Abstract
Background
Side effects of oral contraceptive (OC) pills discourage adherence to and continuation of OC regimens. Strategies to decrease adverse effects led to the introduction of the triphasic OC in the 1980s. Whether triphasic OCs have higher accidental pregnancy rates than monophasic pills is unknown. Nor is it known if triphasic pills give better cycle control and fewer side effects than the monophasic pills.
Objectives
To compare triphasic OCs with monophasic OCs in terms of efficacy, cycle control, and discontinuation due to side effects.
Search methods
We searched the computerized databases of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, POPLINE, EMBASE, and LILACS, as well as clinical trials databases (ClinicalTrials.gov and the World Health Organization Clinical Trials Registry Platform (ICTRP)) in May 2011. Additionally, we searched the reference lists of relevant articles. We also contacted researchers and pharmaceutical companies to identify other trials not found in our search.
Selection criteria
We included randomized controlled trials (RCTs) comparing any triphasic OC with any monophasic pill used to prevent pregnancy. Interventions had to include at least three treatment cycles.
Data collection and analysis
We assessed the studies found in the literature searches for possible inclusion and for their methodological quality. We contacted the authors of all included studies and of possibly randomized trials for supplemental information about the methods used and outcomes studied. We entered the data into RevMan and calculated odds ratios for the outcome measures of efficacy, breakthrough bleeding, spotting, withdrawal bleeding and discontinuation.
Main results
Of 23 trials included, 19 examined contraceptive effectiveness. The triphasic and monophasic preparations did not differ significantly. About half of the included trials reported favorable bleeding patterns, that is less spotting, breakthrough bleeding or amenorrhea, in triphasic versus monophasic OC users. However, meta‐analysis was generally not possible due to differences in measuring and reporting the cycle disturbance data as well as differences in progestogen type and hormone dosages. No significant differences were found in the numbers of women who discontinued due to medical reasons, cycle disturbances, intermenstrual bleeding or adverse events.
Authors' conclusions
The available evidence is insufficient to determine whether triphasic OCs differ from monophasic OCs in effectiveness, bleeding patterns or discontinuation rates. Therefore, we recommend monophasic pills as a first choice for women starting OC use. Large, high‐quality RCTs that compare triphasic and monophasic OCs with identical progestogens are needed to determine whether triphasic pills differ from monophasic OCs. Future studies should follow the recommendations of Belsey or Mishell on recording menstrual bleeding patterns and the CONSORT reporting guidelines.
Plain language summary
Birth control pills with three phases versus one phase
Side effects of birth control pills may keep women from using them as planned. Attempts to decrease side effects led to the three‐phase pill in the 1980s. Pills with three phases provide different amounts of hormones over three weeks. One‐phase pills have the same amount of hormone for three weeks. Whether three‐phase pills lead to more pregnancies is unknown. Nor is it known if the pills give better cycle control or fewer side effects. This review looked at whether three‐phase pills worked as well as one‐phase pills. It also studied whether women had fewer side effects with these pills.
We did a computer search for studies of pills with three phases versus pills with one phase in May 2011. We also wrote to researchers and manufacturers to find other trials. We included randomized trials in any language. The studies had to follow women for at least three treatment cycles.
We found 23 trials that looked at three‐phase versus one‐phase birth control pills. Many studies did not have good methods and the authors did not always report all their methods. The two types of pills did not differ in the numbers of women who got pregnant. About half of the trials found better bleeding patterns with the three‐phase pill. The numbers of women who stopped using the pills were about the same for both types of pills.
The evidence was not strong enough to say whether the three‐phase pill was better than the one‐phase pill for pregnancy prevention, bleeding patterns, or continued use. Therefore, we recommend one‐phase pills for women starting to use birth control pills. Large trials that are of good quality are needed to see if pills with three phases work better than those with one phase.
Background
Side effects of oral contraceptive pills discourage adherence to and continuation of oral contraceptive regimens (Rosenberg 1995; Rosenberg 1998). Three approaches have been used to decrease these adverse effects. These are (a) reduction of the steroid dose; (b) development of new steroids; and (c) new formulas and schedules of administration. These strategies led to the introduction of the triphasic oral contraceptive pill in the 1980s.
Triphasic oral contraceptives allegedly attempt to 'mimic' the rising and falling of estrogen and progesterone levels during the normal menstrual cycle (Upton 1983). This purportedly results in a more 'physiologic' approach and, with some pills, a lower total monthly steroid dosage compared to the older monophasic oral contraceptives. Possible benefits of the triphasic approach are better cycle control and fewer side effects (Guillebaud 1993; Hale 1987). In a cohort study conducted in France, women using 30 to 40 μg pills had similar reports of menstrual symptoms regardless of whether the pills were monophasic, biphasic or triphasic (Moreau 2007). However, potential disadvantages include an increased risk of pill‐taking errors caused by the array of different colored pills, the higher price of the pills and the possible higher incidence of accidental pregnancy (Guillebaud 1993).
Soon after the introduction in Britain of Logynon, a triphasic preparation of levonorgestrel and ethinylestradiol, two case reports described a probable method failure of the pill (Fay 1982; Graham 1982). Studies at abortion clinics in Australia and the Netherlands demonstrated a significant over‐representation of triphasic oral contraceptives used by women with an unplanned pregnancy (Ketting 1988; Kovacs 1989). Whether triphasic oral contraceptives have higher accidental pregnancy rates than monophasic oral contraceptives is unknown. Nor is it known if triphasic contraceptive pills give better cycle control and fewer side effects than the monophasic pills.
Objectives
The aim of this review was to compare triphasic oral contraceptive pills with monophasic oral contraceptive pills. Based on observational studies (Ketting 1988; Kovacs 1989), the a priori hypotheses were: (a) triphasic oral contraceptives are less effective in preventing pregnancy compared to monophasic oral contraceptives; and (b) triphasic oral contraceptives are similar to monophasic pills in terms of cycle control and continuation rates.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials in this review. No language restrictions were placed on the reporting of the trials.
Types of participants
Healthy women of reproductive age were included if they had no contraindications for oral contraceptive use and desired to use oral contraceptives for preventing pregnancy. Women starting oral contraceptives as well as women switching oral contraceptives were included.
Types of interventions
We included any triphasic oral contraceptive pill (OC) compared to any monophasic oral contraceptive pill when used to prevent pregnancy. Both 21‐pill and 28‐pill packages were included. We excluded studies comparing triphasic pills with monophasic pills when the pills were used as a treatment (for example for acne, dysmenorrhea or menorrhagia) and not as a contraceptive. Interventions had to be applied for a minimum of three consecutive cycles to be eligible for inclusion.
Types of outcome measures
We focused on clinically relevant outcome measures. Studies were not included if they primarily looked at metabolic outcome measures or follicular growth. Principal outcomes were contraceptive efficacy, bleeding patterns and trial discontinuation. To be eligible, studies had to report results in a format that could be converted to the outcomes as follow.
Contraceptive efficacy
Proportion of women pregnant.
Cycle control
We used the definitions of spotting and breakthrough bleeding as specified by the authors.
Proportion of cycles with spotting or breakthrough bleeding or intermenstrual bleeding within 3 cycles, 6 cycles and 12 cycles of pill use.
Proportion of cycles with spotting or breakthrough bleeding or intermenstrual bleeding during the third cycle, the sixth cycle and the 12th cycle of pill use.
Proportion of women with spotting or breakthrough bleeding or intermenstrual bleeding within 3 cycles, 6 cycles and 12 cycles of pill use.
Proportion of women with spotting or breakthrough bleeding or intermenstrual bleeding during the third cycle, the sixth cycle and the 12th of pill use.
Proportion of cycles with absence of withdrawal bleeding within 3, 6 and 12 cycles of pill use.
Proportion of women with absence of withdrawal bleeding within 3, 6 and 12 cycles of pill use.
Proportion of women with absence of withdrawal bleeding during the third, the sixth and the 12th cycles of pill use.
Discontinuation
Proportion of women that discontinued within 3, 6 and 12 cycles of pill use.
Proportion of women that discontinued due to bleeding disturbances or adverse events within 3, 6 and 12 cycles of pill use.
Search methods for identification of studies
Electronic searches
We searched the computerized databases of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE using PubMed, POPLINE, EMBASE and LILACS for publications comparing monophasic, biphasic or triphasic oral contraceptives. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). Appendix 1 has the 2011 search strategies along with the search dates. Earlier strategies can be found in Appendix 2.
Searching other resources
We reviewed the reference lists of identified studies for additional trials. For the initial review, we examined the references lists from relevant book chapters and review articles identified with the search strategies. We searched the holdings of the Family Health International (FHI) library for relevant trials, book chapters and review articles. In addition, we attempted to contact the authors of all included trials. We also wrote a letter to pharmaceutical companies in the USA and Europe that market oral contraceptives. In the contact letters, we provided a list of studies identified and asked if they knew of unpublished or published trials we had missed.
Data collection and analysis
Selection of studies
Two authors independently evaluated the titles and abstracts identified during the literature searches under unblinded conditions and all potentially relevant articles were photocopied (Berlin 1997). Family Health International employees translated Russian, Chinese, Norwegian and German articles into English (Chen 1987; Dubnitskaia 1988; Engebretsen 1987; Lachnit‐Fixson (1979) of Zador 1979; Lachnit‐Fixson 1984). Then the authors independently examined the retrieved studies for possible inclusion. We excluded studies that were clearly not randomized, were quasi‐randomized controlled trials, or did not focus on interventions included in this review. Discrepancies were resolved through consensus.
Data extraction and management
One author extracted the data from the included studies under unblinded conditions and entered the data into RevMan 4.2 (Berlin 1997). In addition to the outcome measures and methodological quality of the study, we extracted data on participants, inclusion and exclusion criteria, study sites, duration of study, study medication, method of collecting the data and funding. Correct entry of the data was verified by a second author. No disagreements about the extracted and entered data occurred.
We wrote a letter to the authors of the included trials and to the authors of possibly randomized studies. In the letter we asked for additional information about the study methods and the various outcome measures.
Assessment of risk of bias in included studies
The validity of trials was critically appraised by assessing the potential risk for bias (Higgins 2005). We did not calculate summary quality scores but focused on the method of generating the allocation sequence, the use and method of allocation concealment, the use and method of blinding, exclusion of participants after randomization and loss to follow up (Juni 1999).
Assessment of heterogeneity
Most of the meta‐analyses combined a small number of trials. However, when substantial heterogeneity was evident (I2 > 50%) we examined the studies separately and discussed the results in Effects of interventions.
Data synthesis
The review was limited to the analytic method used in the paper (for example intent‐to‐treat or per‐protocol).
Contraceptive efficacy
All studies that included contraceptive failure data reported the number of women who became pregnant. We used these proportions of women to calculate the odds ratio (OR) and 95% confidence interval (CI) with the random‐effects model.
Cycle control
Due to the possible relationship between (a) progestogen type and (b) dosage of estrogen and progestogen on bleeding patterns, we only combined trials that compared pills with identical contents (Maitra 2004; Rosenberg 1992), as was done in Gallo 2008. Most trials did not report bleeding pattern data according to the World Health Organization (WHO) recommendations (Belsey 1986) or to the Food and Drug Administration Reproductive Health Drugs Advisory Committee (RHDAC) recommendations (Mishell 2007). They generally reported the proportion of women or cycles with spotting, breakthrough bleeding or amenorrhea within three, six or 12 cycles or at the third, sixth or 12th cycle. We used these proportions to calculate the OR and 95% CI with the random‐effects model. Narrative summaries were provided when the reported bleeding data were not compatible with RevMan. For continuous data, we calculated the mean difference and 95% CI with a random‐effects model.
Discontinuation
We used the proportion of women discontinuing, discontinuing due to adverse events, and discontinuing due to cycle disturbances within three, six and 12 cycles to calculate the OR and 95% CI with the random‐effects model.
Sensitivity analysis
We did not conduct a sensitivity analysis based on the methodological quality of the trials.
Results
Description of studies
Included studies
We included 23 studies comparing triphasic oral contraceptives and monophasic oral contraceptives in this review. Six studies were published in duplicate (Carlborg 1983; Dieben 1984; Lachnit‐Fixson 1984; Saxena 1992; Sulak 1999). The Characteristics of included studies has detailed information regarding participants, inclusion and exclusion criteria, study sites, duration of study, study medication and outcome measures. Six trials included more than two intervention groups.
Triphasic levonorgestrel oral contraceptives versus monophasic oral contraceptives
Eight studies compared a triphasic OC composed of 50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) with a monophasic pill composed of 150 μg levonorgestrel and 30 μg ethinylestradiol (Carlborg 1983; Chen 1987; Dunson 1993; Engebretsen 1987; Kashanian 2010; Ramos 1989; Saxena 1992; Zador 1979). Pharmaceutical companies market the triphasic preparation under the brand names Logynon, Trionetta 21 and 28, Triquilar, Trinordiol, and Triphasil. The monophasic preparation is marketed under the names Microgynon 30, Stediril, Neovletta, Rigevidon, Lo‐Femenal, Follimin, and Nordette. Chen 1987 evaluated a third preparation, which was composed of 600 μg norethindrone and 35 μg ethinylestradiol. Ramos 1989 also examined a third preparation, that is 400 μg norethindrone and 35 μg ethinylestradiol (Micropil).
The triphasic pill of 50‐75‐125 μg levonorgestrel and 30‐40‐30 μg ethinylestradiol was compared with a monophasic pill containing 150 μg desogestrel and 30 μg ethinylestradiol (Marvelon) in three studies (Dieben 1984; Ismail 1991; Lachnit‐Fixson 1984).
Percival‐Smith 1990 compared the triphasic 50‐75‐125 μg levonorgestrel and 30‐40‐30 μg ethinylestradiol pill, a triphasic pill containing 500‐750‐1000 μg norethindrone and 35 μg ethinylestradiol, and a monophasic pill of 1500 μg norethindrone acetate and 30 μg ethinylestradiol (Percival‐Smith 1990). Brand names of the triphasic norethindrone oral contraceptive are Ortho Novum 7/7/7 and Trinovum. The monophasic norethindrone pill is Loestrin. Percival‐Smith 1990 also examined a biphasic pill of 500‐1000 μg norethindrone and 35 μg ethinylestradiol but those data were not part of this review. The biphasic pill is known as Ortho 10/11.
One study compared: 1) the 50‐75‐125 μg levonorgestrel and 30, 40, 30 μg ethinylestradiol triphasic OC, and 2) the 500‐750‐1000 μg norethindrone and 35 μg ethinylestradiol triphasic OC with a monophasic OC containing 1000 μg norethindrone and 35 μg ethinylestradiol (Reiter 1990). The monophasic pill is marketed as Ortho‐Novum 1/35.
Triphasic norethindrone oral contraceptives versus monophasic oral contraceptives
Two studies compared a triphasic formulation containing 500‐750‐1000 μg norethindrone (NET) and 35 μg ethinylestradiol with a monophasic formulation containing 100 μg levonorgestrel and 20 μg ethinylestradiol (Chavez 1999; Reisman 1999). The monophasic formulation is marketed as Alesse and Loette.
We also included a study which compared an 'estrophasic' 1000 μg norethindrone acetate (NETA) and 20‐30‐35 μg ethinylestradiol combination with a monophasic 1500 μg norethindrone acetate and 30 μg ethinylestradiol combination (Rowan 1999).
Triphasic gestodene oral contraceptives versus monophasic oral contraceptives
Two trials compared a triphasic formulation composed of 50‐70‐100 μg gestodene (GTD) and 30‐40‐30 μg ethinylestradiol with a monophasic pill of 150 μg desogestrel (DSG) and 30 μg ethinylestradiol (Agoestina 1987; Andrade 1993). Brand names of the triphasic preparation are Trimulet and Triodeen.
The triphasic 50‐70‐100 μg gestodene and 30‐40‐30 μg ethinylestradiol oral contraceptive pill was also compared with: 1) a monophasic 75 μg gestodene and 30 μg ethinylestradiol pill, and 2) a monophasic 150 μg desogestrel and 20 μg ethinylestradiol pill (Bruni 2000). Companies market the monophasic gestodene and ethinylestradiol OC under the name Minulet and the monophasic desogestrel and ethinylestradiol pill under the name Mercilon.
Triphasic norgestimate oral contraceptives versus monophasic oral contraceptives
Sulak 1999 compared a triphasic formulation composed of 180‐215‐250 μg norgestimate (NGM) and 35 μg ethinylestradiol with a monophasic formulation of 1000 μg norethindrone acetate and 20 μg ethinylestradiol (Sulak 1999). Companies market the triphasic pill under the name Ortho Tri‐Cyclen and the monophasic pill under the name Loestrin Fe 1/20.
In Rosenberg 1999, the triphasic formulation of 180‐215‐250 μg norgestimate and 35 μg ethinylestradiol was compared with: 1) a formulation of 100 μg levonorgestrel and 20 μg ethinylestradiol (mentioned above for Chavez 1999 and Reisman 1999), and 2) a monophasic formulation of 150 μg desogestrel and 20 μg ethinylestradiol for 21 days and 10 μg ethinylestradiol for five days (Rosenberg 1999). The brand name of the desogestrel containing monophasic preparation is Mircette.
A triphasic preparation of 180‐215‐250 μg norgestimate and 25 μg ethinylestradiol was compared with a monophasic preparation of 1000 μg norethindrone acetate and 20 μg ethinylestradiol (Hampton 2001). The triphasic oral contraceptive is marketed as Ortho Tri‐Cyclen Lo. Hampton 2001 also compared the triphasic to two 'cyclophasic' preparations but those data were not part of this review. Neither 'cyclophasic' regimen has been put on the market yet. The 'cyclophasic' pills had a fixed daily dose of ethinylestradiol and a dose of norgestimate that alternated every other day.
In Kaunitz 2009, the triphasic preparation of 180‐215‐250 μg norgestimate and 25 μg ethinylestradiol was compared with a monophasic pill containing 3 mg drospirenone and 20 μg ethinylestradiol. The drospirenone containing monophasic preparation is marketed as Yaz.
Excluded studies
We excluded one study described as a randomized controlled trial that proved to be a matched cohort study (Dubnitskaia 1988). Seven studies did not report the method used to generate the allocation sequence. After communication with the author, we excluded Grace 1994 because the allocation sequence was not randomized. We excluded the remaining six studies because we were unable to contact the authors (Christie 1989; Dik 1984; Matsumoto 1988; Otolorin 1989; Perrone 1987; Rubio‐Lotvin 1992). We did not include Bancroft 1987 due to the lack of relevant outcomes for this review.
Risk of bias in included studies
Overall, the description of the study methods was poor (CONSORT 2010; DerSimonian 1982).
Allocation
Only three of the 23 included trials reported the method of generating the allocation sequence (Agoestina 1987; Chen 1987; Kaunitz 2009). In addition, only five studies reported the use and method of concealing the treatment allocation sequence (Agoestina 1987; Ismail 1991; Kashanian 2010; Ramos 1989; Reisman 1999). Communication with the authors provided the method of generating the allocation sequence for eight trials (Carlborg 1983; Dunson 1993; Hampton 2001; Ismail 1991; Kashanian 2010; Reiter 1990; Rosenberg 1999; Saxena 1992). Nine authors informed us on whether allocation concealment was done and, if so, what method was used (Carlborg 1983; Chavez 1999; Dieben 1984; Dunson 1993; Hampton 2001; Reiter 1990; Rosenberg 1999; Rowan 1999; Saxena 1992).
Eight studies appeared to have adequate randomization and concealment of treatment allocation (Schulz 2002c). Randomization was done by a computer (Carlborg 1983; Dunson 1993; Hampton 2001; Ismail 1991; Rosenberg 1999; Saxena 1992) or a random number table (Agoestina 1987; Kashanian 2010). The methods used to conceal the allocation sequence included numbered pharmacy packages (Carlborg 1983); numbered containers (Agoestina 1987); sequentially‐numbered, sealed envelopes opened at the time of admission (Dunson 1993; Ismail 1991; Kashanian 2010; Saxena 1992); sequentially‐numbered randomization cards with an opaque scratch‐off dot (Rosenberg 1999); and a centralized voice‐activated randomization system (Hampton 2001). Only Dunson 1993 mentioned that the envelopes were opaque.
Eight studies provided information on either the randomization method or the allocation concealment. Randomization was done by a random number table in Chen 1987 or a computer in Kaunitz 2009 but both studies did not mention the method used to conceal the allocation sequence. Reiter 1990 generated the allocation sequence by a random number table but did not conceal the allocation sequence. We could not find out the method of randomization in four studies, but the studies appeared to use a proper method to conceal the treatment allocation sequence. Acceptable methods include numbered pharmacy packages (Ramos 1989; Rowan 1999) and sequentially‐numbered, opaque, sealed envelopes (Chavez 1999; Reisman 1999). However, Chavez 1999 did not mention whether the envelopes were sealed and Reisman 1999 did not note if the envelopes were opaque. In Dieben 1984, the method of randomization was unclear and the study featured inadequate concealment of allocation.
The remaining seven trials did not mention either the method used to generate the allocation sequence or the method for concealing the allocation sequence (Andrade 1993; Bruni 2000; Engebretsen 1987; Lachnit‐Fixson 1984; Percival‐Smith 1990; Sulak 1999; Zador 1979).
Blinding
Blinding was not mentioned in five trial reports (Agoestina 1987; Carlborg 1983; Kashanian 2010; Lachnit‐Fixson 1984; Saxena 1992). We obtained details on the use of blinding in four trials from the researchers (Carlborg 1983; Kashanian 2010; Rowan 1999; Saxena 1992). None of the studies provided information regarding successful implementation of blinding (Schulz 2002b). Two studies (Hampton 2001; Ramos 1989) reported information to judge the adequacy of the blinding methods (DerSimonian 1982; Schulz 2002b).
Three trials blinded investigators and participants (Carlborg 1983; Kashanian 2010; Ramos 1989). Furthermore, two trials reported that the study was double‐blinded without specifying who was kept unaware of the oral contraceptives assigned (Chen 1987; Rowan 1999) (DerSimonian 1982; Schulz 2002b). One trial was blinded for the outcome assessor (Percival‐Smith 1990). Fifteen trials were open (Andrade 1993; Bruni 2000; Chavez 1999; Dieben 1984; Dunson 1993; Engebretsen 1987; Hampton 2001; Ismail 1991; Kaunitz 2009; Reisman 1999; Reiter 1990; Rosenberg 1999; Saxena 1992; Sulak 1999; Zador 1979). The remaining two trials did not mention the use of blinding; we were unable to contact one team of researchers (Agoestina 1987) and another investigator could not provide additional information (Lachnit‐Fixson 1984).
Incomplete outcome data
Nine trials described detailed information on the number of and reasons for discontinuation (Andrade 1993; Bruni 2000; Chavez 1999; Chen 1987; Ismail 1991; Kaunitz 2009; Ramos 1989; Reisman 1999; Saxena 1992). We acquired information on the number of and reasons for discontinuation from the authors of three studies (Dieben 1984; Kashanian 2010; Rosenberg 1999). The other trials provided insufficient or no information on withdrawals.
Study discontinuation ranged from 4% to 77%. Thirteen trials included data on losses to follow up. Loss to follow up varied from zero to 39%. In Dunson 1993, more than 20% of the participants were lost to follow up. Losses to follow‐up rates greater than 20% may threaten the validity of trials (Strauss 2005).
Of 23 included studies, 17 excluded participants after randomization for reasons like: failure to start oral contraceptives, failure to appear at the first follow‐up visit, incorrect administration of oral contraceptives, protocol violations such as incorrect pill‐taking or skipping the pill‐free interval, inaccurate recording of data, loss to follow up or cycle disturbances (Andrade 1993; Bruni 2000; Carlborg 1983; Chavez 1999; Chen 1987; Dieben 1984; Engebretsen 1987; Hampton 2001; Ismail 1991; Kaunitz 2009; Kashanian 2010; Percival‐Smith 1990; Ramos 1989; Reisman 1999; Reiter 1990; Saxena 1992; Sulak 1999). Exclusion of participants after randomization may lead to bias (Schulz 2002a).
Two trials reported an analysis based on the intention‐to‐treat principle (Dunson 1993; Hampton 2001). Sulak 1999 stated that the analysis was carried out on the intention‐to‐treat population. However, the population included only participants who had started oral contraceptives and who had at least one cycle control measurement after the baseline (Sulak 1999). In addition, cycles were considered invalid and excluded if they had incorrect pill‐taking or were without a pill‐free interval, lasted longer than 31 days or had inaccurate recording of bleeding data. Kaunitz 2009 stated that the evaluation of bleeding patterns was based on the intention‐to‐treat population, defined as all randomly assigned participants who took the study drug and for whom there were post‐baseline bleeding data after day seven. Another 15 trials did not perform an intention‐to‐treat analysis (Agoestina 1987; Andrade 1993; Bruni 2000; Carlborg 1983; Chavez 1999; Chen 1987; Dieben 1984; Engebretsen 1987; Ismail 1991; Kashanian 2010; Percival‐Smith 1990; Ramos 1989; Reisman 1999; Reiter 1990; Saxena 1992). In four studies, it was unclear whether an analysis based on the intention‐to‐treat approach was performed (Lachnit‐Fixson 1984; Rosenberg 1999; Rowan 1999; Zador 1979).
Four studies continued with a proportion of the participants after six cycles of pill use. In Hampton 2001, women were enrolled for six cycles or 12 cycles of pill use at admittance. In Andrade 1993, Carlborg 1983 and Dieben 1984, we could not find out whether the continuation was decided previously or during the study. The latter may result in selection bias.
Other potential sources of bias
A priori hypothesis and sample size calculation
An a priori hypothesis and sample size calculation were provided in four studies (Kashanian 2010; Kaunitz 2009; Percival‐Smith 1990; Reisman 1999) (DerSimonian 1982). However, Kashanian 2010 was inconsistent regarding the power (80% or 85%). Another study stated that the sample size was developed to meet the US regulatory requirements to evaluate the safety and efficacy of oral contraceptives and provided the power (Hampton 2001). One study reported a sample size without explanation (Lachnit‐Fixson 1984).
Funding
Five of the 23 included trials were conducted or supported by independent organizations: World Health Organization (Chen 1987); Family Health International (Dunson 1993; Ismail 1991); Planned Parenthood Federation (Reiter 1990); and Indian Council of Medical Research (Saxena 1992). Fourteen trials were conducted or sponsored by pharmaceutical companies (Agoestina 1987; Bruni 2000; Carlborg 1983; Chavez 1999; Dieben 1984; Hampton 2001; Kaunitz 2009; Lachnit‐Fixson 1984; Percival‐Smith 1990; Reisman 1999; Rosenberg 1999; Rowan 1999; Sulak 1999; Zador 1979). One study was supported by an international organization (United Nations Population Fund) in combination with a pharmaceutical company (Ramos 1989). We could not identify any assistance for three trials (Andrade 1993; Engebretsen 1987; Kashanian 2010). Studies sponsored by pharmaceutical companies are more likely to have outcomes favoring the sponsor than studies funded by other sources (Als‐Nielsen 2003; Lexchin 2003).
Effects of interventions
Contraceptive effectiveness
Nineteen studies comparing a triphasic formulation with a monophasic formulation assessed contraceptive effectiveness (Agoestina 1987; Andrade 1993; Bruni 2000; Carlborg 1983; Chavez 1999; Chen 1987; Dieben 1984; Dunson 1993; Engebretsen 1987; Hampton 2001; Ismail 1991; Kaunitz 2009; Lachnit‐Fixson 1984; Ramos 1989; Reisman 1999; Rosenberg 1999; Saxena 1992; Sulak 1999; Zador 1979). An additional four studies did not report data regarding pregnancy (Kashanian 2010; Percival‐Smith 1990; Reiter 1990; Rowan 1999). Most studies included pregnancies caused by inadequacy of the method as well as imperfect use in the reported number of pregnancies (Trussell 1991). However, in three reports we could not figure out whether the pregnancies were caused by method failures solely or by both method and user failures (Engebretsen 1987; Kaunitz 2009; Saxena 1992). In the analyses, we considered the number of pregnancies reported in these three studies as method and user failures. There was a discrepancy in the described numbers of pregnancies between Cullberg et al (1982) from Dieben 1984 and the later report of Dieben 1984. The Dieben 1984 paper mentioned two pregnancies, and the paper by Cullberg et al (1982) noted three pregnancies. Communication with the author revealed that three pregnancies occurred in the study period.
No significant differences were found between the various pills in contraceptive effectiveness (Analysis 1.1, Analysis 1.2, Analysis 2.1, Analysis 3.1, Analysis 4.1, Analysis 4.2, Analysis 9.1, Analysis 11.1, Analysis 11.2, Analysis 12.1, Analysis 13.1, Analysis 14.1, Analysis 15.1, Analysis 16.1, Analysis 17.1, Analysis 18.1) (Table 19).
1.1. Analysis.
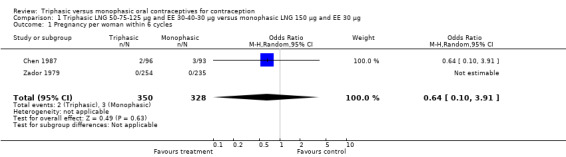
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 1 Pregnancy per woman within 6 cycles.
1.2. Analysis.
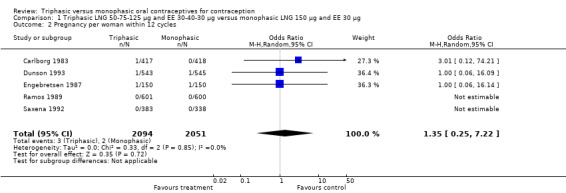
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 2 Pregnancy per woman within 12 cycles.
2.1. Analysis.
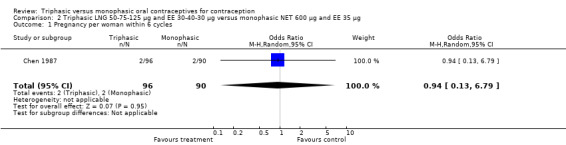
Comparison 2 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 600 μg and EE 35 μg, Outcome 1 Pregnancy per woman within 6 cycles.
3.1. Analysis.
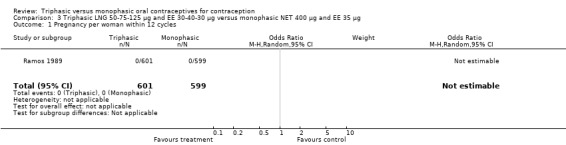
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 1 Pregnancy per woman within 12 cycles.
4.1. Analysis.
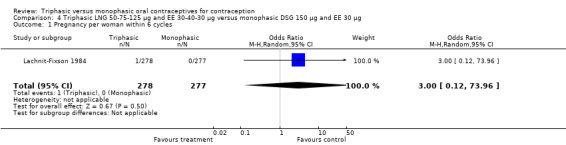
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 1 Pregnancy per woman within 6 cycles.
4.2. Analysis.
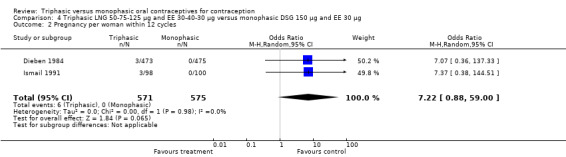
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 2 Pregnancy per woman within 12 cycles.
9.1. Analysis.
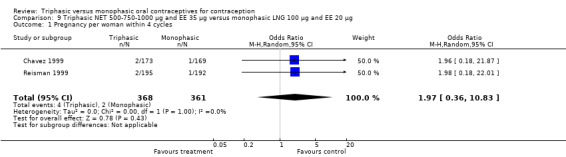
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 1 Pregnancy per woman within 4 cycles.
11.1. Analysis.
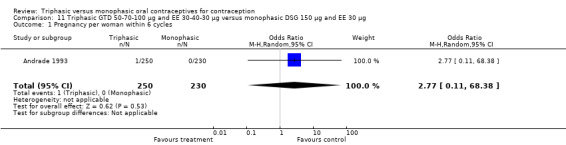
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 1 Pregnancy per woman within 6 cycles.
11.2. Analysis.
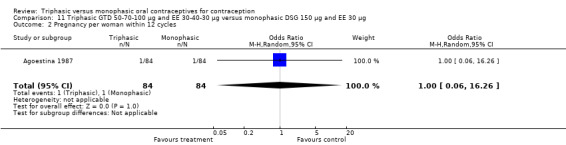
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 2 Pregnancy per woman within 12 cycles.
12.1. Analysis.
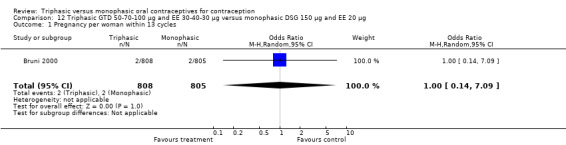
Comparison 12 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 20 μg, Outcome 1 Pregnancy per woman within 13 cycles.
13.1. Analysis.
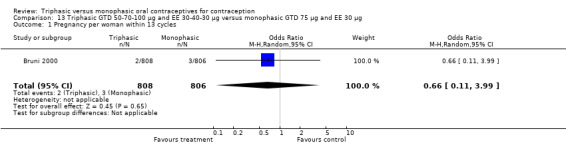
Comparison 13 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic GTD 75 μg and EE 30 μg, Outcome 1 Pregnancy per woman within 13 cycles.
14.1. Analysis.
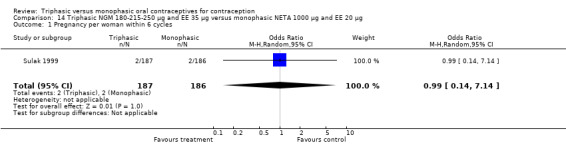
Comparison 14 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 1 Pregnancy per woman within 6 cycles.
15.1. Analysis.
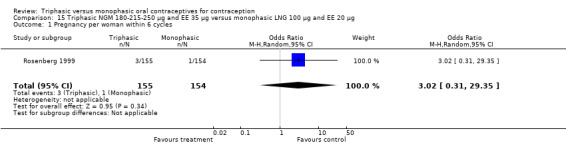
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 1 Pregnancy per woman within 6 cycles.
16.1. Analysis.
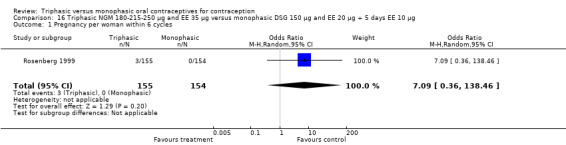
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 1 Pregnancy per woman within 6 cycles.
17.1. Analysis.
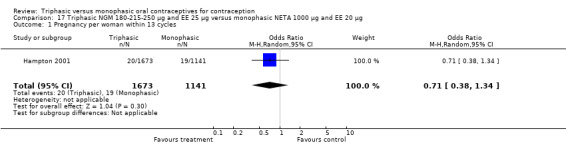
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 1 Pregnancy per woman within 13 cycles.
18.1. Analysis.
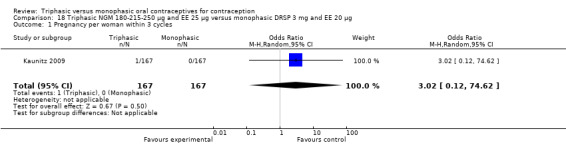
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 1 Pregnancy per woman within 3 cycles.
1. Pregnancies and total study cycles for triphasic and monophasic formulations.
| Triphasic | Monophasic | |||
| Study | Pregnancies | Total cycles | Pregnancies | Total cycles |
| Agoestina 1987 | 1 | 915 | 1 | 903 |
| Andrade 1993 | 1 | 1398 | 0 | 1245 |
| Carlborg 1983 | 1 | 1574 | 0 | 3275 |
| 0 | 1623 | |||
| Chavez 1999 | 2 | 400 | 1 | 384 |
| Chen 1987 | 2 | 492 | 3 | 478 |
| 2 | 474 | |||
| Dieben 1984 | 3 | 2709 | 0 | 2771 |
| Engebretsen 1987 | 1 | 1442 | 1 | 1416 |
| Hampton 2001 | 20 | 11003 | 19 | 7497 |
| Ismail 1991 | 3 | 741 | 0 | 811 |
| Lachnit‐Fixson 1984 | 1 | 1536 | 0 | 1524 |
| Reisman 1999 | 2 | 506 | 1 | 453 |
| Rosenberg 1999 | 3 | 831 | 1 | 819 |
| 0 | 848 | |||
| Saxena 1992 | 0 | 3319 | 0 | 2949 |
| Zador 1979 | 0 | 1440 | 0 | 1343 |
Cycle control
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 150 μg LNG and 30 μg EE (Comparison 1)
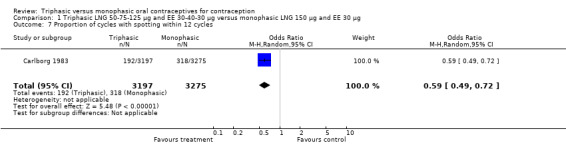
Four studies provided data on intermenstrual bleeding that fulfilled the inclusion criteria (Carlborg 1983; Dunson 1993; Ramos 1989; Zador 1979). In Carlborg 1983 and Zador 1979, users of monophasic LNG oral contraceptives reported more cycles with spotting and breakthrough bleeding within 3, 6 and 12 cycles of pill use compared to users of triphasic LNG oral contraceptives (Analysis 1.3 to Analysis 1.8). For the two studies combined, the OR was 0.57 (95% CI 0.48 to 0.67) for the proportion of cycles with spotting within 6 cycles (Analysis 1.5). For the proportion of cycles with breakthrough bleeding by 6 cycles, the OR was 0.63 (95% CI 0.50 to 0.80) for the two studies combined (Analysis 1.6). In Dunson 1993, which reported the proportion of women with intermenstrual bleeding within 12 cycles, the two formulations did not differ (Analysis 1.14). The sample size of the Ramos 1989 study was too small to assess differences in the number of women with spotting or breakthrough bleeding during cycle 6 or 12 (Analysis 1.10 to Analysis 1.13).
1.3. Analysis.
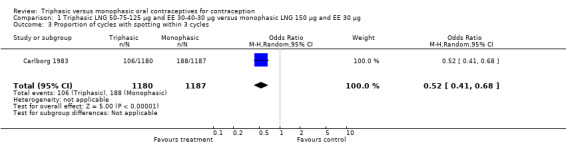
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 3 Proportion of cycles with spotting within 3 cycles.
1.8. Analysis.
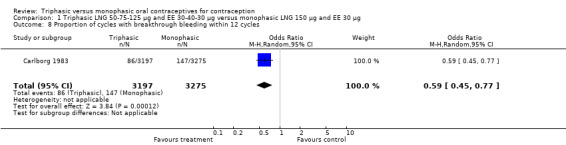
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 8 Proportion of cycles with breakthrough bleeding within 12 cycles.
1.5. Analysis.
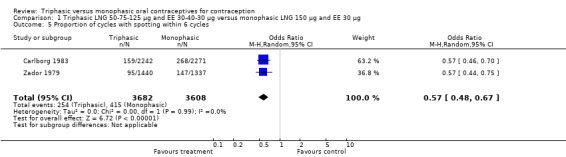
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 5 Proportion of cycles with spotting within 6 cycles.
1.6. Analysis.
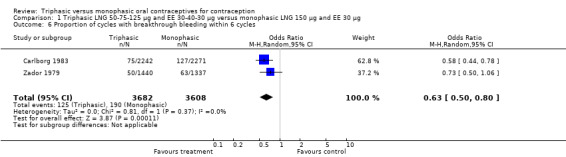
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 6 Proportion of cycles with breakthrough bleeding within 6 cycles.
1.14. Analysis.
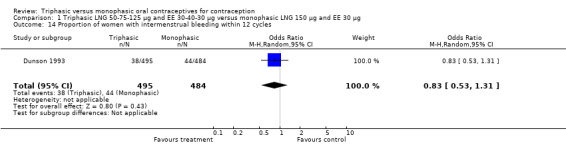
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 14 Proportion of women with intermenstrual bleeding within 12 cycles.
1.10. Analysis.
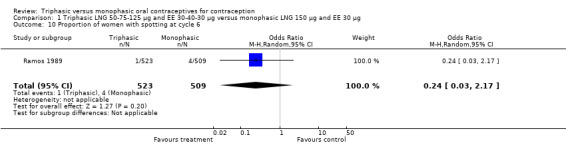
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 10 Proportion of women with spotting at cycle 6.
1.13. Analysis.
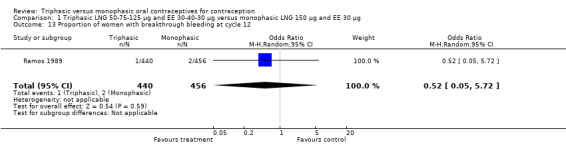
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 13 Proportion of women with breakthrough bleeding at cycle 12.
Saxena 1992 found no bleeding pattern differences between triphasic LNG and monophasic LNG oral contraceptives (Table 20). Chen 1987 observed less spotting in the participants using triphasic pills (Table 20). Engebretsen 1987 reported that triphasic LNG OC and the monophasic LNG OC were similar in the incidence of spotting and breakthrough bleeding.
2. Bleeding pattern 50‐75‐125 μg LNG plus 30‐40‐30 μg EE versus 150 μg LNG plus 30 μg EE.
| Study and COC | Reference period | Number | Acceptable pattern | Infrequent bleeding | Frequent/ prolonged bleeding | No. of bleeding runs | Total bleeding days | Total spotting days |
| Saxena 1992 | ||||||||
| Triphasic LNG | 1 | 289 | 82.7 | 11.8 | 5.5 | 2.9 + 0.6 | 9.9 + 3.5 | 2.8 + 3.6 |
| 2 | 250 | 84.4 | 10.4 | 5.2 | 3.1 + 0.6 | 9.8 + 3.5 | 2.5 + 2.8 | |
| 3 | 195 | 90.3 | 8.2 | 1.5 | 3.0 + 0.5 | 9.4 + 3.1 | 2.7 + 2.9 | |
| 4 | 123 | 83.7 | 10.6 | 5.7 | 3.1 + 0.6 | 9.0 + 2.6 | 3.0 + 3.1 | |
| Monophasic LNG | 1 | 248 | 80.2 | 15.7 | 4.0 | 2.8 + 0.8 | 8.9 + 2.9 | 3.3 + 4.1 |
| 2 | 207 | 85.0 | 12.1 | 2.9 | 3.0 + 0.6 | 9.2 + 2.7 | 2.7 + 3.1 | |
| 3 | 183 | 85.2 | 13.7 | 1.1 | 3.0 + 0.6 | 8.8 + 2.8 | 2.8 + 2.9 | |
| 4 | 129 | 89.1 | 10.1 | 0.8 | 2.9 + 0.5 | 8.5 + 2.5 | 3.5 + 3.5 | |
| Chen 1987 | ||||||||
| Triphasic LNG | 1 | 16.0 + 4.1 | 5.6 + 4.8 | |||||
| 1+2 | 26.2 + 5.8 | 8.7 + 7.5 | ||||||
| Monophasic LNG | 1 | 15.1 + 4.3 | 8.0 + 7.1 | |||||
| 1+2 | 25.0 + 7.0 | 11.2 + 8.4 | ||||||
| Monophasic NET | 1 | 14.8 + 5.0 | 9.4 + 6.3 | |||||
| 1+2 | 25.8 + 8.2 | 14.2 + 8.9 | ||||||
In Kashanian 2010, breakthrough bleeding or spotting was less likely for the triphasic group than the monophasic group at cycle 3 (OR 0.10; 95% CI 0.01 to 0.77) (Analysis 1.15) but no events were reported for either group at cycle 6 (Analysis 1.16).
1.15. Analysis.
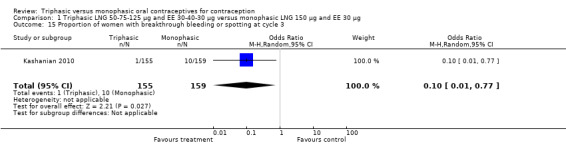
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 15 Proportion of women with breakthrough bleeding or spotting at cycle 3.
1.16. Analysis.
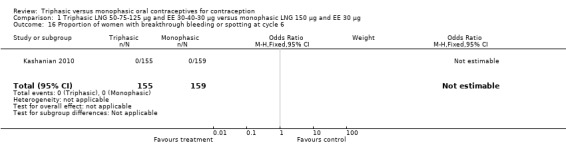
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 16 Proportion of women with breakthrough bleeding or spotting at cycle 6.
Three studies reported data on absence of withdrawal bleeding (Carlborg 1983; Dunson 1993; Zador 1979). Users of triphasic LNG OC were less likely to experience amenorrhea than users of monophasic LNG OC within 12 cycles (OR 0.27; 95% CI 0.17 to 0.45) (Analysis 1.18) (Carlborg 1983). However, the Dunson 1993 (Analysis 1.19) and Zador 1979 studies did not find a difference between the two groups in the proportion of cycles with amenorrhea within six cycles and the proportion of women with amenorrhea within 12 cycles. Ramos 1989 also did not observe a difference between the two groups in the incidence of amenorrhea (Table 21).
1.18. Analysis.
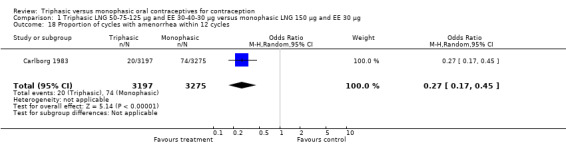
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 18 Proportion of cycles with amenorrhea within 12 cycles.
1.19. Analysis.
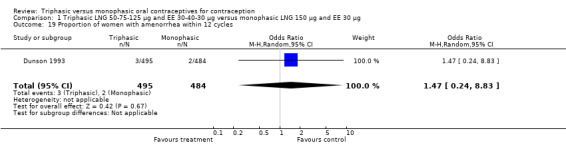
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 19 Proportion of women with amenorrhea within 12 cycles.
3. Withdrawal bleeding 50‐75‐125 μg LNG plus 30‐40‐30 μg EE versus 150 μg LNG plus 30 μg EE (Ramos 1989).
| Months | Triphasic LNG | Monophasic LNG | Monophasic NET |
| 0 to 3 | 39.4 | 42.6 | 45.9 |
| 4 to 6 | 88.1 | 89.7 | 89.7 |
| 7 to 9 | 95.2 | 96.0 | 94.4 |
| 10 to 12 | 93.9 | 93.7 | 94.2 |
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 600 μg norethindrone (NET) and 35 μg EE (Comparison 2)
This comparison was based on a single trial (Chen 1987). Triphasic LNG oral contraceptive users reported less spotting compared to monophasic NET oral contraceptive users (Table 20).
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 400 μg norethindrone (NET) and 35 μg EE (Comparison 3)
One trial was included for this comparison (Ramos 1989). During the sixth cycle, spotting and breakthrough bleeding were less common in women taking triphasic LNG contraceptive pills in comparison with women taking monophasic NET pills. For spotting, the OR was 0.12 (95% CI 0.01 to 0.94) (Analysis 3.2). For breakthrough bleeding, the OR was 0.31 (95% CI 0.11 to 0.86) (Analysis 3.3). This difference did not remain at the 12th cycle (Analysis 3.4, Analysis 3.5).
3.2. Analysis.
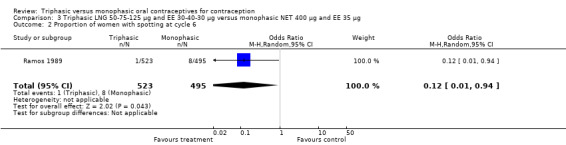
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 2 Proportion of women with spotting at cycle 6.
3.3. Analysis.
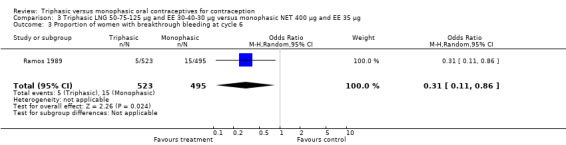
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 3 Proportion of women with breakthrough bleeding at cycle 6.
3.4. Analysis.
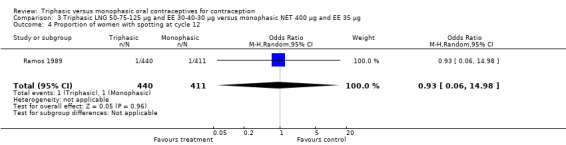
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 4 Proportion of women with spotting at cycle 12.
3.5. Analysis.
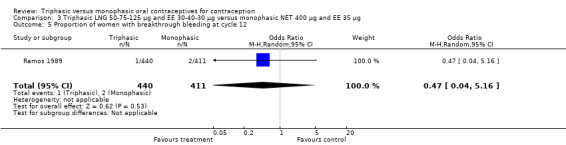
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 5 Proportion of women with breakthrough bleeding at cycle 12.
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 150 μg desogestrel (DSG) and 30 μg EE (Comparison 4)
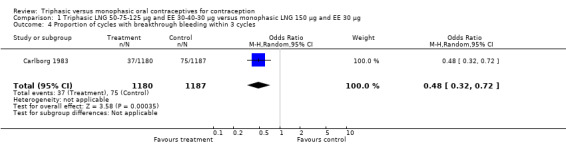
Three studies reported data on intermenstrual bleeding consistent with the inclusion criteria (Dieben 1984; Ismail 1991; Lachnit‐Fixson 1984). Dieben 1984 provided data regarding intermenstrual bleeding that was not described in the paper. Overall, the incidence of spotting or breakthrough bleeding did not differ between women using triphasic LNG OC and monophasic DSG oral contraceptives. Significant heterogeneity was present when Dieben 1984 and Lachnit‐Fixson 1984 were combined in a meta‐analysis for some comparisons. By study, the effects were in different directions. The trials may have used different definitions of bleeding problems since neither provided clear information. Therefore, we divided two meta‐analyses into two separate analyses. When the studies were examined separately, Lachnit‐Fixson 1984 showed that the triphasic group had fewer cycles with spotting (OR 0.34; 95% CI 0.27 to 0.44) (Analysis 4.7) and with breakthrough bleeding (OR 0.41; 95% CI 0.23 to 0.71) (Analysis 4.9) than the monophasic group. In the Dieben 1984 and Lachnit‐Fixson 1984 trials combined, users of triphasic LNG oral contraceptives reported fewer cycles in which breakthrough bleeding and spotting occurred in the same cycle compared to users of monophasic DSG OC during the first six months (OR 0.50; 95% CI 0.29 to 0.86) (Analysis 4.10).
4.7. Analysis.
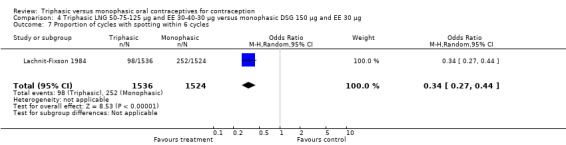
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 7 Proportion of cycles with spotting within 6 cycles.
4.9. Analysis.
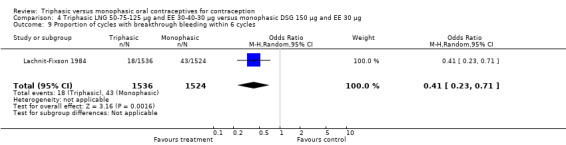
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 9 Proportion of cycles with breakthrough bleeding within 6 cycles.
4.10. Analysis.
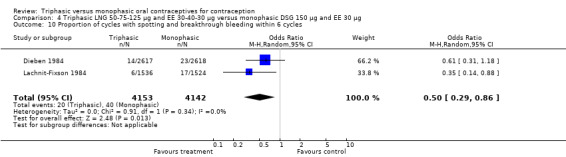
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 10 Proportion of cycles with spotting and breakthrough bleeding within 6 cycles.
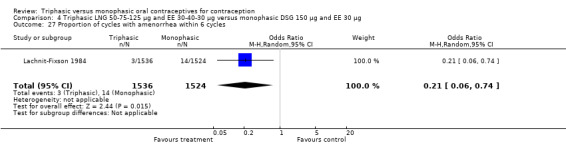
These three studies also described data on withdrawal bleeding. For Ismail 1991, no significant difference between the two preparations was found regarding the outcome of amenorrhea. However, significant heterogeneity was present in a meta‐analysis of Dieben 1984 and Lachnit‐Fixson 1984. When the studies were examined separately, Lachnit‐Fixson 1984 showed that the triphasic group had fewer cycles with amenorrhea than the monophasic group within six cycles (OR 0.21; 95% CI 0.06 to 0.74) (Analysis 4.27).
4.27. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 27 Proportion of cycles with amenorrhea within 6 cycles.
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 1500 μg norethindrone acetate (NETA) and 30 μg EE (Comparison 5)
One trial was included in this comparison (Percival‐Smith 1990). Users of triphasic LNG oral contraceptives were somewhat less likely to experience intermenstrual bleeding and amenorrhea within six cycles of pill use than were users of the monophasic NETA OC. The OR for intermenstrual bleeding was 0.76 (95% CI 0.56 to 1.01) (Analysis 5.1). For amenorrhea, the OR was 0.02 (95% CI 0.00 to 0.18) (Analysis 5.2).
5.1. Analysis.

Comparison 5 Triphasic LNG 50‐75‐125 μg/ EE 30‐40‐30 μg versus monophasic NETA 1500 μg/ EE 30 g, Outcome 1 Proportion of cycles with intermenstrual bleeding within 6 cycles.
5.2. Analysis.
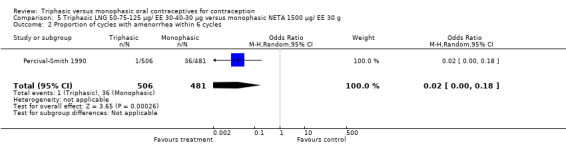
Comparison 5 Triphasic LNG 50‐75‐125 μg/ EE 30‐40‐30 μg versus monophasic NETA 1500 μg/ EE 30 g, Outcome 2 Proportion of cycles with amenorrhea within 6 cycles.
500‐750‐1000 μg norethindrone (NET) and 35 μg ethinylestradiol (EE) versus 1500 μg norethindrone acetate (NETA) and 30 μg EE (Comparison 6)
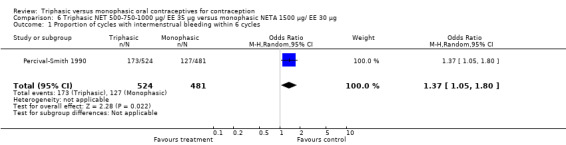
This comparison was based on a single trial (Percival‐Smith 1990). Users of triphasic NET oral contraceptives were more likely to experience intermenstrual bleeding (OR 1.37; 95% CI 1.05 to 1.80) (Analysis 6.1) and less likely to experience amenorrhea (OR 0.59; 95% CI 0.35 to 1.01) (Analysis 6.2) compared to users of the monophasic NETA OC.
6.1. Analysis.
Comparison 6 Triphasic NET 500‐750‐1000 μg/ EE 35 μg versus monophasic NETA 1500 μg/ EE 30 μg, Outcome 1 Proportion of cycles with intermenstrual bleeding within 6 cycles.
6.2. Analysis.
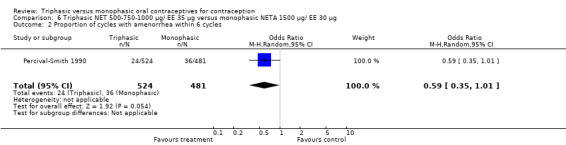
Comparison 6 Triphasic NET 500‐750‐1000 μg/ EE 35 μg versus monophasic NETA 1500 μg/ EE 30 μg, Outcome 2 Proportion of cycles with amenorrhea within 6 cycles.
50‐75‐125 μg levonorgestrel (LNG) and 30‐40‐30 μg ethinylestradiol (EE) versus 1000 μg norethindrone (NET) and 35 μg EE (Comparison 7)
One study provided data for this comparison (Reiter 1990). The numbers of women having intermenstrual bleeding within 12 cycles were similar for the triphasic LNG OC and the monophasic NET OC groups (Analysis 7.1). In the group of triphasic LNG pill users, the incidence of amenorrhea was lower than in the group of monophasic NET pill users (OR 0.03; 95% CI 0.00 to 0.43) (Analysis 7.2).
7.1. Analysis.
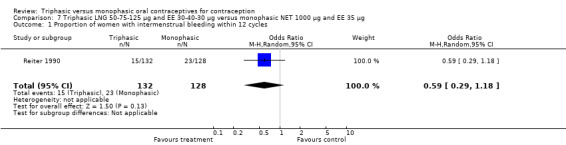
Comparison 7 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 1000 μg and EE 35 μg, Outcome 1 Proportion of women with intermenstrual bleeding within 12 cycles.
7.2. Analysis.
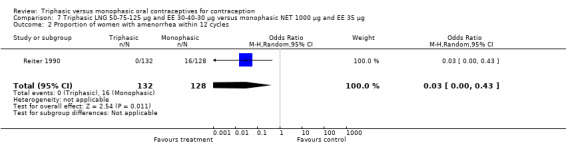
Comparison 7 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 1000 μg and EE 35 μg, Outcome 2 Proportion of women with amenorrhea within 12 cycles.
500‐750‐1000 μg norethindrone (NET) and 35 μg ethinylestradiol (EE) versus 1000 μg NET and 35 μg EE (Comparison 8)
This comparison was based on a single trial (Reiter 1990). The occurrence of intermenstrual bleeding did not differ between triphasic NET and monophasic NET OCs (Analysis 8.1). Women receiving triphasic NET pills experienced amenorrhea less frequently than women using monophasic NET pills (OR 0.25; 95% CI 0.08 to 0.76) (Analysis 8.2).
8.1. Analysis.
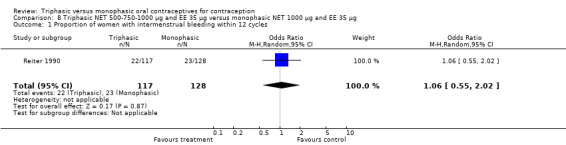
Comparison 8 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic NET 1000 μg and EE 35 μg, Outcome 1 Proportion of women with intermenstrual bleeding within 12 cycles.
8.2. Analysis.
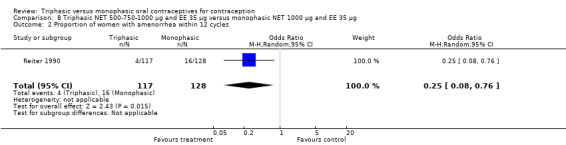
Comparison 8 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic NET 1000 μg and EE 35 μg, Outcome 2 Proportion of women with amenorrhea within 12 cycles.
500‐750‐1000 μg norethindrone (NET) and 35 μg ethinylestradiol (EE) versus 100 μg levonorgestrel (LNG) and 20 μg EE (Comparison 9)
Data on intermenstrual bleeding and amenorrhea were described in two trials (Chavez 1999; Reisman 1999). Reisman 1999 provided us with the number of women that had spotting, breakthrough bleeding and amenorrhea at each treatment cycle. No difference was found in the occurrence of intermenstrual bleeding and amenorrhea between triphasic NET OC and monophasic LNG oral contraceptives.
1000 μg norethindrone acetate (NETA) and 20‐30‐35 μg ethinylestradiol (EE) versus 1500 μg NETA and 30 μg EE (Comparison 10)
One trial was included in this comparison. Rowan 1999 noted that 'estrophasic' NETA pills and monophasic NETA pills had a similar incidence of breakthrough bleeding, but the report did not provide the proportion of women or cycles with breakthrough bleeding. No data regarding the incidence of amenorrhea were provided.
50‐70‐100 μg gestodene (GTD) and 30‐40‐30 μg ethinylestradiol (EE) versus 150 μg desogestrel (DSG) and 30 μg EE (Comparison 11)
This comparison was based on two studies (Agoestina 1987; Andrade 1993). Andrade 1993 observed in the group of women using triphasic GTD OC fewer cycles with breakthrough plus spotting within 6 cycles compared to the group of women using monophasic DSG oral contraceptives (OR 0.49; 95% CI 0.33 to 0.73) (Analysis 11.5). Overall, the two preparations did not differ regarding the outcomes spotting, breakthrough bleeding and amenorrhea.
11.5. Analysis.
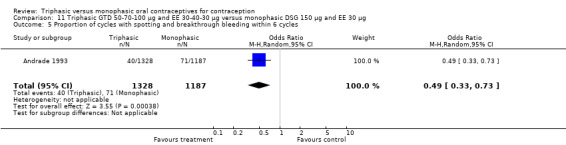
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 5 Proportion of cycles with spotting and breakthrough bleeding within 6 cycles.
50‐70‐100 μg gestodene (GTD) and 30‐40‐30 μg ethinylestradiol (EE) versus 150 μg desogestrel (DSG) and 20 μg EE (Comparison 12)
One trial provided data for this comparison. Bruni 2000 stated that the proportion of women with spotting or breakthrough bleeding was generally lower in the group of women using triphasic GTD pills compared to women using monophasic DSG oral contraceptives. However, the numbers of women with spotting or breakthrough bleeding were not provided in the paper. Triphasic pills were reportedly associated with significantly less spotting than monophasic pills at cycles 1, 2, 4 to 7, 9 and 11 and with significantly less breakthrough bleeding at cycles 1, 3, 4, 6, 9 and 11. From 1% to 6% of the triphasic pill users and 3% to 6% of the monophasic pill users experienced amenorrhea.
50‐70‐100 μg gestodene (GTD) and 30‐40‐30 μg ethinylestradiol (EE) versus 75 μg GTD and 30 μg EE (Comparison 13)
This comparison was based on a single study. Bruni 2000 reported that triphasic and monophasic GTD preparations produced similar patterns of cycle control, but the report did not provide the proportion of women with intermenstrual bleeding and amenorrhea.
180‐215‐250 μg norgestimate (NGM) and 35 μg ethinylestradiol (EE) versus monophasic 1000 μg norethindrone acetate (NETA) and 20 μg EE (Comparison 14)
One study provided data on this comparison (Sulak 1999). During all six treatment cycles the incidence of spotting or breakthrough bleeding was significantly lower among triphasic NGM preparation users compared with monophasic NETA preparation users. However, except for the sixth cycle, the report did not provide the proportion of women or cycles with spotting or breakthrough bleeding. The percentage of cycles with spotting or breakthrough bleeding within the treatment period was 9.6% for the triphasic group and 32.6% for the monophasic group. During the sixth cycle, significantly fewer participants using triphasic NGM contraceptive pills experienced spotting and breakthrough bleeding than did the participants using monophasic NETA pills (OR 0.26; 95% CI 0.14 to 0.51) (Analysis 14.2).
14.2. Analysis.
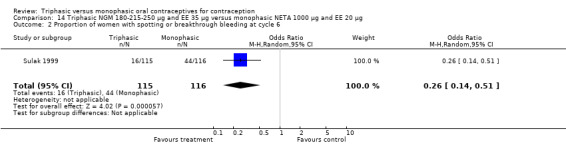
Comparison 14 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 2 Proportion of women with spotting or breakthrough bleeding at cycle 6.
Further, the report mentioned that amenorrhea was significantly less common in the triphasic group compared to the monophasic group during the second to sixth cycles (Sulak 1999). However, except for the sixth cycle, the number of cycles with amenorrhea was not provided. Analysis 14.3 displays the difference in number of women with amenorrhea at cycle six (OR 0.17; 95% CI 0.06 to 0.45).
14.3. Analysis.
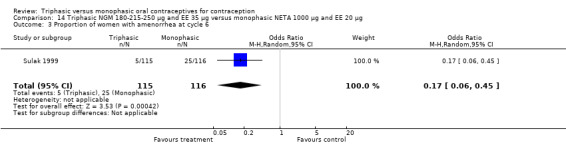
Comparison 14 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 3 Proportion of women with amenorrhea at cycle 6.
180‐215‐250 μg norgestimate (NGM) and 35 μg ethinylestradiol (EE) versus 100 μg levonorgestrel (LNG) and 20 μg EE (Comparison 15)
The comparison was based on one study. Rosenberg 1999 provided us with the number of cycles with spotting, breakthrough bleeding and amenorrhea in the total treatment period. The incidence of spotting was lower among users of triphasic NGM OC compared to users of the monophasic LNG oral contraceptive (OR 0.59; 95% CI 0.42 to 0.81) (Analysis 15.2). The incidence of breakthrough bleeding was similar for the two preparations. Women receiving triphasic NGM pills experienced less amenorrhea than women receiving monophasic LNG pills (OR 0.57; 95% CI 0.34 to 0.96) (Analysis 15.4).
15.2. Analysis.
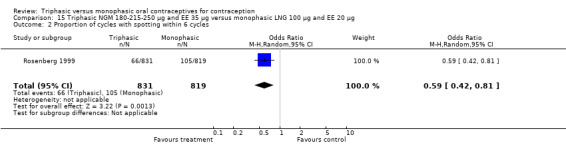
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 2 Proportion of cycles with spotting within 6 cycles.
15.4. Analysis.
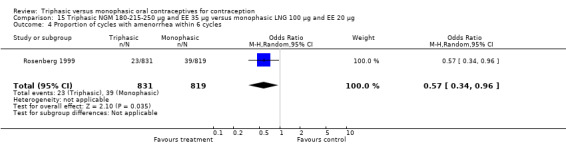
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 4 Proportion of cycles with amenorrhea within 6 cycles.
180‐215‐250 μg norgestimate (NGM) and 35 μg ethinylestradiol (EE) versus 150 μg desogestrel (DSG) and 20 μg EE + 5 days of 10 μg EE (Comparison 16)
One study was included in this comparison. Rosenberg 1999 provided the number of cycles with spotting, breakthrough bleeding and amenorrhea within the treatment period. Spotting was less common in the group of triphasic NGM oral contraceptives users as compared to the monophasic DSG OC users (OR 0.65; 95% CI 0.47 to 0.91) (Analysis 16.2). No difference was found in the occurrence of breakthrough bleeding. The incidence of amenorrhea was lower among women receiving triphasic NGM pills than for women receiving monophasic DSG pills (OR 0.37; 95% CI 0.23 to 0.60) (Analysis 16.4).
16.2. Analysis.
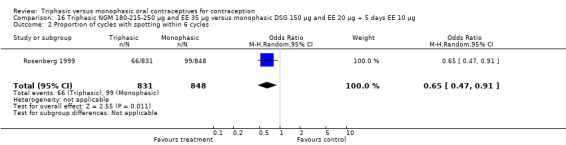
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 2 Proportion of cycles with spotting within 6 cycles.
16.4. Analysis.
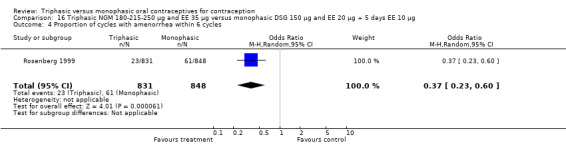
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 4 Proportion of cycles with amenorrhea within 6 cycles.
180‐215‐250 μg norgestimate (NGM) and 25 μg ethinylestradiol (EE) versus 1000 μg norethindrone acetate (NETA) and 20 μg EE (Comparison 17)
This comparison included one study. Hampton 2001 observed that users of triphasic NGM oral contraceptives were less likely to experience intermenstrual bleeding and amenorrhea than users of monophasic NETA OC (Analysis 17.2 to Analysis 17.15). For the proportion of cycles with breakthrough bleeding or spotting within 12 cycles, the OR was 0.45 (95% CI 0.41 to 0.49) (Analysis 17.7). The mean number of days of unscheduled bleeding or spotting was lower for women using triphasic oral contraceptives compared to the women using monophasic oral contraceptives at cycle 3 (MD ‐0.45; 95% CI ‐0.85 to ‐0.05) (Analysis 17.16) but at cycle 6 the mean number of days of bleeding or spotting was comparable (MD 0.20; 95% CI ‐0.22 to 0.62) (Analysis 17.17). The OR for amenorrhea within 13 cycles was 0.05 (95% CI 0.04 to 0.07) (Analysis 17.18).
17.2. Analysis.

Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 2 Proportion of cycles with breakthrough bleeding within 3 cycles.
17.15. Analysis.
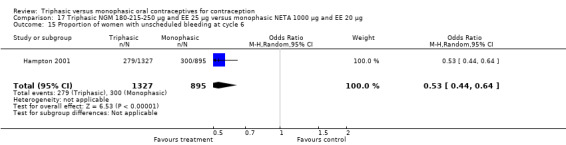
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 15 Proportion of women with unscheduled bleeding at cycle 6.
17.7. Analysis.
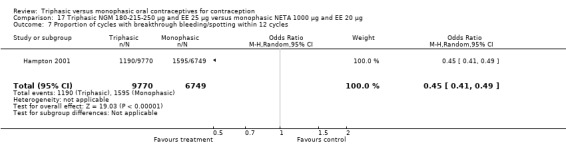
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 7 Proportion of cycles with breakthrough bleeding/spotting within 12 cycles.
17.16. Analysis.
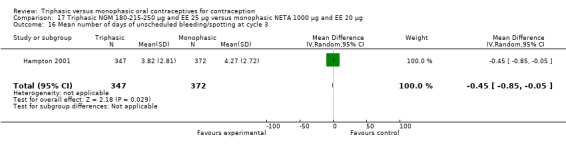
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 16 Mean number of days of unscheduled bleeding/spotting at cycle 3.
17.17. Analysis.
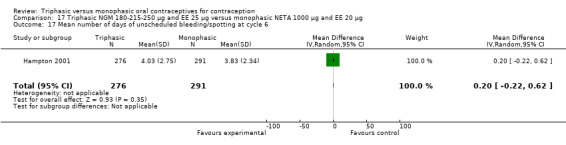
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 17 Mean number of days of unscheduled bleeding/spotting at cycle 6.
17.18. Analysis.
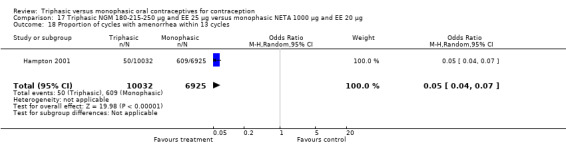
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 18 Proportion of cycles with amenorrhea within 13 cycles.
180‐215‐250 μg norgestimate (NGM) and 25 μg ethinylestradiol (EE) versus drospirenone 3 mg and 20 μg EE (Comparison 18)
One study provided data for this comparison (Kaunitz 2009). During the three cycles of the study period fewer women using triphasic NGM oral contraceptives experienced an unscheduled bleeding episode (OR 0.42; 95% CI 0.25 to 0.70) (Analysis 18.2) and they reported fewer unscheduled bleeding days (MD ‐1.50; 95% CI ‐2.75 to ‐0.25) (Analysis 18.4) in comparison with women using monophasic DRSP oral contraceptives. The report mentioned that an absence of scheduled bleeding was significantly less for the triphasic NGM group compared with the monophasic DRSP group in each cycle (21% versus 42% at cycle 3; P < 0.001).
18.2. Analysis.
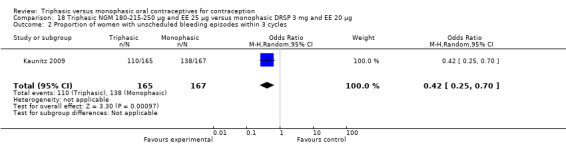
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 2 Proportion of women with unscheduled bleeding episodes within 3 cycles.
18.4. Analysis.
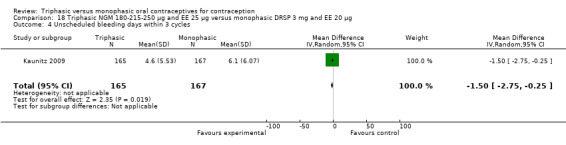
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 4 Unscheduled bleeding days within 3 cycles.
Discontinuation
A total of 21 studies provided data regarding discontinuation of participants. No significant differences were found in the number of women who discontinued or who discontinued due to medical reasons, cycle disturbances, intermenstrual bleeding or adverse events. However, significant heterogeneity was present in Analysis 4.37 and Analysis 9.13. When the studies were examined individually, the triphasic group had fewer discontinuations due to medical reasons than the monophasic group in Lachnit‐Fixson 1984 (Analysis 4.37) and in Reisman 1999 (Analysis 9.13).
4.37. Analysis.
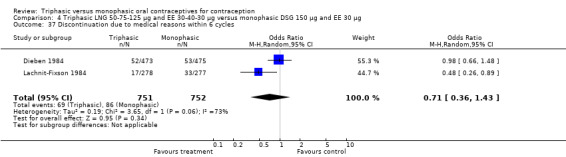
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 37 Discontinuation due to medical reasons within 6 cycles.
9.13. Analysis.
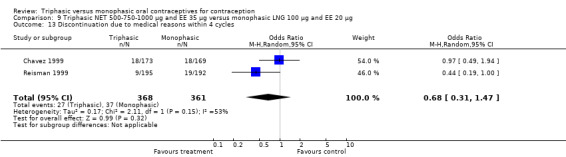
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 13 Discontinuation due to medical reasons within 4 cycles.
Discussion
Summary of main results
Contraceptive effectiveness
The 23 comparative trials included in this systematic review provided insufficient evidence to assess whether the contraceptive effectiveness of triphasic oral contraceptives differs from that of monophasic oral contraceptives. Pooling of the data on contraceptive effectiveness in a meta‐analysis was generally not possible due to differences in (a) progestogen type and (b) dosage of estrogen or progestogen of the studied oral contraceptives. The sample sizes of the individual trials were too small to detect differences in contraceptive effectiveness.
Cycle control
About half of the trials included in this review reported favorable bleeding patterns, that is less spotting, breakthrough bleeding or amenorrhea, in triphasic oral contraceptive users compared to monophasic OC users (Andrade 1993; Bruni 2000; Carlborg 1983; Chen 1987; Hampton 2001; Kashanian 2010; Kaunitz 2009; Lachnit‐Fixson 1984; Percival‐Smith 1990; Reiter 1990; Rosenberg 1999; Sulak 1999; Zador 1979). Combining menstrual bleeding data in a meta‐analysis was generally not possible due to (a) differences between the trials in measuring, analyzing and reporting the data on cycle disturbances, and (b) differences in progestogen type, progestogen dosage and estrogen dosage of the studied contraceptive pills. When interpreting the findings on menstrual bleeding, consideration should be paid to the limitations of the studies. In most trials that reported favorable bleeding patterns in triphasic pill users compared to monophasic pill users, the progestogen type differed between the studied triphasic and monophasic oral contraceptives (Andrade 1993; Bruni 2000; Chen 1987; Hampton 2001; Kaunitz 2009; Lachnit‐Fixson 1984; Percival‐Smith 1990; Reiter 1990; Rosenberg 1999; Sulak 1999). The progestogen type is thought to affect cycle control, so the differences in bleeding pattern might be partially explained by the differences in progestogen content (Maitra 2004; Rosenberg 1992). Further, several trials used the proportion of all cycles with spotting, breakthrough bleeding or amenorrhea as an effect measure (Andrade 1993; Carlborg 1983; Hampton 2001; Percival‐Smith 1990; Rosenberg 1999; Zador 1979). This measure might give a distorted impression as one does not know whether a few women had all the cycles with bleeding problems or lots of women had a few cycles with bleeding problems.
The proportion of women that discontinued due to bleeding problems is as an indicator of how women tolerated the bleeding pattern. No significant differences were found in the number of women who discontinued due to intermenstrual bleeding and cycle disturbances.
Other adverse events
This review did not focus on the incidence of minor adverse events of oral contraceptives like headache, nausea, breast pain and acne. Women may vary in their acceptability of the various minor side effects, so the clinical importance of incidence differences is difficult to assess. We considered discontinuation from the trial as a 'surrogate' outcome for the acceptability of the contraceptive method. No significant differences were observed in the number of women who discontinued due to side effects. The findings on discontinuation may not reflect usage of oral contraceptives in the 'real world'. Participants of prospective comparative trials are not likely to represent the general population of contraceptive users. Free provision of contraceptive methods, financial allowance and regular follow‐up visits all may encourage continuation of the method.
The risk of serious adverse events of oral contraceptives like venous thromboembolism or myocardial infarction was not a subject of our review. Due to the low incidence of these adverse events, the randomized controlled trial does not suit evaluation of the absolute or relative risks. Observational studies, for example case‐control studies and cohort studies, are more appropriate to assess these risks. No difference in the risk of venous thromboembolism between triphasic and monophasic oral contraceptives containing levonorgestrel was observed in a recent case‐control study (van Hylckama 2009).
Quality of the evidence
Overall, the reporting of the study methods and the methodological quality of the studies were poor (CONSORT 2010; DerSimonian 1982). Only three of the 23 trials reported the method of generating the allocation sequence, and only five described the use and method of concealing the treatment allocation sequence. After communication with the researchers, we learned that eight of the included studies appeared to have adequate randomization and allocation concealment. Of 23 trials, 15 were unblinded and 17 studies excluded participants after randomization. Several studies excluded participants because of incorrect pill intake. Bias may result from non‐random methods of generating the allocation sequence, inadequate allocation concealment, not blinding the participants or outcome assessors and exclusion of participants after randomization (DerSimonian 1982; Schulz 2002c; Schulz 2002d; Schulz 2002e). Further, 14 trials were conducted or funded by pharmaceutical companies. Studies sponsored by pharmaceutical companies are more likely to have outcomes favoring the sponsor than studies funded by other sources (Als‐Nielsen 2003; Lexchin 2003).
Authors' conclusions
Implications for practice.
The available evidence is insufficient to determine whether triphasic oral contraceptives differ from monophasic oral contraceptives in important ways, such as efficacy, bleeding patterns and continuation rates. This reflects the generally poor quality of comparative trials to date. Given the often higher cost and greater complexity of triphasic‐pill regimens, monophasic pills should be the first choice in oral contraceptives. According to guidelines of the International Planned Parenthood Federation (IPPF), women should start on a monophasic pill containing 30 to 35 μg of estrogen (IPPF 2004). Pills with 20 μg estrogen cause more breakthrough bleeding and discontinuation because of bleeding than do pills with more estrogen (Gallo 2008).
Implications for research.
Large, adequately reported, high‐quality, randomized controlled trials comparing triphasic and monophasic oral contraceptives with identical progestogens are needed to determine whether triphasic pills differ from monophasic pills in contraceptive effectiveness, menstrual bleeding pattern and continuation rates. Combining the data on menstrual bleeding was complicated by the lack of uniformity in measuring, analyzing and reporting menstrual patterns. Recommendations for the standardization of the collection and analysis of bleeding data for combined hormonal contraceptive clinical trials have been published by Belsey 1986 and by Mishell 2007. Future studies should follow these recommendations to allow comparison of bleeding data. Further, reporting of the study methods and the methodological quality of the studies was poor. Future studies should adhere to the CONSORT guidelines on reporting of randomized controlled trials (CONSORT 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2011 | Amended | minor revision after comment peer‐reviewer. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 11 August 2011 | New citation required but conclusions have not changed | Two new trials added (Kashanian 2010; Kaunitz 2009), along with a 2009 secondary paper for Hampton 2001. An ongoing trial was updated (Bayer 2011). |
| 4 May 2011 | New search has been performed | Searches updated |
| 20 January 2010 | Amended | author order changed |
| 25 November 2008 | New search has been performed | Searches were updated; included a secondary article from earlier trial (Hampton 2001). Also added searches of clinical trials databases. |
| 15 April 2008 | Amended | Converted to new review format. |
| 8 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Carol Manion of FHI 360 who assisted with the literature searches. We gratefully acknowledge all researchers who responded to our inquiry letters and provided additional information on study methods and outcomes.
Appendices
Appendix 1. Search strategies 2011
MEDLINE via PubMed (June 2008 to 02 May 2011)
(("contraceptives, oral"[MeSH Terms] AND (((monophasic[ALL] OR biphasic[ALL]) OR triphasic[ALL]) OR multiphasic[ALL])) AND (((((((((("clinical trials"[MeSH Terms] OR comparative stud*[ALL]) OR ("random allocation"[MeSH Terms] OR random allocation[Text Word])) OR compar*[ALL]) OR clinical trial*[ALL]) OR controlled clinical trial*[ALL]) OR multicenter stud*[ALL]) OR randomized controlled trial*[All]) OR random[ALL]) OR ("double‐blind method"[MeSH Terms] OR double‐blind method[Text Word])) OR ("single‐blind method"[MeSH Terms] OR single‐blind method[Text Word])))
POPLINE (past 5 years to 03 May 2011)
(oral & contracept*) & (monophasic/biphasic/triphasic/multiphasic)
EMBASE (2008 to 04 May 2011)
1. oral contraceptive agent 2. biphasic 3. triphasic 4. multiphasic 5. 2 OR 3 OR 4 6. 1 AND 5 7. monophasic 8. 6 AND 7
LILACS (to 03 May 2011)
(((("contraceptives, oral") or "contraceptive")) or "contraceptives") or "contraception" [Words] and ((("monophasic") or "biphasic") or "triphasic") or "multiphasic" [Words]
CENTRAL (2008 to 02 May 2011)
oral AND contracept* in Title, Abstract or Keywords AND (monophasic OR biphasic OR triphasic OR multiphasic) in Abstract
ClinicalTrials.gov (03 May 2011)
Search terms: biphasic OR triphasic OR multiphasic Condition: NOT diabetes Interventional Studies Studies with Female Participants First received: 01 July 2008 or later
ICTRP (03 May 2011)
Intervention: (monophasic OR biphasic OR triphasic OR multiphasic) NOT insulin Date of registration from 01 Jul 2008
Appendix 2. Previous search strategies
Initial review (2006) and 2008 update
MEDLINE via PubMed
(("contraceptives, oral"[MeSH Terms] AND (((monophasic[ALL] OR biphasic[ALL]) OR triphasic[ALL]) OR multiphasic[ALL])) AND (((((((((("clinical trials"[MeSH Terms] OR comparative stud*[ALL]) OR ("random allocation"[MeSH Terms] OR random allocation[Text Word])) OR compar*[ALL]) OR clinical trial*[ALL]) OR controlled clinical trial*[ALL]) OR multicenter stud*[ALL]) OR randomized controlled trial*[All]) OR random[ALL]) OR ("double‐blind method"[MeSH Terms] OR double‐blind method[Text Word])) OR ("single‐blind method"[MeSH Terms] OR single‐blind method[Text Word])))
CENTRAL
1. (contraceptives and oral) 2. monophasic 3. biphasic 4. triphasic 5. multiphasic 6. (((#2 or #3) or #4) or #5) 7. (#1 and #6)
POPLINE
(kw) oral contraceptives AND (tw) (monophasic OR biphasic OR triphasic OR multiphasic) AND (tw) (compar* OR clinical trials OR comparative studies OR random OR double blind studies)
EMBASE
1. oral contraceptive agent 2. biphasic 3. triphasic 4. multiphasic 5. 2 OR 3 OR 4 6. 1 AND 5 7. monophasic 8. 6 AND 7
LILACS
(((("contraceptives, oral") or "contraceptive")) or "contraceptives") or "contraception" [Words] and ((("monophasic") or "biphasic") or "triphasic") or "multiphasic" [Words]
ClinicalTrials.gov
Search terms: biphasic OR triphasic OR multiphasic Condition: oral contraceptive
ICTRP
Title: monophasic OR biphasic OR triphasic OR multiphasic Intervention or condition: contraception OR contraceptive
Data and analyses
Comparison 1. Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 2 | 678 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.10, 3.91] |
| 2 Pregnancy per woman within 12 cycles | 5 | 4145 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.25, 7.22] |
| 3 Proportion of cycles with spotting within 3 cycles | 1 | 2367 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.68] |
| 4 Proportion of cycles with breakthrough bleeding within 3 cycles | 1 | 2367 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.72] |
| 5 Proportion of cycles with spotting within 6 cycles | 2 | 7290 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.48, 0.67] |
| 6 Proportion of cycles with breakthrough bleeding within 6 cycles | 2 | 7290 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.80] |
| 7 Proportion of cycles with spotting within 12 cycles | 1 | 6472 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.49, 0.72] |
| 8 Proportion of cycles with breakthrough bleeding within 12 cycles | 1 | 6472 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.45, 0.77] |
| 9 Proportion of women with intermenstrual bleeding within 12 cycles | 1 | 979 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.31] |
| 10 Proportion of women with spotting at cycle 6 | 1 | 1032 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.17] |
| 11 Proportion of women with breakthrough bleeding at cycle 6 | 1 | 1032 | Odds Ratio (M‐H, Random, 95% CI) | 2.45 [0.47, 12.67] |
| 12 Proportion of women with spotting at cycle 12 | 1 | 896 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.06, 16.62] |
| 13 Proportion of women with breakthrough bleeding at cycle 12 | 1 | 896 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.72] |
| 14 Proportion of women with intermenstrual bleeding within 12 cycles | 1 | 979 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.31] |
| 15 Proportion of women with breakthrough bleeding or spotting at cycle 3 | 1 | 314 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.77] |
| 16 Proportion of women with breakthrough bleeding or spotting at cycle 6 | 1 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Proportion of cycles with amenorrhea within 6 cycles | 1 | 2777 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.14] |
| 18 Proportion of cycles with amenorrhea within 12 cycles | 1 | 6472 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.45] |
| 19 Proportion of women with amenorrhea within 12 cycles | 1 | 979 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.24, 8.83] |
| 20 Total discontinuation within 6 cycles | 4 | 1829 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.78, 1.37] |
| 21 Discontinuation due to medical reasons within 6 cycles | 3 | 1513 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.61] |
| 22 Discontinuation due to cycle disturbances within 6 cycles | 1 | 189 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.13, 7.02] |
| 23 Total discontinuation within 12 cycles | 4 | 3310 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.97, 1.31] |
| 24 Discontinuation due to medical reasons within 12 cycles | 3 | 3010 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.71, 1.76] |
| 25 Discontinuation due to cycle disturbances within 12 cycles | 3 | 2109 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.21] |
| 26 Discontinuation due to intermenstrual bleeding within 12 cycles | 1 | 1201 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.44, 4.44] |
1.4. Analysis.
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 4 Proportion of cycles with breakthrough bleeding within 3 cycles.
1.7. Analysis.
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 7 Proportion of cycles with spotting within 12 cycles.
1.9. Analysis.
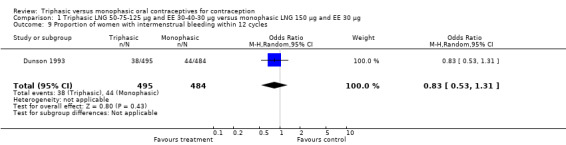
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 9 Proportion of women with intermenstrual bleeding within 12 cycles.
1.11. Analysis.
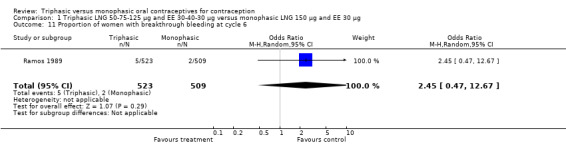
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 11 Proportion of women with breakthrough bleeding at cycle 6.
1.12. Analysis.
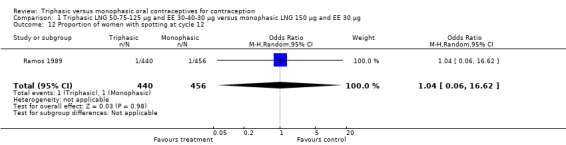
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 12 Proportion of women with spotting at cycle 12.
1.17. Analysis.
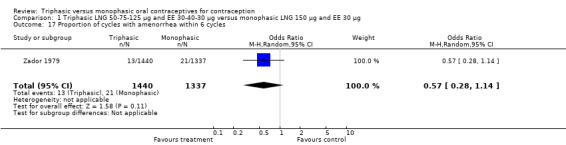
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 17 Proportion of cycles with amenorrhea within 6 cycles.
1.20. Analysis.
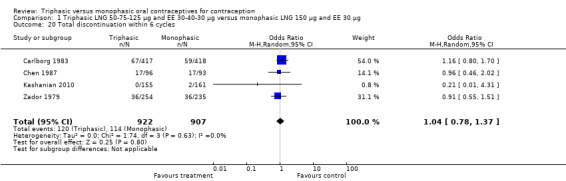
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 20 Total discontinuation within 6 cycles.
1.21. Analysis.
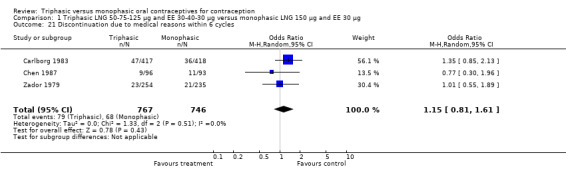
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 21 Discontinuation due to medical reasons within 6 cycles.
1.22. Analysis.
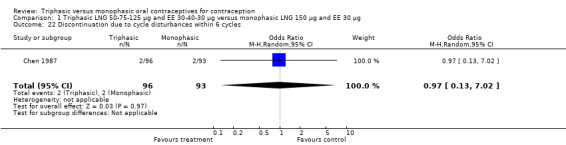
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 22 Discontinuation due to cycle disturbances within 6 cycles.
1.23. Analysis.
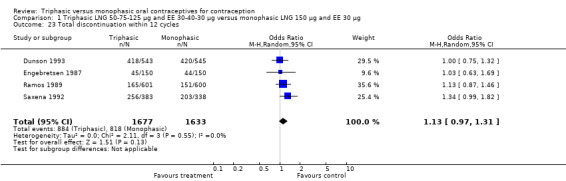
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 23 Total discontinuation within 12 cycles.
1.24. Analysis.

Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 24 Discontinuation due to medical reasons within 12 cycles.
1.25. Analysis.
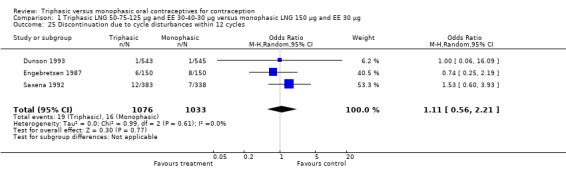
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 25 Discontinuation due to cycle disturbances within 12 cycles.
1.26. Analysis.
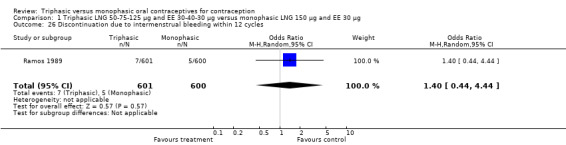
Comparison 1 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic LNG 150 μg and EE 30 μg, Outcome 26 Discontinuation due to intermenstrual bleeding within 12 cycles.
Comparison 2. Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 600 μg and EE 35 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 186 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.13, 6.79] |
| 2 Total discontinuation within 6 cycles | 1 | 186 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.44, 1.94] |
| 3 Discontinuation due to medical reasons within 6 cycles | 1 | 186 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.35, 2.46] |
| 4 Discontinuation due to cycle disturbances within 6 cycles | 1 | 186 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.78] |
2.2. Analysis.
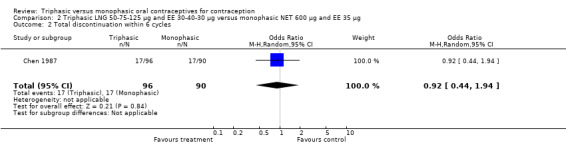
Comparison 2 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 600 μg and EE 35 μg, Outcome 2 Total discontinuation within 6 cycles.
2.3. Analysis.
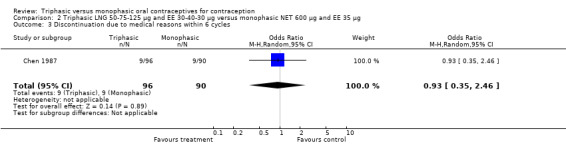
Comparison 2 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 600 μg and EE 35 μg, Outcome 3 Discontinuation due to medical reasons within 6 cycles.
2.4. Analysis.
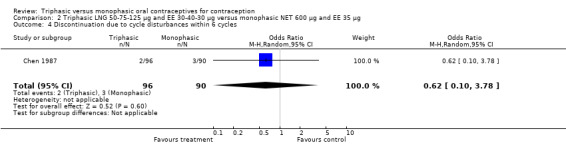
Comparison 2 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 600 μg and EE 35 μg, Outcome 4 Discontinuation due to cycle disturbances within 6 cycles.
Comparison 3. Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 12 cycles | 1 | 1200 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Proportion of women with spotting at cycle 6 | 1 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 0.94] |
| 3 Proportion of women with breakthrough bleeding at cycle 6 | 1 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.86] |
| 4 Proportion of women with spotting at cycle 12 | 1 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.06, 14.98] |
| 5 Proportion of women with breakthrough bleeding at cycle 12 | 1 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.16] |
| 6 Total discontinuation within 12 cycles | 1 | 1200 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.03] |
| 7 Discontinuation due to medical reasons within 12 cycles | 1 | 1200 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.55, 1.10] |
| 8 Discontinuation due to intermenstrual bleeding within 12 cycles | 1 | 1200 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.47] |
3.6. Analysis.
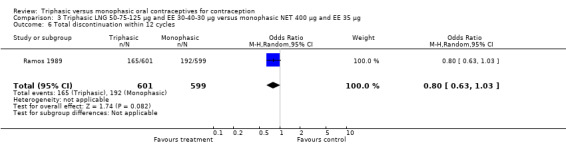
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 6 Total discontinuation within 12 cycles.
3.7. Analysis.
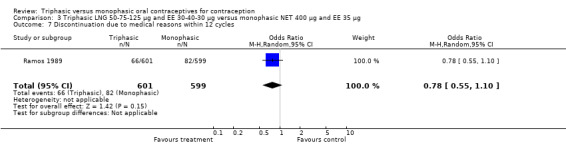
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 7 Discontinuation due to medical reasons within 12 cycles.
3.8. Analysis.
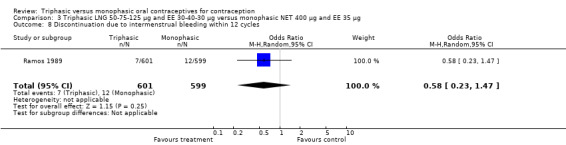
Comparison 3 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 400 μg and EE 35 μg, Outcome 8 Discontinuation due to intermenstrual bleeding within 12 cycles.
Comparison 4. Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 555 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 73.96] |
| 2 Pregnancy per woman within 12 cycles | 2 | 1146 | Odds Ratio (M‐H, Random, 95% CI) | 7.22 [0.88, 59.00] |
| 3 Proportion of cycles with spotting within 3 cycles | 1 | 2763 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.88, 1.41] |
| 4 Proportion of cycles with breakthrough bleeding within 3 cycles | 1 | 2763 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.56] |
| 5 Proportion of cycles with spotting and breakthrough bleeding within 3 cycles | 1 | 2763 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.31, 1.61] |
| 6 Proportion of cycles with spotting within 6 cycles | 1 | 5235 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.97, 1.41] |
| 7 Proportion of cycles with spotting within 6 cycles | 1 | 3060 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.27, 0.44] |
| 8 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 5235 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.42] |
| 9 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 3060 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.71] |
| 10 Proportion of cycles with spotting and breakthrough bleeding within 6 cycles | 2 | 8295 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.86] |
| 11 Proportion of cycles with spotting within 12 cycles | 1 | 5478 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.99, 1.44] |
| 12 Proportion of cycles with breakthrough bleeding within 12 cycles | 1 | 5478 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.88, 1.35] |
| 13 Proportion of cycles with spotting and breakthrough bleeding within 12 cycles | 1 | 5478 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.33, 1.22] |
| 14 Proportion of women with staining/spotting within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.42, 5.67] |
| 15 Proportion of women with moderate flow intermenstrual bleeding within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 2.61 [0.49, 13.77] |
| 16 Proportion of women with spotting at cycle 3 | 1 | 894 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.90] |
| 17 Proportion of women with breakthrough bleeding at cycle 3 | 1 | 894 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.66, 1.82] |
| 18 Proportion of women with spotting and breakthrough bleeding at cycle 3 | 1 | 894 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.04, 5.50] |
| 19 Proportion of women with spotting at cycle 6 | 1 | 797 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.58] |
| 20 Proportion of women with breakthrough bleeding at cycle 6 | 1 | 797 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.80, 2.92] |
| 21 Proportion of women with spotting and breakthrough bleeding at cycle 6 | 1 | 797 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.04, 5.51] |
| 22 Proportion of women with spotting at cycle 12 | 1 | 10 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Proportion of women with breakthrough bleeding at cycle 12 | 1 | 10 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.03, 33.32] |
| 24 Proportion of women with spotting and breakthrough bleeding at cycle 12 | 1 | 10 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Proportion of cycles with amenorrhea within 3 cycles | 1 | 2763 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.82, 1.39] |
| 26 Proportion of cycles with amenorrhea within 6 cycles | 1 | 5235 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.87, 1.31] |
| 27 Proportion of cycles with amenorrhea within 6 cycles | 1 | 3060 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.74] |
| 28 Proportion of cycles with amenorrhea within 12 cycles | 1 | 5478 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.28] |
| 29 Proportion of women with amenorrhea within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.25, 9.37] |
| 30 Proportion of women with amenorrhea at cycle 3 | 1 | 894 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.63, 1.57] |
| 31 Proportion of women with amenorrhea at cycle 6 | 1 | 797 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.76, 2.43] |
| 32 Proportion of women with amenorrhea at cycle 12 | 1 | 10 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.03, 33.32] |
| 33 Total discontinuation within 3 cycles | 1 | 948 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 34 Discontinuation due to medical reasons within 3 cycles | 1 | 948 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.19] |
| 35 Discontinuation due to cycle disturbances within 3 cycles | 1 | 948 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.61] |
| 36 Total discontinuation within 6 cycles | 2 | 1503 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.33] |
| 37 Discontinuation due to medical reasons within 6 cycles | 2 | 1503 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.43] |
| 38 Discontinuation due to cycle disturbances within 6 cycles | 1 | 948 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.58, 1.92] |
| 39 Total discontinuation within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.81, 2.57] |
| 40 Discontinuation due to medical reasons within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.44, 4.72] |
| 41 Discontinuation due to cycle disturbances within 12 cycles | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 7.29 [0.37, 143.08] |
4.3. Analysis.
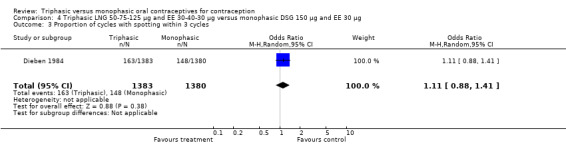
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 3 Proportion of cycles with spotting within 3 cycles.
4.4. Analysis.
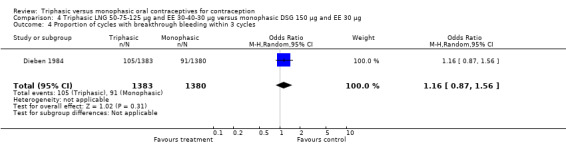
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 4 Proportion of cycles with breakthrough bleeding within 3 cycles.
4.5. Analysis.
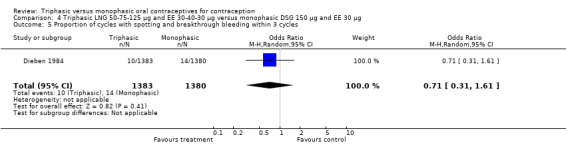
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 5 Proportion of cycles with spotting and breakthrough bleeding within 3 cycles.
4.6. Analysis.
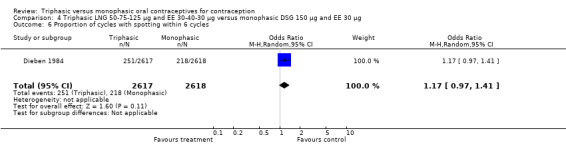
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 6 Proportion of cycles with spotting within 6 cycles.
4.8. Analysis.
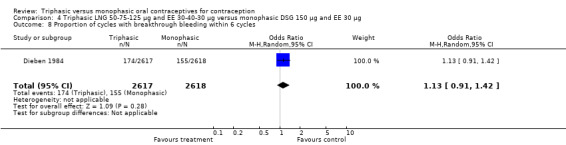
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 8 Proportion of cycles with breakthrough bleeding within 6 cycles.
4.11. Analysis.
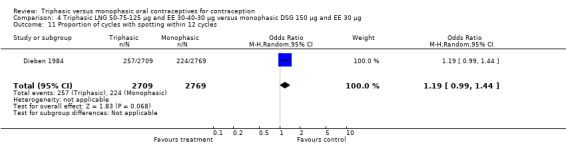
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 11 Proportion of cycles with spotting within 12 cycles.
4.12. Analysis.
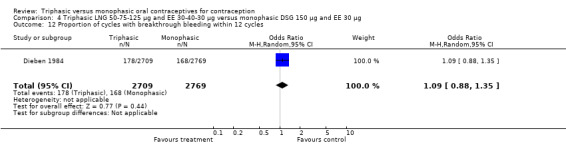
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 12 Proportion of cycles with breakthrough bleeding within 12 cycles.
4.13. Analysis.
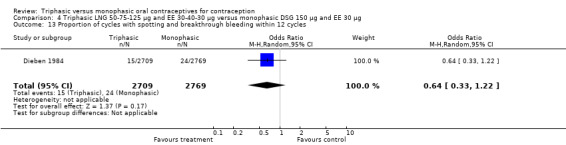
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 13 Proportion of cycles with spotting and breakthrough bleeding within 12 cycles.
4.14. Analysis.
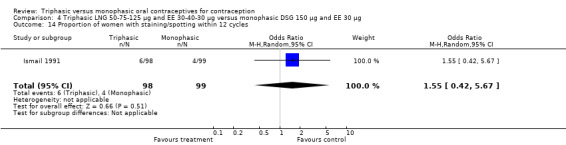
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 14 Proportion of women with staining/spotting within 12 cycles.
4.15. Analysis.
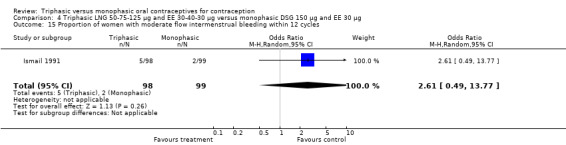
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 15 Proportion of women with moderate flow intermenstrual bleeding within 12 cycles.
4.16. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 16 Proportion of women with spotting at cycle 3.
4.17. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 17 Proportion of women with breakthrough bleeding at cycle 3.
4.18. Analysis.
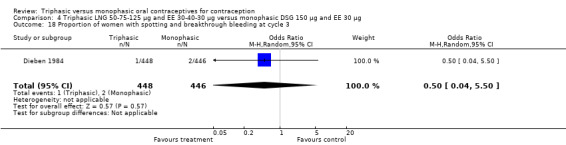
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 18 Proportion of women with spotting and breakthrough bleeding at cycle 3.
4.19. Analysis.
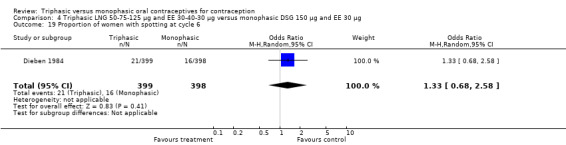
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 19 Proportion of women with spotting at cycle 6.
4.20. Analysis.
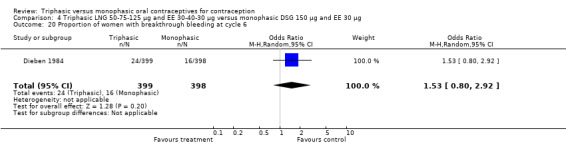
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 20 Proportion of women with breakthrough bleeding at cycle 6.
4.21. Analysis.
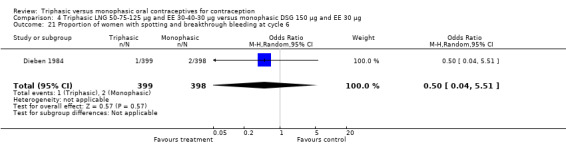
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 21 Proportion of women with spotting and breakthrough bleeding at cycle 6.
4.22. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 22 Proportion of women with spotting at cycle 12.
4.23. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 23 Proportion of women with breakthrough bleeding at cycle 12.
4.24. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 24 Proportion of women with spotting and breakthrough bleeding at cycle 12.
4.25. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 25 Proportion of cycles with amenorrhea within 3 cycles.
4.26. Analysis.
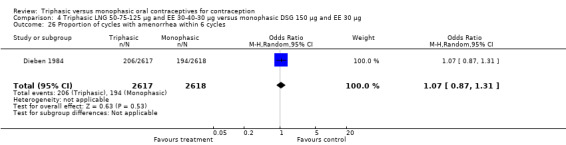
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 26 Proportion of cycles with amenorrhea within 6 cycles.
4.28. Analysis.
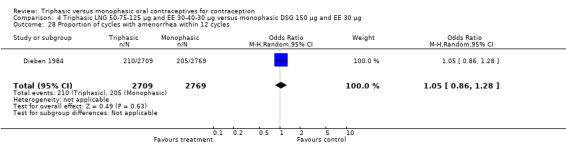
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 28 Proportion of cycles with amenorrhea within 12 cycles.
4.29. Analysis.
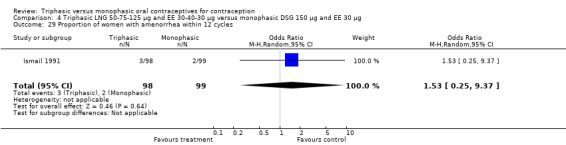
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 29 Proportion of women with amenorrhea within 12 cycles.
4.30. Analysis.
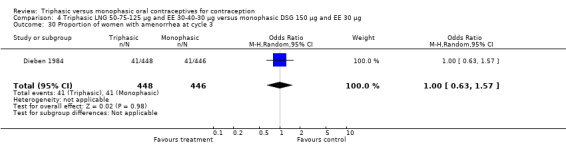
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 30 Proportion of women with amenorrhea at cycle 3.
4.31. Analysis.
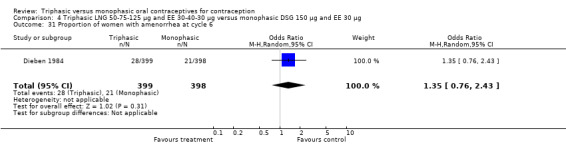
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 31 Proportion of women with amenorrhea at cycle 6.
4.32. Analysis.
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 32 Proportion of women with amenorrhea at cycle 12.
4.33. Analysis.
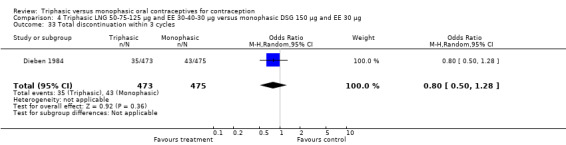
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 33 Total discontinuation within 3 cycles.
4.34. Analysis.
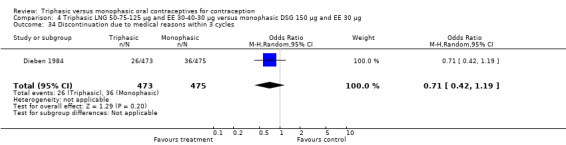
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 34 Discontinuation due to medical reasons within 3 cycles.
4.35. Analysis.
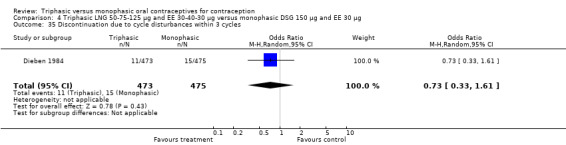
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 35 Discontinuation due to cycle disturbances within 3 cycles.
4.36. Analysis.
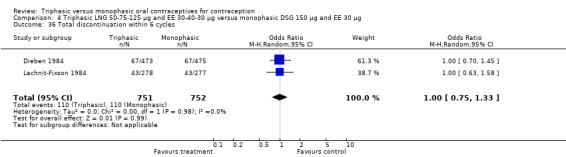
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 36 Total discontinuation within 6 cycles.
4.38. Analysis.
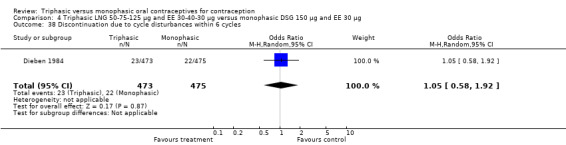
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 38 Discontinuation due to cycle disturbances within 6 cycles.
4.39. Analysis.
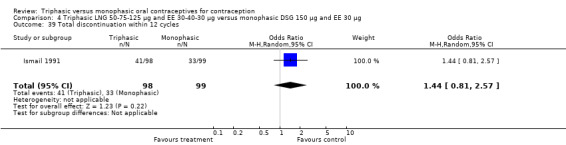
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 39 Total discontinuation within 12 cycles.
4.40. Analysis.
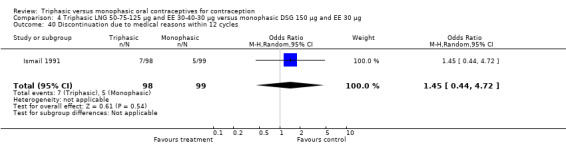
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 40 Discontinuation due to medical reasons within 12 cycles.
4.41. Analysis.
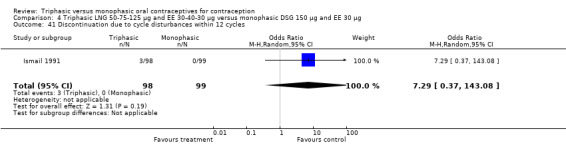
Comparison 4 Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 41 Discontinuation due to cycle disturbances within 12 cycles.
Comparison 5. Triphasic LNG 50‐75‐125 μg/ EE 30‐40‐30 μg versus monophasic NETA 1500 μg/ EE 30 g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cycles with intermenstrual bleeding within 6 cycles | 1 | 987 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.56, 1.01] |
| 2 Proportion of cycles with amenorrhea within 6 cycles | 1 | 987 | Odds Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.18] |
| 3 Total discontinuation within 6 cycles | 1 | 236 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.47] |
5.3. Analysis.
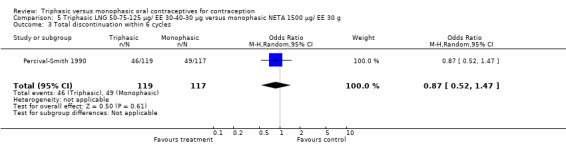
Comparison 5 Triphasic LNG 50‐75‐125 μg/ EE 30‐40‐30 μg versus monophasic NETA 1500 μg/ EE 30 g, Outcome 3 Total discontinuation within 6 cycles.
Comparison 6. Triphasic NET 500‐750‐1000 μg/ EE 35 μg versus monophasic NETA 1500 μg/ EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cycles with intermenstrual bleeding within 6 cycles | 1 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.05, 1.80] |
| 2 Proportion of cycles with amenorrhea within 6 cycles | 1 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 3 Total discontinuation within 6 cycles | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.18] |
6.3. Analysis.
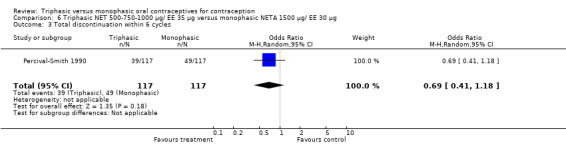
Comparison 6 Triphasic NET 500‐750‐1000 μg/ EE 35 μg versus monophasic NETA 1500 μg/ EE 30 μg, Outcome 3 Total discontinuation within 6 cycles.
Comparison 7. Triphasic LNG 50‐75‐125 μg and EE 30‐40‐30 μg versus monophasic NET 1000 μg and EE 35 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of women with intermenstrual bleeding within 12 cycles | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.18] |
| 2 Proportion of women with amenorrhea within 12 cycles | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.43] |
Comparison 8. Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic NET 1000 μg and EE 35 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of women with intermenstrual bleeding within 12 cycles | 1 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.55, 2.02] |
| 2 Proportion of women with amenorrhea within 12 cycles | 1 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.76] |
Comparison 9. Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 4 cycles | 2 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [0.36, 10.83] |
| 2 Proportion of cycles with spotting within 3 cycles | 1 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.67] |
| 3 Proportion of cycles with breakthrough bleeding within 3 cycles | 1 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.50, 1.78] |
| 4 Proportion of cycles with spotting and breakthrough bleeding within 3 cycles | 1 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.57] |
| 5 Proportion of cycles with intermenstrual bleeding within 3 cycles | 2 | 1367 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.96, 1.54] |
| 6 Proportion of women with spotting at cycle 3 | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 2.03 [0.74, 5.54] |
| 7 Proportion of women with breakthrough bleeding at cycle 3 | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.53, 4.33] |
| 8 Proportion of women with spotting and breakthrough bleeding at cycle 3 | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.38] |
| 9 Proportion of women with intermenstrual bleeding at cycle 3 | 2 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.79, 1.75] |
| 10 Proportion of cycles with amenorrhea within 3 cycles | 2 | 1367 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.48] |
| 11 Proportion of women with amenorrhea at cycle 3 | 2 | 330 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 1.92] |
| 12 Total discontinuation within 4 cycles | 2 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 13 Discontinuation due to medical reasons within 4 cycles | 2 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.47] |
9.2. Analysis.
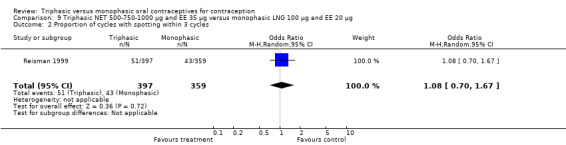
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 2 Proportion of cycles with spotting within 3 cycles.
9.3. Analysis.
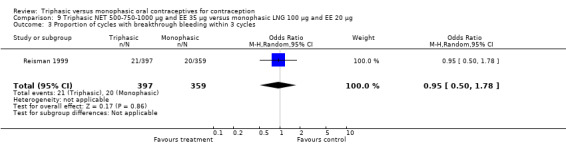
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 3 Proportion of cycles with breakthrough bleeding within 3 cycles.
9.4. Analysis.
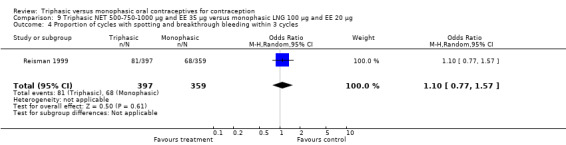
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 4 Proportion of cycles with spotting and breakthrough bleeding within 3 cycles.
9.5. Analysis.
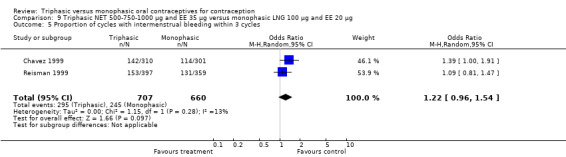
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 5 Proportion of cycles with intermenstrual bleeding within 3 cycles.
9.6. Analysis.
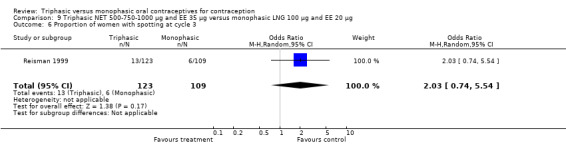
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 6 Proportion of women with spotting at cycle 3.
9.7. Analysis.
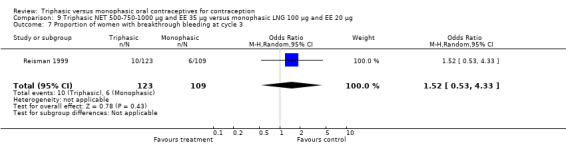
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 7 Proportion of women with breakthrough bleeding at cycle 3.
9.8. Analysis.
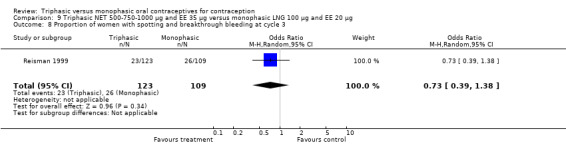
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 8 Proportion of women with spotting and breakthrough bleeding at cycle 3.
9.9. Analysis.
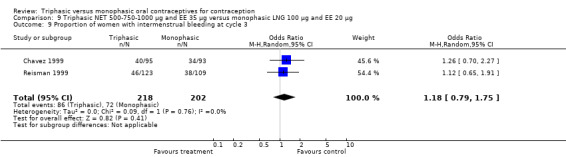
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 9 Proportion of women with intermenstrual bleeding at cycle 3.
9.10. Analysis.
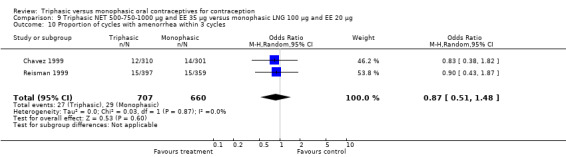
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 10 Proportion of cycles with amenorrhea within 3 cycles.
9.11. Analysis.
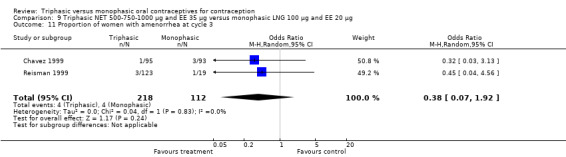
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 11 Proportion of women with amenorrhea at cycle 3.
9.12. Analysis.
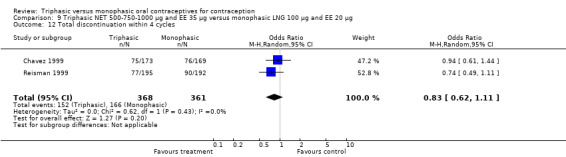
Comparison 9 Triphasic NET 500‐750‐1000 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 12 Total discontinuation within 4 cycles.
Comparison 10. Estrophasic NETA 1000 μg and EE 20‐30‐35 μg versus monophasic NETA 1500 μg and EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to adverse events within 6 cycles | 1 | 1277 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.49, 1.60] |
10.1. Analysis.
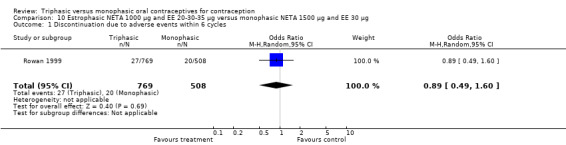
Comparison 10 Estrophasic NETA 1000 μg and EE 20‐30‐35 μg versus monophasic NETA 1500 μg and EE 30 μg, Outcome 1 Discontinuation due to adverse events within 6 cycles.
Comparison 11. Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 68.38] |
| 2 Pregnancy per woman within 12 cycles | 1 | 168 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.26] |
| 3 Proportion of cycles with spotting within 6 cycles | 1 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.72, 1.28] |
| 4 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.43] |
| 5 Proportion of cycles with spotting and breakthrough bleeding within 6 cycles | 1 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.33, 0.73] |
| 6 Proportion of women with spotting at cycle 3 | 2 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.54, 2.33] |
| 7 Proportion of women with breakthrough bleeding at cycle 3 | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 2.85 [0.73, 11.17] |
| 8 Proportion of women with breakthrough bleeding (with or without spotting) at cycle 3 | 1 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.60] |
| 9 Proportion of women with spotting at cycle 6 | 2 | 510 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.50, 2.12] |
| 10 Proportion of women with breakthrough bleeding at cycle 6 | 1 | 158 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.36, 2.81] |
| 11 Proportion of women with breakthrough bleeding (with or without spotting) at cycle 6 | 1 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.20, 1.65] |
| 12 Proportion of women with spotting at cycle 12 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.40, 5.56] |
| 13 Proportion of women with breakthrough bleeding at cycle 12 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.27, 3.51] |
| 14 Proportion of cycles with amenorrhea within 6 cycles | 1 | 2403 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.14] |
| 15 Proportion of cycles with amenorrhea within 12 cycles | 1 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.01] |
| 16 Proportion of women with amenorrhea at cycle 3 | 2 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.15, 11.52] |
| 17 Proportion of women with amenorrhea at cycle 6 | 2 | 510 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.04, 5.56] |
| 18 Proportion of women with amenorrhea at cycle 12 | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.10] |
| 19 Total discontinuation within 6 cycles | 2 | 648 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.35] |
| 20 Discontinuation due to medical reasons within 6 cycles | 1 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.54] |
| 21 Discontinuation due to cycle disturbances within 6 cycles | 1 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.23, 2.53] |
| 22 Total discontinuation within 12 cycles | 1 | 168 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.35, 1.96] |
| 23 Discontinuation due to medical reasons within 12 cycles | 1 | 168 | Odds Ratio (M‐H, Random, 95% CI) | 2.02 [0.18, 22.76] |
11.3. Analysis.
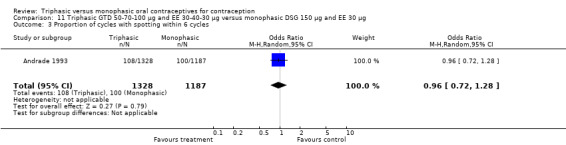
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 3 Proportion of cycles with spotting within 6 cycles.
11.4. Analysis.
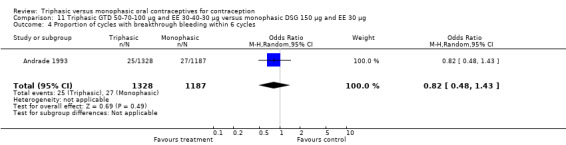
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 4 Proportion of cycles with breakthrough bleeding within 6 cycles.
11.6. Analysis.
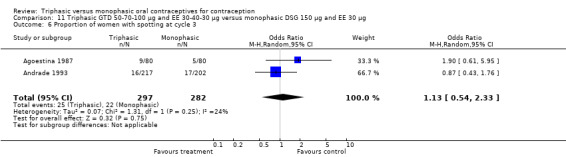
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 6 Proportion of women with spotting at cycle 3.
11.7. Analysis.
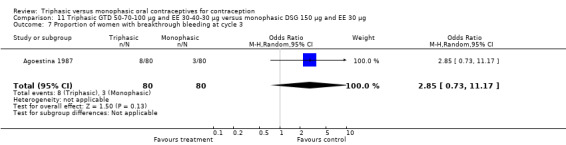
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 7 Proportion of women with breakthrough bleeding at cycle 3.
11.8. Analysis.
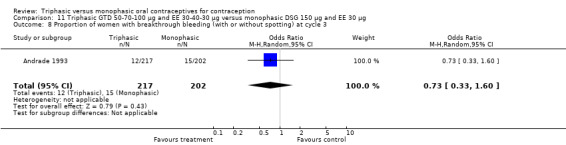
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 8 Proportion of women with breakthrough bleeding (with or without spotting) at cycle 3.
11.9. Analysis.
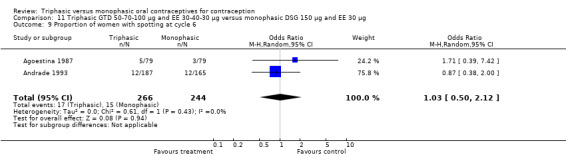
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 9 Proportion of women with spotting at cycle 6.
11.10. Analysis.
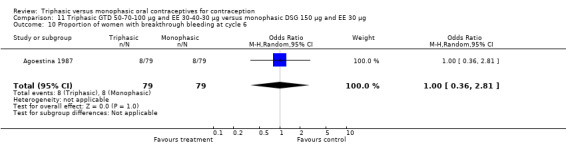
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 10 Proportion of women with breakthrough bleeding at cycle 6.
11.11. Analysis.
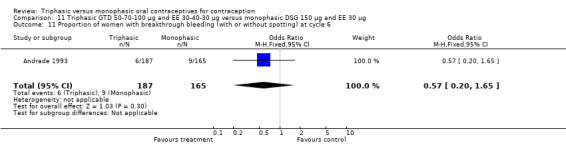
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 11 Proportion of women with breakthrough bleeding (with or without spotting) at cycle 6.
11.12. Analysis.
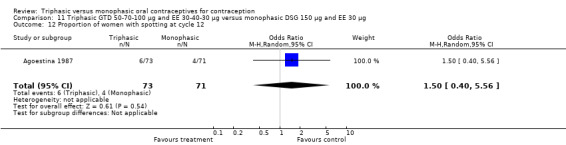
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 12 Proportion of women with spotting at cycle 12.
11.13. Analysis.
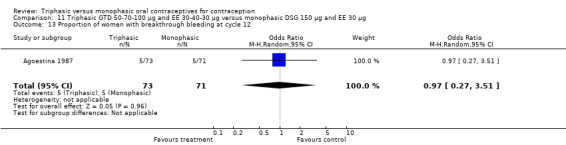
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 13 Proportion of women with breakthrough bleeding at cycle 12.
11.14. Analysis.
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 14 Proportion of cycles with amenorrhea within 6 cycles.
11.15. Analysis.
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 15 Proportion of cycles with amenorrhea within 12 cycles.
11.16. Analysis.
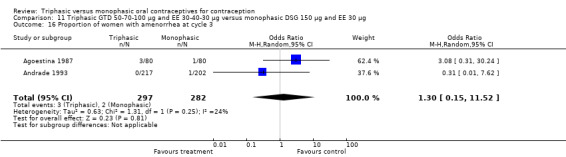
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 16 Proportion of women with amenorrhea at cycle 3.
11.17. Analysis.
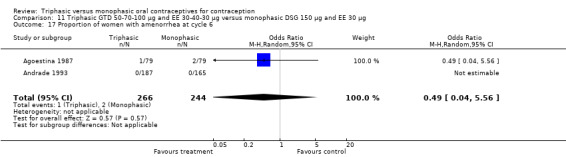
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 17 Proportion of women with amenorrhea at cycle 6.
11.18. Analysis.
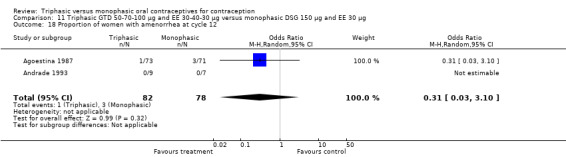
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 18 Proportion of women with amenorrhea at cycle 12.
11.19. Analysis.
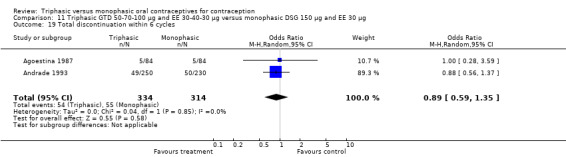
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 19 Total discontinuation within 6 cycles.
11.20. Analysis.
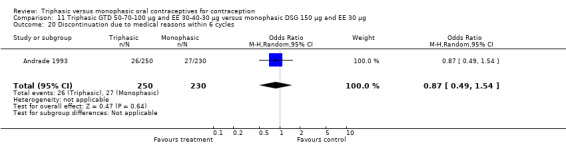
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 20 Discontinuation due to medical reasons within 6 cycles.
11.21. Analysis.
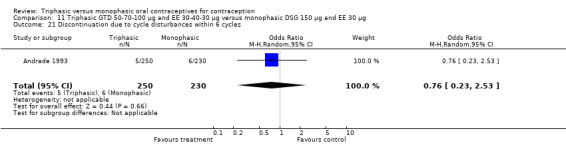
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 21 Discontinuation due to cycle disturbances within 6 cycles.
11.22. Analysis.
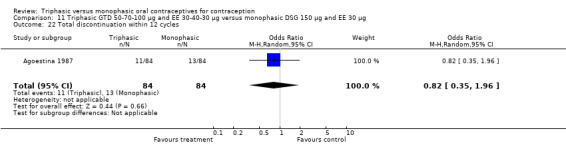
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 22 Total discontinuation within 12 cycles.
11.23. Analysis.
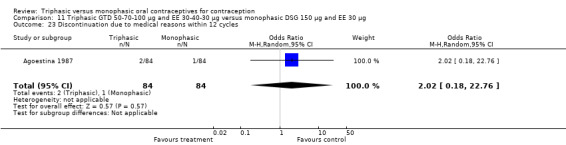
Comparison 11 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 30 μg, Outcome 23 Discontinuation due to medical reasons within 12 cycles.
Comparison 12. Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 13 cycles | 1 | 1613 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.14, 7.09] |
| 2 Total discontinuation within 13 cycles | 1 | 1613 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.88, 1.36] |
| 3 Discontinuation due to medical reasons within 13 cycles | 1 | 1613 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.21] |
12.2. Analysis.
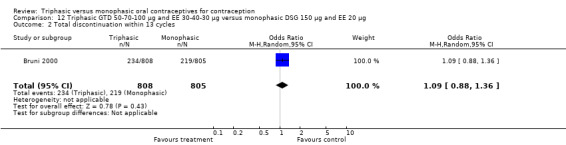
Comparison 12 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 20 μg, Outcome 2 Total discontinuation within 13 cycles.
12.3. Analysis.
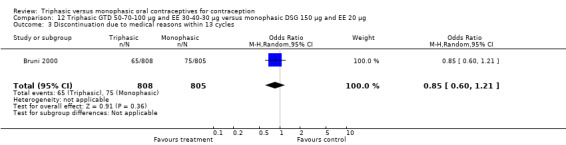
Comparison 12 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic DSG 150 μg and EE 20 μg, Outcome 3 Discontinuation due to medical reasons within 13 cycles.
Comparison 13. Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic GTD 75 μg and EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 13 cycles | 1 | 1614 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.11, 3.99] |
| 2 Total discontinuation within 13 cycles | 1 | 1614 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 3 Discontinuation due to medical reasons within 13 cycles | 1 | 1614 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.77, 1.60] |
13.2. Analysis.
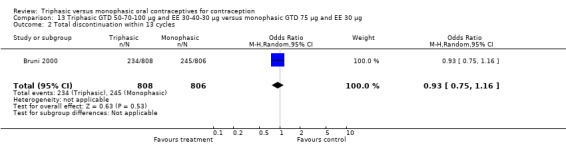
Comparison 13 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic GTD 75 μg and EE 30 μg, Outcome 2 Total discontinuation within 13 cycles.
13.3. Analysis.
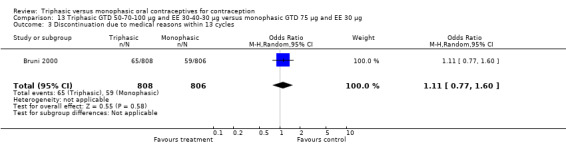
Comparison 13 Triphasic GTD 50‐70‐100 μg and EE 30‐40‐30 μg versus monophasic GTD 75 μg and EE 30 μg, Outcome 3 Discontinuation due to medical reasons within 13 cycles.
Comparison 14. Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 7.14] |
| 2 Proportion of women with spotting or breakthrough bleeding at cycle 6 | 1 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.51] |
| 3 Proportion of women with amenorrhea at cycle 6 | 1 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.06, 0.45] |
| 4 Total discontinuation within 6 cycles | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.68, 1.58] |
| 5 Discontinuation due to adverse events within 6 cycles | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.50, 1.98] |
14.4. Analysis.
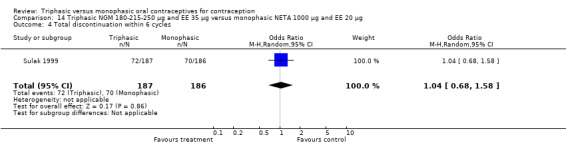
Comparison 14 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 4 Total discontinuation within 6 cycles.
14.5. Analysis.
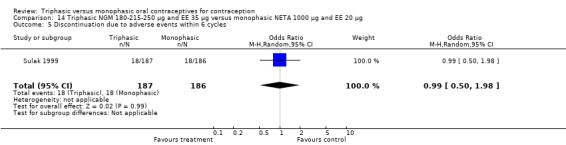
Comparison 14 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 5 Discontinuation due to adverse events within 6 cycles.
Comparison 15. Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 3.02 [0.31, 29.35] |
| 2 Proportion of cycles with spotting within 6 cycles | 1 | 1650 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.42, 0.81] |
| 3 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 1650 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.66, 2.04] |
| 4 Proportion of cycles with amenorrhea within 6 cycles | 1 | 1650 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.96] |
| 5 Total discontinuation within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.73] |
| 6 Discontinuations due to adverse events within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.21, 3.00] |
15.3. Analysis.
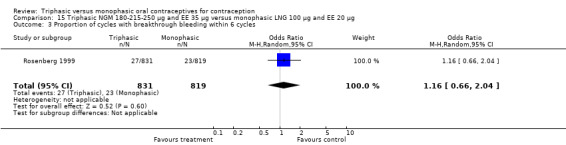
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 3 Proportion of cycles with breakthrough bleeding within 6 cycles.
15.5. Analysis.
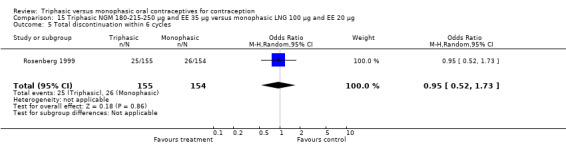
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 5 Total discontinuation within 6 cycles.
15.6. Analysis.
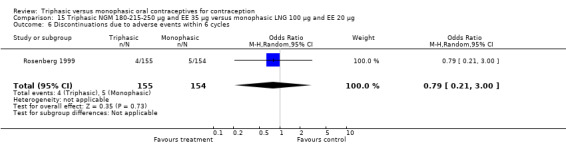
Comparison 15 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic LNG 100 μg and EE 20 μg, Outcome 6 Discontinuations due to adverse events within 6 cycles.
Comparison 16. Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 7.09 [0.36, 138.46] |
| 2 Proportion of cycles with spotting within 6 cycles | 1 | 1679 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.91] |
| 3 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 1679 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.57, 1.68] |
| 4 Proportion of cycles with amenorrhea within 6 cycles | 1 | 1679 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.60] |
| 5 Total discontinuation within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.92] |
| 6 Discontinuations due to adverse events within 6 cycles | 1 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.29, 6.06] |
16.3. Analysis.
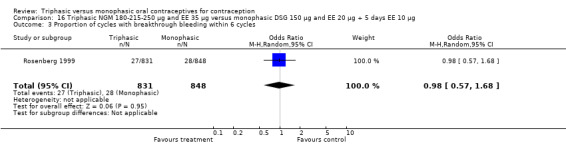
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 3 Proportion of cycles with breakthrough bleeding within 6 cycles.
16.5. Analysis.
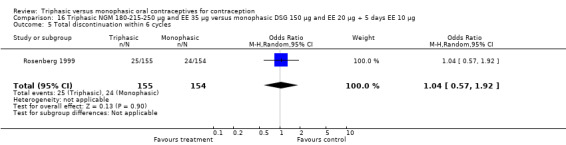
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 5 Total discontinuation within 6 cycles.
16.6. Analysis.
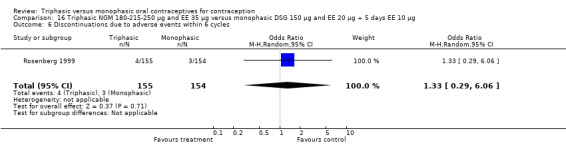
Comparison 16 Triphasic NGM 180‐215‐250 μg and EE 35 μg versus monophasic DSG 150 μg and EE 20 μg + 5 days EE 10 μg, Outcome 6 Discontinuations due to adverse events within 6 cycles.
Comparison 17. Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 13 cycles | 1 | 2814 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.34] |
| 2 Proportion of cycles with breakthrough bleeding within 3 cycles | 1 | 7272 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.47] |
| 3 Proportion of cycles with breakthrough bleeding/spotting within 3 cycles | 1 | 7272 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.45] |
| 4 Proportion of cycles with breakthrough bleeding within 6 cycles | 1 | 13692 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.40, 0.50] |
| 5 Proportion of cycles with breakthrough bleeding/spotting within 6 cycles | 1 | 13692 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.40, 0.48] |
| 6 Proportion of cycles with breakthrough bleeding within 12 cycles | 1 | 16519 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.41, 0.50] |
| 7 Proportion of cycles with breakthrough bleeding/spotting within 12 cycles | 1 | 16519 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.41, 0.49] |
| 8 Proportion of women with breakthrough bleeding at cycle 3 | 1 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.27, 0.49] |
| 9 Proportion of women with breakthrough bleeding/spotting at cycle 3 | 1 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.35, 0.55] |
| 10 Proportion of women with breakthrough bleeding at cycle 6 | 1 | 2118 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.57] |
| 11 Proportion of women with breakthrough bleeding/spotting at cycle 6 | 1 | 2118 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.52] |
| 12 Proportion of women with breakthrough bleeding at cycle 12 | 1 | 444 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.16] |
| 13 Proportion of women with breakthrough bleeding/spotting at cycle 12 | 1 | 444 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.86] |
| 14 Proportion of women with unscheduled bleeding at cycle 3 | 1 | 2478 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.44, 0.62] |
| 15 Proportion of women with unscheduled bleeding at cycle 6 | 1 | 2222 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.44, 0.64] |
| 16 Mean number of days of unscheduled bleeding/spotting at cycle 3 | 1 | 719 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.85, ‐0.05] |
| 17 Mean number of days of unscheduled bleeding/spotting at cycle 6 | 1 | 567 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.22, 0.62] |
| 18 Proportion of cycles with amenorrhea within 13 cycles | 1 | 16957 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.04, 0.07] |
| 19 Total discontinuation within 6 cycles | 1 | 2089 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.82, 1.26] |
| 20 Total discontinuation within 13 cycles | 1 | 805 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.46] |
17.3. Analysis.
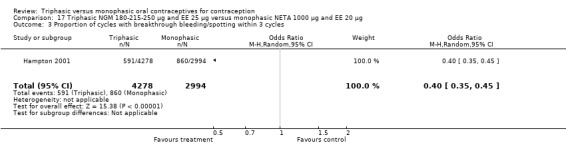
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 3 Proportion of cycles with breakthrough bleeding/spotting within 3 cycles.
17.4. Analysis.
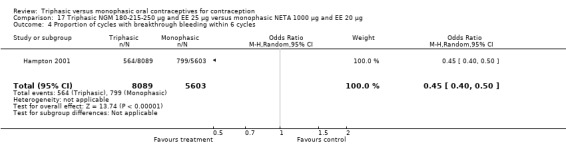
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 4 Proportion of cycles with breakthrough bleeding within 6 cycles.
17.5. Analysis.
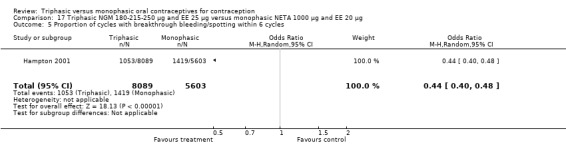
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 5 Proportion of cycles with breakthrough bleeding/spotting within 6 cycles.
17.6. Analysis.
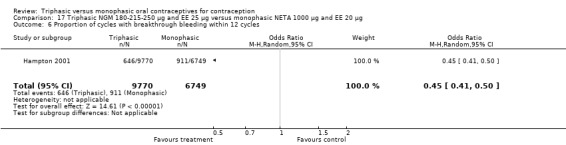
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 6 Proportion of cycles with breakthrough bleeding within 12 cycles.
17.8. Analysis.
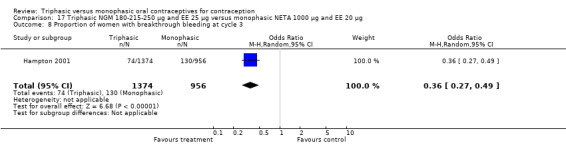
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 8 Proportion of women with breakthrough bleeding at cycle 3.
17.9. Analysis.
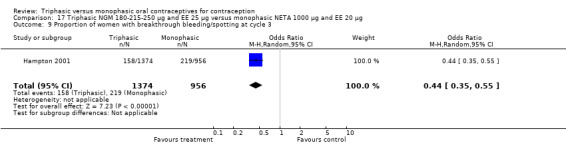
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 9 Proportion of women with breakthrough bleeding/spotting at cycle 3.
17.10. Analysis.

Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 10 Proportion of women with breakthrough bleeding at cycle 6.
17.11. Analysis.
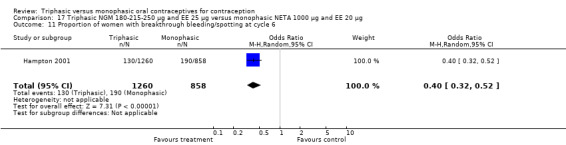
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 11 Proportion of women with breakthrough bleeding/spotting at cycle 6.
17.12. Analysis.
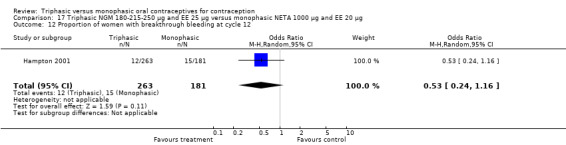
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 12 Proportion of women with breakthrough bleeding at cycle 12.
17.13. Analysis.
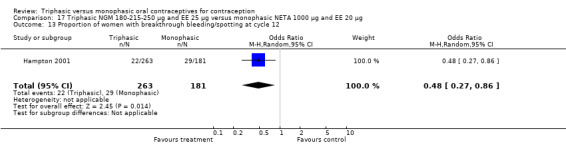
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 13 Proportion of women with breakthrough bleeding/spotting at cycle 12.
17.14. Analysis.
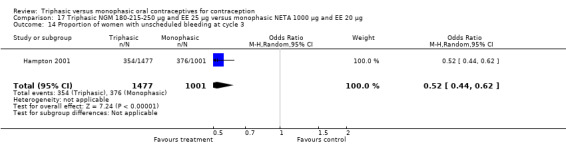
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 14 Proportion of women with unscheduled bleeding at cycle 3.
17.19. Analysis.
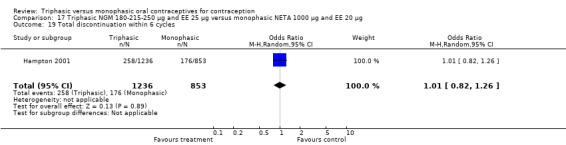
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 19 Total discontinuation within 6 cycles.
17.20. Analysis.
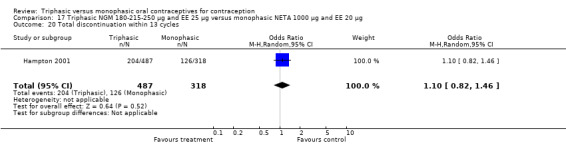
Comparison 17 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic NETA 1000 μg and EE 20 μg, Outcome 20 Total discontinuation within 13 cycles.
Comparison 18. Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman within 3 cycles | 1 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 74.62] |
| 2 Proportion of women with unscheduled bleeding episodes within 3 cycles | 1 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.70] |
| 3 Unscheduled bleeding days in cycle 3 | 1 | 316 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.57, ‐0.43] |
| 4 Unscheduled bleeding days within 3 cycles | 1 | 332 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.75, ‐0.25] |
| 5 Total discontinuation within 3 cycles | 1 | 355 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.62, 2.17] |
| 6 Discontinuations due to adverse events within 3 cycles | 1 | 355 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.16, 3.36] |
18.3. Analysis.
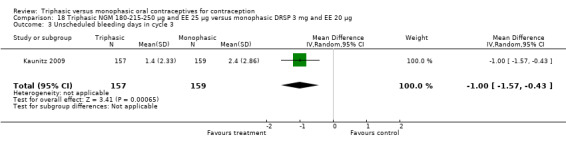
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 3 Unscheduled bleeding days in cycle 3.
18.5. Analysis.
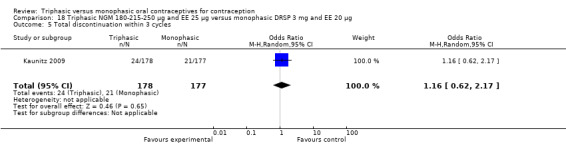
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 5 Total discontinuation within 3 cycles.
18.6. Analysis.
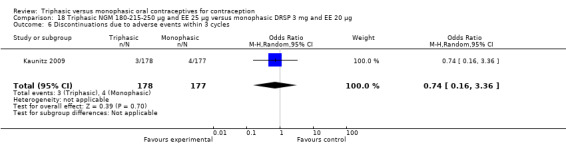
Comparison 18 Triphasic NGM 180‐215‐250 μg and EE 25 μg versus monophasic DRSP 3 mg and EE 20 μg, Outcome 6 Discontinuations due to adverse events within 3 cycles.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agoestina 1987.
| Methods | Randomized controlled trial. Randomization by random number tables. The use of blinding is not described. We were unable to reach the author. | |
| Participants | 170 women at 3 sites in Indonesia. Inclusion criteria were healthy women. Exclusion criteria were contraindications to oral contraceptives, use of hormonal contraceptives within the previous 3 cycles before enrollment and current pregnancy. The mean age of the 2 groups of participants differs. | |
| Interventions | Triphasic gestodene/ethinylestradiol (50‐70‐100 μg GTD and 30‐40‐30 μg EE in a 6/5/10 days regimen) [SHD 415 G] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 30 μg EE for 21 days) [Marvelon]. | |
| Outcomes | Primary outcomes measures are: efficacy; side effects; cycle control; continuation and reasons for discontinuation; pill intake errors. The method to collect data is not described. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 3 women in the triphasic group and 5 women in the monophasic group discontinued early. The reasons for discontinuation are described. The report does not mention loss to follow up or withdrawals because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was supported by the manufacturer of the studied triphasic gestodene/ethinylestradiol pill (Schering). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Numbered containers |
Andrade 1993.
| Methods | Randomized controlled trial without blinding. The method of randomization and the use of allocation concealment are not described. We were unable to reach the author. | |
| Participants | 480 women at 14 study sites in Europe and New Zealand. Inclusion criteria were healthy women under 40 years of age who were at risk of becoming pregnant and had regular 21 to 35 day menstrual cycles. The report does not provide exclusion criteria for the study. Switchers were included in the study. | |
| Interventions | Triphasic gestodene/ethinylestradiol (50‐70‐100 μg GTD and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=250 for 6 cycles of whom N=13 continued for an additional 6 cycles) [no brand name described] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 30 μg EE for 21 days, N=230 for 6 cycles of whom N=8 continued for an additional 6 cycles) [no brand name described]. | |
| Outcomes | Principal outcome measures are: pregnancy; cycle control; cycle length and bleeding intensity; side effects; laboratory and cytology changes; blood pressure; bodyweight; compliance; discontinuation and reasons for discontinuation. The method to collect data is not described. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not describe an a priori hypothesis or sample size or power calculation. Study duration: 6 and 12 cycles. 49 women in the triphasic group and 50 women in the monophasic group discontinued early. The reasons for discontinuation are described. 7 women in the triphasic group and 5 women in the monophasic group were lost to follow up. 6 women in the triphasic group and 8 women in the monophasic group were withdrawn because of protocol violations. Women who missed pills in cycle 6 were excluded from analysis of cycle control. Analysis not according to intention‐to‐treat principle. The paper does not report information on support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Bruni 2000.
| Methods | Randomized controlled trial without blinding. The method of randomization and the use of allocation concealment are not described. We were unable to reach the authors. | |
| Participants | 2419 women in 18 countries worldwide. Inclusion criteria were age 18 to 41 years who had regular menstrual cycles. Exclusion criteria were hypersensitivity to estrogens or progestogens, current pregnancy, breastfeeding, disorders that might interfere with the study protocol. Little information about baseline demographics. The paper does not report if switchers were included in the study. | |
| Interventions | Triphasic gestodene/ethinylestradiol (50‐70‐100 μg GTD and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=808) [Tri‐Minulet] versus monophasic gestodene/ethinylestradiol (75 μg GTD and 30 μg EE for 21 days, N=806) [Minulet] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 20 μg EE for 21 days, N=805) [Mercilon]. | |
| Outcomes | Primary outcome measures are: cycle control; well‐being; side effects and discontinuation. Use of a daily diary card to collect data on cycle control. Use of a modified form of Moos Menstrual Distress Questionnaire (MMDQ) to assess well‐being. The method of collecting data on side effects is unclear. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not describe an a priori hypothesis or sample size or power calculation. Study duration: 13 cycles. 234 women in the triphasic group, 245 women in the monophasic gestodene group and 219 women in the monophasic desogestrel group discontinued early. The reasons for discontinuation are described. 92 women in the triphasic group, 101 women in the monophasic gestodene group and 77 women in the monophasic desogestrel group were lost to follow up. 17 women in the triphasic group, 15 women in the monophasic gestodene group and 10 women in the monophasic desogestrel group were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was sponsored by the manufacturer of the studied triphasic and monophasic gestodene/ethinylestradiol pills (Wyeth‐Ayerst) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Carlborg 1983.
| Methods | Randomized controlled trial. The method of randomization and the use of blinding are not described. Communication with the author indicated a computer‐generated random allocation sequence and blinding of participants and investigators. Method of randomizing is unclear. | |
| Participants | 862 women at 12 sites in Sweden. Inclusion criteria were that women had to fulfill the current recommendations for oral contraceptive use. Limited information on baseline characteristics. Switchers were included in the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐ 40‐30 μg EE in a 6/5/10 days regimen, N=210 for 6 cycles of whom N=89 continued for an additional 6 cycles) [Trionetta 21] versus triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen and 7 days of placebo tablets, N=207 for 6 cycles of whom N=93 continued for an additional 6 cycles) [Trionetta 28] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg ethinylestradiol, N=418 for 6 cycles of whom N=189 continued for an additional 6 cycles) [Neovletta]. | |
| Outcomes | Primary outcomes measures are: pregnancy; side effects; cycle control; continuation rate and reasons for discontinuation. Use of diary cards to collect data on pill‐intake errors and cycle control. Breakthrough bleeding was defined as intermenstrual bleeding which required the use of sanitary protection and spotting as all other cases. Data on side effects were recorded if reported spontaneously. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 and 12 cycles. 67 women in the triphasic group and 60 women in the monophasic group discontinued early in the 1 to 6 cycles trial period. 26 women in the triphasic group and 24 women in the monophasic group discontinued early in the 7 to 12 cycles trial period. Little information concerning the number and reasons for discontinuation. 27 women entered in the trial are not included in the analysis because they were lost to follow up. The report does not describe withdrawals because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was supported by the manufacturer of the studied monophasic and triphasic levonorgestrel/ethinylestradiol pills (Schering). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Report does not mention the use of allocation concealment. Communication with the author indicated allocation concealment by numbered pharmacy packages. |
Chavez 1999.
| Methods | Randomized controlled trial without blinding. The method of randomization is not described. The author could not elucidate the method of randomization. | |
| Participants | 342 women at 11 sites in the USA. 53 women did not start the study after randomization. Inclusion criteria were healthy women aged 18 to 35 years for smokers and no upper age limit for non‐smokers with regular menstrual cycles (25 to 31 days) for the 3 months before enrollment who were at risk of becoming pregnant. Exclusion criteria were the standard contraindications for oral contraceptive studies listed in product class labeling, use of oral contraceptives within the previous 3 cycles before enrollment, use of an IUD or injectable or implantable estrogens, progestins or androgens during the 6 months before enrollment, smoking of more than 15 cigarettes per day and drug or alcohol abuse. | |
| Interventions | Triphasic norethindrone/ethinylestradiol (500‐750‐1000 μg NET and 35 μg ethinylestradiol in a 7/7/7 days regimen and 7 days of placebo tablets, N=173) [Ortho‐Novum 7/7/7] versus monophasic levonorgestrel/ethinylestradiol (100 μg LNG and 20 μg EE for 21 days and 7 days of placebo tablets, N=169) [Alesse/Loette]. | |
| Outcomes | Principal outcome measures are: pregnancy; side effects; cycle control; discontinuation and reasons for discontinuation. Use of a daily diary card to collect data on pill intake, cycle control, side effects and concomitant medication. Spotting was defined as a light flow that did not require sanitary protection; breakthrough bleeding as a heavier flow, similar to normal menstrual flow, that required sanitary protection; withdrawal bleeding as bleeding or spotting that began during the drug‐free interval and stopped by day 4 of the next cycle; intermenstrual bleeding as all other bleeding or spotting; and an amenorrheic cycle as one with no withdrawal bleeding or intermenstrual bleeding. Report describes the results of 4 cycles of exposure. In the article there is a discrepancy in the number of pregnancies. Communication with the authors revealed that one participant in the monophasic group became pregnant before the start of the study. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 4 cycles. 75 women in the triphasic group and 76 women in the monophasic discontinued early. The reasons for discontinuation are described. 23 women in the triphasic group and 30 women in the monophasic group did not start oral contraceptives. 12 women in the triphasic group and 9 women in the monophasic group were lost to follow up. 9 women in the triphasic group and 5 women in the monophasic were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. Breakthrough bleeding includes both breakthrough bleeding and spotting in this review. The trial was sponsored by the manufacturer of the studied monophasic levonorgestrel/ethinylestradiol pill (Wyeth‐Ayerst). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in paper. Communication with an author indicated allocation concealment by sequentially‐numbered opaque envelopes. |
Chen 1987.
| Methods | Double‐blind, randomized controlled trial. Randomization by a WHO random table. The use of allocation concealment and the method of blinding are not described. We were unable to reach the authors. | |
| Participants | 279 women aged 23‐34 years in China. Inclusion criteria were healthy women aged 23 to 34 who have the ability to record menstrual cycle on a diary and have normal physical examination and PAP smear. Exclusion criteria were diabetes mellitus, heart, liver, kidney or nervous system disease, cancer, hypertension, use of hormones 2 months prior to the study, use of injectable contraceptives 6 months prior to the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen and 7 days of placebo tablets, N=96) [no brand name described] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg EE for 21 days and 7 days of placebo tablets, N=93) [Microgynon] versus monophasic norethindrone/ethinylestradiol (600 μg NET and 35 μg ethinylestradiol for 21 days and 7 days of placebo tablets, N=90) [Pill No 1]. | |
| Outcomes | Principal outcomes are: pregnancy; side effects; cycle control; discontinuation and reasons for discontinuation. Use of a diary card to collect data on cycle control. Bleeding pattern was analyzed according to the recommendations of Rodriguez 1976. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 cycles. 17 women in each treatment group discontinued early. The reasons for discontinuation are described. No woman was lost to follow up. One woman in the triphasic group, 2 women in the monophasic levonorgestrel group and one woman in the monophasic norethindrone group were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was conducted by the World Health Organization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Dieben 1984.
| Methods | Randomized controlled trial without blinding. The method of randomization is not described. Communication with the author indicated an allocation sequence in balanced blocks of four. The method of randomizing the blocks of 4 is unclear. | |
| Participants | 948 women at sites in 6 European countries. The report does not provide inclusion/exclusion criteria for the study and scarcely describes the baseline demographics. Communication with the authors provided the inclusion/exclusion criteria. Inclusion criteria were healthy, fertile women with a regular cycle and normally exposed to the risk of pregnancy. Exclusion criteria were history of thromboembolic disease, thrombophlebitis, disturbance of liver function, jaundice or a history of jaundice in pregnancy, mammary carcinoma, estrogen‐dependent tumor, undiagnosed genital bleeding, sickle‐cell anemia, porphyria cutanea tarda, cardiovascular disease, treatment with rifampicin, tetracyclines, phenylhydantoin and phenobarbitone, no spontaneous menstruation postpartum or postabortal, breastfeeding. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=473 for 6 cycles of whom N=38 continued for an additional 6 cycles) [no brand name described] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 30 μg EE for 21 days, N=475 for 6 cycles of whom N=54 continued for an additional 6 cycles) [Marvelon]. | |
| Outcomes | The primary outcome measures are: pregnancy; side effects; cycle control; discontinuation rates. Use of a record to collect data on cycle control and side effects. Withdrawal bleeding was defined as bleeding which begins in the tablet‐free period; spotting as scanty bleeding outside the tablet‐free period that does not require any hygienic measures or at most one sanitary pad per day; and breakthrough bleeding as bleeding that is not spotting and which cannot be considered as withdrawal bleeding. Report describes outcome measures unclearly. Communication with the author revealed that there were 3 pregnancies instead of the reported 2. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 and 12 cycles. The report does not describe the number and reasons for discontinuation. Communication with the author gave information that 67 women in both groups discontinued early in the 1 to 6 cycles trial period and 2 women in both groups discontinued early in the 7 to 12 cycles trial period. Three women in the triphasic group and two women in the monophasic were withdrawn because of protocol violations in the first trial period. No women were withdrawn because of protocol violations in the second trial period. The number of women lost to follow up was not clear. Analysis not according to intent‐to‐treat principle. The trial was supported by the manufacturer of the studied monophasic desogestrel/ethinylestradiol pill (Organon). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Not mentioned in report. Communication with the author indicated no concealment of the allocation sequence. |
Dunson 1993.
| Methods | Randomized controlled trial without blinding. The method of randomization is not described in report. Communication with the authors indicated a computer‐generated random allocation sequence. | |
| Participants | 1088 women aged 18 to 35 years at 5 sites in Sudan, Sri Lanka, Chile, Ecuador and Dominican Republic. Inclusion criteria were healthy women aged 18 to 35 years who were sexually active and had at least one normal menstrual period since the last pregnancy or the last use of a steroidal contraceptive. Exclusion criteria were contraindications to oral contraceptive use, termination of pregnancy less than 42 days prior to admission if not breastfeeding or termination of pregnancy less than 4 months prior to admission if breastfeeding. Switchers were included in the study. The two groups of participants differed in the complaint dizziness at admission. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen and 7 days of placebo tablets, N=543) [Triquilar] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg EE for 21 days and 7 days of placebo tablets, N=545) [Lo‐Femenal]. | |
| Outcomes | Primary outcomes measures are: pregnancy; discontinuation rates and reasons for discontinuation; side effects; cycle control. Use of recall method to collect data on cycle control, side effects and reasons for discontinuation. The report does not describe the definitions of breakthrough bleeding and spotting. Outcome measures cycle control and side effects differ between the various sites. | |
| Notes | The report does not provide an a priori hypothesis or a sample size calculation. Study duration: 12 cycles. 418 women in the triphasic group and 420 women in the monophasic group discontinued early. Reasons for discontinuation are described. The paper reported that 39% of the participants were lost to follow up but provides no breakdown of how many were in each group. The report does not mention withdrawals because of protocol violations. Analysis according to intention‐to‐treat principle. The trial was supported by Family Health International. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in report. Communication with the authors indicated allocation concealment by use of sequentially‐numbered, opaque, sealed envelopes. |
Engebretsen 1987.
| Methods | Randomized controlled trial without blinding. The method of randomization and use of allocation concealment are not described. We were unable to reach the authors. | |
| Participants | 300 women aged 15 to 35 years who did not use oral contraceptives in the month prior to the study at 5 sites in Norway. The participants group had a high rate of abortus provocatus. Exclusion criteria were a history of thrombosis or thrombophlebitis, liver‐disease, cancer, history of herpes gestationis, pregnancy, hypertension and oral contraceptive use in the month prior to the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=150) [Trinordiol] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg EE for 21 days, N=150) [Follimin]. | |
| Outcomes | Primary outcome measures are: pregnancy; cycle control; side effects; continuation rate and reason for discontinuation. Use of a patient diary to collect data on side effects and cycle control. The report does not describe the definitions of spotting and breakthrough bleeding. Limited information on outcome measures. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 45 women in the triphasic group and 44 women in the monophasic group discontinued early. Little information concerning the number and reasons for discontinuation. The report does not describe the number of women lost to follow up or excluded because of protocol violations. Analysis not according to intent‐to‐threat principle. Cycles with incorrect pill‐intake were excluded from the analysis. The paper does not report information on support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Hampton 2001.
| Methods | Randomized controlled trial. Randomization in a 3:3:3:2 ratio in blocks of size 11:9. The method of randomization was not described. NGM/EE regimens were blinded and Loestrin Fe open. Communication with the authors indicated a computer‐generated random allocation sequence. The randomization was balanced using permuted blocks and stratified by study center. | |
| Participants | 6022 women at 110 sites in the USA and Canada. One‐third of the women participated in the study for 13 cycles, two‐thirds of the women participated for 6 cycles. Inclusion criteria were women aged 18 to 45 years who had regular menstrual cycles, were sexually active, at risk of pregnancy and agreed to use only the study drug as contraception. Exclusion criteria were positive serum beta‐hCG pregnancy test, seated systolic/diastolic blood pressure more than 140/90 mm Hg, lactation or pregnancy within 42 days of study admission, any disorders that were contraindications to steroid hormonal therapy, uncontrolled thyroid disorder, cervical dysplasia, smoking in women older than 35 years, exposure to etretinate, receipt of an experimental drug, device, hepatic enzyme‐inducing drug, isotretinoin or tretinoin within 30 days of screening, receipt of Depo‐Provera within 6 months of screening and alcohol or substance abuse within 12 months of screening. More than 60 percent of the women used oral contraceptives less than 2 months before admission. | |
| Interventions | Triphasic norgestimate/ethinylestradiol (180‐215‐250 μg NGM and 25 μg ethinylestradiol in a 7/7/7 days regimen and 7 days of placebo tablets, N=1723 for 6 cycles of whom N=487 continued for an additional 6 cycles) [Ortho Tri‐Cyclen Lo] versus monophasic norethindrone acetate/ethinylestradiol and ferrous fumarate (1000 μg NETA and 20 μg EE for 21 days and 7 days of 75 mg ferrous fumarate, N=1171 of whom N=318 continued for an additional 6 cycles) [Loestrin‐Fe] versus 'cyclophasic' norgestimate/ethinylestradiol (250‐180 μg NGM and 25 μg EE in an alternating 2‐day regimen) [Cyclophasic‐25] versus 'cyclophasic' norgestimate/ethinylestradiol (180‐60 μg NGM and 20 μg EE in an alternating 2‐day regimen) [Cyclophasic‐20]. | |
| Outcomes | Primary outcomes are: pregnancy; cycle control, side effects; laboratory changes; body weight; vital signs; changes in physical examination. Use of daily diary cards to collect data on pill‐intake, cycle control and side effects. Data on side effects were recorded if reported in response to a general question or observed during physical examination. In the original article the recommendations by Belsey 1986 were used for analyzing bleeding patterns. Breakthrough bleeding was defined as bleeding that requires sanitary protection of more than one pad or tampon per day and occurs during the active tablet‐taking interval but is not contiguous with menses. Breakthrough spotting was defined as bleeding that requires one or less pad or tampon during the active tablet‐taking interval but is not contiguous with menses. The definition of amenorrhea was two consecutive cycles without any bleeding or spotting. In a secondary article the bleeding data were re‐analyzed with the new recommendations by Mishell 2007. Unscheduled bleeding was defined as any bleeding that occurs while taking active hormones, regardless of the duration of the regimen, with the exception of bleeding that begins during the hormone‐free interval and continues through days 1‐4 of the subsequent active cycle and bleeding reported during days 1‐7 of the first cycle of combined hormonal contraceptive therapy. The definition of amenorrhea was absence of all bleeding or spotting. A third article examined bleeding patterns by age and weight subgroups. Outcomes for 'cyclophasic' norgestimate/ethinylestradiol groups are not described. | |
| Notes | The report does not provide an a priori hypothesis. The paper states that the sample size was determined to meet the US regulatory requirements of at least 10,000 cycles for the evaluation of the safety and efficacy of oral contraceptives with at least 200 participants evaluated for 13 cycles. Study duration: 6 and 13 cycles. In the group of participants enrolled for a trial period of 6 cycles, 258 women taking triphasic pills and 176 women taking monophasic pills discontinued early. In the group of participants enrolled for a trial period of 13 cycles, 204 women using triphasic pills and 126 women using monophasic pills discontinued early. The reasons for discontinuation are partially described. The paper reports that 6.5% of the women in the triphasic group and 5.8% of the women in the monophasic group were lost to follow up but provides no numerator. The number of women withdrawn because of protocol violations is not mentioned. The paper states that the evaluation of contraceptive efficacy was based on an intent‐to‐treat analysis. The evaluation of cycle control was not according to the intention‐to‐treat principle. Cycles in which data on dosing and bleeding was lacking and cycles with incorrect pill‐intake were excluded from the analysis. The trial was sponsored by the manufacturer of the studied triphasic norgestimate/ethinylestradiol pill (Johnson & Johnson). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in report. Communication with the authors indicated allocation concealment by a centralized voice‐activated randomization system. |
Ismail 1991.
| Methods | Randomized controlled trial without blinding. The method of randomization is not described. Communication with the author indicated a computer‐generated random allocation sequence. | |
| Participants | 200 women in Malaysia. Inclusion criteria were healthy women aged 18 to 35 years who were sexually active, were willing to rely exclusively upon the pills as the only method of contraception and had at least one menstrual period since the last pregnancy. Exclusion criteria were contraindications to oral contraceptives, termination of pregnancy less than 42 days prior to admission and breastfeeding. Switchers were included in the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=100) versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 30 μg EE for 21 days, N=100) [Marvelon]. | |
| Outcomes | Primary outcome measures are: pregnancy; side effects; cycle control; discontinuation and reasons for discontinuation. The method of collecting the data on cycle control and side effects is unclear. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 41 women in the triphasic group and 33 women in the monophasic group discontinued early. The reasons for discontinuation are described. 2 women in the triphasic group did not start oral contraceptives. 9 women in the triphasic group and 6 women in the monophasic group were lost to follow up. 6 women in the triphasic group and 3 women in the monophasic group were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was supported by Family Health International. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by use of preprinted sealed envelopes opened at the time of admission. |
Kashanian 2010.
| Methods | Randomized controlled trial. The method of randomization and the use of blinding are not described. Allocation concealment by sealed, sequentially distributed envelopes. Communication with the author indicated randomization by use of a random number table and blinding of participants, investigators and outcome assessors. | |
| Participants | 342 women at public health centers in Iran. Inclusion criteria were married women aged 17 to 40 years who had regular menstrual cycles, no signs and symptoms similar to adverse effects of pills before using them and no prior oral contraceptive use. Exclusion criteria were contraindication to pills, systemic disorders or drug use, breastfeeding, delivery less than 3 weeks previously, use of injectable contraceptive in past 6 months or implant in past 3 months, abnormal Pap smear, abnormal blood cholesterol and triglycerides, and being illiterate. Further exclusion criteria during the study were omitting one or more pills during the cycles, stop taking pills, using other contraceptives along with oral contraceptive pills, acute severe diarrhea and vomiting, and pregnancy. All participants were first‐time oral contraceptive users. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 µg LNG and 30‐40‐30 µg EE in a 6/5/10 days regimen, N=171) [no brand name described] versus monophasic levonorgestrel/ethinylestradiol (150 µg LNG and 30 µg EE, N=171) [no brand name described]. | |
| Outcomes | Primary outcome measures are: side effects including breakthrough bleeding and spotting; discontinuation and reasons for discontinuation; satisfaction. Use of daily diary method to collect data on side effects. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not provide an a priori hypothesis. The paper describes a sample size calculation which referred to both 80% and 85% power. Correspondence with author indicated 80% power. Study duration: 6 cycles. 16 women in the triphasic group and 12 women in the monophasic group discontinued early. Limited information on reasons for discontinuation. 16 women in the triphasic group and 10 women in the monophasic group were lost to follow‐up. Communication with the author gave information that 2 women in the monophasic group discontinued due to nervousness. Analysis not according to intention‐to‐treat principle. The paper does not report information on trial support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Sealed, sequentially distributed envelopes" with letters A, B, C, D (2 letters assigned to each treatment group). Participant chose an envelope, which investigator opened. |
Kaunitz 2009.
| Methods | Randomized controlled trial without blinding. Randomization by a computer‐generated randomization schedule. Randomization was stratified by center and recent hormonal contraceptive exposure (new user or switcher). The use of allocation concealment is not described. | |
| Participants | 355 women at 20 centers in the USA. Inclusion criteria were healthy, nonpregnant, nonlactating, sexually active women aged 18 to 45 years who had regular menstrual cycles, negative Chlamydia test and a normal Pap test without evidence for moderate or severe dysplasia or any malignancy within the preceding 12 months. Exclusion criteria were contraindications to hormonal therapy, untreated thyroid disorder, body mass index greater than 40 kg/m2, previous discontinuation of one of the treatments due to breakthrough bleeding, receiving an hormonal injectable contraceptive within 6 months of screening, having an implant within 60 days of screening or having an hormonal intrauterine device within 3 months of screening or smoking if aged 35 to 45 years. Switchers were included in the study. The two groups of participants differed in weight and smoking status. More than 50% of the participants used hormonal contraception less than 60days before admission. | |
| Interventions | Triphasic norgestimate/ethinylestradiol (180‐215‐250 µg NGM and 25 µg EE in a 7/7/7 days regimen and 7 days of placebo tablets, N=178) [Ortho Tri‐Cyclen Lo] versus monophasic drospirenone/ethinylestradiol (3 mg DRSP and 20 µg EE for 24 days and 4 days of placebo tablets, N=177) [Yaz]. | |
| Outcomes | Outcomes measure are: cycle control, patient satisfaction, side effects. Use of an interactive voice‐response system‐based diary to collect data on daily cycle control. Bleeding and unscheduled bleeding was defined as per Mishell 2007. Unscheduled bleeding episode was defined as per Belsey 1986. | |
| Notes | The report does not provide an a priori hypothesis. The paper describes an adequate sample size calculation. Study duration: 3 cycles. 24 women in triphasic group and 21 women in monophasic group discontinued early. The reasons for discontinuation are described. 11 women in triphasic group and 10 women in monophasic group did not start oral contraceptives. 7 women in triphasic group and 4 women in monophasic group were lost to follow‐up. 2 women in both treatment groups were withdrawn because of lack of compliance. One woman in monophasic group was withdrawn because of protocol violations. The paper states states that the evaluation of bleeding patterns was based on the intent‐to‐treat population defined as all randomly assigned participants who took the study drug and for whom there was post‐baseline bleeding data after day 7. The trial was sponsored by the manufacturer of the studied triphasic norgestimate/ethinylestradiol pill (Ortho‐McNeil‐Janssen). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Lachnit‐Fixson 1984.
| Methods | Randomized controlled trial. The method of randomization, the use of allocation concealment and the use of blinding are not described. Communication with the author revealed no extra information. | |
| Participants | 555 women at sites in Austria, Germany, the Netherlands and the United Kingdom. The report does not provide inclusion/exclusion criteria for the study. Little information about baseline demographics. The paper does not report if switchers were included in the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=278) [Triquilar/Logynon] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 30 μg EE, N=277) [Marvelon]. | |
| Outcomes | Primary outcome measures are: pregnancy; side effects; cycle control; continuation and reason for discontinuation. Use of a bleeding chart to collect data on cycle control. Data on side effects were recorded if reported spontaneously. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not provide an a priori hypothesis. Report states a sample size, yet the sample size calculation is unclear. Study duration: 6 cycles. Limited information on number and reasons for discontinuation. The paper describes that 15.5% of the participants discontinued early but provides no breakdown of how many were in each group. The report does not mention loss to follow up or withdrawals because of protocol violations. Unclear whether the analysis was according to intention‐to‐treat principle. The trial was supported by the manufacturer of the studied triphasic levonorgestrel/ethinylestradiol pill (Schering). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Percival‐Smith 1990.
| Methods | Randomized controlled trial with blinding of the outcome assessor. The method of randomization, the use of allocation concealment and the method of blinding are not described. Communication with the author revealed no extra information. | |
| Participants | At 4 sites in Canada, 469 women were randomized to one of the pills. However, only 391 women were admitted to the study and used the pills for at least one month. 222 women did not use OC pills at least 90 days before the study, and 247 women did use pills before the study. Inclusion criteria were healthy women aged 15 to 35 years who had a history of regular menses for two months prior to admission. Exclusion criteria were contraindications to oral contraceptives. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=119) [Triphasil] versus triphasic norethindrone/ethinylestradiol (500‐750‐1000 μg NET and 35 μg EE in a 7/7/7 days regimen, N=117)) [Ortho 7/7/7] versus biphasic norethindrone/ethinylestradiol (500‐1000 μg NET and 35 μg ethinylestradiol in a 10/11 days regimen, N=116) [Ortho 10/11] versus monophasic norethindrone acetate/ethinyl estradiol (1500 μg NETA and 30 μg EE, N=117) [Loestrin]. In the pre‐study user group, 16 participants already used Triphasil, 5 Loestrin, 8 Ortho 10/11 and 8 Ortho 7/7/7. | |
| Outcomes | Primary outcomes measures are: side effects; cycle control; continuation, discontinuation rates and reason for discontinuation. Use of daily diary method to collect data on cycle control and side effects. Breakthrough bleeding was defined as free flow, much like menses occurring during the 21 days of active medication and requiring sanitary protection; and spotting as bleeding during the active medication, which is limited to minor staining, whether or not sanitary protection was used. | |
| Notes | The report provides an a priori hypothesis and an adequate sample size calculation. Study duration: 6 cycles. 49 women in the monophasic group, 35 women in the biphasic group, 46 women in the levonorgestrel triphasic group and 39 women in the norethindrone triphasic group discontinued early. The reasons for discontinuation are partially described. The report does not describe the number of women lost to follow up or withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. 78 women who were randomized but did not take the oral contraceptives for at least one cycle were excluded from the analysis. Breakthrough bleeding includes all intermenstrual bleeding except continued menstrual flow in this review. The trial was sponsored by the manufacturer of the monophasic norethindrone acetate/ethinylestradiol pill (Parke‐Davis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Ramos 1989.
| Methods | Randomized controlled trial with blinding of investigators and participants. Allocation concealment by use of numbered pharmacy packages, blinding by repackaging the pills. The method of randomization is not described. Communication with the authors revealed no extra information. | |
| Participants | 1800 women at 18 sites in the Philippines. The report does describe the inclusion and exclusion criteria for the study. Switchers were included in the study. 27% to 32% of the participating women lactated at the time of admission. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen, N=601) [Trinordiol] versus monophasic norethindrone/ethinylestradiol (400 μg NET and 35 μg EE for 21 days, N=599) [Micropil] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg EE for 21 days, N=600) [Nordette]. | |
| Outcomes | Primary outcome measures are: pregnancy; side effects; cycle control; continuation rates and reasons for discontinuation. Use of menstrual diary cards to collect data on cycle control. Data on side effects were recorded if reported spontaneously. Information on side effects was specifically asked at discontinuation or method change. Breakthrough bleeding was defined as intermenstrual bleeding that required the use of sanitary protection, and spotting as intermenstrual bleeding which required no use of pads. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 165 women in the triphasic group, 192 women in the NET monophasic group and 151 women in the LNG monophasic group discontinued early. The reasons for discontinuation are described. 13 participants in the triphasic group, 9 participants in the NET monophasic group and 16 participants in the monophasic LNG group were lost to follow up. 11 women in triphasic group, 12 women in the NET monophasic group and 8 women in the LNG monophasic group were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. The trial was supported by United Nations Population Fund and by the manufacturers of the triphasic levonorgestrel/ethinylestradiol and monophasic levonorgestrel/ethinylestradiol pill (Wyeth‐Ayerst) and monophasic norethindrone/ethinylestradiol pill (Pascual Laboratories). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by use of numbered pharmacy packages |
Reisman 1999.
| Methods | Randomized controlled trial without blinding. The method of randomization is not described. Reports notes stratification using investigational site as the stratification variable. Communication with the investigators revealed no extra information. | |
| Participants | 387 women at 11 sites in the USA. 65 women did not start the study after randomization. Inclusion criteria were healthy women aged 18 to 35 years for smokers and no upper age limit for non‐smokers with regular menstrual cycles (25 to 31 days) for the 3 months before enrollment who were at risk of becoming pregnant. Exclusion criteria were the standard contraindications for oral contraceptive studies listed in product class labeling, use of oral contraceptives within the previous 3 cycles before enrollment, use of an IUD or injectable or implantable estrogens, progestins or androgens during the 6 months before enrollment and smoking of more than 15 cigarettes per day. | |
| Interventions | Triphasic norethindrone/ethinylestradiol (500‐750‐1000 μg NET and 35 μg ethinylestradiol in a 7/7/7 days regimen and 7 days of placebo tablets, N=195) [Ortho‐Novum 7/7/7; TriNovum] versus monophasic levonorgestrel/ethinylestradiol (100 μg LNG and 20 μg ethinylestradiol for 21 days and 7 days of placebo tablets, N=192) [Alesse;Loette]. | |
| Outcomes | Principal outcome measures are: pregnancy; side effects during treatment and after discontinuation; cycle control; discontinuation and reasons for discontinuation; metabolic outcomes. Use of diary cards to collect data on pill intake, cycle control, side effects and concomitant medication. The report describes the results of 4 cycles of exposure. Spotting was defined as a light flow that did not necessitate sanitary protection; breakthrough bleeding as a heavier flow, similar to normal menstrual flow, that did necessitate sanitary protection; withdrawal bleeding as bleeding or spotting that began during the drug‐free interval and stopped by day 4 of the next cycle; intermenstrual bleeding as all other bleeding or spotting; and an amenorrheic cycle as one with neither withdrawal bleeding nor intermenstrual bleeding. | |
| Notes | The report provides an adequate sample size calculation. Study duration: 4 cycles. 77 women in the triphasic group and 90 women in the monophasic group discontinued early. Reasons for discontinuation are described. 28 women in the triphasic group and 37 women in the monophasic group did not take the oral contraceptives. 20 women in the triphasic group and 19 women in the monophasic group were lost to follow up. 6 women in both groups were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat principle. Breakthrough bleeding includes breakthrough bleeding and spotting in this meta‐analysis. The trial was sponsored by the manufacturer of the monophasic levonorgestrel/ethinylestradiol pill (Wyeth‐Ayerst). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by sequentially‐numbered, sealed envelopes opened at the time of admission. |
Reiter 1990.
| Methods | Randomized controlled trial without blinding. The method of randomization was not described. Communication with the authors indicated randomization by use of a random number table. | |
| Participants | 477 women at sites in the U.S.A. Inclusion criteria were women aged 18 years or older. Exclusion criteria were contraindications to oral contraceptive use. Little information about baseline demographics. All participants were first‐time oral contraceptive users. | |
| Interventions | Triphasic norethindrone/ethinylestradiol (500‐750‐1000 μg NET and 35 μg EE in a 7/7/7 days regimen and 7 days of placebo tablets) [Ortho‐Novum 7/7/7] versus triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 days regimen and 7 days of placebo tablets) [Triphasil] versus monophasic norethindrone/ethinylestradiol (1000 μg norethindrone and 35 μg ethinylestradiol for 21 days and 7 days of placebo tablets) [Ortho‐Novum 1/35]. | |
| Outcomes | Outcome measures are: side effects; cycle control; continuation rate; satisfaction; side effects after change of OC. Use of recall method to collect data on cycle control, side effects and satisfaction with the method. Breakthrough bleeding was defined as any spotting or bleeding between menstrual periods, and amenorrhea as the absence of spotting or bleeding during the expected time of the menstrual period. Limited information on outcome measures. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 100 women discontinued early, however the paper does not provide a breakdown of the number in each group. No information on reasons for discontinuation. Analysis not according to intent‐to‐treat principle. The report contains no references to other studies. The trial was conducted by Planned Parenthood Federation of America. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Not described in report. Communication with the authors indicated no concealment of treatment allocation. |
Rosenberg 1999.
| Methods | Randomized controlled trial without blinding. Randomization in balanced blocks of 6. The method of randomization was not described. Communication with the author indicated a computer‐generated randomization sequence | |
| Participants | 463 women at 15 sites in the US. Inclusion criteria were age 18 to 50 years, BMI of 18 to 35, regular menstrual cycles of 21 to 38 days. Exclusion criteria were contraindications to oral contraceptive use, age more than 35 years and smoking more than 15 cigarettes per day, more than 2 alcoholic drinks per day, breastfeeding, fewer than 3 regular cycles after delivery or fewer than 2 regular cycles after an abortion, use of injectable or implant contraceptives within 6 months before enrollment or considered to be poor candidates for follow up or reliability. Analysis of 2 groups: 308 switchers (participants who have used OC in the 2 months before the study); 155 starters. 34 participants were using 20 μg EE preparations and 262 were using 30 or 35 μg EE preparations at study entry. Low percentage of smokers. | |
| Interventions | Triphasic norgestimate/ethinylestradiol (180‐215‐250 μg NGM and 35 μg EE in a 7/7/7 days regimen, N=155) [Tri‐Cyclen] versus monophasic levonorgestrel/ethinylestradiol (100 μg LNG and 20 μg ethinylestradiol for 21 days and 7 hormone‐free days, N=154) [Alesse] versus monophasic desogestrel/ethinylestradiol (150 μg DSG and 20 μg EE for the first 21 days, then 2 hormone‐free days, and 10 μg EE for the last 5 days, N=154) [Mircette]. | |
| Outcomes | Primary outcomes measures are: efficacy; cycle control; side effects; and continuation rates. Use of a daily diary to collect data on pill‐intake, side effects and cycle control. Cycle control was assessed by an index that considered duration and severity of intermenstrual bleeding. The report does not describe the definitions of breakthrough bleeding and spotting. Limited information on outcome measures. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 cycles. The paper describes continuation rates; however, the number of women who continue are not described. Communication with the author indicated that 25 women in the triphasic group, 26 women in the levonorgestrel monophasic group and 24 women in the desogestrel monophasic group discontinued early. Reasons for discontinuation are not described. It is unclear whether the analysis was according to intention‐to‐treat principle. The trial was supported by the manufacturer of the monophasic desogestrel/ethinylestradiol pill (Organon). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in report. Communication with the author indicated allocation concealment by sequentially numbered randomization cards with an opaque scratch‐off dot. |
Rowan 1999.
| Methods | Double‐blind, randomized controlled trial. The method of randomization and the method of blinding are not described. Communication with the author indicated randomization in a 3:2 ratio in blocks of five, and double‐blinding by identical pills and packages. The method of randomizing the blocks of five is unclear. | |
| Participants | 1277 women at 8 sites. Inclusion criteria were women aged 18 to 35 years with a history of regular menstrual cycles (28 + 3 days) for 2 consecutive cycles immediately before study entry. Exclusion criteria were contraindications to oral contraceptives and use of oral contraceptives within 2 months before enrollment. Limited information on baseline demographics. | |
| Interventions | 'Estrophasic' norethindrone acetate/ethinylestradiol (1000 μg NETA and 20‐30‐35 μg EE in a 5/7/9 days regimen, N=769) [Estrostep] versus monophasic norethindrone acetate/ethinylestradiol (1500 μg NETA and 30 μg EE for 21 days, N=508) [Loestrin 1.5/30]. | |
| Outcomes | Primary outcome measures are: efficacy; cycle control; side effects; discontinuation due to side effects. Use of special diaries to collect data on cycle control, side effects, pill‐intake and concomitant medication. Breakthrough bleeding was defined as vaginal bleeding during the medication‐taking period that was not a continuation of menstrual flow and that necessitated pad or tampon protection. | |
| Notes | Report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 cycles. Number of and reasons for discontinuation are not described except discontinuation due to side effects. Unclear whether the analysis was according to intention‐to‐treat principle. Random assignment in a 2:1 ratio. However, 769 women received the 'estrophasic' preparation and 508 the monophasic preparation. Communication with the author indicated a 3:2 allocation ratio. We only included study 1 in this review. Study number 2 compares the 'estrophasic' combination with a triphasic combination in terms of metabolic outcomes. The trial was sponsored by the manufacturer of the studied monophasic and estrophasic norethindrone acetate/ethinylestradiol pills (Parke‐Davis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in report. Communication with the author indicated allocation concealment by numbered pharmacy packages. |
Saxena 1992.
| Methods | Randomized controlled trial. The method of randomization and the use of blinding are not described. Communication with the authors indicated a computer‐generated allocation sequence and no blinding. | |
| Participants | 721 women in reproductive age at 11 sites in India. Inclusion criteria were healthy women in the reproductive age exposed to the risk of pregnancy. Exclusion criteria were contraindications for oral contraceptive use. The paper does not report if switchers were included. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 regimen and 7 days of placebo tablets, N=383) [Triquilar ED] versus monophasic levonorgestrel/ethinylestradiol (150 μg levonorgestrel and 30 μg ethinylestradiol for 21 days and 7 days of placebo tablets, N=338) [MALA‐D]. Report does not describe the composition of the monophasic pill. Communication with the author indicated data described above. | |
| Outcomes | Principal outcome measures are: pregnancy; side effects; cycle control; continuation; discontinuation and reasons for discontinuation; metabolic outcomes. Use of recall method to collect data on pill intake errors, cycle control and side effects. Bleeding pattern was analyzed according to the recommendations by Rodriguez 1976. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 12 cycles. 256 women in the triphasic group and 203 women in the monophasic group discontinued early. The report describes number and reasons for discontinuation. 16 women in the triphasic group and 14 women in the monophasic group were lost to follow up. 9 women in the triphasic group and 14 women in the monophasic group were withdrawn because of protocol violations. Analysis not according to intention‐to‐treat. The trial was conducted by the Indian Council of Medical Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Not described in report. Communication with the authors indicated allocation concealment by sequentially‐numbered sealed envelopes |
Sulak 1999.
| Methods | Randomized controlled trial without blinding. The method of randomization and the use of allocation concealment are not described. Report notes stratification using postpartum status as the stratification variable. We were unable to reach the authors. | |
| Participants | 373 women at 10 sites. Inclusion criteria were healthy women aged 18 to 50 years. Exclusion criteria were disorders considered to be contraindications for steroid hormonal therapy and use of oral contraceptives within 60 days of enrollment. Analysis of 2 groups: safety population (all participants who received at least one dose of study medication); intent‐to‐treat population (all participants who received at least one dose of study medication and who had at least one cycle control measurement). The safety population consisted of 335 women and the intent‐to‐treat population of 328 women. Participants used a nonsteroidal contraceptive method for the first 7 days of cycle 1. | |
| Interventions | Triphasic norgestimate/ethinylestradiol (180‐215‐250 μg NGM and 35 μg EE in a 7/7/7 days regimen and 7 days of placebo tablets, N=187) [Ortho Tri‐Cyclen] versus monophasic norethindrone acetate/ethinylestradiol (1000 μg NETA and 20 μg ethinylestradiol for 21 days and 7 days of placebo tablets, N=186) [Loestrin 1/20]. | |
| Outcomes | Primary outcomes measures are: efficacy; side effects; cycle control; continuation and reasons for discontinuation; pill intake errors. The method to collect data is not described. The report does not describe the definitions of breakthrough bleeding and spotting. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 cycles. 72 women in the triphasic group and 70 women in the monophasic group discontinued early. Limited information on reasons for discontinuation. 16 women in the triphasic group and 22 women in the monophasic group did not start oral contraceptives. The report does not describe the number of women lost to follow up or excluded because of protocol violations. The paper indicated an analysis based on the intent‐to‐treat principle but participants not starting oral contraceptives and invalid cycles were excluded from analysis. Errors in pill intake, a cycle length longer than 31 days, errors in non‐active pill intake and errors in recording cycle information all create invalid cycles. The trial was sponsored by the manufacturer of the triphasic norgestimate/ethinylestradiol pill (Ortho‐McNeil Pharmaceutical). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Zador 1979.
| Methods | Randomized controlled trial without blinding. The method of randomization and the use of allocation concealment are not described. Communication with the author revealed no extra information. | |
| Participants | 489 women at sites in Sweden, Great Britain and Germany. Inclusion criteria were that women had to meet the requirements for the prescription of oral contraceptives in accordance with established medical practice. Limited information about baseline demographics. The paper does not report if switchers were included in the study. | |
| Interventions | Triphasic levonorgestrel/ethinylestradiol (50‐75‐125 μg LNG and 30‐40‐30 μg EE in a 6/5/10 regimen, N=254) [SH B 264 AB] versus monophasic levonorgestrel/ethinylestradiol (150 μg LNG and 30 μg EE for 21 days, N=235) [Neovletta]. | |
| Outcomes | Principal outcome measures are: pregnancy; side effects; cycle control; discontinuation and reasons for discontinuation. Use of a chart for collecting data on side effects and cycle control. Breakthrough bleeding was defined as intermenstrual bleeding that required the use of sanitary protection and spotting as all other cases including slight brownish discharge. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Study duration: 6 cycles. 36 women in both groups discontinued early. Limited information on number and reasons for discontinuation. The report does not mention the number of women lost to follow up or excluded because of protocol violations. Whether the analysis was based on the intention‐to‐treat principle is unclear. The trial was supported by the manufacturer of the studied monophasic and triphasic levonorgestrel/ethinylestradiol pills (Schering). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bancroft 1987 | The study examines mood and sexuality. |
| Christie 1989 | The report does not mention how participants were assigned to groups. We attempted without success to reach the author. |
| Dik 1984 | The report does not mention how participants were assigned to groups. We were unable to contact the authors. |
| Dubnitskaia 1988 | Although described as a randomized controlled trial we learned from the author that the study is a matched cohort study. |
| Grace 1994 | Communication with the author indicated no randomization of the allocation sequence. |
| Kuhl 1985 | Insufficient data for analysis of spotting and breakthrough bleeding. Emphasis was on hormonal and metabolic parameters. |
| Matsumoto 1988 | Report does not mention how participants were assigned to groups. We attempted without success to reach the author. |
| Otolorin 1989 | The report describes allocation as systematical. We were unable to contact the author. |
| Perrone 1987 | Report does not mention how participants were assigned to groups. We attempted without success to reach the author. |
| Rubio‐Lotvin 1992 | The report does not mention how participants were assigned to groups. We could not reach the authors. |
Characteristics of ongoing studies [ordered by study ID]
Bayer 2011.
| Trial name or title | Cycle Control and Safety of E2‐DRSP |
| Methods | Randomized, double‐blind, multicenter |
| Participants | 600 healthy women, 18 to 35 years |
| Interventions | 6 different regimens of drospirenone and ethinyl estradiol (including monophasic and triphasic) over 7 cycles (details not provided) |
| Outcomes | include bleeding patterns and cycle control |
| Starting date | March 2008 |
| Contact information | Bayer Study Director (no more information provided) |
| Notes | Completed June 2009. No report found; searched PubMed and http://www.clinicalstudyresults.org/ |
Contributions of authors
F Helmerhorst developed the idea. H van Vliet and D Grimes wrote the protocol and performed the literature search. H van Vliet, D Grimes and F Helmerhorst abstracted the data. H van Vliet drafted the review. L Lopez drafted the plain language summary and edited the review. For the 2008 update, L Lopez reviewed the search results, incorporated new trials, and updated the text. For the 2011 update, L Lopez reviewed the search results and L Lopez and H van Vliet incorporated new trials and updated the text. All authors edited and advised on the review drafts.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
U.S. Agency for International Development, USA.
Declarations of interest
DA Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
Three of the authors (DA Grimes, KF Schulz, LM Lopez) are employed by FHI 360 (formerly Family Health International), which sponsored two of the trials included in this review. However, they were not employed at FHI at the time of the trials nor were they involved in the trials.
Edited (no change to conclusions)
References
References to studies included in this review
Agoestina 1987 {published data only}
- Agoestina T, Sulaeman M, Rarung M, Sabarudin U. Clinical evaluation of low dose pill triphasic (EE + estoden) versus monophasic (EE + desogestrel). Presented at the XIth Asian and Oceanic Congress of Obstetrics and Gynecology; 1987 Dec 6‐12; Hongkong.
Andrade 1993 {published data only}
- Andrade RP. Clinical comparison of a triphasic gestodene preparation and a monophasic desogestrel preparation. Gynecological Endocrinology 1993;7 Suppl:33‐41. [Google Scholar]
Bruni 2000 {published data only}
- Bruni V, Croxatto H, Cruz J, Dhont M, Durlot F, Fernandes MT, et al. A comparison of cycle control and effect on well‐being of monophasic gestodene‐, triphasic gestodene‐ and monophasic desogestrel‐containing oral contraceptives. Gynecological Endocrinology 2000;14:90‐8. [DOI] [PubMed] [Google Scholar]
- Lacante P. A comparison of cycle control and effect on well‐being of monophasic gestodene‐, triphasic gestodene‐ and monophasic desogestrel‐containing oral contraceptives. Gynecological Endocrinology 1996;10 Suppl 2:33‐41. [DOI] [PubMed] [Google Scholar]
Carlborg 1983 {published and unpublished data}
- Carlborg L. Acceptability of low‐dose oral contraceptives: results of a randomized Swedish multicenter study comparing a triphasic (Trionetta) and a fixed‐dose combination (Neovletta). Benefits and risk of hormonal contraception: Proceedings of an International Symposium; 1982 March 19; Amsterdam. Boston (MA): MTP Press, 1982:78‐92.
- Carlborg L. Comparison of contraceptive acceptability of levonorgestrel and ethinyl oestradiol administered in one three‐phasic (Trionetta) and one monophasic (Neovletta) version. Contraception 1983;27:439‐52. [DOI] [PubMed] [Google Scholar]
Chavez 1999 {published and unpublished data}
- Chavez A, DelConte A. A comparison of cycle control with monophasic levonorgestrel/ethinylestradiol 100 micrograms/20 micrograms versus triphasic norethindrone/ethinylestradiol 500‐750‐1000 micrograms/35 micrograms: a multicenter, randomized, open‐label study. European Journal of Contraception and Reproductive Health Care 1999;4:75‐83. [DOI] [PubMed] [Google Scholar]
Chen 1987 {published data only}
- Chen JK. A comparative clinical study of the effects of three types of low dose estrogen/progestogen oral contraceptives. Reproduction and Contraception 1987;7:11‐6. [PubMed] [Google Scholar]
- Chen JK. A comparative clinical study of the effects of three types of low‐dose estrogen/progestogen oral contraceptives. Shengzhi Yu Biyun 1987;7:11‐6. [PubMed] [Google Scholar]
Dieben 1984 {published and unpublished data}
- Cullberg G. A comparative multicentre study on a triphasic, and a fixed low‐dose oral contraceptive combination. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1983;116:97. [Google Scholar]
- Cullberg G, Samsioe G, Andersen RF, Bredesgaard P, Andersen NB, Ernerot H, et al. Two oral contraceptives, efficacy, serum proteins, and lipid metabolism. A comparative multicentre study on a triphasic and a fixed dose combination. Contraception 1982;26:229‐43. [DOI] [PubMed] [Google Scholar]
- Dieben T. A comparative study of the clinical efficacy of Marvelon and a triphasic combination. Organorama 1984;21:16‐8. [Google Scholar]
- Mattsson LA, Cullberg G. Clinical and metabolic effects of Marvelon: Scandinavian experience. British Journal of Family Planning 1984;10:43‐8. [Google Scholar]
Dunson 1993 {published and unpublished data}
- Dunson TR, McLaurin VL, Aguayo EL, Silva P, Calventi V, Gerais AS, et al. A multicenter comparative trial of triphasic and monophasic, low dose combined oral contraceptives. Contraception 1993;47:515‐25. [DOI] [PubMed] [Google Scholar]
Engebretsen 1987 {published data only}
- Engebretsen T, Thorsen E, Smith CC, Bull‐Njaa T, Christensen A. Triphasic versus monophasic p‐pill. A comparative multicenter study [Trefasisk versus monofasisk p‐pille: en sammenlignende multisenterstudie]. Tidsskrift for den Norske Laegeforening 1987;107:941‐3. [PubMed] [Google Scholar]
Hampton 2001 {published and unpublished data}
- Hampton RM, Fisher AC, Pagano S, LaGuardia KD. Scheduled and unscheduled bleeding patterns with two combined hormonal contraceptives: application of new recommendations for standardization. Fertility and Sterility 2009;92(2):434‐40. [DOI] [PubMed] [Google Scholar]
- Hampton RM, Short M, Bieber E, Bouchard C, Ayotte N, et al. Comparison of a novel norgestimate/ethinyl estradiol oral contraceptive (Ortho Tri‐Cyclen Lo) with the oral contraceptive Loestrin Fe 1/20. Contraception 2001;63:289‐95. [DOI] [PubMed] [Google Scholar]
- Hampton RM, Zhang HF, Barnowski C, Wan GJ. Bleeding patterns with monophasic and triphasic low‐dose ethinyl estradiol combined oral contraceptives. Contraception 2008;77:415‐9. [DOI] [PubMed] [Google Scholar]
Ismail 1991 {published and unpublished data}
- Ismail MTM. A randomised comparative study of Triquilar versus Marvelon: the Malaysian experience. Malaysian Journal of Reproductive Health 1991;9:9‐17. [PubMed] [Google Scholar]
Kashanian 2010 {published data only}
- Kashanian M, Shahpourian F, Zare O. A comparison between monophasic levonorgestrel‐ethinyl estradiol 150/30 and triphasic levonorgestrel‐ethinyl estradiol 50‐75‐125/30‐40‐30 contraceptive pills for side effects and patient satisfaction: a study in Iran. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2010/02/27 2010; Vol. 150, issue 1:47‐51. [DOI] [PubMed]
Kaunitz 2009 {published data only}
Lachnit‐Fixson 1984 {published data only}
- Lachnit‐Fixson U. Progress in oral contraception. Advantages of a levonorgestrel‐containing 3‐stage preparation over low‐dose levonorgestrel and desogestrel containing monophasic combination preparations [Fortschritte in der oralen kontrazeption: vorteille eines levonorgestrel‐haltigen dreistufenpraparates gegenuber niedrigdosierten levonorgestrel‐ und desogestrel‐haltigen monophasischen kombinationspraparaten]. Fortschritte der Medizin 1984;102:825‐30. [PubMed] [Google Scholar]
- Lachnit‐Fixson U, Aydinlik S, Lehnert J. Clinical comparison between a monophasic preparation and a triphasic preparation. In: Rolland R, Harrison RF, Bonnar J, Thompson W editor(s). Advances in fertility control and the treatment of sterility. Proceedings of a special symposium held at the XIth World Congress on Fertility and Sterility; 1983 June; Dublin. Lancaster: MTP Press, 1984:71‐9. [Google Scholar]
Percival‐Smith 1990 {published data only}
- Percival‐Smith RK, Yuzpe AA, Desrosiers JA, Rioux JE, Guilbert E. Cycle control on low‐dose oral contraceptives: a comparative trial. Contraception 1990;42:253‐62. [DOI] [PubMed] [Google Scholar]
Ramos 1989 {published data only}
- Ramos R, Apelo R, Osteria T, Vilar E. A comparative analysis of three different dose combinations of oral contraceptives. Contraception 1989;39:165‐77. [DOI] [PubMed] [Google Scholar]
Reisman 1999 {published and unpublished data}
- Reisman H, Martin D, Gast MJ. A multicenter randomized comparison of cycle control and laboratory findings with oral contraceptive agents containing 100 μg levonorgestrel with 20 μg ethinyl estradiol or triphasic norethindrone with ethinyl estradiol. American Journal of Obstetrics and Gynecology 1999;181:45‐52. [DOI] [PubMed] [Google Scholar]
Reiter 1990 {published and unpublished data}
- Reiter SL, Baer LJ. Initial selection of oral contraceptives. Journal of Reproductive Medicine 1990;35:547‐8. [PubMed] [Google Scholar]
Rosenberg 1999 {published and unpublished data}
- Rosenberg MJ, Meyers A, Roy V. Efficacy, cycle control, and side effects of low‐ and lower‐dose oral contraceptives: a randomized trial of 20 μg and 35 μg estrogen preperations. Contraception 1999;60:321‐9. [DOI] [PubMed] [Google Scholar]
Rowan 1999 {published and unpublished data}
- Rowan JP. "Estrophasic" dosing: A new concept in oral contraceptive therapy. American Journal of Obstetrics and Gynecology 1999;180:302‐6. [DOI] [PubMed] [Google Scholar]
Saxena 1992 {published and unpublished data}
- Datey S, Gaur LN, Saxena BN. Vaginal bleeding patterns of women using different contraceptive methods (implants, injectables, IUDs, oral pills)‐an Indian experience. Contraception 1995;51:155‐65. [DOI] [PubMed] [Google Scholar]
- Saxena B. Randomised clinical trial with Triquilar‐ED and low‐dose combination pill. Journal of Obstetrics and Gynaecology of India 1992;42:71‐7. [Google Scholar]
Sulak 1999 {published data only}
- Lippman J, Siu C, Godwin A, Massaro J. A clinical comparison of two oral contraceptives: triphasic norgestimate/35 μg ethinylestradiol versus monophasic norethindrone acetate/20 μg ethinylestradiol. Obstetrics & Gynecology 1999;93 Suppl:37. [Google Scholar]
- Sulak P, Lippman J, Siu C, Massaro J, Godwin A. Clinical comparison of triphasic norgestimate/35 μg ethinyl estradiol and monophasic norethindrone acetate/20 μg ethinyl estradiol: cycle control, lipid effects, and user satisfaction. Contraception 1999;59:161‐6. [DOI] [PubMed] [Google Scholar]
Zador 1979 {published data only}
- Lachnit‐Fixson U. Clinical investigation with a new triphasic oral contraceptive. In: Greenblatt RB editor(s). The development of a new triphasic oral contraceptive. Proceedings of a Special Symposium held at the 10th World Congress on Fertility and Sterility; 1980 July; Madrid. Lancaster: MTP Press Limited, 1980:99‐107. [Google Scholar]
- Lachnit‐Fixson U. Progress in oral contraception. Advantages of a levonorgestrel‐containing 3‐stage preparation over low‐dose levonorgestrel and desogestrel containing monophasic combination preparations [Fortschritte in der oralen kontrazeption: vorteille eines levonorgestrel‐haltigen dreistufenpraparates gegenuber niedrigdosierten levonorgestrel‐ und desogestrel‐haltigen monophasischen kombinationspraparaten]. Fortschritte der Medizin 1984;102:825‐30. [PubMed] [Google Scholar]
- Lachnit‐Fixson U. The first three‐stage preparation for hormonal contraception. Clinical results [Erstes dreistufenpraeparat zur hormonalen konzeptionsverhutung: klinische ergebnisse]. Munchener Medizinische Wochenschrift 1979;121:1421‐6. [PubMed] [Google Scholar]
- Zador G. Clinical performance of a triphasic administration of ethinyl estradiol and levonorgestrel in comparison with the 30 + 150 μg fixed‐dose regimen. In: Haspels AA, Rolland R editor(s). Benefits and risk of hormonal contraception. Proceedings of an international symposium; 1982 March 19; Amsterdam. Boston (Massachusetts): MTP Press, 1982:43‐55. [Google Scholar]
- Zador G. Fertility regulation using "triphasic" administration of ethinyl estradiol and levonorgestrel in comparison with the 30 plus 150 μg fixed dose regime. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1979;88:43‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bancroft 1987 {published data only}
- Bancroft J, Sanders D, Warner P, Loudon N. The effects of oral contraceptives on mood and sexuality: A comparison of triphasic and combined preparations. Journal of Psychosomatic Obstetrics and Gynaecology 1987;7:1‐8. [Google Scholar]
Christie 1989 {published data only}
- Christie T. A clinical overview of a new triphasic contraceptive containing gestodene. International Journal of Fertility 1989;34 Suppl:40‐9. [PubMed] [Google Scholar]
Dik 1984 {published data only}
- Dik M, Eckert H, Hones S, Schindler AE. Comparison of a 2‐phase preparation (Oviol 22) with a low‐dose 1‐phase preparation (Ovoresta M) [Vergleich eines zweiphasenpraparates (Oviol 22) mit einem niedrig dosierten Einphasenpraparat (Ovoresta M)]. Geburtshilfe und Frauenheilkunde 1984;44:808‐12. [DOI] [PubMed] [Google Scholar]
Dubnitskaia 1988 {published data only}
- Dubnitskaia LV. Acceptability of hormonal contraceptives with a low steroid content. Akusherstvo I Ginekologiia 1988;8:47‐50. [PubMed] [Google Scholar]
Grace 1994 {published data only}
- Grace E, Emans SJ, Havens KK, Merola JL, Woods ER. Contraceptive compliance with a triphasic and a monophasic norethindrone‐containing oral contraceptive pill in a private adolescent practice. Adolescent and Pediatric Gynecology 1994;7:29‐33. [Google Scholar]
Kuhl 1985 {published data only}
- Kuhl H, Gahn G, Romberg G, Marz W, Taubert H‐D. A randomized cross‐over comparison of two low‐dose oral contraceptives upon hormonal and metabolic parameters: 1. Effects upon sexual hormone levels. Contraception 1985;31:583‐93. [DOI] [PubMed] [Google Scholar]
Matsumoto 1988 {published data only}
- Matsumoto S, Sato T, Matsuyama E, Tamada T, Wagatsuma T, Honda H. Results of a clinical study with low‐dose oral contraceptives: investigation with triphasic and monophasic preparations containing levonorgestrel and ethinylestradiol. Current Therapeutic Research, Clinical and Experimental 1988;44:165‐77. [Google Scholar]
Otolorin 1989 {published data only}
- Otolorin EO. Clinical experience with a triphasic oral contraceptive containing ethinyloestradiol and levonorgestrel in Nigerian women. West African Journal of Medicine 1989;8:116‐21. [PubMed] [Google Scholar]
Perrone 1987 {published data only}
- Perrone G, Calzolari E, Mancone M, Masci A, Steffe M, Tesseri E. Oral contraceptives and their minor side effects: comparison of three low‐dose estroprogestinic combinations [Contraccettivi orali ed effetti collaterali minori: confronto fra tre estroprogestinici a basso dosaggio]. Patologia e Clinica Ostetrica e Ginecologica 1987;15:6‐11. [PubMed] [Google Scholar]
Rubio‐Lotvin 1992 {published data only}
- Rubio‐Lotvin B, Ruiz‐Moreno JA, Gonzalez‐Ansorena R. Desogestrel‐ethinylestradiol, an oral monophasic contraceptive. Clinical and lipid metabolic effects: a 5 year experience. Advances in Contraceptive Delivery Systems 1992;8:75‐88. [PubMed] [Google Scholar]
References to ongoing studies
Bayer 2011 {unpublished data only}
- Bayer Schering Pharma AG. Cycle Control and Safety of E2‐DRSP. Available from: http://clinicaltrials.gov/ct2/home (accessed 04 May 2011). [NCT00653614]
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290:921‐8. [DOI] [PubMed] [Google Scholar]
Belsey 1986
- Belsey EM, Machin D, d' Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception 1986;34:253‐61. [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 04 May 2011).
DerSimonian 1982
- DerSimonian R, Charette LJ, McPeek B, Mosteller F. Reporting on methods in clinical trials. New England Journal of Medicine 1982;306:1332‐7. [DOI] [PubMed] [Google Scholar]
Fay 1982
- Fay RA. Failure with the new triphasic oral contraceptive Logynon. British Medical Journal 1982;284:17‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallo 2008
- Gallo MF, Nanda K, Grimes DA, Lopez LM, Schulz KF. 20 μg versus >20 μg estrogen combined oral contraceptives for contraception. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003989] [DOI] [Google Scholar]
Graham 1982
- Graham H. Failure with the new triphasic oral contraceptive Logynon. British Medical Journal 1982;284:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillebaud 1993
- Guillebaud J. Contraception: your questions answered. 2nd Edition. Edinburgh: Churchill Livingstone, 1993:188‐90. [Google Scholar]
Hale 1987
- Hale RW. Phasic approach to oral contraceptives. American Journal of Obstetrics and Gynecology 1987;157:1052‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library 2005, Issue 3. Chichester, UK: John Wiley & Sons, Ltd. Chicester, UK: John Wiley & Sons, Ltd.
IPPF 2004
- Terki F, Malhotra U. In: Powlson M editor(s). Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. Third Edition. London (UK): International Planned Parenthood Federation, 2004. [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282:1054‐60. [DOI] [PubMed] [Google Scholar]
Ketting 1988
- Ketting, E. The relative reliability of oral contraceptives: findings of an epidemiological study. Contraception 1988;37:343‐8. [DOI] [PubMed] [Google Scholar]
Kovacs 1989
- Kovacs, GT, Riddoch G, Duncombe P, Welberry L, Chick P, et al. Inadvertent pregnancies in oral contraceptive users. Medical Journal of Australia 1989;150:549‐51. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maitra 2004
- Maitra N, Kulier R, Bloemenkamp KWM, Helmerhorst FM, Gulmezoglu AM. Progestogens in combined oral contraceptives for contraception. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004861] [DOI] [PubMed] [Google Scholar]
Mishell 2007
- Mishell DR, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception 2007;75(1):11‐5. [DOI] [PubMed] [Google Scholar]
Moreau 2007
- Moreau C, Trussell J, Gilbert F, Bajos N, Bouyer J. Oral contraceptive tolerance. Does the type of pill matter?. Obstetrics & Gynecology 2007;109:1277‐85. [DOI] [PubMed] [Google Scholar]
Rosenberg 1992
- Rosenberg MJ, Long SC. Oral contraceptives and cycle control: a critical review of the literature. Advances in Contraception 1992;8 Suppl:35‐45. [DOI] [PubMed] [Google Scholar]
Rosenberg 1995
- Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception 1995;51:283‐8. [DOI] [PubMed] [Google Scholar]
Rosenberg 1998
- Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. American Journal of Obstetrics and Gynecology 1998;179:577‐82. [DOI] [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781‐5. [DOI] [PubMed] [Google Scholar]
Schulz 2002b
- Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Annals of Internal Medicine 2002;136:254‐9. [DOI] [PubMed] [Google Scholar]
Schulz 2002c
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359:515‐9. [DOI] [PubMed] [Google Scholar]
Schulz 2002d
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2002e
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696‐700. [DOI] [PubMed] [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Trussell 1991
- Trussell J. Methodological pitfalls in the analysis of contraceptive failure. Statistics in Medicine 1991;10:201‐20. [DOI] [PubMed] [Google Scholar]
Upton 1983
- Upton GV. The phasic approach to oral contraception:the triphasic concept and its clinical application. International Journal of Fertility 1983;28:121‐40. [PubMed] [Google Scholar]
van Hylckama 2009
- Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case‐control study. BMJ 2009;339:b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]


