Abstract
Background
About 5% of women experience severe symptoms called premenstrual syndrome (PMS), only in the two weeks before their menstrual periods. Treatment with progesterone may restore a deficiency, balance menstrual hormone levels or reduce effects of falling progesterone levels on the brain or on electrolytes in the blood.
Objectives
The objectives were to determine if progesterone has been found to be an effective treatment for all or some premenstrual symptoms and if adverse events associated with this treatment have been reported.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO to February 2011. We contacted pharmaceutical companies for information about unpublished trials, for the first version of this review.
The search strings are in Appendix 2.
Selection criteria
We included randomised double‐blind, placebo‐controlled trials of progesterone on women with PMS diagnosed by at least two prospective cycles, without current psychiatric disorder.
Data collection and analysis
Two reviewers (BM and OF) extracted data independently and decided which trials to include. OF wrote to trial investigators for missing data.
Main results
From 17 studies, only two met our inclusion criteria. Together they had 280 participants aged between 18 and 45 years. One hundred and fifteen yielded analysable results. Both studies measured symptom severity using subjective scales. Differing in design, participants, dose of progesterone and how delivered, the studies could not be combined in meta‐analysis.
Adverse events which may or may not have been side effects of the treatment were described as mild.
Both trials had defects. They intended to exclude women whose symptoms continued after their periods. When data from ineligible women were excluded from analysis in one trial, the other women were found to have benefited more from progesterone than placebo. The smaller study found no statistically significant difference between oral progesterone, vaginally absorbed progesterone and placebo, but reported outcomes incompletely.
Authors' conclusions
The trials did not show that progesterone is an effective treatment for PMS nor that it is not. Neither trial distinguished a subgroup of women who benefited, nor examined claimed success with high doses.
Keywords: Female, Humans, Premenstrual Syndrome, Premenstrual Syndrome/blood, Premenstrual Syndrome/drug therapy, Progesterone, Progesterone/adverse effects, Progesterone/blood, Progesterone/therapeutic use, Progestins, Progestins/adverse effects, Progestins/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Progesterone for premenstrual syndrome
There is little good evidence for treating premenstrual syndrome with progesterone. Five per cent or more of women experience symptoms, severe enough to damage work and relationships, only in the days leading to their menstrual periods. Blood progesterone levels usually rise after ovulation and fall again before menstruation. It has been suggested that premenstrual syndrome (PMS) might have been caused by too little progesterone or falling levels.
This review found some evidence for relief with progesterone but trials differed in route of administration, dose, duration of treatment and selection of women taking part. Outcomes also differed. The studies had flaws in methods or in handling outcome data or both.
Adverse effects which may or may not have been the result of the treatment were generally mild.
Further research would be needed to test claims for the effectiveness of higher doses of progesterone. They are neither refuted nor borne out as yet. Using each woman's own symptoms to select participants and to judge treatment effects would be more accurate than checklists of largely irrelevant symptoms. Knowing how many women had fewer days with symptoms, fewer or milder symptoms, or the converse, would be more valuable than the calculations based on subjective data for groups of women.
Background
Description of the condition
Premenstrual Syndrome (PMS) is the occurrence, during the luteal phase of the menstrual cycle, of symptoms severe enough to interfere with work or relationships. These symptoms resolve at, or soon after, the beginning of the menstrual period. Although most women experience menstrual cycle change, some are seriously disordered. It has been estimated that approximately 5% of menstruating women experience PMS as defined (O'Brien 1993). Often misunderstood, difficult to treat and of uncertain aetiology, PMS might be several conditions, each with a different cause. Over 100 symptoms have been associated with the luteal phase of the menstrual cycle. None is exclusive to PMS, and few are experienced solely by women. Feeling bloated, breast tenderness, exhaustion, joint pain, anxiety, irritability, depression and mood swings are common, but it is the timing rather than the nature of symptoms which is diagnostic of PMS.
Description of the intervention
Administration of progesterone may alter the menstrual cycle length and, if given before ovulation, may cause breakthrough bleeding. If taken for several days and then stopped, a withdrawal bleed like a menstrual period will follow in a few days. Rarely, women may become sleepy. Progesterone administered as pessaries or suppositories may cause soreness. There may be leakage of the base, particularly with vaginal use, or flatulence with rectal use. Extensive warnings accompany progesterone injections in the UK, where they are not licensed for the treatment of PMS (ABPI 1999).
Many women improve at least temporarily when given a placebo (Freeman 1999; Halbreich 1985).
How the intervention might work
Historically, treatment with progesterone was based on the hypothesis that in PMS sufferers, the ratio of progesterone and its derivatives to other hormones was lower than is usual in women. This allowed oestrogens to cause water retention, because there was insufficient progesterone to oppose them (Greene 1953; Rees 1953).
Early successes in treating premenstrual migraine and premenstrual asthma with progesterone were compatible with this theory (Dalton 1973a; Dalton 1973b; Dalton 1984; Greene 1953). However assays of hormone levels have not confirmed simple deficiency of progesterone in women with PMS (Andersch 1979; Backstrom 1975; Rubinow 1988). Progesterone is not secreted continuously throughout the luteal phase, but in spurts (Collin 1991; Steele 1986). It is rapidly removed from the blood (Chakmakjian 1987). Assessments of progesterone level based on few samples on occasional days in the menstrual cycle should therefore be considered with caution.
More recent studies have suggested that high oestrogen levels are responsible rather than low progesterone (Bjorn 2003) but this was countered by analysis of blood samples drawn on particular cycle days from women with and without premenstrual syndrome (Thys‐Jacobs 2008).
Studies of the frequency, amplitude and duration of progesterone pulses and their relationship to luteinising hormone pulses have suggested that, at the onset of symptoms, the corpora lutea in women with PMS have increased sensitivity to luteinising hormone (Collin 1991; Facchinetti 1990; Facchinetti 1993; Lewis 1995). Treatment with progesterone might overcome changes in the sensitivity of the corpora lutea, as they fail during the luteal phase.
The relationship between the timing of progesterone peak levels, the rate of fall of progesterone and the ratio between the rates of decrease of oestrogen and progesterone were all related to symptoms severity which was worse a few days after peak progesterone level (Halbreich 1986; Hammarbäck 1989; O'Brien 1980; Redei 1995; Seippel 2000).
Gama amino butyric acid (GABA) produced by inhibitory neurons calms symptoms of anxiety, irritability and aggression. Part of the receptors, called GABA(A) on the neurone surface, necessary for GABA to have its effect, cannot be made without the break‐down products of progesterone (Smith 1998). The occurrence of severe symptoms has been correlated with falling levels of progesterone metabolites. Therefore, progesterone could relieve the symptoms of PMS by preventing falling levels of progesterone metabolites and loss of GABA(A) enhancement (Monteleone 2000; Wang 1996).
There are differences between PMS sufferers and women who have no PMS, in the plasma salt levels needed to stimulate the secretion of the hormones which help to regulate the balance of salt and water (Watanabe 1997). These differences might explain luteal water retention. Progesterone promotes excretion of salt in the urine and consequently of water (Corvol 1983; Landau 1958), and might relieve PMS by raising the threshold for the release of the hormone which prevents excretion of water (vasopressin, also called anti‐diuretic hormone, ADH).
Why it is important to do this review
It was considered important to do this review because the efficacy of progesterone for PMS was still in doubt.
Objectives
The three objectives were
1. To find out if progesterone was shown to be an effective treatment for PMS; 2. To find out if progesterone was shown to be effective for a subgroup of women defined by their symptom type; 3. To find out if adverse effects were recorded in trials of progesterone for the treatment of PMS.
Methods
Criteria for considering studies for this review
Types of studies
We looked for randomised controlled trials which compared the effects of progesterone with a placebo or another treatment.
Types of participants
There were three criteria. 1. Participants were of reproductive age. 2. The diagnosis of their PMS was confirmed by at least two cycles of prospectively recorded symptoms. 3. Their symptoms subsided completely at the onset of menstruation or during it.
We excluded studies if their participants had current psychiatric problems, used hormonal preparations (including oral contraception) or used other treatments for PMS during the interventions.
Types of interventions
There were three requirements. 1. Progesterone was compared with placebo or another treatment. 2. Progesterone was given in the luteal phase, in stated doses, by any route of administration. 3. The outcomes for the active intervention and the placebo were recorded in the same study period.
Types of outcome measures
Primary outcomes were
change in the severity of luteal phase symptoms overall, or
change in the severity of particular symptoms.
Symptoms in the luteal phase of the menstrual cycle could be recorded by participants or study personnel by means of charts, visual analogue scales or by any other means.
Secondary outcomes were records of adverse events.
Search methods for identification of studies
Electronic searches
Early in 2000 we searched a cross‐referenced database of the published work on premenstrual syndrome, compiled by OF. The search terms were premenstrual syndrome and its synonyms AND progesterone or manufacturers' product names as summarised in Appendix 1
The Trials Search Co‐ordinator searched MEDLINE, EMBASE and PsycLIT on October 16 2000. MEDLINE and EMBASE were searched again on March 1 2005 and all again on March 3 2008. CINAHL was searched on March 3 2008.
The Trials Search Coordinator searched the CENTRAL database of the Cochrane Library Issue 1 and theTrials Register of the Cochrane Menstrual Disorders and Subfertility Group on March 1 2005 and in March 2008.
The Trials Search Coordinator searched the CENTRAL database of the Cochrane Library Issue 1 and theTrials Register of the Cochrane Menstrual Disorders and Subfertility Group on March 1 2005 and in March 2008.
The Trials Search Coordinator searched the Cochrane Menstrual Disorders and Subfertility Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO in February 2011. See Appendix 2, Appendix 3.
Searching other resources
We searched bibliographies in the articles found, using the same search terms. We also translated two articles in French but they were not relevant. OF wrote to manufacturers of the progesterone products listed above for unpublished trials.
Data collection and analysis
Selection of studies
OF made the first selection of titles found using the search strategy described, and initially included trials which only doubtfully met the selection criteria. She obtained full text copies of the articles and made copies of the methods sections for BM in which the authors' names and their affiliation were blanked out. All randomised controlled trials which compared progesterone with placebo or another treatment were considered. Both reviewers decided independently which studies should be included in the review.
Where details were needed to establish the eligibility of trials, OF wrote to the authors to ask for further information.
Data extraction and management
OF, who was familiar with the published work on PMS, undertook the review. BM helped with the data extraction and HR served as clinical adviser.
Assessment of risk of bias in included studies
The reviewers assessed the risk of bias of all studies that were eligible for inclusion in the review.
Measures of treatment effect
Results in the trials were ordinal scale data and they were clinically diverse. It was planned for any future updates of this review that we would express dichotomous data results for each study as an odds ratio (OR) with 95% confidence interval (95% CI). However, no future updates are planned, unless we become aware of new trials in this area.
Dealing with missing data
OF wrote to trial investigators for missing data.
Assessment of heterogeneity
The reviewers originally intended to assess heterogeneity between the pooled results of different studies by inspecting the scatter in the data points and the overlap in their confidence intervals and more formally, by checking the results of the chi‐squared tests. However, the data from trials were not suitable for such meta‐analysis and heterogeneity was not assessed.
Data synthesis
The reviewers originally intended to pool outcomes statistically. However, the data from trials were not suitable for meta‐analysis.
It was planned for any future updates of this review that we would combine the odds ratios and 95% CIs from individual studies in meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method. We would show continuous differences between groups in the meta‐analysis as a mean difference (MD) and 95% confidence interval. We would use a fixed approach unless there was significant heterogeneity in which case we would confirm results using a random‐effects statistical model. We would investigate sources of the heterogeneity. However, no future updates are planned, unless we become aware of new trials in this area.
Subgroup analysis and investigation of heterogeneity
It was planned for any future updates of this review, if it became possible, to investigate sources of heterogeneity. However no future updates are planned, unless we become aware of new trials in this area.
Results
Description of studies
Results of the search
We considered 16 studies for inclusion.
Only two trials qualified for inclusion (Magill 1995; Vanselow 1996) see Characteristics of included studies.
Included studies
Trial design
Both the included trials compared progesterone with placebo. One trial had a parallel design in which participants were randomised either to progesterone or to placebo (Magill 1995). The other trial compared oral progesterone, vaginal progesterone and placebo in a three‐way crossover design (Vanselow 1996).
Participants
Source of participants
Women were referred by their own doctors in both studies and also responded to publicity in one (Vanselow 1996).
Diagnostic criteria
Both studies looked for participants whose luteal phase symptoms were relieved in the follicular phase, with only one mild occurrence of one symptom (Magill 1995), or one week clear of symptoms after the menstrual period (Vanselow 1996). Symptoms had to be severe enough to disrupt interpersonal relationships or activities (Vanselow 1996).
Cyclicity was indicated by the reported experience of cyclical changes for the last three cycles (Magill 1995), and for at least six cycles (Vanselow 1996) prior to enrolment.
Diagnosis, made first by the participants' GPs, was confirmed by prospective records during two untreated cycles in both studies. In one study, the Moos Menstrual Distress Questionnaire was used on days 6 and 26 (Vanselow 1996). The other study used diary cards designed for the study (Magill 1995).
Inclusion/exclusion criteria other than diagnostic criteria
Both studies required participants to have regular menstrual cycles and recorded their height, weight and blood pressure before treatment began. One made a gynaecological examination (Vanselow 1996). The other noted medical and menstrual history of each woman (Magill 1995).
Women with recent history of psychotic illness, use of antidepressants, benzodiazepines or with suicidal tendency were excluded from one study (Magill 1995), and women with current psychiatric disorder or use of psychotropic drugs were excluded from the other (Vanselow 1996). The latter also excluded women whose main cyclical complaint was of depression with low energy. This study reported a pretreatment profile of each participant using the Eysenck Personality Inventory, Rosenberg's Self Esteem Scale and the Spielberger State‐Trait Personality Inventory.
Both studies forbade hormonal medication including hormonal contraception. Other treatments for PMS were expressly forbidden in one study (Magill 1995) as were drug or alcohol misuse. Women in the other study did not use other treatments (Vanselow 1996).
Women who were experiencing stress like family crises or violence were excluded in one study (Vanselow 1996).
Number of participants
From 281 women identified by their general practitioners, 141 were selected for one study (Magill 1995) and from more than 200 women who applied, 174 were screened and 40 selected in the other (Vanselow 1996).
Interventions
These are summarised in Table 1.
1. Interventions.
| trial | route | vehicle | dose in mg | frequency | beginning | ending | treatment free days | cycles |
| Magill 1995 | vaginal or rectal | Suppocire (a mixture of mono, di and tri‐glycerides and polyoxyethylene glyceride) | 400 | twice a day | 14 days before next expected menses | at menses | none | 4 either placebo or progesterone in parallel design |
| Vanselow 1996 | vaginal and oral | arachis oil in soft gelatine capsules | oral 100 2 x 100 vaginal 2 x 100 | each morning and each night at night | 3 days after ovulation | not reported | not recorded | 1,2 either oral progesterone with vaginal placebo, or oral placebo with vaginal progesterone, or vaginal and oral placebos. 3,4 a different combination. 5,6 remaining combination. |
Preparations
Utrogestan was used for oral and vaginal administration in one study (Vanselow 1996). Cyclogest suppositories were given in the other. Participants were allowed to choose whether they used the medication vaginally or rectally, but the results were not treated separately (Magill 1995).
Duration
The intervention began 14 days before the expected date of the next menstrual period and continued until its onset for each of four cycles (Magill 1995). Two cycles each of the three combinations of oral progesterone with vaginal placebo, vaginal progesterone with oral placebo and both oral and vaginal placebo, were given in the crossover study and a final cycle was recorded without treatment (Vanselow 1996). Treatment was intended to begin three days after ovulation, which was estimated in relation to the date of the last menstrual period and basal temperature records. It is not known if treatment ceased if menstruation did not begin as expected (Vanselow 1996).
Dose
A dose of 400 mg bd was given in one study (Magill 1995). In the other study, two 100 mg capsules were given orally at night and one in the morning, or two 100 mg capsules vaginally at night (Vanselow 1996).
Outcomes
A baseline of symptom severity was recorded by two pre‐treatment cycles in both studies.
Change in symptoms
The diary cards devised for one trial, scored symptoms on a four‐point scale from 'not present' to 'severe' and were used to express outcomes. The sum of the symptoms assessment score from the seven days after menstruation represented the follicular phase; the last seven days before menstruation plus three days of bleeding represented the luteal phase (Magill 1995). The worst symptom for each woman and her average symptoms were used in analysis.
In the other study, daily symptom rating charts were not used to assess outcomes on the grounds that they had not been validated for PMS (Vanselow 1996). Instead, the Menstrual Distress Questionnaire (MDQ) (Moos 1969) was used on day 26, together with two psychiatric rating scales, the Spielberger Anxiety Inventory and the Beck Depression Inventory. These three instruments were used for comparisons made with scores in the second premenstrual assessment month (Vanselow 1996). The MDQ directs attention to 47 different symptoms in eight groups with six degrees of severity for each.
Use of data
All the data recorded were ordinal numbers but subsequent analyses in the publications were made as if they were interval data. Both studies calculated means. One study expressed the results as means and standard deviations as if they were also normally distributed (Vanselow 1996). Standard deviations larger than the means for some outcomes suggested that they were not.
Excluded studies
We excluded 14 studies. One was a follow‐up study of a former trial (Freeman 1990).
We excluded three because they were not randomised controlled trials (Gray 1941) or contained insufficient data (Smith 1975; Vargyas 1985).
We excluded seven because they used only one prospective cycle to confirm the diagnosis (Andersch 1985; Dennerstein 1985; Maddocks 1986; Rapkin 1987; Sampson 1979), did not confirm it at all (Richter 1984) or did not describe diagnosis or its confirmation (van der Meer 1983).
Three studies did not show clearly whether participants were adequately screened for psychiatric disorders. Two did not mention screening at all (Andersch 1985; Sampson 1979) and we excluded both. Another reported current minor psychiatric disorder in 50% of participants and we excluded this also. (Corney 1990).
In three studies, it was doubtful if participants were adequately screened to distinguish between women who had exacerbation of chronic symptoms rather than symptoms confined to the luteal phase (Baker 1995; Freeman 1990; Freeman 1995). The first gave insufficient details in the report to be certain (Baker 1995). The other two depended on an increase of 50% in the scores of symptom severity in the charts used in the diagnosis and assessment of outcomes. It followed that if symptoms were absent in the follicular phase there could not be a 50% increase premenstrually. If symptoms in the luteal phase were moderate or severe, then they must have been more than low or absent in the follicular phase (Freeman 1990; Freeman 1995).
Risk of bias in included studies
1.
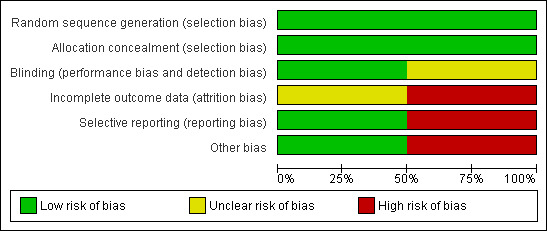
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
We assessed the risk of bias of the studies first on the published reports. Where it was possible to collect extra data from the authors, we did so.
Allocation
Randomisation and concealment of allocation:
The allocation of participants to the experimental or control groups was made by pharmacies and concealed by identical pre‐packed numbered boxes containing doses of progesterone and placebo in both studies. Neither study disclosed the precise method of randomisation but it was not done by the investigators.
Blinding
The active suppositories, pessaries or oral capsules should have been exactly the same in appearance as the placebo versions of each so that neither treatment givers nor participants could distinguish them. They were stated to be so in the parallel study (Magill 2004), but not mentioned at all in the crossover study where adequate blinding was particularly important (Vanselow 1996).
Incomplete outcome data
Neither of the studies was able to analyse data from all the participants. It was considered important that some assessment was made of the likely effect that withdrawals had had on the final outcome levels in the trials (Hollis 1999). In the parallel study, cyclicity was not established for some participants where records were available for only one cycle. Symptom severity did not confirm diagnosis of PMS for others, and some participants used forbidden medications. Distribution between the two study arms of these participants was described and data from them included in an 'intention‐to‐treat' analysis. Data from the rest of the participants (93/141) were analysed separately as 'per protocol' (Magill 1995). Some women (6 who had progesterone and 8 who had the placebo) did not attend all their clinics. There were further losses to analysis during the four cycles.
In the crossover study, participants withdrew after randomisation for personal reasons with one month's data or less (Vanselow 1996). Their distribution across the treatment arms was not described, nor was that of a participant who had a long interval of amenorrhoea before treatment. This puts her diagnosis in doubt and may or may not have affected the results in this small trial. Another participant withdrew because of nausea while using placebo. Four more participants left the trial after three or four cycles. Reasons were given, but no consideration of the effect on the final result made. Data for 22/39 participants were analysed. Because no intention‐to‐treat analysis was made, nor the effect of the distribution of losses to follow‐up considered, this study must be judged less dependable, especially as the attrition was high at 43% (Vanselow 1996).
Selective reporting
Magill 1995 was rated as at low risk of reporting bias, as the study included all the outcomes of interest in the review. Vanselow 1996 was at high risk of reporting bias, as only the second month's records were reported and that for only one day each cycle. Results were reported for each treatment phase but not as the difference from baseline for total MDQ scores, graphically, nor in means (standard deviations) for MDQ total or subscales, or BDI or STPI subscales. Daily ratings were not analysed and raw data were not available.
Other potential sources of bias
Definition and diagnosis of PMS
The concept of menstrual cycle change which has adverse effects on some women's lives has developed from the first descriptions (Frank 1931; Horney 1931). The definitions used have changed and the means of diagnosis has altered accordingly. A definition consistent with the inclusion criteria for this review was given in one study (Magill 1995).
For the selection of participants, the other study (Vanselow 1996) used criteria from the American Psychiatric Association's definition of late luteal phase dysphoric disorder (DSM IIIR 1987). This depended on the kind of symptoms experienced by participants, and required them to complete checklists for the diagnosis and assessment of outcomes. Validity of scales used in the trials
Although it has been a mainstay of research into menstrual cycle change, the Moos Menstrual Distress Questionnaire (MDQ) was originally retrospective (Moos 1968; Moos 1969). It has been suggested that these records were likely to be influenced by cultural differences (Abplanalp 1983; Sveinsdottir 1998). In its development, no tests of internal consistency were conducted and reliability over successive assessments was tested on only 15 participants for two cycles (Haywood 2002). It was used once each cycle to assess outcomes in the crossover study for that day only (Vanselow 1996). The MDQ was developed using means and standard deviations from subjective scores and reliance on its validity is not warranted.
The psychiatric rating scales also used in the latter study (Vanselow 1996) have not been validated for PMS and may be measuring something different from premenstrual symptoms of the same name (Wendestam 1980). The Beck Depression Inventory was found to be sensitive to cyclic changes in women with PMS (Keenen 1992), but does not distinguish them from premenstrual exacerbation (Stout 1985). PMS symptoms may overlap with other mood disorders in the premenstrual phase, and are not simply a brief depression or a short spell of anxiety disorder (Freeman 1996). Mental symptoms of PMS may be a distinct diagnostic entity (Landen 2003). It is possible that psychiatric scales are more likely to pick up a premenstrual exacerbation of subclinical depression (Chisholm 1990).
The use of ordinal data
Assessment of effectiveness of treatments for PMS depended on analysing subjective data. The scales used had four or six categories of severity for each symptom: see Table 2. While scales based on 10 or more severity ratings may be treated as continuous results, these scales yielded ordinal data unsuitable for arithmetical manipulation in both studies. Although statisticians nowadays are less strict about the use of parametric analysis for ordinal scale data, the means and standard deviations calculated from them could not be reliably interpreted ( Vanselow 1996).
2. The nature of primary outcomes in included studies.
| Trial | Scales used | When applied | By whom | Number of items | Degrees of severity | Data Used | Statistic | Significance tests | Comments |
| Magill 1995 | Diary cards | daily | participants | symptoms selected by participants | 4 point scale | baseline score ie mean of eligible symptoms' scores reductions in baseline scores for each cycle in each group | medians and interquartiles of baseline scores for each group medians and interquartiles of reductions in baseline scores for each cycle of each group | Wilcoxon rank sum test | Avoids check lists Cannot distinguish individual women's scores |
| Vanselow 1996 1 | Menstrual Distress Questionnaire MDQ | on day 26 only | trialists | 47 in eight groups | 6 point scale | sum of scores for second month | means and SDs | ANOVA with Bonferroni correction Fisher's Planned Least Significant Difference | unsuitable treatment of ordinal data SDs larger than means |
| 2 | Beck Depression Inventory BDI | on day 26 only | trialists | 21 | 4 point scale | sum of scores for second month | means and SDs | unsuitable treatment of ordinal data May not measure premenstrual depression |
|
| 3 | Spielberger STAI | on day 26 only | trialists | 40 | 4 | sum of scores for second month | means and SDs | unsuitable treatment of ordinal data May not measure premenstrual symptoms |
One study used the median and interquartile range, appropriate measures of location and dispersion for ordinal data (Magill 1995).
Prospective or retrospective records
Retrospective accounts by participants of their PMS symptoms have been shown to be unreliable (Endicott 1982; Halbreich 1985; Taylor 1986). However, the one study using the MDQ made assessments of outcomes on day 26 only, and apparently did not depend on participants' recall of the severity of symptoms on earlier days (Vanselow 1996).
Timing of interventions
The trialists attempted to begin the intervention three days after ovulation (Vanselow 1996), or 14 days before the next menstrual period (Magill 1995). It has been suggested that progesterone therapy is less effective if it is not started before the day that symptoms are expected (Dalton 1984), and it should be continued until menstruation to prevent precipitation of symptoms. Treatment may have stopped before menses began in one study (Vanselow 1996).
Dose
The daily dose administered was 200 mg vaginally and 300 mg orally in one study (Vanselow 1996) and 800 mg either vaginally or rectally in the other study (Magill 1995). Women are known to vary in their ability to absorb progesterone (Dalton 1977; Morville 1982). The vehicle and the route of administration can alter the amount absorbed and the rate of breakdown (Dalton 1977; Hargrove 1989; Price 1983; van der Meer 1982). It has been argued that women vary also in their biological demand for progesterone and that doses of up to six 400 mg suppositories are needed for some women (Dalton 1977). Therefore doses in these trials, although comparable with the amount usually considered adequate, are towards the low end of the range which Dalton used and may possibly have been insufficient for some participants.
Number of participants
A power calculation found that between 45 and 50 women would have been necessary to detect a 20% improvement (Vanselow 1996). Recruitment closed when analysis of data from the first 25 women showed no trends and 39 women were randomised. This study risked type II error and, on its own, was of doubtful value because of its low power.
The parallel study was relatively large, with 141 participants randomised, but no power calculation for the sample size was reported (Magill 1995).
Effects of interventions
Improvement in Symptoms
The studies showed three different outcomes.
Both the experimental and the control groups showed improvement in symptoms but the difference between them was not statistically significant (Vanselow 1996).
Greater improvement was recorded in the experimental group than the control but did not reach statistical significance except in the first cycle in 'intention to treat' analysis (Magill 1995).
Statistically significantly greater improvement was recorded in the experimental group than the control in the per protocol analysis (Magill 1995).
Published numerical data from the trial are shown in Table 3 for Magill 1995 .
3. Published numerical data from trials Magill 1995.
| Changes from baseline in symptoms | |||
| median reductions (interquartile range) | |||
| progesterone | placebo | significance | |
| highest scoring symptom per protocol | |||
| cycle 1 (n=50 /43) | ‐9 (‐16 to ‐5) | ‐4 (‐12 to 0) | P<0.01 |
| cycle 2 (n=49/43) | ‐9 (15 to ‐6) | ‐5 (‐10 to ‐1) | P<0.01 |
| cycle 3 (n=42/38) | ‐10 (‐16 to ‐5) | ‐8 (‐13 to ‐2) | |
| cycle 4 (n=41/31) | ‐10 (‐16 to ‐5) | ‐5 (‐12 to 0) | P< 0.05 |
| highest scoring sympton in intention to treat analysis | not recorded | not recorded | not recorded |
| average symptom score per protocol | |||
| cycle 1(n=50 /43) | ‐7 (‐12 to ‐4) | ‐4 (‐10 to 0) | P<0.01 |
| cycle 2 (n=49/43 | ‐7 (‐12 to ‐5) | ‐5 (‐9 to 0) | P< 0.05 |
| cycle 3 (n=42/38) | ‐10 (‐12 to ‐5) | ‐6 (‐11 to ‐2) | P< 0.05 |
| cycle 4 (41/31) | ‐10 (‐14 to ‐2) | ‐4 (‐10 to 0) | |
| average syptom score in intention to treat analysis | |||
| cycle 1 (n=73/57) | ‐5 (‐9 to ‐1) | ‐2 (‐7 to 2) | P< 0.05 |
| cycle 2 (n=66/57) | ‐5 (‐10 to ‐2) | ‐3 (‐8 to 1) | |
| cycle 3 (n=58/51) | ‐6 (‐10 to 0) | ‐3 (‐8 to 1) | |
| cycle 4 (n=57/43) | ‐4 (‐10 to 1) | ‐4 (‐10 to 2) | |
Skewed data in Table 4 for this small (n=22) trial (Vanselow 1996) cannot be reliably interpreted. Only data from one day in the second cycle for each of the three comparisons was reported. As explained, the BDI and STPI are not designed for PMS and reliance on the MDQ for one day in each cycle, is not warranted. Losses to follow‐up were not adequately addressed.
4. Published data from trial Vanselow 1996.
| Outcome | Mean ± SD | Across all conditions | Between treatments | |||||||
| Before the trial | Placebo | Vaginal progesterone | Oral progesterone | At follow‐up | F | p | F | p | ||
| BDI | total | 12.20±5.37 | 5.25±4.85 | 7.70±6.30 | 6.75±6.26 | 9.50±7.98 | 4.96 | 0.001 | 1.50 | 0.234 |
| STPI | anger | 24.65±10.57 | 15.10±8.53 | 16.15±7.44 | 16.05±7.17 | 17.45±9.09 | 6.14 | 0.0002 | 0.28 | 0.756 |
| anxiety | 29.55±5.63 | 18.85±9.23 | 21.00±7.80 | 22.30±8.41 | 23.05±8.49 | 6.68 | 0.0001 | 1.16 | 0.325 | |
| MDQ | total | 72.05±22.52 | 40.60±24.53 | 40.25±28.92 | 40.90±21.53 | 50.95±32.31 | 10.64 | 0.0001 | 1.66 | 0.998 |
| water | 7.95±3.17 | 5.70±3.50 | 5.80±4.32 | 5.80±3.82 | 6.70±3.70 | 3.84 | 0.0067 | 0.04 | 0.965 | |
| pain | 8.85±4.32 | 4.70±4.05 | 5.60±5.13 | 5.15±4.09 | 6.40±5.17 | 5.32 | 0.0008 | 0.57 | 0.568 | |
| loss of contration | 13.2±6.25 | 6.3±6.83 | 6.40±7.31 | 6.00±5.02 | 8.05±6.40 | 7.93 | 0.0001 | 0.09 | 0.918 | |
| behavioural change | 11.30±4.99 | 5.50±4.5 9 | 5.40±5.05 | 5.65±4.20 | 8.35±6.28 | 10.90 | 0.0001 | 0.02 | 0.979 | |
| negative affect | 21.50±6.13 | 7.95±8.90 | 9.90±9.04 | 10.00±7.55 | 12.55±9.20 | 10.45 | 0.0001 | 0.51 | 0.6058 | |
| autonomic reaction | 2.10±2.83 | 1.30±2.36 | 0.65±1.39 | 1.25±2.05 | 1.85±3.39 | 1.40 | 0.243 | 0.96 | 0.391 | |
| arousal | 4.65±2.48 | 7.95±3.71 | 5.25±3.32 | 6.15±2.72 | 5.35±3.20 | 4.33 | 0.0033 | 3.90 | 0.0283 | |
| control | 2.65±2.32 | 1.35±1.85 | 1.30±2.81 | 0.95±2.31 | 2.05±3.87 | 2.00 | 0.1028 | 0.25 | 0.78 | |
Numerical data from this small (n=22) trial cannot reliably be interpreted. Many of the standard deviations were almost as large, or larger than their means. Only data from one day in the second cycle for each of the three comparisons was reported. As explained, the BDI and STPI are not designed for PMS and reliance on the MDQ for one day in each cycle, is unsafe. Losses to follow‐up were not adequately addressed.
Adverse effects
Both studies recorded adverse events. Neither described them as major. Neither gave sufficient numerical data to allow risk comparison between active and placebo treatments.
Withdrawal from a trial due to adverse events
Some participants withdrew from trials because of what they perceived as side effects. The numbers of withdrawals were 4/141 (Magill 1995) and 3/39 (Vanselow 1996). Particular reasons given for withdrawal were irregular menstruation and an ovarian cyst in the progesterone group, and respiratory infection and depression in the placebo group (Magill 1995). One woman experienced nausea when taking placebo (Vanselow 1996). During treatment with vaginal progesterone, another developed depression and a third suffered a relapse of thyrotoxicosis (Vanselow 1996).
Frequency of adverse events
The studies showed three different outcomes.
In the parallel study, 41/80 participants taking progesterone reported 101 adverse events, while 26/61 participants using placebo reported 53 (Magill 1995). Participants reported menstrual disorder, vaginal pruritus, headache, nausea, abdominal pain, influenza syndrome, dysmenorrhoea, breast pain, rectal pain and diarrhoea. Generally more adverse events occurred in the progesterone group, but only menstrual disorder (mostly changes in cycle length) reached statistical significance, (P<0.05) One participant in the progesterone group in this study became pregnant after a long interval of infertility.
It was not possible to total the incidences from the report of the crossover trial (Vanselow 1996). As well as the withdrawals detailed above, there was one complaint of itchy skin on placebo. Physical tiredness, although the most commonly reported adverse event, was not significantly different between treatment arms. Drowsiness and dizziness were more frequent on oral progesterone. Vaginal irritation was more frequent on vaginal progesterone. Whether these were statistically different between groups was not reported.
Other reported effects
Two participants withdrew from the placeob group of one study (Magill 1995) because they disliked using suppositories. The other study did not mention any objection to them.
Biological parameters
No clinically significant change was noted in severity or duration of menstrual bleeding nor in blood pressure or body weight (Magill 1995). These outcomes were not mentioned in the other study (Vanselow 1996).
Discussion
Summary of main results
Severity of symptoms
These trials have not shown conclusively that progesterone is an effective treatment for premenstrual syndrome, nor that it is not. The review has revealed more about the difficulties inherent in the study of PMS and consequent deficiencies in method than about the efficacy of progesterone in treating it.
Neither of the included studies considered how many days in each cycle the participants experienced symptoms. Calculation of mean scores from the rating scales for each symptom, symptom cluster or total symptom score made it impossible to disentangle one woman's score. So it was not possible to know if any women had milder symptoms, nor was it possible to know whether any of the participants had fewer symptoms.
The two included trials differed in every respect, yielding insufficient suitable data for meta‐analysis. Raw data were unavailable, so the intended re‐working of the data into binary form was not possible.
Adverse effects
Both studies described adverse events which may or may not have been the side effects of the treatment. These were mild and occurred in placebo cycles as well as during active interventions. Women sometimes gave side effects as the reasons for withdrawing. Some of the perceived effects were themselves common symptoms of PMS.
Overall completeness and applicability of evidence
The trials compared the severity of symptoms with progesterone and placebo (Magill 1995) and with progesterone administered by two different routes (Vanselow 1996)
Symptom severity graded by a number is not necessarily equivalent to the same number in another woman's assessment, either for the same symptom or for different symptoms. An increase from 0 (not present) to 1 (mild) is not necessarily the same increase in severity as that from 4 (severe) to 5 (very severe). Yet such assumptions were made in the statistical analyses performed on the results of symptom ratings. Moreover, the combined scores from the Moos Menstrual Distress questionnaire may hide an increase in some items and decrease in others (Vanselow 1996). They are difficult to relate to clinical improvement or worsening.
In one study, narrow diagnostic criteria excluded participants with one overwhelming symptom, several irritating symptoms or a vague feeling of malaise (Vanselow 1996), although they would be found in the general population of PMS sufferers.
With reference to the definition of premenstrual syndrome, the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM IIIR 1987) used in one study (Vanselow 1996) gives stringent qualifying symptoms for Late Luteal Phase Dysphoric Disorder (LLPDD), but is not free from risk of excluding women with relatively few but severe symptoms. Suggestions have been made for its refinement ( Halbreich 2007). The term "premenstrual syndrome" covers a range from slight discomfort to complete disorder, but there is no cut‐off point where PMS ends and LLPDD begins. This is true also of Premenstrual Dysphoric Disorder (PMDD), preferred in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV 1994). Attempts to standardise diagnosis in the interest of scientific comparability serves research needs but not clinical practice. Homogeneous groups of participants make trials more dependable but women presenting with premenstrual symptoms do not fit neatly into precise categories Knaapen 2008. It is also unfortunate that it perpetuates the distinction between mental and physical symptoms. While these may be experienced differently by sufferers, they are not necessarily of different aetiology. At the level of cellular physiology, hormones and neurotransmitters contribute to both Smith 1998.
Quality of the evidence
One of the studies was too small to have sufficient power to detect anything but large universal benefits, so risked type II error (Vanselow 1996). Both trials had potentially biasing attrition levels.
Some studies included design factors which tended to minimise the apparent effect of progesterone:
Use of symptom checklists
Over 100 different symptoms affecting some women in the days leading up to a menstrual period, but not at other times, have been described. Such symptoms are rightly termed 'premenstrual'. They are not exclusive to PMS, nor to women. No woman suffers from them all. Conscientious reporting of mild symptoms would have reduced the difference between a potential participant's follicular and luteal scores, thereby lessening the likelihood of diagnosis and apparently diminishing the effectiveness of treatment (Vanselow 1996).
The parallel study avoided this by allowing women to choose the symptoms they recorded on their diary cards (Magill 1995). The symptoms were then considered eligible if they occurred in the luteal phase, with no more than one mild occurrence in the follicular phase.
Sampling certain days
Sampling on certain days (Vanselow 1996) or pooling records from some days in the luteal phase (Magill 1995) lessens the apparent effect of treatment. Some women will have their worst days then. Others will have moderate symptoms over a longer time and would have a lower score on the sampled days, although their indisposition would have been as much or greater. The length of the symptomatic phase is highly individual (Halbreich 1985).
Length of study period
PMS is known to vary from day to day and from cycle to cycle. Therefore the longer the duration of the trial the better. The cross‐over study administered the progesterone or placebo for two cycles each but recorded from only one (Vanselow 1996). The longer intervention was four cycles in the parallel study (Magill 1995).
Agreements and disagreements with other studies or reviews
Both the included studies referred to the work of Katharina Dalton and to previous studies, few of which seemed to support her claims. Dalton first treated PMS sufferers with progesterone in the late 1940s, and provoked discussion and counter‐claims in the medical literature. She asserted that if PMS were properly diagnosed, with prospective daily record charts showing symptoms only in the luteal phase, progesterone would relieve the symptoms. She objected to conducting controlled trials. She considered them unethical because women whose lives were disrupted by PMS would have no treatment in a control group (Dalton 1984; Dalton 1994).
Dalton advised that administration of progesterone should begin two days before the usual onset of symptoms and be tailored to each woman, increasing the number of 400 mg suppositories to as many as six each day during the luteal phase. If symptoms persisted, intramuscular injections were to be used (Dalton 1977; Dalton 1984). She also recommended that the dose be tapered when bleeding began and not stopped abruptly. The study, which selected participants according to her definition and had the higher dose, found some benefit (Magill 1995). However, the studies reviewed here have neither borne out Dalton's assertions nor refuted them, since the doses were low compared to hers, begun late in the cycle and in one case (Vanselow 1996) may have been of fixed duration.
The randomised controlled trials reviewed here were examined in a systematic review of studies of progesterone and progestogens for PMS (Wyatt 2001). This review did not separate data from studies which had not clearly excluded participants with premenstrual exacerbation of on‐going indisposition, nor with other psychiatric conditions. Studies which had only one cycle of prospective records were also included. This review concluded that exogenous progesterone did not improve symptoms ( Wyatt 2001).
A more recent review (Backstrom 2003) of hormonal treatments for PMS included nine trials (Andersch 1985; Baker 1995; Dennerstein 1985; Freeman 1990; Freeman 1995; Maddocks 1986; Magill 1995; Sampson 1979; Vanselow 1996). It did not perform a meta‐analysis. Benefit from progesterone was not ruled out but the authors were unable to describe a mechanism by which it might alleviate symptoms.
Authors' conclusions
Implications for practice.
Evidence for effectiveness is equivocal because of methodological failings of the trials reviewed, incomplete or inappropriate handling of outcome data, the small numbers of participants or unsuitable psychiatric scales. Although some individual women benefited, there were insufficient data to relate them to particular symptoms.
The review does not suggest that progesterone is unsafe. Mild adverse events occurred during placebo cycles as well as in the progesterone cycles and some were themselves common symptoms of PMS.
Women who have considered themselves infertile could conceive when treated with progesterone for PMS. Some women might notice change in cycle length or sedative effects.
The long‐term effects of progesterone treatment cannot be inferred since the intervention lasted for, at most, four cycles.
Implications for research.
Although 13 trials have been performed, the efficacy of progesterone for PMS is still in doubt. A further randomised controlled trial could examine properly the claims that have been made for higher doses of progesterone matched to individual participants. Since the trials reviewed here were published, advances have been made in trial design and reporting (Altman 2001; Hollis 1999). The difficulties peculiar to the study of PMS have been summarised and suggestions made to avoid the errors stemming from difficulties in diagnosis Halbreich 2007.
Numbers
Many women suffer from PMS. If only a small proportion benefited from progesterone, the actual number would be large.
The definition of PMS and selection of participants
How PMS is defined controls the means of diagnosis and hence selection of participants. There are no specific observations, tests or symptoms. In order to include participants representative of women with various symptoms of different severity, diagnosis must depend on the occurrence of severe symptoms solely in the luteal phase and be confirmed over at least two cycles of prospective records( Abplanalp 1983; Halbreich 2007).
Premenstrual exacerbation of low‐level continual symptoms should be an exclusion factor and so should current psychiatric disorders which could be confused with PMS or premenstrual exacerbation (Endicott 1982; Steiner 1980; Steiner 2000).
Plasma concentrations of progesterone and allopregnanolone following oral progesterone administration support consideration of PMS as a separate entity from other forms of depression and are pertinent to diagnosis of PMS (Klatzkin 2006).
Other medications for PMS or any other hormonal preparations should be forbidden.
Recruitment and the design of trials
Women may be reluctant to commit themselves to studies which inevitably last for months, especially as they must be informed that they may not be having the active intervention. Outcomes of the crossover design, favoured by medical trialists, are difficult to analyse. In theory fewer participants are necessary, because each one is her own control, but crossover trials take longer overall. If each participant has only the active treatment or the placebo, it would be more reasonable to continue for four cycles. With two cycles to confirm diagnosis, this would be six cycles. Crossover trials with the same lengths of interventions would be a minimum of ten cycles, even without a washout cycle.
Premenstrual symptoms vary from one cycle to another so are not ideal for crossover studies even though PMS is a long‐term condition and the treatments are aimed at relieving rather than curing the symptoms.
Parallel studies would therefore be preferable to crossover designs.
Outcomes and the use of checklists
Effective treatment might reduce the severity of symptoms, lead to fewer symptoms or fewer days in the cycle without symptoms. It is important to know if any of these apply but impossible if means are calculated for groups of women.
Lists of symptoms are inappropriate for women with PMS (Taylor 1986). Most women experience only a very few symptoms, and the need to complete checklists each day caused withdrawal from two excluded studies (Maddocks 1986; Richter 1984). Allowing each woman to record only her worst two symptoms, declared at the outset, would also avoid the exclusion of those with uncommon symptoms. It would discourage the reporting of occasional unrelated symptoms, which would confuse diagnosis and lessen the apparent benefit of treatment. Progesterone might be more effective for some of the symptoms than others, but the number of participants who experience a particular symptom may be small.
Similarly, grading symptom severity on a numerical scale is an unnecessary chore likely to increase attrition. There is no certainty that one woman's assessment of a symptom bears any numerical relationship to another woman's assessment of the same symptom. Neither is it sure that a symptom graded identically by the same woman on two consecutive days is at the same level of severity. It is even more doubtful if anyone can make accurate comparisons of the severity of a symptom after a month.
If only one symptom is severe enough to interfere with a woman's life, it would score high on many scales. By contrast, a woman who has several mild symptoms could rate a low score for each with the same total even though she is hardly inconvenienced. Indeed such a score could be the result of successful treatment.
It should be possible to distinguish women who benefit from the intervention from those who do not. Outcomes need only be divided into symptoms relieved or symptoms not relieved, and rating scales designed with this in mind. Numerical grades of severity could be transcribed to 'symptom relieved' or 'not relieved' if the cut off point were decided beforehand. Instruments of this kind are already in use for diagnosis and assesment of treatment Johnson, S 2004.
Symptoms subgroups
Studies have not usually distinguished symptom profiles Halbreich 2006 but it may be that a subgroup of women would benefit from progesterone. For example, symptoms arising from parts of the brain associated with anxiety and panic might be alleviated. Inhibition of these areas depends on neurones which secrete gama amino butyric acid (GABA). Changes in the number, distribution and type of GABA(A) receptor subunits are associated with rising and falling levels of allopregnanolone, a metabolite of progesterone(Lovick 2006; Smith 1998). Further animal work has related the pattern of firing of specific neurones in the midbrain in response to allopregnanalone, to both cycle stage or experimental progesterone withdrawal and also with environmental stressors Lovick 2008. How symptom severity and allopregnanolone levels in women are related remains unclear Nyberg 2007. Researchers should state their intentions of performing such subgroup analysis in their protocols and specify symptoms for separate analysis.
The placebo effect
The non‐specific responses to treatment are known throughout medicine. Improvement without active treatment may have many causes, and the placebo effect as an entity may be illusory (Kienle 1996). It has been recommended that placebo responders be removed before a trial (Halbreich 1985). This is almost certainly unrealistic. It was attempted in one of the excluded trials which none the less reported more improvement in response to the placebo than to progesterone (Freeman 1990). At least some of the observed improvements with active intervention during a trial may be for reasons other than the intervention.
PMS is generally believed to show a big placebo effect. Trials of other treatments for PMS have also reported more apparent effect from the placebo than the active treatment (Halbreich 1985) or the benefit from the placebo outlasting that from the active intervention (Steiner 2000). During a trial of magnesium for PMS, the sorbitol placebo, assumed to be inactive, was found to be effective (Walker 2002).
Parallel studies may allow time for any placebo effect to diminish, and large numbers of participants are more likely to point up differences between active and inactive treatment even if many of the participants in both arms improve for reasons other than the interventions.
Rating scales
It cannot be assumed that a rating scale is reliable because it has been used over many years, especially if it depends on participants' recall. Nor can it be assumed that psychiatric scales developed for other disorders can detect PMS. An electronic method of recording symptom severity based on visual analogue scales (VAS) has been designed (Wyatt 2002). The line lengths in such scales are ordinal data, but can safely be treated as continuous data (Johnson, J 2004).
Copies of charts or diaries used in the trial should be published, together with the instructions given to the participants. As suggested in an analysis of statistical reports in medical journals, all raw data, even that unused in the final analyses, should be preserved electronically and remain available (Garcia‐Berthou 2004).
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2012 | Review declared as stable | As no further studies are expected, this review will no longer be updated. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 15 February 2012 | New citation required but conclusions have not changed | No new studies found |
| 3 February 2012 | New search has been performed | As no new studies are likely, this review can now be considered to be stable. |
| 3 November 2008 | New citation required but conclusions have not changed | Relevant references to the argument were added. Trial data were included. No new studies were included. Risk of bias was re‐examined. |
| 1 April 2008 | Amended | Converted to new review format. |
| 10 July 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Thanks are due to Dr Andersch, Professor Lorrainee Dennerstein, Dr E. W. Freeman, Dr P. J. Magill, Dr Andrea Rapkin, Dr S. S. Shapiro, Dr S. L. Smith and Dr Wendy Vanselow who answered questions about the methods used in their trials.
Ferring Pharmaceuticals, Berkshire, UK, Nordic Pharma UK Ltd and Wyeth Pharmaceuticals UK answered questions about progesterone products and unpublished material.
The staff of the Yeovil Central Branch of Somerset County Council Library took meticulous care in tracing obscure journals and inter‐library loans. Rose Elliot, Jill Lang, Jean Robinson and Dawn Robson deserve special thanks for this work.
Thanks are due also to Jean Hill formerly of the Academy Library, Somerset Academy‐Yeovil, Yeovil District Hospital for help with searches and copies of journal articles.
We are grateful for the support given by the staff of the Menstrual Disorders and Subfertility Group, especially Michelle Proctor, former Review Group Co‐ordinator,and the Search Co‐ordinators.
We thank Peter Gotzsche of the Nordic Cochrane Centre, Andy Vail, Manchester University and Sofia Dias, University of Bristol for advice on presenting data.
We acknowledge help with the assessment of the selective outcome domain in the Risk of Bias Tables, from Jamie Kirkham of the MRC funded project Outcome Reporting Bias in Trials (G0500952) investigating the degree and impact of outcome reporting bias in Cochrane reviews.
Appendices
Appendix 1. Search terms
* Condition of interest ‐ Premenstrual Syndrome or ‐ Premenstrual Tension or ‐ PMS or ‐ LLPDD or ‐ Luteal phase dysphoria or ‐ Late luteal phase dysphoric disorder or ‐ Late luteal premenstrual dysphoric disorder or‐ PMDD or ‐ Premenstrual dysphoria or ‐ Premenstrual dysphoric disorder or ‐ Premenstrual mastalgia or ‐ Premenstrual depression or ‐ Premenstrual asthma or ‐ Premenstrual migraine or ‐ Premenstrual epilepsy
*Intervention ‐ Progesterone administered by suppository, pessary, injection, vaginal gel, transdermal cream, or in oral micronised form. ‐ Cyclogest ‐ suppositories/pessaries ‐ Gestone ‐ injections ‐ Crinone ‐ vaginal gel ‐ Utrogestan ‐ oral micronised progesterone ‐ Prometrium ‐ micronised progesterone in oral gel cap ‐ Progest ‐ transdermal cream ‐ Any other trade names for progesterone products
Appendix 2. Search strings
MEDLINE (Ovid) 1966 to Feb 2011
Keywords CONTAINS "premenstrual " or "premenstrual dysphoric disorder" or "premenstrual symptom scores" or "premenstrual symptoms" or "premenstrual syndrome" or "premenstrual syndrome‐symptoms" or "PMS" or Title CONTAINS "premenstrual " or "premenstrual dysphoric disorder" or "premenstrual symptom scores" or "premenstrual symptoms" or "premenstrual syndrome" or "premenstrual syndrome‐symptoms" or "PMS"
AND
Keywords CONTAINS "Progesterone" or "cyclogest" or "crinone" or "prometrium" or "progesterone cream" or "progesterone gel" or Title CONTAINS "Progesterone" or "cyclogest" or "crinone" or "prometrium" or "progesterone cream" or "progesterone gel"
Search Strategy for CENTRAL Cochrane Central Register of Controlled Trials <Feb 2011>
1 PREMENSTRUAL SYNDROME/ (303)
2 premenstrual$.tw. (550)
3 pms.tw. (229)
4 pmt.tw. (35)
5 pmdd.tw. (93)
6 luteal phase dysphoria.tw. (0)
7 late luteal phase dysphoric disorder.tw. (24)
8 llpdd.tw. (12)
9 lpd.tw. (61)
10 or/1‐9 (789)
11 exp Progesterone/ (1787)
12 cyclogest.tw. (5)
13 gestone.tw. (1)
14 crinone.tw. (30)
15 utrogestan.tw. (15)
16 prometrium.tw. (3)
17 Progesterone$.tw. (1885)
18 or/11‐17 (2904)
19 10 and 18 (95)
EMBASE <1980 to Feb 2011>
Search Strategy:
1 premenstrual dysphoric disorder/ or premenstrual syndrome/ (2923)
2 premenstrua$.tw. (2758)
3 (pms or pmt).tw. (2743)
4 luteal phase dysphoria.tw. (1)
5 late luteal phase dysphoric disorder.tw. (73)
6 llpdd.tw. (33)
7 lpd.tw. (894)
8 or/1‐7 (6668)
9 exp Progesterone/ (37077)
10 cyclogest.tw. (124)
11 gestone.tw. (79)
12 crinone.tw. (142)
13 utrogestan.tw. (433)
14 prometrium.tw. (105)
15 Progesterone.tw. (39229)
16 or/9‐15 (53135)
17 8 and 16 (890)
18 Clinical trial/ (493487)
19 Randomized controlled trials/ (154967)
20 Random Allocation/ (25139)
21 Single‐Blind Method/ (7385)
22 Double‐Blind Method/ (68397)
23 Cross‐Over Studies/ (20005)
24 Placebos/ (110517)
25 Randomi?ed controlled trial$.tw. (27905)
26 RCT.tw. (2178)
27 Random allocation.tw. (604)
28 Randomly allocated.tw. (9576)
29 Allocated randomly.tw. (1309)
30 (allocated adj2 random).tw. (552)
31 Single blind$.tw. (7047)
32 Double blind$.tw. (81097)
33 ((treble or triple) adj blind$).tw. (126)
34 Placebo$.tw. (104083)
35 Prospective Studies/ (72824)
36 or/18‐35 (649713)
37 Case study/ (5332)
38 Case report.tw. (110650)
39 Abstract report/ or letter/ (460220)
40 or/37‐39 (574202)
41 36 not 40 (627179)
42 animal/ (18230)
43 human/ (6043177)
44 42 not 43 (14461)
45 41 not 44 (627083)
46 17 and 45 (248)
Ovid MEDLINE(R) <1950 to February 2011>
Search Strategy:
1 PREMENSTRUAL SYNDROME/ (2971)
2 premenstrual$.tw. (3216)
3 pms.tw. (2441)
4 pmt.tw. (662)
5 pmdd.tw. (232)
6 luteal phase dysphoria.tw. (2)
7 late luteal phase dysphoric disorder.tw. (62)
8 llpdd.tw. (28)
9 lpd.tw. (1009)
10 or/1‐9 (7446)
11 exp Progesterone/ (56201)
12 cyclogest.tw. (4)
13 gestone.tw. (5)
14 crinone.tw. (34)
15 utrogestan.tw. (20)
16 prometrium.tw. (7)
17 Progesterone$.tw. (51878)
18 or/11‐17 (76795)
19 10 and 18 (846)
20 randomised controlled trial.pt. (248340)
21 controlled clinical trial.pt. (76350)
22 randomised controlled trials as topic/ (52334)
23 random allocation/ (59709)
24 double blind method/ (94724)
25 single blind method/ (11622)
26 or/20‐25 (419127)
27 animals/ not (animals/ and humans/) (3157750)
28 26 not 27 (392632)
29 clinical trial.pt. (440652)
30 exp clinical trials as topic/ (198184)
31 (clinic$ adj25 trial$).ti,ab. (140142)
32 cross‐over studies/ (21307)
33 (crossover or cross‐over or cross over).tw. (39734)
34 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (94012)
35 placebos/ (26640)
36 placebo$.ti,ab. (106901)
37 random$.ti,ab. (396364)
38 research design/ (51042)
39 or/29‐38 (898056)
40 39 not 27 (831861)
41 28 or 40 (853075)
42 19 and 41 (156)
CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature <1982 to February Week 4 2008>
Search Strategy:
1 PREMENSTRUAL SYNDROME/ (705)
2 premenstrual$.tw. (475)
3 pms.tw. (382)
4 pmt.tw. (39)
5 pmdd.tw. (50)
6 luteal phase dysphoria.tw. (1)
7 late luteal phase dysphoric disorder.tw. (6)
8 llpdd.tw. (6)
9 lpd.tw. (24)
10 or/1‐9 (968)
11 exp Progesterone/ (753)
12 cyclogest.tw. (0)
13 gestone.tw. (0)
14 crinone.tw. (0)
15 utrogestan.tw. (0)
16 prometrium.tw. (2)
17 Progesterone$.tw. (590)
18 or/11‐17 (1089)
19 10 and 18 (52)
20 exp clinical trials/ (54488)
21 Clinical trial.pt. (27834)
22 (clinic$ adj trial$1).tw. (12548)
23 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw. (7379)
24 Randomi?ed control$ trial$.tw. (10811)
25 Random assignment/ (17267)
26 Random$ allocat$.tw. (1177)
27 Placebo$.tw. (10348)
28 Placebos/ (4067)
29 Quantitative studies/ (3706)
30 Allocat$ random$.tw. (68)
31 or/20‐30 (75750)
32 19 and 31 (5)
33 from 32 keep 1‐5 (5)
PsycINFO <1806 to February 2011>
Search Strategy:
1 PREMENSTRUAL SYNDROME/ (1280)
2 premenstrual$.tw. (1952)
3 pms.tw. (908)
4 pmt.tw. (236)
5 pmdd.tw. (216)
6 luteal phase dysphoria.tw. (6)
7 late luteal phase dysphoric disorder.tw. (107)
8 llpdd.tw. (43)
9 lpd.tw. (39)
10 or/1‐9 (2526)
11 exp Progesterone/ (1386)
12 cyclogest.tw. (0)
13 gestone.tw. (0)
14 crinone.tw. (0)
15 utrogestan.tw. (0)
16 prometrium.tw. (1)
17 Progesterone$.tw. (2469)
18 or/11‐17 (2585)
19 10 and 18 (130)
Appendix 3. Trials Register of the Cochrane Menstrual Disorders and Subfertility Group
1. (Keywords = "premenstrual Syndrome" OR
2. keywords = "Premenstrual Syndrome‐Symptoms" OR
3. keywords = "premenstrual dysphoric disorder" OR
4. keywords = "luteal phase disorders" )
5. and
6. (keywords = "progesterone" OR "crinone" OR "prometrium" OR "cyclogest")
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Magill 1995.
| Methods | RCT. Parallel, 2 arms: progesterone, placebo. Randomisation performed by pharmacy. No power calculation prior to study reported. ITT (mis‐diagnosed and per protocol) | |
| Participants | Nr = 141, Na = 93: Aged 18 to 45 years. Experienced PMS in last three cycles. Agreed not to use oral contraceptives and discontinue other medication for PMS. Had no recent history of menstrual irregularity, psychotic illness or suicidal tendency. Did not misuse drugs or alcohol. Had not recently used antidepressants, benzodiazepines, therapy interfering with normal ovarian function or vitamin B6 preparations. Not eligible if recorded symptoms in only one cycle. PE rigorously excluded by protocol. | |
| Interventions | 400 mg progesterone pessary or identical placebo twice daily from 14 days before expected menstruation and until menstruation for 4 treatment cycles. | |
| Outcomes | Highest scoring PMS symptoms daily on diary cards on four ‐point scale (0 = not present,1 = mild, 2 = moderate, 3 = severe). Average symptom scores. Blood pressure weight and height (in each cycle at surgeries). Eligible patients showed statistically significant improvement in all symptom scores. ITT analysis showed smaller improvement which was not significant at the 5% level except in the first cycle. PE excluded by the protocol. | |
| Notes | 48/141 participants dropped because symptoms recorded in only one cycle (6 progesterone 4 placebo), symptom severity too low in luteal phase or too high in follicular phase (16 progesterone10 placebo), taking medications not permitted by the protocol (8 progesterone 4 placebo). 2 pre‐treatment cycles for prospective records. Diary cards designed for the study. Only each participant's highest scoring symptom used since most significant clinically. AE no clinically significant changes in blood pressure, weight , severity or duration of menstrual bleeding in either group. 41/80 in progesterone group reported a total of 121 AE, 26/61 of placebo group reported a total of 53 AE. Incidence of nausea, breast pain and rectal pain similar in each group. Menstrual disorder (mostly changes in cycle length),vaginal pruritus and headache were more common in the progesterone group but only menstrual disorder statistically significant. AE generally mild. Two from each group withdrew because of AE‐ irregular menstruation and ovarian cyst in progesterone group, respiratory infection and depression in the placebo group. 2 stopped using placebo because they disliked pessaries. 1 using progesterone became pregnant after a long interval of infertility. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote "The randomisation schedule was prepared by Hoechst UK Ltd and, from memory, patient numbers were allocated to active or placebo. Investigators recruited patients in sequence and patients were provided with trial supplies matching their trial numbers." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | quote "Cyclogest and matching placebos were manufactured by Cox and Co. and presented in identical packing." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons were given for withdrawals although they were separated according to treatment group. Changes from baseline for highest scoring symptoms only reported for per protocol subgroup, but changes from baseline for average symptom scores reported for both. Raw data were not available. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but the report includes all the outcomes of interest in the review. |
| Other bias | Low risk | ITT analysis performed. Ineligible participants excluded from per protocol analysis. Large size. (141 participants / 93 eligible) Baseline characteristics of participants in treatment and placebo groups for both ITT and per protocol shown. |
Vanselow 1996.
| Methods | RCT. 3 way crossover: vaginal progesterone/ oral progesterone/ placebo. No washoutcycle. Randomisation and concealment by pharmacy, blocked to max 10. Power calculation 45‐50 women needed to detect 25% difference in outcome with 95% power. | |
| Participants | Nr = 39, Na = 22. Aged 18 to 45 years. Met DSM‐III rev. criteria for LLPDD: severe mood and physical symptoms 7‐10 days before menses included irritability or aggressiveness, alleviated within 3 days of onset of menstruation. Had 1 symptom‐free week. Using adequate non hormonal contraception, menstruating regularly and had experienced symptoms for past 6 cycles. Difference score on MDQ between follicular and premenstrual assessments of at least 20 points (represents two SDs). Had no current psychiatric disorder, nor coexisting medical or gynaecological disorder. Did not use psychotropic drugs or other hormonal preparations. Did not have major cyclical complaint of depression with anergia. PE excluded by symptom‐free week. Excluded menstrual migraine and stress like family crisis or violence. | |
| Interventions | Two 200 mg oral progesterone at night and 1 in the morning with vaginal placebo OR two 200 mg vaginal progesterone at night and 1 in the morning with oral placebo OR both placebo. | |
| Outcomes | Primary measure was MDQ administered on day 26 of second cycle: Follow up after 1 month approx. Secondary STPI, BDI, serum progesterone and metabolites day 26 | |
| Notes | Stopped recruitment when half number needed according to power calculation because no trends were seen. Did not use daily ratings. No major AE. One woman complained of nausea while on placebo and withdrew. One woman developed increasing depression while taking vaginal progesterone. One woman with history of thyrotoxicosis relapsed while taking vaginal progesterone. One woman had itchy skin on placebo but not on progesterone. Physical tiredness was not significantly different between treatment arms. Drowsiness and dizziness more frequent on oral progesterone. Vaginal irritation more frequent on vaginal progesterone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was performed by Besins‐Iscovesco Laboritoires (Paris) for a total of numbered treatment boxes given out sequentially." |
| Allocation concealment (selection bias) | Low risk | By pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Probably done since prepared by pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition (22/39). Distribution of losses between groups not described, nor their effect considered. |
| Selective reporting (reporting bias) | High risk | Only the second month's records reported and that for only one day each cycle. Results were reported for each treatment phase but not as the difference from baseline for total MDQ scores, graphically, nor in means (standard deviations) for MDQ total or subscales, or BDI or STPI subscales. Daily ratings not analysed. Raw data not available. |
| Other bias | High risk | No ITT analysis. Baseline characteristics described only for the participants as a whole. |
AE = adverse effects bd = twice daily BDI = Beck Depression Inventory D = drug DSM IIIR Diagnostic and Statistical Manual of the American Psychiatric Association, third edition revised ITT = intention‐to‐treat analysis MDQ = Moos' Menstrual Distress Questionnaire Na = number of participants analysed Nr = number of participants randomised PE = premenstrual exacerbation of an underlying condition. PMS = premenstrual syndrome. severe symptoms = severe enough to interfere with work or relationships. STAI = Spielberger's State Anxiety Inventory STPI = State Trait Personality Index (Spielberger)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersch 1985 | Only one cycle of prospective records Participants not screened for current psychiatric disorders nor for premenstrual exacerbation of other on‐going conditions |
| Baker 1995 | Exclusion of participants with premenstrual exacerbation of chronic symptoms, in doubt. |
| Corney 1990 | Current minor psychiatric disorder reported in 50% of participants Inclusion and exclusion criteria not reported Duration of intervention not reported |
| Dennerstein 1985 | Only one cycle of prospective records |
| Freeman 1990 | Exclusion of participants with premenstrual exacerbation of chronic symptoms, in doubt. |
| Freeman 1995 | Exclusion of participants with premenstrual exacerbation of chronic symptoms, in doubt |
| Gray 1941 | Not a RCT; case study |
| Maddocks 1986 | Only one cycle of prospective records Ignored data from women whose PMS was not confirmed by subsequent records |
| Rapkin 1987 | Only one cycle of prospective records |
| Richter 1984 | Did not confirm diagnosis with prospective records |
| Sampson 1979 | Only one cycle of prospective records Participants not screened for current psychiatric disorders Inclusion and exclusion criteria not reported. |
| Smith 1975 | Not clearly a RCT; author unable to supply more details. |
| van der Meer 1983 | Did not describe any prospective records for diagnosis |
| Vargyas 1985 | Only abstract available Insufficient data |
Differences between protocol and review
Meta‐analysis was not possible.
Contributions of authors
Ben Mol made suggestions during the writing of the protocol, helped with the extraction of data from the published reports of trials and contributed to the development of the methods for the first publication.
Helen Roberts was the Clinical Adviser and contributed to all sections especially in the final writing and updating.
Anne Lethaby advised during the latter part of the writing of the review particularly on the Description of Studies and Discussion and advised during updating.
Olive Ford took the lead in writing the protocol and review at all stages. She performed initial searches of databases for trials, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Magill 1995 {published and unpublished data}
- Magill PJ. Investigation of the efficacy of progesterone pessaries in the relief of symptoms of premenstrual syndrome. British Journal of General Practice 1995;45(400):589‐93. [PMC free article] [PubMed] [Google Scholar]
Vanselow 1996 {published and unpublished data}
- Vaneslow W, Dennerstein L, Greenwood KM, Lignieres B. Effect of progesterone and its 5 alpha and 5 beta metabolites on symptoms of premenstrual syndrome according to route of administration. Journal of Psychosomatic Obstetrics and Gynaecology 1996;17(1):29‐38. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersch 1985 {published and unpublished data}
- Andersch B Hahn L. Progesterone treatment of premenstrual tension ‐ a double blind study. Journal of Psychosomatic Research 1985;29(5):489‐93. [DOI] [PubMed] [Google Scholar]
Baker 1995 {published data only}
- Baker ER, Best RG, Manfredi RL, Demers LM, Wolf GC. Efficacy of vaginal suppositories in alleviation of nervous symptoms in patients with premenstrual syndrome. Journal of Assisted Reproduction and Genetics 1995;12(3):205‐9. [DOI] [PubMed] [Google Scholar]
Corney 1990 {published data only}
- Corney RH, Stanton R, Newell R. Comparison of progesterone, placebo and behavioural psychotherapy in the treatment of premenstrual syndrome. Journal of Psychosomatic Obstetrics and Gynaecology 1990;11:211‐20. [Google Scholar]
Dennerstein 1985 {published and unpublished data}
- Dennerstein L, Spencer‐Gardener C, Gotts G, Brown JB, Smith MA, Burrows GD. Progesterone and the premenstrual syndrome: a double blind crossover trial. British Medical Journal (Clinical Research Ed.) 1985;290(6482):1617‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1990 {published and unpublished data}
- Freeman E, Rickels K, Sondheimer SJ, Polansky M. Ineffectiveness of progesterone suppository treatment for premenstrual syndrome. JAMA 1990;264(3):349‐53. [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ. Course of premenstrual syndrome symptom severity after treatment. American Journal of Psychiatry 1992;149(4):531‐3. [DOI] [PubMed] [Google Scholar]
Freeman 1995 {published and unpublished data}
- Freeman E W, Rickels K, Sondheimer SJ, Polansky M. A double‐blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA 1995;274(1):51‐7. [PubMed] [Google Scholar]
Gray 1941 {published data only}
- Gray LA. The use of progesterone in nervous tension states. Southern Medical Journal 1941;34(9):1004‐6. [Google Scholar]
Maddocks 1986 {published data only}
- Maddocks S, Hahn P, Moller F, Reid RL. A double‐blind placebo‐controlled trial of progesterone vaginal suppositories in the treatment of premenstrual syndrome. American Journal of Obstetrics and Gynecology 1986;154(3):573‐81. [DOI] [PubMed] [Google Scholar]
Rapkin 1987 {published and unpublished data}
- Rapkin A, Chang L H, Reading AI. Premenstrual syndrome:a double blind trial of treatment with progesterone vaginal suppositories. Journal of Obstetrics and Gynecology 1987;7(3):217‐20. [Google Scholar]
Richter 1984 {published and unpublished data}
- Richter MA, Halvic R, Shapiro SS. Progesterone treatment of premenstrual syndrome. Current Therapeutic Research 1984;36(5):840‐50. [Google Scholar]
Sampson 1979 {published data only}
- Sampson GA. Premenstrual syndrome: a double‐blind controlled trial of progesterone and placebo. British Journal of Psychiatry 1979;135:209‐5. [DOI] [PubMed] [Google Scholar]
Smith 1975 {published and unpublished data}
- Smith SL. Mood and the menstrual cycle. In: Sachar, E J editor(s). Topics in psychoendocrinology. New York: Grune and Stratton, Raven Press, 1975:19‐58. [Google Scholar]
van der Meer 1983 {published data only}
- Meer YG, Benedek‐Jaszmann LJ, Loenan AC. Effect of high dose progesterone on the premenstrual syndrome: a double blind crossover trial. Journal of Psychosomatic Obstetrics and Gynecology 1983;2:220‐2. [Google Scholar]
Vargyas 1985 {published data only}
- Vargyas JM, Marrs RP. The use of progesterone in the premenstrual syndrome. Proceeding of the 41st Annual Meetoing of the American Fertility Society: [Abstract No.2] in Abstracts in Endocrinology.. 1985.
Additional references
ABPI 1999
- Association of the British Pharmaceutical Industry (ABPI). Compendium of data sheets and summaries of product characteristics 1998‐99. London: Datapharm Publications Limited, 1999. [Google Scholar]
Abplanalp 1983
- Abplanalp JM. Psychological components of the premenstrual syndrome. Evaluating the research and choosing the treatment. Journal of Reproductive Medicine 1979;28(8):517‐24. [PubMed] [Google Scholar]
Altman 2001
- Altman DG, Schulz K F, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134(8):663‐94. [DOI] [PubMed] [Google Scholar]
Andersch 1979
- Andersch B, Abrahamsson L, Wendestam C, Ohman R, Hahn L. Hormone profile in premenstrual tension: effects of bromocriptine and diuretics. Clinical Endocrinology 1979;11(6):657‐64. [DOI] [PubMed] [Google Scholar]
Backstrom 1975
- Backstrom T, Mattsson B. Correlation of symptoms in pre‐menstrual tension to oestrogen and progesterone concentrations in blood plasma. A preliminary study. Neuropsychobiology 1975;1(2):80‐6. [DOI] [PubMed] [Google Scholar]
Backstrom 2003
- Backstrom T, Andreen L, Birzniece V, Bjorn I, Johansson IM, Nordenstam‐Haghjo M, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs 2003;17(5):325‐42. [DOI] [PubMed] [Google Scholar]
Bjorn 2003
- Björn I, Sundströme‐Poromaa, Bixo M, Nyberg S, Bäckström G, Bäckström T. Increase of estrogen dose deteriorates mood during progestin phase in sequential hormonal therapy.. The Journal of Clinical Endocrinology and Metabolism 2003;88(5):2026‐30. [DOI] [PubMed] [Google Scholar]
Chakmakjian 1987
- Chakmakjian ZH, Zachariah NY. Bioavailability of progesterone with different methods of administration. Journal of Reproductive Medicine 1987;32:443‐8. [PubMed] [Google Scholar]
Chisholm 1990
- Chisholm G, Jung SO, Cumming CE, Fox EE, Cumming DC. Premenstrual anxiety and depression: comparison of objective psychological tests with a retrospective questionnaire. Acta Psychiatrica Scandinavica 1990;81(1):52‐7. [DOI] [PubMed] [Google Scholar]
Collin 1991
- Collin D, Gandar R. The corpus luteum and progesterone secretion [Corps jaune et secretion de progesterone]. Revue Française de Gynécologie et d Obstétrique 1991;86(10):563‐5. [PubMed] [Google Scholar]
Corvol 1983
- Corvol P, Elkik F, Feneant M, Oblin ME, Michaud A, Claire M, et al. Effect of progesterone and progestins on water and salt metabolism. In: Bardin CW, Milgrom E, Mauvais‐Jarvis P editor(s). Progesterone and Progestins. New York: Raven Press, 1983:179‐86. [Google Scholar]
Dalton 1973a
- Dalton K. Progesterone suppositories and pessaries in the treatment of menstrual migraine. Headache 1973;12(4):151‐9. [DOI] [PubMed] [Google Scholar]
Dalton 1973b
- Dalton K. Migraine in general practice. Journal of the Royal College of General Practitioners 1973;23(127):97‐106. [PMC free article] [PubMed] [Google Scholar]
Dalton 1977
- Dalton K. The premenstrual syndrome and progesterone therapy. London: Heinemann Medical Books, 1977. [Google Scholar]
Dalton 1984
- Dalton. Premenstrual syndrome and progesterone therapy. London: Heinemann Medical, 1984. [Google Scholar]
Dalton 1994
- Dalton K, Holton D. PMS: The essential guide to treatment options. Northampton: Thorsons (imprint of Harper Collins), 1994. [Google Scholar]
DSM IIIR 1987
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders 3rd ed. rev. Washington DC: American Psychiatric Association, 1987. [Google Scholar]
DSM IV 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders 4th ed. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Endicott 1982
- Endicott J, Halbreich U. Retrospective reports of premenstrual changes: factors affecting confirmation of daily ratings. Psychopharmacology Bulletin 1982;16:109‐12. [Google Scholar]
Facchinetti 1990
- Facchinetti F, Genazzani AD, Martignoni E, Fioroni L, Sances G, Genazzani AR. Neuroendocrine correlates of premenstrual syndrome: changes in the pulsatile pattern of plasma LH. Psychoneuroendocrinology 1990;15(4):269‐77. [DOI] [PubMed] [Google Scholar]
Facchinetti 1993
- Facchinetti F, Genazzani AD, Martignoni E, Fioroni L, Nappi G, Genazzani AR. Neuroendocrine changes in luteal function in patients with premenstrual syndrome. Journal of Clinical Endocrinology and Metabolism 1993;76(5):1123‐7. [DOI] [PubMed] [Google Scholar]
Frank 1931
- Frank RT. The hormonal causes of premenstrual tension. Archives of Neurology and Psychiatry 1931;26:1053‐7. [Google Scholar]
Freeman 1996
- Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Research 1996;65(2):97‐106. [DOI] [PubMed] [Google Scholar]
Freeman 1999
- Freeman EW, Rickels K. Characteristics of placebo responses in medical treatment of premenstrual syndrome. American Journal of Psychiatry 1999;156(9):1403‐8. [DOI] [PubMed] [Google Scholar]
Garcia‐Berthou 2004
- Garcia‐Berthou E, Alcaraz C. Incongruence between test statistics and P values in medical papers. Medical Research Methodology 2004;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greene 1953
- Greene R, Dalton K. The premenstrual syndrome. British Medical Journal 1953;1:1007‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halbreich 1985
- Halbreich U, Endicott J. Methodological issues in studies of premenstrual changes. Psychoneuroendocrinology 1985;10(1):15‐32. [DOI] [PubMed] [Google Scholar]
Halbreich 1986
- Halbreich U, Endicott J, Goldstein S, Nee J. Premenstrual changes and changes in gonadal hormones. Acta Psychiatrica Scandinavica 1986;74(6):576‐86. [DOI] [PubMed] [Google Scholar]
Halbreich 2006
- Halbreich U, O'Brien P M S, Erikksson E, Bäckström T, Yonkers K A, Freeman E W. Are there different symptom proflies that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder?. Drugs 2006;20(7):523‐47. [DOI] [PubMed] [Google Scholar]
Halbreich 2007
- Halbreich U, Bäckström T, Eriksson E, O'Brien P M S, Calil H, Ceskova E, Dennerstein L, Douki S, Freeman E, Genazzani A, Heuser I, Kadri N, Rapkin A, Steiner M, Wittchen H‐U, Yonkers K. Clinical diagnostic criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecological Enocrinology 2007;23(3):123‐30. [DOI] [PubMed] [Google Scholar]
Hammarbäck 1989
- Hammarbäck S, Damber JE, Backstrom T. Relationship between symptom severity and hormone changes in women with premenstrual syndrome. Journal of Clinical Endocrinology and Metabolism 1989;68(1):125‐30. [DOI] [PubMed] [Google Scholar]
Hargrove 1989
- Hargrove JT, Maxson WS, Wentz AC. Absorption of oral progesterone is influenced by vehicle and particle size. American Journal of Obstetrics and Gynecology 1989;161(4):948‐51. [DOI] [PubMed] [Google Scholar]
Haywood 2002
- Haywood A, Slade P, King H. Assessing the assessment measures for menstrual cycle symptoms: a guide for researchers and clinicians. Journal of Psychosomatic Research 2002;52(4):223‐37. [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials.. BMJ 1999;319(7211)):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horney 1931
- Horney K. Premenstrual tension. In: Kelman, H editor(s). Feminine Psychology.. New York: WW Norton, 1931:99‐106. [Google Scholar]
Johnson, J 2004
- Johnson JM. Visual Analog scales: Part 1, 2 and 3. DCI Research Review 2004.
Johnson, S 2004
- Johnson, S R. Premenstrual syndrome, premenstrual dysphoric disorder, and beyond: a clinical primer for practitioners. Obstetrics and Gynecology 2004;104(4):845‐59. [DOI] [PubMed] [Google Scholar]
Keenen 1992
- Keenan PA, Lindamer LA, Jong SK. Psychological aspects of premenstrual syndrome. II: Unity of standardized measures. Psychoneuroendocrinology 1992;17(2‐3):189‐94. [DOI] [PubMed] [Google Scholar]
Kienle 1996
- Kienle GS, Kiene H. Placebo effect and placebo concept: a critical methodological and conceptual analysis of reports on the magnitude of the placebo effect.. Alternative Therapies in Health and Medicine 1996;2(6):39‐54. [PubMed] [Google Scholar]
Klatzkin 2006
- Klatzkin R R, Morrow L A, Light K C, Pedersen C A, Girdler S S. Associations of histories of depression and PMDD diagnosis with allopregnanolone concnetrations following the oral administration of micronised progesterone.. Psychoneuroendocrinology 2006;31:1208‐19. [DOI] [PubMed] [Google Scholar]
Knaapen 2008
- Knaapen L, Weisz G. The biomedical standardisation of premenstrual syndrome.. Studies in History and Philosophy of Biological and Biomedical Sciences 2008;39:120‐34. [DOI] [PubMed] [Google Scholar]
Landau 1958
- Landau RL, Lugibihl K. Inhibition of the sodium‐retaining influence of aldosterone by progesterone. Journal of Clinical Endocrinology and Metabolism 1958;18(11):1237‐45. [DOI] [PubMed] [Google Scholar]
Landen 2003
- Landen M, Eriksson E. How does premenstrual dysphoric disorder relate to depression and anxiety disorders?. Depression and Anxiety 2003;17(3):122‐9. [DOI] [PubMed] [Google Scholar]
Lewis 1995
- Lewis LL, Greenblatt EM, Rittenhouse CA, Veldhuis JD, Jaffe RB. Pulsatile release patterns of luteinizing hormone and progesterone in relation to symptom onset in women with premenstrual syndrome. Fertility and Sterility 1995;64(2):288‐92. [PubMed] [Google Scholar]
Lovick 2006
- Lovick, TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Experimental Physiology 2006;91(4):655‐60. [DOI] [PubMed] [Google Scholar]
Lovick 2008
- Lovick T A. GABA in the female brain ‐ Oestrous cycle‐related changes in GABAergic function in the periaqueductal grey matter.. Biochemistry and Behaviour 2008;90:43‐50. [DOI] [PubMed] [Google Scholar]
Magill 2004
- Magill P J. Personal communication 01/11/2004.
Monteleone 2000
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, et al. Allopregnanolone concentrations and premenstrual syndrome. European Journal of Endocrinology 2000;142(3):269‐73. [DOI] [PubMed] [Google Scholar]
Moos 1968
- Moos R H. The development of a menstrual distress questionnaire. Psychosomatic Medicine 1968;30(6):853‐67. [DOI] [PubMed] [Google Scholar]
Moos 1969
- Moos RH. Typology of menstrual cycle symptoms. American Journal of Obstetrics and Gynecology 1969;103(3):390‐402. [DOI] [PubMed] [Google Scholar]
Morville 1982
- Morville R, Dray F, Reynier J, Barratt J. The bioavailability of natural progesterone given by mouth. Measurement of steroid concentration in plasma, endometrium and breast tissue [Biodisponibilitée de la progesterone naturelle administréee par voie orale]. Journal de Gynécologie obstétrique et biologie de reproduction [Paris] 1982;11:355‐63. [PubMed] [Google Scholar]
Nyberg 2007
- Nyberg S, Backstrom T, Zingmark E, Purdy R H, Poromaa I S. Allopregnanolone decrease with symptom improvement during placebo and gonadotropin‐releasing hormone agonist treatment in women with severe premenstrual syndrome.. Gynecological endocrinology 2007;23(5):257‐66. [DOI] [PubMed] [Google Scholar]
O'Brien 1980
- O'Brien PM, Selby C, Symonds EM. Progesterone, fluid and electrolytes in premenstrual syndrome. British Medical Journal 1980;280(6224):1161‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1993
- O'Brien PM. Helping women with premenstrual syndrome. BMJ 1993;307(6917):1471‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 1983
- Price JH, Ismaeil H, Gorwill RH, Sarda IR. Effect of suppository base on progesterone delivery from the vagina.. Fertility and Sterility 1983;39(4):490‐3. [DOI] [PubMed] [Google Scholar]
Redei 1995
- Redei E, Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology 1995;20(3):259‐67. [DOI] [PubMed] [Google Scholar]
Rees 1953
- Rees L. The premenstrual tension syndrome and its treatment. British Medical Journal 1953;1(4818):1014‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinow 1988
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy‐Byrne P, Andersen R, et al. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. American Journal of Obstetrics and Gynecology 1988;158(1):5‐11. [DOI] [PubMed] [Google Scholar]
Seippel 2000
- Seippel L, Eriksson O, Grankvist K, Shoultz B, Backstrom T. Physical symptoms in premenstrual syndrome are related to plasma progesterone and desoxycorticosterone. Gynecological Endocrinology 2000;14(3):173‐81. [DOI] [PubMed] [Google Scholar]
Smith 1998
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench‐Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 1998;392(6679):926‐30. [DOI] [PubMed] [Google Scholar]
Steele 1986
- Steele PA, Braund W, Judd SJ. Regulation of pulsatile secretion of progesterone during the human luteal phase. Clinical Reproduction and Fertility 1986;4(2):117‐24. [PubMed] [Google Scholar]
Steiner 1980
- Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales.. Acta Psychiatrica Scandinavica 1980;62(2):177‐90. [DOI] [PubMed] [Google Scholar]
Steiner 2000
- Steiner M. Premenstrual syndrome and premenstrual dysphoric disorder: guidelines for management. Journal of Psychiatry and Neuroscience 2000;25(5):459‐68. [PMC free article] [PubMed] [Google Scholar]
Stout 1985
- Stout EL, Steege JF. Psychological assessment of women seeking treatment for premenstrual syndrome. Journal of Psychosomatic Research 1985;29(6):621‐9. [DOI] [PubMed] [Google Scholar]
Sveinsdottir 1998
- Sveinsdottir H. Prospective assessment of menstrual and premenstrual experiences of Icelandic women. Health Care for Women International 1998;19(1):71‐82. [DOI] [PubMed] [Google Scholar]
Taylor 1986
- Taylor RJ, Alexander DA, Fordyce ID. A survey of paramenstrual complaints by covert and by overt methods. Journal of the Royal College of General Practitioners 1986;36(292):496‐9. [PMC free article] [PubMed] [Google Scholar]
Thys‐Jacobs 2008
- Thys‐Jacobs S, McMahon D, Bilezikian J P. Differences in free estradiol and sex hormone‐binding globulin in women with and without premenstrual dysphoric disorder. Journal of Clinical Endocrinology and Metabolism 2008;93(1):96‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Meer 1982
- Meer YG, Loenen AC, Loendersloot EW, Jaszmann LJ. Plasma progesterone concentrations after high‐dose suppositories. A preliminary report.. Pharmaceutisch Weekblad Scientific edition 1982;4(5):135‐6. [DOI] [PubMed] [Google Scholar]
Walker 2002
- Walker AF, Souza MC, Marakis G, Robinson PA, Morris AP, Bolland KM. Unexpected benefit of sorbitol placebo in Mg intervention study of premenstrual symptoms: implications for choice of placebo in RCTs. Medical Hypotheses 2002;58(3):213‐20. [DOI] [PubMed] [Google Scholar]
Wang 1996
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha‐pregnane‐3, 20‐dione and 3 alpha‐hydroxy‐5 alpha‐ pregnan‐20‐one. Journal of Clinical Endocrinology and Metabolism 1996;81(3):1076‐82. [DOI] [PubMed] [Google Scholar]
Watanabe 1997
- Watanabe H, Lau DC, Guyn HL, Wong NL. Effect of progesterone therapy on arginine vasopressin and atrial natriuretic factor in premenstrual syndrome. Clinical & Investigative Medicine ‐ Médecine clinique et expérimentale 1997;20(4):211‐23. [PubMed] [Google Scholar]
Wendestam 1980
- Wendestam 1980. Mental changes in the premenstrual phase. A study of the psycho‐pathological profile, its prevalence and its hormonal correlations [Thesis]. Sweden: University of Gotenberg 1980.
Wyatt 2001
- Wyatt K, Dimmock P, Jones P, Obhrai M, O'Brien S. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ 2001;323(7316):776‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyatt 2002
- Wyatt KM, Dimmock PW, Hayes‐Gill B, Crowe J, O'Brien PM. Menstrual symptometrics: a simple computer‐aided method to quantify menstrual cycle disorders. Fertility and Sterility 2002;78(1):96‐101. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ford 2006
- Ford O, Lethaby A, Roberts H, Mol BWJ. Progesterone for premenstrual syndrome DOI: 10.1002/14651858.CD003415.pub2.. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003415.pub2] [DOI] [PubMed] [Google Scholar]


