Abstract
Background
Traditional epidural techniques have been associated with prolonged labour, use of oxytocin augmentation and increased incidence of instrumental vaginal delivery. The combined spinal‐epidural (CSE) technique has been introduced in an attempt to reduce these adverse effects. CSE is believed to improve maternal mobility during labour and provide more rapid onset of analgesia than epidural analgesia, which could contribute to increased maternal satisfaction.
Objectives
To assess the relative effects of CSE versus epidural analgesia during labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (28 September 2011) and reference lists of retrieved studies. We updated the search on 30 June 2012 and added the results to the awaiting classification section.
Selection criteria
All published randomised controlled trials (RCTs) involving a comparison of CSE with epidural analgesia initiated for women in the first stage of labour. Cluster‐randomised trials were considered for inclusion. Quasi RCTs and cross‐over trials were not considered for inclusion in this review.
Data collection and analysis
Three review authors independently assessed the trials identified from the searches for inclusion, assessed trial quality and extracted the data. Data were checked for accuracy.
Main results
Twenty‐seven trials involving 3274 women met our inclusion criteria. Twenty‐six outcomes in two sets of comparisons involving CSE versus traditional epidurals and CSE versus low‐dose epidural techniques were analysed.
Of the CSE versus traditional epidural analyses five outcomes showed a significant difference. CSE was more favourable in relation to speed of onset of analgesia from time of injection (mean difference (MD) ‐2.87 minutes; 95% confidence interval (CI) ‐5.07 to ‐0.67; two trials, 129 women); the need for rescue analgesia (risk ratio (RR) 0.31; 95% CI 0.14 to 0.70; one trial, 42 women); urinary retention (RR 0.86; 95% CI 0.79 to 0.95; one trial, 704 women); and rate of instrumental delivery (RR 0.81; 95% CI 0.67 to 0.97; six trials, 1015 women). Traditional epidural was more favourable in relation to umbilical venous pH (MD ‐0.03; 95% CI ‐0.06 to ‐0.00; one trial, 55 women). There were no data on maternal satisfaction, blood patch for post dural puncture headache, respiratory depression, umbilical cord pH, rare neurological complications, analgesia for caesarean section after analgesic intervention or any economic/use of resources outcomes for this comparison. No differences between CSE and traditional epidural were identified for mobilisation in labour, the need for labour augmentation, the rate of caesarean birth, incidence of post dural puncture headache, maternal hypotension, neonatal Apgar scores or umbilical arterial pH.
For CSE versus low‐dose epidurals, three outcomes were statistically significant. Two of these reflected a faster onset of effective analgesia from time of injection with CSE and the third was of more pruritus with CSE compared to low‐dose epidural (average RR 1.80; 95% CI 1.22 to 2.65; 11 trials, 959 women; random‐effects, T² = 0.26, I² = 84%). There was no significant difference in maternal satisfaction (average RR 1.01; 95% CI 0.98 to 1.05; seven trials, 520 women; random‐effects, T² = 0.00, I² = 45%). There were no data on respiratory depression, maternal sedation or the need for labour augmentation. No differences between CSE and low‐dose epidural were identified for need for rescue analgesia, mobilisation in labour, incidence of post dural puncture headache, known dural tap, blood patch for post dural headache, urinary retention, nausea/vomiting, hypotension, headache, the need for labour augmentation, mode of delivery, umbilical pH, Apgar score or admissions to the neonatal unit.
Authors' conclusions
There appears to be little basis for offering CSE over epidurals in labour, with no difference in overall maternal satisfaction despite a slightly faster onset with CSE and conversely less pruritus with low‐dose epidurals. There was no difference in ability to mobilise, maternal hypotension, rate of caesarean birth or neonatal outcome. However, the significantly higher incidence of urinary retention, rescue interventions and instrumental deliveries with traditional techniques would favour the use of low‐dose epidurals. It is not possible to draw any meaningful conclusions regarding rare complications such as nerve injury and meningitis.
Keywords: Female; Humans; Pregnancy; Labor, Obstetric; Analgesia, Epidural; Analgesia, Epidural/adverse effects; Analgesia, Epidural/methods; Analgesia, Obstetrical; Analgesia, Obstetrical/adverse effects; Analgesia, Obstetrical/methods; Anesthesia, Epidural; Anesthesia, Epidural/adverse effects; Anesthesia, Epidural/methods; Anesthesia, Spinal; Anesthesia, Spinal/adverse effects; Anesthesia, Spinal/methods; Randomized Controlled Trials as Topic
Plain language summary
Combined spinal‐epidural versus epidural analgesia in labour
Regional analgesia has been shown to be effective in providing pain relief in labour. Regional analgesia can be an epidural, a spinal or a combination of the two. An epidural is when the pain‐relieving drugs are injected into the part of the body which surrounds the spinal column (epidural space). It is most common for these drugs to be infused through a very fine tube (catheter) positioned in the epidural space. Traditionally, high concentrations of local anaesthetic drugs were used. These numbed the woman from the waist downwards giving pain relief for most women. However, it also caused leg weakness, poor mobility and difficulty for the mother giving birth. This led to increased instrumental vaginal births with subsequent increased bruising, pain and incontinence later on for the mother. More recently with epidurals, low‐dose local anaesthetic drugs have been used in combination with opioid drugs. Here there is less numbing of the woman's legs but the opioid drugs cross the placenta and may make the baby sleepy.
A spinal is when the analgesic drugs are injected directly into the fluid surrounding the nerves in the spinal column and is quicker to take effect than an epidural. However, because a single spinal injection is only effective for a short period of time, they are not commonly used on their own for pain relief in labour. Also, the use of very fine catheters in the spinal space has been associated with increased injury to nerves. Hence, the combination of a single spinal injection combined with the use of an epidural catheter for ongoing pain relief was developed. This combined spinal‐epidural was thought to have the benefits of being quicker to provide pain relief but with no change to the incidence or severity of side effects for the mother or baby.
This review of trials compared CSE with traditional and with low‐dose epidurals. There were 27 trials, involving 3274 women. The data showed no difference in the mothers' satisfaction between CSE and epidurals. However, CSEs had a slightly faster onset of effective pain relief, but more women itched than with low‐dose epidurals. There was no difference seen for mobility in labour, headaches, caesarean section or adverse effects for the baby. Any differences for rare complications such as nerve injury and meningitis remain unknown. There appears to be little difference overall between these techniques.
Background
This review is one in a series of Cochrane reviews examining pain management in labour. These reviews contribute to an overview of systematic reviews of pain management for women in labour (Jones 2011b).
Epidural analgesia has been shown to be the most effective method of providing pain relief in labour (Glosten 1999) when compared with non‐epidural methods (Anim‐Somuah 2011; Howell 2001). On a national level, an epidural technique is used for pain relief in approximately 25% of labouring women in the UK (Khor 2000; NOAD 2004) and in as many as 58% in the USA (Declercq 2002). Administration of regional analgesia traditionally involves an injection of local anaesthetic through a catheter positioned in the epidural space. Epidural solutions are administered either by bolus or infusion which permits analgesia to be maintained throughout labour. Bolus administration may be at the discretion of the woman in labour in which case it is referred to as patient‐controlled epidural analgesia (PCEA). In addition, a functioning epidural catheter usually gives the option of providing regional anaesthesia for obstetric interventions such as forceps delivery or caesarean section, thus avoiding the risks of general anaesthesia (Hibbard 1996).
Traditional epidural techniques, employing high concentrations of local anaesthetic (at least 0.25% bupivacaine), have been associated with prolonged labour, use of oxytocin augmentation and an increased incidence of instrumental vaginal delivery (Anim‐Somuah 2011). This is probably secondary to a dense motor block which results in leg weakness, poor mobility, decreased pelvic muscle tone and an impaired bearing‐down reflex during the delivery of the baby (Thornton 2001). Newer regional techniques for labour analgesia use a low concentration of local anaesthetic often in combination with an opioid. This low‐dose combination appears to provide the excellent analgesia of higher concentrations of epidural local anaesthetics (Akerman 1988) while maintaining motor function. The mother is therefore more likely to have the ability to walk during her labour or deliver without assistance (COMET 2001a; Russell 2000).
The combined spinal‐epidural involves an injection of an analgesic or local anaesthetic drug, or both, into the intrathecal space immediately before or after epidural catheter placement. A number of variations in the technique have been described (Cook 2000) but typically an epidural needle is first used to identify the epidural space (Brown 1999) at the level of the third lumbar vertebra. A smaller diameter, longer needle is then passed through the epidural needle lumen piercing the dura and arachnoid to allow administration of analgesic medications (e.g. opioids) into the cerebrospinal fluid. The spinal needle is then removed and an epidural catheter is inserted and secured in the normal way. Further analgesia usually in the form of a low‐dose local anaesthetic solution combined with an opioid is then provided through the epidural catheter. Both epidural and spinal drugs are believed to access sites of action within the spinal cord and the peripheral nerve roots (Butterworth 1998), which supply the uterus. Spinal analgesia is not usually used as the sole technique for pain relief in labour because of its relatively short duration. The insertion and use of spinal micro‐catheters has previously been associated with a higher risk of permanent neurological damage (Rigler 1991) and this technique is not in widespread use. CSE is claimed to combine the advantages of both epidural and spinal techniques including: faster onset, more reliable analgesia (due to the spinal component), minimal motor and sensory blockade, improved mobilisation (Collis 1993; Rawal 1997a), lower maternal and cord blood local anaesthetic concentrations (Brown 1999), and higher patient satisfaction (Collis 1994). Since its introduction, CSE has become increasingly popular (Macarthur 1999; Riley 1999) and is used routinely at many institutions for obstetric analgesia (Collis 1994; Rawal 2000).
Although all regional techniques can provide effective pain relief, this needs to be balanced with the risk of potential adverse effects (Bromage 1999). Complications common to both CSE and epidural analgesic techniques include failure to provide satisfactory pain relief, maternal hypotension, post dural puncture headache (PDPH) (Macarthur 2009), urinary retention, itching and transient backache over the injection site. Rare serious complications include meningitis, compression of the spinal cord from a blood clot or abscess and damage to nerve roots causing paraesthesia or weakness. In addition, inadvertent administration of an epidural dose of local anaesthetic intravenously or intrathecally can result in convulsions or total spinal anaesthesia respectively, requiring resuscitation and urgent delivery (Rawal 1997a). The use of two needles in CSE, one epidural and one spinal, may increase the potential for disruption of the protective dural barrier with an associated increase in maternal complications (Macarthur 1999). Modern spinal needles are designed to minimise the incidence of PDPH (Choi 2005), which is approximately 1.5% to 2%. Epidural needles are not designed to enter the intrathecal space and if they do so accidentally, which occurs in approximately 1.5% of women, they are associated with an approximately 50% chance of developing a PDPH (Macarthur 2009). This complication can sometimes be disabling (Weir 2000). If the headache fails to resolve spontaneously an epidural blood patch is a common form of treatment which has been shown to be more effective than conservative management (Boonmak 2010), providing complete relief of headache at seven days in over 80% of women (van Kooten 2007). Although a high block may occur with spinal or epidural anaesthesia alone, CSE may increase the risk of this complication (Macarthur 1999; Rawal 1997; Shaw 2001), which can lead to maternal hypotension, respiratory arrest or loss of consciousness. Neonatal effects such as fetal bradycardia (Nielsen 1996) or the need for resuscitation have been associated with the use of both CSE and epidural techniques (COMET 2001a). Differences in the management of labour (Russell 2000) as well as differences in CSE and epidural techniques (COMET 2001a) themselves may affect the need for other interventions during labour or delivery.
Objectives
To assess the relative efficacy and side effects of combined spinal‐epidural versus epidural analgesia during labour.
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials comparing combined spinal‐epidural with epidural analgesia during labour. Cluster‐randomised trials were considered for inclusion. Quasi RCTs and cross‐over trials were not considered for inclusion in this review.
Types of participants
Women having combined spinal‐epidural or epidural analgesia commenced during the first stage of labour.
Types of interventions
Combined spinal‐epidural analgesia compared with traditional and low‐dose epidural analgesia.
Types of outcome measures
Primary outcomes
The outcomes of interest for the mother are as follows.
Mean time and standard deviation from request of analgesia to the time she felt the level of pain relief was satisfactory.
Mean time and standard deviation from first spinal or epidural injection to the time she felt the level of pain relief was satisfactory.
Number of women after 10 minutes from the time of first spinal or epidural injection experiencing satisfactory pain relief.
Number of women requiring an additional intervention for pain relief at any time after combined spinal‐epidural (CSE)/epidural insertion, e.g. new technique such as intravenous analgesia (e.g. fentanyl) replacing epidural catheter.
Number of women satisfied with their labour analgesia.
Number of women who were mobile. Maternal mobility is defined as the mother demonstrating that she was able to walk during labour on at least one occasion following the CSE or epidural.
Number of women with post dural puncture headache.
Number of women with a known dural tap.
Number of women requiring an epidural blood patch for a post dural puncture headache.
Number of women with any complication requiring treatment/intervention specifically identified: pruritus, urinary retention, nausea or vomiting, or both, hypotension, respiratory depression/arrest, headache (any), sedation.
Number of women with any other complication requiring intervention such as fever, persistent paraesthesia, high block.
Number of women having an instrumental delivery.
Number of women having a caesarean section.
For the neonate
Number of neonates with Apgar scores less than seven at five minutes.
The number of neonates admitted to the neonatal unit and the reason for such admission.
Economic/use of resources
Costs of hospital stay.
Secondary outcomes
The outcomes of interest for the mother are as follows.
Number of women requiring augmentation of labour at any time.
Number of women requiring augmentation after analgesic intervention.
Number of women having a normal delivery including vacuum extraction.
Number of women requiring follow‐up for any reason or with long‐term outcomes, e.g. meningitis, neuropraxia, paralysis, intensive care unit admission, backache, footdrop, unresolved post dural puncture headache.
Number of women requiring general anaesthesia for caesarean section after analgesic intervention.
For the neonate
Mean pH and standard deviation for: umbilical artery, umbilical vein, umbilical cord.
Number of neonates with Apgar scores less than eight at five minutes.
Economic/use of resources
Length of hospital stay.
The number of women re‐admitted to hospital within one month of being discharged home and reason for admission.
The number of women requiring ongoing anaesthetic follow‐up following discharge from hospital.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (28 September 2011). We updated this on 30 June 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Three review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, by consultation with a fourth person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of participants, personnel and outcome assessment (checking for possible performance bias or detection bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials for inclusion in this review.
We intended to include cluster‐randomised trials in the analyses along with individually randomised trials using the methods described in the Cochrane Handbook (Higgins 2011). Their sample sizes would be adjusted using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If we used ICCs from other sources, we would report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We would consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Cross‐over trials
We did not include cross‐over trials.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if T² was greater than zero and either I² was greater than 30% or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry where relevant. For continuous outcomes we used the test proposed by Egger 1997, and for dichotomous outcomes we used the test proposed by Harbord 2006. If asymmetry was detected in any of these tests or was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011).
We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful and, if it was, we used random‐effects analysis to produce it.
We planned, where possible, to carry out the following subgroup analyses based on type of combined spinal‐epidural:
Combined spinal epidural versus opioid combined spinal epidural versus null combined spinal epidural.
We considered all outcomes in subgroup analysis.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2011).
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result. We also planned to explore the effects of fixed‐effect versus random‐effects analyses for outcomes with substantial statistical heterogeneity. Where this was the case relevant comments are made in the body of the text.
Results
Description of studies
Twenty‐seven trials, involving 3274 labouring women, met our criteria for inclusion.
(a) Cochrane Pregnancy and Childbirth Group's Trials Register (September 2011). From the references identified, 54 trials met the criteria for assessment and 27 were included. (b) Manual search: three received, all meeting criteria for assessment, one added as additional reference to study already included. (c) Manual search from reference list in assessed studies: three studies assessed, none included. (d) Personal communications: ongoing.
Results of the search
Twenty‐seven trials, involving 3274 women, were included. We excluded 27 studies. For details of the individual included and excluded studies, see the tables of Characteristics of included studies and Characteristics of excluded studies.
Included studies
Methods and techniques
All included studies reported obtaining informed consent from the participants after prior ethics committee approval. In one study, verbal rather than written consent was obtained (Nickells 2000), the explanation being that the techniques being compared were already in routine use. There was no indication as to the form of the consent in five other studies (Cohen 2006; Goodman 2006; Medina 1994; Patel 2003a; Thomas 2005).
Eighteen studies mentioned a fluid preload with crystalloid before the insertion of epidural or combined spinal‐epidural (CSE); the volumes were either not stated (Medina 1994; Ngamprasertwong 2007) or highly varied: 500 mL (COMET 2001a; Gomez 2001; Price 1998; Roux 1999; Vernis 2004), 500 to 1000 mL (Breen 1999; Parry 1998), or at least 1000 mL (Skupski 2009; Tsen 1999; Zeidan 2004). Four studies related the fluid bolus to parturient weight, giving 10 mL/kg (Abrao 2009; Bhagwat 2008; Cortes 2007) or 15 mL/kg over 15 minutes (Van de Velde 1999).
Almost all studies described a single space, needle‐through‐needle technique for CSE; six studies gave no indication (Abrao 2009; Cohen 2006; Cortes 2007; Goodman 2006; Patel 2003a; Skupski 2009). Where stated, patient position was relatively evenly divided between the sitting (Dunn 1998; Gomez 2001; Hepner 2000; Nickells 2000; Parry 1998; Roux 1999; Sezer 2007; Thomas 2005; Vernis 2004) and lateral (Bhagwat 2008; Kartawiadi 1996; Medina 1994; Ngamprasertwong 2007; Price 1998; Tsen 1999; Van de Velde 1999; Zeidan 2004) alternatives. One study allowed either sitting or lateral position for insertion (COMET 2001a) and in the remaining nine studies (Abouleish 1991; Abrao 2009; Breen 1999; Caldwell 1994; Cohen 2006; Cortes 2007; Goodman 2006; Patel 2003a; Skupski 2009) there is no detail of patient position. Only one study (Skupski 2009) specifically commented on maternal position during labour or ongoing intravenous fluid therapy, both of which could conceivably have had an effect on maternal and fetal parameters subsequently measured. Operators in the included studies were not blinded to the technique although in all studies the assessor was reported as being blinded to group allocation for at least some of the outcome assessments. One further study (Gomez 2001) was described as single‐blinded but this was not further defined.
In all of the included studies a CSE technique was compared with epidural analgesia in the first stage of labour. In five of the included papers (Breen 1999; Dunn 1998; Nickells 2000; Parry 1998; Patel 2003a) the study period involved only the initial intrathecal or epidural bolus with assessments and data collection stopping at the time of request for "top‐up" analgesia. In the remaining studies there was a varied assortment of regimens for epidural maintenance based around timing of commencement relative to initial injection as well as mode of epidural delivery and the types of solutions used. Local anaesthetic boluses of bupivacaine 0.125% or 0.25% for rescue analgesia were specified in a number of studies (Abouleish 1991; Abrao 2009; Cohen 2006; Cortes 2007; Dunn 1998; Gomez 2001; Goodman 2006; Hepner 2000; Ngamprasertwong 2007; Price 1998; Roux 1999; Skupski 2009; Thomas 2005; Tsen 1999; Vernis 2004), while in others this was left up to the discretion of the attending anaesthetist (COMET 2001a; Nickells 2000; Van de Velde 1999). No specific statement was made regarding criteria for intervention in the event of inadequate analgesia in the remaining eight studies (Breen 1999; Caldwell 1994; Kartawiadi 1996; Medina 1994; Parry 1998; Patel 2003a; Sezer 2007; Zeidan 2004).
Participants
All the included trials studied healthy women in labour requesting regional analgesia. Most stipulated a singleton, obstetrically uncomplicated pregnancy at term, as with Ngamprasertwong 2007 and Skupski 2009. Nineteen studies specifically defined the stage of labour by stating the degree of cervical dilatation as an upper limit for inclusion or as an exclusion. In these studies, the acceptable dilatation of the cervix ranged to an upper limit of 4 cm or less (Goodman 2006; Roux 1999; Tsen 1999; Zeidan 2004) or up to 5 cm (Bhagwat 2008; Breen 1999; Cortes 2007; Dunn 1998; Gomez 2001; Hepner 2000; Kartawiadi 1996; Medina 1994; Price 1998; Sezer 2007; Thomas 2005), 6 cm (Abrao 2009; Sezer 2007; Vernis 2004) or 7 cm (Van de Velde 1999). Of the other eight included studies that did not specifically state a degree of cervical dilatation there were less specific or indirect means of determining the stage of labour. Thus 'first stage of labour' was one inclusion (Parry 1998) and 'imminent delivery' was another specific exclusion (COMET 2001a). Exclusion criteria varied greatly with nine included studies not stating any explicit criteria preventing participation (Abouleish 1991; Caldwell 1994; Cohen 2006; Cortes 2007; Medina 1994; Patel 2003a; Nickells 2000; Sezer 2007; Thomas 2005). Only three of the included studies (Gomez 2001; Sezer 2007; Tsen 1999) stipulated spontaneous onset of labour as an entry criterion and no study excluded women on the basis of need for labour augmentation. Eight included studies (Abouleish 1991; Breen 1999; COMET 2001a; Dunn 1998; Kartawiadi 1996; Parry 1998; Vernis 2004; Zeidan 2004) specified previous opioid administration over a range of one to four hours as a criterion for exclusion and in two further studies (Abrao 2009; Van de Velde 1999) women were excluded if they had received "sedative or analgesic drugs".
Interventions
There was considerable heterogeneity between trials with respect to the drug combinations used, both intrathecally and epidurally, the timing of subsequent dosing after initial analgesia and the method of epidural drug delivery. In the context of categorising the epidural drug dose/concentration used, the term traditional was used for trials where the epidural local anaesthetic (LA) concentration was the equivalent of bupivacaine 0.25% or more; lower concentrations were defined as low‐dose. In the CSE groups, there were three types of interventions; LA plus opioid, opioid alone or null CSE where there was a dural puncture with no intrathecal injection of drugs. Using these definitions the comparisons fell into six categories as detailed below:
LA plus opioid CSE versus traditional epidural ‐ three studies, 846 women (COMET 2001a; Gomez 2001; Tsen 1999);
LA plus opioid CSE versus low‐dose epidural ‐ 18 studies, 2086 women (Abrao 2009; Bhagwat 2008; Cohen 2006; COMET 2001a; Goodman 2006; Hepner 2000; Kartawiadi 1996; Medina 1994; Nickells 2000; Parry 1998; Patel 2003a; Price 1998; Skupski 2009; Van de Velde 1999; Vernis 2004; Zeidan 2004);
opioid only CSE versus traditional epidural ‐ four studies, 229 women (Caldwell 1994; Cortes 2007; Ngamprasertwong 2007; Roux 1999);
opioid only CSE versus low‐dose epidural ‐ two studies, 102 women (Abouleish 1991; Sezer 2007);
opioid only CSE versus test LA/opioid epidural ‐ two studies, 111 women (Breen 1999; Dunn 1998);
null CSE versus traditional epidural ‐ one study, 251 women (Thomas 2005).
There were eight trials that included a traditional epidural group (Caldwell 1994; COMET 2001a; Cortes 2007; Gomez 2001; Ngamprasertwong 2007; Roux 1999; Thomas 2005; Tsen 1999). One of these studies (COMET 2001a) involved comparisons of a CSE group with both traditional and a low‐dose epidural group (see below) and so contributed an additional 704 women to category (1) above and 701 women to category (2). With the exception of one study (Thomas 2005), all studies fulfilling this criterion used 0.25% bupivacaine boluses at some time, with volumes ranging from 6 to 12 mL; Thomas 2005 used 2% lignocaine to a total volume of 10 mL. In the three trials with a LA plus opioid group, the CSE technique involved an intrathecal injection of bupivacaine 2.5 mg in combination with either fentanyl (COMET 2001a; Gomez 2001) or sufentanil (Tsen 1999). Of the four trials with an opioid only CSE group, Caldwell 1994 used a combination of fentanyl 25 µg plus morphine 0.25 mg intrathecally, while Roux 1999 used sufentanil 10 µg; the other two trials (Cortes 2007; Ngamprasertwong 2007) used 25 µg of fentanyl only. Whilst the techniques of drug dosing varied between studies, it was noted in these trials that there were essentially two approaches to subsequent epidural management in the CSE groups, either using effectively the same total epidural drug administration as in the epidural group (Caldwell 1994; Cortes 2007; Ngamprasertwong 2007; Roux 1999; Thomas 2005) or using less (COMET 2001a; Gomez 2001; Tsen 1999). Four studies (Caldwell 1994; Gomez 2001; Ngamprasertwong 2007; Tsen 1999) involved the use of a low‐dose infusion down the epidural catheter for maintenance. In these studies the infusions were started immediately after the initial bolus in the epidural groups. In three other studies (COMET 2001a; Cortes 2007; Roux 1999) maintenance was with intermittent epidural boluses at patient request. COMET 2001a used 0.25% bupivacaine in the epidural group but bupivacaine 0.15% plus fentanyl 2 µg/mL in the CSE group; both Cortes 2007 and Roux 1999 used 0.25% bupivacaine in both. In the remaining trial (Thomas 2005) in which no intrathecal drugs were injected as part of the CSE technique, all women received the same epidural management. This was also the only trial in this category which employed a patient‐controlled epidural analgesia (PCEA) technique for maintenance of analgesia. This involved bupivacaine 0.11% plus fentanyl 2 µg/mL at 10 mL/hour with 5 mL bolus and lockout of 10 minutes.
There were 16 included studies that employed a low‐dose LA epidural group compared with CSE. In these trials there was a range of techniques used to establish the epidural block in the epidural groups. All employed bupivacaine as the local anaesthetic in concentrations from 0.0625% to 0.125% and in combination with fentanyl (20 to 75 µg) or sufentanil (5 to 10 µg) to a total volume of between 10 and 20 mL. Seven trials (Abouleish 1991; Abrao 2009; Goodman 2006; Kartawiadi 1996; Medina 1994; Van de Velde 1999; Vernis 2004) used bupivacaine 0.125% with added fentanyl or sufentanil. Eight further studies used even lower concentrations with bupivacaine 0.1% (Nickells 2000; Parry 1998; Price 1998) and 0.0625% (Bhagwat 2008; Hepner 2000; Skupski 2009; Zeidan 2004) and in Cohen 2006 0.04% ropivacaine plus sufentanil was used. In relation to the CSE groups, in all except one trial (Abouleish 1991), the initial intrathecal injection consisted of LA plus opioid using bupivacaine and either sufentanil or fentanyl. In the Abouleish 1991 trial the CSE group consisted of intrathecal morphine 0.2 mg alone and in Cohen 2006, 5 µg of sufentanil and 2 mg of ropivacaine was used. For the other studies the doses employed ranged from 1.25 to 3.75 mg bupivacaine, 5 to 25 µg fentanyl and 1.5 to 5 µg sufentanil. A common technique used in six studies was that of bupivacaine 2.5 mg plus fentanyl 25 µg. In three studies (Nickells 2000; Parry 1998; Patel 2003a) there was no maintenance analgesia stated. In two of the remaining studies intermittent boluses of either 0.1% (Nickells 2000) or 0.125% (Kartawiadi 1996) bupivacaine were delivered down the indwelling epidural catheter for maintenance after return of pain. Six studies (Goodman 2006; Hepner 2000; Medina 1994; Ngamprasertwong 2007; Skupski 2009; Zeidan 2004) used low‐dose LA plus opioid bolus then infusion for maintenance after return of pain in both groups. In Hepner 2000 the first additional analgesia was provided by a bolus of 0.0625% bupivacaine with added fentanyl, bicarbonate and epinephrine. Zeidan 2004 used 0.0625% bupivacaine plus fentanyl 1.5 µg/mL and Medina 1994 used 0.125% bupivacaine plus sufentanil 0.5 µg/mL. In Goodman 2006, Ngamprasertwong 2007 and Skupski 2009 both groups had infusion of bupivacaine 0.0625% plus fentanyl 2 µg/mL at 12 mL/hr.The low‐dose epidural infusion group in COMET 2001a had analgesia established with a bolus of 0.1% bupivacaine with fentanyl and an immediate infusion of the same solution for maintenance. Data from this group were independently compared with the traditional epidural group. Five studies used a PCEA technique for maintenance of analgesia. One (Van de Velde 1999) used boluses of 4 mL 0.125% bupivacaine with sufentanil 0.75 µg/mL and epinephrine 1.25 µg/mL and a lockout time of 15 minutes. The second (Price 1998) used 10 mL 0.1% bupivacaine with added fentanyl 2 µg/mL delivered with a lockout time of 30 minutes. Vernis 2004 used bupivacaine 0.125% plus sufentanil 0.25 µg/mL with a 4 mL bolus and 10 minute lockout. Sezer 2007 used PCEA with 5 mL bolus of bupivacaine 0.1% plus fentanyl 2 µg/mL with a 10 minutes lockout. Bhagwat 2008 used bupivacaine 0.0625% plus fentanyl 2 µg/mL at a rate of 8 to 12 mL/hr to maintain T10 block via PCEA pump. In Abrao 2009 different concentrations of bupivacaine were given based on cervical dilation upon patient request.
In two studies the main epidural bolus consisted of opioid alone, either fentanyl 100 µg (Breen 1999) or sufentanil 4 µg (Dunn 1998). In each case the opioid bolus was only administered after a test dose of 3 mL lignocaine 1.5%. The intrathecal component of the CSE in both trials was sufentanil 10 µg; there was no stated analgesia maintenance in either study.
Maternal outcomes
No study reported time taken from request of maternal analgesia to the time the mother felt the level of pain relief was satisfactory. However, one study (Hepner 2000) commented on the need to take into account the additional time required to prepare certain solutions and the impact this may have on the time from patient request to establishing analgesia. We also evaluated onset of pain relief from time of initial injection, acknowledging that this result comes more from the practical realities of conducting a research trial rather than what may be of interest to consumers. The stated primary outcome of 18 of the included studies was related to the quality of analgesia and data on analgesic efficacy were presented as a secondary outcome in a further eight trials (Bhagwat 2008; Breen 1999; COMET 2001a; Gomez 2001; Hepner 2000; Skupski 2009; Tsen 1999; Zeidan 2004). These data took the form of visual analogue scores in most cases (Abouleish 1991; Breen 1999; Dunn 1998; Gomez 2001; Hepner 2000; Kartawiadi 1996; Medina 1994; Price 1998; Roux 1999; Tsen 1999; Van de Velde 1999; Vernis 2004; Zeidan 2004) and in one study was retrospectively assessed by a postnatal interview (COMET 2001a). Eleven studies (Abouleish 1991; COMET 2001a; Dunn 1998; Gomez 2001; Hepner 2000; Medina 1994; Nickells 2000; Price 1998; Thomas 2005; Vernis 2004; Zeidan 2004) detailed the requirement for additional analgesic intervention. Only two studies (Parry 1998; Patel 2003a) did not quote any data regarding the effectiveness of pain relief but had primary outcomes related to effects on the baby and epidural/CSE effects on dorsal column function respectively.
All but 10 studies (Abouleish 1991; Abrao 2009; Bhagwat 2008; Caldwell 1994; Goodman 2006; Medina 1994; Patel 2003a; Roux 1999; Skupski 2009; Thomas 2005) quoted figures for degree of motor blockade, but only data from papers quoting numbers of women who actually walked during labour were used in the analysis of ability to mobilise (Breen 1999; Cohen 2006; COMET 2001a; Dunn 1998; Parry 1998; Price 1998; Zeidan 2004). In one trial (Collis 1995) only women receiving the CSE technique were assessed for motor block and if able to straight‐leg‐raise satisfactorily, they were encouraged to mobilise. However, the women receiving the traditional epidural analgesia were not assessed and not encouraged to walk. One other study (Nageotte 1997) involved two CSE groups, identical in all other respects except that the women in one group were actively encouraged to walk while those in the second CSE group were discouraged from mobilising. No data on mobility were presented for the women in the epidural group. As there was an actively promoted difference in treatment between epidural and CSE groups in both studies, this cast doubt on the maintenance of blinding, suggesting the possibility of performance bias and a loss of the benefit of randomisation. Sensitivity analysis was performed and both studies (Collis 1995; Nageotte 1997) were excluded. Similarly in COMET 2001a only women in the low‐dose infusion and CSE groups were allowed to mobilise, with no data from the traditional epidural group for comparison. In Cohen 2006 and Cortes 2007 all women in both groups were able to mobilise.
The incidence of short‐term side effects and complications, along with maternal and neonatal effects was also presented in most studies. Maternal satisfaction with analgesia was the primary outcome for one excluded study (Collis 1995) but measurement of satisfaction postdelivery featured as a secondary outcome in seven of the included trials (Gomez 2001; Hepner 2000; Kartawiadi 1996; Price 1998; Van de Velde 1999; Vernis 2004; Zeidan 2004). Typically, the assessment of satisfaction was very simple. For example, Vernis 2004 used a four‐point Likert scale response to the written question, 'Were you satisfied with your labour?'. Gomez 2001 presented satisfaction data as visual analogue scale scores at time of delivery but these could not be included in the data tables. Incidence of headache in the days following delivery was quoted widely, and the use of blood patch for post dural puncture headache was analysed from eight studies (Abouleish 1991; Dunn 1998; Hepner 2000; Kartawiadi 1996; Price 1998; Roux 1999; Vernis 2004; Zeidan 2004). One study quoted dural tap rate but not rate of headache or blood patch requirement (COMET 2001a). One excluded study (Finegold 2003) investigated uterine contraction rates and endogenous oxytocin levels as primary outcomes but these were not included in our measures.
The effect on the progress of labour was the main outcome in four studies, two assessing the rate of cervical dilatation (Bhagwat 2008; Tsen 1999) and two focusing on mode of delivery (COMET 2001a; Zeidan 2004). Other side effects analysed included the occurrence of hypotension, respiratory depression, pruritus, nausea and vomiting, urinary retention and sedation. Data concerning longer‐term outcomes were presented in only one (Medina 1994) of the trials although another (COMET 2001a) explicitly referred to the collection of such data not yet completed. Of note, no study looked at the economics and use of resources involved in the provision of both analgesic regimens, such as comparative cost of consumables, duration and cost of stay in hospital, or need for readmission or follow‐up after discharge. The effect on the progress of labour was the main outcome in four studies, three assessing the rate of cervical dilatation (Bhagwat 2008; Sezer 2007; Tsen 1999) and one focusing on mode of delivery (COMET 2001a).
Neonatal outcomes
Neonatal assessment was carried out in all but six studies (Breen 1999; Gomez 2001; Goodman 2006; Nickells 2000; Price 1998; Tsen 1999). In all other studies, Apgar scores were used as an outcome measure. In Dunn 1998 and Ngamprasertwong 2007 neonatal Apgar scores were stated as not differing between groups but no actual numbers were quoted. In the remaining eight included studies, four quoted numbers of neonates with Apgar scores of less than seven at five minutes, while four others gave data on those scoring less than eight (Abouleish 1991; COMET 2001a; Kartawiadi 1996; Parry 1998). In addition, five studies included cord blood gas analysis (Abouleish 1991; Abrao 2009; Caldwell 1994; Hepner 2000; Van de Velde 1999). These data were presented as mean pH +/‐ SD, rather than the number of neonates demonstrating a predefined degree of acidosis. Only one study (COMET 2001a) included data on the number of neonates requiring admission to a special care neonatal unit. Two studies looked at fetal bradycardia as primary outcome (Abrao 2009; Skupski 2009). In the study by Bhagwat 2008, there was one stillbirth with cord around the neck in the CSE group noted. Four other studies included mean Apgar score and found no difference between groups (Abrao 2009; Bhagwat 2008; Cohen 2006; Sezer 2007).
Excluded studies
Twenty seven studies were excluded. Reasons for exclusion fell into three broad categories. Nine studies (Camann 1992; Camann 1998; Collis 1994; Collis 1999a; Collis 1999b; D'Angelo 1994; Harsten 1997; Pham 1996; Rosenfeld 1998; Van de Velde 2004) consisted of study designs in which all women received a spinal injection or dural puncture plus epidural and as such were not specifically comparing a combined spinal epidural with epidural alone. Six other studies (Cascio 1996; Cooper 2010; Groves 1995; Kassapidis 1997; Patel 2003b; Stocche 2001), although comparing CSE with epidurals for labour analgesia, reported outcomes that were not part of our analysis. A further 12 studies (Backus 1996; Collis 1995; Dresner 1999; Finegold 2003; Fogel 1999; Leighton 1996; Nageotte 1997; Nielson 1996; Norris 1994; Norris 2001; Pan 1996; Pinto 2000) exhibited a variety of methodological and study design issues. For example, Leighton 1996, Nielson 1996, Norris 1994 and Norris 2001 were not randomised studies. Also, Backus 1996 and Fogel 1999 had significant treatment differences within groups, while in Collis 1995 and Nageotte 1997 there were significant differences in the management of patients between groups which are likely to have directly influenced outcomes, notably mobilisation.
Risk of bias in included studies
There was a wide range of methodological quality. Details are shown in the table of Characteristics of included studies. Of the 27 included studies, only four (Breen 1999; Goodman 2006; Hepner 2000; Kartawiadi 1996) were fully consistent with the methodological principles defined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
See Figure 1 and Figure 2 for summary of 'Risk of bias' assessments for all studies.
1.
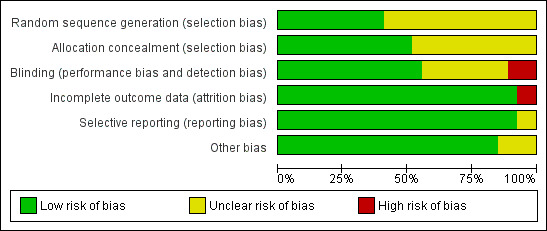
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
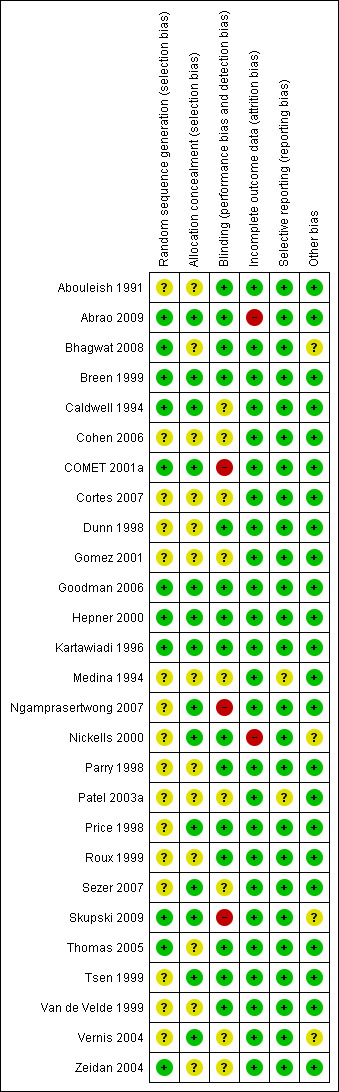
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies stated that women were randomly allocated to treatment groups. Whilst a range of methods for randomisation and concealment of allocation were stated, we assessed 11 studies as being at low risk of bias for randomisation (Abrao 2009; Bhagwat 2008; Breen 1999; Caldwell 1994; COMET 2001a; Goodman 2006; Hepner 2000; Kartawiadi 1996; Skupski 2009; Thomas 2005; Zeidan 2004) and 14 studies as being at low risk of bias for allocation concealment (Abrao 2009; Breen 1999; Caldwell 1994; COMET 2001a; Goodman 2006; Hepner 2000; Kartawiadi 1996; Ngamprasertwong 2007; Nickells 2000; Price 1998; Sezer 2007; Skupski 2009; Tsen 1999; Vernis 2004). Methods employed included computer randomisation and numbered, sealed, opaque envelopes (Caldwell 1994), random‐number tables with sealed envelopes (Hepner 2000) and computerised allocation provided by external sources (COMET 2001a). In the remaining studies, the methods of randomisation and allocation concealment used were not clearly described.
Blinding
We assessed 15 studies as being at low risk of bias for performance and detection bias (Abouleish 1991; Abrao 2009; Bhagwat 2008; Breen 1999; Dunn 1998; Goodman 2006; Hepner 2000; Kartawiadi 1996; Nickells 2000; Parry 1998; Price 1998; Roux 1999; Thomas 2005; Tsen 1999; Van de Velde 1999); in nine studies risk of bias was unclear (Caldwell 1994; Cohen 2006; Cortes 2007; Gomez 2001; Medina 1994; Patel 2003a; Sezer 2007; Vernis 2004; Zeidan 2004); and we assessed three studies as being at high risk of bias (COMET 2001a; Ngamprasertwong 2007; Skupski 2009).
Incomplete outcome data
This was an issue for two studies (Abrao 2009; Nickells 2000). In Abrao 2009, of those originally randomised, 15% were not analysed; for neonatal pH this was 29%. In Nickells 2000, unclear data tables prevented us from using some of their reported data for analysis and it was not clear whether 18 analgesic failures (nine in each group) were included in the analysis of their results. In all the remaining studies, complete outcome data were available.
Selective reporting
We assessed 25 studies as being at low risk of bias for selective reporting (Abouleish 1991; Abrao 2009; Bhagwat 2008; Breen 1999; Caldwell 1994; Cohen 2006; COMET 2001a; Cortes 2007; Dunn 1998; Gomez 2001; Goodman 2006; Hepner 2000; Kartawiadi 1996; Ngamprasertwong 2007; Nickells 2000; Parry 1998; Price 1998; Roux 1999; Sezer 2007; Skupski 2009; Thomas 2005; Tsen 1999; Van de Velde 1999; Vernis 2004; Zeidan 2004) as all expected outcomes were reported. We assessed the remaining two studies as being at unclear risk of bias: in one study primary and secondary outcomes were not stated (Medina 1994); and in the other study reporting of detail of outcomes was unclear (Patel 2003a).
Other potential sources of bias
No other sources of bias were identified in 23 of the included studies (Abouleish 1991; Abrao 2009; Bhagwat 2008; Breen 1999; Caldwell 1994; Cohen 2006; COMET 2001a; Cortes 2007; Dunn 1998; Gomez 2001; Goodman 2006; Hepner 2000; Kartawiadi 1996; Medina 1994; Ngamprasertwong 2007; Nickells 2000; Parry 1998; Patel 2003a; Price 1998; Roux 1999; Sezer 2007; Skupski 2009; Thomas 2005; Tsen 1999; Van de Velde 1999; Vernis 2004; Zeidan 2004). In the other four studies, we assessed other risk of bias as being unclear (Bhagwat 2008; Nickells 2000; Skupski 2009; Vernis 2004): one study (Bhagwat 2008) had not dealt with one still birth in the CSE group (cord around neck); one study (Nickells 2000) was stopped after three months and so there were slightly uneven group sizes; one study (Skupski 2009) was underpowered to detect the difference in fetal heart rate changes; and one study (Vernis 2004) was stopped early.
Effects of interventions
Twenty‐seven trials, involving 3274 labouring women, met our criteria for inclusion.
We performed analyses on all included studies against 26 outcomes. This was performed as two separate sets of comparisons. The first set involved all combined spinal‐epidural (CSE) variants versus traditional epidurals and the second set was all CSE forms versus low‐dose epidurals and variants. For a summary of analyses seeData and analyses.
CSE versus traditional epidural
Of the CSE versus traditional epidural analyses four outcomes showed a significant difference. The time from first injection to effective analgesia was less with CSE (mean difference (MD) ‐2.87 minutes; 95% confidence interval (CI) ‐5.07 to ‐0.67; 129 women (Analysis 1.1)) based on two studies (Ngamprasertwong 2007; Roux 1999). There was a reduced need for rescue analgesia for CSE based on one study (Gomez 2001) with a risk ratio (RR) of 0.31 (95% CI 0.14 to 0.70; 42 women (Analysis 1.3)). CSE was also more favourable with respect to the need for instrumental delivery with RR 0.81 (95% CI 0.67 to 0.97; 1015 women (Analysis 1.19)) based on six studies (COMET 2001a; Cortes 2007; Gomez 2001; Ngamprasertwong 2007; Roux 1999; Tsen 1999). CSE was also associated with less urinary retention based on the COMET 2001a study (RR 0.86; 95% CI 0.79 to 0.95; one study, 704 women (Analysis 1.10)). CSE was associated with a slightly lower umbilical venous pH in comparison to the epidural group, although this result was based on only one study involving morphine and fentanyl for the initial intrathecal injection (Caldwell 1994), and statistical significance was borderline (MD ‐0.03; 95% CI ‐0.06 to ‐0.00; one study, 55 women (Analysis 1.22)).
1.1. Analysis.
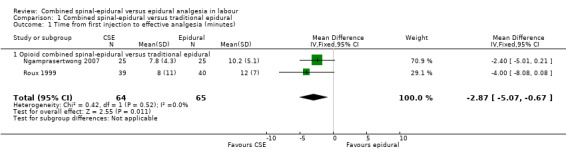
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 1 Time from first injection to effective analgesia (minutes).
1.3. Analysis.
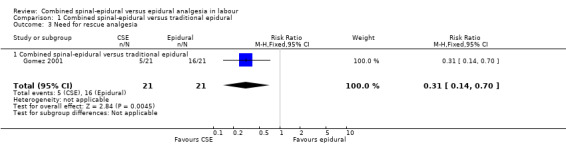
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 3 Need for rescue analgesia.
1.19. Analysis.
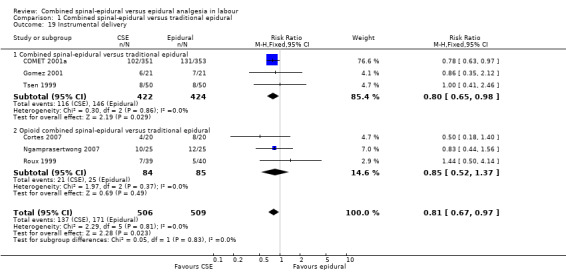
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 19 Instrumental delivery.
1.10. Analysis.
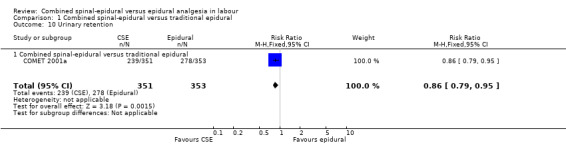
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 10 Urinary retention.
1.22. Analysis.
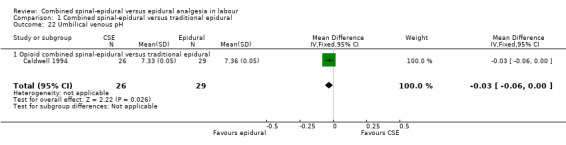
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 22 Umbilical venous pH.
No differences between CSE and epidural were seen for the number of women who mobilised, post dural puncture headache, pruritis, nausea and vomiting, hypotension or the rate of caesarean birth. Also, there were no significant differences for the following neonatal outcomes: umbilical arterial pH, Apgar score less than seven or less than eight at five minutes and the number of admissions to the neonatal unit.
Due to results not being statistically significant or outcomes reported with zero data, it was not possible to draw any conclusions with respect to the following outcomes: number of women with effective analgesia in the first 10 minutes after injection, number of women satisfied with analgesia, number of women requiring a blood patch, maternal respiratory depression and umbilical cord pH.
Subgroup analyses
No subgroup differences between opioid CSE and CSE were observed: known dural tap (Analysis 1.7); pruritus (Analysis 1.9); nausea/vomiting (Analysis 1.11); hypotension (Analysis 1.12); labour augmentation required (Analysis 1.16); normal delivery (Analysis 1.18); instrumental delivery (Analysis 1.19) or caesarean section (Analysis 1.20).
1.7. Analysis.
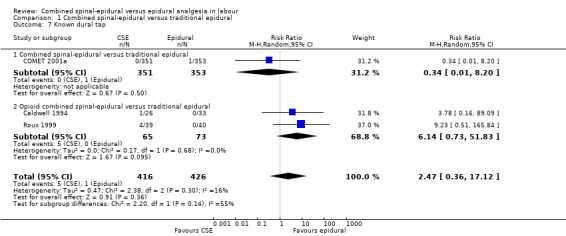
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 7 Known dural tap.
1.9. Analysis.
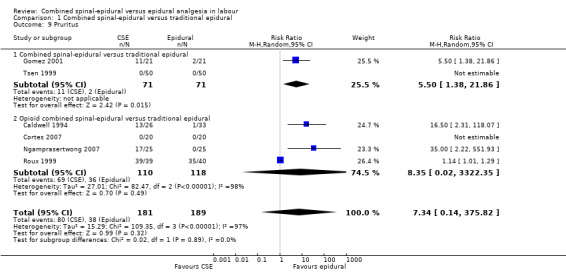
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 9 Pruritus.
1.11. Analysis.
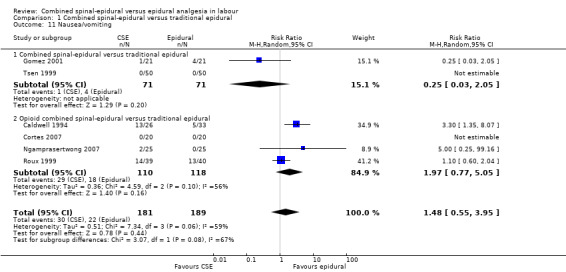
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 11 Nausea/vomiting.
1.12. Analysis.
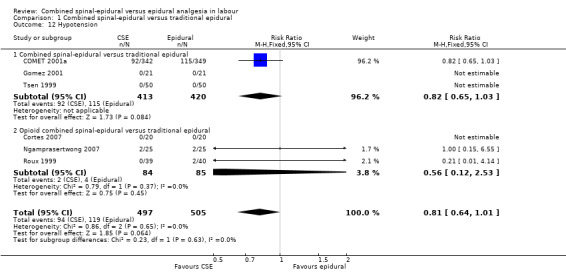
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 12 Hypotension.
1.16. Analysis.
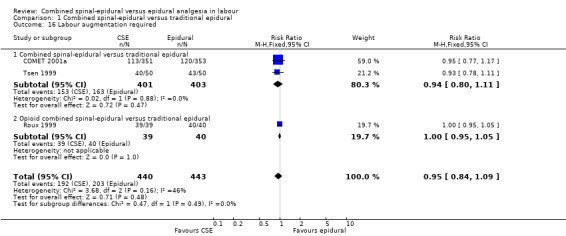
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 16 Labour augmentation required.
1.18. Analysis.
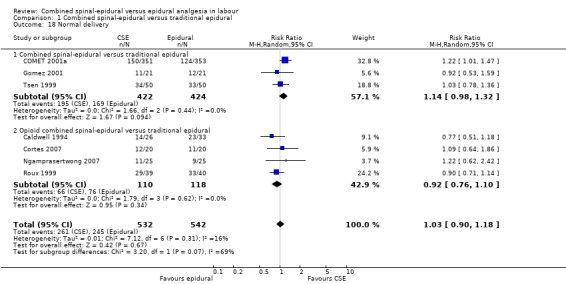
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 18 Normal delivery.
1.20. Analysis.
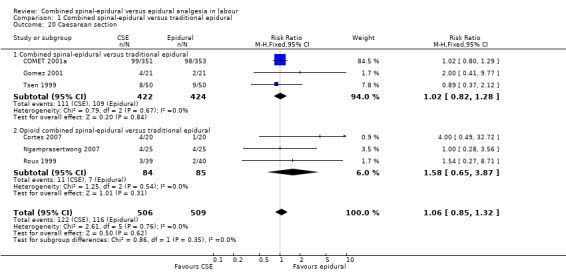
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 20 Caesarean section.
CSE versus low‐dose epidural
For the analyses of CSE versus low‐dose epidurals and variants there were three outcomes which were statistically significant. Both measures of speed of onset of analgesia from the time of injection indicated a faster onset for CSE versus low‐dose epidural. The mean time of onset of effective analgesia was ‐5.42 minutes (95% CI ‐7.26 to ‐3.59; five studies, 461 women; random‐effects,T² = 3.27, I² = 77% (Analysis 2.1)), while the risk ratio of effective analgesia at 10 minutes was 1.94 in favour of CSE (95% CI 1.49 to 2.54) based on a single study with 101 women (Analysis 2.2). Time of onset of effective analgesia showed substantial heterogeneity, but this would be expected given the variation in techniques between studies. However, as has been remarked upon in previous versions of this review, no study reported our primary outcome of interest with regards to time of onset of pain relief in labour from the time of patient request.
2.1. Analysis.
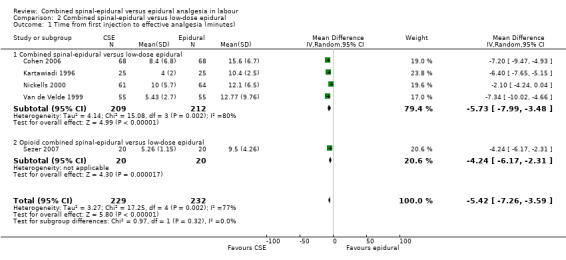
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 1 Time from first injection to effective analgesia (minutes).
2.2. Analysis.
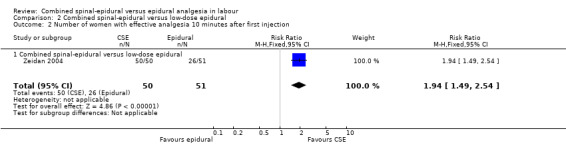
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 2 Number of women with effective analgesia 10 minutes after first injection.
For the analyses of CSE versus low‐dose epidurals, CSE was associated with more pruritus (average RR 1.80; 95% CI 1.22 to 2.65; 11 studies, 959 women; random‐effects, T² = 0.26, I² = 84% (Analysis 2.9)). There was substantial heterogeneity between studies reflecting once again the marked variability in definitions used.
2.9. Analysis.
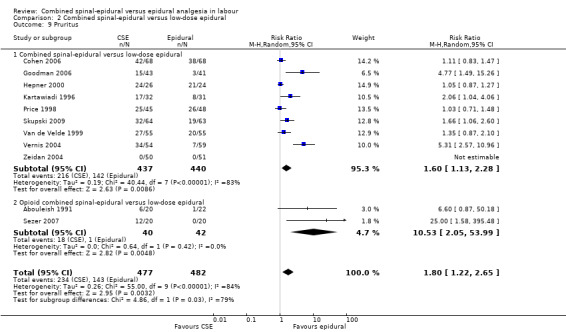
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 9 Pruritus.
There were no significant differences between CSE and low‐dose epidural with regard to modes of delivery and the need for labour augmentation. There was also no significant difference between CSE and low‐dose epidurals with respect to the maternal side effects of urinary retention, nausea and vomiting, post dural puncture headache, known dural tap and need for a blood patch, or for the need of rescue analgesia. Maternal satisfaction with analgesia was also not significantly different based on seven studies (520 women) (see Analysis 2.4). However, there was substantial heterogeneity in this result and this probably reflects the wide variation in and perhaps simplistic methods generally used for assessing this important outcome.
2.4. Analysis.
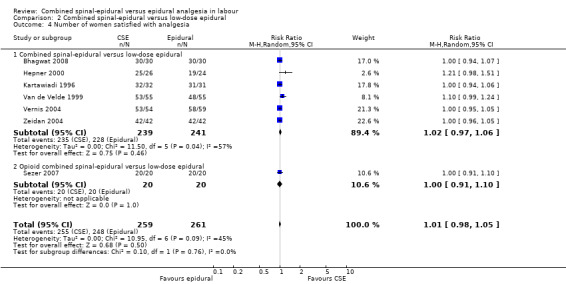
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 4 Number of women satisfied with analgesia.
In relation to all of the neonatal outcomes there was also no difference with umbilical venous pH, umbilical cord pH, Apgar scores at five minutes and the number of neonates requiring neonatal unit admission. It was not possible to draw any conclusions with respect to the following outcomes: maternal respiratory depression and maternal sedation. No women experienced maternal respiratory depression in the studies that reported on this outcome and maternal sedation was not a reported outcome in the included studies. Two other outcomes are worth highlighting specifically. Firstly, mobilisation in labour was reported by seven studies (involving 1200 participants) and there was no difference between the techniques. Secondly, the occurrence of hypotension exhibited substantial heterogeneity, again as a result of the diverse range of definitions used. We have reported this outcome as not significant. However we conducted both subgroup and sensitivity analyses as a result of the wide variation of results seen between different studies and a funnel plot looking for any effects of small studies (Figure 3). The small number of subjects in the different subgroups resulted in there being no discernible effect. However, within the local anaesthetic plus opioid CSE versus low‐dose epidural comparison there appears to be a trend to there being a more favourable outcome for epidural over CSE; a fixed‐effect analysis would produce a significant result.
3.
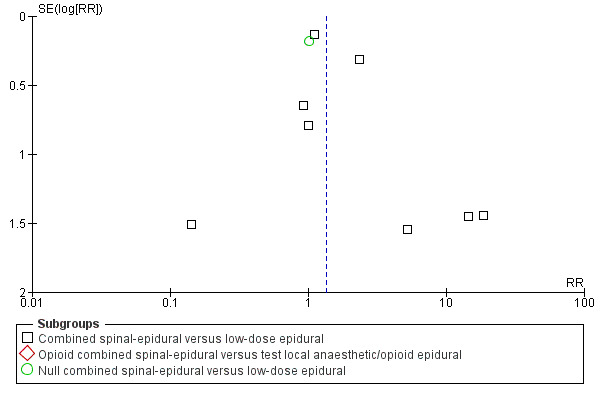
Funnel plot of comparison: 2 Combined spinal‐epidural versus low‐dose epidural, outcome: 2.12 Hypotension.
Adverse events
We included the incidence of longer‐term sequelae (e.g. neurological) as an outcome measure as this has been raised as a source of concern in the past regarding the routine use of CSE analgesia for labour. In Medina 1994, in the CSE group there was one case of reflex sympathetic pain of the foot which required two paravertebral blocks to resolve. In another study (Vernis 2004) there was one woman in the CSE group who developed meningitis which responded effectively to intravenous antibiotics. COMET 2001a made reference to the ongoing collection of data not yet completed.
Subgroup analyses
No subgroup differences between opioid CSE, null CSE and CSE were observed for the following outcomes: time from first injection to effective analgesia (Analysis 2.1); need for rescue analgesia (Analysis 2.3); number of women satisfied with analgesia (Analysis 2.4); number of women who mobilise (Analysis 2.5); post dural puncture headache (Analysis 2.6); known dural tap (Analysis 2.7); number of women requiring blood patch (Analysis 2.8); urinary retention (Analysis 2.10); nausea/vomiting (Analysis 2.11); hypotension (Analysis 2.12); labour augmentation required (Analysis 2.16); normal delivery (Analysis 2.18); instrumental delivery (Analysis 2.19); caesarean section (Analysis 2.20); umbilical arterial pH (Analysis 2.21); umbilical venous pH (Analysis 2.22) or Apgar score less than seven at five minutes (Analysis 2.24).
2.3. Analysis.
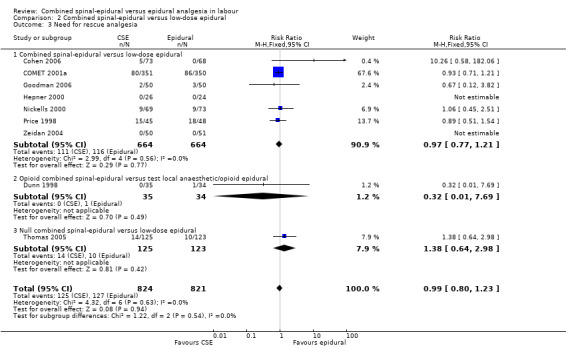
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 3 Need for rescue analgesia.
2.5. Analysis.
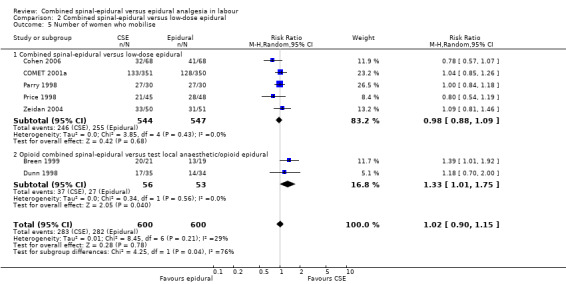
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 5 Number of women who mobilise.
2.6. Analysis.
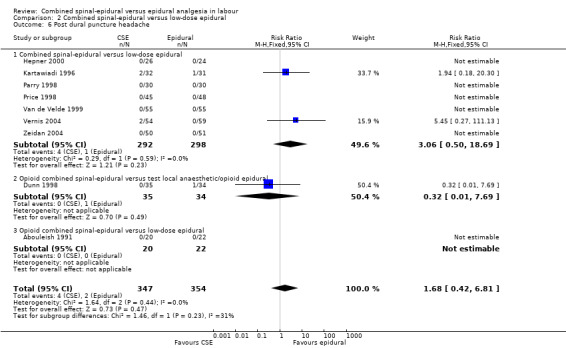
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 6 Post dural puncture headache.
2.7. Analysis.
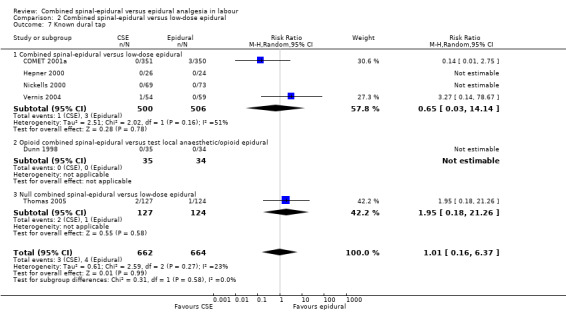
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 7 Known dural tap.
2.8. Analysis.
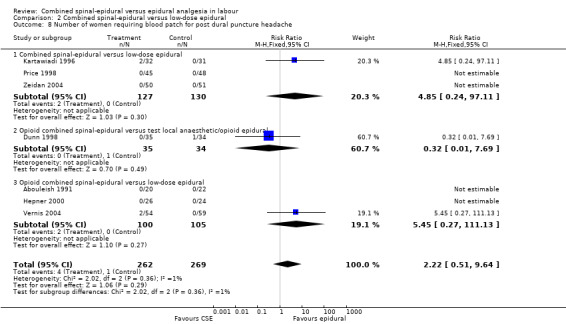
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 8 Number of women requiring blood patch for post dural puncture headache.
2.10. Analysis.
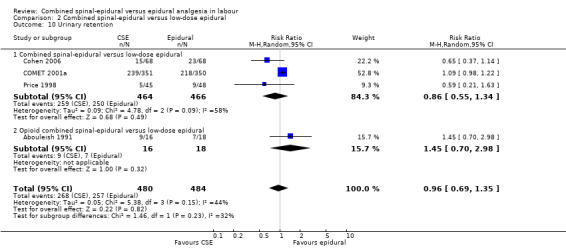
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 10 Urinary retention.
2.11. Analysis.
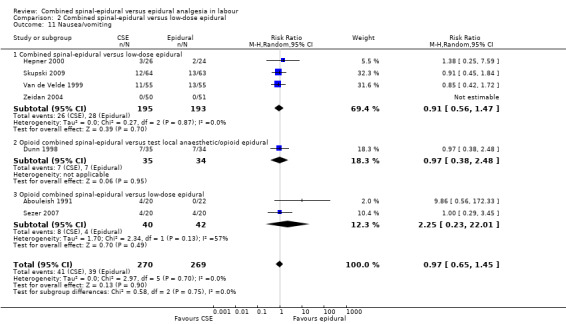
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 11 Nausea/vomiting.
2.12. Analysis.
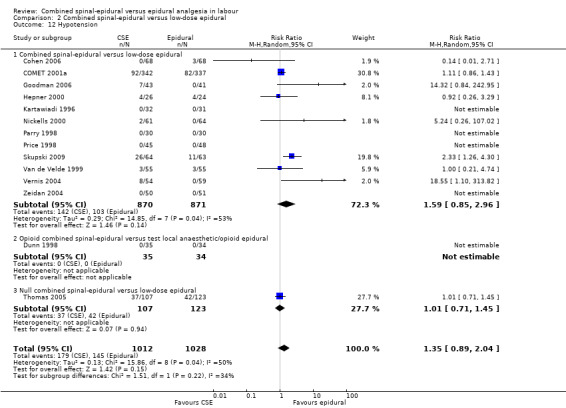
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 12 Hypotension.
2.16. Analysis.
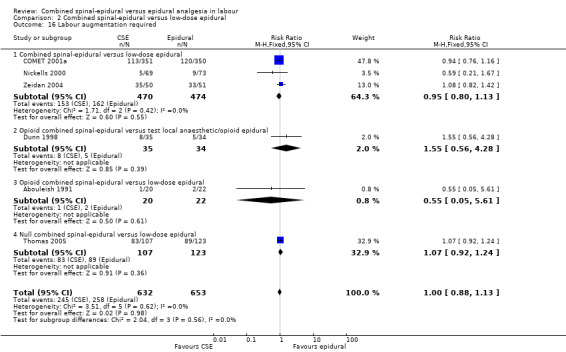
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 16 Labour augmentation required.
2.18. Analysis.
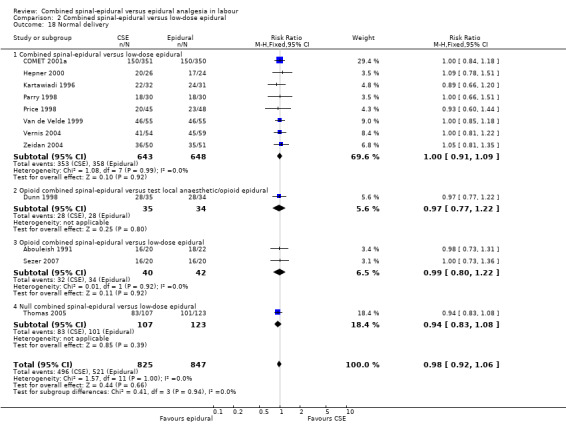
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 18 Normal delivery.
2.19. Analysis.
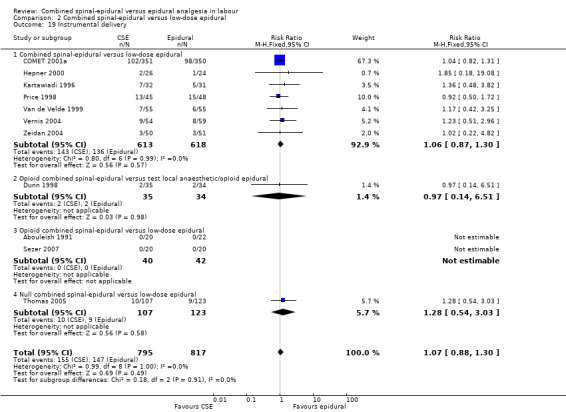
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 19 Instrumental delivery.
2.20. Analysis.
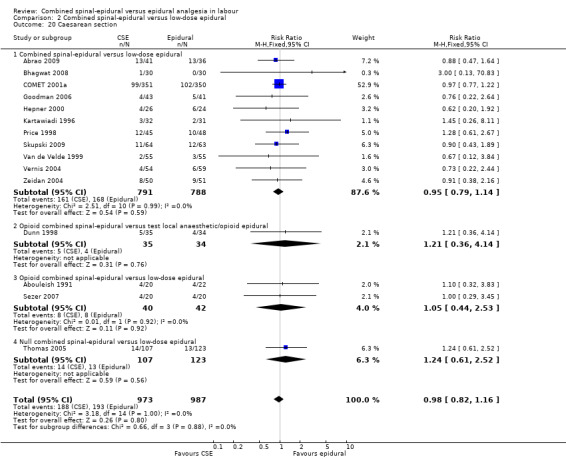
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 20 Caesarean section.
2.21. Analysis.
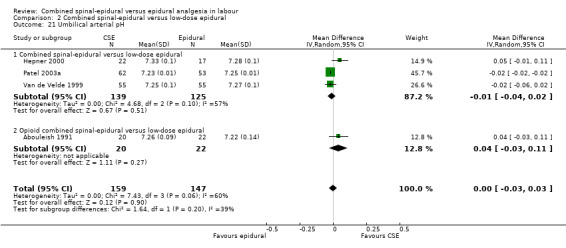
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 21 Umbilical arterial pH.
2.22. Analysis.
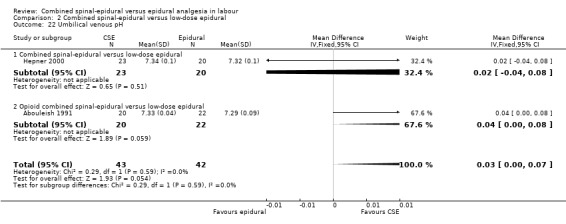
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 22 Umbilical venous pH.
2.24. Analysis.
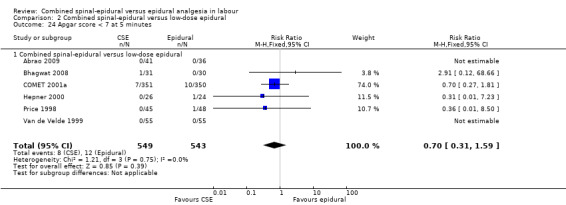
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 24 Apgar score < 7 at 5 minutes.
However, subgroup differences were observed for the following two outcomes: number of women who mobilise (P = 0.04, I² = 76.5%; Analysis 2.5) and pruritus (P = 0.03, I² = 79.4%; Analysis 2.9).
Discussion
There is no standard combined spinal‐epidural (CSE) or epidural technique and this necessitated categorising individual interventions into groups for analysis, and the use of multiple comparisons for individual outcomes. See additional tables, Table 1 and Table 2, for details. Nonetheless, in an attempt to maintain relevance with evolving clinical practice, we performed comparisons separately for CSE versus traditional epidurals and for low‐dose epidurals. This appears to have some clinical relevance supported by this review with there being several outcomes significantly favouring CSE over the traditional forms which was not apparent when CSE was compared with the lower‐dose variants, notably higher urinary retention, higher instrumental delivery rate and the need for rescue analgesia with traditional epidurals.
1. Epidural techniques used ‐ initial dose and subsequent maintenance.
| INITIAL DOSE | Nil maintenance | Immediate infusion | Repeat boluses | Repeat boluses | Bolus/infusion | Repeat boluses | PCEA |
| LA + opioid | High‐dose LA | Low‐dose LA | Low‐dose LA/opioid | Low‐dose LA/opioid | |||
| Low‐dose bupivacaine < 0.25% | Abouleish 1991 | ||||||
| Traditional dose bupivacaine = 0.25% | Gomez 2001 Ngamprasertwong 2007 Tsen 1999 |
COMET 2001a Cortes 2007 |
Thomas 2005 | ||||
| Low‐dose bupivacaine < 0.25% + opioid | Parry 1998Patel 2003a |
Bhagwat 2008 COMET 2001a Goodman 2006 Skupski 2009 |
Hepner 2000 Medina 1994 Zeidan 2004 |
Abrao 2009 Kartawiadi 1996 Nickells 2000 |
Cohen 2006 Price 1998 Sezer 2007 Van de Velde 1999 Vernis 2004 |
||
| Traditional dose bupivacaine + opioid | Caldwell 1994 | Roux 1999 | |||||
| Test lignocaine + opioid |
Breen 1999 Dunn 1998 |
LA: local anaesthetic PCEA: patient‐controlled epidural analgesia
2. CSE techniques used ‐ initial IT injection and subsequent epidural.
| CSE technique | Nil epidural | Immediate infusion | Immediate bolus/infusion | Immediate bolus/es | Delay bolus/infusion | Delayed boluses | PCEA |
| IT INJECTION | |||||||
| IT opioid only |
Breen 1999 Dunn 1998 |
Ngamprasertwong 2007 | Caldwell 1994 |
Abouleish 1991 Cortes 2007 Roux 1999 |
Sezer 2007 | ||
| IT LA + opioid | Parry 1998Patel 2003a |
Bhagwat 2008 Goodman 2006 Skupski 2009 |
Gomez 2001 |
Hepner 2000 Medina 1994 Tsen 1999 Zeidan 2004 |
Abrao 2009 COMET 2001a Kartawiadi 1996 Nickells 2000 |
Cohen 2006 Price 1998 Van de Velde 1999 Vernis 2004 |
|
| IT nil | Thomas 2005 |
CSE: combined spinal‐epidural IT: intrathecal LA: local anaesthetic PCEA: patient‐controlled epidural analgesia
Proposed benefits of CSE analgesia in labour over epidural pain relief are increased mobility and a postulated beneficial effect on mode of delivery. In this update there are now data from eight studies involving 1240 women including one study involving traditional epidurals (Cortes 2007; 40 women) and seven studies (1200 women) involving low‐dose epidurals (Breen 1999; Cohen 2006; COMET 2001a; Dunn 1998; Parry 1998; Price 1998; Zeidan 2004). There was no difference seen in the number of women who actually mobilised. In relation to mode of delivery there is also no apparent benefit attributable to CSE other than for a lower instrumental rate when compared with traditional epidurals. The rate of caesarean section would appear to be influenced by factors that to date have not emerged against these modes of regional analgesia. This is of interest not only in regard to progress of labour but also in relation to concerns for unfavourable fetal heart rate changes that have been attributed to the use of CSE techniques. This review does not include any outcomes specifically linked to fetal heart rate. However, if these effects are real there appears to be no support from this review of a significant translation of concern for the fetus to an increased rate of caesarean delivery. In relation to neonatal outcome there was no difference between CSE and epidural as identified by Apgar scores less than eight or seven at five minutes or the need for neonatal unit admission. Any possible differences between the two techniques in relation to the occurrence of unfavourable fetal heart rate changes during labour were not apparent from these results.
CSE was associated with a higher incidence of pruritus compared with low‐dose epidural techniques. This is almost certainly a result of direct injection of opioids into the subarachnoid space.
From studies included in this review there was no difference in the number of women who expressed satisfaction with CSE analgesia compared with epidural. This was based on seven studies (involving 520 women), all of which involved low‐dose epidurals (Bhagwat 2008; Hepner 2000; Kartawiadi 1996; Sezer 2007; Van de Velde 1999; Vernis 2004; Zeidan 2004). It would appear that the marginally faster onset from the time of injection, potentially favouring CSE, and the higher incidence of pruritus, presumably favouring epidurals, do not significantly impact on the overall sense of satisfaction. It was noted, however, that the measurement of satisfaction was typically very simplistic, using four‐point scales of a seemingly global experience.
Randomised controlled trials are not the best means of assessing differences in the risk of rare complications, such as meningitis, as they invariably fail to recruit the number of participants necessary to show such differences. In this review a total of over 3200 women participated in all 27 included studies combined. This is insufficient to assess the occurrence of very rare events. However, the inclusion of data on long‐term outcomes should still form part of large trials, such as COMET 2001a, so that perhaps in the future some benefit may be gained by collating the evidence from large numbers of studies. In the meantime, clinicians will have to rely on other forms of evidence, such as case reports and large cohort studies, for information on such rare events as meningitis and other neurological complications.
Authors' conclusions
Implications for practice.
Both combined spinal‐epidural and epidural techniques are shown to provide effective pain relief in labour. There appears to be little basis for offering one technique over the other, with no difference in overall maternal satisfaction despite a slightly faster onset with combined spinal‐epidural (CSE) and less pruritus with low‐dose epidurals. There is no difference in ability to mobilise, obstetric outcome or neonatal outcome. The significantly higher incidence with traditional epidural techniques of urinary retention based on one study involving 703 women and of rescue interventions, also with one other study involving 42 women, would favour the use of low‐dose epidurals, although this should be tempered by the knowledge that only single studies have been involved in both instances.
Implications for research.
Future trials should include as an endpoint the time taken from maternal request for pain relief until effective pain relief is established. This may be important as the additional tasks involved in the preparation for, and performance of, a combined spinal‐epidural block may potentially offset some of the advantage of quicker onset of analgesia. The difference in time of onset from the time of injection from this review is of the order of one contraction. In a busy obstetric service there are many factors that contribute to the total time between a woman requesting pain relief and when it is actually delivered and these may be far more relevant to the consumer.
While most papers gave data on numbers of women requiring delivery by caesarean section, none mentioned the type of anaesthesia for the surgery or the need for conversion to general anaesthesia for operative delivery. Avoidance of general anaesthesia still remains an important goal for obstetric anaesthetists. In this setting there are pros and cons for both the CSE and epidural techniques. The untested epidural catheter of a combined regimen may fail when topped up for theatre or result in intravascular injection. On the other hand, there are potential benefits of the intrathecal drugs used in terms of providing good surgical conditions and intra‐operative comfort for the woman. The rate of conversion from regional to general anaesthesia is sufficiently common that a very large study may have sufficient power to provide some answers. This could be the basis of a future review if sufficient studies included these data.
The collection of data on longer‐term sequelae following both CSE and epidural pain relief in labour is important. Rare and long‐term adverse effects of spinal and epidural techniques are difficult to quantify through small randomised trials such as in this review. There are other side effects, however, that can be more specifically addressed. Most papers have focused on obstetric outcomes. In this review we have also attempted to identify other factors through outcome measures such as respiratory depression and maternal sedation but very few papers investigated these issues. Future research work should more specifically address the possible impact of intrathecal and epidural opioids on both mother and baby. One specific area of interest in this regard is a possible link to breastfeeding success which could be different between CSE and epidural variants with differing amounts of opioids.
No reviewed study addressed the economic aspects of both types of regional pain relief. Future research could include data on such aspects as cost of consumables, cost and length of hospital stay, consequences of hospital readmission and resources needed for patient follow‐up after discharge.
Suggested systematic reviews
A number of studies, both included (Breen 1999; Dunn 1998; Parry 1998) and excluded from this review (e.g. Camann 1992) compared an intrathecal injection with an epidural bolus, and ended the study period with the first request for additional analgesia. While the exclusion of some such papers was made on other methodological grounds, the comparison of epidural and intrathecal (as opposed to combined spinal‐epidural) analgesia for labour could form the basis of a future review.
Within this review there were a wide range of interventions included. Not only did the drugs used differ but also the techniques, with studies using repeat boluses or infusions, or both, at different time intervals for the maintenance of analgesia. For the purposes of this review, all have been included together but with an attempt to categorise them into broad groups to assist with interpretation of results. The use of this or a similar structure may facilitate the review of further studies in the future.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2012 | New search has been performed | Search updated in September 2011 and nine new trials identified. Eight new studies have been included (Abrao 2009; Bhagwat 2008; Cohen 2006; Cortes 2007; Goodman 2006; Ngamprasertwong 2007; Sezer 2007; Skupski 2009) and one excluded (Cooper 2010). We updated the search on 30 June 2012 and identified seven new reports for considertion at the next update (Nakamura 2009; Pascual 2011; Pascual‐Ramirez 2010; Pascual‐Ramirez 2011; Patel 2012; Sweed 2011; Wilson 2011) ‐ see Characteristics of studies awaiting classification. The methods have been updated. |
| 31 January 2012 | New citation required but conclusions have not changed | Review updated. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 1 September 2008 | Amended | Converted to new review format. |
| 22 May 2007 | New citation required and conclusions have changed | Comparisons were restructured to be more clinically relevant around combined spinal‐epidural versus either traditional or low‐dose epidural techniques. With this approach there appears to be little basis for recommending one technique over the other with there now being no difference in maternal satisfaction or other key outcomes. |
| 31 December 2006 | New search has been performed | Search updated. An additional five studies were included (Medina 1994; Patel 2003a; Thomas 2005; Vernis 2004; Zeidan 2004). |
Acknowledgements
We thank Jane Brown for her contributions to the development of the first version of the review; Philippa Middleton, Leanne Jones and other members of the Cochrane Pregnancy and Childbirth Group for their valuable feedback.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
Study identification
Types of studies to be considered for review included all published randomised controlled trials involving a comparison of combined spinal‐epidural (CSE) with epidural analgesia initiated for women in the first stage of labour. Trials were identified for inclusion by three review authors independently. Details of reasons for exclusion of any trial considered for review have been clearly stated. If there were any disagreements regarding inclusion of potentially eligible trials these were resolved by discussion and, if necessary, arbitration by a fourth review author.
Quality assessment of included studies
Three review authors independently assessed the quality of all relevant studies.
Details of randomisation were recorded as satisfactory, unclear or unsatisfactory. Studies were excluded from the review if randomisation was clearly unsatisfactory, e.g. by day of the week, case note number, date of birth, etc.
Concealment of allocation was described as adequate, inadequate or unclear.
Blinding of outcome assessments and the number of women lost to follow‐up in included studies was noted.
Data extraction
Data were extracted using a structured form that captured patient demographics (e.g. primipara/multipara), stage of labour, use of oxytocics prior to regional technique.
The technique and drug details of the CSE and the epidural groups were noted and classified.
Three review authors independently extracted the data and differences resolved by referring to the original study.
Data analysis
Dichotomous data were expressed as risk ratios.
An intention‐to‐treat analysis was performed to include all randomised women where possible.
We assessed possible sources of heterogeneity by subgroup analyses and sensitivity analyses. The large diversity of both CSE and epidural techniques used resulted in up to six separate subgroup analyses being conducted. The definition of these groups is covered in detail under the 'Interventions' section below. 'CSE' groups consisted of both local anaesthetic and opioid, 'opioid CSE' groups used only opioids in the CSE, while the 'null CSE' group consisted of studies with a spinal puncture but no intrathecal injection of drugs. 'Low‐dose' epidurals used less than 0.25% bupivacaine or equivalent. Some epidural groups used only a test dose of local anaesthetic, i.e. a relatively small dose at the time of initiating the block. Hence, separate analyses were performed for studies comparing:
CSE with both traditional epidural regimens and also low‐dose epidural techniques;
other types of CSE regimens using an 'opioid only' spinal component with both traditional and low‐dose techniques and also where only local anaesthetic as a test dose had been given.
Sensitivity analyses were performed by excluding trials that:
do not report comparable groups, e.g. with respect to parity, age, use of oxytocics prior to administration of the regional technique; or
where outcomes have not been or may not have been properly blinded.
We tested for publication bias using the funnel plot visually.
We performed statistical analyses with the Review Manager software (RevMan 2008) for calculation of the treatment effect as represented by either the random‐effects or the fixed‐effect models depending upon the status of heterogeneity.
Data and analyses
Comparison 1. Combined spinal‐epidural versus traditional epidural.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time from first injection to effective analgesia (minutes) | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐2.87 [‐5.07, ‐0.67] |
| 1.1 Opioid combined spinal‐epidural versus traditional epidural | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐2.87 [‐5.07, ‐0.67] |
| 2 Number of women with effective analgesia 10 minutes after first injection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Need for rescue analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.70] |
| 3.1 Combined spinal‐epidural versus traditional epidural | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.70] |
| 4 Number of women satisfied with analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number of women who mobilise | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 6 Post dural puncture headache | 3 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.16, 89.09] |
| 6.1 Opioid combined spinal‐epidural versus traditional epidural | 3 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.16, 89.09] |
| 7 Known dural tap | 3 | 842 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [0.36, 17.12] |
| 7.1 Combined spinal‐epidural versus traditional epidural | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.20] |
| 7.2 Opioid combined spinal‐epidural versus traditional epidural | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 6.14 [0.73, 51.83] |
| 8 Number of women requiring blood patch for post dural puncture headache | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Opioid combined spinal‐epidural versus traditional epidural | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Pruritus | 6 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 7.34 [0.14, 375.82] |
| 9.1 Combined spinal‐epidural versus traditional epidural | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [1.38, 21.86] |
| 9.2 Opioid combined spinal‐epidural versus traditional epidural | 4 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 8.35 [0.02, 3322.35] |
| 10 Urinary retention | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.95] |
| 10.1 Combined spinal‐epidural versus traditional epidural | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.95] |
| 11 Nausea/vomiting | 6 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.55, 3.95] |
| 11.1 Combined spinal‐epidural versus traditional epidural | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.05] |
| 11.2 Opioid combined spinal‐epidural versus traditional epidural | 4 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.77, 5.05] |
| 12 Hypotension | 6 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.01] |
| 12.1 Combined spinal‐epidural versus traditional epidural | 3 | 833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 12.2 Opioid combined spinal‐epidural versus traditional epidural | 3 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.53] |
| 13 Respiratory depression | 3 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Opioid combined spinal‐epidural versus traditional epidural | 3 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Headache (any) | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.83] |
| 14.1 Opioid combined spinal‐epidural versus traditional epidural | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.83] |
| 15 Sedation | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.31] |
| 15.1 Opioid combined spinal‐epidural versus traditional epidural | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.31] |
| 16 Labour augmentation required | 3 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.09] |
| 16.1 Combined spinal‐epidural versus traditional epidural | 2 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.11] |
| 16.2 Opioid combined spinal‐epidural versus traditional epidural | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.95, 1.05] |
| 17 Augmentation after analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.24, 1.06] |
| 17.1 Combined spinal‐epidural versus traditional epidural | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.24, 1.06] |
| 18 Normal delivery | 7 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.18] |
| 18.1 Combined spinal‐epidural versus traditional epidural | 3 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.98, 1.32] |
| 18.2 Opioid combined spinal‐epidural versus traditional epidural | 4 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 19 Instrumental delivery | 6 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.97] |
| 19.1 Combined spinal‐epidural versus traditional epidural | 3 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 19.2 Opioid combined spinal‐epidural versus traditional epidural | 3 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| 20 Caesarean section | 6 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.32] |
| 20.1 Combined spinal‐epidural versus traditional epidural | 3 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 20.2 Opioid combined spinal‐epidural versus traditional epidural | 3 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.65, 3.87] |
| 21 Umbilical arterial pH | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 21.1 Opioid combined spinal‐epidural versus traditional epidural | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 22 Umbilical venous pH | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 22.1 Opioid combined spinal‐epidural versus traditional epidural | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 23 Umbilical cord pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Apgar score < 7 at 5 minutes | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.63, 6.97] |
| 24.1 Opioid combined spinal‐epidural versus traditional epidural | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.63, 6.97] |
| 25 Apgar score < 8 at 5 minutes | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.61, 9.00] |
| 25.1 Combined spinal‐epidural versus traditional epidural | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.61, 9.00] |
| 26 Number admitted to neonatal unit | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.37] |
| 26.1 Combined spinal‐epidural versus traditional epidural | 1 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.37] |
1.5. Analysis.
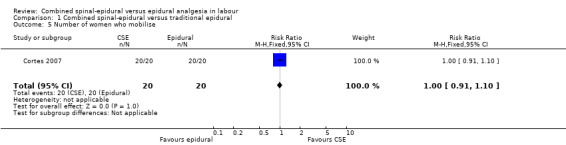
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 5 Number of women who mobilise.
1.6. Analysis.
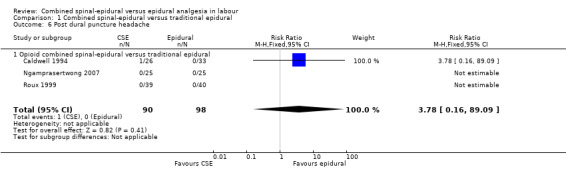
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 6 Post dural puncture headache.
1.8. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 8 Number of women requiring blood patch for post dural puncture headache.
1.13. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 13 Respiratory depression.
1.14. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 14 Headache (any).
1.15. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 15 Sedation.
1.17. Analysis.
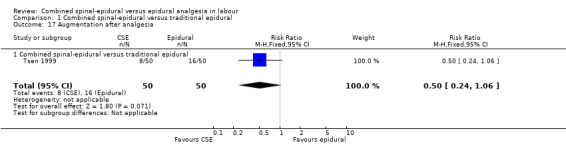
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 17 Augmentation after analgesia.
1.21. Analysis.
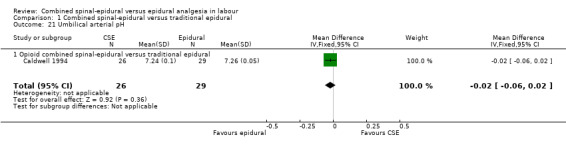
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 21 Umbilical arterial pH.
1.24. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 24 Apgar score < 7 at 5 minutes.
1.25. Analysis.
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 25 Apgar score < 8 at 5 minutes.
1.26. Analysis.
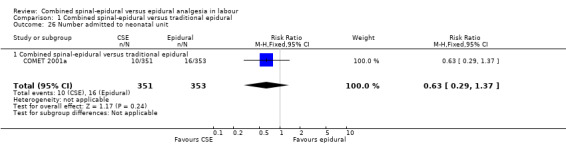
Comparison 1 Combined spinal‐epidural versus traditional epidural, Outcome 26 Number admitted to neonatal unit.
Comparison 2. Combined spinal‐epidural versus low‐dose epidural.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time from first injection to effective analgesia (minutes) | 5 | 461 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐7.26, ‐3.59] |
| 1.1 Combined spinal‐epidural versus low‐dose epidural | 4 | 421 | Mean Difference (IV, Random, 95% CI) | ‐5.73 [‐7.99, ‐3.48] |
| 1.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐4.24 [‐6.17, ‐2.31] |
| 2 Number of women with effective analgesia 10 minutes after first injection | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.49, 2.54] |
| 2.1 Combined spinal‐epidural versus low‐dose epidural | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.49, 2.54] |
| 3 Need for rescue analgesia | 9 | 1645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 3.1 Combined spinal‐epidural versus low‐dose epidural | 7 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.21] |
| 3.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 3.3 Null combined spinal‐epidural versus low‐dose epidural | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.64, 2.98] |
| 4 Number of women satisfied with analgesia | 7 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.98, 1.05] |
| 4.1 Combined spinal‐epidural versus low‐dose epidural | 6 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.97, 1.06] |
| 4.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.91, 1.10] |
| 5 Number of women who mobilise | 7 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.15] |
| 5.1 Combined spinal‐epidural versus low‐dose epidural | 5 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 5.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.01, 1.75] |
| 6 Post dural puncture headache | 9 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.42, 6.81] |
| 6.1 Combined spinal‐epidural versus low‐dose epidural | 7 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.50, 18.69] |
| 6.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 6.3 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Known dural tap | 6 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.16, 6.37] |
| 7.1 Combined spinal‐epidural versus low‐dose epidural | 4 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 14.14] |
| 7.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Null combined spinal‐epidural versus low‐dose epidural | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.18, 21.26] |
| 8 Number of women requiring blood patch for post dural puncture headache | 7 | 531 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.51, 9.64] |
| 8.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.85 [0.24, 97.11] |
| 8.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 8.3 Opioid combined spinal‐epidural versus low‐dose epidural | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.45 [0.27, 111.13] |
| 9 Pruritus | 11 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.22, 2.65] |
| 9.1 Combined spinal‐epidural versus low‐dose epidural | 9 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.13, 2.28] |
| 9.2 Opioid combined spinal‐epidural versus low‐dose epidural | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 10.53 [2.05, 53.99] |
| 10 Urinary retention | 4 | 964 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.35] |
| 10.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 930 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.55, 1.34] |
| 10.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.70, 2.98] |
| 11 Nausea/vomiting | 7 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.65, 1.45] |
| 11.1 Combined spinal‐epidural versus low‐dose epidural | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.56, 1.47] |
| 11.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.38, 2.48] |
| 11.3 Opioid combined spinal‐epidural versus low‐dose epidural | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.23, 22.01] |
| 12 Hypotension | 14 | 2040 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.89, 2.04] |
| 12.1 Combined spinal‐epidural versus low‐dose epidural | 12 | 1741 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.85, 2.96] |
| 12.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Null combined spinal‐epidural versus low‐dose epidural | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.71, 1.45] |
| 13 Respiratory depression | 5 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Headache (any) | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.70] |
| 14.1 Combined spinal‐epidural versus low‐dose epidural | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.70] |
| 15 Sedation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Labour augmentation required | 6 | 1285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 16.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 16.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.56, 4.28] |
| 16.3 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.61] |
| 16.4 Null combined spinal‐epidural versus low‐dose epidural | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 17 Augmentation after analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Normal delivery | 12 | 1672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.06] |
| 18.1 Combined spinal‐epidural versus low‐dose epidural | 8 | 1291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.09] |
| 18.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| 18.3 Opioid combined spinal‐epidural versus low‐dose epidural | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 18.4 Null combined spinal‐epidural versus low‐dose epidural | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.08] |
| 19 Instrumental delivery | 11 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 19.1 Combined spinal‐epidural versus low‐dose epidural | 7 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 19.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.51] |
| 19.3 Opioid combined spinal‐epidural versus low‐dose epidural | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Null combined spinal‐epidural versus low‐dose epidural | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.54, 3.03] |
| 20 Caesarean section | 15 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.16] |
| 20.1 Combined spinal‐epidural versus low‐dose epidural | 11 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 20.2 Opioid combined spinal‐epidural versus test local anaesthetic/opioid epidural | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.36, 4.14] |
| 20.3 Opioid combined spinal‐epidural versus low‐dose epidural | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.44, 2.53] |
| 20.4 Null combined spinal‐epidural versus low‐dose epidural | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.61, 2.52] |
| 21 Umbilical arterial pH | 4 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 21.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 264 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 21.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 22 Umbilical venous pH | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.00, 0.07] |
| 22.1 Combined spinal‐epidural versus low‐dose epidural | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 22.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.00, 0.08] |
| 23 Umbilical cord pH | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 23.1 Combined spinal‐epidural versus low‐dose epidural | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 24 Apgar score < 7 at 5 minutes | 6 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.59] |
| 24.1 Combined spinal‐epidural versus low‐dose epidural | 6 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.59] |
| 25 Apgar score < 8 at 5 minutes | 5 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.12] |
| 25.1 Combined spinal‐epidural versus low‐dose epidural | 4 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.33, 1.97] |
| 25.2 Opioid combined spinal‐epidural versus low‐dose epidural | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.14, 76.33] |
| 26 Number admitted to neonatal unit | 3 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.73] |
| 26.1 Combined spinal‐epidural versus low‐dose epidural | 3 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.73] |
2.13. Analysis.
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 13 Respiratory depression.
2.14. Analysis.
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 14 Headache (any).
2.23. Analysis.
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 23 Umbilical cord pH.
2.25. Analysis.
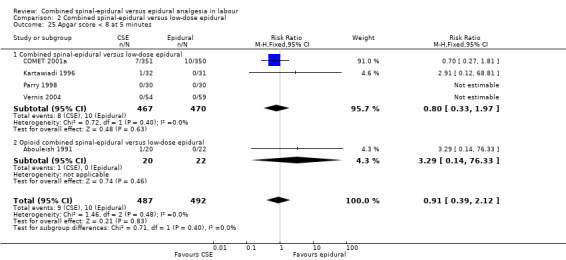
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 25 Apgar score < 8 at 5 minutes.
2.26. Analysis.
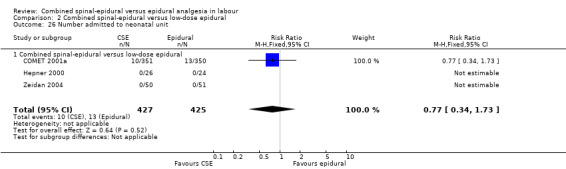
Comparison 2 Combined spinal‐epidural versus low‐dose epidural, Outcome 26 Number admitted to neonatal unit.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abouleish 1991.
| Methods | Randomisation: method not stated "62 women randomly divided into 3 groups". Blinding: investigators were not blinded to the group allocation. Participant, midwife, obstetrician and neonatologist were blinded. Criteria for rescue analgesia: if analgesia inadequate after 40 min 10 mL boluses 0.125% bupivacaine administered until pain relief achieved. Data from all participants used for all outcomes except caesarean section women who were excluded from analysis re duration and urinary catheterisation. |
|
| Participants | Inclusions: 62 women ASA 1 or 2 at term, with singleton cephalic fetus. Exclusions: none mentioned. No. lost to follow‐up: 0. NB: 13 women required caesarean section and these women were excluded from the comparison of urinary retention as they all had an indwelling catheter. |
|
| Interventions | Epidural (n = 22): bolus 10 mL bupivacaine 0.125%. Then as requested further 10 mL boluses given to maintain analgesia. CSE (n = 20): single space, needle‐through‐needle (18 G/26 G). IT injection morphine 0.2 mg, then epidural bolus 10 mL bupivacaine 0.125%. Then boluses of 10 mL bupivacaine 0.125% as requested. IT morphine group (n = 20): CSE technique as above, but only the IT morphine 0.2 mg given. The epidural catheter only used for additional analgesia on request, when 10 mL bupivacaine 0.125% was given as needed. (This arm was not used in the systematic review analysis.) |
|
| Outcomes | VAS pain (0 to 10) every 10 min for 1 hr, then every 20 min. Vital signs monitored at same time intervals as VAS. SpO2 monitored for 24 hr in all women. Mode of birth and duration of labour noted. Occurrence of respiratory depression, nausea/vomiting, pruritus and urinary retention noted. Number with post dural puncture headaches and treatment required recorded. Neonatal assessment by Apgar scores and cord blood gas analysis. | |
| Notes | USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated, "...patients randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Investigators were aware of group allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant, midwife, obstetrician and neonatologist were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all participants used for all outcomes except caesarean section women who were excluded from analysis re duration and urinary catheterisation. |
| Selective reporting (reporting bias) | Low risk | Only with respect to duration and urinary catheterisation for caesarean section women who were excluded from analysis. |
| Other bias | Low risk | No other bias evident. |
Abrao 2009.
| Methods | Randomisation: a computer‐generated random number series.
Allocation concealment: sealed opaque envelopes were generated by a person not related to the protocol and the anaesthetist received the next in a numbered series. Blinding: participant blinded; outcome assessor and obstetrician blinded to allocation. Criteria for rescue analgesia: not stated. Statistics were not performed on an intention‐to‐treat basis; of the 91 participants originally randomised, 14 were excluded from analysis, 8 in the control group and 6 in the treatment group (11 failure to maintain adequate CTG recording, 2 proceeding to vaginal birth in less than 30 minutes, 1 failed spinal); a further 12 did not have umbilical artery pH data. |
|
| Participants | Inclusions: 77 singleton, cephalic, full term, in active labour with cervical dilatation < 7 cm at time of epidural request. Exclusions: regional contraindicated, previous systemic opioids, prostaglandins for cervical ripening, amniotic infection, maternal or fetal medical conditions. | |
| Interventions | All women received 10 mL/kg crystalloid prior to epidural insertion. Epidural (n = 36): initial bolus 10 mL bupivacaine 0.125% + sufentanil 10 µg. After at least 20 minutes, with request for additional analgesia, boluses of bupivacaine of varying concentration were administered; 0.125% until 7 cm cervical dilatation, then 0.25% at 8 to 9 cm and 0.5% in second stage. CSE (n = 41): single space, needle‐through‐needle. IT injection bupivacaine 2.5 mg + sufentanil 2.5 µg. Subsequent epidural boluses as above. |
|
| Outcomes | Primary outcomes: prolonged fetal heart rate decelerations (decrease of 15 bpm for 2 to 10 minutes or baseline < 100) and increase uterine tone (10 mmHg) in the first 15 minutes after injection. Secondary outcome maternal hypotension (systolic < 100 mmHg or 20% fall). VAS (0 to 10) pain scores assessed; mean scores at 5, 10,15 and 20 minutes post‐injection. Intervention failures reported and percentage of caesarean births. Neonatal assessment by Apgar scores at 1 and 5 min and pH. | |
| Notes | Brazil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers is appropriate. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes allocated by independent person via a sequentially numbered series. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Management and outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of those originally randomised, 15% were not analysed; for neonatal pH this was 29%. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Bhagwat 2008.
| Methods | Randomisation: using a computer‐generated randomisation list. Blinding: progress of labour was assessed by an obstetrician who was blinded, observations were made by another anaesthetist who was not present at the time of procedure. |
|
| Participants | Inclusions: 60 nulliparous women in labour, cephalic, singleton with cervical dilatation 4 to 5 cm. Exclusions: cervical dilatation more than 5 cm, non vertex presentation, contraindication to neuraxial analgesia. No. lost to follow‐up: 1 (CSE group) went for emergency caesarean section. |
|
| Interventions | All women received 10 mL/kg crystalloid prior to epidural insertion. Epidural (n = 30): test dose 3 mL 1.5% lidocaine plus 15 µg of epinephrine, followed by 10 mL bupivacaine 0.062% plus 0.0002% fentanyl. After 10 minutes an epidural infusion of 0.0625% bupivacaine plus 0.0002% fentanyl was started using a patient controlled analgesia pump at a rate of 8 to 12 mL/hr and titrated to maintain sensory level of T10. CSE (n = 30): single space, needle‐through‐needle, sitting or lateral position. IT injection of 1.25 mg bupivacaine + fentanyl 25 µg. Then after 10 minutes an epidural infusion of 0.0625% bupivacaine plus 0.0002% fentanyl was started using PCEA pump at the rate of 8 to 12 mL/hr to maintain T10 sensory block. |
|
| Outcomes | Maternal: VAS pain < 3 at 30 minutes post block and the number of women requiring additional analgesia and those who were satisfied on a 4‐point scale. Number requiring caesarean birth and the occurrence of hypotension, nausea/vomiting and pruritus noted. Neonatal assessment by Apgar scores, cord blood gas analysis and need for admission to the neonatal unit. | |
| Notes | One still birth in the CSE group was excluded (cord around the neck). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Progress of labour was assessed by an obstetrician who was blinded, observations were made by another anaesthetist who was not present at the procedure and presumably blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All of the parturients were followed up. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Unclear risk | The study has not dealt with one still birth in the CSE group (cord around neck), excluded from study. |
Breen 1999.
| Methods | Randomisation: computer‐generated randomisation in blocks of 4 with allocation concealed in sealed envelopes. Blinding: procedure performed by an anaesthesiologist not involved in subsequent assessments. Both assessor and participant blinded to allocation. Criteria for rescue analgesia: not specifically stated. Statistics were not performed on an intention‐to‐treat basis. One woman in the epidural group was excluded due to loss of blinding, and data from this participant were not used in analysis. |
|
| Participants | Inclusions: 41 participants were initially enrolled into the study, all ASA class 1 or 2, at least 18 years old, with a singleton fetus with cervical dilatation < 6 cm. Exclusions: inability to give informed consent, allergy to study drugs and contraindication to regional blockade. No. lost to follow‐up: 1 woman in epidural group dropped from analysis due to loss of blinding. |
|
| Interventions | Epidural (n = 20): all women received 750 to 1000 mL crystalloid prior to epidural insertion. The initial epidural bolus was a 3 mL test dose of 1.5% lidocaine with 1:200,000 epinephrine. This was followed 3 min later with a bolus 100 µg fentanyl diluted to a volume of 10 mL with saline down the epidural catheter. The study period ended with the first request for additional analgesia. CSE (n = 21): single space, needle‐through‐needle. An intrathecal injection of sufentanil 10 µg diluted to 2 mL with saline. The epidural catheter was placed in the same manner as for the epidural group, but no drugs were given down it until additional analgesia was requested, at which point the study period ended. |
|
| Outcomes | Primary outcome was time from injection of narcotic until request for additional analgesia (duration of analgesia). VAS pain scores were assessed at 0, 5, 10, 15 and 30 min, and thereafter every 30 min. Motor block on Bromage scale was assessed at 30 min. Ability to walk and void urine was only assessed in those with full motor power. The incidence of itch was assessed by VAS scores. | |
| Notes | Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Used opaque, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, both women and the anaesthesiologist collecting the data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One woman in epidural group dropped from analysis due to loss of blinding. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported except for one epidural group patient. |
| Other bias | Low risk | No other bias evident. |
Caldwell 1994.
| Methods | Randomisation: not stated. "Patients were randomly assigned...".
Blinding: not mentioned at all anywhere in the paper.
Follow‐up request: "Randomisation was by computer‐generated random numbers. Sequentially numbered, sealed envelopes prepared by a non‐investigator. Participant and anesthesiologist‐investigator were blinded to treatment group; upon opening the sealed envelope, a non‐investigator anesthesiologist confidentially prepared the study drug for administration." Criteria for rescue analgesia: no specific mention of rescue analgesia per se. On request for additional analgesia, participants in the CSE group were given 10 mL bupivacaine 0.25% and then an infusion of bupivacaine 0.125% with fentanyl 10 µg/mL was started. Women in the epidural group received an infusion immediately after the initial bolus of bupivacaine 0.125% with sufentanil 100 µg/mL. No mention made of additional rescue analgesia. No participants were lost to follow‐up and data from all were included in the statistical analysis. |
|
| Participants | Inclusions: all women were ASA class 1 in labour with a singleton fetus. Exclusions: none stated. No. lost to follow‐up = 0. |
|
| Interventions | Epidural: 33 women received epidural analgesia, initiated with 10 mL bupivacaine 0.25% and sufentanil 10 µg. This was followed immediately by an infusion of bupivacaine 0.125% with sufentanil 0.2 µg/mL. CSE: 26 women had CSE analgesia initiated in a single space, needle‐through‐needle technique (18 G, 24 G Sprotte). An initial intrathecal injection of morphine sulphate 0.25 mg and fentanyl 25 µg. Nothing was given down the epidural catheter until a request for additional analgesia, at which time a bolus of 10 mL bupivacaine 0.25% was given and followed immediately by an infusion of bupivacaine 0.125% with fentanyl 1 µg/mL. |
|
| Outcomes | Maternal: blood pressure and heart rate recorded every 15 min until delivery. The occurrence of nausea/vomiting, pruritus, PDPH and respiratory depression was noted. Mode of delivery was noted as either "normal" or "operative", i.e. either caesarean or instrumental. Neonatal: Apgar scores and umbilical arterial and venous pH. |
|
| Notes | USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Upon opening the sealed envelope, a non‐investigator anaesthesiologist confidentially prepared the study drug for administration. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported. |
| Other bias | Low risk | No other bias evident. |
Cohen 2006.
| Methods | Parturients who requested epidural analgesia were randomised. | |
| Participants | Inclusions: 136 parturients who requested epidural analgesia for labour pain. Exclusions: none stated. No. lost to follow‐up: 5 CSE women were removed from the study following failure to pierce the dura ‐ and presumably were replaced to leave 68 in both groups. The data from the successful women were included. |
|
| Interventions | Epidural: 68 women received 20 mL ropivacaine 0.04% (8 mg) with adrenaline and sufentanil 1 µg/mL (20 µg) followed by PCEA using same solution with 4 mL bolus and 10 minute lockout plus background infusion of 4 mL/hr. CSE: 68 women received ropivacaine 2 mg with sufentanil 5 µg intrathecally followed by PCEA as above. Both groups received rescue of 0.25% ropivacaine from 20 minutes if VAS > 3. |
|
| Outcomes | Maternal: time from first injection to maximal analgesia; the number who mobilised; the occurrence of hypotension and pruritus and the need for bladder catheterisation. Fetal bradycardia during the fist 30 minutes after block was also noted (this is not an outcome for this review). Neonatal: Apgar scores (actual scores not available). |
|
| Notes | USA. Abstracts only: 2004, 2004 and 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Parturients "were randomized". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for the women who completed the study were available. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
COMET 2001a.
| Methods | Randomisation: using a customised randomisation programme provided by clinical trials experts. Blinding: participant not blinded. Assessor only blinded with respect to obstetric management. Criteria for rescue: in the traditional 0.25% bupivacaine (higher‐dose) group rescue given as fentanyl 50 µg or more concentrated bupivacaine. For CSE and low‐dose infusion (0.1% bupivacaine = fentanyl 2 µg/mL) a further 10 mL of this solution was used or 0.25% bupivacaine if necessary. Statistical analysis was performed on an intention‐to‐treat basis. Separate comparisons between mobile techniques and traditional group. |
|
| Participants | Inclusions: 1054 nulliparous women in labour. Exclusions: contraindication to epidural analgesia, previous epidural or spinal procedure, imminent delivery, injection of pethidine within the previous 4 hours. No. lost to follow‐up (post partum interview): 13. |
|
| Interventions | Epidural (n = 353): test dose 3 mL 2% lidocaine (60 mg) followed after 5 min with 10 mL bupivacaine 0.25%. Subsequent boluses of 10 mL bupivacaine 0.25% on request. Low‐dose infusion (n = 350): bolus of 15 mL bupivacaine 0.1% with fentanyl 2 µg/mL, followed by an infusion of the same at 10 mL/hr. CSE (n = 351): single space, needle‐through‐needle, sitting or lateral position. IT injection of 2.5 mg bupivacaine + fentanyl 25 µg. Then epidural boluses of 15 mL bupivacaine 0.1% + fentanyl 2 µg/mL on request. |
|
| Outcomes | Primary outcome measure mode of delivery. Secondary outcomes progress of labour (duration of first and second stages), oxytocin augmentation, regular pain assessments (VAS), women's perceptions of their ability to push and urinary retention. Neonatal assessments of Apgar scores at 1 and 5 min, resuscitation requirements and admission to the special care unit. Birthweight was recorded after delivery. |
|
| Notes | UK. Funded by grants from the NHS Research and Development Mother and Child Health Programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a customised randomisation programme. |
| Allocation concealment (selection bias) | Low risk | The study group code was not revealed before completion of recruitment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants and those in attendance were not blinded. Trial midwives assessing VAS 24 hours after delivery were blinded to obstetric management. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total number lost 13 (of 1054 randomised). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Cortes 2007.
| Methods | Randomisation: method not stated, "randomly divided into two groups". Blinding: not stated. |
|
| Participants | Inclusions: 40 women, singleton, term in labour at 4 to 5 cm dilatation. Exclusions: none stated. No. lost to follow‐up: 0 |
|
| Interventions | Epidural: 20 women received 8 mL bupivacaine 0.25% (20 mg) with adrenaline and fentanyl 100 µg followed by intermittent bolus of 4 mL bupivacaine 0.25% with adrenaline as required. CSE: 20 women received fentanyl 25 µg intrathecally followed by intermittent epidural boluses as above. Supplementation of 6 mL was available for both groups for delivery. |
|
| Outcomes | Maternal: VAS scores at 0, 5, 10, 15 and 20 minutes post initial injection; the number who mobilised; the occurrence of hypotension, pruritus, respiratory depression and nausea/vomiting. The mode of delivery was also noted. Neonatal: Apgar scores. |
|
| Notes | Brazil ‐ translated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated only, "randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the data are available. |
| Selective reporting (reporting bias) | Low risk | Outcomes are all reported. |
| Other bias | Low risk | No other bias evident. |
Dunn 1998.
| Methods | Randomisation: method not stated. "Patients were randomised into 2 groups." Blinding: outcome assessor blinded. Criteria for rescue analgesia: if inadequate analgesia after 20 min, 15 mL bupivacaine 0.125% given down epidural, followed by 10 mL lidocaine 2% if needed. If this was ineffective then catheter replaced and data excluded from analysis. Statistical analysis not performed on an intention‐to‐treat basis. |
|
| Participants | Inclusions: 70 healthy ASA 1 or 2 women in early labour (cervical dilatation < 5 cm). Exclusions: history of previous caesarean section or IV opioids before requesting epidural analgesia. No. lost to follow‐up: 1. |
|
| Interventions | Epidural (n = 35): test dose 3 mL lidocaine 1.5% + 1:200,000 epinephrine. Then bolus sufentanil 40 µg in 10 mL saline down epidural catheter. Study ended at request for additional analgesia. CSE (n = 34): single space, needle‐through‐needle, sitting. IT injection sufentanil 10 µg diluted to 1 mL. Epidural catheter sited but nothing administered down it until requested, at which point study ended. |
|
| Outcomes | VAS pain scores and severity of side effects assessed at 5, 10, 15, 20 and 30 min, and thereafter every 30 min. Maternal blood pressure, pulse and respiratory rate, and motor block were assessed at the same times. Time of request for additional analgesia noted and study ended. Mode of delivery noted and incidence of PDPH recorded. Neonate assessed by Apgar scores. | |
| Notes | USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated, "randomised into 2 groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated, "observations were made by an individual blinded to the analgesic technique". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported. |
| Other bias | Low risk | No other bias evident. |
Gomez 2001.
| Methods | Randomisation: method not stated. Blinding: stated as "single blinded" study but unclear as to specifically whether the outcome assessor was blinded to allocation. Criteria for rescue analgesia: when VAS pain score 3 or greater, 4 mL bolus bupivacaine 0.25% administered. Minimum 30 min between injections. Nil lost to follow‐up. |
|
| Participants | Inclusions: 42 ASA 1 or 2 women in spontaneous labour with a singleton, vertex presentation fetus. Cervical dilatation 2 to 5 cm. Exclusions: obstetric pathology, meconium stained liquor, ruptured membranes, previous caesarean section. No. lost to follow‐up = 0. |
|
| Interventions | Epidural (n = 21): test dose 3 mL bupivacaine 0.25% + adrenaline 1:200,000 followed by 5 mL of the same solution. Immediate infusion of bupivacaine 0.125% with fentanyl 1 µg/mL at 8 mL/hr. CSE (n = 21): sitting, otherwise technique not stated. IT injection of bupivacaine 2.5 mg + 25 µg fentanyl + adrenaline 1:200,000. Once VAS of 3 to 4, 8 mL bupivacaine 0.125% + adrenaline 1:200,000 and an infusion of the same solution and rate as the epidural group. |
|
| Outcomes | Maternal: VAS pain scores at 5, 10, 15 and 30 min and then hourly. At the same time intervals sensory block to pinprick and motor block (Bromage scale) were assessed. Number of additional rescue analgesia boluses was noted. VAS maternal satisfaction recorded at delivery. Presence of arterial hypotension (decrease from baseline of greater than 20%) was documented. Maternal bradycardia (rate less than 60 bpm) was noted. Adverse effects noted: nausea and vomiting, pruritus. Mode of delivery recorded as normal, instrumental or caesarean. Neonatal: occurrence of fetal bradycardia less than 100 bpm noted. No recorded neonatal assessments. |
|
| Notes | Spanish (translated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were divided randomly into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated, except "prospective, randomised, blinded study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are stated. |
| Other bias | Low risk | No other bias evident. |
Goodman 2006.
| Methods | Randomisation: computer‐generated randomisation table, placed in opaque envelope. Blinding: the women and the nurses caring for the patient and the outcome investigator were blinded. Criteria for rescue analgesia: if after 15 minutes post epidural or spinal dose still had inadequate pain relief, 5 mL bolus of bupivacaine 0.25% was administered via the epidural catheter. |
|
| Participants | Inclusions: 100, ASA 1 to 2, parous (1 or more prior vaginal delivery) women at term in early labour (< 5 cm cervical dilatation). Exclusions: women with severe scoliosis, BMI > 45, any contraindication to neuraxial analgesia, or were taking other pain medication. No. excluded: 16 (9 protocol violations which included 5 in CSE group and 4 in epidural group. 2 women had missing data (1 in each group), 2 spinal anaesthetics resulted in only partial drug administration (CSE group) and 3 epidural catheters failed. |
|
| Interventions | Epidural (n = 41) 3 mL test dose of 0.25% bupivacaine followed 5 min later by 10 mL of bupivacaine 0.125% with fentanyl 50 µg. CSE (n = 43): single space, needle‐through‐needle, 17 gauge Touhy needle and 27 gauge Whitacre needle, intrathecally bupivacaine 2.5 mg and fentanyl 25 µg. Both groups were commenced on a continuous infusion of bupivacaine 0.0625% with fentanyl 2 µg/mL at 12 mL/hr within 15 min of administration of the spinal or epidural dose. |
|
| Outcomes | Maternal: VAS (0 to 10) pain scores at 10 and 30 minutes post procedure and the number with VAS > 3 at 10 minutes; hypotension, pruritus, number requiring caesarean section. Maternal satisfaction with analgesia recorded. Total failures of primary technique were also documented. No neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated, "using computer generated randomisation table". |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used for allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The subject and the investigator were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers were not analysed from both groups ‐ a total of 16 of 100. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes are available. |
| Other bias | Low risk | No other bias evident. |
Hepner 2000.
| Methods | Randomisation: method not stated "randomised to either technique".
Follow‐up request: "1. randomisation: random‐number table, 2. allocation concealment: sequentially‐numbered, sealed, opaque envelopes, 3. blinding: the resident performing the technique was aware of the group assignment but the staff evaluating the outcome was not". Blinding: mother and outcome assessor blinded. Criteria for rescue analgesia: inadequate analgesia at 20 min treated with 13 mL bupivacaine 0.0625% + fentanyl 2 µg/mL + 0.05 mL sodium bicarbonate 8.4% + epinephrine 1:200,000. Data from all participants analysed as no loss from study. |
|
| Participants | Inclusions: "healthy term parturients". Exclusions: pregnancy‐induced hypertension, diabetes, preterm labour, bleeding problems, scoliosis, cervical dilatation > 5 cm, previous IV opioid. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 24): 16 mL bupivacaine 0.0625% + fentanyl 2 µg/mL + 0.05 mL NaHCO3 8.4% + epinephrine 1:200,000 (BFSE). At request for additional analgesia further bolus 13 mL and infusion started of bupivacaine 0.0625% + fentanyl 2 µg/mL + epinephrine 1:400,000 at 10 mL/hr. CSE (n = 26): single space, needle‐through‐needle, sitting. IT LA bupivacaine 2.5 mg + fentanyl 25 µg. On request for additional analgesia 13 mL BFSE (see above) and infusion commenced at 10 mL/hr as above. |
|
| Outcomes | Maternal blood pressure, heart rate and haemoglobin saturation, and fetal heart rate and uterine activity monitored throughout labour. VAS pain scores. Parturient satisfaction at delivery and day 1 postpartum. Motor block at 15 and 30 min. Times: infiltration ‐ catheter taping ‐ initial analgesia ‐ request for additional analgesia. Need for supplemental analgesia, treatment of hypotension, pruritus, nausea/vomiting. Hypotension defined as reduction in systolic blood pressure to < 100 mmHg or reduction of > 30% from baseline. Fetal heart rate changes. Neonatal Apgar scores, umbilical arterial and venous pH. | |
| Notes | USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used. |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered, sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Another anaesthesiologist, blinded to the technique, was in charge of collecting the data". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 in each group delivered before initial block wore off and not included in duration analysis. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported. |
| Other bias | Low risk | No other bias evident. |
Kartawiadi 1996.
| Methods | Randomisation: method not stated "63 ASA class 1‐3 parturients...randomly assigned...".
Blinding: outcome assessor blinded to allocation.
Follow‐up request 2006: "randomisation was done with randomisation tables and the sequence of allocation was put in separate numbered envelopes. After the procedure these were resealed and kept by the third author until the end of the study". Criteria for rescue analgesia: not stated. No loss of participants from the study so all included in statistical analysis. NB 13 delivered before requesting additional analgesia and so were not included in statistics for duration of analgesia. |
|
| Participants | Inclusions: 63 ASA 1‐3, singleton, vertex at 36‐41 weeks' gestation. In active labour with cervical dilatation < 5 cm at time of epidural request.
Exclusions: history of hypertension or pre‐eclampsia. No. lost to follow‐up: 13 for duration data, 0 for all other parameters. |
|
| Interventions | Epidural (n = 31): initial bolus 10 mL bupivacaine 0.125% + sufentanil 10 µg + epinephrine 12.5 µg. On request for additional analgesia 10 mL boluses of same were delivered down the catheter. CSE (n = 32): single space, needle‐through‐needle, lateral. IT injection bupivacaine 1.0 mg + sufentanil 5 µg + epinephrine 25 µg. Immediate epidural bolus 10 mL saline given. On request for additional analgesia, same 10 mL bolus given as for the epidural group (see above). |
|
| Outcomes | Maternal blood pressure and ECG monitoring. VAS (0 to 10) pain scores assessed. Time to VAS < 2.5 or < 50% baseline taken as onset. Total dose of local anaesthetic required and adverse effects. Sensory and motor block assessed post injection. Maternal satisfaction with analgesia recorded. Neonatal assessment by Apgar scores at 1 and 5 min. | |
| Notes | Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used randomisation tables. |
| Allocation concealment (selection bias) | Low risk | Used separate sealed numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The investigating anaesthesiologist was different to the one performing the block. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 (of 63 randomised; 7 in one and 6 in the other group) delivered before requesting additional analgesia and were not included in duration analysis; all others included. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported. |
| Other bias | Low risk | No other bias evident. |
Medina 1994.
| Methods | Randomisation, allocation concealment, blinding and assessment bias are all unclear. | |
| Participants | Inclusions: 28 women 3 to 5 cm dilatation, primigravid. Exclusions: none stated. No. lost to follow‐up: 2, 1 complete failure in each group. | |
| Interventions | Epidural (n = 12): initial bolus 10 mL 0.125% bupivacaine, plus 10 µg sufentanil. CSE: 3.75 mg bupivacaine plus 3 µg sufentanil. Both groups given ED infusion after reappearance of pain: 7 to 10 mL/hour of 0.125% bupivacaine plus 0.5 µg/mL sufentanil. | |
| Outcomes | Maternal: VAS pain scores at 5, 10, 30, 60, 75, 90, 120, 150, 180, 210, 240 minutes. Adverse effects; itch, vomiting, hypotension, high block, bradycardia, reflex sympathetic pain. | |
| Notes | Italy. Poster from ASRA meeting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One in each group lost to follow‐up (one in each group was excluded for complete failure). |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not stated. |
| Other bias | Low risk | No other bias evident. |
Ngamprasertwong 2007.
| Methods | Randomisation: "a sealed‐envelope technique". Blinding: single‐blinded: obstetric residents and labour nurses managed the labours according to standardised protocols and were unaware of the anaesthetic administration. Rescue analgesia 10 mL of 0.25% bupivacaine if inadequate analgesia after 20 min of initial analgesia. No losses to follow‐up and all were included in statistical analysis. |
|
| Participants | Inclusions: 50 women, nulliparous and multiparous, healthy, full term, in labour. Exclusion: twins, pregnancy induced hypertension, placenta praevia, regional contraindication. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 25): crystalloid preload (quantity not stated). Test dose 3 mL of 0.25% bupivacaine plus bolus of 7 mL of 0.25% bupivacaine. Then infusion of 0.0625% bupivacaine with fentanyl at 12 mL/hr started immediately. CSE (n = 25): crystalloid preload. Single space, needle‐through‐needle, using Quicke 27 G needle. 25 µg of fentanyl injected intrathecally. Epidural catheter was inserted and 3 mL of 0.25% bupivacaine given as test dose, 0.0625% bupivacaine with fentanyl 2 µg/mL was infused at 12 mL/hr. |
|
| Outcomes | Primary: onset of effective analgesia. Secondary: motor blockade, mode of delivery and side effects. | |
| Notes | Quinke needles used (27G). Thailand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of the actual randomisation method. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Labour attendants were unaware but assessors were not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Nickells 2000.
| Methods | Randomisation: sealed envelopes. Blinding: participant and midwife blinded to allocation. Assessment of motor block and proprioception made by an unblinded anaesthetist. Criteria for rescue analgesia: any participants with persistently painful contractions at 30 min were treated at the discretion of the anaesthetist to ensure adequate analgesia. Unclear if statistical analysis was performed on an intention‐to‐treat basis. |
|
| Participants | Inclusions: 142 women in established labour, gestation at least 36 weeks, singleton, cephalic fetus. Exclusions: pethidine within 4 hours of requesting epidural analgesia. No. lost to follow‐up: there were 18 analgesic failures (9 in each group) but were included in analysis for outcomes other than time to effective analgesia. |
|
| Interventions | Epidural (n = 73): initial bolus 10 mL bupivacaine 0.125% + fentanyl 50 µg. Further boluses 10 mL bupivacaine 0.1% + fentanyl 2 µg/mL given on request for additional analgesia. CSE (n = 69): single space, needle‐through‐needle, sitting. IT injection bupivacaine 2.5 mg + fentanyl 25 µg. Additional analgesia provided on request as for the epidural group. |
|
| Outcomes | Analgesia onset measured as time to first "comfortable" contraction. Maternal blood pressure every 5 min for 20 min (hypotension defined as decrease in SBP < 100 mgHg). Continuous fetal heart monitoring with fetal bradycardia < 100 bpm noted. At 30 min assessment of motor block and proprioception. | |
| Notes | UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Midwife who assessed the speed of onset and pain was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 18 analgesic failures (9 in each group); all other data outcomes complete. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Unclear risk | Study stopped after 3 months so slightly uneven group sizes. |
Parry 1998.
| Methods | Randomisation: method not stated. "patients....were randomly allocated...". Blinding: assessments made by an anaesthetist blinded to group allocation and treatment. Criteria for rescue analgesia: not mentioned. No participants were lost to follow‐up and data from all participants were included in analysis. |
|
| Participants | Inclusions: ASA 1 or 2 women at term requesting analgesia in the first stage of labour or elective LSCS under regional.
Exclusions: pre‐existing neurological impairment or diabetes mellitus. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 30): all women were preloaded with 500 to 1000 mL crystalloid prior to epidural insertion while sitting. Initial bolus 15 mL bupivacaine 0.1% with fentanyl 2 µg/mL. CSE (n = 30): fluid preloading as for the epidural group. Single space, needle‐through‐needle, sitting. Intrathecal injection of bupivacaine 2.5 mg + fentanyl 25 µg in a total volume 2.5 mL. Epidural catheter placed for subsequent analgesia (after study period). |
|
| Outcomes | 'Routine' measurement of maternal and fetal heart rates and maternal blood pressure were performed in all groups. (Hypotension was defined as a decrease in systolic blood pressure to < 100 mmHg or of > 20% from baseline.)
Assessment of sensory and motor block and dorsal column modalities was performed 20 to 30 min after initial injection. Normal delivery rate was recorded in each group. Assessment was made for spinal headache and neurological complications. LSCS group was analysed as a separate subgroup, enabling comparison of CSE and ED subgroups. Neonatal assessment was by Apgar scores at 5 min. |
|
| Notes | UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated: "patients were randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported. |
| Other bias | Low risk | No other bias evident. |
Patel 2003a.
| Methods | Randomisation: method not stated, "...prospective, double‐blind study and randomised...". Blinding: stated to be "double‐blind". Criteria for rescue: not stated. | |
| Participants | Inclusions: 115 healthy women, 2 to 6 cm dilatation requesting regional. Exclusions: not stated. 2 lost to CTG analysis. | |
| Interventions | Epidural (n = 53): all women received initial bolus 20 mL bupivacaine 0.1% + fentanyl 40 µg. CSE: all women received intrathecal bupivacaine 2.5 mg + fentanyl 5 µg. Subsequent management: not stated. | |
| Outcomes | Maternal: mode of delivery (no results stated). Fetal: umbilical artery pH and base excess; Apgars at 1 and 5 min; CTG abnormalities. | |
| Notes | UK. Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated, "double blind study and randomised", no other information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated to be double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to CTG analysis. |
| Selective reporting (reporting bias) | Unclear risk | Reporting of detail of outcomes is unclear. |
| Other bias | Low risk | No other bias evident. |
Price 1998.
| Methods | Randomisation: sealed and numbered envelopes. Blinding: mother and outcome assessments. Criteria for rescue: additional analgesia delivered as 10 mL bupivacaine 0.25%. Statistics not performed on intention‐to‐treat basis. |
|
| Participants | Inclusions: 100 women in labour cervix < 6 cm dilated. Exclusions: pethidine < 3 hr before epidural request, pregnancy‐induced hypertension. No. lost to follow‐up = 7. | |
| Interventions | Epidural (n = 48): 15 mL bupivacaine 0.1% + fentanyl 75 µg bolus then PCEA 10 mL bupivacaine 0.1% + fentanyl 2 µg/mL with 30 min lockout. CSE (n = 45): single space, needle‐through‐needle, lateral position, IT LA: 2.5 mg bupivacaine + 25 µg fentanyl then PCEA 15 mL 0.1% bupivacaine + fentanyl 2 µg/mL, 30 min. |
|
| Outcomes | Pain scores and motor block at 0, 30, 60, 180 min. Maternal confidence in walking. Time to first epidural top‐up and need for additional analgesia. Adverse effects: hypotension (no definition given), pruritus, need for urinary catheterisation. Maternal satisfaction postpartum #1. | |
| Notes | UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | Used "sealed, numbered envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The patient and the investigator were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up and drop out was similar in both groups (7 in total). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported. |
| Other bias | Low risk | No other bias evident. |
Roux 1999.
| Methods | Randomisation: by drawing lots. Blinding: states "double blinded for all assessments not involving problems at performance of block". Criteria for rescue: not mentioned. Data not analysed on an intention‐to‐treat basis. (NB all participants' data included for complications on insertion.) |
|
| Participants | Inclusions: 80 women between 37 and 42 weeks' gestation, in active labour with cervical dilatation not more than 3 cm. All with singleton, cephalic fetus. Exclusions: any contraindication to CSE or epidural. No. lost to follow‐up: 1 participant not included as failure to site CSE successfully. Data used only in analysis regarding complications at insertion. |
|
| Interventions | Epidural (n = 40): all given 500 mL crystalloid prior to insertion of epidural in the sitting position. Initial bolus given down the catheter of 6 to 8 mL bupivacaine 0.25% with sufentanil 20 µg. Top‐up boluses were delivered on request of 6 to 8 mL bupivacaine 0.25%. CSE (n = 39): sitting, single space, needle‐through‐needle (18 G, 29 G). Initial bolus IT sufentanil 10 µg in total volume 3 mL with isotonic saline. Top‐up bolus given down epidural catheter when requested, as 6 to 8 mL bupivacaine 0.25% in increments of 2 to 3 mL. |
|
| Outcomes | Pain scores assessed on VAS 0 to 10 at injection, then 5, 10, 15, 20, 30 min and thereafter every 30 min. Complete analgesia was defined as a VAS score = 0. Time from initial bolus to first top‐up request was noted as bolus duration. The incidence of complications at time of insertion was recorded. Maternal systolic blood pressure and oxygen saturation were recorded. (Hypotension was defined as SBP of 90 mmHg or lower and was corrected with IV fluid bolus.) Continuous cardiotocograph recording was taken throughout the labour. The duration of first and second stages of labour were noted and the mode of delivery. The occurrence of itch, nausea, vomiting and sedation was noted at the same times as VAS pain. Incidence of headache was assessed on the third postpartum day. Neonatal assessment by Apgar scores at 1 and 5 min. |
|
| Notes | France (translated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded or single‐blinded if problem in performing the block. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant not included as failure to site CSE successfully. Data used only in analysis regarding complications at insertion. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Sezer 2007.
| Methods | Randomisation: "prospectively randomized" using closed envelope allocation into 2 groups. Blinding: not stated. |
|
| Participants | Inclusions: 40 nulliparous women ASA 1 at term with singleton, cephalic fetus, in spontaneous labour with cervical dilatation less than 6 cm. Exclusions: none stated. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 20): test dose of 3 mL of lignocaine 1.5% plus adrenaline, then 7 mL bolus of 0.1% bupivacaine plus fentanyl 50 µg. CSE (n = 20): intrathecal dose of fentanyl 20 µg in 1.5 mL of saline plus epidural test dose of 3 mL of 1.5% lignocaine plus adrenaline after 45 minutes. Both groups were maintained with PCEA of 5 mL bolus of bupivacaine 0.1% plus fentanyl 2 µg/mL on demand with lockout of 10 minutes and maximum of 15 mL in 1 hour. |
|
| Outcomes | Maternal: time from injection to VAS (0 to 10) pain score of less than or equal to 3. Maternal satisfaction with analgesia and number with pruritus and nausea and vomiting; mode of delivery. Neonatal outcomes: mean Apgar score at 5 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospectively randomized". |
| Allocation concealment (selection bias) | Low risk | "Closed envelope allocation". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported. |
| Other bias | Low risk | No other bias evident. |
Skupski 2009.
| Methods | Randomisation: used web‐based randomisation software for 200 women divided into 2 blocks of 100 without stratification. Allocation concealment using opaque, sequentially numbered, sealed envelopes kept off‐site. Blinding: no blinding of subjects or investigators. Statistical analysis was performed on an intention‐to‐treat basis. |
|
| Participants | Inclusions: 127 women, 18 to 50 years old, term, cephalic, singleton, BMI < 40, with or without induction. Exclusions: morbid obesity, non reassuring fetal heart rate pattern, planned caesarean, hypertension for any reason, significant obstetric medical condition. No. lost to follow‐up: 0. |
|
| Interventions | All women received 1 litre of intravenous fluid 30 to 40 min before block. Epidural (n = 63): 15 mL of bupivacaine 0.0625% plus fentanyl 2 µg/mL. CSE (n = 64): 1 mL of 0.25% bupivacaine plus fentanyl 20 µg intrathecally. Both groups received immediate infusion of bupivacaine 0.0625% plus fentanyl 2 µg/mL at 12 mL/hr. |
|
| Outcomes | Primary outcome was prolonged deceleration of fetal heart rate and secondary outcomes of fetal heart rate changes (which are not outcomes for this review). Other maternal: VAS pain scores (1 to 10) every 2 minutes for 10 minutes after block placement, then every 5 minutes for 20 minutes, then every 10 minutes for 30 minutes; hypotension, pruritus, nausea and vomiting, back pain and urinary retention; number requiring caesarean section. No neonatal outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used website random number generator. |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered, sealed, opaque envelopes unavailable to those doing recruitment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of the type of neuraxial block was not performed for either subjects or investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Unclear risk | Study is underpowered to detect the difference in fetal heart rate changes. |
Thomas 2005.
| Methods | Randomisation: computerised random‐number generator. Blinding: outcome assessments blinded. Criteria for rescue: top‐ups of 5 mL 0.25% bupivacaine to 15 mL, then ED catheter withdrawn 1 to 2 cm increments and then replaced. | |
| Participants | Inclusions: 251 healthy, in labour, < 6 cm dilatation, uncomplicated pregnancies, requesting analgesia. Exclusions: not stated. Number lost to follow‐up: 21, 20 from CSE group, 1 from ED group. A subgroup was generated for analysis from 18 CSE participants where no CSF obtained. | |
| Interventions | Epidural (n = 124): all given initial 10 mL divided dose 2% plain lignocaine via ED catheter, then immediately commenced on PCEA. CSE (n = 127): dural puncture with 27 G Whitacre with nil intrathecal drugs; 10 mL lignocaine as per ED group, followed immediately by PCEA as per ED group. PCEA: bupivacaine 0.11% + fentanyl 2 µg/mL at 10 mL per hour with 5 mL bolus and 10 minute lock‐out. |
|
| Outcomes | Maternal: additional interventions, known dural tap, hypotension, labour augmentation, mode of delivery. | |
| Notes | USA. Institutional funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated, "computerized random number generator". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor and patient were blinded; unblinded operator performing the procedure recorded the initial parameters. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 women were excluded (2 because of inadvertent dural puncture and 18 because no CSF return from spinal needle). These 18 women were analysed in another subgroup for separate data analysis and the data are available. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Tsen 1999.
| Methods | Randomisation: sealed and numbered envelopes. Blinding: both participant and outcome assessor blinded. Rescue analgesia as either 6 mL bupivacaine 0.25% or fentanyl 50 µg in 10 mL saline. No losses to follow‐up and all were included in statistical analysis. |
|
| Participants | Inclusions: 100 women, nulliparous, ASA 1 or 2, at term, in spontaneous labour with singleton, cephalic fetus. Cervical dilatation < 5 cm. Exclusion: cervical dilatation > 5 cm. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 50): 1000 mL crystalloid preload. "Dummy" spinal (no dural puncture) before placement of catheter. Bolus 12 mL bupivacaine 0.25% then infusion bupivacaine 0.125% + fentanyl 2 µg/mL at 10 mL/hr. CSE (n = 50): 1000 mL crystalloid preload. Single space, needle‐through‐needle, lateral position. IT injection bupivacaine 2.5 mg + sufentanil 10 µg. When requested, epidural bolus given as 6 mL bupivacaine 0.25% then infusion of bupivacaine 0.125% + fentanyl 2 µg/mL at 10 mL/hr. |
|
| Outcomes | VAS pain scores, sensory level (pin prick) and motor block assessed at onset of analgesia, 60 min and then at 90 min intervals. Incidence of hypotension (no definition), nausea and pruritus noted. Data on labour progress including cervical dilatation, use and maximum dose of oxytocin and mode of delivery. Neonatal assessment of weight, gender and Apgar scores. |
|
| Notes | USA. Funded solely from institutional and departmental sources. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated, "sequentially numbered, opaque, shuffled envelopes", but randomisation method not stated. |
| Allocation concealment (selection bias) | Low risk | Stated, "sequentially numbered, opaque, shuffled envelopes". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, in epidural group the spinal needle placed without puncturing the dura and "dosed" with an empty syringe to blind any observers to the technique being used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Van de Velde 1999.
| Methods | Randomisation: method not stated "110 healthy women....participated in this prospective and randomised clinical trial". Blinding: both mother and outcome assessor were blinded. Criteria for rescue analgesia: if pain relief inadequate (VAS > 25 mm) PCEA lockout time reduced from 15 min to 10 min and additional epidural boluses given manually as needed. No losses to follow‐up and data from all participants were used in statistical analysis. |
|
| Participants | Inclusions: 110 women ASA 1 or 2, > 36 weeks with singleton, vertex fetus. Cervical dilatation 2 to 7 cm. No other sedative or analgesic drugs given. Exclusions: VAS < 60 mm at analgesia request. Substance‐abusing parturients were excluded. No. lost to follow‐up: 0. |
|
| Interventions | Epidural (n = 55): 15 mL/kg crystalloid preload over 15 min. Bolus 10 mL bupivacaine 0.125% + sufentanil 0.75 µg/mL + epinephrine 1.25 µg/mL. Then PCEA connected at first request for additional analgesia. PCEA bolus 4 mL of same solution with lockout time 15 min. CSE (n = 55): 15 mL/kg crystalloid preload given over 15 min. Single space, needle‐through‐needle, lateral position. IT injection bupivacaine 2.5 mg + sufentanil 1.5 µg + epinephrine 2.5 µg. Then immediately 10 mL saline administered down epidural catheter. On request for additional analgesia, PCEA connected as for the epidural group. |
|
| Outcomes | Maternal heart rate, blood pressure and VAS for pain recorded. Onset time (VAS < 25 mm or reduced by > 50% from baseline). Incidence of side effects noted. Hypotension defined as reduction of mean arterial pressure of > 20%. Sensory and motor block assessed. Mode of delivery and fetal heart rate changes recorded. Maternal satisfaction noted. Neonatal assessment by Apgar scores at 1 and 5 min and umbilical artery pH. | |
| Notes | Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both women and outcome assessor were blinded (second anaesthetist entered the room after the completion of the puncture and was unaware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
Vernis 2004.
| Methods | Randomisation: method not stated, "a prospective double‐blind randomised study" and "parturients were randomly included into 1 of 2 study groups, in a blind fashion." Blinding: midwife performing analgesia assessments and women both blinded. |
|
| Participants | Inclusions: 113 healthy women 18 to 40 years, 3 to 6 cm dilatation in active labour, > 37 weeks' gestation, singleton, vertex. Exclusions: contraindications to regional, pre‐eclampsia, psychological, IV opioid administration, labour 'unduly complicated', after‐hours. No. lost to follow‐up: 1 from ED group. |
|
| Interventions | Epidural (n = 60): 0.125% bupivacaine plus sufentanil 7.5 µg, with adrenaline, volume based on height, 4 mL initially as test dose. 5 mL 0.125% bupivacaine given if analgesia inadequate at 15 min. PCEA commenced immediately. CSE (n = 54): needle‐through‐needle with 2.5 mg bupivacaine plus 5 µg sufentanil. Test dose and initial ED followed by PCEA after return of pain. PCEA: bupivacaine 0.125% plus sufentanil 0.25 µg/mL; 4 mL bolus with 10 minute lock‐out. |
|
| Outcomes | Maternal: median time from injection to VAS (0 to 100) less than or equal to 30, satisfaction, dural tap, PDPH and blood patch requirement, major complications, mode of delivery. | |
| Notes | France. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Stated, "parturients were randomly included into one of the two study groups in a blind fashion." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated, "prospective double blind study," no other information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient excluded from epidural group, no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Unclear risk | The study was stopped early (after 113 women rather than 128 that were originally intended because of presumed incidence of adverse effects from CSE). |
Zeidan 2004.
| Methods | Randomisation: random number table; "randomised in a double blinded manner". Blinding: not stated. |
|
| Participants | Inclusions: 104 women, ASA 1 to 2, requesting epidural, singleton, nulliparous, 36 to 41 weeks' gestation, < 4 cm dilatation. Exclusions: multiple pregnancies, estimated fetal weight < 2500 gm, ED contraindicated, suspected fetal abnormalities, IV analgesics within the previous hour. No. lost to follow‐up: 3 in CSE group due to protocol violation; 1 in ED group withdrew. |
|
| Interventions | Epidural (n = 51): 1000 mL Ringer's preload, left lateral; 10 to 20 mL 0.0625% (6.25 to 12.5 mg) bupivacaine plus fentanyl 15 to 30 µg. CSE (n = 50): preload as above: 1.25 mg bupivacaine plus fentanyl 25 µg. Subsequent management same in both groups: ED infusion commenced subsequently at patient request of bupivacaine 0.0625% plus fentanyl 1.5 µg/mL, initial 3 mL bolus followed by 6 to 10 mL/hr. |
|
| Outcomes | Maternal: VAS pain score at 0, 5, 10, 15 and 30 minutes; additional analgesic interventions required, satisfaction, mobilisation, known dural tap, PDPH and blood patching; adverse events needing treatment ‐ hypotension, respiratory depression, nausea and vomiting, pruritus; major complications, mode of delivery. Fetal/neonatal: number admitted to the neonatal unit. |
|
| Notes | Saudi Arabia. Table 4 not in published paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated, "using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated, "double blinded", no other information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women from CSE group were excluded because of protocol deviation, 1 patient from the epidural group chose to discontinue her participation. No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were reported. |
| Other bias | Low risk | No other bias evident. |
ASA: American Society of Anesthesiologists BFSE: mixture of bupivacaine, fentanyl, sodium bicarbonate and epinephrine BMI: body mass index bpm: beats per minute cm: centimetre CSE: combined spinal‐epidural CSF: cerebrospinal fluid CTG: cardiotography ED: epidural hr: hour IJOA: International Journal of Anesthesiology IT: intrathecal IV: intravenous LA: local anaesthetic LSCS: lower segment caesarean section µg: microgram mg: milligram min: minute mL: millilitre NaHCO3: sodium bicarbonate no.: number PCEA: patient‐controlled epidural analgesia PDPH: post dural puncture headache SBP: systolic blood pressure SpO2: saturation of oxygen VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Backus 1996 | There were treatment differences within the groups depending on the degree of cervical dilatation more or less than 5 cm. |
| Camann 1992 | All 24 women in this study had an intrathecal, an epidural and an intravenous injection. Only 1 of the 3 injections contained the active drug, namely sufentanil 10 µg, with the other 2 injections containing saline. The route of sufentanil delivery depended on the group allocation. As all women received a CSE technique, it was felt this did not constitute a direct comparison of CSE with epidural and, therefore, this study was excluded from the review. |
| Camann 1998 | All 100 women entered into the study received an intrathecal injection. In 6 of the 9 groups the intrathecal injection contained sufentanil 2, 5 or 10 µg. The remaining 3 groups received an injection of saline 2 mL. All 100 women also received an epidural bolus containing either bupivacaine or saline. Although the inclusion of groups receiving only saline in the intrathecal injection allowed for complete double‐blinding, it led to all women technically receiving a CSE. As a result it was decided that this was not therefore a comparison of CSE with epidural analgesia and the study was excluded. |
| Cascio 1996 | This study looked only at maternal catecholamine levels and did not address any of our outcomes. |
| Collis 1995 | Although this study was described as randomised and blinded, the women receiving CSE analgesia were assessed for motor weakness and in the absence of motor block were allowed to walk if they wished. Women in the epidural group were not assessed in this way and were not encouraged to mobilise. |
| Collis 1999a | All women received CSE analgesia. |
| Collis 1999b | All women received CSE analgesia. |
| Cooper 2010 | Although this is one of the COMET cluster of reports, this study specifically deals with comparisons involving low‐dose epidurals with traditional epidurals and hence is not relevant to this review. |
| D'Angelo 1994 | All women in the 'epidural' group received an intrathecal injection of saline 2 mL enabling both mother and operator to be blinded to treatment group allocation. However, this means that all women in the trial technically received a CSE and so this study was excluded from analysis. |
| Dresner 1999 | All data relating to motor block and analgesic efficacy were collected retrospectively and took the form of subjective maternal assessments on the first day postpartum. Intrapartum assessments were made by midwives not blinded to treatment allocation. Assessments by the women were potentially biased as subjects were not blinded either. In addition, 50 women withdrawn from the study for a variety of reasons were excluded from all statistical analysis. |
| Finegold 2003 | Abstract only. Methodological quality unclear in relation to randomisation, allocation concealment, blinding and attrition. No data presented against stated review outcomes. |
| Fogel 1999 | Excluded as treatment not the same for all participants within each group. Boluses of drugs given differed according cervical dilatation. |
| Groves 1995 | Only addressed the rate of progress of labour which was not one of outcomes. No other information presented is able to be analysed against our outcomes. |
| Harsten 1997 | All women received a CSE. |
| Kassapidis 1997 | Study looked only at umbilical cord blood flow which was not one of outcomes. |
| Leighton 1996 | Women were not randomised for group allocation. CSE or epidural analgesia was provided on the basis of patient request. |
| Nageotte 1997 | Like the Collis paper, here women who received CSE analgesia were treated differently with regard to mobilisation. There were 2 CSE groups in this study, 1 where mobilisation was encouraged and 1 where walking was actively discouraged. |
| Nielson 1996 | Women were not randomised with regard to group allocation. "The type of anaesthetic technique used was based on patient request, anesthesiologist's preference and obstetrician's choice". |
| Norris 1994 | The women in this study were not randomised with regard to the type of analgesia they received. Allocation was based on patient request. |
| Norris 2001 | This is stated to be "quasi‐randomised", with randomisation actually being in relation to day of week and no allocation concealment. Treatments varied within groups at undefined operator discretion. |
| Pan 1996 | A very small study with insufficient detail regarding randomisation, allocation concealment, blinding and outcome measurement for inclusion. |
| Patel 2003b | This is a dose‐ranging study investigating minimum local analgesic requirements for epidural bupivacaine after CSE or epidural, which was not in our stated outcomes for inclusion. |
| Pham 1996 | The 40 women entered into the study were randomly allocated to 1 of 2 groups. Those in the sufentanil group received intrathecal sufentanil 10 µg followed by an epidural bolus of saline. The women in the bupivacaine group received an intrathecal injection of saline 2 mL followed by an epidural bolus 12 mL bupivacaine 0.25%. As all women in the study had a CSE technique performed, albeit with saline delivered as one of the injections in each case, this study was excluded from data analysis. |
| Pinto 2000 | Randomisation, allocation concealment, blinding and attrition are all unclear. The study is a comparison of 2 CSE techniques with an epidural control that is not valid for inclusion. |
| Rosenfeld 1998 | All women received a CSE. |
| Stocche 2001 | Primary outcomes were not stated outcomes for this review. |
| Van de Velde 2004 | All women received a dural puncture; the epidural control included 2 mL intrathecal saline. |
CSE: combined spinal‐epidural
Characteristics of studies awaiting assessment [ordered by study ID]
Arya 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only. Information regarding the following is inadequate to determine classification: randomisation method, allocation concealment, the reason for the large number excluded; missing data for Apgar scores, satisfaction and the number that actually mobilised. |
Celik 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Poster only. Information regarding the following is inadequate to determine classification: randomisation method, allocation concealment, the number originally allocated, the number lost to follow‐up, the reason for the large number excluded, missing data for Apgar scores, satisfaction and the actual numbers in each group for mode of delivery. |
de Souza 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Gupta 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Kayacan 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Lee 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Lee 2007a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Lian 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only. There is no mention of Ethics approval or consent. The randomisation and allocation processes are not defined. There appears to have been a control group in which no analgesia was offered. There are no usable data presented. Further information may enable inclusion. |
Mantha 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Nakamura 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Olmez 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Turkish; requires translation. Abstract is deficient in ethics approval and informed consent; there is no detail regarding the processes of randomisation or allocation concealment, the inclusion and exclusion criteria and the data pertinent to this review. |
Pascual 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pascual‐Ramirez 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pascual‐Ramirez 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Patel 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Salem 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting clarification of numerous methodological matters. |
Sweed 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wilson 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
We have updated the methods to reflect the latest Cochrane Handbook (Higgins 2011).
Contributions of authors
Planning of review: Allan Cyna. Writing of draft protocol: Allan Cyna. Revision of draft protocol: Allan Cyna, Scott Simmons. Retrieving papers for review: Damien Hughes, Scott Simmons. Extracting data from reviewed papers: Damien Hughes, Scott Simmons, Alicia Dennis, Neda Taghizadeh Checking data prior to entry on Review Manager: Damien Hughes, Scott Simmons, Alicia Dennis, Neda Taghizadeh Checking data entry on Review Manager: Damien Hughes, Scott Simmons, Alicia Dennis. Writing of draft review: Damien Hughes, Scott Simmons, Allan Cyna.
Sources of support
Internal sources
No sources of support supplied
External sources
Department of Health and Ageing, Australia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abouleish 1991 {published data only}
- Abouleish E, Rawal N, Shaw J, Lorenz T, Rashad MN. Intrathecal morphine 0.2 mg versus epidural bupivacaine 0.125% or their combination: effects on parturients. Anesthesiology 1991;74:711‐6. [DOI] [PubMed] [Google Scholar]
- Abouleish E, Rawal N, Shaw J, Rashad MN, Lorenz T. Subarachnoid morphine prolongs labor compared to epidural bupivacaine. Anesthesia & Analgesia 1990;70:S4. [Google Scholar]
Abrao 2009 {published data only}
- Abrao KC, Francisco RP, Miyadahira S, Cicarelli DD, Zugaib M. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstetrics & Gynecology 2009;113(1):41‐7. [DOI] [PubMed] [Google Scholar]
Bhagwat 2008 {published data only}
- Bhagwat AG, Dua CK, Saxena KN, Srinivasan S, Dua K. Comparison of combined spinal epidural technique and low dose epidural technique in progress of labour. Indian Journal of Anaesthesia 2008;52(3):282‐7. [Google Scholar]
Breen 1999 {published data only}
- Breen TW, Giesinger CM, Halpern SH. Comparison of epidural lidocaine and fentanyl to intrathecal sufentanil for analgesia in early labour. International Journal of Obstetric Anesthesia 1999;8:226‐30. [DOI] [PubMed] [Google Scholar]
Caldwell 1994 {published data only}
- Caldwell LE, Rosen MA, Shnider SM. Subarachnoid morphine and fentanyl for labor analgesia. Efficacy and adverse effects. Regional Anesthesia 1994;19:2‐8. [PubMed] [Google Scholar]
Cohen 2006 {published data only}
- Cohen S, Lin A, Pantuck CB, Pantuck EJ, Boxer A. A comparison of combined spinal‐epidural PCA analgesia with continuous epidural‐PCA analgesia alone for labor pain [abstract]. Anesthesiology 2004;101 Suppl:A1200. [Google Scholar]
- Cohen S, Zuker D, Pantuck CB, Hunter CW, Solina A, Prieto N, et al. A comparison of combined spinal‐epidural PCA analgesia with continuous epidural‐PCA analgesia alone for labor pain [abstract]. Anesthesiology 2006;104(Suppl 1):21. [Google Scholar]
COMET 2001a {published data only}
- COMET Study Group. The comparative obstetric mobile epidural trial. Ambulatory epidural analgesia, delivery mode and pain relief: a randomised controlled trial [abstract]. Anesthesiology 2000;92 Suppl:A21. [Google Scholar]
- COMET Study Group, UK. The comparative obstetric mobile epidural trial (C.O.M.E.T.). Ambulatory analgesia, delivery mode and pain relief: a randomised controlled trial. European Journal of Anaesthesiology 2000;17:782‐3. [Google Scholar]
- Comet Study Group, Wilson MJ. A randomised controlled trial comparing traditional with two "mobile" epidural techniques: effect on urinary catheterisation in labor [abstract]. Anesthesiology 2002;96(Suppl 1):Z2. [13436] [DOI] [PubMed] [Google Scholar]
- Comparative Obstetric Mobile Epidural Trial (COMET) Study, Group UK. Effect of low‐dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet 2001;358(9275):19‐23. [DOI] [PubMed] [Google Scholar]
- Duhig K, MacArthur C, May A, Shennan AH. The hypotensive and fetal heart rate response to low‐dose epidurals: analysis of a randomised trial dataset [abstract]. International Journal of Obstetric Anesthesia 2007;16(Suppl 1):S7. [Google Scholar]
- Duhig K, MacArthur C, Shennan AH, The COMET Study Group. The hypotensive and fetal heart rate response to low dose epidurals: analysis of an RCT data set [abstract]. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S63‐4. [Google Scholar]
- Elton C, Bharmal S, May A, COMET Study Group. Does walking in labour with regional blockade affect the mode of delivery? [abstract]. International Journal of Obstetric Anesthesia 2002;11 Suppl:33. [Google Scholar]
- Hussain (for the COMET study group). Haemodynamic changes with 'mobile' epidurals in labour: is it safe for women to ambulate?. Anesthesiology 2001;94(1A):A63. [Google Scholar]
- Shennan A, COMET Study Group. The effect of low‐dose 'mobile' compared with traditional epidural techniques on mode of delivery: a randomised controlled trial [abstract]. Journal of Obstetrics and Gynaecology 2001;21(Suppl 1):S19. [Google Scholar]
- Wilson M, C.O.M.E.T. Study Group. The comparative obstetric mobile epidural trial (C.O.M.E.T.). A randomized controlled trial [abstract]. British Journal of Anaesthesia 2001;87(4):659P. [Google Scholar]
- Wilson MJ, Cooper G, MacArthur C, Shennan A, Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK. Randomized controlled trial comparing traditional with two "mobile" epidural techniques: anesthetic and analgesic efficacy. Anesthesiology 2002;97(6):1567‐75. [12590] [DOI] [PubMed] [Google Scholar]
- Wilson MJ, MacArthur C, Cooper GM, Bick D, Moore PA, Shennan A, et al. Epidural analgesia and breastfeeding: a randomised controlled trial of epidural techniques with and without fentanyl and a non‐epidural comparison group. Anaesthesia 2010;65(2):145‐53. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, MacArthur C, Cooper GM, Shennan A, for the COMET Study Group UK. Ambulation in labour and delivery mode: a randomised controlled trial of high‐dose vs mobile epidural analgesia. Anaesthesia 2009;64(3):266‐72. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Macarthur C, Shennan A, on behalf of the COMET Study Group (UK). Urinary catheterization in labour with high‐dose vs mobile epidural analgesia: a randomized controlled trial. British Journal of Anaesthesia 2009;102(1):97‐103. [DOI] [PubMed] [Google Scholar]
- Wilson MJA, MacArthur C, Shennan A. The effect of epidural analgesia on breast feeding: analysis of a randomised controlled trial. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S7. [Google Scholar]
Cortes 2007 {published data only}
- Cortes C, Sanchez CA, Oliveira AS, Sanchez FM. Labor analgesia: a comparative study between combined spinal‐epidural anesthesia versus continuous epidural anesthesia [Analgesia de parto: estudo comparativo entre anestesia combinada raquiperidural versus anestesia peridural continua]. Revista Brasileira de Anestesiologia 2007;57(1):39‐51. [DOI] [PubMed] [Google Scholar]
Dunn 1998 {published data only}
- Dunn SM, Connelly NR, Parker RK, Steinberg RB, Bazzell CM, Klatt JL, et al. Intrathecal sufentanil vs epidural lidocaine with epinephrine and sufentanil for early labor analgesia. Anesthesiology 1997;87(3A):A891. [DOI] [PubMed] [Google Scholar]
- Dunn SM, Connelly NR, Steinberg RB, Lewis TJ, Bazzell CM, Klatt JL, et al. Intrathecal sufentanil versus epidural lidocaine with epinephrine and sufentanil for early labor analgesia. Anesthesia & Analgesia 1998;87:331‐5. [DOI] [PubMed] [Google Scholar]
Gomez 2001 {published data only}
- Gomez P, Echevarria M, Calderon J, Caba F, Martinez A, Rodriguez R. The efficacy and safety of continuous epidural analgesia versus intradural‐epidural analgesia during labour [Estudio comparativo de la eficacia y securidad de la analgesia epidural continua y la analgesia intradural‐epidural para el trabajo de parto]. Revista Espanola de Anestesiologio y Reanimacion 2001;48:217‐22. [PubMed] [Google Scholar]
Goodman 2006 {published data only}
- Goodman SR, Smiley RM, Negron MA, Freedman PA, Landau R. A randomized trial of breakthrough pain during combined spinal‐epidural versus epidural labor analgesia in parous women. Anesthesia & Analgesia 2009;108(1):246‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SR, Smiley RM, Negron MA, Freedman PA, Landau R. Combined spinal‐epidural versus analgesia in multiparous women [abstract]. Anesthesiology 2006;105:A998. [Google Scholar]
- Goodman SR, Smiley RM, Negron MA, Freedman PA, Landau R. Combined spinal‐epidural versus epidural analgesia in multiparous women [abstract]. Anesthesiology 2006;104(Suppl 1):15. [Google Scholar]
Hepner 2000 {published data only}
- Hepner DL, Gaiser RR, Cheek TG, Gutsche BB. Comparison of combined spinal‐epidural and low dose epidural for labour analgesia. Canadian Journal of Anaesthesia 2000;47:232‐6. [DOI] [PubMed] [Google Scholar]
- Hepner DL, Gaiser RR, Cheek TG, Gutsche BB. Efficacy analysis between the combined spinal‐epidural and epidural for labor analgesia. Anesthesia & Analgesia 1998;86:S373. [Google Scholar]
Kartawiadi 1996 {published data only}
- Kartawiadi L, Vercauteren M, Steenberge A. Extradural (EPI) vs sequential spinal‐extradural (SSE) analgesia during labour: a randomized trial. British Journal of Anaesthesia 1995;74(Suppl 1):106‐7. [Google Scholar]
- Kartawiadi L, Vercauteren MP, Steenberge AL, Adriaensen HA. Spinal analgesia during labor with low‐dose bupivacaine, sufentanil, and epinephrine. A comparison with epidural analgesia. Regional Anesthesia 1996;21:191‐6. [PubMed] [Google Scholar]
Medina 1994 {published data only}
- Medina H, Donadoni R. Combined versus epidural for labor analgesia. ASRA Annual Meeting 1994:95. [8609]
Ngamprasertwong 2007 {published data only}
- Ngamprasertwong P, Kumwilaisakmd K, Indrambarya T, Supbornsug K, Ngarmukos S. Combined spinal‐epidural analgesia and epidural analgesia in labor: effect of intrathecal fentanyl vs. epidural bupivacaine as a bolus. Journal of the Medical Association of Thailand 2007;90(7):1368‐74. [PubMed] [Google Scholar]
Nickells 2000 {published data only}
- Nickells JS, Vaughan DJ, Lillywhite NK, Loughnan B, Hasan M, Robinson PN. Speed of onset of regional analgesia in labour: a comparison of the epidural and spinal routes. Anaesthesia 2000;55:17‐20. [DOI] [PubMed] [Google Scholar]
- Vaughan DJ, Nickells JS, Lillywhite NK, Loughnan B, Hasan M, Robinson PN. Initiation of mobile regional analgesia in labour: a comparison of the spinal and epidural routes [abstract]. International Journal of Obstetric Anesthesia 1999;8:191. [DOI] [PubMed] [Google Scholar]
Parry 1998 {published data only}
- Parry MG, Bawa GPS, Poulton B, Fernando R. Comparison of dorsal column function in parturients receiving epidural and combined spinal epidural (CSE) for labour and elective caesarean section [abstract]. International Journal of Obstetric Anesthesia 1996;5:213. [Google Scholar]
- Parry MG, Fernando R, Bawa GPS, Poulton B. Dorsal column function after epidural and spinal blockade: implications for the safety of walking following low‐dose regional analgesia for labour. Anaesthesia 1998;53:382‐403. [DOI] [PubMed] [Google Scholar]
Patel 2003a {published data only}
- Patel N, Fernando R, Robson S, Columb M, Lyons G. Fetal effects of combined spinal‐epidural (cse) vs epidural labour: a prospective randomised study [abstract]. International Journal of Obstetric Anesthesia 2003;12:193. [13229] [Google Scholar]
Price 1998 {published data only}
- Price C, Lafreniere L, Brosnan C, Findlay I. Regional analgesia in labour: combined spinal epidural v epidural. A double‐blind randomized study [abstract]. International Journal of Obstetric Anesthesia 1996;5:211‐2. [Google Scholar]
- Price C, Lafreniere L, Brosnan C, Findley I. Regional analgesia in early active labour: combined spinal epidural vs epidural. Anaesthesia 1998;53:951‐5. [DOI] [PubMed] [Google Scholar]
Roux 1999 {published data only}
- Roux M, Wattrisse G, Subtil D, Bui Huu Tai R, Krivosic‐Horber R. A comparison of early combined spinal epidural analgesia vs epidural analgesia on labor stage duration and obstetric outcome. Anesthesiology 1996;85:A851. [Google Scholar]
- Roux M, Wattrisse G, Tai RB, Dufossez F, Krivosic‐Horber R. Obstetric analgesia: peridural analgesia versus combined spinal and peridural analgesia [Analgesie obstetricale: analgesie peridurale versus analgesie rachidienne et peridurale combinee]. Annales Francaises d Anesthesie et de Reanimation 1999;18:487‐98. [DOI] [PubMed] [Google Scholar]
Sezer 2007 {published data only}
- Sezer OA, Gunaydin B. Efficacy of patient‐controlled epidural analgesia after initiation with epidural or combined spinal‐epidural analgesia. International Journal of Obstetric Anesthesia 2007;16(3):226‐30. [DOI] [PubMed] [Google Scholar]
Skupski 2009 {published data only}
- Kourpanidis SL. Combined spinal‐epidural versus traditional labor epidural (ongoing trial). www.clinicaltrials.gov (accessed 21 March 2006). [15160]
- Skupski DW, Abramovitz S, Samuels J, Pressimone V, Kjaer K. Adverse effects of combined spinal‐epidural versus traditional epidural analgesia during labor. International Journal of Gynecology & Obstetrics 2009;106(3):242‐5. [DOI] [PubMed] [Google Scholar]
Thomas 2005 {published data only}
- Thomas JA, Pan PH, Harris LC, Owen MD, D'Angelo R. Dural puncture with a 27‐gauge Whitacre needle as part of a combined spinal‐epidural technique does not improve labor epidural catheter function. Anesthesiology 2005;103(5):1046‐51. [14937] [DOI] [PubMed] [Google Scholar]
Tsen 1999 {published data only}
- Tsen L, Segal S, Datta S. Combined spinal/epidural vs epidural analgesia: effects on progression and outcome of labour. Anesthesiology 1996;85:A856. [Google Scholar]
- Tsen LC, Thue B, Datta S, Segal S. Is combined spinal‐epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia?. Anesthesiology 1999;91:920‐5. [DOI] [PubMed] [Google Scholar]
Van de Velde 1999 {published data only}
- Velde M, Mignolet K, Vandermeersch E, Assche A. Prospective, randomized comparison of epidural and combined spinal epidural analgesia during labor. Acta Anaesthesiologica Belgica 1999;50:129‐36. [PubMed] [Google Scholar]
Vernis 2004 {published data only}
- Vernis L, Duale C, Storme B, Mission JP, Rol B, Schoeffler P. Perispinal analgesia for labour followed by patient‐controlled infusion with bupivacaine and sufentanil: combined spinal‐epidural vs. epidural analgesia alone. European Journal of Anaesthesiology 2004;21(3):186‐92. [13198] [DOI] [PubMed] [Google Scholar]
Zeidan 2004 {published data only}
- Zeidan AZ. Combined spinal‐epidural compared with low dose epidural during ambulatory labour analgesia in nulliparous women. Egyptian Journal of Anaesthesia 2004;20:273‐81. [14242] [Google Scholar]
References to studies excluded from this review
Backus 1996 {published data only}
- Backus A, Scanlon J, Calmes S. Epidural bupivacaine compared to intrathecal sufentanil in early labour. Anesthesiology 1996;85(3A):A868. [Google Scholar]
Camann 1992 {published data only}
- Camann WR, Denney RA, Holby ED, Datta S. A comparison of intrathecal, epidural, and intravenous sufentanil for labor analgesia. Anesthesiology 1992;77:884‐7. [DOI] [PubMed] [Google Scholar]
Camann 1998 {published data only}
- Camann W, Abouleish A, Eisenach J, Hood D, Datta S. Intrathecal sufentanil and epidural bupivacaine for labor analgesia: dose‐response of individual agents and in combination. Regional Anesthesia and Pain Medicine 1998;23:457‐62. [DOI] [PubMed] [Google Scholar]
Cascio 1996 {published data only}
- Cascio M, Mandell G, Bernett C, Ramanathan S. Labor analgesia and the stress of labor. Anesthesia & Analgesia 1996;82:S55. [Google Scholar]
Collis 1995 {published data only}
- Collis R, Meares N, Davies W, Aveling W. A randomised study of traditional epidural with 0.25% bupivacaine vs combined spinal epidural (mobile) technique for analgesia in labour: which do mothers prefer?. International Journal of Obstetric Anesthesia 1994;3:172‐3. [Google Scholar]
- Collis RE, Davies DWL, Aveling W. Randomised comparison of combined spinal‐epidural and standard epidural analgesia in labour. Lancet 1995;345:1413‐6. [DOI] [PubMed] [Google Scholar]
Collis 1999a {published data only}
- Collis RE, Plaat FS, Morgan BM. Comparison of midwife top‐ups, continuous infusion and patient‐controlled epidural analgesia for maintaining mobility after a low‐dose combined spinal‐epidural. British Journal of Anaesthesia 1999;82:233‐6. [DOI] [PubMed] [Google Scholar]
Collis 1999b {published data only}
- Collis RE, Harding SA, Morgan BM. Effect of maternal ambulation on labour with low‐dose combined spinal‐epidural analgesia. Anaesthesia 1999;54:535‐9. [DOI] [PubMed] [Google Scholar]
Cooper 2010 {published data only}
- Cooper GM, MacArthur C, Wilson MJ, Moore PA, Shennan A, on behalf of the COMET Study Group UK. Satisfaction, control and pain relief: short‐ and long‐term assessments in a randomised controlled trial of low‐dose and traditional epidurals and a non‐epidural comparison group. International Journal of Obstetric Anesthesia 2010;19(1):31‐7. [DOI] [PubMed] [Google Scholar]
D'Angelo 1994 {published data only}
- Anderson M, D'Angelo R, Philip J, Hood DD, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 1993;79:A970. [DOI] [PubMed] [Google Scholar]
- D'Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 1994;80:1209‐15. [DOI] [PubMed] [Google Scholar]
Dresner 1999 {published data only}
- Dresner M, Bamber J, Calow C, Freeman J, Charlton P. Comparison of low‐dose epidural with combined spinal‐epidural analgesia for labour. British Journal of Anaesthesia 1999;83:756‐60. [DOI] [PubMed] [Google Scholar]
- Dresner M, Bamber J, Freeman J, Calow C. Combined spinal‐epidural or epidural? A randomized comparison of two low dose epidural techniques [abstract]. International Journal of Obstetric Anesthesia 1998;7:195. [Google Scholar]
Finegold 2003 {published data only}
- Finegold H, Rauk P, Mandell G, Golebiewski C, Ramanathan S. The effect of labor analgesia on uterine contraction rates and endogenous oxytocin release [abstract]. Anesthesiology 2003;99:A1214. [Google Scholar]
Fogel 1999 {published data only}
- Fogel ST, Daftary AR, Norris MC, Dalman HM, Holtmann B. Extradural (EPI) vs sequential spinal‐extradural (SSE) analgesia during labour: a randomized trial. Anesthesiology 2000;92 Suppl:A51. [Google Scholar]
- Fogel ST, Daftary AR, Norris MC, Dalman HM, Holtmann B. The incidence of clinically important fetal heart rate abnormalities: combined spinal/epidural vs epidural anesthesia for labor. Regional Anesthesia 1999;24(3 Suppl):75. [Google Scholar]
- Macaulay B, Barton M, Norris M. Interventions during epidural and combined‐spinal epidural labor analgesia [abstract]. Anesthesiology 2000;92 Suppl:A51. [Google Scholar]
- Macaulay B, Barton M, Norris, M. Extradural (EPI) vs sequential spinal‐extradural (SSE) analgesia during labour: a randomized trial. Anesthesiology 2000;92 Suppl:A51. [Google Scholar]
- Norris M, Fogel S, Conway‐Long C. Combined spinal epidural vs. epidural analgesia: part 1: anesthetic outcome [abstract]. Anesthesiology 2000;92 Suppl:A44. [Google Scholar]
- Norris M, Fogel S, Conway‐Long C. Combined spinal epidural vs. epidural analgesia: part 2: obstetric outcome [abstract]. Anesthesiology 2000;92 Suppl:A60. [Google Scholar]
Groves 1995 {published data only}
- Groves PA, Sarna MC, Foley L, Oriol NE. The effect of intrathecal sufentanil and ultra low‐dose epidural bupivacaine on labor progress. Anesthesia & Analgesia 1995;80:S163. [Google Scholar]
Harsten 1997 {published data only}
- Harsten A, Gillberg L, Hakansson L, Olsson M. Intrathecal sufentanil compared with epidural bupivacaine analgesia in labour. European Journal of Anaesthesiology 1997;14:642‐5. [DOI] [PubMed] [Google Scholar]
Kassapidis 1997 {published data only}
- Kassapidis D, Birnbach D, Grunebaum A, Meadows W, Stein D. Placental blood flow as determined by doppler velocimetry: are there differences between combined spinal‐epidural and conventional labor analgesia?. Anesthesiology 1997;87(3A):A893. [Google Scholar]
Leighton 1996 {published data only}
- Leighton BL, Arkoosh VA, Huffnagle S, Huffnagle HJ, Kinsella M, Norris MC. The dermatomal spread of epidural bupivacaine with and without prior intrathecal sufentanil. Anesthesia & Analgesia 1996;83:526‐9. [DOI] [PubMed] [Google Scholar]
Nageotte 1997 {published data only}
- Nageotte M, Larson D, Rumney P, Sidhu M, Hollenback K. A prospective randomized study of intrapartum epidural vs combination intrathecal/epidural anesthesia with or without ambulation. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S22. [Google Scholar]
- Nageotte MP, Larson D, Rumney PJ, Sidhu M, Hollenbach K. Epidural analgesia compared with combined spinal‐epidural analgesia during labor in nulliparous women. New England Journal of Medicine 1997;337:1715‐9. [DOI] [PubMed] [Google Scholar]
Nielson 1996 {published data only}
- Nielsen PE, Erickson JR, Abouleish E, Perriat C, Sheppard C. The effect of intrathecal sufentanil compared to epidural bupivacaine on the fetal heart rate during labor [abstract]. International Journal of Obstetric Anesthesia 1996;5:220. [Google Scholar]
- Nielson PE, Erickson R, Abouleish E, Perriatt S, Sheppard C. Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labor analgesia: incidence and clinical significance. Anesthesia & Analgesia 1996;83:742‐6. [DOI] [PubMed] [Google Scholar]
Norris 1994 {published data only}
- Norris MC, Grieco WM, Borkowski M, Leighton B, Arkoosh VA, Huffnagle HJ, et al. Complications of labor analgesia: epidural versus combined spinal epidural techniques. Anesthesia & Analgesia 1994;79:529‐37. [DOI] [PubMed] [Google Scholar]
Norris 2001 {published data only}
- Norris MC, Fogel ST, Conway‐Long C. Combined spinal‐epidural versus epidural labor analgesia. Anesthesiology 2001;95(4):913‐20. [12456] [DOI] [PubMed] [Google Scholar]
Pan 1996 {published data only}
- Pan P, Fragneto R, Moore C, Ross V. Do obstetric outcomes differ between early combined spinal‐epidural and epidural anesthesia in nulliparous patients receiving intravenous oxytocin?. Anesthesiology 1996;85(3A):A854. [Google Scholar]
- Pan P, Moore C, Fragneto R, Ross V, DiNunzio G. Do obstetric outcomes differ between early combined spinal‐epidural and epidural anesthesia in spontaneously laboring nulliparous paturients?. Anesthesia & Analgesia 1998;86:S382. [9895] [Google Scholar]
- Pan PH, Fragneto R, Moore C. Does combined spinal‐epidural labor analgesia result in better obstetric outcome than epidural anesthesia?. Southern Medical Journal 1996;89:S10. [Google Scholar]
Patel 2003b {published data only}
- Patel N, Fernando R, Columb MO, Bray JK, Sodhi V, Lyons GR. Combined spinal‐epidural (cse) vs epidural labour analgesia: does initial intrathecal analgesia reduce subsequent epidural bupivacaine requirements [abstract]. International Journal of Obstetric Anesthesia 2003;12:197. [13222] [Google Scholar]
Pham 1996 {published data only}
- Pham LH, Camann WR, Smith MP, Datta S, Bader AM. Hemodynamic effects of intrathecal sufentanil compared with epidural bupivacaine in laboring parturients. Journal of Clinical Anesthesia 1996;8:497‐501; discussion 502‐3. [DOI] [PubMed] [Google Scholar]
Pinto 2000 {published data only}
- Pinto G, Collini S, Favaro R, Turkiewicz AM, Pizzicaroli C, Massullo D, et al. Combined spinal‐epidural: selective sequential analgesia during labour. Acta Anaesthesiologica Italica/Anaesthesia & Intensive Care in Italy 2000;51:245‐59. [13219] [Google Scholar]
Rosenfeld 1998 {published data only}
- Rosenfeld DJ, Ackal T, Fournet K, Rosinia FA. A comparison of intrathecal sufentanil and epidural bupivacaine with PCEA throughout labor. Anesthesiology 1998;89:A31. [Google Scholar]
Stocche 2001 {published data only}
- Stocche R, Klamt J, Antunes‐Rodrigues J, Garcia L, Moreira A. Effects of intrathecal sufentanil on plasma oxytocin and cortisol concentrations in women during the first stage of labor. Regional Anaesthesia and Pain Medicine 2001;26(6):545‐50. [DOI] [PubMed] [Google Scholar]
- Stocche RM, Garcia LV, Klamt JG. Effects of analgesic intrathecal sufentanil and 0.25% epidural bupivacaine on oxytocin and cortisol plasma concentration in labor patients. Revista Brasileira de Anestesiologia 2001;51(4):285‐97. [14645] [Google Scholar]
Van de Velde 2004 {published data only}
- Velde M, Teunkens A, Hanssens M, Vandermeersch E, Verhaege J. Intrathecal sufentanil and fetal heart rate abnormalities: a double‐blind, double placebo‐controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesthesia & Analgesia 2004;98(4):1153‐9. [13676] [DOI] [PubMed] [Google Scholar]
- Velde M, Verhaeghe J, Teunkens A, Spitz B, Vandermeersch E. Non‐reassuring fetal heart rate changes and uterine hyperactivity following combined spinal‐epidural or conventional epidural analgesia during labor: the effect of intrathecal opioids. Anesthesiology 2002;96 Suppl:A1048. [Google Scholar]
References to studies awaiting assessment
Arya 2007 {published data only}
- Arya VK, Jain V, Mehdi B, Bhatia A. Comparison of epidural with CSE for 'walking labor analgesia' and fetal fentanyl levels at delivery. Anesthesiology 2007;107:Abstract no: A1766. [Google Scholar]
Celik 2005 {published data only}
- Celik M, Pirbudak, Balat O, Ugur MG, Sahinoz S, Oner U. Comparison of clinical efficacies of combined spinal‐epidural and epidural analgesia techniques with continuous patient controlled infusion method in labor analgesia [abstract]. Regional Anesthesia and Pain Medicine 2005;30(5 Suppl 1):71. [Google Scholar]
de Souza 2009 {published data only}
- Souza MA, Pinto e Silva JL, Maia Filho NL. Combined spinal‐epidural block versus continuous epidural block in labor analgesia for primiparous women: newborns and women outcomes [Bloqueio combinado raquiperidural versus bloqueio peridural continuo para analgesia de parto em primigestas: resultados materos e perinatais]. Revista Brasileira de Ginecologia e Obstetricia 2009;31(10):485‐91. [DOI] [PubMed] [Google Scholar]
Gupta 2002 {published data only}
- Gupta S, Raiger LK, Raman V. Ambulatory labour analgesia ‐ comparison of two regional techniques. Indian Journal of Anaesthesia 2002;46(1):44‐8. [Google Scholar]
Kayacan 2006 {published data only}
- Kayacan N, Ertugrul F, Cete N, Coskunfirat N, Akar M, Karsli B, et al. Comparison of epidural and combined spinal‐epidural analgesia in the management of labour without pain. Journal of International Medical Research 2006;34(6):596‐602. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee SC, Grondin L, Mertz H, Pan PH, Eisenach JC. Effect of spinal vs epidural analgesia on cervical dilation and cytokine expression in laboring women. Anesthesiology 2007;107:Abstract no: A670. [Google Scholar]
Lee 2007a {published data only}
- Lee SC, Pan PH, Mertz H, Smith JG, Grondin L, Harris LC, et al. Effect of spinal versus epidural analgesia on uterine cervical dilution and cytokine expression in laboring women [abstract]. Anesthesiology 2007;106(Suppl 1):9. [Google Scholar]
Lian 2008 {published data only}
- Lian Q, Ye X. The effects of neuraxial analgesia of combination of ropivacaine and fentanyl on uterine contraction. Anesthesiology 2008;109:A1332. [Google Scholar]
Mantha 2007 {published data only}
- Mantha VR, Vallejo MC, Ramesh V, Daftary A, Ramanathan S. Does intermittent epidural analgesia have better labor outcome compared to continuous analgesia? [abstract]. Anesthesiology 2007;106(Suppl 1):37. [Google Scholar]
Nakamura 2009 {published data only}
- Nakamura G, Ganem EM, Rugolo LM, Castiglia YM. Effects on mother and fetus of epidural and combined spinal‐epidural techniques for labor analgesia. Revista Da Associacao Medica Brasileira 2009;55(4):405‐9. [DOI] [PubMed] [Google Scholar]
Olmez 2003 {published data only}
- Olmez G, Dag IH, Ozyilmaz MA, Yalinkaya A. Can combined spinal‐epidural analgesia be an alternative to epidural analgesia alone in labour?. Turk Anesteziyoloji Ve Reanimasyon 2003;31(2):66‐72. [Google Scholar]
Pascual 2011 {published data only}
- Pascual J, Haya J, Perez F, Gil S, Garrido RA, Calatayud L. Combined spinal(morphine)‐epidural analgesia does not shorten labor and delivery compared to epidural analgesia: a randomised study. European Journal of Anaesthesiology 2011;28 Suppl:156. [Google Scholar]
Pascual‐Ramirez 2010 {published data only}
- Pascual Ramirez J, Haya J, Gil S, Calatyud L, Pretel M, Alvarez E, et al. Spinal fentanyl‐morphine in a combined vs epidural labor analgesia does not shorten labor and delivery times. Regional Anesthesia and Pain Medicine 2010;35(5):E154. [Google Scholar]
Pascual‐Ramirez 2011 {published data only}
- Pascual‐Ramirez J, Haya J, Perez‐Lopez FR, Gil‐Trujillo S, Garrido‐Esteban RA, Bernal G. Effect of combined spinal‐epidural analgesia versus epidural analgesia on labor and delivery duration. International Journal of Gynecology and Obstetrics 2011;114(3):246‐50. [DOI] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel NP, Armstrong SL, Fernando R, Columb MO, Bray JK, Sodhi V, et al. Combined spinal epidural vs epidural labour analgesia: does initial intrathecal analgesia reduce the subsequent minimum local analgesic concentration of epidural bupivacaine?. Anaesthesia 2012;67(6):584‐93. [DOI] [PubMed] [Google Scholar]
Salem 2007 {published data only}
- Salem ICF, Fukushima FB, Nakamura G, Ferrari F, Navarro LC, Castiglia YMM, et al. Side effects of subarachnoid and epidural sufentanil associated with a local anesthetic in patients undergoing labor analgesia. Revista Brasileira de Anestesiologia 2007;57(2):125‐35. [DOI] [PubMed] [Google Scholar]
Sweed 2011 {published data only}
- Sweed N, Sabry N, Azab T, Nour S. Regional versus IV analgesics in labor. Minerva Medica 2011;102(5):353‐61. [PubMed] [Google Scholar]
Wilson 2011 {published data only}
- Wilson MJA, Moore PAS, Shennan A, Lancashire RJ, MacArthur C. Long‐term effects of epidural analgesia in labor: a randomized controlled trial comparing high dose with two mobile techniques. Birth 2011;38(2):105‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Akerman 1988
- Akerman B, Arwestrom E. Local anaesthetics potentiate spinal morphine antinociception. Anesthesia & Analgesia 1988;67:943‐8. [PubMed] [Google Scholar]
Anim‐Somuah 2011
- Anim‐Somuah M, Smyth RMD, Jones L. Epidural versus non‐epidural or no analgesia in labour. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000331.pub3] [DOI] [PubMed] [Google Scholar]
Boonmak 2010
- Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post‐dural puncture headache. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001791.pub2] [DOI] [PubMed] [Google Scholar]
Bromage 1999
- Bromage PR. Neurologic complications of labor, delivery, and regional anesthesia. In: Chestnut DH editor(s). Obstetric Anesthesia. 2nd Edition. St. Louis: Mosby, 1999:639‐61. [ISBN 0‐3230‐0383‐4] [Google Scholar]
Brown 1999
- Brown DL. Spinal, epidural and caudal anaesthesia: anatomy, physiology, and technique. In: Chestnut DH editor(s). Obstetric Anaesthesia. 2nd Edition. St. Louis: Mosby, 1999:187‐208. [ISBN 0‐3230‐0383‐4] [Google Scholar]
Butterworth 1998
- Butterworth J. Physiology of spinal anaesthesia: what are the implications for management?. Regional Anesthesia and Pain Medicine 1998;23:370‐3. [DOI] [PubMed] [Google Scholar]
Choi 2005
- Choi PT‐L, Lucas S. Postdural puncture headache. In: Halpern SH, Douglas MJ editor(s). Evidence‐Based Obstetric Anesthesia. Hong Kong: Blackwell, 2005:192‐207. [ISBN 13:978‐0‐7279‐1734‐8] [Google Scholar]
Collis 1993
- Collis RE, Baxandall ML, Srikantharajah ID, Edge G, Kadim MY, Morgan BM. Combined spinal epidural analgesia with ability to walk throughout labour. Lancet 1993;341:767‐8. [DOI] [PubMed] [Google Scholar]
Collis 1994
- Collis RE, Baxandall ML, Srikantharajah ID, Edge G, Kadim MY, Morgan BM. Combined spinal epidural (CSE) analgesia: technique, management, and outcome of 300 mothers. International Journal of Obstetric Anesthesia 1994;3:75‐81. [DOI] [PubMed] [Google Scholar]
Cook 2000
- Cook TM. Combined spinal‐epidural techniques. Anaesthesia 2000;55:42‐64. [DOI] [PubMed] [Google Scholar]
Declercq 2002
- Declercq E, Sakala C, Corry M, Applebaum S, Risher P. Listening to Mothers: Report of the First National Survey of Women's Childbearing Experiences. Maternity Center Association/Harris Interactive: New York, 2002. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glosten 1999
- Glosten B. Epidural and spinal analgesia/anesthesia: local anesthetic techniques. In: Chestnut DH editor(s). Obstetric Anesthesia: Principles and Practice. 2nd Edition. St. Louis: Mosby, 1999:360‐86. [ISBN 0‐3230‐0383‐4] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Hibbard 1996
- Hibbard BM, Anderson MM, Drife JO, Tighe JR, Gordon G, Willatts S, et al. Deaths associated with anaesthesia. In: Rubery E, Bourdillon P editor(s). Report on Confidential Enquiries into Maternal Deaths in the United Kingdom 1991‐1993. Norwich: HMSO, 1996:87‐102. [ISBN 0 11 321983 0] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howell 2001
- Howell CJ, Kidd C, Roberts W, Upton P, Lucking L, Jones PW, et al. A randomised controlled trial of epidural compared with non‐epidural analgesia in labour. BJOG: an International Journal of Obstetrics and Gynaecology 2001;108(1):27‐33. [DOI] [PubMed] [Google Scholar]
Jones 2011b
- Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khor 2000
- Khor LJ, Jeskins G, Cooper GM, Paterson‐Brown S. National obstetric anaesthetic practice in the UK 1997/1998. Anaesthesia 2000;55(12):1168‐72. [DOI] [PubMed] [Google Scholar]
Macarthur 1999
- Macarthur A. Management of controversies in obstetric anaesthesia. Canadian Journal of Anaesthesia 1999;46(5):R111‐6. [DOI] [PubMed] [Google Scholar]
Macarthur 2009
- Macarthur A. Postpartum headache. In: Chestnut DH, Polley LS, Tsen LC, Wong CA editor(s). Obstetric Anesthesia. 4. Philadelphia: Mosby, 2009:677‐700. [ISBN 978‐0‐323‐05541‐3] [Google Scholar]
Nielsen 1996
- Nielsen PE, Erickson JR, Abouleish EI, Perriatt S, Sheppard C. Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labour analgesia: incidence and clinical significance. Anesthesia & Analgesia 1996;83:742‐6. [DOI] [PubMed] [Google Scholar]
NOAD 2004
- Broadway J, Clark V, Plaat F, Velde M. National Obstetric Anaesthetic Database; ongoing OAA audit project, 2002 Report. OAA Newsletter June 2004.
Rawal 1997
- Rawal N, Zundert A, Holmstrom B, Crowhurst J. Combined spinal‐epidural technique. Regional Anesthesia 1997;22(5):406‐23. [DOI] [PubMed] [Google Scholar]
Rawal 1997a
- Rawal N. Problems with combined spinal anaesthesia. In: Russell IF, Lyons G editor(s). Clinical Problems in Obstetric Anaesthesia. 1st Edition. London: Chapman and Hall Medical, 1997:213‐20. [Google Scholar]
Rawal 2000
- Rawal N, Holmstrom B, Crowhurst JA, Zundert A. The combined spinal‐epidural technique. Anesthesiology Clinics of North America 2000;18(2):267‐95. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rigler 1991
- Rigler ML, Drasner K, Krejcie T. Cauda equina syndrome after continuous spinal anaesthesia. Anesthesia & Analgesia 1991;72:275‐81. [DOI] [PubMed] [Google Scholar]
Riley 1999
- Riley ET, Ross BK. Epidural and spinal analgesia/anesthesia: opioid techniques. In: Chestnut DH editor(s). Obstetric Anesthesia: Principles and Practice. 2nd Edition. St. Louis: Mosby, 1999:386‐408. [ISBN 0‐3230‐0383‐4] [Google Scholar]
Russell 2000
- Russell R. The effects of regional analgesia on the progress of labour and delivery. British Journal of Anaesthesia 2000;84(6):709‐12. [DOI] [PubMed] [Google Scholar]
Shaw 2001
- Shaw IC, Birks RJS. A case of extensive block with the combined spinal‐epidural technique during labour. Anaesthesia 2001;56:346‐9. [DOI] [PubMed] [Google Scholar]
Thornton 2001
- Thornton JG. Reducing likelihood of instrumental delivery with epidural anaesthesia. Lancet 2001;358(9275):2. [DOI] [PubMed] [Google Scholar]
van Kooten 2007
- Kooten F, Oedit R, Bakker SLM, Dippel DWJ. Epidural blood patch in post dural puncture headache: a randomised, observer‐blind, controlled clinical trial. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:553‐8. [DOI] [PubMed] [Google Scholar]
Weir 2000
- Weir EC. The sharp end of the dural puncture. BMJ 2000;320:127. [PMC free article] [PubMed] [Google Scholar]


