: cefazolin 2 gr IV. MA: induction. Control: placebo. |
|
| Outcomes | IP= 4; IC= 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Comment: Probably yes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "La administración de la solución (antibiótico o placebo) fue realizada por la enfermera circulante, no informando del contenido de la solución a ninguno de los dos cirujanos implicados en la intervención." Comment: Yes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Yes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "En el seguimiento postoperatorio, los cirujanos, las enfermeras de planta y la de consulta externa desconocían la solución administrada al paciente." Comment: Yes. |
Oteiza 2004.
| Methods | Study: prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 247 patients: 124 P / 123 C. | |
| Interventions |
Hernioplasty ATB: amoxicillin‐clavulanic 2 gr IV. MA: 15‐30 minutes before the incision. Control: no treatment. |
|
| Outcomes | IP= 1; IC= 0 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. |
| Allocation concealment (selection bias) | High risk | Information not provided. Comment: Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Information not provided. Comment: Probably not done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Information not provided. Comment: Probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. Comment: Probably not done. |
Othman 2011.
| Methods | Study: prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 98 patients: 50 P / 48 C. | |
| Interventions |
Hernioplasty ATB: amoxicillin‐clavulanic 1.2 gr IV. MA: 30 minutes before induction. Control: sterile saline |
|
| Outcomes | IP= 4; IC= 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. |
| Allocation concealment (selection bias) | Low risk | Yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Randomization and preparation of drug and placebo were controlled by a surgery clinic nurse without the previous knowledge of the patient or surgeon." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned Comment: Probably not. |
Perez 2005.
| Methods | Study: prospective, randomised. Allocation: computer. Blinding: yes | |
| Participants | 350 patients: 174 P / 176 C. | |
| Interventions |
Hernioplasty ATB: cefazolin 1 gr IV. MA: before incision. Control: placebo. |
|
| Outcomes | IP=4; IC=7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. |
| Allocation concealment (selection bias) | Low risk | Probably yes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Senior surgical residents or consultants, blinded to the study group, performed all operations. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " All incisions were carefully reexamined by an independent surgeon blinded to the study". |
Pessaux 2006.
| Methods | Study: prospective, randomised. Allocation: NM. Blinding: No. | |
| Participants | 2402 patients: 2008 P / 394 C. | |
| Interventions |
Herniorrhaphy ATB: several, IV. MA: induction. Control: not treatment. |
|
| Outcomes | IP=68; IC=20 | |
| Notes | Additional information provided by the first author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A database was established from 3 prospective, randomised, multicenter studies led by the French Associations for Surgical Research on antibiotic prophylaxis in abdominal noncolorectal surgery". Comment: Probably yes. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
Platt 1990.
| Methods | Study: multicenter, prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 612 patients: 301 P / 311 C. | |
| Interventions |
Herniorrhaphy ATB: cefonicid 1 g IV. MA: 90 minutes before surgery. Control: placebo. |
|
| Outcomes | IP= 4; IC= 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Probably yes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Cefonicid and placebo were supplied in identical numbered vials. The treatment codes were not known by anyone at the participating centres, unless the sealed, opaque label attached to each vial was opened". Comment: Yes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " None of the personnel at the data processing or coordinating center knew the treatment codes, and the codes were not revealed to the patients or medical personnel until the last one completed the evaluation." Comment: Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
Shankar 2010.
| Methods | Study: prospective randomised. Allocation: sealed envelope. Blinding: No | |
| Participants | 334 patients: 172 P / 162 C. | |
| Interventions |
Hernioplasty ATB: cefazolin 1 gr IV. MA: induction. Control: sterile saline. |
|
| Outcomes | IP=12; IC=17 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomised into antibiotic group and control group by sealed envelope method on the day before the surgery." Comment: Yes |
| Allocation concealment (selection bias) | High risk | Comment: Randomization on the day before the surgery. Probably not. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned. Comment: Probably not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Comment: Probably not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quoted: "Follow was done by residents who where blinded to the drug used." |
Taylor 1997.
| Methods | Study: multicenter, prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 269 patients: 136 P / 133 C. | |
| Interventions |
Herniorrhaphy ATB: Amoxicillin‐clavulanic 1,2 gr IV. MA: before the incision. Control: placebo. |
|
| Outcomes | IP=1; IC= 12 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated code in blocks of four. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote in abstract: "We have conducted a randomised multicenter, double‐blind prospective trial...". Comment: Information about concealment not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Information about concealment not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
Tzovaras 2007.
| Methods | Study: prospective randomised. Allocation: computer. Blinding: yes. | |
| Participants | 386 patients: 193 P / 193 C. | |
| Interventions |
Hernioplasty ATB: amoxicillin‐clavulanic 1,2 gr. MA: not standardised. Control: sterile saline. |
|
| Outcomes | IP= 5; IC= 9 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Code numbers generated by the Arcus Quiqstat randomisation program using numbered sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Quote: " The list with code numbers generated by the Arcus Quiqstat randomisation program was kept by a secretary, who was not involved in the treatment of the patients at any stage and was opened at the end of the trial for analysis of the results". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
Yerdel 2001.
| Methods | Study: prospective, randomised. Allocation: computer. Blinding: yes. | |
| Participants | 269 patients: 136 P / 133 C. | |
| Interventions |
Hernioplasty ATB: Ampicilln‐sulbactam 1,5 gr IV. MA: before the incision. Control: placebo. |
|
| Outcomes | IP= 1; IC= 12 | |
| Notes | Because of the high rate of wound infections, the code was broken after the discharge of patient 280 (140 patients in each group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code by a resident who also prepared the sealed antibiotic or placebo syringes. He was unaware of the research in progress and was never involved in surgery, data collection or patient follow‐up. |
| Allocation concealment (selection bias) | Low risk | Yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
P = prophylaxis group, C = control group, IP = infected with prophylaxis, IC = infected without prophylaxis, NM = not mentioned, ATB = antibiotic, MA = timing of prophylaxis administration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abo‐Rahmy 1998 | NOT RANDOMIZED. A total of 1524 consecutive hernia patients, divided in 3 groups: A) 606 with ceftriaxone, B) 408 with pefloxacine, C) 510 with several cefalosporins or quinolones different from the A and B groups. One single patient with infection was observed (0,06%). |
| Angio 2001 | UNCONTROLLED TRIAL. Single arm with antibiotic prophylaxis. 112 patients submitted to prosthetic hernioplasty by anterior approach (94 cases) and by transabdominal preperitoneal laparoscopy (18 cases), received levofloxacin 500 mg IV 30 minutes before the surgical operation and 500 mg os in seven days following. Infection rate was zero. |
| Barreca 2000 | RETROSPECTIVE. Study developed in two hospitals, one arm by hospital. Administers cefotaxime 2 gr IV 30' before surgery. A total of 147 patients, 63 with prophylaxis and 84 without; 87% with hernioplasty . Both hospitals show an infection rate of zero. |
| Dazzi 1994 | UNCONTROLLED TRIAL. Single arm with antibiotic prophylaxis. Patients with several pathologies; can be identified 189 hernia patients (100 herniorraphy, 89 hernioplasty), prophylaxis with teicoplanin. Infection rate: zero. |
| Deysine 2005 | UNCONTROLLED TRIAL. Single arm with antibiotic prophylaxis in ventral and inguinal herniorrhaphies. The patients received cefazolin 1 hour before surgery plus frequent wound irrigations with a solution of 80 mg of gentamycin sulphate dissolved in 250 ml of normal saline solution. Infection rate: 0.11% in over 4.000 herniorrhaphies. |
| Dixon 2006 | DOES NOT INCLUDE HERNIA PATOHOLOGY. |
| Escartín 1999 | RETROSPECTIVE. Study with 475 patients. In 277 prosthetic material is used: 144 with antibiotic prophylaxis (3 infected) and 133 with placebo (10 infected). In 198 patients no prosthetic material was used: 14 with antibiotic prophylaxis (0 infected) and 184 with placebo (13 infected). They recommend prophylaxis when using prosthetic material. |
| Esposito 2006 | INCOMPLETE DATA. Prospective randomised trial. The objective of the study is prophylaxis in hernia repair and breast surgery. There were 350 patients underwent to hernia repair, 168 y 162 patients in the prophylaxis and placebo group. Data of infected patients undergoing hernia repair are not provided by the author. |
| Gervino 2000 | UNCONTROLLED TRIAL. Single arm with antibiotic prophylaxis. 1254 patients intervened with prosthetic material, received ceftriaxone IV 2 gr before surgery. Infection rate assessed retrospectively: zero . |
| Gilbert 1993 | RETROSPECTIVE. Large series, that presents methodologic handicaps. Infection rate: a)primary herniorraphy: 1.1% without prophylaxis and 1.3% with prophylaxis; b) primary hernioplasty: 0.34% without prophylaxis and 0.98% with prophylaxis; c) herniorraphy for recurrent hernia: 0% in both groups; d) hernioplasty for recurrent hernia: 2.2% without prophylaxis and 0.43% with prophylaxis. They do not recommend antibiotic prophylaxis on surgery for hernia repair. |
| Gilmore 1977 | PROPHYLAXIS WITH ANTISEPTIC TREATMENT. |
| Hair 2000 | RETROSPECTIVE. Multicentric trial where 71% of 5.506 patients received antibiotic prophylaxis. Global infection rate: 8%, without significant differences between patients with and without antibiotic prophylaxis (RR 0.9, IC95% 0.7 ‐ 1.1). Individualized infection rate is not provided for each group. They recommend not to administer antibiotic prophylaxis. |
| Hedawoo 1995 | REVIEW. In a study of clean surgical techniques, 134 hydroceles and hernias are included, without antibiotic prophylaxis. Infection rate 3,6%. |
| Houck 1989 | INCOMPLETE DATA. The objective of the study is prophylaxis in incisional hernia and patients with inguinal hernia constitute the control group. Data for these patients is not provided. |
| Karran 1992 | INCOMPLETE STUDY. No results provided. |
| Kuzu 2005 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. A total of 408 patient were enrolled in a prospective randomised study which compare the efficacy of oral versus parenteral prophylactic amoxicillin‐clavulanic acid in open mesh hernia repair. Infections rates: 0,5% and 1,5%, respectively. |
| Lewis 1995 | INCOMPLETE DATA. Prospective, randomised, well‐designed study on clean surgical techniques. A total of 165 patients had hernia repair (86 receiving cefotaxime 2 gr IV prior to surgery and 79 receiving placebo), but there's no information on the number of infections on these patients. INCOMPLETE DATA. Prospective, randomised, well‐designed study on clean surgical techniques. A total of 165 patients had hernia repair (86 receiving cefotaxime 2 gr IV prior to surgery and 79 receiving placebo), but there's no information on the number of infections on these patients. |
| Massaioli 1995 | UNCONTROLLED TRIAL. Single arm of antibiotic prophylaxis with vancomycin on patients with incisional hernia or inguinal hernia repair with prosthetic material. Infection rate: 1/40 patients (2,5%). |
| Musella 2001 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. Both groups received prophylaxis with a systemic antibiotic. Intervention group, additionally, is administered a collagen sponge impregnated with gentamicin. Infection rate: 6/284 patients on control group and 1/293 patients on prophylaxis group (OR=0.16; IC95% 0.02‐1.33). |
| Nundy 1983 | INCOMPLETE DATA. Study on clean surgical techniques, not possible to obtain data of patients with hernia repair. Prophylaxis is penicillin 2 to 12 hours prior to the surgery. |
| Platt 1992 | NOT RANDOMISED. Study developed with the patients not included on the published 1990 trial. A total of 1221 patients with herniorraphy. Infection rate: 2/239 patients on prophylaxis, and 15/982 patients without prophylaxis (OR 0.54; IC95% 0,06‐2,07; P= 0.4). They recommend prophylaxis only for high risk patients. |
| Praveen 2009 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. A single blinded prospective randomised trial with locally applied gentamicin against systemic gentamicin in 202 patients underwent to Lichtenteins tension free repair. There were seven SSI in each arm. |
| Reggiori 1996 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. Prospective randomised trial, well designed, administering a single dose of ampicillin 2 gr IV at induction of anaesthesia in one arm and fortified procaine 1,2 megaunits/24 hr IM daily for seven days starting about 3 hr after surgery on the other arm. Infection rate: 0/123 patients on ampicillin and 8/106 patients on penicillin (OR = 0,05; IC 95% 0,0 ‐ 0,82). |
| Ryan 1967 | NOT RANDOMISED. Non homogeneous groups: control group with majority of patients on local anaesthesia, prophylaxis group with majority of patients on general anaesthesia. Children are included on the trial. Control group is sequential in time to the intervention group. Each group is intervened in a different hospital. Infection rate: 82/5335 patients in control group, 2/1183 patients in penicillin group. (OR 0.11; IC95% 0.03‐0.44) |
| Shwed 1991 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. Study of several surgical techniques to evaluate the efficacy of a new antibiotic compared to an already established one. A total of 128 patients, 5 of whom had hernia repair. |
| Spallitta 1999 | UNCONTROLLED TRIAL. Single arm of 100 patients receiving antibiotic prophylaxis and prosthetic material. |
| Sultan 1989 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. |
| Terzi 2005 | BOTH STUDY ARMS RECEIVED PROPHYLAXIS. Prospective randomised study which compare the efficacy of oral ciprofloxacin versus parenteral cefazolin in open mesh hernia repair. Infections rates: 2% in both arms. |
| Van‐Damme 1981 | NOT RANDOMISED. Technical review using different types of antibiotics, both intravenous and topical. |
| Vara 1993 | MISBALANCES IN TREATMENT BETWEEN GROUPS: Patients with hernioplasty received prophylaxis while patients with herniorraphy received placebo . Patients infected: 2/141 patients on prophylaxis and 9 /137 patients on placebo. They recommend antibiotic prophylaxis to all patients. |
| Wantz 1996 | RETROSPECTIVE SERIE. Serie of 1076 patients followed for 6 years. Administers local anaesthesia and prosthetic material (Lichtentein and Gilbert), without antibiotic prophylaxis. One single patient with infection (0,09%). |
Contributions of authors
None mentioned
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersen 1980 {published data only}
- Andersen JR, Burcharth F, Larsen HW, Roder O, Andersen B. Polyglycolic acid, silk, and topical ampicillin. Their use in hernia repair and cholecystectomy. Arch Surg 1980;115:293‐5. [DOI] [PubMed] [Google Scholar]
Aufenacker 2004 {published data only}
- Aufenacker TJ, Geldere D, Mesdag T, Bossers AN, Dekker B, Scheije E, et al. The role of antibiotic prophylaxis in prevention of wound infection after Lichtenstein open mesh repair of primary inguinal hernia. A multicenter double‐blind randomized controlled trial. Ann Surg 2004;240(6):955‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celdran 2004 {published data only}
- Celdran A, Frieyro O, Pinta JC, Souto JL, Esteban J, Rubio JM, et al. The role of antibiotic prophylaxis on wound infection after mesh hernia repair under local anaesthesia on an ambulatory basis. Hernia 2004;8(1):20‐2. [DOI] [PubMed] [Google Scholar]
Ergul 2011 {published data only}
- Ergul Z, Akinci M, Urgulu C, Kulacoglu H, Yilmaz KB. Prophylactic antibiotic use in elective inguinal hernioplasty in a trauma center. Hernia September 2011;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Evans 1973 {published data only}
- Evans C, Pollock AV. The reduction of surgical wound infections by prophylactic parenteral cephaloridine. A controlled clinical trial. Br J Surg 1973;60(6):434‐7. [DOI] [PubMed] [Google Scholar]
Jain 2008 {published data only}
- Jain SK, Jayant M, Norbu C. The role of antibiotic prophylaxis in mesh repair of primary inguinal hernias using prolene hernia system: a randomized prospective double‐blind control trial. Tropical Doctor 2008;38:80‐82. [DOI] [PubMed] [Google Scholar]
Lazorthes 1992 {published data only}
- Lazorthes F, Chiotasso P, Massip P, Materre JP, Sarkissian M. Local antibiotic prophylaxis in inguinal hernia repair. Surg Gynecol Obstet 1992;175:569‐70. [PubMed] [Google Scholar]
Morales 2000 {published data only}
- Morales R, Carmona A, Pagán A, García Menéndez C, Bravo R, Hernández MJ, et al. Utility of antibiotic propyhylaxis in reducing wound infection in inguinal or femoral hernia repair using propypropylene mesh [Utilidad de la profilaxis antibiótica en la reducción de la infección de herida en la reparación de la hernia inguinal o crural mediante malla de prolipropileno]. Cir Esp 2000;67(1):51‐9. [Google Scholar]
Oteiza 2004 {published data only}
- Oteiza F, Ciga MA, Ortiz H. Antibiotic prophylaxis in inguinal hernioplasty [Profilaxis antibiótica en la hernioplastia inguinal]. Cir Esp 2004;75(2):69‐71. [Google Scholar]
Othman 2011 {published data only}
- Othman I. Prospective randomized evaluation of prophylactic antibiotic usage in patients undergoing tension free inguinal hernioplasty. Hernia 2011;15(3):309‐313. [DOI] [PubMed] [Google Scholar]
Perez 2005 {published data only}
- Perez AR, Roxas MF, Hilvano SS. A randomized, double‐blind, placebo‐controlled trial to determine effectiveness of antibiotic prophylaxis for tension‐free mesh herniorraphy. J Am Coll Surg 2005;200(3):393‐7. [DOI] [PubMed] [Google Scholar]
Pessaux 2006 {published and unpublished data}
- Pessaux P, Lermite E, Blezel E, Msika S, Hay J‐M, Flamant Y, et al. Predictive risk score for infection after inguinal hernia repair. Am J Surg 2006;192:165‐171. [DOI] [PubMed] [Google Scholar]
Platt 1990 {published data only}
- Platt R, Zaleznik DF, Hopkins CC, Dellinger EP, Karchmer AW, Bryan CS, et al. Perioperative antibiotic prophylaxis for herniorrhaphy and breast surgery. N Engl J Med 1990;322(3):153‐60. [DOI] [PubMed] [Google Scholar]
Shankar 2010 {published data only}
- Shankar VG, Srinivasan K, Sistla SC, Jagdish S. Prophylactic antibiotics in open mesh repair of inguinal hernia. A randomized controlled trial. Int J Surg 2010;8(6):444‐447. [DOI] [PubMed] [Google Scholar]
Taylor 1997 {published data only}
- Taylor EW, Byrne DJ, Leaper DJ, Karran SJ, Kennedy Browne M, Mitchell KJ. Antibiotic prophylaxis and open groin hernia repair. World J Surg 1997;21(8):811‐5. [DOI] [PubMed] [Google Scholar]
Tzovaras 2007 {published data only}
- Tzovaras G, Delikoukos S, Christodoulides G, Spyridakis M, Mantzos F, Tepetes K, Athanassiou E, Hatzitheofilou C. The role of antibiotic prophylaxis in elective tension‐free mesh inguinal hernia repair: results of a single‐centre prospective randomised trial. Int J Clin Pract 2007;61(2):236‐239. [DOI] [PubMed] [Google Scholar]
Yerdel 2001 {published data only}
- Yerdel MA, Akin EB, Dolalan S, Turkcapar AG, Pehlivan M, Gecim IE, et al. Effect of single‐dose prophylactic ampicillin and sulbactam on wound infection after tension‐free inguinal hernia repair with polypropylene mesh. The randomized, double‐blind, prospective trial. Ann Surg 2001;233(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abo‐Rahmy 1998 {published data only}
- Abo‐Rahmy E. Perioperative antibiotic prophylaxis in abdominal surgery for hernia repair: retrospective study of 1524 consecutive patients. J Chemother 1998;10(3):248‐53. [DOI] [PubMed] [Google Scholar]
Angio 2001 {published data only}
- Angio LG, Versaci A, Rivoli G, Santagati C, Caridi G, Pacile V. The switch prophylaxis with levofloxacin in surgical procedures for prosthetic inguinal hernia repair in one day surgery. [La switch prophylaxis con levofloxacina negli interventi di ernioplastica inguinale con protesi in regime di one day surgery.]. G Chir 2001;22(8‐9):299‐302. [MEDLINE: ] [PubMed] [Google Scholar]
Barreca 2000 {published data only}
- Barreca M, Stipa F, Cardi E, Bianchini L, Lucandri G, Randone B. Antibiotic prophylaxis in the surgical treatment of inguinal hernia: need or habit? [La profilassi antibiotica nel trattamento chirurgico dell'ernia inguinale: necessità o abitudine?]. Minerva Chir 2000;55(9):599‐605. [PubMed] [Google Scholar]
Dazzi 1994 {published data only}
- Dazzi C, Licheri S, Sias F, Secci L, Daniele GM. Wound infection's prophylaxis with teicoplanin in major ambulatory surgery [La teicoplanina nella profilassi delle infezioni della ferita in chirurgia ambulatoriale maggiore]. Ann Ital Chir 1994;65(1):121‐3. [PubMed] [Google Scholar]
Deysine 2005 {published data only}
- Deysine M. Postmesh herniorrhaphy wound infections: can they be eliminated?. Int Surg 2005;90(3 Suppl):S40‐44. [PubMed] [Google Scholar]
Dixon 2006 {published data only}
- Dixon AJ, Dixon MP, Dixon JB. Randomized clinical trial of the effect of applying ointment to surgical wounds before occlusive dressing.. Br J Surg 2006;93:937‐943. [DOI] [PubMed] [Google Scholar]
Escartín 1999 {published data only}
- Escartín A, Pellicer MM, Elía M, Jiménez A, Arribas MD, Lagunas E, et al. Antibiotic prophylaxis in inguinal hernia repair [Profilaxis antibiótica en la cirugía de la hernia inguinal]. Cir Esp 1999;65(1):24‐7. [Google Scholar]
Esposito 2006 {published data only (unpublished sought but not used)}
- Esposito S, Leone S, Noviello S, Ianniello F, Marvaso A, Cuniato V, Bellitti F. Antibiotic prophylaxis in hernia repair and breast surgery: A prospective randomized study comparing piperacillin/tazobactam versus placebo. J Chemother 2006;18(3):278‐289. [DOI] [PubMed] [Google Scholar]
Gervino 2000 {published data only}
- Gervino L, Cangioni G, Renzi F. A retrospective study on the efficacy of short‐term perioperative prophylaxis in abdominal surgery for hernia repair in 1254 patients. J Chemother 2000;12(Suppl 3):34‐7. [DOI] [PubMed] [Google Scholar]
Gilbert 1993 {published data only}
- Gilbert AI, Felton LL. Infection in inguinal hernia repair considering biomaterials and antibiotics. Surg Gynecol Obstet 1993;177:126‐30. [PubMed] [Google Scholar]
Gilmore 1977 {published data only}
- Gilmore OJA, Reid C, Strokon A. A study of the effect of povidone‐iodine on wound healing. Postgrad Med J 1967;53(617):122‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hair 2000 {published data only}
- Hair A, Duffy K, McLean J, Taylor S, Smith H, Walker A, et al. Groin hernia repair in Scotland. Br J Surg 2000;87(12):1722‐6. [DOI] [PubMed] [Google Scholar]
Hedawoo 1995 {published data only}
- Hedawoo JB, Kulkarni VM, Gundeti MS. Role of antibiotics in clean wounds. J Indian Med Assoc 1985;93(8):293‐4. [PubMed] [Google Scholar]
Houck 1989 {published data only}
- Houck JP, Rypins EB, Sarfeh IJ, Juler GL, Shimoda KJ. Repair of incisional hernia. Surg Gynecol Obstet 1989;169:397‐9. [PubMed] [Google Scholar]
Karran 1992 {published data only}
- Karran SJ, Karran SE, Toyn K, Brough P. Antibiotic prophylaxis in clean surgical cases and the role of community surveillance.. Eur J Surg 1992;567 Suppl:31‐2. [PubMed] [Google Scholar]
Kuzu 2005 {published data only}
- Kuzu M A, Jazinedaroglu S, Dolalan S, Özkan N, Yalçin S, Erkek A B, et al. Prevention of surgical site infection after open prosthetic inguinal hernia repair: efficacy of parenteral versus oral prophylaxis with amoxicillin‐clavulanic acid in a randomized clinical trial.. World J Surg 2005;29(6):794‐799. [DOI] [PubMed] [Google Scholar]
Lewis 1995 {published data only}
- Lewis RT, Weigand FM, Mamazza J, Lloyd‐Smith W, Tataryn D. Should antibiotic prophylaxis be used routinely in clean surgical procedures: a tentative yes.. Surgery 1995;118(4):742‐7. [DOI] [PubMed] [Google Scholar]
Massaioli 1995 {published data only}
- Massaioli N, Marchesa P, Bacino A, Galliano R, Borello G, Bonatti L, et al. Antibiotic prophylaxis with vancomycin in reparative surgery using alloplastic material [Antibioticoprofilassi con vancomicina nella chirurgia riparativa con materiale alloplastico.]. Minerva Chir 1995;50(9):827‐9. [PubMed] [Google Scholar]
Musella 2001 {published data only}
- Musella M, Guido A, Musella S. Collagen tampons as aminoglycoside carriers to reduce postoperative infection rate in prosthetic repair of groin hernias. Eur J Surg 2001;167(2):130‐2. [DOI] [PubMed] [Google Scholar]
Nundy 1983 {published data only}
- Nundy S, Ramachandran K. The place of antibiotics in preventing wound infection after clean operations in an Indian hospital: a prospective, randomised, controlled clinical trial.. Ann R Coll Surg Engl 1983;65:400‐2. [PMC free article] [PubMed] [Google Scholar]
Platt 1992 {published data only}
- Platt R, Zucker JR, Zaleznik DF, Hopkins CC, Dellinger EP, Karchmer AW, et al. Prophylaxis against wound infection following herniorrhaphy or breast surgery. [Prophylaxis against wound infection following herniorrhaphy or breast surgery.]. J Infect Dis 1993;166(3):556‐60. [DOI] [PubMed] [Google Scholar]
Praveen 2009 {published data only}
- Praveen S, Rohaizak M. Local antibiotics are equivalent to intravenous antibiotics in the prevention of superficial wound infection in inguinal hernioplasty. Asian J Surg 2009;32(1):59‐63. [DOI] [PubMed] [Google Scholar]
Reggiori 1996 {published data only}
- Reggiori A, Ravera M, Cocozza E, Andreata M, Mukasa F. Randomized study of antibiotic prophylaxis for general and gynaecological surgery from a single centre in rural Africa. Br J Surg 1996;83:356‐9. [DOI] [PubMed] [Google Scholar]
Ryan 1967 {published data only}
- Ryan EA. Wound infection prevention by topical antibiotics. Br J Surg 1967;54(5):324‐9. [DOI] [PubMed] [Google Scholar]
Shwed 1991 {published data only}
- Shwed JA, Danziger LH, Wojtynek J, Rodwold KA. A comparative evaluation of the safety and efficacy of cefotetan and cefoxitin in surgical prophylaxis.. DICP 1991;25:10‐3. [DOI] [PubMed] [Google Scholar]
Spallitta 1999 {published data only}
- Spallitta SI, Temrine G, Zappulla A, Greco V, compagno GM, Lo‐lacono I, et al. Tension‐free hernioplasty in the treatment of inguinal hernia in the adult: our experience with local anesthesia and a review of the literature [Ernioplastica "tension‐free" nel trattamento dell'ernia inguinale dell'adulto: la nostra esperienza in anestesia locale e revisione della letteratura]. Minerva Chir 1999;54(9):573‐89. [PubMed] [Google Scholar]
Sultan 1989 {published data only}
- Sultan S, Ahmed R, Mufti T, Mohammad G, Nawaz M. Use of prophylactic antibiotic in clean surgical cases. A prospective trial of various regimens.. J Pak Med Assoc 1989;39(1):9‐12. [PubMed] [Google Scholar]
Terzi 2005 {published data only}
- Terzi C, Kilic D, Unek T, Hosgorler F, Fuzun M, Ergor G. Single‐dose oral ciprofloxacin compared with single‐dose intravenous cefazolin for prophylaxis in inguinal hernia repair: a controlled randomized clinical study. J Hosp Infect 2005;60(4):340‐347. [DOI] [PubMed] [Google Scholar]
Van‐Damme 1981 {published data only}
- Van‐Damme JP, Timmermans TT. Wound infections in general surgery. A two‐year prospective study in a private hospital. Acta Chir Belg 1981;80(4):161‐9. [PubMed] [Google Scholar]
Vara 1993 {published data only}
- Vara R, Ruiz M, Rosell J, Tovar JL, Moreno A, Guerrero JA, et al. Chemoprophylaxis in inguinal herniorrhaphy? [¿Quimioprofilaxis en cirugía herniaria?]. Cir Esp 1993;53(3):105‐7. [Google Scholar]
Wantz 1996 {published data only}
- Wantz GE. Experience with tension‐free hernioplasty for primary inguinal hernia in men. J Am Coll Surg 1996;183(4):351‐6. [PubMed] [Google Scholar]
Additional references
Altman 1998
- Altman, Douglas G. Confidence intervals for the number need to treat. BMJ 1998;317(7168):1309‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 1992
- Bailey IS, Karran SE, Toyn K, Brough P, Ranaboldo C, Karran SJ. Community surveillance of complications after hernia surgery. BMJ 1992;304(6825):469‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cainzos 1990
- Cainzos M, Lozano F, Balibrea JL, Dávila D, Potel J, Gómez Alonso A, et al. Postoperative infection: multicentric, prospective and controlled study. [La infección postoperatoria: estudio multicéntrico, prospectivo y controlado.]. Cir Esp 1990;48(5):481‐90. [Google Scholar]
Codina 1999
- Codina C, Trilla A, Riera N, Tuset M, Carne X, Ribas J, et al. Perioperative antibiotic prophylaxis in Spanish hospitals: results of a questionnaire survey. Infect Control Hosp Epidemiol 1999;20(6):436‐9. [DOI] [PubMed] [Google Scholar]
Condon 1991
- Condon RE, Wittmann DH. The use of antibiotics in general surgery. Curr Probl Surg 1991;28(12):803‐907. [DOI] [PubMed] [Google Scholar]
Davey 1998
- Davey PG, Nathwani D. What is the value of preventing postoperative infections?. New Horiz 1998;6(2 Suppl):S64‐71. [PubMed] [Google Scholar]
Deeks 1999
- Deeks J, Bradburn M, Localio R, Berlin J. [Much Ado About Nothing: Statistical Methods for Meta‐analysis with Rare Events.]. Presentatioon held at the 2nd Symposium on Systematic Reviews: Beyond the basics.. St. Ctatherine's College, Oxford. UK, 1999.
Dellinger 1994
- Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Clin infect Dis 1994;18:422‐7. [DOI] [PubMed] [Google Scholar]
DerSimonian 1982
- DerSimonian R, Charette J, McPeek B, Mosteller F. Reporting on methods in clinical trials. N Engl J Med 1982;306:1332‐7. [DOI] [PubMed] [Google Scholar]
Haley 1985
- Haley RW, Culver DH, Morgan WM, White JW, Emori TG, Hooton TM. Identifying patients at high risk of surgical wound infection. Am J Epidemiol 1985;121:206‐215. [DOI] [PubMed] [Google Scholar]
Heineck 1999
- Heineck I, Ferreira MB, Schenkel EP. Prescribing practice for antibiotic prophylaxis for 3 commonly performed surgeries in a teaching hospital in Brazil. Am J Infect Control 1999;27(3):296‐300. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Decks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmes 1994
- Holmes J, Readman R. A study of wound infections following inguinal hernia repair. J Hosp Infect 1994;28:153‐6. [DOI] [PubMed] [Google Scholar]
Horan 1992
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;29(Suppl 1):26S‐31S. [PubMed] [Google Scholar]
Imperiale 1999
- Imperiale TE. Meta‐analysis: When and how. Hepatology 1999;29(Suppl 1):26S‐31S. [PubMed] [Google Scholar]
Leaper 1998
- Leaper DJ. Use of antibiotic prophylaxis in clean non‐implant wounds. J Antimicrob Chemother 1998;41:501‐4. [DOI] [PubMed] [Google Scholar]
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, The Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20(4):247‐78. [DOI] [PubMed] [Google Scholar]
Mozillo 1988
- Mozillo N, Greco D, Pescini A, Formato A. Chemoprophylaxis in the surgical ward: results of a national survey in Italy. Eur J Epidemiol 1988;4(3):357‐9. [DOI] [PubMed] [Google Scholar]
NRC 1964
- National Academy of Sciences, National Research Council, Division of Medical Sciences, Ad Hoc Commitee on Trauma. Postoperative wound infections: the influence of ultraviolet irradiation on the operating room and of various other factors.. Ann Surg 1964;16(Suppl 2):1‐196. [PubMed] [Google Scholar]
Page 1993
- Page CP, Bohnen JMA, Fletcher JR, MacManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg 1993;128:79‐88. [DOI] [PubMed] [Google Scholar]
Porcu 1996
- Porcu A, Noya G, Dessanti A, Niolu P, Cottu P, Castiglia P, Dettori G. A new approach to the problem of surgical wound infections in clean operations. Minerva Chir 1996;51(9):691‐6. [PubMed] [Google Scholar]
Ranaboldo 1993
- Ranaboldo CJ, Karran SE, Bailey S, Karran SJ. Antimicrobial prophylaxis in clean surgery: hernia repair. J Antimicrob Chemother 1993;31(Suppl B):35‐41. [DOI] [PubMed] [Google Scholar]
Rodriguez 2005
- Rodriguez‐Cuellar E, Villeta R, Ruiz P, Alcalde J, Landa JI, Porrero JL, et al. National project for the management of clinical processes. Surgical treatment of inguinal hernia [Proyecto nacional para la gestión clínica de procesos asistenciales. Tratamiento quirúrgico de la hernia inguinal]. Cir Esp 2005;77(4):194‐202. [DOI] [PubMed] [Google Scholar]
Rutkow 1993
- Rutkow IM, Robbins AW. Tension‐free inguinal herniorrhaphy: a preliminary report on the mesh‐plug technique. Surgery 1993;114:3‐8. [PubMed] [Google Scholar]
Sackett 1997
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB. Evidence‐based Medicine. How to Practice & Teach EBM. Edinburgh: Churchill Livingstone, 1997. [Google Scholar]
Sacks 1987
- Sacks HS, Berrier J, Reitman D, Ancona‐Berk VA, Chalmers ThC. Meta‐analyses of randomized controlled trials. N Engl J Med 1987;316(8):450‐5. [DOI] [PubMed] [Google Scholar]
Sanderson 1999
- Sanderson PJ. Assessing the role of prophylactic antibiotics in clean surgery. J Hosp Infect 1999;42:7‐9. [DOI] [PubMed] [Google Scholar]
Simons 2009
- Simons MP, Aufenacker T, Bay‐Nielsen M, Bouillot JL, Campanelli G, Conze J, Lange D, Fortelny R, Heikkinen T, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009;13(4):343‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wittmann 1995
- Wittmann DH, Schein M, Condon RE. Antibiotic prophylaxis in abdominal wall hernia surgery: never, always, or selectively?. Probl Gen Surg 1995;12(1):47‐55. [Google Scholar]
Woods 1998
- Woods RK, Dellinger EP. Current guidelines for antibiotic prophylaxis of surgical wounds. Am Fam Physysician 1998;58(11):2731‐40. [PubMed] [Google Scholar]
References to other published versions of this review
Sanchez‐Manuel 2001
- Sánchez‐Manuel FJ, Seco‐Gil JL, Lozano‐García J. [Antibiotic prophylaxis and hernia repair. Systematic quantitative review results]. [Spanish] [Profilaxis antibiótica y reparación herniaria. Resultado de una revisión sistemática cuantitativa.]. Enferm Infecc Microbiol Clin 2001;19(3):107‐13. [DOI] [PubMed] [Google Scholar]
Sanchez‐Manuel 2003
- Sánchez‐Manuel FJ, Seco‐Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI] [PubMed] [Google Scholar]
Sanchez‐Manuel 2004
- Sánchez‐Manuel FJ, Seco‐Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI] [PubMed] [Google Scholar]
Sanchez‐Manuel 2007
- Sánchez‐Manuel FJ, Lozano‐García J, Seco‐Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI] [PubMed] [Google Scholar]
Sanchez‐Manuel 2010
- Sánchez‐Manuel FJ, Lozano‐García J, Seco Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI] [PubMed] [Google Scholar]


