Abstract
Background
Antenatal maternal glucose administration has been suggested to improve the efficiency of antepartum fetal heart rate testing.
Objectives
The objective of this review was to assess the merits or adverse effects of antenatal maternal glucose administration in conjunction with tests of fetal wellbeing.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 July 2012).
Selection criteria
All published and unpublished randomized controlled trials assessing the merits of antenatal maternal (oral or intravenous) glucose administration in conjunction with tests of fetal wellbeing.
Data collection and analysis
Both review authors independently extracted data and assessed trial quality. Authors of published and unpublished trials were contacted for further information.
Main results
A total of two trials, involving 708 participants, were included. Antenatal maternal glucose administration did not decrease the incidence of non‐reactive antenatal cardiotocography tests.
Authors' conclusions
Antenatal maternal glucose administration has not been shown to reduce non‐reactive cardiotocography. More trials are needed to further substantiate this and to determine not only the optimum dose, but also to evaluate the efficacy, predictive reliability, safety and perinatal outcome of glucose administration in conjunction with cardiotocography and also other tests of fetal wellbeing.
Keywords: Female; Humans; Pregnancy; Administration, Oral; Cardiotocography; Cardiotocography/methods; Glucose; Glucose/administration & dosage; Heart Rate, Fetal; Heart Rate, Fetal/drug effects; Heart Rate, Fetal/physiology; Injections, Intravenous; Prenatal Diagnosis; Prenatal Diagnosis/methods; Randomized Controlled Trials as Topic; Single‐Blind Method
Plain language summary
Maternal glucose administration for facilitating tests of fetal wellbeing
There is no evidence that antenatal maternal glucose administration make tests of fetal wellbeing more effective.
Tests on unborn babies such as ultrasound and heart rate testing are carried out to check their wellbeing. As a baby's sleep periods can alter those results, various methods are used to wake the baby. Antenatal maternal glucose administration is one of the methods. The review of two trials, involving 708 participants, did not find this method to be effective. Research on antenatal maternal glucose administration should take into consideration that there have not been any benefits demonstrated as yet.
Background
Various methods of stimulation have been proposed to arouse the fetus from the quiet sleep phase of the rest‐activity cycle. They include a change in maternal position, physical activity, maternal glucose ingestion, acoustic stimulation, stimulation with light and manual manipulation of the fetus. If the fetus can be aroused effectively, such stimulations may be useful when used in conjunction with tests of fetal wellbeing.
A number of studies have reported an increase in fetal activity related to increased serum levels of maternal glucose following glucose administration (Adadjem 1979; Gelman 1980; Miller 1978). Arousing the fetus by administering glucose to the mother (either orally or intravenously) could be useful in conjunction with tests of fetal wellbeing. In particular the time needed to obtain a normal reactive cardiotocograph might be decreased and the number of false positive non‐reactive antepartum fetal heart tracings might be lowered.
Objectives
The objectives are:
to evaluate the effects of antenatal maternal glucose administration (oral glucose ingestion or intravenous glucose) used in conjunction with tests of fetal wellbeing. In particular to assess whether the adjunctive use of glucose administration to alter fetal behavioural states leads to fewer false positive non‐reactive tests.
to assess whether the adjunctive administration of glucose affects: perinatal outcome, maternal satisfaction, time required to complete the test, and cost savings.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomized controlled trials of maternal glucose administration in conjunction with tests of fetal wellbeing. Studies will be included if they meet the following criteria: random allocation to treatment and control groups, with adequate allocation concealment; violations of allocated management and exclusions after allocation not sufficient to materially affect outcomes.
Types of participants
Pregnant women booked for a test of fetal wellbeing.
Types of interventions
Maternal glucose administration versus no administration or placebo.
Maternal glucose administration versus other form of attempted stimulation of the fetus including vibroacoustic stimulation and fetal manipulation.
Maternal glucose administration versus no maternal glucose or placebo, as an adjunct to cardiotocography or other tests of fetal wellbeing, with or without other forms of fetal arousal.
Types of outcome measures
Reactive cardiotocography.
Palpated or visualised fetal movements or those perceived by the mother.
Length of time required to complete the test of fetal wellbeing.
Maternal anxiety and satisfaction.
Clinically relevant maternal, fetal, or neonatal outcomes including operative delivery, perinatal morbidity and mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (6 July 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used in the previous version of this review, see Appendix 1.
For the methods we would have used if we had identified new trials from the updated search, and for the methods we will use in the future if we identify new trials, see Appendix 2.
Results
Description of studies
Only two trials were identified from the search strategy which satisfactorily addressed the effect of glucose ingestion on the incidence of non‐reactive cardiotocography (Eglinton 1984; Richardson 1983). Reactivity was defined as two or more accelerations (Eglinton 1984) or four or more accelerations (Richardson 1983) of at least 15 beats per minute which lasted at least 15 seconds in a 20 minute interval. Patients in the experimental group received an eight ounce preparation of reconstituted orange juice (20 gm of carbohydrate) in Eglinton's trial (Eglinton 1984) and 50 grams of oral glucose drink in Richardson's trial (Richardson 1983). Eglinton 1984 and Richardson 1983 trials, were single blinded studies where the workers who performed the analysis of the cardiotocography did not know whether the patients had received glucose. There was no withdrawal from the two trials after randomization.
Risk of bias in included studies
Randomization were by odd‐even numbers (Eglinton 1984) and alternation (Richardson 1983) with its attendant bias.
Effects of interventions
Two trials with a total of 708 participants were included.
(i) Maternal glucose administration versus no administration or placebo: oral glucose or carbohydrate ingestion did not reduce the incidence of non‐reactive cardiotocography.
(ii) Maternal glucose administration versus no administration or placebo in fasted pregnant women: oral glucose or carbohydrate ingestion did not reduce the incidence of non‐reactive cardiotocography.
(iii) Maternal glucose administration versus no administration or placebo in non‐fasted pregnant women: oral glucose or carbohydrate ingestion did not reduce the incidence of non‐reactive cardiotocography.
Discussion
The benefits of antenatal maternal glucose administration, if any, in conjunction with tests of fetal wellbeing must be weighed against its effect on the predictive reliability of the tests and the safety of the procedure.
There is a paucity in the literature of randomized controlled trials relating to antenatal maternal glucose administration in conjunction with tests of fetal wellbeing. There is currently insufficient evidence from randomized controlled trials upon which to base a recommendation regarding the use of antenatal maternal glucose administration.
Authors' conclusions
Implications for practice.
Current evidence suggests that there is unlikely to be a clinical role for maternal glucose administration in reducing the incidence of non‐reactive cardiotocography. The benefits of the adjunctive use of glucose ingestion with respect to perinatal outcome also remain to be established.
Implications for research.
Further clinical trials on its use in conjunction with tests of fetal wellbeing and its effect on perinatal outcome are needed. However research on antenatal maternal glucose administration should take into consideration that there have not been any clinical benefits demonstrated as yet.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2012 | New citation required but conclusions have not changed | Review updated with a new search. |
| 9 July 2012 | New search has been performed | Search updated. No new trials identified. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 31 May 2009 | New search has been performed | Search updated. No new trials identified. |
| 17 September 2008 | Amended | Converted to new review format. |
| 1 June 2006 | New search has been performed | New search conducted but no new trial reports identified. |
Acknowledgements
None.
Appendices
Appendix 1. Methods used in the previous version of this review
Included trial data were processed as described in Clarke 2000.
Authors of published and unpublished trials were contacted for additional information where needed.
Trials under consideration were evaluated for inclusion and methodological quality, without consideration of their results. Quality scores for concealment of allocation were assigned to each trial, using the criteria described in Section VI of the Cochrane Handbook (Clarke 2000): A = adequate, B = unclear, C = inadequate, D = not used.
Data were extracted from the sources and entered onto the Review Manager computer programme (RevMan 2000), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, risk ratios and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed‐effect model.
All eligible trials were included in the initial analysis and sensitivity analyses were carried out to evaluate the effect of trial quality.
Appendix 2. Methods to be used in future updates
Data collection and analysis
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third person.
Data extraction and management
We will design a form to extract data. For eligible studies, at least two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third person. We will enter data into Review Manager software (RevMan 2011) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We will regard heterogeneity as substantial if I2 is greater than 30% and either T2 is greater than zero, or there is a low P‐value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2011). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Data and analyses
Comparison 1. Maternal glucose administration versus placebo or no administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐reactive cardiotocography | 2 | 708 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 2 Non‐reactive cardiotocography in fasted patients | 1 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.42, 1.72] |
| 3 Non‐reactive cardiotocography in fed patients | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.40, 5.39] |
| 4 Absence of fetal body movements | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Perinatal deaths | 1 | 293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.05, 4.92] |
| 6 Maternal satisfaction | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal anxiety | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
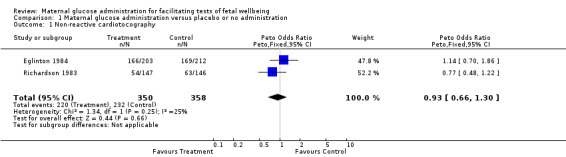
Comparison 1 Maternal glucose administration versus placebo or no administration, Outcome 1 Non‐reactive cardiotocography.
1.2. Analysis.
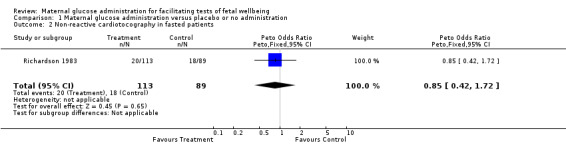
Comparison 1 Maternal glucose administration versus placebo or no administration, Outcome 2 Non‐reactive cardiotocography in fasted patients.
1.3. Analysis.
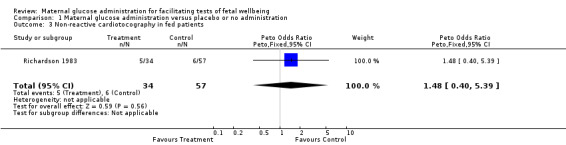
Comparison 1 Maternal glucose administration versus placebo or no administration, Outcome 3 Non‐reactive cardiotocography in fed patients.
1.5. Analysis.
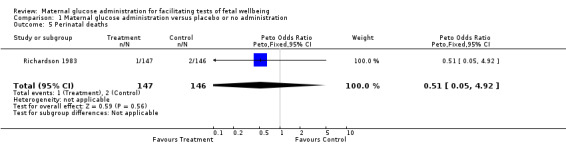
Comparison 1 Maternal glucose administration versus placebo or no administration, Outcome 5 Perinatal deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eglinton 1984.
| Methods | Randomization: randomized by sequential study numbers in order of patient entry with its attendant bias. Effectiveness of randomization was assessed by comparisons of primary indications between the 2 groups. Single blind study in that the physicians who read the non‐stress tests did not know which patients had received orange juice. | |
| Participants | Inpatients and outpatients excluding insulin requiring diabetic patients. Majority of the tests were done because of postdates pregnancy, suspected intrauterine growth retardation, decreased fetal movements and hypertension. Country USA California. 475 women randomized. | |
| Interventions | Patients in the interventional group received a preparation of reconstituted orange juice (20 gm of carbohydrate). | |
| Outcomes | Primary outcome: fetal heart rate reactivity. Reactivity was defined as 2 or more accelerations of at least 15 beats per minute which lasted at least 15 seconds in a 20 minute interval. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
Richardson 1983.
| Methods | Randomization was by alternation with its attendant bias. Effectiveness of randomization was assessed by comparisons of primary indications between the 2 groups (those receiving glucose and those receiving water). Blinded study in that analysis of recordings was performed by 1 of the authors without clinical information and knowledge of administration of glucose or water. Patients were managed by attending physicians who had access to non‐stress test recordings but not to the interpretation of recordings presented in this study. | |
| Participants | Women of high‐risk pregnancy with gestational ages from 28 to 42+ excluding diabetic patients. Majority of the tests were done because of postdates pregnancy, unsuspected intrauterine growth retardation, decreased fetal movements and hypertension. Country USA Oregon. 235 women randomized. | |
| Interventions | Patients in the experimental group received a 50 gram of oral glucose drink while those in the control group were given an equal volume of water 30 minutes prior to the commencement of the test. | |
| Outcomes | Primary outcome: fetal heart rate reactivity. Reactivity was defined as 4 or more accelerations of at least 15 beats per minute which lasted at least 15 seconds in a 20 minute interval. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bocking 1982 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Bocking 1984 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Devoe 1986 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Devoe 1987 | There was a significant discrepancy of numbers after randomization between the experimental and control groups which could bias results. |
| Gelman 1980 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Lewis 1978 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Natale 1983 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Neldam 1982 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Nijhuis 1986 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
Contributions of authors
Kelvin Tan prepared the review with input from Antoinette Sabapathy.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Eglinton 1984 {published data only}
- Eglinton GS, Paul RH, Broussard PM, Walla CA, Platt LD. Antepartum fetal heart rate testing. XI. Stimulation with orange juice. American Journal of Obstetrics and Gynecology 1984;150:97‐9. [DOI] [PubMed] [Google Scholar]
Richardson 1983 {published data only}
- Richardson B, Briggs ML, Toomey C, Burry KJ, O'Grady JP. The effect of maternal glucose administration on the specificity of the nonstress test. American Journal of Obstetrics and Gynecology 1983;145:141‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bocking 1982 {published data only}
- Bocking A, Adamson L, Cousin A, Campbell K, Carmichael L, Natale R, et al. Effects of intravenous glucose injections on human fetal breathing movements and gross fetal body movements at 38 to 40 weeks' gestational age. American Journal of Obstetrics and Gynecology 1982;142:606‐11. [DOI] [PubMed] [Google Scholar]
Bocking 1984 {published data only}
- Bocking A, Adamson L, Carmichael L, Patrick J, Probert C. Effect of intravenous glucose injection on human maternal and fetal heart rate at term. American Journal of Obstetrics and Gynecology 1984;148:414‐20. [DOI] [PubMed] [Google Scholar]
Devoe 1986 {published data only}
- Devoe L, Searle N, Castillo R, Searle J. The effects of maternal administration of trytophane and glucose on fetal trunk movements and fetal heart rate. Proceedings of 6th Annual Meeting of the Society of Perinatal Obstetricians; 1986 Jan 30‐Feb 1; San Antonio, Texas, USA. 1986:44.
- Devoe LD, Castillo RA, Searle NS, Searle JR. Maternal dietary substrates and human fetal biophysical activity. I. The effects of tryptophan and glucose on fetal breathing movements. American Journal of Obstetrics and Gynecology 1986;155:135‐9. [DOI] [PubMed] [Google Scholar]
Devoe 1987 {published data only}
- Devoe LD, Searle N, Castillo RA, Searle J. Fetal biophysical testing. The effects of prolonged maternal fasting and the oral glucose tolerance test. Journal of Reproductive Medicine 1987;32:563‐8. [PubMed] [Google Scholar]
Gelman 1980 {published data only}
- Gelman SR, Spellacy WN, Wood S, Birk SA, Buhi WC. Fetal movements and ultrasound: effect of maternal intravenous glucose administration. American Journal of Obstetrics and Gynecology 1980;137:459‐61. [DOI] [PubMed] [Google Scholar]
Lewis 1978 {published data only}
- Lewis PJ, Trudinger BJ, Mangez J. Effect of maternal glucose ingestion on fetal breathing and body movements in late pregnancy. British Journal of Obstetrics and Gynaecology 1978;85:86‐9. [DOI] [PubMed] [Google Scholar]
Natale 1983 {published data only}
- Natale R, Richardson B, Patrick J. The effect of maternal hyperglycemia on gross body movements in human fetuses at 32‐34 weeks' gestation. Early Human Development 1983;8:13‐20. [DOI] [PubMed] [Google Scholar]
Neldam 1982 {published data only}
- Neldam S, Hornnes PJ, Kuhl C. Effect of maternal triglyceride ingestion on fetal respiratory movements. Obstetrics & Gynecology 1982;59:640‐2. [PubMed] [Google Scholar]
Nijhuis 1986 {published data only}
- Nijhuis JG, Jongsma HW, Crijns IJMJ, Valk IMGM, Velden JWHJ. Effects of maternal glucose ingestion on human fetal breathing movements at weeks 24 and 28 of gestation. Early Human Development 1986;13:183‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Adadjem 1979
- Adadjem S, Feria A, Rest J, Gull K, O'Connor M. Effect of maternal glucose load on fetal activity. American Journal of Obstetrics and Gynecology 1979;134:276. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Miller 1978
- Miller FC, Skiba H, Klapholz H. The effect of maternal blood sugar levels on fetal activity. Obstetrics & Gynecology 1978;52:663. [PubMed] [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.


