Abstract
Background
Status epilepticus is a medical emergency associated with significant mortality and morbidity that requires immediate and effective treatment.
Objectives
(1) To determine whether a particular anticonvulsant is more effective or safer to use in status epilepticus compared to another and compared to placebo. (2) To delineate reasons for disagreement in the literature regarding recommended treatment regimens and to highlight areas for future research.
Search methods
For the latest update of this review, the following electronic databases were searched on 15/08/2013: the Cochrane Epilepsy Group's Specialized Register, CENTRAL The Cochrane Library July 2013, Issue 7, and MEDLINE (Ovid) 1946 to 15/08/2013.
Selection criteria
Randomised controlled trials of participants with premonitory, early, established or refractory status epilepticus using a truly random or quasi‐random allocation of treatments were included.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality and extracted data.
Main results
Eighteen studies with 2755 participants were included. Few studies used the same interventions. Intravenous diazepam was better than placebo in reducing the risk of non‐cessation of seizures (risk ratio (RR) 0.73, 95% confidence interval (CI) 0.57 to 0.92), requirement for ventilatory support (RR 0.39, 95% CI 0.16 to 0.94), or continuation of status epilepticus requiring use of a different drug or general anaesthesia (RR 0.73, 95% CI 0.57 to 0.92). Intravenous lorazepam was better than placebo for risk of non‐cessation of seizures (RR 0.52, 95% CI 0.38 to 0.71) and for risk of continuation of status epilepticus requiring a different drug or general anaesthesia (RR 0.52, 95% CI 0.38 to 0.71). Intravenous lorazepam was better than intravenous diazepam for reducing the risk of non‐cessation of seizures (RR 0.64, 95% CI 0.45 to 0.90) and had a lower risk for continuation of status epilepticus requiring a different drug or general anaesthesia (RR 0.63, 95% CI 0.45 to 0.88). Intravenous lorazepam was better than intravenous phenytoin for risk of non‐cessation of seizures (RR 0.62, 95% CI 0.45 to 0.86). Diazepam gel was better than placebo gel in reducing the risk of non‐cessation of seizures (RR 0.43 95% CI 0.30 to 0.62)
For pre‐hospital treatment, intramuscular midazolam is at least as effective as (probably more effective than) intravenous lorazepam in control of seizures (RR1.16, 95% CI 1.06 to 1.27) and frequency of hospitalisation (RR 0.88, 95% CI 0.79 to 0.97) or intensive care admissions (RR 0.79, 95% CI 0.65 to 0.96). It was uncertain whether Intravenous valproate was better than intravenous phenytoin in reducing risk of non‐cessation of seizures (RR 0.75, 95% CI 0.28 to 2.00). Both levetiracetam and lorazepam were equally effective in aborting seizures (RR 0.97, 95% CI 0.44 to 2.13). Results for other comparisons of anticonvulsant therapies were uncertain due to single studies with few participants.
The body of randomised evidence to guide clinical decisions is small. It was uncertain whether any anticonvulsant therapy was better than another in terms of adverse effects, due to few studies and participants identified. The quality of the evidence from the included studies is not strong but appears acceptable. We were unable to make judgements for risk of bias domains incomplete outcome reporting (attrition bias) and selective outcome reporting (selection bias) due to unclear reporting by the study authors.
Authors' conclusions
Intravenous lorazepam is better than intravenous diazepam or intravenous phenytoin alone for cessation of seizures. Intravenous lorazepam also carries a lower risk of continuation of status epilepticus requiring a different drug or general anaesthesia compared with intravenous diazepam. Both intravenous lorazepam and diazepam are better than placebo for the same outcomes. For pre hospital management, midazolam IM seemed more effective than lorazepam IV for cessation of seizures, frequency of hospitalisation and ICU admissions however,it was unclear whether the risk of recurrence of seizures differed between treatments. The results of other comparisons of anticonvulsant therapies versus each other were also uncertain. Universally accepted definitions of premonitory, early, established and refractory status epilepticus are required. Diazepam gel was better than placebo gel in reducing the risk of non‐cessation of seizures. Results for other comparisons of anticonvulsant therapies were uncertain due to single studies with few participants.
Plain language summary
Anticonvulsant therapy for status epilepticus
Some patients develop abnormal excessive electrical activity of brain nerve cells. This is called seizure activity and may involve a small area of the brain or the whole brain, resulting in sudden dysfunction of the structures involved, such as shaking of the limbs. The seizure activity often results in jerky movements (convulsions) and usually lasts a few minutes. When there is either more than 30 minutes of continuous seizure activity; or there are two or more seizures in a row without recovery of full consciousness between two seizures, the condition is called status epilepticus, which is a medical emergency. Many drugs have been studied in the management of this condition. This review found that intravenous (injected into a vein) lorazepam is better than diazepam or phenytoin for immediate control of status epilepticus. In the treatment of serially occurring seizures, diazepam gel administered rectally is effective in controlling seizures. Intravenous lorazepam is better than intravenous diazepam or phenytoin for immediate control of status epilepticus. For pre‐hospital treatment, intramuscular midazolam is as effective as (probably more effective than) intravenous lorazepam in control of seizures and frequency of hospitalisation or intensive care admissions. There is a need to conduct more studies on other drugs routinely used for this condition.
Summary of findings
Summary of findings for the main comparison. Lorazepam IV versus diazepam IV for status epilepticus.
| Lorazepam IV versus diazepam IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus Settings: Intervention: Lorazepam IV versus diazepam IV | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam IV | |||||
| Non‐cessation of seizures | Study population | RR 0.64 (0.45 to 0.9) | 264 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 381 per 1000 | 244 per 1000 (171 to 343) | |||||
| Moderate | ||||||
| 219 per 1000 | 140 per 1000 (99 to 197) | |||||
| Requirement for ventilatory support | Study population | RR 0.73 (0.36 to 1.49) | 264 (3 studies) | ⊕⊕⊝⊝ low1,2, | ||
| 127 per 1000 | 93 per 1000 (46 to 189) | |||||
| Moderate | ||||||
| 125 per 1000 | 91 per 1000 (45 to 186) | |||||
| Adverse effects | Study population | See comment | 264 (3 studies) | ⊕⊝⊝⊝ very low3,4 | Risks were calculated from pooled risk differences | |
| 82 per 1000 | 51 per 1000 (‐18 to 112) | |||||
| Moderate | ||||||
| 103 per 1000 | 64 per 1000 (‐23 to 141) | |||||
| Continuation of status requiring a different drug or general anaesthesia | Study population | RR 0.63 (0.45 to 0.88) | 264 (3 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 388 per 1000 | 244 per 1000 (175 to 341) | |||||
| Moderate | ||||||
| 250 per 1000 | 158 per 1000 (112 to 220) | |||||
| Death | Study population | See comment | 203 (2 studies) | ⊕⊕⊕⊝ moderate1 | Risks were calculated from pooled risk differences | |
| 30 per 1000 | 51 per 1000 (‐10 to 110) | |||||
| Moderate | ||||||
| 22 per 1000 | 37 per 1000 (‐7 to 81) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IV: intravenous; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Of the three studies, one (Appleton 1995) used odd/even dates for randomisation, clearly incurring high risk of bias. Two studies did not conceal allocation, incurring high risk of bias (Mehta 2007; Misra 2011) . . Furthermore an additional eight studies (Agarwal 2007; Chamberlain 1997; Chen 2011; McCormick 1999; Pellock 1998; Rossetti 2011; Shaner 1988; Sreenath 2010) did not provide clear information regarding generation of random sequence and/or allocation concealment.Taken together, there is enough risk of bias to downgrade the quality of evidence. 2 The summary effect estimate crosses the line of null effect, and hence is consistent with appreciable benefit as well as appreciable harm. 3 No explanation was provided . 4 I SQUARE is 49% 5 The summary effect is consistent with 100 fewer to 30 more patients having an adverse event
Summary of findings 2. Lorazepam IV versus diazepam plus phenytoin IV for status epilepticus.
| Lorazepam IV versus diazepam plus phenytoin IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus Settings: Intervention: Lorazepam IV versus diazepam plus phenytoin IV | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam plus phenytoin IV | |||||
| Non‐cessation of seizures | Study population | RD ‐0.04 (‐0.35 to 0.26) | 370 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | ||
| 227 per 1000 | 179 per 1000 (127 to 257) | |||||
| Moderate | ||||||
| 221 per 1000 | 175 per 1000 (124 to 250) | |||||
| Adverse effects | Study population | RR 0.85 (0.63 to 1.15) | 370 (2 studies) | ⊕⊕⊕⊝ moderate1,3 | ||
| 290 per 1000 | 246 per 1000 (182 to 333) | |||||
| Moderate | ||||||
| 281 per 1000 | 239 per 1000 (177 to 323) | |||||
| Deaths | See comment | See comment | Not estimable | 178 (1) | See comment | Reported in a single study |
| Requirement of Ventilatory support | See comment | See comment | Not estimable | 178 (1) | See comment | Reported in a single study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IV: intravenous; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Even though one study did not have a clear concealment of allocation, this is possibly a lack of reporting. Lack of blinding in this study is unlikely to bias the outcome assessment as the outcomes are clearly detectable and objective. 2 The I square is 95%. 3 The number of events is small and confidence interval is wide.
Summary of findings 3. Diazepam gel versus placebo gel for status epilepticus.
| Diazepam gel versus placebo gel for status epilepticus | ||||||
| Patient or population: patients with status epilepticus Settings: Intervention: Diazepam gel versus placebo gel | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Diazepam gel versus placebo gel | |||||
| Non‐cessation of seizures | Study population | RR 0.43 (0.3 to 0.62) | 165 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 716 per 1000 | 308 per 1000 (215 to 444) | |||||
| Moderate | ||||||
| 716 per 1000 | 308 per 1000 (215 to 444) | |||||
| Adverse effects | Study population | RR 1.5 (0.94 to 2.37) | 165 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 250 per 1000 | 375 per 1000 (235 to 592) | |||||
| Moderate | ||||||
| 248 per 1000 | 372 per 1000 (233 to 588) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One placebo‐controlled study did not describe the method of sequence generation, but probably this is a limitation of writing style. Use of placebo will tend to limit any bias due to this. 2 Only few events recorded.
Background
Description of the condition
Status epilepticus is defined as a condition in which there is either more than 30 minutes of continuous seizure activity; or two or more sequential seizures without recovery of full consciousness between the seizures. Status epilepticus is a medical emergency. It is associated with an overall mortality of 8% in children and 30% in adults (Epilepsy Foundation of America 1993). An additional 5% to 10% of people experiencing this condition have permanent sequelae, such as a permanent vegetative state or cognitive difficulties. Approximately 12% to 30% of adults with a new diagnosis of epilepsy present in status epilepticus (Lowenstein 1998). Some authors, depending upon the duration of seizure activity, stipulate three stages of the condition: early, established and refractory. Early status epilepticus consists of the first 30 minutes of the epileptic state during which physiological mechanisms compensate for the greatly enhanced metabolic activity. Established status epilepticus is defined as the stage beyond 30 minutes, where the status continues despite early stage treatment. It is during this phase that physiological compensation mechanisms begin to fail. If seizures continue for 60 to 90 minutes after the initiation of therapy the stage of refractory status is said to have been reached. Status epilepticus may be convulsive (with limb stiffness and jerking) or non‐convulsive (without limb stiffness and jerking). Though convulsive status epilepticus is associated with a higher mortality and morbidity than non‐convulsive status epilepticus, both require prompt and effective treatment. However, the most effective treatment regimen is not clear from the literature. Different experts give different recommendations regarding the best treatment regimen for status epilepticus (Lowenstein 1998; Prasad 1995; Epilepsy Foundation of America 1993) and the evidence base of many of these recommendations is often unclear. We conducted a systematic review of all the randomised controlled trials that we could identify to summarise the existing evidence, to delineate the reasons for disagreements and to highlight areas requiring further research.
Description of the intervention
Several drugs are available as interventions to treat status epilepticus.
Interventions for the treatment of status epilepticus cover a range of drugs, some of which may be safe, while others may be associated with serious adverse events. As status epilepticus progresses sequentially through premonitory, early, established and refractory stages, accordingly, treatment starts with benzodiazepines but may, in appropriate situations, require anaesthetic agents. The need to terminate the status requires the use of such interventions that enter the brain rapidly, and have a long half‐life to prevent recurrence of seizures. Diazepam, lorazepam and midazolam, being lipid soluble, enter the brain rapidly, but diazepam is rapidly taken up by fatty tissues and hence has a shorter redistribution half‐life (one hour) compared to half‐lives of midazolam (two hours) and lorazepam (14 hours). As a result, duration of action of diazepam is only 0.25 to 0.5 hour, whereas for lorazepam it is 12 to 24 hours. For long‐term maintenance therapy, drugs that can be administered in both parenteral and oral routes are required, such as sodium valproate, phenytoin, phenobarbitone etc.
How the intervention might work
The mechanism of action varies from one drug to another. Benzodiazepines (diazepam, lorazepam, midazolam) mainly potentiate GABA induced chloride influx. The most important mechanism for phenobarbitone is GABA receptor mediated synaptic inhibition. Phenytoin has a stabiliSing influence on the neuronal membrane through prolongation of the inactivated state of voltage sensitive neuronal sodium channel. Sodium valproate acts through multiple mechanisms: frequency dependent prolongation of sodium channel inactivation, attenuation of calcium mediated transient currents, augmentation of GABA. Benzodiazepines have a relatively short duration of action, and hence for maintenance of action, phenytoin, sodium valproate, or phenobarbitone are used or added to benzodiazepines.
Why it is important to do this review
Status epilepticus is a medical emergency with a significant mortality, but like many acute medical emergencies it is a challenging topic for randomised controlled trials. A regularly updated systematic review is required to summarise current evidence in order to inform both treatment guidelines and the research agenda.
Objectives
The primary objective of the review was to synthesise the available evidence from randomised controlled trials (RCTs):
to determine whether a particular anticonvulsant is more effective or safer in controlling status epilepticus compared with another drug or placebo;
to delineate reasons for disagreements regarding recommended treatment and highlight areas for future research.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials using a truly random or quasi‐random allocation of treatment were included in this review if they included people with premonitory, early, established or refractory status epilepticus (see below for definitions).
Types of participants
For a study to be included in the review, study participants were required to have premonitory or early stage status epilepticus or established status epilepticus. The premonitory phase was referred to as the period during which seizures became increasingly frequent or severe but the condition did not meet the definition of status epilepticus. Early status was defined as the first 30 minutes of seizure activity. Established status epilepticus was defined as a condition with either more than 30 minutes of continuous seizure activity or two or more sequential seizures without recovery of full consciousness between the seizures. If seizure activity remains uncontrolled for one to two hours, in spite of first‐line treatment, then the participant is considered to be in refractory status epilepticus (Shorvon 2001).
Both convulsive as well as non‐convulsive status epilepticus were considered for this review.
Types of interventions
Studies comparing any anticonvulsant drug against placebo or another anticonvulsant drug were included in the review. For the premonitory stage of status epilepticus, interventions included diazepam (intravenous or intrarectal); lorazepam (intravenous); paraldehyde (intramuscular or intrarectal) or midazolam (intravenous, intramuscular or intrarectal). For early or established status epilepticus, drug interventions included lorazepam; diazepam; phenytoin; fosphenytoin; lignocaine; paraldehyde or clonazepam. For refractory status epilepticus, thiopentone; propofol; pentobarbitone; isoflurane or etomidate were included.
Types of outcome measures
The outcomes considered depended on the stage of status epilepticus at which the treatment was tested. For the purpose of this review, our intention was to analyse treatment failure as the primary outcome. We intended to define treatment failure for the various stages as any of the following.
For premonitory stage
Development of status epilepticus or death.
For early or established status epilepticus
Death.
Continuation of status epilepticus requiring use of a different drug or general anaesthesia for control.
Long‐term disabling sequelae, defined as persistent neurological deficits severe enough to require dependence on some other person for activities of daily living (walking, toileting, bathing, dressing and eating) at six months after randomisation.
Need for ventilatory support.
Incomplete recovery before discharge, defined as inability to attain pre‐status epilepticus state at the time of discharge. This outcome was included because most people attain their pre‐status epilepticus state before discharge and are not followed up subsequently. People who do not recover need to be followed up in order to judge recovery or persistent deficit.
We used the term 'non‐cessation of seizures' rather than cessation of seizures (used in the various studies) to maintain uniformity since all the other outcomes were unfavourable outcomes. Some of the studies described data on continuation of status epilepticus requiring a different drug or general anaesthesia separately, hence this has been taken as a separate outcome for analysis.
For refractory status epilepticus
Death.
Long‐term sequelae defined as dependence for activities of daily living (walking, toileting, bathing, dressing and eating) assessed at six months or beyond, but if studies reported only one or three months after onset, this was treated as a long‐term outcome.
Other outcomes which were considered for separate analyses in this review
Complications such as infections; renal failure; respiratory failure.
-
Adverse effects of drugs. The following were considered in the safety analysis:
hypotension, defined as a systolic blood pressure below 90 mm Hg recorded while the drug was being administered or within 24 hours of the last dose;
respiratory depression, defined as the occurrence of apnoea or need for intubation;
cardiac arrest (diagnosed clinically) or bradyarrhythmias including heart block, documented on an electrocardiogram.
We intended to analyse outcomes separately and as a composite outcome (treatment failure) because the latter would have allowed more power. This was not possible because many participants had more than one outcome measured during the course of treatment and the studies presented the number of outcomes observed but not the number of participants having one or more of these outcomes. We also considered an analysis of the number of outcomes as a continuous variable but the studies did not present standard deviations for these data and, therefore, did not permit meta‐analysis.
Search methods for identification of studies
This search was run for the original review in July 2005 and subsequent searches have been run in January 2010, January 2011, February 2012, and August 2013. For the initial review, the following databases were searched:
(1) Cochrane Epilepsy Group Specialized Register (December 2009); (2) Cochrane Central Database of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4) using the strategy outlined in Appendix 2; (3) MEDLINE (1950 to December week 4, 2009) using the strategy outlined in Appendix 3; In addition, we searched EMBASE (1966 ‐ January 2003) for the original version of this review, but we no longer have access to this database.
For the most recent update of this review, we searched the following electronic databases for published trials:
(1) Cochrane Epilepsy Group Specialized Register (15 August 2013) using the strategy outlined in Appendix 1; (2) Cochrane Central Database of Controlled Trials (CENTRAL) ,The Cochrane Library July 2013, Issue 7) using the strategy outlined in Appendix 2; (3) MEDLINE (Ovid, 1946 to 15/08/2013) using the strategy outlined in Appendix 3.
We did not apply any language restrictions to our search.
All resulting titles and abstracts were scanned and any relevant articles were followed up.
Data collection and analysis
Two review authors (MP, PRK) independently selected the trials to be included in the review. Any disagreements were resolved by seeking an independent opinion of the third review author (KA‐R).
The methodological quality of each trial was assessed by two review authors (MP, PRK). The following criteria were included: randomisation method; baseline comparability of the trial arms; blinding; and whether the published data permitted an intention‐to‐treat analysis. Data were independently extracted by two review authors and cross‐checked. Data on the number of participants with each outcome event, by allocated treatment group, were sought to allow an intention‐to‐treat analysis.
We assessed the risk of bias as low, high or unclear risk of bias. We evaluated the following characteristics using the Cochrane 'Risk of bias' tool.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
The trials comparing the same drugs were combined, whereas those comparing different drugs were analysed separately.
Our intention was to carry out our primary analysis on the risk of treatment failure. However, it was not possible to extract data on this outcome because the same participants were probably counted more than once in the various outcomes presented in the studies. Clearly some individuals had more than one of the above outcomes. For example, a person might require anaesthesia and ventilation then recover incompletely and may have long‐term disabling sequelae. Our intention was to carry out separate analyses for premonitory stage, early status epilepticus, established status epilepticus and refractory status epilepticus. However, the definitions used in the different studies were both variable (seeTable 4) and often unclear.
1. Definitions of status epilepticus followed in various studies.
| Study | Definition |
| Alldredge 2001 | Continuous or repeated seizure activity > 5 minutes without recovery of consciousness. |
| Leppik 1983 (mixed) | (a) Generalised tonic‐clonic status: three or more generalised tonic‐clonic seizures in one hour; two or more generalised seizures in rapid succession without recovery of consciousness; (b) absence status: confusional state with generalised 3 Hz spike wave pattern on EEG; (c) complex partial status: confusional state, clinical seizure or both with focal EEG abnormality; (d) elementary status: partial seizures without loss of consciousness. |
| Pellock 1998 (premonitory) | Acute repetitive seizures (no definition). |
| Remy 1991 (mixed) | Successive partial seizures for at least 20 minutes or two generalised tonic‐clonic seizures within 20 minutes. |
| Shaner 1988 (established/mixed) | Generalised convulsive status epilepticus: (a) a history of 30 minutes of continuous generalised convulsive seizures and witnessed generalised seizures in the emergency room; (b) a history of 30 minutes of recurrent generalised convulsive seizures but failure to attain baseline mental status between seizures and witnessed generalised seizures in the emergency room; (c) a history of 3 or more generalised convulsive seizures in one hour in patients with obtundation prior to the onset of status epilepticus and witnessed generalised convulsive seizures in the emergency room; (d) uncertain history of seizures but generalised convulsive seizures continuously for more than 5 minutes as witnessed in the emergency room. |
| Singhi 2002 (refractory) | Motor seizures uncontrolled after 2 doses of diazepam and phenytoin infusion. |
| McCormick 1999 (unclear) | No definition. |
| Chamberlain 1997 (premonitory) | Motor seizures of at least 10 minutes duration. |
| Appleton 1995 (unclear) | No definition. |
| Treiman 1998 (premonitory) | Overt generalised convulsive status: two or more generalised convulsions or continuous convulsive activity > 10 minutes. |
| Cereghino 2002 (premonitory) | Multiple seizures of complex partial or generalised type within an observation period between 12 ‐ 24 hours. |
| Misra 2011 | Two or more convulsive seizures without full recovery of consciousness between the seizures or continuous convulsions lasting for more than five minutes. |
| Mehta 2007 | 30 minutes of continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures. |
| Chen 2011 | More than five minutes of continuous seizures or two or more discrete seizures between which there is incomplete recovery of consciousness. |
| Agarwal 2007 | Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. |
| Ahmad 2006 | Generalized convulsions continuing for minimum of five minutes. |
| Rossetti 2011 | Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. |
| Silbergleit 2012 | Continuous convulsions for longer than five minutes or having convulsions at the time of treatment after having intermittent seizures without regaining consciousness for longer than five minutes. |
| Sreenath 2010 | Continuous convulsive activity lasting for 5 minutes or more. |
Potential causes of heterogeneity were assessed by examining differences between trials in respect to trial design; participant population; intervention and outcome. We tested for heterogeneity between trial results for each outcome using a chi squared (Chi2) test. If the test for heterogeneity was statistically non‐significant, then the results from the different trials were combined to obtain a summary estimate of effect (and the corresponding confidence interval (CI)) using a fixed‐effect model. We used risk ratio (RR) as the measure of choice for our analyses, but for some outcomes there were zero events in all the arms of some studies. In such situations we used risk difference (RD) to ensure inclusion of the data in our meta‐analysis. Using RR would exclude such studies from analysis because confidence intervals cannot be calculated around RR when there is a zero event in both the arms of the study.
Assessment of statistical heterogeneity was supplemented using the I2 statistic, which provides an estimate of the percentage of variability due to heterogeneity rather than a sampling error.
Interpretation of I2 for heterogeneity is as follows.
0% to 40%: may not be important.
30% to 60%: represents moderate heterogeneity.
50% to 90%: represents substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
Results
Description of studies
Results of the search
In the original review, the search yielded 28 studies that could potentially be included. After close scrutiny, 10 studies met the inclusion criteria, and the remaining 18 were excluded. For the current update, the search yielded 27 studies that could potentially be included. 17 out of these 27 studies were excluded after screening the abstracts. The full texts of the remaining 10 studies were screened and out of those, eight met the inclusion criteria.The current update includes eight new studies (Agarwal 2007; Ahmad 2006; Chen 2011; Mehta 2007; Misra 2011; Rossetti 2011; Silbergleit 2012; Sreenath 2010).
Included studies
Eighteen studies are included with a total of 2755 participants. Of the 18 studies included in this review, five studied participants with premonitory status (Alldredge 2001; Cereghino 2002; Chamberlain 1997; Pellock 1998; Treiman 1998), one established (Shaner 1988), three refractory (Agarwal 2007; Mehta 2007; Singhi 2002), and one mixed status epilepticus (Leppik 1983). Two studies did not clearly define the status (Appleton 1995; McCormick 1999). This made it difficult to analyse studies according to type of status epilepticus. Ten studies included only adults (Alldredge 2001; Cereghino 2002; Leppik 1983; Pellock 1998; Shaner 1988; Treiman 1998; Chen 2011;Misra 2011; Rossetti 2011), and six only children (Appleton 1995; Chamberlain 1997; McCormick 1999; Singhi 2002;Ahmad 2006;Sreenath 2010). Two studies included both adults and children (Agarwal 2007; Silbergleit 2012) The type of status epilepticus included varied from study to study: twelve generalised tonic‐clonic (Alldredge 2001; Appleton 1995; Shaner 1988; Treiman 1998;Agarwal 2007; Ahmad 2006; Chen 2011;Mehta 2007; Misra 2011; Rossetti 2011; Silbergleit 2012; Sreenath 2010); and three mixed (Cereghino 2002; Leppik 1983; Singhi 2002). Three studies (Chamberlain 1997; McCormick 1999; Pellock 1998) did not describe the type of status epilepticus. Time‐since‐onset of status epilepticus also was variable, from minutes to hours in the different studies.
Three studies had more than two arms: (four arms in two studies (Appleton 1995; Treiman 1998) and three in one (Alldredge 2001)). There was difficulty in data extraction in one of these (Appleton 1995), so that only two arms were included in our analysis. Three studies had placebo arms (Alldredge 2001; Cereghino 2002; Pellock 1998) all in premonitory status epilepticus, described by two authors as acute repetitive seizures (Cereghino 2002; Pellock 1998). Two studies compared intrarectal diazepam gel with placebo (Cereghino 2002; Pellock 1998); and one study had lorazepam, diazepam, and placebo arms (Alldredge 2001), with the interventions all administered intravenously. Eight studies compared two or more active drugs. All studies except three (two intrarectal and one intramuscular (IM) midazolam in one arm) used intravenous (IV) administration of drugs. Twenty different comparisons were available but only five (lorazepam versus diazepam, both administered intravenously; lorazepam versus diazepam plus phenytoin; diazepam plus phenytoin versus phenobarbitone, administered intravenously; diazepam intrarectal gel versus placebo gel, valproate versus diazepam intravenously) included more than one study to permit a meta‐analysis. In three studies (Alldredge 2001; Cereghino 2002; Pellock 1998) drugs were administered in the prehospital phase.
All participants were followed up only during their hospital stay. No study had post‐discharge follow‐up. All studies had cessation of status epilepticus and adverse effects as outcomes. Death was an outcome in five comparisons. Other outcomes studied were requirement for ventilatory support (seven comparisons) and continuation of status epilepticus requiring another drug or general anaesthesia (five comparisons).
Excluded studies
Eleven studies are now excluded from the review. The reasons for exclusion were: poor data presentation (three studies), publication of only the study protocol (one study), a non‐randomised comparative clinical trial (one study), a case study with only one participant (one study), and participants not having status epilepticus (one study), poor randomisation method (one study), comparing two doses of the same drug (one study) (for details seeExcluded studies).
Risk of bias in included studies
Six studies (Alldredge 2001; Cereghino 2002; Leppik 1983; Pellock 1998; Silbergleit 2012; Treiman 1998) used a similar‐looking placebo or comparison drug. With similar‐looking interventions and blinding the recruiting person and the person administering the intervention should not know the group to which the next participant is going to be assigned. Thus, the similarity in appearance concealed the allocation in these studies. In addition, three studies (Rossetti 2011; Shaner 1988; Sreenath 2010) used sealed envelopes to conceal allocation in the randomisation process but whether the envelopes were opaque and serially numbered is unclear from the study reports. The rest of the studies (Agarwal 2007; Appleton 1995; Chamberlain 1997; Chen 2011; McCormick 1999; Mehta 2007; Misra 2011) did not mention any attempt to conceal randomisation. Studies with a similar‐looking placebo or comparison drug were assumed to be blinded but nine studies (Ahmad 2006; Appleton 1995; Chen 2011; Mehta 2007; Misra 2011; Rossetti 2011; Shaner 1988; Singhi 2002; Sreenath 2010) did not have blinding of carers or outcome assessors. The follow‐up was restricted to the period of the hospital admissions in all the studies.
Three studies (Appleton 1995; Leppik 1983; Shaner 1988) did not report the number of participants having the events; rather they counted the number of events in the arms. This made it difficult to extract data from these studies for meta‐analysis.
Please refer to Figure 1 and Figure 2.
1.
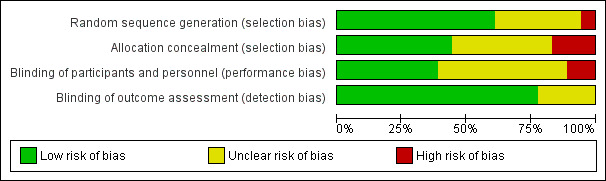
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
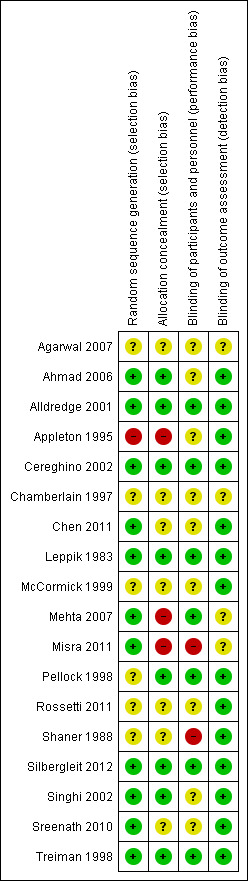
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3
All disagreements were resolved through discussion. Poor data presentation encountered included studies not mentioning the total number of participants studied, or presenting only the number of outcomes observed rather than the number of participants with the outcomes.
The data extraction was difficult because of heterogeneity in the definition of status epilepticus (seeTable 4) and the type of data presented. Very few studies used the same interventions. We could combine data from eleven studies over five different outcomes. Even here, the definitions used by different authors varied and we assumed that the type of participants were similar. We presented the rest of the studies separately.
The results are presented according to the comparisons used.
Comparison 1: Lorazepam IV versus diazepam IV
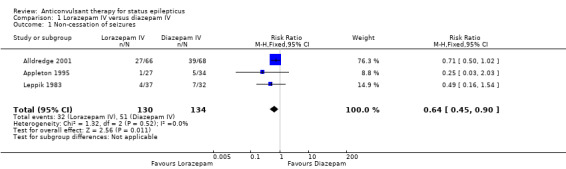
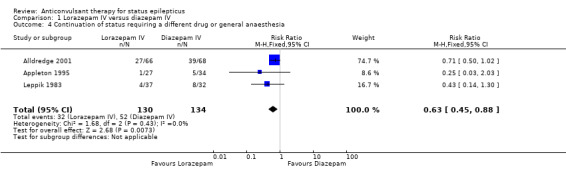
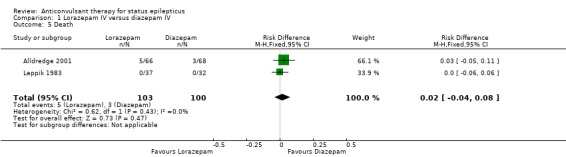
There were three studies with 289 participants (Alldredge 2001; Appleton 1995; Leppik 1983). The outcome of death was available in two studies (203 participants). There was no statistically significant difference in deaths between the two groups (5/103 versus 3/100 participants; risk difference (RD) 0.02, 95% confidence interval (CI) ‐0.04 to 0.08). Compared with diazepam, lorazepam had statistically significant lower risk of non‐cessation of seizures (32/130 versus 51/134 participants; risk ratio (RR) 0.64, 95% CI 0.45 to 0.90) and of continuation of status epilepticus requiring a different drug or general anaesthesia (32/130 participants versus 52/134; RR 0.63, 95% CI 0.45 to 0.88).
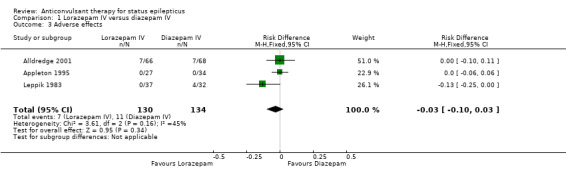
There was a statistically non‐significant trend favouring lorazepam for reducing requirement for ventilatory support (12/130 versus 17/134 participants; RR 0.73, 95% CI 0.36 to 1.49) and adverse effects (7/130 versus 11/134 participants; RD ‐0.03, 95% CI ‐0.10 to 0.03).
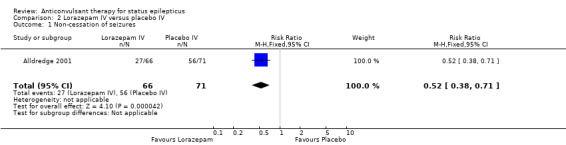
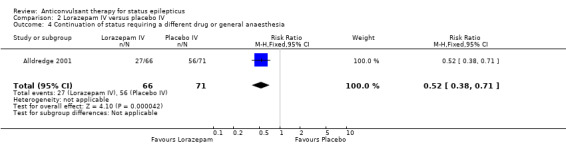
Comparison 2: Lorazepam IV versus placebo IV
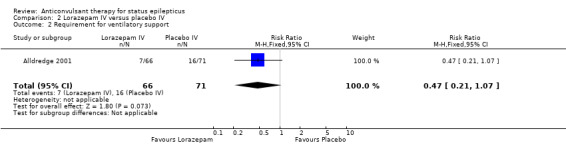
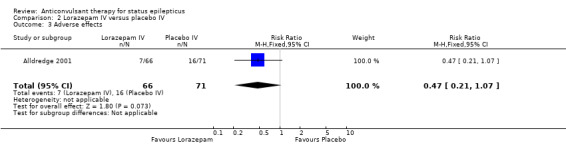
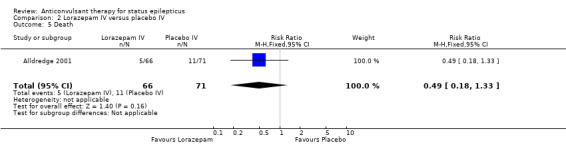
There was one study (Alldredge 2001) with 137 participants. Compared with placebo, lorazepam had a statistically significant lower risk of non‐cessation of seizures (27/66 versus 56/71 participants; RR 0.52, 95% CI 0.38 to 0.71) and of continuation of status epilepticus requiring a different drug or general anaesthesia (27/66 versus 56/71 participants; RR 0.52, 95% CI 0.38 to 0.71). There was a statistically non‐significant but strong trend favouring lorazepam for the following outcomes: death (5/66 versus 11/71 participants; RR 0.49, 95% CI 0.18 to 1.33); requirement for ventilatory support (7/66 versus 16/71 participants; RR 0.47, 95% CI 0.21 to 1.07) and adverse effects (7/66 versus 16/71 participants; RR 0.47, 95% CI 0.21 to 1.07).
Comparison 3: Lorazepam IV versus diazepam plus phenytoin IV
There were two studies (Sreenath 2010,Treiman 1998) with 370 participants. The outcome of non‐cessation of seizures and adverse effects were available in both studies. There was a statistically non‐significant trend favouring lorazepam for both non‐cessation of seizures (34/187 versus 42/183 participants; RD ‐0.04, CI ‐0.35 to 0.26) and adverse effects (46/187 versus 53/183 participants; RR 0.85, 95% CI 0.63 to 1.15). The outcome of death and requirement of ventilatory support were available in one study. There was no statistically significant difference for the outcome of death (0/88 versus 0/90 participants and requirement of ventilatory support (0/88 versus 0/90).
Comparison 4: Lorazepam IV versus phenobarbitone IV
A single study (Treiman 1998) with 188 participants showed no statistically significant difference in the risk of non‐cessation of seizures (34/97 versus 38/91 participants; RR 0.84, 95% CI 0.58 to 1.21) or adverse effects (42/97 versus 46/91 participants; RR 0.86, 95% CI 0.63 to 1.16) between the two drugs.
Comparison 5: Lorazepam IV versus phenytoin IV
There was only one study (Treiman 1998) with 198 participants. Risk of non‐cessation of seizures was less with lorazepam compared with phenytoin (34/97 versus 57/101, RR 0.62, 95% CI 0.45 to 0.86). There was no statistically significant difference between the two groups regarding adverse effects (42/97 versus 44/101, RR 0.99, 95% CI 0.72 to 1.37).
Comparison 6: Midazolam IV versus lorazepam IV
There was a single small study (McCormick 1999) with 27 participants. This study reported a statistically non‐significant trend favouring midazolam regarding the following outcomes: non‐cessation of seizures (1/15 versus 4/12 participants; RR 0.20, 95% CI 0.03 to 1.56); requirement for ventilatory support (1/15 versus 2/12 participants; RR 0.40, 95% CI 0.04 to 3.90) and adverse effects (1/15 versus 2/12 participants; RR 0.40, 95% CI 0.04 to 3.90) and continuation of status epilepticus requiring a different drug or general anaesthesia (1/15 versus 4/12 participants; RR 0.20, 95% CI 0.03 to 1.56).
Comparison 7: Midazolam IV versus diazepam IV
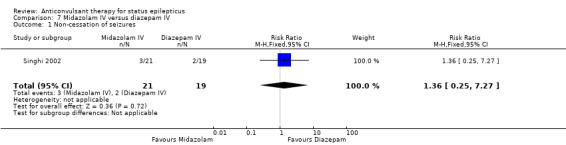
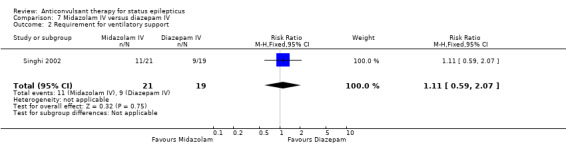
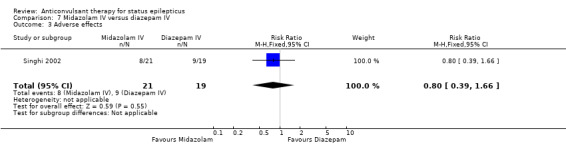
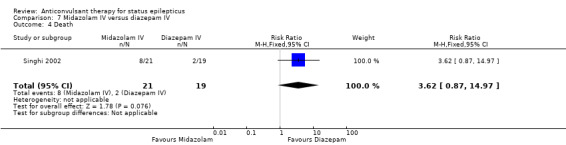
There was a single study (Singhi 2002) with 40 participants. There was no statistically significant difference between the two groups regarding the following outcomes: non‐cessation of seizures (3/21 versus 2/19 participants; RR 1.36, 95% CI 0.25 to 7.27); requirement for ventilatory support (11/21 versus 9/19 participants; RR 1.11, 95% CI 0.59 to 2.07) and adverse effects (8/21 versus 9/19 participants; RR 0.80, 95% CI 0.39 to 1.66). There was a statistically non‐significant trend favouring diazepam for the outcome of death (8/21 versus 2/19 participants; RR 3.62, 95% CI 0.87 to 14.97).
Comparison 8: Midazolam IM versus diazepam IV
A small single study (Chamberlain 1997) of 24 participants showed no statistically significant difference between the two groups for the following outcomes: non‐cessation of seizures (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01); requirement for ventilatory support (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01); adverse effects (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01) and continuation of status epilepticus requiring a different drug or general anaesthesia (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01).
Comparison 9: Diazepam IV versus placebo IV
One hundred and thirty nine participants in a single study (Alldredge 2001) were analysed. Most of the outcomes significantly favoured diazepam: non‐cessation of seizures (39/68 versus 56/71 participants; RR 0.73, 95% CI 0.57 to 0.92); death (3/68 versus 11/71 participants; RR 0.28, 95% CI 0.08 to 0.98); requirement for ventilatory support (6/68 versus 16/71 participants; RR 0.39, 95% CI 0.16 to 0.94) and continuation of status requiring a different drug or general anaesthesia (39/68 versus 56/71 participants; RR 0.73, 95% CI 0.57 to 0.92). There was a non‐significant trend favouring diazepam for adverse effects (7/68 versus 16/71 participants; RR 0.46, 95% CI 0.20 to 1.04).
Comparison 10: Diazepam gel versus placebo gel
There were two studies (Cereghino 2002; Pellock 1998) with a total of 165 participants. The risk of non‐cessation of seizures was significantly less with diazepam gel compared with placebo gel (24/77 versus 63/88 participants; RR 0.43, 95% CI 0.30 to 0.62). For adverse effects there was a strong but statistically non‐significant trend towards the placebo gel (29/77 versus 22/88 participants; RR 1.50, 95% RR 0.94 to 2.37).
Comparison 11: Leviteracetam IV versus Lorazepam IV
There was one study (Misra 2011) with a total of 79 participants. Both levetiracetam and lorazepam were equally effective in aborting seizures (9/38 versus 10/41, RR 0.97, 95% CI 0.44 to 2.13).
Comparison 12: Diazepam plus phenytoin IV versus phenobarbitone IV
There were two studies (Shaner 1988; Treiman 1998) with a total of 222 participants. For the outcomes of death and requirement for ventilatory support, data were available in only one study (36 participants). There was no statistically significant difference between the two groups for the following outcomes: requirement for ventilatory support (6/18 versus 6/18 participants; RR 1.00, 95% CI 0.40 to 2.52); adverse effects (57/113 versus 55/109 participants, RR 1.00, 95% CI 0.77 to 1.30) and death (0/18 versus 0/18 participants; RD 0.00, 95% CI ‐0.10 to 0.10). For non‐cessation of seizures, the test for heterogeneity was significant and the type of status epilepticus studied was different, hence the two studies were analysed separately for this outcome. There was a weak statistically non‐significant trend favouring phenobarbitone in one of the studies (Shaner 1988) (8/18 versus 2/18 participants; RR 4.00, 95% CI 0.98 to 16.30). In the other larger study (Treiman 1998), there was no statistically significant difference between the two groups for non‐cessation of seizures (42/95 versus 38/91 participants; RR 1.06, 95% CI 0.76 to 1.47).
Comparison 13: Diazepam plus phenytoin IV versus phenobarbitone IV (premonitory status)
The was a single study (Treiman 1998) with 196 participants. There was no statistically significant difference between the two groups for cessation of seizures ( 42/95 versus 38/91 RR 1.06, 95% CI 0.76 to 1.47).
Comparison 14: Diazepam plus phenytoin IV versus phenytoin IV
There was a single study (Treiman 1998) with 196 participants. The study reported a statistically non‐significant trend favouring diazepam plus phenytoin for non‐cessation of seizures (42/95 versus 57/101 participants; RR 0.78, 95% CI 0.59 to 1.04). There was no statistically significant difference between the two groups for adverse effects (48/95 versus 44/101 participants; RR 1.16, 95% CI 0.86 to 1.56).
Comparison 15: Phenobarbitone IV versus phenytoin IV
There was a single study ( Treiman 1998) with 186 participants. There was a statistically non‐significant trend favouring phenobarbitone for non‐cessation of seizures (38/91 versus 51/95 participants; RR 0.78, 95% CI 0.57 to 1.06). There was no statistically significant difference between the two groups for adverse effects (46/91 versus 44/95 participants; RR 1.09, 95% CI 0.81 to 1.47).
Comparison 16: Lorazepam intranasal versus paraldehyde IM
There was a single study (Ahmad 2006) with 160 participants. There was no statistically significant difference between two groups for cessation of seizures (60/80 versus 49/80 participants; RR 1.22, 95% CI 0.99 to 1.52). There was no statistically significant difference between two groups for deaths (15/80 versus 13/80 participants; RR 1.15, 95% CI 0.59 to 2.27).
Comparison 17:Valproate IV versus Phenytoin IV
There was one study (Agarwal 2007) with a total of 100 participants. The study reported a statistically non‐significant trend favouring Intravenous valproate for reducing risk of non‐cessation of seizures (6/50 versus 8/50, RR 0.75, 95% CI 0.28 to 2.00).
Comparison 18: Midazolam IM versus lorazepam IV
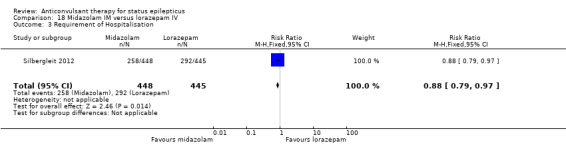
There was a single study (Silbergleit 2012) with 893 participants. The study reported a statistically significant difference favouring midazolam for cessation of seizures (329/448 versus 282/445; RR 1.16, 95% CI 1.06 to 1.27), for requirement for intensive care unit (ICU) admission (128/448 versus 161/445 participants; RR 0.79, 95% CI 0.65 to 0.96), and for requirement for hospitalisation (258/448 versus 292/445 participants; RR 0.88, 95% CI 0.79 to 0.97).There was no statistically significant difference between the two groups for following outcomes: endotracheal Intubation (63/448 versus 64/445 participants; RR 0.98, 95% CI 0.71 to 1.35); recurrence of seizures (51/448 versus 47/445 participants; RR 1.08, 95% CI 0.74 to 1.57); adverse effects (16/448 versus 18/445 participants; RR 0.88, 95% CI 0.46 to 1.71): for ICU stay (mean difference (MD) 1.60, 95% CI ‐0.23 to 3.43, P =0.09); and length of hospital stay (MD 1.20, 95% CI ‐0.24 to 2.64, P =0.10).
Comparison 19: Valproate IV versus diazepam IV infusion
There were two studies (Chen 2011; Mehta 2007) with 106 participants. There was a statistically non‐significant trend favouring diazepam for non‐cessation of seizures (19/50 versus 19/56 participants; RR 1.16, 95% CI 0.71 to 1.89). There was a statistically significant difference between the two groups for the adverse effect of hypotension (0/50 versus 12/56, RR 0.08, 95% CI 0.01 to 0.58)
Comparison 20: Propofol IVversus barbiturates IV
There was a single study (Rossetti 2011) with 23 participants. There was no statistically significant difference between the two groups in any of the following outcomes: refractory status epilepticus (RSE) controlled with first course of drug (6/14 versus 2/9 participants; RR 1.93, 95% CI 0.49 to 7.54); RSE treated subsequently (4/8 versus 5/7 participants; RR 0.70, 95% CI 0.30 to 1.62); thrombotic/embolic complication (0/14 versus 0/9 participants); mortality (6/14 versus 3/9 participants; RR 1.29, 95% CI 0.43 to 3.88); functional outcome at three weeks (5/14 versus 3/9 participants; RR 1.07, 95% CI 0.34 to 3.42); infection requiring antibiotics (7/14 versus 6/9 participants; RR 0.75, 95% CI 0.37 to 1.51).
Discussion
Our review demonstrated that there are few reported randomised studies on drug interventions used in status epilepticus. Considering that the condition is relatively frequent in neurological practice, it is surprising that we found only 18 studies with analysable data. For status epilepticus, carrying out randomised controlled trials (RCTs) in the emergency situation may be difficult; particularly when the patient is unconscious, which makes getting rapid consent to join a trial difficult. This review highlights the need to conduct more randomised studies in status epilepticus.
The review has demonstrated several areas requiring attention in future research in status epilepticus. A universally acceptable definition of premonitory, early, established and refractory status needs to be agreed upon and used consistently by investigators. Agreement on the definition of outcomes and method of data presentation is also desirable to facilitate meta‐analysis. In particular, reports should provide the number of participants having each outcome and the denominator in analyses should be the number of participants rather than the number of episodes of status epilepticus.
The time frame required of the administration of interventions would be an important element for study/comparison in the future. A table has been added depicting the time since onset of status to administration of drug and since administration of drug to cessation of seizures as reported for drugs in respective studies (Table 5).
2. Time elapsed.
| Lorazepam lV | Diazepam‐phenytoin IV | Diazepam IV | Paraldehyde IM | Valproate IV | Levetiracetam IV | Midazolam IV infusion | Midazolam IM | |
| Time since onset of status to administration of drug (min) | 30 (10‐441) (Mehta 2007) | 120 (35.5‐252) (Ahmad 2006) | 30 (5.5‐147) (Mehta 2007) | |||||
| 31.3 (16.8‐45.8) (Alldredge 2001) | ||||||||
| Time since administration of drug to seizure cessation (min) | 0.3 (0.25‐0.38) (Sreenath 2010) | 0.3 (0.255‐0.4) (Sreenath 2010) | 15.8 (2.8‐28.8) (Singhi 2002) (for RSE) | 8 (5‐21) (Ahmad 2006) | ‐ | ‐ | 15.9 (6.3‐25.5) (Singhi 2002) (for RSE) | 3.3 (Silbergleit 2012) |
| 7.5 (4.5‐11.5) (Ahmad 2006) | ||||||||
| 1.6 (Silbergleit 2012) |
IM: intramuscular injection IV: administered intravenously RSE: refractory status epilepticus
Summary of main results
Even with limited data, the results may be summarised as follows.
Diazepam was better than placebo for cessation of seizures: there was a lower risk of requirement for ventilatory support and continuation of status epilepticus requiring a different drug or general anaesthesia with diazepam.
Lorazepam was better than placebo for cessation of seizures and carried a lower risk for continuation of status epilepticus requiring a different drug or general anaesthesia.
Lorazepam was better than diazepam for cessation of seizures and had a lower risk for continuation of status epilepticus requiring a different drug or general anaesthesia.
Lorazepam was better than phenytoin for cessation of seizures.
5. For pre hospital treatment, midazolam IM was as effective and probably better than lorazepam IV for cessation of seizures, frequency of hospitalisation and ICU admissions but not for risk of recurrence of seizures.
No definitive conclusions were possible with other comparisons. However, statistically non‐significant trends were found for the following.
Lorazepam IV versus diazepam IV ‐ trend favouring lorazepam for ventilatory support and adverse effects.
Lorazepam IV versus placebo ‐ trend favouring lorazepam for death, requirement for ventilatory support and adverse effects.
Lorazepam IV versus diazepam plus phenytoin IV ‐ trend favouring lorazepam for cessation of seizures and adverse effects.
Midazolam versus lorazepam IV ‐ trend favouring midazolam for cessation of seizures, need for ventilatory support, adverse effects and continuation of status epilepticus requiring a different drug or general anaesthesia.
Midazolam versus diazepam IV ‐ trend favouring diazepam for reduced number of deaths.
Diazepam versus placebo gel ‐ trend towards fewer adverse effects with placebo gel.
Diazepam plus phenytoin versus phenytoin IV ‐ trend favouring diazepam plus phenytoin for cessation of seizures.
Phenobarbitone versus phenytoin IV ‐ trend favouring phenobarbitone for cessation of seizures.
Overall completeness and applicability of evidence
Considering the frequency and seriousness of the condition, the body of randomised evidence available to guide clinical decisions is small. The only clinically applicable conclusions appear to favour lorazepam or midazolam as initial treatment of status epilepticus. Of these, adequate evidence is available only in favour of midazolam IM. However, this result is applicable only for pre hospital treatment. Once a patient has reached hospital, the intravenous (IV) route is the preferred route, for which evidence seems to favour lorazepam.
Quality of the evidence
The quality of evidence is not strong, but appears acceptable.
Potential biases in the review process
Several criteria to assess risk of bias could not be assessed because of unclear reporting by the authors. Attempts to contact the authors for clarification did not succeed. This may have underestimated/overestimated the risk of bias in the studies.
Agreements and disagreements with other studies or reviews
Seperate systematic reviews for drugs like levetiracetam have appeared in literature but their results lack precision because of the small number of studies and events.
Authors' conclusions
Implications for practice.
Lorazepam is better than diazepam or phenytoin for cessation of seizures and carries a lower risk of continuation of status epilepticus that requires use of a different drug or general anaesthesia. Both lorazepam and diazepam (IV or gel form) are better than placebo for the same outcomes. For pre hospital treatment, midazolam IM seemed more effective than lorazepam IV for cessation of seizures, frequency of hospitalisation and ICU admissions. However, it was unclear whether the risk of recurrence of seizures differed between treatments. The results of other comparisons of anticonvulsant therapies versus each other were also uncertain.
Implications for research.
The review has demonstrated several areas requiring attention in future research in the treatment of status epilepticus. Investigators need to develop and use universally acceptable definitions of premonitory, early, established and refractory status epilepticus. Agreement on the definition of outcomes and method of data presentation is also desirable to facilitate meta‐analysis. In particular, investigators should report the number of participants having each outcome, with one participant included only once in a study. Where counts of seizure episodes are presented, then the standard deviation of the counts should be provided to facilitate meta‐analysis.
A practical difficulty in conducting RCTs in status epilepticus is obtaining consent, because participants are unconscious or not in a state to provide it. Taking consent from next of kin is one option. Such an approach has been used in RCTs with participants experiencing head injury and stroke.
What's new
| Date | Event | Description |
|---|---|---|
| 15 August 2013 | New search has been performed | Searches updated. |
| 15 August 2013 | New citation required but conclusions have not changed | Eight new studies have been added. Conclusions remain the same. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 23 February 2012 | New search has been performed | Searches updated 23 February 2012. |
| 16 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the contribution of Dr Kameshwar Prasad, who led through all the stages of the first version of the review.
We thank the All India Institute of Medical Sciences, New Delhi, India and the Arabian Gulf University, Bahrain for the support extended to them in the preparation of this review.
We acknowledge the support and advice from Sarah Nolan (statistician for the Cochrane Epilepsy Group).
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 diazepam or lorazepam or paraldehyde or midazolam or phenytoin or fosphenytoin or lignocaine or clonazepam or thiopentone or propofol or pentobarbitone or isoflurane or etomidate
#2 MeSH DESCRIPTOR Anticonvulsants Explode All WITH AD AE AG AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR
#3 #1 OR #2
#4 MeSH DESCRIPTOR Status Epilepticus Explode All WITH BL CF CI CL CO CN DI DH DT EC EM EN EP EH ET GE HI IM ME MI MO NU PS PA PP PC PX RA RI RT RH SU TH US UR VE VI
#5 "status epilepticus"
#6 #4 OR #5
#7 #3 AND #6
Appendix 2. CENTRAL search strategy
#1 diazepam OR lorazepam OR paraldehyde OR midazolam
#2 phenytoin or fosphenytoin
#3 lignocaine or clonazepam
#4 thiopentone or propofol or pentobarbitone or isoflurane or etomidate
#5 MeSH descriptor Anticonvulsants explode all trees
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Status Epilepticus explode all trees
#8 (status epilepticus)
#9 (#7 OR #8)
#10 (#6 AND #9)
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2009.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 7 or 5 or 2 or 6 or 1 or 4 or 3
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp Status Epilepticus/
12. status epilepticus.tw.
13. 11 or 12
14. (diazepam or lorazepam or paraldehyde or midazolam).tw.
15. (phenytoin or fosphenytoin or lignocaine or paraldehyde or clonazepam).tw.
16. (thiopentone or propofol or pentobarbitone or isoflurane or etomidate).tw.
17. *Anticonvulsants/
18. 14 or 15 or 16 or 17
19. 10 and 13 and 18
Data and analyses
Comparison 1. Lorazepam IV versus diazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.90] |
| 2 Requirement for ventilatory support | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.49] |
| 3 Adverse effects | 3 | 264 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 4 Continuation of status requiring a different drug or general anaesthesia | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.88] |
| 5 Death | 2 | 203 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
1.1. Analysis.
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 1 Non‐cessation of seizures.
1.2. Analysis.
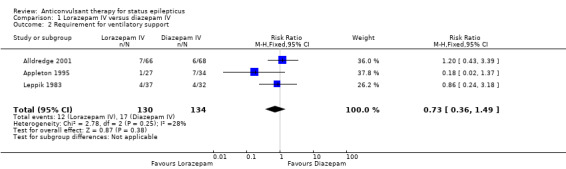
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 2 Requirement for ventilatory support.
1.3. Analysis.
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 3 Adverse effects.
1.4. Analysis.
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.
1.5. Analysis.
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 5 Death.
Comparison 2. Lorazepam IV versus placebo IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 2 Requirement for ventilatory support | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| 3 Adverse effects | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| 4 Continuation of status requiring a different drug or general anaesthesia | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 5 Death | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.33] |
2.1. Analysis.
Comparison 2 Lorazepam IV versus placebo IV, Outcome 1 Non‐cessation of seizures.
2.2. Analysis.
Comparison 2 Lorazepam IV versus placebo IV, Outcome 2 Requirement for ventilatory support.
2.3. Analysis.
Comparison 2 Lorazepam IV versus placebo IV, Outcome 3 Adverse effects.
2.4. Analysis.
Comparison 2 Lorazepam IV versus placebo IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.
2.5. Analysis.
Comparison 2 Lorazepam IV versus placebo IV, Outcome 5 Death.
Comparison 3. Lorazepam IV versus diazepam plus phenytoin IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 2 | 370 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.35, 0.26] |
| 2 Adverse effects | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3 Deaths | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Requirement of Ventilatory support | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
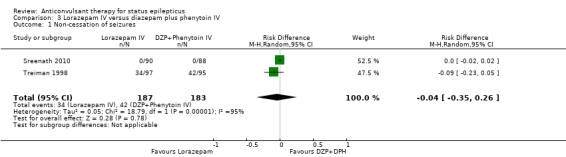
Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 1 Non‐cessation of seizures.
3.2. Analysis.
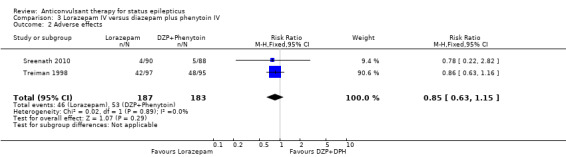
Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 2 Adverse effects.
3.3. Analysis.
Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 3 Deaths.
3.4. Analysis.
Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 4 Requirement of Ventilatory support.
Comparison 4. Lorazepam IV versus phenobarbitone IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.21] |
| 2 Adverse effects | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.16] |
4.1. Analysis.
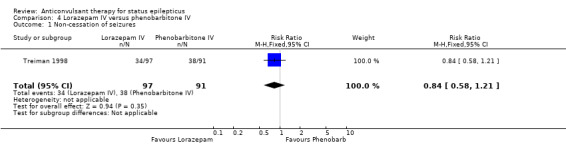
Comparison 4 Lorazepam IV versus phenobarbitone IV, Outcome 1 Non‐cessation of seizures.
4.2. Analysis.
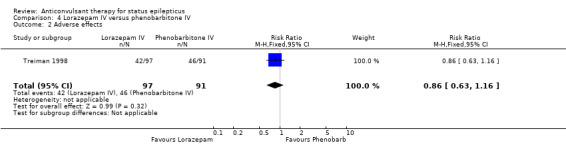
Comparison 4 Lorazepam IV versus phenobarbitone IV, Outcome 2 Adverse effects.
Comparison 5. Lorazepam IV versus phenytoin IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.86] |
| 2 Adverse effects | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.37] |
5.1. Analysis.
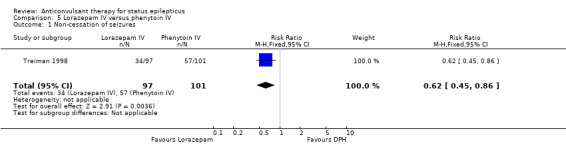
Comparison 5 Lorazepam IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.
5.2. Analysis.
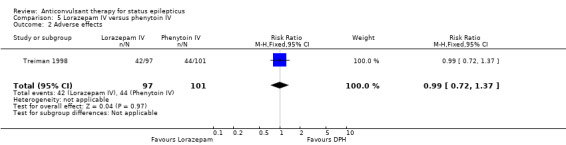
Comparison 5 Lorazepam IV versus phenytoin IV, Outcome 2 Adverse effects.
Comparison 6. Midazolam IV versus lorazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| 2 Requirement for ventilatory support | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 3.90] |
| 3 Adverse effects | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 3.90] |
| 4 Continuation of status requiring a different drug or general anaesthesia | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
6.1. Analysis.
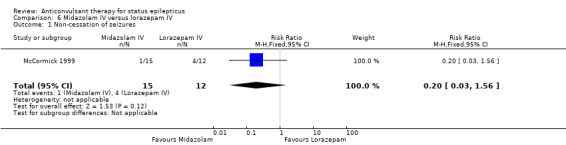
Comparison 6 Midazolam IV versus lorazepam IV, Outcome 1 Non‐cessation of seizures.
6.2. Analysis.
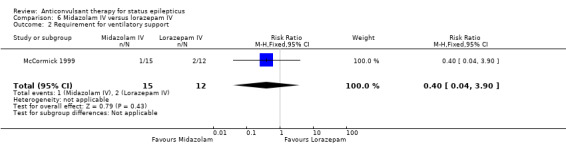
Comparison 6 Midazolam IV versus lorazepam IV, Outcome 2 Requirement for ventilatory support.
6.3. Analysis.
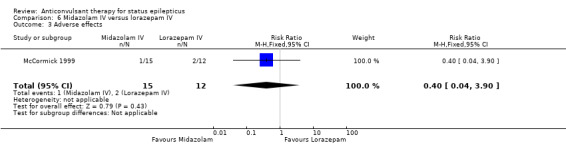
Comparison 6 Midazolam IV versus lorazepam IV, Outcome 3 Adverse effects.
6.4. Analysis.
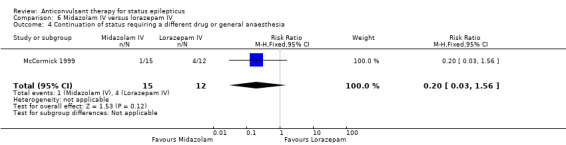
Comparison 6 Midazolam IV versus lorazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.
Comparison 7. Midazolam IV versus diazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.25, 7.27] |
| 2 Requirement for ventilatory support | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.59, 2.07] |
| 3 Adverse effects | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.39, 1.66] |
| 4 Death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [0.87, 14.97] |
7.1. Analysis.
Comparison 7 Midazolam IV versus diazepam IV, Outcome 1 Non‐cessation of seizures.
7.2. Analysis.
Comparison 7 Midazolam IV versus diazepam IV, Outcome 2 Requirement for ventilatory support.
7.3. Analysis.
Comparison 7 Midazolam IV versus diazepam IV, Outcome 3 Adverse effects.
7.4. Analysis.
Comparison 7 Midazolam IV versus diazepam IV, Outcome 4 Death.
Comparison 8. Midazolam IM versus diazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 2 Requirement for ventilatory support | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 3 Adverse effects | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4 Continuation of status requiring a different drug or general anaesthesia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
8.1. Analysis.
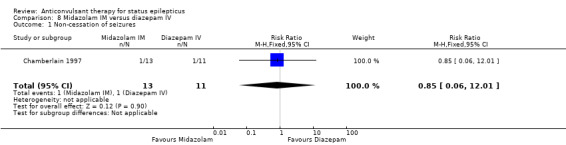
Comparison 8 Midazolam IM versus diazepam IV, Outcome 1 Non‐cessation of seizures.
8.2. Analysis.
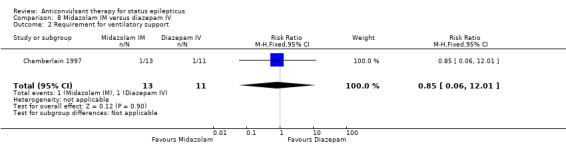
Comparison 8 Midazolam IM versus diazepam IV, Outcome 2 Requirement for ventilatory support.
8.3. Analysis.
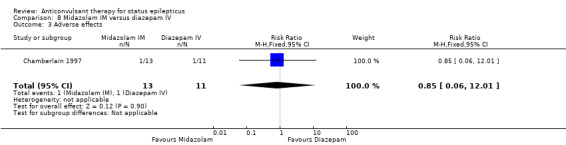
Comparison 8 Midazolam IM versus diazepam IV, Outcome 3 Adverse effects.
8.4. Analysis.
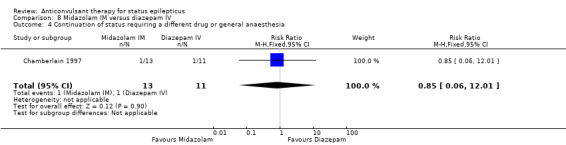
Comparison 8 Midazolam IM versus diazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.
Comparison 9. Diazepam IV versus placebo IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.92] |
| 2 Requirement for ventilatory support | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.94] |
| 3 Adverse effects | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.20, 1.04] |
| 4 Continuation of status requiring a different drug or general anaesthesia | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.92] |
| 5 Death | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
9.1. Analysis.
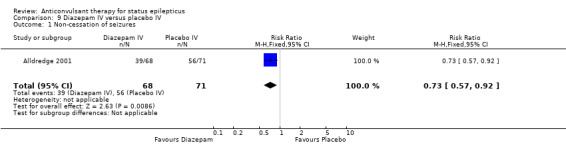
Comparison 9 Diazepam IV versus placebo IV, Outcome 1 Non‐cessation of seizures.
9.2. Analysis.
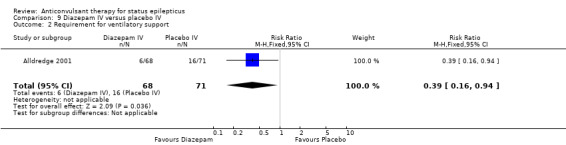
Comparison 9 Diazepam IV versus placebo IV, Outcome 2 Requirement for ventilatory support.
9.3. Analysis.
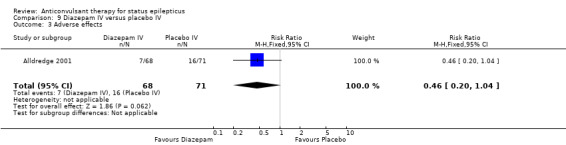
Comparison 9 Diazepam IV versus placebo IV, Outcome 3 Adverse effects.
9.4. Analysis.
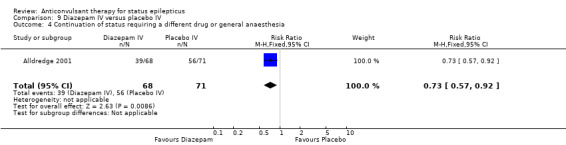
Comparison 9 Diazepam IV versus placebo IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.
9.5. Analysis.
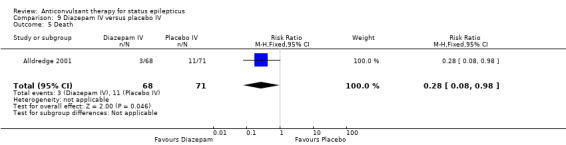
Comparison 9 Diazepam IV versus placebo IV, Outcome 5 Death.
Comparison 10. Diazepam gel versus placebo gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.62] |
| 2 Adverse effects | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.94, 2.37] |
10.1. Analysis.
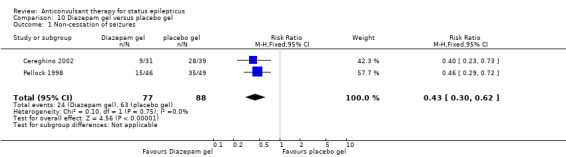
Comparison 10 Diazepam gel versus placebo gel, Outcome 1 Non‐cessation of seizures.
10.2. Analysis.
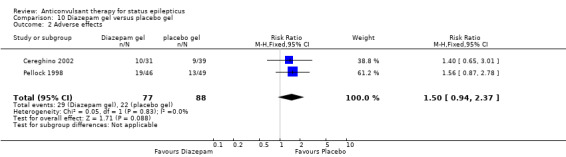
Comparison 10 Diazepam gel versus placebo gel, Outcome 2 Adverse effects.
Comparison 11. Levetiracetam versus lorazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizure | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.44, 2.13] |
| 2 Siezure recurrence at 1‐24 h | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.54] |
| 3 Hypotension | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 0.96] |
| 4 Respiratory failure | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 5 Death | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.51, 2.00] |
| 6 Ventilatory need | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.13, 0.99] |
11.1. Analysis.
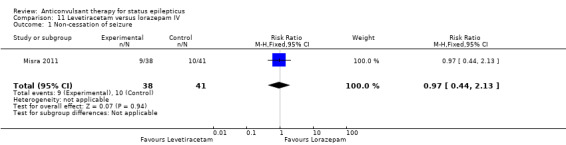
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 1 Non‐cessation of seizure.
11.2. Analysis.
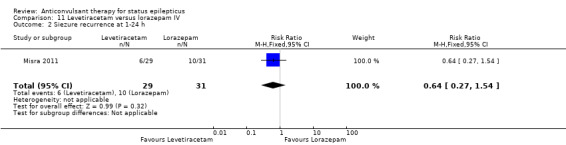
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 2 Siezure recurrence at 1‐24 h.
11.3. Analysis.
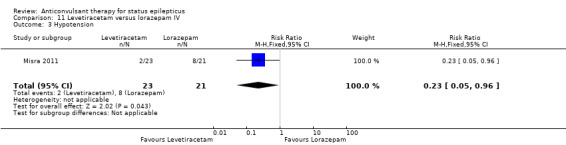
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 3 Hypotension.
11.4. Analysis.
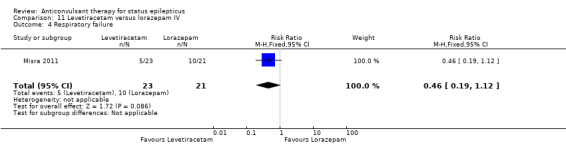
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 4 Respiratory failure.
11.5. Analysis.
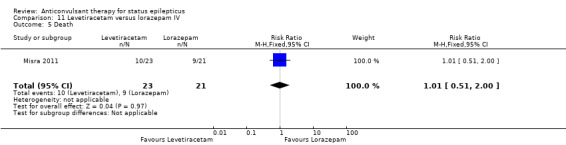
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 5 Death.
11.6. Analysis.
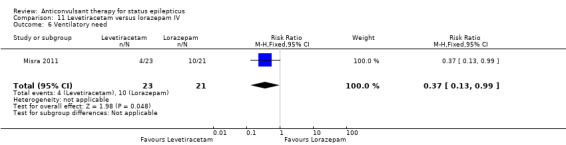
Comparison 11 Levetiracetam versus lorazepam IV, Outcome 6 Ventilatory need.
Comparison 12. Diazepam plus phenytoin IV versus phenobarbitone IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.98, 16.30] |
| 2 Adverse effects | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3 Requirement for ventilatory support | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.40, 2.52] |
| 4 Death | 1 | 36 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
12.1. Analysis.
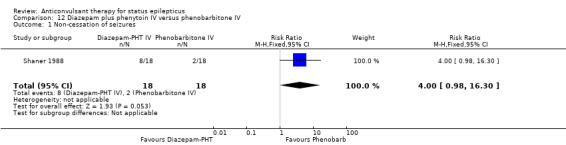
Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 1 Non‐cessation of seizures.
12.2. Analysis.
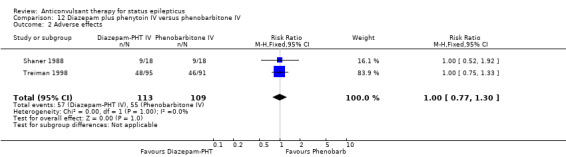
Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 2 Adverse effects.
12.3. Analysis.
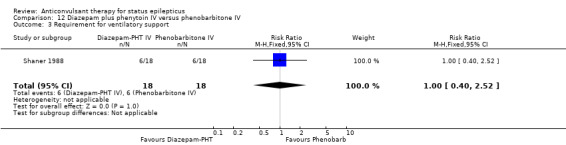
Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 3 Requirement for ventilatory support.
12.4. Analysis.
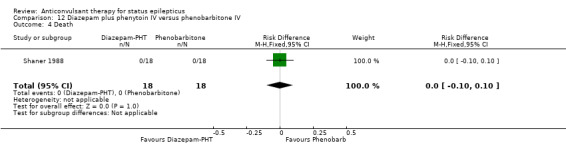
Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 4 Death.
Comparison 13. Diazepam plus phenytoin IV versus phenobarbitone IV (premonitory status).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.47] |
13.1. Analysis.
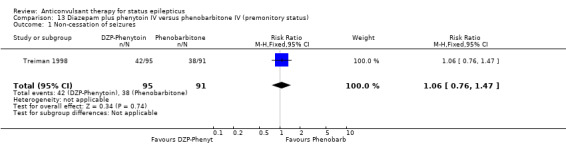
Comparison 13 Diazepam plus phenytoin IV versus phenobarbitone IV (premonitory status), Outcome 1 Non‐cessation of seizures.
Comparison 14. Diazepam plus phenytoin IV versus phenytoin IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 2 Adverse effects | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.86, 1.56] |
14.1. Analysis.
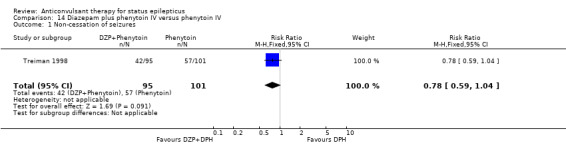
Comparison 14 Diazepam plus phenytoin IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.
14.2. Analysis.
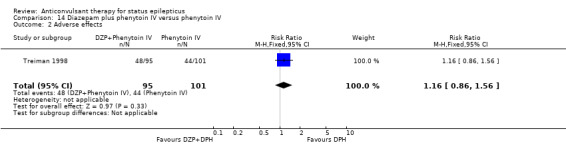
Comparison 14 Diazepam plus phenytoin IV versus phenytoin IV, Outcome 2 Adverse effects.
Comparison 15. Phenobarbitone IV versus phenytoin IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizures | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.06] |
| 2 Adverse effects | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
15.1. Analysis.
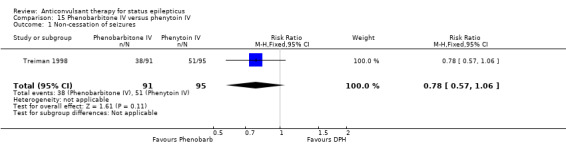
Comparison 15 Phenobarbitone IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.
15.2. Analysis.
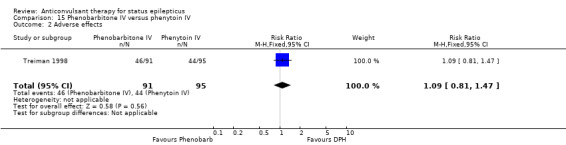
Comparison 15 Phenobarbitone IV versus phenytoin IV, Outcome 2 Adverse effects.
Comparison 16. Lorazepam intranasal versus paraldehyde IM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation of seizures | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
| 2 Deaths | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.27] |
16.1. Analysis.
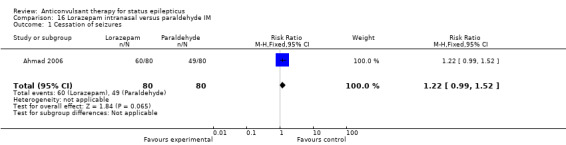
Comparison 16 Lorazepam intranasal versus paraldehyde IM, Outcome 1 Cessation of seizures.
16.2. Analysis.
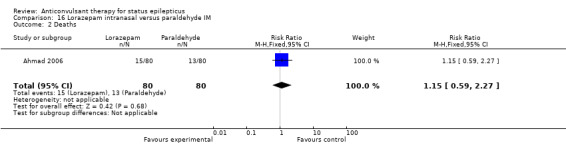
Comparison 16 Lorazepam intranasal versus paraldehyde IM, Outcome 2 Deaths.
Comparison 17. Valproate versus phenytoin IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizure | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.00] |
| 2 Adverse effects | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.16, 1.55] |
| 3 Deaths | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.78] |
17.1. Analysis.
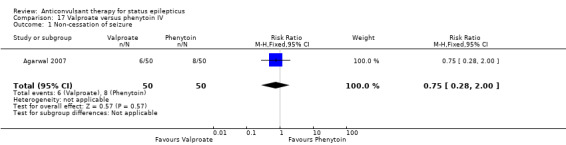
Comparison 17 Valproate versus phenytoin IV, Outcome 1 Non‐cessation of seizure.
17.2. Analysis.
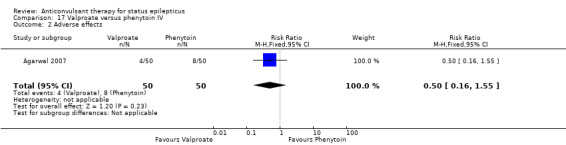
Comparison 17 Valproate versus phenytoin IV, Outcome 2 Adverse effects.
17.3. Analysis.
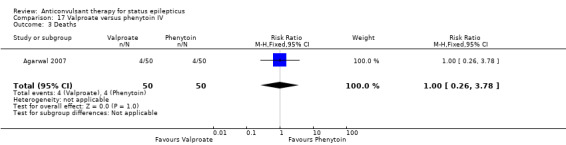
Comparison 17 Valproate versus phenytoin IV, Outcome 3 Deaths.
Comparison 18. Midazolam IM versus lorazepam IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation of seizures | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.06, 1.27] |
| 2 Endotracheal Intubation | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.35] |
| 3 Requirement of Hospitalisation | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| 4 ICU Admissions | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.96] |
| 5 Recurrence of seizure | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.57] |
| 6 Adverse effects | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.71] |
| 7 Length of ICU stay | 1 | 278 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐0.23, 3.43] |
| 8 Length of Hospital stay | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.24, 2.64] |
18.1. Analysis.
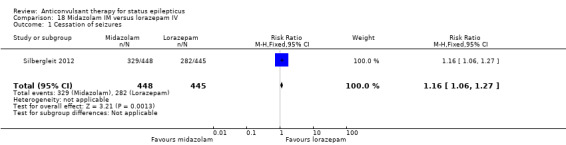
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 1 Cessation of seizures.
18.2. Analysis.
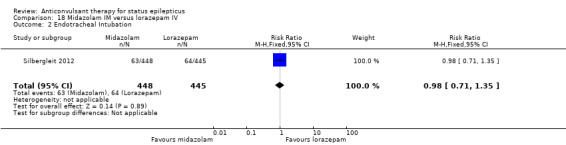
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 2 Endotracheal Intubation.
18.3. Analysis.
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 3 Requirement of Hospitalisation.
18.4. Analysis.
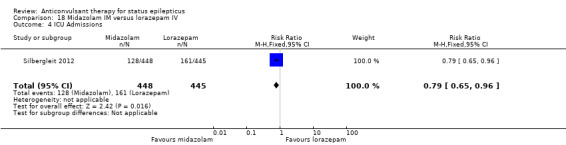
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 4 ICU Admissions.
18.5. Analysis.
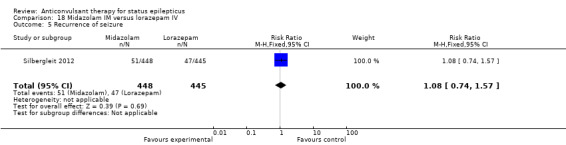
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 5 Recurrence of seizure.
18.6. Analysis.
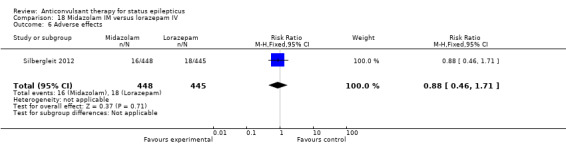
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 6 Adverse effects.
18.7. Analysis.
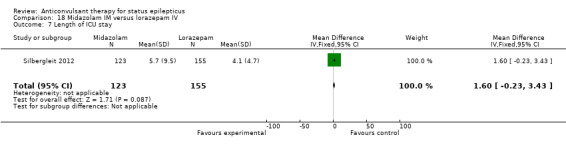
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 7 Length of ICU stay.
18.8. Analysis.
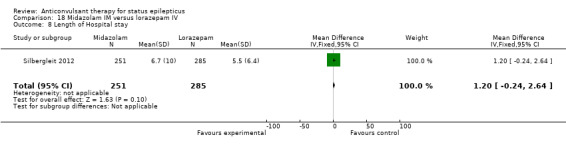
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 8 Length of Hospital stay.
Comparison 19. Valproate IV versus diazepam IV infusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cessation of seizure | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.71, 1.89] |
| 2 Hypotension | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.58] |
| 3 Relapse of seizure | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.83] |
| 4 Respiratory depression | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.76] |
19.1. Analysis.
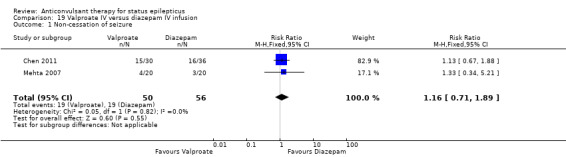
Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 1 Non‐cessation of seizure.
19.2. Analysis.
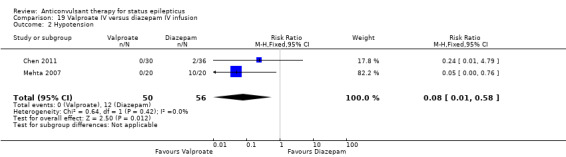
Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 2 Hypotension.
19.3. Analysis.
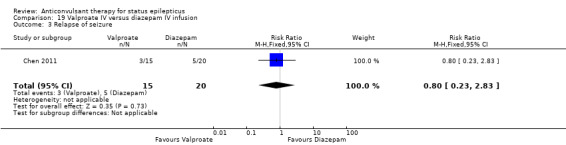
Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 3 Relapse of seizure.
19.4. Analysis.
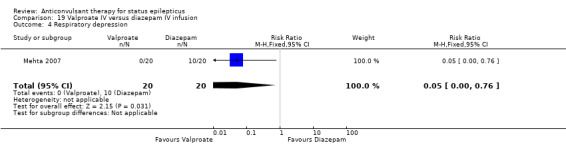
Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 4 Respiratory depression.
Comparison 20. Propofol IV versus barbiturates IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 RSE controlled with first course of drug | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.49, 7.54] |
| 2 RSE treated subsequently | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.30, 1.62] |
| 3 Thrombotic/embolic complications | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.43, 3.88] |
| 5 Functional outcome at 3 weeks | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.34, 3.42] |
| 6 Infections requiring antibiotics | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.37, 1.51] |
20.1. Analysis.
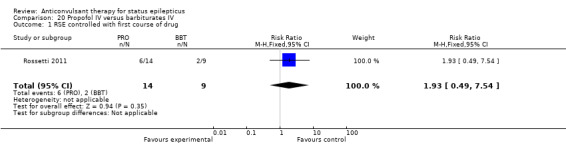
Comparison 20 Propofol IV versus barbiturates IV, Outcome 1 RSE controlled with first course of drug.
20.2. Analysis.
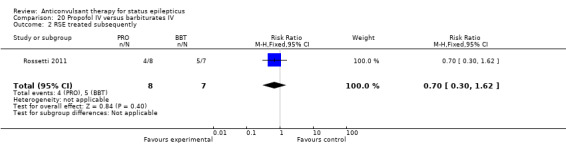
Comparison 20 Propofol IV versus barbiturates IV, Outcome 2 RSE treated subsequently.
20.3. Analysis.
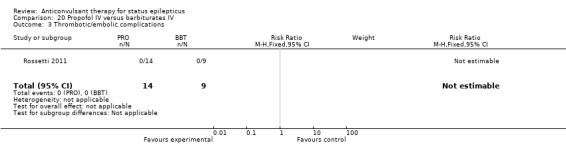
Comparison 20 Propofol IV versus barbiturates IV, Outcome 3 Thrombotic/embolic complications.
20.4. Analysis.
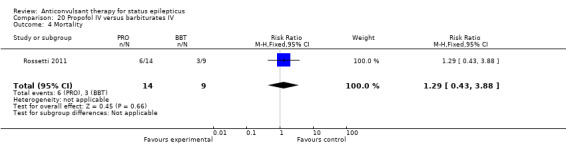
Comparison 20 Propofol IV versus barbiturates IV, Outcome 4 Mortality.
20.5. Analysis.
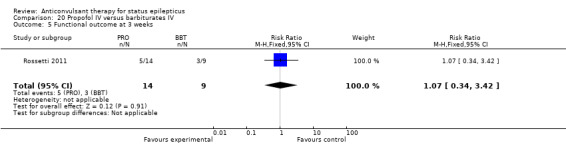
Comparison 20 Propofol IV versus barbiturates IV, Outcome 5 Functional outcome at 3 weeks.
20.6. Analysis.
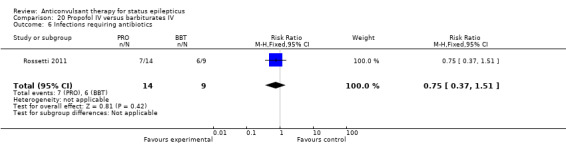
Comparison 20 Propofol IV versus barbiturates IV, Outcome 6 Infections requiring antibiotics.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2007.
| Methods | Randomised parallel group design | |
| Participants | Patients refractory to IV Diazepam | |
| Interventions | Group A; IV Valproate 20 mg/kg loading dose @ 40 mg/min Group B; IV Phenytoin 20 mg/kg @ max. 50 mg/min after dilution with normal saline |
|
| Outcomes | SE controlled, recurrence in 12 h and 24 h, mortality, left against medical advice, hypotension, respiratory depression, mild elevation of SGPT | |
| Notes | Study conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Ahmad 2006.
| Methods | Parallel group, randomised | |
| Participants | Children, mean age 19 years, 55% male, setting: tertiary hospital | |
| Interventions | Arm 1: Intranasal Lorazepam 100 microgram/kg Arm 2: IM Paraldehyde 0.2 mL/kg |
|
| Outcomes | Cessation of seizures within 10 minutes of administration of drug Deaths in hospital |
|
| Notes | Study from Malawi, single centre. Funded by UK college of Emergency Medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation by computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Allocations were sealed in unmarked identical envelopes. Investigators were masked to these allocations before the point of patient treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding may have affected institution of co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though not blinded, outcomes such as seizure activity determination is unlikely to be subject to bias in detection |
Alldredge 2001.
| Methods | Randomised parallel group design | |
| Participants | Adult patients with out‐of‐hospital status epilepticus treated by paramedics. Mean age: 50 yrs, male 63%. Setting: pre hospital treatment by paramedics of Dept of public health, San Francisco, USA | |
| Interventions | Experimental = diazepam (5 mg) or lorazepam (2 mg), IV, pre hospital Control = matching placebo | |
| Outcomes | Cessation of status on arrival to hospital Requirement for ventilatory support on arrival to hospital Status of recovery (neurological outcome) at discharge from hospital Mortality in hospital Complications during transfer Adverse effects of drugs | |
| Notes | Study conducted in USA. Funded by NIH, single‐centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated sequence of random numbers |
| Allocation concealment (selection bias) | Low risk | Coded kits with identical content were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo‐controlled trial |
Appleton 1995.
| Methods | Randomised, parallel group design | |
| Participants | Children (mean age: 5.2 years), male 58%. Emergency services of a tertiary hospital for children | |
| Interventions | Arm 1 = diazepam 0.3 ‐ 0.4 mg/kg IV/rectal, single dose Arm 2 = lorazepam 0.05 ‐ 0.1 mg/kg IV/rectal, single dose | |
| Outcomes | ( I ) the time taken for the initial (presenting) convulsion to stop after administration of the drug; (2) the numbers of doses required to treat the initial convulsion: (3) the use of additional anti‐epileptic drugs to terminate the initial convulsion; (4) the total number of seizures occurring in the first 24 hours of admission: and ( 5 ) the development of respiratory depression, (6) ICU admission, and (6) adverse effects | |
| Notes | Study conducted in UK, No mention of funding source | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised based on date of admission; odd date = diazepam, even date = lorazepam |
| Allocation concealment (selection bias) | High risk | No concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though not blinded, outcomes such as seizure activity determination, requirement of another antiepileptic drug and number of doses are unlikely to be subject to bias in detection |
Cereghino 2002.
| Methods | Randomised parallel group, placebo‐controlled trials | |
| Participants | Adults.(mean 28 years), male 57%. Home treatment by caregivers | |
| Interventions | Experimental = diazepam gel 0.2 mg/kg
Control = identical placebo gel both administered through rectal route No. of doses: single (study 003), three (0, 4 and 12 hours in study 001) |
|
| Outcomes | Cessation of seizures and adverse events over 12 (in study 003) to 24 hours (study 001) | |
| Notes | Study conducted in USA, multi‐centric. Two studies (labelled 001 and 003) have been jointly reported in this report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded study |
Chamberlain 1997.
| Methods | Randomised parallel group | |
| Participants | Birth to age of 18 years. | |
| Interventions | Arm 1 = midazolam 0.2 mg/kg IM Arm 2 = diazepam 0.3 mg/kg IV | |
| Outcomes | Cessation of seizures Requirement for ventilatory support Adverse effects | |
| Notes | Study conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Chen 2011.
| Methods | Randomised, parallel group design | |
| Participants | Adults (mean age 41 years). Male 55%. Setting: University hospital | |
| Interventions | Arm 1: Diazepam bolus 0.2 mg/kg @ 5 mg/min followed by infusion @ 4 mg/hr initially for 3 min and then increased every 3 min by 1 microgram/kg/min Arm 2: Valproate loading dose of 30 mg/kg began @ 6 mg/kg per min followed by a continuous infusion at @ 1–2 mg/kg per h Route: IV |
|
| Outcomes | Effective control of seizures at 24 hours Deaths in‐hospital Recovery to baseline at three months Neurological sequelae at three months Post SE symptomatic epilepsy at three months |
|
| Notes | Study conducted in China, single centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding may have affected institution of co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though not blinded, outcomes such as seizure activity determination is unlikely to be subject to bias in detection |
Leppik 1983.
| Methods | Randomised, placebo‐controlled, parallel group design | |
| Participants | Adults (mean age 53 years), male 71%. Setting: University hospitals | |
| Interventions | Arm 1 = lorazepam 2 mg IV
Arm 2 = diazepam 5 mg IV One or two doses to achieve seizure control |
|
| Outcomes | Cessation of seizures after first dose
Recovery at discharge
Mortality in hospital
Requirement for ventilatory support
Complications
Adverse effects Patients followed up until discharge from hospital |
|
| Notes | Study conducted in USA, multi‐centric (three centres) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly numbered identical kits |
| Allocation concealment (selection bias) | Low risk | Randomly numbered identical kits |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded |
McCormick 1999.
| Methods | Randomised parallel group design | |
| Participants | 1 month to 15 years of age. | |
| Interventions | Arm 1 = midazolam 0.2 mg/kg IV Arm 2 = lorazepam 0.1 mg/kg IV | |
| Outcomes | Cessation of seizures Requirement for ventilatory support Complications Adverse effects | |
| Notes | Study conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There is no mention of 'blinding' in the abstract (full paper not found in our extensive search), but the short period of observation in this study may not be liable to bias in performance of the participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though there was no blinding, the outcome measures are unlikely to be susceptible to high degree of bias |
Mehta 2007.
| Methods | An open‐label, randomised controlled trial | |
| Participants | Children, 5 months to 12 years of age, with refractory convulsive status epilepticus. Mean age in Valproate group‐36.3 months and Diazepam group‐44.5 months | |
| Interventions | Valproate: LoadIng dose: 30 mg/kg diluted 1:1 in normal saline from 2 to 5 mins followed by infusion @ 5 mg/kg/hour continued until seizure‐free period of 6 hours was reached and then reduced @ 1 mg/kg/hour every 2 hours. Maintenance dose: 10 mg/kg IV every 8 hours Diazepam: 10 microgram/kg/min increased every 5 min by 10 mircogram/kg/min |
|
| Outcomes | Control of RSE, time interval for control, dose required to control RSE, breakthrough seizures, respiratory depression, hypotension | |
| Notes | Study conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation method |
| Allocation concealment (selection bias) | High risk | No concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Even though not blinded, outcomes such as seizure activity determination and occurrence of adverse events are unlikely to be subject to bias in detection |
Misra 2011.
| Methods | Randomised, open‐label study | |
| Participants | Adult patients, mean age for Lorazepam group 38.90 and Levetiracetam group 39.16 years Consecutive patients with either convulsive SE or subtle convulsive SE were recruited |
|
| Interventions | Levetiracetam: 20 mg/kg IV over 15 min Lorazepam: 0.1 mg/kg IV over 2‐4 min |
|
| Outcomes | Control of seizure, adverse events such as hypotension, respiratory failure, pneumonia, UTI, ventilatory need, days of ventilation, hepatitis, rash, agitation, thrombocytopenia and deaths | |
| Notes | Study conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used |
| Allocation concealment (selection bias) | High risk | No concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Even though not blinded, outcomes such as seizure activity determination and occurrence of adverse events are unlikely to be subject to bias in detection |
Pellock 1998.
| Methods | Randomised, placebo‐controlled, parallel group design | |
| Participants | Adult patients Premonitory status Patients given the drug at home by caregivers | |
| Interventions | Experimental = diazepam 0.2 mg/kg intrarectal Control = placebo gel intrarectal | |
| Outcomes | Cessation of seizures Complications Adverse effects | |
| Notes | Study conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind placebo‐controlled |
Rossetti 2011.
| Methods | Randomisation was stratified by institution, using blocks with sealed envelopes | |
| Participants | Adults (older than 16 years) Male 43.4%, Median age: Propofol 57 years, Barbiturates 64 years | |
| Interventions | Barbiturate arm: Pentobarbital (USA): bolus of 5 mg/kg IV, then titration toward burst‐suppression or, if no EEG available, toward 2 mg/kg/h until EEG was available OR Thiopental (Switzerland): bolus of 2 mg/kg IV, then titration toward burst‐suppression or, if no EEG available, toward 4 mg/kg/h until EEG was available Propofol arm: Propofol (USA and Switzerland): bolus of 2 mg/kg, then titration toward burst‐suppression or, if no EEG available, toward 5 mg/kg/h until EEG was available In each arm, a BDZ was administered at low dose (lorazepam: 4 mg/24 h, or clonazepam 2 mg/24 h) throughout the study period, to reduce required doses of study drugs |
|
| Outcomes | Efficacy (RSE controlled, mortality, functional outcome) Tolerability (thrombotic/embolic complications, infections, hypotension) Follow‐up: three months Outcomes assessed at one week, three weeks and three months |
|
| Notes | Multi‐centre trial carried out in USA and Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes have been used but it is not clearly mentioned whether they were opaque and serially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were not blinded but there is no clear description of co‐intervention bias due to this |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study is described as single blinded, and outcome measures are unlikely to be subject to bias |
Shaner 1988.
| Methods | Randomisation by sealed envelopes No blinding | |
| Participants | Patients aged over 15 years | |
| Interventions | Arm 1 = diazepam 2 ‐ 20 mg IV + phenytoin 6 ‐ 18 mg/kg IV based on initial drug levels Arm 2 = phenobarbitone 10 ‐ 30 mg/kg IV | |
| Outcomes | Cessation of seizures Requirement for ventilatory support Mortality Complications Adverse effects | |
| Notes | Study from California | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation not given |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes have been used but it is not clearly mentioned whether they were opaque and serially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though there was no blinding, the outcome measures are unlikely to be susceptible to high degree of bias |
Silbergleit 2012.
| Methods | Randomised parallel group design | |
| Participants | Adults and children (mean age 43 years); male 55%, pre hospital treatment by paramedics | |
| Interventions | Arm 1: Midazolam IM 10 mg (for weight > 40 kg) and 5 mg (for weight 13 ‐ 40 kg) Arm 2: Lorazepam IV 4 mg (for weight > 40 kg) and 2 mg (for weight 13 ‐ 40 kg) |
|
| Outcomes | Cessation of seizures Requirement of endotracheal Intubation Requirement of hospitalisation ICU Admissions Recurrence of seizure Hypotension IM Injection site complication IV injection site complication Length of ICU stay Length of hospital stay All outcomes measured in hospital. Follow‐up till discharge from hospital |
|
| Notes | Multi‐centric study by university hospitals, funded by US National Institute of Neurological Disorders and Stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by 'double‐dummy strategy' |
| Allocation concealment (selection bias) | Low risk | Randomisation by 'double‐dummy strategy' in which each kit was randomly assigned at the central pharmacy to contain either group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, randomised trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, randomised trial |
Singhi 2002.
| Methods | Randomised parallel group design | |
| Participants | Children (mean 3.75 years), male 78%, university hospital intensive care setting | |
| Interventions | Arm 1 = midazolam 0.2 mg/kg bolus followed by 2‐10 microgram/kg/min infusion
Arm 2 = diazepam 0.01 ‐ 0.1 mg/kg/min infusion Route of administration: IV |
|
| Outcomes | Cessation of seizures
Recovery at discharge and during follow‐up
Mortality
Complications
Adverse effects Out‐patient follow‐up period: not specified |
|
| Notes | Study from India, single centre, no mention of any funding source | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of six by sealed envelopes |
| Allocation concealment (selection bias) | Low risk | The random assignment was kept sealed in an envelope by a faculty member not directly involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding may have affected institution of co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though not blinded, outcomes such as seizure activity determination are unlikely to be subject to bias in detection |
Sreenath 2010.
| Methods | Randomised parallel group design | |
| Participants | Children (mean age seven years), male 57%, university hospital setting | |
| Interventions | Arm 1: IV Diazepam 0.2 mg/kg (repeated once, if seizure uncontrolled) and IV Phenytoin 18 mg/kg after 15‐30 min Arm 2: IV Lorazepam 0.1 mg/kg (repeated once, if seizure uncontrolled) |
|
| Outcomes | Cessation of seizures Requirement of different drug/GA Requirement of ventilatory support Long‐term disabling sequelae Deaths Adverse effects Follow‐up only in hospital, period: 18 hours |
|
| Notes | Study carried out in India, single centre. Funding source not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Used sealed envelopes but no mention of whether they were opaque and serially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding may have affected institution of co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though not blinded, outcomes such as seizure activity determination is unlikely to be subject to bias in detection |
Treiman 1998.
| Methods | Randomised parallel group design | |
| Participants | Adults (mean age 59 years), male 82%. Setting: University hospitals and Veterans Affairs medical centres | |
| Interventions | Arm 1 = lorazepam IV (Intravenous: 0.1 mg/kg) Arm 2 = phenobarbitone IV (15mg/kg) Arm 3 = diazepam (0.15 mg/kg) + phenytoin IV (18 mg/kg) Arm 4 = phenytoin IV (18 mg/kg) | |
| Outcomes | Cessation of seizures at 20 minutes and no recurrence for next 40 minutes Discharged alive at 30 days Adverse effects over 12 hours |
|
| Notes | Study from USA, multi‐centric | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of sequence generation not described in details but in view of identical kits for the different arms, risk of bias is considered low |
| Allocation concealment (selection bias) | Low risk | Identical and numbered kits were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical kits would blind the participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment would be blinded as identical kits were used |
BDZ: benzodiazepine EEG: electroencephalogram GA: general anaesthesia ICU: intensive care unit IM: intramuscular injection IV: administered intravenously RSE: refractory status epilepticus SE: status epilepticus SGPT: serum glutamic‐pyruvic transaminase UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andermann 1994 | Data analysis not possible due to inadequate information. |
| Czapinski 1995 | Data analysis not possible due to inadequate information. |
| Lowenstein 2001 | This paper provides only details of the study design and methodology of an already included study (Alldredge 2001). |
| Mahmoudian 2006 | This was not a truly randomised study as the allocation was made on the basis of odd or even date of entry into the study. Authors, even in their full paper, did not present the number of patients in each arm of the study and did not respond to emails sent to them for data required to enter into RevMan. |
| Osborn 1987 | Study population did not include patients with status epilepticus. |
| Remy 1992 | This study compares two different doses of the same drug (intrarectal diazepam gel), and hence does not meet the inclusion criteria. |
| Scott 1998 | This study is an abstract of same study excluded (see Scott 1999). |
| Scott 1999 | Only counts of number of seizures in each group are given; the data can be analysed neither as dichotomous nor as continuous variable. |
| Sorel 1981 | Comparative clinical trial. |
| Taverner 1958 | Only one participant in the trial. |
| Van Gestel 2005 | The study was not randomised. |
Contributions of authors
MP was primarily responsible for the update of the review. Selection of studies for inclusion, conducted quality assessment, data extraction, entry to Revman (MP). Creation of risk of bias table for each study (including those included in the first version of the review) and summary of finding tables using GRADEPRO (MP). Duplicate selection of studies for the update, data extraction and commented on the final draft of the review (PK). Contributed to the first version of the review and commented on the final draft of the review (RS, LA‐R).
Sources of support
Internal sources
College of Medicine & Medical Sciences, The Arabian Gulf University, Bahrain.
All India Institute of Medical Sciences, New Delhi, India.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Agarwal 2007 {published data only}
- Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16(6):527‐32. [DOI] [PubMed] [Google Scholar]
Ahmad 2006 {published data only}
- Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet 2006;367(9522):1591‐7. [DOI] [PubMed] [Google Scholar]
Alldredge 2001 {published data only}
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrish S, et al. A comparison of lorazepam, diazepam and placebo for the treatment of out‐of‐hospital status epilepticus. New England Journal of Medicine 2001;345(9):631‐7. [DOI] [PubMed] [Google Scholar]
Appleton 1995 {published data only}
- Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Developmental Medicine and Child Neurology 1995;37(8):682‐8. [DOI] [PubMed] [Google Scholar]
Cereghino 2002 {published data only}
- Cereghino JJ, Cloyd JC, Kuzniecky RI. Rectal diazepam gel for the treatment of acute repetitive seizures in adults. Archives of Neurology 2002;59(12):1915‐20. [DOI] [PubMed] [Google Scholar]
Chamberlain 1997 {published data only}
- Chamberlain JM, Altieri MA, Futtterman C, Young GM, Ochsenschlanger DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatric Emergency Care 1997;13(2):92‐4. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen WB, Gao R, Su YY, Zhao JW, Zhang YZ, Wang L, et al. Valproate versus diazepam for generalized convulsive status epilepticus: a pilot study. European Journal of Neurology 2011;18:1391‐6. [DOI] [PubMed] [Google Scholar]
Leppik 1983 {published data only}
- Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double‐blind study of lorazepam and diazepam in status epilepticus. JAMA 1983;249(11):1452‐4. [PubMed] [Google Scholar]
McCormick 1999 {published data only}
- McCormick EM, Lieh‐lai M, Knazik S, Nigro M. A prospective comparison of midazolam and lorazepam in the initial treatment of status epilepticus in the pediatric patient. Epilepsia 1999;40(Suppl 7):160, Abstract no: G.07. [Google Scholar]
Mehta 2007 {published data only}
- Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. Journal of Child Neurology 2007;22(10):1191‐7. [DOI] [PubMed] [Google Scholar]
Misra 2011 {published data only}
- Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. Journal of Neurology 2012;259(4):645‐8. [DOI] [PubMed] [Google Scholar]
Pellock 1998 {published data only}
- Pellock JM, Mitchell WG, Cloyd JC. Diastat (diazepam rectal gel) in the treatment of acute repetitive seizures in adults. Epilepsia 1998;39(Suppl 6):126‐7, Abstract no: 4.097. [Google Scholar]
Rossetti 2011 {published data only}
- Rossetti AO, Milligan TA, Vulliémoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocritical Care 2011;14(1):4–10. [DOI 10.1007/s12028‐010‐9445‐z] [DOI] [PubMed] [Google Scholar]
Shaner 1988 {published data only}
- Shaner DM, Curdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology 1988;38(2):202‐7. [DOI] [PubMed] [Google Scholar]
Silbergleit 2012 {published data only}
- Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for pre hospital status epilepticus. New England Journal of Medicine 2012;366(7):591‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singhi 2002 {published data only}
- Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. Journal of Child Neurology 2002;17(2):106‐10. [DOI] [PubMed] [Google Scholar]
Sreenath 2010 {published data only}
- Sreenath TG, Gupta P, Sharmab KK, Krishnamurthya S. Lorazepam versus diazepam–phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. European Journal of Paediatric Neurology 2010;14(2):162‐8. [DOI] [PubMed] [Google Scholar]
Treiman 1998 {published data only}
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowman AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. New England Journal of Medicine 1998;339(12):792‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andermann 1994 {published data only}
- Andermann F, Cendes F, Sherwin A, McGoldrick J, Langendorf P, Jones M, et al. A dose‐response study of intravenous lorazepam in the treatment of status epilepticus. Epilepsia 1994;35(Suppl 8):10. [Google Scholar]
Czapinski 1995 {published data only}
- Czapinski P. Prospective randomized study of the efficacy and tolerability of phenytoin and phenobarbital in status epilepticus [abstract]. Journal of Neurology 1995;242(2 Suppl):S83. [Google Scholar]
Lowenstein 2001 {published data only}
- Lowenstein DH, Alldredge BK, Allen F, Neuhaus J, Corry M, Gottwald M, et al. The pre hospital treatment of status epilepticus (PTHSE) study: design and methodology. Controlled Clinical Trials 2001;22(3):290‐309. [DOI] [PubMed] [Google Scholar]
Mahmoudian 2006 {published data only}
- Mahmoudian T, Najafian M. Comparing the effect of intravenous midazolam with rectal sodium valproate in controlling of children with refractory status epilepticus. Journal of Research in Medical Sciences 2006;11(1):1‐5. [Google Scholar]
Osborn 1987 {published data only}
- Osborn HH, Zisfein J, Sparano R. Single dose oral phenytoin loading. Annals of Emergency Medicine 1987;16(4):407‐12. [DOI] [PubMed] [Google Scholar]
Remy 1992 {published data only}
- Remy C, Jourdil N, Villemain D, Favel P, Genton P. Intrarectal diazepam in epileptic adults. Epilepsia 1992;33(2):353‐8. [DOI] [PubMed] [Google Scholar]
Scott 1998 {published data only}
- Scott RC, Besag FM, Neville BG. Buccal midazolam or rectal diazepam for the acute treatment of seizures?. Epilepsia 1998;39(Suppl 6):235, Abstract no: 7.048. [Google Scholar]
Scott 1999 {published data only}
- Scott RC, Besag FMC, Neville BGR. Buccal midazolam and rectal diazepam for the treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999;353(9153):623‐6. [DOI] [PubMed] [Google Scholar]
Sorel 1981 {published data only}
- Sorel L, Mechler L, Harmant J. Comparative trial of intravenous lorazepam and clonazepam in status epilepticus. Clinical Trials 1981;4(4):326‐36. [PubMed] [Google Scholar]
Taverner 1958 {published data only}
- Taverner D, Bain WA. Intravenous lignocaine as an anticonvulsant in status epilepticus and serial epilepsy. Lancet 1958;2(7057):1145‐7. [DOI] [PubMed] [Google Scholar]
Van Gestel 2005 {published data only}
- Gestel JPJ, Blussé van Oud‐Alblas HJ, Malingré M, Ververs FFT, Braun KPJ, Nieuwenhuizen O. Propofol and thiopental for refractory status epilepticus in children. Neurology 2005;65(4):591‐2. [DOI] [PubMed] [Google Scholar]
Additional references
Epilepsy Foundation of America 1993
- Epilepsy Foundation of America's Working Group on Status Epilepticus. Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA 1993;270:854‐9. [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J . Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Lowenstein 1998
- Lowenstein DH, Alldredge BK. Status epilepticus. New England Journal of Medicine 1998;338(14):970‐8. [DOI] [PubMed] [Google Scholar]
Prasad 1995
- Prasad K. Management of convulsive status epilepticus. In: Chaubey PC editor(s). Current Practice in Emergency Care. New Delhi: Saurabh Publishers, 1995:170‐3. [Google Scholar]
Shorvon 2001
- Shorvon S. The management of status epilepticus. Journal of Neurology, Neurosurgery and Psychiatry 2001;70(Suppl 2):ii22‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Prasad 2005
- Prasad K, Al‐Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003723.pub2] [DOI] [PubMed] [Google Scholar]


