Abstract
Background
One‐third of subfertile couples have no identifiable cause for their inability to conceive. In vitro fertilisation (IVF) is a widely accepted treatment for this condition; however, this treatment is invasive and expensive and is associated with risks.
Objectives
To evaluate the effectiveness and safety of IVF compared with expectant management, unstimulated intrauterine insemination (IUI) or intrauterine insemination along with ovarian stimulation with gonadotropins (IUI + gonadotropins) or clomiphene (IUI + CC) or letrozole (IUI + letrozole) in improving pregnancy outcomes.
Search methods
This review has drawn on the search strategy developed by the Cochrane Menstrual Disorders and Subfertility Group. We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (searched May 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, first quarter), MEDLINE (1946 to May 2015), EMBASE (1985 to May 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (May 2015) and reference lists of articles. We searched the following trial registries: clinicaltrials.gov (http://www.clinicaltrials.gov) and the World Health Organization International Trials Registry Platform search portal (http://www.who.int/trialsearch/Default.aspx). We searched the Web of Science (http://wokinfo.com/) as another source of trials and conference abstracts, OpenGrey (http://www.opengrey.eu/) for unpublished literature from Europe and the Latin American Caribbean Health Sciences Literature (LILACS) database (http://regional.bvsalud.org/php/index.php?lang=en). Moreover, we handsearched relevant conference proceedings and contacted study authors to ask about additional publications.
Two review authors independently assessed trial eligibility, extracted data and assessed risk of bias. The primary review outcome was cumulative live birth rate. Multiple pregnancy and other adverse effects were secondary outcomes. We combined data to calculate pooled risk ratios (RRs) and 95% confidence intervals (CIs). We assessed statistical heterogeneity by using the I2 statistic. We assessed the overall quality of evidence for the main comparisons using Grades of Recommendation, Assessment, Development and Evaluation (GRADE) methods.
Selection criteria
We included randomised controlled trials (RCTs) in which the effectiveness of IVF in couples with unexplained subfertility was compared with that of other treatments, including expectant management, unstimulated IUI and stimulated IUI using gonadotropins or clomiphene or letrozole.
Live birth rate (LBR) per woman was the primary outcome.
Data collection and analysis
Two review authors independently assessed the eligibility and quality of trials and evaluated the quality of the evidence by using GRADE criteria.
Main results
IVF versus expectant management (two RCTs):
Live birth rate per woman was higher with IVF than with expectant management (odds ratio (OR) 22.00, 95% confidence interval (CI) 2.56 to 189.37, one RCT, 51 women, very low quality evidence). Multiple pregnancy rates (MPRs), ovarian hyperstimulation syndrome (OHSS) and miscarriage were not reported.
IVF versus unstimulated IUI (two RCTs):
Live birth rate was higher with IVF than with unstimulated IUI (OR 2.47, 95% CI 1.19 to 5.12, two RCTs, 156 women, I2 = 60%, low quality evidence). There was no evidence of a difference between the groups in multiple pregnancy rates (OR 1.03, 95% CI 0.04 to 27.29, one RCT, 43 women, very low quality evidence)
IVF versus IUI + ovarian stimulation with gonadotropins (three RCTs) or clomiphene (one RCT) or letrozole (no RCTs):
Data from these trials could not be pooled because of high statistical heterogeneity (I2 = 93.3%). Heterogeneity was eliminated when studies were stratified by pretreatment status.
In trials comparing IVF versus IUI + gonadotropins among treatment‐naive women, there was no conclusive evidence of a difference between the groups in live birth rates (OR 1.27, 95% CI 0.94 to 1.73, four RCTs, 745 women, I2 = 8.0%, moderate‐quality evidence). In women pretreated with IUI + clomiphene, a higher live birth rate was reported among those who underwent IVF than those given IUI + gonadotropins (OR 3.90, 95% CI 2.32 to 6.57, one RCT, 280 women, moderate‐quality evidence).There was no conclusive evidence of a difference in live birth rates between IVF and IUI + CC in treatment‐naive women (OR 2.51, 95% CI 0.96 to 6.55, one RCT, 103 women, low quality evidence).
In treatment‐naive women, there was no evidence of a difference in rates of multiple pregnancy between women who underwent IVF and those who received IUI + gonadotropins (OR 0.79, 95% CI 0.45 to 1.39, four RCTs, 745 women, I2 = 0%, moderate quality evidence). There was no evidence of a difference in MPRs between women who underwent IVF compared with those given IUI + CC (OR 1.02, 95% CI 0.20 to 5.31, one RCT, 103 women, low‐quality evidence).
There was no evidence of a difference in ovarian hyperstimulation syndrome rate between treatment‐naive women who underwent IVF and those given IUI + gonadotropins (OR 1.23, 95% CI 0.36 to 4.14, two RCTs, 221 women, low quality evidence). There was no evidence of a difference in OHSS rates between groups receiving IVF versus those receiving IUI + CC (OR 1.02, 95% CI 0.20 to 5.31, one RCT, 103 women, low‐quality evidence).
In treatment naive women, there was no evidence of a difference in miscarriage rates between IVF and IUI + CC (OR 1.16, 95% CI 0.44 to 3.02, one RCT, 103 women, low‐quality evidence), nor between women treated with IVF versus those receiving IUI+ gonadotropins (OR 1.16, 95% CI 0.44 to 3.02, one RCT, 103 women).
No studies compared IVF with IUI + letrozole.
The quality of the evidence ranged from very low to moderate. The main limitation was serious imprecision resulting from small study numbers and low event rates.
Authors' conclusions
IVF may be associated with higher live birth rates than expectant management, but there is insufficient evidence to draw firm conclusions. IVF may also be associated with higher live birth rates than unstimulated IUI. In women pretreated with clomiphene + IUI, IVF appears to be associated with higher birth rates than IUI + gonadotropins. However in women who are treatment‐naive there is no conclusive evidence of a difference in live birth rates between IVF and IUI + gonadotropins or between IVF and IUI + clomiphene. Adverse events associated with these interventions could not be adequately assessed owing to lack of evidence.
Plain language summary
In vitro fertilisation (IVF) compared to other options for unexplained subfertility
Review question: Cochrane review authors investigated whether IVF leads to more live births than other management options in women with unexplained subfertility.
Background: IVF is frequently used for couples with unexplained subfertility, as it may bypass a variety of undiagnosed biological problems. However, it is expensive and invasive and can lead to complications. Other management options for unexplained subfertility include trying naturally for a pregnancy, introducing washed sperm within the womb (insemination) and performing insemination after use of drugs ('fertility drugs') to stimulate the ovaries.
Study characteristics: The eight randomised parallel‐group trials included 1622 women. Some were multi‐arm trials with several comparisons. Two compared IVF with expectant management, two compared IVF with insemination alone (IUI) and five compared IVF with insemination plus stimulation of the ovaries. Evidence is current to May 2015.
Key results: IVF may be associated with higher live birth rates than expectant management, but there is insufficient evidence to draw firm conclusions. IVF may also be associated with higher live birth rates than unstimulated IUI. In women pretreated with clomiphene + IUI, IVF appears to be associated with higher birth rates than IUI plus gonadotropins. However in women who are treatment‐naive there is no conclusive evidence of a difference in live birth rates between IVF and IUI + gonadotrophins or between IVF and IUI + clomiphene. Adverse events associated with these interventions could not be adequately assessed owing to lack of evidence.
Quality of the evidence: Quality of the evidence ranged from very low to moderate. The main limitation was serious imprecision resulting from small study numbers and low event rates.
Summary of findings
Background
Description of the condition
Infertility is said to be unexplained when standard investigations fail to reveal any obvious barrier to conception such as absent ovulation, poor semen quality or tubal pathology. The prevalence of unexplained infertility among couples attending a fertility clinic has been shown to be 21% among women younger than 35 years of age and 26% in women older than 35 years (Maheshwari 2008).
In the absence of a known cause for infertility, treatment options have included expectant management, unstimulated intrauterine insemination (IUI), stimulated IUI with clomiphene or gonadotropins and in vitro fertilisation (IVF). IVF is expected to overcome any subtle biological deficiencies that could affect conception. However, it is invasive and is associated with risks such as multiple pregnancy and ovarian hyperstimulation syndrome (OHSS).
NICE 2013 recommends offering IVF to women with unexplained infertility who have not conceived after two years of regular unprotected sexual intercourse. In the UK, estimated live birth rates (LBRs) per IVF treatment for all indications of IVF vary between 32.2% in women younger than 35 years and 13.4% in women between 40 and 42 years of age (HFEA 2012), and the average LBR per cycle started is 25% (HFEA 2012). The Victorian Assisted Reproductive Treatment Authority in Australia (VARTA 2013) and the FIVNAT 2012 report from France have noted pregnancy rates per commenced cycle of 18.3% and 20.8%, respectively. The American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry (ASRM/SART) reported that 40.7% of cycles resulted in a live birth in women younger than 35 years (SART/ASRM 2014).
The chance that pregnancy will lead to live birth is influenced by the prognostic profile of a couple such as female age, duration of infertility and previous pregnancy (Collins 1995). Invasive treatments such as IVF are thought to be more effective than expectant management for couples with limited chances of natural conception, but less so in couples with good prospects of natural conception.
Description of the intervention
In vitro fertilisation involves using standard protocols for controlled ovarian stimulation, oocyte retrieval under ultrasound guidance, insemination, embryo culture and transcervical replacement of embryos at cleavage or blastocyst stage. In comparison with cleavage stage transfer, blastocyst transfer results show a significant increase in LBR per fresh IVF cycle (Glujovsky 2012). IVF is invasive and is associated with several potential complications. The multiple pregnancy rate (MPR) (including twins and triplets) associated with IVF is approximately 18.8% (HFEA 2012). In 2006, the risk of having twins following IVF and intracytoplasmic sperm injection (ICSI) was 19.9%, and that of having triplets was 0.9% (Mouzon 2010). MPRs after single embryo transfer and double embryo transfer have been reported to be 1.5% and 32.4%, respectively (SART/ASRM 2014). The incidence of OHSS in stimulated IVF cycles in Europe was reported to be 0.8% in 2006 (Mouzon 2010). OHSS can present with different grades of severity (mild, moderate, severe). The intravascular depletion associated with OHSS can lead to dehydration, hypovolaemia, electrolyte disturbances and thrombosis due to haemoconcentration.
Other treatments that have been used in unexplained subfertility include IUI (with or without superovulation (SO)) and expectant management (spontaneous pregnancy).
IUI, with or without concomitant use of clomiphene citrate (CC) or gonadotrophins, or letrozole, is a widely used treatment for unexplained infertility (NICE 2013). By bypassing the cervical barrier and increasing the number of motile spermatozoa that reach the uterus and tubes, thereby bringing the sperm in close proximity to one or more eggs, IUI can improve fertilisation and could increase LBRs.
Unstimulated IUI
In a spontaneous cycle, single or dual IUI is normally performed 20 to 30 hours after an endogenous luteinising hormone (LH) surge is detected in the serum or urine. Women are asked to monitor urinary or serum LH levels daily from day 10 to day 12 of the treatment cycle. Normally, a maximum of 0.5 mL suspension of processed spermatozoa is introduced into the uterine cavity with a suitable catheter. Semen is prepared by using a standard pure sperm preparation (a procedure used to prepare semen to isolate a population of sperm with a higher percentage of motile forms and with a more uniform morphology than those found in untreated ejaculates). The procedure involves processing fresh and liquefied ejaculates over a pure sperm gradient of 80/40, followed by centrifugation. Couples are advised to abstain from intercourse from the day of LH monitoring until the day of insemination. Additional luteal support is not required.
IUI + ovarian stimulation with gonadotropins
For ovarian stimulation + IUI cycles, CC (antiestrogen) or gonadotropins are used. The aim is to achieve ovulation from a maximum of two mature follicles. The enhanced fertility induced by ovarian stimulation can be attributed to the increased number of fertilisable oocytes, improved sperm selection and assisted migration. The advantage of this approach is that some of the risks associated with IVF are avoided, particularly those related to oocyte retrieval. However, significant risks of OHSS and multiple pregnancy remain if gonadotropins are used concomitantly.
IUI + gonadotropins
When gonadotropins are used concomitantly with IUI, a baseline ultrasound scan is carried out between days 1 and 3 of the treatment cycle. A daily or alternate‐day dose of 75 IU of gonadotropins is started from day 3, and follicular tracking is carried out from around day 5 of stimulation. Subtle variations in clinical protocol would be found with different clinics. When one or two follicles reach 17 mm in maximum diameter, urinary or serum LH levels are estimated to rule out endogenous surge, a human chorionic gonadotropin (hCG) trigger is given intramuscularly and the IUI is planned 36 to 40 hours later. In the case of excessive response of more than two mature follicles, the cycle is cancelled to avoid risk of high‐order multiple pregnancies. Luteal support generally is not required.
IUI + CC
Clomiphene therapy involves oral administration of CC tablets at a dose of 50 mg to 250 mg daily for five days in the early follicular phase (usually from day 2 to day 6) of the cycle. Follicular tracking is carried out from day 10 to day 12 of the treatment cycle. Once a follicle reaches 17 to 18 mm in maximum diameter, urinary LH or serum LH levels are estimated to rule out endogenous LH surge, an HCG trigger is given intramuscularly and IUI is carried out 36 to 40 hours later.
IUI + letrozole
Letrozole (aromatase inhibitor) therapy involves oral administration of letrozole tablets at a dose of 2.5 mg to 5 mg daily for five days in the early follicular phase (usually from day 2 to day 6) of the cycle. Follicular tracking is carried out from day 8 to day 10 of the treatment cycle. Once a follicle reaches 17 to 18 mm in maximum diameter, an hCG trigger may or may not be given intramuscularly, and IUI is carried out 24 to 40 hours later.
Expectant management
In the absence of an identified cause, couples with unexplained infertility have a relatively high chance of spontaneous pregnancy (Lenton 1977; Collins 1995; Snick 1997; Steures 2006; Steures 2008). A cumulative LBR of 33% at 36 months was estimated from a Canadian multi‐centre cohort study (Collins 1995). Following this report, Snick 1997 presented data from a primary care study in the Netherlands and suggested a cumulative LBR of 60% at 36 months.
In an RCT (Steures 2006) that compared expectant management with IUI plus SO in couples with unexplained subfertility, of the 253 couples enrolled, 127 were assigned IUI with controlled ovarian hyperstimulation, and 126 expectant management. In the intervention group, 42 (33%) women conceived and 29 (23%) pregnancies were ongoing. In the expectant management group, 40 (32%) women conceived and 34 (27%) pregnancies were ongoing (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.63 to 1.1). One twin pregnancy occurred in each study group, and one woman in the intervention group conceived triplets. This study concluded that a large beneficial effect of IUI with controlled ovarian hyperstimulation can be excluded in couples with unexplained subfertility and an intermediate prognosis. Expectant management for six months was therefore justified in these couples and is an efficient way to prevent multiple pregnancies.
In a Scottish multi‐centre trial, 580 couples with unexplained infertility that included mild endometriosis and mild male factor infertility were randomly assigned to three arms: expectant management, CC and IUI (Bhattacharya 2008). Live birth rates of 17% and 23% were obtained after expectant management and IUI, respectively, and no evidence suggested differences (odds ratio (OR) 1.46, 95% CI 0.88 to 2.43). Clinical pregnancy rates were similar in the two groups (expectant group 17% vs 23% in the IUI group) (OR 1.41, 95% CI 0.73 to 2.74). This study suggested that 17 women would need to undergo IUI for one extra live birth to be achieved.
A Cochrane review (Hughes 2010) pooled data from two trials comparing CC with IUI and expectant management and showed no clinical benefit with CC and IUI (OR 2.40, 95% CI 0.70 to 8.19).
How the intervention might work
IVF can potentially circumvent many of the putative causes of unexplained infertility by bypassing several in vivo steps that may be responsible for lack of conception. These include ovarian dysfunction, cervical factors, problems with sperm and egg transport and sperm‐egg interaction.
Why it is important to do this review
IVF is invasive and expensive and is associated with risks. This is an update of a Cochrane review first published in 2002 and updated in 2005 and 2011. This review evaluates current evidence comparing IVF with other, less invasive treatments, including expectant management for unexplained infertility. Comparisons within the review should assist couples and clinicians in choosing the best treatment for unexplained infertility. Current limitations in the literature and future areas of research are highlighted in the review.
Objectives
To evaluate the effectiveness and safety of IVF compared with expectant management, unstimulated intrauterine insemination (IUI) or intrauterine insemination along with ovarian stimulation with gonadotropins (IUI + gonadotropins) or clomiphene (IUI + CC) or letrozole (IUI + letrozole) in improving pregnancy outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs). Cross‐over trials were included if first‐phase results could be extracted.
Types of participants
Couples with unexplained infertility.
Couples with minimal endometriosis (American Fertility Society (AFS) criteria grade I) with subfertility or mild male factor subfertility who have been trying to conceive for one year or longer.
Types of interventions
The study had to include one or more comparisons of effectiveness.
In vitro fertilisation (IVF) versus expectant management.
IVF versus intrauterine insemination (IUI) alone.
IVF versus IUI plus ovarian stimulation with gonadotropins or clomiphene or letrozole.
Types of outcome measures
Primary outcomes
1. Live birth rate (LBR) per woman. Live birth is defined as the delivery of one or more living infants. LBR per woman is defined as the number of live births for each randomly assigned woman over a particular period of time.
Secondary outcomes
2. Pregnancy rate per woman. Demonstration of foetal heart activity on an ultrasound scan defines an ongoing clinical pregnancy. Presence of a gestational sac on ultrasound scan or confirmation of products of conception by pathological examination in the event of spontaneous abortion or ectopic pregnancy defines a clinical pregnancy. Pregnancy rate per woman is defined as the number of pregnancies for each randomly assigned woman over a particular period of time.
3. Multiple pregnancy rate (MPR) per woman. Demonstration of more than one sac with a foetal pole on ultrasound scan defines multiple pregnancy. Multiple pregnancy rate per woman is defined as the number of multiple pregnancies for each randomly assigned woman over a particular period of time.
4. Incidence of ovarian hyperstimulation syndrome (OHSS) per woman.
5. Miscarriage rate per woman, defined as the number of miscarriages for each randomly assigned woman over a particular period of time.
Search methods for identification of studies
The original search was performed in July 2001. Updated searches were completed in August 2004, May 2007, March 2010, July 2011 and May 2015. Updated searches were independently performed by ZP, AG and Marion Showell (Trials Search Co‐ordinator, Cochrane Menstrual Disorders and Subfertility Group (MDSG)).
We used the MDSG search string (Appendix 1).
Electronic searches
We searched the following electronic databases.
Evidence‐based medicine (EBM) Reviews (Appendix 1).
MEDLINE (Appendix 2).
EMBASE (Appendix 3).
The Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 4).
PsycINFO (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (Appendix 6).
Searching other resources
We searched the citation lists of relevant publications, review articles and included studies. We handsearched relevant conference proceedings and sent personal communications to experts and authors in the field.
Data collection and analysis
See Appendix 7.
Selection of studies
One review author (ZP) scanned the titles and abstracts of articles retrieved by the search and removed those that were clearly irrelevant. We retrieved the full texts of all potentially eligible studies. Two review authors (ZP, AG) independently examined full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. ZP corresponded with study investigators, when required, to clarify study eligibility. Review authors resolved disagreements regarding study eligibility by consensus or by discussion with a third review author (SB).
Data extraction and management
Two review authors (ZP, SB) selected trials for inclusion in the review, employing the search strategy described previously. We detailed excluded studies in a table of excluded trials. We analysed included trials for the quality criteria and methodological details outlined below. We presented this information in a table describing the included studies, which provides a context for discussing the reliability of results.
Two review authors (ZP, AG) independently assessed trial quality and extracted data, using forms designed in accordance with Cochrane guidelines. We resolved discrepancies by discussion with a senior review author (SB). We sought additional information on trial methodology or actual original data from the principal authors of trials that appeared to meet eligibility criteria but were unclear in aspects of methodology, or when data were provided in a form that was unsuitable for meta‐analysis. We sent reminders to study authors if we received no reply four weeks after making the initial request.
Assessment of risk of bias in included studies
1.
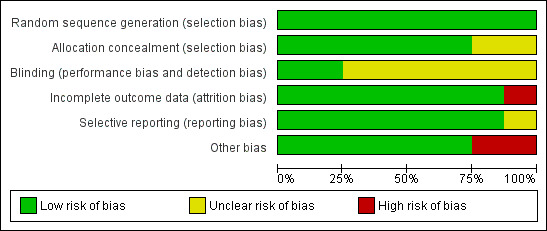
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
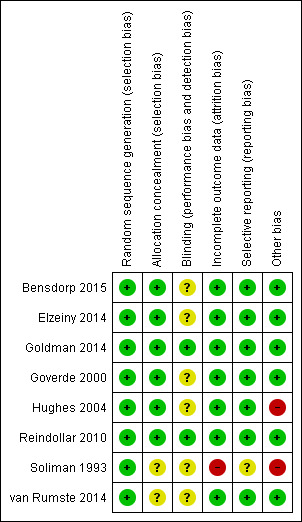
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
We assessed all included studies for risk of bias by using the Cochrane 'Risk of bias' assessment tool (Figure 1) to assess sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias. Two review authors (ZP, AG) assessed these six domains and resolved disagreements by consensus or by discussion with a third review author (SB). We have presented conclusions in the 'Risk of bias' tables (see the Characteristics of included studies table).
When identified studies failed to report the primary outcome of live birth but reported interim outcomes such as pregnancy rate, we informally assessed whether those reporting the primary outcome provided typical values for interim outcomes.
Measures of treatment effect
We expressed results for each study as odds ratios with 95% confidence intervals.
We used dichotomous data for primary and some secondary outcome measures for this review. We expressed results for each study as odds ratios with 95% confidence intervals and combined them for meta‐analysis with RevMan software using a Mantel‐Haenszel fixed‐effect model.
When outcome data were reported as a percentage of the total number of participants, we included this information in the analyses by multiplying the percentage number by the total number of participants (n) in that group and dividing by 100.
We considered pregnancy outcomes as positive consequences of treatment; therefore, we considered a higher proportion of women achieving pregnancy or higher numbers of oocytes to be beneficial. MPRs and OHSS were negative consequences, so that we considered higher numbers to be detrimental. We considered this when designing and viewing summary graphs.
Unit of analysis issues
We performed the primary analysis per woman randomly assigned. When possible, we extracted per‐woman data from trials that reported data per cycle.
We counted multiple live births (e.g. twins, triplets) as one live birth event.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original investigators. When we could not access missing data after attempting to contact the primary authors, we used data that were available.
Assessment of heterogeneity
Review authors considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Even when trials included in a comparison group were statistically homogeneous, we noted potentially large differences in clinical features (clinical heterogeneity). We took these differences into account when analysing and interpreting pooled results. Clinical heterogeneity in subfertility (such as variation in entry criteria and subtle differences in treatments used, which are important from a clinical perspective) cannot be avoided because most centres use their own protocols, which can vary in different aspects. When trials met the inclusion criteria and investigators had provided the same intervention, we considered it appropriate to pool their results. We assessed statistical heterogeneity by inspecting scatter in the data points and overlap in the confidence intervals and, more formally, by checking results of the Chi2 test and measuring the I2 statistic. We considered an I2 value greater than 50% to indicate substantial heterogeneity (Higgins 2011). If we detected substantial heterogeneity, we explored possible explanations by performing sensitivity analyses.
Assessment of reporting biases
In view of the difficulty involved in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data.
Data synthesis
We combined data from primary studies by using the fixed‐effect model in the following comparisons.
IVF versus expectant management.
IVF versus unstimulated IUI.
IVF versus IUI + ovarian stimulation with gonadotropins or IUI + CC or IUI + letrozole.
We graphically displayed an increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. multiple pregnancy), in meta‐analyses to the right of the centre line, and we showed a decrease in the odds of an outcome to the left of the centre line.
We combined results for each study for meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method.
We considered the outcome of clinical pregnancy a positive consequence of treatment; therefore, we regarded a higher proportion of women with pregnancy as a benefit. Outcomes such as OHSS and multiple pregnancy were a negative consequence; therefore, we considered higher numbers to be detrimental. The reader must consider this when viewing summary graphs.
Subgroup analysis and investigation of heterogeneity
We determined possible contributions of differences in trial design to identified heterogeneity.
Sensitivity analysis
We performed sensitivity analysis to determine whether conclusions of the review would have differed if eligibility were restricted to studies without high risk of bias by:
using a funnel plot, if possible, to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies); and
testing the effects of using a random‐effects model and of providing RRs rather than ORs.
Overall quality of the body of evidence: Summary of findings table
We prepared a 'Summary of findings' table to evaluate the overall quality of the body of evidence for main review outcomes (live birth, clinical pregnancy, miscarriage), using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated into reporting of results judgements about evidence quality (high, moderate or low) for each outcome.
Results
Description of studies
Results of the search
We identified 411 articles in our search. When the title or the abstract identified a study as possibly eligible, or if we had any doubt about exclusion of a study, we obtained the full article for further evaluation. We excluded 405/411 articles, as they did not meet the basic inclusion criteria of the review as identified by their titles and abstracts, or because they were duplicated in the different databases searched (Figure 3).
3.
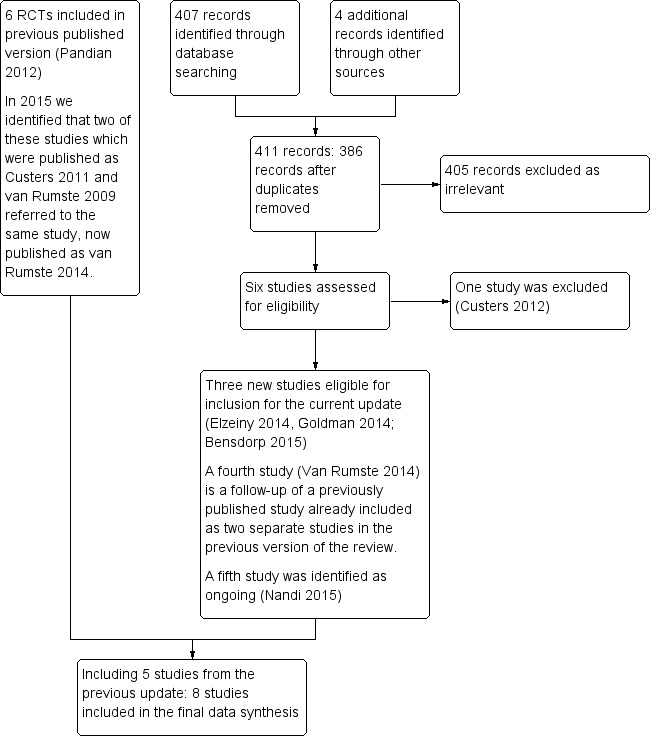
Study flow diagram.
Of the remaining six articles (Custers 2012; Elzeiny 2014; Goldman 2014; van Rumste 2014; Bensdorp 2015; Nandi 2015), three are new trials eligible for inclusion in this update (Bensdorp 2015; Elzeiny 2014; Goldman 2014) and one (van Rumste 2014) is a follow up of Custers et al 2011 and van Rumste 2009 that were included in the previous update of this review as two separate studies. Nandi 2015 is a study registered in the World Health Organization (WHO) trial registry and we classified it as an ongoing study. We excluded one study (Custers 2012: see Excluded studies table). Consequently, we included a total of eight trials in this updated review, comprising three new studies (Bensdorp 2015; Elzeiny 2014; Goldman 2014) and five from the previous version of the review (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; van Rumste 2014).
We sought additional information from study authors when relevant, and we received a response from two authors (Goldman 2014; van Rumste 2014). We have provided a flowchart for the review search results in Figure 3. As relatively few studies were available for analysis, we could not use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) in comparisons 1 and 2. We did not perform subgroup analyses for mild endometriosis as planned because most studies did not identify such subgroups. Sensitivity analysis to determine whether conclusions of the review would have differed if eligibility were restricted to studies without high risk of bias was not required, as we found no significant differences in risk of bias among included trials.
Included studies
We included eight trials in this review (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; Elzeiny 2014; Goldman 2014; van Rumste 2014; Bensdorp 2015).
Trial design characteristics
Design
The eight included studies were randomised parallel‐group trials.
Interventions
Two studies compared in vitro fertilisation (IVF) with expectant management (Soliman 1993; Hughes 2004). The duration of expectant management was three months in one study (Hughes 2004) and six months in the other (Soliman 1993).
Two studies compared IVF with intrauterine insemination (IUI) alone (Goverde 2000; Elzeiny 2014). One of these compared the effectiveness of IVF (six cycles) versus unstimulated IUI (six cycles) (Goverde 2000). The second compared the effectiveness of one cycle of IVF versus one cycle of unstimulated IUI (Elzeiny 2014). Five studies compared IVF with IUI plus ovarian stimulation with gonadotropins (Goverde 2000; Reindollar 2010; Goldman 2014; van Rumste 2014; Bensdorp 2015). One study analysed IUI + CC and IUI + FSH (follicle‐stimulating hormone) separately (Goldman 2014). Both arms of Reindollar 2010 received IUI plus clomiphene citrate (IUI + CC) before going on to IUI + gonadotropins or IVF. No studies compared IVF with IUI + letrozole.
Multi‐centre trials
Five trials were multi‐centre studies (Hughes 2004; Reindollar 2010; Goldman 2014; van Rumste 2014; Bensdorp 2015).
Statistical analysis
Two studies used the Chi2 test for analysis of discrete data on the characteristics of participants and cycles and the Student's t‐test to analyse continuous data (Soliman 1993; Goverde 2000). One study used Fisher's exact test and calculated confidence intervals using the Mantel‐Haenszel method (Hughes 2004). Another study used Fisher's exact test and exact binomial 95% confidence intervals (Reindollar 2010). One study expressed results as risk ratios and 95% confidence intervals (Bensdorp 2015). One study (Elzeiny 2014) used one‐tailed P Fisher's exact tests to compare categorical variables between study groups and represented continuous data as means ± standard deviations and analysed them using Student's t‐test. Another study (Goldman 2014) stated that exact binomial 97.5% confidence intervals were calculated. One study used rate ratios for ongoing pregnancy with corresponding 95% confidence intervals. A formal test of differences in pregnancy rates was performed using Chi2 test statistics (van Rumste 2014).
Financial support or sponsorship
Four trials stated sponsorship. One study (Soliman 1993) was funded by Provincial Health Insurance, Ontario, Canada. Another was supported by a grant from the National Institutes of Health, Rockville, Maryland, USA (Reindollar 2010). One study (Elzeiny 2014) was financially supported by Serono (Geneva, Switzerland) and Melbourne IVF (Melbourne, Australia), another by a grant from ZonMW, the Dutch organisation for Health Research and Development and a grant from Zorgverzekeraars Nederland, the Dutch association of health care insurers.(Bensdorp 2015).
We did not perform subgroup analyses for mild endometriosis because most studies did not identify such subgroups. As data on effectiveness of treatments compared were insufficient, we did not carry out sensitivity analyses.
Baseline characteristics of participants
All studies included couples with unexplained infertility in whom baseline infertility investigations were normal, but inclusion criteria differed among the studies.
One study included women between 21 and 39 years of age (Reindollar 2010), and another included women between 18 and 42 years of age (Elzeiny 2014). Another study included women between 18 and 38 years of age (Bensdorp 2015). One study included women between 38 and 42 years of age (Goldman 2014), and other studies did not mention an age limit for inclusion (Goverde 2000; van Rumste 2014). In one trial, women were included if the duration of infertility was three years (Goverde 2000). A minimum duration of infertility of two years was an inclusion criterion in another trial (Hughes 2004). Infertility for one year was the inclusion criterion in three studies (Soliman 1993; Reindollar 2010; Elzeiny 2014). One study included couples who had a poor prospect of pregnancy, defined as a chance of natural conception within 12 months below 30% (Custers 2011a). One study that included only women between 38 and 42 years of age had an eligibility criterion of six months of attempted conception (Goldman 2014). Four studies included couples with mild male factor infertility (Goverde 2000; Reindollar 2010; van Rumste 2014; Bensdorp 2015), and another included couples with endometriosis American Fertility Society (AFS) stage I (Goverde 2000).
With regard to the studies of expectant management, one (Soliman 1993) included 245 women <40 years of age with varied diagnoses for subfertility and a mean duration of subfertility of 65 months. This study included 35 women with unexplained infertility, who are included in this review. The other 210 women are not included in analysis. The other study of expectant management (Hughes 2004) included women between 18 and 39 years of age with a mean duration of subfertility of 56 months. Most women in this study had unexplained or male factor infertility, and all had patent fallopian tubes. Women in both of these studies had exhausted other treatment options.
Outcomes studied
Primary outcome
Live birth rate (LBR) per woman: Six trials reported LBR per woman or couple as an outcome (Goverde 2000; Hughes 2004; Reindollar 2010; Elzeiny 2014; Goldman 2014; Bensdorp 2015).
Secondary outcomes
Pregnancy rate per woman: Eight trials reported pregnancy rate per woman or couple as an endpoint (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; Elzeiny 2014; Goldman 2014; van Rumste 2014; Bensdorp 2015).
Multiple pregnancy rate (MPR) per woman: Five studies determined MPR per woman (Goverde 2000; Elzeiny 2014; Goldman 2014; van Rumste 2014; Bensdorp 2015).
Ovarian hyperstimulation syndrome (OHSS): Two studies reported incidence of OHSS as an outcome (Goverde 2000; Goldman 2014).
See the Characteristics of excluded studies table.
Excluded studies
See Characteristics of excluded studies.
We excluded eight studies from analysis after checking the full text (Leeton 1987; Crosignani 1991; Jarrell 1993; Raneiri 1995; Zayed 1997; Karande 1998 ; Tanbo 1990; Custers 2012). Two studies did not perform diagnostic stratification before analysis (Jarrell 1993; Karande 1998). One study was a quasi‐randomised trial (Leeton 1987), another study allocated women by pseudo‐randomisation (Zayed 1997), and one did not include an IVF arm. (Custers 2012). We excluded from the current update three studies that had been included in an earlier version of this review: one (Crosignani 1991) because valid pregnancy and LBR data could not be extracted, and two because they compared IVF with gamete intrafallopian transfer (GIFT) (Tanbo 1990; Raneiri 1995), which was not a comparison of interest for this update.
Risk of bias in included studies
See Characteristics of included studies (Figure 1) (Figure 2).
Allocation
Random sequence generation
All eight studies were at low risk of bias for sequence generation.
Of the eight included studies, two used computer‐generated randomisation (Goverde 2000; Elzeiny 2014). One study used a computer‐generated random numbers table (Soliman 1993). Another used an online randomisation programme with biased coin minimisation stratified for study centre (Bensdorp 2015). One study based randomisation on a blocked schedule by using numbered, sealed, opaque envelopes (Hughes 2004). Another study performed randomisation using permuted blocks of varying sizes, stratified by the woman's age (< 35 vs ≥ 35 years), laparoscopy within the past year (yes or no) and study site (Boston IVF or Harvard Vanguard Medical Associates) (Reindollar 2010). One other study performed randomisation using permuted blocks of varying sizes, which were stratified by the woman's age (38th to 41st vs 42nd to 43rd birthday) (Goldman 2014). Another trial used central Internet‐based randomisation, which was stratified for centre (van Rumste 2014).
Allocation concealment
Six studies were at low risk of bias in terms of allocation concealment, and the level of risk was unclear in two. Three studies used sealed envelopes (Goverde 2000; Hughes 2004; Elzeiny 2014). Two studies did not state concealment of allocation (Soliman 1993; van Rumste 2014). Allocation concealment was unclear in one study (Reindollar 2010). One study stated that the allocation sequence was generated by an independent biostatistician and was implemented by an epidemiologist (Goldman 2014). Another study stated that a unique number with allocation code was generated by a Web‐based programme after participant initials and date of birth were entered. Neither recruiters not the trial project group could access the randomisation sequence (Bensdorp 2015).
Blinding
Two studies were at low risk of bias because of blinding, and six were at unclear risk. Blinding of participants and clinicians was not possible because of the nature of the interventions. However, one study stated that investigators were blinded to all outcome determinations (Reindollar 2010), and another study stated that all clinical investigators were blinded to outcome determinations (Goldman 2014). Blinding appears unlikely to affect outcomes measured in the review.
Incomplete outcome data
Seven studies were at low risk of attrition bias, and one was at high risk.
Six trials performed intention‐to‐treat analysis (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; Goldman 2014; Bensdorp 2015). Numbers of withdrawals and dropouts were reported in six trials (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; van Rumste 2014; Bensdorp 2015). One study mentioned the number of women excluded after randomisation but did not perform an intention‐to‐treat analysis (Elzeiny 2014). For this update, we requested from study authors data that were incomplete or that were not clearly reported in the paper (Reindollar 2010; van Rumste 2014).
Selective reporting
To avoid selective reporting and reporting bias, we performed a comprehensive search for eligible studies and ensured that no data were duplicated.
Seven studies were deemed to be at low risk of selective reporting bias, and the risk associated with one was unclear. No evidence suggested that the decision to publish or failure to publish any specific outcomes by authors of included studies was based on perceived statistical significance.
Other potential sources of bias
Six studies were at low risk of other potential biases, and two were at high risk. Seven studies included a priori power calculations in their reports (Soliman 1993; Goverde 2000; Hughes 2004; Reindollar 2010; Elzeiny 2014; Goldman 2014; Bensdorp 2015).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. IVF compared with expectant management for unexplained subfertility.
| IVF compared with expectant management for unexplained subfertility | ||||||
| Population: women with unexplained subfertility Settings: fertility clinic Intervention: IVF Comparison: expectant management | ||||||
| Outcomes | Plain language summary | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| Expectant management | IVF | |||||
| Live birth rate per woman IVF vs expectant management | There is inconclusive evidence to suggest that IVF may result in more births than expectant management | 37 per 1000 | 458 per 1000 (90 to 879) | OR 22 (2.56 to 189.37) | 51 (1 study) | ⊕⊝⊝⊝ Very lowa |
| Pregnancy rate per woman IVF vs expectant management | There is inconclusive evidence to suggest that IVF may result in more clinical pregnancies than expectant management | 127 per 1000 | 320 per 1000 (135 to 588) | OR 3.24 (1.07 to 9.8) | 86 (2 studies) | ⊕⊝⊝⊝ Very lowa |
| Multiple pregnancy rate | Not reported in the included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IVF: In vitro fertilisation; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe GRADE quality rating was downgraded by 3 levels due to very serious imprecision, questionable applicability and (for the analysis of clinical pregnancy) serious inconsistency. Very few events were reported in the included studies (12 births and 18 pregnancies altogether). There was also substantial statistical heterogeneity (I2=80%) in the analysis of clinical pregnancies (with differing directions of effect) and applicability was unclear due to the long duration of unexplained infertility and use of co‐interventions.
Summary of findings 2. IVF compared with unstimulated IUI for unexplained subfertility.
| IVF compared with unstimulated IUI for unexplained subfertility | ||||||
| Population: women with unexplained subfertility Setting: fertility clinic Intervention: IVF Comparison: unstimulated IUI | ||||||
| Outcomes | Plain language summary | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| Unstimulated IUI | IVF | |||||
| Live birth rate IVF vs IUI | Evidence suggests that IVF may result in more births than insemination without using fertility drugs | 160 per 1000 | 320 per 1000 (185 to 494) | OR 2.47 (1.19 to 5.12) | 156 (2 studies) | ⊕⊕⊝⊝ Lowa |
|
Pregnancy rate IVF vs IUI |
It is unclear whether there is a difference in the pregnancy rate resulting from IVF compared with insemination without using fertility drugs, due to insufficient evidence | 121 per 1000 | 400 per 1000 (115 to 775) | OR 4.83 (0.94 to 24.95) | 43 (1 study) | ⊕⊕⊝⊝ Very lowb |
| Multiple pregnancy rate | It is unclear whether there is a difference in the multiple pregnancy rate resulting from IVF compared with insemination without using fertility drugs, due to insufficient evidence | 30 per 1000 | 31 per 1000 (1 to 460) | OR 1.03 (0.04 to 27.29) | 43 (1 study) | ⊕⊝⊝⊝ Very lowc |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IUI: Intrauterine insemination; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe GRADE quality rating was downgraded by 2 levels due to serious imprecision: There were only 44 events. There was also substantial statistical heterogeneity (I2=60%), though the direction of effect was consistent. bThe GRADE quality rating was downgraded by 3 levels due to very serious imprecision, with only 8 events. The confidence interval is compatible with no difference between the groups or with a large benefit in the IVF group.
cThe GRADE quality rating was downgraded by 3 levels due to very serious imprecision: there was only one event in this analysis
Summary of findings 3. IVF compared with IUI + superovulation for unexplained subfertility.
| IVF compared with IUI + superovulation for unexplained subfertility | ||||||
| Population: women with unexplained subfertility Setting: fertility clinic Intervention: IVF Comparison: IUI + superovulation | ||||||
| Outcomes | Plain language summary | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| IUI + superovulation | IVF | |||||
|
Live birth rate in treatment‐naive women IVF vs IUI + gonadotropins |
In treatment‐naive women there is no conclusive evidence of a difference in live birth rates between IVF and insemination using injectable fertility drugs | 273 per 1000 | 308 per 1000 (264 to 360) | OR 1.27 (0.94 to 1.73) |
745 (4 studies) | ⊕⊕⊕⊝ Moderatea |
|
Live birth rate in pretreated women IVF vs IUI + gonadotropins |
In women pretreated with oral fertility drugs IVF leads to more live births than insemination using injectable fertility drugs | 219 per 1000 | 523 per 1000 (374 to 731) | OR 3.90 (2.32 to 6.57) |
280 (1 study) | ⊕⊕⊕⊝ Moderateb |
|
Live birth rate in treatment‐naive women IVF vs IUI + CC |
In treatment‐naive women there is no conclusive evidence of a difference in live birth rates between IVF and insemination using injectable fertility drugs | 154 per 1000 | 314 per 1000 (148 to 668) | OR 2.51 (0.96 to 6.55) |
103 (1 study) | ⊕⊕⊝⊝ Lowc |
| Multiple pregnancy rate | In treatment‐naive women there is no evidence of a difference in multiple pregnancy rates between IVF and insemination using injectable fertility drugs | 58 per 1000 | 47 per 1000 (28 to 78) | OR 0.81 (0.47 to 1.39) | 848 (4 studies) | ⊕⊕⊕⊝ Moderated |
| Incidence of OHSS | In treatment‐naive women there is no evidence of a difference in OHSS rates between IVF and insemination using injectable fertility drugs | 58 per 1000 | 66 per 1000 (26 to 158) | OR 1.15 (0.43 to 3.06) | 324 (2 studies) | ⊕⊕⊝⊝ Lowe |
| *The basis for the assumed risk is the median risk in the control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CC: Clomiphene citrate; CI: Confidence interval; IUI: Intrauterine insemination; IVF: In vitro fertilisation; OHSS: Ovarian hyperstimulation syndrome; OR: Odds ratio ;RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe GRADE quality rating was downgraded by 1 level due to serious imprecision: the confidence interval is compatible with no difference between the interventions or with meaningful benefit from IVF. bThe GRADE quality rating was downgraded by 1 level due to the relatively small number of events (n=97) in the single included trial.
cThe GRADE quality rating was downgraded by 2 levels due to very serious imprecision: there were only 24 events and the confidence interval is compatible with no difference between the interventions or with meaningful benefit from IVF
dThe GRADE quality rating was downgraded by 1 level due to serious imprecision: the confidence interval is compatible with no difference between the interventions or with meaningful benefit in either arm. eThe GRADE quality rating was downgraded by 2 levels due to serious imprecision, risk of bias in one trial and the small number of events in the included trials. The confidence interval is compatible with no difference between the interventions or with meaningful benefit in either arm.
1 IVF versus expectant management
This was tested in two trials (Soliman 1993; Hughes 2004).
Primary outcome
1.1 Live birth rate (LBR)
LBR per woman or couple with a single cycle of IVF was significantly higher than with three months of expectant management (odds ratio (OR) 22.0, 95% confidence interval (CI) 2.56 to 189.38, 51 women). This was tested in a single trial (Hughes 2004) (Analysis 1.1; Figure 4). The quality of evidence was deemed to be very low.
1.1. Analysis.
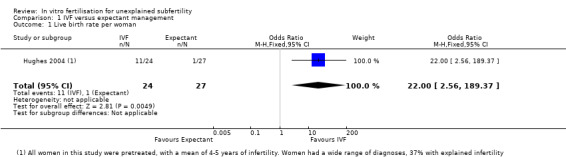
Comparison 1 IVF versus expectant management, Outcome 1 Live birth rate per woman.
4.
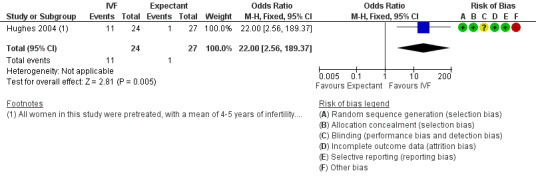
Forest plot of comparison: 1 IVF versus expectant management, outcome: 1.1 Live birth rate per woman.
Secondary outcomes
1.2 Clinical pregnancy rate (CPR)
CPR per woman or couple associated with a single cycle of IVF was significantly higher than with three to six months of expectant management (OR 3.24, 95% CI 1.07 to 9.80, two RCTs, 86 women, I2 = 80%, 28.9% vs 12.2%) (Analysis 1.2; Figure 5). The quality of evidence was deemed to be very low. Heterogeneity was high, as the studies had differing directions of effect.
1.2. Analysis.
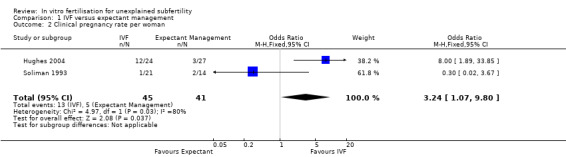
Comparison 1 IVF versus expectant management, Outcome 2 Clinical pregnancy rate per woman.
5.
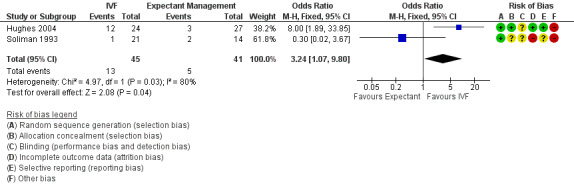
Forest plot of comparison: 1 IVF versus expectant management, outcome: 1.2 Clinical pregnancy rate per woman.
These studies did not report the other review outcomes (MPR, OHSS, miscarriage).
2. IVF versus unstimulated IUI
Two trials compared the effectiveness of IVF versus unstimulated IUI.
One trial compared the effectiveness of IVF (six cycles) versus unstimulated IUI (six cycles) (Goverde 2000). The second trial compared the effectiveness of one cycle of IVF versus one cycle of unstimulated IUI (Elzeiny 2014).
Primary outcome
2.1 Live birth rate (LBR)
IVF was associated with a higher live birth rate than IUI (OR 2.47, 95% CI 1.19 to 5.12, two RCTs, 156 women, I2 = 60%) (Analysis 2.1; Figure 6). The quality of evidence was deemed to be low.
2.1. Analysis.
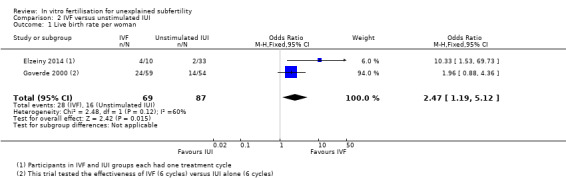
Comparison 2 IVF versus unstimulated IUI, Outcome 1 Live birth rate per woman.
6.
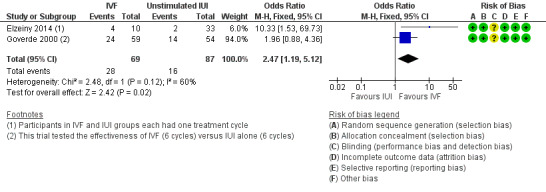
Forest plot of comparison: 2 IVF versus unstimulated IUI, outcome: 2.1 Live birth rate per woman.
Secondary outcomes
2.2 Clinical pregnancy rate (CPR)
There was no evidence of a difference between IVF and IUI in CPR (OR 4.83, 95% CI 0.94 to 24.95, one RCT, 44 women, I2 = 80%) (Analysis 2.2;). The quality of evidence was deemed to be low.
2.2. Analysis.
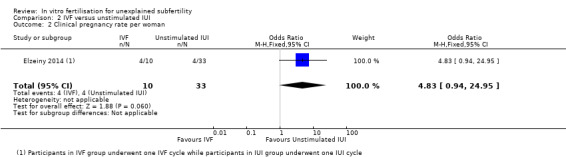
Comparison 2 IVF versus unstimulated IUI, Outcome 2 Clinical pregnancy rate per woman.
2.3 Multiple pregnancy rate (MPR)
There was no evidence of a difference in MPR between the two groups (OR 1.03, 95% CI 0.04 to 27.29, one RCT, 44 women, I2 not applicable (Analysis 2.3)). The quality of evidence was deemed to be very low.
2.3. Analysis.
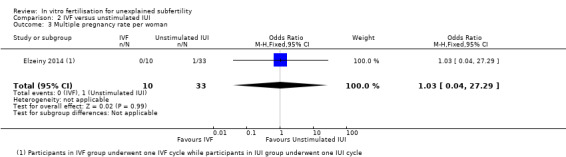
Comparison 2 IVF versus unstimulated IUI, Outcome 3 Multiple pregnancy rate per woman.
These studies did not report the other review outcomes (OHSS, miscarriage).
3. IVF versus IUI + ovarian stimulation with gonadotropins (IUI + gonadotropins) or clomiphene citrate (IUI + CC)
Five trials compared effectiveness of IVF versus IUI + gonadotropins (Goverde 2000; Reindollar 2010; Goldman 2014; van Rumste 2014; Bensdorp 2015).
Goverde 2000: This trial compared effectiveness of a maximum of six cycles of IUI after mild ovarian hyperstimulation with IVF.
Reindollar 2010: This trial compared three cycles of IUI + gonadotropins versus six cycles of IVF in women pretreated with clomiphene + IUI.
Goldman 2014: This trial compared two cycles of clomiphene + IUI versus one cycle of IVF, and two cycles of recombinant FSH + IUI versus one cycle of IVF.
van Rumste 2014: This trial compared three cycles of IUI + gonadotropins versus one cycle of IVF.
Bensdorp 2015: This trial compared three cycles of IVF‐SET(plus subsequent cryo cycles) versus six cycles of IUI + gonadotropins.
Primary outcome
3.1 Live birth rate (LBR)
Five studies reported live birth rates (Goverde 2000; Reindollar 2010; Goldman 2014; van Rumste 2014; Bensdorp 2015). These studies were not pooled because of high statistical heterogeneity (I2 = 93.3%). Heterogeneity was eliminated when studies were stratified by pretreatment status, that is, treatment‐naive women underwent IUI along with gonadotropins (Goverde 2000; van Rumste 2014;Goldman 2014; Bensdorp 2015), or treatment‐naive women underwent IUI along with CC (Goldman 2014) or women were pretreated (Reindollar 2010).
IVF versus IUI + gonadotropins
Among treatment‐naive women, there was no evidence of a difference in LBR between IVF and IUI + gonadotropins (OR 1.27, 95% CI 0.94 to 1.73, four RCTs, 745 women, I2 = 26%, moderate‐quality evidence), but in pretreated women, a significantly higher LBR was noted in those who underwent IVF compared with IUI + gonadotropins (OR 3.90, 95% CI 2.32 to 6.57, one RCT, 280 women) (Analysis 3.1; Figure 7). Evidence was of moderate quality.
3.1. Analysis.
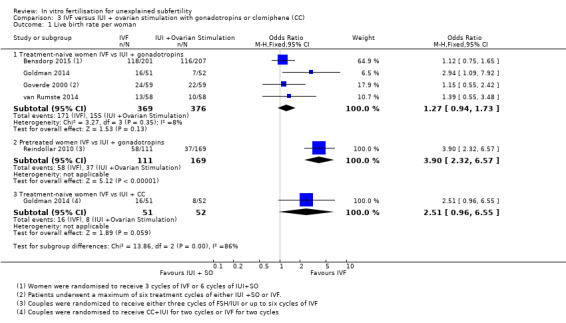
Comparison 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), Outcome 1 Live birth rate per woman.
7.
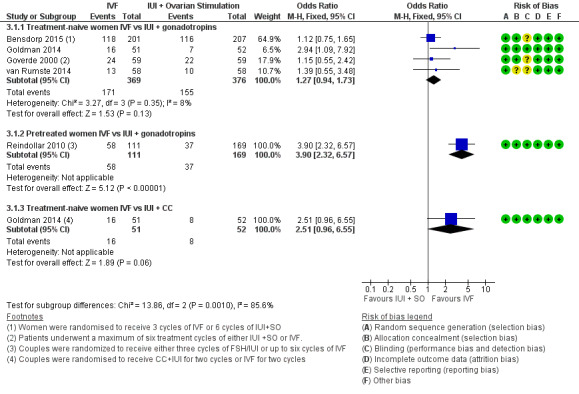
Forest plot of comparison: 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), outcome: 3.1 Live birth rate per woman.
IVF versus IUI + CC
There was no evidence of a difference in LBR between IVF and IUI + CC (OR 2.51, 95% CI 0.96 to 6.55, one RCT, 103 women). Evidence was of low quality.
Secondary outcomes
3.2 Clinical pregnancy rate (CPR)
Four studies reported CPR per woman (Reindollar 2010; Goldman 2014; van Rumste 2014; Bensdorp 2015). These studies were not pooled because statistical heterogeneity was high (I2 = 96.3%). Heterogeneity was eliminated when studies were stratified by pretreatment status, that is, treatment‐naive women underwent IUI with gonadotropins (van Rumste 2014;Goldman 2014; Bensdorp 2015), or treatment‐naive women underwent IUI with CC (Goldman 2014) or women were pretreated (Reindollar 2010).
IVF versus IUI + gonadotropins
Among treatment‐naive women, significant differences between IVF and IUI + gonadotropins were seen in CPR (OR 1.45, 95% CI 1.03 to 2.03, three RCTs, 627 women, I2 = 73%) (Analysis 3.2), but in pretreated women, the pregnancy rate was higher among those who underwent IVF compared with IUI + gonadotropins (OR 14.13, 95% CI 7.57 to 26.38, one RCT, 280 women). These results should be interpreted with caution because of the wide confidence interval (Analysis 3.2; Figure 8). Evidence was of moderate quality.
3.2. Analysis.
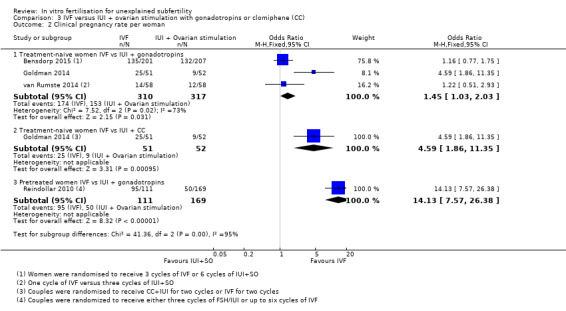
Comparison 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), Outcome 2 Clinical pregnancy rate per woman.
8.
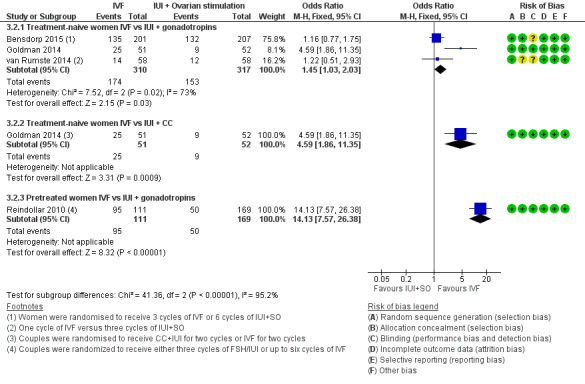
Forest plot of comparison: 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), outcome: 3.2 Clinical pregnancy rate per woman.
IVF versus IUI + CC
For the subgroup of treatment‐naive women who received either IVF or IUI + CC, pregnancy rates were higher in the IVF group (OR 4.59, 95% CI 1.86 to 11.35, one RCT, 103 women)
3.3 Multiple pregnancy rate (MPR)
Four trials reported MPR per woman (Goverde 2000; Goldman 2014; van Rumste 2014; Bensdorp 2015) (Analysis 3.3; Figure 9).
3.3. Analysis.
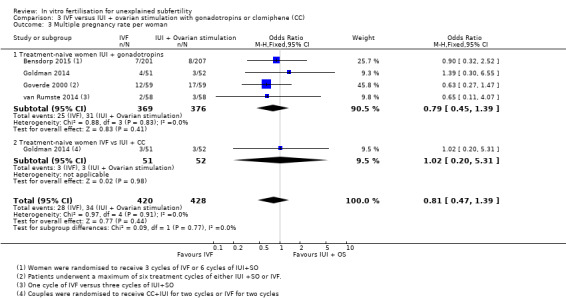
Comparison 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), Outcome 3 Multiple pregnancy rate per woman.
9.
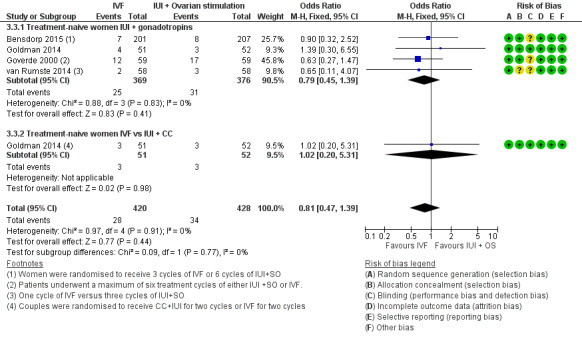
Forest plot of comparison: 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), outcome: 3.3 Multiple pregnancy rate per woman.
IVF versus IUI + gonadotropins
Moderate‐quality evidence showed no evidence of a difference in MPR between treatment‐naive women who underwent IVF and those given IUI + gonadotropins (OR 0.79, 95% CI 0.45 to 1.39, four RCTs, 745 women, I2=0%)
IVF versus IUI + CC
There was no evidence of a difference in MPR between women who had IVF compared with those given IUI + CC (OR 1.02, 95% CI 0.20 to 5.31, one RCT, 103 women) (Analysis 3.3; Figure 9).
3.4 Incidence of ovarian hyperstimulation syndrome (OHSS)
Two studies determined the incidence of OHSS (Goverde 2000; Goldman 2014).
IVF versus IUI + gonadotropins
Low‐quality evidence showed no evidence of a difference in OHSS rate between treatment‐naive women who underwent IVF and those given IUI + gonadotropins (OR 1.23, 95% CI 0.36 to 4.14, two RCTs, 221 women, I2=0%)
IVF versus IUI + CC
Low‐quality evidence showed no evidence of a difference in OHSS rate between the IVF group and the IUI + CC group (OR 1.02, 95% CI 0.20 to 5.31, one RCT, 103 women, I2 = 0%). Evidence was of low quality (Analysis 3.4).
3.4. Analysis.
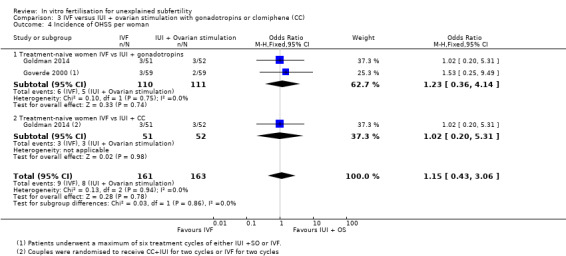
Comparison 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), Outcome 4 Incidence of OHSS per woman.
3.5 Miscarriage rate
One study reported miscarriage rate per woman in treatment‐naive women (Goldman 2014).
IVF versus IUI+ gonadotropins
There was no evidence of a difference in miscarriage rates between the IVF group and the IUI+ gonadotropins group (OR 1.16, 95% CI 0.44 to 3.02, one RCT, 103 women)
IVF versus IUI + CC
There was no evidence of a difference in miscarriage rates between the IVF group and the IUI+ CC group (OR 1.16, 95% CI 0.44 to 3.02, one RCT, 103 women). Evidence was of low quality (Analysis 3.5).
3.5. Analysis.
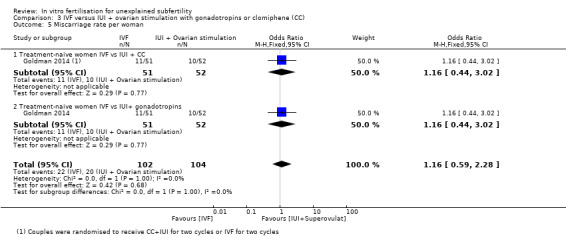
Comparison 3 IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC), Outcome 5 Miscarriage rate per woman.
Discussion
Summary of main results
See Table 1, Table 2 and Table 3.
In vitro fertilisation (IVF) results in higher live birth rates (LBRs) compared with expectant management or unstimulated intrauterine insemination (IUI). LBRs with IVF are also higher compared with those seen with IUI + gonadotropins in women pretreated with clomiphene citrate (CC), but no evidence suggests differences in LBR between IVF and IUI + gonadotropins in treatment‐naive women. Nor does evidence show differences in LBR between IVF and IUI + CC. IVF results in higher clinical pregnancy rates (CPR) compared to IUI + gonadotropins in treatment naive women. Adverse events associated with these interventions have not been adequately reported; additional research is necessary.
Overall completeness and applicability of evidence
Evidence for each comparison was limited. The primary outcome for this review was LBR per woman. Only one study (Bensdorp 2015) followed couples for 12 months after randomisation, during which time they underwent a maximum of three IVF cycles with subsequent transfer of a single fresh and (when appropriate) frozen embryo, or a maximum of six cycles of IUI + gonadotropins. Duration of infertility among couples included in the trials varied significantly. No trials compared IVF with IUI + letrozole. The paucity of trials and possible clinical heterogeneity among included trials suggest that evidence for the effectiveness of IVF is inconclusive.
Meta‐analysis was possible for three comparisons (IVF vs expectant management, IVF vs unstimulated IUI, IVF vs IUI + gonadotropins), but as few outcomes were reported, pooling was limited because data were insufficient. One of the included trials, which compared IVF with expectant management, dates from 1993 (Soliman 1993). IVF versus CC + IUI was represented by a single trial. Although risk of bias was not substantial in the trial included in this comparison, it is difficult to be confident about this, as all trials share similar weaknesses, as discussed above. Adverse events associated with these interventions have not been adequately reported.
The applicability of studies comparing IVF versus expectant management is questionable, as they included extensively pretreated women who had been subfertile for several years (mean 58‐65 months) and the duration of expectant management was only three to six months.
Small studies of treatment‐naive women found no significant differences in LBR per woman between IVF and IUI + gonadotropins.Clinical pregnancy rates were significantly higher with IVF compared with IUI+ gonadotropins. However, a large study of women pretreated with CC + IUI reported a significant increase in pregnancy and LBR rates following IVF. Couples in this study (Reindollar 2010) were randomly assigned to (1) a conventional pathway involving CC plus intrauterine insemination (CC + IUI) followed by IUI + gonadotropins, then IVF, or (2) an accelerated pathway (CC + IUI followed by six cycles of IVF). Randomly assigned groups included similar numbers of women. However, study populations in the other studies in this comparison (Goverde 2000; Goldman 2014; van Rumste 2014; Bensdorp 2015) differed from those of Reindollar 2010, as women in these studies did not undergo CC + IUI treatment before receiving IUI + gonadotropins or IVF. Despite pretreatment with CC + IUI in both randomly assigned arms, we believe the comparison between IUI + gonadotropins and IVF is valid. Thus our analysis suggests that IVF may be more effective than IUI + gonadotropins in terms of pregnancy rate in treatment naive women and IVF may be more effective than IUI+ gonadotropins in terms of pregnancy rate and LBR per woman among pretreated women, but these results should be interpreted with caution. The single study that compared CC + IUI with IVF in women 38 to 42 years of age (Goldman 2014) also showed that pregnancy rates with IVF were significantly higher than with CC + IUI.
Multiple pregnancy, an important adverse effect of superovulation, was seen in four studies that compared IVF with IUI + gonadotropins (Goverde 2000; Goldman 2014; van Rumste 2014; Bensdorp 2015). Results of the analysis suggest higher MPRs in women who underwent IUI + gonadotropins compared with IVF, but findings did not reach statistical significance. The maximum number of embryos transferred was two among women younger than 35 years, and three in women 35 years of age and older in one study (Goverde 2000); up to two embryos were transferred in the second study (van Rumste 2014). One good‐quality embryo was transferred in one study (van Rumste 2014), and two embryos were transferred if no good‐quality embryos were available. Elective single embryo transfer (eSET) was followed in one study (Bensdorp 2015). A further study used American Society for Reproductive Medicine (ASRM) guidelines for day 3 embryo transfers (Goldman 2014). Both twin pregnancies that occurred in the IVF group in one included study occurred after transfer of two non‐top‐quality embryos (van Rumste 2014). Protocols used for ovarian stimulation also differed among the studies that tested this comparison (Goverde 2000; Goldman 2014; van Rumste 2014). A long protocol was followed that included a gonadotropin‐releasing hormone agonist and gonadotropins in two studies (Goverde 2000; van Rumste 2014). One study (Goldman 2014) used an IVF protocol consisting of 21 days of an oral contraceptive followed by a microdose gonadotropin‐releasing hormone agonist, followed by the addition of gonadotropins at a twice‐daily dosage for three days, beginning on day 3 or 4 of the agonist. Standardisation of the number of embryos transferred and the protocols used for ovarian stimulation should be considered in trials related to subfertility.
Quality of the evidence
Few high‐quality randomised controlled trials (RCTs) have conducted head‐to‐head comparisons of relevant interventions in the context of unexplained subfertility. Most studies are methodologically inadequate. Only eight trials were eligible for inclusion in the final analysis. Meta‐analysis was possible in three comparisons. One comparison was represented by a single trial only. This was compounded by insufficient information on some outcomes. All trials reported LBR per woman or couple, although duration of follow‐up in most trials was limited. The method of randomisation was unclear in some trials, and most had small sample sizes. Blinding could not be performed in most studies because of the nature of the interventions, but this was unlikely to affect outcomes measured in the review. One trial was unpublished. Another study reporting only per‐cycle data was excluded from the review (Crosignani 1991).
Existing trials have several limitations. The definition of unexplained infertility and the clinical procedures and protocols used vary among studies. It is unreasonable to expect absolute experimental uniformity among study centres, and different centres inevitably display variation in the application of assisted reproduction treatments (ARTs). Duration of follow‐up is limited and unequal between studies. Sample sizes of the studies included in this review are also limited. Most trials show poor methodological quality. Methods of randomisation and reasons for and numbers of dropouts and withdrawals often are not clearly stated. Inadequate methods of randomisation can lead to bias in estimates of treatment effects (Schulz 1995). Allocation concealment is inadequate in most trials. Intention‐to‐treat analysis is not always performed, possibly leading to exaggerated estimates of treatment effect and possible influence on inferences and clinical decisions. Most trials have determined pregnancy rates per cycle as the endpoint, but LBR per woman is the most important outcome to the couple. The latest updated Cochrane guidelines for analysing and presenting results emphasise the use of pregnancy and LBRs per woman or couple in the final meta‐analysis. However, in practice such data are seldom available. Therefore, only a limited number of trials could be included in this review. Most trials had limited duration of follow‐up. Information on costs associated with various fertility treatments is also very limited. Reported cost‐effectiveness analyses are lacking in their definitions of outcome measures and extent of cost analysis.
Clinical heterogeneity between trials is present as the result of differences between studies in terms of investigation protocols and inclusion criteria. The protocols used for ovarian stimulation also differ. Timing of IUI and method of sperm preparation are not clearly defined in some studies. The sample size of these studies is limited. The following three analyses are included.
IVF versus expectant management for unexplained subfertility
Evidence for live birth or pregnancy per randomly assigned woman was downgraded by three levels because of very serious imprecision: The 95% confidence interval (CI) was too large, and relatively few events were reported in the included studies. Moreover, applicability was questionable (with respect to duration of unexplained infertility and co‐interventions) (Table 1).
IVF versus unstimulated IUI for unexplained subfertility
Evidence for live birth or pregnancy per randomly assigned woman was downgraded by two levels because of serious imprecision: The 95% CI was relatively wide. Besides, only two studies included a limited number of participants (n = 156) (Table 2).
IVF versus IUI + ovarian simulation with gonadotropins or clomiphene for unexplained subfertility
Evidence for outcomes in this comparison was downgraded from one to two levels for various reasons (Table 3), including imprecision, risk of bias in one trial and few events in the included trials.
We identified three studies that determined the incidence of ovarian hyperstimulation syndrome (OHSS) in women who underwent IVF and IUI + gonadotropins (Goverde 2000; Goldman 2014; van Rumste 2014). However, as data were reported per cycle in one of these trials (van Rumste 2014), only two trials were included in the analysis for this outcome (Goverde 2000; Goldman 2014). Although no significant differences were noted in the incidence of OHSS between these two treatment groups, the sample size was too small to allow firm conclusions. In the trial that reported OHSS per cycle (van Rumste 2014), two of 48 couples in the IVF group that reached embryo transfer were cancelled as the result of OHSS, and of the 142 started cycles of IUI + gonadotropins, 14 cycles were cancelled because of the risk of multiple pregnancy (10%).
Potential biases in the review process
Definition of unexplained infertility
Wide inconsistency can be seen in the definition of unexplained infertility. The definition used for this review follows here.
Couples with unexplained infertility were defined as:
couples who have tried to conceive for 1 year;
those with no abnormality identified during the full infertility investigation, laboratory evidence of ovulation (normal luteal progesterone in serum), evidence of tubal patency and exclusion of other tubal or pelvic abnormalities by hysterosalpingography or laparoscopy, or both; and
those producing a normal semen sample according to the definition of normality provided by the World Health Organization (WHO), in accordance with the year the study was performed.
Only three trials reported secondary outcomes such as costs per cycle and costs per couple. Economic evaluation of fertility treatment is an important factor in decision making. Trials evaluating the cost‐effectiveness of available treatments for unexplained infertility are very limited. To date, no studies have compared costs of IVF treatment versus expectant management and CC in the context of RCTs. Only four studies of cost‐effectiveness in ART were based on RCTs (Karande 1998; Goverde 2000; Reindollar 2010; van Rumste 2014). The study of Karande 1998 compared an assumed equity in costs based on mathematical modelling between IVF as first‐line treatment and a traditional treatment algorithm and showed a much higher cost per pregnancy for IVF. Goverde 2000, in a prospective, parallel‐group study, reported that costs of one IVF treatment cycle were 3.5 and 5 times higher than those of one IUI treatment for stimulated and spontaneous cycles, respectively. van Rumste 2014 reported an additional cost of EUR 600 per couple with IVF with eSET compared with IUI + superovulation. Reindollar 2010 also reported cost‐effectiveness of various treatments; however, specific costs for IVF and IUI + superovulation could not be extracted from the data provided.
Agreements and disagreements with other studies or reviews
No other systematic reviews on interventions for unexplained infertility are currently available. Most interventions have been introduced into clinical practice without adequate testing in the context of large RCTs.
Authors' conclusions
Implications for practice.
IVF may be associated with higher live birth rates than expectant management, but there is insufficient evidence to draw firm conclusions. IVF may also be associated with higher live birth rates than unstimulated IUI. In women pretreated with clomiphene + IUI, IVF appears to be associated with higher birth rates than IUI plus gonadotropins. However in women who are treatment‐naive there is no conclusive evidence of a difference in live birth rates between IVF and IUI plus gonadotrophins or between IVF and IUI plus clomiphene. Adverse events associated with these interventions could not be adequately assessed owing to lack of evidence.
Clinicians and couples should balance the invasive nature of IVF and related costs against chances of success with other treatment modalities.
Implications for research.
Some of the difficulties encountered in preparation of this review can be avoided by planning infertility trials with similar study designs and methods and presentation of results. This will allow pooling of data for statistical meta‐analysis.
Large RCTs with sufficient power are warranted. Unexplained infertility should be clearly defined. Participant characteristics should be clear (age, duration of infertility, parity, infertility investigations and previous therapy). These trials should have a prolonged duration of follow‐up (e.g. six cycles of treatment). Treatment protocols, methods of sperm preparation, numbers of embryos transferred and inclusion and exclusion criteria should be clearly stated.
Outcome measures should include LBRs per woman. As comparison of cumulative LBRs is also important, trialists should endeavour to follow participants until frozen transfers accruing from a single oocyte retrieval procedure are completed. In trials in which controlled ovarian hyperstimulation is used, the number of multiple pregnancies and the incidence of OHSS should be stated.
Future trials should use adequate methods of randomisation, and numbers of and reasons for dropouts and withdrawals should be clearly stated. Allocation concealment should be adequate, and intention‐to‐treat analysis performed. A power calculation should be performed with a clear description of the improvement in treatment outcome that is considered clinically significant. Use of parallel‐group rather than cross‐over trials is favoured in the study of events, as the latter may exaggerate the effectiveness of treatment.
IVF versus expectant management and IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene or letrozole require comparison in large RCTs with participants of varying prognostic profiles. It is important to identify the group of patients with certain prognostic profiles who would benefit by proceeding from expectant management to more invasive treatment. The most appropriate time to switch over from expectant management in this group should be identified.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2015 | New search has been performed | Three new studies have been added (Elzeiny 2014; Goldman 2014; Bensdorp 2015). A follow up of a study previously included as two separate studies (Custers et al 2011; van Rumste 2009) has been published and is included in this review (van Rumste 2014). IVF versus clomiphene has been removed from the comparisons. IVF versus IUI + clomiphene has been added to the comparisons. |
| 5 May 2015 | New citation required but conclusions have not changed | The conclusions of this review have not changed with the addition of new evidence. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 23 February 2011 | New citation required but conclusions have not changed | Three studies have been excluded: two (Tanbo 1990; Raneiri 1995) because gamete intrafallopian transfer (GIFT) has been removed from the comparisons, as this treatment is rarely used now, and one (Crosignani 1991) as only per‐cycle data were reported. Per‐cycle data from all comparisons have been deleted. One new study has been added to the comparison IVF versus unstimulated IUI (Elzeiny 2014). Two new studies have been added (Goldman 2014; Bensdorp 2015) to the comparison of in vitro fertilisation (IVF) versus intrauterine insemination plus ovarian stimulation (IUI + SO). One new study has been added to the comparison IVF versus IUI + clomiphene (Goldman 2014) |
| 1 September 2010 | New citation required and conclusions have changed | Substantive amendments have been made |
| 12 November 2008 | Amended | This review has been converted to the new review format |
Acknowledgements
Thanks to the Gynaecology and Fertility Group (CGF) for support provided.
Appendices
Appendix 1. MDSG search string
MDSG search string for ZP672 04/05/2015
Keywords CONTAINS "*Embryo Transfer" or "IVF" or "in vitro fertilisation" or "in‐vitro fertilisation procedure" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection cycle" or "zygote intrafallopian transfer" or "zygote intrafallopian tube transfer" or "zygote transfer" or "ET" or "ZIFT" or Title CONTAINS "*Embryo Transfer" or "IVF" or "in vitro fertilisation" or "in‐vitro fertilisation procedure" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection cycle" or "zygote intrafallopian transfer" or "zygote intrafallopian tube transfer" or "zygote transfer" or "ET" or "ZIFT"
AND
Keywords CONTAINS "expectant management" or "conservative treatment" or "*Clomiphene" or "clomiphene citrate" or "insemination" or "insemination‐fallopian tube sperm perfusion" or "insemination‐utero tubal" or "insemination, intrauterine" or "insemination, intratubal" or "artificial insemination" or "IUI" or "Intrauterine Insemination" or "intrautero tuboperitoneal insemination" or "gamete intrafallopian transfer" or "gamete intrauterine transfer" or "GIFT" or "waiting group" or "conventional insemination" or "conventional" or Title CONTAINS "expectant management" or "conservative treatment" or "*Clomiphene" or "clomiphene citrate" or "insemination" or "insemination‐fallopian tube sperm perfusion" or "insemination‐utero tubal" or "insemination, intrauterine " or "insemination, intratubal" or "artificial insemination" or "IUI" or "Intrauterine Insemination" or "intrautero tuboperitoneal insemination" or "gamete intrafallopian transfer" or "gamete intrauterine transfer" or "GIFT" or "waiting group" or "conventional insemination" or "conventional"
AND
Keywords CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic subfertility" or "idiopathic‐unexplained" or Title CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic subfertility" or "idiopathic‐unexplained"
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp zygote intrafallopian transfer/ (33616) 2 (in Vitro adj2 fertili$).tw. (19493) 3 (ivf or icsi or ZIFT).tw. (20232) 4 (intracytoplas$ adj2 sperm).tw. (5395) 5 zygote intrafallopian transfer$.tw. (85) 6 (embryo transfer$ or ET).tw. (183294) 7 invitro fertili$.tw. (21) 8 or/1‐7 (217509) 9 (expect$ adj2 manage$).tw. (2882) 10 (conservative treat$ or conservative therap$).tw. (29266) 11 exp Clomiphene/ (4871) 12 clomi$.tw. (7512) 13 exp insemination, artificial/ or exp insemination, artificial, homologous/ (10127) 14 (intrauter$ adj5 inseminat$).tw. (1987) 15 (intra‐uter$ adj5 inseminat$).tw. (203) 16 (artificial adj2 inseminat$).tw. (5323) 17 IUI.tw. (1284) 18 (wait adj1 see).tw. (4) 19 (conventional$ adj2 treat$).tw. (18913) 20 (conventional$ adj2 therap$).tw. (16421) 21 or/9‐20 (86888) 22 8 and 21 (4387) 23 randomized controlled trial.pt. (392802) 24 controlled clinical trial.pt. (89296) 25 randomized.ab. (318096) 26 placebo.tw. (165979) 27 clinical trials as topic.sh. (172395) 28 randomly.ab. (229531) 29 trial.ti. (137219) 30 (crossover or cross‐over or cross over).tw. (63820) 31 or/23‐30 (976870) 32 exp animals/ not humans.sh. (4028709) 33 31 not 32 (899597) 34 22 and 33 (412)
Appendix 3. EMBASE search strategy
Database: Embase <1980 to 2015 Week 18> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp fertilization in vitro/ (40286) 2 exp intracytoplasmic sperm injection/ (13555) 3 exp embryo transfer/ (21998) 4 (in?Vitro adj2 fertili$).tw. (131) 5 (ivf or icsi or ZIFT).tw. (31068) 6 (intracytoplas$ adj2 sperm).tw. (6942) 7 zygote intrafallopian transfer$.tw. (94) 8 embryo transfer$.tw. (12285) 9 invitro fertili$.tw. (126) 10 or/1‐9 (62146) 11 exp conservative treatment/ (406649) 12 (expect$ adj2 manage$).tw. (3988) 13 (conservative treatment or conservative therap$).tw. (35481) 14 exp clomifene/ (5121) 15 clomi$.tw. (9200) 16 exp artificial insemination/ (12692) 17 (intrauter$ adj5 inseminat$).tw. (2722) 18 (intra‐uter$ adj5 inseminat$).tw. (326) 19 (artificial adj2 inseminat$).tw. (5059) 20 IUI.tw. (2103) 21 (wait adj1 see).tw. (11) 22 (conventional$ adj2 treat$).tw. (25546) 23 (conventional$ adj2 therap$).tw. (22310) 24 or/11‐23 (501244) 25 Clinical Trial/ (843553) 26 Randomized Controlled Trial/ (369125) 27 exp randomization/ (66088) 28 Single Blind Procedure/ (20079) 29 Double Blind Procedure/ (119899) 30 Crossover Procedure/ (42594) 31 Placebo/ (255278) 32 Randomi?ed controlled trial$.tw. (114897) 33 Rct.tw. (16728) 34 random allocation.tw. (1402) 35 randomly allocated.tw. (22147) 36 allocated randomly.tw. (2011) 37 (allocated adj2 random).tw. (721) 38 Single blind$.tw. (15643) 39 Double blind$.tw. (149839) 40 ((treble or triple) adj blind$).tw. (440) 41 placebo$.tw. (212811) 42 prospective study/ (287679) 43 or/25‐42 (1452441) 44 case study/ (31373) 45 case report.tw. (279675) 46 abstract report/ or letter/ (921238) 47 or/44‐46 (1226085) 48 43 not 47 (1413427) 49 10 and 24 and 48 (908)
Appendix 4. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <May 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp zygote intrafallopian transfer/ (1755) 2 (in Vitro adj2 fertili$).tw. (1600) 3 (ivf or icsi or ZIFT).tw. (2762) 4 (intracytoplas$ adj2 sperm).tw. (531) 5 zygote intrafallopian transfer$.tw. (9) 6 (embryo transfer$ or ET).tw. (11550) 7 invitro fertili$.tw. (3) 8 or/1‐7 (14071) 9 (expect$ adj2 manage$).tw. (407) 10 (conservative treat$ or conservative therap$).tw. (1609) 11 exp Clomiphene/ (387) 12 clomi$.tw. (1581) 13 exp insemination, artificial/ or exp insemination, artificial, homologous/ (294) 14 (intrauter$ adj5 inseminat$).tw. (499) 15 (intra‐uter$ adj5 inseminat$).tw. (43) 16 (artificial adj2 inseminat$).tw. (99) 17 IUI.tw. (380) 18 (wait adj1 see).tw. (88) 19 (conventional$ adj2 treat$).tw. (4206) 20 (conventional$ adj2 therap$).tw. (3089) 21 or/9‐20 (10790) 22 8 and 21 (502)
Appendix 5. PsycINFO search strategy
Database: PsycINFO <1806 to April Week 4 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (in?Vitro adj2 fertili$).tw. (3) 2 (ivf or icsi or ZIFT).tw. (435) 3 (intracytoplas$ adj2 sperm).tw. (43) 4 zygote intrafallopian transfer$.tw. (2) 5 embryo transfer$.tw. (101) 6 invitro fertili$.tw. (3) 7 (expect$ adj2 manage$).tw. (506) 8 (conservative treat$ or conservative therap$).tw. (301) 9 clomi$.tw. (1789) 10 (intrauter$ adj5 inseminat$).tw. (21) 11 (intra‐uter$ adj5 inseminat$).tw. (0) 12 (artificial adj2 inseminat$).tw. (235) 13 IUI.tw. (24) 14 (wait adj1 see).tw. (1) 15 (conventional$ adj2 treat$).tw. (1331) 16 (conventional$ adj2 therap$).tw. (754) 17 or/1‐6 (515) 18 or/7‐16 (4831) 19 17 and 18 (30) 20 random.tw. (43430) 21 control.tw. (337122) 22 double‐blind.tw. (18791) 23 clinical trials/ (8613) 24 placebo/ (4057) 25 exp Treatment/ (613111) 26 or/20‐25 (940087) 27 19 and 26 (8)
Appendix 6. CINAHL search strategy
CINAHL search strategy for ZP672 on 04/05/2015
| # | Query | Results |
| S35 | S22 AND S34 | 86 |
| S34 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 957,665 |
| S33 | TX allocat* random* | 4,263 |
| S32 | (MH "Quantitative Studies") | 13,366 |
| S31 | (MH "Placebos") | 9,206 |
| S30 | TX placebo* | 33,746 |
| S29 | TX random* allocat* | 4,263 |
| S28 | (MH "Random Assignment") | 39,076 |
| S27 | TX randomi* control* trial* | 86,804 |
| S26 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 765,707 |
| S25 | TX clinic* n1 trial* | 171,460 |
| S24 | PT Clinical trial | 77,813 |
| S23 | (MH "Clinical Trials+") | 186,869 |
| S22 | S7 AND S21 | 253 |
| S21 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 10,884 |
| S20 | TX(conventional* N2 therap*) | 2,360 |
| S19 | TX (conventional* N2 treat*) | 2,754 |
| S18 | TX (wait N1 see) | 216 |
| S17 | TX IUI | 79 |
| S16 | TX(artificial* N2 inseminat*) | 455 |
| S15 | TX(intra‐uter* N3 inseminat*) | 9 |
| S14 | TX(intrauter* N3 inseminat*) | 154 |
| S13 | (MM "Insemination, Artificial") | 243 |
| S12 | TX clomi* | 536 |
| S11 | (MM "Clomiphene") | 109 |
| S10 | TX(conservative Therap*) | 904 |
| S9 | TX(conservative treat*) | 3,472 |
| S8 | TX(expect* N2 manage*) | 922 |
| S7 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 | 3,499 |
| S6 | TX (intracytoplas* N2 sperm) | 241 |
| S5 | TX embryo* N3 transfer* | 777 |
| S4 | TX IVF or TX ICSI | 1,252 |
| S3 | (MM "Fertilization in Vitro") | 1,450 |
| S2 | TX vitro fertilization | 2,863 |
| S1 | TX vitro fertilisation | 267 |
Appendix 7. Trial characteristics
(1) Method of randomisation: (a) third party randomisation: for example computer, telephone randomisation (b) true randomisation by trialist: for example sequentially numbered, sealed, opaque envelopes, register, on‐site computer system (c) quasi randomised: for example by alternation, record‐numbers, date of birth, open list of random numbers, open envelopes (d) method not stated
(2) Study design: (a) presence or absence of blinding to randomisation (b) cross‐over or parallel design (c) duration of follow‐up (d) type of follow‐up (3) Size of the studies: (a) numbers of women recruited (b) numbers of women randomised (c) numbers of women excluded (d) numbers of women analysed (e) numbers of women lost to follow‐up (f) details of dropouts given
(4) Study setting: (a) single or multicentre (b) location (c) timing
(5) Analysis: (a) sample size with power calculation (b) whether or not analysed by "intention to treat": (i) done (ii) not done, but possible (iii) not possible (iv) uncertain (c) subgroup analysis for couples with subfertility due to minimal endometriosis
Characteristics of the study participants:
(1) Couples with unexplained infertility in whom full infertility investigations yielded normal results:
normal luteal phase progesterone
tubal patency assessed by hysterosalpingography and/or laparoscopy
normal semen sample according to World Health Organization (WHO) guidelines for the year the study was performed
(2) Baseline characteristics: (a) age of the female partner (b) primary or secondary infertility (c) duration of subfertility (d) previous fertility treatment
(3) Other subgroup criteria: (a) Couples with minimal endometriosis (American Fertility Society criteria grade I), with subfertility Baseline characteristics mentioned in (2) above were considered (4) Interventions used: (a) expectant management (b) clomiphene citrate (c) intrauterine insemination alone (d) intrauterine insemination with controlled ovarian stimulation (e) in vitro fertilisation (f) gamete intrafallopian transfer
(5) Outcomes: Primary: (a) cumulative live birth rate per woman/couple
Secondary: (a) pregnancy rate (i) per woman/couple (ii) cumulative (iii) per treatment cycle commenced
(b) live birth rate (i) cumulative (ii) per cycle commenced
(c) multiple pregnancy rate (i) per woman
(d) ovarian hyperstimulation syndrome (OHSS) (i) per woman
(e) costs (i) per treatment cycle (ii) per couple
Data and analyses
Comparison 1. IVF versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.0 [2.56, 189.37] |
| 2 Clinical pregnancy rate per woman | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [1.07, 9.80] |
Comparison 2. IVF versus unstimulated IUI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.19, 5.12] |
| 2 Clinical pregnancy rate per woman | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.83 [0.94, 24.95] |
| 3 Multiple pregnancy rate per woman | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.04, 27.29] |
Comparison 3. IVF versus IUI + ovarian stimulation with gonadotropins or clomiphene (CC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Treatment‐naive women IVF vs IUI + gonadotropins | 4 | 745 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.94, 1.73] |
| 1.2 Pretreated women IVF vs IUI + gonadotropins | 1 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.90 [2.32, 6.57] |
| 1.3 Treatment‐naive women IVF vs IUI + CC | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.96, 6.55] |
| 2 Clinical pregnancy rate per woman | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment‐naive women IVF vs IUI + gonadotropins | 3 | 627 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.03, 2.03] |
| 2.2 Treatment‐naive women IVF vs IUI + CC | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.59 [1.86, 11.35] |
| 2.3 Pretreated women IVF vs IUI + gonadotropins | 1 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.13 [7.57, 26.38] |
| 3 Multiple pregnancy rate per woman | 4 | 848 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.47, 1.39] |
| 3.1 Treatment‐naive women IUI + gonadotropins | 4 | 745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.39] |
| 3.2 Treatment‐naive women IVF vs IUI + CC | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.31] |
| 4 Incidence of OHSS per woman | 2 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.43, 3.06] |
| 4.1 Treatment‐naive women IVF vs IUI + gonadotropins | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.36, 4.14] |
| 4.2 Treatment‐naive women IVF vs IUI + CC | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.31] |
| 5 Miscarriage rate per woman | 1 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.59, 2.28] |
| 5.1 Treatment‐naive women IVF vs IUI + CC | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.44, 3.02] |
| 5.2 Treatment‐naive women IVF vs IUI+ gonadotropins | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.44, 3.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bensdorp 2015.
| Methods | Multi‐centre open‐label 3‐arm parallel‐group randomised controlled non‐inferiority trial | |
| Participants | 602 couples seeking fertility treatment after ≥ 12 months of unprotected intercourse, with the female partner between 18 and 38 years, an unfavourable prognosis for natural conception and a diagnosis of unexplained or mild male subfertility. Exclusion criteria included anovulation, double‐sided tubal disease, severe endometriosis, premature ovarian failure and known endocrine disorders (e.g. Cushing syndrome, adrenal hyperplasia) | |
| Interventions | Three cycles of IVF‐SET (plus subsequent cryo‐cycles), six cycles of modified natural cycle IVF and six cycles of IUI‐COH within 12 months after randomisation. Any additional treatments provided during this period were included at follow‐up | |
| Outcomes | Main outcome measures: The primary outcome was birth of a healthy child resulting from a singleton pregnancy conceived within 12 months after randomisation. Secondary outcomes included live birth, clinical pregnancy, ongoing pregnancy, multiple pregnancy, time to pregnancy, pregnancy complications and neonatal morbidity and mortality | |
| Notes | States: "During our trial the results of a pilot study, randomising women to three cycles of IUI‐COH or one cycle of IVF‐SET, were published. This pilot study demonstrated that the policy of transferring two embryos when no good quality embryos are available is not effective in preventing multiple pregnancies. The study protocol was amended, and from February 2010, after allocation of 48 women to the IVF‐SET group, a strict single embryo transfer policy (i.e. single embryo transfer was performed irrespective of embryo quality) was implemented" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with an "online randomisation program, using biased coin minimisation, stratified for study centre" |
| Allocation concealment (selection bias) | Low risk | "A web based program generated a unique number with allocation code after entry of the patient’s initials and date of birth. Neither the recruiters nor the trial project group could access the randomisation sequence" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 602/602 randomly assigned women were included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | None was suspected |
Elzeiny 2014.
| Methods | Randomised controlled parallel trial | |
| Participants | 44 couples Inclusion criteria Adults who had primary or secondary infertility ≥ 1 year in duration with evidence of ovulation and tubal patency, aged 18 to 42 years for females and 18 to 60 years for males Exclusion criteria IUI or IVF treatment in the previous 12 months, coital disorder, untreated ovulatory disorders or endometriosis (American Fertility Society criteria grades 2 to 4), tubal obstruction, abnormal semen analyses (concentration < 20 × 106/mL, progressive motility < 25%, abnormal morphology > 95% or positive sperm antibodies) or any contraindication for multiple pregnancy |
|
| Interventions | IVF vs IUI | |
| Outcomes | Live birth rate, clinical pregnancy rate, multiple pregnancy rate, OHSS, cost per live birth | |
| Notes | Financial support provided by a pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated, adaptive‐biased coin randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered opaque sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43/44 randomly assigned women were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study reported primary and secondary treatment outcomes adequately including adverse outcomes |
| Other bias | Low risk | No other potential bias could be observed |
Goldman 2014.
| Methods | Randomised controlled parallel trial, with clinicians blinded to outcome determinations. Intention‐to‐treat analysis performed, numbers of and reasons for withdrawals and dropouts stated, clearly defined interventions applied with standardised protocols, couples followed up until discharge from the hospital of both mother and infant(s), if pregnant, or 1 year after completion of treatment protocol. Tables with permuted blocks of varying sizes, stratified by the woman's age (38th to 41st vs 42nd to 43rd birthday) | |
| Participants | 154 couples Inclusion criteria Couples in which the woman had 38 to 42 years 6 months of attempted conception; at least 1 ovary and ipsilateral patent fallopian tube confirmed by hysterosalpingogram or laparoscopy; regular menstrual cycles of 21 to 45 days; and no pelvic pathology, ectopic pregnancy nor previous infertility treatment (except up to 3 cycles of clomiphene without IUI). Normal prolactin and thyroid‐stimulating hormone levels and body mass index (BMI) < 38 in the woman; sperm concentration > 15 million total motile sperm or > 5 million total motile sperm at reflex IUI preparation in the male partner Exclusion criteria Age outside the range, prior infertility treatment or not a candidate for study treatments, or not covered by a participating insurer |
|
| Interventions | Three‐arm randomised controlled trial. Couples were randomly assigned to treatment with 2 cycles of clomiphene citrate (CC) and intrauterine insemination (IUI), follicle‐stimulating hormone (FSH)/IUI or immediate IVF, followed by by 3 cycles of IVF if not pregnant | |
| Outcomes | Live birth, clinical pregnancy, multiple pregnancy and time to conception were reported | |
| Notes | Population of the study consisted of women with relatively advanced reproductive age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was generated by an independent biostatistician", using tables with permuted blocks of varying sizes, stratified by the woman's age (38th to 41st vs 42nd to 43rd birthday) |
| Allocation concealment (selection bias) | Low risk | Remote allocation: "The allocation sequence was ... implemented by an epidemiologist. Randomization was never conducted by clinical staff" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All clinical investigators were blinded to outcome determinations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 154/154 randomly assigned women were included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | Live birth, clinical pregnancy, multiple pregnancy and time to conception were reported |
| Other bias | Low risk | No other potential bias could be observed |
Goverde 2000.
| Methods | Randomised controlled parallel trial, participants and providers unable to be blinded, intention‐to‐treat analysis performed, numbers of and reasons for withdrawals and dropouts stated, clearly defined interventions applied with standardised protocols, overall duration of follow‐up 6 cycles. Computer‐generated randomisation schedule, administered by numbered masked and sealed envelopes | |
| Participants | 181 women with unexplained or mild male factor infertility of at least 3 years' duration or male subfertility for ≥ 1 year, with no abnormality found during full infertility investigation, which included basal body temperature chart, late luteal phase endometrial biopsy, postcoital test, hysterosalpingogram, diagnostic laparoscopy and ≥ 2 semen analyses. Exclusion criteria included cycle disorders, untreated endometriosis (AFS grade 2 to 4), and bilateral occluded tubes. | |
| Interventions | IVF vs IUI and IVF vs intrauterine insemination plus ovarian stimulation (IUI + SO) | |
| Outcomes | LBR per woman/couple | |
| Notes | Power calculation mentioned Number of dropouts before completion of treatment: IUI, 19 couples out of 86 randomly assigned; IUI + SO, 16 out of 85 randomly assigned; IVF, 39 out of 87 randomly assigned (figures include couples with unexplained subfertility and mild male factor subfertility) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Low risk | "numbered masked and sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 172/181 (95%) randomly assigned women with idiopathic subfertility were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study reported primary and secondary treatment outcomes adequately including adverse outcomes |
| Other bias | Low risk | Pre‐study power calculation was performed, and no other potential bias was observed |
Hughes 2004.
| Methods | 139 women in a multi‐centre randomised controlled trial (RCT). Randomisation was based on a blocked schedule using numbered, sealed, opaque envelopes and stratified by centre; female age (≥ 35 years) and presence or absence of abnormal sperm (total sperm count ≥ 20 million). Power calculation done. Intention‐to‐treat analysis performed. Fisher's exact test used for analysis. Confidence intervals calculated using Mantel‐Haenszel statistics | |
| Participants | Duration of subfertility ≥ 2 years (defined as no live birth during that time), no previous IVF treatment, female age 18 to 39 years, day 3 serum follicle‐stimulating hormone (FSH) level ≥ 15 IU/L or standard level for inclusion in an individual centre's IVF programme, whichever level was lower; semen analysis within past 6 months showing adequate sperm number to perform intracytoplasmic sperm injection (ICSI), evidence of tubal patency by hysterosalpingography or laparoscopy Mean duration of subfertility was 58 months. All couples had exhausted appropriate lower intensity treatment options, such as ovulation induction and intrauterine insemination. |
|
| Interventions | First cycle of IVF compared with 90 days of no treatment (expectant management) | |
| Outcomes | Clinically viable pregnancy rate per couple, LBR per couple | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "Random allocation was based on a blocked schedule using numbered, sealed, opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "Random allocation was based on a blocked schedule using numbered, sealed, opaque envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 68/68 randomly assigned women analysed by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study reported primary and secondary treatment outcomes adequately including adverse outcomes |
| Other bias | High risk | Pre‐study power calculation was performed,.and no other potential bias could be observed |
Reindollar 2010.
| Methods | RCT using permuted blocks of varying sizes, stratified by woman's age (< 35 vs ≥ 35 years), laparoscopy within past year (yes or no) and study site (Boston IVF or Harvard Vanguard Medical Associates). Allocation sequence was produced by random numbers generated by a congruence method. Investigators were blinded to all outcome determinations | |
| Participants | 503 couples; women 21 to 39 years of age with unexplained infertility and mild male factor of 12 months' duration | |
| Interventions | Couples in this study were randomly assigned to conventional pathway involving clomiphene citrate plus intrauterine insemination (CC + IUI) followed by IUI + gonadotropins and then IVF; or accelerated pathway (CC + IUI followed by 6 cycles of IVF) | |
| Outcomes | Pregnancy rate per cycle, pregnancy rate per couple, LBR per cycle, LBR per couple, time to pregnancy, charge data | |
| Notes | Study could not be included for comparison between IVF and IUI + CC, as both arms received CC + IUI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was produced by use of random numbers generated by a congruence method. The sequence was developed by the biostatistician and implemented by the epidemiologist" |
| Allocation concealment (selection bias) | Low risk | Apparently remote allocation: "The sequence was ...implemented by the epidemiologist" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators were blinded to all outcome determinations; allocation was performed by a biostatistician and was implemented by an epidemiologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 503/503 randomly assigned women analysed by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study authors published preliminary results in 2007 and did not appear to publish or failed to publish based on results of the trial |
| Other bias | Low risk | No other potential biases could be detected |
Soliman 1993.
| Methods | RCT; participant and provider could not be blinded. Follow‐up was 1 cycle in the IVF group and 6 months in the expectant management group | |
| Participants | 245 couples with infertility for 1 year, completed investigation for infertility, woman < 40 years. Mean duration of infertility 65 months, all previously treated by conventional means. Only 35 couples had unexplained infertility and are included in analysis in this review |
|
| Interventions | IVF vs expectant management. Duration of expectant management was 6 months, during which time other treatments (apart from IVF) were permitted | |
| Outcomes | Pregnancy rate per woman/couple | |
| Notes | Computer‐generated random number table. 16 cycles (16.2%) cancelled after start of treatment for various reasons For couples randomly assigned to expectant treatment, any form of infertility treatment other than IVF was permitted for the 6‐months expectant management arm. 78% of couples received some form of infertility treatment except IVF while in the expectant arm Despite randomisation, a significant difference was noted between mean ages of participants in the 2 arms of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding was performed because of the nature of the intervention used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis was performed. 19% of participants overall withdrew (unclear how many with unexplained infertility withdrew) |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient for judgement of the trial as low risk or high risk |
| Other bias | High risk | Withdrawals were numerous; exact time of withdrawal was not defined, especially for the expectant management group. Groups were not balanced with regard to prognostic factors: IVF group were older and had higher proportion with endometriosis |
van Rumste 2014.
| Methods | Multi‐centre RCT | |
| Participants | 116 couples with unexplained and mild male factor infertility. All couples had a standard fertility workup, including assessment of ovulation by basal temperature curve or ultrasound, a tubal patency test and sperm analysis. This study included all couples with unexplained or mild male subfertility, female age between 18 and 38 years and poor fertility prospects, defined as a 12‐month prognosis < 30% for natural conception according to the model of Hunault 2004 | |
| Interventions | 1 cycle of IVF–eSET followed by 1 cryocycle or 3 cycles of IUI–ovarian stimulation. Results of freeze–thaw cycles were also included in this study, provided the transfer took place within 4 months after randomisation | |
| Outcomes | Ongoing pregnancy rate per woman/couple, cost per cycle | |
| Notes | Additional data on methods and outcomes were requested from lead author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central Internet‐based randomisation was stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | No other reports on the trial could be retrieved |
| Other bias | Low risk | No other potential bias was noted |
Abbreviations:
AFS: American Fertility Society.
eSET: elective single embryo transfer.
FSH: follicle‐stimulating hormone.
ICSI: intracytoplasmic sperm injection.
IUI: intrauterine insemination.
IUI‐COH: intrauterine insemination‐controlled ovarian hyperstimulation.
IVF: in vitro fertilisation.
IVF‐SET: in vitro fertilisation‐single embryo transfer.
LBR: live birth rate.
SO: ovarian stimulation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Crosignani 1991 | Multi‐centre randomised controlled trial (RCT) comparing effectiveness of in vitro fertilisation (IVF) vs intrauterine insemination plus ovarian stimulation (IUI + gonadotropins) and IVF vs gamete intrafallopian transfer (GIFT). Pregnancy rate per cycle and live birth rate (LBR) per cycle were reported outcomes |
| Custers 2012 | Couples with unexplained subfertility and intermediate prognosis of natural conception were randomly allocated to 6 months EM or immediate start with IUI‐COS: no IVF arm |
| Jarrell 1993 | Diagnostic stratification not done; therefore number of participants with unexplained infertility is not known. Control group could include participants who underwent some form of fertility treatment while awaiting spontaneous pregnancy |
| Karande 1998 | Diagnostic stratification not done. Study population included all categories of infertile couples. Couples with unexplained infertility were not analysed separately |
| Leeton 1987 | Although study authors describe the study as randomised controlled trial (RCT), on closer inspection the method of allocation was found to be non‐random. Every second participant was allocated to the gamete intrafallopian transfer (GIFT) group |
| Raneiri 1995 | No intervention of interest (gamete intrafallopian transfer (GIFT) excluded from 2011 review update) |
| Tanbo 1990 | No intervention of interest (gamete intrafallopian transfer (GIFT) excluded from 2011 review update) |
| Zayed 1997 | Randomisation was not genuine. Study authors describe method of randomisation as pseudo‐randomisation. Allocation of treatment was breached by participant preference. Pregnancy and live birth rate (LBR) per woman/couple has not been reported |
Characteristics of ongoing studies [ordered by study ID]
Nandi 2015.
| Trial name or title | Controlled ovarian stimulation and intrauterine insemination or in vitro fertilisation as first‐line treatment for unexplained infertility: a randomised controlled trial |
| Methods | Randomised controlled trial (RCT) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Randomisation is performed by an independent worker in blocks of 10 and distributed in individual consecutively numbered opaque envelopes. Participants will be randomly assigned to 2 groups:
In IVF group, women will undergo controlled ovarian hyperstimulation after downregulation with gonadotropin releasing hormone (GnRH) agonist in a long protocol starting on day 2. COH is started with FSH, with doses ranging from 150 to 450 IU, depending on initial anti‐Mullerian hormone (AMH) level as decided by attending clinician. Day of embryo transfer will be decided by embryologist base |
| Outcomes | Primary outcome: singleton live birth Secondary outcomes: clinical pregnancy rate, multiple pregnancy rate |
| Starting date | 17/6/2013 |
| Contact information | Anupa Nandi Homerton Fertility Unit Homerton Hospital E9 6SR London United Kingdom |
| Notes | http://isrctn.com/ISRCTN43430382 |
Differences between protocol and review
Gamete intrafallopian transfer (GIFT) has been removed from the comparisons and the review. The primary outcome of cumulative live birth rate per woman has been replaced by live birth rate per woman, and the secondary outcome of cumulative pregnancy rate per woman has been replaced by clinical pregnancy rate per woman. The comparison of in vitro fertilisation (IVF) versus intrauterine insemination (IUI) + letrozole has been added.
Contributions of authors
Zabeena Pandian: development of the protocol, literature search, data extraction, trial selection, quality assessment, data entry and analysis, writing of first draft of the updated review.
Ahmed Gibreel: trial selection, quality assessment for the updated review.
Siladitya Bhattacharya: trial selection, quality assessment, responsible for final draft of the updated review.
Sources of support
Internal sources
Department of Obstetrics & Gynaecology, University of Aberdeen, UK.
External sources
None, Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bensdorp 2015 {published data only}
- Bensdorp AJ, Tjon‐Kon‐Fat RI, Bossuyt PMM, Koks CA, Oosterhuis GJ, Hoek A, et al. Prevention of multiple pregnancies in couples with unexplained or mild male subfertility: a randomised controlled trial comparing in vitro fertilisation with single embryo transfer or in vitro fertilisation in a modified natural cycle to intrauterine insemination with controlled ovarian stimulation. BMJ 2015;9(350):g7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elzeiny 2014 {published data only}
- Elzeiny H, Garrett C, Toledo M, Stern K, McBain J, William H, et al. A randomised controlled trial of intra‐uterine insemination versus in vitro fertilisation in patients with idiopathic or mild male factor. Australian and New Zealand Journal of Obstetrics & Gynaecology 2014;54:156‐61. [DOI] [PubMed] [Google Scholar]
Goldman 2014 {published data only}
- Goldman MB, Thornton KL, Ryley D, Alper MM, June L, Fung JL, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT‐T). Fertility and Sterility 2014;101(6):1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goverde 2000 {published data only}
- Goverde AJ, McDonnell J, Vermeiden JPW, Schats R, Rutten FFH, Schoemaker J. Intrauterine insemination or in vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost‐effectiveness analysis. Lancet 2000;355:13‐8. [DOI] [PubMed] [Google Scholar]
Hughes 2004 {published data only}
- Hughes EG, Beecroft ML, Wilkie V, Burville L, Claman P, Tummon I, et al. A multicentre randomized controlled trial of expectant management versus IVF in women with fallopian tube patency. Human Reproduction 2004;19(5):1105‐9. [DOI] [PubMed] [Google Scholar]
Reindollar 2010 {published data only}
- Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertility and Sterility 2010;94(3):888‐99. [DOI] [PubMed] [Google Scholar]
Soliman 1993 {published data only}
- Soliman S, Daya S, Collins J, Jarrell J. A randomized trial of in vitro fertilization versus conventional treatment for infertility. Fertility and Sterility 1993;59(6):1239‐44. [DOI] [PubMed] [Google Scholar]
van Rumste 2014 {published data only}
- Custers IM, Konig TE, Broekmans FJ, Hompes P, Kaaijk E, Oosterhuis J, et al. Couples with unexplained subfertility and unfavourable prognosis; a randomized pilot trial comparing the effectiveness of IVF‐eSET and IUI‐COS. Fertility & Sterility 2011;96(5):1107‐11. [DOI] [PubMed] [Google Scholar]
- Rumste MME, Custers IM, Koks CA, Weering HGI, Beckers NGM, Scheffer GJ, et al. IVF with planned single‐embryo transfer versus IUI with ovarian stimulation in couples with unexplained subfertility: an economic analysis. Reproductive Biomedicine Online 2014;28(3):336‐42. [DOI] [PubMed] [Google Scholar]
- Rumste MME, Custers IM, Koks CA, Weering HGI, Beckers NGM, Scheffer GJ, et al. IVF with single embryo transfer versus IUI with ovarian hyperstimulation in couples with unexplained subfertility, an economic analysis. Abstracts of the 25th Annual Meeting of ESHRE, Amsterdam, the Netherlands. 28 June‐1 July 2009;25:0‐110 Oral.
References to studies excluded from this review
Crosignani 1991 {published data only}
- Crosignani PG, Walters DE, Soliani A. Addendum to the ESHRE multicentre trial: a summary of the abortion and birth statistics. Human Reproduction 1992;2(7):286‐7. [DOI] [PubMed] [Google Scholar]
Custers 2012 {published data only}
- Custers IM, Rumste ME, Steeg JW, Wely M, Hompes PG, Bossuyt P, et al. Long‐term outcome in couples with unexplained subfertility and an intermediate prognosis initially randomized between expectant management and immediate treatment. Human Reproduction 2012;27(2):444‐50. [DOI] [PubMed] [Google Scholar]
Jarrell 1993 {published data only}
- Jarrell JF, Labelle R, Goeree R, Milner R, Collins J. In vitro fertilisation and embryo transfer: a randomised controlled trial. Online Journal of Current Clinical Trials 1993; Vol. Document number 73:73. [PubMed]
Karande 1998 {published data only}
- Karande VC, Korn A, Morris A, Pao R, Balin M, Rinehart J, et al. Prospective randomized trial comparing the outcome and cost of in vitro fertilisation with that of a traditional treatment algorithm as first line therapy for couples with unexplained infertility. Fertility and Sterility 1998;71(3):468‐75. [DOI] [PubMed] [Google Scholar]
Leeton 1987 {published data only}
- Leeton J, Rogers P, Caro C, Healy D, Yates C. A controlled study between the use of gamete intrafallopian transfer and in vitro fertilisation and embryo transfer in the management of idiopathic and male infertility. Fertility and Sterility 1987;48(4):605‐7. [DOI] [PubMed] [Google Scholar]
Raneiri 1995 {published data only}
- Raneiri M, Beckett VA, Marchant S, Kinis A, Serhal P. Gamete intrafallopian transfer or in vitro fertilisation after failed ovarian stimulation and intrauterine insemination in unexplained infertility. Human Reproduction 1995;10(8):2023‐6. [DOI] [PubMed] [Google Scholar]
Tanbo 1990 {published data only}
- Tanbo T, Dale PO, Jarrell J. Assisted fertilization in infertile women with patent fallopian tubes. A comparison of in‐vitro fertilization, gamete intra‐fallopian transfer and tubal embryo stage transfer. Human Reproduction 1990;3(5):266‐70. [DOI] [PubMed] [Google Scholar]
Zayed 1997 {published data only}
- Zayed F, Lenton EA, Cooke ID. Comparison between stimulated in‐vitro fertilization and stimulated intrauterine insemination for the treatment of unexplained and mild male factor infertility. Human Reproduction 1997;12(11):2408‐13. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Nandi 2015 {published data only}
- Controlled ovarian stimulation and intrauterine insemination or in vitro fertilisation as first‐line treatment for unexplained infertility: a randomised controlled trial. Ongoing study 17/6/2013.
Additional references
Bhattacharya 2008
- Bhattacharya S, Harrild K, Mollison J, Wordsworth S, Tay C, Harrold A, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ 2008 Aug;337. doi: 10.1136/bmj:a716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1995
- Collins JA, Burrows EA, Willan AR. The prognosis for live birth among untreated infertile couples. Fertility and Sterility 1995;64:22‐8. [MEDLINE: ] [PubMed] [Google Scholar]
FIVNAT 2012
- Annual report of FIVNAT. French IVF National Registry, 2012 report.
Glujovsky 2012
- Glujovsky D, Blake D, Bardach A, Farquhar C. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI] [PubMed] [Google Scholar]
HFEA 2012
- Fertility Treatment in 2012: Trends and Figures. http://www.hfea.gov.uk/104.html 2013.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hughes 2010
- Hughes E, Brown J, Collins JJ, Vanderkerchove P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000057.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunault 2004
- Hunault CC, Habbema JD, Eijkemans MJ, Collins JA, Evers JL, Velde ER. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Human Reproduction 2004;19(9):2019‐26. [DOI] [PubMed] [Google Scholar]
Lenton 1977
- Lenton EA, Weston GA, Cooke ID. Long term follow up of apparently normal couple with a complaint of infertility. Fertility and Sterility 1977;28:913‐9. [DOI] [PubMed] [Google Scholar]
Maheshwari 2008
- Maheshwari M, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Human Reproduction 2008;23:538‐42. [DOI] [PubMed] [Google Scholar]
Mouzon 2010
- Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Human Reproduction 2010;25(8):1851‐62. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Collaborating Centre for Women's and Children's Health. Fertility: Assessment and Treatment for People With Fertility Problems. Clinical Guideline. London: RCOG Press, 2013.
SART/ASRM 2014
- Society for Assisted Reproductive Technology and American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2012 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. ASRM Bulletin 2014;16(12). [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Snick 1997
- Snick HKA, Snick TS, Evers JLH, Collins JA. The spontaneous pregnancy prognosis in untreated subfertile couples: the Walcheren primary care study. Human Reproduction 1997;12(7):1582‐8. [DOI] [PubMed] [Google Scholar]
Steures 2006
- Steures P, Steeg JW, Hompes PG, Habbema JD, Eijkemans MJ, Broekmans FJ, et al. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;368(9531):216‐21. [DOI] [PubMed] [Google Scholar]
Steures 2008
- Steures P, Steeg JW, Hompes PG, Bossuyt PM, Veen F, Habbema JD, et al. Intra‐uterine insemination with controlled ovarian hyperstimulation compared to an expectant management in couples with unexplained subfertility and an intermediate prognosis: a randomised study. Nederlands Tijdschrift voor Geneeskunde 2008;152(27):1525‐31. [PubMed] [Google Scholar]
VARTA 2013
- Victorian Assisted Reproductive Treatment Authority. 2013 Annual Report. Melbourne, Australia, 2013.


