Abstract
Background
For specific clinical indications, androgen deprivation therapy (ADT) will induce disease prostate cancer (PC) regression, relieve symptoms and prolong survival; however, ADT has a well‐described range of side effects, which may have a detrimental effect on the patient's quality of life, necessitating additional interventions or changes in PC treatment. The risk‐benefit analysis for initiating ADT in PC patients throughout the PC disease continuum warrants review.
Methods
A 14‐member panel comprised of urologic and medical oncologists were chosen for an expert review panel, to provide guidance on a more judicious use of ADT in advanced PC patients. Panel members were chosen based upon their academic and community experience and expertise in the management of PC patients. Four academic members of the panel served as group leaders; the remaining eight panel members were from Large Urology Group Practice Association practices with proven experience in leading their advanced PC clinics. The panel members were assigned to four separate working groups, and were tasked with addressing the role of ADT in specific PC settings.
Results
This article describes the practical recommendations of an expert panel for the use of ADT throughout the PC disease continuum, as well as an algorithm summarizing the key recommendations. The target for this publication is all providers (urologists, medical oncologists, radiation oncologists, or advanced practice providers) who evaluate and manage advanced PC patients, regardless of their practice setting.
Conclusion
The panel has provided recommendations for monitoring PC patients while on ADT, recognizing that PC patients will progress despite testosterone suppression and, therefore, early identification of conversion from castrate‐sensitive to castration resistance is critical. Also, the requirement to both identify and mitigate side effects of ADT as well as the importance of quality of life maintenance are essential to the optimization of patient care, especially as more combinatorial therapeutic strategies with ADT continue to emerge.
Keywords: androgen deprivation therapy, cancer, consensus, prostate
1. INTRODUCTION
Since the seminal work by Huggins and Hodges 1 establishing that prostate cancer (PC) is an endocrine‐responsive disease, PC patients—those with locally advanced, recurrent, and metastatic disease—may receive treatment with androgen deprivation therapy (ADT). 1 , 2 Castration can be administered surgically (bilateral orchiectomy) or medically (administration of a gonadotropin‐releasing hormone agonist or antagonist). For specific clinical indications, ADT will induce disease PC regression, relieve symptoms and prolong survival; however, ADT has a well‐described range of side effects, including but not limited to, hot flashes, fatigue, depression, sarcopenia, increased visceral and abdominal fat mass, weight gain, increased cholesterol and triglycerides levels, insulin resistance, and loss of bone mineral density with an increased risk of fracture. Any of these side effects may have a detrimental effect on the patient's quality of life, necessitating additional interventions or changes in PC treatment. 3 ADT is often used to manage biochemical recurrence (BCR) or rising levels of prostate‐specific antigen (PSA) after failed primary therapy, despite a paucity of evidence to demonstrate a survival benefit in this clinical setting. Therefore, despite the known risk of ADT side effects, the risk‐benefit analysis for initiating ADT in PC patients throughout the PC disease continuum warrants review.
Guidelines for the management of PC have been systematically reviewed and published by many associations, including the National Comprehensive Cancer Network (NCCN), the American Urological Association (AUA), and the European Association of Urology (EAU). All have evaluated and delineated recommendations for the foundational role of ADT in the management of advanced PC. However, in clinical practice the decision to use ADT is highly variable.
To address the gaps in ADT recommendations, the Large Urology Group Practice Association (LUGPA) organized an expert panel to develop evidence‐based recommendations for the use of ADT throughout the PC disease continuum, with applicability for community urologists, medical oncologists, and radiation oncologists who manage advanced PC patients. The goal of this panel was to provide guidance on a more judicious use of ADT in advanced PC patients, maximizing the benefit‐to‐risk ratio.The initiative was sponsored by an unrestricted grant from Tolmar Pharmaceuticals.
Importantly, the panel's recommendations were derived from a review of clinical trial data, publications, national guidelines, and expert opinion. The clinical trials, in particular, had varying entry criteria or outcomes, so many of the panel's recommendations should be prefaced by the assumption of “generally speaking,” that is, patient‐specific factors will also affect treatment decisions and thus may make the general recommendation suggested by the panel not applicable for a specific patient. Also, the authors recognize the importance of ongoing and future clinical trials and therefore recommend, whenever possible, encouraging patient enrollment in randomized, controlled trials, which are needed to further clarify when evidence‐based data are lacking.
2. MATERIALS AND METHODS
A 14‐member panel comprised of urologists, urologic oncologists, and medical oncologists were chosen based on their experience and expertise in the management of PC patients. Four members of the panel served as group leaders; they were selected from academic institutions, and the remaining eight panel members were from LUGPA practices with experience in advanced PC clinics (Table 1).
Table 1.
Panel members, by state and specialty
| Name | Title/institution | State | Specialty |
|---|---|---|---|
| David M. Albala | Physician—Associated Medical Professionals; | New York | Urology |
| Chief of Urology—Crouse Hospital | |||
| Emmanuel S. Antonarakis | Professor of Oncology and Urology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Maryland | Medical oncology |
| Gordon A. Brown | Medical Director of Advance Therapeutics | New Jersey | Urologic oncology |
| Clinical Associate; Professor of Urology, Rowan‐School of Medicine; Director of Robotic Surgery Jefferson Health New Jersey | |||
| Raoul Concepcion | Urology Chief Clinical Officer, Integra Connect, West Palm Beach | Florida | Urology |
| Michael S. Cookson | Professor and Chairman, Department of Urology | Oklahoma | Urologic oncology |
| University of Oklahoma Stephenson Cancer Center | |||
| E. D. Crawford | Clinical Professor of Urology | California | Urologic oncology |
| Jason Hafron | Associate Professor of Urology, Willam Beaumont School of Medicine, Oakland University, Director of Robotic Surgery Beaumont Health, Royal Oak, MI, Director of Clinical Research | Michigan | Urologic oncology |
| Richard Harris | CEO and President, Uropartners | Illinois | Urology |
| Jonathan Henderson | President, Chief Executive Officer, Regional Urology, LLC | Louisiana | Urology |
| Benjamin Lowentritt | Medical Director, Comprehensive Prostate Cancer Program at Chesapeake Urology Associates | Maryland | Urology |
| Alicia K. Morgans | Associate Professor of Medicine, Northwestern University, Robert H. Lurie Comprehensive Cancer Center | Illinois | Medical oncology |
| Daniel Saltzstein | Medical Director of Research at Urology San Antonio | Texas | Urology |
| Neal D. Shore | Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach | South Carolina | Urology |
| Jeffrey M. Spier | Managing Partner, Rio Grande Urology, El Paso | Texas | Urology |
The panel members were assigned to four separate working groups, and were tasked with addressing the role of ADT in specific PC settings:
-
1.
Primary ADT in newly diagnosed disease.
-
2.
Neoadjuvant ADT before radical prostatectomy (RP) or radiation therapy (RT).
-
3.
ADT as salvage therapy.
-
4.
ADT for BCR postdefinitive therapy.
-
5.
ADT for castrate‐sensitive prostate cancer (CSPC) and castrate‐resistant prostate cancer (CRPC).
-
6.
The management of ADT adverse events (AEs) and prevention strategies.
The subdivided working groups performed a literature review and then presented the published data for their assigned topics, which involved further discussion by the entire 14‐member panel. The 1‐day conference (Atlanta, GA; 23 September 2018) included presentations, debates among all participants, and voting on the key recommendations listed by each working group. The recommendations were voted upon and were tabulated as yes, no, or “indeterminate,” defined if a panelist felt unable to vote clearly for or against the recommendation as it was stated. Consensus was defined as ≥10 of the 14 panelists (≥71%) voting in the affirmative. All panelists have contributed to the review, analysis, and editing of the document and have also approved the final consensus document.
3. RESULTS
Detailed voting results are shown in Table 2. An algorithm summarizing the key recommendations from the panel is shown in Figure 1.
Table 2.
Recommendations and voting results
| Yes | No | Indeterminate | ||||
|---|---|---|---|---|---|---|
| No./14 | % | No./14 | % | No./14 | % | |
| 1. ADT as primary therapy for localized PC | ||||||
| Is there a role for ADT alone as primary therapy for patients with newly diagnosed, localized, asymptomatic PC? | 1 | 7 | 13 | 93 | 0 | 0 |
| 2. ADT as neoadjuvant and adjuvant therapy to RP | ||||||
| Is there a role for neoadjuvant ADT with RP for low‐ to intermediate‐risk patients? | 0 | 0 | 13 | 93 | 1 | 7 |
| Is there a role for neoadjuvant ADT with RP for high‐risk patients? | 1 | 7 | 10 | 71 | 3 | 22 |
| Is there a role for adjuvant ADT following RP (N1, +/− EBR)? | 14 | 100 | ||||
| Is there a role for adjuvant ADT following RP (N0, but other high‐risk features, absence of radiation)? | 0 | 0 | 12 | 86 | 2 | 14 |
| Is there a role for ADT with radiation therapy? | 14 | 100 | ||||
| Low‐risk patients (except for reduction of volume) | 14 | 100 | ||||
| Intermediate‐risk patients (4‐6 months duration) | 14 | 100 | ||||
| High‐risk patients (18‐36 months duration) | 14 | 100 | ||||
| Timing: 1‐2 months before RT | 14 | 100 | ||||
| For intermediate‐ and high‐risk PC patients, pending RT and ADT, we recommend ADT initiation ideally 1‐2 months before initiating RT | 14 | 100 | ||||
| Given the natural history of PC (ie, about 1/3 of patients will go on to progress), the majority of patients with BCR should undergo observation with BCR. For the remaining patients (high risk), ADT would generally be considered for: | 14 | 100 | ||||
|
||||||
| ADT in BCR is generally not recommended in patients with: | 14 | 100 | ||||
|
||||||
| When considering ADT in high‐risk patients with BCR post‐RP, post‐RT, or postsalvage after RP, intermittent ADT is a reasonable alternative to continuous ADT | 12 | 86 | 2 | 14 | ||
| Proposed Intermittent Treatment Pathway (Figure 1) | 13 | 93 | 1 | 7 | ||
| There is level 1 evidence to support ADT use with salvage radiotherapy. It is reasonable to consider ADT in the setting of salvage radiotherapy as part of a shared decision‐making discussion. The duration of ADT in this setting is unclear, but 6‐12 months of ADT would be reasonable | 12 | 86 | 1 | 7 | 1 | 7 |
| 3. ADT for M1 HSPC and M1 CRPC | ||||||
| In men with asymptomatic oligometastatic mCSPC (without visceral metastases), metastasis‐directed therapy may be a reasonable alternative to immediate ADT in select patients | 7 | 50 | 4 | 29 | 3 | 21 |
| In mCSPC, continuous ADT is generally strongly preferred over intermittent ADT, however in certain circumstances (eg, cardiovascular comorbidities, intolerabilities, etc), intermittent may be considered | 13 | 93 | 1 | 7 | ||
| In mCSPC, there are 3 options to achieve castration: bilateral orchiectomy, LHRH agonists, or LHRH antagonists | 13 | 93 | 1 | 7 | ||
| CAB (ie, LHRHa, LHRHantag, +1st‐ or 2nd‐generation antiandrogen) is superior to castration alone | 9 | 64 | 5 | 36 | ||
| In mCSPC, baseline T levels should be obtained before starting ADT | 14 | 100 | ||||
| For ADT administration, testosterone level should be T < 20 ng/dL. Confirm castrate T levels 1‐4 months after surgical/medical castration, regardless of PSA levels. If PSA rises, T levels must be checked. If T levels are >20 ng/dL, clinicians should check luteinizing hormone levels to differentiate whether ADT was administered effectively | 14 | 100 | ||||
| In mCSPC, if T > 20 ng/dL despite low luteinizing hormone levels, consider switching to an alternative agent | 14 | 100 | ||||
| In the broad mCSPC population, ADT + Abi is superior to ADT alone in terms of OS | 14 | 100 | ||||
| In mCSPC, the distinction between low‐ and high‐volume disease has not been studied in this context. Thus, ADT + Abi is a reasonable standard of care irrespective of metastatic burden | 12 | 86 | 2 | 14 | ||
| In the broad mCSPC population, ADT + docetaxel is superior to ADT alone in terms of OS | 14 | 100 | ||||
|
||||||
| In low‐volume mCSPC, there is evidence that ADT + docetaxel does not provide an OS benefit; thus, ADT + Abi is the favored choice | 13 | 93 | 1 | 93 | ||
| In high‐volume mCSPC, there is strong evidence of benefit for ADT + docetaxel. Thus, both ADT + abiraterone or ADT + docetaxel are treatment options | 14 | 100 | ||||
| The panel cannot comment on the comparative efficacy of ADT + Abi in low‐ vs high‐volume mCSPC, because this has not been studied | 14 | 100 | ||||
| mCRPC is defined as: | 14 | 100 | ||||
| mPC (by conventional radiology) | ||||||
| Testosterone level <50 ng/dL | ||||||
| Progressive disease (one or more of): | ||||||
|
||||||
| In mCRPC, continuation of ADT to maintain T < 20 ng/dL is strongly recommended | 14 | 100 | ||||
| Another reasonable option (“value‐based model”) in treating mCRPC is to stop ADT (once castrate levels are reached) and to check T levels q3 months, then restart ADT if T rises above >20 ng/dL | 2 | 14 | 6 | 43 | 6 | 43 |
| For patients with mCRPC on treatment, testosterone levels should be measured: | 14 | 100 | ||||
|
||||||
| Also, PSA and CT/bone scans should be performed at regular intervals | ||||||
| 4. AE Management Strategies with ADT | ||||||
| Should a DEXA scan and FRAX score be obtained for baseline and follow‐up testing of bone fragility during treatment with ADT (pending approval by insurance provider)? | 14 | 100 | ||||
| A DEXA scan should be obtained at baseline (within 6 months of initiating ADT) and at least once every 2 years for follow‐up | 14 | |||||
| Vitamin D levels can be monitored in men taking osteoclast inhibitors, up to annually, or more often if replenishing depleted stores with high‐dose vitamin D. Daily maintenance doses for vitamin D supplementation should be 800‐1000 IU daily, and calcium daily maintenance dosing should not exceed 1200 mg daily | 13 | 93 | 1 | 7 | ||
| The NCCN guidelines on determining which patients are eligible for additional pharmacologic therapy should be followed (ie calculating a patient's individual risk of fracture via FRAX calculator). For those patients who are eligible, zoledronic acid or denosumab can be used. | 14 | 100 | ||||
| Either the urologist or the PCP should monitor blood pressure (at each visit), HbA1c (annually in nondiabetic patients), and lipid profile (annually) in patients receiving ADT | 13 | 93 | 1 | 7 | ||
| Before initiating ADT, patients with CVD comorbidities should be referred to a cardiologist for comanagement | 14 | 100 | ||||
| There is no evidence to support taking metformin to improve PC‐specific outcomes | 12 | 86 | 2 | 14 | ||
| The urologist should communicate with the PCP or endocrinologist when patients with diabetes initiate ADT as they may need closer monitoring of diabetes | 12 | 86 | 2 | 14 | ||
| HbA1c should be monitored up to annually by a cancer care provider or PCP in patients without a history of diabetes | ||||||
| Cancer‐treating physicians should encourage physical activity/exercise, healthy diet, weight control, and smoking cessation in all patients on ADT throughout the course of their treatment | 14 | 100 | ||||
| We recognize the importance of increasing urologists’ awareness of depression risk in patients receiving ADT | 14 | 100 | ||||
| Urologists need to be aware of depression risk in patients receiving ADT | 14 | 100 | ||||
| Consider routine discussions of mental health concerns, including questions about depression and memory concerns, with evaluation performed at least annually, in men receiving ADT | 14 | 100 | ||||
| Patients over the age of 70 who have been on ADT for >2 years should be referred annually for neurocognitive assessment to evaluate for dementia | 4 | 29 | 3 | 21 | 7 | 50 |
| We recognize hot flashes are side effects of ADT that can negatively impact patient quality of life. Of all the agents that are currently being used to treat hot flashes in men receiving ADT, none have an FDA approved indication for this use and each is associated with side effects. Shared decision‐making practices should be used to discuss the pros and cons of off‐label use of medications for hot flashes for men who wish to use medical management strategies | 14 | 100 | ||||
Abbreviations: ADT, androgen deprivation therapy; BCR, biochemical recurrence; CAB, combined androgen blockade; CT, computerized tomography; CVD, cardiovascular disease; DEXA, dexascan ‐ bone densitometry; HSPC, hormone sensitive metastatic prostate cancer; mCRPC, metastatic castrate‐resistant prostate cancer; mCSPC, metastatic castrate‐sensitive prostate cancer; mPC, metastatic PC; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; OS, overall survival; PC, prostate cancer; PCP, primary care provider; PSA, prostate‐specific antigen; PSADT, PSA doubling time; RP, radical prostatectomy.
Figure 1.
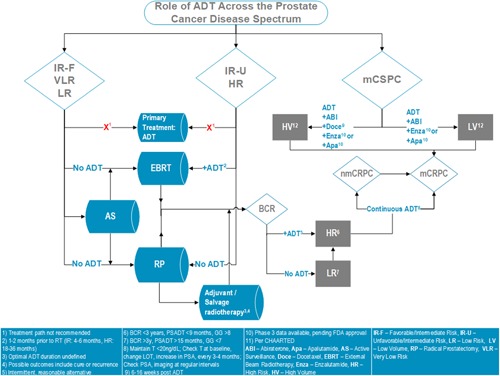
Algorithm summarizing the panel's recommendations. ADT, androgen deprivation therapy; BCR, biochemical recurrence; EBRT, mCSPC, metastatic castrate‐sensitive prostate cancer; RP, radical prostatectomy [Color figure can be viewed at http://wileyonlinelibrary.com]
3.1. The role of ADT as primary therapy for localized PC
The panel members agreed that there is not sufficient evidence to support the use of primary ADT (ie, in the absence of prostatectomy or radiotherapy) in asymptomatic patients with newly diagnosed localized PC and no lymph node involvement. The multiple studies reviewed did not suggest any benefit in decreasing mortality when comparing ADT to observation/no treatment in men with clinically localized PC, thus primary ADT in localized disease is not recommended. 4 , 5 , 6 , 7 , 8 Further studies with the newer androgen blocking agents are indicated. This is in accord with the NCCN guideline on PC. 9
Is there a role for ADT alone as primary therapy for patients with newly diagnosed, localized, asymptomatic PC?
7% Yes, 93% No
3.2. The role of ADT as neoadjuvant and adjuvant therapy to RP
The current AUA guideline states that, “Clinicians should not treat localized PC patients who have elected to undergo RP with neoadjuvant ADT or other systemic therapy outside of clinical trials (Strong Recommendation; Evidence Level: Grade A).” 10 The NCCN guideline supports the use of ADT for M0 PSA persistence/recurrence after RP or external beam radiation therapy (EBRT), and neoadjuvant ADT+/− EBRT post‐RP. The treatment should be individualized, based on PSA levels and rate of change, life expectancy, initial stage, grade, and PSA level at the time of definitive therapy. 9 The panel agreed with this guideline, with the caveat that the current literature demonstrates that there may be benefit with neoadjuvant ADT with RP when restricted to high‐risk patients, and studies assessing this are ongoing. A review of six studies showed that there may be a delay in time to BCR and improvement in surgical margins in high‐risk patients with neoadjuvant ADT before RP, but no overall survival (OS) benefit was observed. 11 , 12 , 13 , 14 , 15 , 16 There were several limitations to these studies in that they were small studies (N ranging from 55 to 297), the definition of “high risk” was not standardized, follow‐up was often short, and they were underpowered to demonstrate a benefit in terms of OS. There may be an issue of patients (particularly high‐risk patients) receiving “temporizing neoadjuvant therapy,” that is, when the patient desires to delay definitive therapy for an important life event. While this is not ideal patient care, the panel recognizes that it is used in unique situations after shared decision making, and the effects on outcomes are unclear.
The panel did not reach consensus on the role of adjuvant ADT after prostatectomy, based on the literature. The panel reviewed four studies, which overall showed some benefit in BCR and OS, but not consistently, in part because of the variable study designs. 17 , 18 , 19 , 20 , 21 The strongest study, in 98 men with node‐positive disease, showed significantly improved OS (HR 1.84 [95% confidence interval—CI, 1.01‐3.35]; P = 0.04), PC‐specific survival (HR 4.09 [1.76‐9.49]; P = .0004), and progression‐free survival (PFS) (HR 3.42 [1.96‐5.98]; P < .0001) after 11.9 years of follow‐up. 17 Given this level 1 evidence, it is rational to consider early ADT in men who are found to be node positive. Future studies are needed to define the value and duration of adjuvant ADT therapies, including novel AR‐directed agents, following RP in men with high‐risk features and without known lymph node metastasis.
Is there a role for neoadjuvant ADT with RP for low‐ to intermediate‐risk patients?
93% No; 7% Indeterminate
Is there a role for neoadjuvant ADT with RP for high‐risk patients?
7% Yes; 71% No; 22% Indeterminate
Is there a role for adjuvant ADT following RP (N1, +/−EBR)?
100% Yes
Is there a role for adjuvant ADT following RP (N0, but other high‐risk features, absence of radiation)?
86% No; 14% Indeterminate
3.3. The role of ADT with RT
The panel reached consensus on the role of ADT with RT: the panel members agreed that ADT should not be used in low‐risk patients being considered for RT, and ADT should be considered for intermediate‐ and high‐risk patients (as defined using the D'Amico criteria). 22 Specifically, the panel agreed upon the role for ADT with RT when the risk of local failure and/or distant disease is high, but not when the local control with radiation alone was expected. The strongest evidence to support adjuvant ADT with RT for intermediate‐risk patients was from the RTOG 94‐08 study in 1979 eligible patients, who received RT alone or 4 months of ADT (starting 2 months before radiotherapy). After a mean follow‐up of 9.1 years, the OS, disease‐specific mortality (DSM), PSA recurrence rates, metastasis‐free survival (MFS), and rates of positive findings on repeat prostate biopsy at 2 years were significantly improved with RT plus short‐term ADT. However, when the results were stratified by risk, only intermediate‐risk patients saw benefit with the addition of ADT to RT, in OS and DSM. 23 A phase III clinical trial to assess whether there is an OS advantage in patients with intermediate‐risk PC treated with dose‐escalated RT with RT alone is ongoing (http://ClinicalTrials.gov identifier: NCT00936390).
The panel reached unanimous consensus on the duration and timing of ADT with RT. Studies have assessed the benefit of 3 to 36 months of ADT in patients with high‐risk disease. In all studies, there was greater benefit with adjuvant ADT given for at least 3 months compared with no ADT, and the panel concluded that optimal dosing of ADT for use with RT is 4 to 6 months for intermediate‐risk patients and 18 to 36 months for high‐risk patients. 23 , 24 , 25 , 26 , 27 , 28 , 29 The first dose of ADT should be administered 1 to 2 months before beginning RT.
Is there a role for ADT with RT?* 100% Yes
Low‐risk patients (except for reduction of volume) 100% No
Intermediate‐risk patients (4‐6 months duration) 100% Yes
High‐risk patients (18‐36 months duration) 100% Yes
Timing: 1 to 2 months before RT 100% Yes
For intermediate‐ and high‐risk PC patients, pending RT and ADT, we recommend ADT initiation ideally 1 to 2 months before initiating RT.
100% Yes
3.4. The role of ADT in patients with BCR after RP and RT
Although there is a paucity of data demonstrating a survival benefit for ADT for BCR patients, ADT can be used to manage BCR or rising levels of PSA after primary therapy. BCR has been defined in the post‐RP setting as two consecutive PSA values ≥0.2 ng/mL and rising, 30 , 31 and in the post‐RT setting as any PSA increase >2 ng/mL higher than the PSA nadir, regardless of nadir. 32 However, as our ability to measure PSA with ultrasensitive assays advances, these definitions may change.
The clinical course of PC in men with BCR post‐RP is highly variable. Pound and colleagues showed that overall, 34% of men who developed BCR post‐RP developed metastases within the 5 years of follow‐up. Yet, BCR is not the only factor that determines risk of developing metastases. Several studies have shown that MFS post‐BCR is strongly influenced by PSA doubling time (PSADT), pathology/Gleason score at the time of RP, interval time to BCR after RP, and age. 33 , 34 , 35 , 36 , 37 , 38 , 39 On the basis of these findings, the panel agreed unanimously that for patients, post‐RP with BCR, PSADT ≤9 months, and Gleason score ≥8 were viewed as significant metrics for consideration for initiating ADT.
Regarding optimal timing of ADT (immediate vs delayed), the literature does not suggest clear evidence to support timing of ADT in patients with BCR post‐RP/XRT, who are node‐positive after RP, with locally advanced, rising PSA or who are not candidates for definitive therapy, or undergoing adjuvant therapy. 17 , 33 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Given the unclear benefit of early ADT in patients with BCR and the known medical sequelae of chronic ADT, this therapy should be reserved for patients exhibiting high‐risk features.
While the NCCN guideline states that intermittent ADT should be considered in this setting, the panel indicated no clear preference for intermittent vs continuous ADT therapy post‐RP/RT. 9 Data from one study suggest that intermittent ADT is not inferior to continuous ADT in nonmetastatic PC in terms of OS and time to progression. The number of PC‐related deaths was higher in the intermittent group but the difference was not statistically significant. The number of PC‐unrelated deaths was higher in the continuous treatment group. Intermittent ADT may offer some benefit in terms of quality of life (sexual desire, less fatigue), as well as less cost and more convenience. 47 The panel reached a consensus that intermittent ADT is a reasonable alternative to continuous ADT in high‐risk patients with BCR post‐RP/RT, or as salvage therapy post‐RP, and they offer a proposed treatment pathway (Figure 2), taking into consideration PSA and testosterone levels.
Figure 2.

Proposed Intermittent Treatment Pathway. *The PSA threshold of 5 ng/mL is arbitrarily chosen for illustrative purposes only. In patients with other high‐risk features, consider restarting treatment at a lower PSA level, but always perform imaging before reinitiation. ADT, androgen deprivation therapy; CRPC, castrate‐resistant prostate cancer; PSA, prostate‐specific antigen [Color figure can be viewed at http://wileyonlinelibrary.com]
Given the natural history of PC (ie, about 1/3 of patients will go on to progress), the majority of patients with BCR should undergo observation with BCR. For the remaining patients (high risk), ADT would generally be considered for:
Early recurrence (BCR <3 years), AND
High‐risk features (PSADT ≤9 months OR Gleason score ≥8)
100% Yes
ADT in BCR is generally not recommended in patients with:
Low‐risk features (PSADT >15 months, AND
Gleason score ≤7, OR
Limited life expectancy, or low risk of metastases, or poor performance status
100% Yes
When considering ADT in high‐risk patients with BCR post‐RP, post‐RT, or postsalvage after RP, intermittent ADT is a reasonable alternative to continuous ADT.
86% Yes, 14% Indeterminate
Proposed Intermittent Treatment Pathway (Figure 1 )
86% Yes, 14% Indeterminate
3.5. The role of ADT in salvage therapy
The NCCN guideline states that some patients are candidates for salvage ADT after M0 PSA persistence/recurrence post‐RP or EBRT. 9 For ADT and salvage RT, the panel agreed that there is level 1 evidence to support ADT use. However, the Shipley study (RTOG 96‐01), which accrued patients more than 15 years ago, may not be applicable, as RT technologies have advanced and high‐dose bicalutamide is not approved within the United States. In the study, which compared RT + bicalutamide (150 mg) vs RT + placebo, the data showed that the addition of ADT resulted in significantly higher rates of OS and MFS and lower incidence of DSM compared with those with RT + placebo. However, in a subgroup analysis, men with PSA <0.7 ng/mL at study entry did not experience a benefit of OS (HR 1.13; 95% CI, 0.77‐1.65; P = .53). 48 Additionally, the 5‐year GETUG‐AFU 16 trial has reported a benefit with 6 months ADT with RT vs no ADT, and the 10‐year MFS data will be reported soon. Thus, it seems reasonable to discuss a short course of ADT in the setting of sXRT, pending additional long‐term data. In the GETUG‐AFU 16 study, comparing RT alone with RT + ADT (goserelin), men who received salvage RT + ADT were significantly more likely to be free of biochemical progression or clinical progression at 5 years vs men who received RT alone if their pretreatment PSA was >0.5 ng/mL, while those with lower PSA did not appear to benefit from the addition of ADT. 49
There is level 1 evidence to support ADT use with salvage radiotherapy. It is reasonable to consider ADT in the setting of salvage radiotherapy as part of a shared decision‐making discussion. The duration of ADT in this setting is unclear, but 6‐12 months of ADT would be reasonable.
86% Yes; 7% No; 7% Indeterminate
3.6. The role ADT in metastatic castrate‐sensitive prostate cancer
While the NCCN guideline identifies ADT as the gold standard treatment for metastatic PC (mPC), the panel did not reach consensus on whether immediate ADT is always necessary in asymptomatic patients with oligometastatic hormone sensitive metastatic prostate cancer without visceral metastases who wish to consider delaying ADT in favor of metastasis‐directed therapy (MDT). 9 The panelists were not able to reach consensus on whether MDT is a reasonable alternative to immediate ADT in select low‐risk patients. Data from a phase II study evaluating MDT for oligorecurrent PC showed a trend toward improved ADT‐free survival at 3 years’ follow‐up (median ADT‐free survival) was 13 months (80% CI, 12‐17 months) for the surveillance group and 21 months (80% CI, 14‐29 months) for the MDT group (HR, 0.60 [80% CI, 0.40‐0.90]; P = .11). Other similar studies to answer this question are ongoing. 50 While these studies alone are not sufficient to warrant a recommendation, MDT is being studied in phase II trials. 51
The panel reached consensus regarding its preference to maintain patients with metastatic castrate‐sensitive prostate cancer (mCSPC) on continuous ADT rather than intermittent ADT, but acknowledged that for certain patients (eg, those with cardiovascular comorbidities), intermittent ADT may be considered to reduce complications. Results from a randomized SWOG study comparing continuous vs intermittent ADT in men with newly diagnosed mCSPC were statistically inconclusive: the CI for survival exceeded the upper boundary for noninferiority with intermittent therapy (HR > 1.20, as per the study design) suggesting that intermittent ADT was inferior to continuous ADT in terms of survival, but the study investigators noted that too few events occurred to rule out significant inferiority of intermittent therapy. Of note, the study included only patients with PSA ≤4.0 ng/mL after 7 months of ADT, which constituted only 60% of those enrolled in the study and suggests that there was not confidence that patients with a higher PSA should receive intermittent therapy. 52 Noninferiority of intermittent therapy with respect to OS, cancer‐specific survival, and PFS was confirmed in a subsequent meta‐analysis including 15 trials. 53 The NCCN guideline discusses the data that indicate no preference for intermittent or continuous ADT in this setting. 9
The panel reached a consensus on the three options to achieve castration (bilateral orchiectomy, LHRH agonists, and LHRG antagonists), as recommended by the American Society of Clinical Oncology. 54 , 55 The panel did not reach consensus on whether combined androgen blockade (CAB) is superior to castration alone, but CAB was considered a reasonable alternative to castration alone, in agreement with the NCCN guideline. 9 , 56
Unanimous consensus was reached on the role of monitoring serum testosterone (T) (Table 3). Obtaining a baseline T level before initiating ADT is essential. Subsequently, in the patient with mCSPC and CRPC, the optimal T level should be <20 ng/dL, based on several studies reviewed. 57 , 58 Of note, the NCCN guideline defines castration as testosterone <50 ng/dL. 9 The panel felt that T levels should be checked 1 to 4 months after initiation of ADT, regardless of PSA levels. Once ADT is initiated and a PSA rise occurs, T levels should be obtained to distinguish noncastrate vs castrate‐resistant progression. If T levels are >20 ng/dL, clinicians should check luteinizing hormone levels to determine whether ADT was administered effectively. If T levels are >20 ng/dL despite low luteinizing hormone, consider switching to an alternative agent.
Table 3.
Monitoring serum testosterone levels in mCSPC
|
Testosterone levels should be checked:
|
Abbreviations: ADT, androgen deprivation therapy; mCSPC, metastatic castrate‐sensitive prostate cancer; PSA, prostate‐specific antigen.
There was unanimous consensus on the superiority of ADT + abiraterone acetate (AA) compared with ADT alone in terms of OS in mCSPC, based on two large clinical trials (LATITUDE and STAMPEDE), consistent with the NCCN recommendation in this setting. 59 , 60 The panel's recommendation was made irrespective of metastatic burden (86% consensus). Recent data from the ARCHES, ENZAMET, and TITAN studies suggest clinical benefit with enzalutamide and apalutamide in this setting as well. 61 , 62 , 63 US FDA approval for these agents in mHSPC is pending.
Likewise, there was unanimous consensus on the superiority of ADT + docetaxel compared with ADT alone in terms of OS for men with mCSPC. In subgroup analyses, there is an unequivocal OS benefit with ADT + docetaxel vs ADT alone in high‐volume disease, defined as ≥4 bone metastases, at least one of which is outside the axial skeleton, and/or visceral (ie, lung or liver) metastases, but not in low‐volume disease. 64 , 65 , 66 The panel recommended that docetaxel should be initiated 6 to 16 weeks after the first ADT dose, to enhance pharmacologic metabolism and tolerability of docetaxel. 67
When considering which up‐front combination for high‐volume mCSPC is superior (ADT + AA or ADT + docetaxel), a prespecified (but not prepowered) analysis of patients from the STAMPEDE trial when recruitment to the two research arms overlapped, showed that ADT + AA was significantly better in failure‐free survival (which included PSA progression) and PFS, with trends to improved MFS, and symptomatic skeletal events (not significant), but there was no significant difference between AA and docetaxel in survival (no significant difference between arms). 68 Cost considerations, access to therapy, duration of therapy, and comorbidities may influence the choice of therapy. 69
When considering optimal systemic therapy for low‐volume and high‐volume disease in mCSPC, the panel reached a consensus that in low‐volume mCSPC, there is strong evidence that ADT + docetaxel does not provide an OS benefit beyond ADT alone; thus, ADT + AA is favored in the low‐volume setting. However, there was unanimous consensus that in high‐volume mCSPC, there is strong evidence of OS benefit for ADT + docetaxel. 70 , 71 Thus, both ADT + AA or ADT + docetaxel are equally preferred treatment options in the high‐volume setting (Table 4). These recommendations are in line with the NCCN guideline. 9
Table 4.
Systemic therapy for mCSPC based on volume
| Preferred agent | ||
|---|---|---|
| ADT + abiraterone acetate | ADT + docetaxel | |
| Low‐volume mCSPC | × | ⋯ |
| High‐volume mCSPC | × | × |
Note: High volume = 4 or more bone metastases (at least one outside of axial skeleton) or visceral (lung, liver) metastases.
Low volume = if criteria for high volume are not met.
Abbreviations: ADT, androgen deprivation therapy; mCSPC, metastatic castrate‐sensitive prostate cancer.
In men with asymptomatic oligometastatic mCSPC (without visceral metastases), metastasis‐directed therapy may be a reasonable alternative to immediate ADT in select patients.
50% Yes, 29% No, 21% Indeterminate
In mCSPC, continuous ADT is generally strongly preferred over intermittent ADT, however in certain circumstances (eg, cardiovascular comorbidities, intolerabilities, etc), intermittent may be considered.
93% Yes, 7% Indeterminate
In mCSPC, there are 3 options to achieve castration: bilateral orchiectomy, LHRH agonists, or LHRH antagonists.
93% Yes, 7% Indeterminate
CAB (ie, LHRHa, LHRHantag, +1st‐ or 2nd‐generation antiandrogen) is superior to castration alone.
64% Yes, 36% No
In mCSPC, baseline T levels should be obtained before starting ADT.
100% Yes
For ADT administration, testosterone level should be T < 20 ng/dL. Confirm castrate T levels 1‐4 months after surgical/medical castration, regardless of PSA levels. If PSA rises, T levels must be checked. If T levels are >20 ng/dL, clinicians should check luteinizing hormone levels to differentiate whether ADT was administered effectively.
100% Yes
In mCSPC, if T > 20 ng/dL despite low luteinizing hormone levels, consider switching to an alternative agent.
100% Yes
In the broad mCSPC population, ADT + Abi is superior to ADT alone in terms of OS.
100% Yes
In mCSPC, the distinction between low‐ and high‐volume disease has not been studied in this context. Thus, ADT + Abi is a reasonable standard of care irrespective of metastatic burden.
86% Yes, 14% Indeterminate
In the broad mCSPC population, ADT + docetaxel is superior to ADT alone in terms of OS.
In high‐volume mCSPC, there is an unequivocal OS benefit to ADT + docetaxel vs ADT alone.
In low‐volume mCSPC, there is no clear OS benefit to ADT + docetaxel vs ADT alone.
Docetaxel treatment can start 6 to 16 weeks after ADT, to avoid toxicities
100% Yes
In low‐volume mCSPC, there is evidence that ADT + docetaxel does not provide an OS benefit; thus, ADT + Abi is the favored choice.
93% Yes, 7% No
In high‐volume mCSPC, there is strong evidence of benefit for ADT + docetaxel. Thus, both ADT + abiraterone or ADT + docetaxel are treatment options.
100% Yes
The panel cannot comment on the comparative efficacy of ADT + Abi in low‐ vs high‐volume mCSPC, because this has not been studied.
100% Yes
3.7. The role of ADT in metastatic castrate‐resistant prostate cancer
Metastatic castrate‐resistant prostate cancer (mCRPC) has been defined as men with PC on ADT with T level <50 ng/dL, a rising PSA (defined as two consecutive rises, ≥4 weeks apart), and radiographic evidence of metastatic disease on imaging studies. 72 However, based on current literature, the panel agreed unanimously that the goal of ADT should be to achieve a T level <20 ng/dL, which differs from the NCCN guideline treatment goal of maintain T levels <50 ng/dL. 9 There was no consensus on a value‐based model of ADT continuation, that is, whether ADT could be stopped once castrate levels are reached and then to check T levels every 3 months (restarting ADT if T rises ≥20 ng/dL). The American Society of Clinical Oncology and NCCN recommend that ADT should be continued indefinitely in the setting of mPC despite the development of castration resistance. 9 , 73 But a survey of PC physicians from five European countries (France, Germany, Italy, Spain, and the United Kingdom) found that up to one third of physicians used chemotherapy as a monotherapy and stopped administering ADT after the development of castration resistance. 74 The panel did not endorse discontinuing ADT after emergence of castration‐resistant disease.
In the context of mCRPC, the panel agreed unanimously that T should be checked at the time of diagnosis of castration resistance, when changing therapy, and if PSA is rising (to confirm castrate levels of T, ie, <20 ng/dL). PSA measures and CT/bone scans should also be obtained at regular intervals (Table 5). These recommendations are in accordance with the Prostate Cancer Clinical Trials Working Group recommendations. 72 , 75
Table 5.
Monitoring serum testosterone levels in mCRPC
|
Testosterone levels should be checked:
|
Abbreviations: ADT, androgen deprivation therapy; mCRPC, metastatic castrate‐resistant prostate cancer; PSA, prostate‐specific antigen.
mCRPC is defined as:
mPC (by conventional radiology)
Testosterone level < 50 ng/dL
Progressive disease (one or more of):
PSA progression (2 rises above a nadir, ≥4 weeks apart)
Radiographic progression (as defined by the PC Working Group 2: at least 2 new bone lesions on a CT or MRI scan)
Unequivocal clinical progression (bone pain progression requiring narcotics or palliative radiotherapy, pathological fracture, urinary obstruction, or spinal cord compression)
100% Yes
In mCRPC, continuation of ADT to maintain T < 20 ng/dL is strongly recommended.
100% Yes
Another reasonable option (“value‐based model”) in treating mCRPC is to stop ADT (once castrate levels are reached) and to check T levels q3 months, then restart ADT if T rises above >20 ng/dL.
14% Yes, 43% No, 43% Indeterminate
For patients with mCRPC on treatment, testosterone levels should be measured:
At baseline
When changing therapy
If PSA rising (to confirm castrate level: <20 ng/dL)
Also, PSA and CT/bone scans should be performed at regular intervals.
100% Yes
3.8. AE management strategies with ADT and combination therapies
With ADT being the most commonly prescribed agent by clinicians for the management of advanced PC, understanding all of the potential effects of therapy is critical to optimizing patient health outcomes. While there are numerous medical and quality‐of‐life adverse events attributable to the use of ADT, the panel focused on the following: bone fragility and associated skeletal events, cardiovascular disease (CVD), diabetes, neurocognitive effects, and hot flashes. A consistent theme of our discussions was that achieving optimal outcomes requires a multidisciplinary approach of cancer care providers, potentially involving primary care providers (PCPs), and other appropriate specialists to coordinate the care of PC patients on ADT to prevent and minimize complications of ADT therapy. The panel noted that the primary cancer provider (urologist, radiation oncologist, or medical oncologist) needs to be fully engaged in identifying these risks and engaging with the overall medical team.
3.9. Bone health
The risk of osteoporosis in aging men on ADT increases due to both advancing age as well as the suppression of T and estrogen, which are both bone protective for fracture risk. ADT has been shown to accelerate bone loss and hypogonadism is a leading cause of osteoporosis in men within the United States. 76 , 77 , 78 , 79 Prolonged exposure to ADT also increases fracture risk in a dose‐dependent manner. 80 The panel reached unanimous consensus on several aspects of risk management of skeletal events with ADT: obtaining a dexascan ‐ bone densitometry (DEXA) scan and calculating a FRAX score at baseline (within 6 months of initiating ADT) and at least once every 2 years for follow‐up; assessing vitamin D levels in men who are taking osteoclast inhibitors, by either the PCP or cancer care provider. All men on ADT should receive appropriate maintenance supplementation of vitamin D and calcium (not to exceed 800‐1000 IU vitamin D daily after restoring vitamin D stores to normal levels and 1200 mg calcium daily), all in accordance with the NCCN guidelines on bone health for men on ADT (Table 6). 9 Likewise, the panel supported the NCCN guidelines on determining which patients are eligible for additional pharmacologic therapies (denosumab or zoledronic acid) to increase bone mineral density in men with osteoporosis, men with osteopenia, and men with an elevated risk of fracture based on personal risk factors as estimated by calculating a patient's FRAX score (eg, FRAX Fracture Risk Assessment Tool, available at: https://www.sheffield.ac.uk/FRAX/tool.aspx?country=9).
Table 6.
Management of bone health during ADT
|
Abbreviations: ADT, androgen deprivation therapy; DEXA, dexascan ‐ bone densitometry; PCP, primary care provider.
Should a DEXA scan and FRAX score be obtained for baseline and follow‐up testing of bone fragility during treatment with ADT (pending approval by insurance provider)?
100% Yes
A DEXA scan should be obtained at baseline (within 6 months of initiating ADT) and at least once every 2 years for follow‐up.
100% Yes
Vitamin D levels can be monitored in men taking osteoclast inhibitors, up to annually, or more often if replenishing depleted stores with high‐dose vitamin D. Daily maintenance doses for vitamin D supplementation should be 800‐1000 IU daily, and calcium daily maintenance dosing should not exceed 1200 mg daily.
93% Yes; 7% Indeterminate
The NCCN guidelines on determining which patients are eligible for additional pharmacologic therapy should be followed (ie, calculating a patient's individual risk of fracture via FRAX calculator). For those patients who are eligible, zoledronic acid or denosumab can be used.
100% Yes
3.10. Cardiovascular risks
Several studies have documented the increased risk of CVD in men on ADT. 81 , 82 , 83 , 84 This increased risk appears to be more pronounced in older men (>75 years of age), for whom prolonged ADT increases the risk of both CVD and diabetes, especially in those with cardiovascular comorbidities. 85 , 86 The NCCN guidelines stress the assessment of traditional risk factors for CVD using the ABCDE approach (Awareness and Aspirin, Blood pressure, Cholesterol and Cigarette smoking, Diet and Diabetes, and Exercise) as well as the importance of a multidisciplinary team approach to address these risks, potentially including the PCP, a geriatrician, and a cardio‐oncologist or cardiologist, when both practical and accessible. 9 , 87 The panel agreed unanimously that either the cancer care provider or the PCP should monitor blood pressure (at each visit), HbA1c (annually to screen for DM, and quarterly by the clinician managing diabetes), and lipid profile (annually) in patients receiving ADT. Obtaining a baseline EKG can be considered in patients initiating ADT, particularly for men with a history of CVD. In addition, before initiating ADT in men with significant CVD comorbidities, referral to a cardiologist for comanagement is recommended.
Either the urologist or the PCP should monitor blood pressure (at each visit), HbA1c (annually in nondiabetic patients), and lipid profile (annually) in patients receiving ADT.
93% Yes; 7% Indeterminate
Before initiating ADT, patients with CVD comorbidities should be referred to a cardiologist for comanagement.
100% Yes
3.11. Diabetes and glucose intolerance
Akin to cardiovascular risks, several studies have shown an increased risk of diabetes with ADT. 85 , 88 , 89 The NCCN guidelines again stress traditional assessment of risk factors for diabetes (HbA1c and/or fasting blood glucose), using a team approach among the PCP, geriatrician, and endocrinologist. 9 , 87 The panel strongly encourages multidisciplinary and integrative care with PCPs and other medical specialists, when possible, to optimize the well‐recognized adverse event profile of ADT. The panel had unanimous consensus that either the cancer care provider or the PCP should monitor diabetes risk factors in diabetic patients receiving ADT, both at baseline and annually. Cancer‐treating physicians should encourage activity/exercise, healthy diet, weight control, and smoking cessation in all patients on ADT throughout the course of their treatment. While some data suggest that metformin may be associated with improved survival in patients on ADT, the data were not strong enough to garner unanimous consensus on recommending metformin for patients on ADT. 90
There is no evidence to support taking metformin to improve PC‐specific outcomes.
86% Yes, 14% Indeterminate
The urologist should communicate with the PCP or endocrinologist when patients with diabetes initiate ADT as they may need closer monitoring of diabetes.
86% Yes; 14% Indeterminate
HbA1c should be monitored up to annually by a cancer care provider or PCP in patients without a history of diabetes.
86% Yes; 7% No; 7% Indeterminate
Cancer‐treating physicians should encourage physical activity/exercise, healthy diet, weight control, and smoking cessation in all patients on ADT throughout the course of their treatment.
100% Yes
3.12. Neurocognitive dysfunction
There is increasing evidence regarding the effects of ADT on psychological and cognitive functioning. Some studies suggest that ADT may be associated with depression and/or cognitive dysfunction. However, specific guidelines for evaluation are lacking. Depression is common in patients with cancer, and men who are taking ADT may have a higher risk of depression. 91 The data suggesting that exposure to ADT is associated with an increased risk of cognitive decline are inconsistent. 92 , 93 , 94 , 95 While effective treatments for depression are available, there are no standardized interventions to reverse cognitive decline for this patient population. Studies are being developed to address this unmet need in men treated with ADT. Management of depression and any potential cognitive dysfunction represent a significant unmet need in men with PC. The panel agreed unanimously on the importance of increasing urologists’ awareness of depression risk in patients receiving ADT, in accordance with the USPSTF recommendations on screening for depression in anyone with a chronic illness. 96 The NCCN guideline notes the potential link between ADT and depression and ADT and cognitive function, with side effects of continuous ADT increasing with duration of treatment. As such, the guideline recommends that patients be advised of these risks before initiating treatment. 9 The panel recommends consideration of routine discussions of mental health concerns, including questions about depression and memory concerns, with evaluation performed at least annually. There was not consensus regarding specific screening tools or treatment for depression or cognitive impairment, although involving primary care and other specialists, psychiatrists, psychologists, and neurologists for further management was supported.
We recognize the importance of increasing urologists’ awareness of depression risk in patients receiving ADT.
100% Yes
Urologists need to be aware of depression risk in patients receiving ADT.
100% Yes
Consider routine discussions of mental health concerns, including questions about depression and memory concerns, with evaluation performed at least annually, in men receiving ADT.
100% Yes
Patients over the age of 70 who have been on ADT for >2 years should be referred annually for neurocognitive assessment to evaluate for dementia.
29% Yes; 21% No; 50% Indeterminate
3.13. Vasomotor symptoms
Recent studies suggest that up to 80% of men on ADT have vasomotor symptoms, principally involving hot flashes, and 27% cited this side effect as the most bothersome. 97 Hot flashes can be associated with sleep disturbance and are a major contributor to the discontinuation of ADT. Currently, there are no approved drugs to treat men with hot flashes and there have been very few placebo‐controlled trials. The panel recognizes that hot flashes are a significant side effect of ADT. Of all the agents that are currently being used to treat hot flashes in men receiving ADT, none have an FDA approved indication for this use and each is associated with side effects (Table 7). Clinicians should have an informed discussion of off‐label options for pharmacologic management of hot flashes in men who wish to use medical therapies to reduce their symptoms. The NCCN guideline notes that vasomotor symptoms are a known side effect of ADT, with side effects of continuous ADT increasing with duration of treatment. As such, the guideline recommends that patients be advised of these risks before initiating treatment. 9
Table 7.
Common medications used to address hot flashes in men with prostate cancer
| Medication | Common doses used for hot flashes | Common adverse effects |
|---|---|---|
| Megace (megestrol acetate) | 20 mg PO qd | Weight gain, CV risk (DVT/PE), cost |
| Effexor XR (venlafaxine) | 75 mg PO qd | Feelings of being activated/jittery if not titrated properly |
| Suicidal ideation | ||
| Withdrawal issues | ||
| Paxil (paroxetine HCl) | ⋯ | Weight gain, loss of libido, suicidal ideation, withdrawal issues |
| Clonidine | ⋯ | ⋯ |
| Gabapentin | ⋯ | Drowsiness, dyspepsia |
| Depo‐Provera (medroxyprogesterone) 150 | 150 mg IM | Increased risk of thrombotic issues |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolus.
We recognize that hot flashes are side effects of ADT and can negatively impact patient quality of life. Of all the agents that are currently being used to treat hot flashes in men receiving ADT, none have an FDA approved indication for this use and each is associated with side effects. Shared decision making practices should be used to discuss the pros and cons of off‐label use of medications for hot flashes for men who wish to use medical management strategies.
100% Yes
4. DISCUSSION
For more than half a century, castration has been the standard of care in the management of advanced PC patients. A major breakthrough for ADT came in the 1980s with the development of LHRH agonists, which were initially approved for patients with metastatic disease. These agents resulted in an increased use of ADT in PC beyond those with documented metastasis. Practicing urologists have used ADT therapies across the entire spectrum of PC, from newly diagnosed localized disease to mCRPC, but its implementation in specific patient populations has evolved. For example, a review of Medicare data from 1991 to 2005 found that 44.8% of men with PC were exposed to ADT within the first year of treatment. Of those, 51.8% were ages 75% to 79% and 60.1% were age ≥80 years old, which raises concern for heightened use of ADT in settings with greater potential geriatric comorbidities. 2
With both retrospective and prospective trials, the urologic oncology community has become aware and more knowledgeable regarding the myriad potential adverse events and side effects associated with T suppression. Thus, there exists a clinical need for more judicious use and management of ADT across the PC spectrum. To develop an effective therapeutic management plan, we have reviewed the literature and delineated a list of pathway recommendations of the optimal uses of ADT as well as the side effect profiles associated with ADT. In this way, we can best identify, manage, and counsel patients who would most benefit from ADT and choose the most effective, risk‐mitigating guidance for ADT management.
Since 2004, and especially within the last 10 years, given the numerous approvals of therapies for advanced PC, multiple treatment guidelines have been developed to aid in the delivery of these agents which, for the most part, were approved within monotherapy phase 3 trials in conjunction with ADT use. Although ADT has been the mainstay of advanced PC therapy, to our knowledge, no consensus statement has been developed to assist practitioners for the optimal use and management of ADT across the PC disease continuum.
5. LIMITATIONS
There were several limitations to this consensus panel and its recommendations. This panel was chosen subjectively, based on clinician experience, research interests, and publication history. We also recognize that the panel did not include a radiation oncologist, which might have added an important perspective to some of the discussions. The studies reviewed for these recommendations was not selected by a formal literature review, but by the knowledge and expertise of the panel subcommittee members. In the medical literature, there is a lack of level 1 evidence to make conclusive statements about ADT use in all settings. In those instances, the panel members based their recommendations on their expertise and clinical experience. Finally, this field is rapidly evolving based on new clinical trial data and developing technologies; hence, these recommendations will likely need to be updated every 2 to 3 years.
6. CONCLUSIONS
The goal of this panel's effort is not to replace existing guidelines that have been published and painstakingly developed by several national and international organizations with input from highly respected experts. Rather, we have attempted to provide a practical and user‐friendly reference for all clinicians who participate in the management of PC, specifically as it involves the utilization of ADT across the various stages of the disease. Of note, the NCCN guideline is broad and includes recommendations for several different treatments in M0 and M1 disease, as the guideline seeks to synthesize all of the published literature. Our goal in this report is to assist physicians in making treatment decisions from among these choices. As we would prefer to have recommendations solely based on level I evidence, we acknowledge that the luxury of having data‐driven information does not always exist, and thus, level III/expert opinion can be solicited and enumerated via a consensus panel of PC experts, thereby providing an educational algorithm to optimize patient care where variability in treatment patterns may exist.
The panel has provided recommendations for monitoring PC patients while on ADT, recognizing that due to the heterogeneity of PC, patients with PC patients will progress despite testosterone suppression and, therefore, early identification of conversion from castrate‐sensitive to castration resistance is critical. Also, the requirement to both identify and mitigate side effects as a result of ADT as well as the importance of quality of life maintenance are essential to the quality of patient care, especially important as more combinatorial ADT therapies emerge throughout the spectrum of disease management. Often, existing guidelines focus upon monitoring workflows for all patients, regardless of their disease stage, and thus we have attempted to improve the granularity of recommendations for all disease states when ADT might be initiated. We have explored, discussed, and debated the current literature and have enumerated our recommendations for our colleagues’ consideration and are hopeful that this will serve as a template for the optimization of patient care.
CONFLICT OF INTERESTS
Neal D. Shore, MD, FACS, Research/consulting: Amgen, Astellas, AstraZeneca, Bayer, BMS, Dendreon, Ferring, Janssen, Merck, Myovant, Nymox, Pfizer, Sanofi‐Genzyme, and Tolmar; Emmanuel S. Antonarakis, MD, Paid consultant/advisor: Janssen, Pfizer, Sanofi, Dendreon, Bayer, Bristol Myers Squibb, Amgen, Merck, AstraZeneca, Clovis Research grants to his institution: Janssen, Johnson&Johnson, Sanofi, Bristol Myers Squibb, Pfizer, AstraZeneca, Celgene, Merck, Bayer, Clovis Inventor of a biomarker technology that has been licensed to Qiagen; Michael S. Cookson, MD, Advisory board: MDxHealth, Janssen Scientific Affairs, LLC; Bayer Healthcare Pharmaceuticals Inc; Ferring Pharmaceuticals Inc; Consulting agreement: Astellas Pharma US Inc; E. D. Crawford, MD, Consultant advisor: Tolmar, Bayer, MDx, Genomic Health, Janssen, Dendreon, Ferring, Spouse employee: Dendreon Meeting participant or lecturer: Bayer Scientific study or trial: NIH, University of Colorado Cancer Center; Alicia K. Morgans, MD, Honoraria for consulting: Astellas, Sanofi, Bayer, AstraZeneca, Janssen, Genentech Research funding: Bayer; David M. Albala, MD, Speaker: Blue Earth Diagnostics, Genomic Health Advisory board: Cellanyx; Jason Hafron, MD, Scientific study/trial: Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc Meeting participant/lecturer: Blue Earth Diagnostics, Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc Consultant/Advisor: Dendreon Pharmaceuticals LLC, Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc; Richard G. Harris, MD, Advisor/speaker: Jansen, Astellas, Bayer. Advisor Dendreon, and Clovis; Daniel Saltzstein, MD, Speaker/advisor: Janssen and Astellas Pharmaceuticals; Jonathan Henderson, MD, Consultant: Myriad Genetics, Clovis Pharmaceuticals Speaker: Janssen; Benjamin Lowentritt, MD, Speaker and/or consultant: Bayer, Dendreon, Janssen, Astellas, Pfizer, Genomic Health and Myriad; Raoul Concepcion, MD, FACS, Consultant: CUSP, Integra Connect, Clovis, Cellay, Merck, Invitae, Astellas, Janssen, Sun Pharma, Dendreon Speaker's Bureau: Astellas, Pfizer, Amgen, Sun Pharma, Dendreon. The remaining authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors wish to thank Mary Gabb, MS, who provided medical writing services and Integra Connect (West Palm Beach, FL) for their assistance in the preparation of this manuscript. Figure 1 was created by Neal Shore, Alicia Morgan, and Raoul Concepcion with technical assistance from Brandon Wang, Integra Connect. Funding for the advisory panel and manuscript was provided by an unrestricted grant from Tolmar Pharmaceuticals.
Shore ND, Antonarakis ES, Cookson MS, et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines. The Prostate. 2020;80:527–544. 10.1002/pros.23967
REFERENCES
- 1. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232‐240. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert SM, Kuo YF, Shaninian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2011;29:647‐653. 10.1016/j.urolonc.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saylor PJ, Lee RJ, Smith MR. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J Clin Oncol. 2011;29:3705‐3714. 10.1200/JCO.2010.34.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu‐Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173‐181. 10.1001/jama.300.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu‐Yao GL, Albertsen PC, Moore DF, et al. Fifteen‐year survival outcomes following primary androgen‐deprivation therapy for localized prostate cancer. JAMA Intern Med. 2014;174:1460‐1467. 10.1001/jamainternmed.2014.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong YN, Freedland SJ, Egleston B, Vapiwala N, Uzzo R, Armstrong K. The role of primary androgen deprivation therapy in localized prostate cancer. Eur Urol. 2009;56:609‐616. 10.1016/j.eururo.2009.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sammon JD, Abdollah F, Reznor G, et al. Patterns of declining use and the adverse effect of primary androgen deprivation on all‐cause mortality in elderly men with prostate cancer. Eur Urol. 2015;68:32‐39. [DOI] [PubMed] [Google Scholar]
- 8. Potosky AL, Haque R, Cassidy‐Bushrow AE, et al. Effectiveness of primary androgen‐deprivation therapy for clinically localized prostate cancer. J Clin Oncol. 2014;32:1324‐1330. 10.1200/JCO.2013.52.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer Version 4. 2019. https://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf. Accessed 17 August 2019.
- 10. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683‐690. 10.1016/j.juro.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 11. Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5‐year results. J Urol. 2002;167:112‐116. [PubMed] [Google Scholar]
- 12. Klotz LH, Goldenberg SL, Jewett MAS, et al. Long‐term follow up of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791‐794. [DOI] [PubMed] [Google Scholar]
- 13. Berglund RK, Tangen CM, Powell IJ, et al. Ten‐year follow‐up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology. 2012;79:633‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKay RR, Montgomery B, Xie W, et al. Post prostatectomy outcomes of patients with high‐risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis. 2018;21:364‐372. 10.1038/s41391-017-0009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long‐term follow‐up of 3‐month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010;105:185‐190. 10.1111/j.1464-410X.2009.08698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pal SK, Ruel N, Vogelzang N, et al. Preoperative androgen deprivation therapy for localized prostate cancer: delayed biochemical recurrence in high‐risk disease. Clin Genitourin Cancer. 2015;12:149‐154. 10.1016/j.clgc.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node‐positive prostate cancer after radical prostatectomy and pelvic lymphadenopathy. Lancet Oncol. 2006;7:472‐479. 10.1016/S1470-2045(06)70700 [DOI] [PubMed] [Google Scholar]
- 18. Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer—evidence for deferred treatment. J Urol. 2001;166:508‐516. [DOI] [PubMed] [Google Scholar]
- 19. Sato YT, Fukuhara H, Suzuki M, et al. Long‐term results of radical prostatectomy with immediate adjuvant androgen deprivation therapy for pT3N0 prostate cancer. BMC Urol. 2014;14:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high‐risk prostate cancer after radical prostatectomy: SWOG S9921 Study. J Clin Oncol. 2011;29:2040‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain M, Tangen CM, Thompson IM, et al. Phase III intergroup trial of adjuvant androgen deprivation with or without mitoxantrone plus prednisone in patients with high‐risk prostate cancer after radical prostatectomy: SWOG S9921. J Clin Oncol. 2018;36:1498‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969‐974. [DOI] [PubMed] [Google Scholar]
- 23. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short‐term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107‐118. 10.1056/NEJMoa1012348 [DOI] [PubMed] [Google Scholar]
- 24. Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86‐10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243‐1252. [DOI] [PubMed] [Google Scholar]
- 25. Denham JW, Steigler A, Lamb DS, et al. Short‐term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans‐Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841‐850. [DOI] [PubMed] [Google Scholar]
- 26. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long‐term results of phase III RTOG 85‐31. Int J Radiat Oncol Biol Phys. 2005;61:1285‐1290. [DOI] [PubMed] [Google Scholar]
- 27. Lawton CAF, Lin X, Hanks GE, et al. Duration of androgen deprivation in locally advanced prostate cancer: long‐term update of NRG Oncology RTOG 9202. Int J Radiat Oncol Biol Phys. 2017;98:296‐303. 10.1016/j.ijrobp.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanks GE, Lu JD, Machtay M, et al. RTOG protocol 92‐02: a phase III trial of the use of long term total androgen supppression following neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2000;48(3 suppl):112. [Google Scholar]
- 29. Bolla M, de Reijke TM, van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516‐2527. 10.1056/NEJMoa0810095 [DOI] [PubMed] [Google Scholar]
- 30. Cornford P, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration‐resistant prostate cancer. Eur Urol. 2017;71:630‐642. 10.1016/j.eururo.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 31. Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441‐449. 10.1016/j.juro.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 32. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG‐ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965‐974. [DOI] [PubMed] [Google Scholar]
- 33. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591‐1597. [DOI] [PubMed] [Google Scholar]
- 34. Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long‐term overall survival and metastasis‐free survival for men with prostate‐specific antigen‐recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108:378‐385. 10.1111/j.1464-410X.2010.09878.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;17(291):1325‐1332. [DOI] [PubMed] [Google Scholar]
- 36. Kim‐Sing C, Pickles T. Prostate cohort outcomes initiative. Intervention after PSA failure: examination of intervention time and subsequent outcomes from a prospective patient database. Int J Radiat Oncol Biol Phys. 2004;60:463‐469. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen T, Boldt RG, Rodrigues G. Prognostic factors for prostate cancer endpoints following biochemical failure: a review of the literature. Cureus. 2015;7:e238 10.7759/cureus.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brockman JA, Alanee S, Vickers AJ, et al. Nomogram predicting prostate cancer‐specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160‐1167. 10.1016/j.eururo.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer‐specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433‐439. [DOI] [PubMed] [Google Scholar]
- 40. Siddiqui SA, Boorjan SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830‐1837. 10.1016/j.juro.2008.01.022 [DOI] [PubMed] [Google Scholar]
- 41. Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen‐deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01‐03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncol. 2016;17:727‐737. 10.1016/S1470-2045(16)00107-8 [DOI] [PubMed] [Google Scholar]
- 42. Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67:1009‐1016. 10.1016/j.eururo.2014.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong YN, Freedland S, Egleston B, Hudes G, Schwartz JS, Armstrong K. Role of androgen deprivation therapy for node‐positive prostate cancer. J Clin Oncol. 2009;27:100‐105. 10.1200/JCO.2007.14.2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Medical Research Council Prostate Cancer Working Party Investigators Group . Immediate versus deferred treatment for advanced prostate cancer: initial results of the Medical Research Council Trial. BJU. 1997;78:235‐246. [DOI] [PubMed] [Google Scholar]
- 45. Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24:1868‐1876. [DOI] [PubMed] [Google Scholar]
- 46. McLeod DG, Iversen P, See WA, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97:247‐254. [DOI] [PubMed] [Google Scholar]
- 47. Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895‐903. 10.1056/NEJMoa1201546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417‐428. 10.1056/NEJMoa1607529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short‐term hormone therapy for rising prostate‐specific antigen concentration after radical prostatectomy (GETUG‐AFU 16): a randomised, multicentre, open‐label phase 3 trial. Lancet Oncol. 2016;17:747‐756. 10.1016/S1470-2045(16)00111-X [DOI] [PubMed] [Google Scholar]
- 50. Radwan N, Phillips R, Ross A, et al. A phase II randomized trial of Observation versus stereotactic ablative RadiatIon for OLigometastatic prostate CancEr (ORIOLE). BMC Cancer. 2017;17:453 10.1186/s12885-017-3455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis‐directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II Trial. J Clin Oncol. 2018;36:446‐453. 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 52. Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314‐1325. 10.1056/NEJMoa1212299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Magnan S, Zarychanski R, Pilote L, et al. Intermittent vs continuous androgen deprivation therapy for prostate cancer: a systematic review and meta‐analysis. JAMA Oncol. 2015;1:1261‐1269. 10.1001/jamaoncol.2015.2895 [DOI] [PubMed] [Google Scholar]
- 54. Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen‐targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24‐38. 10.1038/s41391-018-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen‐sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596‐1605. [DOI] [PubMed] [Google Scholar]
- 56. PCTCG . No authors listed. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. 2000;355:1491‐1498. [PubMed] [Google Scholar]
- 57. Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone‐releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648‐651. 10.1111/j.1464-410X.2009.08814.x [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto S, Sakamoto S, Minhui X, et al. Testosterone reduction of ≥480 ng/dl predicts favorable prognosis of Japanese men with advanced prostate cancer treated with androgen‐deprivation therapy. Clin Genitourin Cancer. 2017;15:e1107‐e1115. 10.1016/j.clgc.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 59. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2017;377:352‐360. 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 60. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163‐1177. 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone‐sensitive prostate cancer. J Clin Oncol. 2019;22:JCO1900799 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first‐line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121‐131. 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 63. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2019;381:13‐24. 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 64. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373:737‐746. 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer: long‐term survival analysis of the randomized phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36:1080‐1087. 10.1200/JCO.2017.75.3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338‐351. 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Franke RM, Carducci MA, Rudek M, Baker SD, Sparreboom A. Castration‐dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol. 2010;28:4562‐4567. 10.1200/JCO.2010.30.7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sydes MR, Spears MR, Mason MD, et al. Adding abiraterone or docetaxel to long‐term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi‐arm, multi‐stage platform protocol. Ann Oncol. 2018;29:1235‐1248. 10.1093/annonc/mdy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McNamara M, Sweeney C, Antonarakis ES, Armstrong AJ. The evolving landscape of metastatic hormone‐sensitive prostate cancer: a critical review of the evidence for adding docetaxel or abiraterone to androgen deprivation. Prostate Cancer Prostatic Dis. 2018;21:306‐318. 10.1038/s41391-017-0014-9 [DOI] [PubMed] [Google Scholar]
- 70. Gravis G, Boher JM, Chen YH, et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG‐AFU15 Studies. Eur Urol. 2018;73:847‐855. 10.1016/j.eururo.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morris MJ, Rumble RB, Basch E, et al. Optimizing anticancer therapy in metastatic non‐castrate prostate cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1521‐1539. 10.1200/JCO.2018.78.0619 [DOI] [PubMed] [Google Scholar]
- 72. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148‐1159. 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Basch E, Loblaw DA, Oliver TK, et al. Systemic therapy in men with metastatic castration‐resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32:3436‐3448. 10.1200/JCO.2013.54.8404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sternberg CN, Baskin‐Bey ES, Watson M, Worsfold A, Rider A, Tombal B. Treatment patterns and characteristics of European patients with castration‐resistant prostate cancer. BMC Urol. 2013;13:58 10.1186/1471-2490-13-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10:e0139440 10.1371/journal.pone.0139440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin‐releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219‐1222. [PubMed] [Google Scholar]
- 77. Falahati‐Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leder BZ, Smith MR, Fallon MA, Lee ML, Finkelstein JS. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2001;86:511‐516. [DOI] [PubMed] [Google Scholar]
- 79. Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen‐deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948‐955. [DOI] [PubMed] [Google Scholar]
- 80. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154‐164. [DOI] [PubMed] [Google Scholar]
- 81. Keating NL, O'Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516‐1524. Epub 9 October 2007 [DOI] [PubMed] [Google Scholar]
- 83. Alibhai SMH, Duong Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta‐analysis of randomized trials. JAMA. 2011;306:2359‐2366. 10.1001/jama.2011.1745 [DOI] [PubMed] [Google Scholar]
- 85. Morgans AK, Fan KH, Koyama T, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol. 2015;193:1226‐1231. 10.1016/j.juro.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Does comorbidity influence the risk of myocardial infarction or diabetes during androgen‐deprivation therapy for prostate cancer? Eur Urol. 2013;64:159‐166. 10.1016/j.eururo.2012.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bhatia N, Santos M, Jones LW, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133:537‐541. 10.1161/CIRCULATIONAHA.115.012519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448‐4456. [DOI] [PubMed] [Google Scholar]
- 89. Keating NL, O'Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39‐46. 10.1093/jnci/djp404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Richards KA, Liou JI, Cryns VL, Downs TM, Abel EJ, Jarrard DF. Metformin use is associated with improved survival for patients with advanced prostate cancer on androgen deprivation therapy. J Urol. 2018;200:1256‐1263. 10.1016/j.juro.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 91. Dinh KT, Reznor G, Muralidhar V, et al. Association of androgen deprivation therapy with depression in localized prostate cancer. J Clin Oncol. 2016;34:1905‐1912. 10.1200/JCO.2015.64.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alibhai SMH, Timilshina N, Duff‐Canning S, et al. Effects of long‐term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer. 2017;123:237‐244. [DOI] [PubMed] [Google Scholar]
- 93. Joly F, Alibhai SMH, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6 pt 1):2443‐2447. [DOI] [PubMed] [Google Scholar]
- 94. Nead KT, Gaskin G, Chester C, Swisher‐McClure S, Leeper NJ, Shah NH. Association between androgen deprivation therapy and risk of dementia. JAMA Oncol. 2017;3:49‐55. 10.1001/jamaoncol.2016.3662 [DOI] [PubMed] [Google Scholar]
- 95. Gonzalez BD, Jim HSL, Booth‐Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen‐deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33:2021‐2027. 10.1200/JCO.2014.60.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Siu AL, US Preventive Services Task Force (USPSTF) , Bibbins‐Domingo K, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380‐387. 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- 97. Holzbeierlein JM, Castle E, Thrasher JB. Complications of androgen deprivation therapy: prevention and treatment. Oncology. 2004;18:303‐309. [PubMed] [Google Scholar]


