Abstract
In vivo and in vitro evidence has shown that mushrooms have the potential to prevent prostate cancer. However, the relationship between mushroom consumption and incident prostate cancer in humans has never been investigated. In the present study, a total of 36,499 men, aged 40–79 years, who participated in the Miyagi Cohort Study in 1990 and in the Ohsaki Cohort Study in 1994 were followed for a median of 13.2 years. Data on mushroom consumption (categorized as <1, 1–2 and ≥3 times/week) was collected using a validated food frequency questionnaire. Cox proportional hazards regression analysis was used to estimate multivariate hazard ratios (HRs) and 95% confidence intervals (CIs) for prostate cancer incidence. During 574,397 person‐years of follow‐up, 1,204 (3.3%) cases of prostate cancer were identified. Compared to participants with mushroom consumption <1 time/week, frequent mushroom intake was associated with a decreased risk of prostate cancer (1–2 times/week: HRs [95% CIs] = 0.92 [0.81, 1.05]; ≥3 times/week: HRs [95% CIs] = 0.83 [0.70, 0.98]; p‐trend = 0.023). This inverse relationship was especially obvious among participants aged ≥50 years and did not differ by clinical stage of cancer and intake of vegetables, fruit, meat and dairy products. The present study showed an inverse relationship between mushroom consumption and incident prostate cancer among middle‐aged and elderly Japanese men, suggesting that habitual mushroom intake might help to prevent prostate cancer.
Keywords: mushroom, prostate cancer, human, cohort study, Japan
Short abstract
What's new?
Mushrooms have long been used as a source of food and medicine in Asian cultures and are suspected of possessing anticancer properties. Whether the consumption of mushrooms can help prevent cancer, however, remains unknown. In the present study, among men who enrolled in the Miyagi and Ohsaki cohort studies in Japan in 1990 and 1994, respectively, long‐term follow‐up indicates that frequent mushroom consumption is associated with reduced prostate cancer risk. The effect was especially pronounced in men age 50 or older and in those with relatively low in fruit and vegetable intake and high in meat and dairy intake.
Introduction
According to Global Cancer Statistics 2018,1 prostate cancer ranks as the second‐most frequent cancer and the fifth leading cause of cancer death in men. Although there is no sure way to prevent prostate cancer, maintaining healthy eating habits (e.g., consuming more vegetables and fruits) has been suggested as an approach that might lower the risk of prostate cancer.2
Mushrooms have a long history of being consumed as food and used in Asian medicines. However, research on the health effects of mushrooms has only emerged and been developed in recent decades. To date, an increasing number of in vivo and in vitro studies have suggested the beneficial effects of mushrooms on health, such as antioxidation, anti‐inflammation, immunomodulation, etc.3
Additionally, mushrooms also reportedly have anticancer properties and effects against tumor development.4 In vivo and in vitro evidence has shown that mushrooms have the potential to prevent several kinds of cancers (e.g., those of the breast, bladder, colon and lung), including prostate cancer. Extracts of mushrooms such as Agaricus blazei Murill,5 Agaricus bisporus,6 Trametes versicolor,7 Cordyceps militaris 8 and Coprinus comatus 9 were suggested to inhibit cell proliferation in human prostate cancer cell lines and to restrict prostate tumorigenic progression from the hormone‐dependent to the hormone‐refractory state.
However, to the best of our knowledge, only one previous human study (a phase I trial) has investigated the biological activity of mushroom intake on prostate cancer recurrence.10 In that study, different doses of white button mushroom (i.e., Agaricus bisporus) powder were taken by biochemically recurrent prostate cancer patients. During the trial, decline in prostate‐specific antigen (PSA) levels, which is used as an indicator of disease recurrence, was observed in 36% of patients, suggesting that mushroom intake might modulate PSA levels in biochemically recurrent prostate cancer.
The above findings were taken to imply the probability that mushroom ingestion might also be promising in the prevention of prostate cancer among the general population. However, the relationship between mushroom consumption and the incident risk of prostate cancer has not been investigated as of yet. The aim of the present study was to estimate this relationship in middle‐aged and elderly Japanese men.
Materials and Methods
Study population
A pooled analysis of our two previous prospective cohort studies, the Miyagi Cohort Study and the Ohsaki Cohort Study, details of which were described elsewhere,11, 12, 13, 14 was carried out in the present study. Both of these cohorts included a focus on associations between lifestyle factors and cancer incidence. Briefly, two similar self‐administered questionnaires that incorporated items on lifestyle including, but not limited to, dietary habits, medical history, family history, physical activity, smoking status, drinking habits and education were distributed to individuals in each cohort. In the Miyagi Cohort Study, we distributed the self‐administered questionnaire to all 25,279 men aged 40–64 years who were residents in 14 municipalities of Miyagi Prefecture (Zao, Kawasaki, Marumori, Rifu, Ohira Village, Onoda, Sanbongi, Kogota, Uguisuzawa, Tome, Kahoku, Kitakami, Onagawa and Karakuwa), northeastern Japan, between June and August 1990. Among them, 22,836 valid responses were collected (response rate: 90.3%).11 For the Ohsaki Cohort Study, we distributed the self‐administered questionnaire to all 26,481 men aged 40–79 years living in the catchment area of the Ohsaki Public Health Center, a local government agency that provides preventive health services to the residents of 14 municipalities in Miyagi Prefecture (Furukawa, Nakaniida, Onoda, Miyazaki, Shikama, Matsuyama, Sanbongi, Kashimadai, Iwadeyama, Naruko, Wakuya, Tajiri, Kogota and Nango) between September and December 1994. We obtained 24,895 valid responses (response rate: 94.0%).12
The study protocol was reviewed and approved by the Institutional Review Board of Tohoku University Graduate School of Medicine (approval number: 2014‐1‐838 for Miyagi and 2014‐1‐839 for Ohsaki). We considered the return of self‐administered questionnaires signed by the participants to indicate their consent to participate in the study.
For the Miyagi Cohort Study, we excluded 414 participants who were identified as having cancer by the Miyagi Prefectural Cancer Registry before follow‐up (June 1, 1990), 1,062 who were aged <40 years at the start of follow‐up, and 43 who self‐reported a cancer history; for the Ohsaki Cohort Study, we excluded 322 participants who had moved out from the study area before the start of follow‐up (January 1, 1995), 1,353 who were identified as having cancer by the Miyagi Prefectural Cancer Registry or died before follow‐up, 2,473 who were common to the Miyagi Cohort Study and 211 who self‐reported a cancer history. Thus, the pooled data of 41,853 participants were obtained. We further excluded 5,354 participants with missing information on mushroom consumption. Finally, 36,499 cancer‐free men were used in our analyses (Fig. 1).
Figure 1.
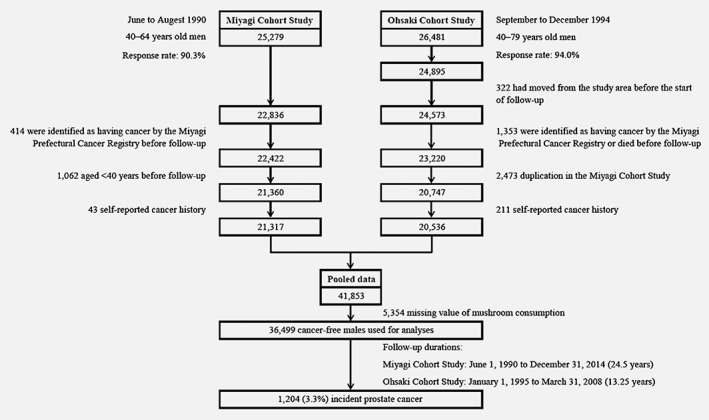
Flow chart of participants: a pooled analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study.
Consumption of mushrooms and other foods
In both the Miyagi Cohort Study and the Ohsaki Cohort Study, data on consumption of mushrooms and other food items were collected using the same valid Food Frequency Questionnaire (FFQ) in the baseline survey. The FFQ included 39 food items and several beverages. The term “meat” was defined as the sum of three food items (i.e., pork, beef, processed meat products), the term “vegetables” was defined as the sum of four food items (i.e., green vegetables, carrot and pumpkin, tomato, cabbage and lettuce), the term “fruit” referred to fruits and fresh juice, and the term “dairy products” referred to milk, yogurt and cheese. For the majority of food items, five frequency categories were applied (almost never, 1–2 times/month, 1–2 times/week, 3–4 times/week and almost every day). For consumption of coffee, five categories (almost never, sometimes, 1–2 cups/day, 3–4 cups/day and more than 5 cups/day) were provided.
We conducted a validation study of the FFQ in which 113 respondents provided four 3‐day food records within 1 year and subsequently responded to the FFQ.15 For men, the Spearman rank correlation coefficient between mushroom consumption according to the FFQ and that according to the food records was 0.32; the correlation between the consumptions measured by the two questionnaires administered 1 year apart was 0.30. The Spearman rank correlation coefficients above were applicable for both the Miyagi Cohort Study and the Ohsaki Cohort Study.
Based on the same validation study, we estimated the average portion sizes of meat, vegetables, fruit, dairy products and coffee. Consumption of each food item was calculated by multiplying the frequency by portion size.
Follow‐up (incident prostate cancer)
The follow‐up period for the Miyagi Cohort Study was from June 1, 1990 to December 31, 2014, and that for the Ohsaki Cohort Study was from January 1, 1995 to March 31, 2008. For both cohorts, we followed the incidence of prostate cancer and residential status using the records of the Miyagi Prefectural Cancer Registry, one of the oldest and most accurate population‐based cancer registries in Japan.16 Data on cancer case registrations were obtained from clinics and hospitals (inpatients and outpatients), radiology and pathology departments, autopay records, mass screening records and death certificates.
The endpoints were incidence of prostate cancer, which was defined according to the International Classification of Diseases for Oncology, 3rd edition (ICD‐O‐3), coded as C61.
During the follow‐up period, the number of individuals lost to follow‐up was 1,395 in the Miyagi Cohort Study (3.8% of the analyzed individuals) and 2,886 in the Ohsaki Cohort Study (7.9%). We counted the person‐years of follow‐up for each participant until the date of incident prostate cancer, death, emigration from study areas or the end of the study period, whichever occurred first.
Statistical analysis
The proportion of each mushroom consumption frequency category was 6.9, 36.8, 36.0, 15.7 and 4.6% for almost never, 1–2 times/month, 1–2 times/week, 3–4 times/week and almost every day, respectively. To ensure a relatively equal number of people in each group, mushroom consumption was categorized into three groups: <1 time/week (combination of almost never and 1–2 times/month), 1–2 times/week and ≥3 times/week (combination of 3–4 times/week and almost every day). Cox proportional hazards regression analysis was performed to estimate multivariate‐adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for incident prostate cancer according to these groups (<1 time/week was treated as a reference), using age as the time scale. The probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model. Other information collected at baseline was adjusted for as confounders, including family history of cancer (yes or no), education level (age at last school graduation; <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol consumption (never, former, current or missing), time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), consumption volume of the five groups of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
Next, the following four sensitivity analyses were conducted: (i) Considering that the chance of having prostate cancer rises rapidly after the age of 50 years, stratified analysis was conducted by age (<50 years and ≥50 years); (ii) Stratified analysis was also conducted by clinical stage (localized [T1–T2], and advanced [T3–T4] or metastatic [N+ and/or M+]), since, in Japan, screening of prostate cancer remains not a mandatory program and individuals need to undergo prostate cancer screening partially at their own expense.17 Hence, we assumed that participants’ characteristics might differ according to the detected clinical stage; (iii) Multivariate‐adjusted Cox regression model analysis was reconducted for vegetable and fruit consumption, meat consumption and dairy product consumption (<median and ≥median for each item), since, although no convincing evidence has been found, other food consumption might have confounded our results; and (iv) Competing‐risk regression analysis was also conducted using death as a competitive event. We modeled the cumulative incidence function by defining the subdistribution hazards, and then imposed the proportional hazards assumption on the subdistribution hazards.18
All p values were two‐sided and differences of p < 0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) software.
Data availability
The data that support the findings of our study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Results
During a total of 574,397 (median 13.2, interquartile range 11.5) person‐years of follow‐up, 1,204 (3.3%) cases of prostate cancer were documented. Table 1 shows the baseline characteristics of the participants according to the categories of mushroom consumption. Participants who consumed mushroom more frequently tended to be older, have a family history of cancer, spend more time walking and have a higher intake of meat, vegetables, fruit, dairy products and energy. They were also less likely to be current smokers.
Table 1.
Baseline characteristics of participants by mushroom consumption (n = 36,499)
| Characteristics | Mushroom consumption | p value1 | ||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| All participants | 15,958 | 13,124 | 7,417 | |
| Age, years, mean ± SD | 54.3 ± 9.9 | 55.3 ± 10.0 | 57.4 ± 9.9 | <0.001 |
| BMI, kg/m2, mean ± SD | 23.5 ± 2.9 | 23.5 ± 2.9 | 23.5 ± 2.9 | 0.830 |
| Family history of cancer, % | 26.2 | 28.7 | 30.1 | <0.001 |
| Current smoker, % | 60.3 | 57.9 | 54.3 | <0.001 |
| Current drinker, % | 75.1 | 75.6 | 74.1 | 0.001 |
| Time spent walking ≥1 hr/day, % | 44.9 | 46.9 | 49.8 | <0.001 |
| Educational level < 16 years, %2 | 89.1 | 88.0 | 88.8 | 0.021 |
| Food intake, mean ± SD | ||||
| Meat, g/day | 13.6 ± 11.2 | 16.0 ± 11.4 | 18.2 ± 14.5 | <0.001 |
| Vegetables, g/day | 41.1 ± 29.8 | 54.9 ± 31.2 | 74.2 ± 35.8 | <0.001 |
| Fruit, g/day | 59.0 ± 50.6 | 80.9 ± 52.8 | 103.1 ± 56.2 | <0.001 |
| Dairy products, g/day | 118.7 ± 95.3 | 134.4 ± 94.7 | 149.3 ± 96.9 | <0.001 |
| Coffee, g/day | 191.7 ± 216.2 | 193.8 ± 211.7 | 189.2 ± 220.3 | 0.368 |
| Energy, kcal/day | 1,875.3 ± 600.0 | 1,996.8 ± 584.9 | 2,100.9 ± 601.4 | <0.001 |
Obtained by using chi‐square test for variables of proportion and 1‐factor ANOVA for continuous variables (missing value excluded).
Age at last school graduation <16 years.
An inverse relationship between mushroom consumption and incident prostate cancer was found, as shown in Table 2. In comparison with participants with mushroom consumption of <1 time/week, multivariate‐adjusted HRs (95% CIs) for incident prostate cancer were 0.92 (0.81, 1.05) for those who consumed mushrooms 1–2 times/week and 0.83 (0.70, 0.98) for those who consumed mushrooms ≥3 times/week (p for trend = 0.023).
Table 2.
Relationships between mushroom consumption and incident prostate cancer (n = 36,499)
| Mushroom consumption | p‐trend1 | |||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| All participants | 15,958 | 13,124 | 7,417 | |
| Person‐years | 261,927 | 204,128 | 108,342 | |
| Incident prostate cancer (%) | 3.42 | 3.26 | 3.11 | |
| Incidence rate/1,000 person‐years | 2.08 | 2.10 | 2.13 | |
| Crude | 1.00 | 0.95 (0.83, 1.07) | 0.84 (0.72, 0.98) | 0.033 |
| Model 12 | 1.00 | 0.94 (0.82, 1.06) | 0.84 (0.72, 0.98) | 0.025 |
| Model 23 | 1.00 | 0.92 (0.81, 1.05) | 0.83 (0.70, 0.98) | 0.023 |
Analysis by the Cox proportional hazards model.
Probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model.
Model 1 was adjusted for family history of cancer (yes or no), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), education level (age at last school graduation: <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol drinking (never, former, current or missing) and time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing).
Model 2 was adjusted as for Model 1 plus five groups of consumption volume of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
In addition, stratified analyses were conducted for age, clinical stage of cancer and other food consumption. The relationship between mushroom consumption and prostate cancer persisted among participants aged ≥50 years (Table 3). The corresponding HRs (95% CIs) were 0.91 (0.79, 1.06) and 0.83 (0.70, 0.998; p for trend = 0.042). However, no significant relationship was observed among participants aged <50 years. When evaluated from the perspective of different clinical stages, although the difference was insignificant, the risk of incident prostate cancer remained lower for participants who consumed mushrooms frequently (Table 4). For localized prostate cancer, the multivariate‐adjusted HRs (95% CIs) were 0.88 (0.72, 1.09) for those who consumed mushrooms 1–2 times/week and 0.83 (0.64, 1.08) for those who consumed mushrooms ≥3 times/week; for advanced and metastatic prostate cancer, the corresponding values were 0.90 (0.69, 1.18) and 0.75 (0.53, 1.06), respectively. Table 5 shows the relationship between mushroom consumption and incident prostate cancer in terms of consumption of vegetables and fruits, meat and dairy products. Overall, the relationship did not change substantially; a higher mushroom consumption was related to a lower risk of incident prostate cancer, especially among participants who had a lower consumption of vegetables and fruits as well as those who had a higher consumption of meat and dairy products. For those who consumed mushrooms ≥3 times/week, multivariate‐adjusted HRs (95% CIs) were 0.69 (0.48, 0.98), 0.70 (0.54, 0.91) and 0.78 (0.61, 0.998), respectively.
Table 3.
Relationships between mushroom consumption and incident prostate cancer by age
| Mushroom consumption | p‐trend1 | |||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| Age < 50 years (n = 13,061) | 6,362 | 4,688 | 2,011 | |
| Person‐years | 118,313 | 83,873 | 34,722 | |
| Incident prostate cancer (%) | 1.70 | 1.69 | 1.59 | |
| Incidence rate/1,000 person‐years | 0.91 | 0.94 | 0.92 | |
| Hazard ratio (95% confidence interval) | ||||
| Crude | 1.00 | 1.08 (0.81, 1.45) | 1.05 (0.71, 1.56) | 0.690 |
| Model 12 | 1.00 | 1.09 (0.82, 1.46) | 1.08 (0.73, 1.61) | 0.587 |
| Model 23 | 1.00 | 1.01 (0.75, 1.36) | 0.98 (0.64, 1.49) | 0.951 |
| Age ≥ 50 years (n = 23,438) | 9,596 | 8,436 | 5,406 | |
| Person‐years | 143,614 | 120,256 | 73,620 | |
| Incident prostate cancer (%) | 4.55 | 4.14 | 3.68 | |
| Incidence rate/1,000 person‐years | 3.04 | 2.90 | 2.70 | |
| Hazard ratio (95% confidence interval) | ||||
| Crude | 1.00 | 0.93 (0.81, 1.07) | 0.84 (0.71, 0.99) | 0.041 |
| Model 12 | 1.00 | 0.91 (0.79, 1.05) | 0.82 (0.69, 0.97) | 0.016 |
| Model 23 | 1.00 | 0.91 (0.79, 1.06) | 0.83 (0.70, 0.998) | 0.042 |
Analysis by the Cox proportional hazards model.
Probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model.
Model 1 was adjusted for family history of cancer (yes or no), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), education level (age at last school graduation: <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol drinking (never, former, current or missing) and time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing).
Model 2 was adjusted as for Model 1 plus five groups of consumption volume of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
Table 4.
Relationships between mushroom consumption and incident prostate cancer by clinical stage (n = 36,499)
| Mushroom consumption | p‐trend1 | |||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| All participants | 15,958 | 13,124 | 7,417 | |
| Person‐years | 261,927 | 204,128 | 108,342 | |
| Localized prostate cancer | ||||
| Incident prostate cancer (%) | 1.36 | 1.31 | 1.25 | |
| Incidence rate/1,000 person‐years | 0.83 | 0.84 | 0.86 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.88 (0.72, 1.09) | 0.83 (0.64, 1.08) | 0.130 |
| Advanced and metastatic prostate cancer | ||||
| Incident prostate cancer (%) | 0.86 | 0.76 | 0.69 | |
| Incidence rate/1,000 person‐years | 0.52 | 0.49 | 0.47 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.90 (0.69, 1.18) | 0.75 (0.53, 1.06) | 0.103 |
Analysis by the Cox proportional hazards model.
Probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model.
Adjusted for family history of cancer (yes or no), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), education level (age at last school graduation: <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol drinking (never, former, current or missing), time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing), and five groups of consumption volume of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
Table 5.
Relationships between mushroom consumption and incident prostate cancer by other food consumption (participants whose food consumption were unavailable were excluded for each item, respectively)
| Mushroom consumption | p‐trend1 | |||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| Vegetables and fruit consumption < median (n = 15,655) | 8,873 | 5,182 | 1,600 | |
| Person‐years | 147,979 | 81,323 | 23,588 | |
| Incident prostate cancer (%) | 3.04 | 3.22 | 2.25 | |
| Incidence rate/1,000 person‐years | 1.82 | 2.05 | 1.53 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 1.04 (0.85, 1.27) | 0.69 (0.48, 0.98) | 0.166 |
| Vegetables and fruit consumption ≥ median (n = 15,636) | 4,580 | 6,235 | 4,821 | |
| Person‐years | 75,796 | 97,978 | 71,154 | |
| Incident prostate cancer (%) | 4.00 | 3.32 | 3.51 | |
| Incidence rate/1,000 person‐years | 2.41 | 2.11 | 2.38 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.87 (0.71, 1.06) | 0.90 (0.72, 1.11) | 0.309 |
| Meat consumption < median (n = 14,257) | 7,006 | 4,763 | 2,488 | |
| Person‐years | 114,624 | 72,898 | 35,226 | |
| Incident prostate cancer (%) | 3.20 | 3.15 | 3.22 | |
| Incidence rate/1,000 person‐years | 1.95 | 2.06 | 2.27 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.93 (0.75, 1.15) | 0.92 (0.70, 1.22) | 0.495 |
| Meat consumption ≥ median (n = 14,876) | 5,431 | 5,966 | 3,409 | |
| Person‐years | 75,796 | 97,978 | 71,154 | |
| Incident prostate cancer (%) | 3.44 | 3.30 | 2.85 | |
| Incidence rate/1,000 person‐years | 2.01 | 2.04 | 1.86 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.92 (0.75, 1.13) | 0.70 (0.54, 0.91) | 0.010 |
| Dairy products consumption < median (n = 14,876) | 7,137 | 5,234 | 2,505 | |
| Person‐years | 147,979 | 81,323 | 23,588 | |
| Incident prostate cancer (%) | 3.01 | 3.19 | 2.87 | |
| Incidence rate/1,000 person‐years | 1.80 | 2.01 | 1.88 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.98 (0.80, 1.21) | 0.80 (0.60, 1.06) | 0.167 |
| Dairy products consumption ≥ median (n = 14,978) | 5,775 | 5,661 | 3,542 | |
| Person‐years | 75,796 | 97,978 | 71,154 | |
| Incident prostate cancer (%) | 3.76 | 3.25 | 3.08 | |
| Incidence rate/1,000 person‐years | 2.23 | 2.05 | 2.10 | |
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.87 (0.71, 1.07) | 0.78 (0.61, 0.998) | 0.041 |
Analysis by the Cox proportional hazards model.
Probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model.
Adjusted for family history of cancer (yes or no), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), education level (age at last school graduation: <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol drinking (never, former, current or missing), time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing) and five groups of consumption volume of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
The results of competing‐risk regression analysis are shown in Table 6. Again, the inverse relationship between mushroom consumption and incident prostate cancer persisted.
Table 6.
Relationships between mushroom consumption and incident prostate cancer when death was treated as competing‐risk event (n = 36,499)
| Mushroom consumption | p‐trend1 | |||
|---|---|---|---|---|
| <1 time/week | 1–2 times/week | ≥3 times/week | ||
| All participants | 15,958 | 13,124 | 7,417 | |
| Event of interest (prostate cancer) | 1,204 | |||
| Incident prostate cancer (%) | 3.42 | 3.26 | 3.11 | |
| Incidence rate/1,000 person‐years | 2.08 | 2.1 | 2.13 | |
| Competing event (death) | 8,884 | |||
| Censored value | 26,411 | |||
| Hazard ratio (95% confidence interval)2 | 1.00 | 0.92 (0.81, 1.05) | 0.83 (0.70, 0.97) | 0.022 |
Analysis by the Cox proportional hazards model.
Probability value for trend was computed by entering the categories as a continuous term (score variable: 1, 2 or 3) in the Cox model.
Adjusted for family history of cancer (yes or no), BMI (<18.5, 18.5–25, 25–30, ≥30 or missing), education level (age at last school graduation: <19 years, ≥19 years or missing), smoking status (never, former, current or missing), alcohol drinking (never, former, current or missing), time spent walking (<0.5 hr/day, 0.5–1 hr/day, ≥1 hr/day or missing) and five groups of consumption volume of meat, vegetables, fruit, dairy products, coffee and energy intake (quartile categories or missing).
Discussion
In the present prospective cohort study that included 36,499 middle‐aged and elderly Japanese men with a median follow‐up period of 13 years, we observed an inverse relationship between mushroom consumption and incident prostate cancer. To the best of our knowledge, this is the first cohort study indicating the prostate cancer‐preventive potential of mushrooms at a population level.
Considering that the risk of prostate cancer increases rapidly after the age of 5019 (since 2010, the Japanese Urological Association has recommended PSA screening for all men over the age of 5020), we conducted age‐stratified analysis (<50 years and ≥50 years). The analysis indicated that there was no relationship between mushroom consumption and incident prostate cancer among men aged <50 years. This could be due to the fact that the incidence rate of prostate cancer in this subgroup was too low (<1 event per 1,000 person‐years in the present study) to provide sufficient statistical power. Furthermore, when an older age (66 years, which is the average age at the time of prostate cancer diagnosis21) was used as the cutoff point in our stratified analysis, a trend of an inverse relationship between mushroom consumption and incident prostate cancer was observed (data not shown).
Results of stratified analysis according to tumor stage also suggested a beneficial effect of habitual mushroom consumption on prevention of prostate cancer regardless of clinical stage (i.e., localized or advanced and metastatic). In Japan, prostate cancer screening is voluntary. Therefore, in our main results, unless affected by a confounding factor that we did not consider, the inverse relationship might have been due to that participant who ate fewer mushrooms also tended to be screened. However, stratified analysis by clinical stage suggested that even considering early stage (i.e., localized) disease alone, participants who consumed mushrooms more frequently still had a lower risk of incident prostate cancer, suggesting that detection bias was not likely to have affected our results.
When stratified by food items, more frequent mushroom intake was related to a lower risk of incident prostate cancer, regardless of consumption levels of vegetables and fruits, meat and dairy products. Although the baseline characteristics indicated that participants who had higher mushroom consumption also had a higher consumption of meat, vegetables, fruit, dairy products and energy intake, the stratified findings (with adjustment for energy intake) suggested that differences in consumption of these foods and energy intake might not affect the relationship observed in the present study.
Additionally, since the participants were middle‐aged and elderly men, death would be a competing‐risk event in our analyses. However, even taking death into account, our results did not change substantially. Thus, our findings are not likely to be attributable to competing risk.
The mechanism of the beneficial effects of mushrooms on prostate cancer remains uncertain. Some biological properties of culinary mushrooms related to prevention of prostate cancer have been reported by in vivo and in vitro studies. One of these components is l‐ergothioneine, an antioxidant, which might mitigate oxidative stress/damage.22 l‐Ergothioneine is reportedly present in large amounts in shiitake mushrooms, oyster mushrooms, maitake mushrooms and king oyster mushrooms.23 These mushrooms also contain considerable amounts of the antioxidant glutathione.24 Additionally, in Phase 1 clinical trial, white button mushrooms also appeared to have antiprostate cancer activity through immune modulation,10 although the active compounds are still unclear. Therefore, we assume these bioactive components might play a role in the observed inverse relationship between mushroom consumption and incident prostate cancer, because shiitake, oyster mushrooms, maitake and white button mushrooms are commonly consumed in the study areas.
Several limitations of our study should be noted: (i) since mushroom consumption was only assessed once at baseline, mushroom intake might have changed during follow‐up; (ii) since information on mushroom species was not collected, it is difficult to know which specific mushroom(s) contributed to our findings; (iii) dietary supplements (e.g., vitamins25, 26, 27) were not considered due to data unavailability in the present study; and (iv) the exact consumption volumes of mushroom for all participants were not obtained in the present study.
In conclusion, the present prospective cohort study with long‐term follow‐up observed an inverse relationship between mushroom consumption and incident prostate cancer among 36,499 middle‐aged and elderly Japanese men. This finding suggests that habitual mushroom intake might help to reduce prostate cancer risk. Further studies in other populations and settings are required to confirm this relationship.
Acknowledgements
We would like to thank Yoshiko Nakata and Yuko Miyoshi for their technical assistance. Permission for publication has been received from all people named in the acknowledgments. Our study was supported by the NARO Bio‐oriented Technology Research Advancement Institution (advanced integration research for agriculture and interdisciplinary fields; recipient: Ichiro Tsuji) and the National Cancer Center Research and Development Fund (30‐A‐15; recipient: Yumi Sugawara). None of the funding organizations or sponsors was involved in the study design; in collection, analysis or interpretation of data; in writing the report; and in making decision to submit the article for publication.
Conflict of interest: Shu Zhang, Yumi Sugawara, Shiuan Chen, Robert B. Beelman, Tsuyoshi Tsuduki, Yasutake Tomata, Sanae Matsuyama, Ichiro Tsuji, no conflict of interest.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kushi LH, Doyle C, McCullough M, et al. American cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 3. Chatterjee S, Sarma MK, Deb U, et al. Mushrooms: from nutrition to mycoremediation. Environ Sci Pollut Res Int 2017;24:19480–93. [DOI] [PubMed] [Google Scholar]
- 4. Patel S, Goyal A. Recent developments in mushrooms as anti‐cancer therapeutics: a review. 3 Biotech 2012;2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu CH, Kan SF, Shu CH, et al. Inhibitory mechanisms of Agaricus blazei Murill on the growth of prostate cancer in vitro and in vivo. J Nutr Biochem 2009;20:753–64. [DOI] [PubMed] [Google Scholar]
- 6. Adams LS, Phung S, Wu X, et al. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr Cancer 2008;60:744–56. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh TC, Wu JM. Cell growth and gene modulatory activities of Yunzhi (Windsor Wunxi) from mushroom Trametes versicolor in androgen‐dependent and androgen‐insensitive human prostate cancer cells. Int J Oncol 2001;18:81–8. [DOI] [PubMed] [Google Scholar]
- 8. Rao YK, Fang SH, Wu WS, et al. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator's production and human cancer cell proliferation. J Ethnopharmacol 2010;131:363–7. [DOI] [PubMed] [Google Scholar]
- 9. Zaidman BZ, Wasser SP, Nevo E, et al. Coprinus comatus and Ganoderma lucidum interfere with androgen receptor function in LNCaP prostate cancer cells. Mol Biol Rep 2008;35:107–17. [DOI] [PubMed] [Google Scholar]
- 10. Twardowski P, Kanaya N, Frankel P, et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: roles of cytokines and myeloid‐derived suppressor cells for Agaricus bisporus‐induced prostate‐specific antigen responses. Cancer 2015;121:2942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukao A, Tsubono Y, Komatsu S, et al. A cohort study on the relation of lifestyle, personality and biologic markers to cancer in Miyagi, Japan : study design, response rate and profiles of the cohort subjects. J Epidemiol 1995;5:153–7. [Google Scholar]
- 12. Tsuji I, Nishino Y, Ohkubo T, et al. A prospective cohort study on National Health Insurance Beneficiaries in Ohsaki, Miyagi prefecture, Japan: study design, profiles of the subjects and medical cost during the first year. J Epidemiol 1998;8:258–63. [DOI] [PubMed] [Google Scholar]
- 13. Sugiyama K, Sugawara Y, Tomata Y, et al. The association between coffee consumption and bladder cancer incidence in a pooled analysis of the Miyagi cohort study and Ohsaki cohort study. Eur J Cancer Prev 2017;26:125–30. [DOI] [PubMed] [Google Scholar]
- 14. Wakamatsu M, Sugawara Y, Zhang S, et al. Weight change since age 20 and incident risk of obesity‐related cancer in Japan: a pooled analysis of the Miyagi cohort study and the Ohsaki cohort study. Int J Cancer 2019;144:967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogawa K, Tsubono Y, Nishino Y, et al. Validation of a food‐frequency questionnaire for cohort studies in rural Japan. Public Health Nutr 2003;6:147–57. [DOI] [PubMed] [Google Scholar]
- 16. Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, vol. X Lyon, France: IARC Scientific Publication, 2014. 1365. [Google Scholar]
- 17. Kitagawa Y, Namiki M. Prostate‐specific antigen‐based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J Androl 2015;17:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. So Y, Lin G, Johnston G. Using the PHREG procedure to analyze competing‐risks data Presented at the SAS Global Forum, Washington, DC, March 23–26, 2014. Available at: http://support.sas.com/rnd/app/stat/papers/2014/competingrisk2014.pdf 2014.
- 19. Leitzmann MF, Rohrmann S. Risk factors for the onset of prostatic cancer: age, location, and behavioral correlates. Clin Epidemiol 2012;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito K, Naito S, Kakehi Y, et al. Updated Japanese Urological Association guidelines on prostate‐specific antigen‐based screening for prostate cancer in 2010. Int J Urol 2010;17:830–8. [DOI] [PubMed] [Google Scholar]
- 21. American Cancer Society . Cancer Facts & Figures 2019, vol. 2019. Atlanta, GA: American Cancer Society, 2019. [Google Scholar]
- 22. Paul BD, Snyder SH. The unusual amino acid L‐ergothioneine is a physiologic cytoprotectant. Cell Death Differ 2010;17:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubost NJ, Beelman RB, Peterson DG, et al. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography mass spectroscopy. Int J Med Mushrooms 2006;8:215–22. [Google Scholar]
- 24. Kalaras MD, Richie JP, Calcagnotto A, et al. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem 2017;233:429–33. [DOI] [PubMed] [Google Scholar]
- 25. Lawson KA, Wright ME, Subar A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health‐AARP diet and health study. J Natl Cancer Inst 2007;99:754–64. [DOI] [PubMed] [Google Scholar]
- 26. Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha‐tocopherol and beta‐carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 1998;90:440–6. [DOI] [PubMed] [Google Scholar]
- 27. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of our study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


