Abstract
Background
Recent data demonstrated that an altered basal membrane, activated melanocytes and secreted factors from keratinocytes but also fibroblasts and endothelial cells are involved in the pathophysiology of melasma.
Objectives
To evaluate the efficacy and tolerability on melasma of a new topical skin‐lightening cosmetic product combination (CCP) targeting several factors identified to be involved in melasma pathogenesis compared to 4% hydroquinone (HQ).
Methods
Forty‐three women with melasma were enrolled in a 12‐week double‐blind, randomized, parallel‐group trial and treated with CCP or 4% HQ cream. Efficacy was evaluated with the modified Melasma Area Severity Index (mMASI) score and colorimetric change. Cutaneous tolerability and patient satisfaction were also investigated.
Results
The mMASI score decreased for both products from baseline and over the study period. At week 12, 90% of the subjects who received the combination products had an improvement in pigmentation vs. 79% with HQ. Similarly, both products significantly increased Individual Typological Angle parameters. For both measures, no statistically significant difference was observed between CCP and HQ in terms of change from baseline. CPP was very well tolerated.
Conclusions
Cosmetic product combination is as effective as HQ in the management of facial dyspigmentation and represents a safe alternative.
Introduction
Melasma is one of the most common pigmentation disorders.1 Data reported the past decade clearly showed that melasma is a much more complex disorder than expected.2 Beyond ultraviolet (UV) exposure, the role of high energy visible light through a specific receptor called Opsin 3 was shown to stimulate pigmentation but also to participate in melasma relapses.3, 4, 5, 6 Importantly, while current approaches mostly target the production of melanin by melanocytes, recent data demonstrated that keratinocytes, fibroblasts and endothelial cells secreted factors which stimulate melanogenesis and strongly impact the development and persistence of melasma lesions.2 Thus, beyond a hyperpigmentation, altered basal membrane,7, 8 elastosis9 and increased vascularization10, 11 are observed in melasma lesions. Fibroblast‐secreted factors, such as WIF112 or sFRP213 are differentially expressed in melasma skin and affect pigmentation. Endothelial cells produce endothelin 1 which consequently acts by stimulating the endothelin receptor B (EDNRB) at the surface of melanocytes, thus activating melanogenic pathways.14 These data have strong therapeutic significance as most of the current approaches focus only on melanocytes.
Despite a strong therapeutic demand, the treatment of melasma remains highly challenging with inconsistent results and almost constant relapses. Topical treatments are typically the first‐line therapies for melasma, among which hydroquinone (HQ) is the most widely used skin depigmenting product and is considered as the gold standard for the treatment of melasma.1 However, several studies have pointed to the long‐term risks of skin damage associated with HQ, thus compromising its use in skin depigmentation products. HQ has been associated with frequently irritant dermatitis and exogenous ochronosis.15, 16 Of note, ochronosis only occurs when HQ is used for long period of time and without photoprotection. Thus, Kligman's trio, combining HQ, topical tretinoin and topical steroids, remains the best therapeutic approach for melasma. However, maintenance therapy with safer products which can be used during the sunny seasons and over a long period of time is lacking. To this respect, several cosmetic formulations were proposed. Surprisingly, despite the increasing data emphasizing the importance of a global approach for treating melasma, most of the products keep on targeting only melanocytes. Moreover, while some compounds showed interesting in vitro or ex vivo results, most of them were not tested in prospective clinical trials. For the rare products that were tested clinically, almost none has been compared to 4% HQ, which remains the gold standard depigmenting agent. A cosmetic depigmenting cream has thus been developed combining compounds targeting several factors identified to be involved in melasma pathogenesis. The objective of the study was to compare the efficacy and tolerability of this skin‐lightening combination cosmetic product (CCP) with 4% HQ in the management of melasma.
Methods
Population
This study was conducted on 43 otherwise healthy women, aged between 18 and 60 years, with a skin phototype IV–V and melasma, as determined by clinical and Wood's light examination. Exclusion criteria included pregnant or nursing women, subjects with a cutaneous pathology on the study zone, individuals who have been excessively exposed to sunlight or UV‐rays within the previous month and subjects having used topical or systemic treatment during the previous 4 weeks liable to interfere with the assessment of the cutaneous acceptability of the study product. All subjects enrolled in another clinical trial during the study period were also excluded from the study.
Study design
This was a 12‐week double‐blind, randomized, parallel‐group clinical trial performed between February and June 2018 in a CRO in Mauritius. Subjects were randomly assigned to one of the two treatment groups (balanced ratio allocation). The randomization scheme was generated using the PLAN procedure in the SAS software (Version 9.4; SAS Institute Inc, Cary, NC, USA) and it used permuted block randomization with a block size of 4. The appropriate containers were labelled sequentially by the project manager as per the randomization list and kept under key until the assignment to subjects. No blinded party had access to the products or randomization list. The enrolment of the participants was performed by the dermatologist and the technician distributed the prerandomized containers in accordance with the generated list. Neither the dermatologist nor the subjects knew which products have been assigned.
In the intervention group (CCP group), subjects received the combination of cosmetic products: Neotone® serum once daily in the evening and Neotone® Radiance SPF 50+ (ISISPHARMA, Lyon, France) once daily in the morning. In the control group (HQ group), subjects received 4% HQ cream once daily in the evening and an SPF 50+ cream once daily in the morning. Importantly, the sunscreen provided in the HQ group was the same as Neotone® Radiance SPF 50+ without the depigmenting compounds. In addition, all the subjects were advised to use a sunscreen without any skin‐lightening components, twice daily.
All the subjects were instructed to apply the products from baseline for a period of 12 weeks and as recommended by the manufacturer on the face, neck and neckline, avoiding eye area. Evaluation of the two treatments modalities was performed at baseline, week 6 and week 12. No changes to methods have been made after trial commencement.
The study received the approbation of a private and independent ethics committee on 01/30/18. The study was conducted according to the Declaration of Helsinki and the International Committee on Harmonization Good Clinical Practice guidelines. Informed consent was obtained from all subjects prior to the study.
Tested products
Products used in the CPP group consisted in a combination of two hypopigmenting formulae:
Neotone® Serum (ISISPHARMA).
Neotone® Radiance (ISISPHARMA) SPF 50+ protection UVA 67.2/UVB 73.4.
Products used in the HQ group consisted in:
HQ serum: same formulation as Neotone® serum but the actives are replaced by 4% HQ as the active ingredient
SPF 50+ cream: same formulation as Neotone® Radiance, without the actives but with the same UV filters.
Both groups also used a SPF50+ invisible fluid (Uveblock Invisible Spf 50+ Light Fluid Cream; ISISPHARMA) twice a day during the rest of day.
Efficacy assessments
Efficacy of the studied products was evaluated at baseline, week 6 and week 12 based on the following parameters.
mMASI score
The main criteria of evaluation were the severity assessed using the modified Melasma Area Severity Index (mMASI) score.17
Facial imaging
Digital images of the face of all subjects were captured under the same visible light and UV light conditions with the Visia® (Canfield Imaging Systems, Fairfield, NJ, USA) complexion analysis system at the different time points to document changes in facial pigmentation.
Colorimetric measurement
The forehead and cheeks were assessed for pigment lightening using the CM‐2500d Spectrophotometer (Minolta, Tokyo, Japan) as a Chromameter. Skin colour measurements were assessed with the L*a*b* system. The Individual Typological Angle (ITA°), which defines the skin pigmentation degree of a subject, was calculated in the lesional and non‐lesional areas.
Assessment of cutaneous tolerability
The dermatologist evaluated the face of each subject at baseline and after product use (week 6 and week 12), for the following clinical signs: erythema, oedema, desquamation, dryness and roughness, using a 5‐point scale (none, very mild, mild, moderate and severe). In addition, the subjects assessed the cutaneous tolerability of the product with physical parameters including tightness, stinging, itching, warmth and burning, using the same 5‐point scale.
Self‐assessment questionnaire
Each subject of the study completed a self‐assessment questionnaire on week 6 and week 12 to evaluate the properties of the studied products, their global efficacy, as well as their future use.
No changes to trial outcomes have been made after trial beginning.
Statistical methods
Considering an expected mean MASI at baseline of 8 and a SD of 2 after 12 weeks of treatment,18 with a α risk of 0.05 and a power of 90%; in order to demonstrate an intragroup improvement of melasma assessed by MASI of at least 25% after 12 weeks, the number of patients in each group was 22. Analyses were performed for both the intention‐to‐treat (ITT) and the per protocol (PP) populations. Standard descriptive statistics including Number of values, Mean, Median, Standard deviation, Standard error of the mean, Minimum and Maximum value, Variation (∆) and Percentage Variation (∆%) were calculated for quantitative data. To assess the change from baseline on week 6 and week 12 in each group and to compare the products for each change from baseline (week 6‐baseline and week 12‐baseline), an unstructured variance‐covariance matrix (UN) was set based on a mixed ANOVA model for repeated measures. The mean changes and proportions were calculated along with their respective 95% confidence intervals (CIs). All differences were considered to be statistically significant at P < 0.05. All analyses were performed using Microsoft Excel (Redmond, WA, USA) and SAS 9.4 (Cary, NC, USA).
Results
Demographics and baseline characteristics
A total of 50 subjects were enrolled in the study, of which seven subjects were excluded because they did not meet the inclusion criteria. Of the 43 subjects who were randomized to the two treatment groups, 39 subjects completed the 12‐week study and were included in the PP analysis. Two patients were lost to follow‐up and two other patients were excluded from the PP analysis due to major protocol violations (Fig. 1). The subjects were all females, with an average age of 51 (±1) years. When assessed using Wood's lamp examination, all the patients had an increased contrast of their lesions and were classified as mostly epidermal. Most of the studied population was Indian (52%). Overall, 62% of the subjects had a skin phototype IV and 38% had a phototype of V (Table 1).
Figure 1.
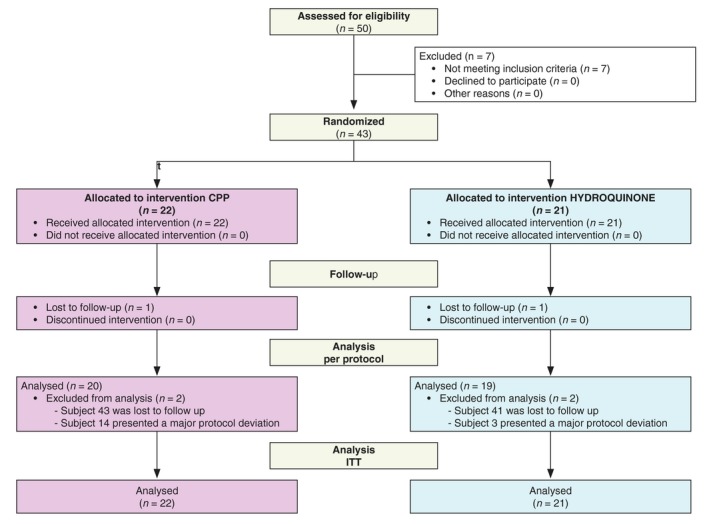
Enrolment flow diagram.
Table 1.
Subject demographics and baseline characteristics of the P.P. population
| Variable | Total | CPP group | HQ group |
|---|---|---|---|
| Age, years | |||
| Mean (SEM) | 51 (±1) | 52 (±2) | 50 (±2) |
| Range | 35; 60 | 37; 59 | 35; 60 |
| Sex, n (%) | |||
| Male | 0 | 0 | 0 |
| Female | 39 (100) | 20 (100) | 19 (100) |
| Ethnicity, n (%) | |||
| Caucasian | 0 | 0 | 0 |
| African | 8 (20) | 5 (25) | 3 (16) |
| Metis | 11 (28) | 4 (20) | 7 (37) |
| Indian | 20 (52) | 11 (55) | 9 (47) |
| Asian | 0 | 0 | 0 |
| Phototype, n (%) | |||
| IV | 24 (62) | 12 (60) | 12 (63) |
| V | 15 (38) | 8 (40) | 7 (37) |
| Skin type, n (%) | |||
| Normal | 8 (20) | 4 (20) | 4 (21) |
| Combination | 14 (36) | 10 (50) | 4 (21) |
| Dry | 3 (8) | 3 (15) | 0 |
| Greasy | 14 (36) | 3 (15) | 11 (58) |
n, number, SEM, standard error of the mean.
Efficacy assessments
mMASI score evaluation
Both CCP and HQ induced a statistically significant decrease in the mMASI score from baseline and over the study period with a confidence interval of 95% (CI 95%). In the CCP group, the mean mMASI score decreased by 43% on week 12 (P = 0.004), vs. 37% in the HQ group (P = 0.0005). No statistically significant difference was observed between CCP and HQ over the study period, in terms of change from baseline (Fig. 2). By week 12, 90% of the subjects in the CCP group had an improvement in pigmentation spots vs. 79% of the subjects in the HQ group (Table S2, Supporting Information). Similar results were obtained for the ITT population analysis, with a slight difference in magnitude (Table S3, Supporting Information).
Figure 2.
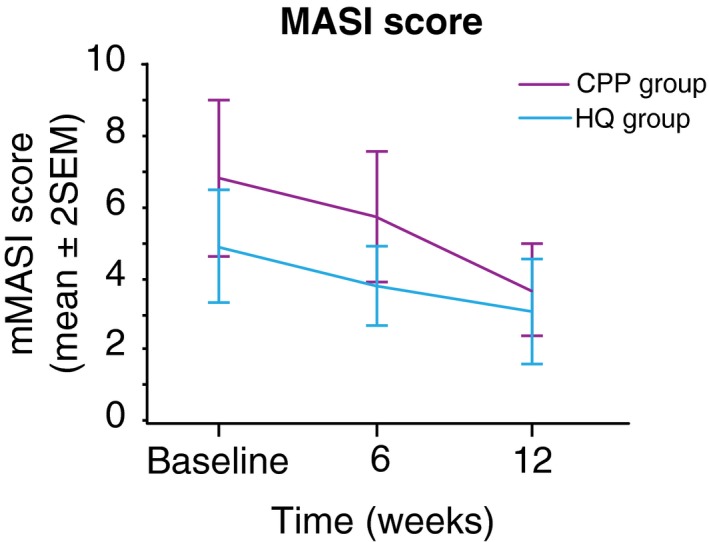
Mean modified Melasma Area Severity Index (mMASI) score over the study period. Improvement in the mMASI score in the cosmetic product combination and hydroquinone groups from baseline to week 12.
Colorimetric assessment
Skin pigmentation degree on melasma lesion, as determined by the ITA° parameter, significantly increased by 25% (P = 0.001) on week 6 (vs. 50% for HQ, P < 0.001) and 51% (P < 0.001) on week 12 with CCP (vs. 71% for HQ, P < 0.001). Comparison between lesional area to unaffected skin was also assessed using ITA parameter and showed a significant increase of 3% (P = 0.012) on week 6 (vs. 19% for HQ, P < 0.0005) and 42% (P < 0.0005) on week 12 with CCP (vs. 17% for HQ, P < 0.0005). However, no statistically significant difference was observed between CCP and HQ over the study period, in terms of change from baseline (Tables 2 and 3). Similar results were obtained for the ITT population analysis (Tables S4 and S5, Supporting Information).
Table 2.
Colorimetric changes of melasma from baseline to week 12 for the two studied groups (P.P. population)–Comparison before/after on lesional area
| ITA° | ||||||
|---|---|---|---|---|---|---|
| CCP group | HQ group | |||||
| Mean ±SEM | Change from baseline | ∆% on the mean | Mean ±SEM | Change from baseline | ∆% on the mean | |
| Baseline | −22.27 (±3.82) | – | −21.37 (±3.66) | – | ||
| Week 6 | −16.52 (±3.32) | 5.75 (±1.44) | +26% | −10.58 (±3.06) | 10.79 (±2.00) | +50% |
| Week 12 | −10.95 (±3.10) | 11.32 (±1.56) | +51% | −6.21 (±2.81) | 15.16 (±1.93) | +71% |
All changes were statistically significant P < 0.005. CCP, cosmetic product combination; HQ, hydroquinone; ITA°, Individual Typological Angle.
Table 3.
Colorimetric changes of melasma from baseline to week 12 for the two studied groups (P.P. population) – Comparison lesional/non‐lesional skin
| ITA° | ||||||
|---|---|---|---|---|---|---|
| CCP group | HQ group | |||||
| Lesional(mean ±SEM) | Non‐lesional(mean ±SEM) | ∆% on the meanlesional/non‐lesional | Lesional(mean ±SEM) | Non‐Lesional(mean ±SEM) | ∆% on the mean lesional/non‐lesional | |
| Baseline | −22.27 (±3.82) | −5.33 (±2.47) | −21.37 (±3.66) | −4.78 (±3.18) | ||
| Week 6 | −16.52 (±3.32) | −4.12 (±2.59) | +3% | −10.58 (±3.06) | −2.87 (±2.94) | +10% |
| Week 12 | −10.95 (±3.10) | −4.87 (±2.50) | +42% | −6.21 (±2.81) | −2.20 (±2.78) | +17% |
All changes were statistically significant P < 0.005. CCP, cosmetic product combination; HQ, hydroquinone; ITA°, Individual Typological Angle.
Cutaneous tolerability
Overall, CCP was very well tolerated at the cutaneous level over the 12 weeks. Using a 5‐point scale (none, very mild, mild, moderate and severe), in the CCP group, only one subject reported functional signs of mild burning sensation on the bilateral cheeks 2‐ to 3‐s after product application in the evening for 5 days in week 6 during <5 min. This was judged as not relevant by the investigator. In the HQ group, only one subject reported physical signs of mild acneiform lesions on the cheeks observed from week 6 to week 12. These lesions were also observed by the investigator during the study and retained as not relevant.
Self‐assessment questionnaires
Subjects appreciated both products for their general characteristics and properties, but also for their efficacy. In each group, they were 90% or more to found that the number and intensity of spots and hyperpigmentation areas were reduced, they noticed that their complexion was unified, brighter and radiant.
Discussion
In the present study, we evaluated the effect of a skin‐lightening combination cosmetic product (CCP) in comparison with the gold standard HQ at 4% in melasma treatment. Our findings demonstrated that CCP is effective in improving melasma severity when used together with daily sun protection. While HQ acts on melanogenesis by inhibiting tyrosinase leading to a defect in melanosomes formation,19, 20 CCP contains five complementary actives acting synergistically to restore the cutaneous homoeostasis and targeting some major pathways involved in melasma: calcium flux regulation, tyrosinase function, WNT pathway, melanosome transfer and endothelin 1 production.14, 21, 22 With the improved understanding of the several complex pathways involved in melanogenesis, newly developed skin‐lightening products should combine more than one agent, targeting multiple pathways and contributing factors.2 Two of the ingredients of the CCP treatment, namely licorice extract and niacinamide, are derived from botanical and natural extracts that proved to be safe and effective in the management of melasma.23 The diacetylboldine is also a natural extract that acts on melanogenesis by decreasing the calcium flux and the PKC pathway. CCP also contains a biomimetic peptide acting as DKK1 thus inhibiting the WNT pathway.21, 22 Additionally, CCP formulated with a biomimetic active that downregulates the production of endothelin 1 which is responsible for the hyperpigmentation induced by endothelial cells.14 Interestingly, ITA° measurements on lesional vs. non‐lesional skin showed a significant increase in both areas with HQ while, with CPP, only the lesional skin had a significant ITA° increase. As a result, the mean difference between lesional and non‐lesional skin improved by 42% with CCP product compared to only 17% in the HQ group (see example in Fig. 3). Such a difference could probably be explained by the fact that HQ is a potent inhibitor of melanogenesis that acts on all melanocytes. Conversely, CCP targets the altered pathways of melasma rather than only decreasing melanogenesis, and thus mostly regulates the lesional skin. Importantly, no adverse events related to CCP treatment were observed throughout the study period and the subjects clearly recognized the beneficial effects, assessed with both the cutaneous tolerability evaluation and the self‐assessment questionnaires. HQ treatment was not associated to any intolerability complaint during this study, although skin reactions with HQ were reported in 18% to 50% of the subjects in the literature, depending on the population.24 This could be explained by the short‐term use of the treatment—12 weeks vs. 12 months in published studies reporting safety issues. Longer studies are therefore required to assess the long‐term tolerance of CCP vs. HQ. In our study, 52% of the subjects were Indian. Management of melasma in darker skin populations is particularly challenging because they are more prone to pigmentary alteration.25 In India, 20–30% of 40–65 years old women present a facial melasma and current treatments remain unsatisfactory.26
Figure 3.
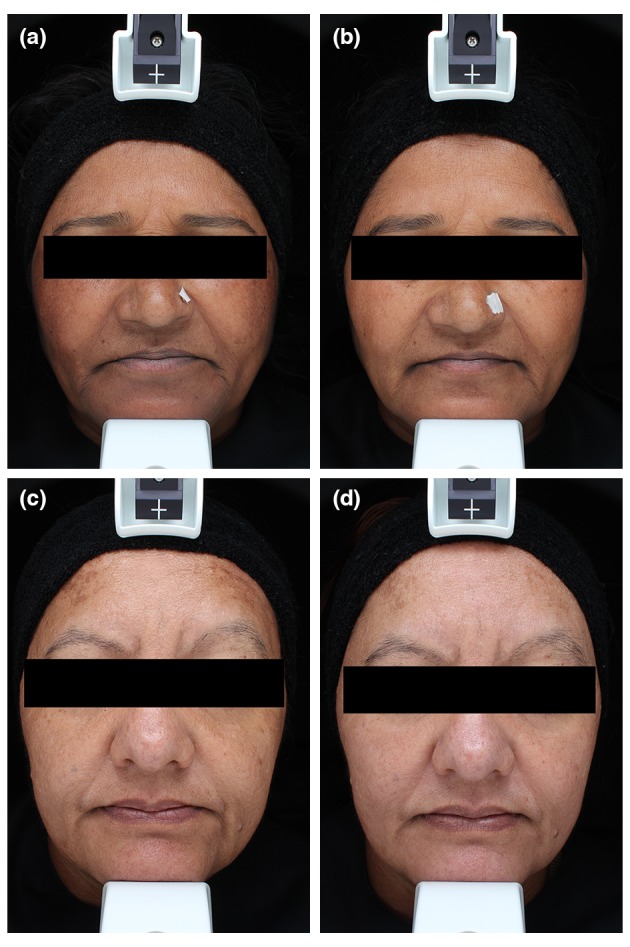
Clinical examples of treated patients. (a) Before CCP, cosmetic product combination treatment. (b) After 12 weeks of cosmetic product combination (CCP) treatment. Note that the overall colour the skin remains the same but that only the hyperpigmentation of the lesional areas has decreased. (c) Before hydroquinone (HQ) treatment. (d) After 12 weeks of 4% HQ treatment. Note that the melasma (i.e. mostly located on the forehead) has significantly faded but the entire pigmentation of the face has also decreased.
The present study was conducted in a single centre on a relative limited number of patients. It would be of interest to determine whether the long‐term use would further improve melasma lesions. Similarly, a longer application of the products would have been of interest as the long‐term use of 4% HQ has proved to be poorly tolerated. We also didn't assess the potential relapses of the lesions after discontinuation of the two treatments. However, the study was designed to assess the efficacy of this combination of cosmetic compounds targeting different pathways involved in melasma to the gold standard 4% HQ. Moreover, such a cosmetic approach is not designed for replacing the Kligman's trio that remains the gold standard care for the first months of treatment but should be positioned for mild cases or for maintenance therapy after the Trio in order to prevent the relapses. Despite the above‐mentioned limitations, we believe that the use the mMASI score, performed on standardized pictures with blinded evaluation, combined with objective colorimetric assessments, provide strong foundation to support the efficacy of this new combination of cosmetic products.
Our findings demonstrate that this new topical cosmetic combination is an effective and well tolerated treatment option for melasma and could be considered as a safe alternative to HQ. These results also support the importance of targeting the several pathways and cellular components involved in melasma to achieve optimal results.
Supporting information
Table S1. Mean mMASI scores of the two groups (P.P. population).
Table S2. Mean mMASI scores of the two groups (I.T.T. population).
Table S3. Colorimetric changes of melasma from baseline to week 12 for the two studied groups (I.T.T. population) – Comparison before/after on lesional area.
Table S4. Colorimetric changes of melasma from baseline to week 12 for the two studied groups (I.T.T. population) – Comparison lesional/non‐lesional skin.
Acknowledgement
The authors acknowledge the editorial assistance of Dr. Emna El Hammi, on behalf of Into‐Evidence (Tunisia).
Conflicts of interest
Elodie Bronzina, Amélie Clement are employees of Isispharma and Philippe Faure is employee of Dewavrin Group. Béatrice Marie is employee of Insight Research. Kim Tiam FOOK has received funding from Insight Research. Thierry Passeron has received honoraria from Isispharma.
Funding sources
This study was funded by Isispharma.
ISRCTN registry: 33310512
Supplementary files are store in Mendeley database: http://dx.doi.org/10.17632/jjbckrzp8k.2
The copyright line for this article was changed on 07 February 2020 after original online publication
References
- 1. Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australas J Dermatol 2015; 56: 151–163. [DOI] [PubMed] [Google Scholar]
- 2. Passeron T, Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res 2018; 31: 461–465. [DOI] [PubMed] [Google Scholar]
- 3. Mahmoud BH, Ruvolo E, Hexsel CL et al Impact of long‐wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol 2010; 130: 2092–2097. [DOI] [PubMed] [Google Scholar]
- 4. Duteil L, Cardot‐Leccia N, Queille‐Roussel C et al Differences in visible light‐induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res 2014; 27: 822–826. [DOI] [PubMed] [Google Scholar]
- 5. Boukari F, Jourdan E, Fontas E et al Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol 2015; 72: 189–190.e181. [DOI] [PubMed] [Google Scholar]
- 6. Regazzetti C, Sormani L, Debayle D et al Melanocytes sense blue light and regulate pigmentation through the Opsin3. J Invest Dermatol 2017; 138: 171–178. [DOI] [PubMed] [Google Scholar]
- 7. Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC Jr. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol 1981; 4: 698–710. [DOI] [PubMed] [Google Scholar]
- 8. Torres‐Alvarez B, Mesa‐Garza IG, Castanedo‐Cazares JP et al Histochemical and immunohistochemical study in melasma: evidence of damage in the basal membrane. Am J Dermatopathol 2011; 33: 291–295. [DOI] [PubMed] [Google Scholar]
- 9. Kang WH, Yoon KH, Lee ES et al Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol 2002; 146: 228–237. [DOI] [PubMed] [Google Scholar]
- 10. Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci 2007; 46: 111–116. [DOI] [PubMed] [Google Scholar]
- 11. Kang HY, Bahadoran P, Suzuki I et al In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol 2010; 19: e228–233. [DOI] [PubMed] [Google Scholar]
- 12. Kim JY, Lee TR, Lee AY. Reduced WIF‐1 expression stimulates skin hyperpigmentation in patients with melasma. J Invest Dermatol 2013; 133: 191–200. [DOI] [PubMed] [Google Scholar]
- 13. Kim M, Han JH, Kim JH, Park TJ, Kang HY. Secreted frizzled‐related protein 2 (sFRP2) functions as a melanogenic stimulator; the role of sFRP2 in UV‐induced hyperpigmentary disorders. J Invest Dermatol 2016; 136: 236–244. [DOI] [PubMed] [Google Scholar]
- 14. Regazzetti C, De Donatis GM, Ghorbel HH et al Endothelial cells promote pigmentation through endothelin receptor B activation. J Invest Dermatol 2015; 135: 3096–3104. [DOI] [PubMed] [Google Scholar]
- 15. Mishra SN, Dhurat RS, Deshpande DJ, Nayak CS. Diagnostic utility of dermatoscopy in hydroquinone‐induced exogenous ochronosis. Int J Dermatol 2013; 52: 413–417. [DOI] [PubMed] [Google Scholar]
- 16. Westerhof W, Kooyers TJ. Hydroquinone and its analogues in dermatology ‐ a potential health risk. J Cosmet Dermatol 2005; 4: 55–59. [DOI] [PubMed] [Google Scholar]
- 17. Pandya AG, Hynan LS, Bhore R et al Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol 2011; 64: 78–83, 83.e71–72. [DOI] [PubMed] [Google Scholar]
- 18. Lajevardi V, Ghayoumi A, Abedini R et al Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. topical hydroquinone 4% alone in melasma: a parallel‐group, assessor‐ and analyst‐blinded, randomized controlled trial with a short‐term follow‐up. J Cosmet Dermatol 2017; 16: 235–242. [DOI] [PubMed] [Google Scholar]
- 19. Ogbechie‐Godec OA, Elbuluk N. Melasma: an up‐to‐date comprehensive review. Dermatol Ther (Heidelb) 2017; 7: 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shankar K, Godse K, Aurangabadkar S et al Evidence‐based treatment for melasma: expert opinion and a review. Dermatol Ther (Heidelb) 2014; 4: 165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaguchi Y, Passeron T, Watabe H et al The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J Invest Dermatol 2007; 127: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi Y, Passeron T, Hoashi T et al Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/beta‐catenin signaling in keratinocytes. FASEB J 2008; 22: 1009–1020. [DOI] [PubMed] [Google Scholar]
- 23. Hollinger JC, Angra K, Halder RM. Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J Clin Aesthet Dermatol 2018; 11: 28–37. [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen FA, Bergfeld WF, Belsito DV et al Final amended safety assessment of hydroquinone as used in cosmetics. Int J Toxicol 2010; 29: 274S–287. [DOI] [PubMed] [Google Scholar]
- 25. Vashi NA, Wirya SA, Inyang M, Kundu RV. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol 2017; 18: 215–230. [DOI] [PubMed] [Google Scholar]
- 26. Hourblin V, Nouveau S, Roy N, de Lacharriere O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol 2014; 80: 395–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean mMASI scores of the two groups (P.P. population).
Table S2. Mean mMASI scores of the two groups (I.T.T. population).
Table S3. Colorimetric changes of melasma from baseline to week 12 for the two studied groups (I.T.T. population) – Comparison before/after on lesional area.
Table S4. Colorimetric changes of melasma from baseline to week 12 for the two studied groups (I.T.T. population) – Comparison lesional/non‐lesional skin.


