Abstract
The assessment and prediction of cognitive performance is a key issue for any discipline concerned with human operators in the context of safety-critical behavior. Most of the research has focused on the measurement of mental workload but this construct remains difficult to operationalize despite decades of research on the topic. Recent advances in Neuroergonomics have expanded our understanding of neurocognitive processes across different operational domains. We provide a framework to disentangle those neural mechanisms that underpin the relationship between task demand, arousal, mental workload and human performance. This approach advocates targeting those specific mental states that precede a reduction of performance efficacy. A number of undesirable neurocognitive states (mind wandering, effort withdrawal, perseveration, inattentional phenomena) are identified and mapped within a two-dimensional conceptual space encompassing task engagement and arousal. We argue that monitoring the prefrontal cortex and its deactivation can index a generic shift from a nominal operational state to an impaired one where performance is likely to degrade. Neurophysiological, physiological and behavioral markers that specifically account for these states are identified. We then propose a typology of neuroadaptive countermeasures to mitigate these undesirable mental states.
Keywords: neuroergonomics, performance prediction, degraded attentional and executive mental states, task engagement, mental workload
Introduction
A study of mental workload is fundamental to understanding the intrinsic limitations of the human information processing system. This area of research is also crucial for investigation of complex teaming relationships especially when interaction with technology necessitates multitasking or a degree of cognitive complexity.
The Growth of Mental Workload
Mental workload has a long association with human factors research into safety-critical performance (Moray, 1979; O’Donnell and Eggemeier, 1986; Hancock and Meshkati, 1988; Hancock and Desmond, 2001; Wickens and Tsang, 2014; Young et al., 2015). Forty years have passed since the publication of the seminal collection edited by Moray (1979) and the study of mental workload remains an active topic in contemporary human factors research; a keyword search based on Google Scholar listed more than 200,000 articles published on the topic since 2000, see also Table 1 in Young et al. (2015). The significance of human mental workload for those technological trends that are forecast during the second machine age (Brynjolfsson and McAfee, 2014) guarantees its importance for human factors research in future decades.
The lineage of mental workload incorporates a number of theoretical perspectives, some of which precede the formalization of the concept itself. Early work linking physiological activation to the prediction of performance (Yerkes and Dodson, 1908; Duffy, 1962) was formalized into an energetical model of attentional resources (Kahneman, 1973) that emphasized a dynamic relationship between finite information processing capacity and variable cognitive demands (Norman and Bobrow, 1975; Navon and Gopher, 1979; Wickens, 1980). The descriptive quality of the early work on attentional resources was sharpened by cognitive models of control (Broadbent, 1971; Schneider et al., 1984; Shallice and Burgess, 1993). Hybrid frameworks that place cognitive processes within a resource framework have been hugely influential in the field, such as the multiple resource model (Wickens, 1984, 2002, 2008; Wickens and Liu, 1988) whereas others introduced agentic features, such as dynamic self-regulation and adaptation, within models of human performance (Hockey et al., 1986; Hockey, 1997). For instance, Hancock and Warm (1989)’s dynamic adaptive theory (DAT) postulates that the brain seeks resource homeostasis and cognitive comfort. However, environmental stressors can progressively shift individual’s adaptive abilities from stability to instability depending on one’s cognitive and psychological resources. The DAT is an extension of the Yerkes and Dodson inverted-U law, in a sense that very low (hypostress) and very high (hyperstress) task demands can both degrade the adaptability and consequently impair performance. All these perspectives are united by a characterization of the human information processing system as a finite resource with limited capacity (Kramer and Spinks, 1991).
Mental Workload Measurement
Research into the measurement of mental workload has outstripped the development of theoretical frameworks. Measures of mental workload can be categorized as performance-based, or linked to the process of subjective self-assessment, or associated with psychophysiology or neurophysiology. Each category has specific strengths and weaknesses (O’Donnell and Eggemeier, 1986; Wierwille and Eggemeier, 1993) and the sensitivity of each measurement type can vary depending on the level of workload experienced by the operator (De Waard, 1996). The development of multidimensional measures led inevitably to an inclusive framework for mental workload. The cost of this integration is dissociation between different measures of mental workload, e.g., Yeh and Wickens (1988), and an integrated workload concept that remains poorly defined from a psychometric perspective (Matthews et al., 2015).
There are a number of reasons that explain why mental workload is easy to quantify but difficult to operationalize. The absence of a unified framework for human mental workload, its antecedents, processes and measures has generated a highly abstract concept, loosely operationalized and supported by a growing database of inconsistent findings (Van Acker et al., 2018). The absence of a general explanatory model is complicated by the fact that mental workload, like stress and fatigue (Matthews, 2002), is a transactional concept representing an interaction between the capacities of the individual and the specific demands of a particular task. Within this transactional framework, mental workload represents a confluence between inter-individual sources of trait variability (e.g., skill, IQ, personality), intra-individual variation (e.g., emotional states, motivation, fatigue), and the specific configuration of the task under investigation (see also Table 2 in Van Acker et al., 2018).
For the discipline of human factors, the study of mental workload serves two primary functions: (a) to quantify the transaction between operators and a range of task demands or technological systems or operational protocols, and (b) to predict the probability of performance impairment during operational scenarios, which may be safety-critical. One challenge facing the field is delineating a consistent relationship between mental workload measurement and performance quality on the basis of complex interactions between the person and the task. The second challenge pertains to the legacy and utility of limited capacity of resources as a framework for understanding those interactions.
In the following sections, we detail some limitations of mental resources and advocate the adoption of a neuroergonomic approach (Sarter and Sarter, 2003; Parasuraman and Rizzo, 2008; Parasuraman and Wilson, 2008; Mehta and Parasuraman, 2013; Ayaz and Dehais, 2018) for the study of mental workload and human performance. The neuroergonomic framework emphasizes a shift from limited cognitive resources to characterizing impaired human performance and associated states with respect to neurobiological mechanisms.
Toward a Limit of the Theory of Limited Resources
The concept of resources represents a foundational challenge to the development of a unified framework for mental workload and prediction of human performance. The conception of a limited capacity for information processing is an intuitive one and has been embedded within several successful models, e.g., multiple resources (Wickens, 2002). But this notion has always been problematic because resources are a general-purpose metaphor with limited explanatory powers (Navon, 1984) that incorporate both cognitive processes (e.g., attention, memory) and energetical constructs (e.g., mental effort) in ways that are difficult to delineate or operationalize. The allegorical basis of resources almost guarantees an abstract level of explanation (Van Acker et al., 2018) that is accompanied by divergent (Matthews et al., 2015), and sometimes contradictory operationalizations (Yeh and Wickens, 1988; Annett, 2002).
For example, the theory of limited cognitive resources predicts that exposure to task demands that are sustained and demanding can impair performance due to resource depletion via self-regulation mechanisms at the neuron-level (i.e., local-sleep state theory, see Van Dongen et al., 2011) or compromise access to resources mechanisms (Borragan Pedraz and Peigneux, 2016). However, this type of explanation fails to clarify why non-challenging tasks, such as passive monitoring (Matthews et al., 2002, 2010) can promote episodes of mind wandering whereby attention drifts from task-related to task-irrelevant thoughts (Smallwood et al., 2008; Durantin et al., 2015; Smallwood and Schooler, 2015). Although some propositions, such as the theory of “malleable resources” (Young and Stanton, 2002), have intuited this paradox, this theory is at a highly descriptive level and remains difficult to operationalize.
Similarly, the occurrence of stressful and unexpected operational scenarios is known to impair executive functioning and provoke perseveration, see Dehais et al. (2019) for review. Perseveration is defined as a tendency to continue an action after cessation of the original stimulation, which is no longer relevant to the goal at hand (Sandson and Albert, 1984). For example, several studies conducted on emergency evacuation situations reported irrational and perseverative behaviors even when tasks were simple and undemanding (Proulx, 2001; Kobes et al., 2010). A paradigmatic situation is the one in which people fail to escape from fire because they push the door instead of pulling it. Perseveration can also have devastating consequences during safety-critical tasks, such as aviation (O’Hare and Smitheram, 1995; Orasanu et al., 1998; Reynal et al., 2017) and in the medical domain (Bromiley, 2008). This category of performance impairment cannot be explained solely through the prism of limited mental resources. Operators who persist with an erroneous strategy, such as an aircrew who attempt to land their craft at all costs despite bad weather conditions, are generally capable of performing the required actions and tend to invest greater effort even as their task goal becomes difficult or even impossible to achieve (Dehais et al., 2010, 2012).
The concept of limited cognitive resources could explain failures of attention such as inattentional blindness (Brand-D’Abrescia and Lavie, 2008) or deafness (Raveh and Lavie, 2015). Both categories describe an inability to detect unexpected stimuli, such as alarms from the interface (Dehais et al., 2011, 2014), and represent breakdown of selective attention due to the presence of competing demands on the human information processing system. It has been demonstrated that individuals with greater information processing capacity (i.e., higher working memory span) exhibit superior ability with respect to divided and sustained attention (Colflesh and Conway, 2007; Unsworth and Engle, 2007), and therefore, should be less susceptible to the effects of inattention during the performance of demanding tasks. However, this hypothesis is contradicted by the absence of any correlation between individual differences in processing capacity and the occurrence of inattentional blindness (Bredemeier and Simons, 2012; Beanland and Chan, 2016; Kreitz et al., 2016a) or deafness (Kreitz et al., 2016b; Dehais et al., 2019).
This research suggests that the limited resource model cannot account for critical lapses of attention and executive functioning that are observed under conditions of high mental workload. Therefore, we must go beyond the limitations of the resource concept as an explanatory model of mental workload and turn our attention to the neural underpinnings of attention and behavior (Parasuraman et al., 1999).
Resources: a Neuroergonomic Perspective
The last three decades have witnessed a revolution in our understanding of neural mechanisms that are fundamental to attention and human performance. Progress in the field has been driven by the development of advanced and portable neuroimaging techniques, which permit non-invasive examination of the “brain at work.” Neuroergonomics is a multidisciplinary field born from these technical innovations that is broadly defined as the study of the human brain in relation to performance at work and in everyday settings (Parasuraman and Rizzo, 2008). The goal of this field is to integrate both theories and principles from ergonomics, neuroscience and human factors in order to provide insights into the relationship between brain function and behavioral outcomes in the context of work and everyday life (Rizzo et al., 2007; Parasuraman and Rizzo, 2008; Parasuraman and Wilson, 2008; Lees et al., 2010; Ayaz and Dehais, 2018).
The Multiple Biological Substrates of Mental Resources
The incorporation of neurophysiological measures of mental workload offers a reductive pathway to the reification of resources and those neurobiological states associated with impaired performance. At a fundamental level, the functioning of neurons within the brain is a form of limited resource (Beatty, 1986), requiring oxygen and glycose to generate cellular energy in the form of adenosine triphosphate (ATP) while having a very limited capacity to store these energy substrates (Saravini, 1999). The same logic holds for ions (e.g., potassium, calcium, sodium) that play a key role in nerve impulses. It is also reasonable to consider neural networks as resources with respect to their supporting glial cells (e.g., astrocytes), which ensure the processing of information (Mandrick et al., 2016). Understanding the interactions between neurobiological resources with reference to fundamental processes in brain physiology represents a crucial approach within neuroergonomic analysis of mental workload (Parasuraman and Rizzo, 2008; Ayaz and Dehais, 2018).
Brain and Inhibitory Mechanisms
The brain must be considered to be a “noisy” organ, whereby assembly of neurons are constantly responsive to environmental stimulations, see Pandemonium architecture as an early example, such as Selfridge (1959). Inhibitory mechanisms are implemented to cancel out cerebral noise by mitigating the activation of distracting neuronal assemblies (Polich, 2007). This process may occur at a local level via lateral inhibition, whereby groups of neurons can attenuate the activity of their neighbors in order to be “better heard” (Coultrip et al., 1992). The same mechanism can also take place via top-down regulation, known as inhibitory control, wherein high-level cortical areas (e.g., prefrontal cortex) reduce task- or stimulus-irrelevant neural activities (Munakata et al., 2011). However, these inhibitory mechanisms can also curtail the capacity of the brain to consider new or alternative information, thus leading to perseveration (Dehais et al., 2019). An appropriate metaphor is to consider a group led by an authoritarian leader who is totally engaged with one specific goal or strategy and does not listen to alternative viewpoints of other members of the group. Within this metaphor, information processing resources are present (i.e., group members) but are disregarded in the presence of an overriding directive (i.e., the leader). In other words, high mental workload leads to impaired performance, not because of limited resources per se, but because of those neurological mechanisms designed to prioritize a specific goal or directive.
The Non-linear Effects of Neuromodulation
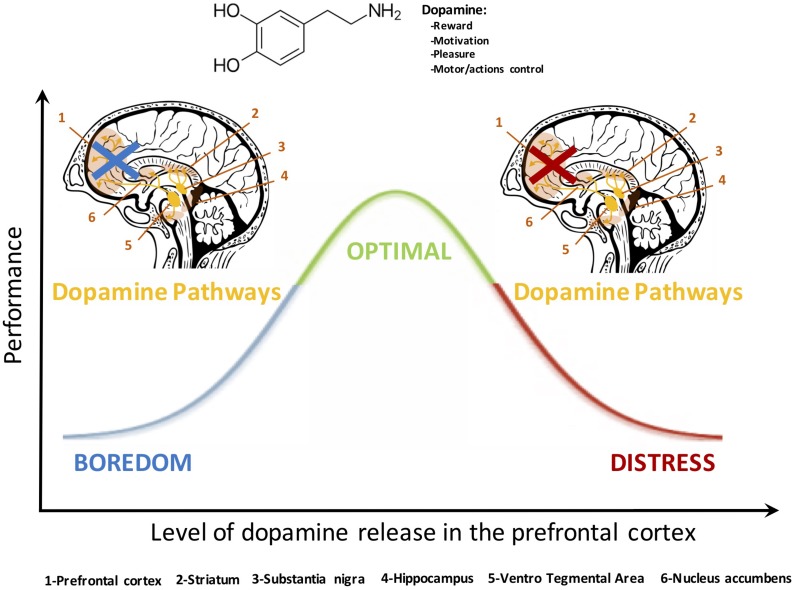
The prefrontal cortex (PFC) is a brain structure often identified as the neurophysiological source of limited resources (Posner and Petersen, 1990; Parasuraman, 2003; Ramsey et al., 2004; Modi et al., 2017). The PFC serves a control function during routine cognitive operations, such as: action selection, retrieval/updating in working memory, monitoring and inhibition (Ramnani and Owen, 2004; Ridderinkhof et al., 2004). It is often activated during high levels of cognitive demand (Ayaz et al., 2012; Herff et al., 2014; Racz et al., 2017; Gateau et al., 2018; Fairclough et al., 2019) and dysfunction of this structure is known to degrade performance (Sandson and Albert, 1984; Dolcos and McCarthy, 2006). However, the PFC is complex and its function is subject to the quadratic influence of neuromodulation via the influence of noradrenaline and dopamine (Arnsten, 2009; Arnsten et al., 2012). Noradrenaline is associated with the mediation of arousal (Chrousos, 2009) whereas dopamine is involved in the processing of reward with regard to the ongoing tasks (Schultz, 2002). Both catecholamines exert an inverted-U relationship with the PFC neurons (Vijayraghavan et al., 2007; Robbins and Arnsten, 2009), a reduction of these neurochemicals will depress the firing rate of noradrenergic and dopaminergic PFC neurons (see Figure 1). This mechanism may explain why unstimulating and non-rewarding tasks (e.g., passive supervisory control over a sustained period) can inhibit executive functioning and induce mind wandering. Conversely, excessive levels can also have a deleterious effect by suppressing PFC neuron firing rate (Birnbaum et al., 1999). In addition to decreasing the activity of the PFC, dopamine and noradrenaline activate subcortical areas, such as basal ganglia, that trigger automated schemes and initiate automatic responses (Wickens et al., 2007). These automated behaviors have an advantage of speed compared to flexible but slower behaviors generated by the prefrontal cortex (Dolan, 2002). This neurological switch from prefrontal to subcortical areas, is presumed to derive from the early age of humanity to ensure survival (Arnsten, 2009). In modern times, it manifests itself as a process of defaulting to well-learned behaviors, which are effective for only operational situations that are simple and familiar. This is the mechanism that promotes perseveration (Dehais et al., 2019) in task scenarios that are complex and novel (Staal, 2004; Ellenbogen et al., 2006) or offer intrinsic, short-term rewards, e.g., landing at all costs after a long transatlantic flight (Causse et al., 2013). These fundamental neurological mechanisms illustrate that impaired operational performance cannot be simply explained in terms of limited resources, such as a concentration of dopamine, but must be viewed from a neuroergonomic perspective that emphasizes the complexity of interactions between brain areas that evolved over thousands of years.
FIGURE 1.
The dopamine pathway exerts a quadratic control over the PFC. A low or a high release of this neurochemical depresses PFC activation whereas an adequate concentration ensures optimal executive functioning (Vijayraghavan et al., 2007; Robbins and Arnsten, 2009). These neurobiological considerations bring interesting highlights to understand the mechanisms underlying the Yerkes and Dodson inverted-U law and the dynamic adaptability theory (Hancock and Warm, 1989). They also provide a relevant prospect to relate motivational aspects to behavioral responses. The noradrenaline pathway mediates the PFC activity and executive functioning in a similar fashion (see Aston-Jones and Cohen, 2005).
Attentional Dynamics and Dominance Effects
The existence of information processing resources can also be conceptualized as functional attentional networks in the brain. Michael Posner was the first to pioneer a network approach to the operationalization of resources in the early days of neuroimaging (Posner and Tudela, 1997). His influential analysis (Posner and Petersen, 1990; Posner and Dehaene, 1994; Petersen and Posner, 2012; Posner, 2012) described how specific networks were dedicated to the particular functions of attentional regulation, e.g., alerting, orientation, focus. This conceptualization developed into the delineation of a dorsal fronto-parietal network (e.g., intraparietal cortex, superior frontal cortex) that supports focused attention on specific task-relevant stimuli and a corresponding ventral fronto-parietal network (e.g., temporo-parietal cortex, inferior frontal cortex) in the right hemisphere, which activates in a bottom-up fashion to reorientate attention to interruptive stimuli (Corbetta and Shulman, 2002; Corbetta et al., 2008). Under nominal conditions, interaction between the dorsal and the ventral pathways ensure optimal trade-off between those attentional strategies associated with exploitation and exploration. However, under conditions of high task demand or stress or fatigue, this mechanism may become biased toward dominance of the dorsal over the ventral network, leading to attentional phenomena associated with inflexibility (Todd et al., 2005; Durantin et al., 2017; Edworthy et al., 2018; Dehais et al., 2019a). A similar dynamic of bias and dominance is apparent in the relationship between the dorsal and ventral pathways and the default mode network (Andrews-Hanna et al., 2014), which is associated with mind-wandering, spontaneous thoughts and disengagement from task-related stimuli (Fox et al., 2015).
Performance Monitoring and Effort Withdrawal
The capacity of the brain to monitor performance quality and progress toward task goals is another important function of the PFC during operational performance. The posterior medial frontal cortex (pMFC) is a central hub in a wider network devoted to performance monitoring, action selection and adaptive behavior (Ullsperger et al., 2014; Ninomiya et al., 2018). The pMFC is sensitive to error and failure to achieve a task goal (Ullsperger et al., 2007); the detection of failure represents an important cue for compensatory strategies, such as increased investment of mental effort (Hockey, 1997). This network is particularly important when the level of task demand experienced by the operator is associated with a high rate of error and increased probability of failure. The model of motivational intensity (Richter et al., 2016) predicts that effort is withdrawn from task performance if success likelihood is appraised to be very low (Hopstaken et al., 2015); similarly, models of behavioral self-regulation (Carver and Scheier, 2000) argue that task goals can be adjusted downward (i.e., lower levels of performance are tolerated as acceptable) or even abandoned if goal attainment is perceived to be impossible. There is evidence that increased likelihood of failure is associated with deactivation of the PFC (Durantin et al., 2014; Ewing et al., 2016; Fairclough et al., 2019), for operational performance where failure can often jeopardize the safety of oneself and others, increased likelihood of failure can also provoke strong emotional responses that are associated with stress and cognitive interference (Sarason et al., 1990), which can function as distractors from task activity in their own right (Dolcos and McCarthy, 2006; Qin et al., 2009; Gärtner et al., 2014).
This neuroergonomic approach provides a biological basis upon which to develop a concept of limited human information processing, with respect to competing neurological mechanisms, the influence of neuromodulation in the prefrontal cortex and antagonist directives between different functional networks in the brain. The prominence of inhibitory control coupled with competition between these neural networks delineate a different category of performance limitations during extremes of low vs. high mental workload, i.e., simultaneous activation of functional networks with biases toward mutually exclusive stimuli (external vs. internal) or contradictory directives (focal attention vs. reorientation of attention).
Understanding Performance Related Mental States
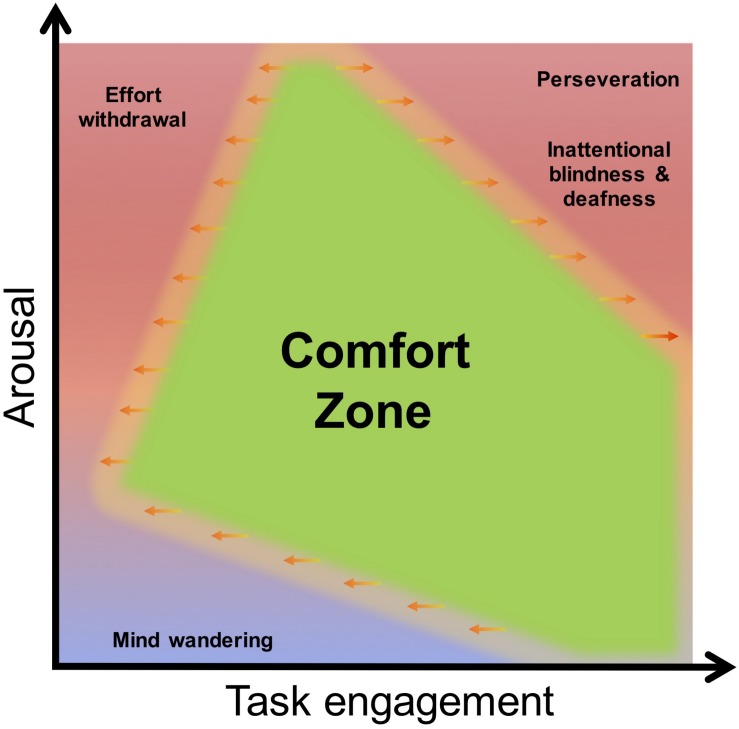
The previous sections have highlighted the complexity of those brain dynamics and networks that can introduce inherent limitations on human information processing. On the basis of this analysis, it is reasonable to target neurophysiological states and their associated mechanisms that account for impaired human performance (see Prinzel, 2002). This review has identified a number of suboptimal neurocognitive states that are predictive of degraded performance such as: mind wandering, effort withdrawal, perseveration, inattentional blindness and deafness. These states may be conceptually mapped along orthogonal dimensions of task engagement and arousal (Figure 2). Engagement is defined as an effortful investment in the service of task/cognitive goals (Pope et al., 1995; Matthews et al., 2002; Stephens et al., 2018), whereas arousal represents a state of physiological readiness to respond to external contingencies (Pribram and McGuinness, 1975).
FIGURE 2.
Performance, arousal and task engagement: the green zone conceptually describes the operator’s “comfort zone” where performance is optimal. The degraded mental states are mapped across a “task engagement” axis and an “arousal” axis. Interestingly, this point of view makes it possible to link the notion of engagement and degraded behavior in a simple way.
The Transactional Dimensions of Engagement and Arousal
The rationale for considering the dimension of task engagement is that performance is driven by goals and motivation (Bedny and Karwowski, 2004; Fairclough et al., 2013; Leontiev, 2014). Goal-oriented cognition theorists argue for the existence of mechanisms dedicated to maintain engagement (Atkinson and Cartwright, 1964), which are associated with an activation of an executive (Baddeley and Hitch, 1974) or task-positive network (Harrivel et al., 2013) within which the dorsolateral prefrontal cortex (DLPFC) exerts a crucial role (Goldman-Rakic, 1987; Curtis and D’Esposito, 2003). This structure plays a key role in the maintenance and updating of information that is relevant for ongoing task performance. The same structure interacts with dorsal and ventral attentional pathways to shift and focus attention to the most relevant stream of task-related information (Johnson and Zatorre, 2006). It is argued that human performance can be assessed in the context of a continuum of task engagement, ranging from disengagement (effort withdrawal, mind wandering) to high-engagement (perseveration, inattentional phenomena Lee, 2014).
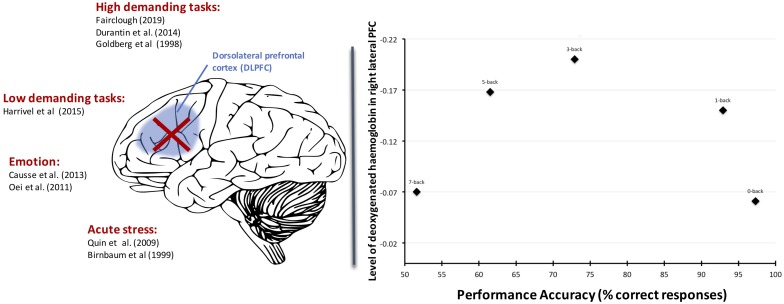
Arousal makes an important contribution to the conceptual space illustrated in Figure 2 because it modulates the homeostasis of the executive (see Arnsten, 2009 for a review) and attentional networks (see Coull, 1998 and Aston-Jones and Cohen, 2005 for review) via the dopaminergic and noradrenergic pathways. For instance, both extremes of low (Harrivel et al., 2013; Durantin et al., 2015) and high arousal can disengage the DLPFC (Goldberg et al., 1998; Arnsten, 2009; Qin et al., 2009; Causse et al., 2013; Durantin et al., 2014; Fairclough et al., 2019) and impair performance (see Figure 3 for summary). Similarly, low (Dehais et al., 2018) and high levels of arousal (Hancock and Warm, 1989; Tracy et al., 2000; Pecher et al., 2011) can alter the interactions between the dorsal and ventral attentional networks and indistinctly that lead either to inattentional phenomena (Molloy et al., 2015; Todd et al., 2005) or effort withdrawal (Oei et al., 2012; Dehais et al., 2015).
FIGURE 3.
Left part: Several types of stressors can yield to the deactivation of the DLPFC and in return drastically induce collapse of performance. Right part: An illustration with the N-Back task: the right-DLPFC deactivates when the task demands exceed mental capacity (7-Back condition) and is associated with reduced performance efficacy and effort withdrawal (from Fairclough et al., 2019).
Monitoring Performance Through Degraded Mental States
Table 1 presents a mapping between extremes of high and low engagement and arousal, their related neurocognitive states and how these states may be operationalized using neurophysiological measures in the laboratory and the field. Monitoring the activation and deactivation of the DLPFC represents a promising generic avenue to predict impaired performance across diverse states such as: mind wandering (Christoff et al., 2009; Harrivel et al., 2013), effort withdrawal (Ayaz et al., 2007; Izzetoglu et al., 2007; Durantin et al., 2014; Modi et al., 2018; Fairclough et al., 2018, 2019) and perseveration (Dehais et al., 2019). However, other neurological networks and sites should be considered as part of this analysis. Mind wandering is characterized by the concomitant activation of the default network, which includes the median prefrontal cortex (Christoff et al., 2009; Harrivel et al., 2013) and areas of the parietal cortex (Christoff et al., 2009).
TABLE 1.
Psycho-physiological and behavioral markers of different mental states related to engagement.
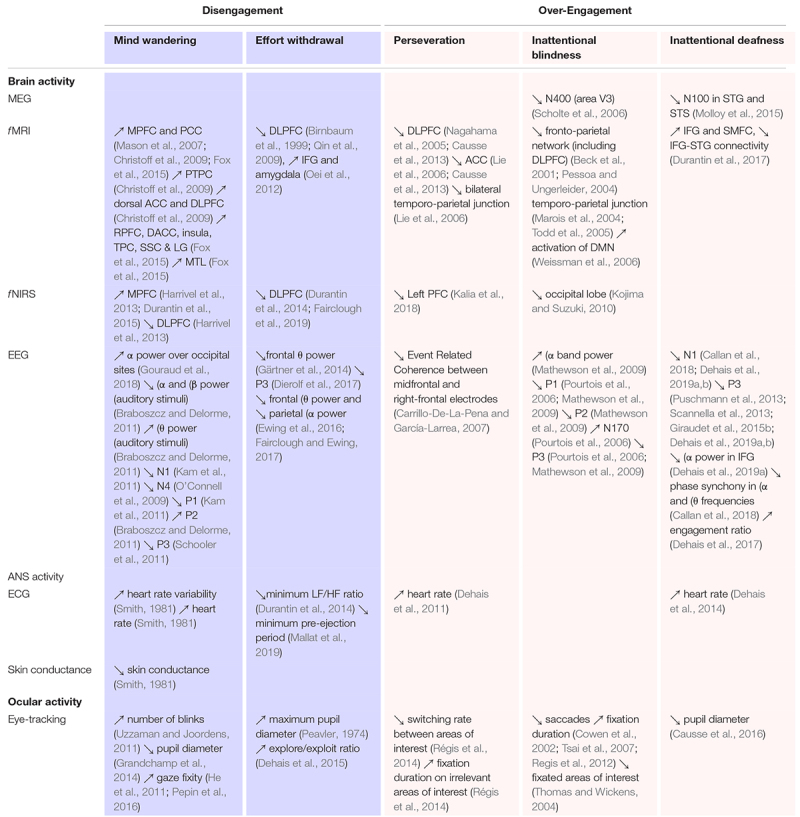
|
The blue and pink color-code respectively tags states induced by low and high task demand. RIFG, right inferior frontal gyrus; DMN, default mode network, MFG, middle frontal gyrus; ACC, anterior cingulate cortex; LFC, lateral frontal cortex; STC, superior temporal cortex; PFC, prefrontal cortex; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; PTPC, posterior temporoparietal cortex; DLPFC, dorsolateral prefrontal cortex; RPFC, rostrolateral prefrontal cortex; DACC, dorsal anterior cingulate cortex; TPC, temporopolar cortex; SSC, secondary somatosensory cortex; LG, lingual gyrus; MTL, medial temporal lobe; SMFC, superior medial frontal cortex; IFG, inferior frontal gyrus; STS, superior temporal sulcus, STG, superior temporal gyrus.
Secondly, attentional states, such as inattentional deafness and blindness, result from the activation of an attentional network involving the inferior frontal gyrus, the insula and the superior medial frontal cortex (Tombu et al., 2011; Callan et al., 2018; Dehais et al., 2019). These regions represent potential candidates upon which to identify attentional failures that can be complemented by monitoring dedicated primary perceptual (see Hutchinson, 2019, for a review) and integrative cortices (Molloy et al., 2015), as well as performing connectivity analyses (Callan et al., 2018). In addition, inattentional phenomena may result from the suppression of activity in the right temporo-parietal junction (TPJ), a part of the ventral network, which also blocks reorientation of attention and the processing of unexpected stimuli (Marois et al., 2004; Todd et al., 2005).
Thirdly, measures of arousal are used to characterize high engagement and delineate distinct mental states within the category of low task engagement (Figure 2). Heart rate (HR) and heart rate variability (HRV) can be used to assess the activation or co-activation of the two branches of the autonomous nervous system (i.e., sympathetic or parasympathetic) (Fairclough, 2008; Qin et al., 2009; Kreibig, 2010). For instance, fluctuations in HR are commonly observed during high task engagement and high arousal (De Rivecourt et al., 2008; Qin et al., 2009; Dehais et al., 2011). Moreover, spectral analyses computed over the EEG signal revealed that shifts in parietal alpha [8–12] Hz and frontal theta [4–8] Hz are relevant markers of arousal (see Borghini et al., 2014, for a review, Senoussi et al., 2017).
Finally, behavioral metrics such as ocular behavior can complement the detection of low and high levels of engagement (Table 1). Hence, eye tracking metrics (e.g., fixation and dwell times, saccadic activity, blink rate) can be used to characterize mind wandering (He et al., 2011; Pepin et al., 2016), inattentional blindness (Thomas and Wickens, 2004; Wickens, 2005), perseveration (Régis et al., 2014), focal vs. diffused attention (Goldberg and Kotval, 1999; Regis et al., 2012; Dehais et al., 2015), and to characterize the level of attentional engagement in a visual task (Cowen et al., 2002; Tsai et al., 2007).
These metrics provide some relevant prospects to identify the targeted deleterious mental states for especially for field studies as long as portable devices are concerned. It is worth noting that the extraction of several features (e.g., time and frequency domains) and the use of several devices is a way for robust diagnosis. Moreover, contextual information (e.g., time of the day, time on task) should be considered as well as actions on the user interface and system parameters (e.g., flight parameters) if available so as to better quantify the user’s mental state.
Solutions to Mitigate Degraded Performance
This review has identified some undesired mental states that account for degraded performance (see section “Understanding Performance Related Mental States” and “Solutions to Mitigate Degraded Performance”). A crucial step is to design cognitive countermeasures to prevent the occurrence of these phenomena. The formal framework that we proposed (see Table 1) paves the way to design neuro-adaptive technology for augmented cognition and enhanced human-machine teaming (Peysakhovich et al., 2018; Krol et al., 2019; Stamp et al., 2019). The implementation of such neuro-adaptive technology relies on a pipeline that consists of a signal acquisition step, a preprocessing step to improve the signal-to-noise ratio, a feature extraction step, a classification step to diagnose the current mental states, and lastly an adaptation step (Zander and Kothe, 2011; Roy and Frey, 2016). This last step implies the implementation of formal decisional unit (Gateau et al., 2018) that dynamically close the loop by triggering the most appropriate cognitive countermeasures (May and Baldwin, 2009). There are currently three types of mitigating solutions to instigate a change in behaviors via: (1) adaptation of the user interface, (2) adaptation of the task and of the level automation, and the (3) “neuro-adaptation” of the end-users.
Adaptation of the User Interface
The first category of neuroadaptive countermeasure consists of triggering new types of notifications via the user interface to alert of impeding hazards. The design of these countermeasures is generally grounded on neuroergonomics basis so that these warning can reach awareness when other means have failed. Following this perspective, Dehais et al. (2010, 2012), Imbert et al. (2014) and Saint Lot et al. (2020) have demonstrated that very brief (∼200 ms) and located information removal was an efficient mean to mitigate perseveration by forcing disengagement from non-relevant tasks. Souza et al. (2016) demonstrated that digital nudging (see Weinmann et al., 2016) could be used to mitigate poor decision making and cognitive bias associated with perseveration. Imbert et al. (2014) designed attention-grabbing stimuli grounded on vision research and demonstrated that yellow chevrons pulsing at a cycle of 1 Hz can re-orientate attention and mitigate inattentional blindness. Jahanpour et al. (2018) has explored the design of pop-up videos that display the gestures to be performed by exploiting the property of mirror neurons. This visual “motor cue” approach was tested and drastically reduced reaction time to alerts during complex situations and appears to be a promising method to prevent effort withdrawal (Causse et al., 2012). In a similar fashion, Navarro et al. (2010) implemented a force-feedback steering wheel to prime the motor response from the driver. This device was found to optimize drivers’ behavior during demanding driving scenario. This latter study demonstrated how tactile notifications can alert human operators of impeding hazards (Lewis et al., 2014; Russell et al., 2016), especially when other sensory channels of information (e.g., visual stream) are saturated (Elliott et al., 2011). However, there are potential limits to the effectiveness of these types of notifications and stimulation (Murphy and Dalton, 2016; Riggs and Sarter, 2019). Other research indicates that multimodal alerts (Giraudet et al., 2015a; Gaspar et al., 2017) increase the likelihood of attentional capture. In addition, Lee et al. (2018) designed a motion seat that modifies the driver’s seat position and posture across time to diminish the potential deleterious effect of mind wandering. Similar concepts have been applied to aviation (Zaneboni and Saint-Jalmes, 2016).
Task and Automation Adaptation
The second category of neuroadaptive countermeasure is the dynamic reallocation of tasks between humans and automation to maintain the performance efficacy of the operators (Freeman et al., 1999; Parasuraman et al., 1999; Prinzel et al., 2000; Scerbo, 2008; Stephens et al., 2018). The underlying concept in this case is to optimize human-human or human(s)-system(s) cooperation according to criteria of availability and skills of human and artificial agents (Gateau et al., 2016). For instance, Prinzel et al. (2000) utilized the continuous monitoring of brain waves that could be used to drive the level of automation and optimize the user’s level of task engagement. Similarly, some authors managed to optimize air traffic controllers’ task demand by triggering different levels of assistance (Aricò et al., 2016; Di Flumeri et al., 2019). These latter studies reported better human performance when neuro-adaptive automation was switched on compared to other conditions. Gateau et al. (2016) implemented an online attentional state estimator coupled with a stochastic decision framework to dynamically adapt authority sharing between human and robots in a search and rescue scenario to prevent effort withdrawal on the part of the human. In a more extreme fashion, Callan et al. (2016) revealed that it is possible to decode user motor intention so automation can perform on behalf of the user to drastically reduce the response time in emergency situations (e.g., collision with terrain). In the future, it is assumed that aircraft designers will implement adaptive automation technology that takes over from the pilots by either inhibiting their inputs on the flight deck or performing automated evasive actions (e.g., automatic pull-up) to prevent from perseveration. A complementary approach is to modulate task difficulty to maintain the task challenging but achievable while preventing the occurrence of task withdrawal (Ewing et al., 2016) or mind wandering (Freeman et al., 2004; Ewing et al., 2016). The online modulation of the tasks does not necessarily reduce the difficulty of the task. For instance, Verwey and colleagues demonstrated that the addition of an entertaining task while driving improved the operator’s ability to maintain their level of task engagement over long period of time (Verwey and Zaidel, 1999). Similarly, it has been suggested that switching the types of tasks presented to the user can prevent the deleterious effect of fatigue and disengagement (Hockey, 2011).
Neuro-Adaptation of the End-User(s)
The third and final category aims to warn the users of their mental state and “stimulate” neurological activity in order to augment performance. One of the most promising approach relies on the implementation of Neurofeedback (see Gruzelier, 2014; Enriquez-Geppert et al., 2017 for reviews). The principle of the latter technique is to provide feedback in real-time to the users of their mental states in the form of a visual, tactile or auditory stimulus. The users can utilize these signals learn to regulate their brain activity and in return improve their executive (Enriquez-Geppert et al., 2013), mental flexibility (Enriquez-Geppert et al., 2014), and attentional abilities (Egner and Gruzelier, 2001) as well as enhance their task engagement (Egner and Gruzelier, 2004). However, the effects of this approach on mind wandering remain unclear (Gonçalves et al., 2018). Transcranial direct current stimulation (tDCS) represents a technique of neuromodulation that can be used to boost executive functioning (see Callan and Perrey, 2019; Cinel et al., 2019). This portable device can be combined with EEG and fNIRS and used in the context of real-life task performance for the purpose of on-line neuromodulation (McKendrick et al., 2015; Gateau et al., 2018). For example, a number of studies support the position that neurostimulation can: enhance mental flexibility and mitigate perseveration (Leite et al., 2011; Jeon and Han, 2012), improve visual attention (Falcone et al., 2012; Nelson et al., 2015), improve executive functioning in multitasking situations (Nelson et al., 2016) and increase alertness (McIntire et al., 2014; Nelson et al., 2014). There are other types of environmental stimulation such as vivid light exposure, especially during night flights, which can promote an optimal level of alertness (see Anund et al., 2015) without altering flight crew performance (see Caldwell et al., 2009). Promising results have also been highlighted by using light exposure in cars (Taillard et al., 2012). The use of light exposure and tDCS should be considered with caution as there is a need to investigate the very long-term efficiency and potential side effects. Alternatively, some authors proposed to use cold-air jet to decrease hypovigilance (Reyner and Horne, 1998), but with contradictory findings.
Synthesis of Neuro-Adaptive Solutions
The following illustration (see Figure 4) depicts the three families of neuro-adaptive based solutions to mitigate performance impairment.
FIGURE 4.
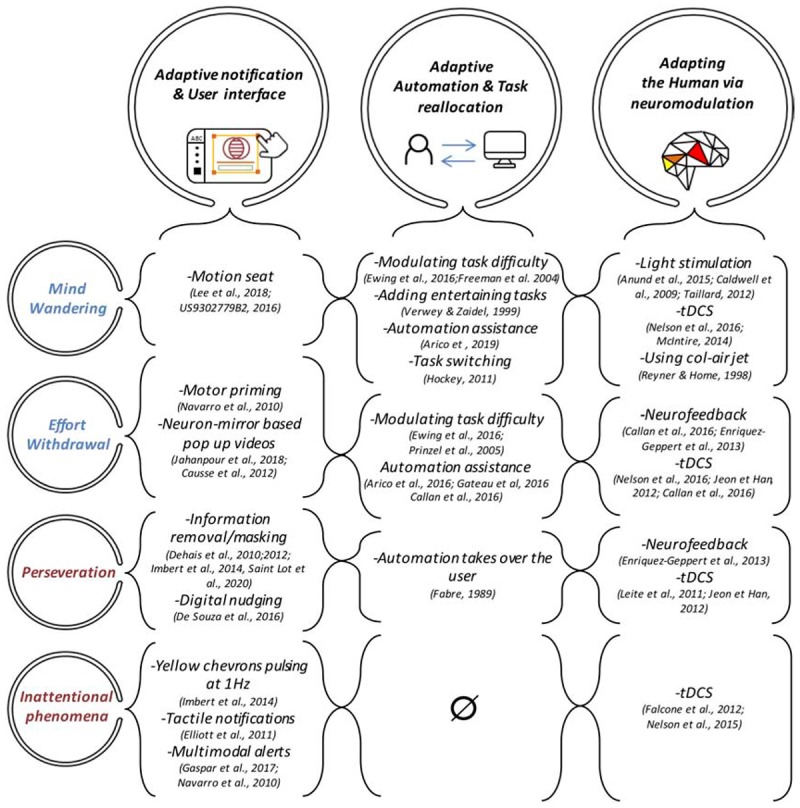
The three types of Neuroadaptive countermeasures dedicated to mitigate the undesirable mental states. Inattentional deafness and Inattentional blindness mental states were merged into “Inattentional phenomena” as no neuroadaptive countermeasure were implemented to explicitly address failure of auditory attention to the exception of multimodal alerts. Moreover, no adaptive automation-based solutions were designed to prevent from inattentional states. This demonstrates the need to conduct more research in this direction.
The three types of neuroadaptive solutions offer promising prospects to mitigate the onset and likelihood of undesirable neurocognitive states. However, they should be delivered in a transparent, meaningful, and timely manner (i.e., when needed) so they are relevant and understood (Dorneich et al., 2016; Sebok et al., 2017), otherwise these types of intervention have the potential for undesirable consequences, such as performance impairment and reduced trust in technology; this point is particularly true for adaptive automation solutions that take over from humans, especially under critical scenarios (see Dorneich et al., 2016; Dehais et al., 2019). One solution is to combine different families of neuroadaptive cognitive countermeasures to maximize their efficiency. Ideally, we would argue to use a gradient of solutions such as (1) the continuous display of the users’ mental states via neurofeedback techniques to give them the opportunity to regulate their brain activity; (2) using notifications to suggest to the users to delegate some tasks to automation in case they don’t manage to modulate their mental states; (3) adapting the user interface (e.g., information removal, flashing yellow chevrons) in case of a critical situation is detected and the previous solutions were inefficient; and (4) taking over if the users do not respond to any of the previous countermeasures.
Conclusion
This paper has argued that the concept of a limited resource provides a limited explanation for the breakdown of operational performance. Our neurophysiological analysis describes a number of additional mechanisms, such as perseveration and effort withdrawal, which do not represent finite resources per se. In both cases, explanations for performance breakdown are based upon neurological processes, such as dominance of specific neural networks or the heightened activity of specific mechanisms. We propose a two-dimensional framework of engagement and arousal that captures the importance of specific degraded mental sates associated with poor performance. The rationale for including the transactional concept of engagement in this scheme is to account for the goal-oriented aspect of cognition. The benefit of including the transactional concept of arousal is to make a distinction between two categories of disengagement, one that is accompanied by high arousal (effort withdrawal) and low arousal (mind wandering) – and to link this conceptual distinction to known neurophysiological effects (see Figure 1). Nonetheless, this approach remains at the conceptual level and minimizes connections to the complexity of brain functioning. To that end, we reviewed and identified several markers at the neurophysiological, physiological and behavioral level of undesirable mental states linked to poor performance.
This neuroergonomic framework encompasses operationali- zations of these undesirable states that can be monitored continuously in an objective fashion. Such considerations eventually lead to propose a typology of neuroadaptive countermeasures and open promising perspectives to mitigate the degradation of human performance. However, to the authors’ very best knowledge, most of the neuroadaptive experimental studies have focused on human-machine dyad situations. We believe that recent research on hyperscanning (Babiloni and Astolfi, 2014), physiological synchrony (Palumbo et al., 2017) and collaborative BCIs (Cinel et al., 2019) have opened promising prospects to improve teaming such as human-human, human(s)-machine(s) interactions. Future research should involve more complex teaming scenarios and enrich the different neuroadaptive solutions. We sincerely hope that this review will encourage research efforts to identify additional degraded mental states and associated neurophysiological markers as well as to implement neuroadaptive solutions for safer and efficient human-human and human(s)-machine(s) interactions.
Author Contributions
All authors have made a substantial and intellectual contribution to this review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by ANITI ANR-19-PI3A-0004 (Neuroadaptive technology for mixed initiative interactions Chair).
References
- Andrews-Hanna J. R., Smallwood J., Spreng R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 1316:29. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett J. (2002). Subjective rating scales: science or art? Ergonomics 45 966–987. 10.1080/00140130210166951 [DOI] [PubMed] [Google Scholar]
- Anund A., Fors C., Kecklund G., Leeuwen W. V., Åkerstedt T. (2015). Countermeasures for Fatigue in Transportation: A Review of Existing Methods for Drivers on Road, Rail, Sea And In Aviation. Linköping: Statens vägoch transportforskningsinstitut. [Google Scholar]
- Aricò P., Borghini G., Di Flumeri G., Colosimo A., Bonelli S., Golfetti A., et al. (2016). Adaptive automation triggered by EEG-based mental workload index: a passive brain-computer interface application in realistic air traffic control environment. Front. Hum. Neurosci. 10:539. 10.3389/fnhum.2016.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10:410. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F., Wang M. J., Paspalas C. D. (2012). Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76 223–239. 10.1016/j.neuron.2012.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28 403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Atkinson J. W., Cartwright D. (1964). Some neglected variables in contemporary conceptions of decision and performance. Psychol. Rep. 14 575–590. 10.2466/pr0.1964.14.2.575 [DOI] [Google Scholar]
- Ayaz H., Dehais F., (eds) (2018). Neuroergonomics: The Brain at Work and in Everyday Life. Cambridge, MA: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz H., Izzetoglu M., Bunce S., Heiman-Patterson T., Onaral B. (2007). “Detecting cognitive activity related hemodynamic signal for brain computer interface using functional near infrared spectroscopy,” in Proceedins of the 3rd International IEEE EMBS conference: CNE’07, Hawaii, 342–345. [Google Scholar]
- Ayaz H., Shewokis P. A., Bunce S., Izzetoglu K., Willems B., Onaral B. (2012). Optical brain monitoring for operator training and mental workload assessment. Neuroimage 59 36–47. 10.1016/j.neuroimage.2011.06.023 [DOI] [PubMed] [Google Scholar]
- Babiloni F., Astolfi L. (2014). Social neuroscience and hyperscanning techniques: past, present and future. Neurosci. Biobehav. Rev. 44 76–93. 10.1016/j.neubiorev.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. D., Hitch G. (1974). “Working memory,” in The Psychology of Learning and Motivation: Advances in Research and Theory, Vol. 8 eds Bower G. H. (New York, NY: Academic Press), 47–89. 10.1016/S0079-7421(08)60452-1 [DOI] [Google Scholar]
- Beanland V., Chan E. H. C. (2016). The relationship between sustained inattentional blindness and working memory capacity. Attent. Percept. Psychophys. 78 808–817. 10.3758/s13414-015-1027-x [DOI] [PubMed] [Google Scholar]
- Beatty J. (1986). “Computation, control and energetics: a biological perspective,” in Energetics and Human Information Processing, ed. Hockey G. R. J. (Dordrecht: Springer; ). [Google Scholar]
- Beck D. M., Rees G., Frith C. D., et Lavie N. (2001). Neural correlates of change detection and change blindness. Nat. Neurosci. 4 645–650. 10.1038/88477 [DOI] [PubMed] [Google Scholar]
- Bedny G. Z., Karwowski W. (2004). Activity theory as a basis for the study of work. Ergonomics 47 134–153. 10.1080/00140130310001617921 [DOI] [PubMed] [Google Scholar]
- Birnbaum S., Gobeske K. T., Auerbach J., Taylor J. R., Arnsten A. F. (1999). A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in the prefrontal cortex. Biol. Psychiatry 46 1266–1274. 10.1016/s0006-3223(99)00138-9 [DOI] [PubMed] [Google Scholar]
- Borghini G., Astolfi L., Vecchiato G., Mattia D., Babiloni F. (2014). Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental work- load, fatigue and drowsiness. Neurosci. Biobehav. Rev. 44 58–75. 10.1016/j.neubiorev.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Borragan Pedraz G., Peigneux P. (2016). Behavioural Bases and Functional Dynamics of Cognitive Fatigue, Doctorat Sciences psychologiques et de l’éducation, Louvain. [Google Scholar]
- Braboszcz C., Delorme A. (2011). Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage 54 3040–3047. 10.1016/j.neuroimage.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Brand-D’Abrescia M., Lavie N. (2008). Task coordination between and within sensory modalities: effects on distraction. Percept. Psychophys. 70 508–515. 10.3758/pp.70.3.508 [DOI] [PubMed] [Google Scholar]
- Bredemeier K., Simons D. J. (2012). Working memory and inattentional blindness. Psychon. Bull. Rev. 19 239–244. 10.3758/s13423-011-0204-8 [DOI] [PubMed] [Google Scholar]
- Broadbent D. E. (1971). Decision and Stress. Oxford: Academic Press. [Google Scholar]
- Bromiley M. (2008). Have you ever made a mistake? RCoA Bull. 48 2442–2445. [Google Scholar]
- Brynjolfsson E., McAfee A. (2014). The Second Machine Age: Work, Progress, and Prosperity in a Time of Brilliant Technologies, 1st Edn New York, NY: W. W. Norton & Company. [Google Scholar]
- Caldwell J. A., Mallis M. M., Caldwell J. L., Paul M. A., Miller J. C., Neri D. F. (2009). Fatigue countermeasures in aviation. Aviat. Space Environ. Med. 80 29–59. 10.3357/asem.2435.2009 [DOI] [PubMed] [Google Scholar]
- Callan D., Perrey S. (2019). “The use of tDCS and rTMS methods in neuroergonomics,” in Neuroergonomics, eds Ayaz H., Dehais F. (Cambridge, MA: Academic Press; ), 31–33. 10.1016/b978-0-12-811926-6.00005-1 [DOI] [Google Scholar]
- Callan D. E., Gateau T., Durantin G., Gonthier N., De-Hais F. (2018). Disruption in neural phase synchrony is related to identification of inattentional deafness in real-world setting. Hum. Brain Mapp. 39 2596–2608. 10.1002/hbm.24026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan D. E., Terzibas C., Cassel D. B., Sato M. A., Parasuraman R. (2016). The brain is faster than the hand in split-second intentions to respond to an impending hazard: a simulation of neuroadaptive automation to speed recovery to perturbation in flight attitude. Front. Hum. Neurosci. 10:187. 10.3389/fnhum.2016.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-De-La-Pena M. T., García-Larrea L. (2007). Right frontal event related EEG coherence (ERCoh) differentiates good from bad performers of the Wisconsin Card Sorting Test (WCST). Clin. Neurophysiol. 37 63–75. 10.1016/j.neucli.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Carver C. S., Scheier M. F. (2000). “On the structure of behavioural self-regulation,” in Handbook of Self-Regulation, eds Boekaerts M., Pintrich P. R., Zeidner M. (San Diego, CA: Academic Press; ), 41–84. 10.1016/b978-012109890-2/50032-9 [DOI] [Google Scholar]
- Causse M., Imbert J. P., Giraudet L., Jouffrais C., Tremblay S. (2016). The role of cognitive and perceptual loads in inattentional deafness. Front. Hum. Neurosci. 10:344. 10.3389/fnhum.2016.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causse M., Péran P., Dehais F., Caravasso C. F., Zeffiro T., Sabatini U., et al. (2013). Affective decision making under uncertainty during a plausible aviation task: an fMRI study. NeuroImage 71 19–29. 10.1016/j.neuroimage.2012.12.060 [DOI] [PubMed] [Google Scholar]
- Causse M., Phan J., Ségonzac T., Dehais F. (2012). Mirror neuron based alerts for control flight into terrain avoidance. Adv. Cognit. Eng. Neuroergon. 16 157–166. [Google Scholar]
- Christoff K., Gordon A. M., Smallwood J., Smith R., Schooler J. W. (2009). Experience sampling during fmri reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U.S.A. 106 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G. P. (2009). Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5:374. 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Cinel C., Valeriani D., Poli R. (2019). Neurotechnologies for human cognitive augmentation: current state of the art and future prospects. Front. Hum. Neurosci. 13:13. 10.3389/fnhum.2019.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colflesh G. J., Conway A. R. (2007). Individual differences in working memory capacity and divided attention in dichotic listening. Psychon. Bull. Rev. 14 699–703. 10.3758/bf03196824 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3:201. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Coull J. T. (1998). Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog. Neurobiol. 55 343–361. 10.1016/s0301-0082(98)00011-2 [DOI] [PubMed] [Google Scholar]
- Coultrip R., Granger R., Lynch G. (1992). A cortical model of winner-take-all competition via lateral inhibition. Neural Netw. 5 47–54. 10.1016/s0893-6080(05)80006-1 [DOI] [Google Scholar]
- Cowen L., Ball L. J., Delin J. (2002). “An eye movement analysis of web page usability,” in People and Computers Xvi-memorable Yet Invisible, eds Faulkner X., Finlay J., Détienne F. (Berlin: Springer; ), 317–335. 10.1007/978-1-4471-0105-5_19 [DOI] [Google Scholar]
- Curtis C. E., D’Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cognit. Sci. 7 415–423. 10.1016/s1364-6613(03)00197-9 [DOI] [PubMed] [Google Scholar]
- De Rivecourt M., Kuperus M. N., Post W. J., Mulder L. J. M. (2008). Cardiovascular and eye activity measures as indices for momentary changes in mental effort during simulated flight. Ergonomics 51 1295–1319. 10.1080/00140130802120267 [DOI] [PubMed] [Google Scholar]
- De Waard D. (1996). The Measurement of Driver Mental Workload, PhD Thesis., Groningen: Rijksuniversiteit Groningen. [Google Scholar]
- Dehais F., Causse M., Tremblay S. (2011). Mitigation of conflicts with automation: use of cognitive countermeasures. Hum. Fact. 53 448–460. 10.1177/0018720811418635 [DOI] [PubMed] [Google Scholar]
- Dehais F., Causse M., Vachon F., Régis N., Menant E., Tremblay S. (2014). Failure to detect critical auditory alerts in the cockpit: evidence for inattentional deafness. Hum. Fact. 56 631–644. 10.1177/0018720813510735 [DOI] [PubMed] [Google Scholar]
- Dehais F., Causse M., Vachon F., Tremblay S. (2012). Cognitive conflict in human–automation interactions: a psychophysiological study. Appl. Ergon. 43 588–595. 10.1016/j.apergo.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Dehais F., Duprès A., Di Flumeri G., Verdière K. J., Borghini G., Babiloni F., et al. (2018). Monitoring Pilots Cognitive Fatigue with Engagement Features in Simulated and actual Flight Conditions Using an Hybrid fNIRS-EEG Passive BCI. IEEE SMC. Availale at: https://hal.archives-ouvertes.fr/hal-01959452. [Google Scholar]
- Dehais F., Hodgetts H. M., Causse M., Behrend J., Durantin G., Tremblay S. (2019). Momentary lapse of control: a cognitive continuum approach to understanding and mitigating perseveration in human error. Neurosci. Biobehav. Rev. 100 252–262. 10.1016/j.neubiorev.2019.03.006 [DOI] [PubMed] [Google Scholar]
- Dehais F., Peysakhovich V., Scannella S., Fongue J., Gateau T. (2015). “Automation surprise in aviation: real- time solutions,” in Proceedings of the 33rd annual ACM conference on human factors in computing systems, New York, NY, 2525–2534. [Google Scholar]
- Dehais F., Rida I., Roy R., Iversen J., Mullen T., Callan D. (2019a). “A pBCI to predict attentional error before it happens in real flight conditions,” in Proceedins of the Conference: 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari. [Google Scholar]
- Dehais F., Roy R. N., Scannella S. (2019b). Inattentional deafness to auditory alarms: inter-individual differences, electrophysiological signature and single trial classification. Behav. Brain Res. 360 51–59. 10.1016/j.bbr.2018.11.045 [DOI] [PubMed] [Google Scholar]
- Dehais F., Roy R. N., Durantin G., Gateau T., Callan D. (2017). “EEG-engagement index and auditory alarm misperception: an inattentional deafness study in actual flight condition,” in Proceedins of the International Conference on Applied Human Factors and Ergonomics, Washington D.C., 227–234. 10.1007/978-3-319-60642-2_21 [DOI] [Google Scholar]
- Dehais F., Tessier C., Christophe L., Reuzeau F. (2010). “The perseveration syndrome in the pilots’ activity: guide- lines and cognitive countermeasures,” in Human Error, Safety and Systems Development, eds Palanque P., Vanderdonckt J., Winkler M. (Berlin: Springer; ), 68–80. 10.1007/978-3-642-11750-3_6 [DOI] [Google Scholar]
- Di Flumeri G., De Crescenzio F., Berberian B., Ohneiser O., Kramer J., Aricò P., et al. (2019). Brain–computer interface-based adaptive automation to prevent out-of-the-loop phenomenon in air traffic controllers dealing with highly automated systems. Front. Hum. Neurosci. 13:296. 10.3389/fnhum.2019.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierolf A. M., Fechtner J., Böhnke R., Wolf O. T., Naumann E. (2017). Influence of acute stress on response inhibition in healthy men: an ERP study. Psychophysiology 54 684–695. 10.1111/psyp.12826 [DOI] [PubMed] [Google Scholar]
- Dolan R. J. (2002). Emotion, cognition, and behavior. Science 298 1191–1194. [DOI] [PubMed] [Google Scholar]
- Dolcos F., McCarthy G. (2006). Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 26 2072–2079. 10.1523/jneurosci.5042-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorneich M. C., Rogers W., Whitlow S. D., DeMers R. (2016). Human performance risks and benefits of adaptive systems on the flight deck. Int. J. Aviat. Psychol. 26 15–35. 10.1080/10508414.2016.1226834 [DOI] [Google Scholar]
- Duffy E. (1962). Activation and Behaviour. New York, NY: Wiley. [Google Scholar]
- Durantin G., Dehais F., Delorme A. (2015). Characterization of mind wandering using fNIRS. Front. Syst. Neurosci. 9:45 10.3389/fnsys.2015.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantin G., Dehais F., Gonthier N., Terzibas C., Callan D. E. (2017). Neural signature of inattentional deafness. Hum. Brain Mapp. 38 5440–5455. 10.1002/hbm.23735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantin G., Gagnon J.-F., Tremblay S., Dehais F. (2014). Using near infrared spectroscopy and heart rate variability to detect mental overload. Behav. Brain Res. 259 16–23. 10.1016/j.bbr.2013.10.042 [DOI] [PubMed] [Google Scholar]
- Edworthy J., Reid S., Peel K., Lock S., Williams J., Newbury C., et al. (2018). The impact of workload on the ability to localize audible alarms. Appl. Ergon. 72 88–93. 10.1016/j.apergo.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Egner T., Gruzelier J. H. (2001). Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. Neuroreport 12 4155–4159. 10.1097/00001756-200112210-00058 [DOI] [PubMed] [Google Scholar]
- Egner T., Gruzelier J. H. (2004). EEG biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related brain potentials. Clin. Neurophysiol. 115 131–139. 10.1016/s1388-2457(03)00353-5 [DOI] [PubMed] [Google Scholar]
- Ellenbogen M. A., Schwartzman A. E., Stewart J., Walker C.-D. (2006). Automatic and effortful emotional informa- tion processing regulates different aspects of the stress response. Psychoneuroendocrinology 31 373–387. 10.1016/j.psyneuen.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Elliott L. R., Schmeisser E. T., Redden E. S. (2011). “Development of tactile and haptic systems for US infantry navigation and communication,” in Proceedins of the Symposium on Human Interface (Berlin: Springer; ), 399–407. 10.1007/978-3-642-21793-7_45 [DOI] [Google Scholar]
- Enriquez-Geppert S., Huster R. J., Figge C., Herrmann C. S. (2014). Self-regulation of frontal-midline theta facilitates memory updating and mental set shifting. Front. Behav. Neurosci. 8:420. 10.3389/fnbeh.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Geppert S., Huster R. J., Herrmann C. S. (2013). Boosting brain functions: improving executive functions with behavioral training, neurostimulation, and neurofeedback. Int. J. Psychophysiol. 88 1–16. 10.1016/j.ijpsycho.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S., Huster R. J., Herrmann C. S. (2017). EEG-neurofeedback as a tool to modulate cognition and behavior: a review tutorial. Front. Hum. Neurosci. 11:51. 10.3389/fnhum.2017.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing K. C., Fairclough S. H., Gilleade K. (2016). Evaluation of an adaptive game that uses EEG measures validated during the design process as inputs to a biocybernetic loop. Front. Hum. Neurosci. 10:223. 10.3389/fnhum.2016.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough S., Ewing K., Burns C., Kreplin U. (2019). “Neural efficiency and mental workload: locating the red line,” in Neuroergonomics, eds Johnson A., Proctor R. W. (Cambridge, MA: Academic Press; ), 73–77. 10.1016/b978-0-12-811926-6.00012-9 [DOI] [Google Scholar]
- Fairclough S. H. (2008). Fundamentals of physiological computing. Interact. Comput. 21 133–145. 10.1016/j.intcom.2008.10.011 [DOI] [Google Scholar]
- Fairclough S. H., Burns C., Kreplin U. (2018). FNIRS activity in the prefrontal cortex and motivational intensity: impact of working memory load, financial reward, and correlation-based signal improvement. Neurophotonics 5 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough S. H., Ewing K. (2017). The effect of task demand and incentive on neurophysiological and cardiovascular markers of effort. Int. J. Psychophysiol. 119 58–66. 10.1016/j.ijpsycho.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Fairclough S. H., Gilleade K., Ewing K. C., Roberts J. (2013). Capturing user engagement via psychophysiology: measures and mechanisms for biocybernetic adaptation. Int. J. Auton. Adapt. Commun. Syst. 6 63–79. [Google Scholar]
- Falcone B., Coffman B. A., Clark V. P., Parasuraman R. (2012). Transcranial direct current stimulation augments perceptual sensitivity and 24-hour retention in a complex threat detection task. PLoS ONE 7:e34993. 10.1371/journal.pone.0034993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. C., Spreng R. N., Ellamil M., Andrews-Hanna J. R., Christoff K. (2015). The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Freeman F. G., Mikulka P. J., Prinzel L. J., Scerbo M. W. (1999). Evaluation of an adaptive automation system using three EEG indices with a visual tracking task. Biol. Psychol. 50 61–76. 10.1016/s0301-0511(99)00002-2 [DOI] [PubMed] [Google Scholar]
- Freeman F. G., Mikulka P. J., Scerbo M. W., Scott L. (2004). An evaluation of an adaptive automation system using a cognitive vigilance task. Biol. Psychol. 67 283–297. 10.1016/j.biopsycho.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Gärtner M., Rohde-Liebenau L., Grimm S., Bajbouj M. (2014). Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology 43 105–113. 10.1016/j.psyneuen.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Gaspar J. G., Brown T. L., Schwarz C. W., Lee J. D., Kang J., Higgins J. S. (2017). Evaluating driver drowsiness countermeasures. Traff. Inj. Prevent. 18 S58–S63. [DOI] [PubMed] [Google Scholar]
- Gateau T., Ayaz H., Dehais F. (2018). In silico versus over the clouds: on-the-fly mental state estimation of aircraft pilots, using a functional near infrared spectroscopy based passive-BCI. Front. Hum. Neurosci. 12:187. 10.3389/fnhum.2018.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateau T., Chanel C. P. C., Le M. H., Dehais F. (2016). “Considering human’s non-deterministic behavior and his availability state when designing a collaborative human-robots system,” in Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Las Vegas, NV, 4391–4397. [Google Scholar]
- Giraudet L., Imbert J. P., Bérenger M., Tremblay S., Causse M. (2015a). The Neuroergonomic evaluation of human machine interface design in air traffic control using behavioral and EEG/ERP measures. Behav. Brain Res. 294 246–253. 10.1016/j.bbr.2015.07.041 [DOI] [PubMed] [Google Scholar]
- Giraudet L., St-Louis M.-E., Scannella S., Causse M. (2015b). P300 event-related potential as an indicator of inat- tentional deafness? PLoS ONE 10:e0118556. 10.1371/journal.pone.0118556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. H., Kotval X. P. (1999). Computer interface evaluation using eye movements: methods and constructs. Int. J. Ind. Ergon. 24 631–645. 10.1016/s0169-8141(98)00068-7 [DOI] [Google Scholar]
- Goldberg T. E., Berman K. F., Fleming K., Ostrem J., Van Horn J. D., Esposito G., et al. (1998). Uncoupling cognitive workload and prefrontal cortical physiology: a PER rCBF study. Neuroimage 7 296–303. 10.1006/nimg.1998.0338 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. (1987). Handbook of Physiology. The Nervous System. Bethesda, MD: American Physiological Society, 373417. [Google Scholar]
- Gonçalves ÓF., Carvalho S., Mendes A. J., Leite J., Boggio P. S. (2018). Neuromodulating attention and mind-wandering processes with a single session real time EEG. Appl. Psychophysiol. Biofeedback 43 143–151. 10.1007/s10484-018-9394-4 [DOI] [PubMed] [Google Scholar]
- Gouraud J., Delorme A., Berberian B. (2018). Out of the loop, in your bubble: mind wandering is independent from automation reliability, but influences task engagement. Front. Hum. Neurosci. 12:383. 10.3389/fnhum.2018.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp R., Braboszcz C., Delorme A. (2014). Oculometric variations during mind wandering. Front. Psychol. 5:31. 10.3389/fpsyg.2014.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzelier J. H. (2014). EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 44 124–141. 10.1016/j.neubiorev.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Hancock P. A., Desmond P. A. (2001). Stress, Workload, and Fatigue. Mahwah, NJ: Erlbaum. [Google Scholar]
- Hancock P. A., Meshkati N. (1988). Human Mental Workload. Amsterdam: North-Holland. [Google Scholar]
- Hancock P. A., Warm J. S. (1989). A dynamic model of stress and sustained attention. Hum. Fact. 31 519–537. 10.1177/001872088903100503 [DOI] [PubMed] [Google Scholar]
- Harrivel A. R., Weissman D. H., Noll D. C., Peltier S. J. (2013). Monitoring attentional state with fnirs. Front. Human Neurosci. 7:861. 10.3389/fnhum.2013.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Becic E., Lee Y.-C., McCarley J. S. (2011). Mind wandering behind the wheel: performance and oculomotor correlates. Human Factors 53 13–21. 10.1177/0018720810391530 [DOI] [PubMed] [Google Scholar]
- Herff C., Heger D., Fortmann O., Hennrich J., Putze F., Schultz T. (2014). Mental workload during n-back task—quantified in the prefrontal cortex using fNIRS. Front. Hum. Neurosci. 7:935. 10.3389/fnhum.2013.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey G. R. J. (1997). Compensatory control in the regulation of human performance under stress and high workload: a cognitive-energetical framework. Biol. Psychol. 45 73–93. 10.1016/s0301-0511(96)05223-4 [DOI] [PubMed] [Google Scholar]
- Hockey G. R. J. (2011). “A motivational control theory of cognitive fatigue,” in Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications, ed. Ackerman P. L. (Washington, DC: American Psychological Association; ). [Google Scholar]
- Hockey G. R. J., Coles M. G., Gaillard A. W. (1986). “Energetical issues in research on human information processing,” in Energetics and Human Information Processing, eds Hockey G. M., Gaillard A. W. K., Coles M. (Berlin: Springer; ), 3–21. 10.1007/978-94-009-4448-0_1 [DOI] [Google Scholar]
- Hopstaken J. F., Van Der Linden D., Bakker A. B., Kompier M. A. (2015). A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 52 305–315. 10.1111/psyp.12339 [DOI] [PubMed] [Google Scholar]
- Hutchinson B. T. (2019). Toward a theory of consciousness: a review of the neural correlates of inattentional blindness. Neurosci. Biobehav. Rev. 104 87–99. 10.1016/j.neubiorev.2019.06.003 [DOI] [PubMed] [Google Scholar]
- Imbert J. P., Hodgetts H. M., Parise R., Vachon F., Dehais F., Tremblay S. (2014). Attentional costs and failures in air traffic control notifications. Ergonomics 57 1817–1832. 10.1080/00140139.2014.952680 [DOI] [PubMed] [Google Scholar]
- Izzetoglu M., Bunce S. C., Izzetoglu K., Onaral B., Pour-rezaei K. (2007). Functional brain imaging using near- infrared technology. IEEE Eng. Med. Biol. Mag. 26:38. 10.1109/memb.2007.384094 [DOI] [PubMed] [Google Scholar]
- Jahanpour E., Fabre E., Dehais F., Causse M. (2018). “Giving a hand to pilots with animated alarms based on mirror system functioning,” in Proceedings of the 2nd International Neuroergonomics Conference, Philadelphia, PA. [Google Scholar]
- Jeon S. Y., Han S. J. (2012). Improvement of the working memory and naming by transcranial direct current stimulation. Ann. Rehabil. Med. 36 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Zatorre R. J. (2006). Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. NeuroImage 31 1673–1681. 10.1016/j.neuroimage.2006.02.026 [DOI] [PubMed] [Google Scholar]
- Kahneman D. (1973). Attention and Effort, Vol. 1063 Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Kalia V., Vishwanath K., Knauft K., Vellen B. V. D., Luebbe A., Williams A. (2018). Acute stress attenuates cognitive flexibility in males only: an fNIRS examination. Front. Psychol. 9:2084. 10.3389/fpsyg.2018.02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam J. W., Dao E., Farley J., Fitzpatrick K., Smallwood J., Schooler J. W., et al. (2011). Slow fluctuations in attentional control of sensory cortex. J. Cognit. Neurosci. 23 460–470. 10.1162/jocn.2010.21443 [DOI] [PubMed] [Google Scholar]
- Kobes M., Helsloot I., De Vries B., Post J. G. (2010). Building safety and human behaviour in fire: a literature review. Fire Saf. J. 45 1–11. 10.1016/j.firesaf.2009.08.005 [DOI] [Google Scholar]
- Kojima H., Suzuki T. (2010). Hemodynamic change in occipital lobe during visual search: visual attention allocation measured with NIRS. Neuropsychologia 48 349–352. 10.1016/j.neuropsychologia.2009.09.028 [DOI] [PubMed] [Google Scholar]
- Kramer A., Spinks J. (1991). “Capacity views of human information processing,” in Handbook of Cognitive Psychophysiology: Central and Nervous Systems Approaches, eds Jennings J. R., Coles M. G. H. (New York: Wiley; ), 179–249. [Google Scholar]
- Kreibig S. D. (2010). Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84 394–421. 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Kreitz C., Furley P., Memmert D., Simons D. J. (2016a). The influence of attention set, working memory capacity, and expectations on inattentional blindness. Perception 45 386–399. 10.1177/0301006615614465 [DOI] [PubMed] [Google Scholar]
- Kreitz C., Furley P., Simons D. J., Memmert D. (2016b). Does working memory capacity predict cross-modally induced failures of awareness? Conscious. Cognit. 39 18–27. 10.1016/j.concog.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Krol L. R., Haselager P., Zander T. O. (2019). Cognitive and affective probing: a tutorial and review of active learning for neuroadaptive technology. J. Neural Eng. 17:012001. 10.1088/1741-2552/ab5bb5 [DOI] [PubMed] [Google Scholar]
- Lee J. D. (2014). Dynamics of driver distraction: the process of engaging and disengaging. Ann. Adv. Automot. Med. 58:24. [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim M., Choi S., You H. (2018). Evaluation of a motion seat system for reduction of a driver’s passive task-related (tr) fatigue. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 62 1843–1847. 10.1177/1541931218621420 [DOI] [Google Scholar]
- Lees M. N., Cosman J. D., Lee J. D., Rizzo M., Fricke N. (2010). Translating cognitive neuroscience to the driver’s operational environment: a neuroergonomics approach. Am. J. Psychol. 123:391 10.5406/amerjpsyc.124.4.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J., Carvalho S., Fregni F., Gonçalves O. F. (2011). Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS ONE 6:e24140. 10.1371/journal.pone.0024140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev A. N. (2014). Activity and Consciousness. Moscow: Progress Publishers. [Google Scholar]
- Lewis B. A., Eisert J. L., Baldwin C. L. (2014). Effect of tactile location, pulse duration, and interpulse interval on perceived urgency. Transport. Res. Rec. 2423 10–14. 10.3141/2423-02 [DOI] [Google Scholar]
- Lie C. H., Specht K., Marshall J. C., Fink G. R. (2006). Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 30 1038–1049. 10.1016/j.neuroimage.2005.10.031 [DOI] [PubMed] [Google Scholar]
- Mallat C., Cegarra J., Calmettes C., Capa R. L. (2019). A curvilinear effect of mental workload on mental effort and behavioral adaptability: an approach with the pre-ejection period. Hum. Fact. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mandrick K., Chua Z., Causse M., Perrey S., Dehais F. (2016). Why a comprehensive understanding of mental workload through the measurement of neurovascular coupling is a key issue for neuroergonomics? Front. Hum. Neurosci. 10:250. 10.3389/fnhum.2016.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R., Yi D. J., Chun M. M. (2004). The neural fate of consciously perceived and missed events in the attentional blink. Neuron 41 465–472. 10.1016/s0896-6273(04)00012-1 [DOI] [PubMed] [Google Scholar]
- Mason M. F., Norton M. I., Van Horn J. D., Wegner D. M., Grafton S. T., Macrae C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315 393–395. 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K. E., Gratton G., Fabiani M., Beck D. M., Ro T. (2009). To see or not to see: prestimulus α phase predicts visual awareness. J. Neurosci. 29 2725–2732. 10.1523/jneurosci.3963-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. (2002). Towards a transactional ergonomics for driver stress and fatigue. Theor. Issues Ergon. Sci. 3 195–211. 10.1080/14639220210124120 [DOI] [Google Scholar]
- Matthews G., Campbell S. E., Falconer S., Joyner L. A., Huggins J., Gilliland K., et al. (2002). Fundamental dimensions of subjective state in performance settings: task engagement, distress, and worry. Emotion 2 315. 10.1037/1528-3542.2.4.315 [DOI] [PubMed] [Google Scholar]
- Matthews G., Reinerman-Jones L. E., Barber D. J., Abich J., IV (2015). The psychometrics of mental work- load: multiple measures are sensitive but divergent. Hum. Fact. 57 125–143. 10.1177/0018720814539505 [DOI] [PubMed] [Google Scholar]
- Matthews G., Warm J. S., Reinerman L. E., Langheim L. K., Saxby D. J. (2010). “Task engagement, attention, and executive control,” in Handbook of Individual Differences in Cognition, eds Gruszka A., Matthews G., Szymura B. (Berlin: Springer; ), 205–230. 10.1007/978-1-4419-1210-7_13 [DOI] [Google Scholar]
- May J. F., Baldwin C. L. (2009). Driver fatigue: the importance of identifying causal factors of fatigue when considering detection and countermeasure technologies. Transport. Res. Part F Traff. Psychol. Behav. 12 218–224. 10.1016/j.trf.2008.11.005 [DOI] [Google Scholar]
- McIntire L. K., McKinley R. A., Goodyear C., Nelson J. (2014). A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 7 499–507. 10.1016/j.brs.2014.04.008 [DOI] [PubMed] [Google Scholar]
- McKendrick R., Parasuraman R., Ayaz H. (2015). Wearable functional near infrared spectroscopy (fNIRS) and transcranial direct current stimulation (tDCS): expanding vistas for neurocognitive augmentation. Front. Syst. Neurosci. 9:27 10.3389/fnsys.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. K., Parasuraman R. (2013). Neuroergonomics: a review of applications to physical and cognitive work. Front. Hum. Neurosci. 7:889. 10.3389/fnhum.2013.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi H. N., Singh H., Orihuela-Espina F., Athanasiou T., Fiorentino F., Yang G. Z., et al. (2018). Temporal stress in the operating room: brain engagement promotes “coping” and disengagement prompts “choking”. Ann. Surg. 267 683–691. 10.1097/sla.0000000000002289 [DOI] [PubMed] [Google Scholar]
- Modi H. N., Singh H., Yang G. Z., Darzi A., Leff D. R. (2017). A decade of imaging surgeons’ brain function (part I): terminology, techniques, and clinical translation. Surgery 162 1121–1130. 10.1016/j.surg.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Molloy K., Griffiths T. D., Chait M., Lavie N. (2015). Inattentional deafness: visual load leads to time-specific suppression of auditory evoked responses. J. Neurosci. 35 16046–16054. 10.1523/jneurosci.2931-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moray N. (1979). Mental Workload: Its Theory and Measurement. New York, NY: Plenum. [Google Scholar]
- Munakata Y., Herd S. A., Chatham C. H., Depue B. E., Banich M. T., O’Reilly R. C. (2011). A unified framework for inhibitory control. Trends Cognit. Sci. 15 453–459. 10.1016/j.tics.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Dalton P. (2016). Out of touch? Visual load induces inattentional numbness. J. Exp. Psychol. 42 761. 10.1037/xhp0000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y., Okina T., Suzuki N., Nabatame H., Matsuda M. (2005). The cerebral correlates of different types of perseveration in the Wisconsin Card Sorting Test. J. Neurol. Neurosurg. Psychiatry 76 169–175. 10.1136/jnnp.2004.039818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J., Mars F., Forzy J. F., El-Jaafari M., Hoc J. M. (2010). Objective and subjective evaluation of motor priming and warning systems applied to lateral control assistance. Accident Anal. Prevent. 42 904–912. 10.1016/j.aap.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Navon D. (1984). Resources - a theoretical soupstone? Psychol. Rev. 91 216–234. [Google Scholar]
- Navon D., Gopher D. (1979). On the economy of the human-processing system. Psychol. Rev. 86 214–225. [Google Scholar]
- Nelson J., McKinley R. A., Phillips C., McIntire L., Goodyear C., Kreiner A., et al. (2016). The effects of transcranial direct current stimulation (tDCS) on multitasking throughput capacity. Front. Hum. Neurosci. 10:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. M., McKinley R. A., McIntire L. K., Goodyear C., Walters C. (2015). Augmenting visual search performance with transcranial direct current stimulation (tDCS). Milit. Psychol. 27 335–347. 10.1037/mil0000085 [DOI] [Google Scholar]
- Nelson J. T., McKinley R. A., Golob E. J., Warm J. S., Parasuraman R. (2014). Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS). Neuroimage 85 909–917. 10.1016/j.neuroimage.2012.11.061 [DOI] [PubMed] [Google Scholar]
- Ninomiya T., Noritake A., Ullsperger M., Isoda M. (2018). Performance monitoring in the medial frontal cortex and related neural networks: from monitoring self actions to understanding others’ actions. Neurosci. Res. 137 1–10. 10.1016/j.neures.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Norman D. A., Bobrow D. G. (1975). On data-limited and resource-limited processes. Cognit. Psychol. 7 44–64. 10.1016/0010-0285(75)90004-3 [DOI] [Google Scholar]
- O’Connell R. G., Dockree P. M., Robertson I. H., Bellgrove M. A., Foxe J. J., Kelly S. P. (2009). Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J. Neurosci. 29 8604–8611. 10.1523/jneurosci.5967-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell R. D., Eggemeier F. T. (1986). “Workload assessment methodology,” in Handbook of Human Perception and Performance, Vol. 2 eds Boff K., Kaufman L., Thomas J. P. (New York, NY: Wiley; ), 42.1–42.49. [Google Scholar]
- Oei N. Y., Veer I. M., Wolf O. T., Spinhoven P., Rombouts S. A., Elzinga B. M. (2012). Stress shifts brain activation towards ventral ‘affective’areas during emotional distraction. Soc. Cognit. Affect. Neurosci. 7 403–412. 10.1093/scan/nsr024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare D., Smitheram T. (1995). Pressing-on into deteriorating conditions: an application of behavioral decision theory to pilot decision making. Int. J. Aviat. Psychol. 5 351–370. 10.1207/s15327108ijap0504_2 [DOI] [Google Scholar]
- Orasanu J., Martin L., Davison J., Null C. H. (1998). Errors in Aviation Decision Making: Bad Decisions or Bad Luck? Moffett Field, CA: NASA Ames Research Center. [Google Scholar]
- Palumbo R. V., Marraccini M. E., Weyandt L. L., Wilder-Smith O., McGee H. A., Liu S., et al. (2017). Interpersonal autonomic physiology : a systematic review of the literature. Personal. Soc. Psychol. Rev. 21 99–141. 10.1177/1088868316628405 [DOI] [PubMed] [Google Scholar]
- Parasuraman R. (2003). Neuroergonomics: research and practice. Theor. Issues Ergon. Sci. 4 5–20. 10.1080/14639220210199753 [DOI] [Google Scholar]
- Parasuraman R., Rizzo M. (2008). Neuroergonomics: The Brain at Work, 1st Edn New York, NY: Oxford University Press, Inc. [Google Scholar]
- Parasuraman R., Wilson G. F. (2008). Putting the brain to work: neuroergonomics past, present, and future. Hum. Fact. 50 468–474. 10.1518/001872008X288349 [DOI] [PubMed] [Google Scholar]
- Parasuraman R., Mouloua M., Hilburn B. (1999). “Adaptive aiding and adaptive task allocation enhance human-machine interaction,” in Automation Technology and Human Performance: Current Research and Trends (Mahwah, NJ: Erlbaum; ), 119–123. [Google Scholar]
- Peavler W. S. (1974). Pupil size, information overload, and performance differences. Psychophysiology 11 559–566. 10.1111/j.1469-8986.1974.tb01114.x [DOI] [PubMed] [Google Scholar]
- Pecher C., Quaireau C., Lemercier C., Cellier J.-M. (2011). The effects of inattention on selective attention: how sad- ness and ruminations alter attention functions evaluated with the attention network test. Rev. Eur. Psychol. Appl. Eur. Rev. Appl. Psychol. 61 43–50. 10.1016/j.erap.2010.10.003 [DOI] [Google Scholar]
- Pepin G., Malin S., Navarro J., Fort A., Jallaiz C., Moreau F., et al. (2016). “Detection of mind-wandering in driving: contributions of cardiac measurement and eye movements,” in Proceedings of the 1st International Neuroergonomics Conference: The Brain at Work and in Everyday Life, Amsterdam: Elsevier. [Google Scholar]
- Pessoa L., Ungerleider L. G. (2004). Neuroimaging studies of attention and the processing of emotion-laden stimuli. Prog. Brain Res. 144 171–182. 10.1016/s0079-6123(03)14412-3 [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Posner M. I. (2012). The attention system of the human brain: 20 years after. Ann. Rev. Neurosci. 35 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peysakhovich V., Lefrançois O., Dehais F., Causse M. (2018). The neuroergonomics of aircraft cockpits: the four stages of eye-tracking integration to enhance flight safety. Safety 4:8 10.3390/safety4010008 [DOI] [Google Scholar]
- Polich J. (2007). Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 118 2128–2148. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope A. T., Bogart E. H., Bartolome D. S. (1995). Biocybernetic system evaluates indices of operator engagement in automated task. Biol. Psychol. 40 187–195. 10.1016/0301-0511(95)05116-3 [DOI] [PubMed] [Google Scholar]
- Posner M. I. (2012). Imaging attention networks. Neuroimage 61 450–456. 10.1016/j.neuroimage.2011.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I., Dehaene S. (1994). Attentional networks. Trends Neurosci. 17 75–79. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E. (1990). The attention system of the human brain. Annu. Rev. Neurosci. 13 25–42. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Tudela P. (1997). Imaging resources. Biol. Psychol. 45 95–107. [DOI] [PubMed] [Google Scholar]
- Pourtois G., De Pretto M., Hauert C. A., Vuilleumier P. (2006). Time course of brain activity during change blindness and change awareness: performance is predicted by neural events before change onset. J. Cognit. Neurosci. 18 2108–2129. 10.1162/jocn.2006.18.12.2108 [DOI] [PubMed] [Google Scholar]
- Pribram K. H., McGuinness D. (1975). Arousal, activation, and effort in the control of attention. Psychol. Review 82:116. 10.1037/h0076780 [DOI] [PubMed] [Google Scholar]
- Prinzel L. J., III (2002). Research on Hazardous States of Awareness and Physiological Factors in Aerospace Operations. Report No. NASA/TM-2002-211444. Washington, DC: NASA. [Google Scholar]
- Prinzel L. J., Freeman F. G., Scerbo M. W., Mikulka P. J., Pope A. T. (2000). A closed-loop system for examining psychophysiological measures for adaptive task allocation. Int. J. Aviat. Psychol. 10 393–410. 10.1207/s15327108ijap1004_6 [DOI] [PubMed] [Google Scholar]
- Proulx G. (2001). “Occupant behaviour and evacuation,” in Proceedings of the 9th International Fire Protection Symposium (Iceland: Iceland Fire Authority; ), 219–232. [Google Scholar]
- Puschmann S., Sandmann P., Ahrens J., Thorne J., Weerda R., Klump G., et al. (2013). Electrophysiological correlates of auditory change detection and change deafness in complex auditory scenes. Neuroimage 75 155–164. 10.1016/j.neuroimage.2013.02.037 [DOI] [PubMed] [Google Scholar]
- Qin S., Hermans E. J., van Marle H. J., Luo J., Fernandez G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry 66 25–32. 10.1016/j.biopsych.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Racz F. S., Mukli P., Nagy Z., Eke A. (2017). Increased prefrontal cortex connectivity during cognitive challenge assessed by fNIRS imaging. Biomed. Opt. Exp. 8 3842–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N., Owen A. M. (2004). Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5:184. 10.1038/nrn1343 [DOI] [PubMed] [Google Scholar]
- Ramsey N. F., Jansma J. M., Jager G., Van Raalten T., Kahn R. S. (2004). Neurophysiological factors in human information processing capacity. Brain 127 517–525. 10.1093/brain/awh060 [DOI] [PubMed] [Google Scholar]
- Raveh D., Lavie N. (2015). Load-induced inattentional deafness. Attent. Percept. Psychophys. 77 483–492. 10.3758/s13414-014-0776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régis N., Dehais F., Rachelson E., Thooris C., Pizziol S., Causse M., et al. (2014). Formal detection of atten- tional tunneling in human operator–automation interactions. IEEE Trans. Hum. Mach. Syst. 44 326–336. 10.1109/thms.2014.2307258 [DOI] [Google Scholar]
- Regis N., Dehais F., Tessier C., Gagnon J.-F. (2012). “Human Factors: a view from an integrative perspective,” in Proceedings HFES Europe Chapter Conference Toulouse 2012, eds De Waard D., Brookhuis K., Dehais F., Weikert C., Röttger S., Manzey D., et al. (Toulouse: HFES; ). Available online at: http://hfes-europe.org [Google Scholar]
- Reynal M., Rister F., Scannella S., Wickens C., Dehais F. (2017). “Investigating pilots decision making when facing an unstabilized approach: an eye-tracking study,” in Proceedings of the 19th International Symposium on Aviation Psychology, Dayton, OH, 335. [Google Scholar]
- Reyner L. A., Horne J. A. (1998). Evaluation of ‘in-car’countermeasures to sleepiness: cold air and radio. Sleep 21 46–51. [PubMed] [Google Scholar]
- Richter M., Gendolla G. H. E., Wright R. A. (2016). “Three decades of research on motivational intensity theory: what we have learned about effort and what we still don’t know,” in Advances in Motivation Science, ed. Elliot A. J. (Cambridge, MA: Academic Press; ), 149–186. [Google Scholar]
- Ridderinkhof K. R., Van Den Wildenberg W. P., Segalowitz S. J., Carter C. S. (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action se- lection, response inhibition, performance monitoring, and reward-based learning. Brain Cognit. 56 129–140. 10.1016/j.bandc.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Riggs S. L., Sarter N. (2019). Tactile, visual, and crossmodal visual-tactile change blindness: the effect of transient type and task demands. Hum. Fact. 61 5–24. 10.1177/0018720818818028 [DOI] [PubMed] [Google Scholar]
- Rizzo M., Robinson S., Neale V. (2007). “The brain in the wild: tracking human behavior in natural and naturalistic settings,” in Neuroergonomics: The Brain at Work, eds Parasuraman R., Rizzo M. (New York, NY: Oxford; ), 113–130. [Google Scholar]
- Robbins T. W., Arnsten A. F. (2009). The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 32 267–287. 10.1146/annurev.neuro.051508.135535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. N., Frey J. (2016). “Neurophysiological markers for passive brain–computer interfaces,” in Brain–Computer Interfaces 1: Foundations and Methods eds Clerc M., Bougrain L., Lotte F. (Hoboken, NJ: John Wiley & Sons; ), 85–100. 10.1002/9781119144977.ch5 [DOI] [Google Scholar]
- Russell D., Statz J. K., Ramiccio J., Henderson M., Still D., Temme L., et al. (2016). Pilot Cueing Synergies for Degraded Visual Environments (No. USAARL-2016-10). Fort Rucker, AL: US Army Aeromedical Research Laboratory Fort Rucker United States. [Google Scholar]
- Saint Lot J., Imbert J.-P., Dehais F. (2020). “Red Altert: a cognitive countermeasure to mitigate attentional tunneling,” in Proceedings CHI 2020, April 25–30 (Honolulu, HI: ). 10.1145/3313831.3376709 [DOI] [Google Scholar]
- Sandson J., Albert M. L. (1984). Varieties of perseveration. Neuropsychologia 22 715–732. 10.1016/0028-3932(84)90098-8 [DOI] [PubMed] [Google Scholar]
- Sarason I. G., Sarason B. R., Pierce G. R. (1990). Anxiety, cognitive interference and performance. J. Soc. Behav. Personal. 5 1–18. [Google Scholar]
- Saravini F. (1999). “Energy and the brain: facts and fantasies,” in Mind Myths, ed. Della Salla E. (Chichester: Wiley; ), 43–58. [Google Scholar]
- Sarter N., Sarter M. (2003). Neuroergonomics: opportunities and challenges of merging cognitive neuroscience with cognitive ergonomics. Theor. Issues Ergon. Sci. 4 142–150. 10.1080/1463922021000020882 [DOI] [Google Scholar]
- Scannella S., Causse M., Chauveau N., Pastor J., Dehais F. (2013). Effects of the audiovisual conflict on auditory early processes. Int. J. Psychophysiol. 89 115–122. 10.1016/j.ijpsycho.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Scerbo M. W. (2008). “Adaptive automation,” in Neuroergonomics: The Brain at Work, eds Parasuraman R., Rizzo M. (New York, NY: Oxford; ), 239–252. 10.1093/acprof:oso/9780195177619.003.0016 [DOI] [Google Scholar]
- Schneider W., Dumais S. T., Shiffen R. M. (1984). “Automatic and control processing and attention,” in Varieties of Attention, eds Parasuraman R., Davies D. R. (Orlando: Academic Press; ), 1–27. [Google Scholar]
- Scholte H. S., Witteveen S. C., Spekreijse H., Lamme V. A. (2006). The influence of inattention on the neural correlates of scene segmentation. Brain Res. 1076 106–115. 10.1016/j.brainres.2005.10.051 [DOI] [PubMed] [Google Scholar]
- Schooler J. W., Smallwood J., Christoff K., Handy T. C., Reichle E. D., Sayette M. A. (2011). Meta-awareness, perceptual decoupling and the wandering mind. Trends Cognit. Sci. 15 319–326. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2002). Getting formal with dopamine and reward. Neuron 36 241–263. 10.1016/s0896-6273(02)00967-4 [DOI] [PubMed] [Google Scholar]
- Sebok A., Wickens C. D., Walters B., Fennell K. (2017). “Alerts on the nextgen flight deck,” in Proceedings of the 19th International Symposium on Aviation Psychology, Dayton, OH, 293. [Google Scholar]
- Selfridge O. G. (1959). “Pandemonium: a paradigm for learning,” in Proceedings of the Symposium on Mechanisation of Thought Processes, eds D. V. Blake and A. M. Uttley (London: H.M. Stationery Office; ), 511–529. [Google Scholar]
- Senoussi M., Verdière K. J., Bovo A., Ponzoni Carvalho, Chanel C., Dehais F., et al. (2017). “Pre- stimulus antero-posterior EEG connectivity predicts performance in a UAV monitoring task,” in Proceedings of 2016 International Conference on Systems, Man, and Cybernetics (Canada: IEEE SMC, 1167–1172. [Google Scholar]
- Shallice T., Burgess P. (1993). “Supervisory control of action and thought selection,” in Attention: Selection, Awareness and Control, eds Baddeley A., Weiskrantz L. (Oxford: Clarendon Press; ), 171–187. [Google Scholar]
- Smallwood J., Beach E., Schooler J. W., Handy T. C. (2008). Going awol in the brain: mind wandering reduces cortical analysis of external events. J. Cognit. Neurosci. 20 458–469. 10.1162/jocn.2008.20037 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J. W. (2015). The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol. 66 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Smith R. P. (1981). Boredom: a review. Hum. Fact. 23 329–340. [DOI] [PubMed] [Google Scholar]
- Souza P. E., Chanel C. P. C., Dehais F., Givigi S. (2016). “Towards human-robot interaction: a framing effect experiment,” in IEEE International Conference on Systems, Man, and Cybernetics, (Budapest: IEEE SMC; ), 001929–001934. [Google Scholar]
- Staal M. A. (2004). Stress, cognition, and human performance: a literature review and conceptual framework. [Google Scholar]
- Stamp K., Fairclough S., Dobbins C., Poole H. (2019). “A neuroadaptive approach to analgesic gaming,” in The Second Neuroadaptive Technology Conference, Liverpool, 19. [Google Scholar]
- Stephens C., Dehais F., Roy R. N., Harrivel A., Last M. C., Kennedy K., et al. (2018). “Biocybernetic adaptation strategies: machine awareness of human engagement for improved operational performance,” in International Conference on Augmented Cognition, Copenhagen, 89–98. 10.1007/978-3-319-91470-1_9 [DOI] [Google Scholar]
- Taillard J., Capelli A., Sagaspe P., Anund A., Akerstedt T., Philip P. (2012). In-car nocturnal blue light exposure improves motorway driving: a randomized controlled trial. PLoS ONE 7:e46750. 10.1371/journal.pone.0046750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. C., Wickens C. D. (2004). Eye-tracking and individual differences in off-normal event detection when flying with a synthetic vision system display. Proc. Hum. Fact. Ergon. Soc. Annu. Meet. 48 223–227. 10.1177/154193120404800148 [DOI] [Google Scholar]
- Todd J. J., Fougnie D., Marois R. (2005). Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 16 965–972. 10.1111/j.1467-9280.2005.01645.x [DOI] [PubMed] [Google Scholar]
- Tombu M. N., Asplund C. L., Dux P. E., Godwin D., Martin J. W., Marois R. (2011). A unified attentional bottleneck in the human brain. Proc. Natl. Acad. Sci. U.S.A. 108 13426–13431. 10.1073/pnas.1103583108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J. I., Mohamed F., Faro S., Tiver R., Pinus A., Bloomer C., et al. (2000). The effect of autonomic arousal on attentional focus. Neuroreport 11 4037–4042. 10.1097/00001756-200012180-00027 [DOI] [PubMed] [Google Scholar]
- Tsai Y.-F., Viirre E., Strychacz C., Chase B., Jung T.-P. (2007). Task performance and eye activity: predicting be- havior relating to cognitive workload. Aviat. Space Environ. Med. 78 B176–B185. [PubMed] [Google Scholar]
- Ullsperger M., Danielmeier C., Jocham G. (2014). Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 94 35–79. 10.1152/physrev.00041.2012 [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Nittono H., von Cramon D. Y. (2007). When goals are missed: dealing with self-generated and externally induced failure. NeuroImage 35 1356–1364. 10.1016/j.neuroimage.2007.01.026 [DOI] [PubMed] [Google Scholar]
- Unsworth N., Engle R. W. (2007). The nature of individ- ual differences in working memory capacity: active main- tenance in primary memory and controlled search from secondary memory. Psychol. Rev. 114 104. 10.1037/0033-295x.114.1.104 [DOI] [PubMed] [Google Scholar]
- Uzzaman S., Joordens S. (2011). The eyes know what you are thinking: eye movements as an objective measure of mind wandering. Conscious. Cognit. 20 1882–1886. 10.1016/j.concog.2011.09.010 [DOI] [PubMed] [Google Scholar]
- Van Acker B. B., Parmentier D. D., Vlerick P., Saldien J. (2018). Understanding mental workload: from a clarifying concept analysis toward an implementable framework. Cognit. Technol. Work 20 351–365. 10.1007/s10111-018-0481-3 [DOI] [Google Scholar]
- Van Dongen H., Belenky G., Krueger J. M. (2011). A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Curr. Top. Med. Chem. 11 2414–2422. 10.2174/156802611797470286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey W. B., Zaidel D. M. (1999). Preventing drowsiness accidents by an alertness maintenance device. Accident Anal. Prevent. 31 199–211. 10.1016/s0001-4575(98)00062-1 [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S., Wang M., Birnbaum S. G., Williams G. V., Arnsten A. F. (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 10:376. 10.1038/nn1846 [DOI] [PubMed] [Google Scholar]
- Weinmann M., Schneider C., vom Brocke J. (2016). Digital nudging. Bus. Inform. Syst. Eng. 58 433–436. 10.1007/s12599-016-0453-1 [DOI] [Google Scholar]
- Weissman D. H., Roberts K. C., Visscher K. M., Woldorff M. G. (2006). The neural bases of momentary lapses in attention. Nat. Neurosci. 9:971. 10.1038/nn1727 [DOI] [PubMed] [Google Scholar]
- Wickens C. D. (1980). The structure of attentional resources. Attent. Perform. VIII 8 239–257. [Google Scholar]
- Wickens C. D. (1984). “Processing resources in attention,” in Varieties of Attention, eds Parasuraman R., Davies D. R. (London: Academic Press; ), 63–101. [Google Scholar]
- Wickens C. D. (2002). Multiple resources and performance prediction. Theor. Issues Ergon. Sci. 3 150–177. [Google Scholar]
- Wickens C. D. (2005). Attentional tunneling and task management. Int. Symp. Aviat. Psychol. 812–817. [Google Scholar]
- Wickens C. D. (2008). Multiple resources and mental work-load. Hum. Fact. 50 449–455. [DOI] [PubMed] [Google Scholar]
- Wickens C. D., Liu Y. (1988). Codes and modalities in multiple resources: a success and a qualification. Hum. Fact. 30 599–616. 10.1177/001872088803000505 [DOI] [PubMed] [Google Scholar]
- Wickens C. D., Tsang P. (2014). “Workload,” in Handbook of Human-Systems Integration, ed. Durso F. (Washington, DC: APA; ). [Google Scholar]
- Wickens J. R., Horvitz J. C., Costa R. M., Killcross S. (2007). Dopaminergic mechanisms in actions and habits. J. Neurosci. 27 8181–8183. 10.1523/jneurosci.1671-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierwille W. W., Eggemeier F. T. (1993). Recommendation for mental workload measurement in a test and evaluation environment. Hum. Fact. 35 263–281. 10.1177/001872089303500205 [DOI] [Google Scholar]
- Yeh Y. Y., Wickens C. D. (1988). Dissociation of performance and subjective measures of workload. Hum. Fact. 30 111–120. 10.1177/001872088803000110 [DOI] [Google Scholar]
- Yerkes R. M., Dodson J. D. (1908). The relation of strength of stimulus to rapidity of habit formation. J. Compar. Physiol. Psychol. 18 459–482. 10.1002/cne.920180503 [DOI] [Google Scholar]
- Young M. S., Brookhuis K. A., Wickens C. D., Hancock P. A. (2015). State of science: mental workload in ergonomics. Ergonomics 58 1–17. 10.1080/00140139.2014.956151 [DOI] [PubMed] [Google Scholar]
- Young M. S., Stanton N. A. (2002). Malleable attentional resources theory: a new explanation for the effects of mental underload on performance. Hum. Fact. 44 365–375. 10.1518/0018720024497709 [DOI] [PubMed] [Google Scholar]
- Zander T. O., Kothe C. (2011). Towards passive brain–computer interfaces: applying brain–computer interface technology to human–machine systems in general. J. Neural Eng. 8:025005. 10.1088/1741-2560/8/2/025005 [DOI] [PubMed] [Google Scholar]
- Zaneboni J., Saint-Jalmes B. (2016). U.S. Patent No. 9,302,779. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]