Abstract
Background and Aims
Anti‐mitochondrial antibodies (AMA) are closely linked to primary biliary cholangitis (PBC). The prevalence of AMA in the general population is low, and AMA positivity may precede PBC. We aimed to determine the natural history of subjects with positive AMA.
Methods
In total, 302 patients were tested AMA‐positive over a ten‐year period. Of these, immunoblotting confirmed specific AMA in 184 (29 male, 155 female, age 59.6 ± 14.1 years). These subjects were invited to our liver outpatient clinic for clinical and biochemical re‐evaluation. Detailed clinical history data were additionally collected from the hospital computer system and by telephone. The subsequent course with regard to mortality, liver‐related morbidity, extrahepatic co‐morbidities and effectiveness of PBC treatment was determined in 150 subjects (81.5%).
Results
After 5.8 ± 5.6 years of follow‐up (FU), of 184 AMA‐positive subjects, 28 subjects (15.2%; liver‐related mortality n = 5) were deceased, and 122 subjects (66.3%) completed FU while 34 subjects (18.5%) were not available for FU. The 122 patients who completed FU were 63 patients with established PBC, six de novo cases of PBC (10.2% of 59 initially at risk), 42 (34.4%) subjects were still AMA‐positive without PBC, and 11 (9.0%) subjects were AMA‐negative at FU.
Conclusions
Anti‐mitochondrial antibodies‐positive patients without PBC at baseline infrequently developed PBC over six years of FU. AMA positivity represented a transient serological autoimmune phenomenon in a significant proportion of subjects.
Keywords: anti‐mitochondrial antibodies, biliary cholangitis, primary biliary cholangitis

Abbreviations
- AIH
autoimmune hepatitis
- AITD
autoimmune thyroiditis
- ALD
alcoholic liver disease
- ALF
acute liver failure
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
anti‐mitochondrial antibody
- ANA
anti‐nuclear antibody
- ASMA
anti‐smooth muscle antibody
- BL
baseline
- BMI
body mass index
- DILI
drug‐induced liver injury
- FU
follow‐up
- GGT
gamma‐glutamyltransferase
- IB
immunoblot
- IgM
immunoglobulin M
- IIF
indirect immunofluorescence
- LC
anti‐liver cytosol antibodies
- LKM
anti‐liver kidney microsomal antibodies
- LSM
liver stiffness measurement
- NAFLD
nonalcoholic fatty liver disease
- PBC
primary biliary cholangitis
- PCR
polymerase chain reaction
- SLE
systemic lupus erythematosus
- UDCA
ursodeoxycholic acid
- ULN
upper limit of normal
Introduction
Anti‐mitochondrial antibodies (AMA) represent a key criterion in the diagnosis for primary biliary cholangitis (PBC) 1, 2. Over 90% of all PBC patients test positive for AMA 3. On the other hand, AMA positivity is a rare finding in the general healthy population, with a prevalence <1% 4, 5, 6, 7. Data on the clinical relevance of AMA positivity outside the PBC context and the subsequent natural course are scarce, and only few studies have dealt with this specific question. AMA positivity may precede the onset of PBC by several years 8, 9. In 1996, Metcalf et al. 10 reported that 76% of 29 initially AMA‐positive patients had developed clinical and biochemical features of PBC 10 years after the initial positive antibody test. Notably, 24 of these patients had histologic findings compatible with or diagnostic for PBC in their baseline liver biopsy. In contrast, a recent analysis in France found a 5‐year‐incidence of PBC of only 16% in 66 AMA‐positive patients 9. An older Norwegian follow‐up study showed that 17 of 48 initially AMA‐positive patients tested AMA‐negative after 1–7 years 11. None of those patients had evidence of liver disease at the time of first AMA testing. No case of new‐onset PBC at follow‐up was reported. Hence, the clinical risk to develop PBC in case of AMA positivity can barely be estimated from these varying and discrepant numbers.
In our study, we aimed to assess the natural course of subjects with AMA positivity with and without PBC by conducting a comprehensive clinical follow‐up of a local cohort of AMA‐positive subjects.
Patients and methods
Study cohort
Baseline data
From January 2006 until December 2015, 302 (out of 15.671 tests performed, 1.9% positive tests) subjects had been tested AMA‐positive by indirect immunofluorescence (IIF) and underwent confirmatory immunoblotting (IB) at the Immunology Laboratory of the Department of Dermatology, Paracelsus Medical University Salzburg, Austria, where all immunological tests for the area are performed. Only subjects with confirmatory IB test performed were counted as having valid test results available. The quality of AMA testing, evaluated by participation in an external quality assessment (ÖQUASTA, Vienna, Austria), has been positively confirmed over the years.
Of 302 AMA‐positive patients, IB was positive in 184/302 (60.9%) and these subjects were re‐evaluated by stratification to one of three groups: (i) 34 (18.5%) subjects who were not recruited for follow‐up with baseline data available only, (ii) 28 (15.2%) deceased subjects and (iii) 122 (66.3%) subjects who completed follow‐up. Mean time to follow‐up was 5.8 ± 5.6 years (Fig. 1 for details).
Figure 1.
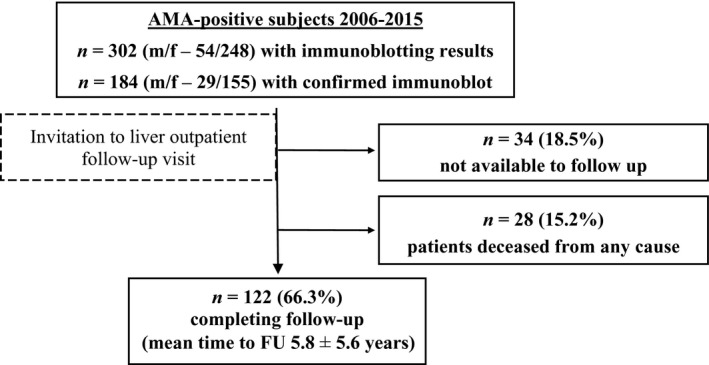
Flow chart of patient cohort. Three hundred and two AMA‐positive patients were invited to follow‐up, 28 of these were deceased, no contact could be established in 34 for follow‐up, and 122 completed follow‐up. AMA, anti‐mitochondrial antibodies; FU, follow‐up.
Data of interest including medical history, baseline laboratory values, medical specialty indicating initial AMA test and state of health at the time of testing were collected using the following strategy: searching the hospital computer database for medical reports, telephone interviews with patients and/or treating physician, as well as written correspondence where no telephone contact could be established.
Follow‐up
All subjects were invited to participate in a detailed follow‐up (FU) examination at the hepatology outpatient clinic. This re‐evaluation included history taking for assessment of current physical status and completion of each medical record, and laboratory tests as indicated below. Additionally, liver stiffness measurement (LSM) was performed using transient elastography (Fibroscan®; Echosens, Paris, France). Treatment response to ursodeoxycholic acid (UDCA) was assessed according to Barcelona criteria, that is decline of alkaline phosphatase (ALP) levels by 40% in subjects where PBC had been established at baseline.
The protocol was approved by the local ethics committee (Ethikkommission für das Bundesland Salzburg) and conducted in accordance to the ethical standards of the 1975 Declaration of Helsinki (revised in 1983). Informed consent was obtained from each patient included in the study.
Laboratory evaluation
Biochemical characteristics were obtained at baseline, that is time point of first AMA test, and at the time of the follow‐up visit (May 2016–December 2016). At baseline and follow‐up, full blood count, electrolytes, liver function tests, serum iron parameters, C‐reactive protein, fasting glucose, lipid profile, hepatitis B and C serology and polymerase chain reaction (PCR), copper, ceruloplasmin, alpha‐1‐antitrypsin and autoantibody screening comprising ANA, ASMA, LC, LKM and AMA including M2‐blot were determined by standardised automated laboratory methods after an overnight fast. AMA detection was performed by IIF and confirmatory immunoblot (IB). For the clinical study, every AMA IIF positive result at baseline was counted, and no cut‐off for low AMA titres was used. Confirmation of specific AMA by immunoblot (184 of 302 subjects) was required for inclusion in the study.
Statistical analysis
Statistical analyses were carried out using spss (SPSS 24.0; IBM Statistics, Armonk, NY, USA). Data are presented as median (range in brackets) unless otherwise stated. Distribution of data sets was assessed by Shapiro–Wilk test. For calculations of inter‐group comparisons, we used the chi‐square test, Kruskal–Wallis H test combined with Dunn–Bonferroni test, Mann–Whitney U test and Wilcoxon signed‐rank test as appropriate. P‐values were adjusted for multiple testing by Benjamini–Hochberg procedure. A P‐value <0.05 was considered significant.
Results
Clinical and biochemical characteristics at baseline
Baseline biochemical and clinical data are given in Table 1.
Table 1.
Biochemical and clinical characteristics of 184 patients at baseline
| Parameter | Available data (n) | Median (range) or n (%) |
|---|---|---|
| Age (years) | 184 | 67 (25–97) |
| Sex (m/f) | 184 | 29/155 (15.8/84.2%) |
| BMI (kg m−2) | 92 | 25.8 (17.8–44.1) |
| Pruritus (n) | 121 | 11 (9.1%) |
| Fatigue (n) | 117 | 9 (7.7%) |
| sp100 or gp210 positive (n) | 172 | 37 (21.5%) |
| Liver biopsy (n) | 160 | 41 (25.6%) |
| Total cholesterol (mmol L−1) | 125 | 5.5 (1.9–11.8) |
| LDL‐cholesterol (mmol L−1) | 129 | 3.3 (0.6–6.2) |
| Fasting glucose (mmol L−1) | 145 | 5.3 (1.3–14.2) |
| Total bilirubin (µmol L−1) | 135 | 7.7 (2.9–99.2) |
| ALT (×ULN) | 157 | 0.8 (0.2–36.5) [35.0% >ULN] |
| ALP (×ULN) | 155 | 0.9 (0.3–10.6) [43.9% >ULN] |
| GGT (×ULN) | 157 | 2.0 (0.3–49.9) [68.8% >ULN] |
| IgM (×ULN) | 104 | 0.7 (0.1–3.3) [25.0% >ULN] |
| Ferritin (pmol L−1) | 123 | 247.2 (20.2–5610.8) |
| Prothrombin index (%) | 130 | 101 (16–140) |
| Platelet count (×109 per L) | 155 | 261.0 (14–616) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BMI, body mass index; GGT, gamma‐glutamyl transpeptidase; IgM, immunoglobulin M; LDL, low‐density lipoprotein; ULN, upper limit of normal.
All subjects were evaluated for the presence of hepatic and extrahepatic diseases at baseline, counted only once according to the clinically predominant condition that initiated the testing for anti‐nuclear and anti‐mitochondrial antibodies. One hundred and fifteen cases were categorised as liver disease, and 69 were counted as extrahepatic disease. Assessment of underlying liver diseases at baseline revealed that PBC represented the largest group with 85 (46.2% of 184) patients. Of these, 34 had a concomitant autoimmune disorder, that is autoimmune thyroid disease (AITD, n = 8), autoimmune hepatitis (AIH, n = 5), type 1 diabetes mellitus and Sjögren’s syndrome (each n = 3), coeliac disease, polymyalgia rheumatica, rheumatoid arthritis, sarcoidosis, systemic lupus erythematosus (SLE), systemic sclerosis (each n = 1), or overlapping conditions comprising PBC plus two or more of the forenamed conditions (n = 9).
Other liver diseases in non‐PBC patients at baseline were nonalcoholic fatty liver disease (NAFLD, n = 10), alcoholic liver disease (ALD, n = 9), AIH (n = 5), drug‐induced liver injury (DILI, n = 3), viral hepatitis (n = 2) or haemochromatosis (n = 1).
Extrahepatic morbidities as the leading entity reported at the time of AMA testing are shown in Table 2. Twelve patients were not categorised to any of the groups mentioned in the table. These included AITD, chronic obstructive pulmonary disease and interstitial lung disease (each n = 2), obstructive cholestasis (n = 1) and other heterogeneous conditions such as coeliac disease, diabetes mellitus type 2, erythema nodosum, ischaemic colitis and smoking‐induced leukocytosis (each n = 1).
Table 2.
Categorisation of extrahepatic diseases of the whole cohort at baseline
| Category (n) | Rheumatic | 19 | Neurologic | 16 |
| Diseases (n) | Systemic sclerosis | 4 | Stroke | 10 |
| Polyarthritis | 4 | Dementia | 2 | |
| Psoriasis | 4 | Epilepsy | 1 | |
| SLE | 2 | Depression | 1 | |
| Rheumatoid arthritis | 2 | ALS | 1 | |
| Sjögren’s syndrome | 1 | Cerebral cavernoma | 1 | |
| UCTD | 1 | |||
| Adult onset Still’s disease | 1 | |||
| Category (n) | Haematologic/oncologic | 11 | Dermatologic | 4 |
| Diseases (n) | Leukaemia/lymphoma | 4 | Cutaneous vasculitis | 1 |
| Solid tumours | 3 | Morphea | 1 | |
| gastrointestinal | 1 | Urticaria | 1 | |
| gynaecologic | 1 | Vitiligo | 1 | |
| pulmonary | 1 | |||
| MGUS | 2 | |||
| Multiple myeloma | 1 | |||
| Myeloproliferative disorder | 1 | |||
| Transient agranulocytosis | 1 | |||
| Category (n) | Renal | 4 | Cardiovascular | 3 |
| Diseases (n) | End‐stage renal disease | 2 | Hypertension | 2 |
| Nephrogenic sepsis | 1 | Coronary artery disease | 1 | |
| Renal artery stenosis | 1 |
ALS, amyotrophic lateral sclerosis; MGUS, monoclonal gammopathy of undetermined significance; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease.
Altogether, 69 nonliver disease patients were counted. Twelve patients were uncategorised.
Deceased patients
At the time of FU, 28 subjects (15.2%) had died and clinical data were available. The baseline AMA test of deceased patients was performed 3.9 ± 4.5 years before death. These patients were significantly older at the time of baseline AMA testing compared to subjects who were still alive [69.5 (50–90) vs. 57 (20–78) years, P < 0.001]. Further clinically significant differences between deceased and alive subjects were a high proportion of subjects with liver cirrhosis (deceased 28.6%, n = 9, vs. alive 9.0%, n = 11; P = 0.005) at a similar proportion of subjects with liver diseases (including PBC) in both groups (alive 63.1% vs. deceased 67.9%, P = 0.637).
Baseline laboratory characteristics of deceased PBC versus non‐PBC patients are given in Table 3.
Table 3.
Baseline laboratory characteristics and causes of death in deceased subjects with PBC versus non‐PBC
| Parameter | PBC (n = 8) | Non‐PBC (n = 20) | P |
|---|---|---|---|
| Age (years) | 66.5 (54–90) | 72.5 (50–89) | 0.633 |
| Sex (m/f) | 0/8 | 7/13 | 0.172 |
| sp100 or gp210 positive (n) | 1 (12.5%) | 3 (15.0%) | 0.864 |
| Total cholesterol (mmol L−1) | 6.4 (6.1–6.6) | 5.4 (1.9–9.0) | 0.193 |
| Fasting glucose (mmol L−1) | 4.3 (3.5–6.3) | 6.0 (4.0–10.9) | 0.065 |
| Total bilirubin (µmol L−1) | 6.8 (5.1–37.6) | 11.1 (5.1–85.5) | 0.633 |
| ALT (×ULN) | 0.8 (0.4–2.5) | 0.6 (0.3–2.1) | 0.633 |
| ALP (×ULN) | 1.6 (1.4–6.0) | 0.7 (0.3–3.6) | 0.065 |
| GGT (×ULN) | 2.3 (0.6–24.4) | 1.8 (0.5–18.0) | 0.633 |
| IgM (×ULN) | 1.1 (0.6–2.3) | 0.6 (0.1–1.0) | 0.065 |
| Ferritin (pmol L−1) | 195.5 (92.1–294.4) | 561.8 (62.9–5610.8) | 0.193 |
| Prothrombin index (%) | 100 (88–122) | 97 (16–112) | 0.633 |
| Platelet count (×109 per L) | 229.5 (165–369) | 225 (60–616) | 0.633 |
| Cause of death | |||
| CV events incl. stroke | n = 0 | n = 8 | |
| Nonhepatic malignancies | n = 1 | n = 5 | |
| Liver‐related events | n = 1 | n = 4 | |
| Not available | n = 6 | n = 3 | |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BMI, body mass index; CV events, cardiovascular events; GGT, gamma‐glutamyl transpeptidase; IgM, immunoglobulin M; PBC, primary biliary cholangitis; ULN, upper limit of normal.
Data shown as median (range in brackets). Levels of significance: *P < 0.05; **P < 0.001 (Mann–Whitney U test, chi‐square test; P‐values adjusted for multiple testing by Benjamini–Hochberg procedure).
Both groups were highly similar except for higher ALP and IgM levels in the PBC group as well as lower fasting glucose in the PBC group (each P = 0.065).
In 20 AMA‐positive patients not diagnosed with PBC, cardiovascular events (n = 8) and nonhepatic malignancies (n = 5) were the most common causes of death.
Subjects with completed follow‐up
Patients with completed FU were very similar to patients lost to FU at baseline except for younger age in the FU group [57 (20–78) vs. 67.5 (17–89), P = 0.001; Table 4 for details]. The 122 patients who completed the FU evaluation were stratified into five groups according to clinical and biochemical patterns at the time of FU as summarised in Table 5. These groups were as follows: established PBC (adequate or inadequate biochemical treatment response), new‐onset PBC, AMA‐positive patients without PBC and AMA‐negative subjects.
Table 4.
Comparison of baseline characteristics for patients completing follow‐up (FU) versus patients not available to follow‐up (n/a to FU)
| Parameter | FU (n = 122) | n/a to FU (n = 34) | P |
|---|---|---|---|
| Age (years) | 57 (20–78) | 67.5 (17–89) | 0.001* |
| Sex (m/f) | 18/104 | 4/30 | 0.658 |
| BMI (kg m−2) | 26.6 (17.9–44.1) | 23.6 (20.4–32) | 0.252 |
| sp100 or gp210 positive (n) | 39 (21.5%) | 8 (12.3%) | 0.343 |
| Total cholesterol (mmol L−1) | 5.5 (3.1–11.8) | 5.0 (2.3–7.2) | 0.370 |
| LDL‐cholesterol (mmol L−1) | 3.3 (0.6–6.2) | 2.8 (1.1–5.0) | 0.640 |
| Fasting glucose (mmol L−1) | 5.3 (1.3–14.2) | 5.3 (3.9–9.3) | 0.853 |
| Total bilirubin (µmol L−1) | 6.8 (2.9–99.2) | 8.6 (5.1–22.2) | 0.176 |
| ALT (×ULN) | 0.8 (0.2–36.5) | 0.8 (0.3–10.3) | 0.292 |
| ALP (×ULN) | 0.9 (0.3–10.6) | 0.8 (0.4–7.7) | 0.574 |
| GGT (×ULN) | 2.3 (0.3–49.9) | 1.2 (0.3–38.9) | 0.179 |
| IgM (×ULN) | 0.7 (0.1–3.1) | 0.7 (0.2–3.3) | 0.987 |
| Ferritin (µg L−1) | 238.2 (20.2–2961.5) | 294.4 (71.9–2368.3) | 0.859 |
| Prothrombin index (%) | 101.5 (38.6–140) | 103 (63.1–124) | 0.640 |
| Platelet count (×109 per L) | 255 (14–615) | 279 (133–559) | 0.315 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BMI, body mass index; GGT, gamma‐glutamyl transpeptidase; IgM, immunoglobulin M; ULN, upper limit of normal.
Data shown as median (range in brackets). Levels of significance: *P < 0.05; **P < 0.001 (Mann–Whitney U test, chi‐square test; P‐values adjusted for multiple testing by Benjamini–Hochberg procedure).
Table 5.
Comparison of biochemical characteristics at baseline (BL) and follow‐up (FU) for each group
| AMA‐negative (n = 11) | AMA‐positive without PBC (n = 42) | New‐onset PBC (n = 6) | Inadequately treated PBC (n = 21) | Adequately treated PBC (n = 42) | |
|---|---|---|---|---|---|
| baseline | |||||
| Age (years) | 66 (54–71) | 56.5 (27–79) | 58.5 (47–71) | 55 (34–70) | 57 (20–76) |
| BMI (m kg−2) | 24.9 (17.9–27.5) | 24.8 (18.6–44.1) | 29.3 (25.0–30.3) | 25.5 (18.3–47.9) | 28.2 (18.0–39.0) |
| Chol (mmol L−1) | 5.7 (4.6–6.7) | 5.2 (3.5–8.5) | 4.7 (4.6–6.3) | 4.9 (3.2–11.8) | 5.9 (3.1–9.0) |
| LDL (mmol L−1) | 3.2 (1.6–4.2) | 3.1 (1.8–6.0) | 3.1 (0.6–4.2) | 2.7 (1.5–6.2) | 3.6 (0.7–5.9) |
| FG (mmol L−1) | 6.1 (4.3–11.0) | 5.1 (1.3–10.5) | 7.2 (4.4–11.0) | 5.0 (3.4–5.5) | 5.3 (3.9–14.2) |
| TBili (µmol L−1) | 6.8 (6.8–13.7) | 6.8 (4.1–18.8) | 10.3 (5.1–18.3) | 6.8 (4.3–24.1) | 7.7 (2.9–99.2) |
| ALT (×ULN) | 0.7 (0.4–2.3) | 0.6 (0.2–1.6) | 1.4 (0.7–2.7) | 1.1 (0.3–2.8) | 1.1 (0.2–36.5)** |
| ALP (×ULN) | 0.7 (0.5–2.6) | 0.7 (0.3–1.1) | 0.7 (0.4–2.1) | 1.2 (0.4–10.6) | 1.5 (0.6–4.3)** |
| GGT (×ULN) | 0.5 (0.5–8.7) | 0.8 (0.3–3.4) | 2.6 (1.4–8.1) | 3.4 (1.2–9.4) | 3.7 (0.5–27.4)** |
| IgM (×ULN) | 0.4 (0.1–0.8) | 0.4 (0.1–0.9) | 0.8 (0.4–1.5) | 0.9 (0.2–2.0) | 1.0 (0.3–3.1) |
| Follow‐up | |||||
| Age (years) | 69 (55–75) | 60.5 (28–79) | 63.5 (51–79) | 62 (41–80) | 60 (25–84) |
| BMI (m kg−2) | 24.6 (16.8–28.7) | 25.1 (18.9–44.1) | 25.1 (22.9–28.6) | 24.4 (17.9–47.9) | 28.1 (19.7–38.5) |
| Chol (mmol L−1) | 5.9 (4.7–9.0) | 5.4 (3.3–9.0) | 4.3 (3.1–6.2) | 5.4 (3.5–13.6) | 5.6 (3.4–9.0) |
| LDL (mmol L−1) | 3.3 (2.1–6.3) | 3.2 (1.7–5.8) | 2.4 (1.2–3.7) | 3.2 (1.7–12.4) | 3.0 (2.0–5.7) |
| FG (mmol L−1) | 5.5 (4.3–8.7) | 5.2 (4.0–8.3) | 5.2 (4.4–6.6) | 5.4 (3.2–13.2) | 5.6 (4.2–10.3) |
| TBili (µmol L−1) | 8.6 (1.7–12.0) | 6.8 (3.4–25.7) | 8.6 (3.4–23.9) | 6.8 (5.1–47.9) | 8.6 (3.4–18.8) |
| ALT (×ULN) | 0.7 (0.4–2.2) | 0.6 (0.2–1.3) | 0.9 (0.5–1.4) | 1.3 (0.3–3.9) | 0.7 (0.4–2.9)** |
| ALP (×ULN) | 0.7 (0.5–0.9) | 0.7 (0.4–1.9) | 1.3 (0.7–1.8) | 1.8 (0.8–11.6) | 0.9 (0.4–5.2)** |
| GGT (×ULN) | 0.7 (0.1–1.8) | 0.9 (0.3–3.8) | 3.2 (2.4–8.0) | 3.7 (0.6–23.9) | 1.2 (0.3–8.1)** |
| IgM (×ULN) | 0.3 (0.1–0.8) | 0.4 (0.1–1.4) | 1.2 (0.4–1.7) | 1.2 (0.2–2.8) | 1.0 (0.4–4.1) |
| LSM (kPa) | 4.3 (3.2–10.2) | 4.8 (2.9–23.4) | 5.1 (3.4–14.5) | 7.2 (3.5–23.4) | 5.7 (3.6–16.3) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti‐mitochondrial antibodies; BMI, body mass index; Chol, cholesterol; FG, fasting glucose; GGT, gamma‐glutamyl transpeptidase; IgM, immunoglobulin M; LDL, low‐density lipoprotein cholesterol; LSM, liver stiffness measurement; PBC, primary biliary cholangitis; TBili, total bilirubin; ULN, upper limit of normal.
Groups were categorised by clinical and biochemical course determined at FU. Intra‐group comparisons (BL versus FU) were calculated for each group. Data shown as median (range in brackets). Levels of significance: *P < 0.05; **P < 0.001 (Wilcoxon signed‐rank test; P‐values adjusted for multiple testing by Benjamini–Hochberg procedure).
Established PBC
At FU, 63 patients with established PBC were re‐evaluated. Of these, 42 patients (67.7%) had an adequate biochemical response to standard therapy (UDCA). Patients in this group showed significant improvements in ALT, ALP (each P < 0.001) and GGT levels (P = 0.003, BL versus FU). Cholesterol and IgM levels had improved, but not statistically significant. LSM results yielded 5.7 kPa (range 3.6–16.3 kPa; corresponding to F0–F1 stage in most subjects). Three patients (7.1% of 42) had cirrhosis in this group.
Twenty‐one PBC patients (33.3% of 63) were classified as having an inadequate treatment response. Those patients showed numerical increases of ALP, GGT levels and IgM serum concentrations although not significant (P = 0.132, P = 0.875, P = 0.077). LSM showed signs of more advanced fibrosis stages as compared to UDCA responders [7.2 (3.5–23.4) kPa; F1–F2]. Four patients in this group were missed PBC diagnoses at baseline and thus untreated until follow‐up. Four out of these 21 patients (19.0%) had been diagnosed with cirrhosis.
The proportion of sp100‐ or gp210‐antibody positivity was not different between the two groups (26.2% vs. 33.3%, P = 0.554).
New‐onset PBC
We diagnosed six new cases of PBC at the time of follow‐up. Diagnosis was made on basis of AMA positivity combined with persistent ALP elevation. Hence 6/59 subjects (10.2%) who had not fulfilled PBC diagnostic criteria at BL fulfilled criteria at FU. Interestingly, BMI of these new‐onset PBC patients was highest among all groups at baseline [29.3 (25.0–30.3) kg m−2] but had decreased at follow‐up [25.1 (22.9–28.6) kg m−2, P = 0.351] while the differences in liver tests were not significant. ALP was normal at baseline but elevated at the FU visit and confirmed thereafter in these 6 subjects. The increase in serum IgM concentrations was not significant [0.8 (0.4–1.5) vs. 1.2 (0.4–1.7) × ULN, P = 0.311]. LSM results were lower than the groups of inadequately and adequately treated PBC [5.1 (3.4–14.5) kPa].
AMA‐positive without PBC
At FU, 42 patients (34.4%) were re‐tested AMA‐positive without clinical or biochemical signs of PBC. ALP, GGT and IgM levels had remained unchanged. Evaluation of baseline disease spectrum in those patients revealed that only a minor proportion (n = 10) had initially suspected liver diseases, mainly NAFLD (n = 6), rarely ALD (n = 2), DILI or hemochromatosis (each n = 1). Most patients had an underlying rheumatic condition (n = 14), which were psoriasis and systemic sclerosis (each n = 3), rheumatoid arthritis and various collagen vascular diseases (n = 8).
AMA‐negative
Eleven patients (9.0%) tested AMA‐negative at FU, and patients in this group showed lowest levels of GGT, IgM and LSM at FU. Interestingly, total cholesterol at FU was highest in these patients [5.9 (4.7–9.0) mg dL−1]. Disease profile was characterized by non‐PBC liver diseases (n = 3) and rheumatic conditions (n = 2). Among the latter, CREST syndrome and psoriasis were found (each n = 1). Hepatic disorders comprised ALD, NAFLD and DILI (each n = 1).
Discussion
Anti‐mitochondrial antibody are clinically useful to establish PBC diagnosis in subjects with persistently elevated ALP; however, AMA are also found in the absence of PBC. The link of AMA with PBC is strong, approximately 90–95% of PBC patients are positive for AMA, while the prevalence is below 1% in the general non‐PBC population 4, 5, 6, 7. It is a rare, but recurring clinical problem to assess the clinical relevance of AMA positivity in non‐PBC subjects 8. The data on this question are highly variable, ranging from a very high risk of developing PBC in older studies to low rates in more recent studies. We therefore aimed to determine the natural course of AMA‐positive subjects detected over a 10‐year period in a Central European population.
We were able to determine the course in 150/184 (81.5%) patients, allowing us to draw representative conclusions for this patient group.
In our investigation we observed (i) a low rate (10.2% of 59 patients at risk) of subjects developing PBC at FU, (ii) a significant proportion of subjects (9.0%) who were AMA‐negative at FU, (iii) a relatively high rate of PBC patients (33.3% of 63) that were inadequately treated with standard medical therapy and (iv) extensive evidence that AMA positivity is frequently found as a collateral phenomenon in other autoimmune or malignant diseases without evidence of liver disease.
The risk to develop PBC in case of AMA positivity can only be inaccurately estimated from the available literature. The high rates in some studies from the UK are contrasted with low rates of newly diagnosed PBC in AMA‐positives reported in France and Norway, which may reflect the chronic and slowly progressive course of the disease but also differences in study design 9, 10, 11. The cohort of Metcalf et al. was of similar age compared to our FU group (54.7 vs. 57 years), but smaller (n = 29 vs. n = 122) and comprised relatively more females (28/29 vs. 104/122 females). Duration of FU was considerably longer in the study of Metcalf et al. (median 17.8 years) than in our cohort (mean 5.8 years). One major difference in study design should be emphasized: 24/29 patients of the UK cohort (82.8%) had already shown histologic findings compatible with or diagnostic for PBC in their baseline liver biopsy, while 28/122 (23.0%) in our cohort were regarded and treated as manifest PBC patients after baseline biopsy. The study subjects from Metcalf et al. were left untreated because UDCA has been officially approved only in 1998, 12 years after the first description of the cohort. The French cohort of Dahlqvist et al. described in 2017 comprised fewer patients (n = 92) than our cohort but duration of follow‐up was similar (mean 4.0 years) and reported a comparably low incidence of new‐onset PBC. Regarding this, our findings in a large group of subjects from a restricted geographic area support a low likelihood for subsequent development of PBC in AMA positivity without liver disease at baseline.
With 9.0%, we observed an unexpectedly high proportion of transient AMA positivity. Data on the ‘loss’ of AMA over time are scarce 11, 12, 13. Transient AMA positivity in the context of non‐PBC liver disease such as DILI, viral hepatitis and acute liver failure has been published 13, 14, 15, 16, 17, 18. In a Norwegian study, 35% (n = 17) of patients turned out AMA‐negative at FU 11. Leung et al. 13 hypothesised oxidative stress might be a possible inducer of AMA in acute liver failure (ALF) when they found only one of 69 ALF patients still testing AMA‐positive 24 months after ALF. In our study, subjects with AMA loss at FU also had some evidence of liver damage at the time of baseline investigation that had largely resolved. Hence, our finding supports and expands data from the ALF cohort that AMA may arise as a nonspecific immune phenomenon also in milder forms of liver damage with subsequent disappearance with the resolution of liver damage.
In summary, the divergent clinical course including resolution of liver damage and loss of AMA positivity on one side and development of PBC on the other side argues that AMA‐positive subjects without established PBC should clinically be followed in order to determine the natural course of these patients.
We identified four subjects who fulfilled PBC diagnostic criteria at BL but the diagnosis had been overlooked. These subjects argue that the awareness for PBC should also be raised among nonhepatologists, that is particularly neurologists in our study who initiated AMA testing as part of an ‘autoimmune screening’ during the etiological work‐up of stroke or stroke‐like episodes.
Although it was not the key aim of our investigation, we obtained data on the clinical course of subjects with established PBC. The results of treatment response in known PBC cases resemble data from study groups in the Netherlands and North America 19, 20. This is clinically important, since in Central Europe the opinion is widely held that the proportion of UDCA nonresponders is lower compared to data reported from Western Europe or Canada. Our findings suggest that this may primarily reflect lack of systematic data in Central Europe. It was our clinical observation that patients who were intolerant or nonresponders to UDCA tended to avoid specialist FU while those who tolerated and responded to treatment had maintained FU visits at specialist clinics. We conclude that PBC subjects need to be actively and systematically followed as those who require specialist care are most likely not to be seen. This is particularly relevant, as novel and effective treatment options have become available 1, 21, 22.
Mortality in AMA‐positive, non‐PBC patients has so far been evaluated in few studies with only small case numbers 4, 10. Nevertheless, our data resemble those of a French study 9. Cardiovascular events including stroke were the most likely cause of death in AMA‐positive patients which reflects the leading causes of mortality in the general population and was different from observations in PBC patients 23, 24. The second most common cause of death in our study, nonhepatic malignancies, corresponds to the leading cause of death of the French PBC cohort.
Mortality due to hepatic causes was infrequently documented in our cohort. This finding corresponds to what is known for PBC in literature as hepatic mortality is generally low in those patients 25, 26, 27.
Limitations of our study include the following: First, some patients’ data were not available for baseline analysis and several patients were not available for follow‐up. Hence, data quality in these subjects relies on the accuracy of baseline medical reports and laboratory data. However, participation rate in follow‐up (122/162, i.e. 75.3% of potentially alive subjects not including deceased subjects) was satisfactory with regard to the high number of deceased subjects (15.2%) by the time of FU. Another limitation was small sample size in the new PBC‐onset group making it difficult to identify ‘hard’ baseline risk factors for future onset of disease.
Conclusion
In conclusion, AMA‐positive patients without PBC at BL only infrequently developed PBC over >6 years of follow‐up at a rate of approximately 10% of subjects at risk. AMA positivity represented a transient serological autoimmune phenomenon in almost twice as many as the number of new‐onset PBC.
Conflict of interest
None of the authors have potential conflicts of interest with regard to this manuscript.
Acknowledgements
Financial grants from PMU‐FFF (E‐18/27/141‐AIS) and Intercept Practice‐to‐Policy Health Awards Program are gratefully acknowledged. Funders had no involvement neither in study design, data collection, analysis, interpretation, writing nor decision of submitting for publication. Support from Spar Austria to Christian Datz is acknowledged.
Zandanell S, Strasser M, Feldman A, Tevini J, Strebinger G, Niederseer D, Pohla‐Gubo G, Huber‐Schönauer U, Ruhaltinger S, Paulweber B, Datz C, Felder T, Aigner E (Paracelsus Medical University, Salzburg; Oberndorf Hospital, Oberndorf, Austria; University of Zurich, Zurich, Switzerland; Paracelsus Medical University, Salzburg, Austria). Low rate of new‐onset primary biliary cholangitis in a cohort of anti‐mitochondrial antibody‐positive subjects over six years of follow‐up. J Intern Med 2020; 10.1111/joim.13005
Contributor Information
T.K. Felder, Email: t.felder@salk.at.
E. Aigner, Email: e.aigner@salk.at.
References
- 1. European Association for the Study of the Liver . EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017; 67: 145–72. [DOI] [PubMed] [Google Scholar]
- 2. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019; 69: 394–419. [DOI] [PubMed] [Google Scholar]
- 3. Van de Water J, Cooper A, Surh CD et al Detection of autoantibodies to recombinant mitochondrial proteins in patients with primary biliary cirrhosis. N Engl J Med 1989; 320: 1377–80. [DOI] [PubMed] [Google Scholar]
- 4. Shibata M, Onozuka Y, Morizane T et al Prevalence of antimitochondrial antibody in Japanese corporate workers in Kanagawa prefecture. J Gastroenterol 2004; 39: 255–9. [DOI] [PubMed] [Google Scholar]
- 5. Mattalia A, Quaranta S, Leung PS et al Characterization of antimitochondrial antibodies in health adults. Hepatology 1998; 27: 656–61. [DOI] [PubMed] [Google Scholar]
- 6. Liu HY, Wang LX, Liu YF. [Frequencies of autoantibodies specific for primary biliary cirrhosis in a general adult population group]. Zhonghua Gan Zang Bing Za Zhi 2008; 16: 922–5. [PubMed] [Google Scholar]
- 7. Turchany JM, Uibo R, Kivik T et al A study of antimitochondrial antibodies in a random population in Estonia. Am J Gastroenterol 1997; 92: 124–6. [PubMed] [Google Scholar]
- 8. Mitchison HC, Bassendine MF, Hendrick A et al Positive antimitochondrial antibody but normal alkaline phosphatase: is this primary biliary cirrhosis? Hepatology 1986; 6: 1279–84. [DOI] [PubMed] [Google Scholar]
- 9. Dahlqvist G, Gaouar F, Carrat F et al Large‐scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology 2017; 65: 152–63. [DOI] [PubMed] [Google Scholar]
- 10. Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet 1996; 348: 1399–402. [DOI] [PubMed] [Google Scholar]
- 11. Jorde R, Rekvig OP, Bostad L. A follow‐up study of 68 patients with anti‐mitochondrial antibodies (AMA). Acta Med Scand 1986; 220: 241–7. [DOI] [PubMed] [Google Scholar]
- 12. Invernizzi P, Alessio MG, Smyk DS et al Autoimmune hepatitis type 2 associated with an unexpected and transient presence of primary biliary cirrhosis‐specific antimitochondrial antibodies: a case study and review of the literature. BMC Gastroenterol 2012; 12: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung PS, Rossaro L, Davis PA et al Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology 2007; 46: 1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernal W, Meda F, Ma Y, Bogdanos DP, Vergani D. Disease‐specific autoantibodies in patients with acute liver failure: the King's College London Experience. Hepatology 2008; 47: 1096–7; author reply 97. [DOI] [PubMed] [Google Scholar]
- 15. Tage‐Jensen U, Permin H, Hardt F et al Circulating autoantibodies in patients with acute viral hepatitis. Relation to etiology and clinical course. Scand J Gastroenterol 1980; 15: 229–35. [DOI] [PubMed] [Google Scholar]
- 16. Liu YM, Yan HP, Han Y et al [Clinical significance of liver function and autoantibodies in patients with acute or chronic drug‐induced liver injury]. Zhonghua Gan Zang Bing Za Zhi 2010; 18: 37–40. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Yu YL, Jin Y, Zhang Y, Zheng CQ. Clinical characteristics of drug‐induced liver injury and primary biliary cirrhosis. World J Gastroenterol 2016; 22: 7579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Volchkova EV, Allenov MN, Umbetova KT, Ivanova IV, Pak SG. [Autoimmune manifestations in acute viral hepatitides]. Ter Arkh 2003; 75: 11–4. [PubMed] [Google Scholar]
- 19. Kumagi T, Guindi M, Fischer SE et al Baseline ductopenia and treatment response predict long‐term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010; 105: 2186–94. [DOI] [PubMed] [Google Scholar]
- 20. Kuiper EM, Hansen BE, de Vries RA et al Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009; 136: 1281–7. [DOI] [PubMed] [Google Scholar]
- 21. Corpechot C, Chazouilleres O, Rousseau A et al A placebo‐controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018; 378: 2171–81. [DOI] [PubMed] [Google Scholar]
- 22. Nevens F, Andreone P, Mazzella G et al A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016; 375: 631–43. [DOI] [PubMed] [Google Scholar]
- 23. Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population‐based study in Canterbury, New Zealand. Hepatology 2012; 55: 522–9. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization . Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000‐2016. Geneva: World Health Organization, 2018. [Google Scholar]
- 25. Floreani A, Caroli D, Variola A et al A 35‐year follow‐up of a large cohort of patients with primary biliary cirrhosis seen at a single centre. Liver Int 2011; 31: 361–8. [DOI] [PubMed] [Google Scholar]
- 26. Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004; 53: 865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boonstra K, Bokelaar R, Stadhouders PH et al Increased cancer risk in a large population‐based cohort of patients with primary biliary cirrhosis: follow‐up for up to 36 years. Hepatol Int 2014; 8: 266–74. [DOI] [PubMed] [Google Scholar]


