Abstract
Background
Maternal embryonic leucine zipper kinase (MELK) is an atypical member of the snf1/AMPK family of serine-threonine kinases, involved in diverse physiological and pathological processes, including cell proliferation, apoptosis, embryogenesis, cancer treatment resistance, and RNA processing. MELK is highly expressed in human cancers and is associated with more aggressive forms of astrocytoma, glioblastoma, breast cancer, and melanoma to date, no information about porcine MELK (pMELK) has been reported.
Methods
In this study, the pMELK coding sequence was cloned from swine spleen and characterized. We also quantitatively determined the expression of MELK in 11 tissues isolated from a piglet and determined its subcellular localization when expressed in swine umbilical vein endothelial cells (SUVEC) as a fusion protein. Moreover, we report the functional characterization of pMELK protein concerning its role in apoptosis.
Results
Sequencing analysis showed that full-length of pMELK is 2,072 bp with 17 exons, encoding 655 amino acids, including an S-TKc conserved domain. Comparison of pMELK with ten other mammalian species of their orthologous sequences showed >91% homology and an evolutionary distance <0.05, demonstrating that MELK is highly conserved in evolution. Relative quantification of MELK expression in 11 tissue samples isolated from 30-day-old piglets showed MELK expression in all tested organs and the highest expression in the superficial inguinal lymph node. Constructed a plasmid named pEGFP-MELK, and the fusion protein GFP-MELK was successfully expressed in SUVECs. Fluorescence microscopy revealed the subcellular distribution of the fusion protein GFP-MELK was limited to the cytoplasm. About function, Flow cytometry analysis showed that overexpression of GFP-pMELK in SUVEC cells enhances staurosporine (STS)—induced apoptosis, but not significantly different. The pMELK protein also was found to interact with porcine BCL-G and transient transfection of the recombinant plasmid pCMV-HA-pMELK into SUVEC cells stably expressing GFP-pBCL-G protein inhibited pBCL-G -induced apoptosis significantly.
Conclusions
The present study provided useful information on pMELK basic details and function in apoptosis offer a potential new molecular model for disease interventions and disease related to human MELK and BCL-G.
Keywords: Porcine MELK (pMELK), cloning, tissue distribution, expression pattern, function
Introduction
Maternal embryonic leucine zipper kinase (MELK) is a member of the Snf1/AMPK family of kinases (1-3), which participates in a wide range of cellular processes related to the cell cycle (4,5), apoptosis, spliceosome assembly (6), and cell proliferation (7). MELK is highly expressed in human cancers and is associated with more aggressive forms of astrocytoma, glioblastoma, breast cancer, and melanoma (8,9), suggesting that MELK is a potential anticancer target in diverse tumor entities (10). According to the studies of oncogenic signal transduction pathways, MELK plays a vital role in the regulation of signal transduction and complex coordination, particularly in pathways relating to cancer cell growth and signaling (11,12). Besides, MELK can covalently attach to various proteins, including MAPK, p53, FOXM1, c-JUN, and BCL-G (a pro-apoptotic member of the BCL-2 family), affect the properties of cancer stem cells (10).
The effects of MELK in apoptosis are controversial, since both promotes and inhibits apoptosis is existing. For example, in mice, the physical association between MELK and apoptosis signal-regulating kinase 1 (ASK1) stimulated H2O2-mediated apoptosis by enhancing ASK1 activity in embryonic kidney and hematopoietic cells (13). In HCT116 colon cancer cells, there is a positive correlation between MELK and p53 expression, and overexpression of MELK increases p53 expression (14). This data supplies evidence that MELK may have a vital role in promoting apoptosis in some kinds of cancer. However, data from other reports support the opposite conclusion. In glioblastoma (a highly malignant brain cancer) cells, gene silencing of MELK resulted in increased p53 expression and consequent p53-dependent apoptosis (15). Lin et al. also confirmed that down-regulation of MELK expression using siRNA markedly inhibits breast cancer cell growth (16). Moreover, MELK has an ambiguous role in promoting both cell division and cell death in Caenorhabditis elegans (17,18). Overall, MELK may play dual roles in different cancers, and further study is required to determine the pro-apoptotic and anti-apoptotic biological functions of MELK accurately.
In this study, the porcine MELK (pMELK) coding sequence was cloned from swine spleen and characterized. We also quantitatively determined the expression of MELK in 11 tissues isolated from a piglet and determined its subcellular localization when expressed in swine umbilical vein endothelial cells (SUVEC) as a fusion protein. Moreover, we report the functional characterization of pMELK protein concerning its role in apoptosis. So, the study provides useful information on pMELK basic details and function in apoptosis, offers a potential new molecular model for disease interventions and disease related to human MELK.
Methods
Swine
Three 30-day-old healthy Landrace piglets were bought from Youhai piglet farm (Shaanxi, China). All animal experiments were conducted in compliance with current Chinese ethical legislation.
Cell lines and reagents
Prof. Yan-Ming Zhang kindly offered SUVEC, College of Veterinary Medicine, Northwest A&F University (19), was cultured in 10% fetal bovine serum (FBS; Gibco) at 5% CO2, 37 °C. Staurosporine (STS) was purchased from Sigma-Aldrich (MO, USA). HA-tag and MYC-tag monoclonal antibodies were bought from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-mouse horseradish peroxidase (HRP)-conjugated antibody was obtained from Sigma-Aldrich.
RNA extraction and real-time qPCR analysis
Heart, liver, spleen, lung, kidney, tonsil, thymus, superficial inguinal lymph node, hilar lymph node, mesenteric lymph node, and chin lymph node were isolated from the piglets and marked seriously. Washed the tissues with Physiological saline three times, and 100 mg of each origin were homogenized and diluted 1:10 with phosphate-buffered saline (PBS; 0.1 M, pH 7.4). Total RNA was isolated from the homogenized using TRIzol reagent (Invitrogen, CA, USA) following the manufacturer’s instructions. The same volume of the RNA samples (0.5 µg) was reverse transcribed into cDNA using a PrimeScript™ RT reagent Kit (TaKaRa, Japan). The qPCR was performed in real-time using an SYBR Green qPCR Master Mix (TransGen, Beijing, China) with primers specific for the MELK gene: PMF1 and PMR1. Detection of the housekeeping gene β-actin was performed with the primers β-actin F1 and β-actin R1, for normalization.
Cloning, plasmid construction, and transfection of MELK
The whole MELK coding sequence was optimized using the MF1 and MR1 primers designed according to human MELK genome Primer Premier 5.0. Each amplification was detected by 1.5% (w/v) agarose gel stained with ethidium-bromide, and the positive amplifications were directly sequenced with primers on an ABI 3370 DNA sequencer at Sangon Company (Shanghai, China). Precise of the date was ensured by sequencing in both directions, and hand assembly using DNAStar software as a sequence editor, the plasmids pEGFP-pMELK and pCMV-HA-pMELK were constructed with primers P-MF1 and P-MR1 (Table 1) designed according to MELK accurate sequence and clone MELK into the EcoRI and KpnI sites of pEGFP-c1 and pCMV-HA (Clontech Laboratories, CA, USA). All transient transfections were performed using 4 µg of recombinant plasmids in 6-well plates of cultured SUVEC cells using Lipofectamine 2000 (Invitrogen, CA, USA), according to the manufacturer’s instructions.
Table 1. The primer designed by Primer Premier 5.0 used in this paper.
| Primers | Sequence (5'-3') |
| PMF1 | TCACAGAAACAACAGGCAAACA |
| PMR1 | TGGCTTGGAGCAGAAGATAAGTAG |
| β-actin F1 | GGATGCAGAAGGAGATCACG |
| β-actin R1 | CTCGTCGTACTCCTGCTTGC |
| MF1 | GACTAAACTTCCAGGAGG |
| MR1 | TTGGTCTTTAGGATAGCC |
| P-MF1 | ATGAATTCATGAAAGATTATGATGAA |
| P-MR1 | TAGGTACCTTAAACCTTGCAGCTAGA |
Indirect immunofluorescence assay
Transfected SUVEC cells with the pCMV-HA-pMELK. After 24 h, cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100/PBS, then incubated with mouse anti-HA tag monoclonal antibody (Sigma-Aldrich), followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary antibody (1:100 dilution; Sigma-Aldrich) for 1 h, and observed under an inverted fluorescence microscope.
Western blot analysis
Cell samples were lysed in lysis buffer and boiled in SDS sample buffer for 10 min. The proteins in equal amounts of total protein were separated by SDS-polyacrylamide gel electrophoresis followed by transfer onto polyvinylidene fluoride membranes (Millipore, MA, USA). After incubation with primary and secondary antibodies, protein bands were visualized using Luminata Classico Western HRP Substrate (Millipore, MA, USA) according to the manufacturer’s instructions.
Co-immunoprecipitation
Co-immunoprecipitation was performed using a Pierce® Mammalian c-Myc Tag IP/Co-IP Kit (Pierce, IL, USA) according to the manufacturer’s instructions. Agarose slurry was washed three times with Tris Buffered Saline plus 0.05% Tween-20, and immunoprecipitates were released from the agarose slurry by boiling in 25 µL non-reducing sample buffer for 5 min. Western blotting was then carried out using the anti-HA tag antibody follow the procedure described above.
Subcellular localization of the protein
SUVEC cells were washed with PBS 36 h after transfection and then incubated in growth medium containing 50 nM MitoTracker Red CMXRos (Invitrogen) for mitochondrial labeling at 37 °C for 30 min. Washed cells with 1 × PBS and incubated with 10 µg/mL Hoechst 33342 (Invitrogen) at 37 °C for 30 min, and then analyzed using an AIR MP Confocal Microscope (Nikon Instruments, Tokyo, Japan). The results were processed with NIS Viexer software (Nikon Instruments).
Cell apoptosis assay
SUVEC cells were transfected with specific plasmids, and the medium replaced with new challenge media supplemented with different doses of STS (0, 500, 1,000 nM) (MO, USA). Cells were harvested after 18 h via detached by trypsin and approximately 5×105 cells from each treatment group and then resuspended in working concentration binding buffer (Abcam, MA, USA) and incubated with annexin V-PE/PI (Abcam, MA, USA) for 15 min at 4 °C. The extent of apoptosis was quantified by flow cytometry (FACS LSRII; Becton Dickinson, San Jose, CA, USA). Dead cells and debris were excluded by selective gating based on electronic cell volume determination. About 20,000 events were collected from each sample.
Statistical analyses
Calculation of means and standard deviations (SD) and other statistical analyses using SPSS 18.0. Differences between test and control groups were assessed using an independent samples test, with P values <0.05 considered statistically significant and <0.01 highly significant.
Results
Cloning of the full-length pMELK gene
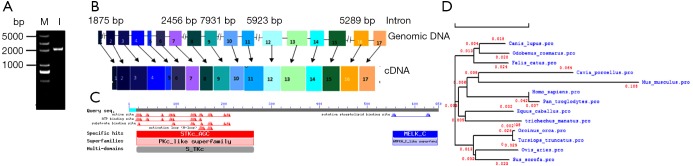
In this study, the pMELK sequence was predicted in silico, and a cDNA containing the whole sequence was obtained for the first time by RT-PCR from the porcine spleen. Using MELK-specific primers PMF1 and PMR1 (Table 1), we amplified a specific band (Figure 1A), sequence analysis found the full length of pMELK is 2,072 bp encoding 655 amino acids, which is entirely consistent with Sus scrofa reference sequence (GenBank access No. XM_013993665.1). According to the sites of predicted intron-exon boundaries (GT - AG sequence motif), the sequence contains 17 exons at 50, 88, 118, 147, 71, 96, 101, 72, 101, 87, 130, 125, 235, 101, 170, 107, and 178 bp (Figure 1B). Prediction of the physical and chemical properties of the pMELK protein using the online ExPASy ProtParam tool (http://www.expasy.ch/cgi-bin/protparam) generated a protein molecular formula C3304H5280N906O967S34, with a relative molecular weight of 74.26 KDa, theoretical PI value was 8.91 and protein half-life was 30 h, the instability index was computed as 38.69, indicating that the protein is stable. Using NCBI CDD predicted pMELK protein conserved domains, as shown in Figure 1C, pMELK contains an S_TKc protein kinase catalytic domain structure. The phylogenetic relationships among pMELK and 10 other mammalian MELK sequences demonstrated MELK is highly evolutionarily conserved, for >91% homology and an evolutionary distance <0.05 (Table 2, Figure 1D).
Figure 1.
The porcine MELK gene. (A) Agarose gel of the porcine MELK cDNA generated by PCR amplification. M, DNA Marker (DL 5000); 1, MELK PCR product. (B) Genomic structure of the porcine MELK gene. (C) Conserved structural domains in the porcine MELK protein predicted by NCBI CDD. (D) Phylogenetic relationship among 11 mammalian species MELK amino acid sequences using DNAMAN.
Table 2. Comparison of pMELK gene and protein sequences with other mammalian species.
| Species | GenBank accession number | Gene similarity | Protein accession number | Protein similarity (%) |
|---|---|---|---|---|
| Ovis aries | XM_004004250.3 | 95% | XP_004004299.1 | 95 |
| Homo sapiens | XM_011518078.1 | 92% | XP_011516380.1 | 91 |
| Equus caballus | XM_001504318.4 | 93% | XP_001504368.2 | 92 |
| Felis catus | XM_003995634.1 | 88% | XP_003995683.1 | 92 |
| Tursiops truncatus | XM_004311138.1 | 95% | XP_004311186.1 | 95 |
| Orcinus orca | XM_004271440.2 | 95% | XP_004271488.1 | 94 |
| Odobenus rosmarus | XM_004392290.2 | 93% | XP_004392347.1 | 92 |
| Trichechus manatus | XM_004372256.2 | 92% | XP_004372316.1 | 92 |
| Sus scrofa | XM_013993665.1 | 100% | XP_013849119.1 | 100 |
| Pan troglodytes | XM_005969963.1 | 95% | XP_005970025.1 | 95 |
| Canis lupus | XM_014117862.1 | 93% | XP_013973337.1 | 92 |
Gene expression of pMELK in different tissues
A total of 11 tissue samples were isolated from a 30-day-old piglet, including heart, liver, spleen, lung, kidney, tonsil, thymus, superficial inguinal lymph node, hilar lymph node, mesenteric lymph node, and chin lymph node. Quantitative RT-PCR (qRT-PCR) results in triplicate that normalized to the housekeeping gene, β-actin, showed that porcine MELK was expressed in all tissues tested; however, there are notable differences among tissues of the expression levels (Figure 2). The highest expression was in the superficial inguinal lymph node, followed by tonsil, heart, liver, kidney, hilar lymph node, spleen, lung, mesenteric lymph node, and thymus; and the lowest expression was in the chin lymph node. The difference in expression levels in different tissues may relate to their function.
Figure 2.
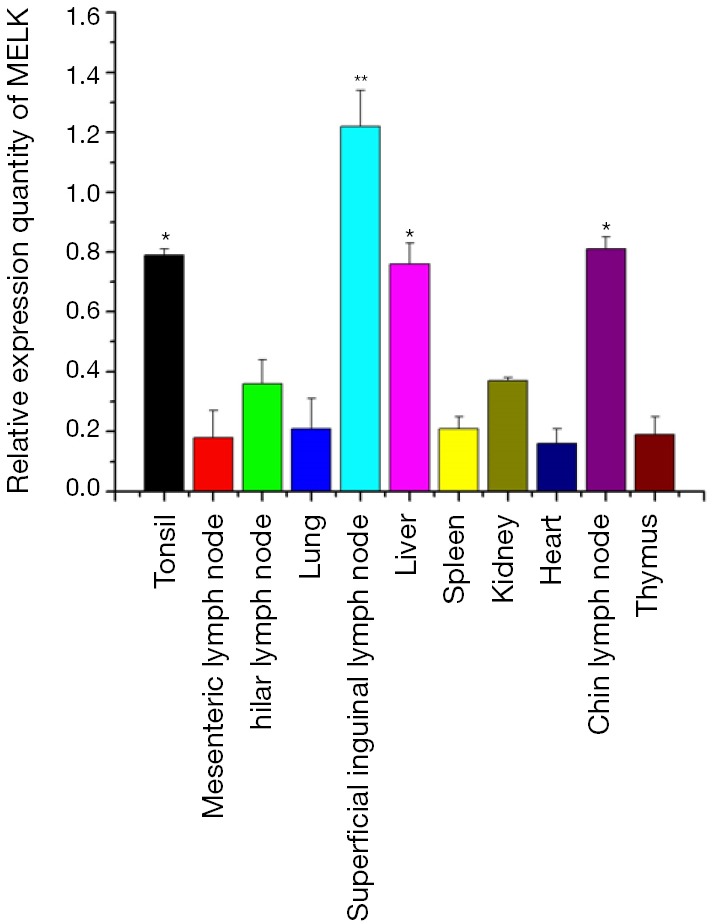
The expression level of MELK mRNA determined by qRT-PCR in different porcine organs.
pMELK plasmid construction, expression, and subcellular localization
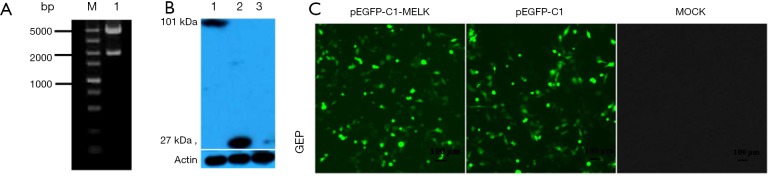
pMELK was cloned into the pEGFP-C1 vector and transformed into Escherichia coli Trans-1 cells (TransGen, Beijing, China); positive clones were selected and verified by sequencing and enzyme digestion, select the clonal that the size, insert location, and the open reading frame was correct (Figure 3A). Green fluorescence in SUVEC cells transfected with pEGFP-C1-MELK and pEGFP-C1 (empty vector) after 24 h was detected by fluorescence microscopy, whereas no fluorescent signals were detected in negative control cells (Figure 3B). After transfection for 48 h, cells were lysed to detect MELK protein expression by Western blotting. As shown in Figure 3C, a band of approximately 101 kDa was detected from cells transfected with pEGFP-C1-MELK plasmid; this is as expected since EGFP-C1 protein is 27 kDa and MELK protein is about 74 kDa. To observe the subcellular localization of MELK protein, the SUVEC cells transfected with pEGFP-C1-MELK plasmid, after 36 h, treat the cells with Hoechst 3,342 dye, under fluorescence microscopy the subcellular localization of MELK was shown to be distributed only in the cytoplasm (Figure 4).
Figure 3.
The identification, expression, subcellular localization of pEGFP-C1-MELK (A) Gel electrophoresis of the recombinant pEGFP-C1-MELK plasmid digested with EcoRI and KpnI. M, DNA Marker (DL 5000); 1, pEGFP-C1-MELK. (B) Western blotting detected the expression of the pMELK fusion protein in SUVEC cells. 1, SUVEC cells transfected with pEGFP-C1-MELK; 2, SUVEC cells transfected with pEGFP-C1; 3, Untransfected SUVEC cells. (C) Fusion proteins detected by indirect immunofluorescence. 1, SUVEC cells transfected with pEGFP-C1-MELK; 2, SUVEC cells transfected with pEGFP-C1; 3, untransfected SUVEC cells.
Figure 4.
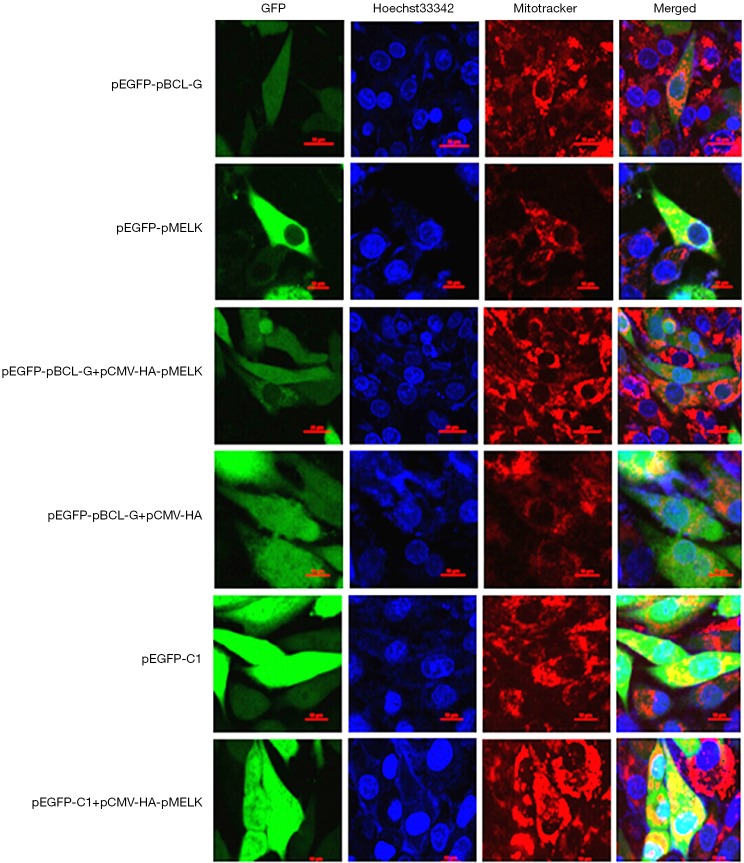
pMELK affects the subcellular localization of the pBCL-G protein. Subcellular localization of GFP-pBCL-G protein was detected by confocal microscopy before and after transfection with pCMV-HA-pMELK or the pCMV-HA empty vector. Cells were stained with Hoechst 33342 and Mito Tracker Red CMXRos. Merged images show the changes in the subcellular localization of the GFP-pBCL-G protein. Scale bar =10 µm in all figures.
Influence on the morphology of SUVEC cells expressing GFP-pMELK protein treated by STS
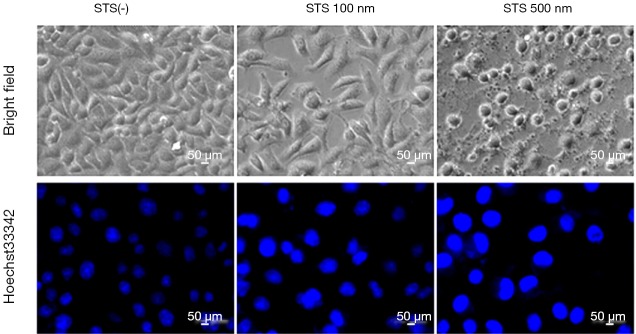
A series of typical apoptotic features were observed by light microscopy 18 h after STS treatment of SUVEC cells, which transiently transfected with GFP-pMELK. Such as cells shrank, became spherical, detached, and smaller, deformed, and exhibited a foaming phenomenon in the cell membrane, all of which are typical characteristics of apoptosis, which were not observed in negative control cells (Figure 5). Treatment with Hoechst 33,342 dye of the cells transiently transfected with GFP-pMELK showed typical chromatin condensation, nucleus pyknosis, bright blue fluorescence in the nuclei, which is enhanced when increasing STS concentration. While in negative control cells, cell and nuclear morphology were normal (Figure 5).
Figure 5.
Morphological changes of SUVEC cells over-expressing GFP-pMELK protein treated with 100, 500 nM STS compared with normal SUVEC cells.
pMELK protein enhance STS-induced apoptosis
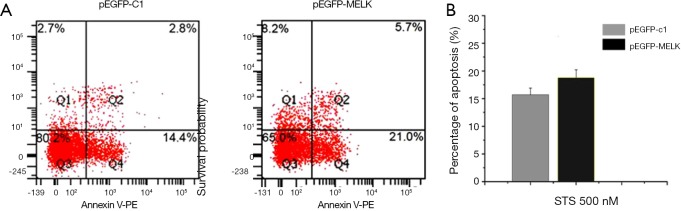
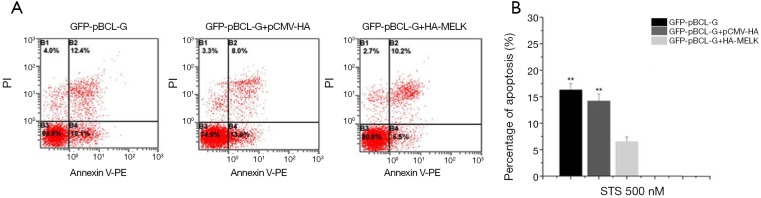
STS appropriate treatment dose is 500 nM to SUVEC cells expressing GFP-pMELK protein from above result, so 500 nM STS was added to the SUVEC cells transiently transfected with the GFP-c1 and SUVEC cells transiently transfected with the GFP-pMELK plasmid, under light microscopy, chromatin condensation and nuclear fragmentation were observed, phenomenon is a little serious in SUVEC cells expressing GFP- pMELK protein. Then cells were stained with Annexin V-PE/PI, and the cell apoptosis rate was analyzed by flow cytometry (Figure 6A). From the result, cells expressing GFP-pMELK protein showed a little higher rate of apoptosis than the control group expressing GFP protein; statistical analysis of the results in triplicate experiments demonstrated that the pMELK protein could enhance STS-induced apoptosis, but the difference is not significant (Figure 6B).
Figure 6.
Apoptosis induced by 500 nM STS in SUVEC cells transfected with GFP-pMELK or GFP-c1. (A) Cells were stained with Annexin V-PE/PI and detected by flow cytometry. The lower right quadrant of the scatter diagram is early apoptotic cells, and the upper left quadrant is necrotic cells. (B) Quantitative analysis of Annexin V-positive cells. Data are expressed as means ± SD, and the experiments were repeated at least three times. There was no statistically significant difference between the two groups (P>0.05).
pMELK interacts with the pBCL-G protein, and the complex is specifically degraded
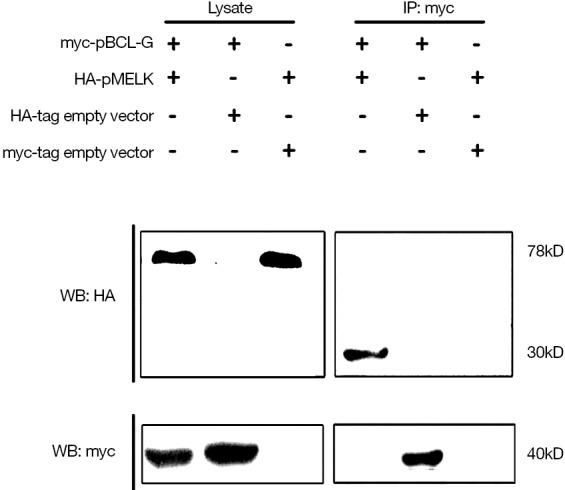
Due to the results of bioinformatics analysis and report from human MELK show, pMELK may interact with BCL-G (16), hypothesized that pMELK could also interact with the porcine BCL-G protein (pBCL-G); therefore, we performed western blotting and co-immunoprecipitation experiments to determine whether such an interaction could be detected. We constructed the plasmid HA-pMELK and co-transfected it with a plasmid expressing the myc-pBCL-G protein in SUVEC cells. Both HA-pMELK and myc-pBCL-G protein bands could be detected in cell lysates; however, identification of the products gained by a Pierce® Mammalian c-Myc Tag IP/Co-IP Kit with anti-HA antibody (to detect HA-pMELK) revealed a band of 30 KDa, rather than the expected 78 kDa. Also, myc-pBCL-G was not detected in the immunoprecipitate. Moreover, the HA-pMELK protein was not detected in two control immunoprecipitates, either from cells co-expressing myc-pBCL-G and HA-pCMA, or HA-pMELK and myc-pCMA. Given these results, we speculate that pBCL-G and pMELK may interact with one another and that the complex may undergo specific degradation (Figure 7).
Figure 7.
Interaction of pMELK with pBCL-G detected by co-immunoprecipitation. The expression of pMELK and pBCL-G proteins co-transfected in SUVEC cells was examined by western blot analysis. The pMELK protein was degraded from 78 to 30 kDa after interaction with pBCL-G.
pMELK interacts with pBCL-G and affects its subcellular localization
Since we determined that pMELK can interact with pBCL-G by co-immunoprecipitation, we performed confocal microscopy using Hoechst 33342 and Mito Tracker® Red CMXRos staining to determine whether pMELK affects the subcellular localization of pBCL-G. As shown in Figure 4, stably expressed GFP-pBCL-G protein was distributed in both nucleus and cytoplasm, whereas when cells were co-transfected with pCMV-HA-pMELK, GFP-pBCL-G protein was explicitly allocated in the cytoplasm, which was consistent with the subcellular localization of pMELK. Cells transfected with pCMV-HA empty vector, the GFP-pBCL-G protein was distributed both in the nucleus and in the cytoplasm. As a control, SUVEC cells stably expressing GFP protein were also transfected with the pCMV-HA-pMELK vector; the GFP protein was always localized both in the nucleus and cytoplasm.
pMELK significantly suppressed pBCL-G induced apoptosis
SUVEC cells stably expressing GFP-pBCL-G from our lab were transiently transfected with pCMV-HA-pMELK or pCMV-HA empty vector and after 24 h, treated with 500 nM STS for 18 h, analyzed by flow cytometry to detect apoptosis. As shown in Figure 8A, compared with cells over-expressing pBCL-G alone, added over-expression of pMELK markedly attenuated STS-induced apoptosis of SUVEC cells stably expressing the GFP-pBCL-G protein. These results indicate that pMELK and pBCL-G interacting complex products may have an opposing role to pBCL-G in apoptosis, which is consistent with humans, so the kinase activity of pMELK could be a promising molecular model for the development of therapy for disease caused by BCL-G.
Figure 8.
The interaction of pMELK with pBCL-G effects pBCL-G -induced apoptosis. (A) FACS scatter plots representing SUVEC cells treated with STS (500 nM) and co-expressing GFP-pBCL-G alone, GFP-pBCL-G, and pCMV-HA (vector control), or GFP-pBCL-G and pCMV-HA-pMELK. (B) Quantification of Annexin V-positive cells. Data are expressed as means ± SD, and the experiments were repeated at least three times. **, statistically significant difference (P<0.05) versus the control group.
Discussion
The MELK gene originally was cloned from Mus musculus, yet MELK is one of the stored maternal mRNAs expressed in developing eggs and embryos (20). MELK protein is classified as part of the AMPK/Snf1 family, due to a conserved serine/threonine kinase domain present in its N-terminal region (21). Similar to other family members, the catalytic domain of MELK is adjacent to an Ubiquitin-Associated (UBA) domain; however, the kinase activity of MELK is activated by autophosphorylation in vitro, whereas other family members are dependent on phosphorylation by upstream kinases (such as LKB1 or CaMKK2) for their activation, shortly after its discovery, MELK, also known as MPK38, was cloned from a murine teratocarcinoma cell line, PCC4 (22). Mouse MELK expresses itself in the spleen and thymus, but not in the muscle, kidney, or liver. MELK expression was also observed in mouse T lineage lymphocytes and macrophage/monocyte cells but could not be detected in a B cell line so that MELK may have an essential role in signal transduction in specific hematopoietic cell lineages (22). Subsequently, the involvement of MELK in underlying biological mechanisms and functions, including cell signaling pathways and tumor progression, has been determined in the organs and cells of different species (23-28), providing a deeper understanding of the role of this protein. However, until now, there has been no report of porcine MELK or its expression, function, and role in apoptosis.
In this study, we cloned the MELK gene from the spleen of a 30-day-old piglet. The full-length sequence is 2,072 bp with 17 exons encoding 655 amino acids, which is like MELK genes from other species. Phylogenetic analysis showed that MELK is highly evolutionarily conserved among 10 other mammalian species, with an evolutionary distance of <0.05. MELK was widely expressed in 11 organ samples isolated from 30-day-old piglets; the highest expression was in the superficial inguinal lymph node and the lowest in the chin lymph node. The observed differential expression levels in various porcine tissues may have functional relevance.
MELK protein structure and function have been studied in various species as model organisms, shedding light on its features in humans. It is conserved throughout species, including the majority of mammalian and non-mammalian species; however, the protein shows slight functional differences among species. Importantly, MELK has been shown to interact with and phosphorylate vital proteins to regulate G2/M cell cycle progression in Xenopus laevis (29). Furthermore, MELK is strongly implicated in cell cycle regulation, proliferation, mitosis, and spliceosome assembly (30). MELK also has a vital role in cell division, cell propagation, and maintenance in organ-specific stem cells from non-mammalian species, whereas, in mammalian systems, MELK is essential for organogenesis, stem cell proliferation, and cell cycle regulation (31). In humans, MELK participates in the development of numerous cancers, including astrocytoma, glioblastoma, breast cancer, and melanoma, through its influence on tumor initiation and propagation. Although many functions of MELK have been elucidated, there is little information available about porcine MELK. In our study, we found that pMELK is 74 kDa in size and distributed solely in the cytoplasm. In SUVEC cells, we discovered that pMELK could enhance STS-induced apoptosis; and pMELK did interact with BCL-G, a pro-apoptotic member of the BCL-2 family and affected its subcellular localization. Also, the interaction production between MELK and pBCL-G significantly inhibited STS-induced apoptosis, indicating that the complex production of pMELK interacted with pBCL-G plays an opposing role to pBCL-G in apoptosis, similar to findings in humans. Porcine models have advantages for scientific research compared to mice since they are more physiologically and anatomically similar to humans (32-34). Therefore, porcine MELK is a potential molecular model for related diseases and inhibition of MELK may be an attractive therapeutic target in several cancers related to humans.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were conducted in compliance with current Chinese ethical legislation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gil M, Yang Y, Lee Y, et al. Cloning and expression of a cDNA encoding a novel protein serine/threonine kinase predominantly expressed in hematopoietic cells. Gene 1997;195:295-301. 10.1016/S0378-1119(97)00181-9 [DOI] [PubMed] [Google Scholar]
- 2.Janostiak R, Rauniyar N, Lam TT, et al. MELK Promotes Melanoma Growth by Stimulating the NF-κB Pathway. Cell Rep 2017;21:2829-41. 10.1016/j.celrep.2017.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cigliano A, Pilo MG, Mela M, et al. Inhibition of MELK Protooncogene as an Innovative Treatment for Intrahepatic Cholangiocarcinoma. Medicina (Kaunas) 2019. doi: . 10.3390/medicina56010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davezac N, Baldin V, Blot J, et al. Human pEg3 kinase associates with and phosphorylates CDC25B phosphatase: a potential role for pEg3 in cell cycle regulation. Oncogene 2002;21:7630-41. 10.1038/sj.onc.1205870 [DOI] [PubMed] [Google Scholar]
- 5.Badouel C, Chartrain I, Blot J, et al. Maternal embryonic leucine zipper kinase is stabilized in mitosis by phosphorylation and is partially degraded upon mitotic exit. Exp Cell Res 2010;316:2166-73. 10.1016/j.yexcr.2010.04.019 [DOI] [PubMed] [Google Scholar]
- 6.Vulsteke V, Beullens M, Boudrez A, et al. Inhibition of spliceosome assembly by the cell cycle-regulated protein kinase MELK and involvement of splicing factor NIPP1. J Biol Chem 2004;279:8642-7. 10.1074/jbc.M311466200 [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Zhang D. Maternal Embryonic Leucine Zipper Kinase (MELK): A Novel Regulator in Cell Cycle Control, Embryonic Development, and Cancer. Int J Mol Sci 2013;14:21551-60. 10.3390/ijms141121551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie SK, Okamoto OK, Uno M, et al. Maternal embryonic leucine zipper kinase transcript abundance correlates with malignancy grade in human astrocytomas. Int J Cancer 2008;122:807-15. 10.1002/ijc.23189 [DOI] [PubMed] [Google Scholar]
- 9.Kig C, Beullens M, Beke L, et al. Maternal embryonic leucine zipper kinase (MELK) reduces replication stress in glioblastoma cells. J Biol Chem 2013;288:24200-12. 10.1074/jbc.M113.471433 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Wang R, Chen Y, Yang B, et al. Design, synthesis, biological evaluation and molecular modeling of novel 1H-pyrrolo[2,3-b]pyridine derivatives as potential anti-tumor agents. Bioorg Chem 2020;94:103474. 10.1016/j.bioorg.2019.103474 [DOI] [PubMed] [Google Scholar]
- 11.Brognard J, Hunter T. Protein kinase signaling networks in cancer. Curr Opin Genet Dev 2011;21:4-11. 10.1016/j.gde.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning BD. Challenges and opportunities in defining the essential cancer kinome. Sci Signal 2009;2:pe15. 10.1126/scisignal.263pe15 [DOI] [PubMed] [Google Scholar]
- 13.Nishitoh H, Saitoh M, Mochida Y, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 1998;2:389-95. 10.1016/S1097-2765(00)80283-X [DOI] [PubMed] [Google Scholar]
- 14.Seong HA, Ha H. Murine protein serine-threonine kinase 38 activates p53 function through ser15 phosphorylation. J Biol Chem 2012;287:20797-810. 10.1074/jbc.M112.347757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu C, Banasavadi-Siddegowda YK, Joshi K, et al. Tumor-specific activation of the C-JUN/MELK pathway regulates glioma stem cell growth in a p53-dependent manner. Stem Cells 2013;31:870-81. 10.1002/stem.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin ML, Park JH, Nishidate T, et al. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res 2007; 9:R17. 10.1186/bcr1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordes S, Frank CA, Garriga G. The C. elegans MELK ortholog PIG-1 regulates cell size asymmetry and daughter cell fate in asymmetric neuroblast divisions. Development 2006;133:2747-56. 10.1242/dev.02447 [DOI] [PubMed] [Google Scholar]
- 18.Denning DP, Hatch V, Horvitz HR. Programmed elimination of cells by caspase-independent cell extrusion in C. elegans. Nature 2012;488:226-30. 10.1038/nature11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong HX, Zhang YM, Xu H, et al. Immortalization of swine umbilical vein endothelial cells with human telomerase reverse transcriptase. Mol Cells 2007;24:358-63. [PubMed] [Google Scholar]
- 20.Cho YS, Yoo J, Park S, et al. The structures of the kinase domain and UBA domain of MPK38 suggest the activation mechanism for kinase activity. Acta Crystallogr D Biol Crystallogr 2014;70:514-21. 10.1107/S1399004713027806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitner MK, Taliaferro JM, Dalby KN, Bartholomeusz C. MELK: a potential novel therapeutic target for TNBC and other aggressive malignancies. Expert Opin Ther Targets 2017;21:849-59. 10.1080/14728222.2017.1363183 [DOI] [PubMed] [Google Scholar]
- 22.Gil M, Yang Y, Lee Y, et al. Cloning and expression of a cDNA encoding a novel protein serine/threonine kinase predominantly expressed in hematopoietic cells. Gene 1997;195:295-301. 10.1016/S0378-1119(97)00181-9 [DOI] [PubMed] [Google Scholar]
- 23.Nakano I, Masterman-Smith M, Saigusa K, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res 2008;86:48-60. 10.1002/jnr.21471 [DOI] [PubMed] [Google Scholar]
- 24.Gray D, Jubb AM, Hogue D, et al. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res 2005;65:9751-61. 10.1158/0008-5472.CAN-04-4531 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Lee YM, Baitsch L, et al. MELK is an oncogenic kinase essential for mitotic progression in basal-like breast cancer cells. Elife 2014;3:e01763. 10.7554/eLife.01763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012;12:133-43. 10.1038/nrc3184 [DOI] [PubMed] [Google Scholar]
- 27.Hebbard LW, Maurer J, Miller A, et al. Maternal embryonic leucine zipper kinase is upregulated and required in mammary tumor-initiating cells in vivo. Cancer Res 2010;70:8863-73. 10.1158/0008-5472.CAN-10-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi K, Banasavadi-Siddegowda Y, Mo X, et al. MELK dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells. Stem Cells 2013;31:1051-63. 10.1002/stem.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HS, Lee SH, Kim H, et al. Germ cell-specific gene 1 targets testis-specific poly(A) polymerase to the endoplasmic reticulum through protein-protein interactions. FEBS Lett 2008;582:1203-9. 10.1016/j.febslet.2008.01.065 [DOI] [PubMed] [Google Scholar]
- 30.Chung S, Suzuki H, Miyamoto T, et al. Development of an orally-administrative MELK-targeting inhibitor that suppresses the growth of various types of human cancer. Oncotarget 2012;3:1629-40. 10.18632/oncotarget.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tassan JP. Cortical localization of maternal embryonic leucine zipper kinase (MELK) implicated in cytokinesis in early xenopus embryos. Commun Integr Biol 2011;4:483-5. 10.4161/cib.15669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codas R, Badet L, Eugene M, Giraud S, et al. Evaluation of pulsatile perfusion machine RM3 for kidney preservation in a swine model of renal autotransplantation. Transplant Proc 2009;41:3296-8. 10.1016/j.transproceed.2009.08.045 [DOI] [PubMed] [Google Scholar]
- 33.Fodor WL, Williams BL, Matis LA, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci USA 1994;91:11153-7. 10.1073/pnas.91.23.11153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozianka J, Kielan W, Waleczek H. Barium peritonitis-a study in pigs. Adv Clin Exp Med 2003;12:569-73. [Google Scholar]