Abstract
Background
Gestational diabetes (GDM) affects 3% to 6% of all pregnancies. Women are often intensively managed with increased obstetric monitoring, dietary regulation, and insulin. However, there has been no sound evidence base to support intensive treatment. The key issue for clinicians and consumers is whether treatment of GDM improves perinatal outcome.
Objectives
To compare the effect of alternative treatment policies for GDM on both maternal and infant outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (January 2009) and bibliographies of relevant papers. We updated this search on 1 July 2011 and added the results to the awaiting classification section of the review.
Selection criteria
Randomised controlled trials comparing alternative management strategies for women with GDM and impaired glucose tolerance in pregnancy.
Data collection and analysis
Two authors and a member of the Cochrane Pregnancy and Childbirth Group's editorial team extracted and checked data independently. Disagreements were resolved through discussion with the third author.
Main results
Eight randomised controlled trials (1418 women) were included.
Caesarean section rate was not significantly different when comparing any specific treatment with routine antenatal care (ANC) including data from five trials with 1255 participants (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.80 to 1.12). However, when comparing oral hypoglycaemics with insulin as treatment for GDM, there was a significant reduction (RR 0.46, 95% CI 0.27 to 0.77, two trials, 90 participants). There was a reduction in the risk of pre‐eclampsia with intensive treatment (including dietary advice and insulin) compared to routine ANC (RR 0.65, 95% CI 0.48 to 0.88, one trial, 1000 participants). More women had their labours induced when given specific treatment compared to routine ANC (RR 1.33, 95% CI 1.13 to 1.57, two trials, 1068 participants). The composite outcome of perinatal morbidity (death, shoulder dystocia, bone fracture and nerve palsy) was significantly reduced for those receiving intensive treatment for mild GDM compared to routine ANC (RR 0.32, 95% CI 0.14 to 0.73, one trial, 1030 infants).
There was a reduction in the proportion of infants weighing more than 4000 grams (RR 0.46, 95% CI 0.34 to 0.63, one trial, 1030 infants) and the proportion of infants weighing greater than the 90th birth centile (RR 0.55, 95% CI 0.30 to 0.99, three trials, 223 infants) of mothers receiving specific treatment for GDM compared to routine ANC. However, there was no statistically significant difference in this proportion between infants of mothers receiving oral drugs compared to insulin as treatment for GDM.
Authors' conclusions
Specific treatment including dietary advice and insulin for mild GDM reduces the risk of maternal and perinatal morbidity. However, it is associated with higher risk of labour induction. More research is needed to assess the impact of different types of intensive treatment, including oral drugs and insulin, on individual short‐ and long‐term infant outcomes.
[Note: the 29 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Plain language summary
Treatments for gestational diabetes
The best way of identifying and treating women with abnormal blood glucose tests in pregnancy is not known. Raised blood glucose levels during pregnancy is known as gestational diabetes. This abnormality may be associated with bigger babies, more difficult births and could be associated with higher rates of operative delivery such as caesarean section. The review of eight studies (1418 women) suggests that offering specific treatment for gestational diabetes may be associated with better baby and mother outcomes, but has not found robust evidence on the best choice of treatment which provides the better outcomes for these women and their babies, even if identified correctly. More research is needed to assess long‐term mother and baby outcomes.
Background
Metabolic changes during pregnancy lower glucose tolerance. Blood glucose levels rise and more insulin is produced in response. As the pregnancy develops, insulin demands increase. For the majority of pregnant women this is a normal physiological process. However, some women develop glucose intolerance resulting in gestational diabetes affecting between 3% and 6% of all pregnancies (Gabbe 1977). Women who had diabetes before becoming pregnant (pre‐existing diabetes) can see their insulin requirements progressively rise throughout pregnancy.
Definition and diagnosis of gestational diabetes (GDM)
GDM is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. The definition applies regardless of whether insulin or only diet modification is used for treatment or whether the condition persists after pregnancy. It does not exclude the possibility that unrecognised glucose intolerance may have antedated or begun concomitantly with the pregnancy (Expert Committee 2003). The diagnosis is generally based on an abnormal oral glucose tolerance test (GTT). A GTT requires women to fast overnight before attending the hospital the following morning. Usually, two blood samples are taken. The first is taken on arrival and the woman is then given a sugary drink containing 75 g of glucose (100 g is more commonly used in the United States). The second blood sample is taken two hours later. The precise diagnostic values of the GTT are controversial (Weiss 1998), making it difficult to provide a clear definition of gestational diabetes. Previously, two distinct diagnostic categories existed; impaired glucose tolerance (IGT) and GDM. However, current World Health Organization recommendations suggest that all abnormal glucose metabolism arising in pregnancy should be defined as gestational diabetes (Alberti 1998). The new National Institute for Clinical Excellence (NICE) Guidelines use the WHO criteria (NICE 2007). However, this does not distinguish between mild and more severe abnormalities of glucose tolerance. Although it has been suggested that the relationship between blood glucose levels in pregnancy and perinatal outcome is a continuous one and no single cut off can separate women into those with high risk and those with no risk at all (Coustan 1998), it is helpful to classify GDM into mild and severe forms (see 'Methods' section).
This review defines gestational diabetes according to the following values:
fasting blood glucose greater than 7 mmol/l; or
two‐hour blood glucose greater than 7.8 mmol/l following a 75 g GTT.
In the USA a 100 g glucose load is commonly used and there is some debate around the precise diagnostic values. This review accepted clearly defined values as described by the American College of Obstetricians and Gynaecologists:
gestational diabetes ‐ fasting greater than 105 mg/dl; one‐hour greater than 190 mg/dl; two‐hour greater than 165 mg/dl; three‐hour greater than 145 mg/dl.
Screening for gestational diabetes
There are various regimens that are used to select women for formal testing with a GTT (Stephenson 1993). Examples of these are a past history of gestational diabetes or IGT, the development of pregnancy complications such as polyhydramnios (increased water around the baby) or a raised random blood glucose. In addition, there are certain predisposing factors which may indicate an increased risk of gestational diabetes such as obesity and ethnicity (Essel 1995). Inevitably, some women diagnosed in pregnancy will have developed true diabetes and women with gestational diabetes should have a postnatal GTT. However, the focus of this review is abnormalities of glucose tolerance associated with pregnancy.
In the past it was pointed out that there was little evidence to support the recommendation that all pregnant women should be screened for gestational diabetes (Enkin 2000). This issue remains controversial. A systematic review of screening for gestational diabetes concluded there was insufficient evidence to justify universal screening (Scott 2002), and a protocol for a Cochrane review of screening for gestational diabetes has been developed (Tieu 2008b). Justification for screening rests on the relevance of gestational diabetes which remains uncertain. The key issue is whether treatment improves outcome or, more importantly, whether treatment is associated with adverse outcome.
Complications of gestational diabetes
Whilst it is recognised that women with pre‐existing diabetes have an increased risk of both maternal and fetal complications (Enkin 2000) including caesarean section, hypoglycaemia, respiratory distress syndrome and stillbirth, there are currently limited data available on the frequency of adverse perinatal outcome resulting from gestational diabetes. The lack of evidence fuels confusion and leads clinicians to conclude that the risk of adverse perinatal outcome is equal to that of pre‐existing diabetes.
The only commonly reported complication of gestational diabetes is macrosomia (birthweight greater than 4000 g) which may increase the risk of caesarean section, shoulder dystocia and birth trauma to the baby (Jarrett 1997). Increased birthweight is indeed associated with increased morbidity (Scott 2002). However, the association between abnormalities of glucose tolerance and increased birthweight remains unclear. It has been suggested that fetal growth acceleration associated with gestational diabetes can be corrected with dietary regulation and insulin (Soares 1997) and there may be long‐term benefits to infants of normalising birthweight (Barker 1992). However, some studies suggest that macrosomia is more closely related to maternal weight or maternal weight gain than blood glucose levels (Green 1991; Essel 1995; Spellacy 1985). Furthermore, there are inconsistencies in defining macrosomia. It is suggested that there may be a continuum of risk in relation to glucose values making dichotomous cut‐off values inappropriate (Scott 2002).
A further consideration is the long‐term consequences for the mother. Women with gestational diabetes may have an increased risk of progression to non‐insulin dependent diabetes in later life, although this is influenced greatly by, again, maternal weight and also ethnicity (Soares 1997).
Current management policies
There has been much confusion surrounding the management of gestational diabetes. Many women are managed according to intensive treatment regimens, including administration of insulin, in the belief that this will prevent pregnancy complications. The rationale being to reduce blood glucose levels in the belief that this will improve maternal and infant outcomes. Interventions include dietary regulation, home blood glucose monitoring and in some cases insulin therapy. Exercise has been suggested as an alternative and less invasive intervention and is now the subject of a Cochrane review (Ceysens 2006). Another Cochrane review assesses the impact of dietary advice on the prevention of GDM (Tieu 2008a). Increased obstetric monitoring often becomes inevitable in the form of more frequent antenatal clinic visits and ultrasound scans which intend to detect abnormalities and avoid macrosomia and stillbirth. However, they also result in an increased level of inconvenience for women compared with standard antenatal care. Furthermore, a diagnosis of gestational diabetes may cause distress in someone who previously thought herself to be healthy (Jarrett 1997).
Rationale for this review
The key issue for both clinicians and consumers is whether identification and treatment of gestational diabetes will improve perinatal outcome, particularly in the case of mild GDM. There is almost clinical consensus to treat severe forms of GDM. However, treating the milder forms of GDM is more controversial; therefore, most of the randomised controlled trials in this area have been done to examine the effect of treatment on mild GDM (previously known as IGT). This review includes the complete range of management options for gestational diabetes from no specific treatment to intensive treatment including individualised dietary advice, close monitoring of blood glucose, insulin therapy and oral hypoglycaemic drugs.
Objectives
To conduct a systematic review of randomised trials, comparing alternative treatment options for women with gestational diabetes and their effect on maternal and infant outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials comparing alternative management strategies for women with gestational diabetes. Quasi‐random designs were excluded.
Types of participants
Pregnant women diagnosed with gestational diabetes mellitus (GDM) or impaired glucose tolerance (IGT) identified by a 75 g or 100 g oral glucose tolerance test (GTT). Studies without a formal GTT were only considered where a fasting blood glucose value greater than seven was used for diagnosis. Women were eligible irrespective of gestation and whether singleton or multiple pregnancy; however, women with pre‐existing diabetes were not.
GDM was defined as fasting blood glucose of at least 7.0 mmol/L or two‐hour blood glucose greater than 7.8 mmol/L. This includes IGT which is defined as two‐hour value between 7.8 mmol/L and 11 mmol/L. Participants with IGT values between 7.8 to 11 mmol/L are classified as having mild GDM. IGT values greater than 11 mmol/ are classified as severe GDM.
Types of interventions
Interventions include any form of management of gestational diabetes on top of routine antenatal care (ANC). These include any form of dietary advice (standard or specific); and drug treatment including insulin and oral drugs. One form of management can be compared to routine ANC, or two forms of management can be compared against each other. Trials that compare two or more types of the same form of treatment (oral drugs or insulin) against each other are not considered in this review.
Types of outcome measures
Maternal outcomes
Risk of operative abdominal operative delivery.
Risk of vaginal operative delivery.
Psychological impact of treatment (assessed by psychometric testing).
Levels of maternal inconvenience assessed by number of hospital visits, number of days in hospital (antenatal), extra investigations, antenatal tests (ultrasound scanning, cardiotocographs, amniocentesis), duration of antenatal admission.
Risk of induction of labour.
Use of insulin.
Risk of pre‐eclampsia.
Risk of abruption.
Risk of postpartum haemorrhage.
Risk of third degree tears.
Risk of postpartum infection.
Risk of postnatal depression.
Risk of hypoglycaemic events.
Risk of future non‐insulin dependent diabetes mellitus.
Infant outcomes
Perinatal death.
Birthweight greater than 90th centile, birthweight greater than 4000 g, and birthweight as a continuous variable.
Gestation at delivery.
Risk of shoulder dystocia (defined as halt to spontaneous delivery because the baby's shoulder is wedged behind the mother's pubis).
Risk of birth trauma.
Risk of nerve palsy or fracture, or both.
Admission to neonatal intensive care unit or special care baby unit and length of stay.
Risk of respiratory distress syndrome and ventilation.
Hypoglycaemia and risk of neonatal infection.
Growth and development.
Adiposity.
Composite outcome (defined as one or more of the following: infant death, shoulder dystocia, bone fracture and nerve palsy). Although this outcome was not included in the original protocol for the review, it has been added as it is the primary outcome used by the ACHOIS trial (Crowther 2005), which includes the biggest sample among all the other studies included in this review.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2009). We updated this search on 1 July 2011 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For additional searching carried out for the previous version of the review, please see Appendix 1.
Searching other resources
We searched bibliographies of relevant papers.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
We assessed for inclusion all potential studies we identified as a result of the search. We resolved any disagreement through discussion.
Assessment of methodological quality of included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence are described for each trial.
(1) Selection bias (allocation concealment)
We assigned a quality score for each trial, using the following criteria: (A) adequate concealment of allocation: such as telephone randomisation, consecutively numbered sealed opaque envelopes; (B) unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach; (C) inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week.
(2) Attrition bias (loss of participants, e.g. withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria: (A) less than 5% loss of participants (10% for long‐term outcomes); (B) 5% to 9.9% loss of participants (10% to 19.9% for long‐term outcomes); (C) 10% to 19.9% loss of participants (20% to 39.9% for long‐term outcomes); (D) more than 20% loss of participants (40% for long‐term outcomes).
Long‐term outcomes are defined as those needing follow up for more than one year.
If attrition bias scored D using the above criteria, data were not used in the main analysis of the review.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria: (A) blinding of participants (yes/no/unclear); (B) blinding of caregiver (yes/no/unclear); (C) blinding of outcome assessment (yes/no/unclear).
Data extraction and management
At least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We used the Review Manager software (RevMan 2003) to double enter all the data.
When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2003). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials are sufficiently similar.
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Available case analysis
We have analysed data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we have attempted to restore them to the correct group.
Assessment of heterogeneity
We have applied tests of heterogeneity between trials, if appropriate, using the I² statistic. A random‐effects meta‐analysis is used as an overall summary if was considered appropriate.
Subgroup analyses
We performed subgroup analyses by:
type of treatment;
severity of GDM.
Sensitivity analyses
We carried out sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment (rated C).
Results
Description of studies
Included studies
The search identified 65 trials; eight are included in the review, involving 1418 women and their babies, and 57 excluded. Of the trials included, the majority were small with the exception of Crowther 2005 (The ACHOIS trial), which randomised 1000 women to either care in which universal screening and treatment of GDM was available or care in which universal screening for GDM was not available. The sample size for the other studies ranged from 32 to 126.
Study interventions
Each study addressed treatments for gestational diabetes. Diagnosis was made either by 75 g or 100 g oral glucose tolerance test. The trials compared different interventions. Three trials compared oral hypoglycaemics to insulin treatment (Bertini 2005; Hague 2003; Moore 2004). Bancroft 2000 compared regular capillary glucose monitoring and fetal growth monitoring, dietary advice and insulin treatment to routine care including standard dietary advice. One study compared specific "diabetic type" dietary advice to standard care (Ford 1997). One study compared specific advice for targeted intake of foods rich in unsaturated fats compared to "diabetic type" dietary advice (Gillen 2004).
Interventions used in the included trials included:
dietary advice, monthly HBA1c, capillary glucose monitoring and insulin therapy if indicated (Bancroft 2000);
universal screening for GDM, individualised dietary advice from qualified dietician, glucose self monitoring, and insulin therapy if indicated (Crowther 2005);
specific "diabetic type" dietary advice (Ford 1997);
dietary advice for targeted intakes of foods rich in unsaturated fats (Gillen 2004);
metformin versus insulin (Hague 2003; Moore 2004);
glyberide versus insulin (Bertini 2005);
acarbose versus insulin (Bertini 2005);
diabetic protocol including dietary advice and insulin therapy if indicated (Langer 1989).
Excluded studies
Many well known and influential studies were excluded from this review. Some were excluded as a consequence of methodological issues. Many were excluded due to the variation in diagnostic criteria which rendered attempts to synthesise data impossible. Others were excluded as they contained an additional screening step that selected from within the population of gestational diabetes.
(Nineteen reports from an updated search in 1 July 2011 have been added to Studies awaiting classification.)
Risk of bias in included studies
All included studies stated that women were randomly allocated to intervention or control groups. Information regarding generation of the randomisation sequence was clearly described in five studies. Of these, three trials used computerised randomisation (Bancroft 2000; Crowther 2005; Langer 1989). Randomisation was achieved with random‐number tables in one study (Gillen 2004), random selection of sealed envelopes was used in two studies (Ford 1997; Moore 2004). Two trials stated women 'were randomised' (Bertini 2005; Hague 2003).
Allocation concealment was adequately described in four trials (Bancroft 2000; Crowther 2005; Ford 1997; Langer 1989). One other trial (Moore 2004) used sealed envelopes that were not described as opaque (classified as B ‐ unclear). One trial (Gillen 2004) used an open table of random numbers (classified C ‐ inadequate). The remaining trials (Hague 2003; Bertini 2005) gave no information. Blinding of clinician was stated for two trials (Bancroft 2000; Crowther 2005), of participant for one trial (Gillen 2004), and of outcome assessor for one trial (Crowther 2005). A number of outcomes are only reported by one trial and many of the outcomes are not addressed by any of the included studies.
Effects of interventions
Eight trials, involving 1418 women, were included in this review.
Comparison one: any specific treatment versus routine antenatal care (ANC)(subgroups by type of specific treatment)
Maternal outcomes
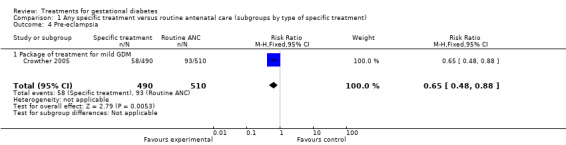
There was a reduction in the risk of pre‐eclampsia with specific treatment for GDM compared to routine ANC (one trial, 1000 women; risk ratio (RR) 0.65, 95% confidence interval (CI) 0.48 to 0.88).
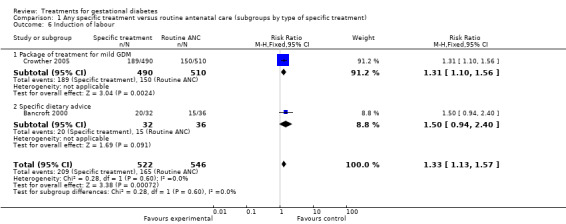
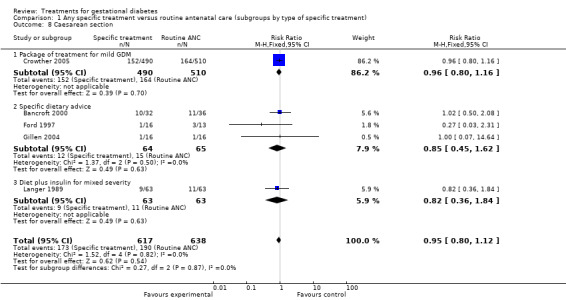
The difference in caesarean section rates was not statistically significant between the two groups (five trials, 1255 women; RR 0.95, 95% CI 0.80 to 1.12). However, women who received specific treatment were more likely to have their labour induced compared to women who received routine ANC only (two trials, 1068 women; RR 1.33, 95% CI 1.13 to 1.57).
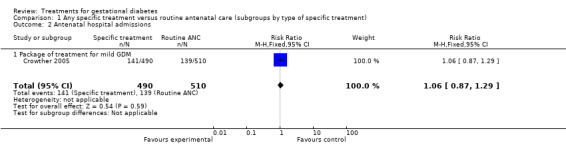
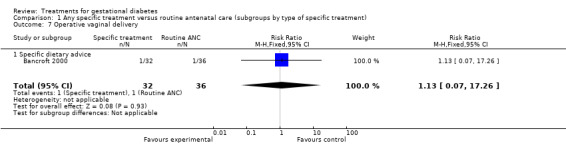
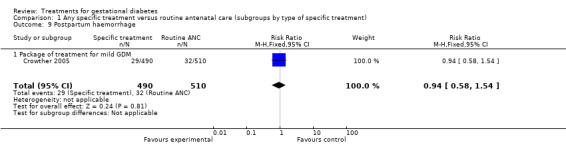
There were no statistically significant differences in antenatal hospital admission rates, operative vaginal delivery, postpartum haemorrhage, or length of hospital stay.
Infant outcomes
The composite outcome of perinatal morbidity used as the main outcome measure in the ACHOIS trial (death, shoulder dystocia, bone fracture, nerve palsy) was significantly reduced for those receiving specific treatment (one trial, 1030 infants; RR 0.32, 95% CI 0.14 to 0.73) (Crowther 2005). For the outcome shoulder dystocia alone, the reduction in its incidence was only marginally significant (two trials, 1098 infants; RR 0.45, 95% CI 0.19 to 1.04).
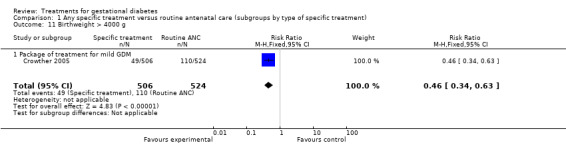
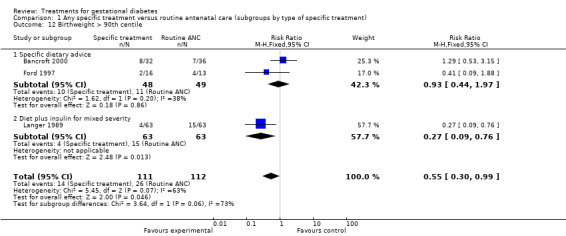
The difference in the proportion of babies with birthweight greater than 4000 g (one trial, 1030 infants, RR 0.46, 95% CI 0.34 to 0.63) or greater than the 90th centile (three trials, 223 infants, RR 0.55, 95% CI 0.30 to 0.99) was statistically significant. Babies born to women receiving a form of specific treatment for GDM were less likely to weigh more than 4000 g or greater than the 90th birth centile compared to those born to women receiving routine ANC.
Infants were more likely to be admitted to the neonatal care unit if their mothers received specific treatment for GDM in the ACHOIS trial (1224 infants, RR 1.13, 95% CI 1.03 to 1.23) (Crowther 2005). However, when combining this result with the results of two other trials examining the same outcome (Bancroft 2000; Langer 1989), the effect lost statistical significance (three trials, RR 0.57, 95% CI 0.18 to 1.86), I2 = 43%).
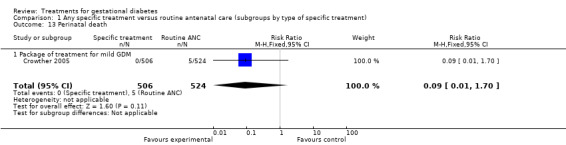
There were no statistically significant differences in gestational age at delivery, incidence of bone fracture in the infant, incidence of nerve palsy in the infant, perinatal death, infant hypoglycaemia, incidence of respiratory distress syndrome or need for ventilation between the two groups.
Comparison two: oral hypoglycaemics versus insulin therapy
Maternal outcomes
There was a significant reduction in caesarean section rates in women receiving oral hypoglycaemics compared to insulin (two trials, 90 women; RR 0.46, 95% CI 0.27 to 0.77).
There were no statistically significant differences detected in the operative vaginal delivery rates, induction of labour and postpartum haemorrhage.
Infant outcomes
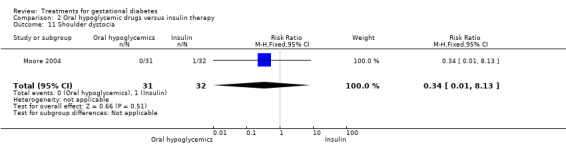
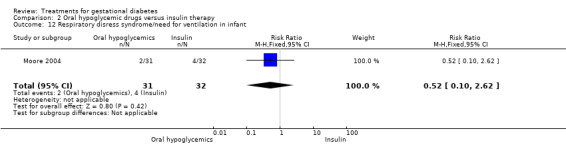
There was no statistically significant difference in the proportion of infants with birthweight greater than 4000 g borne to mothers receiving oral hypoglycemics compared to mother receiving insulin as therapy for GDM (two trials, 93 infants, RR 1.71, 95% CI 0.60 to 4.86). There were also no statistically significant differences in rates of shoulder dystocia, neonatal care admission, respiratory distress syndrome or the need for ventilation.
Infants of mothers treated with insulin were more likely to develop neonatal hypoglycaemia compared to those of mothers treated with oral hypoglycemics (two trials, 114 infants. RR 7.68, 95% CI 1.47 to 40.22).
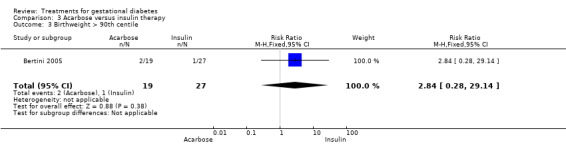
Comparison three: acarbose versus insulin therapy
One trial was included under this comparison (Bertini 2005) with 36 women.
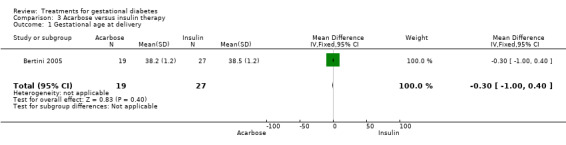
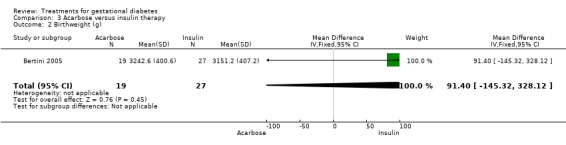
Three infant outcomes were considered under this comparison: birthweight greater than the 90th centile and as a continuous variable, gestational age and neonatal hypoglycaemia. None showed statistically significant differences between the two treatment groups.
Other outcomes
Anxiety scores were assessed at six and three months in Crowther 2005. However, the results were not included in the main analysis as the follow‐up rates did not meet the review's attrition bias criteria. The results are presented separately in Table 4 and Table 5.
1. Anxiety score 6 weeks after enrolment.
| treatment (N) | treatment (mean) | treatment (SD) | control (N) | control (mean) | control (SD) |
| 332 | 11.20 | 3.70 | 350 | 11.50 | 4.00 |
2. Anxiety score 3 months after enrolment.
| treatment (N) | treatment (mean) | treatment (SD) | control (N) | control (mean) | control (SD) |
| 278 | 10.60 | 3.90 | 295 | 10.80 | 3.80 |
Discussion
Intensive treatment including specific dietary advice and insulin therapy for gestational diabetes was found to reduce the risk of serious perinatal morbidity in the infant using a composite measure of death, shoulder dystocia, nerve palsy and bone fracture. It is associated with less proportion of babies born weighing more than 4000 g or more than the 90th centile, However, it is associated with increases in special care baby unit admissions and in labour induction rates. Caesarean section rates were not significantly reduced when comparing intensive treatment with standard care. However, intensive treatment was associated with a reduction in the risk of developing pre‐eclampsia. Caesarean section rates were significantly reduced when comparing oral hypoglycaemics to insulin as a mode of treatment for gestational diabetes mellitus (GDM).
These results are mainly based on the ACHOIS study (Crowther 2005), being the largest trial included, which used a composite outcome of infant death, shoulder dystocia, nerve palsy and bone fracture. This composite outcome is not considered in the other six studies included in this review, which used single end points. It can be criticised on the basis that shoulder dystocia is subjective. Also, there was a lower average gestational age associated with the lower rate of macrosomia. This may have contributed to the outcomes of the ACHOIS study (Crowther 2005). It is difficult to separate elements of the package of care to identify whether the reduced rate of the composite outcome is due to the dietary advice and treatment of raised blood glucose or the intervention which lead to a lower gestational age and reductions in birthweight which reduce the rate of shoulder dystocia.
Macrosomia was reported as an outcome in seven trials, defined as either birthweight over 4000 g or greater than the 90th centile. Babies of mothers receiving specific treatment for GDM were less likely to weigh more than 4000 g. Apart from the increased risk of operative delivery, which is separately assessed in this review, the significance of being born big remains uncertain. This can only by assessed by studies using long‐term end points as primary outcomes.
Caesarean section rate was assessed as an outcome in six trials. The reduction is rate was not significant comparing any specific treatment to standard clinical care (risk ratio 0.94, 95% confidence interval 0.63 to 1.38). However, the difference was significant when comparing oral hypoglycaemic drugs to insulin. This result is based on two trials (Hague 2003; Moore 2004) with a total sample size of 90 women. The risk of pre‐eclampsia was found to be reduced with specific treatment for GDM. This may well be due to better detection of pre‐eclampsia associated with the closer monitoring that women with GDM receive. The review found significantly higher rate of neonatal admission with intensive treatment based on the ACHOIS trial (Crowther 2005). This may be because when it is known that a woman has gestational diabetes, her baby will be more closely observed. This may lead to an over‐diagnosis of hypoglycaemia or a lower threshold for neonatal admission. The important outcome to assess is whether this is associated with an increase in long‐term adverse events.
Overall, the results of this review support the need to offer women with a diagnosis of gestational diabetes specific treatment for GDM for better short‐term outcomes. However, it is important to note that there is a lack of long‐term, follow‐up outcomes for both the mother and the baby. The review does not tell us how to screen or who to screen, nor does it provide enough evidence regarding the best mode of treatment for GDM.
Authors' conclusions
Implications for practice.
When women have a diagnosis of gestational diabetes, they should be considered for specific treatment in addition to routine antenatal care. However, the decision about whether to offer specific dietary advice or more intensive treatment including insulin or oral drugs is not yet clear. The effect of specific treatment on long‐term outcomes in the mother and child is also still unclear.
Implications for research.
The review highlights starkly the confusion in this area of healthcare. Consistent and universal recommendations for the diagnosis of gestational diabetes would improve the generalisability of research findings. The fact that one large trial has shown an improvement in a composite outcome for infant morbidity suggests that women should be offered intensive management. However, further large studies comparing different alternative management strategies, considering different ends of the severity spectrum, and using longer‐term single outcomes would be required to make this recommendation stronger.
[Note: the 29 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 1 July 2011 | Amended | Search updated. Nineteen trial reports added to Studies awaiting classification. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 31 January 2009 | New search has been performed | Search updated in April 2008. Five new trials included: Bertini 2005; Crowther 2005; Gillen 2004; Hague 2003; Moore 2004. The results of the January 2009 search have been added to the Studies awaiting classification section. |
| 12 November 2008 | Amended | Converted to new review format. |
| 31 January 2008 | New citation required and conclusions have changed | There is now evidence that offering specific treatment for gestational diabetes may be associated with better baby and mother outcomes, but has not found robust evidence on the best choice of treatment which provides the better outcome for these women and their babies. |
Notes
This updated review was prepared before the release of RevMan 5 and the new Risk of bias tables. This update was therefore prepared in an earlier version of RevMan and converted to RevMan 5 during the editorial processing of the updated review. The Risk of bias tables have therefore not been completed for this updated review and it does not draw on the Group's updated methodology, as described within the editorial information about the Cochrane Pregnancy and Childbirth Group, which were developed to reflect the new statistical options available in RevMan 5. For future updates, the new risk of bias assessments will be described for new studies (identified through updated searches) that meet the inclusion criteria.
Acknowledgements
Thanks to Stephen A Walkinshaw for his contribution to previous versions of this review.
For the current update: R Smyth (RS), while working as Editorial Assistant at the Cochrane Pregnancy and Childbirth Group's editorial base in Liverpool, updated the following sections: Abstract, Types of participants, Methods section, Descriptions of Studies, Methodological quality of included studies and Results section. RS assessed all new studies for inclusion, extracted and entered all the data independently.
Trial authors that provided additional unpublished information and data: Dr Lynda Gillen; Dr Mark Landon; Dr Christian Briery; Dr Carol Homko; Dr Bridget Hsu‐Hage; Dr Ann Dornhorst; Dr John Kitzmiller; Prof Caroline Crowther.
Thanks to Abigail Elvins for translating Silva 2005.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. CENTRAL search strategy used in previous version of the review
#1 pregnan* #2 pregnancy (MeSH ‐ explode all trees) #3 pregnancy complications (MeSH ‐ explode all trees) #4 antepart* #5 antenatal #6 perinatal #7 postnatal #8 postpart* #9 obstetric* #10 gestation* #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 diabet* #13 diabetes mellitus (MeSH ‐ explode all trees) #14 macrosomi* #15 #12 OR #13 OR #14 #16 #15 AND #11
Data and analyses
Comparison 1. Any specific treatment versus routine antenatal care (subgroups by type of specific treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antenatal hospital visits | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.63, 3.63] |
| 1.1 Specific dietary advice | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.63, 3.63] |
| 2 Antenatal hospital admissions | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 2.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 3 Number of extra blood tests | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Specific dietary advice | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Pre‐eclampsia | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.48, 0.88] |
| 4.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.48, 0.88] |
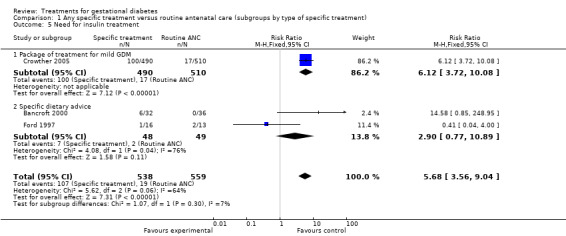
| 5 Need for insulin treatment | 3 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.68 [3.56, 9.04] |
| 5.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.12 [3.72, 10.08] |
| 5.2 Specific dietary advice | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.77, 10.89] |
| 6 Induction of labour | 2 | 1068 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.13, 1.57] |
| 6.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.10, 1.56] |
| 6.2 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.94, 2.40] |
| 7 Operative vaginal delivery | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.07, 17.26] |
| 7.1 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.07, 17.26] |
| 8 Caesarean section | 5 | 1255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| 8.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 8.2 Specific dietary advice | 3 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.62] |
| 8.3 Diet plus insulin for mixed severity | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.36, 1.84] |
| 9 Postpartum haemorrhage | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.58, 1.54] |
| 9.1 Package of treatment for mild GDM | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.58, 1.54] |
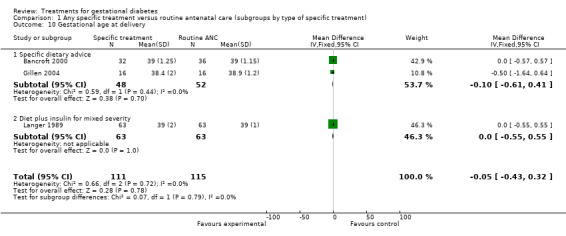
| 10 Gestational age at delivery | 3 | 226 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.43, 0.32] |
| 10.1 Specific dietary advice | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.61, 0.41] |
| 10.2 Diet plus insulin for mixed severity | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.55, 0.55] |
| 11 Birthweight > 4000 g | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.63] |
| 11.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.63] |
| 12 Birthweight > 90th centile | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 0.99] |
| 12.1 Specific dietary advice | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.97] |
| 12.2 Diet plus insulin for mixed severity | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.76] |
| 13 Perinatal death | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.70] |
| 13.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.70] |
| 14 Bone fracture in infant | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.45] |
| 14.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.45] |
| 15 Nerve palsy in infant | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.86] |
| 15.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.86] |
| 16 Shoulder dystocia | 2 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.19, 1.04] |
| 16.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.19, 1.09] |
| 16.2 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.86] |
| 17 Composite outcome in infant (death, shoulder dystocia, nerve palsy, bone fracture) | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.73] |
| 17.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.73] |
| 18 Admission to neonatal care/special baby care unit | 3 | 1224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.03, 1.23] |
| 18.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.26] |
| 18.2 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.73] |
| 18.3 Diet plus insulin for mixed severity | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.86] |
| 19 Respiratory disress syndrome/need for ventilation in infant | 2 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.86, 2.63] |
| 19.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.61] |
| 19.2 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.21, 23.66] |
| 20 Neonatal hypoglycemia | 3 | 1224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.63, 1.48] |
| 20.1 Package of treatment for mild GDM | 1 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.82, 2.18] |
| 20.2 Specific dietary advice | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.73] |
| 20.3 Diet plus insulin for mixed severity | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.97] |
| 21 Length of hospital stay (days) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐0.46, 2.40] |
| 21.1 Specific dietary advice | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐0.46, 2.40] |
1.1. Analysis.

Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 1 Antenatal hospital visits.
1.2. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 2 Antenatal hospital admissions.
1.3. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 3 Number of extra blood tests.
1.4. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 4 Pre‐eclampsia.
1.5. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 5 Need for insulin treatment.
1.6. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 6 Induction of labour.
1.7. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 7 Operative vaginal delivery.
1.8. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 8 Caesarean section.
1.9. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 9 Postpartum haemorrhage.
1.10. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 10 Gestational age at delivery.
1.11. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 11 Birthweight > 4000 g.
1.12. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 12 Birthweight > 90th centile.
1.13. Analysis.
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 13 Perinatal death.
1.14. Analysis.
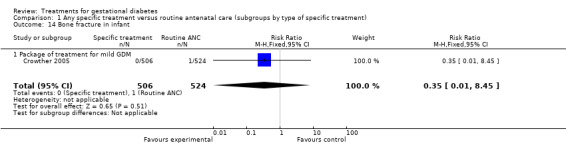
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 14 Bone fracture in infant.
1.15. Analysis.
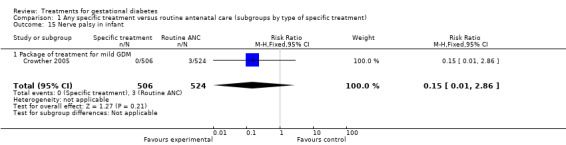
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 15 Nerve palsy in infant.
1.16. Analysis.
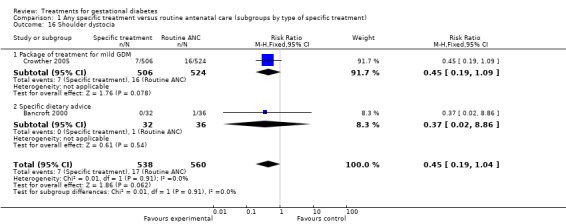
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 16 Shoulder dystocia.
1.17. Analysis.
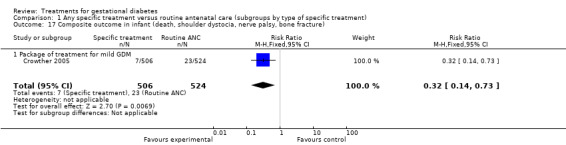
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 17 Composite outcome in infant (death, shoulder dystocia, nerve palsy, bone fracture).
1.18. Analysis.
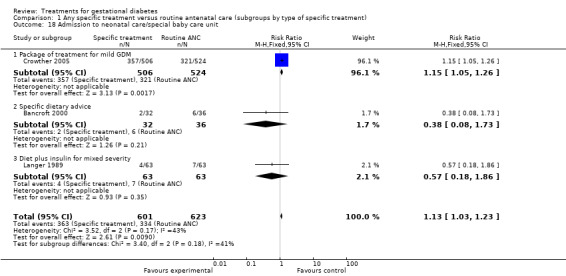
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 18 Admission to neonatal care/special baby care unit.
1.19. Analysis.
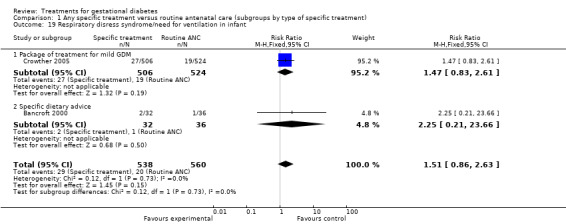
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 19 Respiratory disress syndrome/need for ventilation in infant.
1.20. Analysis.

Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 20 Neonatal hypoglycemia.
1.21. Analysis.
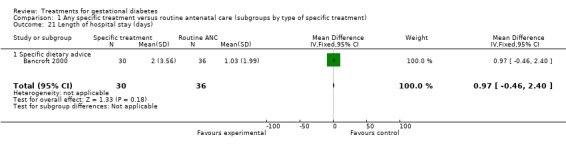
Comparison 1 Any specific treatment versus routine antenatal care (subgroups by type of specific treatment), Outcome 21 Length of hospital stay (days).
Comparison 2. Oral hypoglycemic drugs versus insulin therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.27, 0.77] |
| 2 Operative vaginal delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.59] |
| 3 Induction of labour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.90, 4.69] |
| 4 Postpartum haemorrhage | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 5 Gestational age at delivery | 3 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 6 Birthweight > 4000 g | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.60, 4.86] |
| 7 Birthweight > 90th centile | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [0.87, 52.14] |
| 8 Birthweight (g) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 244.40 [‐15.61, 504.41] |
| 9 Neonatal hypoglycemia | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.68 [1.47, 40.22] |
| 10 Admission to neonatal care/special baby care unit | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.41, 10.47] |
| 11 Shoulder dystocia | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 12 Respiratory disress syndrome/need for ventilation in infant | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.62] |
2.1. Analysis.

Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 1 Caesarean section.
2.2. Analysis.
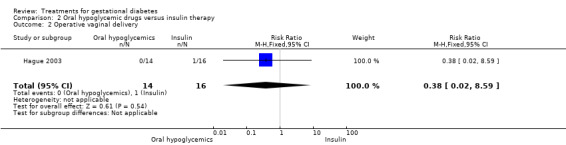
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 2 Operative vaginal delivery.
2.3. Analysis.
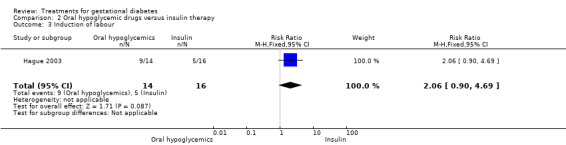
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 3 Induction of labour.
2.4. Analysis.
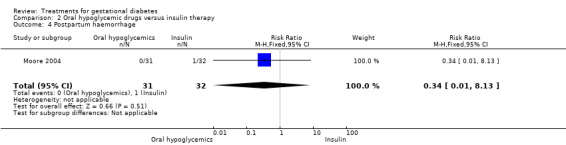
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 4 Postpartum haemorrhage.
2.5. Analysis.
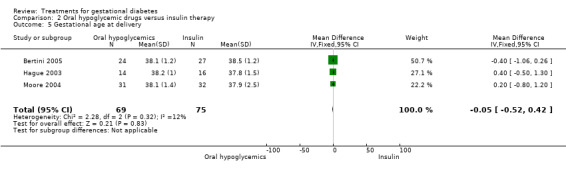
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 5 Gestational age at delivery.
2.6. Analysis.
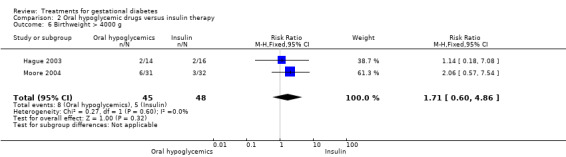
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 6 Birthweight > 4000 g.
2.7. Analysis.
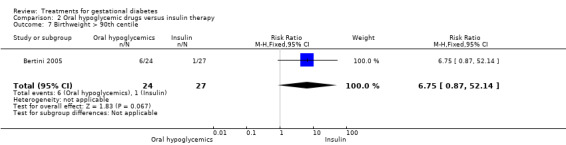
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 7 Birthweight > 90th centile.
2.8. Analysis.
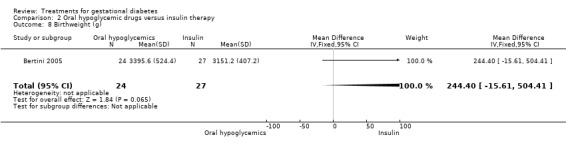
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 8 Birthweight (g).
2.9. Analysis.
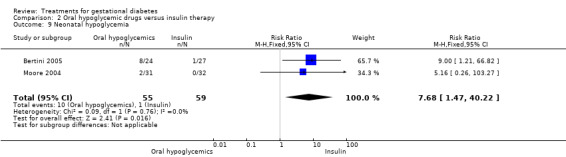
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 9 Neonatal hypoglycemia.
2.10. Analysis.
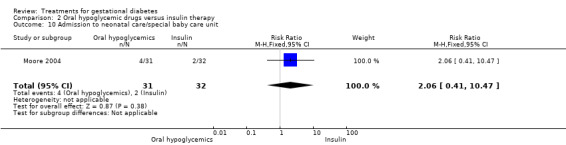
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 10 Admission to neonatal care/special baby care unit.
2.11. Analysis.
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 11 Shoulder dystocia.
2.12. Analysis.
Comparison 2 Oral hypoglycemic drugs versus insulin therapy, Outcome 12 Respiratory disress syndrome/need for ventilation in infant.
Comparison 3. Acarbose versus insulin therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational age at delivery | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.00, 0.40] |
| 2 Birthweight (g) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 91.40 [‐145.32, 328.12] |
| 3 Birthweight > 90th centile | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.28, 29.14] |
| 4 Neonatal hypoglycemia | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.09, 21.34] |
3.1. Analysis.
Comparison 3 Acarbose versus insulin therapy, Outcome 1 Gestational age at delivery.
3.2. Analysis.
Comparison 3 Acarbose versus insulin therapy, Outcome 2 Birthweight (g).
3.3. Analysis.
Comparison 3 Acarbose versus insulin therapy, Outcome 3 Birthweight > 90th centile.
3.4. Analysis.

Comparison 3 Acarbose versus insulin therapy, Outcome 4 Neonatal hypoglycemia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bancroft 2000.
| Methods | Randomised controlled pilot study recruiting from 2 centres. Randomisation method: computer‐generated codes, telephone randomisation service used. Blinding of intervention: "the diabetologist was aware of the group to which each woman was randomised but the obstetrician was blinded". Blinding of outcome assessment: not stated. No drop‐outs but 12 failed to attend follow‐up postnatal measurements. Intention‐to‐treat analysis: not stated (but all women remained in their allocated groups). | |
| Participants | 68 women. Inclusion criteria: impaired glucose tolerance (defined following 75 gm OGTT as fasting < 7.0 mmol/L, 2‐hours between 7.8 mmol/L and 11.0 mmol/L). Exclusions: none stated. Setting: specialist diabetic/AN clinics. | |
| Interventions | Monitored group were given standard dietary advice, glucose metabolism was monitored by capillary glucose series 5 days a week, HbA1c was measured monthly (insulin was introduced if 5 or more capillary measurements > 7.0 mmol/L in 1 week), serial ultrasound for growth and amniotic fluid, Doppler studies, CTG monitoring. Unmonitored group received dietary advice, HbA1c monthly but no capillary glucose measurements. | |
| Outcomes | Primary outcome measure was admission to special care baby unit. Secondary outcomes: perinatal morbidity (including birth trauma, metabolic disturbance, gestation at delivery, birthweight), measures of maternal inconvenience, number of antenatal clinic visits, mode of delivery, frequency of insulin use. | |
| Notes | 2 women in the unmonitored group developed diabetes mellitus, both were diagnosed postnatally and both delivered prematurely. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bertini 2005.
| Methods | Unblinded randomised controlled trial recruited from 1 centre in Brazil. | |
| Participants | 70 women. Inclusion criteria: GDM diagnosed as >= 110 mg/dl fasting plasma glucose and >= 140 mg/dl OGTT after 2 h of 75 g of glucose. | |
| Interventions | 3 groups: insulin as conventional therapy, glyburide, and acarbose. All women received diet and exercise programmes. | |
| Outcomes | Gestational age at birth, Apgar scores, birthweight, neonatal hypoglycaemia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Crowther 2005.
| Methods | Multi centre trial: 18 centres in Australia and UK. Randomisation method: performed centrally with the use of numbers generated by computer with variable block sizes of 6, 8, and 10. Blinding of intervention and outcome assessment: women that were assigned to the intervention group received a slip indicating a diagnosis of glucose intolerance and the plan for intervention, women assigned to routine care received a slip indicating they did not have GD. The full numerical results of the oral glucose‐tolerance test were not released to the women or their providers until after birth, before discharge from hospital. Dropouts: no losses to follow up for primary and secondary clinical outcomes for women. No losses to follow up for primary and secondary outcomes for infants. Intention‐to‐treat analysis was used. | |
| Participants | 1000 women. Inclusion criteria: impaired glucose tolerance (defined as having one or more risk factors for GD on selective screening or a positive 50 g oral glucose challenge test (glucose level 1 hour after glucose challenge at least 7.8 mmol per litre [140 mg per decilitre])), and had a 75 g OGTT at 24 to 34 weeks' gestation in which the venous plasma glucose level was less than 7.8 mmol per litre after an overnight fast and was 7.8 to 11.0 mmol per litre (198 mg per decilitre) at 2 hours). Exclusions: women with previously treated GD or active chronic systemic disease (except essential hypertension), more severe impairment or less than 16 or more than 30 weeks' pregnant. Setting: antenatal clinic. | |
| Interventions | Intervention group: care replicated clinical care in which universal screening and treatment for GD was available, individualised dietary advice from a qualified dietician, instructions on how to self‐monitor glucose levels 4 times a day until fasting glucose levels of at least 3.5 mmol per litre [63 mg per decilitre] and no more than 5.5 mmol per litre [99 mg per decilitre], preprandial levels of no more than 5.5 mmol per litre, and levels 2 hours postprandially that were no more than 7.0 mmol per litre [126 mg per decilitre], followed by daily monitoring at rotating times during the day; and insulin therapy, with the dose adjusted based on glucose levels, if there were 2 capillary‐blood glucose results during the 2‐week period in which the fasting level was at least 5.5 mmol per litre or the postprandial level was at least 7.0 mmol per litre at 35 weeks' gestation or less, if the postprandial level was at least 8.0 mmol per litre (144 mg per decilitre) at more than 35 weeks' gestation, or if one capillary‐blood glucose results during the 2‐week period was at least 9.0 mmol per litre (162 mg per decilitre). Control group: care replicated clinical care in which screening for GD was not available, women and caregivers were not aware of the diagnosis of glucose intolerance, at the discretion of the attending clinician, if indications arose that were suggestive of diabetes, further assessment for GD was permitted, with treatment as considered appropriate. | |
| Outcomes | Primary outcomes ‐ infant: composite measures of serious perinatal complications (defined as 1 or more of death, shoulder dystocia, bone fracture, and nerve palsy), admission to neonatal nursery, and jaundice requiring phototherapy. Primary outcomes ‐ women: need for IOL and caesarean section, health status, and psychological outcomes. Secondary outcomes ‐ infant: gestational age at birth, birthweight, Apgar score of less than 7 at 5 minutes, hypoglycaemia requiring IV therapy, convulsions, RDS. Secondary outcomes ‐ women: number of prenatal visits to a health professional, mode of birth, weight during pregnancy, number of antenatal admissions, presence or absence of PIH, perineal trauma, PPH, breastfeeding at hospital discharge. | |
| Notes | 93% of the women had been found to be at risk of GD on the basis of OGTT, and the remainder on the basis of risk factors. 5 perinatal deaths (3 stillbirths and 2 neonatal deaths) occurred in the control group: 2 stillbirths were unexplained intrauterine deaths at term of appropriately grown infants, and 1 at 35 weeks' gestation, was associated with pre‐eclampsia and intrauterine growth restriction. 1 infant had a lethal congenital anomaly, and 1 infant died after an asphyxial condition during labour with antepartum haemorrhage. After consent had been obtained, a proportion of the women (not fewer than 1 in 5) who had normal oral GTT results were assigned to the routine‐care group to help maintain blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ford 1997.
| Methods | Single centre randomised trial. Randomisation method: sealed opaque envelopes. Blinding of intervention: not stated. Blinding of outcome assessment: not stated. Dropouts: 5 withdrawn from the study ‐ 3 for insulin therapy, other 2 unaccounted for (possibly withdrawn following dietary advice but unclear). Intention‐to‐treat analysis: not stated. | |
| Participants | 29 women. Inclusion criteria: impaired glucose tolerance (defined following a 75 g OGTT as 2‐hour plasma glucose level between 8 mmol/L and 11 mmol/L. Exclusions: none stated. Setting: joint diabetic/AN clinic. | |
| Interventions | Dietary treatment group were given specific 'diabetic type' advice (i.e. 'high fibre, high carbohydrate, low fat and appropriate energy'). The control group received no specific dietary advice. Both groups attended clinic weekly and performed plasma glucose profiles. | |
| Outcomes | Outcomes measured: caesarean section, birthweight, use of insulin. | |
| Notes | If plasma glucose levels were raised in the non‐diet group they were given dietary advice or insulin therapy, or both. An attempt to diagnose macrosomia by ultrasound was made from 30 weeks in both groups. The trial did not recruit its expected sample. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gillen 2004.
| Methods | Single centre randomised controlled trial. Randomisation method: open table of random numbers constructed by an independent person and kept confidential from members of the study team. Women were matched consecutively to the next available number in the table and the study team informed of the result. Blinding of intervention: yes, participants unaware of differences in advice between intervention and control groups. Blinding of outcome assessment: not stated. Intention‐to‐treat analysis was used. Dropouts: data not available for 1 woman from each group. | |
| Participants | 32 women.
Inclusion criteria: GDM (defined on an OGTT defined by the Australian Diabetes in Pregnancy Society).
Exclusions: significant other health concerns, poor English language skills.
Setting: diabetic clinic. Intention‐to‐treat analysis was used. |
|
| Interventions | Control group: standard clinical practice (individualised carbohydrate portion‐controlled meal plan, with low‐fat and low‐glycaemic index dietary strategies and general advice about meeting nutritional requirements of pregnancy). Intervention group: standard clinical practice as above, plus advice for targeted intakes of foods rich in unsaturated fats. | |
| Outcomes | Outcomes: gestation at delivery, mode of delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hague 2003.
| Methods | Pilot study in Australia. Randomisation method: 'women randomised'. Blinding of intervention: not stated. Blinding of outcome assessment: not stated. Dropouts: none reported. Intention‐to‐treat analysis: not stated. | |
| Participants | 30 women. Inclusion criteria: GD diagnosed by the criteria of the Australasian Diabetes in Pregnancy Society. Exclusions: not stated. Setting: not stated. | |
| Interventions | Intervention group: metformin. Control group: insulin. | |
| Outcomes | Outcomes: mode of delivery, pre‐eclampsia, fetal beta cell activity, birthweight, median time in SCBU, neonatal jaundice, need for neonatal phototherapy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Langer 1989.
| Methods | Described as a "randomised trial" ‐ no further information. Method of randomisation: computer‐generated list (information obtained directly from the author). Blinding of intervention: not stated. Blinding of outcome assessment: not stated. Participation rates not given, no dropouts/withdrawals reported. Intention‐to‐treat analysis: not stated. | |
| Participants | 126 women (a further 146 with normal OGTT results were used as a control group but their data have not been included in this review). Inclusion criteria: 1 abnormal value following 100 g OGTT (according to National Diabetes Data Group values fasting > 105 mg/dl, 1‐hour > 10.6 mg/dl, 2‐hour > 165 mg/dl, 3‐hour > 145 mg/dl). Exclusions: none stated. Setting: medical centre. | |
| Interventions | 3 groups in this study ‐ 'treated', 'untreated' and a control group of women with normal screening/OGTT results. (Data for this group are not included in this review.) All participants monitored capillary blood glucose 7 times/day. (This was just for a 4‐week period for the untreated group.) Treated group were managed according to diabetic protocol including dietary advice (determined by pre‐pregnancy body mass index), insulin treatment based on 0.7 units per kg of body weight measured in pregnancy. The untreated group continued normal eating patterns. | |
| Outcomes | Outcomes measured: gestation at delivery, weight gain, caesarean section, hypertensive disorder, birthweight, prematurity, neonatal hypoglycaemia, neonatal hyperbilirubinemia, neonatal polycythemia, NICU admission. | |
| Notes | National diabetes data group criteria include 4 values: fasting, 1‐hour, 2‐hour, 3‐hour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Moore 2004.
| Methods | Single centre randomised trial. Randomisation method: list of tables used, sealed envelopes. Blinding of intervention: not stated. Blinding of outcome assessment: no. Dropouts: none. | |
| Participants | 63 women. Inclusion criteria: impaired glucose tolerance (based on 100 g oral OGTT). Exclusions: insulin‐dependent diabetes mellitus, liver/kidney disease, chronic hypertension or seizure disorders, less than 11 weeks or more than 36 weeks' gestation. Setting: not stated. | |
| Interventions | Intervention group: insulin. Control group: metformin. | |
| Outcomes | Gestational age at birth, mode of delivery, shoulder dystocia, PPH, birthweight, Apgar score at 5 minutes, NICU admission, hypoglycaemia, RDS, hyperbilirubinemia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AN: antenatal CTG: cardiotocography GD: gestational diabetes GDM: gestational diabetes mellitus GTT: glucose tolerance test h: hour IOL: induction of labour IV: intravenous NICU: neonatal intensive care unit OGTT: oral glucose tolerance test PIH: pregnancy‐induced hypertension (defined as blood pressure of at least 140/90) PPH: postpartum haemorrhage RDS: respiratory distress syndrome SCBU: special care baby unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anjalakshi 200 | Not correct outcomes for this review. |
| Bevier 1999 | Not predetermined population; those studied had normal OGTT results. |
| Bonomo 2004 | Not correct population for this review. |
| Bonomo 2005 | Used 50 g glucose challenge test. Not correct population for this review. |
| Brankston 2004 | Not correct intervention for this review ‐ comparing exercise. |
| Buchanan 1994 | Not correct population for this review. Population studied were those with a fetal abdominal circumference above 75th centile. |
| Bung 1991 | Not correct diagnostic criteria for this review. Eligibility was based on persistent fasting plasma glucose at values not matching those specified for this review (5.8 mM‐7.22 mM). |
| Coustan 1978 | Not truly randomised. First 20 women were assigned treatment based on gestation. |
| Cypryk 2007 | Outcomes measures used not under outcomes considered by this review. |
| De Veciana 2002 | Not predetermined population or intervention for this review. |
| Di Cianni 2007 | Compares two types of insulin (short acting versus regular insulin). |
| Dornhorst 1989 | No data available. |
| Dunne 2001 | No published or unpublished information on this trial, unable to trace author. |
| Garner 1997 | Not diagnostic criteria specified for this review. This study used 2‐hour upper limits of 7.5 mmol/l for women in 2nd trimester and 9.6 mmol/l for women in 3rd trimester. |
| Gillmer 1986 | Diagnosis by 50 g OGTT. Not randomised. |
| Graham 2005 | Not predetermined intervention for this review (Cinnamon). |
| Homko 2002 | Eligibility criteria: all women underwent a 3‐hour OGTT and needed to have 2 abnormal values based on the Carpenter‐Coustan criteria but a normal FBS (< 95 mg/dl). |
| Hopp 1996 | Diagnosis by 50 g OGTT. |
| Ilic 1999 | Outcomes measures used not under outcomes considered by this review. |
| Jovanovic 1999 | Not correct intervention for this review ‐ comparing regular human insulin with insulin lispro. |
| Jovanovic‐Peterson 1989 | Not truly randomised, inadequate baseline comparability of groups. |
| Jovanovic‐Peterson 1993 | Not an intervention predetermined by this review. |
| Kitzmiller 1990 | Informed by author that trial never started (March 2006). |
| Kjos 2001 | Inclusion based on 1 fasting glucose result. Therefore not the correct inclusion criteria for this review. |
| Langer 1994 | Not randomised. |
| Langer 2000 | Eligibility criteria: fasting glucose of more than 5.3. |
| Lao 2001 | Correspondence, not a study. |
| Lauszus 2001 | Not the correct outcomes for this review. |
| Lesser 1996 | Crossover design. |
| Li 1987 | Not true randomisation. |
| Li 1999 | Not randomised ‐ allocation by consent to treatment. |
| Magee 1990 | Not predetermined outcomes and specific to obese women. |
| Mecacci 2003 | Not correct intervention for this review ‐ comparing 2 types of insulin. |
| MRC 1955 | Not correct population for this review. |
| Nolan 1984 | Crossover design, no control group. |
| Nor Azlin 2007 | Not correct intervention for this review. Comparing 2 types of insluin (short vs inetrmediate acting). No diagnostic criteria specified. |
| Notelovitz 1971 | Population includes established diabetics ‐ impossible to distinguish between established and gestational in the data. |
| O'Sullivan 1971 | Not randomised, alternate allocation. |
| O'Sullivan 1974 | Primary outcome death. No details of randomisation, no intention‐to‐treat analysis. Endpoints unclear. |
| O'Sullivan 1980 | Not randomised. |
| Pardi 1989 | Study not completed, insufficient data collected to analyse. |
| Persson 1985 | Raw data no longer available for analysis. |
| Pettitt 2003 | Not correct outcomes measures. |
| Pettitt 2007 | Not correct intervention for this review ‐ comparing 2 types of insulin. Not correct diagnostic crietria. |
| Polyhonen‐Alho 2002 | Not correct diagnostic criteria for this review. Diagnosis of GD was made on the following values: fasting 4.8 mmol/l, 2‐hour 8.7 mmol/l. |
| Rae 2000 | Incorrect population for this review ‐ obese women only. |
| Reader 2006 | Cluster RCT. Diagnostic criteria not specified. |
| Reece 1995 | Diagnosis of gestational diabetes not clearly defined. HbA1c levels only given for IDDM. |
| Rey 1995 | Review criteria not met. This was a study of a sweet breakfast screening test therefore, not the appropriate diagnostic criteria for this review. |
| Rey 1997 | Not correct diagnostic criteria for this review. Some participants were included in the study following 50 g glucose screen. OGTT diagnostic values used did not match the criteria for this review. |
| Rossi 2000 | Not correct diagnostic criteria for this review. This study diagnosed GD by the following values: fasting 95 mg/dl, 1‐hour 180 mg/dl, 2‐hour 155 mg/dl, 3‐hour 140 mg/dl. |
| Schaefer‐Graf 2004 | Not correct population for this review. |
| Schuster 1998 | Incorrect population. |
| Silva 2005 | Not predetermined diagnostic criteria of GDM. |
| Snyder 1998 | Eligibility was by OGTT results or 50 g screening test ‐ 50 g screening test not correct eligibility criteria for this review. |
| Thompson 1990 | Gestational diabetes and IGT not divided. Unable to separate data. |
| Todorova 2007 | Economic analysis. |
| Yang 2003 | Attrition rate of 50%. |
FBS: fasting blood sugar GD: gestational diabetes IDDM: insulin‐dependent diabetes mellitus IGT: impaired glucose tolerance OGTT: oral glucose tolerance test RCT: randomised controlled trial vs: versus
Characteristics of ongoing studies [ordered by study ID]
Crowther 2007.
| Trial name or title | Dietary and lifestyle advice for women with borderline gestational glucose intolerance. |
| Methods | |
| Participants | All pregnant women between 24 weeks and 0 day and 29 weeks and 6 days gestation with a singleton pregnancy, attending antenatal clinics at the collaborating hospitals, with a positive OGCT (plasma glucose greater than or equal to 7.8mmol/L) and a normal OGTT (fasting venous plasma glucose less than 5.5 mmol/L and a 2‐hour glucose less than 7.8 mmol/L), who give written, informed consent. There is no age limit criteria for this trial. There is no minimum age limit for the mother. |
| Interventions | Individualised advice regarding their diet from a qualified dietician, based on published recommendations of the Dieticians Association of Australia, that are culturally appropriate and meet the nutritional requirements of pregnancy. Moderate exercise is recognised as an adjunct to dietary advice compared with routine pregnancy care. |
| Outcomes | 1. Risk of infant morbidity (measured by serious perinatal complications, number of large for gestational age infants, neonatal jaundice requiring phototherapy and neonatal hypoglycaemia requiring treatment); measured once, at time of primary hospital discharge or death prior to discharge. 2. Risk of maternal physical morbidity (measured by need for induction of labour, need for caesarean section and pre‐eclampsia); measured up to primary hospital discharge. 3. Maternal psychological outcomes and health status (as measured by anxiety, depression and health related quality of life). |
| Starting date | Not yet recruiting. |
| Contact information | caroline.crowther@adelaide.edu.au |
| Notes |
Golladay 2006.
| Trial name or title | Randomised trial of treatment for gestational diabetes. |
| Methods | |
| Participants | Pregnant women over age 18 who fail to achieve adequate glucose control on diet therapy alone. |
| Interventions | Glyburide. |
| Outcomes | Primary outcome measure: newborn birthweight. Secondary outcome measures: gestational age at delivery; method of delivery (caesarean, forceps, vacuum, spontaneous); complications of delivery (shoulder dystocia, birth injury, 4th degree vaginal laceration); newborn intensive care unit admission; congenital anomalies of the newborn; incidence of neonatal metabolic derangement. |
| Starting date | June 2004. |
| Contact information | Elizabeth C Golladay elizabeth.golladay@us.army.mil |
| Notes |
Kipikasa 2008.
| Trial name or title | Comparison of Glucovance to Insulin for Diabetes During Pregnancy. |
| Methods | |
| Participants | 24‐28 weeks' gestations with diagnostic OGTT. |
| Interventions | Glucovance (glyburide and metformin) versus insulin therapy. |
| Outcomes | Primary outcome measures:
maternal hemoglobin A1C at delivery;
maternal fructosamine at delivery;
maternal glucose at delivery. Secondary outcome measures: mode of delivery; infant birthweight; infant initial glucose; infant complications. |
| Starting date | Recruiting. |
| Contact information | Joseph H Kipikasa, MD Regional Obstetrical Consultants; UT Chattanooga OB‐GYN Department. |
| Notes | Estimated completion date September 2008. |
Landon 2002.
| Trial name or title | A randomised clinical trial of treatment for mild gestational diabetes mellitus. |
| Methods | |
| Participants | Pregnant women, with singleton pregnancies, diagnosed with mild gestational diabetes (1‐hour glucose loading test result between 135 and 200 mg/dl followed by a 3‐hour OGTT with normal fasting, ie less than 95 mg/dl) between 24 and 29 weeks' gestation. |
| Interventions | Diet therapy and insulin as required versus no specific treatment. |
| Outcomes | Composite outcome of neonatal morbidity. |
| Starting date | We do not have this information. |
| Contact information | Dr Mark B Landon. Email address mlandon@columbus.rr.com |
| Notes | 07/03/06 ‐ Information from trialist: the trial is ongoing for approximately 18 additional months. |
Rowan 2005.
| Trial name or title | The Metformin in Gestational Diabetes (MiG) Trial. |
| Methods | |
| Participants | Women aged 18‐45 with singleton pregnancies with fasting glucose > 5.4 or 2 hour GTT > 6.7 mmol/l. |
| Interventions | Metformin versus insulin therapy. |
| Outcomes | Perinatal complications, neonatal anthropometry. |
| Starting date | We do not have this information. |
| Contact information | Janet A Rowan. Email jrowan@internet.co.nz |
| Notes |
GTT: glucose tolerance test OGCT: oral glucose challenge test OGTT: oral glucose tolerance test
Differences between protocol and review
We added the outcome 'composite' (defined as one or more of the following: infant death, shoulder dystocia, bone fracture and nerve palsy) to the list of neonatal outcomes. Although this outcome was not included in the original protocol for the review, it has been added as it is the primary outcome used by the ACHOIS trial (Crowther 2005), which includes the biggest sample among all the other studies included in this review.
Contributions of authors
D Tuffnell (DT), J West (JW), S Walkinshaw (SW) were involved in the planning and approval of the first version of the review. DT and JW wrote the protocol and text of the review incorporating comments from SW. The review was updated by N Alwan, DT and JW with the support of the Cochrane Pregnancy and Childbirth Group editorial base (see 'Acknowledgements').
Sources of support
Internal sources
No sources of support supplied
External sources
The University of Liverpool, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bancroft 2000 {published data only}
- Bancroft K, Tuffnell DJ, Mason GC, Rogerson LJ, Mansfield M. A randomised controlled pilot study of the management of gestational impaired glucose tolerance. BJOG: an international journal of obstetrics and gynaecology 2000;107(8):959‐63. [DOI] [PubMed] [Google Scholar]
- Bancroft K, Tuffnell DJ, Mason GC, Rogerson LJ, Mansfield M. A randomised controlled study of the management of impaired glucose tolerance in pregnancy. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):53‐4. [DOI] [PubMed] [Google Scholar]
Bertini 2005 {published data only}
- Bertini AM, Silva JC, Taborda W, Becker F, Bebber FR, Viesi JM, et al. Perinatal outcomes and the use of oral hypoglycemic agents. Journal of Perinatal Medicine 2005;33(6):519‐23. [DOI] [PubMed] [Google Scholar]
Crowther 2005 {published data only}
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Does antenatal treatment of screen detected impaired glucose tolerance improve health outcomes in women and their infants? The ACHOIS trial. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:26.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477‐86. [DOI] [PubMed] [Google Scholar]
- Moss JR, Crowther CA, Hiller JE, McPhee AJ, Jeffries WS, Willson KJ. Costs and consequences of treatment of gestational diabetes mellitus ‐ evaluation from the ACHOIS randomised trial [abstract]. Journal of Paediatrics and Child Health 2007;43(1):A28‐A29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JR, Crowther CA, Hiller JE, Willson KJ, Robinson JS, for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Costs and consequences of treatment for mild gestational diabetes mellitus ‐ evaluation from the ACHOIS randomised trial. BMC Pregnancy and Childbirth 2007;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirc LK, Owens JA, Crowther CA, Willson K, Blasio MJ, Robinson JS. Mild gestational diabetes in pregnancy and the adipoinsular axis in babies born to mothers in the ACHOIS randomised controlled trial. BMC Pediatrics 2007;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 1997 {unpublished data only}
- Ford FA, Bruce CB, Fraser RB. Preliminary report of a randomised trial of dietary advice in women with mild abnormalities of glucose tolerance in pregnancy. Personal communication 1997.
Gillen 2004 {published and unpublished data}
- Gillen LJ, Tapsell LC. Advice that includes food sources of unsaturated fat supports future risk management of gestational diabetes mellitus. Journal of the American Dietetic Association 2004;104:1863‐7. [DOI] [PubMed] [Google Scholar]
Hague 2003 {published data only}
- Hague WM, Davoren PM, Oliver J, Rowan J. Contraindications to use of metformin. Metformin may be useful in gestational diabetes. BMJ 2003;326:762. [PubMed] [Google Scholar]
Langer 1989 {published data only}
- Langer O, Anyaegbunam A, Brustman L, Divon M. A prospective randomized study: management of women with one abnormal value (OGTT) reduces adverse outcome in pregnancy. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:11.
- Langer O, Anyaegbunam A, Brustman L, Divon M. Management of women with one abnormal oral glucose tolerance test value reduces adverse outcome in pregnancy. American Journal of Obstetrics and Gynecology 1989;161:593‐9. [DOI] [PubMed] [Google Scholar]
Moore 2004 {published and unpublished data}
- Moore L, Briery C, Martin R, Hood E, Bofill J, Morrison J. Metformin (M) vs. insulin (I) in A2 diabetes: a randomized clinical trial [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S8. [Google Scholar]
- Moore L, Clokey D, Robinson A. A randomized trial of metformin compared to glyburide in the treatment of gestational diabetes [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S92. [Google Scholar]
- Moore LE, Briery CM, Clockley D, Martin RW, Williford NJ, Bofill JA et a. Metformin and insulin in the management of gestational diabetes mellitus: Preliminary results of a comparison. Journal of Reproductive Medicine 2007;52(11):1011‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Anjalakshi 200 {published data only}
- Anjalakshi C, Balaji B, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Reserach and Clinical Practice 2007;76(3):474‐5. [DOI] [PubMed] [Google Scholar]
Bevier 1999 {published data only}
- Bevier WC, Fischer R, Jovanovic L. Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. American Journal of Perinatology 1999;16:269‐75. [DOI] [PubMed] [Google Scholar]
Bonomo 2004 {published data only}
- Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes & Metabolism 2004;30(3):237‐44. [DOI] [PubMed] [Google Scholar]
Bonomo 2005 {published data only}
- Bonomo M, Corica D, Mion E, Goncalves D, Motta G, Merati R, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabetic Medicine 2005;22(11):1536‐41. [DOI] [PubMed] [Google Scholar]
Brankston 2004 {published data only}
- Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2004;190(1):188‐93. [DOI] [PubMed] [Google Scholar]
Buchanan 1994 {published data only}
- Buchanan TA, Kjos SL, Montoro MN, Wu PYK, Madrilejo NG, Gonzalez M, et al. Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care 1994;17:275‐83. [DOI] [PubMed] [Google Scholar]
Bung 1991 {published data only}
- Bung P, Artal R, Khodiguian N, Kjos S. Exercise in gestational diabetes. Diabetes 1991;40(Suppl 2):182‐5. [DOI] [PubMed] [Google Scholar]
Coustan 1978 {published data only}
- Coustan DR, Lewis SB. Insulin therapy for gestational diabetes. Obstetrics & Gynecology 1978;51:306‐10. [DOI] [PubMed] [Google Scholar]
Cypryk 2007 {published data only}
- Cypryk K, Kaminska P, Kosinski M, Pertynska‐Marczewska M, Lewinski A. A comparison of the effectiveness, tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynologia Polska 2007; Vol. 58, issue 4:314‐9. [CN‐00621573] [PubMed]
De Veciana 2002 {published data only}
- Veciana M, Trail PA, Evans AT, Dulaney K. A comparison of oral acarbose and insulin in women with gestational diabetes. Obstetrics & Gynecology 2002;99(4 Suppl):5S. [Google Scholar]
Di Cianni 2007 {published data only}
- Cianni G, Volpe L, Ghio A, Lencioni C, Cuccuru I, Benzi L, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes mellitus treated with lispro or aspart insulin: comparison with regular insulin. Diabetes Care 2007;30(4):e11. [DOI] [PubMed] [Google Scholar]
Dornhorst 1989 {unpublished data only}
- Dornhorst A. Trial to assess the effects of dietary manipulation in women with mild gestational diabetes on maternal carbohydrate metabolism and fetal growth. Personal communication 1989.
Dunne 2001 {published data only (unpublished sought but not used)}
- Dunne FP. Randomised trial of twice daily versus four time daily insulin regimens for the treatment of gestational diabetes and gestationally acquired impaired glucose tolerance. National Research Register (www.update‐software.com/nrr) (accessed 10 October 2002).
Garner 1997 {published data only}
- Garner P, Okun N, Keely E, Wells G, Perkins S, Sylvain J, et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. American Journal of Obstetrics and Gynecology 1997;177(1):190‐5. [DOI] [PubMed] [Google Scholar]
- Okun N. Gestational Diabetes Study. Personal communication 1994.
Gillmer 1986 {published data only}
- Gillmer MDG, Maresh M, Beard RW, Elkeles RS, Alderson C, Bloxham B. Low energy diets in the treatment of gestational diabetes. Acta Endocrinologica 1986;277:44‐9. [DOI] [PubMed] [Google Scholar]
- Maresh M, Alderson C, Beard RW, Bloxham B, Elkeles RS, Gillmer MDG. Comparison of insulin against diet treatment in the management of abnormal carbohydrate tolerance in pregnancy. In: Campbell DM, Gillmer MDG editor(s). Nutrition in pregnancy. Proceedings of 10th Study Group of the Royal College of Obstetricians and Gynaecologists. London: Royal College of Obstetricians and Gynaecologists, 1983:255‐67. [Google Scholar]
- Maresh M, Gillmer MDG, Beard RW, Alderson CS, Bloxham BA, Elkeles RS. The effect of diet and insulin on metabolic profiles of women with gestational diabetes mellitus. Diabetes 1985;34:88‐93. [DOI] [PubMed] [Google Scholar]
Graham 2005 {published data only}
- Graham G, Johnson EB, Johnson A, Anderson R, Devine P. Cinnamon for glycemic control in gestational diabetes: a randomized double‐blind placebo controlled pilot study [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S91. [Google Scholar]
Homko 2002 {published and unpublished data}
- Homko CJ, Sivan E, Reece EA. The impact of self‐monitoring of blood glucose on self‐efficacy and pregnancy outcomes in women with diet‐controlled gestational diabetes. Diabetes Educator 2002;28(3):435‐43. [DOI] [PubMed] [Google Scholar]
Hopp 1996 {published data only}
- Hopp H, Vollert W, Ragosch V, Novak A, Weitzel HK, Glockner E, et al. Indication and results of insulin therapy for gestational diabetes mellitus. Journal of Perinatal Medicine 1996;24:521‐30. [DOI] [PubMed] [Google Scholar]
- Novak A, Hopp H, Vollert W, Weitzel H, Glockner E. Fetal indication for insulin therapy in gestational diabetes. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:318.
Ilic 1999 {published data only}
- Ilic S, Jovanovic L, Pettitt DJ. Comparison of the effect of saturated and monounsaturated fat on postprandial plasma glucose and insulin concentration in women with gestational diabetes mellitus. American Journal of Perinatology 1999;16(9):489‐95. [DOI] [PubMed] [Google Scholar]
Jovanovic 1999 {published data only}
- Jovanovic L, Ilic S, Pettitt DJ, Hugo K, Gutierrez M, Bowsher RR, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care 1999;22(9):1422‐7. [DOI] [PubMed] [Google Scholar]
Jovanovic‐Peterson 1989 {published data only}
- Jovanovic‐Peterson L, Durak EP, Peterson CM. Randomized trial of diet vs diet plus cardiovascular conditioning on glucose levels in gestational diabetes. American Journal of Obstetrics and Gynecology 1989;161:415‐9. [DOI] [PubMed] [Google Scholar]
Jovanovic‐Peterson 1993 {published data only}
- Jovanovic‐Peterson L, Palmer JP, Sparks S, Peterson CM. Jet‐injected insulin is associated with decreased antibody production and postprandial glucose variability when compared with needle‐injected insulin in gestational diabetic women. Diabetes Care 1993;16:1479‐83. [DOI] [PubMed] [Google Scholar]
Kitzmiller 1990 {published and unpublished data}
- Kitzmiller JL. Trial of diet vs diet plus insulin for gestational diabetes. Personal communication 1990.
Kjos 2001 {published data only}
- Kjos SL, U Schaefer‐Graf, Sardesi S, Peters RK, Buley A, Xiang AH, et al. A randomised controlled trial using glycaemic plus fetal ultrasound parameters versus glycaemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycaemia. Diabetes Care 2001;24(11):1904‐10. [DOI] [PubMed] [Google Scholar]
Langer 1994 {published data only}
- Langer O, Rodriguez DA, Xenakis EMJ, McFarland MB, Berkus MD, Arredondo F. Intensified versus conventional management of gestational diabetes. American Journal of Obstetrics and Gynecology 1994;170:1036‐47. [DOI] [PubMed] [Google Scholar]
Langer 2000 {published data only}
- Langer O, Conway D, Berkus M, Xenakis E‐J. Oral hypoglycaemic agent is comparable to insulin in GDM management. American Journal of Obstetrics and Gynaecology 1999;180(1 Pt 2):S6. [Google Scholar]
- Langer O, Conway D, Berkus M, Xenakis E‐J, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. New England Journal of Medicine 2000;343(16):1134‐8. [DOI] [PubMed] [Google Scholar]
- Langer O, Yogev Y, Brustman L, Rosenn B. Is there a relationship between severity of gestational diabetes (gdm) and pregnancy outcome in insulin‐ and glyburide‐treated patients [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6):S105. [Google Scholar]
- Langer O, Yogev Y, Rosenn B, Brustman L. Glyburide therapy: the relationship between dosage and level of gestational diabetes (gdm) severity [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6):S105. [Google Scholar]
- Langer O, Yogev Y, Xenakis EMJ, Rosenn B. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. American Journal of Obstetrics and Gynecology 2005;192(1):134‐9. [DOI] [PubMed] [Google Scholar]
- Letonturier P. Gestational diabetes: an alternative to insulin therapy? [Diabete gestationnel: une alternative a l'insulinotherapie]. Presse Medicale 2001;30:169‐70. [PubMed] [Google Scholar]
Lao 2001 {published data only}
- Lao TT, Ho LF. A randomised controlled pilot study of the management of gestational impaired glucose tolerance [letter]. BJOG: an international journal of obstetrics and gynaecology 2001;108:769. [DOI] [PubMed] [Google Scholar]
Lauszus 2001 {published data only}
- Lauszus FF, Rasmussen OW, Henrikson JE, Klebe JG, Jensen L, Lauszus KS, et al. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. European Journal of Clinical Nutrition 2001;55:436‐43. [DOI] [PubMed] [Google Scholar]
Lesser 1996 {published data only}
- Lesser KB, Gruppuso PA, Terry RB, Carpenter MW. Exercise fails to improve postprandial glycaemic excursion in women with gestational diabetes. Journal of Maternal‐Fetal Medicine 1996;5:211‐7. [DOI] [PubMed] [Google Scholar]
Li 1987 {published data only}
- Li DFH, Wong VCW, O'Hoy KMKY, Yeung CY, Ma HK. Is treatment needed for mild impairment of glucose tolerance in pregnancy? A randomized controlled trial. British Journal of Obstetrics and Gynaecology 1987;94:851‐4. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li P, Yang H, Dong Y. Treating women with gestational impaired glucose tolerance reduces adverse outcome of pregnancy. Chinese Journal of Obstetrics and Gynecology 1999;34(8):462‐4. [PubMed] [Google Scholar]
Magee 1990 {published data only}
- Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200‐kcal diet in obese pregnant women with gestational diabetes. Diabetes 1990;39:234‐40. [DOI] [PubMed] [Google Scholar]
Mecacci 2003 {published data only}
- Mecacci F, Carignani L, Cioni R, Bartoli E, Paretti E, Torre P, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non‐diabetic pregnant women. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;111:19‐24. [DOI] [PubMed] [Google Scholar]
MRC 1955 {published data only}
- Medical Research Council. The use of hormones in the management of pregnancy in diabetics. Lancet 1955;2:833‐6. [PubMed] [Google Scholar]
Nolan 1984 {published data only}
- Nolan CJ. Improved glucose tolerance in gestational diabetic women on a low fat, high unrefined carbohydrate diet. Australian and New Zealand Journal of Obstetrics and Gynaecology 1984;24:174‐7. [DOI] [PubMed] [Google Scholar]
Nor Azlin 2007 {published data only}
- Nor Azlin MI, Nor NA, Sufian SS, Mustafa N, Jamil MA, Kamaruddin NA. Comparative study of two insulin regimes in pregnancy complicated by diabetes mellitus. Acta Obstetricia et Gynecologica Scandinavica 2007;86(4):407‐8. [DOI] [PubMed] [Google Scholar]
Notelovitz 1971 {published data only}
- Notelovitz M. Sulphonylurea therapy in the treatment of the pregnant diabetic. South African Medical Journal 1971;45:226‐9. [PubMed] [Google Scholar]
O'Sullivan 1971 {published data only}
- O'Sullivan JB. Prospective study of gestational diabetes and its treatment. In: Stowers JB, Sutherland HW editor(s). Carbohydrate metabolism in pregnancy and the newborn. London: Churchill Livingstone, 1975:195‐204. [Google Scholar]
- O'Sullivan JB, Charles D, Dandrow RV. Treatment of verified prediabetics in pregnancy. Journal of Reproductive Medicine 1971;7:21‐4. [Google Scholar]
- O'Sullivan JB, Gellis SS, Dandrow RV, Tenney BO. The potential diabetic and her treatment in pregnancy. Obstetrics & Gynecology 1966;27:683‐9. [PubMed] [Google Scholar]
O'Sullivan 1974 {published data only}
- O'Sullivan JB, Mahan CM, Charles D, Dandrow RV. Medical treatment of the gestational diabetic. Obstetrics & Gynecology 1974;43:817‐21. [PubMed] [Google Scholar]
O'Sullivan 1980 {published data only}
- O'Sullivan JB, Mahan CM. Insulin treatment and high risk groups. Diabetes Care 1980;3(3):482‐5. [DOI] [PubMed] [Google Scholar]
Pardi 1989 {published data only}
- Pardi G, Buscaglia M, Kustermann A, Bozzetti P, Ferrari MM, Marconi AM. Foetal pulmonary maturation in pregnancies complicated by diabetes and Rh immunization. European Respiratory Journal 1989;2:50s‐52s. [PubMed] [Google Scholar]
Persson 1985 {published data only}
- Persson B, Stangenberg M, Hansson U, Nordlander E. Gestational diabetes mellitus (GDM): comparative evaluation of two treatment regimens, diet vs insulin and diet. Diabetes 1985;34:101‐5. [DOI] [PubMed] [Google Scholar]
Pettitt 2003 {published data only}
- Pettitt DJ, Ospina P, Kolaczynski JW, Jovanovic L. Comparison of an insulin analog, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care 2003;26:183‐6. [DOI] [PubMed] [Google Scholar]
Pettitt 2007 {published data only}
- Anonymous. Safety & efficacy of insulin aspart vs. regular human insulin in gestational diabetes. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 15 September 2004).
- Jovanovic L, Howard C, Pettitt D, Zisser H, Ospina P. Insulin aspart vs. regular human insulin in basal/bolus therapy for patients with gestational diabetes mellitus: safety and efficacy. Diabetologia 2005;48(Suppl 1):A317. [Google Scholar]
- Pettitt DJ, Ospina P, Howard C, Zisser H, Jovanovic L. Efficacy, safety and lack of immunogenicity of insulin aspart compared with regular human insulin for women with gestational diabetes mellitus. Diabetic Medicine 2007;24:1129‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polyhonen‐Alho 2002 {published data only}
- Polyhonen‐Alho M, Teramo K, Kaaja R. Treatment of gestational diabetes with short or long‐acting insulin and neonatal outcome: a pilot study. Acta Obstetricia et Gynecologica Scandinavica 2002;81:258‐9. [PubMed] [Google Scholar]
Rae 2000 {published data only}
- Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2000;40(4):416‐22. [DOI] [PubMed] [Google Scholar]
Reader 2006 {published data only}
- Reader D, Splett P, Gunderson EP. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dietitians on pregnancy outcomes. Journal of the American Dietetics Association 2006;106:1426‐33. [DOI] [PubMed] [Google Scholar]
Reece 1995 {published data only}
- Reece E, Hagey Z, Gay LJ, O'Connor T, DeGennaro N, Homko C, et al. A randomised controlled trial of a fiber‐enriched diabetic diet vs the standard American Diabetes Association‐recommended diet in the management of diabetes mellitus in pregnancy. Journal of Maternal‐Fetal Investigation 1995;5:8‐12. [Google Scholar]
Rey 1995 {published data only}
- Rey E. Gestational diabetes: who needs a tight follow‐up?. American Journal of Obstetrics and Gynecology 1995;172:333. [Google Scholar]
Rey 1997 {published data only}
- Rey E. Usefulness of a breakfast test in the management of gestational diabetes. Obstetrics & Gynecology 1997;89(6):981‐8. [DOI] [PubMed] [Google Scholar]
Rossi 2000 {published data only}
- Rossi G, Somigliani E, Moschetta M, Bottani B, Barbieri M, Vignali M. Adequate timing of fetal ultrasound to guide metabolic therapy in mild gestational diabetes mellitus. Acta Obstetricia et Gynecologica Scandinavica 2000;79:649‐54. [PubMed] [Google Scholar]
Schaefer‐Graf 2004 {published data only}
- Schaefer‐Graf UM, Kjos SL, Fauzan OH, Buhling KJ, Siebert G, Buhrer C, et al. A randomized trial evaluating a predominately fetal growth‐based strategy to guide management of gestational diabetes in caucasian women. Diabetes Care 2004;27(2):297‐302. [DOI] [PubMed] [Google Scholar]
Schuster 1998 {published data only}
- Schuster MW, Chauhan SP, McLaughlin BN, Perry KG Jr, Morrison JC. Comparison of insulin regimens and administration modalities in pregnancy complicated by diabetes. Journal of the Mississippi State Medical Association 1998;39(2):51‐5. [PubMed] [Google Scholar]
Silva 2005 {published data only}
- Silva JC, Taborda W, Becker F, Aquim G, Viese J, Bertini AM. Preliminary results of the use of oral hypoglycemic drugs on gestational diabetes mellitus [Resultados preliminares do uso de anti‐hiperglicemiantes orais no diabete melito gestacional]. Revista Brasileira de Ginecologia y Obstetricia 2005;27(8):461‐6. [Google Scholar]
Snyder 1998 {published data only}
- Snyder J, Morin L, Meltzer S, Nadeau J. Gestational diabetes and glycemic control: a randomized clinical trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):55. [Google Scholar]
Thompson 1990 {published data only}
- Thompson DJ, Porter KB, Gunnells DJ, Wagner PC, Spinnato JA. Prophylactic insulin in the management of gestational diabetes. Obstetrics & Gynecology 1990;75:960‐4. [PubMed] [Google Scholar]
Todorova 2007 {published data only}
- Todorova K, Palaveev O, Petkova VB, Stefanova M, Dimitrova Z. Pharmacoeconomical model for choice of a treatment for pregnant women with gestational diabetes. Acta Diabetologica 2007;33(3):144‐8. [DOI] [PubMed] [Google Scholar]
Yang 2003 {published and unpublished data}
- Yang X, Hsu‐Hage B, Dong L, Shao P, Wang H, Tian H, et al. Intensive diabetes management may improve pregnancy: outcomes in Chinese gravidas with impaired glucose tolerance. Diabetes Care 2003;26:254‐5. [DOI] [PubMed] [Google Scholar]
- Yang X, Hsu‐Hage BH, Dong L, Zhang H, Zhang C, Zhang Y. Postpartum glucose intolerance in Chinese women with gestational diabetes. Diabetic Medicine 2003;20(8):687‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Balaji 2010 {published data only}
- Balaji V, Balaji MS, Alexander C, Ashalata S, Sheela R, Suresh S, et al. Premixed insulin aspart 30 (Biasp 30) vs. premixed human insulin 30 (BHI 30) in gestational diabetes mellitus‐‐a pilot study. Journal of the Association of Physicians of India 2010;58:99‐101. [PubMed] [Google Scholar]
Bertini 2009 {published data only}
- Bertini AM. Up to date treatment of gestational diabetes mellitus. Journal of Perinatal Medicine 2009;37(Suppl 1):3. [Google Scholar]
Cortez 2006 {published data only}
- Cortez J, Tarsa M, Agent S, Chmait R, Moore T. Randomized controlled trial of acarbose vs. placebo in the treatment of gestational diabetes [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S149. [Google Scholar]
Durnwald 2011 {published data only}
- Durnwald CP, Mele L, Spong CY, Ramin SM, Varner MW, Rouse DJ, et al. Glycemic characteristics and neonatal outcomes of women treated for mild gestational diabetes. Obstetrics & Gynecology 2011;117(4):819‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elnour 2008 {published data only}
- Elnour AA, Mugammar IT, Jaber T, Revel T, McElnay JC. Pharmaceutical care of patients with gestational diabetes mellitus. Journal of Evaluation in Clinical Practice 2008;14(1):131‐40. [DOI] [PubMed] [Google Scholar]
Ferrara 2008 {published data only}
- Ferrara A. Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Garcia‐Patterson 2001 {published data only}
- Garcia‐Patterson A, Martin E, Ubeda J, Maria MA, Leiva A, Corcoy R. Evaluation of light exercise in the treatment of gestational diabetes. Diabetes Care 2001;24:2006‐7. [DOI] [PubMed] [Google Scholar]
Gillman 2010 {published data only}
- Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33(5):964‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2011 {published data only}
- Grant SM, Wolever TMS, O'Connor DL, Nisenbaum R, Josse RG. Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Research and Clinical Practice 2011;91(1):15‐22. [DOI] [PubMed] [Google Scholar]
Hickman 2011 {published data only}
- Hickman A. Effectiveness of metformin compared to insulin in pregnant women with mild preexisting or early gestational diabetes (MIPOD). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 15 February 2011) 2011.
Hutchinson 2008 {published data only}
- Hutchinson A, Haugabrook C, Long L, Mason L, Kipikasa J, Adair D. A comparison of glyburide/metformin and insulin for gestational diabetes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S200. [Google Scholar]
Ijas 2011 {published data only}
- Ijas H, Vaarasmaki M, Morin‐Papunen L, Keravuo R, Ebeling T, Saarela T, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG: an international journal of obstetrics and gynaecology 2011;118(7):880‐5. [DOI] [PubMed] [Google Scholar]
Keely 2008 {published data only}
- Keely EJ, Malcolm JC, Hadjiyannakis S, Gaboury I, Lough G, Lawson ML. Prevalence of metabolic markers of insulin resistance in offspring of gestational diabetes pregnancies. Pediatric Diabetes 2008;9(1):53‐9. [DOI] [PubMed] [Google Scholar]
Lain 2008 {published data only}
- Lain K, Garabedian M, Daftary A, Jeyabalan A. Maternal and neonatal metabolic biomarkers in gestational diabetes treated with glyburide compared to insulin. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S199. [DOI] [PubMed] [Google Scholar]
- Lain K, Garabedian M, Daftary A, Jeyabalan A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared to insulin. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S34. [DOI] [PubMed] [Google Scholar]
Lain 2009 {published data only}
- Lain KY, Garabedian MJ, Daftary A, Jeyabalan A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared with insulin. American Journal of Obstetrics and Gynecology 2009;200(5):501.e1‐6. [DOI] [PubMed] [Google Scholar]
Landon 2009 {published data only}
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2010 {published data only}
- Martinez P, Abdulhaj Martinez M, Andres Nunez P, Garcia Leon P, Lopez Sanchez EJ, Gonzalez Ramirez AR. A randomized study comparing metformin and insulin in the treatment of gestational diabetes mellitus. Interim results. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):381. [Google Scholar]
Mendelson 2008 {published data only}
- Mendelson SG, McNeese‐Smith D, Koniak‐Griffin D, Nyamathi A, Lu MC. A community‐based parish nurse intervention program for Mexican American women with gestational diabetes. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2008;37(4):415‐25. [DOI] [PubMed] [Google Scholar]
Moore 2008 {published data only}
- Moore L, Clokey D, Curet L. A randomized controlled trial of metformin and glyburide in gestational diabetes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S34. [DOI] [PubMed] [Google Scholar]
Moore 2010 {published data only}
- Moore LE, Clokey D, Rappaport VJ, Curet LB. Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstetrics & Gynecology 2010;115(1):55‐9. [DOI] [PubMed] [Google Scholar]
Moses 2009 {published data only}
- Moses RG, Barker M, Winter M, Petocz P, Brand‐Miller JC. Can a low‐glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009;32(6):996‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2009 {published data only}
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home‐based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes & Metabolism 2009;35(5):418‐21. [DOI] [PubMed] [Google Scholar]
Refuerzo 2011 {published data only}
- Refuerzo JS. Metformin versus insulin in pregnant women with type 2 diabetes. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 15 February 2011).
Rowan 2008 {published data only}
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, for the MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine 2008;358(19):2003‐15. [DOI] [PubMed] [Google Scholar]
Rowan 2010 {published data only}
- Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care 2010;33(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2007 {published data only}
- Silva JC, Bertini AM, Taborda W, Becker F, Bebber FR, Aquim GM, et al. Glibenclamide in the treatment for gestational diabetes mellitus in a compared study to insulin. Arquivos Brasileiros de Endocrinologia e Metabologia 2007;51(4):541‐6. [DOI] [PubMed] [Google Scholar]
Silva 2010 {published data only}
- Silva JC, Pacheco C, Bizato J, Souza BV, Ribeiro TE, Bertini AM. Metformin compared with glyburide for the management of gestational diabetes. International Journal of Gynecology & Obstetrics 2010;111(1):37‐40. [DOI] [PubMed] [Google Scholar]
Zanganeh 2010 {published data only}
- Zanganeh M. The comparative study of therapeutic effects of insulin and glibenclamide in the gestational diabetes mellitus. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
References to ongoing studies
Crowther 2007 {published data only}
- Crowther C. Dietary and lifestyle advice for women with borderline gestational glucose intolerance. Australian Clinical Trials Registry (www.actr.org.au) (accessed 21 June 2007).
Golladay 2006 {published data only}
- Golladay EC. Glyburide compared to insulin in the management of White's classification A2 gestational diabetes. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Kipikasa 2008 {published data only}
- Kipikasa JH. Comparison of glucovance to insulin for diabetes during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Landon 2002 {published and unpublished data}
- Landon MB. A prospective multicenter randomized treatment trial of mild gestational diabetes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. [Google Scholar]
- Landon MB, Thom E, Spong CY, Carpenter M, Mele L, Johnson F, Tillinghast J, Anderson G. The National Institute of Child Health and Human Development Maternal‐Fetal Medicine Unit Network randomised clinical trial in progress. Diabetes Care 2007;30:S194‐9. [DOI] [PubMed] [Google Scholar]
- Landon MB, Thom E, Spong CY, Gabbe SG, Leindecker S, Johnson F, et al. A planned randomized clinical trial of treatment for mild gestational diabetes mellitus. Journal of Maternal‐Fetal & Neonatal Medicine 2002;11(4):226‐31. [DOI] [PubMed] [Google Scholar]
Rowan 2005 {published data only}
- Rowan J. A trial in progress: gestational diabetes. Diabetes Care 2007;30:S214‐S219. [DOI] [PubMed] [Google Scholar]
- Rowan J. Metformin in gestational diabetes. Australian Clinical Trials Register (http://www.actr.org/actr) (accessed 6 December 2005).
Additional references
Alberti 1998
- Alberti K, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, Part 1 : diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Barker 1992
- Barker DFP. Fetal and infant origins of adult disease. London: BMJ Publishing Group, 1992. [Google Scholar]
Ceysens 2006
- Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 3. [Art. No.: CD004225. DOI: 10.1002/14651858.CD004225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coustan 1998
- Coustan DR, Carpenter MW. The diagnosis of gestational diabetes. Diabetes Care 1998;21 Suppl 2:B5‐B8. [PubMed] [Google Scholar]
Enkin 2000
- Enkin M, Keirse MJNC, Neilson J, Crowther C, Duley L, Hodnett E, et al. A guide to effective care in pregnancy and childbirth. 3rd Edition. Oxford: Oxford University Press, 2000. [Google Scholar]
Essel 1995
- Essel JK, Opai Tetteh ET. Macrosomia – maternal and fetal risk factors. South African Medical Journal 1995;85:43‐6. [PubMed] [Google Scholar]
Expert Committee 2003
- Expert Committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5‐S20. [DOI] [PubMed] [Google Scholar]
Gabbe 1977
- Gabbe SG, Mestman JH, Freeman RK, Anderson GV, Lowensohn RI. Management and outcome of class A diabetes mellitus. American Journal of Obstetrics and Gynecology 1977;127:465‐9. [DOI] [PubMed] [Google Scholar]
Green 1991
- Green JR, Schumacher LB, Pawson IG, Partridge JC, Kretchmer N. Influence of maternal body habitus and glucose tolerance on birth weight. Obstetrics & Gynecology 1991;78:225‐40. [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Jarrett 1997
- Jarrett RJ. The concept of gestational diabetes was popularised before considerations of evidence based medicine came on the scene. BMJ 1997;315:736‐7. [Google Scholar]
NICE 2007
- National Institute for Clinical Excellence (NICE). Diabetes in pregnancy. Draft Guideline 2007.
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Scott 2002
- Scott DA, Loveman E, McIntyre L, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technology Assessment 2002;6(11):1‐161. [DOI] [PubMed] [Google Scholar]
Soares 1997
- Soares JAC, Dornhorstt A, Beard RW. The case for screening for gestational diabetes. BMJ 1997;315:737‐9. [Google Scholar]
Spellacy 1985
- Spellacy WN, Miller S, Winegar A, Peterson PQ. Macrosomia: maternal characteristics and infant complications. Obstetrics & Gynecology 1985;66:158‐61. [PubMed] [Google Scholar]
Stephenson 1993
- Stephenson MJ. Screening for gestational diabetes: a critical review. Journal of Family Practice 1993;37:277‐83. [PubMed] [Google Scholar]
Tieu 2008a
- Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006674.pub2] [DOI] [PubMed] [Google Scholar]
Tieu 2008b
- Tieu J, Crowther CA, Middleton P, McPhee AJ. Screening for gestational diabetes mellitus for improving maternal and infant health. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 1998
- Weiss PA, Hausler M, Kainer F, Purstner P, Haas J. Toward universal criteria for gestational diabetes: relationships between capillary and venous glucose concentrations. American Journal of Obstetrics and Gynecology 1998;178(4):830‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
CDSR 2003
- Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD003395. DOI: 10.1002/14651858.CD003395] [DOI] [PubMed] [Google Scholar]


