Abstract
Background
Anterior cervical discectomy and fusion (ACDF) is an effective treatment for cervical spondylosis. A limitation of ACDF is the risk of adjacent-segment degeneration (ASD), owing to arthrodesis of a motion segment. Cervical disc arthroplasty (CDA) has hence garnered significant attention; yet, compelling evidence of reduction in ASD requiring surgery is lacking. This systematic review and meta-analysis sought to compare long-term longitudinal adjacent-level operation rates with CDA versus ACDF.
Methods
An electronic literature search was conducted. Eligible studies were multi-center randomized controlled trials (RCTs) comparing CDA with ACDF for one- or two-level symptomatic cervical spondylosis. The primary outcome was adjacent-level operation. Index-level reoperation was a secondary outcome. Outcomes were evaluated at 1-year intervals from the index operation to last reported follow-up by random-effects meta-analyses.
Results
Eleven RCTs met criteria. For one-level spondylosis, there was no difference in the rate of adjacent-level operation between CDA (2.3%) and ACDF (3.6%) at 2 years. However, a large difference favoring CDA became evident at 5 years and persisted at 7 years (4.3% vs. 10.8%, P<0.001). Significantly fewer patients who underwent CDA required index-level reoperation at all time points out to 7 years (5.2% vs. 12.7%, P<0.001). Similar to one-level operations, there was no significant difference in adjacent-level operations with two-level CDA (1.7%) versus two-level ACDF (3.4%) at 2 years. At 7 years, a significant difference favoring CDA became apparent (5.1% vs. 10.0%, P=0.014). Two-level CDA resulted in fewer index-level reoperations out to 7 years (4.2% vs. 13.5%, P<0.001).
Conclusions
In this meta-analysis, the short-term rate of adjacent-level operation was similar with CDA or ACDF. However, around 5 years, a statistically significant divergence emerged, where the rate of adjacent-level surgery rose steeply for ACDF. Index-level reoperations were less frequent with CDA in both the short- and long-term. These data indicate CDA may have a superior longevity to ACDF with regard to need for subsequent adjacent-level operation.
Keywords: Anterior cervical discectomy and fusion (ACDF), arthrodesis, arthroplasty, artificial disc, cervical disc arthroplasty (CDA), cervical spine, cervical spondylosis, disc prosthesis, disc replacement, fusion, myelopathy, radiculopathy
Introduction
Anterior cervical discectomy and fusion (ACDF) is a safe and effective treatment for cervical spondylosis causing radiculopathy or myelopathy (1,2). However, the elimination of segmental motion with arthrodesis may increase the risk of adjacent-segment degeneration (ASD) (3,4). Cervical disc arthroplasty (CDA) has hence gained momentum over the last decade in effort to overcome this critical limitation of ACDF (5). CDA achieves the goals of decompression of the neural elements and maintenance of disc height and segmental lordosis, while also preserving physiologic segmental motion.
As a motion-sparing technology, CDA may mitigate the development of symptomatic ASD and need for subsequent reoperation. Nonetheless, clinical studies of CDA compared with ACDF have reported conflicting results regarding the effect on need for secondary adjacent-level surgery, which may be explained by a few reasons (6-8). First, these studies were not specifically powered to evaluate this outcome. Second, the development of symptomatic ASD is most relevant at long-term follow-up, whereas the primary endpoint of the majority of trials was at 2 years. To that end, leveraging statistical power by pooling data from randomized controlled trials (RCTs), we sought to perform a systematic review and meta-analysis of longitudinal reoperation rates out to long-term (≥4-year) follow-up with CDA, as compared with ACDF, for symptomatic one- or two-level cervical spondylosis. Our hypothesis was that a divergence in rates of reoperation may begin to emerge at approximately 4 to 5 years, owing to increased rates of symptomatic ASD with ACDF over a longer time horizon.
Methods
This systematic review and meta-analysis of RCTs was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (9) and the Cochrane Handbook for Systematic Reviews of Interventions (10).
Search strategy
An electronic search of PubMed, EMBASE, and Web of Science was conducted on August 21, 2019 for randomized trials of CDA versus ACDF for cervical spondylosis. The search terms “anterior”, “arthrodesis”, “arthroplasty”, “artificial”, “cervical”, “disc”, “fusion”, “prosthesis”, and “replacement” were used in relevant combinations (Supplementary). References of relevant resources and review articles were manually screened to supplement the search.
Eligibility criteria
Two authors (JH Badhiwala and CD Witiw) evaluated the search results for eligibility. Multi-center RCTs reporting adjacent- or index-level reoperations with CDA, as compared with ACDF, in adult patients with one- or two-level symptomatic (i.e., radiculopathy and/or myelopathy) cervical spondylosis were selected for inclusion. To be eligible, studies must have been published as English-language full-text reports in a peer-reviewed journal; conference abstracts were excluded. Detailed eligibility criteria are summarized in Table 1. Figure 1 provides a flowchart of study eligibility.
Table 1. Detailed inclusion and exclusion criteria.
| Eligibility | Population | Intervention | Control | Outcome | Study design |
|---|---|---|---|---|---|
| Inclusion | Adult patients (≥16 years) | CDA (any device) | ACDF | Index- or adjacent-level reoperations at fixed follow-up time periods (e.g., 2 years) | Multi-center |
| One- or two-level cervical spondylosis | Allograft or cage | Minimum follow-up of 1 year | Randomized controlled trial | ||
| Symptomatic with refractory radiculopathy or progressive myelopathy | Anterior plate | For mixed populations, outcomes reported separately for one- and two-level subgroups | English-language publication as a full-text report | ||
| Exclusion | Cervical spondylosis affecting more than two levels | Anterior cervical discectomy without fusion | Variable follow-up time periods (e.g., mean follow-up, 3 years; range, 1–5 years) | Single-center | |
| Cervical spondylosis at non-contiguous levels | Lack of anterior plate fixation (e.g., stand-alone cage) | Failure to distinguish between index- and adjacent-level reoperations | Non-randomized | ||
| Autologous iliac crest bone graft | For mixed populations, lack of stratification of outcomes by one- or two-level cervical spondylosis | Use of historical controls | |||
| Conference abstract | |||||
| Non-English language publication |
CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 1.
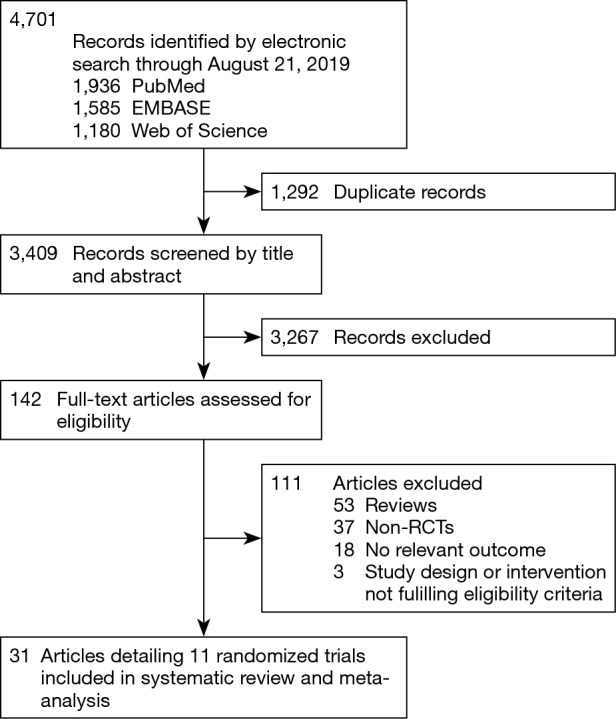
Flowchart of study eligibility.
Data extraction
Data were extracted from the trials’ primary texts and supplementary appendices. For United States Food and Drug Administration (FDA) Investigational Device Exemption (IDE) trials, the corresponding FDA Summary of Safety and Effectiveness Data (SSED) documents were also evaluated for pertinent data. Data fields abstracted included: device name, authors, year of publication, number of centers, enrollment period, number of patients in each treatment arm, eligibility criteria, intervention, control, baseline patient characteristics, and outcomes. The primary outcome was adjacent-level reoperation. Index-level reoperation was evaluated as a secondary outcome. Outcomes were evaluated at 1-year intervals from the index operation to last reported follow-up.
Quality assessment
The methodological quality of included studies was evaluated using the Cochrane tool for assessing risk of bias in randomized trials, version 2 (RoB 2) (11). The RoB 2 provides an evaluation of the risk of bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Scores in these domains are distilled into a global assessment of the overall risk of bias in a given RCT: (I) ‘low risk of bias’; (II) ‘some concerns’; or (III) ‘high risk of bias’.
Statistical analysis
For the purposes of reporting and analysis, multiple publications that reported the results of the same randomized trial at differing time points were grouped to permit longitudinal analysis of outcomes.
Descriptive statistics were by mean and standard deviation (SD) for continuous variables and count and percentage for categorical variables.
Outcomes were analyzed separately for one- and two-level operations; that is, randomized trials of CDA versus ACDF for one-level cervical spondylosis were pooled together in one analysis, whereas those for two-level cervical spondylosis were pooled together in a second separate analysis. Within each treatment arm, aggregate rates of adjacent- and index-level reoperation across trials at each follow-up time point were computed by random-effects meta-analyses based on the number of events and sample size of each trial. These were subsequently plotted over time for CDA and ACDF groups. For comparisons between CDA and ACDF, effect sizes for each trial were summarized by odds ratios (ORs) and corresponding 95% confidence intervals (CIs), which were then pooled by random-effects meta-analyses. Meta-analyses were only performed when at least two studies reported outcomes for a given follow-up time point.
For all meta-analyses, outcomes were pooled by the DerSimonian and Laird method, with weights calculated by the inverse-variance method. Heterogeneity across trials was quantified by the I2, with I2 values exceeding 25%, 50%, and 75% indicating a low, moderate, and high degree of heterogeneity, respectively.
Outcomes were analyzed by intention-to-treat, as reported by each trial (i.e., all randomized patients were included and analyzed according to the treatment group to which they were randomly assigned). All statistical analyses were performed using Comprehensive Meta Analysis version 3.3 (Biostat Inc) with a priori-specified significance level of P=0.05 (two-tailed).
Results
The search yielded 3,409 unique citations, of which 31 reports detailing 11 randomized trials for eight different devices—Bryan (Medtronic Sofamor Danek, Memphis, TN, USA); Kineflex|C (SpinalMotion Inc., Mountain View, CA, USA); Mobi-C (LDR Medical, Troyes, France); PCM (NuVasive Inc., San Diego, CA, USA); Prestige LP (Medtronic Sofamor Danek); Prestige ST (Medtronic Sofamor Danek); ProDisc-C (Synthes Spine, West Chester, PA, USA); and Secure-C (Globus Medical, Audubon, PA, USA)—met criteria (6-8,12-16) for inclusion (17-39). Of the 11 eligible studies, nine compared CDA with ACDF for one-level cervical spondylosis, whereas two examined two-level pathology. Of the nine one-level studies, seven were United States FDA IDE trials and two were multi-center RCTs from China. Both two-level studies were United States FDA IDE trials. A descriptive summary of eligible trials is provided in Table 2. Six trials had a ‘low risk of bias’ (12,17,22,27,30,33), five had ‘some concerns’ (7,15,21,25,38), and none were associated with a ‘high risk of bias’, as assessed by the Cochrane RoB 2 tool (Table 3). The primary source of potential bias in those trials deemed to have ‘some concerns’ generally related to lack of adequate description of the randomization process and allocation concealment. In one trial, there was additionally concern regarding deviation from the intended intervention, as 11 patients withdrew from the study because they desired the treatment to which they were not assigned (15).
Table 2. Descriptive summary of eligible randomized trials of CDA versus ACDF for one- or two-level symptomatic cervical spondylosis.
| Author (year) | Trial | Enrollment | Population | Intervention | Control | Outcomes | Last follow-up | Sponsor/funding |
|---|---|---|---|---|---|---|---|---|
| One-level | ||||||||
| Heller et al. [2009] (12); Sasso et al. [2011] (13); Ghobrial et al. [2019] (14); Lavelle et al. [2019] (8) | Bryan, US FDA IDE | May 2002–October 2004 | N=463 | N=242 | N=221 | NDI | 10 years | Industry: Medtronic |
| 30 centers | CDA (Bryan) | ACDF with allograft and anterior plate | SF-36 | |||||
| One-level spondylosis | Neck/arm pain VAS | |||||||
| Radiculopathy or myelopathy | Neurological exam | |||||||
| Refractory to 6 weeks of non-operative therapy, except myelopathy requiring immediate treatment | Work status | |||||||
| Mean age: 44.4 yrs (CDA); 44.7 yrs (ACDF) | Adverse events | |||||||
| Angular motion | ||||||||
| Zhang et al. [2012] (15) | Bryan, China | May 2004–May 2006 | N=109 | N=56 | N=53 | NDI | 2 years | Non-industry: Chinese Medical Doctor Association |
| 3 centers | CDA (Bryan) | ACDF with allograft and anterior plate | Neck/arm pain VAS | |||||
| One-level spondylosis, C3-7 | ROM | |||||||
| Radiculopathy or myelopathy | Adverse events | |||||||
| Refractory to 6 weeks of non-operative therapy | ||||||||
| Mean age: 44.8 yrs (CDA); 45.6 yrs (ACDF) | ||||||||
| Coric et al. [2011] (7); Coric et al. [2018] (16) | Kineflex|C, US FDA IDE | July 2005–January 2010 | N=269 | N=136 | N=133 | NDI | 5 years | Industry: SpinalMotion |
| 21 centers | CDA (Kineflex|C) | ACDF with allograft and anterior plate | Neck pain VAS | |||||
| One-level spondylosis | Patient satisfaction | |||||||
| Radiculopathy | Neurological exam | |||||||
| Mean age: 43.7 yrs (CDA); 43.9 yrs (ACDF) | Adverse events | |||||||
| Radiographic ASD | ||||||||
| Angular motion | ||||||||
| Activity level | ||||||||
| Hisey et al. [2014] (17); Hisey et al. [2015] (18); Hisey et al. [2016] (19); Jackson et al. [2016] (6); Radcliff et al. [2017] (20) | Mobi-C, US FDA IDE | April 2006–March 2008 | N=245 | N=164 | N=81 | NDI | 7 years | Industry: LDR Spine |
| 23 centers | CDA (Mobi-C) | ACDF with allograft and anterior plate | SF-12 | |||||
| One-level spondylosis, C3-7 | Angular motion | |||||||
| Radiculopathy or myeloradiculopathy | Neurological exam | |||||||
| Refractory to non-operative therapy for 6 weeks or progressive symptoms | Adverse events | |||||||
| Mean age: 43.3 yrs (CDA); 44.0 yrs (ACDF) | ||||||||
| Zhang et al. [2014] (21) | Mobi-C, China | February 2008–November 2009 | N=111 | N=55 | N=56 | JOA | 4 years | None |
| 11 centers | CDA (Mobi-C) | ACDF with cage and anterior plate | NDI | |||||
| One-level spondylosis, C3-7 | Neck pain VAS | |||||||
| Symptomatic | ROM | |||||||
| Refractory to 3 months of non-operative therapy | Adverse events | |||||||
| Mean age: 44.8 yrs (CDA); 46.7 yrs (ACDF) | ||||||||
| Phillips et al. [2013] (25); Phillips et al. [2015] (26) | PCM, US FDA IDE | January 2005-December 2007 | N=403 | N=218 | N=185 | NDI | 7 years | Industry: NuVasive |
| One-level spondylosis, C3-4 to C7-T1 | CDA (PCM) | ACDF with allograft and anterior plate | SF-36 | |||||
| Radiculopathy or myelopathy | Neck/arm pain VAS | |||||||
| Refractory to non-operative therapy | Neurological status | |||||||
| May have undergone prior nonadjacent or adjacent single-level fusion | Adverse events | |||||||
| Angular motion | ||||||||
| Mummaneni et al. [2007] (30); Burkus et al. [2010] (31); Burkus et al. [2014] (32) | Prestige ST, US FDA IDE | October 2002–August 2004 | N=541 | N=276 | N=265 | NDI | 7 years | Industry: Medtronic |
| 32 centers | CDA (Prestige ST) | ACDF with allograft and anterior plate | Neck/arm pain VAS | |||||
| One-level spondylosis, C3-7 | SF-36 | |||||||
| Radiculopathy or myelopathy | Neurological exam | |||||||
| Refractory to non-operative therapy (≥6 weeks) or progressive neurological worsening | Work status | |||||||
| ROM | ||||||||
| Adverse events | ||||||||
| Secondary surgery | ||||||||
| Murrey et al. [2009] (33); Delamarter et al. [2010] (34); Zigler et al. [2013] (36); Delamarter & Zigler [2013] (35); Janssen et al. [2015] (37) | ProDisc-C, US FDA IDE | August 2003–October 2004 | N=209 | N=103 | N=106 | NDI | 7 years | Industry: Synthes |
| 13 centers | CDA (ProDisc-C) | ACDF with allograft and anterior plate | Neck/arm pain VAS | |||||
| One-level spondylosis, C3-7 | Patient satisfaction | |||||||
| Radiculopathy | Neurological exam | |||||||
| Refractory to non-operative therapy for at least 6 weeks | SF-36 | |||||||
| Mean age: 42.1 yrs (CDA); 43.5 yrs (ACDF) | Adverse events | |||||||
| Angular motion | ||||||||
| Vaccaro et al. [2013]; Vaccaro et al. [2018] (38,39) | Secure-C, US FDA IDE | July 2005–April 2008 | N=291 | N=151 | N=140 | NDI | 7 years | Industry: Globus Medical |
| 18 centers | CDA (Secure-C) | ACDF with allograft and anterior plate | Neck/arm pain VAS | |||||
| One-level spondylosis, C3-7 | Neurological status | |||||||
| Radiculopathy or myelopathy | SF-36 | |||||||
| Refractory to non-operative therapy (≥6 weeks) | Range of motion | |||||||
| Mean age: 43.4 yrs (CDA); 44.4 yrs (ACDF) | ||||||||
| Two-level | ||||||||
| Davis et al. [2013] (22); Davis et al. [2015] (23); Jackson et al. [2016] (6); Radcliff et al. [2016] (24); Radcliff et al. [2017] (20) | Mobi-C, US FDA IDE | April 2006–March 2008 | N=330 | N=225 | N=105 | NDI | 7 years | Industry: LDR Medical |
| 24 centers | CDA (Mobi-C) | ACDF with allograft and anterior plate | SF-12 | |||||
| Cervical spondylosis at two contiguous levels, C3-7 | Angular motion | |||||||
| Radiculopathy or myeloradiculopathy | Neurological exam | |||||||
| Refractory to 6 weeks of non-operative therapy | Adverse events | |||||||
| Mean age: 45.3 yrs (CDA); 46.2 yrs (ACDF) | ||||||||
| Gornet et al. [2017] (27); Lanman et al. [2017] (28); Gornet et al. [2019] (29) | Prestige LP, US FDA IDE | June 2006–November 2007 | N=397 | N=209 | N=188 | NDI | 10 years | Industry: Medtronic |
| 30 centers | CDA (Prestige LP) | ACDF with allograft and anterior plate | Neck/arm pain VAS | |||||
| Cervical spondylosis at two contiguous levels, C3-7 | SF-36 | |||||||
| Refractory to 6 weeks of non-operative therapy | Gait abnormality | |||||||
| Mean age: 47.1 yrs (CDA); 47.3 yrs (ACDF) | ROM | |||||||
| Neurological exam | ||||||||
| Adverse events |
ACDF, anterior cervical discectomy and fusion; CDA, cervical disc arthroplasty; JOA, Japanese Orthopaedic Association score; NDI, Neck Disability Index; ROM, range of motion; SF-12, Short-Form 12; SF-36, Short-Form 36; VAS, visual analog scale.
Table 3. Assessment of the methodological quality (risk of bias) in included studies using the Cochrane RoB 2 tool.
| Trial | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|
| One-level | ||||||
| Bryan, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
| Bryan, China | □ | □ | ○ | ○ | ○ | □ |
| Kineflex|C, US FDA IDE | □ | ○ | ○ | ○ | ○ | □ |
| Mobi-C, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
| Mobi-C, China | □ | ○ | ○ | ○ | ○ | □ |
| PCM, US FDA IDE | □ | ○ | ○ | ○ | ○ | □ |
| Prestige ST, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
| ProDisc-C, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
| Secure-C, US FDA IDE | □ | ○ | ○ | ○ | ○ | □ |
| Two-level | ||||||
| Mobi-C, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
| Prestige LP, US FDA IDE | ○ | ○ | ○ | ○ | ○ | ○ |
○, low risk of bias; □, some concerns; △, high risk of bias.
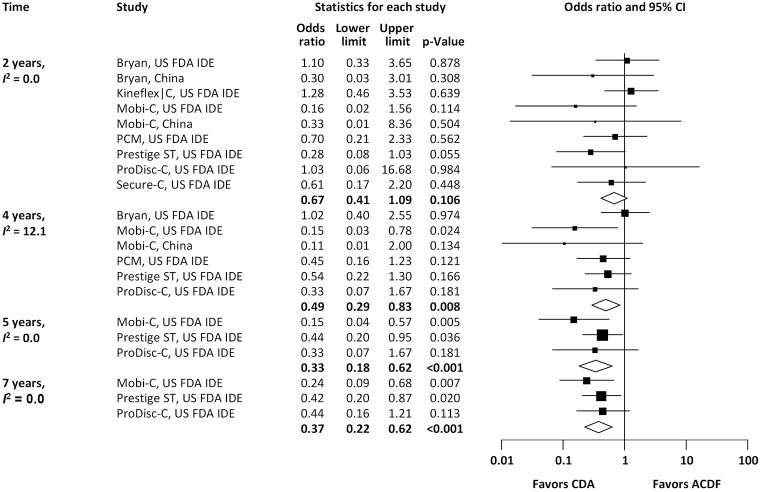
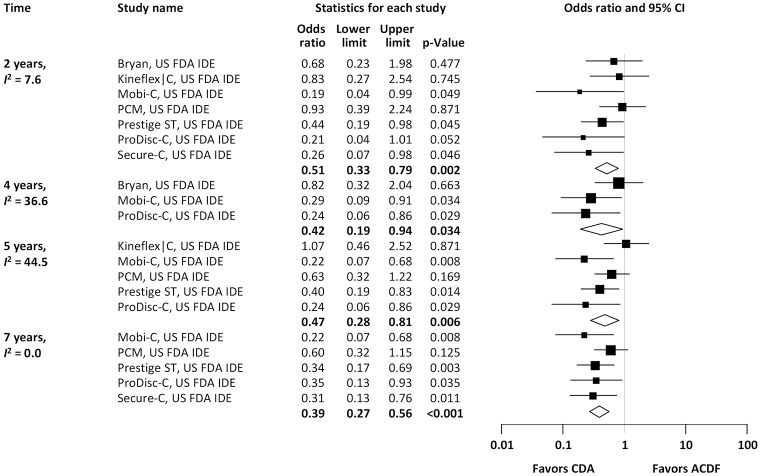
Pooled rates of adjacent-level reoperation for one-level CDA compared with ACDF are summarized in Figure 2; a forest plot of effect sizes appears in Figure 3. There was no difference in the rate of adjacent-level reoperation between CDA (2.3%) and ACDF (3.6%) at 2 years (OR 0.67, 95% CI, 0.41–1.09, P=0.106). However, a very large difference favoring CDA became evident at 5 years. At 7-year follow-up, the rate of adjacent-level reoperation was only 4.3% in the CDA group versus 10.8% in the ACDF cohort (OR 0.37, 95% CI, 0.22–0.62, P<0.001). Rates of index-level reoperation are summarized in Figure 4; a forest plot appears in Figure 5. At 2 years, significantly fewer patients who underwent one-level CDA required index-level reoperation (3.2%), as compared with those treated with one-level ACDF (6.2%) (OR 0.51, 95% CI, 0.33–0.79, P=0.002). At 7 years, index-level reoperations occurred in only 5.2% of CDA patients, compared with 12.7% of the ACDF group (OR 0.39, 95% CI, 0.27–0.56, P<0.001).
Figure 2.
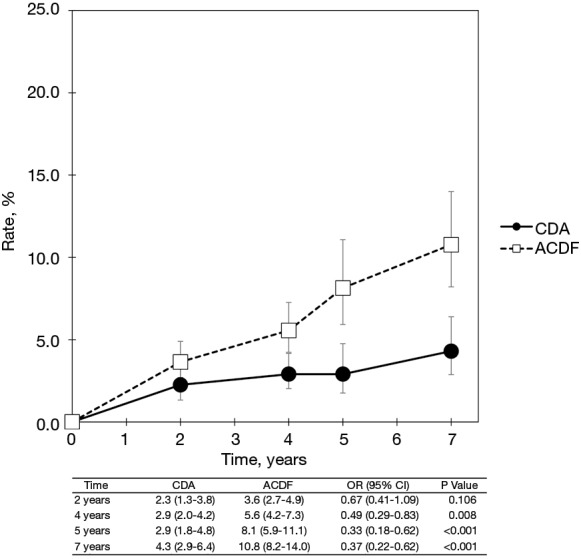
Pooled rates of adjacent-level reoperation for CDA compared with ACDF in patients with one-level symptomatic cervical spondylosis (derived from meta-analysis of nine randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 3.
Forest plot of adjacent-level reoperations for CDA compared with ACDF in patients with one-level symptomatic cervical spondylosis (derived from meta-analysis of nine randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 4.
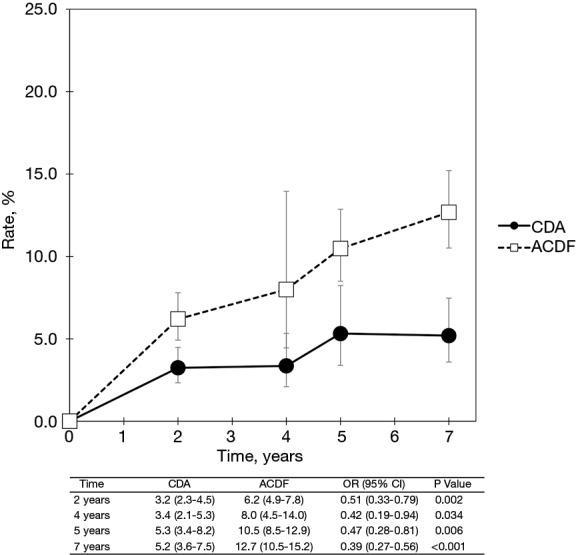
Pooled rates of index-level reoperation for CDA compared with ACDF in patients with one-level symptomatic cervical spondylosis (derived from meta-analysis of nine randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 5.
Pooled rates of index-level reoperation for CDA compared with ACDF in patients with one-level symptomatic cervical spondylosis (derived from meta-analysis of nine randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
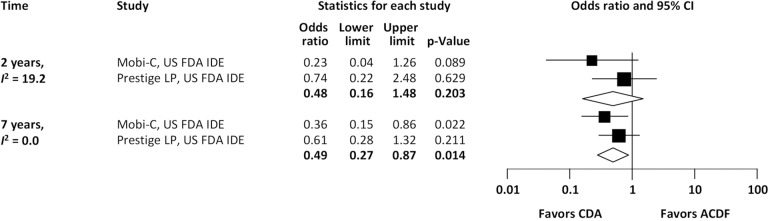
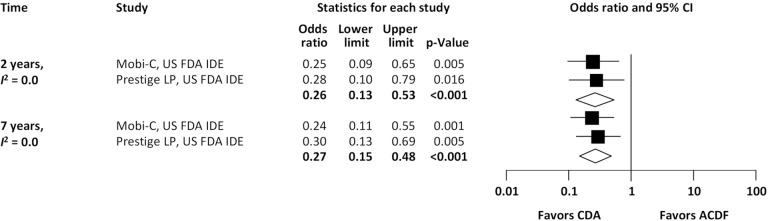
With regard to two-level cervical spondylosis, rates of adjacent-level reoperation are provided in Figure 6; a forest plot appears in Figure 7. Similar to the one-level analysis, there was no significant difference in adjacent-level reoperations with two-level CDA (1.7%) versus two-level ACDF (3.4%) at 2 years. Nonetheless, at 7 years, a large and statistically significant difference favoring CDA became apparent (5.1% vs. 10.0%; OR 0.49, 95% CI, 0.27–0.87, P=0.014). Pooled rates of index-level reoperation are plotted in Figure 8; a forest plot of effect sizes is provided in Figure 9. Again, like the corresponding analysis of one-level studies, CDA resulted in fewer index-level reoperations at 2 years (2.8% vs. 9.3%; OR 0.26, 95% CI, 0.13–0.53, P<0.001) and also 7 years (4.2% vs. 13.5%; OR 0.27, 95% CI, 0.15–0.48, P<0.001).
Figure 6.
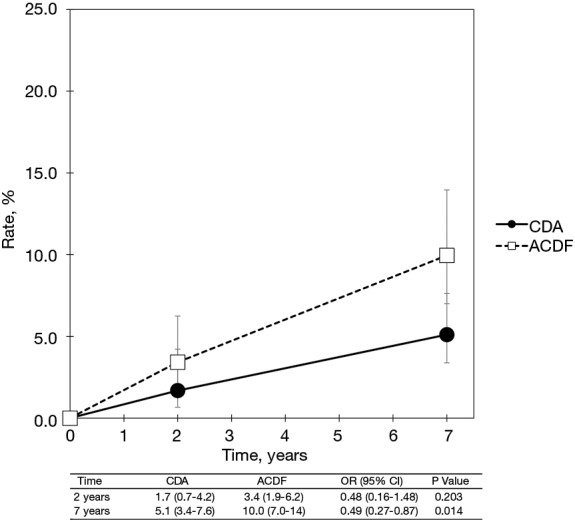
Pooled rates of index-level reoperation for CDA compared with ACDF in patients with one-level symptomatic cervical spondylosis (derived from meta-analysis of nine randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 7.
Forest plot of adjacent-level reoperations for CDA compared with ACDF in patients with two-level symptomatic cervical spondylosis (derived from meta-analysis of two randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 8.
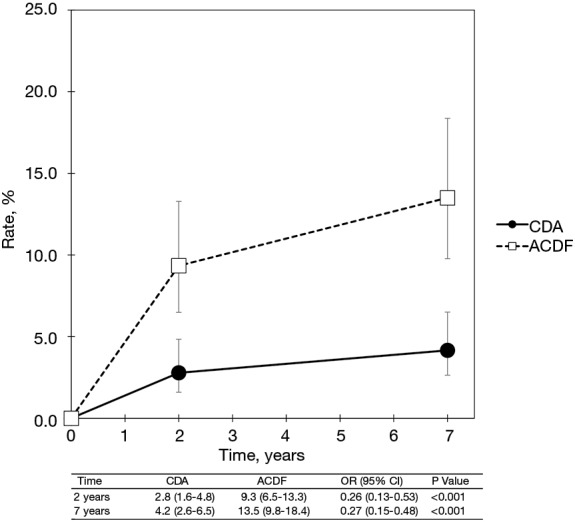
Pooled rates of index-level reoperation for CDA compared with ACDF in patients with two-level symptomatic cervical spondylosis (derived from meta-analysis of two randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Figure 9.
Forest plot of index-level reoperations for CDA compared with ACDF in patients with two-level symptomatic cervical spondylosis (derived from meta-analysis of two randomized trials). CDA, cervical disc arthroplasty; ACDF, anterior cervical discectomy and fusion.
Discussion
This paper calculates and provides longitudinal reoperation rates following CDA, as compared with ACDF, based on a rigorous systematic review and meta-analysis of published RCTs. For both one- and two-level symptomatic cervical spondylosis, this study found that in the short-term, up to 2 years, there was no statistically significant difference in adjacent-level reoperations with CDA versus ACDF. Nonetheless, rates of adjacent-level reoperation diverged over time, with reoperation rates with ACDF being significantly greater than CDA at long-term (≥4-year) follow-up, likely owing to higher rates of symptomatic ASD. By contrast, index-level reoperations occurred significantly less frequently with CDA at both short- and long-term follow-up. The findings of this study support a superior longevity with CDA versus ACDF with regard to need for reoperation.
It has been demonstrated that fusion alters spinal kinematics, such that motion, along with intradiscal pressures and shear strain, are increased at adjacent levels (3,4,40). These factors may predispose adjacent levels to accelerated degeneration, thus providing the rationale for CDA, which preserves physiologic segmental motion and may thereby mitigate ASD. The contemporary era of CDA devices began in 1989 with the design of a steel metal-on-metal artificial disc by Cummins, which was later redesigned and reintroduced as the Frenchay disc, and eventually purchased by Medtronic and marketed as the Prestige disc (41,42). Around the same time, American neurosurgeon, Vincent Bryan, designed the Bryan cervical disc (43). Since then, a number of additional devices have been developed and the evidence base has rapidly expanded. The current literature supports equivalent or superior outcomes with CDA compared with ACDF depending on the specific outcome examined (44,45). Despite this, compelling evidence of benefit with regard to reduction in ASD requiring further surgery is lacking. One issue to consider is that individual trials in isolation are likely underpowered to detect a difference in adjacent-level reoperations. The primary outcome in United States FDA IDE trials of CDA, which sample size calculations are based on, is ‘overall success’, which is a composite outcome defined by: Neck Disability Index (NDI) score improvement ≥15 points; maintenance or improvement in neurological status; no additional surgical procedure classified as a ‘failure’; and no serious adverse event classified as implant-associated (30,46). Further, the primary outcome is evaluated at 2 years, when the cumulative rate of ASD is expectedly relatively low, making sample size requirements even greater. Long-term data with follow-up durations over 5 years have emerged only recently. These issues provided the impetus for the current meta-analysis examining longitudinal reoperation rates from RCTs of CDA versus ACDF.
There are multiple definitions of ASD (47,48). Imaging evidence of degenerative changes at adjacent levels (i.e., radiographic ASD) after ACDF has been reported in up to 92% of patients at long-term follow-up (49). A subset of these patients will develop symptoms, radiculopathy and/or myelopathy, referable to the adjacent level (i.e., clinical ASD); a subset of these latter patients, still, will require secondary surgery to address the adjacent-segment pathology (50). Hilibrand et al. (4) retrospectively studied the incidence of symptomatic ASD following ACDF in 374 patients with up to 21-year follow-up. This study found that 25.6% of patients who had an anterior cervical fusion would develop clinical ASD at 10 years. Lee et al. (51) retrospectively studied 1,038 patients who underwent anterior cervical fusion; Kaplan-Meier analysis predicted that 22.2% of patients would require reoperation at adjacent segments by 10 years. While smoking, female sex, number of levels fused, and the specific levels fused have been identified as risk factors (4,51), the most important and obvious risk factor for development of symptomatic ASD, requiring surgery or not, is time. Hilibrand et al. (4) found symptomatic ASD developed at a relatively constant rate of 2.9% per year. Similarly, Lee et al. (51) noted that symptomatic ASD requiring operation occurred at a relatively consistent rate of 2.4% per year. Therefore, if CDA reduced the rate of development of symptomatic ASD, it would take several years to become apparent. In a consecutive cohort of 888 patients, Xu et al. (52) found ASD warranting secondary operation occurred at a mean of 47 months after the index ACDF in 108 patients (12.2%). Other series have reported an even longer time lag. Wang et al. (53) reported the average time to onset of ASD requiring subsequent surgery to be 8.5 years. In the current meta-analysis, the rate of adjacent-level reoperation following single-level ACDF at 7 years was 10.8%; this represents an average rate of 1.5% per year. At 2 years, none of the one-level randomized trials included in this meta-analysis noted a significant difference in adjacent-level reoperations. By 4 years, a significant reduction with CDA occurred in the Mobi-C trial (US FDA IDE) and non-significant trends were noted for Mobi-C (China), PCM, Prestige ST, and ProDisc-C, such that the pooled treatment effect was statistically significant in favor of CDA. At 5 years, both the Mobi-C (US FDA IDE) and Prestige ST trials found a significant effect on adjacent-level reoperations, whereas a trend was observed for ProDisc-C; the pooled treatment effect of CDA in reducing adjacent-level reoperations was statistically significant and the magnitude of the effect was quite large. These findings persisted at the 7-year mark. Neither two-level study found a significant difference in adjacent-level reoperations at 2 years, but a significantly lower rate was seen for CDA in the Mobi-C trial at 7 years; the pooled treatment effect at 7 years was statistically significant in favor of CDA.
With regard to index-level reoperations, for both one- and two-level symptomatic cervical spondylosis, we observed a statistically significant lower rate for CDA compared with ACDF at all follow-up time points that could be examined, from 2 to 7 years. The majority of index-level reoperations in the ACDF group were likely due to persistent radiculopathy or symptomatic pseudarthrosis. Indeed, in an in-depth examination of subsequent surgery rates after CDA with the Mobi-C disc, Jackson et al. (6) reported that the most common reasons for reoperations at the index level for one- and two-level ACDF were radiculopathy, neck pain, and pseudarthrosis; by contrast, radiculopathy was the most common indication for secondary surgery among patients who underwent CDA.
The key strengths of this study include the greater statistical power derived from performing meta-analyses that pool data multiple trials; the longitudinal evaluation of reoperation rates out to 7-year follow-up; and the application of rigorous and uniform eligibility criteria to select only high-quality RCTs for analysis. Nonetheless, this study does have notable limitations. Any meta-analysis is limited to some degree by heterogeneity. Although all included studies used relatively similar patient selection criteria and outcome definitions, there remain important sources of heterogeneity. The most obvious and important source of heterogeneity would be that the artificial disc tested in each trial was a different device, with inherently different biomechanical properties and potential for efficacy. Despite this, we observed only low-to-moderate heterogeneity in meta-analyses of outcomes. Second, it should be recognized that the results of this systematic review and meta-analysis are inherently generalizable only to patients fulfilling the relatively specific indications and eligibility criteria used in the included studies.
Conclusions
This systematic review and meta-analysis of RCTs comparing CDA with ACDF for one- or two-level symptomatic cervical spondylosis found similar rates of adjacent-level reoperation with either intervention at 2 years. However, at 4 to 5 years, there developed a clear and statistically significant divergence, with the rate of adjacent-level reoperation rising steeply for ACDF. By contrast, CDA was associated with a statistically significant lower rate of index-level reoperation at all follow-up time points examined. These findings support the superior longevity of CDA, as compared with ACDF, with regard to need for subsequent surgical intervention.
Acknowledgments
Funding: None.
Supplementary
Search strategy
PubMed
(cervical OR “Cervical Vertebrae”[Mesh]) AND (“Intervertebral Disc Displacement”[Mesh] OR “Total Disc Replacement”[Mesh] OR ((arthroplasty OR replacement OR artificial OR prosthesis OR prosthetic) AND (disc OR disk))) AND (fusion OR ACDF OR arthrodesis OR fixation OR “Arthrodesis”[Mesh] OR “Spinal Fusion”[Mesh])
EMBASE
1. exp cervical spine/or cervical.mp.
2. exp total disc replacement/or exp disk prosthesis/
3. (arthroplasty or replacement or artificial or prosthesis or prosthetic).mp.
4. (disc or disk).mp.
5. 3 and 4
6. 2 or 5
7. exp anterior spine fusion/or exp spine fusion/
8. (fusion or ACDF or arthrodesis or fixation).mp. or exp arthrodesis/
9. 7 or 8
10. 1 and 6 and 9
Web of Science
#4 #3 AND #2 AND #1
Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years
#3 TOPIC: (fusion OR ACDF OR arthrodesis OR fixation)
Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years
#2 TOPIC: ((arthroplasty OR replacement OR artificial OR prosthesis OR prosthetic) AND (disc OR disk))
Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years
#1 TOPIC: (cervical)
Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Traynelis is a paid consultant for Medtronic, NuVasive, and Thompson Surgical, and receives IP royalties from Medtronic. The other authors have no conflicts of interest to declare.
References
- 1.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. 10.2106/00004623-195840030-00009 [DOI] [PubMed] [Google Scholar]
- 2.Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993;75:1298-307. 10.2106/00004623-199309000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976) 2002;27:2431-4. 10.1097/00007632-200211150-00003 [DOI] [PubMed] [Google Scholar]
- 4.Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519-28. 10.2106/00004623-199904000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Nunley PD, Coric D, Frank KA, et al. Cervical Disc Arthroplasty: Current Evidence and Real-World Application. Neurosurgery 2018;83:1087-106. 10.1093/neuros/nyx579 [DOI] [PubMed] [Google Scholar]
- 6.Jackson RJ, Davis RJ, Hoffman GA, et al. Subsequent surgery rates after cervical total disc replacement using a Mobi-C Cervical Disc Prosthesis versus anterior cervical discectomy and fusion: a prospective randomized clinical trial with 5-year follow-up. J Neurosurg Spine 2016;24:734-45. 10.3171/2015.8.SPINE15219 [DOI] [PubMed] [Google Scholar]
- 7.Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 2011;15:348-58. 10.3171/2011.5.SPINE10769 [DOI] [PubMed] [Google Scholar]
- 8.Lavelle WF, Riew KD, Levi AD, et al. Ten-year Outcomes of Cervical Disc Replacement With the BRYAN Cervical Disc: Results From a Prospective, Randomized, Controlled Clinical Trial. Spine (Phila Pa 1976) 2019;44:601-8. 10.1097/BRS.0000000000002907 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane. 2019. Available online: www.training.cochrane.org/handbook
- 11.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12.Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976) 2009;34:101-7. 10.1097/BRS.0b013e31818ee263 [DOI] [PubMed] [Google Scholar]
- 13.Sasso RC, Anderson PA, Riew KD, et al. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am 2011;93:1684-92. 10.2106/JBJS.J.00476 [DOI] [PubMed] [Google Scholar]
- 14.Ghobrial GM, Lavelle WF, Florman JE, et al. Symptomatic Adjacent Level Disease Requiring Surgery: Analysis of 10-Year Results From a Prospective, Randomized, Clinical Trial Comparing Cervical Disc Arthroplasty to Anterior Cervical Fusion. Neurosurgery 2019;84:347-54. 10.1093/neuros/nyy118 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang X, Chen C, et al. Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine (Phila Pa 1976) 2012;37:433-8. 10.1097/BRS.0b013e31822699fa [DOI] [PubMed] [Google Scholar]
- 16.Coric D, Guyer RD, Nunley PD, et al. Prospective, randomized multicenter study of cervical arthroplasty versus anterior cervical discectomy and fusion: 5-year results with a metal-on-metal artificial disc. J Neurosurg Spine 2018;28:252-61. 10.3171/2017.5.SPINE16824 [DOI] [PubMed] [Google Scholar]
- 17.Hisey MS, Bae HW, Davis R, et al. Multi-center, prospective, randomized, controlled investigational device exemption clinical trial comparing Mobi-C Cervical Artificial Disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. Int J Spine Surg 2014. doi: . 10.14444/1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisey MS, Bae HW, Davis RJ, et al. Prospective, Randomized Comparison of Cervical Total Disk Replacement Versus Anterior Cervical Fusion: Results at 48 Months Follow-up. J Spinal Disord Tech 2015;28:E237-43. 10.1097/BSD.0000000000000185 [DOI] [PubMed] [Google Scholar]
- 19.Hisey MS, Zigler JE, Jackson R, et al. Prospective, Randomized Comparison of One-level Mobi-C Cervical Total Disc Replacement vs. Anterior Cervical Discectomy and Fusion: Results at 5-year Follow-up. Int J Spine Surg 2016;10:10. 10.14444/3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radcliff K, Davis RJ, Hisey MS, et al. Long-term Evaluation of Cervical Disc Arthroplasty with the Mobi-C(c) Cervical Disc: A Randomized, Prospective, Multicenter Clinical Trial with Seven-Year Follow-up. Int J Spine Surg 2017;11:31. 10.14444/4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HX, Shao YD, Chen Y, et al. A prospective, randomised, controlled multicentre study comparing cervical disc replacement with anterior cervical decompression and fusion. Int Orthop 2014;38:2533-41. 10.1007/s00264-014-2497-5 [DOI] [PubMed] [Google Scholar]
- 22.Davis RJ, Kim KD, Hisey MS, et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled multicenter clinical trial: clinical article. J Neurosurg Spine 2013;19:532-45. 10.3171/2013.6.SPINE12527 [DOI] [PubMed] [Google Scholar]
- 23.Davis RJ, Nunley PD, Kim KD, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine 2015;22:15-25. 10.3171/2014.7.SPINE13953 [DOI] [PubMed] [Google Scholar]
- 24.Radcliff K, Coric D, Albert T. Five-year clinical results of cervical total disc replacement compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled, multicenter investigational device exemption clinical trial. J Neurosurg Spine 2016;25:213-24. 10.3171/2015.12.SPINE15824 [DOI] [PubMed] [Google Scholar]
- 25.Phillips FM, Lee JY, Geisler FH, et al. A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2-year results from the US FDA IDE clinical trial. Spine (Phila Pa 1976) 2013;38:E907-18. 10.1097/BRS.0b013e318296232f [DOI] [PubMed] [Google Scholar]
- 26.Phillips FM, Geisler FH, Gilder KM, et al. Long-term Outcomes of the US FDA IDE Prospective, Randomized Controlled Clinical Trial Comparing PCM Cervical Disc Arthroplasty With Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2015;40:674-83. 10.1097/BRS.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 27.Gornet MF, Lanman TH, Burkus JK, et al. Cervical disc arthroplasty with the Prestige LP disc versus anterior cervical discectomy and fusion, at 2 levels: results of a prospective, multicenter randomized controlled clinical trial at 24 months. J Neurosurg Spine 2017;26:653-67. 10.3171/2016.10.SPINE16264 [DOI] [PubMed] [Google Scholar]
- 28.Lanman TH, Burkus JK, Dryer RG, et al. Long-term clinical and radiographic outcomes of the Prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial. J Neurosurg Spine 2017;27:7-19. 10.3171/2016.11.SPINE16746 [DOI] [PubMed] [Google Scholar]
- 29.Gornet MF, Lanman TH, Burkus JK, et al. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine 2019. doi: . 10.3171/2019.4.SPINE19157 [DOI] [PubMed] [Google Scholar]
- 30.Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 2007;6:198-209. 10.3171/spi.2007.6.3.198 [DOI] [PubMed] [Google Scholar]
- 31.Burkus JK, Haid RW, Traynelis VC, et al. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. J Neurosurg Spine 2010;13:308-18. 10.3171/2010.3.SPINE09513 [DOI] [PubMed] [Google Scholar]
- 32.Burkus JK, Traynelis VC, Haid RW, Jr, et al. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine 2014;21:516-28. 10.3171/2014.6.SPINE13996 [DOI] [PubMed] [Google Scholar]
- 33.Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 2009;9:275-86. 10.1016/j.spinee.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 34.Delamarter RB, Murrey D, Janssen ME, et al. Results at 24 months from the prospective, randomized, multicenter Investigational Device Exemption trial of ProDisc-C versus anterior cervical discectomy and fusion with 4-year follow-up and continued access patients. SAS J 2010;4:122-8. 10.1016/j.esas.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976) 2013;38:711-7. 10.1097/BRS.0b013e3182797592 [DOI] [PubMed] [Google Scholar]
- 36.Zigler JE, Delamarter R, Murrey D, et al. ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine (Phila Pa 1976) 2013;38:203-9. 10.1097/BRS.0b013e318278eb38 [DOI] [PubMed] [Google Scholar]
- 37.Janssen ME, Zigler JE, Spivak JM, et al. ProDisc-C Total Disc Replacement Versus Anterior Cervical Discectomy and Fusion for Single-Level Symptomatic Cervical Disc Disease: Seven-Year Follow-up of the Prospective Randomized U.S. Food and Drug Administration Investigational Device Exemption Study. J Bone Joint Surg Am 2015;97:1738-47. 10.2106/JBJS.N.01186 [DOI] [PubMed] [Google Scholar]
- 38.Vaccaro A, Beutler W, Peppelman W, et al. Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine (Phila Pa 1976) 2013;38:2227-39. 10.1097/BRS.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 39.Vaccaro A, Beutler W, Peppelman W, et al. Long-Term Clinical Experience with Selectively Constrained SECURE-C Cervical Artificial Disc for 1-Level Cervical Disc Disease: Results from Seven-Year Follow-Up of a Prospective, Randomized, Controlled Investigational Device Exemption Clinical Trial. Int J Spine Surg 2018;12:377-87. 10.14444/5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang UK, Kim DH, Lee MC, et al. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine 2007;7:33-9. 10.3171/SPI-07/07/033 [DOI] [PubMed] [Google Scholar]
- 41.Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg 1998;88:943-8. 10.3171/jns.1998.88.6.0943 [DOI] [PubMed] [Google Scholar]
- 42.Wigfield CC, Gill SS, Nelson RJ, et al. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine (Phila Pa 1976) 2002;27:2446-52. 10.1097/00007632-200211150-00006 [DOI] [PubMed] [Google Scholar]
- 43.Basho R, Hood KA. Cervical total disc arthroplasty. Global Spine J 2012;2:105-8. 10.1055/s-0032-1315453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAfee PC, Reah C, Gilder K, et al. A meta-analysis of comparative outcomes following cervical arthroplasty or anterior cervical fusion: results from 4 prospective multicenter randomized clinical trials and up to 1226 patients. Spine (Phila Pa 1976) 2012;37:943-52. 10.1097/BRS.0b013e31823da169 [DOI] [PubMed] [Google Scholar]
- 45.Gao Y, Liu M, Li T, et al. A meta-analysis comparing the results of cervical disc arthroplasty with anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical disc disease. J Bone Joint Surg Am 2013;95:555-61. 10.2106/JBJS.K.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Food and Drug Administration (FDA). Preparation and Review of Investigational Device Exemption Applications (IDEs) for Total Artificial Discs. 2008. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preparation-and-review-investigational-device-exemption-applications-ides-total-artificial-discs
- 47.Anderson PA, Andersson GB, Arnold PM, et al. Terminology. Spine (Phila Pa 1976) 2012;37:S8-9. 10.1097/BRS.0b013e31826d62ed [DOI] [PubMed] [Google Scholar]
- 48.Kraemer P, Fehlings MG, Hashimoto R, et al. A systematic review of definitions and classification systems of adjacent segment pathology. Spine (Phila Pa 1976) 2012;37:S31-9. 10.1097/BRS.0b013e31826d7dd6 [DOI] [PubMed] [Google Scholar]
- 49.Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech 2004;17:79-85. 10.1097/00024720-200404000-00001 [DOI] [PubMed] [Google Scholar]
- 50.Kong L, Cao J, Wang L, et al. Prevalence of adjacent segment disease following cervical spine surgery: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4171. 10.1097/MD.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JC, Lee SH, Peters C, et al. Adjacent segment pathology requiring reoperation after anterior cervical arthrodesis: the influence of smoking, sex, and number of operated levels. Spine (Phila Pa 1976) 2015;40:E571-7. 10.1097/BRS.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 52.Xu R, Bydon M, Macki M, et al. Adjacent segment disease after anterior cervical discectomy and fusion: clinical outcomes after first repeat surgery versus second repeat surgery. Spine (Phila Pa 1976) 2014;39:120-6. 10.1097/BRS.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Hou HT, Wang P, et al. Symptomatic adjacent segment disease after single-lever anterior cervical discectomy and fusion: Incidence and risk factors. Medicine (Baltimore) 2017;96:e8663. 10.1097/MD.0000000000008663 [DOI] [PMC free article] [PubMed] [Google Scholar]